Abstract:
ST-elevation myocardial (STEMI) is frequently associated with conduction disorders. Regional myocardial ischemia or injury may affect the cardiac conduction system at various locations, and neural reflexes or changes in the balance of the autonomous nervous system may be involved. Sinoatrial and atrioventricular blocks are more frequent in inferior than anterior STEMI, while new left anterior fascicular block and right bundle branch block indicate proximal occlusion of the left anterior descending coronary artery. New left bundle branch block is associated with multi-vessel disease.
Most conduction disorders associated with STEMI are reversible with reperfusion therapy, but they may still impair prognosis because they indicate a large area at risk, extensive myocardial infarction or severe coronary artery disease.
Acute STEMI recognition is possible in patients with a fascicular or right bundle branch block, but future studies need to define the cut-off values for ST depression in the leads V1-V3 in inferolateral MI and for ST elevation in the same leads in anterior STEMI. In the left bundle branch block, concordant ST elevation is a specific sign of acute coronary artery occlusion, but the ECG feature has low sensitivity.
Keywords: Conduction disorder, acute coronary syndrome, acute myocardial infarction, bundle branch block, atrioventricular block, pacemaker
1. Introduction
1.1. Bradyarrhythmias
1.1.1. Sinoatrial Block
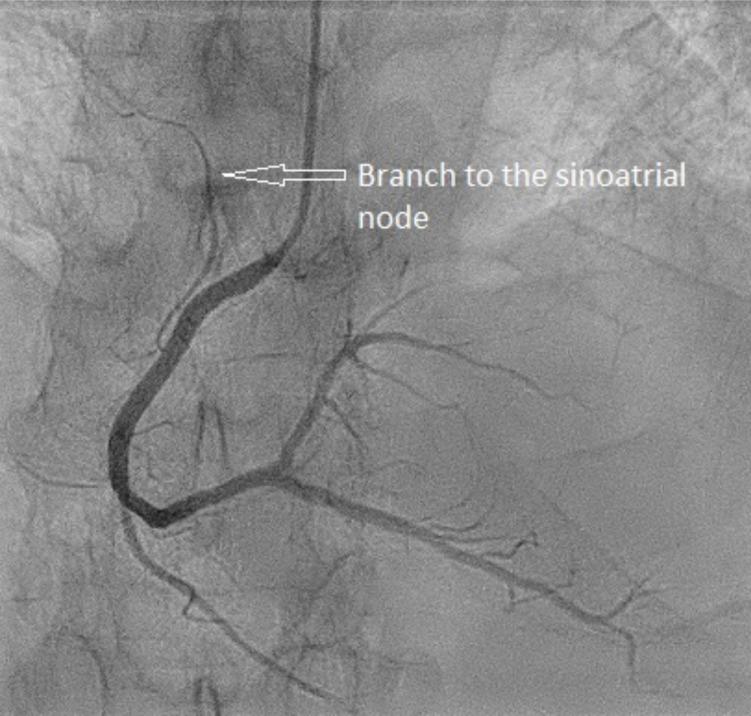
Sinus bradycardia is frequent in acute inferior ST-elevation myocardial infarction (STEMI), especially during the first hours after occlusion of the coronary artery [1, 2]. Sinus bradycardia was three times more common in inferior than in anterior acute myocardial infarction (MI) [3]. Bradycardia may be caused by depressed automatism. The Bezold-Jarisch reflex includes a triad of bradycardia, hypotension and vasodilation, and inferior wall myocardial ischemia may elicit this reflex [4]. The reflex is thought to result from the stimulus of cardiopulmonary mechanoreceptors that are associated with unmyelinated vagal afferent nerve fibers, classified as C-fibers. The sinoatrial (SA) nodal side branch (or artery) originates from the right coronary artery (RCA) in about two thirds (Fig. 1) and from the left circumflex artery
Fig. (1).
Coronary angiography of the right coronary artery to illustrate the typical take-off of the side branch to the sinoatrial node. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
(LCX) in about one fifth of individuals [5]. The side branch may also take off directly from the aorta. Very proximal occlusion of the RCA caused by thrombus or aortic dissection may result in occlusion of the SA nodal branch, but occlusion may also result from stenting over the side branch ostium or from embolization. Ischemia of the cardiac conduction system may be the result of these pathophysiological processes.
SA nodal branch occlusion may result in sinus bradycardia, sinoatrial block or even asystole. Because the SA nodal branch also irrigates parts of the atrial walls, bradycardia may be accompanied by atrial MI. In practice, it is not possible to distinguish between abnormalities in impulse formation, impulse conduction, or a combination, in the standard 12-lead ECG in patients with intermittent absence of sinus P waves. In sinus bradycardia and SA block, vagally induced bradycardia will disappear with atropine while ischemic bradycardia persists.
Serrano et al. compared inferior STEMI patients based on the culprit artery and the level of occlusion [2]. Sinus bradycardia in the admission ECG was noted in 15% of the patients with RCA occlusion, but in none of the patients with LCX occlusion, and proximal RCA occlusion was more often associated with sinus bradycardia than mid or distal occlusion.
1.1.2. Interatrial Block
Interatrial block (IAB) is a distinct ECG pattern caused by conduction delay between the right and left atrium, probably resulting from local fibrosis reflected in the surface ECG as a biphasic morphology of the P wave in the inferior leads (II, III, aVF) [6, 7]. This, together with a P-wave duration of ≥120 ms is considered as advanced IAB. P-wave duration ≥120 ms with normal P-wave morphology is defined as partial IAB. In STEMI patients (n=198) without a history of atrial arrhythmias treated successfully with primary percutaneous coronary intervention (PCI), about half of the patients had partial or advanced IAB at presentation, while six hours after PCI, the prevalence of IAB had decreased to one quarter [8]. RCA stenosis and diffuse coronary artery disease were associated with IAB at admission. Partial IAB on admission and six hours post-PCI were independent predictors of atrial fibrillation at 12-month follow-up. In a registry study (n=972) of STEMI patients in sinus rhythm at hospital discharge, 21.3% had partial and 5.9% advanced IAB [9]. Patients with IAB had higher all-cause mortality than patients without IAB, but the association was explained by older age and other variables, while the multivariable analysis did not show any independent association between IAB and prognosis. In patients (n=109) undergoing elective PCI of the RCA or LCX, atrial branch occlusion was associated with more frequent intra-atrial conduction delay, atrial tachycardia and atrial fibrillation [10]. After adjustment by a propensity score, atrial branch occlusion was an independent predictor of periprocedural infarction and atrial arrhythmias. The authors speculated that atrial ischemic episodes might be considered as a potential cause of atrial fibrillation in patients with coronary artery disease.
1.1.3. Atrioventricular Block
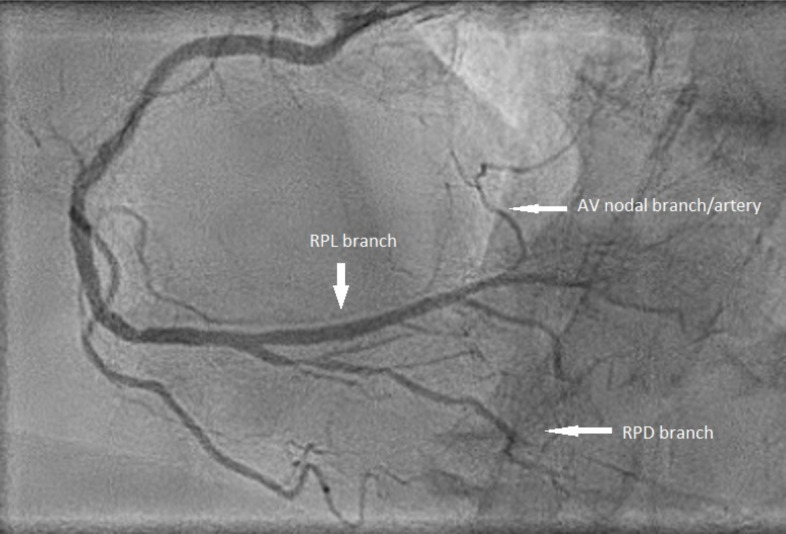
Atrioventricular (AV) block is a rather frequent finding in acute inferior STEMI. This is partly explained by the fact that the side branch to the AV node takes off distally from the posterolateral branch of the RCA (Fig. 2). In left-dominant circulation, the AV nodal branch arises from the LCX. The mechanism of AV conduction disturbance in acute STEMI is different depending on the infarct location. In a recent study of STEMI patients, RCA culprit predicted second degree Mobitz 2 or third-degree AV block (high degree AV block, HDAVB) with an odds ratio of 3.80 [11]. RCA occlusion increases acetylcholine release from the myocardium of the inferior wall, and this contributes to the AV block. As for ischemia of the SV node, vagal overdrive disappears following the administration of intravenous atropine, while AV block of ischemic origin persists. Ischemic AV block usually presents a fast heart rate, while vagally induced block does not [12]. According to Lie et al., in patients with acute inferior MI and HDAVB, escape beats of left bundle branch block (LBBB) morphology represent conducted beats (AV junctional escape beats), while those with right bundle branch block (RBBB) morphology most probably represent fascicular of ventricular escape rhythm requiring pacemaker implantation [13].
Fig. (2).
Coronary angiography of the right coronary artery shows the typical location of a branch to the atrioventricular node taking off from the right posterolateral (RPL) branch in the right-dominant coronary circulation. There is some atherosclerotic narrowing in the proximal part of the artery before the take-off of a large acute marginal branch and the right posterior descending branch (RPD). (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Mobitz 1 (Wenckebach) second-degree AV block is most frequent in inferior STEMI caused by RCA occlusion, and it is usually transient and supra-Hisian, while Mobitz 2 -block is usually infra-Hisian and implies a worse prognosis. In infra-Hisian Mobitz 2 -block, the PQ-interval prolongation is usually mild, or the PQ interval may even be normal [12, 14].
The prognostic implications of third-degree (complete) AV block depend on the STEMI location. When it occurs in association with inferior STEMI, it usually evolves from a first-degree block, the QRS complex is narrow and the block is supra-Hisian [12]. Advanced AV block presenting in an anteroapical infarction is usually accompanied by an infra-Hisian escape rhythm with a wide QRS complex, which may result in hemodynamic deterioration. In this scenario, pacemaker implantation is indicated, but the outcome of the patient is dependent on the extent of the infarction and the degree of left ventricular dysfunction. In inferior STEMI, advanced AV block is more often seen in patients with terminal QRS distortion (Sclarovsky-Birnbaum grade III ischemia) than in those with grade II ischemia in the leads with ST-segment elevation [15].
Berger et al. reported a 19% incidence of second- or third-degree AV block in acute inferior MI by combining results from studies published in the 1960’s - 1980’s [16]. In the recent HORIZONS-AMI trial, where almost all 3,115 study STEMI patients had primary PCI, HDAVB was found in 1.5% of the patients [11]. Of the patients with HDAVB, 60.9% had a temporary pacemaker implanted in the catheterization laboratory. The incidence of HDAVB in a recent national survey from 2010 was 2.1% [17]. In a recent large STEMI registry study (n=16,536), the incidence of HDAVB was 6.6% in inferior STEMI, but only 0.3% in anterior STEMI [18]. In a recent study of 4,799 patients with the acute coronary syndrome (ACS) (STEMI in 55.7%), 1.9% presented with complete AV block [19].
HDAVB has been associated with an increased risk for in-hospital complications and higher mortality rates during follow-up; in the old study by Berger et al., the mean in-hospital mortality rate was 23% [16]. In the recent HORIZONS-AMI study, 30-day mortality in HDAVB was 8.8% compared with 2.3% (p=0.005) in the patients without HDAVB.
In ACS patients with complete AV block in the previously mentioned publication by Aguiar Rosa et al., 79.1% had inferior STEMI compared with 21.9% in the patients without complete AV block, and in-hospital mortality was almost eightfold higher in patients with complete AV block (23.1% vs. 3.5%) [19]. In the large STEMI registry study referred to previously, anterior, but not inferior location was associated with increased in-hospital mortality in HDAVB patients after multivariate adjustment [18].
The HORIZONS-AMI investigators also studied the incidence and prognostic implications of worsening AV block post-primary PCI [20]. They reported a 1.5% progression of both second and third degree AV block after one year. Anterior STEMI was associated with worsened AV block, which was an independent predictor of all-cause death and major adverse cardiac events. The authors speculated that HDAVB probably was the result of chronic damage to the AV conduction system, related to left anterior descending (LAD) coronary artery culprit lesions associated with extensive myocardial injury or infarction in these patients.
2. Which Patients Need a Temporary Pacemaker
Although AV block associated with inferior STEMI is usually temporary, benign and nearly always resolves with reperfusion therapy, temporary pacemaker therapy may be necessary in case of hemodynamic compromise or bradycardia-related ventricular arrhythmias. According to the 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation, temporary pacing is required in cases of sinus bradycardia with hemodynamic intolerance or HDAVB without stable escape rhythm if the arrhythmia does not respond to positive chronotropic medication [21]. AV sequential pacing should be considered in patients with complete AV block, right ventricular infarction, and hemodynamic compromise. According to the 2013 ACCF/AHA STEMI guidelines, temporary pacing is indicated for symptomatic bradyarrhythmias unresponsive to medical treatment, and application of transcutaneous pacing pads for potential use is reasonable treatment in HDAVB caused by inferior STEMI [22]. Prophylactic placement of a temporary pacing system is recommended for HDAVB in patients with anterior/lateral MI.
3. Intraventricular block
3.1. Fascicular Block
The presence of a new left anterior fascicular block (LAFB) associated with anterior STEMI strongly supports a culprit artery location in the proximal part of the LAD, because the left anterior fascicle receives its blood supply from the first major septal branch of the LAD [23]. The development of LAFB in the course of an inferior infarction (RCA or LCX occlusion) indicates significant concomitant stenosis of the LAD before the first major septal branch.
The appearance of the left posterior fascicular block, which causes clear rightward deviation of the frontal plane QRS axis, is extremely rare in STEMI patients, and they are typically published as case reports. The left posterior fascicle is the least vulnerable division of the intraventricular conduction system. Compared with the left anterior fascicle, the left posterior fascicle is larger, has faster de- and repolarization, receives its blood supply from two coronary systems (the LAD and the RCA), and runs through a more protected area, the left ventricular inflow tract with less mechanical pressure impact [24]. Transient rightward shift of the frontal QRS axis in patients with acute anterior MI has been associated with severe two- or three-vessel disease, typically with collateral circulation between the LAD and the RCA [25]. Ischemia of the left posterior fascicle was suggested as the etiologic factor.
The existence of a tetrafascicular intraventricular conduction system, including a left septal fascicle, remains debatable. A consensus statement ended up with some discrepancies and, despite agreeing on the possible existence of an anatomical left septal fascicle, the electrocardiographic and vectorcardiographic characteristics of a left septal fascicular block (LSFB) were not universally accepted [7]. The most important criteria to indicate the existence of LSFB is its intermittent nature because other causes of prominent anterior QRS forces, such as right ventricular hypertrophy, septal hypertrophy, lateral wall myocardial infarction, and lead switch have to be considered in the ECG diagnosis. The proposed ECG criteria for LSFB include normal or slightly increased (up to 110 ms) QRS duration, R-wave voltage of V1 ≥ 5 mm, R wave of V2 > 15 mm, and absence of a q wave in the left precordial leads V5, V6 and in the lead I [26]. Two case reports from patients with ST-elevation ACS, one with anterior and one with inferior ST elevation, and prominent anterior forces, indicating LSFB, were reported [27, 28].
3.2. Right Bundle Branch Block
The right bundle branch receives its blood supply from an anterior septal branch of the LAD alone or jointly with the AV nodal artery [29]. Therefore, a proximal LAD occlusion may result in a new RBBB, which is often accompanied by LAFB, because the right bundle branch and the left anterior fascicle receive essentially the same blood supply (Fig. 3). RBBB + LAFB is a type of bifascicular block. These intraventricular blocks are mostly reversible with reperfusion therapy.
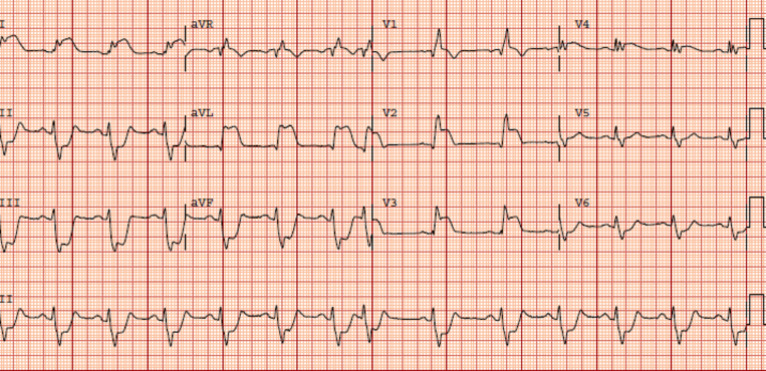
Fig. (3).
The ECG of a patient with proximal left anterior descending coronary artery occlusion: ST elevation in V1-V4, I and aVL and reciprocal ST depression in II, III, aVF, and V5-V6. There is a right bundle branch block and left anterior fascicular block. In addition, there is grade III ischemia (J-point /R wave ratio of >0.5 in I, aVL, V2-V3). (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Transient RBBB in inferior STEMI is rare, and is probably explained by ischemia of the proximal His bundle, selectively inhibiting conduction to the right bundle branch; the His bundle is dually supplied by the AV nodal branch (proximal His bundle) and the first septal branch of the LAD (distal His bundle) [14, 29].
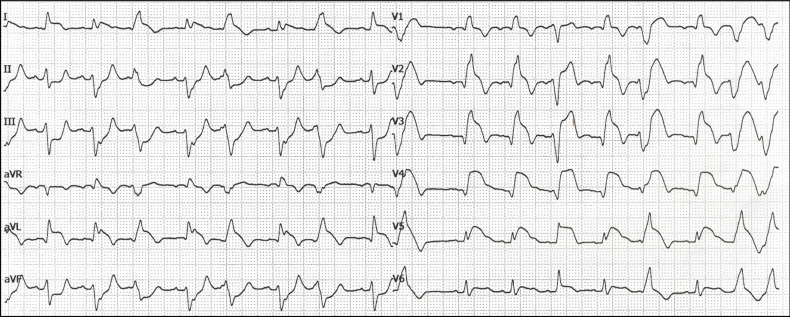
RBBB and LAFB were demonstrated in more than half of the patients with acute total left main occlusion in a small patient series (Fig. 4) [30]. In addition, there was typically ST-segment elevation in the precordial leads from V2 to V4-6 and in leads I and aVL, accompanied by ST-segment depression in the inferior leads.
Fig. (4).
A patient with acute total occlusion of the left main coronary artery. The ECG shows ST elevation in V2-V6, I and aVL and reciprocal ST depression in II, III, aVF. There is no ST elevation in leads aVR or V1, probably caused by the cancellation of electrical forces due to transmural ischemia from myocardial segments of both the left anterior descending and the left circumflex coronary artery. There is right bundle branch block associated with left anterior fascicular block and ventricular extrasystoles. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
A unique situation occurs when LAFB obscures RBBB, abolishing the terminal S waves in leads I and aVL and the terminal R (R’) wave in V1 [31]. The existence of an RBBB is indicated by a broad QRS, wide R’ in lead aVR and a wide S wave in leads V5 and V6.
RBBB does not interfere with the diagnosis of acute STEMI, and the ECG criteria for STEMI are the same as for patients with a narrow QRS. Diagnosing ST elevation in the anterior, inferior and lateral leads can easily be done in patients with RBBB. However, the diagnosis of inferolateral STEMI equivalent in patients with RBBB and baseline ST depression in V1-V3 is a challenge, and prospective studies are needed to define the threshold for ST deviations in this setting. In acquired RBBB, there is typically an initial Q wave, while in pre-existing RBBB, there is rRS’ configuration [14] (Figs. 3 and 4).
RBBB was associated with increased mortality in acute MI patients in a meta-analysis, which included a considerable proportion of acute STEMI patients [32]. A radical change has recently taken place in the treatment recommendations for patients with new or presumably new RBBB and suspicion of acute MI. The Fourth Universal Definition of Myocardial Infarction (UDMI) states: “New, or presumed new, RBBB without associated ST-segment or T wave changes is associated with thrombolysis in myocardial infarction (TIMI) 0-2 flow in as many as 66% of patients (compared with >90% in those with ST-segment or T wave changes)” [33]. The 2017 ESC guidelines for STEMI also specify: “Patients with myocardial infarction and RBBB have a poor prognosis. It may be difficult to detect transmural ischaemia in patients with chest pain and RBBB. Therefore, a primary percutaneous coronary intervention strategy (emergent coronary angiography and percutaneous coronary intervention if indicated) should be considered when persistent ischaemic symptoms occur in the presence of RBBB” [21]. These recommendations are mainly based on a retrospective study by Widimsky et al. [34]. That study included 6,742 patients with AMI and showed that among the 427 patients with RBBB (53% with concomitant ST elevation), TIMI 0 flow in the infarct related artery was found in 51.7%, and primary PCI was performed in 80.1% of the patients. TIMI flow 0 in the infarct-related artery was found in significantly more patients with new or presumably new RBBB (55%) than in the group with old RBBB (34.9%), old LBBB (28%) or new or presumably new LBBB (41.1%). It is somewhat surprising that also RBBB without ST elevation has been added as a STEMI equivalent ECG manifestation in the new guidelines. The study results by Widimsky et al. apply to patients with adjudicated acute MI, not necessarily to patients in the emergency care settings, and not all study patients underwent emergent angiography according to a primary PCI protocol. Significant coronary artery lesions in patients with RBBB are not necessarily new culprit lesions. Therefore, outcome data are needed in patients with chest pain, presumably new RBBB and no significant ST deviation treated with or without primary PCI. Also the usefulness of primary PCI for patients presenting with RBBB and atypical symptoms (shortness of breath, acute heart failure, etc.) should be prospectively tested.
A more recent article by Neumann et al. included 4,067 patients with suspected acute MI [35]. RBBB was found in 3.1% of the patients, and of them, only 20.8% had a final diagnosis of acute MI (six had STEMI and 17 non-ST elevation MI). Mortality for patients with RBBB at 1-year follow-up was 10.7% compared to 3.2% in the patients without broad QRS. The study data challenge the concept of RBBB as an indication for emergent coronary angiography, as the likelihood of MI was similar to that of patients without bundle branch block.
Another clinical point related to RBBB is, whether a lower threshold for ST elevation than for narrow QRS should be used in anterior STEMI because of the baseline secondary ST depression in the right precordial leads associated with this conduction disorder. Future studies should address this issue, and also the cut-off values for ST depression in the leads V1-V3 in inferolateral MI need to be established.
3.3. Left bundle Branch Block
The left bundle branch is a larger and less vulnerable structure than the right bundle branch. The blood supply to the left bundle branch is clearly of dual origin as the anterior fascicle is supplied mainly by anterior septal branches of the LAD and the posterior fascicle from the AV nodal artery, which originates from the RCA or the LCX depending on coronary artery dominance. Accordingly, severe two- or three-vessel disease should typically be present for a new LBBB to develop.
Sgarbossa et al. introduced three criteria to help in identifying acute MI in patients with LBBB [36]. They were: (1) ST-segment elevation ≥1 mm and concordant with the QRS complex (5 points); (2) ST-segment depression ≥1 mm in leads V1, V2 or V3 (3 points); and (3) ST-segment elevation ≥5 mm and discordant with the QRS complex (2 points). A total score of three or more points was considered diagnostic of acute MI, whereas a score of 2 points suggests an acute MI. A meta-analysis reported the Sgarbossa criteria to be specific (98%) but with low sensitivity (20%) [37]. Concordant ST elevation has proved to be the single most specific criterion for the diagnosis of acute MI in the presence of LBBB, and improves the detection of this “STEMI equivalent” [38] (Fig. 5). An adaptation of the Sgarbossa criteria, including ST-elevation to S-wave ratio may be somewhat complicated for use in the emergency setting [39]. Also, serial ECG recordings may be helpful for acute MI diagnosis in patients with acute chest pain or equivalent and LBBB [40].
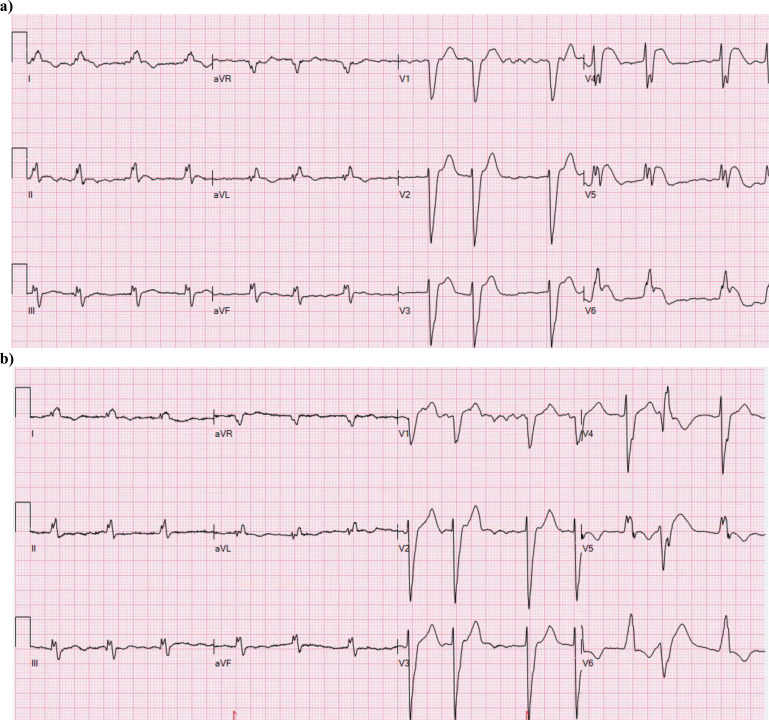
Fig. (5).
The ECG of a 74-year old man recorded 2.5 h after the initiation of chest pain. The medical history contains permanent atrial fibrillation with poorly controlled warfarin therapy, peripheral atherosclerosis, chronic obstructive pulmonary disease and frequent ventricular extrasystoles. The ECG in (a) shows LBBB with concordant ST elevation in I, and V5-V6. There is discordant ST elevation in V3-V4, but less than five mm. Coronary angiography showed single vessel disease with an occluded diagonal branch, possibly of embolic origin. (b) A previous ECG of the patient showing atrial fibrillation and LBBB (QRS 168 ms) with the “normal” secondary discordant ST/T changes associated with the conduction disorder. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
In a study from the Minneapolis Heart Institute STEMI protocol (n=3,903), new or presumably new LBBB was present in 3.3% of the patients [41]. The LBBB patients were older, more commonly women, with a lower ejection fraction, and more often presented with cardiac arrest or heart failure than those without new LBBB. The patients with new LBBB had fewer culprit arteries (54.2% vs. 86.4%, p<0.001), but at one-year follow-up they had higher all-cause mortality. The authors tested a hierarchical algorithm based on hemodynamic instability and Sgarbossa concordance criteria. The algorithm yielded high sensitivity (97%) and negative predictive value (94%) for identification of a culprit lesion, while specificity was 48%.
Another study from the US reported new or presumably new LBBB in 69/802 (8.6%) patients in the hospital primary PCI laboratory activation database [42]. Less than 30% of the patients with new or presumably new LBBB had troponin elevation, and 54% underwent emergent coronary angiography. Of these, 22% had a culprit vessel occlusion, while the emergent revascularization rate was 11.6%.
In a recent large registry study of patients with a definite diagnosis of acute MI, crude in-hospital mortality of patients with LBBB was 16.2% versus 6.5% for patients with STEMI [43] The patients with LBBB were older, with a greater burden of risk factors and comorbidity, and they were less likely to receive medication and invasive therapy.
In patients (n=8,830) with suspected acute MI, LBBB was present in 2.8%, and of these 30% had a final MI diagnosis, with similar incidence in those with known LBBB vs. those with presumably new LBBB [44]. ECG criteria had low sensitivity (1%-12%) but high specificity (95%–100%) for acute MI. The study showed that combining ECG criteria with high sensitive troponin testing allows early and accurate diagnosis of acute MI in LBBB.
The fourth UDMI states: “In patients with LBBB, ST-segment elevation ≥1 mm concordant with the QRS complex in any lead may be an indicator of acute myocardial ischemia” [31]. The conclusion was that since the detection of ischemia by the ECG in LBBB is difficult, decisions concerning urgent reperfusion therapy should be based mainly on symptoms and hemodynamic parameters.
According to the 2013 ACCF/AHA STEMI guidelines, “New or presumably new LBBB has been considered a STEMI equivalent. Most cases of LBBB at time of presentation, however, are “not known to be old” because of prior electrocardiogram (ECG) is not available for comparison. New or presumably new LBBB at presentation occurs infrequently, may interfere with ST-elevation analysis, and should not be considered diagnostic of acute myocardial infarction (MI) in isolation” [22]. The 2017 ESC STEMI guidelines specify: “In the presence of LBBB, the ECG diagnosis of acute myocardial infarction is difficult but often possible if marked ST-segment abnormalities are present. Somewhat complex algorithms have been offered to assist the diagnosis, but they do not provide diagnostic certainty. The presence of concordant ST-segment elevation (i.e. in leads with positive QRS deflections) appears to be one of the best indicators of ongoing MI with an occluded infarct artery. Patients with a clinical suspicion of ongoing myocardial ischaemia and LBBB should be managed in a way similar to STEMI patients, regardless of whether the LBBB is previously known. It is important to remark that the presence of a (presumed) new LBBB does not predict an MI per se”. [20].
Hence, recognition of an impending acute myocardial infarction in patients with LBBB remains a challenge. Decision making regarding emergent coronary angiography cannot be based solely on 12-lead ECG findings. Clinical presentation, and probably also, sensitive troponin testing are important additional clinical tools.
Conclusion
STEMI is frequently associated with conduction disorders. SA and AV block are more frequent in inferior than anterior STEMI, while new LAFB and RBBB indicate proximal occlusion of the LAD. Most conduction disorders associated with STEMI are reversible with reperfusion therapy, but they may still impair prognosis, because they indicate a large area at risk, extensive myocardial infarction or severe coronary artery disease.
Acknowledgements
Declared none.
List oF aBBrivations
- ACS
Acute Coronary Syndrome
- AV
Atrioventricular
- HDAVB
High Degree Atrioventricular Block
- IAB
Interatrial Block
- LAD
Left Anterior Descending Artery
- LAFB
Left Anterior Fascicular Block
- LBBB
Left Bundle Branch Block
- LCX
Left Circumflex Artery
- LSFB
Left Septal Fascicular Block
- MI
Myocardial Infarction
- PCI
Percutaneous Coronary Intervention
- RBBB
Right Bundle Branch Block
- RCA
Right Coronary Artery
- SA
Sinoatrial
- STEMI
ST-elevation Myocardial Infarction
- TIMI
Thrombolysis In Myocardial Infarction
- UDMI
Universal Definition of Myocardial Infarction
Consent for Publication
Not applicable.
Funding
None.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Zipes D.P. The clinical significance of bradycardic rhythms in acute myocardial infarction. Am. J. Cardiol. 1969;24(6):814–825. doi: 10.1016/0002-9149(69)90470-6. [DOI] [PubMed] [Google Scholar]
- 2.Serrano C.V., Jr, Bortolotto L.A., César L.A., et al. Sinus bradycardia as a predictor of right coronary artery occlusion in patients with inferior myocardial infarction. Int. J. Cardiol. 1999;68(1):75–82. doi: 10.1016/S0167-5273(98)00344-1. [DOI] [PubMed] [Google Scholar]
- 3.Liem K.L., Lie K.I., Louridtz W.J., Durrer D., Wellens H.J. Sinus bradycardia in acute myocardial infarct. Ned. Tijdschr. Geneeskd. 1976;120(14):604–608. [PubMed] [Google Scholar]
- 4.Shah S.P., Waxman S. Two cases of Bezold-Jarisch reflex induced by intra-arterial nitroglycerin in critical left main coronary artery stenosis. Tex. Heart Inst. J. 2013;40(4):484–486. [PMC free article] [PubMed] [Google Scholar]
- 5.Vikse J., Henry B.M., Roy J., et al. Anatomical Variations in the Sinoatrial Nodal Artery: A Meta-Analysis and Clinical Considerations. PLoS One. 2016;11(2):e0148331. doi: 10.1371/journal.pone.0148331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bayes de Luna A.J. Block at the auricular level. Rev. Esp. Cardiol. 1979;32(1):5–10. [PubMed] [Google Scholar]
- 7.Bayés de Luna A., Platonov P., Cosio F.G., et al. Interatrial blocks. A separate entity from left atrial enlargement: a consensus report. J. Electrocardiol. 2012;45(5):445–451. doi: 10.1016/j.jelectrocard.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 8.Çinier G., Tekkeşin A.I., Genç D., et al. Interatrial block as a predictor of atrial fibrillation in patients with ST-segment elevation myocardial infarction. Clin. Cardiol. 2018;41(9):1232–1237. doi: 10.1002/clc.23029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruña V., Velásquez-Rodríguez J., Valero-Masa M.J., et al. Prognostic of Interatrial Block after an Acute ST-Segment Elevation Myocardial Infarction. Cardiology. 2019;142(2):109–115. doi: 10.1159/000499501. [DOI] [PubMed] [Google Scholar]
- 10.Álvarez-García J., Vives-Borrás M., Gomis P., et al. Electrophysiological Effects of Selective Atrial Coronary Artery Occlusion in Humans. Circulation. 2016;133(23):2235–2242. doi: 10.1161/CIRCULATIONAHA.116.021700. [DOI] [PubMed] [Google Scholar]
- 11.Kosmidou I., Redfors B., Dordi R., et al. Incidence, Predictors, and Outcomes of High-Grade Atrioventricular Block in Patients With ST-Segment Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention (from the HORIZONS-AMI Trial). Am. J. Cardiol. 2017;119(9):1295–1301. doi: 10.1016/j.amjcard.2017.01.019. [DOI] [PubMed] [Google Scholar]
- 12.Bayés de Luna A., Fiol-Sala M. 2008. Acute coronary syndrome: unstable angina and acute myocardial infarction. [Google Scholar]
- 13.Lie K.I., Wellens H.J., Schuilenburg R.M., Durrer D. Mechanism and significance of widened QRS complexes during complete atrioventricular block in acute inferior myocardial infarction. Am. J. Cardiol. 1974;33(7):833–839. doi: 10.1016/0002-9149(74)90629-8. [DOI] [PubMed] [Google Scholar]
- 14.Wellens H.J.J., Gorgels A.P.M., Doevendans P.A. Wellens HJJ, Gorgels APM, Doevendans PA The ECG in acute myocardial infarction and unstable angina. Diagnosis and risk stratification. Dordrecht, the Netherlands: Kluwer Academic Publishers; 2003. Conduction disturbances in acute myocardial infarction. pp. 43–64. [Google Scholar]
- 15.Birnbaum Y., Sclarovsky S., Herz I., et al. Admission clinical and electrocardiographic characteristics predicting in-hospital development of high-degree atrioventricular block in inferior wall acute myocardial infarction. Am. J. Cardiol. 1997;80(9):1134–1138. doi: 10.1016/S0002-9149(97)00628-0. [DOI] [PubMed] [Google Scholar]
- 16.Berger P.B., Ryan T.J. Inferior myocardial infarction. High-risk subgroups. Circulation. 1990;81(2):401–411. doi: 10.1161/01.CIR.81.2.401. [DOI] [PubMed] [Google Scholar]
- 17.Alnsasra H., Ben-Avraham B., Gottlieb S., et al. High-grade atrioventricular block in patients with acute myocardial infarction. Insights from a contemporary multi-center survey. J. Electrocardiol. 2018;51(3):386–391. doi: 10.1016/j.jelectrocard.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Kim K.H., Jeong M.H., Ahn Y., Kim Y.J., Cho M.C., Kim W. Other Korea acute myocardial infarction registry investigators. differential clinical implications of high-degree atrioventricular block complicating st-segment elevation myocardial infarction according to the location of infarction in the Era of Primary Percutaneous Coronary Intervention. Korean Circ. J. 2016;46(3):315–323. doi: 10.4070/kcj.2016.46.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aguiar Rosa S., Timóteo A.T., Ferreira L., et al. Complete atrioventricular block in acute coronary syndrome: prevalence, characterisation and implication on outcome. Eur. Heart J. Acute Cardiovasc. Care. 2018;7(3):218–223. doi: 10.1177/2048872617716387. [DOI] [PubMed] [Google Scholar]
- 20.Kosmidou I., Redfors B., McAndrew T., et al. Worsening atrioventricular conduction after hospital discharge in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention: the HORIZONS-AMI trial. Coron. Artery Dis. 2017;28(7):550–556. doi: 10.1097/MCA.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 21.Ibanez B., James S., Agewall S., et al. ESC Scientific Document Group 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018;39(2):119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 22.O’Gara P.T., Kushner F.G., Ascheim D.D., et al. American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127(4):e362–e425. doi: 10.1161/CIR.0b013e3182742c84. [DOI] [PubMed] [Google Scholar]
- 23.Engelen D.J., Gorgels A.P., Cheriex E.C., et al. Value of the electrocardiogram in localizing the occlusion site in the left anterior descending coronary artery in acute anterior myocardial infarction. J. Am. Coll. Cardiol. 1999;34(2):389–395. doi: 10.1016/S0735-1097(99)00197-7. [DOI] [PubMed] [Google Scholar]
- 24.Pérez-Riera A.R., Barbosa-Barros R., Daminello-Raimundo R., de Abreu L.C., Tonussi Mendes J.E., Nikus K. Left posterior fascicular block, state-of-the-art review: A 2018 update. Indian Pacing Electrophysiol. J. 2018;18(6):217–230. doi: 10.1016/j.ipej.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sclarovsky S., Sagie A., Strasberg B., Lewin R.F., Rehavia E., Agmon J. Transient right axis deviation during acute anterior wall infarction or ischemia: electrocardiographic and angiographic correlation. J. Am. Coll. Cardiol. 1986;8(1):27–31. doi: 10.1016/S0735-1097(86)80087-0. [DOI] [PubMed] [Google Scholar]
- 26.Pérez-Riera A.R., Barbosa-Barros R., Daminello-Raimundo R., de Abreu L.C., Nikus K. The tetrafascicular nature of the intraventricular conduction system. Clin. Cardiol. 2019;42(1):169–174. doi: 10.1002/clc.23093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pérez-Riera A.R., Barbosa-Barros R., Lima Aragão W., et al. Transient left septal fascicular block in the setting of acute coronary syndrome associated with giant slurring variant J-wave. Ann. Noninvasive Electrocardiol. 2018;23(6):e12536. doi: 10.1111/anec.12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pérez-Riera A.R., Barbosa-Barros R. Fernandes Silva E Sousa Neto A, Daminello-Raimundo R, de Abreu LC, Nikus K. Transient prominent anterior QRS forces in the setting ST segment elevation coronary syndrome: Left septal fascicular block. J. Electrocardiol. 2018;51(5):798–800. doi: 10.1016/j.jelectrocard.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 29.Frink R.J., James T.N. Normal blood supply to the human His bundle and proximal bundle branches. Circulation. 1973;47(1):8–18. doi: 10.1161/01.CIR.47.1.8. [DOI] [PubMed] [Google Scholar]
- 30.Fiol M., Carrillo A., Rodríguez A., Pascual M., Bethencourt A., Bayés de Luna A. Electrocardiographic changes of ST-elevation myocardial infarction in patients with complete occlusion of the left main trunk without collateral circulation: differential diagnosis and clinical considerations. J. Electrocardiol. 2012;45(5):487–490. doi: 10.1016/j.jelectrocard.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 31.Sclarovsky S., Lewin R.F., Strasberg B., Agmon J. Left anterior hemiblock obscuring the diagnosis of right bundle branch block in acute myocardial infarction. Circulation. 1979;60(1):26–32. doi: 10.1161/01.CIR.60.1.26. [DOI] [PubMed] [Google Scholar]
- 32.Xiang L., Zhong A., You T., Chen J., Xu W., Shi M. Prognostic Significance of Right Bundle Branch Block for Patients with Acute Myocardial Infarction: A Systematic Review and Meta-Analysis. Med. Sci. Monit. 2016;22:998–1004. doi: 10.12659/MSM.895687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thygesen K., Alpert J.S., Jaffe A.S., et al. ESC Scientific Document Group Fourth universal definition of myocardial infarction (2018). Eur. Heart J. 2019;40(3):237–269. doi: 10.1093/eurheartj/ehy462. [DOI] [PubMed] [Google Scholar]
- 34.Widimsky P., Rohác F., Stásek J., et al. Primary angioplasty in acute myocardial infarction with right bundle branch block: should new onset right bundle branch block be added to future guidelines as an indication for reperfusion therapy? Eur. Heart J. 2012;33(1):86–95. doi: 10.1093/eurheartj/ehr291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neumann J.T., Sörensen N.A., Rübsamen N., et al. Right bundle branch block in patients with suspected myocardial infarction. Eur. Heart J. Acute Cardiovasc. Care. 2019;8(2):161–166. doi: 10.1177/2048872618809700. [DOI] [PubMed] [Google Scholar]
- 36.Sgarbossa E.B., Pinski S.L., Barbagelata A., et al. Electrocardiographic diagnosis of evolving acute myocardial infarction in the presence of left bundle-branch block. GUSTO-1 (Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries) Investigators. N. Engl. J. Med. 1996;334(8):481–487. doi: 10.1056/NEJM199602223340801. [DOI] [PubMed] [Google Scholar]
- 37.Tabas J.A., Rodriguez R.M., Seligman H.K., et al. Electrocardiographic criteria for detecting acute myocardial infarction in patients with left bundle branch block: a meta-analysis. Ann. Emerg. Med. 2008;52(4):329–336. doi: 10.1016/j.annemergmed.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 38.Lopes R.D., Siha H., Fu Y., et al. Diagnosing acute myocardial infarction in patients with left bundle branch block. Am. J. Cardiol. 2011;108(6):782–788. doi: 10.1016/j.amjcard.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 39.Smith S.W., Dodd K.W., Henry T.D., Dvorak D.M., Pearce L.A. Diagnosis of ST-elevation myocardial infarction in the presence of left bundle branch block with the ST-elevation to S-wave ratio in a modified Sgarbossa rule. Ann. Emerg. Med. 2012;60(6):766–776. doi: 10.1016/j.annemergmed.2012.07.119. [DOI] [PubMed] [Google Scholar]
- 40.Wackers F.J.T., Lie K.J., David G., et al. Assessment of the value of electrocardiographic signs for myocardial infarction in left bundle branch block. In: Wellens H.J.J., Kulbertus H.E., editors. What’s new in electrocardiography? Springer, Dordrecht. The Hague, The Netherlands: Martinus Nijhoff; 1981. pp. 37–57. [Google Scholar]
- 41.Pera V.K., Larson D.M., Sharkey S.W., et al. New or presumed new left bundle branch block in patients with suspected ST-elevation myocardial infarction. Eur. Heart J. Acute Cardiovasc. Care. 2018;7(3):208–217. doi: 10.1177/2048872617691508. [DOI] [PubMed] [Google Scholar]
- 42.Mehta N., Huang H.D., Bandeali S., Wilson J.M., Birnbaum Y. Prevalence of acute myocardial infarction in patients with presumably new left bundle-branch block. J. Electrocardiol. 2012;45(4):361–367. doi: 10.1016/j.jelectrocard.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 43.Erne P., Iglesias J.F., Urban P., et al. Left bundle-branch block in patients with acute myocardial infarction: Presentation, treatment, and trends in outcome from 1997 to 2016 in routine clinical practice. Am. Heart J. 2017;184:106–113. doi: 10.1016/j.ahj.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 44.Nestelberger T., Cullen L., Lindahl B., et al. APACE, ADAPT and TRAPID-AMI Investigators. Diagnosis of acute myocardial infarction in the presence of left bundle branch block. Heart. 2019;105(20):1559–1567. doi: 10.1136/heartjnl-2018-314673. [DOI] [PubMed] [Google Scholar]