Abstract
Simple Summary
Since 2016, the fall armyworm, an important economic pest native to tropical and subtropical regions of the Western Hemisphere, has invaded Africa and further spread rapidly into most Asian countries. The fall armyworm is highly polyphagous, but two of its major strains, the corn and the rice strains, cause severe damage in the Western Hemisphere. However, the invaded populations in Africa and Asia mostly infested the corn fields. Studies on the genetic identity of the species using two molecular markers, one nuclear gene and one mitochondrial gene, showed that the major genetic group is a heterogeneous hybrid of males from the corn strain and females from the rice strain. Moreover, a minor group of homogenous individuals from the corn strain but no homogenous individuals from the rice strain were also detected. A geographic distribution analysis at the subpopulation level indicated similar genetic diversity in Africa and Asia, suggesting fall armyworm in Africa spread into Asia without significant genetic change.
Abstract
The fall armyworm, Spodoptera frugiperda, is an important agricultural pest native to tropical and subtropical regions of the Western Hemisphere, and has invaded Africa and further spread into most countries of Asia within two years. Here, we analyzed the genetic variation of invaded populations by comparing the nucleotide sequences of two genes: the nuclear Z-chromosome linked gene triose phosphate isomerase (Tpi) and the mitochondrial gene cytochrome oxidase subunit I (COI) of 27 specimens collected in Africa (DR Congo, Tanzania, Uganda, and Zimbabwe) and Asia (Bangladesh, Korea, Nepal, and Vietnam). The results revealed that 25 specimens were from a heterogeneous hybrid (Tpi-corn strain and COI-rice strain; Tpi-C/COI-R) of the corn strain male and rice strain female, but two specimens were from a homogenous corn strain (Tpi-corn strain and COI-corn strain; Tpi-C/COI-C). The further analysis of the fourth exon and the fourth intron sequences of the Tpi gene identified at least four subgroups of the corn strain. These four genetic subgroups were identified in Africa and Asia, suggesting no significant genetic change due to the rapid migration within two years. Our study provides essential information for understanding the genetic diversity of fall armyworm in new habitats.
Keywords: Spodoptera frugiperda, invasive pest, corn, invasion, COI, Tpi gene
1. Introduction
The fall armyworm (FAW) Spodoptera frugiperda (J. E. Smith, 1797) (Lepidoptera: Noctuidae) is an important agricultural pest native in tropical and subtropical regions of the Western Hemisphere [1]. Due to the lack of diapause mechanism, FAW cannot overwinter in the northern areas over Florida and Texas of the United States, but they can disperse across thousands of kilometers into the north in the growing season [2]. In 2016, its invasion into Western Africa was first reported and it rapidly spread into most Sub-Saharan Africa countries [3,4,5,6]. In 2018–2019, the invasion into India was firstly reported and further spread into most Asia-Pacific countries, including Korea, Japan, and Australia, within an year [7,8,9,10,11,12,13,14]. The enormous migratory power of the FAW is a severe threat to new habitats in Africa and Asia and poses as a significant concern related to the potential economic damage of crop plants [15,16,17].
The FAW is a polyphagous species, consuming at least 353 species of plants, and it is a significant pest of corn, rice, and forage grasses [18,19]. Pashley et al. [20] showed at least two host plant strains in the southeastern United States: one of them feeding on corn, cotton, and sorghum (corn strain, C-strain) and the other feeding on rice and various pasture grasses, preferentially (rice strain; R-strain) [18,21,22]. The two FAW strains are morphologically indistinguishable and are distributed in sympatric patterns [23]. Further studies identified their different genetic characteristics in mating behaviors and zygotic reproductive incompatibility [24,25], pheromone composition [26], and differential susceptibility in xenobiotics [27].
Molecular markers can be used to diagnose the genetic identity of each strain of FAW [28,29]. Polymorphic variation of mitochondrial cytochrome oxidase subunit I (COI) gene sequence was identified between C- and R- strains but was not always consistent with host plant preference [30,31]. For example, some populations collected from the cornfields possess an R-strain marker in the COI gene. The group of Nagoshi and collaborators developed another genetic marker using a nuclear triosephosphate isomerase (Tpi) gene linked with Z-chromosome [32,33]. Therefore, the Tpi gene is hemizygous in females (ZW), whereas in males it is either homozygous or heterozygous (ZZ) [32]. Two genotypes, Tpi-C and Tpi-R, were identified on different host plants in the Western Hemisphere [31,32]. The group of Nagoshi and collaborators found that significant corn field populations are a hybrid (Tpi-C/COI-R) that possesses a nuclear Tpi-C marker but a mitochondrial COI-R marker. This finding indicates that the host plant preference of the hybrid is associated with the nuclear Tpi marker rather than the mitochondrial COI marker [34]. Therefore, it suggests that the Tpi gene is a suitable molecular marker compared with the COI gene to identify the FAW genetic characteristics associated with the host plant preference of the species.
Here, we assessed the genetic variation of FAW specimens collected from eight African and Asian countries based on a Tpi gene and compared with their variation of COI gene markers. Moreover, we discussed the relationship between genetic diversity and the potential population dynamic of FAW populations that invaded the new African and Asian habitats.
2. Materials and Methods
2.1. Collection
The FAW larvae were collected from corn fields (Zea mays L.) in Gyeongsan, Gyeongbuk Province, and adult moths were caught using the sex pheromone traps (GreenAgrotech, Gyeongsan, Korea) in Jeju Island of Korea from 2019 to 2020. Other specimens were obtained as larvae and adults from corn plants at various locations in Africa (DR Congo, Tanzania, Uganda, and Zimbabwe) and Asia (Bangladesh, Korea, Nepal, and Vietnam) from October 2017 to August 2020 (Figure 1; Table 1). In the field, FAW was identified based on the morphological characteristics of larva and adults. Specimens were stored 70% ethanol. Then, the vials were stored at −20 °C until further analysis.
Figure 1.
Map showing the collection sites of Spodoptera frugiperda specimens in the different African and Asian countries.
Table 1.
Specimens’ details of Spodoptera frugiperda collected from different African and Asian countries. DR, Democratic Republic.
| Regions/ Countries |
Locations | Specimen Names | Collection Dates | Insect Stages | Accession Numbers | |
|---|---|---|---|---|---|---|
| Tpi | COI | |||||
| Africa | ||||||
| DR Congo | Katana, Kabare | Con-11 | 11/29/2018 | Larva | MT894220 | MT103350 |
| Miti, Kabare | Con-12 | 11/29/2018 | Larva | MT894221 | MT933052 | |
| Minova, Kalehe | Con-21 | 11/29/2018 | Larva | MT894222 | MT933053 | |
| Luvungi, Uvira | Con-31 | 12/15/2018 | Larva | MT894223 | MT933054 | |
| Sange, Uvira | Con-41 | 12/15/2018 | Larva | MT894224 | MT933055 | |
| Nduba, Walungu | Con-42 | 12/15/2018 | Larva | MT894225 | MT103349 | |
| Tanzania | Arusha, Tengeru | Tan-1 | 1/10/2019 | Larva | MT894226 | MT103348 |
| Mlali, Morogoro | Tan-2 | 1/17/2019 | Larva | MT894227 | MT933056 | |
| Sri, Pwani | Tan-3 | 1/10/2019 | Larva | MT894228 | MT933057 | |
| Sua, Morogoro | Tan-4 | 1/14/2019 | Larva | MT894229 | MT933058 | |
| Uganda | Mbale | Uga-1 | 1/10/2018 | Larva | MT894230 | MT933059 |
| Masindi | Uga-2 | 10/17/2017 | Larva | MT894231 | MT933060 | |
| Kole | Uga-3 | 10/18/2018 | Larva | MT894232 | MT933061 | |
| Luwero | Uga-4 | 10/15/2018 | Larva | MT894233 | MT933062 | |
| Zimbabwe | Harare research station, Harare | Zim-1 | 2/8/2019 | Larva | MT894234 | MT103346 |
| Chipinge, Manicaland | Zim-2 | 2/22/2019 | Larva | MT894235 | MT103347 | |
| Asia | ||||||
| Bangladesh | Dhaka | Ban-1 | 8/14/2019 | Larva | MT894236 | MT933063 |
| Korea | Jeju | Kor-1 | 9/19/2019 | Adult | MT894237 | MT933064 |
| Gyeongsan | Kor-2 | 8/29/2019 | Larva | MT894238 | MT103342 | |
| Gyeongsan | Kor-3 | 6/10/2020 | Larva | MT894239 | MT933065 | |
| Jeju | Kor-4 | 6/9/2020 | Adult | MT894240 | MT933066 | |
| Nepal | Bhakundebesi, Kavre | Nep-1 | 9/24/2019 | Larva | MT894241 | MT103345 |
| Khumaltar, Lalitpur | Nep-2 | 7/30/2019 | Larva | MT894242 | MT933067 | |
| Khaira, Pyathan | Nep-3 | 8/6/2019 | Larva | MT894243 | MT933068 | |
| Vietnam | Ninh Binh | Vie-1 | 9/30/2019 | Adult | MT894244 | MT103334 |
| Vinh Phuc | Vie-2 | 9/30/2019 | Adult | MT894245 | MT103335 | |
| Hanoi | Vie-3 | 9/30/2019 | Larva | MT894246 | MT103336 | |
2.2. DNA Preparation
Genomic DNA was extracted from a portion of each specimen and homogenized using the pure link genomic DNA mini kit (Invitrogen, Carlsbad, CA, USA). The specimens were placed in a 1.5 mL centrifuge tube containing 180 µL of digestion buffer and 20 µL of proteinase K (50 µg/mL) and then incubated at 55 °C for 4 h. The DNA samples were extracted and purified using genomic spin columns, as described in the kit. DNA concentration was determined using a NanoPhotometer™ (Implen GmbH, Schatzbogen, Germany).
2.3. Polymerase Chain Reaction (PCR) Amplification
PCR was performed in a total reaction volume of 30 µL, containing 15 µL SolgTM 2 × Taq PreMix (Solgent, Daejeon, Korea), 2 µL of each primer (10 pmol/µL), 3 μL of the DNA solution, and 8 μL distilled water. A partial sequence (444 bp) of the Tpi gene was amplified using the primer pair TPI412F (5′-CCGGACTGAAGGTTATCGCTTG-3′) and TPI1140R (5′-GCGGAAGCATTCGCTGACAACC-3′) [15], whereas the partial sequence (658 bp) of the COI gene was amplified using the primer pair LCO1490 (5′-GGTCAACAAATCATAAAGATATTGG-3′) and HCO2198 (5′-TAAACTTCAGGGTGACCAAAAAATCA-3′) [35]. The reaction mixtures were amplified under the following conditions: Tpi gene (initial denaturation at 94 °C for 1 min; followed by 33 cycles of 92 °C for 30 s, 56 °C for 45 s, and 72 °C for 45 s; and a final segment of 72 °C for 3 min], and mtCOI (initial denaturation at 92 °C for 5 min; followed by 35 cycles 92 °C for 60 s, 55 °C for 60 s, and 72 °C for 60 s; and a final extension at 72 °C for 5 min in SimpliAmp 96-Well Thermal Cycler [Applied Biosystems, Foster City, CA, USA]). The PCR products were separated using a 1% agarose gel electrophoresis, stained with ethidium bromide solution, and visualized under ultraviolet (UV) light. The amplified PCR products were excised from the gel and purified using the Wizard® PCR Preps DNA Purification System (Wizard® SV Gel, Promega Co., Madison, WI, USA).
2.4. DNA Sequence Analysis
The purified DNA was sequenced using the BigDye® Terminator Cycle Sequencing Kit and ABI Prism 3730XL DNA Analyzer (50 cm capillary) (DNA Sequencer) (Applied Biosystems, Foster City, CA, USA) at the Solgent Sequencing Facility (Solgent Co., Daejeon, Korea). The GenBank database in the National Center for Biotechnology Information (NCBI) was searched using the BLAST algorithm [36], and the nucleotide sequences were aligned using CLUSTAL W [37].
2.5. Phylogenetic Analysis
A phylogenetic tree for the COI gene was constructed using the maximum likelihood method implemented in MEGA 6.0 software [38] with reference sequences obtained in the GenBank. We used 1000 bootstrap replicates to test the robustness of each of the phylogeny with the Hasegawa–Kishnio–Yano (HKY850) model and gamma distribution rate of variation among sites [39].
2.6. Characterization of the Tpi and COI Gene Segments
Single nucleotide substitutions of Tpi and COI genes were used for strain diagnostic markers. The Tpi gene was designated by a “g” (genomic), whereas the COI gene was designated by an “m” (mitochondria followed by gene name, base pairs number from the predicted translational start site). In both Tpi and COI genes, we aligned our FAW specimen sequences with the previously identified NCBI sequences of C-strain and R-strain FAW in CLUSTAL W to find polymorphic nucleotides to identify both the C and R strains. Nagoshi et al. [15] reported various polymorphic nucleotides in the exon (Tpi-E4) and intron region (Tpi-I4) of the Tpi gene to identify the C- and R-strains. The gTpi183 (C for C-strain, T for R-strain) is used to identify C- and R-strains [15]. Both gTpi192 and gTpi198 were used to identify subgroups (Tpi-Ca1, Tpi-Ca2, and Tpi-Ca1/Ca2) of C-strain. In addition, various polymorphic nucleotides in Tpi-I4 were used to identify genetic variation of FAW.
2.7. Genetic Analyses
Genetic parameters, such as the number of segregating sites, haplotype numbers, haplotype diversity, nucleotide diversity, theta/site, and Tajima’s D [40], were analyzed using the DnaSP software v.5.10 [41,42]. The TCS software v.1.21 was used to generate the haplotype network [43]. We excluded the Kor-1 specimen, the hybrid of Tpi-Ca1/Ca2, from all genetic analyses with the Tpi gene.
3. Results
3.1. Analysis of the Tpi Gene Sequence
The partial nucleotide sequence (444 bp), including 166 bp of the fourth exon (Tpi-E4) and 278 bp of the fourth intron (Tpi-I4) region of the Tpi gene, was determined from the 27 specimens of the FAW specimens collected from eight different African and Asian countries. Single nucleotide polymorphism (SNP) characteristics of the Tpi gene were analyzed separately in Tpi-E4 and Tpi-I4 regions (Figure 2).
Figure 2.
Polymorphic sites of the Tpi gene segments used for strain identification and haplotype diagnosis of Spodoptera frugiperda collected from different African and Asian countries.
In the Tpi-E4 region, the gTpi183 of all 27 specimens was C, but not T, which indicated that all of them were the corn strain. Furthermore, nucleotides of both the gTpi192 and the gTpi198 consisted of three different types, such as C and C, T and T, and Y (C/T) and Y (C/T), which indicates the three subgroups of corn strain, Tpi-Ca1, Tpi-Ca2, and Tpi-Ca1/Ca2, respectively (Figure 2). The heterozygote Tpi-Ca1/Ca2 specimen (Kor-1) was identified only from Jeju, Korea, in 2019.
In the Tpi-I4 region, among 17 polymorphic nucleotides, we found three different polymorphic sequences of Tpi-C in our samples but did not identified Tpi-R sequences reported by Nagoshi et al. [15]. Ten polymorphic nucleotides (31, 38, 53, 55, 58, 70, 77, 87, 96, and 148) were identified between Tpi-Ca1 and Tpi-Ca2 subgroups. Among them, the nucleotide 148 was distinct polymorphic nucleotide between Tpi-Ca2a and Tpi-Ca2b. In addition, nucleotide variation within subgroup was identified in some specimens of Tpi-Ca1 and Tpi-Ca2b but was not detected in Tpi-Ca2a. For example, in Tpi-Ca1 subgroup, Kor-1 and Tan-1 specimens were substituted the nucleotides 70 and 96 into “C” but Con-11 specimen was substituted only the nucleotide 96 into “C”. In Tpi-Ca2b, three types of variation in the nucleotides 87 and 96 were identified, for example, T and T, T and C, and C and T, respectively. Moreover, in the Tpi-Ca1/Ca2 heterozygote specimen, all ten polymorphic nucleotides were heterozygous into S (C/G), M (C/A), W (A/T), and Y (C/T). Therefore, our 27 specimens were classified into four subgroups as Tpi-Ca1a, Tpi-Ca2a, Tpi-Ca2b, and Tpi-Ca1/Tpi-Ca2.
The SNP pattern of the Tpi gene was analyzed according to geographic distribution (Figure 2). The results showed that each subgroup was distributed in both Africa and Asia. For example, the Tpi-Ca1a subgroup was identified in DR Congo (Con-11, 42), Tanzania (Tan-1, 3), Uganda (Uga-1, 4), and Zimbabwe (Zim-1, 2) in Africa as well as in Nepal (Nep-1, 2, 3), Vietnam (Vie-1, 3), and Korea (Kor-2, 4) in Asia. Tpi-Ca2a was identified in DR Congo (Con-21, 31) and Uganda (Uga-3) in Africa, as well as Vietnam (Vie-2) and Korea (Kor-3) in Asia, whereas Tpi-Ca2b was identified from DR Congo (Con-12, 41), Tanzania (Tan-2, 4) and Uganda (Uga-2) in Africa, as well as Bangladesh (Ban-1) in Asia. The Tpi-Ca1/Tpi-Ca2 was identified only in Korea (Kor-1). The results showed that each subgroup was widely distributed in both continents in a mixed pattern.
3.2. Analysis of the COI Gene Sequence
The partial sequence (658 bp) of the COI gene was determined and phylogenetic relationship was compared with those of previously known sequences from the GenBank database using the maximum likelihood phylogenetic tree (Figure 3). The result showed that 92.6% (25/27) were clustered to the COI-rice strain, whereas only 7.4% (Tan-3, Vie-3; 2/27) specimens were clustered to the COI-corn strain of FAW. All the specimens were collected from the cornfields. All the COI-rice strain sequences (25 specimens) were 100% identical but 98.33–98.48% similar with two sequences of the COI-corn strain (two specimens). Two COI-corn strain specimens were 99.85% identical (Table A1 and Table A3 in Appendix A).
Figure 3.
The maximum likelihood phylogenetic tree of the COI sequences of Spodoptera frugiperda collected from the different African and Asian countries. The color indicates the COI sequences from collected samples in this study, and the others are reference sequences obtained from the GenBank database. Hasegawa-Kishnio-Yano HKY850 model and gamma distribution rate of variation among sites were implemented to construct the phylogenetic tree in MEGA6.
The SNP analysis from the COI gene fragment alignment showed that ten nucleotides (mCOI72, mCOI117, mCOI171, mCOI207, mCOI258, mCOI564, mCOI570, mCOI600, mCOI634, and mCOI663) were different between the corn and the rice strains. Based on this comparison, the specimens Tan-3 and Vie-3 belong to the C-strain, and the remaining specimens belong to the R-strain (Figure 4).
Figure 4.
Individual nucleotide differences of the COI gene in the corn and the rice strains of Spodoptera frugiperda. We used 658 bp from 39 to 696 positions of 1,531 bp of S. frugiperda COI gene sequence (MN599981, Korea) from NCBI.
3.3. Genetic Diversity of Tpi and COI Genes of FAW
The nucleotide sequence variation of the Tpi gene was slightly higher in the African specimens (0.23–3.15%) than the Asian specimens (0.23–2.93%), and its variation between Africa and Asia was 0.23–3.38% (Table A2). The numbers of segregating sites, haplotype numbers, haplotype diversity, and nucleotide diversity were higher in Africa than in Asia (Table 2). The nucleotide sequence variation of the COI gene was higher in the Asian specimens (1.67%) than in the African ones (1.52%), and its variation between Africa and Asia was 0.15–1.67% (Table A3). The numbers of segregating sites, haplotype diversity, and nucleotide diversity were almost similar between African and Asian specimens (Table 2).
Table 2.
Genetic variability analysis of Tpi and COI gene of Spodoptera frugiperda in Africa and Asia.
| Genes | Regions | Number of Sequences | Segregating Sites | Haplotypes | Haplotype Diversity |
Nucleotide Diversity |
Theta/Site | Tajima’s D |
|---|---|---|---|---|---|---|---|---|
| Tpi | Africa | 16 | 18 | 10 | 0.933 | 0.016911 | 0.012 | 1.531362 |
| Asia | 10 | 14 | 5 | 0.667 | 0.011671 | 0.011 | 1.11681 | |
| COI | Africa | 16 | 10 | 2 | 0.125 | 0.0019 | 0.005 | −2.182611 ** |
| Asia | 11 | 11 | 2 | 0.182 | 0.00304 | 0.006 | −2.011459 * |
* p < 0.05, ** p < 0.01.
The population genetic study of FAW in Africa and Asia was assessed by Tajima’s neutrality test for the Tpi and the COI genes (Table 2). The results showed that Tajima’s D was positive and non-significant for the Tpi gene in both regions, whereas Tajima’s D is negative but significant for the COI gene in both Africa and Asia regions suggesting the recent population expansion.
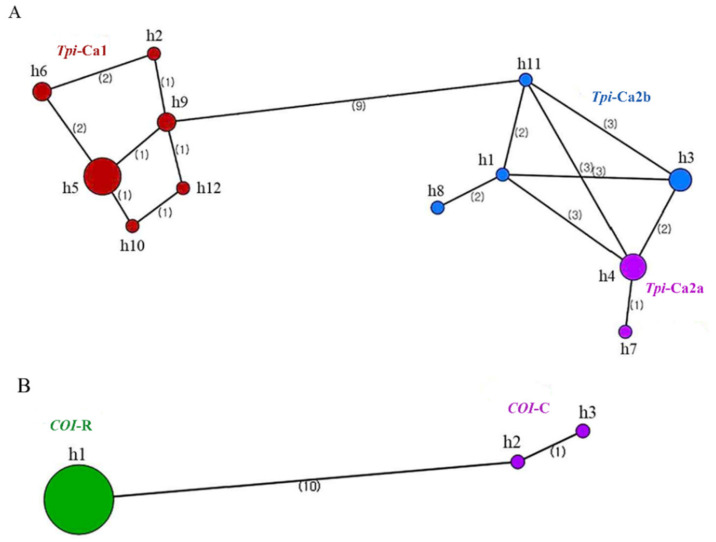
The evolutionary relationship of both Tpi and COI gene haplotypes from FAW was assessed using the minimum spanning network. In the Tpi gene, 12 haplotypes were identified and separated into two distinct groups, Tpi-Ca1 and Tpi-Ca2, by nine mutational steps (Figure 5A). The Tpi-Ca1 consisted of six haplotypes. Among Tpi-Ca2, two haplotypes (h4 and h7) belong to the subgroup Tpi-Ca2a, whereas four haplotypes (h1, h3, h8, and h11) belong to the subgroup Tpi-Ca2b (Table 3). Some identical haplotypes were identified in both Africa and Asia. For example, the h5, which is the most frequent haplotype, was found in two African (Con-42 and Zim-1) and six Asian specimens (Kor-4, Nep-1, Nep-2, Nep-3, Vie-1, and Vie-3). The h6 haplotype was found in one African (Tan-1) and one Asian specimen (Kor-1). The h4 haplotype was found in three African (Con-21, Con-31, and Uga-3) and one Asian specimen (Vie-2).
Figure 5.
Minimum spanning network of the Tpi gene (A) and the COI gene (B) haplotypes of Spodoptera frugiperda from different African and Asian countries.
Table 3.
Specimens and haplotypes of Spodoptera frugiperda Tpi gene.
| Sn | Speamens | Haplotypes | Strains |
|---|---|---|---|
| 1 | Con-11 | h2 | Tpi-Ca1 |
| 2 | Con-42, Kor-4, Nep-1, Nep-2, Nep-3, Vie-1, Vie-3, Zim-1 | h5 | |
| 3 | Kor-2, Tan-1 | h6 | |
| 4 | Tan-3, Zim-2 | h9 | |
| 5 | Uga-1 | h10 | |
| 6 | Uga-4 | h12 | |
| 7 | Con-21, Con-31, Uga-3, Vie-2 | h4 | Tpi-Ca2a |
| 8 | Kor-3 | h7 | |
| 9 | Ban-1 | h1 | Tpi-Ca2b |
| 10 | Con-12, Con-14, Tan-4 | h3 | |
| 11 | Tan-2 | h8 | |
| 12 | Uga-2 | h11 |
Only three haplotypes were identified in the COI gene, and h1 was differed by ten mutational steps with h2 and h3 (Figure 5B). The h1 haplotype contained 25 specimens from Africa and Asia and belonged to COI-R, whereas h2 and h3 haplotypes had a single specimen, Vie-3, and Tan-3, respectively. Both of these haplotypes belonged to the COI-C (Table 4). The haplotype analysis of both Tpi and COI genes indicated that FAW populations invaded in Africa and Asia are genetically diverse at a similar rate.
Table 4.
Specimens and haplotypes of Spodoptera frugiperda COI gene.
| Sn | Speamens | Haplotypes | Strains |
|---|---|---|---|
| 1 | Ban-1, Kor-1, Kor-2, Kor-3, Kor-4, Nep-1, Nep-2, Nep-3, Vie-1, Vie-2, Con-11, Con-12, Con-21, Con-31, Con-41, Con-42, Tan-1, Tan-2, Tan-4, Uga-1, Uga-2, Uga-3, Uga-4, Zim-1, Zim-2 | h1 | COI-R |
| 2 | Vie-3 | h2 | COI-C |
| 3 | Tan-3 | h3 |
4. Discussion
In this study, FAW collected from cornfields of eight African and Asian countries were genetically characterized using molecular markers of both the Tpi and the COI genes. Our Tpi gene analysis showed that all the specimens had the Tpi-C genotype, whereas the COI gene analysis showed that 92.6% had the COI-R and 7.4% had the COI-C genotypes. Therefore, the hybrid (Tpi-C/COI-R) was predominant, but the homogenous corn strain (Tpi-C/COI-C) was a minor genetic group in our survey. This result is similar to previous studies wherein the Tpi gene is a predictable molecular marker compared with the COI gene for the diagnosis of the FAW strain associated with host plant preference [15,16,17,44]. Another study in Myanmar and Southern China indicated that most of the strain is hybrid (Tpi-C/COI-R) [45]. It is worth investigating the nuclear Tpi gene, a more reliable host strain marker compared with the mitochondrial COI marker in invaded populations in Africa and Asia, to prevent further uncertainty on host plant preference analysis.
The genetic variation of both the Tpi and the COI genes showed that Tpi is more diverse compared with COI. Moreover, those values were higher for the African specimens than for the Asian specimens. Our data indicated the African populations of FAW are more diversified compared with the Asian ones, especially in the nuclear Tpi gene. Nagoshi et al. [6] compared the frequency of both the Tpi and the COI haplotype combination in the Western Hemisphere and Africa. The homogeneous corn strain (Tpi-C/COI-C) is predominant in the Western Hemisphere and Western Africa. Both Tpi-C/COI-C and a hybrid strain (Tpi-C/COI-R) are similarly distributed in Central Africa, but a hybrid strain predominates in Eastern Africa. Further studies indicated that the hybrid strain predominates in South Africa and India [15,44]. The rice strain (Tpi-R/COI-R) is found in the Western Hemisphere, but it is rare in Africa [34]. Our data is consistent with previous studies, suggesting that the hybrid strain is predominantly distributed in Africa and Asia while spreading into the east of continents.
Polymorphism of the fourth exon and intron region of the Tpi gene is useful for the subgroup identification of FAW [15,44]. Our analysis showed four subgroups of corn strain, such as Tpi-Ca1a, Tpi-Ca2a, Tpi-Ca2b, and Tpi-Ca1/Tpi-Ca2. Similar profiles are shown in Africa and India, which showed that Tpi-Ca1a is the major group, and other subgroups, such as Tpi-Ca2a and Tpi-Ca2b, are a minor group [15,44]. We found a hybrid (Tpi-Ca1/Tpi-Ca2) of two subgroups only in one region, Jeju, which is an island located in the southern region of Korea. However, this hybrid was already identified in India at a high frequency [44]. This finding indicates the great potential of further invasion of hybrid strain from India into other Asian countries.
The FAW is a highly polyphagous species that feeds on at least 353 species of plants worldwide [19]. However, the FAW that invaded Africa and Asia mostly prefer corns but not rice and other host plants in the fields, although their major genotype is a hybrid, possessing the nuclear corn strain Tpi gene and mitochondrial rice strain COI gene [6]. The genetic characteristic of their corn preference is highly associated with the genetic marker of the Tpi gene compared with the COI gene. Besides, this host plant preference phenotype is not discriminated in the subgroup level, Tpi-Ca1, and Tpi-Ca2. There are no studies on the relationship between Tpi genotype and phenotypic host plant preference. The Tpi gene product acts as an essential metabolic enzyme in glycolysis, which catalyzes the reversible reaction of the triose phosphate isomers, dihydroxyacetone phosphate, and D-glyceraldehyde 3-phosphate in the cytosol [46]. The Tpi C-strain of FAW may have adaptative mechanisms on the feeding, digestion, and metabolic efficiency of corn plants. It is interesting to study the relationship between the genetic mutation of the Tpi gene and metabolic adaptation related to host plant preference.
5. Conclusions
In conclusion, the genetic characterization of the Tpi and the COI genes of African and Asian specimens showed that the Tpi gene is a more suitable molecular marker of host plant preference phenotype compared with the COI gene. From 2016 to 2020, at least four genetic subgroups of the Tpi-corn strain were geographically distributed in Africa and Asia in a similar profile, indicating the limited genetic variation of invaded FAW populations. However, we do not exclude that invaded FAW populations have a great potential to develop genetic adaptations to new environments.
Acknowledgments
The authors would like to thank Enago (www.enago.co.kr, accessed on 14 March 2021) for the English language review.
Appendix A
Table A1.
Accession numbers of Tpi and COI gene sequences of Spodoptera frugiperda from different countries of Africa and Asia their identity searched in the GenBank database.
| Specimens | Highest Sequence Identity with GenBank Database | |||||
|---|---|---|---|---|---|---|
| Tpi | COI | |||||
| % Identity | Accession Numbers |
Countries | % Identity | Accession Numbers |
Countries | |
| Con-11 | 99.77 | KT336237 | USA | 100 | MT605970 | India |
| Con-12 | 99.54 | KT336239 | USA | 100 | MT605970 | India |
| Con-21 | 100 | KT336239 | USA | 100 | MT605970 | India |
| Con-31 | 100 | KT336239 | USA | 100 | MT605970 | India |
| Con-41 | 99.54 | KT336239 | USA | 100 | MT605970 | India |
| Con-42 | 100 | KT336236 | USA | 100 | MT605970 | India |
| Tan-1 | 99.54 | KT336236 | USA | 100 | MT605970 | India |
| Tan-2 | 99.86 | KT336239 | USA | 100 | MT605970 | India |
| Tan-3 | 100 | KT336237 | USA | 99.85 | MN541574 | India |
| Tan-4 | 99.54 | KT336239 | USA | 100 | MT605970 | India |
| Uga-1 | 99.77 | KT336236 | USA | 100 | MT605970 | India |
| Uga-2 | 99.54 | KT336229 | USA | 100 | MT605970 | India |
| Uga-3 | 100 | KT336239 | USA | 100 | MT605970 | India |
| Uga-4 | 99.77 | KT336237 | USA | 100 | MT605970 | India |
| Zim-1 | 100 | KT336236 | USA | 100 | MT605970 | India |
| Zim-2 | 100 | KT336237 | USA | 100 | MT605970 | India |
| Ban-1 | 100 | KT336229 | USA | 100 | MT605970 | India |
| Kor-1 | 97.3 | FO681385 | France | 100 | MT605970 | India |
| Kor-2 | 99.54 | KT336236 | USA | 100 | MT605970 | India |
| Kor-3 | 99.77 | KT336239 | USA | 100 | MT605970 | India |
| Kor-4 | 100 | KT336236 | USA | 100 | MT605970 | India |
| Nep-1 | 100 | KT336236 | USA | 100 | MT605970 | India |
| Nep-2 | 100 | KT336236 | USA | 100 | MT605970 | India |
| Nep-3 | 100 | KT336236 | USA | 100 | MT605970 | India |
| Vie-1 | 100 | KT336236 | USA | 100 | MT605970 | India |
| Vie-2 | 100 | KT336239 | USA | 100 | MT605970 | India |
| Vie-3 | 100 | KT336236 | USA | 100 | MN541574 | India |
Table A2.
Percentage identity matrix of Spodoptera frugiperda Tpi gene analysis from different African and Asian countries.
| SN | Specimens | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Ban-1 | ||||||||||||||||||||||||||
| 2 | Kor-1 | 97.07 | |||||||||||||||||||||||||
| 3 | Kor-2 | 97.30 | 97.07 | ||||||||||||||||||||||||
| 4 | Kor-3 | 99.10 | 97.30 | 97.30 | |||||||||||||||||||||||
| 5 | Kor-4 | 97.30 | 97.07 | 99.55 | 97.30 | ||||||||||||||||||||||
| 6 | Nep-1 | 97.30 | 97.07 | 99.55 | 97.30 | 100.00 | |||||||||||||||||||||
| 7 | Nep-2 | 97.30 | 97.07 | 99.55 | 97.30 | 100.00 | 100.00 | ||||||||||||||||||||
| 8 | Nep-3 | 97.30 | 97.07 | 99.55 | 97.30 | 100.00 | 100.00 | 100.00 | |||||||||||||||||||
| 9 | Vie-1 | 97.30 | 97.07 | 99.55 | 97.30 | 100.00 | 100.00 | 100.00 | 100.00 | ||||||||||||||||||
| 10 | Vie-2 | 99.32 | 97.30 | 97.52 | 99.77 | 97.07 | 97.07 | 97.07 | 97.07 | 97.07 | |||||||||||||||||
| 11 | Vie-3 | 97.30 | 97.07 | 99.55 | 97.30 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 97.07 | ||||||||||||||||
| 12 | Con-11 | 97.30 | 97.30 | 99.55 | 97.75 | 99.55 | 99.55 | 99.55 | 99.55 | 99.55 | 97.52 | 99.55 | |||||||||||||||
| 13 | Con-12 | 99.32 | 97.07 | 97.52 | 99.32 | 97.07 | 97.07 | 97.07 | 97.07 | 97.07 | 99.55 | 97.07 | 97.52 | ||||||||||||||
| 14 | Con-21 | 99.32 | 97.30 | 97.52 | 99.77 | 97.07 | 97.07 | 97.07 | 97.07 | 97.07 | 100.00 | 97.07 | 97.52 | 99.55 | |||||||||||||
| 15 | Con-31 | 99.32 | 97.30 | 97.52 | 99.77 | 97.07 | 97.07 | 97.07 | 97.07 | 97.07 | 100.00 | 97.07 | 97.52 | 99.55 | 100.00 | ||||||||||||
| 16 | Con-41 | 99.32 | 97.07 | 97.52 | 99.32 | 97.07 | 97.07 | 97.07 | 97.07 | 97.07 | 99.55 | 97.07 | 97.52 | 100.00 | 99.55 | 99.55 | |||||||||||
| 17 | Con-42 | 97.30 | 97.07 | 99.55 | 97.30 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 97.07 | 100.00 | 99.55 | 97.07 | 97.07 | 97.07 | 97.07 | ||||||||||
| 18 | Tan-1 | 97.30 | 97.07 | 100.00 | 97.30 | 99.55 | 99.55 | 99.55 | 99.55 | 99.55 | 97.52 | 99.55 | 99.55 | 97.52 | 97.52 | 97.52 | 97.52 | 99.55 | |||||||||
| 19 | Tan-2 | 99.55 | 96.62 | 96.85 | 98.65 | 96.85 | 96.85 | 96.85 | 96.85 | 96.85 | 98.87 | 96.85 | 96.85 | 98.87 | 98.87 | 98.87 | 98.87 | 96.85 | 96.85 | ||||||||
| 20 | Tan-3 | 97.52 | 97.30 | 99.32 | 97.52 | 99.77 | 99.77 | 99.77 | 99.77 | 99.77 | 97.30 | 99.77 | 99.77 | 97.30 | 97.30 | 97.30 | 97.30 | 99.77 | 99.32 | 97.07 | |||||||
| 21 | Tan-4 | 99.32 | 97.07 | 97.52 | 99.32 | 97.07 | 97.07 | 97.07 | 97.07 | 97.07 | 99.55 | 97.07 | 97.52 | 100.00 | 99.55 | 99.55 | 100.00 | 97.07 | 97.52 | 98.87 | 97.30 | ||||||
| 22 | Uga-1 | 97.07 | 96.85 | 99.32 | 97.07 | 99.77 | 99.77 | 99.77 | 99.77 | 99.77 | 96.85 | 99.77 | 99.32 | 96.85 | 96.85 | 96.85 | 96.85 | 99.77 | 99.32 | 96.62 | 99.55 | 96.85 | |||||
| 23 | Uga-2 | 99.55 | 97.30 | 97.75 | 99.10 | 97.75 | 97.75 | 97.75 | 97.75 | 97.75 | 99.32 | 97.75 | 97.75 | 99.32 | 99.32 | 99.32 | 99.32 | 97.75 | 97.75 | 99.10 | 97.97 | 99.32 | 97.52 | ||||
| 24 | Uga-3 | 99.32 | 97.30 | 97.52 | 99.77 | 97.07 | 97.07 | 97.07 | 97.07 | 97.07 | 100.00 | 97.07 | 97.52 | 99.55 | 100.00 | 100.00 | 99.55 | 97.07 | 97.52 | 98.87 | 97.30 | 99.55 | 96.85 | 99.32 | |||
| 25 | Uga-4 | 97.30 | 97.07 | 99.10 | 97.30 | 99.55 | 99.55 | 99.55 | 99.55 | 99.55 | 97.07 | 99.55 | 99.55 | 97.07 | 97.07 | 97.07 | 97.07 | 99.55 | 99.10 | 96.85 | 99.77 | 97.07 | 99.77 | 97.75 | 97.07 | ||
| 26 | Zim-1 | 97.30 | 97.07 | 99.55 | 97.30 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 97.07 | 100.00 | 99.55 | 97.07 | 97.07 | 97.07 | 97.07 | 100.00 | 99.55 | 96.85 | 99.77 | 97.07 | 99.77 | 97.75 | 97.07 | 99.55 | |
| 27 | Zim-2 | 97.52 | 97.30 | 99.32 | 97.52 | 99.77 | 99.77 | 99.77 | 99.77 | 99.77 | 97.30 | 99.77 | 99.77 | 97.30 | 97.30 | 97.30 | 97.30 | 99.77 | 99.32 | 97.07 | 100.00 | 97.30 | 99.55 | 97.97 | 97.30 | 99.77 | 99.77 |
Table A3.
Percentage identity matrix of Spodoptera frugiperda COI gene analysis from different African and Asian countries.
| SN | Specimens | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Ban-1 | ||||||||||||||||||||||||||
| 2 | Kor-1 | 100 | |||||||||||||||||||||||||
| 3 | Kor-2 | 100 | 100 | ||||||||||||||||||||||||
| 4 | Kor-3 | 100 | 100 | 100 | |||||||||||||||||||||||
| 5 | Kor-4 | 100 | 100 | 100 | 100 | ||||||||||||||||||||||
| 6 | Nep-1 | 100 | 100 | 100 | 100 | 100 | |||||||||||||||||||||
| 7 | Nep-2 | 100 | 100 | 100 | 100 | 100 | 100 | ||||||||||||||||||||
| 8 | Nep-3 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | |||||||||||||||||||
| 9 | Vie-1 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | ||||||||||||||||||
| 10 | Vie-2 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | |||||||||||||||||
| 11 | Vie-3 | 98.33 | 98.33 | 98.33 | 98.33 | 98.33 | 98.33 | 98.33 | 98.33 | 98.33 | 98.33 | ||||||||||||||||
| 12 | Con-11 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 98.33 | |||||||||||||||
| 13 | Con-12 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 98.33 | 100 | ||||||||||||||
| 14 | Con-21 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 98.33 | 100 | 100 | |||||||||||||
| 15 | Con-31 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 98.33 | 100 | 100 | 100 | ||||||||||||
| 16 | Con-41 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 98.33 | 100 | 100 | 100 | 100 | |||||||||||
| 17 | Con-42 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 98.33 | 100 | 100 | 100 | 100 | 100 | ||||||||||
| 18 | Tan-1 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 98.33 | 100 | 100 | 100 | 100 | 100 | 100 | |||||||||
| 19 | Tan-2 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 98.33 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | ||||||||
| 20 | Tan-3 | 98.48 | 98.48 | 98.48 | 98.48 | 98.48 | 98.48 | 98.48 | 98.48 | 98.48 | 98.48 | 99.85 | 98.48 | 98.48 | 98.48 | 98.48 | 98.48 | 98.48 | 98.48 | 98.48 | |||||||
| 21 | Tan-4 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 98.33 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 98.48 | ||||||
| 22 | Uga-1 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 98.33 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 98.48 | 100 | |||||
| 23 | Uga-2 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 98.33 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 98.48 | 100 | 100 | ||||
| 24 | Uga-3 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 98.33 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 98.48 | 100 | 100 | 100 | |||
| 25 | Uga-4 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 98.33 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 98.48 | 100 | 100 | 100 | 100 | ||
| 26 | Zim-1 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 98.33 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 98.48 | 100 | 100 | 100 | 100 | 100 | |
| 27 | Zim-2 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 98.33 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 98.48 | 100 | 100 | 100 | 100 | 100 | 100 |
Author Contributions
Conceptualization, R.A., A.A.A., I.K., M.N., S.-J.L., S.-M.K., and K.-Y.L.; methodology, R.A., A.A.A., M.J.M., P.K., K.B.N., M.K.M., Y.K.S., J.S.M.H., T.X.H., H.T.D., J.-H.P., H.-S.H., and K.-Y.L.; formal analysis, R.A.; investigation, Y.K.S., and K.-Y.L.; resources, M.J.M., P.K., K.B.N., M.K.M., Y.K.S., J.S.M.H., T.X.H., H.T.D., J.-H.P., H.-S.H., and K.-Y.L.; writing—original draft preparation, R.A.; writing—review and editing, R.A., I.K., M.N., S.-J.L., S.-M.K., and K.-Y.L.; supervision, K.-Y.L.; project administration, I.K., M.N., S.-J.L., S.-M.K., and K.-Y.L.; funding acquisition, K.-Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Research Program for Exportation Support of Agricultural Products, Animal, and Plant Quarantine Agency, in the Republic of Korea (Grant #Z-1543086-2017-21-01).
Data Availability Statement
The genetic data presented in this study are publicly available on GenBank, and the accession numbers are reported in Table 1.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sparks A.N. A review of the biology of the fall armyworm. Fla. Entomol. 1979;62:82–87. doi: 10.2307/3494083. [DOI] [Google Scholar]
- 2.Luginbill P. The fall armyworm. Us Dept. Agric. Tech. Bull. 1928;34:1–91. [Google Scholar]
- 3.Goergen G., Kumar P.L., Sankung S.B., Togola A., Tamò M. First report of outbreaks of the fall armyworm Spodoptera frugiperda (J E Smith) (Lepidoptera, Noctuidae), a new alien invasive pest in West and Central Africa. PLoS ONE. 2016;11:e0165632. doi: 10.1371/journal.pone.0165632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cock M.J.W., Beseh P.K., Buddie A.G., Cafá G., Crozier J. Molecular methods to detect Spodoptera frugiperda in Ghana, and implications for monitoring the spread of invasive species in developing countries. Sci. Rep. 2017;7:4103. doi: 10.1038/s41598-017-04238-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobs A., Van Vuuren A., Rong I.H. Characterisation of the fall armyworm (Spodoptera frugiperda J.E. Smith) (Lepidoptera: Noctuidae) from South Africa. Afr. Entomol. 2018;26:45–49. doi: 10.4001/003.026.0045. [DOI] [Google Scholar]
- 6.Nagoshi R.N., Goergen G., Tounou K.A., Agboka K., Koffi D., Meagher R.L. Analysis of strain distribution, migratory potential, and invasion history of fall armyworm populations in northern Sub-Saharan Africa. Sci. Rep. 2018;8:3710. doi: 10.1038/s41598-018-21954-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ganiger P.C., Yeshwanth H.M., Muralimohan K., Vinay N., Kumar A.R.V., Chandrashekara K. Occurrence of the new invasive pest, fall armyworm, Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae), in the maize fields of Karnataka, India. Curr. Sci. 2018;115:621–623. doi: 10.18520/cs/v115/i4/621-623. [DOI] [Google Scholar]
- 8.Sharanabasappa, Chandrashekara K., Kalleshwaraswamy C.M., Asokan R., Maruthi M.S., Pavithra H.B., Hegbe K., Navi S., Prabhu S.T., Goergen G.E. First report of the fall armyworm, Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae), an alien invasive pest on maize in India. Pest Manag. Hortic. Ecosyst. 2018;24:23–29. [Google Scholar]
- 9.Shylesha A.N., Jalali S.K., Gupta A., Varshney R., Venkatesan T., Shetty P., Ojha R., Ganiger P.C., Navik O., Subaharan K., et al. Studies on new invasive pest Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae) and its natural enemies. J. Biol. Control. 2018;32:145–151. doi: 10.18311/jbc/2018/21707. [DOI] [Google Scholar]
- 10.Swamy H.M.M., Asokan R., Kalleshwaraswamy C.M., Sharanabasappa, Prasad Y.G., Maruthi M.S., Shashank P.R., Devi N.I., Surakasula A., Adarsha S., et al. Prevalence of “R” strain and molecular diversity of fall army worm Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) in India. Indian J. Entomol. 2018;80:544–553. doi: 10.5958/0974-8172.2018.00239.0. [DOI] [Google Scholar]
- 11.Lee G., Seo B.Y., Lee J., Kim H., Song J.H., Lee W. First report of the fall armyworm, Spodoptera frugiperda (Smith, 1797) (Lepidoptera: Noctuidae), a new migratory pest in Korea. Korean J. Appl. Entomol. 2020;59:73–78. doi: 10.5656/KSAE.2020.02.0.006. [DOI] [Google Scholar]
- 12.Vennila S., Wang Z., Young K., Khurana J., Cruz I., Chen J., Reynaud B., Delatte H., Baufeld P., Rajan R.P., et al. G20 discussion group on fall armyworm Spodoptera frugiperda (J.E.Smith) [Lepidoptera: Noctuidae]; Proceedings of the International Workshop on Facilitatng International Research Collaboration on Transboundary Plant Pests; Tsukuba, Japan. 27–29 November 2019. [Google Scholar]
- 13.Food and Agriculture Organization Global Action for Fall Armyworm Control. [(accessed on 10 March 2021)]; Available online: http://www.fao.org/fall-armyworm/global-action/en/
- 14.EPPO Spodoptera Frugiperda (LAPHER) [(accessed on 12 October 2020)]; Available online: https://gd.eppo.int/taxon/LAPHFR/distribution.
- 15.Nagoshi R.N., Dhanani I., Asokan R., Mahadevaswamy H.M., Kalleshwaraswamy C.M., Sharanabasappa, Meagher R.L. Genetic characterization of fall armyworm infesting South Africa and India indicate recent introduction from a common source population. PLoS ONE. 2019;14:e0217755. doi: 10.1371/journal.pone.0217755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagoshi R.N., Nagoshi B.Y., Cañarte E., Navarrete B., Solórzano R., Garcés-Carrera S. Genetic characterization of fall armyworm (Spodoptera frugiperda) in Ecuador and comparisons with regional populations identify likely migratory relationships. PLoS ONE. 2019;14:e0222332. doi: 10.1371/journal.pone.0222332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagoshi R.N., Goergen G., Du Plessis H., van den Berg J., Meagher R. Genetic comparisons of fall armyworm populations from 11 countries spanning sub-Saharan Africa provide insights into strain composition and migratory behaviors. Sci. Rep. 2019;9:8311. doi: 10.1038/s41598-019-44744-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pashley D.P. Host-associated genetic differentiation in fall armyworm (Lepidoptera: Noctuidae): A sibling species complex? Ann. Entomol. Soc. Am. 1986;79:898–904. doi: 10.1093/aesa/79.6.898. [DOI] [Google Scholar]
- 19.Montezano D.G., Specht A., Sosa-Gómez D.R., Roque-Specht V.F., Sousa-Silva J.C., Paula-Moraes S.V., Peterson J.A., Hunt T.E. Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018;26:286–300. doi: 10.4001/003.026.0286. [DOI] [Google Scholar]
- 20.Pashley D.P., Johnson S.J., Sparks A.N. Genetic population structure of migratory moths: The fall armyworm (Lepidoptera: Noctuidae) Ann. Entomol. Soc. Am. 1985;78:756–762. doi: 10.1093/aesa/78.6.756. [DOI] [Google Scholar]
- 21.Pashley D.P. Quantitative genetics, development, and physiological adaptation in host strains of fall armyworm. Evolution. 1988;42:93–102. doi: 10.1111/j.1558-5646.1988.tb04110.x. [DOI] [PubMed] [Google Scholar]
- 22.Nagoshi R.N., Meagher R.L. Behavior and distribution of the two fall armyworm host strains in Florida. Fla. Entomol. 2004;87:440–449. doi: 10.1653/0015-4040(2004)087[0440:BADOTT]2.0.CO;2. [DOI] [Google Scholar]
- 23.Dumas P., Legeai F., Lemaitre C., Scaon E., Orsucci M., Labadie K., Gimenez S., Clamens A.L., Henri H., Vavre F., et al. Spodoptera frugiperda (Lepidoptera: Noctuidae) host-plant variants: Two host strains or two distinct species? Genetica. 2015;143:305–316. doi: 10.1007/s10709-015-9829-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pashley D.P., Martin J.A. Reproductive incompatibility between host strains of the fall armyworm (Lepidoptera: Noctuidae) Ann. Entomol. Soc. Am. 1987;80:731–733. doi: 10.1093/aesa/80.6.731. [DOI] [Google Scholar]
- 25.Groot A.T., Marr M., Heckel D.G., SchÖfl G. The roles and interactions of reproductive isolation mechanisms in fall armyworm (Lepidoptera: Noctuidae) host strains. Ecol. Entomol. 2010;35:105–118. doi: 10.1111/j.1365-2311.2009.01138.x. [DOI] [Google Scholar]
- 26.Groot A.T., Marr M., Schöfl G., Lorenz S., Svatos A., Heckel D.G. Host strain specific sex pheromone variation in Spodoptera frugiperda. Front. Zool. 2008;5 doi: 10.1186/1742-9994-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hay-Roe M.M., Meagher R.L., Nagoshi R.N. Effects of cyanogenic plants on fitness in two host strains of the fall armyworm (Spodoptera frugiperda) J. Chem. Ecol. 2011;37:1314–1322. doi: 10.1007/s10886-011-0049-7. [DOI] [PubMed] [Google Scholar]
- 28.Prowell D.P. Sex linkage and speciation in Lepidoptera. In: Howard D.J., Berlocher S.H., editors. Endless Forms: Species and Speciation. Oxford University Press; Oxford, UK: 1998. [Google Scholar]
- 29.Prowell D.P., McMichael M., Silvain J.F. Multilocus genetic analysis of host use, introgression, and speciation in host strains of fall armyworm (Lepidoptera: Noctuidae) Ann. Entomol. Soc. Am. 2004;97:1034–1044. doi: 10.1603/0013-8746(2004)097[1034:MGAOHU]2.0.CO;2. [DOI] [Google Scholar]
- 30.Nagoshi R.N. Improvements in the identification of strains facilitate population studies of fall armyworm subgroups. Ann. Entomol. Soc. Am. 2012;105:351–358. doi: 10.1603/AN11138. [DOI] [Google Scholar]
- 31.Murúa M.G., Nagoshi R.N., Santos D.A.D., Hay-Roe M.M., Meagher R.L., Vilardi J.C. Demonstration using field collections that Argentina fall armyworm populations exhibit strain-specific host plant preferences. J. Econ. Entomol. 2015;108:2305–2315. doi: 10.1093/jee/tov203. [DOI] [PubMed] [Google Scholar]
- 32.Nagoshi R.N. The fall armyworm triose phosphate isomerase (Tpi) gene as a marker of strain identity and interstrain mating. Ann. Entomol. Soc. Am. 2010;103:283–292. doi: 10.1603/AN09046. [DOI] [Google Scholar]
- 33.Nagoshi R.N., Meagher R.L. Using intron sequence comparisons in the triose phosphate isomerase gene to study the divergence of the fall armyworm host strains. Insect Mol. Biol. 2016;25:324–337. doi: 10.1111/imb.12223. [DOI] [PubMed] [Google Scholar]
- 34.Nagoshi R.N. Evidence that a major subpopulation of fall armyworm found in the Western Hemisphere is rare or absent in Africa, which may limit the range of crops at risk of infestation. PLoS ONE. 2019;14:e0208966. doi: 10.1371/journal.pone.0208966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Folmer O., Black M., Hoeh W., Lutz R., Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994;3:294–299. doi: 10.1071/ZO9660275. [DOI] [PubMed] [Google Scholar]
- 36.Schäffer A.A., Aravind L., Madden T.L., Shavirin S., Spouge J.L., Wolf Y.I., Koonin E.V., Altschul S.F. Improving the accuracy of PSI-BLAST protein database searches with composition-based statistics and other refinements. Nucleic Acids Res. 2001;29:2994–3005. doi: 10.1093/nar/29.14.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thompson J.D., Higgins D.G., Gibson T.J. CLUSTAL W (improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice) Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Felsenstein J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 40.Tajima F. Evolutionary relationship of DNA sequences in finite populations. Genetics. 1983;105:437–460. doi: 10.1093/genetics/105.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tajima F. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics. 1989;123:585–595. doi: 10.1093/genetics/123.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Librado P., Rozas J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25:1451–1452. doi: 10.1093/bioinformatics/btp187. [DOI] [PubMed] [Google Scholar]
- 43.Clement M., Posada D., Crandall K.A. TCS: A computer program to estimate gene genealogies. Mol. Ecol. 2000;9:1657–1659. doi: 10.1046/j.1365-294x.2000.01020.x. [DOI] [PubMed] [Google Scholar]
- 44.Nayyar N., Gracy R.G., Ashika T.R., Mohan G., Swathi R.S., Mohan M., Chaudhary M., Bakthavatsalam N., Venkatesan T. Population structure and genetic diversity of invasive fall armyworm after 2 years of introduction in India. Sci. Rep. 2021;11:7760. doi: 10.1038/s41598-021-87414-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nagoshi R.N., Htain N.N., Boughton D., Zhang L., Xiao Y., Nagoshi B.Y., Mota-Sanchez D. Southeastern Asia fall armyworms are closely related to populations in Africa and India, consistent with common origin and recent migration. Sci. Rep. 2020;10:1421. doi: 10.1038/s41598-020-58249-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Albery W., Knowles J. Evolution of enzyme function and the development of catalytic efficiency. Biochemistry. 1976;15:5631–5640. doi: 10.1021/bi00670a032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The genetic data presented in this study are publicly available on GenBank, and the accession numbers are reported in Table 1.