Abstract
Exposure to ambient PM2.5 pollution has been linked to multiple adverse health effects. Additional effects have been identified in the literature and there is a need to understand its potential role in high prevalence diseases. In response to recent indications of PM2.5 as a risk factor for dementia, we examine the evidence by systematically reviewing the epidemiologic literature, in relation to exposure from ambient air pollution, household air pollution, secondhand smoke, and active smoking. We develop preliminary exposure‐response functions, estimate the uncertainty, and discuss sensitivities and model selection. We estimate the likely impact to be 2.1 M (1.4 M, 2.5 M; 5%–95% confidence) global incident dementia cases and 0.6 M (0.4 M, 0.8 M) deaths attributable to ambient PM2.5 pollution in 2015. This implies a combined toll from morbidity and mortality of dementia of 7.3 M (5.0 M, 9.1 M) lost disability‐adjusted life years. China, Japan, India, and the United States had the highest estimated total burden, and the per capita burden was highest in developed countries with large elderly populations. Compared to 2000, most countries in Europe, the Americas, and Southern Africa reduced the burden in 2015, while other regions had a net increase. Based on a recent systematic review of cost of illness studies for dementia, our estimates imply economic costs of US$ 26 billion worldwide in 2015. Based on this estimation, ambient PM2.5 pollution may be responsible for 15% of premature deaths and 7% of DALYs associated with dementia. Our estimates also indicate substantial uncertainty in this relationship, and future epidemiological studies at high exposure levels are especially needed.
Keywords: air pollution, dementia, exposure, meta‐analysis, particulate matter
Key Points
Our work indicates PM2.5 is a potential risk factor of dementia, but showed high uncertainty with exposure‐response functions
We estimate a likely global impact of PM2.5 pollution on dementia to be 2.1 M (1.4–2.5 M) incident cases and 0.6 M (0.4–0.8 M) deaths in 2015
Our study indicates future studies in high‐exposure regions are especially valuable in reducing the uncertainty
1. Introduction
Particulate matter with a diameter less than or equal to 2.5 microns (PM2.5) in aerodynamic diameter damages human health via multiple diseases with subsequent economic costs. Previous health impact studies have attributed acute lower respiratory infections, chronic obstructive pulmonary disease, lung cancer, ischemic heart disease, and stroke to PM2.5, while the Global Burden of Disease (GBD) 2017 study added Type 2 diabetes mellitus (Stanaway et al., 2018) and impacts mediated by low birthweight and short gestation in 2019 (Murray et al., 2020). The GBD cause‐specific burdens of PM2.5 pollution have been quantified by exposure‐response functions, which integrate epidemiologic effect estimates from cohort and case‐control studies of ambient air pollution (AAP), household air pollution (HAP), second‐hand smoke (SHS), and, until recently, active smoking (AS) (Murray et al., 2020). However, updated epidemiological studies have continued to suggest that the adverse health impacts of PM2.5 are underestimated and that there are additional diseases beyond those currently included in the GBD (Schraufnagel et al., 2019). One such cause appears to be dementia. The recent Lancet Commission report on Dementia identified three additional dementia risk factors with “newer, convincing evidence,” namely air pollution, excessive alcohol consumption, and traumatic brain injury (Livingston et al., 2020). According to the report, reducing exposure to AAP and SHS throughout the life course is an action to prevent dementia. Based on the report, “although the higher levels of dementia from air pollutants are still subject to the potential for residual confounding, the effects on animal models are evidence of physiological effects over and above those driven by life‐course deprivation.” As such, studying the potential magnitude of the contributions of additional risk factors such as air pollution is important for better understanding and prevention of this important disease. Here, we investigate the potential magnitude and characteristics of the burden of dementia attributable to long‐term exposure to ambient PM2.5 pollution, by developing and applying a preliminary integrated exposure‐response function. We test the robustness of this model, and analyze uncertainties based on current literature. This model can be used for estimation of the burden of dementia associated with air pollution, yet it should also be further improved with additional epidemiological results as studies become available, including critical appraisal of the overall strength of evidence supporting this relationship.
Globally, the incidence and mortality due to dementia have increased by 88% and 106%, respectively, from 2000 to 2019 (Global Burden of Disease Collaborative Network, 2020), partly due to aging population and increased longevity. In the near future, the global burden of dementia is expected to triple from 2015 to 2050 (Prince et al., 2015). In addition, dementia costs are estimated to account for up to 35% of the total economic costs of all noncommunicable diseases due to the relatively long survival time of those affected (Mateen et al., 2013; Trautmann et al., 2016). Moreover, the “indirect costs” of dementia, mainly informal care costs and labor productivity losses associated with informal care, can be significant (Trautmann et al., 2016).
Previous systematic reviews of this topic present consistent possible linkages, while expressing some reservations. Oudin (2020), Paul et al. (2019), and Carey et al. (2018) reviewed the epidemiologic evidence and concluded that air pollution was a potential risk factor, but described the evidence as either “limited” (Paul et al., 2019) or lacking a “multi‐exposure response” (when noise and greenness were also considered), thus suggesting residual cofounding (Oudin, 2020). Existing studies also were limited in identifying whether the linkage between air pollution and dementia was direct or may be mediated by other conditions, such as cardiovascular disease, stroke, and systematic inflammation (Weuve, 2020). Other systematic reviews that focused on ambient air pollution suggests the current evidence is heterogeneous and not conclusive, and that future studies are needed, especially focused on longer‐term exposure (Choi & Kim, 2019; Power et al., 2016; Schikowski & Altug, 2020; Tsai et al., 2019). Despite the limitations, as Weuve (2020) commented, and as reiterated by the recent Lancet Commission (Livingston et al., 2020) the possible linkage between dementia and air pollution suggests a potential opportunity for dementia prevention, through environmental policy and regulation.
Both epidemiologic and animal toxicological studies have begun to address the above limitations, while recent studies have extended that range of PM2.5 exposures included in the epidemiological evidence base (Jung et al., 2015; Lee et al., 2019b; Qiu et al., 2019). Yet additional studies from more heavily polluted regions are still required (Fu & Yung, 2020). Within the exposure ranges of the United States, effect size appears to be linearly increase below 16 μg/m3, followed by a plateaued pattern at higher levels (Shi et al., 2020). Grande et al. (2020) found that heart failure and ischemic heart disease enhanced the association between air pollution and incidence of dementia, and that stroke can be an important intermediate condition. Crous‐Bou et al. (2020) examined different types of urban environmental exposures, and found higher exposure to air pollutants, but not noise, is potentially linked to cerebral vulnerability to Alzheimer's disease, whereas greenness can be beneficial. Since the most recent systematic reviews, several more studies have been published that seem to provide more pathological and physical evidence on this linkage. As mentioned above, the most recent Lancet Commission's report on dementia prevention, intervention, and care summarized that “the effects on animal models are evidence of physiological effects over and above those driven by life‐course deprivation” as one of the reasons to identify air pollution as a risk factor. A variety of evidence has been discovered by mechanistic studies to demonstrate the potential pathways to development of dementia from PM2.5 exposure. These were recently summarized by Shou et al. (2019) as four known pathways through which PM2.5 may impact the central nerve system and development of dementia: epigenetic mechanisms related to neuronal dysfunctions, peripheral inflammations that indirectly influence the central nerve system, through olfactory neurons to cause neuroinflammation, or influences on microbiota and functioning through the gut‐brain axis. Younan et al. (2020) and Crous‐Bou et al. (2020) used brain imaging changes to show that PM2.5 exposures are related to higher risk of dementia, although other studies using such an approach are too sparse and heterogeneous to confirm that finding (Power, 2020).
Although the linkage between dementia and PM2.5 exposure remains subject to substantial uncertainty. It is valuable to examine the consistency and robustness of existing evidence, and more importantly, what it means to our understanding of the adverse impacts of PM2.5. We therefore used a meta‐analysis approach to quantitatively study the effects reported in previous studies regarding this linkage. In particular, one challenge in estimating such functions for dementia has been the relatively limited number of currently available evidence included in the meta‐analysis. We therefore used a randomized bootstrap approach to resample within the confidence intervals of the effects reported in the epidemiological studies, and developed preliminary exposure‐response function based on Monte Carlo simulations. We also used results from the Monte Carlo simulations to quantify uncertainty associated with these functions. In addition, we performed a series of sensitivity tests to evaluate its robustness. Additionally, we applied this relationship to estimate the incidence and death of dementia likely attributable to PM2.5 exposure, analyzing its trend and characteristics. Finally, we estimated the potential economic costs associated with PM2.5‐attributable dementia, based on a data set of region‐specific unit cost of dementia (Wimo et al., 2017).
2. Methods
2.1. Search and Selection of Studies
We conducted a search for cohort and case‐control epidemiologic studies reporting a quantitative measure of risk of dementia due to long term exposure to AAP, HAP, SHS, or AS in four major databases (PubMed, Sciverse Scopus, Web of Knowledge, and Science Direct), based on the search keywords (Table S1) and following standardized procedures (Figure S1). We included all four types of exposures that have been used in prior PM2.5 exposure‐response studies at this initial state, while examining the assumptions and uncertainty involved in Section 2.4. Studies of source‐specific PM2.5 or other PM measures were excluded, while we included all major types of dementia, including Alzheimer's disease, vascular dementia, and “other dementia.” We did not include Parkinson's disease, because of its distinct differences in pathology (Emre, 2003). Further details were provided in the supplementary information.
We compared our final search results with seven previous systematic review papers (five focused on AAP [Killin et al., 2016; Paul et al., 2019; Peters et al., 2019; Power et al., 2016; Tsai et al., 2019]; one focused on SHS [Stirland et al., 2018]; one on AS [Anstey et al., 2007]), while including the most recent studies until October 2020. All studies that were selected in previous review papers were identified in our search, but some were subsequently excluded based on our inclusion criteria (“Criteria of inclusion” in supplementary information; Table S2). Our search results were more inclusive than some of the systematic reviews. For example, we included different types of exposures. We also included studies with different diagnosis approaches of dementia, such as outpatient diagnosis, first hospital admission because of dementia, or in the case of SHS and AS, diagnosis based on a standardized evaluation questionnaire, as a more comprehensive indicator of the onset of dementia. Thus we used a relatively broad inclusion criterion as to how the relationship between dementia and PM2.5 was evaluated, but then examined potential heterogeneity as sensitivity tests of the results. We then discuss model selection based on sensitivity analysis of the C‐R functions.
2.2. Data Extraction and Transformation
In our meta‐analysis, we incorporated nine AAP studies, two SHS studies, and 10 AS studies (see Table S3 for a list of studies used in this meta‐analysis). No studies of HAP met our inclusion criteria. Information on RR or HR of dementia incidence or first hospitalization and the corresponding PM2.5 concentrations and their ranges were extracted from each study.
For the SHS studies, exposures were transformed into PM2.5 exposures of 30–60 µg/m3, based on the categorization of high, middle, and low SHS smokers as defined in epidemiological studies. The equivalent PM2.5 exposures are computed as the sum of the exposures due to SHS and the annual average ambient PM2.5 exposures at the year and location of the studied cohorts. Specifically, 88 μg/m3 is used for the annual ambient PM2.5 exposure for the China four provinces studies in 2007–2009, based on Yao and Lu (2014), and 12 μg/m3 is used for the annual ambient PM2.5 exposure for the US cohorts in 1989–1999 (Barnes et al., 2010), based on Yanosky et al. (2014). This approach is consistent with GBD 2017 (Stanaway et al., 2018).
For AS cohorts, PM2.5 exposures were estimated based on the number of cigarettes consumed. Some studies report cigarettes per day, for which we transformed to PM2.5 exposures assuming an equivalent 667 µg/m3 per cigarette per day, following GBD 2017 (Stanaway et al., 2018). For studies that did not report cigarette use per day, we tried to find an average cigarette use per day data for the years and the countries where the studies were conducted. We found this information from a study that used cigarette consumption data to estimate smoking prevalence in 187 countries over 1980 to 2012 (Ng et al., 2014).
Table S3 shows the 20 studies included and the associated HR we extracted. For four of the studies, two HR estimates were extracted for distinct PM2.5 exposure ranges since the effects were estimated separately for the different ranges. The two HR estimates from sipngle studies were treated as independent data points in our analysis as the degree of systematic bias across the two samples is unknown. In this way, we explicitly included at least a portion of any nonlinearity that was reported in those epidemiological studies.
Most studies included in the systematic review focused on “any dementia” and did not include details regarding different subtypes. One AAP study (Kioumourtzoglou et al., 2016), however, derived HRs for Alzheimer's disease, dementia, and Parkinson's disease, with HR of 1.15 (1.11, 1.19), 1.08 (1.05, 1.11), and 1.08 (1.04, 1.12) per 1 μg/m3 increase in PM2.5, respectively. In addition, four AS studies also examined the risks for different types of dementia (Juan et al., 2004; Reitz et al., 2007; Wang et al., 1999; Yoshitake et al., 1995). However, there is no consistency as to whether any individual subtype has a systematically higher risk because of PM2.5 than others, and the current evidence regarding effects for subtypes of dementia is still too limited for reliable quantification. As such, we do not distinguish the risks for different subtypes of dementia. Instead, we use the effect for “any dementia” whenever available. When a study does not report effect for “any dementia,” but instead by subtypes, we calculated a weighted average of effect size, using the fraction of patients within the entire sample as weights. Therefore, all the final data points used from our meta‐analysis are for “any dementia.”
2.3. Exposure‐Response Function
Based on the AAP studies, the theoretical minimum risk exposure level, that is, counterfactual PM2.5 concentrations xcf, were derived to reflect the lowest levels for which increased risks of dementia had been observed. PM2.5 exposures below the counterfactual levels are considered, by definition, to have no increased risk, or a HR of 1. Here we defined xcf as a uniform distribution between the minimum (2.7 μg/m3) and median (7.6 μg/m3) of the fifth percentiles of PM2.5 concentrations, of all AAP studies. These assumptions were slightly different from the GBD 2018 approach (Stanaway et al., 2018) that used the average of the minimum and fifth percentiles of the AAP studies conducted in the US as the lower and upper bound of the uniform distribution. We used the minimum of the fifth percentile because some cohorts report only the fifth percentiles of exposures, instead of the minimum exposures. We used the median instead of the mean of the fifth percentiles of AAP studies because the AAP cohorts in our meta‐analysis had a very wide spread in exposure levels, so that the median was less sensitive to the values of the minimum and maximum of that large range.
To examine the numerical relationship to the three types of exposure studies, we fit C‐R functions with (1) only AAP studies, (2) AAP and SHS, and (3) AAP, SHS, and AS studies. We used log likelihood (LL) as a metric for the fit to the data—where the larger the value of LL, the better the fit of a model. By this criterion, models with only AAP and with AAP and SHS perform similarly well (LL = 1.05 and 1.00 in Figure 1b), and significantly better than the one including the AS studies (LL = −3.95).
Figure 1.
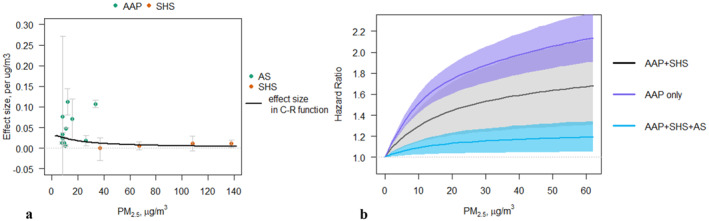
(a) Collected effect sizes from the meta‐analysis for ambient air pollution (AAP) and second‐hand smoke (SHS), where the gray error bars are the 95% confidence intervals in each study, and the black line shows the effect size modeled as in the AAP + SHS C‐F function on the right. (b) Concentration‐response models for AAP‐only, AAP and SHS, and AAP, SHS, and active smoking (AS). Shaded areas show the 95% confidence intervals from the Monte Carlo simulations.
Effect size estimation from each study was collected for the meta‐analysis. Studies included in the meta‐analysis usually report either HR or beta, which are related using the function below:
| (1) |
where β is the effect size estimated from the regression in the study while controlling for other confounding factors, and HR, if reported, is associated with a unit range of exposures, usually per 10 μg/m3. For each study, the effect size applies to the range of exposures in the specific cohort, which we characterized by the contrast between the 95th and the fifth percentiles of the PM2.5 distribution in each study.
To construct the C‐R functions, we adopted the mathematical form of the GEMM model (Burnett et al., 2018). This model is helpful because its mathematical form can be directly implemented in most major tools for air‐quality health impact assessment (Burnett et al., 2018), such as the ones used by the US Environmental Protection Agency (USEPA, 2015), Health Canada (Judek et al., 2012), and the WHO (WHO, 2018a, 2018b) R(x) = exp{θ f(x) ω(x)}, where R is HR at exposure x. x is calculated as max (0, PM 2.5 – x cf), f(x) = log(x+1) and ω(x) = 1/(1+exp{‐(x‐μ)/(τ r)}) so that ω is a logistic weighting function of x. In ω, parameters μ and τ control the shape and curvature respectively. r represents the range of PM2.5 exposure, for which we used the difference in exposures between the lowest fifth percentiles and the highest 95th percentiles among all the studies examined. θ is the coefficient that defines general rate of change of the C‐R function. In practice, to find θ given different combinations of (μ,τ), and to quantify uncertainty, 20 models were fit and ranked by their LL value (Nasari et al., 2016). The construction of 20 models was to create good coverage of different levels of μ and τ to address different possibilities of optimized θ. The “best three” models of the 20 with highest LL values were extracted and used to quantify the uncertainty (discussed in Section 2.4). We used GEMM for fitting, instead of the simpler log‐linear function as in Equation 1, because the fitting process of GEMM also includes Equation 1. Specifically, when ω(x) = 1 and f(x) = x, the GEMM function becomes identical to Equation 1. In addition, when ω(x) = 1 and f(x) = log(x+1), this functional form produces a pure linear model (i.e., HR = exp(θ) (x+1)). Since GEMM compares the best 20 models in all the functional forms it allows, if the log‐linear functional form in Equation 1 results in the best‐fit, it will show up as the result of GEMM.
2.4. Randomization and Quantifying Uncertainty
To include the uncertainty originating from the epidemiological studies in our meta‐analysis, we first created an expanded set of randomized data, and implemented Monte Carlo simulations to estimate the C‐F functions and quantify the uncertainties associated with the functions.
As a first step, we created a randomization of the effect sizes (β in Equation 1, or within the confidence intervals in Figure 1a) to include full distribution of the reported effects. We bootstrapped for 10,000 sets of effect size values for each study, randomizing β using a normal distribution with the mean and standard deviations obtained from the studies. Then by using and Equation 1, we created a larger set of HR values and their associated range of exposures.
As a second step, using this new data set of HR, we used the GEMM model to produce an ensemble of C‐R functions. For each of the 10,000 randomized set of resampled β, we extracted the “best three” model fit, instead of only one “optimal” model fit. This was because sometimes differently formed nonlinear models can reach similar goodness of fit. In practice, the “best three” and the “optimal” model fits are very similar (see Figure S2 for an example using AAP‐only data). Within this sample of 30,000 models, we computed the population mean and standard deviation as estimates for the main nonlinear ERFs. In addition, we found the “mode” (the most frequently result) for parameters μ and τ, to capture the most common shape and curvature of the ERFs. To compare “goodness of fit,” we also calculated the mean of LL.
As a third step, to better characterize the uncertainty of the C‐R functions, we conducted another set of Monte Carlo simulations. At each specific level of PM2.5 exposure, we used the ensemble estimates from the second step to make 10,000 predictions of HR based on the GEMM model. We then found the 5th and 95th percentiles for these predictions to use as the 95% confidence intervals.
The approach described above was used to generate the main model, as well as all the models in this paper for each sensitivity case we examined, as explained in Section 2.5.
2.5. Sensitivity Analyses of Exposure‐Response Function Robustness
To examine the robustness of our approach of integrating three different types of exposure studies, sensitivity analyses of the C‐R functions were conducted. They were aimed at three goals: (1) to understand sensitivity related to nonlinearity; (2) to show sensitivity to inclusion of individual studies; (3) to examine if there are systematic biases due to heterogeneity of studies included in our review. For each of the sensitivity case examined, we used the randomized Monte Carlo approach described above.
For the first goal, we compare the C‐R function estimated using the GEMM to a simple log‐linear relationship (as in Equation 1), since the latter is a commonly used functional form for C‐F functions. We thus examined it as a sensitivity case. Since the functional form in GEMM that we used for our main model included the possibility of a log‐linear function, we also calculate the probability of the log‐linear function showing up among the ensemble of the “best three” models (i.e., among the 30,000 best model fits). This examination allows us to explore the influence of allowing nonlinearity in the effect size β varying across the entire exposure range.
For the second goal, we conducted one‐at‐a‐time sensitivity analysis. We excluded one study at a time and re‐fit the C‐R function, and compared the differences in estimation of effect size.
For the third goal, we found that the major source of heterogeneity between the studies included in the meta‐analysis lies in how each study determined the incidence of dementia. Most studies use a dementia diagnosis to determine incidence. Some studies, however, used first hospitalization due to dementia as the indicator for dementia incidence (Kioumourtzoglou et al., 2016; Lee et al., 2019a; Shi et al., 2020). We constructed a C‐R function excluding those three studies, and compared with the main model. In addition, while all the AAP studies used a dementia diagnosis from medical records (either incidence or first hospitalization), the two SHS studies used more qualitative methods to determine dementia incidence (Chen et al., 2013), used a global standard tool conducted by trained interviewers to evaluate incidence of dementia (Barnes et al., 2010), used an adjudication committee of experts with experience to decide dementia incidence. To test the influence of this inconsistency in diagnosis of dementia, we compared the C‐R models with and without these two studies. In practice, this latter test was virtually the same as the comparison between AAP only and AAP + SHS.
2.6. Analysis of Dementia Attributable to PM2.5
To estimate the burden of dementia attributed to PM2.5, we essentially followed the protocol in the most recent GBD analysis (Murray et al., 2020). We first calculated the RR of dementia incidence due to PM2.5 exposure by applying the C‐R function to a global 0.1° by 0.1° gridded annual estimate of surface PM2.5 concentrations (Shaddick et al., 2018), the same data set used in GBD 2017. These RRs were then used to calculate the grid‐level attributable fractions (AF) of dementia due to PM2.5 exposure (), and population attributable fraction (, because we considered that only the population older than 65 years old was at risk for dementia. Next, we calculated country‐level annual dementia incidence attributable to PM2.5 as the sum‐product of each grid's AF, and age‐specific baseline incidence rates and population, summed over national areas. Incidence rates per 100K population were also calculated. The Baseline incidence rates of dementia by age groups were obtained from the GBD database (Global Burden of Disease Collaborative Network, 2018) at the country level, and then mapped onto the same 0.1° by 0.1° grid as the surface PM2.5 concentrations. As such, the incidence rates for each age group were the same across all the grid boxes within a country. For grid boxes at the boundary of countries, the incidence rates for each age group were calculated as the average rates weighted by fractions of population of each countries sharing the border. We used the age‐specific incidence rates for our calculation, but also contrast the results to calculations using the age‐standardized incidence rates during the study period. Age‐standardized rates excludes the impact of changes in population age structure. Global age‐specific population data are from the UN Population Division and were distributed to grids using population density from the Gridded Population of the World (GPW) version 4 (CIESIN, 2016). Years lost due to disability (YLD) associated with these incidences were then calculated as the product of incidence, disability weight, and average numbers of years lived with the disease before death. The latter two variables come from Appendix one of the GBD (Vos et al., 2017).
To estimate dementia‐related deaths attributable to PM2.5, we modeled the ratio of incidence and deaths for dementia, δ i(t), as a function of age t (in years) for each country i, based on the modeling framework of Commenges et al. (2004):
where is the dementia incidence rate for the 60–64 baseline age group, and is the mortality rate for dementia for the 60–64 baseline age group (since age 65 is used as the lower age limit for dementia in most literature). The age‐specific mortality rate of dementia was also obtained from the GBD database (Global Burden of Disease Collaborative Network, 2018) at the country level. Using this approach, φ i and γ i are coefficients estimated from the country‐level regression between δ (the ratio of dementia incidence and death) and age t (see Figure S3, e.g., of the relationship between incidence rate and mortality rate). We then applied this function to the country‐level age‐specific attributable incidence of dementia to calculate premature deaths from dementia attributable to PM2.5. Finally, years of life lost (YLL) for each country were calculated as the sum‐product of deaths at each age group and the standard life expectancy for that age group in each country. Country‐specific life expectancy by age group was obtained from the GBD 2017 database (Global Burden of Disease Collaborative Network, 2018) (life expectancy for post‐95 population was calculated as the weighted average of 95–99, 100–104, 105–109, and post‐110 age groups, assuming weights of 0.4, 0.3, 0.2, and 0.1, respectively). Disability‐adjusted life years (DALYs) were then calculated as the sum of YLD and YLL. All results were reported only for the post‐65 population.
In addition, we quantified the contributions of major variables to the total toll of dementia attributable to PM2.5 pollution involved in the above calculations. These variables include the PM2.5 exposure (represented by estimated RR), the total post‐65 population, the age structure of the population (which we represent using the fraction of post‐80 out of post‐65 population), and the baseline incidence rate. To isolate the role of individual variables, we conducted a decomposition analysis in which we assessed the influence of each variable on the associated changes in the attributable incidence rate. Due to the nonlinear nature of the calculations above, these effects are not distributed evenly, but instead vary across the ranges of each variable's distribution. As such, to compare across variables, we used a linear regression to obtain the “average” contributions over the range of each variable to the incidence rates attributable to PM2.5. Moreover, because both the variables and the attributable incidence rate were log‐normally distributed, we performed a linear regression between the logarithm of attributable incidence rate and the logarithm of each of the four above variables. The log‐log regression was calculated at the country level, with coefficients indicating the responsive percentage changes in the dependent variable for a unit percentage change in each independent variable.
Finally, economic costs associated with the incidence of dementia attributable to the ambient PM2.5 pollution were estimated using a “unit cost” approach. “Unit costs” are evidence‐based estimates of the cost of a resource for people subject to an incidence of dementia in a country/region. A variety of cost‐of‐illness studies have estimated the “unit cost” of dementia worldwide. We used the results from a recent systematic review of 42 cost‐of‐illness studies (Wimo et al., 2017), that has compiled a database including the direct cost in social care and medical expenses from 21 countries (49% of worldwide dementia population), as well as indirect informal care costs from time spent assisting with basic daily activities, time spent with instrumental daily activities, and time spent in supervision of behavioral symptoms. They then did a statistical imputation to estimate an average cost for the above three cost types for each of the 25 World Health Organization (WHO) categorized regions. In each region, we used their estimated average unit value for each type of costs, and apply to our incidence estimates to obtain the total economic costs in 2015. These estimates should represent a comprehensive set of both direct and indirect costs associated with both the dementia patients and their care‐givers.
3. Results and Discussion
3.1. C‐R Functions, Their Sensitivity, and Model Selection
Figure 1a shows effect sizes collected from literature. Effect sizes from AAP literature are much greater than those from SHS literature. However, because all AAP studies from the meta‐analysis are from regions with relatively low annual exposures, we cannot determine if the divergence in effect sizes is due to different exposure types (AAP and SHS) having different impacts, or if it stems from a nonlinear relationship between effect and exposures. We thus further explored how the different data influence C‐R functions.
Fitting of the GEMM model leads to a nonlinear function as the best fit for the relationship between increased risk of dementia incidence and PM2.5 exposure, for all the three models using each type of exposure data (Figure 1b). All the three models show a plateaued pattern in higher exposure levels, consistent with Shi et al. (2020). The model with both AAP and SHS data estimates an effect size of ∼0.12 per 1 μg/m3 (i.e., up to 12% increase in risk per 1 μg/m3 increase in exposure when above the counterfactual level, depending on the exposure level, SE = 0.03), while the AAP‐only model estimate an effect size of ∼0.18 per 1 μg/m3 (SE = 0.01). The goodness‐of‐fit of these two analyses are similar (LL = −5 and −3, respectively). While the AAP model (Figure 1b, purple line) directly reflects the relationship between increased dementia risk and ambient exposure, the exposure range covered is limited to evidence collected below 39 μg/m3 (highest among the 95th percentiles of all the studies included in our meta‐analysis). The AAP and SHS model, however covers a wider range of exposure (up to 146 μg/m3), but includes the assumption that the two types of exposure have a similar exposure‐response relationship. While the AAP‐only model more accurately reflects the evidence on the effect of AAP exposure on incidence of dementia, the AAP and SHS model captures the potential nonlinearity in higher‐exposure ranges. As such, model selection should be based on the specific case for which these functions are used. For example, if the application case is limited to regions having an annual average exposure lower than ∼40 μg/m3, the AAP model may be a better choice. However, if the analysis includes regions having a higher annual exposure, the AAP and SHS model should be considered, while acknowledging that such application inherently assumes that the effects of SHS are equivalent to those of AAP.
In addition, we also constructed a C‐R function with AAP, SHS, and AS (Figure 1b, blue line), which has a lower effect estimate and also a lower LL (−14). In addition, the equivalent PM2.5 concentrations of AS were two orders of magnitude larger than the AAP and SHS studies, and thus were far beyond the range of ambient exposures. This model therefore does not well represent the effects when evaluating AAP exposures, but may be more appropriate for estimating impacts of household air pollution from the use of solid fuels for cooking, which can exceed 500 µg/m3 in some settings. However, similar with the use of AAP and SHS model, any consideration of the C‐R function with AAP, SHS, and AS should caution the important assumption that all exposure types are treated as equivalent AAP, and the potential bias caused by doing so.
Notably, the uncertainty range of the AAP and SHS model includes the central estimates and most of the 95% confidence intervals of the other two C‐R functions. This is because the randomization approach in this study incorporates the uncertainty when combining the AAP and SHS data, as discussed above. In practice, when using the AAP and SHS model, we can approximately refer to the upper estimate as only considering AAP data, given the overlap between the two (Figure 1b). In this study, given our goals of providing a preliminary global estimate of the possible dementia burden associated with PM pollution, the AAP and SHS model is most appropriate given the global distribution of PM2.5 exposure. For regions with lower exposures, the upper estimates from the AAP and SHS model can provide an approximation to the AAP‐only model. As such, we interpret our central result in this study as being conservative, whereas our upper estimates as being a potential alternative, especially for low‐exposure regions.
In our sensitivity analysis, we developed log‐linear models from only AAP data (Figure S4a), and from AAP and SHS data (Figure S4b). We found that the log‐linear functional form is much more sensitive to the application of a different range of exposure compared to the nonlinear functional form. Including SHS data at higher exposures changes the effect size drastically. When comparing to the nonlinear C‐R functions, the log‐linear functions have lower goodness‐of‐fit.
Next, we examined one‐at‐a‐time sensitivity analysis in “AAP and SHS” and “AAP only” (Figure S5). We removed each study and re‐conducted the randomization and regressions. Among the 10 one‐at‐a‐time sensitivity cases examined for AAP and SHS, regression coefficients for the effect size (Table S5) appear to be robust, varying between 0.10 to 0.13, lying within the range of the estimated 95% confidence intervals of the main estimates. However, C‐R functions developed using only AAP data show more sensitivity to single studies (Figure S6, regression coefficients in Table S5). In general, exclusion of individual studies leads to consistent C‐R functions with effect size between 0.18 and 0.21, except for the exclusion of Jung et al. (2015). When excluding Jung et al. (2015), the estimated effect size for the C‐R function of AAP studies is reduced to 0.13, in the same range as the C‐R functions that include AAP and SHS studies. This again implies the uncertainty between the “AAP only” and “AAP and SHS” C‐R funcitons with current evidence. As such, more AAP evidence, especially from regions with relatively moderate to high PM2.5 exposure levels, will be crucial to improve estimation of the C‐R relationship for dementia.
Finally, we examined whether the different approaches used to determine incidence of dementia influence estimation of C‐R functions. Figure S7 and Table S5 show that there is hardly any difference when excluding studies using the first hospital admission rather than first diagnosis to determine incidence of dementia. The influence of using a survey/expert approach to determine dementia is unclear: excluding the two SHS studies using a survey/expert approach would transform the analysis into the model with only AAP data (purple line in Figure 1b), such that we cannot separate the different exposure types from the determination of dementia.
Overall, we found the nonlinear model with AAP and SHS to be the most suitable for our preliminary global analysis of the burden of dementia attributable to PM2.5 pollution, because it includes evidence from a wide range of exposures and is robust to individual observations and major heterogeneities between these studies.
3.2. Incidence and Mortality of Dementia Associated With PM2.5 Exposure
We estimate that in 2015, globally there were 2.1 million (M) (1.5 M, 2.6 M, 5%–95% confidence interval) new cases of dementia in the post‐65 population and 0.62 M (0.44 M, 0.77 M) dementia deaths attributable to PM2.5 exposure. The distribution of incidence and deaths was unbalanced geographically (Figure 2). The largest burden was in China: 0.55 M (0.37 M, 0.69 M) new cases in 2015, and 163K (110K, 205K) deaths, accounting for about 26% of the global toll in 2015. The second and third largest burden was in Japan and India, each with 8% of the global toll, followed by the United States (around 6%). In Europe (47 countries, except for San Marino, Liechtenstein, and Kosovo) there were 0.43 M (0.31 M, 0.54 M) incident cases and 130K (93K, 162K) deaths. On a per capita basis, the highest rate of incidence and death occurred in high income European and Asian countries (Figure 3a), due to an older population and therefore a higher baseline incidence rate of dementia (more discussion in this section).
Figure 2.
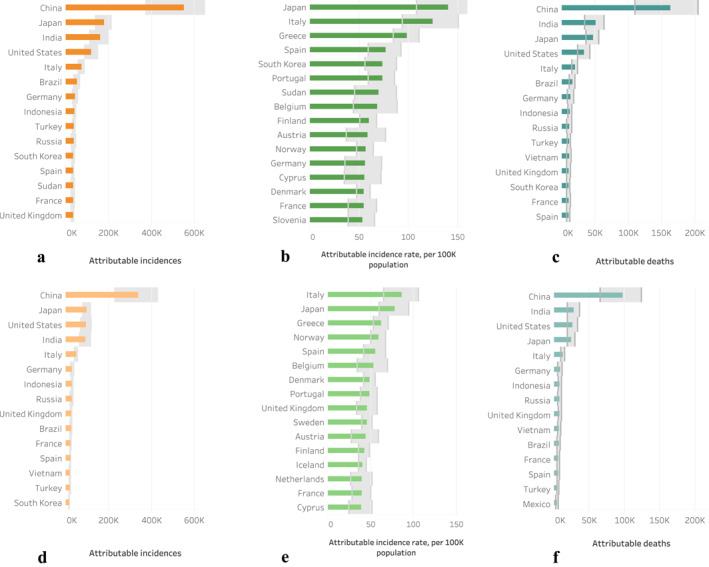
Top 15 world countries with the highest (a) attributable incidence, (b) attributable incidence rate, and (c) attributable dementia deaths in 2015 and the same metrics (d–f) in 2000. Bands show uncertainty range of 95% confidence intervals.
Figure 3.
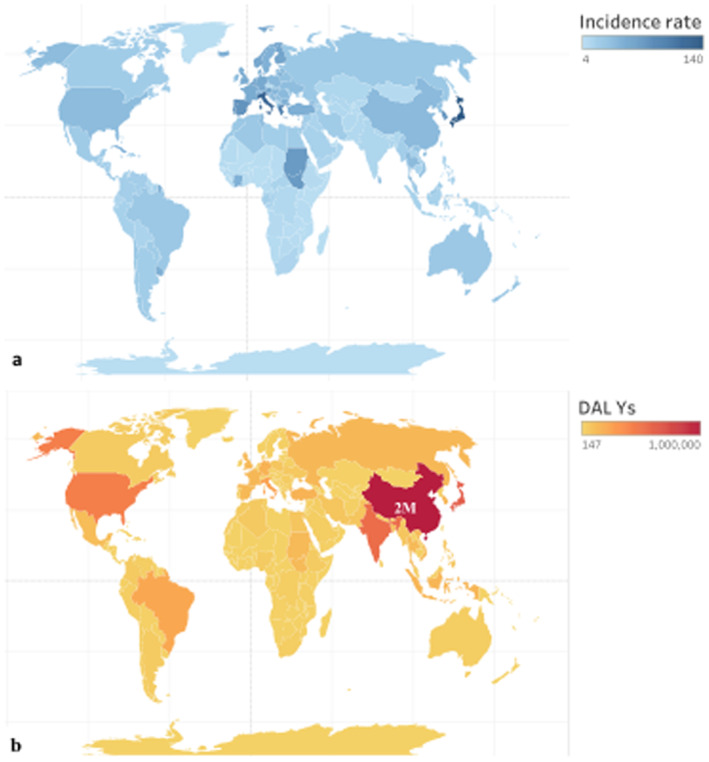
Global map of incidence rate (a), and DALYs (b) attributable to PM2.5 pollution in 2015, where colors indicate the values. Major countries and those with high values are radiolabeled. In (b), the DALYs for China are much above the color range of other countries, so is annotated with its estimate.
For comparison, we calculated results for year 2000 and the average annual rate of change in dementia attributable ambient PM2.5 pollution during the 2000–2015 period. On global scale, the total burden of dementia attributable to PM2.5 has a 62% net increase (equivalent to 3.3% per year), with ∼790K more incidence in dementia and ∼250K more deaths in 2015. Yet countries varied significantly in the trend of dementia since 2000. The countries that bear the major burden from air‐pollution‐associated dementia do not change much over the 15 years (Figures 2a, 2d, and 2c, 2f). On a per capita basis, countries having the largest burdens in dementia associated with PM2.5 pollution mostly stay stable in the pollution‐related incidence rates in 2015 compared to 2000 (Figures 2b and 2e). These countries are mostly in Europe. This correspond to a decline in both the age‐specific and the age‐standardized baseline incidence rate worldwide since 1990, except for East Asia and High‐Income Asia Pacific (Global Burden of Disease Collaborative Network, 2018; Wolters et al., 2020). In East Asia and High‐Income Asia Pacific, as well as in northern and central Africa, incidence rates attributable to air pollution mostly increased, which could be related to increases in ambient PM2.5 pollution and baseline incidence rates, and an aging population. On subnational level, we found divided changes within countries (Figure S8). For example, in China, the number of incidence attributable to PM2.5 pollution increased in most regions in the East, but decreased in the middle and the south in 2015 compared to 2000. Similarly, the US also had significant decreases in the middle and Eastern regions, while showing some increases on the Western coast.
From the perspective of the change in exposure alone, significant increases occurred in Asia, Middle East and Africa, while high income countries had either a small decrease, of about 1% in AF, or stayed stable (Figure S9). The magnitude of change in AF ranged from −4% to 5% between 2000 and 2015.
In the above calculations of trends, several factors are at play. To explore these, we decompose their relative impacts on the estimated incidence rate results. Because these factors are involved nonlinearly throughout the calculations, we did a linear regression on a log‐log scale to compare the “average” contributions of them over their respective ranges. The log‐log regression (Table S6) shows that the largest impact comes from the fraction of post‐80 population (age structure), followed by baseline incidence rate, RR (which is a proxy for exposure levels here, because its value is only dependent on exposure in the C‐R function), and total post‐65 population (population size). A 1% increment in each of the above variables results in a 1.2%, 0.9%, 0.9%, and 0.1% increase in attributable incidence rate, respectively. This implies that the per capita burden of dementia attributable to PM2.5 pollution is very sensitive to the age structure. While the portion of “senior elderly” (post‐80) has a high influence (1.2% change in incidence rate in response to 1% change, i.e., elasticity = 1.2) on our results, the total number of elderly people (post‐65) is much less influential. A 1% change in the baseline incidence rate (age‐specific here) changes the attributable incidence rate by 0.9% (i.e., elasticity <1), and is therefore still very influential. These variable‐specific contributions are a result of the model structure in the equations in Section 2.
While the top 15 countries with the highest number of attributable incidences were largely determined by the size of the post‐65 population (Figures 2a and 2d), incidence rates are more complicated to interpret (Figures 2b and 2e). We use the results for 2015 as an example (Figure 2b). First, many countries listed have a relatively high PM2.5 exposure (higher than 20 µg/m3 on annual average), leading to a comparatively high RR (>1.4) relative to the counterfactual exposure levels (e.g., Hungary, Bulgaria, Cyprus, etc.). In addition, Japan, Italy, Spain, and Belgium were the top four countries with the highest post‐65 baseline incidence rate of dementia among all global countries, and Austria and Switzerland were also among the top 15 countries for dementia incidence. United States, Japan, Germany, Italy, United Kingdom, and Spain in total had 22% of global post‐65 population. Furthermore, the average fraction of post‐80 population out of post‐65 was 27%, significantly higher than the global average of 17%. In summary, on a per capita basis, PM2.5 exposure leads to the highest burden of dementia in high income countries with aging populations.
To quantify the morbidity effects and provide additional information related to mortality, we calculated YLD and YLL, respectively. The sum of YLD and YLL, DALY, interpreted as the lost years of “healthy” life, reaches 7.4 M (5.2 M, 9.2 M) globally in 2015 (Figure 3b). China, Japan, the US, and India had the largest total burden in DALYs, together accounting for 50% of the global burden of dementia attributable to ambient PM2.5. Italy and Brazil were the next, each with more than 2 M DALYs. The ratio between YLD and YLL varied across countries from 0.22 to 0.55, with a global average of 0.33. This same ratio is 0.2 for the other PM2.5‐related (Global Burden of Disease Collaborative Network, 2018), indicating a higher portion of morbidity in the total burden of dementia in our analysis. Globally, net changes in YLD, YLL, and DALY from 2000 to 2015 are above 60%, because of increases in exposures and an aging population. The largest increments occurred in China, with 758K additional DALYs, followed by Japan and India.
In 2015, the deaths from dementia attributable to ambient PM2.5 represent about 15% (11%–19%) of the total deaths from ambient PM2.5 pollution, and the DALYs from dementia were 7% (5%–9%) of the all‐cause PM2.5‐associated DALYs. This fraction was higher in older populations. However, the fraction relative to total PM2.5‐related YLD reached 30% (22%–39%), indicating a substantial burden of morbidity incurred by dementia, compared to other causes affected by air pollution. Ambient PM2.5 pollution was responsible for about 28% (20%–35%) of deaths and 30% (21%–37%) of DALY from the total burden of dementia in 2015 globally, which indicates that ambient PM2.5 pollution is potentially a significant contributor to the incidence of and death due to dementia. The burden of dementia associated with PM2.5 also varied across countries (Table S7). In general, the estimated DALY burden was relatively higher as a fraction of a country's total PM2.5‐related DALY burden in high income countries, reaching the highest in Japan, Finland, Italy, etc. This is because many of these countries have lowered the baseline incidence and mortality rates of the other PM2.5‐related diseases. By contrast, the DALY burdens represent higher fractions of the total dementia DALY burden in many low and middle‐income countries in Asia and Africa, indicating that air pollution is a relatively more important contributor to dementia in these locations.
Cost of illness studies focused on dementia have shown that it causes high socioeconomic burdens, and has been rapidly expanding worldwide. In 2015, the PM2.5‐associated dementia burden induced costs of $26 billion (USD in 2010) according to our estimates, among which $15 billion were direct economic costs, that is, direct care cost and direct medical costs, while the remaining $11 billion were from informal care costs, mostly provided by family members. The high informal care costs are a specific characteristic of dementia compared to other diseases. Regionally, the highest market costs occur in Western Europe, North America, and Asia (Table 1), mainly due to their significantly higher unit costs. It has been estimated that the costs associated with dementia have been growing at an annual rate of 16% since 2000 globally (Xu et al., 2017). With the aging population and a longer survival time, the economic burden associated with PM2.5‐induced dementia is very likely continue to increase in future.
Table 1.
Economic Costs of Dementia Attributable to Air Pollution by Regions, in Million 2010 USD
| Regions | Informal care cost | Direct care cost | Direct medical cost |
|---|---|---|---|
| Asia Pacific high income | 1,917 | 3,296 | 408 |
| Asia, central | 15 | 9 | 10 |
| Asia, east | 1,550 | 289 | 440 |
| Asia, south | 109 | 27 | 55 |
| Asia, southeast | 106 | 44 | 88 |
| Australasia | 104 | 123 | 17 |
| Caribbean | 57 | 27 | 30 |
| Europe, central | 359 | 123 | 112 |
| Europe, eastern | 261 | 96 | 112 |
| Europe, western | 3,678 | 3,934 | 1,279 |
| Latin America, Andean | 13 | 10 | 11 |
| Latin America, central | 70 | 105 | 115 |
| Latin America, southern | 60 | 33 | 36 |
| Latin America, tropical | 114 | 127 | 140 |
| North Africa/Middle East | 216 | 61 | 233 |
| North America high income | 2,320 | 2,871 | 1,085 |
| Oceania | 5 | 1 | 1 |
| Sub‐Saharan Africa, central | 6 | 1 | 3 |
| Sub‐Saharan Africa, east | 19 | 3 | 5 |
| Sub‐Saharan Africa, southern | 46 | 5 | 10 |
| Sub‐Saharan Africa, west | 36 | 6 | 13 |
| Global | 11,068 | 11,189 | 4,202 |
3.3. Strengths and Limitations
Our analysis includes several important features. First, we systematically reviewed evidence from different types of exposures to PM2.5 (i.e., AAP, HAP, SHS, and AS), and examined the sensitivity of the C‐R functions to each of them. In general, evidence for a link between incidence of dementia and AS is more mixed, while effects with SHS are more significant. Previous studies have separately reviewed the relationship between dementia and different exposure types, but have not examined across exposure types. Given that current AAP studies are mostly conducted in less‐polluted, wealthier regions, we examined effects from SHS and AS literature as an indirect way to learn possible effects in higher exposure ranges.
We discussed pros and cons of multiple fitting functions from the perspective of model selection. In addition, we conducted various sensitivity tests on the extended data set, with respect to major sources of heterogeneity and sensitivity, such as different exposure types, heterogeneous diagnosis standard for dementia, sensitivity to single study, and the choice of function forms. We selected one of these C‐R functions for a preliminary global application because its evidence base covers an exposure range that is well‐suited to our global scope, it is the most robust across all the above sensitivity tests, and is likely to be conservative. Our analysis of temporal trends in dementia attributable to PM2.5 and associated economic impacts can inform future research.
As our study is an initial analysis based on a limited number of primary epidemiologic studies it includes important limitations. First, there were no AAP studies identified at the highest ambient exposure levels. Most of the AAP studies were based in North America and Europe, while the majority of the estimated attributable burden was in China and India. We found no eligible studies indicating the effects of PM2.5 exposure from HAP on dementia, and therefore caution against application of estimates from this study to HAP. Moreover, there is not yet a thorough examination of the pathological linkage between tobacco smoke exposure and dementia. Some caveats may include the build‐up of cardiovascular diseases from smoking will indirectly increase the chance of dementia (Anstey et al., 2007; Peters et al., 2008), and other toxins in tobacco may cause neuronal damage (Ghosh et al., 2009; Heffernan et al., 2014; Heffernan & O'Neill, 2013). Nonetheless, if we relax this assumption and consider only AAP evidence, essentially all the estimates we present below would be higher. The above points largely limited our confidence in estimating the C‐R functions. Further research in these areas would be highly beneficial.
Finally, knowledge of co‐pollution effects from other ambient air pollutants and a multi‐exposure approach to simultaneously consider the effect of all air pollution is still insufficient. For example, noise and greenness likely influence the risk of demential, together with air pollution. A recent study found that road proximity was associated with all types of dementia, likely partially mediated by air pollution, and that greenness could have protective effects from incidence of dementia (Yuchi et al., 2020). Additional potential environmental and socioeconomic risk factors that may be correlated with air pollution may also be relevant. Other than the exposure‐response relationship, we used the national level incidence and age structure, although there can be important subnational variation. Moreover, these data can have different level of uncertainties, which should also be further quantified. We used global PM2.5 concentration estimates from Shaddick et al. (2018), which was also used by the GBD 2017 studies and therefore eliminated the variations caused by different exposures. However, more work to further improve the exposures estimation has been done since, by incorporating more monitoring observations and improving algorithms to merge satellite and ground monitoring data.
In addition, the effect estimates in evidence for the PM2.5–dementia relationship, and thus in our effect estimates, are influenced by methodological limitations and heterogeneities. For instance, we did not look into the sub‐types of dementia, lacking enough information. For example, Cerza et al. (2019) reported significantly variations in the effect estimates among vascular dementia (VaD), Alzheimer's disease, and senile dementia, highlighting that VaD tends to have a more significant risk increase in response to PM2.5 exposures. This may be related to the methodological bias that patients with VaD are more likely to be hospitalized (Cerza et al., 2019). But it may also be due to a different pathological pathway of VaD. However, this limitation is not unique to dementia. Some other diseases that were determined to be equally or less confident in terms of evidence of causality from air pollution by the EPA (2019), such as Type 2 diabetes, have been included in the GBD 2017 database (Global Burden of Disease Collaborative Network, 2018).
In summary, increasing experimental and epidemiological evidence indicates a relationship between increased risk of dementia and exposure to PM2.5 pollution, yet development of a consistent quantitative relationship is difficult, due to the limited number of epidemiological studies and the heterogeneity amongst them. However, based on the existing evidence and our randomized statistical approach, we found a significant increase in the risk of dementia with increased exposure to air pollution. Our results show that dementia attributable to air pollution can be a costly health consequence, with incidence expanding rapidly around the world given an aging population and increased longevity. The potentially large impacts estimated in our study should motivate further work on this linkage between air pollution and dementia, including improved understanding of biological mechanisms and additional epidemiological studies to help further evaluate the exposure‐response relationship and the overall strength of evidence.
4. Conclusion
We reviewed the evidence for the linkage between dementia and PM2.5 exposures. We found that the current evidence is still limited, especially in AAP studies from high‐exposure regions. We developed preliminary exposure‐response functions based on the available epidemiologic literature, and examined their sensitivities to a variety of factors.
Using the more conservative C‐R function developed with AAP and SHS data, we estimated 2.1 M (1.5 M, 2.6 M) cases of incident dementia and 0.62 M (0.44 M, 0.77 M) dementia deaths in 2015. This indicates a 62% net increase (or 3.3% per year). Countries in Europe and North America mostly had reduced total burdens, while those in Asia, the Middle East, and North and Central Africa had increased total burdens. Overall, developed countries with an aging population and a high‐to‐moderate level of PM2.5 pollution, as well as developing countries with high PM2.5 pollution, tend to have the highest burdens of dementia attributable to ambient PM2.5.
Furthermore, our analysis suggests that ambient PM2.5 pollution could be a major risk factor for dementia, responsible for ∼15% of dementia deaths and ∼7% of DALYs. This burden was estimated to lead to $26 billion (USD in 2010) in costs in 2015 globally. As such, reducing PM2.5 exposure may be an important measure to reduce the global burden and the high costs associated with dementia. Accounting for the impact of PM2.5 on dementia may substantially increase the benefits associated with clean air or climate change mitigation policies.
Conflict of Interest
The authors declare no conflicts of interest relevant to this study.
Supporting information
Supporting Information S1
Table S2
Table S3
Acknowledgments
The authors thank NASA GISS as the funding source under the contract 80NSSC19M0138, and the National Center for Atmospheric Research (NCAR), which is a major facility sponsored by the National Science Foundation (NSF) under Cooperative Agreement 1852977. We thank Prasad Kasibhatla and Neal Fann for their support and suggestions during the development of the project. We also thank Richard Burnett for his help and explanations about the methodology. Gridded estimates of PM2.5 exposure data can be obtained at DOI: 10.1021/acs.est.8b02864, where supporting information of Shaddick et al. (2018) is stored.
Ru, M. , Brauer, M. , Lamarque, J.‐F. , & Shindell, D. (2021). Exploration of the global burden of dementia attributable to PM2.5: What do we know based on current evidence? GeoHealth, 5, e2020GH000356. 10.1029/2020GH000356
Contributor Information
Muye Ru, Email: muye.ru@duke.edu, Email: muye.ru@columbia.edu.
Michael Brauer, Email: michael.brauer@ubc.ca.
Jean‐François Lamarque, Email: lamar@ucar.edu.
Drew Shindell, Email: drew.shindell@duke.edu.
Data Availability Statement
Population data can be obtained at http://sedac.ciesin.columbia.edu/gpw, and baseline mortality data from the Global Burden of Disease at http://ghdx.healthdata.org/gbd-results-tool. Parameters of the exposure‐response functions developed in this paper is in Table S4.
References
- Anstey, K. J. , Von Sanden, C. , Salim, A. , & O'Kearney, R. (2007). Smoking as a risk factor for dementia and cognitive decline: A meta‐analysis of prospective studies. American Journal of Epidemiology, 166, 367–378. 10.1093/aje/kwm116 [DOI] [PubMed] [Google Scholar]
- Barnes, D. E. , Haight, T. J. , Mehta, K. M. , Carlson, M. C. , Kuller, L. H. , & Tager, I. B. (2010). Secondhand smoke, vascular disease, and dementia incidence: Findings from the cardiovascular health cognition study. American Journal of Epidemiology, 171, 292–302. 10.1093/aje/kwp376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett, R. , Chen, H. , Szyszkowicz, M. , Fann, N. , Hubbell, B. , Pope, C. A. , et al. (2018). Global estimates of mortality associated with long‐term exposure to outdoor fine particulate matter. Proceedings of the National Academy of Sciences, 115, 9592–9597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey, I. M. , Anderson, H. R. , Atkinson, R. W. , Beevers, S. D. , Cook, D. G. , Strachan, D. P. , et al. (2018). Are noise and air pollution related to the incidence of dementia? A cohort study in London, England. BMJ Open, 8, e022404. 10.1136/bmjopen-2018-022404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerza, F. , Renzi, M. , Gariazzo, C. , Davoli, M. , Michelozzi, P. , Forastiere, F. , & Cesaroni, G. (2019). Long‐term exposure to air pollution and hospitalization for dementia in the Rome longitudinal study. Environmental Health, 18, 72. 10.1186/s12940-019-0511-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, R. , Wilson, K. , Chen, Y. , Zhang, D. , Qin, X. , He, M. , et al. (2013). Association between environmental tobacco smoke exposure and dementia syndromes. Occupational and Environmental Medicine, 70, 63–69. 10.1136/oemed-2012-100785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, H. , & Kim, S. H. (2019). Air Pollution and Dementia. Dementia and Neurocognitive Disorders, 18, 109–112. 10.12779/dnd.2019.18.4.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commenges, D. , Joly, P. , Letenneur, L. , & Dartigues, J. (2004). Incidence and mortality of Alzheimer's disease or dementia using an illness‐death model. Statistics in Medicine, 23, 199–210. 10.1002/sim.1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous‐Bou, M. , Gascon, M. , Gispert, J. D. , Cirach, M. , Sánchez‐Benavides, G. , Falcon, C. , et al. (2020). Impact of urban environmental exposures on cognitive performance and brain structure of healthy individuals at risk for Alzheimer's dementia. Environment International. [DOI] [PubMed] [Google Scholar]
- EMRE (2003). Dementia associated with Parkinson's disease. The Lancet Neurology, 2, 229–237. 10.1016/s1474-4422(03)00351-x [DOI] [PubMed] [Google Scholar]
- EPA (2019). Integrated Science assessment (ISA) for particulate matter (final report). U.S. Environmental Protection Agency. [PubMed] [Google Scholar]
- Fu, P. , & Yung, K. K. L. (2020). Air pollution and Alzheimer's Disease: A systematic review and meta‐analysis. Journal of Alzheimer's Disease, 1–14. [DOI] [PubMed] [Google Scholar]
- Ghosh, D. , Mishra, M. K. , Das, S. , Kaushik, D. K. , & Basu, A. (2009). Tobacco carcinogen induces microglial activation and subsequent neuronal damage. Journal of Neurochemistry, 110, 1070–1081. 10.1111/j.1471-4159.2009.06203.x [DOI] [PubMed] [Google Scholar]
- Global Burden of Disease Collaborative Network . (2018). Global Burden of Disease Study 2017 (GBD 2017) Results. Seattle. [Google Scholar]
- Global Burden of Disease Collaborative Network . (2020). Global Burden of Disease Study 2019 (GBD 2019) Results. Seattle. [Google Scholar]
- Grande, G. , Ljungman, P. L. , Eneroth, K. , Bellander, T. , & Rizzuto, D. (2020). Association between cardiovascular disease and long‐term exposure to air pollutION WITH THE RISK OF DEMENTIA. JAMA Neurology 77, 801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffernan, T. M. , Carling, A. , O’Neill, T. S. , & Hamilton, C. (2014). Smoking impedes executive function and related prospective memory. Irish Journal of Psychological Medicine, 31, 159–165. 10.1017/ipm.2014.17 [DOI] [PubMed] [Google Scholar]
- Heffernan, T. M. , & O’Neill, T. S. (2013). Exposure to second‐hand smoke damages everyday prospective memory. Addiction, 108, 420–426. 10.1111/j.1360-0443.2012.04056.x [DOI] [PubMed] [Google Scholar]
- Juan, D. , Zhou, D. H. D. , Li, J. , Wang, J. Y. J. , Gao, C. , & Chen, M. (2004). A 2‐year follow‐up study of cigarette smoking and risk of dementia. European Journal of Neurology, 11, 277–282. 10.1046/j.1468-1331.2003.00779.x [DOI] [PubMed] [Google Scholar]
- Judek, S. , Stieb, D. , Jovic, B. , & Edwards, B. (2012). Air quality benefits assessment tool user guide. Health Canada. [Google Scholar]
- Jung, C.‐R. , Lin, Y.‐T. , & Hwang, B.‐F. (2015). Ozone, particulate matter, and newly diagnosed Alzheimer's disease: A population‐based cohort study in Taiwan. Journal of Alzheimer's Disease, 44, 573–584. 10.3233/jad-140855 [DOI] [PubMed] [Google Scholar]
- Killin, L. O. , Starr, J. M. , Shiue, I. J. , & Russ, T. C. (2016). Environmental risk factors for dementia: A systematic review. BMC Geriatrics, 16, 175. 10.1186/s12877-016-0342-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kioumourtzoglou, M.‐A. , Schwartz, J. D. , Weisskopf, M. G. , Melly, S. J. , Wang, Y. , Dominici, F. , & Zanobetti, A. (2016). Long‐term PM 2.5 Exposure and Neurological HospitalAdmissions in the Northeastern United States. Environmental Health Perspectives, 124, 23–29. 10.1289/ehp.1408973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, M. , Schwartz, J. , Wang, Y. , Dominici, F. , & Zanobetti, A. (2019). Long‐term effect of fine particulate matter on hospitalization with dementia. Environmental Pollution, 254, 112926. 10.1016/j.envpol.2019.07.094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S. , Lee, W. , Kim, D. , Kim, E. , Myung, W. , Kim, S.‐Y. , & Kim, H. (2019). Short‐term PM2.5 exposure and emergency hospital admissions for mental disease. Environmental Research, 171, 313–320. 10.1016/j.envres.2019.01.036 [DOI] [PubMed] [Google Scholar]
- Livingston, G. , Huntley, J. , Sommerlad, A. , Ames, D. , Ballard, C. , Banerjee, S. , et al. (2020). Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet, 396, 413–446. 10.1016/s0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateen, F. , Oh, J. , Tergas, A. I. , Bhayani, N. H. , & Kamdar, B. B. (2013). Titles versus titles and abstracts for initial screening of articles for systematic reviews. Clinical Epidemiology, 5, 89–95. 10.2147/clep.s43118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, C. J. , Aravkin, A. Y. , Zheng, P. , Abbafati, C. , Abbas, K. M. , Abbasi‐Kangevari, M. , et al. (2020). Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. The Lancet, 396, 1223–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasari, M. M. , Szyszkowicz, M. , Chen, H. , Crouse, D. , Turner, M. C. , Jerrett, M. , et al. (2016). A class of non‐linear exposure‐response models suitable for health impact assessment applicable to large cohort studies of ambient air pollution. Air Quality, Atmosphere & Health, 9, 961–972. 10.1007/s11869-016-0398-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng, M. , Freeman, M. K. , Fleming, T. D. , Robinson, M. , Dwyer‐Lindgren, L. , Thomson, B. , et al. (2014). Smoking prevalence and cigarette consumption in 187 countries, 1980‐2012. Journal of the American Medical Association, 311, 183–192. 10.1001/jama.2013.284692 [DOI] [PubMed] [Google Scholar]
- Oudin, A. (2020). Short review: Air pollution, noise and lack of greenness as risk factors for Alzheimer's disease‐ epidemiologic and experimental evidence. Neurochemistry International, 134, 104646. 10.1016/j.neuint.2019.104646 [DOI] [PubMed] [Google Scholar]
- Paul, K. C. , Haan, M. , Mayeda, E. R. , & Ritz, B. R. (2019). Ambient air pollution, noise, and late‐life cognitive decline and dementia risk. Annual Review of Public Health, 40, 203–220. 10.1146/annurev-publhealth-040218-044058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters, R. , Ee, N. , Peters, J. , Booth, A. , Mudway, I. , & Anstey, K. J. (2019). Air pollution and dementia: A systematic review. Journal of Alzheimer's Disease, 70, S145–S163. 10.3233/jad-180631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters, R. , Poulter, R. , Warner, J. , Beckett, N. , Burch, L. , & Bulpitt, C. (2008). Smoking, dementia and cognitive decline in the elderly, a systematic review. BMC Geriatrics, 8, 36. 10.1186/1471-2318-8-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power, M. C. (2020). Growing evidence links air pollution exposure to risk of Alzheimer's disease and related dementia. Brain, 143, 8–10. 10.1093/brain/awz396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power, M. C. , Adar, S. D. , Yanosky, J. D. , & Weuve, J. (2016). Exposure to air pollution as a potential contributor to cognitive function, cognitive decline, brain imaging, and dementia: A systematic review of epidemiologic research. Neurotoxicology, 56, 235–253. 10.1016/j.neuro.2016.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince, M. J. , Wimo, A. , Guerchet, M. , Ali, G. C. , Wu, Y. T. , & Prina, M. (2015). World Alzheimer Report 2015: The Global Impact of Dementia: An Analysis of Prevalence. Incidence, Cost and Trends. [Google Scholar]
- Qiu, H. , Zhu, X. , Wang, L. , Pan, J. , Pu, X. , Zeng, X. , et al. (2019). Attributable risk of hospital admissions for overall and specific mental disorders due to particulate matter pollution: A time‐series study in Chengdu, China. Environmental Research, 170, 230–237. 10.1016/j.envres.2018.12.019 [DOI] [PubMed] [Google Scholar]
- Reitz, C. , Den Heijer, T. , Van Duijn, C. , Hofman, A. , & Breteler, M. M. B. (2007). Relation between smoking and risk of dementia and Alzheimer disease: The Rotterdam Study. Neurology, 69, 998–1005. 10.1212/01.wnl.0000271395.29695.9a [DOI] [PubMed] [Google Scholar]
- Schikowski, T. , & Altug, H. (2020). The role of air pollution in cognitive impairment and decline. Neurochemistry International. [DOI] [PubMed] [Google Scholar]
- Schraufnagel, D. E. , Balmes, J. R. , Cowl, C. T. , De Matteis, S. , Jung, S.‐H. , Mortimer, K. , et al. (2019). Air pollution and noncommunicable diseases. Chest, 155, 417–426. 10.1016/j.chest.2018.10.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaddick, G. , Thomas, M. L. , Amini, H. , Broday, D. , Cohen, A. , Frostad, J. , et al. (2018). Data integration for the assessment of population exposure to ambient air pollution for global burden of disease assessment. Environmental Science and Technology, 52, 9069–9078. 10.1021/acs.est.8b02864 [DOI] [PubMed] [Google Scholar]
- Shi, L. , Wu, X. , Yazdi, M. D. , Braun, D. , Awad, Y. A. , Wei, Y. , et al. (2020). Long‐term effects of PM2· 5 on neurological disorders in the American Medicare population: A longitudinal cohort study. The Lancet Planetary Health. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou, Y. , Huang, Y. , Zhu, X. , Liu, C. , Hu, Y. , & Wang, H. (2019). A review of the possible associations between ambient PM2.5 exposures and the development of Alzheimer's disease. Ecotoxicology and Environmental Safety, 174, 344–352. 10.1016/j.ecoenv.2019.02.086 [DOI] [PubMed] [Google Scholar]
- Stanaway, J. D. , Afshin, A. , Gakidou, E. , Lim, S. S. , Abate, D. , Abate, K. H. , et al. (2018). Global, regional, and national comparative risk assessment of 84 behavioral, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. The Lancet, 392, 1923–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirland, L. E. , O'shea, C. I. , & Russ, T. C. (2018). Passive smoking as a risk factor for dementia and cognitive impairment: Systematic review of observational studies. International Psychogeriatrics, 30, 1177–1187. 10.1017/s1041610217002824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautmann, S. , Rehm, J. , & Wittchen, H. U. (2016). The economic costs of mental disorders: Do our societies react appropriately to the burden of mental disorders? EMBO Reports, e201642951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai, T. L. , Lin, Y. T. , Hwang, B. F. , Nakayama, S. F. , Tsai, C. H. , Sun, X. L. , et al. (2019). Fine particulate matter is a potential determinant of Alzheimer's disease: A systemic review and meta‐analysis. Environmental Research, 177. 10.1016/j.envres.2019.108638 [DOI] [PubMed] [Google Scholar]
- USEPA . (2015). Environmental benefits Mapping and analysis Program–Community Edition (BenMAP‐CE) [online]. US Environmental Protection Agency. [Google Scholar]
- Vos, T. , Abajobir, A. A. , Abate, K. H. , Abbafati, C. , Abbas, K. M. , Abd‐Allah, F. , et al. (2017). Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. The Lancet, 390, 1211–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H.‐X. , Fratiglioni, L. , Frisoni, G. B. , Viitanen, M. , & Winblad, B. (1999). Smoking and the occurence of Alzheimer's disease: Cross‐sectional and longitudinal data in a population‐based study. American Journal of Epidemiology, 149, 640–644. 10.1093/oxfordjournals.aje.a009864 [DOI] [PubMed] [Google Scholar]
- Weuve, J. (2020). Are we ready to call exposure to air pollution a risk factor for dementia?. AAN Enterprises. [DOI] [PubMed] [Google Scholar]
- WHO (2018a). AirQ+: Software tool for health risk assessment of air pollution. [Google Scholar]
- WHO (2018b). Metrics: Disability‐Adjusted Life Year (DALY) [Online]. World Health Organization. Retrieved from http://www.who.int/healthinfo/global_burden_disease/metrics_daly/en/ [Google Scholar]
- Wimo, A. , Guerchet, M. , Ali, G.‐C. , Wu, Y.‐T. , Prina, A. M. , Winblad, B. , et al. (2017). The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimer's and Dementia, 13, 1–7. 10.1016/j.jalz.2016.07.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters, F. J. , Chibnik, L. B. , Waziry, R. , Anderson, R. , Berr, C. , Beiser, A. , et al. (2020). 27‐year time trends in dementia incidence in Europe and the US. The Alzheimer Cohorts Consortium. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, J. , Zhang, Y. , Qiu, C. , & Cheng, F. (2017). Global and regional economic costs of dementia: A systematic review. The Lancet, 390, S47. 10.1016/s0140-6736(17)33185-9 [DOI] [Google Scholar]
- Yanosky, J. D. , Paciorek, C. J. , Laden, F. , Hart, J. E. , Puett, R. C. , Liao, D. , & Suh, H. H. (2014). Spatio‐temporal modeling of particulate air pollution in the conterminous United States using geographic and meteorological predictors. Environmental Health, 13, 63. 10.1186/1476-069x-13-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao, L. , & Lu, N. (2014). Spatiotemporal distribution and short‐term trends of particulate matter concentration over China, 2006‐2010. Environmental Science & Pollution Research, 21, 9665–9675. 10.1007/s11356-014-2996-3 [DOI] [PubMed] [Google Scholar]
- Yoshitake, T. , Kiyohara, Y. , Kato, I. , Ohmura, T. , Iwamoto, H. , Nakayama, K. , et al. (1995). Incidence and risk factors of vascular dementia and Alzheimer's disease in a defined elderly Japanese population: The Hisayama Study. Neurology, 45, 1161–1168. 10.1212/wnl.45.6.1161 [DOI] [PubMed] [Google Scholar]
- Younan, D. , Petkus, A. J. , Widaman, K. F. , Wang, X. , Casanova, R. , Espeland, M. A. , et al. (2020). Particulate matter and episodic memory decline mediated by early neuroanatomic biomarkers of Alzheimer's disease. Brain, 143, 289–302. 10.1093/brain/awz348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuchi, W. , Sbihi, H. , Davies, H. , Tamburic, L. , & Brauer, M. (2020). Road proximity, air pollution, noise, green space and neurologic disease incidence: A population‐based cohort study. Environmental Health, 19, 8. 10.1186/s12940-020-0565-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Table S2
Table S3
Data Availability Statement
Population data can be obtained at http://sedac.ciesin.columbia.edu/gpw, and baseline mortality data from the Global Burden of Disease at http://ghdx.healthdata.org/gbd-results-tool. Parameters of the exposure‐response functions developed in this paper is in Table S4.


