Abstract
We identified 1218 Campylobacter coli isolates from fecal and carcass samples of pigs (n = 643) and chickens (n = 575) between 2010 and 2018. About 99% of the isolates were resistant to at least one antimicrobial agent. The isolates exhibited high resistance rates (>75%) to ciprofloxacin, nalidixic acid, and tetracycline. Azithromycin and erythromycin resistance rates were the highest in isolates from pigs (39.7% and 39.2%, respectively) compared to those of chickens (15.8% and 16.3%, respectively). Additionally, a low-to-moderate proportion of the isolates were resistant to florfenicol, gentamicin, clindamycin, and telithromycin. Multidrug resistance (MDR) was found in 83.1% of the isolates, and profiles of MDR usually included ciprofloxacin, nalidixic acid, and tetracycline. We found point mutation (A2075G) in domain V of the 23S rRNA gene in the majority of erythromycin-resistant isolates. Multilocus sequence typing of 137 erythromycin-resistant C. coli isolates revealed 37 previously reported sequence types (STs) and 8 novel STs. M192I, A103VI, and G74A substitutions were frequently noted in the ribosomal proteins L4 or L22. Further, we identified a considerable proportion (>90%) of erythromycin-resistant isolates carrying virulence factor genes: flaA, cadF, ceuE, and VirB. The prudent use of antimicrobials and regular microbiological investigation in food animals will be vital in limiting the public health hazards of C. coli in Korea.
Keywords: C. coli, food animals, macrolide, mutation, resistance, virulence
1. Introduction
Campylobacter species are commensal bacteria that reside within the gastrointestinal tract of many wild and domestic animals. They are among the most important foodborne pathogens that cause human gastroenteritis worldwide [1]. Campylobacter was associated with 236 foodborne outbreaks between 2010 and 2017 in the United States [2]. In European countries, human cases of campylobacteriosis have exceeded those caused by classic enteric bacteria such as Salmonella or Escherichia coli, with about 214,000 confirmed cases reported in 2016 [3]. Additionally, data from low and middle-income countries indicated that the rate of Campylobacter infection has increased over the past decade [4,5,6].
Although most cases of Campylobacter enteritis are self-limiting, severe or prolonged cases of enteritis, septicemia, and other extraintestinal infections may require antibiotic treatment [4]. Fluoroquinolones are commonly used to treat human campylobacteriosis. In addition, macrolides such as azithromycin and erythromycin are drugs of choice for infections caused by fluoroquinolone-resistant Campylobacter strains. However, the increase in the consumption of antimicrobials in food animals has contributed to the emergence of antimicrobial-resistant Campylobacter strains. Indeed, the observation of Campylobacter strains that are resistant to critically important antimicrobials in food animals has raised concerns that treatment of human infections will be compromised [1,4].
Macrolides inhibit bacterial RNA-dependent protein synthesis by targeting the 50S ribosomal subunit. The binding of macrolides leads to conformational changes in the ribosome and subsequent termination of the elongation of the peptide chain [7,8]. Base substitutions in multiple alleles of the 23S rRNA gene are the most common mutations conveying macrolide resistance in Campylobacter species [9]. Mutations at positions 2074 and 2075 in the peptidyl transferase region in domain V of the 23S rRNA target gene are associated with high-level macrolide resistance (MIC > 128 µg/mL) in C. coli [8,9]. Mutations have also been identified in the ribosomal proteins L4 and L22, both of which form portions of the polypeptide exit tunnel within the bacterial 70S ribosome and have been described in C. coli [8,10]. In addition, macrolide resistance has been associated with the chromosomally-encoded multidrug-resistant efflux system and ribosomal methylation encoded by the erythromycin ribosome methylase B-erm(B) gene [9,10]. Notably, the CmeABC multidrug efflux pump is reported to work in synergy with specific mutations, even in the absence of any other factor affecting resistance [11].
The incidence of Campylobacter infection is increasing worldwide [6]. C. coli is considered the second most common Campylobacter species responsible for human infections, next to C. jejuni, and continues to present a significant threat to food safety and public health. In the past decade, macrolide-resistant C. coli isolates of animal origin were reported in many countries [12,13,14,15]. Despite frequent reports of macrolide resistance in C. coli isolated from food animals and humans in South Korea (Korea) [16,17,18,19,20], only a few studies were performed to determine the resistance mechanisms [21,22]. The studies were conducted in a relatively small number of isolates collected from some specific parts of the country before 2016. Considering the global public health risk posed by macrolide-resistant C. coli in food animals and the increase in the total consumption of macrolides in food animals in Korea [23], continuous investigation of the resistance profiles and the mechanisms of macrolide resistance in C. coli isolated from food animals is vital to safeguard public health. Therefore, we performed extensive evaluations of the antimicrobial resistance profiles, the mechanism(s) of macrolide resistance, and virulence factor genes in C. coli isolated from fecal and carcass samples of chickens and pigs in Korea from 2010 to 2018.
2. Materials and Methods
2.1. Collection and Identification of C. coli
Altogether 1218 C. coli isolates (643 pig and 575 chicken isolates) were obtained from 16 laboratories/centers participating in the Korean Veterinary Antimicrobial Resistance Monitoring System from 2010 to 2018 (Table S1). C. coli was isolated from the feces and carcasses of pigs, and chickens. One to five isolates were collected from each farm. The isolation of C. coli was performed using Bolton broth (Thermo Scientific, Basingstoke, UK) and Campylobacter blood-free selective agar (Thermo Scientific, Basingstoke, UK), as previously described [19]. Isolates were then confirmed using the polymerase chain reaction as described by Denis et al. [24]. However, we do not have information about the number of slaughterhouses, animals, and samples considered for this study.
2.2. Antimicrobial Susceptibility Test
Antimicrobial susceptibility was determined via the broth microdilution method according to the Clinical and Laboratory Standards Institute [25] guideline, using commercially available antibiotic-containing CAMPY plates (Sensititre, Trek Diagnostics, Cleveland, OH, USA). C. jejuni ATCC 33560 was used as a reference strain. The following nine antimicrobials were tested: azithromycin (0.015–64 µg/mL), ciprofloxacin (0.015–64 µg/mL), clindamycin (0.03–16 µg/mL), erythromycin (0.03–64 µg/mL), florfenicol (0.03–64 µg/mL), gentamicin (0.12–32 µg/mL), nalidixic acid (4–64 µg/mL), telithromycin (0.015–8 µg/mL), and tetracycline (0.06–64 µg/mL). Antimicrobial resistance breakpoints were determined based on the National Antimicrobial Resistance Monitoring System [26]. Multi-drug resistance (MDR) was defined as resistance to three or more antimicrobial subclasses. One erythromycin-resistant isolate per farm was considered for further characterization.
2.3. Analysis of Macrolide Resistance Mechanisms
A total of 137 isolates (87 from pigs and 50 from chickens) were selected from different farms for analysis of macrolide resistance mechanisms and subsequent characterization. A PCR assay was used to investigate the presence of erm(B) gene as previously described [27]. Mutations in the genes encoding domain V of the 23S rRNA and ribosomal proteins L4 and L22 were determined as previously described [10,11]. Briefly, PCR reactions were performed in a final volume of 20 µL containing genomic DNA, PCR mix, and each of the forward and reverse primers (Solgent, Daejeon, Korea). After an initial denaturation of 5 min at 95 °C, amplification was performed over 30 cycles each consisting of 95 °C for 1 min, annealing temperature for 1 min, and 72 °C for 1 min with a final extension of 7 min at 72 °C. PCR products were purified (Solgent, Daejeon, Korea) and sequenced using an ABI prism 3100 analyzer (Genotech, Daejeon, Korea). Analysis of the nucleotide sequence and comparison with C. coli JV20 genome (GeneBank accession number NZ_AEER01000024) were performed using the BLAST program (http://www.ncbi.nlm.nih.gov/BLAST (accessed on 12 Auguts 2020)) and ExPASY proteomics tools (http://www.expasy.ch/tools/#similarity (accessed on 12 Auguts 2020)).
2.4. Detection of Virulence Factor Genes
We analyzed virulence factor genes linked with Campylobacter motility (flaA), adhesion and invasion (cadF, dnaJ, pldA, racR, virB, ceuE, and ciaB), cytotoxic production (cdtA, cdtB, and cdtC), and Guillain-Barré syndrome (wlaN) using PCR, as previously described [28,29,30].
2.5. Multilocus Sequence Typing (MLST) and eBURST Analysis
MLST was performed according to Dingle et al. [31]. Specific primers (Genotech, Daejeon, Korea) were used to amplify and sequence the following 7 housekeeping genes: aspA, glnA, gltA, glyA, pgm, tkt, and uncA. PCR products were purified (Solgent, Daejeon, Korea) and sequenced using an ABI prism 3100 analyzer (Genotech, Daejeon, Korea). Allele profiles and sequence types (ST)s were designated using the MLST website for Campylobacter (https://pubmlst.org/organisms/campylobacter-jejunicoli) (accessed on 14 April 2021). Each sequence is assigned with an allele number, and the combination of alleles yields an ST. In addition, the relatedness of the sequence types was determined using goeBURST software (http://goeBURST.phyloviz.net (accessed on 19 April 2021))
2.6. Statistical Analysis
Antimicrobial resistance rates and Pearson correlation were analyzed using Excel (Microsoft Excel, 2016, Microsoft Corporation, Redmond, WA, USA). P values less than 0.05 were considered statistically significant.
3. Results
3.1. Antimicrobial Resistance
The majority of C. coli isolates (>75%) recovered from pigs and chickens exhibited high resistance rates to ciprofloxacin, nalidixic acid, and tetracycline (Table 1). Indeed, the highest resistance rates were observed in chicken isolates compared to that of pigs. More than one-third of the pig isolates were resistant to azithromycin, clindamycin, erythromycin, and telithromycin. Additionally, relatively small percentages (<18%) of chicken isolates exhibited resistance to these antimicrobials. Florfenicol resistance was noted only in 8.4% and 1.7% of pig and chicken isolates, respectively.
Table 1.
Antimicrobial resistance profiles of C. coli isolated from pigs and chickens from 2010 to 2018 in Korea.
| Antimicrobials | % (No.) of Resistant Isolates | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pigs | Chickens | Total | |||||||||
| 2010–2012 | 2013–2015 | 2016–2018 | Subtotal | p-Value | 2010–2012 | 2013–2015 | 2016–2018 | Subtotal | p-Value | (n = 1218) | |
| (n = 268) | (n = 263) | (n = 112) | (n = 643) | (n = 196) | (n = 103) | (n = 276) | (n = 575) | ||||
| Azithromycin | 35.8 (96) | 42.2 (111) | 42.9 (48) | 39.7 (255) | 0.2763 | 16.3 (32) | 12.6 (13) | 16.7 (46) | 15.8 (91) | 0.9436 | 28.4 (346) |
| Ciprofloxacin | 91.4 (245) | 86.3 (227) | 88.4 (99) | 88.8 (571) | 0.6020 | 99 (194) | 98.1 (101) | 99.3 (274) | 98.8 (568) | 0.8456 | 93.5 (1139) |
| Clindamycin | 40.7 (109) | 46 (121) | 42.9 (48) | 43.2 (278) | 0.7289 | 16.8 (33) | 11.7 (12) | 17.8 (49) | 17.2 (99) | 0.9023 | 31 (377) |
| Erythromycin | 35.1 (94) | 42.2 (111) | 42 (47) | 39.2 (252) | 0.3491 | 15.8 (31) | 11.7 (12) | 16.3 (45) | 16.3 (94) | 0.9368 | 28.4 (346) |
| Florfenicol | 5.2 (14) | 10.3 (27) | 11.6 (13) | 8.4 (54) | 0.2102 | 0.5 (1) | 1.9 (2) | 2.5 (7) | 1.7 (10) | 0.1445 | 5.3 (64) |
| Gentamicin | 11.6 (31) | 14.4 (38) | 12.5 (14) | 12.9 (83) | 0.7961 | 13.3 (26) | 19.4 (20) | 28.6 (79) | 21.7 (125) | 0.0741 | 17.1 (208) |
| Nalidixic acid | 91.4 (245) | 85.9 (226) | 83.9 (94) | 87.9 (565) | 0.1675 | 98.5 (193) | 97.1 (100) | 99.3 (274) | 98.6 (567) | 0.7661 | 93 (1132) |
| Telithromycin | 46.6 (125) | 40.7 (107) | 33.9 (38) | 42 (270) | 0.0260 | 12.2 (24) | 12.6 (13) | 14.9 (41) | 13.6 (78) | 0.2457 | 28.6 (348) |
| Tetracycline | 77.6 (208) | 82.5 (217) | 70.5 (79) | 78.4 (504) | 0.5995 | 78.6 (154) | 89.3 (92) | 81.2 (224) | 80.9 (465) | 0.8503 | 79.6 (969) |
| MDR | 84.3 (226) | 85.8 (226) | 76.9 (86) | 83.8 (539) | 77.7 (151) | 85.4 (88) | 84.8 (234) | 82.3 (473) | 83.1 (1012) | ||
MDR, multidrug resistance.
3.2. Antimicrobial Resistance Trends
The resistance rates of most of the tested antimicrobials in pig and chicken isolates remained stable (Table 1). The gentamicin resistance rate in chicken isolates relatively peaked in 2016–2018. Despite fluctuations, we noted a trend of decreasing resistance (P < 0.05) to telithromycin in pig isolates. Additionally, the florfenicol resistance rate remained very low throughout the study period, especially in chicken isolates.
3.3. Antimicrobial Resistance Patterns
In this study, 98.9% (1204/1218) of the isolates were resistant to one or more of the tested antimicrobials (Table 2). MDR was noted in the majority of pig (83.8%) and chicken (82.3%) isolates (Table 1 and Table 2). We identified 57 and 27 different resistance patterns in chicken and pig isolates, respectively (Table S2). Resistance to seven of the tested antimicrobials, except to florfenicol and gentamicin, was the major MDR pattern in pig isolates, whereas resistance to ciprofloxacin, nalidixic acid, and tetracycline was the predominant MDR pattern in chicken isolates. Notably, five isolates from pigs and three isolates from chickens exhibited resistance to all of the tested antimicrobials.
Table 2.
Frequent resistance patterns in C. coli isolated from pigs (n = 643) and chickens (n = 575) from 2010 to 2018 in Korea.
| Pigs | ||
|---|---|---|
| Number of Antimicrobials |
% (No.) of Isolates | Most Frequent Resistance Pattern |
| 0 | 1.6 (10) | - |
| 1 | 4.2 (27) | TET (n = 23) |
| 2 | 10.4 (67) | CIP NAL (n = 56) |
| 3 | 33.7 (217) | CIP NAL TET (n = 187) |
| 4 | 8.7 (56) | CIP GEN NAL TET (n = 14) |
| 5 | 6.2 (40) | CIP CLI FFC NAL TET (n = 13) |
| 6 | 6.1 (39) | AZM CIP CLI ERY NAL TEL (n = 16) |
| 7 | 21.5 (138) | AZM CIP CLI ERY NAL TEL TET (n = 131) |
| 8 | 6.8 (44) | AZM CIP CLI ERY GEN NAL TEL TET (n = 37) |
| 9 | 0.8 (5) | AZM CIP CLI ERY FFC GEN NAL TEL TET (n = 5) |
| MDR | 83.8 (539) | |
| Chickens | ||
|
Number of
Antimicrobials |
% (No.) of Isolates | Most Frequent Resistance Pattern |
| 0 | 0.7 (4) | - |
| 1 | 0.3 (2) | CIP (n = 2) |
| 2 | 16.7 (96) | CIP NAL (n = 94) |
| 3 | 50.4 (290) | CIP NAL TET (n = 269) |
| 4 | 16 (92) | CIP GEN NAL TET (n = 79) |
| 5 | 0.5 (3) | CIP FFC GEN NAL TET (n = 1) |
| AZM CIP GEN NAL TET (n = 1) | ||
| AZM CIP CLI ERY NAL (n = 1) | ||
| 6 | 4 (23) | AZM CIP CLI ERY NAL TET (n = 12) |
| 7 | 8 (46) | AZM CIP CLI ERY NAL TEL TET (n = 42) |
| 8 | 2.8 (16) | AZM CIP CLI ERY GEN NAL TEL TET (n = 15) |
| 9 | 0.5 (3) | AZM CIP CLI ERY FFC GEN NAL TEL TET (n = 3) |
| MDR | 82.3 (473) | |
Abbreviations: AZM, azithromycin; CIP, ciprofloxacin; clindamycin, CLI; ERY, erythromycin; FFC, florfenicol; GEN, gentamicin; NAL, nalidixic acid; TEL, telithromycin; TET, tetracycline; MDR, multidrug resistance.
3.4. Detection of Mutation and ermB Gene
Analysis of the 23S rRNA gene among erythromycin-resistant isolates (n = 137) demonstrated an A2075G mutation in 83 (95.4%) and 46 (92%) pig and chicken isolates, respectively (Table 3). Erythromycin-resistant isolates from pigs (A2075G and C2097T, n = 2) and chickens (A2075G and T2114C, n = 4; A2074M and A2075Y, n = 1) exhibited double mutations. However, no mutation was found in the 23S rRNA gene in three of the pig and chicken isolates, each.
Table 3.
Mutations in erythromycin-resistant C. coli isolated from pigs and chickens from 2010 to 2018 in Korea.
| Source | ERY a MIC Range (µg/mL) |
Nucleotide/Amino Acid Substitution | Sequence Types | |||
|---|---|---|---|---|---|---|
| 23s rRNA b | L4c | L22 c | No. of Isolates | |||
| Pigs (n = 87) |
≥64 | A2075G | WT | WT | 38 | 7 ST854, 7 ST1016, 2 ST2715, 2 ST2733, 2 ST11050, 2 ST1556, 2 ST4172, 2 ST828, and each of ST860, ST900, ST1062, ST1068, ST1104, ST1142, ST1446, ST11056, ST11049, ST11061, ST2716, and ST890 |
| >64 | A2075G | WT | A103V | 1 | ST854 | |
| >64 | A2075G | WT | no band | 1 | ST10826 | |
| ≥64 | A2075G | M192I | WT | 13 | 4 ST829, 2 ST1055, and each of ST828, ST872, ST1142, ST2715, ST7168, and ST11054, ST11062 | |
| 64 | A2075G | M192I | A103V | 3 | ST1131, ST1450, and ST2715 | |
| >64 | A2075G | P28S | WT | 6 | 2 ST854, 2 ST9575, and each of ST1556 and ST11050 | |
| >64 | A2075G | V121A | WT | 2 | ST1096 and ST11054 | |
| >64 | A2075G | V121A, V176I, T177S, V184I, M192I | WT | 4 | ST1016, ST1096, ST7123, and ST11054 | |
| >64 | A2075G | V121A, V176I, T177S, V184I, M192I | A103V | 4 | ST1417, ST1450, ST1556, and ST11054 | |
| >64 | A2075G | V176I, T177S | WT | 2 | ST1058 and ST1117 | |
| >64 | A2075G | V176I, T177S, V184I | WT | 1 | ST2715 | |
| >64 | A2075G | V176I, T177S, V184I, M192I | WT | 2 | ST854 and ST2715 | |
| >64 | A2075G | V176I, T177S, V184I, M192I | A103V | 1 | ST1131 | |
| >64 | A2075G | V184I, M192I | WT | 2 | ST829 and ST2715 | |
| >64 | A2075G | no band | no band | 1 | ST829 | |
| >64 | A2075Y | WT | WT | 1 | ST4809 | |
| >64 | A2075G, C2097T | V121A, V176I, T177S, V184I, M192I | A103V | 2 | ST890 | |
| >64 | WT | V121A, A140T | WT | 1 | ST2718 | |
| >64 | WT | V121A | WT | 1 | ST1055 | |
| >64 | WT | P28S | WT | 1 | ST854 | |
| Chickens (n = 50) |
≥64 | A2075G | WT | WT | 32 | 10 ST860, 7 ST9867, 4 ST9201, 2 ST5675, 2 ST6148, 2 ST11051, 2ST1016, and each of ST828, ST1587, and ST1556 |
| >64 | A2075G | WT | Q24R, V65I, G74A, T109A | 2 | ST11052 and ST860 | |
| 64 | A2075G | WT | V65I, G74A, T109A | 1 | ST5675 | |
| >64 | A2075G | M192I | G74A, T109A | 1 | ST2711 | |
| >64 | A2075G | P28S | WT | 1 | ST854 | |
| >64 | A2075G | V184I | WT | 1 | ST1096 | |
| 64 | A2075G | V121A, M192I | V65M | 1 | ST11051 | |
| >64 | A2075G | V121A, M192I | V65I, G74A, T109S | 1 | ST6148 | |
| 64 | A2075G | V184I, M192I | WT | 1 | ST2715 | |
| >64 | A2075G | no band | WT | 1 | ST860 | |
| ≥32 | A2075G, T2114C | WT | WT | 4 | ST860 | |
| >64 | A2074M, A2075Y | WT | WT | 1 | ST6148 | |
| ≥32 | WT | WT | WT | 3 | 2 ST9867 and an ST829 | |
a Abbreviations: ERY, erythromycin; WT, wild type; ST, b position according to Escherichia Coli numbering. c Position of amino acids changes. DNA sequences of rplD and rplV genes coding L4 and L22 ribosomal proteins, respectively, were compared with the sequence in the C. coli JV20 genome.
We identified various types of mutations in the ribosomal proteins L4 and L22 in erythromycin-resistant isolates from chickens and pigs (Table 3). Pig isolates exhibited seven types of amino acid substitutions in the ribosomal proteins L4 (M192I, n = 31; V176I, n = 16, T177S, n = 16; V184I, n = 16; V121A, n = 14; P28S, n = 7; and A140T, n = 1) and one in ribosomal protein L22 (A103V, n = 11). In addition, chicken isolates presented four types of amino acid substitutions in the ribosomal proteins L4 (M192I, n = 4; V184I, n = 2; P28S, n = 1; and V121A, n = 2) and six in ribosomal protein L22 (G74A, n = 5; V65I, n = 4; T109A, n = 4; Q24R, n = 2; T109S, n = 1; and V65M, n = 1). Notably, we identified mutations in both the 23S rRNA gene and L4 and/or L22 ribosomal protein (s) in 43 pig and nine chicken isolates. However, none of the investigated isolates from chickens and pigs harbored the erm(B) gene.
3.5. Virulence Factor Genes
The erythromycin-resistant isolates were investigated for the presence of various virulence factor genes that are associated with Campylobacter motility, adhesion, and invasion into human intestinal cells, and cytotoxin production. More than 85% of the isolates carried at least three virulence factor genes (Table 4). The majority (>93%) of isolates from pigs carried the cadF, ceuE, flaA, and virB genes. Similarly, the cadF, ceuE, and flaA genes were detected in at least 90% of the isolates from chicken. However, the virB gene was detected in only 4.1% of chicken isolates. None of the isolates carried genes associated with cytotoxin production, expression of Guillain–Barré syndrome, and most of the invasion-associated virulence factors.
Table 4.
Prevalence of virulence marker genes in erythromycin-resistant C. coli isolated from pigs and chickens from 2010 to 2018 in Korea.
| Source | No. of Isolates |
Distribution (%) of Virulence Factor Genes | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| flaA | cadF | dnaJ | pldA | racR | virB | ceuE | ciaB | cdtA | cdtB | cdtC | wlaN | 1 | 2 | 3 | 4 | ||
| Pigs | 87 | 93.1 | 97.7 | 0 | 0 | 0 | 93.1 | 98.8 | 0 | 0 | 0 | 0 | 0 | 1.1 | 4.6 | 89.6 | 4.6 |
| Chickens | 50 | 90 | 100 | 0 | 0 | 0 | 4 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 86 | 4 |
3.6. MLST and eBURST Analysis
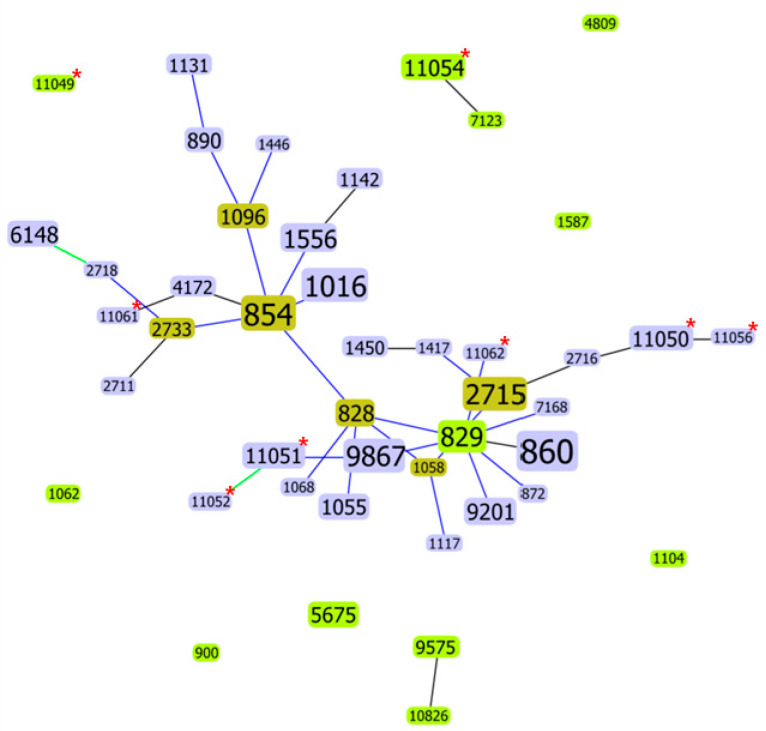
We identified 37 and 16 different STs from the 87 pig and 50 chicken isolates, respectively (Table 3). The dominant STs in pigs were ST854 (n = 12), ST1016 (n = 8), ST2715 (n = 7), and ST829 (n = 6), whereas ST860 (n = 16) and ST9867 (n = 9) were frequent in chickens. Thirteen STs in pigs (four ST1556, four ST11054, three ST828, three ST890, three ST1055, three ST11050, two ST1096, two ST1131, two ST1142, two ST1450, two ST2733, two ST9575, and two ST4172) and five STs in chickens (four ST6148, four ST9201, three ST5675, three ST11051, and two ST1016) were each represented by fewer than five isolates. In addition, 20 STs in pigs and 9 STs in chickens were represented by only a single isolate each. Among the identified STs, six from pigs (ST11049, ST11050, 11054, ST11056, ST11061, and ST11062) and two from chickens (ST11051 and 11052) were reported for the first time. Moreover, eight of the STs (ST860, ST828, ST829, ST854, ST1016, ST1096, ST1556, and ST2715) were identified in both chicken and pigs. Further, the goeBURST algorithm revealed 38 STs with five or more allele matches, whereas seven STs are singletons and are unrelated to any other within the single locus variant collection (Figure 1).
Figure 1.
goeBURST analysis conducted at a single locus variant level, with double locus variants added to show further relatedness. Light green ST nodes, group founder; dark green ST nodes, subgroup founder; light blue ST nodes, common node; and red asterisks, novel STs. Colored links: black, link drawn without recourse to tiebreak rules; blue, link drawn using tiebreak rule 1; and green, link drawn using tiebreak rule 2.
4. Discussion
Our data demonstrated that a considerable proportion of chicken and pig C. coli isolates were resistant to some of the clinically important antimicrobials. We found diverse STs, mutations, and virulence factor genes in the majority of erythromycin-resistant isolates.
The macrolide resistance rate was high in pig isolates compared to that of chicken isolates. C. coli isolated from various food animals and carcasses in Korea presented variable resistance rates to azithromycin (30–43%) and erythromycin (6–30%) [16,17,18,19,20]. In this study, the azithromycin and erythromycin resistance rates in pig and chicken isolates were higher than those reported in Europe [32,33,34]. In contrast, our findings in chicken isolates were lower than those described in Africa [5,9], China [35,36], and some European countries [15,37]. The use of macrolides for the prevention and control of various diseases in food animals, particularly pigs, in Korea could be associated with the emergence of erythromycin and azithromycin-resistant C. coli. Indeed, about 68% of the total macrolide sold for livestock in Korea is used in pig husbandry [23]. These observations are concerning because macrolides, especially erythromycin and azithromycin, are the drug of choice for the treatment of human Campylobacter infections [1,4].
In agreement with previous studies in Korea [16,17,18,19] and China [35,36], C. coli isolated from pigs and chickens exhibited very high resistance rates to ciprofloxacin, nalidixic acid, and tetracycline. However, our findings were much higher than previous reports in Africa [5,38], Europe [32,34], and North America [39,40]. The frequent use of fluoroquinolones and tetracyclines in food animals can select resistant strains that could be readily transferred to humans through the food chain [41].
The gentamicin resistance rate in Campylobacter species has been reported to be low [34]. In this study, the gentamicin resistance rates in chicken and pig isolates were higher than previous reports in Africa [12,42], the EU [32], and North America [40], but it was low compared to those reported in China [35,36]. Gentamicin is normally considered for serious bacteremia and other systemic infections due to Campylobacter [8]. Thus, the observation of resistance to this antibiotic in considerable proportions of isolates from food animals has a potential public health implication.
Globally, the incidences of resistance to several key antibiotics useful in the treatment of Campylobacter disease are increasing and multiple resistance patterns to several classes of antibiotics are emerging [8]. High levels of multidrug resistance among C. coli isolates have been observed within the food chain [43]. In our study, about 83% of the isolates were resistant to at least three antimicrobial classes. Previous studies in Thailand [44], Poland [43], and France [45] revealed that 99%, 95%, and 54% of C. coli isolates, respectively, from various food animals and carcasses were resistant to multiple antimicrobials. In China, 42% to 98% of C. coli isolated from retail chicken exhibited MDR [14,36]. Concordant with previous reports in Guatemala [46], Europe [33], and the United States [47], profiles of MDR usually included ciprofloxacin, nalidixic acid, tetracycline, and to some extent erythromycin. Alfredson et al. [8] revealed that trends in antimicrobial resistance have shown a clear association between the use of antibiotics in food animals and resistant isolates of Campylobacter in humans. The increasing incidence of resistance to several key antibiotics in C. coli presented a public health threat [8].
The study showed that antimicrobial resistance rates of C. coli isolated from pigs and chickens in Korea differed from those described previously from various geographical regions. However, comparing and contrasting data between studies is often difficult due, for example, to different origins, duration of studies, number of isolates studied, and laboratory analysis. The differences in antimicrobial use in livestock husbandry among countries could also contribute to the variation in the prevalence of antimicrobial resistance.
Base substitutions at positions 2074 and 2075 of the adenine residues in the 23S rRNA gene in Campylobacter spp. are the most common mutations associated with erythromycin resistance [48]. In this study, we identified A2075G mutation in the majority of erythromycin-resistant isolates. A2075G mutation in all three copies of the 23S rRNA gene is associated with high-level macrolide resistance [48], as it has been indicated in previous studies in many countries, including Korea [9,21,22,49]. In addition, this mutation has been shown to provide stability to Campylobacter in culture and maintain their ability to colonize their host [50]. We also detected double mutations in two pig (A2075G and C2097T) and five chicken (A2075G and T2114C, A2074M, and A2075Y) isolates. Despite previous reports on the detection of double mutation in erythromycin-resistant C. jejuni and C. coli isolated from humans [51,52], these types of double mutations have been rarely associated with erythromycin-resistant Campylobacter species isolated from food animals.
Amino acid substitutions in the ribosomal proteins L4 and L22 are linked with a low level of macrolide resistance in Campylobacter species. Amino acids at positions 63–74 are a part of the most important target region in ribosomal protein L4 [11]. However, in this study, no variation was found in this region. Most of the changes were concentrated in the region at amino acid 121-192, except in eight strains that harbored the P-28→S replacement. Among the seven types (V121A, V176A, T177S, V184I, M192I, A140T, and P28S) of substitutions identified in the ribosomal protein L4, M192I was the most frequent change observed in C. coli isolates recovered from food animals, especially pigs. Previous studies [21,53] have also identified V121A, T177S, and M192I substitutions in ribosomal protein L4 in erythromycin-resistant isolates from various sources. In this study, mutation in ribosomal protein L22 was not common compared to ribosomal protein L4. We noted seven types of mutations in ribosomal protein L22: A103V, Q24R, V65I, G74A, T109A, V65M, and T109S. A103V was the predominant type of substitution found only in pig isolates. Consistent with this study, V65I, A74G, and A103V substitutions were identified in erythromycin-susceptible and -resistant isolates in Korea and other countries [10,21,53]. Substitutions in the ribosomal proteins L4 and L22 are known to confer low-level resistance to macrolides [10,11,50]. Further, we observed the coexistence of mutation in the 23S rRNA gene and amino acid substitutions in L4 and/or L22, although the significance of the coexistence is unknown.
Three erythromycin-resistant isolates from chickens identified in this study did not harbor any mutation. Furthermore, none of the isolates carried the erm(B) gene. Although we did not investigate other resistance mechanisms, the presence of efflux pumps could be linked with erythromycin resistance [49]. Wei and Knag [21] identified erythromycin-resistant Campylobacter strains that did not exhibit any of the currently identified resistance mechanisms, indicating the presence of unidentified mechanisms. Therefore, further studies are needed to elucidate mechanisms underlying the development of resistance.
MLST followed by eBURST clustering is useful for assessing major changes of the lineages among isolates and is suitable for periodic typing and global epidemiology [14]. We found 45 various STs; 37 were from pigs and 16 were from chickens. Indeed, 8 of the STs (ST860, ST828, ST829, ST854, ST1016, ST1096, ST1556, and ST2715) were identified in both chicken and pigs. Previous studies in Korea [14,22,54] and other countries [55,56,57,58,59] have reported diverse STs in C. coli isolated from food animals and humans, and ST827, ST828, ST829, and ST855 were the predominant STs. The most frequent STs identified in this study (ST854, ST1016, ST2715, ST829, ST860, and ST9867) differed from those described in previous reports [55,56,57,58,59], except for ST829 C. coli, which was frequently detected in pigs in Korea [22]. Genetic diversity in the Campylobacter population might emerge through mutation and recombination events [59]. Chicken isolates shared the same STs with that of pigs, indicating the dissemination of identical clones in the poultry and pig industry. In addition, the identification of new STs in pigs (ST11049, ST11050, ST11054, ST11056, ST11061, and ST11062) and chickens (ST11051 and ST11052) might suggest the emergence of new clones in the poultry and pig industry. Among the identified C. coli STs, ST828, ST829, ST860, ST872, ST1055, ST1058, and ST1446 were frequently reported in patients with campylobacteriosis [55,56]. Thus, food animals may serve as an important reservoir and source of human infection. Furthermore, the presence of closely related STs may indicate the evolutionary relationship between isolates [54,59].
The expression of genes involved in Campylobacter motility, adhesion and invasion into intestinal epithelial cells, as well as toxin production, is vital for the establishment of infection in humans. Motility of the bacterium is fundamental for adhesion into intestinal epithelial cells in the early stage of pathogenesis. We identified the flagellin-coding flaA gene, which is primarily responsible for bacterial motility [60], in at least 90% of the erythromycin-resistant isolates recovered from pigs and chickens. These findings are in agreement with previous reports in Poland [61] and Vietnam [62]. We also detected the cadF gene, which encodes for a fibronectin-binding outer membrane protein, in almost all of the erythromycin-resistant isolates. The cadF gene is responsible for bacterial adhesion and influencing microfilament organization in host cells [28]. Many virulence factors have been associated with the invasion of Campylobacter into intestinal epithelial cells, including the pldA, virB, iam, ceuE, and ciaB [63]. The ceuE gene was identified in almost all of the erythromycin-resistant isolates. Although the exact mechanism remains obscure, it is one of the most important genes encoding for Campylobacter invasion [30]. Further, we noted the virB gene in 93.1% of pig and 4% of chicken isolates. The virB gene encodes a putative type IV secretion system involved in adhesion and invasion of Campylobacter to the intestinal epithelial cells [64].
In conclusion, the present study demonstrated long-term trends that C. coli isolated from food animals exhibits resistance to multiple clinically important antimicrobials. We identified erythromycin-resistant C. coli isolates with diverse STs and various mutations in the 23S rRNA gene and ribosomal proteins L4 and L22. Our observations highlight the need for proper food safety practices to prevent the spread of antimicrobial-resistant and virulent strains of Campylobacter spp. Additionally, the prudent use of antimicrobials in food animals and constant monitoring of resistance among Campylobacter isolates in food animals and animal products are urgently needed.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/microorganisms9051077/s1. Table S1. The number of Campylobacter coli isolates recovered from fecal and carcass of apparently healthy and diseased pig and chicken between 2010 and 2118 in Korea. Table S2. Antimicrobial resistance patterns in C. coli isolated from pigs and chickens from 2010 to 2018 in Korea.
Author Contributions
Conceptualization, S.-K.L., J.-H.C., and S.-S.Y.; methodology, J.-H.C., H.Y.K. and D.C.M.; software, A.F.M., J.-H.C., and S.-J.K.; validation, A.F.M., S.-J.K., and J.-H.C.; formal analysis, J.-H.C. and H.Y.K.; investigation, H.Y.K., H.-J.S., J.-H.C., and S.-J.K.; data curation, D.C.M., S.-S.Y., and A.F.M.; writing—original draft preparation, A.F.M. and J.-H.C.; writing—review and editing, A.F.M., S.-S.Y., D.C.M., and S.-K.L.; supervision, S.-S.Y. and S.-K.L.; project administration, D.C.M. and H.Y.K.; funding acquisition; S.-K.L. and D.C.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Animal and Plant Quarantine Agency, Ministry of Agriculture, Food, and Rural Affairs, South Korea, grant N-1543081-2017-24-01.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nachamkin I., Szymanski M.C., Blaser J.M. Campylobacter. 3rd ed. ASM Press; Washington, DC, USA: 2008. pp. 31–57. [Google Scholar]
- 2.Centers for Disease Control and Prevention . Campylobacter, General Information. CDC; Atlanta, GA, USA: 2019. [(accessed on 21 January 2021)]. Available online: https://www.cdc.gov/campylobacter/outbreaks.html. [Google Scholar]
- 3.European Food Safety Authority. European Center for Disease Prevention and Control The European Union summary report on trends and sources of zoonoses, zoonotic agents, and food-borne outbreaks in 2016. EFSA J. 2017;16:14–19. doi: 10.2903/j.efsa.2017.5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Igwaran A., Okoh A.I. Human campylobacteriosis: A public health concern of global importance. Heliyon. 2019;5:e02814. doi: 10.1016/j.heliyon.2019.e02814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kouglenou S.D., Agbankpe A.J., Dougnon V., Djeuda A.D., Deguenon E., Hidjo M., Baba-Moussa L., Bankole H., Bankole H. Prevalence and susceptibility to antibiotics from Campylobacter jejuni and Campylobacter coli isolated from chicken meat in Southern Benin, West Africa. BMC Res. Notes. 2020;13:305. doi: 10.1186/s13104-020-05150-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Platts-Mills J.A., Kosek M. Update on the burden of Campylobacter in developing countries. Curr. Opin. Infect. Dis. 2014;27:444–450. doi: 10.1097/QCO.0000000000000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wieczorek K., Osek J. Antimicrobial resistance mechanisms among Campylobacter. Biomed Res. Int. 2013;2013:340605. doi: 10.1155/2013/340605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alfredson D.A., Korolik V. Antibiotic resistance and resistance mechanisms in Campylobacter jejuni and Campylobacter coli. FEMS Microbiol. Lett. 2007;277:123–132. doi: 10.1111/j.1574-6968.2007.00935.x. [DOI] [PubMed] [Google Scholar]
- 9.Gibreel A., Kos V.N., Keelan M., Trieber C.A., Levesque S., Michaud S., Taylor D.E. Macrolide resistance in Campylobacter jejuni and Campylobacter coli: Molecular mechanism and stability of the resistance phenotype. Antimicrob. Agents Chemother. 2005;49:2753–2759. doi: 10.1128/AAC.49.7.2753-2759.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corcoran D., Quinn T., Cotter L., Fanning S. An investigation of the molecular mechanisms contributing to high-level erythromycin resistance in Campylobacter. Int. J. Antimicrob. Agents. 2006;27:40–45. doi: 10.1016/j.ijantimicag.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 11.Cagliero C., Mouline C., Cloeckaert A., Payot S. Synergy between efflux pump CmeABC and modifications in ribosomal proteins L4 and L22 in conferring macrolide resistance in Campylobacter jejuni and Campylobacter coli. Antimicrob. Agents Chemother. 2006;50:3893–3896. doi: 10.1128/AAC.00616-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gharbi M., Béjaoui A., Ben Hamda C., Jouini A., Ghedira K., Zrelli C., Hamrouni S., Aouadhi C., Bessoussa G., Ghram A., et al. Prevalence and antibiotic resistance patterns of Campylobacter spp. isolated from broiler chickens in the North of Tunisia. Biomed. Res. Int. 2018:7943786. doi: 10.1155/2018/7943786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hungaro H.M., Mendonça R.C.S., Rosa V.O., Badaró A.C.L., Moreira M.A.S., Chaves J.B.P. Low contamination of Campylobacter spp. on chicken carcasses in Minas Gerais state, Brazil: Molecular characterization and antimicrobial resistance. Food Control. 2015;51:15–22. doi: 10.1016/j.foodcont.2014.11.001. [DOI] [Google Scholar]
- 14.Wang Y., Dong Y., Deng F., Liu D., Yao H., Zhang Q., Shen J., Liu Z., Gao Y., Wu C., et al. Species shift and multidrug resistance of Campylobacter from chicken and swine, China, 2008–14. J. Antimicrob. Chemother. 2016;71:666–669. doi: 10.1093/jac/dkv382. [DOI] [PubMed] [Google Scholar]
- 15.Wo’zniak-Biel A., Wieliczko A. Tetracycline, erythromycin, and gentamicin resistance of Campylobacter jejuni and Campylobacter coli isolated from poultry in Poland. Bvet. I Pulawy. 2011;55:51–54. [Google Scholar]
- 16.Chae M. Antimicrobial resistance in Campylobacter jejuni and Campylobacter coli isolated from food animals and raw meats in slaughterhouse in Korea during 2010. Kor. J. Vet. Publ. Health. 2011;35:239–245. [Google Scholar]
- 17.Chon J.W., Lee S.K., Yoon Y., Yoon K.S., Kwak H.S., Joo I.S., Seo K.H. Quantitative prevalence and characterization of Campylobacter from chicken and duck carcasses from poultry slaughterhouses in South Korea. Poult. Sci. 2018;97:2909–2916. doi: 10.3382/ps/pey120. [DOI] [PubMed] [Google Scholar]
- 18.Kim J.M., Hong J., Bae W., Koo C., Kim S.H., Park Y.H. Prevalence, antibiograms, and transferable tet(O) plasmid of Campylobacter jejuni and Campylobacter coli isolated from raw chicken, pork, and human clinical cases in Korea. J. Food Prot. 2010;73:1430–1437. doi: 10.4315/0362-028X-73.8.1430. [DOI] [PubMed] [Google Scholar]
- 19.Kim H.J., Kim J.H., Kim Y.I., Choi J.S., Park M.Y., Nam H.M., Jung S.C., Kwon J.W., Lee C.H., Kim Y.H., et al. Prevalence and characterization of Campylobacter spp. isolated from domestic and imported poultry meat in Korea, 2004–2008. Foodborne Pathog. Dis. 2010;7:1203–1209. doi: 10.1089/fpd.2010.0553. [DOI] [PubMed] [Google Scholar]
- 20.Wei B., Cha S.Y., Kang M., Roh J.H., Seo H.S., Yoon R.H., Jang H.K. Antimicrobial susceptibility profiles and molecular typing of Campylobacter jejuni and Campylobacter coli isolates from ducks in South Korea. Appl. Environ. Microbiol. 2014;80:7604–7610. doi: 10.1128/AEM.02469-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei B., Kang M. Molecular basis of macrolide resistance in Campylobacter strains isolated from poultry in South Korea. Biomed Res. Int. 2018;2018:4526576. doi: 10.1155/2018/4526576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shin E., Lee Y. Characterization of erythromycin-resistant porcine isolates of Campylobacter coli. Microb. Drug Resist. 2010;16:231–239. doi: 10.1089/mdr.2010.0039. [DOI] [PubMed] [Google Scholar]
- 23.Animal and Plant Quarantine Agency . Korean Veterinary Antimicrobial Resistance Monitoring System. APQA Annual Report; Animal and Plant Quarantine Agency; Gimcheon, Korea: 2019. [Google Scholar]
- 24.Denis M., Soumet C., Rivoal K., Ermel G., Blivet D., Salvat G., Colin P. Development of a m-PCR assay for simultaneous identification of Campylobacter jejuni and C. coli. Lett. Appl. Microbiol. 1999;29:406–410. doi: 10.1046/j.1472-765X.1999.00658.x. [DOI] [PubMed] [Google Scholar]
- 25.Clinical and Laboratory Standards Institute (CLSI) Performance Standards for Antimicrobial Susceptibility Testing. Twentieth Informational Supplement; Wayne, PA, USA: 2018. CLSI Document M100. [Google Scholar]
- 26.National Antimicrobial Resistance Monitoring System . NARMS Integrated Report: The National Antimicrobial Resistance Monitoring System: Enteric Bacteria. U.S. Food and Drug Administration; Rockville, MD, USA: 2014. [Google Scholar]
- 27.Bolinger H.K., Zhang Q., Miller W.G., Kathariou S. Lack of evidence for erm(B) infiltration into erythromycin-resistant Campylobacter coli and Campylobacter jejuni from commercial turkey production in Eastern North Carolina: A major turkey-growing region in the United States. Foodborne Pathog Dis. 2018;15:698–700. doi: 10.1089/fpd.2018.2477. [DOI] [PubMed] [Google Scholar]
- 28.Bang D.D., Nielsen E.M., Scheutz F., Pedersen K., Handberg K., Madsen M. PCR detection of seven virulence and toxin genes of Campylobacter jejuni and Campylobacter coli isolates from Danish pigs and cattle and cytolethal distending toxin production of the isolates. J. Appl. Microbiol. 2003;94:1003–1014. doi: 10.1046/j.1365-2672.2003.01926.x. [DOI] [PubMed] [Google Scholar]
- 29.Datta S., Niwa H., Itoh K. Prevalence of 11 pathogenic genes of Campylobacter jejuni by PCR in strains isolated from humans, poultry meat and broiler, and bovine faeces. J. Med. Microbiol. 2003;52:345–348. doi: 10.1099/jmm.0.05056-0. [DOI] [PubMed] [Google Scholar]
- 30.Koolman L., Whyte P., Burgess C., Bolton D. Distribution of virulence-associated genes in a selection of Campylobacter isolates. Foodborne Pathog. Dis. 2015;12:424–432. doi: 10.1089/fpd.2014.1883. [DOI] [PubMed] [Google Scholar]
- 31.Dingle K.E., Colles F.M., Wareing D.R.A., Ure R., Fox A.J., Bolton F.E., Bootsma H.J., Willems R.J.L., Urwin R., Maiden M.C.J. Multilocus sequence typing system for Campylobacter jejuni. J. Clin. Microbiol. 2001;39:14–23. doi: 10.1128/JCM.39.1.14-23.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bywater R., Deluyker H., Deroover E., de Jong A., Marion H., McConville M., Rowan T., Shryock T., Shuster D., Thomas V., et al. A European survey of antimicrobial susceptibility among zoonotic and commensal bacteria isolated from food-producing animals. J. Antimicrob. Chemother. 2004;54:744–754. doi: 10.1093/jac/dkh422. [DOI] [PubMed] [Google Scholar]
- 33.European Food Safety Authority. European Center for Disease Prevention and Control The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2017. EFSA J. 2019;17:5598. doi: 10.2903/j.efsa.2019.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fallon R., O’Sullivan N., Maher M., Carroll C. Antimicrobial resistance of Campylobacter jejuni and Campylobacter coli isolates from broiler chickens isolated at an Irish poultry processing plant. Lett. Appl. Microbiol. 2003;36:277–281. doi: 10.1046/j.1472-765X.2003.01308.x. [DOI] [PubMed] [Google Scholar]
- 35.Chen X., Naren G.W., Wu C.M., Wang Y., Dai L., Xia L.N., Luo P.J., Zhang Q., Shen J.Z. Prevalence and antimicrobial resistance of Campylobacter isolates in broilers from China. Vet. Microbiol. 2010;144:133–139. doi: 10.1016/j.vetmic.2009.12.035. [DOI] [PubMed] [Google Scholar]
- 36.Li B., Ma L., Li Y., Jia H., Wei J., Shao D., Liu K., Shi Y., Qiu Y., Ma Z. Antimicrobial resistance of Campylobacter species isolated from broilers in live bird markets in Shanghai, China. Foodborne Pathog. Dis. 2017;14:96–102. doi: 10.1089/fpd.2016.2186. [DOI] [PubMed] [Google Scholar]
- 37.Parisi A., Lanzilotta S.G., Addante N., Normanno G., Di Modugno G., Dambrosio A., Montagna C.O. Prevalence, molecular characterization and antimicrobial resistance of thermophilic Campylobacter isolates from cattle, hens, broilers, and broiler meat in south-eastern Italy. Vet. Res. Commun. 2007;31:113–123. doi: 10.1007/s11259-006-3404-3. [DOI] [PubMed] [Google Scholar]
- 38.Hlashwayo D.F., Sigaúque B., Bila C.G. Epidemiology and antimicrobial resistance of Campylobacter spp. in animals in Sub-Saharan Africa: A systematic review. Heliyon. 2020;6:e03537. doi: 10.1016/j.heliyon.2020.e03537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Englen M.D., Hill A.E., Dargatz D.A., Ladely S.R., Fedorka-Cray P.J. Prevalence and antimicrobial resistance of Campylobacter in US dairy cattle. J. Appl. Microbiol. 2007;102:1570–1577. doi: 10.1111/j.1365-2672.2006.03189.x. [DOI] [PubMed] [Google Scholar]
- 40.Varga C., Guerin M.T., Brash M.L., Slavic D., Boerlin P., Susta L. Antimicrobial resistance in Campylobacter jejuni and Campylobacter coli isolated from small poultry flocks in Ontario, Canada: A two-year surveillance study. PLoS ONE. 2019;14:0221429. doi: 10.1371/journal.pone.0221429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garcia-Migura L., Hendriksen R.S., Fraile L., Aarestrup F.M. Antimicrobial resistance of zoonotic and commensal bacteria in Europe: The missing link between consumption and resistance in veterinary medicine. Vet. Microbiol. 2014;170:1–9. doi: 10.1016/j.vetmic.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 42.Kassa T., Gebre-Selassie S., Asrat D. Antimicrobial susceptibility patterns of thermotolerant Campylobacter strains isolated from food animals in Ethiopia. Vet. Microbiol. 2007;119:82–87. doi: 10.1016/j.vetmic.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 43.Wieczorek K., Osek J. A five-year study on prevalence and antimicrobial resistance of Campylobacter from poultry carcasses in Poland. Food Microbiol. 2015;49:161–165. doi: 10.1016/j.fm.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 44.Thomrongsuwannakij T., Blackall P.J., Chansiripornchai N. A Study on Campylobacter jejuni and Campylobacter coli through commercial broiler production chains in Thailand: Antimicrobial resistance, the characterization of DNA gyrase subunit a mutation, and genetic diversity by flagellin a gene restriction fragment length polymorphism. Avian Dis. 2017;61:186–197. doi: 10.1637/11546-120116-Reg.1. [DOI] [PubMed] [Google Scholar]
- 45.Châire P., Haenni M., Meunier D., Botree M.A., Calavas D., Madec J.Y. Prevalence and antimicrobial resistance of Campylobacter jejuni and Campylobacter coli isolated from cattle between 2002 and 2006 in France. J. Food Prot. 2010;73:825–831. doi: 10.4315/0362-028x-73.5.825. [DOI] [PubMed] [Google Scholar]
- 46.Benoit S.R., Lopez B., Arvelo W., Henao O., Parsons M.B., Reyes L., Moir J.C., Lindblade K. Burden of laboratory-confirmed Campylobacter infections in Guatemala 2008–2012: Results from a facility-based surveillance system. J. Epidemiol. Glob. Health. 2014;4:51–59. doi: 10.1016/j.jegh.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ricotta E.E., Palmer A., Wymore K., Clogher P., Oosmanally N., Robinson T., Lathrop S., Karr J., Hatch J., Dunn J., et al. Epidemiology and antimicrobial resistance of international travel-associated Campylobacter infections in the United States, 2005–2011. Am. J. Public Health. 2014;104:301867. doi: 10.2105/AJPH.2013.301867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jeon B., Muraoka W., Sahin O., Zhang Q. Role of Cj1211 in natural transformation and transfer of antibiotic resistance determinants in Campylobacter jejuni. Antimicrob. Agents Chemother. 2008;52:2699–2708. doi: 10.1128/AAC.01607-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bolinger H., Kathariou S. The current state of macrolide resistance in Campylobacter spp.: Trends and impacts of resistance mechanisms. Appl. Environ. Microbiol. 2017;83:00416–17. doi: 10.1128/AEM.00416-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Caldwell D.B., Wang Y., Lin J. Development, stability, and molecular mechanisms of macrolide resistance in Campylobacter jejuni. Antimicrob. Agents Chemother. 2008;52:3947–3954. doi: 10.1128/AAC.00450-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vacher S., Menard A., Bernard E., Santos A., Megraud F. Detection of mutations associated with macrolide resistance in thermophilic Campylobacter spp. by real-time PCR. Microb. Drug Resist. 2005;11:41–46. doi: 10.1089/mdr.2005.11.40. [DOI] [PubMed] [Google Scholar]
- 52.Perez-Boto D., Lopez-Portoles J.A., Simon C., Valdezate S., Echeita M.A. Study of the molecular mechanisms involved in high-level macrolide resistance of Spanish Campylobacter jejuni and campylobacter coli strains. J. Antimicrob. Chemother. 2010;65:2083–2088. doi: 10.1093/jac/dkq268. [DOI] [PubMed] [Google Scholar]
- 53.Lehtopolku M., Kotilainen P., Haanperä-Heikkinen M., Nakari U.M., Hänninen M.L., Huovinen P., Siitonen A., Eerola E., Jalava J., Hakanen A.J. Ribosomal mutations as the main cause of macrolide resistance in Campylobacter jejuni and Campylobacter coli. Antimicrob. Agents Chemother. 2011;55:5939–5941. doi: 10.1128/AAC.00314-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guk J.H., Kim J., Song H., Kim J., An J.K., Kim J., Ryu S., Jeon B., Cho S. Hyper-aerotolerant Campylobacter coli from duck sources and its potential threat to public health: Virulence, antimicrobial resistance, and genetic relatedness. Microorganisms. 2019;7:579. doi: 10.3390/microorganisms7110579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang L., Li Y., Shao Y., Hu Y., Lou H., Chen X., Wu Y., Mei L., Zhou B., Zhang X., et al. Molecular characterization and antibiotic-resistant profiles of Campylobacter species isolated from poultry and diarrheal patients in Southeastern China 2017–2019. Front. Microbiol. 2020;11:1244. doi: 10.3389/fmicb.2020.01244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang P., Zhang X., Liu Y., Jiang J., Shen Z., Chen Q., Ma X. Multilocus sequence types and antimicrobial resistance of Campylobacter jejuni and C. coli isolates of human patients from Beijing, China, 2017–2018. Front. Microbiol. 2020;191:554784. doi: 10.3389/fmicb.2020.554784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thaker S., Gebreyes W.A. Campylobacter coli in swine production: Antimicrobial resistance mechanisms and molecular epidemiology. J. Clin. Microbiol. 2005;43:5705–5714. doi: 10.1128/JCM.43.11.5705-5714.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ocejo M., Oporto B., Hurtado A. Occurrence of Campylobacter jejuni and Campylobacter coli in cattle and sheep in Northern Spain and changes in antimicrobial resistance in two studies 10-years Apart. Pathogens. 2019;8:98. doi: 10.3390/pathogens8030098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Litrup E., Torpdahl M., Nielsen E.M. Multilocus sequence typing performed on Campylobacter coli isolates from humans, broilers, and cattle originating in Denmark. J. Appl. Microbiol. 2006;103:210–218. doi: 10.1111/j.1365-2672.2006.03214.x. [DOI] [PubMed] [Google Scholar]
- 60.Little C.L., Richardson J.F., Owen R.J., de Pinna E., Threlfall E.J. Campylobacter and Salmonella in raw red meats in the United Kingdom: Prevalence, characterization and antimicrobial resistance pattern, 2003–2005. Food Microbiol. 2008;25:538–543. doi: 10.1016/j.fm.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 61.Andrzejewska M., Szczepańska B., Śpica D., Klawe J.J. Trends in the occurrence and characteristics of Campylobacter jejuni and Campylobacter coli isolates from poultry meat in Northern Poland. Food Control. 2015;51:190–194. doi: 10.1016/j.foodcont.2014.11.014. [DOI] [Google Scholar]
- 62.Nguyen T.N.M., Hotzel H., El-Adawy H., Tran H.T., Le M.T.H., Tomaso H., Neubauer H., Hafez H.M. Genotyping and antibiotic resistance of thermophilic Campylobacter isolated from chicken and pig meat in Vietnam. Gut Pathog. 2016;8:19. doi: 10.1186/s13099-016-0100-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wysok B., Wojtacka J., Wiszniewska-łaszczych A., Szteyn J. Antimicrobial resistance and virulence properties of Campylobacter spp. Originating from domestic geese in Poland. Animals. 2020;10:742. doi: 10.3390/ani10040742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bacon D.J., Alm R., Burr D.H., Hu L., Kopecko D.J., Ewing C.P., Trust T.J., Guerry P. Involvement of a plasmid in virulence of Campylobacter jejuni 81–176. Infect. Immun. 2000;68:4384–4390. doi: 10.1128/IAI.68.8.4384-4390.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.