Abstract
The health effects of saturated fat, particularly tropical oil, on cardiovascular disease are unclear. We investigated the effect of tropical oil (palm and coconut oils), lard, and other common vegetable oils (soybean and rice bran oils) that are widely used in tropical and Asian countries on lipid profiles. We performed an umbrella review of meta-analyses and systematic reviews. Electronic databases (Medline, Scopus, Embase, and Cochrane) were searched up to December 2018 without language restriction. We identified nine meta-analyses that investigated the effect of dietary oils on lipid levels. Replacement of polyunsaturated fatty-acid-rich oils (PUFAs) and monounsaturated FA-rich oils (MUFAs) with palm oil significantly increased low-density lipoprotein cholesterol (LDL-c), by 3.43 (0.44–6.41) mg/dL and 9.18 (6.90–11.45) mg/dL, respectively, and high-density lipoprotein cholesterol (HDL-c), by 1.89 (1.23–2.55) mg/dL and 0.94 (−0.07–1.97) mg/dL, respectively. Replacement of PUFAs with coconut oil significantly increased HDL-c and total cholesterol –by 2.27 (0.93–3.6) mg/dL and 5.88 (0.21–11.55) mg/dL, respectively—but not LDL-c. Substituting lard for MUFAs and PUFAs increased LDL-c–by 8.39 (2.83–13.95) mg/dL and 9.85 (6.06–13.65) mg/dL, respectively—but not HDL-c. Soybean oil substituted for other PUFAs had no effect on lipid levels, while rice bran oil substitution decreased LDL-c. Our findings show the deleterious effect of saturated fats from animal sources on lipid profiles. Replacement of unsaturated plant-derived fats with plant-derived saturated fats slightly increases LDL-c but also increases HDL-c, which in turn may exert a neutral effect on cardiovascular health.
Keywords: tropical oil, vegetable oil, palm oil, coconut oil, lard, soybean oil, rice bran oil, cardiovascular disease, lipid
1. Introduction
Cardiovascular disease (CVD) is responsible for the largest proportion of deaths worldwide [1], and dyslipidemia is an important modifiable risk factor for the development of CVD [2,3,4]. One non-pharmacologic intervention that can reduce the risk of CVD is the modification of dietary fat, whereby a reduction in the intake of saturated fats (SFs) and replacement with unsaturated fat may reduce the risk of CVD. Several studies have shown that high intake of SFs increases low-density lipoprotein cholesterol (LDL-c) level [5]. However, there is some evidence to indicate that consumption of SFs, particularly tropical oils (e.g., coconut and palm oils), might not increase the risk of CVD [6,7]. According to the United States Department of Agriculture (USDA), the most commonly consumed oils worldwide are palm oil and soybean oil [8]. Lard, coconut oil, and rice bran oil (RBO) are also popular in tropical region and throughout Asia. Based on their major FA components, palm oil, coconut oil, and lard are classified as SFs, while soybean oil and RBO are classified as polyunsaturated fatty acid (PUFA)-rich oil and monounsaturated fatty acid (MUFA)-rich oil, respectively. Palm oil and coconut oil are commonly used oils in tropical countries and both contain high amounts of SFs, which can increase LDL-cholesterol and may increase the risk of CVD. However, the potential benefits of coconut oil, which also contains medium chain triglycerides (MCT), have led to an increase in its popularity. Palm oil is unique in that it contains high amounts of SFs and MUFAs, and has increasingly been used as an alternative to partially hydrogenated fats [9]. Lard is an animal-derived fat that has received much attention in the popular media because of its positioning as a naturally sourced, heat-stable cooking fat which contains both SFAs and MUFAs. Although dietary guidelines generally recommend restricting the intake of SFs, there is no clear consensus on the health effects of SFs from current evidence, particularly with respect to the effects of oils from tropical regions on CVD. We, therefore, conducted an umbrella review to systemically assess the existing evidence and evaluate the effect on lipid parameters of tropical oil (palm oil and coconut oil), lard, and other common vegetable oils (soybean oil and RBO) that are widely used in Asian and tropical countries.
2. Materials and Methods
2.1. Literature Search and Selection Criteria
The study protocol was registered in the international prospective register of systematic reviews (PROSPERO; CRD42019130581). We conducted a review of multiple systematic reviews (SR) and meta-analyses (MAs) in compliance with standardized procedures [10,11]. Relevant SRs and MAs were identified from electronic databases (MEDLINE/PubMed, Scopus, Embase, and Cochrane central register of controlled trials (CENTRAL)) up to December 2018 without any language restriction using the search terms and search strategies described in Supplementary Data S1.
2.2. Eligibility Criteria
Studies were selected independently by two authors (C.U. and P.C.S.) and disagreements were resolved through consensus with a third author (V.K.). Studies were eligible if they met all of the following criteria: SRs and MAs of randomized controlled trials (RCT) or observational studies; inclusion of adult patients; comparison of coconut oil, palm oil, RBO, soybean oil, or lard as a dietary intervention with any other edible oils on iso-caloric exchange; and reporting of any lipid outcomes (LDL-c, HDL-c, triglyceride, total cholesterol (TC), or TC-to-HDL-c ratio). SRs or MAs that evaluated oils in the form of dietary supplements or drugs or the postprandial effect of oil intake on blood lipids were excluded.
2.3. Data Extraction
One reviewer (C.U.) extracted the following data for each study: first author, year of publication, origin (country), number and type of included studies (N), number of participant (n), sex, mean age, body mass index (BMI, kg/m2), baseline TC, study population, dietary interventions and comparators and duration (days), outcomes, pooling method, effect size (ES, e.g., mean difference (MD)), 95% confidence interval (95% CI), conflicts of interest, and funding source. The senior reviewer (A.T.) evaluated and verified the extracted data, and any disagreement was solved by consensus.
2.4. Quality Assessment
Methodological quality was independently assessed by two reviewers (C.U. and P.C.S.) using the AMSTAR2 tool [12]. Grading was classified into critically low (more than one critical flaw with or without non-critical weaknesses), low (one critical flaw with or without non-critical weakness), moderate (more than one non-critical weakness), or high (no more than one non-critical weakness) confidence. Disagreements were resolved by consensus between authors.
2.5. Data Analysis
Characteristics of MAs (e.g., setting, type of included studies, N, n) and their findings (ESs and 95% CIs) were described. An overlap of primary included studies was estimated across included MAs using a corrected covered area (CCA) [13]. CCA was classified as slight overlap, moderate, high, or very high if the percent overlap was 0–5%, 6–10%, 11–14%, or >15%, respectively. Data from individual included RCTs/cohorts were extracted, and ESs along with their variances were estimated and re-pooled using a random-effect model. Heterogeneity was assessed using Cochran’s Q test and Higgin’s I² statistic, and was deemed present for a p value < 0.1 or I2 ≥ 50% [14]. All analyses were performed using STATA version 16.0 (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC)].
3. Results
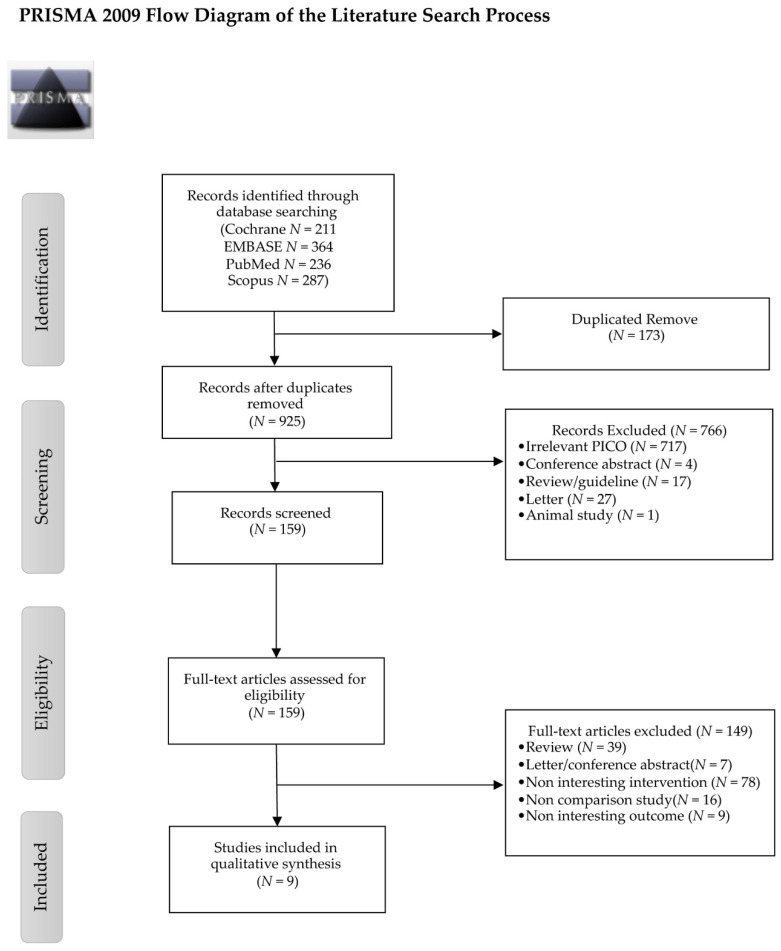
A total of 1098 studies were identified, of which 9 met the inclusion criteria (Figure 1) [15,16,17,18,19,20,21,22,23].
Figure 1.
PRISMA 2009 flow diagram of the literature search process.
The characteristics of the included studies are summarized in Table 1 and Supplementary Table S2. The degree of overlap of included primary studies on lipid outcome was assessed (Supplementary Table S3), and the resulting CCA of 7.4% indicated moderate overlap among the primary studies. The AMSTAR2 assessment results are summarized in Supplementary Table S4 for lipid outcomes and Supplementary Table S5 for clinical outcomes. Among the 9 studies with fasting lipid outcomes, the number of included primary studies ranged from 2 to 51, with sample sizes of 34 to 2065 (Table 1). The studies were published between 2009 and 2018 and had study durations of 2–27 weeks. Mean ages and BMI were 16–84 years and 17–37.4 kg/m2, respectively. The included populations varied from healthy individuals to patients with CVD risk factors or established CVD. For all studies, intervention oils were replaced with comparator oils in an isocaloric fashion. Indirect comparison from network meta-analysis [23] is also included in the present review. Where there was more than one comparison, the largest study was selected for the present review and indirect comparison of the effect of individual oils was shown on the forest plot with an asterisk (*). Re-pooling of ESs on lipid markers is described in the following sections.
Table 1.
Baseline characteristics of included systematic reviews and meta-analyses.
| Author, Year | Country | Number of Included Studies |
n | % Male | Mean Age (years) | Intervention Oil | Comparator Oil | Duration of Intervention (days) | Outcome | Baseline Serum Total Cholesterol (mg/dL) |
Conflict of Interest Funding Source |
AMSTAR2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Harland et al., 2009 [15] |
UK | 2 | 34 | 52.9 | 18–78 | Mixed saturated fat Palm oil (22 g/d) (*30%–39% of total calorie intake from fat) |
Canola oil (23 g/d) |
18–56 | LDL-c, TC, HDL-c, TAG | 170 to 275 | CI: Food industry organizations FS: Dow AgroSciences, Hertfordshire, UK. |
Critically low |
| Mozaffarian et al., 2009 [16] | USA | 13 for lipid parameters | 528 | 53 | 25–63 | Palm oil Lard Soybean oil (* 7.5% of total calorie intake from fat) |
20%Trans fatty acid PHO 35% Trans fatty acid PHO 45% Trans fatty acid PHO |
17–70 | TC/HDL-c, Lap(a) |
116 to 278 |
CI: no conflict of interest FS: University of Otago Research and Enterprise Unit. |
Critically low |
| 4 prospective cohorts for clinical outcomes |
139,836 | 43.6 | 30–84 (min to max) |
5–20 years | Adjusted risk reduction in coronary heart disease (nonfatal myocardial infarction or CHD death) |
190 to 282 | Critically low | |||||
| Fattore et al., 2014 [17] | Italy | 51 | 1526 | 66 | 16–75 | Palm oil (*4%–43% of total calorie intake from fat) |
Stearic acid Myristic/lauric oil MUFA PUFA Trans fatty acid PHO Interestified palm oil |
14–112 | LDL-c, TC, HDL-c, TAG, VLDL-c, apo B, apo A-I, TC/HDL-c, LDL-c/HDL-c, Lap(a) |
108 to 271 | CI: no conflict of interest FS: Universita Bocconi, Soremartec Italia s.r.l. |
Critically low |
| Sun et al., 2015 [18] | Singapore | 32 | 1073 |
65.4 | 16–68 | Palm oil (*12%–43% of total energy intake from fat) |
Vegetable oil low in saturated fat Trans fat-containing oil Animal fat |
14–112 | LDL-c, TC, HDL-c, TAG |
120 to 341 | CI: no conflict of interest FS: The National Medical Research Council, Singapore |
Low |
| Jolfaie et al., 2016 [19] |
Iran | 11 | 344 | 36 | 34–61 | Rice bran oil | Other oils | 21–90 | LDL-c, TC, HDL-c, TAG, VLDL-c, apo B, apo A, TC/HDL-c, LDL-c/HDL-c, Lap(a) | 134 to 325 | CI: no conflict of interest FS: NR |
Low |
| Ghobadi et al., 2018 [20] |
Iran | 9 | 292 | 49.31 | 22–65 | Saturated fat (*7%–20% of total energy intake from fat) |
Canola oil (12–50 g/d) |
21–180 | LDL-c, TC, HDL-c, TAG, apo B, apo A-I, LDL/HDL, TC/HDL | 130 to 309 | CI: no conflict of interest FS: no funding source |
Moderate |
| Ghobadi et al., 2018 [21] | Iran | 3 | 198 | 58.08 | 23–84 | Palm oil (*3%–81% of total energy intake from fat) |
Olive oil (25–60 g/d) |
21–180 | LDL-c, TC, HDL-c, TAG, apo B, apo A-I | 167 to 257 | CI: no conflict of interest FS: Shiraz University of Medical Sciences |
High |
| Panth et al., 2018 [22] | Australia | 10 | 299 | 53.5 | 21–66 | Naturally occurring medium chain fatty acid (*14.2–108 g/d) |
Long-chain fatty acid | 21–42 | LDL-c, TC, HDL-c, TAG, VLDL-c apo A-I, apo B |
113 to 274 | CI: no conflict of interest FS: no funding source |
High |
| Schwingshackl et al., 2018 [23] | Germany | 28 |
2065 | 54 | 22–84 | Soy oil, palm oil, coconut oil, lard | Other oils and solid fat | 21–189 | LDL-c, TC, HDL-c, TAG | 130 to 274 | CI: no conflict of interest FS: no funding source |
High |
Apo A-1: Apolipoprotein A-1; Apo B: Apolipoprotein B; CI: Conflict of interest; FS: Funding source; HDL-c: High-density lipoprotein-cholesterol; Lap (a): Lipoprotein (a); LDL-c: Low-density lipoprotein-cholesterol; MUFA: Monounsaturated fatty acid; NA: not reported; NIDDM: Non-insulin dependent diabetes mellitus; PHO: Partially hydrogenated oil; PUFA: Polyunsaturated fatty acid; TAG: Triacylglycerol; TC: Total cholesterol; TFA: Trans fatty acid.
3.1. LDL–c Outcome
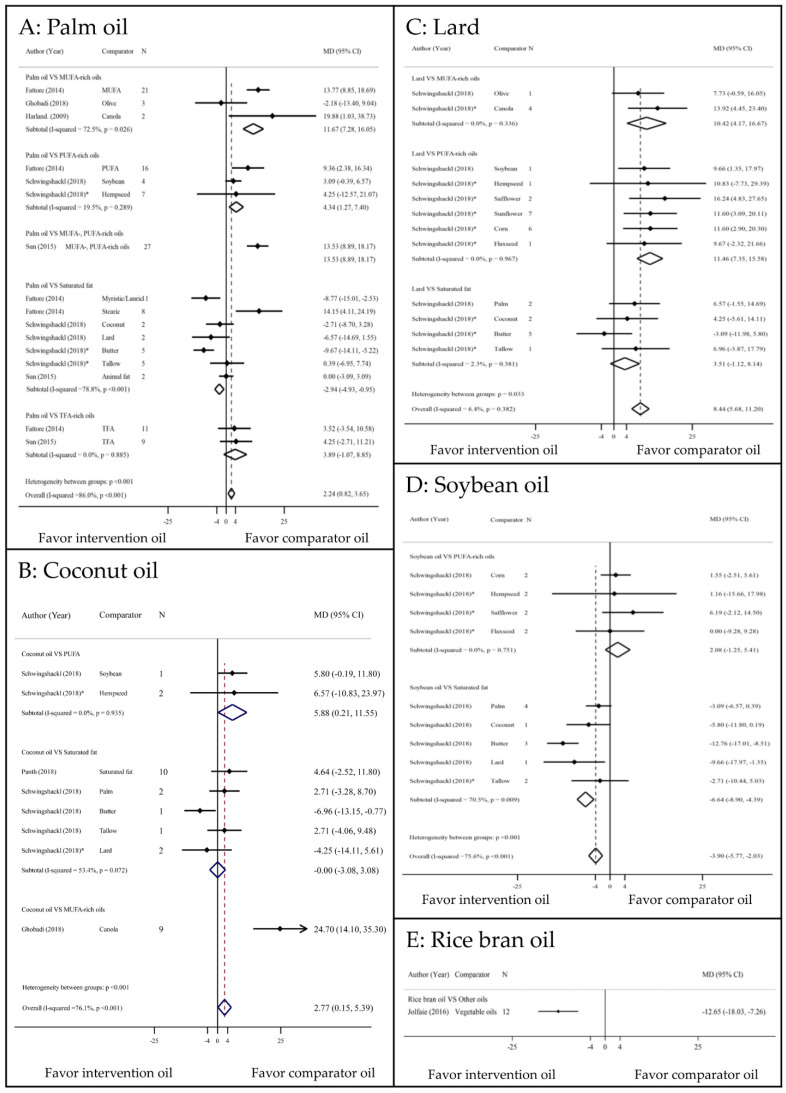
ESs of all LDL-c comparisons are displayed in Figure 2. Palm oil significantly increased LDL-c concentration compared with MUFA and PUFA with the corresponding MDs (95% CIs, I2) of 9.18 mg/dL (6.90 to 11.45, 84.3%) and 3.43 mg/dL (0.44 to 6.41, 19.9%) (Figure 2a). In addition, palm oil was associated with a significantly lower LDL-c than those of other SFs, with MDs of −2.32 (−4.24 to −0.40, 70.3%), except for trans-fatty acid (TFA) rich oils. Coconut oil increased LDL-c compared with PUFA, although this effect was not significant (Figure 2b). Conversely, coconut oil was associated with a lower LDL-c than other SFs, though this difference was not statistically significant. Compared with MUFA- and PUFA-rich oils, lard was associated with significant increases in LDL-c (8.39 mg/dL increase; 2.83 to 13.95, 0% and 9.85 mg/dL increase; 6.06 to 13.65, 0%, respectively). Lard also caused higher LDL-c levels than other SFs, although this difference was not significant (3.82 mg/dL increase; −0.42 to 8.06, 23.2%) (Figure 2c). Soybean oil caused an insignificant increase in LDL-c compared with PUFA-rich oils (2.34 mg/dL increase; −1.01 to 5.7, 0%) [23]. Conversely, soybean oil was associated with a 5.01 mg/dL decrease in LDL-c (−7.22 to −2.81, 69.3%) compared with other SFs (Figure 2d). Only one study indicated that RBO significantly decreased LDL-c compared with other vegetable oils (−6.91 mg/dL decrease; −10.24 to −3.57) [19] (Figure 2e).
Figure 2.
Comparisons of LDL–c levels among PUFA, MUFA, and SFAs. CI: confidence interval; HL: high linoleic; HO: high oleic; MD: mean difference; MUFA: monounsaturated fatty acid; PUFA: polyunsaturated fatty acid; TFA: trans fatty acid; * indirect comparison.
3.2. Total Cholesterol Outcome
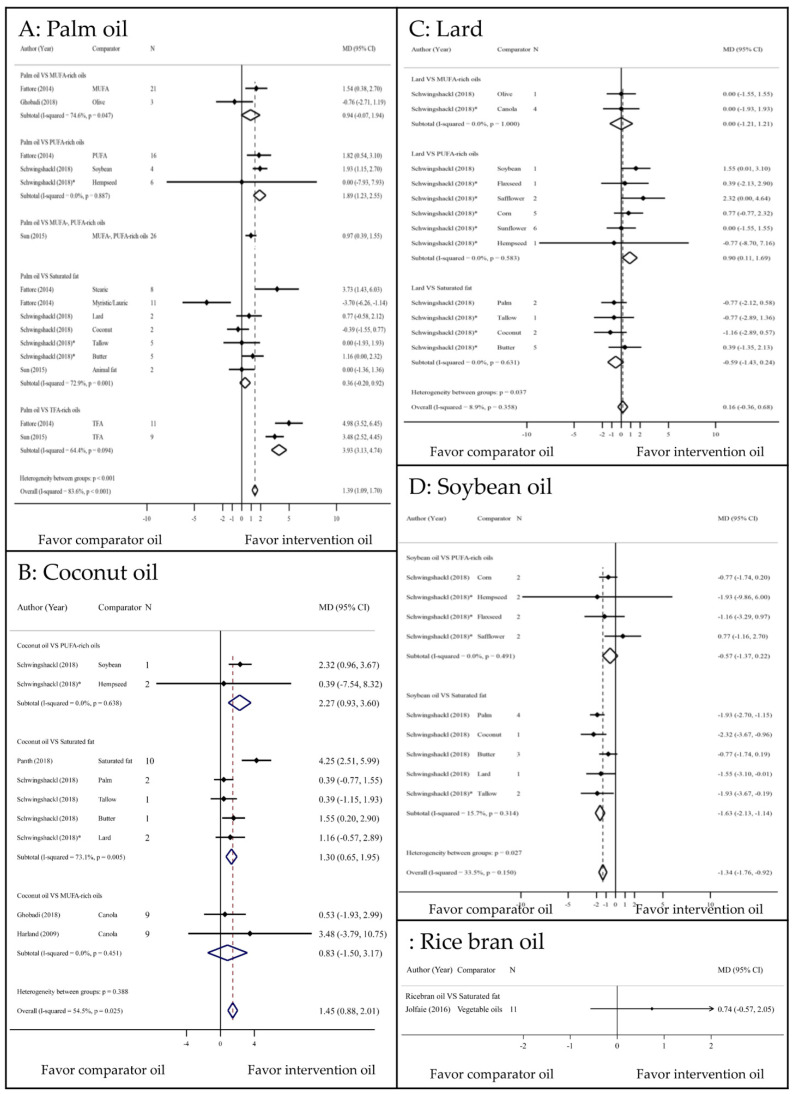
Compared with MUFA and PUFA-rich oils, palm oil significantly increased TC (11.67 mg/dL increase; 7.28 to 16.05, 72.5% and 4.34 mg/dL increase; 1.27 to 7.4, 19.5%, respectively) (Figure 3a). Conversely, palm oil caused decrease TC of −2.94 (−4.93 to −0.95, 78.8%) which was significantly lower than those of other SFs. Coconut oil had a significantly increased TC of 5.88 mg/dL (0.21 to 11.55, 0%) compared with PUFA, although this difference was not significant compared with other SFs (Figure 3b). Other SFs (palm oil, coconut oil, and dairy products) significantly increased TC of 24.7 mg/dL (14.1 to 35.3) [20] compared with canola oil. Lard significantly increased TC of 10.42 mg/dL (4.17 to 16.67, 0%) and 11.46 mg/dL (7.35 to 15.58, 0%) compared with MUFA- and PUFA-rich oils, respectively. Lard also had higher TC than other SFs, but this was not significant. Soybean oil significantly decreased TC by −6.64 mg/dL (−8.9 to −4.39, 70.5%) compared with SFs, but there was no difference compared with PUFA-rich oils [23]. Similar to the LDL-c outcome, RBO significantly decreased TC by 12.65 (−18.03 to −7.26) [19] (Figure 3e).
Figure 3.
Comparisons of TC levels among PUFA, MUFA, and SFAs. CI: confidence interval; MD: mean difference; MUFA: monounsaturated fatty acid; PUFA: polyunsaturated fatty acid; TFA: trans fatty acid; * indirect comparison.
3.3. HDL–c Outcome
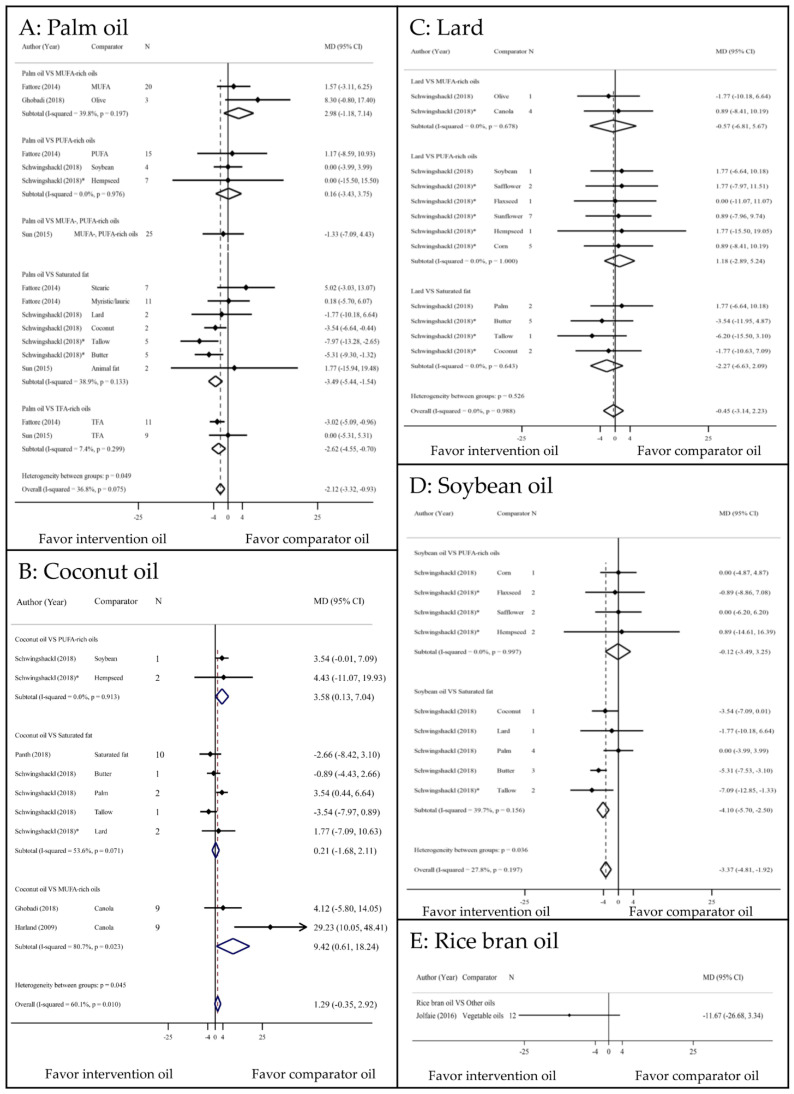
Palm oil significantly increased HDL-c levels compared with PUFA and TFA-rich oils–by 1.89 mg/dL (1.23 to 2.55, 0%) and 3.93 mg/dL (3.13–4.74, 64.4%), respectively—but there was no significantly difference compared with MUFA-rich oils and other SFs (Figure 4a). In addition, compared with PUFA-rich oils and other SFs, coconut oil significantly increased HDL-c (2.27 mg/dL increase; 0.93 to 3.6, 0% and 1.3 mg/dL increase; 0.65 to 1.95, 73.1%), respectively (Figure 4b). Replacement of butter [23] with coconut oil significantly increased HDL-c (1.55 mg/dL increase; 0.2 to 2.9) (Figure 4b). Lard increased HDL-c compared with PUFA-rich oils–by 0.90 mg/dL (0.11 to 1.69, 0%)—but not MUFA-rich oils and other SFs. Overall, palm oil and coconut oil significantly increased HDL compared with comparator oils; however, lard had no significant effect on HDL (Figure 4c). Soybean oil significantly lowered HDL-c compared with SFs—by −1.63 mg/dL (−2.13 to −1.14, 15.7%)—but not PUFA-rich oils (Figure 4d). RBO had no effect on HDL-c compared with other vegetable oils (Figure 4e) [19].
Figure 4.
Comparisons of HDL–c levels among PUFA, MUFA, and SFAs. CI: confidence interval; MD: mean difference; MUFA: monounsaturated fatty acid; PUFA: polyunsaturated fatty acid; TFA: trans fatty acid; * indirect comparison.
3.4. Triacylglycerol (TAG) Outcome
Palm oils had higher TAG levels than MUFAs and PUFAs, but the differences were not significant (Figure 5a). However, palm oils had −3.49 (−5.44 to −1.54, 38.9%) and −2.62 (−4.55 to −0.70, 7.4%) mg/dL lower TAG levels than SFs and TFA-rich oils, respectively. Replacement of PUFA-rich oils with coconut oil significantly increased the TAG level (3.58 mg/dL increase; 0.13 to 7.04, 0%), although replacement of other SFs with coconut oil had no effect on TAG levels (Figure 5b). Soybean oil reduced the TAG level by −4.10 mg/dL (−5.70 to −2.50, 39.7%) compared with SFs (Figure 5d) [23]. When lard and RBO [19] were substituted for other oils, no significant effect on TAG levels was observed (Figure 5c,e).
Figure 5.
Comparisons of TAG levels among PUFA, MUFA, and SFAs. CI: confidence interval; MD: mean difference; MUFA: monounsaturated fatty acid; PUFA: polyunsaturated fatty acid; TFA: trans fatty acid; * indirect comparison.
3.5. Total Cholesterol to HDL-c Ratio Outcome
In the current study, palm oil and RBO [19] had no significant effect on the TC/HDL-c ratio when substituted for MUFA- or PUFA-rich oil or other SFs. However, palm oil substituted for TFA-rich oil consumption significantly decreased the TC/HDL-c ratio [16,17]. Substituting TFA-rich oils with palm oil, lard, and soybean oil also decreased the TC/HDL-c ratio (Table 2).
Table 2.
Ratio of total cholesterol to HDL-cholesterol.
| Studies | Intervention | Comparator | n | Effect Size (95%CI) | Heterogeneity I2 (%) |
|---|---|---|---|---|---|
| Fattore et al., 2014 | Palm oil | MUFA-rich oils | 5 | 0.02 (−0.1, 0.14) | 0.00% |
| Harland et al.,2009 | Canola oil | 2 | 0.77 | ||
| Fattore et al., 2014 | Palm oil | PUFA-rich oils | 5 | −0.19 (−0.43, 0.06) | 22.71% |
| Fattore et al., 2014 | Palm oil | Stearic acid | 3 | −0.12 (−0.4, 0.16) | 18.43% |
| Fattore et al., 2014 | Palm oil | TFA | 3 | −0.45 (−0.58, −0.31) | 0.00% |
| Mozaffarian et al., 2009 | 20% TFA PHO | 13 | −0.02 | ||
| Mozaffarian et al., 2009 | 35% TFA PHO | 13 | −0.1 | ||
| Mozaffarian et al., 2009 | 45% TFA PHO | 13 | −0.14 | ||
| Ghobadi et al., 2018 [20] | Palm oil, animal fat | Canola oil | 8 | 0.07 (−0.15, 0.3) | 23.2% |
| Mozaffarian et al., 2009 | Lard | 20% TFA PHO | 13 | −0.02 | |
| Mozaffarian et al., 2009 | 35% TFA PHO | 13 | −0.09 | ||
| Mozaffarian et al., 2009 | 45% TFA PHO | 13 | −0.14 | ||
| Mozaffarian et al., 2009 | Soybean oil | 20% TFA PHO | 13 | −0.12 | |
| Mozaffarian et al., 2009 | 35% TFA PHO | 13 | −0.20 | ||
| Mozaffarian et al., 2009 | 45% TFA PHO | 13 | −0.25 | ||
| Jolfaie et al., 2016 | Rice bran oil | Vegetable oils | 4 | −0.08 (−0.22, 0.07) | 13% |
4. Discussion
We performed an umbrella review to summarize the findings of previous SRs and MAs that explored the association of dietary fat intake with lipid profiles and CVD. Our study confirmed the deleterious effects of SFs and showed that SFs derived from animal and plant sources had different effects on lipid profiles. Although the use of palm oil and coconut oil in place of MUFA- and PUFA-rich oils increased LDL-c and TC (for palm oil) and increased TC (for coconut oil), both oils significantly increased HDL-c. Furthermore, substituting lard for comparator oils increased LDL-c and TC, but not HDL-c. Several studies [24,25,26] have indicated that not all SFs have equal effects on lipid profiles, with differences especially clear between animal-derived and plant-derived fats. Our study supported the current recommendations to reduce dietary SF intake, particularly animal-derived fat, and to replace this source of fat with foods rich in unsaturated FAs from plants to lower the risk of CVD [27].
Previous dietary guidelines from 1980 recommending the limitation of dietary fat intake to less than 30% of total calories were later revised in 2005 to 20–35% of calories with a suggestion to reduce SF intake [28]. However, the prevalence of obesity, diabetes, and CVD has risen substantially despite a reduction in dietary fat intake [29]. In 2015, the Dietary Guidelines Advisory Committee [30] removed the upper limit on dietary fat intake and instead focused on the type of dietary fat. Several studies have indicated that MUFA derived from plant (e.g., olive oil) or animal (e.g., lard) sources is not equivalent to PUFA with respect to the effect on CVD [24,25,26]. The 2019 American College of Cardiology (ACC) and American Heart Association (AHA) guideline on the primary prevention of CVD recommended the replacement of SFs with dietary MUFA- and PUFA-rich oils [31] and also recommended plant-based diets, which are associated with lower mortality than animal-based diets [32,33]. This guidance is similar to that issued by the European Society of Cardiology (ESC) [34]. In tropical countries, palm oil, coconut oil, and lard, categorized as SFs, have been widely used for centuries [35], and the effect of SFs from tropical oils on CVD thus remains controversial [36].
Palm oil is among the most widely used plant-derived SFs in many countries [37], and of its major FA constituent is palmitic acid (C16:0) (45%) [38]. Although other vegetable oils are available, palm oil is inexpensive and therefore affordable for the majority of the population within developing countries [39] and in the food industry. We demonstrated that the replacement of PUFA- and MUFA-rich oils with palm oil significantly increased LDL-c and HDL-c levels. Furthermore, our findings confirmed the deleterious effect of TFA-rich oils, even compared with SFs [17,18]. Palm oil substituted for TFA-rich oils not only decreased TAG and TC/HDL-c ratio but also increased HDL-c level. Compared with coconut oil, palm oil had no effect on LDL-c, TC, or HDL-c, while one study reported that substituting palm oil for coconut oil decreased TAG [23]. Conversely, one MA demonstrated that substituting palm oil for butter, an animal-derived fat, significantly decreased TC, LDL-c, and TAG [23]. This result may be attributable to the large proportion of palmitic acid in palm oil, which is primarily esterified to the terminal carbons of the triglyceride glycerol (sn-1 and sn-3), thus limiting its absorption and its excretion primarily in feces as calcium salts compared with other SFs [40,41].
Coconut oil has been heavily promoted as a healthy oil with benefits for cardiovascular outcomes. It is rich in SFs (82%), including lauric acid (47%) and myristic acid (16.5%). Our study demonstrated that replacement of PUFA-rich oils with coconut oil significantly increased TC and HDL-c but not LDL-c, while coconut oil substituted for other SFs significantly increased HDL-c. Moreover, replacement of butter with coconut oil significantly decreased LDL-c and TC and increased HDL-c. Myristic acid (C14:0) is the most potent cholesterol-raising SF followed by palmitic acid and lauric acid [42]. A major source of myristic acid is dairy fat, which is found in butter and milk [43]. These results emphasized the different effects of animal- and plant-derived SF on lipid profiles. Coconut oil mainly contains lauric acid (C12:0), which behaves predominantly like a long-chain FA from a metabolic standpoint [44]. Thus, research and health claims on MCTs cannot be applied to coconut oil because most FAs in coconut oil differ from MCTs in their structure, absorption, and metabolism [44]. The effect of lauric acid on blood lipids is an increase in both TC and HDL-c, with a greater effect on HDL-c. Thus, lauric-acid rich oils may decrease the ratio of TC to HDL-c [16]. However, no prospective studies or RCTs to date have specifically assessed the effect of coconut oil on CVD outcomes and mortality. Observational data have indicated that indigenous populations consuming coconut as their staple food have a low incidence of CVD [45]; nevertheless, the diets of these populations did not contain high proportions of SFs. Moreover, these populations consumed coconut flesh or squeezed coconut as part of a traditional diet, rather than coconut oil.
Lard is popular in Thai and other Asian cuisines as a cooking oil for fried or deep-fried food. Even though palm oil and lard are rich in MUFA (containing 37% and 45% MUFA, respectively) [36] and lard contains stearic acid (C18:0) (11%), which has no cholesterol-raising effect [46], our study shows that lard did not increase HDL-c compared with comparator oils. Moreover, our findings indicate that substituting lard for MUFAs and PUFAs increased LDL-c and TC. A recent study demonstrated that MUFA intake from plants but not from animals was associated with lower CHD risk [24]. Therefore, consumption of SF-rich oil, particularly animal-derived fat, should be limited to decrease the risk of CVD.
We combined the effect of lipid profile change after substitution of SF with MUFA and PUFA by estimating the 10-year atherosclerotic cardiovascular disease (ASCVD) risk (http://tools.acc.org/ASCVD-Risk-Estimator-Plus/, accessed on 5 January 2020). The result revealed that substituting lard for PUFA and MUFA could increase the 10-year ASCVD risk by approximately 1% in patients with high CV risk (diabetes, hypertension, and smoking). Similarly, substituting palm oil for MUFA-rich oil increased the 10-year ASCVD risk by approximately 1%. However, substituting palm oil for PUFA and substituting coconut oil for PUFA and MUFA did not increase the ASCVD risk. Thus, it may be appropriate to recommend the intake of plant-derived SFs either from palm oil or coconut oil instead of those from animal-derived SFs for health-related outcomes [31].
Soybean oil is the second most commonly consumed oil worldwide [8]. In our analysis, replacement of SF with soybean oil significantly improved all lipid parameters. Substituting soybean oil for lard and butter significantly decreased LDL-c. However, substituting soybean oil for other PUFA-rich oils had no effect on lipid levels.
RBO is a rich source of MUFA (44%), PUFA (33.6%), tocopherols, tocotrienols, and phytosterol [47], and is thus a popular cooking oil in Asia and tropical countries. RBO also contains γ-oryzanol, which has antioxidant properties [48] and has been shown to improve lipid profiles [49,50,51]. Our study findings indicate the favorable effect of RBO in decreasing both LDL-c and TC levels. Our umbrella review included only one MA of the effect of RBO on lipid levels [19].
Our study had some strengths, including the umbrella review design, which is an efficient approach to summarize comprehensive evidence from SRs and MAs. SFAs, PUFAs, and MUFAs from animal and plant sources were included, and their effects on all lipid profiles were pooled. Limitations of our study included variation in dosages of intervention oils and baseline diets across the studies, meaning that most SRs and MAs had substantially heterogeneous findings. Most MAs evaluating groups of oils, such as SFs, PUFAs, and MUFAs, with unidentified individual oils were excluded. Further research into the effect of consumption of tropical oils compared with other common vegetable oils on the number and size of LDL and HDL particles, CVD incidence, and mortality is therefore warranted.
Our study findings support current recommendations to reduce animal-derived SF intake and replace it with soybean oil, RBO, or other PUFA- and MUFA-rich vegetable oils to improve lipid profiles and reduce the risk of CVD. However, the health impact of plant-derived SFs, which are widely used in Asia, remains inconclusive because both ‘good’ and ‘bad’ cholesterol are elevated following their consumption. Animal-derived (lard and butter) and plant-derived (palm and coconut oil) SFs have different effects on lipid profiles. Future guidelines should therefore address this issue and give specific recommendations.
Acknowledgments
The authors would like to thank Naphat Taonam and Nantaporn Sittikho for technical support and We thank Edanz (https://www.edanz.com/ac, accessed on 23 April 2021) for editing a draft of this manuscript.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/nu13051549/s1, Supplementary Data S1: Search Strategy, Table S2: Additional baseline included systematic reviews and meta-analyses, Table S3: Degree of Overlapping Individual Studies in LDL Outcome, Table S4: AMSTARII of Studies with Lipid Outcomes.
Author Contributions
Conceptualization and methodology, C.U., P.C.S., V.K. and A.T.; investigation and data curation, C.U., P.C.S., V.K., S.S. and A.T.; formal analysis, C.U., S.S. and A.T; writing—original draft preparation, C.U. and P.C.S.; writing—review and editing, C.U., P.C.S., V.K., D.W., S.M., P.V., P.S. and A.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Thai Health Promotion Foundation, Thailand.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Roth G.A., Abate D., Abate K.H., Abay S.M., Abbafati C., Abbasi N., Abbastabar H., Abd-Allah F., Abdela J., Abdelalim A., et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong N.D. Epidemiological studies of CHD and the evolution of preventive cardiology. Nat. Rev. Cardiol. 2014;11:276–289. doi: 10.1038/nrcardio.2014.26. [DOI] [PubMed] [Google Scholar]
- 3.Yusuf S., Hawken S., Ounpuu S., Dans T., Avezum A., Lanas F., McQueen M., Budaj A., Pais P., Varigos J., et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (The INTERHEART Study): Case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 4.GBD 2017 Risk Factor Collaborators Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1923–1994. doi: 10.1016/s0140-6736(18)32225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joris P.J., Mensink R.P. Role of cis-Monounsaturated fatty acids in the prevention of coronary heart disease. Curr. Atheroscler. Rep. 2016;18:38. doi: 10.1007/s11883-016-0597-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siri-Tarino P.W., Sun Q., Hu F.B., Krauss R.M. Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am. J. Clin. Nutr. 2010;91:535–546. doi: 10.3945/ajcn.2009.27725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamley S. The effect of replacing saturated fat with mostly n-6 polyunsaturated fat on coronary heart disease: A meta-analysis of randomised controlled trials. Nutr. J. 2017;16:30. doi: 10.1186/s12937-017-0254-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.United States Department of Agcriculture [Internet]. Washington: Global Oilseed Demand Growth Forecast to Outpace Production (Again). Oilseeds: World Markets and Trade. [(accessed on 23 April 2021)];2018 May 3; Available online: https://www.fas.usda.gov/data/oilseeds-world-markets-and-trade.
- 9.L’Abbé M.R., Stender S., Skeaff C.M., Ghafoorunissa. Tavella M. Approaches to removing trans fats from the food supply in industrialized and developing countries. Eur. J. Clin. Nutr. 2009;63:S50–S67. doi: 10.1038/ejcn.2009.14. [DOI] [Google Scholar]
- 10.Aromataris E., Fernandez R., Godfrey C.M., Holly C., Khalil H., Tungpunkom P. Summarizing systematic reviews: Methodological development, conduct and reporting of an umbrella review approach. Int. J. Evid. Based Healthc. 2015;13:132–140. doi: 10.1097/XEB.0000000000000055. [DOI] [PubMed] [Google Scholar]
- 11.Ioannidis J.P. Integration of evidence from multiple meta-analyses: A primer on umbrella reviews, treatment networks and multiple treatments meta-analyses. CMAJ. 2009;181:488–493. doi: 10.1503/cmaj.081086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shea B.J., Reeves B.C., Wells G., Thuku M., Hamel C., Moran J., Moher D., Tugwell P., Welch V., Kristjansson E., et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pieper D., Antoine S.L., Mathes T., Neugebauer E.A., Eikermann M. Systematic review finds overlapping reviews were not mentioned in every other overview. J. Clin. Epidemiol. 2014;67:368–375. doi: 10.1016/j.jclinepi.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harland J.I. An Assessment of the economic and heart health benefits of replacing saturated fat in the diet with monounsaturates in the form of rapeseed (canola) oil. Nutr. Bull. 2009;34:174–184. doi: 10.1111/j.1467-3010.2009.01756.x. [DOI] [Google Scholar]
- 16.Mozaffarian D., Clarke R. Quantitative effects on cardiovascular risk factors and coronary heart disease risk of replacing partially hydrogenated vegetable oils with other fats and oils. Eur. J. Clin. Nutr. 2009;63(Suppl. 2):S22–S33. doi: 10.1038/sj.ejcn.1602976. [DOI] [PubMed] [Google Scholar]
- 17.Fattore E., Bosetti C., Brighenti F., Agostoni C., Fattore G. Palm oil and blood lipid-related markers of cardiovascular disease: A systematic review and meta-analysis of dietary intervention trials. Am. J. Clin. Nutr. 2014;99:1331–1350. doi: 10.3945/ajcn.113.081190. [DOI] [PubMed] [Google Scholar]
- 18.Sun Y., Neelakantan N., Wu Y., Lote-Oke R., Pan A., van Dam R.M. Palm oil consumption increases LDL cholesterol compared with vegetable oils low in saturated fat in a meta-analysis of clinical trials. J. Nutr. 2015;145:1549–1558. doi: 10.3945/jn.115.210575. [DOI] [PubMed] [Google Scholar]
- 19.Jolfaie N.R., Rouhani M.H., Surkan P.J., Siassi F., Azadbakht L. Rice bran oil decreases total and LDL cholesterol in humans: A systematic review and meta-analysis of randomized controlled clinical trials. Horm. Metab. Res. 2016;48:417–426. doi: 10.1055/s-0042-105748. [DOI] [PubMed] [Google Scholar]
- 20.Ghobadi S., Hassanzadeh-Rostami Z., Mohammadian F., Zare M., Faghih S. Effects of canola oil consumption on lipid profile: A systematic review and meta-analysis of randomized controlled clinical trials. J. Am. Coll. Nutr. 2019;38:185–196. doi: 10.1080/07315724.2018.1475270. [DOI] [PubMed] [Google Scholar]
- 21.Ghobadi S., Hassanzadeh-Rostami Z., Mohammadian F., Nikfetrat A., Ghasemifard N., Raeisi Dehkordi H., Faghih S. Comparison of blood lipid-lowering effects of olive oil and other plant oils: A systematic review and meta-analysis of 27 randomized placebo-controlled clinical trials. Crit. Rev. Food Sci. Nutr. 2018;59:2110–2124. doi: 10.1080/10408398.2018.1438349. [DOI] [PubMed] [Google Scholar]
- 22.Panth N., Abbott K.A., Dias C.B., Wynne K., Garg M.L. Differential effects of medium- and long-chain saturated fatty acids on blood lipid profile: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2018;108:675–687. doi: 10.1093/ajcn/nqy167. [DOI] [PubMed] [Google Scholar]
- 23.Schwingshackl L., Bogensberger B., Bencic A., Knuppel S., Boeing H., Hoffmann G. Effects of oils and solid fats on blood lipids: A systematic review and network meta-analysis. J. Lipid Res. 2018;59:1771–1782. doi: 10.1194/jlr.P085522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zong G., Li Y., Sampson L., Dougherty L.W., Willett W.C., Wanders A.J., Alssema M., Zock P.L., Hu F.B., Sun Q. Monounsaturated fats from plant and animal sources in relation to risk of coronary heart disease among US men and women. Am. J. Clin. Nutr. 2018;107:445–453. doi: 10.1093/ajcn/nqx004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guasch-Ferré M., Zong G., Willett W.C., Zock P.L., Wanders A.J., Hu F.B., Sun Q. Associations of monounsaturated fatty acids from plant and animal sources with total and cause-specific mortality in two US prospective cohort studies. Circ. Res. 2019;124:1266–1275. doi: 10.1161/circresaha.118.313996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mao L., Zhang Y., Wang W., Zhuang P., Wu F., Jiao J. Plant-sourced and animal-sourced monounsaturated fatty acid intakes in relation to mortality: A prospective nationwide cohort study. Eur. J. Nutr. 2020;59:1989–1998. doi: 10.1007/s00394-019-02048-8. [DOI] [PubMed] [Google Scholar]
- 27.Nettleton J.A., Brouwer I.A., Mensink R.P., Diekman C., Hornstra G. Fats in foods: Current evidence for dietary advice. Ann. Nutr. Metab. 2018;72:248–254. doi: 10.1159/000488006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mozaffarian D., Ludwig D.S. The 2015 US dietary guidelines: Lifting the ban on total dietary fat. JAMA. 2015;313:2421–2422. doi: 10.1001/jama.2015.5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lahey R., Khan S.S. Trends in obesity and risk of cardiovascular disease. Curr. Epidemiol. Rep. 2018;5:243–251. doi: 10.1007/s40471-018-0160-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGuire S. Scientific Report of the 2015 Dietary Guidelines Advisory Committee. Washington, DC: US departments of agriculture and health and human services, 2015. Adv. Nutr. 2016;7:202–204. doi: 10.3945/an.115.011684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arnett D.K., Blumenthal R.S., Albert M.A., Buroker A.B., Goldberger Z.D., Hahn E.J., Himmelfarb C.D., Khera A., Lloyd-Jones D., McEvoy J.W., et al. 2019 ACC/AHA Guideline on the primary prevention of cardiovascular disease: A report of the American college of cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2019;140:e596–e646. doi: 10.1161/CIR.0000000000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song M., Fung T.T., Hu F.B., Willett W.C., Longo V.D., Chan A.T., Giovannucci E.L. Association of animal and plant protein intake with all-cause and cause-specific mortality. JAMA Int. Med. 2016;176:1453–1463. doi: 10.1001/jamainternmed.2016.4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tharrey M., Mariotti F., Mashchak A., Barbillon P., Delattre M., Fraser G.E. Patterns of plant and animal protein intake are strongly associated with cardiovascular mortality: The Adventist Health Study-2 cohort. Int. J. Epidemiol. 2018;47:1603–1612. doi: 10.1093/ije/dyy030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piepoli M.F., Abreu A., Albus C., Ambrosetti M., Brotons C., Catapano A.L., Corra U., Cosyns B., Deaton C., Graham I., et al. Update on cardiovascular prevention in clinical practice: A position paper of the European Association of Preventive Cardiology of the European Society of Cardiology. Eur. J. Prev. Cardiol. 2020;27:181–205. doi: 10.1177/2047487319893035. [DOI] [PubMed] [Google Scholar]
- 35.Boateng L., Ansong R., Owusu W.B., Steiner-Asiedu M. Coconut oil and palm oil’s role in nutrition, health and national development: A review. Ghana Med. J. 2016;50:189–196. [PMC free article] [PubMed] [Google Scholar]
- 36.Szajewska H., Szajewski T. Saturated fat controversy: Importance of systematic reviews and meta-analyses. Crit. Rev. Food Sci. Nutr. 2016;56:1947–1951. doi: 10.1080/10408398.2015.1018037. [DOI] [PubMed] [Google Scholar]
- 37.Obahiagbon F.I. A Review: Aspects of the African Oil Palm (Elaeis guineesis jacq.) and the implications of its bioactives in human health. Am. J. Biochem. Mol. Biol. 2012;2:106–119. doi: 10.3923/ajbmb.2012.106.119. [DOI] [Google Scholar]
- 38.Ismail S.R., Maarof S.K., Siedar Ali S., Ali A. Systematic review of palm oil consumption and the risk of cardiovascular disease. PLoS ONE. 2018;13:e0193533. doi: 10.1371/journal.pone.0193533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen B.K., Seligman B., Farquhar J.W., Goldhaber-Fiebert J.D. Multi-Country analysis of palm oil consumption and cardiovascular disease mortality for countries at different stages of economic development: 1980–1997. Global Health. 2011;7:45. doi: 10.1186/1744-8603-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berry S.E., Sanders T.A. Influence of triacylglycerol structure of stearic acid-rich fats on postprandial lipaemia. Proc. Nutr. Soc. 2005;64:205–212. doi: 10.1079/PNS2005422. [DOI] [PubMed] [Google Scholar]
- 41.Bracco U. Effect of triglyceride structure on fat absorption. Am. J. Clin. Nutr. 1994;60:1002S–1009S. doi: 10.1093/ajcn/60.6.1002S. [DOI] [PubMed] [Google Scholar]
- 42.Kris-Etherton P.M., Yu S. Individual fatty acid effects on plasma lipids and lipoproteins: Human studies. Am. J. Clin. Nutr. 1997;65:1628s–1644s. doi: 10.1093/ajcn/65.5.1628S. [DOI] [PubMed] [Google Scholar]
- 43.Zock P.L., de Vries J.H., Katan M.B. Impact of myristic acid versus palmitic acid on serum lipid and lipoprotein levels in healthy women and men. Arterioscler. Thromb. 1994;14:567–575. doi: 10.1161/01.ATV.14.4.567. [DOI] [PubMed] [Google Scholar]
- 44.Eyres L., Eyres M.F., Chisholm A., Brown R.C. Coconut oil consumption and cardiovascular risk factors in humans. Nutr. Rev. 2016;74:267–280. doi: 10.1093/nutrit/nuw002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prior I.A., Davidson F., Salmond C.E., Czochanska Z. Cholesterol, coconuts, and diet on Polynesian atolls: A natural experiment: The Pukapuka and Tokelau island studies. Am. J. Clin. Nutr. 1981;34:1552–1561. doi: 10.1093/ajcn/34.8.1552. [DOI] [PubMed] [Google Scholar]
- 46.Berry S.E., Miller G.J., Sanders T.A. The solid fat content of stearic acid-rich fats determines their postprandial effects. Am. J. Clin. Nutr. 2007;85:1486–1494. doi: 10.1093/ajcn/85.6.1486. [DOI] [PubMed] [Google Scholar]
- 47.Orsavova J., Misurcova L., Ambrozova J.V., Vicha R., Mlcek J. Fatty acids composition of vegetable oils and its contribution to dietary energy intake and dependence of cardiovascular mortality on dietary intake of fatty acids. Int. J. Mol. Sci. 2015;16:12871–12890. doi: 10.3390/ijms160612871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Juliano C., Cossu M., Alamanni M.C., Piu L. Antioxidant activity of gamma-oryzanol: Mechanism of action and its effect on oxidative stability of pharmaceutical oils. Int. J. Pharm. 2005;299:146–154. doi: 10.1016/j.ijpharm.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 49.Bumrungpert A., Chongsuwat R., Phosat C., Butacnum A. Rice bran oil containing gamma-oryzanol improves lipid profiles and antioxidant status in hyperlipidemic subjects: A randomized double-blind controlled trial. J. Altern. Complement. Med. 2019;25:353–358. doi: 10.1089/acm.2018.0212. [DOI] [PubMed] [Google Scholar]
- 50.Shakib M.-C., Gabrial S., Gabrial G. Rice bran oil compared to atorvastatin for treatment of dyslipidemia in patients with type 2 diabetes. Open Access Maced. J. Med. Sci. 2014;2:95–102. doi: 10.3889/oamjms.2014.017. [DOI] [Google Scholar]
- 51.Lichtenstein A.H., Ausman L.M., Carrasco W., Gualtieri L.J., Jenner J.L., Ordovas J.M., Nicolosi R.J., Goldin B.R., Schaefer E.J. Rice bran oil consumption and plasma lipid levels in moderately hypercholesterolemic humans. Arterioscler. Thromb. 1994;14:549–556. doi: 10.1161/01.ATV.14.4.549. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.