Abstract
Diabetic kidney disease (DKD) is a common and severe complication of diabetes mellitus. If left untreated, DKD can advance to end stage renal disease that requires either dialysis or kidney replacement. While numerous mechanisms underlie the pathogenesis of DKD, oxidative stress driven by NADH/NAD+ redox imbalance and mitochondrial dysfunction have been thought to be the major pathophysiological mechanism of DKD. In this review, the pathways that increase NADH generation and those that decrease NAD+ levels are overviewed. This is followed by discussion of the consequences of NADH/NAD+ redox imbalance including disruption of mitochondrial homeostasis and function. Approaches that can be applied to counteract DKD are then discussed, which include mitochondria-targeted antioxidants and mimetics of superoxide dismutase, caloric restriction, plant/herbal extracts or their isolated compounds. Finally, the review ends by pointing out that future studies are needed to dissect the role of each pathway involved in NADH-NAD+ metabolism so that novel strategies to restore NADH/NAD+ redox balance in the diabetic kidney could be designed to combat DKD.
Keywords: diabetic kidney disease, caloric restriction, NADH/NAD+, redox imbalance, mitochondrial homeostasis, mitophagy, oxidative stress
1. Introduction
Constituting approximately 0.51% to 1.08% of the body weight, the kidneys are an energy-demanding organ and receive approximately 20–25% of the cardiac output [1,2,3]. Every 24 min, it filters a volume equal to that of whole plasma volume; and every 6 h, it filters a volume equal to that of total body water [3]. Given this workload, the kidney needs a large amount of ATP produced by mitochondria, which, unfortunately, also generate reactive oxygen species (ROS) as metabolic byproducts [4,5,6]. Therefore, the kidney is under constant attack from ROS. Such is indeed the case in diabetic kidney disease (DKD) whereby oxidative stress is elevated and mitochondrial dysfunction is aggravated, leading to renal injury [7,8]. DKD, also known as diabetic nephropathy [9,10,11,12,13], is a common complication of diabetic mellitus, including both type 1 and type 2 diabetes. While type 1 diabetes is caused by lack of insulin due to pancreatic β cell destruction [14,15,16], type 2 diabetes could be caused by insulin resistance or insulin deficiency [17,18,19,20,21,22]. The hallmark of diabetes is a persistent high blood glucose content (hyperglycemia) that can damage a variety of tissues and cells [15,21,22,23]. In the kidney, renal microvascular structures are the major targets of high blood glucose [24,25,26,27]. Additionally, given the facts that the kidney is the organ where mature or active form of vitamin D is made [28,29,30] and erythropoiesis erythropoietin is produced [31,32,33], DKD can also lead to vitamin D deficiency and anemia [34,35,36,37,38,39,40]. Therefore, while there is great understanding of the pathophysiology and progression of DKD, novel and effective treatment approaches are still needed as current therapeutic options remain limited.
While many mechanisms underlie the pathogenesis of DKD including protein kinase C pathway [41,42], hexosamine pathway [43,44], formation of advanced glycation end products [45,46] and the polyol pathway [47,48]; at the molecular level, redox imbalance of NADH/NAD+ caused by deranged glucose metabolism [49,50,51] may stand out as a distinct mechanism of diabetic kidney injury [52,53,54,55]. This is because electrons from breakdown of glucose and other nutrients such as fatty acids and amino acids are stored in NADH using NAD+ as the electron acceptor [56,57,58]. Therefore, a key feature of diabetes mellitus is oversupply of NADH and under supply of NAD+ [48,51,59].
2. Sources of Elevated NADH in Diabetes
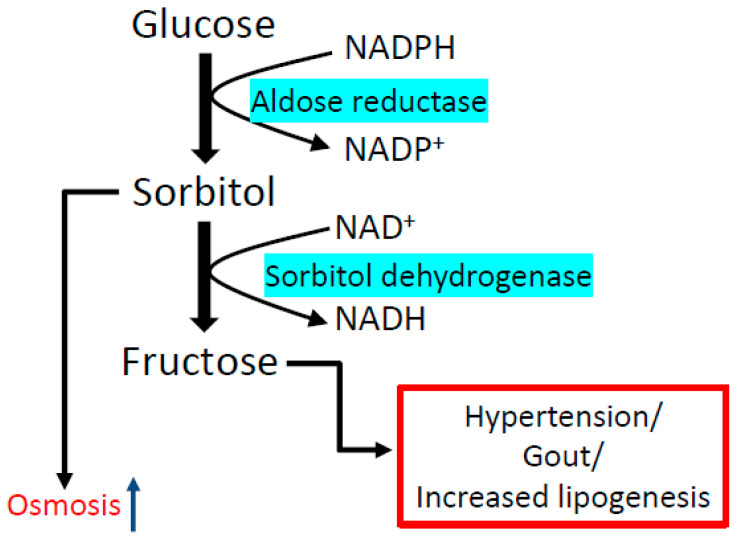
In addition to the conventional metabolic pathways that extract electrons by breaking the chemical bonds in carbohydrates and fatty acids (Figure 1), other glucose utilization pathways are activated by hyperglycemia [47]. One of these pathways is the polyol pathway [60,61] (Figure 2), which can burn up to 30% of the glucose pool in a diabetic patient [62]. This pathway converts glucose to fructose and also converts NADPH to NADH. There is also an intermediate product known as sorbitol, which could accumulate and impair cellular osmosis in the kidney [63,64]. While the first reaction from glucose to sorbitol is catalyzed by aldose reductase, the second reaction from sorbitol to fructose is catalyzed by sorbitol dehydrogenase. In this pathway, aldose reductase is the rate-limiting enzyme that has a high Km value for glucose [65]. Therefore, numerous studies have focused on aldose reductase as a potential therapeutic target in diabetes [66,67,68,69,70,71]. In particular, attention has been paid to develop small molecule compounds that can inhibit aldose reductase [72,73,74,75,76] to prevent accumulation of sorbitol and fructose and to prevent build-up of NADH, the elevation of which can perturb NADH/NAD+ redox balance, initiating reductive stress and oxidative stress. Furthermore, the contribution of the polyol pathway to diabetes development has been demonstrated by the use of aldose reductase animal models whereby lack of aldose reductase prevents the development of diabetes [76]. It should be noted that this NADH/NAD+ redox imbalance is also termed as pseudohypoxia in diabetes [77,78] because hypoxia and ischemia often leads to NADH accumulation and NAD+ depletion [79,80,81]. It should also be noted that endogenous production of fructose via the polyol pathway has been shown to cause increased fructose and fructose-1-phosphate contents in the kidney, leading to aggravation of DKD [82].
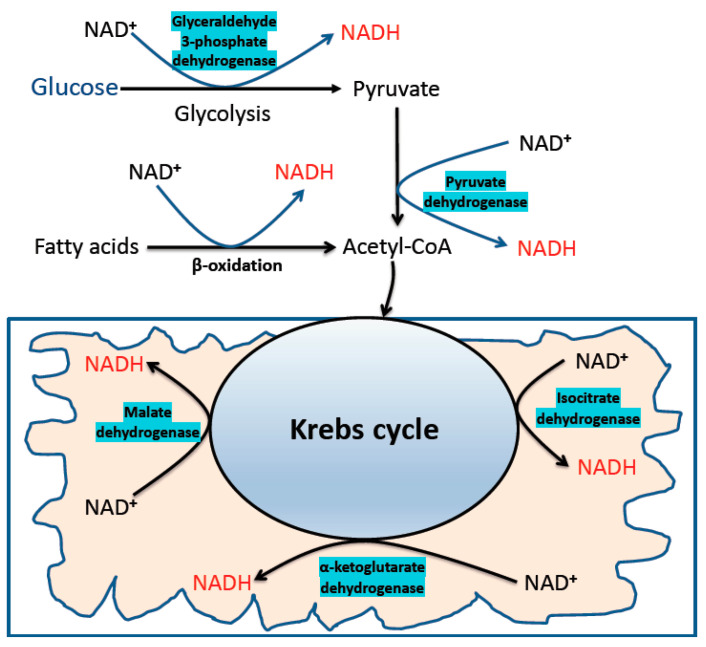
Figure 1.
The conventional metabolic pathways that generate NADH from NAD+. Shown are the glycolytic pathway, fatty acid oxidation, and the Krebs cycle. These are the major pathways that store electrons in NADH by breaking the chemical bonds in dietary components including glucose, fatty acids. Enzymes involved in direct production of NADH are also indicated in the diagram.
Figure 2.
The polyol pathway. This pathway contains two reactions. The first reaction converting glucose to sorbitol is catalyzed by aldose reductase. This enzyme is rate-limiting for the whole pathway. The second reaction converting sorbitol to fructose is catalyzed by sorbitol dehydrogenase. The final products are NADH and fructose, and sorbitol is an intermediate product. Note that NADPH is consumed by aldose reductase in the first reaction. Additionally, accumulation of sorbitol in the kidney could cause osmotic problems for nephrons [63,64].
3. Pathways of NAD+ Consumption in Diabetes
3.1. The Poly ADP Ribosylase Pathway
While NADH is over-supplied in diabetes, NAD+ could be depleted in diabetes. One major pathway utilizing NAD+ is the poly ADP ribosylase catalyzed reaction (Figure 3A), which is activated due to DNA damage by ROS in diabetes and uses NAD+ as a substrate thereby leading to degradation of NAD+ [83,84,85]. The contribution of this pathway to the pathogenesis of diabetes has been confirmed by studies using poly ATP ribosylase deficient mouse, in which lack of the enzyme prevents development of diabetes [86,87], demonstrating the detrimental effects of NAD+ depletion in diabetes.
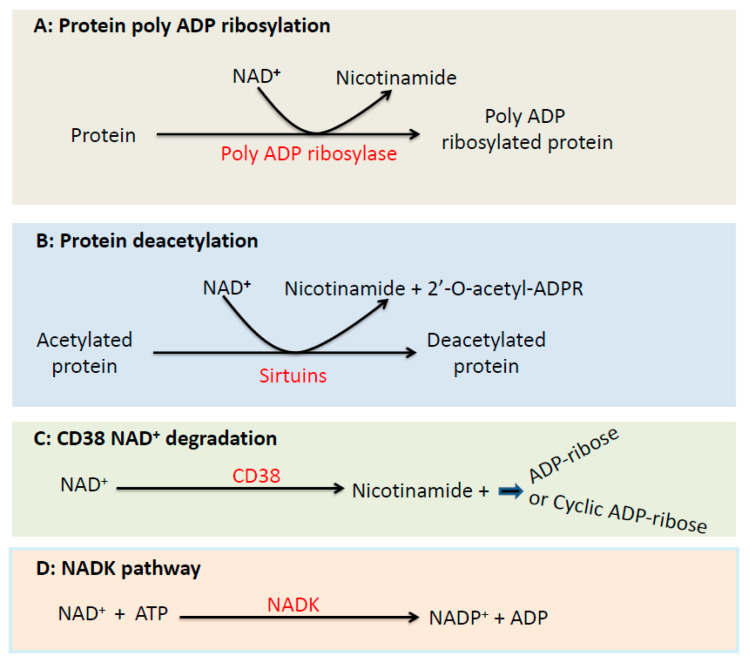
Figure 3.
Major pathways that consume NAD+. Shown are (A) the poly ADP ribosylase reaction; (B) the sirtuin-catalyzed deacetylation reaction; (C) the CD38 NAD+ degradation pathway; (D) the NAD kinase pathway converting NAD+ to NADP+. All the shown pathways or reactions use NAD+ as the respective enzyme’s substrate.
3.2. The Sirtuins Pathway
Another pathway that also consumes NAD+ is the sirtuin proteins [88,89] (Figure 3B), which remove acetyl groups from acetylated proteins using NAD+ as a substrate. This pathway may play an important role in lowering NAD+ levels in early stages of diabetes, but at advanced stages of diabetes, sirtuin protein contents tend to be down regulated [90]. Therefore, it is likely that sirtuin deficiency in advanced stages of diabetes contributes less to NAD+ depletion in diabetes.
3.3. The CD38 Pathway
CD38 is an NADase that catalyzes the degradation of NAD+ [91,92,93] (Figure 3C). This enzyme has been shown to be upregulated in a variety of diseases as well as aging [92,94], leading to decreased content of NAD+ that would impair the function of sirtuins and poly ADP ribosylase [95,96]. CD38-driven NAD+ deficiency has been shown to be responsible for organ fibrosis and diabetic kidney dysfunction [97]. Conversely, CD38 inhibitors have been shown to mitigate mitochondrial oxidative stress in DKD via restoration of NADH/NAD+ redox balance [98].
3.4. The NAD Kinase Pathway
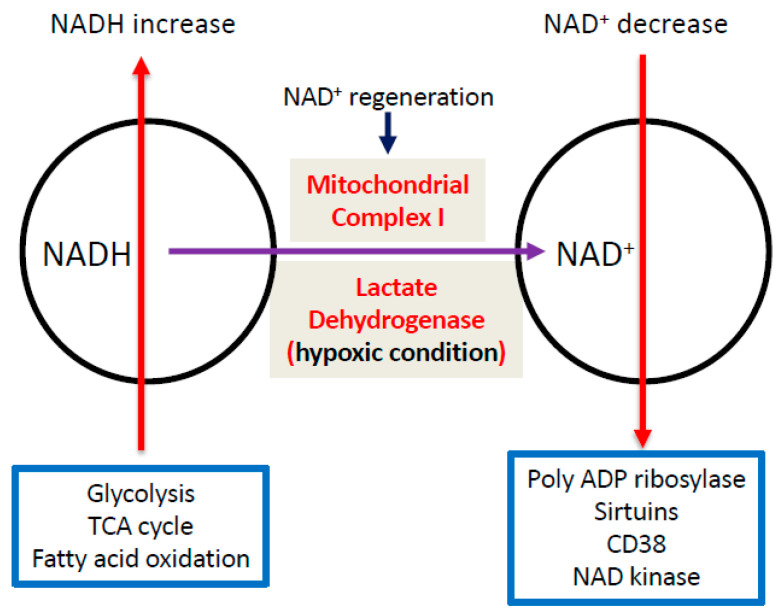
NAD kinase (NADK) exists both in the cytosol and in the mitochondria [99,100]. This protein is the sole enzyme responsible for conversion of NAD+ to NADP+ [101,102] (Figure 3D). Given the key role of NADP+ in maintaining the levels of cellular antioxidant glutathione [103,104,105], NADK is an indispensable element in the redox metabolic pathways. Although many studies have been conducted on NADK in a variety of experimental systems, the role of this protein in DKD has yet to be explored. Furthermore, as NADK consumes NAD+, how it is involved in maintaining or perturbing NADH/NAD+ redox balance in DKD will also need to be investigated. The major pathways causing increase in NADH and decrease in NAD+ as well as NAD+ regeneration by mitochondrial complex I and lactate dehydrogenase (under hypoxia) are summarized in Figure 4.
Figure 4.
Diagram summarizing the pathways that cause NADH increase and NAD+ decrease in the diabetic kidneys. Regeneration of NAD+ from NADH by either mitochondrial complex I or lactate dehydrogenase (under hypoxic conditions) is also shown.
4. Redox Imbalance-linked Mitochondrial Dysfunction in DKD
One of the major consequences of NADH/NAD+ redox imbalance is mitochondrial oxidative stress due to oversupply of NADH to the mitochondrial electron transport chain [47,106,107]. This is caused by electron leakage from the electron transport chain [6,108,109,110,111,112], as it cannot use all of the NADH for ATP production [90,113]. As such, oxygen is partially reduced to form superoxide anion via the electron transport chain, mainly through complexes I, III and IV [114,115]. This mitochondrial superoxide, regardless of the exact site of its generation, is the original source of oxidative stress that can cause oxidative damage to DNA, proteins and lipids [116,117,118,119]. Accumulation of these oxidatively damaged macromolecule adducts can eventually lead to cell death and kidney failure [120,121].
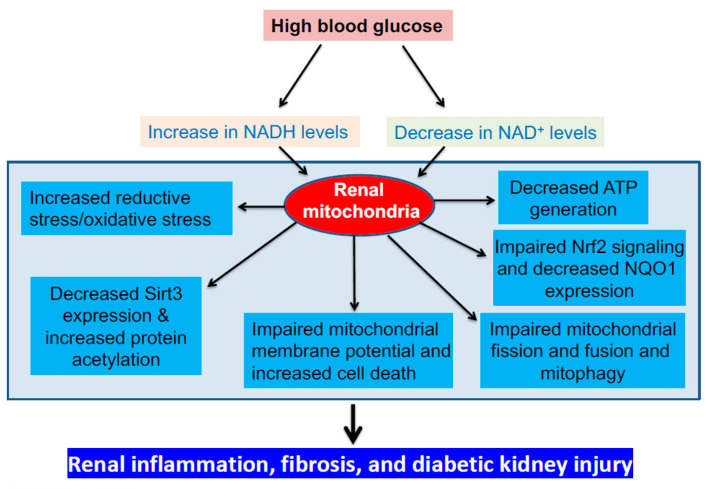
While NADH/NAD+ redox imbalance drives the initial event of superoxide production in mitochondria [47,48,122], other abnormalities of mitochondria could also manifest in DKD, culminating in decreased oxygen consumption and ATP production. As mitochondrion is a dynamic organelle, disruption of its fission and fusion processes [123], also knowns as mitochondrial homeostasis [123,124,125], can also worsen diabetic kidney injury [126]. Indeed, dynamin-related protein 1 (Drp1), well known for its role in regulating mitochondrial fission, has been shown to be upregulated to cause mitochondrial fragmentation in DKD [127,128,129]. Conversely, mitochondrial fusion regulating proteins such as optic atrophy-1 (opa1) and mitochondrial fusion proteins, in particular, mitochondrial fusion protein 2 (Mfn2), have been shown to be down regulated to impair mitochondrial fusion in DKD [130,131,132]. This disruption of mitochondrial homeostasis is linked with redox imbalance and oxidative stress accompanied with impairment of mitochondrial membrane potential and release of apoptosis stimulating factors such as cytochrome c and apoptosis inducing factor (AIF) [133,134,135,136]. These deranged mitochondrial dynamics, if left unattended or uncorrected, would eventually lead to accumulation of damaged mitochondria, which could overwhelm the mitophagy capacity that is regulated by key proteins such as PINK1 and Parkin [137,138,139,140], resulting in cell death and worsened diabetic kidney injury. Therefore, mitochondrial homeostasis and dynamics can also serve as targets for renal therapy in DKD. Figure 5 outlines the potential deleterious mitochondrial consequences of NADH/NAD+ redox imbalance implicated in the pathogenesis of DKD.
Figure 5.
Mitochondrial dysfunction driven by NADH/NAD+ redox imbalance and the potential mitochondrial mechanisms underlying pathophysiology of DKD. These mechanisms include increased mitochondrial oxidative damage, decreased ATP production, perturbed mitochondrial membrane potential and deranged mitochondrial homeostasis and impaired sirt3 pathway as well as Nrf2 signaling pathway. The ultimate manifestation of these mitochondrial dysfunctional mechanisms is renal inflammation, fibrosis and diabetic kidney injury.
It is also worth mentioning that a hallmark of the diabetic kidney is hyperfiltration, so that the energy demands of the proximal tubule are greatly increased [141,142,143]. This may temporarily ameliorate the NADH/NAD+ redox imbalance as NADH utilization is increased for ATP production. However, as increased NADH consumption means more oxygen consumption and more electron leakage from mitochondria for superoxide production [114,144,145], tubular cells could exhibit increased oxidative stress, which could eventually lead to hyperfiltration linked diabetic nephropathy [53,146]. Nonetheless, whether there is an increased mitochondrial superoxide production linked to hyperfiltration and increased ATP demands remains to be determined.
5. Therapeutic Approaches to Counteracting DKD
5.1. Superoxide Dismutation and Suppression
There are at least 11 mitochondrial sites that are involved in superoxide generation [147]. Therefore, overall approaches of dismutating superoxide could alleviate DKD [148]. Recently, small molecules that can suppress or inhibit mitochondrial superoxide production have been developed. Typical examples of these small molecules are S1QELs and S3QELs [145,149], which do not interfere with the process of oxidative phosphorylation or ATP production [144]. S1QELs acts at the site IQ of complex I [145,147] while S3QELs acts at the Q site of complex III [150,151]. The usefulness of these suppressors in combating oxidative stress has been tested in certain experimental systems [151,152,153,154]. However, studies of these suppressors in alleviation of DKD have yet to be conducted. It is anticipated that these compounds could attenuate the severity of DKD in diabetic subjects.
5.2. Mitochondria-targeted Antioxidants and Superoxide Dismutase (SOD) Mimetics
Antioxidants that go into mitochondria are a class of compounds that can be used to counteract mitochondrial oxidative stress. These are generally purposely synthesized for targeting mitochondria. One well-known compound is mitoQ that has been investigated in a variety of diseases including kidney disease [155,156,157,158,159,160,161,162]. Using type 1 diabetic Akita mouse model, Chacko et al. [163] demonstrated that mitoQ administration over a 12-week period improved tubular and glomerular function in the Akita diabetic mice and decreased urinary albumin content to the level as observed in healthy controls. Moreover, mitoQ-treated Akita mice yielded mitochondria that functioned similar to those isolated from healthy control animals, resulting in attenuation of interstitial fibrosis and glomerular damage. MitoQ could also ameliorate tubular injury by enhancing mitophagy via the Nrf2/PINK1 pathway [164]. In fact, the efficacy of mitoQ in diabetic renal protection is nearly equal to that of angiotensin converting enzyme inhibition [165]. MitoQ could also decrease mitochondrial fragmentation mediated by the JNK signaling pathway in DKD [166]. All these protective effects of mitoQ on DKD can be attributed to its capacity in destroying ROS [167]. It should be noted that while the protective effects of an SOD mimetic tempol has been investigated in DKD [168,169,170,171], the protective effects of other SOD mimetics such as GC4419 [172,173] and EUK189 [174,175,176] are yet to be evaluated in DKD.
5.3. Plant and Herb Derived Antioxidants
Numerous plant- or herbal extracts or plant/herb-derived natural products have been tested for their capacity in fighting DKD. A major representative of these extracts is polyphenols that can scavenge ROS [177], leading to correction of redox imbalance and enhancement of mitochondrial function [178,179,180]. Moreover, these plants extracts can also activate the Nrf2 signaling pathway thereby leading to upregulation of the so-called second cellular defense system including antioxidant proteins such as heme oxygenase-1 and NQO1 [181]. As chronic inflammation is implicated in the pathogenesis of DKD, many studies involving plant extracts have also demonstrated their anti-inflammation properties in preclinical DKD [182]. Table 1 shows selected representatives of plant/herb extracts or plant/herb-derived compounds and their redox balanced-related anti-DKD mechanisms.
Table 1.
Selected representatives of plant/herbal extracts/components in DKD from the literature. Experimental models and the major underlying renoprotective mechanisms are also given in the table.
| Extracts/Components | Experimental Model | Major Mechanisms | Refs. |
|---|---|---|---|
| Azuki bean extract | * STZ-rat | Autophagy stimulation | [183] |
| Acacia nilotica | STZ-rat | Antioxidant/anti-hyperglycemia | [184] |
| Anogeissus acuminate leaf | STZ-rat | Antioxidation | [185] |
| Broccoli | STZ-rat | Mitigating oxidative damage | [186] |
| Curcumin | STZ-rat | Inhibiting PKC beta | [187] |
| Coccinia indica | STZ-rat | Increased antioxidant enzymes | [188] |
| Coffea arabica pulp | HFD/STZ | Antioxidation upregulation | [189] |
| Ganoderma lucidum | STZ-rat | TGFβ-1, NFkB | [190] |
| Garlic extract | STZ-rat | Anti-glycation | [191] |
| Geraniin | * HFD | Inhibiting oxidative stress | [192] |
| Ginger extract | STZ-rat | Apoptosis attenuation | [193] |
| Ginkgo biloba EGB761 | HFD/STZ mouse | Mitigating ECM * accumulation | [194] |
| Berberine | db/db mouse | Mitochondrial fission | [195] |
| Cupuacu extract | STZ-rat | Mitigating nitrosation | [196] |
| Anchomanes difformis (leaf) | STZ-rat | Nrf2 activation | [197] |
| Abelmoschus manihot | HFD/STZ mouse | Autophagy activation | [198] |
| Hibiscus sabdariffa Linnaeus | STZ-rat | Akt regulating | [199] |
| Mulberry leaf | HFD/STZ rat | Inhibiting TGF-β1 | [200] |
| Liriope spicata var. prolifera | STZ-rat | Suppressing inflammation | [201] |
| Nelumbo nucifera leaf | HFD/STZ rat | Antioxidative | [202] |
| Coreopsis tinctoria nutt | High glucose/HFD/STZ | Anti-fibrotic | [203] |
| Oil palm | STZ-rat | Attenuating oxidative stress | [204] |
| Armillariella tabescens | STZ-mouse | Anti-inflammation | [205] |
| Red ginseng | STZ-rat | Autophagy acceleration | [206] |
| Paederia foetida leaf | Alloxan-rat | Antioxidative effects | [207] |
| Tiliacora triandra | HFD/STZ rat | Redox imbalance modulation | [208] |
| Flavonoids (review article) | Numerous models | Miscellaneous mechanisms | [209] |
| Grape seed | STZ-rat | Reduce apoptosis | [210] |
| Grape seed/proanthocyanidins | STZ-rat | Mitigating ER stress | [211] |
| Grape seed procyanidin B2 | db/db mouse | Targeting MFG-E8* | [212] |
| Grape seed polyphenols | Cell culture | Mitigating oxidative stress | [213] |
| Catlpol | db/db mouse | Improving lipid metabolism | [214] |
| Cudrania tricuspidata root | Human kidney cells | Preventing inflammation | [215] |
| Hyperoside | HFD/STZ mouse | Targeting miR-499-5p?APC | [216] |
| Phyllanthus niruri leaf | STZ/nicotinamide rat | Anti-fibrosis/apoptosis | [217] |
| Pomegranate peel extract | STZ-mouse | Nrf2 signaling pathway | [218] |
| Quercetin | STZ-mouse | Anti-apoptosis/oxidative stress | [219] |
| Resveratrol | STZ-mouse | Sirt1 activation | [220] |
* Abbreviations: HFD, high fat diet; STZ, streptozotocin; ECM, extracellular matrix; MFG-E8, milk fat globule EGF-8. Please note that this table is not meant to exhaust the literature on plant/herbal extracts and DKD.
5.4. Caloric Restriction
Caloric restriction (CR) [221,222,223], sometimes also called energy restriction [224,225], is a well-established approach for extending the lifespan of many species. CR can also prolong the health span of many organs including the kidney [226,227,228,229]. As CR has a direct impact on energy supply that involves NADH and NAD+, it thus is involved in eliciting antioxidative responses in DKD by restoring redox balance and mitigating diabetic kidney injury [230,231]. Such responses include AMPK activation, autophagy, ROS elimination, Nrf2 signaling pathway activation and enhancement of antioxidative capacity in the kidney [231,232,233,234,235]. In certain studies, exercise has been shown to have a synergistic effect on CR [236,237]. Therefore, CR and exercise may be applied simultaneously to enhance kidney function in diabetes [238,239]. Moreover, intermittent fasting, a different version of CR, has also been demonstrated to prevent progression of DKD via NAD+ dependent sirtuin pathway [230]. Additionally, the restriction of single element in a given diet such as iron can also afford renoprotection in diabetes via attenuation of oxidative stress [240].
6. Magnitude of Redox Imbalance and Progression of DKD
While it is now known that NADH/NAD+ redox imbalance is one of the underlying mechanisms of DKD and this redox imbalance drives reductive stress to oxidative stress [47], culminating in renal dysfunction in DKD, whether the magnitude of NADH/NAD+ redox imbalance can be associated with the indices of DKD progression has not been established. DKD progression can be determined by the ratio of urinary albumin to urinary creatinine [127] and by estimated glomerular flow rate (eGFR) [241,242], but whether NADH/NAD+ ratio would also advance from low to high during DKD progression is unknown at this time and needs to be investigated. It is conceivable that with the progression of DKD quantitated by the above-mentioned parameters, values of the NADH/NAD+ ratio would also increase gradually to reflect the severity of DKD. Conversely, the value of the NADH/NAD+ ratio should go down upon remission of DKD after treatment. Regardless, this would need to be evaluated using proper animal models that can show clearly an association of the value of NADH/NAD+ to progression of DKD until the end stage of renal disease.
7. Conclusions
NADH/NAD+ redox imbalance, driven by persistent hyperglycemia and oversupply of other nutrients, is the initiator of reductive stress and oxidative stress in DKD [47]. More studies would be needed to dissect the role of each and every player in this cascade of redox imbalance biochemistry mechanism. Complete and comprehensive studies not only will shed insights into the mechanisms of DKD but will also facilitate identification of targets that can be explored for DKD therapy. As indicated in a recent review article by Matoba et al. [243], targeting NADH/NAD+ redox imbalance would be a valuable approach for combating DKD. Finally, it should be pointed out that in terms of potential injury caused by redox imbalance, which part of the kidney or what type of cells that sustain the most damage have not been comprehensively evaluated. Therefore, future efforts should be made to assess redox imbalance-induced damage to endothelial cells of the renal vasculature, the podocytes and mesangial cells of the glomerulus and the epithelial cells of the tubule. Additionally, how redox imbalance differs within the tubule should also be measured.
Acknowledgments
The author’s work was supported in part by UNTHSC seed grants RI10015 and RI10039.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Radi Z.A. Kidney Pathophysiology, Toxicology, and Drug-Induced Injury in Drug Development. Int. J. Toxicol. 2019;38:215–227. doi: 10.1177/1091581819831701. [DOI] [PubMed] [Google Scholar]
- 2.Koeppen B.M., Stanton B.A. Renal Physiology. 5th ed. Elsevier; Philadelphia, PA, USA: 2013. [Google Scholar]
- 3.Carroll R.G. Elsevier’s Integrated Physiology. Elsevier; Philadelphia, PA, USA: 2007. [Google Scholar]
- 4.Zorov D.B., Filburn C.R., Klotz L.O., Zweier J.L., Sollott S.J. Reactive oxygen species (ROS)-induced ROS release: A new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J. Exp. Med. 2000;192:1001–1014. doi: 10.1084/jem.192.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim J., Jang H.S., Park K.M. Reactive oxygen species generated by renal ischemia and reperfusion trigger protection against subsequent renal ischemia and reperfusion injury in mice. Am. J. Physiol. Renal Physiol. 2010;298:F158–F166. doi: 10.1152/ajprenal.00474.2009. [DOI] [PubMed] [Google Scholar]
- 6.Rosca M.G., Vazquez E.J., Chen Q., Kerner J., Kern T.S., Hoppel C.L. Oxidation of fatty acids is the source of increased mitochondrial reactive oxygen species production in kidney cortical tubules in early diabetes. Diabetes. 2012;61:2074–2083. doi: 10.2337/db11-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei P.Z., Szeto C.C. Mitochondrial dysfunction in diabetic kidney disease. Clin. Chim. Acta. 2019;496:108–116. doi: 10.1016/j.cca.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Hallan S., Sharma K. The Role of Mitochondria in Diabetic Kidney Disease. Curr. Diab. Rep. 2016;16:61. doi: 10.1007/s11892-016-0748-0. [DOI] [PubMed] [Google Scholar]
- 9.Cui J., Bai X., Chen X. Autophagy and Diabetic Nephropathy. Adv. Exp. Med. Biol. 2020;1207:487–494. doi: 10.1007/978-981-15-4272-5_36. [DOI] [PubMed] [Google Scholar]
- 10.Ji J., Tao P., Wang Q., Li L., Xu Y. SIRT1: Mechanism and Protective in Diabetic Nephropathy. Endocr. Metab. Immune Disord. Drug Targets. 2020 doi: 10.2174/1871530320666201029143606. [DOI] [PubMed] [Google Scholar]
- 11.Zoja C., Xinaris C., Macconi D. Diabetic Nephropathy: Novel Molecular Mechanisms and Therapeutic Targets. Front. Pharmacol. 2020;11:586892. doi: 10.3389/fphar.2020.586892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y., Tian J., Mi Y., Ren X., Lian S., Kang J., Wang J., Zang H., Wu Z., Yang J., et al. Experimental study on renoprotective effect of intermedin on diabetic nephropathy. Mol. Cell. Endocrinol. 2021;528:111224. doi: 10.1016/j.mce.2021.111224. [DOI] [PubMed] [Google Scholar]
- 13.Lodhi A.H., Ahmad F.U., Furwa K., Madni A. Role of Oxidative Stress and Reduced Endogenous Hydrogen Sulfide in Diabetic Nephropathy. Drug Des. Devel. Ther. 2021;15:1031–1043. doi: 10.2147/DDDT.S291591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Powers A.C. Type 1 diabetes mellitus: Much progress, many opportunities. J. Clin. Investig. 2021;131 doi: 10.1172/JCI142242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tuch B., Dunlop M., Proietto J. Diabetes Research: A Guide for Postgraduates. Harwood Academic Publishers; Reading, UK: 2000. [Google Scholar]
- 16.Volfson-Sedletsky V., Jones A., IV, Hernandez-Escalante J., Dooms H. Emerging Therapeutic Strategies to Restore Regulatory T Cell Control of Islet Autoimmunity in Type 1 Diabetes. Front. Immunol. 2021;12:635767. doi: 10.3389/fimmu.2021.635767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol. Res. 2001;50:537–546. [PubMed] [Google Scholar]
- 18.Szkudelski T. Streptozotocin-nicotinamide-induced diabetes in the rat. Characteristics of the experimental model. Exp. Biol. Med. 2012;237:481–490. doi: 10.1258/ebm.2012.011372. [DOI] [PubMed] [Google Scholar]
- 19.Kahn S.E., Cooper M.E., Del Prato S. Pathophysiology and treatment of type 2 diabetes: Perspectives on the past, present, and future. Lancet. 2014;383:1068–1083. doi: 10.1016/S0140-6736(13)62154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeFronzo R.A. Pathogenesis of type 2 diabetes mellitus. Med. Clin. N. Am. 2004;88:787–835. doi: 10.1016/j.mcna.2004.04.013. ix. [DOI] [PubMed] [Google Scholar]
- 21.DeFronzo R.A. Insulin resistance: A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidaemia and atherosclerosis. Neth. J. Med. 1997;50:191–197. doi: 10.1016/S0300-2977(97)00012-0. [DOI] [PubMed] [Google Scholar]
- 22.Barnett A.H. Type 2 Diabetes. 2nd ed. Oxford University Press; New York, NY, USA: 2012. p. 162. [Google Scholar]
- 23.Abdul-Ghani M.A., DeFronzo R.A. Oxidative stress in type 2 diabetes. In: Miwa S., Beckman K.B., Muller F.L., editors. Oxidative Stress in Aging. Humana Press; Totowa, NJ, USA: 2008. pp. 191–212. [Google Scholar]
- 24.Maqbool M., Cooper M.E., Jandeleit-Dahm K.A.M. Cardiovascular Disease and Diabetic Kidney Disease. Semin. Nephrol. 2018;38:217–232. doi: 10.1016/j.semnephrol.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Su J., Ye D., Gao C., Huang Q., Gui D. Mechanism of progression of diabetic kidney disease mediated by podocyte mitochondrial injury. Mol. Biol. Rep. 2020;47:8023–8035. doi: 10.1007/s11033-020-05749-0. [DOI] [PubMed] [Google Scholar]
- 26.Sharma K., McCue P., Dunn S.R. Diabetic kidney disease in the db/db mouse. Am. J. Physiol. Renal Physiol. 2003;284:F1138–F1144. doi: 10.1152/ajprenal.00315.2002. [DOI] [PubMed] [Google Scholar]
- 27.Tesch G.H., Lim A.K. Recent insights into diabetic renal injury from the db/db mouse model of type 2 diabetic nephropathy. Am. J. Physiol. Renal Physiol. 2011;300:F301–F310. doi: 10.1152/ajprenal.00607.2010. [DOI] [PubMed] [Google Scholar]
- 28.Martin K.J., Gonzalez E.A. Vitamin D and the kidney. Mo. Med. 2012;109:124–126. [PMC free article] [PubMed] [Google Scholar]
- 29.Dusso A.S. Kidney disease and vitamin D levels: 25-hydroxyvitamin D, 1,25-dihydroxyvitamin D, and VDR activation. Kidney Int. Suppl. 2011;1:136–141. doi: 10.1038/kisup.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iida K., Shinki T., Yamaguchi A., DeLuca H.F., Kurokawa K., Suda T. A possible role of vitamin D receptors in regulating vitamin D activation in the kidney. Proc. Natl. Acad. Sci. USA. 1995;92:6112–6116. doi: 10.1073/pnas.92.13.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Souma T., Suzuki N., Yamamoto M. Renal erythropoietin-producing cells in health and disease. Front. Physiol. 2015;6:167. doi: 10.3389/fphys.2015.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki N., Yamamoto M. Roles of renal erythropoietin-producing (REP) cells in the maintenance of systemic oxygen homeostasis. Pflugers Arch. 2016;468:3–12. doi: 10.1007/s00424-015-1740-2. [DOI] [PubMed] [Google Scholar]
- 33.Hu X., Xie J., Chen N. Hypoxia-Inducible Factor-Proline Hydroxylase Inhibitor in the Treatment of Renal Anemia. Kidney Dis. 2021;7:1–9. doi: 10.1159/000510587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galuska D., Pacal L., Kankova K. Pathophysiological Implication of Vitamin D in Diabetic Kidney Disease. Kidney Blood Press Res. 2021;46:152–161. doi: 10.1159/000514286. [DOI] [PubMed] [Google Scholar]
- 35.Xiao X., Wang Y., Hou Y., Han F., Ren J., Hu Z. Vitamin D deficiency and related risk factors in patients with diabetic nephropathy. J. Int. Med. Res. 2016;44:673–684. doi: 10.1177/0300060515593765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xie S., Huang L., Cao W., Hu Y., Sun H., Cao L., Liu K., Liu C. Association between serum 25-hydroxyvitamin D and diabetic kidney disease in Chinese patients with type 2 diabetes. PLoS ONE. 2019;14:e0214728. doi: 10.1371/journal.pone.0214728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsai S.F., Tarng D.C. Anemia in patients of diabetic kidney disease. J. Chin. Med. Assoc. 2019;82:752–755. doi: 10.1097/JCMA.0000000000000175. [DOI] [PubMed] [Google Scholar]
- 38.Sonkar S.K., Singh H.P., Sonkar G.K., Pandey S. Association of Vitamin D and secondary hyperparathyroidism with anemia in diabetic kidney disease. J. Family Med. Prim. Care. 2018;7:815–818. doi: 10.4103/jfmpc.jfmpc_174_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomas M., Tsalamandris C., MacIsaac R., Jerums G. Anaemia in diabetes: An emerging complication of microvascular disease. Curr. Diabetes Rev. 2005;1:107–126. doi: 10.2174/1573399052952587. [DOI] [PubMed] [Google Scholar]
- 40.Ravanan R., Spiro J.R., Mathieson P.W., Smith R.M. Impact of diabetes on haemoglobin levels in renal disease. Diabetologia. 2007;50:26–31. doi: 10.1007/s00125-006-0514-y. [DOI] [PubMed] [Google Scholar]
- 41.Feng B., Ruiz M.A., Chakrabarti S. Oxidative-stress-induced epigenetic changes in chronic diabetic complications. Can. J. Physiol. Pharmacol. 2013;91:213–220. doi: 10.1139/cjpp-2012-0251. [DOI] [PubMed] [Google Scholar]
- 42.Naruse K., Rask-Madsen C., Takahara N., Ha S.W., Suzuma K., Way K.J., Jacobs J.R., Clermont A.C., Ueki K., Ohshiro Y., et al. Activation of vascular protein kinase C-beta inhibits Akt-dependent endothelial nitric oxide synthase function in obesity-associated insulin resistance. Diabetes. 2006;55:691–698. doi: 10.2337/diabetes.55.03.06.db05-0771. [DOI] [PubMed] [Google Scholar]
- 43.Gurel Z., Sieg K.M., Shallow K.D., Sorenson C.M., Sheibani N. Retinal O-linked N-acetylglucosamine protein modifications: Implications for postnatal retinal vascularization and the pathogenesis of diabetic retinopathy. Mol. Vis. 2013;19:1047–1059. [PMC free article] [PubMed] [Google Scholar]
- 44.McLarty J.L., Marsh S.A., Chatham J.C. Post-translational protein modification by O-linked N-acetyl-glucosamine: Its role in mediating the adverse effects of diabetes on the heart. Life Sci. 2013;92:621–627. doi: 10.1016/j.lfs.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lovestone S., Smith U. Advanced glycation end products, dementia, and diabetes. Proc. Natl. Acad. Sci. USA. 2014;111:4743–4744. doi: 10.1073/pnas.1402277111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mellor K.M., Brimble M.A., Delbridge L.M. Glucose as an agent of post-translational modification in diabetes—New cardiac epigenetic insights. Life Sci. 2014 doi: 10.1016/j.lfs.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 47.Yan L.J. Pathogenesis of Chronic Hyperglycemia: From Reductive Stress to Oxidative Stress. J. Diabetes Res. 2014;2014:137919. doi: 10.1155/2014/137919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yan L.J. Redox imbalance stress in diabetes mellitus: Role of the polyol pathway. Animal Model. Exp. Med. 2018;1:7–13. doi: 10.1002/ame2.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berg D., Youdim M.B., Riederer P. Redox imbalance. Cell Tissue Res. 2004;318:201–213. doi: 10.1007/s00441-004-0976-5. [DOI] [PubMed] [Google Scholar]
- 50.Hayden M.R., Sowers J.R. Redox imbalance in diabetes. Antioxid. Redox Signal. 2007;9:865–867. doi: 10.1089/ars.2007.1640. [DOI] [PubMed] [Google Scholar]
- 51.Wu J., Jin Z., Zheng H., Yan L.J. Sources and implications of NADH/NAD+ redox imbalance in diabetes and its complications. Diabetes Metab. Syndr. Obes. 2016;9:145–153. doi: 10.2147/DMSO.S106087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhan M., Usman I.M., Sun L., Kanwar Y.S. Disruption of renal tubular mitochondrial quality control by Myo-inositol oxygenase in diabetic kidney disease. J. Am. Soc. Nephrol. 2015;26:1304–1321. doi: 10.1681/ASN.2014050457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miranda-Diaz A.G., Pazarin-Villasenor L., Yanowsky-Escatell F.G., Andrade-Sierra J. Oxidative Stress in Diabetic Nephropathy with Early Chronic Kidney Disease. J. Diabetes Res. 2016;2016:7047238. doi: 10.1155/2016/7047238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yanar K., Coskun Z.M., Beydogan A.B., Aydin S., Bolkent S. The effects of delta-9-tetrahydrocannabinol on Kruppel-like factor-4 expression, redox homeostasis, and inflammation in the kidney of diabetic rat. J. Cell. Biochem. 2019;120:16219–16228. doi: 10.1002/jcb.28903. [DOI] [PubMed] [Google Scholar]
- 55.Amorim R.G., Guedes G.D.S., Vasconcelos S.M.L., Santos J.C.F. Kidney Disease in Diabetes Mellitus: Cross-Linking between Hyperglycemia, Redox Imbalance and Inflammation. Arq. Bras. Cardiol. 2019;112:577–587. doi: 10.5935/abc.20190077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tilton R.G., Baier L.D., Harlow J.E., Smith S.R., Ostrow E., Williamson J.R. Diabetes-induced glomerular dysfunction: Links to a more reduced cytosolic ratio of NADH/NAD+ Kidney Int. 1992;41:778–788. doi: 10.1038/ki.1992.121. [DOI] [PubMed] [Google Scholar]
- 57.Luo X., Li R., Yan L.J. Roles of Pyruvate, NADH, and Mitochondrial Complex I in Redox Balance and Imbalance in β Cell Function and Dysfunction. J. Diabetes Res. 2015;2015:512618. doi: 10.1155/2015/512618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luo X., Wu J., Jing S., Yan L.J. Hyperglycemic stress and carbon stress in diabetic glucotoxicity. Aging Dis. 2016;7:90–110. doi: 10.14336/AD.2015.0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ido Y. Pyridine nucleotide redox abnormalities in diabetes. Antioxid. Redox Signal. 2007;9:931–942. doi: 10.1089/ars.2007.1630. [DOI] [PubMed] [Google Scholar]
- 60.Chung S.S., Ho E.C., Lam K.S., Chung S.K. Contribution of polyol pathway to diabetes-induced oxidative stress. J. Am. Soc. Nephrol. 2003;14:S233–S236. doi: 10.1097/01.ASN.0000077408.15865.06. [DOI] [PubMed] [Google Scholar]
- 61.Dunlop M. Aldose reductase and the role of the polyol pathway in diabetic nephropathy. Kidney Int. Suppl. 2000;77:S3–S12. doi: 10.1046/j.1523-1755.2000.07702.x. [DOI] [PubMed] [Google Scholar]
- 62.Fantus I.G. The pathogenesis of the chronic complications of the diabetes mellitus. Endocrinol. Rounds. 2002;2:1–8. [Google Scholar]
- 63.Chatzilias A.A., Whiteside C.I. Cellular mechanisms of glucose-induced myo-inositol transport upregulation in rat mesangial cells. Am. J. Physiol. 1994;267:F459–F466. doi: 10.1152/ajprenal.1994.267.3.F459. [DOI] [PubMed] [Google Scholar]
- 64.Yancey P.H., Haner R.G., Freudenberger T.H. Effects of an aldose reductase inhibitor on organic osmotic effectors in rat renal medulla. Am. J. Physiol. 1990;259:F733–F738. doi: 10.1152/ajprenal.1990.259.5.F733. [DOI] [PubMed] [Google Scholar]
- 65.Brownlee M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 66.Veeresham C., Rama Rao A., Asres K. Aldose reductase inhibitors of plant origin. Phytother. Res. 2014;28:317–333. doi: 10.1002/ptr.5000. [DOI] [PubMed] [Google Scholar]
- 67.El Gamal H., Eid A.H., Munusamy S. Renoprotective Effects of Aldose Reductase Inhibitor Epalrestat against High Glucose-Induced Cellular Injury. Biomed. Res. Int. 2017;2017:5903105. doi: 10.1155/2017/5903105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shukla K., Pal P.B., Sonowal H., Srivastava S.K., Ramana K.V. Aldose Reductase Inhibitor Protects against Hyperglycemic Stress by Activating Nrf2-Dependent Antioxidant Proteins. J. Diabetes Res. 2017;2017:6785852. doi: 10.1155/2017/6785852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Artasensi A., Pedretti A., Vistoli G., Fumagalli L. Type 2 Diabetes Mellitus: A Review of Multi-Target Drugs. Molecules. 2020;25:1987. doi: 10.3390/molecules25081987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sonowal H., Ramana K.V. Development of aldose reductase inhibitors for the treatment of inflammatory disorders and cancer: Current drug design strategies and future directions. Curr. Med. Chem. 2020 doi: 10.2174/0929867327666201027152737. [DOI] [PubMed] [Google Scholar]
- 71.Jannapureddy S., Sharma M., Yepuri G., Schmidt A.M., Ramasamy R. Aldose Reductase: An Emerging Target for Development of Interventions for Diabetic Cardiovascular Complications. Front. Endocrinol. 2021;12:636267. doi: 10.3389/fendo.2021.636267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou X., Liu Z., Ying K., Wang H., Liu P., Ji X., Chi T., Zou L., Wang S., He Z. WJ-39, an Aldose Reductase Inhibitor, Ameliorates Renal Lesions in Diabetic Nephropathy by Activating Nrf2 Signaling. Oxid. Med. Cell. Longev. 2020;2020:7950457. doi: 10.1155/2020/7950457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Suzen S., Buyukbingol E. Recent studies of aldose reductase enzyme inhibition for diabetic complications. Curr. Med. Chem. 2003;10:1329–1352. doi: 10.2174/0929867033457377. [DOI] [PubMed] [Google Scholar]
- 74.Reddy A.B., Ramana K.V. Aldose reductase inhibition: Emerging drug target for the treatment of cardiovascular complications. Recent Pat. Cardiovasc. Drug Discov. 2010;5:25–32. doi: 10.2174/157489010790192683. [DOI] [PubMed] [Google Scholar]
- 75.Ohmura C., Watada H., Azuma K., Shimizu T., Kanazawa A., Ikeda F., Yoshihara T., Fujitani Y., Hirose T., Tanaka Y., et al. Aldose reductase inhibitor, epalrestat, reduces lipid hydroperoxides in type 2 diabetes. Endocr. J. 2009;56:149–156. doi: 10.1507/endocrj.K08E-237. [DOI] [PubMed] [Google Scholar]
- 76.Tang J., Du Y., Petrash J.M., Sheibani N., Kern T.S. Deletion of aldose reductase from mice inhibits diabetes-induced retinal capillary degeneration and superoxide generation. PLoS ONE. 2013;8:e62081. doi: 10.1371/journal.pone.0062081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Williamson J.R., Chang K., Frangos M., Hasan K.S., Ido Y., Kawamura T., Nyengaard J.R., van den Enden M., Kilo C., Tilton R.G. Hyperglycemic pseudohypoxia and diabetic complications. Diabetes. 1993;42:801–813. doi: 10.2337/diab.42.6.801. [DOI] [PubMed] [Google Scholar]
- 78.Ido Y., Williamson J.R. Hyperglycemic cytosolic reductive stress ‘pseudohypoxia’: Implications for diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 1997;38:1467–1470. [PubMed] [Google Scholar]
- 79.Yang L., Garcia Canaveras J.C., Chen Z., Wang L., Liang L., Jang C., Mayr J.A., Zhang Z., Ghergurovich J.M., Zhan L., et al. Serine Catabolism Feeds NADH when Respiration Is Impaired. Cell. Metab. 2020;31:809–821. doi: 10.1016/j.cmet.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Harden W.R., III, Barlow C.H., Simson M.B., Harken A.H. Temporal relation between onset of cell anoxia and ischemic contractile failure. Myocardial ischemia and left ventricular failure in the isolated, perfused rabbit heart. Am. J. Cardiol. 1979;44:741–746. doi: 10.1016/0002-9149(79)90296-0. [DOI] [PubMed] [Google Scholar]
- 81.He M.D., Xu S.C., Zhang X., Wang Y., Xiong J.C., Zhang X., Lu Y.H., Zhang L., Yu Z.P., Zhou Z. Disturbance of aerobic metabolism accompanies neurobehavioral changes induced by nickel in mice. Neurotoxicology. 2013;38:9–16. doi: 10.1016/j.neuro.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 82.Doke T., Ishimoto T., Hayasaki T., Ikeda S., Hasebe M., Hirayama A., Soga T., Kato N., Kosugi T., Tsuboi N., et al. Lacking ketohexokinase-A exacerbates renal injury in streptozotocin-induced diabetic mice. Metabolism. 2018;85:161–170. doi: 10.1016/j.metabol.2018.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zakaria E.M., El-Maraghy N.N., Ahmed A.F., Ali A.A., El-Bassossy H.M. PARP inhibition ameliorates nephropathy in an animal model of type 2 diabetes: Focus on oxidative stress, inflammation, and fibrosis. Naunyn Schmiedebergs Arch. Pharmacol. 2017;390:621–631. doi: 10.1007/s00210-017-1360-9. [DOI] [PubMed] [Google Scholar]
- 84.Massudi H., Grant R., Guillemin G.J., Braidy N. NAD+ metabolism and oxidative stress: The golden nucleotide on a crown of thorns. Redox Rep. 2012;17:28–46. doi: 10.1179/1351000212Y.0000000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nikiforov A., Kulikova V., Ziegler M. The human NAD metabolome: Functions, metabolism and compartmentalization. Crit. Rev. Biochem. Mol. Biol. 2015;50:284–297. doi: 10.3109/10409238.2015.1028612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pieper A.A., Brat D.J., Krug D.K., Watkins C.C., Gupta A., Blackshaw S., Verma A., Wang Z.Q., Snyder S.H. Poly (ADP-ribose) polymerase-deficient mice are protected from streptozotocin-induced diabetes. Proc. Natl. Acad. Sci. USA. 1999;96:3059–3064. doi: 10.1073/pnas.96.6.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Masutani M., Suzuki H., Kamada N., Watanabe M., Ueda O., Nozaki T., Jishage K., Watanabe T., Sugimoto T., Nakagama H., et al. Poly (ADP-ribose) polymerase gene disruption conferred mice resistant to streptozotocin-induced diabetes. Proc. Natl. Acad. Sci. USA. 1999;96:2301–2304. doi: 10.1073/pnas.96.5.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vedantham S., Thiagarajan D., Ananthakrishnan R., Wang L., Rosario R., Zou Y.S., Goldberg I., Yan S.F., Schmidt A.M., Ramasamy R. Aldose reductase drives hyperacetylation of Egr-1 in hyperglycemia and consequent upregulation of proinflammatory and prothrombotic signals. Diabetes. 2014;63:761–774. doi: 10.2337/db13-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sauve A.A. Sirtuin chemical mechanisms. Biochim. Biophys. Acta. 2010;1804:1591–1603. doi: 10.1016/j.bbapap.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wu J., Jin Z., Yan L.J. Redox imbalance and mitochondrial abnormalities in the diabetic lung. Redox Biol. 2017;11:51–59. doi: 10.1016/j.redox.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chini C.C.S., Tarrago M.G., Chini E.N. NAD and the aging process: Role in life, death and everything in between. Mol. Cell. Endocrinol. 2017;455:62–74. doi: 10.1016/j.mce.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Benzi A., Sturla L., Heine M., Fischer A.W., Spinelli S., Magnone M., Sociali G., Parodi A., Fenoglio D., Emionite L., et al. CD38 downregulation modulates NAD+ and NADP (H) levels in thermogenic adipose tissues. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids. 2021;1866:158819. doi: 10.1016/j.bbalip.2020.158819. [DOI] [PubMed] [Google Scholar]
- 93.Peclat T.R., Shi B., Varga J., Chini E.N. The NADase enzyme CD38: An emerging pharmacological target for systemic sclerosis, systemic lupus erythematosus and rheumatoid arthritis. Curr. Opin. Rheumatol. 2020;32:488–496. doi: 10.1097/BOR.0000000000000737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Covarrubias A.J., Kale A., Perrone R., Lopez-Dominguez J.A., Pisco A.O., Kasler H.G., Schmidt M.S., Heckenbach I., Kwok R., Wiley C.D., et al. Senescent cells promote tissue NAD+ decline during ageing via the activation of CD38+ macrophages. Nat. Metab. 2020;2:1265–1283. doi: 10.1038/s42255-020-00305-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zuo W., Liu N., Zeng Y., Liu Y., Li B., Wu K., Xiao Y., Liu Q. CD38: A Potential Therapeutic Target in Cardiovascular Disease. Cardiovasc. Drugs Ther. 2020:1–14. doi: 10.1007/s10557-020-07007-8. [DOI] [PubMed] [Google Scholar]
- 96.Sun L., Adebanjo O.A., Moonga B.S., Corisdeo S., Anandatheerthavarada H.K., Biswas G., Arakawa T., Hakeda Y., Koval A., Sodam B., et al. CD38/ADP-ribosyl cyclase: A new role in the regulation of osteoclastic bone resorption. J. Cell. Biol. 1999;146:1161–1172. doi: 10.1083/jcb.146.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shi B., Wang W., Korman B., Kai L., Wang Q., Wei J., Bale S., Marangoni R.G., Bhattacharyya S., Miller S., et al. Targeting CD38-dependent NAD+ metabolism to mitigate multiple organ fibrosis. iScience. 2021;24:101902. doi: 10.1016/j.isci.2020.101902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ogura Y., Kitada M., Xu J., Monno I., Koya D. CD38 inhibition by apigenin ameliorates mitochondrial oxidative stress through restoration of the intracellular NAD+/NADH ratio and Sirt3 activity in renal tubular cells in diabetic rats. Aging. 2020;12:11325–11336. doi: 10.18632/aging.103410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tedeschi P.M., Bansal N., Kerrigan J.E., Abali E.E., Scotto K.W., Bertino J.R. NAD+ Kinase as a Therapeutic Target in Cancer. Clin. Cancer Res. 2016;22:5189–5195. doi: 10.1158/1078-0432.CCR-16-1129. [DOI] [PubMed] [Google Scholar]
- 100.Zhang R. MNADK, a Long-Awaited Human Mitochondrion-Localized NAD Kinase. J. Cell. Physiol. 2015;230:1697–1701. doi: 10.1002/jcp.24926. [DOI] [PubMed] [Google Scholar]
- 101.Shi F., Li Y., Li Y., Wang X. Molecular properties, functions, and potential applications of NAD kinases. Acta Biochim. Biophys. Sin. 2009;41:352–361. doi: 10.1093/abbs/gmp029. [DOI] [PubMed] [Google Scholar]
- 102.Hoxhaj G., Ben-Sahra I., Lockwood S.E., Timson R.C., Byles V., Henning G.T., Gao P., Selfors L.M., Asara J.M., Manning B.D. Direct stimulation of NADP+ synthesis through Akt-mediated phosphorylation of NAD kinase. Science. 2019;363:1088–1092. doi: 10.1126/science.aau3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen L., Zhang Z., Hoshino A., Zheng H.D., Morley M., Arany Z., Rabinowitz J.D. NADPH production by the oxidative pentose-phosphate pathway supports folate metabolism. Nat. Metab. 2019;1:404–415. doi: 10.1038/s42255-019-0043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Spencer N.Y., Stanton R.C. Glucose 6-phosphate dehydrogenase and the kidney. Curr. Opin. Nephrol. Hypertens. 2017;26:43–49. doi: 10.1097/MNH.0000000000000294. [DOI] [PubMed] [Google Scholar]
- 105.Yan L.J., Christians E.S., Liu L., Xiao X., Sohal R.S., Benjamin I.J. Mouse heat shock transcription factor 1 deficiency alters cardiac redox homeostasis and increases mitochondrial oxidative damage. EMBO J. 2002;21:5164–5172. doi: 10.1093/emboj/cdf528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ogura Y., Kitada M., Monno I., Kanasaki K., Watanabe A., Koya D. Renal mitochondrial oxidative stress is enhanced by the reduction of Sirt3 activity, in Zucker diabetic fatty rats. Redox Rep. 2018;23:153–159. doi: 10.1080/13510002.2018.1487174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Forbes J.M., Coughlan M.T., Cooper M.E. Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes. 2008;57:1446–1454. doi: 10.2337/db08-0057. [DOI] [PubMed] [Google Scholar]
- 108.Turrens J.F. Superoxide production by the mitochondrial respiratory chain. Biosci. Rep. 1997;17:3–8. doi: 10.1023/A:1027374931887. [DOI] [PubMed] [Google Scholar]
- 109.Turrens J.F., Alexandre A., Lehninger A.L. Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Arch. Biochem. Biophys. 1985;237:408–414. doi: 10.1016/0003-9861(85)90293-0. [DOI] [PubMed] [Google Scholar]
- 110.Vujic A., Koo A.N.M., Prag H.A., Krieg T. Mitochondrial redox and TCA cycle metabolite signaling in the heart. Free Radic. Biol. Med. 2021;166:287–296. doi: 10.1016/j.freeradbiomed.2021.02.041. [DOI] [PubMed] [Google Scholar]
- 111.Lee S.R., An E.J., Kim J., Bae Y.S. Function of NADPH Oxidases in Diabetic Nephropathy and Development of Nox Inhibitors. Biomol. Ther. 2020;28:25–33. doi: 10.4062/biomolther.2019.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Granata S., Dalla Gassa A., Tomei P., Lupo A., Zaza G. Mitochondria: A new therapeutic target in chronic kidney disease. Nutr. Metab. 2015;12:49. doi: 10.1186/s12986-015-0044-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wu J., Luo X., Thangthaeng N., Sumien N., Chen Z., Rutledge M.A., Jing S., Forster M.J., Yan L.J. Pancreatic mitochondrial complex I exhibits aberrant hyperactivity in diabetes. Biochem. Biophys. Rep. 2017;11:119–129. doi: 10.1016/j.bbrep.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Murphy M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Drose S., Brandt U. Molecular mechanisms of superoxide production by the mitochondrial respiratory chain. Adv. Exp. Med. Biol. 2012;748:145–169. doi: 10.1007/978-1-4614-3573-0_6. [DOI] [PubMed] [Google Scholar]
- 116.Yan L.J., Sumien N., Thangthaeng N., Forster M.J. Reversible inactivation of dihydrolipoamide dehydrogenase by mitochondrial hydrogen peroxide. Free Radic. Res. 2013;47:123–133. doi: 10.3109/10715762.2012.752078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zheng H., Wu J., Jin Z., Yan L.J. Protein Modifications as Manifestations of Hyperglycemic Glucotoxicity in Diabetes and Its Complications. Biochem. Insights. 2016;9:1–9. doi: 10.4137/BCI.S36141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yan L.J., Lodge J.K., Traber M.G., Matsugo S., Packer L. Comparison between copper-mediated and hypochlorite-mediated modifications of human low density lipoproteins evaluated by protein carbonyl formation. J. Lipid Res. 1997;38:992–1001. doi: 10.1016/S0022-2275(20)37223-0. [DOI] [PubMed] [Google Scholar]
- 119.Ames B.N., Shigenaga M.K. Oxidants are a major contributor to aging. Ann. N. Y. Acad. Sci. 1992;663:85–96. doi: 10.1111/j.1749-6632.1992.tb38652.x. [DOI] [PubMed] [Google Scholar]
- 120.Stefek M., Gajdosik A., Tribulova N., Navarova J., Volkovova K., Weismann P., Gajdosikova A., Drimal J., Mihalova D. The pyridoindole antioxidant stobadine attenuates albuminuria, enzymuria, kidney lipid peroxidation and matrix collagen cross-linking in streptozotocin-induced diabetic rats. Methods Find. Exp. Clin. Pharmacol. 2002;24:565–571. [PubMed] [Google Scholar]
- 121.Wang W.X., Luo S.B., Jiang P., Xia M.M., Hei A.L., Mao Y.H., Li C.B., Hu G.X., Cai J.P. Increased Oxidative Damage of RNA in Early-Stage Nephropathy in db/db Mice. Oxid. Med. Cell. Longev. 2017;2017:2353729. doi: 10.1155/2017/2353729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wu J., Luo X., Jing S., Yan L.J. Two-dimensional gel electrophoretic detection of protein carbonyls derivatized with biotin-hydrazide. J. Chromatogr. B. 2016;1019:128–131. doi: 10.1016/j.jchromb.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bhargava P., Schnellmann R.G. Mitochondrial energetics in the kidney. Nat. Rev. Nephrol. 2017;13:629–646. doi: 10.1038/nrneph.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Higgins G.C., Coughlan M.T. Mitochondrial dysfunction and mitophagy: The beginning and end to diabetic nephropathy? Br. J. Pharmacol. 2014;171:1917–1942. doi: 10.1111/bph.12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yi H.S., Chang J.Y., Shong M. The mitochondrial unfolded protein response and mitohormesis: A perspective on metabolic diseases. J. Mol. Endocrinol. 2018;61:R91–R105. doi: 10.1530/JME-18-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Galvan D.L., Green N.H., Danesh F.R. The hallmarks of mitochondrial dysfunction in chronic kidney disease. Kidney Int. 2017;92:1051–1057. doi: 10.1016/j.kint.2017.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ayanga B.A., Badal S.S., Wang Y., Galvan D.L., Chang B.H., Schumacker P.T., Danesh F.R. Dynamin-Related Protein 1 Deficiency Improves Mitochondrial Fitness and Protects against Progression of Diabetic Nephropathy. J. Am. Soc. Nephrol. 2016;27:2733–2747. doi: 10.1681/ASN.2015101096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Galvan D.L., Long J., Green N., Chang B.H., Lin J.S., Schumacker P., Truong L.D., Overbeek P., Danesh F.R. Drp1S600 phosphorylation regulates mitochondrial fission and progression of nephropathy in diabetic mice. J. Clin. Investig. 2019;129:2807–2823. doi: 10.1172/JCI127277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang Y., Lu M., Xiong L., Fan J., Zhou Y., Li H., Peng X., Zhong Z., Wang Y., Huang F., et al. Drp1-mediated mitochondrial fission promotes renal fibroblast activation and fibrogenesis. Cell. Death Dis. 2020;11:29. doi: 10.1038/s41419-019-2218-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang Y., Zhang X., Wang P., Shen Y., Yuan K., Li M., Liang W., Que H. Sirt3 overexpression alleviates hyperglycemia-induced vascular inflammation through regulating redox balance, cell survival, and AMPK-mediated mitochondrial homeostasis. J. Recept. Signal Transduct. 2019;39:341–349. doi: 10.1080/10799893.2019.1684521. [DOI] [PubMed] [Google Scholar]
- 131.Wang Y., Katayama A., Terami T., Han X., Nunoue T., Zhang D., Teshigawara S., Eguchi J., Nakatsuka A., Murakami K., et al. Translocase of inner mitochondrial membrane 44 alters the mitochondrial fusion and fission dynamics and protects from type 2 diabetes. Metabolism. 2015;64:677–688. doi: 10.1016/j.metabol.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 132.Agil A., Chayah M., Visiedo L., Navarro-Alarcon M., Rodriguez Ferrer J.M., Tassi M., Reiter R.J., Fernandez-Vazquez G. Melatonin Improves Mitochondrial Dynamics and Function in the Kidney of Zucker Diabetic Fatty Rats. J. Clin. Med. 2020;9:2916. doi: 10.3390/jcm9092916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhan M., Usman I., Yu J., Ruan L., Bian X., Yang J., Yang S., Sun L., Kanwar Y.S. Perturbations in mitochondrial dynamics by p66Shc lead to renal tubular oxidative injury in human diabetic nephropathy. Clin. Sci. 2018;132:1297–1314. doi: 10.1042/CS20180005. [DOI] [PubMed] [Google Scholar]
- 134.Mishra R., Emancipator S.N., Kern T., Simonson M.S. High glucose evokes an intrinsic proapoptotic signaling pathway in mesangial cells. Kidney Int. 2005;67:82–93. doi: 10.1111/j.1523-1755.2005.00058.x. [DOI] [PubMed] [Google Scholar]
- 135.Coughlan M.T., Higgins G.C., Nguyen T.V., Penfold S.A., Thallas-Bonke V., Tan S.M., Ramm G., van Bergen N.J., Henstridge D.C., Sourris K.C., et al. Deficiency in Apoptosis-Inducing Factor Recapitulates Chronic Kidney Disease via Aberrant Mitochondrial Homeostasis. Diabetes. 2016;65:1085–1098. doi: 10.2337/db15-0864. [DOI] [PubMed] [Google Scholar]
- 136.Kostic S., Hauke T., Ghahramani N., Filipovic N., Vukojevic K. Expression pattern of apoptosis-inducing factor in the kidneys of streptozotocin-induced diabetic rats. Acta Histochem. 2020;122:151655. doi: 10.1016/j.acthis.2020.151655. [DOI] [PubMed] [Google Scholar]
- 137.Zhao Y., Guo Y., Jiang Y., Zhu X., Liu Y., Zhang X. Mitophagy regulates macrophage phenotype in diabetic nephropathy rats. Biochem. Biophys. Res. Commun. 2017;494:42–50. doi: 10.1016/j.bbrc.2017.10.088. [DOI] [PubMed] [Google Scholar]
- 138.Zuo Z., Jing K., Wu H., Wang S., Ye L., Li Z., Yang C., Pan Q., Liu W.J., Liu H.F. Mechanisms and Functions of Mitophagy and Potential Roles in Renal Disease. Front. Physiol. 2020;11:935. doi: 10.3389/fphys.2020.00935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nguyen T.N., Padman B.S., Lazarou M. Deciphering the Molecular Signals of PINK1/Parkin Mitophagy. Trends Cell Biol. 2016;26:733–744. doi: 10.1016/j.tcb.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 140.Springer M.Z., Macleod K.F. In Brief: Mitophagy: Mechanisms and role in human disease. J. Pathol. 2016;240:253–255. doi: 10.1002/path.4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Persson M.F., Franzen S., Catrina S.B., Dallner G., Hansell P., Brismar K., Palm F. Coenzyme Q10 prevents GDP-sensitive mitochondrial uncoupling, glomerular hyperfiltration and proteinuria in kidneys from db/db mice as a model of type 2 diabetes. Diabetologia. 2012;55:1535–1543. doi: 10.1007/s00125-012-2469-5. [DOI] [PubMed] [Google Scholar]
- 142.Fabris B., Candido R., Armini L., Fischetti F., Calci M., Bardelli M., Fazio M., Campanacci L., Carretta R. Control of glomerular hyperfiltration and renal hypertrophy by an angiotensin converting enzyme inhibitor prevents the progression of renal damage in hypertensive diabetic rats. J. Hypertens. 1999;17:1925–1931. doi: 10.1097/00004872-199917121-00023. [DOI] [PubMed] [Google Scholar]
- 143.Tan A.L., Sourris K.C., Harcourt B.E., Thallas-Bonke V., Penfold S., Andrikopoulos S., Thomas M.C., O’Brien R.C., Bierhaus A., Cooper M.E., et al. Disparate effects on renal and oxidative parameters following RAGE deletion, AGE accumulation inhibition, or dietary AGE control in experimental diabetic nephropathy. Am. J. Physiol. Renal Physiol. 2010;298:F763–F770. doi: 10.1152/ajprenal.00591.2009. [DOI] [PubMed] [Google Scholar]
- 144.Brand M.D. Riding the tiger—Physiological and pathological effects of superoxide and hydrogen peroxide generated in the mitochondrial matrix. Crit. Rev. Biochem. Mol. Biol. 2020;55:592–661. doi: 10.1080/10409238.2020.1828258. [DOI] [PubMed] [Google Scholar]
- 145.Brand M.D., Goncalves R.L., Orr A.L., Vargas L., Gerencser A.A., Borch Jensen M., Wang Y.T., Melov S., Turk C.N., Matzen J.T., et al. Suppressors of Superoxide-H2O2 Production at Site IQ of Mitochondrial Complex I Protect against Stem Cell Hyperplasia and Ischemia-Reperfusion Injury. Cell Metab. 2016;24:582–592. doi: 10.1016/j.cmet.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Apakkan Aksun S., Ozmen B., Ozmen D., Parildar Z., Senol B., Habif S., Mutaf I., Turgan N., Bayindir O. Serum and urinary nitric oxide in Type 2 diabetes with or without microalbuminuria: Relation to glomerular hyperfiltration. J. Diabetes Complicat. 2003;17:343–348. doi: 10.1016/S1056-8727(02)00196-4. [DOI] [PubMed] [Google Scholar]
- 147.Fang J., Wong H.S., Brand M.D. Production of superoxide and hydrogen peroxide in the mitochondrial matrix is dominated by site IQ of complex I in diverse cell lines. Redox Biol. 2020;37:101722. doi: 10.1016/j.redox.2020.101722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kuo C.W., Shen C.J., Tung Y.T., Chen H.L., Chen Y.H., Chang W.H., Cheng K.C., Yang S.H., Chen C.M. Extracellular superoxide dismutase ameliorates streptozotocin-induced rat diabetic nephropathy via inhibiting the ROS/ERK1/2 signaling. Life Sci. 2015;135:77–86. doi: 10.1016/j.lfs.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 149.Watson M.A., Wong H.S., Brand M.D. Use of S1QELs and S3QELs to link mitochondrial sites of superoxide and hydrogen peroxide generation to physiological and pathological outcomes. Biochem. Soc. Trans. 2019;47:1461–1469. doi: 10.1042/BST20190305. [DOI] [PubMed] [Google Scholar]
- 150.Plecita-Hlavata L., Engstova H., Jezek J., Holendova B., Tauber J., Petraskova L., Kren V., Jezek P. Potential of Mitochondria-Targeted Antioxidants to Prevent Oxidative Stress in Pancreatic beta-cells. Oxid. Med. Cell. Longev. 2019;2019:1826303. doi: 10.1155/2019/1826303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Homma T., Kobayashi S., Sato H., Fujii J. Superoxide produced by mitochondrial complex III plays a pivotal role in the execution of ferroptosis induced by cysteine starvation. Arch. Biochem. Biophys. 2021;700:108775. doi: 10.1016/j.abb.2021.108775. [DOI] [PubMed] [Google Scholar]
- 152.Heslop K.A., Rovini A., Hunt E.G., Fang D., Morris M.E., Christie C.F., Gooz M.B., DeHart D.N., Dang Y., Lemasters J.J., et al. JNK activation and translocation to mitochondria mediates mitochondrial dysfunction and cell death induced by VDAC opening and sorafenib in hepatocarcinoma cells. Biochem. Pharmacol. 2020;171:113728. doi: 10.1016/j.bcp.2019.113728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wong H.S., Mezera V., Dighe P., Melov S., Gerencser A.A., Sweis R.F., Pliushchev M., Wang Z., Esbenshade T., McKibben B., et al. Superoxide produced by mitochondrial site IQ inactivates cardiac succinate dehydrogenase and induces hepatic steatosis in Sod2 knockout mice. Free Radic. Biol. Med. 2021;164:223–232. doi: 10.1016/j.freeradbiomed.2020.12.447. [DOI] [PubMed] [Google Scholar]
- 154.Hatinguais R., Pradhan A., Brown G.D., Brown A.J.P., Warris A., Shekhova E. Mitochondrial Reactive Oxygen Species Regulate Immune Responses of Macrophages to Aspergillus fumigatus. Front. Immunol. 2021;12:641495. doi: 10.3389/fimmu.2021.641495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dare A.J., Bolton E.A., Pettigrew G.J., Bradley J.A., Saeb-Parsy K., Murphy M.P. Protection against renal ischemia-reperfusion injury in vivo by the mitochondria targeted antioxidant MitoQ. Redox Biol. 2015;5:163–168. doi: 10.1016/j.redox.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dare A.J., Logan A., Prime T.A., Rogatti S., Goddard M., Bolton E.M., Bradley J.A., Pettigrew G.J., Murphy M.P., Saeb-Parsy K. The mitochondria-targeted anti-oxidant MitoQ decreases ischemia-reperfusion injury in a murine syngeneic heart transplant model. J. Heart Lung Transplant. 2015;34:1471–1480. doi: 10.1016/j.healun.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.James A.M., Sharpley M.S., Manas A.R., Frerman F.E., Hirst J., Smith R.A., Murphy M.P. Interaction of the mitochondria-targeted antioxidant MitoQ with phospholipid bilayers and ubiquinone oxidoreductases. J. Biol. Chem. 2007;282:14708–14718. doi: 10.1074/jbc.M611463200. [DOI] [PubMed] [Google Scholar]
- 158.Saretzki G., Murphy M.P., von Zglinicki T. MitoQ counteracts telomere shortening and elongates lifespan of fibroblasts under mild oxidative stress. Aging Cell. 2003;2:141–143. doi: 10.1046/j.1474-9728.2003.00040.x. [DOI] [PubMed] [Google Scholar]
- 159.Yang M.Y., Fan Z., Zhang Z., Fan J. MitoQ protects against high glucose-induced brain microvascular endothelial cells injury via the Nrf2/HO-1 pathway. J. Pharmacol. Sci. 2021;145:105–114. doi: 10.1016/j.jphs.2020.10.007. [DOI] [PubMed] [Google Scholar]
- 160.Hou L., Zhang J., Liu Y., Fang H., Liao L., Wang Z., Yuan J., Wang X., Sun J., Tang B., et al. MitoQ alleviates LPS-mediated acute lung injury through regulating Nrf2/Drp1 pathway. Free Radic. Biol. Med. 2021;165:219–228. doi: 10.1016/j.freeradbiomed.2021.01.045. [DOI] [PubMed] [Google Scholar]
- 161.Cen M., Ouyang W., Zhang W., Yang L., Lin X., Dai M., Hu H., Tang H., Liu H., Xia J., et al. MitoQ protects against hyperpermeability of endothelium barrier in acute lung injury via a Nrf2-dependent mechanism. Redox Biol. 2021;41:101936. doi: 10.1016/j.redox.2021.101936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fortner K.A., Blanco L.P., Buskiewicz I., Huang N., Gibson P.C., Cook D.L., Pedersen H.L., Yuen P.S.T., Murphy M.P., Perl A., et al. Targeting mitochondrial oxidative stress with MitoQ reduces NET formation and kidney disease in lupus-prone MRL-lpr mice. Lupus Sci. Med. 2020;7:e000387. doi: 10.1136/lupus-2020-000387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chacko B.K., Reily C., Srivastava A., Johnson M.S., Ye Y., Ulasova E., Agarwal A., Zinn K.R., Murphy M.P., Kalyanaraman B., et al. Prevention of diabetic nephropathy in Ins2+/- AkitaJ mice by the mitochondria-targeted therapy MitoQ. Biochem. J. 2010;432:9–19. doi: 10.1042/BJ20100308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Xiao L., Xu X., Zhang F., Wang M., Xu Y., Tang D., Wang J., Qin Y., Liu Y., Tang C., et al. The mitochondria-targeted antioxidant MitoQ ameliorated tubular injury mediated by mitophagy in diabetic kidney disease via Nrf2/PINK1. Redox Biol. 2017;11:297–311. doi: 10.1016/j.redox.2016.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ward M.S., Flemming N.B., Gallo L.A., Fotheringham A.K., McCarthy D.A., Zhuang A., Tang P.H., Borg D.J., Shaw H., Harvie B., et al. Targeted mitochondrial therapy using MitoQ shows equivalent renoprotection to angiotensin converting enzyme inhibition but no combined synergy in diabetes. Sci. Rep. 2017;7:15190. doi: 10.1038/s41598-017-15589-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gao P., Yang M., Chen X., Xiong S., Liu J., Sun L. DsbA-L deficiency exacerbates mitochondrial dysfunction of tubular cells in diabetic kidney disease. Clin. Sci. 2020;134:677–694. doi: 10.1042/CS20200005. [DOI] [PubMed] [Google Scholar]
- 167.Han Y., Xu X., Tang C., Gao P., Chen X., Xiong X., Yang M., Yang S., Zhu X., Yuan S., et al. Reactive oxygen species promote tubular injury in diabetic nephropathy: The role of the mitochondrial ros-txnip-nlrp3 biological axis. Redox Biol. 2018;16:32–46. doi: 10.1016/j.redox.2018.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fujita H., Fujishima H., Chida S., Takahashi K., Qi Z., Kanetsuna Y., Breyer M.D., Harris R.C., Yamada Y., Takahashi T. Reduction of renal superoxide dismutase in progressive diabetic nephropathy. J. Am. Soc. Nephrol. 2009;20:1303–1313. doi: 10.1681/ASN.2008080844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Peixoto E.B., Pessoa B.S., Biswas S.K., Lopes de Faria J.B. Antioxidant SOD mimetic prevents NADPH oxidase-induced oxidative stress and renal damage in the early stage of experimental diabetes and hypertension. Am. J. Nephrol. 2009;29:309–318. doi: 10.1159/000163767. [DOI] [PubMed] [Google Scholar]
- 170.Asaba K., Tojo A., Onozato M.L., Goto A., Fujita T. Double-edged action of SOD mimetic in diabetic nephropathy. J. Cardiovasc. Pharmacol. 2007;49:13–19. doi: 10.1097/FJC.0b013e31802b6530. [DOI] [PubMed] [Google Scholar]
- 171.Rafikova O., Salah E.M., Tofovic S.P. Renal and metabolic effects of tempol in obese ZSF1 rats—Distinct role for superoxide and hydrogen peroxide in diabetic renal injury. Metabolism. 2008;57:1434–1444. doi: 10.1016/j.metabol.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 172.El-Mahdy M.A., Alzarie Y.A., Hemann C., Badary O.A., Nofal S., Zweier J.L. The novel SOD mimetic GC4419 increases cancer cell killing with sensitization to ionizing radiation while protecting normal cells. Free Radic. Biol. Med. 2020;160:630–642. doi: 10.1016/j.freeradbiomed.2020.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Anderson C.M., Lee C.M., Saunders D.P., Curtis A., Dunlap N., Nangia C., Lee A.S., Gordon S.M., Kovoor P., Arevalo-Araujo R., et al. Phase IIb, Randomized, Double-Blind Trial of GC4419 Versus Placebo to Reduce Severe Oral Mucositis Due to Concurrent Radiotherapy and Cisplatin for Head and Neck Cancer. J. Clin. Oncol. 2019;37:3256–3265. doi: 10.1200/JCO.19.01507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Langan A.R., Khan M.A., Yeung I.W., van Dyk J., Hill R.P. Partial volume rat lung irradiation: The protective/mitigating effects of Eukarion-189, a superoxide dismutase-catalase mimetic. Radiother. Oncol. 2006;79:231–238. doi: 10.1016/j.radonc.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 175.Garcia-Quintans N., Prieto I., Sanchez-Ramos C., Luque A., Arza E., Olmos Y., Monsalve M. Regulation of endothelial dynamics by PGC-1alpha relies on ROS control of VEGF-A signaling. Free Radic. Biol. Med. 2016;93:41–51. doi: 10.1016/j.freeradbiomed.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 176.Hosakote Y.M., Komaravelli N., Mautemps N., Liu T., Garofalo R.P., Casola A. Antioxidant mimetics modulate oxidative stress and cellular signaling in airway epithelial cells infected with respiratory syncytial virus. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012;303:L991–L1000. doi: 10.1152/ajplung.00192.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Torbati F.A., Ramezani M., Dehghan R., Amiri M.S., Moghadam A.T., Shakour N., Elyasi S., Sahebkar A., Emami S.A. Ethnobotany, Phytochemistry and Pharmacological Features of Centella asiatica: A Comprehensive Review. Adv. Exp. Med. Biol. 2021;1308:451–499. doi: 10.1007/978-3-030-64872-5_25. [DOI] [PubMed] [Google Scholar]
- 178.Liu I.M., Tzeng T.F., Liou S.S., Chang C.J. Angelica acutiloba root attenuates insulin resistance induced by high-fructose diet in rats. Phytother. Res. 2011;25:1283–1293. doi: 10.1002/ptr.3403. [DOI] [PubMed] [Google Scholar]
- 179.Wang H., Guan Y., Widlund A.L., Becker L.B., Baur J.A., Reilly P.M., Sims C.A. Resveratrol ameliorates mitochondrial dysfunction but increases the risk of hypoglycemia following hemorrhagic shock. J. Trauma Acute Care Surg. 2014;77:926–933. doi: 10.1097/TA.0000000000000358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bao L., Cai X., Zhang Z., Li Y. Grape seed procyanidin B2 ameliorates mitochondrial dysfunction and inhibits apoptosis via the AMP-activated protein kinase-silent mating type information regulation 2 homologue 1-PPARgamma co-activator-1alpha axis in rat mesangial cells under high-dose glucosamine. Br. J. Nutr. 2015;113:35–44. doi: 10.1017/S000711451400347X. [DOI] [PubMed] [Google Scholar]
- 181.Guerrero-Hue M., Rayego-Mateos S., Vazquez-Carballo C., Palomino-Antolin A., Garcia-Caballero C., Opazo-Rios L., Morgado-Pascual J.L., Herencia C., Mas S., Ortiz A., et al. Protective Role of Nrf2 in Renal Disease. Antioxidants. 2020;10:39. doi: 10.3390/antiox10010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Menini S., Iacobini C., Vitale M., Pugliese G. The Inflammasome in Chronic Complications of Diabetes and Related Metabolic Disorders. Cells. 2020;9:1812. doi: 10.3390/cells9081812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sato S., Kataoka S., Kimura A., Mukai Y. Azuki bean (Vigna angularis) extract reduces oxidative stress and stimulates autophagy in the kidneys of streptozotocin-induced early diabetic rats. Can. J. Physiol. Pharmacol. 2016;94:1298–1303. doi: 10.1139/cjpp-2015-0540. [DOI] [PubMed] [Google Scholar]
- 184.Omara E.A., Nada S.A., Farrag A.R., Sharaf W.M., El-Toumy S.A. Therapeutic effect of Acacia nilotica pods extract on streptozotocin induced diabetic nephropathy in rat. Phytomedicine. 2012;19:1059–1067. doi: 10.1016/j.phymed.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 185.Navale A.M., Paranjape A. Antidiabetic and renoprotective effect of Anogeissus acuminata leaf extract on experimentally induced diabetic nephropathy. J. Basic Clin. Physiol. Pharmacol. 2018;29:359–364. doi: 10.1515/jbcpp-2017-0190. [DOI] [PubMed] [Google Scholar]
- 186.Cho E.J., Lee Y.A., Yoo H.H., Yokozawa T. Protective effects of broccoli (Brassica oleracea) against oxidative damage in vitro and in vivo. J. Nutr. Sci. Vitaminol. 2006;52:437–444. doi: 10.3177/jnsv.52.437. [DOI] [PubMed] [Google Scholar]
- 187.ALTamimi J.Z., AlFaris N.A., Al-Farga A.M., Alshammari G.M., BinMowyna M.N., Yahya M.A. Curcumin reverses diabetic nephropathy in streptozotocin-induced diabetes in rats by inhibition of PKCβ/p66Shc axis and activation of FOXO-3a. J. Nutr. Biochem. 2021;87:108515. doi: 10.1016/j.jnutbio.2020.108515. [DOI] [PubMed] [Google Scholar]
- 188.Gurukar M.S., Mahadevamma S., Chilkunda N.D. Renoprotective effect of Coccinia indica fruits and leaves in experimentally induced diabetic rats. J. Med. Food. 2013;16:839–846. doi: 10.1089/jmf.2012.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Boonphang O., Ontawong A., Pasachan T., Phatsara M., Duangjai A., Amornlerdpison D., Jinakote M., Srimaroeng C. Antidiabetic and Renoprotective Effects of Coffea arabica Pulp Aqueous Extract through Preserving Organic Cation Transport System Mediated Oxidative Stress Pathway in Experimental Type 2 Diabetic Rats. Molecules. 2021;26:1907. doi: 10.3390/molecules26071907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hassan H.M., Mahran Y.F., Ghanim A.M.H. Ganoderma lucidum ameliorates the diabetic nephropathy via down-regulatory effect on TGFβ-1 and TLR-4/NFκB signalling pathways. J. Pharm. Pharmacol. 2021 doi: 10.1093/jpp/rgab058. [DOI] [PubMed] [Google Scholar]
- 191.Shiju T.M., Rajesh N.G., Viswanathan P. Renoprotective effect of aged garlic extract in streptozotocin-induced diabetic rats. Indian J. Pharmacol. 2013;45:18–23. doi: 10.4103/0253-7613.106429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Chung A., Gurtu S., Chakravarthi S., Moorthy M., Palanisamy U.D. Geraniin Protects High-Fat Diet-Induced Oxidative Stress in Sprague Dawley Rats. Front. Nutr. 2018;5:17. doi: 10.3389/fnut.2018.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Al Hroob A.M., Abukhalil M.H., Alghonmeen R.D., Mahmoud A.M. Ginger alleviates hyperglycemia-induced oxidative stress, inflammation and apoptosis and protects rats against diabetic nephropathy. Biomed. Pharmacother. 2018;106:381–389. doi: 10.1016/j.biopha.2018.06.148. [DOI] [PubMed] [Google Scholar]
- 194.Han J., Pang X., Shi X., Zhang Y., Peng Z., Xing Y. Ginkgo Biloba Extract EGB761 Ameliorates the Extracellular Matrix Accumulation and Mesenchymal Transformation of Renal Tubules in Diabetic Kidney Disease by Inhibiting Endoplasmic Reticulum Stress. Biomed. Res. Int. 2021;2021:6657206. doi: 10.1155/2021/6657206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Qin X., Zhao Y., Gong J., Huang W., Su H., Yuan F., Fang K., Wang D., Li J., Zou X., et al. Berberine Protects Glomerular Podocytes via Inhibiting Drp1-Mediated Mitochondrial Fission and Dysfunction. Theranostics. 2019;9:1698–1713. doi: 10.7150/thno.30640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Punaro G.R., Lima D.Y., Rodrigues A.M., Pugliero S., Mouro M.G., Rogero M.M., Higa E.M.S. Cupuacu extract reduces nitrosative stress and modulates inflammatory mediators in the kidneys of experimental diabetes. Clin. Nutr. 2019;38:364–371. doi: 10.1016/j.clnu.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 197.Alabi T.D., Brooks N.L., Oguntibeju O.O. Leaf Extracts of Anchomanes difformis Ameliorated Kidney and Pancreatic Damage in Type 2 Diabetes. Plants. 2021;10:300. doi: 10.3390/plants10020300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kim H., Dusabimana T., Kim S.R., Je J., Jeong K., Kang M.C., Cho K.M., Kim H.J., Park S.W. Supplementation of Abelmoschus manihot Ameliorates Diabetic Nephropathy and Hepatic Steatosis by Activating Autophagy in Mice. Nutrients. 2018;10:1703. doi: 10.3390/nu10111703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wang S.C., Lee S.F., Wang C.J., Lee C.H., Lee W.C., Lee H.J. Aqueous Extract from Hibiscus sabdariffa Linnaeus Ameliorate Diabetic Nephropathy via Regulating Oxidative Status and Akt/Bad/14-3-3γ in an Experimental Animal Model. Evid. Based Complement. Alternat. Med. 2011;2011:938126. doi: 10.1093/ecam/nep181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zhang Q., Lu Y., Ma Z., Li Y., Guo J., Meng Q., Bian H. A novel formula from mulberry leaf ameliorates diabetic nephropathy in rats via inhibiting the TGF-β1 pathway. Food Funct. 2015;6:3307–3315. doi: 10.1039/C5FO00711A. [DOI] [PubMed] [Google Scholar]
- 201.Lu H.J., Tzeng T.F., Hsu J.C., Kuo S.H., Chang C.H., Huang S.Y., Chang F.Y., Wu M.C., Liu I.M. An Aqueous-Ethanol Extract of Liriope spicata var. prolifera Ameliorates Diabetic Nephropathy through Suppression of Renal Inflammation. Evid. Based Complement. Alternat. Med. 2013;2013:201643. doi: 10.1155/2013/201643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Chen H.W., Yang M.Y., Hung T.W., Chang Y.C., Wang C.J. Nelumbo nucifera leaves extract attenuate the pathological progression of diabetic nephropathy in high-fat diet-fed and streptozotocin-induced diabetic rats. J. Food Drug Anal. 2019;27:736–748. doi: 10.1016/j.jfda.2018.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Yao L., Li L., Li X., Li H., Zhang Y., Zhang R., Wang J., Mao X. The anti-inflammatory and antifibrotic effects of Coreopsis tinctoria Nutt on high-glucose-fat diet and streptozotocin-induced diabetic renal damage in rats. BMC Complement. Altern. Med. 2015;15:314. doi: 10.1186/s12906-015-0826-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Rajavel V., Abdul Sattar M.Z., Abdulla M.A., Kassim N.M., Abdullah N.A. Chronic Administration of Oil Palm (Elaeis guineensis) Leaves Extract Attenuates Hyperglycaemic-Induced Oxidative Stress and Improves Renal Histopathology and Function in Experimental Diabetes. Evid. Based Complement. Alternat. Med. 2012;2012:195367. doi: 10.1155/2012/195367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Yang R., Li Y., Mehmood S., Yan C., Huang Y., Cai J., Ji J., Pan W., Zhang W., Chen Y. Polysaccharides from Armillariella tabescens mycelia ameliorate renal damage in type 2 diabetic mice. Int. J. Biol. Macromol. 2020;162:1682–1691. doi: 10.1016/j.ijbiomac.2020.08.006. [DOI] [PubMed] [Google Scholar]
- 206.Karunasagara S., Hong G.L., Park S.R., Lee N.H., Jung D.Y., Kim T.W., Jung J.Y. Korean red ginseng attenuates hyperglycemia-induced renal inflammation and fibrosis via accelerated autophagy and protects against diabetic kidney disease. J. Ethnopharmacol. 2020;254:112693. doi: 10.1016/j.jep.2020.112693. [DOI] [PubMed] [Google Scholar]
- 207.Borgohain M.P., Chowdhury L., Ahmed S., Bolshette N., Devasani K., Das T.J., Mohapatra A., Lahkar M. Renoprotective and antioxidative effects of methanolic Paederia foetida leaf extract on experimental diabetic nephropathy in rats. J. Ethnopharmacol. 2017;198:451–459. doi: 10.1016/j.jep.2017.01.035. [DOI] [PubMed] [Google Scholar]
- 208.Song P., Sun C., Li J., Long T., Yan Y., Qin H., Makinde E.A., Famurewa A.C., Jaisi A., Nie Y., et al. Tiliacora triandra extract and its major constituent attenuates diabetic kidney and testicular impairment by modulating redox imbalance and pro-inflammatory responses in rats. J. Sci. Food Agric. 2021;101:1598–1608. doi: 10.1002/jsfa.10779. [DOI] [PubMed] [Google Scholar]
- 209.Vargas F., Romecin P., Garcia-Guillen A.I., Wangesteen R., Vargas-Tendero P., Paredes M.D., Atucha N.M., Garcia-Estan J. Flavonoids in Kidney Health and Disease. Front. Physiol. 2018;9:394. doi: 10.3389/fphys.2018.00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Guclu A., Yonguc N., Dodurga Y., Gundogdu G., Guclu Z., Yonguc T., Adiguzel E., Turkmen K. The effects of grape seed on apoptosis-related gene expression and oxidative stress in streptozotocin-induced diabetic rats. Ren. Fail. 2015;37:192–197. doi: 10.3109/0886022X.2014.991996. [DOI] [PubMed] [Google Scholar]
- 211.Gao Z., Liu G., Hu Z., Shi W., Chen B., Zou P., Li X. Grape seed proanthocyanidins protect against streptozotocininduced diabetic nephropathy by attenuating endoplasmic reticulum stressinduced apoptosis. Mol. Med. Rep. 2018;18:1447–1454. doi: 10.3892/mmr.2018.9140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zhang Z., Li B.Y., Li X.L., Cheng M., Yu F., Lu W.D., Cai Q., Wang J.F., Zhou R.H., Gao H.Q., et al. Proteomic analysis of kidney and protective effects of grape seed procyanidin B2 in db/db mice indicate MFG-E8 as a key molecule in the development of diabetic nephropathy. Biochim. Biophys. Acta. 2013;1832:805–816. doi: 10.1016/j.bbadis.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 213.Fujii H., Yokozawa T., Kim Y.A., Tohda C., Nonaka G. Protective effect of grape seed polyphenols against high glucose-induced oxidative stress. Biosci. Biotechnol. Biochem. 2006;70:2104–2111. doi: 10.1271/bbb.60053. [DOI] [PubMed] [Google Scholar]
- 214.Jiang P., Xiang L., Chen Z., Lu H., Zhou L., Yang L., Ji Y., Liu Y., Sun X., Deng Y., et al. Catalpol alleviates renal damage by improving lipid metabolism in diabetic db/db mice. Am. J. Transl. Res. 2018;10:1750–1761. [PMC free article] [PubMed] [Google Scholar]
- 215.Kim D., Cheon J., Yoon H., Jun H.S. Cudrania tricuspidata Root Extract Prevents Methylglyoxal-Induced Inflammation and Oxidative Stress via Regulation of the PKC-NOX4 Pathway in Human Kidney Cells. Oxid. Med. Cell. Longev. 2021;2021:5511881. doi: 10.1155/2021/5511881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Zhou J., Zhang S., Sun X., Lou Y., Bao J., Yu J. Hyperoside ameliorates diabetic nephropathy induced by STZ via targeting the miR-499-5p/APC axis. J. Pharmacol. Sci. 2021;146:10–20. doi: 10.1016/j.jphs.2021.02.005. [DOI] [PubMed] [Google Scholar]
- 217.Giribabu N., Karim K., Kilari E.K., Salleh N. Phyllanthus niruri leaves aqueous extract improves kidney functions, ameliorates kidney oxidative stress, inflammation, fibrosis and apoptosis and enhances kidney cell proliferation in adult male rats with diabetes mellitus. J. Ethnopharmacol. 2017;205:123–137. doi: 10.1016/j.jep.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 218.Manna K., Mishra S., Saha M., Mahapatra S., Saha C., Yenge G., Gaikwad N., Pal R., Oulkar D., Banerjee K., et al. Amelioration of diabetic nephropathy using pomegranate peel extract-stabilized gold nanoparticles: Assessment of NF-κB and Nrf2 signaling system. Int. J. Nanomed. 2019;14:1753–1777. doi: 10.2147/IJN.S176013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Gomes I.B., Porto M.L., Santos M.C., Campagnaro B.P., Pereira T.M., Meyrelles S.S., Vasquez E.C. Renoprotective, anti-oxidative and anti-apoptotic effects of oral low-dose quercetin in the C57BL/6J model of diabetic nephropathy. Lipids Health Dis. 2014;13:184. doi: 10.1186/1476-511X-13-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Zhang T., Chi Y., Kang Y., Lu H., Niu H., Liu W., Li Y. Resveratrol ameliorates podocyte damage in diabetic mice via SIRT1/PGC-1alpha mediated attenuation of mitochondrial oxidative stress. J. Cell. Physiol. 2019;234:5033–5043. doi: 10.1002/jcp.27306. [DOI] [PubMed] [Google Scholar]
- 221.Sohal R.S., Forster M.J. Caloric restriction and the aging process: A critique. Free Radic. Biol. Med. 2014;73:366–382. doi: 10.1016/j.freeradbiomed.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Sohal R.S., Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Diaz-Ruiz A., Di Francesco A., Carboneau B.A., Levan S.R., Pearson K.J., Price N.L., Ward T.M., Bernier M., de Cabo R., Mercken E.M. Benefits of Caloric Restriction in Longevity and Chemical-Induced Tumorigenesis Are Transmitted Independent of NQO1. J. Gerontol. A. 2019;74:155–162. doi: 10.1093/gerona/gly112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Wang S.Y., Cai G.Y., Chen X.M. Energy restriction in renal protection. Br. J. Nutr. 2018;120:1149–1158. doi: 10.1017/S0007114518002684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Gray K.L., Clifton P.M., Keogh J.B. The effect of intermittent energy restriction on weight loss and diabetes risk markers in women with a history of gestational diabetes: A 12-month randomized control trial. Am. J. Clin. Nutr. 2021 doi: 10.1093/ajcn/nqab058. [DOI] [PubMed] [Google Scholar]
- 226.Estrela G.R., Wasinski F., Batista R.O., Hiyane M.I., Felizardo R.J., Cunha F., de Almeida D.C., Malheiros D.M., Camara N.O., Barros C.C., et al. Caloric Restriction Is More Efficient than Physical Exercise to Protect from Cisplatin Nephrotoxicity via PPAR-Alpha Activation. Front. Physiol. 2017;8:116. doi: 10.3389/fphys.2017.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Ning Y.C., Cai G.Y., Zhuo L., Gao J.J., Dong D., Cui S.Y., Shi S.Z., Feng Z., Zhang L., Sun X.F., et al. Beneficial effects of short-term calorie restriction against cisplatin-induced acute renal injury in aged rats. Nephron Exp. Nephrol. 2013;124:19–27. doi: 10.1159/000357380. [DOI] [PubMed] [Google Scholar]
- 228.Stern J.S., Gades M.D., Wheeldon C.M., Borchers A.T. Calorie restriction in obesity: Prevention of kidney disease in rodents. J. Nutr. 2001;131:913S–917S. doi: 10.1093/jn/131.3.913S. [DOI] [PubMed] [Google Scholar]
- 229.Singh G., Krishan P. Dietary restriction regimens for fighting kidney disease: Insights from rodent studies. Exp. Gerontol. 2019;128:110738. doi: 10.1016/j.exger.2019.110738. [DOI] [PubMed] [Google Scholar]
- 230.Tikoo K., Lodea S., Karpe P.A., Kumar S. Calorie restriction mimicking effects of roflumilast prevents diabetic nephropathy. Biochem. Biophys. Res. Commun. 2014;450:1581–1586. doi: 10.1016/j.bbrc.2014.07.039. [DOI] [PubMed] [Google Scholar]
- 231.Pall M.L., Levine S. Nrf2, a master regulator of detoxification and also antioxidant, anti-inflammatory and other cytoprotective mechanisms, is raised by health promoting factors. Sheng Li Xue Bao. 2015;67:1–18. [PubMed] [Google Scholar]
- 232.Juszczak F., Caron N., Mathew A.V., Decleves A.E. Critical Role for AMPK in Metabolic Disease-Induced Chronic Kidney Disease. Int. J. Mol. Sci. 2020;21:7994. doi: 10.3390/ijms21217994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Nowak K.L., Hopp K. Metabolic Reprogramming in Autosomal Dominant Polycystic Kidney Disease: Evidence and Therapeutic Potential. Clin. J. Am. Soc. Nephrol. 2020;15:577–584. doi: 10.2215/CJN.13291019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Kume S., Koya D. Autophagy: A Novel Therapeutic Target for Diabetic Nephropathy. Diabetes Metab. J. 2015;39:451–460. doi: 10.4093/dmj.2015.39.6.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Sharma K. Mitochondrial hormesis and diabetic complications. Diabetes. 2015;64:663–672. doi: 10.2337/db14-0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Aydemir N., Pike M.M., Alsouqi A., Headley S.A.E., Tuttle K., Evans E.E., Milch C.M., Moody K.A., Germain M., Lipworth L., et al. Effects of diet and exercise on adipocytokine levels in patients with moderate to severe chronic kidney disease. Nutr. Metab. Cardiovasc. Dis. 2020;30:1375–1381. doi: 10.1016/j.numecd.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Malin S.K., Navaneethan S.D., Fealy C.E., Scelsi A., Huang H., Rocco M., Kirwan J.P. Exercise plus caloric restriction lowers soluble RAGE in adults with chronic kidney disease. Obes. Sci. Pract. 2020;6:307–312. doi: 10.1002/osp4.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Doshi S.M., Friedman A.N. Diagnosis and Management of Type 2 Diabetic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2017;12:1366–1373. doi: 10.2215/CJN.11111016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Ikizler T.A., Robinson-Cohen C., Ellis C., Headley S.A.E., Tuttle K., Wood R.J., Evans E.E., Milch C.M., Moody K.A., Germain M., et al. Metabolic Effects of Diet and Exercise in Patients with Moderate to Severe CKD: A Randomized Clinical Trial. J. Am. Soc. Nephrol. 2018;29:250–259. doi: 10.1681/ASN.2017010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Ikeda Y., Enomoto H., Tajima S., Izawa-Ishizawa Y., Kihira Y., Ishizawa K., Tomita S., Tsuchiya K., Tamaki T. Dietary iron restriction inhibits progression of diabetic nephropathy in db/db mice. Am. J. Physiol. Renal Physiol. 2013;304:F1028–F1036. doi: 10.1152/ajprenal.00473.2012. [DOI] [PubMed] [Google Scholar]
- 241.Lin C.H., Chang Y.C., Chuang L.M. Early detection of diabetic kidney disease: Present limitations and future perspectives. World J. Diabetes. 2016;7:290–301. doi: 10.4239/wjd.v7.i14.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Ibarra-Gonzalez I., Cruz-Bautista I., Bello-Chavolla O.Y., Vela-Amieva M., Pallares-Mendez R., Ruiz de Santiago Y.N.D., Salas-Tapia M.F., Rosas-Flota X., Gonzalez-Acevedo M., Palacios-Penaloza A., et al. Optimization of kidney dysfunction prediction in diabetic kidney disease using targeted metabolomics. Acta Diabetol. 2018;55:1151–1161. doi: 10.1007/s00592-018-1213-0. [DOI] [PubMed] [Google Scholar]
- 243.Matoba K., Takeda Y., Nagai Y., Yokota T., Utsunomiya K., Nishimura R. Targeting Redox Imbalance as an Approach for Diabetic Kidney Disease. Biomedicines. 2020;8:40. doi: 10.3390/biomedicines8020040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.