Preface
Since nearly everyone who experiences a spinal cord injury (SCI) experiences neurogenic bowel dysfunction (NBD), the information contained in this clinical practice guideline (CPG) is of great relevance both to the community of persons with SCI and to the clinicians who help them manage the secondary conditions of SCI, of which controlled NBD remains a major determinant of quality of life (QOL).
This CPG, similar to its predecessor version, is anticipated to be one of the most important CPGs of the Consortium for Spinal Cord Medicine regarding potential impact on the care of persons with SCI. The scope of this most recent update has been expanded to include a review of the different types of oral and rectally administered medications, of procedures such as transanal irrigation, and of surgical options.
During the development and peer review of this CPG, we were fortunate to have the representation of an international team of various stakeholders, including the subspecialties affected by these recommendations, ranging from experts in gastroenterology, gastrointestinal surgery, and nutrition to rehabilitation professionals, including physiatrists, nurses, psychologists, and occupational and physical therapists. We hope that this wide-ranging representation translates into uniform quality practice through the widespread use of this CPG to guide NBD in all settings, which can only result in the best outcomes and QOL for those who experience SCI.
On behalf of the consortium steering committee, I want first to acknowledge the leadership of the Chair, Jeffery Johns, MD, in guiding this panel through the development process. Next, I would like to acknowledge the panel members themselves for keeping to task, as well as the many reviewers who provided valuable feedback from all areas and are to be commended. All of these people, including the panel Chair, have volunteered their time to help produce this superb document. In addition, I wish to acknowledge the ongoing support of the Paralyzed Veterans of America, especially President David Zurfluh, Executive Director Carl Blake, and Director of Research and Education Cheryl Vines, as well as the rest of the leadership team, without whose support these guidelines would not exist.
Finally, we thank the Craig H. Neilsen Foundation and Executive Director Kym Eisner for their commitment to improving the QOL for those living with SCIs and for their generous financial support of this CPG.
Thomas Bryce
Chair
Consortium for Spinal Cord Medicine
Foreword
On behalf of this panel, I would like to thank the authors of the previous version of the clinical practice guideline (CPG) for neurogenic bowel management in adults with spinal cord injury (SCI), originally published in 1998. That CPG served as an invaluable resource for health care providers, administrators, and third-party payors over the past 2 decades and has helped to improve the health and quality of life for individuals living with SCI. The present guidelines are written not as a revision of the previous guidelines, but as a fresh review and critical analysis of the available literature and practice in this area, and we are confident that they will similarly serve as a trusted source to guide management decisions related to neurogenic bowel dysfunction (NBD).
This panel wants to emphasize the fact that the management of NBD is necessarily holistic and must be uniquely tailored to each affected individual. We are therefore hopeful that the appropriate use of the terms bowel program versus bowel care will integrate more accurately into the minds of health care providers and individuals with SCI. This CPG emphasizes the functional terms reflexic and areflexic NBD rather than an anatomic definition in an effort to focus on the pathophysiology of this condition, as well as to recognize the evolution in understanding of the neurological influences of bowel function beyond spinal cord innervation.
I am honored and grateful to have served as the Chair of this expert multidisciplinary international panel. The dedication, collaboration, and contributions of these individuals is greatly appreciated. I would also like to personally thank the Spinal Cord Medicine Consortium and its Chair, Thomas Bryce, for their leadership and support throughout this development process. In addition, I am thankful to all of the expert field reviewers who provided valuable insight and feedback to help fine-tune these guidelines. Finally, I want to thank the Paralyzed Veterans of America and especially Cheryl Vines for their support and leadership through this entire process.
Jeffery Johns, MD
Panel Chair
Acknowledgments
Paralyzed Veterans of America (PVA) is proud to sponsor the development and dissemination of the spinal cord injury (SCI) clinical practice guidelines (CPGs). For over 25 years, we have partnered with the Consortium of Spinal Cord Medicine in a shared mission to improve the health of individuals living with SCI. Today, hundreds of thousands of copies of the guidelines are used around the world by physicians and other medical professionals who provide care to individuals living with SCI at every level, from the emergency department to acute care, rehabilitation to community services.
We thank Dr. Jeffery Johns for his leadership and perseverance in guiding this important new guideline into practice. Sincere thanks is also extended to each of the panel members who worked tirelessly, without remuneration, to bring this project to fruition. Dr. Thomas Bryce and the members of the SCI Consortium have provided vision, leadership, and support in bringing this and many other CPGs to completion. Their efforts and those of the field reviewers assure the high quality of the recommendations.
This CPG is based on a comprehensive search of the latest evidence. We are grateful for the collaboration of Dr. Janice Eng and the Spinal Cord Injury Research Evidence (www.scireproject.com) research team in searching, extracting, and grading the literature.
PVA is grateful to our partner, the Craig H. Neilsen Foundation, and Executive Director Kym Eisner. Out of their shared interest in improving the quality of life for those living with SCI, they have provided significant funding to support this guideline.
Within PVA, work on this guideline benefitted from the efforts of nearly every department. But special appreciation goes to medical editor Barbara Every and graphic designers Kevin Johnson and Jonathan Franklin.
Finally, it is only with the significant mission-driven support of PVA, our leadership and our members, that we are able to provide these services. Sincere thanks to PVA President David Zurfluh, Executive Director Carl Blake, and Deputy Executive Director Shaun Castle for their support.
Abbreviations
- ACG
American College of Gastroenterology
- AD
autonomic dysreflexia
- AGA
American Gastroenterological Association
- AIS
American Spinal Injury Association Impairment Scale
- ARM
anorectal manometry
- BBM
basic bowel management
- CDAD
Clostridium difficile-associated diarrhea
- CFTR
cystic fibrosis transmembrane conductance regulator
- cGMP
cyclic guanosine monophosphate
- CNS
central nervous system
- CPG
clinical practice guideline
- CT
computed tomography
- DGNS
dorsal genital nerve stimulation
- DRS
digital rectal stimulation
- FDA
U.S. Food and Drug Administration
- FES
functional electrical stimulation
- FMS
functional magnetic stimulation
- FODMAPs
fermentable oligosaccharides, disaccharides, monosaccharides, and polyols
- GI
gastrointestinal
- GRADE
Grading of Recommendations Assessment, Development and Evaluation
- IBS
irritable bowel syndrome
- IBS-C
constipation-predominant irritable bowel syndrome
- ISNCSCI
International Standards for Neurological Classification of Spinal Cord Injury
- MACE
Malone anterograde continence enema
- MASCIP
Multidisciplinary Association of Spinal Cord Injury Professionals
- NBD
neurogenic bowel dysfunction
- PEG
polyethylene glycol
- PICOTS
population, interventions, comparators, outcomes, timing, setting, study designs
- PIE
pulsed irrigation evacuation
- PTNS
posterior tibial nerve stimulation
- PVA
Paralyzed Veterans of America
- QOL
quality of life
- RAIR
rectoanal inhibitory reflex
- RCT
randomized controlled trial
- SARS
sacral anterior root stimulation
- SCI
spinal cord injury
- SCI-QOL
Spinal Cord Injury-Quality of Life (measurement system)
- SCIRE
Spinal Cord Injury Research Evidence
- SNS
sacral nerve stimulation
- TAI
transanal irrigation
Grading of the Recommendations
The overall objective of this guideline is to improve the care of individuals with spinal cord injury (SCI) by guiding clinicians and policy makers with its recommendations. The following recommendations use available evidence and—where evidence is limited—panel experience and consensus. The panel based its evidence ratings primarily on research in which the focus of the study was SCI. This information was supplemented by using evidence from trials, guidelines, and expert opinions contained in the scientific literature of non-SCI populations.
For individual patients, decisions are best made by considering these recommendations combined with clinical judgment, the latter based on specific knowledge about each patient’s risk factors, the potential for adverse effects, and the availability of various options within one’s center. The ratings in parentheses below each recommendation in the sections that follow refer to the level of scientific evidence, the strength of the evidence, and the level of panel agreement with the recommendations (Tables 1–3).
Table 1.
Levels of Scientific Evidence
| Level | Description |
|---|---|
| I | Evidence based on randomized controlled clinical trials (or meta-analysis of such trials) of adequate size to ensure a low risk of incorporating false-positive or false-negative results. |
| II | Evidence based on randomized controlled trials that are too small to provide Level I evidence. These may show either positive trends that are not statistically significant or no trends and are associated with a high risk of false-negative results. |
| III | Evidence based on nonrandomized, controlled, or cohort studies; case series; case-controlled studies; or cross-sectional studies. |
| IV | Evidence based on the opinion of respected authorities or expert committees as indicated in published consensus conferences or guidelines. |
| V | Evidence that expresses the opinion of those individuals who have written and reviewed this guideline, based on experience, knowledge of the relevant literature, and discussions with peers. |
Sources: Adapted from Sackett DL. Rules of evidence and clinical recommendation on the use of antithrombotic agents. Chest. 1989 95(suppl 2):2S–4S; and U.S. Preventive Health Services Task Force. Guide to Clinical Preventive Services. 2nd ed. Baltimore, MD: Williams and Wilkins; 1996.
Table 3.
Levels of Panel Agreement with the Recommendations
| Level | Mean Agreement Score |
|---|---|
| Low | 1.0 to less than 2.33 |
| Moderate | 2.33 to less than 3.87 |
| Strong | 3.87 to 5.0 |
Table 2.
Categories of the Strength of Evidence Associated with the Recommendations
| Category | Description |
|---|---|
| A | The guideline recommendation is supported by one or more Level I studies. |
| B | The guideline recommendation is supported by one or more Level II studies. |
| C | The guideline recommendation is supported by only one or more Level III, IV or V studies |
Executive Summary of the Recommendations
1. Assessment of Neurogenic Bowel Dysfunction (NBD)
-
1.1
Define the level and completeness of spinal cord injury (SCI) according to the current International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI) scale.
(Level - IV; Strength - C; Agreement - strong)
-
1.2
A systematic comprehensive evaluation of bowel function, impairment, and possible problems should be completed at the onset of SCI and at least annually throughout the continuum of care.
(Level - IV; Strength - C; Agreement - strong)
-
1.3
A comprehensive, detailed gastrointestinal (GI) history should be completed at the onset of SCI, annually, and as needed when any significant GI changes occur.
(Level - IV; Strength - C; Agreement - strong)
-
1.4
A physical examination should be done at the onset of SCI, annually, and upon any significant change in bowel function or health. This should include thorough abdominal and rectal examinations.
(Level - IV; Strength - C; Agreement - strong)
-
1.5
An abdominal x-ray/computed tomography scan can be used to evaluate the extent of fecal loading, fecal incontinence due to stool overflow, and other bowel problems such as fecal impaction, bowel obstruction, megacolon, and megarectum.
(Level - III; Strength - C; Agreement - strong)
-
1.6
Colonic transit time testing with radiopaque markers or scintigraphy can be used to provide more information on NBD.
(Level - III; Strength - C; Agreement - strong)
-
1.7
A wireless motility capsule can be used to provide more information on NBD by evaluating gastric emptying time, small intestinal transit time, and colonic transit time.
(Level - III; Strength - C; Agreement - strong)
-
1.8
Anorectal manometry can be used for detailed assessment of pelvic floor dysfunction in individuals with motor incomplete SCI.
(Level - III; Strength - C; Agreement - moderate)
2. Basic Bowel Management (BBM)
-
2.1
A BBM program should be used in individuals with both reflexic and areflexic NBD.
(Level - III; Strength - C; Agreement - strong)
-
2.2
The optimal frequency of bowel movements per week should account for an individual’s lifestyle and premorbid bowel history.
(Level - IV; Strength - C; Agreement - strong)
-
2.3
Mechanical rectal stimulation should be used for individuals with reflexic NBD.
(Level - III; Strength - C; Agreement - strong)
-
2.4
Manual evacuation of stool should be used for individuals with areflexic NBD.
(Level - III; Strength - C; Agreement - strong)
-
2.5
Abdominal massage should not be used for NBD emptying.
(Level - III; Strength - C; Agreement - strong)
-
2.6
The Valsalva maneuver should not be used for NBD emptying.
(Level - V; Strength - C; Agreement - strong)
3. Adaptive Equipment
-
3.1
Use of adaptive equipment, including a suppository inserter and adaptive digital stimulator, should be considered for individuals with limited hand function or difficulty with reach.
(Level - IV; Strength - C; Agreement - strong)
-
3.2
A clinical evaluation of a commode/shower chair should be performed with a focus on the individual’s current bowel care routine and transfer ability, goals of the individual and caregiver, and individual functionality, including postural stability, reach, and skin integrity.
(Level - III; Strength - C; Agreement - strong)
4. Diet, Supplements, Fiber, Fluids, and Probiotics
-
4.1
Providers should inquire about and document diet history, including all dietary supplements that an individual with SCI is taking.
(Level - V; Strength - C; Agreement - strong)
-
4.2
Providers should refer to a registered dietitian if the individual has poor appetite, poor oral intake, or significant weight changes.
(Level - V; Strength - C; Agreement - strong)
-
4.3
Individuals with SCI should not be uniformly placed on high-fiber diets. Increases in fiber intake from food or a supplement should be done gradually to assess tolerance.
Level III Strength C Agreement strong
-
4.4
Foods that cause an individual with SCI to experience excessive flatulence, bloating, abdominal distension, and/or altered bowel movements should be identified and either limited or avoided.
(Level - V; Strength - C; Agreement - strong)
-
4.5
Providers should recommend that an individual with SCI maintain euhydration and avoid dehydration to reduce the tendency to experience constipation. The amount of fluid needed to promote optimal stool consistency must be balanced with the amount needed for bladder management.
(Level - V; Strength - C; Agreement - strong)
-
4.6
Providers should not routinely recommend probiotics to an individual with SCI.
(Level - V; Strength - C; Agreement - strong)
-
4.7
Probiotics may be advantageous to an individual with SCI who is taking antibiotics by reducing antibiotic-associated diarrhea and Clostridium difficile-associated diarrhea.
(Level - I; Strength - A; Agreement - strong)
5. Oral Medications
-
5.1
Providers can use oral medications for bowel management; however, the evidence for their use is limited and there are no data to suggest the use of one medication over another.
(Level - IV; Strength - C; Agreement - strong)
6. Use of Suppositories, Enemas, and Irrigation
-
6.1
Providers can use rectal medications for bowel management.
(Level - III; Strength - C; Agreement - strong)
-
6.2
A polyethylene glycol (PEG)-based bisacodyl suppository is recommended over a hydrogenated vegetable oil-based bisacodyl suppository.
(Level - II; Strength - B; Agreement - strong)
-
6.3
Docusate mini enemas are recommended over glycerin, mineral oil, or vegetable oil-based bisacodyl suppositories.
(Level - III; Strength - C; Agreement - strong)
-
6.4
The routine use of enema formulations such as sodium phosphate (Phospho-Soda), soapsuds, or milk and molasses is not recommended; however, in select individuals, intermittent use for constipation may be helpful.
(Level - V; Strength - C; Agreement - strong)
-
6.5
Transanal irrigation is recommended in individuals with NBD who have insufficient results with BBM.
(Level - I; Strength - A; Agreement - strong)
-
6.6
Pulsed irrigation evacuation (PIE) in a hospital/clinic setting can be used to relieve fecal impaction.
(Level - III; Strength - C; Agreement - strong)
7. Impact of Posture and Activity on NBD
-
7.1
Regular physical activity should be encouraged as part of a healthy lifestyle.
(Level - III; Strength - C; Agreement - strong)
-
7.2
For some individuals, a standing program may be beneficial for bowel function but should be weighed against other means of physical activity, as well as against precautions to undertake the activity safely.
(Level - III; Strength - C; Agreement - strong)
8. Use of Functional Magnetic Stimulation (FMS)
-
8.1
Routine use of FMS for NBD is not recommended.
(Level - III; Strength - C; Agreement - strong)
9. Use of Functional Electrical Stimulation (FES)
-
9.1
FES systems are not recommended for regular clinical use in NBD.
(Level - III; Strength - C; Agreement - strong)
10. Surgical Intervention to Manage NBD
-
10.1
Malone antegrade continence enema (MACE) procedures can be used for individuals with SCI with severe NBD for whom other treatment modalities have failed.
(Level - III; Strength - C; Agreement - strong)
-
10.2
The MACE procedure can be a choice for individuals with NBD who prefer the option after thorough education regarding risks, benefits, and complications and after shared decision making with their providers.
(Level - III; Strength - C; Agreement - strong)
-
10.3
Colostomy is recommended for individuals with severe NBD for whom other treatment modalities have failed or who have had significant complications.
(Level - III; Strength - C; Agreement - strong)
-
10.4
Colostomy can be a choice for individuals with NBD who prefer the option after thorough education regarding risks, benefits, and complications and after shared decision making with their providers.
(Level - III; Strength - C; Agreement - strong)
11. Managing Medical Complications of NBD
-
11.1
Providers must assess and monitor for the unique clinical presentation of GI and intra-abdominal complications related to NBD in individuals with SCI.
(Level - IV; Strength - C; Agreement - strong)
-
11.2
Providers must assess and monitor for complications that primarily affect areas outside the abdomen but that are related to NBD, such as autonomic dysreflexia (AD) and skin breakdown.
(Level - IV; Strength - C; Agreement - strong)
-
11.3
Treatment for hemorrhoids is conservative; if bleeding is refractory, non-excisional techniques are warranted. Excisional hemorrhoidectomy should be avoided.
(Level - III; Strength - C; Agreement - strong)
12. Education for Individuals with SCI and Caregivers
-
12.1
Education for individuals with SCI, caregivers, and health care providers should be provided and comprehensive to all levels of learners.
(Level - IV; Strength - C; Agreement - strong)
-
12.2
The components of the bowel program should be taught to individuals with an SCI as well as to caregivers.
(Level - IV; Strength - C; Agreement - strong)
-
12.3
Education on potential complications should be completed.
(Level - IV; Strength - C; Agreement - strong)
-
12.4
Education and support for the caregiver should be considered and completed when appropriate.
(Level - IV; Strength - C; Agreement - strong)
-
12.5
Sexual intimacy and considerations related to bowel program management should be discussed.
(Level - IV; Strength - C; Agreement - strong)
13. Psychosocial Aspects of NBD
-
13.1
Assessments of NBD should include psychosocial aspects that are barriers to learning the bowel program, such as cognition (ability to learn and to direct others), depression, anxiety, pain, literacy, language, and ethnic or cultural issues.
(Level - III; Strength - C; Agreement - strong)
-
13.2
If an individual with SCI is having multiple problems with NBD or is noncompliant with the bowel program, a formal screening tool should be used to assess depression, anxiety, and quality of life.
(Level - I; Strength - C; Agreement - strong)
The Consortium for Spinal Cord Medicine
The Consortium of Spinal Cord Medicine is a collaboration of professional and consumer organizations with a common interest in health care for individuals living with spinal cord injury (SCI). The Consortium’s mission is to direct the development and dissemination of evidence-based clinical practice guidelines (CPGs) and companion consumer guides. This mission is solely intended to improve the health care and quality of life for individuals with SCI.
The Consortium is funded and administered by Paralyzed Veterans of America (PVA) through their Research and Education Department. The Steering Committee is made up of one representative from each Consortium member organization, appointed by the member organization and approved by the Consortium members.
Summary of Guidelines Development Process
The development of these guidelines involved the following major steps: creation of a list of formal, key questions to be addressed; systematic searches of published literature related to these questions; critical appraisal of the quality of the retrieved studies; abstraction of relevant study results; creation of evidence-based recommendations; development of rationale that explain the recommendations; and review and agreement by panel members. The SCI Consortium’s CPG development process also involves extensive field review and a legal review to ensure that the recommendations are evidence based and applicable and that they can be implemented in a variety of health care settings.
Funding & Potential Conflicts of Interest
PVA contracted the literature searches and evidence reviews for these guidelines to an independent firm, the Spinal Cord Injury Research Evidence program at the University of British Columbia, Vancouver, BC, and provided administrative support for the process. Panel members received no compensation for their participation and declared all potential financial or other conflicts of interest.
Methodology
Literature Search
Members of the Spinal Cord Injury Research Evidence (SCIRE) methodology team searched Ovid MEDLINE, EMBASE, CINAHL, and PsycINFO for literature published from 1980 through June 2018 by using search terms related to bowel dysfunction (e.g., constipation, bowel incontinence) and spinal cord injury (SCI) (e.g., paraplegia, tetraplegia, spinal cord injury/dysfunction) and to the topic of inquiry (e.g., assessment, prevalence, treatment). The SCIRE methodology team also used the same search terms to search the Cochrane Database of Systematic Reviews and Google Scholar for additional studies, systematic reviews, and guidelines in the area of neurogenic bowel dysfunction (NBD) after SCI. The SCIRE methodology team identified additional studies by hand searching the reference lists of the included studies and reviews.
Study Selection
The selection of studies was based on the inclusion criteria created in consultation with the Paralyzed Veterans of America Management of Neurogenic Bowel Dysfunction in Adults after SCI expert panel. Two reviewers independently assessed the titles and abstracts of citations identified through literature searches for inclusion by using the criteria described in the Inclusion Criteria subsection. Full-text articles of potentially relevant citations were retrieved and assessed for inclusion by both reviewers. Disagreements were resolved by consensus. Review articles were included only if bowel management or NBD was the focus of discussion and it was a systematic review, meaning that it was designed to find articles on the study of NBD after SCI, rather than finding articles that described current opinions or research in the area (e.g., in a book chapter).
All articles were limited to English only. Animal studies and articles that described the neurophysiology of the bowel were excluded. Studies that reviewed pediatric populations only were also excluded.
Inclusion Criteria
Studies were included if they principally dealt with NBD after SCI. Two principles guided study inclusion: Studies were included if the population of interest comprised individuals with SCI and if they measured outcomes related to bowel dysfunction or bowel-related dysfunction.
Modifications to inclusion criteria were as follows:
In prevalence studies (e.g., frequency of NBD within a sample population), N had to be >50 to ensure the validity of the findings.
Results published only in abstract form or in conference proceedings could be included if adequate details were available for quality assessment (e.g., risk of bias) and if the area of inquiry had relatively little published information, and so the unpublished study would be making a contribution to the field.
Mixed populations were acceptable if at least 20% of individuals with SCI were included in the sample.
Key Questions
In consultation with the expert panel, we formulated key questions (to guide study inclusion) related to prevalence, assessment, and treatment of NBD in the SCI population. Key questions generated by the expert panel, as well as additional questions that arose as the literature was extracted, are described below.
Guidelines
What are the existing NBD guidelines (or sections of guidelines) that focus on the SCI population?
What are the recommendations from major guideline groups for NBD beyond SCI?
Prevalence
What are the prevalence rates of NBD or bowel complications after SCI?
What are the prevalence rates of NBD for tetraplegia vs. paraplegia?
What are the prevalence rates of NBD after traumatic vs. non-traumatic SCI?
Screening
What screening/classification/assessment tools or outcome measures are used to assess NBD after SCI?
What imaging techniques are used to assess NBD after SCI?
Treatment
Conservative Bowel Management
What are the indications/contraindications/complications/methods for the use of conservative bowel management for NBD after SCI?
Supplements and Fluid
What are the effects of daily supplements on NBD after SCI?
What is the effect of daily fiber on NBD after SCI?
What is the effect of daily fluid intake on NBD after SCI?
What are the effects of probiotics on NBD after SCI?
Components of Bowel Management
What are the indications/contraindications/complications/methods for digital rectal stimulation (DRS) for NBD after SCI?
What are the indications/contraindications/complications/methods for manual evacuation in the management of NBD after SCI?
What are the indications/contraindications/complications/methods for functional electrical stimulation (FES) in the management of NBD after SCI?
What are the indications/contraindications/complications/methods for functional magnetic stimulation in the management of NBD after SCI?
What are the indications/contraindications/complications/methods for abdominal massage in the management of NBD after SCI?
What are the indications/contraindications/complications/methods for transanal irrigation in the management of NBD after SCI?
What are the indications/contraindications/complications/methods for the Valsalva maneuver in the management of NBD after SCI?
Standing and Mobility Training
What are the effects of routine standing or upright posturing on the management of NBD?
What are the effects of lateral decubitus on the management of NBD?
What are the effects of body weight-supported treadmill training on the management of NBD?
What are the effects of exoskeleton training on the management of NBD?
What are the indications/contraindications/complications/methods for the use of FES cycling in the management of NBD?
Assistive Technology and Equipment
What are the indications/contraindications/complications/methods for the use of assistive equipment in the management of NBD?
What are the indications/contraindications/complications/methods for the use of commodes for NBD after SCI?
Oral Medications
What are the indications/contraindications/complications/methods for the use of metoclopramide in the management of NBD after SCI?
What are the indications/contraindications/complications/methods for the use of neostigmine in the management of NBD after SCI?
What are the indications/contraindications/complications/methods for the use of prucalopride in the management of NBD after SCI?
What are the indications/contraindications/complications/methods for the use of fampridine in the management of NBD after SCI?
What are the indications/contraindications/methods for the use of polyethylene glycol (PEG) 3350 (MiraLAX) in the management of NBD after SCI?
Suppositories and Enemas
What are the indications/contraindications/methods for the use of suppositories and enemas that are effective for NBD management after SCI?
What are the indications/contraindications/complications/methods for the use of bisacodyl in the management of NBD after SCI?
What are the indications/contraindications/methods for the use of PEG in the management of NBD after SCI?
What are the indications/contraindications/methods for the use of glycerin in the management of NBD after SCI?
What are the indications/contraindications/methods for the use of a bisacodyl enema (Fleet Enema) in the management of NBD after SCI?
What are the indications/contraindications/methods for the use of soapsuds enemas in the management of NBD after SCI?
What are the indications/contraindications/methods for the use of milk and molasses in the management of NBD after SCI?
What are the indications/contraindications/methods for the use of a docusate enema (Enemeez) in the management of NBD after SCI?
Surgery or Stoma Formation
What are the indications/contraindications/complications/methods for stoma formation in the management of NBD after SCI?
Education
What educational programs or techniques are used for caregivers regarding NBD after SCI?
What educational programs or techniques are used for individuals regarding NBD after SCI?
Psychosocial Effects
What is the effect of NBD after SCI on health-related quality of life (QOL)?
What is the effect of NBD after SCI on restrictions on social activities and QOL?
What is the effect of NBD after SCI on sexual health (e.g., sexual intimacy concerns)?
What is the caregiver burden effect of NBD (e.g., bowel care time disruption on caregiver’s schedule) after SCI?
What is the effect of noncompliance with a bowel management program to manage NBD after SCI?
What are the effects of depression/mental health regarding NBD after SCI?
Consultation Process
The SCIRE methodology team sent relevant articles and evidence tables to the expert panel for study and as the basis for decision making for the construction of this CPG. Subsequently, the SCIRE team responded to queries for additional study from the panel chair and panel members. Tables and text of supplemental evidence were created and included in the final documents to address the additional areas requested.
PICOTS
(Population, Interventions, Comparators, Outcomes, Timing, Setting, Study Designs)
The PICOTS framework was used to develop literature search strategies and to frame and answer a clinical or health care-related question in evidence-based practice (Huang et al. 2006). The PICOTS indicators that the SCIRE methodology team searched for and found include the following:
Population
The population consisted of adults (18 years and older) with non-acute traumatic SCI or spinal cord dysfunction resulting in paralysis (excluding individuals with spinal stroke). In studies with mixed populations, the sample needed to have at least 50% of participants with SCI to be included. If the sample only included people with NBD, then at least 20% of the sample needed to include participants with SCI to be included.
Assessments and Interventions
-
Screening, assessment, or outcome measures
International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI), including the American Spinal Injury Association Impairment Scale (AIS)
International Standards to Document Remaining Autonomic Function after Spinal Cord Injury
Neurogenic Bowel Dysfunction score
International SCI Bowel Function Basic and Extended Data Sets
Cleveland Clinic Constipation Score
Wexner Fecal Incontinence score
St. Mark’s and Pescatori Incontinence scores
Spinal Cord Injury Secondary Conditions Scale
Health Utilities Index-Mark III
Bristol Stool Form Scale
Full body screening
Note: Imaging techniques (e.g., wireless motility capsule, abdominal radiograph, scintigraphy) were collected and assessed for their reliability and validity only.
-
Treatment
Lifestyle modification (e.g., diet, education, and counseling)
DRS and/or manual evacuation
Abdominal massage
Assistive devices (e.g., standing table, modified toilet seat)
Electrical, magnetic, anorectal, or epidural stimulation
Conservative bowel management: a combined stepwise approach to NBD treatment, from least to most invasive (doesn’t include medication or surgery)
Exercise, including physical activity, exoskeleton, body weight-supported treadmill training, or FES cycling
Medication: prokinetic agents (prucalopride, metoclopramide, neostigmine, and fampridine) Suppositories and enemas with or without pharmacological stimulants
Surgery: colostomy and ileostomy, stoma formation
Comparators
Adults without SCI or matched controls (people of the same age, gender, physical characteristics)
Adults with other neurological dysfunctions (e.g., amyotrophic lateral sclerosis, multiple sclerosis, spina bifida)
Another included intervention (head-to-head study in SCI population)
Usual care: Participants in the control group undertook their usual type, number, and order of interventions to achieve evacuation
Placebo
Outcomes
Fecal Incontinence: occurrence and/or frequency
Constipation
Frequency of bowel movements
Duration of bowel movement
Level or type of intervention required to complete evacuation
Time to stool
Colonic transit time
Psychosocial effects of NBD, including QOL, participation/activities, sexual health or participation in sexual relationships, depression, anxiety, mental health, or compliance to treatment regimens
Caregiver burden
Mortality
Fecal impaction
Autonomic dysreflexia
Adverse events
Timing
Timing or duration of NBD was measured in a variety of intervals (days, weeks, months) and was documented at varying points in time from onset of SCI or spinal cord dysfunction. Some studies measured participant recall of bowel problems over the past weeks, months, or year.
Setting
Settings included inpatient, outpatient, and in the community.
Study Designs
Study designs included randomized controlled trials, matched controlled trials, crossover trials, prospective controlled trials, cohort studies, longitudinal studies, case-control studies, pre-post designs, posttests, case series, and observational and cross-sectional studies (surveys). Qualitative studies and case reports (n=1) were included only in areas in which no other credible information existed.
Data Extraction
The SCIRE methodology team extracted information from included studies on population characteristics and demographics, interventions, prevalence, measurement, outcomes, and any adverse effects reported. Data abstraction was performed by one reviewer and independently checked by a second reviewer; any differences were resolved by discussion and/or by involving a third reviewer. The data extraction forms were used to compile information from the approximately 300 articles found in the primary and secondary searches. Extracted information was compiled into evidence tables according to subject area, including epidemiology, comparisons of bowel management programs, dietary intake and nutrition, educational interventions, oral medications, QOL, oral laxatives and rectal stimulants, and surgical interventions.
Data Synthesis
The SCIRE methodology team constructed evidence tables that show the study characteristics, outcomes, and quality ratings/risk of bias for all included studies. The team presents the studies by using a hierarchy-of-evidence approach in which the best evidence is presented first in the tables and is the focus of any results, point estimates, or conclusions.
Validity Assessment (Risk of Bias)
The SCIRE methodology team assessed the internal validity (risk of bias) of trials, observational studies, and systematic reviews on the basis of the methods used for randomization, allocation concealment, blinding, similarity of compared groups at baseline, loss to follow-up, and accounting of statistical confounds. The results were then accumulated to assess the trials as high, moderate, or low risk of bias. A survey study with a low response rate (lower than 50%) was automatically rated as a high risk of bias. Observational studies were rated for non-biased selection, loss to follow-up, prespecification of outcomes, well-described and adequate ascertainment techniques, statistical analysis of potential confounders, and adequate duration of follow-up. Systematic reviews were rated for clarity of review question, specification of inclusion and exclusion criteria, use of multiple databases for searching, sufficient detail of included studies, adequate assessment of risk of bias in included studies, and provision of an adequate summary of primary studies.
Two reviewers independently assessed the quality of each study and differences were resolved by consensus.
Grading the Quality of Evidence
The SCIRE methodology team assessed the quality of evidence by using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach. The GRADE approach provides a system for rating the strength and quality of evidence that is intended to be objective, transparent, and comprehensive, and it is increasingly the standard by which clinical guidelines are constructed.2 The approach incorporates 4 key domains: risk of bias, consistency, directness, and precision of the evidence. For example, the body of evidence in a particular area would be highly rated if there were multiple studies with control groups, the results were in a consistent direction, the outcomes offered direct measurement of the area of interest, and these outcomes were reported consistently.
Grades do not refer to the general efficacy or effectiveness of treatments, but assess the quality of the evidence and thus the confidence one could have in the findings.
Results Overview
The SCIRE methodology team identified 5,603 potentially relevant records from our searches and reviewed their titles and abstracts. The team then assessed 571 articles for eligibility at the full-text level and ultimately included 333 studies (132 studies measured multiple outcomes and thus appear in multiple sections) (Figure 1). Most of the studies pertained to key questions that address different treatments for NBD and specific consequences of NBD in the SCI population.
Figure 1.
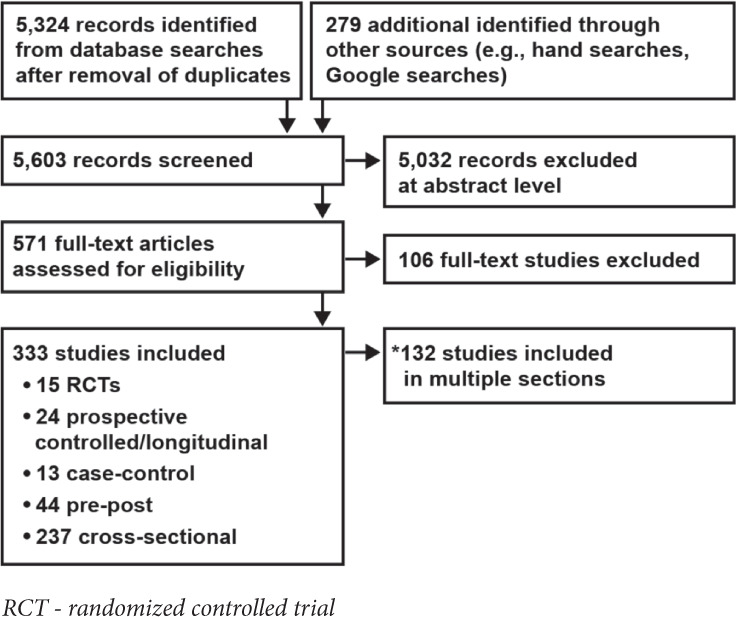
Results of literature search.
Introduction
Most individuals with spinal cord lesions have chronic gastrointestinal (GI) symptoms. The term “neurogenic bowel dysfunction” (NBD) is commonly used to describe bowel dysfunction resulting from trauma or disease within the spinal cord. Usually, NBD includes constipation, fecal incontinence, and, in some individuals, abdominal pain. The pathophysiology behind NBD has been the focus of much research during the last decades. In spite of this, treatment of NBD is often empirical or based on a low level of evidence.
Innervation and Physiology of the Colorectum and the Anal Canal
NBD mainly affects the colorectum and the anal canal. Peristalsis and secretion within the gut are primarily controlled by the enteric nervous system located in the submucosa (Meissner’s plexus) and between the 2 layers of smooth muscle cells in the gut (Auerbach’s plexus).3 The vagus nerve innervates the gut down to the splenic flexure of the colon. The distal part of the colon and the whole rectum receive parasympathetic innervation from the second to the fourth sacral segments of the spinal cord (S2–S4). Sympathetic innervation to the colon and rectum originates from the ninth thoracic segment to the second lumbar segment of the spinal cord (T9-L2). In general, parasympathetic activity enhances secretion and peristalsis while relaxing the GI sphincters. In contrast, sympathetic activity reduces secretion and peristalsis while contracting GI sphincters.
The anal canal is surrounded by the striated external anal sphincter muscle and the smooth internal anal sphincter muscle. The upper part of the anal canal is also surrounded by the puborectalis muscle. The internal anal sphincter muscle is a continuation of the circular muscle layer of the rectum. It is under reflex control of the enteric nervous system and the sacral spinal cord. The external anal sphincter muscle is partly under voluntary control of the pudendal nerve (S3–S5). The tone of the puborectalis muscle creates the anorectal angle, which prohibits the movement of rectal contents into the anal canal.
Normal defecation is preceded by a mass movement of stool from the colon to the rectum. Stretch of the rectal wall initiates the defecation reflex, which stimulates contraction of the rectal wall through a reflex arch between the rectum and the sacral spinal cord. Furthermore, the rectoanal inhibitory reflex (RAIR) causes relaxation of the internal anal sphincter muscle during rectal distension. It is mediated by intramural nerve fibers but enhanced by the parasympathetic nerve fibers from the sacral spinal cord (S2–S4). Defecation can be interrupted by voluntary contraction of the external anal sphincter muscle.
Normal continence for stools depends on complex interactions between the consistency of stools, colorectal transit time, rectal tone, anorectal sensibility, tone of the puborectalis and anal sphincter muscles, and voluntary contraction of the external anal sphincter muscle.
The Pathophysiology of NBD
NBD results from a variety of impairments, including autonomic dysfunction, sensory deficits, paralysis of motor function, and immobility. The contribution of each depends on the level and completeness of SCI, as well as other factors such as age, time since injury, medication, and concomitant disease.
The enteric nervous system makes the innervation of the gut distinct from the innervation of other organs. Sympathetic and parasympathetic nerves mainly act through stimulation or inhibition of the nerves within the enteric nervous system rather than through direct innervation of the smooth muscle cells. Furthermore, parasympathetic innervation of the stomach, small intestine, and proximal colon is from the vagus nerve and thereby unaffected by SCI. Hence, the commonly used terminology upper motor neuron vs. lower motor neuron has generally been abandoned for NBD.
Individuals with lesions above the S2 tend to have increased tone of the external anal sphincter muscle and also increased tone and contractility of the rectum.4,5,6,7 This may cause reflex defecation.4 In contrast, those with lesions at or below the S2 usually have reduced tone of the external anal sphincter muscle, as well as reduced tone and contractility of the rectum. This may cause fecal impaction and incontinence.8,9,10 Therefore, the physiological terms reflexic and areflexic bowel are commonly used even though many individuals with lesions above S2 show no sign of reflexic bowel, and some with lesions at the conus medullaris or cauda equina have remaining reflexes.
In the first days after acute SCI, the gut wall is hypotonic and unresponsive to stimuli.11 Accordingly, most individuals with SCI have severely prolonged colonic transit time during the first weeks after injury. In the chronic phase, the majority of individuals continue to have prolonged transit through the colon.8,12,13,14 The pattern of prolonged colorectal transit varies significantly between people and even within the same person.15 Nonetheless, individuals with reflexic NBD tend to have prolonged transit throughout the colon, but less so in the rectum, whereas those with areflexic NBD tend to have more prolonged transit in the descending colon and the rectosigmoid.12
The underlying pathophysiology in constipation in NBD remains to be described in detail. Emptying of the rectosigmoid during defecation is severely reduced both in individuals with reflexic NBD16 and in those with areflexic NBD.17 Furthermore, lack of parasympathetic stimuli, immobility, and side effects of medication may all contribute to constipation. Prolonged transit time in individuals with SCI is not limited to the colon and rectum. Hence, gastric emptying time18,19 and orocecal transit time7 may also be prolonged.
NBD from SCI leads to defecatory disorders that present as impaired rectal emptying due to poor rectal muscle propulsion and/or increased resistance to evacuation. This obstructed defecation may occur from high anal resting pressure and/or incomplete relaxation or dyssynergia characterized by paradoxical contraction of the pelvic floor and external sphincters during defecation. Decreased rectal sensation is typically associated with impaired motor function.7, 20, 21 Other structural abnormalities may be present such as rectocele; rectal prolapse may be present as well.
In individuals with SCI, several factors contribute to fecal incontinence. Anorectal sensibility and voluntary contraction of the external anal sphincter muscle are reduced or absent.5,16,17 Individuals with reflexic NBD tend to have increased tone and contractility of the rectum,5,7,8,22 causing reflex defecation.4 In those with areflexic NBD, poor emptying of the rectum, hypotonic rectum, and poor sphincter function may cause fecal impaction and incontinence.8,9,10 Fecal incontinence in NBD depends on several factors, including reduced or absent anorectal sensibility, lack of voluntary contraction of the external anal sphincter muscle, fecal impaction, and reflex defecation.9,10,22 Overflow incontinence from significant constipation should always be a consideration in both reflexic and areflexic NBD.
Epidemiology and Clinical Course of NBD
The prevalence of NBD varies between studies and depends on the setting and definitions used. However, most studies have found that more than 80% of individuals with SCI have some degree of bowel dysfunction.23, 24, 25, 26 The most commonly reported symptoms include constipation (reported by 32% to 56%), fecal incontinence (27% to 86%), need for digital stimulation or evacuation of the rectum (66%), abdominal distension or discomfort (22% to 33%), and hemorrhoids (31% to 36%).23, 24, 27, 28, 29,30, 31, 32, 33, 34, 35 The average time used for each defecation has been reported to be more than 30 minutes in 25% of individuals and more than an hour in 9%.24
Bowel symptoms are considered moderate to severe by 39% to 50% of individuals with SCI,24,33,36 and the severity of bowel dysfunction is associated with depression and reduced quality of life (QOL).23,36 The symptom having the most severe negative impact on QOL is daily episodes of fecal incontinence.37
Sensory dysfunction after SCI and the commonly reported presence of pain, constipation, anorectal bleeding, and other alarm symptoms of serious GI pathology are significant problems in daily clinical practice.
Consequently, GI disease remains a frequent cause of hospitalization and even mortality after SCI.
NBD and Type of SCI
Bowel symptoms are more severe in individuals with complete SCI than in those with incomplete SCI.24,36,38,39 The extent to which the level of SCI affects symptoms of NBD is not fully established. Most studies have found that bowel symptoms are more common in those with cervical and upper thoracic lesions than in those with lesions at a lower spinal level.25,28,29,36 However, the opposite pattern has been reported in other studies.31,35 Symptoms of autonomic dysreflexia (AD) are common in individuals with cervical or upper thoracic lesions.24 No clear difference has been shown in the prevalence or pattern of NBD between individuals with traumatic SCI and those with nontraumatic SCI.31,34, 40
NBD and Time Since SCI
Symptoms of NBD may become more severe with time since injury.38 Although most researchers have found that the risk of fecal incontinence does not increase with time since injury,26,30,35,41,42 others have reported that it increases by a factor 1.5 per 10 years.43 There are indications that the time needed for bowel management increases significantly with time since injury26,41 and that the use of a stoma as the primary method of bowel care becomes more common.41,44
Recommendations and Rationales
1. Assessment of NBD
1.1 Define the level and completeness of SCI according to the current International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI) scale.
To fully understand the expected type of NBD that an individual is likely to experience, a provider must either complete a thorough neurological examination or have information and understanding about a recent and reliable examination to define the individual’s SCI. The most reliable and consistent way to complete an examination and communicate this understanding is by using the ISNCSCI (see Appendix C). This standardized assessment tool is used to evaluate the level and degree of completeness of an individual’s motor and sensory function following an SCI. Although a thorough review of the ISNCSCI examination is beyond the scope of this clinical practice guideline (CPG), readers seeking additional information about it are referred to the American Spinal Injury Association website. Knowledge and understanding of the individual’s neurological status will not only allow a better understanding of the NBD, but it will also direct the provider toward other aspects of management detailed later in these guidelines, including positioning, equipment needs, and possible complications such as AD (for individuals with an injury level at or above T6).
Although not formally a component of the ISNCSCI examination, it is also important that a provider assess sacral reflexes and pelvic floor tone and function in order to further understand an individual’s NBD. These assessments should include anal tone, anocutaneous reflex, and bulbocavernosus reflex. The absence of these reflexes and anal tone indicates the presence of areflexic bowel dysfunction.
1.2 A systematic comprehensive evaluation of bowel function, impairment, and possible problems should be completed at the onset of SCI and at least annually throughout the continuum of care.
Options for such evaluations may include the following:
The International SCI Bowel Function Basic Data Set is a standardized assessment tool that has been validated for individuals with SCI (see Appendix D).
The Bristol Stool Form Scale can be used for assessment of stool consistency (see Appendix E).
The Bowel Management subscale of the Spinal Cord Injury-Quality of Life (SCI-QOL) measurement system can be used for assessment of the impact of NBD on daily living and QOL (see Appendix F).
Symptoms of NBD are often underestimated in clinical practice, and standardized questionnaires may be useful for clinical assessment.46 It is also important to include this as a reassessment at least annually, as such things as diet and bowel physiology and circumstances such as family/work demands and availability of caregiver assistance are likely to change with time and aging.
The International Standards to Document Remaining Autonomic Function after Spinal Cord Injury include basic information about bowel function in terms of sensation for bowel movement, ability to prevent stool leakage, and voluntary sphincter contraction, all graded as 0 (absent), 1 (impaired), or 2 (normal).3 The degree of sacral sparing can be determined from a simple 5-item questionnaire.47
Several questionnaires have been used to assess bowel function after SCI. They each capture different aspects of NBD and none are universally accepted.48,49
The Wexner50 and the St. Mark’s scores51 have been developed as simple and easy-to-use tools to grade the severity of fecal incontinence in nonneurogenic populations. They are commonly used in colorectal surgery and in gastroenterology, but their validity in individuals with SCI needs to be proven. The Cleveland Clinic Constipation Scoring System is a questionnaire that measures the severity of constipation on a scale from 0 to 30.52 It is also commonly used but has never been validated in individuals with SCI. Furthermore, all of these scores describe either fecal incontinence or constipation but not the combined impact of both conditions. The Bristol Stool Form Scale (See Appendix 4) describes the consistency of stools on a scale from 1 (hard) to 7(watery).53 It corresponds to colonic transit time in able-bodied persons but remains to be validated for NBD.
The SCI-QOL measurement system includes an item bank of 26 questions that have been developed and thoroughly validated in a large population of American individuals with SCI.54 The items include assessment of specific symptoms of NBD, as well as their impact on daily living and QOL. The items do not add to a total score.
The NBD score includes 10 items that describe bowel dysfunction.37 They add to a total score, which is associated with the self-reported impact of NBD on QOL. The NBD score was developed among Danish individuals with SCI and subsequently validated and translated into several languages.37,38,39,55,56,57 This score has mainly been used for comparison of groups rather than for clinical decision making in individuals with SCI.58
The International SCI Bowel Function Basic Data Set (Version 2.0)59 was developed by an international group of experts on NBD and endorsed by both the American Spinal Injury Association and the International Spinal Cord Injury Society. It was developed from work described in the International SCI Bowel Function Basic and Extended Data Sets.60,61,62 Version 2.0 consists of 16 items that describe background information on bowel function, as well as details of NBD and bowel management. It includes the NBD score.
1.3 A comprehensive, detailed GI history should be completed at the onset of SCI, annually, and as needed when any significant GI changes occur.
A detailed GI history should include the following: Description of current bowel status63,64,65,66,67
frequency of defecation, stool consistency (using the Bristol Stool Form Scale), approximate volume of stool evacuated per bowel movement, time of day of bowel movement, presence of urge or awareness to defecate, ability to control passage of stool (in various circumstances of stress such as laughing, sneezing, coughing, or with transfers or pressure relief), symptoms of urgency
associated symptoms of GI pain and discomfort, abdominal bloating or distension, early satiety
occurrence of incontinence and whether these were small, moderate, or large episodes, and if there was an awareness of these episodes
a daily log of all of the preceding information is helpful to determine the need for current or future modifications to the bowel program
Description of current bowel care64,65,66,68
use of oral and rectal bowel medications
facilitative techniques and methods used for initiation of defecation and/or bowel emptying, such as digital rectal stimulation (DRS), digital disimpaction of stool, and flushing enemas, as well as the devices used
schedule of medications and methods
duration from initiation to completion of bowel care
functional level, positioning, and need for assistance or caregiver support
Premorbid GI function and medical conditions64,65,66
List of medications typically used to treat SCI conditions that can affect bowel function63,64
opiates, anticholinergics, tricyclics, antispasmodics, anticonvulsants, benzodiazepines, etc.
type and amount of fiber consumed per meal
type and amount of fluids taken per 24 hours, and indication of associated caffeine intake
participation in therapies and gym or home exercise programs, mention of type of exercises (ambulatory vs. non-ambulatory exercises)
1.4 A physical examination should be done at the onset of SCI, annually, and upon any significant change in bowel function or health. This should include thorough abdominal and rectal examinations.
The physical examination is fundamental to the assessment of NBD. Signs of malnutrition and dehydration must be noted: weight loss, dry skin, orthostasis, and tachycardia. The clinician can assess the abdomen for distension, hernias, and other abnormalities. Auscultation of bowel sounds and percussion can be used to evaluate for chronic constipation, obstruction, or pseudo-obstruction when a tympanitic and hypoactive abdomen is identified. Abdominal palpation is important in inspecting for pain, tenderness, discomfort, masses, and other lesions.64,66,70
A physical examination must include perineal inspection, rectal examination, and at least several key features of the ISNCSCI examination. Inspection can reveal fissures, hemorrhoids, and a gaping anus. The clinician should observe perineal descent while the individual strains, in addition to his or her ability to squeeze the anal sphincter. Sensation to light touch and pinprick around the anus must be assessed. The presence of bulbocavernosus reflex and anal wink can show emergence from spinal shock and/or identification of reflexic NBD, in contrast to hyporeflexia while in spinal shock and with areflexic NBD. The presence or absence of deep anal pressure can denote completeness or incompleteness of the SCI. Digital examination and palpation of the anal canal and rectum aids the clinician in identifying hemorrhoids, rectoceles, and rectal prolapse. Digital examination of sphincter tone at rest and with anal squeeze, as well as simulated defecation with bearing down and attempted expulsion of the examiner’s finger, can be used to assist with identification of muscle weakness or hypertonicity of the sphincter muscles and pelvic floor muscles. Paradoxical contraction of muscles suggests dyssynergia, and voluntary anal squeeze indicates motor incompleteness.64,66,71,72
1.5 An abdominal x-ray/computed tomography (CT) scan can be used to evaluate the extent of fecal loading, fecal incontinence due to stool overflow, and other bowel problems such as fecal impaction, bowel obstruction, megacolon, and megarectum.
An abdominal x-ray is recommended as the initial method of radiological evaluation in the workup of people who have progressive problems with NBD specifically related to severity of fecal loading. Park et al.73 reported that the abdominal x-ray can be useful in showing the presence and degree of stool retained throughout the colon and in each colon segment as measured by the Leech and Starreveld radiograph scores. The degree of stool retention shown on an abdominal radiograph can be reliably correlated with total and segmental colon transit times and can indicate the degree of GI dysfunction.73 Aggressive bowel cleansing and treatment of constipation can be immediately recommended when significant fecal loading is identified to prevent progression to serious complications. Furthermore, abdominal radiography can be used to follow up and evaluate effects of bowel treatment and management.73 If fecal loading is left untreated, it can result in conditions that may warrant surgical intervention, such as severe fecal impaction and bowel obstruction, megacolon, and megarectum, which can also be evaluated by abdominal radiography.73
Small and large bowel obstruction are medical emergencies that require immediate diagnosis and treatment due to their high morbidity and mortality rates if not addressed promptly. Aside from a thorough history and physical examination, abdominal radiography is the initial imaging of choice for evaluation. However, diagnosis is not determined in almost half of cases with these methods alone. Moreover, individuals with SCI may have vague clinical symptoms, nonspecific physical findings, and confusing laboratory results.74,75,76,77,78
When further diagnostic testing is indicated, abdominal CT is a quick, accurate, specific, and sensitive tool in delineating small or large bowel obstruction and other lesions. CT of the abdomen is the preferred study for facilitating diagnosis of small or large intestinal obstruction by determining cause, defining the site and extent of obstruction, distinguishing non-emergent (adynamic ileus) from emergent obstructions (closed loop and strangulated obstructions), demonstrating the presence of ischemia, and ultimately, informing proper treatment, intervention, and the need for surgery.74,75,76,77,78
1.6 Colonic transit time testing with radiopaque markers or scintigraphy can be used to provide more information on NBD.
Individuals with SCI present with chronic issues of constipation and/or fecal incontinence related to defecation disorders with normal or slow colonic transit. Individuals with defecation issues can present with prolonged colonic motility. Evaluation of colonic transit with radiopaque markers, scintigraphy, or a wireless motility capsule is endorsed in the American Gastroenterological Association (AGA) and American College of Gastroenterology (ACG) guidelines72,79 for individuals with persistent GI symptoms and chronic constipation in which symptoms do not respond to laxatives or first-line pharmacological therapy. Findings of slow colonic transit may educate individuals about the pathophysiology of their symptoms. Such findings may also provide good information for response to therapy and afford the physician with justification for prescribing newer agents, which may be questioned or denied by payers because of their higher cost. Long-term side effects and risks of these newer medications are unknown at this time and thus their use is better reserved for those who have severe constipation with slow transit.
Colonic Transit Time with Radiopaque Markers and Scintigraphy
Colonic transit testing involves ingestion of radiopaque markers with one or more subsequent abdominal radiographs that track the passage of the markers along the colon. Colon transit time can be calculated from the distribution of markers in the right, transverse, and rectosigmoid segments of the colon.72
Several studies have reported colonic transit testing in people with NBD. Krogh et al.17 demonstrated that total GI transit time was prolonged in subjects with acute supraconal lesions (reflexic) (n=15) and acute conus medullaris or cauda equina lesions (areflexic) (n=11) compared with that in controls (n=24). With acute supraconal lesions (n=15), transit times of the ascending colon, transverse colon, and descending colon, but not the rectosigmoid, were prolonged. In individuals with acute conus medullaris or cauda equina lesions (n=11), all segmental colonic transit times were prolonged. Total GI transit time was prolonged in those with chronic supraconal lesions (n=10), as well as in those with chronic conal/cauda equina lesions (n=9) compared with that in controls (n=24). Similarly, Emmanuel et al. showed that individuals with chronic supraconal lesions (n=10) had prolonged transit time in the transverse and descending colon, but not in the rectosigmoid.
In subjects with chronic conal or cauda equina lesions (n=9), transit times were prolonged in the transverse colon, descending colon, and rectosigmoid, but not in the ascending colon. No statistical differences were found between acute and chronic lesions. Slow colonic transit time was found in 32 of 55 subjects and all 32 had symptomatic constipation.
Colonic Transit Time with Scintigraphy
In scintigraphy testing, a radionuclide (e.g., 99m-Tc sulfur colloid) is administered with a standardized meal (e.g., scrambled egg) and immediately afterward, images of the anterior and posterior abdomen are taken by a gamma camera every 30 minutes for 2 hours to gastric emptying. Colonic transit time can be determined by imaging at 24, 48, and 72 hours after the meal is eaten.82 Comparison of 4 different methods to analyze scintigrams in SCI showed that visual assessment was unreliable, whereas a combination of analog images and one of the quantitative methods was the best option for evaluating transit time (n=16 SCI).83 Scintigraphy performed just before and after defecation showed that the median position of the contents was more prolonged and the velocity of the median position of the contents was lower.84 Scintigraphy showed that median antegrade transport was 27% in sacral SCI vs. 82% in controls (n=16) for the rectosigmoid, and 4% in SCI (n=10) vs. 38% in controls for the descending colon; however, it was not significantly different in the transverse colon and ascending colon. Scintigraphy showed that rectosigmoid emptying at defecation was longer in individuals with SCI than in controls.17
1.7 A wireless motility capsule can be used to provide more information on NBD by evaluating gastric emptying time, small intestinal transit time, and colonic transit time.
A wireless motility capsule (SmartPill™ motility capsule, Medtronic) is ingested with food and excreted to provide clinicians with information about the individual’s gastric pH, segmental transit times, and GI motility. Williams et al.18 showed that gastric emptying, colonic transit time, and whole gut transit time is delayed in individuals with paraplegia (n=8) and tetraplegia (n=12) compared with that in controls (n=10). There were no recorded adverse events.
In comparison, Fynne et al.7 demonstrated that GI tract motility can also be monitored by a magnetic pill that is ingested. The pill travels through the GI and its position is detected by a plate over the abdomen as the individual remains immobile in bed for approximately 6 hours. Orocecal transit time was found to be longer in those with SCI than in controls. There were no differences found in gastric emptying between individuals with SCI and controls. Subjects with high lesions were shown to have slower gastric emptying than were those with conal/cauda equina lesions (n=19 SCI).
1.8 Anorectal manometry (ARM) can be used for detailed assessment of pelvic floor dysfunction in individuals with motor incomplete SCI.
Pelvic floor dysfunction and defecation disorders are known to contribute to NBD from SCI. This occurs because of loss of motor and sensory innervation of the pelvic floor and anorectum,7,20,87 which is also the crux of the problem with stool evacuation for individuals with NBD. This impairment can include dyssynergic defecation, pelvic floor dyssynergia, or obstructive defecation. There is discoordination between the abdominal, pelvic, rectal, and sphincter muscles, which results in deficient propulsive forces, with increased resistance to evacuation, high rectal pressures, and/or paradoxical contraction of the pelvic floor and sphincter muscles, as well as poor muscle relaxation during attempts at defecation.
ARM is recommended by the AGA and the ACG to elucidate the pattern of dysfunction in defecatory disorders by assessing the functional performance of the pelvic floor musculature.72 A high-resolution probe with sensors can measure puborectalis and anal sphincter pressures at rest, with squeeze, during a cough maneuver, and during attempted defecation; rectal sensation and compliance; and the presence of the rectoanal inhibitory reflex (RAIR). The probe is inserted into the length of the anal canal while an individual is in the left lateral decubitus position. The balloon expulsion test is usually performed with the ARM; more than a 1-minute delay in expulsion of a balloon filled with 50 mL of water or air is considered to be a positive result. Pelvic electromyography and pudendal nerve latency testing may accompany ARM to complete anorectal physiological testing.71,72
Anorectal physiological testing in individuals with SCI who have NBD is useful in those with incomplete motor lesions. Using water perfusion and balloon ARM, Thiruppathy et al.21 found that the severity of constipation significantly correlated with an abnormally elevated urge volume (r=0.68, p=0.002) and maximal volume (r=0.39, p=0.03). They also found that ARM could differentiate between individuals with constipation and those with fecal incontinence; individuals with SCI and constipation tended to have diminished relaxation of the sphincters, whereas those with SCI and fecal incontinence had prolonged duration of the RAIR (r=0.33, p=0.009) and recovery phase (r=0.37, p=0.05).21
Using perfusion ARM, Tjandra et al.88 found that individuals with SCI who had severe bowel symptoms had significantly lower anal canal pressures than did healthy controls. There were no significant differences in anorectal physiological parameters between traumatic SCI (n=8) and nontraumatic spinal cord lesions (n=4), or between those with cervical/thoracic lesions (C4-T12; n=7) and those with lumbar lesions (L2–L5; n=5). Eleven (92%) individuals had prolonged pudendal nerve terminal motor latency (9 bilateral and 2 unilateral), whereas RAIR was abolished in all 9 individuals tested. The researchers concluded that individuals with SCI who had severe bowel symptoms tended to have lower anal canal pressures than did healthy controls. Pudendal neuropathy and impaired RAIR are common and may be important in the pathogenesis of bowel dysfunction in individuals with spinal cord lesions.
Krogh et al.6 demonstrated with ARM that rectal tone was significantly higher (p<0.05) than normal in individuals with acute and chronic supraconal (reflexic) lesions but significantly lower (p<0.05) in those with acute and chronic conal/cauda equina lesions (areflexic). The proportion of subjects with single giant rectal contractions was significantly higher than normal (33%) with acute supraconal SCI (77%; p=0.02) but not in subjects with acute conal/cauda equina lesions (45%; p=0.69).
Phasic giant contractions occurred only in individuals with SCI (once or more in 8 of 25 subjects), but they were not correlated with the level of the lesion. Rectal tone and the number of giant rectal contractions did not change significantly from the acute to the chronic phase of SCI. The amplitude of RAIR at distension pressures of 5 and 10 cm H2O was significantly lower than normal in individuals with acute and chronic conal/cauda equina lesions (acute: 5% and 44% vs. normal: 37% and 82%; chronic: 6% and 66%), but not in individuals with supraconal SCI (acute: 32% and 83%; chronic: 61% and 85%).6
Defecography is recommended by the AGA and ACG when the ARM and balloon expulsion test is inconclusive. This is typically performed with barium instilled in the anorectum and under fluoroscopy, with dynamic evaluation before, during, and after attempted defecation. The relaxation or contraction of the puborectalis can be observed. Anatomic causes of outlet obstruction such as rectal prolapse, rectocele, or enterocoele can be identified. Magnetic resonance imaging defecography can also be performed, which shows better resolution of soft tissue structures around the rectum and anal canal (bladder, uterus, small intestine) and improved visualization of the anal sphincter and levator ani muscles, with decreased radiation exposure.71,72
2. Basic Bowel Management (BBM)
2.1 A BBM program should be used in individuals with both reflexic and areflexic NBD.
A bowel program is the treatment plan that is designed to minimize or eliminate the occurrence of unplanned or difficult evacuations; to evacuate stool at a regular, predictable time within 60 minutes of bowel care; and to minimize GI complications. BBM is indicated as first-line treatment for all individuals with SCI diagnosed with NBD who do not require surgical intervention because of the severity of their GI status or secondary complications. The GI history, physical examination, and diagnostic testing are used to create a customized plan for a bowel program.
Components include the following:
diet and fluid management
physical activity
oral medications (stimulants and/or softeners)
rectal medications
scheduled bowel care
rectal evacuation methods
Bowel care is the process of assisted defecation, typically at a scheduled interval, which can include rectal stimulation (chemical, mechanical, or both), manual evacuation of stool, positioning, and adaptive equipment.
Working on a framework of goals for the bowel program with each person – individualized to personal goals, life schedules, role obligations, attendant care, and self-rated QOL – is critical for success. Response to medications and methods to manage constipation and fecal incontinence is unique for each individual. Therefore, an effective bowel program will need trial and evaluation, close monitoring, and careful adjustments that can take up to months to establish. It is imperative that conscientious supervision and modification as needed continues regularly. To achieve an effective BBM program, consistent and regular bowel emptying are crucial.64,66
Goals for BBM should include the following64,66:
Regular passage of stool on a daily or every other day basis
Adequate amounts of stool (moderate to large) with every bowel movement
Bowel evacuation at a consistent time of day (am or pm)
Complete emptying of the rectal vault with every bowel care session
Soft, formed, bulky stools
Completion of bowel care ideally in less than 30 minutes, but no longer than 1 hour
These goals guide the ongoing and necessary adjustments in oral and/or rectal medications, timing/scheduling, techniques/methods, and other factors such as diet and fluids that greatly impact bowel function.64,66,68 As an individual transitions out of neurogenic shock after an acute SCI, their NBD status and associated management needs will require continual evaluation. BBM components for those with reflexic and areflexic NBD differ and individuals may use some or all components of the program (Table 4).
Table 4.
Overview of Basic Bowel Management (BBM) According to NBD Classification
| Reflexic Neurogenic Bowel Dysfunction | Areflexic Neurogenic Bowel Dysfunction |
|---|---|
| Lifestyle modifications: adequate fluid and fiber intake, physical exercise, and an individualized bowel care plan | |
| Daily but can be a minimum of 3 times per week | One or more times each day |
| Lifestyle +/− medication regimen to achieve a Bristol Stool Form Scale score of 3 (firm) or 4 (smooth soft) | Lifestyle +/− medication regimen to achieve a Bristol Stool Form Scale score of 3 (firm) or 4 (smooth soft) |
| Rectal stimulants (suppository or mini enema) | |
| Digital rectal stimulation and manual evacuation of stool | Manual removal of stool |
| Medication options: oral laxatives (stimulants, bulk-forming agents, and stool softeners) and prokinetics | |
Source: Adapted and modified from the Multidisciplinary Association of Spinal Cord Injury Professionals (MASCIP) Guidelines for Management of Neurogenic Bowel Dysfunction in Individuals with Central Neurological Conditions.89 The order of the components in the table is not necessarily chronological, but represents only the general order in which basic bowel management components appear in the literature.65,67,89,90,91
While BBM is the standard of care for NBD following SCI, a very low level of evidence supports its use. This is because the body of evidence consists of 2 randomized controlled trials (RCTs) and a limited number of observational studies. Overall, this evidence demonstrates generally that BBM increases evacuation frequency81,92 while reducing bowel care time,55,92,93,94 need for invasive interventions,55,91,93,94,95 and NBD symptoms.91,92
Complications
Across studies, the most common complications experienced by individuals who use BBM were constipation, incontinence, abdominal pain, anorectal pain, and autonomic dysreflexia (AD) symptoms. Whether the first 3 complications increase during BBM is unclear, as some studies indicate that they were reduced with BBM.91,92,94
2.2 The optimal frequency of bowel movements per week should account for an individual’s lifestyle and premorbid bowel history.
For most individuals, a minimum of 3 adequate bowel movements per week is recommended to avoid constipation.
In the setting of areflexic NBD, at least daily bowel care is typically needed in order to minimize the risk of unplanned bowel evacuations.
Bowel care for assisted defecation is needed to assist the individual in having predictable bowel evacuation, to decrease incontinence and constipation, and to help improve QOL.90 BBM should be initiated in the acute phase of care to help establish the bowel program and to prevent complications. When designing a bowel program, it is important to include the individual with the SCI as well as their possible caregivers; providers must also consider which portions of a bowel program will be beneficial for the individual based on the level of neurological injury. Both types of NBD should have their bowel care set for the same time of day for each cycle of bowel care to facilitate habituation. Timing and frequency of bowel care for optimal bowel movements can match premorbid patterns. Adequate rectal emptying that aims for every day or every other day (at least 3 times per week) reduces the risk of constipation and fecal incontinence.
The colon absorbs water from the lumen to prepare for stool evacuation; therefore, with each day that passes, stool becomes harder and drier. This can result in incomplete emptying of the rectum and various degrees of constipation, which can then lead to fecal incontinence during the course of the day. Efforts to complete rectal emptying consistently assist with planning and reduce episodes of incontinence. Oral medications are useful to regulate stools toward the ideal consistency (soft, formed, bulky), ease passage, and/or stimulate colon motility to propel stool to the rectum, which prepares for improved evacuation.64,66,69 If rectal reflex methods do not efficiently evacuate stool, manual evacuation or irrigating techniques can be used.67,68,69
Bowel Care for Reflexic NBD
Individuals deemed to have reflexic NBD may benefit from mechanical and chemical rectal stimulation because of an intact defecation reflex. Some people may require the use of manual evacuation of stool prior to the use of mechanical and/or chemical stimulation to assist with more effective elimination. Bowel care is typically performed at the same time of day for each cycle of bowel care that best fits the needs of the individual with SCI, the availability of caregiver(s), and the premorbid time of previous bowel movements. It is recommended that the bowel care occur daily in the acute phase of care and during the establishment of a bowel program. As the bowel program becomes more established and efficient, individuals may choose to decrease their bowel care to a minimum of 3 times a week because of different life considerations. The risk of constipation and complications can increase with fewer bowel movements per week. Individuals may also decrease medication and other interventions as their programs become more established.
Bowel Care for Areflexic NBD
Individuals deemed to have areflexic NBD may benefit from manual evacuation of stool because of the absence of the defecation reflex. These individuals may benefit from completing their bowel care one or more times each day. Stool consistency for those with an areflexic NBD should be firmer to assist stool retention between bowel care sessions. Diet, fluid, and activity also impact stool consistency. An assessment should be performed to evaluate the appropriate positioning of the individual for bowel care on the basis of level of injury, sitting balance, and functional skills. Individuals with higher levels of injury will likely need more assistance with their bowel care from a caregiver than will those with lower levels of injury. The ability of an individual with SCI to learn should also be evaluated prior to implementing a bowel program; education about the components of a bowel program and the GI system should be conducted with the affected individual and appropriate co-learners.90
2.3 Mechanical rectal stimulation should be used for individuals with reflexic NBD.
Mechanical rectal stimulation is used to trigger anorectal reflexes to increase motility and relax sphincters in reflexic NBD.90 It is one of the most common techniques, with 15.5% to 72% of individuals with SCI using it for bowel management.96,97 Digital rectal stimulation (DRS) is a form of mechanical rectal stimulation that is most commonly used for those with reflexic NBD. DRS users were found to be more likely to have the greatest QOL sustained over time vs. users of suppositories, enemas, or manual evacuation.32,98 Haas et al.99 reported that mechanical rectal stimulation and manual evacuation users had 70% less unplanned bowel evacuation than non-users did. Complications associated with mechanical rectal stimulation include potential for AD for those at risk, hemorrhoids, abdominal distension, and anal fissures.28 Individuals with a T6 level of injury and above are at risk for AD. If during mechanical rectal stimulation an individual has signs and symptoms of AD, such as bradycardia, cardiac arrhythmia, pounding headache, anxiety, sweating above the level of their SCI, flushing, blurry vision, nasal congestion, and/or piloerection, rectal stimulation should be discontinued immediately.100,101 Lidocaine gel for lubrication is commonly used to reduce the risk of AD episodes caused by mechanical rectal stimulation.
The individual or caregiver should perform bowel care at the same time daily. Providers should educate the individual and caregiver, if pertinent, about the importance of consistent bowel care timing. Without that consistency, the individual is at increased risk of unplanned bowel movements, with or without urge. Consider the affected individual’s preinjury bowel evacuation routine and the availability of assistance with bowel care if needed:
Evaluate the individual’s physical abilities, including sitting tolerance, sitting balance, and upper extremity strength, and incorporate these abilities into decision making about positioning during bowel care tasks. Occupational therapist and/or physical therapist evaluation and input in this area are suggested. Position the affected individual on his or her side, or up on a padded commode, based on the positioning that is appropriate for that individual.
Use the gastrocolic reflex by having the affected individual consume food or a beverage 30 minutes prior to bowel care, which can help facilitate a bowel movement.90
The affected individual or caregiver should check the rectal vault for stool by placing a gloved, lubricated finger into the rectum. If stool is present, it should be gently removed from the vault.
If using a rectal medication for chemical stimulation such as a suppository or mini enema, it should be placed into the rectum. The suppository should be placed directly against the rectal wall. Allow the suppository or mini enema to sit for 10 to 15 minutes prior to mechanical rectal stimulation.102
DRS is performed by the affected individual or caregiver by placing a gloved, lubricated finger into the rectum and performing slow rotation in a circular movement for no longer than 10 to 20 seconds at a time. Repeat the rectal stimulation sequence every 5 to 10 minutes until evacuation of the stool is achieved.90,103 Using additional fingers or excessive dilation has shown no benefit and can contribute to complications.103
Monitor those at risk for AD for signs and symptoms during bowel care. If symptoms are present, discontinue rectal stimulation immediately for the current cycle of bowel care. The reader is referred to the autonomic dysreflexia and dysfunction CPG for further management recommendations of the episode in relation to the bowel.100,101
For the individual who is at risk for AD and/or has had an episode during bowel care, lidocaine should be used routinely as a lubricant prior to bowel care.102,105
Individuals with SCI and/or their caregivers should keep a log of the bowel program’s effectiveness and record any complications as recommended in section 1.3.
2.4 Manual evacuation of stool should be used for individuals with areflexic NBD.
Manual evacuation of stool is indicated as treatment for areflexic bowel management and fecal impaction.90 It is the most common bowel management technique, with 29.8% to 56% of individuals with SCI using it.106 Manual evacuation is performed with the affected individual side-lying in bed or positioned on a padded commode if prescribed to be performed in an upright position. The individual with SCI or caregiver inserts a single, gloved, lubricated finger into the rectum to disimpact and remove stool that is present in the rectal vault.81 Manual evacuation may need to be performed daily or more often in areflexic NBD.
The risk of AD for individuals with an SCI T6 and above leads experts to advise against the use of manual evacuation as the primary technique for bowel care for these individuals with reflexic NBD.101,105,106,107 If manual evacuation needs to be used in individuals at risk for AD, application of a topical anesthetic to the rectum is suggested prior to implementation.105 Caution is advised when using manual evacuation for an individual who is at risk for AD.
Users of manual evacuation were shown to have an increased community participation and occupation score over time.98 A very low level of evidence supports manual evacuation treatment of NBD in individuals with SCI, as primary sources of evidence are uncontrolled observational studies.96,107,108 Risks associated with manual evacuation include AD for those at risk, hemorrhoids in thoracic and lumbosacral injuries,28 and anal fissures.
2.5 Abdominal massage should not be used for neurogenic bowel emptying.
Abdominal massage is the technique of using the back or heel of the hand or a tennis ball to apply firm palpation starting from the lower right region of the abdomen and continuously moving in a clockwise pattern along the region of the colon toward the rectum.89 This technique is thought to increase peristalsis in the colon and can be used with other conservative bowel management techniques.89 No specific indications or contraindications have been found for abdominal massage. In a pre-post study performed by Janssen et al.,109 no benefit was shown from abdominal massage performed with an electromechanical device. A very low level of evidence supports the use of abdominal massage for treatment of NBD for SCI because the studies performed have been observational with no RCTs. The potential benefits of abdominal massage are unclear, however, because one study showed positive results and one showed negative results for effects on abdominal distension, constipation, and bowel care time.28,110,111 Those with a cervical level of SCI who used abdominal massage were 1.5 and 1.8 times more likely to report hemorrhoids and abdominal pain than non-users were, and those with thoracic levels of SCI were 2.3 times more likely to report anal fissures than non-users were.28 Given the increased risk of complications, along with unclear documented benefits, it is recommended that this technique not be used routinely in the management of NBD.
2.6 The Valsalva maneuver should not be used for neurogenic bowel emptying.
The Valsalva maneuver is defined as the closing of the throat/glottis and forcefully exhaling to increase intraabdominal and intrarectal pressure to assist defecation.89 It should be performed gently, as the excessive force can lead to contraction of the pelvic floor, which introduces resistance that can hinder defecation.112 There is a very low level of evidence from a single cross-sectional study that suggests that those who use the Valsalva maneuver have a higher rate of rectal abscess and a lower rate of hemorrhoids than do those who use rectal stimulation and manual evacuation.113 No other indications or contraindications for the Valsalva maneuver have been provided other than from expert opinions, which indicate that this maneuver should not be used in reflexic NBD.89 The effect on constipation, incontinence, and duration of defecation is unclear.
3. Adaptive Equipment
3.1 Use of adaptive equipment, including a suppository inserter and adaptive digital stimulator, should be considered for individuals with limited hand function or difficulty with reach.
The adjustable, extended handle on adaptive equipment, such as a suppository inserter or an adaptive digital stimulator, may be suitable for individuals with C6–C8 tetraplegia with grip and dexterity limitations. It may also be used by individuals with paraplegia, if they experience difficulty with reach and balance. Use of these devices should be part of a comprehensive evaluation of potential for independent bowel care.
3.2 A clinical evaluation of a commode/shower chair should be performed with a focus on the individual’s current bowel care routine and transfer ability, goals of the individual and caregiver, and individual functionality, including postural stability, reach, and skin integrity.
A clinical evaluation of a commode/shower chair should be performed by skilled health care professionals, with a focus on the individual’s current bowel care routine and transfer ability, goals of the individual and caregiver, and functionality, including postural stability, reach, and skin integrity.114 The individual with SCI should be able to tolerate upright sitting and have a plan for managing clothing. This equipment should also be clinically evaluated according to its structural features. This should include overall frame support, adequate access to the perianal area, robust and effective braking, adjustability, stability, and backward sloping seating.114,115,116,117 The commode seat design should maximize the contact surface area to prevent pressure injuries, distribute pressure through the thighs and greater trochanters, and position the ischial tuberosities over the chair or let them float.114,115,116,117 These features, along with the individual’s size, level of injury, and clinical evaluation, should be taken into account when selecting an appropriate commode or shower chair. Safety straps, lateral supports, and seat padding should be considered for safety and skin protection. A tilt-in-space feature in a commode or shower chair, helpful for weight shifts, postural stability, and blood pressure management, should be considered for individuals who require more dependent physical assistance (see Table 5).
Table 5.
Potential Functional Performance and Adaptive Equipment by Level of Injury
| Level of Injury | Potential Functional Performance Outcome for Bowel Carea | Bathroom Equipment Options | Assistive Device Optionsb |
|---|---|---|---|
| C1–5 | Independent in providing verbal instruction; dependent for performance of bowel care; dependent for transfers |
|
|
| C6 | Independent in providing verbal instruction; assistance with clothing; modified independent performance of bowel care; possibly independent with transfers |
|
|
| C7 | Modified independent with all components |
|
|
| C8-T1 | Modified independent to independent with all components |
|
|
| T2–T6 | Independent with all components |
|
|
| T7-L2 | Independent with all components |
|
|
a Potential functional performance outcomes are considered to be optimal functional outcomes by level of injury. However, completeness of injury; other physical, cognitive, and environmental factors; and the amount of time, energy, and resources available to complete bowel care may limit or enhance achievement of performance outcomes.
b Additional supplies for bowel care (individuals may not require every item listed): gloves, suppository, water-soluble lubricant, plastic-lined pads, and washcloths or wipes for cleanup.
Designing A Neurogenic Bowel Management Program For Individuals With Sci
Guiding Principles for the Management of NBD
A systematic and comprehensive evaluation of bowel function and impairments is completed at onset of injury and continues on an annual basis.
Bowel management starts during acute care and is revised as needed.
Bowel management program provides predictable and effective elimination and reduces gastrointestinal and evacuation complaints.
Knowledge, cognition, motor performance, and function are important assessments in determining the ability of the individual to complete a bowel care program or instruct a caregiver.
Attendant care needs, personal goals, life schedules, role obligations, developmental needs, and self-rated quality of life are to be considered in the development of bowel care programs.
The design of effective interventions includes an awareness of social and emotional support, as well as impairments, disabilities, and handicaps.
Establishing a consistent schedule for defecation, based on factors that influence elimination, preinjury patterns of elimination, and anticipated life demands, is essential when designing a bowel care program.
Prescriptions for appropriate adaptive equipment for bowel care should be based on the individual’s functional status and discharge environment.
All aspects of the bowel management program are designed to be easily replicated in the individual’s home and community environments.
Adherence to treatment recommendations is assessed when evaluating bowel complaints and problems.
Knowledge of the unique clinical presentation and a prompt diagnosis of common complaints is necessary for the effective treatment of neurogenic bowel conditions.
Effective treatment of common neurogenic bowel complications, including fecal impaction, constipation, and hemorrhoids, is necessary to minimize potential longterm morbidities.
4. Diet, Supplements, Fiber, Fluids, and Probiotics
4.1 Providers should inquire about and document diet history, including all dietary supplements that an individual with SCI is taking.
Greater use of dietary supplements has been observed in the SCI population compared with that in the general population.118 A recent systematic review found moderate quality evidence that supported the use of vitamin D, alpha lipoic acid, and omega-3 supplementation in individuals with SCI, but found no studies that measured bowel-related outcomes.119 Providers should be aware that limited information is available on the clinical effectiveness of dietary supplement use for NBD in SCI. In a small, non-controlled study, Kim et al.120 administered 1,600 mg of Poncirus fructus to 31 individuals with SCI for 2 weeks and found statistically significant improvements in Bristol Stool Form Scale scores, stool retention, and colon transit time. Of the 25 subjects who completed the study, 28% experienced increased GI side effects, 2 noted soft stools, and 5 reported diarrhea. Kim et al.120 demonstrated that Poncirus fructus enhances colon motility and improves constipation symptoms in individuals with SCI with NBD; however, a larger study with a control group is needed prior to recommending routine use of this supplement in practice.
Despite the lack of available literature on vitamin, mineral, and protein supplements for NBD, preventing and correcting nutrient deficiencies in individuals with SCI is essential for optimal long-term health. Providers should supplement an individual with SCI who has a nutritional deficiency with the appropriate vitamin(s), mineral(s), and/or protein supplement. It should be noted there can be complications related to supplementation, mainly related to toxicity, as well as side effects. Clinicians should review an individual’s supplements to ensure that they are not being taken in excess and should discuss potential side effects.
4.2 Providers should refer to a registered dietitian if the individual has poor appetite, poor oral intake, or significant weight changes.
Individuals with SCI who are in the acute phase, in a rehabilitation setting, or in a community setting may experience unintentional weight gain or weight loss for various reasons, including, but not limited to, varying energy needs, appetite, and food choices and availability. Medical nutrition therapy provided to individuals with SCI by a registered dietitian has been shown to improve nutrition-related outcomes, such as adequate nutrient intake and management of serum lipids, weight, dysphagia, bowel function, and pressure injuries.121
4.3 Individuals with SCI should not be uniformly placed on high-fiber diets. Increases in fiber intake from food or a supplement should be done gradually to assess tolerance.
Fiber intake from food and fiber supplements should be assessed in individuals with SCI, as interventions to alter the quantity or type of fiber can be used to influence bowel management. Increases in fiber intake from food or a supplement should be done gradually to assess tolerance and to avoid undesirable side effects from increasing fiber too quickly. If symptoms of intolerance occur, a reduction or change in the type of fiber that is being used is recommended.
The use of fiber supplementation has been studied in the general population, and specific fiber supplements have been shown to be effective for those with constipation. However, well-designed placebo-controlled trials regarding both dietary fiber and fiber supplementation are lacking in the SCI population.
Daily Fiber Quantity Recommendations
The Consortium for Spinal Cord Medicine66 suggests that individuals with SCI consume at least 15 g of fiber per day initially and that they adjust it gradually as tolerated.
The Association of Rehabilitation Nurses practice guidelines suggest including 20 to 35 g of fiber per day for adults without SCI who have constipation.122
The Institute of Medicine123 recommends 14 g of fiber intake per every 1,000 calories consumed. This recommendation is based on fiber intake levels that are observed to protect against coronary heart disease.
Adequate intake for daily recommended fiber is 25 g for women and 38 g for men under 50 years of age. To account for decreased food intake with aging, for men and women over 50 the daily recommended amount is 21 g for women and 30 g for men.123
Fiber Defined124
Dietary: nondigestible carbohydrates and lignin from plants that is intrinsic and intact
-
Functional: isolated, nondigestible carbohydrates that have been shown to have beneficial physiological effects on humans
Fiber supplements: confers some but not all health benefits found in dietary fibers
Fiber-containing foods contain a mixture of fibers, both soluble and insoluble, as well as other beneficial nutrients such as vitamins and minerals. Regarding fiber supplements, the solubility, degree/rate of fermentation, and viscosity/gel-forming capability are the characteristics that drive clinical efficacy and should be taken into consideration when recommending a fiber supplement to provide a health benefit, such as improving constipation.125,126
A fiber supplement that resists fermentation throughout the large intestine, is present in stool, and increases stool water content will be efficacious in improving regularity or in having a laxative effect.126 Although the mechanism of action differs, both insoluble and soluble fiber supplements have been shown to improve constipation by increasing stool water content, thereby creating bulky, soft, easy-to-pass stools.126 For example, psyllium is a soluble gel-forming fiber that has a high water-holding capacity, resists dehydration in the large intestine, and has been shown to be effective in helping both constipation and diarrhea.126 Insoluble fiber can also be effective for constipation, depending on the fiber’s particle size. A large course fiber such as wheat bran will have a mechanically irritating effect on large bowel mucosa and will stimulate water and mucous secretion, leading to increased stool water content and easier-to-pass stools.126 Small, smooth, insoluble fiber particles may have the opposite effect and worsen constipation, as they are unable to mechanically irritate the gut mucosa, add to the dry mass of stool, or decrease the water content of stools, which results in harder stools.126
In considering fiber intake specifically in the population of individuals with SCI, few studies have provided a baseline of pre-intervention. Although many individuals with SCI report that adjusting their diet improves bowel function,93 there is little evidence to support this. Sabour et al.127 found a significant positive correlation of age and time since injury with fiber intake. A higher fiber intake was associated with less bowel dysfunction in a cross-sectional observational study in individuals who were >5 years postinjury.39 Yim et al.128 showed that more individuals with SCI with areflexic NBD reported benefits with a high-fiber diet than did those with reflexic NBD; however, the fiber quantity was not reported. Lynch et al.35 surveyed individuals with SCI and randomly selected controls with a mailed questionnaire and found increasing age corresponded to more frequent fiber use in both groups. Menardo et al.13 reported that subjects with chronic SCI who were receiving the usual hospital diet of 16 g of dietary fiber per day showed delayed left colonic transit. Cameron et al.129 examined the effect of increased dietary fiber intake on mean colonic transit time and concluded that dietary fiber did not have the same effect on bowel function in individuals with SCI as observed in individuals whose bowels functioned normally. The Multidisciplinary Association of Spinal Cord Injury Professionals (MASCIP)89 group highlighted that the results of the small study by Cameron et al.129 may not be particularly relevant to clinical practice, given that the individuals at baseline were already consuming a high-fiber diet that was then supplemented with ground bran cereal and little information was provided about their fluid intake. In addition, wheat bran is high in insoluble fiber and if the bran particles were not coarse or large enough, a constipating effect could be anticipated.
4.4 Foods that cause an individual with SCI to experience excessive flatulence, bloating, abdominal distension, and/or altered bowel movements should be identified and limited or avoided.
Certain foods increase gas production, which may increase or influence GI symptoms in individuals with SCI. A diet that is low in fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) may be useful for improving GI symptoms, particularly in individuals with SCI who had irritable bowel syndrome (IBS) prior to injury. The low FODMAP diet has not been studied in the SCI population, but it has been shown to improve GI symptoms in 50% to 75% of people with IBS.130,131,132 FODMAPs are short-chain carbohydrates that exert osmotic properties in the small bowel and are rapidly fermentable by gut bacteria in the large bowel, which can result in increased gas, bloating, cramping, abdominal distension, pain, and/or altered bowel movements. This diet is best taught by a knowledgeable dietitian and occurs in 3 phases: elimination, reintroduction, and personalization. The elimination phase is ~2 to 6 weeks long, during which low FODMAP foods are consumed and foods high in FODMAPs are avoided (see Table 6 for examples).
Table 6.
Partial List of High and Low FORMAP Foods
| High FODMAPs | Apples, Blackberries, Cherries, Dates, Grapefruit, Mango, Pear, Watermelon | Artichoke, Asparagus, Cauliflower, Garlic, Mushrooms, Onion/shallots/leeks, Sugar snap peas | Barley, Rye, Wheat | Cottage Cheese, Frozen yogurt, Ice cream, Milk, Soy milk, Yogurt | Most beans/legumes, Processed meatsa | Sodas & juices containing high-fructose corn syrup, Rum, Tea: chamomile, oolong, fennel, and chai |
| Low FODMAPs | Banana (unripe), Grapes, Kiwifruit, Lemon, Lime, Mandarin Orange, Papaya, Pineapple | Bok choy, Broccoli, Carrots, Chives, Cucumber, Eggplant, Kale, Lettuce, Olives, Radish, Spinach, Tomato | Corn tortilla, Gluten free-pastas, crackers and breadsa Oatmeal, Potato, Popcorn, Rice, Sourdough bread, Quinoa | Almond milk, Cheese, Coconut Yogurt, Lactose-free ice cream, milk, yogurt,a cottage cheese | Beans/legumes: edamame, lentils, Canned/rinsed: Coffee, Sucrose chickpeas, black beans, diet soft drinks, Beef, Chicken, Egg, Fish/Seafood, Pork, Turkey, Tempeh, Tofu: firm | Alcohol: wine (most), beer, spirits, sweetened or Tea (except those listed above), Water |
Abbreviation: FODMAPs, fermentable oligosaccharides, disaccharides, monosaccharides, and polyols.
aRead ingredients on packaged foods to ensure that they do not have added high-FODMAP ingredients (e.g., high-fructose corn syrup, wheat, onion, garlic). This is not a complete list of foods. Portion size matters when it comes to FODMAPs, as several foods have a specific serving size to determine high vs. low in FODMAPs.
A comprehensive list of foods and their portion sizes can be found in the Monash University FODMAP Diet phone app and The Monash University Low FODMAP Diet Booklet,134 which are regularly updated.
The challenge phase involves reintroducing high-FODMAP foods into the diet in a systematic way over 6 to 8 weeks to identify which FODMAPs cause symptoms and which are well tolerated. The final phase, personalization, involves reintroducing the high-FODMAP foods that are well tolerated to expand variety in the diet while maintaining long-term symptom improvement.
4.5 Providers should recommend that an individual with SCI maintain euhydration and avoid dehydration to reduce the tendency to experience constipation. The amount of fluid needed to promote optimal stool consistency must be balanced with the amount needed for bladder management.
No research has been reported to support the recommendation that individuals with NBD require more fluid than do individuals in the general population.
Increasing fluid intake to improve constipation has been shown to benefit individuals who were in a hypohydrated state, with minimal efficacy demonstrated for individuals who were in a euhydrated state.135 General population guidelines published by the Association of Rehabilitation Nurses suggests including 2 L of fluids per day for people with constipation.122 A cross-sectional study of 125 individuals with SCI who reported their levels of fluid intake, duration of bowel care program, and episodes of incontinence found that participants who reported a water intake of more than 2 L/day tended to have longer durations of bowel care.136 This research was survey based and nonexperimental, and so no causation can be inferred.
Standard guidelines indicate that adult fluid needs can be estimated by 1 of the following 3 formulas:
1 mL/kcal energy consumed137
100 mL/kg for the first 10 kg, 50 mL/kg for the next 10 kg, and 15 mL for each additional kilogram of body weight (calculation: 1,500 mL + ((weight in kg-20) x 15))140
Clinicians should use these calculations to provide an estimate of fluid needs as a starting point and adjust as needed based on the individual. Conditions that increase sweating such as hot weather or a fever can increase an individual’s fluid needs and should be taken into consideration.
4.6 Providers should not routinely recommend probiotics to an individual with SCI.
By definition, probiotics are “live microorganisms, which, when consumed in adequate amounts, confer a health benefit on the host.”141 The efficacy of probiotic use is strain-, dose-, and disease-specific, and should be taken into consideration when recommending a probiotic to an individual. RCTs have shown therapeutic efficacy for probiotic use in antibiotic-associated diarrhea, Clostridium difficile infection, IBS, inflammatory bowel disease, and reduction of risk for neonatal sepsis and necrotizing enterocolitis. Given the concern and potential for commercial probiotics to cause harm, clinicians should not routinely prescribe them and should limit their use to established indications.141
Limited research is available in the SCI population to demonstrate the benefits of routine probiotic use unless an individual is taking antibiotics.
Currently, there are no clear indications or contraindications for the routine use of probiotics for individuals with SCI.
4.7 Probiotics may be advantageous to an individual with SCI who is taking antibiotics by reducing antibiotic-associated diarrhea and Clostridium difficile-associated diarrhea (CDAD).
Wong et al.142 conducted an RCT to assess the efficacy of a commercial probiotic (Lactobacillus casei Shirota) for the prevention of antibiotic-associated diarrhea and CDAD in adults with SCI. They found significantly lower incidences of antibiotic-associated diarrhea in those who had ingested the probiotic drink. A 2017 Cochrane review that examined probiotics for the prevention of CDAD in both adults and children found that probiotics given with antibiotics reduce the risk of developing CDAD by 60% on average.143
5. Oral Medications
5.1 Providers can use oral medications for bowel management; however, the evidence is limited and there are no data to suggest the use of one medication over another.
Management of NBD involves a hierarchical approach to a personalized bowel routine that aims to achieve regular, consistent, predictable bowel movements with adequate stool evacuation; no episodes of incontinence; and prevention of GI and perianal problems. Basic bowel care begins with conservative therapy that includes a combination of oral stimulants and/or medications and rectal laxatives (e.g., suppositories, mini enemas), coupled with mechanical strategies such as DRS or digital evacuation of stool.67
The American Gastroenterological Association Medical Position Statement on Constipation79 recommends treatment of normal or slow-transit constipation to initially include minimizing medications that are constipating (opiates, anticholinergics, etc.) and then gradually increasing dietary fiber or supplemental fiber intake and/or an inexpensive osmotic agent (Milk of Magnesia or polyethylene glycol [PEG]). Depending on stool consistency, the recommended next step is to supplement the osmotic agent with a stimulant (senna, bisacodyl) that is preferably administered 30 minutes after a meal to synergize the pharmacological agent with the gastrocolonic response. Newer agents such as lubiprostone or linaclotide should be considered if there is insufficient or poor response to simple laxatives.
Prucalopride is a serotonin4 receptor agonist with enterokinetic properties approved for chronic constipation. In a randomized, double-blind, placebo-controlled study among 23 individuals with SCI, prucalopride 2 mg once daily increased the weekly frequency of bowel movements and reduced colonic transit time. The standard dose of prucalopride is 2 mg, but in individuals older than 60 years, the dose is usually 1 mg daily. Common side effects of prucalopride are headache, abdominal pain, and diarrhea. The headache often disappears after a few days’ treatment. In the case of diarrhea, the dose can be reduced.144
Many individuals with SCI take opiate medications and are affected not only by NBD, but also by opiate-induced constipation. Newer medications such as naloxegol, methylnaltrexone, and alvimopan are peripherally acting μ-opioid receptor antagonists that selectively block μ-receptors outside the central nervous system and improve constipation without reversing analgesia or prompting opioid withdrawal.145
A few studies show that neostigmine (intravenous, intramuscular, transdermal) facilitates defecation in individuals with NBD by enhancing parasympathetic activity and peristaltic contractions of the left bowel.146 Since neostigmine is an acetylcholinesterase inhibitor, it is co-administered with glycopyrrolate, a selective anticholinergic agent, to attenuate neostigmine’s cardiopulmonary side effects, such as bradycardia and bronchospasms.147 However, the use of neostigmine with a glycopyrrolate as a bowel-cleansing additive should be limited to severe cases of constipation and should be administered only in a closely monitored setting in the hospital.
Multiple studies in the non-SCI population show good efficacy of osmotic and stimulant laxatives in treating constipation.94,148,149,150,151,152,153,154,155 Table 7. (See page 39) describes currently available and emerging agents for constipation.
Table 7.
Luminally Acting Agents for Constipation
| Category/Agent | Mechanism of Action | Clinical Considerations |
|---|---|---|
| Currently available agents | ||
| Bulk laxatives: soluble fiber (e.g., psyllium, methylcellulose, calcium polycarbophil, partially hydrolyzed guar gum, wheat dextrin) and insoluble fiber (e.g., bran, flaxseed, rye) | Increases stool water content to soften stool; increased stool mass might stimulate peristalsis | Use in mild constipation; soluble fiber is more effective than insoluble fiber; psyllium and ispaghula husk most studied; avoid when dyssynergia present |
| Surfactant laxatives (e.g., docusate sodium, docusate calcium) | Anionic detergents lower surface tension of stool; allows water to penetrate stool | Use in mild constipation; psyllium is more effective than docusate |
| Osmotic laxatives (e.g., PEG, lactulose, sorbitol, magnesium salts) | Generation of an osmotic gradient in gut lumen; promotes movement of water into lumen; luminal water softens stool and stimulates secondary peristalsis | PEG and lactulose effective for intermittent and chronic constipation; PEG is more effective than lactulose; might not benefit pain in IBS-C; avoiduse of magnesium in individuals with renal dysfunction |
| Stimulant laxatives such as diphenylmethanes (bisacodyl, sodium picosulfate), anthraquinones (senna, cascara), misoprostol, castor oil | Direct colonic wall irritant; stimulation of sensory nerves on colonic mucosa; possible inhibition of water absorption; prostaglandin-induced effects on motility and secretion with misoprostol | Efficacy for intermittent constipation; diphenylmethanes are effective for chronic constipation; long-term safety not established |
| Chloride channel activation (e.g., lubiprostone) | Secretion of chloride ions into intestinal lumen through direct activation of Cl–C2 chloride channels on enterocytes; results in passive movement of sodium and water into intestine | Short-and long-term efficacy and safety data in chronic constipation and in women with IBS-C; main adverse event is dose-dependent nausea |
| Prucalopride | Serotonin4 receptor agonist with enterokinetic properties | Common side effects are headache, abdominal pain, or diarrhea; headacheoften disappears after a few days of treatment; in case of diarrhea, dose can be reduced |
| Probiotics (e.g., Bifidobacterium lactis, Lactobacillus paracasei) | Hypothesized effects on gut transit and secretion through alteration of gut microbiota | Possible role in chronic constipation and IBS-C; no long-term efficacy or safety data; quality control issues (regulatedas food additives, not drugs) |
| Emerging agents | ||
| Chloride-channel activation (e.g., linaclotide, plecanatide) | Activation of guanylate cyclase c receptor generating cGMP; secretion of chloride ions into intestinal lumen through cGMP-mediated activation of CFTR; results in passive movement of sodium and water into intestine; inhibition of visceral pain fiber firing by cGMP in animals | Effective in chronic constipation and IBS-C in phase II (linaclotide and plecanatide) and phase III clinical trials (linaclotide); main adverse event is diarrhea |
| Bile-acid analogs (e.g., chenodeoxycholic acid) | Increases colonic motor activity; increases luminal secretory activity | Effective in IBS-C, as shown in phase II trial; risk of abdominal pain and/or cramps |
| Inhibitors of bile-acid resorption (e.g., elobixibat) | Partial inhibition of ileal bile acid transporter; increases colonic bile acid concentrations, promoting colonic motility and secretion | Effective for chronic constipation, as shown in phase II trial; dose-dependent abdominal pain reported |
| Peripherally acting μ-opioid receptor antagonists (e.g., methylnaltrexone, alvimopan) | Improve constipation without reversing analgesia or prompting opioid withdrawal by selectively blocking μ-receptors outside the CNS | Effective in controlling constipation in chronic opioid users; methylnaltrexone (6 RCTs) and alvimopan (4 RCTs) were shown to be superior to placebo; adverseeffects are abdominal pain and diarrhea |
Abbreviations: CFTR - cystic fibrosis transmembrane conductance regulator; cGMP - cyclic guanosine monophosphate; CNS - central nervous system; IBS-C - constipation-predominant irritable bowel syndrome; PEG - polyethylene glycol; RCT -randomized controlled trial.
6. Use of Suppositories, Enemas, and Irrigation
6.1 Providers can use rectal medications for bowel management.
Rectal medications are a key component of conservative bowel care for individuals with SCI who have reflexic NBD.89 Rectal medications are currently one of the most commonly used bowel management programs.98,106 These medications can also be used as rectal irritants in the management of areflexic NBD.
Chemical Rectal Agents
Bisacodyl and Glycerin Suppositories
Glycerin and bisacodyl are commonly used active ingredients in suppositories for bowel care. The glycerin suppository acts as a mild local stimulus and lubricating agent.
Bisacodyl Suppository
Brand Names: Dulcolax, Correctol, Magic Bullet, Carter’s Little Pills, Fleet Bisacodyl
A bisacodyl suppository is a contact irritant that enhances gastric motility, increases fecal water content, and reduces transit time in the large intestine.156 The 2 variants available for bisacodyl suppositories are hydrogenated vegetable oil based (e.g., Dulcolax) and PEG based (e.g., Magic Bullet).90 The bases act as vehicles for delivering bisacodyl, the active ingredient.
No clear indications or contraindications for bisacodyl suppositories or enemas were found in the SCI literature. Contraindications for their use in the general population are ileus, intestinal obstruction, acute abdominal conditions (including appendicitis), acute inflammatory bowel diseases, severe abdominal pain associated with nausea and vomiting, severe dehydration, and anal fissures or ulcerative proctitis with mucosal damage.157
Glycerin Suppository
Brand Names: Colace Glycerin Suppositories, Fleet Babylax, Fleet Glycerin Suppositories Adult, Glycerin Suppositories Maximum Strength, Sani-Supp Glycerin is one of the main suppository types used in SCI bowel management. It facilitates defecation by acting as a lubricant and stimulating rectal contractions via local hyperosmotic activity and mild irritation. It takes approximately 15 to 30 minutes following insertion of the suppository for a bowel movement to occur.89 No clear contraindications for glycerin were found.67
Suppositories are used to facilitate initiation and completion of rectal emptying of stool. Studies have investigated the use of suppositories in bowel management in SCI. Use of suppositories has been shown to decrease duration of time spent on bowel care and the need for nursing/caregiver assistance, specifically for PEG-based suppositories.158,159,160,161,162 There is at least one good quality RCT study by House and Stiens that showed effective response to PEG-based bisacodyl suppositories for bowel management.158 There are other studies with lower levels of evidence that support this finding.160,161,162
Amir et al.162 showed that for segmental colonic transit time, glycerin suppositories and mineral oil enemas were better than docusate sodium and bisacodyl suppositories in reducing right colonic transit time. Furthermore, bisacodyl suppositories, a contact stimulant laxative that stimulates sensory nerve endings to increase colonic peristaltic activity, is as effective as docusate sodium in shortening the rectosigmoid colonic transit time. In the same study, however, bisacodyl decreased difficulties in evacuation better than glycerin suppositories did.162
Stiens et al.66 suggest that glycerin suppositories are often used during the transition from bisacodyl suppositories to digital stimulation in individuals with SCI because they provide a less potent chemical stimulus. Individuals with SCI can be put on a schedule of alternating bisacodyl and glycerin suppositories. Stiens et al.66 recommend that the frequency of DRS may need to be increased with the glycerin suppositories in order to achieve similar results. In many people, transitioning to glycerin as the sole chemical triggering agent is possible, alternating with just DRS. Thereafter, similar results can be achieved with DRS alone to trigger and maintain the progress of defecation.66
6.2 A PEG-based bisacodyl suppository is recommended over a hydrogenated vegetable oil-based bisacodyl suppository.
PEG-based bisacodyl outperformed hydrogenated vegetable oil-based bisacodyl across multiple outcomes and studies. Individuals who received PEG-based bisacodyl had flatus 12.8 to 15 minutes after administration,158,161 20- to 32-minute-long defecation sessions,158,161 and total bowel care times of 43 to 66 minutes.158,161 These outcomes were 44.8% to 58.7% faster than when hydrogenated vegetable oil-based bisacodyl was given to the same individuals to initiate bowel care. Stiens et al.161 attributed this difference to the more effective ability of PEG-based suppositories to readily dissolve from body heat, to distribute bisacodyl on mucous membranes, and to sustain reflex propulsion of stool.
6.3 Docusate mini enemas are recommended over glycerin, mineral oil, or vegetable oil-based bisacodyl suppositories.
Docusate Sodium Mini Enemas
Docusate sodium is a stool softener that emulsifies fat in the intestines and reduces water reabsorption.66 The 2 brand names of docusate sodium mini enemas searched for were Therevac SB and Enemeez, both of which contain docusate sodium (283 mg) combined with glycerin in a PEG solution.163 Therevac SB has since been discontinued, but Enemeez is still in production with an additional formulation called Enemeez Plus. This version of Enemeez contains benzocaine 20 mg, which the manufacturer claims reduces the risk of autonomic dysreflexia (AD) in susceptible individuals.
As no research was found pertaining to Enemeez, these results focus on Therevac and may not necessarily translate to the use of Enemeez in the SCI population. In addition, only comparative studies were extracted for Therevac. Within the examined studies, individuals receiving Therevac had flatus 15 minutes after administration,158 17- to 31-minute-long defecation sessions,158,162 and total bowel care times of 37 minutes.158
Therevac SB was more efficacious at reducing difficulties in evacuation and produced shorter evacuation times than did glycerin, mineral oil, and bisacodyl suppositories of an undisclosed base type.162 The study by Dunn and Galka160 suggests that Therevac facilitates quicker bowel evacuation times than bisacodyl suppositories do, although the base type was not stated. The base type of bisacodyl could alter interpretations of these results. House and Stiens158 found that Therevac outperformed hydrogenated vegetable oil-based bisacodyl suppositories, but had comparatively similar results to PEG-based bisacodyl. Notably, in this study, participants were given Therevac only if they routinely used it for bowel management before the study. This methodology introduces significant biases in favor of Therevac.
6.4 The routine use of enema formulations such as sodium phosphate (Phospho-Soda), soapsuds, or milk and molasses is not recommended; however, in select individuals, intermittent use for constipation may be helpful.
Enemas
Fleet Enema
Only one study on the use of oral Fleet Phospho-Soda for colonoscopy preparation was extracted. The evidence from this study is potentially indirect because the intervention was not an enema, not given more than once, and not used for bowel care treatment. Subsequently, the evidence described below may not reflect the efficacy or complications of repeated use in an appropriate clinical setting or bowel care program.
The standard Fleet Enema is a saline laxative that contains monobasic and biphasic sodium phosphate. It draws water into the small intestine, causing distension and peristaltic action, which assists in bowel evacuation.66 No specific parameters for Fleet administration for individuals with SCI were found. Although Fleet Enema packaging instructs that bowel movement can be expected 2 to 5 minutes following administration,164 Stiens et al.66 advise that phosphate enemas have an unpredictable onset. Because of severe adverse reactions within the general population, the U.S. Food and Drug Administration (FDA) strongly supports adherence to the recommended dose of 1 sodium phosphate product per 24 hours.165 The FDA also states that individuals who are taking sodium phosphate products should be well hydrated and have their electrolyte balance and renal function assessed if they are at high risk, vomit, have signs of dehydration, or retain a rectal dose for more than 30 minutes.165
Expert opinion does not generally support the use of phosphate enemas (such as Fleet) for individuals with SCI for bowel management. Stiens et al.66 recommend that more gentle stimulants or treatment options should be considered before using phosphate enemas. MASCIP guidelines89 cite the difficulty of retention, unpredictable onset, and risk of AD, watery stool, or abdominal cramping as reasons that the use of large-volume phosphate enemas is uncommon. Stiens et al.66 also mention that long-term use can cause an enema-dependent bowel. If alternative treatments are ineffective or inappropriate, phosphate enemas can be used in combination with oral medication.66,89 In 2014, the FDA released a warning that physicians should be consulted prior to the use of sodium phosphate enemas for individuals who are over the age of 55, have an inflamed colon, or are taking drugs that alter kidney function, such as angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and nonsteroidal anti-inflammatory drugs.165 The FDA also states that sodium phosphate enemas should be recommended cautiously to those with hypovolemia, decreased intravascular volume, baseline kidney disease, decreased bowel transit time, bowel obstruction, or active colitis.165
Contraindications specific to the Fleet Enema include appendicitis (or symptoms such as abdominal pain, nausea, fever, or vomiting), intestinal blockage, ulcerative colitis, ileitis, heart disease, rectal bleeding, high blood pressure, kidney disease, severe dehydration, and debility.164
Soapsuds Enema
No SCI-related research studies were extracted, but a description of soapsuds was obtained from studies of the general population and of individuals with liver transplant. As hypotonic solutions, soapsuds enemas are believed to enhance defecation by chemically irritating the rectal mucosa and introducing a large volume into the rectum.166 The following information pertains to the use of soapsuds enemas in the adult general population reported by Schmelzer et al.166,167 Because of the lack of consensus on the administration of soapsuds enemas even in the general population, a description is included here. In these studies, soapsuds enemas were prepared by using 6 g of castile soap per 1,000 g of deionized water.166,167 Schmelzer described warming the enema solution to 40° C (105° F) prior to administration to avoid hypothermia.
Soapsuds enemas are primarily used to treat constipation; however, in Schmelzer et al.,166,167 soapsuds enemas were used for colonic cleansing. Although there are no clear contraindications for these enemas, it is uncertain whether they produce a greater stool output than tap water enemas of equivalent volume.166 Nonetheless, the single administration of a soapsuds enema produced significantly greater net mean stool output than a PEG enema did.
Milk and Molasses Enemas
A description of these enemas was provided from the study of a population treated in a hospital emergency department, although no SCI-related studies were extracted regarding milk and molasses enemas. Sugars present in milk and molasses enemas are speculated to interact with the intestinal lining to produce gas, facilitating defecation by increasing intestinal pressure, distension, and peristalsis.168 Vilke et al.168 reported this prospective cohort study conducted at the University of California Emergency Department. Additional information, including how to prepare this enema, can be found in the article by Vilke et al.168
6.5 Transanal irrigation (TAI) is recommended in individuals with NBD who have insufficient results with BBM.
During TAI, irrigation fluid is electrically or gravity pumped from a reservoir into the colon via a rectal cone or rectal catheter that has been inserted into the anus.169 Experts recommend performing TAI 20 to 30 minutes after a meal to take advantage of the gastrocolic reflex.170 TAI should also begin after the user is positioned over a toilet or commode, so that the rectum can be emptied or digitally checked for emptiness if the user has lost sensory awareness. After the device has been inserted, a user or caregiver should hold it in place for the duration of irrigation.169 Balloon catheters, if used, should be inflated with care to avoid triggering reflex contractions or AD. Repeated catheter expulsion and balloon bursting are the 2 most commonly cited reasons for individuals to reject TAI.55,171,172,173,174,175 Experts suggest that irrigation fluid should be clean water at 36 to 38°C pumped at a rate of 200 to 300 mL/min.170,175 Starting at 500 mL, the total volume can be increased by 100-mL increments during each session until irrigation is successful without leakage.175 If electrolyte balance is a concern, saline should be used instead of water. In the event of cramping, discomfort, or pain during irrigation, pumping should be paused and then continued at a slower rate.170 When single irrigation sessions fail, 2 half sessions with a 10-to 15-minute break in between is recommended. If pain or fecal incontinence persists, constipation should be investigated, along with reducing the irrigation volume or using constipation agents.176 No clear parameters for TAI frequency have been defined.
Indications and Contraindications
Experts recommend TAI for individuals who are refractory to conservative methods, and who have a low rectal volume at defecation urge and at maximal capacity.170 Faaborg et al.173 demonstrated that the following factors positively influence TAI success: male gender, dual constipation and incontinence symptoms, and prolonged colorectal transit. Individuals with full or restricted hand function experienced improvements with TAI. It is unclear whether a user’s dependence on assistance during bowel care or SCI etiology affects the success of TAI.55,172 Emmanuel et al.170 suggest the following absolute contraindications for TAI: anal or rectal stenosis, active inflammatory bowel disease, acute diverticulitis, colorectal cancer, ischemic colitis, rectal surgery within the previous 3 months, or endoscopic polypectomy within the previous 4 weeks. Relative contraindications for TAI are severe diverticulosis; dense sigmoid disease; history of diverticulitis, diverticular abscess, or rectal surgery; long-term steroid medication; fecal impactions; painful anal conditions; planned or current pregnancy; bleeding diathesis or anticoagulant therapy (except aspirin or clopidogrel); and severe AD.170
Efficacy
Evidence supports the success of TAI in treating constipation (40% to 63% of cases), fecal incontinence (47% to 72.7% of cases), and prolonged defecation time.171,173,177 In addition, TAI improves symptom-related quality of life (QOL), with 2 studies indicating increased satisfaction and opinion of intestinal function.171,172,174,177 In an observational study, TAI reduced or eliminated pharmaceutical use in 28.6% of subjects.177 Although TAI is considered a second-line treatment for conservative bowel management, it outperformed or matched conservative treatment in all parameters in a comparative RCT.55 Furthermore, a computer-modeled cost analysis of TAI against conservative bowel management estimated significant lifetime financial savings.178 A German study affirmed that although TAI had a higher product cost compared with conservative bowel management, the overall cost was offset by lower caregiver costs, bowel care time, and frequency of urinary tract infection.179 Notably, 92% of TAI studies were affiliated with Coloplast, a manufacturer of TAI-related products.
Complications
The most common adverse events in the single TAI RCT were abdominal pain (15.7% of all bowel observations), sweating (10.5%), chills (7.0%), dizziness (5.4%), and pronounced general discomfort (5.9%).55 A global audit of TAI (Peristeen)-related bowel perforation found the overall average risk to be 6 per million procedures, with 83% of perforations resulting in emergency surgery.180 Caution is necessary when using this method in individuals at risk for AD.
6.6 Pulsed irrigation evacuation (PIE) in a hospital/clinic setting can be used to relieve fecal impaction.
PIE involves widening of the anus with a lubricated speculum and the application of 5 mL/second pulses of tap water from a cuffed tube.181 These pulses are used for up to 1 minute until stool disimpaction or bowel peristalsis is triggered to assist evacuation. The treatment is considered complete when anal outflow is clear with no visible fecal matter after evacuation. The speculum should be inserted cautiously in individuals with a history of rectal bleeding from hemorrhoids, as it is possible to cause rectal bleeding.181
Indications and Contraindications
In the single study of PIE, it was used for individuals who had an ineffective bowel routine and were symptomatic. Caution is necessary when using this method in individuals at risk for AD, impaction, or asymptomatic impaction.181 The stated absolute contraindications for PIE were colon surgery within the past year, evidence of acute abdomen, and evidence of acute diverticulitis. The relative contraindications for PIE were histories of colon surgery longer than 1 year ago, rectal or lower GI bleeding, or diverticular disease.
Efficacy
PIE successfully removed stool for 98% of individuals who used it for ineffective bowel routine, symptomatic impaction, or asymptomatic impaction.181
Complications
Complications reported to be associated with PIE were minimal, except for rectal bleeding during speculum insertion in individuals with a history of rectal bleeding from hemorrhoids.181
7. Impact Of Posture And Activity On NBD Physical Activity
7.1 Regular physical activity should be encouraged as part of a healthy lifestyle.
The literature on the relationship between physical activity and bowel outcomes in SCI is sparse. A cross-sectional study showed that sports participation is associated with better bowel continence in SCI.182 In the general population, lack of exercise or physical activity may play a role in constipation, especially in those who are very sedentary.183 Several potential mechanisms may explain the impact of exercise on bowel function, including an increase in fluids due to thirst after exercise or stimulation of the muscle and nervous systems.
Regular physical activity is recommended as part of a healthy lifestyle, which may in turn positively affect bowel function. Individuals with SCI may engage in a variety of physical activities, including sports, wheeling, lifting weights, or structured exercise. Although therapeutic devices such as functional electrical stimulation (FES) leg cycle,184 body weight-supported walking systems,185 or robotic walking exoskeletons186,187,188,189 can facilitate physical activity, it is not known whether these activities affect bowel function beyond that of standing activities or other standard physical activities such as an aerobic arm ergometer or wheeling exercise.
Standing
7.2 For some individuals, a standing program may be beneficial for bowel function but should be weighed against other means of physical activity, as well as against precautions to undertake the activity safely.
A standing program may facilitate bowel management in some individuals who do not stand or walk regularly.190,191,192,193 The evidence is supported by cross-sectional surveys that reported that people who participated in standing programs perceived benefits to their bowel management. The use of assistive devices, such as orthoses, standing frames, or standing wheelchairs, can facilitate upright posture to standing. However, a cross-over study of 17 individuals with SCI did not show an effect on any bowel outcomes of a 6-week, 5 days-per-week tilt-table standing program, although 8 people perceived an improvement in bowel function. Furthermore, it is unknown whether general physical activity of the upper extremities (e.g., wheeling) better facilitates bowel function over that of standing. It is critical that individuals have appropriate equipment and physical assistance to stand safely and to avoid adverse events such as skin breakdown, falls and fractures, orthostatic hypotension, and pain. A minimum of 30 minutes of standing 3 times per week has been recommended by MASCIP193 for general health after SCI. Upright posture of the individual during bowel management (i.e., using a toilet) has been significantly associated with successful bowel evacuation.195 However, this finding could be confounded by individuals who are less severely affected (physically and in bowel function) being more able to achieve an upright posture during bowel care.
8. Use Of Functional Magnetic Stimulation (FMS)
8.1 Routine use of FMS for NBD is not recommended.
In FMS, a magnetic field from a coil is used to stimulate spinal nerves.196 In individuals with SCI, FMS has been applied over the suprapubic region; the thoracic, lumbar, and lumbosacral spinal cord; and the pudendal nerve. Small observational studies have indicated that transabdominal196 or thoracic and lumbosacral FMS can reduce colonic transit time and alleviate NBD.197 In addition, FMS reduced gastric emptying time.196 Applying it to the sacral roots198 or the pudendal nerve199 was found to increase anal resting pressure. However, all studies of FMS in persons with NBD were small and observational in design. Hence, the level of evidence for efficacy is low, and FMS is therefore not recommended for the treatment of NBD.
9. Use of Functional Electrical Stimulation (FES)
9.1 FES systems are not recommended for regular clinical use in NBD.
FES of bowel function can be performed by using one of several methods. Some are based on direct stimulation of the efferent nerve fibers to the bowel or the pelvic floor, whereas others act through stimulation of the afferent nerves or by stimulation of both efferent and afferent pathways.
For sacral anterior root stimulation (SARS), a posterior laminectomy is performed. Sleeved electrodes are placed for direct stimulation of the anterior roots, usually S2–S4, while bilateral posterior rhizotomy is performed for S2–S5. The electrodes are connected to a transmitter box placed in the subcutaneous fat tissue of the abdominal wall. The individual can activate stimulation by means of wireless technology.200,201 Observational studies among individuals with SCI have shown that SARS reduces symptoms of constipation in general,200,202 increases the frequency of defecation,200,203 and reduces time spent on bowel management.201,204 SARS also reduces the number of other methods used for bowel care, as 67% of individuals use it as their primary method for bowel evacuation.205 The use of SARS is limited by the need for spinal surgery, concerns about posterior rhizotomy, and the fact that the technology is available at only a few centers.
For sacral nerve stimulation (SNS), an electrode is placed through the posterior foramina of the sacral bone, usually S3.206 If symptoms are significantly reduced during a 3-week evaluation period, a permanent electrode is placed and connected to a battery placed in the subcutaneous fat of the gluteal region. The stimulation parameters can be adjusted by hospital staff to achieve the best possible effect. The exact mode of action is unknown, but SNS is likely to have both direct effects on the anal sphincter complex and afferent effects through the sacral nerves and the spinal cord.206 Small observational studies among individuals with incomplete SCI have shown that SNS reduces fecal incontinence,207,208,209,210 reduces the use of pads for fecal incontinence,209,210 decreases constipation,209,211 and improves QOL.207,208,209,210,212,213 SNS is a minor invasive surgical method with few complications. However, its use among individuals with SCI is still limited by lack of controlled clinical trials and the fact that few data have been published from individuals with complete SCI.
For posterior tibial nerve stimulation (PTNS), a temporary electrode is placed close to the posterior tibial nerve to provide afferent stimulation of the sacral spinal cord. A single observational study among individuals with incomplete SCI has indicated that PTNS can reduce fecal incontinence, increase anal squeeze pressure, and improve fecal incontinence-related QOL.214 PTNS is without risk, but the evidence for efficacy is still limited.
For dorsal genital nerve stimulation (DGNS), a plaster electrode is placed over the dorsal genital nerve of the penis or clitoris to provide afferent stimulation to the sacral spinal cord. In a small study of 7 individuals with SCI, acute DGNS caused minor contractions of the rectum.215 However, the effects on bowel symptoms remain obscure.
Electrical stimulation of the abdominal muscles, either by surface electrodes located above the external oblique and rectus muscles or by electrodes worn in a belt around the abdomen, may aid defecation by stimulation of the muscles of the abdominal wall. In a small RCT that included 8 subjects with SCI, electrical abdominal stimulation reduced the time needed for bowel care.216 In another small RCT that included 10 individuals with SCI, electrical stimulation of the abdominal muscles applied for 25 minutes each day for 8 weeks reduced colonic transit time.217
In another study, perianal electrical stimulation caused acute contractions of the anal canal in 4 of the 5 individuals with SCI, and Praxis implantation improved evacuation in a study of a single individual. However, none of the methods have been further studied in individuals with SCI.
Epidural electrical stimulation is currently being explored as a method for restoring some neural function in individuals with SCI. Case reports have indicated that this technique may improve bowel function.218
Several studies have found that FES may improve bowel function in those with SCI. However, the level of evidence for effect remains low, as no sufficiently powered placebo-controlled studies have been performed.
10. Surgical Intervention to Manage NBD
10.1 Malone antegrade continence enema (MACE) procedures can be used for individuals with SCI with severe NBD for whom other treatment modalities have failed.
10.2 The MACE procedure can be a choice for individuals with NBD who prefer the option after thorough education regarding risks, benefits, and complications and after shared decision making with their providers.
Individuals with SCI have several options to manage severe NBD. Colostomy is the most commonly performed surgical option and can be routinely performed today by using a minimally invasive approach. However, it may be complicated by stoma prolapse or stricture and parastomal hernia, and individuals with SCI may also be plagued with frequent rectal discharge following the colostomy. The MACE procedure is a safe and effective treatment for NBD when conservative management, including TAI, is unsuccessful or contraindicated. MACE involves the surgical creation of an entry by using the affected individual’s native appendix connected to the abdominal wall. Through the small stoma (appendicostomy), a catheter is introduced to administer an enema that irrigates the colon and rectum. Although the valve mechanism is surgically created to allow catheterization of the appendix for irrigation, it also avoids leakage of stool outside the appendiceal stoma. In addition, MACE does not require an external device to be worn for waste, and there is therefore also less odor. Potential complications of the MACE procedure include stomal stenosis, stomal site infection, leakage through the stoma, and difficulty with stomal catheterization.
A decision analysis study used systematic reviews, utilities catalogues, and life table analyses to compare 4 surgical strategies for bowel care after failure of conservative clinical management.219 The analysis included colostomy, ileostomy, the MACE procedure, and SARS. The results demonstrated that the MACE procedure had the highest quality-adjusted life expectancy and may also provide the best long-term outcomes in terms of the probability of improving bowel function and reducing complication rates and the incidence of AD. While several studies have demonstrated proof-of-concept of these treatments, larger randomized studies are lacking, and long-term effects should continuously be evaluated and reevaluated.219
The overall success rate of using MACE in individuals with neurological diseases, including SCI, was 75% and 85% after a mean follow-up of 38 and 75 months, respectively. Complication rates vary, often depending on the skill of the surgeon, and include wound infections, bowel obstructions, and stomal stenosis. There is level 4 evidence that the MACE successfully treats NBD.49,171,220,221,222,223
10.3 Colostomy is recommended for individuals with severe NBD for whom other treatment modalities have failed or who have had significant complications.
10.4 Colostomy can be a choice for individuals with NBD who prefer the option after thorough education regarding risks, benefits, and complications and after shared decision making with their providers.
There is no general consensus as to when a colostomy should be performed. Individuals with relatively recent SCI may choose colostomy as a preferred option for bowel management. The optimal timing of instituting the colostomy is open for discussion. In a retrospective study, Boucher et al.224 evaluated patients who were identified as having a colostomy “early,” in the first few months after SCI, and those who chose “later,” usually more than a year after injury and being discharged home. Early complication rates in both groups were low. Long-term complications were higher in the early group. Parastomal hernia rates were low in both groups, as was the need for further surgery. Following colostomy formation, 9 of the early group and 6 of the later group achieved independence with bowel care. Overall, 20.8% of the individuals in this study who had previously been reliant on caregivers gained independence.224
Co-management with an enterostomal therapist prior to surgery and in follow-up optimizes outcome. Colostomy has been compared with conservative management bowel programs and found to have equivalent or superior QOL outcomes. Colostomy decreases time spent on bowel care, decreases the number of hospitalizations, improves physical and psychosocial health, and improves independence. It becomes a more favorable option with increasing age. Solid stool is generally considered easier to manage than more liquid stool. Dissatisfaction is higher among individuals with SCI who have an ileostomy than among those who have a colostomy. Keeping the left colon as distal as possible (sigmoid colostomy) best maximizes water absorption and prevents dehydration. Right-sided colostomies are less likely to have problems with emptying, but they result in more watery stools, more frequent stoma care, and a risk for left-sided colonic diversion colitis. It is uncommon to have the stoma reversed after it has been created. Complications of colostomies are leakage of mucus via the rectum, stomal prolapse, parastomal hernias, and bowel obstructions. It is paramount that individuals be well informed of the short- and long-term complications.
Six studies demonstrated that colostomy reduces the number of hours spent on bowel care225,226,227,228,229,230; 1 that colostomy reduces the number of hours spent on bowel care and that it simplifies bowel care routines225; and 1 that colostomy reduces the number of hospitalizations, improves physical and psychosocial health, and reduces the need for laxative use and dietary manipulations to assist bowel care.228 There is also evidence that colostomy reduces the need for laxative use and dietary manipulation to assist bowel care.231
Individuals with SCI are prone to chronic infections in the pelvis and perineum secondary to pressure injuries. If conservative management fails, surgery with wide resection of the ulcer with a myocutaneous flap and a diverting colostomy can be used to optimize healing of the flap. The decision on whether to use fecal diversion to optimize flap healing for pressure injury repair is often dependent on the plastic surgeon’s preference. It is impossible to determine how often this occurs because each individual has other comorbidities and different flap reconstructive options. The surgeon may be more likely to choose to use a diverting colostomy in the setting of coexisting osteomyelitis and diabetes. In addition, if the ulcer is close to the anus and the flap is in close proximity to its orifice, a colostomy is best performed.
A study that compared the outcomes of individuals with SCI and pressure injuries who electively underwent fecal diversion with those who did not demonstrated that stoma construction remains a safe procedure with low morbidity and mortality. It also showed that the pressure injury recurrence rate was lower in the colostomy group compared with that in the non-colostomy group. In addition, noncolostomy individuals had longer healing times and required more surgical procedures to address their ulcers.232,233
11. Managing Medical Complications of NBD
11.1 Providers must assess and monitor for the unique clinical presentation of GI and intra-abdominal complications related to NBD in individuals with SCI.
Depending on an individual’s level of SCI and associated altered visceral sensation, the typical signs and symptoms associated with intra-abdominal pathology may be altered or absent following SCI. Pain may or may not be a presenting symptom; when it is present, it may be dull, poorly localized, or oppressive. Anorexia is a common presenting feature of abdominal pathology in an individual with SCI. Nausea and/or emesis with or without abdominal distension should also raise concern, as intra-abdominal tenderness is not common in individuals with injuries above T5. Abdominal pathology in individuals with injuries at or above T6 may present with AD, vague nonlocalized discomfort, increased spasticity, and/or a rigid abdomen. An injury level between T6 and T10 may allow some localization of pain via sympathetic visceral afferent innervation and/or somatic afferent innervation from the abdominal wall. A level below T12 yields abdominal symptoms and findings similar to those of neurologically intact individuals.
Given the atypical and variable presentations of GI complications in the setting of NBD, it is imperative that health care providers have a high index of suspicion to initiate timely diagnostic evaluation and treatment for complications such as severe constipation, bowel obstruction, ileus, small intestine bacterial overgrowth, and ischemic bowel syndrome. In a large national database review, Tseng et al.234 found that individuals with SCI were at significantly higher risk for ischemic bowel syndrome than were matched controls without SCI.
NBD with prolonged colonic transit time can lead to marked abdominal distension with chronic constipation and a dilated colon, further aggravating bowel evacuation. The presence of an obstructing lesion can be delineated with a CT scan or possibly a barium contrast enema. If there is evidence of a proximal bowel impaction, oral stimulants may be required. However, use of such stimulants in the setting of, or suspicion of, colonic obstruction could result in intestinal perforation.
11.2 Providers must assess and monitor for complications that primarily affect areas outside the abdomen but that are related to NBD, such as AD and skin breakdown.
Individuals with NBD are at increased risk for skin breakdown and pressure injury. Fecal incontinence can lead to overgrowth of perianal microorganisms, which weakens the skin, resulting in increased risk of skin breakdown. In addition, prolonged sitting on an inadequately padded bowel care seat without frequent pressure relief can result in pressure injury.
Individuals with a T6 level of injury and above are at risk for AD, a syndrome of autonomic dysregulation in which there is a partially or completely unopposed outflow of sympathetic stimulation in response to a noxious stimulus below the neurological level of injury. That stimulation could result from intra-abdominal pathology, or from bowel care positioning or emptying techniques such as manual evacuation, digital stimulation, or suppository insertion. If the individual with SCI has signs and symptoms of AD such as bradycardia, cardiac arrhythmia, pounding headache, anxiety, sweating above the level of their SCI, flushing, blurry vision, nasal congestion, or piloerection, a survey must be quickly undertaken to identify and remove or correct the underlying cause. Inciting factor(s) for the AD may or may not be related directly to the NBD or its management, and so other potential causes must be considered as part of the acute assessment of the individual. A thorough discussion of AD and its potential causes and management are beyond the scope of this CPG; the reader is encouraged to review the guidelines in the Consortium of Spinal Cord Medicine Evaluation and Management of Autonomic Dysreflexia and Other Autonomic Dysfunctions: Preventing the Highs and Lows.104
11.3 Treatment for hemorrhoids is conservative; if bleeding is refractory, non-excisional techniques are warranted. Excisional hemorrhoidectomy should be avoided.
Individuals with SCI develop common benign anorectal conditions similar to those in neurologically intact individuals. These conditions may include hemorrhoids, anorectal abscesses and fistulae, rectal prolapse, and pilonidal disease, as well as other entities. If there is any uncertainty in diagnosis or if symptoms cannot be explained, flexible sigmoidoscopy and pelvic magnetic resonance imaging should be used. Chronic constipation, straining, pelvic and perineal pressure, stasis, hygiene, and poor blood flow often contribute to the development of these entities. Hemorrhoids are best managed conservatively except for chronic blood loss, in which case rubber band ligation, infrared coagulation, and sclerotherapy are options.235,236 Excisional hemorrhoidectomy should be avoided unless the pedicle has evidence of a necrotizing infection. Perirectal and perianal abscesses should be adequately drained. Anal fistulae are best managed with non-cutting setons and these setons may be required long term.
12. Education for Individuals with SCI and Caregivers
12.1 Education for individuals with SCI, caregivers, and health care providers should be provided and be comprehensive to all levels of learners.
Education for individuals with SCI is a priority of care and a key factor in how well they transition from hospital to community and how they live with a disability.237,238 Little research has been conducted on measuring bowel education programs even though bowel education has been reported as a top research priority.239,240,241 Research studies that have focused on nursing have shown that 50% of nursing care was directed toward psychosocial support, with medication, skin care, and bladder, bowel, and pain management being the main education topics.242
Individuals with SCI may have difficulty adjusting to the effects and care needs resulting from their SCI. Their level of willingness to learn and difficulty in coping may have a direct relationship to their reluctance to learn.243 Some individuals may also be resistant or not ready to discuss bowel care education, or not ready to assume the responsibility of performing or directing their bowel care.244 For individuals with SCI who are not ready to receive education in the hospital setting, it is important that it be available to them after they are out in the community.245
Educational needs, as well as learners, should be identified. Learners can be considered individuals with SCI, caregivers, family members, or anyone else involved their care. Addressing the level of education of the affected individual or caregiver is important in discerning the type of educational resources that will be needed. Providing individuals with SCI and their caregivers with education on the anatomy and physiology of the GI system and the definitions of NBD should be completed prior to implementing a bowel program.
12.2 The components of the bowel program should be taught to individuals with an SCI as well as to their caregivers.
Defining what a bowel program is in relation to the SCI should be completed. Definitions can include rectal stimulation, manual evacuation of stool, reflexic and areflexic NBD, and any other process that is to be included. Directions on how to perform bowel care from the perspective of the affected individual and the caregiver should also be discussed and educational material made available, as well as a return demonstration of the bowel care. The suggestion should be made that individuals with SCI and/or their caregivers keep a log of the bowel program, including total time for bowel care, results, complications, and dietary intake (see Recommendation 1.3).
Additional discussion should include clothing management, bathroom accessibility, positioning in bed and/or on equipment, adaptive tools, post-program hygiene, and skin protection (see Recommendations 2 and 3). If the individual with SCI can transfer or has a lift, he or she is encouraged to sit upright on a padded toilet seat, commode, or shower chair for bowel care. Peristaltic activity is greater when sitting upright and gravity can aid the expulsion of stool from the rectum. All individuals with SCI and caregivers should be educated on how to perform bowel care in bed if sitting upright is not feasible or not an option.
Individuals with SCI and their caregivers should be educated in monitoring for signs of skin breakdown or pressure injury by performing daily skin checks; they should receive education on the importance of incontinence management, perineal care, and not using briefs because of the risk of skin breakdown from moisture retention.
Prescribed bowel medications and timing should be discussed, including the type, purpose, dose, frequency, side effects, and potential drug interactions. Both individuals with SCI and caregivers should understand the difference between laxatives, stool softeners, and enemas.
Education on nutrition and fluid management should be provided to individuals with SCI and caregivers. Nutritional assessment may be beneficial so that individuals with SCI can assist with their own best needs (see section 4 for further recommendations).
Individuals with SCI who have an ostomy placed will benefit from education on how to care for it, as well as on signs and symptoms of complications. Supplies are needed for an ostomy or ileostomy, and maintenance of these supplies is key for ostomy care.
Individuals with SCI and their caregivers should be evaluated and educated by an enterostomal nurse before being discharged, and they must return demonstration of ostomy care. Individuals can establish care with either home care services or their primary care clinic to reinforce education. Education should include instructions on emptying the pouch and how to perform skin care around the stoma.231,246
12.3 Education on potential complications should be provided.
Individuals with SCI and their caregivers should be educated on how to monitor for complications such as foul odor for more than 1 week, erythema and irritation around the stoma, no bowel movements, nausea, vomiting, pain, cramping, bloating, or change in the size of the stoma. Individuals should notify their provider about any complications or issues.246
Individuals with SCI and caregivers should be educated about potential complications and possible issues with the bowel program, including (but not limited to) constipation, incontinence, impaction, ileus, diarrhea, hemorrhoids, AD, anal fissures, and skin breakdown. Information about whom they should contact, and when, should be provided to individuals with SCI and their caregivers.
12.4 Education and support for the caregiver should be considered and provided when appropriate.
Willingness to learn, understanding and retention of the procedure, and competency of the caregiver or those who will be assisting the individual with SCI with care needs should be assessed during bowel care training. Mutual goal setting and planning should take place between the individual and caregiver, ensuring that caregivers who have a personal relationship with the individual with SCI have time for their own care needs. It is important to consider how bowel care for an individual with SCI may affect the personal relationship with the caregiver. If the individual with SCI and caregiver share an intimate physical relationship, it is important to discuss bowel care timing and hygiene considerations.
12.5 Sexual intimacy and considerations related to bowel program management should be discussed.
Sexual intimacy and considerations related to bowel care should be discussed with all individuals with SCI and their partners. Education should include pre-and post-intercourse perineal care, infection risk, and timing of sexual activity to reduce the risk of incontinence and optimize the intimate experience. Resources on emotional support should also be provided.
Table 8 outlines education topics for individuals with SCI and their caregivers. (See to the right)
Table 8.
NBD Content Topic List for Education
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
13. Psychosocial Aspects of NBD
13.1 Assessments of NBD should include psychosocial aspects that are barriers to learning the bowel program, such as cognition (ability to learn and to direct others), depression, anxiety, pain, literacy, language, and ethnic or cultural issues.
NBD is common following SCI and has the potential to influence the emotional, social, and physical well-being of individuals living with SCI and their caregiver. Bowel dysfunction can be stressful and/or the source of significant depression or anxiety; some research has shown that moderate-to-severe depression was associated with reduced bowel function.38 Bowel management has been identified as interfering with social activities, personal relationships, and working, thus affecting an individual’s mental state and QOL.38,108,247,248 Increased depressive symptoms have been identified with NBD.108,204,249 It is possible that depression or mental health issues resulting from SCI are so formidable that a person’s bowel management program suffers, or the person becomes noncompliant. Yet, to date we have found no research that addresses this exact issue in SCI. Nevertheless, it is not just the motivation, anxiety, depression, anger, fear, and beliefs about the medical condition of the individual with SCI that can affect readiness to learn the bowel program, but also those of the caregiver.243
To minimize negative outcomes, bowel programs should be designed and revised with the participation of the individual with SCI and his or her caregiver. It is important to identify whether there are any problems with understanding and the retention of the protocol for the NBD for both the individual with SCI and the caregiver. Potential barriers toward an individual’s or caregiver’s willingness to learn the bowel program must be identified and mitigated to the fullest extent possible. Factors that might block learning could include ethnicity, gender, sexual orientation, culture, religion, or socioeconomic status. In addition to physical and physiological factors, the personal goals, life schedules, and role obligations of the individual and the family caregiver, as well as the need for and availability of attendant care, should be considered in the bowel program design. Individuals with SCI and caregivers may be resistant to learning about bowel care or not ready to assume the responsibility of directing or conducting it.217,244,249 Burns et al.249 reported that caregivers are often inadequately trained and prepared, have issues with the intimate nature of the work and discomfort with the physical experience, and lack time to complete the program properly. Assistance of an attendant for daily needs is preferable for some individuals in order to maintain their privacy and their intimate and family relationships.
Research has noted that bowel education is a top research priority.239,240,241 However, the amount of research that has been conducted to measure bowel education programs after SCI is to date is limited, indicating that this is an area for further research. Through inpatient education programs, the individual with SCI can learn an entire bowel routine (including information on timing, diet, positioning) that can help with his or her physical, emotional, and social well-being.237 Evidence shows that having a bowel management program prevents rehospitalization in the post-acute rehabilitation period.97 After baseline information has been obtained, mutual goal setting and planning are essential next steps. If the individual with SCI appears not to be ready to receive information in the hospital setting, it is important that the information presented be available in the community after he or she is discharged.245 It is essential that everyone on the team be educated on all aspects of spinal cord care with the involvement of the individual with SCI. Education during initial rehabilitation is recognized as the key by which individuals with SCI effectively transition from hospital to community and guides the lifelong process of living with a disability.237,238 A website to assist rehabilitation professionals in delivering person- and family-centered care by using motivational interviewing has been created, called “Rehabilitation Engagement Collaborative” (rehabilitationengagementcollaborative.org). This website has a helpful video that demonstrates how to address the important components of a successful bowel program.
Providing care for an individual with SCI can be a significant source of stress. Most of the research on the caregiving burden in SCI does not mention or measure bowel dysfunction. Caregivers can display more depression and more stress and have more of a burden than their non-caregiving counterparts. Characteristics most frequently mentioned with increased burden for caregivers were not having the time for socializing and thus having lower social/community support and social integration.250,251,252 Therefore, it is important to include the caregiver in the assessment and develop avenues of social support for them. Interventions to improve the health status of caregivers have had some modestly positive results. For example, Elliott and Berry found that caregivers in the intervention group (those receiving problem-solving training) reported a significant decrease in dysfunctional problem-solving style scores over time. However, there were no significant effects of the problem-solving training on caregiver depression.253
One must also consider and incorporate the QOL, preparedness, willingness, diversity, and mental status of the individual with SCI and the caregiver into the assessment of post-injury individuals with SCI. NBD after SCI has a negative effect on health-related QOL. Individuals with SCI reported bowel dysfunction as being more problematic than bladder dysfunction, sexual dysfunction, pain, fatigue, and perception of body image. Glickman and Kamm,23 Pires et al.,33 and Kim et al.32 reported in their research a strong relationship between severity of bowel dysfunction and the person’s QOL. Researchers studying bowel dysfunction and sexual activity report that high proportions of both males and females had fecal incontinence, constipation, and some form of sexual dysfunction.254 Fear of bowel incontinence compromises sexual activity, even though research suggests that people’s partners respond to fecal leakage with understanding.255 Many studies that presented descriptive results generally reported bowel function improvements on aspects of QOL, including greater independence (range 29% to 67%), general health improvements, less worry, and greater satisfaction. Liu et al.36 reported that neurogenic bowel function was related to health-related QOL, while Pardee et al.97 reported that those dissatisfied with their bowel program perceived a lower QOL. Kim et al.32 reported that QOL was reduced by fecal incontinence, perianal skin problem, flatus, incontinence, and hemorrhoids.
Some studies indicate that bowel management and bowel dysfunction limit people’s participation in social and other daily living activities. This lower ability to socialize can lead to lower QOL, which can lead to depression. There was a wide range in how much bowel dysfunction limited activities (8% to 90%). More commonly, limitations were reported in the 50% to 70% range. Notably, one study reported that half of the participants felt bowel management limited their ability to participate even though 90% had performed an evacuation before going out.256 The fear at times is that they will have a bowel accident before they return home. Cobb et al.257 found an increased risk ratio for most participants in not participating in a wide range of activities, including performing bowel care, social activities, and working outside the home. Bowel management can be a major life-limiting problem, affecting participation in daily life activities.258 Continued evaluation of the impact of NBD on physical, social, psychological, sexual, and QOL over time will assist the health professional in providing guidance. It is important that these components be included in the development of interventions and as an outcome in program evaluation and future research. Therefore, questions to include in the assessment should address barriers to learning such as depression, anxiety, pain, cognition, literacy, language, embarrassment, readiness, and cultural issues. Health disparities associated with race, ethnicity, and preferences are critical to minimize complications in bowel programs.39
In conclusion, these findings suggest the need to consider and incorporate the psychological stresses, QOL, preparedness, willingness, diversity, and mental status of individuals with SCI and caregivers into the assessment of post-injury individuals with SCI. To minimize negative outcomes, bowel programs should be designed and revised with the participation of the individual with SCI and his or her caregiver.
13.2 If an individual with SCI is having multiple problems with NBD or is noncompliant with the bowel program, a formal screening tool should be used to assess depression, anxiety, and QOL.
Byrne259 reported that the most frequent QOL concern was the ability to get out of the home, to socialize outside of the home, to go shopping, and to not have to worry about the location of the nearest toilet while out of the home. At least 1 of these 4 objectives was stated by 72% of subjects (85 of 118), and 31% (37 of 118) identified an objective related to the physical act of soiling. In another cross-sectional study, Prysak et al.260 found that 71.4% of those reporting bowel problems also reported being unable to do important activities. Individuals with SCI wanted to leave home without an accident.
It is important to address the emotional toll on the individual when NBD is a chronic issue and to refer the person to counseling if he or she appears depressed. Inskip et al.108 reported that for up to 70% of participants, their daily activities were affected by bowel dysfunction, which can lead to lower QOL and life satisfaction.
Two simple outcome measures can be used in the clinic to identify an individual with SCI who is at risk. The Patient Health Questionnaire and the Generalized Anxiety Scale-7 are efficient in that they are brief and can be completed entirely by individuals and validated for individuals with SCI.261,262 This latter feature is particularly important, given the time constraints and competing demands of busy clinicians. Although studies have not examined the effectiveness of supportive counseling in managing bowel-related distress, reports by Kannisto and Rintala263 on the extent of social discomfort; Inskip et al.108 on the length of bowel care decreasing QOL and increasing depression; Julia and Othman,264 Kreuter et al.,255 and Otero-Villaverde et al.265 on sexuality concerns; and Lui et al.38 and Glickman and Kamm23 on depression related to bowel function all suggest that psychosocial difficulties are common. Overall, these findings suggest the need to consider and incorporate QOL, depression, and anxiety questionnaires into the assessment of postinjury individuals with SCI. In addition, it is important that QOL be included in the development of interventions and as an outcome in program evaluation and research.
Future Research
As noted in the Methodology section of this manuscript, these guidelines are based on a thorough review of pertinent available literature. Although there is broad appreciation in the field of SCI medicine that the issue of NBD and its management are of great importance to individuals with SCI, there continues to be significant opportunity and need for further research on this topic. The following list roughly follows the outline of this CPG in summarizing some of the research needs and priorities that can begin to fill the gaps and then be used to inform changes in patient care.
In addition, a few comments are included below to guide the reader in some areas that would benefit from enhanced advocacy. Such efforts from health care providers knowledgeable about NBD following SCI should have a positive impact on access to improved care and QOL for individuals living with this condition.
1. Pathophysiology
-
Delineate the pathophysiology of NBD and defecation disorders.
There is much that is not known about how SCI and AD affect the GI tract and its segments. Additional research should help to define alterations in, and provide greater understanding about, the GI tract’s microbiome in NBD, as well as the patterns of NBD dysmotility and its various humoral, hormonal, and enzymatic influences and receptors.
2. Assessment of NBD
-
Investigate the use of electronic medical records to gather and study data related to NBD.
Access to such a large volume of data could help us to better understand many aspects of NBD, including demographics, bowel histories, symptoms, constipating medications, and treatment and use of oral and rectal medications, as well as methods used for bowel evacuation.
Develop better diagnostic tools to more accurately define NBD and assess the effectiveness bowel program components. These tools could also help us to better understand how medications used to treat complications of SCI (e.g., gabapentin, baclofen, anticholinergics) affect NBD.
Develop better methods to evaluate and treat symptoms associated with NBD, such as abdominal pain/discomfort, bloating, distension, and rectal pain/discomfort.
3. Basic Bowel Management
-
Determine the most effective bowel care procedure for reflexic NBD.
This should include studies to determine the optimal technique, duration, and frequency to perform digital rectal stimulation.
-
Describe in more definitive detail the outcomes (e.g., QOL, wounds, admission for constipation) of individuals who receive bowel care at a suboptimal frequency.
Given the frequent lack of coverage for skilled nurses to assist individuals with bowel care in the home setting or lack of staffing and/or training for nursing assistance in settings such as skilled nursing facilities, such a description could help providers advocate for escalation of covered care for this population.
Investigate the efficacy and safety of alternate digital rectal techniques such as gentle posterior puborectalis muscle stretch to determine their effectiveness in stimulating adequate colonic contraction, as well as the possibility of causing less AD.
4. Diet, Supplements, Fiber, Fluids, and Probiotics
-
Enhance understanding of the impact of diet, fiber, and fluid on the management NBD.
These studies should include randomized controlled trials on levels of dietary fiber intake, fiber supplementation, and dietary supplementation in individuals with NBD.
Compare the effects of the commercially available fiber products on stool consistency (Bristol Stool Form Scale score), client satisfaction with bowel care, and incontinence.
Study in additional detail the use of probiotics in the SCI population beyond prevention of antibiotic-associated diarrhea and Clostridium difficile colitis.
5. Oral Medications
Investigate the determinants of effective bowel emptying after SCI, including the most commonly used medications and laxatives on gastric emptying and small intestine and colonic transit time.
6. Impact of Posture and Activity on NBD
Determine the efficacy of activity-based therapy (e.g., body-weight supported treadmill training) and other standing/locomotor training technologies (e.g., exoskeletons, electrical stimulation) on bowel management compared with active controls (e.g., standing, recreation, traditional exercise).
Evaluate the effect of positioning (i.e., left-side lying vs. right side-lying, side-lying vs. sitting, knees level with hips vs. knees higher than hips) on bowel management.
7. Use of Functional Electrical Stimulation
Investigate the effects of nerve modulation/stimulation for NBD in placebo-controlled clinical trials.
8. Psychosocial Aspects of NBD
-
Evaluate the impact of NBD on physical, social, and psychological QOL over time.
It is important that these components be included in the development of interventions and as an outcome in program evaluation and future research in order to assist the health professional in providing guidance for individuals with SCI.
Determine the long-term effects of NBD on life satisfaction and bowel-related problems in older individuals with chronic SCI.
9. Advocacy
Encourage payer coverage for appropriate U.S. Food and Drug Administration (FDA)-approved technology (e.g., wireless motility capsule, high-resolution anal rectal manometry) to assist in the assessment of NBD that directs providers toward more optimal and specific management recommendations.
Encourage payer coverage for appropriate FDA-approved medication (e.g., linaclotide, prucalopride) and non-medication treatments (e.g., transanal irrigation) to enhance the management of NBD.
Appendices
Appendix 1: Glossary
- anorectal manometry:
a diagnostic procedure that measures the muscle tone of the sphincters and other muscles in the anus and rectum.
- areflexic neurogenic bowel dysfunction:
a type of neurogenic bowel dysfunction generally resulting from an injury at the sacral segments in which spinal reflexes are reduced or lost.
- autonomic dysreflexia:
an uninhibited sympathetic nervous system response to a variety of noxious stimuli occurring in people with spinal cord injury at or above the thoracic 6 level.
- bowel care:
the process of assisted defecation, which includes one or more of the following components: rectal stimulation, positioning and assistive techniques, and adaptive equipment.
- bowel program:
treatment plan designed to minimize or eliminate the occurrence of unplanned, inadequate, or difficult evacuations; to evacuate stool at a regular, predictable time within 60 minutes of bowel care; and to minimize gastrointestinal complications. Components include a routine schedule for bowel care, diet and fluid management, and physical activity, as well as possibly including rectal stimulation and oral and/or rectal medication.
- chemical rectal stimulation:
the use of chemical agents inserted rectally in the form of suppositories or enemas.
- constipation:
infrequent or incomplete defecation (even with rectal stimulation) characterized by small amounts of hard, dry stool that is difficult to pass.
- digital rectal stimulation:
the insertion of a gloved, lubricated finger into the rectal vault followed by rotation to relax the internal anal sphincter. The procedure is used to facilitate evacuation in the setting of reflexic neurogenic bowel dysfunction.
- fecal impaction:
a large mass of stool in distal or proximal colon that cannot be evacuated. A finding of diarrheal stool may indicate the presence of an impaction.
- fiber:
carbohydrate that is not hydrolyzed or absorbed in the upper part of the gastrointestinal tract.
- dietary fiber:
nondigestible carbohydrates and lignin from plants that is intrinsic and intact.
- functional fiber:
isolated, nondigestible carbohydrates that have been shown to have beneficial physiological effects on humans.
- fiber supplements:
confers some but not all health benefits found in dietary fibers.
- functional electrical stimulation:
modality for several methods of improving motor function in paralyzed limbs by stimulation of the nerves and muscles.
- functional magnetic stimulation:
stimulation of the spinal nerves and contraction of deep muscles to facilitate bowel elimination without surgical procedures or unnecessary tissue damage.
- ileus:
a dynamic state of the intestine precipitated by infection, injury, or medication.
- incontinence:
inability to control defecation to achieve voluntary and predictable fecal evacuation.
- manual evacuation:
digital removal of stool from the rectum, which is the usual bowel care treatment choice for an areflexic bowel.
- mechanical rectal stimulation:
manual procedures to stimulate bowel evacuation in the setting of reflexic neurogenic bowel dysfunction.
- osmotic laxative:
a laxative that contains dissolved products that are not absorbed by the gut and that retain water to moisten stool and promote peristalsis.
- paraplegia:
impairment or loss of motor and/or sensory function resulting from a spinal cord injury at or below the first thoracic neurological level.
- peristalsis:
intestinal motion, characterized by waves of alternate circular contraction and relaxation by which contents are propelled forward.
- probiotics:
live microorganisms that are intended to have health benefits when consumed or applied to the body. They can be found in yogurt and other fermented foods, dietary supplements, and beauty products.
- prokinetic medication:
chemical agents that stimulate gastrointestinal motility.
- pulsed irrigation evacuation:
intermittent propulsion of a small volume of water into the rectum through a speculum to break up fecal impaction.
- reflexic neurogenic bowel dysfunction:
type of neurogenic bowel dysfunction generally resulting from a spinal cord injury above the sacral segments in which the spinal reflexes are intact or, in some cases, enhanced.
- tetraplegia:
impairment or loss of motor and/or sensory function resulting from a spinal cord injury above the first thoracic neurologic level, or within the cervical levels of the spinal cord.
- transanal irrigation:
designed to assist the evacuation of feces from the bowel by introducing water into the rectum via a catheter inserted thru the anus.
- unplanned bowel evacuation:
an incontinence episode in which stool is passed outside of a regular bowel care session.
- Valsalva maneuver:
any forced expiratory effort (strain) against a closed glottis.
Appendix 2: International Standards for Neurological Classification of Spinal Cord Injury
Appendix 3: International Spinal Cord Injury Bowel Function Basic Data Set (Version 2.1)
DATA COLLECTION FORM


Appendix 4: Bristol Stool Scale
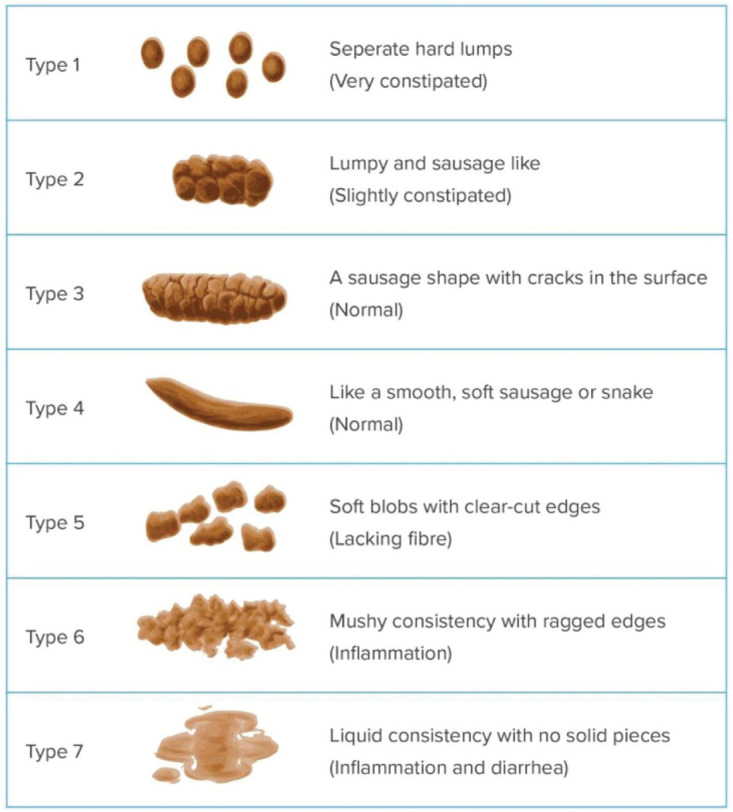
Appendix 5:

Appendix 6: Panel Conflict of Interest Statement
Consortium for Spinal Cord Medicine
Steering Committee Member and Guideline Development Panel Member please read the following policies on Conflicts of Interest and Confidentiality and sign below to indicate acceptance.
Policy on Conflicts of Interest
The Consortium for Spinal Cord Medicine (hereafter referred to as “the Consortium”) is a collaboration of professional and consumer organizations funded and administered through Paralyzed Veterans of America (hereafter referred to as “PVA”). PVA wants to ensure that regular business of the Consortium’s Steering Committee and the guideline development process are free from conflicts of interest. PVA recognizes that those on the Steering Committee and Guidelines Development Panels are involved in a variety of organizations and projects, and may hold financial investments which might create actual or potential conflicts of interest or the appearance of a conflict (each a “conflict” or “conflict of interest”).
To achieve that result, the following policy is adopted:
1. Applicability.
This Policy applies to the Consortium’s Steering Committee Members, including the Chair and Vice-Chair, in addition to those members on the Guideline Development Panels (collectively, “Covered Persons”).
2. Term.
This agreement is effective for the term the Covered Person is a member of the Steering Committee and/or a Guideline Development Panel, notwithstanding how active or passive a role he or she may play as a member of the Steering Committee or a Guideline Development Panel.
3. Determining the Existence of a Conflict.
The guidelines set forth below shall be used to determine the existence of a conflict. The guidelines are meant to be illustrative and not exclusive; a conflict may exist even though the situation in question is not included below. Each Covered Person bears the personal responsibility for initially determining if a conflict of interest exists with respect to such Covered Person. If a Covered Person has any questions regarding the existence of a conflict, such Covered Person should promptly contact the Steering Committee Chair.
4. Guidelines for Determining Existence of Conflict.
A conflict may exist if the Covered Person is unduly influenced by others (i.e. his/her spouse, parent, child, or other individual with whom such Covered Person has a close personal, business or professional relationship (including persons with whom such Covered Person is a partner, shareholder in a closely held corporation, coauthor or other close professional coworker or colleague) to the detriment of and against the mission of the Consortium, the Steering Committee, the Guideline Development Panels, and PVA.
5. Disclosure of Conflict: Recusal.
immediately the Steering Committee Chair or the Director of PVA’s Research and Education Department. The Chair, with input from the Director of Research and Education, shall determine whether a conflict exists (except that in cases of conflicts involving the Chair, the Vice Chair shall decide). The decision on conflicts and the basis of that decision shall be reported to the Steering Committee and recorded in the minutes. Unless otherwise determined by the Chair (or, as appropriate, the Vice Chair) in individual cases, if a conflict is found to exist, the affected person shall recuse himself/herself from all discussions, determinations and votes with respect to the matter with which the conflict exists, and shall excuse him/herself from all meetings at which any discussions regarding the matter take place. Following the termination of such determinations and discussions involving the conflict, such Covered Person may rejoin the meeting.
Policy on Confidentiality
In the course of conducting regular business for the Consortium and/or Guideline Development Panel(s), Steering Committee Members and Panel Members may receive and be given access to confidential information concerning PVA or another entity working with the Consortium. To ensure that the confidentiality of the information will be maintained, the following Policy on Confidentiality is adopted.
1. Applicability.
This Policy applies to the Consortium’s Steering Committee Members, including the Chair and Vice-Chair, in addition to those members on the Guideline Development Panels (collectively, “Covered Persons”).
2. Term.
This agreement is effective for the term the Covered Person is a member of the Steering Committee and/or a Guideline Development Panel, notwithstanding how active or passive a role they may play as a member of the Steering Committee or a Guideline Development Panel.
3. Definition of Confidential Information.
“Confidential Information” means (i) all written business, financial, technical and scientific information relating to the Consortium and which PVA has marked conspicuously “CONFIDENTIAL,” “PROPRIETARY,” or similar marking; or (ii) oral information which is specified as confidential by the Steering Committee and/or PVA. All documents derived during the guideline development process are confidential, and they remain so until 1) the document has been approved for publication by a vote of the Steering Committee and 2) the document is released by PVA as a printed document.
“Confidential Information” shall exclude information which (a) is in the public domain at the time of disclosure; (b) is in the possession of the Consortium (including any Covered Person) free of any obligation of confidence prior to the time of disclosure; (c) though originally within the definition of “Confidential Information”, subsequently becomes part of the public knowledge through no fault of the Consortium (including any Covered Person), as of the date of its becoming part of the public knowledge; (d) though originally within the definition of “Confidential Information”, subsequently is received by the Consortium (including any Covered Person) without any obligation of confidentiality from a third party who is free to disclose the information, as of the date of such third-party disclosure; or (e) is independently developed by the Consortium without the use of any Confidential Information.
4. Nondisclosure of Confidential Information.
Each Covered Person agrees not to disclose to any person outside the Consortium or its affiliates (including for these purposes Chapters and International Affiliates) any Confidential Information, except as provided below. Each Covered Person agrees that he/she will use the Confidential Information only for the purpose of Consortium business. Notwithstanding the foregoing, a Covered Person may disclose the Confidential Information (i) to employees, professional advisors, volunteer scientists and other Covered Persons asked to participate in Consortium business, consultants and agents of the Consortium who have a need to know and who have been informed of this Policy on Confidentiality; or (ii) to the extent required by a court order or by law. Each Covered Person shall use the same degree of care, but not less than a reasonable degree of care, that he/she uses to protect the Consortium’s own most highly confidential information to prevent any unauthorized or inadvertent disclosure of Confidential Information.
Any individual having question(s) concerning this policy or its applicability in a given situation(s) should address those question(s) to the Director of Research and Education (PVA).
5. Return of Confidential Information.
Each Covered Person agrees to return to the Chair of the Steering Committee or the Director of Research and Education, all tangible materials incorporating Confidential Information made available or supplied to such Covered Person and all copies and reproductions thereof upon request of the Chair of the Committee and/or the Director of Research and Education (PVA).
Disclosures
In the interest of full disclosure, panel member Klaus Krogh, MD, PhD, DMSc, serves as an advisor to Wellspect Inc., Sweden and Coloplast, Inc., Denmark; however, he has no financial or personal interest in either concern.
Certification Regarding Conflicts Of Interest And Confidentiality Of Information
Each Covered Person agrees to comply with the provisions of these Policies so long as he/she is a Covered Person. By signing, you are confirming that you have read and understand the above Policy on Conflicts of Interest and Confidentiality and agree to abide by same during all times that you are a Covered Person, as defined in the Policy.
Signed:

Certification Regarding Consortium Policies And Procedures
Each Covered Person agrees to comply with the provisions of the policies and procedures outlined in the Clinical Practice Guideline Orientation Manual so long as he/she is a Covered Person. By signing, you are confirming that you have read and understand the Clinical Practice Guidelines Orientation Manual Policies and Procedures and agree to abide by same during all times that you are a Covered Person.
Signed:

References
- 1.Huang X, Lin J, Demner-Fushman D. Evaluation of PICO as a knowledge representation for clinical questions. AMIA Annu Symp Proc. 2006:359–363. [PMC free article] [PubMed] [Google Scholar]
- 2.Guyatt GH, Oxman AD, Vist GE, et al. GRADE Working Group GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krassioukov A, Biering-Sørensen F, Donovan W, et al. Autonomic Standards Committee of the American Spinal Injury Association/International Spinal Cord Society International standards to document remaining autonomic function after spinal cord injury. J Spinal Cord Med. 2012;35(4):201–210. doi: 10.1179/1079026812Z.00000000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glick ME, Meshkinpour H, Haldeman S, Hoeler F, Downey N, Bradley WE. Colonic dysfunction in patients with spinal cord injury. Gastroenterology. 1984;86(2):287–294. [PubMed] [Google Scholar]
- 5.Meshkinpour H, Nowroozi F, Glick ME. Colonic compliance in patients with spinal cord injury. Arch Phys Med Rehabil. 1983;64(3):111–112. [PubMed] [Google Scholar]
- 6.Krogh K, Mosdal C, Gregersen H, Laurberg S. Rectal wall properties in patients with acute and chronic spinal cord lesions. Dis Colon Rectum. 2002;45(5):641–649. doi: 10.1007/s10350-004-6261-6. [DOI] [PubMed] [Google Scholar]
- 7.Fynne L, Worsøe J, Gregersen T, Schlageter V, Laurberg S, Krogh K. Gastric and small intestinal dysfunction in spinal cord injury patients. Acta Neurol Scand. 2012;125(2):123–128. doi: 10.1111/j.1600-0404.2011.01508.x. [DOI] [PubMed] [Google Scholar]
- 8.Beuret-Blanquart F, Weber J, Gouverneur JP, Demangeon S, Denis P. Colonic transit time and anorectal manometric anomalies in 19 patients with complete transection of the spinal cord. J Auton Nerv Syst. 1990;30(3):199–207. doi: 10.1016/0165-1838(90)90251-d. [DOI] [PubMed] [Google Scholar]
- 9.Longo WE, Woolsey RM, Vernava AM, Virgo KS, McKirgan L, Johnson FE. Cisapride for constipation in spinal cord injured patients: a preliminary report. J Spinal Cord Med. 1995;18(4):240–244. doi: 10.1080/10790268.1995.11719403. [DOI] [PubMed] [Google Scholar]
- 10.Sun WM, Read NW, Donnelly TC. Anorectal function in incontinent patients with cerebrospinal disease. Gastroenterology. 1990;99(5):1372–1379. doi: 10.1016/0016-5085(90)91164-2. [DOI] [PubMed] [Google Scholar]
- 11.Denny-Brown D, Graeme Robertson E. An investigation on the nervous control of defaecation. Brain. 1935;58:256–310. [Google Scholar]
- 12.Krogh K, Mosdal C, Laurberg S. Gastrointestinal & segmental colonic transit times in patients with acute and chronic spinal cord lesions. Spinal Cord. 2000;38(10):615–621. doi: 10.1038/sj.sc.3101066. [DOI] [PubMed] [Google Scholar]
- 13.Menardo G, Bausano G, Corazziari E, et al. Large-bowel transit in paraplegic patients. Dis Colon Rectum. 1987;30(12):924–928. doi: 10.1007/BF02554277. [DOI] [PubMed] [Google Scholar]
- 14.Nino-Murcia M, Stone JM, Chang PJ, Perkash I. Colonic transit in spinal cord-injured patients. Invest Radiol. 1990;25(2):109–112. doi: 10.1097/00004424-199002000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Media S, Christensen P, Lauge I, Al-Hashimi M, Laurberg S, Krogh K. Reproducibility and validity of radiographically determined gastrointestinal and segmental colonic transit times in spinal cord-injured patients. Spinal Cord. 2009;47(1):72–75. doi: 10.1038/sc.2008.88. [DOI] [PubMed] [Google Scholar]
- 16.Rasmussen MM, Krogh K, Clemmensen D, Bluhme H, Rawashdeh Y, Christensen P. Colorectal transport during defecation in subjects with supraconal spinal cord injury. Spinal Cord. 2013;51(9):683–687. doi: 10.1038/sc.2013.58. [DOI] [PubMed] [Google Scholar]
- 17.Krogh K, Olsen N, Christensen P, Madsen JL, Laurberg S. Colorectal transport during defecation in patients with lesions of the sacral spinal cord. Neurogastroenterol Motil. 2003;15(1):25–31. doi: 10.1046/j.1365-2982.2003.00381.x. [DOI] [PubMed] [Google Scholar]
- 18.Williams RE, 3rd, Bauman WA, Spungen AM, et al. SmartPill technology provides safe and effective assessment of gastrointestinal function in persons with spinal cord injury. Spinal Cord. 2012;50(1):81–84. doi: 10.1038/sc.2011.92. [DOI] [PubMed] [Google Scholar]
- 19.Kao CH, Ho YJ, Changlai SP, Ding HJ. Gastric emptying in spinal cord injury patients. Dig Dis Sci. 1999;44(8):1512–1515. doi: 10.1023/a:1026690305537. [DOI] [PubMed] [Google Scholar]
- 20.Wheatley IC, Hardy KJ, Dent J. Anal pressure studies in spinal patients. Gut. 1977;18(6):488–490. doi: 10.1136/gut.18.6.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thiruppathy K, Roy A, Preziosi G, Pannicker J, Emmanuel A. Morphological abnormalities of the recto-anal inhibitory reflex reflects symptom pattern in neurogenic bowel. Dig DisSci. 2012;57(7):1908–1914. doi: 10.1007/s10620-012-2113-8. [DOI] [PubMed] [Google Scholar]
- 22.Freckner B. Function of the anal sphincters in spinal man. Gut. 1975;16(8):638–644. doi: 10.1136/gut.16.8.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glickman S, Kamm MA. Bowel dysfunction in spinal-cord-injury patients. Lancet. 1996;347(9016):1651–1653. doi: 10.1016/s0140-6736(96)91487-7. [DOI] [PubMed] [Google Scholar]
- 24.Krogh K, Nielsen J, Djurhuus JC, Mosd9.al C, Sabroe S, Laurberg S. Colorectal function in patients with spinal cord lesions. Dis Colon Rectum. 1997;40(10):1233–123. doi: 10.1007/BF02055170. [DOI] [PubMed] [Google Scholar]
- 25.Vallès M, Vidal J, Clavé P, Mearin F. Bowel dysfunction in patients with motor complete spinal cord injury: clinical, neurological, and pathophysiological associations. Am J Gastroenterol. 2006;101(10):2290–2299. doi: 10.1111/j.1572-0241.2006.00729.x. [DOI] [PubMed] [Google Scholar]
- 26.Faaborg PM, Christensen P, Finnerup N, Laurberg S, Krogh K. The pattern of colorectal dysfunction changes with time since spinal cord injury. Spinal Cord. 2008;46(3):234–238. doi: 10.1038/sj.sc.3102121. [DOI] [PubMed] [Google Scholar]
- 27.Bloemen-Vrencken JHA, Post MWM, Hendriks JMS, De Reus ECE, De Witte LP. Health problems of persons with spinal cord injury living in the Netherlands. Disabil Rehabil. 2005;27(22):1381–1389. doi: 10.1080/09638280500164685. [DOI] [PubMed] [Google Scholar]
- 28.Coggrave MJ, Norton C, Wilson-Barnett J. Management of neurogenic bowel dysfunction in the community after spinal cord injury: a postal survey in the United Kingdom. Spinal Cord. 2009;47(4):323–330. doi: 10.1038/sc.2008.137. [DOI] [PubMed] [Google Scholar]
- 29.De Looze D, Van Laere M, De Muynck M, Beke R, Elewaut A. Constipation and other chronic gastrointestinal problems in spinal cord injury patients. Spinal Cord. 1998;36(1):63–66. doi: 10.1038/sj.sc.3100531. [DOI] [PubMed] [Google Scholar]
- 30.Han TR, Kim JH, Kwon BS. Chronic gastrointestinal problems and bowel dysfunction in patients with spinal cord injury. Spinal Cord. 1998;36(7):485–490. doi: 10.1038/sj.sc.3100616. [DOI] [PubMed] [Google Scholar]
- 31.Hitzig SL, Tonack M, Campbell KA, et al. Secondary health complications in an aging Canadian spinal cord injury sample. Am J Phys Med Rehabil. 2008;87(7):545–555. doi: 10.1097/PHM.0b013e31817c16d6. [DOI] [PubMed] [Google Scholar]
- 32.Kim JY, Koh ES, Leigh J, Shin H. Management of bowel dysfunction in the community after spinal cord injury: a postal survey in the Republic of Korea. Spinal Cord. 2012;50(4):303–308. doi: 10.1038/sc.2011.124. [DOI] [PubMed] [Google Scholar]
- 33.Pires JM, Ferreira AM, Rocha F, et al. Assessment of neurogenic bowel dysfunction impact after spinal cord injury using the International Classification of Functioning, Disability and Health. Eur J Phys Rehabil Med. 2018;54(6):873–879. doi: 10.23736/S1973-9087.18.04991-2. [DOI] [PubMed] [Google Scholar]
- 34.New PW. Secondary conditions in a community sample of people with spinal cord damage. J Spinal Cord Med. 2016;39(6):665–670. doi: 10.1080/10790268.2016.1138600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lynch AC, Wong C, Anthony A, Dobbs BR, Frizelle FA. Bowel dysfunction following spinal cord injury: a description of bowel function in a spinal cord-injured population and comparison with age and gender matched controls. Spinal Cord. 2000;38(12):717–723. doi: 10.1038/sj.sc.3101058. [DOI] [PubMed] [Google Scholar]
- 36.Liu CW, Huang CC, Yang YH, Chen SC, Weng MC, Huang MH. Relationship between neurogenic bowel dysfunction and health-related quality of life in persons with spinal cord injury. J Rehabil Med. 2009;41(1):35–40. doi: 10.2340/16501977-0277. [DOI] [PubMed] [Google Scholar]
- 37.Krogh K, Christensen P, Sabroe S, Laurberg S. Neurogenic bowel dysfunction score. Spinal Cord. 2006;44(10):625–631. doi: 10.1038/sj.sc.3101887. [DOI] [PubMed] [Google Scholar]
- 38.Liu CW, Huang CC, Chen CH, Yang YH, Chen TW, Huang MH. Prediction of severe neurogenic bowel dysfunction in persons with spinal cord injury. Spinal Cord. 2010;48(7):554–559. doi: 10.1038/sc.2009.181. [DOI] [PubMed] [Google Scholar]
- 39.Tate DG, Forchheimer M, Rodriguez G, et al. Risk factors associated with neurogenic bowel complications and dysfunction in spinal cord injury. Arch Phys Med Rehabil. 2016;97(10):1679–1686. doi: 10.1016/j.apmr.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 40.Jörgensen S, Iwarsson S, Lexell J. Secondary health conditions, activity limitations, and life satisfaction in older adults with long-term spinal cord injury. PM R. 2017;9(4):356–366. doi: 10.1016/j.pmrj.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 41.Nielsen SD, Faaborg PM, Finnerup NB, Christensen P, Krogh K. Ageing with neurogenic bowel dysfunction. Spinal Cord. 2017;55(8):769–773. doi: 10.1038/sc.2017.22. [DOI] [PubMed] [Google Scholar]
- 42.Adriaansen JJ, Ruijs LE, van Koppenhagen CF, et al. Secondary health conditions and quality of life in persons living with spinal cord injury for at least ten years. J Rehabil Med. 2016;48(10):853–860. doi: 10.2340/16501977-2166. [DOI] [PubMed] [Google Scholar]
- 43.Weitzenkamp DA, Jones RH, Whiteneck GG, Young DA. Ageing with spinal cord injury: cross-sectional and longitudinal effects. Spinal Cord. 2001;39(6):301–309. doi: 10.1038/sj.sc.3101146. [DOI] [PubMed] [Google Scholar]
- 44.Adriaansen JJ, van Asbeck FW van Kuppevelt MD, Snoek GJ, Post MW. Outcomes of neurogenic bowel management in individuals living with a spinal cord injury for at least 10 years. Arch Phys Med Rehabil. 2015;96(5):905–912. doi: 10.1016/j.apmr.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 45.American Spinal Injury Association. International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI) https://asia-spinalinjury.org/product/international-standards-for-neurological-classification-of-spinal-cord-injury-isncsci-revised-2019 Revised 2019. Accessed April 29,2020.
- 46.Johanson JF, Lafferty J. Epidemiology of fecal incontinence: the silent affliction. Am J Gastroenterol. 1996;91(1):33–36. [PubMed] [Google Scholar]
- 47.Liu N, Xing H, Zhou MW, Biering-Sørensen F. Development and validation of a bowel-routine-based self-report questionnaire for sacral sparing after spinal cord injury. Spinal Cord. 2017;55(11):1010–1015. doi: 10.1038/sc.2017.77. [DOI] [PubMed] [Google Scholar]
- 48.Patel DP, Elliott SP, Stoffel JT, Brant WO, Hotaling JM, Myers JB. Patient reported outcomes measures in neurogenic bladder and bowel: a systematic review of the current literature. Neurourol Urodyn. 2016;35(1):8–14. doi: 10.1002/nau.22673. [DOI] [PubMed] [Google Scholar]
- 49.Stoffel JT, Van der Aa F, Wittmann D, Yande S, Elliott S. Neurogenic bowel management for the adult spinal cord injury patient. World J Urol. 2018;36(10):1587–1592. doi: 10.1007/s00345-018-2388-2. [DOI] [PubMed] [Google Scholar]
- 50.Jorge JM, Wexner SD. Etiology and management of fecal incontinence. Dis Colon Rectum. 1993;36(1):77–97. doi: 10.1007/BF02050307. [DOI] [PubMed] [Google Scholar]
- 51.Vaizey CJ, Carapeti E, Cahill JA, Kamm MA. Prospective comparison of faecal incontinence grading systems. Gut. 1999;44(1):77–80. doi: 10.1136/gut.44.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Agachan F, Chen T, Pfeifer J, Reissman P, Wexner SD. A constipation scoring system to simplify evaluation and management of constipated patients. Dis Colon Rectum. 1996;39(6):681–685. doi: 10.1007/BF02056950. [DOI] [PubMed] [Google Scholar]
- 53.Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32(9):920–924. doi: 10.3109/00365529709011203. [DOI] [PubMed] [Google Scholar]
- 54.Tulsky DS, Kisala PA, Tate DG, Spungen AM, Kirshblum SC. Development and psychometric characteristics of the SCI-QOL Bladder Management Difficulties and Bowel Management Difficulties item banks and short forms and the SCI-QOL Bladder Complications scale. J Spinal Cord Med. 2015;38(3):288–302. doi: 10.1179/2045772315Y.0000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Christensen P, Bazzocchi G, Coggrave M, et al. A randomized, controlled trial of transanal irrigation versus conservative bowel management in spinal cord injured patients. Gastroenterology. 2006;131(3):738–747. doi: 10.1053/j.gastro.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 56.Mallek A, Elleuch MH, Ghroubi S. Neurogenic bowel dysfunction (NBD) translation and linguistic validation to classical Arabic. Prog Urol. 2016;26(10):553–557. doi: 10.1016/j.purol.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 57.Erdem D, Hava D, Keskinoglu P, et al. Reliability, validity and sensitivity to change of neurogenic bowel dysfunction score in patients with spinal cord injury. Spinal Cord. 2017;55(12):1084–1087. doi: 10.1038/sc.2017.82. [DOI] [PubMed] [Google Scholar]
- 58.Leibold S. Neurogenic bowel and continence programs for the individual with spina bifida. J Pediatr Rehabil Med. 2008;1(4):325–336. [PubMed] [Google Scholar]
- 59.Krogh K, Emmanuel A, Perrouin-Verbe B, Korsten MA, Mulcahey MJ, Biering-Sørensen F. International spinal cord injury bowel function basic data set (Version 2.0) Spinal Cord. 2017;55(7):692–698. doi: 10.1038/sc.2016.189. [DOI] [PubMed] [Google Scholar]
- 60.Krogh K, Perkash I, Stiens SA, Biering-Sørensen F. International bowel function basic spinal cord injury data set. Spinal Cord. 2009;47(3):230–234. doi: 10.1038/sc.2008.102. [DOI] [PubMed] [Google Scholar]
- 61.Krogh K, Perkash I, Stiens SA, Biering-Sørensen F. International bowel function extended spinal cord injury data set. Spinal Cord. 2009;47(3):235–241. doi: 10.1038/sc.2008.103. [DOI] [PubMed] [Google Scholar]
- 62.Juul T, Bazzocchi G, Coggrave M, et al. Reliability of the international spinal cord injury bowel function basic and extended data sets. Spinal Cord. 2011;49(8):886–891. doi: 10.1038/sc.2011.23. [DOI] [PubMed] [Google Scholar]
- 63.Cotterill N, Madersbacher H, Wyndaele JJ, et al. Neurogenic bowel dysfunction: clinical management recommendations of the Neurologic Incontinence Committee of the Fifth International Consultation on Incontinence 2013. Neurourol Urodyn. 2018;37(1):46–53. doi: 10.1002/nau.23289. [DOI] [PubMed] [Google Scholar]
- 64.Rodriguez G, Stiens SA. Neurogenic bowel: dysfunction and rehabilitation. In: Cifu DX, editor. Braddom’s Physical Medicine and Rehabilitation. 5th ed. Philadelphia, PA: Elsevier; 2016. pp. 449–468. [Google Scholar]
- 65.Consortium for Spinal Cord Medicine Neurogenic bowel management in adults with spinal cord injury: clinical practice guidelines. Paralyzed Veterans of America. 1998 doi: 10.1080/10790268.1998.11719536. [DOI] [PubMed] [Google Scholar]
- 66.Stiens SA, Bergman SB, Goetz LL. Neurogenic bowel dysfunction after spinal cord injury: clinical evaluation and rehabilitative management. Arch Phys Med Rehabil. 1997;78(3 suppl):S86–102. doi: 10.1016/s0003-9993(97)90416-0. [DOI] [PubMed] [Google Scholar]
- 67.Krassioukov A, Eng JJ, Claxton G, Sakakibara BM, Shum S. Neurogenic bowel management after spinal cord injury: a systematic review of the evidence. Spinal Cord. 2010;48(10):718–733. doi: 10.1038/sc.2010.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Coggrave MJ, Norton C. The need for manual evacuation and oral laxatives in the management of neurogenic bowel dysfunction after spinal cord injury: a randomized controlled trial of a stepwise protocol. Spinal Cord. 2010;48(6):504–510. doi: 10.1038/sc.2009.166. [DOI] [PubMed] [Google Scholar]
- 69.Preziosi G, Emmanuel A. Neurogenic bowel dysfunction: pathophysiology, clinical manifestations and treatment. Expert Rev Gastroenterol Hepatol. 2009;3(4):417–423. doi: 10.1586/egh.09.31. [DOI] [PubMed] [Google Scholar]
- 70.Ebert E. Gastrointestinal involvement in spinal cord injury: a clinical perspective. J Gastrointestin Liver Dis. 2012;21(1):75–82. [PubMed] [Google Scholar]
- 71.Brenner DM, Shah M. Chronic constipation. Gastroenterol Clin North Am. 2016;45(2):205–216. doi: 10.1016/j.gtc.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 72.Wald A, Bharucha AE, Cosman BC, et al. ACG clinical guideline: management of benign anorectal disorders. Am J Gastroenterol. 2014;109(8):1141–1157. doi: 10.1038/ajg.2014.190. [DOI] [PubMed] [Google Scholar]
- 73.Park HJ, Noh SE, Kim GD, Joo MC. Plain abdominal radiograph as an evaluation method of bowel dysfunction in patients with spinal cord injury. Ann Rehabil Med. 2013;37(4):547–555. doi: 10.5535/arm.2013.37.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jaffe T, Thompson WM. Large-bowel obstruction in the adult: classic radiographic and CT findings, etiology, and mimics. Radiology. 2015;275(3):651–663. doi: 10.1148/radiol.2015140916. [DOI] [PubMed] [Google Scholar]
- 75.Godfrey EM, Addley HC, Shaw AS. The use of computed tomography in the detection and characterisation of large bowel obstruction. N Z Med J. 2009;122(1305):57–73. [PubMed] [Google Scholar]
- 76.Romano S, Bartone G, Romano L. Ischemia and infarction of the intestine related to obstruction. Radiol Clin North Am. 2008;46(5):925–942. doi: 10.1016/j.rcl.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 77.Maglinte DD, Kelvin FM, Sandrasegaran K, et al. Radiology of small bowel obstruction: contemporary approach and controversies [erratum in Abdom Imaging. 2005;30(5):651] Abdom Imaging. 2005;30(2):160–178. doi: 10.1007/s00261-004-0211-6. [DOI] [PubMed] [Google Scholar]
- 78.Macari M, Megibow A. Imaging of suspected acute small bowel obstruction. Semin Roentgenol. 2001;36(2):108–117. doi: 10.1053/sroe.2001.22827. [DOI] [PubMed] [Google Scholar]
- 79.American Gastroenterological Association. Bharucha AE, Dorn SD, Lembo A, Pressman A. American Gastroenterological Association medical position statement on constipation. Gastroenterology. 2013;144(1):211–217. doi: 10.1053/j.gastro.2012.10.029. [DOI] [PubMed] [Google Scholar]
- 80.Emmanuel AV, Chung EA, Kamm MA, Middleton F. Relationship between gut-specific autonomic testing and bowel dysfunction in spinal cord injury patients. Spinal Cord. 2009;47(8):623–627. doi: 10.1038/sc.2009.14. [DOI] [PubMed] [Google Scholar]
- 81.Ranjan P, Bansal N, Sachdeva M, Jain P, Arora A. Colonic transit time: current methodology and its clinical implications. JIMSA. 2012;25(1):35–37. [Google Scholar]
- 82.Nino-Murcia M, Friedland GW. Functional abnormalities of the gastrointestinal tract in patients with spinal cord injuries: evaluation with imaging procedures. AJR Am J Roentgenol. 1992;158(2):279–281. doi: 10.2214/ajr.158.2.1729781. [DOI] [PubMed] [Google Scholar]
- 83.Freedman PN, Goldberg PA, Fataar AB, Mann MM. A comparison of methods of assessment of scintigraphic colon transit. J Nucl Med Technol. 2006;34(2):76–81. [PubMed] [Google Scholar]
- 84.Keshavarzian A, Barnes WE, Bruninga K, Nemchausky B, Mermall H, Bushnell D. Delayed colonic transit in spinal cord-injured patients measured by indium-111 amberlite scintigraphy. Am J Gastroenterol. 1995;90(8):1295–1300. [PubMed] [Google Scholar]
- 85.Medtronic. SmartPill™ motility testing system. www.medtronic.com/covidien/en-us/products/motility-testing/smartpill-motility-testing-system.html#smartpill-motility-capsule Accessed April 29, 2020. [Google Scholar]
- 86.Rao SS, Camilleri M, Hasler WL, et al. Evaluation of gastrointestinal transit in clinical practice: position paper of the American and European Neurogastroenterology and Motility Societies. Neurogastroenterol Motil. 2011;23(1):8–23. doi: 10.1111/j.1365-2982.2010.01612.x. [DOI] [PubMed] [Google Scholar]
- 87.MacDonagh R, Sun WM, Thomas DG, Smallwood R, Read NW. Anorectal function in patients with complete supraconal spinal cord lesions. Gut. 1992;33(11):1532–1538. doi: 10.1136/gut.33.11.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tjandra JJ, Ooi BS, Han WR. Anorectal physiologic testing for bowel dysfunction in patients with spinal cord lesions. Dis Colon Rectum. 2000;43(7):927–931. doi: 10.1007/BF02237352. [DOI] [PubMed] [Google Scholar]
- 89.Multidisciplinary Association of Spinal Cord Injury Professionals (MASCIP) Guidelines for management of neurogenic bowel dysfunction in individuals with central neurological conditions. www.mascip.co.uk/wp-content/uploads/2015/02/CV653N-Neurogenic-Guidelines-Sept-2012.pdf Published 2012. Accessed April 28, 2020. [Google Scholar]
- 90.Srikumar V. Management of neurogenic bowel. In: Chhabra HS, editor. ISCoS Textbook on Comprehensive Management of Spinal Cord Injuries. New Delhi, India: Wolters Kluwer; 2015. pp. 423–432. [Google Scholar]
- 91.Ozisler Z, Koklu K, Ozel S, Unsal-Delialioglu S. Outcomes of bowel program in spinal cord injury patients with neurogenic bowel dysfunction. Neural Regen Res. 2015;10(7):1153–1158. doi: 10.4103/1673-5374.160112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Correa GI, Rotter KP. Clinical evaluation and management of neurogenic bowel after spinal cord injury. Spinal Cord. 2000;38(5):301–308. doi: 10.1038/sj.sc.3100851. [DOI] [PubMed] [Google Scholar]
- 93.Coggrave M, Burrows D, Durand MA. Progressive protocol in the bowel management of spinal cord injuries. Br J Nurs. 2006;15(20):1108–1113. doi: 10.12968/bjon.2006.15.20.22295. [DOI] [PubMed] [Google Scholar]
- 94.Badiali D, Bracci F, Castellano V, et al. Sequential treatment of chronic constipation in paraplegic subjects. Spinal Cord. 1997;35(2):116–120. doi: 10.1038/sj.sc.3100355. [DOI] [PubMed] [Google Scholar]
- 95.Coggrave M, Mills P, Willms R, Eng JJ. Bowel dysfunction and management following spinal cord injury. In: Eng JJ, Teasell RW, Miller WC, et al., editors. Spinal Cord Injury Rehabilitation Evidence Version 50. Vancouver, BC: Spinal Cord Injury Research Evidence (SCIRE) Project; 2014. pp. 1–48. [Google Scholar]
- 96.Adriaasen JJ, van Asbeck FW, van Kuppevelt D, Snoek GJ, Post MW. Outcomes of neurogenic bowel management in individuals living with a spinal cord injury for at least 10 years. Arch Phys Med Rehabil. 2015;96(5):905–912. doi: 10.1016/j.apmr.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 97.Pardee C, Bricker D, Rundquist J, MacRae C, Tebben C. Characteristics of neurogenic bowel in spinal cord injury and perceived quality of life. Rehabil Nurs. 2012;37(3):128–135. doi: 10.1002/RNJ.00024. [DOI] [PubMed] [Google Scholar]
- 98.Hwang M, Zebracki K, Vogel LC. Long-term outcomes and longitudinal changes of neurogenic bowel management in adults with pediatric-onset spinal cord injury. Arch Phys Med Rehabil. 2017;98(2):241–248. doi: 10.1016/j.apmr.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 99.Haas U, Geng V, Evers GC, Knecht H. Bowel management in patients with spinal cord injury: a multicenter study of the German speaking society of paraplegia (DMGP) Spinal Cord. 2005;43(12):724–730. doi: 10.1038/sj.sc.3101795. [DOI] [PubMed] [Google Scholar]
- 100.Furusawa K, Sugiyama H, Ikeda A, et al. Autonomic dysreflexia during a bowel program in patients with cervical spinal cord injury. Acta Med Okayama. 2007;61(4):211–227. doi: 10.18926/AMO/32867. [DOI] [PubMed] [Google Scholar]
- 101.Faaborg PM, Christensen P, Krassioukov A, Laurberg S, Frandsen E, Krogh K. Autonomic dysreflexia during bowel evacuation procedures and bladder filling in subjects with spinal cord injury. Spinal Cord. 2014;52(6):494–498. doi: 10.1038/sc.2014.45. [DOI] [PubMed] [Google Scholar]
- 102.Craig Hospital Spinal Cord Injury Resource Library Bowel program: upper motor neuron injury. https://craighospital.org/resources/bowel-program-upper-motor-neuron-injury Revised January 2015. Accessed April 28,2020.
- 103.Shafik A, El-Sibai O, Shafik IA. Physiologic basis of digital-rectal stimulation for bowel evacuation in patients with spinal cord injury: identification of an anorectal excitatory reflex. J Spinal Cord Med. 2000;23(4):270–275. doi: 10.1080/10790268.2000.11753536. [DOI] [PubMed] [Google Scholar]
- 104.Consortium for Spinal Cord Medicine Evaluation and management of autonomic dysreflexia and other autonomic dysfunctions: preventing the highs and lows: clinical practice guidelines. Paralyzed Veterans of America. in press. [Google Scholar]
- 105.Furusawa K, Sugiyama H, Tokuhior A, Takahashi M, Nakamura T, Tajima F. Topical anesthesia blunts the pressor response induced by bowel manipulation in subjects with cervical spinal cord injury. Spinal Cord. 2009;47(2):144–148. doi: 10.1038/sc.2008.86. [DOI] [PubMed] [Google Scholar]
- 106.Furusawa K, Tokuhiro A, Sugiyama H, et al. Incidence of symptomatic autonomic dysreflexia varies according to the bowel and bladder management techniques in patients with spinal cord injury. Spinal Cord. 2011;49(1):49–54. doi: 10.1038/sc.2010.94. [DOI] [PubMed] [Google Scholar]
- 107.Solomons J, Woodward S. Digital removal of faeces in the bowel management of patients with spinal cord injury: a review. Br J Neurosci Nurs. 2013;9(5):216–222. [Google Scholar]
- 108.Inskip JA, Lucci VM, McGrath MS, Willms R, Claydon VE. A community perspective on bowel management and quality of life after spinal cord injury: the influence of autonomic dysreflexia. J Neurotrauma. 2018;35(9):1091–1105. doi: 10.1089/neu.2017.5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Janssen TWJ, Prakken ES, Heniks JM, Lourens C, van der Vlist J, Smit CA. Electromechanical abdominal massage and colonic function in individuals with a spinal cord injury and chronic bowel problems. Spinal Cord. 2014;52(9):693–696. doi: 10.1038/sc.2014.101. [DOI] [PubMed] [Google Scholar]
- 110.Ayas S, Leblebici B, Sozay S, Bayramoglu M, Niron EA. The effect of abdominal massage on bowel function in patients with spinal cord injury. Am J Phys Med Rehabil. 2006;85(12):951–955. doi: 10.1097/01.phm.0000247649.00219.c0. [DOI] [PubMed] [Google Scholar]
- 111.Hu C, Ye M, Huang Q. Effects of manual therapy on bowel function of patients with spinal cord injury. J Phys Ther Sci. 2013;25(6):687–688. doi: 10.1589/jpts.25.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Trivedi PM, Griffiths DJ. Neurological control of the bowel in health and disease. In: Fowler CJ, Panicker JN, Emmanuuel A, editors. Pelvic Organ Dysfunction in Neurological Disease. Cambridge, United Kingdom: Cambridge University Press; 2010. pp. 25–39. [Google Scholar]
- 113.Menter R, Weitzenkamp D, Cooper D, Bingley J, Charlifue S, Whiteneck G. Bowel management outcomes in individuals with longer-term spinal cord injuries. Spinal Cord. 1997;35(9):608–612. doi: 10.1038/sj.sc.3100461. [DOI] [PubMed] [Google Scholar]
- 114.Friesen E, Theodoros D, Russell T. Clinical assessment, design and performance testing of mobile shower commodes for adults with spinal cord injury: an exploratory review. Disabil Rehabil Assist Technol. 2013;8(4):267–274. doi: 10.3109/17483107.2012.704656. [DOI] [PubMed] [Google Scholar]
- 115.Friesen E, Theodoros D, Russell T. Use, performance and features of mobile shower commodes: perspectives of adults with spinal cord injury and expert clinicians. Disabil Rehabil Assist Technol. 2015;10(1):38–45. doi: 10.3109/17483107.2013.832413. [DOI] [PubMed] [Google Scholar]
- 116.Nelson A, Malassigné P, Murray J. Comparison of seat pressures on 3 bowel care/shower chairs in spinal cord injury. SCI Nurs. 1994;11(4):105–107. [PubMed] [Google Scholar]
- 117.Malassigné P, Nelson A, Amerson T, Salzstein R, Binard J. Toward the design of a new bowel care chair for the spinal cord injury: a pilot study. SCI Nurs. 1993;10(3):84–90. [PubMed] [Google Scholar]
- 118.Opperman EA, Buchholz AC, Darlington GA, Martin Ginis KA, The SHAPE-SCI Research Group Dietary supplement use in the spinal cord injury population. Spinal Cord. 2010;48(1):60–64. doi: 10.1038/sc.2009.86. [DOI] [PubMed] [Google Scholar]
- 119.Navarrete-Opazo A, Cuitiño P, Salas I. Effectiveness of dietary supplements in spinal cord injury subjects. Disabil Health J. 2017;10(2):183–197. doi: 10.1016/j.dhjo.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 120.Kim JH, Lee SK, Joo MC. Effects and safety of aqueous extract of Poncirus fructus in spinal cord injury with neurogenic bowel. Evid Based Complement Alternat Med. 2016;2016:7154616. doi: 10.1155/2016/7154616. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Academy of Nutrition and Dietetics Evidence Analysis Library. 2009 Spinal cord injury evidence-based nutrition practice guideline. https://www.andeal.org/topic.cfm?menu=3485&cat=3486 Published 2009. Accessed March 10, 2019.
- 122.Folden SL. Practice guidelines for the management of constipation in adults. Rehabil Nurs. 2002;27(5):169–176. doi: 10.1002/j.2048-7940.2002.tb02005.x. [DOI] [PubMed] [Google Scholar]
- 123.Institute of Medicine Dietary Reference Intakes for Energy Carbohydrate Fiber Fat Fatty Acids Cholesterol Protein and Amino Acids. Washington, DC: The National Academies Press; 2005. Accessed April 29, 2020. [DOI] [Google Scholar]
- 124.In Dietary Reference Intakes Proposed Definition of Dietary Fiber. Washington, DC: National Academies Press (US); 2001. Institute of Medicine (US) Panel on the Definition of Dietary Fiber and the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. IV. Proposed definition of dietary fiber. https://www.ncbi.nlm.nih.gov/books/NBK223591/ Accessed April 29, 2020. [PubMed] [Google Scholar]
- 125.Mcrorie JW. Evidence-based approach to fiber supplements and clinically meaningful health benefits, Part 1: What to look for and how to recommend an effective fiber therapy. Nutr Today. 2015;50(2):82–89. doi: 10.1097/NT.0000000000000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mcrorie JW, Mckeown NM. Understanding the physics of functional fibers in the gastrointestinal tract: an evidence-based approach to resolving enduring misconceptions about insoluble and soluble fiber. J Acad Nutr Diet. 2017;117(2):251–264. doi: 10.1016/j.jand.2016.09.021. [DOI] [PubMed] [Google Scholar]
- 127.Sabour H, Javidan AN, Vafa MR, et al. Calorie and macronutrients intake in people with spinal cord injuries: an analysis by sex and injury-related variables. Nutrition. 2012;28(2):143–147. doi: 10.1016/j.nut.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 128.Yim SY, Yoon SH, Lee IY, Rah EW, Moon HW. A comparison of bowel care patterns in patients with spinal cord injury: upper motor neuron bowel vs lower motor neuron bowel. Spinal Cord. 2001;39(4):204–207. doi: 10.1038/sj.sc.3101131. [DOI] [PubMed] [Google Scholar]
- 129.Cameron KJ, Nyulasi IB, Collier GR, Brown DJ. Assessment of the effect of increased dietary fibre intake on bowel function in patients with spinal cord injury. Spinal Cord. 1996;34(5):277–283. doi: 10.1038/sc.1996.50. [DOI] [PubMed] [Google Scholar]
- 130.Eswaran SL, Chey WD, Han-Markey T, Ball S, Jackson K. A randomized controlled trial comparing the low FODMAP diet vs. modified NICE guidelines in US adults with IBS-D. Am J Gastroenterol. 2016;111(12):1824–1832. doi: 10.1038/ajg.2016.434. [DOI] [PubMed] [Google Scholar]
- 131.Böhn L, Störsrud S, Liljebo T, et al. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: a randomized controlled trial. Gastroenterology. 2015;149(6):1399–1407.e2. doi: 10.1053/j.gastro.2015.07.054. [DOI] [PubMed] [Google Scholar]
- 132.Halmos EP, Power VA, Shepherd SJ, Gibson PR, Muir JG. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology. 2014;146(1):67–75. doi: 10.1053/j.gastro.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 133. Monash University. The Monash University FODMAP Diet app. https://www.monashfodmap.com/ibs-central/i-have-ibs/get-the-app/ Accessed April 29, 2020.
- 134.Monash University The Monash University Low FODMAP Diet Booklet. 6th ed. Melbourne, Australia: Department of Gastroenterology, Central Clinical School; https://www.monashfodmap.com/i-am-a-health-professional/fodmap-resources/ Accessed April 29, 2020. [Google Scholar]
- 135.Arnaud MJ. Mild dehydration: a risk factor of constipation? Eur J Clin Nutr. 2003;57(suppl 2):S88–95. doi: 10.1038/sj.ejcn.1601907. [DOI] [PubMed] [Google Scholar]
- 136.Engakasan JP, Sudin SS. Neurogenic bowel management after spinal cord injury: Malaysian experience. J Rehabil Med. 2013;45(2):141–144. doi: 10.2340/16501977-1074. [DOI] [PubMed] [Google Scholar]
- 137.Food and Nutrition Board Recommended Dietary Allowances. 10th ed. Washington, DC: National Academy Press; 1989. [Google Scholar]
- 138.Chernoff R. Meeting the nutritional needs of the elderly in the institutional setting. Nutr Rev. 1994;52(4):132–136. doi: 10.1111/j.1753-4887.1994.tb01405.x. [DOI] [PubMed] [Google Scholar]
- 139.Grant A, DeHoog S. 4th ed. Seattle, WA: Grant/DeHoog; 1991. Nutritional Assessment and Support. [Google Scholar]
- 140.Skipper A, editor. Dietitian’s Handbook of Enteral and Parenteral Nutrition. Rockville, MD: Aspen Publishers; 1993. 298 pp. [Google Scholar]
- 141.Abid MB, Koh CJ. Probiotics in health and disease: fooling Mother Nature? Infection. 2019;47(6):911–917. doi: 10.1007/s15010-019-01351-0. [DOI] [PubMed] [Google Scholar]
- 142.Wong S, Jamous A, O’Driscoll J, et al. A Lactobacillus casei Shirota probiotic drink reduces antibiotic-associated diarrhoea in patients with spinal cord injuries: a randomised controlled trial. Br J Nutr. 2014;111(4):672–678. doi: 10.1017/S0007114513002973. [DOI] [PubMed] [Google Scholar]
- 143.Goldenberg JZ, Yap C, Lytvyn L, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;(12):CD006095. doi: 10.1002/14651858.CD006095.pub4. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Krogh K, Jensen MB, Gandrup P, et al. Efficacy and tolerability of prucalopride in patients with chronic constipation due to spinal cord injury. Scand J Gastroenterol. 2002;37(4):431–436. doi: 10.1080/003655202317316060. [DOI] [PubMed] [Google Scholar]
- 145.Menees S, Saad R, Chey WD. Agents that act luminally to treat diarrhoea and constipation. Nat Rev Gastroenterol Hepatol. 2012;9(11):661–674. doi: 10.1038/nrgastro.2012.162. [DOI] [PubMed] [Google Scholar]
- 146.Korsten MA, Rosman AS, Ng A, et al. Infusion of neostigmine-glycopyrrolate for bowel evacuation in persons with spinal cord injury. Am J Gastroenterol. 2005;100(7):1560–1565. doi: 10.1111/j.1572-0241.2005.41587.x. [DOI] [PubMed] [Google Scholar]
- 147.Rosman AS, Chaparala G, Monga A, Spungen AM, Bauman WA, Korsten MA. Intramuscular neostigmine and glycopyrrolate safely accelerated bowel evacuation in patients with spinal cord injury and defecatory disorders. Dig Dis Sci. 2008;53(10):2170–2173. doi: 10.1007/s10620-008-0216-z. [DOI] [PubMed] [Google Scholar]
- 148.Corazziari E. Need of the ideal drug for the treatment of chronic constipation. Ital J Gastroenterol Hepatol. 1999;31(suppl 3):S232–233. [PubMed] [Google Scholar]
- 149.Müller-Lissner S. Pharmacokinetic and pharmacodynamic considerations for the current chronic constipation treatments. Expert Opin Drug Metab Toxicol. 2013;9(4):391–401. doi: 10.1517/17425255.2013.773972. [DOI] [PubMed] [Google Scholar]
- 150.Müller-Lissner S. The pathophysiology, diagnosis, and treatment of constipation. Dtsch Arztebl Int. 2009;106(25):424–431. doi: 10.3238/arztebl.2009.0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tack J, Müller-Lissner S, Stanghellini V, et al. Diagnosis and treatment of chronic constipation: a European perspective. Neurogastroenterol Motil. 2011;23(8):697–710. doi: 10.1111/j.1365-2982.2011.01709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kamm MA, Mueller-Lissner S, Wald A, Richter E, Swallow R, Gessner U. Oral bisacodyl is effective and well-tolerated in patients with chronic constipation. Clin Gastroenterol Hepatol. 2011;9(7):577–583. doi: 10.1016/j.cgh.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 153.Corazziari E, Materia E, Bausano G, et al. Laxative consumption in chronic nonorganic constipation. J Clin Gastroenterol. 1987;9(4):427–430. doi: 10.1097/00004836-198708000-00014. [DOI] [PubMed] [Google Scholar]
- 154.DiPalma JA, McGowan J, Cleveland MV. Clinical trial: an efficacy evaluation of reduced bisacodyl given as part of a polyethylene glycol electrolyte solution preparation prior to colonoscopy. Aliment Pharmacol Ther. 2007;26(8):1113–1119. doi: 10.1111/j.1365-2036.2007.03459.x. [DOI] [PubMed] [Google Scholar]
- 155.DiPalma JA. Current treatment options for chronic constipation. Rev Gastroenterol Disord. 2004;4(suppl 2):S34–42. [PubMed] [Google Scholar]
- 156.Kienzle-Horn S, Vix JM, Schuijt C, Peil H, Jordan CC, Kamm MA. Efficacy and safety of bisacodyl in the acute treatment of constipation: double-blind, randomized, placebo-controlled study. Aliment Pharmacol Ther. 2006;23(10):1479–1488. doi: 10.1111/j.1365-2036.2006.02903.x. [DOI] [PubMed] [Google Scholar]
- 157. The electronic Medicines Compendium (eMC). Bisacodyl 10mg suppositories. https://www.medicines.org.uk/emc/product/8462/smpc Revised February 12, 2019. Accessed April 29, 2020.
- 158.House JG, Stiens SA. Pharmacologically initiated defecation for persons with spinal cord injury: effectiveness of three agents. Arch Phys Med Rehabil. 1997;78(10):1062–1065. doi: 10.1016/s0003-9993(97)90128-3. [DOI] [PubMed] [Google Scholar]
- 159.Frisbie JH. Improved bowel care with a polyethylene glycol based bisacadyl suppository. J Spinal Cord Med. 1997;20(2):227–229. doi: 10.1080/10790268.1997.11719473. [DOI] [PubMed] [Google Scholar]
- 160.Dunn KL, Galka ML. A comparison of the effectiveness of Therevac SB and bisacodyl suppositories in SCI patients’ bowel programs. Rehabil Nurs. 1994;19(6):334–338. doi: 10.1002/j.2048-7940.1994.tb01336.x. [DOI] [PubMed] [Google Scholar]
- 161.Stiens SA, Luttrel W, Binard JE. Polyethylene glycol versus vegetable oil based bisacodyl suppositories to initiate side-lying bowel care: a clinical trial in persons with spinal cord injury. Spinal Cord. 1998;36(11):777–781. doi: 10.1038/sj.sc.3100702. [DOI] [PubMed] [Google Scholar]
- 162.Amir I, Sharma R, Bauman WA, Korsten MA. Bowel care for individuals with spinal cord injury: comparison of four approaches. J Spinal Cord Med. 1998;21(1):21–24. doi: 10.1080/10790268.1998.11719506. [DOI] [PubMed] [Google Scholar]
- 163. National Drug Codes List. NDC 17433–9877 Enemeez Plus. Docusate sodium and benzocaine. https://ndclist.com/ndc/17433-9877 Accessed July 7, 2018.
- 164.Lynchburg, VA: C.B. Fleet Co, Inc; 2020. Fleet Enema [package insert] [Google Scholar]
- 165.U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA warns of possible harm from exceeding recommended dose of over-the-counter sodium phosphate products to treat constipation. https://www.fda.gov/Drugs/DrugSafety/ucm380757.htm Published January 8, 2014. Accessed July 7, 2018.
- 166.Schmelzer M, Case P, Chappell SM, Wright KB. Colonic cleansing, fluid absorption, and discomfort following tap water and soapsuds enemas. Appl Nurs Res. 2000;13(2):83–91. [PubMed] [Google Scholar]
- 167.Schmelzer M, Schiller LR, Meyer R, Rugari SM, Case P. Safety and effectiveness of large-volume enema solutions. Appl Nurs Res. 2004;17(4):265–274. [PubMed] [Google Scholar]
- 168.Vilke GM, DeMers G, Patel N, Castillo EM. Safety and efficacy of milk and molasses enemas in the emergency department. J Emerg Med. 2015;48(6):667–670. doi: 10.1016/j.jemermed.2015.01.035. [DOI] [PubMed] [Google Scholar]
- 169.Wilson M. A review of transanal irrigation in adults. Br J Nurs. 2017;26(15):846–856. doi: 10.12968/bjon.2017.26.15.846. [DOI] [PubMed] [Google Scholar]
- 170.Emmanuel AV, Krogh K, Bazzochi G, et al. Members of the working group on Transanal Irrigation from UK, Denmark, Italy, Germany, France and the Netherlands Consensus review of best practice of transanal irrigation in adults. Spinal Cord. 2013;51(10):732–738. doi: 10.1038/sc.2013.86. [DOI] [PubMed] [Google Scholar]
- 171.Christensen P, Kvitzau B, Krogh K, Buntzen S, Laurberg S. Neurogenic colorectal dysfunction: use of new antegrade and retrograde wash-out methods. Spinal Cord. 2000;38(4):255–261. doi: 10.1038/sj.sc.3100991. [DOI] [PubMed] [Google Scholar]
- 172.Christensen P, Bazzocchi G, Coggrave M, et al. Outcome of transanal irrigation for bowel dysfunction in patients with spinal cord injury. J Spinal Cord Med. 2008;31(5):560–567. doi: 10.1080/10790268.2008.11754571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Faaborg PM, Christensen P, Kvitsau B, Buntzen S, Laurberg S, Krogh K. Long-term outcome and safety of transanal colonic irrigation for neurogenic bowel dysfunction. Spinal Cord. 2009;47(7):545–549. doi: 10.1038/sc.2008.159. [DOI] [PubMed] [Google Scholar]
- 174.Kim HR, Lee BS, Lee JE, Shin HI. Application of transanal irrigation for patients with spinal cord injury in South Korea: a 6-month follow-up study. Spinal Cord. 2013;51(5):389–394. doi: 10.1038/sc.2012.171. [DOI] [PubMed] [Google Scholar]
- 175.Coggrave M, Norton C, Cody JD. Management of faecal incontinence and constipation in adults with central neurological diseases. Cochrane Database Syst Rev. 2014;(1):CD002115. doi: 10.1002/14651858.CD002115.pub5. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Christensen P, Krogh K. Transanal irrigation for disordered defecation: a systematic review. Scand J Gastroenterol. 2010;45(5):517–527. doi: 10.3109/00365520903583855. [DOI] [PubMed] [Google Scholar]
- 177.Del Popolo G, Mosiello G, Pilati C, et al. Treatment of neurogenic bowel dysfunction using transanal irrigation: a multicenter Italian study. Spinal Cord. 2008;46(7):517–522. doi: 10.1038/sj.sc.3102167. [DOI] [PubMed] [Google Scholar]
- 178.Emmanuel A, Kumar G, Christensen P, et al. Long-term cost-effectiveness of transanal irrigation in patients with neurogenic bowel dysfunction. PLoS One. 2016;11(8):e0159394. doi: 10.1371/journal.pone.0159394. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Christensen P, Andreasen J, Ehlers L. Cost-effectiveness of transanal irrigation versus conservative bowel management for spinal cord injury patients. Spinal Cord. 2009;47(2):138–143. doi: 10.1038/sc.2008.98. [DOI] [PubMed] [Google Scholar]
- 180.Christensen P, Krogh K, Perrouin-Verbe B, et al. Global audit on bowel perforations related to transanal irrigation. Tech Coloproctol. 2016;20(2):109–115. doi: 10.1007/s10151-015-1400-8. [DOI] [PubMed] [Google Scholar]
- 181.Puet TA, Jackson H, Amy S. Use of pulsed irrigation evacuation in the management of the neuropathic bowel. Spinal Cord. 1997;35(10):694–699. doi: 10.1038/sj.sc.3100491. [DOI] [PubMed] [Google Scholar]
- 182.Sale P, Mazzarella F, Pagliacci MC, Aito S, Agosti M, Franceschini M. Sport, free time and hobbies in people with spinal cord injury. Spinal Cord. 2012;50(6):452–456. doi: 10.1038/sc.2011.161. [DOI] [PubMed] [Google Scholar]
- 183.Müller-Lissner SA, Kamm MA, Scarpignato C, Wald A. Myths and misconceptions about chronic constipation. Am J Gastroenterol. 2005;100(1):232–242. doi: 10.1111/j.1572-0241.2005.40885.x. [DOI] [PubMed] [Google Scholar]
- 184.Sadowsky CL, Hammond ER, Strohl AB, et al. Lower extremity functional electrical stimulation cycling promotes physical and functional recovery in chronic spinal cord injury. J Spinal Cord Med. 2013;36(6):623–631. doi: 10.1179/2045772313Y.0000000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hubscher CH, Herrity AN, Williams CS, et al. Improvements in bladder, bowel and sexual outcomes following task-specific locomotor training in human spinal cord injury. PLoS One. 2018;13(1):e0190998. doi: 10.1371/journal.pone.0190998. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Baunsgaard CB, Nissen UV, Brust AK, et al. Exoskeleton gait training after spinal cord injury: an exploratory study on secondary health conditions. J Rehabil Med. 2018;50(9):806–813. doi: 10.2340/16501977-2372. [DOI] [PubMed] [Google Scholar]
- 187.Esquenazi A, Talaty M, Packel A, Saulino M. The ReWalk powered exoskeleton to restore ambulatory function to individuals with thoracic-level motor-complete spinal cord injury. Am J Phys Med Rehabil. 2012;91(11):911–921. doi: 10.1097/PHM.0b013e318269d9a3. [DOI] [PubMed] [Google Scholar]
- 188.Huang Q, Yu L, Gu R, Zhou Y, Hu C. Effects of robot training on bowel function in patients with spinal cord injury. J Phys Ther Sci. 2015;27(5):1377–1378. doi: 10.1589/jpts.27.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kozlowski AJ, Bryce TN, Dijkers MP. Time and effort required by persons with spinal cord injury to learn to use a powered exoskeleton for assisted walking. Top Spinal Cord Inj Rehabil. 2015;21(2):110–121. doi: 10.1310/sci2102-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Eng JJ, Levins SM, Townson AF, Mah-Jones D, Bremmer J, Huston G. Use of prolonged standing for individuals with spinal cord injuries. Phys Ther. 2001;81(8):1392–1399. doi: 10.1093/ptj/81.8.1392. [DOI] [PubMed] [Google Scholar]
- 191.Dunn RB, Walter JS, Lucero Y, et al. Follow-up assessment of standing mobility device users. Assistive Tech. 1998;10(2):84–93. doi: 10.1080/10400435.1998.10131966. [DOI] [PubMed] [Google Scholar]
- 192.Walter JS, Sola PG, Sacks J, Lucero Y, Langbein E, Weaver F. Indications for a home standing program for individuals with spinal cord injury. J Spinal Cord Med. 1999;22(3):152–158. doi: 10.1080/10790268.1999.11719564. [DOI] [PubMed] [Google Scholar]
- 193.Multidisciplinary Association of Spinal Cord Injury Professionals (MASCIP) Clinical guideline for standing adults following spinal cord injury. https://www.mascip.co.uk/wp-content/uploads/2015/05/Clinical-Guidelines-for-Standing-Adults-Following-Spinal-Cord-Injury.pdf Published April 2013. Accessed June 10, 2020.
- 194.Kwok S, Harvey L, Glinsky J, Bowden JL, Coggrave M, Tussle D. Does regular standing improve bowel function in people with spinal cord injury? A randomised crossover trial. Spinal Cord. 2015;53(1):36–41. doi: 10.1038/sc.2014.189. [DOI] [PubMed] [Google Scholar]
- 195.Coggrave M, Burrows D, Durand MA. Progressive protocol in the bowel management of spinal cord injuries. Br J Nurs. 2006;15(20):1108–1113. doi: 10.12968/bjon.2006.15.20.22295. [DOI] [PubMed] [Google Scholar]
- 196.Lin VW, Kim KH, Hsiao I, Brown W. Functional magnetic stimulation facilitates gastric emptying. Arch Phys Med Rehabil. 2002;83(6):806–810. doi: 10.1053/apmr.2002.32644. [DOI] [PubMed] [Google Scholar]
- 197.Tsai PY, Wang CP, Chiu FY, Tsai YA, Chang YC, Chuang TY. Efficacy of functional magnetic stimulation in neurogenic bowel dysfunction after spinal cord injury. J Rehabil Med. 2009;41(1):41–47. doi: 10.2340/16501977-0280. [DOI] [PubMed] [Google Scholar]
- 198.Morren GL, Walter S, Hallböök O, Sjödahl R. Effects of magnetic sacral root stimulation on anorectal pressure and volume. Dis Colon Rectum. 2001;44(12):1827–1833. doi: 10.1007/BF02234462. [DOI] [PubMed] [Google Scholar]
- 199.Shafik A. Magnetic stimulation: a novel method for inducing evacuation of the neuropathic rectum and urinary bladder in a canine model. Urology. 1999;54(2):368–372. doi: 10.1016/s0090-4295(99)00083-7. [DOI] [PubMed] [Google Scholar]
- 200.Vallès M, Rodríguez A, Borau A, Mearin F. Effect of sacral anterior root stimulator on bowel dysfunction in patients with spinal cord injury. Dis Colon Rectum. 2009;52(5):986–992. doi: 10.1007/DCR.0b013e31819ed459. [DOI] [PubMed] [Google Scholar]
- 201.Creasey GH, Grill JH, Korsten M, et al. ; Implanted Neuroprosthesis Research Group An implantable neuroprosthesis for restoring bladder and bowel control to patients with spinal cord injuries: a multicenter trial. Arch Phys Med Rehabil. 2001;82(11):1512–1519. doi: 10.1053/apmr.2001.25911. [DOI] [PubMed] [Google Scholar]
- 202.MacDonagh RP, Sun WM, Smallwood R, Forster D, Read NW. Control of defecation in patients with spinal injuries by stimulation of sacral anterior nerve roots. BMJ. 1990;300(6738):1494–1497. doi: 10.1136/bmj.300.6738.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Binnie NR, Smith AN, Creasey GH, Edmond P. Constipation associated with chronic spinal cord injury: the effect of pelvic parasympathetic stimulation by the Brindley stimulator. Paraplegia. 1991;29(7):463–439. doi: 10.1038/sc.1991.63. [DOI] [PubMed] [Google Scholar]
- 204.Kachourbos MJ, Creasey GH. Health promotion in motion: improving quality of life for persons with neurogenic bladder and bowel using assistive technology. SCI Nurs. 2000;17(3):125–129. [PubMed] [Google Scholar]
- 205.Rasmussen MM, Kutzenberger J, Krogh K, et al. Sacral anterior root stimulation improves bowel function in subjects with spinal cord injury. Spinal Cord. 2015;53(4):297–301. doi: 10.1038/sc.2015.2. [DOI] [PubMed] [Google Scholar]
- 206.Worsøe J, Rasmussen M, Christensen P, Krogh K. Neurostimulation for neurogenic bowel dysfunction. Gastroenterol Res Pract. 2013;2013:563294. doi: 10.1155/2013/563294. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Jarrett ME, Matzel KE, Christiansen J, et al. Sacral nerve stimulation for faecal incontinence in patients with previous partial spinal injury including disc prolapse. Br J Surg. 2005;92(6):734–739. doi: 10.1002/bjs.4859. [DOI] [PubMed] [Google Scholar]
- 208.Holzer B, Rosen HR, Novi G, Ausch C, Hölbling N, Schiessel R. Sacral nerve stimulation for neurogenic faecal incontinence. Br J Surg. 2007;94(6):749–753. doi: 10.1002/bjs.5499. [DOI] [PubMed] [Google Scholar]
- 209.Lombardi G, Del Popolo G, Cecconi F, Surrenti E, Macchiarella A. Clinical outcome of sacral neuromodulation in incomplete spinal cord-injured patients suffering from neuro-genic bowel dysfunctions. Spinal Cord. 2010;48(2):154–159. doi: 10.1038/sc.2009.101. [DOI] [PubMed] [Google Scholar]
- 210.Lombardi G, Nelli F, Mencarini M, Del Popolo G. Clinical concomitant benefits on pelvic floor dysfunctions after sacral neuromodulation in patients with incomplete spinal cord injury. Spinal Cord. 2011;49(5):629–636. doi: 10.1038/sc.2010.176. [DOI] [PubMed] [Google Scholar]
- 211.Chen G, Liao L. Sacral neuromodulation for neurogenic bladder and bowel dysfunction with multiple symptoms secondary to spinal cord disease. Spinal Cord. 2015;53(3):204–208. doi: 10.1038/sc.2014.157. [DOI] [PubMed] [Google Scholar]
- 212.Gstaltner K, Rosen H, Hufgard J, Märk R, Schrei K. Sacral nerve stimulation as an option for the treatment of faecal incontinence in patients suffering from cauda equina syndrome. Spinal Cord. 2008;46(9):644–647. doi: 10.1038/sc.2008.6. [DOI] [PubMed] [Google Scholar]
- 213.Sievert KD, Amend B, Gakis G, et al. Early sacral neuromodulation prevents urinary incontinence after complete spinal cord injury. Ann Neurol. 2010;67(1):74–84. doi: 10.1002/ana.21814. [DOI] [PubMed] [Google Scholar]
- 214.Mentes BB, Yüksel O, Aydin A, Tezcaner T, Leventoglu A, Aytaç B. Posterior tibial nerve stimulation for faecal incontinence after partial spinal injury: preliminary report. Tech Coloproctol. 2007;11(2):115–119. doi: 10.1007/s10151-007-0340-3. [DOI] [PubMed] [Google Scholar]
- 215.Worsøe J, Fynne L, Laurberg S, Krogh K, Rijkhoff NJ. Acute effect of electrical stimulation of the dorsal genital nerve on rectal capacity in patients with spinal cord injury. Spinal Cord. 2012;50(6):462–466. doi: 10.1038/sc.2011.159. [DOI] [PubMed] [Google Scholar]
- 216.Korsten MA, Fajardo NR, Rosman AS, Creasey GH, Spungen AM, Bauman WA. Difficulty with evacuation after spinal cord injury: colonic motility during sleep and effects of abdominal wall stimulation. J Rehabil Res Dev. 2004;41(1):95–100. doi: 10.1682/jrrd.2004.01.0095. [DOI] [PubMed] [Google Scholar]
- 217.Hascakova-Bartova R, Dinant JF, Parent A, Ventura M. Neuromuscular electrical stimulation of completely paralyzed abdominal muscles in spinal cord-injured patients: a pilot study. Spinal Cord. 2008;46(6):445–450. doi: 10.1038/sj.sc.3102166. [DOI] [PubMed] [Google Scholar]
- 218.Walter M, Lee AHX, Kavanagh A, Phillips AA, Krassioukov AV. Epidural spinal cord stimulation acutely modulates lower urinary tract and bowel function following spinal cord injury: a case report. Front Physiol. 2018;9:1816. doi: 10.3389/fphys.2018.01816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Furlan JC, Urbach DR, Fehlings MG. Optimal treatment for severe neurogenic bowel dysfunction after chronic spinal cord injury: a decision analysis. Br J Surg. 2007;94(9):1139–1150. doi: 10.1002/bjs.5781. [DOI] [PubMed] [Google Scholar]
- 220.Teichman JMH, Harris JM, Currie DM, Barber DB. Malone antegrade continence enema for adults with neurogenic bowel disease. J Urol. 1998;160(4):1278–1281. [PubMed] [Google Scholar]
- 221.Teichman JM, Zabihi N, Kraus SR, Harris JM, Barber DB. Long-term results for Malone antegrade continence enema for adults with neurogenic bowel disease. Urology. 2003;61(3):502–506. doi: 10.1016/s0090-4295(02)02282-3. [DOI] [PubMed] [Google Scholar]
- 222.Worsøe J, Christensen P, Krogh K, Buntzen S, Laurberg S. Long-term results of antegrade colonic enema in adults: assessment of functional results. Dis Colon Rectum. 2008;51(10):1523–1528. doi: 10.1007/s10350-008-9401-6. [DOI] [PubMed] [Google Scholar]
- 223.Smith PH, Decker RM. Antegrade continence enema procedure: impact of QOL in patients with spinal cord injury. Spinal Cord. 2015;53(3):213–215. doi: 10.1038/sc.2014.223. [DOI] [PubMed] [Google Scholar]
- 224.Boucher M, Dukes S, Bryan S, Branagan G. Early colostomy formation can improve independence following spinal cord injury and increase acceptability of bowel management. Top Spinal Cord Inj Rehabil. 2019;25(1):23–30. doi: 10.1310/sci18-00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Frisbie JH, Tun CG, Nguyen CH. Effect of enterostomy on quality of life in spinal cord injury patients. J Am Paraplegia Soc. 1986;9(1–2):3–5. doi: 10.1080/01952307.1986.11785936. [DOI] [PubMed] [Google Scholar]
- 226.Stone JM, Wolfe VA, Nino-Murcia M, Perkash I. Colostomy as treatment for complications of spinal cord injury. Arch Phys Med Rehabil. 1990;71(7):514–518. [PubMed] [Google Scholar]
- 227.Kelly SR, Shashidharan M, Bonwell Tromans AM, Finnis D, Grundy DJ. The role of intestinal stoma in patients with spinal cord injury. Spinal Cord. 1999;37(3):211–214. doi: 10.1038/sj.sc.3100764. [DOI] [PubMed] [Google Scholar]
- 228.Rosito O, Nino-Murcia M, Wolfe VA, Kiratli BJ, Perkash I. The effects of colostomy on the quality of life in patients with spinal cord injury: a retrospective analysis. J Spinal Cord Med. 2002;25(3):174–183. doi: 10.1080/10790268.2002.11753619. [DOI] [PubMed] [Google Scholar]
- 229.Branagan G, Tromans A, Finnis D, et al. Effect of stoma formation on bowel care and quality of life in patients with spinal cord injury. Spinal Cord. 2003;41(12):680–683. doi: 10.1038/sj.sc.3101529. [DOI] [PubMed] [Google Scholar]
- 230.Munck J, Simoens Ch, Thill V, et al. Intestinal stoma in patients with spinal cord injury: a retrospective analysis of 23 patients. Hepatogastroenterology. 2008;55(88):2125–2129. [PubMed] [Google Scholar]
- 231.Coggrave MJ, Ingram RM, Gardner BP, Norton CS. The impact of stoma for bowel management after spinal cord injury. Spinal Cord. 2012;50(11):848–852. doi: 10.1038/sc.2012.66. [DOI] [PubMed] [Google Scholar]
- 232.de la Fuente SG, Levin LS, Reynolds JD, et al. Elective stoma construction improves outcomes in medically intractable pressure ulcers. Dis Colon Rectum. 2003;46(11):1525–1530. doi: 10.1007/s10350-004-6808-6. [DOI] [PubMed] [Google Scholar]
- 233.Kruger EA, Pires M, Ngann Y, Sterling M, Rubayi S. Comprehensive management of pressure ulcers in spinal cord injury: current concepts and future trends. J Spinal Cord Med. 2013;36(6):572–584. doi: 10.1179/2045772313Y.0000000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Tseng CW, Lin CL, Chen YT, Jeng LB. Ischemic bowel syndrome in patients with spinal cord injury: a nationwide study. PLoS One. 2017;12:e0169070. doi: 10.1371/journal.pone.0169070. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Scott D, Papa MZ, Sareli M, Velano A, Ben-Ari GY, Koller M. Management of hemorrhoidal disease in patients with chronic spinal cord injury. Tech Coloproctol. 2002;6(1):19–22. doi: 10.1007/s101510200003. [DOI] [PubMed] [Google Scholar]
- 236.Cosman BC, Cajas-Monson LC, Ramamoorthy SL. Twenty years of a veterans spinal cord colorectal clinic: flexible sigmoidoscopy and multiple hemorrhoid ligation. Dis Colon Rectum. 2017;60(4):398–404. doi: 10.1097/DCR.0000000000000773. [DOI] [PubMed] [Google Scholar]
- 237.May L, Day R, Warren S. Evaluation of patient education in spinal cord injury rehabilitation: knowledge, problem-solving and perceived importance. Disabil Rehabil. 2006;28(7):405–413. doi: 10.1080/09638280500192439. [DOI] [PubMed] [Google Scholar]
- 238.Potter PJ, Wolfe DL, Burkell JA, Hayes KC. Challenges in educating individuals with SCI to reduce secondary conditions. Top Spinal Cord Inj Rehabil. 2004;10(1):30–40. [Google Scholar]
- 239.Anderson K. Targeting recovery: priorities of the spinal cord injured population. J Neurotrauma. 2004;21(10):1371–1383. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- 240.Ditunno PL, Patrick M, Stineman M, Dittuno JF. Who wants to walk? Preferences for recovery after SCI: a longitudinal and cross-sectional study. Spinal Cord. 2008;46(3):500–506. doi: 10.1038/sj.sc.3102172. [DOI] [PubMed] [Google Scholar]
- 241.Braaf S, Lennon A, Nunn A, Gabbe B. Social activity and relationship changes experienced by people with bowel and bladder dysfunction following spinal cord injury. Spinal Cord. 2017;55(7):679–686. doi: 10.1038/sc.2017.19. [DOI] [PubMed] [Google Scholar]
- 242.Rundquist J, Gassaway J, Bailey J, Lingefelt P, Reyes IA, Thomas J. The SCIRehab project: treatment time spent in SCI rehabilitation. Nursing bedside education and care management time during inpatient spinal cord injury rehabilitation. J Spinal Cord Med. 2011;34(2):205–215. doi: 10.1179/107902611X12971826988255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Olinzock BJ. A model for assessing learning readiness for self-direction of care in individuals with spinal cord injuries: a qualitative study. SCI Nurs. 2004;21(2):69–74. [PubMed] [Google Scholar]
- 244.Cabigon RD, Wojciechowski E, Rosen L, Miller D, Mix C, Chen D. Interprofessional collaboration and peer mentors for bowel education in spinal cord injury: a case consultation. Rehabil Nurs. 2019;44(2):123–127. doi: 10.1097/rnj.0000000000000123. [DOI] [PubMed] [Google Scholar]
- 245.Pryor J, Jannings W. Preparing patients to self-manage faecal continence following spinal cord injury. Int J Ther Rehabil. 2004;11(2):79–82. [Google Scholar]
- 246.Wound, Ostomy and Incontinence Nurse Society Peristomal skin assessment guide for clinicians and consumers. https://www.wocn.org/page/PSAG Published 2018. Accessed April 29, 2020. [Google Scholar]
- 247.Park SE, Elliott S, Noonan VK, et al. Impact of bladder, bowel and sexual dysfunction on health status of people with thoracolumbar spinal cord injuries living in the community. J Spinal Cord Med. 2017;40(5):548–559. doi: 10.1080/10790268.2016.1213554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Bartlett L, Nowak M, Ho Y. Impact of fecal incontinence on quality of life. World J Gastroenterol. 2009;15(26):3276–3282. doi: 10.3748/wjg.15.3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Burns AS, St-Germain D, Connolly M, et al. Neurogenic bowel after spinal cord injury from the perspective of support providers: a phenomenological study. PM R. 2015;7(4):407–416. doi: 10.1016/j.pmrj.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 250.Castellano-Tejedor C, Lusilla-Palacios P. A study of burden of care and its correlates among family members supporting relatives and loved ones with traumatic spinal cord injuries. Clin Rehabil. 2017;31(7):948–956. doi: 10.1177/0269215517709330. [DOI] [PubMed] [Google Scholar]
- 251.Dickson A, O’Brien G, Ward R, Allan D, O’Carroll RE. The impact of assuming the primary caregiver role following traumatic spinal cord injury: an interpretative phenomenological analysis of the spouse’s experience. Psychol Health. 2010;25(9):1101–1120. doi: 10.1080/08870440903038949. [DOI] [PubMed] [Google Scholar]
- 252.Boschen KA, Tonack M, Gargaro J. The impact of being a support provider to a person living in the community with a spinal cord injury. Rehabil Psychol. 2005;50(4):397–407. [Google Scholar]
- 253.Elliott TR, Berry JW. Brief problem-solving training for family caregivers of persons with recent-onset spinal cord injuries: a randomized controlled trial. J Clin Psychol. 2009;65(4):406–422. doi: 10.1002/jclp.20527. [DOI] [PubMed] [Google Scholar]
- 254.Bozan BC, Karamehmetoglu SS, Koyuncu H. The sex effect on the perceived significance of functional loss due to spinal cord injury. Neurosurg Q. 2015;25(3):388–391. [Google Scholar]
- 255.Kreuter M, Taft C, Siösteen A, Biering-Sørensen F. Women’s sexual functioning and sex life after spinal cord injury. Spinal Cord. 2011;49(1):154–160. doi: 10.1038/sc.2010.51. [DOI] [PubMed] [Google Scholar]
- 256.Naicker AS, Roohi SA, Naicker MS, Zaleha O. Bowel dysfunction in spinal cord injury. Med J Malaysia. 2008;63(2):104–108. [PubMed] [Google Scholar]
- 257.Cobb JE, Leblond J, Dumont FS, Noreau L. Perceived influence of intrinsic/extrinsic factors on participation in life activities after spinal cord injury. Disabil Health J. 2018;11(4):583–590. doi: 10.1016/j.dhjo.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 258.Nevedal A, Kratz AL, Tate DG. Women’s experiences of living with neurogenic bladder and bowel after spinal cord injury: life controlled by bladder and bowel. Disabil Rehabil. 2016;38(6):573–81. doi: 10.3109/09638288.2015.1049378. [DOI] [PubMed] [Google Scholar]
- 259.Byrne CM, Pager CK, Rex J, Roberts R, Solomon MJ. Assessment of quality of life in the treatment of patients with neuropathic fecal incontinence. Dis Colon Rectum. 2002;45(11):1431–1436. doi: 10.1007/s10350-004-6444-1. [DOI] [PubMed] [Google Scholar]
- 260.Prysak G, Andresen E, Meyers A. Prevalence of secondary conditions in veterans with spinal cord injury and their interference with life activities. Top Spinal Cord Inj Rehabil. 2000;6(1):34–42. [Google Scholar]
- 261.Bombardier CH, Richards JS, Krause JS, Tulsky D, Tate DG. Symptoms of major depression in people with spinal cord injury: implications for screening. Arch Phys Med Rehabil. 2004;85(11):1749–1756. doi: 10.1016/j.apmr.2004.07.348. [DOI] [PubMed] [Google Scholar]
- 262.Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 263.Kannisto M, Rintala R. Bowel function in adults who have sustained spinal cord injury in childhood. Paraplegia. 1995;33(12):701–703. doi: 10.1038/sc.1995.147. [DOI] [PubMed] [Google Scholar]
- 264.Julia PE, Othman AS. Barriers to sexual activity: counselling spinal cord injured women in Malaysia. Spinal Cord. 2011;49(7):791–794. doi: 10.1038/sc.2011.4. [DOI] [PubMed] [Google Scholar]
- 265.Otero-Villaverde S, Ferreiro-Velasco ME, Montoto-Marqués A, Salvador de la Barrera S, Arias-Pardo AI, Rodriguez-Sotillo A. Sexual satisfaction in women with spinal cord injuries. Spinal Cord. 2015;53(7):557–560. doi: 10.1038/sc.2015.53. [DOI] [PubMed] [Google Scholar]