Abstract
The intracellular infection thread initiated in a root hair cell is a unique structure associated with Rhizobium-legume symbiosis. It is characterized by inverted tip growth of the plant cell wall, resulting in a tunnel that allows invasion of host cells by bacteria during the formation of the nitrogen-fixing root nodule. Regulation of the plant-microbial interface is essential for infection thread growth. This involves targeted deposition of the cell wall and extracellular matrix and tight control of cell wall remodeling. This review describes the potential role of different actors such as transcription factors, receptors, and enzymes in the rearrangement of the plant-microbial interface and control of polar infection thread growth. It also focuses on the composition of the main polymers of the infection thread wall and matrix and the participation of reactive oxygen species (ROS) in the development of the infection thread. Mutant analysis has helped to gain insight into the development of host defense reactions. The available data raise many new questions about the structure, function, and development of infection threads.
Keywords: arabinogalactan protein, cell wall, extensin, infection thread, legume, pectin, Rhizobium, symbiosis
1. Introduction
During the course of evolution, plants have exploited certain properties of microorganisms to expand their functional capabilities. Legumes and actinorhizal plants belong to the nitrogen-fixing clade Fabids or Eurosid I, collectively termed the FaFaCuRo [1,2]. These plants have acquired the ability to develop endosymbiotic relationships with various proteobacteria, collectively called rhizobia, and with actinobacteria from the genus Frankia [3,4,5]. In these symbioses, prokaryotes fix nitrogen derived from the air, which is provided to host plants in exchange for carbon sources derived from photosynthesis. One of the features associated with the symbiotic interaction is the formation of specialized organs called root nodules which provide a suitable microenvironment for bacterial nitrogenase activity. Nodule development requires the synthesis and recognition of signal molecules and structural components that are produced by both bacterial and plant partners [6].
To penetrate plant roots, bacteria follow different routes and use a variety of entry mechanisms that are determined by the host plant [7]. Probably the most primitive mechanism is intercellular root penetration, which is present in at least 25% of all legume genera [7,8]. This type of infection is morphologically simpler than the formation of intracellular infection threads occurring through the root hair cell, a process that apparently arose later in evolution. In the case of intercellular invasion, rhizobia can penetrate into the host tissue by several different routes: through the middle lamellae between adjacent root hair cells; through wounds arising during lateral root appearance (‘crack entry’); and directly between cells of the intact epidermis [7,8,9,10,11]. In this case, the subsequent colonization of the nodule primordium occurs as a result of intercellular infection, which is accompanied by the formation of tubular intercellular structures that resemble intracellular infection threads but lack the property of polar cell wall growth [12]. In some species of legumes with an intercellular infection, the bacteria within infection threads develop the capacity for nitrogen fixation. Such structures are called fixation threads [10].
The most well-characterized process of infection involves intracellular infection threads and occurs through root hair cells. About 75% of all legumes studied form infection threads in this way [7,13]. In 1887, Ward [14] described small ‘hyphae’ (which we now describe as infection threads) passing through the lumen of cells and through their walls. This was observed during the infection of clover, pea, vetch, beans, and other legumes. Early investigators believed that infection threads represented bacteria trapped in mucous threads. However, McCoy [15] observed that the mucous thread was encased in a sheath having the same general composition (cellulose, hemicellulose, and possibly some pectin) as the walls of young plant cells [16]. It is now considered that rhizobia penetrate the root hair cells through tubular structures bounded by plant cell wall material. Infection threads serve as a channel for the colonization of bacterial cells that grow and divide in their lumen, which is filled with a plant-derived extracellular matrix [17].
Infection threads are unique cell wall invaginations of plant origin. In many cases, they are able to traverse host cells, apparently fusing with the wall on the opposite side of the cell, thereby releasing bacteria into the intercellular matrix. Apical growth of an intracellular infection thread resembles tip growth of root hairs and pollen tubes [18], except that the orientation is ‘inside-out.’ This means that tubular growth proceeds into the cell [19,20,21]. In summary, in different legumes, infection threads can either grow through the intercellular space (intercellular infection threads) or through cells (intracellular or transcellular infection threads). The present review is concerned with the structure and development of intercellular and transcellular infection threads, which are characterized principally by the remodeling of plant cell wall growth and differentiation.
An infection thread is not just an ingrowth of a plant cell wall but a complex symbiotic structure [22,23]. It includes components of plant origin (cell wall polysaccharides, extracellular matrix glycoproteins such as arabinogalactan proteins (AGP), hydroxyproline-rich glycoproteins (HRGP), glycine-rich glycoproteins, extensins and others, as well as, various enzymes, receptors, and structural proteins) and also components of bacterial origin (both polysaccharides and proteins). During the infection of host tissue, the physical interaction between the bacterial and plant cell surfaces becomes progressively more intimate [24,25]. At each stage, the symbiotic interface must adapt thus that bacteria can exist in the new environment and avoid the development of defense reactions by the host plant [17,22].
Underpinning the infection process is a network of species-specific plant-microbial signal exchanges that involve lipochito-oligosaccharide Nod factors. These interactions have been extensively described [6,26,27,28]. Using model legumes for genetics and genomics, an ever-increasing range of plant genes has been identified that apparently contribute to the infection process. These genes encode transcription factors, LysM receptor kinases, E3 ubiquitin ligases, the Suppressor of cAMP receptor defect/WASP family verpolin homologous protein (SCAR/WAVE) actin regulatory complex, nitrate transporters, remorins, flotillins, proteins involved in biogenesis and membrane movement, and numerous other components [29,30,31]. Very little is known about the direct relevance of these components to the structure and development of infection threads, the dynamics of the polysaccharides of the of cell walls, and the extracellular matrix [17].
In this review, we will explore the sequential development of the symbiotic interface, which involves the remodeling of the cell wall and extracellular matrix during the growth of infection threads. This process stretches from the early stages of tissue invasion in root hairs through to the stage when biological nitrogen fixation develops within the host cells of mature nodules.
2. Molecular Dialogue
The study of chemical signaling in the interaction of plants and microorganisms has shown the existence of a precise molecular dialogue before and during direct contact between plant and bacterial cells [28,30,32]. Nod factors of rhizobia bind in the cell wall of root hairs [33] and cause a number of physiological and morphological plant reactions, such as depolarization of the host cell membrane [34], production of reactive oxygen species (ROS) [35], spiking of intracellular Ca2+ [36,37], and reorganization of actin microfilaments and endoplasmic microtubules in the tip of the root hair [38,39,40,41]. The first morphological changes observed under the action of Nod factors on legumes are deformations and curling of root hairs.
3. Attachment
3.1. Attachment of Rhizobia
Although deformation of root hairs can occur in response to Nod factor, intense curling is only possible after attachment of rhizobia [42,43]. As a prelude to infection, rhizobial cells accumulate on the surface of legume roots, forming a biofilm [44,45]. The increased population of bacterial cells in the biofilm amplifies the bacterial signal, thereby increasing the response from the host plant. The first phase of attachment is through weak, reversible, and nonspecific binding. The second stage is associated with the synthesis of bacterial cellulose microfibrils, which further strengthens the binding of bacteria to the roots. Other factors, such as pH, Ca2+, and Mg2+ concentrations, specific growth conditions, and root pretreatment, can also affect the attachment of rhizobia to the root surface [46].
It had previously been suggested that the binding of plant lectins to bacterial cell surface polysaccharides was part of the mechanism that determines the specificity between rhizobia and their legume hosts [47]. However, it is now generally accepted that this ‘lectin hypothesis’ was incorrect. The main determinants of host specificity are Nod factors, not surface polysaccharides [43,48]. Attachment of rhizobial cells to roots is considered to be a non-specific process and independent of symbiotic properties [42,49].
Rhizobium leguminosarum bv. viciae can use at least two mechanisms for attachment to root hairs of Pisum sativum L. and Vicia sativa L. One of them involves plant lectin, while the other is mediated by bacterial rhicadhesin [50]. In an acidic environment, lectin on the surface of root hairs binds to the polarized surface polysaccharide glucomannan produced by R. leguminosarum [50,51]. Thus, plant lectins can influence the extent of nodulation in legumes [52]. However, a complicating factor is that lectins can also modulate plant defense responses. During pathogenic interactions, lectins may become associated with receptor proteins in membrane microdomains. This strengthens the host defense response, but this effect is apparently weakened in a mutualistic symbiosis [53,54]. Nod factor could play a role in suppressing the defense response through either a direct or indirect interaction with the Lectin receptor kinase (LecRK) [55,56].
Rhicadhesin, a Ca2+-binding protein of R. leguminosarum, has been shown to increase adherence to root hairs under alkaline conditions [43,57]. A similar protein has been identified in Bradyrhizobium spp. [58]. Using a phage display library from R. leguminosarum, several Rhizobial attachment proteins (RAPs) were identified [59,60]. These proteins are secreted through the inner and outer membranes via a Type I secretion system, encoded by the prsD and prsE genes [61]. Calcium-binding adherence proteins (cadherins) are also secreted through the PrsDE system [62].
Legume annexins can also play a role during the early stages of infection [63]. The symbiotic annexins MtANN1 and MtANN2 have been shown to be associated with individual symbiotic events [64], in particular, with Ca2+ spiking [63,65]. Another possible participant in rhizobial attachment is the arabinogalactan protein (AGP). This new mode of binding may be important for the growth of rhizobia on the roots of both legumes and non-legumes [66].
3.2. Curling of Root Hairs
Rhizobial attachment is closely associated with Nod factor-induced deformation of the root hairs, which undergoes curling through 360-degrees [22,67]. Curling requires living bacterial cells [68]. It disrupts the normal pattern of polar growth of the root hair tip, resulting in the trapping of bacteria within the curl, called the ’shepherd’s crook.’ Here, the bacteria multiply to form a microcolony [67,69].
The actively growing tip of the root hair cell has a characteristic polarized organization [70,71]. It is enveloped by the cristalline layer of the cell wall, behind which is a dense cytoplasm filled mainly with secretory vesicles that are located along the actin microfilaments or microtubules [72]. The nucleus follows the advancing tip of the root hair at a fixed distance [72,73].
Successful invasion involves a reorientation of plant cell wall growth to allow initiation of an infection thread by inward growth into the root hair cell. After the Nod factor is recognized by receptor kinases, Ca2+ spiking in root hairs initiates the downstream signaling events [30,32]. Calcium spiking is also involved in the initiation of the tip growth of root hair cells and pollen tubes [18,71]. In root hairs, the nuclear envelope and the endoplasmic reticulum associated with the nucleus are potential internal stores of Ca2+ to be released during Ca2+ spiking [74]. The creation of artificial Ca2+ gradients in the root hair using ultraviolet-activated ionophores indicates that the establishment of the Ca2+ gradient is sufficient to initiate root hair growth. In this case, a temporary shift occurs in the direction of growth from the tip to the site of the induced gradient [75].
Curling of root hairs and a change in the direction of growth are correlated with and probably caused by changes in the plant cytoskeleton [76]. Actin depolymerization and reorganization of both endoplasmic and cortical microtubules are some of the earliest effects observed in root hairs following exposure to the Nod factors [38,40,41]. Following root hair curling, the tips of the root hairs swell, the number of subapical fine bundles of actin filaments (FB-actin) increases [39], and the microtubular cytoskeleton is re-formed [40,41]. Recently, it was demonstrated that microtubule reorganization during rhizobial infection in Medicago truncatula Gaertn. is regulated by Developmentally regulated plasma membrane polypeptide (DREPP), a member of the DREPP/PCaP family of microtubule-binding proteins [77].
Based on the phenotypes of legume mutants defective at successive stages of nodule development, substantial progress has been made in elucidating the mechanisms controlling the processes of infection [29,31,78,79]. Inhibition of the M. truncatula Phosphatidylinositol 3 kinase (MtPI3K) gene (regulating vesicle trafficking and the oxidative burst) led to decreased root hair curling and infection thread initiation. This indicated an important role for the vesicle trafficking system and for ROS in the initial steps of rhizobial colonization [80]. The curling of root hairs is also mediated by the Rho family of small GTPases (ROP). In M. truncatula, ROP10 is localized on the plasma membrane at the tips of root hairs. Interactions between ROP10 and Nod factor receptors are required for root hair deformations and curling during rhizobial infection [81].
4. Invasion of Host Cells
4.1. Initiation of the Infection Thread
As a result of root hair curling, rhizobia are trapped in a confined space [82]. Continued growth and division lead to the formation of a microcolony, which develops within the infection chamber (pocket). Rhizobia inhabiting the infection thread are the descendants of only a few founder cells derived from the initial infection event [83]. Gradual enlargement of the microcolony is accompanied by a rearrangement of the infection chamber. The entrapped bacteria generate a high local concentration of Nod factor, which may stimulate the initiation of an infection thread [84,85]. Thus, localized production of Nod factor within the infection pocket may act as a morphogenic organizing center, providing positional information for cell wall remodeling through reorientation of the underlying plant cytoskeleton.
During the modification of the infection chamber and initiation of the infection thread, many different proteins are involved. For example, upon inoculation of M. truncatula with Sinorhizobium meliloti, rearrangement of the infection chamber is accompanied by accumulation of a marker for exocytosis, the Vesicle-associated membrane protein 721e (MtVAMP721e) [20,86]. Intensive synthesis of the infection-associated secreted protein Early nodulin 11 (MtENOD11) begins around the rhizobia trapped in the chamber. This may increase the cell wall plasticity required to reduce turgor and radial expansion, followed by the initiation of inward polar growth of the infection thread [20]. ENOD11 is a proline-rich protein that contains a reduced amount of tyrosine, which probably limits its cross-linking with other cell wall components.
Other plant components involved in the maturation of the infection chamber and the initiation of the infection thread were identified in different legumes: E3 ubiquitin ligase Cerberus [87], the SCAR/WAVE complex [76], two flotillins [88], vapirin [89], a nonspecific lipid transfer protein N5 protein (MtN5) [90], Lack of symbiont accommodation (LAN), acting as a subunit of the mediator complex [91], transcription factors CYCLOPS/IPD3 (Interacting protein with DMI3) [92], NSP1 (Nodulation signaling pathway 1) [93], NSP2 [94], ERF required for nodulation (ERN) [95], Nodule inception (NIN) [96], CCAAT-box-binding Nuclear factor YA1 (NF-YA1) and NF-YA2 [97]. Growth of the infection thread in the root hair requires the movement of nuclei and recently the involvement of Linker of nucleoskeleton and cytoskeleton (LINC) complexes was demonstrated [98].
Three different explanations have been proposed for structural changes leading to bacterial penetration into the root hair cell as part of an incipient infection thread. First, Nutman [99] proposed invagination of the root hair cell wall, in which the growth direction of the plant cell wall changes at a localized point so that it grows back into the root hair, forming a tubular infection thread. Second, Ljunggren and Fåhraeus [100] proposed the ‘polygalacturonase’ hypothesis, according to which rhizobial exopolysaccharide increases the activity of plant polygalacturonase, and an individual bacterial cell dissolves cell wall pectins and subsequently penetrates through it without obvious structural damage. The infection thread is formed as an encapsulation response upon contact of the rhizobia with the plasmalemma. Finally, Dart and Mercer [101] proposed the penetration of small coccoid forms of rhizobia through cracks in cellulose microfibrils.
Currently, it is thought that the initiation occurs by remodeling of the cell wall and ingrowth of the infection thread using some form of inverted tip growth mechanism [17,83]. Structural disorganization of the cell wall of root hairs has been demonstrated at the site of infection thread initiation and is associated with direct contact between rhizobial cells and the plasma membrane [102,103,104]. Subsequent initiation of an infection thread wall probably involves the participation of bacterial and plant enzymes that modify cell wall polysaccharides. Rhizobia have enzymes that can degrade cellulose and other polysaccharides of the plant cell wall [54]. In addition, to initiate an altered growth process, rhizobia can induce the production of plant polygalacturonases (PGs) and pectin methylesterases (PMEs) [105,106,107]. Plants are also able to modify the composition of their cell walls [108,109]. In response to S. meliloti, the gene for M. sativa polygalacturonase (MsPG3) is induced [110,111]. The Nodulation pectate lyase gene (LjNPL) was identified in Lotus japonicus (Regel) K. Larsen, [112], and expansins are also involved in the infection process [113]. It should be emphasized that the wall of the infection thread is topologically continuous with the host cell wall and encapsulates the rhizobia [102,103,104]. There is no direct penetration through the plasmalemma: Bacteria always remain in the apoplastic space of root hair cells [102].
Both actin [80] and microtubular [114] cytoskeletons are involved in the initiation of infection threads. When the actin cytoskeleton is disturbed, and in particular when the fine F-actin is disorganized, it results in defective growth of infection threads, pollen tubes and root hairs. This phenotype was observed in the mutant crinkle of L. japonicus [115]. Mutants in L. japonicus Actin-related protein component 1 (LjARPC1) gene, which encodes the Actin-related protein 2/3 (APR2/3) subunit of the complex that controls the nucleation of Y-shaped branched actin microfilaments, formed a reduced number of microcolonies [116]. L. japonicus mutants 121F-specific p53 inducible RNA (Ljpir1) and nck-associated protein 1 (Ljnap1) [76], as well as the M. truncatula required for infection thread (Mtrit–1) mutant (ortholog Ljnap1) [117] were characterized by a similar phenotype (disorganization of the actin cytoskeleton, no reorganization of F–actin in response to inoculation, a decrease in the number of microcolonies in curled root hairs) [76,116]. In addition, Actin reorganization is regulated by the activation of the ROP GTPase family [118], inositol phospholipids [119], and actin depolymerization factor (PvADFE) in the Phaseolus vulgaris L.-rhizobia symbiosis [120].
ROS and NO also play an important role in the initiation and growth of infection threads [121]. Nod factors can activate the first wave of ROS production, which is involved in nodule development, and they also inhibit the second wave, which is involved in defense responses [122,123]. The first wave modulates the expression of plant genes and/or the redox status of proteins involved in root hair deformation [123], infection thread progression, and nodule formation [124,125,126]. For the second wave, suppression of immune responses (ROS production, and accumulation of salicylic acid) was observed in the roots of M. truncatula and M. sativa upon addition of Nod factors [122,127]. In addition to ROS effects, there is an initial release of NO at the early stage of symbiotic interaction. This induces the expression of non-symbiotic hemoglobin (ns-Hb), which, in turn, traps NO and reduces the plant defense response [128,129,130].
Class III peroxidases (Prx-III) are considered as potential sources of enzymatic ROS. Examples include Rhizobium-induced peroxidases (Rip1-10) [131,132] and NADPH oxidases, also called Respiratory burst oxidase homologues (Rbohs) [123,125,126].
4.2. The Nodule Primordium and Nodule Meristem
Simultaneous with the initiation of infection threads in root hairs, cells of the root cortex and the pericycle begin to divide, creating a nodule primordium [133,134]. The synchronized occurrence of host cell infection and nodule organogenesis suggests that there is some form of long-range transmission of symbiotic signals [135,136]. In cells of the outer cortex, the nucleus migrates to the center of the host cell, and a cytoplasmic bridge is formed with longitudinal microtubules connecting opposite sides of the cell [134]. The orientation of this cytoplasmic strand sets the path for the subsequent formation of an infection thread. Therefore, it has been termed a pre-infection thread (PIT) [133].
Temperate legumes such as M. truncatula, M. sativa, P. sativum, and Trifolium sp. have a permanent meristem at the tip of elongated nodules even after full maturation. These are termed indeterminate nodules (or, more accurately, nodules with indeterminate meristems). In this case, the development and growth of infection threads continues in the post-meristematic tissue of mature nodules, and the bacteria are continuously released into host cells [83]. Glycine max (soybean), Vicia faba (bean), and L. japonicus are tropical legumes that usually form round determinate nodules. Determinate nodules lack a persistent meristem and do not display an obvious developmental gradient [137].
5. Propagation of the Infection Thread
Direct interaction between plant and bacterial cell surfaces plays a critical role in the formation of the infection thread [138]. Morphologically, the infection thread is a tubular ingrowth of the cell wall, surrounded by a plasma membrane and containing a matrix with enclosed bacteria (Figure 1) [17,83]. Rhizobia in the infection thread are in the apoplast and remain physically separated from plant cell cytoplasm [19,83]. The distance between the tip of the infection thread and the rhizobia is constant and does not normally exceed 10 μm [17]. Growth of the infection thread apparently occurs in discrete steps. Rapid cell wall growth at the tip is followed by a phase of bacterial division and sliding growth in the extracellular matrix within the lumen of the thread [19,69,139]. Rhizobia in the infection thread are surrounded by an exopolysaccharide capsule, which may play an important role in facilitating this movement [140,141].
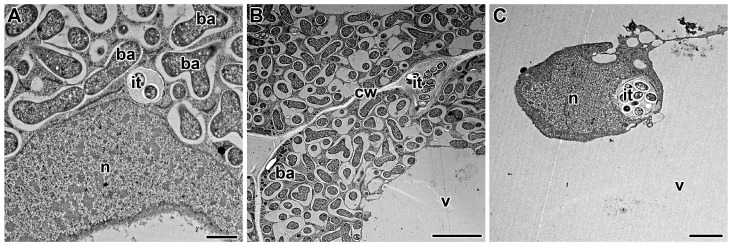
Figure 1.
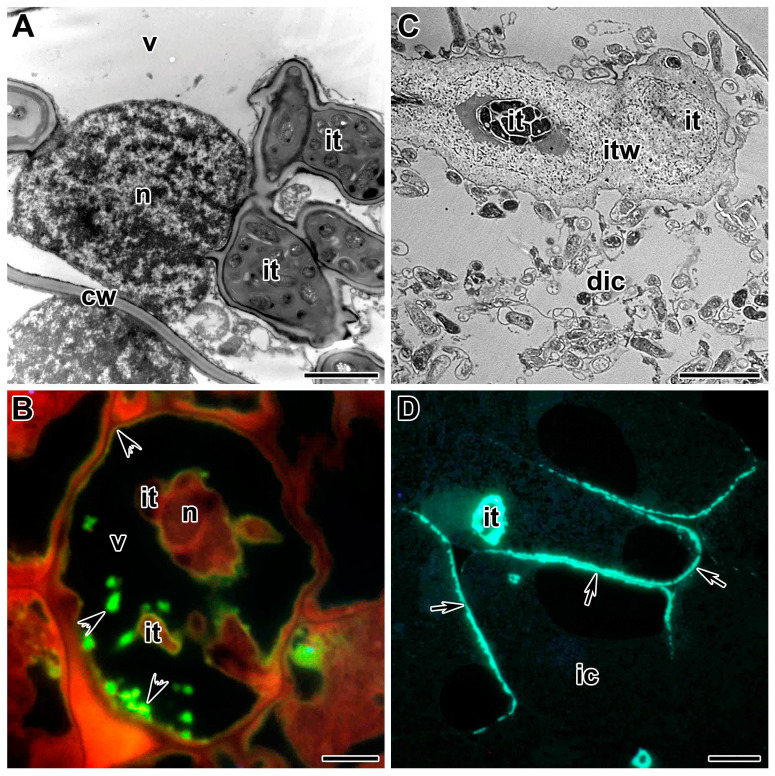
Infection threads in the symbiotic nodules of Pisum sativum. (A) Intracellular infection thread in the nodules of wild-type line SGE. (B) Intercellular infection thread in the nodules of wild-type line SGE. (C) Localization of the infection thread in close proximity to the nucleus in the nodules of mutant line SGEFix–-1 (Pssym40-1). Low-temperature embedding in LR White and transmission electron microscopy. n—nucleus, v—vacuole, cw—cell wall, it—infection thread, ba—bacteroid. Bar (A) = 1 µm, (C) = 2 µm, (B) = 5 µm.
It has also been suggested that the growth and biophysical properties of the infection thread are associated with a transition of the thread matrix from a fluid to a solid-state [17]. Such a transition might result from peroxide-driven cross-linking of tyrosine residues in a composite matrix glycoprotein termed arabinogalactan protein-extensins (AGPEs) [17,142,143,144,145].
Thus, the growth and development of the infection thread are controlled by the host plant with a constant signal exchange between symbionts. Nod factors and low molecular mass exopolysaccharides [146] have been proposed as such signaling molecules. Rhizobia continue to express nod genes and synthesize Nod-factor in the infection threads [147,148]. It is thought that different host cell receptor complexes are associated with the initiation of infection, progression of infection thread [86,149,150,151], and even for bacterial release [152].
Extracellular polysaccharides (EPS) can act as signaling molecules that promote the development of infection threads. Indeed, L. japonicus was found to have an EPS receptor 3 (LjEPR3) that recognize EPS [153]. Its function is apparently to control the rate of bacterial infection during the growth of an infection thread, both in the epidermis and in subsequent layers of root tissue towards the nodule primordium [154]. Direct binding of EPS to the receptor has been demonstrated [155]. In M. truncatula LysM domain-containing receptor-like kinase (MtLYK10), an ortholog of LjERP3 was identified; however, MtLYK10 is not involved in EPS recognition (at least in recognition of succinoglycan), but it is required for infection thread growth [156]. In legumes that form determinate nodules (such as soybean and L. japonium), the production of EPS by rhizobia is required for the curling of root hairs, correct formation of infection threads, bacterial release, differentiation of bacteroids, and effective nodulation [25,157,158].
In legumes that form nodules with indeterminate meristems, capsular polysaccharides (KPS) may also play a role in the infection process [159,160]. It appears to stimulate the initiation and development of the infection thread [161]. Bacterial proteins may also play a role in the growth and development of the infection thread. For example, RosR encodes a protein belonging to the Ros/MucR family of rhizobial transcriptional regulators, and the mutant rosR is impaired in development of infection threads, bacterial release, and differentiation of bacteroids [162].
Many components of the host plant have also been shown to play a role. These include enzymes such as the E3 ubiquitin ligase in L. japonicus [87], the putative E3 ubiquitin ligase with the Nodule specific RING finger domain (LjnsRING) in L. japonicus [163], the E3 ubiquitin ligase with ‘zinc finger’ type domain, Seven in absentia (MtSINA) in M. truncatula [164] and Cystathionine-β-synthase-like1 (MtCBS1) in M. truncatula [165]. Recently, using pea gibberellin-deficient and della-deficient mutants, it was shown that the phytohormone gibberellin suppresses the formation of infection threads [166], and its amount in infection threads is much lower than in bacteroids [167], on the contrary, phytohormones cytokinins and auxins play an important role in the development and propagation of infection threads, as well as in the release of bacteria from infection droplets [132,168,169,170]. A putative role of ethylene in infection thread maturation was also suggested [171]. In P. vulgaris, a small heat shock protein Nodulin 22 (PvNod22) was implicated in nodule development. Its function is associated with the expansion of the infection thread, probably due to the maintenance of protein homeostasis in the ER, since the lack of this protein leads to overloading of ER’s capacity for protein folding [172].
The protein Symbiotic remorin 1 (MtSYMREM1), flotillins MtFLOT2 and MtFLOT4 [88] present in specific microdomains of the infection thread membrane are involved in the regulation of polar growth in the infection thread [173] and possibly interact with MtLYK3 [32]. For M. truncatula, it has been shown that vapyrin (MtVPY), putative E3 ligase Lumpy infections (MtLIN), and cytoplasmic exocyst subunit EXO70H4 are part of a symbiosis-specific mechanism required for polar growth of infection threads [21]. Recently, it was shown that LjCerberus stabilizes LjVPY1 and LjVPY2 into trans-Golgi network/early endosome vesicles [174]. In addition, the Rhizobium-directed polar growth (MtRPG) gene is involved in the spatial subcellular reorganization in M. truncatula, encoding a protein belonging to the family of plant-specific proteins with a specific RPG-related proteins (RRP) domain and coiled-coil domain [175]. Infection thread growth also involves the action of small GTPases MtROP6 and MtROP10 [81], and monomeric GTPase RabA2 in P. vulgaris [176]. Recently, it was demonstrated that MtROP6 is activated with SPIKE 1 (LjSPK1), a DOCK family guanine nucleotide exchange factor (GEF), and that their interaction is necessary for polar infection thread growth [177]. Transcription factors of the APETALA 2/ethylene-responsive element binding factor (AP2/ERF) family in L. japonicus are also important for infection thread growth [178].
The growth and development of the infection thread are accompanied by the movement of the nucleus and the rearrangement of cytoskeletal elements. The nucleus is apparently an active participant in the infection process (Figure 1C). Accompanied by a significant pool of cytoplasm with various organelles, the nucleus moves to the site of contact with the penetrating agent [179], be it pathogen or symbiotic partner. Through this repositioning of the nucleus, signal transduction pathways can perhaps activate gene expression more effectively [180].
Microtubules form a dense cytoplasmic network surrounding the growing infection thread [134,147,181]. This network controls polar growth and serves as a template for the formation of an infection thread. The role of actin filaments is suggested by the presence of a panel of mutants showing the impaired organization of the actin cytoskeleton and impaired polar growth of infection threads. L. japonius mutants Ljarpc1 [116], 121F-specific p53 inducible RNA (Ljpir1) and nck-associated protein 1 (Ljnap1) [76], as well as the M. truncatula mutant Mtrit–1 (ortholog Ljnap1) [117] were characterized by a decreased number of infection threads and their disintegration. This led to the formation of ‘empty’ nodules, into which the infection threads did not penetrate [116]. L. japonicus SCAR-Nodulation (LjSCARN) encodes another component of the SCAR/WAVE complex [182]. Ljscarn mutants were blocked at the stage of initiation of infection thread growth. In contrast to the Ljarpc1, Ljnap1, and Ljpir1 mutants, in the Ljscarn mutants, the organization of actin cytoskeleton was not impaired at the early stages of nodule development. LjSCARN is likely to function at later stages of the actin cytoskeleton reorganization during the development of an infection thread [182].
The endoplasmic reticulum and Golgi apparatus (GA) direct material to the active sites of biosynthesis and remodeling of the infection thread wall [138,183,184,185]. Moreover, the secretion of the components apparently proceeds in two different ways, depending on the stage of cell infection. In a young cell, the route is: ER → Golgi (packaging) → exit → wall; whereas, in a differentiated cell, the route is: ER → vesicle formation → wall. The smooth endoplasmic reticulum tends to be adjacent to the cell wall [183,185]. The GA also has at least two different export pathways, one for pectin-containing vesicles and the other for vesicles containing extracellular membrane and matrix components. Examples include the membrane glycoprotein antigen identified by the antibody MAC207 and the matrix AGPE identified by MAC265 [186]. Vesicles moving out of the Golgi also contain xyloglucan precursors.
5.1. Infection Thread Wall
The structure and development of infection threads in the root cortex and in the infected nodule tissue have been extensively studied using microscopic techniques [138]. Having crossed the host cell cytoplasm, the tip of the infection thread fuses with the mother cell wall at the site of exit. Penetration into the adjacent cell involves the local degradation of its cell wall and the re-initiation of a new infection thread [187,188]. This repetitive cell-autonomous process facilitates the overall process of tissue invasion by Rhizobium [189]. Infection threads are intracellular and transcellular when they cross plant cells (Figure 1A) and intercellular, when they pass between cells, in which case the plant cell walls effectively serve as the boundary of the intercellular infection thread (Figure 1B).
Intensive genetic studies in various legumes have led to the identification of mutants with defects in the growth and development of infection threads: for L. japonicus [87,92,115]; for M. truncatula [164,190,191,192]; and for P. sativum [166,193,194,195,196]. By using these mutants in combination with monoclonal antibodies and other probes that react with components of the plant-rhizobial interface, it has become possible to analyze surface interactions between symbiotic partners in infection threads (Table 1).
Table 1.
List of molecular probes (antibody, cytochemical reagent, and enzyme) used to detect infection thread cell wall and matrix components.
| Probe a | Component b | Epitope Recognized c | References |
|---|---|---|---|
| Antibody | |||
| JIM5 | low methyl-esterified HG | α-MeGalA(2)-(1→4)-α-GalA(3)- (1→4)-α-MeGalA |
[186,197,198,199,200,201,202,203,204] |
| JIM7 | high methyl-esterified HG | α-GalA-(1→4)-α- MeGalA(4)-(1→4)-α-GalA |
[197,202,203,204] |
| LM19 | low methyl-esterified HG | α-GalA-(1→4)(4) | [205] |
| LM20 | high methyl-esterified HG | α-MeGalA-(1→4)(4) | [205] |
| 2F4 | calcium cross-linked HG | dimer of α-MeGalA-(1→4)(9) and Ca2+(5) | [202] |
| LM5 | (1→4)-β-D-galactan (RG-I) | β-Gal-(1→4)(3) | [203,204] |
| anti-RG- II | monomeric and dimeric RG-II | unknown | [199,200,205,206] |
| anti- XyG | XyG | unknown | [197,207] |
| anti-callose | callose ((1→3)-β-D-glucan) | β-Glc-(1→3)(5) | [205] |
| MAC265 | 95kDa AGPE | unknown | [141,186,197,205,208,209,210,211] |
| MAC204 | 95kDa AGPE | unknown | [186,212,213] |
| MAC236 | 95kDa AGPE | unknown | [186,213] |
| JIM13 | AGP | unknown | [208] |
| anti-HRGP | hydroxyproline-rich glycoproteins (HPGPs) | unknown | [214] |
| anti-ENOD2 | early nodulin2/hydroxyproline-rich glycoproteins (ENOD2/HPGPs) | unknown | [215,216] |
| anti-VAMP721d/VAMP721e | vesicle-associated membrane proteins (VAMPs) | peptide QKLPSTNNKFTYNC | [205] |
| anti- EGL1 | endo-β-1,4-glucanase | peptide CYFPKRIHHRGSSLP | [217] |
| anti-LOX | lipoxygenase (LOX) | unknown | [218] |
| anti-SOD | superoxide dismutase (SOD) | unknown | [219] |
| anti-DAO | diamine oxidase (DAO) | unknown | [220] |
| Cytochemical reagent | |||
| chlor-zinc-iodide | cellulose | na | [15] |
| I2KI (I2, KI, H2SO4) | cellulose | na | [15] |
| cerium chloride (CeCl4) | H2O2 | na | [219,220,221,222,223] |
| ruthenium red | unesterified HG | na | [202,204] |
| aniline blue | callose ((1→3)-β-D-glucan) | na | [201,204] |
| neutral red | suberin | na | [201,204] |
| I2KI (I2, KI, H2SO4) | suberin | na | [204] |
| Protein | |||
| CBH-I | cellulose | na | [197] |
a JIM, John Innes Monoclonal, LM, Leeds Monoclonal, MAC, Monoclonal Antibody Centre (Babraham), RG-II. Rhamnogalacturonan-II, CBH-I. Cellobiohydrolase-I. b XyG. Xyloglucan, HG. Homogalacturonan, RG-I. Rhamnogalacturonan-I. c Gal, galactose, GalA, galacturonic acid, MeGalA, 6-O-methyl-galacturonate, na. not applicable.
5.1.1. Enzymes Involved in the Growth of the Infection Thread
The growth of an infection thread involves a range of enzymes both for the synthesis of the infection thread wall and for the local degradation of the host cell wall when the infection thread passes through it. There are two possible mechanisms for this process. The first possibility is that bacterial enzymes degrade the cell wall, thus allowing bacteria to penetrate into plant cells [54,106,107]. In R. etli, forming nodules on P. vulgaris, the gene HrpW was isolated, encoding a component of the Type III secretory system. It exhibited pectate lyase activity and may be involved in the degradation of the cell wall during infection thread development [224].
A second possible mechanism for cell wall degradation involves plant enzymes. Cell wall degrading enzymes are produced in nodule cells in response to rhizobial infection and are possibly induced by Nod factors [188]. In M. sativa, the polygalacturonase gene (MsPG3) is specifically expressed during symbiosis [110]. Another degradative enzyme is Nodulation pectate lyase (LjNPL) [112]. The identified pectate lyase is probably only one of several proteins associated with the initiation and growth of an infection thread. In addition, PMEs are apparently involved in the modification of the pectin matrix of the infection thread wall [106]. Thus, it was described in nodules on the adventitious roots of Sesbania rostrata Bremek. and Oberm. after inoculation with Azorhizobium caulinodans [225]. In M. truncatula, after inoculation with S. meliloti, the expression of symbiotic Pectin methylesterase (MtPER) was identified [226]. In pea nodules, the participation of endo-β-1,4-glucanases in the maturation of the infection thread and cell walls was shown [217].
5.1.2. Polysaccharides and Proteins of the Infection Thread Wall
The infection thread wall is an extension of the host cell wall. It includes esterified and de- esterified homogalacturonan (HG), substituted pectins, xyloglucans and cellulose microfibrils [15,197,207] as well as extensin. In the nodules of P. vulgaris and M. truncatula, the presence of proline- and hydroxyproline-rich glycoproteins has been demonstrated [214,227]. In addition, rhizobial infection has been shown to modulate the gene expression for extensins and expansins, both structural proteins of the cell wall [228,229,230]. In M. truncatula, in response to infection, genes of early nodulins, such as Early nodulin 5 (ENOD5), Early nodulin 12 (ENOD12), Early nodulin 16/20 (ENOD16/20), which are proline- and hydroxyproline-rich proteins, begin to be expressed in cells with actively growing infection threads [231]. The involvement of ENOD5 at the late stages of infection was demonstrated using a large set of symbiotic pea mutants blocked at different stages of infection [232].
The pectins of the infection thread wall are diverse [233], and their detailed localization has been examined by immunocytochemical microscopy (Figure 2). The distribution of HG was studied in various legumes. Infection thread walls were shown to have a high content of low methyl-esterified HG in P. sativum, M. truncatula, Vicia hirsuta (L.) Gray, and P. vulgaris, (Figure 2A,B) [186,197,203,216]. There was also evidence for high methyl-esterified HG (Figure 2C,D) [197,203]. When vesicular transport was impaired in M. truncatula and G. max in nodules with partially silenced VAMP721d and VAMP721e, large clusters of bacteria were found immersed in a matrix of high and low methyl-esterified HG, surrounded by a membrane [205,234]. At the same time, GmVAMP721d partially co-localized with pectate lyase, and abnormal endocytosis of low methyl-esterified HG was observed [205].
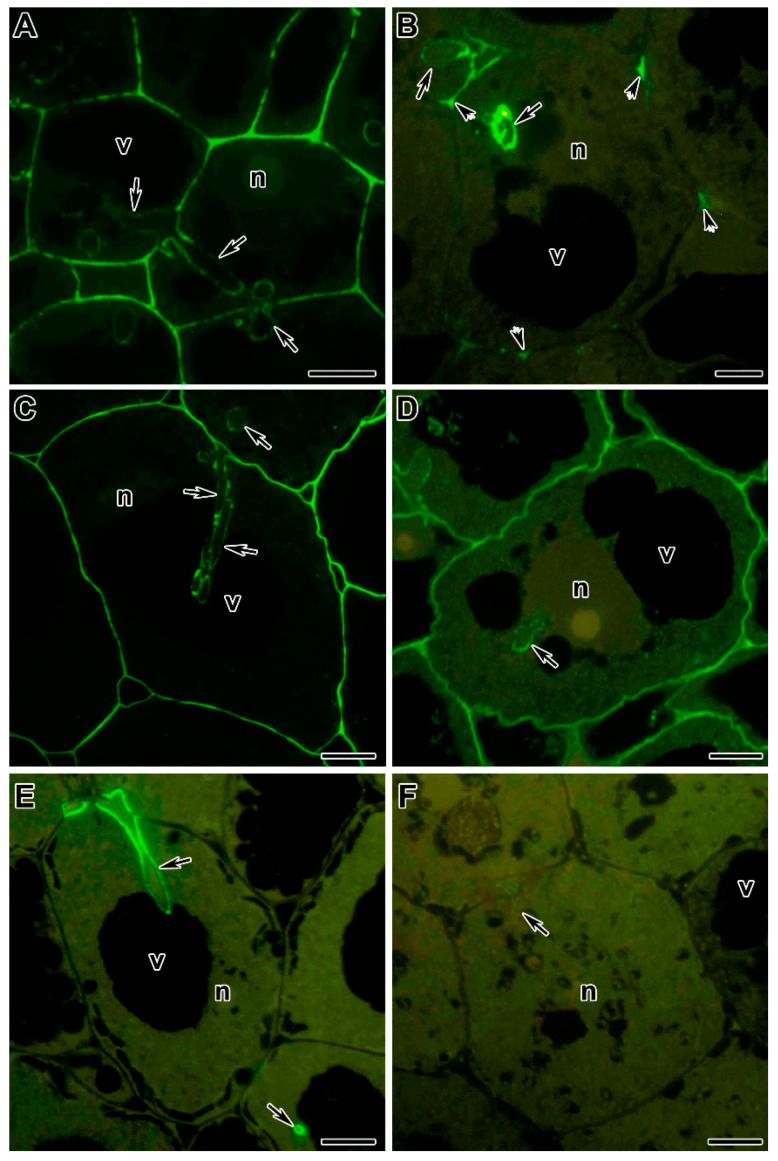
Figure 2.
Pectins in the infection thread walls in the symbiotic nodules of Pisum sativum and Medicago truncatula. (A,B) Low methyl-esterified homogalacturonan (HG) labelled with JIM5. (C,D) High methyl-esterified HG labelled with JIM7. (E,F) (1→4)-β-D-galactan sidechain of rhamnogalacturonan I labelled with LM5. (A,C,E) Nodules of the wild-type line SGE of P. sativum. (B,D,F) Nodules of the wild-type line A-17 of M. truncatula. n—nucleus, v—vacuole. Arrows indicate infection threads, arrowheads indicate ‘three-way’ junctions. Low temperature embedding in LR White, semi-thin sections (0.5 µm), fluorescent immunolocalization. Bars = 5 µm.
Mutants of P. sativum and M. truncatula with defects in infection thread development have been investigated for possible changes in the composition of HG in infection thread walls. Mutants in the gene PsSym33 and the orthologous gene MtIPD3, characterized by ‘locked’ infection threads, showed a strong accumulation of low methyl-esterified HG in the infection thread walls [201,203]. By contrast, in symbiotically-defective mutants without abnormalities in infection thread development, the distribution of low methyl-esterified HG did not differ from that in wild-type plants [203].
Rhamnogalacturonan I (RG-I) consists of alternating (1,2)-linked α-L-rhamnose residues and (1,4)-linked α-D-galacturonic acid residues. Its role in the development of the legume-rhizobial symbiosis has not been studied until recently [203]. Using the LM5 antibody raised against galactan side chain epitope, it was shown to be present in infection thread walls in P. sativum nodules but was not detected M. truncatula nodules (Figure 2E,F). At the same time, in the pea mutant SGEFix−-2 (Pssym33-3), the LM5 epitope was absent from the walls of some infection threads [203].
Rhamnogalacturonan II (RG-II) is structurally the most complex but also the most conserved pectin polysaccharide. RG-II macromolecules are self-conjugated as dimers through a diester bond with boron [235]. When studying the effect of boron on nodulation, the localization of RG-II at the interface between the plasma membrane and the cell wall was shown [199]. However, in plants deficient in boron, RG-II was evenly distributed over the entire thickness of the cell wall [199]. Later, it was shown that RG-II can form a complex with AGPEs and was localized in the infection thread matrix [200,206].
5.2. Infection Thread Matrix
Production and secretion of plant extracellular matrix material are stimulated in response to rhizobial infection. Most of these compounds accumulate in the lumen of the infection thread. According to some estimates, for P. sativum and other legumes that form indeterminate nodules, the volume of matrix material in the infection thread lumen is approximately five times the volume of rhizobial cells [236].
The main components of the matrix within the infection thread lumen are plant glycoproteins, basically similar to those of the extracellular matrix. AGPs are found widely in plants, but legume nodules contain a tissue-specific set of AGPs. This class of hydroxyproline-rich glycoproteins is found in infected tissues of symbiotic nodules of legumes, in actinorhizal symbiosis, and in arbuscular mycorrhiza [17,237,238]. AGPs apparently play a significant role in the infection process, most likely in the symbiotic interface [237,238,239].
Legumes are apparently unique in their ability to synthesize a complex copolymer that contains alternating AGP and extensin motifs [17,141,144,238]. AGPE molecules appear to combine the biophysical properties of soluble gums (characteristic of AGPs) with the more structural properties of extensins (which usually serve to strengthen plant cell walls). The high content of tyrosine residues in AGPEs suggests the possibility of cross-linking of these molecules with H2O2 and Prx-III, as with the extensin network in many plant cells [144,145]. This cross-linking may serve to regulate the growth of the infection thread itself [17,142,206,240].
AGPE, with an apparent molecular weight of 95 kDa or larger, was identified in extracts from symbiotic nodules using three monoclonal antibodies MAC204, MAC236, and MAC265. These antibodies apparently recognize different epitopes on the same group of glycoprotein macromolecules [186,212]. The two epitopes recognized by MAC236 and MAC265 were mutually exclusive as seen from isoelectric focusing, while MAC204 recognized a periodate-sensitive epitope common to both the acidic and neutral forms. AGPEs were immunolocalized to the infection thread matrix. Although its abundance is increased in nodule tissue extracts, it is not a classic nodulin [241]. It is accumulated in uninfected root tissue, in particular in intercellular spaces bounded by three or more cells [186,207,208,212,242]. In addition, AGPE recognized by MAC265 was also found in the intercellular spaces of pseudo-nodules induced by the LPS-defective mutant of R. leguminosarum [208].
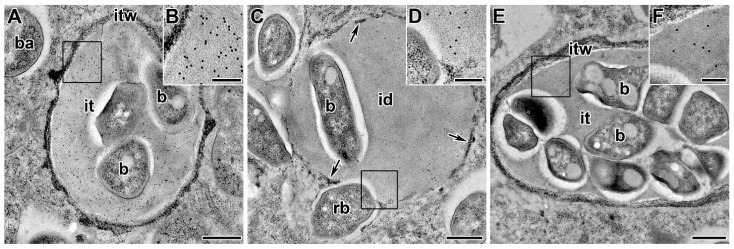
The exact function of AGPEs in the growth of infection threads is unknown. Immunocytological analysis showed that AGPE recognized by MAC265, is localized in the infection pocket of curled root hairs, in young infection threads in the infection zone, and in mature infection threads in the nitrogen fixation zone of symbiotic nodules of P. sativum (Figure 3A), V. sativa, P. vulgaris [141,186,197,210,211]. A similar distribution of AGPE epitopes is observed using MAC204 and MAC236 in P. sativum (Figure 3C–F) [186,213]. However, in pea mutants with abnormalities in infection thread development and evidence of bacterial degradation in the lumen, the MAC265 and MAC236 epitopes were observed in excessive amounts in the intercellular spaces of the infected nodule tissue. There was also accumulation of the MAC204 epitope in the cell wall, perhaps as a result of abnormal infection thread development [211,213]. These studies indicate that the nature of AGPE macromolecules may be subject to change during the infection process [213].
Figure 3.
Arabinogalactan protein-extensins (AGPEs) in the infection thread matrix in the symbiotic nodules of Pisum sativum. (A) AGPE labeled with MAC265. (B) High magnification of the boxed area in (A). (C) AGPE labeled with MAC204. (D) High magnification of the boxed area in (C). (E) AGPE labeled with MAC236. (F) High magnification of the boxed area in (E). Low-temperature embedding in LR White, immunogold localization, transmission electron microscopy. id—infection droplet, it—infection thread, itw—infection thread wall, b—bacterium, rb—released bacterium, ba—bacteroid. Arrows indicate remnants of the infection thread wall. Bars = 500 nm.
Borate is an essential micronutrient for legume nodule development. An effect of boron deficiency on the distribution of matrix AGPE in P. sativum nodules has been demonstrated [209]. In P. sativum plants, the RG-II complex with boron and AGPE was observed in the infection thread matrix, while the rhizobial cells were separated from the matrix by an exopolysaccharide capsule. In nodules of plants deficient in boron, the complex of AGPE with RG-II was strongly associated with the surface of rhizobia in the infection thread lumen [206].
5.3. ROS and NO
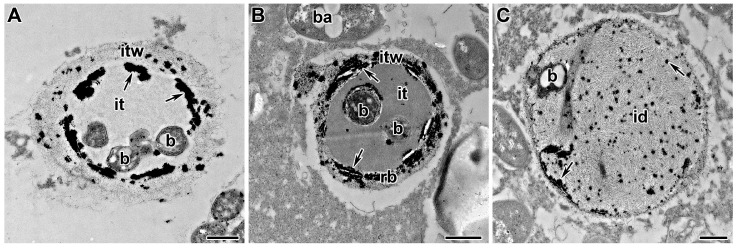
Reactive oxygen species (ROS) are produced during infection by rhizobia [243]. Rbohs have been identified and characterized in legume genomes [123,244,245]. It has been shown that RbohA and RbohB can play a key role in the successful colonization of rhizobia and the correct growth and shape of infection threads, apparently because they stimulate ROS production [244,245]. Prx-III, as well as rhizobial catalases [222], affect the rigidity of the infection thread wall and matrix [142]. During infection, the production of superoxide anion (O2-) and H2O2 were localized in infection threads and infected cells [219,221]. At the same time, it is possible to trace the accumulation of H2O2, first on the inner surface of the infection thread wall (Figure 4A), then throughout the entire thickness (Figure 4B), and then inside the infection thread matrix (Figure 4C), possibly promoting its hardening as a result of crosslinking of tyrosine residues of AGPE molecules [17,144]. Thus, it was suggested that the role of H2O2 during extension of the infection thread is associated with the rigidity of the infection thread [125,141,145,246] or with the signaling role of H2O2 for the regulation of symbiotic function [125].
Figure 4.
Sequential steps of hardening of the infection thread wall and matrix during development and growth in the wild-type nodules of Pisum sativum. (A) Localization of H2O2 in the inner side of infection thread wall. Incomplete hardening of the wall of the infection thread makes it possible to form an infection droplet and release the Rhizobium into the plant cell. (B) Localization of H2O2 across the entire infection thread wall thickness. Complete hardening of the infection thread wall prevents the formation of an infection droplet and the release of bacteria. (C) Localization of H2O2 inside infection droplet matrix. Hardening of the infection droplet prevents the growth and division of bacteria inside the lumen. Cytochemical localization of H2O2 as electron-dense precipitate formed in the presence of cerium chloride. id—infection droplet, it—infection thread, itw—infection thread wall, b—bacterium. Arrows indicate electron-dense precipitates. Bars = 500 nm.
In addition to Rbohs, there are several other potential sources of reactive oxygen species. Diamine oxidase (DAO) is an important source of hydrogen peroxide in intercellular spaces in legume tissues, both in intact plants and in plants exposed to various stresses [142,220]. Peroxide distribution and DAO activity in nodules have been demonstrated in plant cell walls, intercellular spaces, and infection threads. Similarly, in symbiotic nodules of P. sativum the localization of lipoxygenase (LOX) in the infection thread matrix was demonstrated [218]. This enzyme is involved in lipid peroxidation, and its accumulation may indicate a hypersensitivity reaction that develops in response to rhizobial infection. However, EPS-I of rhizobia can reduce the effects of H2O2 on bacteria [247]. Nitric oxide production was also observed along growing infection threads and in nodule primordia [129].
5.4. Defense Reactions
Bacterial colonization triggers non-specific plant defense responses [248,249]. Mutants of S. meliloti, R. leguminosarum bv. trifolii, R. leguminosarum bv. viciae, B. japonicum, and A. caulinodans, unable to produce EPS, induce defense reactions in their respective hosts M. sativa, Trifolium sp., V. sativa sp. nigra, G. max and S. rostrata [42,250]. In such cases, later colonization and histochemical changes in the cortical cell walls of the pseudo-nodule are observed. They are abnormally thickened, encrusted with autofluorescent phenolic compounds, and contain callose [251,252]. In addition, phytoalexins (glyceolin) accumulate in pseudo-nodules, peroxidase activity is increased, as well as the levels of phenylalanine ammonium lyase, 3-O-methylesterase, and isoflavone reductase transcripts, which indicates the occurrence of typical defense reactions [248]. It is likely that EPS produced at an early stage of infection is necessary as a diffusion barrier protecting bacteria from toxic H2O2 generated in plant cells [42,253,254].
Allelic mutants of lateral root organ-defective (latd) [255] and numerous infections and polyphenolics (nip) were identified in M. truncatula with defects in the architecture of the root and nodules [191,192]. NIP/LATD gene encodes a putative nitrate transporter [256]. In latd and nip mutants, the accumulation of polyphenolic compounds and abortion of infection were observed either at the stage of propagation of the infection thread or during the release of rhizobia into the cytoplasm of plant cells [256]. A similar phenotype was also observed for TE7 mutant [190] in the MtIPD3 gene [257].
Later, it was found that, for pea mutants in the Pssym33 gene (ortholog to MtIPD3), the deposition of suberin was observed in the infection thread walls (Figure 5A,B) [201]. In addition, in this mutant, suberin was present in the cell walls of colonized cells. It was also found in the infection thread walls and around the vacuole of infected cells (Figure 5B) in the nodules of the weak allele SGEFix–-2 (Pssym33-3) [204]. In some infection threads of this pea mutant, an electron-dense matrix was also observed when labeled with the LM5 antibody, recognizing the galactan side chain of RG-I [204]. There was also deposition of de-esterified HG in the infection thread walls and an increase in the expression level of a gene encoding peroxidase 7RA84 [201]. The deposition of cell wall material inside the vacuole and the formation of a pectin gel in the infection thread matrix is another manifestation of the host plant’s defense reaction and the perception of rhizobia as pathogens.
Figure 5.
Defense reactions in the nodules of symbiotic Pisum sativum mutants. (A) Transmission electron micrograph of abnormal infection threads in the nodule of mutant SGEFix–-2 (Pssym33-3). (B) Suberin depositions in the infection thread walls and around vacuole in the nodule of mutant SGEFix–-2 (Pssym33-3), which is characterized with ‘locked’ infection threads. Neutral red staining for detection of suberin. (C) Transmission electron micrograph of an abnormal infection thread in the nodule of mutant RisFixV (Pssym42). (D) Callose (β-1,3-glucan) depositions in the infection thread wall and cell wall of infected cells in the nodule of mutant RisFixV (Pssym42), which is characterized with abnormal infection threads and early senescence of symbiotic structures. Callose depositions detected by staining with Aniline blue. ic—infected cell, dic—degenerated infected cell, n—nucleus, v—vacuole, cw—cell wall, it—infection thread, itw—infection thread wall. Arrows indicate callose depositions in cell walls of infected cells, arrowheads indicate suberin depositions in the vacuole. Bars (A) = 2 µm, (B–D) = 5 µm.
The deposition of callose (β-1,3-glucan) in plant cell walls is an important aspect of many processes associated with developmental physiology, pathogenesis, or stress. In the P. sativum symbiotically-defective mutant RisFixV (Pssym42), callose deposition (Figure 5C,D) was associated with Rhizobium infection as part of the defense response. Another striking feature of RisFixV (Pssym42) was the encapsulation of ineffective bacteroids with de-esterified HG, as detected by JIM5 immunolabelling. This mutant demonstrates unique defense reactions for symbiotic mutants [201].
6. Release of Bacteria from Infection Threads
The process of tissue and cellular infection is accompanied by the differentiation of plant cells originating from the apical meristem. The differentiation of host cells in the nodule cortex is associated with the release of bacteria into the plant cell as organelle-like structures termed symbiosomes, which are still bounded by a plant membrane that is structurally equivalent to the plasma membrane [258].
The exact mechanism triggering the transition of rhizobia from the extracellular space (apoplast) to intracellular existence within organelle-like symbiosomes surrounded by a plant membrane is not yet known, but it is associated with further remodeling of the host cell wall and cell membrane. In indeterminate nodules of temperate legumes, the release of rhizobia occurs from infection droplets that lack a covering of cell wall material [17,236,259]. These droplets are confined by a membrane that is an extension of the plant plasma membrane [138]. They contain a matrix similar in composition to the matrix of the infection thread. This includes AGPEs, which is recognized by the monoclonal antibodies MAC265, MAC236, and MAC204 (Figure 3B) [197,211,213].
Sometimes, for example, in Phaseolus sp., bacterial release occurs at the tips of short intracellular infection threads [260]. A study of the tips of infection threads in the cytoplasm of host cells of Lupinus angustifolius L. showed that rhizobia bud off from infection threads and are enclosed in membranes of plant origin [261]. The released bacterial cells eventually stop dividing and differentiate into an endosymbiotic, nitrogen-fixing form (bacteroids). In nodules of some legumes, differentiation of bacteroids occurs as a result of the action of antimicrobial nodule cysteine-rich (NCR) peptides [262].
Presumably, the release of bacteria occurs due to the absence of a cell wall in the infection droplet and the possibility of close contact between the plasma membranes of the plant and rhizobia [17,197,263]. The physical interaction of isolated symbiosomal and bacterial membranes has been demonstrated in vitro [264]; moreover, when rhizobia enter the cytoplasm of plant cells, they lose their exopolysaccharide capsule (Figure 3B) [140]. Many components associated with symbiosomal and plasma membranes may play a direct role in surface interactions with rhizobia [265].
On the part of rhizobia, the gene BacA controls the modification of the bacterial cell wall, including the development of Lipid -A derivatives with long-chain fatty acids [266]. During bacteroid development, modifications to Lipid-A and O-antigen sidechains cause rhizobial LPSs to become more hydrophobic [267,268], which may facilitate interaction with plant membrane glycoproteins [239]. Evidence that the O-antigen of LPS plays an important role in this process comes from the observation that rhizobial mutants defective in its production are not able to release from infection droplets [269].
7. Nodule Senescence and Release of Bacteria
Senescence of nodule tissue is the final stage of symbiosis. There is autolysis of infected cells, and the nutrients stored in the nodules are recycled by the host plant [270]. Proximal to the senescence zone, a network of intercellular infection threads develops among degenerating cells [271,272]. Rhizobia multiply as saprophytic organisms, thus enhancing the population of bacteria that can be released into the soil [271]. A similar infection network is observed during ineffective symbiotic associations leading to early senescence [273,274]. Infection threads in the saprophytic zone sometimes form large infection droplets filled with a matrix of unknown nature [272].
8. Environmental Influences
The extent of nodulation and the efficiency of symbiotic nitrogen fixation can be influenced by environmental conditions, such as temperature, humidity, aeration, pH, salinity, soil structure, imbalance of nutrients in the soil (for example, nitrogen, phosphorus, calcium, boron, potassium, and magnesium). Other factors affecting nodulation include diseases and insects, as well as anthropogenic influences in the form of fertilization, soil and water pollution with pesticides, chemicals, and heavy metal ions [275,276]. Environmental stresses often have diverse effects: For example, pesticides can improve plant viability by suppressing pests, or they can have a direct toxic effect on nodule metabolism [277]. The stress sensitivity of the legume-rhizobial symbiosis has been extensively studied, although the growth dynamics of an infection thread under abiotic stress are not easy to analyze. It should be noted that stress sensitivity can sometimes be reduced by inoculation with strains of rhizobia resistant to various stresses [278,279,280].
9. Conclusions and Perspectives
Most plants have two types of tip-growing cells: Pollen tubes and root hairs. Infection threads represent a third type of tip growth for the deposition of cell wall material. The infection thread is an inwardly growing tube in which polar (apical) growth is topologically inverted relative to the tip growth of root hairs or pollen tubes. The following model (Figure 6) summarizes basic concepts of infection thread growth.
Figure 6.
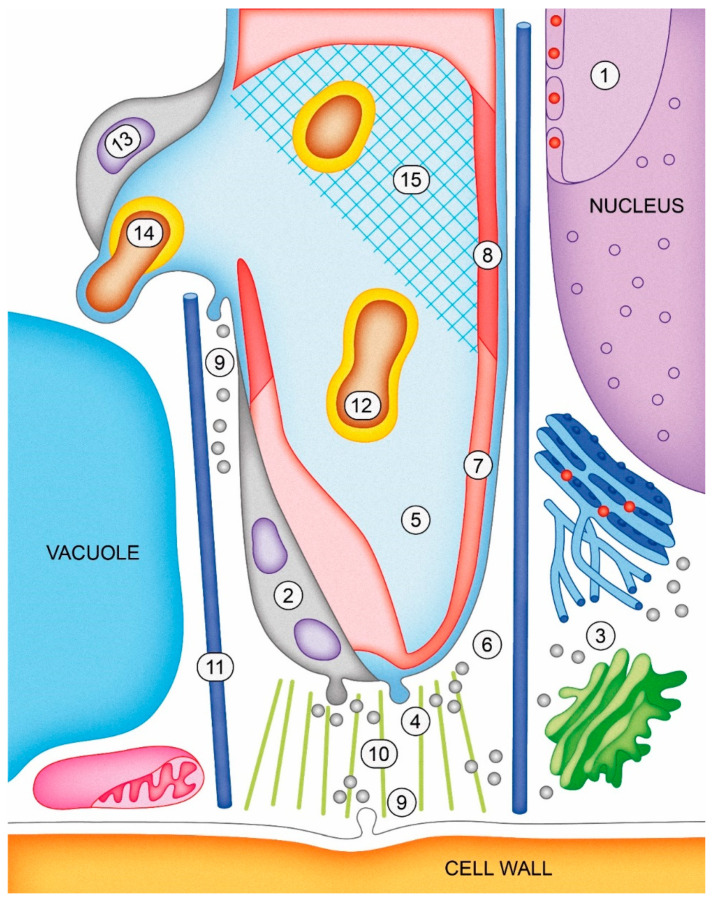
Structure and development of the legume-rhizobial symbiotic interface in infection threads (see text for details). (1) Transcription factors modify the course of host cell development. (2) Plant proteins and glycoproteins are localized in the microdomains of the plasma membrane of the infection thread. (3 and 4) Vesicles derived from the endoplasmic reticulum and Golgi apparatus are directed to the plasma membrane at the tip of the infection thread, releasing their contents into the wall and extracellular matrix. This secretory mechanism is the driving force for the growth of the infection thread, coupled with the growth of bacterial cells within the luminal matrix. (5) Glycoproteins, proteins, and polysaccharides influence the biophysical properties of the infection thread wall and luminal matrix. (6) Cell wall proteins and polysaccharides are transferred in vesicles from the Golgi apparatus. (7) In the nascent wall of the infection thread, there is a layer of α-cellulose, and HG is predominantly in the highly methyl-esterified form. (8) The mature wall of the infection thread contains cellulose, xyloglucan, and HG with decreasing degrees of methyl-esterification. This creates a more rigid structure reinforced by bridging with Ca2+ ions. Also present is RG-I and RG-II (in the form of dimers with the borate ion), extensins, AGPs, and expansins. (9) During the development of the infection droplet, enzymes are involved in the modification and degradation of the cell wall. (10) The actin cytoskeleton is involved in the organization of polar growth. (11) The microtubular cytoskeleton forms a tunnel for orientation. (12) Polysaccharides and proteins of the bacterial cell wall and capsule play an important role in the progression of the infection thread. (13) The symbiosome membrane contains a new range of proteins associated with nitrogen-fixing endosymbiosis. (14) Within the symbiosomes, rhizobial cells lose their exopolysaccharide capsule, and the structure of LPS is modified. (15) H2O2 plays a role in cross-linking of AGPEs and hardening of the infection thread matrix. HG, homogalacturonan; RG-I, rhamnogalacturonan I; RG-II, rhamnogalacturonan II. Green lines are actin microfilaments; blue lines are microtubules;  —Ca2+; light blue extracellular matrix is fluid; shaded blue matrix is solid; pink cell wall is newly synthesized; red cell wall is mature or modified. Objects are not scaled.
—Ca2+; light blue extracellular matrix is fluid; shaded blue matrix is solid; pink cell wall is newly synthesized; red cell wall is mature or modified. Objects are not scaled.
The initiation and growth of the infection thread are the consequence of signal exchange with the infecting Rhizobium bacteria and altered transcriptional activity in the host cell nucleus. Among other things, this leads to the synthesis and deposition of new proteins in the microdomains of the plasma membrane of the infection thread. Vesicles derived from the ER and GA fuse with the plasma membrane at the tip of the infection thread, releasing their contents into the wall and extracellular matrix. This process is apparently controlled by a range of proteins, including: PI3K, GmVAMP721d, EXO7OH4, PvNod22. Targeted secretion from vesicles (together with the growth and division of bacteria within the luminal matrix) is the driving force behind the growth of the infection thread. Glycoproteins, proteins, and polysaccharide components of the infection thread matrix include the following: AGPEs, HRGPs, ENOD2/11, LOX, DAO and RG-II. In addition, H2O2 probably plays a role in cross-linking AGPEs and changing the biophysical properties of the infection thread matrix. Vesicles derived from the GA also contain cellulose synthases, xyloglucan and pectins (HG, RG-I, and RG-II). HG is synthesized in a highly esterified methyl form and may be transported along with PME/PMEI complexes (pectin methylesterase/pectin methylesterase inhibitor). All these components and other cell wall remodeling enzymes are released into the apoplast.
In the nascent wall of the infection thread, cellulose synthases are incorporated into the membrane and deposit the crystalline cellulose. At this stage, highly methyl-esterified HG is the major component of the infection thread wall. At its mature stage, the main polysaccharide components are: cellulose, xyloglucan, and HG with varying degrees of methyl-esterification. HG having a low level of methyl-esterification binds with Ca2+ ions, thus increasing its rigidity. RG-I is present in the infection thread wall, and RG-II is also present in the form of dimers with the borate ion. The wall also contains extensins, AGPs, and expansins. Additionally, callose and phenolic compounds such as suberin can accumulate as part of defense reactions in response to an ineffective symbiosis.
Many enzymes are involved in the modification and degradation of the cell wall during the growth of an infection thread and the formation of an infection droplet. These include: MsPG3, LjNPL, MtPER, and endo-β-1,4-glucanases. Polar growth of the infection thread is mediated by the actin cytoskeleton, and the alignment of microtubules creates a constraining tunnel for infection thread growth. Within the lumen, bacterial polysaccharides play an important role: EPS, KPS, LPS, cyclic β-glucan, RosR, and Rhizobia-induced peroxidases (Rip1-10).
Following the release of bacterial cells from the infection droplet, several plant proteins are associated with the symbiosomal membrane. These include: Lectin-like glycoprotein (PsNLEC1), synaptotagmin (MtSyt1/2/3), syntaxin (MtSYP132), inositol-containing phospholipid (JIM18 antigen), and AGP with a GPI anchor (JIM1 antigen). Within the symbiosome compartment, rhizobial cells differentiate into nitrogen-fixing bacteroids: They lose their exopolysaccharide capsule, and the structure Lipid-A and O-antigen groups of LPS becomes modified, partly as a result of the action of the protein BacA.
From an evolutionary perspective, the nature of the legume-rhizobial symbiotic interface has become progressively more intimate and complex. On the one hand, it incorporates novel aspects of cellular morphogenesis, in particular the infection thread and the symbiosome compartments. On the other hand, there is a precise system for suppression of host defense responses. As with the other forms of tip-growth observed in root hair cells and pollen tubes, growth of the infection thread is due to the targeted deposition of cell wall and cell membrane material at the apex. However, there is an important distinction. Whereas the extension of root hairs and pollen tubes is driven by cell turgor, the driving force behind the growth of an infection thread is the synthesis and directed secretion of the extracellular matrix material into the lumen of the infection thread and the division of bacteria inside. Polar growth of the infection thread requires a high degree of coordination between many cellular and extracellular processes, including calcium dynamics, apoplastic reactive oxygen species, the cytoskeleton, and vesicular transport.
The cell wall that surrounds the infection thread is a dynamic structure that performs both structural and defense functions. While the localization and distribution of the main pectins of the infection thread wall have been recently studied [203], the role of other components of the cell wall, including numerous proteins, remains poorly understood. Many questions arise. How are internal and external processes coordinated during the growth of infection threads? What controls the progressive change in composition of the infection thread wall? What is the role of cell wall proteins? What is the role of bacterial components? How are the cell wall and matrix of the infection thread modified in response to abiotic stresses?
Experiments designed to investigate these questions will provide new insights into how an infection thread grows. Furthermore, these studies will help to elucidate the more general patterns of plant cell wall development during growth and differentiation. New probes targeting cell wall components will permit a more detailed analysis of the biochemistry and biomechanics of the cell wall of the infection thread. A major problem in studying the infection thread is that it is a dynamic and continually changing system. Its structure at an early stage in a root hair or root cortical cell may be very different from that in a host cell embedded deep in the tissue of a maturing nodule. These differences could affect the remodelling of the cell wall (Figure 6). The challenge for further research will be to use genetics, genomics, and cytological studies to integrate the many parameters involved in the development of infection threads, ranging from signaling and protein transport to deposition and remodeling of the plant cell wall.
One of the intriguing questions regarding the infection of legume tissues by Rhizobium is the relationship between intercellular and intracellular modes of infection. An interesting model has recently been developed based on interaction of L. japonicus with different strains capable of infecting the host plant either via intracellular or via intercellular modes [281]. Furthermore, the presence of infection threads in root hairs during actinorhizal symbiosis [151] and the existence of common genes controlling Rhizobium infection and endomycorrhizal symbiosis [282,283] clearly indicate the early origin of infection threads during the course of the evolution of plants.
Acknowledgments
We are very grateful to Olga Marchenko (the Komarov Botanical Institute, St. Petersburg, Russia) for her assistance with 3D-design of Figure 6. The research was performed using equipment belonging to the Core Centrum “Genomic Technologies, Proteomics and Cell Biology” in the All-Russia Research Institute for Agricultural Microbiology and the “Molecular and Cell Technologies” Research Resource Centre at Saint Petersburg State University.
Author Contributions
Writing—original draft preparation, A.V.T.; writing—review and editing, V.E.T., N.J.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ministry of Science and Higher Education of the Russian Federation in accordance with agreement № 075-15-2020-920 date 16 November 2020 on providing a grant in the form of subsidies from the Federal budget of Russian Federation. The grant was provided for state support for the creation and development of a World-class Scientific Center “Agrotechnologies for the Future”.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Liu S., Ratet P., Magne K. Nodule diversity, evolution, organogenesis and identity. In: Frendo P., Frugier F., Masson-Boivin C., editors. Advances in Botanical Research. Vol. 94. Academic Press; London, UK: San Diego, CA, USA: Waltham, MA, USA: Oxford, UK: 2020. pp. 119–148. [Google Scholar]
- 2.Forest F., Chase M.W., Eurosid I. In: The Timetree of Life. Hedges S.B., Kumar S., editors. Oxford University Press; New York, NY, USA: 2009. pp. 188–196. [Google Scholar]
- 3.Van Rhijn P., Vanderleyden J. The Rhizobium-plant symbiosis. Microbiol. Rev. 1995;59:124–142. doi: 10.1128/MR.59.1.124-142.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pawlowski K., Sirrenberg A. Symbiosis between Frankia and actinorhizal plants: Root nodules of non-legumes. Indian J. Exp. Biol. 2003;41:1165–1183. [PubMed] [Google Scholar]
- 5.Franche C., Lindström K., Elmerich C. Nitrogen-fixing bacteria associated with leguminous and non-leguminous plants. Plant Soil. 2009;321:35–59. doi: 10.1007/s11104-008-9833-8. [DOI] [Google Scholar]
- 6.Zipfel C., Oldroyd G.E.D. Plant signalling in symbiosis and immunity. Nature. 2017;543:328–336. doi: 10.1038/nature22009. [DOI] [PubMed] [Google Scholar]
- 7.Ibáñez F., Wall L., Fabra A. Starting points in plant-bacteria nitrogen-fixing symbioses: Intercellular invasion of the roots. J. Exp. Bot. 2016;68:1905–1918. doi: 10.1093/jxb/erw387. [DOI] [PubMed] [Google Scholar]
- 8.Sprent J.I. Evolving ideas of legume evolution and diversity: A taxonomic perspective on the occurrence of nodulation. New Phytol. 2007;174:11–25. doi: 10.1111/j.1469-8137.2007.02015.x. [DOI] [PubMed] [Google Scholar]
- 9.Sprent J.I., de Faria S.M. Mechanisms of infection of plants by nitrogen fixing organisms. Plant Soil. 1988;110:157–165. doi: 10.1007/BF02226795. [DOI] [Google Scholar]
- 10.Sprent J.I., James E.K. Legume evolution: Where do nodules and mycorrhizas fit in? Plant Physiol. 2007;144:575–581. doi: 10.1104/pp.107.096156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma V., Bhattacharyya S., Kumar R., Kumar A., Ibañez F., Wang J., Guo B., Sudini H.K., Gopalakrishnan S., DasGupta M., et al. Molecular basis of root nodule symbiosis between Bradyrhizobium and ‘crack-entry’ legume groundnut (Arachis hypogaea L.) Plants. 2020;9:276. doi: 10.3390/plants9020276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Subba-Rao N.S., Mateos P.F., Baker D., Stuart Pankratz H., Palma J., Dazzo F.B., Sprent J.I. The unique root-nodule symbiosis between Rhizobium and the aquatic legume, Neptunia natans (L. f.) Druce. Planta. 1995;196:311–320. doi: 10.1007/BF00201390. [DOI] [Google Scholar]
- 13.Sprent J.I., Ardley J., James E.K. Biogeography of nodulated legumes and their nitrogen-fixing symbionts. New Phytol. 2017;215:40–56. doi: 10.1111/nph.14474. [DOI] [PubMed] [Google Scholar]
- 14.Ward H.M. On the tubercular swellings on the roots of Vicia faba. Proc. R. Soc. Lond. 1887;42:539–562. doi: 10.1098/rspl.1887.0075. [DOI] [Google Scholar]
- 15.McCoy E. Infection by bact. radicicola in relation to the microchemistry of the host’s cell walls. Proc. R. Soc. Lond. Ser. B Contain. Pap. Biol. Character. 1932;110:514–533. doi: 10.1098/rspb.1932.0040. [DOI] [Google Scholar]
- 16.Allen E.K., Allen O.N. Biological aspects of symbiotic nitrogen fixation. In: Allen E.K., editor. Der Stickstoffumsatz/Nitrogen Metabolism. Vol. 8. Springer; Berlin/Heidelberg, Germany: 1958. pp. 48–118. [Google Scholar]
- 17.Brewin N.J. Plant cell wall remodelling in the Rhizobium–legume symbiosis. Crit. Rev. Plant Sci. 2004;23:293–316. doi: 10.1080/07352680490480734. [DOI] [Google Scholar]
- 18.Cole R.A., Fowler J.E. Polarized growth: Maintaining focus on the tip. Curr. Opin. Plant Biol. 2006;9:579–588. doi: 10.1016/j.pbi.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 19.Fournier J., Timmers A.C.J., Sieberer B.J., Jauneau A., Chabaud M., Barker D.G. Mechanism of infection thread elongation in root hairs of Medicago truncatula and dynamic interplay with associated rhizobial colonization. Plant Physiol. 2008;148:1985–1995. doi: 10.1104/pp.108.125674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fournier J., Teillet A., Chabaud M., Ivanov S., Genre A., Limpens E., de Carvalho-Niebel F., Barker D.G. Remodeling of the infection chamber before infection thread formation reveals a two-step mechanism for rhizobial entry into the host legume root hair. Plant Physiol. 2015;167:1233–1242. doi: 10.1104/pp.114.253302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu C.-W., Breakspear A., Stacey N., Findlay K., Nakashima J., Ramakrishnan K., Liu M., Xie F., Endre G., de Carvalho-Niebel F., et al. A protein complex required for polar growth of rhizobial infection threads. Nat. Commun. 2019;10:2848. doi: 10.1038/s41467-019-10029-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brewin N.J. Development of the legume root nodule. Ann. Rev. Cell Biol. 1991;7:191–226. doi: 10.1146/annurev.cb.07.110191.001203. [DOI] [PubMed] [Google Scholar]
- 23.Perret X., Staehelin C., Broughton W.J. Molecular basis of symbiotic promiscuity. Microbiol. Mol. Biol. Rev. 2000;64:180–201. doi: 10.1128/MMBR.64.1.180-201.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spaink H.P. Root nodulation and infection factors produced by rhizobial bacteria. Ann. Rev. Microbiol. 2000;54:257–288. doi: 10.1146/annurev.micro.54.1.257. [DOI] [PubMed] [Google Scholar]
- 25.Fraysse N., Couderc F., Poinsot V. Surface polysaccharide involvement in establishing the rhizobium–legume symbiosis. Eur. J. Biochem. 2003;270:1365–1380. doi: 10.1046/j.1432-1033.2003.03492.x. [DOI] [PubMed] [Google Scholar]
- 26.Denarie J., Debelle F., Prome J.-C. Rhizobium lipo-chitooligosaccharide nodulation factors: Signaling molecules mediating recognition and morphogenesis. Ann. Rev. Biochem. 1996;65:503–535. doi: 10.1146/annurev.bi.65.070196.002443. [DOI] [PubMed] [Google Scholar]
- 27.Buhian W.P., Bensmihen S. Mini-Review: Nod factor regulation of phytohormone signaling and homeostasis during rhizobia-legume symbiosis. Front. Plant Sci. 2018;9:1247. doi: 10.3389/fpls.2018.01247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mbengue M.D., Hervé C., Debellé F. Nod factor signaling in symbiotic nodulation. In: Frendo P., Frugier F., Masson-Boivin C., editors. Advances in Botanical Research. Vol. 94. Academic Press; London, UK: San Diego, CA, USA: Waltham, MA, USA: Oxford, UK: 2020. pp. 1–39. [Google Scholar]
- 29.Tsyganova A.V., Tsyganov V.E. Plant genetic control over infection thread development during legume-Rhizobium symbiosis. In: Rigobelo E.C., editor. Symbiosis. IntechOpen; London, UK: 2018. pp. 23–52. [DOI] [Google Scholar]
- 30.Roy S., Liu W., Nandety R.S., Crook A., Mysore K.S., Pislariu C.I., Frugoli J., Dickstein R., Udvardi M.K. Celebrating 20 years of genetic discoveries in legume nodulation and symbiotic nitrogen fixation. Plant Cell. 2020;32:15–41. doi: 10.1105/tpc.19.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsyganov V.E., Tsyganova A.V. Symbiotic regulatory genes controlling nodule development in Pisum sativum L. Plants. 2020;9:1741. doi: 10.3390/plants9121741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oldroyd G.E. Speak, friend, and enter: Signalling systems that promote beneficial symbiotic associations in plants. Nat. Rev. Microbiol. 2013;11:252–263. doi: 10.1038/nrmicro2990. [DOI] [PubMed] [Google Scholar]
- 33.Goedhart J., Hink M.A., Visser A.J.W.G., Bisseling T., Gadella T.W.J., Jr. In Vivo fluorescence correlation microscopy (FCM) reveals accumulation and immobilization of Nod factors in root hair cell walls. Plant J. 2000;21:109–119. doi: 10.1046/j.1365-313x.2000.00656.x. [DOI] [PubMed] [Google Scholar]
- 34.Felle H.H., Kondorosi E., Kondorosi A., Schultze M. The role of ion fluxes in Nod factor signalling in Medicago sativa. Plant J. 1998;13:455–463. doi: 10.1046/j.1365-313X.1998.00041.x. [DOI] [Google Scholar]
- 35.Damiani I., Drain A., Guichard M., Balzergue S., Boscari A., Boyer J.-C., Brunaud V., Cottaz S., Rancurel C., Da Rocha M., et al. Nod factor effects on root hair-specific transcriptome of Medicago truncatula: Focus on plasma membrane transport systems and reactive oxygen species networks. Front. Plant Sci. 2016;7:794. doi: 10.3389/fpls.2016.00794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ehrhardt D.W., Wais R., Long S.R. Calcium spiking in plant root hairs responding to Rhizobium nodulation signals. Cell. 1996;85:673–681. doi: 10.1016/S0092-8674(00)81234-9. [DOI] [PubMed] [Google Scholar]
- 37.Sieberer B.J., Chabaud M., Timmers A.C., Monin A., Fournier J., Barker D.G. A nuclear-targeted cameleon demonstrates intranuclear Ca2+ spiking in Medicago truncatula root hairs in response to rhizobial nodulation factors. Plant Physiol. 2009;151:1197–1206. doi: 10.1104/pp.109.142851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cárdenas L., Vidali L., Domınguez J., Pérez H., Sánchez F., Hepler P.K., Quinto C. Rearrangement of actin microfilaments in plant root hairs responding to Rhizobium etli nodulation signals. Plant Physiol. 1998;116:871–877. doi: 10.1104/pp.116.3.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Ruijter N.C., Bisseling T., Emons A.M.C. Rhizobium Nod factors induce an increase in sub-apical fine bundles of actin filaments in Vicia sativa root hairs within minutes. Mol. Plant Microbe Interact. 1999;12:829–832. doi: 10.1094/MPMI.1999.12.9.829. [DOI] [Google Scholar]
- 40.Sieberer B., Emons A.M.C. Cytoarchitecture and pattern of cytoplasmic streaming in root hairs of Medicago truncatula during development and deformation by nodulation factors. Protoplasma. 2000;214:118–127. doi: 10.1007/BF02524268. [DOI] [Google Scholar]
- 41.Sieberer B.J., Timmers A.C., Emons A.M.C. Nod factors alter the microtubule cytoskeleton in Medicago truncatula root hairs to allow root hair reorientation. Mol. Plant Microbe Interact. 2005;18:1195–1204. doi: 10.1094/MPMI-18-1195. [DOI] [PubMed] [Google Scholar]
- 42.D’Haeze W., Glushka J., De Rycke R., Holsters M., Carlson R.W. Structural characterization of extracellular polysaccharides of Azorhizobium caulinodans and importance for nodule initiation on Sesbania rostrata. Mol. Microbiol. 2004;52:485–500. doi: 10.1111/j.1365-2958.2004.03989.x. [DOI] [PubMed] [Google Scholar]
- 43.Downie J.A. The roles of extracellular proteins, polysaccharides and signals in the interactions of rhizobia with legume roots. FEMS Microbiol. Rev. 2010;34:150–170. doi: 10.1111/j.1574-6976.2009.00205.x. [DOI] [PubMed] [Google Scholar]
- 44.Wisniewski-Dyé F., Downie J.A. Quorum-sensing in Rhizobium. Antonie Leeuwenhoek. 2002;81:397–407. doi: 10.1023/A:1020501104051. [DOI] [PubMed] [Google Scholar]
- 45.Rinaudi L.V., Giordano W. An integrated view of biofilm formation in rhizobia. FEMS Microbiol. Lett. 2010;304:1–11. doi: 10.1111/j.1574-6968.2009.01840.x. [DOI] [PubMed] [Google Scholar]
- 46.Rodríguez-Navarro D.N., Dardanelli M.S., Ruíz-Saínz J.E. Attachment of bacteria to the roots of higher plants. FEMS Microbiol. Lett. 2007;272:127–136. doi: 10.1111/j.1574-6968.2007.00761.x. [DOI] [PubMed] [Google Scholar]
- 47.Hirsch A.M. Role of lectins (and rhizobial exopolysaccharides) in legume nodulation. Curr. Opin. Plant Biol. 1999;2:320–326. doi: 10.1016/S1369-5266(99)80056-9. [DOI] [PubMed] [Google Scholar]
- 48.Lagarda-Diaz I., Guzman-Partida A.M., Vazquez-Moreno L. Legume lectins: Proteins with diverse applications. Int. J. Mol. Sci. 2017;18:1242. doi: 10.3390/ijms18061242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Albareda M., Dardanelli M.S., Sousa C., Megías M., Temprano F., Rodríguez-Navarro D.N. Factors affecting the attachment of rhizospheric bacteria to bean and soybean roots. FEMS Microbiol. Lett. 2006;259:67–73. doi: 10.1111/j.1574-6968.2006.00244.x. [DOI] [PubMed] [Google Scholar]
- 50.Laus M.C., Logman T.J., Lamers G.E., Van Brussel A.A.N., Carlson R.W., Kijne J.W. A novel polar surface polysaccharide from Rhizobium leguminosarum binds host plant lectin. Mol. Microbiol. 2006;59:1704–1713. doi: 10.1111/j.1365-2958.2006.05057.x. [DOI] [PubMed] [Google Scholar]
- 51.Williams A., Wilkinson A., Krehenbrink M., Russo D.M., Zorreguieta A., Downie J.A. Glucomannan-mediated attachment of Rhizobium leguminosarum to pea root hairs is required for competitive nodule infection. J. Bacteriol. 2008;190:4706–4715. doi: 10.1128/JB.01694-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lodeiro A.b.R., Favelukes G. Early interactions of Bradyrhizobium japonicum and soybean roots: Specificity in the process of adsorption. Soil Biol. Biochem. 1999;31:1405–1411. doi: 10.1016/S0038-0717(99)00058-9. [DOI] [Google Scholar]
- 53.Gibson K.E., Kobayashi H., Walker G.C. Molecular determinants of a symbiotic chronic infection. Annu. Rev. Genet. 2008;42:413–441. doi: 10.1146/annurev.genet.42.110807.091427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Robledo M., Jiménez-Zurdo J.I., Velázquez E., Trujillo M.E., Zurdo-Piñeiro J.L., Ramírez-Bahena M.H., Ramos B., Díaz-Mínguez J.M., Dazzo F., Martínez-Molina E., et al. Rhizobium cellulase CelC2 is essential for primary symbiotic infection of legume host roots. Proc. Natl. Acad. Sci. USA. 2008;105:7064–7069. doi: 10.1073/pnas.0802547105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Navarro-Gochicoa M.-T., Camut S., Timmers A.C.J., Niebel A., Hervé C., Boutet E., Bono J.-J., Imberty A., Cullimore J.V. Characterization of four lectin-like receptor kinases expressed in roots of Medicago truncatula. Structure, location, regulation of expression, and potential role in the symbiosis with Sinorhizobium meliloti. Plant Physiol. 2003;133:1893–1910. doi: 10.1104/pp.103.027680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Hoff P.L., Brill L.M., Hirsch A.M. Plant lectins: The ties that bind in root symbiosis and plant defense. Mol. Genet. Genom. 2009;282:1–15. doi: 10.1007/s00438-009-0460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Swart S., Logman T.J., Smit G., Lugtenberg B.J., Kijne J.W. Purification and partial characterization of a glycoprotein from pea (Pisum sativum) with receptor activity for rhicadhesin, an attachment protein of Rhizobiaceae. Plant Mol. Biol. 1994;24:171–183. doi: 10.1007/BF00040583. [DOI] [PubMed] [Google Scholar]
- 58.Dardanelli M., Angelini J., Fabra A. A calcium-dependent bacterial surface protein is involved in the attachment of rhizobia to peanut roots. Can. J. Microbiol. 2003;49:399–405. doi: 10.1139/w03-054. [DOI] [PubMed] [Google Scholar]
- 59.Ausmees N., Jacobsson K., Lindberg M. A unipolarly located, cell-surface-associated agglutinin, RapA, belongs to a family of Rhizobium-adhering proteins (Rap) in Rhizobium leguminosarum bv. trifolii. Microbiology. 2001;147:549–559. doi: 10.1099/00221287-147-3-549. [DOI] [PubMed] [Google Scholar]
- 60.Mongiardini E.J., Ausmees N., Pérez-Giménez J., Althabegoiti M.J., Ignacio Quelas J., López-García S.L., Lodeiro A.R. The rhizobial adhesion protein RapA1 is involved in adsorption of rhizobia to plant roots but not in nodulation. FEMS Microbiol. Ecol. 2008;65:279–288. doi: 10.1111/j.1574-6941.2008.00467.x. [DOI] [PubMed] [Google Scholar]
- 61.Russo D.M., Williams A., Edwards A., Posadas D.M., Finnie C., Dankert M., Downie J.A., Zorreguieta A. Proteins exported via the PrsD-PrsE type I secretion system and the acidic exopolysaccharide are involved in biofilm formation by Rhizobium leguminosarum. J. Bacteriol. 2006;188:4474–4486. doi: 10.1128/JB.00246-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Krehenbrink M., Downie J.A. Identification of protein secretion systems and novel secreted proteins in Rhizobium leguminosarum bv. viciae. BMC Genom. 2008;9:55. doi: 10.1186/1471-2164-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Talukdar T., Gorecka K.M., de Carvalho-Niebel F., Downie J.A., Cullimore J., Pikula S. Annexins - calcium- and membrane-binding proteins in the plant kingdom: Potential role in nodulation and mycorrhization in Medicago truncatula. Acta Biochim. Pol. 2009;56:199–210. doi: 10.18388/abp.2009_2451. [DOI] [PubMed] [Google Scholar]
- 64.Manthey K., Krajinski F., Hohnjec N., Firnhaber C., Pühler A., Perlick A.M., Küster H. Transcriptome profiling in root nodules and arbuscular mycorrhiza identifies a collection of novel genes induced during Medicago truncatula root endosymbioses. Mol. Plant Microbe Interact. 2004;17:1063–1077. doi: 10.1094/MPMI.2004.17.10.1063. [DOI] [PubMed] [Google Scholar]
- 65.Edwards A., Heckmann A.B., Yousafzai F., Duc G., Downie J.A. Structural implications of mutations in the pea SYM8 symbiosis gene, the DMI1 ortholog, encoding a predicted ion channel. Mol. Plant Microbe Interact. 2007;20:1183–1191. doi: 10.1094/MPMI-20-10-1183. [DOI] [PubMed] [Google Scholar]
- 66.Xie F., Williams A., Edwards A., Downie J.A. A plant arabinogalactan-like glycoprotein promotes a novel type of polar surface attachment by Rhizobium leguminosarum. Mol. Plant Microbe Interact. 2012;25:250–258. doi: 10.1094/MPMI-08-11-0211. [DOI] [PubMed] [Google Scholar]
- 67.Esseling J.J., Lhuissier F.G., Emons A.M.C. Nod factor-induced root hair curling: Continuous polar growth towards the point of Nod factor application. Plant Physiol. 2003;132:1982–1988. doi: 10.1104/pp.103.021634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dazzo F.B., Orgambide G.G., Philip-Hollingsworth S., Hollingsworth R.I., Ninke K.O., Salzwedel J.L. Modulation of development, growth dynamics, wall crystallinity, and infection sites in white clover root hairs by membrane chitolipooligosaccharides from Rhizobium leguminosarum biovar trifolii. J. Bacteriol. 1996;178:3621–3627. doi: 10.1128/JB.178.12.3621-3627.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gage D.J., Margolin W. Hanging by a thread: Invasion of legume plants by rhizobia. Curr. Opin. Microbiol. 2000;3:613–617. doi: 10.1016/S1369-5274(00)00149-1. [DOI] [PubMed] [Google Scholar]
- 70.Carol R.J., Dolan L. Building a hair: Tip growth in Arabidopsis thaliana root hairs. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2002;357:815–821. doi: 10.1098/rstb.2002.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mollet J.-C., Leroux C., Dardelle F., Lehner A. Cell wall composition, biosynthesis and remodeling during pollen tube growth. Plants. 2013;2:107–147. doi: 10.3390/plants2010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Galway M.E., Heckman J.W., Schiefelbein J.W. Growth and ultrastructure of Arabidopsis root hairs: The rhd3 mutation alters vacuole enlargement and tip growth. Planta. 1997;201:209–218. doi: 10.1007/BF01007706. [DOI] [PubMed] [Google Scholar]
- 73.Grierson C.S., Roberts K., Feldmann K.A., Dolan L. The COW1 locus of Arabidopsis acts after RHD2, and in parallel with RHD3 and TIP1, to determine the shape, rate of elongation, and number of root hairs produced from each site of hair formation. Plant Physiol. 1997;115:981–990. doi: 10.1104/pp.115.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Peiter E., Sun J., Heckmann A.B., Venkateshwaran M., Riely B.K., Otegui M.S., Edwards A., Freshour G., Hahn M.G., Cook D.R., et al. The Medicago truncatula DMI1 protein modulates cytosolic calcium signaling. Plant Physiol. 2007;145:192–203. doi: 10.1104/pp.107.097261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bibikova T.N., Zhigilei A., Gilroy S. Root hair growth in Arabidopsis thaliana is directed by calcium and an endogenous polarity. Planta. 1997;203:495–505. doi: 10.1007/s004250050219. [DOI] [PubMed] [Google Scholar]
- 76.Yokota K., Fukai E., Madsen L.H., Jurkiewicz A., Rueda P., Radutoiu S., Held M., Hossain M.S., Szczyglowski K., Morieri G. Rearrangement of actin cytoskeleton mediates invasion of Lotus japonicus roots by Mesorhizobium loti. Plant Cell. 2009;21:267–284. doi: 10.1105/tpc.108.063693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Su C., Klein M.-L., Hernández-Reyes C., Batzenschlager M., Ditengou F.A., Lace B., Keller J., Delaux P.-M., Ott T. The Medicago truncatula DREPP protein triggers microtubule fragmentation in membrane nanodomains during symbiotic infections. Plant Cell. 2020;32:1689–1702. doi: 10.1105/tpc.19.00777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Murray J.D. Invasion by invitation: Rhizobial infection in legumes. Mol. Plant Microbe Interact. 2011;24:631–639. doi: 10.1094/MPMI-08-10-0181. [DOI] [PubMed] [Google Scholar]
- 79.Janczarek M., Rachwał K., Marzec A., Grządziel J., Palusińska-Szysz M. Signal molecules and cell-surface components involved in early stages of the legume–rhizobium interactions. Appl. Soil Ecol. 2015;85:94–113. doi: 10.1016/j.apsoil.2014.08.010. [DOI] [Google Scholar]
- 80.Peleg-Grossman S., Volpin H., Levine A. Root hair curling and Rhizobium infection in Medicago truncatula are mediated by phosphatidylinositide-regulated endocytosis and reactive oxygen species. J. Exp. Bot. 2007;58:1637–1649. doi: 10.1093/jxb/erm013. [DOI] [PubMed] [Google Scholar]
- 81.Lei M.-J., Wang Q., Li X., Chen A., Luo L., Xie Y., Li G., Luo D., Mysore K.S., Wen J., et al. The small GTPase ROP10 of Medicago truncatula is required for both tip growth of root hairs and Nod factor-induced root hair deformation. Plant Cell. 2015;27:806–822. doi: 10.1105/tpc.114.135210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sahlman K., Fåhraeus G. An electron microscope study of root-hair infection by Rhizobium. Microbiology. 1963;33:425–427. doi: 10.1099/00221287-33-3-425. [DOI] [PubMed] [Google Scholar]
- 83.Gage D.J. Infection and invasion of roots by symbiotic, nitrogen-fixing rhizobia during nodulation of temperate legumes. Microbiol. Mol. Biol. Rev. 2004;68:280–300. doi: 10.1128/MMBR.68.2.280-300.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Walker S.A., Viprey V., Downie J.A. Dissection of nodulation signaling using pea mutants defective for calcium spiking induced by Nod factors and chitin oligomers. Proc. Natl. Acad. Sci. USA. 2000;97:13413–13418. doi: 10.1073/pnas.230440097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Catoira R., Timmers A., Maillet F., Galera C., Penmetsa R.V., Cook D., Dénarié J., Gough C. The HCL gene of Medicago truncatula controls Rhizobium-induced root hair curling. Development. 2001;128:1507–1518. doi: 10.1242/dev.128.9.1507. [DOI] [PubMed] [Google Scholar]
- 86.Sogawa A., Yamazaki A., Yamasaki H., Komi M., Manabe T., Tajima S., Hayashi M., Nomura M. SNARE Proteins LjVAMP72a and LjVAMP72b are required for root symbiosis and root hair formation in Lotus japonicus. Front. Plant Sci. 2019;9:1992. doi: 10.3389/fpls.2018.01992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yano K., Shibata S., Chen W.-L., Sato S., Kaneko T., Jurkiewicz A., Sandal N., Banba M., Imaizumi-Anraku H., Kojima T., et al. CERBERUS, a novel U-box protein containing WD-40 repeats, is required for formation of the infection thread and nodule development in the legume–Rhizobium symbiosis. Plant J. 2009;60:168–180. doi: 10.1111/j.1365-313X.2009.03943.x. [DOI] [PubMed] [Google Scholar]
- 88.Haney C.H., Long S.R. Plant flotillins are required for infection by nitrogen-fixing bacteria. Proc. Natl. Acad. Sci. USA. 2010;107:478–483. doi: 10.1073/pnas.0910081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Guan D., Stacey N., Liu C., Wen J., Mysore K.S., Torres-Jerez I., Vernié T., Tadege M., Zhou C., Wang Z.-y., et al. Rhizobial infection is associated with the development of peripheral vasculature in nodules of Medicago truncatula. Plant Physiol. 2013;162:107–115. doi: 10.1104/pp.113.215111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pii Y., Molesini B., Pandolfini T. The involvement of Medicago truncatula non-specific lipid transfer protein N5 in the control of rhizobial infection. Plant Signal. Behav. 2013;8:e24836. doi: 10.4161/psb.24836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Suzaki T., Takeda N., Nishida H., Hoshino M., Ito M., Misawa F., Handa Y., Miura K., Kawaguchi M. LACK OF SYMBIONT ACCOMMODATION controls intracellular symbiont accommodation in root nodule and arbuscular mycorrhizal symbiosis in Lotus japonicus. PLoS Genet. 2019;15:e1007865. doi: 10.1371/journal.pgen.1007865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yano K., Yoshida S., Müller J., Singh S., Banba M., Vickers K., Markmann K., White C., Schuller B., Sato S., et al. CYCLOPS, a mediator of symbiotic intracellular accommodation. Proc. Natl. Acad. Sci. USA. 2008;105:20540–20545. doi: 10.1073/pnas.0806858105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Smit P., Raedts J., Portyanko V., Debellé F., Gough C., Bisseling T., Geurts R. NSP1 of the GRAS protein family is essential for rhizobial Nod factor-induced transcription. Science. 2005;308:1789–1791. doi: 10.1126/science.1111025. [DOI] [PubMed] [Google Scholar]
- 94.Kaló P., Gleason C., Edwards A., Marsh J., Mitra R.M., Hirsch S., Jakab J., Sims S., Long S.R., Rogers J., et al. Nodulation signaling in legumes requires NSP2, a member of the GRAS family of transcriptional regulators. Science. 2005;308:1786–1789. doi: 10.1126/science.1110951. [DOI] [PubMed] [Google Scholar]
- 95.Middleton P.H., Jakab J., Penmetsa R.V., Starker C.G., Doll J., Kaló P., Prabhu R., Marsh J.F., Mitra R.M., Kereszt A., et al. An ERF transcription factor in Medicago truncatula that is essential for Nod factor signal transduction. Plant Cell. 2007;19:1221–1234. doi: 10.1105/tpc.106.048264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Borisov A.Y., Madsen L.H., Tsyganov V.E., Umehara Y., Voroshilova V.A., Batagov A.O., Sandal N., Mortensen A., Schauser L., Ellis N., et al. The Sym35 gene required for root nodule development in pea is an ortholog of Nin from Lotus japonicus. Plant Physiol. 2003;131:1009–1017. doi: 10.1104/pp.102.016071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Laloum T., Baudin M., Frances L., Lepage A., Billault-Penneteau B., Cerri M.R., Ariel F., Jardinaud M.-F., Gamas P., de Carvalho-Niebel F., et al. Two CCAAT-box-binding transcription factors redundantly regulate early steps of the legume-rhizobia endosymbiosis. Plant J. 2014;79:757–768. doi: 10.1111/tpj.12587. [DOI] [PubMed] [Google Scholar]
- 98.Newman-Griffis A.H., del Cerro P., Charpentier M., Meier I. Medicago LINC complexes function in nuclear morphology, nuclear movement, and root nodule symbiosis. Plant Physiol. 2019;179:491–506. doi: 10.1104/pp.18.01111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nutman P.S. The influence of the legume in root-nodule symbiosis. Biol. Rev. 1956;31:109–151. doi: 10.1111/j.1469-185X.1956.tb00650.x. [DOI] [Google Scholar]
- 100.Ljunggren H., Fåhraeus G. Effect of Rhizobium polysaccharide on the formation of polygalacturonase in lucerne and clover. Nature. 1959;184:1578–1579. doi: 10.1038/1841578a0. [DOI] [PubMed] [Google Scholar]
- 101.Dart P.J., Mercer F.V. The legume rhizosphere. Archiv. Mikrobiol. 1964;47:344–378. doi: 10.1007/BF00406359. [DOI] [Google Scholar]
- 102.Napoli C.A., Hubbell D.H. Ultrastructure of Rhizobium-induced infection threads in clover root hairs. Appl. Microbiol. 1975;30:1003–1009. doi: 10.1128/AM.30.6.1003-1009.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Callaham D.A., Torrey J.G. The structural basis for infection of root hairs of Trifolium repens by Rhizobium. Can. J. Bot. 1981;59:1647–1664. doi: 10.1139/b81-223. [DOI] [Google Scholar]
- 104.Turgeon B.G., Bauer W.D. Ultrastructure of infection-thread development during the infection of soybean by Rhizobium japonicum. Planta. 1985;163:328–349. doi: 10.1007/BF00395142. [DOI] [PubMed] [Google Scholar]
- 105.Fåhraeus G. The infection of clover root hairs by nodule bacteria studied by a simple glass slide technique. J. Gen. Microbiol. 1957;16:374–381. doi: 10.1099/00221287-16-2-374. [DOI] [PubMed] [Google Scholar]
- 106.Fåhraeus G., Ljunggren H. The possible significance of pectic enzymes in root hair infection by nodule bacteria. Physiol. Plant. 1959;12:145–154. doi: 10.1111/j.1399-3054.1959.tb07893.x. [DOI] [Google Scholar]
- 107.Mateos P.F., Jimenez-Zurdo J.I., Chen J., Squartini A.S., Haack S.K., Martinez-Molina E., Hubbell D.H., Dazzo F.B. Cell-associated pectinolytic and cellulolytic enzymes in Rhizobium leguminosarum biovar trifolii. Appl. Environ. Microbiol. 1992;58:1816–1822. doi: 10.1128/AEM.58.6.1816-1822.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Roberts J.A., Whitelaw C.A., Gonzalez-Carranza Z.H., McManus M.T. Cell separation processes in plants—Models, mechanisms and manipulation. Ann. Bot. 2000;86:223–235. doi: 10.1006/anbo.2000.1203. [DOI] [Google Scholar]
- 109.Cosgrove D.J., Li L.C., Cho H.-T., Hoffmann-Benning S., Moore R.C., Blecker D. The growing world of expansins. Plant Cell Physiol. 2002;43:1436–1444. doi: 10.1093/pcp/pcf180. [DOI] [PubMed] [Google Scholar]
- 110.Muñoz J.A., Coronado C., Pérez-Hormaeche J., Kondorosi A., Ratet P., Palomares A.J. MsPG3, a Medicago sativa polygalacturonase gene expressed during the alfalfa–Rhizobium meliloti interaction. Proc. Natl. Acad. Sci. USA. 1998;95:9687–9692. doi: 10.1073/pnas.95.16.9687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rodríguez-Llorente I.D., Pérez-Hormaeche J., Dary M., Caviedes M.A., Kondorosi A., Ratet P., Palomares A.J. Expression of MsPG3-GFP fusions in Medicago truncatula ‘hairy roots’ reveals preferential tip localization of the protein in root hairs. Eur. J. Biochem. 2003;270:261–269. doi: 10.1046/j.1432-1033.2003.03384.x. [DOI] [PubMed] [Google Scholar]
- 112.Xie F., Murray J.D., Kim J., Heckmann A.B., Edwards A., Oldroyd G.E.D., Downie J.A. Legume pectate lyase required for root infection by rhizobia. Proc. Natl. Acad. Sci. USA. 2012;109:633–638. doi: 10.1073/pnas.1113992109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li X., Zhao J., Tan Z., Zeng R., Liao H. GmEXPB2, a cell wall β-expansin, affects soybean nodulation through modifying root architecture and promoting nodule formation and development. Plant Physiol. 2015;169:2640–2653. doi: 10.1104/pp.15.01029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Perrine-Walker F.M., Lartaud M., Kouchi H., Ridge R.W. Microtubule array formation during root hair infection thread initiation and elongation in the Mesorhizobium-Lotus symbiosis. Protoplasma. 2014;251:1099–1111. doi: 10.1007/s00709-014-0618-z. [DOI] [PubMed] [Google Scholar]
- 115.Tansengco M.L., Hayashi M., Kawaguchi M., Imaizumi-Anraku H., Murooka Y. crinkle, a novel symbiotic mutant that affects the infection thread growth and alters the root hair, trichome, and seed development in Lotus japonicus. Plant Physiol. 2003;131:1054–1063. doi: 10.1104/pp.102.017020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hossain M.S., Liao J., James E.K., Sato S., Tabata S., Jurkiewicz A., Madsen L.H., Stougaard J., Ross L., Szczyglowski K. Lotus japonicus ARPC1 is required for rhizobial infection. Plant Physiol. 2012;160:917–928. doi: 10.1104/pp.112.202572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Miyahara A., Richens J., Starker C., Morieri G., Smith L., Long S., Downie J.A., Oldroyd G.E.D. Conservation in function of a SCAR/WAVE component during infection thread and root hair growth in Medicago truncatula. Mol. Plant Microbe Interact. 2010;23:1553–1562. doi: 10.1094/MPMI-06-10-0144. [DOI] [PubMed] [Google Scholar]
- 118.Liu W., Chen A.-M., Luo L., Sun J., Cao L.-P., Yu G.-Q., Zhu J.-B., Wang Y.-Z. Characterization and expression analysis of Medicago truncatula ROP GTPase family during the early stage of symbiosis. J. Integr. Plant Biol. 2010;52:639–652. doi: 10.1111/j.1744-7909.2010.00944.x. [DOI] [PubMed] [Google Scholar]
- 119.Saarikangas J., Zhao H., Lappalainen P. Regulation of the actin cytoskeleton-plasma membrane interplay by phosphoinositides. Physiol. Rev. 2010;90:259–289. doi: 10.1152/physrev.00036.2009. [DOI] [PubMed] [Google Scholar]
- 120.Ortega-Ortega Y., Carrasco-Castilla J., Juárez-Verdayes M.A., Toscano-Morales R., Fonseca-García C., Nava N., Cárdenas L., Quinto C. Actin depolymerizing factor modulates rhizobial infection and nodule organogenesis in common bean. Int. J. Mol. Sci. 2020;21:1970. doi: 10.3390/ijms21061970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Andrio E., Marino D., Marmeys A., de Segonzac M.D., Damiani I., Genre A., Huguet S., Frendo P., Puppo A., Pauly N. Hydrogen peroxide-regulated genes in the Medicago truncatula–Sinorhizobium meliloti symbiosis. New Phytol. 2013;198:179–189. doi: 10.1111/nph.12120. [DOI] [PubMed] [Google Scholar]
- 122.Shaw S.L., Long S.R. Nod factor inhibition of reactive oxygen efflux in a host legume. Plant Physiol. 2003;132:2196–2204. doi: 10.1104/pp.103.021113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lohar D.P., Haridas S., Gantt J.S., VandenBosch K.A. A transient decrease in reactive oxygen species in roots leads to root hair deformation in the legume–rhizobia symbiosis. New Phytol. 2007;173:39–49. doi: 10.1111/j.1469-8137.2006.01901.x. [DOI] [PubMed] [Google Scholar]
- 124.Cárdenas L., Quinto C. Reactive oxygen species (ROS) as early signals in root hair cells responding to rhizobial nodulation factors. Plant Signal. Behav. 2008;3:1101–1102. doi: 10.4161/psb.3.12.7004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Puppo A., Pauly N., Boscari A., Mandon K., Brouquisse R. Hydrogen peroxide and nitric oxide: Key regulators of the legume–Rhizobium and mycorrhizal symbioses. Antioxid. Redox Signal. 2013;18:2202–2219. doi: 10.1089/ars.2012.5136. [DOI] [PubMed] [Google Scholar]
- 126.Montiel J., Arthikala M.-K., Cárdenas L., Quinto C. Legume NADPH oxidases have crucial roles at different stages of nodulation. Int. J. Mol. Sci. 2016;17:680. doi: 10.3390/ijms17050680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Martínez-Abarca F., Herrera-Cervera J.A., Bueno P., Sanjuan J., Bisseling T., Olivares J. Involvement of salicylic acid in the establishment of the Rhizobium meliloti-alfalfa symbiosis. Mol. Plant Microbe Interact. 1998;11:153–155. doi: 10.1094/MPMI.1998.11.2.153. [DOI] [Google Scholar]
- 128.Nagata M., Hashimoto M., Murakami E.-i., Shimoda Y., Shimoda-Sasakura F., Kucho K.-i., Suzuki A., Abe M., Higashi S., Uchiumi T. A possible role of class 1 plant hemoglobin at the early stage of legume-rhizobium symbiosis. Plant Signal. Behav. 2009;4:202–204. doi: 10.4161/psb.4.3.7796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Del Giudice J., Cam Y., Damiani I., Fung-Chat F., Meilhoc E., Bruand C., Brouquisse R., Puppo A., Boscari A. Nitric oxide is required for an optimal establishment of the Medicago truncatula–Sinorhizobium meliloti symbiosis. New Phytol. 2011;191:405–417. doi: 10.1111/j.1469-8137.2011.03693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Murakami E.-i., Nagata M., Shimoda Y., Kucho K.-i., Higashi S., Abe M., Hashimoto M., Uchiumi T. Nitric oxide production induced in roots of Lotus japonicus by lipopolysaccharide from Mesorhizobium loti. Plant Cell Physiol. 2011;52:610–617. doi: 10.1093/pcp/pcr020. [DOI] [PubMed] [Google Scholar]
- 131.Bindschedler L.V., Dewdney J., Blee K.A., Stone J.M., Asai T., Plotnikov J., Denoux C., Hayes T., Gerrish C., Davies D.R., et al. Peroxidase-dependent apoplastic oxidative burst in Arabidopsis required for pathogen resistance. Plant J. 2006;47:851–863. doi: 10.1111/j.1365-313X.2006.02837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Breakspear A., Liu C., Roy S., Stacey N., Rogers C., Trick M., Morieri G., Mysore K.S., Wen J., Oldroyd G.E., et al. The root hair “infectome” of Medicago truncatula uncovers changes in cell cycle genes and reveals a requirement for auxin signaling in rhizobial infection. Plant Cell. 2014;26:4680–4701. doi: 10.1105/tpc.114.133496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.van Brussel A.A.N., Bakhuizen R., van Spronsen P.C., Spaink H.P., Tak T., Lugtenberg B.J.J., Kijne J.W. Induction of pre-infection thread structures in the leguminous host plant by mitogenic lipo-oligosaccharides of Rhizobium. Science. 1992;257:70–72. doi: 10.1126/science.257.5066.70. [DOI] [PubMed] [Google Scholar]
- 134.Timmers A.C., Auriac M.C., Truchet G. Refined analysis of early symbiotic steps of the Rhizobium-Medicago interaction in relationship with microtubular cytoskeleton rearrangements. Development. 1999;126:3617–3628. doi: 10.1242/dev.126.16.3617. [DOI] [PubMed] [Google Scholar]
- 135.Tsyganov V.E., Voroshilova V.A., Priefer U.B., Borisov A.Y., Tikhonovich I.A. Genetic dissection of the initiation of the infection process and nodule tissue development in the Rhizobium-pea (Pisum sativum L.) symbiosis. Ann. Bot. 2002;89:357–366. doi: 10.1093/aob/mcf051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Oldroyd G.E., Downie J.A. Coordinating nodule morphogenesis with rhizobial infection in legumes. Ann. Rev. Plant Biol. 2008;59:519–546. doi: 10.1146/annurev.arplant.59.032607.092839. [DOI] [PubMed] [Google Scholar]
- 137.Madsen L.H., Tirichine L., Jurkiewicz A., Sullivan J.T., Heckmann A.B., Bek A.S., Ronson C.W., James E.K., Stougaard J. The molecular network governing nodule organogenesis and infection in the model legume Lotus japonicus. Nat. Commun. 2010;1:10. doi: 10.1038/ncomms1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Robertson J.G., Wells B., Brewin N.J., Wood E., Knight C.D., Downie J.A. The legume-Rhizobium symbiosis: A cell surface interaction. J. Cell Sci. Suppl. 1985;2:317–331. doi: 10.1242/jcs.1985.Supplement_2.17. [DOI] [PubMed] [Google Scholar]
- 139.Gage D.J. Analysis of infection thread development using Gfp-and DsRed-expressing Sinorhizobium meliloti. J. Bacteriol. 2002;184:7042–7046. doi: 10.1128/JB.184.24.7042-7046.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Roth L., Stacey G. Bacterium release into host cells of nitrogen-fixing soybean nodules: The symbiosome membrane comes from three sources. Eur. J. Cell Biol. 1989;49:13–23. [PubMed] [Google Scholar]
- 141.Rathbun E.A., Naldrett M.J., Brewin N.J. Identification of a family of extensin-like glycoproteins in the lumen of Rhizobium-induced infection threads in pea root nodules. Mol. Plant Microbe Interact. 2002;15:350–359. doi: 10.1094/MPMI.2002.15.4.350. [DOI] [PubMed] [Google Scholar]
- 142.Wisniewski J.-P., Rathbun E.A., Knox J.P., Brewin N.J. Involvement of diamine oxidase and peroxidase in insolubilization of the extracellular matrix: Implications for pea nodule initiation by Rhizobium leguminosarum. Mol. Plant Microbe Interact. 2000;13:413–420. doi: 10.1094/MPMI.2000.13.4.413. [DOI] [PubMed] [Google Scholar]
- 143.Hérouart D., Baudouin E., Frendo P., Harrison J., Santos R., Jamet A., Van de Sype G., Touati D., Puppo A. Reactive oxygen species, nitric oxide and glutathione: A key role in the establishment of the legume–Rhizobium symbiosis? Plant Physiol. Biochem. 2002;40:619–624. doi: 10.1016/S0981-9428(02)01415-8. [DOI] [Google Scholar]
- 144.Brewin N., Khodorenko A., Tsyganov V.E., Borisov A.Y., Tikhonovich I.A., Rathbun E. Legume AGP-extensins in Rhizobium infection. In: Dakora F.D., Chimphango S.B.M., Valentine A.J., Elmerich C., Newton W.E., editors. Biological Nitrogen Fixation: Towards Poverty Alleviation through Sustainable Agriculture. Vol. 42. Springer; Dordrecht, The Netherlands: 2008. pp. 185–187. [Google Scholar]
- 145.Rathbun E.A., Brewin N.J. Gum arabic glycoprotein and the infection of legumes by Rhizobium: Evidence for tyrosine cross-linking by peroxidase and by inorganic catalysis. Asp. Appl. Biol. 2009;96:241–246. [Google Scholar]
- 146.Cheng H.-P., Walker G.C. Succinoglycan is required for initiation and elongation of infection threads during nodulation of alfalfa by Rhizobium meliloti. J. Bacteriol. 1998;180:5183–5191. doi: 10.1128/JB.180.19.5183-5191.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Timmers A.C., Auriac M.C., de Billy F., Truchet G. Nod factor internalization and microtubular cytoskeleton changes occur concomitantly during nodule differentiation in alfalfa. Development. 1998;125:339–349. doi: 10.1242/dev.125.3.339. [DOI] [PubMed] [Google Scholar]
- 148.Tsyganov V.E., Voroshilova V.A., Herrera-Cervera J.A., Sanjuan-Pinilla J.M., Borisov A.Y., Tikhonovich I.A., Priefer U.B., Olivares J., Sanjuan J. Developmental downregulation of rhizobial genes as a function of symbiosome differentiation in symbiotic root nodules of Pisum sativum. New Phytol. 2003;159:521–530. doi: 10.1046/j.1469-8137.2003.00823.x. [DOI] [PubMed] [Google Scholar]
- 149.Arrighi J.-F., Barre A., Amor B.B., Bersoult A., Soriano L.C., Mirabella R., de Carvalho-Niebel F., Journet E.-P., Ghérardi M., Huguet T., et al. The Medicago truncatula lysine motif-receptor-like kinase gene family includes NFP and new nodule-expressed genes. Plant Physiol. 2006;142:265–279. doi: 10.1104/pp.106.084657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Haney C.H., Riely B.K., Tricoli D.M., Cook D.R., Ehrhardt D.W., Long S.R. Symbiotic rhizobia bacteria trigger a change in localization and dynamics of the Medicago truncatula receptor kinase LYK3. Plant Cell. 2011;23:2774–2787. doi: 10.1105/tpc.111.086389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Huisman R., Geurts R. A roadmap toward engineered nitrogen-fixing nodule symbiosis. Plant Commun. 2020;1:100019. doi: 10.1016/j.xplc.2019.100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kirienko A.N., Porozov Y.B., Malkov N.V., Akhtemova G.A., Le Signor C., Thompson R., Saffray C., Dalmais M., Bendahmane A., Tikhonovich I.A., et al. Role of a receptor-like kinase K1 in pea Rhizobium symbiosis development. Planta. 2018;248:1101–1120. doi: 10.1007/s00425-018-2944-4. [DOI] [PubMed] [Google Scholar]
- 153.Kawaharada Y., Kelly S., Nielsen M.W., Hjuler C.T., Gysel K., Muszyński A., Carlson R.W., Thygesen M.B., Sandal N., Asmussen M.H., et al. Receptor-mediated exopolysaccharide perception controls bacterial infection. Nature. 2015;523:308–312. doi: 10.1038/nature14611. [DOI] [PubMed] [Google Scholar]
- 154.Kawaharada Y., Nielsen M.W., Kelly S., James E.K., Andersen K.R., Rasmussen S.R., Füchtbauer W., Madsen L.H., Heckmann A.B., Radutoiu S., et al. Differential regulation of the Epr3 receptor coordinates membrane-restricted rhizobial colonization of root nodule primordia. Nat. Commun. 2017;8:14534. doi: 10.1038/ncomms14534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Muszyński A., Heiss C., Hjuler C.T., Sullivan J.T., Kelly S.J., Thygesen M.B., Stougaard J., Azadi P., Carlson R.W., Ronson C.W. Structures of exopolysaccharides involved in receptor-mediated perception of Mesorhizobium loti by Lotus japonicus. J. Biol. Chem. 2016;291:20946–20961. doi: 10.1074/jbc.M116.743856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Maillet F., Fournier J., Mendis H.C., Tadege M., Wen J., Ratet P., Mysore K.S., Gough C., Jones K.M. Sinorhizobium meliloti succinylated high-molecular-weight succinoglycan and the Medicago truncatula LysM receptor-like kinase MtLYK10 participate independently in symbiotic infection. Plant J. 2020;102:311–326. doi: 10.1111/tpj.14625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Skorupska A., Janczarek M., Marczak M., Mazur A., Król J. Rhizobial exopolysaccharides: Genetic control and symbiotic functions. Microb. Cell Factories. 2006;5:7. doi: 10.1186/1475-2859-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zgadzaj R., James E.K., Kelly S., Kawaharada Y., de Jonge N., Jensen D.B., Madsen L.H., Radutoiu S. A legume genetic framework controls infection of nodules by symbiotic and endophytic bacteria. PLoS Genet. 2015;11:e1005280. doi: 10.1371/journal.pgen.1005280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kiss E., Kereszt A., Barta F., Stephens S., Reuhs B.L., Kondorosi Á., Putnoky P. The rkp-3 gene region of Sinorhizobium meliloti Rm41 contains strain-specific genes that determine K antigen structure. Mol. Plant Microbe Interact. 2001;14:1395–1403. doi: 10.1094/MPMI.2001.14.12.1395. [DOI] [PubMed] [Google Scholar]
- 160.Le Quéré A.J.L., Deakin W.J., Schmeisser C., Carlson R.W., Streit W.R., Broughton W.J., Forsberg L.S. Structural characterization of a K-antigen capsular polysaccharide essential for normal symbiotic infection in Rhizobium sp. NGR234: Deletion of the rkpMNO locus prevents synthesis of 5,7-diacetamido-3,5,7,9-tetradeoxy-non-2-ulosonic acid. J. Biol. Chem. 2006;281:28981–28992. doi: 10.1074/jbc.M513639200. [DOI] [PubMed] [Google Scholar]
- 161.Pellock B.J., Cheng H.-P., Walker G.C. Alfalfa root nodule invasion efficiency is dependent on Sinorhizobium meliloti polysaccharides. J. Bacteriol. 2000;182:4310–4318. doi: 10.1128/JB.182.15.4310-4318.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Rachwał K., Boguszewska A., Kopcińska J., Karaś M., Tchórzewski M., Janczarek M. The regulatory protein RosR affects Rhizobium leguminosarum bv. trifolii protein profiles, cell surface properties, and symbiosis with clover. Front. Microbiol. 2016;7:1302. doi: 10.3389/fmicb.2016.01302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Shimomura K., Nomura M., Tajima S., Kouchi H. LjnsRING, a novel RING finger protein, is required for symbiotic interactions between Mesorhizobium loti and Lotus japonicus. Plant Cell Physiol. 2006;47:1572–1581. doi: 10.1093/pcp/pcl022. [DOI] [PubMed] [Google Scholar]
- 164.Den Herder G., De Keyser A., De Rycke R., Rombauts S., Van de Velde W., Clemente M.R., Verplancke C., Mergaert P., Kondorosi E., Holsters M., et al. Seven in Absentia proteins affect plant growth and nodulation in Medicago truncatula. Plant Physiol. 2008;148:369–382. doi: 10.1104/pp.108.119453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sinharoy S., Liu C., Breakspear A., Guan D., Shailes S., Nakashima J., Zhang S., Wen J., Torres-Jerez I., Oldroyd G., et al. A Medicago truncatula Cystathionine-β-synthase-like domain-containing protein is required for rhizobial infection and symbiotic nitrogen fixation. Plant Physiol. 2016;170:2204–2217. doi: 10.1104/pp.15.01853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.McAdam E.L., Reid J.B., Foo E. Gibberellins promote nodule organogenesis but inhibit the infection stages of nodulation. J. Exp. Bot. 2018;69:2117–2130. doi: 10.1093/jxb/ery046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Serova T.A., Tsyganova A.V., Tikhonovich I.A., Tsyganov V.E. Gibberellins inhibit nodule senescence and stimulate nodule meristem bifurcation in pea (Pisum sativum L.) Front. Plant Sci. 2019;10:285. doi: 10.3389/fpls.2019.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kiss E., Oláh B., Kaló P., Morales M., Heckmann A.B., Borbola A., Lózsa A., Kontár K., Middleton P., Downie J.A., et al. LIN, a novel type of U-Box/WD40 protein, controls early infection by rhizobia in legumes. Plant Physiol. 2009;151:1239–1249. doi: 10.1104/pp.109.143933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Qiao Z., Zogli P., Libault M. Plant hormones differentially control the sub-cellular localization of plasma membrane microdomains during the early stage of soybean nodulation. Genes. 2019;10:1012. doi: 10.3390/genes10121012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Dolgikh E.A., Kusakin P.G., Kitaeva A.B., Tsyganova A.V., Kirienko A.N., Leppyanen I.V., Dolgikh A.V., Ilina E.L., Demchenko K.N., Tikhonovich I.A., et al. Mutational analysis indicates that abnormalities in rhizobial infection and subsequent plant cell and bacteroid differentiation in pea (Pisum sativum) nodules coincide with abnormal cytokinin responses and localization. Ann. Bot. 2020;125:905–923. doi: 10.1093/aob/mcaa022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Serova T.A., Tikhonovich I.A., Tsyganov V.E. Analysis of nodule senescence in pea (Pisum sativum L.) using laser microdissection, real-time PCR, and ACC immunolocalization. J. Plant Physiol. 2017;212:29–44. doi: 10.1016/j.jplph.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 172.Rodríguez-López J., López A.H., Estrada-Navarrete G., Sánchez F., Díaz-Camino C. The noncanonical heat shock protein PvNod22 is essential for infection thread progression during rhizobial endosymbiosis in common bean. Mol. Plant Microbe Interact. 2019;32:939–948. doi: 10.1094/MPMI-02-19-0041-R. [DOI] [PubMed] [Google Scholar]
- 173.Liang P., Stratil T.F., Popp C., Marín M., Folgmann J., Mysore K.S., Wen J., Ott T. Symbiotic root infections in Medicago truncatula require remorin-mediated receptor stabilization in membrane nanodomains. Proc. Natl. Acad. Sci. USA. 2018;115:5289–5294. doi: 10.1073/pnas.1721868115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Liu M., Jia N., Li X., Liu R., Xie Q., Murray J.D., Downie J.A., Xie F. CERBERUS is critical for stabilization of VAPYRIN during rhizobial infection in Lotus japonicus. New Phytol. 2021;229:1684–1700. doi: 10.1111/nph.16973. [DOI] [PubMed] [Google Scholar]
- 175.Arrighi J.-F., Godfroy O., de Billy F., Saurat O., Jauneau A., Gough C. The RPG gene of Medicago truncatula controls Rhizobium-directed polar growth during infection. Proc. Natl. Acad. Sci. USA. 2008;105:9817–9822. doi: 10.1073/pnas.0710273105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Dalla Via V., Traubenik S., Rivero C., Aguilar O.M., Zanetti M.E., Blanco F.A. The monomeric GTPase RabA2 is required for progression and maintenance of membrane integrity of infection threads during root nodule symbiosis. Plant Mol. Biol. 2017;93:549–562. doi: 10.1007/s11103-016-0581-5. [DOI] [PubMed] [Google Scholar]
- 177.Liu J., Liu M.X., Qiu L.P., Xie F. SPIKE1 activates the GTPase ROP6 to guide the polarized growth of infection threads in Lotus japonicus. Plant Cell. 2020;32:3774–3791. doi: 10.1105/tpc.20.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yano K., Aoki S., Liu M., Umehara Y., Suganuma N., Iwasaki W., Sato S., Soyano T., Kouchi H., Kawaguchi M. Function and evolution of a Lotus japonicus AP2/ERF family transcription factor that is required for development of infection threads. DNA Res. 2016;24:193–203. doi: 10.1093/dnares/dsw052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jaffe M.J., Leopold A.C., Staples R.C. Thigmo responses in plants and fungi. Am. J. Bot. 2002;89:375–382. doi: 10.3732/ajb.89.3.375. [DOI] [PubMed] [Google Scholar]
- 180.Zhu J.-K. Abiotic stress signaling and responses in plants. Cell. 2016;167:313–324. doi: 10.1016/j.cell.2016.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kitaeva A.B., Demchenko K.N., Tikhonovich I.A., Timmers A.C.J., Tsyganov V.E. Comparative analysis of the tubulin cytoskeleton organization in nodules of Medicago truncatula and Pisum sativum: Bacterial release and bacteroid positioning correlate with characteristic microtubule rearrangements. New Phytol. 2016;210:168–183. doi: 10.1111/nph.13792. [DOI] [PubMed] [Google Scholar]
- 182.Qiu L., Lin J.-s., Xu J., Sato S., Parniske M., Wang T.L., Downie J.A., Xie F. SCARN a novel class of SCAR protein that is required for root-hair infection during legume nodulation. PLoS Genet. 2015;11:e1005623. doi: 10.1371/journal.pgen.1005623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Roland J.-C. The relationship between the plasmalemma and plant cell wall. In: Bourne G.H., Danielli J.F., Jeon K.W., editors. International Review of Cytology. Vol. 36. Academic Press; London, UK: San Diego, CA, USA: Waltham, MA, USA: Oxford, UK: 1973. pp. 45–92. [DOI] [PubMed] [Google Scholar]
- 184.Hirsch A.M., Bang M., Ausubel F.M. Ultrastructural analysis of ineffective alfalfa nodules formed by nif::Tn5 mutants of Rhizobium meliloti. J. Bacteriol. 1983;155:367–380. doi: 10.1128/JB.155.1.367-380.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Tsyganova A.V., Tsyganov V.E. Organization of the endoplasmic reticulum in cells of effective and ineffective pea nodules (Pisum sativum L.) Ekol. Genet. 2019;17:5–14. doi: 10.17816/ecogen1745-14. [DOI] [Google Scholar]
- 186.VandenBosch K.A., Bradley D.J., Knox J.P., Perotto S., Butcher G.W., Brewin N.J. Common components of the infection thread matrix and the intercellular space identified by immunocytochemical analysis of pea nodules and uninfected roots. EMBO J. 1989;8:335–341. doi: 10.1002/j.1460-2075.1989.tb03382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Dixon R.O.D. The structure of infection threads, bacteria and bacteroids in pea and clover root nodules. Archiv. Mikrobiol. 1964;48:166–178. doi: 10.1007/BF00408456. [DOI] [Google Scholar]
- 188.van Spronsen P.C., Bakhuizen R., van Brussel A.A., Kijne J.W. Cell wall degradation during infection thread formation by the root nodule bacterium Rhizobium leguminosarum is a two-step process. Eur. J. Cell Biol. 1994;64:88–94. [PubMed] [Google Scholar]
- 189.Monahan-Giovanelli H., Pinedo C.A., Gage D.J. Architecture of infection thread networks in developing root nodules induced by the symbiotic bacterium Sinorhizobium meliloti on Medicago truncatula. Plant Physiol. 2006;140:661–670. doi: 10.1104/pp.105.072876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Benaben V., Duc G., Lefebvre V., Huguet T. TE7, an inefficient symbiotic mutant of Medicago truncatula Gaertn. cv Jemalong. Plant Physiol. 1995;107:53–62. doi: 10.1104/pp.107.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Veereshlingam H., Haynes J.G., Penmetsa R.V., Cook D.R., Sherrier D.J., Dickstein R. nip, a symbiotic Medicago truncatula mutant that forms root nodules with aberrant infection threads and plant defense-like response. Plant Physiol. 2004;136:3692–3702. doi: 10.1104/pp.104.049064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Teillet A., Garcia J., de Billy F., Gherardi M., Huguet T., Barker D.G., de Carvalho-Niebel F., Journet E.-P. api, a novel Medicago truncatula symbiotic mutant impaired in nodule primordium invasion. Mol. Plant Microbe Interact. 2008;21:535–546. doi: 10.1094/MPMI-21-5-0535. [DOI] [PubMed] [Google Scholar]
- 193.Tsyganov V.E., Morzhina E.V., Stefanov S.Y., Borisov A.Y., Lebsky V.K., Tikhonovich I.A. The pea (Pisum sativum L.) genes sym33 and sym40 control infection thread formation and root nodule function. Mol. Gen. Genet. 1998;259:491–503. doi: 10.1007/s004380050840. [DOI] [PubMed] [Google Scholar]
- 194.Morzhina E.V., Tsyganov V.E., Borisov A.Y., Lebsky V.K., Tikhonovich I.A. Four developmental stages identified by genetic dissection of pea (Pisum sativum L.) root nodule morphogenesis. Plant Sci. 2000;155:75–83. doi: 10.1016/S0168-9452(00)00207-7. [DOI] [PubMed] [Google Scholar]
- 195.Voroshilova V.A., Boesten B., Tsyganov V.E., Borisov A.Y., Tikhonovich I.A., Priefer U.B. Effect of mutations in Pisum sativum L. genes blocking different stages of nodule development on the expression of late symbiotic genes in Rhizobium leguminosarum bv. viciae. Mol. Plant Microbe Interact. 2001;14:471–476. doi: 10.1094/MPMI.2001.14.4.471. [DOI] [PubMed] [Google Scholar]
- 196.Voroshilova V.A., Demchenko K.N., Brewin N.J., Borisov A.Y., Tikhonovich I.A. Initiation of a legume nodule with an indeterminate meristem involves proliferating host cells that harbour infection threads. New Phytol. 2009;181:913–923. doi: 10.1111/j.1469-8137.2008.02723.x. [DOI] [PubMed] [Google Scholar]
- 197.Rae A.L., Bonfante-Fasolo P., Brewin N.J. Structure and growth of infection threads in the legume symbiosis with Rhizobium leguminosarum. Plant J. 1992;2:385–395. doi: 10.1111/j.1365-313X.1992.00385.x. [DOI] [Google Scholar]
- 198.Bolaños L., El-Hamdaoui A., Bonilla I. Recovery of development and functionality of nodules and plant growth in salt-stressed Pisum sativum-Rhizobium leguminosarum symbiosis by boron and calcium. J. Plant Physiol. 2003;160:1493–1497. doi: 10.1078/0176-1617-01003. [DOI] [PubMed] [Google Scholar]
- 199.Redondo-Nieto M., Wilmot A.R., El-Hamdaoui A., Bonilla I., Bolaños L. Relationship between boron and calcium in the N2-fixing legume–rhizobia symbiosis. Plant Cell Environ. 2003;26:1905–1915. doi: 10.1046/j.1365-3040.2003.01107.x. [DOI] [Google Scholar]
- 200.Redondo-Nieto M., Pulido L., Reguera M., Bonilla I., Bolaños L. Developmentally regulated membrane glycoproteins sharing antigenicity with rhamnogalacturonan II are not detected in nodulated boron deficient Pisum sativum. Plant Cell Environ. 2007;30:1436–1443. doi: 10.1111/j.1365-3040.2007.01721.x. [DOI] [PubMed] [Google Scholar]
- 201.Ivanova K.A., Tsyganova A.V., Brewin N.J., Tikhonovich I.A., Tsyganov V.E. Induction of host defences by Rhizobium during ineffective nodulation of pea (Pisum sativum L.) carrying symbiotically defective mutations sym40 (PsEFD), sym33 (PsIPD3/PsCYCLOPS) and sym42. Protoplasma. 2015;252:1505–1517. doi: 10.1007/s00709-015-0780-y. [DOI] [PubMed] [Google Scholar]
- 202.Sujkowska-Rybkowska M., Borucki W. Pectins esterification in the apoplast of aluminum-treated pea root nodules. J. Plant Physiol. 2015;184:1–7. doi: 10.1016/j.jplph.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 203.Tsyganova A.V., Seliverstova E.V., Brewin N.J., Tsyganov V.E. Comparative analysis of remodelling of the plant–microbe interface in Pisum sativum and Medicago truncatula symbiotic nodules. Protoplasma. 2019;256:983–996. doi: 10.1007/s00709-019-01355-5. [DOI] [PubMed] [Google Scholar]
- 204.Tsyganova A.V., Seliverstova E.V., Brewin N.J., Tsyganov V.E. Bacterial release is accompanied by ectopic accumulation of cell wall material around the vacuole in nodules of Pisum sativum sym33-3 allele encoding transcription factor PsCYCLOPS/PsIPD3. Protoplasma. 2019;256:1449–1453. doi: 10.1007/s00709-019-01383-1. [DOI] [PubMed] [Google Scholar]
- 205.Gavrin A., Chiasson D., Ovchinnikova E., Kaiser B.N., Bisseling T., Fedorova E.E. VAMP721a and VAMP721d are important for pectin dynamics and release of bacteria in soybean nodules. New Phytol. 2016;210:1011–1021. doi: 10.1111/nph.13837. [DOI] [PubMed] [Google Scholar]
- 206.Reguera M., Abreu I., Brewin N.J., Bonilla I., Bolaños L. Borate promotes the formation of a complex between legume AGP-extensin and rhamnogalacturonan II and enhances production of Rhizobium capsular polysaccharide during infection thread development in Pisum sativum symbiotic root nodules. Plant Cell Environ. 2010;33:2112–2120. doi: 10.1111/j.1365-3040.2010.02209.x. [DOI] [PubMed] [Google Scholar]
- 207.Moore P.J., Staehelin L.A. Immunogold localization of the cell-wall-matrix polysaccharides rhamnogalacturonan I and xyloglucan during cell expansion and cytokinesis in Trifolium pratense L.; implication for secretory pathways. Planta. 1988;174:433–445. doi: 10.1007/BF00634471. [DOI] [PubMed] [Google Scholar]
- 208.Rae A., Perotto S., Knox J., Kannenberg E., Brewin N. Expression of extracellular glycoproteins in the uninfected cells of developing pea nodule tissue. Mol. Plant-Microbe Interact. 1991;4:563–570. doi: 10.1094/MPMI-4-563. [DOI] [Google Scholar]
- 209.Bolanos L., Brewin N.J., Bonilla I. Effects of Boron on Rhizobium-legume cell-surface interactions and nodule development. Plant Physiol. 1996;110:1249–1256. doi: 10.1104/pp.110.4.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Olsson P.A., Kjellbom P., Rosendahl L. Rhizobium colonization induced changes in membrane-bound and soluble hydroxyproline-rich glycoprotein composition in pea. Physiol. Plant. 2002;114:652–660. doi: 10.1034/j.1399-3054.2002.1140420.x. [DOI] [PubMed] [Google Scholar]
- 211.Tsyganova A.V., Tsyganov V.E., Findlay K.C., Borisov A.Y., Tikhonovich I.A., Brewin N.J. Distribution of legume arabinogalactan protein-extensin (AGPE) glycoproteins in symbiotically defective pea mutants with abnormal infection threads. Cell Tissue Biol. 2009;3:93–102. doi: 10.1134/S1990519X09010131. [DOI] [PubMed] [Google Scholar]
- 212.Bradley D.J., Wood E.A., Larkins A.P., Galfre G., Butcher G.W., Brewin N.J. Isolation of monoclonal antibodies reacting with peribacteriod membranes and other components of pea root nodules containing Rhizobium leguminosarum. Planta. 1988;173:149–160. doi: 10.1007/BF00403006. [DOI] [PubMed] [Google Scholar]
- 213.Tsyganova A.V., Brewin N., Tsyganov V.E. Analysis of epitope distribution of arabinogalactan protein-extensins in pea (Pisum sativum) nodules of wild-type and mutants impaired in infection thread growth. Ekol. Genet. 2019;17:5–12. doi: 10.17816/ecogen1735-12. [DOI] [Google Scholar]
- 214.Benhamou N., Lafontaine P.J., Mazau D., Esquerré-Tugayé M.-T. Differential accumulation of hydroxyproline-rich glycoproteins in bean root nodule cells infected with a wild-type strain or a C4-dicarboxylic acid mutant of Rhizobium leguminosarum bv. phaseoli. Planta. 1991;184:457–467. doi: 10.1007/BF00197893. [DOI] [PubMed] [Google Scholar]
- 215.Bonilla I., Mergold-Villasenor C., Campos M.E., Sanchez N., Perez H., Lopez L., Castrejon L., Sanchez F., Cassab G.I. The aberrant cell walls of boron-deficient bean root nodules have no covalently bound hydroxyproline/proline-rich proteins. Plant Physiol. 1997;115:1329–1340. doi: 10.1104/pp.115.4.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Sherrier D.J., Taylor G.S., Silverstein K.A.T., Gonzales M.B., VandenBosch K.A. Accumulation of extracellular proteins bearing unique proline-rich motifs in intercellular spaces of the legume nodule parenchyma. Protoplasma. 2005;225:43–55. doi: 10.1007/s00709-005-0090-x. [DOI] [PubMed] [Google Scholar]
- 217.Sujkowska M., Górska-Czekaj M., Bederska M., Borucki W. Vacuolar organization in the nodule parenchyma is important for the functioning of pea root nodules. Symbiosis. 2011;54:1. doi: 10.1007/s13199-011-0126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Gardner C.D., Sherrier D.J., Kardailsky I.V., Brewin N.J. Localization of lipoxygenase proteins and mRNA in pea nodules: Identification of lipoxygenase in the lumen of infection threads. Mol. Plant Microbe Interact. 1996;9:282–289. doi: 10.1094/MPMI-9-0282. [DOI] [Google Scholar]
- 219.Rubio M.C., James E.K., Clemente M.R., Bucciarelli B., Fedorova M., Vance C.P., Becana M. Localization of superoxide dismutases and hydrogen peroxide in legume root nodules. Mol. Plant Microbe Interact. 2004;17:1294–1305. doi: 10.1094/MPMI.2004.17.12.1294. [DOI] [PubMed] [Google Scholar]
- 220.Sujkowska-Rybkowska M., Borucki W. Localization of hydrogen peroxide accumulation and diamine oxidase activity in pea root nodules under aluminum stress. Micron. 2014;57:13–22. doi: 10.1016/j.micron.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 221.Santos R., Hérouart D., Sigaud S., Touati D., Puppo A. Oxidative burst in alfalfa-Sinorhizobium meliloti symbiotic interaction. Mol. Plant Microbe Interact. 2001;14:86–89. doi: 10.1094/MPMI.2001.14.1.86. [DOI] [PubMed] [Google Scholar]
- 222.Jamet A., Mandon K., Puppo A., Hérouart D. H2O2 is required for optimal establishment of the Medicago sativa/Sinorhizobium meliloti symbiosis. J. Bacteriol. 2007;189:8741–8745. doi: 10.1128/JB.01130-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Tsyganova A.V., Tsyganov V., Borisov A.Y., Tikhonovich I.A., Brewin N.J. Comparative cytochemical analysis of hydrogen peroxide distribution in pea ineffective mutant SGEFix−-1 (sym40) and initial line SGE. Ekol. Genet. 2009;7:3–9. doi: 10.17816/ecogen733-9. [DOI] [Google Scholar]
- 224.Fauvart M., Verstraeten N., Dombrecht B., Venmans R., Beullens S., Heusdens C., Michiels J. Rhizobium etli HrpW is a pectin-degrading enzyme and differs from phytopathogenic homologues in enzymically crucial tryptophan and glycine residues. Microbiology. 2009;155:3045–3054. doi: 10.1099/mic.0.027599-0. [DOI] [PubMed] [Google Scholar]
- 225.Lievens S., Goormachtig S., Herman S., Holsters M. Patterns of pectin methylesterase transcripts in developing stem nodules of Sesbania rostrata. Mol. Plant Microbe Interact. 2002;15:164–168. doi: 10.1094/MPMI.2002.15.2.164. [DOI] [PubMed] [Google Scholar]
- 226.Rodríguez-Llorente I.D., Pérez-Hormaeche J., Mounadi K.E., Dary M., Caviedes M.A., Cosson V., Kondorosi A., Ratet P., Palomares A.J. From pollen tubes to infection threads: Recruitment of Medicago floral pectic genes for symbiosis. Plant J. 2004;39:587–598. doi: 10.1111/j.1365-313X.2004.02155.x. [DOI] [PubMed] [Google Scholar]
- 227.Frueauf J.B., Dolata M., Leykam J.F., Lloyd E.A., Gonzales M., VandenBosch K., Kieliszewski M.J. Peptides isolated from cell walls of Medicago truncatula nodules and uninfected root. Phytochemistry. 2000;55:429–438. doi: 10.1016/S0031-9422(00)00336-8. [DOI] [PubMed] [Google Scholar]
- 228.Arsenijevic-Maksimovic I., Broughton W.J., Krause A. Rhizobia modulate root-hair-specific expression of extensin genes. Mol. Plant Microbe Interact. 1997;10:95–101. doi: 10.1094/MPMI.1997.10.1.95. [DOI] [PubMed] [Google Scholar]
- 229.Giordano W., Hirsch A.M. The expression of MaEXP1, a Melilotus alba expansin gene, is upregulated during the sweetclover-Sinorhizobium meliloti interaction. Mol. Plant Microbe Interact. 2004;17:613–622. doi: 10.1094/MPMI.2004.17.6.613. [DOI] [PubMed] [Google Scholar]
- 230.Lee A., Giordano W., Hirsch A.M. Cytokinin induces expansin gene expression in Melilotus alba Desr. wild-type and the non-nodulating, non-mycorrhizal (Nod-Myc-) mutant Masym3. Plant Signal. Behav. 2008;3:218–223. doi: 10.4161/psb.3.4.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Greene E.A., Erard M., Dedieu A., Barker D.G. MtENOD16 and 20 are members of a family of phytocyanin-related early nodulins. Plant Mol. Biol. 1998;36:775–783. doi: 10.1023/A:1005916821224. [DOI] [PubMed] [Google Scholar]
- 232.Dolgikh E.A., Leppyanen I.V., Osipova M.A., Savelyeva N.V., Borisov A.Y., Tsyganov V.E., Geurts R., Tikhonovich I.A. Genetic dissection of Rhizobium-induced infection and nodule organogenesis in pea based on ENOD12A and ENOD5 expression analysis. Plant Biol. 2011;13:285–296. doi: 10.1111/j.1438-8677.2010.00372.x. [DOI] [PubMed] [Google Scholar]
- 233.Tsyganova A.V., Tsyganov V.E. Plant cell wall in symbiotic interactions. Pectins. Agric. Biol. 2019;54:446–457. doi: 10.15389/agrobiology.2019.3.446eng. [DOI] [Google Scholar]
- 234.Ivanov S., Fedorova E.E., Limpens E., De Mita S., Genre A., Bonfante P., Bisseling T. Rhizobium–legume symbiosis shares an exocytotic pathway required for arbuscule formation. Proc. Natl. Acad. Sci. USA. 2012;109:8316–8321. doi: 10.1073/pnas.1200407109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Atmodjo M.A., Hao Z., Mohnen D. Evolving views of pectin biosynthesis. Ann. Rev. Plant Biol. 2013;64:747–779. doi: 10.1146/annurev-arplant-042811-105534. [DOI] [PubMed] [Google Scholar]
- 236.Brewin N.J., Rae A.L., Perotto S., Knox J.P., Roberts K., LeGal M.F., Sindhu S.S., Wood E.A., Kannenberg E.L. Immunological dissection of the plant-microbe interface in pea nodules. In: Gresshoff P.M., Roth L.E., Stacey G., Newton W.E., editors. Nitrogen Fixation. Springer; Boston, MA, USA: 1990. pp. 227–234. [DOI] [Google Scholar]
- 237.Cassab G.I. Arabinogalactan proteins during the development of soybean root nodules. Planta. 1986;168:441–446. doi: 10.1007/BF00392262. [DOI] [PubMed] [Google Scholar]
- 238.Nguema-Ona E., Vicré-Gibouin M., Cannesan M.-A., Driouich A. Arabinogalactan proteins in root–microbe interactions. Trends Plant Sci. 2013;18:440–449. doi: 10.1016/j.tplants.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 239.Perotto S., Vandenbosch K.A., Butcher G.W., Brewin N.J. Molecular composition and development of the plant glycocalyx associated with the peribacteroid membrane of pea root-nodules. Development. 1991;112:763–773. doi: 10.1242/dev.112.3.763. [DOI] [Google Scholar]
- 240.Gucciardo S., Rathbun E.A., Shanks M., Jenkyns S., Mak L., Durrant M.C., Brewin N.J. Epitope tagging of legume root nodule extensin modifies protein structure and crosslinking in cell walls of transformed tobacco leaves. Mol. Plant Microbe Interact. 2005;18:24–32. doi: 10.1094/MPMI-18-0024. [DOI] [PubMed] [Google Scholar]
- 241.Legocki R.P., Verma D.P.S. Identification of “nodule-specific” host proteins (nodulins) involved in the development of Rhizobium-legume symbiosis. Cell. 1980;20:153–163. doi: 10.1016/0092-8674(80)90243-3. [DOI] [PubMed] [Google Scholar]
- 242.Jeffree C.E., Dale J.E., Fry S.C. The genesis of intercellular spaces in developing leaves of Phaseolus vulgaris L. Protoplasma. 1986;132:90–98. doi: 10.1007/BF01275795. [DOI] [Google Scholar]
- 243.Peleg-Grossman S., Melamed-Book N., Levine A. ROS production during symbiotic infection suppresses pathogenesis-related gene expression. Plant Signal. Behav. 2012;7:409–415. doi: 10.4161/psb.19217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Montiel J., Nava N., Cárdenas L., Sánchez-López R., Arthikala M.-K., Santana O., Sánchez F., Quinto C. A Phaseolus vulgaris NADPH oxidase gene is required for root infection by rhizobia. Plant Cell Physiol. 2012;53:1751–1767. doi: 10.1093/pcp/pcs120. [DOI] [PubMed] [Google Scholar]
- 245.Arthikala M.-K., Montiel J., Sánchez-López R., Nava N., Cárdenas L., Quinto C. Respiratory burst oxidase homolog gene A is crucial for Rhizobium infection and nodule maturation and function in common bean. Front. Plant Sci. 2017;8:2003. doi: 10.3389/fpls.2017.02003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Provorov N.A., Tsyganova A.V., Brewin N.J., Tsyganov V.E., Vorobyov N.I. Evolution of symbiotic bacteria within the extra- and intra-cellular plant compartments: Experimental evidence and mathematical simulation (Mini-review) Symbiosis. 2012;58:39–50. doi: 10.1007/s13199-012-0220-0. [DOI] [Google Scholar]
- 247.Lehman A.P., Long S.R. Exopolysaccharides from Sinorhizobium meliloti can protect against H2O2-dependent damage. J. Bacteriol. 2013;195:5362–5369. doi: 10.1128/JB.00681-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Mithöfer A. Suppression of plant defence in rhizobia–legume symbiosis. Trends Plant Sci. 2002;7:440–444. doi: 10.1016/S1360-1385(02)02336-1. [DOI] [PubMed] [Google Scholar]
- 249.Soto M.J., Sanjuán J., Olivares J. Rhizobia and plant-pathogenic bacteria: Common infection weapons. Microbiology. 2006;152:3167–3174. doi: 10.1099/mic.0.29112-0. [DOI] [PubMed] [Google Scholar]
- 250.van Workum W.A.T., van Slageren S., van Brussel A.A.N., Kijne J.W. Role of exopolysaccharides of Rhizobium leguminosarum bv. viciae as host plant-specific molecules required for infection thread formation during nodulation of Vicia sativa. Mol. Plant Microbe Interact. 1998;11:1233–1241. doi: 10.1094/mpmi.1998.11.12.1233. [DOI] [Google Scholar]
- 251.Niehaus K., Kapp D., Pühler A. Plant defence and delayed infection of alfalfa pseudonodules induced by an exopolysaccharide (EPS I)-deficient Rhizobium meliloti mutant. Planta. 1993;190:415–425. doi: 10.1007/BF00196971. [DOI] [Google Scholar]
- 252.Beck S., Marlow V.L., Woodall K., Doerrler W.T., James E.K., Ferguson G.P. The Sinorhizobium meliloti MsbA2 protein is essential for the legume symbiosis. Microbiology. 2008;154:1258–1270. doi: 10.1099/mic.0.2007/014894-0. [DOI] [PubMed] [Google Scholar]
- 253.Albus U., Baier R., Holst O., Pühler A., Niehaus K. Suppression of an elicitor-induced oxidative burst reaction in Medicago sativa cell cultures by Sinorhizobium meliloti lipopolysaccharides. New Phytol. 2001;151:597–606. doi: 10.1046/j.0028-646x.2001.00214.x. [DOI] [PubMed] [Google Scholar]
- 254.Scheidle H., Groß A., Niehaus K. The lipid A substructure of the Sinorhizobium meliloti lipopolysaccharides is sufficient to suppress the oxidative burst in host plants. New Phytol. 2005;165:559–566. doi: 10.1111/j.1469-8137.2004.01214.x. [DOI] [PubMed] [Google Scholar]
- 255.Bright L.J., Liang Y., Mitchell D.M., Harris J.M. The LATD gene of Medicago truncatula is required for both nodule and root development. Mol. Plant Microbe Interact. 2005;18:521–532. doi: 10.1094/MPMI-18-0521. [DOI] [PubMed] [Google Scholar]
- 256.Yendrek C.R., Lee Y.-C., Morris V., Liang Y., Pislariu C.I., Burkart G., Meckfessel M.H., Salehin M., Kessler H., Wessler H., et al. A putative transporter is essential for integrating nutrient and hormone signaling with lateral root growth and nodule development in Medicago truncatula. Plant J. 2010;62:100–112. doi: 10.1111/j.1365-313X.2010.04134.x. [DOI] [PubMed] [Google Scholar]
- 257.Ovchinnikova E., Journet E.-P., Chabaud M., Cosson V., Ratet P., Duc G., Fedorova E., Liu W., den Camp R.O., Zhukov V., et al. IPD3 controls the formation of nitrogen-fixing symbiosomes in pea and Medicago Spp. Mol. Plant Microbe Interact. 2011;24:1333–1344. doi: 10.1094/MPMI-01-11-0013. [DOI] [PubMed] [Google Scholar]
- 258.Tsyganova A.V., Kitaeva A.B., Tsyganov V.E. Cell differentiation in nitrogen-fixing nodules hosting symbiosomes. Funct. Plant Biol. 2018;45:47–57. doi: 10.1071/FP16377. [DOI] [PubMed] [Google Scholar]
- 259.Bassett B., Goodman R.N., Novacky A. Ultrastructure of soybean nodules. I: Release of rhizobia from the infection thread. Can. J. Microbiol. 1977;23:573–582. doi: 10.1139/m77-083. [DOI] [PubMed] [Google Scholar]
- 260.Cermola M., Fedorova E., Taté R., Riccio A., Favre R., Patriarca E.J. Nodule invasion and symbiosome differentiation during Rhizobium etli–Phaseolus vulgaris symbiosis. Mol. Plant Microbe Interact. 2000;13:733–741. doi: 10.1094/MPMI.2000.13.7.733. [DOI] [PubMed] [Google Scholar]
- 261.Robertson J.G., Lyttleton P., Bullivant S., Grayston G.F. Membranes in lupin root nodules. I. The role of Golgi bodies in the biogenesis of infection threads and peribacteroid membranes. J. Cell Sci. 1978;30:129–149. doi: 10.1242/jcs.30.1.129. [DOI] [PubMed] [Google Scholar]
- 262.Kereszt A., Mergaert P., Montiel J., Endre G., Kondorosi É. Impact of plant peptides on symbiotic nodule development and functioning. Front. Plant Sci. 2018;9:1026. doi: 10.3389/fpls.2018.01026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Ivanov S., Fedorova E., Bisseling T. Intracellular plant microbe associations: Secretory pathways and the formation of perimicrobial compartments. Curr. Opin. Plant Biol. 2010;13:372–377. doi: 10.1016/j.pbi.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 264.Bradley D.J., Butcher G.W., Galfre G., Wood E.A., Brewin N.J. Physical association between the peribacteroid membrane and lipopolysaccharide from the bacteroid outer membrane in Rhizobium-infected pea root nodule cells. J. Cell Sci. 1986;85:47–61. doi: 10.1242/jcs.85.1.47. [DOI] [PubMed] [Google Scholar]
- 265.Venado R.E., Liang J., Marín M. Rhizobia infection, a journey to the inside of plant cells. In: Frendo P., Frugier F., Masson-Boivin C., editors. Advances in Botanical Research. Vol. 94. Academic Press; London, UK: San Diego, CA, USA: Waltham, MA, USA: Oxford, UK: 2020. pp. 97–118. [Google Scholar]
- 266.Ferguson G.P., Roop R.M., Walker G.C. Deficiency of a Sinorhizobium meliloti bacA mutant in alfalfa symbiosis correlates with alteration of the cell envelope. J. Bacteriol. 2002;184:5625–5632. doi: 10.1128/JB.184.20.5625-5632.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Kannenberg E.L., Perotto S., Bianciotto V., Rathbun E.A., Brewin N.J. Lipopolysaccharide epitope expression of Rhizobium bacteroids as revealed by in situ immunolabelling of pea root nodule sections. J. Bacteriol. 1994;176:2021–2032. doi: 10.1128/JB.176.7.2021-2032.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Bourassa D.V., Kannenberg E.L., Sherrier D.J., Buhr R.J., Carlson R.W. The lipopolysaccharide lipid A long-chain fatty acid is important for Rhizobium leguminosarum growth and stress adaptation in free-living and nodule environments. Mol. Plant Microbe Interact. 2017;30:161–175. doi: 10.1094/MPMI-11-16-0230-R. [DOI] [PubMed] [Google Scholar]
- 269.Perotto S., Brewin N., Kannenberg E. Cytological evidence for a host defense response that reduces cell and tissue invasion in pea nodules by lipopolysaccharide-defective mutants of Rhizobium leguminosarum strain 3841. Mol. Plant Microbe Interact. 1994;7:99–112. doi: 10.1094/MPMI-7-0099. [DOI] [Google Scholar]
- 270.Serova T.A., Tsyganov V.E. Symbiotic nodule senescence in legumes: Molecular-genetic and cellular aspects (review) Agric. Biol. 2014;5:3–15. doi: 10.15389/agrobiology.2014.5.3eng. [DOI] [Google Scholar]
- 271.Timmers A.C.J., Soupène E., Auriac M.-C., de Billy F., Vasse J., Boistard P., Truchet G. Saprophytic intracellular rhizobia in alfalfa nodules. Mol. Plant Microbe Interact. 2000;13:1204–1213. doi: 10.1094/MPMI.2000.13.11.1204. [DOI] [PubMed] [Google Scholar]
- 272.Skawińska M., Sańko-Sawczenko I., Dmitruk D., Czarnocka W., Łotocka B. Organization and ultrastructure of Medicago truncatula root apical meristem. In: de Bruijn F., editor. The Model Legume Medicago Truncatula. John Wiley & Sons; Hoboken, NJ, USA: 2020. p. 709. [DOI] [Google Scholar]
- 273.Xi J., Chen Y., Nakashima J., Wang S.-m., Chen R. Medicago truncatula esn1 defines a genetic locus involved in nodule senescence and symbiotic nitrogen fixation. Mol. Plant Microbe Interact. 2013;26:893–902. doi: 10.1094/MPMI-02-13-0043-R. [DOI] [PubMed] [Google Scholar]
- 274.Melino V.J., Drew E.A., Ballard R.A., Reeve W.G., Thomson G., White R.G., O’Hara G.W. Identifying abnormalities in symbiotic development between Trifolium spp. and Rhizobium leguminosarum bv. trifolii leading to sub-optimal and ineffective nodule phenotypes. Ann. Bot. 2012;110:1559–1572. doi: 10.1093/aob/mcs206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Zahran H.H. Rhizobium-legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiol. Mol. Biol. Rev. 1999;63:968–989. doi: 10.1128/MMBR.63.4.968-989.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Chaudhary P., Dudeja S.S., Kapoor K.K. Effectivity of host-Rhizobium leguminosarum symbiosis in soils receiving sewage water containing heavy metals. Microbiol. Res. 2004;159:121–127. doi: 10.1016/j.micres.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 277.Gorshkov A.P., Tsyganova A.V., Vorobiev M.G., Tsyganov V.E. The fungicide tetramethylthiuram disulfide negatively affects plant cell walls, infection thread walls, and symbiosomes in pea (Pisum sativum L.) symbiotic nodules. Plants. 2020;9:1488. doi: 10.3390/plants9111488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Delgadillo J., Lafuente A., Doukkali B., Redondo-Gómez S., Mateos-Naranjo E., Caviedes M.A., Pajuelo E., Rodríguez-Llorente I.D. Improving legume nodulation and Cu rhizostabilization using a genetically modified rhizobia. Environ. Technol. 2015;36:1237–1245. doi: 10.1080/09593330.2014.983990. [DOI] [PubMed] [Google Scholar]
- 279.Fagorzi C., Checcucci A., DiCenzo G.C., Debiec-Andrzejewska K., Dziewit L., Pini F., Mengoni A. Harnessing rhizobia to improve heavy-metal phytoremediation by legumes. Genes. 2018;9:542. doi: 10.3390/genes9110542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Tsyganov V.E., Tsyganova A.V., Gorshkov A.P., Seliverstova E.V., Kim V.E., Chizhevskaya E.P., Belimov A.A., Serova T.A., Ivanova K.A., Kulaeva O.A., et al. Efficacy of a plant-microbe system: Pisum sativum (L.) cadmium-tolerant mutant and Rhizobium leguminosarum strains, expressing pea metallothionein genes PsMT1 and PsMT2, for cadmium phytoremediation. Front. Microbiol. 2020;11:15. doi: 10.3389/fmicb.2020.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Montiel J., Reid D., Grønbæk T.H., Benfeldt C.M., James E.K., Ott T., Ditengou F.A., Nadzieja M., Kelly S., Stougaard J. Distinct signaling routes mediate intercellular and intracellular rhizobial infection in Lotus japonicus. Plant Physiol. 2020;185:1131–1147. doi: 10.1093/plphys/kiaa049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Borisov A.Y., Danilova T.N., Koroleva T.A., Kuznetsova E.V., Madsen L., Mofett M., Naumkina T.S., Nemankin T.A., Ovchinnikova E.S., Pavlova Z.B., et al. Regulatory genes of garden pea (Pisum sativum L.) controlling the development of nitrogen-fixing nodules and arbuscular mycorrhiza: A review of basic and applied aspects. Appl. Biochem. Microbiol. 2007;43:237–243. doi: 10.1134/S0003683807030027. [DOI] [PubMed] [Google Scholar]
- 283.Radhakrishnan G.V., Keller J., Rich M.K., Vernié T., Mbadinga Mbadinga D.L., Vigneron N., Cottret L., Clemente H.S., Libourel C., Cheema J., et al. An ancestral signalling pathway is conserved in intracellular symbioses-forming plant lineages. Nat. Plants. 2020;6:280–289. doi: 10.1038/s41477-020-0613-7. [DOI] [PubMed] [Google Scholar]