Abstract
Any patient with a herpes zoster infection of the mandibular branch of the trigeminal nerve should benefit from early oral monitoring, especially in elderly population where traumatic dental prostheses are common, because osteonecrosis can occur.
Keywords: herpes zoster, jaw osteonecrosis, oral ulceration, trigeminal nerve, zona
Herpes zoster infection is a rare and little‐known etiology of osteonecrosis of the maxilla. Only a few cases have reported in the international literature. We present a rare case of right mandibular osteonecrosis following herpes zoster infection in the mandibular branch of the trigeminal nerve.

1. INTRODUCTION
Mandibular osteonecrosis is a well‐known phenomenon in oral pathology. It is due to a local ischemia and generally occurs in patients with a history of local irradiation (osteoradionecrosis), 1 anti‐osteoclastic treatments such as bisphosphonates, therapeutic monoclonal antibodies like denosumab and tocilizumab 2 (osteochimionecrosis), or chronic osteitis. 1
However, exceptionally, other etiologies can be found. We are reporting a rare case of right mandibular osteonecrosis following herpes zoster infection in the mandibular branch of the trigeminal nerve according to CARE Guidelines. Only a few cases have reported in the international literature. 3
2. CASE PRESENTATION
An 87‐year‐old Caucasian man was referred by his attending dentist to the Oral Surgery Department of the Metz Regional Hospital about a gingival ulceration that had appeared one month earlier. The patient's medical history revealed an uncontrolled diabetes (HbA1c: 8, 8%), complicated by microangiopathy and macroangiopathy, myocardial infarction and IOMA that required a femoral bypass. His usual treatment included Kardegic®, Clopidogrel, Metformin, Nebivolol, Perindopril, and Atorvastatin.
Immediately after his last hospital discharge, the patient presented anesthesia of the right preauricular area and of the right antero‐inferior gingiva, followed by systematized vesicles in the territory of the mandibular branch of the trigeminal nerve (V.3: responsible of mandibular sensitivity), typical of herpes zoster infection of the right mandibular nerve (Figure 1).
FIGURE 1.

Herpes zoster infection in the territory of the right mandibular nerve
One month later, while he was still presenting a zone of persistent anesthesia in this same territory, a large right gingival ulceration under his complete removable mandibular prosthesis appeared.
The mucosal biopsy was not contributory because it evoked a pseudoepitheliomatous hyperplasia that could correspond to an irritative origin (rubbing of his removable prosthesis).
When he consulted the Oral Surgery Department two weeks later, the patient reported discomfort in wearing his prosthesis, responsible for feeding difficulties. Intraoral examination revealed a large bone exposure in the previously ulcerated area, measuring 2cm x 1cm, and showing necrotic alveolar bone associated with peripheral suppuration. There was no regional superinfection and the cutaneous herpes zoster was at the end of its course despite the persistence of hypoesthesia in the V.3 innervation territory (Figures 2 and 3).
FIGURE 2.

Cutaneous aspect at the end of the infection
FIGURE 3.
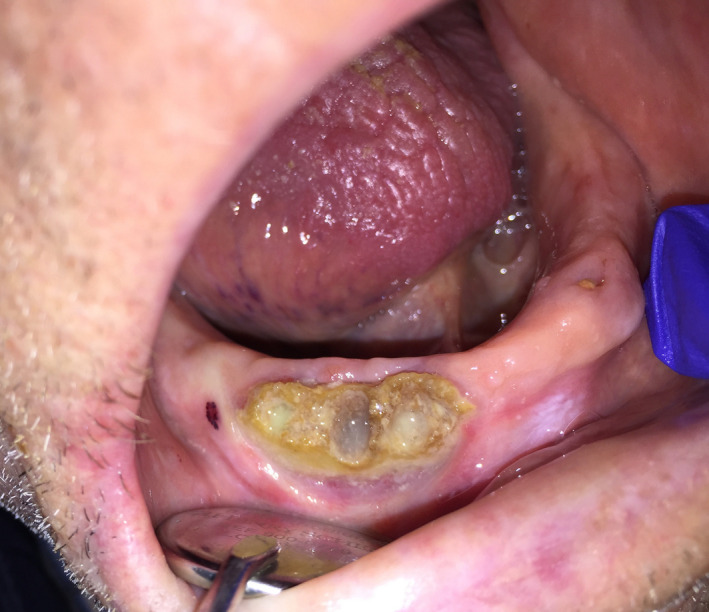
Large bone exposure of the right mandible
CBCT (Cone Beam Computed Tomography, Figure 4) objectified an inhomogeneous aspect of this mandibular region, mixing osteolysis, and dense radiographic areas. The necrotic zone was poorly defined in the periphery, with no periosteal apposition and no border that could suggest sequestration.
FIGURE 4.

CBCT: Inhomogeneous aspect of this bone region showing osteolysis
In light of this medical history, topographical arguments, and literature reviews, a right mandibular osteonecrosis occurring following herpes zoster infection in the mandibular branch of the trigeminal nerve was retained.
A surgical resection of the necrotic zone during a local anesthesia has been realized in one month later (Figures 5 and 6) and followed by an antibiotic therapy (Amoxicillin 1g q8hr for 7 days and Metronidazole 500mg q8hr for 7 days). Anatomopathological analysis objectified uninhabited osteocyte cubicles and the presence of necrotic material areas, confirming osteonecrosis (Figures 7 and 8).
FIGURE 5.

Intraoperative aspect before resection of the necrotic bone
FIGURE 6.

Intraoperative aspect after resection of the necrotic bone
FIGURE 7.

Areas of necrotic material within bone trabeculae (hematoxylin‐eosin, 4x)
FIGURE 8.

Uninhabited osteocyte cubicles within bone trabeculae (hematoxylin‐eosin, 40x)
One week later, postoperative control was satisfactory, the oral mucosa was in the healing process and the patient had no symptoms. Regular clinical follow‐up has been done, which confirmed a good evolution with an optimal cicatrization and no recurrence of the symptoms after 3 months (Figure 9).
FIGURE 9.

Mucosal healing three months after surgery
Currently, the patient is also being followed for the renewal of his prosthesis by our prosthodontist colleagues and has been followed for more than 4 months with a very satisfactory evolution.
This case of osteonecrosis, although quite typical in its oral presentation, is highly original due to its postherpetic etiology.
3. DISCUSSION
Herpes zoster infection (HZI) is caused by the reactivation of the VZV virus, 4 originally responsible for the primary infection of chicken pox, occurring usually in childhood. Then, the virus remains latent in the sensory nerve ganglia 5 and during some episode of trauma, immunosuppression (neoplasia, hematological pathology, HIV, diabetes, immunosuppressive treatment), or postradiotherapy, 1 , 4 , 5 it can reactivate with the appearance of a unilateral vesiculobullous skin rash in a metameric territory. The most commonly affected sites are the thoracic dermatomes (T3‐L3) in 56% of cases and the trigeminal ganglia in 18%‐22% of cases (most frequently ophthalmic branch). 3 , 4 When there is maxillary or mandibular branch involvement, dental pain, root resorption, pulp necrosis or pulp calcifications, periapical lesions, or tooth exfoliation are reported to occur. 4 , 5
Some rare cases of maxilla osteonecrosis following herpes zoster infection have been described in the literature. 4 , 5
Rose et al 3 first described bone alterations related to an episode of shingles in 1908 and in 1922, Gonnet et al 6 described the first case of bone alveolar osteonecrosis associated with dental exfoliations following zoster infection. Since 1922, only 48 cases have been recorded worldwide, 3 which clearly shows that this is a rare and little‐known etiology of osteonecrosis of the maxilla.
Samprati et al 1 explains that tooth exfoliation is an early sign of postherpetic osteonecrosis because there is a loss of dental proprioception associated with impaired periosteal blood flow, leading to necrosis of the periodontal ligament prior to necrosis of the alveolar bone.
In our case, sensitivity disorders could be explained by herpes zoster infection or directly bone destruction next to the canal of the inferior alveolar nerve, although postherpetic algae are more frequently found than sensory deficits. 3 We suspect that his gingival ulceration could have been caused by a prosthetic trauma on an insensitive gingiva. This feeling was confirmed by the patient himself and our examination of his prosthesis confirmed that it was largely maladjusted.
In a recent literature review, Gupta et al 3 reported 46 similar cases. The mean age found was 52 years (wide age range of 6‐79 years), with a slight male predilection. Among the 46 cases described, 26 patients were a priori immunocompetent, and 20 patients presented various comorbidities such as hematological or oncological pathologies, immunosuppression, viral or bacterial infection (HIV, tuberculosis, CMV) or chronic hepatitis, and acute rheumatic fever.
More recently, Gholami et al 5 reported that bone necrosis appeared between 9 and 150 days after the onset of shingles, with an average of 30 days and that infection was most often severe. Our patient presented gingival ulceration one month after shingles's symptoms. This variable time may, therefore, justify the regular and prolonged follow‐up for several months of these at‐risk patients.
This author also reported a case of left mandibular osteonecrosis occurring after herpes zoster infection in a patient with only a history of high blood pressure and unbalanced diabetes, such as our patient's case. Uncontrolled diabetes leads to a state of relative immunosuppression with increased susceptibility to infections (and recurrences) and microangiopathy can play a role in local ischemia.
Nevertheless, the pathologic mechanisms behind HZI‐involving osteonecrosis remains a contentious subject.
Mendieta et al 7 suggests that the direct invasion of blood vessels by virus spreading from adjacent cranial nerves causes segmental granulomatous vasculitis. As this mechanism has already been reported with multifocal infarcts in the brain and spinal cord, it could be the same mechanism in the cranial nerves. Furthermore, in the maxillary region, the neurovascular relationships are very close. In addition, a pre‐existing infection could aggravate this phenomenon. 8 Our patient was wearing a removable mandibular prosthesis responsible for a daily trauma on the mucous membrane and underlying bone that could further compromise an already impaired vascularization.
Owotade et al 9 suggests that this could rather result from a compression of the alveolar artery within its narrow bone canal, by the nervous edema triggered by the infection, leading to arterial ischemia and then necrosis of the supplied territory.
More recently, Gholami et al 5 noted that chronic mucosal ulceration most often goes unnoticed in an immunocompromised patient, thus leading to delayed diagnosis and bacterial colonization responsible for osteomyelitis.
In our clinical case, postherpetic osteonecrosis has been probably triggered or at least aggravated by the wearing of a traumatic mandibular removable prosthesis on a hypoesthetized area and favored by the existence of uncontrolled diabetes. The prosthetic trauma can, thus, be assimilated to the traumatic factor frequently found in the triggering of osteochimionecrosis and osteoradionecrosis.
Omolehinwa 10 reported that trauma mainly creates a portal of entry for microorganisms to invade a hypoxic‐hypocellular‐hypovascular bone (3H theory) in osteoradionecrosis. He also reported that local risk factors like trauma may predispose to osteochimionecrosis, in addition to drugs and systemic risk factors like diabetes.
Many authors 11 , 12 have similarly reported the role of trauma in triggering or aggravating the onset of osteoradionecrosis or osteochimionecrosis.
This type of case report could allow a better understanding of the mechanisms in the phenomena of osteonecrosis, all etiologies combined.
In the recent studies, complementary treatments, including the application of platelet concentrates in solid and liquid form, are being developed and studied both to prevent jaws osteonecrosis and to improve healing after surgical treatment of bone lesions. 13 , 14
4. CONCLUSION
Herpes zoster infection is a rare and little‐known etiology of osteonecrosis of the maxilla.
Uncontrolled diabetes could be one of the risk factors for the development of this pathology, but we suspect that traumatic dental prosthesis could be the main triggering factor, as it is in numerous similar diseases. This highlights the primordial importance of conscientious dental care in this elderly population, especially those living in nursing homes, because potential consequences can largely overflow the dental domain. On the other hand, more studies would be necessary to specify the physiopathology of this phenomenon and thus identify the associated risk factors in order to permit a global multidisciplinary management.
Consequently, any patient with a herpes zoster infection of the maxillary or mandibular branch of the trigeminal nerve should benefit from early oral monitoring, especially in elderly population where traumatic dental prostheses are common, because osteonecrosis can occur.
ETHICS STATEMENT
We obtained the patient's written consent for this article.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
AUTHOR CONTRIBUTIONS
EF: wrote and drafted the manuscript. RC, MED, EG: helped draft the manuscript. EAP: provided the histological photographs. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
The authors are grateful to Dr Cuny J. F. (Department of Dermatology and Venerology, Metz Regional Hospital, France) for extremely valuable comments and suggestions. We would also like to thank our patient and his family for the courtesy of giving us all the information we needed.
Faure E, Engels‐Deutsch M, Paraschiv E‐A, Gérard E, Curien R. Mandibular osteonecrosis following herpes zoster infection: Report of a rare case with a literature review. Clin Case Rep. 2021;9:e04196. 10.1002/ccr3.4196
Funding information
This article received no funding
DATA AVAILABILITY STATEMENT
No data are associated with this article.
REFERENCES
- 1. Badjate SJ, Cariappa KM, Shenoi SR, Nakhate S. Ramsay‐Hunt syndrome complicating osteonecrosis of edentulous maxilla and mandible: report of a rare case. J Maxillofac Oral Surg. 2009;8(2):188‐191. 10.1007/s12663-009-0046-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bennardo F, Buffone C, Giudice A. New therapeutic opportunities for COVID‐19 patients with Tocilizumab: possible correlation of interleukin‐6 receptor inhibitors with osteonecrosis of the jaws. Oral Oncol. 2020;106:104659. 10.1016/j.oraloncology.2020.104659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gupta S, Sreenivasan V, Patil PB. Dental complications of herpes zoster: two case reports and review of literature. Indian J Dent Res. 2015;26(2):214‐219. [DOI] [PubMed] [Google Scholar]
- 4. Song J‐M, Seo J‐S, Lee J‐Y. Mandibular osteonecrosis following herpes zoster infection in the mandibular branch of the trigeminal nerve: a case report and literature review. J Korean Assoc Oral Maxillofac Surg. 2015;41(6):357. 10.5125/jkaoms.2015.41.6.357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gholami M, Shahakbari R, Abdolahpour S, Hatami M, Roshanmir A. Herpes zoster induced alveolar bone necrosis in immunocompromised patients; two case reports. Iran J Otorhinolaryngol. 2016;28(5):369‐373. 10.22038/ijorl.2016.7446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kamarthi N, LA Narasimha GE. An unusual case of osteonecrosis and spontaneous tooth exfoliation following trigeminal herpes zoster in a HIV seropositive patient. Int J Oral Med Sci. 2009;8:52‐59. [Google Scholar]
- 7. Mendieta C, Miranda J, Brunet LI, Gargallo JBL. Alveolar bone necrosis and tooth exfoliation following herpes zoster infection: a review of the literature and case report. J Periodontal. 2005;76(1):148‐153. [DOI] [PubMed] [Google Scholar]
- 8. Volvoikar P, DA Patil S. Tooth exfoliation, osteonecrosis and neuralgia following herpes zoster of trigeminal nerve. Ind J Dent Res. 2002;13(1):11‐14. [PubMed] [Google Scholar]
- 9. Owotade FJ, Ugboko VIBK. Herpes zoster infection of maxilla. J Oral Maxillofac Surg. 1999;57(10):1249‐1251. [DOI] [PubMed] [Google Scholar]
- 10. Omolehinwa TT, SOA . Chemical and radiation associated jaw lesions. Dent Clin North Am. 2016;60(1):265‐277. 10.1016/j.cden.2015.08.009.Chemical [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nicolatou‐Galitis O, Schiødt M, Mendes RA, et al. Medication‐related osteonecrosis of the jaw: definition and best practice for prevention, diagnosis, and treatment. Oral Surg Oral Med Oral Pathol Oral Radiol. 2019;127(2):117‐135. 10.1016/j.oooo.2018.09.008 [DOI] [PubMed] [Google Scholar]
- 12. Khan AA, Morrison A, Hanley DA, et al. Diagnosis and management of osteonecrosis of the jaw: a systematic review and international consensus. J Bone Miner Res. 2015;30(1):3‐23. 10.1002/jbmr.2405 [DOI] [PubMed] [Google Scholar]
- 13. Fortunato L, Bennardo F, Buffone C, Giudice A. Is the application of platelet concentrates effective in the prevention and treatment of medication‐related osteonecrosis of the jaw? A systematic review. J Cranio‐Maxillofacial Surg. 2020;48(3):268‐285. 10.1016/j.jcms.2020.01.014 [DOI] [PubMed] [Google Scholar]
- 14. Bennardo F, Bennardo L, Del Duca E, et al. Autologous platelet‐rich fibrin injections in the management of facial cutaneous sinus tracts secondary to medication‐related osteonecrosis of the jaw. Dermatol Ther. 2020;33(3):e13334. 10.1111/dth.13334 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data are associated with this article.


