Abstract
Diabetes mellitus (DM) is known to cause reproductive impairment. In men, it has been linked to altered sperm quality and testicular damage. Oxidative stress (OS) plays a pivotal role in the development of DM complications. Glutathione (GSH) is a part of a nonenzymatic antioxidant defense system that protects lipid, protein, and nucleic acids from oxidative damage. However, the protective effects of exogenous GSH on the male reproductive system have not been comprehensively examined. This study determined the impact of GSH supplementation in ameliorating the adverse effect of type 1 DM on sperm quality and the seminiferous tubules of diabetic C57BL/6NTac mice. GSH at the doses of 15 mg kg−1 and 30 mg kg−1 was given intraperitoneally to mice weekly for 6 consecutive weeks. The mice were then weighed, euthanized, and had their reproductive organs excised. The diabetic (D Group) showed significant impairment of sperm quality and testicular histology compared with the nondiabetic (ND Group). Diameters of the seminiferous lumen in diabetic mice treated with 15 mg kg−1 GSH (DGSH15) were decreased compared with the D Group. Sperm motility was also significantly increased in the DGSH15 Group. Improvement in testicular morphology might be an early indication of the protective roles played by the exogenous GSH in protecting sperm quality from effects of untreated type 1 DM or diabetic complications. Further investigation using different doses and different routes of GSH is necessary to confirm this suggestion.
Keywords: diabetes mellitus, glutathione, mice, sperm quality, testis histology
INTRODUCTION
Diabetes mellitus (DM) is the most common human endocrine disorder in the world. In 2019, the overall prevalence of DM among adults aged 18 years and above in Malaysia was 18.3%.1 The current trend worldwide shows that DM tends to manifest early, afflicting younger population.2,3,4,5
Oxidative stress (OS) plays a pivotal role in the development of DM complications. It results from the overproduction of free radicals and reactive oxygen species (ROS) and depletion of antioxidant defenses in cells and tissues, which may perturb cellular redox balance.6,7 OS leads to structural changes and functional modulation of macromolecules, including nucleic acids, lipids, and proteins. Oxidative degradation of lipids yields malondialdehyde (MDA) and 4-hydroxynonenal (4-HNE), while protein damage may occur with thiol oxidation, leading to loss of biological activity in the affected cells. This may underlie the pathogenesis of several diseases including DM.7 DM has been shown to be associated with male infertility, resulting from changes in sperm quality and testicular tissue damage.2,8 Excessive ROS generated in the testes and epididymides have been found to induce sperm DNA damage in murine models of diabetes.9 Thus, interventions to counteract OS may be of potential therapeutic use for protection against diabetes-associated male infertility.
The increasing incidence of type 1 DM in young males of reproductive age is a critical concern in this regard, as the altered infertility rates may compromise the quality of life. Type 1 DM lowers seminal fluid volume.10 The concentration, motility, vitality, and proportion of morphologically normal spermatozoa are also lowered in diabetes.11 Furthermore, DM has been associated with reproductive impairment in men, by causing subtle molecular changes in sperm quality and function. Diabetes-related alterations occur in the nucleus of the spermatozoa, and it has also been reported to cause mitochondrial DNA fragmentation, alter metabolism of glucose, glucose transport, and glucose transporters (GLUTs), as well as result in programmed cell death.12
It has been reported that primary exogenous antioxidants in fruits and vegetables, such as vitamins C and E, carotenoids, and phenolics complement the activity of the endogenous antioxidant defense system.7 Clinical studies have shown that a diet rich in fruit, vegetables, whole grains, legumes, and omega-3 fatty acids may prevent the occurrence of cardiovascular diseases, DM, obesity, cancers, and neurodegenerative disorders.13 Other sources of exogenous antioxidants may be dietary supplements such as vitamins, minerals, fibers, fatty acids, or amino acids. Food supplements may also comprise several antioxidants such as Vitamin A (retinoids and carotenes), Vitamins C and E (tocopherols), lycopene, lutein, ubiquinone, polyphenols (flavonoids and nonflavonoids), resveratrol, N-acetylcysteine, and glutathione (GSH).14 Among these antioxidants, GSH or its building blocks can be found in many vegetables such as asparagus, potatoes, broccoli, and bean sprouts.15
GSH plays a vital role in the nonenzymatic antioxidant defense system of the body. It is a natural antioxidant found in humans, animals, fungi, and bacteria. It is an excellent free radical scavenger for a wide variety of free radicals.16 It protects lipids, protein, and nucleic acids from oxidative damage.17 GSH is a tripeptide with a gamma-peptide linkage between the carboxyl group of the glutamate side chain and the amine group of cysteine. Thiol groups reduce disulfide bonds that are formed within the cytoplasmic proteins to cysteines by serving as an electron donor. In the process, GSH is converted to its oxidized form, glutathione disulfide (GSSG). Several studies reported the loss of endogenous GSH from the blood of diabetic patients.18,19,20 In addition, GSH deficiency has been shown to lead to the instability of the spermatozoa midpiece, thus resulting in defective motility and morphology of the spermatozoa.21,22 These changes must affect male fertility potential, although the underlying molecular mechanisms of such disturbances are not fully understood. Hence, it is necessary to explore therapeutic options. In the current study, we evaluate the efficacy of different doses of exogenous GSH in protecting against the detrimental effect of type 1 DM on the spermatozoa and testes of mice. The determination of the effective dose of GSH in this study is expected to provide a baseline for further exploration into the mechanisms underlying the possible protective effects of GSH and its development for clinical application.
MATERIALS AND METHODS
Animal care
A total of 24 male C57BL/6NTac mice (8 weeks old to 12 weeks old, 25–30 g) were maintained at the Laboratory Animal Care Unit (LACU), Universiti Teknologi MARA Sungai Buloh Campus, Sungai Buloh, Malaysia. Sample size was determined on the Resource Equation method.23 The mice were acclimatized for at least 2 weeks before treatments were started. They were housed in plastic cages containing wood chip bedding, provided with water and standard rodent maintenance diet (Altromin Spezialfutter GmbH and Co., Lage, Germany) ad libitum. Temperature and humidity were maintained at 24°C ± 3°C and 50% ± 5%, respectively, in a controlled light environment (12 h light:12 h dark). Mouse bedding was changed daily. The study was approved by the Committee of Animal Research and Ethics (UiTM CARE), Universiti Teknologi MARA, Puncak Alam, Malaysia (UiTM CARE: 240/2/2018 [6/4/2018]).
Induction of type 1 DM with streptozotocin (STZ; day 1 to day 5)
The C57BL/6NTac mice were divided into four experimental groups. Each group comprised six mice. The nondiabetic control (ND Group) received intraperitoneal 0.1 mol l−1 sodium citrate buffer (vehicle) daily for 5 consecutive days. To induce type 1 DM, the mice were injected with STZ (Sigma-Aldrich, St. Louis, MO, USA). Intraperitoneal (IP) injection of STZ at a dose of 50 mg kg−1 body weight (BW) was administered once daily for 5 consecutive days. Animals with blood glucose levels of >16.7 mmol l−1, 72 h after the final STZ injection, were considered diabetic.24 After the onset of diabetes, 18 mice were divided into three groups: D Group, DGSH15 Group and DGSH30 Group (details see below).
Exogenous GSH treatment (day 8 to day 50)
Mice in the DGSH15 and DGSH30 Groups were then treated weekly, for 6 weeks, with reduced L-glutathione (Sigma-Aldrich) starting from experimental day 8 until day 50. In these groups, 0.1 ml of GSH was administered at 15 mg kg-1 and 30 mg kg-1 BW, respectively. The doses were chosen based on the previous studies.25,26 At the same time, mice in the ND Group and D Group were injected with 0.1 ml of distilled water.
Body weight, relative organ weights, and blood glucose levels
The body weight and blood glucose levels were monitored weekly for 50 days (experimental day 1 to day 50). Nonfasting blood glucose levels were estimated via tail vein prick at noon using a handheld glucometer (Accu-Chek Active, Roche Diagnostics, Mannheim, Germany). Mice were euthanized on day 50. Their testes, seminal vesicles, and epididymides were excised and weighed. Relative organ weight was calculated by dividing organ weight by body weight.
Sperm parameter evaluation
Sperm parameters were determined on spermatozoa retrieved from the cauda epididymidis. The parameters examined were sperm number, motility, and morphology. For each parameter, three replicates were examined. All measurements were carried out by two investigators unaware of the treatment. Average of the readings from two independent investigators was taken as the final estimate for statistical comparison.
Sperm number
For spermatozoa collection, each epididymis was minced separately in 1 ml of 0.9% NaCl. Then, 10 μl epididymal suspension was transferred onto a Makler counting chamber (Sefi Medical Instruments LTD, Haifa, Israel) and observed in a light microscope (×200; BX53, Olympus, Tokyo, Japan). The number of spermatozoa in ten squares was counted and the average from triplicates determined. Sperm number was expressed in million per ml (×106 ml−1).
Sperm motility
A total of 200 spermatozoa were observed in random fields for motility and expressed as percentage of motile cells. This was recorded for all groups and reported as mean.
Sperm morphology
For sperm morphology, 200 spermatozoa per replicate per animal were assessed to determine their normality. Smears were stained with Eosin Y (Leica Biosystems Richmond Inc., Buffalo Grove, IL, USA) and spermatozoa with the following morphologies were considered to be abnormal: (i) headless, (ii) hookless, (iii) double headed, (iv) broken tail, (v) coiled tail, and (vi) double tailed.27,28
Testicular morphological evaluation
The excised testes were fixed in 10% formalin and kept at room temperature overnight. The fixed testicular tissues were dehydrated through a series of graded ethanol baths in ascending concentrations (70%, 80%, 90%, 95%, and 100%) to displace the water. The tissues were then infiltrated with wax. Tissue processing was run overnight in an automated tissue processor. The processed tissues were embedded into paraffin wax for subsequent tissue sectioning. The testicular tissue samples were sectioned into 5-μm slices on a rotary microtome and transferred onto slides. The slides were then stained with hematoxylin and eosin (H and E; Leica Biosystems Richmond Inc.) and examined. Fifty seminiferous tubules that were round or nearly round were chosen from five sections, ten tubules for each section. The diameter of the seminiferous tubule and seminiferous lumen was measured across the minor and major axes, and the mean diameter was obtained.29 The tubular diameter, luminal diameter, and height of the seminiferous tubule epithelium were captured at ×100 and ×400 magnifications using Cell D software Olympus Soft Imaging Solutions GmbH (BX53, Olympus), and the length was measured using ImageJ software (US National Institutes of Health, Bethesda, MD, USA). These data were used for morphometric measurements of the testicular morphology. All measurements were done by two investigators blind to the treatment. The average of the two readings was taken as the final estimate for statistical comparison.
Statistical analyses
One-way analysis of variance (ANOVA) with Tukey's post hoc test was performed using GraphPad Prism 8.0.1 for Windows (GraphPad Software, San Diego, CA, USA). The data were expressed as mean ± standard deviation (s.d.). A significant difference was accepted when P < 0.05.
RESULTS
Body weight, relative organ weight, and blood glucose levels
Diabetic mice treated with 30 mg kg−1 GSH showed a reduction in body weight at the end of the treatment period compared with the body weight at the start of treatment (P < 0.05; Figure 1a). None of the other groups showed a significant change in the body weight throughout the experimental period. The relative weight for both left and right testes in all treatment groups did not show difference from the ND Group (Table 1). However, a decrease was observed in the weight of seminal vesicle of the diabetic mice treated with GSH at 15 mg kg−1 and 30 mg kg−1 BW compared with the ND Group. The relative weights for the left and right epididymides in the DGSH30 Group were higher than those of the ND Group (P < 0.05). Blood glucose levels were increased in D and both DGSH-treated groups from day 15 to day 50 compared with the ND Group (Figure 1b).
Figure 1.
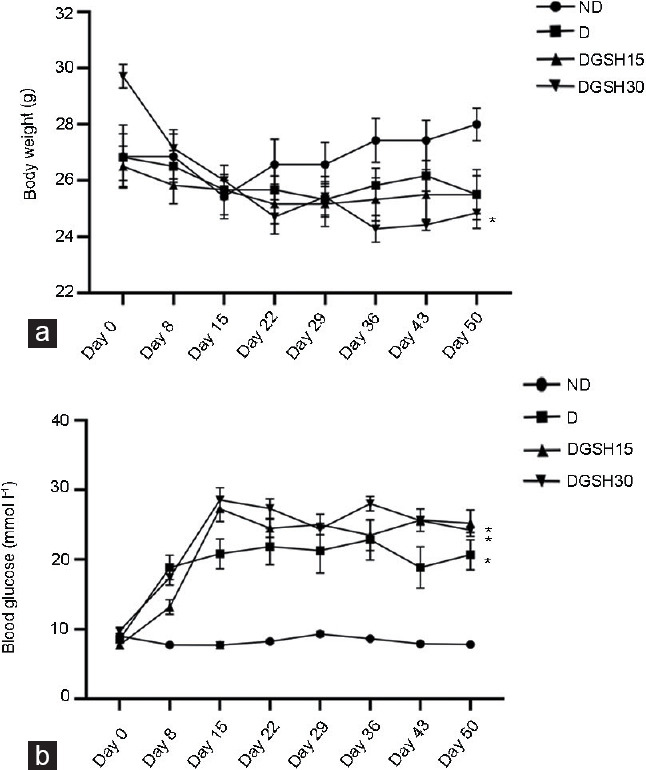
Effect of GSH on (a) body weight and (b) blood glucose level in diabetic mice after 50 days of treatment (n = 6). Values are presented as mean ± s.d. *P < 0.05, the indicated group compared to day 0. ND: nondiabetic control group; D: diabetic control group; DGSH15: diabetic mice treated with GSH at 15 mg kg−1 BW; DGSH30: diabetic mice treated with GSH at 30 mg kg−1 BW; GSH: glutathione; s.d.: standard deviation; BW: body weight.
Table 1.
Body and organ weight in mice of experimental groups (n = 6) after 50 days of treatment (mean±standard deviation)
| Group | ND | D | DGSH15 | DGSH30 |
|---|---|---|---|---|
| Initial body weight (g) | 26.91±2.97 | 28.52±2.04 | 26.25±1.76 | 29.13±1.11 |
| Final body weight (g) | 28.02±1.53 | 26.33±1.64 | 23.03±2.17 | 24.91±1.46 |
| Weight gain (%) | 3.9±1.6 | −4.3±6.5 | −6.5±4.6 | −16.3±4.6 |
| Left testis (g) | 0.09±0.01 | 0.08±0.04 | 0.08±0.01 | 0.09±0.02 |
| Relative left testicular weight (%) | 0.3±0.0 | 0.3±0.1 | 0.4±0.0 | 0.4±0.1 |
| Right testis (g) | 0.09±0.01 | 0.08±0.04 | 0.08±0.01 | 0.09±0.01 |
| Relative right testicular weight (%) | 0.3±0.0 | 0.3±0.1 | 0.4±0.0 | 0.4±0.0 |
| Seminal vesicle (g) | 0.28±0.04 | 0.24±0.16 | 0.13±0.12 | 0.21±0.04 |
| Relative seminal vesicle weight (%) | 1.0±0.2 | 0.9±0.5 | 0.6±0.2* | 0.8±0.2* |
| Left epididymis (g) | 0.04±0.01 | 0.04±0.02 | 0.04±0.01 | 0.05±0.02 |
| Relative left epididymis weight (%) | 0.1±0.0 | 0.2±0.0 | 0.2±0.0 | 0.2±0.1* |
| Right epididymis (g) | 0.04±0.01 | 0.04±0.02 | 0.04±0.01 | 0.05±0.01 |
| Relative right epididymis weight (%) | 0.1±0.0 | 0.2±0.1 | 0.2±0.0 | 0.2±0.0* |
*P < 0.05, the indicated group compared with ND Group. ND: nondiabetic control group; D: diabetic control group; DGSH15: diabetic mice treated with GSH at 15 mg kg−1 BW; DGSH30: diabetic mice treated with GSH at 30 mg kg−1 BW; GSH: glutathione; BW: body weight
Sperm number
A significant reduction was observed in the extracted sperm number of the D Group (14.43 × 106 ± 2.20 × 106 ml-1) compared with the ND Group (24.58 × 106 ± 2.84 ×106 ml−1; P < 0.05), as shown in Figure 2a. Neither GSH-treated group showed a difference from nondiabetic or diabetic groups.
Figure 2.
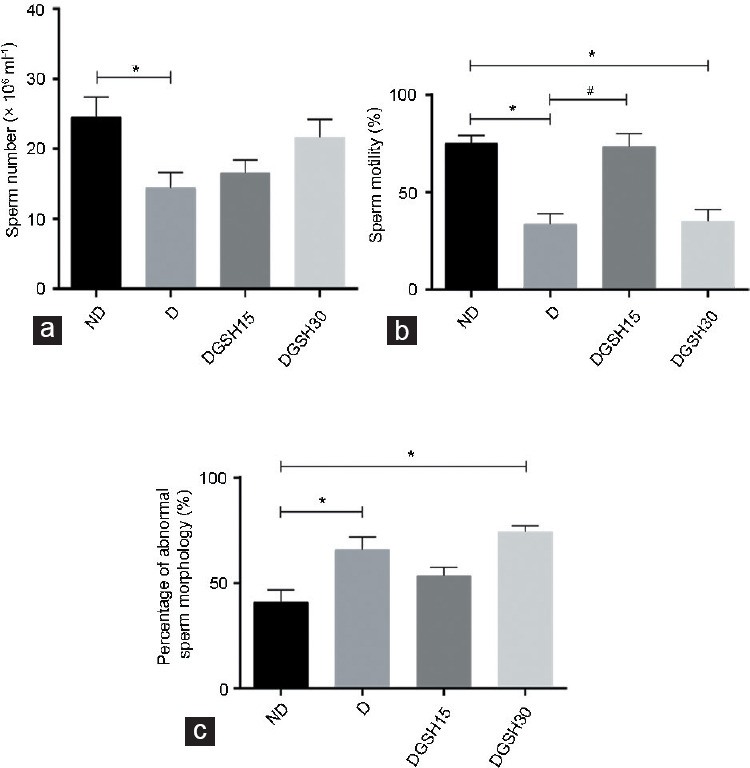
Effect of GSH on sperm parameters after 50 days of treatment (n = 6) on (a) sperm number, (b) sperm motility, and (c) abnormal sperm morphology. Data are represented as mean ± s.d. *P < 0.05, the indicated group compared to ND Group, #P < 0.05, the indicated group compared to D Group. ND: nondiabetic control group; D: diabetic control group; DGSH15: diabetic mice treated with GSH at 15 mg kg−1 BW; DGSH30: diabetic mice treated with GSH at 30 mg kg−1 BW; s.d.: standard deviation; ANOVA: analysis of variance; GSH: glutathione; BW: body weight.
Sperm motility
The D Group had a lower percentage of motile spermatozoa (33.7% ± 5.3%) than the ND Group (75.1% ± 4.0%; P < 0.05). The sperm motility was greater in the DGSH15 Group (73.3% ± 6.8%) than that in the D Group (33.7% ± 5.3%; P < 0.05). However, the DGSH30 Group of diabetic mice showed no improvement in sperm motility compared with the D Group (Figure 2b).
Sperm morphology
The D Group showed a higher percentage of spermatozoa with abnormal morphology (65.9% ± 6.1%) than the ND Group (41.0% ± 5.8%; P < 0.05), as shown in Figure 2c. The proportion of spermatozoa with abnormal morphology in DGSH30 mice was higher (74.5% ± 1.6%) than that in the ND Group (P < 0.05) and was comparable to the D Group. Spermatozoa with different morphologies are shown in Figure 3.
Figure 3.
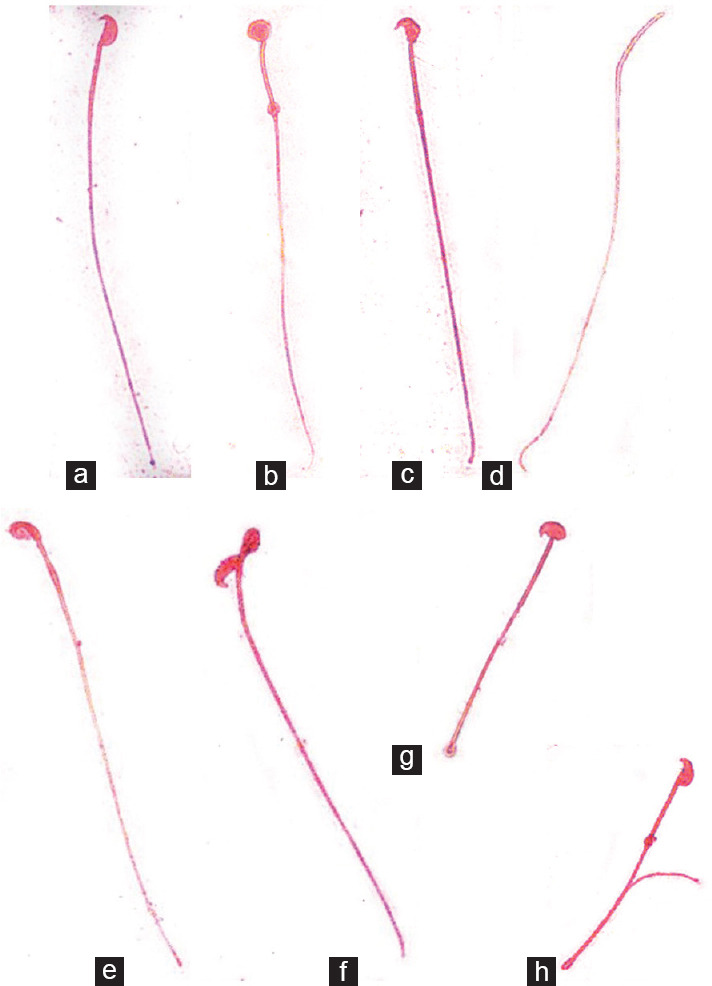
Smears of sperms were stained with Eosin Y (×1000). (a) Normal. (b) Amorphous. (c) Bent head. (d) Headless. (e) Defective head. (f) Folded. (g) Short tail. (h) Coiled tail.
Testicular morphology
The testicular morphology from various groups is shown in Figure 4. The mean diameter of seminiferous tubules was higher in the DGSH30 Group (617.20 ± 11.49 μm) than that in the ND Group (529.00 ± 17.75 μm) and D Group (522.30 ± 6.50 μm; P < 0.05 for both groups), as shown in Figure 5. The diameter of the seminiferous tubular lumen of mice in the D Group (203.00 ± 5.64 μm) and DGSH30 Group (217.20 ± 3.67 μm) was greater than that in the ND Group (185.60 ± 3.59 μm; P < 0.05). However, the mean diameter of seminiferous tubular lumen in the DGSH15 Group (179.40 ± 1.59 μm) was lower than that in the D Group (203.00 ± 5.64 μm; P < 0.05). No significant differences were seen in the epithelial height of seminiferous tubules in any groups. In the DGSH30 Group, the increase in seminiferous tubule lumen was accompanied by increase in diameter, hence preserving the epithelial height.
Figure 4.
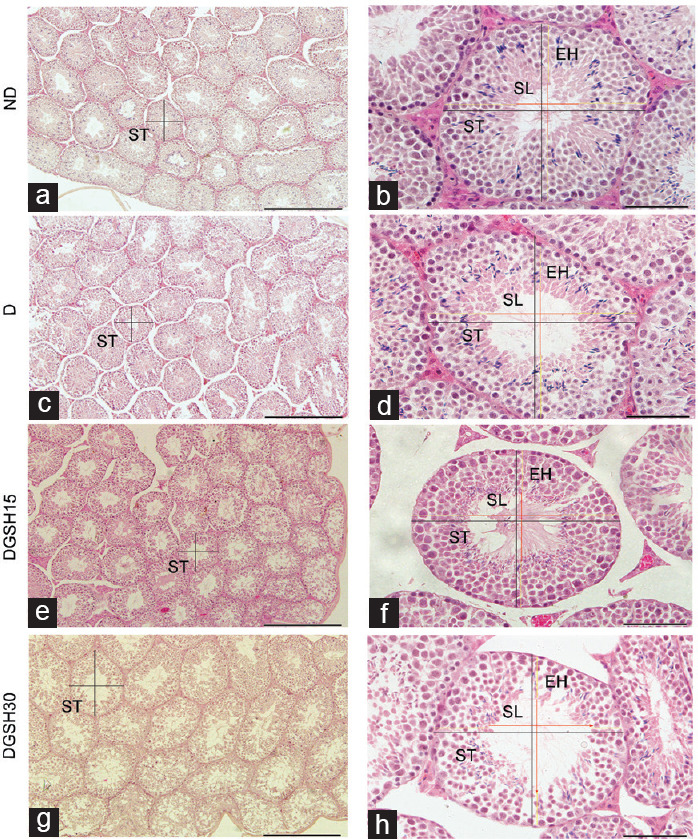
Effect of exogenous GSH on mice testes after 50 days of treatment (n = 6). Embedded testis tissue blocks were cut into 5-μm sections and stained with H and E. Black lines indicate diameter of seminiferous tubule, red lines indicate diameter of seminiferous lumen, and yellow lines indicate epithelial height of seminiferous tubule. Representative images of testicular morphology at different magnifications. Scale bars in the left column (a, c, e and g) represent 1000 μm, while scale bars in the right column (b, d, f and h) represent 200 μm. ST: seminiferous tubule; SL: seminiferous lumen; EH: epithelial height; ND: nondiabetic control group; D: diabetic control group; DGSH15: diabetic mice treated with GSH at 15 mg kg−1 BW; DGSH30: diabetic mice treated with GSH at 30 mg kg−1 BW; GSH: glutathione; H and E: hematoxylin and eosin; BW: body weight.
Figure 5.
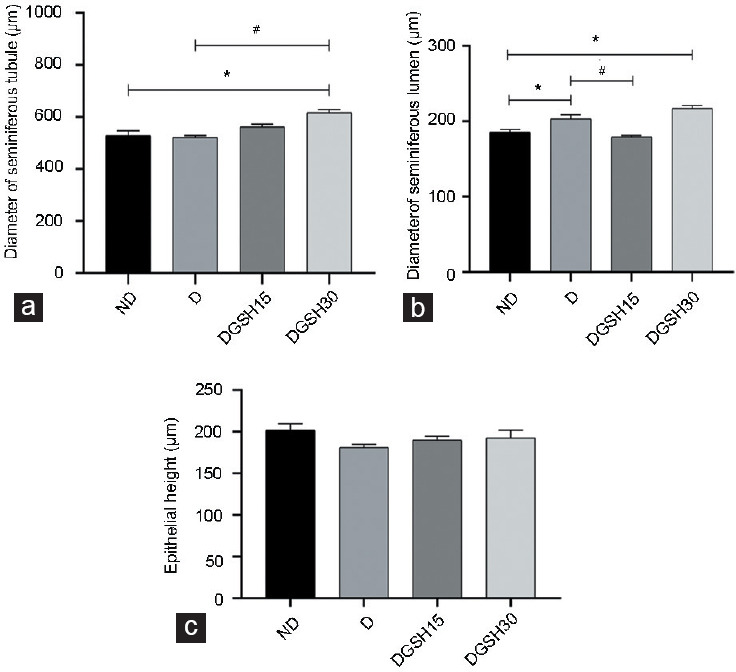
Effect of exogenous GSH on changes in the testicular morphology of seminiferous tubules in diabetic mice after 50 days of treatment (n = 6). (a) Diameter of seminiferous tubules, (b) diameter of seminiferous lumen, and (c) epithelial height. All measurements were done under the same magnification. Data are represented as mean ± s.d. *P < 0.05, the indicated group compared to ND Group, #P<0.05, the indicated group compared to D Group. ND: nondiabetic control group; D: diabetic control group; DGSH15: diabetic mice treated with GSH at 15 mg kg−1 BW; DGSH30: diabetic mice treated with GSH at 30 mg kg−1 BW. GSH: glutathione; s.d.: standard deviation; ANOVA: analysis of variance; BW: body weight.
DISCUSSION
This study demonstrates protection against diabetes-induced changes in sperm quality and testicular morphology through exogenous administration of GSH in mice with type 1 diabetes. DM is a chronic disease characterized by abnormally high blood sugar levels. It is linked to increased production of ROS.30,31 Even though ROS is needed at low concentration for sperm capacitation and acrosomal reaction, at high concentrations, it is capable of causing damage in a cascade of reactions involving a wide range of biomolecules.27,32,33,34 Male reproductive organs and gametes are particularly vulnerable to oxidative damage, as testicular tissue and sperm plasma membranes are rich in polyunsaturated fatty acids (PUFA).35,36 When targeting lipids, oxidant compounds can induce a peroxidation chain reaction that produces several toxic lipid aldehyde species including MDA and 4-HNE. Proteins and DNA are susceptible to alterations from aldehydes, which play a critical role in multiple cellular processes and may engage in secondary deleterious reactions through intramolecular or intermolecular protein/DNA crosslinking.37,38
Although it has been documented that GSH is capable of protecting cells from the harmful effects of excessive ROS, the ability of GSH and its metabolites to promote oxidative processes by participating in metal ion-mediated reactions leading to the formation of ROS and free radicals has also been reported.39 GSH supplementation at a dose of 5 g kg−1 has been shown to increase myocardial GSH content, to protect the heart from oxidative damage, and to protect kidney cells against oxidative injury in rats.40,41 Pimson et al.26 demonstrated that the coadministration of insulin and GSH at 30 mg kg−1 normalizes GSH content and the GSH/GSSG ratio and also restores superoxide dismutase (SOD) and catalase (CAT) activities in STZ-induced type 1 DM (Institute of Cancer Research [ICR]) mice. However, the impact of exogenous GSH treatment alone on type 1 DM remains unclear. Thus far, there have been no reports on the doses of GSH that may improve fertility in males.
This research explores the effects of exogenous GSH at two doses on sperm parameter values and testicular morphology in diabetic mice. In line with the findings of the current study, Pourentezari et al.42 reported reduction in sperm concentration and motility of diabetic mice compared to normal mice, owing to chromatin damage. We have clearly demonstrated that GSH at a dose of 15 mg kg−1 BW of GSH provides significant protection against the diabetes-induced reduction in sperm motility. Although the proportion of spermatozoa with abnormal morphology in the DGSH15 Group was not significantly improved over the D Group, it was notably maintained at the same level as the ND Group, showing that GSH was able to reduce the number of spermatozoa with abnormal morphology. GSH, therefore, confers an improvement in sperm morphology in type 1 DM to some degree at a dosage of 15 mg kg−1. As the GSH/GSSG ratio decreases in diabetes, exogenous administration of GSH appears to restore this ratio.43 It is presumably due to the availability of GSH to scavenge free radicals and reduce OS. It seems to contribute to this protective impact of GSH. Our observations are in line with a previous study that showed that the restoration of GSH/GSSG ratio in diabetic Wistar rats prevented diabetes-induced changes in sperm quality.44 In the current study, however, we did not observe a similar protective effect of GSH at a dose of 30 mg kg−1 BW.
Another important finding in the current study was that the increase of GSH dose to 30 mg kg−1 BW did not cause similar changes in sperm parameters and testicular morphology, as in the case of the 15 mg kg−1 bw dose. It is important to note that the use of high doses of antioxidants may result in adverse effects. At higher doses, antioxidants may scavenge even physiologically required amounts of free radicals, thus resulting in deleterious effects on cell survival. Furthermore, higher dosage of antioxidants may yield pro-oxidant effects by exerting proglycation, disrupting cellular signaling, promoting expression of inflammatory cytokines, and endocrine disrupting activities.45 The effects of high doses of antioxidants have also been referred as anti-OS.14 Hence, one of the highlights of the current study is also the use of appropriate doses of antioxidants for desired outcomes.
Some other significant finding to note in the current study is that the exogenous GSH at a dose of 15 mg kg−1 improved the sperm parameters, particularly sperm motility and testicular morphology without any effect on blood sugar and body weight of the animals. Clearly, this is evident that exogenous GSH produces organ-specific effects without affecting the basic metabolic abnormalities of diabetes. It is likely that if combined with antidiabetic drug therapy, GSH can provide additional benefits against organ-specific complications of diabetes. However, this needs confirmation in further studies.
In the DGSH15 Group, it was noted that lumen of seminiferous tubules was smaller than those in the D Group. The reduction in lumen diameter without change in the overall diameter of seminiferous tubules as observed in the DGSH15 Group was most likely resulted from an increased epithelial height. While changes in epithelial height were not large enough to show statistical differences from the D Group, significant luminal diameter reduction was observed. The significant increase of luminal diameter in the D Group seems to accompany relatively smaller, nonsignificant reductions in epithelial height. Although there were no changes in epithelial height of mice in the DGSH15 Group compared with the D Group, we observed that an epithelial height increases in the DGSH15 Group. In line with our observations, a previous study has shown that increased OS is associated with an increase in the diameter of seminiferous tubule lumen and deterioration of sperm quality.28
In both GSH-treated groups, relative testicular weights did not change in comparison to nondiabetic or diabetic control groups. In contrast to our finding, another study has reported decreased testicular weight and density, reduced diameter and height of germ epithelium in seminiferous tubules, and decreased Leydig cell count in STZ-induced diabetic rats.46 This is a pertinent finding as Leydig cells function to synthesize and release testosterone that acts on peritubular cells and Sertoli cells within the testis to stimulate spermatogenesis.47,48 The disparities in results may be attributable to several factors. Testicular changes reported in the study mentioned above were observed after 10 weeks (70 days) of experimental duration, which is much longer than the 50-day duration in the current study. Moreover, differences in outcomes may reflect the different species used. From the observations made in the current study, GSH at 15 mg kg−1 BW seems to preserve sperm morphology in diabetic mice close to normal.
To the best of our knowledge, this is the first study documenting the effects of exogenous GSH on sperm parameters and testicular morphology of C57BL/6NTac mice with type 1 diabetes. In conclusion, our results confirmed that diabetes impairs the quality of spermatozoa and testicular histomorphology. Intraperitoneal injection of GSH at 15 mg kg−1 BW resulted in enhanced sperm motility and improved seminiferous tubule morphology, probably due to reduced OS via its antioxidative property. Future work involving GSH at a dose of 15 mg kg−1 BW should require the elucidation of molecular mechanisms, so that their ability to minimize type 1 DM adverse reproductive effects can be identified.
AUTHOR CONTRIBUTIONS
MNKN-A obtained the funding. FA and MNKN-A contributed to the conception and design of this study, carried out the search for the articles, and drafted the manuscript. FA, MNKN-A, RA, YSK, MAM, NSB, A-AMK, M-SS, N-SAR, and NHM were responsible for analysis and interpretation of data and helped draft the manuscript. FA wrote the manuscript. MNKN-A, RA, YSK, and NSB participated in the article screening and critically revising the manuscript. MNKN-A edited and approved the final manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declared no competing interests.
ACKNOWLEDGMENTS
This work was supported by the Ministry of Higher Education Malaysia (grant FRGS 5/3 [273/2019]) and the Universiti Teknologi MARA (grant MYRA 5/3/MITRA [008/2017]-2). The authors acknowledge staff of the Institute of Medical Molecular Biotechnology (IMMB), Laboratory Animal Care Unit (LACU), Center of Pathology Diagnostics & Research Laboratories (CPDRL), and the Anatomy Lab of the Faculty of Medicine, Universiti Teknologi MARA, for their contributions.
REFERENCES
- 1.Institute for Public Health (IPH), National Institutes of Health, Ministry of Health Malaysia. National Health and Morbidity Survey (NHMS) 2019: Vol.I: NCDs-Non-Communicable Diseases: Risk Factors and Other Health Problems. Selangor: Ministry of Health Malaysia; 2020. pp. 26–67. [Google Scholar]
- 2.Agbaje I, Rogers DA, McVicar CM, McClure N, Atkinson AB, et al. Insulin dependant diabetes mellitus: implications for male reproductive function. Hum Reprod. 2007;22:1871–7. doi: 10.1093/humrep/dem077. [DOI] [PubMed] [Google Scholar]
- 3.Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, et al. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103:137–49. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Maresch CC, Stute DC, Ludlow H, Hammes HP, de Kretser DM, et al. Hyperglycemia is associated with reduced testicular function and activin dysregulation in the Ins2Akita+/− mouse model of type 1 diabetes. Mol Cell Endocrinol. 2017;446:91–101. doi: 10.1016/j.mce.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. World Health Statistics 2016: Monitoring Health for the SDGs Sustainable Development Goals. Geneva: World Health Organization; 2016. [Google Scholar]
- 6.Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;5:9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pisoschi AM, Pop A. The role of antioxidants in the chemistry of oxidative stress: a review. Eur J Med Chem. 2015;97:55–74. doi: 10.1016/j.ejmech.2015.04.040. [DOI] [PubMed] [Google Scholar]
- 8.Ding GL, Liu Y, Liu ME, Pan JX, Guo MX, et al. The effects of diabetes on male fertility and epigenetic regulation during spermatogenesis. Asian J Androl. 2015;17:948–53. doi: 10.4103/1008-682X.150844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rato L, Oliveira PF, Sousa M, Silva BM, Alves MG. Role of reactive oxygen species in diabetes-induced male reproductive dysfunction. In: Henkel R, Samanta L, Agarwal A, editors. Oxidants, Antioxidants and Impact of the Oxidative Status in Male Reproduction. Cambridge: Academic Press; 2018. pp. 135–47. [Google Scholar]
- 10.Ghasemi H, Karimi J, Goodarzi MT, Khodadadi I, Tavilani H, et al. Seminal plasma zinc and magnesium levels and their relation to sperm parameters in semen of diabetic men. Int J Diabetes Dev Ctries. 2016;36:34–9. [Google Scholar]
- 11.Alves MG, Martins AD, Rato L, Moreira PI, Socorro S, et al. Molecular mechanisms beyond glucose transport in diabetes-related male infertility. Biochim Biophys Acta Mol Basis Dis. 2013;1832:626–35. doi: 10.1016/j.bbadis.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi MK. Structure-function relationship of transporters in the glutamate–glutamine cycle of the central nervous system. Int J Mol Sci. 2018;19:1177. doi: 10.3390/ijms19041177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wichansawakun S, Buttar HS. Antioxidant diets and functional foods promote healthy aging and longevity through diverse mechanisms of action. In: Watson RR, Singh RB, Takahashi T, editors. The Role of Functional Food Security in Global Health. Cambridge: Academic Press; 2018. pp. 541–63. [Google Scholar]
- 14.Poljsak B, Šuput D, Milisav I. Achieving the balance between ROS and antioxidants: when to use the synthetic antioxidants. Oxid Med Cell Longev. 2013;2013:956792. doi: 10.1155/2013/956792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zacharis CK, Tzanavaras PD, Zotou A. Ethyl propiolate as a post-column derivatization reagent for thiols: development of a green liquid chromatographic method for the determination of glutathione in vegetables. Anal Chim Acta. 2011;690:122–8. doi: 10.1016/j.aca.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Galano A, Alvarez-Idaboy JR. Glutathione: mechanism and kinetics of its non-enzymatic defense action against free radicals. RSC Adv. 2011;1:1763–71. [Google Scholar]
- 17.Robaczewska J, Kedziora-Kornatowska K, Kozakiewicz M, Zary-Sikorska E, Pawluk H, et al. Role of glutathione metabolism and glutathione-related antioxidant defense systems in hypertension. J Physiol Pharmacol. 2016;67:331–7. [PubMed] [Google Scholar]
- 18.Kalkan IH, Suher M. The relationship between the level of glutathione, impairment of glucose metabolism and complications of diabetes mellitus. Pak J Med Sci. 2013;29:938–42. doi: 10.12669/pjms.294.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lagman M, Ly J, Saing T, Singh MK, Tudela EV, et al. Investigating the causes for decreased levels of glutathione in individuals with type II diabetes. PloS One. 2015;10:e0118436. doi: 10.1371/journal.pone.0118436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lutchmansingh FK, Hsu JW, Bennett FI, Badaloo AV, McFarlane-Anderson N, et al. Glutathione metabolism in type 2 diabetes and its relationship with microvascular complications and glycemia. PLoS One. 2018;13:e0198626. doi: 10.1371/journal.pone.0198626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adeoye O, Olawumi J, Opeyemi A, Christiania O. Review on the role of glutathione on oxidative stress and infertility. JBRA Assist Reprod. 2018;22:61–6. doi: 10.5935/1518-0557.20180003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rastrelli G, O'Neill TW, Ahern T, Bártfai G, Casanueva FF, et al. Symptomatic androgen deficiency develops only when both total and free testosterone decline in obese men who may have incident biochemical secondary hypogonadism: prospective results from the EMAS. Clin Endocrinol. 2018;89:459–69. doi: 10.1111/cen.13756. [DOI] [PubMed] [Google Scholar]
- 23.Festing MF. On determining sample size in experiments involving laboratory animals. Lab Anim. 2018;52:341–50. doi: 10.1177/0023677217738268. [DOI] [PubMed] [Google Scholar]
- 24.Furman BL. Streptozotocin-induced diabetic models in mice and rats. Curr Protoc Pharmacol. 2015;70:5–47. doi: 10.1002/0471141755.ph0547s70. [DOI] [PubMed] [Google Scholar]
- 25.Aw TY, Wierzbicka G, Jones DP. Oral glutathione increases tissue glutathione in vivo. Chem Biol Interact. 1991;80:89–97. doi: 10.1016/0009-2797(91)90033-4. [DOI] [PubMed] [Google Scholar]
- 26.Pimson C, Chatuphonprasert W, Jarukamjorn K. Improvement of antioxidant balance in diabetes mellitus type 1 mice by glutathione supplement. Pak J Pharm Sci. 2014;27:1731–7. [PubMed] [Google Scholar]
- 27.Almabhouh FA, Osman K, Ibrahim SF, Gupalo S, Gnanou J, et al. Melatonin ameliorates the adverse effects of leptin on sperm. Asian J Androl. 2017;19:647–54. doi: 10.4103/1008-682X.183379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dobrzyńska MM. Combined action of X-rays and nonylphenol on mouse sperm. Cent Eur J Biol. 2011;6:320–9. [Google Scholar]
- 29.Mehraein F, Negahdar F. Morphometric evaluation of seminiferous tubules in aged mice testes after melatonin administration. Cell J. 2011;13:1–4. [PMC free article] [PubMed] [Google Scholar]
- 30.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–20. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 31.Gil-del Valle L, Milian LD, Toledo A, Vilaró N, Tápanes R, et al. Altered redox status in patients with diabetes mellitus type I. Pharmacol Res. 2005;51:375–80. doi: 10.1016/j.phrs.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 32.Tafuri S, Ciani F, Iorio EL, Esposito L, Cocchia N. Reactive oxygen species (ROS) and male fertility. In: Bin Wu., editor. New Discoveries in Embryology. London: Intech Open Limited; 2015. pp. 19–33. [Google Scholar]
- 33.Makker K, Agarwal A, Sharma R. Oxidative stress & male infertility. Indian J Med Res. 2009;129:357–67. [PubMed] [Google Scholar]
- 34.Maartens PJ, Peng J, Agarwal A, Vaamonde D, du Plessis SS. Oxidative stress and infertility: A possible link to exercise. In: Garcia-Manso JM, Esteve TV, editors. Exercise and Human Reproduction. New York: Springer; 2016. pp. 303–15. [Google Scholar]
- 35.Radák Z, Sasvári M, Nyakas C, Pucsok J, Nakamoto H, et al. Exercise preconditioning against hydrogen peroxide-induced oxidative damage in proteins of rat myocardium. Arch Biochem Biophys. 2000;376:248–51. doi: 10.1006/abbi.2000.1719. [DOI] [PubMed] [Google Scholar]
- 36.Ghosh D, Das UB, Ghosh S, Mallick M, Debnath J. Testicular gametogenic and steroidogenic activities in cyclophosphamide treated rat: a correlative study with testicular oxidative stress. Drug Chem Toxicol. 2002;25:281–92. doi: 10.1081/dct-120005891. [DOI] [PubMed] [Google Scholar]
- 37.Taso OV, Philippou A, Moustogiannis A, Zevolis E, Koutsilieris M. Lipid peroxidation products and their role in neurodegenerative diseases. Ann Res Hosp. 2019;3:1–10. [Google Scholar]
- 38.Ayala A, Muñoz MF, Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014;2014:360438. doi: 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pompella A, Visvikis A, Paolicchi A, De Tata V, Casini AF. The changing faces of glutathione, a cellular protagonist. Biochem Pharmacol. 2003;66:1499–503. doi: 10.1016/s0006-2952(03)00504-5. [DOI] [PubMed] [Google Scholar]
- 40.Ramires PR, Ji LL. Glutathione supplementation and training increases myocardial resistance to ischemia-reperfusion in vivo. Am J Physiol Heart Circ Physiol. 2001;281:H679–88. doi: 10.1152/ajpheart.2001.281.2.H679. [DOI] [PubMed] [Google Scholar]
- 41.Akerboom T, Sies H. Glutathione transport and its significance in oxidative stress. In: Vina J, editor. Glutathione (1990) Boca Raton: CRC Press; 2017. pp. 45–56. [Google Scholar]
- 42.Pourentezari M, Talebi AR, Mangoli E, Anvari M, Rahimipour M. Additional deleterious effects of alcohol consumption on sperm parameters and DNA integrity in diabetic mice. Andrologia. 2016;48:564–9. doi: 10.1111/and.12481. [DOI] [PubMed] [Google Scholar]
- 43.Đorđević M, Mihailović M, Jovanović JA, Grdović N, Uskoković A, et al. Centaurium erythraea methanol extract protects red blood cells from oxidative damage in streptozotocin-induced diabetic rats. J Ethnopharmacol. 2017;202:172–83. doi: 10.1016/j.jep.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 44.Syed Imam R, Kshama D, Salma K. Pioglitazone, a PPAR-gamma ligand inhibited the nicotinamide streptozotocin induced sperm abnormalities in type-2 diabetic Wistar rats. Pak J Pharm Sci. 2010;23:326–31. [PubMed] [Google Scholar]
- 45.Bouayed J, Bohn T. Exogenous antioxidants—double-edged swords in cellular redox state: health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid Med Cell Longev. 2010;3:228–37. doi: 10.4161/oxim.3.4.12858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kianifard D, Sadrkhanlou RA, Hasanzadeh S. The ultrastructural changes of the Sertoli and Leydig cells following streptozotocin induced diabetes. Iran J Basic Med Sci. 2012;15:623–35. [PMC free article] [PubMed] [Google Scholar]
- 47.Schlatt S, Meinhardt A, Nieschlag E. Paracrine regulation of cellular interactions in the testis: factors in search of a function. Eur J Endocrinol. 1997;137:107–17. doi: 10.1530/eje.0.1370107. [DOI] [PubMed] [Google Scholar]
- 48.Weinbauer GF, Luetjens CM, Simoni M, Nieschlag E. Physiology of testicular function. In: Nieschlag E, Behre HM, Nieschlag S, editors. Andrology: Male Reproductive Health and Dysfunction. Berlin Heidelberg: Springer-Verlag; 2010. pp. 11–59. [Google Scholar]


