Abstract
Objective
To improve the echocardiographic assessment of heart failure in patients with atrial fibrillation (AF) by comparing conventional averaging of consecutive beats with an index-beat approach, whereby measurements are taken after two cycles with similar R-R interval.
Methods
Transthoracic echocardiography was performed using a standardised and blinded protocol in patients enrolled in the RATE-AF (RAte control Therapy Evaluation in permanent Atrial Fibrillation) randomised trial. We compared reproducibility of the index-beat and conventional consecutive-beat methods to calculate left ventricular ejection fraction (LVEF), global longitudinal strain (GLS) and E/e’ (mitral E wave max/average diastolic tissue Doppler velocity), and assessed intraoperator/interoperator variability, time efficiency and validity against natriuretic peptides.
Results
160 patients were included, 46% of whom were women, with a median age of 75 years (IQR 69–82) and a median heart rate of 100 beats per minute (IQR 86–112). The index-beat had the lowest within-beat coefficient of variation for LVEF (32%, vs 51% for 5 consecutive beats and 53% for 10 consecutive beats), GLS (26%, vs 43% and 42%) and E/e’ (25%, vs 41% and 41%). Intraoperator (n=50) and interoperator (n=18) reproducibility were both superior for index-beats and this method was quicker to perform (p<0.001): 35.4 s to measure E/e’ (95% CI 33.1 to 37.8) compared with 44.7 s for 5-beat (95% CI 41.8 to 47.5) and 98.1 s for 10-beat (95% CI 91.7 to 104.4) analyses. Using a single index-beat did not compromise the association of LVEF, GLS or E/e’ with natriuretic peptide levels.
Conclusions
Compared with averaging of multiple beats in patients with AF, the index-beat approach improves reproducibility and saves time without a negative impact on validity, potentially improving the diagnosis and classification of heart failure in patients with AF.
Keywords: atrial fibrillation, echocardiography, heart failure, diastolic, systolic
Introduction
The prevalence of atrial fibrillation (AF) is climbing,1 and around 50% of patients with AF have or will develop heart failure.2 3 Assessment of systolic and diastolic ventricular function using echocardiography is essential for AF management, including stroke risk stratification, choice of rate and rhythm control therapy, and the identification of heart failure.4 However, assessment of systolic and diastolic function is challenging in AF due to variable R-R intervals leading to beat-to-beat changes in left ventricular function. This may explain why the association of echocardiography parameters with adverse events is weaker in AF compared with sinus rhythm.5 In clinical practice, multiple beats are averaged despite a lack of any evidence base for this approach.6 Multiple beat acquisition and analysis is time-consuming and may not adequately compensate for the variable stroke volume and filling time seen in AF.7 To correctly classify heart failure in the context of AF and manage it appropriately, it is crucial to have accurate and reproducible measurements of systolic and diastolic function.8
The aim of this study was to systematically compare the reproducibility and efficiency of a more physiological approach to improve the assessment of heart failure in patients with AF. The index-beat method takes into account time-dependent processes involved in contractility and relaxation.9 We compared this approach with conventional averaging of 3 beats (clinical routine) and 5 beats and 10 beats (as recommended in guidelines), with complete blinding of observers. Unlike previous studies, there was no preselected exclusion of patients according to image quality.10 We hypothesised that the index-beat method would be more reproducible and time-efficient, facilitating better management of patients with AF and heart failure.
Methods
Patient population
Baseline echocardiograms were evaluated for all participants enrolled in the RATE-AF (RAte control Therapy Evaluation in permanent Atrial Fibrillation) randomised trial after obtaining written informed consent (NCT02391337). The trial received oversight from a trial steering committee and data monitoring committee with independent chairs. The rationale and methods for the RATE-AF trial have previously been published.11 In brief, outpatients aged 60 years or older with permanent AF and symptoms of heart failure (New York Heart Association class II or above) were randomised to digoxin or beta-blockers for rate control of AF. Permanent AF was defined as a physician’s decision for rate control and no plans for antiarrhythmic drugs or interventional rhythm control. Patients were excluded if their heart rate was <60 beats per minute or had prior evidence of second-degree or third-degree heart block. Other exclusion criteria were minimised to enable generalisable ‘real-world’ results. There were no exclusion criteria related to known heart failure or according to left ventricular ejection fraction (LVEF), apart from those with decompensated heart failure in the last 14 days, evidenced by the need for intravenous inotropes, vasodilators or diuretics.11 The trial was publicly funded by the UK National Institute for Health Research and the main results have been published.10
Echocardiography protocol
AF was confirmed on 12-lead ECG. All patients then underwent transthoracic echocardiography using Philips EPIQ 7 and X5-1 transducer, by an experienced echocardiographer accredited with the British Society of Echocardiography. Patients were positioned in left lateral decubitus position and images were acquired during quiet respiration according to an echocardiography protocol ratified prior to the trial commencing.
A minimum of 30-beat loops were obtained of the apical two-chamber, three-chamber and four-chamber views. All images were optimised to maximise frame rate while ensuring all left ventricular segments were visible. A minimum of 30 traces of tissue Doppler imaging (TDI)-derived lateral and septal e’ were obtained and mitral inflow using pulse wave Doppler acquired at a sweep speed of 50 mm/s. The recorded images were given a unique identification number and all patient-identifiable features (including trial number) were removed, blinding the operator to patient, trial and clinical details.
All measurements were analysed offline by the same operator a minimum of 3 months after the scan date. To minimise selection bias, there were no predefined exclusions made on the quality of imaging; however, if image quality was insufficient to accurately obtain measurements, these images were excluded from analysis. All analyses were performed on Philips Q-Station (V.3.5; Philips Healthcare, Andover, Massachusetts). For LVEF, Simpson’s biplane LVEF was measured from the apical four-chamber and two-chamber views. Longitudinal strain was taken from the apical two-chamber, three-chamber and four-chamber views and then averaged to generate overall global longitudinal strain (GLS). The mean frame rate for GLS acquisition was 57 Hertz (SD 7.0). For E/e’, mitral valve peak E velocity was measured and then divided by the TDI-derived e’ (averaged from the lateral and septal walls).
Index-beat and conventional averaging method
The index-beat was identified as the cardiac cycle which followed a preceding and pre-preceding R-R interval of similar duration (within 60 ms of each other; figure 1). R-R intervals were measured using a calliper in the reporting software. The index-beats were selected consecutively from the beginning of each set of echocardiogram data to avoid selection bias. Conventional analysis involved averaging of 3, 5 and 10 consecutive cardiac cycles from a 10-beat data set.
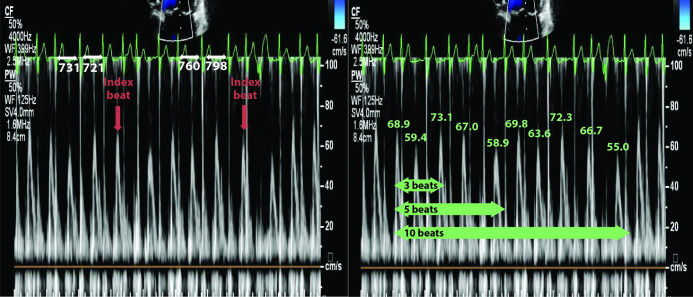
Figure 1.
Index-beat approach versus conventional averaging of consecutive beats. Left panel: pulse wave mitral inflow Doppler using the index-beat method, with similar preceding and pre-preceding R-R intervals indicated by the red arrows. Values are the R-R interval length of the preceding cardiac cycle. Right panel: 3, 5 and 10 consecutive beats. Values are the peak E velocity measurement in cm/s.
Intraoperator reproducibility
The same operator took a second set of 10 images to measure GLS and E/e’ (using the methods described above) with a single index-beat and an average of 3, 5 and 10 beats.
Interoperator reproducibility and time comparison
A second accredited operator obtained images in 18 randomly selected patients to measure GLS and E/e’ using a single index-beat and average of 3, 5 and 10 beats. The operator was blinded to the previously recorded measurements as well as all clinical details of each echocardiogram. The time taken to select and measure E/e’ using a single index-beat and guideline-recommended 5 and 10 consecutive beats was measured, with time commencing from the first visualisation of Doppler images.
Validity
All patients had N-terminal pro-B-type natriuretic peptide (NT-proBNP) measured on the same day as their echocardiogram using an Abbott Alinity platform. This was correlated with the parameters LVEF Simpson’s biplane, GLS and E/e’.
Patient and public involvement
A patient and public involvement team were involved in the design and conduct of this study and assisted with the plain English summary.
Statistical analysis
Summary results are presented as percentage, mean with SD, or median with IQR (displayed as 25th–75th quartiles). All echocardiographic measurements were transformed to their natural log value. A multilevel mixed effects linear regression model (adjusting for the random effects of the patient) was used to calculate the intraclass correlation coefficient (ICC) and derive the SD (σ), with 95% CI for the within-beat variability. Post-hoc analysis indicated >90% assurance that the sample size was sufficient for ICC comparison.12 The coefficient of variation (CV) within three index-beats and within each set of consecutive beats (3, 5 and 10) was calculated using the following formula: √exp(SD2)−1×100; 95% CIs were calculated for each CV. For intraoperator and interoperator reproducibility, Bland and Altman analysis was used to obtain the mean bias and limits of agreement, and the ICC with 95% CI was calculated at the patient level for each measurement method using a multilevel mixed effects linear regression model (adjusting for the random effects of the patient and the time the measurement was taken). The statistical comparison of time taken was performed using a two-tailed t-test accounting for unequal variance. The association with NT-proBNP was compared using Spearman’s r and univariate linear regression analysis. The difference in correlation coefficient between measuring on an index-beat and averaging 3, 5 and 10 consecutive beats was calculated using the method of Meng et al.13 Statistical analysis was performed using Stata V.14.2. A two-tailed p value of 0.05 was considered statistically significant.
Results
One hundred and sixty patients were included, with a median age of 75 years (IQR 69–82), heart rate of 100 beats per minute at time of acquisition (86–112) and blood pressure of 134/84 mm Hg (123/76–148/93) (see table 1). The median LVEF was 59% (52–64), GLS −14% (−12 to −15) and E/e’ 9.4 (7.8–11.7); variables measured by consecutive beats are listed in online supplemental table 2. Image quality was insufficient for LVEF measurement in 18 patients (11.3%) and for GLS in 21 patients (13.1%), with strain assessment more challenging at higher heart rates. All patients had sufficient image quality to measure E/e’. Other echocardiogram parameters of interest are summarised in table 2. Online supplemental figure 1 depicts the flow diagram of the study population. A plain English summary of results is presented in online supplemental table 1.
Table 1.
Baseline demographics
| Characteristics | N=160 |
| Age, median years (IQR) | 75 (69–82) |
| Women, n (%) | 74 (46) |
| Years in AF, mean years (SD) | 3.8 (6) |
| Ethnicity: white British or Irish, n (%) | 149 (93.1) |
| Ethnicity: black African, Caribbean or black British, n (%) | 3 (1.9) |
| Ethnicity: Asian or Asian British, n (%) | 8 (5.01) |
| Previous rhythm control, n (%) | 23 (14) |
| Modified EHRA class 3 or 4, n (%) | 77 (48) |
| Previous heart failure clinical diagnosis, n (%) | 59 (37) |
| Signs of heart failure at randomisation, n (%) | 84 (53) |
| NYHA class III or IV, n (%) | 61 (38) |
| Previous myocardial infarction, n (%) | 13 (8) |
| Previous stroke, n (%) | 19 (12) |
| Previous TIA, n (%) | 15 (9) |
| COPD, n (%) | 29 (18) |
| Diabetes mellitus, n (%) | 38 (24) |
| Heart rate, median bpm (IQR) | 100 (86–112) |
| Systolic BP, median mm Hg (IQR) | 134 (123–148) |
| Diastolic BP, median mm Hg (IQR) | 84 (76–93) |
| Body mass index, median kg/m2 (IQR) | 30 (26–34) |
| NT-proBNP, median pg/mL (IQR) | 1057 (744–1522) |
| Estimated GFR, median mL/min (IQR) | 67 (55–77) |
| Already receiving anticoagulant medication, n (%) | 135 (84) |
| Antihypertensive medication, n (%) | 116 (73) |
| Inhalers for airway disease, n (%) | 40 (25) |
AF, atrial fibrillation; BP, blood pressure; bpm, beats per minute; COPD, chronic obstructive pulmonary disorder; EHRA, European Heart Rhythm Association; GFR, glomerular filtration rate; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Failure Association functional classification; TIA, transient ischaemic attack.
Table 2.
Echocardiography parameters
| Echocardiographic measurement | Baseline |
| Left ventricular end diastolic volume, median mL (IQR) | 76 (57–99) |
| Left ventricular end systolic volume, median mL (IQR) | 30 (22–42) |
| Stroke volume, median mL (IQR) | 55 (45–64) |
| Left ventricular ejection fraction, median % (IQR) | 59 (52–64) |
| Global longitudinal strain, median % (IQR) | −14 (−12 to −15) |
| Lateral s’, median cm/s (IQR) | 6.7 (5.6–7.9) |
| Septal s’, median cm/s (IQR) | 6.1 (5.1–7.2) |
| Average e’, median cm/s (IQR) | 9.3 (8.1–10.9) |
| Mitral E velocity, median cm/s (IQR) | 89.7 (77.1–102.8) |
| Mitral deceleration time, median ms (IQR) | 212 (188–234) |
| Average E/e’, median (IQR) | 9.4 (7.8–11.7) |
| Isovolumic relaxation time, median ms (IQR) | 97 (89–108) |
| Pulmonary vein ratio, mean (SD) | 0.7 (0.1) |
| Pulmonary vein deceleration time, median (IQR) | 242 (223–258) |
| Left atrial volume indexed to BSA, median mL/m2 (IQR) | 38 (32–49) |
| Left atrial ejection fraction, median % (IQR) | 23 (15–33) |
| TAPSE mm, median (IQR) | 18.7 (17.1–21.8) |
BSA, body surface area; TAPSE, tricuspid annular plane systolic excursion.
heartjnl-2020-318557supp001.pdf (554.3KB, pdf)
Within-beat reproducibility
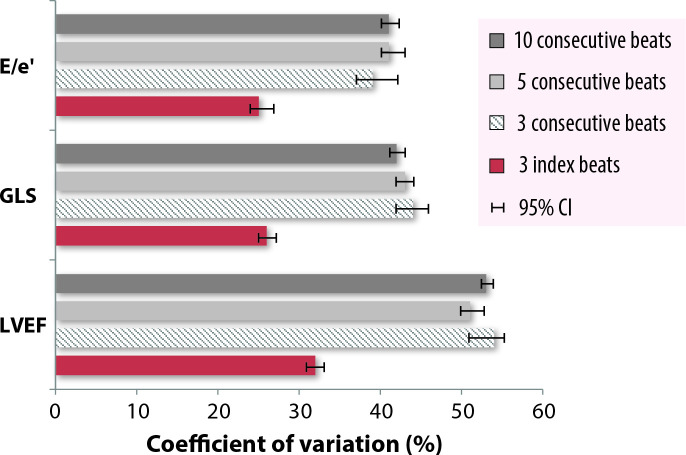
The index-beat approach had less variability compared with conventional consecutive beat averaging for all comparisons of CV for LVEF, GLS and E/e’ (figure 2 and table 3). In all cases, the difference between the index-beat method and the variability of 3, 5 and 10 consecutive beats was statistically significant with no overlap of 95% CIs. Similarly, the index-beat approach demonstrated higher ICC values for LVEF, GLS and E/e’ (figure 3 and table 3).
Figure 2.
Comparison of the coefficient of variation of within-beat variability. Within-beat coefficient of variation for Simpson’s biplane LVEF, GLS and E/e’ between 3 index-beats, compared with 3, 5 and 10 consecutive beats with 95% CI. E/e’, mitral E wave max/average diastolic tissue Doppler velocity from the septal and lateral annulus; GLS, global longitudinal strain; LVEF, left ventricular ejection fraction.
Table 3.
Within-beat variability of 3 index-beats versus 3, 5 and 10 consecutive beats
| LVEF Simpson’s biplane | GLS | E/e’ | ||||
| Coefficient of variation, % (95% CI) |
Intraclass correlation coefficient (95% CI) | Coefficient of variation, % (95% CI) |
Intraclass correlation coefficient (95% CI) | Coefficient of variation, % (95% CI) |
Intraclass correlation coefficient (95% CI) | |
| 3 index-beats | 32 (31 to 34) | 0.94 (0.93 to 0.96) | 26 (25 to 27) | 0.88 (0.85 to 0.91) | 25 (24 to 26) | 0.96 (0.95 to 0.97) |
| 3 consecutive beats | 54 (52 to 57) | 0.71 (0.64 to 0.77) | 44 (42 to 46) | 0.81 (0.76 to 0.85) | 40 (37 to 41) | 0.81 (0.76 to 0.85) |
| 5 consecutive beats | 51 (50 to 53) | 0.76 (0.71 to 0.81) | 43 (42 to 44) | 0.82 (0.77 to 0.85) | 41 (40 to 43) | 0.77 (0.72 to 0.81) |
| 10 consecutive beats | 53 (52 to 54) | 0.74 (0.69 to 0.79) | 42 (41 to 43) | 0.80 (0.75 to 0.84) | 41 (41 to 42) | 0.78 (0.73 to 0.81) |
E/e', mitral E wave max/average diastolic tissue Doppler velocity from the septal and lateral annulus; GLS, global longitudinal strain; LVEF, left ventricular ejection fraction.
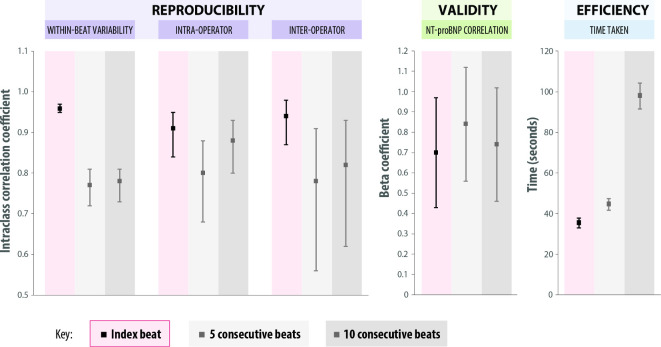
Figure 3.
Reproducibility, validity and time efficiency of the index-beat approach for E/e’. Comparison of the index-beat method verses averaging of 5 and 10 consecutive beats for: (1) Reproducibility (ICC and 95% CI for within-beat, intra- and inter-operator variability); (2) Validity (beta coefficient for NT-proBNP with 95% CI); and (3) Time taken to measure E/e’ (mean seconds, with 95% CI). E/e’, mitral E wave max / average diastolic tissue Doppler velocity from the septal and lateral annulus; ICC, intraclass correlation coefficient; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
For LVEF, the index-beat approach had the smallest CV of 32% (95% CI 31 to 34) and strongest ICC of 0.94 (0.93 to 0.96), compared with 54% (52 to 57) and 0.71 (0.64 to 0.77) for 3 consecutive beats, 51% (50 to 53) and 0.76 (0.71 to 0.81) for 5 consecutive beats, and 53% (52 to 54) and 0.74 (0.69 to 0.79) for 10 consecutive beats. The mean RR1 of the index-beat (pre-preceding R-R interval) was 552 ms and the mean RR2 (preceding R-R interval) was 555 ms, with a mean RR1 to RR2 ratio of 1.00.
For GLS, the index-beat approach had the smallest CV of 26% (95% CI 25 to 27) and strongest ICC of 0.88 (0.85 to 0.91), compared with 44% (42 to 46) and 0.81 (0.76 to 0.85) for 3 consecutive beats, 43% (42 to 44) and 0.82 (0.77 to 0.85) for 5 consecutive beats, and 42% (41 to 43) and 0.80 (0.75 to 0.84) for 10 consecutive beats. The mean RR1 of the index-beat was 568 ms and the mean RR2 was 570 ms, with a mean RR1 to RR2 ratio of 1.09.
For E/e’, the index-beat approach had the smallest CV of 25% (95% CI 24 to 26) and strongest ICC of 0.96 (0.95 to 0.97), compared with 40% (37 to 41) and 0.81 (0.76 to 0.85) for the average of 3 beats, 41% (40 to 43) and 0.77 (0.72 to 0.81) for the average of 5 beats, and 41% (41 to 42) and 0.78 (0.73 to 0.81) for the average of 10 beats. The mean RR1 of the index-beat was 653 ms and the mean RR2 was 655 ms, with a mean RR1 to RR2 ratio of 1.00.
Intraoperator and interoperator reproducibility
Intraoperator reproducibility of the index-beat method compared with consecutive beat averaging was assessed in 50 patients for E/e’ and GLS (with similar patient characteristics to the main cohort; online supplemental table 3). For E/e’, the index-beat had the smallest bias at −0.2 with similar limits of agreement to the average of 10 beats (−4.2 to 3.9) and the highest ICC of 0.91 (95% CI 0.84 to 0.95) (table 4 and online supplemental figure 2). Similar findings were seen for GLS, with the index-beat method having the smallest bias at −0.5 with narrow limits of agreement (−3.6 to 2.6) and the highest ICC of 0.82 (95% CI 0.72 to 0.90) (online supplemental table 4 and online supplemental figure 2).
Table 4.
Intraoperator and interoperator reproducibility and time efficiency for E/e’
| Intraoperator reproducibility, n=50 | Interoperator reproducibility, n=18 | Time taken (s) to select and measure E/e’ (95% CI), n=18 | |||
| Bias (limits of agreement) | ICC (95% CI) | Bias (limits of agreement) | ICC (95% CI) | ||
| Single index-beat | −0.2 (−4.2 to 3.9) | 0.91 (0.84 to 0.95) | −0.3 (−2.9 to 2.2) | 0.94 (0.87 to 0.98) | 35.4 (33.1 to 37.8) |
| 3 consecutive beats | −0.7 (−6.2 to 4.8) | 0.74 (0.60 to 0.85) | −1.1 (−5.4 to 3.2) | 0.83 (0.64 to 0.93) | Not performed |
| 5 consecutive beats | −0.6 (−5.3 to 4.2) | 0.80 (0.68 to 0.88) | −1.1 (−6.5 to 4.2) | 0.78 (0.56 to 0.91) | 44.7 (41.8 to 47.5) |
| 10 consecutive beats | −0.4 (−4.2 to 3.4) | 0.88 (0.80 to 0.93) | −0.9 (−6.1 to 4.2) | 0.82 (0.62 to 0.93) | 98.1 (91.7 to 104.4) |
E/e’, mitral E wave max/average diastolic tissue Doppler velocity from the septal and lateral annulus; ICC, intraclass correlation coefficient.
Interoperator reproducibility was tested in 18 randomly selected patients for GLS and E/e’, with a new set of images taken by a second operator (with similar patient characteristics as seen in online supplemental table 3). The index-beat demonstrated only a small degree of bias for both GLS and E/e’. ICC values for the index-beat were 0.72 for GLS (95% CI 0.45 to 0.88) and 0.94 for E/e’ (95% CI 0.87 to 0.98) (table 4, online supplemental table 4 and supplemental figure 3). Comparison of the second operator’s assessment of E/e’ with measurements taken by the first operator (from a different set of images) demonstrated that the index-beat method had similar or higher levels of reproducibility than averaging 5 or 10 consecutive beats: Bland and Altman bias (limits of agreement) of −0.6 (−3.5 to 4.6), vs 0.8 for 5 beats (−3.3 to 4.9) and 0.4 for 10 beats (−2.7 to 3.6).
Efficiency and validity of the index-beat method
The index-beat method took significantly less time to measure E/e’ (mean 35.4 s; 95% CI 33.1 to 37.8), compared with averaging 5 consecutive beats (44.7 s; 41.8 to 47.5; p<0.001) or 10 consecutive beats (98.1 s; 91.7 to 104.4; p<0.001) (figure 3 and table 4). Using the index-beat method saved 9.3 and 62.7 s, respectively (absolute difference in means).
Despite only using a single index-beat, there was no evidence that this impacted on the validity; there were no significant differences in the correlation of NT-proBNP with echocardiographic variables when comparing index-beats with conventional averaging (figure 3). For example, the correlation of LVEF with NT-proBNP was 0.11 using a single index-beat (p<0.001) vs 0.10 for 10 consecutive beats (p<0.001), with no significant difference between the two methods (p=0.84). Additional correlations for LVEF, GLS and E/e’ are presented in online supplemental table 5. Using the index-beat method led to reclassification of LVEF compared with 10-beat analysis (online supplemental table 6).
Discussion
This study has demonstrated that the index-beat method produces more reproducible quantification of systolic and diastolic left ventricular function in patients with AF than conventional averaging of consecutive beats, while saving time and without compromising validity. The within-beat coefficient of variability and intraobserver/interobserver reproducibility were all favourable using the index-beat approach to imaging in patients who have a variable stroke volume and filling time. Using the index-beat method routinely in clinical practice has the potential to improve workflow and productivity, enhance the reliability of echocardiography, and provide more confidence in the diagnosis and classification of heart failure in patients with AF.
Heart failure is common in patients with AF, and accurate assessment of systolic and diastolic left ventricular function is essential for patient management.4 Although current guidelines recommend averaging 5–10 consecutive beats,6 14 15 this is based on consensus opinion and lacks reliable evidence.16 Measuring consecutive beats is time-consuming and the overall measurement obtained will vary according to which beats are selected, making reliability in clinical practice uncertain.17 18 AF is characterised by a loss of atrial contraction and so ventricular filling relies heavily on the length of the R-R interval, with variation in intervals leading to considerable challenges to achieve reproducible measurements.19 In addition to cycle length, stroke volume is critically dependent on preload, and this is also variable in the setting of AF.9
It is important to note the very high levels of variation we demonstrate using the guideline-recommended 10 consecutive-beat analysis; the CV of 41% for E/e’ should be considered unacceptable, highlighting the limitations of current practice in patients with AF. The influence of the R-R interval on contractility is believed to be caused by preload and uptake of calcium during the relaxation phase. The longer the R-R interval, the more time for calcium uptake and the greater the amount released in response to the sequential action potential, triggering a greater force of contraction.20 As ventricular filling and stroke volume for a particular beat are determined by the previous two R-R intervals, the index-beat method selects a cardiac cycle for analysis where the preceding and pre-preceding R-R interval are of similar duration. Hence, the real value of the index-beat method may be to achieve a more physiologically appropriate measurement. In this context, the end diastolic volume should be similar and so the contractility will also be similar, producing less variability between index-beats when assessing systolic function.19 21 22 The reliance on previous cycle lengths will also apply to GLS for detection of early myocardial dysfunction17 23 and diastolic filling indices such as E/e’.24 25
Intraoperator reproducibility for both GLS and E/e’ was shown to be highest when measuring on a single index-beat. Previous studies assessing strain rate have found a strong correlation between the index- beat and averages of 10 and 15 beats with high levels of agreement.17 26 We also demonstrated that the index-beat method has a high level of reproducibility between different operators, an important contribution to value within the clinical setting, enhancing the practicality and usefulness of serial scans.
Strengths and limitations
A major concern with nearly all previous studies is the preselection of patients with ‘good echocardiography windows’.7 27 This is the first study to our knowledge in which the index-beat method has been interrogated in all patients with no pre-exclusions to image quality or heart rate, and all patients confirmed as being in AF at the time of echocardiography. The trial-based setting allowed us to ‘double-blind’ the imaging process; anonymised analyses were performed offline with a separate random code to the study identification number, and operators were blinded to patient details, clinical status and therapy. When compared with consecutive beats, our study showed no compromise in the correlation with NT-proBNP, a useful prognostic marker for both heart failure and coronary disease.28 However, this is clearly a limited analysis, and as the weak correlations between LVEF and NT-proBNP show, natriuretic peptide levels can be influenced by a variety of factors, including AF itself.29 When measured on an-index beat, LVEF was higher, on average, than using consecutive beats, and we also demonstrated that the index-beat led to reclassification of LVEF (online supplemental table 6). However, as we lack ‘ground truth’, further studies are warranted to compare the association of index-beat measurements with long-term clinical events in AF, and validation against invasive haemodynamic measurements. Our study was limited by non-simultaneous acquisition of Doppler and chamber images, and although this is currently standard practice globally there are single-beat options available for E/e’ that can also be valuable in AF.30 With pressure on echocardiography services increasing due to growing patient populations and wider indications, the index-beat method could increase the efficiency of echocardiography in AF; however, this was tested in a limited number of patients and only for E/e’. Qualitative assessment is also desirable to establish how to effectively introduce the index-beat approach in cardiology departments and the training required to aid clinical productivity.
Conclusion
In a blinded analysis without preselection for image quality, assessing left ventricular function using the index-beat method provides a more reproducible and quicker method of assessing heart function in patients with AF. Pending independent validation, our results suggest that echocardiography departments should change to the index-beat method to diagnose and characterise heart failure in patients with AF.
Key messages.
What is already known on this subject?
Accurate assessment of ventricular function is essential to diagnose the type of heart failure in patients with atrial fibrillation (AF) and guide treatment strategies.
However, measuring ventricular function in patients with AF is challenging due to the irregular cardiac cycle length.
What might this study add?
The index-beat approach, whereby measurements of ventricular function are taken after two cycles with a similar R-R interval, was compared with conventional averaging of consecutive beats.
We demonstrated that the index-beat approach was more reproducible, more time-efficient and did not compromise clinical validity against natriuretic peptide levels.
How might this impact on clinical practice?
Pending external validation, use of the index-beat in clinical practice could improve the assessment of ventricular function, leading to more precise treatment strategies for patients with AF and the potential for fewer hospital admissions and better patient quality of life.
Acknowledgments
We are grateful to the patients who gave their time to take part in the RATE-AF trial. We would like to thank the trial team, particularly Patience Domingos (Research Nurse; Sandwell and West Birmingham Hospitals NHS Trust, Birmingham, UK), staff at the Wellcome/NIHR Clinical Research Facility (University Hospitals Birmingham NHS Foundation Trust, Birmingham, UK), the Birmingham Clinical Trials Unit (University of Birmingham, Birmingham, UK), and the Patient and Public Involvement Team (Mary Stanbury, Jacqueline Jones and Sandra Haynes). This paper presents independent research supported by the NIHR Birmingham Biomedical Research Centre at the University Hospitals Birmingham NHS Foundation Trust and the University of Birmingham.
Footnotes
Twitter: @RichardSteeds
Contributors: All authors are justifiably credited with authorship, according to the authorship criteria. In detail KVB, RPS and DK are responsible for overall content, conception and design; KVB, SG, KO, MG, JNT, RPS and PK for acquisition of data; KVB, DK, SM and AS for analysis and interpretation of data; KVB, DK, MS and RPS for drafting the manuscript and final approval; and KVB, SG, AS, SM, KO, GL, PK, VYS, JC, KR, MS, JNT, MG, AK, GVG, RPS and DK for revision of manuscript and final approval.
Funding: KVB, DK and the RATE-AF trial were funded by a National Institute for Health Research (NIHR) Career Development Fellowship, awarded to DK (CDF-2015-08-074). The work is also supported by a British Heart Foundation (BHF) Accelerator Award to the University of Birmingham Institute of Cardiovascular Sciences (AA/18/2/34218). The opinions expressed are those of the authors and do not represent the NIHR, BHF or the UK Department of Health and Social Care.
Competing interests: KVB reports she was the research fellow for the RATE-AF trial funded by a National Institute for Health Research (NIHR) Career Development Fellowship awarded to DK. SG: none directly relevant to this work; funding through the BigData@Heart Innovative Medicines Initiative (grant no 116074). GL: none directly relevant to this work; consultant for Bayer/Janssen, BMS/Pfizer, Medtronic, Boehringer Ingelheim, Novartis, Verseon and Daiichi Sankyo; and speaker for Bayer, BMS/Pfizer, Medtronic, Boehringer Ingelheim and Daiichi Sankyo; no fees are directly received personally. PK reports grants from NIHR, European Union and British Heart Foundation, during the conduct of the study; grants and non-financial support from the European Union, British Heart Foundation, Leducq Foundation, Medical Research Council (UK) and German Centre for Heart Research, from several drug and device companies active in atrial fibrillation, and has received honoraria from several such companies, outside the submitted work; in addition, PK is listed as inventor on two patents held by the University of Birmingham (Atrial Fibrillation Therapy WO 2015140571, Markers for Atrial Fibrillation WO 2016012783). JC: none directly relevant to this work; reports grants and personal fees from Bayer, Boehringer Ingelheim, Daiichi Sankyo and Pfizer/BMS; and personal fees from Medtronic, Boston Scientific and Abbott. KR reports grants from NIHR Oxford Biomedical Research Centre, University of Oxford; grants from UKRI’s Global Challenge Research Fund (ESRC), PEAK Urban Programme Grant Ref: ES/P011055/1; grants from the British Heart Foundation (grant numbers: FS/19/36/34346 and PG/18/65/33872); grants from Oxford Martin School, University of Oxford, during the conduct of the study; and personal fees from PLOS Medicine and from BMJ Heart, outside the submitted work. DK reports grants from the National Institute for Health Research (NIHR CDF-2015-08-074 and NIHR HTA-130280), the British Heart Foundation (PG/17/55/33087 and AA/18/2/34218), the European Society of Cardiology supported by educational grants from Boehringer Ingelheim/BMS-Pfizer Alliance/Bayer/Daiichi Sankyo/Boston Scientific, the NIHR/University of Oxford Biomedical Research Centre and British Heart Foundation/University of Birmingham Accelerator Award (STEEER-AF NCT04396418), EU/EFPIA Innovative Medicines Initiative (BigData@Heart 116074) and IRCCS San Raffaele/Menarini (Beta-blockers in Heart Failure Collaborative Group NCT0083244), in addition to personal fees from Bayer (Advisory Board), AtriCure (Speaker fees), Amomed (Advisory Board), Protherics Medicines Development (Advisory Board) and Myokardia (Advisory Board), all outside the submitted work.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data sharing requests are required to meet the funder's policies; enquiries to be made to the corresponding author for Steering Committee review.
Ethics statements
Patient consent for publication
Patients have consented to publication of anonymised group results.
Ethics approval
The trial received favourable ethical review from the UK Health Research Authority (East Midlands - Derby Research Ethics Committee; 16/EM/0178).
References
- 1. Lane DA, Skjøth F, Lip GYH, et al. Temporal trends in incidence, prevalence, and mortality of atrial fibrillation in primary care. J Am Heart Assoc 2017;6:e005155. 10.1161/JAHA.116.005155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kotecha D, Lam CSP, Van Veldhuisen DJ, et al. Heart Failure With Preserved Ejection Fraction and Atrial Fibrillation: Vicious Twins. J Am Coll Cardiol 2016;68:2217–28. 10.1016/j.jacc.2016.08.048 [DOI] [PubMed] [Google Scholar]
- 3. Kotecha D, Piccini JP. Atrial fibrillation in heart failure: what should we do? Eur Heart J 2015;6:ehv513–3257. 10.1093/eurheartj/ehv513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893–962. 10.1093/eurheartj/ehw210 [DOI] [PubMed] [Google Scholar]
- 5. Cleland JGF, Bunting KV, Flather MD, et al. Beta-blockers for heart failure with reduced, mid-range, and preserved ejection fraction: an individual patient-level analysis of double-blind randomized trials. Eur Heart J 2018;39:26–35. 10.1093/eurheartj/ehx564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr 2015;28:1–39. 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 7. Kotecha D, Mohamed M, Shantsila E, et al. Is echocardiography valid and reproducible in patients with atrial fibrillation? A systematic review. Europace 2017;19:1427–38. 10.1093/europace/eux027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bunting KV, Steeds RP, Slater LT, et al. A practical guide to assess the reproducibility of echocardiographic measurements. J Am Soc Echocardiogr 2019;32:1505–15. 10.1016/j.echo.2019.08.015 [DOI] [PubMed] [Google Scholar]
- 9. Belenkie I. Beat-To-Beat variability of echocardiographic measurements of left ventricular end diastolic diameter and performance. J Clin Ultrasound 1979;7:263–8. 10.1002/jcu.1870070405 [DOI] [PubMed] [Google Scholar]
- 10. Kotecha D, Bunting KV, Gill SK, et al. Effect of digoxin vs bisoprolol for heart rate control in atrial fibrillation on patient-reported quality of life: the RATE-AF randomized clinical trial. JAMA 2020;324:2497–508. 10.1001/jama.2020.23138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kotecha D, Calvert M, Deeks JJ, et al. A review of rate control in atrial fibrillation, and the rationale and protocol for the RATE-AF trial. BMJ Open 2017;7:e015099. 10.1136/bmjopen-2016-015099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zou GY. Sample size formulas for estimating intraclass correlation coefficients with precision and assurance. Stat Med 2012;31:3972–81. 10.1002/sim.5466 [DOI] [PubMed] [Google Scholar]
- 13. Meng X-li, Rosenthal R, Rubin DB. Comparing correlated correlation coefficients. Psychol Bull 1992;111:172–5. 10.1037/0033-2909.111.1.172 [DOI] [Google Scholar]
- 14. Mathew TSR, Jones R, Kanagala P. Wheeler R. a guideline protocol for the echocardiographic assessment of diastolic dysfunction. British Society of Echocardiography 2013. https://www.bsecho.org/media/112942/dd_protocol_final.pdf [Google Scholar]
- 15. Donal E, Lip GYH, Galderisi M, et al. EACVI/EHRA expert consensus document on the role of multi-modality imaging for the evaluation of patients with atrial fibrillation. Eur Heart J Cardiovasc Imaging 2016;17:355–83. 10.1093/ehjci/jev354 [DOI] [PubMed] [Google Scholar]
- 16. Wu VC-C, Otani K, Yang C-H, et al. Optimal number of Heartbeats required for representing left chamber volumes and function in patients with Rate-Controlled atrial fibrillation. J Am Soc Echocardiogr 2019;32:495–502. 10.1016/j.echo.2018.12.004 [DOI] [PubMed] [Google Scholar]
- 17. Lee C-S, Lin T-H, Hsu P-C, et al. Measuring left ventricular peak longitudinal systolic strain from a single beat in atrial fibrillation: validation of the index beat method. J Am Soc Echocardiogr 2012;25:945–52. 10.1016/j.echo.2012.06.006 [DOI] [PubMed] [Google Scholar]
- 18. Wang C-L, Ho W-J, Luqman N, et al. Biplane assessment of left ventricular function during atrial fibrillation at beats with equal subsequent cycles. Int J Cardiol 2006;113:54–60. 10.1016/j.ijcard.2005.10.018 [DOI] [PubMed] [Google Scholar]
- 19. Wang C-L, Lin K-H, Luqman N, et al. Simultaneous biplane single-beat assessment of left ventricular systolic function in patients with atrial fibrillation. Am J Cardiol 2004;94:942–4. 10.1016/j.amjcard.2004.06.035 [DOI] [PubMed] [Google Scholar]
- 20. Eisner DA, Caldwell JL, Kistamás K, et al. Calcium and excitation-contraction coupling in the heart. Circ Res 2017;121:181–95. 10.1161/CIRCRESAHA.117.310230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kerr AJ, Simmonds MB, Stewart RA. Influence of heart rate on stroke volume variability in atrial fibrillation in patients with normal and impaired left ventricular function. Am J Cardiol 1998;82:1496–500. 10.1016/S0002-9149(98)00693-6 [DOI] [PubMed] [Google Scholar]
- 22. Kong L-Y, Sun L-L, Chen L-L, et al. Value of index beat in evaluating left ventricular systolic and diastolic function in patients with atrial fibrillation: a dual Pulsed-Wave Doppler study. Ultrasound Med Biol 2020;46:255–62. 10.1016/j.ultrasmedbio.2019.10.028 [DOI] [PubMed] [Google Scholar]
- 23. Nafati C, Gardette M, Leone M, et al. Use of speckle-tracking strain in preload-dependent patients, need for cautious interpretation! Ann Intensive Care 2018;8:29. 10.1186/s13613-018-0376-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kusunose K, Yamada H, Nishio S, et al. Clinical utility of single-beat E/e' obtained by simultaneous recording of flow and tissue Doppler velocities in atrial fibrillation with preserved systolic function. JACC Cardiovasc Imaging 2009;2:1147–56. 10.1016/j.jcmg.2009.05.013 [DOI] [PubMed] [Google Scholar]
- 25. Tabata T, Grimm RA, Asada J, et al. Determinants of LV diastolic function during atrial fibrillation: beat-to-beat analysis in acute dog experiments. Am J Physiol Heart Circ Physiol 2004;286:H145–52. 10.1152/ajpheart.00588.2003 [DOI] [PubMed] [Google Scholar]
- 26. Kusunose K, Yamada H, Nishio S, et al. Index-beat assessment of left ventricular systolic and diastolic function during atrial fibrillation using myocardial strain and strain rate. J Am Soc Echocardiogr 2012;25:953–9. 10.1016/j.echo.2012.06.009 [DOI] [PubMed] [Google Scholar]
- 27. Bunting KV, O'Connor K, Steeds RP, et al. Cardiac imaging to assess left ventricular systolic function in atrial fibrillation. Am J Cardiol 2021;139:40–9. 10.1016/j.amjcard.2020.10.012 [DOI] [PubMed] [Google Scholar]
- 28. Kotecha D, Flather MD, Atar D, et al. B-type natriuretic peptide trumps other prognostic markers in patients assessed for coronary disease. BMC Med 2019;17:72. 10.1186/s12916-019-1306-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chua W, Purmah Y, Cardoso VR, et al. Data-driven discovery and validation of circulating blood-based biomarkers associated with prevalent atrial fibrillation. Eur Heart J 2019;40:1268–76. 10.1093/eurheartj/ehy815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li C, Zhang J, Zhou C, et al. Will simultaneous measurement of E/e' index facilitate the non-invasive assessment of left ventricular filling pressure in patients with non-valvular atrial fibrillation? Eur J Echocardiogr 2010;11:296–301. 10.1093/ejechocard/jep218 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
heartjnl-2020-318557supp001.pdf (554.3KB, pdf)
Data Availability Statement
Data sharing requests are required to meet the funder's policies; enquiries to be made to the corresponding author for Steering Committee review.