Abstract
Asymptomatic bradyarrhythmias involving sinus node dysfunction and atrioventicular blocks are frequently noted in clinical practice. Its prevalence is expected to rise as devices that are developed for monitoring cardiac rhythm for longer duration become more widely available. Episodes of bradyarrhythmia that are asymptomatic are considered to have a benign course compared with those that cause symptoms and do not necessitate further treatment. However, in certain cases, they can be a harbinger of future symptoms or cardiac manifestations of systemic diseases. The evaluation and risk stratification of individuals presenting with asymptomatic bradyarrhythmias is important not only for preventing implantation of unnecessary permanent pacing devices but also for reducing significant morbidity by implementing proper treatment as required. In this article, we will review the current evidence on the pathophysiology, diagnosis, evaluation and management of patients with asymptomatic bradyarrhythmias.
Keywords: Asymptomatic, atrioventricular block, bradyarrhythmias, cardiac pacing, sinus node dysfunction, ECG
1. INTRODUCTION
Asymptomatic bradyarrhythmias are frequently noted in clinical practice in individuals who are undergoing clinical evaluation or diagnostic workup for another cardiac or non- cardiac disorder. Recently, there has been an increase in the number of medical devices and accessories available for long-term cardiac rhythm monitoring. Holter monitoring has long been considered as the only strategy in ambulatory rhythm monitoring for patients with suspected cardiac arrhythmias and infrequent symptoms. Despite its clinical utility, limitations in diagnostic yield due to allowing only short time of monitoring motivated the development of devices that enabled cardiac rhythm monitoring remotely and for longer durations. These devices include external loop recorders, electrocardiographic (ECG) patches, implantable loop recorders, wearable consumer electronics and smartphone applications. Findings from several studies have indicated that the use of long-term cardiac rhythm monitoring devices increases the likelihood of diagnosing cardiac arrhythmias correlating with symptoms in patients with unexplained syncope [1-3]. However, these devices also have the potential to increase the diagnostic yield of asymptomatic bradyarrhythmias. Even more challenging issue is the quick adoptation of wearable technologies such as smart-watches for rhythm monitoring that already began changing the healthcare system from physician-directed into consumer- directed.
The European and American guidelines recommend permanent pacemaker (PM) implantation for symptomatic bradyarrhythmia with very few exceptions [4, 5]; however, the recommendations for the management of asymptomatic bradyarrhythmias are not straightforward. The evaluation and risk stratification of individuals presenting with asymptomatic bradyarrhythmias is important in order to prevent unnecessary implantation of pacing devices, which can carry considerable procedural and long-term complications [6, 7]. In this review, we will summarize the current evidence on the diagnosis, evaluation and management of asymptomatic bradyarrhythmias associated with sinus node dysfunction (SND) and atrioventricular block (AVB).
2. EVALUATION AND DIAGNOSIS OF ASYMPTOMATIC BRADYARRHYTHMIAS
The main physiological effect of bradyarrhythmias is the decrease in cardiac output which is determined by the left ventricular stroke volume multiplied by the heart rate. Patients with bradyarrhythmias can be asymptomatic if changes in stroke volume compensate for the decrease in heart rate. The symptoms associated with bradyarrhythmias are mostly nonspecific and include syncope, fatigue, reduced exercise capacity, dizziness, dyspnea; subtle symptoms include irritability, lassitude, inability to concentrate and forgetfulness (Table 1). Findings of asymptomatic bradyarrhythmias during routine evaluation or diagnostic work-up are not infrequent and are usually identified on ECG recordings and ambulatory holter recordings obtained for other cardiac and noncardiac indications.
Table 1.
Common Symptoms Associated with Bradyarrhythmias.
| Syncope | Dyspnea | Inability to Concentrate |
|---|---|---|
| Pre-syncope | Angina | Forgetfulness |
| Lightheadness | Fatigue | Irritability |
| Vertigo | Exertion intolerance | Lassitude |
| Dizziness | Weakness | - |
In most instances, asymptomatic bradyarrhythmias are considered to have a benign course compared to those causing symptoms, and not require any treatment. However, there are several exceptions and clinicians should recognize the asymptomatic cases that necessitate detailed evaluation or treatment, if required. It should be kept in mind that death related to AVB does not always depend on heart failure but also on sudden cardiac death associated with prolonged asystole or bradycardia triggered ventricular tachycardia/fibrillation.
Factors that are considered to determine whether bradyarrhythmias cause symptoms or not are not well understood and depend on several conditions, including individual patient characteristics and hemodynamics, presence of any cardiovascular disease and autonomic tonus. For instance, while first degree AVB does not cause any symptoms and can be frequently noted in well-trained athletes, it can cause or aggravate heart failure symptoms in an individual who has concomitant cardiovascular disease impairing left ventricular filling such as left ventricular hypertrophy or heart failure with preserved ejection fraction [5]. Likewise, sinus bradycardia can be tolerated by young patients without causing symptoms, but it may deteriorate hemodynamics in an old patient with anemia. Thus, it is crucial to obtain a detailed medical history and to perform systematic physical examination in a patient with bradyarrhythmia irrespective of symptom status.
Physicians should not solely focus on cardiac symptoms, as bradyarrhythmias can be a manifestation of a more systemic disease, such as sarcoidosis and Lyme carditis. Cardiac conduction disturbances in young patients with extracardiac symptoms involving multi-organs should raise suspicion for cardiac sarcoidosis [8]. Accordingly, Lyme carditis should be investigated in cases of AVB in young otherwise healthy males who have a history of spending time in an endemic area and constitutional symptoms including fever, fatigue and malaise [9, 10]. In patients presenting with asymptomatic, intermittent nocturnal bradyarrhythmias should prompt screening for sleep apnea, as high proportion of patient with sleep apnea develops bradyarrhythmia and conduction disturbances particularly during apneic episodes [11, 12]. It is important to recognize these conditions since treating the underlying disease with immunosuppressive medications, antibiotics or continuous positive airway pressure is likely to resolve bradyarrhythmias and conduction disturbances, precluding unnecessary PM implantation.
During a routine evaluation of patients with asymptomatic bradyarrhythmias, clinicians should be careful to differentiate those who are truly asymptomatic from individuals who have yet to notice subtle symptoms or restrain themselves to reduce the burden of symptoms. For further evaluation, functional test including treadmill or stationary bicycle exercise tests can be performed to unmask the symptoms. In addition, detailed history of medications that the patient has used should be obtained, since many agents for treating cardiovascular and non-cardiovascular conditions may produce bradyarrhythmia. Laboratory tests should include electrolytes and thyroid function tests.
3. DEFINITION AND PATHOPHYSIOLOGY OF SINUS NODE DYSFUNCTION
The term “sinus node dysfunction” was first coined in 1967 and it was initially used to illustrate the delayed return of sinoatrial node activity in patients following electrical cardioversion [13, 14]. Currently, SND is a heterogeneous clinical entity that is commonly employed to describe the inability of the sinus node and surrounding atrial myocardium to generate a heart rate that meets the physiologic needs of an individual. Popular simple thresholds for the definition of SND include a sinus rate of < 50 beats per minute (bpm) or a sinus pause of >3 seconds [5]. However, these criteria alone are not sufficient for the diagnosis of SND. For example, these parameters are prevalent in athletes but warrant neither exercise restriction nor therapeutic intervention if secondary to physical conditioning [15]. Rather, the more nuanced concept of chronotropic incompetence, often defined as the failure to attain 80% of the expected heart rate reserve during exercise, better captures the inability of the heart to modulate rate with increased activity or demand. SND may be the result of several pathological mechanisms (Table 2). Rather than a single entity, SND is better conceptualized as a spectrum of disorders, where varied pathophysiologic mechanisms lead to a very similar disease phenotype. The most common cause of SND is progressive fibrosis of the sinus node and atrial myocardium, which ultimately hinders impulse formation and propagation [16, 17]. Connective tissue surrounds and electrically insulates the specialized pacemaker cardiomyocytes of the sinus node; the age-dependent increase in the collagen content of the heart is associated with slower heart rate and sinoatrial conduction times [18]. Histological studies further support the association between fibrosis and SND [17]. Besides structural remodeling from fibrosis, age-related molecular remodeling also contributes to the pathophysiology of SND by changes in the expression of ion channels and clock genes in the sinus node [19]. Moreover, SND is associated with diseases causing atrial myopathies — cardiomyocyte-predominant, fibrosis-predominant, a combination of both, or noncollagen infiltration [20]. Secondary, potentially reversible, or treatable causes of SND are presented in (Table 3). In terms of acute myocardial ischemia or infarction, the sinus node is perfused by branches of the right coronary artery (55%) or the left circumflex artery (45%). Thirty-three percent of SND cases are estimated to be related to coronary artery disease [21]. In addition, approximately 5 percent of patients with myocardial infarction (usually inferior) have SND that is reversible [22].
Table 2.
Definition of SND.
| Sinus node arrest | No Evidence of Sinus Node Depolarization |
|---|---|
| Sinoatrial exit block | Blocked conduction between the sinus node and adjacent atrial tissue |
| Ectopic atrial bradycardia | Atrial pacemaker other than the sinus node with a rate <50 beats per minute |
| Tachycardia-bradycardia syndrome | Pathophysiological mechanisms responsible for SND also modify the atrial myocardium to generate arrhythmogenic substrates |
| Isorhythmic dissociation | Atrial depolarization is slower than ventricular depolarization |
Table 3.
Common Secondary Causes of SND.
| Cardiac | Acute Myocardial Ischemia or Infarction |
|---|---|
| - | Atrial fibrillation |
| - | Cardiac surgery: Valve replacement, maze procedure, coronary artery bypass graft |
| - | Heart transplant: Acute rejection, chronic rejection, remodeling |
| Physiologic derangements | Electrolyte abnormalities: Hyperkalemia, hypokalemia, hypoglycemia, hypocalcemia |
| - | Hypothermia: Environmental or Therapeutic (e.g. post- cardiac arrest cooling) |
| - | Hypoxemia, Hypercarbia, Acidosis: Including sleep apnea and respiratory insufficiency |
| - | Hypovolemic Shock |
| - | Hypervagotonia |
| - | Hypothyroidism |
| Infection | Lyme, legionella, psittacosis, typhoid fever, typhus, listeria (29), malaria, leptospirosis, dengue fever, viral hemorrhagic fevers, Guillain-Barre |
| Medications or toxins | Cardiac: Antiarrhythmic medication (class I and III), β-blockers, calcium channel blockers, digoxin |
| - | Acetylcholinesterase inhibitors: Used in the treatment of Alzheimer's disease, such as donepezil and rivastigmine |
| - | Parasympathomimetic and sympatholytic agents: methyldopa, clonidine, cimetidine, lithium, ivabradine |
| - | Other example medications: Risperidone, cisplatin, interferon |
| - | Toxins: Toluene, organophosphates, tetrodotoxin, cocaine |
Abbreviation: SND: Sinus node dysfunction
4. TREATMENT OF ASYMPTOMATIC SINUS NODE DYSFUNCTION
The treatment for bradycardia depends on the type of electrical conduction disturbance, the severity of the symptoms and the cause of the decreased heart rate. If there are no symptoms, a treatment regimen may not be warranted. However, secondary causes of bradycardia should be carefully evaluated. Compared to the general population, asymptomatic bradycardia is frequently found in trained athletes (Fig. 1A) [23]. The European Heart Rhythm Association (EHRA) recommends that an asymptomatic patient with a resting heart rate above the lower limit of 30 bpm may be considered normal for an athlete, and requires no further examination [15]. The presence of bradycardia alone does not merit treatment. In nonathletes, it is still unclear if asymptomatic sinus bradycardia reflects cardiovascular fitness or masked sinus node abnormalities. Patients with asymptomatic bradycardia were found to have different electrophysiologic properties compared to patients with symptomatic sinus bradycardia and/or sinoatrial block and patients with the bradycardia-tachycardia syndrome [24]. Specifically, mean values of corrected sinus node recovery time, atrial effective refractory period and atrial functional refractory period differentiated asymptomatic subjects from the other two subgroups of patients with sinoatrial disease while mean sinoatrial conduction time was not found to differ significantly. Several studies have investigated the association of asymptomatic sinus bradycardia with major outcomes. Tresch and Fleg [25] showed that sinus bradycardia defined as heart rate <50 bpm in apparently healthy, nonathletic individuals older than 40 years was not associated with cardiovascular morbidity and mortality. Furthermore, a retrospective analysis which included 6733 participants from the Multi-Ethnic Study of Atherosclerosis showed that sinus bradycardia (heart rate < 50 bpm) in patients not receiving heart rate modifying medications was not associated with mortality while sinus bradycardia mediated by heart rate modifying medications was associated with a 2.5-fold increase in the risk of mortality [26].
Fig. (1).
Sample ECG recordings from asymptomatic individuals. (A): A 26 years old male endurance athlete presented to the outpatient clinic for routine control. He denied any symptoms. Marked sinus bradycardia (35-40 bpm) was observed in his ECG recordings. No treatment was necessary. (B): A 75 years old female was referred to cardiology clinic for preoperative assessment before elective knee surgery. She did not report any symptoms related to low heart rate. Her ECG recordings showed second degree type 2 block with 3 to 1 and 2 to 1 conduction to the ventricles (HR: 35-40 bpm). The surgery was postponed after the discussion with orthopedics and dual chamber pacemaker was implanted. (C): An 80 years old male patient presented to the clinic for his routine control. His ECG recordings showed complete AV block (HR: 40-45 bpm). The patient denied any symptoms. Dual chamber pacemaker was implanted. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Regarding the management of patients with asymptomatic bradycardia and the role of pacemaker implantation, Goldberger et al. evaluated the clinical need for subsequent pacemaker implantation and mortality rate in outpatients >60 years of age with versus without asymptomatic bradycardia [27]. The authors found that bradycardia patients have significantly higher rates of PM implantation compared to no bradycardia patients while the higher incidence of PM implantation did not appear in the first 4 years. In addition, overall annual PM implantation rate was found very low (<1% per year) [27]. Subsequent analysis showed that pacemaker implantation was significantly associated with all-cause mortality while bradycardia itself was found to have a protective role regarding mortality. However, the increased mortality associated with PM implantation may be attributed to the disease progression that led to the device implantation and as a result device implantation can be recognized as a marker of sicker population. In the same study [27], factors such as baseline atrial fibrillation or flutter, PR interval, and QRS width were significantly associated with the subsequent PM implantation.
Another finding that may influence the clinical decision for a PM implantation is the duration of cardiac pauses. Recently, a systematic review on cardiac pauses in competitive athletes concluded that the accepted ‘3 second’ pause threshold does not adequately discriminate between potentially asymptomatic and symptomatic competitive athletes [28]. Cardiac pauses of ≤ 3 seconds had a low-positive predictive value (35.7%) and low sensitivity (26.3%), but good negative predictive value (86.7%) and specificity (91%) to predict symptoms. Accordingly, ventricular pauses of ‘3 s’ or longer usually do not cause symptoms, and the presence of these pauses does not necessarily indicate a poor prognosis or the need for pacing in asymptomatic healthy patients [29].
AV: Atrioventricular; Bpm: Beats per minute; ECG: Electrocardiography.
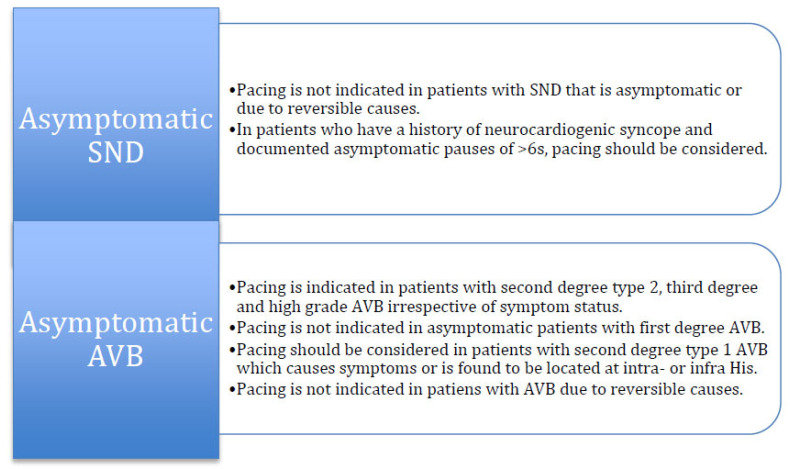
In patients with a history of neurocardiogenic syncope and asymptomatic pauses > 6 seconds, there is weak evidence for PM implantation, although it should be considered as a finding for treatment initiation [30]. In the ISSUE-2 [31] and ISSUE-3 [32] studies, the average duration of pause during syncope was found 9 (range 8-18 s) and 12 ± 10 seconds, respectively. Importantly in the ISSUE-3 study, patients without syncope had an asystolic pause of 10 ± 6 seconds. Furthermore, in AF patients, pauses between 3-5 sec are normally observed and there is no need for treatment if it remains asymptomatic [15]. Current recommendations from the guidelines for the management of patients with asymptomatic SND are illustrated in (Fig. 2).
Regarding nighttime bradycardia episodes, sleep apnea should be evaluated and, if present, should be treated with continuous positive airway pressure that have been found to correct bradycardia and AVB [33]. According to the current guidelines, in patients with sleep-related sinus bradycardia or transient sinus pauses occurring during sleep, permanent pacing should not be performed unless other indications for pacing are present [5].
5. DEFINITION AND PATHOPHYSIOLOGY OF ATRIOVENTRICULAR BLOCKS
Atrioventricular conduction disturbances include first-, second- and third-degree AVB. First-degree AVB is defined as an abnormal prolongation of the PR interval (>200 ms) with a 1:1 AV conduction ratio. First-degree AVB is not a true block in conduction, as each P wave is followed by a QRS complex, but rather slowed conduction through the AV node or the infranodal conduction system [5]. Second-degree AVB is subclassified into two types: type I (Mobitz I or Wenckebach) and type II (Mobitz II). Type I second-degree AVB is defined as the occurrence of a single nonconducted P wave associated with inconstant PR intervals before and after the blocked impulse with at least 2 consecutive conducted P waves (e.g., 3:2 AV block) to determine the behavior of the PR intervals [34, 35]. Type II second-degree AVB is defined as the occurrence of a single nonconducted P wave associated with constant PR intervals before and after a single blocked impulse (PP and RR intervals are constant). Mobitz I AVB occurs after a gradual PR prolongation whereas Mobitz II AVB does not. A 2:1 AVB cannot be classified as type I or II second-degree AV block and therefore it is important to deduce the level of block. Advanced, high-grade or high-degree AVB refers to instances where ≥ 2 consecutive P waves at a constant physiologic rate do not conduct to the ventricles without evidence for some AV conduction. Third-degree or complete AVB is characterized by the failure of each P wave to conduct to the ventricles, resulting in complete AV dissociation and no evidence of AV conduction.
AVB may result from both congenital and acquired forms of disease states. Congenital AVB is rare and has a prevalence of 1 per 15,000 to 20,000 live births [36]. Acquired AVB is more common and can include inflammatory, infectious, degenerative, ischemic and iatrogenic causes. Degenerative causes are associated with advanced age, hypertension, and diabetes mellitus. Infectious and ischemic causes may be reversible and not warrant cardiac pacing [37, 38]. Common secondary causes of AVB are presented in (Table 4).
Table 4.
Common Secondary Causes of AVB.
| Inflammatory | Cardiac sarcoidosis |
|---|---|
| - | Myocarditis |
| - | Rheumatologic Diseases |
| - | Amyloidosis |
| Infectious | Lyme Carditis |
| - | Infective Endocarditis |
| - | Toxoplasmosis |
| - | Chagas Disease |
| - | Acute Rheumatic Fever |
| Ischemia | Acute Coronary Syndrome |
| Medications | Beta Blockers |
| - | Verapamil, Diltiazem |
| - | Digoxin |
| - | Anti-arrhythmics |
| Metabolic | Thyroid Disease |
| - | Adrenal Disease |
| - | Acid-base Disorders |
| Vagatonic | Sleep Apnea |
| - | Athlete’s Heart |
| - | Neurocardiogenic |
Abbreviation: AVB: Atrioventricular block
6. TREATMENT OF ASYMPTOMATIC ATRIOVENTRICULAR BLOCKS
First-degree AVB and type I second-degree AVB, when above or at the level of the AV node, are not considered to be a concern for progression to a higher degree. Patients with first-degree AV block are typically asymptomatic. However, in cases of severe first-degree AVB (PR interval of > 0.3 seconds), patients can suffer from symptoms owing to the occurrence of atrial contraction very early in diastole at the expense of ventricular filling, diastolic mitral regurgitation and loss of AV synchrony. These patients may become symptomatic particularly during exercise, as the PR interval does not shorten in accordance to the decrease in the R-R interval [39, 40]. Type I second-degree AVB (Wenckebach) is often asymptomatic and can be observed in active, healthy patients or in well-trained athletes with no history of cardiovascular disease. If it occurs frequently or during exercise, there can be symptoms of exertional intolerance or dizziness. The 2018 ACC/AHA/HRS Bradycardia and Cardiac Conduction Delay Guideline recommend that in asymptomatic patients with first-degree AV block, type I second-degree AV block (Wenckebach) or 2:1 AV block believed to be at the level of the AV node, permanent pacing should not be performed (COR: III, harm; LOE: C-LD). There is some controversy on the need for pacing of type I second-degree AV block in selected asymptomatic elderly individuals [41, 42]. Asymptomatic Type I second degree AV block is considered to have a benign course and pacemaker implantation is not typically indicated. However, in the presence of wide QRS complexes, an infra-Hisian localization should be considered, and an exercise testing or electrophysiologic study may be an indication [15]. On the other hand, evidence from the Devon Heart study showed that Mobitz I block seems to not have a benign course in patients ≥ 45 years of age and therefore the authors proposed that a PM implantation should be considered, even in the absence of symptomatic bradycardia or organic heart disease [41, 42]. In view of these findings, the 2019 EHRA consensus document on the management of asymptomatic arrhythmias suggests a need for further investigation before making conclusive recommendations for Mobitz I block [15]. In contrast to patients with SND, PM may indicate for prognostic reasons in patients with AVB. Thus, it is recommended that for third-degree AV Block, in the absence of correctable causes, and type II second-degree AV block should be treated with pacing even in the absence of symptoms (Fig. 1B and Fig. 1C) [15]. Current recommendations from the guidelines for the management of patients with asymptomatic AVB are illustrated in (Fig. 2).
Fig. (2).
A figure illustrating the recommendations for the management of asymptomatic sinus node dysfunctions and atrioventricular blocks. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
There are specific conditions that may influence the decision for device implantation. In patients with neuromuscular diseases associated with conduction disorders, including muscular dystrophy (e.g., myotonic dystrophy type 1) or Kearns-Sayre syndrome, who have evidence of second-degree AVB, third-degree AVB, or an HV interval of ≥70 ms, regardless of symptoms, permanent pacing, with additional defibrillator capability if needed and meaningful survival of >1 year is expected, is recommended [5].The choice for implanting a permanent PM for asymptomatic congenital heart block in the absence structural heart disease depends on several parameters such as ventricular impairment (LV dilatation and/or dysfunction), average heart rate (below 50 bpm), pauses (more than 3.0 secs), QRS duration, frequency of premature ventricular complexes, and prolonged QT interval. Patients fulfilling the aforementioned parameters should be treated with a permanent PM, regardless of symptoms [43].
CONCLUSION
Individuals presenting with asymptomatic bradyarrhythmias often have a benign course and generally do not warrant further treatment apart from being followed. Nevertheless, a detailed evaluation and diagnostic workup are required to rule out cases that may progress to a more advanced conduction disease or those that are a cardiac manifestation of a systematic disease. In patients with AVB, deducing the degree/level of disease is important to management decisions.
ACKNOWLEDGEMENTS
Declared none.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors have no conflicts of interest, financial or otherwise.
REFERENCES
- 1.Brignole M., Vardas P., Hoffman E., Huikuri H., Moya A., Ricci R., Sulke N., Wieling W., Auricchio A., Lip G.Y., Almendral J., Kirchhof P., Aliot E., Gasparini M., Braunschweig F., Lip G.Y., Almendral J., Kirchhof P., Botto G.L. Task Force members; EHRA Scientific Documents Committee; Document Reviewers; EHRA Scientific Documents Committee. Indications for the use of diagnostic implantable and external ECG loop recorders. Europace. 2009;11(5):671–687. doi: 10.1093/europace/eup097. [DOI] [PubMed] [Google Scholar]
- 2.Rockx MA, Hoch JS, Klein GJ, et al. Is ambulatory monitoring for “community-acquired” syncope economically attractive? A cost-effectiveness analysis of a randomized trial of external loop recorders versus Holter monitoring. Am Heart J. 2005;150(5):e1–e5. doi: 10.1016/j.ahj.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Locati E.T., Moya A., Oliveira M., Tanner H., Willems R., Lunati M., Brignole M. External prolonged electrocardiogram monitoring in unexplained syncope and palpitations: Results of the SYNARR-Flash study. Europace. 2016;18(8):1265–1272. doi: 10.1093/europace/euv311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brignole M, Auricchio A, Baron-Esquivias G, et al. ESC guidelines on cardiac pacing and cardiac resynchronization therapy. Revista española de cardiología-English Edition. 2014;67(1):58e1–e60. doi: 10.1016/j.rec.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Kusumoto F.M., Schoenfeld M.H., Barrett C., Edgerton J.R., Ellenbogen K.A., Gold M.R., et al. ACC/AHA/HRS guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2018;2018:25701. doi: 10.1016/j.jacc.2018.10.043. [DOI] [PubMed] [Google Scholar]
- 6.Gadler F., Valzania C., Linde C. Current use of implantable electrical devices in Sweden: Data from the Swedish pacemaker and implantable cardioverter-defibrillator registry. Europace. 2015;17(1):69–77. doi: 10.1093/europace/euu233. [DOI] [PubMed] [Google Scholar]
- 7.Kusumoto F.M., Schoenfeld M.H., Wilkoff B.L., Berul C.I., Birgersdotter-Green U.M., Carrillo R., Cha Y.M., Clancy J., Deharo J.C., Ellenbogen K.A., Exner D., Hussein A.A., Kennergren C., Krahn A., Lee R., Love C.J., Madden R.A., Mazzetti H.A., Moore J.C., Parsonnet J., Patton K.K., Rozner M.A., Selzman K.A., Shoda M., Srivathsan K., Strathmore N.F., Swerdlow C.D., Tompkins C., Wazni O. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm. 2017;14(12):e503–e551. doi: 10.1016/j.hrthm.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Birnie D.H., Nery P.B., Ha A.C., Beanlands R.S. Cardiac sarcoidosis. J. Am. Coll. Cardiol. 2016;68(4):411–421. doi: 10.1016/j.jacc.2016.03.605. [DOI] [PubMed] [Google Scholar]
- 9.Besant G., Wan D., Yeung C., Blakely C., Branscombe P., Suarez-Fuster L., Redfearn D., Simpson C., Abdollah H., Glover B., Baranchuk A. Suspicious index in Lyme carditis: Systematic review and proposed new risk score. Clin. Cardiol. 2018;41(12):1611–1616. doi: 10.1002/clc.23102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yeung C, Al-Turki M, Baranchuk A. The value of the surface ECG for the diagnosis and management of lyme carditis. Curr Cardiol Rev. 2020;17(1):5–9. doi: 10.2174/1573403X16666200312101751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehra R., Benjamin E.J., Shahar E., Gottlieb D.J., Nawabit R., Kirchner H.L., Sahadevan J., Redline S. Sleep Heart Health Study. Association of nocturnal arrhythmias with sleep-disordered breathing: The Sleep Heart Health Study. Am. J. Respir. Crit. Care Med. 2006;173(8):910–916. doi: 10.1164/rccm.200509-1442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffstein V., Mateika S. Cardiac arrhythmias, snoring, and sleep apnea. Chest. 1994;106(2):466–471. doi: 10.1378/chest.106.2.466. [DOI] [PubMed] [Google Scholar]
- 13.Ferrer M.I. The sick sinus syndrome in atrial disease. JAMA. 1968;206(3):645–646. doi: 10.1001/jama.1968.03150030101028. [DOI] [PubMed] [Google Scholar]
- 14.Lown B. Electrical reversion of cardiac arrhythmias. Br. Heart J. 1967;29(4):469–489. doi: 10.1136/hrt.29.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arnar D.O., Mairesse G.H., Boriani G., Calkins H., Chin A., Coats A., et al. Management of asymptomatic arrhythmias: A European Heart Rhythm Association (EHRA) consensus document, endorsed by the Heart Failure Association (HFA), Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), Cardiac Arrhythmia Society of Southern Africa (CASSA), and Latin America Heart Rhythm Society (LAHRS). EP Europace; 2019. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan B.M., Langendorf R., Lev M., Pick A. Tachycardia-bradycardia syndrome (so-called “sick sinus syndrome”). Pathology, mechanisms and treatment. Am. J. Cardiol. 1973;31(4):497–508. doi: 10.1016/0002-9149(73)90302-0. [DOI] [PubMed] [Google Scholar]
- 17.Thery C., Gosselin B., Lekieffre J., Warembourg H. Pathology of sinoatrial node. Correlations with electrocardiographic findings in 111 patients. Am. Heart J. 1977;93(6):735–740. doi: 10.1016/S0002-8703(77)80070-7. [DOI] [PubMed] [Google Scholar]
- 18.Csepe T.A., Kalyanasundaram A., Hansen B.J., Zhao J., Fedorov V.V. Fibrosis: A structural modulator of sinoatrial node physiology and dysfunction. Front. Physiol. 2015;6:37. doi: 10.3389/fphys.2015.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tellez J.O., Mczewski M., Yanni J., Sutyagin P., Mackiewicz U., Atkinson A., Inada S., Beresewicz A., Billeter R., Dobrzynski H., Boyett M.R. Ageing-dependent remodelling of ion channel and Ca2+ clock genes underlying sino-atrial node pacemaking. Exp. Physiol. 2011;96(11):1163–1178. doi: 10.1113/expphysiol.2011.057752. [DOI] [PubMed] [Google Scholar]
- 20.Goette A., Kalman J.M., Aguinaga L., Akar J., Cabrera J.A., Chen S.A., Chugh S.S., Corradi D., D’Avila A., Dobrev D., Fenelon G., Gonzalez M., Hatem S.N., Helm R., Hindricks G., Ho S.Y., Hoit B., Jalife J., Kim Y.H., Lip G.Y., Ma C.S., Marcus G.M., Murray K., Nogami A., Sanders P., Uribe W., Van Wagoner D.R., Nattel S. Document Reviewers. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: Definition, characterization, and clinical implication. Europace. 2016;18(10):1455–1490. doi: 10.1093/europace/euw161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monfredi O., Dobrzynski H., Mondal T., Boyett M.R., Morris G.M. The anatomy and physiology of the sinoatrial node--a contemporary review. Pacing Clin. Electrophysiol. 2010;33(11):1392–1406. doi: 10.1111/j.1540-8159.2010.02838.x. [DOI] [PubMed] [Google Scholar]
- 22.Hatle L., Bathen J., Rokseth R. Sinoatrial disease in acute myocardial infarction. Long-term prognosis. Br. Heart J. 1976;38(4):410–414. doi: 10.1136/hrt.38.4.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D’Souza A., Sharma S., Boyett M.R. CrossTalk opposing view: Bradycardia in the trained athlete is attributable to a downregulation of a pacemaker channel in the sinus node. J. Physiol. 2015;593(8):1749–1751. doi: 10.1113/jphysiol.2014.284356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franchi F., Padeletti L., Brat A., Michelucci A., Arcangeli C., Fantini F. Assessment of sinus node function in asymptomatic subjects with sinus bradycardia and in symptomatic patients with sino-atrial disease. Acta Cardiol. 1979;34(6):385–399. [PubMed] [Google Scholar]
- 25.Tresch D.D., Fleg J.L. Unexplained sinus bradycardia: clinical significance and long-term prognosis in apparently healthy persons older than 40 years. Am. J. Cardiol. 1986;58(10):1009–1013. doi: 10.1016/S0002-9149(86)80029-7. [DOI] [PubMed] [Google Scholar]
- 26.Dharod A., Soliman E.Z., Dawood F., Chen H., Shea S., Nazarian S., Bertoni A.G. MESA Investigators. Association of asymptomatic bradycardia with incident cardiovascular disease and mortality: The Multi-Ethnic Study of Atherosclerosis (MESA). JAMA Intern. Med. 2016;176(2):219–227. doi: 10.1001/jamainternmed.2015.7655. [DOI] [PubMed] [Google Scholar]
- 27.Goldberger J.J., Johnson N.P., Gidea C. Significance of asymptomatic bradycardia for subsequent pacemaker implantation and mortality in patients >60 years of age. Am. J. Cardiol. 2011;108(6):857–861. doi: 10.1016/j.amjcard.2011.04.035. [DOI] [PubMed] [Google Scholar]
- 28.Senturk T., Xu H., Puppala K., Krishnan B., Sakaguchi S., Chen L.Y., Karim R., Dickinson O., Benditt D.G. Cardiac pauses in competitive athletes: A systematic review examining the basis of current practice recommendations. Europace. 2016;18(12):1873–1879. doi: 10.1093/europace/euv373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hilgard J., Ezri M.D., Denes P. Significance of ventricular pauses of three seconds or more detected on twenty-four-hour Holter recordings. Am. J. Cardiol. 1985;55(8):1005–1008. doi: 10.1016/0002-9149(85)90735-0. [DOI] [PubMed] [Google Scholar]
- 30.Moya A., Brignole M., Sutton R., Menozzi C., Garcia-Civera R., Wieling W., Andresen D., Benditt D.G., Garcia-Sacristán J.F., Beiras X., Grovale N., Vardas P. International Study on Syncope of Uncertain Etiology 2 (ISSUE 2) Group. Reproducibility of electrocardiographic findings in patients with suspected reflex neurally-mediated syncope. Am. J. Cardiol. 2008;102(11):1518–1523. doi: 10.1016/j.amjcard.2008.07.043. [DOI] [PubMed] [Google Scholar]
- 31.Brignole M., Sutton R., Wieling W., Lu S.N., Erickson M.K., Markowitz T., Grovale N., Ammirati F., Benditt D.G. Analysis of rhythm variation during spontaneous cardioinhibitory neurally-mediated syncope. Implications for RDR pacing optimization: an ISSUE 2 substudy. Europace. 2007;9(5):305–311. doi: 10.1093/europace/eum017. [DOI] [PubMed] [Google Scholar]
- 32.Brignole M., Menozzi C., Moya A., Andresen D., Blanc J.J., Krahn A.D., Wieling W., Beiras X., Deharo J.C., Russo V., Tomaino M., Sutton R. International Study on Syncope of Uncertain Etiology 3 (ISSUE-3) Investigators. Pacemaker therapy in patients with neurally mediated syncope and documented asystole: Third International Study on Syncope of Uncertain Etiology (ISSUE-3): A randomized trial. Circulation. 2012;125(21):2566–2571. doi: 10.1161/CIRCULATIONAHA.111.082313. [DOI] [PubMed] [Google Scholar]
- 33.Koehler U., Fus E., Grimm W., Pankow W., Schäfer H., Stammnitz A., Peter J.H. Heart block in patients with obstructive sleep apnoea: Pathogenetic factors and effects of treatment. Eur. Respir. J. 1998;11(2):434–439. doi: 10.1183/09031936.98.11020434. [DOI] [PubMed] [Google Scholar]
- 34.Vogler J., Breithardt G., Eckardt L. Bradyarrhythmias and conduction blocks. Rev. Esp. Cardiol. (Engl. Ed.) 2012;65(7):656–667. doi: 10.1016/j.rec.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 35.Surawicz B., Uhley H., Borun R., et al. Task Force I: standardization of terminology and interpretation. Am. J. Cardiol. 1978;41(1):130–145. doi: 10.1016/0002-9149(78)90147-9. [DOI] [PubMed] [Google Scholar]
- 36.Baruteau A-E., Pass R.H., Thambo J-B., Behaghel A., Le Pennec S., Perdreau E., Combes N., Liberman L., McLeod C.J. Congenital and childhood atrioventricular blocks: Pathophysiology and contemporary management. Eur. J. Pediatr. 2016;175(9):1235–1248. doi: 10.1007/s00431-016-2748-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yeung C., Baranchuk A. Diagnosis and treatment of lyme carditis: JACC review topic of the week. J. Am. Coll. Cardiol. 2019;73(6):717–726. doi: 10.1016/j.jacc.2018.11.035. [DOI] [PubMed] [Google Scholar]
- 38.Brady W.J., Jr, Harrigan R.A. Diagnosis and management of bradycardia and atrioventricular block associated with acute coronary ischemia. Emerg. Med. Clin. North Am. 2001;19(2):371–384, xi-xii. doi: 10.1016/S0733-8627(05)70189-9. [DOI] [PubMed] [Google Scholar]
- 39.Barold S.S. Indications for permanent cardiac pacing in first-degree AV block: Class I, II, or III? Pacing Clin. Electrophysiol. 1996;19(5):747–751. doi: 10.1111/j.1540-8159.1996.tb03355.x. [DOI] [PubMed] [Google Scholar]
- 40.Carroz P., Delay D., Girod G. Pseudo-pacemaker syndrome in a young woman with first-degree atrio-ventricular block. Europace. 2010;12(4):594–596. doi: 10.1093/europace/eup373. [DOI] [PubMed] [Google Scholar]
- 41.Coumbe A.G., Naksuk N., Newell M.C., Somasundaram P.E., Benditt D.G., Adabag S. Long-term follow-up of older patients with Mobitz type I second degree atrioventricular block. Heart. 2013;99(5):334–338. doi: 10.1136/heartjnl-2012-302770. [DOI] [PubMed] [Google Scholar]
- 42.Shaw D.B., Gowers J.I., Kekwick C.A., New K.H., Whistance A.W. Is Mobitz type I atrioventricular block benign in adults? Heart. 2004;90(2):169–174. doi: 10.1136/hrt.2003.017806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bordachar P., Zachary W., Ploux S., Labrousse L., Haissaguerre M., Thambo J-B. Pathophysiology, clinical course, and management of congenital complete atrioventricular block. Heart Rhythm. 2013;10(5):760–766. doi: 10.1016/j.hrthm.2012.12.030. [DOI] [PubMed] [Google Scholar]