Abstract
The activation of mitochondrial large conductance calcium-activated potassium (mitoBKCa) channels increases cell survival during ischemia/reperfusion injury of cardiac cells. The basic biophysical and pharmacological properties of mitoBKCa correspond to the properties of the BKCa channels from the plasma membrane. It has been suggested that the VEDEC splice variant of the KCNMA1 gene product encoding plasma membrane BKCa is targeted toward mitochondria. However there has been no direct evidence that this protein forms a functional channel in mitochondria. In our study, we used HEK293T cells to express the VEDEC splice variant and observed channel activity in mitochondria using the mitoplast patch-clamp technique. For the first time, we found that transient expression with the VEDEC isoform resulted in channel activity with the conductance of 290 ± 3 pS. The channel was voltage-dependent and activated by calcium ions. Moreover, the activity of the channel was stimulated by the potassium channel opener NS11021 and inhibited by hemin and paxilline, which are known BKCa channel blockers. Immunofluorescence experiments confirmed the partial colocalization of the channel within the mitochondria. From these results, we conclude that the VEDEC isoform of the BKCa channel forms a functional channel in the inner mitochondrial membrane. Additionally, our data show that HEK293T cells are a promising experimental model for expression and electrophysiological studies of mitochondrial potassium channels.
Subject terms: Biochemistry, Biophysics
Introduction
Mitochondrial potassium (mitoK) channels play an important role in the physiology of mitochondria. The activation of mitoK channels results in K+ influx into the mitochondrial matrix which decreases the mitochondrial membrane potential and can thus affect various processes such as oxidative phosphorylation and reactive oxygen species synthesis1,2.
Several potassium channels have been identified in the inner mitochondrial membrane1,3. The basic biophysical and pharmacological properties of mitoK channels are very similar to those of their plasma membrane counterparts. One of the best-described channels in mitochondria is the large conductance calcium-activated potassium (mitoBKCa) channel. For the first time mitoBKCa was identified by the patch-clamp technique in the mitochondria of glioma cells4. Later, the channel was described in other tissues, including the heart, brain, endothelium, skin fibroblasts or skeletal muscle and in the mitochondria of lower organisms1,5–11.
The mitoBKCa channel plays a cytoprotective role that is most apparent during hypoxia/reperfusion injury of heart tissue11–15. The detailed mechanism of this phenomenon is unclear; however it was suggested that the mild uncoupling of the mitochondrial membrane potential induced by mitoBKCa opening might influence mitochondrial reactive oxygen species synthesis and/or prevent mitochondrial calcium overload11,13,15–18. It was also observed that mitoBKCa might be the target for multiple signaling pathways including phosphorylation and redox regulation, which might be critical for the induction of cytoprotective mechanisms15,19,20.
The biophysical properties of mitoBKCa expressed natively were described using electrophysiological tools including mitoplast patch clamping and reconstitution of submitochondrial particles into bulk lipid membranes21–23. Usually the mitoBKCa conductance is close to 300 pS however in heart tissue, it was also shown to be below 200 pS12. The open probability of the channel depends on the voltage and concentration of calcium ions. The channel is activated by several potassium channel openers such as NS1619, NS110021 or CGS7184 and inhibited by iberiotoxin, charybdotoxin and paxilline24–26. Similar to the plasma membrane BKCa channels, mitoBKCa channel activity is reduced by heme and hemin27–29. All of the above properties correspond to the biophysical and pharmacological properties of BKCa channels in the plasma membrane30,31.
The BKCa channels in the plasma membrane are tetramers formed by pore-forming α subunits encoded by the KCNMA1 gene. The properties of the channels can be modified by β and γ auxiliary subunits31,32. Based on similarities between mitoBKCa and BKCa in the plasma membrane, it was proposed that the α subunit of both channels is encoded by the same gene. These observations were supported by identification of the α protein in the mitochondrial fraction with antibodies recognizing the subunit of the plasma membrane BKCa5,6,9,33. However, the KCNMA1 gene was reported to produce multiple splice variants. Several recent studies proposed that one of the mitochondrial isoforms of the α subunit might be a protein containing the VEDEC tail motif. This isoform was shown to colocalize with mitochondria in the mouse cochlea34,35 and rat heart36. Similarly, expression of this isoform in HEK293T cells resulted in partial localization of this protein to the mitochondria37; however, there was no direct evidence that this splice variant forms a functional channel in mitochondrial membranes. Here, we provide a set of experimental data describing the channel activity formed by the VEDEC isoform in the mitochondrial inner membrane. In our study, we used HEK293T cells transiently transfected with a plasmid encoding this splice variant36,37 followed by a patch-clamp of isolated mitochondria. Electrophysiological recordings of detected activity correspond with known properties of the mitoBKCa described previously. Therefore, we conclude that the VEDEC splice variant is able to form the mitoBKCa channel in the inner mitochondrial membrane. Additionally, our data indicate that HEK293T cells are a very useful experimental model for the expression and electrophysiological recordings of mitochondrial potassium channels.
Results
The main aim of this study was to measure activity and to characterize BKCa channel previously described as mitochondrial isoform of BKCa channel, namely, VEDEC channel.
To achieve the intended goal, we used the following approach:
expression of the VEDEC BKCa isoform in cells lacking any background K+ channel activity in the inner mitochondrial membrane;
confirming mitochondrial localization of the a VEDEC isoform;
describing basic BKCa channel properties such as regulation by known channel activators and inhibitors.
Biophysical properties of mitochondrial channels recorded after transfection with VEDEC-encoding plasmid
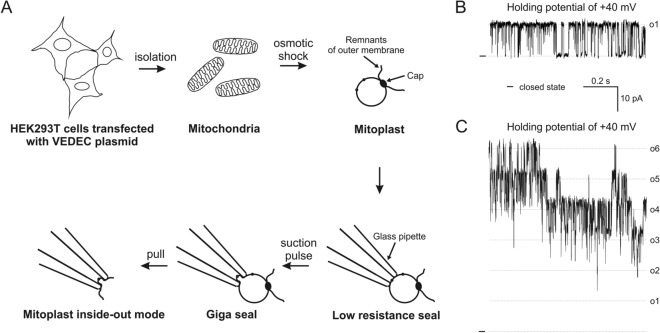
In the present study, we used HEK293T cells as a model system for the expression of the VEDEC isoform of the protein encoded by the KCNMA1 gene. This cell line is a known model system for plasma membrane BKCa research. To verify the hypothesis that HEK293T cells could be an appropriate experimental model for mitoK channel studies, we decided to perform a series of experiments using mitoplast patch-clamp measurements. In our studies, we used mitochondria isolated from HEK293T cells transiently transfected with a construct encoding the VEDEC isoform of BKCa described previously36,37. A schematic depiction of the experimental protocol used in the study is presented in Fig. 1A. The ion current was measured in symmetrical 150/150 mM KCl isotonic solution in the presence of 100 µM Ca2+ unless otherwise stated. We found that transient transfection of HEK293T cells with a plasmid encoding VEDEC resulted in recordings of single or multiple channels with kinetics shown in the Fig. 1B and C. By contrast, we did not detect similar ion channel activity in mitochondria isolated from untransfected HEK293T cells (n = 104 patches, 10 mitochondrial isolations).
Figure 1.
Mitoplast patch-clamp measurement and single-channel recordings of channels observed after transient transfection with the BKCa VEDEC-encoding plasmid. (A) Schematic depiction of the patch-clamp channel measurement of the inner mitochondrial membrane: mitoplast preparation and single-channel patches in the inside-out configuration. (B) Representative recording of single- and (C) multiple-channel activity in the patch of the inner mitochondrial membranes after transient transfection of HEK293T cells with the BKCa VEDEC-encoding plasmid. Single- and six-mitoBKCa channel activities are presented. Recordings were performed at holding potential + 40 mV.
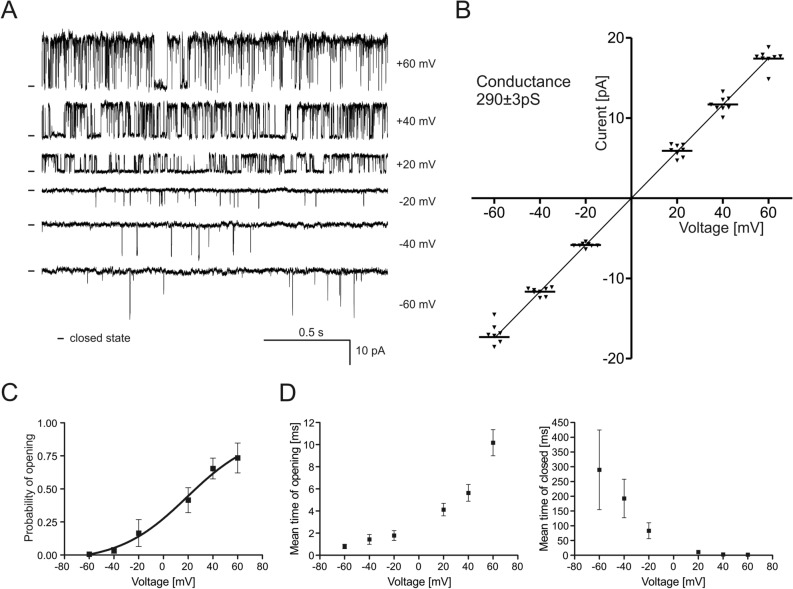
The calculated conductance of the channel based on the current/voltage dependence (Fig. 2A,B) was equal to 290 ± 3 pS (n = 7). Interestingly, recordings indicating the activity of multiple channels (up to approximately 30 channels in the single patch) were frequently detected. In the first set of experiments we analyzed the dependence of the channel activity on the applied voltage (Fig. 2C). The opening probability of (Po) of the channel was approximately 0.006 at − 60 mV and a gradual increase in Po was observed when a more positive voltage was applied. The maximal Po was observed at + 60 mV and was equal to 0.73. Experimental points presented in the Fig. 2C were fitted using Boltzmann function. Based on fitting results, calculated value of potential at which open probability is halfway (V1/2) was 19.2 mV and gating charge was equal to 0.95e0.
Figure 2.
Biophysical properties of the channel recorded in mitoplasts isolated from HEK293T cells transiently transfected with the VEDEC isoform of the BKCa channel. (A) Single-channel recordings in symmetrical 150/150 mM KCl at different voltages ranging from -60 to + 60 mV. (B) Current–voltage characteristics of the single-channel events in symmetrical 150/150 mM KCl. The conductance of the channel, as calculated based on the presented I-V curve, was equal to 290 ± 3 pS. (C) Analysis of the channel open probability (Po) at different potentials. (D) Distribution of the mean open and closed dwell times of the channel at different voltages in symmetrical 150/150 mM KCl solution. The results are presented as the means ± SD (n = 7).
Application of wider range of voltages resulted in patch instability and disruption of the membrane. Additionally, the mean open time and mean closed time dwell times at various voltages were calculated (Fig. 2D). The maximal average τopen was observed at + 60 mV and was equal to 10.2 ms, and the maximal average τclosed was observed at − 60 mV and was equal to approximately 289.7 ms. These biophysical properties of the channel were conducted base on single-channel recordings (n = 7).
Based on the above data we concluded that the biophysical properties of the mitochondrial ion channel recorded after transfection with the VEDEC encoding plasmid correspond with the properties of the previously described mitoBKCa channel in various tissues1.
The channel formed by VEDEC in mitochondria is Ca2+-regulated
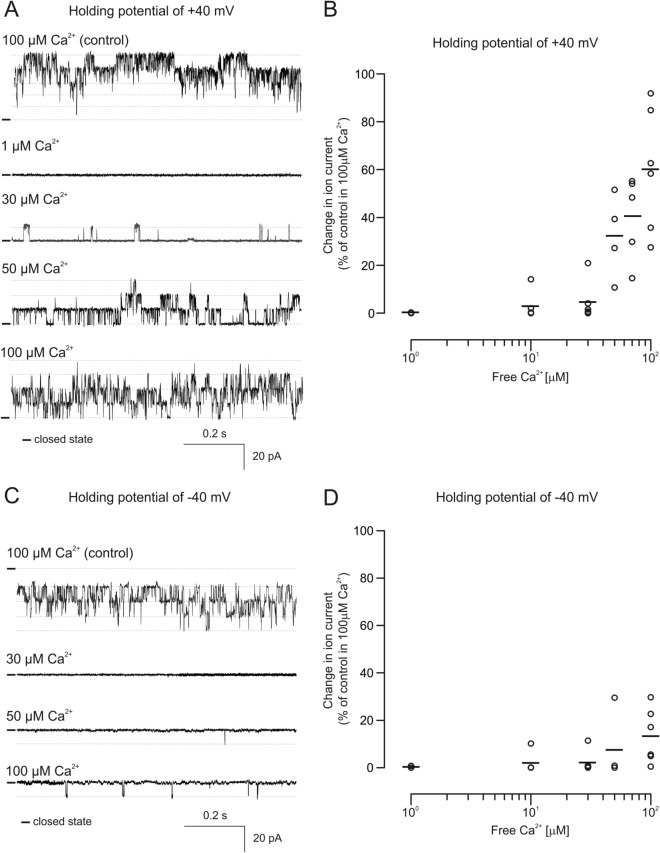
In the second set of experiments, we verified the dependence of the channel on the presence of calcium ions, which is the canonical property of mitoBKCa channels. For this purpose, the channel activity was recorded in a buffer containing various concentrations of free calcium ions (Fig. 3). The activity of the channels was recorded at holding potentials equal to + 40 mV or − 40 mV. Perfusion of the channel with buffer containing 1 µM free Ca2+ resulted in a virtual lack of channel activity (0.07% of control at + 40 mV and 0.28% of control at − 40 mV). Increasing the free Ca2+ in the buffer to 50 µM resulted in an ion current boost to approximately 32.2% of that of the control at + 40 mV and 7.6% at − 40 mV. When the level of free Ca2+ returned to the control level (100 μM) observed ion current increased up to 60.2% of that of the control at + 40 mV and only 13.5% at − 40 mV. We also noticed large variability in channel activity in response to increasing calcium ion concentrations in the applied buffer. Nevertheless our data clearly show that the channel recorded after transient transfection with the VEDEC plasmid shows strong Ca2+ regulation.
Figure 3.
Modulation of the channel activity by calcium ions. (A) Sample of the single-channel recordings in symmetrical 150/150 mM KCl solution at different Ca2+ concentrations at a holding potential of + 40 mV. (B) Analysis of changes in the ion current in the presence of various Ca2+ concentrations recorded at holding potentials of + 40 mV. (C) Representative single-channel recordings in symmetrical 150/150 mM KCl solution in the presence of different Ca2+ concentrations recorded at a holding potential of − 40 mV. (D) Analysis of changes in the ion current in the presence of various Ca2+ concentrations recorded at a holding potentials of − 40 mV.
Regulation of the channel formed by the VEDEC splice variant by mitoBKCa modulators
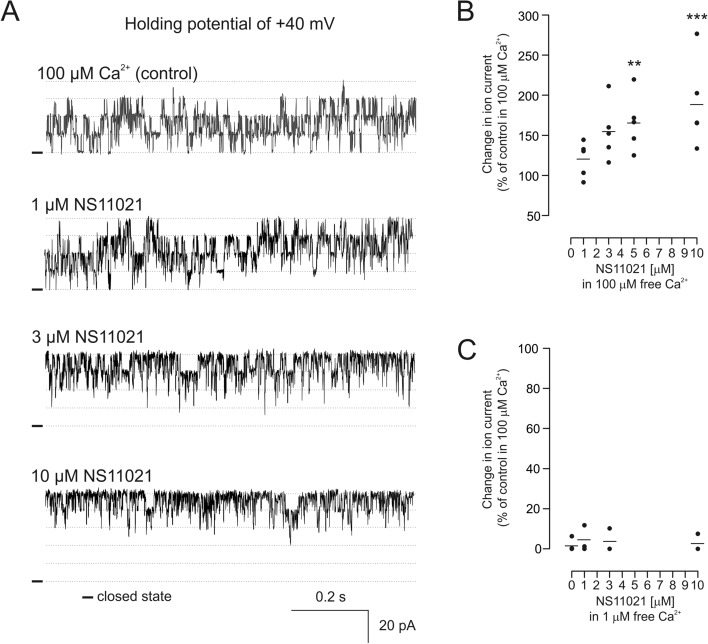
In the next step, we characterized the pharmacological properties of the channel formed by the VEDEC isoform. Therefore, typical modulators of the mitoBKCa channel were applied. In our research, we used NS11021, which was shown to activate channels both from the plasma membrane and from mitochondria7,38,39. The first set of experiments was performed in the presence of 100 µM calcium ions in the buffer. The application of NS11021 induced channel activation in a concentration-dependent manner (Fig. 4A). A maximal increase in the ion current was observed after the application of 10 µM NS11021 at + 40 mV (approximately 175% of the control) (Fig. 4B). Interestingly, when the free calcium ion concentration was reduced from 100 µM to 1 µM, the application of NS11021 resulted in very modest activation of the channel (Fig. 4C).
Figure 4.
Regulation of channel activity by the BKCa channel activator NS11021. (A) Single-channel recordings in symmetrical 150/150 mM KCl solution at different NS11021 concentrations in the presence of 100 µM Ca2+ in experimental buffer at a holding potential of + 40 mV. (B) Analysis of changes in the ion current induced by increasing NS11021 concentrations in the presence of 100 µM Ca2+ recorded at holding potential of + 40 mV. (C) Analysis of changes in the ion current induced by increasing concentrations of NS11021 recorded in the presence of 1 μM Ca2+. **p < 0.01, ***p < 0.001 vs control activity in 100 µM Ca2+.
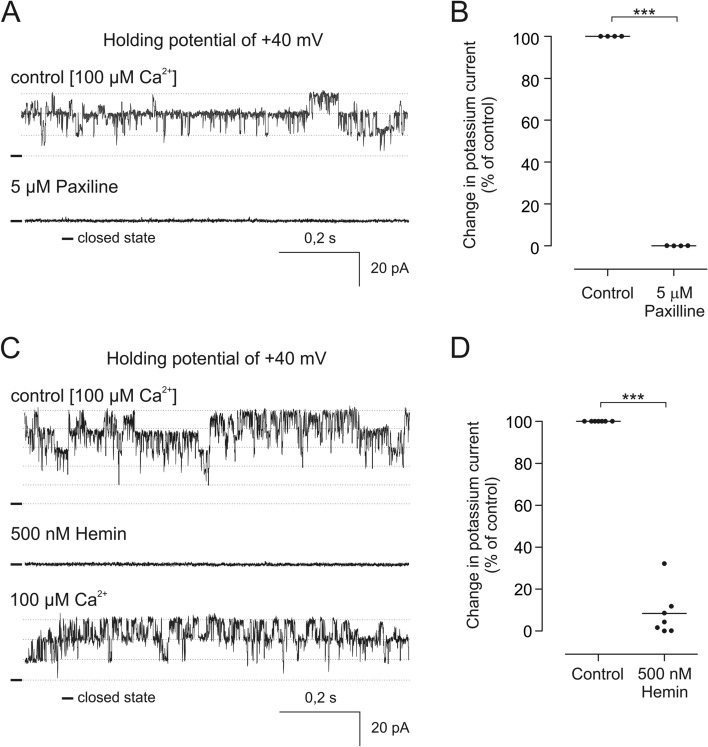
Contrary to the potassium channel opener NS11021, the fungal tremorgenic alkaloid paxilline is the canonical inhibitor of BKCa channels23,40–42. Therefore, we applied this blocker to the channel recorded in our experimental system. All recorded channels with pharmacological and biophysical properties corresponding to mitoBKCa were blocked by 5 µM paxilline (Fig. 5).
Figure 5.
Inhibitory effect of the BKCa channel blockers on the channel recorded after transfection with VEDEC. (A) Single channel recording in symmetrical 150/150 mM KCl of the channel under the control condition and after 5 µM paxilline application. (B) Statistical analysis of the paxilline inhibitory effect on the recorded channel. (C) Representative single- channel recording of the channel in symmetrical 150/150 mM KCl under the control condition and after 500 nM hemin application (D) Analysis of changes in the ion current under the control condition and after hemin application. ***p < 0.001 vs control activity.
Furthermore, it has been shown that BKCa channels contain a heme-binding motif in their C-terminal part, and the application of hemin results in the inhibition of these channels, including mitoBKCa27,43. Our experiments revealed that the application of hemin indeed resulted in clear inhibition of the channels recorded after transfection with the VEDEC isoform. When the channel was recorded at + 40 mV in the presence of 100 µM free calcium ions, perfusion of the patch with 500 nM hemin resulted in a decrease in the ion current to approximately 8,3% of the control value (Fig. 5A,B).
Analysis of the BKCa expression in HEK293T cells
To verify our electrophysiological mitoplast patch-clamp experiments we decided to analyze the expression of pore-forming BKCa channel subunits and, specifically, VEDEC isoform in wild-type and transiently transfected HEK293T cells (Fig. 6A,B). Previous reports suggested either that the expression of the BKCa channel pore-forming subunit in wild-type HEK293 cells is not detectable40 or that the BKCa currents were at very low levels in comparison to transfected cells41. However, recently, it was also reported that natively expressed BKCa might be present in the mitochondrial fraction of HEK293 cells44. In our study we used HEK293T cells which are a modified subclone of the original HEK293 cell line45. In our experiments, we used primers recognizing all splice variants of the pore-forming subunit (marked as “α”) and VEDEC isoform (marked as “VEDEC”). Standard qualitative PCR experiments suggested that in wild-type cells, the expression of the KCNMA1 gene product was virtually undetectable, whereas transient transfection of HEK293T cells resulted in high expression of the BKCa α subunit (Fig. 6A, S1). However, quantitative PCR suggested endogenous expression of the α component in wild-type cells (Fig. 6B). This result was obtained using both sets of primers. Transfection with VEDEC resulted in multiple increases in the expression of the BKCa α subunit (shift in Ct value). For comparison, the expression of control genes (GAPDH and MT-CO1) did not change significantly under all tested conditions. Due to ambiguous quantitative PCR results in wild-type HEK293T cells, we decided to perform western blot analysis of α protein levels. In the nontransfected cells, we were unable to detect the α subunit of the BKCa channel. On the other hand, transfection of HEK293T cells with the VEDEC splice variant resulted in the presence of the BKCa channel pore-forming subunit in the crude mitochondrial fraction (Fig. 6C, S2).
Figure 6.
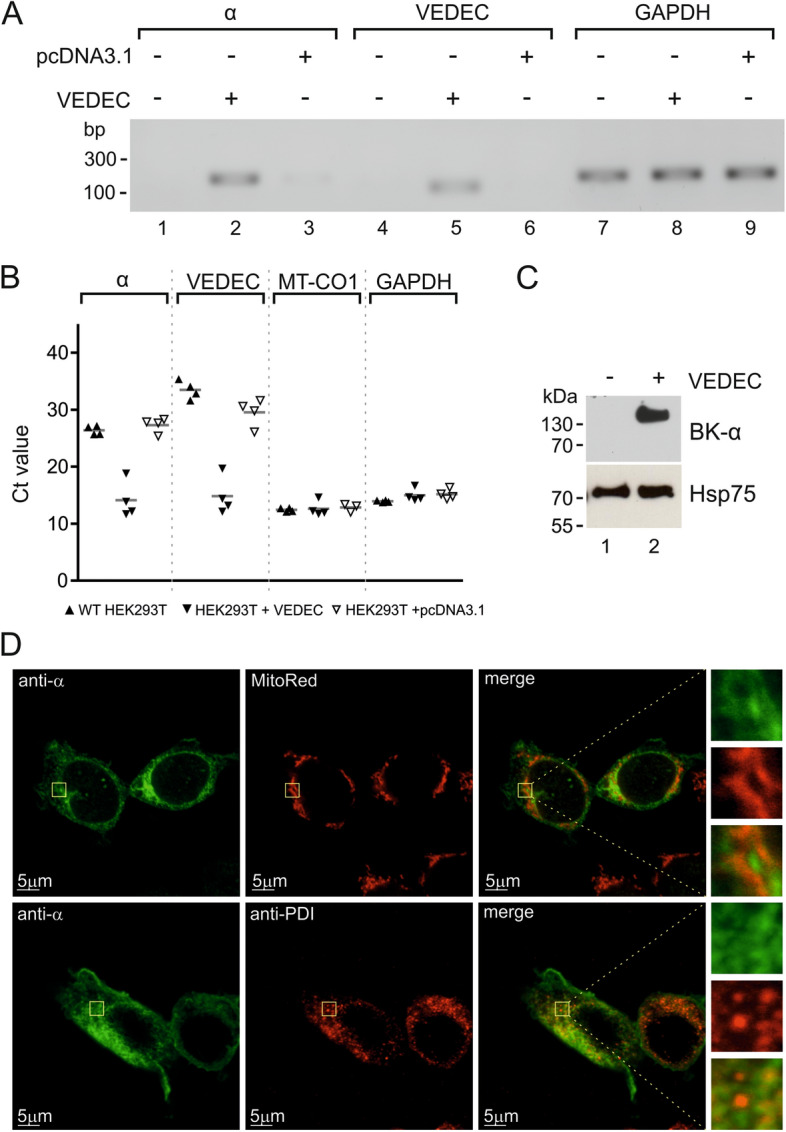
Analysis of the presence of the BKCa α subunit in wild-type and VEDEC transiently transfected HEK293T cells. (A) Analysis of selected gene expression using qualitative PCR. (B) Real-time PCR analysis of the expression levels of selected genes. (C) Western blot analysis of the BKCa pore-forming subunit in the mitochondrial fraction of wild-type and transfected cells. (D) Localization of VEDEC in HEK293T cells after transient transfection. Confocal images of cultured cells stained with MitoRed as a mitochondrial marker (upper panel, red channel) and an anti-PDI antibody as an endoplasmic reticulum marker (lower panel, red channel). The pore-forming α subunit was stained with an anti-BK α antibody (green channel). The superimposition of the two signals revealed the partial mitochondrial and ER localization of the BKCa α subunit (orange).
Subcellular localization of VEDEC in HEK293T cells
In the last set of experiments we wanted to verify the mitochondrial localization of BKCa α expressed in transiently transfected HEK293T cells. Immunostaining followed by confocal microscopy revealed that mitochondria are one of the targets of the VEDEC protein (Fig. 6D). Additionally, we observed colocalization of the α subunit with the endoplasmic reticulum. In our opinion, immunostaining experiments confirmed that the expression product of the VEDEC plasmid is partially targeted toward mitochondria.
Discussion
Despite many years of research, many questions remain concerning the structure and function of the mitoBKCa channel3. It has been shown that the VEDEC isoform of the BKCa channel is located in the mitochondria of heart tissue36. However, there was no evidence that this protein could form an active channel in the inner mitochondrial membrane. The research presented in this paper aimed to verify this hypothesis.
In our study, we used a mitoplast patch-clamp to detect ion channel activity resulting from transfection of HEK293T cells with a plasmid encoding a VEDEC isoform of the BKCa channel.
Based on the data obtained, two main conclusions can be drawn. First, the expression of the VEDEC isoform leads to mitoBKCa activity in the mitochondrial inner membrane. Second, our data show that HEK293T cells are potentially a suitable model for expression and electrophysiological studies of mitoK channels.
The channel observed after transfection with VEDEC showed a conductance of approximately 290 pS which is close to the conductance previously described for the BKCa channel. In brain tissue and several glioma cell lines the average conductance of the channel recorded under the same experimental conditions ranged between ~ 275 pS and ~ 295 pS4,8,23,46–48. Similar values were reported for mitoBKCa from skeletal muscle and endothelial cells6,7. The conductance of the recorded channel was also close to the values observed in the plasma membrane in stably transfected HEK293 cells where a 250pS channel was reported after the expression of BKCa49. However, the conductance of mitoBKCa can differ from that indicated above. For example, in mouse heart tissue, conductance of the channel was significantly smaller and did not exceed 190 pS depending on the experimental conditions12,19. On the other hand, a conductance of 308 pS was reported for the mitoBKCa channel from rat heart cardiomyocytes22.
Other analyzed biophysical features of the recorded channels are also similar to those reported for mitoBKCa. Both the dependence of the open probability on the applied voltage and the values of the mean open and closed dwell times correspond to the mitoBKCa channel from cardiac tissue and are close to the values observed for endothelial and glioma mitoBKCa7,12,22.
Apart from that the activity of the channel was dependent on the presence of calcium ions. This property is one of the key features of BKCa channels50. Furthermore, application of the BKCa activator NS11021 induced an increase in the ion current in the presence of 100 µM calcium ions. NS110121 was previously shown to be a potent activator of BKCa from the plasma membrane when channels were expressed in oocytes of Xenopus laevis and HEK293 cells38. It was also shown that activation of cardiac mitoBKCa after application of NS11021 leads to the induction of cytoprotection mechanisms51,52. This compound activates BKCa channels over a range of calcium ion concentrations including nominally Ca2+-free medium and truncated channels lacking calcium-sensing domain39. Contrary to the aforementioned studies, in our experiments no increase in channel activity was observed after the administration of NS11021 in the presence of 1 µM Ca2+ in experimental buffer. One of the possible explanations for the observed differences could be mitochondrial localization of the channel and potential interactions with the mitochondrial proteome and/or differences in lipid composition between mitochondria and the plasma membrane. On the other hand, it is possible that recordings in the wider range of voltages would confirm the channel activation by NS11021 in the presence of 1 µM Ca2+. However, in our system, the use of higher voltages during mitoplast patch-clamp experiment results in membrane instability. Additionally, NS11021 was shown to modulate mitochondrial functions, and these effects were reversed by the application of paxilline, proving the involvement of the mitoBKCa channel7. In our experiments, paxilline inhibited channel activity. Similarly, the application of the mitoBKCa inhibitor hemin resulted in a decrease in the probability of channel opening. Previously, heme and hemin were shown to block mitoBKCa activity in glioma and endothelial cells27–29. Importantly, both inhibitors block plasma membrane BKCa channels41,53.
All of the above data strongly support the hypothesis that the VEDEC splice variant of the BKCa channels forms a functional channel in the inner mitochondrial membrane. The expression of VEDEC in HEK293T cells results in the presence of the α subunit in various cellular compartments, including the endoplasmic reticulum and mitochondria. This observation is in line with previously published data35,37,54. Equally importantly, in contrast to HEK293T cells in cardiac tissue, the VEDEC splice variant is exclusively targeted to mitochondria35. However, the VEDEC isoform is not the only splice variant that could be targeted toward mitochondria. In astrocytoma U87-MG cells, the full-length VEDEC isoform was not detectable, but mitoBKCa activity was present55. Additionally, it was shown that the VYR and ERL isoforms of BKCa might be located in mitochondria after expression in HEK293 cells44,54. These observations might suggest that in cardiac cells, a specific VEDEC sorting mechanism exists.
Additionally, our study shows that HEK293T cells could be a promising experimental model for expression and electrophysiological measurements of mitochondrial potassium channels. HEK293 cells are a common model in studies of BKCa channels in the plasma membrane. Some previous studies suggested s lack of BKCa pore-forming subunit expression in these cells40. On the other hand, native expression of BKCa in HEK293 cells was shown recently, and patch-clamp recordings suggested the existence of minimal currents corresponding to BKCa activity in the plasma membrane41,44. In our cells we did not observe any known mitoK channel electrophysiological activity in mitoplasts isolated from wild-type HEK293T cells. Therefore, in our opinion, HEK293T cells could be a suitable model for studies of these issues related to mitoBKCa in the future.
Methods
Cell culture
HEK293T cells were a kind gift from the laboratory of Prof. Mike Ryan (Monash University, Australia) and Dr. David Stroud (currently The University of Melbourne, Australia). The cells were grown in Dulbecco’s modified essential medium (Hirszfeld Institute of Immunology and Experimental Therapy, Wrocław, Poland) supplemented 10% FBS (Gibco), GlutaMAX (Gibco), uridine 50 μg/ml, 100 U/ml penicillin, and 100 µg/ml streptomycin (Sigma-Aldrich) at 37 °C in a humidified atmosphere with 5% CO2.
Cell transfection for electrophysiological studies
Plasmid DNA encoding the VEDEC splice variant (previously described by Singh et al.36 was transiently introduced into HEK293T cells by transfection with polyethylenimine (PEI, Sigma-Aldrich). Briefly, the cells were seeded on 150 mm plastic dishes and after reaching 60 – 80% of confluency transfection was performed. First, 20 µg of plasmid DNA was resuspended in 1 ml of Optimem (Invitrogen). In parallel 60 µg of PEI was resuspended in 1 ml Optimem. After 5–15 min of incubation both solutions were mixed and incubated for 30 min. Next, the culture medium was replaced, and a mixture of DNA/PEI was added to the cells. After 3–4 h incubation, the Optimem was replaced with culture medium. After 20–24 h, mitochondria were isolated for patch-clamp experiments.
Mitochondria isolation
Mitochondria were prepared as previously described56. For the patch-clamp experiments, HEK293T cells were washed and collected in PBS buffer and centrifuged for 8 min at 800×g. Next, the pellet was resuspended in ice-cold isolation buffer (sucrose 250 mM, HEPES 5 mM, EDTA 1 mM, pH 7.2) and gently homogenized with a Dounce homogenizer. To isolate the mitochondrial fraction, the homogenate was centrifuged for 10 min at 9200×g and 4 °C. Next, the pellet was resuspended and centrifuged at 750×g for 10 min, and 4 °C. The resulting supernatant was centrifuged in a new tube for 10 min at 9200×g, 4 °C. The pelleted mitochondrial fraction was resuspended in an isotonic solution (150 mM KCl, 10 mM HEPES, pH 7.2). The tubes and homogenizer were cooled on ice. Each mitochondrial isolation was performed using between 1 and 3 million cells.
Electrophysiological single-channel recordings
Patch-clamp experiments using HEK293T cell mitoplasts were performed as previously described23,57. In brief, a patch-clamp pipette was filled with an isotonic solution containing 150 mM KCl, 10 mM HEPES, and 100 µM CaCl2 at pH 7.2. All of the modulators of the mitoBKCa channel were added as dilutions in isotonic solution. For the calcium-dependence experiments, the concentration of free Ca2+ was controlled with EGTA, and appropriate concentrations of CaCl2 were calculated with MaxChelator software58 (Stanford University, Stanford, CA, USA). To apply channel modulators and isotonic solutions with different calcium concentrations, a perfusion system was used. The mitoplasts at the tip of the measuring pipette were transferred into the openings of a multibarrel “sewer pipe” system in which their outer faces were rinsed with the test solutions (Fig. 1A)23. The current–time traces of the experiments were recorded in single-channel mode. The pipettes were made of borosilicate glass and had a resistance of 10–20 MΩ (Harvard Apparatus GC150-10)23. A PC-10 puller (Narishige) was used. The currents were low-pass filtered at 1 kHz and sampled at a frequency of 100 kHz (amplifiers: Axopatch 200B, digidata: Axon 1440A, Molecular Devices)23. The traces of the experiments were recorded in single-channel mode. For data analysis, Clampfit 10.7 software (Axon Instruments, Molecular Devices) was used. The conductance of the channel was calculated from the current–voltage relationship (Fig. 2B)23. The open probability (Po) of the channels was determined using the single-channel search mode. Changes in the ion current were determined by event statistics. For multichannel recordings, the area (pA*ms) under the curve was calculated. The baseline was fixed individually for each experiment based on the closed state of the channels (pA). The maximum number of channels in the patch was obtained by dividing the peak amplitude (pA) by the current of a single channel (pA). Fitting the Boltzmann function to the data from the open probability/voltage plot (Fig. 2C) and the calculation of the V1/2 value and the gating charge were prepared according to GraphPad Prism software.
All modulators of the mitoBKCa channels used in the study were from Sigma-Aldrich.
The electrophysiological data for transiently transfected HEK293T cells were obtained from 56 independent mitochondrial isolations (approx. 600 patches). A mitoplast patch-clamp of untransfected HEK293T cells was acquired from 10 independent mitochondrial isolations (104 patches).
Reverse-transcription, qualitative and quantitative PCR
RNA from HEK293T cells was isolated using the RNAesy Mini Kit (QIAGEN) according to the manufacturer’s instructions. Reverse-transcription reactions were performed using RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific). For analysis of the expression levels of selected genes, SYBR Select master mix (Applied Biosystems) was used. The reaction was performed with a 7900HT real-time PCR system (Applied Biosystems). For standard PCR, REDTaq polymerase (Sigma-Aldrich) was used, and a Bio-Rad C1000 Thermal Cycler was applied. Primer sequences used in the study were as follows:
BK α (recognizing all isoforms) forward 5′-CCCGCAGACACTGGCCAATAG-3′, reverse 5′-GAGCATCTCTCAGCCGGTAA-3′;
VEDEC, forward 5′-GGGACAAACAGAATGCAACA-3′, reverse 5′-GGTACTCATGGGCTTGATTT-3′;
GAPDH forward 5′-TCAGACACCATGGGGAAGGTGAA-3′, reverse 5′-GAATCATATTGGAACATGTAAACCATG-3′;
MT-CO1 forward 5′-TTAGCTGACTCGCCACACTC-3′, reverse 5′-GGCCACCTACGGTGAAAAGA-3′.
Western blot analysis
Mitochondrial fractions isolated from HEK293T cells were isolated as described above. First, a 50 µg sample solubilized in Laemmli buffer (Bio-Rad) was separated by 10% Tris-Tricine gel electrophoresis and transferred onto polyvinylidene difluoride (PVDF) membranes (Bio-Rad). After protein transfer, the membranes were exposed to an anti-BK α antibody (NeuroMab, clone L6/60, diluted 1:200) and an anti-mHSP75 antibody (Abcam, 1:1000). The blots were developed using a secondary anti-rabbit or anti-mouse antibody (both GE Healthcare) coupled to horseradish peroxidase in conjunction with an enhanced chemiluminescence solution (GE Healthcare). The results are the summary of analysis of six independent mitochondrial isolations.
Immunostaining and confocal microscopy
HEK293T cells were seeded onto a 35 mm glass-bottom dish (IBL Baustoff + Labor GmbH) in standard culture medium. After reaching 50–80% of confluency, the culture medium was replaced with DMEM supplemented with 10% FBS and glutamine (as above) but without antibiotics. Next, the cells were transiently transfected with the appropriate plasmid DNA. DNA (2.5 µg VEDEC-encoding plasmid) and 2.5 µl of PEI (1 µg/µl in water) were resuspended in 50 µl of Optimem (Invitrogen). After 15 min of incubation at room temperature, the mixture was added to the cells. After approximately 24 h, the cells were fixed for 15 min with an ice-cold 4% solution of paraformaldehyde (Cell Signaling Technology). For mitochondrial visualization prior to fixation the cells were incubated with 100 nM MitoRed (Sigma-Aldrich) for 30 min at 37 °C in a humidified atmosphere with 5% CO2. After fixation, the cells were washed with PBS and permeabilized with 0.05% Triton X-100 solution in PBS (2 × 3 min). Next, the cells were washed with PBS and incubated overnight at 4 °C with a primary antibody resuspended in PBS supplemented with 4% FBS. For indication of the BKCa α subunit, a 1:200 anti-BK α antibody was used (Alomone Labs, APC021). For indication of the endoplasmic reticulum, a 1:500 anti-PDI (protein disulfide isomerase) antibody was applied (Abcam). Next, the cells were washed with PBS and incubated with a secondary antibody for 1 h at RT (1:1000, anti-mouse Alexa 555, anti-rabbit Alexa 488). Finally, the cells were washed with PBS and mounted with Vectashield medium (Vector Laboratories). Confocal images of stained cells were acquired using an Olympus FV 1200.
Statistical analysis
The results are presented as means ± SD obtained from at least three independent experiments. Unpaired two-tailed Student’s t-test was used to identify significant differences between two groups of samples. For analysis of differences among at least three groups the one-way ANOVA followed by Tukey’s multiple comparison test was used. Differences were considered to be statistically significant if p < 0.05 (*), p < 0.01 (**), or p < 0.001 (***).
Supplementary Information
Acknowledgements
This work was supported by Polish National Science Center Grant Nos. 2015/18/E/NZ1/00737 (to BK) and 2019/34/A/NZ1/00352 (to AS). We would like to thank Dr. Antoni Wrzosek and Aleksandra Sęk for valuable discussions and Prof. Mike Ryan and Dr. David Stroud for providing the HEK293T cell line.
Abbreviations
- Po
Open probability of the channel
- mitoBKCa
Mitochondrial large conductance calcium-activated potassium channel
- mitoK
Mitochondrial potassium channels
- KCOs
Potassium channel openers
- BKCa
Large conductance calcium-activated potassium channel
Author contributions
B.K., P.B., and A.S. designed research; S.K., P.B. and B.K. performed research; S.K., P.B., B.K. and A.S. analyzed data; All authors wrote the manuscript together.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Shur Gałecka and Bogusz Kulawiak.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-90465-3.
References
- 1.Szabo I, Zoratti M. Mitochondrial channels: Ion fluxes and more. Physiol. Rev. 2014;94:519–608. doi: 10.1152/physrev.00021.2013. [DOI] [PubMed] [Google Scholar]
- 2.Brand MD. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic. Biol. Med. 2016;100:14–31. doi: 10.1016/j.freeradbiomed.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Laskowski M, et al. What do we not know about mitochondrial potassium channels? Biochim. Biophys. Acta. 1857;1247–1257:2016. doi: 10.1016/j.bbabio.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Siemen D, Loupatatzis C, Borecky J, Gulbins E, Lang F. Ca2+-activated K channel of the BK-type in the inner mitochondrial membrane of a human glioma cell line. Biochem. Biophys. Res. Commun. 1999;257:549–554. doi: 10.1006/bbrc.1999.0496. [DOI] [PubMed] [Google Scholar]
- 5.Douglas RM, et al. The calcium-sensitive large-conductance potassium channel (BK/Maxi K) is present in the inner mitochondrial membrane of rat brain. Neuroscience. 2006;139:1249–1261. doi: 10.1016/j.neuroscience.2006.01.061. [DOI] [PubMed] [Google Scholar]
- 6.Skalska J, et al. A novel potassium channel in skeletal muscle mitochondria. BBA-Bioenergetics. 2008;1777:651–659. doi: 10.1016/j.bbabio.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Bednarczyk P, Koziel A, Jarmuszkiewicz W, Szewczyk A. Large-conductance Ca2+-activated potassium channel in mitochondria of endothelial EAhy926 cells. Am. J. Physiol. Heart Circ. Physiol. 2013;304:H1415–H1427. doi: 10.1152/ajpheart.00976.2012. [DOI] [PubMed] [Google Scholar]
- 8.Skalska J, et al. Calcium ions regulate K+ uptake into brain mitochondria: The evidence for a novel potassium channel. Int. J. Mol. Sci. 2009;10:1104–1120. doi: 10.3390/ijms10031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kicinska A, et al. A large-conductance calcium-regulated K+ channel in human dermal fibroblast mitochondria. Biochemical Journal. 2016;473:4457–4471. doi: 10.1042/bcj20160732. [DOI] [PubMed] [Google Scholar]
- 10.Laskowski M, Kicinska A, Szewczyk A, Jarmuszkiewicz W. Mitochondrial large-conductance potassium channel from Dictyostelium discoideum. Int. J. Biochem. Cell Biol. 2015;60:167–175. doi: 10.1016/j.biocel.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Xu W, et al. Cytoprotective role of Ca2+- activated K+ channels in the cardiac inner mitochondrial membrane. Science. 2002;298:1029–1033. doi: 10.1126/science.1074360. [DOI] [PubMed] [Google Scholar]
- 12.Soltysinska E, et al. KCNMA1 encoded cardiac BK channels afford protection against ischemia-reperfusion injury. PLoS ONE. 2014;9:e103402. doi: 10.1371/journal.pone.0103402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borchert GH, Yang C, Kolar F. Mitochondrial BKCa channels contribute to protection of cardiomyocytes isolated from chronically hypoxic rats. Am. J. Physiol. Heart Circ. Physiol. 2011;300:H507–513. doi: 10.1152/ajpheart.00594.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bentzen BH, et al. Activation of big conductance Ca(2+)-activated K (+) channels (BK) protects the heart against ischemia-reperfusion injury. Pflugers Arch. 2009;457:979–988. doi: 10.1007/s00424-008-0583-5. [DOI] [PubMed] [Google Scholar]
- 15.Sato T, Saito T, Saegusa N, Nakaya H. Mitochondrial Ca2+-activated K+ channels in cardiac myocytes: A mechanism of the cardioprotective effect and modulation by protein kinase A. Circulation. 2005;111:198–203. doi: 10.1161/01.CIR.0000151099.15706.B1. [DOI] [PubMed] [Google Scholar]
- 16.Kulawiak B, Kudin AP, Szewczyk A, Kunz WS. BK channel openers inhibit ROS production of isolated rat brain mitochondria. Exp. Neurol. 2008;212:543–547. doi: 10.1016/j.expneurol.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Stowe DF, et al. Cardiac mitochondrial preconditioning by Big Ca2+-sensitive K+ channel opening requires superoxide radical generation. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H434–H440. doi: 10.1152/ajpheart.00763.2005. [DOI] [PubMed] [Google Scholar]
- 18.Heinen A, et al. Reverse electron flow-induced ROS production is attenuated by activation of mitochondrial Ca2+-sensitive K+ channels. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H1400–H1407. doi: 10.1152/ajpheart.00198.2007. [DOI] [PubMed] [Google Scholar]
- 19.Frankenreiter S, et al. cGMP-elevating compounds and ischemic conditioning provide cardioprotection against ischemia and reperfusion injury via cardiomyocyte-specific BK channels. Circulation. 2017;136:2337–2355. doi: 10.1161/CIRCULATIONAHA.117.028723. [DOI] [PubMed] [Google Scholar]
- 20.Rotko D, Kunz WS, Szewczyk A, Kulawiak B. Signaling pathways targeting mitochondrial potassium channels. Int. J. Biochem. Cell Biol. 2020;125:10572. doi: 10.1016/j.biocel.2020.105792. [DOI] [PubMed] [Google Scholar]
- 21.Kulawiak B, Bednarczyk P. Reconstitution of brain mitochondria inner membrane into planar lipid bilayer. Acta Neurobiol. Exp. 2005;65:271–276. doi: 10.55782/ane-2005-1562. [DOI] [PubMed] [Google Scholar]
- 22.Balderas E, et al. MitoBK(Ca) channel is functionally associated with its regulatory beta 1 subunit in cardiac mitochondria. J. Physiol. Lond. 2019;597:3817–3832. doi: 10.1113/jp277769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bednarczyk P, et al. Putative structural and functional coupling of the mitochondrial BKCa channel to the respiratory chain. PLoS ONE. 2013;8:e68125. doi: 10.1371/journal.pone.0068125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Augustynek B, K. W., Szewczyk A. in Handbook of Experimental Pharmacology Vol. 240, 103–127 (2017). [DOI] [PubMed]
- 25.Bentzen BH, Olesen SP, Ronn LC, Grunnet M. BK channel activators and their therapeutic perspectives. Front. Physiol. 2014;5:389. doi: 10.3389/fphys.2014.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Augustynek B, et al. Mitochondrial BK channel openers CGS7181 and CGS7184 exhibit cytotoxic properties. Int. J. Mol. Sci. 2018;19:353. doi: 10.3390/ijms19020353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Augustynek B, Kudin AP, Bednarczyk P, Szewczyk A, Kunz WS. Hemin inhibits the large conductance potassium channel in brain mitochondria: A putative novel mechanism of neurodegeneration. Exp. Neurol. 2014;257:70–75. doi: 10.1016/j.expneurol.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 28.Kaczara P, et al. Carbon monoxide released by CORM-401 uncouples mitochondrial respiration and inhibits glycolysis in endothelial cells: A role for mitoBKCa channels. Biochim. Biophys. Acta. 1847;1297–1309:2015. doi: 10.1016/j.bbabio.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Rotko D, et al. Heme is required for carbon monoxide activation of mitochondrial BKCa channel. Eur. J. Pharmacol. 2020;881:173191. doi: 10.1016/j.ejphar.2020.173191. [DOI] [PubMed] [Google Scholar]
- 30.Hou SW, Heinemann SH, Hoshi T. Modulation of BKCa channel gating by endogenous signaling molecules. Physiology. 2009;24:26–35. doi: 10.1152/physiol.00032.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoshi, T. & Heinemann, S. H. Modulation of BK channels by small endogenous molecules and pharmaceutical channel openers. Big on Bk: Current Insights into the Function of Large Conductance Voltage- and Ca2+- Activated K+ Channels at the Molecular, Cellular, and Systemic Levels, Vol 128128, 193–23. 10.1016/bs.irn.2016.03.020 (2016). [DOI] [PubMed]
- 32.Li Q, Yan J. Modulation of BK channel function by auxiliary beta and gamma subunits. Int. Rev. Neurobiol. 2016;128:51–90. doi: 10.1016/bs.irn.2016.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bednarczyk P, et al. Large conductance potassium ion channel from rat brain mitochondria. Biochim. Biophys. Acta Bioenerg. 2006;461:1–12. [Google Scholar]
- 34.Sakai Y, Harvey M, Sokolowski B. Identification and quantification of full-length BK channel variants in the developing mouse cochlea. J. Neurosci. Res. 2011;89:1747–1760. doi: 10.1002/jnr.22713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kathiresan T, Harvey M, Orchard S, Sakai Y, Sokolowski B. A protein interaction network for the large conductance Ca2+-activated K+ channel in the mouse cochlea. Mol. Cell. Proteomics. 2009;8:1972–1987. doi: 10.1074/mcp.M800495-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh H, et al. mitoBK(Ca) is encoded by the Kcnma1 gene, and a splicing sequence defines its mitochondrial location. Proc. Natl. Acad. Sci. U.S.A. 2013;110:10836–10841. doi: 10.1073/pnas.1302028110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh H, et al. MaxiK channel interactome reveals its interaction with GABA transporter 3 and heat shock protein 60 in the mammalian brain. Neuroscience. 2016;317:76–107. doi: 10.1016/j.neuroscience.2015.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bentzen BH, et al. The small molecule NS11021 is a potent and specific activator of Ca2+-activated big-conductance K+ channels. Mol. Pharmacol. 2007;72:1033–1044. doi: 10.1124/mol.107.038331. [DOI] [PubMed] [Google Scholar]
- 39.Rockman ME, Vouga AG, Rothberg BS. Molecular mechanism of BK channel activation by the smooth muscle relaxant NS11021. J. Gen. Physiol. 2020;152:6. doi: 10.1085/jgp.201912506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahring PK, Strøbaek D, Christophersen P, Olesen S-P, Johansen TE. Stable expression of the human large-conductance Ca2+-activated K+ channel α- and β-subunits in HEK293 cells. FEBS Lett. 1997;415:67–70. doi: 10.1016/s0014-5793(97)01096-x. [DOI] [PubMed] [Google Scholar]
- 41.Gribkoff VK, et al. Effects of channel modulators on cloned large-conductance calcium-activated potassium channels. Mol. Pharmacol. 1996;50:206–217. [PubMed] [Google Scholar]
- 42.Knaus HG, et al. Tremorgenic indole alkaloids potently inhibit smooth-muscle high-conductance calcium-activated potassium chann.els. Biochemistry. 1994;33:5819–5828. doi: 10.1021/bi00185a021. [DOI] [PubMed] [Google Scholar]
- 43.Tang XD, et al. Haem can bind to and inhibit mammalian calcium-dependent Slo1 BK channels. Nature. 2003;425:531–535. doi: 10.1038/nature02003. [DOI] [PubMed] [Google Scholar]
- 44.Du X, et al. Loss-of-function BK channel mutation causes impaired mitochondria and progressive cerebellar ataxia. Proc. Natl. Acad. Sci. USA. 2020;117:6023–6034. doi: 10.1073/pnas.1920008117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DuBridge RB, et al. Analysis of mutation in human cells by using an Epstein-Barr virus shuttle system. Mol. Cell Biol. 1987;7:379–387. doi: 10.1128/mcb.7.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gu XQ, Pamenter ME, Siemen D, Sun X, Haddad GG. Mitochondrial but not plasmalemmal BK channels are hypoxia-sensitive in human glioma. Glia. 2014;62:504–513. doi: 10.1002/glia.22620. [DOI] [PubMed] [Google Scholar]
- 47.Gu XQ, et al. Hypoxia increases BK channel activity in the inner mitochondrial membrane. Biochem. Biophys. Res. Commun. 2007;358:311–316. doi: 10.1016/j.bbrc.2007.04.110. [DOI] [PubMed] [Google Scholar]
- 48.Cheng Y, Gulbins E, Siemen D. Activation of the permeability transition pore by Bax via inhibition of the mitochondrial BK channel. Cell. Physiol. Biochem. 2011;27:191–200. doi: 10.1159/000327944. [DOI] [PubMed] [Google Scholar]
- 49.Strobaek D, et al. Modulation of the Ca(2+)-dependent K+ channel, hslo, by the substituted diphenylurea NS 1608, paxilline and internal Ca2+ Neuropharmacology. 1996;35:903–914. doi: 10.1016/0028-3908(96)00096-2. [DOI] [PubMed] [Google Scholar]
- 50.Maqoud F, Cetrone M, Mele A, Tricarico D. Molecular structure and function of big calcium-activated potassium channels in skeletal muscle: Pharmacological perspectives. Physiol. Genom. 2017;49:306–317. doi: 10.1152/physiolgenomics.00121.2016. [DOI] [PubMed] [Google Scholar]
- 51.Borchert GH, Hlavackova M, Kolar F. Pharmacological activation of mitochondrial BK(Ca) channels protects isolated cardiomyocytes against simulated reperfusion-induced injury. Exp. Biol. Med. 2013;238:233–241. doi: 10.1177/1535370212474596. [DOI] [PubMed] [Google Scholar]
- 52.Frankenreiter S, et al. Cardioprotection by ischemic postconditioning and cyclic guanosine monophosphate-elevating agents involves cardiomyocyte nitric oxide-sensitive guanylyl cyclase. Cardiovasc. Res. 2018;114:822–829. doi: 10.1093/cvr/cvy039. [DOI] [PubMed] [Google Scholar]
- 53.Jaggar JH, et al. Heme is a carbon monoxide receptor for large-conductance Ca2+-activated K+ channels. Circ. Res. 2005;97:805–812. doi: 10.1161/01.RES.0000186180.47148.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang J, et al. The mitochondrial BKCa channel cardiac interactome reveals BKCa association with the mitochondrial import receptor subunit Tom22, and the adenine nucleotide translocator. Mitochondrion. 2017;33:84–101. doi: 10.1016/j.mito.2016.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walewska A, Kulawiak B, Szewczyk A, Koprowski P. Mechanosensitivity of mitochondrial large-conductance calcium-activated potassium channels. Biochim. Biophys. Acta Bioenerg. 1859;797–805:2018. doi: 10.1016/j.bbabio.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 56.Laskowski M, et al. Single-channel properties of the ROMK-pore-forming subunit of the mitochondrial ATP-sensitive potassium channel. Int. J. Mol. Sci. 2019;20:21. doi: 10.3390/ijms20215323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kampa RP, et al. Naringenin as an opener of mitochondrial potassium channels in dermal fibroblasts. Exp. Dermatol. 2019;28:543–550. doi: 10.1111/exd.13903. [DOI] [PubMed] [Google Scholar]
- 58.Bers DM, Patton CW, Nuccitelli R. A practical guide to the preparation of Ca2+ a buffers. Calcium Living Cells. 2010;99:1–26. doi: 10.1016/s0091-679x(10)99001-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.