Abstract
Nontypeable Haemophilus influenzae (NTHi) is a major respiratory pathogen that initiates infection by colonising the upper airways. Strategies that interfere with this interaction may therefore have a clinically significant impact on the ability of NTHi to cause disease. We have previously shown that strains of the commensal bacterium Haemophilus haemolyticus (Hh) that produce a novel haem-binding protein, haemophilin, can prevent NTHi growth and interactions with host cells in vitro. We hypothesized that natural pharyngeal carriage of Hh strains with the hpl open reading frame (Hh-hpl+) would be associated with a lower prevalence and/or density of NTHi colonisation in healthy individuals. Oropharyngeal swabs were collected from 257 healthy adults in Australia between 2018 and 2019. Real-time PCR was used to quantitatively compare the oropharyngeal carriage load of NTHi and Hh populations with the Hh-hpl+ or Hh-hpl− genotype. The likelihood of acquiring/maintaining NTHi colonisation status over a two- to six-month period was assessed in individuals that carried either Hh-hpl− (n = 25) or Hh-hpl+ (n = 25). Compared to carriage of Hh-hpl− strains, adult (18–65 years) and elderly (>65 years) participants that were colonised with Hh-hpl+ were 2.43 or 2.67 times less likely to carry NTHi in their oropharynx, respectively. Colonisation with high densities of Hh-hpl+ correlated with a low NTHi carriage load and a 2.63 times lower likelihood of acquiring/maintaining NTHi colonisation status between visits. Together with supporting in vitro studies, these results encourage further investigation into the potential use of Hh-hpl+ as a respiratory probiotic candidate for the prevention of NTHi infection.
Keywords: nontypeable Haemophilus influenzae, Haemophilus haemolyticus, haem-binding protein, haemophilin, respiratory probiotic
1. Introduction
Nontypeable Haemophilus influenzae (NTHi) is a major bacterial cause of opportunistic infections in the respiratory tract, most notably otitis media in infants and young children, community-acquired pneumonia in the elderly, and acute exacerbations in individuals with chronic obstructive pulmonary disease (COPD) [1,2]. Collectively, these infections and subsequent long-term health complications, such as hearing loss or decline in lung function, impart a significant global disease burden [3,4]. Asymptomatic pharyngeal colonisation occurs in 20–30% of healthy adults and 20–80% of children under the age of 5 and is characterised by the simultaneous carriage of multiple strains and a rapid turnover rate of constituent genotypes [1,2,5,6,7,8]. Week to week turnover rates of NTHi genotypes as high as 62% have been reported from healthy children attending day care [9]. In the majority of cases, NTHi strains are typically replaced within three months of acquisition, but the persistent colonisation of a single strain up to six or seven months has been reported [6,9]. Although the mechanisms that lead from colonisation to infection are poorly understood, the frequency of pharyngeal colonisation, especially with immunologically new NTHi strains, has been directly linked to an increased risk of developing acute otitis media [10,11] and acute exacerbations of chronic obstructive pulmonary disease [8,12]. Higher bacterial loads in the nasopharynx have also been correlated with an increased risk of developing acute otitis media [11,13,14] and a clinically significant increase in respiratory symptoms in COPD, even in the absence of a clinical exacerbation [15]. Thus, eradicating or reducing the pharyngeal load of NTHi may have a clinically significant impact on the ability of NTHi to cause infection.
The need for alternative preventative therapies for NTHi infections has become apparent following ineffective vaccination attempts and a rapidly evolving antibiotic resistance profile that has resulted in treatment failure with first- and second-line antibiotic regimens [4,16,17,18,19,20]. One avenue gaining attention is the use of upper respiratory tract commensals, which may have utility in altering the pharyngeal habitability for pathogens such as NTHi [21]. We have previously shown that some strains of the closely related respiratory tract commensal Haemophilus haemolyticus (Hh) produce a haem-binding protein, haemophilin (Hpl), that has been shown to inhibit NTHi growth in vitro [22,23] and interactions with host cells in model respiratory cell lines [24]. NTHi-inhibitory activity in these models was speculated to be mediated by Hpl haem binding that limited NTHi access to the essential nutrient haem [22], which is required for growth, survival, and pathogenicity [25].
We hypothesised that natural pharyngeal carriage of Hh strains with the hpl open reading frame (Hh-hpl+) would be associated with a lower prevalence and/or density of NTHi colonisation in healthy individuals. To explore this hypothesis, real-time PCR was used to quantitatively compare the carriage load of NTHi and Hh populations with the Hh-hpl+ or Hh-hpl− genotype from the oropharynx of 257 healthy adults in Australia collected between 2018 and 2019. Carriage of Hh-hpl+ was associated with a significantly lower likelihood of concurrent NTHi carriage, long-term maintenance, or acquisition of NTHi colonisation status. Additionally, NTHi density was negatively correlated with Hh-hpl+ carriage density. This work supports further investigation into the potential use of Hh-hpl+ as a respiratory probiotic candidate for the prevention of NTHi colonisation and disease.
2. Results and Discussion
2.1. Carriage Profile of NTHi and Hh Varied between Participant Age Groups
NTHi was detected by real-time PCR of the siaT gene target in 29% of adult (18–65 years) participants compared to 53% of elderly (>65 years) participants (Table S1). Conversely, the prevalence of Hh carriage was higher in adults (77%) compared to the elderly (52%) participants. Among Hh carriers, the frequency of detecting the Hh-hpl+ genotype was slightly higher in the adults (55%) than in the elderly participants (39%). Together, the lower propensity for Hh and Hh-hpl+ carriage in elderly participants may describe an environment that favours NTHi carriage.
NTHi carriage rates observed in this study are largely consistent with previous reports of nasopharyngeal carriage rates of 23–31% in healthy adults in the UK and Indigenous communities in Australia [5,26,27,28,29]. However, carriage rates as low as 3–15% have also been reported in Kenya and Nepal [30,31,32], highlighting geographical differences in NTHi carriage. Despite their predisposition to NTHi-associated infections [33], information surrounding carriage rates in elderly demographics is limited to studies in Germany (2012–2013) and Brazil (2017), reporting rates of 1.9–2.5% [1,34]. It is unclear whether the substantially higher NTHi carriage rates detected in elderly participants is representative of the local population or of sampling bias. Although the SiaT PCR target may also be detected in capsular H. influenzae [35], carriage of these strains is uncommon and collectively accounts for around 1.0% of H. influenzae isolates [36,37]. Thus, the false detection rate of NTHi strains is likely to be extremely low and unlikely to affect statistical findings presented in this study. This does not compromise the clinical utility of Hh-hpl+, as Hpl also exhibits inhibitory activity against capsular strains in vitro [22]. This is the first study to assess Hh carriage prevalence among adults at a community level.
In addition to geographical and age-related variances, NTHi carriage rates vary considerably between studies, largely owing to the different culture- and molecular-based methods employed and the difficulty of distinguishing NTHi from Hh [38,39]. Although the nasopharynx is the preferred collection site for H. influenzae isolation in culture-based carriage studies [38], several studies have reported similar or improved detection of NTHi and Hh from oropharyngeal (OP) collections using qPCR-based methods [27,40] that can reliably differentiate the two species, such as the hypD and siaT targets employed in this study [35]. Therefore, collection site is unlikely to impact carriage rates determined in this study.
2.2. Carriage of hpl-Positive Hh Is Correlated with Reduced Prevalence and Density of NTHi Cocolonisation
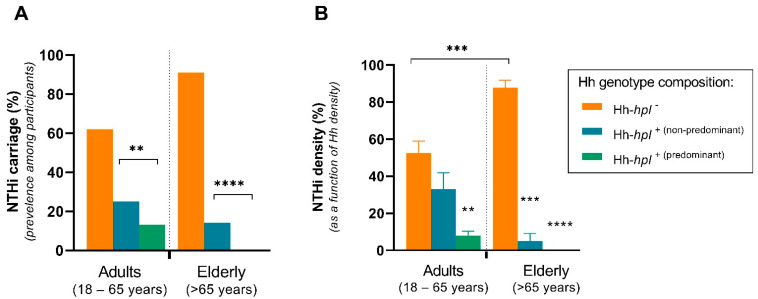
Elderly participants were 2.43 times (95% CI, 1.95–2.61; p < 0.0001) and adult participants were 2.67 times (95% CI, 2.63–2.70; p = 0.0036) less likely to carry NTHi if Hh-hpl+ strains were detected, compared to the carriage of Hh-hpl− strains. NTHi carriage prevalence was highest in adult (62%) and elderly (91%) participants that concurrently carried Hh strains that did not possess the hpl ORF (Hh-hpl−) in their oropharynx. Among participants carrying Hh-hpl+ strains, NTHi carriage rates were 25% (adults) and 14% (elderly) or 13% (adults) and 0% (elderly) in participants where Hh-hpl+ represented the predominant Hh genotype (Figure 1A).
Figure 1.
NTHi dominance in oropharyngeal swabs of healthy adult (18–65 years) or elderly (>65 years) participants co-colonised with Hh. NTHi oropharyngeal carriage prevalence (A) or proportion of NTHi (as a function of total Hh) (B) among participants concurrently carrying Hh strains that possess the hpl ORF (Hh-hpl+) or do not possess the hpl ORF (Hh-hpl−). Hh-hpl+ (predominant) denotes instances where hpl+ is the predominant Hh genotype (>0.5 of total Hh). Error bars represent ±SEM; statistical significance was determined by simple logistic regression (A) or nonparametric Spearman correlation (B); ** p < 0.005, *** p < 0.001, **** p < 0.0001.
Comparison of Hh densities determined by qPCR of the hpl and hypD gene targets suggested concurrent carriage of multiple Hh genotypes, where not all possessed the hpl ORF. Simultaneous colonisation with multiple Hh and NTHi genotypes has previously been described in a longitudinal study of healthy adults [6]. Hh-hpl+ represented the predominating genotype in 49% (39/79) and 53% (8/15) of cases when the hpl gene was detected in adult and elderly participants, respectively (Table S1). The proportionate density of Hh-hpl+ (as a function of total Hh carriage) was negatively correlated with NTHi density among adult (rs = −0.16; 95% CI, −0.314–−0.006; p = 0.0366) and, to a larger degree, elderly (rs = −0.53; 95% CI, −0.7106–0.2851; p < 0.0001) participants that carried either species. In the adult age group, the average proportion of NTHi density decreased by 20% among individuals who concurrently carried Hh-hpl+ as the non-predominant Hh genotype or by 47% if Hh-hpl+ represented the predominant Hh genotype. This trend was more pronounced in the elderly age group where the average proportion of NTHi density decreased by 83% among individuals who concurrently carried Hh-hpl+ or by 88% if Hh-hpl+ represented the predominant Hh genotype (Figure 1B). Together these data suggest that carriage of Hh-hpl+, but not Hh strains lacking the hpl ORF, lowers the incidence and density of concurrent NTHi carriage, particularly if Hh-hpl+ represents the predominant Hh genotype.
2.3. Carriage of hpl-Positive Hh Prevents Persistent Colonisation or Acquisition of NTHi Carriage Status
To investigate the risk of acquiring/maintaining NTHi colonisation status, follow-up OP swabs were collected from individuals that carried hpl+ (n = 25) or hpl− (n = 25) strains of Hh. At visit 2, Hh-hpl+ colonisation status was maintained in 19/25 of individuals, with an additional 11 participants gaining colonisation status, resulting in a total Hh-hpl+ carriage rate of 60% (30/50) at visit 2. Maintenance or acquisition of NTHi carriage status at visit 2 was associated with the carriage of Hh-hpl− strains at visit 1 and at visit 2 in 75% (12/16) of cases (Table 1). The remaining four NTHi carriers were co-colonised with Hh-hpl+ strains; however, in all cases, Hh-hpl+ was not the predominant Hh genotype. In contrast, of participants who were not colonised with NTHi (either by loss of NTHi or who were never colonised), 88% (30/36) were carrying Hh-hpl+ (Table 1). The likelihood of being colonised with NTHi at visit 2 (either by maintaining or acquiring NTHi colonisation status) was 2.63 times (95% CI, 2.56–2.70, p = 0.0112) lower in individuals colonised with Hh-hpl+ strains at either visit 1 or visit 2. These results suggest that Hh-hpl+ colonisation may have a protective effect against NTHi colonisation in vivo. However, the data can only account for total changes in carriage status between two visits and cannot assess the characteristically diverse and dynamic turnover of individual NTHi/Hh genotypes. Therefore, these findings may underestimate the protective capacity of Hh-hpl+ carriage and additional longitudinal studies with genotypic resolution are warranted. The findings presented by this study are only correlative and, as such, cannot rule out other unmeasured host-derived factors that may affect an individual’s susceptibility to NTHi colonisation, such as smoking, airway dysbiosis, and underlying chronic respiratory diseases [41,42,43,44].
Table 1.
NTHi colonisation status in participants between visit 1 and visit 2 (n = 50).
| NTHi Colonisation at Visit 1 -> Visit 2 Frequency (%) | ||||
|---|---|---|---|---|
| Hh Genotype | NTHi+ | NTHi− (Colonisation Loss) |
NTHi− | NTHi− (Never Colonised) |
NTHi+ | NTHi+ (Consistently Colonised) |
NTHi− | NTHi+ (Colonisation Gain) |
| Total | 8/50 (16) | 26/50 (52) | 4/50 (8) | 12/50 (24) |
| hpl+ | 7/8 (88) | 23/26 (88) | 2/4 (50) | 2/12 (17) |
| hpl− | 1/8 (12) | 3/26 (12) | 2/4 (50) | 10/12 (83) |
+/− Detection by PCR for corresponding gene targets siaT (NTHi), hypD (Hh) and the hpl ORF.
2.4. Potential Therapeutic Utility of hpl-Positive Strains of Hh
In summary, the carriage of Hh-hpl+ was associated with a significantly lower proportionate density and prevalence of concurrent NTHi carriage and long-term maintenance or acquisition of NTHi colonisation status. These findings suggest a potential protective role of Hh-hpl+ strains against NTHi pharyngeal colonisation, particularly in an elderly population with a predisposition to NTHi colonisation in the absence of Hh-hpl+. The frequency and density of NTHi colonisation or the acquisition of immunologically new strains are factors associated with an increased risk of disease onset and severity [8,12]. Thus, a commensal bacterium that can prevent NTHi pharyngeal colonisation incidence and/or bacterial load has compounded therapeutic utility and may be favourable over immunogenic approaches that are hampered by the highly variable expression of NTHi surface proteins and immunogenicity that does not protect against reinfection [45]. However, clinical trials are required to determine if Hh-hpl+ can eradicate or protect against direct NTHi challenge in vivo, particularly in populations predisposed to NTHi infections.
Production of the Hpl haemophore has previously been shown to mediate the in vitro inhibitory capacity of Hh against NTHi growth and the adherence/invasion of model respiratory cell lines by restricting NTHi access to the essential nutrient haem [22,23,24]. Although we postulate that the same mechanism may be involved here, the study design reports on the presence of the hpl coding region and does not assess phenotypic production of the Hpl protein. We have previously shown that even among Hh strains containing identical hpl ORF sequences, some strains lack the capacity to express hpl and produce the Hpl protein that mediates NTHi inhibitory activity in vitro [23,24,44]. However, the majority (16/17) of Hh-hpl+ detectable by our PCR assay from our culture collection produce Hpl and elicit NTHi-inhibitory activity (Table S4), and there are no incidences of Hpl production in strains that do not contain the hpl ORF. Further work is underway to determine the genetic determinants of hpl expression and Hpl production. The involvement of other host-mediated responses or interactions with other microbial communities in the oropharynx also cannot be excluded. Immune modulation, rather than physical competition, was shown to play an important role in the protective capacity of intranasal Muribacter muris (Hh surrogate) against NTHi colonisation and infection in a murine NTHi otitis media model [46]. Additionally, other microbial upper respiratory tract commensals capable of producing bacteriocins against common pathogens have been reported [47,48]. However, the substantial effect sizes correlating Hh-hpl+ and NTHi density despite potential confounders support a protective role of Hh-hpl+ against NTHi pharyngeal colonisation in healthy adults.
The in vivo correlations from the current study, together with previously published causative in vitro evidence, support further investigation into the potential use of hpl-positive strains of Hh as a respiratory probiotic candidate for the prevention of NTHi colonisation and disease.
3. Materials and Methods
3.1. Study Population
Participants (n = 257) were comprised of community groups and university staff and students in Tasmania, Australia. Recruitment and sample collection was conducted between June 2018 and November 2019. All participants were briefed on study details and received written information prior to giving informed consent to participate. Participants were included in the study if they fulfilled the following criteria: (i) over 18 years of age, (ii) not currently taking antibiotics, and (iii) not experiencing respiratory-related symptoms. The study was approved by the Tasmanian Health and Medical Human Research Ethics Committee (Ref No: H0016835, approved 11 December 2017) in accordance with the National Statement on the Ethical Conduct in Human Research (NHMRC 2007, updated 2014).
3.2. Oropharyngeal Swab Collection
Oropharyngeal (OP) swabs were collected due to ease of collection and participant tolerance. Several studies have reported similar or improved qPCR detection of NTHi and Hh from OP swabs compared to nasopharyngeal swabs [1,27,38,40]. OP swabs were collected by two investigators by depressing the tongue and rolling the tip of a sterile cotton swab on the posterior wall of either side of the oropharynx for 2 seconds, avoiding contact with the surface of the mouth to minimise contamination with mouth flora. Follow-up swabs were collected 2–6 months following the initial visit from a randomly selected subset of this population (all ages) that carried either Hh-hpl− (n = 25) or Hh-hpl+ (n = 25) at the first visit.
Immediately following collection, swabs were stored in 1 mL of room temperature transport media containing skim milk, tryptone, glucose, and glycerin (STGG) and transported to the laboratory within 2 h. STGG has previously been described and evaluated for optimal storage [49] and PCR detection of Haemophilus influenzae from nasopharyngeal swabs [50]. Specimens were subject to a vigorous vortex for 1 min to disperse organisms from the swab tip, and the tip was removed from the media by pressing the swab against the wall. Media suspensions were frozen at −80 °C until analysis.
3.3. Real-Time PCR Quantification of NTHi, Hh, and Hh-hpl+
Template gDNA was prepared from thawed 500 µL OP suspension aliquots using the DNeasy Blood & Tissue kit (Qiagen) following the standard proteinase K extraction protocol. NTHi and Hh strains containing the hpl ORF were simultaneously detected and quantified from OP swab gDNA by using a previously optimised and validated triplex real-time PCR assay [23]. Briefly, this PCR assay adapted the use of previously validated genes for the discrimination of Hh (hypD) and H. influenzae (siaT) [35] as well as primers specific for the detection of the hpl ORF (GenBank MN720274) in Hh strains. This assay was validated for the detection of hpl sequences within 85–100% similarity to the PCR amplicon, which accounts for all known Hpl-producing (and thus NTHi-inhibitory) isolates. Hh strains with more divergent sequences have been isolated; however, none are known to produce Hpl based on bioactivity assays (data not shown). Further details of PCR validation, including the limits of detection and quantification of gene targets, are supplied in the Supplementary Materials. PCRs were performed using the CFX96 TouchTM real-time PCR system (Bio-Rad) in 96-well optical plates. The complete details of PCR thermocycling conditions, reagents, primer/probe design, and assay optimisation/validation have previously been described [23]. Each run included a negative control (H. parainfluenzae ATCC 7901), no template control, and 10-fold dilutions of a standard containing 2 × 10−8 ng NTHi ATCC 49247 and Hh-BW1 (known Hh-hpl+ strain) gDNA. The quantification of NTHi and Hh was expressed as genome equivalents calculated from the appropriate standard, as previously described [35]. Bacterial carriage status was considered negative for Ct values above 35 or if genome equivalents fell below the limit of quantification for the corresponding gene target. The Hh-hpl+ genotype was considered predominant if genome equivalents calculated from the hpl target accounted for more than 50% of the total calculated Hh (from the hypD target).
3.4. Statistical Analysis
Based on reported age-associated variation in the pharyngeal carriage rate and density of NTHi, analyses were stratified by age (18–65 years and >65 years). Logistic regression models were used to assess whether Hh carriage status (either absolute presence of Hh-hpl+ or Hh-hpl−) or age predicted NTHi colonisation by measuring odds ratios (ORs) for each bacterium associated with the density of the other species. A simple logistic regression was also used to measure the OR between Hh-hpl+ carriage and NTHi colonization at follow up. A nonparametric Spearman correlation was conducted to determine the correlation between the proportion Hh-hpl+ carriage density (as a function of total Hh) and the proportion of NTHi carriage in the swabs.
Acknowledgments
We are grateful to all individuals who volunteered for participation in this study and to the Launceston General Hospital, University of Tasmania, and community groups that facilitated these collections. We also thank Erin Price for advice regarding collection and bacterial quantification using PCR.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/pathogens10050577/s1. Supplemental information includes Table S1: raw oropharyngeal colonisation frequencies of NTHi and Hh strains; Table S2: raw oropharyngeal colonisation density of NTHi and Hh strains; Text S1: triplex real-time PCR assay design, optimisation, and validation; Figure S1: PCR efficiency and measure of LoQ and LoD; Table S3: summary of primer and LNA probe sequences and expected amplicon size for the hypD, siaT, and hpl targets; Table S4: genotypic and phenotypic characteristics of Hh strains containing the hpl ORF.
Author Contributions
Conceptualization, B.A., S.T. and D.K.; methodology, B.A., S.T. and D.K.; validation, B.A.; formal analysis, B.A.; investigation, B.A. and S.T.; resources, S.T. and D.K.; data curation, B.A.; writing—original draft preparation, B.A., S.T. and D.A.G.; writing—review and editing, B.A., S.T. and D.A.G.; visualization, B.A.; supervision, S.T., D.K. and D.A.G.; project administration, S.T.; funding acquisition, S.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Clifford Craig Foundation, Launceston, Tasmania (Grant number CCF192).
Institutional Review Board Statement
The study was approved by the Tasmanian Health and Medical Human Research Ethics Committee (Ref No: H0016835, approved 11 December 2017) in accordance with the National Statement on the Ethical Conduct in Human Research (NHMRC 2007, updated 2014).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are contained within the article or Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zanella R.C., Brandileone M.C.d.C., Almeida S.C.G., de Lemos A.P.S., Sacchi C.T., Gonçalves C.R., Gonçalves M.G., Fukasawa L.O., Saraiva M.D., Rangel L.F. Nasopharyngeal carriage of Streptococcus pneumoniae, Haemophilus influenzae, and Staphylococcus aureus in a Brazilian elderly cohort. PLoS ONE. 2019;14:e0221525. doi: 10.1371/journal.pone.0221525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slack M.P. A review of the role of Haemophilus influenzae in community-acquired pneumonia. Pneumonia. 2015;6:26. doi: 10.15172/pneu.2015.6/520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cerquetti M., Giufrè M. Why we need a vaccine for non-typeable Haemophilus influenzae. Hum. Vaccines Immunother. 2016;12:2357–2361. doi: 10.1080/21645515.2016.1174354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Eldere J., Slack M.P., Ladhani S., Cripps A.W. Non-typeable Haemophilus influenzae, an under-recognised pathogen. Lancet Infect. Dis. 2014;14:1281–1292. doi: 10.1016/S1473-3099(14)70734-0. [DOI] [PubMed] [Google Scholar]
- 5.Mackenzie G.A., Leach A.J., Carapetis J.R., Fisher J., Morris P.S. Epidemiology of nasopharyngeal carriage of respiratory bacterial pathogens in children and adults: Cross-sectional surveys in a population with high rates of pneumococcal disease. BMC Infect. Dis. 2010;10:304. doi: 10.1186/1471-2334-10-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mukundan D., Ecevit Z., Patel M., Marrs C.F., Gilsdorf J.R. Pharyngeal colonization dynamics of Haemophilus influenzae and haemophilus haemolyticus in healthy adult carriers. J. Clin. Microbiol. 2008;46:1575. doi: 10.1128/JCM.00124-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Puig C., Marti S., Fleites A., Trabazo R., Calatayud L., Linares J., Ardanuy C. Oropharyngeal colonization by nontypeable Haemophilus influenzae among healthy children attending day care centers. Microb. Drug Resist. 2014;20:450–455. doi: 10.1089/mdr.2013.0186. [DOI] [PubMed] [Google Scholar]
- 8.Van Kempen M., Dhooge I., Vaneechoutte M., Claeys G. Turnover of Haemophilus influenzae isolates in otitis-prone children. Acta Otorhinolaryngol. Belg. 2001;55:83. [PubMed] [Google Scholar]
- 9.Kaur R., Chang A., Xu Q., Casey J.R., Pichichero M.E. Phylogenetic relatedness and diversity of non-typable Haemophilus influenzae in the nasopharynx and middle ear fluid of children with acute otitis media. J. Med. Microbiol. 2011;60:1841. doi: 10.1099/jmm.0.034041-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harabuchi Y., Faden H., Yamanaka N., Duffy L., Wolf J., Krystofik D., Pediatrics T.W. Nasopharyngeal colonization with nontypeable Haemophilus influenzae and recurrent otitis media. J. Infect. Dis. 1994;170:862–866. doi: 10.1093/infdis/170.4.862. [DOI] [PubMed] [Google Scholar]
- 11.Kirkham L.-A.S., Wiertsema S.P., Mowe E.N., Bowman J.M., Riley T.V., Richmond P.C. Nasopharyngeal carriage of Haemophilus haemolyticus in otitis-prone and healthy children. J. Clin. Microbiol. 2010;48:2557–2559. doi: 10.1128/JCM.00069-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sethi S., Evans N., Grant B.J., Murphy T.F. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N. Engl. J. Med. 2002;347:465–471. doi: 10.1056/NEJMoa012561. [DOI] [PubMed] [Google Scholar]
- 13.Smith-Vaughan H., Byun R., Nadkarni M., Jacques N.A., Hunter N., Halpin S., Morris P.S., Leach A.J. Measuring nasal bacterial load and its association with otitis media. BMC Ear Nose Throat Disord. 2006;6:10. doi: 10.1186/1472-6815-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith-Vaughan H.C., Binks M.J., Marsh R.L., Kaestli M., Ward L., Hare K.M., Pizzutto S.J., Thornton R.B., Morris P.S., Leach A.J. Dominance of Haemophilus influenzae in ear discharge from Indigenous Australian children with acute otitis media with tympanic membrane perforation. BMC Ear Nose Throat Disord. 2013;13:12. doi: 10.1186/1472-6815-13-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pettigrew M.M., Laufer A.S., Gent J.F., Kong Y., Fennie K.P., Metlay J.P. Upper respiratory tract microbial communities, acute otitis media pathogens, and antibiotic use in healthy and sick children. Appl. Environ. Microbiol. 2012;78:6262–6270. doi: 10.1128/AEM.01051-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Witherden E.A., Tristram S.G. Prevalence and mechanisms of β-lactam resistance in Haemophilus haemolyticus. J. Antimicrob. Chemother. 2013;68:1049–1053. doi: 10.1093/jac/dks532. [DOI] [PubMed] [Google Scholar]
- 17.Ito M., Hotomi M., Maruyama Y., Hatano M., Sugimoto H., Yoshizaki T., Yamanaka N. Clonal spread of β-lactamase-producing amoxicillin–clavulanate-resistant (BLPACR) strains of non-typeable Haemophilus influenzae among young children attending a day care in Japan. Int. J. Pediatr. Otorhinolaryngol. 2010;74:901–906. doi: 10.1016/j.ijporl.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 18.Dabernat H., Delmas C. Epidemiology and evolution of antibiotic resistance of Haemophilus influenzae in children 5 years of age or less in France, 2001–2008: A retrospective database analysis. Eur. J. Clin. Microbiol. Infect. Dis. 2012;31:2745–2753. doi: 10.1007/s10096-012-1623-9. [DOI] [PubMed] [Google Scholar]
- 19.Hare K.M., Leach A.J., Morris P.S., Smith-Vaughan H., Torzillo P., Bauert P., Cheng A.C., McDonald M., Brown N., Chang A.B. Impact of recent antibiotics on nasopharyngeal carriage and lower airway infection in Indigenous Australian children with non-cystic fibrosis bronchiectasis. Int. J. Antimicrob. Agents. 2012;40:365–369. doi: 10.1016/j.ijantimicag.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 20.Atkinson C., Tristram S. Antimicrobial resistance in cystic fibrosis isolates of Haemophilus influenzae. Br. J. Biomed. Sci. 2016;73:87–89. doi: 10.1080/09674845.2016.1165408. [DOI] [PubMed] [Google Scholar]
- 21.Clark S.E. Commensal bacteria in the upper respiratory tract regulate susceptibility to infection. Curr. Opin. Immunol. 2020;66:42–49. doi: 10.1016/j.coi.2020.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Latham R.D., Gell D.A., Fairbairn R.L., Lyons A.B., Shukla S.D., Cho K.Y., Jones D.A., Harkness N.M., Tristram S.G. An isolate of Haemophilus haemolyticus produces a bacteriocin-like substance that inhibits the growth of nontypeable Haemophilus influenzae. Int. J. Antimicrob. Agents. 2017;49:503–506. doi: 10.1016/j.ijantimicag.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 23.Atto B., Latham R., Kunde D., Gell D.A., Tristram S. In vitro anti-NTHi activity of haemophilin-producing strains of Haemophilus haemolyticus. Pathogens. 2020;9:243. doi: 10.3390/pathogens9040243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atto B., Kunde D., Gell D.A., Tristram S. Haemophilin-Producing Strains of Haemophilus haemolyticus Protect Respiratory Epithelia from NTHi Colonisation and Internalisation. Pathogens. 2021;10:29. doi: 10.3390/pathogens10010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang L., Xie J., Patel M., Bakhtyar A., Ehrlich G.D., Ahmed A., Earl J., Marrs C.F., Clemans D., Murphy T.F. Nontypeable Haemophilus influenzae genetic islands associated with chronic pulmonary infection. PLoS ONE. 2012;7:e44730. doi: 10.1371/journal.pone.0044730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shak J.R., Cremers A.J., Gritzfeld J.F., de Jonge M.I., Hermans P.W., Vidal J.E., Klugman K.P., Gordon S.B. Impact of experimental human pneumococcal carriage on nasopharyngeal bacterial densities in healthy adults. PLoS ONE. 2014;9:e98829. doi: 10.1371/journal.pone.0098829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenberg D., Broides A., Blancovich I., Peled N., Givon-Lavi N., Dagan R. Relative importance of nasopharyngeal versus oropharyngeal sampling for isolation of Streptococcus pneumoniae and Haemophilus influenzae from healthy and sick individuals varies with age. J. Clin. Microbiol. 2004;42:4604–4609. doi: 10.1128/JCM.42.10.4604-4609.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rawlings B.A., Higgins T.S., Han J.K. Bacterial pathogens in the nasopharynx, nasal cavity, and osteomeatal complex during wellness and viral infection. Am. J. Rhinol. Allergy. 2013;27:39–42. doi: 10.2500/ajra.2013.27.3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chi D.H., Hendley J.O., French P., Arango P., Hayden F.G., Winther B. Nasopharyngeal reservoir of bacterial otitis media and sinusitis pathogens in adults during wellness and viral respiratory illness. Am. J. Rhinol. 2003;17:209–214. doi: 10.1177/194589240301700406. [DOI] [PubMed] [Google Scholar]
- 30.Abdullahi O., Nyiro J., Lewa P., Slack M., Scott J.A.G. The descriptive epidemiology of Streptococcus pneumoniae and Haemophilus influenzae nasopharyngeal carriage in children and adults in Kilifi district, Kenya. Pediatr. Infect. Dis. J. 2008;27:59. doi: 10.1097/INF.0b013e31814da70c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Subramanya S.H., Thapa S., Dwedi S.K., Gokhale S., Sathian B., Nayak N., Bairy I. Streptococcus pneumoniae and Haemophilus species colonization in health care workers: The launch of invasive infections? BMC Res. Notes. 2016;9:66. doi: 10.1186/s13104-016-1877-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adegbola R.A., DeAntonio R., Hill P.C., Roca A., Usuf E., Hoet B., Greenwood B.M. Carriage of Streptococcus pneumoniae and other respiratory bacterial pathogens in low and lower-middle income countries: A systematic review and meta-analysis. PLoS ONE. 2014;9:e103293. doi: 10.1371/journal.pone.0103293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blain A., MacNeil J., Wang X., Bennett N., Farley M.M., Harrison L.H., Lexau C., Miller L., Nichols M., Petit S. Invasive Haemophilus influenzae disease in adults ≥65 years, United States, 2011. OFID. 2014;1 doi: 10.1093/ofid/ofu044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drayß M., Claus H., Hubert K., Thiel K., Berger A., Sing A., van der Linden M., Vogel U., Lâm T.-T. Asymptomatic carriage of Neisseria meningitidis, Haemophilus influenzae, Streptococcus pneumoniae, Group A Streptococcus and Staphylococcus aureus among adults aged 65 years and older. PLoS ONE. 2019;14:e0212052. doi: 10.1371/journal.pone.0212052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Price E.P., Harris T.M., Spargo J., Nosworthy E., Beissbarth J., Chang A.B., Smith-Vaughan H.C., Sarovich D.S. Simultaneous identification of Haemophilus influenzae and Haemophilus haemolyticus using real-time PCR. Future Microbiol. 2017;12:585–593. doi: 10.2217/fmb-2016-0215. [DOI] [PubMed] [Google Scholar]
- 36.Yang P., Zhang J., Peng A. The pharyngeal carriage of Haemophilus influenzae among healthy population in China: A systematic review and meta-analysis. BMC Infect. Dis. 2019;19:547. doi: 10.1186/s12879-019-4195-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dabernat H., Plisson-Sauné M.-A., Delmas C., Séguy M., Faucon G., Pélissier R., Carsenti H., Pradier C., Roussel-Delvallez M., Leroy J. Haemophilus influenzae carriage in children attending French day care centers: A molecular epidemiological study. J. Clin. Microbiol. 2003;41:1664–1672. doi: 10.1128/JCM.41.4.1664-1672.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hare K.M., Binks M.J., Grimwood K., Chang A.B., Leach A.J., Smith-Vaughan H. Culture and PCR detection of Haemophilus influenzae and Haemophilus haemolyticus in Australian Indigenous children with bronchiectasis. J. Clin. Microbiol. 2012;50:2444–2445. doi: 10.1128/JCM.00566-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pickering J., Richmond P.C., Kirkham L.-A.S. Molecular tools for differentiation of non-typeable Haemophilus influenzae from Haemophilus haemolyticus. Front. Microbiol. 2014;5:664. doi: 10.3389/fmicb.2014.00664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lieberman D., Shleyfer E., Castel H., Terry A., Harman-Boehm I., Delgado J., Peled N., Lieberman D. Nasopharyngeal versus oropharyngeal sampling for isolation of potential respiratory pathogens in adults. J. Clin. Microbiol. 2006;44:525–528. doi: 10.1128/JCM.44.2.525-528.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang J., Zhu Z., Zuo X., Pan H., Gu Y., Yuan Y., Wang G., Wang S., Zheng R., Liu Z. The role of NTHi colonization and infection in the pathogenesis of neutrophilic asthma. Respir. Res. 2020;21:1–12. doi: 10.1186/s12931-019-1261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pickering J., Smith-Vaughan H., Beissbarth J., Bowman J., Wiertsema S., Riley T., Leach A.J., Richmond P., Lehmann D., Kirkham L.-A. Diversity of nontypeable Haemophilus influenzae strains colonizing Australian Aboriginal and non-Aboriginal children. J. Clin. Microbiol. 2014;52:1352–1357. doi: 10.1128/JCM.03448-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Voss M., Wonnenberg B., Honecker A., Kamyschnikow A., Herr C., Bischoff M., Tschernig T., Bals R., Beisswenger C. Cigarette smoke-promoted acquisition of bacterial pathogens in the upper respiratory tract leads to enhanced inflammation in mice. Respir. Res. 2015;16:1–11. doi: 10.1186/s12931-015-0204-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Atto B., Eapen M.S., Sharma P., Frey U., Ammit A.J., Markos J., Chia C., Larby J., Haug G., Weber H.C. New therapeutic targets for the prevention of infectious acute exacerbations of COPD: Role of epithelial adhesion molecules and inflammatory pathways. Clin. Sci. 2019;133:1663–1703. doi: 10.1042/CS20181009. [DOI] [PubMed] [Google Scholar]
- 45.Sethi S., Wrona C., Grant B.J., Murphy T.F. Strain-specific immune response to Haemophilus influenzae in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2004;169:448–453. doi: 10.1164/rccm.200308-1181OC. [DOI] [PubMed] [Google Scholar]
- 46.Granland C.M., Scott N.M., Lauzon-Joset J.-F., Langereis J.D., De Gier C., Sutherland K.M., Clark S.L., Pickering J.L., Thornton R.B., Richmond P.C. Nasal delivery of a commensal Pasteurellaceae species inhibits nontypeable Haemophilus influenzae colonization and delays onset of otitis media in mice. Infect. Immun. 2020;88 doi: 10.1128/IAI.00685-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Boeck I., van den Broek M.F., Allonsius C.N., Spacova I., Wittouck S., Martens K., Wuyts S., Cauwenberghs E., Jokicevic K., Vandenheuvel D. Lactobacilli have a niche in the human nose. Cell Rep. 2020;31:107674. doi: 10.1016/j.celrep.2020.107674. [DOI] [PubMed] [Google Scholar]
- 48.Hols P., Ledesma-García L., Gabant P., Mignolet J. Mobilization of microbiota commensals and their bacteriocins for therapeutics. Trends Microbiol. 2019;27:690–702. doi: 10.1016/j.tim.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 49.Kaijalainen T., Ruokokoski E., Ukkonen P., Herva E. Survival of Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis frozen in skim milk-tryptone-glucose-glycerol medium. J. Clin. Microbiol. 2004;42:412–414. doi: 10.1128/JCM.42.1.412-414.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hare K.M., Smith-Vaughan H.C., Beissbarth J., Leach A.J. Haemophilus influenzae isolates survive for up to 20 years at −70 °C in skim milk tryptone glucose glycerol broth (STGGB) if thawing is avoided during re-culture. J. Microbiol. Methods. 2015;119:132–133. doi: 10.1016/j.mimet.2015.10.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are contained within the article or Supplementary Materials.