Abstract
Our team previously demonstrated that Ganoderma lucidum spores (GLS) and resistant starch (RS) had hypoglycemic effects separately on type 2 diabetic mellitus (T2DM) rats. This work was to explore the effects of administering encapsulated GLS within RS (referred to as EGLS) in the T2DM rats, which were induced by streptozotocin (STZ). The EGLS was orally administered to rats for 28 days. The parameters of glycometabolism and lipometabolism were evaluated, and fecal microbiota composition was investigated. The results showed that EGLS significantly enhanced glycometabolism and lipometabolism parameters in T2DM rats, which might be associate with the enhancement of the glucose and lipid metabolism, insulin secretion, and glycogen synthesis and reduced lipogenesis. Furthermore, the intervention of EGLS also reduced the Proteobacteria community and improved dysfunctional gut microbiota. This study indicated EGLS may be a potential candidate for dietary intervention to modulate diabetes.
Keywords: Ganoderma lucidum spores, Resistant starch, Encapsulation, Diabetes, Gut microbiota
Introduction
Diabetes mellitus (DM), one of representative chronic metabolic diseases, showed fasting and postprandial hyperglycemia in the body. In accordance with the World Health Organization (2014), disease of diabetes is an ever-increasing global problem with a presence of 9% among adults, and type 2 diabetes mellitus (T2DM) represents 90% of global diabetes. With increasing incidence especially in Asia and Europe, current treatment strategies for T2DM to reduce hyperglycemia and insulin resistance include the use of synthetic based recombinant insulin and pharmacotherapy. However, these medicines may be associated with drug-resistance, hypoglycemia, weight gain dropsy and other side effects. Alternative or synergistic therapies may be beneficial. Traditional Chinese medicines have been widely used for the treatment of diabetes with a long history. Traditional remedies have been assessed in rat models of diabetes remedies, which are relatively cheaper and more effective with almost no side effects compared to synthetic agents (Pan et al., 2013). Such medicinal plants could also serve as a good source for drug design and herbal medicines.
Ganoderma lucidum (GL), commonly known as Lingzhi, was the most studied medicinal mushroom species all over the world. It has been used to cure various kind diseases in China, Korea, Japan and many other countries for many years. Intervention of GL exerted hypoglycemic and hypolipidimic effects in streptozotocin-induced diabetic rats (Bach et al., 2018). Ganoderma lucidum spores (GLS) are the mature germ cells of Ganoderma lucidum and have been associated with the control of blood glucose levels, decrease of plasma glucose concentrations and release of insulin in vivo (Pan et al., 2013). However, therapeutic-benefits mechanisms have not been fully elucidated. Complexing GLS with other bioactive ingredients is a novel approach to enhance the therapeutic benefit through synergistic action. Among them, resistant starch (RS) is a type of cereal fiber fraction. It cannot be digested in the small intestine but can be fermented by microflora in the colon (Jiang et al., 2019). The RS improved blood glucose parameters through direct and indirect mechanisms, such as delayed gastric emptying and improved gut microbiota. The RS seems to be the most beneficial source for maintaining normoglycemia(Marques et al., 2020). We have previously demonstrated that GLS and RS had hypoglycemic effects separately on diabetic rats (Wang et al., 2015). Encapsulation can increase the stability and also modify the release characteristics of essential contents. It was reported that starch based delivery of the bioactive compounds to the gut may prolong their release (Janaswamy, 2014). Thus, in this study, encapsulated GLS was prepared using RS as a coating material and the final product was termed as encapsulated Ganoderma lucidum spores (EGLS). The dietary change and nutritional intervention could modify the human microbiome (Wilson et al., 2020). In the last few decades, there were growing evidences that the gut microbiome played an important role in the development of insulin resistance, T2DM and obesity (Xu et al., 2017). In the upcoming future, one of the emerging strategies for the prevention and control of diabetes may modulate gut microbiota through precise dietary strategies (Conteh and Huang, 2020). To evaluate the efficacy of the combined application of GLS and RS on the regulation of glucose metabolisms and lipids metabolisms and gut microbiota in T2DM rats, we explored the influence of EGLS on blood glucose level, blood lipid compositions, the expression of gene relating with glucose and lipid metabolisms and gut microbiota in STZ induced T2DM rats.
Materials and methods
Materials
STZ was obtained from Sigma-Aldrich (St. Louis, MO, USA). The wall-broken spore powder of GL was obtained from Chongqing Biotechnology Institute, Chongqing, China. RS, from high amylose maize starch (Hi-maizeTM), was provided by the National Starch and Chemical Company, NSW, Australia.
Preparation of RS encapsulated GLS
10% of RS slurry was prepared using Reverses Osmosis (RO)water, followed by thermal treatment in an autoclave at 121 °C for 2 h. After cooling down to 50 °C, GLS powder was slowly added to the slurry with vigorous agitation. The ratio of the two substrates (RS/GLS) used in this study was 3/1 (w/w). After complete dispersion, the mixture was then spray dried with 230 °C for air intake, 180 °C for air output. The final product (ECLS) was collected (Fig. 1) and used in the STZ-induced T2DM rats for intervention.
Fig. 1.
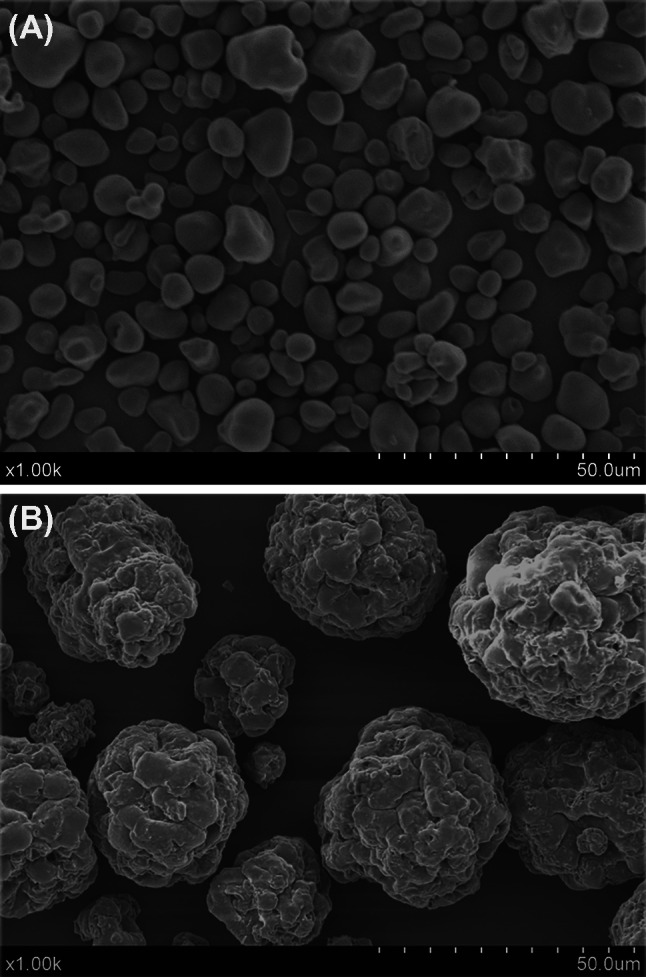
Morphology of EGLS with scanning electron microscope. (A) Before encapsulation; (B) after encapsulation
Animals and experimental design
Twenty-four healthy Sprague–Dawley rats (male, 190 ± 10 g weight) were provided by Chinese Military Medical Science Academy (Beijing, China). Rats were randomly divided into three different groups:NC: normal control, normal rats with basal diet;MC: model control, diabetic rats with basal diet and IG: Intervention group, diabetic rats with an intervention of EGLS (10.5 g/kg bw/day). The basal diet consisted of the AIN-76 diet (Si et al., 2017).
The SD rats were housed in plastic cages (4 rats/cage) in controlled conditions of light (12/12 h light/dark cycle), humidity (55 ± 5%) and temperature (at 25 °C) with free access to drinking water. After fed with the basal diet for one week, the rats were fasted for 12 h. STZ was dissolved in citrate buffer (0.1 mol/L, pH 4.0). The STZ (50 mg/kg b.w) was intravenous injected in rats except for the normal control group. Normal control rats were injected with the same volume of citrate buffer. After the injection, food was not provided to these animals until 2 h later. After 72 h STZ injection, blood was drawn from the rat tail vein, and fasting blood glucose (FBG) was detected with a glucometer (ACCU-CHEK, ROCHE, Basel, Switzerland). The FBG above 16.7 mmol/L in rats was considered as the diagnostic standard of diabetes and these diabetic rats were included in this experiment (Zhou et al., 2015). During the next four weeks, FBG and body mass was measured every week (Zhou et al., 2015). After 28 days, samples of feces, liver tissue and serum were collected and stored at − 80 °C until further analysis. During the experiments, all rats survived. No casual or obvious sign of toxicity was observed (Si et al., 2017).
Biochemical analysis
Before sacrifice, rat blood samples were taken from the femoral artery. High-density lipoprotein-cholesterol (HDL-c) (Beijing North Kangtai clinical Reagent Co., China, F003-2), total cholesterol (TC) (Dong'ou diagnosis Products Co Ltd, F002-1), and triglyceride (TG) (Dong'ou diagnosis Products Co Ltd, Zhejiang, China, F001-1) concentrations were measured according to the instructions of their corresponding kits (Jiancheng Biological Engineering Institute, Nanjing, China), respectively.
Expression analysis of genes involved in glucose and lipid metabolism
After being immediately frozen in liquid nitrogen, rat liver was stored at − 80 °C before RNA extraction. TRIzol (Invitrogen, Carlsbad, CA, USA) was used to extract total RNA from liver tissues, and DNase I (Ambion, Austin, USA) was used to remove the contaminating genomic DNA. Total RNA was transcribed to cDNA using a High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA, USA) (Shang et al., 2017). Real-time quantitative PCR (RT-PCR) reactions were performed using a DyNAmo Flash SYBR Green Kit (Finnzymes, Espoo, Finland) on CFX96 RT-PCR Detection System (Bio-Rad, Rich-mond, CA, USA) (Shang et al., 2017). RT-PCR was performed under the following conditions: at 95 °C for 10 min for initial denaturation, 40 cycles at 95 °C for 30 s, 58 ~ 60 °C for 30 s, 72 °C for 30 s, and 72 °C for 10 min. To normalize target gene expression, the 18S rRNA gene was measured as an internal control. The relative transcript quantity was calculated according to the method of 2-ΔΔCT (Shang et al., 2017). Each reaction was carried out for three replicates. The primer sequences of the genes used for RT-PCR were shown in Table 1.
Table 1.
Primer sequences for gene amplification
| Gene | Primer sequence (5′-3′) | Product size (bp) | Accession No | |
|---|---|---|---|---|
| 18S rRNA |
Forward: Reverse: |
AAACGGCTACCACATCCAAG TTGCCCTCCAATGGATCCT | 159 | NR_046237 |
| ACC |
Forward: Reverse: |
CAACCACTACGGCATGACTCA CGCAGAAGCAGCCCATTACTT |
155 | NM_022193 |
| FAS |
Forward: Reverse: |
TGCTCCCAGCTGCAG GCCCGGTAGCTCTGGGTGTA |
107 | NM_017332 |
| Acox1 |
Forward: Reverse: |
CAAGGAGAGTGCTACGGGTTA TTCAGGTAGCCGTTATCCAT |
137 | NM_017340 |
| SREBP-1 |
Forward: Reverse: |
GCAAGGCCATCGACTACATC TTTCATGCCCTCCATAGACAC |
161 | XM_213329 |
| Insig 1 |
Forward: Reverse: |
TTGTCGGCTTATTGTATCCCT GCACATTATTGGCGAAATCT |
147 | NM_022392 |
| Insig 2 |
Forward: Reverse: |
GGCGGAAGGAGAGACGGAGTC AAGCCAGGAACACGCCAATGA |
128 | NM_178091 |
| Acly |
Forward: Reverse: |
GCAGACCAGAAGGGCGTGAC CACACTGCCTGGGCGATACAG |
135 | NM_016987 |
| Fads1 |
Forward: Reverse: |
GTTTGTGTGGGTGACGCAGAT TTGAAGGCTGACTGGTGAACG |
117 | NM_053445 |
| Gpam |
Forward: Reverse: |
CCT GTG GGC ATC TCG TAT GAT TTC CGC AGC ATT CTG ATA AC |
122 | NM_017274 |
| Dgat1 |
Forward: Reverse: |
CAGATGGGGCTGCTGCTACAT GGCGGCACCACAGATTGACAT |
175 | NM_053437 |
| GS2 |
Forward: Reverse: |
GACACTGAGCAGGGCTTTTCC GAGGAGGGCCTGGGATACTT |
90 | NM_013089 |
| GYG1 |
Forward: Reverse: |
TCGCCAGCCCACAGGTT CACCACTGTCCAAGACATCTACCA |
90 | NM_031043.2 |
| PEPCK |
Forward: Reverse: |
GAAAGTTGAATGTGTGGGTGAT TTCTGGGTTGATGGCCCTTA |
79 | NM_198780 |
| G6PC1 |
Forward: Revers: |
GTATGGATTCCGGTGCTT AATGCCTGACAAGACTCCA |
132 | NM_013098 |
Determination of fecal microbial composition
After twenty-eight days of the feeding, the rat feces were collected within half an hour in fresh sterilized cages and frozen immediately in liquid nitrogen. Feces were stored at − 80 °C until further experiments.
16S rDNA amplicon sequencing of gut microbiota and operational taxonomic unit (OTU) analysis were performed in Shanghai Majorbio Bio-Pharm Technology Co., Ltd. PCR primers flanking the V1-V3 hyper variable region of bacterial 16S rDNA were amplified. The barcoded fusion forward primer was 27F 5′-AGAGTTTGATCCTGGCTCAG-3′ and the reverse primer was 533R 5′-TTACCGCGGCTGCTGGCAC-3′. High quality sequence length was archived and uploaded to QIIME for further study. Sequences sharing > 97% V1–V3 gene sequence identity were defined as an OTU using UCLUST de novo clustering in Quantitative Insights into Microbial Ecology (QIME). Shannon diversity indices and Chao 1 richness, describing the alpha diversity were determined by the OTUs. The Venn diagram showed the similarity and differences among the three groups. Unweighted clustering was performed using principal component analysis (PCA) to show beta diversity. Cluster analysis, venn diagram, heatmap representation and PCA were performed via R Project. Taxonomic classification (genus, family, order, class, and phylum) was performed using Mothur via the silva database.
Statistical analysis
Statistical analysis was performed by using SPSS 11.0 statistical software. One-way analysis of variance (chi-square test) was used for biochemical results. Pooled t-tests were used for bacterial abundance. P < 0.05 was considered significant.
Results and discussion
Effects of EGLS on body mass in diabetic rats
As a useful experimental animal model for studying the hypoglycemic activity, the STZ-induced diabetes in rats is characterized by a severe weight loss and some associated diabetic complications. The initial body mass among the three groups were similar. During four weeks experimental period, the body mass of diabetic rats (MC group) was significantly lower than that of NC group (P < 0.05). Unusual weight loss is the characteristic of diabetes and may be related to the fact that carbohydrates are not used as an energy source(Clark et al., 2007).Although there was no statistically significant difference in the two diabetic groups from groups between MC and IG (P > 0.05), the body mass recovery was most evident for IG group.
Effects of EGLS on blood glucose levels
Hyperglycemia was observed after the injection of STZ. Glucose level was usually above 30 mmol/L within 72 h of STZ injection, which was the symptom of insulin-dependent diabetes. In the following four weeks, the blood glucose level of MC group was consistently higher than that of NC group (P < 0.05) (Table 2). During the four weeks, the blood glucose level of NC and MC rats did not change significantly (P > 0.05). The blood glucose level in the IG-treated animals had decreased (21.9% reduction) comparing with its initial level from the third week, and it also statistically significantly lower than the blood glucose level of MC group (P < 0.05). This was consistent with the findings of our previous work, in which the administration of either RS or GLS contributed to an anti-hyperglycemic effect (REF). Nevertheless, the intervention time needed to be extended for further research (Wang et al., 2015; Zhou et al., 2015).
Table 2.
Effect of EGLS administrations on blood glucose level in diabetic rats
| Group | Blood glucose concentration (mmol/L) | ||||
|---|---|---|---|---|---|
| 72 h | 1 W | 2 W | 3 W | 4 W | |
| NC | 5.9 ± 0.6a | 5.8 ± 0.8a | 5.7 ± 0.3a | 5.8 ± 0.8a | 6.2 ± 0.5a |
| MC | 32.3 ± 1.0b | 30.0 ± 3.3b | 30.1 ± 1.6b | 31.0 ± 3.0b | 32.2 ± 1.7b |
| IG | 31.5 ± 2.2b | 30.1 ± 2.7b | 30.2 ± 4.1b | 27.2 ± 5.5b | 24.6 ± 2.8C |
Results were expressed as means ± SD (n = 8, one-way ANOVA). Different superscript lowercase letters on the table indicate significant difference (P < 0.05)
Effect of EGLS on the expression of glucose metabolism genes
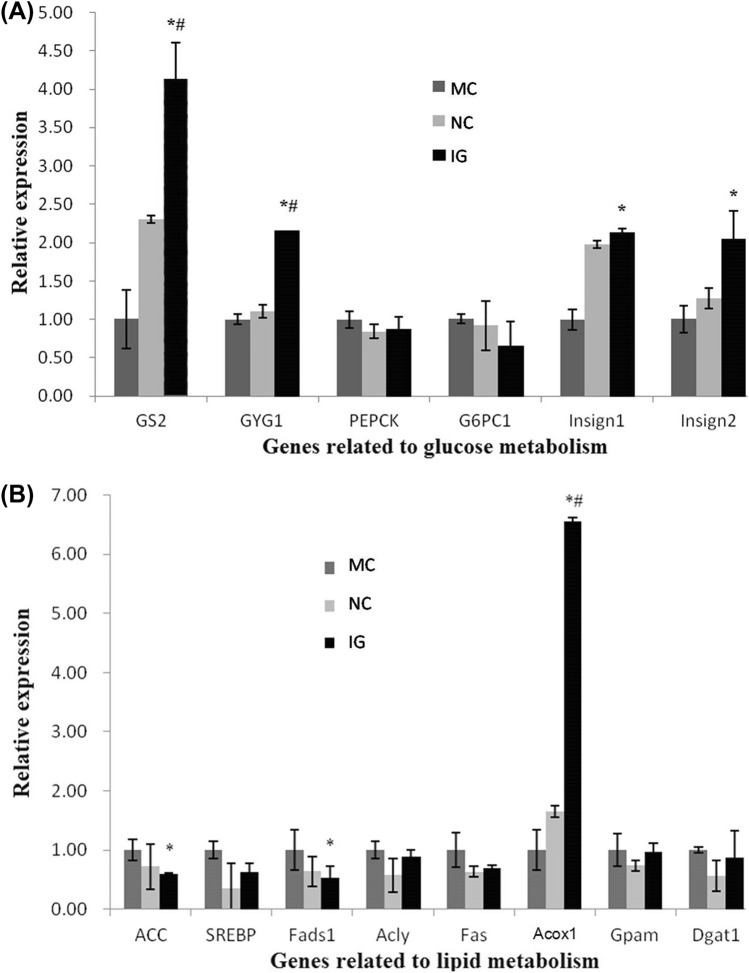
Results presented in Fig. 2A demonstrated the effects of EGLS on the expression of glucose-metabolism-related genes in diabetic rats. Hepatic glycogen synthesis and gluconeogensis are the two main metabolic pathways associated with maintaining FBG levels. The storage of glucose as glycogen is controlled partly by the glycogen synthesis related enzymes-glycogenin 1 (GYG1) and glycogen synthase 2 (GS). In this study, GS expression in the diabetic control rats showed prominently to be reduced comparing with non-diabetic rats, while a slight reduction was also observed in GYG1 expression (Fig. 2A). Glycogenin is a core protein in glycogen particles and GYG1 is involved in the initial steps of glycogen synthesis(Laforêt et al., 2017). Hence, in this T2DM model, the suppressed of glycogen synthesis in the liver may be related with the sequential steps of glycogen synthesis, especially the decreased expression the GS. The decreased activity or expression of GS in diabetes was previously reported (Mukundwa et al., 2015; Oyenihi et al., 2019).This study found that, after intervening with EGLS, the glycogen-synthesis-related genes, such as GS2 and GYG1 mRNA levels, were all significantly up-regulated compared to those of diabetic rats and normal rats (P < 0.05), indicating a progressive stimulation of glycogen synthesis during EGLS administration. In T2DM, the dysregulation of glycogen and glucose metabolism is crucial mechanisms. It was proposed the activation of GS activity was the therapeutic mechanisms of drugs for T2DM (Kaidanovich-Beilin and Eldar-Finkelman, 2006). Our result also suggested that the hypoglycemic activity of EGLS may be related with the inhibition of glycogen synthesis by reducing GS2 and GYG1 expression.
Fig. 2.
Hepatic gene expression profiles for glucose metabolism and lipid metabolism 2A: Hepatic gene expression profiles for glucose metabolism. Hepatic gene expression profiles for glucose metabolism. 2B: Hepatic gene expression profiles for lipid metabolism. Hepatic gene expression profiles for lipid metabolism. Changes in gene expression were determined in triplicate by qPCR. Data are presented as mean ± SD. *P < 0.05 vs. MC, #P < 0.05 vs. NC. NC: normal control; MC: model control; IG: interventional group utilizing EGLS
Isoform 1 of the catalytic subunit of glucose-6-phosphatase (G6PC1) and phosphenolpyruvate carboxykinase (PEPCK) are the two key regulatory enzymes in gluconeogenesis. In this study, G6PC1 mRNA levels were shown to be lower in the IG compared to the MC and NC groups, but there was no significant difference among the three groups. Similarly, previous researches had reported that the hepatic mRNA level of G6PC was also lower in GL or RS treated groups compared with a diabetic MC group (Sun et al., 2018; Xiao et al., 2012). However, no related report regarding the effect of GL and RS was found. G6Pase has catalytic effect on the ultimate reaction in hepatic glucose production, of which the inhibition is expected to reduce hepatic glucose production, irrespective of the contribution of gluconeogenesis or glycogenolysis (Zheng et al., 2012). Consequently, EGLS could also suppress the hepatic glucose output by reducing G6PC expression production in diabetic rats, which may be enhanced by a synergistic effect of RS and GLS. PEPCK catalyzes the rate-controlling step of gluconeogenesis, and is a key enzyme in maintaining blood glucose homeostasis (Hanson and Reshef, 1997). In this study, the PEPCK mRNA level was similar in the MC and NC groups (1.00 ± 0.11 and 0.84 ± 0.09). This result was in contrast to the previous reports that hepatic PEPCK activity of diabetic mice was prominently higher comparing with the normal mice (Gómez-Valadés et al., 2006; Pan et al., 2013). The reason may be related with the difference of animal. The influence of PEPCK to the diabetic rats needs further research. In contrast, PEPCK mRNA level was slightly down-regulated in IG group comparing with the MC group, although the difference was not significant. This result was partly in accordance with other studies that the administration of GLS alone could significantly reduce the expression of PEPCK in diabetic mice (Pan et al., 2013; Xiao et al., 2012).
Insulin-induced gene (Insig) play an important role in glucose homeostasis. In this study, T2DM caused a reduction in Insig1 and Insig2 expression in the diabetic control rats, and the decline of Insig1 was more obvious. The blood glucose level reduction of diabetic rats may be related with the relatively low expression of Insig1 and Insig2, which suggested an inhibition of insulin production in the rats. However, there was no significant difference in comparison to normal rats. After taking the EGLS, the mRNA expressions of insulin induced genes (Insig1 and Insig2) in IG were significantly up-regulated by a twofold increase comparing with the MC group (P < 0.05). Pancreas can produce insulin and promote the glucose uptake and synthesis of liver glycogen and muscle glycogen responding to insulin-stimulation. Some dietary fibers have the hypoglycemic properties, which are mainly induced by improving insulin secretion and protecting the pancreatic islet cells (Kabir et al., 2014). Enhanced activity and secretion of insulin is beneficial to alleviate diabetic symptoms and pancreatic repair (Sun et al., 2018). This study showed that EGLS treatment directly affected glucose influx by significantly increasing the expression levels of Insig1 and Insig2 in T2DM rats. EGLS may interfere in T2DM with insufficient insulin secretion.
In conclusion, the gene expression of GS2, GYG1, G6PC1, PEPCK, Insig1 and Insig2 suggested that the hypoglycemic effects of EGLS may be related with the regulation of these key glucose metabolism enzymes involved in both promoting insulin production (Insig1 and Insig2) and glycogen synthesis (GS2 and GYG1) at the mRNA expression level.
Effects of EGLS on HDL-c, TC and TG concentrations
Following four weeks treatment, the HDL-c, TC and TG concentrations in the blood were detected and results were shown in Table 3. Among all three experimental groups, diabetic rats had the highest value of TC and TG and the lowest HDL-c value (P < 0.05). It is well known that dyslipidemia is associated with uncontrolled diabetes mellitus. The blood levels of TC and TG increased, while the HDL-C levels declined, contributing to secondary complications of diabetes (Elberry et al., 2015). In this research, diabetic rats exhibited a significant elevation of TG and TC and decline of HDL-C. The HDL-c value in the IG increased to 2.79 mmol/L, close to the standard level of 2.99 mmol/L (P > 0.05). Some earlier studies have also reported that RS and GL treatment can significantly increase the levels of HDL-c (Wang et al., 2015; Zheng et al., 2012). Our results further confirmed and the improvements in blood lipid profile could be associated with the reduced blood glucose levels seen in diabetic rats (Teng et al., 2012). The EGLS supplementation resulted in the elevation of HDL-C levels and reduction of TC and TG levels. These effects may be caused by lowering the activity of lipolysis level or cholesterol biosynthesis enzymes (Gong et al., 2009).
Table 3.
Effect of EGLS administrations on blood TC, TG and HDL-c levels in diabetic rats (mmol/L)
| Group | TG | TC | HDL-c |
|---|---|---|---|
| NC | 0.285 ± 0.0a | 2.96 ± 1.2a | 2.99 ± 0.2b |
| MC | 2.915 ± 1.2b | 5.57 ± 1.9a | 1.32 ± 0.2a |
| IG | 0.644 ± 1.7a | 3.96 ± 0.3a | 2.79 ± 0.1b |
Results were expressed as means ± SD (n = 8, one-way ANOVA). Different superscript lowercase letters on the table indicate significant difference (P < 0.05)
TG triglyceride, TC total cholesterol, HDL-C high density lipoprotein cholesterol
Effects of EGLS on expression of lipid metabolism related genes
The effects of EGLS on the gene expression in diabetic rats were investigated (Fig. 2B). Comparing to MC, feeding with EGLS resulted in a decreased expression of lipogenesis associated genes, including acetyl-CoA carboxylse glycogen (ACC), fatty acid syntheses (FAS), ATP citrate lyase (Acly) and fatty acid desaturase (Fads1). In particular, ACC and Fads1 gene expression was significantly decreased (0.59 ± 0.02, 0.53 ± 0.20 fold). We propose that EGLS may efficiently inhibit the expression of FAS and subsequently reduce lipogenesis.
Fatty acid degradation occurs primarily via the β-oxidation cycle in most organisms, and Acox1 is the rate-limiting enzyme of lipid degradation. In this study, the expression level of Acox1 mRNA in EGLS-treated rats was significantly higher than the MC group (6.56 ± 0.06 fold). The up-regulation of Acox1 expression in the liver may suggest that EGLS could reduce TG levels through promoting lipid oxidation via activation of β-oxidation pathway. Up-regulation of Acox1 is associated with increased β-oxidation in diabetes animal models and obesity animal models. Chan et al. (2013) have shown that high fructose fed mice treated with a PPARgamma agonist had reduced insulin resistance and steatosis associated with increased Acox1 (Chan et al., 2013). Up-regulation of Acox1 and increased beta-oxidation were also seen in a protein kinase SKT25 KO mouse with an improvement in diet induced diabetes (Amrutkar et al., 2015).
For triglyceride-synthesis-related genes Gpam and Dgat1, rats in the IG showed decreases in their mRNA levels, although this difference in the expression was not statistically significant. In terms of gene expression related to cholesterol homeostasis, changes in SREBP-1 mRNA levels were minor. However,Insig1 and Insig2 gene expressions improved greatly in EGLS-treated rats comparing with the diabetic model controls, and Insig1 and Insig2 had a great influence on the regulation of intracellular cholesterol and fat metabolism (Goldstein et al., 2006).
In summary, our biochemical and gene expression results suggest that EGLS may exert an anti-hyperlipidemic effect through enhancing lipid oxidation, suppressing triglyceride synthesis and regulating cholesterol homeostasis.
Effects of EGLS on gut microbiota composition
In total, 297,913 high-quality sequences were obtained through MiSeq sequencing analysis (Zhou et al., 2016). 6418 OTUs were identified per sample. All sequences were assigned to 14 different phyla and 142 different genus. The data was available through the NCBI/EBI/DDBJ Short Read Archive (accession number ERA043547; http://www.ebi.ac.uk/ena/data/view/ ERP000842) (Shi et al., 2012).
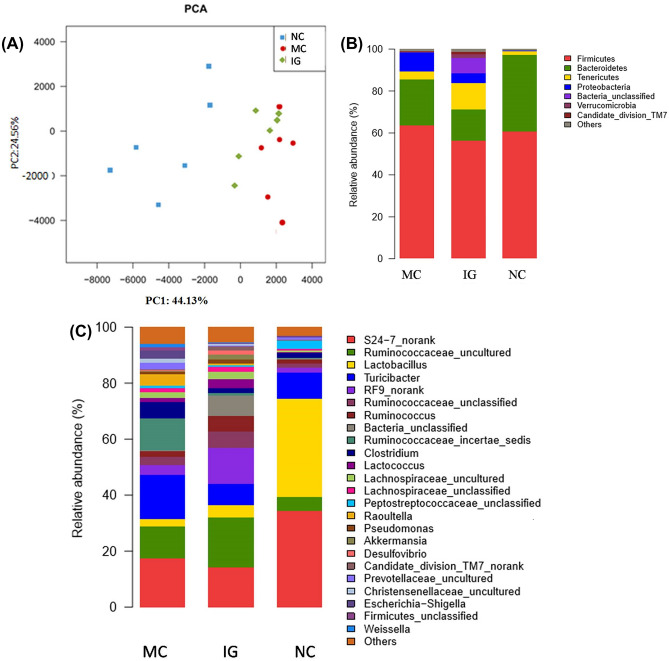
The abundance of intestinal community was measured with the Chao index. The diversity of intestinal community was measured with the Shannon index. PCA showed a visual cluster of intestinal flora composition in different group (Fig. 3A).
Fig. 3.
Compositions analysis of the mocrobial communities. 3A: Principal component analysis (PCA) of samples based on the compositions of the microbial communities. 3B: Relative abundance of sequences belonging to different bacterial phyla. 3C: Relative abundance of sequences belonging to different bacterial genus. NC: normal control; MC: model control; IG: interventional group utilizing EGLS
The PCA analysis of gut community indicated that there was an obvious difference between normal rats and diabetic rats. Detailed analysis showed that both community abundance and diversity of diabetic model control group were the highest, which was in accordance with the previous works. The quantity, composition and diversity of the intestinal flora changed significantly in diabetic rats (Larsen et al., 2010; Wirth et al., 2014). Following the administrations of EGLS, both community abundance and diversity decreased comparing with model control group, indicating that the EGLS intervention can effectively perturb the microflora diversity and abundance in diabetic rats, and this might contribute to an improvement of intestinal microflora.
Firmicutes and Bacteroidetes were dominant bacterial phyla in the three groups (Zhou et al., 2016). The abundance of Proteobacteria (the most identified as pathogenic bacteria) in diabetic rats was the highest (Fig. 3B) (Larsen et al., 2010; Wirth et al., 2014). The increased levels of Proteobacteria may suggest an inflammatory reaction in the gut of MC group,which may be involved in the onset of T2DM. The enrichment of Proteobacteria in the gut might be applied to predict T2DM (Karlsson et al., 2013). However, the ratio of Proteobacteria in EGLS treated rats was significantly reduced when compared with MC group (4.78% vs. 9.03%). Both RS and GL have shown previously to promote anti-inflammatory activity and the inflammation in the host can disrupt the intestinal microflore(Claudia et al., 2007; Haenen et al., 2013; Yan et al., 2014).It could be postulated that the reduction in blood glucose and improvements in cholesterol metabolism in the EGLS treated diabetic rats may be related with changes in faecal microbial community which promoted anti-inflammatory response.
The abundance of Lactobacillus was predominant in the normal rats (35.01%) at the genus level, and the data showed a sharp drop to 2.69% in MC group, which was also lower in the IG group (4.49%) (Fig. 3C). Lactobacillus is the most extensively studied probiotic strain, and has been shown to exert an anti-hyperglycemic effect in several rodent models (Park et al., 2015; Zhou et al., 2016). Park et al. (2015) also systematically demonstrated beneficial effects of Lactobacillus in the treatment of diabetes. The intervention of EGLS may increase the proportion of Lactobacillus, which may then contribute to an anti-hyperglycemic effect. The enrichment of Lactobacillus may be due to the GL component feeding (Xu et al., 2017).
Conclusion
This study suggests that the administration of EGLS may exert a synergistic influence on modulating hypoglycemic and hypolipidemic activities in the liver of diabetic rats by promoting gene expression which is associated with of glycogen synthesis (GS2 and GYG1), glucose homeostasis and cholesterol homeostasis (Insig1, Insig2), lipid oxidation (Acox1), and lipogenesis suppression (ACC, Fads1). The gut microbiota of diabetic rats was significantly different from that of normal rats. The gut micorobiota in T2DM rats contributed to inflammation. EGLS treatment had a positive effect on the inflammation by reducing the abundance of Proteobacteria. The synergism of the RS and GLS could exert a positive effect on human metabolism. Thus, EGLS is a potential candidate for the prevention and therapy of T2DM.
Acknowledgements
This work was financially supported by Tianjin One Belt and One Road Technological Innovation Project 583(18PTZWHZ00080), the Foundation (No. xnc201605) of Tianjin University of Science and Technology, Institute for New Rural Development, P. R. China. The contents are the authors' sole responsibility.
Declarations
Conflict of interest
All authors declare no conflict of interest.
Ethics statement
All national guidelines of laboratory animals were followed. The animals were approved by the Animal Care and Use Committee.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yumei Jiang, Email: jiangyumei@tust.edu.cn.
Na Zhang, Email: 1756671786@qq.com.
Yawen Zhou, Email: 2018208017@njau.edu.cn.
Zhongkai Zhou, Email: zkzhou@tust.edu.cn.
Yu Bai, Email: baiyu95014@126.com.
Padraig Strappe, Email: p.strappe@cqu.edu.au.
Chris Blanchard, Email: cblanchard@csu.edu.au.
References
- Amrutkar M, Cansby E, Chursa U, Nuñez-Durán E, Chanclón B, Ståhlman M, Fridén V, Mannerås-Holm L, Wickman A, Smith U. Genetic disruption of protein kinase STK25 ameliorates metabolic defects in a diet-induced type 2 diabetes model. Diabetes. 2015;64:2791–2804. doi: 10.2337/db15-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach EM, Martins, Cristina AM, Nascimento, Paloma AM, Wadt, Yamashita NS. Hypoglicemic and Hypolipedimic Effects of Ganoderma lucidum in Streptozotocin-Induced Diabetic Rats. Medicines (2018) [DOI] [PMC free article] [PubMed]
- Chan S, Sun R, Zeng X, Choong Z, Wang H, Watt M, Ye J. Activation of PPARa ameliorates hepatic insulin resistance and steatosis in high fructose-fed mice despite increased ER stress. Diabetes. 2013;62:1–11. doi: 10.2337/db12-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark NG, Fox KM, Grandy S. Symptoms of diabetes and their association with the risk and presence of diabetes: findings from the Study to Help Improve Early evaluation and management of risk factors Leading to Diabetes (SHIELD) Diabetes Care. 2007;30:2868–2873. doi: 10.2337/dc07-0816. [DOI] [PubMed] [Google Scholar]
- Claudia, Lupp, and, Marilyn, L., Robertson, and, Mark, E., Wickham. Host-Mediated Inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host and Microbe (2007) [DOI] [PubMed]
- Conteh A, Huang R. Targeting the gut microbiota by Asian and Western dietary constituents: a new avenue for diabetes. Toxicology Research. 2020;9:569–577. doi: 10.1093/toxres/tfaa065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elberry AA, Harraz FM, Ghareib SA, Gabr SA, Nagy AA, Abdelsattar E. Methanolic extract of Marrubium vulgare ameliorates hyperglycemia and dyslipidemia in streptozotocin-induced diabetic rats. International Journal of Diabetes Mellitus. 2015;3:37–44. doi: 10.1016/j.ijdm.2011.01.004. [DOI] [Google Scholar]
- Goldstein JL, DeBose-Boyd RA, Brown MS. Protein sensors for membrane sterols. Cell. 2006;124:35–46. doi: 10.1016/j.cell.2005.12.022. [DOI] [PubMed] [Google Scholar]
- Gómez-Valadés AG, Vidal-Alabró A, Molas M, Boada J, Bermúdez J, Bartrons R, Perales JC. Overcoming Diabetes-Induced Hyperglycemia through Inhibition of Hepatic Phosphoenolpyruvate Carboxykinase (GTP) with RNAi. Molecular Therapy. 2006;13:401–410. doi: 10.1016/j.ymthe.2005.08.026. [DOI] [PubMed] [Google Scholar]
- Gong F, Li F, Zhang L, Li J, Zhang Z, Wang G. Hypoglycemic effects of crude polysaccharide from purslane. International Journal of Molecular Sciences. 2009;10:880–888. doi: 10.3390/ijms10030880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenen D, Zhang J, Souza dS, C., Bosch G, Van dM, I. M., Van Arkel J, van den Borne JJGC, Perez Gutierrez O, Smidt H, Kemp B. A Diet High in Resistant Starch Modulates Microbiota Composition, SCFA Concentrations, and Gene Expression in Pig Intestine. Journal of Nutrition 143: 274-283 (2013) [DOI] [PubMed]
- Hanson RW, Reshef L. Regulation of phosphoenolpyruvate carboxykinase (GTP) gene expression. Annual Review of Biochemistry. 1997;66:581–611. doi: 10.1146/annurev.biochem.66.1.581. [DOI] [PubMed] [Google Scholar]
- Janaswamy S. Encapsulation altered starch digestion: Toward developing starch-based delivery systems. Carbohydrate Polymers. 2014;101:600–605. doi: 10.1016/j.carbpol.2013.09.094. [DOI] [PubMed] [Google Scholar]
- Jiang F, Du C, Jiang W, Wang L, Du SK. The preparation, formation, fermentability, and applications of resistant starch. International Journal of Biological Macromolecules 150 (2019) [DOI] [PubMed]
- Kabir AU, Samad MB, D’Costa NM, Akhter F, Ahmed A, Hannan JMA. Anti-hyperglycemic activity of Centella asiatica is partly mediated by carbohydrase inhibition and glucose-fiber binding. BMC Complementary and Alternative Medicine. 2014;14:31. doi: 10.1186/1472-6882-14-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaidanovich-Beilin O, Eldar-Finkelman H. Long-term treatment with novel glycogen synthase kinase-3 inhibitor improves glucose homeostasis in ob/ob mice: molecular characterization in liver and muscle. Journal of Pharmacology and Experimental Therapeutics. 2006;316:17–24. doi: 10.1124/jpet.105.090266. [DOI] [PubMed] [Google Scholar]
- Karlsson F, Tremaroli V, Nielsen J, Baeckhed F. Assessing the human gut microbiota in metabolic diseases. Diabetes. 2013;62:3341–3349. doi: 10.2337/db13-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laforêt P, Malfatti E, Vissing J. Update on new muscle glycogenosis. Current opinion in neurology. 2017;30:449–456. doi: 10.1097/WCO.0000000000000484. [DOI] [PubMed] [Google Scholar]
- Larsen N, Vogensen FK, Den Berg FV, Nielsen DS, Andreasen AS, Pedersen BK, Alsoud WA, Sorensen SJ, Hansen LH, Jakobsen M. Gut Microbiota in Human Adults with Type 2 Diabetes Differs from Non-Diabetic Adults. PLOS ONE 5 (2010) [DOI] [PMC free article] [PubMed]
- Marques AM, Linhares BS, Novaes RD, Freitas MB, Gonalves RV. Effects of the amount and type of carbohydrates used in type 2 diabetes diets in animal models: a systematic review. PLoS ONE. 2020;15:e0233364. doi: 10.1371/journal.pone.0233364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukundwa A, Mukaratirwa S, Masola B. Effects of oleanolic acid on the insulin signaling pathway in skeletal muscle of streptozotocin-induced diabetic male Sprague-Dawley rats. Journal of Diabetes (2015) [DOI] [PubMed]
- Oyenihi AB, Langa SOP, Mukaratirwa S, Masola B. Effects of Centella asiatica on skeletal muscle structure and key enzymes of glucose and glycogen metabolism in type 2 diabetic rats. Biomedicine and Pharmacotherapy. 2019;112:108715. doi: 10.1016/j.biopha.2019.108715. [DOI] [PubMed] [Google Scholar]
- Pan D, Zhang D, Wu J, Chen C, Xu Z, Yang H, Zhou P. Antidiabetic, antihyperlipidemic and antioxidant activities of a novel proteoglycan from Ganoderma lucidum fruiting bodies on db/db mice and the possible mechanism. PloS ONE. 2013;8:e68332. doi: 10.1371/journal.pone.0068332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K-Y, Kim B, Hyun C-K. Lactobacillus rhamnosus GG improves glucose tolerance through alleviating ER stress and suppressing macrophage activation in db/db mice. Journal of Clinical Biochemistry and Nutrition. 2015;56:240–246. doi: 10.3164/jcbn.14-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang W, Si X, Zhou Z, Wang J, Strappe P, Blanchard C. Studies on the unique properties of resistant starch and chito-oligosaccharide complexes for reducing high-fat diet-induced obesity and dyslipidemia in rats. Journal of Functional Foods. 2017;38:20–27. doi: 10.1016/j.jff.2017.08.032. [DOI] [Google Scholar]
- Shi Z, Wang L, Zhang H. Low Diversity Bacterial Community and the Trapping Activity of Metabolites from Cultivable Bacteria Species in the Female Reproductive System of the Oriental Fruit Fly, Bactrocera dorsalis Hendel (Diptera: Tephritidae) International Journal of Molecular Sciences. 2012;13:6266–6278. doi: 10.3390/ijms13056266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si X, Zhou Z, Strappe P, Blanchard C. A comparison of RS4-type resistant starch to RS2-type resistant starch in suppressing oxidative stress in high-fat-diet-induced obese rats. Food and Function. 2017;8:232–240. doi: 10.1039/C6FO01225F. [DOI] [PubMed] [Google Scholar]
- Sun H, Ma X, Zhang S, Zhao D, Liu X. Resistant starch produces antidiabetic effects by enhancing glucose metabolism and ameliorating pancreatic dysfunction in type 2 diabetic rats. International Journal of Biological Macromolecules. 2018;110:276–284. doi: 10.1016/j.ijbiomac.2017.11.162. [DOI] [PubMed] [Google Scholar]
- Teng BS, Wang CD, Zhang D, Wu JS, Pan D, Pan LF, Yang HJ, Zhou P. Hypoglycemic effect and mechanism of a proteoglycan from Ganoderma lucidum on streptozotocin-induced type 2 diabetic rats. European Review for Medical and Pharmacological Sciences. 2012;16:166. [PubMed] [Google Scholar]
- Wang F, Zhou Z, Ren X, Wang Y, Yang R, Luo J, Strappe P. Effect of Ganoderma lucidum spores intervention on glucose and lipid metabolism gene expression profiles in type 2 diabetic rats. Lipids in Health and Disease. 2015;14:49. doi: 10.1186/s12944-015-0045-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AS, Koller KR, Ramaboli MC, Nesengani LT, O'Keefe SJD. Diet and the human gut microbiome: an international review. Digestive Diseases and Sciences 65 (2020) [DOI] [PMC free article] [PubMed]
- Wirth R, Bódi N, Maróti G, Bagyánszki M, Talapka P, Fekete É, Bagi Z, Kovács KL. Regionally distinct alterations in the composition of the gut microbiota in rats with streptozotocin-induced diabetes. Plos One 9 (2014) [DOI] [PMC free article] [PubMed]
- Xiao C, Wu Q-P, Cai W, Tan J-B, Yang X-B, Zhang J-M. Hypoglycemic effects of Ganoderma lucidum polysaccharides in type 2 diabetic mice. Archives of Pharmacal Research. 2012;35:1793–1801. doi: 10.1007/s12272-012-1012-z. [DOI] [PubMed] [Google Scholar]
- Xu S, Dou Y, Ye B, Wu Q, Wang Y, Hu M, Ma F, Rong X, Guo J. Ganoderma lucidum polysaccharides improve insulin sensitivity by regulating inflammatory cytokines and gut microbiota composition in mice. Journal of Functional Foods. 2017;38:545–552. doi: 10.1016/j.jff.2017.09.032. [DOI] [Google Scholar]
- Yan G, Shuiling Z, Zhenming L, Hongyu X, Jin-Song S. Anti-inflammatory activity of mycelial extracts from medicinal mushrooms. International Journal of Medicinal Mushrooms (2014) [DOI] [PubMed]
- Zheng J, Yang B, Yu Y, Chen Q, Huang T, Li D. Ganoderma lucidum polysaccharides exert anti-hyperglycemic effect on streptozotocin-induced diabetic rats through affecting β-cells. Combinatorial Chemistry and High Throughput Screening. 2012;15:542–550. doi: 10.2174/138620712801619168. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Wang F, Ren X, Wang Y, Blanchard C. Resistant starch manipulated hyperglycemia/hyperlipidemia and related genes expression in diabetic rats. International Journal of Biological Macromolecules. 2015;75:316–321. doi: 10.1016/j.ijbiomac.2015.01.052. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Ren X, Jiang Y, Zhang Q, Zhang M, Strappe P, Blanchard C. Responses of fecal bacterial communities to resistant starch intervention in diabetic rats. Starch - Stärke. 2016;68:1008–1015. doi: 10.1002/star.201500139. [DOI] [Google Scholar]