Abstract
Heme oxygenase 1 (HO-1) plays a key role in cell adaptation to stressors through the antioxidant, antiapoptotic, and anti-inflammatory properties of its metabolic products. For these reasons, in cancer cells, HO-1 can favor aggressiveness and resistance to therapies, leading to poor prognosis/outcome. Genetic polymorphisms of HO-1 promoter have been associated with an increased risk of cancer progression and a high degree of therapy failure. Moreover, evidence from cancer biopsies highlights the possible correlation between HO-1 expression, pathological features, and clinical outcome. Indeed, high levels of HO-1 in tumor specimens often correlate with reduced survival rates. Furthermore, HO-1 modulation has been proposed in order to improve the efficacy of antitumor therapies. However, contrasting evidence on the role of HO-1 in tumor biology has been reported. This review focuses on the role of HO-1 as a promising biomarker of cancer progression; understanding the correlation between HO-1 and clinical data might guide the therapeutic choice and improve the outcome of patients in terms of prognosis and life quality.
Keywords: HO-1, Nrf2, cancer progression, patients, therapy, prognosis, biomarker
1. Introduction
Heme oxygenase (HO) is an evolutionarily conserved enzyme that, in the presence of molecular oxygen (O2) and reduced nicotinamide adenine dinucleotide phosphate (NADPH), catalyzes the degradation of heme into equimolar amounts of biliverdin, carbon monoxide (CO), and free iron (Fe2+), releasing NADP+ and H2O [1].
Two different isoforms of HO have been described in mammalian cells (HO-1 and HO-2) and, heme oxygenase 1 (HO-1) represents the inducible form [2]. The HMOX-1 gene maps on the human chromosome 22q12.3 [3], on a region of approximately 13,148 bp, containing five exons and four introns [4], and codifies for a 32 kDa stress protein present at low levels in physiological conditions in most mammalian tissues [2]. HO-1 induction generally occurs in response to different endogenous and exogenous stimuli, mainly related to oxidative stress and inflammation, as well as to iron metabolism imbalance [5,6,7,8]. In tissues responsible for heme metabolism, such as spleen, liver, and bone marrow, HO-1 is highly expressed [9].
The induction of HO-1 exerts pleiotropic effects. It is well known that HO-1 is involved in the adaptive response to cellular stress and in attenuating inflammation, and, in healthy cells, HO-1 maintains redox homeostasis and prevents carcinogenesis. Importantly, in cancer cells, its expression correlates with tumor growth, aggressiveness, metastatic and angiogenetic potential. Recently, a crucial role of HO-1 in tumor immune escape has also been highlighted [10,11,12,13].
All the above-mentioned functions are ascribed mainly to the activity of HO-1 metabolic products [14,15,16]. Bilirubin (BR), derived by biliverdin reduction catalyzed by biliverdin reductase (BVRA), is a powerful antioxidant [17,18,19,20], able to scavenge reactive oxygen species (ROS) [21], therefore preventing protein and lipid peroxidation [17,22,23,24]. BR plays a key role in the regulation of inflammation and adaptive immunity, exerting immunosuppressive effects and promoting immune tolerance [25,26,27]. It is important to remark that BR is an important modulator of endothelial cell activity also in the microvasculature. Indeed, BR is able to reduce leukocyte transmigration and to prevent leucocyte rolling by decreasing the expression of P- and E-selectin, VCAM, and ICAM [28,29,30,31]. CO is well known as an antiapoptotic, anti-inflammatory, antiproliferative, and anticoagulant factor [32,33,34,35] and modulates the mitogen-activated protein kinase pathway (MAPK), soluble guanylyl cyclase (sGC) and the level of intracellular cGMP [36,37,38]. HO-1-derived CO is involved in blood vessel development [39] and VEGF synthesis [37], and enhances the proliferation of endothelial cells [38]. In addition, CO is able to attenuate inflammation [40,41], acting on both T cells [42] and antigen-presenting cells [11,12,33,43]. Finally, HO-1-derived free iron induces the synthesis of the heavy chain of the iron-chelating protein ferritin and activates the membrane transporter Fe-ATPase, which is crucial for decreasing the intracellular concentration of free Fe2+ and for preventing ROS production through the Fenton reaction [44,45]. Notably, HO-1 overactivation, if not balanced by the induction of ferritin and iron transporters or quenching systems, can trigger ferroptosis. Indeed, in this condition, iron accumulation leads to cell death through excessive ROS production and consequent lipid peroxidation [46,47].
Among HO-1 metabolic products, only CO has been recognized to be directly involved in tumor progression, promoting cancer cell proliferation, migration, angiogenesis, and immune escape [11]. The role of HO-1-derived bilirubin in cancer biology has been hypothesized considering its pro-surviving, pro-angiogenetic, and anti-inflammatory activity [31,48]. Instead, the generation of free iron due to HO-1 activation has been proved to favor non-canonical ferroptosis and is considered a therapeutic approach.
This review touches on the relevance of HO-1 expression in cancer progression, with a particular interest in the correlation with clinical features of tumors, taking into account data from histopathological analysis of tumor specimens.
2. HO-1 Gene Transcription and Protein Localization
2.1. HO-1 Transcriptional and Post-Transcriptional Regulation
The regulation of HO-1 expression occurs mainly at the transcriptional level (Figure 1). The promoter region of HO-1 contains several binding sites for different transcription factors activated in oxidative stress conditions, such as AP-1, HIF-1, NF-kB, and Nrf2 [49,50]. Thus, HO-1 is under the control of different signaling pathways. Moreover, two kinds of polymorphisms are present in its promoter region: the length of (GT)n repeats and the single nucleotide polymorphism (SNP) at the codon −413. Further, HO-1 protein levels can be regulated post-transcriptionally. Here, the main aspects of HO-1 synthesis regulation will be in brief as they are already reviewed elsewhere [51,52]; in particular, we will focus on the roles of HO-1 in cancer biology.
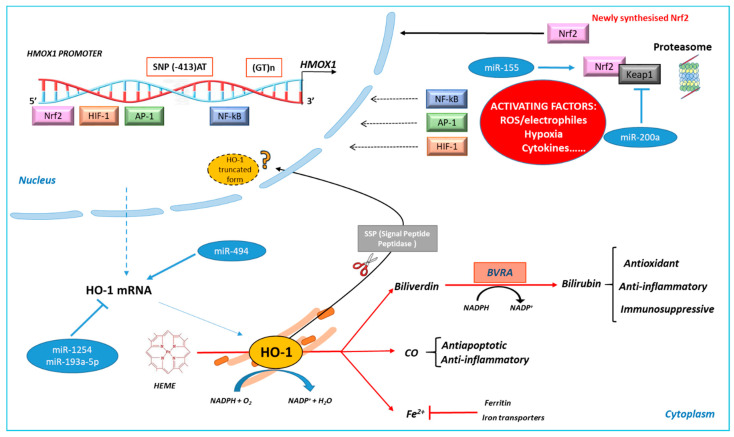
Figure 1.
Schematic representation of heme oxygenase 1 (HO-1) activity and regulation. HO-1 induction can be regulated at the transcriptional level by several stress-related transcription factors (Nrf2, AP-1, NF-kB, and HIF-1). Two polymorphisms that modify HO-1 inducibility have been indicated. Post-transcriptional regulation can involve miRNA. HO-1 regulates intracellular heme level catalyzing its degradation into biliverdin, carbon monoxide (CO), and ferrous iron (Fe2+). Biliverdin is converted into bilirubin by biliverdin reductase A (BVRA). Free iron activates iron transporters and induces the expression of ferritin. HO-1 metabolic products exert pro-survival activities, as indicated. A truncated form of HO-1, formed by signal peptide peptidase (SSP) cleavage, with nuclear localization and no enzymatic activity, has been described.
Among the HO-1 promoter polymorphisms, the (GT)n microsatellite repeats are crucial in modulating HO-1 expression. In particular, (GT)n polymorphisms are usually classified as short and long according to the number of the GT repeats: individuals with long (GT)n repeats show lower HO-1 inducibility due to a decreased promoter activity compared to individuals with short (GT)n repeats who have higher transcriptional activity, higher HO-1 inducibility and thus higher HO-1 levels [53]. The presence of this polymorphism correlates with the development of various pathologies, such as cardiovascular diseases, pulmonary disease [53,54,55], and cancer. However, contrasting results have been reported in different types of cancers [56].
Moreover, the SNP rs2071746 (−413A > T) polymorphism can also modulate HO-1 inducibility, being the higher HO-1 expression associated with the 413-A variant [57]. This polymorphism correlates with a reduced incidence of ischemic heart disease [58] and with graft survival after liver transplantation when present in the donor [59]. To our knowledge, only recently, the role of SNPs −413A > T in cancer risk has been analyzed by Bukowska [60]. The role of HO-1 polymorphisms in cancer will be discussed later.
The transcription factor nuclear erythroid 2-related factor-2 (Nrf2) is recognized to be the master regulator of HO-1 activation. Under nonstressed conditions, Nrf2 is bound to Kelch-like ECH-associated protein 1 (Keap1), which continuously targets Nrf2 for proteasome degradation. When cells are exposed to electrophiles and/or oxidants, Keap1 is inactivated and the newly synthetized Nrf2 is free to move into the nucleus, where it dimerizes with small Maf proteins and binds to the antioxidant/electrophile responsive elements (ARE/EpRE), leading to HO-1 gene transcription [5,61].
Of note, in cancer cells, genetic and epigenetic modifications of Nrf2/Keap1 have been described [5,62,63]. Indeed, gain-of-function mutations in Nrf2 or loss-of-function mutations in Keap1 lead to constitutive activation of Nrf2 and of its downstream target genes [5,62]. In particular, Nrf2 gain-of-function mutations have been identified in lung, head and neck, and bladder cancer, while Keap1 loss-of-function mutations have been identified in esophageal, head and neck, liver, gastric, and colorectal cancer [64]. In addition, epigenetic, especially TET-dependent demethylation of the Nrf2 promoter or Keap1 and CUL3 hypermethylation, favors Nrf2 activation, as demonstrated in lung, colorectal, and ovarian cancer [65,66,67,68].
Furthermore, HO-1 transcriptional regulation specifically involves the BTB domain and CNC homolog 1 (Bach1), a heme-binding protein that represents a major transcriptional repressor of HO-1. Indeed, Bach1 competes with Nrf2 for the binding to ARE sequences and impairs Nrf2-DNA binding activity. In response to oxidative stress, and in particular, to high levels of intracellular heme, Bach1 detaches from ARE sequences and is degraded by proteasome; in this condition, HO-1 transcription is allowed [10,69,70]. Of note, it has been demonstrated that in lung cancer metastasis, Bach1 can be stabilized in terms of protein expression and correlates with poor overall survival [71,72]. In the same works, high levels of HO-1 have been observed, meaning that the activity of HO-1 can halt Bach1 proteasomal degradation by reducing heme content. Thus, Bach1 stabilization can be observed both in the absence of Nrf2 activity or in the presence of Nrf2 activity, being dependent on the content of intracellular free heme.
Different kinase pathways (i.e., MAPKs and PI3K/AKT) are involved in HO-1 induction in cancer cells, not only by acting on Nrf2 but also by favoring Nrf2 independent HO-1 activation. p38 MAPK is responsible for Nrf2-dependent HO-1 activation in human MCF-7 breast cancer cells exposed to cadmium chloride [73] and cooperates with ERK for Nrf2-independent HO-1 activation in MKN-45 and in MKN-28 human gastric cancer cells [74]. Moreover, PI3K/AKT has been proved to be involved in HO-1 induction in SH-SY5Y neuroblastoma cancer cells in response to guanosine [75] and in cholangiocarcinoma cells treated with piperlongumine [76].
The regulation of HO-1 expression also occurs at the post-transcriptional level and microRNAs (miRNAs) play a key role. miRNAs can directly regulate HO-1 or indirectly modulate Nrf2, as already reviewed by Cheng and coworkers [77]. More recently, the involvement of miRNAs in regulating HO-1 in cancer cells has been proved. In particular, miR-155 favors lung cancer resistance to arsenic trioxide through Nrf2/HO-1 activation [78]. miR200a, in breast cancer, regulates HO-1 via Nrf2 activation by targeting Keap1 mRNA [79]. miR-1254 or miR-193a-5p, in non-small cell lung cancer (NSCLC) and prostate cancer, respectively, act on HO-1, reducing its expression and contributing to decreasing cancer cell growth [80,81]. We also demonstrated the involvement of miR494 in favoring neuroblastoma cell adaptation to oxidative stress through HO-1 up-regulation [82].
2.2. HO-1 Sub-Cellular and Extra-Cellular Localization
As far as HO-1 localization is concerned, HO-1 is mainly present at the endoplasmic reticulum (ER), where co-localizes with cytochrome P-450 reductase [83,84]. In addition, HO-1 can co-localize with caveolin 1/2 on plasma membrane caveolae [85] and a mitochondrial localization has been also demonstrated [86]. Of note, HO-1 can move into the nucleus, and nuclear translocation is favored by the signal peptide peptidase (SSP)-mediated intra-membrane cleavage, which leads to a C-terminal truncated form of HO-1 without catalytic activity but with transcriptional function [87,88,89]. Indeed, the truncated form of HO-1 interacts with Nrf2, increasing its stabilization [90]. Moreover, it has been demonstrated that the acetylation of the truncated form of HO-1 significantly enhances JunD-mediated AP-1 transcriptional activity leading to cancer cell proliferation, invasion, and resistance to therapy [91], indicating that post-translational modification of nuclear HO-1 plays an important role in cell proliferation, migration, and metastasis [92]. HO-1 nuclear compartmentalization is associated with cancer progression and chemoresistance, as demonstrated in chronic myeloid leukemia (CML) [93,94]; however, some opposite observations are reported in the literature [95,96,97,98]. A deeper review of the significance of HO-1 nuclear-truncated form has been recently published [92].
Furthermore, an extracellular localization of HO-1 in body fluids, including plasma, serum, milk, and cerebrospinal fluid, has been described [99,100,101]. In this context, a potential role of HO-1 as a disease biomarker has been suggested [94]. To date, the mechanisms of HO-1 release in biological fluids have not been understood. It has been hypothesized that plasma levels of HO-1 are the result of an active secretion and not the consequence of cell necrosis since it has been demonstrated, in patients with acute myocardial infarction, that HO-1 plasma levels are independent of necrosis biomarkers [102]. Interestingly, in acute kidney injury (AKI), HO-1 plasma and urinary levels parallel the level of HO-1 expression in renal tissue in response to damage [103]. Moreover, in both serum and urine, a truncated form of HO-1 was detected, suggesting that proteolytic cleavage occurs, even though the causes and consequences of this cleavage remain unknown [103]. More recently, the involvement of extracellular vesicles (EVs), such as exosomes and micro-vesicles, as potential sources of extracellular biomarkers has been considered [104,105]. In this context, HO-1 mRNA and protein have been detected in exosomes isolated from peripheral blood mononuclear cells (PMBC) of psoriasis patients [106]. Schipper and coworkers detected HO-1 protein in EVs from various human bio fluids [107]. With regard to cancer, HO-1 protein is found in EVs from the culture medium of several types of cancer cells, such as breast, lung, melanoma, and kidney [108]. However, this aspect needs further investigation.
3. Role of HO-1 in Cancer Progression
HO-1 overexpression has been described in several types of cancers and is associated with cancer cell proliferation, angiogenesis, invasiveness, immune escape, and resistance to therapy. However, opposite evidence has been reported as well, correlating HO-1 expression with inhibition of cancer cell proliferation, induction of apoptosis, and reduction of invasiveness; this suggests that the role of HO-1 in tumors could be tissue- and cell-specific [10].
3.1. HO-1 in Cancer Cell Growth, Metastasis, and Angiogenesis
The overexpression of HO-1 correlates with an increase in proliferation of cell viability in many types of cancer, such as human renal adenocarcinoma and in murine melanoma [109,110]. It favors the proliferation of malignant prostate tissues [111], pancreatic cancer, hepatoma, and lymphosarcoma [112], as well as brain and hematological cancers, as widely reviewed [11,113,114].
The acquisition of a metastatic phenotype, characterized by more aggressive features, is a key step in cancer growth and progression. In this context, HO-1 overexpression has been shown to favor metastasis development in melanoma [110], pancreatic cancer [115], oral squamous cell carcinoma [116], and prostate cancer [117]. In non-small cell lung cancer (NSCLC), the invasive and migratory abilities of cancer cells significantly increase after HO-1 overexpression, decrease after HO-1 silencing and correlate with the expression of metastasis-associated protein EGFR, CD147, and MMP9 [118]. In gastric cancer, the Nrf2-dependent HO-1 activation is involved in metastatic potential both in vitro and in vivo models [119]. Furthermore, HO-1 is involved in the epithelial-to-mesenchymal transition, a critical step in the metastasis process. Indeed, in ovarian cancer cells, HO-1 inhibition by zinc II protoporphyrin IX (ZnPPIX) down-regulates the expression of the mesenchymal markers vimentin, N-cadherin, and Zeb1, while up-regulates the expression of epithelial markers [120]. Consistently, it has been demonstrated that the down regulation of GRP78 increases the migration and invasiveness of colon cancer cells by the activation of Nrf2/HO-1, the induction of vimentin, and the reduction of E-cadherin expression [121].
Moreover, tumor invasiveness and metastasis development are strictly related to the stimulation of angiogenesis. In this regard, the role played by HO-1 in pathological angiogenesis of cancer is well documented both in vitro and in vivo. The up-regulation of VEGF expression in response to prostaglandin in human microvascular endothelial cells (HMEC-1) is mediated by the activation of HO-1 [122], and CO seems to be the main mediator in stimulating blood vessel formation [39]. It has been shown that HO-1 overexpression promotes angiogenesis in urothelial carcinoma cells [123] as well as in human pancreatic cancer [115]; in bladder cancer, HO-1 overexpression correlates with HIF-1α and VEGF expression [124]. Moreover, HO-1 inhibition by ZnPPIX suppresses VEGF production in GC9811-P gastric cancer cells, a cellular line characterized by high peritoneal metastatic potential [125], and in HCT-15-induced xenografts model of colorectal cancer reduces VEGF release and tumor angiogenesis [126]. In addition, inhibition of the Nrf2/HO-1 pathway by oxysophocarpine treatment suppresses the migration, the invasion potential, and the angiogenesis of oral squamous cells carcinoma [127].
3.2. HO-1 in Cancer Immune Escape
Recently, an important role of HO-1 in cancer immune escape has been highlighted. Indeed, HO-1 expression in infiltrating immune cells, including macrophages, dendritic cells (DC), neutrophils, natural killer cells (NK), and T and B lymphocytes, leads to their polarization toward a tumor-promoting and immunosuppressive phenotype. Moreover, HO-1 expression in cancer cells can be associated with the recruitment of specific subsets of infiltrating leucocytes and to the generation of specific cytokines that favor tumor progression.
Indeed, HO-1 expression is involved in macrophages polarization towards a pro-tolerogenic, pro-angiogenic, IL-10 producing, M2 phenotype [128], and HO-1-derived CO keeps DCs immature and modulates their cytokines secretion towards a tolerogenic phenotype [129].
In particular, it has been demonstrated that HO-1 is highly expressed in monocytes within the tumor microenvironment once they differentiate to TAMs, which indicates that HO-1 promotes their immunosuppressive function [130]. Furthermore, HO-1 detection in TAMs of prostate and breast cancers correlates with accelerated tumor growth [131,132].
Interestingly, in aggressive and metastatic prostate cancer, both in vivo and in ex vivo models, HO-1 positive macrophages were mainly detected outside the tumor tissue at the invasive zone of prostate tumors. These data suggest that extra tumor HO-1 positive macrophages could be involved in cancer aggressiveness, probably by playing a prominent role in stimulating tumor growth and metastasis [117].
Furthermore, in HO-1 overexpressing solid tumors, as well as in hematological malignancies, a high number of T regulatory cells (Treg) are present and act to suppress the immune response against the tumor mass [133,134,135]. For instance, in 4T1 breast cancer and in breast and melanoma bearing mice, it has been demonstrated that Treg recruitment is increased in an HO-1 dependent manner [136], and HO-1 expressing Treg accumulates during glioma progression [137].
Regarding the role played by HO-1 in regulating NK lymphocytes, crucially involved in the early immune response to tumor cells [138], little data are available in the literature. In a co-culture of an HO-1 positive cervical cancer cell (CCC) line and NK cells, pretreatment with various HO-1 inhibitors, tin II protoporphyrin IX (SnPPIX) and ZnPPIX, restores the expression of NKG2D, NKp30, and NKp46, markers of NK activation, and increases the production of IFN-ɣ and TNF-α, enhancing NK killing activity towards cancer cells [139]. Furthermore, we have recently demonstrated in BRAFv600 melanoma cells that HO-1 inhibition with tin mesoporphyrin IX (SnMPIX) and HO-1 siRNAdown-regulation favors cell death induced by vemurafenib, and increases NK cancer cell recognition by up-regulating B7H6 and ULBP3 ligands of NK cells [140]. To the best of our knowledge, no studies have been reported so far on the expression of HO-1 in NK cells.
3.3. HO-1 in the Resistance to Therapy
An important aspect of HO-1 expression in cancer cells is the gain of a resistant phenotype. It is well known that conventional anticancer treatments such as chemo- and radio- therapies can act to induce oxidative stress by increasing intracellular ROS levels [141] in order to favor apoptosis, as recently reviewed by Aggarwal and co-workers [142]. However, cancer cells, by up-regulating their antioxidant defenses, including HO-1, can counteract oxidative stress. Thus, the increase in HO-1 expression attenuates the efficacy of anticancer therapy as shown in different types of tumor where high levels of HO-1 are associated with a lower sensitivity to anticancer treatment. For instance, HO-1 overexpression is involved in resistance to chemo- and radio-therapy in central nervous system malignancies [113] and in resistance to cisplatin in hepatoma cells and ovarian cancer cells [143,144]. This aspect will be discussed later in Section 5, in the context of the possible modulation of HO-1 to favor antitumor therapies [145,146,147,148,149,150,151,152,153,154].
4. HO-1 Promoter Polymorphisms and Cancer Risk
As reported above, two major polymorphisms in the HO-1 promoter have been identified and linked to the modulation of HO-1 transcription: the (−413A > T) SNP and the presence of long/short (GT)n repeats. So far, no association between SNP-413 and cancers has been demonstrated, as indicated by Wang et al., who analyzed studies conducted on digestive neoplasms [155]. Moreover, recently, no prognostic significance has been shown for (−413A > T) SNP in children with acute lymphoblastic leukemia (ALL) [60].
Considering the length of GT repeats, an association has been found considering only East-Asian carriers of long (GT)n repeats, who show a high incidence of cancers in the digestive tract compared to carriers of short repeats. In fact, in Caucasian, American, and West-Asian populations, this association has not been demonstrated. Notwithstanding the small number of samples and the lack of uniformity of the studies analyzed, it seems evident that for the East-Asian populations, the presence of long (GT)n repeats is a risk factor for digestive tract cancers, probably in association with environmental factors. Indeed, in some studies, an association with alcohol consumption has been shown for the development of laryngeal squamous cell carcinoma (LSCC) for l-allele carriers in male Chinese [156]. Exposure to carcinogenic chemical compounds is a determinant to be considered; for instance, the role of smoking in male Japanese carriers of long repeats (GT)n who developed lung adenocarcinoma has been proven [157]; moreover, in asbestos-exposed Japanese subjects, the frequency of l-genotype correlates with an increased risk of developing mesothelioma [158].
An interesting study from Wu and collaborators, conducted in a cohort of patients in the area of Taiwan in which arsenic poisoning is endemic, demonstrated that (GT)n polymorphisms modify the risk of cancer due to arsenic exposure. Indeed, the risk of developing the different subtypes of arsenic-dependent tumors (skin cancer and urothelial carcinomas) is differently affected by (GT)n length. In particular, the S/S genotype carriers show a high risk of skin cancer, while no association is found for the risk of developing urothelial carcinoma among the three genotypes (S/S, L/S, and L/L) [159].
Based on this evidence, the analysis of (GT)n polymorphisms may represent a tool for evaluating an individual risk profile for a specific type of cancer, also considering the specific patient ethnicity.
5. HO-1 Expression, Tumor Aggressiveness, and Disease Outcome. Evidence from Immunohistochemistry
To date, the most consistent data regarding the correlation among HO-1 expression, cancer progression, patient prognosis, and outcome derive from immunohistochemistry studies on specimens from surgical patients. The data available in the literature are synthesized in Table 1 at the end of this paragraph. It is important to underline that, since Nrf2 is crucially involved in the regulation of HO-1 transcription, its expression has been considered as well. Both solid and hematopoietic malignancies have been taken into consideration, and the possible existence of negative association has also been analyzed.
Table 1.
Correlation among HO-1 expression, aggressiveness, and outcomes in histological specimens.
| Tumor | HO-1 | Nrf2 | Grade and Stage | Additional Markers |
Metastasis, Lymph Node, Angiogenesis |
Clinical and Pathological Features |
Disease Outcome/Prognosis |
Ref. |
|---|---|---|---|---|---|---|---|---|
| Positive correlation among HO-1 expression and tumor aggressiveness/poor prognosis | ||||||||
| -Solid tumors | ||||||||
| Astrocytoma | High level | n.e. | Grade II and III | n.e. | n.e. | n.e. | Poor OS | [163] |
| Clear cell renal cell carcinoma |
High level | High level | No correlation with ISUP grade and T stage |
n.e. | No correlation with lymph node metastasis |
No significant correlation with age, gender |
Poor prognosis Low MST Low post operative OS |
[165] |
| Colangiocarcinoma | High level | n.e. | n.e. | n.e. | No association with metastasis |
No significant association with age, gender, histological type |
Poor OS | [166] |
| Gastric cancer | High level | High level | Poor differentiated tumors |
Positive correlation with VEGF |
Positive correlation with MVD |
n.e. | n.e. | [174] |
| Gallbladder cancer | High level | High level | Moderately differentiated and poorly differentiated tumors (G2-G3) Correlation with Nevin classification (III-IV-V) |
Positive correlation with MRP3 |
Metastasis | No significant correlation with gender, age, and histology type (SCC and AD) |
Poor OS | [164] |
| Hepatocellular carcinoma |
High level | n.e. | Poor differentiated tumors Edmondson-Steiner grade 2–4 |
n.e. | Microvascular and capsular invasion |
High levels of preoperative AFP |
No significant correlation with OS and recurrence |
[175] |
| Hormone refractory prostate cancer |
High level | n.e. | n.e. | n.e. | n.e. | Cancer progression | n.e. | [188] |
| Laryngeal cancer | High level | High level | No correlation with tumor stage (clinical stage III and IV), size tumor |
High level Keap1 and NQO1 |
No correlation with lymph node metastasis |
No correlation with age |
n.e. | [189] |
| Melanoma | High level | n.e. | n.e. | Positive correlation with B-Raf and ERK |
n.e. | n.e. | n.e. | [176] |
| Neuroblastoma | High level | n.e. | n.e. | n.e. | n.e. | n.e. | Poor OS | [168] |
| Non-muscle-invasive bladder cancer | High level | n.e. | Tumor grade G3 tumor stage pT1 |
Ki-67 and p53 | n.e. | No significant correlation with age and gender |
Poor prognosis No correlation with RFS and PFS |
[161] |
| High level | n.e. | Tumor grade G3 Tumor stage T1 |
Positive correlation with S100A4 |
Lymph vascular invasion |
n.e. | Low RFS Low PFS |
[162] | |
| High level | n.e. | n.e. | Positive correlation with HIF-1α |
High MVD | n.e. | n.e. | [123] | |
| High level | High level | n.e. | Correlation with HIF-1α, HIF-2α, VEGF | n.e. | Increased serum/plasma level of IL-6, IL-8, VEGF |
n.e. | [124] | |
| Non-small cell lung cancer |
High level | n.e. | Stage III-IV | Positive correlation with MMP-9 |
High metastatic rate |
No correlation with age and gender |
Poor prognosis Low OS High mortality risk |
[118] |
| High level | n.e. | Stage III-IV T status (T3-T4) |
n.e. | Lymph node metastasis |
No correlation with gender |
No significant difference in patient survival between high and low staining group |
[98] | |
| Ovarian cancer | High level | n.e. | Serous undifferentiated tumors Correlation with FIGO stage (III-IV) |
n.e. | Lymph node metastasis |
Non optimal-debulking | Poor OS | [160] |
| Prostate cancer | High level | n.e. | Localized tumor | PTEN deletion |
n.e. | n.e. | Relapse after radical prostatectomy |
[173] |
| Thyroid cancer | High level | n.e. | Positive correlation with TNM (1,2,3,4) and with MACIS score |
BRAFV600E mutation | No significant association with lymph node metastasis |
Correlation with age and tumor aggressiveness |
n.e. | [172] |
| -Hematopoietic tumors | ||||||||
| Acute myeloid leukemia | High level | n.e. | n.e. | Positive correlation with HIF-1α and GLUT-1 |
n.e. | n.e. | Correlation with relapse and refractory |
[170] |
| High level | n.e. | Correleation with M5 patients |
Correlation with RET gene | n.e. | Correlation with leukocytosis at diagnosis |
n.e. | [167] | |
| Chronic myeloid leukemia |
Higher level in peripheral blood cells |
n.e. | n.e. | n.e. | n.e. | Tumor progression | Correlation with relapse |
[169] |
| Myelodysplastic Syndrome |
High level | n.e. | Correlation with high-risk and very high-risk patients | Positive correlation with EZH2 |
n.e. | Progression to AML and decreased response to decitabine |
n.e. | [171] |
| Positive correlation among HO-1 expression in tumor-associated cells and tumor aggressiveness/poor prognosis | ||||||||
| Colorectal cancer | High level in cancer cells and in macrophages |
n.e. | Stage III | n.e. | Lymph node metastasis |
No significant difference between the HO-1-positive and negative with gender, age, tumor size, histological type, and depth of tumor invasion |
Poor prognosis Short DSF |
[179] |
| Glioblastoma | High level in infiltrating macrophages |
n.e. | Grade IV | n.e. | Positive correlation with vascular density |
n.e. | n.e. | [178] |
| Glioma | HO-1 positive Treg | n.e. | Correlation with grade glioma (II-III-IV) |
n.e. | n.e. | n.e. | n.e. | [137] |
| Non-muscle-invasive bladder cancer | High level in cancer cells and fibroblast-like, tumor- infiltrating, and endothelial cells |
n.e. | Correlation with high grade tumors and with stage (T1) |
COX-1 | MVD, LVD, PI, increased risk of metastasis |
No association with age and gender | No association with recurrence |
[177] |
| Prostate cancer | HO-1 positive macrophages infiltrate and in bone metastasis |
n.e. | High-grade tumors Gleason score 7–10 |
n.e. | Bone metastasis | n.e. | n.e. | [117] |
| Negative correlation among HO-1 expression and tumor aggressiveness/poor prognosis | ||||||||
| Colorectal cancer | High level | n.e. | Invasive CRC | Significant correlation with K-ras |
n.e. | Significant correlation with normal CEA level |
Better prognosis, increased MTS |
[181] |
| High level | n.e. | n.e. | n.e. | Low vascular invasion and lymph node metastasis |
n.e. | Better survival rate | [180] | |
| Gastric cancer | High level | n.e. | Well and moderate differentiated |
n.e. | Negative lymph node metastasis | n.e. | Better prognosis |
[182] |
| Oral squamous cell carcinoma |
High level | n.e. | Well-differentiated Grade G1 No association with T stage |
n.e. | Low lymph node metastasis |
No association with age and sex No association with clinical stage |
n.e. | [184] |
| Small intestinal adenocarcinoma |
High level | n.e. | Low T stage (T1, T2, T3) |
n.e. | Low pancreatic invasion |
n.e. | Tend to have longer OS (difference not significative) |
[183] |
| Different correlation among HO-1 expression and tumor aggressiveness/poor prognosis depending on HO-1 subcellular localization | ||||||||
| Breast cancer | High level in malignant epithelial cells |
n.e. | Grade I-II (>80%) | Positive correlation with E-cadherin |
Negative correlation with lymph node metastasis |
Reduced tumor size |
Longer OS with increased MST |
[186] |
| Colorectal cancer | High level in cancer cells and in stromal cells (fibroblasts, neutrophils, and macrophages) |
n.e. | Well-differentiated adenocarcinoma Nuclear HO-1 localization in moderate and poor differentiated No association with TNM |
n.e. | No correlation with lymph node and liver metastasis | n.e. | n.e. | [187] |
| Head and neck squamous cell carcinoma |
High level | n.e. | High rate of HO-1 positivity in well-differentiated and moderately differentiated (<90%) Poor-differentiated high rate of nuclear HO-1 |
n.e. | n.e. | No association with age, gender, tumor location |
n.e. | [185] |
Tumors are listed alphabetically. List of table abbreviations. n.e., not evaluated; AD, adenocarcinoma; AFP, alpha feto protein; CEA, carcinoempryonic antigen; ISUP, International Society of Urologic Pathologists; LVD, lymph vascular density; MTS, median survival time; MVD, microvascular density; OS, overall survival; PI, proliferation index: PFS, progression free survival; RFS, recurrence free survival; SCC, squamous cell carcinoma.
5.1. HO-1 Expression and Disease Outcome
HO-1 expression in tumor mass is associated with poor prognosis/outcome and with high grade/stage in several types of tumors. In serous ovarian cancer, the association of HO-1 expression with FIGO stage III-IV and with poor overall survival has been proven [160]. In non-muscle-invasive bladder cancer (NMIBC), HO-1 expression is associated with grade 3, and poor prognosis or low recurrence/progression-free survival [161,162]. Similarly, in astrocytoma, high levels of HO-1 have been associated with tumor grade II and III and poor overall survival [163], and NSCLC at stage III-IV, high levels of HO-1 have been associated with high mortality risk and short overall survival [118]. In gallbladder cancer, the positivity for Nrf2, together with high expression of HO-1, has been shown to correlate with high grade/stage and poor prognosis [164], highlighting the role played by Nrf2 in the induction of HO-1 during tumor progression. Similar observations have been provided for clear cell renal cell carcinoma (ccRCC) [165], even though without correlation with tumor grade or stage. Indeed, patients with ccRCC showing high levels of HO-1 and Nrf2 have lower median survival time and shorter post-operative overall survival, with no proven correlation with tumor grade/stage.
In some studies, the expression level of HO-1 in tumors has been associated with clinical outcomes but without reference to the histopathological analysis. Thus, cholangiocarcinoma [166], acute myeloid leukemia (AML) [167], and neuroblastoma [168] show a correlation between high HO-1 expression and poor disease outcomes. Furthermore, HO-1 positivity in chronic myeloid leukemia [169], acute myeloid leukemia [170], and myelodysplastic syndrome [171] correlate with disease progression, resistance to therapy, and relapse.
5.2. HO-1 Expression and Tumor Grade/Stage
Vice versa, in other reports, HO-1 expression has been correlated with grade and stage and with invasion potential, but the clinical outcomes have not been analyzed. For instance, HO-1 overexpression in papillary thyroid cancer positively correlates with the TNM stage and cancer progression [172].
The intensity of HO-1 positivity has also been analyzed in order to find a possible correlation with the progression of a disease or with clinical outcomes. Interestingly, in NSCLC, the levels of HO-1 correlates with advanced stage (III-IV), T3, and T4 status and with lymph node metastasis; however, no association with overall survival has been demonstrated when patients were divided into two different subgroups related to HO-1 intensity of expression. Thus, no differences in patient survival were observed with regard to HO-1 intensity, highlighting that HO-1 positivity also at a low degree correlates with disease severity [98].
5.3. Correlation between HO-1 Expression and Tumor Markers
In many studies, HO-1 positivity has been correlated with other tumor markers. In localized prostatic cancer, HO-1 positivity associates with relapse frequency and PTEN deletion [173]. In NMIBC bladder cancers, HO-1 expression in tumor mass correlates with HIF-1α expression and microvessel density [123], and in particular, Nrf2 and HO-1 positivity correlates with HIF-1α, HIF-2α, and VEGF expression in the tumor, and with VEGF and interleukin levels in the plasma [124]. Similarly, in gastric cancer [174] and hepatocellular carcinoma [175], HO-1 positivity is associated with VEGF expression, poor differentiation, and microvascular density.
It is worth noting, in melanoma [176], thyroid cancer [172], and acute myeloid leukemia [167], HO-1 positivity correlates with the gain of function mutations of specific oncogenes B-Raf and RET. Moreover, in high-risk and very high-risk myelodysplastic syndrome, HO-1 expression correlates with overexpression of the enhancer of the zeste homologue 2 (EZH2) gene [171].
It is remarkable to note that HO-1 expression can be detected not only in tumor cells but also in cancer-associated cells, where it can contribute to the generation of a tumor-permissive environment. The number of HO-1 positive cancer-associated cells correlates with the tumor grade, metastatic competence, and neoangiogenesis. Indeed, in NMIBC bladder cancer HO-1 positivity has been detected not only in tumor cells but also in infiltrating fibroblasts and endothelial cells, in association with an increased risk of metastasis but without association to recurrence [177]. Further, high levels of HO-1 in infiltrating macrophages show a positive correlation with vascular density and high tumor grade in glioblastoma [178], with stage II, lymph node metastasis, and poor prognosis in colorectal cancer [179], and with a high Gleason score and bone metastasis in prostate cancer [117]. High HO-1 expression in lymphocyte Treg shows a correlation with a high tumor grade in glioma [137].
5.4. Contrasting Evidence
Although a great deal of literature highlights the correlation between HO-1 overexpression and cancer progression and often with the poor clinical outcomes, it seems important to consider that opposite evidence has also been provided. Indeed, it has been demonstrated that high HO-1 expression level correlates with a better prognosis and better overall survival in colorectal cancer [180,181], in gastric cancer [182], in small intestinal adenocarcinoma [183], and in oral squamous carcinoma [184].
An important observation concerning HO-1 subcellular localization comes from three different studies on head and neck cancer [185], breast cancer [186], and colorectal cancer [187] that analyzed the correlation of histological features with HO-1 positivity in cytosol or nuclei. In these studies, high expression of HO-1 in cytosol correlated with low grade and differentiation without correlation with invasiveness. However, nuclear localization of HO-1 was associated with a high grade and poor differentiation. Moreover, in breast cancer, Gandini showed that cytosolic HO-1 is enzymatically active, while the nuclear form is truncated and with no catalytic activity [186]. These observations appear to be interesting and helpful in understanding the contrasting observation of the role of HO-1 in tumor progression and lead to speculation that HO-1 pro- or antitumor activity may depend on its subcellular localization and catalytic activity.
6. HO-1 and Tumor Therapies
It has been widely reported that the induction of HO-1 in response to anticancer treatments can attenuate the efficacy of therapy, increasing cancer cell survival. Indeed, HO-1 expression is increased in response to different chemotherapeutic agents that act through the imbalance of intracellular oxidative state. For instance, in neuroblastoma cells, HO-1 expression is induced by exposure to etoposide through the activation of Nrf2 [145], and by the exposure to proteasome inhibitors bortezomib or carfilzomib [148,149,150], and mediates cell survival. To note, doxorubicin or pharmorubicin promote HO-1 expression increasing cell survival in breast cancers through the activation of Src/STAT3 or PI3K/AKT, respectively [146,147].
Remarkably, HO-1 induction mediates cancer cell resistance not only to chemotherapeutic agents but also to radio-, photodynamic-, and non-thermal-plasma (NTP) therapies, as demonstrated in non-small cell lung carcinoma [152,153,154].
As far as hematological malignancies are concerned, HO-1 expression significantly increases in myeloid neoplasms both in chronic and acute myeloid leukemia. Its overexpression occurs mainly after therapeutic intervention and induces chemoresistance. Recently, it has been demonstrated that PI3K/AKT-dependent HO-1 induction drives drug resistance to imatinib in CML [190] as well as to panobinostat in AML [191] by modulating the expression of HDACs. HO-1 overexpression enhances the viability and decreases the apoptotic rate in AML cell lines treated with cytarabine. Accordingly, the derived xenograft mouse model shows a significantly shorter survival and a great extent of organ invasion, while HO-1 down regulation significantly increases the survival rate [192]. Moreover, HO-1 up-regulation in myelodysplastic syndromes is closely related to resistance to decitabine-induced apoptosis [193], and in multiple myeloma, HO-1 up-regulation is involved in bortezomib chemoresistance [194].
In this context, pharmacological and genetic tools to reduce HO-1 activity have been proposed, and their use has been hypothesized in therapy, as described later and summarized in Table 2.
Table 2.
HO-1 inhibitory tools.
| Pharmacological Inhibitors | Benefits | Drawbacks | Ref. | |
|---|---|---|---|---|
| Porphyrin-Based Compounds | ||||
Metalloporphyrins
|
|
|
[196,201,202] | |
Modified protoporphyrins
|
|
[206,207,208,209,210] | ||
| Imidazole-based compounds | ||||
|
|
|
[211,212] | |
| Genetic tools | ||||
| Small interfering RNA and short hairpin RNA |
|
|
[195] | |
| CRISPR/Cas9 |
|
|
[195] |
6.1. Inhibition of HO-1 by Pharmacological Compounds
Among the pharmacological tools, metalloporphyrins and imidazole-based compounds are the most well-known and have been recently reviewed [195].
Briefly, metalloporphyrins represent the first generation of HO-1 inhibitors and include deuteroporphyrin, mesoporphyrin, and protoporphyrin [196]. Structurally similar to heme, metalloporphyrins strongly inhibit HO-1 by a competitive mechanism [197]. The most used metalloporphyrins are ZnPPIX, SnPPIX, and SnMPIX, and their efficacy in favoring conventional tumor therapies has been widely demonstrated in vitro and in vivo. For instance, ZnPPIX favors the sensitivity of nasopharyngeal carcinoma cells to radiotherapy [198] and of neuroblastoma to glutathione depletion and etoposide [145]. Moreover, ZnPPIX sensitizes C-26 colon and MDAH2774 ovarian carcinoma cells to photodynamic therapy-mediated cytotoxicity [199] and increases the effects of cisplatin in liver cancers [143]. It has also been demonstrated that treatment with ZnPPIX reduces cell growth in hepatoma, sarcoma, lung cancer, and B cell lymphoma [52,125]. Furthermore, in melanoma cells, SnPPIX enhances the efficacy of photodynamic therapy [200] and in BRAFV600-mutated melanoma cells SnMPIX increases cell death induced by vemurafenib/PLX4032 [140].
Unfortunately, metalloporphyrins are able to act on other heme-dependent enzymes, such as nitric oxide synthase (NOS), sGC, and cytochrome P450 [201,202]. Moreover, even though they efficiently inhibit HO-1 activity, they can often favor HO-1 protein synthesis, as demonstrated in liver cells and fibroblasts, and more recently, in prostate cancer PC-3 cells by a compensatory mechanism [203,204,205]. Of note, another important disadvantage of using metalloporphyrins is related to their photo reactivity, which is responsible for side effects and even tissue and organ damage [196]. Another strong drawback for the potential clinical use of some metalloporphyrins (e.g., ZnPPIX) is represented by their poor solubility in aqueous solutions, which limits translational applicability. However, this inconvenience has been overcome by conjugation with specific molecules, e.g., polyethylene-glycol or amphiphilic styrene-maleic acid copolymer, generating water-soluble molecules [206,207,208,209,210].
Imidazole-based compounds represent the second generation of HO-1 inhibitors. These molecules are non porphyrin-based and non competitive water-soluble inhibitors of HO-1 and exhibit low or even no inhibitory action on NOS, sGC, and CYP [211,212]. The first reported was Azalanstat [213], but other molecules and novel azole-based compounds derived from the structural modification of Azalanstat have been recently discovered [214,215]. Imidazole-based compounds have shown potent antitumor activity in prostate and breast cancer cell lines [216]; in a preclinical model of hormone-refractory prostate cancer, the small molecule imidazole-derived OB-24 acts in synergism with the conventional chemotherapy drug Taxol, preventing tumor growth and formation of lymph node and lung metastasis [188]. However, imidazole-based compounds have not been tested in clinical studies so far.
6.2. Inhibition of HO-1 by RNA Interference and CRIPR/Cas9 Technology
With regard to genetic tools to modulate HO-1 activity, the most consistent data derive from studies on RNA interference, including small interfering RNA and short hairpin RNA, able to inhibit HO-1 activity by targeting HO-1 transcription and consequently protein synthesis. Thus, HO-1 silencing increases the effect of chemotherapeutic drugs in pancreatic cancer [217], neuroblastoma [148,149], and melanoma cancer cells [140], as well as in myeloid leukemia [170]. Moreover, HO-1 silencing sensitizes cancer cells to apoptosis, as demonstrated in lung, colon, and leukemic cancer cells [195]. Similar results have been obtained in an in vivo experimental mouse model of hepatocellular carcinoma, where injection of siRNA-HO-1 results in the diminished growth of the tumor [218]. Furthermore, HO-1 is considered a survival factor in ALL, regardless of Philadelphia chromosome positivity; indeed, the down-regulation of HO-1 expression by siRNA increases apoptosis and arrests cell growth [219]. Consistently, in chronic lymphocytic leukemia (CLL), it has been demonstrated that HO-1 silencing directly leads to apoptosis of MEC-1 cells and enhances the effects of the combined therapy fludarabine plus entinostat [220].
A new approach in the inhibition of HO-1 activity is represented by genetic ablation of HO-1 with the CRISPR/Cas9 editing system. It has been recently demonstrated that homozygous HO-1 knock-out in BRAF-WT melanoma cells is able to decrease clone formation and to lower tumor cell growth [176]; further, in pancreatic ductal adenocarcinoma cells, HO-1 CRISPR/Cas9 is able to suppress cell proliferation and improve the efficacy of gemcitabine treatment [151]. Importantly, in in vivo experiments on C57/BL6 mice, HO-1 CRISPR/Cas9 editing blocks lymphocyte B development [221].
6.3. Induction of HO-1 as a Therapeutic Strategy
Thus, a great deal of literature shows a direct correlation between the overexpression of HO-1 and the gain of resistance of cancer cells and tumor progression. However, it must be taken into account that in some tumors, the over expression of HO-1 exerts opposite effects by inhibiting tumor growth and cancer progression. In particular, it has been shown in some types of prostate cancer that HO-1 expression and carbon monoxide generation are associated with significant inhibition of cell proliferation and invasiveness [96]. Moreover, in non-small-cell lung carcinoma NCI-H292 cells, the stable HO-1 overexpression is able to up-regulate tumor-suppressive miRNAs and to down-regulate the expression of oncomirs and angiomirs, leading to the inhibition of cell proliferation, invasiveness, and angiogenesis [222]. It has been highlighted that this tumor-suppressive phenotype is characterized by the attenuation of the metastatic potential mainly by down regulating MMP-9 and MMP-13 [223]. Similarly, stable overexpression of HO-1 retards hepatocellular carcinoma progression [224]. The antitumorigenic effects of HO-1 have also been demonstrated in human and rat breast cancer, where its overexpression correlates with inhibition of cell proliferation [225] and in pancreatic and prostate cancer, where it is associated with a decrease in cell proliferation and invasiveness by a down regulation of the proangiogenic mediators VEGF and MMP-9 [97,195,226]. In this context, the induction of HO-1 has been proposed to increase conventional cancer therapies, and some “natural” compounds derived from plants have shown interesting properties. In colorectal cancer, it has been demonstrated that treatment with extracts from Sageretia thea, a medicinal plant used for treating hepatitis and fevers in Korea and China, decreases cell viability by inducing GSK3β-dependent cyclin D1 degradation and increasing HO-1 expression via activation of Nrf2 [227]. In addition, Ginnalin A, a polyphenolic compound isolated from red maple (Acer rubrum), inhibits cell viability and colony formation in colorectal cancer, inducing cell cycle arrest by activating the Nrf2/HO-1 pathway through the up-regulation of p62 and the inhibition of Keap1 [228]. Similarly, treatment with fisetin, a bioactive flavonol molecule abundantly found in strawberries, decreases the level of MMPs and cell migration in metastatic breast cancer with a mechanism depending on Nrf2 nuclear translocation and HO-1 up-regulation [229].
Since ferroptosis may be a way to kill cancer cells, and it can be enhanced by HO-1 overactivation, the pharmacological induction of HO-1 has been proposed. Indeed, HO-1-dependent intracellular Fe2+ overload induces lipid peroxidation and triggers a noncanonical ferroptosis [230]. Phytochemicals are often used for this purpose [231]. Neuroblastoma cell treatment with withaferin A, a steroidal lactone derived from Withania somnifera (Indian ginseng), directly targets Keap1, leading to Nrf2 release and HO-1 up-regulation and consequently increasing intracellular Fe2+ and inducing ferroptosis [232]. Similarly, in human colon cancer cells, a high concentration of extract of Betula etnensis extract induces HO-1 leading to ferroptotic cell death through an increase of ROS production and in lipid peroxidation mediated by iron accumulation [233]. Moreover, HO-1 up-regulation has been proved to be the primary factor for curcumin-induced ferroptosis in human breast adenocarcinoma-derived MCF7 cells and in human triple-negative MDA-MB-231 cell line [234]. In addition, β-elemene, a sesquiterpene found in a variety of plants, is able to induce ferroptosis by enhancing HO-1 activity in KRAS mutant colorectal HCT116 cancer cells [235]. In addition, in this work, the presence of possible side effects of β-elemene were tested in the derived orthotopic murine colon cancer model, and no toxicity was found relatively the different organs analyzed (lung, heart, liver, kidney, and spleen) by H&E staining.
Thus, the evaluation of HO-1 expression in cancer samples from patients may help to define a therapeutic strategy where inhibition or induction of HO-1 could improve the efficacy of the standard antineoplastic therapy used.
7. Future Perspectives and Conclusions
The chance to analyze HO-1 expression in cancer patients seems to be a useful tool to improve tumor diagnosis and to better define prognosis and therapy. On the one hand, the analysis of (GT)n length polymorphisms seems a very promising approach to assess the risk of treatment failure as recently proved in ALL patients carrier of short (GT) repeats [60]. On the other hand, the characterization of HO-1 expression in tumors may be a useful tool to improve tumor diagnosis and prognosis because it can correlate with tumor grade/stage, invasiveness, and clinical outcomes. However, contrasting data are reported, and larger analyses need to be performed. Importantly, it has been recently highlighted the role played by the truncated form of HO-1 in favoring cell growth, opening to a new scenario in which HO-1 can be involved in tumor biology [92].
As a future perspective, in order to better assess tumor progression, the correlation between tissue expression of HO-1 and its levels in a blood sample could be taken into consideration, even though no evidence has been reported so far. However, the analysis of HO-1 level may be proposed in other biological fluids such as urine, peritoneal or pleural fluids, if directly related to the tissue bearing neoplastic cells. It is important to remember that in other diseases, HO-1 levels in bio fluids correlate with HO-1 expression levels in tissues [103].
Moreover, a great amount of data support the efficacy of HO-1 modulation in order to improve cancer response to therapies (Figure 2). Different approaches have been proposed, using either pharmacological agents or genetic tools. Unfortunately, concerning HO-1 pharmacological inhibitors, the translational applicability is not completely elucidated, even though both SnPPIX and SnMPIX have been already tested in humans [236] and approved for the treatment of hyperbilirubinemia [237]. Instead, genetic tools have been tested only in experimental animal models. Therefore, HO-1 modulation may represent an important strategy also to prevent cancer immune escape. However, we must consider that, so far, little data in the literature are available on the role played by HO-1 in the function of tumor-related immune cells. This is still an open field of research.
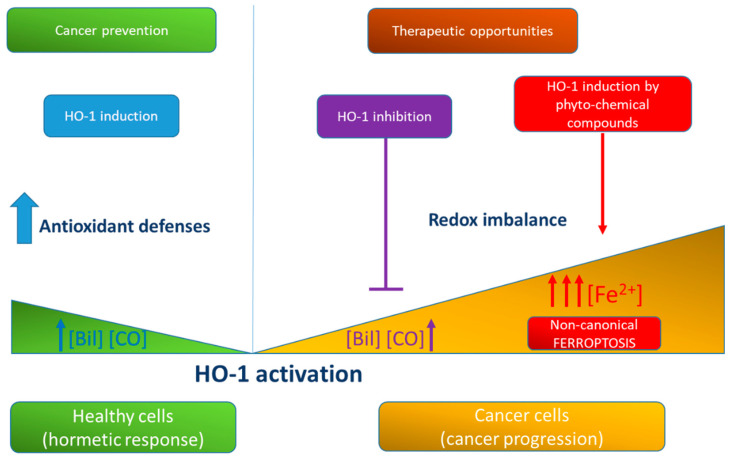
Figure 2.
Schematic representation of the effects of HO-1 activation and generation of its metabolic products in healthy and cancer cells. HO-1 activation is involved in antioxidant defenses and in healthy cells promotes the hormetic response and cancer prevention through the generation of bilirubin and CO. In cancer cells, HO-1 favors cancer progression, and its inhibition represents a therapeutic opportunity. However, also HO-1 over-activation can be proposed as a therapeutic option, as it can favor unconventional ferroptosis through the accumulation of pro-oxidant-free iron.
Conversely, molecules able to induce HO-1 may be used in order to favor cancer cell death due to iron imbalance. About this issue, as mentioned before, many natural compounds have been tested and showed their efficacy in this sense, but even in this case, translational applicability in humans seems to be still far away.
In conclusion, a deeper investigation of the specific multifaceted role played by HO-1 in different types of cancers, in the tumor microenvironment and bio fluids is needed in order to customize therapy and improve the outcome of cancer patients. Thus, HO-1 could become in the future an important clinical tool for cancer management.
Acknowledgments
Grants from Genoa University.
Abbreviations
| ALL | acute lymphoblastic leukemia |
| AML | acute myeloid leukemia |
| BR | bilirubin |
| BVRA | biliverdin reductase A |
| ccRCC | clear cell Renal cell carcinoma |
| CML | chronic myeloid leukemia |
| DC | dendritic cells |
| EV | extracellular vescicles |
| HO-1 | heme oxygenase 1 |
| MAPK | mitogen-activated protein kinase pathway |
| NK | natural killer cells |
| NMIBC | non-muscle-invasive bladder cancer |
| NOS | nitric oxide synthase |
| PMBC | peripheral blood mononuclear cells |
| ROS | reactive oxygen species |
| sGC | soluble guanylyl cyclase |
| SnMPPIX | tin mesoporphyrin IX |
| SnPPIX | tin protoporphyrin IX |
| SSP | signal peptide peptidase |
| VSMC | vascular smooth muscle cells |
| ZnPPIX | zinc(II) protoporphyrin IX |
Author Contributions
M.N. and A.L.F. conceived and wrote the manuscript; M.N., C.I., N.T., and A.L.F. revised the literature; M.N. and N.T. provided financial support. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Maines M.D. Heme Oxygenase: Function, Multiplicity, Regulatory Mechanisms, and Clinical Applications. FASEB J. 1988;2:2557–2568. doi: 10.1096/fasebj.2.10.3290025. [DOI] [PubMed] [Google Scholar]
- 2.Waza A.A., Hamid Z., Ali S., Bhat S.A., Bhat M.A. A Review on Heme Oxygenase-1 Induction: Is It a Necessary Evil. Inflamm. Res. 2018;67:579–588. doi: 10.1007/s00011-018-1151-x. [DOI] [PubMed] [Google Scholar]
- 3.Kutty R.K., Nagineni C.N., Kutty G., Hooks J.J., Chader G.J., Wiggert B. Increased Expression of Heme Oxygenase-1 in Human Retinal Pigment Epithelial Cells by Transforming Growth Factor-Beta. J. Cell Physiol. 1994;159:371–378. doi: 10.1002/jcp.1041590221. [DOI] [PubMed] [Google Scholar]
- 4.Bian C., Zhong M., Nisar M.F., Wu Y., Ouyang M., Bartsch J.W., Zhong J.L. A Novel Heme Oxygenase-1 Splice Variant, 14kDa HO-1, Promotes Cell Proliferation and Increases Relative Telomere Length. Biochem. Biophys Res. Commun. 2018;500:429–434. doi: 10.1016/j.bbrc.2018.04.096. [DOI] [PubMed] [Google Scholar]
- 5.Furfaro A.L., Traverso N., Domenicotti C., Piras S., Moretta L., Marinari U.M., Pronzato M.A., Nitti M. The Nrf2/HO-1 Axis in Cancer Cell Growth and Chemoresistance. Oxid. Med. Cell. Longev. 2016;2016:1958174. doi: 10.1155/2016/1958174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keyse S.M., Tyrrell R.M. Heme Oxygenase Is the Major 32-KDa Stress Protein Induced in Human Skin Fibroblasts by UVA Radiation, Hydrogen Peroxide, and Sodium Arsenite. Proc. Natl. Acad. Sci. USA. 1989;86:99–103. doi: 10.1073/pnas.86.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alam J., Shibahara S., Smith A. Transcriptional Activation of the Heme Oxygenase Gene by Heme and Cadmium in Mouse Hepatoma Cells. J. Biol. Chem. 1989;264:6371–6375. doi: 10.1016/S0021-9258(18)83358-0. [DOI] [PubMed] [Google Scholar]
- 8.Foresti R., Clark J.E., Green C.J., Motterlini R. Thiol Compounds Interact with Nitric Oxide in Regulating Heme Oxygenase-1 Induction in Endothelial Cells. Involvement of Superoxide and Peroxynitrite Anions. J. Biol. Chem. 1997;272:18411–18417. doi: 10.1074/jbc.272.29.18411. [DOI] [PubMed] [Google Scholar]
- 9.Ayer A., Zarjou A., Agarwal A., Stocker R. Heme Oxygenases in Cardiovascular Health and Disease. Physiol. Rev. 2016;96:1449–1508. doi: 10.1152/physrev.00003.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nitti M., Piras S., Marinari U.M., Moretta L., Pronzato M.A., Furfaro A.L. HO-1 Induction in Cancer Progression: A Matter of Cell Adaptation. Antioxidants. 2017;6:29. doi: 10.3390/antiox6020029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loboda A., Jozkowicz A., Dulak J. HO-1/CO System in Tumor Growth, Angiogenesis and Metabolism-Targeting HO-1 as an Anti-Tumor Therapy. Vasc. Pharmacol. 2015;74:11–22. doi: 10.1016/j.vph.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Riquelme S.A., Carreño L.J., Espinoza J.A., Mackern-Oberti J.P., Alvarez-Lobos M.M., Riedel C.A., Bueno S.M., Kalergis A.M. Modulation of Antigen Processing by Haem-Oxygenase 1. Implications on Inflammation and Tolerance. Immunology. 2016;149:1–12. doi: 10.1111/imm.12605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vijayan V., Wagener F.A.D.T.G., Immenschuh S. The Macrophage Heme-Heme Oxygenase-1 System and Its Role in Inflammation. Biochem. Pharmacol. 2018;153:159–167. doi: 10.1016/j.bcp.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Siow R.C., Sato H., Mann G.E. Heme Oxygenase-Carbon Monoxide Signalling Pathway in Atherosclerosis: Anti-Atherogenic Actions of Bilirubin and Carbon Monoxide? Cardiovasc. Res. 1999;41:385–394. doi: 10.1016/S0008-6363(98)00278-8. [DOI] [PubMed] [Google Scholar]
- 15.Otterbein L.E., Foresti R., Motterlini R. Heme Oxygenase-1 and Carbon Monoxide in the Heart: The Balancing Act Between Danger Signaling and Pro-Survival. Circ. Res. 2016;118:1940–1959. doi: 10.1161/CIRCRESAHA.116.306588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kishimoto Y., Kondo K., Momiyama Y. The Protective Role of Heme Oxygenase-1 in Atherosclerotic Diseases. Int. J. Mol. Sci. 2019;20:3628. doi: 10.3390/ijms20153628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stocker R., Yamamoto Y., McDonagh A.F., Glazer A.N., Ames B.N. Bilirubin Is an Antioxidant of Possible Physiological Importance. Science. 1987;235:1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 18.Stocker R. Antioxidant Activities of Bile Pigments. Antioxid. Redox Signal. 2004;6:841–849. doi: 10.1089/ars.2004.6.841. [DOI] [PubMed] [Google Scholar]
- 19.Tenhunen R., Marver H.S., Schmid R. The Enzymatic Conversion of Heme to Bilirubin by Microsomal Heme Oxygenase. Proc. Natl. Acad. Sci. USA. 1968;61:748–755. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tenhunen R., Marver H.S., Schmid R. The Enzymatic Conversion of Hemoglobin to Bilirubin. Trans. Assoc. Am. Physicians. 1969;82:363–371. [PubMed] [Google Scholar]
- 21.Neuzil J., Stocker R. Bilirubin Attenuates Radical-Mediated Damage to Serum Albumin. FEBS Lett. 1993;331:281–284. doi: 10.1016/0014-5793(93)80353-V. [DOI] [PubMed] [Google Scholar]
- 22.Sedlak T.W., Saleh M., Higginson D.S., Paul B.D., Juluri K.R., Snyder S.H. Bilirubin and Glutathione Have Complementary Antioxidant and Cytoprotective Roles. Proc. Natl. Acad. Sci. USA. 2009;106:5171–5176. doi: 10.1073/pnas.0813132106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu T.W., Fung K.P., Yang C.C. Unconjugated Bilirubin Inhibits the Oxidation of Human Low Density Lipoprotein Better than Trolox. Life Sci. 1994;54:P477–P481. doi: 10.1016/0024-3205(94)90140-6. [DOI] [PubMed] [Google Scholar]
- 24.He M., Nitti M., Piras S., Furfaro A.L., Traverso N., Pronzato M.A., Mann G.E. Heme Oxygenase-1-Derived Bilirubin Protects Endothelial Cells against High Glucose-Induced Damage. Free Radic. Biol. Med. 2015;89:91–98. doi: 10.1016/j.freeradbiomed.2015.07.151. [DOI] [PubMed] [Google Scholar]
- 25.Wu B., Wu Y., Tang W. Heme Catabolic Pathway in Inflammation and Immune Disorders. Front. Pharmacol. 2019;10:825. doi: 10.3389/fphar.2019.00825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Canesin G., Hejazi S.M., Swanson K.D., Wegiel B. Heme-Derived Metabolic Signals Dictate Immune Responses. Front. Immunol. 2020;11:66. doi: 10.3389/fimmu.2020.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ryter S.W. Heme Oxygenase-1/Carbon Monoxide as Modulators of Autophagy and Inflammation. Arch. Biochem. Biophys. 2019;678:108186. doi: 10.1016/j.abb.2019.108186. [DOI] [PubMed] [Google Scholar]
- 28.Keshavan P., Deem T.L., Schwemberger S.J., Babcock G.F., Cook-Mills J.M., Zucker S.D. Unconjugated Bilirubin Inhibits VCAM-1-Mediated Transendothelial Leukocyte Migration. J. Immunol. 2005;174:3709–3718. doi: 10.4049/jimmunol.174.6.3709. [DOI] [PubMed] [Google Scholar]
- 29.Grochot-Przeczek A., Dulak J., Jozkowicz A. Haem Oxygenase-1: Non-Canonical Roles in Physiology and Pathology. Clin. Sci. 2012;122:93–103. doi: 10.1042/CS20110147. [DOI] [PubMed] [Google Scholar]
- 30.Mazzone G.L., Rigato I., Ostrow J.D., Bossi F., Bortoluzzi A., Sukowati C.H.C., Tedesco F., Tiribelli C. Bilirubin Inhibits the TNFalpha-Related Induction of Three Endothelial Adhesion Molecules. Biochem. Biophys. Res. Commun. 2009;386:338–344. doi: 10.1016/j.bbrc.2009.06.029. [DOI] [PubMed] [Google Scholar]
- 31.Nitti M., Furfaro A.L., Mann G.E. Heme Oxygenase Dependent Bilirubin Generation in Vascular Cells: A Role in Preventing Endothelial Dysfunction in Local Tissue Microenvironment? Front. Physiol. 2020;11:23. doi: 10.3389/fphys.2020.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petrache I., Otterbein L.E., Alam J., Wiegand G.W., Choi A.M. Heme Oxygenase-1 Inhibits TNF-Alpha-Induced Apoptosis in Cultured Fibroblasts. Am. J. Physiol. Lung Cell Mol. Physiol. 2000;278:L312–L319. doi: 10.1152/ajplung.2000.278.2.L312. [DOI] [PubMed] [Google Scholar]
- 33.Ryter S.W., Alam J., Choi A.M. Heme Oxygenase-1/Carbon Monoxide: From Basic Science to Therapeutic Applications. Physiol. Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 34.Ryter S.W., Ma K.C., Choi A.M.K. Carbon Monoxide in Lung Cell Physiology and Disease. Am. J. Physiol. Cell Physiol. 2018;314:C211–C227. doi: 10.1152/ajpcell.00022.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Motterlini R., Foresti R., Bassi R., Green C.J. Curcumin, an Antioxidant and Anti-Inflammatory Agent, Induces Heme Oxygenase-1 and Protects Endothelial Cells against Oxidative Stress. Free Radic. Biol. Med. 2000;28:1303–1312. doi: 10.1016/S0891-5849(00)00294-X. [DOI] [PubMed] [Google Scholar]
- 36.Dennery P.A. Heme Oxygenase in Neonatal Lung Injury and Repair. Antioxid. Redox Signal. 2014;21:1881–1892. doi: 10.1089/ars.2013.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dulak J., Jozkowicz A., Foresti R., Kasza A., Frick M., Huk I., Green C.J., Pachinger O., Weidinger F., Motterlini R. Heme Oxygenase Activity Modulates Vascular Endothelial Growth Factor Synthesis in Vascular Smooth Muscle Cells. Antioxid. Redox Signal. 2002;4:229–240. doi: 10.1089/152308602753666280. [DOI] [PubMed] [Google Scholar]
- 38.Jozkowicz A., Huk I., Nigisch A., Weigel G., Dietrich W., Motterlini R., Dulak J. Heme Oxygenase and Angiogenic Activity of Endothelial Cells: Stimulation by Carbon Monoxide and Inhibition by Tin Protoporphyrin-IX. Antioxid. Redox Signal. 2003;5:155–162. doi: 10.1089/152308603764816514. [DOI] [PubMed] [Google Scholar]
- 39.Loboda A., Jazwa A., Grochot-Przeczek A., Rutkowski A.J., Cisowski J., Agarwal A., Jozkowicz A., Dulak J. Heme Oxygenase-1 and the Vascular Bed: From Molecular Mechanisms to Therapeutic Opportunities. Antioxid. Redox Signal. 2008;10:1767–1812. doi: 10.1089/ars.2008.2043. [DOI] [PubMed] [Google Scholar]
- 40.Rochette L., Cottin Y., Zeller M., Vergely C. Carbon Monoxide: Mechanisms of Action and Potential Clinical Implications. Pharmacol. Ther. 2013;137:133–152. doi: 10.1016/j.pharmthera.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 41.Ryter S.W., Choi A.M.K. Targeting Heme Oxygenase-1 and Carbon Monoxide for Therapeutic Modulation of Inflammation. Transl. Res. 2016;167:7–34. doi: 10.1016/j.trsl.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pae H.O., Choi B.M., Oh G.S., Lee M.S., Ryu D.G., Rhew H.Y., Kim Y.M., Chung H.T. Roles of Heme Oxygenase-1 in the Antiproliferative and Antiapoptotic Effects of Nitric Oxide on Jurkat T Cells. Mol. Pharmacol. 2004;66:122–128. doi: 10.1124/mol.66.1.122. [DOI] [PubMed] [Google Scholar]
- 43.Otterbein L.E., Bach F.H., Alam J., Soares M., Tao Lu H., Wysk M., Davis R.J., Flavell R.A., Choi A.M. Carbon Monoxide Has Anti-Inflammatory Effects Involving the Mitogen-Activated Protein Kinase Pathway. Nat. Med. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- 44.Balla G., Jacob H.S., Balla J., Rosenberg M., Nath K., Apple F., Eaton J.W., Vercellotti G.M. Ferritin: A Cytoprotective Antioxidant Strategem of Endothelium. J. Biol. Chem. 1992;267:18148–18153. doi: 10.1016/S0021-9258(19)37165-0. [DOI] [PubMed] [Google Scholar]
- 45.Baker H.M., Anderson B.F., Baker E.N. Dealing with Iron: Common Structural Principles in Proteins That Transport Iron and Heme. Proc. Natl. Acad. Sci. USA. 2003;100:3579–3583. doi: 10.1073/pnas.0637295100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chiang S.-K., Chen S.-E., Chang L.-C. A Dual Role of Heme Oxygenase-1 in Cancer Cells. Int. J. Mol. Sci. 2018;20:39. doi: 10.3390/ijms20010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Song X., Long D. Nrf2 and Ferroptosis: A New Research Direction for Neurodegenerative Diseases. Front. Neurosci. 2020;14:267. doi: 10.3389/fnins.2020.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Di Biase S., Longo V.D. Fasting-Induced Differential Stress Sensitization in Cancer Treatment. Mol. Cell. Oncol. 2016;3:e1117701. doi: 10.1080/23723556.2015.1117701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lavrovsky Y., Schwartzman M.L., Levere R.D., Kappas A., Abraham N.G. Identification of Binding Sites for Transcription Factors NF-Kappa B and AP-2 in the Promoter Region of the Human Heme Oxygenase 1 Gene. Proc. Natl. Acad. Sci. USA. 1994;91:5987–5991. doi: 10.1073/pnas.91.13.5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lavrovsky Y., Schwartzman M.L., Abraham N.G. Novel Regulatory Sites of the Human Heme Oxygenase-1 Promoter Region. Biochem. Biophys. Res. Commun. 1993;196:336–341. doi: 10.1006/bbrc.1993.2253. [DOI] [PubMed] [Google Scholar]
- 51.Medina M.V., Sapochnik D., Garcia Solá M., Coso O. Regulation of the Expression of Heme Oxygenase-1: Signal Transduction, Gene Promoter Activation, and Beyond. Antioxid. Redox Signal. 2020;32:1033–1044. doi: 10.1089/ars.2019.7991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Was H., Dulak J., Jozkowicz A. Heme Oxygenase-1 in Tumor Biology and Therapy. Curr. Drug Targets. 2010;11:1551–1570. doi: 10.2174/1389450111009011551. [DOI] [PubMed] [Google Scholar]
- 53.Exner M., Minar E., Wagner O., Schillinger M. The Role of Heme Oxygenase-1 Promoter Polymorphisms in Human Disease. Free Radic. Biol. Med. 2004;37:1097–1104. doi: 10.1016/j.freeradbiomed.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 54.Zhang M.-M., Zheng Y.-Y., Gao Y., Zhang J.-Z., Liu F., Yang Y.-N., Li X.-M., Ma Y.-T., Xie X. Heme Oxygenase-1 Gene Promoter Polymorphisms Are Associated with Coronary Heart Disease and Restenosis after Percutaneous Coronary Intervention: A Meta-Analysis. Oncotarget. 2016;7:83437–83450. doi: 10.18632/oncotarget.13118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Daenen K.E.L., Martens P., Bammens B. Association of HO-1 (GT)n Promoter Polymorphism and Cardiovascular Disease: A Reanalysis of the Literature. Can. J. Cardiol. 2016;32:160–168. doi: 10.1016/j.cjca.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 56.Zhang L., Song F.-F., Huang Y.-B., Zheng H., Song F.-J., Chen K.-X. Association between the (GT)n Polymorphism of the HO-1 Gene Promoter Region and Cancer Risk: A Meta-Analysis. Asian Pac. J. Cancer Prev. 2014;15:4617–4622. doi: 10.7314/APJCP.2014.15.11.4617. [DOI] [PubMed] [Google Scholar]
- 57.Horio T., Morishita E., Mizuno S., Uchino K., Hanamura I., Espinoza J.L., Morishima Y., Kodera Y., Onizuka M., Kashiwase K., et al. Donor Heme Oxygenase-1 Promoter Gene Polymorphism Predicts Survival after Unrelated Bone Marrow Transplantation for High-Risk Patients. Cancers. 2020;12:424. doi: 10.3390/cancers12020424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ono K., Goto Y., Takagi S., Baba S., Tago N., Nonogi H., Iwai N. A Promoter Variant of the Heme Oxygenase-1 Gene May Reduce the Incidence of Ischemic Heart Disease in Japanese. Atherosclerosis. 2004;173:315–319. doi: 10.1016/j.atherosclerosis.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 59.Buis C.I., van der Steege G., Visser D.S., Nolte I.M., Hepkema B.G., Nijsten M., Slooff M.J.H., Porte R.J. Heme Oxygenase-1 Genotype of the Donor Is Associated with Graft Survival after Liver Transplantation. Am. J. Transplant. 2008;8:377–385. doi: 10.1111/j.1600-6143.2007.02048.x. [DOI] [PubMed] [Google Scholar]
- 60.Bukowska-Strakova K., Włodek J., Pitera E., Kozakowska M., Konturek-Cieśla A., Cieśla M., Gońka M., Nowak W., Wieczorek A., Pawińska-Wąsikowska K., et al. Role of HMOX1 Promoter Genetic Variants in Chemoresistance and Chemotherapy Induced Neutropenia in Children with Acute Lymphoblastic Leukemia. Int. J. Mol. Sci. 2021;22:988. doi: 10.3390/ijms22030988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paladino S., Conte A., Caggiano R., Pierantoni G.M., Faraonio R. Nrf2 Pathway in Age-Related Neurological Disorders: Insights into MicroRNAs. Cell Physiol. Biochem. 2018;47:1951–1976. doi: 10.1159/000491465. [DOI] [PubMed] [Google Scholar]
- 62.Mitsuishi Y., Motohashi H., Yamamoto M. The Keap1-Nrf2 System in Cancers: Stress Response and Anabolic Metabolism. Front. Oncol. 2012;2:200. doi: 10.3389/fonc.2012.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shibata T., Kokubu A., Gotoh M., Ojima H., Ohta T., Yamamoto M., Hirohashi S. Genetic Alteration of Keap1 Confers Constitutive Nrf2 Activation and Resistance to Chemotherapy in Gallbladder Cancer. Gastroenterology. 2008;135:1358–1368.e1-4. doi: 10.1053/j.gastro.2008.06.082. [DOI] [PubMed] [Google Scholar]
- 64.Na H.K., Surh Y.J. Oncogenic Potential of Nrf2 and Its Principal Target Protein Heme Oxygenase-1. Free Radic. Biol. Med. 2014;67:353–365. doi: 10.1016/j.freeradbiomed.2013.10.819. [DOI] [PubMed] [Google Scholar]
- 65.Muscarella L.A., Parrella P., D’Alessandro V., la Torre A., Barbano R., Fontana A., Tancredi A., Guarnieri V., Balsamo T., Coco M., et al. Frequent Epigenetics Inactivation of KEAP1 Gene in Non-Small Cell Lung Cancer. Epigenetics. 2011;6:710–719. doi: 10.4161/epi.6.6.15773. [DOI] [PubMed] [Google Scholar]
- 66.Hanada N., Takahata T., Zhou Q., Ye X., Sun R., Itoh J., Ishiguro A., Kijima H., Mimura J., Itoh K., et al. Methylation of the KEAP1 Gene Promoter Region in Human Colorectal Cancer. BMC Cancer. 2012;12:66. doi: 10.1186/1471-2407-12-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhao X.-Q., Zhang Y.-F., Xia Y.-F., Zhou Z.-M., Cao Y.-Q. Promoter Demethylation of Nuclear Factor-Erythroid 2-Related Factor 2 Gene in Drug-Resistant Colon Cancer Cells. Oncol. Lett. 2015;10:1287–1292. doi: 10.3892/ol.2015.3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van der Wijst M.G., Brown R., Rots M.G. Nrf2, the Master Redox Switch: The Achilles’ Heel of Ovarian Cancer? Biochim. Biophys. Acta. 2014;1846:494–509. doi: 10.1016/j.bbcan.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 69.Ogawa K., Sun J., Taketani S., Nakajima O., Nishitani C., Sassa S., Hayashi N., Yamamoto M., Shibahara S., Fujita H., et al. Heme Mediates Derepression of Maf Recognition Element through Direct Binding to Transcription Repressor Bach1. EMBO J. 2001;20:2835–2843. doi: 10.1093/emboj/20.11.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Davudian S., Mansoori B., Shajari N., Mohammadi A., Baradaran B. BACH1, the Master Regulator Gene: A Novel Candidate Target for Cancer Therapy. Gene. 2016;588:30–37. doi: 10.1016/j.gene.2016.04.040. [DOI] [PubMed] [Google Scholar]
- 71.Lignitto L., LeBoeuf S.E., Homer H., Jiang S., Askenazi M., Karakousi T.R., Pass H.I., Bhutkar A.J., Tsirigos A., Ueberheide B., et al. Nrf2 Activation Promotes Lung Cancer Metastasis by Inhibiting the Degradation of Bach1. Cell. 2019;178:316–329.e18. doi: 10.1016/j.cell.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wiel C., Le Gal K., Ibrahim M.X., Jahangir C.A., Kashif M., Yao H., Ziegler D.V., Xu X., Ghosh T., Mondal T., et al. BACH1 Stabilization by Antioxidants Stimulates Lung Cancer Metastasis. Cell. 2019;178:330–345.e22. doi: 10.1016/j.cell.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 73.Alam J., Wicks C., Stewart D., Gong P., Touchard C., Otterbein S., Choi A.M., Burow M.E., Tou J. Mechanism of Heme Oxygenase-1 Gene Activation by Cadmium in MCF-7 Mammary Epithelial Cells. Role of P38 Kinase and Nrf2 Transcription Factor. J. Biol. Chem. 2000;275:27694–27702. doi: 10.1074/jbc.M004729200. [DOI] [PubMed] [Google Scholar]
- 74.Liu Z.-M., Chen G.G., Ng E.K.W., Leung W.-K., Sung J.J.Y., Chung S.C.S. Upregulation of Heme Oxygenase-1 and P21 Confers Resistance to Apoptosis in Human Gastric Cancer Cells. Oncogene. 2004;23:503–513. doi: 10.1038/sj.onc.1207173. [DOI] [PubMed] [Google Scholar]
- 75.Dal-Cim T., Molz S., Egea J., Parada E., Romero A., Budni J., Martín de Saavedra M.D., del Barrio L., Tasca C.I., López M.G. Guanosine Protects Human Neuroblastoma SH-SY5Y Cells against Mitochondrial Oxidative Stress by Inducing Heme Oxigenase-1 via PI3K/Akt/GSK-3β Pathway. Neurochem. Int. 2012;61:397–404. doi: 10.1016/j.neuint.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 76.Talabnin C., Talabnin K., Wongkham S. Enhancement of Piperlongumine Chemosensitivity by Silencing Heme Oxygenase-1 Expression in Cholangiocarcinoma Cell Lines. Oncol. Lett. 2020;20:2483–2492. doi: 10.3892/ol.2020.11784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cheng X., Ku C.H., Siow R.C. Regulation of the Nrf2 Antioxidant Pathway by MicroRNAs: New Players in Micromanaging Redox Homeostasis. Free Radic. Biol. Med. 2013;64:4–11. doi: 10.1016/j.freeradbiomed.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 78.Gu S., Lai Y., Chen H., Liu Y., Zhang Z. MiR-155 Mediates Arsenic Trioxide Resistance by Activating Nrf2 and Suppressing Apoptosis in Lung Cancer Cells. Sci. Rep. 2017;7:12155. doi: 10.1038/s41598-017-06061-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Eades G., Yang M., Yao Y., Zhang Y., Zhou Q. MiR-200a Regulates Nrf2 Activation by Targeting Keap1 MRNA in Breast Cancer Cells. J. Biol. Chem. 2011;286:40725–40733. doi: 10.1074/jbc.M111.275495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pu M., Li C., Qi X., Chen J., Wang Y., Gao L., Miao L., Ren J. MiR-1254 Suppresses HO-1 Expression through Seed Region-Dependent Silencing and Non-Seed Interaction with TFAP2A Transcript to Attenuate NSCLC Growth. PLoS Genet. 2017;13:e1006896. doi: 10.1371/journal.pgen.1006896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang Z., Chen J.-S., Wen J.-K., Gao H.-T., Zheng B., Qu C.-B., Liu K.-L., Zhang M.-L., Gu J.-F., Li J.-D., et al. Silencing of MiR-193a-5p Increases the Chemosensitivity of Prostate Cancer Cells to Docetaxel. J. Exp. Clin. Cancer Res. 2017;36:178. doi: 10.1186/s13046-017-0649-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Piras S., Furfaro A.L., Caggiano R., Brondolo L., Garibaldi S., Ivaldo C., Marinari U.M., Pronzato M.A., Faraonio R., Nitti M. MicroRNA-494 Favors HO-1 Expression in Neuroblastoma Cells Exposed to Oxidative Stress in a Bach1-Independent Way. Front. Oncol. 2018;8:199. doi: 10.3389/fonc.2018.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Durante W. Targeting Heme Oxygenase-1 in the Arterial Response to Injury and Disease. Antioxidants. 2020;9:829. doi: 10.3390/antiox9090829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Huber W.J., Backes W.L. Expression and Characterization of Full-Length Human Heme Oxygenase-1: The Presence of Intact Membrane-Binding Region Leads to Increased Binding Affinity for NADPH Cytochrome P450 Reductase. Biochemistry. 2007;46:12212–12219. doi: 10.1021/bi701496z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jung N.-H., Kim H.P., Kim B.-R., Cha S.H., Kim G.A., Ha H., Na Y.E., Cha Y.-N. Evidence for Heme Oxygenase-1 Association with Caveolin-1 and -2 in Mouse Mesangial Cells. IUBMB Life. 2003;55:525–532. doi: 10.1080/15216540310001620968. [DOI] [PubMed] [Google Scholar]
- 86.Slebos D.J., Ryter S.W., van der Toorn M., Liu F., Guo F., Baty C.J., Karlsson J.M., Watkins S.C., Kim H.P., Wang X., et al. Mitochondrial Localization and Function of Heme Oxygenase-1 in Cigarette Smoke-Induced Cell Death. Am. J. Respir. Cell Mol. Biol. 2007;36:409–417. doi: 10.1165/rcmb.2006-0214OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 87.Lin Q., Weis S., Yang G., Weng Y.H., Helston R., Rish K., Smith A., Bordner J., Polte T., Gaunitz F., et al. Heme Oxygenase-1 Protein Localizes to the Nucleus and Activates Transcription Factors Important in Oxidative Stress. J. Biol. Chem. 2007;282:20621–20633. doi: 10.1074/jbc.M607954200. [DOI] [PubMed] [Google Scholar]
- 88.Lin Q.S., Weis S., Yang G., Zhuang T., Abate A., Dennery P.A. Catalytic Inactive Heme Oxygenase-1 Protein Regulates Its Own Expression in Oxidative Stress. Free Radic. Biol. Med. 2008;44:847–855. doi: 10.1016/j.freeradbiomed.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hsu F.F., Yeh C.T., Sun Y.J., Chiang M.T., Lan W.M., Li F.A., Lee W.H., Chau L.Y. Signal Peptide Peptidase-Mediated Nuclear Localization of Heme Oxygenase-1 Promotes Cancer Cell Proliferation and Invasion Independent of Its Enzymatic Activity. Oncogene. 2015;34:2410–2411. doi: 10.1038/onc.2014.464. [DOI] [PubMed] [Google Scholar]
- 90.Biswas C., Shah N., Muthu M., La P., Fernando A.P., Sengupta S., Yang G., Dennery P.A. Nuclear Heme Oxygenase-1 (HO-1) Modulates Subcellular Distribution and Activation of Nrf2, Impacting Metabolic and Anti-Oxidant Defenses. J. Biol. Chem. 2014;289:26882–26894. doi: 10.1074/jbc.M114.567685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hsu F.-F., Chiang M.-T., Li F.-A., Yeh C.-T., Lee W.-H., Chau L.-Y. Acetylation Is Essential for Nuclear Heme Oxygenase-1-Enhanced Tumor Growth and Invasiveness. Oncogene. 2017;36:6805–6814. doi: 10.1038/onc.2017.294. [DOI] [PubMed] [Google Scholar]
- 92.Mascaró M., Alonso E.N., Alonso E.G., Lacunza E., Curino A.C., Facchinetti M.M. Nuclear Localization of Heme Oxygenase-1 in Pathophysiological Conditions: Does It Explain the Dual Role in Cancer? Antioxidants. 2021;10:87. doi: 10.3390/antiox10010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dennery P.A. Signaling Function of Heme Oxygenase Proteins. Antioxid. Redox Signal. 2014;20:1743–1753. doi: 10.1089/ars.2013.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tibullo D., Barbagallo I., Giallongo C., La Cava P., Parrinello N., Vanella L., Stagno F., Palumbo G.A., Li Volti G., Di Raimondo F. Nuclear Translocation of Heme Oxygenase-1 Confers Resistance to Imatinib in Chronic Myeloid Leukemia Cells. Curr. Pharm. Des. 2013;19:2765–2770. doi: 10.2174/1381612811319150012. [DOI] [PubMed] [Google Scholar]
- 95.Elguero B., Gueron G., Giudice J., Toscani M.A., De Luca P., Zalazar F., Coluccio-Leskow F., Meiss R., Navone N., De Siervi A., et al. Unveiling the Association of STAT3 and HO-1 in Prostate Cancer: Role beyond Heme Degradation. Neoplasia. 2012;14:1043–1056. doi: 10.1593/neo.121358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gueron G., De Siervi A., Ferrando M., Salierno M., De Luca P., Elguero B., Meiss R., Navone N., Vazquez E.S. Critical Role of Endogenous Heme Oxygenase 1 as a Tuner of the Invasive Potential of Prostate Cancer Cells. Mol. Cancer Res. MCR. 2009;7:1745–1755. doi: 10.1158/1541-7786.MCR-08-0325. [DOI] [PubMed] [Google Scholar]
- 97.Ferrando M., Gueron G., Elguero B., Giudice J., Salles A., Leskow F.C., Jares-Erijman E.A., Colombo L., Meiss R., Navone N., et al. Heme Oxygenase 1 (HO-1) Challenges the Angiogenic Switch in Prostate Cancer. Angiogenesis. 2011;14:467–479. doi: 10.1007/s10456-011-9230-4. [DOI] [PubMed] [Google Scholar]
- 98.Degese M.S., Mendizabal J.E., Gandini N.A., Gutkind J.S., Molinolo A., Hewitt S.M., Curino A.C., Coso O.A., Facchinetti M.M. Expression of Heme Oxygenase-1 in Non-Small Cell Lung Cancer (NSCLC) and Its Correlation with Clinical Data. Lung Cancer. 2012;77:168–175. doi: 10.1016/j.lungcan.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vanella L., Barbagallo I., Tibullo D., Forte S., Zappalà A., Li Volti G. The Non-Canonical Functions of the Heme Oxygenases. Oncotarget. 2016;7:69075–69086. doi: 10.18632/oncotarget.11923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Signorelli S.S., Li Volsi G., Fiore V., Mangiafico M., Barbagallo I., Parenti R., Rizzo M., Li Volti G. Plasma Heme Oxygenase-1 Is Decreased in Peripheral Artery Disease Patients. Mol. Med. Rep. 2016;14:3459–3463. doi: 10.3892/mmr.2016.5644. [DOI] [PubMed] [Google Scholar]
- 101.Serpero L.D., Frigiola A., Gazzolo D. Human Milk and Formulae: Neurotrophic and New Biological Factors. Early Hum. Dev. 2012;88(Suppl. 1):S9–S12. doi: 10.1016/j.earlhumdev.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 102.Novo G., Cappello F., Rizzo M., Fazio G., Zambuto S., Tortorici E., Marino Gammazza A., Gammazza A.M., Corrao S., Zummo G., et al. Hsp60 and Heme Oxygenase-1 (Hsp32) in Acute Myocardial Infarction. Transl. Res. 2011;157:285–292. doi: 10.1016/j.trsl.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 103.Zager R.A., Johnson A.C.M., Becker K. Plasma and Urinary Heme Oxygenase-1 in AKI. J. Am. Soc. Nephrol. 2012;23:1048–1057. doi: 10.1681/ASN.2011121147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.van Niel G., D’Angelo G., Raposo G. Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2018;19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 105.Margolis L., Sadovsky Y. The Biology of Extracellular Vesicles: The Known Unknowns. PLoS Biol. 2019;17:e3000363. doi: 10.1371/journal.pbio.3000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.El-Rifaie A.-A.A., Sabry D., Doss R.W., Kamal M.A., Abd El Hassib D.M. Heme Oxygenase and Iron Status in Exosomes of Psoriasis Patients. Arch. Dermatol. Res. 2018;310:651–656. doi: 10.1007/s00403-018-1852-6. [DOI] [PubMed] [Google Scholar]
- 107.Cressatti M., Galindez J.M., Juwara L., Orlovetskie N., Velly A.M., Eintracht S., Liberman A., Gornitsky M., Schipper H.M. Characterization and Heme Oxygenase-1 Content of Extracellular Vesicles in Human Biofluids. J. Neurochem. 2020 doi: 10.1111/jnc.15167. [DOI] [PubMed] [Google Scholar]
- 108.Hurwitz S.N., Rider M.A., Bundy J.L., Liu X., Singh R.K., Meckes D.G. Proteomic Profiling of NCI-60 Extracellular Vesicles Uncovers Common Protein Cargo and Cancer Type-Specific Biomarkers. Oncotarget. 2016;7:86999–87015. doi: 10.18632/oncotarget.13569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Goodman A.I., Choudhury M., da Silva J.L., Schwartzman M.L., Abraham N.G. Overexpression of the Heme Oxygenase Gene in Renal Cell Carcinoma. Proc. Soc. Exp. Biol. Med. 1997;214:54–61. doi: 10.3181/00379727-214-44069. [DOI] [PubMed] [Google Scholar]
- 110.Was H., Cichon T., Smolarczyk R., Rudnicka D., Stopa M., Chevalier C., Leger J.J., Lackowska B., Grochot A., Bojkowska K., et al. Overexpression of Heme Oxygenase-1 in Murine Melanoma: Increased Proliferation and Viability of Tumor Cells, Decreased Survival of Mice. Am. J. Pathol. 2006;169:2181–2198. doi: 10.2353/ajpath.2006.051365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Maines M.D., Abrahamsson P.A. Expression of Heme Oxygenase-1 (HSP32) in Human Prostate: Normal, Hyperplastic, and Tumor Tissue Distribution. Urology. 1996;47:727–733. doi: 10.1016/S0090-4295(96)00010-6. [DOI] [PubMed] [Google Scholar]
- 112.Schacter B.A., Kurz P. Alterations in Microsomal Drug Metabolism and Heme Oxygenase Activity in Isolated Hepatic Parenchymal and Sinusoidal Cells in Murphy-Sturm Lymphosarcoma-Bearing Rats. Clin. Investig. Med. Med. Clin. Exp. 1986;9:150–155. [PubMed] [Google Scholar]
- 113.Sferrazzo G., Di Rosa M., Barone E., Li Volti G., Musso N., Tibullo D., Barbagallo I. Heme Oxygenase-1 in Central Nervous System Malignancies. J. Clin. Med. 2020;9:1562. doi: 10.3390/jcm9051562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li Volti G., Tibullo D., Vanella L., Giallongo C., Di Raimondo F., Forte S., Di Rosa M., Signorelli S.S., Barbagallo I. The Heme Oxygenase System in Hematological Malignancies. Antioxid. Redox Signal. 2017;27:363–377. doi: 10.1089/ars.2016.6735. [DOI] [PubMed] [Google Scholar]
- 115.Sunamura M., Duda D.G., Ghattas M.H., Lozonschi L., Motoi F., Yamauchi J., Matsuno S., Shibahara S., Abraham N.G. Heme Oxygenase-1 Accelerates Tumor Angiogenesis of Human Pancreatic Cancer. Angiogenesis. 2003;6:15–24. doi: 10.1023/A:1025803600840. [DOI] [PubMed] [Google Scholar]
- 116.Lee S.S., Yang S.F., Tsai C.H., Chou M.C., Chou M.Y., Chang Y.C. Upregulation of Heme Oxygenase-1 Expression in Areca-Quid-Chewing-Associated Oral Squamous Cell Carcinoma. J. Formos. Med. Assoc. 2008;107:355–363. doi: 10.1016/S0929-6646(08)60100-X. [DOI] [PubMed] [Google Scholar]
- 117.Halin Bergström S., Nilsson M., Adamo H., Thysell E., Jernberg E., Stattin P., Widmark A., Wikström P., Bergh A. Extratumoral Heme Oxygenase-1 (HO-1) Expressing Macrophages Likely Promote Primary and Metastatic Prostate Tumor Growth. PLoS ONE. 2016;11:e0157280. doi: 10.1371/journal.pone.0157280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tsai J.R., Wang H.M., Liu P.L., Chen Y.H., Yang M.C., Chou S.H., Cheng Y.J., Yin W.H., Hwang J.J., Chong I.W. High Expression of Heme Oxygenase-1 Is Associated with Tumor Invasiveness and Poor Clinical Outcome in Non-Small Cell Lung Cancer Patients. Cell Oncol. 2012;35:461–471. doi: 10.1007/s13402-012-0105-5. [DOI] [PubMed] [Google Scholar]
- 119.Wang X., Ye T., Xue B., Yang M., Li R., Xu X., Zeng X., Tian N., Bao L., Huang Y. Mitochondrial GRIM-19 Deficiency Facilitates Gastric Cancer Metastasis through Oncogenic ROS-NRF2-HO-1 Axis via a NRF2-HO-1 Loop. Gastric. Cancer. 2020 doi: 10.1007/s10120-020-01111-2. [DOI] [PubMed] [Google Scholar]
- 120.Zhao Z., Zhao J., Xue J., Zhao X., Liu P. Autophagy Inhibition Promotes Epithelial-Mesenchymal Transition through ROS/HO-1 Pathway in Ovarian Cancer Cells. Am. J. Cancer Res. 2016;6:2162–2177. [PMC free article] [PubMed] [Google Scholar]
- 121.Chang Y.J., Chen W.Y., Huang C.Y., Liu H.H., Wei P.L. Glucose-Regulated Protein 78 (GRP78) Regulates Colon Cancer Metastasis through EMT Biomarkers and the NRF-2/HO-1 Pathway. Tumour Biol. 2015;36:1859–1869. doi: 10.1007/s13277-014-2788-x. [DOI] [PubMed] [Google Scholar]
- 122.Jozkowicz A., Huk I., Nigisch A., Weigel G., Weidinger F., Dulak J. Effect of Prostaglandin-J(2) on VEGF Synthesis Depends on the Induction of Heme Oxygenase-1. Antioxid. Redox Signal. 2002;4:577–585. doi: 10.1089/15230860260220076. [DOI] [PubMed] [Google Scholar]
- 123.Miyake M., Fujimoto K., Anai S., Ohnishi S., Kuwada M., Nakai Y., Inoue T., Matsumura Y., Tomioka A., Ikeda T., et al. Heme Oxygenase-1 Promotes Angiogenesis in Urothelial Carcinoma of the Urinary Bladder. Oncol. Rep. 2011;25:653–660. doi: 10.3892/or.2010.1125. [DOI] [PubMed] [Google Scholar]
- 124.Kozakowska M., Dobrowolska-Glazar B., Okoń K., Józkowicz A., Dobrowolski Z., Dulak J. Preliminary Analysis of the Expression of Selected Proangiogenic and Antioxidant Genes and MicroRNAs in Patients with Non-Muscle-Invasive Bladder Cancer. J. Clin. Med. 2016;5:29. doi: 10.3390/jcm5030029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shang F.T., Hui L.L., An X.S., Zhang X.C., Guo S.G., Kui Z. ZnPPIX Inhibits Peritoneal Metastasis of Gastric Cancer via Its Antiangiogenic Activity. Biomed. Pharmacother. 2015;71:240–246. doi: 10.1016/j.biopha.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 126.Cheng C.C., Guan S.S., Yang H.J., Chang C.C., Luo T.Y., Chang J., Ho A.S. Blocking Heme Oxygenase-1 by Zinc Protoporphyrin Reduces Tumor Hypoxia-Mediated VEGF Release and Inhibits Tumor Angiogenesis as a Potential Therapeutic Agent against Colorectal Cancer. J. Biomed. Sci. 2016;23:18. doi: 10.1186/s12929-016-0219-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu R., Peng J., Wang H., Li L., Wen X., Tan Y., Zhang L., Wan H., Chen F., Nie X. Oxysophocarpine Retards the Growth and Metastasis of Oral Squamous Cell Carcinoma by Targeting the Nrf2/HO-1 Axis. Cell Physiol. Biochem. 2018;49:1717–1733. doi: 10.1159/000493615. [DOI] [PubMed] [Google Scholar]
- 128.Hao N.B., Lu M.H., Fan Y.H., Cao Y.L., Zhang Z.R., Yang S.M. Macrophages in Tumor Microenvironments and the Progression of Tumors. Clin. Dev. Immunol. 2012;2012:948098. doi: 10.1155/2012/948098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Blancou P., Anegon I. Editorial: Heme Oxygenase-1 and Dendritic Cells: What Else? J. Leukoc. Biol. 2010;87:185–187. doi: 10.1189/jlb.0909636. [DOI] [PubMed] [Google Scholar]
- 130.Alaluf E., Vokaer B., Detavernier A., Azouz A., Splittgerber M., Carrette A., Boon L., Libert F., Soares M., Le Moine A., et al. Heme Oxygenase-1 Orchestrates the Immunosuppressive Program of Tumor-Associated Macrophages. JCI Insight. 2020;5 doi: 10.1172/jci.insight.133929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nemeth Z., Li M., Csizmadia E., Döme B., Johansson M., Persson J.L., Seth P., Otterbein L., Wegiel B. Heme Oxygenase-1 in Macrophages Controls Prostate Cancer Progression. Oncotarget. 2015;6:33675–33688. doi: 10.18632/oncotarget.5284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Deng R., Wang S.M., Yin T., Ye T.H., Shen G.B., Li L., Zhao J.Y., Sang Y.X., Duan X.G., Wei Y.Q. Inhibition of Tumor Growth and Alteration of Associated Macrophage Cell Type by an HO-1 Inhibitor in Breast Carcinoma-Bearing Mice. Oncol. Res. 2013;20:473–482. doi: 10.3727/096504013X13715991125684. [DOI] [PubMed] [Google Scholar]
- 133.Beyer M., Schultze J.L. Regulatory T Cells in Cancer. Blood. 2006;108:804–811. doi: 10.1182/blood-2006-02-002774. [DOI] [PubMed] [Google Scholar]
- 134.Chattopadhyay S., Chakraborty N.G., Mukherji B. Regulatory T Cells and Tumor Immunity. Cancer Immunol. Immunother. CII. 2005;54:1153–1161. doi: 10.1007/s00262-005-0699-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kohno T., Yamada Y., Akamatsu N., Kamihira S., Imaizumi Y., Tomonaga M., Matsuyama T. Possible Origin of Adult T-Cell Leukemia/Lymphoma Cells from Human T Lymphotropic Virus Type-1-Infected Regulatory T Cells. Cancer Sci. 2005;96:527–533. doi: 10.1111/j.1349-7006.2005.00080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Di Biase S., Lee C., Brandhorst S., Manes B., Buono R., Cheng C.W., Cacciottolo M., Martin-Montalvo A., de Cabo R., Wei M., et al. Fasting-Mimicking Diet Reduces HO-1 to Promote T Cell-Mediated Tumor Cytotoxicity. Cancer Cell. 2016;30:136–146. doi: 10.1016/j.ccell.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.El Andaloussi A., Lesniak M.S. CD4+ CD25+ FoxP3+ T-Cell Infiltration and Heme Oxygenase-1 Expression Correlate with Tumor Grade in Human Gliomas. J. Neuro-Oncol. 2007;83:145–152. doi: 10.1007/s11060-006-9314-y. [DOI] [PubMed] [Google Scholar]
- 138.Orange J.S. Formation and Function of the Lytic NK-Cell Immunological Synapse. Nat. Rev. Immunol. 2008;8:713–725. doi: 10.1038/nri2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gomez-Lomeli P., Bravo-Cuellar A., Hernandez-Flores G., Jave-Suarez L.F., Aguilar-Lemarroy A., Lerma-Diaz J.M., Dominguez-Rodriguez J.R., Sanchez-Reyes K., Ortiz-Lazareno P.C. Increase of IFN-Gamma and TNF-Alpha Production in CD107a + NK-92 Cells Co-Cultured with Cervical Cancer Cell Lines Pre-Treated with the HO-1 Inhibitor. Cancer Cell Int. 2014;14:100. doi: 10.1186/s12935-014-0100-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Furfaro A.L., Ottonello S., Loi G., Cossu I., Piras S., Spagnolo F., Queirolo P., Marinari U.M., Moretta L., Pronzato M.A., et al. HO-1 Downregulation Favors BRAFV600 Melanoma Cell Death Induced by Vemurafenib/PLX4032 and Increases NK Recognition. Int. J. Cancer. 2020;146:1950–1962. doi: 10.1002/ijc.32611. [DOI] [PubMed] [Google Scholar]
- 141.Wang Y., Qi H., Liu Y., Duan C., Liu X., Xia T., Chen D., Piao H.-L., Liu H.-X. The Double-Edged Roles of ROS in Cancer Prevention and Therapy. Theranostics. 2021;11:4839–4857. doi: 10.7150/thno.56747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Aggarwal V., Tuli H.S., Varol A., Thakral F., Yerer M.B., Sak K., Varol M., Jain A., Khan M.A., Sethi G. Role of Reactive Oxygen Species in Cancer Progression: Molecular Mechanisms and Recent Advancements. Biomolecules. 2019;9:735. doi: 10.3390/biom9110735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Liu Y.-S., Li H.-S., Qi D.-F., Zhang J., Jiang X.-C., Shi K., Zhang X.-J., Zhang X.-H. Zinc Protoporphyrin IX Enhances Chemotherapeutic Response of Hepatoma Cells to Cisplatin. World J. Gastroenterol. 2014;20:8572–8582. doi: 10.3748/wjg.v20.i26.8572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sun X., Wang S., Gai J., Guan J., Li J., Li Y., Zhao J., Zhao C., Fu L., Li Q. SIRT5 Promotes Cisplatin Resistance in Ovarian Cancer by Suppressing DNA Damage in a ROS-Dependent Manner via Regulation of the Nrf2/HO-1 Pathway. Front. Oncol. 2019;9:754. doi: 10.3389/fonc.2019.00754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Furfaro A.L., Macay J.R., Marengo B., Nitti M., Parodi A., Fenoglio D., Marinari U.M., Pronzato M.A., Domenicotti C., Traverso N. Resistance of Neuroblastoma GI-ME-N Cell Line to Glutathione Depletion Involves Nrf2 and Heme Oxygenase-1. Free Radic. Biol. Med. 2012;52:488–496. doi: 10.1016/j.freeradbiomed.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 146.Tan Q., Wang H., Hu Y., Hu M., Li X., Aodengqimuge, Ma Y., Wei C., Song L. Src/STAT3-Dependent Heme Oxygenase-1 Induction Mediates Chemoresistance of Breast Cancer Cells to Doxorubicin by Promoting Autophagy. Cancer Sci. 2015;106:1023–1032. doi: 10.1111/cas.12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pei L., Kong Y., Shao C., Yue X., Wang Z., Zhang N. Heme Oxygenase-1 Induction Mediates Chemoresistance of Breast Cancer Cells to Pharmorubicin by Promoting Autophagy via PI3K/Akt Pathway. J. Cell Mol. Med. 2018;22:5311–5321. doi: 10.1111/jcmm.13800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Furfaro A.L., Piras S., Passalacqua M., Domenicotti C., Parodi A., Fenoglio D., Pronzato M.A., Marinari U.M., Moretta L., Traverso N., et al. HO-1 up-Regulation: A Key Point in High-Risk Neuroblastoma Resistance to Bortezomib. Biochim. Biophys. Acta. 2014;1842:613–622. doi: 10.1016/j.bbadis.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 149.Furfaro A.L., Piras S., Domenicotti C., Fenoglio D., De Luigi A., Salmona M., Moretta L., Marinari U.M., Pronzato M.A., Traverso N., et al. Role of Nrf2, HO-1 and GSH in Neuroblastoma Cell Resistance to Bortezomib. PLoS ONE. 2016;11:e0152465. doi: 10.1371/journal.pone.0152465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Barbagallo I., Giallongo C., Volti G.L., Distefano A., Camiolo G., Raffaele M., Salerno L., Pittalà V., Sorrenti V., Avola R., et al. Heme Oxygenase Inhibition Sensitizes Neuroblastoma Cells to Carfilzomib. Mol. Neurobiol. 2019;56:1451–1460. doi: 10.1007/s12035-018-1133-6. [DOI] [PubMed] [Google Scholar]
- 151.Abdalla M.Y., Ahmad I.M., Rachagani S., Banerjee K., Thompson C.M., Maurer H.C., Olive K.P., Bailey K.L., Britigan B.E., Kumar S. Enhancing Responsiveness of Pancreatic Cancer Cells to Gemcitabine Treatment under Hypoxia by Heme Oxygenase-1 Inhibition. Transl. Res. 2019;207:56–69. doi: 10.1016/j.trsl.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 152.Ma J., Yu K.N., Cheng C., Ni G., Shen J., Han W. Targeting Nrf2-Mediated Heme Oxygenase-1 Enhances Non-Thermal Plasma-Induced Cell Death in Non-Small-Cell Lung Cancer A549 Cells. Arch. Biochem. Biophys. 2018;658:54–65. doi: 10.1016/j.abb.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 153.Zhang W., Qiao T., Zha L. Inhibition of Heme Oxygenase-1 Enhances the Radiosensitivity in Human Nonsmall Cell Lung Cancer A549 Cells. Cancer Biother. Radiopharm. 2011;26:639–645. doi: 10.1089/cbr.2010.0939. [DOI] [PubMed] [Google Scholar]
- 154.Chen N., Wu L., Yuan H., Wang J. ROS/Autophagy/Nrf2 Pathway Mediated Low-Dose Radiation Induced Radio-Resistance in Human Lung Adenocarcinoma A549 Cell. Int. J. Biol. Sci. 2015;11:833–844. doi: 10.7150/ijbs.10564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang R., Shen J., Yang R., Wang W.-G., Yuan Y., Guo Z.-H. Association between Heme Oxygenase-1 Gene Promoter Polymorphisms and Cancer Susceptibility: A Meta-Analysis. Biomed. Rep. 2018;8:241–248. doi: 10.3892/br.2018.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hu J.L., Li Z.Y., Liu W., Zhang R.G., Li G.L., Wang T., Ren J.H., Wu G. Polymorphism in Heme Oxygenase-1 (HO-1) Promoter and Alcohol Are Related to the Risk of Esophageal Squamous Cell Carcinoma on Chinese Males. Neoplasma. 2010;57:86–92. doi: 10.4149/neo_2010_01_086. [DOI] [PubMed] [Google Scholar]
- 157.Kikuchi A., Yamaya M., Suzuki S., Yasuda H., Kubo H., Nakayama K., Handa M., Sasaki T., Shibahara S., Sekizawa K., et al. Association of Susceptibility to the Development of Lung Adenocarcinoma with the Heme Oxygenase-1 Gene Promoter Polymorphism. Hum. Genet. 2005;116:354–360. doi: 10.1007/s00439-004-1162-2. [DOI] [PubMed] [Google Scholar]
- 158.Murakami A., Fujimori Y., Yoshikawa Y., Yamada S., Tamura K., Hirayama N., Terada T., Kuribayashi K., Tabata C., Fukuoka K., et al. Heme Oxygenase-1 Promoter Polymorphism Is Associated with Risk of Malignant Mesothelioma. Lung. 2012;190:333–337. doi: 10.1007/s00408-012-9371-2. [DOI] [PubMed] [Google Scholar]
- 159.Wu M.-M., Lee C.-H., Hsu L.-I., Cheng W.-F., Lee T.-C., Wang Y.-H., Chiou H.-Y., Chen C.-J. Effect of Heme Oxygenase-1 Gene Promoter Polymorphism on Cancer Risk by Histological Subtype: A Prospective Study in Arseniasis-Endemic Areas in Taiwan. Int. J. Cancer. 2016;138:1875–1886. doi: 10.1002/ijc.29926. [DOI] [PubMed] [Google Scholar]
- 160.Zhao Z., Xu Y., Lu J., Xue J., Liu P. High Expression of HO-1 Predicts Poor Prognosis of Ovarian Cancer Patients and Promotes Proliferation and Aggressiveness of Ovarian Cancer Cells. Clin. Transl. Oncol. 2018;20:491–499. doi: 10.1007/s12094-017-1738-7. [DOI] [PubMed] [Google Scholar]
- 161.Miyake M., Fujimoto K., Anai S., Ohnishi S., Nakai Y., Inoue T., Matsumura Y., Tomioka A., Ikeda T., Okajima E., et al. Inhibition of Heme Oxygenase-1 Enhances the Cytotoxic Effect of Gemcitabine in Urothelial Cancer Cells. Anticancer Res. 2010;30:2145–2152. [PubMed] [Google Scholar]
- 162.Kim J.H., Park J. Prognostic Significance of Heme Oxygenase-1, S100 Calcium-Binding Protein A4, and Syndecan-1 Expression in Primary Non-Muscle-Invasive Bladder Cancer. Hum. Pathol. 2014;45:1830–1838. doi: 10.1016/j.humpath.2014.04.020. [DOI] [PubMed] [Google Scholar]
- 163.Gandini N.A., Fermento M.E., Salomon D.G., Obiol D.J., Andres N.C., Zenklusen J.C., Arevalo J., Blasco J., Lopez Romero A., Facchinetti M.M., et al. Heme Oxygenase-1 Expression in Human Gliomas and Its Correlation with Poor Prognosis in Patients with Astrocytoma. Tumour Biol. 2014;35:2803–2815. doi: 10.1007/s13277-013-1373-z. [DOI] [PubMed] [Google Scholar]
- 164.Wang J., Zhang M., Zhang L., Cai H., Zhou S., Zhang J., Wang Y. Correlation of Nrf2, HO-1, and MRP3 in Gallbladder Cancer and Their Relationships to Clinicopathologic Features and Survival. J. Surg. Res. 2010;164:e99–e105. doi: 10.1016/j.jss.2010.05.058. [DOI] [PubMed] [Google Scholar]
- 165.Deng Y., Wu Y., Zhao P., Weng W., Ye M., Sun H., Xu M., Wang C. The Nrf2/HO-1 Axis Can Be a Prognostic Factor in Clear Cell Renal Cell Carcinoma. Cancer Manag. Res. 2019;11:1221–1230. doi: 10.2147/CMAR.S188046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kongpetch S., Puapairoj A., Ong C.K., Senggunprai L., Prawan A., Kukongviriyapan U., Chan-On W., Siew E.Y., Khuntikeo N., Teh B.T., et al. Haem Oxygenase 1 Expression Is Associated with Prognosis in Cholangiocarcinoma Patients and with Drug Sensitivity in Xenografted Mice. Cell Prolif. 2016;49:90–101. doi: 10.1111/cpr.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yu M., Wang J., Ma D., Chen S., Lin X., Fang Q., Zhe N. HO-1, RET and PML as Possible Markers for Risk Stratification of Acute Myelocytic Leukemia and Prognostic Evaluation. Oncol. Lett. 2015;10:3137–3144. doi: 10.3892/ol.2015.3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fest S., Soldati R., Christiansen N.M., Zenclussen M.L., Kilz J., Berger E., Starke S., Lode H.N., Engel C., Zenclussen A.C., et al. Targeting of Heme Oxygenase-1 as a Novel Immune Regulator of Neuroblastoma. Int. J. Cancer. 2016;138:2030–2042. doi: 10.1002/ijc.29933. [DOI] [PubMed] [Google Scholar]
- 169.Wei S., Wang Y., Chai Q., Fang Q., Zhang Y., Lu Y., Wang J. Over-Expression of Heme Oxygenase-1 in Peripheral Blood Predicts the Progression and Relapse Risk of Chronic Myeloid Leukemia. Chin. Med. J. 2014;127:2795–2801. [PubMed] [Google Scholar]
- 170.Zhe N., Wang J., Chen S., Lin X., Chai Q., Zhang Y., Zhao J., Fang Q. Heme Oxygenase-1 Plays a Crucial Role in Chemoresistance in Acute Myeloid Leukemia. Hematology. 2015;20:384–391. doi: 10.1179/1607845414Y.0000000212. [DOI] [PubMed] [Google Scholar]
- 171.He Z., Zhang S., Ma D., Fang Q., Yang L., Shen S., Chen Y., Ren L., Wang J. HO-1 Promotes Resistance to an EZH2 Inhibitor through the PRB-E2F Pathway: Correlation with the Progression of Myelodysplastic Syndrome into Acute Myeloid Leukemia. J. Transl. Med. 2019;17:366. doi: 10.1186/s12967-019-2115-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wang T.-Y., Liu C.-L., Chen M.-J., Lee J.-J., Pun P.C., Cheng S.-P. Expression of Haem Oxygenase-1 Correlates with Tumour Aggressiveness and BRAF V600E Expression in Thyroid Cancer. Histopathology. 2015;66:447–456. doi: 10.1111/his.12562. [DOI] [PubMed] [Google Scholar]
- 173.Li Y., Su J., DingZhang X., Zhang J., Yoshimoto M., Liu S., Bijian K., Gupta A., Squire J.A., Alaoui Jamali M.A., et al. PTEN Deletion and Heme Oxygenase-1 Overexpression Cooperate in Prostate Cancer Progression and Are Associated with Adverse Clinical Outcome. J. Pathol. 2011;224:90–100. doi: 10.1002/path.2855. [DOI] [PubMed] [Google Scholar]
- 174.Xu Y., Yang Y., Huang Y., Ma Q., Shang J., Guo J., Cao X., Wang X., Li M. Inhibition of Nrf2/HO-1 Signaling Pathway by Dextran Sulfate Suppresses Angiogenesis of Gastric Cancer. J. Cancer. 2021;12:1042–1060. doi: 10.7150/jca.50605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Park C.-S., Eom D.-W., Ahn Y., Jang H.J., Hwang S., Lee S.-G. Can Heme Oxygenase-1 Be a Prognostic Factor in Patients with Hepatocellular Carcinoma? Medicine. 2019;98:e16084. doi: 10.1097/MD.0000000000016084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Liu L., Wu Y., Bian C., Nisar M.F., Wang M., Hu X., Diao Q., Nian W., Wang E., Xu W., et al. Heme Oxygenase 1 Facilitates Cell Proliferation via the B-Raf-ERK Signaling Pathway in Melanoma. Cell Commun. Signal. 2019;17:3. doi: 10.1186/s12964-018-0313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Matsuo T., Miyata Y., Mitsunari K., Yasuda T., Ohba K., Sakai H. Pathological Significance and Prognostic Implications of Heme Oxygenase 1 Expression in Non-Muscle-Invasive Bladder Cancer: Correlation with Cell Proliferation, Angiogenesis, Lymphangiogenesis and Expression of VEGFs and COX-2. Oncol. Lett. 2017;13:275–280. doi: 10.3892/ol.2016.5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Nishie A., Ono M., Shono T., Fukushi J., Otsubo M., Onoue H., Ito Y., Inamura T., Ikezaki K., Fukui M., et al. Macrophage Infiltration and Heme Oxygenase-1 Expression Correlate with Angiogenesis in Human Gliomas. Clin. Cancer Res. 1999;5:1107–1113. [PubMed] [Google Scholar]
- 179.Kimura S., Aung N.Y., Ohe R., Yano M., Hashimoto T., Fujishima T., Kimura W., Yamakawa M. Increasing Heme Oxygenase-1-Expressing Macrophages Indicates a Tendency of Poor Prognosis in Advanced Colorectal Cancer. Digestion. 2020;101:401–410. doi: 10.1159/000500225. [DOI] [PubMed] [Google Scholar]
- 180.Becker J.C., Fukui H., Imai Y., Sekikawa A., Kimura T., Yamagishi H., Yoshitake N., Pohle T., Domschke W., Fujimori T. Colonic Expression of Heme Oxygenase-1 Is Associated with a Better Long-Term Survival in Patients with Colorectal Cancer. Scand. J. Gastroenterol. 2007;42:852–858. doi: 10.1080/00365520701192383. [DOI] [PubMed] [Google Scholar]
- 181.Andrés N.C., Fermento M.E., Gandini N.A., Romero A.L., Ferro A., Donna L.G., Curino A.C., Facchinetti M.M. Heme Oxygenase-1 Has Antitumoral Effects in Colorectal Cancer: Involvement of P53. Exp. Mol. Pathol. 2014;97:321–331. doi: 10.1016/j.yexmp.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 182.Yin Y., Liu Q., Wang B., Chen G., Xu L., Zhou H. Expression and Function of Heme Oxygenase-1 in Human Gastric Cancer. Exp Biol. Med. 2012;237:362–371. doi: 10.1258/ebm.2011.011193. [DOI] [PubMed] [Google Scholar]
- 183.Jun S.-Y., Hong S.-M., Bae Y.K., Kim H.K., Jang K.Y., Eom D.W. Clinicopathological and Prognostic Significance of Heme Oxygenase-1 Expression in Small Intestinal Adenocarcinomas. Pathol. Int. 2018;68:294–300. doi: 10.1111/pin.12657. [DOI] [PubMed] [Google Scholar]
- 184.Tsuji M.H., Yanagawa T., Iwasa S., Tabuchi K., Onizawa K., Bannai S., Toyooka H., Yoshida H. Heme Oxygenase-1 Expression in Oral Squamous Cell Carcinoma as Involved in Lymph Node Metastasis. Cancer Lett. 1999;138:53–59. doi: 10.1016/S0304-3835(98)00372-3. [DOI] [PubMed] [Google Scholar]
- 185.Gandini N.A., Fermento M.E., Salomon D.G., Blasco J., Patel V., Gutkind J.S., Molinolo A.A., Facchinetti M.M., Curino A.C. Nuclear Localization of Heme Oxygenase-1 Is Associated with Tumor Progression of Head and Neck Squamous Cell Carcinomas. Exp. Mol. Pathol. 2012;93:237–245. doi: 10.1016/j.yexmp.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 186.Gandini N.A., Alonso E.N., Fermento M.E., Mascaró M., Abba M.C., Coló G.P., Arévalo J., Ferronato M.J., Guevara J.A., Núñez M., et al. Heme Oxygenase-1 Has an Antitumor Role in Breast Cancer. Antioxid. Redox Signal. 2019;30:2030–2049. doi: 10.1089/ars.2018.7554. [DOI] [PubMed] [Google Scholar]
- 187.Yin H., Fang J., Liao L., Maeda H., Su Q. Upregulation of Heme Oxygenase-1 in Colorectal Cancer Patients with Increased Circulation Carbon Monoxide Levels, Potentially Affects Chemotherapeutic Sensitivity. BMC Cancer. 2014;14:436. doi: 10.1186/1471-2407-14-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Alaoui-Jamali M.A., Bismar T.A., Gupta A., Szarek W.A., Su J., Song W., Xu Y., Xu B., Liu G., Vlahakis J.Z., et al. A Novel Experimental Heme Oxygenase-1-Targeted Therapy for Hormone-Refractory Prostate Cancer. Cancer Res. 2009;69:8017–8024. doi: 10.1158/0008-5472.CAN-09-0419. [DOI] [PubMed] [Google Scholar]
- 189.Li C., Wu H., Wang S., Zhu J. Expression and Correlation of NRF2, KEAP1, NQO-1 and HO-1 in Advanced Squamous Cell Carcinoma of the Larynx and Their Association with Clinicopathologic Features. Mol. Med. Rep. 2016;14:5171–5179. doi: 10.3892/mmr.2016.5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wei D., Lu T., Ma D., Yu K., Li X., Chen B., Xiong J., Zhang T., Wang J. Heme Oxygenase-1 Reduces the Sensitivity to Imatinib through Nonselective Activation of Histone Deacetylases in Chronic Myeloid Leukemia. J. Cell Physiol. 2019;234:5252–5263. doi: 10.1002/jcp.27334. [DOI] [PubMed] [Google Scholar]
- 191.Cheng B., Tang S., Zhe N., Ma D., Yu K., Wei D., Zhou Z., Lu T., Wang J., Fang Q. Low Expression of GFI-1 Gene Is Associated with Panobinostat-Resistance in Acute Myeloid Leukemia through Influencing the Level of HO-1. Biomed. Pharmacother. 2018;100:509–520. doi: 10.1016/j.biopha.2018.02.039. [DOI] [PubMed] [Google Scholar]
- 192.Lin X., Fang Q., Chen S., Zhe N., Chai Q., Yu M., Zhang Y., Wang Z., Wang J. Heme Oxygenase-1 Suppresses the Apoptosis of Acute Myeloid Leukemia Cells via the JNK/c-JUN Signaling Pathway. Leuk. Res. 2015;39:544–552. doi: 10.1016/j.leukres.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 193.Ma D., Fang Q., Wang P., Gao R., Sun J., Li Y., Hu X.Y., Wang J.S. Downregulation of HO-1 Promoted Apoptosis Induced by Decitabine via Increasing P15INK4B Promoter Demethylation in Myelodysplastic Syndrome. Gene Ther. 2015;22:287–296. doi: 10.1038/gt.2015.1. [DOI] [PubMed] [Google Scholar]
- 194.Barrera L.N., Rushworth S.A., Bowles K.M., MacEwan D.J. Bortezomib Induces Heme Oxygenase-1 Expression in Multiple Myeloma. Cell Cycle. 2012;11:2248–2252. doi: 10.4161/cc.20343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Podkalicka P., Mucha O., Józkowicz A., Dulak J., Łoboda A. Heme Oxygenase Inhibition in Cancers: Possible Tools and Targets. Contemp. Oncol. 2018;22:23–32. doi: 10.5114/wo.2018.73879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Vreman H.J., Ekstrand B.C., Stevenson D.K. Selection of Metalloporphyrin Heme Oxygenase Inhibitors Based on Potency and Photoreactivity. Pediatr. Res. 1993;33:195–200. doi: 10.1203/00006450-199302000-00021. [DOI] [PubMed] [Google Scholar]
- 197.Schulz S., Wong R.J., Vreman H.J., Stevenson D.K. Metalloporphyrins—An Update. Front. Pharmacol. 2012;3:68. doi: 10.3389/fphar.2012.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Shi L., Fang J. Implication of Heme Oxygenase-1 in the Sensitivity of Nasopharyngeal Carcinomas to Radiotherapy. J. Exp. Clin. Cancer Res. 2008;27:13. doi: 10.1186/1756-9966-27-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Nowis D., Legat M., Grzela T., Niderla J., Wilczek E., Wilczynski G.M., Glodkowska E., Mrowka P., Issat T., Dulak J., et al. Heme Oxygenase-1 Protects Tumor Cells against Photodynamic Therapy-Mediated Cytotoxicity. Oncogene. 2006;25:3365–3374. doi: 10.1038/sj.onc.1209378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Frank J., Lornejad-Schäfer M.R., Schöffl H., Flaccus A., Lambert C., Biesalski H.K. Inhibition of Heme Oxygenase-1 Increases Responsiveness of Melanoma Cells to ALA-Based Photodynamic Therapy. Int. J. Oncol. 2007;31:1539–1545. doi: 10.3892/ijo.31.6.1539. [DOI] [PubMed] [Google Scholar]
- 201.Appleton S.D., Chretien M.L., McLaughlin B.E., Vreman H.J., Stevenson D.K., Brien J.F., Nakatsu K., Maurice D.H., Marks G.S. Selective Inhibition of Heme Oxygenase, without Inhibition of Nitric Oxide Synthase or Soluble Guanylyl Cyclase, by Metalloporphyrins at Low Concentrations. Drug Metab. Dispos. 1999;27:1214–1219. [PubMed] [Google Scholar]
- 202.Kinobe R.T., Dercho R.A., Nakatsu K. Inhibitors of the Heme Oxygenase-Carbon Monoxide System: On the Doorstep of the Clinic? Can. J. Physiol. Pharmacol. 2008;86:577–599. doi: 10.1139/Y08-066. [DOI] [PubMed] [Google Scholar]
- 203.Sardana M.K., Kappas A. Dual Control Mechanism for Heme Oxygenase: Tin(IV)-Protoporphyrin Potently Inhibits Enzyme Activity While Markedly Increasing Content of Enzyme Protein in Liver. Proc. Natl. Acad. Sci. USA. 1987;84:2464–2468. doi: 10.1073/pnas.84.8.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Yang G., Nguyen X., Ou J., Rekulapelli P., Stevenson D.K., Dennery P.A. Unique Effects of Zinc Protoporphyrin on HO-1 Induction and Apoptosis. Blood. 2001;97:1306–1313. doi: 10.1182/blood.V97.5.1306. [DOI] [PubMed] [Google Scholar]
- 205.Kwok S.C.M. Zinc Protoporphyrin Upregulates Heme Oxygenase-1 in PC-3 Cells via the Stress Response Pathway. Int. J. Cell Biol. 2013;2013:162094. doi: 10.1155/2013/162094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sahoo S.K., Sawa T., Fang J., Tanaka S., Miyamoto Y., Akaike T., Maeda H. Pegylated Zinc Protoporphyrin: A Water-Soluble Heme Oxygenase Inhibitor with Tumor-Targeting Capacity. Bioconjugate Chem. 2002;13:1031–1038. doi: 10.1021/bc020010k. [DOI] [PubMed] [Google Scholar]
- 207.Iyer A.K., Greish K., Seki T., Okazaki S., Fang J., Takeshita K., Maeda H. Polymeric Micelles of Zinc Protoporphyrin for Tumor Targeted Delivery Based on EPR Effect and Singlet Oxygen Generation. J. Drug Target. 2007;15:496–506. doi: 10.1080/10611860701498252. [DOI] [PubMed] [Google Scholar]
- 208.Iyer A.K., Greish K., Fang J., Murakami R., Maeda H. High-Loading Nanosized Micelles of Copoly(Styrene-Maleic Acid)-Zinc Protoporphyrin for Targeted Delivery of a Potent Heme Oxygenase Inhibitor. Biomaterials. 2007;28:1871–1881. doi: 10.1016/j.biomaterials.2006.11.051. [DOI] [PubMed] [Google Scholar]
- 209.Fang J., Sawa T., Akaike T., Akuta T., Sahoo S.K., Khaled G., Hamada A., Maeda H. In Vivo Antitumor Activity of Pegylated Zinc Protoporphyrin: Targeted Inhibition of Heme Oxygenase in Solid Tumor. Cancer Res. 2003;63:3567–3574. [PubMed] [Google Scholar]
- 210.Herrmann H., Kneidinger M., Cerny-Reiterer S., Rülicke T., Willmann M., Gleixner K.V., Blatt K., Hörmann G., Peter B., Samorapoompichit P., et al. The Hsp32 Inhibitors SMA-ZnPP and PEG-ZnPP Exert Major Growth-Inhibitory Effects on D34+/CD38+ and CD34+/CD38- AML Progenitor Cells. Curr. Cancer Drug Targets. 2012;12:51–63. doi: 10.2174/156800912798888992. [DOI] [PubMed] [Google Scholar]
- 211.Kinobe R.T., Vlahakis J.Z., Vreman H.J., Stevenson D.K., Brien J.F., Szarek W.A., Nakatsu K. Selectivity of Imidazole-Dioxolane Compounds for in Vitro Inhibition of Microsomal Haem Oxygenase Isoforms. Br. J. Pharmacol. 2006;147:307–315. doi: 10.1038/sj.bjp.0706555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Pittala V., Salerno L., Romeo G., Modica M.N., Siracusa M.A. A Focus on Heme Oxygenase-1 (HO-1) Inhibitors. Curr. Med. Chem. 2013;20:3711–3732. doi: 10.2174/0929867311320300003. [DOI] [PubMed] [Google Scholar]
- 213.Vlahakis J.Z., Kinobe R.T., Bowers R.J., Brien J.F., Nakatsu K., Szarek W.A. Synthesis and Evaluation of Azalanstat Analogues as Heme Oxygenase Inhibitors. Bioorg. Med. Chem. Lett. 2005;15:1457–1461. doi: 10.1016/j.bmcl.2004.12.075. [DOI] [PubMed] [Google Scholar]
- 214.Salerno L., Floresta G., Ciaffaglione V., Gentile D., Margani F., Turnaturi R., Rescifina A., Pittalà V. Progress in the Development of Selective Heme Oxygenase-1 Inhibitors and Their Potential Therapeutic Application. Eur. J. Med. Chem. 2019;167:439–453. doi: 10.1016/j.ejmech.2019.02.027. [DOI] [PubMed] [Google Scholar]
- 215.Ciaffaglione V., Intagliata S., Pittalà V., Marrazzo A., Sorrenti V., Vanella L., Rescifina A., Floresta G., Sultan A., Greish K., et al. New Arylethanolimidazole Derivatives as HO-1 Inhibitors with Cytotoxicity against MCF-7 Breast Cancer Cells. Int. J. Mol. Sci. 2020;21:1923. doi: 10.3390/ijms21061923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Salerno L., Pittalà V., Romeo G., Modica M.N., Marrazzo A., Siracusa M.A., Sorrenti V., Di Giacomo C., Vanella L., Parayath N.N., et al. Novel Imidazole Derivatives as Heme Oxygenase-1 (HO-1) and Heme Oxygenase-2 (HO-2) Inhibitors and Their Cytotoxic Activity in Human-Derived Cancer Cell Lines. Eur. J. Med. Chem. 2015;96:162–172. doi: 10.1016/j.ejmech.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 217.Berberat P.O., Dambrauskas Z., Gulbinas A., Giese T., Giese N., Kunzli B., Autschbach F., Meuer S., Buchler M.W., Friess H. Inhibition of Heme Oxygenase-1 Increases Responsiveness of Pancreatic Cancer Cells to Anticancer Treatment. Clin. Cancer Res. 2005;11:3790–3798. doi: 10.1158/1078-0432.CCR-04-2159. [DOI] [PubMed] [Google Scholar]
- 218.Sass G., Leukel P., Schmitz V., Raskopf E., Ocker M., Neureiter D., Meissnitzer M., Tasika E., Tannapfel A., Tiegs G. Inhibition of Heme Oxygenase 1 Expression by Small Interfering RNA Decreases Orthotopic Tumor Growth in Livers of Mice. Int. J. Cancer. 2008;123:1269–1277. doi: 10.1002/ijc.23695. [DOI] [PubMed] [Google Scholar]
- 219.Cerny-Reiterer S., Meyer R.A., Herrmann H., Peter B., Gleixner K.V., Stefanzl G., Hadzijusufovic E., Pickl W.F., Sperr W.R., Melo J.V., et al. Identification of Heat Shock Protein 32 (Hsp32) as a Novel Target in Acute Lymphoblastic Leukemia. Oncotarget. 2014;5:1198–1211. doi: 10.18632/oncotarget.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Zhou Z., Fang Q., Li P., Ma D., Zhe N., Ren M., Chen B., He Z., Wang J., Zhong Q., et al. Entinostat Combined with Fludarabine Synergistically Enhances the Induction of Apoptosis in TP53 Mutated CLL Cells via the HDAC1/HO-1 Pathway. Life Sci. 2019;232:116583. doi: 10.1016/j.lfs.2019.116583. [DOI] [PubMed] [Google Scholar]
- 221.Zhou Z., Ma D., Liu P., Wang P., Wei D., Yu K., Li P., Fang Q., Wang J. Deletion of HO-1 Blocks Development of B Lymphocytes in Mice. Cell Signal. 2019;63:109378. doi: 10.1016/j.cellsig.2019.109378. [DOI] [PubMed] [Google Scholar]
- 222.Skrzypek K., Tertil M., Golda S., Ciesla M., Weglarczyk K., Collet G., Guichard A., Kozakowska M., Boczkowski J., Was H., et al. Interplay between Heme Oxygenase-1 and MiR-378 Affects Non-Small Cell Lung Carcinoma Growth, Vascularization, and Metastasis. Antioxid. Redox Signal. 2013;19:644–660. doi: 10.1089/ars.2013.5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Tertil M., Golda S., Skrzypek K., Florczyk U., Weglarczyk K., Kotlinowski J., Maleszewska M., Czauderna S., Pichon C., Kieda C., et al. Nrf2-Heme Oxygenase-1 Axis in Mucoepidermoid Carcinoma of the Lung: Antitumoral Effects Associated with down-Regulation of Matrix Metalloproteinases. Free Radic. Biol. Med. 2015;89:147–157. doi: 10.1016/j.freeradbiomed.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 224.Zou C., Cheng W., Li Q., Han Z., Wang X., Jin J., Zou J., Liu Z., Zhou Z., Zhao W., et al. Heme Oxygenase-1 Retards Hepatocellular Carcinoma Progression through the MicroRNA Pathway. Oncol. Rep. 2016;36:2715–2722. doi: 10.3892/or.2016.5056. [DOI] [PubMed] [Google Scholar]
- 225.Hill M., Pereira V., Chauveau C., Zagani R., Remy S., Tesson L., Mazal D., Ubillos L., Brion R., Asghar K., et al. Heme Oxygenase-1 Inhibits Rat and Human Breast Cancer Cell Proliferation: Mutual Cross Inhibition with Indoleamine 2,3-Dioxygenase. FASEB J. 2005;19:1957–1968. doi: 10.1096/fj.05-3875com. [DOI] [PubMed] [Google Scholar]
- 226.Gueron G., Giudice J., Valacco P., Paez A., Elguero B., Toscani M., Jaworski F., Leskow F.C., Cotignola J., Marti M., et al. Heme-Oxygenase-1 Implications in Cell Morphology and the Adhesive Behavior of Prostate Cancer Cells. Oncotarget. 2014;5:4087–4102. doi: 10.18632/oncotarget.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Kim H.N., Park G.H., Park S.B., Kim J.D., Eo H.J., Son H.-J., Song J.H., Jeong J.B. Extracts from Sageretia Thea Reduce Cell Viability through Inducing Cyclin D1 Proteasomal Degradation and HO-1 Expression in Human Colorectal Cancer Cells. BMC Complement. Altern. Med. 2019;19:43. doi: 10.1186/s12906-019-2453-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Bi W., He C.-N., Li X.-X., Zhou L.-Y., Liu R.-J., Zhang S., Li G.-Q., Chen Z.-C., Zhang P.-F. Ginnalin A from Kujin Tea (Acer tataricum subsp. ginnala) Exhibits a Colorectal Cancer Chemoprevention Effect via Activation of the Nrf2/HO-1 Signaling Pathway. Food Funct. 2018;9:2809–2819. doi: 10.1039/C8FO00054A. [DOI] [PubMed] [Google Scholar]
- 229.Tsai C.-F., Chen J.-H., Chang C.-N., Lu D.-Y., Chang P.-C., Wang S.-L., Yeh W.-L. Fisetin Inhibits Cell Migration via Inducing HO-1 and Reducing MMPs Expression in Breast Cancer Cell Lines. Food Chem. Toxicol. 2018;120:528–535. doi: 10.1016/j.fct.2018.07.059. [DOI] [PubMed] [Google Scholar]
- 230.Kim M.J., Yun G.J., Kim S.E. Metabolic Regulation of Ferroptosis in Cancer. Biology. 2021;10:83. doi: 10.3390/biology10020083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Greco G., Catanzaro E., Fimognari C. Natural Products as Inducers of Non-Canonical Cell Death: A Weapon against Cancer. Cancers. 2021;13:304. doi: 10.3390/cancers13020304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Hassannia B., Wiernicki B., Ingold I., Qu F., Van Herck S., Tyurina Y.Y., Bayır H., Abhari B.A., Angeli J.P.F., Choi S.M., et al. Nano-Targeted Induction of Dual Ferroptotic Mechanisms Eradicates High-Risk Neuroblastoma. J. Clin. Investig. 2018;128:3341–3355. doi: 10.1172/JCI99032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Malfa G.A., Tomasello B., Acquaviva R., Genovese C., La Mantia A., Cammarata F.P., Ragusa M., Renis M., Di Giacomo C. Betula Etnensis Raf. (Betulaceae) Extract Induced HO-1 Expression and Ferroptosis Cell Death in Human Colon Cancer Cells. Int. J. Mol. Sci. 2019;20:2723. doi: 10.3390/ijms20112723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Li R., Zhang J., Zhou Y., Gao Q., Wang R., Fu Y., Zheng L., Yu H. Transcriptome Investigation and In Vitro Verification of Curcumin-Induced HO-1 as a Feature of Ferroptosis in Breast Cancer Cells. Oxid. Med. Cell. Longev. 2020;2020:3469840. doi: 10.1155/2020/3469840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Chen P., Li X., Zhang R., Liu S., Xiang Y., Zhang M., Chen X., Pan T., Yan L., Feng J., et al. Combinative Treatment of β-Elemene and Cetuximab Is Sensitive to KRAS Mutant Colorectal Cancer Cells by Inducing Ferroptosis and Inhibiting Epithelial-Mesenchymal Transformation. Theranostics. 2020;10:5107–5119. doi: 10.7150/thno.44705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Zager R.A., Johnson A.C.M., Guillem A., Keyser J., Singh B. A Pharmacologic “Stress Test” for Assessing Select Antioxidant Defenses in Patients with CKD. Clin. J. Am. Soc. Nephrol. 2020;15:633–642. doi: 10.2215/CJN.15951219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Bhutani V.K., Poland R., Meloy L.D., Hegyi T., Fanaroff A.A., Maisels M.J. Clinical Trial of Tin Mesoporphyrin to Prevent Neonatal Hyperbilirubinemia. J. Perinatol. 2016;36:533–539. doi: 10.1038/jp.2016.22. [DOI] [PubMed] [Google Scholar]