Abstract
Opportunistic infections from pathogenic fungi present a major challenge to healthcare because of a very limited arsenal of antifungal drugs, an increasing population of immunosuppressed patients, and increased prevalence of resistant clinical strains due to overuse of the few available antifungals. Cryptococcal meningitis is a life-threatening opportunistic fungal infection caused by one of two species in the Cryptococcus genus, Cryptococcus neoformans and Cryptococcus gattii. Eighty percent of cryptococcosis diseases are caused by C. neoformans that is endemic in the environment. The standard of care is limited to old antifungals, and under a high standard of care, mortality remains between 10 and 30%. We have identified a series of 5-nitro-6-thiocyanatopyrimidine antifungal drug candidates using in vitro and computational machine learning approaches. These compounds can inhibit C. neoformans growth at submicromolar levels, are effective against fluconazole-resistant C. neoformans and a clinical strain of C. gattii, and are not antagonistic with currently approved antifungals.
Keywords: C. neoformans, C. gattii, 4-thiocyano-5-nitropyrimidines, small-molecule antifungal agents, machine learning
The basidiomycete yeast, Cryptococcus neoformans, is a fungal pathogen of immunocompromised people that each year causes up to 1 million pulmonary infections and meningoencephalitis, which are fatal if untreated and result in up to 250 000 deaths annually.1C. neoformans was first observed clinically in the 1960s with the advent of organ transplant and aggressive treatment of cancers and other diseases that resulted in immunosuppression.2C. neoformans is the third leading cause of infections in solid organ transplant patients, where up to 3% develop an invasive fungal infection within the first year, with an overall mortality of 25–40%.3,4 Transplant patients remain susceptible to C. neoformans for 5 years due to its presence in the environment.5C. neoformans infections can be successfully treated with a combination of amphotericin B (AmB) and flucytosine, but the treatment regimen is long and has significant toxicity. The mortality rate for cryptococcal infections remains 15–30%, even in the context of antiviral treatments for human immunodeficiency virus (HIV).6−8 The number of AIDS patients infected with C. neoformans peaked in the mid-1990s, driven by increased HIV infections. The advent of effective antiretroviral therapies (ART) in 1997 significantly reduced the number of HIV-positive patients with cryptococcosis,9 with ca. 2.9% of AIDS patients in the U.S. positive for the cryptococcal antigen.10 This reduction has not been observed in resource-limited countries, especially in areas with high disease burden such as sub-Saharan Africa. There, the prevalence of cryptococcal meningitis (CM) in HIV-infected patients is between 25 and 45%.11 CM causes high mortality among AIDS patients, surpassing tuberculosis deaths in some areas of sub-Saharan Africa.1
A closely related Cryptococcus species, C. gattii, lives in soil and in association with certain trees and can affect the lungs (pneumonia) and nervous system (causing meningitis and focal brain lesions called cryptococcomas) in humans. The main complication of lung infection is respiratory failure. Central nervous system infection may lead to hydrocephalus, seizures, and focal neurological deficit. It can infect immunocompetent people and is endemic in tropical areas. It was first observed in temperate regions in an outbreak on Vancouver Island that has since expanded geographically.12,13C. gattii causes ongoing infections among immunocompromised patients in southern California and in the southeastern U.S.2,14−16 Antifungals alone are often insufficient to cure C. gattii infections. Cryptococcal infections are therefore difficult to treat, have high mortality, and no new antifungal drugs have been approved since 2003. Hence, there is a dire need to develop new safe and effective drugs for treating cryptococcal species infections.
There are only three approved drugs effective for cryptococcosis, all of which were developed decades ago. AmB, which binds ergosterol, is highly specific for fungi but has very significant toxicity, and its use in resource-poor areas is limited because it is administered intravenously.17 5-Fluorocytosine (5-FC) targets pyrimidine biosynthesis, but it is used only in combination with other drugs because of the high rate of resistance evolution. Fluconazole (FLC) also targets ergosterol biosynthesis but is only fungistatic and leaves patients susceptible to relapse. The current standard of treatment is a combination of 5-FC and AmB, but efficacy is limited even in resource-rich settings, where mortality from CM among all patients, regardless of the underlying condition, is between 10 and 35%.6,7,18,19 Finally, the newest class of antifungals, the echinocandins, is not active against Cryptococcus.20 The current situation has been termed a titanic drug resistance threat.21 Therefore, there is an urgent need for novel and more effective anticryptococcal therapies.
Multiple research groups are engaged in preclinical studies of anticryptococcal agents and have identified some promising scaffolds.22−27 In addition, the development of a chemical probe ML40728 targeting the cell wall may be a useful starting point for drug discovery. Viamet Pharmaceuticals has developed a CYP51 inhibitor, VT-1129, that inhibits the growth of multiple Cryptococcal isolates (as low as 0.015 μg/mL for 50% inhibition and 0.06 μg/mL for 100% inhibition, equivalent to 30–120 nM),29,30 including C. neoformans and C. gattii. VT-1129 also shows activity in a mouse model of CM.31 VT-1129 has been granted Qualified Infectious Disease Product designation and is currently in phase I clinical trials for treatment of cryptococcal meningitis. However, since the recent acquisition of this company by NovaQuest Capital, the status of this program is uncertain. Among active phase II or phase III clinical trials for treating cryptococcosis, there are only two exploratory treatments that use drugs other than FLC, AmB, or 5-FC. A phase III trial to determine the efficacy of a repurposed serotonin uptake inhibitor, sertraline, for patients with CM (NCT 01802385), was completed in 2019 but saw no improvement in mortality over placebo controls, possibly due to insufficient duration of therapeutic sertraline concentrations.32 A phase II study to examine efficacy of combinations of tamoxifen, an estrogen receptor agonist, and FLC or AmB (NCT 03112031) was completed in 2019, but no results have yet been posted.23 No other novel compounds for treatment of cryptococcosis and CM have reached IND status. Thus, it is imperative that we increase the number of new classes of antifungals drug candidates and stimulate the pipeline for treatment of cryptococcosis in general.
In the current study, we have utilized a Bayesian machine learning approach, which classifies data as active or inactive based on user-defined thresholds using a simple probabilistic classification model based on Bayes’ theorem. Three early studies by us used Bayesian machine learning models to identify molecules with promising in vitro activity for Mycobacterium tuberculosis,33Trypanosoma cruzi,34 and Ebola virus.35 Most recently, we have used this software to assist in drug discovery for chordoma,36Neisseria gonorrheae,37 HIV,38 and Staphylococcus aureus.39 This study represents our first attempt at applying this approach to antifungal drug discovery.
We have now identified a series of 5-nitro-6-thiocyanatopyrimidine antifungal drug candidates using in vitro and computational machine learning approaches that have been shown to inhibit C. neoformans and C. gattii growth at submicromolar levels (Figure 1). These may represent a starting point for further hit-to-lead optimization and target identification.
Figure 1.
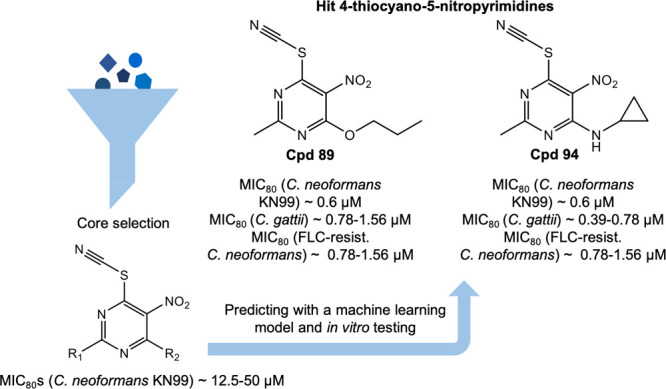
4-Thiocyano-5-nitropyrimidine core selection and two hit compounds of this class active against Cryptococcus neoformans and Cryptococcus gattii at submicromolar levels.
Synthesis of 5-Nitro-6-thiocyanatopyrimidines
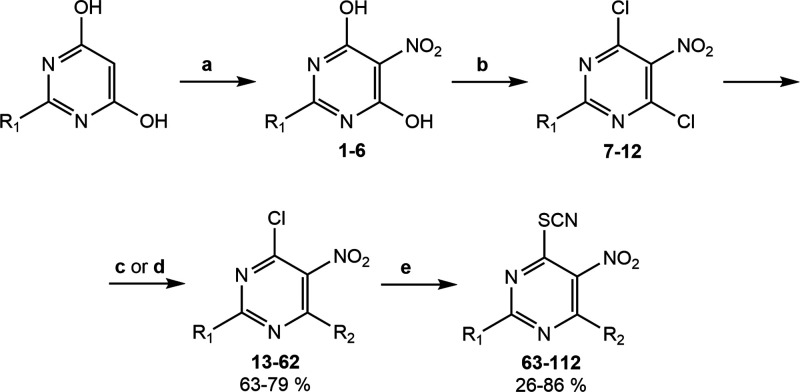
2,4-Disubstituted 5-nitro-6-thiocyanatopyrimidines studied here were synthesized in four steps according to Figure 2. The starting 2-substituted 4,6-dihydroxypyrimidines were nitrated at the 5-position using fuming nitric acid and sulfuric acid in a catalytic amount. Further treatment of these intermediates with phosphorus oxychloride resulted in the formation of 2-substituted 4,6-dichloro-5-nitropyrimidines 7–12. At the next stage, 4,6-dichloropyrimidines were reacted with the corresponding amines in the acetate form in dioxane (Figure 2c) or with the corresponding sodium alkoxides in alcohol (Figure 2d), which led to the replacement of only one chlorine atom in the 4-position to amines or alcohols. The final 5-nitro-6-thiocyanatopyrimidines 63–112 (Table 1) were obtained by nucleophilic substitution of the second chlorine atom with potassium thiocyanate in good yields.
Figure 2.
General synthetic scheme of 5-nitro-6-thiocyanatopyrimidines. Reagents and conditions: (a) HNO3, H2SO4cat; (b) POCl3, Et3N, HCl; (c) corresponding amine solution, AcOH, dioxane; or (d) corresponding sodium alkoxide, alcohol; (e) KSCN, alcohol
Table 1. In Vitro Activity of 2,4-Disubstituted 5-Nitro-6-thiocyanatopyrimidines 63–112 against Cryptococcus neoformans and Cryptococcus gattii.
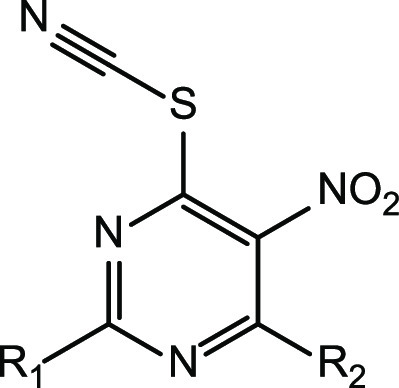
| molecule no. | R1 | R2 | CC50 μM (μg/mL) | C. neoformans MIC80 μM (μg/mL) | C. gattia MIC80 μM (μg/mL) | C. neoformansa MIC80 (FLC-resistance) μM (μg/mL) |
|---|---|---|---|---|---|---|
| 63 | H | MeO | 9.37 ± 3.12 (1.98 ± 0.66) | >25 (>5.3) | ||
| 64 | H | PrO | 31.8 (7.64) | 3.9 ± 0 (0.93 ± 0) | 4.68 ± 1.56 (1.12 ± 0.38) | |
| 65 | H | iPrO | 23.3 (5.6) | 1.9 ± 0 (0.45 ± 0) | 1.17 ± 0.39 (0.27 ± 0.09) | |
| 66 | H | NH2 | 4.68 ± 1.56 (0.92 ± 0.3) | 1.17 ± 0.39 (0.22 ± 0.07) | ||
| 67 | H | iPrNH | 12 (2.87) | 2.34 ± 0.78 (0.55 ± 0.18) | 0.58 ± 0.19 (0.13 ± 0.04) | 2.34 ± 1.56 (0.55 ± 0.37) |
| 68 | H | 3-methylbutan-2-yl-NH | 3 ± 0 (0.8 ± 0) | |||
| 69 | H | pentan-3-yl-NH | 18.5 ± 0 (4.94 ± 0) | >25 (>6.678) | ||
| 70 | H | cyclopentyl-NH | 4.9 ± 0 (1.29 ± 0) | 1.17 ± 0.39 (0.3 ± 0.1) | 2.34 ± 1.56 (0.62 ± 0.41) | |
| 71 | H | cyclohexyl-NH | 6 ± 0 (1.67 ± 0) | 1.17 ± 0.39 (0.32 ± 0.11) | ||
| 72 | H | piperidinyl | 11.9 (3.16) | 2.34 ± 0.78 (0.61 ± 0.2) | 1.17 ± 0.39 (0.3 ± 0.1) | 4.68 ± 3.13 (1.24 ± 0.83) |
| 73 | H | 2-Me-piperidinyl | 9.37 ± 3.12 (2.61 ± 0.86) | 1.17 ± 0.39 (0.32 ± 0.11) | ||
| 74 | H | 2,6-di-Me-piperidinyl | 9.37 ± 3.12 (2.74 ± 0.9) | 15.62 ± 9.37 (4.58 ± 2.75) | ||
| 75 | H | 3,5-di-Me-piperidinyl | 33.6 (9.86) | 2.34 ± 0.78 (0.68 ± 0.22) | 9.37 ± 3.12 (2.74 ± 0.91) | |
| 76 | H | azepanyl | 17.7 (4.94) | 2.34 ± 0.78 (0.65 ± 0.22) | 2.34 ± 0.78 (0.65 ± 0.22) | 2.34 ± 1.56 (0.65 ± 0.43) |
| 77 | H | azocanyl | 4.68 ± 1.56 (1.37 ± 0.46) | 2.34 ± 0.78 (0.68 ± 0.23) | ||
| 78 | H | benzyl-NH | 19.6 (5.63) | 2.34 ± 0.78 (0.66 ± 0.22) | 1.17 ± 0.39 (0.33 ± 0.11) | 1.17 ± 0.78 (0.33 ± 0.22) |
| 79 | H | (mCF3-benzyl)-NH | 25.91 (9.21) | 9.37 ± 12.5 (3.32 ± 4.44) | 4.68 ± 1.56 (1.66 ± 0.56) | |
| 80 | H | (pCl-benzyl)-NH | 22.53 (7.25) | 5.85 ± 7.8 (1.88 ± 2.5) | 1.17 ± 0.39 (0.37 ± 0.12) | |
| 81 | H | Ph-(CH2)2-NH | 15.09 (4.55) | 3.51 ± 4.68 (1.05 ± 1.4) | 2.34 ± 0.78 (0.7 ± 0.23) | |
| 82 | H | Me2N | 4.68 ± 1.56 (1.05 ± 0.34) | 1.17 ± 0.39 (0.26 ± 0.09) | ||
| 83 | H | di-Et-N | 9.37 ± 3.12 (2.37 ± 0.78) | 0.14 ± 0.04 (0.03 ± 0.01) | ||
| 84 | H | iPrEtN | 10.7 (2.86) | 4.55 ± 4.24 (1.4 ± 1.12) | 1.17 ± 0.39 (0.3 ± 0.1) | |
| 85 | H | cyclohexylMeN | 25.3 (7.42) | 6 ± 0 (1.76 ± 0) | 4.68 ± 1.56 (1.37 ± 0.46) | |
| 86 | H | benzylMeN | 18.8 (5.66) | 2.34 ± 0.78 (0.7 ± 0.22) | 1.17 ± 0.39 (0.35 ± 0.12) | |
| 87 | H | benzyl-N-iPr | >50 (>16.46) | >25 (>8.23) | ||
| 88 | Me | EtO | 19.8 (4.76) | 1.17 ± 0.38 (0.27 ± 0.08) | 0.29 ± 0.1 (0.06 ± 0.02) | 2.34 ± 1.56 (0.56 ± 0.37) |
| 89 | Me | PrO | 15.8 (4.02) | 0.6 ± 0 (0.15 ± 0) | 1.17 ± 0.39 (0.29 ± 0.1) | 1.17 ± 0.78 (0.29 ± 0.19) |
| 90 | Me | iPrO | 14.6 (3.71) | 1 ± 0 (0.25 ± 0) | 1.17 ± 0.39 (0.29 ± 0.1) | 1.17 ± 0.78 (0.29 ± 0.19) |
| 91 | Me | NH2 | 24.5 (5.17) | 2.34 ± 0.78 (0.48 ± 0.16) | 0.58 ± 0.19 (0.12 ± 0.04) | 2.34 ± 1.56 (0.49 ± 0.32) |
| 92 | Me | MeNH | >100 (>22.53) | 2.34 ± 0.78 (0.52 ± 0.16) | 1.17 ± 0.39 (0.26 ± 0.09) | 2.34 ± 1.56 (0.52 ± 0.35) |
| 93 | Me | pentan-3-yl-NH | 9 ± 0 (2.53 ± 0) | 9.37 ± 3.12 (2.63 ± 0.88) | ||
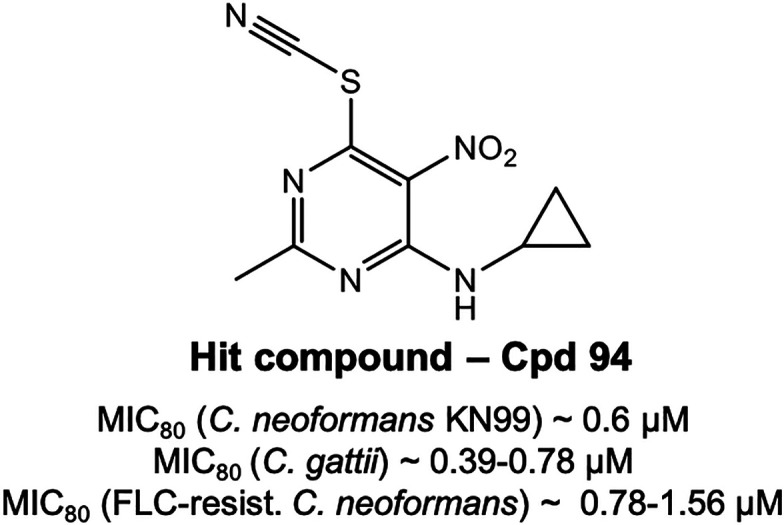
| 94 | Me | cyclopropyl-NH | 12.7 (3.19) | 0.6 ± 0 (0.15 ± 0) | 0.58 ± 0.19 (0.14 ± 0.05) | 1.17 ± 0.78 (0.29 ± 0.19) |
| 95 | Me | cyclopentyl-NH | 9 ± 0 (2.51 ± 0) | 2.34 ± 0.78 (0.65 ± 0.22) | ||
| 96 | Me | cyclohexyl-NH | >50 (>24.62) | >25 (>12.31) | ||
| 97 | Me | pyrrolidinyl | 4.68 ± 1.56 (1.23 ± 0.4) | 1.17 ± 0.39 (0.3 ± 0.1) | ||
| 98 | Me | 2-Me-piperidinyl | 18.75 ± 6.24 (5.49 ± 1.82) | 0.58 ± 0.19 (0.16 ± 0.05) | ||
| 99 | Me | 3,5-di-Me-piperidinyl | 18.75 ± 6.24 (5.76 ± 1.92) | 9.37 ± 3.12 (2.88 ± 0.96) | ||
| 100 | Me | azepanyl | 18.75 ± 6.24 (5.49 ± 1.82) | 4.68 ± 1.56 (1.37 ± 0.46) | ||
| 101 | Me | azocanyl | 18.75 ± 6.24 (5.76 ± 1.92) | 2.34 ± 0.78 (0.71 ± 0.24) | ||
| 102 | Me | benzyl-NH | 15.87 (4.78) | 50 ± 0 (15.06 ± 0) | 18.75 ± 6.25 (5.64 ± 1.88) | |
| 103 | Me | (pMe-benzyl)-NH | 14.73 (4.65) | 50 ± 0 (15.76 ± 0) | 1.17 ± 0.39 (0.36 ± 0.12) | |
| 104 | Me | Ph-(CH2)2-NH | 12.27 (3.87) | 50 ± 0 (15.76 ± 0) | 2.34 ± 0.78 (0.73 ± 0.24) | |
| 105 | Me | Me2N | 4.68 ± 1.56 (1.11 ± 0.36) | 2.34 ± 0.78 (0.55 ± 0.18) | ||
| 106 | Me | di-Et-N | 9.37 ± 3.12 (2.5 ± 0.82) | 2.34 ± 0.78 (0.62 ± 0.21) | ||
| 107 | SMe | benzyl-NH | 18.59 (6.2) | 4.68 ± 4.68 (1.56 ± 1.56) | 4.68 ± 1.56 (1.56 ± 0.52) | |
| 108 | SMe | (pMe-benzyl)-NH | 19.2 (6.67) | 9.37 ± 3.12 (3.25 ± 1.08) | 4.68 ± 1.56 (1.62 ± 0.54) | |
| 109 | SMe | Ph-(CH2)2-NH | 15.39 (5.35) | 9.37 ± 9.36 (3.25 ± 3.24) | 1.17 ± 0.39 (0.4 ± 0.13) | |
| 110 | CF3 | iPrNH | 42.8 (13.15) | 12.25 ± 6.5 (4.4 ± 2) | >25 (>7.68) | 18.75 ± 12.5 (5.76 ± 3.84) |
| 111 | Ph | iPrNH | 50 ± 0 (15.76 ± 0) | |||
| 112 | styryl | MeNH | >50 (>15.66) | >25 (>7.83) |
The C. neoformans fluconazole-resistant DUMC-158.03, and C. gattii, RSA-3615, clinical strains were obtained from Dr. John Perfect, Duke University.40
In Vitro Assays and Preliminary Structure–Activity Relationships Study
Using a whole-cell phenotypic screen approach, we tested a set of 121 chemically diverse compounds from our laboratory library and measured the MIC80 of these compounds against the pathogenic C. neoformans laboratory strain KN99.28 We identified that 17 out of 121 hits show activity with the MIC80 values less than or equal to 50 μM. Among them, the 5-nitro-6-thiocyanatopyrimidines represent the most interesting series for further research. Next, we synthesized a number of compounds based on the scaffold for the primary study of the structure–activity relationship. We found that only compounds with small substituents, such as a hydrogen atom or a methyl group at position 2 of the scaffold, exhibit favorable in vitro activity, while compounds with bulky groups at the same position were less active. Moreover, the volume and length of the substituents at position 4 also influence the antifungal activity. So, compound 94 with 2-methyl group and 4-cyclopropyl-NH substituent appears to have the most promising MIC80 of 0.6 μM for C. neoformans and MIC 0.39–0.78 μM for C. gattii. The MIC80 was 0.79–1.56 μM against a fluconazole (FLC) resistant strain with a CC50 of 9.6 μM in human hepatoma cells resulting in a specificity index, SI = 16, for C. neoformans. Compound 94 was also tested for synergy in a checkerboard assay with AmB and FLC, where the FICIs were calculated to be 1.25 and 1.5, respectively (data not shown). These data suggest an indifference but, importantly, no antagonism between compound 94 and either AmB or FLC.
Machine Learning
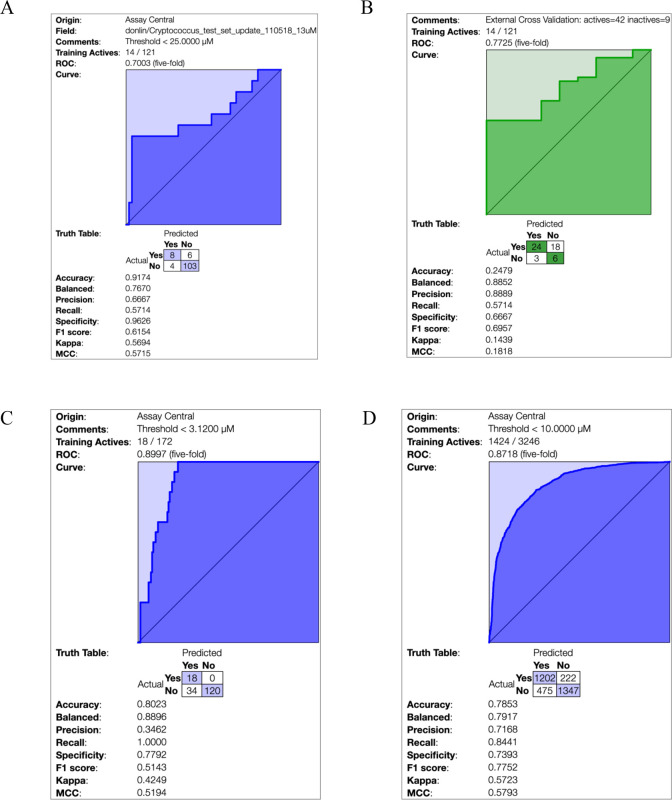
Assay Central was used to generate machine learning models that used a Bayesian algorithm and ECFP6 fingerprints alone, for the 121 compounds with C. neoformans data. The threshold for actives was MIC80 12.5–25.0 μM. The 5-fold cross validation ROC was 0.70, and other statistics (Figure 3A, Supporting Information (SI), Table S1) were acceptable for such a relatively small data set. This data was then used to score a set of 51 additional analogues (external set test ROC 0.77, Figure 3B). From past experience, as we add more data to the machine learning model the cross-validation statistics generally improve (172 compounds, ROC 0.90, Figure 3C) and generate better predictive models for optimization of the molecules. We are thus able to lower the threshold for an active to MIC80 < 3.12 μM in order to predict more potent compounds using this iterative approach.
Figure 3.
Bayesian model statistics for (A) Cryptococcus model with an MIC80 threshold for activity threshold of MIC80 12.5–25.0 μM. (B) “Subvalidation” model statistics for Cryptococcus model using new activity data as a test set. (C) Updated model statistics with new compounds/activity data added with an increased threshold for activity of MIC80 1.52–3.12 μM. (D) Literature cryptococcus model derived from the NIAID ChemDB database.
We have also generated a Bayesian model with over 3000 compounds with data from the NIAID ChemDB HIV, Opportunistic Infection and Tuberculosis Therapeutics Database, with excellent statistics overall at a threshold of 10 μM (ROC 0.87, Figure 3D), which is potentially useful for exploring more chemical diversity because of the broader makeup of the training set. We used these data from the 172 compounds tested as an external test set for this training model, which yielded reasonable statistics (ROC 0.81; Figure 4).
Figure 4.
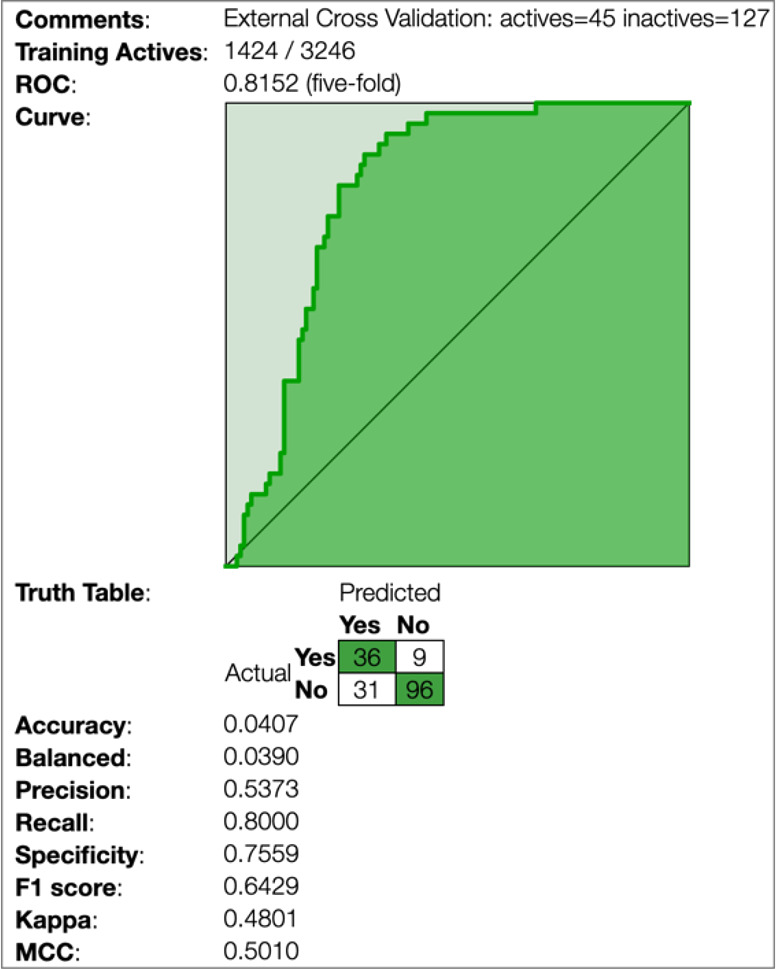
Statistics for the Cryptococcus data from this study when used as an external test set using the literature cryptococcus model derived from the NIAID ChemDB database as a training set (10 μM activity threshold).
The molecules of most interest highlighted in this study (Table 1) were used with this literature C. neoformans model with a cutoff of 10 μM (ROC 0.62, SI, Figure S1A) or 3.2 μM (ROC 0.82, SI, Figure S1B). The predictions and model domains (a measure of applicability) can be seen in more detail in SI, Table S2. As we also generated in vitro data for C. gattii in this study, we used this set from Table 1 to construct a preliminary model with a cutoff at MIC 1.52–3.12 μM (ROC 0.62, SI, Figure S2). These machine learning models will be put to further use for virtual screening of compound libraries of commercial molecules in order to find additional molecules for testing against these fungi. These Bayesian models can also be used to help us further optimize the current chemical series alongside models for ADME/Tox properties.
During recent patent preparation, we became aware of a Russian patent describing 2-nitroheterylthiocyanates with activity against Candida, Aspergillus, and Fusarium strains only.41 Interestingly, they did not test against Cryptococcus strains, and we also describe many more derivatives as well as the structure–activity relationship.
We have now described new 5-nitro-6-thiocyanatopyrimidine antifungals including compound 94, which has a submicromolar MIC80 for C. neoformans, a FLC-resistant strain of C. neoformans, and C. gattii as a starting point for future optimization and evaluation. Thiocyanate ions are ubiquitous,42,43 and our antifungals contain an isothiocyanate group which may be important for this activity and may not be cytotoxic. We have shown that we can separate antifungal activity from cytotoxicity (Table 1) and understanding the mechanism of action of these molecules and whether the thiocyanate represents a leaving group responsible for the activity will be addressed in future studies.
Experimental Procedures
All experimental procedures are described in Supporting Information.
Acknowledgments
We kindly acknowledge Dr. Alex M. Clark (Molecular Materials Informatics, Inc.) for Assay Central support and Anna Egorova for help in manuscript preparation. We kindly acknowledge NIH NIGMS funding to develop the software from R44GM122196-02A1, NIH NINDS: 1R01NS102164-01. The National Institute of Allergy and Infectious Diseases of the National Institutes of Health provided funding to M.J.D. under award number R01AI123407. The Saint Louis University Research Growth Fund provided support for J.L and the work conducted in the M.J.D. laboratory.
Glossary
Abbreviations
- (AmB)
amphotericin B
- (ART)
antiretroviral therapies
- (CM)
cryptococcal meningitis
- (FBS)
fetal bovine serum
- (FICI)
fractional inhibitory concentration index
- (FLC)
fluconazole
- (5-FC)
5-fluorocytosine
- (HIV)
human immunodeficiency virus
- (MIC)
minimal inhibitory concentration
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.1c00038.
Full experimental procedures, analytical data of all the compounds, description of machine learning software methods, supplemental references, supplemental figures and tables describe the machine learning model results as well as comparisons with in vitro data PDF)
Author Contributions
O.R., A.L., and V.M. synthesized the molecules. E.X., J.L., and M.D. generated biological data, T.R.L. performed all machine learning work. M.D., V.M., and SE designed the study and wrote the manuscript.
The authors declare the following competing financial interest(s): S.E. is owner, and T.R.L. is an employee of Collaborations Pharmaceuticals, Inc. All others have no competing interests. We have filed a provisional patent on this work.
Supplementary Material
References
- Rajasingham R.; Smith R. M.; Park B. J.; Jarvis J. N.; Govender N. P.; Chiller T. M.; Denning D. W.; Loyse A.; Boulware D. R. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect. Dis. 2017, 17 (8), 873–881. 10.1016/S1473-3099(17)30243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyrgos V.; Seitz A. E.; Steiner C. A.; Prevots D. R.; Williamson P. R. Epidemiology of Cryptococcal Meningitis in the US: 1997–2009. PLoS One 2013, 8 (2), e56269. 10.1371/journal.pone.0056269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoham S.; Marr K. A. Invasive fungal infections in solid organ transplant recipients. Future Microbiol. 2012, 7 (5), 639–55. 10.2217/fmb.12.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George I. A.; Santos C. A. Q.; Olsen M. A.; Powderly W. G. Epidemiology of Cryptococcosis and Cryptococcal Meningitis in a Large Retrospective Cohort of Patients After Solid Organ Transplantation. Open forum infectious diseases 2017, 4 (1), ofx004. 10.1093/ofid/ofx004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neofytos D.; Fishman J. A.; Horn D.; Anaissie E.; Chang C. H.; Olyaei A.; Pfaller M.; Steinbach W. J.; Webster K. M.; Marr K. A. Epidemiology and outcome of invasive fungal infections in solid organ transplant recipients. Transplant Infect. Dis. 2010, 12 (3), 220–9. 10.1111/j.1399-3062.2010.00492.x. [DOI] [PubMed] [Google Scholar]
- Lortholary O. Management of cryptococcal meningitis in AIDS: the need for specific studies in developing countries. Clin. Infect. Dis. 2007, 45 (1), 81–3. 10.1086/518583. [DOI] [PubMed] [Google Scholar]
- Mdodo R.; Brown K.; Omonge E.; Jaoko W.; Baddley J.; Pappas P.; Kempf M. C.; Aban I.; Odera S.; Suleh A.; Jolly P. E. The prevalence, clinical features, risk factors and outcome associated with cryptococcal meningitis in HIV positive patients in Kenya. East Afr. Med. J. 2010, 87 (12), 481. [PMC free article] [PubMed] [Google Scholar]
- Bratton E. W.; El Husseini N.; Chastain C. A.; Lee M. S.; Poole C.; Sturmer T.; Weber D. J.; Juliano J. J.; Perfect J. R. Approaches to antifungal therapies and their effectiveness among patients with cryptococcosis. Antimicrob. Agents Chemother. 2013, 57 (6), 2485–95. 10.1128/AAC.01800-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza S. A.; Phelan M.; Rimland D.; Graviss E.; Hamill R.; Brandt M. E.; Gardner T.; Sattah M.; Ponce de Leon G.; Baughman W.; Hajjeh R. A. The changing epidemiology of cryptococcosis: an update from population-based active surveillance in 2 large metropolitan areas, 1992–2000. Clin. Infect. Dis. 2003, 36 (6), 789. 10.1086/368091. [DOI] [PubMed] [Google Scholar]
- McKenney J.; Smith R. M.; Chiller T. M.; Detels R.; French A.; Margolick J.; Klausner J. D.; Prevalence and correlates of cryptococcal antigen positivity among AIDS patients--United States, 1986–2012. MMWR Morbidity and Mortality Weekly Report 2014, 63 (27), 585. [PMC free article] [PubMed] [Google Scholar]
- Sloan D. J.; Parris V. Cryptococcal meningitis: epidemiology and therapeutic options. Clinical epidemiology 2014, 6, 169–82. 10.2147/CLEP.S38850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes E. J. 3rd; Li W.; Lewit Y.; Ma H.; Voelz K.; Ren P.; Carter D. A.; Chaturvedi V.; Bildfell R. J.; May R. C.; Heitman J. Emergence and pathogenicity of highly virulent Cryptococcus gattii genotypes in the northwest United States. PLoS Pathog. 2010, 6 (4), e1000850. 10.1371/journal.ppat.1000850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta K.; Bartlett K. H.; Baer R.; Byrnes E.; Galanis E.; Heitman J.; Hoang L.; Leslie M. J.; MacDougall L.; Magill S. S.; Morshed M. G.; Marr K. A. Spread of Cryptococcus gattii into Pacific Northwest region of the United States. Emerging Infect. Dis. 2009, 15 (8), 1185. 10.3201/eid1508.081384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes E. J. 3rd; Li W.; Ren P.; Lewit Y.; Voelz K.; Fraser J. A.; Dietrich F. S.; May R. C.; Chatuverdi S.; Chatuverdi V.; Heitman J. A diverse population of Cryptococcus gattii molecular type VGIII in southern Californian HIV/AIDS patients. PLoS Pathog. 2011, 7 (9), e1002205. 10.1371/journal.ppat.1002205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart S. R.; Iqbal N.; Harris J. R.; Grossman N. T.; DeBess E.; Wohrle R.; Marsden-Haug N.; Vugia D. J. Cryptococcus gattii in the United States: genotypic diversity of human and veterinary isolates. PLoS One 2013, 8 (9), e74737. 10.1371/journal.pone.0074737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer D. J.; Billmyre R. B.; Filler E. E.; Voelz K.; Pursall R.; Mieczkowski P. A.; Larsen R. A.; Dietrich F. S.; May R. C.; Filler S. G.; Heitman J. Cryptococcus gattii VGIII Isolates Causing Infections in HIV/AIDS Patients in Southern California: Identification of the Local Environmental Source as Arboreal. PLoS Pathog. 2014, 10 (8), e1004285. 10.1371/journal.ppat.1004285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill R. J. Amphotericin B formulations: a comparative review of efficacy and toxicity. Drugs 2013, 73 (9), 919–34. 10.1007/s40265-013-0069-4. [DOI] [PubMed] [Google Scholar]
- Bratton E. W.; El Husseini N.; Chastain C. A.; Lee M. S.; Poole C.; Sturmer T.; Juliano J. J.; Weber D. J.; Perfect J. R. Comparison and temporal trends of three groups with cryptococcosis: HIV-infected, solid organ transplant, and HIV-negative/non-transplant. PLoS One 2012, 7 (8), e43582. 10.1371/journal.pone.0043582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brizendine K. D.; Baddley J. W.; Pappas P. G. Predictors of mortality and differences in clinical features among patients with Cryptococcosis according to immune status. PLoS One 2013, 8 (3), e60431. 10.1371/journal.pone.0060431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller M. A.; Messer S. A.; Rhomberg P. R.; Castanheira M. Activity of a Long-Acting Echinocandin (CD101) and Seven Comparator Antifungal Agents Tested against a Global Collection of Contemporary Invasive Fungal Isolates in the SENTRY 2014 Antifungal Surveillance Program. Antimicrob. Agents Chemother. 2017, 61, e02045-16. 10.1128/AAC.02045-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafar H.; Altamirano S.; Ballou E. R.; Nielsen K. A titanic drug resistance threat in Cryptococcus neoformans. Curr. Opin. Microbiol. 2019, 52, 158–164. 10.1016/j.mib.2019.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts A.; DiDone L.; Koselny K.; Baxter B. K.; Chabrier-Rosello Y.; Wellington M.; Krysan D. J. A repurposing approach identifies off-patent drugs with fungicidal cryptococcal activity, a common structural chemotype, and pharmacological properties relevant to the treatment of cryptococcosis. Eukaryotic Cell 2013, 12 (2), 278–87. 10.1128/EC.00314-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts A.; Koselny K.; Chabrier-Rosello Y.; Semighini C. P.; Brown J. C.; Wang X.; Annadurai S.; DiDone L.; Tabroff J.; Childers W. E. Jr.; Abou-Gharbia M.; Wellington M.; Cardenas M. E.; Madhani H. D.; Heitman J.; Krysan D. J. Estrogen receptor antagonists are anti-cryptococcal agents that directly bind EF hand proteins and synergize with fluconazole in vivo. mBio 2014, 5, e00765-13. 10.1128/mBio.00765-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartland K.; Pu J.; Palmer M.; Dandapani S.; Moquist P. N.; Munoz B.; DiDone L.; Schreiber S. L.; Krysan D. J. High-Throughput Screen in Cryptococcus neoformans Identifies a Novel Molecular Scaffold That Inhibits Cell Wall Integrity Pathway Signaling. ACS Infect. Dis. 2016, 2 (1), 93–102. 10.1021/acsinfecdis.5b00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koselny K.; Green J.; DiDone L.; Halterman J. P.; Fothergill A. W.; Wiederhold N. P.; Patterson T. F.; Cushion M. T.; Rappelye C.; Wellington M.; Krysan D. J. The Celecoxib Derivative AR-12 Has Broad-Spectrum Antifungal Activity In Vitro and Improves the Activity of Fluconazole in a Murine Model of Cryptococcosis. Antimicrob. Agents Chemother. 2016, 60 (12), 7115–7127. 10.1128/AAC.01061-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabjohns J. L. A.; Park Y.-D.; Dehdashti J.; Sun W.; Henderson C.; Zelazny A.; Metallo S. J.; Zheng W.; Williamson P. R. A high-throughput screening assay for fungicidal compounds against Cryptococcus neoformans. J. Biomol. Screening 2014, 19, 270–277. 10.1177/1087057113496847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor V.; Rella A.; Farnoud A. M.; Singh A.; Munshi M.; Bryan A.; Naseem S.; Konopka J. B.; Ojima I.; Bullesbach E.; Ashbaugh A.; Linke M. J.; Cushion M.; Collins M.; Ananthula H. K.; Sallans L.; Desai P. B.; Wiederhold N. P.; Fothergill A. W.; Kirkpatrick W. R.; Patterson T.; Wong L. H.; Sinha S.; Giaever G.; Nislow C.; Flaherty P.; Pan X.; Cesar G. V.; de Melo Tavares P.; Frases S.; Miranda K.; Rodrigues M. L.; Luberto C.; Nimrichter L.; Del Poeta M. Identification of a New Class of Antifungals Targeting the Synthesis of Fungal Sphingolipids. mBio 2015, 6, e00647-15. 10.1128/mBio.00647-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartland C. L.; Pu J.; Krysan D.; Didone L.; Moquist P. N.; Dandapani S.; Munoz B.; Palmer M.; Schreiber S., Discovery and Evaluation of Fungicidal Anti-Cryptococcal Molecules. Probe Reports from the NIH Molecular Libraries Program; NIH: Bethesda MD, 2010. [PubMed]
- Wiederhold N. P.; Xu X.; Wang A.; Najvar L. K.; Garvey E. P.; Ottinger E. A.; Alimardanov A.; Cradock J.; Behnke M.; Hoekstra W. J.; Brand S. R.; Schotzinger R. J.; Jaramillo R.; Olivo M.; Kirkpatrick W. R.; Patterson T. F. In Vivo Efficacy of VT-1129 against Experimental Cryptococcal Meningitis with the Use of a Loading Dose-Maintenance Dose Administration Strategy. Antimicrob. Agents Chemother. 2018, 62, e01315-18. 10.1128/AAC.01315-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart S. R.; Fothergill A. W.; Iqbal N.; Bolden C. B.; Grossman N. T.; Garvey E. P.; Brand S. R.; Hoekstra W. J.; Schotzinger R. J.; Ottinger E.; Patterson T. F.; Wiederhold N. P. The Investigational Fungal Cyp51 Inhibitor VT-1129 Demonstrates Potent In Vitro Activity against Cryptococcus neoformans and Cryptococcus gattii. Antimicrob. Agents Chemother. 2016, 60 (4), 2528–31. 10.1128/AAC.02770-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen K.; Vedula P.; Smith K. D.; Meya D. B.; Garvey E. P.; Hoekstra W. J.; Schotzinger R. J.; Boulware D. R. Activity of VT-1129 against Cryptococcus neoformans clinical isolates with high fluconazole MICs. Med. Mycol. 2017, 55, 453–456. 10.1093/mmy/myw089. [DOI] [PubMed] [Google Scholar]
- Rhein J.; Huppler Hullsiek K.; Tugume L.; Nuwagira E.; Mpoza E.; Evans E. E.; Kiggundu R.; Pastick K. A.; Ssebambulidde K.; Akampurira A.; Williams D. A.; Bangdiwala A. S.; Abassi M.; Musubire A. K.; Nicol M. R.; Muzoora C.; Meya D. B.; Boulware D. R.; et al. Adjunctive sertraline for HIV-associated cryptococcal meningitis: a randomised, placebo-controlled, double-blind phase 3 trial. Lancet Infect. Dis. 2019, 19 (8), 843–851. 10.1016/S1473-3099(19)30127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekins S.; Reynolds R.; Kim H.; Koo M.-S.; Ekonomidis M.; Talaue M.; Paget S. D.; Woolhiser L. K.; Lenaerts A. J.; Bunin B. A.; Connell N.; Freundlich J. S. Bayesian Models Leveraging Bioactivity and Cytotoxicity Information for Drug Discovery. Chem. Biol. 2013, 20, 370–378. 10.1016/j.chembiol.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. M.; Dole K.; Coulon-Spektor A.; McNutt A.; Grass G.; Freundlich J. S.; Reynolds R. C.; Ekins S. Open Source Bayesian Models. 1. Application to ADME/Tox and Drug Discovery Datasets. J. Chem. Inf. Model. 2015, 55 (6), 1231–45. 10.1021/acs.jcim.5b00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekins S.; Freundlich J.; Clark A.; Anantpadma M.; Davey R.; Madrid P. Machine learning models identify molecules active against Ebola virus in vitro. F1000Research 2015, 4, 1091. 10.12688/f1000research.7217.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson E.; Havener T. M.; Zorn K. M.; Foil D. H.; Lane T. R.; Capuzzi S. J.; Morris D.; Hickey A. J.; Drewry D. H.; Ekins S. Synergistic drug combinations and machine learning for drug repurposing in chordoma. Sci. Rep. 2020, 10, 12982. 10.1038/s41598-020-70026-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira J. C.; Daher S. S.; Zorn K. M.; Sherwood M.; Russo R.; Perryman A. L.; Wang X.; Freundlich M. J.; Ekins S.; Freundlich J. S. Machine Learning Platform to Discover Novel Growth Inhibitors of Neisseria gonorrhoeae. Pharm. Res. 2020, 37, 141. 10.1007/s11095-020-02876-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorn K. M.; Lane T. R.; Russo D. P.; Clark A. M.; Makarov V.; Ekins S. Multiple Machine Learning Comparisons of HIV Cell-based and Reverse Transcriptase Data Sets. Mol. Pharmaceutics 2019, 16 (4), 1620–1632. 10.1021/acs.molpharmaceut.8b01297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalecki A. G.; Zorn K. M.; Clark A. M.; Ekins S.; Narmore W. T.; Tower N.; Rasmussen L.; Bostwick R.; Kutsch O.; Wolschendorf F. High-throughput screening and Bayesian machine learning for copper-dependent inhibitors of Staphylococcus aureus. Metallomics 2019, 11 (3), 696–706. 10.1039/C8MT00342D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw K. J.; Schell W. A.; Covel J.; Duboc G.; Giamberardino C.; Kapoor M.; Moloney M.; Soltow Q. A.; Tenor J. L.; Toffaletti D. L.; Trzoss M.; Webb P.; Perfect J. R. In Vitro and In Vivo Evaluation of APX001A/APX001 and Other Gwt1 Inhibitors against Cryptococcus. Antimicrob. Agents Chemother. 2018, 62, e00523-18. 10.1128/AAC.00523-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rjabova S. J. E.; Surovtsev V. V. E.. 2-Nitroheterylthiocyanates for treating fungal infections, pharmaceutical composition for local application. Russian Patent RU2504541C1, 2012.
- Weuffen W.; Bergmann H.; Blohm H.; Bohland H.; Hiepe T.; Schonfeld P. Thiocyanate--a biologically active ion of veterinary and medical relevance. Berl. Munch. Tierarztl. Wochenschr. 2003, 116 (3–4), 144–156. [PubMed] [Google Scholar]
- Bezsudnova E. Y.; Sorokin D. Y.; Tikhonova T. V.; Popov V. O. Thiocyanate hydrolase, the primary enzyme initiating thiocyanate degradation in the novel obligately chemolithoautotrophic halophilic sulfur-oxidizing bacterium Thiohalophilus thiocyanoxidans. Biochim. Biophys. Acta, Proteins Proteomics 2007, 1774 (12), 1563–70. 10.1016/j.bbapap.2007.09.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.