Abstract
Thousands of observational studies have linked vitamin D deficiency with numerous diseases, but randomised controlled trials (RCTs) often fail to show benefit of supplementation. Population characteristics and trial design have long been suspected to undermine power but were not systematically investigated. We propose a flexible generative model to characterise benefit of vitamin D supplementation at the individual level, and use this to quantify power in RCTs. The model can account for seasonality and population heterogeneity. In a simulated 1-year trial with 1000 participants per arm and assuming a 25-hydroxyvitamin D (25OHD) increase of 20 nmol/L due to the intervention, with baseline 25OHD in the population of 15, 35, 50, 60 and 75 nmol/L, the power to detect intervention effect was 77%, 99%, 95%, 68% and 19%, respectively. The number of participants required per arm to achieve 80% power according to baseline 25OHD of 15–60 nmol/L was 1200, 400, 600 and 1400, respectively. As expected, larger increases in 25OHD due to supplementation improved power in certain scenarios. For a population baseline of 50 nmol/L, with 1500 participants in each arm, there was 100% power to detect a 20 nmol/L 25OHD increase while it was 76% for a 10 nmol/L increase. Population characteristics and trial design, including temporal considerations, have a dramatic impact on power and required sample size in vitamin D RCTs.
Subject terms: Biomarkers, Diseases, Endocrinology, Medical research, Risk factors
Introduction
A large number of observational studies have linked vitamin D deficiency with cancer, cognition, cardio-vascular, metabolic, autoimmune, infectious diseases, mortality, and many other illnesses1; most recently vitamin D has been implicated in COVID-19 infection and severity2,3. Vitamin D deficiency is very common world-wide: it is estimated that over 1 billion people are vitamin D deficient4. If disease associations are real, tackling deficiency could have an enormous impact on public health globally. Therefore it is not surprising that there has been a considerable interest in vitamin D in the last two decades. However, randomised controlled trials (RCTs) often fail to show benefit of vitamin D supplementation.
Vitamin D status is assessed by measuring 25-hydroxyvitamin D (25OHD) concentration in the circulation: levels below 25 nmol/L are generally considered to indicate “deficiency”, levels between 25 and 50 nmol/L “inadequacy”, and above 50 nmol/L “sufficiency”5. However, these cut-offs are still under debate and some advocate much higher levels for optimal health6. Interestingly, there might not be a single definition of vitamin D deficiency. To prevent rickets, levels of 50 nmol/L are “sufficient”7, but much higher concentrations may be required for other conditions. For example, levels of 100–150 nmol/L may be needed for prevention of cancer8 or multiple sclerosis9.
It is well-documented, and unsurprising, that the benefit of vitamin D supplementation is greatest in deficient individuals. In the context of RCTs where no benefit was found, common concerns invariably relate to the study population being vitamin D sufficient at baseline (limiting the extent of benefit supplementation could achieve), or treatment being too short or dose too low to have a meaningful impact on vitamin D status.
In addition to this, we note that an individual’s vitamin D status is determined primarily by exposure to ultraviolet B (UVB) radiation exposure in their environment10,11, and hence follows a strong seasonal pattern12. Given that an individual will benefit most from supplementation when they are deficient, any potential usefulness of vitamin D in prevention will also be affected by this seasonality: while supplementation may contribute the majority of vitamin D in winter, the same treatment may be dwarfed by the abundance of skin-synthesised vitamin D in the summer, as it has been reported that 30 min of whole skin surface exposure to summer sunshine is equivalent to 10,000–20,000 IU, even in Norway13. The impact of season on vitamin D status14,15 and association with disease traits16–18 is well established and lifestyle factors may strongly affect this19 or even reverse it20. Thus, the supplementation dose, duration and time of year in which a trial is carried out, as well as individual baseline status and other factors, may impact the comparisons of supplemented and placebo arms in trials21.
When planning a trial, one hopes at the very least that the assumptions made for the power calculation will roughly approximate the conditions expected in the wild, so that conclusions from analysed trial data can be made with a high degree of confidence. With regard to vitamin D, our claim is that obtaining the power for comparisons of supplementation schemes is non-trivial, and hence traditional RCT planning protocols cannot represent the specific nature of vitamin D trials. As is widely applied in biomedical research, power calculations are based on detection of an expected difference between groups (e.g. means) and variation in outcomes. Elicitation of this variation in a vitamin D context is challenging because variation within a subject (e.g. across seasons) is compounded with variation between subjects (e.g. across trial arms), the impact of which has also been observed in vitamin D RCTs11. We advocate exploring variation in a different way. Frameworks such as the DELTA guidance on choosing the target difference for RCTs22 highlight the importance of appropriate elicitation of effect sizes for sample size and power calculations. We demonstrate through a model characterisation of vitamin D supplementation, that effect sizes are not easily articulated in the context of vitamin D trials. The simulation tools our proposal offers, allow a gateway to reclaiming prior knowledge and intuition for designing complex trials where there is a strong temporal within and between subject variability. Obtaining power through simulation is a common approach for complex study designs, see for example23,24. This is critical, as design decisions can have detrimental impact on the reliability of RCT conclusions in the context of vitamin D25. Given the widespread interest in vitamin D, a bespoke flexible planning tool is timely, both for ensuring adequate sample size in future trials, and better informed interpretation of reported findings. This is particularly relevant for trials that found no benefit, as the likelihood of a false negative results can be interrogated.
In this paper, we develop a simulation-based approach for sample size and power calculation in RCTs of vitamin D supplementation, and we show the impact of population characteristics and trial design on the power. By simulating individual vitamin D status trajectories, dominant sources of variability and heterogeneity in status (and consequently potential benefit of supplementation) can be accounted for. Each component of the simulation model can be parameterised to harness any available domain expertise (e.g. cancer or bone researchers may define “optimal” vitamin D status differently), specific characteristics of the population (e.g. baseline level), and trial-related factors (e.g. duration or the increase in 25OHD in treatment group). The tools discussed in this paper are easily used through the R package SimVitD26 available on the Comprehensive R Archive Network (CRAN).
For the sake of exposition, the paper considers a respiratory infection. Simulation of exposures at an individual level are used to approximate the potential effect of supplementation in protecting against these, accounting for natural cyclic patterns in status. The power is approximated via simulation and this can be used to determine the required sample size to obtain a given power. The text focuses on vitamin D, however the methods presented have the potential to be quite flexible and could be extended to other nutrient studies where the nutrient level varies according to some pattern e.g. vitamin shots to supplement vegetarian diets, or in prevention of thromboembolic or bleeding events in patients who are on vitamin K antagonists and other.
Methods
Modelling individual vitamin D status trajectories
As most of an individual’s vitamin D is derived from synthesis in skin following UVB exposure10,11, vitamin D status will naturally vary throughout the year. 25OHD concentration (marker of vitamin D status) tends to peak late in the summer, following the period with the strongest UVB radiation; the status trough will follow the period of lowest exposure27,28. It is useful to note that the peak and trough in an individual’s status will depend on geolocation; there will be variability within, say, the northern hemisphere28. This paper works on the assumption of a northern hemisphere seasonal schedule with summer months being June to August. Cyclic status profiles follow the assumed yearly periodic curve with a trough in February or March and peak in August or September27,28. The peak 25OHD occurs with 1–2 month time lag following the peak UVB radiation (here we assume 2 months); this reflects the period of pronounced vitamin D accumulation arising from abundant production of the nutrient in the skin.
Consider a group of trial participants, indexed by i. A phase shifted cosine curve with a lower threshold is used to model a participant’s vitamin D status12 derived from non-supplement sources (UVB) over time,
| 1 |
Here, is a mean level that could be described by participant specific characteristics i.e. with a vector of covariates. Models of vitamin D involving parameterised cosine functions have been used previously to describe seasonal variation in 25OHD12, and this would appear a natural choice to characterise these seasonal patterns. UVB is by far the most dominant source of vitamin D, as food sources are largely scarce29. The lower threshold of 10 nmol/L of circulating 25OHD is a detectability threshold. In (1) t is time measured in years i.e. the interval [0, 1] corresponds to 1 year. The parameter gives a perturbation of the mean around similar to a random effect, and controls the change in status between periods with and without significant UVB exposure. The phase adjustment accounts for a lag effect from UVB exposure to expressed circulating vitamin D level. This can be used to make adjustments for geolocation effects e.g. northern/southern hemisphere. Here, is assumed, meaning that time is counted from the beginning of March , and at that point 25OHD is lowest.
Variation in individual 25OHD concentration is accounted for by generating the amplitude and a height perturbation in (1) via
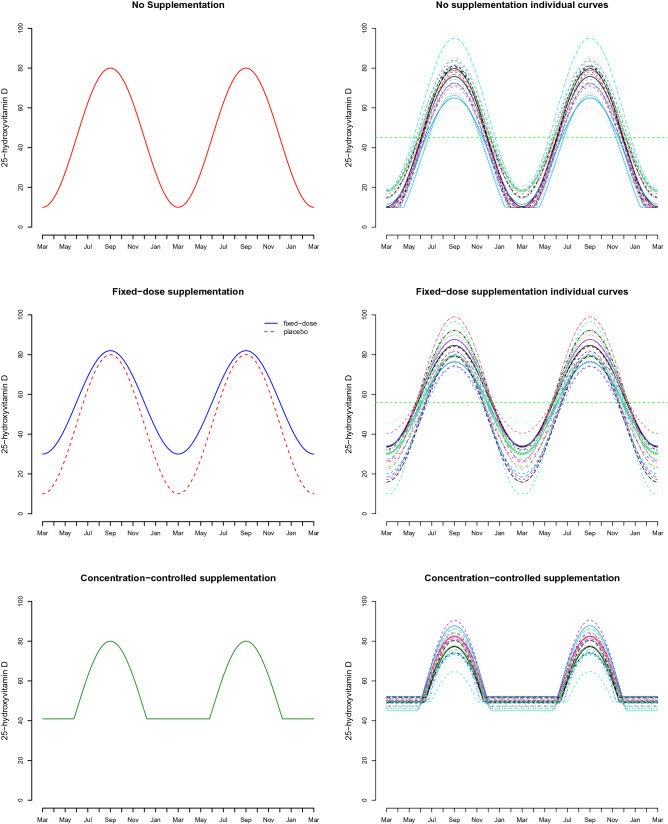
independently drawn for each individual. The shape and rate parameters are chosen to have a specified expected value and standard deviation . The height perturbation standard deviation will also be specified. These specifications should be made to sufficiently represent typical population variation in trial participants. The top panel of Fig. 1 shows what may be representative of a target cohort.
Figure 1.
25-hydroxyvitamin D (25OHD) status profiles for placebo, fixed-dose supplementation and concentration-controlled intervention schemes. The middle panel on the left compares the fixed-dose scheme with more uptake during deficient periods. Horizontal green dashed lines in the top two panels indicate effective overall mean levels of 25OHD assumed in the model.
Intervention: randomised controlled trials (RCT) and randomised concentration-controlled trials (RCCT)
A number of possible approaches may be under consideration when planning a prospective trial. The curve in (1) corresponds to no supplementation and is referred to as placebo in what follows. Two potential supplementation schemes are considered in this paper and determined by the nature of the trial. The first of these, an RCT, would be the more common approach. The second, RCCT, gives an example of frequent 25OHD measurement during the trial and a responsive dosing scheme design that one might employ had they the necessary resources.
Fixed-dose scheme
A fixed-dose scheme corresponds to an individual taking a daily supplement of a fixed amount, and this is the prevailing approach in RCTs. Supplementation might provide more of a boost when vitamin D levels are low. To allow for this, the no supplement curve is modified by adding a flexible function
where
Here represents an individual’s overall derived benefit from that dosage, accounting for variation in overall assimilation. The parameter gives the proportion of the fixed-dose which is always utilised. As supplementation may have more impact in periods of deficiency, we allow the uptake from the remaining proportion of the fixed dose to vary according to a complementary cosine function. Individual values are sampled from a beta distribution with expected value and standard deviation . The value of may also be simulated at an individual level using a truncated distribution capped at the administered dose level. A distribution for this purpose is given in the supplementary material (S.2).
Concentration-controlled scheme
A concentration-controlled scheme allows an individual to be monitored regularly and their status kept above a 25OHD threshold , for example in a randomised concentration-controlled trial (RCCT) design30. That is
A comparison of the placebo, fixed-dose supplementation and concentration-controlled schemes can be made from Fig. 1. The individual target level is simulated via with determined to give a specified expectation and standard deviation .
A model for benefit of vitamin D supplementation
Individuals’ exposures (e.g. to infection, or other risk factors) are assumed to arise independently from their vitamin D status profiles. Benefit of supplementation is assumed here to correspond to heightened immune defences, giving protection against illness. The proposed generative model for supplementation benefit is:
simulation of an individual’s vitamin D status profile (introduced in “Modelling individual vitamin D status trajectories”)
simulation of times of exposure (to infection in this example)
get probability of developing illness conditional on vitamin D status at exposure
simulation of occurrence of event (illness) using the result of step 3.
Exposures to infection
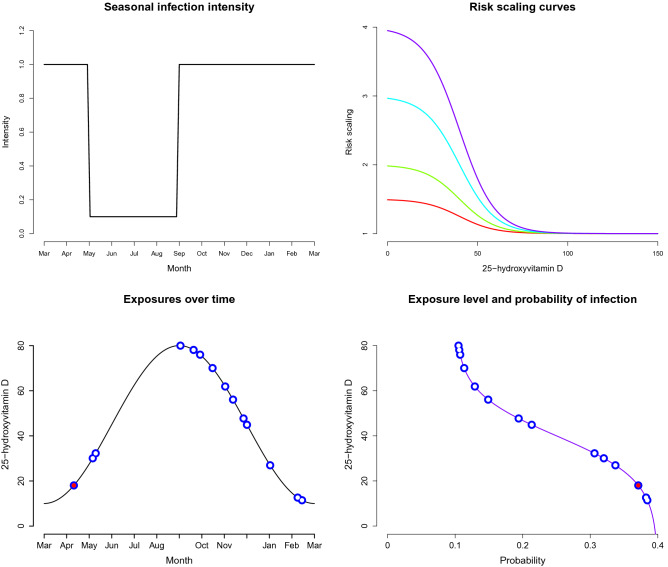
Exposure to infection is taken as the lead example in this paper (one could, for example, be examining protection against allergic reaction following exposure to allergens, asthma attack, relapse of autoimmune disease). An individual’s exposures over the period of a prospective trial are simulated from a Poisson process. In the case of seasonally concentrated infections (e.g. flu, see Fig. 2), a non-homogeneous Poisson process (NHPP) with rate function is used31. Simulations from an NHPP can be carried out conveniently in R using the poisson32 package. The function can represent different kinds of exposure (for example, respiratory infections are common in winter; pollen allergies in late spring and summer). It can also, of course, represent a constant rate of exposure. Instituting this function is an opportunity to incorporate domain expertise into the planning stage of the trial. Modelling exposures in this way would appear reasonable considering the varying social mixing and other lifestyle habits and individual circumstances that participants might typically have. In general, the expected number of exposures over a time window is given by . For step intensity functions where for some interval of t values, this can conveniently be re-scaled to an exposures-per-week rate over that interval.
Figure 2.
Top row left: intensity function for a seasonal infection shown in units of exposures per week. Top row right: generalised logistic curves giving the risk scalings for and . Bottom row left: example of an individual’s exposures shown in blue, with red centre indicating an infection developed following exposure. Bottom row right: corresponding 25OHD status at exposure, shown as a function of the probability of infection for that status level.
Likelihood of infection
The likelihood an individual contracts infection after exposure depends on the vitamin D status at exposure. This is modulated by a baseline prevalence and a relative risk scaling curve. As one’s status moves towards insufficient from sufficient 25OHD concentration, their risk increases. Whether an individual contracts infection from a given exposure is assumed to be independent of other exposures conditional on the individual’s status curve. A sigmoid shaped dose–response curve is justified in studying effectiveness of nutrients33, including vitamin D. This thinking is adopted through the risk scaling curve which is taken as a member of the generalised logistic family
where x represents status. The parameters l, u give the lowest and highest relative risk scaling values. The difference represents how much more likely one is to get infection with completely insufficient 25OHD compared to being fully sufficient. The values of a and b are determined by providing reference values between which one observes the steepest change of the relative risk scaling curve. The points 10 and 70 nmol/L are used for the results presented in this paper; these are the status levels between which one sees the greatest change in protection attributable to vitamin D. See Fig. 2 for an example showing scaling curves where the probability would vary between and 1.5, 2, 3, 4 times . The probability gives the probability of a fully sufficient vitamin D individual contracting infection after exposure. There is much debate around the reference values chosen here as 10 and 70 nmol/L and this is another opportunity to explicitly represent domain experience in trial planning.
Summary of simulation steps
Before progressing further, we give an overall summary of the generative model of exposures and infections. Consider individual i and let denote the times at which they are exposed. Exposure times only within the time frame of the trial are used: , .
In the case of infections (), one may also wish to impose a non-susceptible period, for example, an exponentially distributed amount of time where the infected individual is not susceptible to a new infection. The package SimVitD provides this option. S.3 in the supplementary material gives a glossary of all parameters involved in the simulation.
Power of detecting benefit of supplementation
Determining whether there is a benefit of supplementation will ordinarily be carried out by investigation of the number of events (e.g. infections) that occurred in participants assigned to each arm over the trial duration, or some function thereof.
Types of comparisons
Define
The power of the tests
| 2 |
| 3 |
will be of interest.
Theorem 1
Consider a two-armed vitamin D trial, where individuals in the first arm receive a placebo and those in the second all receive an intervention (supplement) following the same scheme (e.g. all fixed dose). Then making the same assumptions as outlined in “Modelling individual vitamin D status trajectories” and “A model for benefit of vitamin D supplementation” we have that:
the propensities to contract at least one infection in each group satisfy
the expected number of infections in the placebo group is larger than that in the supplement group,
That is, the simulation model in “Modelling individual vitamin D status trajectories” and “A model for benefit of vitamin D supplementation” will correctly generate samples under for (2) and (3) when there is indeed an intervention (supplement) administered.
A proof is provided in the supplementary material. This result says that if one considers the proposed models to be reasonable for the purposes of study planning, then approximating the power using them should serve as a proxy for a trial in the wild.
To approximate the power of detecting treatment effects, tests of (2) and (3) are carried out using a non-parametric Bootstrap34. Two sample tests from the R package wBoot35 are used. These comparisons are extensible to other planning scenarios. For example, to account for age differences, one could have an age range specific logistic curve when carrying out the simulation steps in “Summary of simulation steps”. Then a logistic regression incorporating age range could be used to approximate the power of any comparisons. Again, there is scope for domain knowledge to be incorporated in such considerations.
Traditional power calculations return a sample size for a specified effect size, Type I error and target power. Here the concept of a catch-all prescriptive effect size cannot be characterised directly through a univariate quantity. It will depend on dosage and risk scaling. However, an implied effect size for tests (2) and (3) can be approximated and returned as a by-product of our approach.
Approximating power
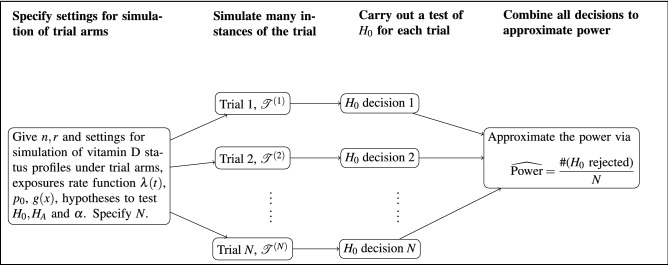
Let there be and participants in each of the two arms “” and “”. Here the supplement arm will be of size r : 1 to the placebo arm. Often will be investigated and in cases where necessary . Power to detect group differences is approximated by simulating the trial a large number of times using the models outlined in “Modelling individual vitamin D status trajectories” and “A model for benefit of vitamin D supplementation” and carrying out a bootstrap hypothesis test on each of these. Let denote N simulated trial realisations, each simulated under (i.e. where there is a difference). For each realisation, the test of hypothesis is applied to that data and the decision to reject or not is recorded. This gives the process illustrated in Fig. 3. Rejection in any case is based on a significance level .
Figure 3.
Simulation process for estimating the power of a study.
A simulation-based estimate of the power is given by
the proportion of times was rejected when corresponds to the true generating process. Since trial instances are generated independently, the law of large numbers guarantees convergence in probability to the true power
as . The precision of the simulation based power estimate will scale as . The R package SimVitD implements this approximation for the comparisons outlined in “Types of comparisons” including convenient utilities for visualisation.
This procedure to estimate the power can be repeated for a range of possible values of n to construct a power function for the comparison. At first glance, this may appear to have a heavy computational load. Note however that these simulations may be easily parallelized using, for example, the R package parallel36. Also, the computations need only be carried out in the planning stages of a trial, and so any computational latency may not be a crucial issue.
Examples
Examining the impact of baseline 25OHD concentration in the population and of average 25OHD change due to intervention
Here we examine the RCT power for a two-armed trial with five different populations having baseline 25OHD concentrations of: 15, 35, 50, 60, 75 nmol/L (in this context, this is annual average 25OHD in the population, i.e. the horizontal dashed green line in the top right panel of Fig. 1), and assuming minimum detectable levels of 10 nmol/L. This translates to . The researchers aim to enrol a heterogeneous cohort of participants, so a large scatter around the expected maximum and minimum levels would be anticipated and is reflected as , . The primary endpoint is the number of infections contracted over the trial duration.
Participants receive either a placebo or a fixed-dose vitamin D supplement. For each population we run three trials: those in the intervention arm receive a dose that is equivalent to 10, 20 or 40 nmol/L increase in 25OHD, with little variability in the derived uptake (i.e. a large value of is assumed and we take , see supplementary material S.2.).
A seasonal infection is considered where the intensity function is expressed through a step function. The intensity is shown in Fig. 2 with its equivalent per week exposure rate during the corresponding period. That is having 1 expected exposure per week from September to April end, and 0.1 per week from May to August end. Two different levels of risk scaling are considered following “Likelihood of infection”. In each case and then we consider relative risk comparing highly insufficient and fully sufficient of either 2 or 4, i.e. (Fig. 2). The infection probability which gives the probability for a fully 25OHD sufficient individual becoming infected on exposure is taken to be in all cases. A non-susceptible period is simulated after each infection. This is exponentially distributed, with an expected duration of two weeks. Here the quantity to compare the two arms is the expected number of infections i.e. test (3) with . The power approximation uses simulations of each trial condition with 500 bootstrap replications for each hypothesis test. Each configuration is run five times independently to explore the Monte Carlo error.
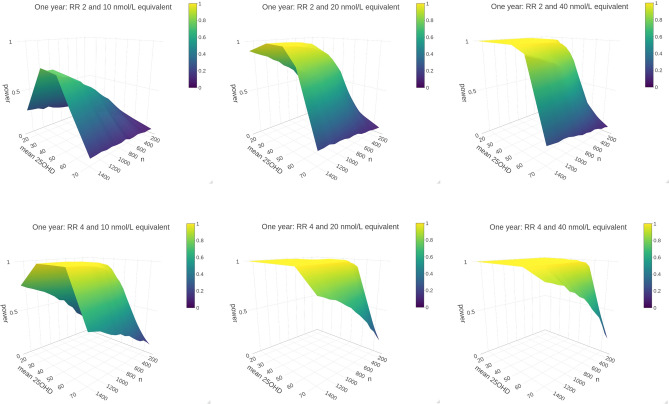
Figure 4 shows power surfaces constructed by taking the Monte Carlo average over the five replications of each trial condition with in each trial arm ().
Figure 4.
Power surfaces are shown for intervention that achieves a 25OHD increase of 10 nmol/L (left), 20 nmol/L (centre),and 40 nmol/L (right) versus placebo, against the sample size n and population baseline vitamin D status . The top row shows surfaces when relative risk of 2 is assumed between fully insufficient and fully sufficient i.e. , and the bottom row shows the same for . Here n gives the number of participants in each trial arm.
Examining the impact of the start date of trials for 6 month trials
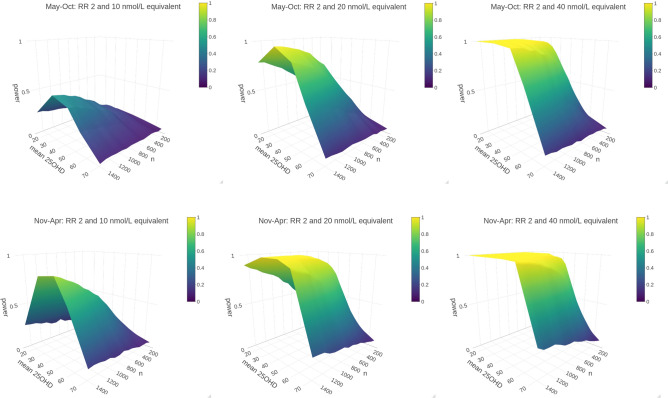
Since status level varies naturally throughout the calendar year, the date of trial initiation could conceivably have an impact on the trial power. Anecdotally, this has been observed, and a number of vitamin D trials have been conducted over winter months. To investigate this approach through our simulation model, we repeat the experiments above , but this time make the duration of the trial 6 months rather than 1 year and restrict our investigations to . Two 6 month periods are considered; beginning of May to the end of October and beginning of November to the end of April. The estimated power surfaces are shown in Fig. 5.
Figure 5.
Six month trials. Top row: power surfaces for “summer trial”, running from May until end of October. Bottom row: “winter trial” running from November through April. All experiments assume relative risk between fully depleted and fully replete of 2, . n represents the number of participants in each trial arm.
Results
As expected, we found that the trial power was very strongly linked with the relative risk scaling: at least 80% power was achieved in majority of experiments when relative risk of 4 () between fully sufficient and fully insufficient was assumed, but this was not the case when the relative risk was taken to be . Details are given in the supplementary material (Section S.4, Tables S1–S5). The increase in 25OHD attributable to intervention also played a major role: sufficient power was not reached with an equivalent increase of 10 nmol/L in any of the test cases when . For 20 and 40 nmol/L increases (Table 1; S.4 supplementary material), we observed a dramatic impact of population baseline 25OHD concentration. When annual average baseline 25OHD surpasses 50 nmol/L, the power deteriorates sharply. For example, while a trial with 1400 participants per arm (Table 1) would have close to full power to detect an intervention effect when the population baseline concentration is 35 nmol/L, the power would drop to 21.24% if this baseline was 75 nmol/L. Interestingly, for the case, we also have power plunges at 10 nmol/L intervention. This implies that the effect of intervention is less detectable when participants are at extreme ends of the 25OHD sufficiency spectrum.
Table 1.
Power (in percentage) for 20 nmol/L equivalent dose at for a range of sample sizes and baseline population 25OHD concentrations.
| n (per arm) | 15 nmol/L | 35 nmol/L | 50 nmol/L | 60 nmol/L | 75 nmol/L |
|---|---|---|---|---|---|
| 100 | 19.1 | 36.6 | 28.9 | 16.8 | 8.1 |
| 200 | 25.6 | 54.2 | 42.1 | 23.3 | 8.9 |
| 300 | 35.2 | 70.5 | 54.6 | 28.9 | 10.0 |
| 400 | 44.2 | 81.8 | 67.3 | 37.8 | 11.6 |
| 500 | 49.8 | 88.2 | 74.8 | 41.3 | 12.1 |
| 600 | 56.1 | 93.3 | 81.8 | 49.1 | 13.3 |
| 700 | 64.0 | 96.6 | 87.6 | 54.8 | 16.0 |
| 800 | 68.2 | 97.3 | 90.5 | 60.4 | 16.7 |
| 900 | 71.4 | 98.7 | 92.8 | 63.8 | 16.5 |
| 1000 | 76.5 | 99.1 | 95.3 | 67.5 | 18.5 |
| 1100 | 79.2 | 99.5 | 96.8 | 72.8 | 19.4 |
| 1200 | 83.3 | 99.8 | 97.7 | 75.1 | 19.2 |
| 1300 | 85.5 | 99.9 | 98.6 | 77.5 | 21.7 |
| 1400 | 88.0 | 99.9 | 98.8 | 81.0 | 21.2 |
| 1500 | 90.8 | 100.0 | 99.1 | 84.6 | 21.8 |
Designs having power are in bold font.
Figure 5 shows the estimated power surfaces for the 6 month trials. We note here that the power surface for the trial conducted from November to April is higher overall, even at the 10 nmol/L equivalent dose. The intervention effect is less detectable for those with high baseline vitamin D levels as noted previously.
Discussion
This paper proposes a bespoke approach to establishing power and sample size required for randomised controlled trials investigating the benefit of vitamin D supplementation. The tools we present aim to account for dominant sources of variability and heterogeneity in vitamin D status that impact on the potential benefit of supplementation, and enable quantification of the impact different factors may have on power, an investigation that cannot be conducted in vivo. The specific issue with vitamin D is that 25OHD naturally fluctuates in both arms11. This makes articulation of a vitamin D supplementation effect more difficult since treatment effect can vary within and between individuals and is impacted by other factors (e.g. skin exposure to UVB).
Our results highlight four considerations regarding vitamin D RCTs. Firstly, how well powered a trial is depends strongly on the population that participants are drawn from; vitamin D deficient populations will have more detectable effects in certain instances. Secondly, even quite large increases in 25OHD in the intervention arm might not achieve sufficient power if the population is already 25OHD sufficient. Thirdly, trial power is closely connected with the expected magnitude of the effect of vitamin D, as illustrated by the different surface shapes in the top and bottom rows of Fig. 4. Finally, our results support benefit of conducting trials in the winter time as the start date of the trial has a demonstrable impact on the overall power. In practice, participants may be enrolled to such trials on an ongoing basis; what our simulations highlight is that the schedule for the roll-out of a trial lasting less than a year should be mindful of seasonal change.
The issue of high baseline 25OHD concentrations is an increasingly important consideration. Vitamin D supplementation is becoming more prevalent in general and in diseased populations37,38. Additionally, research ethics committees often request that potential trial participants who are vitamin D deficient should not be permitted to participate in the trial. Summarily, those who would benefit the most are excluded, and baseline 25OHD in the trial population is further increased artificially.
Two key aspects of the work need to be highlighted. First, as seasonal variation in solar radiation is pronounced, there will be a natural, cyclic variation in vitamin D status12; consequently, the relative contribution of supplementation to the overall vitamin D status, and it’s impact, will vary seasonally. Therefore, there may be a large within-person heterogeneity in the general effectiveness of supplementation depending on the time of year. Simulating individual trajectories allows for varied benefit of supplementation both between and within individuals; both are accounted for in the power approximations. Second, the relationship between nutrient status and health is often best represented by a sigmoid curve, and evidence suggests this might also be the case for vitamin D33. This implies that below a certain threshold further deterioration in status won’t further worsen health (fully insufficient), and similarly, above a certain threshold no extra benefit will be achieved (fully sufficient). The approach we propose allows investigators to hypothesise what these thresholds are. By modelling individual vitamin D trajectories, an individual’s relative risk can vary, as their 25OHD fluctuates between fully insufficient and fully sufficient. It is important to appreciate that within-arm the risk profile varies over the trial, and risk is not constant. In simple terms, this means that observations from a more deficient participant will contribute more to the study power, than observations from vitamin D sufficient participant.
After decades of research, the interest in vitamin D is not winding down, and the issue of vitamin D supplementation remains strongly divisive. Findings from trials are consequential. Our proposal provides a utility to aid sample size calculation in prospective vitamin D trials (and can be adapted for nutrients where status might change over time). The solution we give is a flexible model which characterises the main components of variability in a vitamin D trial, and key parameters can be chosen by the user and easily visualised. The findings we present are also relevant for post–hoc assessment of power for past trials (we provide an illustrative analysis in the supplementary material S.5)39–42. On foot of null-findings, treatment cannot be recommended and investment in future trials becomes less likely. Therefore, it is critical to appreciate the likelihood of false-negative findings. The key role of 25OHD concentration when evaluating trial findings has long been speculated. This was recently supported by findings from a large trial that found benefit in those who maintained high intra-trial 25OHD concentration43. Two other large trials found benefit of supplementation in normal weight individuals ( same dose of treatment achieves greater increase in 25OHD concentration in non-overweight or obese)44,45.
A number of studies have examined the protective properties of vitamin D in acute respiratory tract infections in different populations, as detailed in a thorough individual-patient data (IPD) meta-analysis46. The IPD meta-analysis found that vitamin D supplementation was effective at reducing the risk of these infections. However, the majority (16 out of 24) of original research studies included in this meta-analysis did not detect significant benefit of vitamin D (7 found significant protective effects and one concluded increased risk). In the light of these findings, it should be considered whether the null-findings from these 16 studies are false-negatives. Since it is reasonable to assume that investigators conducted sample size calculation prior to conducting a trial, it would be instructive to investigate the calculations deployed and their implied power.
A key novelty of our approach lies in modelling vitamin D status and exposures, and propagating these through the power calculation. Primary sources of variability in vitamin D trials are characterised in such a way as to allow domain expertise and knowledge of characteristics of the population trial participants to flexibly inform their parametrisation. In addition to looking purely at a traditional fixed-dose intervention (allowing the 25OHD response to treatment to vary between individuals), there is scope to investigate the concentration-controlled design aimed at achieving target 25OHD concentration in intervention arm. The power approximation presented relies on assuming that the model proposed for status and infections provides a reasonable approximation of a trial in the wild. If this is the case, the theorem of “Approximating power” justifies the approximation. The flexibility of the model allows for incorporation of potential covariate effects (e.g. BMI, skin tone) at the planning stage. If there are 25OHD characteristics that are known to impact specific groups of individuals in the cohort being targeted, then these effects can be included in the simulation model. As much information as is available at the time of trial conception can be incorporated into the power and sample size determination and this is particularly important in the case of vitamin D where extensive domain experience may be harnessed.
It is certain that individual 25OHD trajectories do not follow a smooth annual curve, as a number of factors might cause departures from this in either direction. For example, a sun holiday may boost vitamin D status or a surgery might deplete it47. However, the purpose of this model is to characterise the predominant sources leading to large variability in 25OHD level and treatment effect in vitamin D RCTs. What we propose is native to the cyclic variation expected in vitamin D. There are adjustments that would make the generative model of disease more realistic; for example, one may expect disease events within an individual to be correlated beyond just the vitamin D status curve. However, for the sake of planning, the model we propose should serve as a representative scheme of the phenomena one could conceive unfolding throughout a vitamin D trial. This study primarily considers outcomes that are acutely affected by contemporaneous vitamin D status. Many outcomes, for example cancer, take years to develop, and the role of vitamin D supplementation in such cases will be more difficult to model.
There is much interest in vitamin D supplementation as an inexpensive way to improve quality of life in general. Establishing evidence-based guidelines on the use of vitamin D supplements requires conclusions from appropriately powered trials. Recently impact of deficiency on the immune system is being debated with respect to COVID-19 infection. This work is timely in proposing power determination tools in this direction, where considerations about the natural cycle of solar radiation native to vitamin D uptake have been incorporated.
While developed for vitamin D trials, the strategy presented may be extended to other nutrient studies. For example, one could instead think of a status curve of the form
which allows incorporation of a trend and a given cyclic period with individual specific parameters, with a detectability threshold. This could be useful to plan trials involving nutrients with cumulative benefit, or supplements that are administered periodically (e.g. vitamin B12 shots or vitamin K antagonists).
In conclusion, understanding the population characteristics and trial features is key to accurately discerning the power of vitamin D trials.
Supplementary Information
Acknowledgements
RM was supported by Enterprise Ireland grant to LZ (CS20182076). JW acknowledges support from HELICAL (HEalth data LInkage for ClinicaL benefit) EU Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie Grant agreement no. 813545. The authors would like to thank Dr. James Sweeney for helpful discussions on this work and giving feedback on an earlier draft.
Author contributions
L.Z. identified problem. J.W. and L.Z. developed generative model. R.M. and J.W. implemented models. All authors contributed to interpretation of results. L.Z. and J.W. wrote the manuscript. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
The original online version of this Article was revised: The original version of this Article contained an error in the Introduction section, where “The tools discussed in this paper are easily used through the undefined R package SimVitD26 available on the Comprehensive R Archive Network (CRAN)” now reads: “The tools discussed in this paper are easily used through the R package SimVitD26 available on the Comprehensive R Archive Network (CRAN).”
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jason Wyse, Rebecca Mangan and Lina Zgaga.
Change history
6/22/2021
A Correction to this paper has been published: 10.1038/s41598-021-92486-4
Contributor Information
Jason Wyse, Email: wyseja@tcd.ie.
Lina Zgaga, Email: zgagal@tcd.ie.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-90019-7.
References
- 1.Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JPA. Vitamin D and multiple health outcomes: Umbrella review of systematic reviews and meta-analyses of observational studies. Br. Med. J. 2014;348:2035. doi: 10.1136/bmj.g2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grant WB, et al. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12:34. doi: 10.3390/nu12061620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Entrenas Castillo M, et al. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study. J. Steroid Biochem. Mol. Biol. 2020;203:105751. doi: 10.1016/j.jsbmb.2020.105751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holick MF. Vitamin D deficiency. N. Engl. J. Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 5.Ross AC, et al. The 2011 dietary reference intakes for calcium and vitamin D: What dietetics practitioners need to know. J. Am. Diet. Assoc. 2011;111:524–527. doi: 10.1016/j.jada.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Holick MF, et al. Evaluation, treatment, and prevention of vitamin D deficiency: An endocrine society clinical practice guideline. J. Endocrinol. Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 7.Arnaud SB, Stickler GB, Hamworth JC. Serum 25-hydroxyvitamin D in infantile rickets. Pediatrics. 1976;57:221–225. [PubMed] [Google Scholar]
- 8.Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: Results of a randomized trial. Am. J. Clin. Nutr. 2007;85:1586–1591. doi: 10.1093/ajcn/85.6.1586. [DOI] [PubMed] [Google Scholar]
- 9.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296:2832–2838. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 10.Webb AR, Holick MF. The role of sunlight in the cutaneous production of vitamin D3. Annu. Rev. Nutr. 1988;8:375–399. doi: 10.1146/annurev.nu.08.070188.002111. [DOI] [PubMed] [Google Scholar]
- 11.O’Sullivan F, et al. Sunshine is an important determinant of vitamin D status even among high-dose supplement users: Secondary analysis of a randomized controlled trial in Crohn’s disease patients. Photochem. Photobiol. 2019;20:20. doi: 10.1111/php.13086. [DOI] [PubMed] [Google Scholar]
- 12.Degerud E, Hoff R, Nygard O, et al. Cosinor modelling of seasonal variation in 25-hydroxyvitamin D concentrations in cardiovascular patients in Norway. Eur. J. Clin. Nutr. 2016;70:517–522. doi: 10.1038/ejcn.2015.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cicarma E, et al. Sun and sun beds: Inducers of vitamin D and skin cancer. Anticancer Res. 2009;29:3495–3500. [PubMed] [Google Scholar]
- 14.Gu Y, Zhu Z, Luan X, He J. Vitamin d status and its association with season, depression in stroke. Neurosci. Lett. 2019;690:99–105. doi: 10.1016/j.neulet.2018.09.046. [DOI] [PubMed] [Google Scholar]
- 15.Ziff, O. J., Penny, H., Frame, S., Cronin, A. & Goldsmith, D. Impact of seasonality on the dynamics of native Vitamin D repletion in long-term renal transplant patients. Clin. Kidney J.10, 411–418. 10.1093/ckj/sfw136 (2017). https://academic.oup.com/ckj/article-pdf/10/3/411/17689190/sfw136.pdf. [DOI] [PMC free article] [PubMed]
- 16.RohrThomsen C, BrinkHenriksen T, Uldbjerg N, Milidou I. Seasonal variation in the hypertensive disorders of pregnancy in Denmark. Acta Obstetr. Gynecol. Scand. 2020;99:623–630. doi: 10.1111/aogs.13786. [DOI] [PubMed] [Google Scholar]
- 17.Nikooyeh B, et al. Healthy changes in some cardiometabolic risk factors accompany the higher summertime serum 25-hydroxyvitamin D concentrations in Iranian children: National Food and Nutrition Surveillance. Public Health Nutr. 2018;21:2013–2021. doi: 10.1017/S1368980018000630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cannistraci CV, et al. “Summer Shift”: A potential effect of sunshine on the time onset of ST-elevation acute myocardial infarction. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 2018 doi: 10.1161/JAHA.117.006878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nowak J, et al. Lack of seasonal variations in vitamin D concentrations among hospitalized elderly patients. Int. J. Environ. Res. Public Health. 2021 doi: 10.3390/ijerph18041676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al Zarooni A, Al Marzouqi F, Al Darmaki S, Prinsloo E, Nagelkerke N. Prevalence of vitamin D deficiency and associated comorbidities among Abu Dhabi Emirates population. BMC Res. Notes. 2019 doi: 10.1186/s13104-019-4536-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zgaga L. Heterogeneity of the effect of vitamin D supplementation in randomized controlled trials on cancer prevention. JAMA Netw. Open. 2020;3:e2027176. doi: 10.1001/jamanetworkopen.2020.27176. [DOI] [PubMed] [Google Scholar]
- 22.Cook, J. A. et al. Delta2 guidance on choosing the target difference and undertaking and reporting the sample size calculation for a randomised controlled trial. BMJ363. 10.1136/bmj.k3750 (2018). https://www.bmj.com/content/363/bmj.k3750.full.pdf. [DOI] [PMC free article] [PubMed]
- 23.Moineddin R, Matheson F, Glazier R. A simulation study of sample size for multilevel logistic regression models. BMC Med. Res. Methodol. 2007;7:34. doi: 10.1186/1471-2288-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arnold BF, Hogan DR, Colford JM, Hubbard AE. Simulation methods to estimate design power: An overview for applied research. BMC Med. Res. Methodol. 2011 doi: 10.1186/1471-2288-11-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grant WB, Boucher BJ. Randomized controlled trials of vitamin D and cancer incidence. PLoS One. 2017;12:0176448. doi: 10.1371/journal.pone.0176448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mangan, R., Wyse, J. & Zgaga, L. SimVitD: Simulation Tools for Planning Vitamin D Studies (2020). R package version 1.0.
- 27.Kelly D, et al. The contributions of adjusted ambient ultraviolet B radiation at place of residence and other determinants to serum 25-hydroxyvitamin D concentrations. Br. J. Dermatol. 2016;174:1068–1078. doi: 10.1111/bjd.14296. [DOI] [PubMed] [Google Scholar]
- 28.O’Sullivan F, et al. Ambient UVB dose and sun enjoyment are important predictors of vitamin D status in an older population. J. Nutr. 2017;147:858–868. doi: 10.3945/jn.116.244079. [DOI] [PubMed] [Google Scholar]
- 29.Crowe FL, et al. Plasma concentrations of 25-hydroxyvitamin D in meat eaters, fish eaters, vegetarians and vegans: Results from the EPIC-Oxford study. Public Health Nutr. 2011;14:340–346. doi: 10.1017/S1368980010002454. [DOI] [PubMed] [Google Scholar]
- 30.Kraiczi H, Jang T, Ludden T, Peck CC. Randomized concentration-controlled trials: Motivations, use, and limitations. Clin. Pharmacol. Ther. 2003;74:203–214. doi: 10.1016/S0009-9236(03)00169-3. [DOI] [PubMed] [Google Scholar]
- 31.Lewis PAW, Shedler GS. Simulation of nonhomogeneous Poisson processes by thinning. Naval Res. Logist. Q. 1979;26:403–413. doi: 10.1002/nav.3800260304. [DOI] [Google Scholar]
- 32.Brock, K. & Slade, D. poisson: Simulating Homogenous and Non-Homogenous Poisson Processes (2015). R package version 1.0.
- 33.Heaney RP. Guidelines for optimizing design and analysis of clinical studies of nutrient effects. Nutr. Rev. 2014;72:48–54. doi: 10.1111/nure.12090. [DOI] [PubMed] [Google Scholar]
- 34.Efron B, Tibshirani RJ. An Introduction to the Bootstrap. No. 57 in Monographs on Statistics and Applied Probability. Chapman & Hall; 1993. [Google Scholar]
- 35.Weiss, N. A. wBoot: Bootstrap Methods (2016). R package version 1.0.3.
- 36.R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2018. [Google Scholar]
- 37.Rooney, M. R. et al. Trends in use of high-dose vitamin D Supplements exceeding 1000 or 4000 international units daily, 1999–2014. JAMA317, 2448–2450, 10.1001/jama.2017.4392 (2017). https://jamanetwork.com/journals/jama/articlepdf/2632494/jama_rooney_2017_ld_170014.pdf. [DOI] [PMC free article] [PubMed]
- 38.Madden JM, Duffy MJ, Zgaga L, Bennett K. Trends in vitamin D supplement use in a general female and breast cancer population in Ireland: A repeated cross-sectional study. PLoS One. 2018;13:1–7. doi: 10.1371/journal.pone.0209033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li-Ng M, et al. A randomized controlled trial of vitamin D3 supplementation for the prevention of symptomatic upper respiratory tract infections. Epidemiol. Infect. 2009;137:1396–1404. doi: 10.1017/S0950268809002404. [DOI] [PubMed] [Google Scholar]
- 40.Laaksi, I. et al. Vitamin D supplementation for the prevention of acute respiratory tract infection: A randomized, double-blinded trial among young Finnish Men. J. Infect. Dis.202, 809–814. 10.1086/654881 (2010). https://academic.oup.com/jid/article-pdf/202/5/809/18063181/202-5-809.pdf. [DOI] [PubMed]
- 41.Bergman, P. et al. Vitamin D3 supplementation in patients with frequent respiratory tract infections: A randomised and double-blind intervention study. BMJ Open2. 10.1136/bmjopen-2012-001663 (2012). https://bmjopen.bmj.com/content/2/6/e001663.full.pdf. [DOI] [PMC free article] [PubMed]
- 42.Urashima M, Mezawa H, Noya M, Camargo CA. Effects of vitamin D supplements on influenza A illness during the 2009 H1N1 pandemic: A randomized controlled trial. Food Funct. 2014;5:2365–2370. doi: 10.1039/C4FO00371C. [DOI] [PubMed] [Google Scholar]
- 43.Dawson-Hughes, B. et al. Intratrial exposure to vitamin d and new-onset diabetes among adults with prediabetes: A secondary analysis from the vitamin d and type 2 diabetes (d2d) study. Diabetes Care43, 2916–2922. 10.2337/dc20-1765 (2020). https://care.diabetesjournals.org/content/43/12/2916.full.pdf. [DOI] [PMC free article] [PubMed]
- 44.Pittas AG, et al. Vitamin d supplementation and prevention of type 2 diabetes. N. Engl. J. Med. 2019;381:520–530. doi: 10.1056/NEJMoa1900906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manson JE, et al. Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N. Engl. J. Med. 2019;380:23–32. doi: 10.1056/NEJMoa1811403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martineau AR, et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. Bri. Med. J. 2017;356:25. doi: 10.1136/bmj.i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reid D, et al. The relation between acute changes in the systemic inflammatory response and plasma 25-hydroxyvitamin D concentrations after elective knee arthroplasty. Am. J. Clin. Nutr. 2011;93:1006–1011. doi: 10.3945/ajcn.110.008490. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.