Abstract
Simple Summary
Colorectal cancer (CRC) is suggested to be preventable by certain food intakes. Fucoxanthin (Fx) is an anticancer agent contained abundantly in edible brown algae. However, epidemiological studies, in vivo and in vitro experiments for CRC, using Fx and Fx-rich foods, have not been fully outlined. To date, it has been reported that Fx, its metabolite of fucoxanthinol (FxOH) and Fx-rich algal extracts exerted anticancer potentials in human CRC cell lines, their cancer stem-cells-like spheroids and CRC animal models through a number of molecular mechanisms. Moreover, many in vivo experiments and interventional human trials have demonstrated that Fx, Fx-rich algal extracts and brown alga itself may improve CRC and/or certain risks, such as obesity, diabetes, metabolic syndrome, inflammation, oxidation, tumor microenvironment and/or gut microbiota. This review is the first report that summarizes the improving effects by Fx, FxOH and its rich brown algae for CRC and the risk factors.
Abstract
Colorectal cancer (CRC), which ranks among the top 10 most prevalent cancers, can obtain a good outcome with appropriate surgery and/or chemotherapy. However, the global numbers of both new cancer cases and death from CRC are expected to increase up to 2030. Diet-induced lifestyle modification is suggested to be effective in reducing the risk of human CRC; therefore, interventional studies using diets or diet-derived compounds have been conducted to explore the prevention of CRC. Fucoxanthin (Fx), a dietary carotenoid, is predominantly contained in edible brown algae, such as Undaria pinnatifida (wakame) and Himanthalia elongata (Sea spaghetti), which are consumed particularly frequently in Asian countries but also in some Western countries. Fx is responsible for a majority of the anticancer effects exerted by the lipophilic bioactive compounds in those algae. Interventional human trials have shown that Fx and brown algae mitigate certain risk factors for CRC; however, the direct mechanisms underlying the anti-CRC properties of Fx remain elusive. Fx and its deacetylated type “fucoxanthinol” (FxOH) have been reported to exert potential anticancer effects in preclinical cancer models through the suppression of many cancer-related signal pathways and the tumor microenvironment or alteration of the gut microbiota. We herein review the most recent studies on Fx as a potential candidate drug for CRC prevention.
Keywords: fucoxanthin, colorectal cancer, cancer prevention, carotenoid, tumor microenvironment, gut microbiota
1. Introduction
Colorectal cancer (CRC) is a major global cancer, accounting for about 6% of total cases of new cancer (1.1 million per 18.1 million) and cancer death (0.6 million per 9.6 million), as estimated by the GLOBOCAN 2018 database [1]. The incidence and mortality of CRC have been declining in highly developed countries, such as the USA, Australia, Russia and Japan, where an early diagnosis, surgical resection and drug treatments are easy to receive. However, both the global incidence and mortality of CRC are expected to increase by 2030 due to an increasing trend in the CRC burden in many other countries [2].
The diagnosed types of CRC typically include CRC derived from polyp and inflammatory bowel disease (IBD)-derived CRC, with a low incidence of hereditable CRCs, such as Lynch syndrome (about 2–4%), familial adenomatous polyposis (FAP, about 1%), Peutz–Jeghers syndrome and MUTYH-associated polyposis [3]. The 5-year survival rate in CRC is 60–68% with racial diversity in all stages; the survival rate is about 90% when CRC is detected at an early stage before spreading [3]. Convincing and probable risk factors for CRC are as follows: IBD, Lynch syndrome, FAP, Peutz-Jeghers syndrome, MUTYH-associated polyposis, processed meat, alcoholic drinks, body fatness, adult attained height and red meat [4,5,6,7,8]. The key triggers involved in human colorectal carcinogenesis are high frequencies of gene mutations (e.g., adenomatous polyposis coli (APC), Kirsten-ras (KRAS), and TP53) and gene fusions, aberrant expressions of mRNA, micro RNA and long non-coding RNA, alterations of splicing event, core signal transduction, DNA repair, extracellular matrix construction and metabolism, microsatellite instability, hypermethylation, copy number variation, immune dysregulation and gut microbiota alteration [9,10,11,12,13,14,15]. Pathological diagnoses have revealed that CRC arises through multistep carcinogenesis from dysplastic crypts to adenocarcinoma. Mutated APC, KRAS, and TP53 are strongly associated with the malignant progression of CRC [16,17,18,19]. In addition, the formation of immunosuppressive tumor microenvironment (TME) is essential for the onset of adenocarcinoma in colorectal mucosal tissue. The colorectal TME is composed of colorectal cancer stem cells (CCSCs), cancer-associated fibroblasts (CAFs), many immune cells, including tumor-associated macrophages (TAMs) and dendritic cells (DCs), modified extracellular matrix (ECM), stromal collagen enhancement and abnormal neovessels [20,21,22].
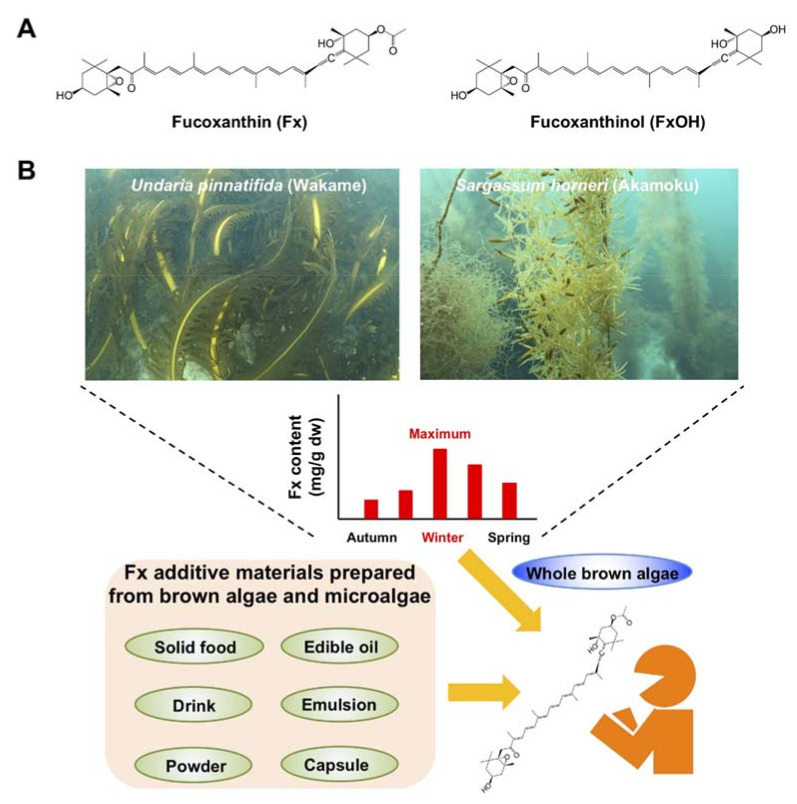
Fucoxanthin (Fx, Figure 1), a non-provitamin A carotenoid, is found abundantly in brown algae and microalgae. Fx binds the chlorophyll a/c-protein and contributes to efficient light harvesting for photosynthetic organisms as well as the body color. Fx has been estimated to account for >10% of total biogenic carotenoids [23]. It has an unusual allenic bond and a 5,6-monoepoxide, and its molecular weight is 658.9 g/mol (C42H58O6). Fx has been cleared as a safe carotenoid without any adverse effects at 0.5% (w/v) on human skin and at 20–2000 mg Fx/kg body weight (BW) in rodents [24,25,26]. Fx is easily metabolized to cis-Fx, fucoxanthinol (FxOH, Figure 1) in the intestine and then to amarouciaxanthin A (Amx A) and cis-Amx A in the liver. FxOH and cis-FxOH occur as the main plasma metabolites of human-ingested brown algae or its extract [27,28]. Hashimoto et al. showed that a single administration of an algal extract (31 mg Fx) resulted in a maximum concentration of 44.2 nmol/l, time at maximum concentration of 4.0 h and terminal half-life of 7.0 h for plasma FxOH [28]. In contrast, FxOH, Amx A and cis-Amx A are the predominant forms in the blood and various organs of mice administered Fx [29,30]. Several early studies have shown that Fx exerts important anti-inflammation [31], anti-obesity [32], anti-diabetes [33], anti-hypertension [34], anti-cardiovascular diseases [34], antimicrobial [35], antioxidation [36], photoprotection [37], anti-angiogenesis [38], anti-brain injury [39,40] and anticancer [41,42] effects in human, animal models and culture cells.
Figure 1.
Ingestible routes of Fx in humans. (A) Chemical structures of fucoxanthin (Fx) and fucoxanthinol (FxOH). (B) Ingestible routes of Fx in humans. Whole brown algae, such as Undaria pinnatifida (Wakame) and Sargassum horneri (Akamoku), collected in the winter, when the content of Fx is the highest, may be beneficial for human health. Furthermore, Fx extracted from brown algae and microalgae can be added to various edible items to customize their route of ingestion by humans.
Although many epidemiological studies have been conducted on nutritional approaches to CRC prevention, carotenoids—particularly non-polar carotenoids, such as α-carotene, β-carotene and lycopene—are still ranked as having limited evidence or no conclusion. However, carotenoids are abundantly contained in vegetables and fruits whose effects on CRC prevention are described as limited evidence to suggestive [8]. Furthermore, the effects of carotenoids vary widely depending on the polarity and include Fx, neoxanthin, violaxanthin, β-cryptoxanthin and lutein, α-carotene, β-carotene and lycopene. To date, epidemiological studies involving in vivo and in vitro experiments for CRC, using Fx itself and its rich foods, have not been fully outlined.
We herein review the most recent studies on Fx as a potential CRC preventive agent. There is little information available on a direct evidence of anti-CRC properties of Fx for patients; however, Fx and FxOH have been reported to exert potential anticancer effects in many CRC cell lines (summarized in Section 3), as well as in many preclinical CRC animal models (summarized in Section 4). In addition, many interventional human trials and in vivo studies have suggested that Fx and its rich brown algae may improve CRC and/or certain risks for CRC such as obesity, diabetes, metabolic syndrome, inflammation, oxidation, TME, and gut microbiota (summarized in Section 4 and Section 5). This is the first report integrated the improving effects by Fx and its rich brown algae for CRC and the risk factors.
2. Fx Sources in Foods and Other Materials
Brown algae, such as Heterokontophyta and Ochrophyta, are traditionally and most widely consumed in Asian countries, such as Japan, China and Korea [43]. However, brown algae are expanding steadily as novel foods in North America, South America and Europe [44]. The European market, in particular, is one of the most rapidly growing regions regarding the consumption of brown algae [45,46].
Four brown algae species—Undaria pinnatifida (Wakame), Sargassum horneri (Akamoku), Hizikia fusiforme (Hiziki) and Saccharina japonica (Makombu)—are habitually consumed as representative seafood sources in Asia. In Europe, nine species of edible brown algae—Fucus vesiculosus, Fucus serratus, Himanthalia elongata (Sea spaghetti), U. pinnatifida, Ascophyllum nodosum, Laminaria digitate, Laminaria saccharina, Saccharina japonica and Alaria esculenta—are generally consumed [47].
Data on the collection site and Fx content of 24 major brown algae are presented in Table 1 [48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65]. The Fx profile of U. pinnatifida has been investigated by many researchers worldwide. Among these reports, seasonal variations in Fx content have been well observed in U. pinnatifida collected from Japan, New Zealand and Australia: Japan, 0.3–5.3 mg Fx/g dry weight (dw); New Zealand, 0.8–6.2 mg Fx/g dw; Australia, 2.1–3.0 mg Fx/g dw in juvenile and 1.3–2.9 mg Fx/g dw in adult sporophytes [48,50,51,52,53]. The highest Fx content in U. pinnatifida was observed in the winter in Japan, New Zealand and Australia. Furthermore, the Fx content of S. horneri peaked at 10.8 mg Fx/g dw in winter in Japan [50]. Moreover, although the collection period was unknown, the Fx level of H. elongata was surprisingly high at 18.6 mg Fx/g dw [62]. In general, the winter season, which is characterized by low levels of both sunshine duration and seawater temperature, facilitates increased Fx production in brown algae through the xanthophyll-cycle pathway, involving the formation of Fx by upstream carotenoids [66,67]. These results suggest that U. pinnatifida, S. horneri and H. elongata collected in the winter period may be particularly good sources of Fx for human consumption (Figure 1).
Table 1.
Fucoxanthin content in 24 major edible brown algae worldwide.
| Family | Species | Common Name | Synonym Name | Collected Location | Body Part of Alga | Fx (mg/g dw) a |
Reference |
|---|---|---|---|---|---|---|---|
| Alariaceae | Undaria pinnatifida | Wakame | Japan New Zealand Australia |
Blade Blade --- b |
0.3–5.3 0.8–6.2 1.3–3.0 |
[48,50,51] [52] [53] |
|
| Alaria esculenta | Dabberlocks | Ireland | Blade | 0.9 | [54] | ||
| Alaria crassifolia | Chigaiso | Japan | Blade | 1.1 | [49] | ||
| Sargassaceae | Sargassum horneri | Akamoku | Japan Korea |
Lateral branch --- |
0.8–10.8 0.8 |
[49,50] [55] |
|
| Sargassum fusiforme | Hiziki | Japan | Lateral branch | 1.1 | [49] | ||
| Sargassum wightii | India | --- | 0.1 | [56] | |||
| Sargassum binderi | India | --- | 0.7 | [57] | |||
| Sargassum duplicatum | India | --- | 1.0 | [57] | |||
| Nizamuddinia zanardinii | Iran | --- | 0.6–1.7 | [58] | |||
| Cystoseira indica | Iran | --- | 2.3–3.6 | [58] | |||
| Turbinaria ornata | Indonesia | --- | 1.3 | [59] | |||
| Laminariaceae | Saccharina japonica | Makombu | Laminalia japonica | Japan China Korea |
--- --- --- |
0.2 0.4 0.5 |
[60] [61] [55] |
| Saccharina sculpera | Gagomekombu | Kjellmaniella crassifolia, Saccharina crassifolia | Japan | Lateral branch | 0.7 | [49] | |
| Laminaria digitata | Ireland | Blade | 0.7 | [54] | |||
| Laminaria saccharina | Sugar kelp, sea belt | Fucus saccharinus, Saccharina latissima | Ireland | Blade | 0.5 | [54] | |
| Lessoniaceae | Ecklonia kurome | Kurome | Japan | Blade | 1.7 | [59] | |
| Fucaceae | Fucus vesiculosus | sea oak | Ireland | Blade | 0.7 | [54] | |
| Fucus serratus | Toothed wrack | Ireland | Blade | 0.3 | [54] | ||
| Ascophyllum nodosum | Rockweed | Ireland | Blade | 0.4 | [54] | ||
| Ralfsiaceae | Analipus japonicus | Matsumo | Japan | Lateral branch | 1.4 | [49] | |
| Chordariaceae | Sphaerotrichia divaricata | Kusamozuku | Japan | Whole body | 0.2 | [49] | |
| Himanthaliaceae | Himanthalia elongata | Sea spaghetti |
Fucus elongatus, Himanthalia lorea
|
Ireland Spain |
Blade |
0.3–18.6 1.1 |
[54,62] [63] |
| Chordariaceae | Cladosiphon okamuranus | Okinawamozuku | Japan | --- | 0.3 | [64] | |
| Dictyotaceae | Padina australis | Malaysia Indonesia |
--- --- |
0.4 1.3 |
[65] [59] |
a Variations of fucoxanthin (Fx) content in major edible brown algae include seasonal change and different location {value: minimum–max mg/g dry weight (dw)}. b The body part for extraction in the alga is unknown.
While Fx can be synthesized chemically, the harmless extraction of Fx from biological materials is extremely promising from the perspective of accessibility, economy, environmental load and safety for food additive, cosmetic and pharmaceutical applications. When obtaining Fx through natural extraction, not only brown algae but also microalgae are convenient for use in the large-scale preparation of various materials requiring Fx addition. For instance, the Fx levels of Mallomonas sp. SBV13, Phaeodactylum tricornutum, Odontella aurita and Isochrisis affinis galbana, all microalgae, are 26.6, 8.6–24.2, 21.7 and 18.2 mg/g dw, respectively [68,69,70]. Edible oil-, emulsion- or encapsulation-based Fx products in addition to pure Fx powder have been successfully commercialized worldwide. Their Fx-loading materials correspond to the protective shell are good options for achieving high bioavailability and health benefits of Fx in humans compared with a free body of Fx powder that get easily be degraded by light, temperature and oxidation. Edible oils, such as palm, olive and soybean oils, have low toxicity for humans and are often used to the extraction of Fx from brown algae or microalgae [71]. Long- and medium-chain triacylglycerols (TAGs), indigestible oils, Arabic gum and lecithin are frequently used as emulsifiers of Fx. Encapsulation has been constructed by the individual or combination with proteins, oligosaccharides, polysaccharides or glycolipids [72].
Many researchers have described the stability and functional properties of Fx-incorporated drinks, edible oils and foods [73,74,75,76,77]. Taken together, these findings suggest that the ingestion approaches of Fx for humans include not just plain brown algae but also Fx-incorporated products intended for human health benefits (Figure 1).
3. Effect by Brown Algae and Fx in CRC Cellular Lines
Many studies have shown an antiproliferative effect and the induction of apoptosis by brown algal extract, Fx and FxOH in CRC cells. It was suggested that ethanol extract of U. pinnatifida sporophyll induced apoptosis in human CRC HCT116 cells with the activation of caspase-3, unlike molecular mechanisms of cell death due to two anti-carcinostatic drugs (5-fluorouracil (5-FU) and irinotecan) [78]. Ethanol extract from the brown algae Dictyopteris undulata augmented endoplasmic reticulum stress, abrogated mitochondrial membrane potential and induced apoptosis in human CRC SW480 cells through the enhancement of Bax; active caspase-3, caspase-9 and caspase-12; phospho-PERK; phospho-IRE1; cleaved ATF6; CAAT/enhancer-binding protein-homologous protein; and attenuation of Bcl-2 [79,80]. Furthermore, methanol extract of the brown algae Pylaiella littoralis also had an apoptosis-inducing effect in HT-29 cells, with downregulation of Bcl-2, and upregulations of Bax, active caspase-3, cleaved PARP, phospho-JNK, phospho-ERK and p38 was observed [81]. Ethanol extracts of the two brown algae T. ornata and P. pavonia significantly suppressed the growth of HCT116 cells in a dose-dependent manner [82]. Mhadhebi et al. showed that the organic fraction from Cystoseira sedoides exerted an anti-proliferative effect in human CRC HCT15 cells in a dose-dependent manner (25–500 μg/mL), along with antioxidation and anti-inflammation effects [83]. Our group recently investigated the transcriptome profile and protein expression in human CRC DLD-1 cells with FxOH treatment. FxOH (5.0 μM) significantly reduced cell growth and induced apoptosis. Gene hierarchical clustering of the cells revealed a significant difference in 807 genes compared with control cells. The genes belonging to cancer-related pathways, including the cell cycle, integrin, PI3K/AKT, MAPK, nuclear factor erythroid 2 [NF-E2]-related factor 2 (NRF2), adipogenesis, TGF-β, signal transducer and activators of transcription (STAT) and wingless/integrated (WNT)/β-catenin signals, were remarkably altered. In addition, the protein expression of cyclin D1, cyclin D2, integrin α5, integrin β1, phospho-Paxillin(Tyr31), phospho-AKT(Ser473), phospho-C-Raf (Ser338), phospho-MEK1/2(Ser217/221), PPARγ and phospho-Smad2(Ser465/467) was downregulated, while that of phospho-ERK1/2(Thr202/Tyr204) and NRF2 was upregulated [84]. We also demonstrated the suppressive effect of CLIC4 signal and induction of anoikis due to attenuation of an integrin signal by FxOH in DLD-1 cells [85,86]. Tamura et al. showed that FxOH (10 μM) induced apoptosis in HCT116 cells by increasing the NF-κB activity. However, the co-treatment of FxOH and an NF-κB inhibitor enhanced apoptosis induction [87]. Lopes-Costa et al. reported that Fx reduced cell viability and induced DNA damage in HCT116 and HT-29 cells, while little apparent effect on normal human colon CCD-18Co cells was observed, except at high concentrations (50 and 100 μM). This report also showed that Fx enhanced the cytotoxic effect of 5-FU in HCT116 cells [88]. Fx treatment (50 and 75 μM) induced apoptosis in human CRC WiDr cells through the arrest of the G0/G1 phase of the cell cycle and upregulation of p21WAF1/Cip1, an inhibitor of cyclin D [89]. Both Fx and FxOH addition induced apoptosis in human CRC Caco-2 cells. The Bcl-2 protein expression was decreased on cells treated with Fx [90,91]. Fx suppressed cell growth in human CRC SW-620 s via the loss of adhesion and invasion activities and of MMP-9 expression and amplified the 5-FU-induced anti-proliferative effect [92]. Treatment with FxOH and Fx (both 20 μM) significantly inhibited and tended to inhibit cell growth, respectively, of primary cells isolated from tumor tissue from patients with CRC [93].
CCSCs are involved in the development of CRC and have properties that include self-renewal, pluripotency, chemotherapy resistance, sphere formation and tumorigenesis [94,95]. Sphere-forming cells prepared by using stem cell medium with slight growth factors from CRC cells, colonospheres (Csps), are known to possess CCSCs-like features [96]. Our previous study demonstrated that FxOH treatment significantly disintegrated Csps in a dose-dependent manner. In addition, FxOH downregulated phospho-AKT(Ser473), PPARβ/δ and PPARγ in the Csps and significantly suppressed subcutaneous tumorigenesis in NOD-SCID (NOD.CB17-Prkdcscid/J) mice [97]. FxOH also inhibited cell migration and invasion and induced apoptosis under both normoxia and hypoxia conditions by altering certain signals, including EMT, integrin, MAPK, WNT/β-catenin, apoptosis and/or STAT signals [98,99]. Similarly, Fx treatment exerted a sphere-forming activity in spheroid prepared from human breast cancer MCF-7 cells, although its molecular mechanisms remain unknown [100]. Further studies are needed to confirm the underlying mechanisms on the suppression of sphere formation in Csps by Fx and FxOH treatments. Table 2 summarizes the molecular mechanisms underlying the effects of brown algae and Fx in human CRC cells.
Table 2.
Effect of fucoxanthin (Fx)-rich brown algal extract, Fx and fucoxanthinol (FxOH) in human colorectal cancer cell lines.
| Brown Algal Extract or Compound (Additive Concentration) |
Cell Line (Cell Type) |
Promoted Molecular Mechanism(s) | Involved Intracellular Component | Final Outcome (Cell Function) |
Reference |
|---|---|---|---|---|---|
| Ethanol extract of Undaria pinnatifida sporophyll (~2.0%) | HCT116 (PCs) |
Caspase-3 activation, and non-oxidative mechanisms differed from those of 5-fluorouracil and irinotecan treatments | NA | Apoptosis | [78] |
| Ethanol extract of Dictyopteris undulata sporophyll (~200 μg/mL) |
SW480 (PCs) |
Augmentation of endoplasmic reticulum stress; attenuation of mitochondrial membrane potential; increases of Bax, caspase-3, caspase-9, caspase-12, phospho-PERK, phospho-IRE1, cleaved ATF6, and CAAT/enhancer-binding protein-homologous protein; and decrease of Bcl-2 | Endoplasmic reticulum, and mitochondria | Apoptosis | [79,80] |
| Methanol extract of Pylaiella littoralis (~100 μg/mL) | HT-29 (PCs) |
Attenuation of mitochondrial membrane potential, decrease of Bcl-2, and increases of Bax, active caspase-3 form, cleaved PARP, phospho-JNK, phospho-ERK and p38 | Mitochondria | Apoptosis | [81] |
| Ethanol extracts of Turbinaria ornata and Padina pavonia
(~50 μg/mL) |
HCT116 (PCs) |
NA | Growth inhibition | [82] | |
| Organic fraction of Cystoseira sedoides (~500 μg/mL) | HCT115 (PCs) |
NA | Growth inhibition, antioxidation and anti-inflammation | [83] | |
| FxOH (~5.0 μM) | DLD-1 (PCs) |
Alterations of gene set belonging cell cycle, integrin, PI3K/AKT, MAPK, NRF2, adipogenesis, TGF-β, STAT and WNT/β-catenin signals, decreases of cyclin D1, cyclin D2, integrin α5, integrin β1, phospho-Paxillin(Tyr31), phospho-AKT(Ser473), phospho-C-Raf (Ser338), phospho-MEK1/2(Ser217/221), PPARγ and phospho-Smad2(Ser465/467), and increases of phospho-ERK1/2(Thr202/Tyr204) and NRF2 | NA | Apoptosis | [84] |
| FxOH (~5.0 μM) | DLD-1 (PCs) |
Arrest of G2/M cell cycle phase, decreases of CLIC4, integrin β1, phospho-Smad2(Ser465/467) and NHERF2 | NA | Apoptosis | [85] |
| FxOH (~2.5 μM) | DLD-1 (PCs) |
Alteration on cellular distribution of integrin β1, and decreases of phospho-FAK(Tyr397), phospho-AKT(Ser473) and PPARγ | NA | Anoikis | [86] |
| FxOH (~10 μM) | HCT116 (PCs) |
Arrest of G0/G1 cell cycle phase, activations of NF-κB and caspase-3, and increases of XIAP and cIAP-1 | NA | Apoptosis | [87] |
| Fx (~100 μM) | HCT116 and HT-29 (Both PCs) |
Increase of p53 and decrease of Bcl-2 in HCT116 cells, and increase of Bax and decrease of pro-caspase-9 in HT-29 cells | NA | Growth inhibition | [88] |
| Fx (~75 μM) | WiDr | Arrest of G0/G1 cell cycle phase, and increases of p21WAF1/Cip1 and p27Kip1, and decreases of phospho-pRb(Ser780), phospho-pRb(Ser807/811), cyclin D1, cyclin D2 and cyclin D3 | NA | Apoptosis | [89] |
| Fx (~15.2 μM) | Caco-2 | Decrease of Bcl-2 and activation of caspases | NA | Apoptosis | [90] |
| FxOH (~25 μM) | Caco-2 | NA | Growth inhibition | [91] | |
| Fx (~30 μM) | SW-620 | Loss of adhesion and invasion activities, and decrease of MMP-9 | NA | Growth inhibition | [92] |
| Fx and FxOH (~20 μM) | Primary cells in CRC patients | NA | Growth inhibition | [93] | |
| FxOH (~5.0 μM in vitro, 5 mg/kg body weight in vivo) | HT-29 (Csps) |
Decreases of phospho-AKT(Ser473), PPARβ/δ and PPARγ, suppression of tumorigenesis in NOD/SCID mice | NA | Apoptosis | [97] |
| FxOH (~50 μM) | HT-29 (Csps) |
Suppressions of cell migration and invasion; attenuations of EMT, integrin, MAPK and STAT signal proteins; decrease of p53; and increase of active caspase-3 form | NA | Apoptosis under normoxia condition | [98] |
| FxOH (~50 μM) | HT-29 (Csps) |
Attenuations of EMT, integrin, MAPK and STAT signal proteins; decreases of HIF-1α, cyclin D1and p53; and increases of phospho-β-catenin(Ser31/37/Thr42) and active caspase-3 form | NA | Apoptosis under hypoxia condition | [99] |
Parent cells (PCs) indicate each intact cell line, most of which are the adherent types. Colonospheres (Csps) indicate a spheroid prepared from the parent cells by stem cell medium and some growth factors. Fx, fucoxanthin; FxOH, fucoxanthinol; NA, not available; PERK, protein kinase RNA (PKR)-like ER kinase; IRE1, inositol-requiring enzyme 1; ATF6, activating transcription factor 6; PARP, poly(ADP-ribose) polymerase; JNK, c-Jun NH2-terminal kinase; ERK, mitogen-activated protein kinase 1; PI3K/AKT, phosphatidylino-sitol-3 kinase/protein kinase B; MAPK, mitogen-activated protein kinase; NRF2, nuclear factor erythroid 2 [NF-E2]-related factor 2; TGF-β, transforming growth factor beta; STAT, signal transducers and activators of transcription; WNT, wingless/integrated; MEK, mitogen-activated protein/extracellular signal-regulated kinase; PPARγ, peroxisome proliferator activated receptor gamma; CLIC4, chloride intracellular channel 4; NHERF2, Na+/H+ exchanger regulatory factor 2; FAK, focal adhesion kinase; NF-κB, nuclear factor-κB; XIAP, X-linked inhibitor of apoptosis protein; cIAP-1, cellular inhibitor of apoptosis protein-1; MMP-9, matrix metallopeptidase 9; PPARβ/δ, peroxisome proliferator activated receptor beta/delta; NOD/SCID, NOD.CB17-Prkdcscid/J; EMT, epithelial-mesenchymal transition; HIF-1α, hypoxia-inducible factor-1 alpha.
Clinical data on humans are the most important to support both the experimental findings and the hypothesized relationship between Fx and CRC. However, it is necessary to accumulate evidence on the anticancer effect of Fx for CRC in both in vivo and in vitro experiments as the basic mechanism for discussing the implications on potential effect of Fx in human.
4. Cancer Preventive Effect of Brown Algae and Fx in CRC Model Animals
4.1. Cancer Chemopreventive Effect of Whole Brown Algae and Fx-Containing Extract
Some researchers have reported on the anticancer properties of whole brown algae and Fx-containing extracts in CRC model animals.
Recently, we cultivated U. pinnatifida on the Nesaki coast of Hokkaido, Japan, for 3 months and collected algae with the highest Fx content in February 2017 (Fx-high wakame, >5.0 mg Fx/g dw). Using a CRC murine model (azoxymethane–dextran sodium sulfate [AOM/DSS] mice with an ICR background), we investigated the effects of whole Fx-high wakame feeding on colorectal TME. The administration of Fx-high wakame (equivalent to Fx 30 mg/kg bw) for 7 weeks significantly decreased the number of CCSCs-like CD44high/EpCAMhigh cells, CAFs-like αSMAhigh cells, TAMs- and DCs-like CD206high cells and increased apoptotic-cells-like cleaved caspase-3high cells in colorectal mucosal tissue in the mice. In addition, the salivary glycine level was found to be a predictor correlated with the chemopreventive efficacy of Fx-high wakame in the mice [51]. Kong et al. showed that the administration of Fx-rich extract (1–5 g extract/kg bw) from S. muticum exerted antioxidation, anti-inflammation and/or anticancer effects in DSS-derived colitis-induced and AOM/DSS-induced BALB/c mice by reducing the disease activity score, nitric oxide (NO), malondialdehyde, TNF-α and IL-6 and increasing the level of SOD. In addition, the extract prevented the onset of colorectal tumors and restored lymphocyte proliferation and survival rates [101]. Son et al. revealed that diet feeding both 2% and 6% whole H. fusiforme for 8 weeks significantly reduced the number of aberrant crypt foci (ACF) and the rate of proliferating cell nuclear antigen labeling index in mucosal tissue of an AOM-induced F344 rat CRC model [102]. Mahmoud et al. investigated the CRC chemopreventive effect using ethanol extracts of two brown algae, T. ornata and Padina pavonia, collected from the shore of the Red Sea in Egypt. The administration of 100 mg/kg bw of either T. ornata or P. pavonia extract for 8 weeks in colorectal tissue of AOM-induced albino Swiss CRC mice significantly prevented colorectal inflammation and oxidation by reducing the lipid peroxidation and NO levels and downregulating the NF-κB expression and upregulating the peroxisome proliferator-activated receptor gamma (PPARγ) and p53 expression. In addition, the colorectal levels of GSH, SOD and GPx activities in the mice were significantly increased by the feeding of both T. ornata and P. pavonia extracts [82].
Of note, Das et al. prepared ethanol extract containing 15 mg Fx/g from S. japonica and administered it as an ad libitum dimethyl sulfoxide solution (final Fx concentration: 0.075 mg/mL) to AOM-initiated ddY mice for 4 weeks to investigate the difference in CRC chemoprevention from other groups given 0.05 and 0.1 mg Fx/mL solutions. Consequently, the administrations of S. japonica extract, 0.05 and 0.1 mg Fx/mL solutions all significantly decreased the number of colorectal ACF in the mice to similar degrees. These findings suggested that S. japonica extract exerted an inhibitory effect on the onset of colorectal ACF with the same potency as Fx alone [103]. Further complicating matters, Reddy et al. showed that the dietary intake of Laminaria angustata (Mitsuishikombu) (diet containing 10% algae) for 28 weeks significantly increased the incidence and multiplicity of colorectal tumors in an AOM-induced F344 rat CRC model [104].
4.2. Cancer Chemopreventive Effect of Fucoxanthin Itself
Some researchers have studied the direct anticancer property of Fx in CRC model animals. Our group previously found a cell death mechanism by which FxOH treatment induced anoikis in human CRC cells [86]. Anoikis is an apoptotic mechanism that occurs following cell detachment due to a lack of integrin anchor points between the cell and the extracellular matrix (ECM) or the cell and neighbor cells, and it is physiologically significant for tissue homeostasis and disease. During the anoikis process, certain core mechanisms, such as phosphatidylinositol-3 kinase/protein kinase B (PI3K/AKT), MAPK and transforming growth factor-beta (TGF-β) signals, are first inhibited by changes in some transmembrane receptors, and cells subsequently become detached from the ECM, finally inducing caspase activation, which triggers anoikis. Cancer cells frequently suppress this stepwise anoikis induction. Therefore, anoikis resistance in cancer cells is indispensable for the cell survival, epithelial–mesenchymal transition (EMT), invasion and metastasis [105,106,107].
Following our anoikis findings in vitro, we explored whether or not Fx administration in an AOM/DSS-induced ICR mouse model of CRC induced anoikis in the colorectal tissue. As a result, oral Fx administration (30 mg/kg bw) for 2 weeks before sacrifice significantly suppressed the number and size of colorectal tumors by enhancing anoikis-like integrin β1low/-, phospho(p)-FAK(Tyr397)low/- and pPaxillin(Tyr31)low/- with cleaved caspase-3high cells in the colorectal mucosal crypts [108]. Furthermore, Fx treatment also markedly increased the number of anoikis-like integrin β1low/-/cleaved caspase-3high cells in colorectal adenocarcinoma in the mice [109]. These two reports suggest that the induction of anoikis by Fx was the relevant anticancer mechanism in AOM/DSS mice. CCSCs often promote an EMT accompanied by the alterations of related proteins. EMT is considered to mediate their migration and invasion of the cells and to cause CRC recurrence and distant metastasis [110]. Therefore, the inhibition of the EMT phenotype in CCSCs would be a promising approach for cancer prevention. We speculate that the anoikis induction in colorectal normal mucosa and adenocarcinoma of AOM/DSS mice with Fx treatment lead to suppress the occurrence and EMT activation of CCSCs.
The formation of TME is a significant process in the onset of adenocarcinoma in colorectal mucosal tissue [20,21,22]. We previously showed that Fx administration (30 mg/kg bw) for 8 and 14 weeks significantly prevented colorectal carcinogenesis and decreased the number of CCSCs-like CD44high/EpCAMhigh cells, CAFs-like αSMAhigh cells, TAMs- and DCs-like CD206high cells and/or increased apoptotic cell-like cleaved caspase-3high cells in colorectal mucosal tissue in AOM/DSS mice. In addition, the salivary glycine level was found to be a predictor of the chemopreventive efficacy of Fx in the mice [111,112]. These findings suggested that Fx was a promising inducer for the occurrence suppression in CCSCs of the mice.
Notably, changes in the gut microbiota can also function as a key trigger in human colorectal carcinogenesis [15,113,114]. We investigated the chemopreventive potency of Fx and its effect on the gut microbiota in AOM/DSS mice. Oral Fx administration (30 mg/kg bw) for 14 weeks significantly prevented the onset of colorectal adenocarcinoma in mice with attenuations of Bacteroidlales and Rikenellaceae and enhancement of Lachnospiraceae in the fecal microbiota composition. Of note, the oral administration of a fecal suspension from the Fx-treated mouse suppressed the number of colorectal adenocarcinoma in another AOM/DSS mice with a successful increase of gut Lachnospiraceae. These findings suggested that the induction of changes in the gut microbiota by Fx is a significant mechanism underlying the cancer chemopreventive effects of Fx in AOMDSS mice [115].
CCSCs occupy only a small subset of CRC tissue, but they are thought to play an important role in cancer progression. Self-renewal, sphere formation, differentiation, and tumorigenicity in animals have been characterized as biological properties for CCSCs [94,95]. Therefore, we especially focused on the effects of Fx and FxOH on the tumorigenesis from CCSCs in two immunodeficiency murine models. Consequently, we have revealed that the administration of Fx and FxOH inhibited tumorigenesis in xenograft NOD/SCID and BALB/c mice, respectively, subcutaneously injected with CCSCs-like cells prepared from human CRC HT-29 cells. In addition, the administration of Fx to BALB/c mice significantly downregulated the cyclin D1 expression in the tumor [97,116]. Furthermore, we investigated the effect of Fx in a ApcMin/+ mouse model of human FAP. The intake of Fx diet (1000 ppm) for 5 weeks significantly attenuated the number of colorectal adenocarcinoma lesions in DSS-treated ApcMin/+ mice with downregulation of cyclin D1 expression in mucosal tissues [117]. One group described the effects of Fx in another animal model: Kim et al. showed that Fx administration in drinking water (0.01%) for 7 weeks significantly suppressed the onset of colorectal ACF and the BrdU Labeling Index in the colorectal crypt compartment in a 1,2-dimethylhydrazine-induced murine CRC model (B6C3F1) [41].
Many reports have described the cancer chemopreventive effects seen in animal CRC models treated with whole brown algae, extract containing Fx and Fx itself. However, the detailed molecular mechanisms underlying these effects in the colorectal tissue of animal models remain elusive. Further investigations are needed to determine the CRC chemopreventive effects of brown algae and Fx.
4.3. Effect by Brown Algae and Fx in CRC Risks
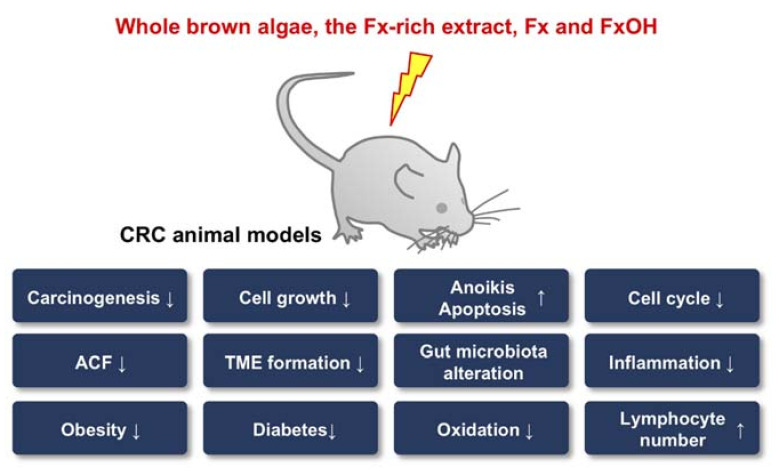
Many researchers have reported the attenuation of CRC risk factors, such as anti-obesity, anti-metabolic syndrome and anti-inflammation effects, and changes in gut microbiota by whole brown algae, Fx-containing extracts and Fx itself in animal models. Diets containing U. pinnatifida ethanol extracts, equivalent to 0.05% and 0.2% Fx, which has already demonstrated anti-obesity effects when administered through the diet in C57BL/6N mice, or another diet containing S. horneri powders (2% and 6%) reduced both the body and WAT weights in C57BL/6J mice fed a high-fat diet, with enhancement of the UCP-1 mRNA expression in WAT [118,119,120]. Okada et al. also reported on the anti-obesity effects, characterized by reductions in the body and WAT weights, changes in serum lipid profiles and upregulation of the UCP-1 protein and mRNA expression in KK-Ay mice with U. pinnatifida lipid treatment (0.2% as a drink; 1.0% as a diet) [73]. Grasa-López et al. revealed the anti-obesity and anti-inflammation effects of both U. pinnatifida (400 mg/kg bw) and Fx (1 mg/kg bw) in Wister rats given a high-fat diet, along with the upregulation of PPARα, PPARγ coactivator-1α, PPARγ and UCP-1 and downregulation of IL-6 [34]. Diet containing S. polycystum (2.5–10.0%) induced significant decreases in the BW and plasma LDL-cholesterol and TAG levels and increased the high-density lipoprotein-cholesterol levels in Sprague-Dawley rats fed a high-fat diet [121]. The oral administration of extracts from two brown algae—P. pavonia and T. ornata (100 mg/kg bw)—for 21 d exerted antioxidation and anti-inflammation effects in streptozotocin/nicotinamide-induced type 2 diabetic rats (albino) through the reductions in glucose, TNF-α and malondialdehyde, and increases of insulin, GSH, GPx and SOD [122]. An ethanol extract of the brown algae Petalonia binhamiae (150 mg/kg bw) for 70 d induced an anti-obesity effect with many obesity-related markers in high-fat-diet-fed C57BL/6 mice [123]. Maeda et al. showed that the administration of diets containing Fx-rich U. pinnatifida extract (2.0%) significantly reduced the WAT weight in both Wister rats and KK-Ay mice, but not the BAT weight. In addition, both a 2.0% U. pinnatifida-containing diet and 0.4% Fx diet induced the marked upregulation of the UCP1 expression in WAT of KK-Ay mice compared with that in control mice [124]. Similarly, those authors further demonstrated the anti-obesity and anti-diabetic effects of Fx in KK-Ay and C57BL/6J mice [125,126,127,128,129,130]. Other researchers have described the anti-diabetic effect of Fx and U. pinnatifida extract in C57BL/6N and C57BL/6J mice, respectively [131,132]. Furthermore, treatment with Fx (50 and 100 mg/kg) could exert the anti-inflammatory effect with BW reduction and colorectal mucosal damage, decreases of PGE2, COX-2 and NF-κB in a DSS-induced colitis murine model [133]. Another group reported on changes in the gut microbiota in the administration of whole brown algae. The intake of a diet containing 10% dried U. pinnatifida or S. japonica for 4 weeks increased the Prevotella, Alistipes and Bacteroides genera and decreased the Roseburia, Mollicute and Oscillibacter genera in feces of Sprague-Dawley rats [134]. Another two groups explored the alteration of the gut microbiota in obese mice by Fx. Sun et al. showed that the administration of a high-fat diet containing Fx (1 mg Fx/g diet) decreased the rate of Lachnospiraceae and Erysipelotrichaceae and increased the rates of Lactobacillus, Lactococcus, Bifidobacterium and several butyrate-producing bacteria in feces of an obese murine C57BL/6J model, along with decreasing serum levels of TNF-α and IL-6. In addition, the gut bacterial taxa were significantly associated with obesity phenotypes and the degree of inflammation [135]. Another group reported that Fx administration (125 mg/kg bw) for 4 weeks reduced BW and changed the cecal and fecal microbiota in BALB/c mice given a normal or a high-fat diet. In particular, changes in the ratio of Firmicutes/Bacteroidetes and the composition of S24-7 and Akkermansia were observed in the cecal contents [136]. Furthermore, Liu et al. prepared a fecal bacterial suspension from C57BL/6 mice served a normal diet. The bacteria were cultured under anaerobic conditions and treated with 0.1 mg/mL Fx. The Fx treatment consequently suppressed the growth of Escherichia coli but promoted that of Lactobacilli [137]. Figure 2 summarizes the molecular mechanisms underlying the effects of whole brown algae, Fx-rich extract and Fx on CRC itself or its risk factors in animal models.
Figure 2.
Possible mechanisms underlying the cancer chemopreventive effects of whole brown algae, fucoxanthin (Fx)-rich extract, Fx and fucoxanthinol (FxOH) against colorectal cancer (CRC). ACF, aberrant crypt foci; TME, tumor microenvironment. ↑, induction or increase; ↓, inhibition or decrease.
5. Beneficial Effects of Brown Algae and Fx in Human
5.1. Effects of Fx and Fx-Containing Materials on the Risk Factors Associated with CRC
Little information is available on the direct mechanisms underlying the anti-CRC properties of Fx and Fx-containing materials. However, interventional studies have shown that whole brown algae, Fx extracts and Fx itself can improve the risk factors associated with CRC, such as obesity, diabetes, metabolic syndrome, inflammation and oxidation.
Obesity is a chronic metabolic disorder defined as excessive visceral fat accumulation, typically characterized by a body mass index (BMI) of ≥ 30 kg/m2 (≥25 kg/m2 in Japan), an increased waist circumference and/or a reduced energy expenditure [138]. Large cohort studies have described a positive association between obesity and CRC [139,140] and between diabetes and CRC [141,142]. Obesity is mainly caused by overeating, low physical activity and heritable features. The disease leads to low-level inflammation and metabolic syndrome, such as hyperlipidemia, hypertension, diabetes mellitus, non-alcoholic fatty liver disease (NAFLD) and arteriosclerosis. The excessive fat accumulation is accompanied by invasion of inflammatory cells into adipose tissue, which secretes cancer-related adipokines/chemokines including adiponectin, plasminogen activator inhibitor-1 (PAI-1), leptin, resistin, tumor necrosis factor-α (TNF-α), monocyte chemoattractant protein-1 (MCP-1), nicotinamide phosphoribosyltransferase (NAMPT), interleukine-6 (IL-6) and lipocalin 2 [143,144,145,146,147].
Inflammation is a protective response that causes injured tissues to heal. However, chronic inflammation induced by the persistent activation of signaling pathways without normal healing is a major CRC risk factor [148,149,150]. The core inflammatory mediators in white blood cells and tissue cells, such as nuclear factor-κB (NF-κB), cyclooxygenase-2 (COX-2), prostaglandin E2 (PGE2), peroxisome proliferator-activated receptor δ (PPARδ), IL-6, mitogen-activated protein kinase (MAPK), c-Jun NH2-terminal kinases and activating protein-1, lead to carcinogenesis; therefore, the suppression of these inflammatory activators is a significant target for anti-inflammatory agents [151,152,153].
Oxidative stress resulting from increased production of reactive oxygen species (ROS) by various stimuli is also strongly associated with the development of CRC and IBD [154,155,156,157,158,159,160]. Enhanced oxidative stress induces increased DNA base oxidation (8OHdG-8hydroxy-2’-deoxyguanosine) and lipid peroxidation under conditions of inflammation and carcinogenesis in human subjects [161,162]. The ROS superoxide (O2-), hydroxyl radicals (HO・) and hydrogen peroxide (H2O2) are mainly generated through the mitochondrial electron transport chain via enzymatic and non-enzymatic pathways, and their byproducts are balanced by glutathione (GSH) and antioxidant enzymes, such as superoxide dismutase (SOD), catalase, glutathione peroxidase (GPx), peroxiredoxin or antioxidant agents, to achieve a normal cell function [163]. However, excessive ROS generation frequently plays a major role in tumorigenesis [164,165].
As an early experiment, Adidov et al. investigated the anti-obesity effect of the Fx-containing oil XanthigenTM (PL Thomas & Co., Morristown, NJ, USA) in humans. The consumption of Xanthigen-600, which is composed of an algal extract (equivalent to 2.4 mg Fx) and plant oil, by obese premenopausal women with NAFLD or normal liver fat content for 16 weeks significantly decreased their BW, waist circumference, body and liver fat content, liver enzymes, serum TAG and/or C-reactive protein levels [166]. Mikami et al. prepared 1 and 2 mg Fx capsules composed of an algal oil extracted from S. horneri, medium-chain triacylglycerol, lecithin and vitamin E. The administration of 2 mg Fx/d for 8 weeks significantly decreased the HbA1c level compared with the placebo control. They investigated the polymorphism status of uncoupling protein 1 (UCP1) in the subjects [33]. UCP1, a mitochondrial membrane protein, plays a central role in the metabolic thermogenesis process for inhibiting excessive fat accumulation [167]. The UCP1 molecule is strongly expressed in brown adipose tissue (BAT), which promotes whole body energy expenditure, and its protein aberration leads to the development of obesity, although most fat is accumulated in the white adipose tissue (WAT), where UCP1 is absent. Of note, UCP-1 can be induced by various intrinsic and extrinsic stimuli [168,169,170]. Maeda et al. demonstrated that the intake of a diet containing Fx-rich U. pinnatifida extract significantly reduced the WAT weight with the enhancement of UCP1 expression in KK-Ay mice [124]. Interestingly, the reduction of HbA1c in subjects with the UCP1-3826 G/G genotype was significantly greater than that in those with an A/A or A/G genotype. No significant change in the HbA1c level was observed following 1 mg Fx administration [33]. A capsule of phlorotannin (107.3 mg) and Fx (36.5 mg)-rich content was prepared from ethanol extract of A. nodosum collected on the coast of the United Kingdom and administered to individuals with a BMI ≥ 25 for 8 weeks. As a result, the algal extract slightly reduced the DNA damage in these obese individuals [171].
However, some researchers have described the beneficial effects for humans of whole brown algae or its extract with unknown Fx contents. The intake of 0.9 g A. nodosum for 6 weeks reduced the BW, BMI and TAG level and enhanced the adiponectin levels in healthy volunteers [172]. A single dose of both U. pinnatifida itself (4 g) and its sporophylls (70 g) significantly decreased the postprandial glucose concentration in plasma of healthy volunteers at 0.5 h after a meal [173,174]. Dosage plan of 4 g/d for 4 weeks plus 6 g/d for 4 weeks of U. pinnatifida powder-packed capsules brought about reductions in the systolic blood pressure and waist circumference in participants with at least one symptom of metabolic syndrome [175]. A daily intake of 50 g of a snack food containing 64 mg of whole U. pinnatifida for 8 weeks significantly reduced the total cholesterol, low density lipoprotein (LDL)-cholesterol and resistin levels [176]. The administration of fermented S. japonica (1.5 g/d) for 4 weeks significantly downregulated the activities of catalase and SOD [177]. The intake of S. japonica (6 g/d for 4 weeks) also improved the molecular species profiles of phosphatidylcholine (PC), phosphatidylethanolamine (PE), lysophosphatidylcholine and lysophosphatidylethanolamine as well as the free fatty acid levels, and algal ingestion increased the plasmanyl and plasmenyl forms of PC and PE in the serum of participants with abnormal serum triacylglycerol levels [178,179]. Furthermore, dietary intervention of S. japonica (2 g/d for 6 weeks) reduced the body fat proportion and enhanced the adiponectin level in healthy volunteers [180].
Brown algae contain many attractive hydrophilic and lipophilic compounds with nutritional benefits for both humans and animals. The promising agents considered to be involved in these effects include Fx, fucosterol, polyunsaturated fatty acids, polysaccharides, peptides, bromophenols, phlorotannins and assorted minerals [181,182,183]. Therefore, it must be noted that the beneficial effects of whole brown algae and its extract in human subjects are not due to Fx alone.
5.2. Effects of Fucoxanthin Itself on CRC Risk
Abidov et al. have demonstrated that administration of 2.4–8.0 mg Fx for 16 weeks enhanced the resting energy expenditure in obese volunteers with NAFLD but had no effect at 1.6 mg [166]. Hitoe et al. investigated the anti-obesity effect of the commercial Fx oil Fucoxanthin-P1 containing a powder with 1% Fx (Oryza Oil & Fat Chemical Co. Ltd., Osaka, Japan) in Japanese adults with a BMI ≥ 25 kg/m2. Consequently, the interventional intake of 3 mg Fx/d for 4 weeks significantly reduced the BMI, fat areas (total, subcutaneous and visceral parts) and waist circumference compared with placebo groups. In addition, the administration of 1 mg Fx/d for 4 weeks decreased the total fat area alone [32].
In summary, interventional studies, a prospective cohort study and two case-control studies on the association between the intake of seaweed likely containing brown algae and CRC have shown promising findings concerning the effect of brown algae intake on preventing CRC. In addition, the daily intake of >0.9 g of whole brown algae or 2 mg of Fx may result in anti-obesity, anti-metabolic syndrome, antioxidation and anti-inflammation effects. To date, there is little information available concerning interventional studies of brown algae and Fx for gut microbiota and hereditary CRC syndromes. Further work is needed to confirm the response in patients with CRC or CRC risks.
The interventional results concerning whole brown algae, Fx-rich extracts and purified Fx are summarized in Table 3.
Table 3.
Effect of brown algae and fucoxanthin on CRC risk factors in human interventional studies.
| Brown Algae Source | Fx Dosage | Administration Type | Study Design | Effect | Reference |
|---|---|---|---|---|---|
| Unknown | 2.4 mg/d | A capsule of algal lipid-rich extract containing 300 mg pomegranate seed oil and 300 mg dw brown algal extract (XanthigenTM) | Double-blind, placebo-controlled, randomized trial in 151 women with non-diabetic and obese premenopausal (period, 16 weeks) | Reductions of BW, waist circumference, body and liver fat contents, liver enzymes, serum TAG and C-reactive protein | [166] |
| Sargassum horneri | 2.0 mg/d | A capsule containing 220 mg dw S. horneri Fx-rich extract | Single-blind, placebo-controlled, randomized trial in 60 adults (30–77 y) with normal-weight and obese (period, 8 weeks) | Reductions of blood HbA1c level | [33] |
| Ascophyllum nodosum | 36.5 mg/d | A capsule containing 100 mg dw A. nodosum ethanol/water extract | Double-blind, placebo-controlled, randomized trial in 80 women (30–65 y) with obese (period, 8 weeks) | Weak inhibition of DNA damage | [171] |
| Ascophyllum nodosum | Unknown/d | A capsule containing 900 mg dw of whole A. nodosum | Double-blind, placebo-controlled, randomized trial in 43 healthy adults (21–63y) (period, 6 weeks) | Reductions of BW, BMI, TAG, and TNF-α levels, and increase of adiponectin | [172] |
| Undaria pinnatifida | Unknown | Meat containing 70 g ww of U. pinnatifida sporophyll | Interventional study in 12 healthy adults (average 25.4y) (period, 180 min) | Reductions of plasma glucose and its AUC | [173] |
| Undaria pinnatifida | Unknown | 4 g dw of U. pinnatifida (FUERU WAKAME-CHAN®) with rice | An open-label, two-period, placebo-controlled, randomized trial in 26 healthy adults (average 51.5 y) (period, 120 min) | Reductions of blood glucose and insulin levels, and those AUC | [174] |
| Undaria pinnatifida | Unknown/d | A capsule containing 4 g dw of U. pinnatifida sporophyll/d for 4 weeks plus 6 g the alga/d for 4 weeks | Double-blind, placebo-controlled, randomized trial in 27 adults (average 46.2 y) with at least one symptom of the metabolic syndrome (period, 8 weeks) | Reductions of systolic blood pressure and waist circumference | [175] |
| Undaria pinnatifida | Unknown/d | A snack containing 32 mg dw of U. pinnatifida | Double-blind, placebo-controlled, randomized trial in 32 adults (average 51.1 y) with obese (period, 8 weeks) | Reductions of LDL-cholesterol, total-cholesterol and resistin level | [176] |
| Saccharina japonica | Unknown/d | 1.5 g dw of fermented S. japonica | Double-blind, placebo-controlled, randomized trial in 48 healthy volunteers (period, 4 weeks) | Reductions of serum γ-GT and MDA, increases of SOD and CAT activities | [177] |
| Saccharina japonica | Unknown/d | 6 g dw of roasted S. japonica | Interventional study in 52 adults (39–86 y) with normal and abnormal serum TAG levels (period, 4 weeks) | Reduction of serum TAG, Improvements of molecular species of PC, PE, LPC, LPE and FFA in the subjects with abnormal serum TAG level | [178,179] |
| Saccharina japonica | Unknown/d | A capsule containing 2.0 g dw of S. japonica | Double-blind, placebo-controlled, randomized trial in 70 healthy adults (average 56.6 y) (period, 6 weeks) | Reduction of body fat and improvement of adiponectin level | [180] |
| Unknown | 2.4–8.0 mg/d | A capsule of 100 mg algal lipid-rich extract | Double-blind, placebo-controlled, randomized trial in 41 women with non-diabetic obese and NAFLD (period, 16 weeks) | Increase of resting energy expenditure | [166] |
| Undaria pinnatifida and Saccharina japonica | 3.0 mg/d | A capsule containing 1.5 mg Fx powder (Fucoxanthin-P1®) | Double-blind, placebo-controlled, randomized trial in 33 adults (average 42.8 y) with a BMI ≥25 (period, 4 weeks) | Reductions of BMI, fat area, waist circumference | [32] |
Fx, fucoxanthin; Dw, dry weight; BW, body weight; TAG, triacylglycerol; HOMA, homeostatic model assessment; BMI, body mass index; AUC, Area under the curve; ww, wet weight; LDL, lipoprotein; γ-GT, γ-glutamyltransferase; MDA, malondialdehyde; SOD, superoxide dismutase; CAT, catalase; NAFLD, non-alcoholic fatty liver disease.
5.3. Clinical Studies with Whole Brown Algae and Fx-Containing Extracts in CRC
Hoshiyama et al. showed that a high intake of seaweed, likely including U. pinnatifida, showed a significant trend toward an inverse association with the risk of overall CRC (odds ratio (OR), 95% confidence interval (CI) = 0.2 [0.0–0.9], Ptrend < 0.01) and rectal cancer (OR, 95% CI = 0.4 [0.1–1.1], Ptrend = 0.01) as a case-control study in Japan [184]. In addition, Minami et al. investigated the association between the Japanese food intake and major digestive tract cancers from 1997 to 2013 as a prospective cohort study at an institution in Japan. As a result, the intake of seaweed, likely including U. pinnatifida, tended to be inversely associated with death in patients with rectal cancer (Ptrend = 0.02) [185]. Kim et al. reported a significant inverse association between dietary intake of algae containing U. pinnatifida and CRC in a case-control study of Korean patients (OR, 95% CI = 0.65 [0.50–0.85]). In addition, the rs6983267 polymorphism of c-MYC, an oncogene, was associated with a significant interaction between the dietary algae intake and both the overall CRC and rectal cancer risk [186]. Epidemiological data in humans supporting the role of Fx in CRC prevention are few and not well-addressed. They involve the same geographic area, Japan [184,185] and Korea [186]. Although there are no or few specific algae among those listed in Table 1, they contain Fx at different amounts. Indeed, the sample size is relatively small, but epidemiological studies [185,186] may suggest that dietary seaweed containing Fx have a positive benefit as a chemoprevention and/or chemotherapeutic agent for CRC risk.
6. Conclusions
The high-polarity xanthophyll Fx is specific to brown algae, and microalgae and has been verified as safe without side effects in human [24,32,166]. This review discussed the most recent studies available concerning Fx as a potentially useful agent for CRC prevention.
Edible brown algae are often high in Fx content, and their consumption has been expanding in Asia, North and South America and Europe. In particular, the three algae, U. pinnatifida, S. horneri and H. elongata, are not only commonly eaten all around the world, but they have also been proven considerable Fx sources (Table 1). Brown algae are characterized by increased Fx production under low-sunlight conditions with cold seawater temperatures. Therefore, U. pinnatifida, S. horneri and H. elongata collected during the period of high Fx production may be useful as potent sources of Fx. In addition, the acquisition of Fx from not only brown algae but also microalgae can be convenient on large-scale preparation for food, cosmetic and pharmaceutical applications (Figure 1).
Fx-rich extract, Fx and FxOH can induce apoptosis and anoikis in human CRC cells and their spheroids through a number of molecular mechanisms. Such treatments can alter the activities and protein expression of caspases, Bax, Bcl-2, PARP, JNK, MAPK, PI3K/AKT, integrin, WNT/β-catenin, STAT and PPARs and induce changes in the cell cycle, DNA damage and the CRC cellular functions of adhesion, migration and invasion, as well as the mitochondria and endoplasmic reticulum of these cells (Table 2). A few molecular alterations in human CRC cells were correspondingly observed in CRC animal models treated with whole brown algae, its Fx-rich extract or Fx itself. Consecutive studies in vitro will be important as the basis for clarifying the molecular mechanisms underlying cancer prevention in humans with CRC and CRC animal models by brown algae and Fx. Further studies are needed to confirm the anticancer mechanisms in both CRC animal models and cells.
CRC animal models have been the vehicle for many discoveries concerning the anticancer effects of whole brown algae, its Fx-rich extract and Fx itself. The TME, inflammation, oxidation and gut microbiota, which are significant factors associated with colorectal carcinogenesis, have been reported to be prime targets of Fx and were found to be altered by this agent’s cancer chemopreventive properties. In addition, the administration of Fx induced anoikis in CRC animal models (Figure 2). However, the detailed molecular mechanisms underlying the cancer chemopreventive effect in animals remain poorly understood.
Few reports on the direct anticancer effects of Fx intake on human CRC have been published. However, the negative association between the intake of seaweed likely including brown algae and human CRC has been reported. IBD, heritable factors, obesity, diabetes, metabolic syndrome, inflammation and oxidation are suggested as key risks triggering colorectal carcinogenesis. Interventional approaches have revealed that the administration of whole brown algae, its Fx-rich extract and Fx itself can ameliorate most of these CRC risks (Table 3). However, the underlying mechanisms remain elusive. Further clinical investigations are needed to assess the anticancer effect of Fx in humans.
Finally, this review suggests that whole brown algae, its Fx-rich extract, Fx and FxOH may be potential candidates as beneficial agents for preventing CRC.
Author Contributions
Conceptualization, M.T.; writing—original draft preparation, M.T. and T.T.; writing—review and editing, A.K., H.K., H.M., K.M., C.K. and M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part by the Japan Society for the Promotion of Science KAKENHI (nos. 16K07880 and 20K05879).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ferlay J., Colombet M., Soerjomataram I., Mathers C., Parkin D.M., Piñeros M., Znaor A., Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer. 2019;144:1941–1953. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 2.Arnold M., Sierra M.S., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683–691. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 3.American Cancer Society Colorectal Cancer Facts & Figures 2020–2022. [(accessed on 20 February 2021)]; Available online: https://www.cancer.org/research/cancer-facts-statistics/colorectal-cancer-facts-figures.html.
- 4.Eaden J.A., Abrams K.R., Mayberry J.F. The risk of colorectal cancer in ulcerative colitis: A meta-analysis. Gut. 2001;48:526–535. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernstein C.N., Blanchard J.F., Kliewer E., Wajda A. Cancer risk in patients with inflammatory bowel disease: A population-based study. Cancer. 2001;91:854–862. doi: 10.1002/1097-0142(20010215)91:4<854::AID-CNCR1073>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 6.Boland P.M., Yurgelun M.B., Boland C.R. Recent progress in Lynch syndrome and other familial colorectal cancer syndromes. CA Cancer. J. Clin. 2018;68:217–231. doi: 10.3322/caac.21448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jasperson K.W., Tuohy T.M., Neklason D.W., Burt R.W. Hereditary and familial colon cancer. Gastroenterology. 2010;138:2044–2058. doi: 10.1053/j.gastro.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Cancer Research Fund/American Institute for Cancer Research . Diet, Nutrition, Physical Activity and Colorectal Cancer. World Cnacer Research Fund International; London, UK: 2018. pp. 1–111. [Google Scholar]
- 9.Cancer Genome Atlas Network Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pearlman R., Frankel W.L., Swanson B., Zhao W., Yilmaz A., Miller K., Bacher J., Bigley C., Nelsen L., Goodfellow P.J., et al. Prevalence and spectrum of germline cancer susceptibility gene mutations among patients with early-onset colorectal cancer. JAMA Oncol. 2017;3:464–471. doi: 10.1001/jamaoncol.2016.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fearon E.R. Molecular genetics of colorectal cancer. Annu. Rev. Pathol. 2011;6:479–507. doi: 10.1146/annurev-pathol-011110-130235. [DOI] [PubMed] [Google Scholar]
- 12.Yuan W., Li X., Liu L., Wei C., Sun D., Peng S., Jiang L. Comprehensive analysis of lncRNA-associated ceRNA network in colorectal cancer. Biochem. Biophys. Res. Commun. 2019;508:374–379. doi: 10.1016/j.bbrc.2018.11.151. [DOI] [PubMed] [Google Scholar]
- 13.Wu Y., Wang X., Wu F., Huang R., Xue F., Liang G., Tao M., Cai P., Huang Y. Transcriptome profiling of the cancer, adjacent non-tumor and distant normal tissues from a colorectal cancer patient by deep sequencing. PLoS ONE. 2012;7:e41001. doi: 10.1371/journal.pone.0041001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fehlker M., Huska M.R., Jöns T., Andrade-Navarro M.A., Kemmner W. Concerted down-regulation of immune-system related genes predicts metastasis in colorectal carcinoma. BMC Cancer. 2014;14:64. doi: 10.1186/1471-2407-14-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Keefe S.J. Diet, microorganisms and their metabolites, and colon cancer. Nat. Rev. Gastroenterol. Hepatol. 2016;13:691–706. doi: 10.1038/nrgastro.2016.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Margetis N., Kouloukoussa M., Pavlou K., Vrakas S., Mariolis-Sapsakos T. K-ras mutations as the earliest driving force in a subset of colorectal carcinomas. In Vivo. 2017;31:527–542. doi: 10.21873/invivo.11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vogelstein B., Papadopoulos N., Velculescu V.E., Zhou S., Diaz L.A., Jr., Kinzler K.W. Cancer genome landscapes. Science. 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leslie A., Carey F.A., Pratt N.R., Steele R.J.C. The colorectal adenoma-carcinoma sequence. Br. J. Surg. 2002;89:845–860. doi: 10.1046/j.1365-2168.2002.02120.x. [DOI] [PubMed] [Google Scholar]
- 19.Fearon E.R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-I. [DOI] [PubMed] [Google Scholar]
- 20.Medema J.P., Vermeulen L. Microenvironmental regulation of stem cells in intestinal homeostasis and cancer. Nature. 2011;474:318–326. doi: 10.1038/nature10212. [DOI] [PubMed] [Google Scholar]
- 21.Mariani F., Sena P., Roncucci L. Inflammatory pathways in the early steps of colorectal cancer development. World J. Gastroenterol. 2014;20:9716–9731. doi: 10.3748/wjg.v20.i29.9716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Legitimo A., Consolini R., Failli A., Orsini G., Spisni R. Dendritic cell defects in the colorectal cancer. Hum. Vaccin. Immunother. 2014;10:3224–3235. doi: 10.4161/hv.29857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liaaen-Jensen S. Marine Carotenoids. In: Scheuer P.J., editor. Marine Natural Products; Chemical and Biological Perspectives. Academic Press; New York, NY, USA: 1978. pp. 1–73. [Google Scholar]
- 24.Tavares R.S.N., Maria-Engler S.S., Colepicolo P., Debonsi H.M., Schäfer-Korting M., Marx U., Gaspar L.R., Zoschke C. Skin irritation testing beyond tissue viability: Fucoxanthin effects on inflammation, homeostasis, and metabolism. Pharmaceutics. 2020;12:136. doi: 10.3390/pharmaceutics12020136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beppu F., Niwano Y., Tsukui T., Hosokawa M., Miyashita K. Single and repeated oral dose toxicity study of fucoxanthin (FX), a marine carotenoid, in mice. J. Toxicol. Sci. 2009;34:501–510. doi: 10.2131/jts.34.501. [DOI] [PubMed] [Google Scholar]
- 26.Iio K., Okada Y., Ishikura M. Single and 13-week oral toxicity study of fucoxanthin oil from microalgae in rats. Sokuhin Eiseigaku Zasshi. 2011;52:183–189. doi: 10.3358/shokueishi.52.183. [DOI] [PubMed] [Google Scholar]
- 27.Asai A., Yonekura L., Nagao A. Low bioavailability of dietary epoxyxanthophylls in humans. Br. J. Nutr. 2008;100:273–277. doi: 10.1017/S0007114507895468. [DOI] [PubMed] [Google Scholar]
- 28.Hashimoto T., Ozaki Y., Mizuno M., Yoshida M., Nishitani Y., Azuma T., Komoto A., Maoka T., Tanino Y., Kanazawa K. Pharmacokinetics of fucoxanthinol in human plasma after the oral administration of kombu extract. Br. J. Nutr. 2012;107:1566–1569. doi: 10.1017/S0007114511004879. [DOI] [PubMed] [Google Scholar]
- 29.Hashimoto T., Ozaki Y., Taminato M., Das S.K., Mizuno M., Yoshimura K., Maoka T., Kanazawa K. The distribution and accumulation of fucoxanthin and its metabolites after oral administration in mice. Br. J. Nutr. 2009;102:242–248. doi: 10.1017/S0007114508199007. [DOI] [PubMed] [Google Scholar]
- 30.Yonekura L., Kobayashi M., Terasaki M., Nagao A. Keto-carotenoids are the major metabolites of dietary lutein and fucoxanthin in mouse tissues. J. Nutr. 2010;140:1824–1831. doi: 10.3945/jn.110.126466. [DOI] [PubMed] [Google Scholar]
- 31.Shiratori K., Ohgami K., Ilieva I., Jin X.H., Koyama Y., Miyashita K., Yoshida K., Kase S., Ohno S. Effects of fucoxanthin on lipopolysaccharide-induced inflammation in vitro and in vivo. Exp. Eye Res. 2005;81:422–428. doi: 10.1016/j.exer.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Hitoe S., Shimoda H. Seaweed fucoxanthin supplementation improves obesity parameters in mild obese Japanese subjects. Func. Foods Health Dis. 2017;7:246–262. doi: 10.31989/ffhd.v7i4.333. [DOI] [Google Scholar]
- 33.Mikami N., Hosokawa M., Miyashita K., Sohma H., Ito Y.M., Kokai Y. Reduction of HbA1c levels by fucoxanthin-enriched akamoku oil possibly involves the thrifty allele of uncoupling protein 1 (UCP1): A randomised controlled trial in normal-weight and obese Japanese adults. J. Nutr. Sci. 2017;6:e5. doi: 10.1017/jns.2017.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grasa-López A., Miliar-Garcia Á., Quevedo-Corona L., Paniagua-Castro N., Escalona-Cardoso G., Reyes-Maldonado E., Jaramillo-Flores M.E. Undaria pinnatifida and fucoxanthin ameliorate lipogenesis and markers of both inflammation and cardiovascular dysfunction in an animal model of diet-induced obesity. Mar. Drugs. 2016;14:148. doi: 10.3390/md14080148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karpiński T.M., Adamczak A. Fucoxanthin-An antibacterial carotenoid. Antioxidants. 2019;8:239. doi: 10.3390/antiox8080239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sachindra N.M., Sato E., Maeda H., Hosokawa M., Niwano Y., Kohno M., Miyashita K. Radical scavenging and singlet oxygen quenching activity of marine carotenoid fucoxanthin and its metabolites. J. Agric. Food Chem. 2007;55:8516–8522. doi: 10.1021/jf071848a. [DOI] [PubMed] [Google Scholar]
- 37.Chen S.J., Lee C.J., Lin T.B., Peng H.Y., Liu H.J., Chen Y.S., Tseng K.W. Protective effects of fucoxanthin on Ultraviolet B-induced corneal denervation and inflammatory pain in a rat model. Mar. Drugs. 2019;17:152. doi: 10.3390/md17030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sugawara T., Matsubara K., Akagi R., Mori M., Hirata T. Antiangiogenic activity of brown algae fucoxanthin and its deacetylated product, fucoxanthinol. J. Agric. Food Chem. 2006;54:9805–9810. doi: 10.1021/jf062204q. [DOI] [PubMed] [Google Scholar]
- 39.Ikeda K., Kitamura A., Machida H., Watanabe M., Negishi H., Hiraoka J., Nakano T. Effect of Undaria pinnatifida (Wakame) on the development of cerebrovascular diseases in stroke-pone spontaneously hypertensive rats. Clin. Exp. Pharmacol. Physiol. 2003;30:44–48. doi: 10.1046/j.1440-1681.2003.03786.x. [DOI] [PubMed] [Google Scholar]
- 40.Zhang L., Wang H., Fan Y., Gao Y., Li X., Hu Z., Ding K., Wang Y., Wang X. Fucoxanthin provides neuroprotection in models of traumatic brain injury via the Nrf2-ARE and Nrf2-autophagy pathways. Sci. Rep. 2017;7:46763. doi: 10.1038/srep46763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim J.M., Araki S., Kim D.J., Park C.B., Takasuka N., Baba-Toriyama H., Ota T., Nir Z., Khachik F., Shimidzu N., et al. Chemopreventive effects of carotenoids and curcumins on mouse colon carcinogenesis after 1,2-dimethylhydrazine initiation. Carcinogenesis. 1998;19:81–85. doi: 10.1093/carcin/19.1.81. [DOI] [PubMed] [Google Scholar]
- 42.Nishino H., Murakoshi M., Tokuda H., Satomi Y. Cancer prevention by carotenoids. Arch. Biochem. Biophys. 2009;483:165–168. doi: 10.1016/j.abb.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 43.Dawes C.J., editor. Marine Botany. John Wiley & Sons, Inc.; New York, NY, USA: 1998. p. 480. [Google Scholar]
- 44.McHugh D.J. A Guide to the Seaweed Industry. Food and Agriculture Organization of the United Nations; Rome, Italy: 2003. p. 105. FAO Fisheries Technical Paper No.441. [Google Scholar]
- 45.Holland J. Seaweed on Track to Become Europe’s Next Big Superfood Trend. [(accessed on 27 February 2021)];2016 Available online: https://www.seafoodsource.com/news/food-safety-health/seaweed-on-track-to-become-europe-s-next-big-superfood-trend.
- 46.Mintel Press Team Seaweed-Flavoured Food and Drink Launches Increased by 147% in Europe between 2011 and 2015. [(accessed on 27 February 2021)];2016 Available online: https://www.mintel.com/press-centre/food-and-drink/seaweed-flavoured-food-and-drink-launches-increased-by-147-in-europe-between-2011-and-2015.
- 47.CEVA Edible seaweed and microalgae-regulatory status in France and Europe. 19 March 2020 update. [(accessed on 27 February 2021)]; Available online: https://www.ceva-algues.com/en/document/edible-algae-regulatory-update/
- 48.Mori K., Ooi T., Hiraoka M., Oka N., Hamada H., Tamura M., Kusumi T. Fucoxanthin and its metabolites in edible brown algae cultivated in deep seawater. Mar. Drugs. 2004;2:63–72. doi: 10.3390/md202063. [DOI] [Google Scholar]
- 49.Terasaki M., Hirose A., Narayan B., Baba Y., Kawagoe C., Yasui H., Saga N., Hosokawa M., Miyashita K. Evaluation of recoverable functional lipid components of several brown seaweeds (phaeophyta) from Japan with special reference to fucoxanthin and fucosterol contents. J. Phycol. 2009;45:974–980. doi: 10.1111/j.1529-8817.2009.00706.x. [DOI] [PubMed] [Google Scholar]
- 50.Terasaki M., Narayan B., Kamogawa H., Nomura M., Stephen N.M., Kawagoe C., Hosokawa M., Miyashita K. Carotenoid profile of edible Japanese seaweeds: An improved HPLC method for separation of major carotenoids. J. Aquat. Food Prod. Technol. 2012;21:468–479. doi: 10.1080/10498850.2011.610025. [DOI] [Google Scholar]
- 51.Terasaki M., Kuramitsu Y., Kojoma M., Kim S.Y., Tanaka T., Maeda H., Miyashita K., Kawagoe C., Kohno S., Mutoh M. High fucoxanthin wakame (Undaria pinnatifida) prevents tumor microenvironment formation in an AOM/DSS mouse carcinogenic model. J. Func. Foods. 2020;64:103709. doi: 10.1016/j.jff.2019.103709. [DOI] [Google Scholar]
- 52.Fung A., Hamid N., Lu J. Fucoxanthin content and antioxidant properties of Undaria pinnatifida. Food Chem. 2013;136:1055–1062. doi: 10.1016/j.foodchem.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 53.Campbell S.J., Bité J.S., Burridge T.R. Seasonal patterns in the photosynthetic capacity, tissue pigment and nutritional content of different developmental stages of Undaria pinnatifida (Phaeophyta: Laminariales) in Port Phillip Bay, south-eastern Australia. Bot. Mar. 1999;42:231–241. doi: 10.1515/BOT.1999.027. [DOI] [Google Scholar]
- 54.Shannon E., Abu-Ghannam N. Optimisation of fucoxanthin extraction from Irish seaweeds by response surface methodology. J. Appl. Phycol. 2017;29:1027–1036. doi: 10.1007/s10811-016-0983-4. [DOI] [Google Scholar]
- 55.Sivagnanam S.P., Yin S., Choi J.H., Park Y.B., Woo H.C., Chun B.S. Biological properties of fucoxanthin in oil recovered from two brown seaweeds using supercritical CO2 extraction. Mar. Drugs. 2015;13:3422–3442. doi: 10.3390/md13063422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sudhakar M.P., Ananthalakshmi J.S., Beena B.N. Extraction, purification and study on antioxidant properties of fucoxanthin from brown seaweeds. J. Chem. Pharm. Res. 2013;5:169–175. [Google Scholar]
- 57.Jaswir I., Noviendri D., Salleh H.M., Miyashita K. Fucoxanthin extractions of brown seaweeds and analysis of their lipid fraction in methanol. Food Sci. Technol. Res. 2012;18:251–257. doi: 10.3136/fstr.18.251. [DOI] [Google Scholar]
- 58.Fariman G.A., Shastan S.J., Zahedi M.M. Seasonal variation of total lipid, fatty acids, fucoxanthin content, and antioxidant properties of two tropical brown algae (Nizamuddinia zanardinii and Cystoseira indica) from Iran. J. Appl. Phycol. 2016;28:1323–1331. doi: 10.1007/s10811-015-0645-y. [DOI] [Google Scholar]
- 59.Susanto E., Fahmi A.S., Abe M., Hosokawa M., Miyashita K. Lipids, fatty acids, and fucoxanthin content from template and tropical brown seaweeds. Aquat. Procedia. 2016;7:66–75. doi: 10.1016/j.aqpro.2016.07.009. [DOI] [Google Scholar]
- 60.Kanazawa K., Ozaki Y., Hashimoto T., Das S.K., Matsushita S., Hirano M., Okada T., Komoto A., Mori N., Nakatsuka M. Commercial-scale preparation of biofunctional fucoxanthin from waste parts of brown sea algae Laminalia japonica. Food Sci. Technol. Res. 2008;14:573–582. doi: 10.3136/fstr.14.573. [DOI] [Google Scholar]
- 61.Xu S., Liao W.C., Chen W., Kang B., Chen J., Lin Y. Study of microwave synergistic enzyme method for extraction from Laminaria Japonica by response surface methodology. Earth Environ. Sci. 2018;146:012077. doi: 10.1088/1755-1315/146/1/012077. [DOI] [Google Scholar]
- 62.Rajauria G., Foley B., Abu-Ghannam N. Characterization of dietary fucoxanthin from Himanthalia elongata brown seaweed. Food Res. Int. 2017;99:995–1001. doi: 10.1016/j.foodres.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 63.Amorim-Carrilho K., Lage-Yusty M.A., López-Hernández J. Variation of bioactive compounds in dried seaweed Himanthalia elongata subjected to different culinary processes. CyTA-J. Food. 2014;12:336–339. doi: 10.1080/19476337.2013.877082. [DOI] [Google Scholar]
- 64.Mise T., Ueda M., Yasumoto T. Production of fucoxanthin-rich powder from Cladosiphon okamuranus. Adv. J. Food Sci. Technol. 2011;3:73–76. [Google Scholar]
- 65.Jaswir I., Noviendri D., Salleh H.M., Taher M., Miyashita K. Isolation of fucoxanthin and fatty acids analysis of Padina australis and cytotoxic effect of fucoxanthin on human lung cancer (H1299) cell lines. African J. Biotechnol. 2011;10:18855–18862. [Google Scholar]
- 66.Miyashita K., Takagi T. Tocopherol content of Japanese algae and its seasonal variation. Agric. Biol. Chem. 1987;51:3115–3118. doi: 10.1080/00021369.1987.10868522. [DOI] [Google Scholar]
- 67.Lohr M., Wilhelm C. Algae displaying the diadinoxanthin cycle also possess the violaxanthin cycle. Proc. Natl. Acad. Sci. USA. 1999;96:8784–8789. doi: 10.1073/pnas.96.15.8784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Petrushkina M., Gusev E., Sorokin B., Zotko N., Mamaeva A., Filimonova A., Kulikovskiy M., Maltsev Y., Yampolsky I., Guglya E., et al. Fucoxanthin production by heterokont microalgae. Algal Res. 2017;24:387–393. doi: 10.1016/j.algal.2017.03.016. [DOI] [Google Scholar]
- 69.Kim S.M., Kang S.W., Kwon O., Chung D., Pan C.H. Fucoxanthin as a major carotenoid in Isochrysis aff. Galbana: Characterization of extraction for commercial application. J. Korean Soc. Appl. Biol. Chem. 2012;55:477–483. doi: 10.1007/s13765-012-2108-3. [DOI] [Google Scholar]
- 70.Xia S., Wang K., Wan L., Li A., Hu Q., Zhang C. Production, characterization, and antioxidant activity of fucoxanthin from the marine diatom Odontella Aurita. Mar. Drugs. 2013;11:2667–2681. doi: 10.3390/md11072667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Teramukai K., Kakui S., Beppu F., Hosokawa M., Miyashita K. Effective extraction of carotenoids from brown seaweeds and vegetable leaves with edible oils. Innov. Food Sci. Emerg. Technol. 2020;60:102302. doi: 10.1016/j.ifset.2020.102302. [DOI] [Google Scholar]
- 72.Lourenço-Lopes C., Garcia-Oliveira P., Carpena M., Fraga-Corral M., Jimenez-Lopez C., Pereira A.G., Prieto M.A., Simal-Gandara J. Scientific approaches on extraction, purification and stability for the commercialization of fucoxanthin recovered from brown algae. Foods. 2020;9:1113. doi: 10.3390/foods9081113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Okada T., Mizuno Y., Sibayama S., Hosokawa M., Miyashita K. Antiobesity effects of Undaria lipid capsules prepared with scallop phospholipid. J. Food Sci. 2011;76:H2–H6. doi: 10.1111/j.1750-3841.2010.01878.x. [DOI] [PubMed] [Google Scholar]
- 74.Mok I.K., Yoon J.R., Pan C.H., Kim S.M. Development, quantification, method validation, and stability study of a novel fucoxanthin-fortified milk. J. Agric. Food Chem. 2016;64:6196–6202. doi: 10.1021/acs.jafc.6b02206. [DOI] [PubMed] [Google Scholar]
- 75.Sasaki K., Ishihara K., Oyamada C., Sato A., Fukushi A., Arakane T., Motoyama M., Yamazaki M., Mitsumoto M. Effects of fucoxanthin addition to ground chicken breast meat on lipid and colour stability during chilled storage, before and after cooking. Asian-Australas. J. Anim. Sci. 2008;21:1067–1072. doi: 10.5713/ajas.2008.70670. [DOI] [Google Scholar]
- 76.Sugimura R., Suda M., Sho A., Takahashi T., Sashima T., Abe M., Hosokawa M., Miyashita K. Stability of fucoxanthin in dried Undaria pinnatifida (wakame) and baked products (scones) containing wakame powder. Food Sci. Technol. Res. 2012;18:687–693. doi: 10.3136/fstr.18.687. [DOI] [Google Scholar]
- 77.Robertson R.C., Gracia Mateo M.R., O’Grady M.N., Guihéneuf F., Stengel D.B., Ross R.P., Fitzgerald G.F., Kerry J.P., Stanton C. An assessment of the techno-functional and sensory properties of yoghurt fortified with a lipid extract from the microalga Pavlova lutheri. Innov. Food Sci. Emerg. Technol. 2016;37:237–246. doi: 10.1016/j.ifset.2016.03.017. [DOI] [Google Scholar]
- 78.Nishibori N., Itoh M., Kashiwagi M., Arimochi H., Morita K. In vitro cytotoxic effect of ethanol extract prepared from sporophyll of Undaria pinnatifida on human colorectal cancer cells. Phytother. Res. 2012;26:191–196. doi: 10.1002/ptr.3527. [DOI] [PubMed] [Google Scholar]
- 79.Kim A.D., Kang K.A., Piao M.J., Kim K.C., Zheng J., Yao C.W., Cha J.W., Hyun C.L., Boo S.J., Lee N.H., et al. Dictyopteris undulata extract induces apoptosis in human colon cancer cells. Biotechnol. Bioproc. Eng. 2014;19:419–425. doi: 10.1007/s12257-014-0200-8. [DOI] [Google Scholar]
- 80.Kang K.A., Kim J.K., Jeong Y.J., Na S.Y., Hyun J.W. Dictyopteris undulata extract induces apoptosis via induction of endoplasmic reticulum stress in human colon cancer cells. J. Cancer Prev. 2014;19:118–124. doi: 10.15430/JCP.2014.19.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ye B.R., Kim J., Kim M.S., Jang J., Oh C., Kang D.H., Qian Z.J., Jung W.K., Choi I.W., Heo S.J. Induction of apoptosis by the tropical seaweed Pylaiella littoralis in HT-29 cells via the mitochondrial and MAPK pathways. Ocean Sci. J. 2013;48:338–348. doi: 10.1007/s12601-013-0032-z. [DOI] [Google Scholar]
- 82.Mahmoud A.M., Abdella E.M., El-Derby A.M., Abdella E.M. Protective effects of Turbinaria ornata and Padina pavonia against azoxymethane-induced colon carcinogenesis through modulation of PPAR gamma, NF-κB and oxidative stress. Phytother. Res. 2015;29:737–748. doi: 10.1002/ptr.5310. [DOI] [PubMed] [Google Scholar]
- 83.Mhadhebi L., Laroche-Clary A., Robert J., Bouraoui A. Antioxidant, anti-inflammatory, and antiproliferative activities of organic fractions from the Mediterranean brown seaweed Cystoseira sedoides. Can. J. Physiol. Pharmacol. 2011;89:911–921. doi: 10.1139/y11-093. [DOI] [PubMed] [Google Scholar]
- 84.Terasaki M., Takahashi S., Nishimura R., Kubota A., Kojima H., Ohta T., Hamada J., Kuramitsu Y., Maeda H., Miyashita K., et al. A marine carotenoid of fucoxanthinol accelerates the growth of human pancreatic cancer PANC-1 cells. Nutr. Cancer. 2021:1–16. doi: 10.1080/01635581.2020.1863994. [DOI] [PubMed] [Google Scholar]
- 85.Yokoyama R., Kojima H., Takai R., Ohta T., Maeda H., Miyashita K., Mutoh M., Terasaki M. Effects of CLIC4 on fucoxanthinol-induced apoptosis in human colorectal cancer cells. Nutr Cancer. 2021;73:889–898. doi: 10.1080/01635581.2020.1779760. [DOI] [PubMed] [Google Scholar]
- 86.Terasaki M., Maeda H., Miyashita K., Mutoh M. Induction of anoikis in human colorectal cancer cells by fucoxanthinol. Nutr. Cancer. 2017;69:1043–1052. doi: 10.1080/01635581.2017.1339814. [DOI] [PubMed] [Google Scholar]
- 87.Tamura S., Narita T., Fujii G., Miyamoto S., Hamoya T., Kurokawa Y., Takahashi M., Miki K., Matsuzawa Y., Komiya M., et al. Inhibition of NF-kappaB transcriptional activity enhances fucoxanthinol-induced apoptosis in colorectal cancer cells. Genes Environ. 2019;41:1. doi: 10.1186/s41021-018-0116-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lopes-Costa E., Abreu M., Gargiulo D., Roche E., Ramos A.A. Anticancer effects of seaweed compounds fucoxanthin and phloroglucinol, alone and in combination with 5-fluorouracil in colon cells. J. Toxicol. Environ. Health A. 2017;80:776–787. doi: 10.1080/15287394.2017.1357297. [DOI] [PubMed] [Google Scholar]
- 89.Das S.K., Hashimoto T., Shimizu K., Yoshida T., Sakai T., Sowa Y., Komoto A., Kanazawa K. Fucoxanthin induces cell cycle arrest at G0/G1 phase in human colon carcinoma cells through up-regulation of p21WAF1/Cip1. Biochim. Biophys. Acta. 2005;1726:328–335. doi: 10.1016/j.bbagen.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 90.Hosokawa M., Kudo M., Maeda H., Kohno H., Tanaka T., Miyashita K. Fucoxanthin induces apoptosis and enhances the antiproliferative effect of the PPARγ ligand, troglitazone, on colon cancer cells. Biochim. Biophys. Acta. 2004;1675:113–119. doi: 10.1016/j.bbagen.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 91.Konishi I., Hosokawa M., Sashima T., Kobayashi H., Miyashita K. Halocynthiaxanthin and fucoxanthinol isolated from Halocynthia roretzi induce apoptosis in human leukemia, breast and colon cancer cells. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2006;142:53–59. doi: 10.1016/j.cbpc.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 92.Manmuan S., Manmuan P. Fucoxanthin enhances 5-FU chemotherapeutic efficacy in colorectal cancer cells by affecting MMP-9 invasive proteins. J. Appl. Pharm. Sci. 2019;9:007–014. doi: 10.7324/JAPS.2019.91202. [DOI] [Google Scholar]
- 93.Takahashi K., Hosokawa M., Kasajima H., Hatanaka K., Kudo K., Shimoyama N., Miyashita K. Anticancer effects of fucoxanthin and fucoxanthinol on colorectal cancer cell lines and colorectal cancer. Oncol. Lett. 2015;10:1463–1467. doi: 10.3892/ol.2015.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dalerba P., Dylla S.J., Park I.K., Liu R., Wang X., Cho R.W., Hoey T., Gurney A., Huang E.H., Simeone D.M., et al. Phenotypic characterization of human colorectal cancer stem cells. Proc. Natl. Acad. Sci. USA. 2007;104:10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vermeulen L., Todaro M., de Sousa Mello F., Sprick M.R., Kemper K., Alea M.P., Richel D.J., Stassi G., Medema J.P. Single-cell cloning of colon cancer stem cells reveals a multi-lineage differentiation capacity. Proc. Natl. Acad. Sci. USA. 2008;105:13427–13432. doi: 10.1073/pnas.0805706105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kanwar S.S., Yu Y., Nautiyal J., Patel B.B., Majumdar A.P.N. The Wnt/β-catenin pathway regulates growth and maintenance of colonospheres. Mol. Cancer. 2010;9:212. doi: 10.1186/1476-4598-9-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Terasaki M., Maeda H., Miyashita K., Tanaka T., Miyamoto S., Mutoh M. A marine bio-functional lipid, fucoxanthinol, attenuates human colorectal cancer stem-like cell tumorigenicity and sphere formation. J. Clin. Biochem. Nutr. 2017;61:25–32. doi: 10.3164/jcbn.16-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Terasaki M., Mima M., Kudoh S., Endo T., Maeda H., Hamada J., Osada K., Miyashita K., Mutoh M. Glycine and succinic acid are effective indicators of the suppression of epithelial-mesenchymal transition by fucoxanthinol in colorectal cancer stem-like cells. Oncol. Rep. 2018;40:414–424. doi: 10.3892/or.2018.6398. [DOI] [PubMed] [Google Scholar]
- 99.Terasaki M., Ogawa Y., Endo T., Maeda H., Hamada J., Osada K., Miyashita K., Mutoh M. Glycine is a predictor for a suppressive effect of fucoxanthinol on colonosphere formation under hypoxia. Anticancer Res. 2018;38:2169–2179. doi: 10.21873/anticanres.12458. [DOI] [PubMed] [Google Scholar]
- 100.De la Mare J., Sterrenberg J.N., Sukhthankar M.G., Chiwakata M.T., Beukes D.R., Blatch G.L., Edkins A.L. Assessment of potential anti-cancer stem cell activity of marine algal compounds using an in vitro mammosphere assay. Cancer Cell Int. 2013;13:39. doi: 10.1186/1475-2867-13-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kong Z.L., Kao N.J., Hu J.Y., Wu C.S. Fucoxanthin-rich brown algae extract decreases inflammation and attenuates colitis-associated colon cancer in mice. J. Food Nutr. Res. 2016;4:137–147. doi: 10.12691/jfnr-4-3-2. [DOI] [Google Scholar]
- 102.Son Y.S., Ullah H.M.A., Elfadl A.K., Ghim S.G., Chung M.J., Kim Y.D., Lee E.J., Kang K.K., Jeong K.S. Inhibition of formation of azoxymethane-induced colonic aberrant crypt foci in rats by edible green algae Capsosiphon fulvescens and brown algae Hizikia fusiforme. In Vivo. 2018;32:101–108. doi: 10.21873/invivo.11210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Das S.K., Hasshimoto T., Baba M., Nishino H., Komoto A., Kanazawa K. Japanese kelp (kombu) extract suppressed the formation of aberrant crypt foci in azoxymethane challenged mouse colon. J. Clin. Biochem. Nutr. 2006;38:119–125. doi: 10.3164/jcbn.38.119. [DOI] [Google Scholar]
- 104.Reddy B.S., Numoto S., Choi C.I. Effects of dietary Laminaria angustata (brown seaweed) on azoxymethane-induced intestinal carcinogenesis in male F344 rats. Nutr. Cancer. 1985;7:59–64. doi: 10.1080/01635588509513840. [DOI] [PubMed] [Google Scholar]
- 105.Buchheit C.L., Weigel K.J., Schafer Z.T. Cancer cell survival during detachment from the ECM: Multiple barriers to tumour progression. Nat. Rev. Cancer. 2014;14:632–641. doi: 10.1038/nrc3789. [DOI] [PubMed] [Google Scholar]
- 106.Horbinski C., Mojesky C., Kyprianou N. Live free or die: Tales of homeless (cell) in cancer. Am. J. Pathol. 2010;177:1044–1052. doi: 10.2353/ajpath.2010.091270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Paoli P., Giannoni E., Chiarugi P. Anoikis molecular pathways and its role in cancer progression. Biochim. Biophys. Acta. 2013;1833:3481–3498. doi: 10.1016/j.bbamcr.2013.06.026. [DOI] [PubMed] [Google Scholar]
- 108.Terasaki M., Iida T., Kikuchi F., Tamura K., Endo T., Kuramitsu Y., Tanaka T., Maeda H., Miyashita K., Mutoh M. Fucoxanthin potentiates anoikis in colon mucosa and prevents carcinogenesis in AOM/DSS model mice. J. Nutr. Biochem. 2019;64:198–205. doi: 10.1016/j.jnutbio.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 109.Terasaki M., Ikuta M., Kojima H., Tanaka T., Maeda H., Miyashita K., Mutoh M. Dietary fucoxanthin induces anoikis in colorectal adenocarcinoma by suppressing integrin signaling in a murine colorectal cancer model. J. Clin. Med. 2020;9:90. doi: 10.3390/jcm9010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Findlay V.J., Wang C., Watson D.K., Camp E.R. Epithelial to mesenchymal transition and the cancer stem cell phenotype: Insights from cancer biology with therapeutic implications for colorectal cancer. Cancer Gene Ther. 2014;21:181–187. doi: 10.1038/cgt.2014.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Terasaki M., Masaka S., Fukada C., Houzaki M., Endo T., Tanaka T., Maeda H., Miyashita K., Mutoh M. Salivary glycine is a significant predictor for the attenuation of polyp and tumor microenvironment formation by fucoxanthin in AOM/DSS mice. In Vivo. 2019;33:365–374. doi: 10.21873/invivo.11483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Terasaki M., Kimura R., Kubota A., Kojima H., Tanaka T., Maeda H., Miyashita K., Mutoh M. Continuity of tumor microenvironmental suppression in AOM/DSS mice by fucoxanthin may be able to track with salivary glycine. In Vivo. 2020;34:3205–3215. doi: 10.21873/invivo.12156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Garrett W.S. The gut microbiota and colon cancer. Science. 2019;364:1133–1135. doi: 10.1126/science.aaw2367. [DOI] [PubMed] [Google Scholar]
- 114.Gagnière J., Raisch J., Veziant J., Barnich N., Bonnet R., Buc E., Bringer M.A., Pezet D., Bonnet M. Gut microbiota imbalance and colorectal cancer. World J. Gastroenterol. 2016;22:501–518. doi: 10.3748/wjg.v22.i2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Terasaki M., Uehara O., Ogasa S., Sano T., Kubota A., Kojima H., Tanaka T., Maeda H., Miyashita K., Mutoh M. Alteration of fecal microbiota by fucoxanthin results in prevention of colorectal cancer in AOM/DSS mice. Carcinogenesis. 2021;42:210–219. doi: 10.1093/carcin/bgaa100. [DOI] [PubMed] [Google Scholar]
- 116.Terasaki M., Matsumoto N., Hashimoto R., Endo T., Maeda H., Hamada J., Osada K., Miyashita K., Mutoh M. Fucoxanthin administration delays occurrence of tumors in xenograft mice by colonospheres, with an anti-tumor predictor of glycine. J. Clin. Biochem. Nutr. 2019;64:52–58. doi: 10.3164/jcbn.18-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Terasaki M., Hamoya T., Kubota A., Kojima H., Tanaka T., Maeda H., Miyashita K., Mutoh M. Fucoxanthin prevents colorectal cancer development in dextran sodium sulfate-treated ApcMin/+ mice. Anticancer Res. 2021;41:1299–1305. doi: 10.21873/anticanres.14887. [DOI] [PubMed] [Google Scholar]
- 118.Jeon S.M., Kim H.J., Woo M.N., Lee M.K., Shin Y.C., Park Y.B., Choi M.S. Fucoxanthin-rich seaweed extract suppresses body weight gain and improves lipid metabolism in high-fat-fed C57BL/6J mice. Biotechnol. J. 2010;5:961–969. doi: 10.1002/biot.201000215. [DOI] [PubMed] [Google Scholar]
- 119.Woo M.N., Jeon S.M., Shin Y.C., Lee M.K., Kang M.A., Choi M.S. Anti-obese property of fucoxanthin is partly mediated by altering lipid-regulating enzymes and uncoupling proteins of visceral adipose tissue in mice. Mol. Nutr. Food Res. 2009;53:1603–1611. doi: 10.1002/mnfr.200900079. [DOI] [PubMed] [Google Scholar]
- 120.Murakami S., Hirazawa C., Ohya T., Yoshikawa R., Mizutani T., Ma N., Moriyama M., Ito T., Matsuzaki C. The edible brown seaweed Sargassum horneri (Turner) C. Agardh ameliorates high-fat diet-induced obesity, diabetes, and hepatic steatosis in mice. Nutrients. 2021;13:551. doi: 10.3390/nu13020551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Awang A.N., Ng J.L., Matanjun P., Sulaiman M.R., Tan T.S., Ooi Y.B.H. Anti-obesity property of the brown seaweed Sargassum polycystum using an in vivo animal model. J. Appl. Phycol. 2014;26:1043–1048. doi: 10.1007/s10811-013-0149-6. [DOI] [Google Scholar]
- 122.Germoush M.O. Antioxidant and anti-inflammatory effects of Padina pavonia and Turbenaria ornate in streptozotocin/nicotinamide diabetic rats. Life Sci. J. 2013;10:1265–1271. [Google Scholar]
- 123.Kang S.I., Shin H.S., Kim H.M., Yoon S.A., Kang S.W., Kim J.H., Ko H.C., Kim S.J. Petalonia binghamiae extract and its constituent fucoxanthin ameliorate high-fat diet-induced obesity by activating AMP-activated protein kinase. J. Agric. Food Chem. 2012;60:3389–3395. doi: 10.1021/jf2047652. [DOI] [PubMed] [Google Scholar]
- 124.Maeda H., Hosokawa M., Sashima T., Funayama K., Miyashita K. Fucoxanthin from edible seaweed, Undaria pinnatifida, shows antiobesity effect through UCP1 expression in white adipose tissues. Biochem. Biophys. Res. Commun. 2005;332:392–397. doi: 10.1016/j.bbrc.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 125.Maeda H., Hosokawa M., Sashima T., Funayama K., Miyashita K. Effect of medium-chain triacylglycerols on anti-obesity effect of fucoxanthin. J. Oleo Sci. 2007;56:615–621. doi: 10.5650/jos.56.615. [DOI] [PubMed] [Google Scholar]
- 126.Maeda H., Hosokawa M., Sashima T., Miyashita K. Dietary combination of fucoxanthin and fish oil attenuates the weight gain of white adipose tissue and decreases blood glucose in obese/diabetic KK-Ay mice. J. Agric. Food Chem. 2007;55:7701–7706. doi: 10.1021/jf071569n. [DOI] [PubMed] [Google Scholar]
- 127.Maeda H., Hosokawa M., Sashima T., Murakami-Funayama K., Miyashita K. Anti-obesity and anti-diabetic effects of fucoxanthin on diet-induced obesity conditions in a murine model. Mol. Med. Rep. 2009;2:897–902. doi: 10.3892/mmr_00000189. [DOI] [PubMed] [Google Scholar]
- 128.Hosokawa M., Miyashita T., Nishikawa S., Emi S., Tsukui T., Beppu F., Okada T., Miyashita K. Fucoxanthin regulates adipocytokine mRNA expression in white adipose tissue of diabetic/obese KK-Ay mice. Arch. Biochem. Biophys. 2010;504:17–25. doi: 10.1016/j.abb.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 129.Beppu F., Hosokawa M., Yim M.J., Shinoda T., Miyashita K. Down-regulation of hepatic stearoyl-CoA desaturase-1 expression by fucoxanthin via leptin signaling in diabetic/obese KK-A(y) mice. Lipids. 2013;48:449–455. doi: 10.1007/s11745-013-3784-4. [DOI] [PubMed] [Google Scholar]
- 130.Nishikawa S., Hosokawa M., Miyashita K. Fucoxanthin promotes translocation and induction of glucose transporter 4 in skeletal muscles of diabetic/obese KKA(y) mice. Phytomedicine. 2012;19:389–394. doi: 10.1016/j.phymed.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 131.Woo M.N., Jeon S.M., Kim H.J., Lee M.K., Shin S.K., Shin Y.C., Park Y.B., Choi M.S. Fucoxanthin supplementation improves plasma and hepatic lipid metabolism and blood glucose concentration in high-fat fed C57BL/6N mice. Chem. Biol. Interact. 2010;186:316–322. doi: 10.1016/j.cbi.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 132.Park H.J., Lee M.K., Park Y.B., Shin Y.C., Choi M.S. Beneficial effects of Undaria pinnatifida ethanol extract on diet-induced-insulin resistance in C57BL/6J mice. Food Chem. Toxicol. 2011;49:727–733. doi: 10.1016/j.fct.2010.11.032. [DOI] [PubMed] [Google Scholar]
- 133.Yang Y.P., Tong Q.Y., Zheng S.H., Zhou M.D., Zeng Y.M., Zhou T.T. Anti-inflammatory effect of fucoxanthin on dextran sulfate sodium-induced colitis in mice. Nat. Prod. Res. 2020;34:1791–1795. doi: 10.1080/14786419.2018.1528593. [DOI] [PubMed] [Google Scholar]
- 134.Kim J.Y., Yu D.Y., Kim J.A., Choi E.Y., Lee C.Y., Hong Y.H., Kim C.W., Lee S.S., Choi I.S., Cho K.K. Effects of Undaria pinnatifida and Laminaria japonica on rat’s intestinal microbiota and metabolite. J. Nutr. Food Sci. 2016;6:3. doi: 10.4172/2155-9600.1000502. [DOI] [Google Scholar]
- 135.Sun X., Zhao H., Liu Z., Sun X., Zhang D., Wang S., Xu Y., Zhang G., Wang D. Modulation of gut microbiota by fucoxanthin during alleviation of obesity in high-fat diet-fed mice. J. Agric. Food Chem. 2020;68:5118–5128. doi: 10.1021/acs.jafc.0c01467. [DOI] [PubMed] [Google Scholar]
- 136.Guo B., Yang B., Pang X., Chen T., Chen F., Cheng K.W. Fucoxanthin modulates cecal and fecal microbiota differently based on diet. Food Funct. 2019;10:5644–5655. doi: 10.1039/C9FO01018A. [DOI] [PubMed] [Google Scholar]
- 137.Liu Z., Sun X., Sun X., Wang S., Xu Y. Fucoxanthin isolated from Undaria pinnatifida can interact with Escherichia coli and lactobacilli in the intestine and inhibit the growth of pathogenic bacteria. J. Ocean Univ. China. 2019;18:926–932. doi: 10.1007/s11802-019-4019-y. [DOI] [Google Scholar]
- 138.Haslam D., Sattar N., Lean M. ABC of obesity. Obesity-time to wake up. BMJ. 2006;333:640–642. doi: 10.1136/bmj.333.7569.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Calle E.E., Rodriguez C., Walker-Thurmond K., Thun M.J. Overweight, obesity, and mortality from cancer in a prospective cohort of U.S. adults. N. Engl. J. Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 140.Renehan A.G., Tyson M., Egger M., Heller R.F., Zwahlen M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 141.Gallagher E.J., LeRoith D. Obesity and diabetes: The increased risk of cancer and cancer-related mortality. Physiol. Rev. 2015;95:727–748. doi: 10.1152/physrev.00030.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Larsson S.C., Orsini N., Wolk A. Diabetes mellitus and risk of colorectal cancer: A meta-analysis. J. Natl. Cancer Inst. 2005;97:1679–1687. doi: 10.1093/jnci/dji375. [DOI] [PubMed] [Google Scholar]
- 143.Blüher M. Adipokines-removing road blocks to obesity and diabetes therapy. Mol. Metab. 2014;3:230–240. doi: 10.1016/j.molmet.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ohashi K., Shibata R., Murohara T., Ouchi N. Role of anti-inflammatory adipokines in obesity-related diseases. Trends Endocrinol. Metab. 2014;25:348–355. doi: 10.1016/j.tem.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 145.Ouchi N., Parker J.L., Lugus J.J., Walsh K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Matsuzawa Y. The metabolic syndrome and adipocytokines. FEBS Lett. 2006;580:2917–2921. doi: 10.1016/j.febslet.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 147.Ferrante A.W., Jr. Obesity-induced inflammation: A metabolic dialogue in the language of inflammation. J. Intern. Med. 2007;262:408–414. doi: 10.1111/j.1365-2796.2007.01852.x. [DOI] [PubMed] [Google Scholar]
- 148.Brennan C.A., Garrett W.S. Gut microbiota, inflammation, and colorectal cancer. Annu. Rev. Microbiol. 2016;70:395–411. doi: 10.1146/annurev-micro-102215-095513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Beaugerie I., Itzkowitz S.H. Cancers complicating inflammatory bowel disease. N. Engl. J. Med. 2015;372:1441–1452. doi: 10.1056/NEJMra1403718. [DOI] [PubMed] [Google Scholar]
- 150.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 151.Dray A. Inflammatory mediators of pain. Br. J. Anaesth. 1995;75:125–131. doi: 10.1093/bja/75.2.125. [DOI] [PubMed] [Google Scholar]
- 152.Hotamisligil G.S. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 153.Newton K., Dixit V.M. Signaling in innate immunity and inflammation. Cold Spring Harb Perspect. Biol. 2012;4:a006049. doi: 10.1101/cshperspect.a006049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Matosevic P., Klepac-Pulanic T., Kinda E., Augustin G., Brcic I., Jakic-Razumovic J. Immunohistochemical expression of 8-oxo-7,8-dihydro-2’-deoxyguanosine in cytoplasm of tumour and adjacent normal mucosa cells in patients with colorectal cancer. World J. Surg. Oncol. 2015;13:241. doi: 10.1186/s12957-015-0667-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Guz J., Foksinski M., Siomek A., Gackowski D., Rozalski R., Dziaman T., Szpila A., Olinski R. The relationship between 8-oxo-7,8-dihydro-2’-deoxyguanosine level and extent of cytosine methylation in leukocytes DNA of healthy subjects and in patients with colon adenomas and carcinomas. Mut. Res. 2008;640:170–173. doi: 10.1016/j.mrfmmm.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 156.Van der Logt E.M.J., Roelofs H.M.J., Wobbes T., Nagengast F.M., Peters W.H.M. High oxygen radical production in patients with sporadic colorectal cancer. Free Radic. Biol. Med. 2005;39:182–187. doi: 10.1016/j.freeradbiomed.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 157.Moret-Tatay I., Iborra M., Cerrillo E., Tortosa L., Nos P., Beltrán B. Possible biomarkers in blood for Crohn’s disease: Oxidative stress and microRNAs-Current evidences and further aspects to unravel. Oxid. Med. Cell Longev. 2016;2016:2325162. doi: 10.1155/2016/2325162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Piechota-Polanczyk A., Fichna J. Review article: The role of oxidative stress in pathogenesis and treatment of inflammatory bowel diseases. Naunyn Schmiedebergs Arch. Pharmacol. 2014;387:605–620. doi: 10.1007/s00210-014-0985-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Basak D., Uddin M.N., Hancock J. The role of oxidative stress and its counteractive utility in colorectal cancer (CRC) Cancers. 2020;12:3336. doi: 10.3390/cancers12113336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Parisi E., Sorolla A., Montal R., González-Resina R., Novell A., Salud A., Sorolla M.A. Prognostic factors involved in the epithelial-mesenchymal transition process in colorectal cancer have a preponderant role in oxidative stress: A systematic review and meta-analysis. Cancers. 2020;12:3330. doi: 10.3390/cancers12113330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kawanishi S., Hiraku Y., Pinlaor S., Ma N. Oxidative and nitrative DNA damage in animals and patients with inflammatory diseases in relation to inflammation-related carcinogenesis. Biol. Chem. 2006;387:365–372. doi: 10.1515/BC.2006.049. [DOI] [PubMed] [Google Scholar]
- 162.Szatrowski T.P., Nathan C.F. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51:794–798. [PubMed] [Google Scholar]
- 163.Oberley T.D., Oberley L.W. Antioxidant enzyme levels in cancer. Histol. Histopathol. 1997;12:525–535. [PubMed] [Google Scholar]
- 164.Kumar B., Koul S., Khandrika L., Meacham R.B., Koul H.K. Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res. 2008;68:1777–1785. doi: 10.1158/0008-5472.CAN-07-5259. [DOI] [PubMed] [Google Scholar]
- 165.Martinez-Sánchez G., Giuliani A. Cellular redox status regulates hypoxia inducible factor-1 activity. Role in tumour development. J. Exp. Clin. Cancer Res. 2007;26:39–50. [PubMed] [Google Scholar]
- 166.Adibov M., Ramazanov Z., Seifulla R., Grachev S. The effects of Xanthigen in the weight management of obese premenopausal women with non-alcoholic fatty liver disease and normal liver fat. Diabetes Obes. Metab. 2010;12:72–81. doi: 10.1111/j.1463-1326.2009.01132.x. [DOI] [PubMed] [Google Scholar]
- 167.Miyashita K., Beppu F., Hosokawa M., Liu X., Wang S. Nutraceutical characteristics of the brown seaweed carotenoid fucoxanthin. Arch. Biochem. Biophys. 2020;686:108364. doi: 10.1016/j.abb.2020.108364. [DOI] [PubMed] [Google Scholar]
- 168.Seale P., Kajimura S., Spiegelman B.M. Transcriptional control of brown adipocyte development and physiological function-of mice and men. Genes Dev. 2009;23:788–797. doi: 10.1101/gad.1779209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Brestoff J.R., Artis D. Immune regulation of metabolic homeostasis in health and disease. Cell. 2015;161:146–160. doi: 10.1016/j.cell.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Harms M., Seale P. Brown and beige fat: Development, function and therapeutic potential. Nat. Med. 2013;19:1252–1263. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- 171.Baldrick F.R., McFadden K., Ibars M., Sung C., Moffatt T., Megarry K., Thomas K., Mitchell P., Wallace J.M.W., Pourshahidi L.K., et al. Impact of a (poly)phenol-rich extract from the brown algae Ascophyllum nodosum on DNA damage and antioxidant activity in an overweight or obese population: A randomized controlled trial. Am. J. Clin. Nutr. 2018;108:688–700. doi: 10.1093/ajcn/nqy147. [DOI] [PubMed] [Google Scholar]
- 172.Lacoviello L., Zito F., Rago L., Di Castelnuovo A., De Curtis A., Zappacosta B., de Gaetano G., Donati M.B., Cerletti C. Prolong administration of Ascophyllum nodosum to healthy human volunteers and cardiovascular risk. Nutrafoods. 2013;12:137–144. doi: 10.1007/s13749-013-0059-x. [DOI] [Google Scholar]
- 173.Tanemura Y., Yamanaka-Okumura H., Sakuma M., Nii Y., Taketani Y., Takeda E. Effects of the intake of Undaria pinnatifida (Wakame) and its sporophylls (Mekabu) on postprandial glucose and insulin metabolism. J. Med. Investig. 2014;61:291–294. doi: 10.2152/jmi.61.291. [DOI] [PubMed] [Google Scholar]
- 174.Yoshinaga K., Mitamura R. Effects of Undaria pinnatifida (Wakame) on postprandial glycemia and insulin levels in humans: A randomized crossover trial. Plant Foods Hum. Nutr. 2019;74:461–467. doi: 10.1007/s11130-019-00763-5. [DOI] [PubMed] [Google Scholar]
- 175.Teas J., Baldeón M.E., Chiriboga D.E., Davis J.R., Sarriés A.J., Braverman L.E. Could dietary seaweed reverse the metabolic syndrome? Asia Pac. J. Clin. Nutr. 2009;18:145–154. [PubMed] [Google Scholar]
- 176.Izaola O., Primo D., Bargués D.R., Martín-Diana A.B., Villaluenga C.M., Miranda J., de Luis Román D. Effects of a snack enriched with carob and Undaria pinnatifida (wakame) on metabolic parameters in a double blind, randomized clinical trial in obese patient. Nutr. Hosp. 2020;34:465–473. doi: 10.20960/nh.02906. [DOI] [PubMed] [Google Scholar]
- 177.Kang Y.M., Lee B.J., Kim J.I., Nam B.H., Cha J.Y., Kim Y.M., Ahn C.B., Choi J.S., Choi I.S., Je J.Y. Antioxidant effects of fermented sea tangle (Laminaria japonica) by Lactobacillus brevis BJ20 in individuals with high level of γ-GT: A randomized, double-blind, and placebo-controlled clinical study. Food Chem. Toxicol. 2012;50:1166–1169. doi: 10.1016/j.fct.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 178.Nishiumi S., Izumi Y., Kobayashi T., Yoshida M. Possible involvement of lipids in the effectiveness of kombu in individuals with abnormally high serum triglyceride levels. J. Nutr. Sci. Vitaminol. 2020;66:185–190. doi: 10.3177/jnsv.66.185. [DOI] [PubMed] [Google Scholar]
- 179.Nishiumi S., Izumi Y., Kobayashi T., Yoshida M. A pilot study: Effects of kombu intake on lifestyle-related diseases-possibility that kombu intake is effective in individuals with abnormally high serum triacylglycerol levels. Food Sci. Technol. Res. 2019;25:827–834. doi: 10.3136/fstr.25.827. [DOI] [Google Scholar]
- 180.Nishimura M., Sugawara M., Kudo M., Kinoshita Y., Yoshino H., Nishihira J. Effects of daily intake of Harudori-kombu: A randomized, double-blind, placebo-controlled, parallel-group study. Func. Foods Health Dis. 2019;9:205–223. doi: 10.31989/ffhd.v9i4.594. [DOI] [Google Scholar]
- 181.Rosa G.P., Tavares W.R., Sousa P.M.C., Pagès A.K., Seca A.M.L., Pinto D.C.G.A. Seaweed secondary metabolites with beneficial health effects: An overview of successes in in vitro studies and clinical trials. Mar. Drugs. 2020;18:8. doi: 10.3390/md18010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Afonso N.C., Catarino M.D., Silva A.M.S., Cardoso S.M. Brown macroalgae as valuable food ingredients. Antioxidants. 2019;8:365. doi: 10.3390/antiox8090365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wan-Loy C., Siew-Moi P. Marine algae as a potential source for anti-obesity agents. Mar. Drugs. 2016;14:222. doi: 10.3390/md14120222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hoshiyama Y., Sekine T., Sasaba T. A case-control study of colorectal cancer and its relation to diet, cigarettes, and alcohol consumption in Saitama Prefecture, Japan. Tohoku J. Exp. Med. 1993;171:153–165. doi: 10.1620/tjem.171.153. [DOI] [PubMed] [Google Scholar]
- 185.Minami Y., Kanemura S., Oikawa T., Suzuki S., Hasegawa Y., Nishino Y., Fujiya T., Miura K. Associations of Japanese food intake with survival of stomach and colorectal cancer: A prospective patient cohort study. Cancer Sci. 2020;111:2558–2569. doi: 10.1111/cas.14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kim J., Lee J., Oh J.H., Chang H.J., Sohn D.K., Shin A., Kim J. Associations among dietary seaweed intake, c-MYC rs6983267 polymorphism, and risk of colorectal cancer in a Korean population: A case-control study. Eur. J. Nutr. 2020;59:1963–1974. doi: 10.1007/s00394-019-02046-w. [DOI] [PubMed] [Google Scholar]