Abstract
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus responsible for coronavirus disease 2019 (COVID-19), enters affected cells through the angiotensin-converting enzyme 2 (ACE2) receptor, which is highly expressed in type II alveolar cells, enterocytes, and cholangiocytes. SARS-CoV-2 infection causes fever, dry cough, and breathing difficulty, which can progress to respiratory distress due to interstitial pneumonia, and hepatobiliary injury due to COVID-19 is increasingly recognized. The hepatobiliary injury may be evident at presentation of the disease or develop during the disease progression. The development of more severe clinical outcomes in patients with chronic liver diseases (CLD) with or without cirrhosis infected with SARS-CoV-2 has not been elucidated. Moreover, there is limited data related to common medications that affect the disease severity of COVID-19 patients. Additionally, ACE2 receptor expression of hepatobiliary tissue related to the disease severity also have not been clarified. This review summarized the current situation regarding the clinical outcomes of COVID-19 patients with chronic liver diseases who were treated with common medications. Furthermore, the association between ACE2 receptor expression and disease severity in these patients is discussed.
Keywords: SARS-CoV-2, COVID-19, Hepatobiliary tissue, Angiotensin converting enzyme 2, Chronic liver disease, Common medications, Clinical outcome
Core Tip: With more than 100 million confirmed cases worldwide, hepatobiliary injury has been reported in many coronavirus disease 2019 (COVID-19) patients. The association between COVID-19 and hepatobiliary injury refers to any hepatobiliary damage during disease progression and treatment in COVID-19 patients with or without chronic liver diseases or common medications. Angiotensin-converting enzyme 2 receptor may be a significant factor in hepatobiliary derangement due to its high expression in cholangiocytes, and it is also an entry point of severe acute respiratory syndrome coronaviruses 2. Moreover, drug-induced liver injury and cytokine storm may be an added risk in severe clinical outcomes. Close monitoring of liver function in COVID-19 patients is mandatory.
INTRODUCTION
Knowledge of the fundamental physiology of angiotensin-converting enzyme 2 (ACE2) has cumulated more than 20 years since its discovery in 2000 and has greatly increased our understanding of the renin-angiotensin system (RAS)[1,2]. The RAS is an essential hormone system with critical roles in blood pressure regulation, vascular biology, nervous system, electrolyte homeostasis, tissue injury, and lipid homeostasis[3,4]. ACE is the key-driven enzyme in classical RAS. On the other hand, the protective RAS is regulated by ACE2 and counterbalances many of the classical deleterious effects of the RAS[5,6]. ACE2 has definite roles ranging from catalytic activities with numerous substrates, as the receptors for severe acute respiratory syndrome coronaviruses (SARS-CoV) and SARS-CoV-2, and as an amino acid transporter[7-10]. ACE2 regulates the RAS by converting angiotensin (Ang) I and II into Ang 1-9 and Ang 1–7, respectively. Clinical and animal studies demonstrated a physiological and pathophysiological aspect of ACE2 in cardiovascular disease (CVD), and activating ACE2 may evoke protective outcomes against hypertension and CVD[11-13].
Since the end of 2019, ACE2 has amassed interest as the cellular receptor of SARS-CoV-2, the causative virus of the coronavirus disease 2019 (COVID-19) pandemic that emerged from Wuhan, China. It has rapidly spread through China, crossed the global borders of 221 countries, and infected 101529722 people, with 2186606 deaths resulting in a 2.15% mortality rate[14]. The clinical manifestations of COVID-19 patients include cough, fever, sore throat, diarrhea, and loss of sense of taste or smell. More than 80% of infected patients have mild symptoms, 14% have severe symptoms, and 5% have a critical illness. Older patients and those with medical co-morbidities are at risk of a severe disease course[15]. Previous studies demonstrated liver damage in nearly 60% of patients suffering from SARS. They also found SARS-CoV virus particles in the hepatocytes of patients[16]. Moreover, SARS-CoV-2 is associated with hepatic dysfunction ranging from 14% to 53% with abnormal levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) without known liver disease[17-19]. Patients with severe or critical outcomes showed higher frequency and degree of liver dysfunction, while in milder patients, the liver injury was transient[20]. Liver injury in COVID-19 patients included psychological stress, systemic inflammation response, drug toxicity, the progression of pre-existing chronic liver diseases (CLD), and other factors[21]. Hence, three possible scenarios have been postulated. Firstly, patients with CLD and pre-existing co-morbidity diseases may be more prone to the severe clinical outcomes of COVID-19, including oxygen desaturation and hypoxemia due to severe pneumonia or the cytokine storm. Secondly, liver enzyme abnormalities are the consequence of drug toxicity. Thirdly, SARS-CoV-2 directly or indirectly causes liver injury[22-24]. Although ACE2 receptors are abundantly present in type 2 alveolar cells, they are also expressed in the gastrointestinal tract, vascular endothelium, hepatocytes, and cholangiocytes and may be the significant factors in disease severity. This review will clarify the relationship between CLD, common medications, and the expression of ACE2 with the clinical outcomes in COVID-19 patients.
ACE2 RECEPTOR
Physiology of ACE2 receptor
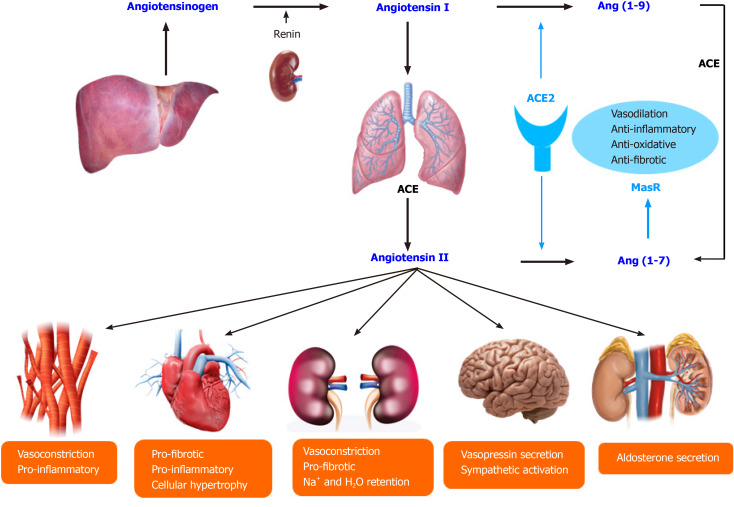
ACE2 receptor resembles the ACE receptor and plays a crucial role in the renin-angiotensin-aldosterone system (RAAS), including blood pressure control and electrolyte homeostasis. The liver produced angiotensinogen, which is cleaved by renin from the kidney, results in Ang I. After that, ACE catalyzes the conversion of Ang I to Ang II. Ang II is the significant active RAAS portion and exerts its effects via Ang II type 1 receptor. Furthermore, Ang II's main effects include vasoconstriction, renal sodium reabsorption, potassium excretion, aldosterone synthesis, blood pressure elevation, and induction of pro-inflammatory and pro-fibrotic pathways. ACE2 splits Ang II to Ang (1-7) and Ang I to Ang (1-9). Furthermore, Ang (1-9) is cleaved by ACE to Ang (1-7). Ang (1-7) exerts vasodilatation, anti-inflammatory, and anti-fibrotic effects through the Mas receptor to counterbalance Ang II's action. Notably, ACE2 functionally counteracts the physiological role of ACE and creates the tissue balance of ACE and ACE2, which determines the pro-inflammatory, pro-fibrotic, or anti-inflammatory and anti-fibrotic pathways[25,26] (Figure 1). The common drugs prescribed for RAAS blockade in several disease conditions can affect this balance. Moreover, many dietary factors (high sodium, high fat, and high fructose intake) can also shift the ACE/ACE2 balance towards pro-inflammatory and pro-fibrotic[27-29].
Figure 1.
The renin-angiotensin-aldosterone system and the physiology of angiotensin-converting enzyme 2. ACE: Angiotensin-converting enzyme.
Expression of ACE2 receptor in hepatobiliary tissue
In 2004, Hamming et al[30] investigated the immuno-localization of ACE2 in 93 human specimens and found that ACE2 was present in endothelial cells from small arteries, large arteries, and veins in the studied tissues. Marked ACE2 immuno-staining was found in type I and typed II alveolar epithelial cells in normal lungs. ACE2 was abundantly demonstrated in enterocytes of all small intestine but not in the enterocytes of the large intestine. ACE2 was not found in lymphoid tissues and hepatocytes. Recently, Xu et al[31] investigated ACE2 expression in the oral cavity mucosa and various organs, including the intestine, kidney, stomach, bile duct, liver, lungs, thyroid, esophagus, bladder, breasts, uterus, and prostate. They found that ACE2 could be expressed in various organs. The mean expression of ACE2 in the liver, bile duct, and lungs was 6.86 ± 1.35, 7.23 ± 1.16, 5.83 ± 0.71, respectively. This result demonstrated that the expression of ACE2 in the lungs and the liver was not different. Moreover, Zhao et al[32] identified ACE2 expression sparsely in cholangiocytes of human liver ductal organoids cells. Anti-ACE2 immuno-staining further confirmed the presence of ACE2 receptors on those cells. Furthermore, Li et al[33] explored the underlying liver injury mechanism by profiling ACE2 expression with CLD expression data. They found that the liver tissues with chronic diseases, such as cirrhosis, non-alcoholic steatohepatitis, simple steatosis, and dysplasia, could express higher levels of ACE2 than normal liver tissues.
The relationship between common medications and ACE2 expression
Sinha et al[34] performed in vitro and in vivo studies to identify the clinically approved drugs that could modify ACE2 expression. They found that ACE inhibitors (ACEIs) but not angiotensin II type-I receptor blockers (ARBs) tend to upregulate ACE2 expression, and anti-adrenergic drugs other than alpha/beta-blockers tend to down-regulate ACE2 expression. Moreover, calcium channel blockers (CCBs) do not significantly change ACE2 expression, consistent with the finding that they do not act on the RAAS. This evidence provides preliminary in vitro support for the use of CCBs as an alternative to ACEIs in COVID-19 patients with hypertension. They also studied the 13 approved anti-diabetic drugs related to ACE2 expression, and they could not demonstrate that the drugs significantly altered ACE2 expression. Surprisingly, they reported that intravenous dexamethasone injection could increase ACE2 expression. They also demonstrated the effect of vancomycin, which increased an ACE2 expression. Saheb SharifAskari et al[35] studied the effect of common medications on the expression of ACE2 receptors in human primary hepatocytes. They found that the top three drugs that increased ACE2 expression were penicillamine, ethambutol, and vitamin A. The top five drugs that decreased ACE2 expression were colchicine, acetaminophen, sulindac, diazepam, and nimesulide. The top five drugs that did not change ACE2 expression were ibuprofen, lornoxicam, mefenamic acid, meloxicam, and methyltestosterone.
COVID-19 AND HEPATOBILIARY INJURY
Laboratory evidence of hepatobiliary injury
Previous studies have shown that nearly 60% of SARS patients developed a hepatobiliary injury and that SARS-CoV antigens were detected in liver tissues by reverse transcription-polymerase chain reaction[36,37]. Hepatobiliary injury in COVID-19 patients was also demonstrated by abnormal transaminase levels linked to the disease severity and the clinical outcome. Abnormal liver enzymes in COVID-19 patients were first reported by Chen et al[38]. They analyzed data of 99 COVID-19 patients from Wuhan and found that 43 cases (43.4%) had elevated ALT, AST, and lactic dehydrogenase. Most of them had a mild elevation of AST and ALT, and only one patient had very high ALT levels of 7590 U/L and AST levels of 1445 U/L. Recently, Kulkarni et al[39] conducted a systematic review with meta-analysis to evaluate the liver manifestations and clinical outcomes in 20874 COVID-19 patients. They found that the pooled incidence of elevated AST and ALT in COVID-19 was 23.1% (19.3%-27.3%) at initial presentation. Moreover, 24.4% (13.5%-40%) of the patients developed elevated AST and ALT during the illness. They also reported the prevalence of underlying CLD as 3.6% among the 15407 COVID-19 patients. The pooled incidence of drug-induced hepatobiliary injury was 25.4% (14.2%-41.4%). They found that the development of severe COVID-19 in CLD patients had an odds ratio (OR) of 0.81 [95% confidence interval (CI): 0.31-2.09] compared with non-CLD patients. Furthermore, COVID-19 patients with elevated AST and ALT had increased risk of mortality (OR = 3.46, 95%CI: 2.42-4.95, P < 0.001) and severe disease (OR = 2.87, 95%CI: 2.29-3.6, P < 0.001) when compared with the patients without elevated AST and ALT.
Recently, Del Zompo et al[40] conducted a systematic review with meta-analysis to elucidate the prevalence of hepatobiliary injury in 20724 COVID-19 patients with or without pre-existing CLD. They found that the pooled prevalence of abnormal liver function tests (LFTs) on admission was 46.9% [AST 26.5%, ALT 22.8%, gamma-glutamyl transferase (GGT) 22.5%, alkaline phosphatase (ALP) 5.7%, and total bilirubin (tBIL) 8.0%]. The elevation of ALT, AST, and tBIL were independent predictors of disease severity and in-hospital mortality. Wong et al[41] conducted another systematic review with meta-analysis to evaluate the prevalence and degree of liver injury in 5961 severe and non-severe COVID-19. They found that the OR for elevated ALT was 2.5, AST was 3.4, hyperbilirubinemia was 1.7, and hypoalbuminemia was 7.1, which were higher in critical COVID-19. They concluded that hepatobiliary injury is more common in COVID-19 patients with severe clinical outcomes than in COVID-19 patients with non-severe clinical outcomes.
Mao et al[42] conducted another meta-analysis to evaluate the prevalence and prognosis of gastrointestinal symptoms and hepatobiliary injury in 6686 patients with COVID-19. They found that the pooled prevalence of liver co-morbidities was 3%, including chronic hepatitis and liver cirrhosis. The pooled prevalence of liver injury from 12 studies (n = 1267) was 19%. The prevalence of elevated ALT was 18%, AST was 21%, tBIL was 6%, and decreased albumin was 6%. They also reported a higher risk of abnormal LFT in patients with severe COVID-19 than those with the non-severe disease.
Kumar-M et al[43] conducted another meta-analysis to evaluate the overall prevalence, stratified prevalence based on severity, estimated risk ratio (RR), and estimated standardized mean difference (SMD) of liver function parameters in severe compared to non-severe COVID-19 patients with a total number of 28659 subjects. They found that the most frequent abnormalities were hypoalbuminemia (61.27%), elevated GGT = 27.94%, elevated ALT = 23.28%, and elevated AST = 23.41%. Furthermore, the relative risk (RR) of these abnormalities was higher in the patients with severe COVID-19 when compared to non-severe disease (hypoalbuminemia RR = 2.65; GGT RR = 2.31; AST RR = 2.30; and ALT RR = 1.76). The pooled prevalence and RR of CLD as a pre-existing co-morbidity were 2.64% and 1.69%, respectively. They concluded that the most frequent hepatobiliary injury was hypoalbuminemia followed by elevated GGT, elevated AST, and elevated ALT, which were more common in severe COVID-19 patients.
Youssef et al[44] conducted a meta-analysis of 3428 COVID-19 patients to elucidate the relationship between hepatobiliary injuries and the severity of COVID-19 disease. They found that the patients who had severe presentations of COVID-19 had hypoalbuminemia (SMD = 0.68), elevated AST (SMD = 0.36), elevated ALT (SMD = 0.44), and elevated tBIL (SMD = 0.40). They also reported that severe COVID-19 patients had a higher OR of developing acute hepatobiliary injury (OR = 1.93). They concluded that hepatobiliary injury was related to a critical outcome of COVID-19 patients. Close monitoring of the development of liver dysfunction is beneficial in early warning of unfavorable outcomes.
Wang et al[45] conducted a meta-analysis to evaluate the association of liver injury and gastrointestinal symptoms (GIS) with the progression of COVID-19 in 3024 patients. They found that 53% of patients had a hepatobiliary injury, and the degree of hepatobiliary damage was associated with disease severity. The prevalence of GIS was relatively low and was not associated with disease progression, with diarrhea of 9.1%, nausea/vomiting of 5.2%, and abdominal pain of 3.5%.
Wu et al[46] conducted a meta-analysis to explore the probable clinical severity and mortality of COVID-19 patients and their liver dysfunction in 3722 COVID-19 patients. They found a significant connection between hepatobiliary dysfunction and mortality in COVID-19 patients with a pooled OR of 1.98. There was a significant association between elevated AST and severity of COVID-19 with a pooled OR of 4.48 and a pooled weighted mean difference of 3.35. They also found a significant difference between elevated tBIL and severe COVID-19 (pooled OR = 1.91 and pooled weighted mean difference = 1.18). They concluded that the mortality and severity of COVID-19 patients are significantly associated with hepatobiliary dysfunction.
Samidoust et al[47] conducted a meta-analysis study to investigate the incidence of liver injury among 4191 COVID-19 patients. They found that the pooled prevalence of liver injury was 19.5%. They concluded that hepatobiliary system is the most frequently damaged outside of the respiratory system. Wu et al[48] conducted the meta-analysis to explore the incidence, risk factors, and prognosis of abnormal liver biochemical tests in 7228 COVID-19 patients. They found that the pooled prevalence of any abnormal liver biochemistry parameters on admission and during hospitalization was 27.2% and 36%, respectively. The most common prevalence was hypoalbuminemia followed by GGT, AST, ALT, tBIL, and ALP (39.8%, 35.8%, 21.8%, 20.4%, 8.8%, and 4.7%). Moreover, severe or critical patients had a significantly higher pooled incidence of abnormal liver biochemistry parameters on admission than mild or moderate patients. Non-survival patients also had a significantly higher incidence of abnormal liver biochemical indicators than survival patients (RR = 1.34). They concluded that abnormal liver biochemical tests are common and are closely related to the severity and prognosis of COVID-19 patients.
Mantovani et al[49] conducted the meta-analysis to assess the overall prevalence of CLD among 2034 COVID-19 patients. They found that the overall prevalence of CLD at baseline was 3%, and patients with severe COVID-19 disease had relevant increases of liver enzymes and coagulation profile due to the innate immune response against the SAR-CoV-2 virus. Sultan et al[50] conducted the meta-analysis to summarize international data on the gastrointestinal (GI) and liver manifestations of SAR-CoV-2 infection and treatment in 10890 COVID-19 patients. They found that elevated AST, elevated ALT, and elevated tBIL are observed in approximately 15%-20% of COVID-19 patients. These findings inform that the clinician should perform a careful evaluation of patients with new-onset GI symptoms for classic and atypical symptoms of COVID-19. All hospitalized COVID-19 patients may benefit from liver enzyme monitoring, particularly in drug treatment with known hepatotoxic potential.
Pathological finding of hepatobiliary injury
Xu et al[51] reported the first post-mortem findings of a patient who succumbed to severe COVID-19. They found that the liver histology showed moderate microvesicular steatosis and mild inflammatory infiltrates in the hepatic lobule and portal tract. They do not know whether these changes were from the direct viral injury or drug toxicity. Wichmann et al[52] conducted a prospective cohort study to perform the autopsies of 12 consecutive COVID-19 deaths, including post-mortem computed tomography and histopathologic and virologic analyses. The median patient age was 73 years (52 to 87 years), 75% of patients were male, and death occurred in the hospital (n = 10) or outpatient department (n = 2). They did not report the histopathology of the hepatobiliary system; however, they could demonstrate the detection of SARS-CoV-2 ribonucleic acid in the lungs of 12 patients (1.2 × 104 to 9 × 109 copies/mL) and the pharynx of nine patients. In five of these patients, viral ribonucleic acid was also detected in the heart, liver, and kidney. They concluded that SARS-CoV-2 might spread via the bloodstream and infect other organs, including the hepatobiliary system. Tian et al[53] performed post-mortem needle core biopsies of lung, liver, and heart in four patients who died of COVID-19 pneumonia. They found that the liver histopathology showed mild lobular infiltration by small lymphocytes, centrilobular sinusoidal dilatation, focal macrovesicular steatosis, and patchy hepatic necrosis in the periportal and centrilobular areas. Tabary et al[54] reviewed multiple organs, including lung, GI tract, liver, kidney, skin, heart, blood, spleen, lymph nodes, brain, blood vessels, and placenta, in COVID-19-related pathological alterations. The liver found hepatocyte degeneration with lobular focal necrosis, congestion of hepatic sinuses with microthrombus, fibrosis of portal tract, the proliferation of portal vein branches, mononuclear leukocyte, and neutrophil infiltration within the portal area and moderate microvascular steatosis. Yao et al[55] conducted another histopathology of the hepatobiliary system. They found that the liver exhibits mild sinusoidal dilation, with mildly increased small lymphocytes infiltration in sinusoidal spaces. Mild to moderate steatosis and multifocal hepatic necrosis have been reported. These findings confirmed that the hepatocellular injury in COVID-19 patients should be considered as a significant factor in disease severity.
CLD AND CLINICAL OUTCOME
The COVID-19 patients with pre-existing CLD usually face a relatively high risk of poor clinical outcomes. Li et al[33] established that patients with CVDs could express higher ACE2 expression than those without heart diseases. Furthermore, ACE2 was upregulated in patients with type 2 diabetes (T2D) compared to the individuals without T2D. For CLD such as cirrhosis, non-alcoholic steatohepatitis, and simple steatosis, ACE2 could express higher levels than normal liver tissues. The upregulation of ACE2 expression in patients with CLD may result in greater susceptibility to SARS-CoV-2 infection of hepatobiliary tissues. Sarin et al[56] conducted The APASL COVID-19 Liver Injury Spectrum Study (APCOLIS Study) to evaluate the liver injury patterns of SARS-CoV-2 in 185 CLD patients without cirrhosis compared with 43 CLD patients with cirrhosis. They found that pre-existing CLD, like metabolic associate fatty liver disease, obesity, and diabetes, was present in nearly 80% of the patients. Moreover, SARS-CoV-2 infection produces acute liver injury in 43% of CLD patients without cirrhosis. Nearly half of decompensated cirrhosis patients develop liver-related complications, which were more severe and had higher mortality. The liver injury pattern in CLD patients was mostly a hepatocellular injury. Notably, elevated serum ALP and elevated GGT were detected, indicating virus-related injury to hepatobiliary tissue due to the overexpression of ACE2 on cholangiocytes. They also found acute, chronic liver failure (ACLF) or acute decompensation in 20% of the cirrhotic patients, which indicated that SARS-CoV-2, a non-hepatotropic virus, can directly precipitate a severe hepatic injury to cause liver failure in cirrhotic patients. They concluded that pre-existing CLD is an added risk in severe COVID-19 patients. Liver-related complications, overall complications, and clinical outcomes correlated with the existing hepatic reserve. Moreover, acute liver injury is more severe and more progressive with higher mortality in COVID-19 patients with decompensated cirrhosis.
Marjot et al[57] conducted an international registry study to evaluate the impact of COVID-19 on patients with pre-existing CLD. They recruited 745 patients with CLD who were infected with SARS-CoV-2 (386 with cirrhosis and 359 without cirrhosis) and compared them to non-CLD patients with SARS-CoV-2 infection. They found that the mortality rate was 32% in COVID-19 patients with cirrhosis compared to 8% in those without cirrhosis. Mortality in cirrhosis patients increased according to Child-Pugh Class [A (19%), B (35%), and C (51%)] and 71% of death was an acute respiratory distress syndrome. Compared to COVID-19 patients without CLD (n = 620), the propensity-score-matched analysis revealed a significant increase in mortality in those with Child-Pugh B cirrhosis (+ 20.0%) and Child-Pugh C cirrhosis (+ 38.1%). Acute hepatic decompensation developed in 46% of cirrhosis patients, of whom 21% had no respiratory symptoms. Half of those with hepatic decompensation had ACLF. They concluded that baseline liver disease and alcohol-related liver disease are independent risk factors for death from COVID-19. Another group of investigators from Korea conducted a multicenter study to evaluate the clinical outcomes in 1005 COVID-19 patients related to pre-existing CLD and the predictors of disease severity and mortality. They found that liver cirrhosis was more common in COVID-19 patients with severe pneumonia than in non-severe pneumonia (4.5% vs 0.9%). The overall survival rate significantly decreased in COVID-19 patients with liver cirrhosis than in those without liver cirrhosis. The presence of liver cirrhosis was found to be an independent predictor of severe clinical outcome. They suggested that more robust personal protection and more intensive treatment for COVID-19 with pre-existing CLD should be highly recommended[58].
Del Zompo et al[40] conducted the meta-analysis to elucidate the prevalence of hepatobiliary injury in COVID-19 patients with or without pre-existing CLD. They explored 36 studies, including 20724 patients with SARS-CoV-2 infection, and found that LFTs alterations were reported in up to 47% of unselected patients with COVID-19 and were associated with severe clinical outcomes or in-hospital mortality. COVID-19 was associated with a high risk of liver decompensation or mortality. Váncsa et al[59] conducted the meta-analysis to evaluate the prognostic value of on-admission LFTs and pre-existing CLD on the clinical course of COVID-19. They evaluated 50 studies with 17205 COVID-19 patients. They reported that the decreased platelet count, elevated ALT, elevated AST, increased C-reactive protein, and the presence of acute or CLDs at the time of admission could predict severe clinical outcomes of COVID-19 patients. Significantly, the pre-existing CLD or acute liver injury combined with SARS-CoV-2 infection was an important factor in predicting mortality rate.
COMMON MEDICATIONS TREATMENT AND CLINICAL OUTCOME IN COVID-19 PATIENTS
Several publications reviewed the role of RAS inhibitors in COVID-19 patients and found that there is no definitive evidence indicating harmful effects of RAS inhibitors. Because ACE and ACE2 are different enzymes, ACEIs do not inhibit ACE2, making this class' harmful effect unlikely[60-62]. Other common anti-hypertensive drugs are ARBs, which have been shown to upregulate ACE2 in animal studies, but these findings do not translate into clinical observations related to COVID-19[63]. Drager et al[64] summarized that the available clinical evidence points to a neutral or even beneficial effect on clinical outcomes in COVID-19 patients who received ACEIs or ARBs. Luo et al[65] conducted a retrospective analysis to compare the outcome of metformin users and non-users in 283 hospitalized COVID-19 patients with diabetes (104 used metformin, and 179 did not use metformin). They found that in-hospital mortality was significantly lower in the metformin group [3/104 (2.9%) vs 22/179 (12.3%), P = 0.01]. They concluded that metformin might offer benefits in COVID-19 patients. However, they did not mention the relationship between metformin and hepatobiliary injury in their study. Treatment of common co-morbidities such as cardiovascular, hepatobiliary, and metabolic disorders often requires continuous use of several medications, which may result in an additive increase in the expression of ACE2. Furthermore, the combined effect of chronic use of these medications could affect liver susceptibility in COVID-19 patients. Although the increased risk of developing severe clinical outcomes in COVID-19 patients should not be the direct effect of common medications, we should be vigilant about the possible effects of those medications.
CONCLUSION
Several factors have been associated with the alteration of ACE2 expression and COVID-19 severity and progression. Although ACE2 is widely expressed in various human tissues and most of its determinants have been well recognized, ACE2-expressing organs do not equally participate in COVID-19 pathophysiology, implicating that other factors are involved orchestrating cellular infection resulting in several organs injury. Abnormal LFTs are reported in up to half of the patients with COVID-19 infection. The disease severity, pre-existing CLD, and some common medications presented risks for hepatobiliary injury in COVID-19 patients. It has been demonstrated that SARS-CoV-2 may directly bind to ACE2 positive cholangiocytes and cause severe hepatic injury. However, pre-existing CLD and some common medications could also upregulate ACE2 expression in the hepatobiliary tissues and cause more severe clinical outcomes in COVID-19 patients. Furthermore, other contributing mechanisms such as drug-induced liver injury, activation of the immune system, and cytokine storm may be the other contributing factors in severe clinical outcomes.
Footnotes
Conflict-of-interest statement: The author declares no conflict of interest for this article.
Manuscript source: Invited manuscript
Peer-review started: January 28, 2021
First decision: February 24, 2021
Article in press: April 26, 2021
Specialty type: Medicine, general and internal
Country/Territory of origin: Thailand
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Deng K S-Editor: Zhang L L-Editor: Filipodia P-Editor: Xing YX
References
- 1.Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem . 2000;275:33238–33243. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- 2.Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R, Breitbart RE, Acton S. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res . 2000;87:E1–E9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 3.Patel S, Rauf A, Khan H, Abu-Izneid T. Renin-angiotensin-aldosterone (RAAS): The ubiquitous system for homeostasis and pathologies. Biomed Pharmacother . 2017;94:317–325. doi: 10.1016/j.biopha.2017.07.091. [DOI] [PubMed] [Google Scholar]
- 4.Miller AJ, Arnold AC. The renin-angiotensin system in cardiovascular autonomic control: recent developments and clinical implications. Clin Auton Res . 2019;29:231–243. doi: 10.1007/s10286-018-0572-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santos RAS, Sampaio WO, Alzamora AC, Motta-Santos D, Alenina N, Bader M, Campagnole-Santos MJ. The ACE2/Angiotensin-(1-7)/MAS Axis of the Renin-Angiotensin System: Focus on Angiotensin-(1-7) Physiol Rev . 2018;98:505–553. doi: 10.1152/physrev.00023.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paz Ocaranza M, Riquelme JA, García L, Jalil JE, Chiong M, Santos RAS, Lavandero S. Counter-regulatory renin-angiotensin system in cardiovascular disease. Nat Rev Cardiol . 2020;17:116–129. doi: 10.1038/s41569-019-0244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grace JA, Herath CB, Mak KY, Burrell LM, Angus PW. Update on new aspects of the renin-angiotensin system in liver disease: clinical implications and new therapeutic options. Clin Sci (Lond) . 2012;123:225–239. doi: 10.1042/CS20120030. [DOI] [PubMed] [Google Scholar]
- 8.Herath CB, Lubel JS, Jia Z, Velkoska E, Casley D, Brown L, Tikellis C, Burrell LM, Angus PW. Portal pressure responses and angiotensin peptide production in rat liver are determined by relative activity of ACE and ACE2. Am J Physiol Gastrointest Liver Physiol . 2009;297:G98–G106. doi: 10.1152/ajpgi.00045.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hashimoto T, Perlot T, Rehman A, Trichereau J, Ishiguro H, Paolino M, Sigl V, Hanada T, Hanada R, Lipinski S, Wild B, Camargo SM, Singer D, Richter A, Kuba K, Fukamizu A, Schreiber S, Clevers H, Verrey F, Rosenstiel P, Penninger JM. ACE2 Links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature . 2012;487:477–481. doi: 10.1038/nature11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turner AJ, Hiscox JA, Hooper NM. ACE2: from vasopeptidase to SARS virus receptor. Trends Pharmacol Sci . 2004;25:291–294. doi: 10.1016/j.tips.2004.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang K, Gheblawi M, Oudit GY. Angiotensin Converting Enzyme 2: A Double-Edged Sword. Circulation . 2020;142:426–428. doi: 10.1161/CIRCULATIONAHA.120.047049. [DOI] [PubMed] [Google Scholar]
- 12.Patel VB, Zhong JC, Grant MB, Oudit GY. Role of the ACE2/Angiotensin 1-7 Axis of the Renin-Angiotensin System in Heart Failure. Circ Res . 2016;118:1313–1326. doi: 10.1161/CIRCRESAHA.116.307708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKinney CA, Fattah C, Loughrey CM, Milligan G, Nicklin SA. Angiotensin-(1-7) and angiotensin-(1-9): function in cardiac and vascular remodelling. Clin Sci (Lond) . 2014;126:815–827. doi: 10.1042/CS20130436. [DOI] [PubMed] [Google Scholar]
- 14.Worldometer COVID-19 coronavirus pandemic 2021. [cited 20 March 2021]. Available from: https://www.worldometers.info/coronavirus/?fbclid=IwAR3fVhmfOSlgc6Hm_hwkpJv7-MjFFwfmHBlHbIcQQC80wFA_15DQ2RHMZho .
- 15.Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA . 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 16.Ding Y, He L, Zhang Q, Huang Z, Che X, Hou J, Wang H, Shen H, Qiu L, Li Z, Geng J, Cai J, Han H, Li X, Kang W, Weng D, Liang P, Jiang S. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol . 2004;203:622–630. doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol . 2020;5:428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int . 2020;40:998–1004. doi: 10.1111/liv.14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie H, Zhao J, Lian N, Lin S, Xie Q, Zhuo H. Clinical characteristics of non-ICU hospitalized patients with coronavirus disease 2019 and liver injury: A retrospective study. Liver Int . 2020;40:1321–1326. doi: 10.1111/liv.14449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, Zhang Y, Huang S, Liu Z, Cheng J. Clinical Features of COVID-19-Related Liver Functional Abnormality. Clin Gastroenterol Hepatol . 2020;18:1561–1566. doi: 10.1016/j.cgh.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li J, Fan JG. Characteristics and Mechanism of Liver Injury in 2019 Coronavirus Disease. J Clin Transl Hepatol . 2020;8:13–17. doi: 10.14218/JCTH.2020.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheung KS, Hung IFN, Chan PPY, Lung KC, Tso E, Liu R, Ng YY, Chu MY, Chung TWH, Tam AR, Yip CCY, Leung KH, Fung AY, Zhang RR, Lin Y, Cheng HM, Zhang AJX, To KKW, Chan KH, Yuen KY, Leung WK. Gastrointestinal Manifestations of SARS-CoV-2 Infection and Virus Load in Fecal Samples From a Hong Kong Cohort: Systematic Review and Meta-analysis. Gastroenterology . 2020;159:81–95. doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Zheng L, Liu L, Zhao M, Xiao J, Zhao Q. Liver impairment in COVID-19 patients: A retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int . 2020;40:2095–2103. doi: 10.1111/liv.14455. [DOI] [PubMed] [Google Scholar]
- 24.Pirola CJ, Sookoian S. SARS-CoV-2 virus and liver expression of host receptors: Putative mechanisms of liver involvement in COVID-19. Liver Int . 2020;40:2038–2040. doi: 10.1111/liv.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tikellis C, Thomas MC. Angiotensin-Converting Enzyme 2 (ACE2) Is a Key Modulator of the Renin Angiotensin System in Health and Disease. Int J Pept . 2012;2012:256294. doi: 10.1155/2012/256294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamming I, Cooper ME, Haagmans BL, Hooper NM, Korstanje R, Osterhaus AD, Timens W, Turner AJ, Navis G, van Goor H. The emerging role of ACE2 in physiology and disease. J Pathol . 2007;212:1–11. doi: 10.1002/path.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamming I, van Goor H, Turner AJ, Rushworth CA, Michaud AA, Corvol P, Navis G. Differential regulation of renal angiotensin-converting enzyme (ACE) and ACE2 during ACE inhibition and dietary sodium restriction in healthy rats. Exp Physiol . 2008;93:631–638. doi: 10.1113/expphysiol.2007.041855. [DOI] [PubMed] [Google Scholar]
- 28.Bernardi S, Toffoli B, Zennaro C, Tikellis C, Monticone S, Losurdo P, Bellini G, Thomas MC, Fallo F, Veglio F, Johnston CI, Fabris B. High-salt diet increases glomerular ACE/ACE2 ratio leading to oxidative stress and kidney damage. Nephrol Dial Transplant . 2012;27:1793–1800. doi: 10.1093/ndt/gfr600. [DOI] [PubMed] [Google Scholar]
- 29.Gupte M, Boustany-Kari CM, Bharadwaj K, Police S, Thatcher S, Gong MC, English VL, Cassis LA. ACE2 is expressed in mouse adipocytes and regulated by a high-fat diet. Am J Physiol Regul Integr Comp Physiol . 2008;295:R781–R788. doi: 10.1152/ajpregu.00183.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol . 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, Li T, Chen Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci . 2020;12:8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao B, Ni C, Gao R, Wang Y, Yang L, Wei J, Lv T, Liang J, Zhang Q, Xu W, Xie Y, Wang X, Yuan Z, Zhang R, Lin X. Recapitulation of SARS-CoV-2 infection and cholangiocyte damage with human liver ductal organoids. Protein Cell . 2020;11:771–775. doi: 10.1007/s13238-020-00718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y, Xu Q, Ma L, Wu D, Gao J, Chen G, Li H. Systematic profiling of ACE2 expression in diverse physiological and pathological conditions for COVID-19/SARS-CoV-2. J Cell Mol Med . 2020;24:9478–9482. doi: 10.1111/jcmm.15607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sinha S, Cheng K, Schäffer AA, Aldape K, Schiff E, Ruppin E. In vitro and in vivo identification of clinically approved drugs that modify ACE2 expression. Mol Syst Biol . 2020;16:e9628. doi: 10.15252/msb.20209628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saheb Sharif-Askari N, Saheb Sharif-Askari F, Mdkhana B, Al Heialy S, Ratemi E, Alghamdi M, Abusnana S, Kashour T, Hamid Q, Halwani R. Effect of common medications on the expression of SARS-CoV-2 entry receptors in liver tissue. Arch Toxicol . 2020;94:4037–4041. doi: 10.1007/s00204-020-02869-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsang KW, Ho PL, Ooi GC, Yee WK, Wang T, Chan-Yeung M, Lam WK, Seto WH, Yam LY, Cheung TM, Wong PC, Lam B, Ip MS, Chan J, Yuen KY, Lai KN. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N Engl J Med . 2003;348:1977–1985. doi: 10.1056/NEJMoa030666. [DOI] [PubMed] [Google Scholar]
- 37.Chau TN, Lee KC, Yao H, Tsang TY, Chow TC, Yeung YC, Choi KW, Tso YK, Lau T, Lai ST, Lai CL. SARS-associated viral hepatitis caused by a novel coronavirus: report of three cases. Hepatology . 2004;39:302–310. doi: 10.1002/hep.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet . 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kulkarni AV, Kumar P, Tevethia HV, Premkumar M, Arab JP, Candia R, Talukdar R, Sharma M, Qi X, Rao PN, Reddy DN. Systematic review with meta-analysis: liver manifestations and outcomes in COVID-19. Aliment Pharmacol Ther . 2020;52:584–599. doi: 10.1111/apt.15916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Del Zompo F, De Siena M, Ianiro G, Gasbarrini A, Pompili M, Ponziani FR. Prevalence of liver injury and correlation with clinical outcomes in patients with COVID-19: systematic review with meta-analysis. Eur Rev Med Pharmacol Sci . 2020;24:13072–13088. doi: 10.26355/eurrev_202012_24215. [DOI] [PubMed] [Google Scholar]
- 41.Wong YJ, Tan M, Zheng Q, Li JW, Kumar R, Fock KM, Teo EK, Ang TL. A systematic review and meta-analysis of the COVID-19 associated liver injury. Ann Hepatol . 2020;19:627–634. doi: 10.1016/j.aohep.2020.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mao R, Qiu Y, He JS, Tan JY, Li XH, Liang J, Shen J, Zhu LR, Chen Y, Iacucci M, Ng SC, Ghosh S, Chen MH. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol . 2020;5:667–678. doi: 10.1016/S2468-1253(20)30126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumar-M P, Mishra S, Jha DK, Shukla J, Choudhury A, Mohindra R, Mandavdhare HS, Dutta U, Sharma V. Coronavirus disease (COVID-19) and the liver: a comprehensive systematic review and meta-analysis. Hepatol Int . 2020;14:711–722. doi: 10.1007/s12072-020-10071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Youssef M, H Hussein M, Attia AS, M Elshazli R, Omar M, Zora G, S Farhoud A, Elnahla A, Shihabi A, Toraih EA, S Fawzy M, Kandil E. COVID-19 and liver dysfunction: A systematic review and meta-analysis of retrospective studies. J Med Virol . 2020;92:1825–1833. doi: 10.1002/jmv.26055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang H, Qiu P, Liu J, Wang F, Zhao Q. The liver injury and gastrointestinal symptoms in patients with Coronavirus Disease 19: A systematic review and meta-analysis. Clin Res Hepatol Gastroenterol . 2020;44:653–661. doi: 10.1016/j.clinre.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu ZH, Yang DL. A meta-analysis of the impact of COVID-19 on liver dysfunction. Eur J Med Res . 2020;25:54. doi: 10.1186/s40001-020-00454-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Samidoust P, Samidoust A, Samadani AA, Khoshdoz S. Risk of hepatic failure in COVID-19 patients. A systematic review and meta-analysis. Infez Med . 2020;28:96–103. [PubMed] [Google Scholar]
- 48.Wu Y, Li H, Guo X, Yoshida EM, Mendez-Sanchez N, Levi Sandri GB, Teschke R, Romeiro FG, Shukla A, Qi X. Incidence, risk factors, and prognosis of abnormal liver biochemical tests in COVID-19 patients: a systematic review and meta-analysis. Hepatol Int . 2020;14:621–637. doi: 10.1007/s12072-020-10074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mantovani A, Beatrice G, Dalbeni A. Coronavirus disease 2019 and prevalence of chronic liver disease: A meta-analysis. Liver Int . 2020;40:1316–1320. doi: 10.1111/liv.14465. [DOI] [PubMed] [Google Scholar]
- 50.Sultan S, Altayar O, Siddique SM, Davitkov P, Feuerstein JD, Lim JK, Falck-Ytter Y, El-Serag HB AGA Institute. AGA Institute Rapid Review of the Gastrointestinal and Liver Manifestations of COVID-19, Meta-Analysis of International Data, and Recommendations for the Consultative Management of Patients with COVID-19. Gastroenterology 2020; 159: 320-334. :e27. doi: 10.1053/j.gastro.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med . 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wichmann D, Sperhake JP, Lütgehetmann M, Steurer S, Edler C, Heinemann A, Heinrich F, Mushumba H, Kniep I, Schröder AS, Burdelski C, de Heer G, Nierhaus A, Frings D, Pfefferle S, Becker H, Bredereke-Wiedling H, de Weerth A, Paschen HR, Sheikhzadeh-Eggers S, Stang A, Schmiedel S, Bokemeyer C, Addo MM, Aepfelbacher M, Püschel K, Kluge S. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19: A Prospective Cohort Study. Ann Intern Med . 2020;173:268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tian S, Xiong Y, Liu H, Niu L, Guo J, Liao M, Xiao SY. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol . 2020;33:1007–1014. doi: 10.1038/s41379-020-0536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tabary M, Khanmohammadi S, Araghi F, Dadkhahfar S, Tavangar SM. Pathologic features of COVID-19: A concise review. Pathol Res Pract . 2020;216:153097. doi: 10.1016/j.prp.2020.153097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yao XH, Li TY, He ZC, Ping YF, Liu HW, Yu SC, Mou HM, Wang LH, Zhang HR, Fu WJ, Luo T, Liu F, Guo QN, Chen C, Xiao HL, Guo HT, Lin S, Xiang DF, Shi Y, Pan GQ, Li QR, Huang X, Cui Y, Liu XZ, Tang W, Pan PF, Huang XQ, Ding YQ, Bian XW. [A pathological report of three COVID-19 cases by minimal invasive autopsies] Zhonghua Bing Li Xue Za Zhi . 2020;49:411–417. doi: 10.3760/cma.j.cn112151-20200312-00193. [DOI] [PubMed] [Google Scholar]
- 56.Sarin SK, Choudhury A, Lau GK, Zheng MH, Ji D, Abd-Elsalam S, Hwang J, Qi X, Cua IH, Suh JI, Park JG, Putcharoen O, Kaewdech A, Piratvisuth T, Treeprasertsuk S, Park S, Wejnaruemarn S, Payawal DA, Baatarkhuu O, Ahn SH, Yeo CD, Alonzo UR, Chinbayar T, Loho IM, Yokosuka O, Jafri W, Tan S, Soo LI, Tanwandee T, Gani R, Anand L, Esmail ES, Khalaf M, Alam S, Lin CY, Chuang WL, Soin AS, Garg HK, Kalista K, Batsukh B, Purnomo HD, Dara VP, Rathi P, Al Mahtab M, Shukla A, Sharma MK, Omata M APASL COVID Task Force. APASL COVID Liver Injury Spectrum Study (APCOLIS Study- NCT 04345640) Pre-existing liver disease is associated with poor outcome in patients with SARS CoV2 infection; The APCOLIS Study (APASL COVID-19 Liver Injury Spectrum Study) Hepatol Int . 2020;14:690–700. doi: 10.1007/s12072-020-10072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marjot T, Moon AM, Cook JA, Abd-Elsalam S, Aloman C, Armstrong MJ, Pose E, Brenner EJ, Cargill T, Catana MA, Dhanasekaran R, Eshraghian A, García-Juárez I, Gill US, Jones PD, Kennedy J, Marshall A, Matthews C, Mells G, Mercer C, Perumalswami PV, Avitabile E, Qi X, Su F, Ufere NN, Wong YJ, Zheng MH, Barnes E, Barritt AS 4th, Webb GJ. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: An international registry study. J Hepatol . 2021;74:567–577. doi: 10.1016/j.jhep.2020.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee YR, Kang MK, Song JE, Kim HJ, Kweon YO, Tak WY, Jang SY, Park JG, Lee C, Hwang JS, Jang BK, Suh JI, Chung WJ, Kim BS, Park SY. Clinical outcomes of coronavirus disease 2019 in patients with pre-existing liver diseases: A multicenter study in South Korea. Clin Mol Hepatol . 2020;26:562–576. doi: 10.3350/cmh.2020.0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Váncsa S, Hegyi PJ, Zádori N, Szakó L, Vörhendi N, Ocskay K, Földi M, Dembrovszky F, Dömötör ZR, Jánosi K, Rakonczay Z Jr, Hartmann P, Horváth T, Erőss B, Kiss S, Szakács Z, Németh D, Hegyi P, Pár G. Pre-existing Liver Diseases and On-Admission Liver-Related Laboratory Tests in COVID-19: A Prognostic Accuracy Meta-Analysis With Systematic Review. Front Med (Lausanne) . 2020;7:572115. doi: 10.3389/fmed.2020.572115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin-Angiotensin-Aldosterone System Inhibitors in Patients with Covid-19. N Engl J Med . 2020;382:1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Danser AHJ, Epstein M, Batlle D. Renin-Angiotensin System Blockers and the COVID-19 Pandemic: At Present There Is No Evidence to Abandon Renin-Angiotensin System Blockers. Hypertension . 2020;75:1382–1385. doi: 10.1161/HYPERTENSIONAHA.120.15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong JC, Turner AJ, Raizada MK, Grant MB, Oudit GY. Angiotensin-Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System: Celebrating the 20th Anniversary of the Discovery of ACE2. Circ Res . 2020;126:1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang X, Ye Y, Gong H, Wu J, Yuan J, Wang S, Yin P, Ding Z, Kang L, Jiang Q, Zhang W, Li Y, Ge J, Zou Y. The effects of different angiotensin II type 1 receptor blockers on the regulation of the ACE-AngII-AT1 and ACE2-Ang(1-7)-Mas axes in pressure overload-induced cardiac remodeling in male mice. J Mol Cell Cardiol . 2016;97:180–190. doi: 10.1016/j.yjmcc.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 64.Drager LF, Pio-Abreu A, Lopes RD, Bortolotto LA. Is Hypertension a Real Risk Factor for Poor Prognosis in the COVID-19 Pandemic? Curr Hypertens Rep . 2020;22:43. doi: 10.1007/s11906-020-01057-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Luo P, Qiu L, Liu Y, Liu XL, Zheng JL, Xue HY, Liu WH, Liu D, Li J. Metformin Treatment Was Associated with Decreased Mortality in COVID-19 Patients with Diabetes in a Retrospective Analysis. Am J Trop Med Hyg . 2020;103:69–72. doi: 10.4269/ajtmh.20-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]