Abstract
Alzheimer’s disease (AD) is characterized by the accumulation in the brain of extracellular amyloid β (Aβ) plaques as well as intraneuronal inclusions (neurofibrillary tangles) consisting of total tau and phosphorylated tau. Also present are dystrophic neurites, loss of synapses, neuronal death, and gliosis. AD genetic studies have highlighted the importance of inflammation in this disease by identifying several risk associated immune response genes, including TREM2. TREM2 has been strongly implicated in basic microglia function including, phagocytosis, apoptosis, and the inflammatory response to Aβ in mouse brain and primary cells. These studies show that microglia are key players in the response to Aβ and in the accumulation of AD pathology. However, details are still missing about which apoptotic or inflammatory factors rely on TREM2 in their response to Aβ, especially in human cell lines. Given these previous findings our hypothesis is that TREM2 influences the response to Aβ toxicity by enhancing phagocytosis and inhibiting both the BCL-2 family of apoptotic proteins and pro-inflammatory cytokines. Aβ42 treatment of the human microglial cell line, HMC3 cells, was performed and TREM2 was overexpressed or silenced and the phagocytosis, apoptosis and inflammatory response were evaluated. Results indicate that a robust phagocytic response to Aβ after 24 hours requires TREM2 in HMC3 cells. Also, TREM2 inhibits Aβ induced apoptosis by activating the Mcl-1/Bim complex. TREM2 is involved in activation of IP-10, MIP-1a, and IL-8, while it inhibits FGF-2, VEGF and GRO. Taken together, TREM2 plays a role in enhancing the microglial functional response to Aβ toxicity in HMC3 cells. This novel information suggests that therapeutic strategies that seek to activate TREM2 may not only enhance phagocytosis and inhibit apoptosis, but may also inhibit beneficial inflammatory factors, emphasizing the need to define TREM2-related inflammatory activity in not only mouse models of AD, but also in human AD.
Keywords: Aβ, TREM2, Microglia, Phagocytosis, Apoptosis, Inflammatory factors
1. Introduction
Alzheimer’s disease (AD) is the leading cause of dementia worldwide (Alzheimer’s 2016). The histopathological hallmarks of AD are extracellular amyloid plaques composed predominantly of the amyloid-β1–42 (Aβ42) peptide and neurofibrillary tangles (NFTs) within neurons consisting of abnormally aggregated, hyperphosphorylated tau protein (Castellani et al. 2010, Serrano-Pozo et al. 2011). Microglia are the resident innate immune cells in the central nervous system (CNS) that survey the surrounding environment (Wang and Colonna 2019). Genome-wide association studies (GWAS) have identified the loss of function genetic variants of triggering receptor expressed on myeloid cell 2 (TREM2), immunoreceptor primarily present on microglia, are associated with Alzheimer’s disease risk, in which chronic inflammatory responses can occur (Guerreiro et al. 2013, Jonsson et al. 2013, Abduljaleel et al. 2014, Gallardo and Holtzman 2019),
Microglia survey the brain for abnormalities and are rapidly activated to phagocytose cellular debris and are thus key players in maintaining brain homeostasis (Priller and Prinz 2019, Afridi et al. 2020). Microglial cells come in close proximity of the senile plaques in AD and undergo morphological change from resting ramified appearance to an activated amoeboid phenotype (Mandrekar-Colucci and Landreth 2010). The microglial phenotypic plasticity and the impact of microglial phagocytosis play important role in disease progression (Lull and Block 2010). Mouse models and mouse microglia studies have revealed that TREM2 is involved in a variety of physiological processes, such as phagocytosis of Aβ and the pro-inflammatory response (Gao et al. 2013, Sieber et al. 2013, Kawabori et al. 2015). TREM2 suppresses the pro-inflammatory response (Takahashi et al. 2005, Takahashi et al. 2007) and TREM2 deletion abolishes Aβ-induced microglial proliferation and apoptosis (Zhao et al. 2018).
Apoptosis is an essential physiological process that plays a critical role tissue homeostasis. At least two major apoptotic pathways have been described in mammalian cells: the intrinsic pathway via Caspase-9 activation, in which death arises from mitochondrial dysfunction, and the extrinsic pathway via Caspase-8 activation, in which death is initiated from the activation of cell surface receptors. Bcl-2 family members play an important role in the intrinsic apoptotic pathway in several AD models (Paradis et al. 1996, Akhter et al. 2015, Akhter et al. 2018). The Bcl-2 family constitutes pro-survival (e.g. Bcl-2 and BclxL), multidomain proapoptotic (e.g. BAX and BAK), and BH3-only proapoptotic (e.g. Bim, Puma, Bid) proteins (Youle and Strasser 2008, Akhter et al. 2014). Interactions of Bcl-2 family members function as a ‘life/death switch’ that integrates diverse inter- and intracellular cues to determine whether or not the stress apoptosis pathway should be activated. The Bcl-2 family has been involved in the microglia response to Aβ (Shang et al. 2012). The Bcl family of proteins have been implicated in the Bcl family Akt signaling response to amyloid (Clementi et al. 2006, Yin et al. 2011, Zhu et al. 2015). However, even though TREM2-related Akt signaling has been described, (He et al. 2019, Chen et al. 2020) to our knowledge a link between TREM2 specific signaling and Bcl-2 family mediated apoptosis has not been studied.
In addition, TREM2 inhibits the inflammatory response in mouse microglia, but less is known about the response to Aβ in human microglia. TREM2 inhibits the inflammatory response to LPS in mouse microglia by suppressing the PI3K/NF-κB signaling (Li et al. 2019). TREM2 silencing restrained the replication of PRRSV, whereas TREM2 overexpression facilitated viral replication while TREM2 downregulation led to early activation of PI3K/NF-κB signaling, thus reinforcing the expression of proinflammatory cytokines and type I interferons (Zhu et al. 2020). Furthermore, TREM2 overexpression downregulates the expression of the TLR family (TLR2, TLR4 and TLR6) in BV-2 cells and LPS, as an agonist of the TLR family, up-regulated the expression of inflammatory cytokines (IL-1β, TNF-α, and IL-6) in BV-2 cells overexpressing TREM2 (Long et al. 2019). Interestingly, in human studies, the levels of TREM2, IL-6, and TNF-α significantly increase in peripheral blood of spinal cord injury patients as well as in LPS-stimulated mouse primary microglia cells (Wang et al. 2019). The cleaved soluble TREM2 isoform has been associated with alterations in the immune response in Down syndrome (Weber et al. 2020), after exercise (Jensen et al. 2019), and AD (Bekris et al. 2018, Brosseron et al. 2018, Rauchmann et al. 2020). Interestingly, CSF sTREM2 has been recently been described as positively associated with the pro-inflammatory proteins and anti-inflammatory proteins (Rauchmann et al. 2020). Indeed, it has been suggested that sTREM2 cerebrospinal fluid levels are a potential biomarker for microglia activity in early-stage Alzheimer’s disease and associated with neuronal injury markers (Suárez-Calvet et al. 2016). However, which inflammatory factors are influenced by TREM2 after Aβ treatment in human microglia cell lines has not been fully elucidated.
Therefore, the aim of this study is to identify key factors influenced by TREM2 in the response to Aβ42 treatment. Given previous evidence the hypothesis is that TREM2 enhances the response to Aβ toxicity by enhancing phagocytosis and inhibiting both the Bcl family of apoptotic proteins and pro-inflammatory cytokines. In this investigation three critical microglia activities, phagocytosis, the production of intrinsic apoptotic proteins and the production of inflammatory factors were evaluated to understand whether TREM2 alters the response to Aβ toxicity in a human microglia cell line. Indeed, the results suggest that phagocytic, apoptotic and inflammatory response to Aβ requires TREM2.
2. Materials and Methods
Cell Culture and Transfections
To select a cell line with a moderate level of TREM2 expression, immortalized human cell lines were obtained from American Type Culture Collection (ATCC®) to represent an array of cell types while focusing primarily on myeloid cell types since these cell types are known to express TREM2 and are the primary cell type of interest in AD studies of TREM2. Cells lines of non-glial cell types were selected as control comparisons to glial cell types and included a hepatocyte cell line; HepG2, two neuronal cell lines; IMR-32, CHP-212, and two monocyte cell lines; THP-1, U937. Three glial cell lines; U87, U118, HMC3 were included for selection of a moderate TREM2 expressing glial cell line to test hypotheses. HepG2, IMR-32, U87, U118, U138 cell lines were cultured in DMEM medium, HMC3 with EMEM, THP-1 with RPMI and U937 with RPMI-1640. All cells line culture conditions also included 10% FBS and 1% penicillin/streptomycin at 37 °C in a humidified atmosphere containing 95% air and 5% CO2, and the medium was changed every two days. The cells were split with 0.05% trypsin when they reached 80% confluence and sub-cultured for further passages. HMC3 (ATCC®: CRL-3304™) have been reviewed (Janabi et al. 1995, Li et al. 2009, Jadhav et al. 2014, Nakagawa and Chiba 2015, Dello Russo et al. 2018). Transfections were performed as previously described (Akhter et al. 2018) and included a human TREM2 ORF mammalian expression plasmid vector (6158 bp) tagged with Flag (pCMV3-C-Flag) under the promoter CMV for TREM2 overexpression experiments per manufacturer protocols (Sino Biological Inc, Cat# HG11084-CF) as well as a human TREM2 siRNA for TREM2 silencing experiments (ThermoFisher Scientific; Assay ID: 289814).
Quantitative RT-PCR and Immunoblot Analysis
RNA and protein were extracted from cell lines using the Qiagen AllPrep DNA/RNA Mini Kit (Qiagen, Valencia, CA, USA). Total RNA was also DNase treated using the TURBO DNA-free Kit (Applied Biosystems, Austin, TX, USA) to further deplete DNA content. Protein was extracted using a modification of the Qiagen AllPrep DNA/RNA Mini Kit (Qiagen) according to manufacturer instructions. Total cell line extracts (36 μg protein) from the different treatments were separated by 12.5% SDS-PAGE and electroblotted onto supported nitrocellulose. Each blot was blocked for 1 h in Tris-buffered saline/Tween-20 (10 mM Tris base, 150 mM NaCl, 0.05% Tween-20) containing 5% fat-free milk, and then incubated with a primary TREM2 antibody (TREM2 Ab MAB1828: R&D Systems) overnight at 4°C. Washing of membranes three times (10 min each) with Tris-buffered saline/Tween-20 was followed by incubation for 1 h at room temperature with the appropriate secondary antibody (Anti-mouse IgG horseradish peroxidase cat# NA931V from GE Healthcare, Uk). Western blots of at least duplicate experiments were developed using the ECL Prime western blotting detection Reagent (RPN 2232: GE Healthcare) and were reprobed with anti-actin (Actin A4700 mouse monoclonal: Sigma) to verify that proteins were uniformly loaded across the gel. Integrated density of the western blot bands was normalized to actin. Band integrated density was measured with ImageJ (US National Institutes of Health, rsb.info.nih.gov/ij/). TREM2 mRNA was measured in triplicate by quantitative real-time PCR gene expression assay spanning exons 2–3 (TaqMan® Assays ThermoFisher Scientific: Hs01003898_m1) using the 7500 Real Time PCR System (Applied Biosystems, Foster City, CA, USA), with β-actin (ACTB) as a loading control (Applied Biosystems). All qRT-PCR results are presented as a TREM2/ACTB (ΔCT).
Aβ42 treatment
Solution of oligomeric Aβ1–42 from lyophilized, high performance liquid chromatography-purified Aβ1–42 was prepared as described previously (Barghorn et al. 2005). First, 100% HFIP (1,1,1,3,3,3-hexafluoro-2-propanol) was used to reconstitute Aβ1–42 (1 mM), and then HFIP was removed by evaporation using a Speed Vac (Eppendorf, Hamburg, Germany). The obtained pellet was then resuspended to 5 μM in anhydrous dimethyl sulfoxide. This stock was diluted with phosphate-buffered saline to a final concentration of 400 μM, and sodium dodecyl sulfate was added to a final concentration of 0.2%. The resulting solution was then incubated at 37 °C for 18–24 hours. The preparation was again incubated at 37 °C for 18–24 hours after further dilution with phosphate-buffered saline to a final concentration of 100 μM. The nature of the Aβ1–42 oligomers of the preparation was then checked by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (Akhter et al. 2015). Cells were treated with 1 – 20 μM Aβ42 for 2–72 hours (data not shown). A 5 μM Aβ42 treatment for 24 hours was selected for phagocytosis, apoptosis and inflammatory panel analyses because of moderate effect on cell survival and phagocytosis parameters.
Immunocytochemistry, Morphology and Phagocytosis Assay
HMC3 cells were fixed with 4% paraformaldehyde for 10 min and then were washed with PBS three times for 5 min each. The cells were then blocked in 3% goat serum in PBS containing 0.1% Triton X-100 for 2 h at room temperature. The cells were incubated with anti-TREM2 antibody (R&D, 1:100) and microglial marker IBA-1 antibody (Wako, 1:300) in a blocking solution overnight at 4 °C. Alexa Fluor 546 (Invitrogen Life technologies, 1:500) was used as secondary antibody, and the nuclei were stained with Hoechst. Cells were examined under the fluorescence microscope (20x Leica DFC3000G).
HMC3 morphology was examined under the microscope (20x Leica DFC3000G) with and without 5 μM Aβ42 treatment for 24 hours to visualize activated morphology. Vybrant™ Phagocytosis Assay Kit (Invitrogen) was utilized to evaluate phagocytosis alterations in HMC3 cells with and without Aβ42 treatment and TREM2 overexpression or silencing in at least duplicate experiments. Cells were harvested from tissue culture plate and transferred to 96 well microplate at a concentration of 10^6/ml. A Negative control was included consisting of only 200 μl of cell culture medium. A Positive control was included consisting of 200 μl of adjusted cell suspension. Also 200 ul of adjusted cell suspension was added to the experimental wells. The plates were incubated overnight optimum adherence. The next day cells were treated with 5 μM Aβ42. After 0, 8 24, 48 and 72 hour incubation, media was removed and 100 μl was added of prepared fluorescent bioparticles to all the negative control, positive control and experimental wells and incubated for 2 hours. Bioparticles were removed and 100 μl of the prepared trypan blue suspension was added to all of the wells and incubated for 1 min at room temp. After removal of the trypan blue suspension phagocytosis status was immediately evaluated by fluorescence plate reader at 480 nm excitation, 520 nm emission (Synergy HTX multi-mode reader by BioTeK) (Gopallawa et al. 2020).
Cell Survival and Apoptosis Assays
HMC3 cell viability in response to Aβ42 treatment was determined using MTT (0.5 mg/ml) assay and trypan blue exclusion (TBE) from at least duplicate experiments. HMC3 cells were cultivated at a density of 10,000 cells/well in 96-well plates for 0, 8 24, 48 and 72 hours. Cells were then stained with 20 μl MTT 5 mg/ml stock solution (Thiazolyl Blue Tetrazolium Bromide: Sigma-Aldrich Cat: 5655) for 4 hours and then dissolved in DMSO. The optical density was determined at 590 nm (Plate Reader Synerty MX by BioTek). To evaluate whether the intrinsic apoptotic pathway is activated by 24 hours of 5 μM Aβ42 treatment with TREM2 overexpression or silencing, the MILLIPLEX® MAP 6-plex Bcl-2 Family Apoptosis Panel 1 Magnetic Bead Kit (Millipore: Cat. No. 48–682MAG) was utilized. Six analytes were measured utilizing cell lystates from at least duplicate experiments (Luminex): pBAD (Ser112), BAD (total), BIM (total), BAX (total), Bcl-xL/BAD, and Mcl-1/BIM according to manufacturer instructions. This panel was selected because these Bcl-2 family members together provide a marker of the activation of a ‘life/death switch’ in cells (Tsujimoto and Shimizu 2000).
Inflammatory Factor Assay
In at least duplicate experiments 25 μl of HMC3 cell culture media to evaluate inflammatory factor concentration utilizing a MILLIPLEX MAP multiplex kit (HCYTMAG60PMX41BK; EMD Millipore) following the manufacturer’s instructions for human cytokine/chemokine analyte detection and the Luminex LX-200 system. The 38 inflammatory markers in the panel were as follows: epidermal growth factor (EGF), fibroblast growth factor 2 (FGF-2), eotaxin, TGF-α, G-CSF, FMS-like tyrosine kinase 3 ligand (Flt-3L), GM-CSF, fractalkine (also known as CX3CL1), IFN-α2, IFN-γ, growth-regulated oncogene (GRO), IL-10, monocyte chemotactic protein-3 (MCP-3, also known as CCL7), IL-12 40 kDa (IL-12p40), macrophage-derived chemokine (MDC), IL-12 70 kDa (IL-12P70), IL-13, IL-15, soluble CD40-ligand (sCD40L), IL-17A, IL-1 receptor agonist (IL-1RA), IL-1α, IL-9, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IFN-γ inducible protein 10 kDa (IP-10), MCP-1 (also known as CCL2), macrophage inflammatory protein (MIP) 1α (MIP-1α, also known as CCL3), MIP-1β (also known as CCL4), TNF-α, TNF-β, and vascular endothelial growth factor (VEGF).
3. Results
HMC3 cells express moderately low TREM2 levels relative to other cell lines
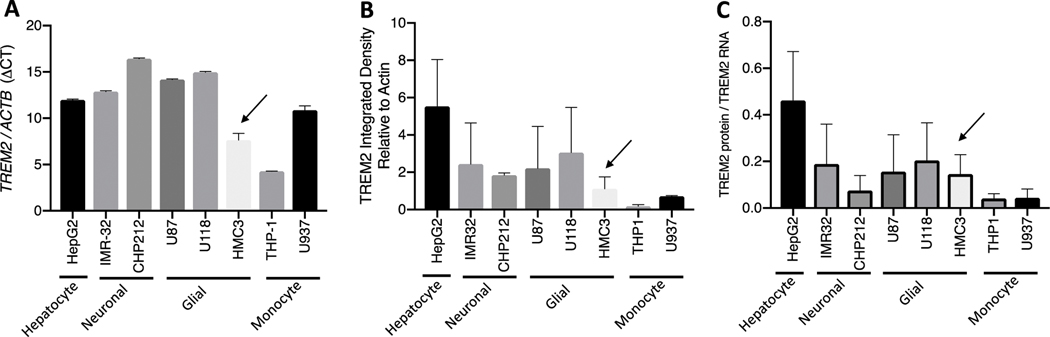
For selection of human cell line with low to moderate TREM2 expression, TREM2 RNA and protein expression was evaluated in eight human untreated cell lines: HepG2 hepatocytes; neuronal IMR-32 and CHP212; glial U87, U118, HMC3; monocytes, THP1, U937. TREM2 relative to ACTB RNA expression was lower in HMC3 than most other cell lines (Fig1A). TREM2 relative to Actin B was lower than many of the other cell lines (Fig1B). TREM2 protein relative to RNA was moderately low in HMC3 cells compared to the other cell lines (Fig1C).
Fig 1. HMC3 cells express low to moderate TREM2 levels relative to other cell lines.
TREM2 RNA ΔCT levels (Relative to ACTB) are lower than most other cell lines (A). TREM2 protein (Relative to Actin B) are lower than most other cell lines (B). TREM2 protein relative to TREM2 RNA is lower the HepG2 cell line (C).
Aβ42 treatment of HMC3 cells increases TREM2
To determine if Aβ42 treatment influences TREM2 expression in HMC3 cells, cells were treated and evaluated using immunostaining and Western blot. DAPI (blue), IBA1 (green) and TREM2 (red) immunostain of HMC3 cells treated with 5 μM Aβ42 indicates an increase in membrane cell surface TREM2 after 24 hours (Fig2A). TREM2 full length 28 kDa was significantly elevated after 24 hours 5 μM Aβ42 treatment on Western blot (Fig2B). TREM2 overexpression in HMC3 significantly increases TREM2 protein levels with or without Aβ42 treatment while TREM2 siRNA knockdown depletes TREM2 with or without Aβ42 treatment (Fig2C). These results demonstrate that the Aβ42 peptide stimulates TREM2 protein expression in HMC3 cells.
Fig 2. Aβ42 treatment of HMC3 cells increases TREM2.
Treatment of HMC3 cells with 5 μM Aβ for 24 hours increases cell surface TREM2 expression (A). TREM2 full length protein expression is significantly increase after 24 hour 5 μM Aβ42 treatment (25 kDa: p-value = 0.0247) (B). In a separate experiment, full length TREM2 relative to Actin (Western Blot: 25 kDa) is significantly increased, upon 24 hour 5 μM Aβ42 treatment while overexpression of TREM2 (OE) plus Aβ42 treatment does not significantly increase TREM2. Knockdown of TREM2 (siRNA) significantly depletes TREM2 with and without 24 hour 5 μM Aβ42 treatment (C). Fisher’s LSD test without correction for multiple comparisons significant p-value <0.05.
Lack of TREM2 decreases Aβ42 stimulated phagocytosis in HMC3 cells
To determine if TREM2 influences HMC3 Aβ42 stimulated phagocytosis, HMC3 cells were treated with 5 μM Aβ42 for 24 hours and phagocytosis assays performed. Aβ42 treatment changed HMC3 cell morphology from ramified to amoeboid after 24 hours (Fig3A–B). Aβ42 treatment significantly increased %phagocytosis at 8–24 hours and declined after 48–72 hours (Fig3C). TREM2 overexpression significantly increased phagocytosis but does not enhance phagocytosis after 24 hours of 5 μM Aβ42 treatment while TREM2 knockdown decreases phagocytosis with or without 24 hour 5 μM Aβ42 treatment (Fig3D). These results demonstrate that a lack of TREM2 depletes Aβ42 stimulated phagocytosis in HMC3 cells.
Fig 3. Lack of TREM2 decreases Aβ42 stimulated phagocytosis in HMC3 cells.
Treatment of HMC3 cells with 5 μM Aβ42 for 24 hours changes cell morphology from resting ramified microglial (A) to activated amoeboid microglia (B). Maximum phagocytosis is observed after 24 hours of 5 μM Aβ42 treatment (lines represent significant p- values <0.01) (C). After 24 hours of 5 μM Aβ42 treatment there is a significant increase in phagocytosis with or without overexpression of TREM2 and upon TREM2 knockdown there is a decrease in phagocytosis with or without 24 hour 5 μM Aβ42 treatment (D).
Aβ42 treatment alters cell viability in HMC3 cells
To understand the influence of Aβ42 treatment on HMC3 cell survival over time. Cells were treated with 5 μM Aβ42 for 72 hours and an MTT assay for cell viability was performed at 8, 24, 48 and 72 hours. After 8 hours there was a significant decline in viability (p-value <0.001) suggesting that both cell death and functional phagocytosis occurs at 24 hours of 5 μM Aβ42 treatment in HMC3 cells (Fig4).
Fig 4. Aβ42 treatment alters cell survival, pro-apoptotic and anti-apoptotic factors in HMC3 cells.
HMC3 cell viability significantly declines after 8 hours of 5 μM Aβ42 treatment Bars represent Fisher’s LSD test without correction for multiple comparisons significant p-value <0.05.
TREM2 modulates apoptotic machinery in HMC3 cells exposed to the Aβ42 peptide
To determine if TREM2 plays a role Bcl-2 family intrinsic apoptotic response to 24 hours of 5 μM Aβ42 in HMC3 cells, the apoptosis-related proteins BAX, BAD, Bcl-xL/BAD, BIM, pBAD and MCL-1/BIM were evaluated. At 24 hours of Aβ42 treatment Bax, Bad, and pBad were reduced (Fig5A–B). TREM2 overexpression (TREM2 OE) alone did not influence these apoptotic proteins while TREM2 silencing (TREM2 siRNA) alone increased Bax. (4) When TREM2 was overexpressed, 24 hour of Aβ42 treatment resulted in a significant increase in Mcl-1/Bim. When TREM2 was silenced, Bax, Bcl-xL/Bad are decreased in response to 24 hour Aβ42 treatment. In addition, at 24 hour Aβ42 treatment TREM2 silencing resulted in a decrease in Bax compared to TREM2 overexpression. These results suggest that Bax related apoptosis is influenced by TREM2-related activation of the Mcl-1/Bim complex.
Fig 5. TREM2 modulates apoptotic machinery in HMC3 cells.
(1) Bax, Bad, and pBad are reduced in response to 24 hour Aβ42 treatment. (2) TREM2 overexpression (TREM2 OE) does not influence these apoptotic proteins. (3) TREM2 silencing (TREM2 siRNA) increases Bax. (4) Mcl-1/Bim is increased in response to 24 hour Aβ42 treatment in the context of TREM2 overexpression. (5) Bax, Bcl-xL/Bad are decreased in response to 24 hour Aβ42 treatment in the context of TREM2 silencing. (6) In response to 24 hour Aβ42 treatment TREM2 silencing results in a decrease in Bax compared to TREM2 overexpression. (A) Significance (p-values) are Tukey adjusted for multiple comparisons and mean difference (Mean Diff.) is shown for each apoptotic factor. (B)
Proinflammatory factors generally increased upon TREM2 overexpression in HMC3 cells
To determine if TREM2 plays a role in HMC3 inflammatory response to Aβ42 HMC3 cell media was collected and a panel of 38 cytokines and chemokines were evaluated after 24 hours of 5 μM Aβ42 treatment both with and without TREM2. Generally, anti-inflammatory and immunomodulatory factors (on the inflammatory panel) were below or near the lower limit of detection in HMC3 cells. Only 6 inflammatory factors; FGF-2, IP-10, VEGF, MIP-1a, GRO and IL-8, were significantly associated with TREM2 mediated response to 24 hours of 5 μM Aβ42 treatment in HMC3 cells (Fig6A–B). There was no change in inflammatory factors after 24 hour Aβ42 treatment alone. In addition, there was not a significant change in inflammatory factor level with TREM2 overexpression or silencing. Furthermore, when TREM2 is overexpressed there was not a significant difference in inflammatory factor level after 24 hour Aβ42 treatment. In contrast, when TREM2 was silenced, FGF-2 was increased and MIP-1a was decreased after 24 hour Aβ42 treatment. In addition, TREM2 silencing resulted in a decrease in IP-10, MIP-1a, IL-8 and an increase in FGF-2, VEGF, GRO, compared to TREM2 overexpression, in response to Aβ42 treatment. This suggests that in HMC3 cells TREM2 plays a role in Aβ induced activation of IP-10, MIP-1a, and IL-8 as well as the inhibition of the FGF-2, VEGF, GRO.
Fig 6. Proinflammatory factors generally increased upon TREM2 overexpression in HMC3 cells.
(1) No change in inflammatory factors after 24 hour Aβ42 treatment. (2) No change in inflammatory factors with TREM2 overexpression. (3) No change in inflammatory factors when TREM2 silenced. (4) No change in inflammatory factors with TREM2 overexpression after 24 hour Aβ42 treatment. (5) FGF-2 is increased and MIP- 1a is decreased when TREM2 silenced after 24 hour Aβ42 treatment. (6) In response to 24 hour Aβ42 treatment TREM2 silencing results in a decrease in IP-10, MIP-1a, IL- 8 and a increase in FGF-2, VEGF, GRO, compared to TREM2 overexpression. (A) Significance (p-values) are Tukey adjusted for multiple comparisons and mean difference (Mean Diff.) is shown for each apoptotic factor. (B)
4. Discussion
TREM2 has been strongly associated with the microglia response to amyloid in mouse models of AD. Increasing evidence supports the role of TREM2 in microglial function where phagocytosis, apoptosis and the inflammatory response have all been linked to TREM2. However, there is missing information about which specific apoptotic and inflammatory proteins are influenced by TREM2 in response to Aβ42 in human cell lines. To test the hypothesis that TREM2 influences the response to Aβ toxicity by enhancing phagocytosis and inhibiting both the BCL-2 family of apoptotic proteins and pro-inflammatory cytokines, the human HMC3 microglial cell line was selected because it has a moderate expression level of endogenous TREM2. This is necessary for experiments that involve both overexpression and silencing of TREM2.
Given previous evidence that there is an increase in TREM2 with Aβ42 treatment in mouse microglia or in AD mouse models (Brendel et al. 2017, Ulrich et al. 2017, Zhao et al. 2018), but TREM2 has not been characterized in HMC3 cells, we first addressed the question of the whether Aβ42 treatment increased TREM2 in this human microglia cell line. TREM2 immunohistochemistry indicates that TREM2 is elevated upon Aβ42 treatment after 24 hours. This increase in TREM2 was also observed by western blot derived protein expression. When TREM2 is overexpressed in HMC3 cells, this overexpression is maintained upon 24 hour Aβ42 treatment and depleted upon TREM2 silencing. This confirmed that we could effectively overexpress and silence TREM2 in this cell line under conditions of Aβ42 toxicity as previously demonstrated in mouse cell lines (Jiang et al. 2014, Zhao et al. 2018).
To demonstrate that HMC3 cells undergo alterations in morphology upon exposure to Aβ42 treatment as previously described in other cell lines (Pan et al. 2011, Hansen et al. 2018), they were treated for 24 hours with 5 μM Aβ42. The cells normally had a resting ramified phenotype and then when stimulated by 24 hours of 5 μM Aβ42 they developed an activated amoeboid phenotype. TREM2 regulates microglial cholesterol metabolism upon chronic phagocytic challenge (Nugent et al. 2020) and recognizes anionic ligands on bacteria and some eukaryotic cells (Daws et al. 2003). This amoeboid phenotype in response to Aβ has been described in mouse cell lines (Krabbe et al. 2013, Caldeira et al. 2017), but has not been described previously in HMC3 cell lines. To quantify changes in phagocytic activity over time, a phagocytosis assay using fluorescein labeled bioparticles was performed. The results indicated that in HMC3 cells there is a significant increase in phagocytosis in response to 5 μM Aβ42 treatment until 24 hours. However, there was an eventual decline in this phagocytic response to Aβ in this cell line. Interestingly, when TREM2 is overexpressed for 24 hours, there was a significant increase in phagocytosis compared to the control, both with and without Aβ42 treatment. In contrast, when TREM2 was silenced, phagocytosis was inhibited and only reached the level of the control upon 24 hour Aβ42 treatment. Together, this suggests that TREM2 is involved in phagocytosis in HMC3 cells. However, given the observed low level of phagocytosis upon TREM2 silencing in conjunction with Aβ42 treatment, it is possible there is another active phagocytosis mechanism in this cell line. This is not novel information in the context of myeloid cell TREM2 (Hsieh et al. 2009, Kawabori et al. 2015). Indeed, other Aβ-related, non-TREM2, phagocytic mechanisms have been described in myeloid cells (Lai and McLaurin 2012, Yamanaka et al. 2012). However, this is new information about HMC3 cells. Together this indicates that without TREM2 there is a significantly reduced phagocytic response to Aβ. This is supported by previous studies describing TREM2 mutation disruption of phagocytic capabilities in microglia and other cell types (Kleinberger et al. 2014, Kawabori et al. 2015, Schlepckow et al. 2017, Yao et al. 2019).
Since the Bcl-2 family of apoptotic proteins have been implicated in the Bcl-2 family Akt signaling response to amyloid (Clementi et al. 2006, Yin et al. 2011, Shang et al. 2012, Zhu et al. 2015) and TREM2-related Akt signaling has been described (He et al. 2019, Chen et al. 2020), but to our knowledge a link between TREM2 specific signaling and Bcl-2 apoptosis has not been studied, we addressed the question of whether Aβ42 treatment influences cell survival in HMC3 cells. After 8 hours a significant decline in viability was observed. Interestingly, at 24 hours there was also an increase in phagocytosis suggesting that even though there is a decline in viability at 24 hours there is still functional phagocytosis. Taken together, this suggests that cell death occurs between 8 and 24 hours. But, HMC3 cells are still functional in that timeframe where they are phagocytic until somewhere between 24 and 48 hours. Therefore, next to understand if the intrinsic Bcl-2 apoptotic machinery is influenced by Aβ42 treatment, the Bcl-2 family of apoptotic factors were evaluated at this time point of declining cell viability and remaining phagocytic capability. Indeed, Bax, Bad, and pBad are reduced in response to 24 hours of Aβ42 treatment. However, TREM2 overexpression alone does not influence the Bcl-2 family of apoptotic proteins in HMC3 cells. Interestingly, Mcl-1/Bim was increased in response to 24 hour Aβ42 treatment in the context of TREM2 overexpression suggesting reduced apoptosis due to sequestration of pro-apoptotic Bim by antiapoptotic Mcl-1. In contrast, TREM2 silencing increased Bax while Bad and Bcl-xL/Bad were decreased in response to 24 hour Aβ42 treatment in the context of TREM2 silencing. Furthermore, in response to 24 hour Aβ42 treatment TREM2 silencing resulted in a decrease in Bax compared to TREM2 overexpression. Taken together, this novel information suggests that TREM2 inhibits Aβ induced apoptosis by activating the Mcl-1/Bim complex and thus inhibiting the production of Bax in HMC3 cells.
Given that these findings implicate a relationship between TREM2 and apoptotic proteins, further study of other apoptotic proteins, such as caspase −3/7, will shed more light on how TREM2 might impact apoptosis in microglia. Autophagy is also a critical component of microglial function and was not examined here. TREM2-deficient mice with AD-like pathology have abundant autophagic vesicles (Ulland et al. 2017) and resveratrol relieves neuropathic pain through suppressing microglia-mediated neuroinflammation via regulating the TREM2-autophagy axis (Wang et al. 2020). Given these previous reports, autophagy is another likely critical TREM2-related microglia function that warrants further investigation and has not been studied in HMC3 cells.
TREM2 inhibits the inflammatory response to LPS in mouse microglia by suppressing the PI3K/NF-κB signaling (Li et al. 2019), but less is known about the response to Aβ in human microglia. TREM2 rare genetic variants located in the phagocytic receptor triple the risk of developing AD (Jonsson et al. 2013, Abduljaleel et al. 2014) highlighting the importance of TREM2 in brain homeostasis. Critical cellular biology studies link TREM2 downstream signaling to inflammatory factors (Li et al. 2019, Long et al. 2019, Wang et al. 2019, Zhu et al. 2020). However, which inflammatory factors are influenced by TREM2 after Aβ treatment has not been fully elucidated. Since a main function of microglia is to secrete inflammatory factors and the inflammatory factors influenced by TREM2 and Aβ42 treatment have not been characterized in HMC3 cells, we analyzed a panel of 38 inflammatory factors. Interestingly, there was not a significant change in inflammatory factors after 24 hour Aβ42 treatment. In addition, there was not a significant change in inflammatory factors when TREM2 was overexpressed or silenced. Furthermore, there was no change in inflammatory factors with TREM2 overexpression after 24 hour Aβ42 treatment. In contrast, FGF-2 was significantly increased and MIP-1a was decreased after 24 hour Aβ42 treatment upon silencing of TREM2. Furthermore, in response to 24 hour Aβ42 treatment TREM2 silencing resulted in a decrease in IP-10, MIP-1a, IL-8 and an increase in FGF-2, VEGF, GRO, compared to TREM2 overexpression. These results suggest that under conditions of Aβ toxicity TREM2 is involved in activation of IP-10, MIP-1a and IL-8 and the inhibition of FGF-2, VEGF, and GRO, in HMC3 cells. Interestingly, IP-10, MIP-1a and IL-8 all play a role in chemoattraction and cell recruitment (Engelhardt et al. 1998, Turner et al. 2014) while FGF-2, VEGF, and GRO play a role in response to injury, angiogenesis, wound healing, and the immune response (Werner and Grose 2003, Frantz et al. 2005) suggesting that TREM2 is involved in activating chemoattraction and recruitment based cell biology, while inhibiting the response to injury.
In conclusion, this study has elucidated the relationship between TREM2 and HMC3 microglial biology in response to neurotoxic Aβ. A robust phagocytic response to Aβ requires TREM2 in these cells. TREM2 inhibits Aβ induced Bcl-2 related apoptosis by activating the Mcl-1/Bim complex and thus inhibiting the production of Bax in HMC3 cells. Furthermore, in response to Aβ toxicity, TREM2 is involved in activation of IP-10, MIP-1a and IL-8 which play a role in chemoattraction and cell recruitment. In contrast, TREM2 is involved in inhibition of the FGF-2, VEGF, and GRO which play a role in response to injury, angiogenesis, wound healing, and the immune response. This study reveals novel insights into contribution of TREM2 in the response to Aβ in microglia and suggests that therapeutics that seek to target TREM2 biology may have a broad influence on microglial function that warrants further investigation.
Highlights.
A robust phagocytic response to Aβ requires TREM2 in HMC3 cells.
TREM2 inhibits Aβ induced apoptosis by activating the Mcl-1/Bim complex and thus inhibiting the production of Bax in HMC3 cells.
Under conditions of Aβ toxicity, in HMC3 cells, TREM2 is involved in activation of IP- 10, MIP-1a, and IL-8 which play a role in chemoattraction and cell recruitment.
Under conditions of Aβ toxicity, in HMC3 cells, TREM2 is involved in inhibition of the FGF-2, VEGF, and GRO which play a role in response to injury, angiogenesis, wound healing, and the immune response.
Taken together, TREM2 plays a role in enhancing the microglial functional response to Aβ toxicity in HMC3 human microglia.
Acknowledgements
This study was supported by the National Institute of Health R00AG034214 and R56 AG063870.
Footnotes
Authorship
All authors contributed to the design of the experiments, analysis of the data, data interpretation and writing the manuscript.
Declaration of Competing Interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abduljaleel Z, Al-Allaf FA, Khan W, Athar M, Shahzad N, Taher MM, Elrobh M, Alanazi MS and El-Huneidi W. (2014). “Evidence of trem2 variant associated with triple risk of Alzheimer’s disease.” PLoS One 9(3): e92648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afridi R, Lee WH and Suk K. (2020). “Microglia Gone Awry: Linking Immunometabolism to Neurodegeneration.” Front Cell Neurosci 14: 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhter R, Saleem S, Saha A and Biswas SC (2018). “The pro-apoptotic protein Bmf co-operates with Bim and Puma in neuron death induced by beta-amyloid or NGF deprivation.” Mol Cell Neurosci 88: 249–257. [DOI] [PubMed] [Google Scholar]
- Akhter R, Sanphui P and Biswas SC (2014). “The essential role of p53-up-regulated modulator of apoptosis (Puma) and its regulation by FoxO3a transcription factor in beta-amyloid-induced neuron death.” J Biol Chem 289(15): 10812–10822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhter R, Sanphui P, Das H, Saha P and Biswas SC (2015). “The regulation of p53 up-regulated modulator of apoptosis by JNK/c-Jun pathway in beta-amyloid-induced neuron death.” J Neurochem 134(6): 1091–1103. [DOI] [PubMed] [Google Scholar]
- Akhter R, Sanphui P, Das H, Saha P and Biswas SC (2015). “The regulation of p53 up-regulated modulator of apoptosis by JNK/c-Jun pathway in β-amyloid-induced neuron death.” J Neurochem 134(6): 1091–1103. [DOI] [PubMed] [Google Scholar]
- Akhter R, Shao Y, Shaw M, Formica S, Khrestian M, Leverenz JB and Bekris LM (2018). “Regulation of ADAM10 by miR-140–5p and potential relevance for Alzheimer’s disease.” Neurobiol Aging 63: 110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer’s A. (2016). “2016 Alzheimer’s disease facts and figures.” Alzheimers Dement 12(4): 459–509. [DOI] [PubMed] [Google Scholar]
- Barghorn S, Nimmrich V, Striebinger A, Krantz C, Keller P, Janson B, Bahr M, Schmidt M, Bitner RS, Harlan J, Barlow E, Ebert U and Hillen H. (2005). “Globular amyloid beta-peptide oligomer - a homogenous and stable neuropathological protein in Alzheimer’s disease.” J Neurochem 95(3): 834–847. [DOI] [PubMed] [Google Scholar]
- Bekris LM, Khrestian M, Dyne E, Shao Y, Pillai JA, Rao SM, Bemiller SM, Lamb B, Fernandez HH and Leverenz JB (2018). “Soluble TREM2 and biomarkers of central and peripheral inflammation in neurodegenerative disease.” J Neuroimmunol 319: 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brendel M, Kleinberger G, Probst F, Jaworska A, Overhoff F, Blume T, Albert NL, Carlsen J, Lindner S, Gildehaus FJ, Ozmen L, Suarez-Calvet M, Bartenstein P, Baumann K, Ewers M, Herms J, Haass C and Rominger A. (2017). “Increase of TREM2 during Aging of an Alzheimer’s Disease Mouse Model Is Paralleled by Microglial Activation and Amyloidosis.” Front Aging Neurosci 9: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosseron F, Traschütz A, Widmann CN, Kummer MP, Tacik P, Santarelli F, Jessen F and Heneka MT (2018). “Characterization and clinical use of inflammatory cerebrospinal fluid protein markers in Alzheimer’s disease.” Alzheimers Res Ther 10(1): 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldeira C, Cunha C, Vaz AR, Falcao AS, Barateiro A, Seixas E, Fernandes A and Brites D. (2017). “Key Aging-Associated Alterations in Primary Microglia Response to Beta-Amyloid Stimulation.” Front Aging Neurosci 9: 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani RJ, Rolston RK and Smith MA (2010). “Alzheimer disease.” Dis Mon 56(9): 484–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Peng J, Sherchan P, Ma Y, Xiang S, Yan F, Zhao H, Jiang Y, Wang N, Zhang JH and Zhang H. (2020). “TREM2 activation attenuates neuroinflammation and neuronal apoptosis via PI3K/Akt pathway after intracerebral hemorrhage in mice.” J Neuroinflammation 17(1): 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementi ME, Pezzotti M, Orsini F, Sampaolese B, Mezzogori D, Grassi C, Giardina B and Misiti F. (2006). “Alzheimer’s amyloid beta-peptide (1–42) induces cell death in human neuroblastoma via bax/bcl-2 ratio increase: an intriguing role for methionine 35.” Biochem Biophys Res Commun 342(1): 206–213. [DOI] [PubMed] [Google Scholar]
- Daws MR, Sullam PM, Niemi EC, Chen TT, Tchao NK and Seaman WE (2003). “Pattern recognition by TREM-2: binding of anionic ligands.” J Immunol 171(2): 594–599. [DOI] [PubMed] [Google Scholar]
- Dello Russo C, Cappoli N, Coletta I, Mezzogori D, Paciello F, Pozzoli G, Navarra P and Battaglia A. (2018). “The human microglial HMC3 cell line: where do we stand? A systematic literature review.” J Neuroinflammation 15(1): 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt E, Toksoy A, Goebeler M, Debus S, Brocker EB and Gillitzer R. (1998). “Chemokines IL-8, GROalpha, MCP-1, IP-10, and Mig are sequentially and differentially expressed during phase-specific infiltration of leukocyte subsets in human wound healing.” Am J Pathol 153(6): 1849–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz S, Vincent KA, Feron O and Kelly RA (2005). “Innate immunity and angiogenesis.” Circ Res 96(1): 15–26. [DOI] [PubMed] [Google Scholar]
- Gallardo G and Holtzman DM (2019). “Amyloid-β and Tau at the Crossroads of Alzheimer’s Disease.” Adv Exp Med Biol 1184: 187–203. [DOI] [PubMed] [Google Scholar]
- Gao X, Dong Y, Liu Z and Niu B. (2013). “Silencing of triggering receptor expressed on myeloid cells-2 enhances the inflammatory responses of alveolar macrophages to lipopolysaccharide.” Mol Med Rep 7(3): 921–926. [DOI] [PubMed] [Google Scholar]
- Gopallawa I, Freund JR and Lee RJ (2020). “Bitter taste receptors stimulate phagocytosis in human macrophages through calcium, nitric oxide, and cyclic-GMP signaling.” Cell Mol Life Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, Cruchaga C, Sassi C, Kauwe JS, Younkin S, Hazrati L, Collinge J, Pocock J, Lashley T, Williams J, Lambert JC, Amouyel P, Goate A, Rademakers R, Morgan K, Powell J, St George-Hyslop P, Singleton A, Hardy J and Alzheimer Genetic Analysis G. (2013). “TREM2 variants in Alzheimer’s disease.” N Engl J Med 368(2): 117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen DV, Hanson JE and Sheng M. (2018). “Microglia in Alzheimer’s disease.” J Cell Biol 217(2): 459–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He GL, Luo Z, Shen TT, Wang ZZ, Li P, Luo X, Yang J, Tan YL, Wang Y, Gao P and Yang XS (2019). “TREM2 Regulates Heat Acclimation-Induced Microglial M2 Polarization Involving the PI3K-Akt Pathway Following EMF Exposure.” Front Cell Neurosci 13: 591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh CL, Koike M, Spusta SC, Niemi EC, Yenari M, Nakamura MC and Seaman WE (2009). “A role for TREM2 ligands in the phagocytosis of apoptotic neuronal cells by microglia.” J Neurochem 109(4): 1144–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav VS, Krause KH and Singh SK (2014). “HIV-1 Tat C modulates NOX2 and NOX4 expressions through miR-17 in a human microglial cell line.” J Neurochem 131(6): 803–815. [DOI] [PubMed] [Google Scholar]
- Janabi N, Peudenier S, Héron B, Ng KH and Tardieu M. (1995). “Establishment of human microglial cell lines after transfection of primary cultures of embryonic microglial cells with the SV40 large T antigen.” Neurosci Lett 195(2): 105–108. [DOI] [PubMed] [Google Scholar]
- Jensen CS, Bahl JM, Østergaard LB, Høgh P, Wermuth L, Heslegrave A, Zetterberg H, Heegaard NHH, Hasselbalch SG and Simonsen AH (2019). “Exercise as a potential modulator of inflammation in patients with Alzheimer’s disease measured in cerebrospinal fluid and plasma.” Exp Gerontol 121: 91–98. [DOI] [PubMed] [Google Scholar]
- Jiang T, Tan L, Zhu XC, Zhang QQ, Cao L, Tan MS, Gu LZ, Wang HF, Ding ZZ, Zhang YD and Yu JT (2014). “Upregulation of TREM2 ameliorates neuropathology and rescues spatial cognitive impairment in a transgenic mouse model of Alzheimer’s disease.” Neuropsychopharmacology 39(13): 2949–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, Snaedal J, Bjornsson S, Huttenlocher J, Levey AI, Lah JJ, Rujescu D, Hampel H, Giegling I, Andreassen OA, Engedal K, Ulstein I, Djurovic S, Ibrahim-Verbaas C, Hofman A, Ikram MA, van Duijn CM, Thorsteinsdottir U, Kong A and Stefansson K. (2013). “Variant of TREM2 associated with the risk of Alzheimer’s disease.” N Engl J Med 368(2): 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabori M, Kacimi R, Kauppinen T, Calosing C, Kim JY, Hsieh CL, Nakamura MC and Yenari MA (2015). “Triggering receptor expressed on myeloid cells 2 (TREM2) deficiency attenuates phagocytic activities of microglia and exacerbates ischemic damage in experimental stroke.” J Neurosci 35(8): 3384–3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinberger G, Yamanishi Y, Suárez-Calvet M, Czirr E, Lohmann E, Cuyvers E, Struyfs H, Pettkus N, Wenninger-Weinzierl A, Mazaheri F, Tahirovic S, Lleó A, Alcolea D, Fortea J, Willem M, Lammich S, Molinuevo JL, Sánchez-Valle R, Antonell A, Ramirez A, Heneka MT, Sleegers K, van der Zee J, Martin JJ, Engelborghs S, Demirtas-Tatlidede A, Zetterberg H, Van Broeckhoven C, Gurvit H, Wyss-Coray T, Hardy J, Colonna M and Haass C. (2014). “TREM2 mutations implicated in neurodegeneration impair cell surface transport and phagocytosis.” Sci Transl Med 6(243): 243ra286. [DOI] [PubMed] [Google Scholar]
- Krabbe G, Halle A, Matyash V, Rinnenthal JL, Eom GD, Bernhardt U, Miller KR, Prokop S, Kettenmann H and Heppner FL (2013). “Functional impairment of microglia coincides with Beta-amyloid deposition in mice with Alzheimer-like pathology.” PLoS One 8(4): e60921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai AY and McLaurin J. (2012). “Clearance of amyloid-beta peptides by microglia and macrophages: the issue of what, when and where.” Future Neurol 7(2): 165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Bedard K, Sorce S, Hinz B, Dubois-Dauphin M and Krause KH (2009). “NOX4 expression in human microglia leads to constitutive generation of reactive oxygen species and to constitutive IL-6 expression.” J Innate Immun 1(6): 570–581. [DOI] [PubMed] [Google Scholar]
- Li C, Zhao B, Lin C, Gong Z and An X. (2019). “TREM2 inhibits inflammatory responses in mouse microglia by suppressing the PI3K/NF-κB signaling.” Cell Biol Int 43(4): 360–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long H, Zhong G, Wang C, Zhang J, Zhang Y, Luo J and Shi S. (2019). “TREM2 Attenuates Aβ1–42-Mediated Neuroinflammation in BV-2 Cells by Downregulating TLR Signaling.” Neurochem Res 44(8): 1830–1839. [DOI] [PubMed] [Google Scholar]
- Lull ME and Block ML (2010). “Microglial activation and chronic neurodegeneration.” Neurotherapeutics 7(4): 354–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrekar-Colucci S and Landreth GE (2010). “Microglia and inflammation in Alzheimer’s disease.” CNS Neurol Disord Drug Targets 9(2): 156–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa Y and Chiba K. (2015). “Diversity and plasticity of microglial cells in psychiatric and neurological disorders.” Pharmacol Ther 154: 21–35. [DOI] [PubMed] [Google Scholar]
- Nugent AA, Lin K, van Lengerich B, Lianoglou S, Przybyla L, Davis SS, Llapashtica C, Wang J, Kim DJ, Xia D, Lucas A, Baskaran S, Haddick PCG, Lenser M, Earr TK, Shi J, Dugas JC, Andreone BJ, Logan T, Solanoy HO, Chen H, Srivastava A, Poda SB, Sanchez PE, Watts RJ, Sandmann T, Astarita G, Lewcock JW, Monroe KM and Di Paolo G. (2020). “TREM2 Regulates Microglial Cholesterol Metabolism upon Chronic Phagocytic Challenge.” Neuron 105(5): 837–854 e839. [DOI] [PubMed] [Google Scholar]
- Pan XD, Zhu YG, Lin N, Zhang J, Ye QY, Huang HP and Chen XC (2011). “Microglial phagocytosis induced by fibrillar beta-amyloid is attenuated by oligomeric beta-amyloid: implications for Alzheimer’s disease.” Mol Neurodegener 6: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis E, Douillard H, Koutroumanis M, Goodyer C and LeBlanc A. (1996). “Amyloid beta peptide of Alzheimer’s disease downregulates Bcl-2 and upregulates bax expression in human neurons.” J Neurosci 16(23): 7533–7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priller J and Prinz M. (2019). “Targeting microglia in brain disorders.” Science 365(6448): 32–33. [DOI] [PubMed] [Google Scholar]
- Rauchmann BS, Sadlon A, Perneczky R and Alzheimer’s Disease Neuroimaging I. (2020). “Soluble TREM2 and Inflammatory Proteins in Alzheimer’s Disease Cerebrospinal Fluid.” J Alzheimers Dis 73(4): 1615–1626. [DOI] [PubMed] [Google Scholar]
- Schlepckow K, Kleinberger G, Fukumori A, Feederle R, Lichtenthaler SF, Steiner H and Haass C. (2017). “An Alzheimer-associated TREM2 variant occurs at the ADAM cleavage site and affects shedding and phagocytic function.” EMBO Mol Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano-Pozo A, Frosch MP, Masliah E and Hyman BT (2011). “Neuropathological alterations in Alzheimer disease.” Cold Spring Harb Perspect Med 1(1): a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang YC, Chong ZZ, Wang S and Maiese K. (2012). “Prevention of β-amyloid degeneration of microglia by erythropoietin depends on Wnt1, the PI 3-K/mTOR pathway, Bad, and Bcl-xL.” Aging (Albany NY) 4(3): 187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieber MW, Jaenisch N, Brehm M, Guenther M, Linnartz-Gerlach B, Neumann H, Witte OW and Frahm C. (2013). “Attenuated inflammatory response in triggering receptor expressed on myeloid cells 2 (TREM2) knock-out mice following stroke.” PLoS One 8(1): e52982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suárez-Calvet M, Kleinberger G, Araque Caballero M, Brendel M, Rominger A, Alcolea D, Fortea J, Lleó A, Blesa R, Gispert JD, Sánchez-Valle R, Antonell A, Rami L, Molinuevo JL, Brosseron F, Traschütz A, Heneka MT, Struyfs H, Engelborghs S, Sleegers K, Van Broeckhoven C, Zetterberg H, Nellgård B, Blennow K, Crispin A, Ewers M and Haass C. (2016). “sTREM2 cerebrospinal fluid levels are a potential biomarker for microglia activity in early-stage Alzheimer’s disease and associate with neuronal injury markers.” EMBO Mol Med 8(5): 466–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Prinz M, Stagi M, Chechneva O and Neumann H. (2007). “TREM2-transduced myeloid precursors mediate nervous tissue debris clearance and facilitate recovery in an animal model of multiple sclerosis.” PLoS Med 4(4): e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Rochford CD and Neumann H. (2005). “Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2.” J Exp Med 201(4): 647–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto Y and Shimizu S. (2000). “Bcl-2 family: life-or-death switch.” FEBS Lett 466(1): 6–10. [DOI] [PubMed] [Google Scholar]
- Turner MD, Nedjai B, Hurst T and Pennington DJ (2014). “Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease.” Biochim Biophys Acta 1843(11): 25632582. [DOI] [PubMed] [Google Scholar]
- Ulland TK, Song WM, Huang SC, Ulrich JD, Sergushichev A, Beatty WL, Loboda AA, Zhou Y, Cairns NJ, Kambal A, Loginicheva E, Gilfillan S, Cella M, Virgin HW, Unanue ER, Wang Y, Artyomov MN, Holtzman DM and Colonna M. (2017). “TREM2 Maintains Microglial Metabolic Fitness in Alzheimer’s Disease.” Cell 170(4): 649–663 e613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich JD, Ulland TK, Colonna M and Holtzman DM (2017). “Elucidating the Role of TREM2 in Alzheimer’s Disease.” Neuron 94(2): 237–248. [DOI] [PubMed] [Google Scholar]
- Wang M, Gao X, Zhao K, Chen H, Xu M and Wang K. (2019). “Effect of TREM2 on Release of Inflammatory Factor from LPS-stimulated Microglia and Its Possible Mechanism.” Ann Clin Lab Sci 49(2): 249–256. [PubMed] [Google Scholar]
- Wang S and Colonna M. (2019). “Microglia in Alzheimer’s disease: A target for immunotherapy.” J Leukoc Biol. [DOI] [PubMed] [Google Scholar]
- Wang Y, Shi Y, Huang Y, Liu W, Cai G, Huang S, Zeng Y, Ren S, Zhan H and Wu W. (2020). “Resveratrol mediates mechanical allodynia through modulating inflammatory response via the TREM2-autophagy axis in SNI rat model.” J Neuroinflammation 17(1): 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber GE, Koenig KA, Khrestian M, Shao Y, Tuason ED, Gramm M, Lal D, Leverenz JB and Bekris LM (2020). “An Altered Relationship between Soluble TREM2 and Inflammatory Markers in Young Adults with Down Syndrome: A Preliminary Report.” J Immunol 204(5): 1111–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner S and Grose R. (2003). “Regulation of wound healing by growth factors and cytokines.” Physiol Rev 83(3): 835–870. [DOI] [PubMed] [Google Scholar]
- Yamanaka M, Ishikawa T, Griep A, Axt D, Kummer MP and Heneka MT (2012). “PPARgamma/RXRalpha-induced and CD36-mediated microglial amyloid-beta phagocytosis results in cognitive improvement in amyloid precursor protein/presenilin 1 mice.” J Neurosci 32(48): 17321–17331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Coppola K, Schweig JE, Crawford F, Mullan M and Paris D. (2019). “Distinct Signaling Pathways Regulate TREM2 Phagocytic and NFκB Antagonistic Activities.” Front Cell Neurosci 13: 457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin G, Li LY, Qu M, Luo HB, Wang JZ and Zhou XW (2011). “Upregulation of AKT attenuates amyloid-β-induced cell apoptosis.” J Alzheimers Dis 25(2): 337–345. [DOI] [PubMed] [Google Scholar]
- Youle RJ and Strasser A. (2008). “The BCL-2 protein family: opposing activities that mediate cell death.” Nat Rev Mol Cell Biol 9(1): 47–59. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Wu X, Li X, Jiang LL, Gui X, Liu Y, Sun Y, Zhu B, Pina-Crespo JC, Zhang M, Zhang N, Chen X, Bu G, An Z, Huang TY and Xu H. (2018). “TREM2 Is a Receptor for beta-Amyloid that Mediates Microglial Function.” Neuron 97(5): 1023–1031 e1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Wu X, Li X, Jiang LL, Gui X, Liu Y, Sun Y, Zhu B, Piña-Crespo JC, Zhang M, Zhang N, Chen X, Bu G, An Z, Huang TY and Xu H. (2018). “TREM2 Is a Receptor for βAmyloid that Mediates Microglial Function.” Neuron 97(5): 1023–1031 e1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N, Lin J, Wang K, Wei M, Chen Q and Wang Y. (2015). “Huperzine A protects neural stem cells against Aβ-induced apoptosis in a neural stem cells and microglia co-culture system.” Int J Clin Exp Pathol 8(6): 6425–6433. [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Zhang X, Dong W, Wang X, He S, Zhang H, Wang X, Wei R, Chen Y, Liu X and Guo C. (2020). “TREM2 suppresses the proinflammatory response to facilitate PRRSV infection via PI3K/NF-κB signaling.” PLoS Pathog 16(5): e1008543. [DOI] [PMC free article] [PubMed] [Google Scholar]