Abstract
Olfactory impairment is a common clinical motif across neurodevelopmental disorders, suggesting olfactory circuits are particularly vulnerable to disease processes and can provide insight into underlying disease mechanisms. The mouse olfactory bulb is an ideal model system to study mechanisms of neurodevelopmental disease due to its anatomical accessibility, behavioral relevance, ease of measuring circuit input and output, and the feature of adult neurogenesis. Despite the clinical relevance and experimental benefits, olfactory testing across animal models of neurodevelopmental disease has been inconsistent and non-standardized. Here we performed a systematic literature review of olfactory function testing in mouse models of neurodevelopmental disorders, and identified intriguing inconsistencies that include evidence for both increased and decreased acuity in odor detection in various mouse models of Autism Spectrum Disorder (ASD). Based on our identified gaps in the literature, we recommend direct comparison of different mouse model of ASD using standardized tests for odor detection and discrimination. This review provides a framework to guide future olfactory function testing in mouse models of neurodevelopmental diseases.
Keywords: Neurodevelopmental disorders, Olfaction, Sensory circuits, Mouse models of neurological disease, Anosmia, Hyposmia
1. Olfaction as a window to neurological disease
1.1. Introduction
Olfactory dysfunction has been noted in a variety of neurological diseases ranging from neurodevelopmental (Hornix et al., 2018) to neurodegenerative disorders (Ruan et al., 2012). Intriguingly, these deficits are not minor components, but critical features of disease phenotypes across a wide range of neurological diseases (Demarguay et al., 2007). For example, hyposmia is one of the earliest clinical signs of Parkinson disease (Doty, 2012). Similarly, hyposmia and hyperosmia are included within the diagnostic criteria for Autism spectrum disorder which is associated with changes in sensory sensitivity (Tonacci et al., 2017). The consistent presence of an olfactory phenotype across otherwise divergent disorders indicates olfactory systems are uniquely vulnerable to neurological disease, and suggests the olfactory system is an important key to elucidating specific disease mechanisms. Notably, prior work has reviewed the clinical importance of olfaction in a number of neurological disorders, including linking insights revealed from studies of mouse olfactory bulb (Parkinson disease Doty, 2012; Epilepsy Khurshid et al., 2019; Autism Tonacci et al., 2017; Alzheimer’s disease Jung et al., 2019). However, a comparison of olfaction across models of different neurodevelopmental diseases is lacking.
By systemically comparing olfactory function across various models of neurodevelopmental disorders, we can identify common patterns and targeted differences that reveal shared mechanisms among different disease-causing mutations and linked olfactory functions (Hunsaker, 2012). These findings may, in turn, lead to better assays for early detection of neurological disease in humans. Therefore, to better understand shared mechanisms and identify distinguishing pathways causing circuit dysfunction in neurodevelopmental disorders, and specifically the consequences for olfactory processing, a comprehensive overview of olfactory function across animal models is needed.
This review begins with a discussion of olfactory bulb neuroanatomy with a focus on the unique neurodevelopmental features of this sensory system and a description of olfactory mechanics, followed by a brief summary of olfactory impairments within specific neurological clinical phenotypes in neurodevelopmental diseases. We then provide a comprehensive review of known olfactory deficits in mouse models of neurodevelopmental diseases obtained through a formal literature search. Finally, we critically evaluate the range of available olfactory testing, and include key areas that require further investigation.
1.2. Methods
In order to provide a comprehensive overview of olfactory function across animal models of neurological disease, a systematic literature review was performed in Pubmed (https://pubmed.ncbi.nlm.nih.gov/) using the search term: ((((olfactory) OR olfaction) OR odor)) AND (((neurodevelopment) OR neurodevelopmental disorder) AND mouse) in any field on 1/19/20 which yielded 160 manuscripts including 12 reviews and 148 primary articles. The results were very specific, with most (82/148) describing olfactory behavior testing. Based on our experience with this literature, we were aware of additional relevant articles and realized that olfactory testing is often not mentioned in abstracts or in tagged search terms. Therefore, we performed a broader search with the terms: (((developmental) AND olfactory) AND mice) on 4/1/20, which yielded 1445 manuscripts with 43 overlapping results. From the broad search, 66 were review articles, 1316 were primary literature, 17 were methods papers, and 3 were commentaries. We reviewed the full text of the 78 review articles and the abstracts of articles citing the 17 methods papers, and identified 34 additional primary studies of olfactory function in mouse models of neurodevelopmental disorders which are included in our analysis. In total, the abstracts of 1498 primary studies were reviewed to determine the species, experimental model, associated disease, and olfactory testing (supplementary materials). We reviewed in more detail those manuscripts where this information could not be obtained from the abstract. All abstracts that mentioned behavioral assays, functional testing, or any disease state were reviewed in full to ensure no relevant manuscripts were missed. As expected, given our broad search terms, the majority of articles (1273/1498) did not describe olfactory behavioral testing. Of the 224 articles that reported olfactory behavioral testing, 65 were not relevant to disease models, 38 were done in non-neurodevelopmental diseases such as Alzheimer’s and Parkinson disease, and two were not in rodent models. These were not included in our analysis. Figure 1 shows a flow chart of the process.
Figure 1.
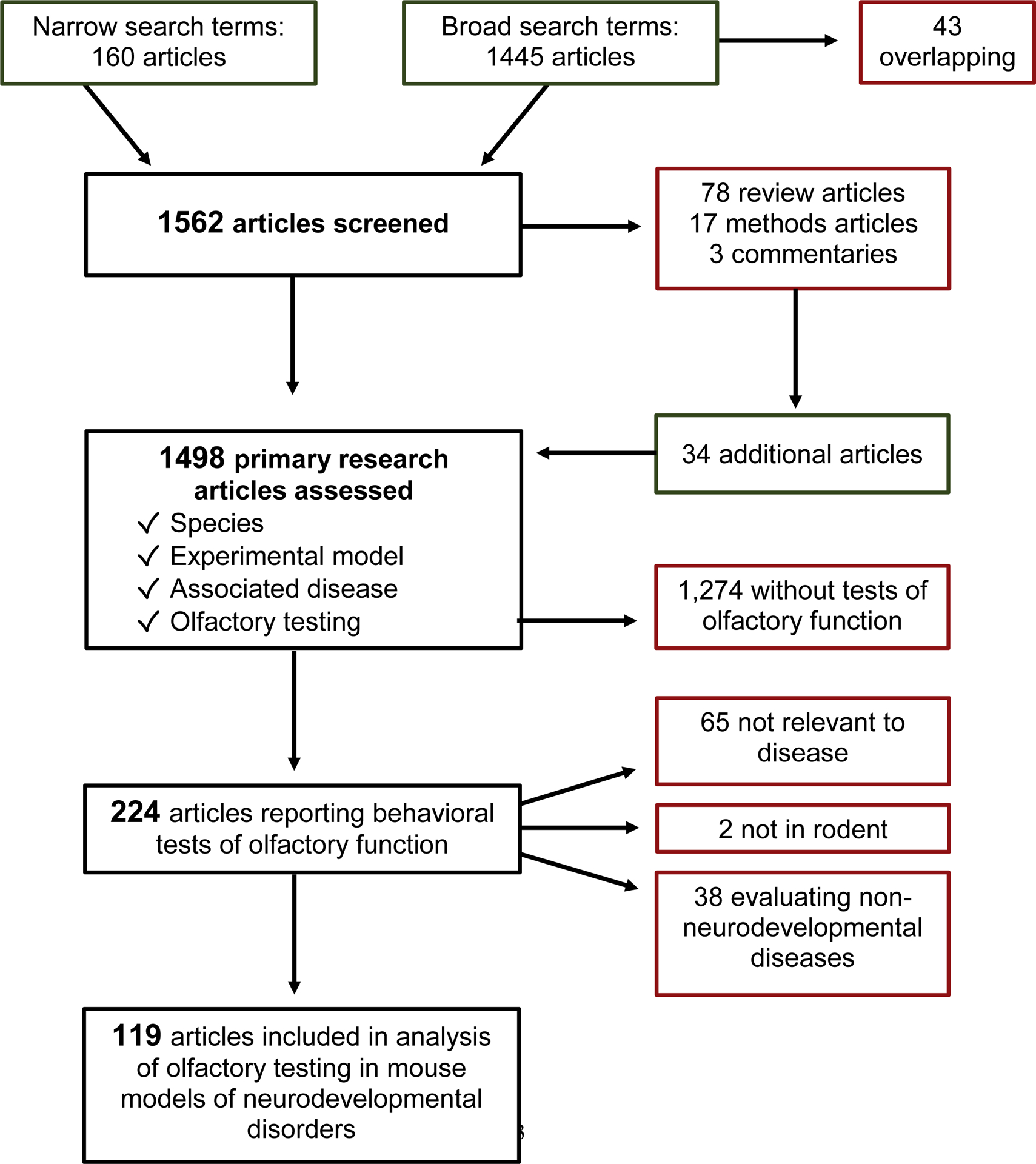
Flowsheet of systematic literature review.
2. Developmental Neuroanatomy of the Olfactory Bulb
2.1. Mammalian olfactory system development
The mammalian olfactory system is a complex structure that develops early during embryogenesis. Olfactory bulb development is similar among species. In mice, the olfactory system has two distinct components, the main olfactory system, which is responsible for the sense of smell, and the accessory olfactory system, also known as the vomeronasal system, which is essential for pheromone-based communication. During development, the olfactory placode (OP) invaginates to form the nasal epithelium and vomeronasal organ (Cho et al., 2019). OPs form bilateral transient thickenings of non-neuronal ectoderm at the ventrorostral region of the developing embryonic head and arise from pre-placodal ectoderm. The OP forms as an epithelial sheet at E9.5 during mouse embryogenesis (Figure 2A). Over 24 hours, this epithelial sheet invaginates to form the olfactory pit, which constitutes the beginning of the nasal cavity, and is comprised of olfactory epithelium (OE) and surrounding nasal mesenchyme. Intermixing of migratory neural crest cells and ectodermal cells occurs in the OPs. These migratory neural crest cells give rise to OE cells. The olfactory pit contains neural and non-neural structures and forms sensory and respiratory epithelium. As development progresses, the OE forms into the vomeronasal organ (VNO) and turbinates of the main OE. The VNO is formed medially in the ventral portion of the OE and gives rise to pheromone receptor expressing cells that project to the accessory olfactory bulb (AOB), whereas the main OE generates olfactory sensory neurons (OSNs) that project to the main olfactory bulb (OB). In the OE, globose basal cells are actively dividing progenitors that give rise to neurons, while horizontal basal cells give rise to neuronal and non-neuronal cells. As they mature, OSNs extend their cilia into the nasal cavity on their apical surface for odor detection and project their OE-wrapped axons to the OB via the olfactory nerve (Huilgol and Tole, 2016).
Figure 2. Olfactory bulb development and local circuitry.
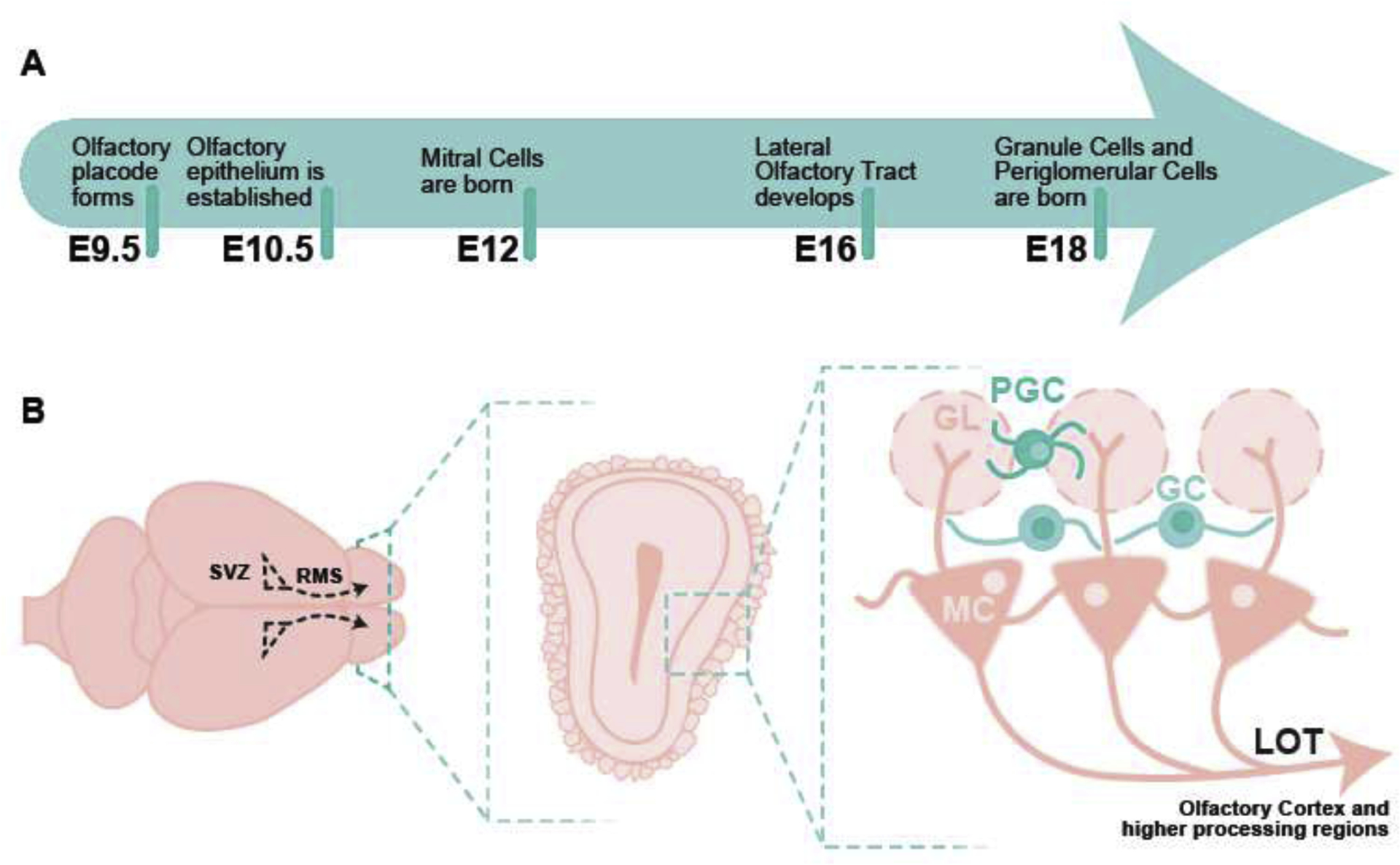
A) Timeline of olfactory bulb development. B) View of whole mouse brain. Olfactory bulbs are labeled and coronal section of mouse olfactory bulb highlighting different layers and local circuitry. E-embryonic day, MC-mitral cells, LOT-lateral olfactory tract, GC-granule cells, GL-glomerular layer, PGC-periglomerular cells.
The OB develops from the telencephalon, which also gives rise to the cerebral cortex, basal ganglia, and amygdala (Huilgol and Tole, 2016). OB development occurs in two steps. The projection neurons (mitral/tufted cells) are born first in the ventricular zone from where they migrate outward by E12–13 (Figure 2A). This is followed by entry of granule and periglomerular cells (the interneurons of the OB) on E14 (Huilgol and Tole, 2016). At E15, the OB projection neurons form a compact bundle that constitutes the primordial lateral olfactory tract, which is formed by E16 and extends to the olfactory cortex. OB interneurons are generated in the subventricular zone and migrate through the rostral migratory stream (Alvarez-Buylla, 1997). The majority of these interneurons are produced between E18 and P5 (Ravi et al, 2017), however this also remains a site of ongoing adult neurogenesis (Alvarez-Buylla, 1997). Cells of the OB display a laminar arrangement, such that mitral/tufted cells, granule cells, and periglomerular cells all occupy distinct layers, which is common across different vertebrate species (Figure 2B). The OB is unique in that it directly projects to the olfactory cortex without thalamic relay via efferent mitral/tufted cell axonal projections. These projections follow and comprise the lateral olfactory tract.
2.2. Mechanisms of olfaction
In the mammalian olfactory system, odorants are detected by olfactory receptors expressed on OSNs. The olfactory system is a highly plastic region where continuous remodeling of neuronal circuits in the olfactory bulb takes place throughout life as a result of constant turnover of OSNs (Plendl and Sinowatz, 1998). Each OSN expresses a single type of olfactory receptor, and OSNs expressing the same receptor converge their axons onto the same glomeruli in the OB (Imai 2014; Nishizumi and Sakano 2015; Ravi et al., 2017). Within a glomerulus, odor information is relayed to second order mitral/tufted cells, which are modulated by intrabulbar circuits and centrifugal inputs. These modulations shape olfactory circuitry to establish unique odor codes. OSNs release glutamate onto postsynaptic mitral tufted cells, which in turn are glutamatergic and efferent to the olfactory cortex. Additionally, GABAergic periglomerular cells and short axon cells located in the juxtaglomerular area also receive direct inputs from OSNs and help shape olfactory information via mitral/tufted cell signaling (Figure 2B).
2.3. The murine olfactory bulb as a model
The mouse OB is an excellent model system to study neurodevelopmental diseases because it provides a unique intersection of three key features; circuit development, phenotypic relevance, and experimental accessibility. Specifically, the OB is one of two locations in the adult brain to exhibit ongoing generation and integration of adult-born neurons (Altman, 1969; Alvarez-Buylla, 1997). OB neurogenesis thus provides a substrate to investigate how specific disease mutations impact neuronal development, as was done for the fragile X mental retardation protein (Scotto-Lomassese et al., 2011). Secondly, olfaction is behaviorally salient to mice, thus allowing for a wide range of behavioral assays, while also being phenotypically relevant to neurological disease. Finally, the OB is anatomically accessible due to its anterior position, which is optimal for a wide array of technologies, including in vivo imaging and viral tracing (Murphey et al., 2014). Further, the olfactory circuit is well described, including clearly defined outputs to piriform cortex by mitral cells (Nagayama et al., 2014) (Figure 2B). Together, these features make the mouse OB an ideal model system for studying the impact of neurological disease mutations on circuit function.
2.4. Olfaction in the human fetus
The neuroanatomy and axonal connections of the human olfactory system were defined the late 19th century by Golgi and Ramon y Cajal. In the human embryo, discrimination of odorous molecules occurs in the amniotic fluid after 28–30 weeks gestation, at which time the olfactory bulbs are identifiable by MRI (Sarnat and Sarnat, 2019; Sarnat and Sarnat 2017; Sarnat, Sarnat, and Wei, 2017). A normal OB is usually accompanied by an olfactory sulcus or groove. Continuous flow of amniotic fluid through fetal nasal passages enables human fetuses to detect odors of strong foods ingested by the mother such as garlic, onion, and spices; the odorant molecules of which pass the placental circulation to enter the amniotic fluid (Sarnat and Sarnat, 2019). After 28–30 weeks gestation, fetuses and preterm infants exhibit reproducible olfactory reflexes that reliably test the integrity of the olfactory system. Hypoplasia and aplasia of the olfactory bulbs is diagnosable in the third trimester and postnatally. Histologic lamination of the OB is present at 14 weeks of gestation but maturation remains incomplete at term, and neuronal differentiation, synaptogenesis, and myelination continue into the infantile period (Sarnat and Sarnat 2017; Sarnat and Sarnat, 2019). Enriched neonatal exposure to odors increases the number of neurons in the olfactory bulb and enhances olfactory memory (Woo et al., 1987). In neonates with certain cerebral malformations, endocrinopathies, hypoxic or metabolic encephalopathies, chromosomal abnormalities and other genetic diseases, olfactory reflexes may be severely diminished or absent if the olfactory bulbs are hypoplastic or aplastic (Sarnat and Sarnat, 2019). Absence of the olfactory bulbs can be bilateral or unilateral (Sarnat and Sarnat, 2017). Total absence of the olfactory bulb is a characteristic finding in alobar and semilobar holoprosencephaly (Dubourg et al., 2007).
3. Clinical relevance of olfaction in neurodevelopmental disorders
Olfactory sensory processing abnormalities include difficulties with odor identification, discrimination, and odor detection threshold. Interestingly, all are common clinical findings in neurodevelopmental disorders including Autism spectrum disorder (ASD) (Little et al., 2018), hypogonadotropic hypogonadism (Kallmann syndrome), ciliopathies, epilepsy (Khurshid et al., 2019), Tourette syndrome (Kronenbuerger et al., 2018), 22q11 deletion (Tang et al., 2018), and Fragile X (Rogers et al., 2003) (Table 1).
Table 1. Comparison of olfactory deficits in humans and mouse models of disease.
Odor identification, discrimination, or threshold deficits, as well as morphological changes in humans or mice with various neurological diseases are shown. In mice, odor identification was tested using a hole board assay in which number of nose pokes into each hole was measured with no odor, social odors, or appetitive odors. Odor discrimination was measured using a place preference assay in which time spent exploring the second odor is expected to increase over baseline if mice can discriminate that it is a distinct odor as well as various bedding-odor based tasks. Odor detection and threshold was measured most commonly using a buried food retrieval task, and in the fmr1−/y mouse a cross habituation task using decreasing odor concentrations. In humans, olfactory function was tested using Sniffin’ Sticks or the University of Pennsylvania Smell Identification Test (UPSIT).
| Disease | Odor Identification | Odor Discrimination | Odor Detection Threshold | Morphological Change in OB | ||||
|---|---|---|---|---|---|---|---|---|
| Mouse | Human | Mouse | Human | Mouse | Human | Mouse | Human | |
| Fragile X | − | − | mixed | − | + | − | + | − |
| Rett Syndrome | − | − | − | − | − | − | + | + |
| Niemann Pick Disease | − | − | − | − | + | − | + | − |
| Prader-Willi | − | − | + | − | + | − | − | − |
| Autism Spectrum Disorders | + | + | + | − | mixed | + | + | − |
| Tourette Syndrome | − | + | − | + | − | O | − | − |
| 22q11.2 deletion syndrome | − | + | − | + | − | − | − | − |
(+) = deficit, (o) = no deficit, (−) = not tested
3.1. Olfactory deficits in Autism Spectrum Disorder
Given the role of sensory dysfunction in the diagnostic criteria for ASD, it is not surprising that the most clinical research of sensory abnormalities in neurodevelopmental disorders has been done in the ASD population. Altered sensitivity with both hyper- and hypo-sensitivity phenotypes have been reported in vision, hearing, taste, and tactile senses of ASD patients (Baum et al., 2015). Interestingly, mixed phenotypes in which children score high on both hypersensitivity and hyposensitivity metrics have been reported (Ausderau et al., 2014). Prior attempts to identify subgroups based on sensory phenotype have been mixed (Simpson et al., 2019), likely because most data sets do not separate hyper- and hypo-sensitivity for each sensory modality. Using questionnaires, studies of olfactory deficits in ASD patients consistently identify subjective deficits (Simpson et al., 2019). However, with olfactory testing, only five out of seven studies found deficits in odor identification, two out of four studies identified deficits in odor sensitivity, and two out of three studies found differences in odor pleasantness (Tonacci et al., 2017). A recent meta-analysis looking at 17 studies with a total of 346 subjects found a moderate but significant (95% CI −0.68 to −0.16) difference in olfactory performance amongst individuals with ASD compared to typically developing subjects (Crow et al., 2020). Notably, results were very heterogeneous except for sub-analysis of odor identification which was more consistently impaired in ASD. Another recent study of olfactory function using Sniffin’ Sticks in 51 children with ASD further supports the results of the meta-analysis, demonstrating that children with ASD have intact odor detection but impaired odor identification (Sweigert et al., 2020). Identification of fewer deficits on objective testing compared to parent surveys may be due to assay limitations in the setting of developmentally delayed patients. Alternatively, these results may also reflect phenotypic heterogeneity in which all patients have some degree of olfactory deficit, with significant variability in the manifestation of that deficit. Understanding variability in clinical presentation is essential for dissecting mechanistic variations in this complex syndrome.
3.2. Neurodevelopmental disorders with olfactory deficits as part of the diagnostic criteria
A total of 60% of patients with hypogonadotropic hypogonadism also present with anosmia or hyposmia, and are diagnosed with Kallmann Syndrome (Balasubramanian et al., 2007). Like ASD, Kallmann syndrome is a heterogeneous disorder associated with numerous genetic mutations (Stamou and Georgopoulos, 2017). A systematic study of types of olfactory deficits in Kallmann syndrome has not been published. However, Ragancokova et al. nicely demonstrate the power of linking animal models with patient mutations. In their study, a role for Tshz1 in OB development was identified. Mice lacking Tshz1 have impaired odor detection, but no deficits in odor discrimination. Similarly, patients with Tshz1 mutations have reduced odor sensitivity, as well as difficulties with odor discrimination (Ragancokova et al., 2014). Similar to ASD and Kallmann syndrome, Bardet-Biedl syndrome (BBS) is a clinically heterogeneous, multigene ciliopathy and neurodevelopmental disorder associated with deficits in olfaction (Priya et al., 2016). In one study, 7 out of 19 individuals with BBS were anosmic, and 2 showed impaired olfactory ability using the Brief Smell Identification Test (Kulaga et al., 2004).
3.3. Olfactory deficits in Epilepsy
Epilepsy is another broad diagnostic category within neurodevelopmental disorders for which patients exhibit olfactory impairments. In a recent meta-analysis, Khurshid et al. reported 21 publications evaluating olfactory function in patients with epilepsy (Khurshid et al., 2019), and that olfactory deficits are most commonly reported in patients with temporal lobe epilepsy. Overall, the authors concluded that epilepsy was associated with impairment in odor detection threshold, identification, discrimination, and memory. However, effect size was variable. Interestingly, in one study, impairment in odor identification using a University of Pennsylvania Smell Identification Test (UPSIT) was observed with right but not left temporal lobe epilepsy, suggesting a mechanism based on anatomical proximity to olfactory centers (Kohler et al 2001). Interestingly, olfactory deficits have also been reported in patients with transient epileptic amnesia (Savage et al., 2017).
3.4. Olfactory deficits in other neurodevelopmental disorders
Intriguingly, olfactory deficits have been reported in other neurodevelopmental disorders which do not have an obvious link to olfaction. For example, in a study of 28 individuals with Tourette syndrome, patients had reduced odor discrimination and odor identification abilities, but showed no difference in odor threshold compared to controls (Kronenbuerger et al., 2018). Similarly, odor identification and discrimination were impaired in patients with 22q11.2 deletion syndrome (Tang et al., 2018). Sensory impairments in patients with Fragile X have also been reported by parents using the Short Sensory Profile, although we are not aware of any studies where direct tests of olfactory function have been conducted (Rogers et al., 2003). However, in a study of 41 adult premutation carriers of Fragile X, 61% exhibited difficulty with odor identification using UPSIT, compared to 29% of controls (Juncos et al., 2012). Finally, mild to severe hyposmia and anosmia have been reported in 13 patients with PAX6 haploinsufficiency, which is traditionally associated with eye abnormalities (Sisodiya et al., 2001).
4. Olfaction in mouse models of neurodevelopmental disorders
4.1. Olfactory deficits in mouse models
Animal models, and particularly genetic mouse models, offer invaluable tools for studying human disease. Unlike in vitro systems, animal models allow researchers to assay for functional relevance of circuit components, and to test the efficacy of potential therapeutic interventions. Given the clinical relevance of olfactory deficits, and the importance of olfaction to rodent survival, reliable olfactory testing in animal models is highly beneficial. Through a systematic literature review, we identified 119 manuscripts that reported olfactory testing in mouse models of neurodevelopmental disease (Figure 1). The specific neurodevelopmental diseases for which olfactory testing in mouse models has been reported include ASD (56), toxic prenatal exposures like fetal alcohol syndrome (23), epilepsy (4), environmental factors (4), and genetic neurodevelopmental disorders (32) such as Rett-like phenotypes, adult-onset leukodystrophy, Down syndrome, Fragile X (4), Phelan-McDermid syndrome (4), Huntington Disease, Kallmann syndrome (2), PKU, Prader Willi, Angelman syndrome, Tuberous Sclerosis, lissencephaly, CHARGE syndrome, Niemann-Pick disease C1, Spinocerebellar ataxia 38, Williams-Beuren syndrome, and α-mannosidosis. A full summary of testing results is available in tables 2 and 3. Importantly, we find that testing methodology is inconsistent among different disease models. Not surprisingly, the results are often contradictory and difficult to interpret. Here we focus our comparison on the three most common neurodevelopmental categories: ASD, other monogenetic causes of intellectual disability, and prenatal exposures.
Table 2. Task-specific olfactory deficits in mouse models of autism spectrum disorder.
Types of mouse models included single gene defects, micro-deletions and duplications, inbred mouse strains, and external factors and manipulations. Gray bars indicate testing performed, and there was no difference. Red bars indicate deficits, green bars indicate improved performance compared to controls, white bars indicate testing was not performed.
| Type of Model | Model | Buried food (Detection) | Hole Board or Place Preference (Preference) | Habituation/Dishabituation (Discrimination) | Other |
|---|---|---|---|---|---|
| Single gene disruption | AC5−/− | ||||
| Adnp−/− | |||||
| Brinp1−/− | No difference in odor sensitivity | ||||
| Casp3−/− | |||||
| Celf6−/− | |||||
| Clstn2−/− | |||||
| Cntnap2tlacz/tlacZ or Cntnap2−/− | No preference | ↓ approach of social odors | |||
| Crmp4−/− | ↓ discrimination of bedding | ||||
| Dhcr7−/− | |||||
| En2−/− | |||||
| Igf-1−/− | |||||
| Itgb3+/− | |||||
| Jakmip1−/− | |||||
| MALTT | ↓ exploration time of social odors | ||||
| Neph2−/− | |||||
| Nlgn-2−/− | |||||
| NL-3−/− or NL3R451C | ↓ acuity (KO) | (Knock In) | |||
| NR1neo/neo (NMDAR deficient) | No preference | ||||
| Nrxn1α−/− | |||||
| βNrx1ΔC | ↑ exploration time of odors | ||||
| Nrcam−/− | |||||
| OCAM−/− | ↑ acuity | ||||
| Pcdh10+/− | |||||
| Pten cKO | |||||
| PX-RICS−/− | ↓ exploration time of social odors | ||||
| Shank1−/− | |||||
| Slc6a4−/− | |||||
| Slc13a4+/− | |||||
| Tbr1+/− | ↓ sensitivity | ||||
| Tbx1+/− | No difference in odor exploration | ||||
| TPH2−/− | No preference | ||||
| Tshz3+/lacZ | |||||
| Ube3a2x | |||||
| VIP−/− |
| Type of Model | Model | Buried food (Detection) | Hole Board or Place Preference (Preference) | Habituation/Dishabituation (Discrimination) | Other |
|---|---|---|---|---|---|
| Micro-deletions and duplications | 15q11–13 duplication | ||||
| 15q13.3 deletion | |||||
| 16p11.2 deletion | ↑ acuity | ||||
| In-bred strain | Balb/c | ↑ exploration | Differences in exploration time | ||
| BTBR | |||||
| C58/J | ↓ exploration | ||||
| Manipulations | Balb/c + electromagnetic field stimulation | ||||
| hM4Di increased firing in cerebellum | |||||
| Maternal infection | No difference in odor exploration | ||||
| Zinc deficiency | |||||
| VIP antagonist exposure | |||||
| SKF105111 exposure | |||||
| GFA exposure | ↓ exploration time of social odors | ||||
| Valproate exposure |
Table 3. Task-specific olfactory deficits in mouse models of neurodevelopmental disorders.
Disease conditions include genetic etiologies, prenatal exposures, environmental exposures, and epilepsy. Gray bars indicate testing performed, and there was no difference. Red bars indicate deficits, green bars indicate improved performance compared to controls, white bars indicate testing was not performed.
| Disease | Model | Buried food (Detection) | Hole board or Place Preference (Preference) | Habituation/Dishabituation (Discrimination) | Other |
|---|---|---|---|---|---|
| Fragile X | fmr1−/y | No difference | |||
| No difference in discrimination using go-no-go | |||||
| Down Syndrome | Ts65Dn | ↓ learned discrimination using go-no-go | |||
| Prader-Willi | Magel2-null | ↓ acuity | ↓ exploration time of odors | ||
| Angelman Syndrome | Upe3a−/p+ | ↓ acuity | ↓ detection sensitivity using dehabituation ↓ learned discrimination in a digging task |
||
| Phelan-McDermid Syndrome |
Shank3Δ4−22 Shank3 null |
↓ acuity | |||
| Rett like | Prune2Ex16−/− | No preference | ↓ exploration time of odors Impaired odor memory in paired aversion task | ||
| Adult-onset leukodystrophy | Csf1r+/− | ↓ acuity | |||
| CHARGE | Chd7whi/+ | ↓ sensitivity | |||
| Niemann Pick | NPC1−/− | ↓ acuity | |||
| Huntington disease | HTT CAG knock-in | ↓ learned discrimination in a digging task | |||
| Phenylketonuria | Pahenu2 | ↓ acuity | ↓ learned discrimination in a digging task | ||
| Spinocerebellar Atrophy 38 | Elovl5 KO | ↓ acuity | |||
| Tuberous Sclerosis Complex | Tsc1 cKO | ||||
| Williams-Beuren syndrome |
Gtf2i+/− Gtf2i+/dup |
||||
| α-mannosidosis | EαM−/− | ↓ learned discrimination in a digging task | |||
| Lissencephaly | Lis1+/− | ||||
| Kallmann Syndrome | Tshz1 cKO | ↓ acuity | |||
| Jacob/Nsmf KO | No differences in behavioral response to odor | ||||
| Other monogenic causes of intellectual disability | Brinp1–3 | No difference in odor exploration time | |||
| Tlr7 KO | ↑ acuity | ||||
| Visc-2−/− | |||||
| OPHN1−/y | ↓ sensitivity | ||||
| Nrp2−/− | |||||
| ArxF/X | |||||
| EPAC2−/− | |||||
| BRD7 KO |
| Disease | Model | Buried food (Detection) | Hole board or Place Preference (Preference) | Habituation/Dishabituation (Discrimination) | Other |
|---|---|---|---|---|---|
| Prenatal Exposures | Fetal Alcohol Syndrome | ||||
| ↓ learned discrimination in a digging task Impairment in a paired aversion task in pups | |||||
| Ethiprole | Earlier orientation to bedding by pups | ||||
| Amaranth | Later orientation to bedding by pups | ||||
| BMAA | Males ↓ odor memory | ||||
| BPA | ↓ sensitivity | ||||
| Cocaine | ↓ learned discrimination using go-no-go | ||||
| DBP | Later orientation to bedding by pups | ||||
| Exhaust | No preference | Difficulty in a learned odor association task | |||
| Lead | ↓ sensitivity | ||||
| Piperonyl butotxide | Later orientation to bedding by pups | ||||
| Methamphetamines | ↓ sensitivity | ||||
| Organophosphates | |||||
| Propofol | ↓ acuity | ||||
| SAD | No preference | ||||
| Sevofluorane | |||||
| Tartrazine | |||||
| Environmental Factors | Low iron diet (rat) | ↑ acuity | ↑ preference | ↑ exploration time of odors | |
| Vitamin D deficiency | ↓ odor learning in a tube maze | ||||
| Maternal obesity | ↓ acuity | ↓ sensitivity | |||
| Very low birth weight | ↑ time to find home bedding | ||||
| Epilepsy | |||||
| Scn1a+/− | No preference | ↓ sensitivity | |||
| Scn2a+/− | |||||
| mTOR cKO | ↓ exploration time of odors |
4.2. Olfactory deficits in models of ASD
Following the plethora of research in human ASD, olfactory testing has been reported in 48 rodent models of ASD (Table 2). Of these models, 37 are based on genetic mutations identified in patient populations. Eight of the models are interventions, including valproate exposure- which is known to cause ASD in humans (Rasalam et al., 2005), and a model with genetically altered cerebellar firing patterns based on an established role for cerebellar dysfunction in ASD (Becker and Stoodley, 2013). Inbred mouse strains including Balb/c, BTBR, and C58/J also have been shown to exhibit numerous features of ASD (Teng et al., 2013). Notably, only three models showed a change in odor detection as measured by the buried food test, and in two cases the animals exhibited an increased acuity (Penagarikano et al., 2011; Pucilowska et al., 2018) compared to controls. However, only 20 of the 48 models evaluated odor detection using a buried food test. The most common olfactory task performed was odor discrimination via a dehabituation paradigm, which was done in 28 models. In these experiments mice were habituated to one odor, then presented with a different odor. Exploration of the new odor indicates dehabituation, and suggests the animal can discriminate the two odors. Only the Tbr1−/− mouse exhibited a deficit. One possible explanation for the lack of identifiable deficits could be the use of complex and distinct odors such as vanilla vs banana, rather than molecularly similar odor pairs such as isoamyl acetate vs isoamylbutyrate. In fact, notable variation was found in the specific odors tested, the length of odor exposure, and the quantification methods. Nine studies evaluated odor preference using either a hole board or a place preference assay. Three of the models showed no preference (Levy et al., 2019,; Moy et al., 2008; Kane et al., 2012) suggesting an olfactory deficit, while Balb/c mice showed increased exploration on the hole board task (Burket et al., 2016). Ten models were evaluated using other variations on olfactory testing, with three showing no difference from controls, five with deficits in function, and two simply being different from controls. This discrepancy likely reflects that clinical heterogeneity in the patient population, although it is impossible to compare results across such dissimilar testing paradigms. Understanding which subgroups of ASD patients have a predominantly hypersensitivity phenotype, and those that have a hyposensitivity phenotype may help separate out underlying mechanistic subgroups. A total of 34 of the models have only been tested with a single olfactory behavioral assay, resulting in an incomplete picture of the sensory impairments in those animal models. More uniform sensory testing across mouse models, rather than simply identifying presence or absence of sensory abnormalities may identify overlapping circuit mechanisms across animal models.
4.3. Olfactory deficits in monogenic NDD models
Olfactory testing has also been reported in 27 monogenetic neurodevelopmental disorders with established animal models. Unlike in Autism models, the buried food test of odor detection is the most common assay used, and was performed in 12 of the models. Of these, eight showed impairment, three were no different from controls, and the Tlr7 KO mouse exhibited improved odor detection compared to littermate controls (Hung et al., 2018). Interestingly, for eight of those models, buried food was the only olfactory test performed, limiting the ability to compare olfactory abilities across models. The importance of broader testing is illustrated by comparing Phelan-McDermid syndrome (PMS) to Phenylketonuria (PKU). In the Shank3Δ4−22 model for PMS (Drapeau et al., 2018) and Pahenu2 model for PKU (Zagreda et al., 1999), mice demonstrated impaired odor detection on a buried food task. However, PMS mice had no deficits in odor discrimination (Bozdagi et al., 2010; Dhamme et al., 2017; Yang et al., 2012), while PKU mice did (Zagreda et al., 1999). Thus, olfactory function is not a uniform phenotype, but like in humans, odor detection, discrimination, and preference can be uniquely impacted.
We identified four manuscripts which evaluated olfactory function in a mouse model of Fragile X. These mice exhibit deficits in cross habituation at low odor concentrations, without alterations in habituation (Schilit Nitenson et al., 2015). Confusingly, these mice showed no difficulty with learned odor discrimination in a go-no-go task (Larson et al., 2008), but demonstrated impairment in learned odor discrimination when tested using olfactory habituation/dishabituation (Daroles et al., 2016). This discrepancy illustrates the importance of uniform testing methodology, and the difficulties of interpreting a single olfactory behavior in any given model. Interestingly, both the mouse model for Prader-Willi Syndrome, harboring the Magel2-null mutation, and the model for Angelman Syndrome, Upe3a−/P+, exhibit deficits in all attempted olfactory tasks, including decreased acuity in finding buried food (Mercer and Wevrick, 2009; Koyavski et al., 2019). Olfactory ability has also been evaluated in a mouse model for Down Syndrome, Ts65Dn. These mice exhibited deficits in learned odor discrimination using a go-no-go task (de Souza et al., 2011). Similarly, the mouse model for Niemann Pick Disease, NPC1−/−, exhibited decreased acuity in the buried food test, which improved following treatment with 2-hydroxypropyl-β-cyclodextrin, which led the authors to suggest that olfactory function could be used to monitor treatment efficacy in Niemann Pick Disease (Meyer et al., 2018; Seo et al., 2014). Olfactory function testing has also been performed in mouse models of tuberous sclerosis (Tsai et al., 2012) and Williams-Beuren Syndrome (Martin et al., 2017) with no deficits identified.
A thoughtful approach to testing in the context of the NDD is important. For example, Kallman syndrome which is associated with anosmia in humans, has been shown to have an isolated odor detection deficit (Ragancokova et al., 2014), in contrast to the ASD models described earlier which rarely showed deficits in gross odor detection, mimicking the human population.
Surprisingly, no olfactory behavioral assays have been reported in the Mecp2 mouse model for Rett Syndrome, despite the known abnormal olfactory bulb organization. Interestingly however, when Mecp2 was removed from olfactory sensory neurons, olfactory sensory axons fail to converge, and instead contact more than twice as many glomeruli (Lee et al., 2014; Degano et al., 2014). Further, nasal epithelial biopsies in patients with Rett Syndrome showed abnormal structure with fewer mature olfactory receptor neurons (Ronnett et al., 2003). Given the observed olfactory bulb structural abnormalities in Mecp2 mice, we predict that olfactory testing would reveal functional deficits. While Mecp2 mice are ataxic, which could make a habituation/dehabituation task challenging, olfactory testing could be performed in pups. Indeed, The Prune2Ex16/− mouse which has a Rett-like phenotype, exhibits numerous olfactory deficits (Islam et al., 2018). Importantly, no olfactory testing has been reported in mouse models of several important neurodevelopmental disorders, including Tourette syndrome, Ebf3, Cyfip, or Chd8. Yet the clinical phenotypes of many of these diseases include sensory impairment, suggesting sensory circuits will be affected. Some olfactory assays have been done in other model systems associated with neurodevelopmental delays in humans, such as bats with FOXP2 mutations (Chen et al., 2013).
4.4. Olfactory deficits in neurodevelopmental disorders caused by prenatal exposures
Environmental exposures are also known to lead to neurodevelopmental delays. Most commonly, fetal alcohol syndrome caused by prenatal exposure to ethanol has been shown to cause deficits in odor identification in children (Bower et al., 2013). Interestingly, mouse models of fetal alcohol syndrome have deficits in learned odor discrimination using a digging task (Akers et al., 2011) and an odor paired aversion task (Barron et al., 1988), but no difficulty with odor detection (Holman et al., 2018) or odor discrimination using habituation/dehabituation (Wilson et al., 2011). Again, using these results to understand circuit mechanisms is more challenging given the discrepancy between odor discrimination testing methods. Of the 15 other prenatal exposures evaluated, two were tested for odor detection using buried food, three were tested for differences in odor preference, and five were tested for odor discrimination using habituation-dehabituation. Exposure to amaranth (Tanaka, 1993), dibutyl phthalate (Lee et al., 2019), and piperonyl butotxide (Tanaka, 1992) caused pups to be unable to orient to home bedding preferentially over clean bedding until older ages. In contrast, exposure to ethiprole resulted in orientation at an earlier age (Tanaka et al., 2018).
In addition to prenatal exposures, the impact of environmental factors on olfactory function such as low iron, vitamin D deficiency, maternal obesity, and very low birth weight, have also been evaluated. Interestingly, low iron diet was reported to cause improved acuity on buried food (Ruvin Kumara et al., 2018). These mice also showed increased preference and increased time exploring odors, suggesting a more general active seeking phenotype, and illustrating the importance of diversified olfactory testing.
5. Testing olfaction in humans and mice
Clinically, sensory impairments are typically identified during personal history taking or via a questionnaire. Direct testing of olfactory function in the neurology clinic is often overlooked with 63% of child neurologists reporting they rarely or never test olfaction in their neurological exam (Sarnat and Flores-Sarnat, 2020), suggesting olfactory deficits may be more common than currently reported. However, olfaction can be readily tested (Figure 3) using Sniffin’ Sticks, an assay in which patients are asked to smell felt pens that contain a volatile odorant (Kobal et al., 1996). This test can be used to measure odor detection threshold, discrimination, and identification. Briefly, for odor detection threshold, the subject is presented with three choices, and must correctly identify which pen has odorant. Odorant concentration is slowly increased until a correct choice is made. Similarly, for odor discrimination, the participant must select which of the three choices has a different odorant compared to the other two. Finally, odor identification is assayed by presenting one pen at a time and asking the participant to identify the odor from a list (Rumeau et al., 2016). Alternatively, the UPSIT measures odor identification by asking participants to select from a four-question multiple choice answer sheet after smelling a scratch-and-sniff (Doty et al., 1984). A direct comparison of Sniffin’ Sticks with UPSIT demonstrated that both tests could be used as early as age 5, but that scores were better on Sniffin’ Sticks (Hugh et al., 2015). There is also a shorter version of this task called the Brief Smell Identification Test (BSIT) with only 12 odors (Doty et al., 1996), as well as a three odor version (Jackman and Doty, 2005). Finally, a less commonly used test called the Candy Smell Test in which the patient identifies the smell of 23 aromatic candies has been validated against Sniffin’ Sticks (Haxel et al., 2011).
Figure 3. Olfactory skill testing in mice and humans.
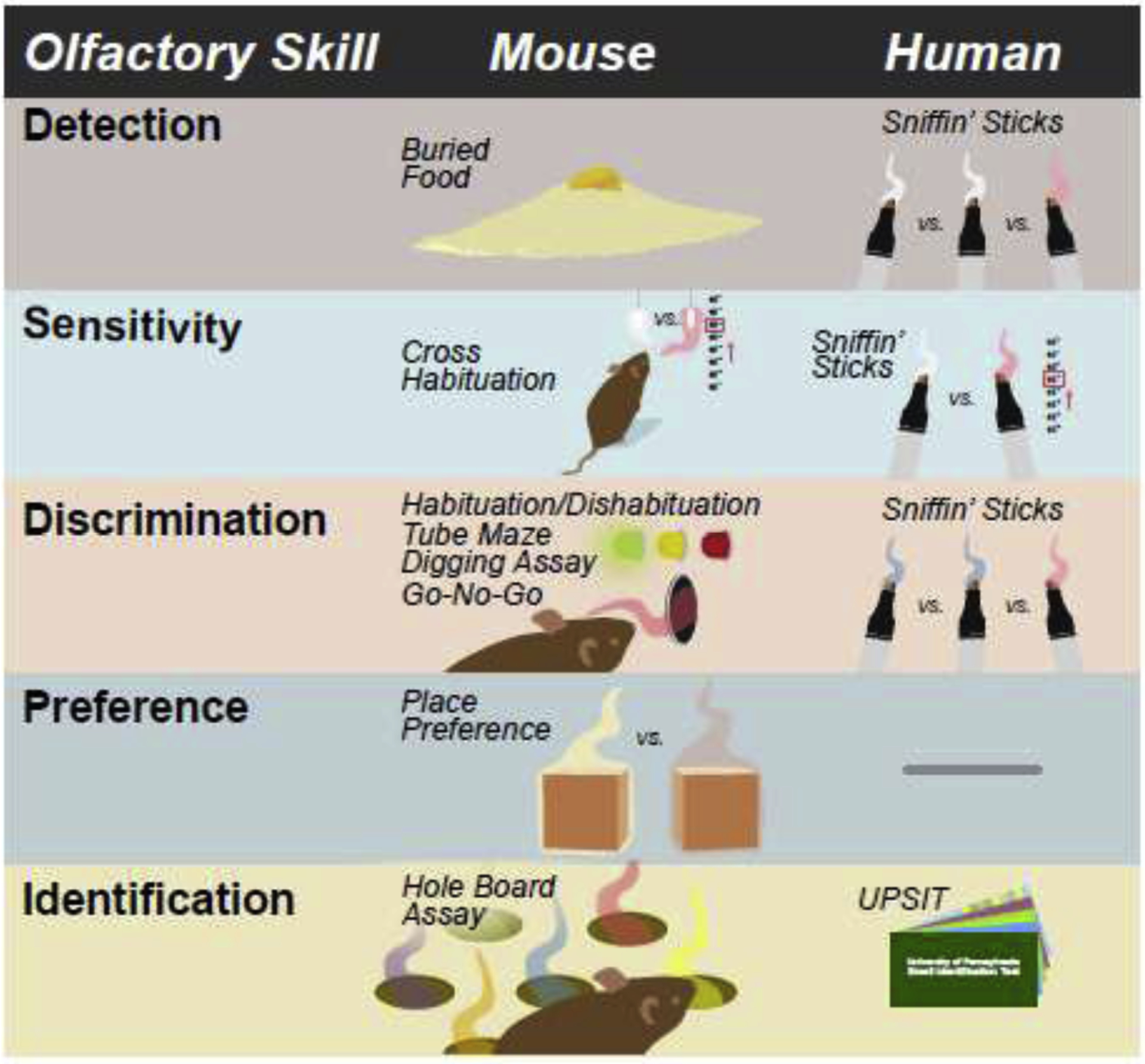
Figure shows the different types of olfactory tests in mice and humans for each testable olfactory skill. Note that for humans, the Sniffin’ sticks test evaluates olfactory sensitivity, discrimination, and identification. In the mouse, each olfactory skill is evaluated by different types of tests. There is no test for olfactory preference in the human. UPSIT-University of Pennsylvania Smell Identification Test. BSIT – Brief Smell Identification Test
In mice, odor detection is most commonly measured using a buried food retrieval task. For this task the mouse is food restricted for 24 hours, and then tested to see how quickly it can find a buried food pellet. While relatively easy to perform and well validated, the buried food test is mostly useful for detecting gross anosmia, and does not reveal subtle differences in odor detection threshold or discrimination. A cross habituation place-preference task using increasing odor concentrations can be used to determine odor detection threshold. In this task, mice are presented with a neutral stimulus such as mineral oil alongside odor stimuli at increasing concentrations across trials. Mice with intact olfactory function will spend equal time exploring both stimuli until odor concentration reaches detection threshold. Once an odor is detected, mice will spend significantly more time exploring the odor stimulus. This task is analogous to the Sniffin’ Sticks odor threshold detection assay in humans. Similarly, a cross habituation place-preference task can be used to test odor discrimination by measuring the time spent exploring a novel odor compared to a habituated odor. This test is similar to the odor discrimination component of Sniffin’ Sticks, although slightly different in that the mouse is not directly comparing odors simultaneously (Papes et al., 2018). Place preference habituation tasks can also be performed using bedding (Mercer and Wevrick, 2009), or wood blocks left in the home cages for 24 hours to pick up the cage smell (Lehmkuhl et al., 2014). Bedding based odor assays have the advantage of utilizing more behaviorally relevant odors, but as odors are more complex, they may involve more complex circuit mechanisms. Odor identification is the most commonly tested olfactory function in humans, and the most difficult to assay in mice. One method for odor identification is to quantify the number of nose pokes into each hole of a hole board in which each hole harbors a different odor (Chang et al., 2017). Finally, odor ‘memory’ has been tested by pairing an odor with a reward for several days, and then measuring the amount of time the animal spends digging in the presence of the odor without the reward, compared to a non-paired odor (Powell et al., 2007). However, this test does not discriminate between deficits in odor association or odor memory.
6. Conclusions
6.1. Summary
Hyposomia is a common but critical feature of many neurological disorders. Odor identification, detection threshold, and discrimination can be rigorously tested in patients using established tests such as Sniffin’ Sticks. In mouse models of neurological disease, the buried food test is the most commonly used assay of olfactory function. While relatively easy to perform and well validated, the buried food test is only useful for detecting gross anosmia, and thus does not reveal subtle differences in odor detection or discrimination. Even with this simple assay, however, some variation is noted across models for ASD. The next most common assay is a place-preference based odor discrimination task. We noted considerable variability in odor choice, which may account for inconsistent results across different models of the same disease. Odor identification, while the most commonly tested phenotype in humans, has been largely ignored in animal models. This is likely due to the absence of well validated assays.
6.2. Applying mouse models to human disorders
Mouse olfaction is a uniquely well-suited tool for investigating mechanisms of circuit dysfunction in neurological disease. Currently, olfactory testing in mouse models of neurodevelopmental disorders other than ASD is limited, and largely restricted to the buried food test, which only assays gross odor detection, or odor discrimination using a place preference assay for which the sensitivity is dependent on specific odor choices (Table 2). In non-ASD models of neurodevelopmental disorders, testing methodology is even more varied (Table 3). Thus, we first recommend comprehensive, consistent testing across models of the same disease. Specifically, direct comparison of odor detection threshold and odor discrimination ability for each model using consistent cross-habituation assays with the same odors and concentrations. Such rigorous investigations will identify phenotypic subgroups within models which will be particularly helpful for ASD. We hypothesize that models based around similar genetic mechanisms should exhibit similar phenotypic patterns of olfactory dysfunction. For example, odor detection threshold and odor discrimination could both be modulated via inhibitory signaling mechanisms. If the same groups of inhibitory interneurons are necessary for both functions, we would expect these two phenotypes to occur together. Thus, comparison of patterns of deficits can help clarify circuit mechanisms and/or dysfunction in disease. Alternatively, impaired odor identification could be due to olfactory receptor abnormalities, or disruption in sensory neuron axon guidance programs. Comparison across different monogenic models with established molecular findings can help discriminate which mechanisms are necessary for specific olfactory functions. Differences in phenotypes between similar genetic mutations would suggest multiple molecular roles for that gene. Inter-model comparison can then be used to delineate these differences, which could then potentially translate clinically to identify patient sub-populations.
In addition to inter-model comparisons, we also recommend evaluating across models. This approach is particularly important for monogenetic neurodevelopmental disorders that share overlapping clinical phenotypes, and are broadly due to circuit dysfunction. In these cases, specific circuit mechanisms such as lateral inhibition, gating, or axonal guidance – which are challenging to dissect – can be elucidated through direct comparison between phenotypic patterns in diseases such as Fragile X, for which molecular mechanisms are better understood. Overall, we hope this review provides a framework to guide future olfactory function testing in mouse models of neurological disease.
Supplementary Material
Highlights:
Hyposmia is a common feature of neurodevelopmental and neurodegenerative disorders
The buried food test is the most commonly used assay of olfactory function in mice
Although olfactory testing is more commonly performed in mouse models of Autism than other neurodevelopmental disorders, it is less likely to be abnormal.
Rigorous and uniform olfactory testing in models of neurological disease is needed
Acknowledgements
The authors would like to thank Elizabeth Hanson for providing feedback on this manuscript.
Funding Sources
This work was supported through NINDS (1K12NS098482) to A.L-W., BRASS; Baylor Research Advocates for Student Scientists and the AHA (20PRE35040011) award to P.J.H., and the McNair Medical Institute, DOD (PR180451-PRMP), NIDDK (R01DK109934) and NINDS (4R01NS078294) (U01NS111692) awards to B.R.A. The project described was supported in part by the Neuroconnectivity Core at Baylor College of Medicine, which is supported by IDDRC Grant Number 1 U54 HD083092 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akers KG, Kushner SA, Leslie AT, et al. Fetal alcohol exposure leads to abnormal olfactory bulb development and impaired odor discrimination in adult mice. Mol Brain. 2011;4:29. Published 2011 Jul 7. doi: 10.1186/1756-6606-4-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J, 1969. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J. Comp. Neurol 137, 433–457. 10.1002/cne.901370404 [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A Mechanism of migration of olfactory bulb interneurons. Semin Cell Dev Biol. 1997. April;8(2):207–13. [DOI] [PubMed] [Google Scholar]
- Ausderau K, Sideris J, Furlong M, Little LM, Bulluck J, Baranek GT. National survey of sensory features in children with ASD: factor structure of the sensory experience questionnaire (3.0). J Autism Dev Disord. 2014;44(4):915–925. doi: 10.1007/s10803-013-1945-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian R, Crowley WF. Isolated Gonadotropin-Releasing Hormone (GnRH) Deficiency. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, Amemiya A, editors. GeneReviews® [Internet]. University of Washington, Seattle; Seattle (WA): May 23, 2007. [Google Scholar]
- Barron S, Gagnon WA, Mattson SN, Kotch LE, Meyer LS, Riley EP. The effects of prenatal alcohol exposure on odor associative learning in rats. Neurotoxicol Teratol. 1988;10(4):333–339. doi: 10.1016/0892-0362(88)90036-0 [DOI] [PubMed] [Google Scholar]
- Baum SH, Stevenson RA, Wallace MT, 2015. Behavioral, perceptual, and neural alterations in sensory and multisensory function in autism spectrum disorder. Prog. Neurobiol 134, 140–160. 10.1016/j.pneurobio.2015.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker EBE, Stoodley CJ, 2013. Autism spectrum disorder and the cerebellum. Int. Rev. Neurobiol 113, 1–34. 10.1016/B978-0-12-418700-9.00001-0 [DOI] [PubMed] [Google Scholar]
- Bower E, Szajer J, Mattson SN, Riley EP, Murphy C. 2013. Impaired odor identification in children with histories of heavy prenatal alcohol exposure. Alcohol; 47(4):275–8. doi: 10.1016/j.alcohol.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozdagi O, Sakurai T, Papapetrou D, et al. Haploinsufficiency of the autism-associated Shank3 gene leads to deficits in synaptic function, social interaction, and social communication. Mol Autism. 2010;1(1):15. Published 2010 Dec 17. doi: 10.1186/2040-2392-1-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burket JA, Young CM, Green TL, Benson AD, Deutsch SI, 2016. Characterization of gait and olfactory behaviors in the Balb/c mouse model of autism spectrum disorders. Brain Res. Bull 122, 29–34. 10.1016/j.brainresbull.2016.02.017 [DOI] [PubMed] [Google Scholar]
- Chang YC, Cole TB, Costa LG, 2017. Behavioral Phenotyping for Autism Spectrum Disorders in Mice. Curr. Protoc. Toxicol 72, 11.22.1–11.22.21. 10.1002/cptx.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Wang L, Jones G, Metzner W, Xuan FJ, Yin J, Sun Y, 2013. FoxP2 and olfaction: divergence of FoxP2 expression in olfactory tubercle between different feeding habit bats. Acta Biol. Hung 64, 426–437. 10.1556/ABiol.64.2013.4.3 [DOI] [PubMed] [Google Scholar]
- Cho HJ, Shan Y, Whittington NC, Wray S. Nasal Placode Development, GnRH Neuronal Migration and Kallmann Syndrome. Front Cell Dev Biol. 2019. July 11;7:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow AJD, Janssen JM, Vickers KL, et al. Olfactory Dysfunction in Neurodevelopmental Disorders: A Meta-analytic Review of Autism Spectrum Disorders, Attention Deficit/Hyperactivity Disorder and Obsessive-Compulsive Disorder. J Autism Dev Disord. 2020; 50(8):2685–2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daroles L, Gribaudo S, Doulazmi M, et al. Fragile X Mental Retardation Protein and Dendritic Local Translation of the Alpha Subunit of the Calcium/Calmodulin-Dependent Kinase II Messenger RNA Are Required for the Structural Plasticity Underlying Olfactory Learning. Biol Psychiatry. 2016; 80(2):149–159. doi: 10.1016/j.biopsych.2015.07.023 [DOI] [PubMed] [Google Scholar]
- de Souza FM, Busquet N, Blatner M, Maclean KN, Restrepo D. Galantamine improves olfactory learning in the Ts65Dn mouse model of Down syndrome. Sci Rep. 2011;1:137. doi: 10.1038/srep00137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degano AL, Park MJ, Penati J, Li Q, Ronnett GV, 2014. MeCP2 is required for activity-dependent refinement of olfactory circuits. Mol. Cell. Neurosci 59, 63–75. 10.1016/j.mcn.2014.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarguay G, Ryvlin P, Royet J, 2007. Olfaction and neurological diseases: a review of the literature. Rev Neurol 163, 155–167. [DOI] [PubMed] [Google Scholar]
- Dhamne SC, Silverman JL, Super CE, et al. Replicable in vivo physiological and behavioral phenotypes of the Shank3B null mutant mouse model of autism. Mol Autism. 2017;8:26. Published 2017 Jun 15. doi: 10.1186/s13229-017-0142-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RL, 2012. Olfaction in Parkinson’s disease and related disorders. Neurobiol. Dis 46, 527–552. 10.1016/j.nbd.2011.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RL, Marcus A, Lee WW. Development of the 12-Item Cross-Cultural Smell Identification Test (CC-SIT). Laryngoscope. 1996;106:353–356. [DOI] [PubMed] [Google Scholar]
- Doty R, Shaman P, Kimmelman CP, Dann MS, 1984. University of Pennsylvania Smell Identification Test: a rapid quantitative olfactory function test for the clinic. Laryngoscope 94, 176–178. [DOI] [PubMed] [Google Scholar]
- Drapeau E, Riad M, Kajiwara Y, Buxbaum JD. Behavioral Phenotyping of an Improved Mouse Model of Phelan-McDermid Syndrome with a Complete Deletion of the Shank3 Gene. eNeuro. 2018;5(3):ENEURO.0046–18.2018. Published 2018 Oct 5. doi: 10.1523/ENEURO.0046-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubourg C, Bendavid C, Pasquier L, Henry C, Odent S, David V. Holoprosencephaly. Orphanet J Rare Dis 2007;2:8. Published 2007 Feb 2. doi: 10.1186/1750-1172-2-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxel BR, Bertz-Duffy S, Faldum A, et al. The Candy Smell Test in clinical routine. Am J Rhinol Allergy. 2011; 25(4):e145–8. [DOI] [PubMed] [Google Scholar]
- Holman PJ, Ellis L, Morgan E, Weinberg J. Prenatal alcohol exposure disrupts male adolescent social behavior and oxytocin receptor binding in rodents. Horm Behav. 2018;105:115–127. doi: 10.1016/j.yhbeh.2018.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornix BE, Havekes R, Kas MJH. Multisensory cortical processing and dysfunction across the neuropsychiatric spectrum. Neurosci Biobehav Rev. 2018. 10.1016/j.neubiorev.2018.02.010 [DOI] [PubMed] [Google Scholar]
- Hugh SC, Siu J, Hummel T, et al. Olfactory testing in children using objective tools: comparison of Sniffin’ Sticks and University of Pennsylvania Smell Identification Test (UPSIT). J Otolaryngol Head Neck Surg. 2015; 44(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huilgol D, Tole S. Cell migration in the developing rodent olfactory system. Cell Mol Life Sci. 2016. July;73(13):2467–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung YF, Chen CY, Li WC, Wang TF, Hsueh YP. Tlr7 deletion alters expression profiles of genes related to neural function and regulates mouse behaviors and contextual memory. Brain Behav Immun. 2018;72:101–113. doi: 10.1016/j.bbi.2018.06.006 [DOI] [PubMed] [Google Scholar]
- Hunsaker MR. Comprehensive neurocognitive endophenotyping strategies for mouse models of genetic disorders. Prog Neurobiol. 2012;96(2):220–241. doi: 10.1016/j.pneurobio.2011.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai T. Construction of functional neuronal circuitry in the olfactory bulb. Semin Cell Dev Biol 2014. 35:180–8. [DOI] [PubMed] [Google Scholar]
- Islam S, Ueda M, Nishida E, et al. Odor preference and olfactory memory are impaired in Olfaxin-deficient mice. Brain Res. 2018;1688:81–90. doi: 10.1016/j.brainres.2018.03.025 [DOI] [PubMed] [Google Scholar]
- Jackman AH, Doty RL. Utility of a three-item smell identification test in detecting olfactory dysfunction. Laryngoscope. 2005;115(12):2209–2212. doi: 10.1097/01.mlg.0000183194.17484.bb [DOI] [PubMed] [Google Scholar]
- Juncos JL, Lazarus JT, Rohr J, et al. Olfactory dysfunction in fragile X tremor ataxia syndrome. Mov Disord. 2012;27(12):1556–1559. doi: 10.1002/mds.25043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung HJ, Shin IS, Lee JE, 2019. Olfactory function in mild cognitive impairment and Alzheimer’s disease: A meta-analysis. Laryngoscope 129, 362–369. 10.1002/lary.27399 [DOI] [PubMed] [Google Scholar]
- Kane MJ, Angoa-Peréz M, Briggs DI, et al. Mice genetically depleted of brain serotonin display social impairments, communication deficits and repetitive behaviors: possible relevance to autism. PLoS One. 2012;7(11):e48975. doi: 10.1371/journal.pone.0048975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobal G, Hummel T, Sekinger B, Barz S, Roscher S, Wolf S, 1996. “Sniffin” sticks”: screening of olfactory performance.” Rhinology 34, 222–226. [PubMed] [Google Scholar]
- Kohler CG, Moberg PJ, Gur RE, O’Connor MJ, Sperling MR, Doty RL. Olfactory dysfunction in schizophrenia and temporal lobe epilepsy. Neuropsychiatry Neuropsychol Behav Neurol. 2001;14(2):83–88. [PubMed] [Google Scholar]
- Koyavski L, Panov J, Simchi L, et al. Sex-Dependent Sensory Phenotypes and Related Transcriptomic Expression Profiles Are Differentially Affected by Angelman Syndrome. Mol Neurobiol. 2019;56(9):5998–6016. doi: 10.1007/s12035-019-1503-8 [DOI] [PubMed] [Google Scholar]
- Kronenbuerger M, Belenghi P, Ilgner J, Freiherr J, Hummel T, Neuner I, 2018. Olfactory functioning in adults with Tourette syndrome. PLoS One 13, e0197598. 10.1371/journal.pone.0197598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurshid K, Crow AJD, Rupert PE, Minniti NL, Carswell MA, Mechanic-Hamilton DJ, Kamath V, Doty RL, Moberg PJ, Roalf DR. 2019. A Quantitative Meta-analysis of Olfactory Dysfunction in Epilepsy. Neuropsychol Rev. 29(3):328–337. doi: 10.1007/s11065-019-09406-7. Epub 2019 May 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulaga HM, Leitch CC, Eichers ER, et al. Loss of BBS proteins causes anosmia in humans and defects in olfactory cilia structure and function in the mouse. Nat Genet. 2004;36(9):994–998. doi: 10.1038/ng1418 [DOI] [PubMed] [Google Scholar]
- Larson J, Kim D, Patel RC, Floreani C. Olfactory discrimination learning in mice lacking the fragile X mental retardation protein. Neurobiol Learn Mem. 2008;90(1):90–102. doi: 10.1016/j.nlm.2008.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SM, Jeon S, Jeong HJ, Kim BN, Kim Y. Dibutyl phthalate exposure during gestation and lactation in C57BL/6 mice: Maternal behavior and neurodevelopment in pups. Environ Res. 2020;182:109025. doi: 10.1016/j.envres.2019.109025 [DOI] [PubMed] [Google Scholar]
- Lee W, Yun JM, Woods R, Dunaway K, Yasui DH, Lasalle JM, Gong Q, 2014. MeCP2 regulates activity-dependent transcriptional responses in olfactory sensory neurons. Hum. Mol. Genet 23, 6366–6374. 10.1093/hmg/ddu358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmkuhl AM, Dirr ER, Fleming SM, 2014. Olfactory Assays for Mouse Models of Neurodegenerative Disease. J Vis Exp 90, 51804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DR, Tamir T, Kaufman M, et al. Dynamics of social representation in the mouse prefrontal cortex [published correction appears in Nat Neurosci. 2020 Apr;23(4):594]. Nat Neurosci. 2019;22(12):2013–2022. doi: 10.1038/s41593-019-0531-z [DOI] [PubMed] [Google Scholar]
- Little LM, Dean E, Tomchek S, Dunn W, 2018. Sensory Processing Patterns in Autism, Attention Deficit Hyperactivity Disorder, and Typical Development. Phys. Occup. Ther. Pediatr 38, 243–254. 10.1080/01942638.2017.1390809 [DOI] [PubMed] [Google Scholar]
- Martin LA, Iceberg E, Allaf G. Consistent hypersocial behavior in mice carrying a deletion of Gtf2i but no evidence of hyposocial behavior with Gtf2i duplication: Implications for Williams-Beuren syndrome and autism spectrum disorder. Brain Behav. 2017;8(1):e00895. Published 2017 Dec 19. doi: 10.1002/brb3.895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer RE, Wevrick R, 2009. Loss of magel2, a candidate gene for features of Prader-Willi syndrome, impairs reproductive function in mice. PLoS One 4, e4291. 10.1371/journal.pone.0004291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Glaser A, Brauer AU, Wree A, Strotmann J, Rolfs A, Witt M, 2018. Olfactory Performance as an Indicator for Protective Treatment Effects in an Animal Model of Neurodegeneration. Front. Integr. Neurosci 12, 35. 10.3389/fnint.2018.00035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Poe MD, et al. Development of a mouse test for repetitive, restricted behaviors: relevance to autism. Behav Brain Res. 2008;188(1):178–194. doi: 10.1016/j.bbr.2007.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphey DK, Herman AM, Arenkiel BR, 2014. Dissecting inhibitory brain circuits with genetically-targeted technologies. Front. Neural Circuits 8, 124. 10.3389/fncir.2014.00124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagayama S, Homma R, Imamura F, 2014. Neuronal organization of olfactory bulb circuits. Front Neural Circuits 8, 98. 10.3389/fncir.2014.00098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizumi H, Sakano H. Developmental regulation of neural map formation in the mouse olfactory system. Dev Neurobiol. 2015. June;75(6):594–607. [DOI] [PubMed] [Google Scholar]
- Papes F, Nakahara TS, Camargo AP, 2018. Behavioral Assays in the Study of Olfaction: A Practical Guide. Methods Mol. Biol 1820, 289–388. 10.1007/978-1-4939-8609-5_21 [DOI] [PubMed] [Google Scholar]
- Penagarikano O, Abrahams BS, Herman EI, Winden KD, Gdalyahu A, Dong H, et al. 2011. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell 147, 235–246. 10.1016/j.cell.2011.08.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plendl J, Sinowatz F. Glycobiology of the olfactory system. Acta Anat (Basel). 1998;161(1–4):234–53. [DOI] [PubMed] [Google Scholar]
- Powell KJ, Hori SE, Leslie R, Andrieux A, Schellinck H, Thorne M, Robertson GS, 2007. Cognitive impairments in the STOP null mouse model of schizophrenia. Behav. Neurosci 121, 826–835. 10.1037/0735-7044.121.5.826 [DOI] [PubMed] [Google Scholar]
- Priya S, Nampoothiri S, Sen P, Sripriya S. Bardet-Biedl syndrome: Genetics, molecular pathophysiology, and disease management. Indian J Ophthalmol. 2016;64(9):620–627. doi: 10.4103/0301-4738.194328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucilowska J, Vithayathil J, Pagani M, Kelly C, Karlo JC, et al. 2018. Pharmacological Inhibition of ERK Signaling Rescues Pathophysiology and Behavioral Phenotype Associated with 16p11.2 Chromosomal Deletion in Mice. J. Neurosci 38, 6640–6652. 10.1523/JNEUROSCI.0515-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragancokova D, Rocca E, Oonk AM, et al. TSHZ1-dependent gene regulation is essential for olfactory bulb development and olfaction. J Clin Invest. 2014;124(3):1214–1227. doi: 10.1172/JCI72466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasalam AD, Hailey H, Williams JHG, Moore SJ, Turnpenny PD, Lloyd DJ, Dean JCS, 2005. Characteristics of fetal anticonvulsant syndrome associated autistic disorder. Dev. Med. Child Neurol 47, 551–555. [DOI] [PubMed] [Google Scholar]
- Ravi N, Sanchez-Guardado L, Lois C, Kelsch W. Determination of the connectivity of newborn neurons in mammalian olfactory circuits. Cell Mol Life Sci. 2017. March;74(5):849–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SJ, Hepburn S, Wehner E. Parent reports of sensory symptoms in toddlers with autism and those with other developmental disorders. J Autism Dev Disord. 2003;33(6):631–642. doi: 10.1023/b:jadd.0000006000.38991.a7 [DOI] [PubMed] [Google Scholar]
- Ronnett GV, Leopold D, Cai X, Hoffbuhr KC, Moses L, Hoffman EP, Naidu S, 2003. Olfactory biopsies demonstrate a defect in neuronal development in Rett’s syndrome. Ann. Neurol 54, 206–218. 10.1002/ana.10633 [DOI] [PubMed] [Google Scholar]
- Ruan Y, Zheng XY, Zhang HL, Zhu W, Zhu J, 2012. Olfactory dysfunctions in neurodegenerative disorders. J. Neurosci. Res 90, 1693–1700. 10.1002/jnr.23054 [DOI] [PubMed] [Google Scholar]
- Rumeau C, Nguyen D, Jankowski R, 2016. How to assess olfactory performance with the Sniffin’ Sticks test. Eur Ann Otorhinolaryngol Head Neck Dis 133, 203–206. [DOI] [PubMed] [Google Scholar]
- Ruvin Kumara VM, Wessling-Resnick M. Influence of Iron Deficiency on Olfactory Behavior in Weanling Rats. J Behav Brain Sci. 2012;2(2):19552. doi: 10.4236/jbbs.2012.22020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnat HB, Flores-Sarnat L, Wei XC. Olfactory Development, Part 1: Function, From Fetal Perception to Adult Wine-Tasting. J Child Neurol. 2017. May;32(6):566–578. [DOI] [PubMed] [Google Scholar]
- Sarnat HB, Flores-Sarnat L. Olfactory Development, Part 2: Neuroanatomic Maturation and Dysgeneses. J Child Neurol. 2017. May;32(6):579–593. [DOI] [PubMed] [Google Scholar]
- Sarnat HB, Flores-Sarnat L. Development of the human olfactory system. Handb Clin Neurol. 2019;164:29–45. [DOI] [PubMed] [Google Scholar]
- Sarnat HB, Flores-Sarnat L. Survey on Olfactory Testing by Pediatric Neurologists: Is the Olfactory a “True” Cranial Nerve? J Child Neurol. 2020; 35(5):317–321. [DOI] [PubMed] [Google Scholar]
- Savage SA, Butler CR, Milton F, Han Y, Zeman AZ. On the nose: Olfactory disturbances in patients with transient epileptic amnesia. Epilepsy Behav. 2017;66:113–119. doi: 10.1016/j.yebeh.2016.09.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilit Nitenson A, Stackpole EE, Truszkowski TLS, Midroit M, Fallon JR, Bath KG, 2015. Fragile X mental retardation protein regulates olfactory sensitivity but not odorant discrimination. Chem. Senses 40, 345–350. 10.1093/chemse/bjv019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotto-Lomassese S, Nissant A, Mota T, Neant-Fery M, Oostra BA, Greer CA, Lledo PM, Trembleau A, Caille I, 2011. Fragile X mental retardation protein regulates new neuron differentiation in the adult olfactory bulb. J. Neurosci 31, 2205–2215. 10.1523/JNEUROSCI.5514-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo Y, Kim HS, Shin Y, Kang I, Choi SW, Yu KW, Kang KS, 2014. Excessive microglial activation aggravates olfactory dysfunction by impeding the survival of newborn neurons in the olfactory bulb of Niemann-Pick disease type C1 mice. Biochim Biophys Acta 1842, 2193–2203. [DOI] [PubMed] [Google Scholar]
- Simpson K, Adams D, Alston-Knox C, Heussler HS, Keen D. Exploring the Sensory Profiles of Children on the Autism Spectrum Using the Short Sensory Profile-2 (SSP-2). J Autism Dev Disord. 2019;49(5):2069–2079. doi: 10.1007/s10803-019-03889-2 [DOI] [PubMed] [Google Scholar]
- Sisodiya SM, Free SL, Williamson KA, et al. PAX6 haploinsufficiency causes cerebral malformation and olfactory dysfunction in humans. Nat Genet. 2001;28(3):214–216. doi: 10.1038/90042 [DOI] [PubMed] [Google Scholar]
- Stamou MI, Georgopoulos NA. Kallmann syndrome: phenotype and genotype of hypogonadotropic hypogonadism. Metabolism. 2018;86:124–134. doi: 10.1016/j.metabol.2017.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweigert JR, St John T, Begay KK, et al. Characterizing Olfactory Function in Children with Autism Spectrum Disorder and Children with Sensory Processing Dysfunction. Brain Sci. 2020; 10(6):362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T. Reproductive and neurobehavioral effects of amaranth administered to mice in drinking water. Toxicol Ind Health. 1993;9(6):1027–1035. doi: 10.1177/074823379300900603 [DOI] [PubMed] [Google Scholar]
- Tanaka T Effects of piperonyl butoxide on F1 generation mice. Toxicol Lett. 1992;60(1):83–90. doi: 10.1016/0378-4274(92)90050-t [DOI] [PubMed] [Google Scholar]
- Tanaka T, Suzuki T, Inomata A. Reproductive and neurobehavioral effects of maternal exposure to ethiprole in F1 -generation mice. Birth Defects Res. 2018;110(3):259–275. doi: 10.1002/bdr2.1162 [DOI] [PubMed] [Google Scholar]
- Tang SX, Moberg PJ, Yi JJ, Wiemken AS, Dress EM, Moore TM., et al. 2018. Olfactory deficits and psychosis-spectrum symptoms in 22q11.2 deletion syndrome. Schizophr. Res 202, 113–119. 10.1016/j.schres.2018.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng BL, Nonneman RJ, Agster KL, Nikolova VD, Davis TT, Riddick NV, et al. 2013. Prosocial effects of oxytocin in two mouse models of autism spectrum disorders. Neuropharmacology 72, 187–196. 10.1016/j.neuropharm.2013.04.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonacci A, Billeci L, Tartarisco G, Ruta L, Muratori F, Pioggia G, Gangemi S, 2017. [Formula: see text]Olfaction in autism spectrum disorders: A systematic review. Child Neuropsychol. 23, 1–25. 10.1080/09297049.2015.1081678 [DOI] [PubMed] [Google Scholar]
- Tsai PT, Hull C, Chu Y, et al. Autistic-like behaviour and cerebellar dysfunction in Purkinje cell Tsc1 mutant mice. Nature. 2012;488(7413):647–651. doi: 10.1038/nature11310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DA, Peterson J, Basavaraj BS, Saito M. Local and regional network function in behaviorally relevant cortical circuits of adult mice following postnatal alcohol exposure. Alcohol Clin Exp Res. 2011;35(11):1974–1984. doi: 10.1111/j.1530-0277.2011.01549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo CC, Coopersmith R, Leon M. Localized changes in olfactory bulb morphology associated with early olfactory learning. J Comp Neurol. 1987. September 1;263(1):113–25. [DOI] [PubMed] [Google Scholar]
- Yang M, Bozdagi O, Scattoni ML, et al. Reduced excitatory neurotransmission and mild autism-relevant phenotypes in adolescent Shank3 null mutant mice. J Neurosci. 2012;32(19):6525–6541. doi: 10.1523/JNEUROSCI.6107-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagreda L, Goodman J, Druin DP, McDonald D, Diamond A. Cognitive deficits in a genetic mouse model of the most common biochemical cause of human mental retardation. J Neurosci. 1999;19(14):6175–6182. doi: 10.1523/JNEUROSCI.19-14-06175.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


