Abstract
Background
The clinical outcomes of patients who decline anti-spike monoclonal antibody therapies for coronavirus disease-2019 (COVID-19) is not known. Factors associated with the decision to accept or decline the offer for anti-spike monoclonal antibody therapies are not established. This study aimed to identify factors impacting the decision to consent for monoclonal antibody therapies and assess the differences in clinical outcomes of patients who accepted compared to those who declined these therapies.
Methods
This retrospective cohort study enrolled 2820 adult patients who were offered monoclonal antibody therapies, bamlanivimab and casirivimab-imdevimab, for COVID-19 at Mayo Clinic in the Midwest between 11/19/2020 and 12/31/2020. The primary endpoint is the decision to accept or decline monoclonal antibody treatment. Secondary endpoints were patient-level factors that could have impacted the decision to accept treatment (age, gender, race, ethnicity, primary language spoken, and medical comorbidities). The main clinical endpoint was hospitalization within 28 days of COVID-19 diagnosis.
Results
59.1% (n = 1669) chose to accept monoclonal antibody therapy, and 40.9% (n = 1151) chose to decline the offer for treatment. Patients were more likely to accept treatment if they were non-Hispanic White, English speaking, identified a spouse or life partner, had a religious affiliation, and possessed more medical comorbidities. Overall, 28-day hospitalization rate was 2.6% (n = 72/2820) and was higher among those who declined (3.3%) than those who accepted monoclonal antibody therapy (2.0%; Rate Ratio = 0.62, 95% Confidence Interval, 0.39-0.98).
Conclusions
Despite having more comorbidities, patients who accepted monoclonal antibody treatments had a lower rate of hospitalization compared to patients who declined treatment. Several social and cultural factors were associated with the decision to decline therapy, including race, language, ethnicity, and lack of social support. These findings can inform public health efforts to reduce social disparities in the treatment of COVID-19 and increase utilization of monoclonal antibody therapies in high risk populations.
Keywords: covid_19, sars_cov-2, monoclonal antibodies, patient outcomes, bamlanivimab, casirivimab, imdevimab
Introduction
The management of coronavirus disease-19 (COVID-19) has evolved since the start of the pandemic in December 2019. Clinical trials have rapidly defined novel therapeutic agents for inpatients such as remdesivir that halts viral replication, and dexamethasone to reduce pro-inflammatory cytokine syndrome.1 In November 2020, the United States (US) Food and Drug Administration (FDA) granted emergency use authorizations (EUA) for 2 anti-spike monoclonal antibody therapies (Bamlanivimab and Casirivimab-Imdevimab) for outpatient treatment of high-risk patients with mild to moderate COVID-19.2,3 The EUA was based on evidence gathered from early-phase clinical trials that showed reduced viral load and rates of hospitalization among high risk patients who received these antibodies.4
Despite these EUAs, there was a slow uptake in the use of anti-spike monoclonal antibodies in the clinical setting. The logistical difficulties of establishing dedicated infusion therapy centers and the skepticism of medical providers in recommending these therapies due to a lack of solid evidence on their efficacy have limited their use.1,5,6 Likewise, patients have not actively sought out these therapies, and despite our proactive efforts to identify, contact and educate eligible patients, many of them have declined our offer for these potentially life-saving treatments.
We hypothesized that there may be social, cultural and clinical factors that influence the decision to accept or decline the offer for experimental monoclonal antibody therapies. Differences in the social determinants of health and the resultant disparities within populations have been previously demonstrated to impact the likelihood of acceptance of novel therapies.7 The primary aim of our study was to investigate the patient-level factors associated with patient decision to accept or decline infusion of monoclonal antibodies for COVID-19 in our large outpatient program. Identifying and understanding these patient-level factors may assist in improving the acceptance of these therapies. We also sought to compare the rates of hospitalization in patients who accepted or declined the monoclonal antibodies.
Methods
Setting
This study took place in a large, integrated healthcare delivery system with several primary locations situated in the Midwestern region of the United States. Within the Mayo Clinic Midwest practice, our health care facility has large medical centers situated in 4 locations within 2 states. Outside of these city locations are many primary, acute, and hospital-based care facilities that serve catchments within 3 states. The Mayo Clinic Midwest practice serves an estimated 600 000 unique patients each year.
Monoclonal Antibody Treatment Program
The Mayo Clinic monoclonal antibody treatment (MATRx) program was established on November 7, 2020, in anticipation of the issuance of EUA by the US FDA for anti-spike monoclonal antibody therapies for COVID-19. Mayo Clinic established dedicated outpatient COVID-19 infusion therapy centers across its Midwestern sites. The 7 infusion centers were geographically situated to serve the populations of 2 states. In addition, a mobile infusion team was created to serve patients in long-term care facilities across our regions. The first patients were infused with bamlanivimab (700 mg dose) on November 19, 2020, and later, the combination of casirivimab (1200 mg dose) and imdevimab (1200 mg dose) after they were granted EUA on November 21, 2020.
The MATRx providers proactively screened patients using automated tools within our electronic health record (EHR) to identify patients who fulfilled the eligibility criteria of the FDA EUA for the monoclonal antibodies. Patients were eligible for monoclonal antibodies if they had positive SARS-CoV-2 PCR or antigen test, had mild to moderate COVID-19, were within 10 days of symptom onset, and had at least one of the following criteria: age ≥65 years, body mass index (BMI) ≥35, diabetes, chronic kidney disease, immunosuppressive medication use, or an immunocompromising condition. Patients 55 years and older also qualified if they had hypertension, cardiovascular disease, or chronic lung disease. The Monoclonal Antibody Screening Score (a weighted score reflecting the relative risk and the number of additional comorbidity) was subsequently developed to identify eligible patients and stratify their risk profiles.8
A multidisciplinary team reviewed all patients identified by a registry with positive SARS-CoV-2 PCR tests as well as self- and provider-referred patients. All eligible patients were approached by the MATRx team for education about monoclonal antibodies, and discussion about potential benefits and adverse reactions of treatment. All team members used the same standardized script for education and consenting of patients. Language interpreters and translators were available to patients who needed them.
Patients who consented for monoclonal antibody treatment were scheduled for infusion at the nearest Outpatient COVID-19 Infusion Therapy Center. The patients received either bamlanivimab or the combination of casirivimab and imdevimab, depending on available supply and allocation at the infusion center. Patients who were undecided or initially declined treatment were provided a copy of the education materials to review and given 48 h to reconsider their decision.
Patients
After approval by our Institutional Review Board, the study population was identified. It included all patients with mild to moderate COVID-19 who were eligible to receive monoclonal antibody therapies and were contacted by the MATRx team for education and consent during the first 45 days since the inception of the program on November 19, 2020. Based on the patient decision on the monoclonal antibody therapies, the study population was divided into 2 groups—the accept and the decline populations. By virtue of the strict FDA EUA guidance, all patients in both groups had at least 1 condition or characteristic that identified them as high-risk for progression to severe and critical COVID-19. Only patients who had provided research authorization are included in this study.
Measures
The primary outcome of interest was the decision to accept or decline outpatient monoclonal antibody treatment. In order to identify patient-level factors which could be associated with patient decision to accept or decline enrollment in our monoclonal antibody infusion program, the study team performed a literature review and created a directed acyclic graph.9 Patient factors selected for investigation within our study included patient age, gender, race, ethnicity, and primary language spoken. We also included clinical patient factors including comorbidities that are listed under the FDA EUA criteria for eligibility for anti-spike monoclonal antibody therapies. Patient level information was collected as part of routine clinical care at 1 of our Institution sites and was stored within the EHR. As a measure of progression of COVID-19 illness, we assessed for hospital admission within 28 days of the onset of COVID-19 symptoms via assessment within our integrated EMR.
Statistical Analysis
Patient-level characteristics were reported as simple counts (n) and proportions (%). Bivariate analyses were performed to understand the demographic and clinical differences between patients who accepted and who declined outpatient infusion of monoclonal antibodies for COVID-19. The accept and decline groups were compared using Chi Square test for the association of 2 categorical variables unless the assumptions of the Chi Square test were unmet, then the Fischer Exact test was deployed. In order to understand the hospital admission rate of the 2 study populations, we calculated the crude rate of hospital admissions within 28-days over the total population size for each group. We also reported crude hospitalization rates by patient factor and compared rates using a calculated rate ratio (rate in the exposed / rate in the unexposed) with associated 95% confidence intervals (95% CIs). We calculated risk differences by subtracting the risk of 28-day hospitalization among those in MASS group 0-1 to each subsequent group. Factors were considered significant if P < .05 or the 95% CI did not span the null. All data management and statistical analyses were performed in Statistical Analysis Software (SAS) version 9.4 (Cary, North Carolina).
Results
During the first 45 days of the MATRx program, there were a total of 2820 EUA-eligible patients approached by the providers to offer anti-spike monoclonal antibody therapy for mild to moderate COVID-19. Of those, 59.1% (n = 1669) chose to accept monoclonal antibody therapy, and 40.9% (n = 1151) chose to decline the offer of treatment. The median time to monoclonal antibody infusion was 3 days from a positive SARS-CoV-2 PCR. Patients offered monoclonal antibody therapy were 51.2% female and majority non-Hispanic White (92.6%). The majority (65.7%) identified themselves as married or with a life partner, 97.1% identified English as their primary spoken language, and 59.8% reported a religious affiliation. Most patients had 2 or fewer comorbidities ( Table 1 ).
Table 1.
Patient Characteristics between Patients Who Accepted and Declined Monoclonal Antibody Therapy for COVID-19.
| Accept (N = 1669) | Decline (N = 1151) | P value | |
|---|---|---|---|
| Gender | .9981 | ||
| Female | 854 (51.2%) | 589 (51.2%) | |
| Male | 815 (48.8%) | 562 (48.8%) | |
| Race | .006 | ||
| Missing | 0 | 1 | |
| Asian descent | 18 (1.1%) | 12 (1.0%) | |
| Black/African American | 19 (1.1%) | 21 (1.8%) | |
| Other | 64 (3.8%) | 74 (6.4%) | |
| White | 1568 (93.9%) | 1043 (90.7%) | |
| Ethnicity | .0006 | ||
| Missing | 0 | 1 | |
| Hispanic or Latino | 74 (4.4%) | 61 (5.3%) | |
| Not Hispanic or Latino | 1568 (93.9%) | 1045 (90.9%) | |
| Unknown | 27 (1.6%) | 44 (3.8%) | |
| Marital status | .0002 | ||
| Married/life partner | 1128 (67.6%) | 726 (63.1%) | |
| Separated/divorced | 141 (8.4%) | 96 (8.3%) | |
| Single | 279 (16.7%) | 254 (22.1%) | |
| Unknown | 5 (0.3%) | 13 (1.1%) | |
| Widowed | 116 (7.0%) | 62 (5.4%) | |
| Language | .0277 | ||
| Missing | 2 | 2 | |
| English | 1629 (97.7%) | 1109 (96.5%) | |
| Other | 13 (0.8%) | 22 (1.9%) | |
| Spanish | 25 (1.5%) | 18 (1.6%) | |
| Religious affiliation | <.0001 | ||
| Missing | 127 | 146 | |
| No | 465 (30.2%) | 397 (39.5%) | |
| Yes | 1077 (69.8%) | 608 (60.5%) | |
| Weighted comorbidity score* | <.0001 | ||
| 0-1 | 763 (45.7%) | 695 (60.4%) | |
| 2 | 378 (22.6%) | 237 (20.6%) | |
| 3 | 249 (14.9%) | 103 (8.9%) | |
| 4 | 162 (9.7%) | 67 (5.8%) | |
| 5-11 | 117 (7.0%) | 49 (4.3%) | |
Weighted Comorbidity Score (Monoclonal Antibody Selection Score): age ≥65 years (1 point), body mass index ≥35 (1 point), diabetes mellitus (1 point), chronic kidney disease (2 points), immunosuppressive condition or medication use (3 points). Patients 55 years and older qualified if they had hypertension (0 point), cardiovascular disease (1 point), or chronic lung disease (2 points).
Differences in Patient Characteristics between Those who Accepted and Declined Monoclonal Antibody Therapy for COVID-19
Patients who chose to accept monoclonal antibody therapy for COVID-19 differed from those who chose to decline by race, ethnicity, marital status, primary language spoken, report of a religious affiliation, and comorbidity burden ( Table 1 ). Patients who accepted monoclonal antibody therapy were more likely to be White and non-Hispanic, be married or have a life partner, identify English as their primary spoken language and report a religious affiliation. The 2 groups did not differ by gender. Patients who accepted monoclonal antibody were more likely to have greater weighted comorbidity (P < .0001).
Hospital Admission Rate by Patient Characteristics and Decision to Accept or Decline Monoclonal Antibody Therapy for COVID-19
The overall 28-day hospitalization rate was 2.6% (n = 72/2820) for the total population. There was a higher 28-day hospitalization rate among those who declined (3.3%) than those who accepted monoclonal antibody therapy (2.0%; rate ratio (RR) = .62, 95% CI 0.39-0.98).
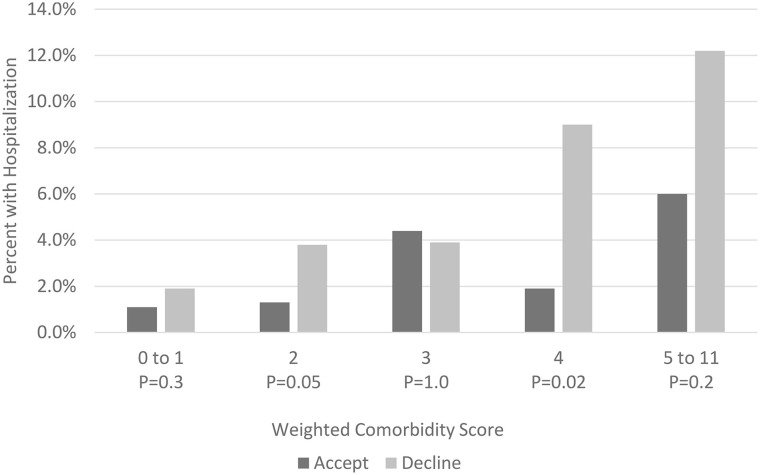
There were significantly different 28-day hospitalization rates between those who accepted and declined monoclonal antibody therapy for COVID-19 among males (accept 2.0% vs decline 4.1%, RR = 0.48, 95% CI 0.24-0.91), patients identifying as not Hispanic or Latino (accept 2.2% vs decline 3.5%, RR = 0.61, 95% CI 0.38-0.98), and those identifying as married or with a life partner (accept 2.0% vs decline 2.9%, RR = 0.49, 95% CI 0.28-0.85) ( Table 2 ). The rates of 28-day hospitalization varied among the MASS groups and were different between those who accepted or declined monoclonal antibody therapies (Figure 1).
Table 2.
Hospital Admission Rates (%) among Patients Who Accepted/Declined Monoclonal Antibody Therapies for Mild to Moderate COVID-19.
| Accept (34/1669) | Decline (n = 38/1151) | Rate ratio (95% CI) | |
|---|---|---|---|
| Gender | |||
| Female | 2.1% | 2.5% | 0.82 (0.41-1.67) |
| Male | 2.0% | 4.1% | 0.48 (0.24-0.91) |
| Race | |||
| Asian descent | 5.6% | 16.7% | 0.33 (0.01-4.38) |
| Black/African American | 0.0% | 14.3% | 0.00 (0.00-1.90) |
| White | 2.1% | 3.2% | 0.66 (0.41-1.08) |
| Other | 0.0% | 0.0% | - |
| Ethnicity | |||
| Hispanic | 0.0% | 0.0% | - |
| Not Hispanic or Latino | 2.2% | 3.5% | 0.61 (0.38-0.98) |
| Unknown | 0.0% | 2.3% | 0.00 (0.00-44.8) |
| Marital status | |||
| Married/life partner | 2.0% | 2.9% | 0.49 (0.28-0.85) |
| Separated/divorced | 4.3% | 7.3% | 0.56 (0.18-9.26) |
| Single | 1.4% | 2.4% | 0.61 (0.15-2.22) |
| Widowed | 1.7% | 6.5% | 0.27 (0.03-1.51) |
| Primary language spoken | |||
| English | 2.1% | 3.1% | 0.68 (0.42-1.10) |
| Other | 0.0% | 18.2% | 0.00 (0.00-1.78) |
| Spanish | 0.0% | 0.0% | - |
| Religious affiliation | |||
| No | 1.3% | 3.0% | 0.43 (0.15-1.13) |
| Yes | 2.4% | 3.8% | 0.64 (0.36-1.13) |
| Weighted comorbidity score* | |||
| 0 | 0.0% | 2.6% | 0.00 (0.00-21.2) |
| 1 | 1.1% | 1.8% | 0.61 (0.23-1.48) |
| 2 | 1.3% | 3.8% | 0.34 (0.11-1.04) |
| 3 | 4.4% | 3.9% | 1.14 (0.37-4.13) |
| 4 | 1.9% | 9.0% | 0.21 (0.04-0.83) |
| 5-11 | 6.0% | 12.2% | 0.49 (0.16-1.55) |
Weighted Comorbidity Score (Monoclonal Antibody Selection Score): age ≥65 years (1 point), body mass index ≥35 (1 point), diabetes mellitus (1 point), chronic kidney disease (2 points), immunosuppressive condition or medication use (3 points). Patients 55 years and older qualified if they had hypertension (0 point), cardiovascular disease (1 point), or chronic lung disease (2 points).
Figure 1.
Rates of hospitalizations for patients with mild to moderate coronavirus disease-19 who accepted or declined monoclonal antibody treatment stratified by weighted comorbidity score.
Discussion
In this retrospective study of a large cohort of high-risk patients, we identified several social, cultural and clinical factors associated with the decision to accept or decline monoclonal antibody therapies for mild to moderate COVID-19. First, we observed that patients who declined anti-spike monoclonal antibody infusions were more likely to belong to underrepresented populations by race, language, and ethnicity. Patients who identified their race as Black/African American or Other and patients who identified their ethnicity as Latino/Hispanic or Other had a higher rate of declining the therapy than the predominantly White non-Hispanic population. This difference extended to primary language where patients who primarily spoke a language other than English had a higher rate of declining therapy.
This finding highlights the health disparities affecting the underrepresented populations during the COVID-19 pandemic. Some factors that may contribute to this higher decline rate in accepting new therapies among underrepresented groups include a mistrust in the healthcare system, lack of resources (for example, concern for cost and travel means), concerns about residence and immigration status, medical misinformation and misconceptions, poor health literacy, lack of community engagement, and lack of appropriate language and cultural interpretation.10-17 The inadequate representation of underrepresented populations in clinical trials may have also led to a lack of understanding of the effect of these factors on acceptance of treatment and resultant healthcare inequities.18
Second, we observed that the social support system appears to be associated with the decision to accept or decline monoclonal antibody therapies. Social capital may be defined as how reciprocal social networks provide support and opportunities for achievement of mutual goals.19 In our study, patients who were partnered tended to accept the therapies at a higher rate than those who were single or had an unknown relationship status. This may mean that those who had a partner have a stronger support system and increased social capital, including a fear of death or hospitalization separating them from their partner.19 This may extend to practical benefits, such as easier access to transportation. We also observed a higher rate of acceptance among patients who reported a religious affiliation compared to those who did not. This could again reflect the greater social capital in those who are involved in a religious group and are part of a community.20
Third, we observed an association between acceptance of monoclonal antibody therapy and the degree of medical complexity. Patients with a higher degree of medical comorbidity or complexity, as reflected by the MASS, accepted the offer for monoclonal antibody infusions at a higher rate than patients with a single risk factor. This observation may be accounted for by a patients’ familiarity with our healthcare system, as these medically complex patients may have a more longitudinal relationship with our physicians. Additionally, these medically complex patients could be more concerned about their increased risk of COVID-19 complications and therefore more receptive to the potential benefit of the experimental monoclonal antibody therapies.21
Finally, we observed that hospitalization rates were significantly lower among patients who accepted monoclonal antibody therapy compared to those who declined the therapy, despite having a higher medical complexity. The absolute difference in the all-cause hospitalization rate is relatively small but this likely reflects an overall low hospitalization rate at baseline in our clinical practice, and given the continued prevalence of COVID-19, even small absolute differences add up to represent significant public health benefit.22 This study highlights the potential impact on clinical outcomes that can come from addressing social and cultural factors influencing medical decisions. Interestingly, the difference in hospitalization was magnified in non-White and non-English speaking populations. While we are unable to determine the cause of this finding, our data shows the health disparities that have been highlighted by the COVID-19 pandemic extend to outcomes associated with the acceptance of monoclonal antibody therapies.23,24
Our findings should be interpreted in the context of several study limitations. Our population was predominantly Caucasian, preventing us from analyzing individual populations as subgroups. Accordingly, we were unable to identify if a specific group accounted for the higher decline rates. The decision to decline therapies may be related to a lack of established rapport between our newly created MATRx team and the patients despite our efforts to work with language services, translators and interpreters. We also used crude hospitalization rate, which did not account for other confounders associated with admission to the hospital. Moreover, patients may have been hospitalized outside of our institution, as we could not completely account for loss to follow-up. We also did not evaluate the specific reasons for declining therapy since many patients did not provide specific reasons for their decision. Despite these limitations, we believe that data gathered from this large patient population provide important information that should guide public health policies addressing health inequities in both monoclonal antibody allocation and the broader COVID-19 pandemic.
Conclusion
This study demonstrates the potential benefits of anti-spike monoclonal antibodies in the real-world setting. Patients who accepted monoclonal antibody therapies had significantly lower rates of all-cause hospitalization when compared to those who declined treatment. Social and cultural factors were associated with the decision to accept or decline the monoclonal antibody infusion for COVID-19. These findings highlighted healthcare disparities that exist related to race, ethnicity, and other social determinants. Delineation of the relationship between social and demographic factors and acceptance of new therapies can be used to promote the development of better strategies for delivering high value therapies to the underrepresented populations. These strategies are essential to ensuring equity across all populations affected by the COVID-19 pandemic.
Acknowledgments
Contributors: We would like to thank the MATRx team members: Nicole C. E. Aloia, M.A., M.H.A.; Ryan J. Anderson, Pharm.D., R.Ph.; Gokhan Anil, M.D.; Lori L. Arndt, P.A.-C; Richard Arndt, Pharm.D., R.Ph.; Sara E. Ausman, Pharm.D., R.Ph.; Andrew D. Badley, M.D.; Sarah Bell, M.S.N., M.H.A., R.N.; Marcie L. Billings, M.D.; Rachel K. Bishop, R.N.; Carl H. Cramer, M.D.; Tracy L. Culbertson, M.S.N., R.N.; Ala S. Dababneh, M.D.; Molly Destro Borgen, M.A.; Amber N. Derr, M.B.A; Susan M. Flaker, Pharm.D., R.Ph.; Mary A. Gilmer, Pharm.D., R.Ph.; Eric Gomez Urena, M.D.; Christopher R. Gulden, M.A.; Tamara L. Haack; Jenna R. Herzog; Alexander Heyliger Pharm.D., R.Ph.; Lex D. Hokanson, D.N.P., R.N,22 Laura H. Hopkins, M.S.N., R.N.; Richard J. Horecki, M.D.; Bipinchandra Hirisave Krishna, M.D.; W. Charles Huskins, M.D., M.Sc.; Ryan R. Johnson; Betty Jorgenson, M.S.N., R.N.; Cory Kudrna; Brian D. Kennedy, R.Ph.; Mary K. Klingsporn, M.S.N., R.N. COCN; Brian Kottke, M.B.A; Sarah R. Lessard, Pharm.D., R.Ph.; Larry I. Lutwick, M.D.; Edward J. Malone III, M.D.; Jennifer A. Matoush, APRN, CNS, M.S.; Ivana N. Micallef, M.D.; Muhanad Mohamed M.B.B.S.; Colleena N. Ness; Shelly M. Olson, M.S.N., R.N.; Raj Palraj, M.B.B.S.; Janki Patel, D.O.; Damian J. Paulson; David Phelan, M.D.; Margaret T. Peinovich, Pharm.D., R.Ph.; Wilford L. Ramsey, M.H.A.; Taunya J. Rau-Kane; Kevin I. Reid, D.M.D.; Karen J. Reinschmidt, M.S.; Erin C. Skold, J.D.; Jill M. Smith, APRN, C.N.P.; Laurie A. Spielman, M.S.N., R,N; Donna J. Springer, APRN, CNS, M.S.; Perry W. Sweeten, Pharm.D.; Jennifer M. Tempelis, Pharm.D.; Sidna M. Tulledge-Scheitel, M.D., M.P.H.; Paschalis Vergidis, M.D.; Daniel C Whipple, M.S.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Raymund Razonable received external industry research grant (funds given to the institution) from Roche, Regeneron and Gilead, and received honorarium as a member of the Data and Safety Monitoring Board (Novartis). All other authors declare no support from any organization for the submitted work.
All authors declare no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; and no other relationships or activities that could appear to have influenced the submitted work.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was supported in part by an award from Mayo Clinic to Raymond Razonable. Mayo Clinic supported the conduct of this research but did not have any role in study design; in the collection, analysis and interpretation of data; in writing the report; and in the decision to submit this article for publication.
Ethics and Consent to Participate: In accordance with the Declaration of Helsinki, this study was reviewed and approved (ID 16-004817) by the Mayo Clinic Institutional Review Board (IRB). Mayo Clinic IRB approved waiver of informed consent. This study was also reviewed and approved by The Mayo Clinic COVID-19 Taskforce.
Ethical Standards: All authors assert that all procedures contributing to this work comply with the ethical standards of the Mayo Clinic.
ORCID iDs: Darcie E. Moehnke  https://orcid.org/0000-0002-5952-6029
https://orcid.org/0000-0002-5952-6029
Lindsey M Philpot  https://orcid.org/0000-0002-0462-6233
https://orcid.org/0000-0002-0462-6233
Availability of Data and Materials: All data supporting the study findings are contained within this manuscript
References
- 1. Bhimraj AM, Morgan RL, Shumaker AH, et al. Guidelines on the Treatment and Management of Patients with COVID-19. Published 2021. Updated April 14, 2021. Accessed February 4, 2021. https://www.idsociety.org/globalassets/idsa/practice-guidelines/covid-19/treatment/idsa-covid-19-gl-tx-and-mgmt-v4.2.02.pdf
- 2. Chen P, Nirula A, Heller B, et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med. 2021;384:229-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med. 2021;384:238-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. An EUA for bamlanivimab-a monoclonal antibody for COVID-19. Med Lett Drugs Ther. 2020;62:185-186. [PubMed] [Google Scholar]
- 5. Pediatric Infectious Diseases Society (PIDS). SARS-COV-2 neutralizing antibody LY-COV555 in outpatients with COVID-19. Published 2021. Updated January 13, 2021. Accessed January 12, 2021. https://pids.org/2020/11/19/sars-cov-2-neutralizing-antibody-ly-cov555-in-outpatients-with-covid-19/
- 6. Statement on Bamlanivimab EUA. COVID-19 treatment guidelines. Updated January 13, 2021. Accessed January 12, 2021. https://www.covid19treatmentguidelines.nih.gov/statement-on-bamlanivimab-eua/
- 7. Wheeler SM, Bryant AS. Racial and ethnic disparities in health and health care. Obstet Gynecol Clin North Am. 2017;44:1-11. [DOI] [PubMed] [Google Scholar]
- 8. Bierle DM, Buckmeier J, Kudrna C, O’Horo JC, Razonable RR. Monoclonal antibody selection score for allocation of anti-spike monoclonal antibodies to high risk patients with COVID-19. Personal Communication, 2021. [Google Scholar]
- 9. Pearce N, Lawlor DA. Causal inference-so much more than statistics. Int J Epidemiol. 2016;45:1895-1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rasi S. Impact of language barriers on access to healthcare services by immigrant patients: a systematic review. Asia Pac J Health Manage. 2020;15:35-48. [Google Scholar]
- 11. Patel JA, Nielsen FBH, Badiani AA, et al. Poverty, inequality and COVID-19: the forgotten vulnerable. Public Health. 2020;183:110-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Clarke SK, Kumar GS, Sutton J, et al. Potential impact of COVID-19 on recently resettled refugee populations in the United States and Canada: perspectives of refugee healthcare providers. J Immigr Minor Health. 2021;23:184-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alang S, McAlpine DD, Hardeman R. Police brutality and mistrust in medical institutions. J Racial Ethn Health Disparities 2020;7:760-768. [DOI] [PubMed] [Google Scholar]
- 14. Vernice NA, Pereira NM, Wang A, Demetres M, Adams LV. The adverse health effects of punitive immigrant policies in the United States: a systematic review. PLoS One. 2020;15:e0244054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Love JS, Blumenberg A, Horowitz Z. The parallel pandemic: medical misinformation and COVID-19: primum non nocere. J Gen Intern Med. 2020;35:2435-2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jaiswal J, Halkitis PN. Towards a more inclusive and dynamic understanding of medical mistrust informed by science. Behav Med. 2019;45:79-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Paakkari L, Okan O. COVID-19: health literacy is an underestimated problem. Lancet Public Health. 2020;5:e249-e250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miller HN, Charleston J, Wu B, et al. Use of electronic recruitment methods in a clinical trial of adults with gout. Clin Trials. 2021;18:92-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eriksson M. Social capital and health–implications for health promotion. Glob Health Action. 2011;4:5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kawachi I. Invited commentary: religion as a social determinant of health. Am J Epidemiol. 2020;189:1461-1463. [DOI] [PubMed] [Google Scholar]
- 21. Smith L, Jacob L, Yakkundi A, et al. Correlates of symptoms of anxiety and depression and mental wellbeing associated with COVID-19: a cross-sectional study of UK-based respondents. Psychiatry Res. 2020;291:113138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. O’Horo JC, Cerhan JR, Cahn EJ, et al. Outcomes of COVID-19 with the mayo clinic model of care and research. Mayo Clin Proc 2020;96:601-6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim EJ, Marrast L, Conigliaro J. COVID-19: magnifying the effect of health disparities. J Gen Intern Med. 2020;35:2441-2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hooper MW, Nápoles AM, Pérez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA. 2020;323:2466-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]