Abstract
Research examining whether intentions to get a COVID-19 vaccine change over time is scarce. Moreover, the deep and pervasive history of medical racism in the U.S. has created a context in which some racial and ethnic groups exhibit greater levels of COVID-19 vaccine hesitancy; yet few researchers have attempted to determine whether these patterns persist with time. The purpose of this study was twofold: (a.) assess the role of time in COVID-19 vaccine intentions from April 2020 to January 2021, and (b.) examine whether race and ethnicity shape COVID-19 vaccine intention trajectories. Data were drawn from 9 waves of the Understanding America Study (n = 5023), a national probability panel study of U.S. adults. Multilevel logistic regression models were used to assess overall COVID-19 vaccine intention trajectories and trajectories by race and ethnicity. Results demonstrate intentions to get a COVID-19 vaccine significantly decreased from April 2020 to November 2020, but by January 2021, intentions to get a COVID-19 vaccine slightly increased. Findings also show trajectories significantly differed by racial and ethnic background. Asian/Pacific Islanders had the highest probability of likely getting a COVID-19 vaccine at baseline, followed by Whites and Latina/os. Black Americans exhibited the lowest probability of likely getting vaccinated, and, in most cases, the gap between Black Americans and other racial groups grew over time. Key findings from this study demonstrate that, among U.S. adults, time and race and ethnicity play significant roles in COVID-19 vaccine intentions. Understanding the role of time and race and racism in shaping COVID-19 vaccine intention trajectories can help government agencies and public health experts tasked with administrating vaccines better understand disparities in vaccine uptake.
Keywords: COVID-19, Vaccine intentions, Race, Racism, Infectious diseases
Highlights
-
•
Intentions to receive a COVID-19 vaccine decreased from April to November 2020.
-
•
Vaccine intentions increased slightly from November 2020 to January 2021.
-
•
Asian/Pacific Islanders had the highest probability of likely getting a COVID-19 vaccine at baseline.
-
•
Black Americans exhibited the lowest probability of likely getting vaccinated.
-
•
In most cases, the gap between Black Americans and other racial groups grew over time.
1. Introduction
Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2), the novel coronavirus that causes COVID-19 disease, has quickly spread throughout the globe. By late January 2021, more than 100 million cases and approximately 2.1 million COVID-19 related deaths have been reported worldwide. The United States has been particularly impacted by the current pandemic, recording more than 25 million cases and 423,000 COVID-19 related deaths by late January 2021. With no clear signs of abatement, scholars argue that the development, distribution, and administration of vaccines will be the most effective approach to addressing the COVID-19 pandemic (Cory et al., 2020; Shin et al., 2020).
Safe, effective, and accessible vaccines are foundational to eradicating or significantly reducing morbidity and mortality associated with a range of infectious diseases (Orenstein & Ahmed, 2017), including the elimination of smallpox and continued reduction of polio cases, tetanus, and measles (Serdobova & Kieny, 2006). Several COVID-19 vaccines are currently under development, two of which received emergency use authorization by the Food and Drug Administration (FDA) in December 2020. While safety, efficacy, and dissemination of vaccines are critical to the goal of reducing morbidity and mortality associated with COVID-19, an equally important area is vaccine acceptance and uptake. Without widespread acceptance and uptake of COVID-19 vaccines, the goal of herd immunity will be difficult to achieve, leading to the further spread of the novel coronavirus. Scholars argue that approximately 70 percent (69.6%) of the U.S. population needs to have protective immunity from SARS-CoV-2 either through prior infections or vaccinations to reach herd immunity (Kwok et al., 2020). Herd immunity is essential, given that it not only provides direct protection to the immunized but also indirectly protects those who are potentially unable to receive COVID-19 vaccinations, such as young children and the immunocompromised (Randolph & Barreiro, 2020).
A significant barrier to reaching herd immunity is vaccine hesitancy, which is defined as the refusal or delay in acceptance of a safe and effective vaccine, despite widespread availability (MacDonald, 2015). In recent years, vaccine hesitancy has increased substantially across the globe (de Figueiredo et al., 2020) and has contributed to recent preventable infectious disease outbreaks, such as the reemergence of measles in the U.S. in 2019 (Hotez et al., 2020; Sarkar et al., 2019). The prevalence of vaccine hesitancy and the reemergence of preventable infectious diseases, not only in the U.S. but globally, has become such a concern that the World Health Organization (WHO) formally named vaccine hesitancy a top ten threat to global health in 2019 (WHO, 2019).
Given the significant challenge vaccine hesitancy poses to herd immunity, an emerging body of research has attempted to better understand COVID-19 vaccine intentions in the U.S. More specifically, scholars have focused on identifying structural and cultural factors associated with intentions to receive COVID-19 vaccines. One of the most consistent predictors of vaccine intentions within this small but growing body of research is race and ethnicity. Across a number of studies, scholars find Black Americans show greater hesitancy to receive COVID-19 vaccines (Carpiano, 2020; Fisher et al., 2020; Reiter et al., 2020), which aligns with broader bodies of work on vaccine hesitancy (Freimuth et al., 2017; Jamison et al., 2019; Quinn et al., 2017).
While scholars find significant differences in vaccine intentions between Black Americans and other racial and ethnic groups, few properly contextualize the role medical racism has played in these observed patterns (Jaiswal & Halkitis, 2019; Jamison et al., 2019). Numerous studies find medical racism has led to deep distrust of medical institutions within Black communities, shaping how Black Americans perceive and engage with medical research (Evans & Hargittai, 2020), physicians (Hagiwara et al., 2016), and vaccines (Freimuth et al., 2017; Harris et al., 2006). The Tuskegee Syphilis Study is the most notable example of medical racism in the U.S. and remains a part of collective memory among Black Americans (Fraizier, 2020). In 1932, the U.S. Public Health Service, in concert with the Tuskegee Institute, conducted an experiment designed to observe untreated syphilis in Black men. Participants were told they were being treated for "bad blood" and went untreated for syphilis for 40 years, well after effective treatments were available.
Contemporary research also shows that the legacies of medical racism continue to impact access and quality of care for Black Americans. Black patients are less likely to receive effective treatments for leading causes of death, including heart disease, stroke, and lung cancer, when compared to their White counterparts (Bach et al., 1999; Fincher et al., 2004; Mayberry et al., 2000; Schulman et al., 1999). Black women are also three times more likely to die from pregnancy-related complications than other racial groups (Oparah & Bonaparte, 2015). Similarly, Black children are twice as likely to die before age 1 compared to White children (CDC, 2019). Notably, Black families are able to mitigate the risk of infant death by more than half (58%) when Black physicians care for their children (Greenwood et al., 2020). Taken as a whole, the historical and contemporary nature of medical racism is a key contributor to observed inequities in vaccine intentions and uptake observed among Black Americans.
While the extant literature has identified important patterns in intention to receive COVID-19 vaccinations, several critical gaps remain. First, to date, little to no research has attempted to investigate whether time shapes COVID-19 vaccine intentions. Prior studies find time plays a significant role in hesitancy to vaccinate. For example, recently developed vaccines receive substantially more resistance from the general public than more established vaccines (Dubé et al., 2013; Larson et al., 2011). Moreover, when examining vaccine intentions for a myriad of specific infectious diseases, including types of influenza (Holm et al., 2007) and human papillomavirus (Reiter et al., 2013; Thompson et al., 2017), evidence suggests intention to vaccinate significantly changes over time. For instance, Gidengil et al. (2012) find that among a sample of U.S. adults, the intention to receive the H1N1 vaccine was the highest during the early months of the pandemic in 2009, but as time progressed, intention to vaccinate steadily decreased, even as infections increased. Gindengil and colleagues argue, as with other rare events, people tended to overestimate the risk of hospitalization and death related to H1N1 at the onset of the pandemic, which led to high intentions to receive an H1N1 vaccine. However, as evidence emerged showing that the risk of mortality was comparable to seasonal influenza, intentions to receive an H1N1 vaccine declined substantially over time. Given that the U.S. continues to report record COVID-19 infections and hospitalizations, understanding whether intentions to receive a COVID-19 vaccine increase, decrease, or remain stable is of vital importance as public health practitioners and policymakers develop COVID-19 distribution and administration plans.
Second, the history of differential medical treatment based on race and ethnicity has created a context in which some racial and ethnic groups are more hesitant to receive vaccines; yet whether these patterns change over time is less known. This is of particular importance given the continued disproportionate impact of COVID-19 on historically marginalized racial and ethnic groups. For instance, Black and Latina/o adults are more likely to become infected; be hospitalized due to severe illness and die from COVID-19 complications when compared to Whites. While Asian Americans do not present an increased risk of death from COVID-19, virologic surveillance data indicate Asian Americans are more likely to become infected and be hospitalized when compared to their White counterparts (CDC, 2020).
Using a large, national longitudinal sample of U.S. adults, we address these critical gaps by first assessing the timing and shape of intentions to receive a COVID-19 vaccine from April 2020 to January 2021. We then model trajectories of vaccine intention by race and ethnicity. More specifically, we assess whether vaccine intention trajectories vary between Black, Latina/o, Asian/Pacific Islander, and White adults from April 2020 to January 2021. In doing so, we are able to determine whether trajectories by race and ethnicity diverge, remain stable, or converge during one of the most significant public health crises in modern history.
2. Material and methods
Data for this study were drawn from the Understanding America Study (UAS), a national probability-based panel survey of U.S. adults, managed by the Center for Economic and Social Research at the University of Southern California. The UAS, which began in 2014, selected panel members using address-based sampling, a sequential sample procedure where a simple random sample is first generated using addresses from the Computerized Delivery Sequence (CDS) file; a file containing every postal address in the U.S. Sequential Importance Sampling (SIS), a type of adaptive sampling, was then used to generate unequal sampling probabilities with the overall goal of moving closer to the general U.S. population. Invitations to participate in UAS surveys are sent via email, and panelists receive approximately $20 for every 30 min of survey time. Panelists were also provided with broadband internet and a tablet when needed.
On March 10, 2020, UAS panelists were invited to participate in an ongoing survey related to the COVID-19 pandemic. Approximately 7000 out of the 9000 eligible UAS respondents agreed to participate. UAS surveys focused on a range of issues related to the COVID-19 pandemic, including, but not limited to, vaccine intentions, perceived COVID-19 symptoms, protective behaviors, mental health, perceived discrimination, and other social behaviors (e.g., alcohol and drug use). For this study, we draw from 9 Waves of the UAS between April 1, 2020, to January 6, 2021 - all of which capture vaccine intention responses at the time of interview.
2.1. Measures
COVID-19 vaccine intention was assessed using the following question “how likely are you to get vaccinated for coronavirus once a vaccination is available to the public?” Original response categories included: (1) very unlikely, (2) somewhat unlikely, (3) somewhat likely, (4) very likely, and (5) unsure. Given that we are interested in changes in COVID-19 vaccine intentions, and to simplify and ease the interpretation of results, we construct a binary measure of COVID-19 vaccine intention: (0) very unlikely/somewhat unlikely/unsure, (1) somewhat likely/very likely. As stated above, COVID-19 vaccine intention was measured repeatedly on the same study participants across all 9 UAS Waves.
Race and ethnicity was measured using a respondent's self-reported racial and ethnic identity. The original race and ethnicity measure included in the UAS captured the following self-reported racial and ethnic identities: Black, Latina/o, Asian/Pacific Islander, and Native American. Unfortunately, due to small sample sizes across UAS waves, we were unable to keep respondents that self-identified as Native American in the final analytic sample. Furthermore, UAS surveys did not provide opportunities for respondents to select more than one racial and ethnic identity. Therefore, we were unable to identify and include a category for respondents that held more than one racial and/or ethnic identity. Due to these data limitations, our final race and ethnicity measure included categories for Black, Latina/o, Asian/Pacific Islander, and White (reference).
We also include a series of measures that have been shown to play a role in vaccine hesitancy. The measures include gender, age, immigrant generation, work status, marital status, education level, household income, and perceived risk of death from COVID-19. Gender was measured using a dummy variable for male, with female as the reference. Age was measured in years and ranged from ages 18–101. Immigrant generation includes three categories: first-generation (foreign-born), second-generation (children of immigrants), and third-generation plus. Respondents that were born in a foreign country to at least one foreign-born parent were classified as first-generation. Individuals were considered second-generation if they were born in the United States to at least one foreign-born parent. Those born in the United States to parents also born in the United States were considered third-generation plus. Work status is a dummy variable that denotes whether respondents were working or not. Marital status was measured using a dummy variable for married, with not married as the reference. Education level was measured using four dummy variables: less than high school, high school, some college, and bachelor's degree or higher. Annual household income was divided into four categories: less than $5000-$34,999, $35,000-$59,999, $60,000-$74,999, and greater than or equal to $75,000. Perceived risk of death from COVID-19 was measured using the following question “if you do get the coronavirus, what is the percent chance you will die from it. Responses for this measure ranged from 0 to 100%.
2.2. Analytic approach
To estimate vaccine intention trajectories from early April 2020 to January 2021, we adopt a multilevel logistic regression model with an individual-level random effect to control for the repeated measurements of respondents. For example, the probability of likely to vaccinate by individual i at wave t, is predicted by:
such that = , where represents the estimated slope; represents the row vector of estimated coefficients and the timing of the corresponding UAS wave t; represents the vector of control variables and estimated coefficients; is the individual-level intercept | . The general trajectories for COVID-19 vaccine intention are described by time and time2 and their respective parameters.
To test whether COVID-19 vaccine intentions differ by racial and ethnic background, we include race/ethnicity-time interactions. The following equation describes the basic structure of the interaction model:
We utilized maximum likelihood estimation to account for missing data under the assumption that non-responses were missing at random.
2.3. Sensitivity analyses
Given that there is a potential loss of information when including the unsure category with responses for very unlikely and somewhat unlikely, we estimated a series of regression models with alternative operations of the COVID-19 vaccine intention measure. In the first series of supplemental models (S1), unsure responses were removed from the final analytic sample, and results produced results that were similar in statistical significance, direction, and magnitude. The second set of supplemental models (S2) provide estimates for a series of repeated measures multinomial logistic regressions that operationalized COVID-19 vaccine intentions with three categories: (0) unsure, (1) very unlikely/somewhat unlikely, (2) somewhat likely/very likely. Results from the first model show the probability respondents were unsure of getting a vaccine was low from the onset of the COVID-19 pandemic (<1%) and remained low throughout the duration of this study. When compared to unsure respondents, model 2 shows racial and ethnic groups have statistically indistinguishable rates of change in likely and unlikely to get a COVID-19 vaccine. Taken together, estimates from our sensitivity analysis provide little to no evidence of a meaningful loss of information when including unsure responses with unlikely responses.
3. Results
Table 1 provides descriptive statistics for all measures included in this study, pooled across UAS waves and by racial and ethnic background. Table 1 also shows that the majority of respondents in the final analytic sample were White (75%), female (57%), third generation-plus (75%), working at the time of the interview (54%), and married (57%). Across all racial and ethnic groups, results show vaccine intention steadily declined from April to November of 2020, but as vaccines became available across the country in early December, we observed an increase in vaccine intentions. These findings align with recent reports that demonstrate the dynamic nature of COVID-19 vaccine intentions using cross-sectional data (Guidry et al., 2021; Robinson et al., 2020; Wong et al., 2020). Results also show vaccine intention is lowest among Black respondents, which remains consistent over time. In contrast, Asian/Pacific Islanders present the highest proportion of likely to get a COVID-19 vaccine among the racial and ethnic groups included in this study.
Table 1.
Proportions of COVID-19 vaccine intention from early April 2020 to late November 2020 by race and ethnicity
Source: Understanding America Study.
| Overall |
Latina/o |
Asian/Pacific Islander |
Black |
White |
|
|---|---|---|---|---|---|
| Mean/(S.D.) | Mean(S.D.) | Mean(S.D.) | Mean(S.D.) | Mean(SD) | |
| COVID-19 Vaccine Intention | |||||
| Wave 1 (4/1–4/29/20) | 0.78(.42) | 0.74(.44) | 0.86(.35) | 0.51(.50) | 0.81(.40) |
| Wave 2 (4/29-5/26/20) | 0.70(.46) | 0.67(.47) | 0.86(.35) | 0.45(.50) | 0.73(.45) |
| Wave 3 (5/27-6/23/20) | 0.67(.47) | 0.65(.48) | 0.83(.38) | 0.40(.49) | 0.69(.46) |
| Wave 4 (6/24-7/22/20) | 0.66(.47) | 0.63(.48) | 0.80(.40) | 0.42(.49) | 0.69(.46) |
| Wave 5 (7/22-8/19/20) | 0.65(.48) | 0.63(.48) | 0.83(.38) | 0.43(.50) | 0.67(.47) |
| Wave 6 (8/19-9/16/20) | 0.63(.48) | 0.60(.49) | 0.75(.43) | 0.39(.49) | 0.65(.48) |
| Wave 7 (9/30-10/27/20) | 0.58(.49) | 0.54(.50) | 0.73(.45) | 0.30(.46) | 0.60(.49) |
| Wave 8 (10/28-11/25/20) | 0.60(.49) | 0.55(.50) | 0.73(.45) | 0.38(.48) | 0.62(.49) |
| Wave 9 (12/9/20-1/6/21) | 0.61(.49) | 0.58(.49) | 0.73(.45) | 0.40(.49) | 0.64(.48) |
| Gender | |||||
| Male | 0.43(.49) | 0.36(.48) | 0.47(.50) | 0.31(.46) | 0.45(.50) |
| Age | 52.06(16.08) | 40.84(13.72) | 44.84(15.78) | 50.33(14.25) | 55.04(15.51) |
| Immigrant generation | |||||
| First generation | 0.11(.31) | 0.27(.44) | 0.64(.48) | 0.04(.21) | 0.04(.20) |
| Second generation | 0.14(.35) | 0.51(.50 | 0.29(.45) | 0.04(.21) | 0.07(.25) |
| Third generation plus | 0.75(.43) | 0.22(.42) | 0.07(.26) | 0.91(.29) | 0.89(.31) |
| Work status | |||||
| Working | 0.54(.50) | 0.62(.48) | 0.67(.47) | 0.54(.50) | 0.52(.50) |
| Marital status | |||||
| Married | 0.57(.49) | 0.49(.50) | 0.52(.50) | 0.29(.45) | 0.62(.48) |
| Education level | |||||
| Bachelor's or higher | 0.43(.49) | 0.31(.46) | 0.67(.47) | 0.28(.45) | 0.45(.50) |
| Some college | 0.36(.48) | 0.43(.49) | 0.22(.42) | 0.44(.50) | 0.35(.48) |
| High school | 0.16(.37) | 0.18(.39) | 0.09(.29) | 0.22(.41) | 0.16(.36) |
| Less than high school | 0.05(.21) | 0.08(.27) | 0.01(.12) | 0.07(.25) | 0.04(.19) |
| Household income | |||||
| less than $5000-$34,999 | 0.28(.45) | 0.34(.47) | 0.27(.45) | 0.51(.50) | 0.24(.43) |
| $35,000-$59,999, | 0.21(.40) | 0.23(.42) | 0.18(.39) | 0.22(.42) | 0.20(.40) |
| $60,000-$74,999 | 0.11(.31) | 0.10(.31) | 0.11(.31) | 0.06(.24) | 0.11(.32) |
| $75,000 and above | 0.41(.49) | 0.32(.47) | 0.44(.50) | 0.20(.40) | 0.45(.50) |
| Perceived risk of death | 24.70(26.84) | 26.04(25.04) | 18.10(22.23) | 24.37(27.16) | 24.93(27.39) |
| Number of respondents | 5023 | 752 | 252 | 352 | 3667 |
| Number of observations | 40854 | 6039 | 2106 | 3003 | 29,706 |
We present estimates from the multilevel logistic regression models for COVID-19 vaccine intention in Table 2. Model 1 contains estimates on the initial level and rate of change in COVID-19 vaccine intention, net controls. Results indicate both the negative fixed linear growth component and the positive fixed quadratic term were statistically significant, suggesting the average population rate of change is non-linear. More specifically, the negative coefficient for time (−0.64, p < .001) and the positive coefficient for time-squared (0.04, p < .001) indicates COVID-19 vaccine intention declined substantially during the early months of the pandemic, but over time, the decline in vaccine intention slowed. In other words, the overall rate of change for vaccine intention is decreasing at an increasing rate.
Table 2.
Estimates for repeated measures logistic regression
Source: Understanding America Study.
| Model 1 |
Model 2 |
|
|---|---|---|
| β(S.E.) | β(S.E.) | |
| Intercept | 1.37(.35)*** | 1.54(.35)*** |
| Race and Ethnicity | ||
| Latina/o | -.12(.18) | -.52(.25)* |
| Asian/Pacific Islander | .86(.28)** | .16(.43) |
| Black | −1.95(.20)*** | −2.58(.28)*** |
| Linear Slope (Wave) | -.64(.03)*** | -.71(.04)*** |
| Latina/o | .19(.08)* | |
| Asian/Pacific Islander | .38(.14)** | |
| Black | .21(.10)* | |
| Quadratic Slope (Wave2) | .04(.00)*** | .05(.00)*** |
| Latina/o | -.02(.01)* | |
| Asian | -.05(.02)** | |
| Black | -.01(.00)* | |
| Immigrant generation | ||
| First generation | .42(.21)* | .42(.21)* |
| Second generation | .23(.18) | .24(.18) |
| Age | .02(.00)*** | .02(.00)*** |
| Work status | ||
| Working | -.23(.12)** | -.23(.12)* |
| Marital status | ||
| Married | -.33(.11)** | -.33(.11)** |
| Education level | ||
| High school | .30(.25) | .29(.25) |
| Some college | .76(.24)** | .76(.24)** |
| Bachelor's or higher | 2.13(.25)*** | 2.13(.25)*** |
| Household income | ||
| less than $5000-$34,999 | −1.19(.15)*** | −1.19(.15)*** |
| $35,000-$59,999 | -.66(.15)*** | -.66(.15)*** |
| $60,000-$74,999 | -.51(.18)** | -.51(.18)** |
| Gender | ||
| Male | .70(.11)*** | .70(.11)*** |
| Perceived risk of death | .01(.00)*** | .01(.00)*** |
| Random intercept | 3.11(.06)*** | 3.11(.06)*** |
| Number of respondents | 5023 | 5023 |
| Number of observations | 40,854 | 40,854 |
*p < .05, **p < .01, ***p < .001.
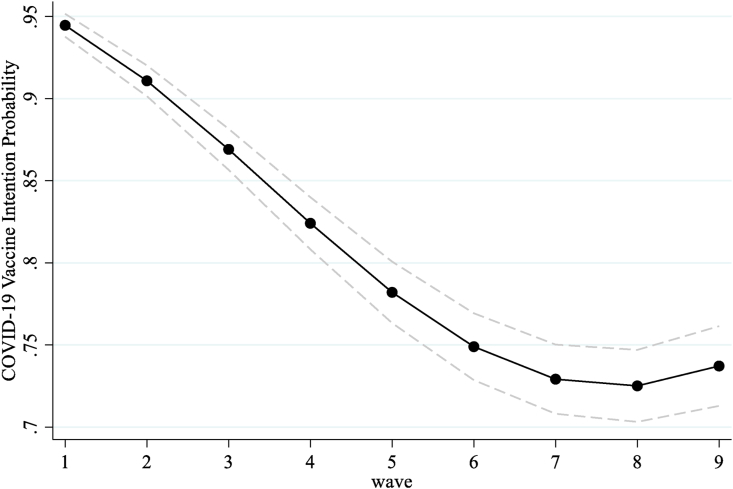
To better illustrate overall COVID-19 vaccine intentions trajectories, Fig. 1 shows the plotted predicted probabilities for vaccine intention from early April 2020 to January 2021. Results from Fig. 1 show that the probability of likely getting a COVID-19 vaccine was over 90% in early April but, by late July/August 2020 (wave 5), the probability decreased by 16%. By late October/November 2020 (wave 8), we also observe that the probability of likely getting a vaccine dropped by another six percentage points, but as the U.S. transitioned into 2021, intentions to receive a COVID-19 vaccine increased slightly. Results from model 1 in Table 2 also show significant differences in COVID-19 vaccine intentions by race and ethnicity. More specifically, when compared to Whites, the odds of likely getting a COVID-19 vaccine were lower for Black respondents and higher for Asian and Pacific Islanders at baseline.
Fig. 1.
Overall COVID-19 vaccine intention trajectories.
Model 1 assumes COVID-19 vaccine intention trajectories from early April 2020 to January 2021 are the same across racial and ethnic background. Model 2 relaxes this assumption by introducing time*race/ethnicity interactions. Results from model 2 show vaccine intention intercepts, and slopes significantly vary by race and ethnicity. To ease the interpretation of results, Fig. 2 presents the model-implied group-specific COVID-19 vaccine intention trajectories by race and ethnicity, illustrating their magnitude and shape, net of controls. At baseline, Asian/Pacific Islanders had the highest probability of intention to vaccinate (0.98), followed by White (0.96), Latina/o (0.91), and Black Americans (0.52). Fig. 2 also shows that vaccine intentions decreased from April 2020 to January 2021 for all racial and ethnic groups, with two notable exceptions. During the final wave (i.e., December 2020/January 2021), we observe a slight increase in the probability of likely getting vaccinated for Black Americans and Whites at wave 9.
Fig. 2.
COVID 19 vaccine intention trajectories by race and ethnicity.
From wave 1 to wave 9, results also demonstrate that Black Americans and Latina/os had the largest decrease in vaccine intentions over time. More specifically, from April 2020 to January 2021, vaccine intentions decreased by 28% for Black Americans and 27% for Latina/o, respectively. Moreover, while we observed a slight increase in vaccine intentions for Black Americans at wave 9, the gap in vaccine intentions between Black Americans and other racial and ethnic groups is particularly stark and, in most cases, grew over time. For instance, for Black Americans, there was almost a 50% (46%) gap in intent to vaccinate between Black and Asian and Pacific Islanders in late April 2020, and by January 2021, the gap increased by 19%–65%.
4. Discussion
The U.S. is currently experiencing unprecedented rates of COVID-19 infections, hospitalizations, and deaths. As a result, scholars argue that widespread uptake of COVID-19 vaccines may be the most effective strategy to reduce COVID-19 related morbidity and mortality. However, an emerging body of research finds a sizable portion of the U.S. population is hesitant to receive COVID-19 vaccines, with notable differences across racial and ethnic lines. While the extant literature provides important insights into the social patterning of COVID-19 vaccine intentions in the U.S., far less is known about whether intentions to get a COVID-19 vaccination change over time and whether potential changes in vaccine intentions vary by racial and ethnic background.
Using panel data from the Understanding America Study, the present study examines how time and race and ethnicity shape vaccine intention patterns from April 2020 to January 2021. In doing so, we advance prior knowledge on vaccine intentions in two key respects. First, to our knowledge, this is the only study to examine whether COVID-19 vaccine intentions remain stable, converge, or diverge over time. Documenting trajectories of COVID-19 vaccine intentions during this timeframe can provide health professionals with a better understanding of what strategies will be necessary to increase widespread vaccine uptake during one of the most significant public health crises in modern U.S. history. Second, regardless of the infectious disease, this study is among the first to conceptualize and model vaccine intention trajectories by racial and ethnic background, providing new insights into whether vaccine intentions change over time for Black, Latina/o, Asian/Pacific Islander, and White adults.
Consistent with prior longitudinal research on vaccine intentions for other infectious disease outbreaks, results revealed intentions to get a COVID-19 vaccine significantly changed over time. More specifically, we find the probability of likely getting a COVID-19 vaccine steadily declined from April to November 2020. By January 2021, however, vaccine intentions increased slightly. A number of potential mechanisms may explain the overall COVID-19 vaccine intentions trajectory observed in the following study. First, the unprecedented pace at which COVID-19 vaccines have been developed and tested left little time to clarify a myriad of concerns related to newly developed vaccines. For instance, reports indicate a majority of U.S. adults have concerns over the cost, side effects, and effectiveness of COVID-19 vaccines – all of which would reduce the likelihood of getting vaccinated (Tyson et al., 2020). Lack of confidence in government and government officials may also contribute to the overall vaccine trajectory patterns observed in this study (Evans & Hargittai, 2020). According to a Kaiser Family Foundation report, more than half of U.S. adults do not trust the federal government to ensure the safety and efficacy of COVID-19 vaccines (Hamel et al., 2020), which ties to broader patterns showing less than 20% of U.S. adults trust the federal government to do the right thing always or most of the time (Wong et al., 2020).
Online misinformation concerning vaccines' safety and efficacy may also partially explain the declines in vaccine intentions observed in this study. A recent study of approximately 100 million Facebook users found anti-vaccination clusters were dominant in network patches and heavily entangled in clusters of users with undecided stances on vaccines. In the same study, researchers also found anti-vaccination individuals form twice as many clusters as pro-vaccination individuals, providing more opportunities for engagement and ultimately increasing their network centrality (Johnson and et al., 2020). Consequently, the authors forecast that anti-vaccination views will dominate the platform within ten years without proper interventions.
This study also draws on literatures that highlight inequities in vaccine intention and vaccine uptake across racial and ethnic background. Our analysis shows significant differences in vaccine intentions by racial and ethnic background at the onset of the COVID-19 pandemic. More specifically, in April of 2020, Black Americans had the lowest probability of likely getting a COVID-19 vaccination, while Asian/Pacific Islanders had the highest probability of getting a vaccination. Moreover, while the gap in COVID-19 vaccine intentions between Black Americans and other racial groups was substantial at the onset of the pandemic, results demonstrate the gap between Black Americans and other racial groups widened over time in most cases. Our finding that there is a substantial gap in vaccine intentions is consistent with a broad body of literature on racial and ethnic disparities in vaccine hesitancy and vaccine uptake. However, this is the first study to document that the gap between Black Americans and other racial groups may be widening as the U.S. continues to move through the COVID-19 pandemic.
A plausible explanation for the widening gap in vaccine intentions between Black Americans and other racial groups is trust. Studies find that if people do not trust the sources of information concerning vaccine efficacy and safety, they are likely to be vaccine-hesitant (Brownlie & Howson, 2005; Goldenberg, 2016; Yaqub et al., 2014). As discussed previously, medical racism in the U.S. is deep and pervasive, disproportionately impacting Black communities both historically and presently. Evidence of contemporary medical racism has also been documented in recent reports that show almost 7 out 10 Black Americans believe patients are treated unfairly in medical settings due to their race and ethnicity (Hamel et al., 2020). This is coupled with the fact that during the time of this study, Black Americans reported significant levels of distrust in political leadership and government institutions. For instance, in September 2020, (Baum et al., 2020) find Black Americans had the lowest level of distrust in President Donald Trump and the White House when compared to other racial and ethnic groups. Finally, as evidenced earlier, studies continue to show health and social inequities among Black American have not only persisted, but in some cases, have widened during the pandemic (Perry et al., 2021; Snowden & Graff, 2021), furthering distrust in health systems and government institutions tasked at vaccine distribution.
Given these realities, Black Americans must grapple with the complex relationship between racism and mistrust when considering whether to get the COVID-19 vaccine when eligible (Harris et al., 2006). Moreover, the pace at which vaccines have been developed, approved, and disseminated has left little time to address concerns related to vaccine efficacy and safety within Black communities. Therefore, the growing gap in vaccine intentions between Black Americans and other racial groups may be due to differential exposures to medical racism, both historically and presently, the lack of trust in government and health institutions, and the pace at which vaccines have been developed, tested, and distributed.
To begin to meaningfully address the gap in COVID-19 vaccine intentions between Black Americans and other racial and ethnic groups, scholars and health professionals argue that we must move beyond traditional vaccine public education campaigns designed to increase vaccine uptake. More specifically, health experts (e.g. South, 2021) argue that those responsible for increasing vaccine uptake should abandon traditional shame tactics and shift to normalizing vaccine hesitancy when new vaccines are developed and administered, especially for Black Americans. Given the long history of medical racism and mistrust, Black Americans are likely to have questions and exhibit some hesitancy regarding receiving a new vaccine. Moreover, medical and governmental institutions must begin to publicly acknowledge and address how racist structures and interactions have historically and presently fostered deep distrust among Black Americans. Finally, public health campaigns should partner with Black medical professionals, especially those with a deep understanding of and experiences with vaccine hesitancy in communities of color, to address the valid concerns of Black vaccine-hesitant patients (Craven, 2020).
This study is not without limitations. First, while this is one of the first studies to examine COVID-19 vaccine intention trajectories, the measure for vaccine intentions does not capture important contextual elements of vaccine hesitancy, such as whether respondents would get the COVID-19 vaccine if recommended by a physician, employer, spouse/partner, and/or community leaders. Given this limitation, future research should investigate general trajectories and trajectories by race and ethnicity using more nuanced vaccine intentions measures. Second, due to data limitations, we were unable to include more inclusive measures of racial and ethnic background. For instance, reports continue to show the COVID-19 pandemic has particularly impacted Native American communities, yet little is known about vaccine intentions within this community. We were also unable to account for a number of other factors that have been shown to play a role in vaccine hesitancy, such as vaccine attitudes, personal experience with the medical system, and broader aspects of family structure. Future panel studies should adopt sampling strategies that will allow scholars to better understand vaccine intention trajectories among racial and ethnic groups that are often overlooked and include a broad set of measures that have been shown to shape vaccine intention patterns. Third, results from this study are only reflective of intentions to get a COVID-19 vaccine in the U.S. Given the varied countrywide responses to COVID-19, as well as the unique history of race and racism in the U.S., results from this study may not be applicable to other countries. Finally, recent intersectional scholarship also demonstrates that, due to structured systems that systematically disadvantage minoritized communities, race/ethnicity, gender, and social class jointly and simultaneously shape economic, social, and health disparities related to the COVID-19 pandemic (Gezici & Ozay, 2020; Calarco et al., 2020; Hearne & Niño, 2021; Moen et al., 2020). Given that structurally racist and sexist systems, in conjunction with socioeconomic drivers, may be jointly shaping vaccine intention patterns, future research would benefit from adopting an intersectional framework when examining race/ethnicity-COVID-19 vaccine intention trajectories.
CRediT authorship contribution statement
Michael D. Niño: Conceptualization, Methodology, Formal analysis, Writing – original draft, Writing – review & editing. Brittany N. Hearne: Writing – original draft, Writing – review & editing. Tianji Cai: Methodology, Formal analysis, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare no financial conflicts of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ssmph.2021.100824.
Contributor Information
Michael D. Niño, Email: mnino@uark.edu.
Brittany N. Hearne, Email: bnhearne@uark.edu.
Tianji Cai, Email: tjcai@um.edu.mo.
Ethics statement
The authors declare that there are no financial or personal relationships with other people or organizations that could have inappropriately influenced or biased their work.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Multimedia component 1
References
- Bach P.B., Cramer L.D., Warren J.L., Begg C.B. Racial differences in the treatment of early-stage lung cancer. New England Journal of Medicine. 1999;341:1198–1205. doi: 10.1056/NEJM199910143411606. [DOI] [PubMed] [Google Scholar]
- Baum M.A., Lin J., Ognyanova K., Chwe H., Quintana A., Lazer D., Druckman J., Perlis R., Santillana M., Volpe J., Simonson M., Green J. The COVID-19 Consortium for Understanding the Publics Policy Preferences Across States. 2020. The State of the Nation: A 50-state COVID-19 survey Report #13: Public trust in institutions and vaccine acceptance.http://www.kateto.net/covid19/COVID19%20CONSORTIUM%20REPORT%2013%20TRUST%20SEP%202020.pdf Retrieved from. [Google Scholar]
- Brownlie J., Howson A. ‘Leaps of faith’and MMR: An empirical study of trust. Sociology. 2005;39:221–239. [Google Scholar]
- Calarco J.M., Meanwell E., Anderson E., Knopf A. “‘My husband thinks I'm crazy’: COVID-19-Related conflict in couples with young children. SocArXiv. 2020 doi: 10.31235/osf.io/cpkj6. October 9. [DOI] [Google Scholar]
- Carpiano R.M. medRxiv; 2020. Demographic differences in U.S. adult intentions to receive a potential coronavirus vaccine and implications for ongoing study.https://www.medrxiv.org/content/10.1101/2020.09.07.20190058v1.article-metrics Retrieved from. [DOI] [Google Scholar]
- Centers of Disease Control (CDC) 2019. Infant mortality in the United States, 2017: Data from the period linked birth/infant death file.https://stacks.cdc.gov/view/cdc/80304 Retrieved from. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) 2020. Health equity considerations and racial and ethnic minority groups.https://www.cdc.gov/coronavirus/2019-ncov/community/health-equity/race-ethnicity.html Retrieved from. [Google Scholar]
- Corey L., Mascola J.R., Fauci A.S., Collins F.S. A strategic approach to COVID-19 vaccine R&D. Science. 2020;368:948–950. doi: 10.1126/science.abc5312. [DOI] [PubMed] [Google Scholar]
- Craven J. Slate; 2020. Black doctors explain how to overcome reluctance toward the COVID vaccine.https://slate.com/technology/2021/01/covid-vaccine-trust-black-doctors.html Retrieved from. [Google Scholar]
- Dubé E., Laberge C., Guay M., Bramadat P., Roy R., Bettinger J.A. Vaccine hesitancy: An overview. Human Vaccines & Immunotherapeutics. 2013;9:1763–1773. doi: 10.4161/hv.24657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J.H., Hargittai E. Who doesn't trust fauci? The public's belief in the expertise and shared values of scientists in the COVID-19 pandemic. Socius. 2020;6:1–13. [Google Scholar]
- de Figueiredo A., Simas C., Karafillakis E., Paterson P., Larson H.J. Mapping global trends in vaccine confidence and investigating barriers to vaccine uptake: A large-scale retrospective temporal modelling study. Lancet. 2020;396:898–908. doi: 10.1016/S0140-6736(20)31558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fincher C., Williams J.E., MacLean V., Allison J.J., Kiefe C.I., Canto J. Racial disparities in coronary heart disease: A sociological view of the medical literature on physician bias. Ethnicity & Disease. 2004;14:360–371. [PubMed] [Google Scholar]
- Fisher K.A., Bloomstone S.J., Walder J., Crawford S., Fouayzi H., Mazor K.M. Attitudes toward a potential SARS-CoV-2 vaccine: A survey of U.S. Adults. Annals of Internal Medicine. 2020;173:964–973. doi: 10.7326/M20-3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier C. It's more than just news: Print media, the tuskegee syphilis study and collective memory among african Americans. The Journal of Historical Sociology. 2020;33:280–296. [Google Scholar]
- Freimuth V.S., Jamison A.M., An J., Hancock G.R., Quinn S.C. Determinants of trust in the flu vaccine for African Americans and Whites. Social Science & Medicine. 2017;193:70–79. doi: 10.1016/j.socscimed.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gezici A., Ozay O. An Intersectional Analysis of COVID-19 Unemployment. J. Econ. Race Pol. 2020;3:270–281. doi: 10.1007/s41996-020-00075-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidengil C.A., Parker A.M., Zikmund-Fisher B.J. Trends in risk perceptions and vaccination intentions: A longitudinal study of the first year of the H1N1 pandemic. American Journal of Public Health. 2012;102:672–679. doi: 10.2105/AJPH.2011.300407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg M.J. Public misunderstanding of science? Reframing the problem of vaccine hesitancy. Perspect. Sci. 2016;24:552–581. [Google Scholar]
- Greenwood B.N., Hardeman R.R., Huang L. Sojourner. Physician-patient racial concordance and disparities in birthing mortality for newborns. Proceedings of the National Academy of Sciences. 2020;117:21194–21200. doi: 10.1073/pnas.1913405117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidry J.P., Laestadius L.I., Vraga E.K., Miller C.A., Perrin P.B., Burton C.W., Ryan M., Fuemmeler B.F., Carlyle K.E. Willingness to get the COVID-19 vaccine with and without emergency use authorization. American Journal of Infection Control. 2021;49:137–142. doi: 10.1016/j.ajic.2020.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara N., Dovidio J.F., Eggly S., Penner L.A. The effects of racial attitudes on affect and engagement in racially discordant medical interactions between non-Black physicians and Black patients. Group Processes & Intergroup Relations. 2016;19:509–527. doi: 10.1177/1368430216641306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel L., Kearney A., Kirzinger A., Lopes L., Muñana C., Brodie M. Kaiser Family Foundation; 2020. KFF health tracking poll–june 2020.https://www.kff.org/racial-equity-and-health-policy/report/kff-health-tracking-poll-june-2020/ Retrieved from. [Google Scholar]
- Hamel L, Lopes L, Muñana C, Artiga S, Brodie M. Kaiser Family Foundation; 2020. Race, Health, and COVID-19: The Views and Experiences of African Americans.https://files.kff.org/attachment/Report-Race-Health-and-COVID-19-The-Views-and-Experiences-of-Black-Americans.pdf Retrieved from. [Google Scholar]
- Harris L.M., Chin N.P., Fiscella K., Humiston S. Barriers to pneumococcal and influenza vaccinations in Black elderly communities: Mistrust. Journal of the National Medical Association. 2006;98:1678–1684. [PMC free article] [PubMed] [Google Scholar]
- Hearne B.N., Niño M.D. Understanding how race, ethnicity, and gender shape mask-wearing adherence during the COVID-19 pandemic: Evidence from the COVID impact survey. J. Racial Ethn. Health Disparities. 2021:1–8. doi: 10.1007/s40615-020-00941-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm M.V., Blank P.R., Szucs T.D. Trends in influenza vaccination coverage rates in Germany over five seasons from 2001 to 2006. BMC Infectious Diseases. 2007;7:1–8. doi: 10.1186/1471-2334-7-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotez P.J., Nuzhath T., Colwell B. Combating vaccine hesitancy and other 21st century social determinants in the global fight against measles. Curr. Opin. Virol. 2020;41:1–7. doi: 10.1016/j.coviro.2020.01.001. [DOI] [PubMed] [Google Scholar]
- Jaiswal J., Halkitis P.N. Towards a more inclusive and dynamic understanding of medical mistrust informed by science. Journal of Behavioral Medicine. 2019;45:79–85. doi: 10.1080/08964289.2019.1619511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamison A.M., Quinn S.C., Freimuth V.S. “You don't trust a government vaccine”: Narratives of institutional trust and influenza vaccination among African American and white adults. Social Science & Medicine. 2019;221:87–94. doi: 10.1016/j.socscimed.2018.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson N.F., Velásquez N., Restrepo N.J., Leahy R., Gabriel N., El O.S., Zheng M., Manrique P., Wuchty S., Lupu Y. The online competition between pro-and anti-vaccination views. Nature. 2020;582:230–233. doi: 10.1038/s41586-020-2281-1. [DOI] [PubMed] [Google Scholar]
- Kwok K.O., Lai F., Wei W.I., Wong Y.S., Tang J.W. Herd immunity–estimating the level required to halt the COVID-19 epidemics in affected countries. Am. J. Infect. 2020;80:e32–e33. doi: 10.1016/j.jinf.2020.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson H.J., Cooper L.Z., Eskola J., Katz S.L., Ratzan S. Addressing the vaccine confidence gap. Lancet. 2011;378:526–535. doi: 10.1016/S0140-6736(11)60678-8. [DOI] [PubMed] [Google Scholar]
- MacDonald N.E. Vaccine hesitancy: Definition, scope and determinants. Vaccine. 2015;33:4161–4164. doi: 10.1016/j.vaccine.2015.04.036. [DOI] [PubMed] [Google Scholar]
- Mayberry R.M., Mili F., Ofili E. Racial and ethnic differences in access to medical care. Medical Care Research and Review. 2000;57:108–145. doi: 10.1177/1077558700057001S06. [DOI] [PubMed] [Google Scholar]
- Moen P., Pedtke J.H., Flood S. Disparate disruptions: Intersectional COVID-19 employment effects by age, gender, education, and race/ethnicity. Work, aging and retire. 2020;6:207–228. doi: 10.1093/workar/waaa013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oparah J.C., Bonaparte A.D. Routledge; New York: 2015. Birthing justice: Black women, pregnancy, and childbirth. [Google Scholar]
- Orenstein W.A., Ahmed R. Simply put: Vaccination saves lives. Proceedings of the National Academy of Sciences. 2017;114:4031–4033. doi: 10.1073/pnas.1704507114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry BL, Aronson B, Pescosolido BA. Pandemic precarity: COVID-19 is exposing and exacerbating inequalities in the American heartland. Proceedings of the National Academy of Sciences. 2021;118:1–6. doi: 10.1073/pnas.2020685118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn S.C., Jamison A.M., Freimuth V.S., An J., Hancock G.R. Determinants of influenza vaccination among high-risk Black and White adults. Vaccine. 2017;35:7154–7159. doi: 10.1016/j.vaccine.2017.10.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph H.E., Barreiro L.B. Herd immunity: Understanding COVID-19. Immunity. 2020;52:737–741. doi: 10.1016/j.immuni.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter P.L., McRee A.L., Pepper J.K., Gilkey M.B., Galbraith K.V., Brewer N.T. Longitudinal predictors of human papillomavirus vaccination among a national sample of adolescent males. American Journal of Public Health. 2013;103:1419–1427. doi: 10.2105/AJPH.2012.301189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter P.L., Pennell M.L., Katz M.L. Acceptability of a COVID-19 vaccine among adults in the United States: How many people would get vaccinated? Vaccine. 2020;38:6500–6507. doi: 10.1016/j.vaccine.2020.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson E., Jones A., Daly M. International estimates of intended uptake and refusal of COVID-19 vaccines: A rapid systematic review and meta-analysis of large nationally representative samples. medRxiv. 2020 doi: 10.1101/2020.12.01.20241729. https://www.medrxiv.org/content/10.1101/2020.12.01.20241729v1 [preprint]. Retrieved from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S., Zlojutro A., Khan K., Gardner L. Measles resurgence in the USA: How international travel compounds vaccine resistance. The Lancet Infectious Diseases. 2019;19:684–686. doi: 10.1016/S1473-3099(19)30231-2. [DOI] [PubMed] [Google Scholar]
- Schulman K.A., Berlin J.A., Harless W., Kerner J.F., Sistrunk S., Gersh B.J., Dube R., Taleghani C.K., Burke J.E., Williams S., Eisenberg J.M. The effect of race and sex on physicians' recommendations for cardiac catheterization. New England Journal of Medicine. 1999;340:618–626. doi: 10.1056/NEJM199902253400806. [DOI] [PubMed] [Google Scholar]
- Serdobova I., Kieny M.P. Assembling a global vaccine development pipeline for infectious diseases in the developing world. American Journal of Public Health. 2006;96:1554–1559. doi: 10.2105/AJPH.2005.074583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin M.D., Shukla S., Chung Y.H., Beiss V., Chan S.K., Ortega-Rivera O.A., Wirth D.M., Chen A., Sack M., Pokorski J.K., Steinmetz N.F. COVID-19 vaccine development and a potential nanomaterial path forward. Nature Nanotechnology. 2020;15:646–655. doi: 10.1038/s41565-020-0737-y. [DOI] [PubMed] [Google Scholar]
- Snowden LR, Graff G. COVID-19, social determinants past, present, and future, and African Americans’ health. Journal of Racial Ethnic Health Disparities. 2021;8:12–20. doi: 10.1007/s40615-020-00923-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South E. NBC News; 2021. I'm a Black doctor who didn't trust the COVID vaccine. Here's what changed my mind.https://www.nbcnews.com/think/opinion/i-m-black-doctor-who-didn-t-trust-covid-vaccine-ncna1255085 Retrieved from. [Google Scholar]
- Thompson E.L., Rosen B.L., Vamos C.A., Kadono M., Daley E.M. Human papillomavirus vaccination: What are the reasons for nonvaccination among U.S. Adolescents? Journal of Adolescent Health. 2017;61:288–293. doi: 10.1016/j.jadohealth.2017.05.015. [DOI] [PubMed] [Google Scholar]
- Tyson A., Johnson C., Funk C. Pew Research Center; 2020. U.S. Public now divided over whether to get COVID-19 Vaccine.https://www.pewresearch.org/science/2020/09/17/u-s-public-now-divided-over-whether-to-get-covid-19-vaccine/ Retrieved from. [Google Scholar]
- Wong L.P., Alias H., Wong P.F., Lee H.Y., AbuBakar S. The use of the health belief model to assess predictors of intent to receive the COVID-19 vaccine and willingness to pay. Human Vaccines & Immunotherapeutics. 2020;16:2204–2214. doi: 10.1080/21645515.2020.1790279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO) 2019. Ten threats to global health in 2019.https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019 Retrieved from. [Google Scholar]
- Yaqub O., Castle-Clarke S., Sevdalis N., Chataway J. Attitudes to vaccination: A critical review. Social Science & Medicine. 2014;112:1–11. doi: 10.1016/j.socscimed.2014.04.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multimedia component 1