Abstract
Background
Punica granatum L. (pomegranate) with astringent activities and Matricaria chamomilla L. (chamomile) with anti-inflammatory and antioxidant properties are natural remedies used for various skin disorders, including wound healing.
Objectives
This study was conducted to evaluate the individual and combined wound healing activity of the methanol extracts of pomegranate and chamomile flowers.
Methods
After preparing the menthol fraction of pomegranate and chamomile flowers, the content of total phenols, total tannins, and total flavonoids of fractions was measured. For standardization of pomegranate and chamomile fractions, Gallic acid and apigenin-7-O-glucoside contents of them were determined using high-performance liquid chromatography (HPLC). Moreover, their antioxidant activities were examined using DPPH and FRAP tests. The antimicrobial assay was performed against Staphylococcus aureus, Staphylococcus epidermidis, and Pseudomonas aeruginosa. Three different concentrations of methanol fraction of each plant and one combination dose of fractions were investigated for their wound healing activities in an excision wound model on the rats’ dorsum. Finally, histopathological studies were done at the end of the experiment.
Results
Phytochemical examinations showed high amounts of phenolic compounds in pomegranate flowers, while chamomile flower fractions contained a high amount of total flavonoids. Both fractions, especially pomegranate, had potent antioxidant activity. The best results for wound closure were observed 7 days after wound induction. All treated groups exhibited superior wound contraction compared to their placebo at all measurement times. The combined form of pomegranate and chamomile had better wound healing properties compared to a single therapy, especially on time earlier to wound induction.
Conclusion
This study represented high antioxidant and wound healing activities for methanol fraction of pomegranate and chamomile flowers, which could be related to their high content of phytochemicals. In comparison with single herb treatment, the combined form of these two fractions in lower concentrations accelerated wound closure.
Graphical abstract
Keywords: Punica granatum L., Matricaria chamomilla L., Persian medicine, Wound healing, Antioxidant activity, Antibacterial activity
Introduction
Wound healing is a complex, dynamic repair response to tissue injury achieved through four highly programmed overlapping phases: hemostasis, inflammation, proliferation, and remodeling. Wound care provides a favorable condition for these processes within a reasonable timeframe [1]. Phytotherapy is highly preferred by the general population worldwide, and wound dressing with certain herbs is a prehistoric tradition. Many healing effects of the botanical extracts are well studied and accepted by modern medicine. It has been established that anti-inflammatory, antimicrobial, and anesthetics agents, in addition to protective modalities, will be helpful for promoting the healing cascade. Herbal extracts can affect different curative wound healing phases and accelerate ulcer healing using various phytochemicals [2].
Pomegranate (Punica granatum L.) is an ancient Iranian medicinal plant. The antioxidant, anti-inflammatory, antibacterial, and anticarcinogenic properties of the extracts of different parts of the plant (flower, juice, skin, and tree bark) have been investigated and confirmed in several studies [3]. The rind of its edible fruit is the main part of the plant that has been considered for wound healing activity [4–6]. In this study, the flowers were used because of their unique astringent quality. Polyphenols (tannins, punicalagin, and ellagic acid), phenolic compounds (gallic acid), and triterpenoids (maslinic, ursolic acid, and asiatic acid) are the major constituents of the flowers [7]. Gallic acid and tannins act as an astringent, boost wound contraction, and reduce ulcer exudation. Besides, the polyphenols stimulate dermal fibroblast proliferation, enhance type I procollagen synthesis, and inhibit matrix metalloproteinase-1 [8].
Chamomile (Matricaria chamomilla L.) is a medicinal plant widely used for several pharmacological applications. Topical preparations of the chamomile extract have significant efficacy for treating various skin diseases, including UV-induced erythema, contact dermatitis, phlebitis, atopic eczema, radiodermatitis, and wound healing [9–11]. The hydroalcoholic extract of the flowers contains flavonoids (quercetin and apigenin), coumarins, and terpenoids (α-bisabolol and chamazulene) [12]. The flavonoids inhibit histamine release from leukocytes [13], and bisabolol acts as a granulation tissue promoter [14, 15]. A limited number of studies have evaluated the effect of chamomile on wound healing. However, its medicinal properties, such as anti-inflammatory [16, 17], antioxidant [18, 19], and antimicrobial effects [20, 21], are eligible features for promoting the wound healing process.
The presence of a mixture of phytochemicals is the most critical characteristic of herbal extracts offering multitarget properties. Because of the complexity of the wound healing nature and the contribution of different components and processes, the integration of various plant extracts into one formulation is a new approach in wound healing modalities that has superior advantages over individual use [22]. Polyherbal topical preparations like “Dragon’s blood” and “Jena” are two successful examples [23, 24]. The present study is the first work conducted to investigate the wound healing activity of the methanol fractions of flower extracts of chamomile and pomegranate individually and in combination and to evaluate the association between their healing effects and the antioxidant and antibacterial properties of their phytochemicals.
Materials and methods
Chemicals
Methanol, Acetonitrile (Duksan, Gyeonggi-do, Korea), Sodium carbonate (Ca2CO3), Aluminium chloride (AlCl3), Butylated hydroxyanisole (BHA), Ferrous chloride (FeCl3), Trifluoroacetic acid, Eosin, Hematoxylin (Merck, Darmstadt, Germany), Gallic acid, Polyvinylpolypyrrolidone (PVPP), Apigenin-7-O-glucoside (Sigma-Aldrich Chemie GmbH, Steinheim, Germany), 2,2-diphenyl-1-picrylhydrazyl (DPPH), Catechin, Folin-ciocalteau (Fluka, Buchs, Switzerland), (Sigma Chemical Co., Saint Louis, MO), Ciprofloxacin (Temad, Tehran, Iran), Semisolid paraffin (Rose polymer Co., Esfahan, Iran), Ketamine (Rotexmedica Pharmaceutical Co., Allemagne, Germany), Xylazine (Caspian Tamin Co., Rasht, Iran).
Plant material, extraction, and preparation of methanol fraction
The flowers of Matricaria chamomilla L. and Punica granatum L. were purchased from a market in Tehran, Iran. The plant samples were identified, and voucher specimens were deposited at the Herbarium of the Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran (PMP-339 and PMP-520, respectively).
One kilogram of each plant was powdered and extracted with methanol (80%) at four 24 h rounds at room temperature separately. The extracts were concentrated using the Heidolph Laboratora 4000 vacuum rotary evaporator under reduced pressure at 40 °C to obtain a gummy residue (crude extract). Then, they were suspended in water and partitioned with chloroform to achieve a residue named methanol fraction. The solvent volume was reduced again by evaporation under a vacuum. Then, the final methanol fractions were lyophilized by a laboratory freeze dryer (CHRIST ALPHA 1–2 LDPLUS, Germany) to reach a dry, puffy powder. The percent yield of extract and fractions was calculated using the following formula:
Phytochemical analysis
The presence of phytochemicals (total phenols, total tannins, and total flavonoids) was determined in each plant’s methanol fraction.
Measurement of total phenolic content
Total phenolic compounds of each fraction were determined by colorimetry using the modified Folin-Ciocalteau method [25]. Then, 0.1 mL of each fraction was mixed with 0.25 ml of Folin- Ciocalteau reagent (previously diluted 1:10 with distilled water) and allowed to stand at 25 °C for 3 min. Next, 1.25 mL of sodium carbonate solution (20% w/v) was added to the mixture. After 40 min, the absorbance was measured against a prepared blank reagent at 725 nm using a UV visible spectrophotometer (OPTIZEN 2120UV, South Korea). The results were obtained in triplicate. The total phenolic content of each plant is expressed as gallic acid equivalent in dry fraction (μg GA/mg FR) using the gallic acid calibration curve (Y = 0.0076x, R2 = 0.9993).
Measurement of total tannin content
To measure tannins, PVPP was applied to bind to tannins and remove them by precipitation. The free phenolic content of the supernatant solution after precipitation of tannin-phenolics by PVPP was calculated using the Folin-Ciocalteaus method. The total tannin content of each fraction was calculated by deducting the amount of free phenols from the total phenolic content [25]. All the results were obtained in triplicate. The total tannin content of the methanol fraction of each plant is expressed as gallic acid equivalent in dry fraction (mg GA/μg FR) using the gallic acid calibration curve (Y = 0.0076x, R2 = 0.9993).
Measurement of total flavonoid content
The flavonoid content was measured with the aluminum chloride procedure using catechin as a reference compound [26]. Briefly, an aliquot (1 mL) of each extract solution (100 mg/mL) or catechin solution (50, 100, 150, 200, 250 and 300 mg/L) was mixed with 4 mL of double-distilled water. Then, 0.3 mL of NaNO3 5% was added. After 5 min, 0.3 mL of AlCl3 (10%) was added. The next step was to add 2 mL of NaOH 1 M and increase the volume of the mixture to reach a total volume of 10 mL using double distilled water. The absorbance of the solution was measured against a prepared blank reagent at 510 nm after 30 min. All the results were obtained in triplicate. The total flavonoid content is expressed as catechin equivalent (mg of CE/g FRE) using the catechin calibration curve (Y = 0.003x + 0.0054, R2 = 0.983).
Chemical marker analysis with RP-HPLC-DAD
Pomegranate and chamomile methanol fractions were standardized based on apigenin-7-O-glucoside and gallic acid as markers of their phytoconstituents, respectively, using the RP-HPLC-DAD.
The reversed-phase high-performance liquid chromatography with a diode array detector (RP-HPLC-DAD) was performed using the Knauer-Azura HPLC system (Knauer Association, Germany) and a photodiode array detector (Azura DAD 2.1 L) using the ClarityChrom software (version 6.1.0). The analysis was performed using a VP-ODS C18 column (250 × 4.9 mm, 5 휇m particle size; Knauer LC-column, Germany). All the solvents were filtered and degassed before injection into the column.
For gallic acid quantification, the isocratic mode of the mobile phase of 0.05% v/v trifluoroacetic acid (TFA) in water: methanol (10:90% v/v) was used at a flow rate of 1.0 mL/min and detection wavelength of 272 nm. For measurement of apigenin-7-O-glucoside, gradient solvent system, 80% purified water (with 0.02% acetic acid) and 20% acetonitrile to 20% acetonitrile and 80% water (with 0.02% acetic acid) within 30 min was used as mobile phases at a flow rate of 0.8 mL/m and detection wavelength of 335 nm. The injection volume was 20 μl, and all the measurements were performed at ambient temperature.
Known quantities of the standards were dissolved in methanol, and the stocks were serially diluted to seven different concentrations separately to prepare working solutions (5–100 μg/ml) for calibration curves. The system suitability test was assessed by three replicates of standard gallic acid and apigenin-7-O-glucoside in a particular concentration. The peak area of each solution was plotted against the concentration to obtain a calibration curve. The concentrations of gallic acid and apigenin-7-O-glucoside are expressed as μg/mg of extract dry weight (DW).
Antioxidant activity
DPPH radical scavenging activity
The fractions’ antioxidant capacity for scavenging free radicals was determined using 2,2-Diphenyl-1-picrylhydrazyl (DPPH) as a standard method [27, 28]. Five concentrations of each fraction solution (100, 50, 25, 12.5, 6.2 μg/mL) were prepared with the serial dilution method. One milliliter of each concentration plus 1 mL of methanol and 1 mL of DPPH solution (70 μg/mL methanol) was added to a suitable glass tube. The negative control contained 1 mL methanol fraction, 1 mL methanol, and 1 mL DPPH solution. The absorbance of the samples and blank solutions (including 2 mL methanol and 1 mL DPPH solution) was measured at 517 nm after 30 min. Free radical 50% inhibition concentration (IC50) of the fractions was reported compared to vitamin E and BHA as conventional antioxidants using the following formula.
All samples were analyzed in triplicate.
FRAP test
The Ferric Reducing Antioxidant Power (FRAP) assay is another test for evaluating antioxidant activity [28]. Antioxidants reduce the 2,4,6-ferric-tripyridyl triazine (TPTZ) complex to its ferrous colored form, which is detectable by spectrophotometry. Briefly, after preparing fresh FRAP reagent (5 mL of a TPTZ (10 mmol/L), 5 mL FeCl3 (20 mmol/L), 50 mL acetate buffer (0.3 mol/L, pH = 3.6), and 0.1 mL of each fraction were mixed with 3 mL of FRAP reagent. Then, the absorbance of the solution was measured against the prepared blank reagent at 593 nm. The results were calculated from the standard curve of FeSO4 .7H2O (y = 0.001x + 0.049, R2 = 0.925) expressed in mmol Fe+2 / 100 g of sample and compared with vitamin E and BHA.
Antimicrobial assay
The antimicrobial activity of the fractions was investigated against Staphylococcus aureus ATCC 6538p, Streptococcus epidermidis ATCC 12228, and Pseudomonas aeruginosa ATCC 9027 as three major bacterial strains causing skin diseases or wound infections, which were obtained from the Department of Biotechnology, Faculty of Pharmacy, Tehran University of Medical Sciences.
The organisms were subcultured on a sterile nutrient agar plate and incubated aerobically for 18–24 h at 37 ± 2 °C. The colonies of each organism were homogenized in sterile normal saline (0.9%), and the turbidity of the initial suspension was adjusted by comparing with 0.5 McFarland’s standard.
According to the microdilution method, susceptibility was determined for all the bacteria using 96 U-shaped well plates [29]. A 200-μL aliquot of the stock solution (2 mg/mL) of each fraction in Mueller–Hinton broth (MHB) was transferred into the first well in each row and serially diluted by mixing with 100 μL of MHB in subsequent wells. Then, 100 μL of the bacterial suspension (1 × 106 CFU/mL) was added to each well to reach a final inoculum size of about 5 × 105 CFU/well. After 24-h incubation at 37 ± 2 °C, the microdilution trays were tested for the absence or presence of visible growth in comparison to the growth of bacteria in ciprofloxacin control wells. The lowest concentration (highest dilution) of each fraction that produced no visible bacterial growth (no turbidity) was regarded as the minimum inhibitory concentration (MIC).
Moreover, the antimicrobial activity of fractions was surveyed in specific concentrations (25 mg/mL of pomegranate and 50 mg/mL of chamomile) selected for the combination form for wound healing assessment using the agar well diffusion method [30]. Muller-Hinton agar was used as a medium for bacterial growth. The plates were incubated at 37 °C for 24–48 h. The inhibition zones were measured with a standard scale compared to a positive control (ciprofloxacin 1 mg/mL).
Wound healing evaluation
Experimental animals
Forty-two Wistar male rats (200–220 g) of approximately 2 months of age were used as experimental animals and were randomly divided into seven groups of six rats. The animals were housed in standard environmental conditions (temperature: 22 ± 3 °C, humidity: 60 ± 5%, and a 12 h light/dark cycle). During the experiment, the rats were fed a standard pellet diet (Pastor Institute, Iran) and water ad libitum. All procedures were carried out according to the institutional guidelines for animal care and use. The study was approved by the Ethics Committee of the Institute of Pharmaceutical Sciences (TIPS) of Tehran University of Medical Sciences, with the code number of IR.TUMS.PSRC.REC.1396.4521.
Preparation of ointment
To make the fraction applicable, semisolid paraffin was used as an ointment base for fraction delivery, and three concentrations of each herb was integrated to examine the optimum wound healing concentration. Moreover, the middle concentration of each fraction was included in one ointment to assay their combined effect. An ointment base without any extracts was used as a placebo (negative control). For ointment preparation, the required amount of the extract was dissolved in 10 mL of distilled water. The herbal solution was then gradually added to the required amount of an anhydrous hydrocarbon base (Eucerin 200%-EMAD, Emad Darman Pars Co.) and mixed well until a homogenous preparation was achieved.
Excision wound model
Excision wound models were used to evaluate the wound healing activity of the methanol fractions of plant extracts. After inducing anesthesia with 2% xylazine (5 mg/kg, IP) and 10% ketamine (90 mg/kg, IP), the rats were fixed in a ventral posture on a surgery table. Then, the dorsal area from the scapula to the ilium was shaved and prepared for surgery. Two square (2 × 2), full-thickness wounds (about 2 mm deep), 1 cm away from both sides of the backbone and 2 cm away from each other, were made using a Bistoury surgical knife. The epidermal, dermal, hypodermal, and panniculus carnosus layers were removed.
Grouping animals
The animals were numbered, weighed, and randomly divided into seven groups of six rats:
C 2.5% group: Rats treated with a topical ointment containing 2.5% (w/w) methanol fraction of Matricaria chamomilla L. flowers.
C 5% group: Rats treated with a topical ointment containing 5% (w/w) methanol fraction of Matricaria chamomilla L. flowers.
C 10% group: Rats treated with a topical ointment containing 10% (w/w) methanol fraction of Matricaria chamomilla L. flowers.
P 1% group: Rats treated with a topical ointment containing 1% (w/w) methanol fraction of Punica granatum L. flowers.
P 2.5% group: Rats treated with a topical ointment containing 2.5% (w/w) methanol fraction of Punica granatum L. flowers.
P 5% group: Rats treated with a topical ointment containing 5% (w/w) methanol fraction of Punica granatum L. flowers.
C 5% + P 2.5% group: Rats treated with a combined topical ointment containing 5% (w/w) methanol fraction of Matricaria chamomilla L. flowers plus 2.5% (w/w) methanol fraction of Punica granatum L. flowers.
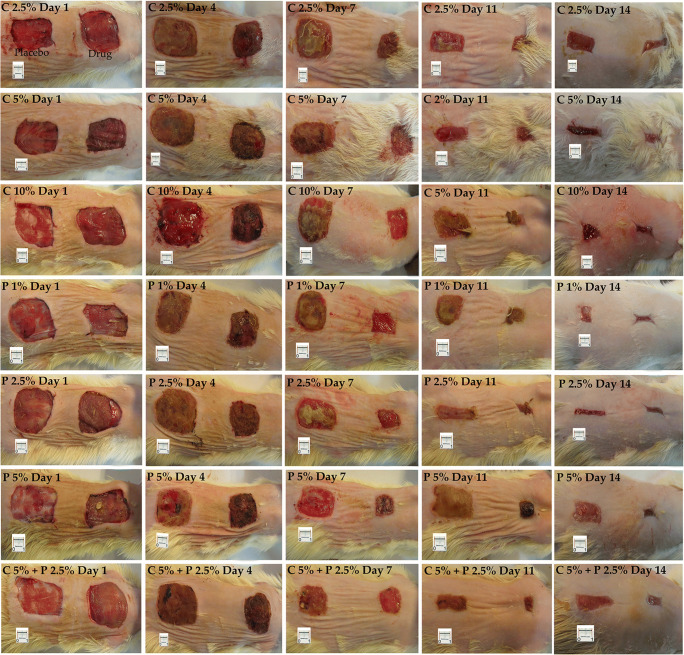
By creating two wound areas in the dorsum of animals in this method (Fig. 1), the ointment containing fraction (drug) was applied to the surface of the inferior wound, and the ointment vehicle (placebo) was applied to the superior wounds in each group.
Fig. 1.
Example of a wound healing process during 14 days. C: chamomile; P: Pomegranate
The drug and placebo were applied to the wounds using supportive sterile gauze. The wound dressing was changed every 24 h. All rats were monitored daily for 14 days, and any wound discharge or evidence of infection or other abnormalities were noted. The wound area was calculated using Adobe Photoshop CS (version 15) software (Adobe Systems Inc.).
Histopathological study
The skin specimens of the healed wound bed were surgically removed for histological evaluation at the end of the experiment (day 14). The samples were fixed in 10% formalin, embedded in paraffin, and sectioned (5 and 20 μm thick sections) using a microtome. Eight sections were randomly selected and stained with hematoxylin and eosin to evaluate histopathological changes [31].
Estimation of the volume of epidermis, dermis, and fibrous tissue
Cavalieri’s principle was applied to estimate the total volume of epidermis, dermis, and fibrous tissue using the following formula [31]:
where t is the distance between the sampled sections, ΣP is estimated using the point-counting method, and a/p is the area associated with each point projected on the skin tissue.
Estimation of the number of fibroblasts, neutrophil, and basal cells
The total number of fibroblasts/myofibroblasts, neutrophils, and new basal cells was determined using the optical dissector method [31]. The positions of the microscopic fields were selected by equal intervals of stage movements and systematic uniform random sampling. A microcator was used to measure the Z-axis movement of the microscope stage. An unbiased counting frame with inclusion and exclusion borders was superimposed on the images of the sections viewed on the monitor. A nucleus was counted if it was placed completely or partially within the counting frame and did not reach the exclusion line. Numerical density (Nv) was calculated using the following formula:
where “ΣQ-” is the number of the nuclei, “h” is the height of the dissector, “a/f” is the frame area, “ΣP” is the total number of the unbiased counting frame in all fields, “t” is the whole section thickness measured in every field using the microcator, and BA is the block advance of the microtome which was set at 10 μm.
The total number of the fibroblasts/myofibroblasts, neutrophils, and basal cells was estimated by multiplying the numerical density (Nv) by the total volume (V).
Statistical analysis
All the experiments were done in triplicate. The results are presented as mean ± standard error of the mean (SEM). One-way analysis of variance (one-way ANOVA) was applied to assess the antimicrobial activity and histopathological data using the IBM SPSS. Tukey HSD was used as the post hoc test. For wound healing assay, all the parameters were analyzed using paired T-test and one-way ANOVA followed by the Holm-Sidak post hoc test. P values <0.05 (*), <0.01 (**), and < 0.001 (***) were considered significant. The Bonferroni adjustment was used to compensate for that increase by testing each individual hypothesis at a significance level of α.
Results and discussion
Extraction yield and phytochemical analysis
The extraction and methanol fraction yield were 10.3% and 4.2% for the chamomile flower and 37.8% and 27.3% for the pomegranate flower. The high extraction yield of pomegranate flowers suggests that they contain highly methanol soluble secondary metabolites.
Total phenols, total tannins, and total flavonoid content of methanol fractions of chamomile and pomegranate flowers are shown in Table 1.
Table 1.
Bioactive contents of methanol fractions of Matricaria chamomilla L. and Punica granatum L. flower extracts
| Methanol fraction | Matricaria chamomilla L. | Punica granatum L. |
|---|---|---|
|
Total flavonoids contents (mg of CA/g of FR) |
212.1 ± 4.2 | 13.2 ± 2.3 |
|
Total phenols contents (mg of GA/g of FR) |
656.1 ± 56.4 | 1728.1 ± 28.9 |
|
Total tannins contents (mg of GA/g of FR) |
198.2 ± 28.4 | 1157.7 ± 24.5 |
| Total tannin/ Total phenol | 0.30 | 0.67 |
CA Catechin, FR Fraction, GA Galic acid
Gallic acid and apigenin-7-O-glucoside were considered chemical markers of pomegranate and chamomile, and their concentrations were measured in both fractions by HPLC. The calibration curve equation obtained by standard gallic acid solution was y = 0.47.18x + 20.41, R2 = 0.9993, and the gallic acid content of herbal fractions was estimated. The mean weight of gallic acid (mg) in All samples were pomegranate and chamomile (g) was 73.25 ± 2.01 mg/g (N = 3) and 14.01 ± 0.76 mg/g (x = 3), respectively. The calibration curve equation of standard apigenin-7-O-glucoside solution was y = 12.33x + 13.38, R2 = 0.9955. The mean weight of apigenin-7-O-glucoside (mg) in the methanol fractions of pomegranate and chamomile (g) was 0.39 ± 1.72 mg/g (N = 3) and 89.82 ± 4.21 mg/g (N = 3), respectively.
As expected, methanol fractions of chamomile flowers contained high amounts of apigenin-7-O- glucoside, which was near zero in methanol fractions of pomegranate flowers. Therefore, apigenin-7-O-glucoside could be considered a good chemical marker for standardizing the chamomile extract. However, this monopoly was not seen in gallic acid in two herbal fractions.
The phytochemical analysis showed that the pomegranate fraction contained a higher content of phenolic compounds than chamomile (more than fivefold). These phenolic chemicals are in the form of tannins (2/3 of the total phenolic content), whereas this ratio was one-third in the methanol fraction of chamomile. Since some studies reported the beneficial effects of tannins on pain relief, prevention of infections, and increased epithelialization rate [32], the potent antioxidant and antimicrobial properties of pomegranate reported in this research could be attributed to the high level of tannins.
As expected, the methanol fraction of the chamomile flowers contained high amounts of flavonoids, which was more than five times higher than the pomegranate fraction. Kashchenko et al. quantitatively analyzed the flavonoids content of chamomile flowers by HPLC-UV [33]. Apigenin and its derivatives are the main well-known antioxidant flavonoid of chamomile. Flavonoids inhibit the complement system, reduce the metabolism of arachidonic acid, and decrease cyclooxygenase, which leads to an anti-inflammatory effect [34]. Chamomile contains 36 identified flavonoid substances that could play a substantial role in wound healing by justifying the inflammation phase in the wound healing process. Ardakani et al. [35] surveyed the phytochemical content of the peel and pulp extract of Persian pomegranate subspecies. Based on the findings, the flavonoids content of flowers was less than the peel and more than the pulp extract.
Interestingly, in the present study, the flavonoids content of flowers was more than in previous studies, which may be related to the measurement of phytochemicals in the methanol fraction instead of the total extract. The good solubility of phenolic compounds in methanol confirmed that the methanol fractions were the major source of phenolic compositions.
According to these herbal fractions’ phytochemical contents, a combination of pomegranate, which is rich in tannins as astringent and antimicrobial compounds, and chamomile, which is rich in flavonoids with anti-inflammatory properties, could be an appropriate combination for wound treatment.
Antioxidant assay
The IC50 of the methanol fraction of the flower extract of chamomile and pomegranate estimated by the DPPH assay was 84.2 and 8.6 μg/mL, respectively. Pomegranate flowers showed potent antioxidant activity comparable with BHA (7.8 μg/mL) and vitamin E (14.1 μg/mL). The FRAP test confirmed these findings. The FRAP values for chamomile and pomegranate were 13 and 356 mmol Fe+2/ 100 g, respectively. The reducing power of BHA and vitamin E was 880 and 313 mmol Fe+2/ 100 g, respectively.
Several studies have shown the antioxidant activity of the extracts of different parts of pomegranate (peel, seed, fruit, juice) [36, 37]. However, the values found in this study for pomegranate flowers were much higher than in previous reports. DPPH and FRAP assay are two standard tests for measuring the antioxidant activity of the extracts. The DPPH assay demonstrates the free radical scavenging capacity of compounds, whereas the FRAP test shows iron-reducing capacity. As expected, there was a significant correlation between the phenolic content of fractions and the results of DPPH and FRAP assays. Polyphenols in the form of tannins can scavenge and trap free radicals more efficiently than free phenols due to the presence of several free and combined hydroxyl groups. This theory legitimizes the high amount of observed antioxidant results.
Antimicrobial screening
The antibacterial activity of the fractions was investigated individually against three microbial species and defined as minimum inhibitory concentration (MIC) value (Table 2). Both pomegranate and chamomile fractions had substantially larger MIC values than the positive control (ciprofloxacin), but pomegranate was markedly more effective than chamomile on all of the three bacterial strains. Both fractions showed higher inhibitory effects against gram-positive bacteria, especially S. aureus.
Table 2.
Antibacterial effect of methanol fractions of Punica granatum L. and Matricaria chamomilla L. flower extracts
| Minimum Inhibitory concentration (MIC) (μg/mL) | |||
| Methanol fraction of flowers’ extract | S. aureus | S. epidermidis | P. aeruginosa |
| Matricaria chamomilla L. | 62.5 | 125 | 500 |
| Punica granatum L. | 31.5 | 125 | 250 |
| Ciprofloxacin | 0.195 | 0.39 | 0.78 |
| The diameter of the Inhibition zones (Mean ± SEM) (mm) | |||
| S. aureuse | S. epidermidis | P. aeruginosa | |
| Matricaria chamomilla L. (50 mg/mL) | 1.3 ± 0.3 | 1.0 ± 0.6 | 0.3 ± 0.3 |
| Punica granatum L. (25 mg/mL) | 4.0 ± 0.6 * | 3.3 ± 0.5 * | 2.5 ± 0.5 * |
| Ciprofloxacin (1 mg/mL) | 10.3 ± 0.9 | 12 ± 0.6 | 7.7 ± 0.7 |
Statistically significant differences are indicated: * p < 0.05
The agar diffusion method was used to determine the antibacterial effect of fractions in the concentrations used in combination therapy (group 7 in the rat model). The results showed that the methanol fraction of Punica granatum L. flower extract possessed a significantly more antibacterial effect than the methanol fraction of the Matricaria chamomilla L flower extract.
The antimicrobial effects of Punica granatum L. peel have been investigated in several studies. Yussif et al. [38] evaluated the antimicrobial effect of the phenolic extract of pomegranate peel on eight microorganisms in different concentrations. Similar to this study, they found that it was more effective against gram-positive bacteria. In the present study, the MIC value of the methanol fraction of the pomegranate flower extract was lower than a previous report on the hydroalcoholic extract of pomegranate peel (2 and 0.5 mg/mL for S. aureus ATCC 25923 and P. aeruginosa ATCC 9027, respectively) [39]. It has been established that polyphenolic compounds and tannins have toxic properties against several bacteria. The mechanism of action speculates that polyphenols bind to superficial proteins and receptors on the bacterial cell wall and disturb its barrier function [40]. Moreover, tannins with astringent properties may be made complexion with bacterial enzymes or substrates [41]. It is a presumable antimicrobial activity of pomegranate due to a high concentration of these phytochemicals in the methanol fraction of its extract.
There was no report of antimicrobial activity of pomegranate flower, but according to the similarity of chemical composition, the observed result is related to the high concentration of phenolic and tannin content of extract that previously mentioned.
Wound healing activity
The decrease in the wound area was evaluated in different groups for 14 days. The wound area was measured on days 4, 7, 11, and 14 post-surgery in all groups. In the beginning, each treated zone was compared with its own placebo control zone. The wound contraction results showed that the methanol fractions of pomegranate and chamomile flower extracts in the topical form had significant wound healing activities compared to placebo (negative control group) (Table 3).
Table 3.
Wound healing effect of methanol fractions of Matricaria chamomilla L. and Punica granatum L. flower extracts at different concentrations in an excision wound model (N = 6). Percentage (%) of wound contraction (Mean ± SEM)
| Group (Treatment) | Percentage (%) of wound contraction (Mean ± SEM) | |||
|---|---|---|---|---|
| Post wounding (days) | ||||
| 4 | 7 | 11 | 14 | |
| C 2.5% | 6.51 ± 2.46 | 62.99 ± 2.72* | 82.77 ± 1.19** | 94.93 ± 1.44*** |
| C 5% | 18.89 ± 6.74 | 52.90 ± 6.105* | 86.22 ± 1.352** | 94.53 ± 0.87* |
| C 10% | 21.58 ± 4.80 | 67.44 ± 3.29*** | 84.072 ± 2.97* | 96.27 ± 0.72** |
| P 1% | 16.03 ± 4.77 | 60.18 ± 3.70* | 84.24 ± 1.86* | 94.70 ± 0.70*** |
| P 2.5% | 7.553 ± 2.82 | 61.17 ± 4.06* | 86.58 ± 2.18* | 94.27 ± 1.55* |
| P 5% | 21.28 ± 4.20 | 51.90 ± 7.69 | 83.13 ± 2.81* | 93.44 ± 2.89* |
| C 5% + P 2.5% | 21.70 ± 3.95 | 61.52 ± 4.92* | 89.43 ± 2.66** | 96.06 ± 1.41*** |
N (Number of rats in each group) = 6; Percentage (%) of wound contraction (Mean ± SEM); SEM Standard error of the mean, P Pomegranate, C Chamomile
Mean values within each group against placebo followed by significantly different by paired T-test at:* p < 0.05, ** p < 0.01, and ***p < 0.001
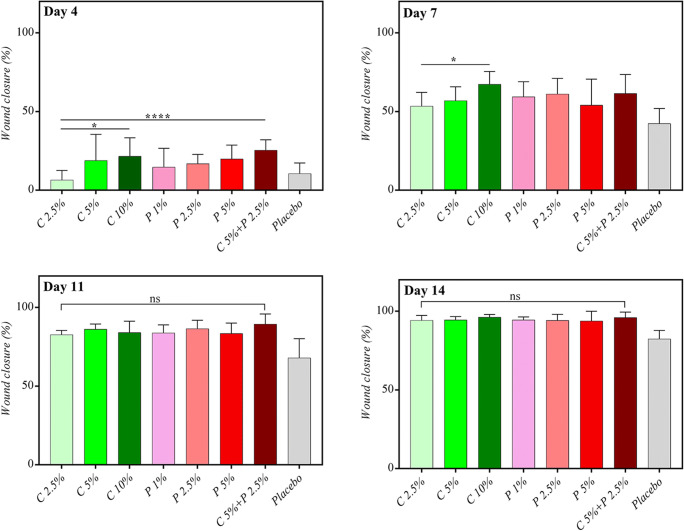
There were no significant differences between treatment and placebo in the study groups until the 4th day. On day 7, a significant difference was seen between treatment and placebo in all groups except the group receiving pomegranate 5%. The highest percentage of wound closure was seen in the C 10% group, followed by C 5% + P 2.5% group. A significant wound closure compared to placebo was observed on days 11 and 14 in all groups. Figure 1 shows an example of the wound healing process in different rat groups during this period.
Figure 2 illustrates the difference in the percentage of wound closure between groups on different days. The results showed that these herbal extracts had productive wound healing activities in low concentrations. A significant difference was observed between a low concentration (2.5%) and high concentration (10%) of chamomile at earlier times (day 4 and day 7), while C 2.5% was as effective as C 10% in the subsequent days. Since the wound is in the inflammatory phase in earlier stages, chamomile with considerable amounts of anti-inflammatory compounds can be more beneficial in this phase. The combined form of extracts (C 5% + P 2.5%) has a more effective wound closure property on the fourth day.
Fig. 2.
Pattern of wound closure on the 4th, 7th, 11th, and 14th post-surgery day. C: chamomile; P: Pomegranate, ns: not significant. * p < 0.05, ** p < 0.01, and ***p < 0.001
On days 11 and 14, despite the superiority of all drug groups over the placebo, there was no significant difference between treatment groups.
Miraj et al. [42] systematically reviewed the effect of chamomile in detail. Among several therapeutic effects, anti-inflammatory, cell-protective, antigenic, antioxidant, and antimicrobial effects of chamomile were effective in the wound healing process. According to the findings of the present study, chamomile can accelerate the wound closure process. Rezaie et al. [43] assessed chamomile’s extract wound healing effect combined with zinc oxide (20% ointment). They achieved satisfactory healing results, but they applied the chamomile extract at 10% and 20% concentrations. The present study showed that the methanol fraction of the chamomile extract was applicable at lower concentrations. Jarrahi et al. [44] reported the effectiveness of chamomile in a burn wound model in the rubbing oil dosage form, but they did not mention the exact concentration of the extract.
Several studies investigated the wound healing effects of the extracts of different parts of pomegranate. Most of them were done on the peel of the fruits. In the previous studies, the applied concentrations of the extracts were between 2.5% and 10%. Ointments, gels, and solutions were the final dosage forms that were studied. Pomegranate extracts were applied to excision, incision, dead space, and burn wound models in diabetic and nondiabetic animals. All of the studies found efficacious wound healing properties of different pomegranate extracts. Some hypotheses have been presented to explain the mechanisms of its effects, including the antimicrobial effect of its tannins, matrix metalloproteinase-1, inhibitory effects of its polyphenols, and anti-inflammatory properties of its flavonoids [45]. Pirbalouti et al. [46] evaluated the combined wound healing effect of diethyl ether fraction of Malva sylvestris and Punica granatum L. flower extracts. The study was done on diabetic rats, and the dressing was applied to different groups at a concentration of 0.2% individually and in the combined dosage form. The authors reported good wound contraction effects for Malva sylvestris and Punica granatum L. The combined form did not offer any added values versus single therapy. In this study, three concentrations of each plant’s methanol fraction were used to find the minimum effective dose with healing properties. The findings showed that both fractions were effective at lower doses than previously reported concentrations. Moreover, it was found that Matricaria chamomilla L. and Punica granatum L. had synergistic effects on wound healing.
C 5% + P 2.5% treatment group represents more wound closure percent in almost all evaluation days. Still, the differences did not reach the significance level, which may be related to fractions’ high potency.
The chosen concentration of each plant for combined treatment (C 5% + P 2.5%) was appointed based on the minimum effective concentration reported in previous studies. Also, one lower and one higher concentration of each plant were evaluated to find the relation between concentration and wound healing efficacy. As mentioned, the result showed some dose-dependent wound healing activity for chamomile fraction in the fourth and seventh days after wound induction day. However, there were no significant differences in different concentrations in all evaluation days for pomegranate fraction against other studies. It may be related to the high potency of methanol fraction, which suggested more studies on lower concentrations.
One of the limitations of animal wound healing study is the rapid wound healing process in young, healthy rates. These phenomena impress and mask the little significant differences in compared treatment groups. This limitation affects the ratio of generalizing the results to human skin. Therefore, the impaired wound healing model, like the diabetic rate model, is recommended for accurate results.
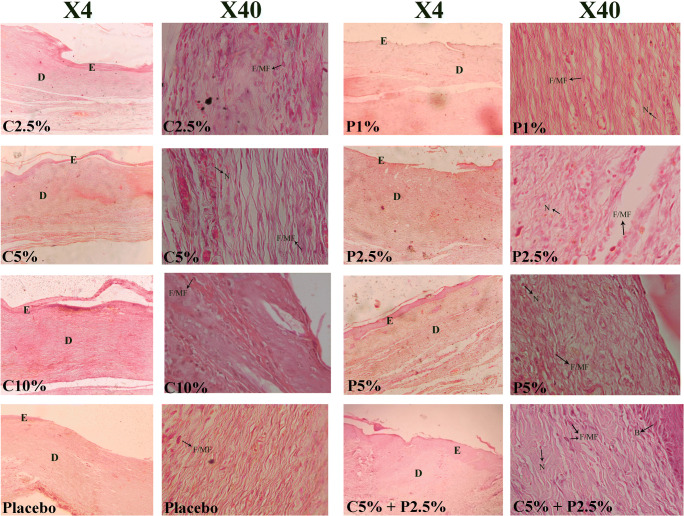
Histopathology
The upper layers of healed tissues were collected on day 14 post-surgery for histopathology analysis to determine the epidermal and dermal tissue regeneration and skin cell number in all groups (Fig. 3). More tissue regeneration was seen in the skin wound treated with a combination of extracts.
Fig. 3.
Histopathology of wounds in all groups on day 14 in X4 and X40 magnifications; Hematoxylin and Eosin; E: Epidermis; D: Dermis; F/MF: Fibroblast/myofibroblast; N: Neutrophil; B: Basal Cell; C: Chamomile; P: Pomegranate
There was a significant difference in the number of skin cells, which have a pivotal role in the wound healing process. As shown in Table 4, the number of fibroblasts/myofibroblasts, as the main producer of the extracellular matrix, was significantly higher in C 10% and P 5% groups. The P 1% group had the smallest number of fibroblasts/myofibroblasts after the placebo group, whereas the number of neutrophils was significantly higher in this group compared to other treatment groups, which may suggest that these groups are at higher risk of infections. The number of basal cells was significantly higher in P 5% and C 5% + P 2.5% groups, while P 1% and placebo had a smaller number of new basal cells.
Table 4.
Histological properties of wounds receiving different concentrations of methanol fractions of Matricaria chamomilla L. and Punica granatum L. flower extract
| N.F/MF | N N |
NB | VE (mm3) | VD (mm3) | VC (mm3) | |
|---|---|---|---|---|---|---|
| C 2.5% | 208.0 ± 6.7* | 11.3 ± 2.1* | 134.9 ± 3.8 | 88.1 ± 12.6 | 130.9 ± 3.3 | 59.0 ± 11.3 |
| C 5% | 218.8 ± 1.7** | 10.3 ± 1.5* | 140.6 ± 2.5* | 94.0 ± 3.4 | 144.7 ± 8.9 | 73.8 ± 5.3* |
| C 10% | 209.9 ± 10.2 | 11.0 ± 3.5* | 142.4 ± 4.9* | 86.1 ± 8.3 | 145.1 ± 6.1* | 77.1 ± 6.0* |
| P 1% | 183.4 ± 13.6 | 14.0 ± 3.2 | 124.4 ± 3.9 | 83.1 ± 9.8 | 124.1 ± 3.7 | 50.8 ± 6.9 |
| P 2.5% | 202.6 ± 4.0 | 15.3 ± 2.8 | 132.8 ± 4.7 | 81.9 ± 4.4 | 133.9 ± 6.8 | 67.2 ± 6.3 |
| P 5% | 219.8 ± 5.0** | 21.7 ± 3.0 | 153.1 ± 2.5*** | 101.5 ± 4.4* | 157.3 ± 5.0*** | 78.2 ± 4.5* |
| C 5% + P 2.5% | 219.0 ± 8.3* | 11.5 ± 0.5* | 147.8 ± 4.5** | 100.6 ± 1.9* | 151.4 ± 2.4*** | 85.0 ± 2.7** |
| Placebo | 182.9 ± 6.7 | 21.5 ± 2.7 | 126.9 ± 2.9 | 82.3 ± 4.7 | 124.4 ± 2.6 | 43.9 ± 6.4 |
NF Number of fibroblasts/myofibroblasts × 103, NN Number of neutrophils × 103, NB Number of basal cells of epidermis × 103, VE Volume of the epidermis, VD Volume of the dermis, VC Volume of collagen, SEM Standard error of mean, P Pomegranate, C Chamomile
Mean values within each group against placebo were significantly different: * p < 0.05, ** p < 0.01, and ***p < 0.001
The epidermis volume was not different between groups, but the dermis was thicker in P 5% and C 5% + P 2.5% groups. The amount of collagen fibers as an indicator of the granulation tissue formation was significantly higher in all treatment groups compared to the placebo group. Among them, C 10% had the highest amount of synthesized collagen.
Among different modalities being developed for wound care, herbal extracts have maintained their position for wound dressing as biocompatible and nontoxic agents due to their multi constituent nature that can contribute to the complex orchestrated cascade of wound healing. The present study started with the hypothesis that chamomile, with its well-known anti-inflammatory properties and pomegranate with its unique astringent activity, may have complementary effects on wound repair. The inflammation phase, which starts almost immediately after injury, is necessary for initiating the healing process but its prolongation that is not favorable. The anti-inflammatory effect of these plants may be related to their high total phenolic content, which was confirmed by antioxidant tests, especially for pomegranate. Overall, combined treatment with both chamomile and pomegranate (C 5% + P 2.5%) showed better wound healing activity. Moreover, in comparison with previous studies on pomegranate and chamomile, findings of this study for phytochemical contents, the antioxidant and antimicrobial effect of fractions were higher and more in amount, which could be due to the use of methanol fraction instead of total extract that is rich in hydrophilic compounds like flavonoids and tannins. All groups treated with herbal fractions showed faster wound closure than placebo, indicating the potency of the fractions.
Conclusion
Herbal extracts that contain phytoconstituents with anti-inflammatory, antioxidant, and antimicrobial properties can promote high speed, uncomplicated wound healing. The present study was the first work surveying the combined effect of methanol fraction of pomegranate and chamomile flowers. The results demonstrated that while the methanol fraction of chamomile and pomegranate flower extracts exhibit an acceleratory effect in wound enclosures individually, combining them in one formulation can be suggestible for dressing.
Acknowledgments
This research was supported by Tehran University of Medical Sciences and Health Services grant (No.43415).
Authors’ contributions
The study is a part of the Ph.D. thesis of S. Niknam. All practical investigations were managed and done by T. Toliyat and S. Niknam, including; purchasing required materials and instruments, designing the experiments, analyzing data, and reviewing the manuscript. Z. Tofighi was the supervisor in botanical studies and designed the methods for herbal extracts preparations and specifications. M.A. Faramarzi designed and supervised microbial evaluations. M.A. Abdollahifar was the supervisor in the animal and histopathological studies. Ensieh Sajadi was the co-worker for histopathology assessments. R. Dinarvand designed and involved in pharmaceutical preparation methods. All authors read and approved the final manuscript.
Declarations
Conflict of interest
None.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Reinke J, Sorg H. Wound repair and regeneration. Eur Surg Res. 2012;49(1):35–43. doi: 10.1159/000339613. [DOI] [PubMed] [Google Scholar]
- 2.Pazyar N, Yaghoobi R, Rafiee E, Mehrabian A, Feily A. Skin wound healing and phytomedicine: a review. Skin Pharmacol Physiol. 2014;27(6):303–310. doi: 10.1159/000357477. [DOI] [PubMed] [Google Scholar]
- 3.Rahimi HR, Arastoo M, Ostad SN. A comprehensive review of Punica granatum (pomegranate) properties in toxicological, pharmacological, cellular, and molecular biology researches. Iran J Pharm Res. 2012;11(2):385–400. [PMC free article] [PubMed] [Google Scholar]
- 4.Ma K, Du M, Liao M, Chen S, Yin G, Liu Q. Evaluation of wound healing effect of Punica granatum L Peel extract on deep second-degree burns in rats. Trop J Pharm Res. 2015;14(1):73–78. [Google Scholar]
- 5.Nayak SB, Rodrigues V, Maharaj S, Bhogadi VS. Wound healing activity of the fruit skin of Punica granatum. J Med Food. 2013;16(9):857–861. doi: 10.1089/jmf.2012.0229. [DOI] [PubMed] [Google Scholar]
- 6.Zekavat O, Amanat A, Karami M, Paydar S, Gramizadeh B, Zareian-Jahromi M. Wound healing studies using Punica granatum peel: an experimental animal study. Adv Skin Wound Care. 2016;29(5):217–225. doi: 10.1097/01.ASW.0000481116.16998.55. [DOI] [PubMed] [Google Scholar]
- 7.Bekir J, Mars M, Vicendo P, Fterrich A, Bouajila J. Chemical composition and antioxidant, anti-inflammatory, and antiproliferation activities of pomegranate (Punica granatum) flowers. J Med Food. 2013;16(6):544–550. doi: 10.1089/jmf.2012.0275. [DOI] [PubMed] [Google Scholar]
- 8.Aslam MN, Lansky EP, Varani J. Pomegranate as a cosmeceutical source: pomegranate fractions promote proliferation and procollagen synthesis and inhibit matrix metalloproteinase-1 production in human skin cells. J Ethnopharmacol. 2006;103(3):311–318. doi: 10.1016/j.jep.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 9.Beikert F, Schönfeld B, Frank U, Augustin M. Antiinflammatory potential of seven plant extracts in the ultraviolet erythema test. A randomized, placebo-controlled study. Hautarzt. 2013;64(1):40–46. doi: 10.1007/s00105-012-2505-x. [DOI] [PubMed] [Google Scholar]
- 10.Charousaei F, Dabirian A, Mojab F. Using chamomile solution or a 1% topical hydrocortisone ointment in the management of peristomal skin lesions in colostomy patients: results of a controlled clinical study. Ostomy Wound Manage. 2011;57(5):28–36. [PubMed] [Google Scholar]
- 11.Reis PEDd, Carvalho ECd, Bueno PCP, Bastos JK. Clinical application of Chamomilla recutita in phlebitis: dose response curve study. Rev Lat Am Enfermagem. 2011;19(1):03–10. doi: 10.1590/s0104-11692011000100002. [DOI] [PubMed] [Google Scholar]
- 12.Stanojevic LP, Marjanovic-Balaban ZR, Kalaba VD, Stanojevic JS, Cvetkovic DJ. Chemical composition, antioxidant, and antimicrobial activity of chamomile flowers essential oil (Matricaria chamomilla L.) J Essent Oil-Bear Plants. 2016;19(8):2017–2028. [Google Scholar]
- 13.Brown DJ, Dattner AM. Phytotherapeutic approaches to common dermatologic conditions. Arch Dermatol. 1998;134(11):1401–1404. doi: 10.1001/archderm.134.11.1401. [DOI] [PubMed] [Google Scholar]
- 14.McKay DL, Blumberg JB. A review of the bioactivity and potential health benefits of chamomile tea (Matricaria recutita L.) Phytother Res. 2006;20(7):519–530. doi: 10.1002/ptr.1900. [DOI] [PubMed] [Google Scholar]
- 15.Duarte CME, Quirino MRS, Patrocínio MC, Anbinder AL. Effects of Chamomilla recutita (L.) on oral wound healing in rats. Med Oral Patol Oral Cir Bucal. 2011;16(6):716–721. [PubMed] [Google Scholar]
- 16.Drummond EM, Harbourne N, Marete E, Jacquier J, O’Riordan D, Gibney ER. An in vivo study examining the anti-inflammatory effects of chamomile, meadowsweet, and willow bark in a novel functional beverage. J Diet Suppl. 2013;10(4):370–380. doi: 10.3109/19390211.2013.830680. [DOI] [PubMed] [Google Scholar]
- 17.Srivastava JK, Pandey M, Gupta S. Chamomile, a novel and selective COX-2 inhibitor with anti-inflammatory activity. Life Sci. 2009;85(19–20):663–669. doi: 10.1016/j.lfs.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duman F, Ocsoy I, Kup FO. Chamomile flower extract-directed CuO nanoparticle formation for its antioxidant and DNA cleavage properties. Mater Sci Eng C. 2016;60:333–338. doi: 10.1016/j.msec.2015.11.052. [DOI] [PubMed] [Google Scholar]
- 19.Kolodziejczyk-Czepas J, Bijak M, Saluk J, Ponczek MB, Zbikowska HM, Nowak P. Radical scavenging and antioxidant effects of Matricaria chamomilla polyphenolic–polysaccharide conjugates. Int J Biol Macromol. 2015;72:1152–1158. doi: 10.1016/j.ijbiomac.2014.09.032. [DOI] [PubMed] [Google Scholar]
- 20.Mekinić IG, Skroza D, Ljubenkov I, Krstulović L, Možina SS, Katalinić V. Phenolic acids profile, the antioxidant and antibacterial activity of chamomile, common yarrow, and immortelle (Asteraceae) Nat Prod Commun. 2014;9(12):1745–1748. [PubMed] [Google Scholar]
- 21.Móricz ÁM, Ott PG, Alberti Á, Böszörményi A, Lemberkovics É, Szőke É. Applicability of preparative overpressured layer chromatography and direct bioautography in search of antibacterial chamomile compounds. J AOAC Int. 2013;96(6):1214–1221. doi: 10.5740/jaoacint.sgemoricz. [DOI] [PubMed] [Google Scholar]
- 22.Budovsky A, Yarmolinsky L, Ben-Shabat S. Effect of poly-herbal preparations on wound healing. Wound Repair Regen. 2016;24(1):196–197. doi: 10.1111/wrr.12382. [DOI] [PubMed] [Google Scholar]
- 23.Fahimi S, Abdollahi M, Mortazavi SA, Hajimehdipoor H, Abdolghaffari AH, Rezvanfar MA. Wound healing activity of a traditionally used polyherbal product in a burn wound model in rats. Iran Red Crescent Med J. 2015;17(9):e19960. doi: 10.5812/ircmj.19960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta D, Bleakley B, Gupta RK. Dragon’s blood: botany, chemistry, and therapeutic uses. J Ethnopharmacol. 2008;115(3):361–380. doi: 10.1016/j.jep.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 25.Makkar HP. Quantification of tannins in tree and shrub foliage: a laboratory manual. 2003. [Google Scholar]
- 26.Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64(4):555–559. [Google Scholar]
- 27.Goodarzi S, Hadjiakhoondi A, Yassa N, Khanavi M, Tofighi Z. Essential oils chemical composition, antioxidant activities, and total phenols of Astrodaucus persicus. Iran J Basic Med Sci. 2016;19(2):159–165. [PMC free article] [PubMed] [Google Scholar]
- 28.Benzie IF, Strain J. [2] Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration, Methods in enzymology: Elsevier; 1999. p. 15–27. [DOI] [PubMed]
- 29.Wikler M. Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically. 2009. [Google Scholar]
- 30.Al-Hadethi H, Al-Saimeri I. Practical bacteriology. 1993. [Google Scholar]
- 31.Gundersen H, Bendtsen TF, Korbo L, Marcussen N, Møller A, Nielsen K, et al. Some new, simple, and efficient stereological methods and their use in pathological research and diagnosis. Apis. 1988;96(1–6):379–394. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- 32.Chen Y, Tian L, Yang F, Tong W, Jia R, Zou Y. Tannic acid accelerates cutaneous wound healing in rats via activation of the erk 1/2 signaling pathways. Adv Wound Care. 2019;8(7):341–354. doi: 10.1089/wound.2018.0853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kashchenko N, Olennikov D. Quantitative analysis of flavonoids in chamomile flowers (Matricaria chamomilla L.) by microcolumn HPLC-UV. Russ J Bioorg Chem. 2017;43(7):783–789. [Google Scholar]
- 34.Srivastava JK, Shankar E, Gupta S. Chamomile: a herbal medicine of the past with a bright future. Mol Med Rep. 2010;3(6):895–901. doi: 10.3892/mmr.2010.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ardekani MRS, Hajimahmoodi M, Oveisi MR, Sadeghi N, Jannat B, Ranjbar AM, et al. Comparative antioxidant activity and total flavonoid content of Persian pomegranate (Punica granatum L.) cultivars. Iran J Pharm Res. 2011;10(3):519–524. [PMC free article] [PubMed] [Google Scholar]
- 36.Fischer UA, Carle R, Kammerer DR. Identification and quantification of phenolic compounds from pomegranate (Punica granatum L.) peel, mesocarp, aril and differently produced juices by HPLC-DAD–ESI/MSN. Food Chem. 2011;127(2):807–821. doi: 10.1016/j.foodchem.2010.12.156. [DOI] [PubMed] [Google Scholar]
- 37.Naveena BM, Sen AR, Kingsly RP, Singh DB, Kondaiah N. Antioxidant activity of pomegranate rind powder extract in cooked chicken patties. Int J Food Sci Technol. 2008;43(10):1807–1812. [Google Scholar]
- 38.Yussif BK, Jasim AS, Abdul-wahid AT, Gabar LAA. Evaluation of the antimicrobial activity of phenolic extract from Punica granatum L. peel. Bas J Vet Res. 2014;1(1):26–32. [Google Scholar]
- 39.Hayouni E, Miled K, Boubaker S, Bellasfar Z, Abedrabba M, Iwasaki H, et al. Hydroalcoholic extract based-ointment from Punica granatum L. peels with enhanced in vivo healing potential on dermal wounds. Phytomedicine. 2011;18(11):976–984. doi: 10.1016/j.phymed.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 40.Brantner A, Grein E. Antibacterial activity of plant extracts used externally in traditional medicine. J Ethnopharmacol. 1994;44(1):35–40. doi: 10.1016/0378-8741(94)90096-5. [DOI] [PubMed] [Google Scholar]
- 41.Akiyama H, Fujii K, Yamasaki O, Oono T, Iwatsuki K. Antibacterial action of several tannins against Staphylococcus aureus. J Antimicrob Chemother. 2001;48(4):487–491. doi: 10.1093/jac/48.4.487. [DOI] [PubMed] [Google Scholar]
- 42.Miraj S, Alesaeidi S. A systematic review study of therapeutic effects of Matricaria recuitta chamomile (chamomile) Electron Physician. 2016;8(9):3024–3031. doi: 10.19082/3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rezaie A, Mohajeri D, Zarkhah A, Nazeri M. Comparative assessment of Matricaria chamomilla and zinc oxide on healing of experimental skin wounds on rats. Ann Biol Res. 2012;3(1):550–560. [Google Scholar]
- 44.Jarrahi M. An experimental study of the effects of Matricaria chamomilla extract on cutaneous burn wound healing in albino rats. Nat Prod Res. 2008;22(5):422–427. doi: 10.1080/14786410701591713. [DOI] [PubMed] [Google Scholar]
- 45.Nasiri E, Hosseinimehr SJ, Akbari J, Azadbakht M, Azizi S. The effects of Punica granatum flower extract on skin injuries induced by the burn in rats. Adv Pharmacol Pharm Sci. 2017;2017:305974e5. [DOI] [PMC free article] [PubMed]
- 46.Pirbalouti AG, Azizi S, Koohpayeh A, Hamedi B. Wound healing activity of Malva sylvestris and Punica granatum in alloxan-induced diabetic rats. Acta Pol Pharm. 2010;67(5):511–516. [PubMed] [Google Scholar]