SUMMARY
Components of the proteostasis network malfunction in aging, and reduced protein quality control in neurons has been proposed to promote neurodegeneration. Here, we investigate the role of chaperone-mediated autophagy (CMA), a selective autophagy shown to degrade neurodegeneration-related proteins, in neuronal proteostasis. Using mouse models with systemic and neuronal-specific CMA blockage, we demonstrate that loss of neuronal CMA leads to altered neuronal function, selective changes in the neuronal metastable proteome, and proteotoxicity, all reminiscent of brain aging. Imposing CMA loss on a mouse model of Alzheimer’s disease (AD) has synergistic negative effects on the proteome at risk of aggregation, thus increasing neuronal disease vulnerability and accelerating disease progression. Conversely, chemical enhancement of CMA ameliorates pathology in two different AD experimental mouse models. We conclude that functional CMA is essential for neuronal proteostasis through the maintenance of a subset of the proteome with a higher risk of misfolding than the general proteome.
In brief
Chaperone-mediated autophagy in neurons regulates a subset of the proteome at risk of aggregation, and manipulation of its activity in mice modulates Alzheimer’s-disease-related brain proteotoxicity.
Graphical Abstract

INTRODUCTION
All cells rely on intracellular surveillance systems to maintain their proteome’s homeostasis (proteostasis). These systems are especially important in neurons that, due to their postmitotic status, are highly sensitive to proteotoxic insults (Nixon, 2013; Scrivo et al., 2018). Decreased neuronal protein quality control with age increases the risk of neurodegenerative diseases (Douglas and Dillin, 2010; Kaushik and Cuervo, 2018; Morimoto and Cuervo, 2014). In fact, the presence of protein aggregates is a common feature in neurodegenerative patient brains (Bourdenx et al., 2017; Jellinger, 2009). Interestingly, most elderly brains also display protein aggregation, even in the absence of disease (Nixon, 2013).
Defective autophagy, one of the components of the proteostasis network, associates with neurodegenerative diseases, including Parkinson’s disease (PD) and Alzheimer’s disease (AD) (Menzies et al., 2017; Scrivo et al., 2018). Macroautophagy has been proven necessary for maintenance of neuronal proteostasis and protection against neurodegeneration (Hara et al., 2006; Komatsu et al., 2006). This work focuses on chaperone-mediated autophagy (CMA), a pathway for selective degradation of single proteins in lysosomes (Kaushik and Cuervo, 2018). Lysosomal targeting is triggered when the cytosolic chaperone heat-shock cognate 71 kDa (Hsc70) recognizes a pentapeptide (KFERQ-like motif) in the substrate protein sequence. Once at the lysosome, the substrate/chaperone complex binds the cytosolic tail of the lysosome-associated membrane protein type 2A (L2A), which triggers L2A multimerization into a translocation complex for lysosomal internalization of substrate proteins for degradation. L2A is the only isoform of the three spliced protein variants of the lamp2 gene (Gough et al., 1995) that participates in CMA (Cuervo and Dice, 2000).
CMA contributes to the degradation of pathogenic proteins involved in neurodegenerative disorders such as α-synuclein (α-syn) or tau (Caballero et al., 2018; Cuervo et al., 2004). Pathogenic variants of these proteins (mutations, or aberrant posttranslational modifications) are targeted to lysosomes via CMA, but they fail to translocate and ultimately block CMA (Caballero et al., 2018; Cuervo et al., 2004; Orenstein et al., 2013; Xilouri et al., 2009). However, little is known about the consequences of the reduced neuronal CMA activity observed in the aging brain and neurodegenerative diseases. Furthermore, considering that multiple types of autophagy coexist in all cells, it is not clear if there is some level of redundancy or if each of them maintains a specific subproteome.
In this work, we knocked out L2A in mice systemically or specifically in neurons to analyze the contribution of CMA to maintenance of the neuronal proteome. We found that neuronal CMA loss leads to profound quantitative and qualitative alterations of the neuronal proteome that result in deregulation of important neuronal functions. Using comparative quantitative proteomics, we identified the subproteome dependent on CMA activity as different from the one depending on functional macroautophagy. We show that CMA is inhibited in experimental models of tauopathies and AD patient brains at an early disease stage and that experimental inhibition of CMA in a mouse model of AD-related proteotoxicity accelerates disease progression through a synergistic negative impact on the proteome at risk of aggregation. Furthermore, we demonstrate that chemical upregulation of CMA in two different mouse models of AD, with tau pathology or combined tau and β-amyloid pathologies, reduces brain pathology and improves disease phenotype. Our findings highlight the contribution of CMA to neuronal proteostasis, demonstrate that CMA deficiency in the aging brain is an aggravating factor in the onset of neurodegenerative diseases, and provide proof of concept for the value of targeting CMA as therapeutic strategy in these conditions.
RESULTS
Neuronal-specific CMA deficiency leads to behavioral impairments
We generated a neuronal-specific CMA-deficient mouse model (CKL2A−/− mice) by removing L2A in excitatory neurons (expressing calcium/calmodulin-dependent protein kinase II [α – CaMKIIα]). We bred L2Aflox/flox mice (Schneider et al., 2014) with a CaMKIIα-driven Cre recombinase transgenic mouse line (Alberi et al., 2011; Choi et al., 2009; Feng et al., 2001; Wang et al., 2013) for selective deletion of the Lamp2 gene exon encoding for the cytosolic and transmembrane domain of the L2A protein (Gough et al., 1995) (Figure S1A). As comparison, we used whole-body L2A knockout (hereafter named L2A−/−) mice. We confirmed the selective deletion of L2A and not other Lamp2 isoforms in both mouse models (Figures S1B–S1D; the remaining L2A signal in CKL2A−/− mice originates from other brain cell types). Both mouse models were born at Mendelian frequency and proportional male/female ratios, and only L2A−/− mice showed an initial delay in body weight gain that normalized by 6 months (Figure S1E). Transgenic mice displayed a discrete reduction in life expectancy that was more noticeable for CKL2A−/− mice (Figure S1F).
Behavioral testing revealed higher scores and faster hindlimb clasping progression, a phenotype of neurological disorder models (Mangiarini et al., 1996), in L2A−/− and CKL2A−/− mice than control (CTR) littermates as early as 3 months of age (Figures S1G–S1I). Both mouse models showed higher latency in the negative geotaxis test, indicative of vestibular/sensorimotor dysfunction (Figure S1J) and reduced short-term memory in a Y-maze (Figures S1K and S1L). Interestingly, only L2A−/− mice displayed impaired locomotion, as evidenced by (1) hyperlocomotion in the open field, reminiscent of some AD models (Min et al., 2015; Takeuchi et al., 2011) (Figures S1M and S1N) and (2) reduction in stride length (Figures S1O and S1P), a feature of PD gait (Fernagut et al., 2002). In addition, CKL2A−/− mice displayed a trend toward reduced spatial working memory (Figure S1Q) and significant reduction in nest building (Figures S1R and S1S), a common phenotype of neurodegeneration in mice (Hernandez et al., 2019). Overall, loss of CMA in only excitatory neurons reproduced most of the behavioral outputs observed upon systemic loss of CMA.
Neuronal CMA deficiency leads to proteostasis collapse
By 6 months of age, both mice with systemic or neuronal-specific CMA loss showed thicknesses of CA1, dentate gyrus, and cortex as well as hippocampal surface undistinguishable from their respective CTR littermates (Figures 1A–1D and S2A–S2D). Number of astrocytes and microglia was unchanged in the hippocampus of L2A−/− and CKL2A−/− mice (Figures S2E and S2F), and only a small increase of astrocyte area was noticeable in CKL2A−/− mice (Figure S3A).
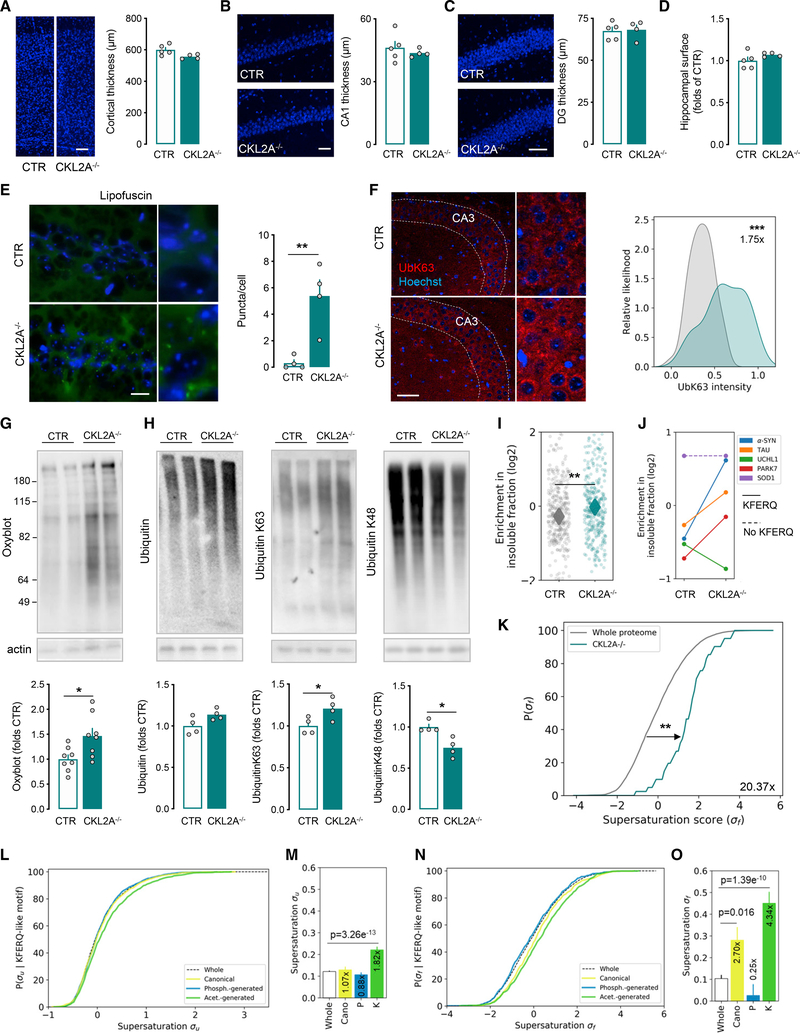
Figure 1. CMA deficiency in excitatory neurons leads to proteostasis collapse.
(A–C) Thickness of somatosensory cortex (A), CA1 (B), and dentate gyrus (DG) (C) in control (CTR) and CKL2A−/− mice assessed by Hoechst nuclear staining. Shown are representative images (left) and quantification (right).
(D) Hippocampal surface in CKL2A−/− relative to CTR mice.
(E) Lipofuscin autofluorescence in the hippocampus of CTR and CKL2A−/− mice. Shown are representative images (left) and quantification of puncta per cell (right) (related quantification in the stratum radiatum are shown in Figure S3C).
(F) Immunostaining for K63-linkage ubiquitin in CTR and CKL2A−/− mice hippocampal neurons (left) and staining intensity distribution per cell body (right). Insert highlights the CA3 region.
(G and H) Immunoblot for carbonyl groups (to detect oxidized proteins; G) and ubiquitinated proteins (total, K63- and K48-linkage; H) in the hippocampus of CTR and CKL2A−/− mice. Shown are representative immunoblots (top) and normalized densitometric quantification (bottom).
(I–K) Comparative quantitative proteomics of CTR and CKL2A−/− mice cortex. Enrichment of total proteins (I) and prone-to-aggregate proteins (J) in the insoluble fraction (solid and discontinuous lines represent presence and absence of KFERQ-like motifs, respectively) and fold increase of the σf supersaturation score of proteins in the insoluble fraction (K).
(L–O) Cumulative distribution of the supersaturation score σu (L) σf (N) of putative CMA substrates compared the whole proteome. Mean value of supersaturation score σu (M) σf (O) for different types of KFERQ-like motifs. Whole, whole proteome; Cano, canonical motif; P, phosphorylation (phosph.)-generated motif; K, acetylation (acet.)-generated motif.
Scale bars represent 50 μm (A, C, and F), 40 μm (B), and 20 μm (E). Data represent mean ± SEM and individual values (A–E and G–I). Comparisons were made with an unpaired t test (A–I) and one-way ANOVA followed by Tukey’s post hoc test (M and O). *p < 0.05, **p < 0.005, and ***p < 0.0005. See also Figure S3, Data S1, and Table S1.
Analysis of overall cellular proteostasis revealed lipofuscin deposits (cross-linked oxidized proteins and lipids) (Figure S2G) and K63-linked ubiquitinated proteins inclusions, which are usually targets of lysosomal degradation (Kraft et al., 2010) (Figures S2H and S2I), in the hippocampus of L2A−/− mice at 6 months of age. Similar features were noticeable at this early age in excitatory pyramidal neurons in the hippocampus of CKL2A−/− mice (Figures 1E and 1F; CaMKIIα staining shown in Figure S3B), but not in regions occupied mostly by interneurons (i.e., the stratum radiatum) or in glial cells (Figures S3C–S3E). Immunoblot analysis of CKL2A−/− mice cortex revealed accumulation of oxidized proteins (Figure 1G), hydroxynonenal protein adducts (Figure S3F), and ubiquitinated proteins, specifically with K63 linkage (Figure 1H), further supporting that loss of CMA results in profound disruption of neuronal proteostasis.
To analyze the extent of proteome compromise upon neuronal CMA blockage, we isolated the sarkosyl-insoluble fraction from the cortex of CTR and CKL2A−/− mice and performed comparative quantitative proteomics. We found a significant enrichment of proteins in the insoluble fraction of CKL2A−/− mice brains (Cohen’s d = 0.24; Figure 1I; Table S1). Most insoluble proteins (76%) contained KFERQ-like motifs, mainly those that do not require posttranslational modifications for hsc70 recognition (~52% in CKL2A−/− sarkosyl-insoluble fraction versus ~47% in the whole murine proteome; Kirchner et al., 2019) (Figure S3G). Prone-to-aggregate proteins bearing KFERQ-like motifs such as α-syn, tau, UCHL1, and PARK7 displayed a shift toward insolubility in CKL2A−/− brains, whereas this was not the case for SOD1, which lacks the motif (Figure 1J). The only exception was UCHL1 (Figure 1J), which, interestingly, bears a phosphorylation-generated motif. To investigate the status of the supersaturated proteome (the one at risk of aggregation due to protein concentrations close to their solubility limit; Ciryam et al., 2013; Kundra et al., 2017), we analyzed the supersaturation score (expression of a protein relative to its solubility limit) and found a remarkable increase (20.37-fold) of the supersaturation score σf of proteins enriched in the insoluble fraction of CKL2A−/− mice compared to the whole proteome (Figure 1K). These findings support that the proteome that shift to insolubility upon CMA loss is part of the supersaturated proteome. In fact, proteins bearing constitutive or acetylation-generated KFERQ-like motifs showed higher σu and σf supersaturation scores, indicative of their tendency to form aggregates from unfolded or folded state, respectively (Figures 1L–1O).
Taken together, these results demonstrate a profound collapse of the proteome upon neuronal CMA blockage.
Neuronal CMA deficiency leads to functional dysregulation
Neuronal protein aggregates have been described upon blockage of macroautophagy (Hara et al., 2006; Komatsu et al., 2006). However, the loss of proteostasis in CMA-deficient brains was not due to macroautophagy malfunction. Contrary to the macroautophagy failure observed when all three spliced variants of LAMP2 were eliminated (Rothaug et al., 2015; Tanaka et al., 2000), steady-state levels of autophagosome components (LC3 and GATE16), macroautophagy adaptors (p62), and lysosomal membrane proteins (LAMP1) were comparable in cortex and hippocampus of L2A−/− ανδ CKL2A−/− mice and their corresponding CTR littermates (Figures S2J, S2K, S3H, and S3I; note the marked p62 accumulation in macroautophagy-defective mice in the same neurons [CKATG7−/− mice]). Ultrastructural analysis revealed that number and state of maturation of autophagic compartments was indistinguishable between CKL2A−/− and CTR mice (Figure S3J), while perinuclear electrodense material (likely protein inclusions) was only observed in CKL2A−/− neurons (Figure S3K).
We used comparative proteomics of the insoluble proteome of CKL2A−/− and CKATG7−/− mice to elucidate the specific contribution of CMA and macroautophagy to neuronal proteostasis (Figure 2A; large functional multiprotein complexes present in the sarkosyl-insoluble fraction of CTR mice were eliminated from the transgenic mice analysis). Approximately half of the proteins in the sarkosyl-insoluble fractions were the same, independently of the autophagic pathway blocked, whereas the rest were distinctive for CKL2A−/− or CKATG7−/− brains (Figure 2B; Table S2). Rank-rank hypergeometric overlap (RRHO) analysis (Plaisier et al., 2010) showed that the most enriched proteins were the less similar (Figures 2C and S4A). We propose that proteins present in both CKL2A−/− and CKATG7−/− aggregates are metastable proteins trapped in the insoluble fraction independently of the cause of proteostasis failure, whereas proteins specific for the autophagic pathway blocked reflect the subproteome selectively regulated by each of them.
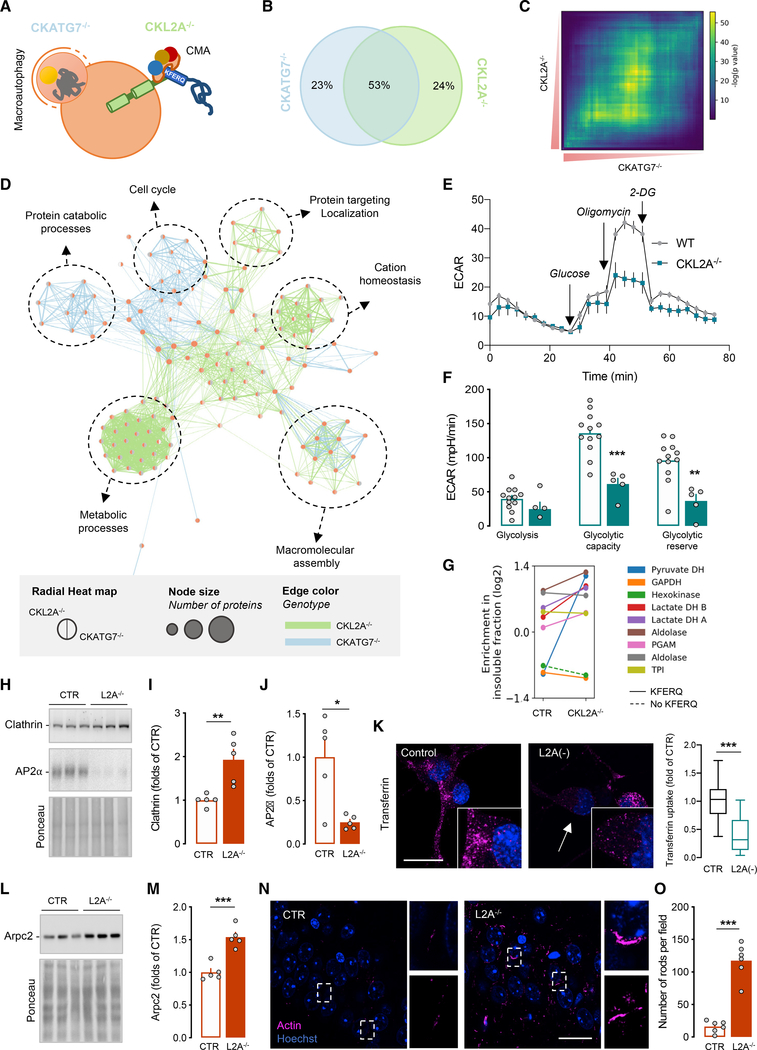
Figure 2. Blockage of CMA and macroautophagy in excitatory neurons leads to collapse of different subsets of the neuronal proteome.
(A) Diagram of the two autophagic pathways blocked in excitatory neurons this study, macroautophagy (blue) and CMA (green).
(B–D) Comparative quantitative proteomics of the insoluble fractions of CKL2A−/− and CKATG7−/− mice brains. Venn diagram of proteins in the insoluble fractions (B), rank-rank hypergeometric overlap (RRHO) plot comparing enriched proteins in the insoluble fractions (C), and network visualization of Gene Ontology enrichment of insoluble proteins (D) (blue edges show similarity between nodes in CKATG7−/−, and green nodes show similarity between nodes in CKL2A−/− mice).
(E and F) Extracellular acidification rates (ECARs) in primary cortical neurons from CTR and CKL2A−/− mice upon addition of the indicated compounds (E) and glycolytic properties calculated from the areas under the curve in ECAR (F).
(G) Shift from soluble to insoluble fraction of individual glycolysis-related enzymes in CTR and L2A−/− mice. Solid and dashed lines indicate presence or absence of KFERQ-like motifs, respectively.
(H–J) Clathrin-mediated endocytosis related proteins in cortex of CTR and L2A−/− mice at 6 months. Representative immunoblots (H) and quantifications (I and J).
(K) Transferrin uptake at 10 min in differentiated neuroblastoma cell lines transduced with empty vector (control) or shL2A construct (L2A(−)). Representative images of transferrin (magenta) and Hoechst (blue) (left). Inset: higher magnification and quantification of transferrin uptake expressed as folds of control (CTR) cells. n = 15–25 cells per condition (right). Scale bar, 20 μm. See also Figure S4P.
(L and M) Arpc2 in cortex of CTR and L2A−/− mice at 6 months. Representative immunoblots (L) and quantification (M).
(N and O) Immunostaining for actin in hippocampal neurons of CTR and L2A−/− mice at 6 months. Representative images with higher magnification illustrating actin-rich rod-like structures (N) and quantification (O).
Scale bars represent 20 μm (K and O). Data represent mean ± SEM, and individual values are shown in (F), (I), (J), (M), and (O). Comparisons were made using an unpaired t test (F, I–K,M, and O). *p < 0.05, **p < 0.005, and ***p < 0.0005. See also Figure S4, Data S1, and Tables S2 and S3.
Gene set enrichment and Enrichment Map analyses (Subramanian et al., 2005; Merico et al., 2010) of proteins in the insoluble fractions of only CKL2A−/− or CKATG7−/− mice brains (Figure 2D) revealed that clusters associated with macroautophagy deficiency were related with cell cycle and ubiquitin-proteasome catabolic processes (Figures 2D and S4B), while those associated with CMA deficiency were related with protein trafficking, cation (mostly calcium) homeostasis, and metabolism (Figures 2D and S4C).
We experimentally confirmed that the presence of these proteins in the insoluble fraction had functional consequences in the CMA-defective neurons. Measurement of the extracellular acidification rate (ECAR) revealed pronounced decrease of glycolysis in CKL2A−/− neurons (Figures 2E and 2F). Although macroautophagy blockage has been shown to also affect neuronal glycolysis (Esteban-Martínez et al., 2017), side-by-side comparison demonstrated that L2A and ATG7 knockdown affected glycolysis properties differently (Figures S4D–S4F). Most glycolytic enzymes, confirmed as bona fide CMA substrates in other cell types and tissues (Aniento et al., 1993; Lv et al., 2011; Schneider et al., 2014), also increased in the insoluble fraction of CKL2A−/− mice, with pyruvate dehydrogenase (PDH), which catalyzes formation of acetyl-coenzyme A (acetyl-CoA) from pyruvate, showing the most dramatic increase (Figures 2G and S4G–S4J). Reduced acetyl-CoA levels due to PDH entrapment in aggregates could explain the alterations in the acetylome of CMA-defective neurons detected by mass spectrometry. The brain proteome of CKL2A−/− mice contains lower number of unique acetylation sites (−11%) and of acetylation events per site (−30%) compared to CTR littermates (Figure S4K; Table S3). As described in other conditions with limited acetyl-CoA availability such as aging (Pietrocola et al., 2015), we also found that a subset (30%) of the CKL2A−/− proteome was hyperacetylated (Figures S4L–S4N). The hypoacetylated proteome was functionally associated with glycolysis-related terms and the hyperacetylated with ion transport (Figure S4O).
To further confirm the functional consequences of the shift toward insolubility of the CMA-deficient neuronal proteome, we analyzed a second protein cluster (“protein targeting and localization”) enriched in proteins related to endocytosis and to actin remodeling (Figure 2D). We found significant accumulation of clathrin and marked reduction of the clathrin adaptor protein AP2α in L2A−/− mice brains (Figures 2H–2J), which are likely responsible for the significant decrease in endocytosis observed in L2A-defficient cells (Figures 2K and S4P). Among the actin remodeling proteins, L2A−/− mice accumulate Arpc2, an essential regulator of actin polymerization (Figures 2L and 2M), which could be responsible for the actin cytoskeleton changes observed in the hippocampus of L2A−/− mice (Figures 2N, 2O, S4Q, and S4R), including the formation of actin-rich rods, reminiscent of Hirano bodies described in AD and other tauopathies (Fulga et al., 2007; Hirano, 1994),
These results demonstrate that defective neuronal function upon CMA blockage is a combination of a direct impact on the solubility of the neuronal proteome (Figure S4S) and indirect effects due to the functional disruption of aggregate-entrapped CMA substrate proteins.
CMA is inhibited in early AD stages
The alterations in proteostasis and neuronal function upon direct neuronal CMA blockage make us propose that reduced CMA activity (as described in aging and in some experimental models of neurodegeneration; Caballero et al., 2018; Cuervo et al., 2004; Mak et al., 2010; Orenstein et al., 2013; Wang et al., 2009; Xilouri et al., 2009), could increase vulnerability and accelerate disease progression in neurodegenerative disorders.
To test this possibility, we measured CMA in a mouse model of tau-mediated proteotoxicity expressing mutant human tau (hTauP301L) under the Thy1.2 promotor (line pR5) (Grueninger et al., 2010; Kins et al., 2001). We crossed hTauP301L mice with a transgenic mouse model that expresses systemically a KFERQ-tagged fluorescent protein Dendra2 (KFERQ-Dendra), recently developed by our group (Dong et al., 2020). This reporter protein, when targeted for degradation via CMA, highlights lysosomes as fluorescent puncta (Koga et al., 2011) that can be counted as read out of CMA activity. Hippocampal neurons of KFERQ-Dendra-hTauP301L mice displayed a significant reduction in the number of fluorescent puncta compared to control KFERQ-Dendra mice (Figures 3A and 3B). CMA inhibition, at least at this stage of the pathology, preferentially occurred in neurons, while astrocytes showed no difference in number of KFERQ-Dendra+ puncta per cell (Figures 3C and 3D).
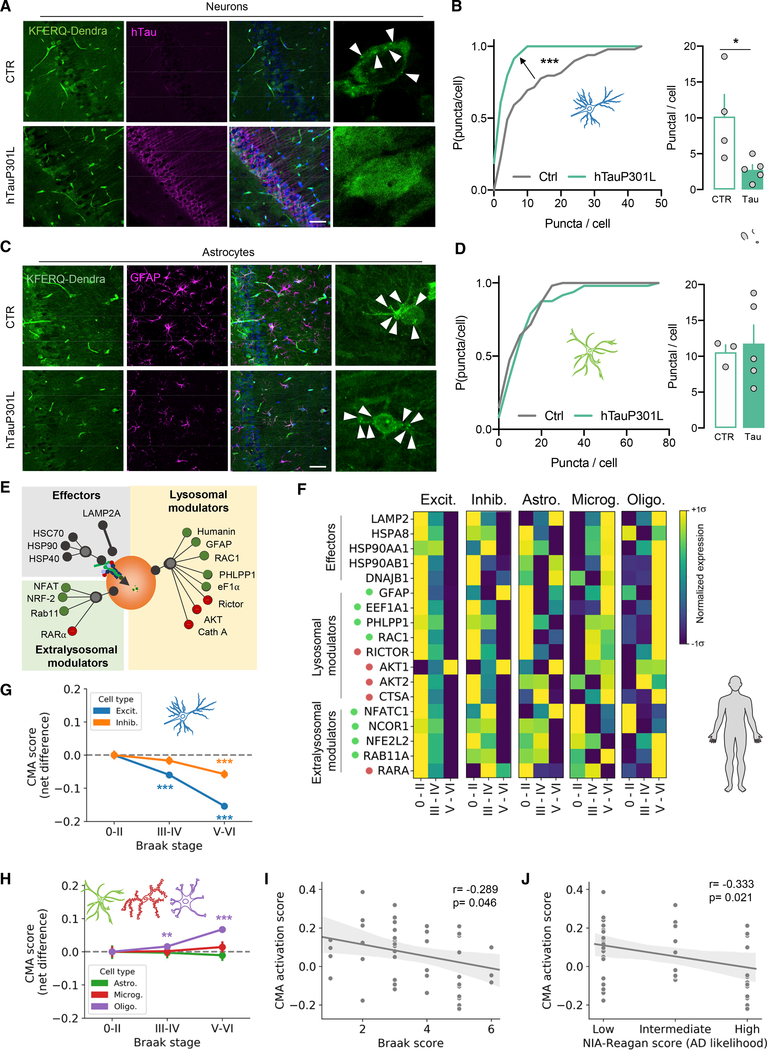
Figure 3. CMA activity is inhibited in a tauopathy mouse model and Alzheimer’s disease patient brains.
(A–D) CMA (measured as the number of fluorescent puncta per cell) in CA1 pyramidal neurons (A and B) and GFAP-positive astrocytes (D and E) in the hippocampus of CTR and hTauP301L-expressing mice (Tau) at 12 months. (A and C) KFERQ-Dendra fluorescence (green), immunostaining for human tau (magenta), and Hoechst staining (blue). Arrowheads: KFERQ-Dendra+ puncta. (B and D) Distribution of the number of KFERQ-Dendra+ puncta per cell in CTR and Tau mice (left) and mean number of puncta per cell per animal (right) (right). Dendra values are from 9–17 (B) or 10–19 (D) individual cell from three to five animals per genotype. Scale bar, 50 μm.
(E) Schematic representation of the CMA network. Proteins involved in CMA are grouped based on function (effectors and modulators) and localization (lysosomal and extra-lysosomal) (modified from Kirchner et al., 2019).
(F) Normalized expression (Z scoring within each cell type) of CMA network components (organized in functional groups and colored dots indicate the effect of a given element on CMA activity; green, positive element; red, negative element).
(G and H) CMA activation score of excitatory (Excit.) and inhibitory (Inhib.) neurons (G) and astrocytes (Astro.), microglia (Microg.), and oligodendrocytes (Oligo.)
(H) in brains with low (Braak stage 0–II), medium (Braak stage III to IV), and advanced (Braak score V to VI) pathology.
(I and J) Negative correlation between CMA activation score in excitatory neurons and pathology markers using Braak pathology staging (I) and NIA-Reagan score (J).
Data represent mean ± SEM in (B), (D), (G), and (H) and individual values in (B), (D), (I), and (J). Comparisons were made using a Kruskal-Wallis test followed by Dunn’s post hoc test (B and D), two-way ANOVA followed by Sidak’s multiple comparisons test (G and H), or Pearson correlation test (I and J). **p < 0.005 and ***p < 0.0005. See also Figure S5.
Insights on the CMA status of the neurodegenerating human brain have been so far limited to analysis of L2A expression in PD blood cells or whole-brain extracts (Alvarez-Erviti et al., 2013; Murphy et al., 2015; Pang et al., 2012). We took advantage of a recently published single-nucleus RNA sequencing (snRNA-seq) dataset from prefrontal cortex of 24 healthy individuals and 24 AD patients grouped according to their Braak score in three categories: low (Braak 0, I, and II), medium (Braak III and IV), and high (Braak V and VI) (Mathys et al., 2019). We then extracted the expression level of every element in the CMA network (Figure 3E) (Kirchner et al., 2019). Although mRNA and protein levels correlate weakly in single bases (Vogel and Marcotte, 2012), considering a group of genes, as a functional network, is a robust approach to infer functional outputs (Freer et al., 2016). Gene expression analysis revealed transcriptional inhibition of almost the entire CMA network in excitatory neurons, whereas in inhibitory neurons, we observed increased expression of two CMA negative regulatory elements, AKT1 and retinoic acid receptor α (RARα) (Figure 3F). In comparison, we only detected modest changes in expression levels of the transcription factor EB (TFEB), a master regulator of macroautophagy and lysosomal biogenesis (Settembre et al., 2011) (Figures S5A and S5B).
To infer the impact of the transcriptional differences on CMA output and compare CMA activity among cell types and pathological stages, we next defined a CMA activation score. This score is a weighted average of the expression level of every element of the CMA network. Higher scores could result from (1) increased expression of effectors or positive modulators or (2) decreased expression of negative modulators, whereas changes in opposite direction will render lower CMA activation scores. We experimentally validated this score in cultured cells exposed to pro-oxidant conditions (known to activate CMA; Kiffin et al., 2004) or a chemical CMA activator (Anguiano et al., 2013) (Figures S5C–S5F).
Using the CMA activation score in the snRNA-seq dataset from the control and AD human brains, we found an inhibition of the CMA network already at early pathology stages in both excitatory and inhibitory neurons, followed by deeper inhibition at later disease stages (Figure 3G). In agreement with our observations in KFERQ-Dendra-hTauP301L mice (Figures 3C and 3D), we observed no changes in astrocytes and microglia and an increase in CMA score in oligodendrocytes, mostly driven by higher expression of CMA effectors and positive modulators (Figures 3H and 3F).
The CMA activation score of excitatory neurons revealed significant negative correlation with different quantitative pathology markers such as the Braak stage (Figure 3I) and the NIA-Reagan score (which combines neurofibrillary tangles and neuritic plaques) (Figure 3J). Inhibitory neurons, despite reduced CMA at late Braak stages, did not show correlation with these neuropathology markers (Figures S5G and S5H). The CMA activation index was reduced in most of the 13 subclusters of excitatory neurons identified in this dataset (Mathys et al., 2019), although in some of them, CMA inhibition was more gradual (Ex4 neurons) or only at a late stage (Ex9 population), and in others, there was no inhibition (Ex8 population) or even CMA activation (Ex6 population), supporting the recently described diversity within neuronal population (Fan et al., 2018) and neuronal-type-specific differences in vulnerability to CMA loss in AD (Figure S5I). When considering the distribution of these neuron subpopulations into cortical layers (Mathys et al., 2019), layers II and III excitatory neurons displayed the highest inhibition of CMA early in the disease (Figure S5J). These results suggest spatial-temporal decrease in CMA activity in the cortex coincident with the development of pathology, especially in excitatory neurons, which are known to be highly vulnerable in tauopathies (Fu et al., 2019).
Altogether, these findings reveal a previously unknown neuron-specific transcriptional downregulation of CMA early in AD, that could add to the direct toxic effect of pathogenic AD proteins on CMA, as reported before (Caballero et al., 2018).
CMA deficiency accelerates pathology in a mouse model of AD
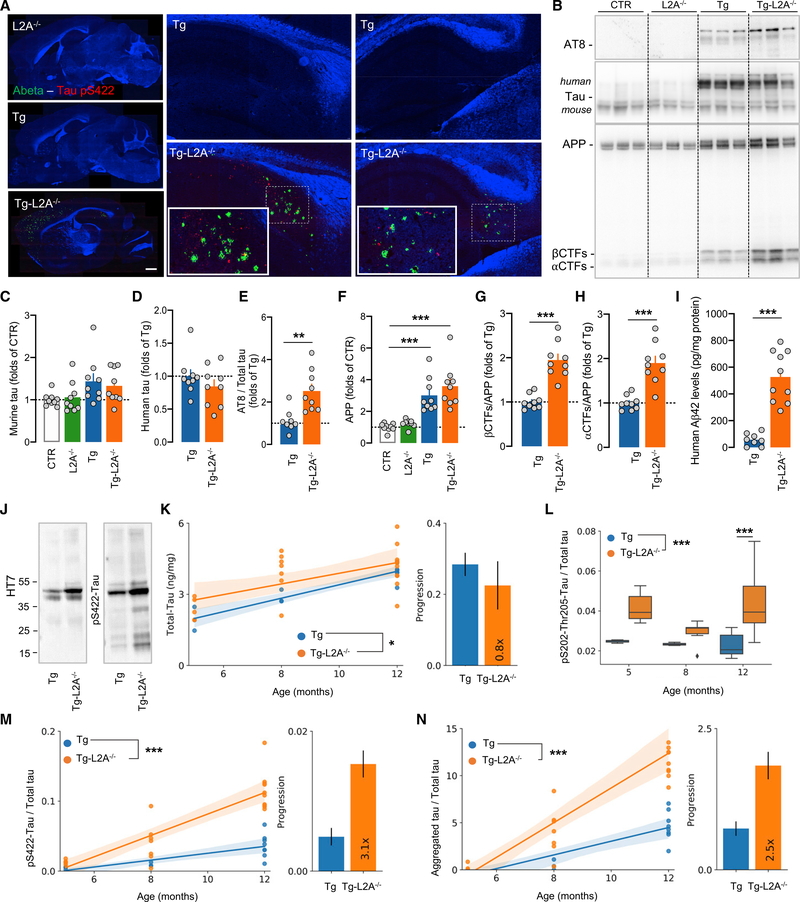
To evaluate the contribution to disease progression of the observed early inhibition of CMA in AD, we imposed CMA loss to a mouse model of AD-related proteotoxicity by breeding triple-transgenic (Tg) AD mice (expressing APPSwe, PS2N141I, and hTauP301L) (Grueninger et al., 2010) with L2A−/− mice (hereafter named Tg-L2A−/−) (Figure S6A). At 8 months, when Tg mice displayed modest Aβ pathology, Tg-L2A−/− mice already showed profound Aβ deposition and accumulation of S422 phosphorylated Tau (pS422-tau) (Figure 4A). Immunoblot analyses revealed that despite comparable levels of murine, human tau (Figures 4B–4D), and K63-linked ubiquitinated proteins (Figure S6B), Tg-L2A−/− mice displayed significantly higher accumulation of phosphorylated tau (Figures 4B and 4E), aggregated tau (HT7 epitope), and pS422-tau in the sarkosyl-insoluble fraction (Figure 4J). CMA loss did not increased accumulation of full-length APP in Tg mice but significantly increased levels of APP C-terminal fragments (CTFs) and of Aβ42 peptide (Figures 4B and 4F–4I).
Figure 4. Loss of CMA in neurons accelerates pathology in a mouse model of AD-related proteotoxicity.
(A) Immunostaining for Aβ (green) and S422-phosphorylated (pS422) tau (red) and Hoechst staining (blue) in the hippocampus of Tg and Tg-L2A−/− mice. Montages of individual images from the scanning of whole-brain slices are shown. Right shows higher magnification images of the dorsal hippocampus. Insets show boxed areas at higher magnification. Scale bar, 1,500 μm.
(B–H) Immunoblots for the indicated proteins in brains of 12-month-old CTR, L2A−/−, Tg, and Tg-L2A−/− mice (B) and densitometric quantifications expressed as fold of CTR (C and F) or fold of Tg (D, E, G, and H) for endogenous murine tau (C), human tau (D), S202/T205-phosphorylated tau (AT8)(E), APP (F), C-terminal fragments (CTFs) of APP (G), and αCTFs (H). n = 9 mice per genotype.
(I) ELISA for Aβ42 of low-speed supernatants of hippocampus from Tg and Tg-L2A−/− mice. n = 8–10 mice per genotype.
(J) Immunoblot for oligomeric tau (HT7, left) and pS422 tau (right) of the low-speed sarkosyl-insoluble fraction of brains from the indicated mouse genotypes.
(K–N) AlphaLISA of low-speed supernatants of hippocampus from Tg and Tg-L2A−/− mice for total tau (K), S202/T205-phosphorylated (pS202-T205) tau (L), S422-phosphorylated (pS422) tau (M), and aggregated tau (N). Left panels show time course and right panels show regression coefficient (±95% confidence interval [CI]). n = 3–10 mice per genotype/time point.
Data represent mean ± SEM (linear regression panels) and individual values (all other panels). Comparisons were made using a Kruskal-Wallis test followed by Dunn’s post hoc test (C and F), unpaired t test (D, E, and G–I), or two-way ANOVA followed by Sidak’s post hoc analysis (J–M). *p < 0.05, **p < 0.005, and ***p < 0.0005. See also Figure S6 and Data S1.
Total tau levels displayed similar progression in Tg and Tg-L2A−/− mice brains at 5, 8, and 12 months of age, while pS202/T205 tau levels were constantly higher in Tg-L2A−/− mice and both pS422 and aggregated tau showed higher levels and faster accumulation rates (2.5- to 3.1-fold) (Figure 4K–4N). These findings suggest that reduced CMA activity, observed early in AD patients and further accentuated with aging, could aggravate brain proteotoxicity and accelerate pathology progression.
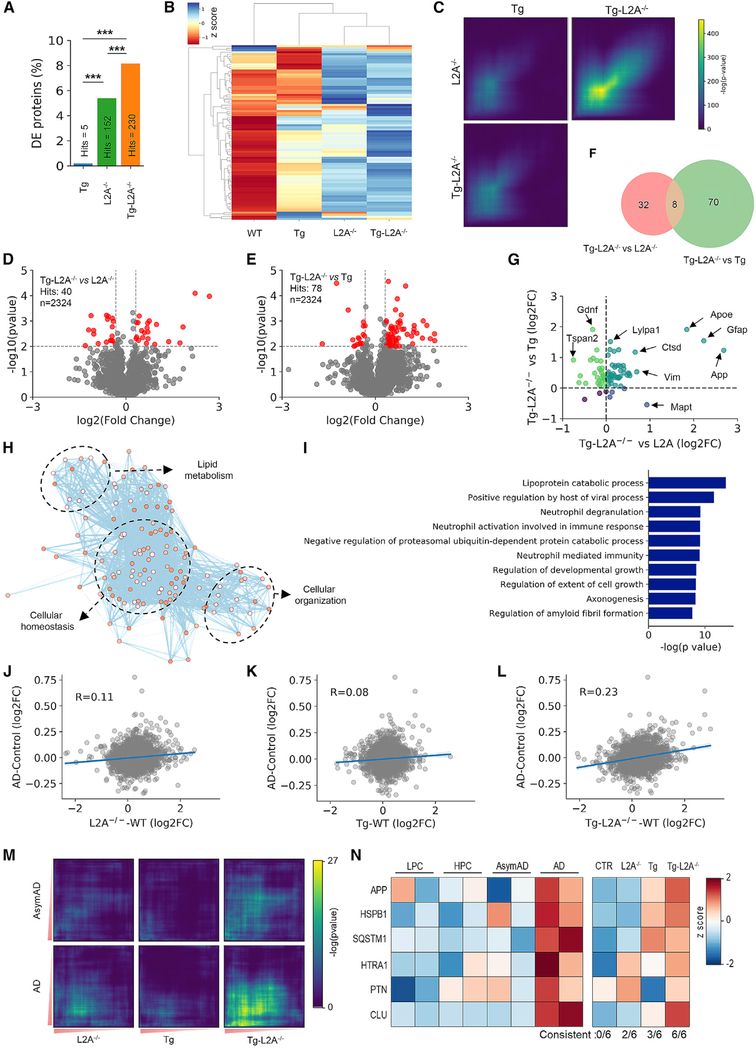
To identify how CMA deficiency aggravates AD, we used comparative quantitative proteomic of brain cortex from CTR, Tg, L2A−/−, and Tg-L2A−/− mice. We found 152 differentially expressed proteins between CTR and L2A−/− mice (Figures 5A and S6C; Table S4) and only five differentially expressed proteins between CTR and Tg mice (Figures 5A and S6D). Interestingly, Tg-L2A−/− mice showed higher number of differentially expressed proteins than Tg and L2A−/− mice combined (Figures 5A and S6E). A hierarchical clustering analysis grouped L2A−/− and Tg-L2A−/− mice together (Figure 5B), and a proteome-wide transition mapping approach (Stein et al., 2014) revealed strong overlap between L2A−/− and Tg-L2A−/− signatures (Figure 5C), both supporting dominance of the CMA deficiency phenotype in Tg-L2A−/− mice.
Figure 5. Neuronal loss of CMA has a synergistic deleterious impact on AD-related brain proteotoxicity.
(A) Number of differentially expressed (DE) proteins between Tg, L2A−/−, and Tg-L2A−/− mice brains compared to CTR. Comparisons were made with two-way ANOVA followed by Sidak’s post hoc analysis. ***p < 0.0005.
(B) Heatmap and hierarchical clustering analysis based on changes in protein abundance between genotypes.
(C) RRHO plots show similarity in enriched proteins between genotypes.
(D and E) Volcano plot of the quantitative proteomic analysis of brain from Tg-L2A−/− compared with L2A−/− (D) or Tg (E) mice brains. Top left: number of significant hits. Red dots indicate DE proteins (p < 0.01 and |fold change| > 1.25).
(F and G) Venn diagram of significantly DE nonoverlapping and overlapping proteins in Tg-L2A−/− compared to L2A−/− and Tg mice (F) and fold and directionality of changes of the eight overlapping proteins (G).
(H) Network visualization of Gene Ontology enrichment analysis of proteins specifically modified in Tg-L2A−/− mice brains.
(I) Gene Ontology analysis of proteins specifically increased in Tg-L2A−/− mice brains.
(J–L) Comparison between AD human cases (Johnson et al., 2020) and L2A−/−, Tg, and Tg-L2A−/− mice proteomes. The human cases were normalized to cognitively control subjects (“AD-Control”), whereas L2A−/−, Tg, and Tg-L2A−/− mice results were normalized to CTR mice. Blue line indicates the linear regression (±95% CI) between human and mouse values.
(M) RRHO plots to show similarity in enriched proteins between both asymptomatic (AsymAD) and symptomatic AD (AD) cases and L2A−/−, Tg, and Tg-L2A−/− mice.
(N) Human Aβ-correlated proteins (from Bai et al., 2020) validated in L2A−/−, Tg, and Tg-L2A−/− mice. Numbers at the bottom indicated the number of consistent changes between AD and mouse proteome. See also Table S4.
Imposing CMA blockage in the context of AD-related brain proteotoxicity resulted in quantitative and qualitative proteome changes not recapitulated by any of the genotypes separately. Direct comparison of Tg-L2A−/− and L2A−/− proteomes (Figure 5D) and Tg-L2A−/− and Tg proteomes (Figure 5E) revealed little overlap in differentially expressed proteins (Figures 5F and 5G). Gene Ontology and network analysis to identify biological processes altered only in Tg-L2A−/− mice (Figure 5H) revealed clusters related with cellular metabolism (including calcium homeostasis), lipid metabolism, and cellular organization (mostly of actin cytoskeleton), which suggest that metabolic and architectural dysfunctions of CKL2A−/− mice could be further altered in the context of AD pathology. Additional Tg-L2A−/− selective changes included proteins associated with lipoprotein catabolism, ubiquitin/proteasome, and regulation of amyloid processing, which contained APP and the well-known AD risk factor apolipoprotein E (Figure 5I).
We reasoned that our mouse model of AD with defective CMA may recapitulate, at least in part, human disease progression, since CMA activity will further decrease in AD patients as they age. Comparison of the proteomic changes in brains of low- and high-pathology controls, asymptomatic, and symptomatic AD patients from the Consensus study (Johnson et al., 2020), with those in our Tg-L2A−/− mice supports this possibility, as we found (1) the highest positive correlation between proteome changes in AD patients and Tg-L2A−/− mice brains (Figures 5J–5L and S6F–S6H), (2) stronger overlap between AD patients and Tg-L2A−/− mice signatures from proteome-wide transition mapping than with Tg or L2A−/− mice (Figure 5M), and (3) higher similarity in changes in levels of regulatory proteins of amyloid fibril (Bai et al., 2020) between AD patients and Tg-L2A−/− mice (Figure 5N).
Reduced CMA may also accelerate the underlying autophagy/lysosomal defect reported in AD brains (Nixon and Yang, 2011). Contrary to Tg and L2A−/− mice, Tg-L2A−/− mice showed accumulation of p62 and GATE-16 (suggestive of reduced autophagic flux; Figure S6I), marked increase in lysosomal hexosaminidase activity, and accumulation and mislocalization of cathepsin D, which are also described in the AD brain (Cataldo and Nixon, 1990; Cataldo et al., 1991; Vidoni et al., 2016) (Figures S6I–S6K; note that cathepsin D levels increase in homogenate, but not in lysosomes). As expected, elimination of L2A alone or in the Tg background did not have a major effect on levels of other CMA components (Figures S6L and S6M).
Overall, these results support the aggravating effect of CMA loss with age on neurodegenerative disease progression due to synergistic, and not merely additive, alterations of the brain proteome.
Chemical activation of CMA improves pathology in complementary AD mouse models
To test if CMA activation could be protective against AD-related neuroproteotoxicity, we performed extensive medicinal chemistry on AR7, one of the generated CMA activators (Anguiano et al., 2013), to make derivatives suitable for in vivo administration (see STAR Methods). The derivative used in this study (CA77.1, hereafter CA) activates CMA in vitro in dose- and time-dependent manner without affecting macroautophagy (Figures S7A–S7D) and when administered in vivo demonstrated brain penetrance with favorable pharmacokinetics (Figures S7E and S7F), activation of CMA in brain of KFERQ-Dendra mice (Dong et al., 2020), and absence of blood or major organs toxicity after 3 months of daily treatment (Figures S7G–S7J).
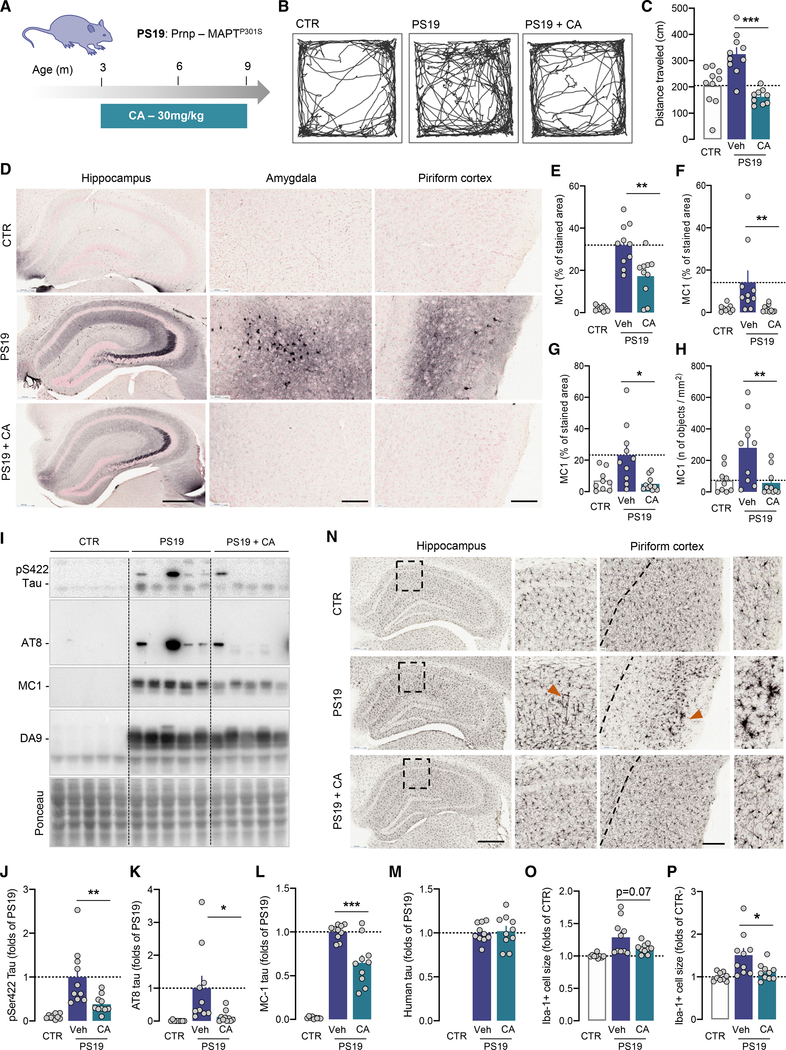
We first administered CA to PS19 mice, which express tau with the frontotemporal dementia mutation P301S (Yoshiyama et al., 2007). We used a clinically relevant administration design (Figure 6A) with oral daily doses of CA (30 mg/kg body weight) for 6 months starting at 3 months of age, when PS19 mice exhibit synaptic loss and tau seeding activity, but before overt tau pathology (Holmes et al., 2014; Yoshiyama et al., 2007). CA administration normalized the previously described locomotor hyperactivity of PS19 mice to control levels (Figures 6B and 6C) and reduced the levels and number of neurons containing pathogenic tau conformations (recognized by the MC1 antibody) in the hippocampus, amygdala, and piriform cortex (Jicha et al., 1997) (Figures 6D–6H). A semiquantitative analysis of hyperphosphorylated tau (S202/T205, AT8 antibody) (Shi et al., 2017) also revealed less aggressive tau pathology in the hippocampus of CA-treated mice, with tau type III pathology observed in 60% of vehicle- and 10% of CA-treated PS19 mice (Figure S7K). Immunoblot analyses confirmed that CA-treated mice showed lower levels of conformationally aberrant, S422 and AT8 phosphorylated, oligomeric, and insoluble forms of tau (Figures 6I–6L and S7L–S7N). CA did not reduce total levels of human tau, indicating improved processing rather than reduction of expressed levels (Figure 6M). The higher number of microglial cells and presence of large Iba1-positive cells with rod-like dystrophic morphology in vehicle-treated PS19 mice (Kaufman et al., 2016) were reduced upon CA treatment (Figures 6N–6P, S7O, and S7P).
Figure 6. Chemical activation of CMA improves behavior and neuropathology in a mouse model of frontotemporal-dementia-related proteotoxicity.
(A) Schematic of regimen of CMA activator (CA) administration to mice overexpressing human P301S tau (PS19).
(B and C) Spontaneous locomotion in an open field of 9-month-old CTR or PS19 mice administered vehicle (Veh) or CA. Shown are representative tracks (B) and total distance traveled in 10 min (C).
(D–H) Immunostaining for MC1 tau in the hippocampus, the amygdala, and the piriform cortex of 9-month-old CTR, PS19 mice ±CA. Representative images of the indicated brain regions (D). Scale bars represent 500 μm (hippocampus) and 200 μm (amygdala and piriform cortex). Quantification of the area stained in the hippocampus (E), piriform cortex (F), and amygdala (G). Quantification of the number of MC1-positive neurons per mm2 in the amygdala (H).
(I–M) Immunoblots for tau and phosphorylated tau. Representative immunoblots and densitometric quantifications for the indicated proteins: S422-phosphorylated tau (J), S202/T205-phosphorylated tau (AT8) (K), MC1 tau (L), and total tau (M).
(N–P) Immunostaining for Iba1 (N) and quantification of average cell size in the hippocampus (O) and the piriform cortex (P). Scale bars represent 500 μm (hippocampus) and 200 μm (piriform cortex).
Data are mean ± SEM. Individual values are shown for n = 9–10 mice per genotype and treatment (C, E–H, and J–P). Comparisons were made using one-way ANOVA followed by Tukey’s post hoc analysis (C, E, G, H, J, and L–P) or Kruskal-Wallis test followed by Dunn’s post hoc test (F and K). *p < 0.05, **p < 0.005, and ***p < 0.0005. See also Figure S7 and Data S1.
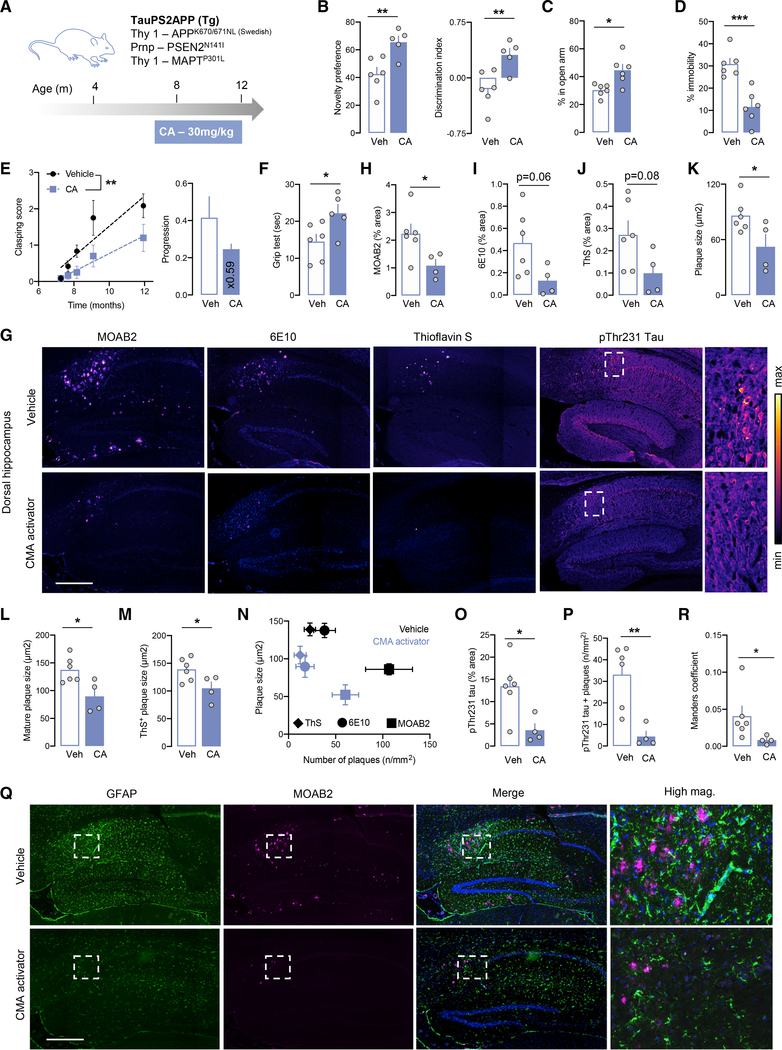
Next, we used the triple-transgenic mouse model of AD (Tg) (Grueninger et al., 2010) to explore the effect of pharmacological activation of CMA on combined tau and Aβ proteotoxicity. Tg mice were given daily oral doses of CA (30 mg/kg body weight) for 4 months starting at 8 months of age (after symptom onset and when β-amyloid plaques are already detectable; Grueninger et al., 2010). CA-treated Tg mice had better visual memory, decreased anxiety- and depression-like behaviors, slower clasping progression and increased performance in horizontal grid test than those receiving the vehicle (Figures 7A–7F). Histopathology revealed significantly reduced β-amyloid and tau-related pathologies in the hippocampus and cortex of CA-treated mice, as evidenced by the reduced area and size of immature (MOAB2-positive) and mature (6E10- and thioflavin-S-positive) amyloid plaques (Figures 7G–7M and S8A–S8D), lower number of deposits depending on the deposit maturity (Figure 7N), and reduced levels of the early T231 phosphorylated Tau (Figures 7K, 7O, S8E, and S8F), especially in 6E10-positive areas, in support of lower number of neurons bearing pathology (Figure 7P). Immunoblot analysis demonstrated significant reduction of the mature phosphosite AT8 in CA-treated Tg mice (Figure S8G). We also found a lower number of microglia and astrocytes in the dorsal hippocampus and less association of glial cells with amyloid-plaque-like deposits (Figures 7Q, 7R, and S8H–S8K), revealing reduced gliosis in the CA-treated group.
Figure 7. Chemical activation of CMA improves behavior and neuropathology in a mouse model of AD-related proteotoxicity.
(A) Schematic of regimen of CMA activator (CA) administration to TauPS2APP (Tg) mice.
(B–D) Performance of Tg mice administered vehicle (Veh) or CMA activator (CA) in the novel object recognition test (B) (left plot: percentage of time dedicated to the novel object; right plot: discrimination index), the elevated plus maze (C), and the forced swim test (D).
(E) Clasping score in Tg mice ±CA: time course of clasping score increase (left) and mean linear regression coefficient (right).
(F) Performance of Tg mice ±CA in horizontal grid test.
(G–P) Immunostaining of immature amyloid plaques (MOAB2), mature amyloid depositions (6E10), β sheet marker (thioflavin S), and threonine-231-phosphorylated tau (pThr231 tau) in the dorsal hippocampus of Tg mice ±CA. (G) Representative images. Montages of individual images from the scanning of whole-brain slices are shown. Inset: higher magnification of pThr231 tau staining. Scale bar, 200 μm. Quantification of the percentage of area positive and plaque size for MOAB2 (H and K), 6E10 (I and L), or thioflavin S (J and M). Relationship between number of plaques and average plaque size for different markers of plaque maturity (ThS > 6E10 > MOAB2) (N). Quantification of percentage of area positive for pThr231 tau (O) and the overlap between pThr231 tau staining and 6E10 staining (P).
(Q and R) Immunostaining of astrocytes (GFAP, green), amyloid pathology (MOAB2, magenta), and nuclei (Hoechst, blue) in the dorsal hippocampus of Tg mice ±CA (Q) and colocalization coefficient (R). Montages of individual images from the scanning of whole-brain slices are shown. Inset: higher magnification of the boxed area. Scale bar, 200 μm (Q).
All quantifications (except clasping; E) were done at 12 months in four to six mice per group. Data represent mean ± SEM. Comparisons were made using unpaired t test (B–D, F, and H–P), Mann-Whitney U test (Q), or two-way ANOVA test followed by Sidak’s post hoc analysis (E). *p < 0.05, **p < 0.005, and ***p < 0.0005. See also Figure S8.
Altogether, our results highlight that pharmacological CMA activation using a clinically relevant design has a beneficial effect on AD-related pathology.
DISCUSSION
We have identified in this study a direct contribution of CMA to the maintenance of the neuronal proteome in vivo in physiological and pathological conditions and provide evidence of the potential value of chemical enhancement of CMA activity as therapeutic strategy for age-related neurodegenerative disorders.
The loss of proteostasis upon CMA blockage noted in neurons did not occur when CMA was disrupted in other cell types and tissues (Massey et al., 2006; Schneider et al., 2014; Valdor et al., 2014). A notable difference is that the compensatory activation of macroautophagy observed in other cells upon CMA blockage did not occur in neurons. This absence of compensation highlights the importance of intact CMA function in maintenance and fine-tuning of part of the neuronal proteome. The solubility shift of the proteome upon CMA inhibition, only observed until now in neurons, can also explain the functional differences between CMA-deficient neurons and peripheral cells. For example, while the blockage of CMA in hepatocytes leads to the accumulation of soluble fully functional glycolytic enzymes and increased glycolysis (Schneider et al., 2014), the entrapment of glycolytic enzymes in protein aggregates in CMA-defective neurons accounts for their lower glycolytic activity.
While macroautophagy can directly sequester and degrade protein inclusions (Hara et al., 2006; Komatsu et al., 2006), only soluble proteins can be degraded by CMA (Salvador et al., 2000). Consequently, accumulation of protein aggregates in CMA-defective neurons occurs by inability to timely remove soluble proteins that, as they accumulate, promote the observed shift toward aggregation of the CMA-regulated proteome. This tight relation between CMA and the recently proposed supersaturated proteome (Ciryam et al., 2013; Kundra et al., 2017) identifies that putative CMA substrates are at a higher risk of misfolding than the general proteome and places CMA in a central position for the maintenance of this high-risk proteome.
In clear contrast with the overt neurodegeneration observed upon acute viral-mediated silencing of L2A in rat dopaminergic neurons (Xilouri et al., 2016), the more gradual but sustained neuronal CMA blockage of our models induces proteome alterations in the absence of actual neurodegeneration (suggestive of early pre-degenerative stages) and makes them ideal models to study early disease events and disease progression. In fact, we show here that inhibition of the neuronal CMA network is an early event in AD patient brains, and that neuron subclusters with earlier and more marked CMA failure (Ex4) are those previously shown to have high tau pathology (Mathys et al., 2019), further strengthening the potential value of CMA enhancement in these conditions.
Activation of CMA in a clinically relevant design and with compounds that could be developed into drugs, has been possible in part by the brain penetrance and mechanism of action of the CA molecules (Anguiano et al., 2013), which preserve most of the RARα-dependent signaling known to be neuroprotective (Colas et al., 2020). Even at a symptomatic stage, activation of CMA led to strikingly reduced β-amyloid and tau pathologies as well as glial activation. Changes in β-amyloid pathology were unexpected, as APP, as a transmembrane protein, is not anticipated to undergo direct degradation via CMA. Although improvement of the b-amyloid pathology could originate from the positive effect of CMA restoration on the functioning of other proteostasis systems (Zhang and Cuervo, 2008), the presence of a KFERQ-like motif (763KFFEQ768) in the cytosolic region of APP could make CMA clearance of APP cytosolic fragments possible. The observed reduction in number and size of mature amyloid depositions (6E10 and thioflavin S positive) upon administration of the CMA activator is strongly suggestive that this intervention could also have a beneficial effect on preexistent pathology.
Limitations of the study
We confirmed reduced L2A brain levels in our CKL2A−/− mice by biochemical procedures and showed that proteotoxicity was mostly limited to neurons, but we could not produce direct evidence of L2A depletion only in excitatory neurons, because the only available antibody against mouse L2A also recognizes a second nonselective protein in brain. Although expression of Cre in the Cre-driver transgenic mouse used in this study has shown to be restricted to excitatory neurons (Alberi et al., 2011; Choi et al., 2009; Feng et al., 2001; Wang et al., 2013), isolation of pure excitatory neurons (enough for immunoblotting) will be necessary for direct confirmation of L2AKO selectivity. Although we demonstrate that L2A deletion leads to specific proteostasis impairments in neurons and not astrocytes, it is still possible that our proteomic studies capture changes in other cell types. Development of single-cell proteomics (Marx, 2019) may assist in the future to directly study cell-type-specific proteostasis impairments.
STAR★METHODS
RESOURCE AVAILABILITY
Lead contact
Further information and requests for reagents may be directed to and will be fulfilled by the lead author Ana Maria Cuervo (ana-maria.cuervo@einsteinmed.org).
Materials availability
All reagents generated in this study are available from the Lead Contact with a completed Material Transfer Agreement. Because our synthesis capabilities for CA are limited, request for this compound will be honored in reasonable amounts and depending on availability at the time of request.
Data and code availability
There are not restrictions on data availability in this manuscript. All the information is included in the manuscript. All Main and Supplementary Figures have associated raw data that is provided as an Excel worksheet organized by figures. Proteomic data is included as Data S1. Raw data files and code is available from the authors upon request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Animal models
Male wild-type mice (C57BL/6J) or transgenic for CaMKIIα-Cre (Alberi et al., 2011; Choi et al., 2009; Feng et al., 2001; Wang et al., 2013) (B6.Cg-Tg(Camk2a-cre)T29–1Stl/J, Jackson Laboratory), LAMP-2Aflox/flox (Schneider et al., 2014), TauP301L (line pR5) (Kins et al., 2001), Tg (APPSwe; PS2N141I; TauP301L) (Grueninger et al., 2010), PS19 (TauP301S) (Yoshiyama et al., 2007) and Atg7f/f (Komatsu et al., 2006) were used in the study. Conditional LAMP-2A deletion was generated as previously described (Schneider et al., 2014) by breeding LAMP-2Aflox/flox with the transgenic Cre mice of interest. Knockout for L2A in the whole body (L2A−/−) was generated by insemination of a wild-type female with spermatozoids with L2A floxed to excise this gene in all tissues in the offspring. Male littermate wild-type and only L2Af/f were separately analyzed for each test and because no differences were detected among them, they were grouped in the results as “control” (CTR) for the experimental group (CamKIIαCreL2Af/f or L2A−/−). All mice were genotyped at weaning and genotyping was re-confirmed postmortem to correct for any possible misplacement during husbandry. Mice were all in the C57BL/6J background and maintained under specific pathogen-free conditions in ventilated cages with no more than 5 mice per cage. Only males were used in this study due to the complexity in generating the quadruple transgenic mouse model with L2A knock out in homozygosis (for which we took advantage of the location of the Lamp2 gene in the X chromosome). Age of the animals was 4–6 months in most experiments except when otherwise indicated in text, figures and figure legends. Animals were maintained at 19–23°C in 12h light/dark cycle. Mice were fed ad libitum. CA77.1 was administered as sucralose jelly pellets for a daily dose of 30mg/Kg body weight whereas the vehicle treated group received the same sucralose jelly pellet without drug. Briefly, for preparation of the jelly pellets the final amount of the compound per day (adjusted per animal weight) was dissolved in ethanol and then mixed with a warm gelatin solution (100mg/ml, 10mg/ml sucralose in water), that was poured into 24 well flat bottom plates for solidification. To avoid animal stress and competition for the pellets, animals were separated with a grid spacer in the same cage that they were housed, eating of the pellet was monitored and the spacer was removed as soon as all mice consumed the pellet (average time 2min). Sentinel animals were included in each study to determine brain exposure at the end of treatment. These animals received the same batch of jelly pellets in parallel to the experimental group. Animals were assigned randomly to the vehicle and placebo groups and no animals were eliminated from the study. All genotyping, breeding, handling and treatments in this study were done according to protocol and all animal studies were under an animal study protocol approved by the Institutional Animal Care and Use Committee of Albert Einstein College of Medicine.
Cortical primary cultures
Cortical neurons were obtained from control (CTR) and L2AKO P0-P1 postnatal mice and neuronal cultures were prepared as follows: brain cortices were dissected and enzymatically digested (0.36 mg/ml papain in phosphate buffered saline (PBS) with D-glucose (6 mg/ml) and 1% bovine serum albumin for 15 minutes 37°C). Neurons collected by centrifugation, were resuspended in Neurobasal Medium (ThermoFisher 10888022), supplemented with 2% B27-Supplement (GIBCO-Invitrogen, 17504044), 1% Penicillin/Streptomycin and 1% GlutaMAX (Fisher, 35050–061) and plated at a density of 2.5 × 105 cells/cm2 into 24-well Seahorse Bioscience plates (Agilent, 100777–004) pre-coated with CELL-TAK (CORNING, 354240) or in coverslips. The first 24 h the media contained fetal calf serum 15% (v/v) heat-inactivated. Cells in coverslips were co-stained with NeuN, GFAP and Hoechst to assess level of glial presence in the primary neuronal cultures.
Cell lines
Mouse embryonic NIH 3T3 fibroblasts from the American Type Culture Collection (ATCC) and mouse neuroblastoma CAD cell lines (gift from Dr. Duncan Wilson, Albert Einstein College of Medicine) were maintained in DMEM (Sigma-Aldrich) in the presence of 10% newborn calf serum (NCS) (Atlanta Biologicals). CAD cells were differentiated by serum removal and used at 5 days post-starvation. Lentivirus expressing the shRNA constructs against LAMP-2A and Atg7 were generated by the same protocol using the shRNA previously described (Caballero et al., 2018; Massey et al., 2006).
METHOD DETAILS
Reagents
Source of chemicals are indicated in the Key resources table.
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Goat anti Mouse Alexa Fluor 488 | ThermoFisher Scientific | Cat# A-10680, RRID: AB_2534062 |
| Goat anti mouse Alexa Fluor 555 | ThermoFisher Scientific | Cat# A32727; RRID: AB_2633276 |
| Goat anti Rabbit Alexa Fluor 488 | ThermoFisher Scientific | Cat# A-11008, RRID: AB_143165 |
| Goat anti Rabbit Alexa Fluor 555 | ThermoFisher Scientific | Cat# A-21429, RRID: AB_2535850 |
| Goat anti-GFAP | Abcam | Cat# ab53554; RRID:AB_880202 |
| Goat anti-Mouse HRP | KPL | Cat# 5067205 |
| Goat anti-Rabbit HRP | KPL | Cat# 5067202 |
| Mouse anti-β-actin | Sigma | Cat# A4700; RRID: AB_476730 |
| Mouse anti-α-synuclein | BD Biosciences | Cat# 610787; RRID: AB_398108 |
| Mouse anti-Aβ | Novus Biologicals | Cat# NB2-50055AF488 |
| Mouse anti-Aβ [Clone 6E10] | Biolegend | Cat#803003; RRID:AB_2564652 |
| Mouse anti-Aβ [Clone MOAB2] | Abcam | Cat# ab126649 |
| Mouse anti-humanTau [Clone Tau13] | Abcam | Cat# ab19030; RRID: AB_778252 |
| Mouse anti-phosphoS422-Tau | Custom generated antibody (Grueninger et al., 2010) | N/A |
| Mouse anti-phosphoSer202-Thr205-Tau [Clone AT8] | Thermo Fisher Scientific | Cat# MN1020; RRID: AB_223647 |
| Mouse anti misconformed tau [Clone MC1] | Custom generated antibody (P. Davies) | RRID: AB_2314773 |
| Mouse anti-Tau [Clone HT7] | Thermo Fisher Scientific | Cat# MN1000; RRID: AB_2314654 |
| Rabbit anti-4HNE | Abcam | Cat# ab46545; RRID: AB_722490 |
| Rabbit anti-GATE16 | MBL | Cat# PM038; RRID: AB_10210502 |
| Rabbit anti-GFAP | Dako | Cat# Z0334; RRID: AB_10013382 |
| Rabbit anti-GFAP | Abcam | Cat# ab5804; RRID: AB_2109645 |
| Rabbit anti-Iba1 | Wako | Cat#019-19741; RRID:AB_839504 |
| Rabbit anti-K48ubiquitin | Millipore | Cat# 05-1307; RRID: AB_1587578 |
| Rabbit anti-K63ubiquitin | Millipore | Cat# 05-1308; RRID: AB_1587580 |
| Rabbit anti-LAMP-2A | ThermoFisher Scientific | Cat# 512200; RRID: AB_2533900 |
| Rabbit anti-LC3 | Cell Signaling | Cat# 2775; RRID: AB_915950 |
| Rabbit anti-LRRK2 | Abcam | Cat# ab133518 |
| Rabbit anti-p62 | EnzoLife Sciences | Cat# BMLPW98600100; RRID: AB_2052149 |
| Rabbit anti-Parkin | Millipore | Cat# ab9244; RRID: AB_570711 |
| Rabbit anti-phosphoT231-Tau [Clone EPR2488] | Abcam | Cat#ab151559 |
| Rabbit anti-Tyrosine Hydroxylase | Abcam | Cat# Ab112; RRID: AB_297840 |
| Rabbit anti-ubiquitin | Dako | Cat# 0458; RRID: AB_2315524 |
| Rabbit anti-UCHL1 | Enzo | Cat# PG9500; RRID: AB_11181886 |
| Rat anti-Dopamine Transporter | Millipore | Cat# MAB369; RRID: AB_2190413 |
| Rat anti-LAMP1 | Hybridoma Bank | Cat# 1D4B; RRID: AB_2134500 |
| Rabbit anti-Clathrin [Clone D3C6] | Cell Signaling | Cat# 4796, RRID:AB_10828486 |
| Mouse anti-AP2α [Clone 3B5] | ThermoFisher Scientific | Cat# MA1-872, RRID:AB_2199412 |
| Rabbit anti-Arpc2 | Novus Biological | Cat# NBP1-88852, RRID:AB_11040464 |
| Mouse anti-APP | Biolegend | Cat# 802803, RRID:AB_2715853 |
| Mouse anti-PDH | ThermoFisher Scientific | Cat# 459400, RRID:AB_2532238 |
| Rabbit anti-Cathepsin D | Abcam | Cat# ab75852, RRID:AB_1523267 |
| Mouse anti-Hsc70 | Novus Biological | Cat# NB120-2788, RRID:AB_2120309 |
| Rat anti-Hsp90 | Stressgen / Enzo Life sciences | Cat# ADI-SPA-835-F, RRID:AB_11181205 |
| Rabbit anti-Hsp40 | Stressgen / Enzo Life sciences | Cat# ADI-SPA-400, RRID:AB_10631418 |
| Mouse anti-Rac1 | Millipore | Cat# 05-389, RRID:AB_309712 |
| Rabbit anti-Phlipp1 | Bethyl | Cat# A300-660A, RRID:AB_2283757 |
| Rabbit anti-Rictor | Bethyl | Cat# A300-459A, RRID:AB_2179967 |
| Rabbit anti-CathepsinA | Abcam | Cat#ab184553 |
| Rabbit anti-RARα | Cell Signaling | Cat# 2554, RRID:AB_2253585 |
| Mouse anti-CaMKIIα | Millipore | Cat# 05-532, RRID:AB_309787 |
| Chemicals, peptides, and recombinant proteins | ||
| Ammonium Chloride | Sigma-Aldrich | Cat #A9434 |
| B27 Supplement | GIBCO-Invitrogen | Cat# 17504044 |
| DMEM | Sigma-Aldrich | Cat# D5648 |
| Formaldehyde solution, 37wt | Sigma-Aldrich (Merck) | Cat#252549 |
| GlutaMAX | ThermoFisher Scientific | Cat# 35050-061 |
| Hoechst 33342 | ThermoFisher Scientific | Cat# H3570 |
| Leupeptin | Sigma-Aldrich | Cat# L5793 |
| Methanol | Sigma-Aldrich (Merck) | Cat#322415 |
| Neurobasal medium | ThermoFisher Scientific | Cat# 10888022 |
| Newborn Calf Serum | Atlanta Biologicals | Cat# S11250 |
| Oxyblot Protein Oxidation Detection Kit | Merck-Millipore | Cat# S7150 |
| Papain | Sigma-Aldrich | Cat# P3125 |
| Pierce ECL Western Blotting Substrate | Thermo Fisher Scientific | Cat#32106 |
| Prolong Diamond Antifade Mountant | ThermoFisher Scientific | Cat# P36961 |
| Sarkosyl | Sigma-Aldrich | Cat# L9150 |
| ThioflavineS | Santa Cruz | Cat#sc391005 |
| CyQuant | ThermoFisher Scientific | Cat#C7026 |
| Alexa555-conjugated Transferrin | Life Technologies | Cat# T35352 |
| Amyloid beta 42 Human ELISA | ThermoFisher Scientific | Cat # KHB3442 |
| Experimental models: cell lines | ||
| NIH 3T3 | ATCC | Cat# CRL-1658 |
| CAD | ECACC | Cat# 08100805, RRID:CVCL_0199 |
| Experimental models: organisms/strains | ||
| Mouse: B6.Cg-Tg(Camk2a-cre)T29-1Stl/J | The Jackson Laboratory | IMSR Cat# JAX:005359, RRID:IMSR_JAX:005359 |
| Mouse: B6.Cg-7630403G23RikTg(Th-cre)1Tmd/J | The Jackson Laboratory, Savitt et al., 2005 | IMSR Cat# JAX:008601, RRID:IMSR_JAX:008601 |
| Mouse: LAMP-2Aflox/flox | Schneider et al., 2014 | N/A |
| Mouse: Atg7flox/flox | Komatsu et al., 2006 | N/A |
| Mouse: Tg (APPSwe; PS2N141I; TauP301L) | Grueninger et al., 2010 | N/A |
| Mouse: TauP301L (line pR5) | Kins et al., 2001 | N/A |
| Mouse; TauP301S (line PS19) | Yoshiyama et al., 2007 | Cat# JAX:008169, RRID:IMSR_JAX:008169 |
| Software and algorithms | ||
| Adobe Photoshop 6.0 | Adobe Systems | https://www.adobe.com/ RRID:SCR_014199 |
| AxioVision Rel. 4.8 | Carl Zeiss | https://www.zeiss.com/ RRID:SCR_002677 |
| Cytoscape 3.7.1 | Cytoscape, RRID: SCR_003032 | |
| Enrichr | Chen et al., 2013; Kuleshov et al., 2016 | Enrichr, RRID:SCR_001575 |
| Fiji | NIH | https://fiji.sc/ RRID:SCR_002285 |
| Gene Set Enrichment Analysis | Subramanian et al., 2005 | Gene Set Enrichment Analysis, RRID:SCR_003199 |
| GraphPad Prism 8.0 | GraphPad Software | https://www.graphpad.com/scientific-software/prism/ RRID:SCR_002798 |
| KFERQ-finder | Kirchner et al., 2019 https://doi.org/10.1371/journal.pbio.3000301 | http://ec2-18-188-198-152.us-east-2.compute.amazonaws.com:3838/kferq/ |
| LAS AF Lite | Leica | https://www.leica-microsystems.com/ RRID:SCR_013673 |
| Matplotlib 3.1.1 | Hunter et al., 2007 | MatPlotLib, RRID: SCR_008624 |
| Numpy 1.17.2 | van der Walt et al., 2011 | NumPy, RRID: SCR_008633 |
| Python 3.7.4 | Python Software Foundation | Python Programming Language, RRID: SCR_008394 |
| SciPy 1.3.1 | Jones et al., 2001 | SciPy, RRID: SCR_008058 |
| ezTrack | Pennigton et al., 2019 | N/A |
| Seahorse Wase Desktop Software | Agilent Techonologies | Seahorse Wave, RRID:SCR_014526 |
| Other | ||
| Axiovert 200 with ApoTome 0.2 system fluorescence microscope | Carl Zeiss, Albert Einstein College of Medicine | https://www.zeiss.com/ |
| Fujifilm LAS-3000 Imager | Fujifilm, Albert Einstein College of Medicine | https://www.fujifilm.com/products/medical/ |
| Operetta system | Perkin Elmer, Albert Einstein College of Medicine | https://www.perkinelmer.com:443/product/operetta-cls-system-hh16000000 |
| Seahorse Biosciences Extracellular Flux Analyzer XFe24 | Agilent, Albert Einstein College of Medicine | https://www.agilent.com |
| SP8-LIA confocal microscope | Leica Microsystems, Albert Einstein College of Medicine | https://www.leica-microsystems.com/ |
Antibodies
Primary antibodies were from the following sources (dilutions, commercial source and catalog number indicated in brackets): rabbit anti-LAMP-2A (1:5000, ThermoFisher Scientific, 512200), rabbit anti-LC3 (1:1000, Cell Signaling, 2775), rabbit anti-p62 (1:1000, Enzo Life Sciences, BMLPW98600100), mouse anti-β-actin (1:10000, Sigma, A4700), rat anti-LAMP1 (1:1000, Hybridoma Bank, 1D4B), rabbit anti-4HNE (1:1000, abcam, ab46545), rabbit anti-GFAP (1:1000, Dako, Z0334), rabbit anti-GFAP (1:1000, abcam, ab5804), rabbit anti-ubiquitin (1:1000, Dako, Z0458), rabbit anti-K48-ubiquitin (1:1000, Millipore, 05–1307), rabbit anti-K63-ubiquitin (1:1000, Millipore, 05–1308), mouse anti-Aβ (1:1000, Novus Biologicals, MOAB2-AF488), mouse anti-human-Tau (1:1000, Abcam, Tau13 ab19030), mouse anti-Tau (HT7 clone) (1:1000, ThermoFisher Scientific, MN1000), mouse anti-phosphoTau Ser202-Thr205 (AT8 clone) (1:1000, ThermoFisher Scientific, MN1020), mouse anti-phosphoTau Ser422 (1:1000, generated in house using the phosphopeptide CSIDMVD-pS-PQLATLAD as antigen (Grueninger et al., 2010)), rabbit anti-GATE16 (1:1000, MBL, PM038), mouse anti-misconformed tau (MC1) (1:1000, a generous gift from Dr. Peter Davis), rabbit anti-Clathrin (Clone D3C6 – 1:1000 – Cell Signaling 4796S), mouse anti-AP2α (Clone 3B5 – 1:1000 - ThermoFisher Scientific, MA1–872), rabbit anti-Arpc2 (1:1000 – Novus Biological NBP188852), mouse anti-APP (1:1000 – Biolegend 802803), mouse anti-PDH (1:1000 - ThermoFisher Scientific, 459400), rabbit anti-CathepsinD (CathD – 1:1000 – Abcam #ab75852), rabbit anti-GBA (1:1000 – Sigma Aldrich, G4171), mouse anti-Hsc70 (1:1000 – Novus Biological NB120–2788), rat anti-Hsp90 (1:1000 – Stressgen ADI-SPA-835-F), rabbit anti-Hsp40 (1:1000 – Stressgen ADI-SPA-400), mouse anti-Rac1 (Clone23A8 – 1:1000 – Millipore 05–389), rabbit anti-Phlpp1 (1:1000 – Bethyl A300–660A), anti-Rictor (1:1000 – Bethyl A300–459A), anti-CathepsinA (Ctsa – 1:1000 – Abcam #ab184553), rabbit anti-RARα (1:1000 – Cell Signaling 2554), mouse anti-CaMKIIα (1:200 – Millipore #05–532). All the secondary antibodies were from ThermoFisher Scientific. All antibodies used in this study were from commercial sources and were validated following the multiple dilution method and, where available, using cell lines or tissues from animals knock-out for the antigen.
Behavioral procedures
All behavioral procedures were performed by investigators blind to genotype of each group or nature of intervention. To decrease stress related to procedures, all animals were first habituated to handling by the experimenter and to the procedure room for at least 1hour prior to testing. Limb Clasping. Clasping was assessed for 5 s and scored as previously described (Miedel et al., 2017). Spontaneous alternation in a Y-maze. Mice were allowed to freely explore the maze for 10 min. Number and order of arm entries were quantified. Alternation index was calculated as previously described (Miedel et al., 2017). Stride length. Stride length was measured as previously described (Fernagut et al., 2002) in a 4.5cm x 40cm corridor following inking of hindlimb paws. The three longest stride lengths (corresponding to maximum velocity) were considered. Short-term memory test in Y-maze. Test was performed in a Y-shaped maze with three arms angled at 120°. Visual cues were placed on surrounding walls. On the first trial (learning), animals explored the maze for 8 minutes with only two arms opened (‘start’ and ‘familiar’ arms). Access to the third arm (‘novel’) was blocked by an opaque door. After a 1hour retention time, mice were placed again in the maze for 5 minutes with all arms accessible (test). Exploration was recorded and an automated tracking system was used (Pennington et al., 2019). Data are reported as fraction of time spent in the novel arm. Negative geotaxis. Mice are placed on the sloped platform (50°) facing in a downward direction. The latency to turn and orient themselves to be facing up the slope was recorded. Novel object recognition. Novel object recognition was performed as before (Leger et al., 2013) after training mice in an open field arena with identical objects for 4 minutes, followed by 2 hours retention time. Mice were placed in the same arena after replacing one of the familiar objects by a novel object and exploration of both objects was quantified for 4 minutes. Novelty preference is quantified as amount of time dedicated to the exploration of the novel object. Discrimination index is the difference between the exploration time of the novel and familiar object over the total exploration time. Elevated plus maze. Anxiety-like behavior was quantified as previously described (Walf and Frye, 2007). Briefly, mice were allowed to freely explore an elevated plus maze with two open arms and two closed arms. Quantification of the % of time spent to explore the open arms versus the closed arms was done. Forced Swim test. Mice were placed in cylinder tank (30cm x 20cm) filled with water at room temperature. Animals were gently placed in water and immobility was quantified over a total time of 9 minutes. Open field. Mice were allowed to freely move in an open field arena (50×50cm) for 10 min. Tracking was performed using ezTrack (Pennington et al., 2019). The number of animals selected for each behavior test was determined by power analysis. In those cases, in which test allowed for repetition without risking a co-funding effect of “learning the test” or where multiple tests in the same animal were possible, we performed testing in higher number of animals than the minimal determined by power analysis in order to further strengthen confidence in the findings.
Tissue dissection and histological procedures
Mice were euthanized with pentobarbital overdose (100 mg/kg i.p.) and intracardially perfused with 0.9% saline solution. Brains were removed quickly after death. Each brain was then dissected along the midline. The right hemisphere was post-fixed overnight in 4% paraformaldehyde, cryoprotected in PBS containing 20% sucrose before being freeze by immersion in a cold isopentane bath (−50°C), and stored immediately at −80°C until sectioning. Brains were sectioned in a Leica CM3050S cryostat (Leica Microsystem, Wetzlar, Germany) at −20°C in either coronal or sagittal 40 μm-thick free-floating sections and stored in PBS containing 0.2% sodium azide at 4°C until use. The left hemisphere was dissected, and several brain regions were collected for further analysis: cortex, hippocampus, midbrain, striatum and cerebellum. Samples were stored at −80°C until use. Prior to staining, sections of appropriate levels (e.g., striatum, midbrain or hippocampus) were selected. Immunostainings were performed as previously described (Bourdenx et al., 2015; Schneider et al., 2014; Schneider et al., 2015). Briefly, selected sections were washed, incubated in blocking buffer and then incubated overnight with the appropriate primary antibody. The following day, sections were washed 3 times for 5 minutes in PBS, incubated with the appropriate anti-specie secondary antibody (1:2000), washed 3 times in PBS and mounted. Cell nuclei were stained using Hoechst (Life Technologies, 33342) at 1:5,000 for 30 s prior to mounting. ProLong Diamond mounting medium was used (ThermoFisher Scientific P36965). For the immunostainings in the Tg and Tg-L2A−/− immunofluorescent detection of phosphorylated tau aggregates and amyloid plaques was performed as described in Grueninger et al. (2010) with the exception that the amyloid-specific BAP-2 antibody was replaced by MOAB2-AF488 (Novus Biologicals). Images were acquired with an Axiovert 200 fluorescence microscope (Carl Zeiss Microscopy), or when full brain sections were imaged individual images from the scanning of brain slices were mounted with an ApoTome.2 slider, or a Leica confocal TCS-SP8 (Leica Microsystem) and prepared using ImageJ Software (NIH). A perceptually uniform lookup table (Magma) was used to enhance contrast and highlight pattern and intensity differences between experimental groups in Figures 7G, S4D, S4E, and S5Q (Crameri et al., 2020).
Thioflavin S staining
Thioflavin S staining was performed prior to incubation in the blocking buffer using a 0.5% Thioflavin S solution (Santa Cruz, sc391005) in water for 7 minutes at room temperature.
Histopathology of peripheral organs and blood cell count
Where indicated mouse liver, lung and kidneys were dissected and fixed in 1% PFA overnight and paraffin embedded. Tissues were sectioned, stained with hematoxylin and eosin (H&E) and analyzed by an expert pathologist, blind to the treatment groups, to score for possible presence of toxicity in these organs. Blood cell count in the groups of mice administered vehicle or CA was analyzed in tail blood drawn monthly and at the moment of tissue dissection using an Oxford Science Forcyte Blood Analysis Unit.
Western blotting
Protein concentration was determined using the Lowry method with bovine serum albumin as a standard (Lowry et al., 1951). Dissected brain regions were solubilized on ice with RIPA buffer (1% Triton X-100, 1% sodiumdeoxycholate, 0.1% SDS, 0.15M NaCl, 0.01M sodium phosphate, pH7.2) followed by sonication. Immunoblotting was performed after transferring SDS-PAGE gels to nitrocellulose membrane and blocking with 5% low-fat in milk 0.01% Tween-TBS for 1h at room temperature. The proteins of interest were visualized after incubation with primaries by chemiluminescence using peroxidase-conjugated secondary antibodies in LAS-3000 Imaging System (Fujifilm, Tokyo, Japan). Densitometric quantification of the immunoblotted membranes was performed using ImageJ (NIH). All protein quantifications were done upon normalization of protein levels to a loading control (β-actin) or Ponceau staining and expressed as fold of the relevant control group.
Autophagy measurements in cultured cells
Macroautophagic flux was measured in protein lysates using immunoblot for LC3-II in cells treated or not with lysosomal protease inhibitors (20 mM ammonium chloride and 100 μM leupeptin). Flux was calculated as the increase in levels of LC3-II in protease inhibitors-treated cells relative to untreated cells. CMA activity was measured in cells stably transduced with lentivirus carrying the KFERQ-PS-Dendra reporter (Koga et al., 2011)) and plated in glass-bottom 96-well. Sixteen hours after photoswitching with a LED lamp (405nm for 3 minutes), cells were fixed with 4% PFA and imaged using high-content microscopy (Operetta system, Perkin Elmer). Images were quantified using the manufacturer’s software in a minimum of 800 cells.
Transferrin uptake in differentiated CAD cells
Transferrin internalization was performed as previously (Yu et al., 2014). Briefly, CAD cells grown in serum-free DMEM were incubated for 10min using Alexa555-conjugated transferrin (25μg/ml; Life Technologies). The cells were then transferred on ice and wash 3 times with ice-cold PBS. Cells were then fixed for immunofluorescence.
Isolation of sarkosyl insoluble fraction
Brain homogenates were prepared as described in Grueninger et al. (2010). Homogenates from several mice of each genotype were pooled and diluted to a final protein concentration of 1 mg/ml. Sarkosyl was then added to a final concentration of 1% and the homogenates incubated for 30 min at 4°C. The homogenates were subsequently centrifuged at 100,000xg for 1 hr. Pelleted proteins were sent for proteomic analysis or were resuspended directly in SDS-PAGE sample buffer and boiled for 2 min. For each genotype, equal volumes of resuspended pellet were used for SDS-PAGE/western blotting.
AlphaLISA immunoassays
Tau, pS422-Tau, pS202/T205-Tau, and aggregated tau were measured by immunoassay in brain extracts as described in Grueninger et al. (2010), except that the MSD assay format was replaced by AlphaLISA immunoassay technology (Perkin-Elmer).
Aβ42 enzyme-linked immunosorbent assay (ELISA)
Aβ42 levels were measured by ELISA using a commercial kit (ThermoFisher #KHB3442). Mouse brain lysates were diluted 1:50 in the provided diluent and assay was performed following manufacturer’s recommendations.
Extracellular flux analysis
Oxygen consumption rates and extracellular acidification rates were measured using a 24-well Seahorse Bioanalyzer XF 24 according to the manufacturer’s instructions (Agilent Technologies). Briefly, neurons were plated into 24-well plates pre-coated with CELL-TAK (CORNING, 354240at a concentration of 1.8×1014 cells/well and used at 14 days-in-vitro. Once in the reader, plates were sequentially injected 10mM glucose, 1.0μM oligomycin and 50mM 2-Deoxyglucose (2-DG) in artificial cerebrospinal fluid (aCSF, 120mM NaCl, 3.5mM KCl, 1.3mM CaCl2, 0.4mM KH2PO4, 1mM MgCl2, 5mM HEPES) + 2mM glutamine, pH: 7.4. Differentiated CAD cells plated on gelatin-coated plates were switched to manufacture provided base medium supplemented with 2mM L-glutamine and sequentially injected with 10mM glucose, 2 μM oligomycin and 100mM 2DG in the bioanalyzer. Quantifications were performed using Seahorse Wave Desktop software. Data were normalized to cell number using CyQuant (ThermoFisher, C7026).
Materials and methods for the chemical synthesis of CMA activators
All chemical reagents and solvents were obtained from commercial sources (Aldrich, Acros, Fisher) and used without further purification unless otherwise noted. Chromatography was performed on a Teledyne ISCO CombiFlash Rf 200i using disposable silica cartridges (4, 12, and 24 g). Analytical thin layer chromatography (TLC) was performed on aluminum-backed Silicycle silica gel plates (250 μm film thickness, indicator F254). Compounds were visualized using a dual wavelength (254 and 365 nm) UV lamp, and/or staining with CAM (cerium ammonium molybdate) or KMnO4 stains. NMR spectra were recorded on Bruker DRX 300 and DRX 600 spectrometers. 1H and 13C chemical shifts (d) are reported relative to tetramethyl silane (TMS, 0.00/0.00 ppm) as internal standard or to residual solvent (CDCl3: 7.26/77.16 ppm; dmso-d6: 2.50/39.52 ppm). Mass spectra (ESI-MS) were recorded on a Shimadzu LCMS 2010EV (direct injection unless otherwise noted). High resolution mass spectra (HRMS) were recorded on an Orbitrap Velos high resolution mass spectrometer at the Proteomics Facility of Albert Einstein College of Medicine.
Synthesis of 7-chloro-3-(p-tolyl)-2H-benzo[b][1,4]oxazine (AR7)
Preparation of AR7 was as described before (Anguiano et al., 2013). Briefly, 2-bromo-4-chloroacetophenone (0.01mol) in dichloromethane was added drop-wise to a solution of 2-aminophenol in dichloromethane, aqueous potassium carbonate (20% w/v) and tetrabutylammonium hydrogen sulfate (0.0005 mol). The resulting mixture was refluxed till completion for 4–6 h and the organic layer was extracted with dichloromethane and dried over sodium sulfate evaporated in vacuum to give a crude solid product. The solid was then recrystallized with hot ethanol to obtain pure yield 95%.
AR7 : 1H NMR (300 MHz, CDCl3): δ = 2.44 (s, 3H), 5.09 (s, 2H), 6.91–6.98 (m, 2H), 7.12 (d, J = 12Hz, 2H), 7.33–7.38 (d, J = 8 Hz, 1H), 7.82–7.84 (d, J = 12 Hz, 2H); 13C NMR (75 MHz, CDCl3): δ = 21.56, 62.82, 76.58, 115.94, 122.46, 126.41, 128.34, 132.15, 132.44, 133.02, 141.97, 146.82, 158.94; HRMS (for C15H12ClNO ([M+H]+): calculated 258.0641, found: 258.0702.
Synthesis of N-(4-(6-chloroquinoxalin-2-yl)phenyl)acetamide (CA77.1)
In a dry and Ar-flushed 50 mL round-bottom flask, 2,6-dichloroquinoxaline (250 mg, 1.25 mmol), (4-acetamidophenyl)boronic acid (293 mg, 1.64 mmol, 1.30 equiv), and potassium carbonate (1.0 M; 1.25 mL, 1.25 mmol, 1.00 equiv) were dissolved in dioxane (8.40 mL). The mixture was degassed (argon bubbling for 20 min), then palladium tetrakis (73 mg, 63 μmol, 5.0 mol-%), was added, the flask purged with more argon and a reflux condenser was placed on top. The mixture was heated under an argon atmosphere at 100°C over night. TLC analysis indicated complete conversion. The mixture was cooled to RT and the solvents were evaporated in vacuo. The residue was taken up in CH2Cl2/acetone and absorbed on silica gel. The silica gel was dried in high vacuum before being loaded on top of a loading cartridge into the CombiFlash. We found that product batches, particularly in scale-up batches of g-scale, after column chromatography and re-crystallization still contained > 500 ppm of Pd impurity. We optimized a purification protocol with respect to reduction of Pd impurity, but also overall yield, using a combined Smopex/recrystallization protocol as follows: Purified N-(4-(6-chloroquinoxalin-2-yl)phenyl)acetamide (189 mg, 0.635 mmol, 50%) was suspended in CH2Cl2 (34 ml) and MeOH (3.7 mL) and RT. To this was added Smopex 110 (37.8 mg) and the resulting mixture was stirred at RT for 7 days. The solution was filtered through a paper filter, the solvents were evaporated in vacuo. The residue was re-crystallized from acetone to obtain pure N-(4-(6-chloroquinoxalin-2-yl)phenyl)acetamide (80.0 mg) with a Pd content of < 50ppm. Repeated crystallization from acetone can lower the Pd content to < 25ppm.
CA77.1: 1H-NMR (600 MHz, dmso-d6): 10.20 (s, 1H), 9.58 (s, 1H), 8.32–8.31 (m, 2H), 8.18 (d, J = 2.4 Hz, 1H), 8.13 (d, J = 8.9 Hz, 1H), 7.89 (dd, J = 8.9, 2.4 Hz, 1H), 7.82 (d, J = 8.8 Hz, 2H), 2.11 (s, 3H). 13C-NMR (151 MHz, CDCl3): δ 168.6, 150.9, 144.5, 141.6, 141.0, 140.1, 133.5, 131.0, 130.8, 129.9, 128.1, 127.5, 119.0, 24.1. HRMS (for C16H12ClN3O, M+H): calculated: 298.0742, found: 298.0743.
Pharmacokinetic properties
ICR (CD-1) male mice were fasted at least three hours and water was available ad libitum before the study. Animals were housed in a controlled environment, target conditions: temperature 18 to 29°C, relative humidity 30 to 70%. Temperature and relative humidity were monitored daily. An electronic time-controlled lighting system was used to provide a 12 hr light/12 hr dark cycle. 3 mice for each indicated time point were administered 10 mg/Kg CA77.1 by oral gavage or 1mg/Kg by intravenous injection using 30% PEG-400, 65% D5W (5% dextrose in water), 5% Tween-80 vehicle. Mice were sacrificed, and brain samples were harvested at 0 hr, 0.25 hr, 0.5 hr, 1 hr, 2 hr, 4 hr, 8 hr, 24 hr, and analyzed for CA77.1 levels using LC-MS/MS. Pharmacokinetics parameters were calculated using Phoenix WinNonlin 6.3. Experiments performed at SIMM-SERVIER joint Biopharmacy Laboratory.
Quantitative proteomics and protein pathway analysis
Insoluble fractions of autophagy-deficient mice.
Sarkosyl-insoluble fractions from three different animals per genotype (Ctr, CKL2A−/−, and CKATG7−/−) were pooled. Quantitative proteomic analysis was performed using iTRAQ multiplex (Applied Biomics). For each protein hit the average ratio(s) for the protein, the number of peptide ratios that contributed and the geometric standard deviation were determined. Values in the experimental groups were compared to Ctr and are represented as folds. For the comparative analysis between the sarkosyl-insoluble fractions of CKL2A−/− and CKATG7−/− mice (Figure 3), we first selected proteins with a significant enrichment over that in the same fraction in CTR mice (as presented in Figure 2). This allowed us to exclude the large functional multi-protein complexes and membrane-associated structures detectable in the sarkosyl-insoluble fraction that are not relevant to protein aggregation.
Alzheimer’s disease – CMA deficient mice.
Protein was precipitated from lysates (cortex and hippocampus pooled) from three mice of each genotype (WT, L2A−/−, Tg, Tg-L2A−/−), solubilized in 8M urea, 0.1 M ammonium bicarbonate pH 8.0, 150 mM NaCl, complete mini protease and phosphatase inhibitors (Roche) and cysteine residues were reduced and alkylated with TCEP and iodoacetamide, followed by a 5-fold dilution with 0.1M ammonium bicarbonate. Proteins were digested into peptides by the addition of trypsin over night at 37°C (1 μg trypsin per 100 μg lysate). Samples were desalted on C18 cartridges (NEST), lyophilized, resuspended in 4% formic acid, 3% acetonitrile and approximately 1 μg of digested peptides per sample were loaded onto a 75 μm ID column packed with 25cm of Reprosil C18 1.9 μm, 12Å particles (Dr. Maisch GmbH HPLC, Germany). Peptides were eluted into an Orbitrap Fusion Lumos Tribrid mass spectrometer (Thermo Fisher Scientific) over the course of a 120 minute acquisition by gradient elution delivered by an Easy1200 nLC system (Thermo Fisher Scientific). The composition of mobile phase A and B were 0.1% formic acid in water and 0.1% formic acid in 80% acetonitrile, respectively. All MS spectra were collected with orbitrap detection and the most abundant ions were fragmented by higher energy collision dissociation and detected in the ion trap, with a 3 s cycle time between MS1 spectra. All data were searched against the Uniprot mouse database (downloaded 7/19/2016). Peptide and protein identification searches were performed using the MaxQuant data analysis algorithm (Cox et al., 2014), and all peptide and protein identifications were filtered to a 1% false-discovery rate. Label-free quantification and statistical testing was performed using the MSstats statistical R-package (Choi et al., 2014). Significantly modified proteins were selected by p < 0.05 followed correction using the Benjamini-Hochberg protocol (FDR 5%). When FDR correction led to no hit, inspection of uncorrected p values distribution was performed: if an anticonservative distribution was observed, we applied alternative method of false discovery rate control by lowering threshold for significance (p < 0.01) and using fold change cutoff (|fold change| > 1.25), as previously suggested (Pascovici et al., 2016). The mass spectrometry data files (raw and search results) have been deposited to the ProteomeXchange Consortium (Deutsch et al., 2017) (http://proteomecentral.proteomexchange.org) via the PRIDE partner repository with dataset identifier PXD017108.
Rank-rank hypergeometric overlap test.
Relevant protein lists were ranked according to fold change compared to Ctr mice and submitted to rank-rank hypergeometric overlap test in R (v. 3.6.2) (Team, 2018) using default settings (Plaisier et al., 2010). Colormap indicates -log10 p value of the exact Fisher test by bin. We also used proteome-wide transition mapping (Stein et al., 2014), that consists of serialized differential expression analysis between genotypes using overlap quantification by RRHO and has the advantage of being threshold free and allowing a statistical assessment of the overlap extent. In our studies with Tg, Tg-L2A−/− and L2A−/− mice, we used comparison with this method of a fold change-ranked list of proteins of each genotype (normalized to CTR).
Gene-ontology analysis and EnrichmentMap.
Proteomic results were ranked according based on fold change and submitted to a GSEA Preranked analysis in GSEA (v. 4.0.2) with 1000 permutations (Subramanian et al., 2005). Terms smaller than 15 genes or bigger than 500 were discarded as previously reported. The enrichment map was generated in Cytoscape (3.7.1) using Enrichment map plugin (3.2.0) (Merico et al., 2010) using the following thresholds: p value < 0.05, FDR < 0.001. Alternatively, proteomic results were submitted to ontology analysis using Enrichr (Chen et al., 2013; Kuleshov et al., 2016). Node size indicates the number of proteins per node. Major clusters are circled, and the associated name represent the major functional association.
KFERQ-like motif enrichment
Analysis of content in KFERQ-like motif was performed as previously described (Kirchner et al., 2019) using the publicly available tool: http://ec2-18-188-198-152.us-east-2.compute.amazonaws.com:3838/kferq/.
Calculation of CMA activation score
Dataset used were from Grubman et al. (2019) and Mathys et al. (2019). Raw counts per cell and metadata (cell type identification and patients’ information) were obtained from the Synapse portal (https://www.synapse.org/#!Synapse:syn18485175). Filtered counts provided by Mathys et al. (2019) were used. Single cell gene expression data and metadata were obtained from http://adsn.ddnetbio.com/ for the dataset (Grubman et al., 2019)
Prior to calculations, counts were log normalized using SCANPY (v.1.4.5) (Wolf et al., 2018). For each cell, a CMA activation score was calculated. To do so, each element of the CMA network (Kirchner et al., 2019) was attributed a weight. As LAMP-2A is the rate limiting component of CMA, it was given a weight of 2. Every other element received a weight of 1. Then, every element was attributed direction score that is +1 or −1 based on the known effect of a given element on CMA activity. The score was then calculated as the weighted/directed average of expression counts of every element of the CMA network. To ease understanding of transcriptional CMA activity changes between conditions, the CMA score was expressed as fold of healthy individuals within a given cell type.
QUANTIFICATION AND STATISTICAL ANALYSIS
All data presented are mean ± s.e.m. with *p < 0.05, **p < 0.005, ***p < 0.0005. Prior to statistical testing, normality was assessed using the Shapiro Wilk test. Parameters with two groups were compared using unpaired, two-tailed t test or Mann Whitney U test. Parameters with more than two groups were compared using one-way ANOVA and Tukey’s post hoc analysis, Kruskal-Wallis test followed by Dunn’s post hoc analysis or two-way ANOVA followed by Sidak post hoc analysis. The number of animals used per experiment was calculated through power analysis based in previous results. Statistical analyses were performed either in GraphPad Prism 8.0 or using Python (Python software foundation v.3.7.4 available at https://www.python.org/) and the scientific python stack: scipy (v.1.3.1) (Jones et al., 2001), numpy (v.1.17.2) (van der Walt et al., 2011), and matplotlib (v.3.1.1) (Hunter, 2007).
Supplementary Material
Figure S1. Mice with CMA blockage display behavioral impairments, related to Figures 1 and S2
(A) Schematic of the generation of mice with systemic (whole body; L2A−/−) or neuronal-specific (CaMKinase-II a Cre; CKL2A−/−) deletion of LAMP2A (L2A).
(B and C) Immunoblots for L2A, L2B and LAMP1 (L1, loading control) in cortex from control (CTR), L2A−/− and CKL2A−/− mice (B). Quantification of L2A (left) and LA2B (right) after normalization for L1 (C). [Genotype effect: F2,13 = 214.3; p < 0.0001].
(D) RT-PCR quantification of L2A and L2C mRNA in cortex from CTR, L2A−/− and CKL2A−/− mice. [Genotype effect: F2, 25 = 19.32, p < 0.0001].
(A) Body weight change over time in WT, L2A−/−, and CKL2A−/− mice.
(B) Kaplan-Meier curve of survival of WT, L2A−/−, and CKL2A−/− mice.
(G-I) Clasping in CTR, L2A−/− and CKL2A−/− mice. Representative pictures of the indicated scores (G), age-dependent change in clasping score [genotype effect: F2, 63 = 18.24, p < 0.0001] (H) and mean linear regression coefficient (±95% C.I.) on clasping scores (I).
(J) Negative geotaxis test in CTR, L2A−/− and CKL2A−/− mice at 6 months. Quantification of latency. [Kruskal-Wallis statistic = 9.987, p = 0.0018].
(K,L) Short-term memory Y test in 6 months old CTR, L2A−/− and CKL2A−/− mice. Quantification of the fraction of time spent in the novel arm (J) [F2, 12 = 4.775, p = 0.0298] (K) and representative tracks (L).
(M, N) Spontaneous locomotion in an open field in CTR, L2A−/− and CKL2A−/− mice at 6months. Representative tracks (M) and quantification of the distance walked over 10minutes (N). [Kruskal-Wallis statistic = 9.006, p = 0.0046].
(O, P) Stride length in CTR, L2A−/− and CKL2A−/− mice at 6months. Representative pictures of stride length (O) and quantification (P). [Genotype effect: F2, 79 = 35.84, p < 0.0001].
(Q) Spatial working memory measured at 6 months by the percentage of spontaneous alternation in the Y maze fro WT, L2A−/−, and CKL2A−/− mice.
(R, S) Nest building test in 6 months old WT, L2A−/−, and CKL2A−/− mice. Representative pictures per genotype (R) and quantification (S) are shown. [Genotype effect, Kruskal-Wallis statistic = 8.814; p = 0.0035]
All data are mean ± s.e.m. and individual values are shown in C, D, N, J, K, P, Q, and S. n = 4-8 per time point/genotype. Comparisons were made using Kruskal-Wallis test followed by Dunn’s post hoc test (J, N, Q, S), one-way ANOVA followed by Tukey’s post hoc test (C, D, K, P) or two way ANOVA followed by Sidak’s post hoc test (H). *p < 0.05, **p < 0.005, ***p < 0.0005. See also Data S1.
Figure S2. Consequence of systemic CMA blockage in brain proteostasis, related to Figures 1 and S1
(A-D) Nuclear staining with DAPI to assess thickness and area of different brain regions in 6 months old CTR and L2A−/− mice. Representative images (left) and quantification of region thickness of somatosensory cortex (A), CA1 (B) and dentate gyrus (C). Hippocampal surface in L2A−/− mice relative to CTR mice is shown in D. Montages of individual images from the scanning of the region of interests in brain slices are shown in A-C.
(E, F) Astrocyte (GFAP) (E) and microglial (Iba1) (F) staining in CA1 of 6 months old WT and L2A−/− mice. Left: representative images. Right: quantifications of number of positive cells per field. Scale bar: 50μm.
(G-I) Lipofuscin autofluorescence (G) and immunostaining for K63-linkage ubiquitin (H,I) in hippocampal neurons of 6 months old CTR and L2A−/− mice. Representative images (left) and quantification of number of fluorescent puncta per cell [t8 = 4.012, p = 0.0039] (right G) or staining intensity distribution per cell body [t198 = 9.945, p = p < 0.0001] (right H). Scale bar: 20μm (G) 50μm (H). n = 20 cells per mice, five mice per genotype. (I) Higher magnification and XZ/YZ projections of immunostaining for K63-linkage ubiquitin to illustrate presence of protein aggregates (white arrowhead) in the CA3 region (left) and cellular area occupied by ubiquitin-positive aggregates (right). Overall cumulative distribution [Mann Whitney U = 1209, p < 0.0001] and mean per animal is shown as inset [t8 = 4.398, p = 0.023]. Scale bar: 10μm.
(J, K) Status of macroautophagy in cortex form WT and L2A−/− mice. Immunoblot for the indicated autophagosome components (LC3, GATE16), the macroautophagy receptor (p62) and the lysosomal membrane protein (LAMP1) (J) and densitometric quantification for the indicated proteins (K). Ponceau staining is shown as loading control.
Data are mean ± s.e.m and individual values from n = 5 per group. Comparison were made using Mann Whitney U test (H) or unpaired t test (all the rest). *p < 0.05, **p < 0.005, ***p < 0.0005. See also Data S1.
Figure S3. Neuronal CMA blockage did not induce gliosis or changes in macroautophagy, related to Figure 1
(A) Astrocyte (GFAP) and microglial (Iba1) staining in CA1 of 6 months old WT and CKL2A−/− mice. Left: representative pictures. Right: quantifications of number of positive cells per field and area of positive cells (n = 30 cells per animal). Scale bar: 50μm.
(B) Immunostaining for CaMKIIα (magenta), autofluorescent lipofuscin (green) and Hoechst nuclear staining (blue) in the hippocampus of CKL2A−/− mice. Top: widefield view of CA1 region in the hippocampus; scale bar: 50μm. Bottom: (left) high magnification picture of pyramidal CaMKIIα-positive neurons in the stratum pyramidale and (right) CaMKIIα-negative cell in the stratum radiatum; scale bar: 10μm. White arrowheads indicate lipofuscin puncta.
(C) Quantification of number of fluorescent lipofuscin puncta per cell in the stratum radiatum of CTR and CKL2A−/− mice at 6 months (related to Figure 2E).
(D, E) Immunostaining for K63-linkage ubiquitin in astrocytes (D) and microglial cells (E) in the hippocampus of 6 months old CTR and CKL2A−/− mice. Representative images for the indicated antibodies and overlay of cell mask on ubiquitin staining (left). Staining intensity distribution per cell body (right top) and mean intensity per cell body per animal (right bottom). Scale bar: 10μm. n = 50-70 cells per mice, 4-5 mice per genotype.
(F) Immunoblot for 4-hydroxynonenal protein adducts in cortex of WT and CKL2A−/− mice at 6 months. Left: representative immunoblot (two mice/ genotype). Right: densitometric quantification.
(G) Distribution of KFERQ-like motifs in the insoluble protein fraction of CKL2A−/− mice compared to the whole mouse proteome. Gray: no KFERQ-like motifs; Yellow: only canonical motifs; Blue: post-translational-generated motifs (phosphorylation and acetylation).
(H-K) Status of macroautophagy in cortex from 6 months old WT and CKL2A−/− mice. Immunoblot (left) and quantification (right) for the indicated autophagosome components (LC3, GATE16), the macroautophagy receptor (p62) and the lysosomal membrane protein (LAMP1) (H). Samples from cortex and hippocampus of CKATG7−/− mice are shown as positive control of disrupted macroautophagy (I). Representative electron microscopy images of cortex (left, full field; right, examples of autophagosomes (APG) and autolysosomes (AUT)). Bottom shows morphometric quantification of number of vesicles per field (left) and percentage of autophagic vacuoles (AV) at the APG and AUT state (right) (J). Low (left) and high magnification (right) images of CKL2A−/− mice cortex to highlight accumulation of electrodense material (yellow arrows) and presence of autophagic vacuoles (green arrows) in the proximity (K). Bottom shows examples of cytosolic and membrane surrounded (inside vesicles) electrodense material (K). Scale bar: 1μm for full fields and 0.20μm for insets.
Data are mean ± s.e.m. and individual values from n = 4-5 per group. Comparisons were made using unpaired t test. See also Data S1.
Figure S4. CMA deficiency induce proteome alterations, related to Figure 2
(A) Diagonal plot of the RRHO matrix (shown in Figure 3C) showing low similarity of highly enriched proteins and high similarity of middle ranked proteins in the insoluble fraction of CMA-deficient CKL2A−/− mice compared to macroautophagy-deficient CKATG7−/− mice.
(B-C) Examples of GSEA plots associated with macroautophagy (B) or CMA (C) deficiency.
(D) Immunoblots for the indicated proteins of differentiated neuroblastoma cells transduced with lentiviral carrying empty vector (Control) or shRNA against L2A or ATG7, to confirm the knock-down in the cells used for the experiments in main Figures 3K and S4E, S4F, and S4P.$$PARABREAKHERE$$(E-F) Extracellular acidification rate (ECAR) in differentiated neuroblastoma cells control (Ctrl) or knocked down for L2A or ATG7. Changes in average ECAR values upon addition of the indicated compounds (E) and glycolytic properties calculated as the areas under the curve in ECAR plots upon the addition of the indicated compounds (F). [Knockdown effect: F2,30 = 25.45, p < 0.0001]. n = 3-5 independent experiments.
(G-J) Shift of the glycolytic enzyme PDH from the soluble to insoluble fraction. Representative immunoblot for the indicated protein/fraction from 5 CTR or L2A−/− mice at 6 months of age (G). Densitometric quantification, normalized to ponceau for: soluble PDH [t8 = 2.423, p = 0.0417] (H), insoluble PDH [Mann Whitney U = 0, p = 0.0079] (I) and ratio insoluble to soluble PDH [t8 = 3.742, p = 0.0057] (J).
(K-O) Mass spectrometry analysis of the acetylation status of the proteome of CTR and L2A−/− mice brains. Overall acetylation of the proteome; x axis error bar was estimated using bootstrap (K), overall distribution of changes in acetylation levels (l) and plots of 22 proteins that showed decreased acetylation (M) and 16 proteins that showed increased acetylation (N) in CMA-deficient mice. (O) Gene-ontology terms associated with proteins showing altered acetylation profile in CMA-deficient mice (L2A−/−). Green bars: hypoacetylated proteins in L2A−/− mice. Orange: proteins hyperacetylated in L2A−/− mice.
(P, Q) Transferrin uptake at 10 minutes in differentiated neuroblastoma cell lines transduced with empty vector (control) or shL2A construct. Representative images of GFP (Green), Transferrin (Magenta) and DAPI (Blue). Non infected cells (GFP negative) (*) and infected cells (arrowhead) are marked. Boxed areas are shown at higher magnification in main Figure 3K. Scale bar 20μm.
(Q, R) Immunostaining for actin in hippocampal neurons of 6 months old CTR and L2A−/− mice. Representative images with higher magnification inset (Q) and cumulative distribution of the mean staining intensity per cell [t118 = 6.970, p < 0.0001] (R). Scale bar: 20μm.
(S) Schematic representation illustrating the proposed mechanism. In the context of CMA deficiency, functional proteins are trapped in protein aggregates leading to loss of function.
Data are mean ± s.e.m. Comparison were made using one-way ANOVA (F), unpaired t test (H-J) or Kruskal-Wallis test followed by Dunn’s post hoc test (R). *p < 0.05, **p < 0.005, ***p < 0.0005. See also Data S1.
Figure S5. Alteration of CMA network in the brain of AD patients and validation of the CMA score, related to Figure 3
(A,B) Levels of the master transcriptional regulator of macroautophagy and lysosomes (TFEB) in major brain cell types of the frontal cortex of brains with low (Braak stage 0-II), medium (Braak stage III-IV) and advanced (Braak score V-VI) pathology. Mean TFEB count per cell (A) and normalized TFEB counts expressed as fold change between major brain cell types (B).
(C-F) Validation of the CMA score by comparing CMA activity (C) measured using the fluorescent KFERQ-Dendra reporter in NIH 3T3 cells in response to paraquat (PQ) (left) and the CMA activator (right) with the CMA score calculated from the changes in the mRNA levels of components of the CMA network at the concentration indicated with the arrow (D) n = 3 experiments. Non-linear fit curve shown in C. Transcriptional profile (E) and CMA score (F) in livers of KFERQ-Dendra mice treated with the CA activator. n = 3 mice.
(G, H) Negative correlation between CMA activation score in inhibitory neurons and pathology markers at patient level: Braak pathology staging (G), and NIA-Reagan score (H).
(I, J) CMA activation score in excitatory neuron subclusters (I) and distribution of early pathology-associated inhibition of CMA network in excitatory neurons depending on their localization within specific cortical layers (J). d is Cohen’s d measure of effect size. p indicates the p value of the unpaired t test between control and early pathology stage within a given subtype of excitatory neuron.
Data are mean ± s.e.m. Comparison were made using one-way ANOVA (B) or Kruskal-Wallis test followed by Dunn’s post hoc test (G,H). **p < 0.005, ***p < 0.0005.
Figure S6. Mouse model of CMA deficiency AD-related proteotoxicity with CMA, related to Figures 4 and 5
(A) Immunoblot for the indicated proteins in brains from control, L2A−/−, 3xTg and 3xTg-L2A−/− mice at 12 months of age. Ponceau staining of the membrane is shown as protein loading control.
(B) Immunoblot for K63-linkage of ubiquitinated proteins (left) and densitometric quantification (right) in brains of CTR, L2A−/−, Tg and Tg-L2A−/− mice at 12 months [Genotype effect: F3,32 = 5.615, p = 0.0033]. n = 9 mice per genotype.
(C-E) Volcano plot of the quantitative proteomic analysis of brain from L2A−/− (C), Tg (D) and Tg-L2A−/− (E) compared with CTR mice brains. The number in the top left corner indicates the number of significant hits. Red dots: differentially expressed proteins (adj. p < 0.05).
(F-H) Whole proteome comparison between human asymptomatic AD cases from the Consensus study and L2A−/−, Tg and Tg-L2A−/− mice. The human cases were normalized to cognitively control subjects (AD-Control), whereas L2A−/−, Tg and Tg-L2A−/− results were normalized to CTR mice. Blue line indicates the linear regression (+/− 95% confidence interval) between human and mouse values. Numbers in the top left corner are the regression coefficient. P values: (F) p = 0.0014, (G) p = 0.024, (H) p = 1.83×10−8
(I) Status of macroautophagy in cortex from 6 months old CTR, L2A−/−, Tg and Tg-L2A−/− mice. Immunoblot for the indicated autophagosome components (LC3, GATE16), the macroautophagy receptor (p62) and the lysosomal components (LAMP1, GBA, Pro and mature CathD)(left). Densitometric quantification for the indicated proteins normalized to ponceau (right). (n = 9 mice per genotype).
(J) beta-Hexosaminidase assay in total homogenates from cortex of 12 months old L2A−/−, Tg and Tg-L2A−/− mice [F3,20 = 9.833, p = 0.0003]. n = 6 mice per genotype.
(K) Immunoblots for the indicated proteins from subcellular fractionation of cortex from L2A−/−, Tg and Tg-L2A−/− mice. Fractions are total homogenates (Hom), cytosol (Cyt.), a population of lysosome active for CMA (CMA+) and a population of lysosomes inactive for CMA (CMA−).
(L,M) Status of the CMA network in cortex from 6 months old CTR, L2A−/−, Tg and Tg-L2A−/− mice. Immunoblot for the indicated CMA effectors (Hsc70, Hsp90 and Hsp40), CMA lysosomal modulators (Rac1, PHLPP1, Rictor and CathA) and CMA extralysosomal modulators (Rab11 and RARα) (L). Densitometric quantification for the indicated proteins normalized to ponceau (M). (n = 6 mice per genotype).
Data are mean ± s.e.m. and individual values. Comparisons were made using One-way ANOVA followed by Tukey post hoc analysis (B, I, J, L) or Kruskal Wallis test followed by Dunn’s post hoc analysis (C-H). *p < 0.05, **p < 0.005. See also Data S1.
Figure S7. Characterization of the brain-permeable chemical activator of CMA and effect in a mouse model of frontotemporal-dementia-related proteotoxicity, related to Figures 6 and 7
(A) Chemical structure of AR7 and CA77.1.
(B) Quantification of the effect of adding increasing concentrations of AR7 and CA77.1 to NIH 3T3 cells stably expressing the KFERQ-PS-Dendra reporter. CMA activity was quantified after 16h treatment as the average of fluorescent puncta per cell. (n > 800 cells per condition).
(C) Autophagic flux in NIH 3T3 cells incubated at 37°C for 16h with 20 μM AR7 or CA77.1 or without additions (none). Where indicated lysosomal protease inhibitors (PI) were added to the cells 6h before lysis. Left:: representative immunoblot. Actin is shown as loading control. Right: quantification of LC3-II flux as the difference in LC3-II levels in cells treated with PI relative to untreated. Values are presented as folds those in untreated cells that were given an arbitrary value of 1. n = 5 independent experiments.
(D) Quantification of the effect of adding increasing concentrations of AR7 and CA77.1 to NIH 3T3 cells stably expressing the KFERQ-PS-Dendra reporter. CMA activity was quantified after 12h or 24h treatment as the average of fluorescent puncta per cell. (n > 800 cells per condition).
(E) Pharmacokinetic analysis of CA77.1 after p.o. (oral, 10mg/Kg bw) and i.v. (intravenous, 1mg/kg bw) administration in mice. n = 3 mice per time point.
(F) Summary of pharmacokinetic parameters of CA77.1 in mice brain.
(G) Mouse blood count at the end of a 4 months daily oral administration of vehicle (Veh) or CA77.1 (CA). n = 6 mice in each group.
(H-J) Representative images of sections of H&E stained liver (B), lung (C) and kidney (D) sections from the same mice. Two magnifications are shown. In D, details of glomeruli and tubules are shown in the higher magnification images.
(K) Distribution of four types of S202/T205-phosphorylated tau stainings described in Shi et al., 2017. Numbers indicate the number of mice belonging to each group. n = 10 mice per group.
(L-N) Immunoblot for AT8 tau on the Sarkosyl-insoluble fraction of CTR and PS19 mice administered Veh or CA for 6 months. HMW: high-molecular weight species of AT8. Representative immunoblot of 4-5 mice per genotype and treatment (L) and densitometric quantification of AT8 as monomer [Kruskal-Wallis statistic = 20.48, p < 0.0001] (M) and as HMW-species [F2,26 = 7.183, p = 0.0033] (N).
(O, P) Quantification of the number of Iba1-positive microglial cells per mm2 in the hippocampus [Kruskal-Wallis statistic = 10.55, p = 0.0051] (O) and piriform cortex (P) of CTR and PS19 mice treated with Veh or CA for 6 months. Images shown in Figure 7N.
Mean ± s.e.m and individual values. Comparisons were made using One-way ANOVA followed by Tukey post hoc analysis *p < 0.05. See also Data S1.
Figure S8. Effect of chemical activation of CMA in a mouse model of AD-related proteotoxicity, related to Figure 7
Representative images of immunostaining of immature amyloid plaques (MOAB2), mature amyloid depositions (6E10), β sheet marker (Thioflavin S) and Threonine231-phosphorylated tau (pThr231 tau) in the frontal cortex of Tg mice administered Veh or CA for 4 months. Montages of individual images of the frontal cortex from the scanning of whole-brain slices are shown. Scale bar: 200μm.
(B-E) Quantification of size of plaques positive for MOAB2 (t7 = 3.181, p = 0.0155) (B), 6E10 (C) or Thioflavin S (ThS) (D) and of T231-phosphorylated tau pathology (t8 = 2.382, p = 0.0444) (E) in brain cortex from Tg mice administered Veh or CA for 4 months.
(F) Summary heatmap highlighting the extent of pathology reduction upon CMA activation in cortex and dorsal hippocampus. * highlights significant difference between Veh and CA administered Tg mice.
(G) Immunoblot for S202/T205-phosphorylated (AT8) tau in the brain of Tg mice treated with Veh or CA. Montages of individual images of the dorsal hippocampus from the scanning of whole-brain slices are shown. Representative immunoblot (left) and densitometric quantification (t8 = 2.425, p = 0.0415) (right). Values were normalized to ponceau and are expressed as folds of Veh treated mice.
(H) Number of GFAP-positive astrocytes per area unit (t8 = 4.058, p = 0.0036) (related to Figure 8Q)
(I-K) Representative images of immunostaining of inflammation (microglial marker: Iba1 - Green), amyloid pathology (MOAB2 – Magenta) and nuclear staining with DAPI (blue) in the dorsal hippocampus of Tg mice administered Veh or CA. Scale bar: 200μm (I). Quantification of number of Iba1-positive microglial cells per area unit (t8 = 2.041, p = 0.0755) (left) and mean cell size (t8 = 2.079, p = 0.0713) (right) (J) and colocalization coefficient between microglia (Iba1) and amyloid plaques (MOAB2) (K) (Mann Whitney U = 6, p = 0.2571).
Mean ± s.e.m. and individual values are shown. Comparisons were made using unpaired t test analysis. *p < 0.05, **p < 0.005. See also Data S1.
Highlights.
Blockage of CMA in neurons leads to proteotoxicity and neuronal dysfunction
CMA regulates a specific neuronal subproteome at risk of aggregation
CMA loss and disease-driven proteotoxicity are synergistic on proteome collapse
Chemical enhancement of CMA ameliorates proteotoxicity-driven neurodegeneration
ACKNOWLEDGMENTS
We appreciated help from Dr. Y. Cooper with the CMA activation score, Dr. P. Kirchner with dataset handling, Dr. J.J. Bravo-Cordero with actin cytoskeleton analysis, Dr. A.R. Saez with confocal microscopy, Dr. E. Bejarano with immunoblotting, and Dr. L. Ozmen with breeding design. This work was supported by the National Institutes of Health (grants AG054108, AG021904, AG017617, and AG038072 to A.M.C.; grant AG031782 to A.M.C., L.S., and E.G.; grant NS100717 to A.M.C. and N.J.K.; grant NS095435 to D.S.; and grants P30 CA013330 and 1S10OD016305 [for chemical synthesis] and T32 GM007491 [for support] to Y.R.J.). This work was also supported by the JPB Foundation (A.M.C. and D.S.), the Rainwater Charitable Foundation (A.M.C. and E.G.), the Michael J. Fox Foundation (A.M.C. and E.G.), the Backus Foundation (to A.M.C.), Ramon Areces (fellowships to A.M.-S.), MINECO (grant SAF78666R), Charles H. Revson (grant CP13–00234 to J.A.R.-N.), Fulbright MINECC (I.T.), EMBO (N.J.S.), the Danish Council DFF (N.J.S.), and Fundación Tatiana Pérez de Guzmán el Bueno (E.L.).
Footnotes
DECLARATION OF INTERESTS
A.M.C. and E.G. are cofounders and scientific advisors for Life Bioscience. A.M.C. consults for Generian Therapeutics and Cognition. N.J.K. consults for Maze Therapeutics and Interline Therapeutics, received research support from Vir Biotechnology and F. Hoffmann-La Roche, and is a shareholder of Tenaya Therapeutics. The remaining authors declare no competing interests. CA compound is under US patent US9512092 (E.G., A.M.C., and Q.X.).
SUPPLEMENTAL INFORMATION
Supplemental information can be found online at https://doi.org/10.1016/j.cell.2021.03.048.
REFERENCES
- Alberi L, Liu S, Wang Y, Badie R, Smith-Hicks C, Wu J, Pierfelice TJ, Abazyan B, Mattson MP, Kuhl D, et al. (2011). Activity-induced Notch signaling in neurons requires Arc/Arg3.1 and is essential for synaptic plasticity in hippocampal networks. Neuron 69, 437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Seow Y, Schapira AH, Rodriguez-Oroz MC, Obeso JA, and Cooper JM (2013). Influence of microRNA deregulation on chaperone-mediated autophagy and a-synuclein pathology in Parkinson’s disease. Cell Death Dis. 4, e545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anguiano J, Garner TP, Mahalingam M, Das BC, Gavathiotis E, and Cuervo AM (2013). Chemical modulation of chaperone-mediated autophagy by retinoic acid derivatives. Nat. Chem. Biol. 9, 374–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aniento F, Roche E, Cuervo AM, and Knecht E (1993). Uptake and degradation of glyceraldehyde-3-phosphate dehydrogenase by rat liver lysosomes. J. Biol. Chem. 268, 10463–10470. [PubMed] [Google Scholar]
- Bai B, Wang X, Li Y, Chen PC, Yu K, Dey KK, Yarbro JM, Han X, Lutz BM, Rao S, et al. (2020). Deep Multilayer Brain Proteomics Identifies Molecular Networks in Alzheimer’s Disease Progression. Neuron 106, 700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdenx M, Dovero S, Engeln M, Bido S, Bastide MF, Dutheil N, Vollenweider I, Baud L, Piron C, Grouthier V, et al. (2015). Lack of additive role of ageing in nigrostriatal neurodegeneration triggered by α-synuclein overexpression. Acta Neuropathol. Commun. 3, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdenx M, Koulakiotis NS, Sanoudou D, Bezard E, Dehay B, and Tsarbopoulos A (2017). Protein aggregation and neurodegeneration in prototypical neurodegenerative diseases: Examples of amyloidopathies, tauopathies and synucleinopathies. Prog. Neurobiol. 155, 171–193. [DOI] [PubMed] [Google Scholar]
- Caballero B, Wang Y, Diaz A, Tasset I, Juste YR, Stiller B, Mandelkow EM, Mandelkow E, and Cuervo AM (2018). Interplay of pathogenic forms of human tau with different autophagic pathways. Aging Cell 17, e12692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataldo AM, and Nixon RA (1990). Enzymatically active lysosomal proteases are associated with amyloid deposits in Alzheimer brain. Proc. Natl. Acad. Sci. USA 87, 3861–3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataldo AM, Paskevich PA, Kominami E, and Nixon RA (1991). Lysosomal hydrolases of different classes are abnormally distributed in brains of patients with Alzheimer disease. Proc. Natl. Acad. Sci. USA 88, 10998–11002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen EY, Tan CM, Kou Y, Duan Q, Wang Z, Meirelles GV, Clark NR, and Ma’ayan A (2013). Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinformatics 14, 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Drombosky KW, Hou Z, Sari L, Kashmer OM, Ryder BD, Perez VA, Woodard DR, Lin MM, Diamond MI, and Joachimiak LA (2019). Tau local structure shields an amyloid-forming motif and controls ag gregation propensity. Nat. Commun. 10, 2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Li Y, Parada LF, and Sisodia SS (2009). Regulation of hippocampal progenitor cell survival, proliferation and dendritic development by BDNF. Mol. Neurodegener. 4, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi M, Chang CY, Clough T, Broudy D, Killeen T, MacLean B, and Vitek O (2014). MSstats: an R package for statistical analysis of quantitative mass spectrometry-based proteomic experiments. Bioinformatics 30, 2524–2526. [DOI] [PubMed] [Google Scholar]
- Ciryam P, Tartaglia GG, Morimoto RI, Dobson CM, and Vendruscolo M (2013). Widespread aggregation and neurodegenerative diseases are associated with supersaturated proteins. Cell Rep. 5, 781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colas J, Chessel N, Ouared A, Gruz-Gibelli E, Marin P, Herrmann FR, and Savioz A (2020). Neuroprotection against Amyloid-β-Induced DNA Double-Strand Breaks Is Mediated by Multiple Retinoic Acid-Dependent Pathways. Neural Plast. 2020, 9369815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J, Hein MY, Luber CA, Paron I, Nagaraj N, and Mann M (2014). Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Mol. Cell. Proteomics 13, 2513–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crameri F, Shephard GE, and Heron PJ (2020). The misuse of colour in science communication. Nat. Commun. 11, 5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuervo AM, and Dice JF (2000). Regulation of lamp2a levels in the lysosomal membrane. Traffic 1, 570–583. [DOI] [PubMed] [Google Scholar]
- Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, and Sulzer D (2004). Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science 305, 1292–1295. [DOI] [PubMed] [Google Scholar]
- Dehay B, Bourdenx M, Gorry P, Przedborski S, Vila M, Hunot S, Singleton A, Olanow CW, Merchant KM, Bezard E, et al. (2015). Targeting α-synuclein for treatment of Parkinson’s disease: mechanistic and thera peutic considerations. Lancet Neurol. 14, 855–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch EW, Csordas A, Sun Z, Jarnuczak A, Perez-Riverol Y, Ternent T, Campbell DS, Bernal-Llinares M, Okuda S, Kawano S, et al. (2017). The ProteomeXchange consortium in 2017: supporting the cultural change in proteomics public data deposition. Nucleic Acids Res. 45 (D1), D1100–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong S, Aguirre-Hernandez C, Scrivo A, Eliscovich C, Arias E, Bravo-Cordero JJ, and Cuervo AM (2020). Monitoring spatiotemporal changes in chaperone-mediated autophagy in vivo. Nat. Commun. 11, 645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas PM, and Dillin A (2010). Protein homeostasis and aging in neurodegeneration. J. Cell Biol. 190, 719–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban-Martínez L, Sierra-Filardi E, McGreal RS, Salazar-Roa M, Mariño G, Seco E, Durand S, Enot D, Graña O, Malumbres M, et al. (2017). Programmed mitophagy is essential for the glycolytic switch during cell differentiation. EMBO J. 36, 1688–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Dong J, Zhong S, Wei Y, Wu Q, Yan L, Yong J, Sun L, Wang X, Zhao Y, et al. (2018). Spatial transcriptomic survey of human embryonic cerebral cortex by single-cell RNA-seq analysis. Cell Res. 28, 730–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng R, Rampon C, Tang YP, Shrom D, Jin J, Kyin M, Sopher B, Miller MW, Ware CB, Martin GM, et al. (2001). Deficient neurogenesis in forebrain-specific presenilin-1 knockout mice is associated with reduced clearance of hippocampal memory traces. Neuron 32, 911–926. [DOI] [PubMed] [Google Scholar]
- Fernagut PO, Diguet E, Labattu B, and Tison F (2002). A simple method to measure stride length as an index of nigrostriatal dysfunction in mice. J. Neurosci. Methods 113, 123–130. [DOI] [PubMed] [Google Scholar]
- Freer R, Sormanni P, Vecchi G, Ciryam P, Dobson CM, and Vendruscolo M (2016). A protein homeostasis signature in healthy brains recapitulates tissue vulnerability to Alzheimer’s disease. Sci. Adv. 2, e1600947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H, Possenti A, Freer R, Nakano Y, Hernandez Villegas NC, Tang M, Cauhy PVM, Lassus BA, Chen S, Fowler SL, et al. (2019). A tau homeostasis signature is linked with the cellular and regional vulnerability of excitatory neurons to tau pathology. Nat. Neurosci. 22, 47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulga TA, Elson-Schwab I, Khurana V, Steinhilb ML, Spires TL, Hyman BT, and Feany MB (2007). Abnormal bundling and accumulation of F-actin mediates tau-induced neuronal degeneration in vivo. Nat. Cell Biol. 9, 139–148. [DOI] [PubMed] [Google Scholar]
- Gough NR, Hatem CL, and Fambrough DM (1995). The family of LAMP-2 proteins arises by alternative splicing from a single gene: characterization of the avian LAMP-2 gene and identification of mammalian homologs of LAMP-2b and LAMP-2c. DNA Cell Biol. 14, 863–867. [DOI] [PubMed] [Google Scholar]
- Grubman A, Chew G, Ouyang JF, Sun G, Choo XY, McLean C, Simmons RK, Buckberry S, Vargas-Landin DB, Poppe D, et al. (2019). A single-cell atlas of entorhinal cortex from individuals with Alzheimer’s disease reveals cell-type-specific gene expression regulation. Nat. Neurosci. 22, 2087–2097. [DOI] [PubMed] [Google Scholar]
- Grueninger F, Bohrmann B, Czech C, Ballard TM, Frey JR, Weidensteiner C, von Kienlin M, and Ozmen L (2010). Phosphorylation of Tau at S422 is enhanced by Abeta in TauPS2APP triple transgenic mice. Neurobiol. Dis. 37, 294–306. [DOI] [PubMed] [Google Scholar]
- Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, and Mizushima N (2006). Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 441, 885–889. [DOI] [PubMed] [Google Scholar]
- Hernandez I, Luna G, Rauch JN, Reis SA, Giroux M, Karch CM, Boctor D, Sibih YE, Storm NJ, Diaz A, et al. (2019). A farnesyltransferase inhibitor activates lysosomes and reduces tau pathology in mice with tauopathy. Sci. Transl. Med. 11, eaat3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano A (1994). Hirano bodies and related neuronal inclusions. Neuropathol. Appl. Neurobiol. 20, 3–11. [DOI] [PubMed] [Google Scholar]
- Holmes BB, Furman JL, Mahan TE, Yamasaki TR, Mirbaha H, Eades WC, Belaygorod L, Cairns NJ, Holtzman DM, and Diamond MI (2014). Proteopathic tau seeding predicts tauopathy in vivo. Proc. Natl. Acad. Sci. USA 111, E4376–E4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter JD (2007). Matplotlib: A 2D graphics environment. Comput. Sci. Eng. 9, 90–95. [Google Scholar]
- Jellinger KA (2009). Recent advances in our understanding of neurodegeneration. J. Neural Transm. (Vienna) 116, 1111–1162. [DOI] [PubMed] [Google Scholar]
- Jicha GA, Bowser R, Kazam IG, and Davies P (1997). Alz-50 and MC-1, a new monoclonal antibody raised to paired helical filaments, recognize conformational epitopes on recombinant tau. J. Neurosci. Res. 48, 128–132. [DOI] [PubMed] [Google Scholar]
- Johnson ECB, Dammer EB, Duong DM, Ping L, Zhou M, Yin L, Higginbotham LA, Guajardo A, White B, Troncoso JC, et al. (2020). Large-scale proteomic analysis of Alzheimer’s disease brain and cerebrospinal fluid reveals early changes in energy metabolism associated with microglia and astrocyte activation. Nat. Med. 26, 769–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E, Oliphant T, and Peterson P (2001). SciPy: Open Source Scientific Tools for Python.. [Google Scholar]
- Kaufman SK, Sanders DW, Thomas TL, Ruchinskas AJ, Vaquer-Alicea J, Sharma AM, Miller TM, and Diamond MI (2016). Tau Prion Strains Dictate Patterns of Cell Pathology, Progression Rate, and Regional Vulnerability In Vivo. Neuron 92, 796–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik S, and Cuervo AM (2018). The coming of age of chaperone-mediated autophagy. Nat. Rev. Mol. Cell Biol. 19, 365–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiffin R, Christian C, Knecht E, and Cuervo AM (2004). Activation of chaperone-mediated autophagy during oxidative stress. Mol. Biol. Cell 15, 4829–4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kins S, Crameri A, Evans DR, Hemmings BA, Nitsch RM, and Gotz J (2001). Reduced protein phosphatase 2A activity induces hyperphosphorylation and altered compartmentalization of tau in transgenic mice. J. Biol. Chem. 276, 38193–38200. [DOI] [PubMed] [Google Scholar]
- Kirchner P, Bourdenx M, Madrigal-Matute J, Tiano S, Diaz A, Bartholdy BA, Will B, and Cuervo AM (2019). Proteome-wide analysis of chaperone-mediated autophagy targeting motifs. PLoS Biol. 17, e3000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga H, Martinez-Vicente M, Macian F, Verkhusha VV, and Cuervo AM (2011). A photoconvertible fluorescent reporter to track chaperone-mediated autophagy. Nat. Commun. 2, 386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, and Tanaka K (2006). Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 441, 880–884. [DOI] [PubMed] [Google Scholar]
- Kraft C, Peter M, and Hofmann K (2010). Selective autophagy: ubiquitin-mediated recognition and beyond. Nat. Cell Biol. 12, 836–841. [DOI] [PubMed] [Google Scholar]
- Kuleshov MV, Jones MR, Rouillard AD, Fernandez NF, Duan Q, Wang Z, Koplev S, Jenkins SL, Jagodnik KM, Lachmann A, et al. (2016). Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 44 (W1), W90–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundra R, Ciryam P, Morimoto RI, Dobson CM, and Vendruscolo M (2017). Protein homeostasis of a metastable subproteome associated with Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 114, E5703–E5711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leger M, Quiedeville A, Bouet V, Haelewyn B, Boulouard M, Schumann-Bard P, and Freret T (2013). Object recognition test in mice. Nat. Protoc. 8, 2531–2537. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, and Randall RJ (1951). Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–275. [PubMed] [Google Scholar]
- Lv L, Li D, Zhao D, Lin R, Chu Y, Zhang H, Zha Z, Liu Y, Li Z, Xu Y, et al. (2011). Acetylation targets the M2 isoform of pyruvate kinase for degradation through chaperone-mediated autophagy and promotes tumor growth. Mol. Cell 42, 719–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak SK, McCormack AL, Manning-Bog AB, Cuervo AM, and Di Monte DA (2010). Lysosomal degradation of alpha-synuclein in vivo. J. Biol. Chem. 285, 13621–13629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiarini L, Sathasivam K, Seller M, Cozens B, Harper A, Hetherington C, Lawton M, Trottier Y, Lehrach H, Davies SW, and Bates GP (1996). Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell 87, 493–506. [DOI] [PubMed] [Google Scholar]
- Marx V (2019). A dream of single-cell proteomics. Nat. Methods 16, 809–812. [DOI] [PubMed] [Google Scholar]
- Massey AC, Kaushik S, Sovak G, Kiffin R, and Cuervo AM (2006). Consequences of the selective blockage of chaperone-mediated autophagy. Proc. Natl. Acad. Sci. USA 103, 5805–5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathys H, Davila-Velderrain J, Peng Z, Gao F, Mohammadi S, Young JZ, Menon M, He L, Abdurrob F, Jiang X, et al. (2019). Single-cell transcriptomic analysis of Alzheimer’s disease. Nature 570, 332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies FM, Fleming A, Caricasole A, Bento CF, Andrews SP, Ashkenazi A, Füllgrabe J, Jackson A, Jimenez Sanchez M, Karabiyik C, et al. (2017). Autophagy and Neurodegeneration: Pathogenic Mechanisms and Therapeutic Opportunities. Neuron 93, 1015–1034. [DOI] [PubMed] [Google Scholar]
- Merico D, Isserlin R, Stueker O, Emili A, and Bader GD (2010). Enrichment map: a network-based method for gene-set enrichment visualization and interpretation. PLoS ONE 5, e13984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miedel CJ, Patton JM, Miedel AN, Miedel ES, and Levenson JM (2017). Assessment of Spontaneous Alternation, Novel Object Recognition and Limb Clasping in Transgenic Mouse Models of Amyloid-b and Tau Neuropathology. J. Vis. Exp. (123), 55523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min S-W, Chen X, Tracy TE, Li Y, Zhou Y, Wang C, Shirakawa K, Minami SS, Defensor E, Mok SA, et al. (2015). Critical role of acetylation in tau-mediated neurodegeneration and cognitive deficits. Nat. Med. 21, 1154–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto RI, and Cuervo AM (2014). Proteostasis and the aging proteome in health and disease. J. Gerontol. A Biol. Sci. Med. Sci. 69 (Suppl 1), S33–S38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L (2013). Glucose metabolism in normal aging and Alzheimer’s disease: Methodological and physiological considerations for PET studies. Clin. Transl. Imaging 1, e1–e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KE, Gysbers AM, Abbott SK, Spiro AS, Furuta A, Cooper A, Garner B, Kabuta T, and Halliday GM (2015). Lysosomal-associated membrane protein 2 isoforms are differentially affected in early Parkinson’s disease. Mov. Disord. 30, 1639–1647. [DOI] [PubMed] [Google Scholar]
- Nixon RA (2013). The role of autophagy in neurodegenerative disease. Nat. Med. 19, 983–997. [DOI] [PubMed] [Google Scholar]
- Nixon RA, and Yang DS (2011). Autophagy failure in Alzheimer’s disease–locating the primary defect. Neurobiol. Dis. 43, 38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orenstein SJ, Kuo SH, Tasset I, Arias E, Koga H, Fernandez-Carasa I, Cortes E, Honig LS, Dauer W, Consiglio A, et al. (2013). Interplay of LRRK2 with chaperone-mediated autophagy. Nat. Neurosci. 16, 394–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang S, Chen D, Zhang A, Qin X, and Yan B (2012). Genetic analysis of the LAMP-2 gene promoter in patients with sporadic Parkinson’s disease. Neurosci. Lett. 526, 63–67. [DOI] [PubMed] [Google Scholar]
- Pascovici D, Handler DC, Wu JX, and Haynes PA (2016). Multiple testing corrections in quantitative proteomics: A useful but blunt tool. Proteomics 16, 2448–2453. [DOI] [PubMed] [Google Scholar]
- Pennington ZT, Dong Z, Feng Y, Vetere LM, Page-Harley L, Shuman T, and Cai DJ (2019). ezTrack: An open-source video analysis pipeline for the investigation of animal behavior. Sci. Rep. 9, 19979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrocola F, Galluzzi L, Bravo-San Pedro JM, Madeo F, and Kroemer G (2015). Acetyl coenzyme A: a central metabolite and second messenger. Cell Metab. 21, 805–821. [DOI] [PubMed] [Google Scholar]
- Plaisier SB, Taschereau R, Wong JA, and Graeber TG (2010). Rank-rank hypergeometric overlap: identification of statistically significant overlap between gene-expression signatures. Nucleic Acids Res. 38, e169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothaug M, Stroobants S, Schweizer M, Peters J, Zunke F, Allerding M, D’Hooge R, Saftig P, and Blanz J (2015). LAMP-2 deficiency leads to hippocampal dysfunction but normal clearance of neuronal substrates of chaperone-mediated autophagy in a mouse model for Danon disease. Acta Neuropathol. Commun. 3, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador N, Aguado C, Horst M, and Knecht E (2000). Import of a cytosolic protein into lysosomes by chaperone-mediated autophagy depends on its folding state. J. Biol. Chem. 275, 27447–27456. [DOI] [PubMed] [Google Scholar]
- Schneider JL, Suh Y, and Cuervo AM (2014). Deficient chaperone-mediated autophagy in liver leads to metabolic dysregulation. Cell Metab. 20, 417–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JL, Villarroya J, Diaz-Carretero A, Patel B, Urbanska AM, Thi MM, Villarroya F, Santambrogio L, and Cuervo AM (2015). Loss of hepatic chaperone-mediated autophagy accelerates proteostasis failure in aging. Aging Cell 14, 249–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrivo A, Bourdenx M, Pampliega O, and Cuervo AM (2018). Selective autophagy as a potential therapeutic target for neurodegenerative disorders. Lancet Neurol. 17, 802–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settembre C, Di Malta C, Polito VA, Garcia Arencibia M, Vetrini F, Erdin S, Erdin SU, Huynh T, Medina D, Colella P, et al. (2011). TFEB links autophagy to lysosomal biogenesis. Science 332, 1429–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Yamada K, Liddelow SA, Smith ST, Zhao L, Luo W, Tsai RM, Spina S, Grinberg LT, Rojas JC, et al. ; Alzheimer’s Disease Neuroimaging Initiative (2017). ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature 549, 523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JL, de la Torre-Ubieta L, Tian Y, Parikshak NN, Hernández IA, Marchetto MC, Baker DK, Lu D, Hinman CR, Lowe JK, et al. (2014). A quantitative framework to evaluate modeling of cortical development by neural stem cells. Neuron 83, 69–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, and Mesirov JP (2005). Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 102, 15545–15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Iba M, Inoue H, Higuchi M, Takao K, Tsukita K, Karatsu Y, Iwamoto Y, Miyakawa T, Suhara T, et al. (2011). P301S mutant human tau transgenic mice manifest early symptoms of human tauopathies with dementia and altered sensorimotor gating. PLoS ONE 6, e21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Guhde G, Suter A, Eskelinen EL, Hartmann D, Lüllmann-Rauch R, Janssen PM, Blanz J, von Figura K, and Saftig P (2000). Accumulation of autophagic vacuoles and cardiomyopathy in LAMP-2-deficient mice. Nature 406, 902–906. [DOI] [PubMed] [Google Scholar]
- Team, R.C. (2018). R: A language and environment for statistical computing (R Foundation for Statistical Computing). [Google Scholar]
- Valdor R, Mocholi E, Botbol Y, Guerrero-Ros I, Chandra D, Koga H, Gravekamp C, Cuervo AM, and Macian F (2014). Chaperone-mediated autophagy regulates T cell responses through targeted degradation of negative regulators of T cell activation. Nat. Immunol. 15, 1046–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Walt S, Colbert SC, and Varoquaux G (2011). The NumPy Array: A Structure for Efficient Numerical Computation. Comput. Sci. Eng. 13, 22–30. [Google Scholar]
- Vidoni C, Follo C, Savino M, Melone MAB, and Isidoro C (2016). The Role of Cathepsin D in the Pathogenesis of Human Neurodegenerative Disorders. Med. Res. Rev. 36, 845–870. [DOI] [PubMed] [Google Scholar]
- Vogel C, and Marcotte EM (2012). Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 13, 227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, and Frye CA (2007). The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2, 322–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Martinez-Vicente M, Krüger U, Kaushik S, Wong E, Mandelkow EM, Cuervo AM, and Mandelkow E (2009). Tau fragmentation, aggregation and clearance: the dual role of lysosomal processing. Hum. Mol. Genet. 18, 4153–4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhang C, Szábo G, and Sun QQ (2013). Distribution of CaMKIIα expression in the brain in vivo, studied by CaMKIIα-GFP mice. Brain Res. 1518, 9–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf FA, Angerer P, and Theis FJ (2018). SCANPY: large-scale single-cell gene expression data analysis. Genome Biol. 19, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xilouri M, Vogiatzi T, Vekrellis K, Park D, and Stefanis L (2009). Abberant alpha-synuclein confers toxicity to neurons in part through inhibition of chaperone-mediated autophagy. PLoS ONE 4, e5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xilouri M, Brekk OR, Landeck N, Pitychoutis PM, Papasilekas T, Papadopoulou-Daifoti Z, Kirik D, and Stefanis L (2013). Boosting chaperone-mediated autophagy in vivo mitigates α-synuclein-induced neurodegeneration. Brain 136, 2130–2146. [DOI] [PubMed] [Google Scholar]
- Xilouri M, Brekk OR, Polissidis A, Chrysanthou-Piterou M, Kloukina I, and Stefanis L (2016). Impairment of chaperone-mediated autophagy induces dopaminergic neurodegeneration in rats. Autophagy 12, 2230–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiyama Y, Higuchi M, Zhang B, Huang SM, Iwata N, Saido TC, Maeda J, Suhara T, Trojanowski JQ, and Lee VM (2007). Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron 53, 337–351. [DOI] [PubMed] [Google Scholar]
- Yu A, Shibata Y, Shah B, Calamini B, Lo DC, and Morimoto RI (2014). Protein aggregation can inhibit clathrin-mediated endocytosis by chaperone competition. Proc. Natl. Acad. Sci. USA 111, E1481–E1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, and Cuervo AM (2008). Restoration of chaperone-mediated autophagy in aging liver improves cellular maintenance and hepatic function. Nat. Med. 14, 959–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Mice with CMA blockage display behavioral impairments, related to Figures 1 and S2
(A) Schematic of the generation of mice with systemic (whole body; L2A−/−) or neuronal-specific (CaMKinase-II a Cre; CKL2A−/−) deletion of LAMP2A (L2A).
(B and C) Immunoblots for L2A, L2B and LAMP1 (L1, loading control) in cortex from control (CTR), L2A−/− and CKL2A−/− mice (B). Quantification of L2A (left) and LA2B (right) after normalization for L1 (C). [Genotype effect: F2,13 = 214.3; p < 0.0001].
(D) RT-PCR quantification of L2A and L2C mRNA in cortex from CTR, L2A−/− and CKL2A−/− mice. [Genotype effect: F2, 25 = 19.32, p < 0.0001].
(A) Body weight change over time in WT, L2A−/−, and CKL2A−/− mice.
(B) Kaplan-Meier curve of survival of WT, L2A−/−, and CKL2A−/− mice.
(G-I) Clasping in CTR, L2A−/− and CKL2A−/− mice. Representative pictures of the indicated scores (G), age-dependent change in clasping score [genotype effect: F2, 63 = 18.24, p < 0.0001] (H) and mean linear regression coefficient (±95% C.I.) on clasping scores (I).
(J) Negative geotaxis test in CTR, L2A−/− and CKL2A−/− mice at 6 months. Quantification of latency. [Kruskal-Wallis statistic = 9.987, p = 0.0018].
(K,L) Short-term memory Y test in 6 months old CTR, L2A−/− and CKL2A−/− mice. Quantification of the fraction of time spent in the novel arm (J) [F2, 12 = 4.775, p = 0.0298] (K) and representative tracks (L).
(M, N) Spontaneous locomotion in an open field in CTR, L2A−/− and CKL2A−/− mice at 6months. Representative tracks (M) and quantification of the distance walked over 10minutes (N). [Kruskal-Wallis statistic = 9.006, p = 0.0046].
(O, P) Stride length in CTR, L2A−/− and CKL2A−/− mice at 6months. Representative pictures of stride length (O) and quantification (P). [Genotype effect: F2, 79 = 35.84, p < 0.0001].
(Q) Spatial working memory measured at 6 months by the percentage of spontaneous alternation in the Y maze fro WT, L2A−/−, and CKL2A−/− mice.
(R, S) Nest building test in 6 months old WT, L2A−/−, and CKL2A−/− mice. Representative pictures per genotype (R) and quantification (S) are shown. [Genotype effect, Kruskal-Wallis statistic = 8.814; p = 0.0035]
All data are mean ± s.e.m. and individual values are shown in C, D, N, J, K, P, Q, and S. n = 4-8 per time point/genotype. Comparisons were made using Kruskal-Wallis test followed by Dunn’s post hoc test (J, N, Q, S), one-way ANOVA followed by Tukey’s post hoc test (C, D, K, P) or two way ANOVA followed by Sidak’s post hoc test (H). *p < 0.05, **p < 0.005, ***p < 0.0005. See also Data S1.
Figure S2. Consequence of systemic CMA blockage in brain proteostasis, related to Figures 1 and S1
(A-D) Nuclear staining with DAPI to assess thickness and area of different brain regions in 6 months old CTR and L2A−/− mice. Representative images (left) and quantification of region thickness of somatosensory cortex (A), CA1 (B) and dentate gyrus (C). Hippocampal surface in L2A−/− mice relative to CTR mice is shown in D. Montages of individual images from the scanning of the region of interests in brain slices are shown in A-C.
(E, F) Astrocyte (GFAP) (E) and microglial (Iba1) (F) staining in CA1 of 6 months old WT and L2A−/− mice. Left: representative images. Right: quantifications of number of positive cells per field. Scale bar: 50μm.
(G-I) Lipofuscin autofluorescence (G) and immunostaining for K63-linkage ubiquitin (H,I) in hippocampal neurons of 6 months old CTR and L2A−/− mice. Representative images (left) and quantification of number of fluorescent puncta per cell [t8 = 4.012, p = 0.0039] (right G) or staining intensity distribution per cell body [t198 = 9.945, p = p < 0.0001] (right H). Scale bar: 20μm (G) 50μm (H). n = 20 cells per mice, five mice per genotype. (I) Higher magnification and XZ/YZ projections of immunostaining for K63-linkage ubiquitin to illustrate presence of protein aggregates (white arrowhead) in the CA3 region (left) and cellular area occupied by ubiquitin-positive aggregates (right). Overall cumulative distribution [Mann Whitney U = 1209, p < 0.0001] and mean per animal is shown as inset [t8 = 4.398, p = 0.023]. Scale bar: 10μm.
(J, K) Status of macroautophagy in cortex form WT and L2A−/− mice. Immunoblot for the indicated autophagosome components (LC3, GATE16), the macroautophagy receptor (p62) and the lysosomal membrane protein (LAMP1) (J) and densitometric quantification for the indicated proteins (K). Ponceau staining is shown as loading control.
Data are mean ± s.e.m and individual values from n = 5 per group. Comparison were made using Mann Whitney U test (H) or unpaired t test (all the rest). *p < 0.05, **p < 0.005, ***p < 0.0005. See also Data S1.
Figure S3. Neuronal CMA blockage did not induce gliosis or changes in macroautophagy, related to Figure 1
(A) Astrocyte (GFAP) and microglial (Iba1) staining in CA1 of 6 months old WT and CKL2A−/− mice. Left: representative pictures. Right: quantifications of number of positive cells per field and area of positive cells (n = 30 cells per animal). Scale bar: 50μm.
(B) Immunostaining for CaMKIIα (magenta), autofluorescent lipofuscin (green) and Hoechst nuclear staining (blue) in the hippocampus of CKL2A−/− mice. Top: widefield view of CA1 region in the hippocampus; scale bar: 50μm. Bottom: (left) high magnification picture of pyramidal CaMKIIα-positive neurons in the stratum pyramidale and (right) CaMKIIα-negative cell in the stratum radiatum; scale bar: 10μm. White arrowheads indicate lipofuscin puncta.
(C) Quantification of number of fluorescent lipofuscin puncta per cell in the stratum radiatum of CTR and CKL2A−/− mice at 6 months (related to Figure 2E).
(D, E) Immunostaining for K63-linkage ubiquitin in astrocytes (D) and microglial cells (E) in the hippocampus of 6 months old CTR and CKL2A−/− mice. Representative images for the indicated antibodies and overlay of cell mask on ubiquitin staining (left). Staining intensity distribution per cell body (right top) and mean intensity per cell body per animal (right bottom). Scale bar: 10μm. n = 50-70 cells per mice, 4-5 mice per genotype.
(F) Immunoblot for 4-hydroxynonenal protein adducts in cortex of WT and CKL2A−/− mice at 6 months. Left: representative immunoblot (two mice/ genotype). Right: densitometric quantification.
(G) Distribution of KFERQ-like motifs in the insoluble protein fraction of CKL2A−/− mice compared to the whole mouse proteome. Gray: no KFERQ-like motifs; Yellow: only canonical motifs; Blue: post-translational-generated motifs (phosphorylation and acetylation).
(H-K) Status of macroautophagy in cortex from 6 months old WT and CKL2A−/− mice. Immunoblot (left) and quantification (right) for the indicated autophagosome components (LC3, GATE16), the macroautophagy receptor (p62) and the lysosomal membrane protein (LAMP1) (H). Samples from cortex and hippocampus of CKATG7−/− mice are shown as positive control of disrupted macroautophagy (I). Representative electron microscopy images of cortex (left, full field; right, examples of autophagosomes (APG) and autolysosomes (AUT)). Bottom shows morphometric quantification of number of vesicles per field (left) and percentage of autophagic vacuoles (AV) at the APG and AUT state (right) (J). Low (left) and high magnification (right) images of CKL2A−/− mice cortex to highlight accumulation of electrodense material (yellow arrows) and presence of autophagic vacuoles (green arrows) in the proximity (K). Bottom shows examples of cytosolic and membrane surrounded (inside vesicles) electrodense material (K). Scale bar: 1μm for full fields and 0.20μm for insets.
Data are mean ± s.e.m. and individual values from n = 4-5 per group. Comparisons were made using unpaired t test. See also Data S1.
Figure S4. CMA deficiency induce proteome alterations, related to Figure 2
(A) Diagonal plot of the RRHO matrix (shown in Figure 3C) showing low similarity of highly enriched proteins and high similarity of middle ranked proteins in the insoluble fraction of CMA-deficient CKL2A−/− mice compared to macroautophagy-deficient CKATG7−/− mice.
(B-C) Examples of GSEA plots associated with macroautophagy (B) or CMA (C) deficiency.
(D) Immunoblots for the indicated proteins of differentiated neuroblastoma cells transduced with lentiviral carrying empty vector (Control) or shRNA against L2A or ATG7, to confirm the knock-down in the cells used for the experiments in main Figures 3K and S4E, S4F, and S4P.$$PARABREAKHERE$$(E-F) Extracellular acidification rate (ECAR) in differentiated neuroblastoma cells control (Ctrl) or knocked down for L2A or ATG7. Changes in average ECAR values upon addition of the indicated compounds (E) and glycolytic properties calculated as the areas under the curve in ECAR plots upon the addition of the indicated compounds (F). [Knockdown effect: F2,30 = 25.45, p < 0.0001]. n = 3-5 independent experiments.
(G-J) Shift of the glycolytic enzyme PDH from the soluble to insoluble fraction. Representative immunoblot for the indicated protein/fraction from 5 CTR or L2A−/− mice at 6 months of age (G). Densitometric quantification, normalized to ponceau for: soluble PDH [t8 = 2.423, p = 0.0417] (H), insoluble PDH [Mann Whitney U = 0, p = 0.0079] (I) and ratio insoluble to soluble PDH [t8 = 3.742, p = 0.0057] (J).
(K-O) Mass spectrometry analysis of the acetylation status of the proteome of CTR and L2A−/− mice brains. Overall acetylation of the proteome; x axis error bar was estimated using bootstrap (K), overall distribution of changes in acetylation levels (l) and plots of 22 proteins that showed decreased acetylation (M) and 16 proteins that showed increased acetylation (N) in CMA-deficient mice. (O) Gene-ontology terms associated with proteins showing altered acetylation profile in CMA-deficient mice (L2A−/−). Green bars: hypoacetylated proteins in L2A−/− mice. Orange: proteins hyperacetylated in L2A−/− mice.
(P, Q) Transferrin uptake at 10 minutes in differentiated neuroblastoma cell lines transduced with empty vector (control) or shL2A construct. Representative images of GFP (Green), Transferrin (Magenta) and DAPI (Blue). Non infected cells (GFP negative) (*) and infected cells (arrowhead) are marked. Boxed areas are shown at higher magnification in main Figure 3K. Scale bar 20μm.
(Q, R) Immunostaining for actin in hippocampal neurons of 6 months old CTR and L2A−/− mice. Representative images with higher magnification inset (Q) and cumulative distribution of the mean staining intensity per cell [t118 = 6.970, p < 0.0001] (R). Scale bar: 20μm.
(S) Schematic representation illustrating the proposed mechanism. In the context of CMA deficiency, functional proteins are trapped in protein aggregates leading to loss of function.
Data are mean ± s.e.m. Comparison were made using one-way ANOVA (F), unpaired t test (H-J) or Kruskal-Wallis test followed by Dunn’s post hoc test (R). *p < 0.05, **p < 0.005, ***p < 0.0005. See also Data S1.
Figure S5. Alteration of CMA network in the brain of AD patients and validation of the CMA score, related to Figure 3
(A,B) Levels of the master transcriptional regulator of macroautophagy and lysosomes (TFEB) in major brain cell types of the frontal cortex of brains with low (Braak stage 0-II), medium (Braak stage III-IV) and advanced (Braak score V-VI) pathology. Mean TFEB count per cell (A) and normalized TFEB counts expressed as fold change between major brain cell types (B).
(C-F) Validation of the CMA score by comparing CMA activity (C) measured using the fluorescent KFERQ-Dendra reporter in NIH 3T3 cells in response to paraquat (PQ) (left) and the CMA activator (right) with the CMA score calculated from the changes in the mRNA levels of components of the CMA network at the concentration indicated with the arrow (D) n = 3 experiments. Non-linear fit curve shown in C. Transcriptional profile (E) and CMA score (F) in livers of KFERQ-Dendra mice treated with the CA activator. n = 3 mice.
(G, H) Negative correlation between CMA activation score in inhibitory neurons and pathology markers at patient level: Braak pathology staging (G), and NIA-Reagan score (H).
(I, J) CMA activation score in excitatory neuron subclusters (I) and distribution of early pathology-associated inhibition of CMA network in excitatory neurons depending on their localization within specific cortical layers (J). d is Cohen’s d measure of effect size. p indicates the p value of the unpaired t test between control and early pathology stage within a given subtype of excitatory neuron.
Data are mean ± s.e.m. Comparison were made using one-way ANOVA (B) or Kruskal-Wallis test followed by Dunn’s post hoc test (G,H). **p < 0.005, ***p < 0.0005.
Figure S6. Mouse model of CMA deficiency AD-related proteotoxicity with CMA, related to Figures 4 and 5
(A) Immunoblot for the indicated proteins in brains from control, L2A−/−, 3xTg and 3xTg-L2A−/− mice at 12 months of age. Ponceau staining of the membrane is shown as protein loading control.
(B) Immunoblot for K63-linkage of ubiquitinated proteins (left) and densitometric quantification (right) in brains of CTR, L2A−/−, Tg and Tg-L2A−/− mice at 12 months [Genotype effect: F3,32 = 5.615, p = 0.0033]. n = 9 mice per genotype.
(C-E) Volcano plot of the quantitative proteomic analysis of brain from L2A−/− (C), Tg (D) and Tg-L2A−/− (E) compared with CTR mice brains. The number in the top left corner indicates the number of significant hits. Red dots: differentially expressed proteins (adj. p < 0.05).
(F-H) Whole proteome comparison between human asymptomatic AD cases from the Consensus study and L2A−/−, Tg and Tg-L2A−/− mice. The human cases were normalized to cognitively control subjects (AD-Control), whereas L2A−/−, Tg and Tg-L2A−/− results were normalized to CTR mice. Blue line indicates the linear regression (+/− 95% confidence interval) between human and mouse values. Numbers in the top left corner are the regression coefficient. P values: (F) p = 0.0014, (G) p = 0.024, (H) p = 1.83×10−8
(I) Status of macroautophagy in cortex from 6 months old CTR, L2A−/−, Tg and Tg-L2A−/− mice. Immunoblot for the indicated autophagosome components (LC3, GATE16), the macroautophagy receptor (p62) and the lysosomal components (LAMP1, GBA, Pro and mature CathD)(left). Densitometric quantification for the indicated proteins normalized to ponceau (right). (n = 9 mice per genotype).
(J) beta-Hexosaminidase assay in total homogenates from cortex of 12 months old L2A−/−, Tg and Tg-L2A−/− mice [F3,20 = 9.833, p = 0.0003]. n = 6 mice per genotype.
(K) Immunoblots for the indicated proteins from subcellular fractionation of cortex from L2A−/−, Tg and Tg-L2A−/− mice. Fractions are total homogenates (Hom), cytosol (Cyt.), a population of lysosome active for CMA (CMA+) and a population of lysosomes inactive for CMA (CMA−).
(L,M) Status of the CMA network in cortex from 6 months old CTR, L2A−/−, Tg and Tg-L2A−/− mice. Immunoblot for the indicated CMA effectors (Hsc70, Hsp90 and Hsp40), CMA lysosomal modulators (Rac1, PHLPP1, Rictor and CathA) and CMA extralysosomal modulators (Rab11 and RARα) (L). Densitometric quantification for the indicated proteins normalized to ponceau (M). (n = 6 mice per genotype).
Data are mean ± s.e.m. and individual values. Comparisons were made using One-way ANOVA followed by Tukey post hoc analysis (B, I, J, L) or Kruskal Wallis test followed by Dunn’s post hoc analysis (C-H). *p < 0.05, **p < 0.005. See also Data S1.
Figure S7. Characterization of the brain-permeable chemical activator of CMA and effect in a mouse model of frontotemporal-dementia-related proteotoxicity, related to Figures 6 and 7
(A) Chemical structure of AR7 and CA77.1.
(B) Quantification of the effect of adding increasing concentrations of AR7 and CA77.1 to NIH 3T3 cells stably expressing the KFERQ-PS-Dendra reporter. CMA activity was quantified after 16h treatment as the average of fluorescent puncta per cell. (n > 800 cells per condition).
(C) Autophagic flux in NIH 3T3 cells incubated at 37°C for 16h with 20 μM AR7 or CA77.1 or without additions (none). Where indicated lysosomal protease inhibitors (PI) were added to the cells 6h before lysis. Left:: representative immunoblot. Actin is shown as loading control. Right: quantification of LC3-II flux as the difference in LC3-II levels in cells treated with PI relative to untreated. Values are presented as folds those in untreated cells that were given an arbitrary value of 1. n = 5 independent experiments.
(D) Quantification of the effect of adding increasing concentrations of AR7 and CA77.1 to NIH 3T3 cells stably expressing the KFERQ-PS-Dendra reporter. CMA activity was quantified after 12h or 24h treatment as the average of fluorescent puncta per cell. (n > 800 cells per condition).
(E) Pharmacokinetic analysis of CA77.1 after p.o. (oral, 10mg/Kg bw) and i.v. (intravenous, 1mg/kg bw) administration in mice. n = 3 mice per time point.
(F) Summary of pharmacokinetic parameters of CA77.1 in mice brain.
(G) Mouse blood count at the end of a 4 months daily oral administration of vehicle (Veh) or CA77.1 (CA). n = 6 mice in each group.
(H-J) Representative images of sections of H&E stained liver (B), lung (C) and kidney (D) sections from the same mice. Two magnifications are shown. In D, details of glomeruli and tubules are shown in the higher magnification images.
(K) Distribution of four types of S202/T205-phosphorylated tau stainings described in Shi et al., 2017. Numbers indicate the number of mice belonging to each group. n = 10 mice per group.
(L-N) Immunoblot for AT8 tau on the Sarkosyl-insoluble fraction of CTR and PS19 mice administered Veh or CA for 6 months. HMW: high-molecular weight species of AT8. Representative immunoblot of 4-5 mice per genotype and treatment (L) and densitometric quantification of AT8 as monomer [Kruskal-Wallis statistic = 20.48, p < 0.0001] (M) and as HMW-species [F2,26 = 7.183, p = 0.0033] (N).
(O, P) Quantification of the number of Iba1-positive microglial cells per mm2 in the hippocampus [Kruskal-Wallis statistic = 10.55, p = 0.0051] (O) and piriform cortex (P) of CTR and PS19 mice treated with Veh or CA for 6 months. Images shown in Figure 7N.
Mean ± s.e.m and individual values. Comparisons were made using One-way ANOVA followed by Tukey post hoc analysis *p < 0.05. See also Data S1.
Figure S8. Effect of chemical activation of CMA in a mouse model of AD-related proteotoxicity, related to Figure 7
Representative images of immunostaining of immature amyloid plaques (MOAB2), mature amyloid depositions (6E10), β sheet marker (Thioflavin S) and Threonine231-phosphorylated tau (pThr231 tau) in the frontal cortex of Tg mice administered Veh or CA for 4 months. Montages of individual images of the frontal cortex from the scanning of whole-brain slices are shown. Scale bar: 200μm.
(B-E) Quantification of size of plaques positive for MOAB2 (t7 = 3.181, p = 0.0155) (B), 6E10 (C) or Thioflavin S (ThS) (D) and of T231-phosphorylated tau pathology (t8 = 2.382, p = 0.0444) (E) in brain cortex from Tg mice administered Veh or CA for 4 months.
(F) Summary heatmap highlighting the extent of pathology reduction upon CMA activation in cortex and dorsal hippocampus. * highlights significant difference between Veh and CA administered Tg mice.
(G) Immunoblot for S202/T205-phosphorylated (AT8) tau in the brain of Tg mice treated with Veh or CA. Montages of individual images of the dorsal hippocampus from the scanning of whole-brain slices are shown. Representative immunoblot (left) and densitometric quantification (t8 = 2.425, p = 0.0415) (right). Values were normalized to ponceau and are expressed as folds of Veh treated mice.
(H) Number of GFAP-positive astrocytes per area unit (t8 = 4.058, p = 0.0036) (related to Figure 8Q)
(I-K) Representative images of immunostaining of inflammation (microglial marker: Iba1 - Green), amyloid pathology (MOAB2 – Magenta) and nuclear staining with DAPI (blue) in the dorsal hippocampus of Tg mice administered Veh or CA. Scale bar: 200μm (I). Quantification of number of Iba1-positive microglial cells per area unit (t8 = 2.041, p = 0.0755) (left) and mean cell size (t8 = 2.079, p = 0.0713) (right) (J) and colocalization coefficient between microglia (Iba1) and amyloid plaques (MOAB2) (K) (Mann Whitney U = 6, p = 0.2571).
Mean ± s.e.m. and individual values are shown. Comparisons were made using unpaired t test analysis. *p < 0.05, **p < 0.005. See also Data S1.
Data Availability Statement
There are not restrictions on data availability in this manuscript. All the information is included in the manuscript. All Main and Supplementary Figures have associated raw data that is provided as an Excel worksheet organized by figures. Proteomic data is included as Data S1. Raw data files and code is available from the authors upon request.