Abstract

Nitrosamine impurities in angiotensin II receptor antagonists (sartans) containing a tetrazole group represent an urgent concern for active pharmaceutical ingredient (API) manufacturers and global regulators. Regarding safety, API manufacturers must develop methods to monitor the levels of each nitrosamine impurity before individual batch release. In this study, we developed and validated a sensitive, selective, and high-throughput method based on headspace gas chromatography–mass spectrometry (HS-GC–MS) for the simultaneous determination of four nitrosamines in losartan potassium API with simple sample preparation. N-Nitrosodimethylamine (NDMA, m/z 74), N-nitrosodiethylamine (NDEA, m/z 102), N-nitrosoethylisopropylamine (EIPNA, m/z 116), and N-nitrosodiisopropylamine (DIPNA, m/z 130) levels were quantified using an electron impact, single quadrupole mass spectrometer under a selected-ion-monitoring acquisition method. The method was validated according to the Q2(R1) ICH guidelines. The calibration curves of the assay ranged from 25 to 5000 ng/mL with limits of quantitation of 25 ppb for NDMA and NDEA and 50 ppb for DIPNA and EIPNA. The accuracy of the developed method ranged from −7.04% to 7.25%, and the precision %CV was ≤11.5. Other validation parameters, including specificity, stability, carryover, and robustness, met the validation criteria. In conclusion, the developed method was successfully applied for the determination of nitrosamines in losartan potassium APIs.
1. Introduction
Angiotensin II receptor antagonists (sartans) such as valsartan, losartan, irbesartan, and olmesartan have been widely used to treat hypertension, diabetic nephropathy, and congestive heart failure.1−3 In July 2018, the United States Food and Drug Administration (US FDA)4 and the European Medicines Agency (EMA)5 initially announced a recall of valsartan tablets because of contamination by the nitrosamine N-nitrosodimethylamine (NDMA). Subsequently, other nitrosamine impurities, including N-nitrosodiethylamine (NDEA), N-nitrosodiisopropylamine (DIPNA), and N-nitrosoethylisopropylamine (EIPNA), have been found in several sartan drugs including losartan, irbesartan, and candesartan.6 In addition, other nitrosamines, such as N-nitrosomethylphenylamine (NMPhA), N-nitrosodibutylamine (NDBA), and N-nitromethylbutyric acid (NMBA), have been detected in sartan APIs.7 These nitrosamine impurities are classified as probable human carcinogens3−7 and are believed to be present in finished sartan products because of the manufacturing process for sartan active pharmaceutical ingredients (APIs). Sartans containing a tetrazole ring carry a potential risk of nitrosamine contamination because the excess amount of sodium nitrite used to quench unreacted sodium azide in the synthesis of sartan APIs can react with residual secondary amines, which inadvertently generates nitrosamines.8,9 One example of contaminating secondary amines is dimethylamine, which may be present in the dimethylformamide solvent. Nitrosative dealkylation of triethylamine and diisopropylethylamine is a possible pathway to generate residual secondary amines.9
Because of possible nitrosamine formation during API manufacturing, it is essential to monitor nitrosamine impurities in sartan APIs before individual batch release. Since July 2018, the US FDA, EMA, and other national regulatory agencies have released various methods for the determination of nitrosamine contaminants in sartan APIs based on gas chromatography coupled to mass spectrometry (GC–MS) or tandem mass spectrometry (GC–MS/MS), high-performance liquid chromatography with ultraviolet detection (HPLC–UV), and liquid chromatography coupled to mass spectrometry (LC–MS) or tandem mass spectrometry (LC–MS/MS).10−16 The French National Agency for Medicines and Health Products Safety developed an HPLC–UV method to determine the NDMA content in valsartan APIs and drug products.10 The German Official Medicines Control Laboratory published a validated LC–MS/MS method to analyze NDMA levels in valsartan products.11 The technique had the linearity range of 0.1–3.0 ppm and a limit of quantification (LOQ) of 0.2 ppm, which is slightly less than the interim limit at 0.3 ppm for NDMA in valsartan. The Public Analyst’s Laboratory (Galway, Ireland) developed a headspace gas chromatography–mass spectrometry (HS-GC–MS) method for the quantitative analysis of NDMA in valsartan APIs and drug products without reporting the detection and quantitation limits.12 In 2019, the US FDA initially reported an HS-GC–MS method for NMDA determination in valsartan APIs and drug products with an LOQ at the interim limit of NDMA at 0.3 ppm.13 The US FDA reported a simultaneous HS-GC–MS analysis method for NDMA and NDEA in valsartan with improved sensitivity (LOQs of 0.10 and 0.05 ppm, respectively).14 Other nitrosamines, including DIPNA and EIPNA, have recently been identified as impurities in the sartan drug class by the EMA.9 In addition to the US FDA and EMA report, at least 12 nitrosamines were reported in an application note published by the Taiwanese FDA.15,16 Among the 12 nitrosamines,16 only three nitrosamines including NDMA, NDEA, and NMBA were found in valsartan, losartan, and irbesartan. The evidence basis of the recall reports of nitrosamine contamination from US FDA and EMA press releases suggests that NDMA, NDEA, NMBA, DIPNA, and EIPNA are most commonly found in sartans. Therefore, it is essential to develop a method for detecting these potential nitrosamine contaminants in sartans. The US FDA recently proposed the simultaneous analysis of NDMA, NDEA, DIPNA, and EIPNA using a GC–MS triple quadrupole coupled with either sample direct injection or a headspace autosampler.16−18 Nonetheless, the published GC–MS methods were developed and validated for valsartan APIs and drug products with particular acceptable sensitivity.
In addition to valsartan, losartan is one of the most commonly used sartans,19 and it has been extensively reported to contain nitrosamine impurities above the US FDA20 and EMA6,21,22 limit criteria. The US FDA released an application note on the analytical method for determining six nitrosamines in losartan using liquid chromatography coupled to high-resolution, accurate mass (HRAM) mass spectrometry (LC–HRMS).23 Although HRAM mass spectrometers provide better resolution and selectivity for NDMA impurity analysis than single-quadrupole, triple-quadrupole, and time-of-flight mass spectrometers,24 the HRAM system suggested by the US FDA is not commonly used in routine quality control laboratories. Another concern is that directly injecting samples into GC or LC–MS systems without sample cleanup may lead to machine contamination from high sample matrix loading, resulting in a loss of sensitivity and robustness. Headspace injection is a simple sample preparation technique for preventing high levels of sample matrix contamination in a mass spectrometer. Therefore, HS-GC–MS was developed and optimized for volatile nitrosamine impurity analysis with high sample matrix loading. Literature reviews, some application notes from the FDA in several countries,10−16 and a few published studies25 have revealed methods for nitrosamine determination, and such methods require further validation for specific purposes by users.
Based on US FDA recall events, there are seven reported nitrosamines, including NDMA, NDEA, NMBA, DIPNA, EIPNA, NDBA, and NMPhA. Three of those nitrosamines, including NDMA, NDEA, and NMBA, have caused most often recalled in losartan, while DIPNA and EIPNA have insufficient evidence. Moreover, the recall caused by NDBA and NMPhA contaminations has rarely occurred. However, the number of nitrosamine contamination may be relevant to the manufacturing process and different sartans. In this work, we focused on determining the potential contamination risk of four nitrosamines, e.g., NDMA, NDEA, DIPNA, and EIPNA, in losartan raw materials using HS-GC–MS. Although NMBA is a potential contaminating nitrosamine, it could not be included in this analytical method because of volatile property limitations. This work is the first report on the development and validation of a robust, simple, sensitive, and specific HS-GC–MS method for the simultaneous determination of nitrosamines in losartan APIs in accordance with the Q2(R1) ICH guidelines.26 The method proved applicable for identifying nitrosamine contamination in different batches of losartan APIs.
2. Results and Discussion
2.1. Method Development
The stationary phase, column length, film thickness, and column diameter have a large impact on the retention and peak shape of the analytes. Based on the trial-and-error methodology with the literature review, the optimal separation of nitrosamines was achieved when the capillary surface was modified by polyethylene glycol residues at the length of 30 m.29−31 The optimal chromatographic separation and retention were achieved using a column dimension of 30 × 0.32 mm2 i.d. and a film thickness of 1 μm coated with polyethylene glycol stationary phase. The temperature programing is also a very important parameter to influence peak separation. Yang et al. stated that 15N-DMF representing residual solvents in API has the same molecular mass of 74, which must be discriminated from NDMA using an HRMS in cases of poor chromatographic separation.24 An initial constant temperature at 40 °C for 2 min followed by an increase at a rate of 20 °C can provide the best separation with a resolution greater than 2 between NDMA and 15N-DMF at the retention times of 7.47 and 7.67 min, respectively, as presented in Figure S1. The temperature of the sample and transfer lines was set above 200 °C, and the temperature of the transfer line should be slightly higher than that of the sample line to prevent a carryover effect from the previous sample. The optimal temperatures of sample and transfer lines were controlled at 230 and 250 °C, respectively. The filament was turned off after all nitrosamines had entered into the mass spectrometer to prevent filament damage from an excessive dimethyl sulfoxide (DMSO) concentration.
The selection of a suitable sample introduction technique requiring no complicated sample preparation is an essential key for GC–MS analysis. Direct and headspace injection techniques are commonly used because they can be fully and easily automated. The concern of the direct injection technique is that the sample matrices can cause variability and inaccuracy of the GC–MS analysis. The injected matrix, which co-elutes with the analyte, can deteriorate the column shelf life and cause mass spectrometer contamination. Additional sample cleanup can resolve these problems, leading to a decrease in the matrix interference, an extension of the column lifespan, and a reduction in machine contamination. The samples often need to be preconcentrated before analysis by direct injection due to a deficient level of the target analyte. In this study, the headspace injection technique was chosen because it can avoid loading nonvolatile sample matrices into the column, leading to significantly reduced matrix interference.32 The analyte in the sample solution is vaporized and concentrated during the sample heating process, increasing the sensitivity of the method.33 Compared to the direct injection, the headspace sampling technique may be inappropriate for thermolabile samples. In addition, reinjection of the pierced sample is not possible if the headspace sampling technique is applied. The headspace condition was optimized to determine the proper headspace oven temperature and incubation period. Typically, the oven temperature should be set below the boiling point of the solvent. The sensitivity of the method is poor at excessively low incubation temperatures, e.g., 50–100 °C, whereas extremely high incubation temperatures, e.g., 165–180 °C, can generate substantial baseline noise from the solvent. The optimal incubation temperature is achieved at 150 °C. Regarding the incubation time, the highest sensitivity was achieved at an incubation time of 15 ± 1 min. Shorter incubation periods result in lower sensitivity, whereas longer periods do not affect the sensitivity. It is noteworthy that losartan potassium is thermally stable up to 275 °C,34 whereas the autosampler oven was set at 150 °C, indicating that losartan potassium is stable under the autosampler temperature used in this study. Identifying the proper solvent for sample dilution before analysis is challenging during the headspace technique. Low boiling point solvents such as methanol, ethanol, and isopropanol should be avoided because their high vapor pressure properties can significantly dilute the sample concentration in the headspace. High boiling point solvents such as DMSO, dimethylacetamide, or DMF are often chosen. In this study, DMSO was used, and advantages in terms of volatility and polarity were achieved. More importantly, losartan and other sartans are soluble in DMSO, and nitrosamine impurities in the APIs can be extracted entirely and dissolved in DMSO. The high boiling point of DMSO also prevents its interference with the nitrosamine peak. The headspace injection technique using DMSO as the sample solvent provided sufficient chromatographic performance, and it was, therefore, chosen in this study.
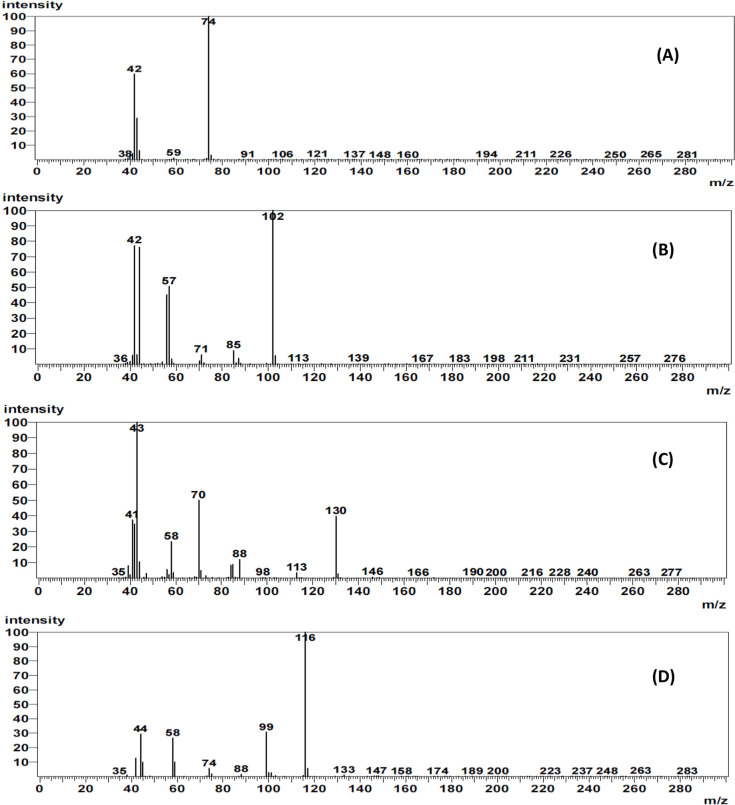
The GC–MS condition for the simultaneous determination of four nitrosamines in losartan was optimized to ensure reliability, selectivity, and sensitivity. Initially, the mass parameters were optimized to achieve the highest sensitivity with a consistent response. NDMA (m/z 74), NDEA (m/z 102), DIPNA (m/z 130), and EIPNA (m/z 116) were tuned on a single-quadrupole mass spectrometer in the electron ionization (EI) mode under the selected ion monitoring (SIM) acquisition method. These particular ions were used for quantification. In addition to the parent ions, the major daughter ions of NDMA (m/z 42), NDEA (m/z 57), DIPNA (m/z 70), and EIPNA (m/z 99) shown in Figure 1 were qualitatively monitored for confirmation. Alternatively, the ion ratio calculation between the parent and daughter ions was also quantitatively used in the confirmatory objective. The GC chromatograms shown in Figure 2A indicate sufficient peak separation with appropriate retention times of 7.535 ± 0.050, 8.168 ± 0.050, 8.646 ± 0.050, and 8.443 ± 0.050 min for NDMA, NDEA, DIPNA, and EIPNA, respectively.
Figure 1.
Mass spectra of (A) NDMA, (B) NDEA, (C) DIPNA, and (D) EIPNA.
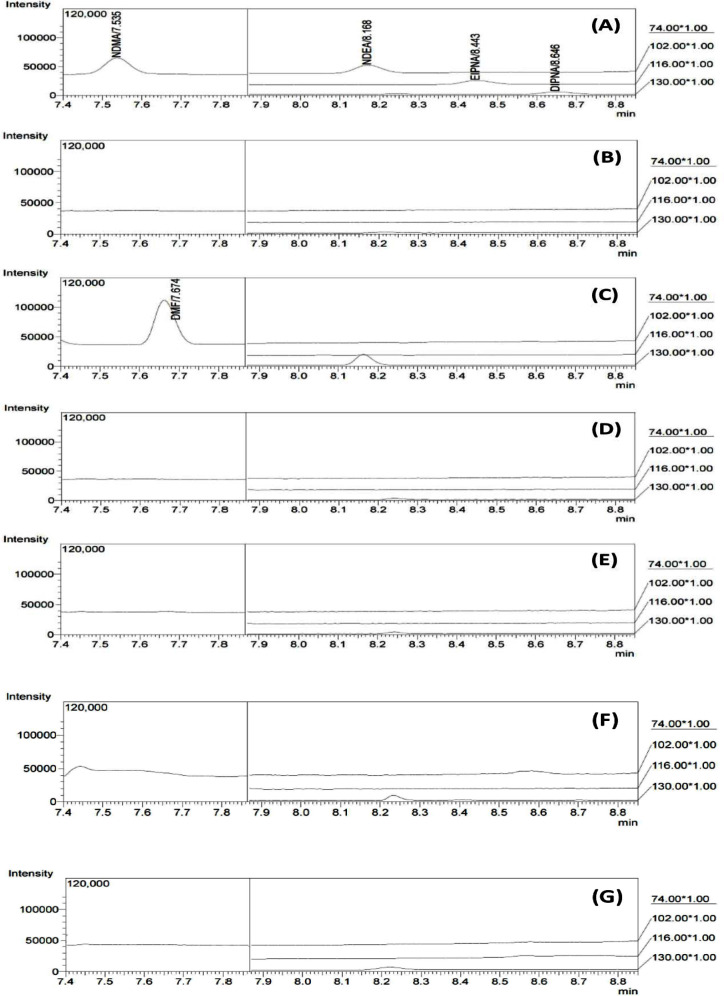
Figure 2.
Representative GC chromatograms of (A) the standard mixture of four nitrosamines, (B) DMSO, (C) DMF, (D) methanol, (E) losartan potassium, (F) olmesartan medoxomil, and (G) valsartan sacubitril.
2.2. Method Validation
2.2.1. System Suitability
Suitability was evaluated before the validation experiments and impurity determination to verify the reproducibility and performance of the chromatographic system. A system reproducibility solution containing a mixture of NDMA, NDEA, DIPNA, and EIPNA was prepared at a concentration of 250 ng/mL for each compound by appropriately aliquoting the working standard solution (1000 μg/mL). The system suitability solution was injected with five replicates. The system performance was evaluated using the tailing factor of analytes (T < 2), and the system reproducibility was assessed by the variation of peak area (%CV < 11%) and retention time (%CV < 2%) to indicate the precision of injections.
2.2.2. Specificity and Selectivity
The specificity of the method for nitrosamine determination focused on the diluents involved in sample preparation, e.g., DMSO and methanol. Contamination of losartan by residual solvents, such as DMF,24 which may affect analysis reliability, was investigated. Furthermore, the selectivity of matrices focused on various sartans. Figure 2A presents a representative GC chromatogram of a standard mixture of four nitrosamines. Figure 2B–D presents the chromatograms of DMSO, DMF, and methanol, respectively. The results revealed the absence of coeluting peaks at the retention times of nitrosamines. The adjacent interfering components from 15N-DMF were observed, but they were resolved from NDMA with a resolution exceeding 2 (see Figure S1). In addition to the solvents, no interference from the matrices of various sartans, e.g., losartan potassium, olmesartan medoxomil, and valsartan sacubitril, was observed, as demonstrated in Figure 2E–G.
2.2.3. Carryover
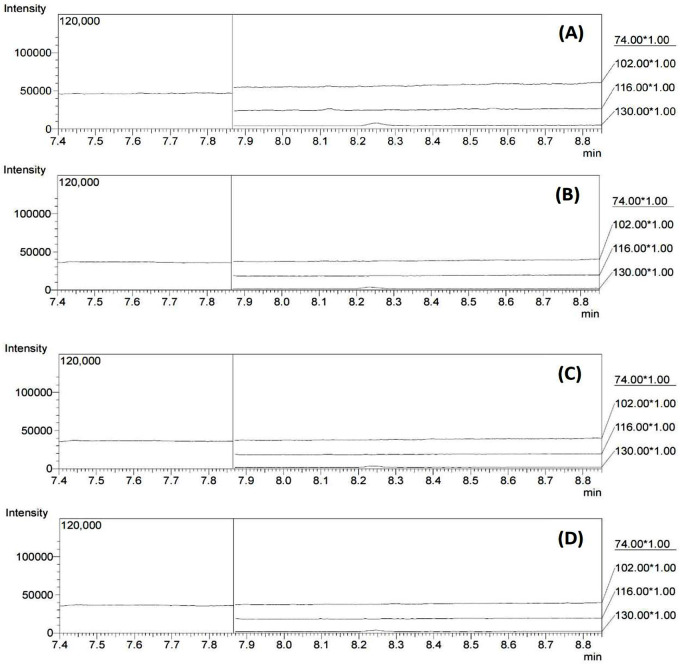
Carryover was assessed to ensure that no analyte was present from the previously analyzed sample to prevent contamination of the subsequent sample. In this study, DMSO (blank) was injected and evaluated after injecting the highest concentration of the calibration standard. The experiment was performed in triplicate. The peak response in the blank sample was compared to that in the lowest calibration sample. The percentage of interference must be less than 20%. The results revealed no peak at the retention times of NDMA, NDEA, DIPNA, and EIPNA, demonstrating that the method has no carryover effect, as shown in Figure 3.
Figure 3.
Representative GC chromatograms of the carryover effect on blank solutions after injecting (A) NDMA, (B) NDEA, (C) DIPNA, and (D) EIPNA at a concentration of 5000 ng/mL.
2.2.4. Calibration Curve
The linear relationships between analyte concentrations and signal responses were assessed at concentration ranges of 25–5000 ng/mL for NDMA and NDEA and 50–5000 ng/mL for DIPNA and EIPNA. The calibration curves were constructed by plotting different concentrations of NDMA, NDEA, DIPNA, and EIPNA against their corresponding peak areas. The regression line and homoscedasticity of the calibration curve were assessed to determine whether the ordinary or weighted least square is appropriate for the robust calibration model. The calibration curve was linear over the tested concentration range (r2 > 0.995) using the weighted-linear least square model with a weighting factor of 1/x2. The percent deviations of the mean back-calculated concentration and actual spiked plasma concentrations of NDMA, NDEA, DIPNA, and EIPNA were in the ranges of −1.38 to 2.32, −4.31 to 4.50, −1.73 to 1.55, and −2.33 to 4.19, respectively. The %CV (n = 3) of the back-calculated concentration was less than 7.63%. The calibration curve results are summarized in Table 1.
Table 1. Mean Interday Back-Calculated Standard and Calibration Curve Results (n = 3).
| compound | nominal concentration (ng/mL) | back-calculated
concentration (ng/mL) |
mean back-calculated concentration (ng/mL) | % deviation | %CV | ||
|---|---|---|---|---|---|---|---|
| day 1 | day 2 | day 3 | |||||
| NDMA | 25.0 | 25.1 | 24.6 | 25.9 | 25.2 | 0.72 | 2.58 |
| 50.0 | 51.5 | 52.2 | 48.1 | 50.6 | 1.25 | 4.32 | |
| 100.0 | 98.2 | 102.6 | 98.9 | 99.9 | –0.09 | 2.38 | |
| 250.0 | 250.5 | 263.4 | 253.6 | 255.8 | 2.32 | 2.64 | |
| 500.0 | 518.2 | 485.1 | 494.3 | 499.2 | –0.17 | 3.43 | |
| 1000.0 | 995.7 | 950.9 | 1040.2 | 995.6 | –0.44 | 4.48 | |
| 5000.0 | 4864.1 | 4965.6 | 4963.1 | 4931.0 | –1.38 | 1.17 | |
| r2 | 0.999 | 0.998 | 0.999 | ||||
| Fcal | 12 945.996 | ||||||
| FANOVA | 2.158 × 10–28 | ||||||
| p value | slope | 2.158 × 10–28 | |||||
| y-intercept | 0.425 | ||||||
| NDEA | 25.0 | 24.7 | 24.6 | 24.1 | 24.5 | –2.12 | 1.31 |
| 50.0 | 49.6 | 51.0 | 51.1 | 50.6 | 1.17 | 1.67 | |
| 100.0 | 105.0 | 102.5 | 105.9 | 104.5 | 4.50 | 1.69 | |
| 250.0 | 244.8 | 232.3 | 264.2 | 247.1 | –1.16 | 6.51 | |
| 500.0 | 531.4 | 505.3 | 489.8 | 508.8 | 1.77 | 4.14 | |
| 1000.0 | 905.8 | 965.0 | 999.7 | 956.9 | –4.31 | 4.96 | |
| 5000.0 | 5075.5 | 5284.3 | 4548.5 | 4969.4 | –0.61 | 7.63 | |
| r2 | 0.996 | 0.997 | 0.996 | ||||
| Fcal | 17 667.266 | ||||||
| FANOVA | 1.129 × 10–29 | ||||||
| p value | slope | 1.129 × 10–29 | |||||
| y-intercept | 0.941 | ||||||
| DIPNA | 50.0 | 46.2 | 51.8 | 51.1 | 49.7 | –0.55 | 6.11 |
| 100.0 | 96.7 | 101.5 | 99.6 | 99.3 | –0.73 | 2.45 | |
| 250.0 | 248.1 | 257.1 | 241.0 | 248.7 | –0.52 | 3.25 | |
| 500.0 | 508.4 | 494.3 | 520.5 | 507.7 | 1.55 | 2.58 | |
| 1000.0 | 1015.4 | 965.3 | 978.2 | 986.3 | –1.37 | 2.64 | |
| 5000.0 | 5200.9 | 4954.9 | 5042.8 | 5066.2 | 1.32 | 2.46 | |
| r2 | 0.999 | 0.999 | 0.999 | ||||
| Fcal | 794.423 | ||||||
| FANOVA | 4.573 × 10–15 | ||||||
| p value | slope | 4.573 × 10–15 | |||||
| y-intercept | 0.920 | ||||||
| EIPNA | 50.0 | 51.9 | 49.4 | 47.3 | 49.5 | –0.93 | 4.64 |
| 100.0 | 94.1 | 97.3 | 101.6 | 97.7 | –2.33 | 3.84 | |
| 250.0 | 263.0 | 255.9 | 227.2 | 248.7 | –0.53 | 7.63 | |
| 500.0 | 499.9 | 485.7 | 517.1 | 500.9 | 0.19 | 3.14 | |
| 1000.0 | 984.9 | 993.8 | 975.2 | 984.6 | –1.54 | 0.95 | |
| 5000.0 | 4973.3 | 5200.5 | 5454.9 | 5209.6 | 4.19 | 4.62 | |
| r2 | 0.998 | 0.999 | 0.996 | ||||
| Fcal | 1671.131 | ||||||
| FANOVA | 1.290 × 10–17 | ||||||
| p value | slope | 1.290 × 10–17 | |||||
| y-intercept | 0.945 | ||||||
According to the residual plots and regression generated using one-way analysis of variance, the results demonstrated that the F values (FANOVA) of all regression lines were significantly less than the calculated F value (Fcal), indicating a good linear relationship between the peak response from the instrument (y) and the concentration of the analyte (x). The p values of the slope and y-intercept were also calculated, as summarized in Table 1. The p values of the slope and intercept represent whether the slope and intercept significantly differ from zero.28 The results demonstrated that the p values of the slope were less than 0.05, indicating a significant difference versus zero. However, the p values of the intercept exceeded 0.05, indicating that the intercepts of all regression lines were insignificantly different from zero. Consequently, a single-point calibration standard can be applied for the routine analysis of nitrosamine determination.
2.2.5. Linearity and Range
The range of an analytical procedure evaluates its ability to elicit test results that are directly, or by a well-defined mathematical transformation, proportional to the concentration of an analyte in sample matrices with a given dynamic range. Thus, the linear relationships between the spiked concentration of four nitrosamines in the losartan matrix and the back-calculated concentration results were demonstrated via the dynamic range of analysis. As presented in Table 2, the results were linear over the concentration ranges of 25–5000 ppb for NDMA and NDEA and 50–5000 ppb for DIPNA and EIPNA (r2 > 0.995).
Table 2. Range of Nitrosamines.
| nitrosamine | dynamic range (ppb) | slope | intercept | r2 |
|---|---|---|---|---|
| NDMA | 25–5000 | 1.038 | –15.178 | 0.9998 |
| NDEA | 25–5000 | 1.038 | –15.791 | 0.9997 |
| DIPNA | 50–5000 | 0.983 | 8.498 | 0.9999 |
| EIPNA | 50–5000 | 0.968 | 17.140 | 0.9999 |
2.2.6. Accuracy and Precision
The intraday and interday accuracy and precision were evaluated at LOQ QC, MCQ, and HQC. The accuracy and precision results are summarized in Table 3. The spiked QC samples of NDMA for intraday and interday accuracy exhibited percent deviation ranging from −4.19 to 4.89 (%CV = 1.3–4.8) and from −2.57 to 5.57 (%CV = 1.3–5.9), respectively. The spiked QC samples of NDEA for the intraday and interday accuracy exhibited % deviation ranging from −6.47 to 7.15 (%CV = 0.4–1.3) and from −7.04 to 5.82 (%CV = 1.4–2.4), respectively. The spiked QC samples of DIPNA for intraday and interday accuracy exhibited % deviation ranging from −0.58 to 6.42 (%CV = 0.9–4.6) and from 2.79 to 3.98 (%CV = 2.9–4.8), respectively. The spiked QC samples of EIPNA for intraday and interday accuracy exhibited % deviation ranging from 4.34 to 7.25 (%CV = 1.1–2.0) and from −4.19 to 5.13 (%CV = 2.5–11.5), respectively. The accuracy and precision results showed good accuracy and precision of the proposed method.
Table 3. Intraday and Interday Accuracy and Precision.
| compound | nominal concentration (ppb) | intraday
(n = 3) |
interday
(n = 6) |
||||
|---|---|---|---|---|---|---|---|
| mean back-calculated concentration (ppb) | accuracy (% deviation) | precision (%CV) | mean back-calculated concentration (ppb) | accuracy (% deviation) | precision (%CV) | ||
| NDMA | 25.0 | 24.0 | –4.19 | 4.8 | 24.4 | –2.57 | 5.9 |
| 250.0 | 262.2 | 4.89 | 1.5 | 262.9 | 5.01 | 1.3 | |
| 1000.0 | 1047.8 | 4.78 | 1.3 | 1063.6 | 5.57 | 1.8 | |
| NDEA | 25.0 | 23.4 | –6.47 | 0.9 | 23.2 | –7.04 | 1.4 |
| 250.0 | 267.9 | 7.15 | 0.4 | 264.5 | 5.82 | 1.8 | |
| 1000.0 | 1062.6 | 6.26 | 1.3 | 1045.4 | 4.54 | 2.4 | |
| DIPNA | 50.0 | 53.2 | 6.42 | 0.9 | 52.0 | 3.98 | 2.9 |
| 250.0 | 247.2 | –1.12 | 4.0 | 257.0 | 2.79 | 4.8 | |
| 1000.0 | 994.2 | –0.58 | 4.6 | 1030.4 | 3.04 | 4.8 | |
| EIPNA | 50.0 | 52.2 | 4.34 | 2.0 | 47.9 | –4.19 | 11.5 |
| 250.0 | 268.1 | 7.25 | 1.9 | 255.4 | 2.17 | 6.5 | |
| 1000.0 | 1068.7 | 6.87 | 1.1 | 1051.3 | 5.13 | 2.5 | |
2.2.7. Limit of Detection and LOQ
The experiments demonstrated the sensitivity of the method via limit of detection (LOD) and LOQ. Compared to the GC–MS from the US FDA application note,17 the developed method is more sensitive in NDMA and NDEA analysis. Recently, the US FDA has published a more specific HPLC–HRMS method23 with an LOQ of 50 ppb for nitrosamine analysis. However, the developed method demonstrated a more sensitive LOQ at 25 ppb for NDMA and NDEA, while the sensitivity of DIPNA and EIPNA for both methods is comparable. The quantification limits obtained from the developed method are much lower than the interim limit criteria proposed by the US FDA4 (0.96 ppm for NDMA and 0.27 ppm for NDEA, DIPNA, and EIPNA) and the EMA6 (0.640 ppm for NDMA and 0.177 ppm for NDEA, DIPNA, and EIPNA), indicating that the method can be applied for nitrosamine analysis in losartan APIs. The LOD and LOQ of the method for nitrosamine determination are summarized in Table 4.
Table 4. LOD and LOQ of Nitrosamines.
| compound | LOD
(n = 5) |
LOQ
(n = 5) |
|||||
|---|---|---|---|---|---|---|---|
| nominal concentration (ppb) | %CV | S/N | nominal concentration (ppb) | accuracy (% deviation) | precision (%CV) | S/N | |
| NDMA | 5 | 6.8 | 6.5 | 25 | 3.3 | 2.9 | 36.8 |
| NDEA | 5 | 3.7 | 3.8 | 25 | –5.2 | 1.0 | 15.4 |
| DIPNA | 25 | 5.6 | 4.3 | 50 | 3.8 | 0.8 | 27.3 |
| EIPNA | 25 | 6.5 | 3.7 | 50 | –2.4 | 3.5 | 39.9 |
2.2.8. Robustness and Stability
The robustness of the developed method was investigated to determine any impacts on the result in terms of the small variations of chromatographic parameters and the stability profile of the test sample. The headspace equilibrium time was varied in the range of ±1 min from the proposed method condition. The transfer line temperature was also varied in the range of ±5 °C from the proposed method condition. The robustness results (see Tables S1–S8) demonstrated the %CV of peak area and retention time of ≤7.49 and ≤0.03, respectively, with the tailing factor of ≤1.27, indicating that the efficiency of the method was not affected upon small changes in the investigating parameters. Moreover, there is no significant impact on the separation between 15N-DMF and NDMA with the resolution of >2 (see Figure S2) under the variations of the flow rate (±0.1 mL/min).
In addition to the chromatographic parameter variations, the sample stability in the headspace autosampler at room temperature was studied to estimate the appropriate analysis time after sample preparation. The percent deviation of all nitrosamines at 6, 12, and 24 h was <−6.54%, demonstrating that the sample was stable in the autosampler for up to 24 h after preparation.
2.3. Applications of the Method for Nitrosamine Determination in Losartan Potassium APIs
The validated method was applied to investigate four contaminating nitrosamines, namely, NDMA, NDEA, DIPNA, and EIPNA, in 10 batches of losartan potassium samples from two manufacturers. In this study, triplicate analyses were performed for each production batch. The repeatability (%CV) of the results was found to be less than 1%. The results are summarized in Table 5. Based on the current interim limit criteria of nitrosamines, all samples were found to meet the acceptable levels of the four nitrosamines. It is of note that the EU has proposed a limit for all nitrosamines at 30 ppb with an effective date of April 1, 2021. Therefore, there are five out of 10 batches that contain NDMA exceeding the proposed limit. In contrast, two other batches were detected with NDEA at a level higher than the LOQ of 25 ppb but less than 30 ppb.
Table 5. Results of Four Nitrosamines in 10 Batches of Losartan Potassium (n = 3).
| batch no. | nitrosamine
content (ppb) (%CV) |
|||
|---|---|---|---|---|
| NDMA | NDEA | DIPNA | EIPNA | |
| 1 | 81.0 (0.75) | <5 | <25 | <25 |
| 2 | 30.5 (0.38) | <5 | <25 | <25 |
| 3 | 35.1 (0.16) | <5 | <25 | <25 |
| 4 | 55.4 (0.28) | <5 | <25 | <25 |
| 5 | 42.8 (0.54) | <5 | <25 | <25 |
| 6 | <5 | 25.4 (0.82) | <25 | <25 |
| 7 | <5 | 28.6 (0.35) | <25 | <25 |
| 8 | <5 | <5 | <25 | <25 |
| 9 | <5 | <5 | <25 | <25 |
| 10 | <5 | <5 | <25 | <25 |
3. Materials and Methods
3.1. Drugs and Chemicals
An NDMA certified reference standard at a concentration of 1004.0 μg/mL (lot no. A0141467) was purchased from Restek (Bellefonte, PA, USA). NDEA (lot no. H5GMI, purity 100.0%) was purchased from Tokyo Chemical Industry Co, Ltd. (Tokyo, Japan). DIPNA (lot no. 9-AQL-128-2, purity 98.0%) and EIPNA (lot no. 2-AMR-151-1, purity 95.0%) were purchased from Toronto Research Chemicals (Toronto, Canada). Losartan potassium, valsartan sacubitril, and olmesartan medoxomil were provided by Siam Bheasach Co., Ltd. (Bangkok, Thailand). A preliminary screening test for selecting nitrosamine-free sartans was performed for losartan potassium, valsartan sacubitril, and olmesartan medoxomil to ensure the lack of interference in the sartan standards used in the validation experiments. Ten batches of losartan potassium APIs were purchased from two manufacturers (IPCA, Mumbai, India; Hetero Labs Limited, Hyderabad, India). DMSO was purchased from Thermo Fisher Scientific (Leics, UK). All reagents were of at least analytical grade. The purified water was prepared from a Milli-Q water purification system (Millipore; S.A.S, France).
3.2. Instruments and Chromatographic Conditions
GC was performed on a Nexis GC-2030 system (Shimadzu Corp., Kyoto, Japan) with an HS-20 headspace autosampler. An analytical separation was performed using an SH-Rtx-Wax (polyethylene glycol) GC column (30 × 0.32 mm2 i.d., 1 μm, Shimadzu America, MD, USA) placed in a column oven built-in GC system. Helium was used as a carrier gas. The temperature programing was set in a gradient mode using an initial constant temperature at 40 °C for 2 min, a ramping temperature of 20 °C/min to 240°C, and a holding temperature at 240 °C for 3.5 min. The carrier gas flow rate was set at 2.0 mL/min. The oven sample was heated up to 150 °C under a vibration level of 5 with an appropriate sample equilibrating time of 15 min. After equilibration, the sample vapor was pressurized for 1 min before 1 μL of the sample was injected under a gas pressure of 103 kPa with a split ratio of 3:1. The temperatures of the sample and transfer lines were set at 230 and 250 °C, respectively. The solvent cut time was set at 1 min. The filament was turned off at 9.0 min. The chromatographic run time was 15.5 min, and the cool down period was 1.7 min. Thus, the total analysis time was 17.2 min. The entire flow was directed into the detector.
Quantitation was achieved via MS detection in the EI mode for nitrosamine detection using a QP2020 NX mass spectrometer (Shimadzu Corp., Kyoto, Japan). The detector voltage was set at +0.6 kV. Both the ion source and the interface temperatures were set at 230 °C. NDMA (m/z 74.0), NDEA (m/z 102.0), DIPNA (m/z 130.0), and EIPNA (m/z 116.0) were quantified on a single-quadrupole mass spectrometer in the EI mode under the SIM acquisition method. The dwell time or event time was set at 300 ms. Quantitation of NDMA, NDEA, DIPNA, and EIPNA was based on their peak areas. Data were processed using Lab Solutions Software (GCMS Solution Version 4.50, Shimadzu Corp., Kyoto, Japan).
3.3. Preparation of Nitrosamine Stock and Working Standard Solutions
The NDMA certified reference standard (1000 μg/mL) was used as an NDMA stock standard solution. Other nitrosamine stock standard solutions were separately prepared by dissolving NDEA, DIPNA, or EIPNA standards in methanol at a concentration of 1000 μg/mL. Nitrosamine working standard solutions of NDMA, NDEA, DIPNA, and EIPNA were prepared at a concentration of 5 μg/mL by diluting each stock standard solution with an appropriate amount of DMSO.
3.4. Preparation of Calibration Standards and Spiked Quality control Samples
Calibration standards for NDMA, NDEA, DIPNA, and EIPNA were prepared by aliquoting the working standard solutions in DMSO to yield final concentrations of 25 (LOQ of NDMA and NDEA), 50 (LOQ of DIPNA and EIPNA), 100, 250, 500, 1000, and 5000 ng/mL. Spiked QC samples for NDMA and NDEA were prepared by adding the working standard solutions to the nitrosamine-free losartan API at concentrations of 25, 250, and 1000 ppb, representing QC at LOQ (LOQ QC), medium QC (MQC), and high QC (HQC) samples, respectively. For DIPNA and EIPNA, spiked QC samples were prepared at concentrations of 50 (LOQ), 250, and 1000 ppb, representing LOQ QC, MQC, and HQC, respectively.
3.5. Sample Preparation
Five milliliters of DMSO were transferred to a glass vial for headspace injection containing 1 g losartan potassium. The sample was then gently vortexed using a vortex mixer at the maximum rotation speed for 5 min. The clear sample solution was placed into the HS-20 headspace autosampler. The sample was incubated in the headspace oven at 150 °C under the vibration level of 5, with the appropriate sample equilibrating time of 15 min. The residual nitrosamines were then vaporized into the vial headspace and directly injected into the column.
3.6. Method Validation
The method was validated according to the Q2(R1) ICH guidelines for analytical method validation.25 The validation parameters included system suitability, specificity, carryover, linearity, accuracy, precision, LOD, LOQ, and robustness.
3.6.1. System Suitability
The suitability of the system was evaluated before the validation experiments and impurity determination. A system suitability solution containing a mixture of NDMA, NDEA, DIPNA, and EIPNA was prepared at 250 ng/mL for each compound by appropriately aliquoting the working standard solutions (1000 μg/mL). The system suitability solution was injected with five replicates. The system performance was evaluated on the tailing factor of analytes (T ≤ 2), and the system reproducibility was assessed as the variation of the peak area (%CV ≤ 11%) and retention time (%CV ≤ 2%) to indicate the precision of injections.
3.6.2. Specificity and Selectivity
Specificity is the ability to discriminate the analyte in the presence of interferences such as solvents, matrices, and impurities.26 The specificity was investigated by separately injecting diluents, e.g., DMSO and methanol, an authentic standard of nitrosamine solution (250 ng/5 mL in DMSO), and blank sartan matrices including losartan potassium, olmesartan medoxomil, and valsartan sacubitril (1 g/5 mL in DMSO). According to Yang et al.,24 a 15N isotope of dimethylformamide (DMF) with a molecular mass per charge (m/z) of 74 may interfere with the analysis of NDMA. The concern of 15N-DMF contamination becomes significant if the chromatographic condition cannot separate DMF from NDMA. Therefore, DMF at a concentration of 500 ng/5 mL in DMSO also caused interference. The specificity was determined as the presence or absence of interfering peaks at the same m/z value and retention time of NDMA, NDEA, DIPNA, and EIPNA on the chromatograms.
3.6.3. Carryover
The carryover effect was evaluated as the percentage of carryover by analyzing a blank DMSO after injecting the upper LOQ concentration of 5000 ng/mL of the calibration standard curve. The percentage of carryover in the blank sample was calculated compared to the LOQ and considered acceptable at ≤20%. Three independent experiments were performed on three different days.
3.6.4. Calibration Curves
Seven-point calibration curves were constructed in triplicate by plotting the analyte peak areas versus different analyte concentrations of NDMA, NDEA, DIPNA, or EIPNA. The weighted-linear least square model with a 1/x2 weighting factor was selected using a statistical test of homoscedasticity.27 The coefficient of determination (r2 ≥ 0.995) was determined to verify the linearity of the plot. The percent deviation of the mean back-calculated concentrations of NDMA, NDEA, DIPNA, and EIPNA should be within ±10%. The residual plot was demonstrated whether the slope and y-intercept were significantly different from zero at a 95% confidence interval. Statistically, a p value of the slope should be less than 0.05, meaning that it is significantly different from zero, whereas the p value of the y-intercept should be more than 0.05, meaning that it is insignificant from zero. Fcal should be greater than FANOVA demonstrating the linear regression.28
3.6.5. Linearity and Range
The range describes the concentration interval between the lower and upper quantitative levels. The range of the method was established using the spiked calibration samples by constructing a calibration curve using a losartan matrix spiked with the corresponding nitrosamines. The equation model was designed in the same manner as the calibration curve experiment (Section 3.6.4). The slope, intercept, and r2 were calculated.
3.6.6. Accuracy and Precision
Accuracy and precision were determined by analyzing the spiked losartan samples at 25 (LOQ), 250, and 1000 ppb for NDMA and NDEA and 50 (LOQ), 250, and 1000 ppb for DIPNA and EIPNA. Each concentration level was prepared and analyzed in triplicate on the same day and for two different days for intraday (within-run) and interday (between-run) analyses, respectively. Accuracy was assessed using the percent deviation between the mean back-calculated concentration and nominal (actual) concentration. The percent deviation should be within ±10%. The intraday and interday precision values were determined using the %CV of the back-calculated concentration. The %CV should be ≤11% for all concentrations excluding the LOQ, which should be ≤15%.
3.6.7. LOD and LOQ
The LOD is the lowest amount of analyte in a sample matrix that could be detected but not necessarily quantitated as an exact value. The LOD can be derived from the signal-to-noise (S/N) ratio, and it is usually expressed as the concentration of an analyte in the sample matrix. It is determined by injecting samples that generate the S/N ratio and %CV of replicate injections. The LOD was verified in terms of S/N ≥ 3 and %CV of five replicate injections of ≤15.
The LOQ is the lowest amount of analyte in a sample matrix that can be quantitatively determined with appropriate precision and accuracy. Typically, the LOQ is estimated by determining the S/N ratio and %CV of replicate injections. The LOQ established using spiked samples independent of calibration standards with five replicate injections was satisfied with accuracy and precision of percent deviation within ±10 and %CV ≤ 15, respectively.
3.6.8. Robustness and Stability
Robustness is an indicator of method tolerability when small variations occur in method parameters to ensure the reliability of the method during routine analysis. In this study, the robustness of the method was determined as the suitability of the system to permit changes in transfer temperature and headspace equilibration time. The precision values of the peak response and retention time for four nitrosamines were determined after slight variation in the method parameters. The %CV of the peak area and retention time should be ≤11 and 2, respectively, whereas the tailing factor of each injection should be lower than 2. Moreover, the influence of the flow rate on the resolution between 15N-DMF and NDMA was studied. The resolution should be >2. In addition, the stability of the sample solutions was investigated. To confirm the good stability of the sample solution, the percent deviation of the analyte concentration between the initial time point (0 h) and the storage time of 6, 12, or 24 h should not be greater than 10%.
3.7. Application for Nitrosamine Determination in Losartan Potassium APIs
The developed and validated method was applied to determine residual contamination by NDMA, NDEA, DIPNA, and EIPNA in ten batches of losartan potassium API samples. Five milliliters of DMSO was transferred into a glass vial for headspace injection containing 1 g of losartan potassium, and the mixture was vortexed until it was completely dissolved. The sample solution was placed into the built-in headspace autosampler of the oven. The sample was incubated in the headspace oven at 150 °C under a vibration level of 5, with an appropriate sample equilibrating time of 15 min. The residual nitrosamines were then vaporized into the vial headspace and directly injected into the column.
4. Conclusions
In this work, the HS-GC–MS method was developed and validated for the quantitation of NDMA, NDEA, DIPNA, and EIPNA in losartan potassium APIs. The headspace injection technique was applied to serve as an online sample preparation in residual impurity analysis, thereby offering benefits concerning the efficient removal of potential interference and high-throughput routine analysis. The complete method validation was performed using the Q2(R1) ICH guidelines.26 The overall results indicated that the developed method is reliable and useful for determining the levels of four nitrosamines in losartan APIs. For other sartans, we confirmed the selectivity of the method based on the absence of interfering matrix co-elution at the retention times of individual nitrosamines. To use the method to analyze nitrosamine contamination in other sartan APIs and drug products, the method should be further fully investigated for its accuracy, precision, and other validation parameters using the acceptable limits of nitrosamines in each substance. The LOQ and LOD of the developed method were far below the US FDA and EMA interim limits of the corresponding nitrosamines in losartan potassium. In addition, the established LOD and LOQ can support a tighter limit of 30 ppb for NDMA and NDEA, which will be implemented in the forthcoming year.7 Although the developed method cannot be applied in the quantitative sense under this stricter limit, it can also serve as a screening test for DIPNA and EIPNA. In a broader sense, the methodology can also be adapted for the simultaneous analysis of additional nitrosamines in other sartans.
Acknowledgments
The authors thank Chukkapong Comsup, Bara Scientific Co., Ltd., Assistant Product Manager of Analytical Product for providing the technical support and helpful discussion for this research. The authors’ complement is also due to the demo version of the Shimadzu GCMS instrument and software kindly offered by Bara Scientific Co., Ltd, Thailand.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c00982.
Chromatograms of spiked NDMA (m/z 74), spiked DMF (15N isotopic ion at m/z 74), and co-spiked between NDMA and DMF in losartan (Figure S1); results of method robustness under small variations in the HS equilibration time and transfer line temperature (Tables S1–S8); and chromatograms of co-spiked between NDMA and DMF in losartan under the variations of the flow rate (Figure S2) (PDF)
Author Contributions
W.W. conceived and designed the project methodology. O.S. managed all resources used in all experiments and performed graphic preparation. P.S. and K.C. conducted the method validation, data acquisition, and evaluation. S.W. wrote the original draft of manuscript. P.R. reviewed the manuscript. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Barreras A.; Gurk-Turner C. Angiotensin II receptor blockers. Baylor Univ. Med. Cent. Proc. 2003, 16, 123–126. 10.1080/08998280.2003.11927893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnier M.; Brunner H. R. Angiotensin II receptor antagonists. Lancet 2000, 355, 637–645. 10.1016/S0140-6736(99)10365-9. [DOI] [PubMed] [Google Scholar]
- Park J.; Seo J.; Lee J.; Kwon H. Distribution of seven N-nitrosamines in food. Toxicol. Res. 2015, 31, 279–288. 10.5487/TR.2015.31.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US FDA press announcements . FDA announces voluntary recall of several medicines containing valsartan following detection of an impurity. http:\\FDA.gov (accessed Jul 13, 2018)
- EMA release . CHMP list of questions. EMA/CHMP/467845/2018. https://www.ema.europa.eu/en/documents/referral/angiotensin-ii-receptor-antagonists-sartans-article-31-referral-chmp-list-questions-be-addressed-api_en.pdf (accessed Jul 16, 2018).
- EMA release . Temporary interim limits for NMBA, DIPNA and EIPNA impurities in sartan blood pressure medicines. EMA/351053/2019 rev 1. https://www.ema.europa.eu/en/documents/other/temporary-interim-limits-nmba-dipna-eipna-impurities-sartan-blood-pressure-medicines_en.pdf (accessed Aug 20, 2019).
- EMA release . Overview and recommendation: Lessons learnt from presence of N-nitrosamine impurities in sartan medicines. EMA/CHMP/526934/2019. https://www.ema.europa.eu/en/documents/report/lessons-learnt-presence-n-nitrosamine-impurities-sartan-medicines_en.pdf (accessed Jun 23, 2019).
- Oruganti S.A viewpoint on the presence of N-nitrosodimethylamine (NDMA) impurity in certain generic samples of Valsartan, Drils Perspectives; Dr. Reddy’s Institute of Life Sciences, 2018. [Google Scholar]
- EMA release . Assessment report. EMA/CHMP/217823/2019. https://www.ema.europa.eu/en/documents/variation-report/angiotensin-ii-receptor-antagonists-sartans-article-31-referral-chmp-assessment-report_en.pdf (accessed Feb 14, 2019).
- European network of OMCLs, OMCLs Release . Determination of NDMA in valsartan active substances and finished products by HPLC/UV; French National Agency for Medicines and Health Products Safety, 2018. [Google Scholar]
- European network of OMCLs, OMCLs Release . Test method for the determination of NDMA by LC/MS/MS in Valsartan finished products; Chemisches und Veterinäruntersuchungsamt: Karlsruhe, Germany, 2018. [Google Scholar]
- European network of OMCLs, OMCLs Release . Determination of NDMA (HS-GC–MS) method 3/30, issue no. 1; Public Analyst’s Laboratory: Galway, Ireland, 2018. [Google Scholar]
- USFDA . GC/MS Headspace Method for NDMA in Valsartan Drug Substance and Drug Products; US Food and Drug Administration: Washington, DC, 2019. [Google Scholar]
- USFDA . Combined N-Nitrosodimethylamine (NDMA) and N-Nitrosodiethylamine (NDEA) Impurity Assay by GC/MS-Headspace; US Food and Drug Administration: Washington, DC, 2019. [Google Scholar]
- Taiwan FDA . Method of Test for Nitrosamines in Medicines Multiple Analysis (GC–MS/MS Method); Taiwan Food and Drug Administration: Nangang, Taipei City, 2020. [Google Scholar]
- Chang S.; Chang C.; Wang L.; Chen W.; Fan S.; Zang C.; Hsu Y.; Lin M.; Tseng S.; Wang D. A multi-analyte LC-MS/MS method for screening and quantification of nitrosamines in sartans. J. Food Drug Anal. 2020, 28, 98–107. 10.38212/2224-6614.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USFDA . Combined Direct Injection N-Nitrosodimethylamine (NDMA), N-Nitrosodiethylamine (NDEA), N-Nitrosoethylisopropylamine (NEIPA), N-Nitrosodiisopropylamine (NDIPA), and N-Nitrosodibutylamine (NDBA) Impurity Assay by GC–MS/MS; US Food and Drug Administration: Washington, DC, 2019. [Google Scholar]
- USFDA . Combined headspace N-Nitrosodimethylamine (NDMA), N-Nitrosodiethylamine (NDEA), N-Nitrosoethylisopropylamine (NEIPA), N-Nitrosodiisopropylamine (NDIPA), and N-Nitrosodibutylamine (NDBA) Impurity Assay by GC–MS/MS; US Food and Drug Administration: Washington, DC, 2019. [Google Scholar]
- Brian Byrd J.; Chertow G. M.; Bhalla V. Hypertension hot potato—Anatomy of the angiotensin-receptor blocker recalls. N. Engl. J. Med. 2019, 380, 1589–1591. 10.1056/NEJMp1901657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA Updates and Press Announcements on Angiotensin II Receptor Blocker (ARB) Recalls (Valsartan, Losartan, and Irbesartan): FDA updates table of interim limits for nitrosamine impurities in ARBS. https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-and-press-announcements-angiotensin-ii-receptor-blocker-arb-recalls-valsartan-losartan (accessed Feb 28, 2019).
- EMA release . Questions and answers for marketing authorization holder/applicants on the CHMP Opinion for the Article 5 (3) of Regulation (EC) No 726/2004 referral on nitrosamine impurities in human medicinal products. EMA/CHMP/409815/2020. https://www.ema.europa.eu/en/documents/referral/nitrosamines-emea-h-a53-1490-questions-answers-marketing-authorisation-holders/applicants-chmp-opinion-article-53-regulation-ec-no-726/2004-referral-nitrosamine-impurities-human-medicinal-products_en.pdf (accessed Aug 03, 2020).
- EMA release . Assessment report: Procedure under Article 5(3)of Regulation EC (No) 726/2004 Nitrosamine impurities in human medicinal products. EMA/CHMP/369136/2020. https://www.ema.europa.eu/en/documents/referral/nitrosamines-emea-h-a53-1490-assessment-report_en.pdf (accessed June 25, 2020).
- USFDA . Liquid Chromatography-High Resolution Mass Spectrometry (LC-HRMS) Method for the Determination of Six Nitrosamine Impurities in ARB Drugs; US Food and Drug Administration: Washington, DC, 2019. https://www.fda.gov/media/125478/download
- Yang J.; Andres Marzan T.; Ye W.; Sommers C. D.; Rodriguez J. D.; Keire D. A. A cautionary tale: Quantitative LC-HRMS analytical procedures for the analysis of N-Nitrosodimethylamine in metformin. AAPS J. 2020, 20, 89 10.1208/s12248-020-00473-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi T.; Akiyama H.; Demizu Y.; Uchiyama N.; Masada S.; Tsuji G.; Arai R.; Abe Y.; Hakamatsuka T.; Izutsu K.; Goda Y.; Okuda H. Analysis of an impurity, N-Nitrosodimethylamine, in valsartan drug substances and associated products using GC–MS. Biol. Pharm. Bull. 2019, 42, 547–551. 10.1248/bpb.b19-00006. [DOI] [PubMed] [Google Scholar]
- ICH Harmonised tripartite guideline: Validation of Analytical Procedures: Text and methodology Q2(R1). Current Step 4 version (accessed Oct 27, 1994). [Google Scholar]
- Danzer K.; Currie L. A. Guidelines for calibration in analytical chemistry. Pure Appl. Chem. 1998, 70, 993–1014. 10.1351/pac199870040993. [DOI] [Google Scholar]
- Barwick V. A.Preparation of Calibration Curves: A Guide to Best Practice. LGC/VAM/2003/032, 2003, 1–27. [Google Scholar]
- Crews C. The determination of N-nitrosamines in food. Qual. Assur. Saf. Crops Foods 2010, 2, 2–12. 10.1111/j.1757-837X.2010.00049.x. [DOI] [Google Scholar]
- Seyler T. H.; Kim J. G.; Hodgson J. A.; Cowan E. A.; Blount B. C.; Wang L. Quantitation of urinary volatile nitrosamines from exposure to tobacco smoke. J. Anal. Toxicol. 2013, 37, 195–202. 10.1093/jat/bkt020. [DOI] [PubMed] [Google Scholar]
- Munch J. W.; Bassett M. V.. Method 521. Determination of Nitrosamines in Drinking Water by Solid-phase Extraction and Capillary Column Gas Chromatography with Large Volume Injection and Chemical Ionization Tandem Mass Spectrometry (MS/MS); U.S. Environmental Protection Agency: Cincinnati, Ohio, 2004. [Google Scholar]
- Parr M. K.; Joseph J. F. NDMA impurity in valsartan and other pharmaceutical products: Analytical methods for the determination of N-nitrosamines. J. Pharm. Biomed. Anal. 2019, 164, 536–549. 10.1016/j.jpba.2018.11.010. [DOI] [PubMed] [Google Scholar]
- Şerban E. S.; Socaci S. A.; Tofană M.; Maier S. C.; Bojiţă M. Advantages of “headspace” technique for GC/MS analysis of essential oils. Farmacia 2012, 60, 249–256. [Google Scholar]
- Ibrahim M. M. Investigation on thermal stability and purity determination of two antihypertensive drugs, valsartan and losartan potassium. Int. J. Curr. Pharm. Res. 2015, 7, 64–69. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.