Abstract
There is a large number of bioactive polyketides well-known for their anticancer, antibiotic, cholesterol-lowering, and other therapeutic functions, and hispidin is among them. It is a highly abundant secondary plant and fungal metabolite, which is investigated in research devoted to cancer, metabolic syndrome, cardiovascular, neurodegenerative, and viral diseases. This review summarizes over 20 years of hispidin studies of its antioxidant, anti-inflammatory, anti-apoptotic, antiviral, and anti-cancer cell activity.
Keywords: hispidin, polyketides, antioxidant, therapeutic potential
1. Introduction
People have been using fungi as nutrients and the source of natural bioactive compounds since ancient times. Fungi have become a part of folk and traditional medicine due to the unique medicinal properties of some species [1,2]. Thus, the extracts of mushrooms that belong to the Phellinus and Inonotus genera have been used for the treatment of various diseases including malignant tumors, cardiovascular and liver diseases, diabetes, and others [3,4]. The exact mechanisms underlying the medicinal activity of fungal extracts are still mostly unknown. However, the main role of treating agents was attributed to the number of polysaccharides [3,4,5] and, more recently, to the low-molecular-weight bioactive compounds. Among the latter, there are styrylpyrones—polyphenol pigments that display a large number of biological effects. One of the first discovered styrylpyrones was polyketide hispidin isolated from Inonotus hispidus fungi in 1889, its structure was identified in 1961 [5,6]. Hispidin was found in various related fungi species of the Hymenochaetaceae family (genus Inonotus (I. obliquus, I. xeranticus), Phellinus (P. linteus also known as Sanghuangporus sanghuang [7], P. ignarius, P. baumii, P. harmala, P. sensulato)) and the Australian fungus Cortinarius [8]. Hispidin was also identified in luminescent mushrooms Neonothopanus nambi as a key precursor of fungal luciferin [9]. Moreover, hispidin and its derivatives were discovered in many plants such as the horsetail (Equisetum arvense) [10], piper (Piper methysticum) [11], the Compositae plant achirocline (Achyrocline bogotensis) [12], pistachio (Pistacia atlantica) [13], and others.
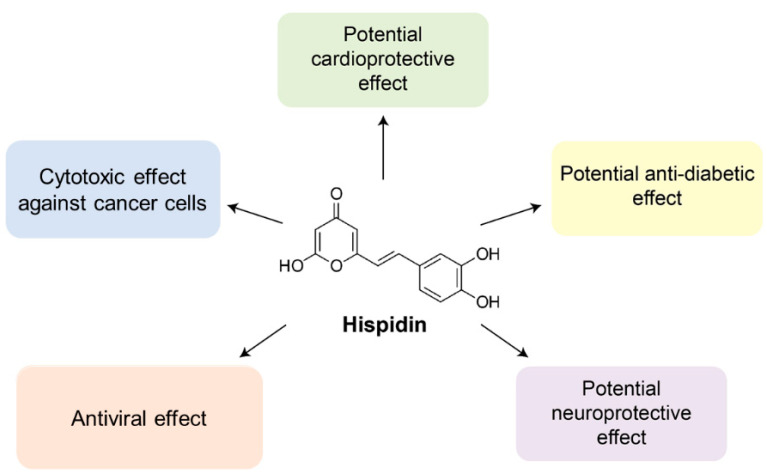
To date, hispidin is one of the best-studied polyketides among other fungal polyphenols. It can neutralize free radicals [3,14,15,16,17]. Recent studies have shown that hispidin but not polysaccharides or flavonols determine the antioxidant and antitumor properties of Phenllinus mushroom extracts [18]. Besides antioxidant activity hispidin displays cytotoxic [19], potentially hypoglycemic [20], anti-inflammatory [21], antiviral [22], and neuroprotective properties [23]. At the same time, the Ames test, in vitro chromosome aberration test, acute oral toxicity test, and bone marrow micronucleus test demonstrate a very low toxicity for human consumptions [7]. As hispidin is a promising bioactive compound for the potential application in biomedicine, herein we summarize the available data on the effects of hispidin (Figure 1).
Figure 1.
Hispidin exhibits cytotoxic, anti-diabetic, anti-inflammatory, antiviral, cardioprotective, and neuroprotective effects covered in the present review.
2. Cytotoxic Effect of Hispidin on Cancer Cells
The World Health Organization represents cancer as one of the main death causes of the past and ongoing centuries [24]. As a universal approach to cancer treatment is absent, the search for novel compounds exhibiting anticancer activities is still relevant [25]. Natural compounds are particularly attractive due to the virtual lack of side effects compared to synthetic analogs [26]. Secondary metabolites of plants and fungi, including polyketides, can serve as a bioactive compound source [27].
Gonindard and colleagues in 1997 have shown the hispidin-induced in vitro cytotoxic effects on two different cancer cell lines including skin squamous cell carcinoma SCL-1 and pancreatic ductal adenocarcinoma Capan-1 [19]. Later a similar cytotoxic effect of hispidin was observed for other cancer cell lines. The list of cell lines susceptible to hispidin can be found in Table 1. According to published data cytotoxic effect of hispidin is achieved by activating various mechanisms, which will be considered below.
Table 1.
Semilethal hispidin dose (IC50) for various cancer cell lines.
| Cell Line | Approximate Semilethal Hispidin Dose, mol/L |
|---|---|
| Skin squamous cell carcinoma SCL-1 [19] | 1 × 10−4 |
| Pancreatic ductal adenocarcinoma Capan-1 [19] | Between 1 × 10−4 and 1 × 10−3 |
| Rectal carcinoma CMT-93 [28] | 7 ± 1 × 10−4 |
| Colorectal carcinoma HCT 116 [28] | 7 ± 1 × 10−4 |
| Lung carcinoma A549 [29] | 2.5 × 10−4 |
| Endocervical adenocarcinoma SGC-7901 [30] | 6.1 ± 1.1 × 10−3 |
| Pancreatic ductal adenocarcinoma BxPC-3 [31] | 1 × 10−4 |
| Pancreatic ductal adenocarcinoma AsPC-1 [31] | 2 × 10−4 |
One of the mechanisms is related to protein kinase C (PKC), which isoforms are among the key participants of carcinogenesis. Despite under normal circumstances PKC is responsible for cell homeostasis maintenance, an increase in the expression level of PKC isoforms is often associated with malignant tumor growth [32]. The cytotoxic effect of hispidin is presumably achieved via inhibition of the activity of PKC isoform β through an unclear exact mechanism [19,33]. This ability of hispidin to inhibit PKC activity was successfully applied in the studies of PKC activation-dependent cell processes [34,35].
The alternative mechanism of cytotoxic effect of hispidin is described for the human papillomavirus-related endocervical adenocarcinoma SGC-7901 cell line. According to Lv and co-authors, this polyketide induces autophagic and necrotic death of adenocarcinoma cells but does not show the cytotoxic effect on control cells such as human liver cell line L02 and stomach cell line GES-1 [30]. Hispidin activates phosphorylation of stathmin-1 at Ser16 which causes depolymerization of microtubules in SGC-7901. Cancer cell microtubules oscillate and increase the membrane permeability of peripheral lysosomes, which become large and fragile compared to the normal ones [36]. This mechanism determines the cytotoxic effect of hispidin on SGC-7901, human lung adenocarcinoma A549 and human hepatocellular carcinoma HepG2 [30].
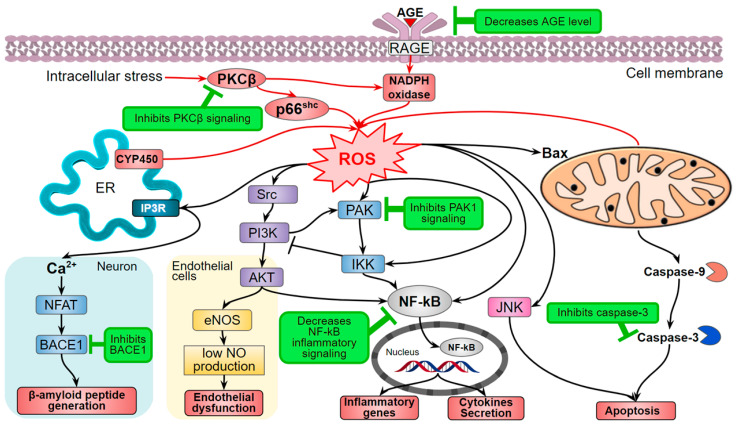
The Shinkichi Tawata research group suggested the mechanism of indirect influence of hispidin and a number of other natural compounds through inhibtion of serine/threonine-protein kinase 1 (PAK1)-dependent signaling. This assumption was corroborated with the experiments on mouse B16F10 melanoma and human A549 lung adenocarcinoma cell lines [29,37]. Hispidin decreases ROS production and ROS-dependent signaling, which is consistent with other studies (Figure 2).
Figure 2.
The hispidin effect on ROS production and ROS-dependent signaling (marked green). AGE—Advanced glycation end-product; AKT—Protein kinase B; BACE1—β-site amyloid precursor protein cleaving enzyme 1 (β-secretase); Bax—Bcl-2-associated X protein; CYP450—cytochrome P450; eNOS—Endothelial nitric oxide synthase; ER—Endoplasmic reticulum; IKK—IκB kinase; JNK—Jun N-Terminal protein kinase; NF-kB—Nuclear factor-kappa B; NFAT—Nuclear factor of activated T-cells; p66Shc—isoform of SHC1 adaptor protein; PAK—Serine/threonine-protein kinase; PKCβ—protein kinase C β-isoform; RAGE—Receptor for AGE; ROS—reactive oxygen species; Src—Proto-oncogene tyrosine-protein kinase.
The antioxidant properties of hispidin are noted in many studies [38,39,40,41]. Despite this fact, the study by Lim et al. describes that hispidin-induced ROS-dependent apoptosis in CMT-93 mouse colon cancer cells and HCT 116 human colon cancer cells [28]. According to the experimental results, hispidin increases ROS level in a dose-dependent manner. In addition, hispidin-dependent apoptosis manifests in chromatin condensation, nuclear fragmentation, and the cells acquiring an abnormal spherical shape. It is worth noting that a similar effect was never described by other research groups. In this regard, it is possible that the ROS level increase is hispidin-independent, and the exact mechanism of apoptosis, in this case, remains unknown.
Another mechanism of impact of hispidin on cancer cell viability comprises influence on the key transcription factor Nuclear factor-kappa B (NF-kB), which is crucial for cycle maintenance, stress adaptation, inflammation, and apoptosis. The malfunctioning of NF-kB can cause oncogenesis, malignant tumor invasion, autoimmune disorders, metastasis, and resistance to antitumor therapy [42,43]. Chandimali and colleagues have shown that hispidin induced apoptosis and inhibited proliferation of pancreatic ductal adenocarcinoma BxPC-3 and AsPC1 cells. Hispidin inhibited NF-kB and enhanced the activity of p53 (known as a malignant tumor suppressor), caspase-3, and poly-ADP-ribose polymerase expression [31]. Thus, hispidin shows cytotoxic effects on cancer cells.
Hispidin increased sister chromatid exchange frequency and decreased replication index and nuclear division index values in human lymphocytes in vitro without other DNA damage [44]. Perhaps, the same mechanism partially underlies the cytotoxic effect of hispidin on cancer cells.
Furthermore, hispidin enhances the biological activities of other compounds such as chemotherapy drug gemcitabine: hispidin sensitized pancreatic cancer stem cells to gemcitabine and promoted its therapeutic efficacy [31,45].
3. The Effect of Hispidin on the Metabolic Syndrome
Metabolic syndrome is a worldwide condition comprising carbohydrate metabolism disorders, abdominal obesity, hypertension, and dyslipidemia that characterize diabetes mellitus and accompanying cardiovascular complications [46]. This disorder is strongly associated with pathological processes like oxidative stress and inflammation [47]. Therefore, scientists struggle to discover new natural compounds and to derive novel drugs applicable for the treatment and prevention of obesity, insulin resistance, and other accompanying complications.
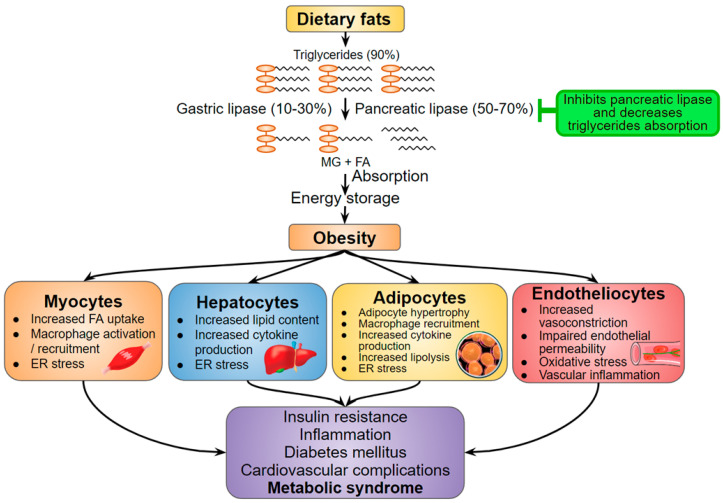
Abdominal obesity and increased free fatty acids lead to inflammation and oxidative stress that provoke insulin resistance and β-cells dysfunction [48]. To avoid ectopic fat accumulation and, therefore, prevent obesity, the treatment should stimulate lipolysis and inhibit the absorption of food triglycerides [49,50]. Hispidin in vitro inactivates pancreatic lipase — an important anti-inflammatory target (Figure 3) that decreases triglyceride absorption in the small intestine. In addition, hispidin inhibits glycerol-3-phosphate dehydrogenase, which serves as an important link between carbohydrate and lipid metabolism, and, as a consequence, decreases intracellular triglyceride levels in a dose-dependent manner [51]. Hispidin is also able to increase the intracellular cAMP level [51], which is supposedly associated with enhanced lipolytic signaling [52]. Concluding, hispidin can suppress lipohypertrophy and triglycerides absorption.
Figure 3.
Hispidin as a potential anti-obesity compound (marked green) in the context of metabolic syndrome developing. Adapted from [50,53]. MG—Monoacylglycerol; FA—Fatty acids; ER stress—Endoplasmic reticulum stress.
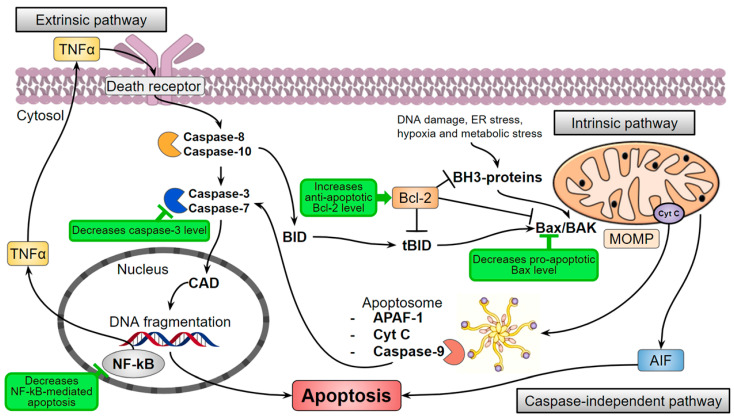
Triglycerides accumulation causes elevated oxidative stress that can lead to the death of ROS-sensitive cells. It is especially important for skeletal muscles that utilize about 75% of available in the organism glucose in their normal postprandial state [54] and are very sensitive to lipotoxicity, inflammation, and oxidation [55]. For instance, the palmitate-induced obesity model of immortalized mouse myoblasts (myotubes C2C12) provoked cell death but hispidin increased the cell survival through the inhibition of the key effector protease in apoptosis-caspase-3 [38] (Figure 4). In addition, the pro-apoptotic protein Bax level is decreased, and the anti-apoptotic protein Bcl-2 level is increased. Moreover, hispidin inhibited the translocation of the nuclear factor NF-kB—the major inflammation regulator—and prevented cell apoptosis through the NF-kB signaling pathway in the hyperglycemic model of rat pheochromocytoma PC12 cells [40]. The antioxidant effect of hispidin contributes to the leveling of oxidative stress caused by ROS, but the mechanism remains unknown.
Figure 4.
Hispidin (marked green) can influence the levels of apoptotic pathways participants. AIF—Apoptosis-inducing factor; APAF-1—Apoptotic protease activating factor-1; BAK—Bcl-2 homologous antagonist/killer; Bax—Bcl-2-associated X protein, pro-apoptotic regulator; Bcl-2—Anti-apoptotic regulator protein; BID—Pro-apoptotic protein of the Bcl-2 family; CAD—Caspase-activated DNase; Cyt C—Cytochrome C; ER stress—Endoplasmic reticulum stress; MOMP—Mitochondrial outer membrane permeabilization; NF-kB—Nuclear factor-kappa B; TNFα—Tumor necrosis factor-alpha.
Elevated oxidative stress and inflammation also induce insulin resistance: the inability of insulin-dependent cells to respond to insulin stimulation, which normally enhances the glucose uptake from the blood. Thus, insulin resistance results in excessive glucose accumulation in the blood—hyperglycemia, a key component of diabetes mellitus pathogenesis. Hyperglycemia leads to even more elevated oxidative and carbonyl stress and further progression of metabolic disorders. The increased ROS concentration follows an increased level of advanced glycation end-product (AGE), activation of AGE receptor (RAGE), PKC activity, and the hexosamine and polyol metabolic pathways [56,57]. Enhanced non-enzymatic protein glycation is one of the major hyperglycemia problems that provokes the activation of RAGE, NF-kB pro-inflammation signaling, and increases oxidative stress (Figure 2) [58]. In this context, hispidin and its fungal derivatives demonstrate both direct free radical activity and antiglycation effect that not only reduces the oxidative stress but also compensates the damage caused by AGE and ROS [59]. In addition, hispidin is better than unnatural AGE inhibitors that exhibit poor pharmacokinetics [60].
The increase in glucose levels also stimulates its consumption through the polyol pathway—two-step conversion of glucose to fructose. High activity of its key enzyme—aldose reductase—leads to the depletion of the cellular NADPH pool, which shifts the oxidative-antioxidant balance and elevates oxidative stress [57]. Hispidin inhibits aldose reductase, which is a potential therapeutic target for the prevention of hyperglycemia-associated complications [20].
The treatment of diabetes mellitus and insulin resistance also targets tyrosine phosphatase 1β (PTP1β) [61] which blocks insulin-dependent signaling by insulin receptor dephosphorylation [62]. Presumably, PTP1β inhibitors increase insulin receptor sensitivity, recover its autophosphorylation and activate the downstream signaling cascades [63]. A number of low-molecular-weight PTP1β inhibitors have been described including ones undergone clinical trials [64] but the search for more effective pharmaceuticals is still ongoing. Thus, the ability of hispidin isolated from Phellinus linteus to inhibit PTP1β [65] arouses great interest in further study of mechanisms of hispidin interaction with the target.
Decreased sensitivity of muscle, fat, and liver cells to insulin stimulation forces pancreatic β-cells to synthesize more insulin that inevitably leads to their depletion. This pathologic process is enhanced by developing inflammation and oxidative stress that causes the apoptosis of β-cells. Therefore, the dysfunction and death of pancreatic β-cells are other important factors in pathophysiology of diabetes mellitus [66]. Hispidin showed the protective effect in the model of pancreatic β-cells RINm5F treated with hydrogen peroxide. H2O2 induces oxidative stress, which, in turn, suppresses insulin production by pancreatic β-cells in the RINm5F and MIN6N lines. However, hispidin-reduced cell apoptosis and also promoted the maintenance of insulin secretion even in the presence of hydrogen peroxide [39,41]. The hispidin-dependent reduction of free radicals and intracellular ROS levels are comparable to other antioxidants such as N-acetyl-L-cysteine and vitamin C [39]. Besides, unlike synthetic antioxidants such as butylated hydroxyanisole and hydroxytoluene, hispidin is not toxic and therefore is a prospective insulin production-associated protective agent [67].
The mentioned protective effects of hispidin should provoke a further molecular mechanisms comprehensive study of this influence on inflammation processes, oxidative and carbonyl stress, as well as lipid metabolism. Potentially, it would be effective for the development of metabolic syndrome therapy methods.
4. Hispidin as a Potential Cardiovascular Protector
Cardiovascular diseases can evolve as an independent pathological process or as complications of metabolic disturbance such as diabetes mellitus. Oxidative stress is an important risk factor for the development of cardiovascular diseases [68]. ROS provoke apoptosis in the cardiomyocytes and, as a consequence, lead to ischemia and myocardial infarction [69]. They also cause and enhance endothelial dysfunction that leads to vascular damage and the development of atherosclerosis [70].
Hispidin demonstrated the antioxidant effect on H9c2 cardiomyoblast cells treated with hydrogen peroxide. Hispidin stimulated a decrease of intracellular ROS, an increase of antioxidant enzymes (heme oxygenase-1 and catalase), and activation of Akt, GSK-3β, and ERK1/2, and whole phosphatidylinositol-3-kinase signaling pathway in H9c2 cells [71]. These kinases control the metabolism and death of the cells, so they could be considered as potential therapeutic targets for the treatment of cardiovascular diseases [72].
Restoration of anti-apoptotic Bcl-2 and pro-apoptotic Bax proteins ratio suppresses the death of cardiomyoblasts H9c2 and likely increases cell survival under the influence of hispidin [73,74]. Hispidin also inhibits caspase-3 in H9c2 in a dose-dependent manner that increases the survival of the cells (Figure 4) [71].
During hyperlipidemia, free fatty acids accumulate in cells and lead to the formation of diacylglycerols—activators of PKC. Stimulation of PKC signaling in blood vessels provokes the development of insulin resistance, impaired NO production, and the development of endothelial dysfunction [75]. A number of studies rely on the hispidin ability to specifically inhibit the β isoform of PKC, including endothelial cell models HUVEC and HCAEC [76,77]. Another research group analyzed hispidin influence on the endothelial barrier function regulated by PKC signaling [78]. Unfortunately, no one has yet studied the effect of this polyketide on the functioning of vascular cells, NO production, and intracellular signal transmission.
Cancer patients are prone to the risk of cardiovascular diseases [79]. Antitumor therapy can lead to ischemia and oxidative stress-induced reperfusion development [80]. Therefore, the authors investigated the effect of hispidin in the doxorubicin-induced cytotoxic model cardiomyoblast cells H9c2 and showed that hispidin suppresses the apoptotic activation of caspase-9 (Figure 4) [81]. In addition, PKCβ isoform activates adapter protein and oxidative stress sensor—p66Shc—that leads to the formation of ROS [82] (Figure 2). Sampaio and coauthors showed that hispidin inhibits the subcellular movement of p66Shc thus decreasing oxidative stress. Thus, the protective effect of hispidin is based on its antioxidant PKS inhibition activity [81]. Nevertheless, this is not always sufficient to suppress the cytotoxic influence of the oncosuppressors, presumably due to a caspase-independent pathway of cell death [83]. Hispidin also inhibited phosphorylation of p66Shc Ser36 in fibroblasts of Leigh syndrome patients, which led to decreased ROS production and intracellular oxidative stress [84].
Thereby, the cardioprotective effect of hispidin is mainly associated with its antioxidant properties and the ability to specifically inhibit PKC activity as well as to suppress cardiomyoblasts apoptosis by various mechanisms.
5. Potential Neuroprotective Effect of Hispidin
Neurodegenerative diseases are a group of disorders with complex dysfunction of the nervous system and sensory organs. Commonly, these pathological processes are associated with metabolic disorders, deficiency of several compounds (thiamine, B12), or exogenous toxins effects [85]. They can also occur as a result of mitochondrial dysfunctions and oxidative stress caused by ROS [86,87].
Age is a risk factor for neurodegeneration accompanied by increased oxidative stress. Thus, high ROS levels provoke retinal pigment epithelial cells macular degeneration—leading cause of progressive blindness [88]. Hispidin incubated with adult retinal pigment epithelial ARPE-19 cells enhanced the expression of nuclear factor erythroid 2-related factor 2 (Nrf2) and its target genes, coding antioxidant enzymes (HO-1, NQO-1, GCLM, and GCLC) [89]. These results demonstrate hispidin antioxidative activity by activation of the JNK-pathway and Nrf2 signaling.
Oxidative stress impairs mitochondrial metabolism. Patients with such disorders suffer from multiple metabolic dysfunctions such as mitochondrial encephalopathy, deafness, retinitis pigmentosa, etc. Hispidin exhibits antioxidant activity and acts as an active substance in antioxidant defense reactions in fibroblasts, derived from patients with mitochondrial disorders. Long-term incubation of fibroblast cells with hispidin did not change mitochondrial bioenergetic parameters significantly, but the production of cytosolic superoxide decreased [90].
Oxidative stress also causes Parkinson’s disease, though its pathogenesis is poorly studied [91]. Hispidin activity in suppression of p66Shc 6-hydroxydopamine-induced phosphorylation reduces hydroxydopamine cytotoxicity [92]. Inhibition of β-site amyloid precursor protein cleaving enzyme 1 (BACE1) is one of the ways of dementia and Alzheimer’s disease treatment. BACE1 stimulates the release of a toxic β-amyloid peptide in the brain [93]. Hispidin inhibits BACE1 and as a consequence decreases the accumulation of β-amyloid peptide [94] (Figure 2).
Nitrative stress also provokes pathogenic mechanisms that lead to neuronal degeneration. A high concentration of peroxynitrite mediates this stress and causes neuron degeneration [95]. Oxidation induces such a dangerous initial injury as DNA breaks, which, furthermore, cause necrosis and apoptosis of neurons [96]. Hispidin is reported to reduce cytotoxicity and inhibit peroxynitrite-induced DNA damage [97]. Shin and colleagues recommend using hispidin for cell protection against methamphetamine-induced neurotoxicity [98].
6. Antiviral Effects of Hispidin
The medicinal properties of fungi have been described a long time ago, however, the antiviral effect of hispidin is poorly studied and is mentioned only in several works. Neuraminidase is one of the first described targets that hispidin inhibits. This viral enzyme is responsible for the hydrolysis of terminal neuraminic acid residues of virions and host cell receptors that lead to the release of virions from the infected cell. Hispidin obtained from the extract of P. baumii fruit bodies demonstrated inhibitory activity to H1N1, H5N1, and H3N2 neuraminidases at the same level as commonly used neuraminidase inhibitor zanamivir [99]. Inhibition of neuraminidases was also shown using hispidin extracted from I. hispidus fruit body [22] and P. linteus culture [100].
Hispidin was predicted as an inhibitor of the SARS-Cov2 main protease. Serseg and co-authors showed that hispidin forms highly affinable hydrogen bonds with 2019-nCoV protease residues. This group also recommended using hispidin in medical practice and supposed that hispidin could become a candidate for antiCOVID-19 drug [101,102].
7. Conclusions
Free radicals are involved in the pathogenesis of cardiovascular, neurodegenerative, metabolic diseases, as well as in cancer initiation and progression [103]. Many studies highlight that hispidin scavenges free radicals that raises the interest for further study of the mechanisms of this phenomenon as well as its medicinal properties (Table 2). Experimental and theoretical studies of the correlation between the structure of polyphenols and their antioxidant activity have shown that it mainly depends on phenolic hydroxyl groups and their ability to donate a hydrogen atom to quench free radicals [104,105]. Thermodynamic and kinetic studies have shown that the proton-coupled electron transfer is the main mechanism for getting rid of free radicals by hispidin and its analogs, which were found in the Hymenochaetaceae family of fungi [16]. The anti-diabetic, cardioprotective, and cytoprotective properties of hispidin are likely caused by the reduction of oxidative damage and activation of signaling cascades that stimulate the internal protective resources of cells.
Table 2.
Biological activities of hispidin mentioned in the review. Abbreviations of the proteins: BACE1—β-site amyloid precursor protein cleaving enzyme 1, GPDH—glycerol-3-phosphate dehydrogenase, NF-kB—Nuclear factor-kappa B, PAK1—Serine/threonine-protein kinase 1, p66shc—isoform of SHC1 adaptor protein, PTP1β—protein tyrosine phosphatase 1β.
| Bioactive Property of Hispidin | Cytotoxic Effect |
The Effect on Carbohydrate and Lipid Metabolism | Possible Neuroprotective Effect | Possible Cardioprotective Effect | Antiviral Effect |
|---|---|---|---|---|---|
| Free radical scavenger | Direct antioxidant activity and reduction of oxidative stress | ||||
| ↓PAK1 and NF-kB signaling—anti-inflammatory activity | |||||
| ↑Gene expression of antioxidant enzymes that (but not only) control apoptosis | |||||
| Inhibitor of activities of proteins | ↓Protein kinase C | ↓Caspase-3 ↓Caspase-9 |
↓Neuraminidases | ||
| ↓Aldose reductase ↓GPDH ↓PTP1β |
↓p66Shc | ||||
| ↓BACE1 | |||||
| Regulator of pro- and anti-apoptotic proteins interplay | Tumor cell death | Survival of myoblasts | Survival of cardiomyoblasts | ||
| Anti-apoptotic proteins levels | ↓ | ↑ | ↑ | ||
| Pro-apoptotic proteins levels | ↑ | ↓ | ↓ | ||
The other crucial activity of hispidin is its ability to inhibit the activities of proteins such as PKC, neuraminidases, BACE1, and others (Table 2). In the case of PKC, it results in the dysregulation of signaling pathways associated with the homeostasis of the cells and cancer development (Figure 2). Even though the exact mechanisms of PKC activity inhibition by hispidin are unclear, it certainly acts as a non-competitive inhibitor and has high selectivity against PKC [19]. Hispidin also inhibits the activity of a number of other proteins which cause an overall reduction of both oxidative stress and inflammation (Figure 2).
In addition, hispidin affects the levels of pro- and anti-apoptotic proteins from the Bcl-2 family (Table 2). Hispidin may manipulate their ratio that results in the controlled death of cancer cells and the survival/protection of healthy cells. However, the exact mechanisms of hispidin action on the regulation of apoptosis in different cells still remain unknown and need to be elucidated.
Hispidin is often studied as a fungal extract component in combination with other substances. Thus, the first report about the anti-allergic effect of hispidin for rat basophilic leukemia-2H3 (RBL2H3) cells was published several years ago [106]. It was demonstrated that the anti-allergic characteristics of fungal extracts become stronger with hispidin presence. These investigations reveal new properties of hispidin that should be studied more comprehensively.
Author Contributions
Conceptualization and connecting the text chapters, tables, and figures, K.A.P.; proofreading, first chapter and conclusion preparation, A.V.B.; second, third, and fourth chapter preparation, D.A.I.; fifth and sixth chapter preparation, E.S.S.; supervision and funding acquisition, N.M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the President of the Russian Federation grant for leading scientific schools number LS-2605.2020.4 and by the Russian Foundation for Basic Research grant number 18-29-08049.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available in publicly accessible sources, which are listed in References.
Conflicts of Interest
The authors declare no conflict of interest, financial or otherwise.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hobbs C. Medicinal Mushrooms: An Exploration of Tradition, Healing, and Culture. Botanica Press; Summertown, TN, USA: 2002. [Google Scholar]
- 2.Chen H., Tian T., Miao H., Zhao Y.-Y. Traditional uses, fermentation, phytochemistry and pharmacology of Phellinus linteus: A review. Fitoterapia. 2016;113:6–26. doi: 10.1016/j.fitote.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 3.Lee I.-K., Yun B.-S. Styrylpyrone-class compounds from medicinal fungi Phellinus and Inonotus spp., and their medicinal importance. J. Antibiot. 2011;64:349–359. doi: 10.1038/ja.2011.2. [DOI] [PubMed] [Google Scholar]
- 4.He P., Zhang Y., Li N. The phytochemistry and pharmacology of medicinal fungi of the genus Phellinus: A review. Food Funct. 2021;12:1856–1881. doi: 10.1039/D0FO02342F. [DOI] [PubMed] [Google Scholar]
- 5.Edwards R.L., Lewis D.G., Wilson D.V. 983. Constituents of the higher fungi. Part I. Hispidin, a new 4-hydroxy-6-styryl-2-pyrone from polyporus hispidus (Bull.) Fr. J. Chem. Soc. 1961:4995–5002. doi: 10.1039/jr9610004995. [DOI] [Google Scholar]
- 6.Edwards R.L., Wilson D.V. 984. Constituents of the higher fungi. Part II. The synthesis of hispidin. J. Chem. Soc. (Resumed) 1961:5003–5004. doi: 10.1039/jr9610005003. [DOI] [Google Scholar]
- 7.Li I.-C., Chen C.C., Sheu S.-J., Huang I.-H., Chen C.-C. Optimized production and safety evaluation of hispidin-enriched Sanghuangporus sanghuang mycelia. Food Sci. Nutr. 2020;8:1864–1873. doi: 10.1002/fsn3.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watling R., Gill M., Giménez A., May T.W. A new styrylpyrone-containing Cortinarius from Australia. Mycol. Res. 1992;96:743–748. doi: 10.1016/S0953-7562(09)80443-7. [DOI] [Google Scholar]
- 9.Kotlobay A.A., Sarkisyan K.S., Mokrushina Y.A., Marcet-Houben M., Serebrovskaya E.O., Markina N.M., Gonzalez Somermeyer L., Gorokhovatsky A.Y., Vvedensky A., Purtov K.V., et al. Genetically encodable bioluminescent system from fungi. Proc. Natl. Acad. Sci. USA. 2018;115:12728–12732. doi: 10.1073/pnas.1803615115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beckert C., Horn C., Schnitzler J.-P., Lehning A., Heller W., Veit M. Styrylpyrone biosynthesis in Equisetum arvense. Phytochemistry. 1997;44:275–283. doi: 10.1016/S0031-9422(96)00543-2. [DOI] [Google Scholar]
- 11.Pluskal T., Torrens-Spence M.P., Fallon T.R., De Abreu A., Shi C.H., Weng J.-K. The biosynthetic origin of psychoactive kavalactones in kava. Nat. Plants. 2019;5:867–878. doi: 10.1038/s41477-019-0474-0. [DOI] [PubMed] [Google Scholar]
- 12.Tian L.-W., Feng Y., Tran T.D., Shimizu Y., Pfeifer T., Vu H.T., Quinn R.J. Achyrodimer F, a tyrosyl-DNA phosphodiesterase I inhibitor from an Australian fungus of the family Cortinariaceae. Bioorg. Med. Chem. Lett. 2017;27:4007–4010. doi: 10.1016/j.bmcl.2017.07.062. [DOI] [PubMed] [Google Scholar]
- 13.Yousfi M., Djeridane A., Bombarda I., Chahrazed-Hamia, Duhem B., Gaydou E.M. Isolation and characterization of a new hispolone derivative from antioxidant extracts ofPistacia atlantica. Phytother. Res. 2009;23:1237–1242. doi: 10.1002/ptr.2543. [DOI] [PubMed] [Google Scholar]
- 14.Jung J.-Y., Lee I.-K., Seok S.-J., Lee H.-J., Kim Y.-H., Yun B.-S. Antioxidant polyphenols from the mycelial culture of the medicinal fungi Inonotus xeranticus and Phellinus linteus. J. Appl. Microbiol. 2008;104:1824–1832. doi: 10.1111/j.1365-2672.2008.03737.x. [DOI] [PubMed] [Google Scholar]
- 15.Park I.-H., Chung S.-K., Lee K.-B., Yoo Y.-C., Kim S.-K., Kim G.-S., Song K.-S. An antioxidant hispidin from the mycelial cultures of Phellinus linteus. Arch. Pharm. Res. 2004;27:615–618. doi: 10.1007/BF02980159. [DOI] [PubMed] [Google Scholar]
- 16.El Hassane A., Shah S.A.A., Hassan N.B., El Moussaoui N., Ahmad R., Zulkefeli M., Weber J.-F.F. Antioxidant activity of hispidin oligomers from medicinal fungi: A DFT study. Molecules. 2014;19:3489–3507. doi: 10.3390/molecules19033489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zan L.-F., Qin J.-C., Zhang Y.-M., Yao Y.-H., Bao H.-Y., Li X. Antioxidant hispidin derivatives from medicinal mushroom Inonotus hispidus. Chem. Pharm. Bull. 2011;59:770–772. doi: 10.1248/cpb.59.770. [DOI] [PubMed] [Google Scholar]
- 18.Zhang H., Chen R., Zhang J., Bu Q., Wang W., Liu Y., Li Q., Guo Y., Zhang L., Yang Y. The integration of metabolome and proteome reveals bioactive polyphenols and hispidin in ARTP mutagenized Phellinus baumii. Sci. Rep. 2019;9:16172. doi: 10.1038/s41598-019-52711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonindard C., Bergonzi C., Denier C., Sergheraert C., Klaebe A., Chavant L., Hollande E. Synthetic hispidin, a PKC inhibitor, is more cytotoxic toward cancer cells than normal cells in vitro. Cell Biol. Toxicol. 1997;13:141–153. doi: 10.1023/A:1007321227010. [DOI] [PubMed] [Google Scholar]
- 20.Lee Y.S., Kang Y.-H., Jung J.-Y., Kang I.-J., Han S.-N., Chung J.-S., Shin H.-K., Lim S.S. Inhibitory constituents of aldose reductase in the fruiting body of Phellinus linteus. Biol. Pharm. Bull. 2008;31:765–768. doi: 10.1248/bpb.31.765. [DOI] [PubMed] [Google Scholar]
- 21.Wangun H.V.K., Härtl A., Tam Kiet T., Hertweck C. Inotilone and related phenylpropanoid polyketides from Inonotus sp. and their identification as potent COX and XO inhibitors. Org. Biomol. Chem. 2006;4:2545–2548. doi: 10.1039/B604505G. [DOI] [PubMed] [Google Scholar]
- 22.Ali N.A.A., Awadh Ali N.A., Mothana R.A.A., Lesnau A., Pilgrim H., Lindequist U. Antiviral activity of Inonotus hispidus. Fitoterapia. 2003;74:483–485. doi: 10.1016/s0367-326x(03)00119-9. [DOI] [PubMed] [Google Scholar]
- 23.Park I.-H., Jeon S.-Y., Lee H.-J., Kim S.-I., Song K.-S. A beta-secretase (BACE1) inhibitor hispidin from the mycelial cultures of Phellinus linteus. Planta Med. 2004;70:143–146. doi: 10.1055/s-2004-815491. [DOI] [PubMed] [Google Scholar]
- 24.WHO . Key Facts about Cancer. WHO; Geneva, Switzerland: 2018. [Google Scholar]
- 25.Roy P.S., Saikia B.J. Cancer and cure: A critical analysis. Indian J. Cancer. 2016;53:441–442. doi: 10.4103/0019-509X.200658. [DOI] [PubMed] [Google Scholar]
- 26.Joseph T.P., Chanda W., Padhiar A.A., Batool S., LiQun S., Zhong M., Huang M. A Preclinical Evaluation of the Antitumor Activities of Edible and Medicinal Mushrooms: A Molecular Insight. Integr. Cancer Ther. 2018;17:200–209. doi: 10.1177/1534735417736861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen W., Tan H., Liu Q., Zheng X., Zhang H., Liu Y., Xu L. A Review: The Bioactivities and Pharmacological Applications of Phellinus linteus. Molecules. 2019;24:1888. doi: 10.3390/molecules24101888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim J.-H., Lee Y.-M., Park S.R., Kim D.H., Lim B.O. Anticancer activity of hispidin via reactive oxygen species-mediated apoptosis in colon cancer cells. Anticancer Res. 2014;34:4087–4093. [PubMed] [Google Scholar]
- 29.Nguyen B.C.Q., Taira N., Maruta H., Tawata S. Artepillin C and other herbal PAK1-blockers: Effects on hair cell proliferation and related PAK1-dependent biological function in cell culture. Phytother. Res. 2016;30:120–127. doi: 10.1002/ptr.5510. [DOI] [PubMed] [Google Scholar]
- 30.Lv L.-X., Zhou Z.-X., Zhou Z., Zhang L.-J., Yan R., Zhao Z., Yang L.-Y., Bian X.-Y., Jiang H.-Y., Li Y.-D., et al. Hispidin induces autophagic and necrotic death in SGC-7901 gastric cancer cells through lysosomal membrane permeabilization by inhibiting tubulin polymerization. Oncotarget. 2017;8:26992–27006. doi: 10.18632/oncotarget.15935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chandimali N., Huynh D.O.L., Jin W.Y., Kwon T. Combination Effects of Hispidin and Gemcitabine via Inhibition of Stemness in Pancreatic Cancer Stem Cells. Anticancer Res. 2018;38:3967–3975. doi: 10.21873/anticanres.12683. [DOI] [PubMed] [Google Scholar]
- 32.Isakov N. Protein kinase C (PKC) isoforms in cancer, tumor promotion and tumor suppression. Semin. Cancer Biol. 2018;48:36–52. doi: 10.1016/j.semcancer.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 33.Pellicano F., Copland M., Jorgensen H.G., Mountford J., Leber B., Holyoake T.L. BMS-214662 induces mitochondrial apoptosis in chronic myeloid leukemia (CML) stem/progenitor cells, including CD34 38−cells, through activation of protein kinase Cβ. Blood. 2009;114:4186–4196. doi: 10.1182/blood-2009-05-219550. [DOI] [PubMed] [Google Scholar]
- 34.Grosso S., Volta V., Sala L.A., Vietri M., Marchisio P.C., Ron D., Biffo S. PKCβII modulates translation independently from mTOR and through RACK1. Biochem. J. 2008;415:77–85. doi: 10.1042/BJ20080463. [DOI] [PubMed] [Google Scholar]
- 35.Hernández-Méndez A., Alcántara-Hernández R., Acosta-Cervantes G.C., Martínez-Ortiz J., Avendaño-Vázquez S.E., García-Sáinz J.A. Conventional protein kinase C isoforms mediate phorbol ester-induced lysophosphatidic acid LPA1 receptor phosphorylation. Eur. J. Pharmacol. 2014;723:124–130. doi: 10.1016/j.ejphar.2013.11.043. [DOI] [PubMed] [Google Scholar]
- 36.Piao S., Amaravadi R.K. Targeting the lysosome in cancer. Ann. N. Y. Acad. Sci. 2016;1371:45–54. doi: 10.1111/nyas.12953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tu P.T.B., Chompoo J., Tawata S. Hispidin and related herbal compounds from Alpinia zerumbet inhibit both PAK1-dependent melanogenesis in melanocytes and reactive oxygen species (ROS) production in adipocytes. Drug Discov. Ther. 2015;9:197–204. doi: 10.5582/ddt.2015.01038. [DOI] [PubMed] [Google Scholar]
- 38.Park J.M., Lee J.S., Song J.E., Sim Y.C., Ha S.-J., Hong E.K. Cytoprotective effect of hispidin against palmitate-induced lipotoxicity in C2C12 myotubes. Molecules. 2015;20:5456–5467. doi: 10.3390/molecules20045456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jang J.S., Lee J.S., Lee J.H., Kwon D.S., Lee K.E., Lee S.Y., Hong E.K. Hispidin produced from Phellinus linteus protects pancreatic β-cells from damage by hydrogen peroxide. Arch. Pharm. Res. 2010;33:853–861. doi: 10.1007/s12272-010-0607-5. [DOI] [PubMed] [Google Scholar]
- 40.Song T.-Y., Yang N.-C., Chen C.-L., Thi T.L.V. Protective Effects and Possible Mechanisms of Ergothioneine and Hispidin against Methylglyoxal-Induced Injuries in Rat Pheochromocytoma Cells. Oxid. Med. Cell. Longev. 2017;2017:4824371. doi: 10.1155/2017/4824371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee J.H., Lee J.S., Kim Y.R., Jung W.C., Lee K.E., Lee S.Y., Hong E.K. Hispidin Isolated from Phellinus linteus Protects Against Hydrogen Peroxide–Induced Oxidative Stress in Pancreatic MIN6N β-Cells. J. Med. Food. 2011;14:1431–1438. doi: 10.1089/jmf.2010.1493. [DOI] [PubMed] [Google Scholar]
- 42.Arlt A., Gehrz A., Müerköster S., Vorndamm J., Kruse M.-L., Fölsch U.R., Schäfer H. Role of NF-κB and Akt/PI3K in the resistance of pancreatic carcinoma cell lines against gemcitabine-induced cell death. Oncogene. 2003;22:3243–3251. doi: 10.1038/sj.onc.1206390. [DOI] [PubMed] [Google Scholar]
- 43.Xie C., Liu D., Chen Q., Yang C., Wang B., Wu H. Soluble B7-H3 promotes the invasion and metastasis of pancreatic carcinoma cells through the TLR4/NF-κB pathway. Sci. Rep. 2016;6:27528. doi: 10.1038/srep27528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smolskaite L., Slapšyte G., Mierauskiene J., Dedonyte V., Venskutonis P.R. Antioxidant and Genotoxic Properties of Hispidin Isolated from the Velvet-Top Mushroom, Phaeolus schweinitzii (Agaricomycetes) Int. J. Med. Mushrooms. 2017;19:967–980. doi: 10.1615/IntJMedMushrooms.2017024522. [DOI] [PubMed] [Google Scholar]
- 45.Zhao H., Duan Q., Zhang Z., Li H., Wu H., Shen Q., Wang C., Yin T. Up-regulation of glycolysis promotes the stemness and EMT phenotypes in gemcitabine-resistant pancreatic cancer cells. J. Cell. Mol. Med. 2017;21:2055–2067. doi: 10.1111/jcmm.13126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saklayen M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018;20:12. doi: 10.1007/s11906-018-0812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCracken E., Monaghan M., Sreenivasan S. Pathophysiology of the metabolic syndrome. Clin. Dermatol. 2018;36:14–20. doi: 10.1016/j.clindermatol.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 48.Yazıcı D., Sezer H. Insulin Resistance, Obesity and Lipotoxicity. In: Engin A.B., Engin A., editors. Obesity and Lipotoxicity. Springer International Publishing; Cham, Switzerland: 2017. pp. 277–304. [Google Scholar]
- 49.Wang Y.W., Jones P.J.H. Conjugated linoleic acid and obesity control: Efficacy and mechanisms. Int. J. Obes. Relat. Metab. Disord. 2004;28:941–955. doi: 10.1038/sj.ijo.0802641. [DOI] [PubMed] [Google Scholar]
- 50.Liu T.-T., Liu X.-T., Chen Q.-X., Shi Y. Lipase Inhibitors for Obesity: A Review. Biomed. Pharmacother. 2020;128:110314. doi: 10.1016/j.biopha.2020.110314. [DOI] [PubMed] [Google Scholar]
- 51.Tu P.T.B., Tawata S. Anti-obesity effects of hispidin and Alpinia zerumbet bioactives in 3T3-L1 adipocytes. Molecules. 2014;19:16656–16671. doi: 10.3390/molecules191016656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holm C., Osterlund T., Laurell H., Contreras J.A. Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Annu. Rev. Nutr. 2000;20:365–393. doi: 10.1146/annurev.nutr.20.1.365. [DOI] [PubMed] [Google Scholar]
- 53.De Luca C., Olefsky J.M. Inflammation and insulin resistance. FEBS Lett. 2008;582:97–105. doi: 10.1016/j.febslet.2007.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Björnholm M., Zierath J.R. Insulin signal transduction in human skeletal muscle: Identifying the defects in Type II diabetes. Biochem. Soc. Trans. 2005;33:354–357. doi: 10.1042/BST0330354. [DOI] [PubMed] [Google Scholar]
- 55.Wei Y., Chen K., Whaley-Connell A.T., Stump C.S., Ibdah J.A., Sowers J.R. Skeletal muscle insulin resistance: Role of inflammatory cytokines and reactive oxygen species. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;294:R673–R680. doi: 10.1152/ajpregu.00561.2007. [DOI] [PubMed] [Google Scholar]
- 56.Giardino I., Edelstein D., Brownlee M. BCL-2 expression or antioxidants prevent hyperglycemia-induced formation of intracellular advanced glycation endproducts in bovine endothelial cells. J. Clin. Investig. 1996;97:1422–1428. doi: 10.1172/JCI118563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 58.Stern D., Yan S.D., Yan S.F., Schmidt A.M. Receptor for advanced glycation endproducts: A multiligand receptor magnifying cell stress in diverse pathologic settings. Adv. Drug Deliv. Rev. 2002;54:1615–1625. doi: 10.1016/S0169-409X(02)00160-6. [DOI] [PubMed] [Google Scholar]
- 59.Lee Y.S., Kang Y.-H., Jung J.-Y., Lee S., Ohuchi K., Shin K.H., Kang I.-J., Park J.H.Y., Shin H.-K., Lim S.S. Protein glycation inhibitors from the fruiting body of Phellinus linteus. Biol. Pharm. Bull. 2008;31:1968–1972. doi: 10.1248/bpb.31.1968. [DOI] [PubMed] [Google Scholar]
- 60.Reddy V.P., Prakash Reddy V., Beyaz A. Inhibitors of the Maillard reaction and AGE breakers as therapeutics for multiple diseases. Drug Discov. Today. 2006;11:646–654. doi: 10.1016/j.drudis.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 61.Johnson T.O., Ermolieff J., Jirousek M.R. Protein tyrosine phosphatase 1B inhibitors for diabetes. Nat. Rev. Drug Discov. 2002;1:696–709. doi: 10.1038/nrd895. [DOI] [PubMed] [Google Scholar]
- 62.Liu G., Trevillyan J.M. Protein tyrosine phosphatase 1B as a target for the treatment of impaired glucose tolerance and type II diabetes. Curr. Opin. Investig. Drugs. 2002;3:1608–1616. [PubMed] [Google Scholar]
- 63.Taylor S.D., Hill B. Recent advances in protein tyrosine phosphatase 1B inhibitors. Expert Opin. Investig. Drugs. 2004;13:199–214. doi: 10.1517/13543784.13.3.199. [DOI] [PubMed] [Google Scholar]
- 64.Cho S.-Y., Ahn J.-H., Ha J.-D., Kang S.-K., Baek J.-Y., Han S.-S., Shin E.-Y., Kim S.-S., Kim K.-R., Cheon H.-G., et al. Protein Tyrosine Phosphatase 1B Inhibitors: Heterocyclic Carboxylic Acids. Bull. Korean Chem. Soc. 2003;24:1455–1464. doi: 10.1002/chin.200407105. [DOI] [Google Scholar]
- 65.Lee Y.S., Kang I.-J., Won M.H., Lee J.-Y., Kim J.K., Lim S.S. Inhibition of Protein Tyrosine Phosphatase 1β by Hispidin Derivatives Isolated from the Fruiting Body of Phellinus linteus. Nat. Prod. Commun. 2010;5 doi: 10.1177/1934578X1000501218. [DOI] [PubMed] [Google Scholar]
- 66.Remedi M.S., Emfinger C. Pancreatic β-cell identity in diabetes. Diabetes Obes. Metab. 2016;18:110–116. doi: 10.1111/dom.12727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rolo A.P., Palmeira C.M. Diabetes and mitochondrial function: Role of hyperglycemia and oxidative stress. Toxicol. Appl. Pharmacol. 2006;212:167–178. doi: 10.1016/j.taap.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 68.Incalza M.A., D’Oria R., Natalicchio A., Perrini S., Laviola L., Giorgino F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vasc. Pharmacol. 2018;100:1–19. doi: 10.1016/j.vph.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 69.Takano H., Zou Y., Hasegawa H., Akazawa H., Nagai T., Komuro I. Oxidative stress-induced signal transduction pathways in cardiac myocytes: Involvement of ROS in heart diseases. Antioxid. Redox Signal. 2003;5:789–794. doi: 10.1089/152308603770380098. [DOI] [PubMed] [Google Scholar]
- 70.Endemann D.H., Schiffrin E.L. Endothelial dysfunction. J. Am. Soc. Nephrol. 2004;15:1983–1992. doi: 10.1097/01.ASN.0000132474.50966.DA. [DOI] [PubMed] [Google Scholar]
- 71.Kim D.-E., Kim B., Shin H.-S., Kwon H.J., Park E.-S. The protective effect of hispidin against hydrogen peroxide-induced apoptosis in H9c2 cardiomyoblast cells through Akt/GSK-3β and ERK1/2 signaling pathway. Exp. Cell Res. 2014;327:264–275. doi: 10.1016/j.yexcr.2014.07.037. [DOI] [PubMed] [Google Scholar]
- 72.Amaravadi R., Thompson C.B. The survival kinases Akt and Pim as potential pharmacological targets. J. Clin. Investig. 2005;115:2618–2624. doi: 10.1172/JCI26273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yamaguchi H., Wang H.G. The protein kinase PKB/Akt regulates cell survival and apoptosis by inhibiting Bax conformational change. Oncogene. 2001;20:7779–7786. doi: 10.1038/sj.onc.1204984. [DOI] [PubMed] [Google Scholar]
- 74.Weston C.R., Balmanno K., Chalmers C., Hadfield K., Molton S.A., Ley R., Wagner E.F., Cook S.J. Activation of ERK1/2 by ΔRaf-1: ER* represses Bim expression independently of the JNK or PI3K pathways. Oncogene. 2003;22:1281–1293. doi: 10.1038/sj.onc.1206261. [DOI] [PubMed] [Google Scholar]
- 75.Naruse K., Rask-Madsen C., Takahara N., Ha S.-W., Suzuma K., Way K.J., Jacobs J.R.C., Clermont A.C., Ueki K., Ohshiro Y., et al. Activation of Vascular Protein Kinase C- Inhibits Akt-Dependent Endothelial Nitric Oxide Synthase Function in Obesity-Associated Insulin Resistance. Diabetes. 2006;55:691–698. doi: 10.2337/diabetes.55.03.06.db05-0771. [DOI] [PubMed] [Google Scholar]
- 76.Li D., Liu L., Chen H., Sawamura T., Ranganathan S., Mehta J.L. LOX-1 mediates oxidized low-density lipoprotein-induced expression of matrix metalloproteinases in human coronary artery endothelial cells. Circulation. 2003;107:612–617. doi: 10.1161/01.CIR.0000047276.52039.FB. [DOI] [PubMed] [Google Scholar]
- 77.Li Y.-B., Zhang Q.-H., Chen Z., He Z.-J., Yi G.-H. Oxidized low-density lipoprotein attenuated desmoglein 1 and desmocollin 2 expression via LOX-1/Ca(2+)/PKC-β signal in human umbilical vein endothelial cells. Biochem. Biophys. Res. Commun. 2015;468:380–386. doi: 10.1016/j.bbrc.2015.10.079. [DOI] [PubMed] [Google Scholar]
- 78.Yuan S.Y., Ustinova E.E., Wu M.H., Tinsley J.H., Xu W., Korompai F.L., Taulman A.C. Protein kinase C activation contributes to microvascular barrier dysfunction in the heart at early stages of diabetes. Circ. Res. 2000;87:412–417. doi: 10.1161/01.RES.87.5.412. [DOI] [PubMed] [Google Scholar]
- 79.Curigliano G., Cardinale D., Dent S., Criscitiello C., Aseyev O., Lenihan D., Cipolla C.M. Cardiotoxicity of anticancer treatments: Epidemiology, detection, and management. CA Cancer J. Clin. 2016;66:309–325. doi: 10.3322/caac.21341. [DOI] [PubMed] [Google Scholar]
- 80.Carvalho F.S., Burgeiro A., Garcia R., Moreno A.J., Carvalho R.A., Oliveira P.J. Doxorubicin-Induced Cardiotoxicity: From Bioenergetic Failure and Cell Death to Cardiomyopathy. Med. Res. Rev. 2014;34:106–135. doi: 10.1002/med.21280. [DOI] [PubMed] [Google Scholar]
- 81.Sampaio S.F., Branco A.F., Wojtala A., Vega-Naredo I., Wieckowski M.R., Oliveira P.J. p66Shc signaling is involved in stress responses elicited by anthracycline treatment of rat cardiomyoblasts. Arch. Toxicol. 2016;90:1669–1684. doi: 10.1007/s00204-015-1583-9. [DOI] [PubMed] [Google Scholar]
- 82.Pinton P., Rizzuto R. p66Shc, oxidative stress and aging: Importing a lifespan determinant into mitochondria. Cell Cycle. 2008;7:304–308. doi: 10.4161/cc.7.3.5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Moreira A.C., Branco A.F., Sampaio S.F., Cunha-Oliveira T., Martins T.R., Holy J., Oliveira P.J., Sardão V.A. Mitochondrial apoptosis-inducing factor is involved in doxorubicin-induced toxicity on H9c2 cardiomyoblasts. Biochim. Biophys. Acta. 2014;1842:2468–2478. doi: 10.1016/j.bbadis.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 84.Wojtala A., Karkucinska-Wieckowska A., Sardao V.A., Szczepanowska J., Kowalski P., Pronicki M., Duszynski J., Wieckowski M.R. Modulation of mitochondrial dysfunction-related oxidative stress in fibroblasts of patients with Leigh syndrome by inhibition of prooxidative p66Shc pathway. Mitochondrion. 2017;37:62–79. doi: 10.1016/j.mito.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 85.Dugger B.N., Dickson D.W. Pathology of Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2017;9:a028035. doi: 10.1101/cshperspect.a028035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lin M.T., Beal M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 87.Golpich M., Amini E., Mohamed Z., Azman Ali R., Mohamed Ibrahim N., Ahmadiani A. Mitochondrial Dysfunction and Biogenesis in Neurodegenerative diseases: Pathogenesis and Treatment. CNS Neurosci. Ther. 2017;23:5–22. doi: 10.1111/cns.12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Khandhadia S., Lotery A. Oxidation and age-related macular degeneration: Insights from molecular biology. Expert Rev. Mol. Med. 2010;12:e34. doi: 10.1017/S146239941000164X. [DOI] [PubMed] [Google Scholar]
- 89.Huang S.-Y., Chang S.-F., Chau S.-F., Chiu S.-C. The Protective Effect of Hispidin against Hydrogen Peroxide-Induced Oxidative Stress in ARPE-19 Cells via Nrf2 Signaling Pathway. Biomolecules. 2019;9:380. doi: 10.3390/biom9080380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lebiedzinska M., Karkucinska-Wieckowska A., Giorgi C., Karczmarewicz E., Pronicka E., Pinton P., Duszynski J., Pronicki M., Wieckowski M.R. Oxidative stress-dependent p66Shc phosphorylation in skin fibroblasts of children with mitochondrial disorders. Biochim. Biophys. Acta. 2010;1797:952–960. doi: 10.1016/j.bbabio.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 91.Sherer T.B., Betarbet R., Greenamyre J.T. Pathogenesis of Parkinson’s disease. Curr. Opin. Investig. Drugs. 2001;2:657–662. [PubMed] [Google Scholar]
- 92.Yamamori T., Mizobata A., Saito Y., Urano Y., Inanami O., Irani K., Noguchi N. Phosphorylation of p66shc mediates 6-hydroxydopamine cytotoxicity. Free Radic. Res. 2011;45:342–350. doi: 10.3109/10715762.2010.532496. [DOI] [PubMed] [Google Scholar]
- 93.Citron M. Alzheimer’s disease: Treatments in discovery and development. Nat. Neurosci. 2002;5:1055–1057. doi: 10.1038/nn940. [DOI] [PubMed] [Google Scholar]
- 94.Bennett L., Sheean P., Zabaras D., Head R. Heat-stable components of wood ear mushroom, Auricularia polytricha (higher Basidiomycetes), inhibit in vitro activity of beta secretase (BACE1) Int. J. Med. Mushrooms. 2013;15:233–249. doi: 10.1615/IntJMedMushr.v15.i3.20. [DOI] [PubMed] [Google Scholar]
- 95.Ischiropoulos H., Beckman J.S. Oxidative stress and nitration in neurodegeneration: Cause, effect, or association? J. Clin. Investig. 2003;111:163–169. doi: 10.1172/JCI200317638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Szabó C. Multiple pathways of peroxynitrite cytotoxicity. Toxicol. Lett. 2003;140–141:105–112. doi: 10.1016/S0378-4274(02)00507-6. [DOI] [PubMed] [Google Scholar]
- 97.Chen W., Feng L., Huang Z., Su H. Hispidin produced from Phellinus linteus protects against peroxynitrite-mediated DNA damage and hydroxyl radical generation. Chem. Biol. Interact. 2012;199:137–142. doi: 10.1016/j.cbi.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 98.Shin E.-J., Duong C.X., Nguyen X.-K.T., Li Z., Bing G., Bach J.-H., Park D.H., Nakayama K., Ali S.F., Kanthasamy A.G., et al. Role of oxidative stress in methamphetamine-induced dopaminergic toxicity mediated by protein kinase Cδ. Behav. Brain Res. 2012;232:98–113. doi: 10.1016/j.bbr.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hwang B.S., Lee I.-K., Choi H.J., Yun B.-S. Anti-influenza activities of polyphenols from the medicinal mushroom Phellinus baumii. Bioorg. Med. Chem. Lett. 2015;25:3256–3260. doi: 10.1016/j.bmcl.2015.05.081. [DOI] [PubMed] [Google Scholar]
- 100.Yeom J.-H., Lee I.-K., Ki D.-W., Lee M.-S., Seok S.-J., Yun B.-S. Neuraminidase Inhibitors from the Culture Broth of Phellinus linteus. Mycobiology. 2012;40:142–144. doi: 10.5941/MYCO.2012.40.2.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Serseg T., Benarous K., Yousfi M. Hispidin and Lepidine E: Two Natural Compounds and Folic acid as Potential Inhibitors of 2019-novel coronavirus Main Protease (2019-nCoVMpro), molecular docking and SAR study. Curr. Comput. Aided Drug Des. 2020;16:1–14. doi: 10.2174/1573409916666200422075440. [DOI] [PubMed] [Google Scholar]
- 102.Lehrer S., Rheinstein P.H. Ivermectin Docks to the SARS-CoV-2 Spike Receptor-binding Domain Attached to ACE2. In Vivo. 2020;34:3023–3026. doi: 10.21873/invivo.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Maddu N. Diseases Related to Types of Free Radicals. In: Shalaby E., editor. Antioxidants. IntechOpen; Rijeka, Croatia: 2019. [Google Scholar]
- 104.Kozlowski D., Marsal P., Steel M., Mokrini R., Duroux J.-L., Lazzaroni R., Trouillas P. Theoretical investigation of the formation of a new series of antioxidant depsides from the radiolysis of flavonoid compounds. Radiat. Res. 2007;168:243–252. doi: 10.1667/RR0824.1. [DOI] [PubMed] [Google Scholar]
- 105.Priyadarsini K.I., Indira Priyadarsini K., Maity D.K., Naik G.H., Sudheer Kumar M., Unnikrishnan M.K., Satav J.G., Mohan H. Role of phenolic O-H and methylene hydrogen on the free radical reactions and antioxidant activity of curcumin. Free Radic. Biol. Med. 2003;35:475–484. doi: 10.1016/S0891-5849(03)00325-3. [DOI] [PubMed] [Google Scholar]
- 106.Tamrakar S., Fukami K., Parajuli G.P., Shimizu K. Antiallergic Activity of the Wild Mushrooms of Nepal and the Pure Compound Hispidin. J. Med. Food. 2019;22:225–227. doi: 10.1089/jmf.2018.4267. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available in publicly accessible sources, which are listed in References.