Key Points
Question
What is the efficacy of 2 inactivated SARS-CoV-2 vaccines for prevention of symptomatic COVID-19?
Findings
This prespecified interim analysis of a randomized clinical trial included 40 382 participants who received at least 1 dose of a 2-dose inactivated vaccine series developed from either SARS-CoV-2 WIV04 (5 µg/dose) or HB02 (4 µg/dose) strains or an aluminum hydroxide–only control, with a primary end point of the incidence of symptomatic COVID-19 at least 14 days after the second injection. The efficacy for the 2 vaccines, compared with an aluminum hydroxide–only control, was 72.8% in the WIV04 group and 78.1% in the HB02 group; both comparisons were statistically significant.
Meaning
Two inactivated SARS-CoV-2 vaccines demonstrated efficacy against symptomatic COVID-19 compared with an aluminum hydroxide–only control.
Abstract
Importance
Although effective vaccines against COVID-19 have been developed, additional vaccines are still needed.
Objective
To evaluate the efficacy and adverse events of 2 inactivated COVID-19 vaccines.
Design, Setting, and Participants
Prespecified interim analysis of an ongoing randomized, double-blind, phase 3 trial in the United Arab Emirates and Bahrain among adults 18 years and older without known history of COVID-19. Study enrollment began on July 16, 2020. Data sets used for the interim analysis of efficacy and adverse events were locked on December 20, 2020, and December 31, 2020, respectively.
Interventions
Participants were randomized to receive 1 of 2 inactivated vaccines developed from SARS-CoV-2 WIV04 (5 µg/dose; n = 13 459) and HB02 (4 µg/dose; n = 13 465) strains or an aluminum hydroxide (alum)–only control (n = 13 458); they received 2 intramuscular injections 21 days apart.
Main Outcomes and Measures
The primary outcome was efficacy against laboratory-confirmed symptomatic COVID-19 14 days following a second vaccine dose among participants who had no virologic evidence of SARS-CoV-2 infection at randomization. The secondary outcome was efficacy against severe COVID-19. Incidence of adverse events and reactions was collected among participants who received at least 1 dose.
Results
Among 40 382 participants randomized to receive at least 1 dose of the 2 vaccines or alum-only control (mean age, 36.1 years; 32 261 [84.4%] men), 38 206 (94.6%) who received 2 doses, contributed at least 1 follow-up measure after day 14 following the second dose, and had negative reverse transcriptase–polymerase chain reaction test results at enrollment were included in the primary efficacy analysis. During a median (range) follow-up duration of 77 (1-121) days, symptomatic COVID-19 was identified in 26 participants in the WIV04 group (12.1 [95% CI, 8.3-17.8] per 1000 person-years), 21 in the HB02 group (9.8 [95% CI, 6.4-15.0] per 1000 person-years), and 95 in the alum-only group (44.7 [95% CI, 36.6-54.6] per 1000 person-years), resulting in a vaccine efficacy, compared with alum-only, of 72.8% (95% CI, 58.1%-82.4%) for WIV04 and 78.1% (95% CI, 64.8%-86.3%) for HB02 (P < .001 for both). Two severe cases of COVID-19 occurred in the alum-only group and none occurred in the vaccine groups. Adverse reactions 7 days after each injection occurred in 41.7% to 46.5% of participants in the 3 groups; serious adverse events were rare and similar in the 3 groups (WIV04: 64 [0.5%]; HB02: 59 [0.4%]; alum-only: 78 [0.6%]).
Conclusions and Relevance
In this prespecified interim analysis of a randomized clinical trial, treatment of adults with either of 2 inactivated SARS-CoV-2 vaccines significantly reduced the risk of symptomatic COVID-19, and serious adverse events were rare. Data collection for final analysis is pending.
Trial Registration
ClinicalTrials.gov Identifier: NCT04510207; Chinese Clinical Trial Registry: ChiCTR2000034780
This interim analysis of an ongoing randomized trial evaluates the efficacy of 2 inactivated coronavirus vaccines for preventing symptomatic COVID-19 in healthy adults and adverse events after immunization.
Introduction
Effective vaccines against COVID-19 are urgently needed to control the global pandemic. More than 280 candidate vaccines are in development worldwide, 23 of which are already in phase 3 trials using different platforms.1 Among the platforms, inactivated vaccines have been extensively studied. They are generally safe and are widely used for prevention of respiratory infections, such as influenza, as well as other infectious diseases, such as hepatitis A, polio, and rabies.2 The inactivated vaccines also have the advantage of being easily stored and shipped at 2 °C to 8 °C for years, making them suitable for many low-income countries and places with limited cold-storage capacity.
Two inactivated vaccines against COVID-19 have been shown to be generally safe and have induced antibody responses in adults 18 years and older in phase 1/2 trials.3,4 However, their efficacy has yet to be demonstrated and their safety needs to be evaluated in a larger sample size. Therefore, a multicenter phase 3 randomized clinical trial is being conducted to assess the efficacy and safety of these vaccines against COVID-19 in healthy adults. The results presented here represent a prespecified interim analysis of the trial findings.
Methods
Study Design
This double-blind, randomized, phase 3 trial was designed by the Wuhan Institute of Biological Products Co, Ltd, and the Beijing Institute of Biological Products Co, Ltd, both of which belong to the China National Biotec Group Company Limited. The ongoing trial is being performed and data are being collected by the investigators at the Sheikh Khalifa Medical City in Abu Dhabi and the Al Qarain Health Center in Sharjah, both in the United Arab Emirates (UAE); the Salmanyia Medical Complex in Bahrain; Vacsera Medical Center and Katameya Medical Center in Egypt; and Prince Hamza Hospital in Jordan. An independent data and safety monitoring board is monitoring safety data and evaluating risks among the participants. The study protocol (Supplement 1) was approved by the institutional review board of each participating country (Abu Dhabi Health COVID19 Research Ethics Committee, research ethics committee of the UAE Ministry of Health and Prevention, independent research ethics committee at the National Health Regulatory Authority of Bahrain, institutional review board at the Prince Hamza Hospital in Jordan, and research ethics committee at the Central Directorate for Research and Health Development, Egypt Ministry of Health and Population). Written informed consent was obtained from all participants before enrollment.
Study Participants
Adults 18 years and older without prior known history of SARS-CoV, SARS-CoV-2, or Middle East respiratory syndrome infection (via on-site inquiry) were eligible for enrollment. Participants with respiratory symptoms within 14 days before enrollment and those with confirmed or suspected serious respiratory diseases or various acute or chronic diseases that may affect adherence were excluded. Detailed inclusion and exclusion criteria are shown in the eMethods in Supplement 2.
Randomization
Participants were randomly assigned to 1 of the 2 vaccine candidate groups or a control group receiving aluminum hydroxide (alum) adjuvant only (in a 1:1:1 ratio), according to unique serial numbers generated by an independent statistician. A stratified block randomization method was used, with study site as the stratification factor and block size in each stratum of 15. The concealed random grouping allocation and blind codes were kept in signed and sealed envelopes and were blinded to the investigators, participants, and statisticians. The participants received 2 intramuscular injections 21 days apart.
Interventions
Development of the 2 inactivated vaccines was described previously.3,4,5,6 In brief, 2 SARS-CoV-2 strains (WIV04 and HB02) were isolated from 2 patients in Jinyintan Hospital, Wuhan, China, and separately used to develop the 2 vaccines (referred to as WIV04 and HB02 vaccines hereafter). The virus strains were cultivated in qualified Vero cell lines for proliferation, followed by purification and β-propanolide inactivation. The WIV04 vaccine was adsorbed with 0.5 mg of alum and the HB02 was adsorbed with 0.45 mg of alum, and each was packed into prefilled syringes in 0.5-mL sterile phosphate-buffered saline without preservative. The total protein content, determined by the Lowry method, was 5 μg in the WIV04 vaccine and 4 μg in the HB02 vaccine. Because the final product of the vaccines contained adjuvant, same-dose alum adjuvant (0.5 mg) was selected as placebo control and packed into prefilled syringes in 0.5 mL of sterile phosphate-buffered saline without preservative. The vaccines and controls were approved by the National Institutes for Food and Drug Control of China, and were supplied in coded, identical-appearing, single-dose vials.
Assessments
Participants were educated about COVID-19 and related symptoms and were monitored through active follow-up by the investigators (phone call every week and face-to-face examination at the time of vaccine injection), active reports of the participants and sentinel hospitals, and passive linkage to local medical systems. Suspected COVID-19 cases were defined if participants had (1) at least 2 of the following symptoms lasting for at least 2 days: fever (axillary temperature ≥37.5 °C), chills, sore throat, stuffy nose, myalgia, fatigue, headache, nausea or vomiting, or diarrhea or (2) at least 1 respiratory sign or symptom (including cough, shortness of breath), new olfactory or taste disorder, or radiographic evidence of COVID-19–like pneumonia. Participants with SARS-CoV-2 infection–like symptoms were requested to come to the designated hospitals immediately for laboratory tests, including real-time reverse transcriptase–polymerase chain reaction (RT-PCR) assay of nasal or nasopharyngeal swab specimens and specific IgG antibody tests of serum samples by commercial ELISA (enzyme-linked immunosorbent assay) kits. A laboratory-confirmed case was defined if the patient had a positive RT-PCR test result. Cases were diagnosed and severity status was categorized as mild, moderate, severe, or critical according to the Diagnosis and Treatment Scheme for COVID-19 released by the National Health Commission of China,7 with modifications from the World Health Organization (WHO) and the US Centers for Disease Control and Prevention criteria (eMethods in Supplement 2).
An end point adjudication committee (EAC) in the UAE comprising 3 content experts independently determined case diagnosis and severity. The results were then submitted to a separate EAC in China comprising 5 experts to make the final decision. EAC members were unaware of group assignment at the time of adjudication. Adjudication of suspected cases is shown in a flow diagram in eFigure 1 in Supplement 2. Nasopharyngeal swabs for RT-PCR testing were collected from all participants before each dose and on day 14 following the second dose. The local medical systems were also linked to identify participants with symptomatic and asymptomatic COVID-19. Asymptomatic cases were defined as those in which patients had positive PCR test results indicating virologic evidence of SARS-CoV-2 infection but no prespecified symptoms.
Efficacy End Points
The primary efficacy end point was laboratory-confirmed symptomatic COVID-19 cases that occurred at least 14 days after receipt of the second vaccine dose. The secondary efficacy end point was severe cases of COVID-19 and/or death occurring at least 14 days after receipt of the second dose. Three exploratory post hoc efficacy analyses included asymptomatic cases of COVID-19 after day 14 following the second dose, cases that occurred between the first injection and day 14 following the second dose, and cases by subgroup (site, sex, age, baseline IgG status).
Safety End Points
Participants were requested to record any injection site–specific adverse reactions (eg, pain, redness, swelling) and systemic adverse reactions (eg, fever, headache, fatigue) on diary cards within 7 days of each injection. Any other unsolicited symptoms and signs were also recorded during follow-up. The grading criteria of adverse events and the relationship with receipt of an injection were decided by the investigators before unblinding, and details are shown in the protocol (Supplement 1) as well as previous publications from the phase 1/2 trials.3,4
Exploratory Immunogenicity End Point
Blood samples were collected before the first injection and on day 14 after the second injection. Serum samples of the first 900 participants in the 3 centers (Sheikh Khalifa Medical City, Abu Dhabi, and Al Qarain Health Center, Sharjah, UAE; the Salmanyia Medical Complex in Bahrain) were selected as the immunogenicity subcohort for neutralization capacity testing using infectious SARS-CoV-2 virus (strain 19nCoV-CDC-Tan-Strain04 [QD01]) by the 50% cell culture infectious dose. Details of the immunogenicity assays were described previously4 and are provided in the eMethods in Supplement 2. A positive antibody response (seroconversion) was defined as postinjection titer of at least 1:16 if the baseline titer was below 1:4 or at least a 4-fold increase in postinjection titer from baseline if the baseline titer was at least 1:4.
Sample Size and Power Analysis
Sample size was calculated according to the WHO recommendation of achieving 150 cases across the vaccine and control groups, with a target vaccine efficacy of 60% and a lower bound of 30%.8 The probability of type I error was set at 1-sided 0.025 and the statistical power was 0.90. Based on the estimated incidence rate of 850 per 100 000 person-years in the study sites, a total of 44 488 participants were required, anticipating a 15% dropout rate.9 Details of the sample size calculation are shown in the protocol (Supplement 1).
Statistical Analysis
The statistical analysis plan is included in Supplement 3. To accelerate the vaccine application process, 2 interim analyses were planned in the protocol when the combined number of incident cases of COVID-19 in the alum-only group and either of the vaccine groups reached 50 (one-third of the planned cases required for final analysis) or 100 (two-thirds of the planned cases), as recommended by WHO.8 In the interim analysis, the O’Brien-Fleming spending function10 was used to control the family-wise type I error to be within a 2-sided α of .05. With the planned cases of 50 or 100, the nominal significance level was calculated to be .0001 (1-sided) and .006 (1-sided) for the first and second interim analyses. However, the precise nominal significance level used was to be calculated based on the observed cases (eMethods in Supplement 2). Based on the number of events at the time of the analyses reported here, the significance threshold was set at .0124.
Poisson regression was used to calculate the efficacy of the 2 vaccine groups compared with the alum-only group, with the number of incident cases as the dependent variable, treatment group as the independent variable, and the person-years as the offset. The primary efficacy analysis was performed on data from a modified full analysis population, which included participants who received 2 doses, contributed at least 1 efficacy follow-up visit after day 14 following the second dose, and had negative RT-PCR test results at enrollment. Given the size of the study population of this randomized clinical trial, missing covariates at baseline were not likely to have affected the primary efficacy calculation and were not imputed. Participants who withdrew from the trial were censored at the time of loss to follow-up.
A number of prespecified sensitivity analyses were performed to test the robustness of the results. First, efficacy was calculated among participants who received at least 1 injection, contributed at least 1 efficacy follow-up visit after the first dose, and had negative RT-PCR test results at enrollment (full analysis population-1); among participants who received at least 2 injections and contributed at least 1 efficacy follow-up visit regardless of the RT-PCR test results at enrollment (modified full analysis population-2); and among participants who completely followed the protocol (per-protocol population-1 and -2). Definitions of and the number of participants in each analysis are shown in eTable 1 in Supplement 2.
Three post hoc exploratory analyses were also performed. First, asymptomatic cases after 14 days following the second dose were included in the efficacy calculation. Second, symptomatic cases that occurred between the first injection and day 14 following the second dose were included in the efficacy calculation, with the purpose of testing the overall efficacy against symptomatic cases after the first dose. Third, subgroup analyses were performed by baseline characteristics, including study sites, sex, age groups (aged <60 and ≥60 years), and baseline IgG status. Tests for interaction among subgroups were not conducted due to limited power.
Incidence rates of adverse events and reactions in each group are described in the total cohort of participants who received at least 1 injection. The exploratory immunogenicity analysis was performed in the per-protocol immunogenicity subcohort. χ2 tests or Fisher exact tests (when data were sparse) were used to analyze categorical data, and t tests or Mann-Whitney U tests (for nonnormally distributed data) were used to analyze log-transformed antibody titers between vaccine and control groups. The significance threshold for the secondary and immunogenicity end points was set at 2-sided .05, but because of the potential for type I error, these analyses should be interpreted as exploratory. Analyses were conducted by independent statisticians using SAS software, version 9.4 (SAS Institute Inc).
Results
Study Participants
At the time of data set lock for the interim analysis on December 20, 2020, data on incident cases were not yet available from the Egypt and Jordan sites, where enrollment began later and totaled 3469 participants; thus, the 2 sites were not included in the current analysis. Follow-up data are continuing to be collected at all study sites, and data from the Egypt and Jordan sites will be included in the final analysis.
In the UAE and Bahrain sites, 46 270 volunteers were screened for qualification and 40 411 were randomized: 13 470 to the WIV04 group, 13 470 to the HB02 group, and 13 471 to the alum-only group (Figure 1). Among all participants, 40 382 received the first dose (13 459 in the WIV04 group, 13 465 in the HB02 group, and 13 458 in the alum-only group). Of these participants, 39 223 received the second dose (13 066 in the WIV04 group, 13 086 in the HB02 group, and 13 071 in the alum-only group), among whom 38 206 (12 743 in the WIV04 group, 12 726 in the HB02 group, and 12 737 in the alum-only group) had negative PCR test results before the first dose and were included in the modified full analysis population for the primary efficacy analysis.
Figure 1. Flow of Participants in a Study of the Effect of 2 Inactivated SARS-CoV-2 Vaccines on Symptomatic COVID-19 Infection in Adults.
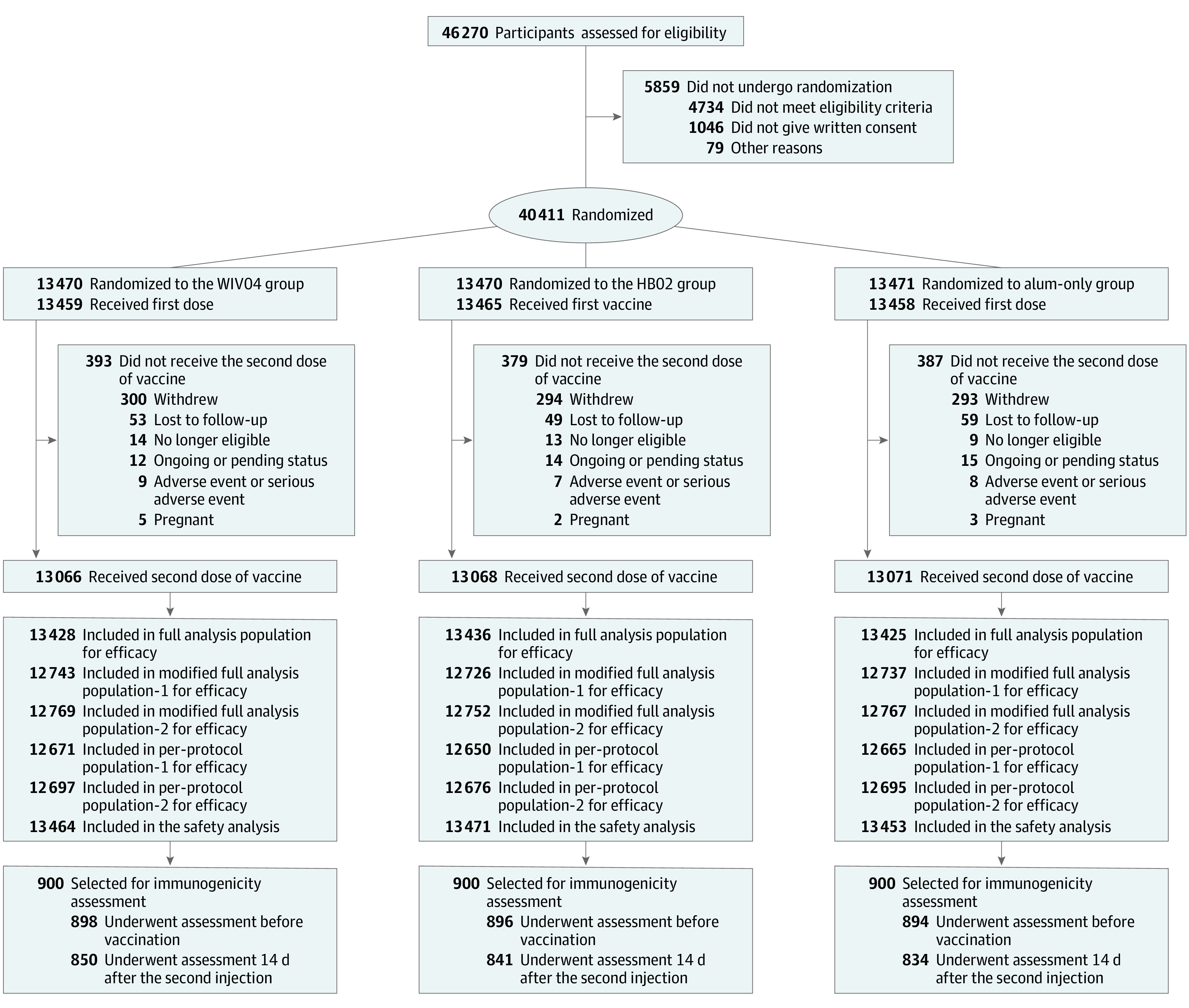
See eTable 1 in Supplement 2 for definitions of each analysis population. Among those receiving aluminum hydroxide (alum) for the first dose, 5 participants received WIV04 (n = 2) and HB02 (n = 3) vaccines for the second dose and were not included in the safety analysis population of the alum-only group (13 458 – 5 = 13 453), but were included in the WIV04 and HB02 groups, respectively. Three participants who received WIV04 for the first dose and HB02 for the second dose and 3 participants who received HB02 for the first dose and WIV04 for the second dose were included in both groups (WIV04: 13 459 + 2 + 3 = 13 464; HB02: 13 465 + 3 + 3 = 13 471). To measure neutralization antibody levels, the first 900 participants from each study site were selected. There were 9 participants in the WIV04 group, 7 in the HB02 group, and 8 in alum-only group who had adverse events or serious adverse events (SAEs) after the first dose and did not receive the second dose, and only 1 SAE in the HB02 group was deemed to be vaccine-related. See eTables 7 and 8 in Supplement 3 for a list of serious adverse events.
Baseline characteristics in the modified full analysis population are shown in Table 1. The mean (SD) age of participants was 36.1 (9.3) years, and 32 261 (84.4%) were men. A total of 25 634 participants (67.1%) were recruited from the study site in Abu Dhabi and 5135 (13.4%) from the study site in Sharjah, both in the UAE, and 7437 (19.5%) were recruited from the study site in Bahrain. Participant countries of origin included the UAE (23.6%), India (13.9%), Bangladesh (10.0%), China (9.7%), Pakistan (9.4%), Bahrain (7.1%), Egypt (5.1%), Philippine (3.9%), Nepal (2.3%), and Syria (2.2%). The demographics in the study samples approximated the population structure in the UAE, where 72.0% of individuals are males and 88.5% are expatriates; the male predominance of trial participants likely also reflected exclusion of pregnant women and women with intention of pregnancy. Among the participants, 30 035 underwent IgG antibody tests before receiving the first dose and 1925 (6.4%) had positive results. Baseline characteristics of participants who received at least 1 dose (safety analysis set) are shown in eTable 2 in Supplement 2, and similar patterns were found as with the modified full analysis population. Daily counts of administered doses at UAE and Bahrain trial sites and daily national number of incident COVID-19 cases from July 16, 2020, to December 20, 2020, are shown in eFigure 2 in Supplement 2.
Table 1. Baseline Characteristics of Participants in a Study of the Effect of 2 Inactivated SARS-CoV-2 Vaccines on Symptomatic COVID-19 Infection in Adultsa.
| Characteristic | No. (%) | ||
|---|---|---|---|
| WIV04 vaccine group (n = 12 743) | HB02 vaccine group (n = 12 726) | Alum-only group (n = 12 737) | |
| Age, mean (SD), y | 36.2 (9.2) | 36.1 (9.3) | 36.1 (9.3) |
| Age groups | |||
| <60 y | 12 530 (98.3) | 12 525 (98.4) | 12 539 (98.4) |
| ≥60 y | 213 (1.7) | 201 (1.6) | 198 (1.6) |
| Sexb | |||
| Male | 10 706 (84.0) | 10 750 (84.5) | 10 805 (84.8) |
| Female | 2037 (16.0) | 1976 (15.5) | 1932 (15.2) |
| Study sites | |||
| Abu Dhabi | 8552 (67.1) | 8538 (67.1) | 8544 (67.1) |
| Sharjah | 1705 (13.4) | 1717 (13.5) | 1713 (13.4) |
| Bahrain | 2486 (19.5) | 2471 (19.4) | 2480 (19.5) |
| National originb | |||
| United Arab Emirates | 3040 (23.9) | 2992 (23.5) | 2978 (23.4) |
| India | 1787 (14.0) | 1781 (14.0) | 1724 (13.5) |
| Bangladesh | 1332 (10.4) | 1228 (9.6) | 1278 (10.0) |
| China | 1247 (9.8) | 1219 (9.6) | 1245 (9.8) |
| Pakistan | 1159 (9.1) | 1189 (9.3) | 1248 (9.8) |
| Bahrain | 875 (6.9) | 921 (7.2) | 899 (7.1) |
| Egypt | 660 (5.2) | 653 (5.1) | 647 (5.1) |
| Philippines | 476 (3.7) | 501 (3.9) | 504 (4.0) |
| Nepal | 280 (2.2) | 279 (2.2) | 319 (2.5) |
| Syria | 261 (2.0) | 292 (2.3) | 287 (2.3) |
| Others | 1626 (12.8) | 1671 (13.1) | 1608 (12.6) |
| Height, mean (SD), cmb | 170.5 (8.4) [n = 12 736] | 170.7 (8.4) [n = 12 717] | 170.6 (8.4) [n = 12 731] |
| Weight, mean (SD), kgb | 78.6 (16.8) [n = 12 736] | 78.9 (16.9) n = [12 717] | 78.7 (16.8) [n = 12 731] |
| BMI, mean (SD)b | 27.0 (5.0) [n = 12 736] | 27.0 (5.1) [n = 12 717] | 27.0 (5.0) [n = 12 731] |
| Positive baseline IgG antibodyc | 640/10 065 (6.4) | 666/9969 (6.7) | 619/10 001 (6.2) |
Abbreviations: alum, aluminum hydroxide; BMI, body mass index.
Modified full analysis population-1 included those who received 2 doses, contributed at least 1 efficacy follow-up visit, and had negative polymerase chain reaction test results at enrollment.
There were 7 participants in the WIV04 group, 9 in the HB02 group, and 6 in the alum-only group who had missing information on height, weight and body mass index.
There were 2678 participants in the WIV04 group, 2757 in the HB02, and 2736 in the alum-only group who had missing information on IgG antibody levels.
Primary Efficacy End Point
On November 12, 2020, the first interim analysis was performed with a total of 70 incident cases. On December 20, 2020, the efficacy data set was locked and a second interim analysis was planned. Details of the 2 interim analyses and the nominal significance levels calculated by the O’Brien-Fleming spending function are described in the eMethods in Supplement 2.
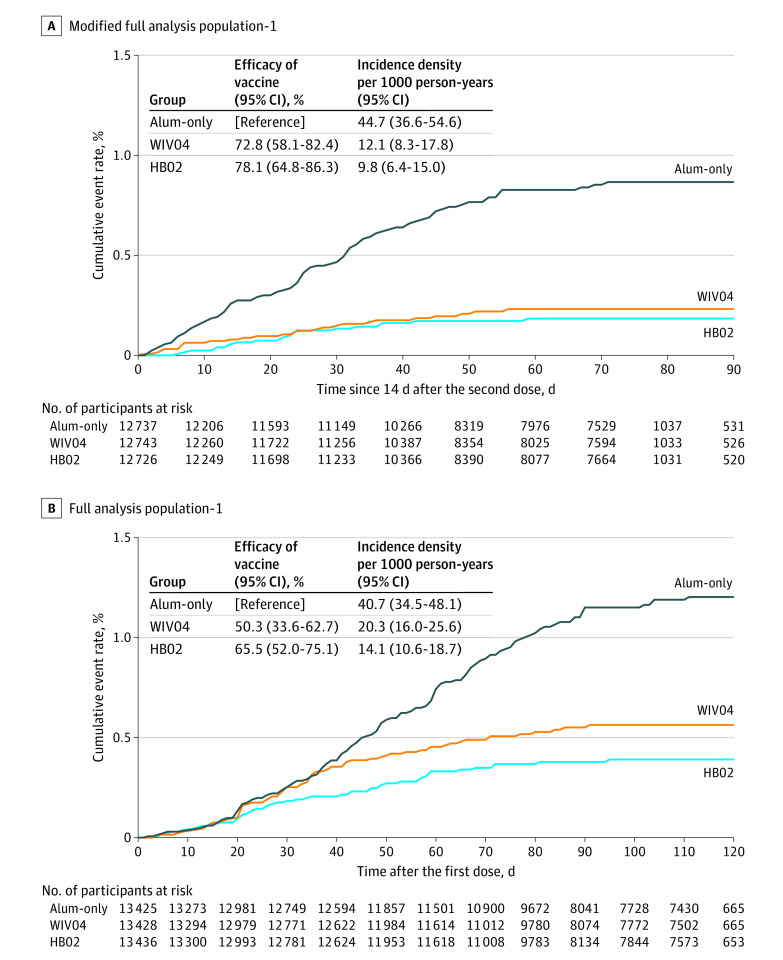
As of December 20, 2020, participants had a median (range) follow-up duration of 77 (1-121) days, beginning 14 days after the second dose (the beginning of the primary outcome case monitoring period). A total of 962 participants had prespecified SARS-CoV-2 infection–like symptoms after receiving the first dose and were subsequently assessed for case diagnosis and adjudication (eFigure 1 in Supplement 2). After review by the 2 EACs, 255 symptomatic COVID-19 cases were confirmed, with 142 in the primary case monitoring period and 113 outside of the monitoring period. Among the 142 cases in the monitoring period, 26 were in the WIV04 group, 21 were in the HB02 group, and 95 were in the alum-only group (Table 2; Figure 2A). The incidence rate (per 1000 person-years) was 12.1 (95% CI, 8.3-17.8) in the WIV04 group, 9.8 (95% CI, 6.4-15.0) in the HB02 group, and 44.7 (95% CI, 36.6-54.6) in the alum-only group. This resulted in a vaccine efficacy, compared with the alum-only group, of 72.8% ([95% CI 58.1%-82.4%]; P < .001) for the WIV04 group and 78.1% ([95% CI, 64.8%-86.3%]; P < .001) for the HB02 group (Table 2 Figure 2A). Efficacy was similar in the sensitivity analyses among various study populations (eTable 3 in Supplement 2).
Table 2. Incident COVID-19 Cases and Vaccine Efficacy 14 Days After 2 Doses of Immunizationa.
| Outcome | WIV04 vaccine group | HB02 vaccine group | Alum-only group |
|---|---|---|---|
| Primary analysis: incident symptomatic cases | |||
| No. of participants | 12 743 | 12 726 | 12 737 |
| No. of incident cases | 26 | 21 | 95 |
| Person-years | 2140.2 | 2143.3 | 2125.6 |
| Incidence density per 1000 person-years (95% CI) | 12.1 (8.3-17.8) | 9.8 (6.4-15.0) | 44.7 (36.6-54.6) |
| Vaccine efficacy (95% CI), % | 72.8 (58.1-82.4) | 78.1 (64.8-86.3) | [Reference] |
| Secondary analysis: incident severe cases | |||
| No. of participants | 12 743 | 12 726 | 12 737 |
| No. of incident cases | 0 | 0 | 2 |
| Person-years | 2140.2 | 2143.3 | 2125.6 |
| Incidence density per 1000 person-years (95% CI) | 0 (NA) | 0 (NA) | 9 (1.0-34.0) |
| Vaccine efficacy (95% CI), % | 100 (NA) | 100 (NA) | [Reference] |
| Post hoc analysis: incident symptomatic and asymptomatic casesb | |||
| No. of participants | 12 727 | 12 713 | 12 722 |
| No. of incident cases | 42 | 31 | 116 |
| Person-years | 2135.5 | 2139.6 | 2121.0 |
| Incidence density per 1000 person-years (95% CI) | 19.7 (14.5-26.6) | 14.5 (10.2-20.6) | 54.7 (45.6-65.6) |
| Vaccine efficacy (95% CI), % | 64.0 (48.8-74.7) | 73.5 (60.6-82.2) | [Reference] |
Abbreviations: alum, aluminum hydroxide; NA, not applicable.
The analyses were conducted in the modified full analysis population-1, which included those who received 2 doses, contributed at least 1 efficacy follow-up visit, and had negative polymerase chain reaction test results at enrollment. A Poisson regression model with log-link function was used, with the number of incident cases as the dependent variable, treatment group as the independent variable, and person-years as the offset. Incidence density with its 95% CI was estimated using the least-square method. If the number of cases in any of the groups was less than 5, the exact method was used to estimate the incidence rate, vaccine efficacy, and 95% CI using StatXact software.
Forty-four participants were excluded from the analysis because they had asymptomatic COVID-19 between the first dose and 14 days after the second dose.
Figure 2. Efficacy of 2 Inactivated Vaccines Against Symptomatic COVID-19.
A, Cumulative event rates of confirmed symptomatic COVID-19 cases 14 days following a second vaccine dose among participants who received 2 doses, contributed at least 1 efficacy follow-up visit, and had negative polymerase chain reaction test results at enrollment (modified full analysis population-1). Median (interquartile range) observation times for all groups was 77 (47-78) days. B, Cumulative event rates of confirmed symptomatic COVID-19 cases after the first dose among participants who received at least 1 dose, contributed at least 1 efficacy follow-up visit, and had negative polymerase chain reaction test results at enrollment (full analysis population-1). Median (interquartile range) observation times for all the groups were 112 (82-113) days. Alum indicates aluminum hydroxide.
Secondary Efficacy End Point
Among the incident cases of COVID-19, 2 severe cases were identified in the alum-only group and none were identified in the 2 vaccine groups, resulting in an efficacy of 100% in the vaccine groups against severe COVID-19 (Table 2). However, these results should be interpreted with caution given the small number of incident severe cases. No mortality events occurred during follow-up.
Post Hoc Analyses
A total of 47 asymptomatic cases of COVID-19 were identified 14 days following the second dose: 16 in the WIV04 group, 10 in the HB02 group, and 21 in the alum-only group. Thus, the total number of cases of COVID-19 (including both symptomatic and asymptomatic cases) was 42 in the WIV04 group, 31 in the HB02 group, and 116 in the alum-only group. A post hoc analysis adding the asymptomatic cases resulted in a vaccine efficacy of 64.0% (95% CI, 48.8%-74.7%) for the WIV04 vaccine and 73.5% (95% CI 60.6%-82.2%) for the HB02 vaccine (Table 2).
A total of 113 symptomatic cases of COVID-19 were identified between receipt of the first dose and 14 days after receipt of the second dose: 43 in the WIV04 group, 27 in the HB02 group, and 43 in the alum-only group (Figure 2B). Thus, the total number of cases after the first dose was 69 in the WIV04 group, 48 in the HB02 group, and 138 in the alum-only group. A post hoc analysis that additionally included those cases resulted in a vaccine efficacy of 50.3% (95% CI 33.6%-62.7%) for the WIV04 vaccine and 65.5% (95% CI 52.0%-75.1%) for the HB02 vaccine against symptomatic cases of COVID-19 after the first dose. The incidence rate was similar across groups until the date of the second dose for the HB02 group and until 14 days after the second dose for the WIV04 group, compared with the alum-only group, after which the incidence rates were lower in the vaccine groups compared with the alum-only group.
In post hoc subgroup analyses, similar efficacy rates were observed at different study sites and for men and women (eTable 4 in Supplement 2). Participants 60 years and older were recruited starting late October 2020; there were 213 participants in the WIV04 group, 201 in the HB02 group, and 198 in the alum-only group. No incident cases of COVID-19 occurred in either group, thus efficacy was not calculated. Among participants with positive IgG antibody test results at enrollment (640 in the WIV04 group, 666 in the HB02 group, and 619 in the alum-only group), only 1 incident case occurred in the alum-only group and efficacy was not calculated. Interpretation of these subgroup findings is limited in the absence of tests for interaction, which could not be conducted because of limited statistical power.
Exploratory Immunogenicity End Points
The geometric mean titers of the neutralizing antibody at baseline were 2.3 in the 2 vaccine groups and 2.4 in the alum-only group, while the geometric mean titers on day 14 after the second dose were 94.5 (95% CI, 89.7-99.5) in the WIV04 group, 156.0 (95% CI, 149.6-162.7) in the HB02 group, and 2.7 (95% CI, 2.6-2.8) in the alum-only group (Table 3). The seroconversion rate was 99.3% in the WIV04 group, 100.0% in the HB02 group, and 2.3% in the alum-only group.
Table 3. Neutralizing Antibodies to Live SARS-CoV-2 Before the First Dose and 14 Days After the Second Dosea.
| Neutralizing antibodies to live SARS-CoV-2 | WIV04 vaccine group | HB02 vaccine group | Alum-only group |
|---|---|---|---|
| Geometric mean titers (95% CI) | |||
| Before the first dose | 2.3 (2.2-2.4) [n = 898] | 2.3 (2.2-2.3) [n = 896] | 2.4 (2.3-2.5) [n = 894] |
| 14 d after the second dose | 94.5 (89.7-99.5) [n = 850] | 156.0 (149.6-162.7) [n = 841] | 2.7 (2.6-2.8) [n = 834] |
| Geometric mean ratio (95% CI) | 41.0 (38.9-43.2) [n = 848] | 68.7 (65.5-72.1) [n = 837] | 1.1 (1.1-1.1) [n = 828] |
| Seroconversion rate (95% CI), % | 99.3 (98.5-99.7) [n = 848] | 100.0 (99.6-100.0) [n = 837] | 2.3 (1.4-3.6) [n = 828] |
Abbreviation: alum, aluminum hydroxide.
Geometric mean ratio was calculated as the geometric mean titer on day 14 after the second dose over the geometric mean titer before the first dose. Seroconversion was defined as postinjection titer of at least 1:16 if the baseline titer was below 1:4 or at least a 4-fold increase in postinjection titer from baseline if the baseline titer was at least 1:4. The 95% CIs were calculated using the Clopper-Pearson method. Geometric mean ratio and seroconversion were calculated in participants with data both at baseline and on day 14 after the second dose.
Adverse Reactions and Events
Systemic and injection site reaction symptoms (according to solicited reports) are shown in Figure 3 and eTable 5 in Supplement 2. Within 7 days after each injection, total adverse reactions were reported by 5957 participants (44.2%) in the WIV04 group, 5623 (41.7%) in the HB02 group, and 6250 (46.5%) in the alum-only group. The most common adverse reaction in the WIV04 group, HB02 group, and alum-only group was pain at the injection site (24.3%, 19.4%, and 27.9%), followed by headache (12.9%, 13.1%, and 12.6%). Most adverse reactions were mild in severity (grade 1 or 2) and were transient and self-limiting, without need for special treatment. During 8 to 28 days after injection, solicited adverse reactions did not increase, while unsolicited adverse reactions increased from 11.1% to 16.1% in the WIV04 group, 10.7% to 15.5% in the HB02 group, and 10.6% to 15.4% in the alum-only group.
Figure 3. Common Adverse Reactions and Grades Within 7 Days After 2 Doses in the Safety Analysis Set.
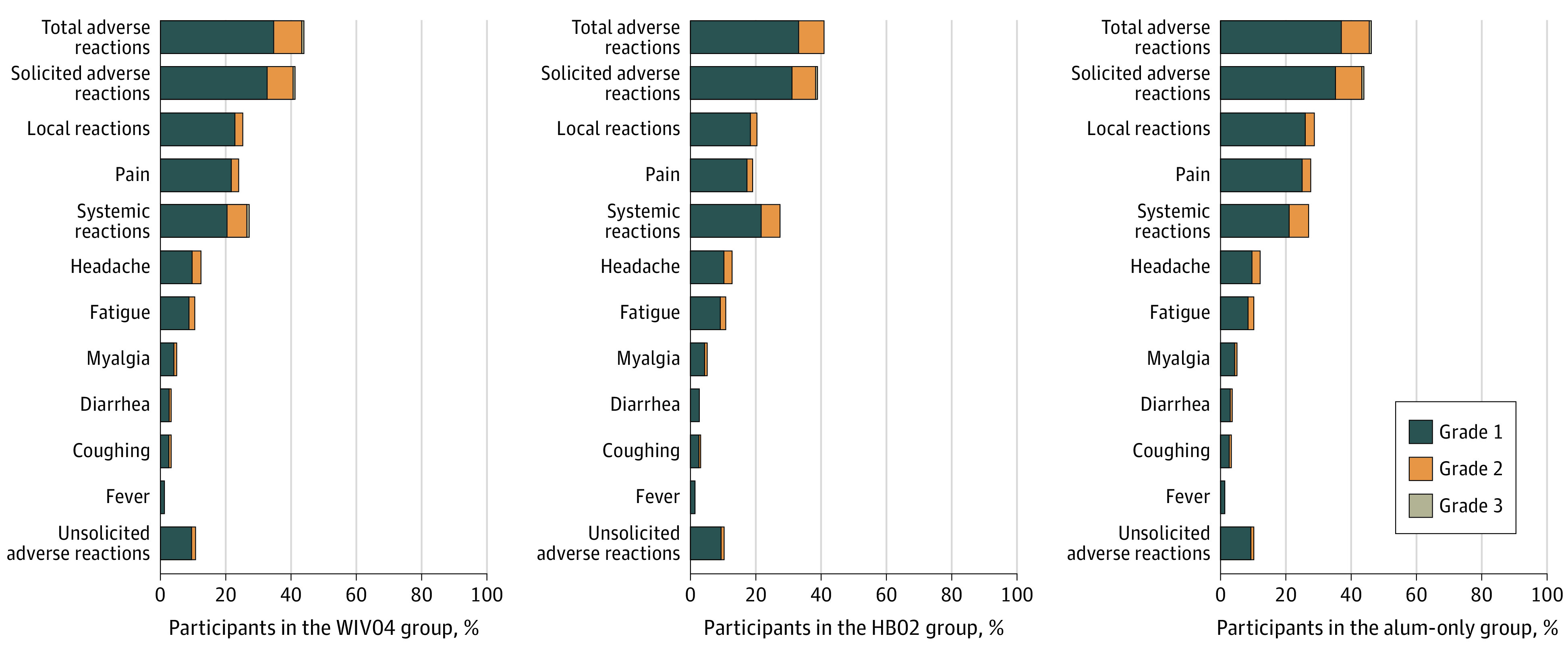
The safety analysis population included all participants who received at least 1 dose. Only adverse reactions that occurred in at least 2% of participants are included; see eTable 5 in Supplement 2 for details of all adverse reactions (including common and less common adverse reactions). Participants with more than 1 adverse reaction in a specific reaction category were only counted once; for example, if they had the same symptom (eg, injection-site pain) after each dose or if they had more than 1 symptom in the reaction class (total, systemic, and local), they were only counted once in that adverse reaction class. Participants with both lower- and higher-grade adverse events were counted once in the higher-grade total adverse events. Grading scales for systemic and local adverse events are detailed in the protocol in Supplement 1. Alum indicates aluminum hydroxide.
Unsolicited adverse events (related or unrelated to injection) are shown in eTable 6 in Supplement 2; total adverse events were reported by 48.3% of the participants in the WIV04 group, 46.1% in the HB02 group, and 50.5% in the alum-only group. A total of 201 serious adverse events occurred during follow-up with similar rates in the 3 groups: 0.5% in the WIV04 group, 0.4% in the HB02 group, and 0.6% in the alum-only group (eTable 7 in Supplement 2). Two adverse events were considered to be possibly related to the injections, and both were in the HB02 group (eTable 8 in Supplement 2): a man aged 30 years was diagnosed with possible demyelinating myelitis after receiving the first dose, but later pathophysiological tests excluded the possibility of multiple sclerosis and identified that the man was heterozygous for very long-chain acyl-CoA dehydrogenase deficiency variant, and a woman aged 35 years had severe emesis (grade 3) after receiving the second dose, which resulted in emergency visits, but was relieved after receiving ondansetron.
Discussion
In this phase 3 randomized trial in adults, 2 whole-virus inactivated vaccines showed efficacy of 72.8% and 78.1% against symptomatic COVID-19 cases. The 2 vaccines had rare serious adverse events at a frequency similar to the alum-only control, and the majority were not related to the vaccinations. An exploratory analysis found that the 2 vaccines induced measurable neutralizing antibodies, similar to results in the phase 1/2 trials.4,5
Interim results of phase 3 trials of vaccines using other platforms have been recently published, including the 2 mRNA vaccines (BNT162b2 and mRNA-1273) and 3 adenoviral vector-based vaccines (ChAdOx1 nCoV-19, Gam-COVID-Vac [Sputnik V], and Ad26.COV2.S).11,12,13,14,15 Efficacy was reported to be 95.0% for the BNT162b2 (Pfizer-BioNTech) vaccine; 94.1% for mRNA-1273 (Moderna) vaccine; 62.1% and 90.0% for the ChAdOx1 nCoV-19 vaccine, depending on the dose scheme (2 standard doses or a low dose followed by a standard dose); 91.6% for the Gam-COVID-Vac vaccine; and 66.9% for the Ad26.COV2.S vaccine. All vaccines, including the 2 inactivated vaccines examined in the current study, have met the WHO and US Food and Drug Administration recommendations of being at least 50% more effective than the inactive comparator.16 A direct comparison of the point estimates of the efficacy cannot be made because differences in efficacy could be due to variations in the manufacture platforms (and underlying mechanisms of how different vaccines work in human body),2 characteristics of the study populations, infection prevalence, and predominant virus variants at study sites. The current trial was conducted in the Middle East, where the SARS-CoV-2 incidence rate (44.7 per 1000 person-years in the alum-only group) was lower than in other studies (79.8 per 1000 person-years in the mRNA-1273 trial,11 72.9 per 1000 person-years in the BNT162b2 trial,12 149.2 per 1000 person-years in the ChAdOx1 nCoV-19 trial,14 , and 112.4 per 1000 person-years Ad26.COV2.S trial15). The current study included mainly healthy and younger adults from Middle Eastern and other Asian countries, while the other studies primarily included populations from Western countries and had a higher proportion of older participants.
An increasing concern is whether the investigational vaccines can protect against emerging SARS-CoV-2 variants,17,18,19,20,21 particularly the variants first detected in the UK (B.1.1.7 lineage [501Y.V1]), South Africa (B.1.351 lineage [501Y.V2]), and Brazil (P.1 lineage [501Y.V3]), with variations in the S gene. A recent study found no protection of the ChAdOx1 nCoV-19 vaccine against the B.1.351 variant.22 The efficacy of the 2 inactivated vaccines against these emerging variants could not be tested given that the variants were not common in the Middle Eastern countries at the time of the trial. A 2021 study assessed the neutralizing activity of serum samples from participants who received 2 doses of the HB02 vaccine against emerging SARS-CoV-2 variants.23 Results of the study found similar or slightly higher neutralizing capacity of the HB02 vaccinee serum samples against the D614G and B.1.1.7 variants compared with wild-type pseudovirus, but the B.1.351 variant showed more resistance to the neutralization of vaccinee serum (by a factor of 2.5) than the wild-type virus. In the same study, another inactivated vaccine, the CoronaVac, was also tested, and similar results were found.23 More studies are needed to investigate the neutralizing capacity as well as the efficacy of the inactivated vaccines against emerging SARS-CoV-2 variants with increased transmission.
Limitations
This study has several limitations. First, the study did not include pregnant women or those younger than 18 years; thus, the efficacy and safety of the inactivated vaccines in these groups remain unknown. Results from phase 1/2 trials among these groups have not been reported, but evaluation of the safety and immunogenicity of the 2 vaccines among children and adolescents is ongoing. Second, the trial was mainly conducted in generally healthy, young men in the Middle East, and there was insufficient power to test the efficacy among those with chronic diseases, women, older adults, those in other geographic populations, and those with previous SARS-CoV-2 infections; this includes people who are most vulnerable to severe COVID-19 cases and mortality.24 Third, there were only 2 severe cases of COVID-19 among participants, so conclusions about prevention of severe cases cannot be made. Fourth, the study could not address the question of whether the inactivated vaccines prevent against asymptomatic infection, which requires formal study-wide surveillance via virologic and serologic tests. Fifth, 2 study sites (Egypt and Jordan) were not included in the current interim analysis because of a data availability issue; the 2 sites will be included in the final analysis of the trial. However, it is unlikely that the results will be materially altered given that only 3469 participants were recruited from the 2 sites. Sixth, the trial is ongoing, and data continue to be collected as specified in the protocol to further refine long-term efficacy estimates, potential adverse events, protection against severe disease and mortality, and durability of immunity. Although no antibody-dependent enhancement phenomenon or vaccine-associated enhanced respiratory disease was observed in the preclinical studies in different animal models,5,6 phase 1/2 trials,3,4 and the current phase 3 trial with a large sample size, longer follow-up is needed to provide firm conclusions about the possibility of vaccine-enhanced illness.
Conclusions
In this prespecified interim analysis of a randomized clinical trial, treatment of adults with either of 2 inactivated SARS-CoV-2 vaccines significantly reduced the risk of symptomatic COVID-19. Data collection for the final analysis is pending.
Trial protocol
Statistical analysis plan
eMethods
eTable 1. Explanation of the Changes in Denominator Numbers in Various Analyses
eTable 2. Baseline Characteristics of the Study Participants in the Safety Set
eTable 3. Efficacy after 14 Days Following the Second Dose: Sensitivity Analysis
eTable 4. Efficacy after 14 Days Following the Second Dose: Subgroup Analysis
eTable 5. Total Adverse Reactions after Two Doses in the Safety Set
eTable 6. Total Adverse Events after Two Doses in the Safety Set
eTable 7. The Number of Serious Adverse Events (SAEs) in the Safety Set
eTable 8. The Number of Serious Adverse Events Considered as Possible Related to Vaccination in the Safety Set
eFigure 1. Handling of the Suspected Cases
eFigure 2. Daily Count of Administered Vaccines and Number of Incident COVID-19 Cases in the United Arab Emirates and Bahrain Between July 16, 2020 and December 20, 2020
Data sharing statement
References
- 1.Draft landscape and tracker of COVID-19 candidate vaccines. World Health Organization . Accessed May 18, 2021. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines
- 2.Kyriakidis NC, López-Cortés A, González EV, Grimaldos AB, Prado EO. SARS-CoV-2 vaccines strategies: a comprehensive review of phase 3 candidates. NPJ Vaccines. 2021;6(1):28. doi: 10.1038/s41541-021-00292-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xia S, Duan K, Zhang Y, et al. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA. 2020;324(10):951-960. doi: 10.1001/jama.2020.15543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xia S, Zhang Y, Wang Y, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21(1):39-51. doi: 10.1016/S1473-3099(20)30831-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang H, Zhang Y, Huang B, et al. Development of an inactivated vaccine candidate, BBIBP-CorV, with potent protection against SARS-CoV-2. Cell. 2020;182(3):713-721. doi: 10.1016/j.cell.2020.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang ZJ, Zhang HJ, Lu J, et al. Low toxicity and high immunogenicity of an inactivated vaccine candidate against COVID-19 in different animal models. Emerg Microbes Infect. 2020;9(1):2606-2618. doi: 10.1080/22221751.2020.1852059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Interim diagnosis and treatment of 2019. Novel Coronavirus Pneumonia (8th edition). National Health Commission of the People’s Republic of China . Accessed March 9, 2021. http://www.gov.cn/zhengce/zhengceku/2020-08/19/content_5535757.htm
- 8.An international randomised trial of candidate vaccines against COVID-19. World Health Organization . Accessed March 17, 2021. https://www.who.int/publications/i/item/an-international-randomised-trial-of-candidate-vaccines-against-covid-19
- 9.Chan ISF, Bohidar NR. Exact power and sample size for vaccine efficacy studies. Commun Stat Theory Methods. 1998;27(6):1305-1322. doi: 10.1080/03610929808832160 [DOI] [Google Scholar]
- 10.Lan KKG, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika 1983;70: 659-663. doi: 10.2307/2336502 [DOI] [Google Scholar]
- 11.Baden LR, El Sahly HM, Essink B, et al. ; COVE Study Group . Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403-416. doi: 10.1056/NEJMoa2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polack FP, Thomas SJ, Kitchin N, et al. ; C4591001 Clinical Trial Group . Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. doi: 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Logunov DY, Dolzhikova IV, Shcheblyakov DV, et al. ; Gam-COVID-Vac Vaccine Trial Group . Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397(10275):671-681. doi: 10.1016/S0140-6736(21)00234-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Voysey M, Clemens SAC, Madhi SA, et al. ; Oxford COVID Vaccine Trial Group . Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99-111. doi: 10.1016/S0140-6736(20)32661-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sadoff J, Gray G, Vandebosch A, et al. ; ENSEMBLE Study Group . Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021. doi: 10.1056/NEJMoa2101544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WHO target product profiles for COVID-19 vaccines. World Health Organization . Accessed March 9, 2021. https://www.who.int/who-documents-detail/who-target-product-profiles-for-covid-19-vaccines
- 17.Liu Y, Liu J, Xia H, et al. Neutralizing activity of BNT162b2-elicited serum. N Engl J Med. 2021;384(15):1466-1468. doi: 10.1056/NEJMc2102017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Supasa P, Zhou D, Dejnirattisai W, et al. Reduced neutralization of SARS-CoV-2 B.1.1.7 variant by convalescent and vaccine sera. Cell. 2021;184(8):2201-2211.e7. doi: 10.1016/j.cell.2021.02.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang P, Nair MS, Liu L, et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature. 2021;593(7857):130-135. doi: 10.1038/s41586-021-03398-2 [DOI] [PubMed] [Google Scholar]
- 20.Wang Z, Schmidt F, Weisblum Y, et al. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature. 2021;592(7855):616-622. doi: 10.1038/s41586-021-03324-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu K, Werner AP, Koch M, et al. Serum neutralizing activity elicited by mRNA-1273 vaccine. N Engl J Med. 2021;384(15):1468-1470. doi: 10.1056/NEJMc2102179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madhi SA, Baillie V, Cutland CL, et al. ; NGS-SA Group Wits–VIDA COVID Group . Efficacy of the ChAdOx1 nCoV-19 Covid-19 vaccine against the B.1.351 variant. N Engl J Med. 2021. doi: 10.1056/NEJMoa2102214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang GL, Wang ZY, Duan LJ, et al. Susceptibility of circulating SARS-CoV-2 variants to neutralization. N Engl J Med. 2021. doi: 10.1056/NEJMc2103022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fakhroo AD, Al Thani AA, Yassine HM. Markers associated with COVID-19 susceptibility, resistance, and severity. Viruses. 2020;13(1):45. doi: 10.3390/v13010045 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
Statistical analysis plan
eMethods
eTable 1. Explanation of the Changes in Denominator Numbers in Various Analyses
eTable 2. Baseline Characteristics of the Study Participants in the Safety Set
eTable 3. Efficacy after 14 Days Following the Second Dose: Sensitivity Analysis
eTable 4. Efficacy after 14 Days Following the Second Dose: Subgroup Analysis
eTable 5. Total Adverse Reactions after Two Doses in the Safety Set
eTable 6. Total Adverse Events after Two Doses in the Safety Set
eTable 7. The Number of Serious Adverse Events (SAEs) in the Safety Set
eTable 8. The Number of Serious Adverse Events Considered as Possible Related to Vaccination in the Safety Set
eFigure 1. Handling of the Suspected Cases
eFigure 2. Daily Count of Administered Vaccines and Number of Incident COVID-19 Cases in the United Arab Emirates and Bahrain Between July 16, 2020 and December 20, 2020
Data sharing statement