Abstract
Sleep apnea is highly associated with atrial fibrillation (AF), and both diseases are highly prevalent in the United States. The mechanistic underpinnings that contribute to their association remain uncertain, but numerous possible mechanisms have been proposed, including dysfunction of the cardiac autonomic nervous system (ANS). Studies have reported that apnea induces hyperactivity of the ANS, leading to increases in AF susceptibility. This review compiles the latest evidence on the role of the ANS in sleep-apnea-induced AF.
Keywords: atrial fibrillation, sleep apnea, obstructive sleep apnea, central sleep apnea, autonomic nervous system, sensory neurons
INTRODUCTION
Atrial fibrillation (AF), the most common sustained arrhythmia in adults,1, 2, 3, 4 is highly associated with both obstructive and central sleep apnea.5, 6, 7, 8 Individuals with sleep apnea have 2- to 4-fold higher odds of having AF than those without sleep apnea, even after adjustment for potential confounders.5 Sleep apnea is characterized by intermittent cessation or attenuation of breathing of at least 10 seconds during sleep, leading to oxygen desaturation. In obstructive sleep apnea (OSA), this results from a partial or complete collapse of the upper airways, whereas in central sleep apnea, this results from a decline or absence of adequate respiratory effort.1, 8 Sleep apnea increases the risk of hypertension, coronary artery disease, congestive heart failure, stroke, diabetes, and heart arrhythmias.9 AF increases the risk of stroke, heart failure, and dementia by 5-fold, 3-fold, and 2-fold, respectively,10, 11, 12, 13, 14, 15 as well as increased overall mortality and healthcare costs.16, 17 Sleep apnea has been identified as a risk factor for initiation, maintenance, and recurrence of AF, and its presence reduces the efficacy of current AF therapies, including catheter ablation.2, 8, 18, 19 A study investigating whether continuous positive airway pressure (CPAP) improved success rates of AF catheter ablation found that OSA was an independent predictor of ablation failure and that patients not treated with CPAP were 8 times more likely to fail the procedure.20
Although the mechanistic underpinnings that contribute to the association between sleep apnea and AF remain uncertain, numerous possible mechanisms have been proposed, including dysfunction of the cardiac autonomic nervous system (ANS). Apnea induces hyperactivity of the ANS, which is thought to contribute to the genesis of sleep apnea-induced AF.21, 22, 23, 24, 25 This literature review summarizes the latest evidence on the role of the ANS in sleep apnea-induced AF.
PREVALENCE OF AF AND SLEEP APNEA
The prevalence of AF in the United States ranges from 2.7 million to 6.1 million and is predicted to increase to 12.1 million cases by 2030.26 Approximately 15 to 20 million American adults suffer from OSA. It is estimated that roughly 20% of adults have at least mild OSA, and 7% of adults may have moderate-to-severe OSA. The prevalence of OSA is higher in men, older adults, and obese individuals.27, 28 Central sleep apnea is much less common than OSA; in fact, the overall prevalence in a population-based study on polysomnography was 0.9% in adults aged 40 and older.29 In a more recent cohort of patients undergoing diagnostic polysomnography, OSA prevalence was 53.3% while central sleep apnea was 8.4%. The prevalence of central sleep apnea is higher in men, older adults, people with heart failure, those who chronically use opioids, and those who had a stroke.8
CARDIAC ANS ACTIVITY DURING APNEA AND INCREASED AF SUSCEPTIBILITY
Growing evidence points to the cardiac ANS as a significant contributor to the association between OSA and AF.21, 22, 30 The cardiac ANS (Figure 1 , first panel) comprises the extrinsic cardiac autonomic system, centrally derived parasympathetic and sympathetic nerves, and the intrinsic cardiac autonomic system, which consists of epicardial ganglionated plexi (GP) embedded in fat pads that contain efferent parasympathetic and sympathetic neurons, interneurons, afferent sensory neurons, and others.24, 31, 32 Apnea induces acidosis, hypoxia, hypercarbia, and increased pulmonary pressure, all powerful stimuli for increasing cardiac ANS activity. This causes hemodynamic and electrophysiological changes that culminate in increased AF susceptibility.21, 24, 33
Figure 1.
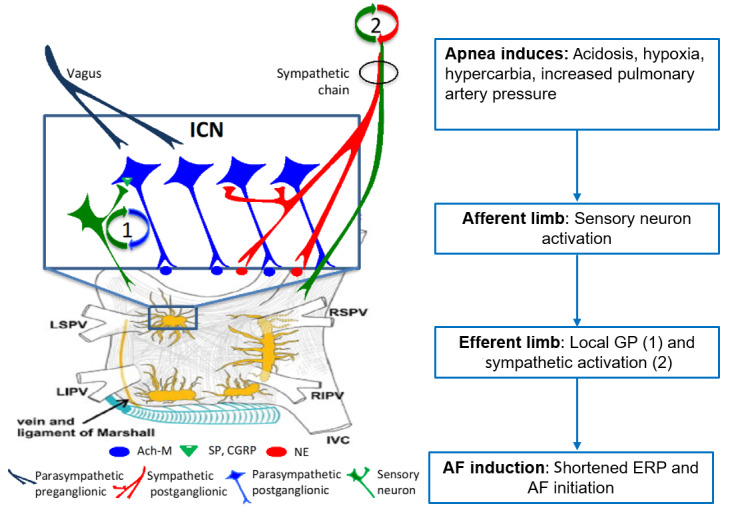
Autonomic response to apnea that contributes to initiation of atrial fibrillation (AF). Proposed integrated autonomic response to apnea: upon apnea, sensory neurons respond to hypoxia and hypercapnia, thus activating a local reflex loop. The loop (1) activates postganglionic parasympathetic neurons that, via muscarinic receptors, lead to decreased ERP. Sympathetic afferents increase sympathetic outflow in a central loop, which leads to (2) increased blood pressure and contributes to AF inducibility. Ach-M: acetylcholine-muscarinic; CGRP: calcitonin gene-related peptide; ERP: effective refractory period; ICN: intrinsic cardiac nervous system; IVC: inferior vena cava; GP: ganglionated plexi; LIPV: left inferior pulmonary vein; LSPV: left superior pulmonary vein; NE: norepinephrine; RIPV: right inferior pulmonary vein; RSPV: right superior pulmonary vein; SP: substance P
In an acute canine model of OSA published in 2019,24 we performed simultaneous nerve recordings from bilateral vagal nerves, left stellate ganglion (SG), and anterior right GP (ARGP) before and during apnea and before and after resiniferatoxin (RTX) injection in the ARGP before and during apnea and before and after resiniferatoxin (RTX) injection in the ARGP. RTX is an ultrapotent analogue of capsaicin—the active compound of chili peppers that causes the transient receptor potential vanilloid 1 (TRPV1), present in sensory neurons, to become permanently permeable to cations34, 35, 36—and causes neuronal death, desensitization, and analgesia.37, 38, 39 The atrial effective refractory period (ERP) and AF inducibility upon single extra stimulation were also assessed before and during apnea and before and after RTX, and we showed that GP sensory neurons play a significant role in apnea-induced AF (Figure 1 , second panel, and Figure 2 ). A consistent sequence of events occurred with oxygen desaturation, including (1) increases in GP activity; (2) progressively increasing phasic bursts of vagal activity, which closely correlate with heart rate (HR) and blood pressure (BP) oscillations; and (3) tonic increase in sympathetic activity, which correlates with steady increases in HR and SBP. When GP sensory neurons were denervated with RTX, decreases in sympathetic and GP nerve activity were seen. In addition, apnea’s electrophysiological response (ERP shortening) was also abolished, and AF inducibility during apnea no longer occurred (Figure 2 ).24 This study expanded on previous experimental studies that demonstrated a close mechanistic association between the cardiac ANS and apnea-induced AF21, 22, 30by recording from both intrinsic and extrinsic cardiac ANS and showing the orchestrated intrinsic (GP) vs extrinsic ANS (vagal and SG) response to apnea and their changes after sensory denervation with RTX. These results support the fundamental role of the cardiac ANS in mediating the atrial myocardium’s electrophysiological responses to apnea and suggest cardiac afferents as a possible therapeutic target for autonomic modulation.24
Figure 2.
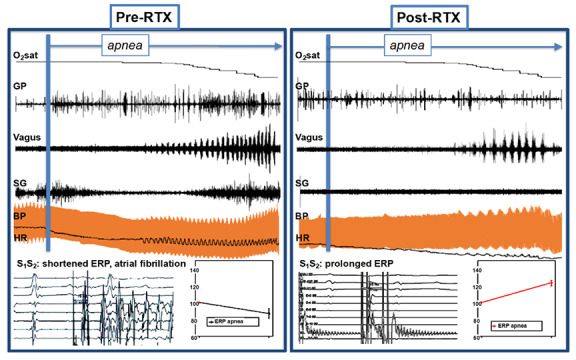
Extrinsic and intrinsic autonomic response to apnea before and after GP sensory denervation. Top: examples of GP, vagal, and SG responses to apnea along with their hemodynamic and electrophysiological correlates. O2 sat: oxygen saturation; RTX: resiniferatoxin; GP: ganglionated plexi; SG: stellate ganglion; BP: blood pressure; HR: heart rate; S1 and S2 : stimulus 1 and 2; ERP: effective refractory period
Further studies are needed to provide a more detailed description of the mechanistic underpinnings that contribute to the impact of the ANS on OSA and AF and to clarify which component of the ANS could more effectively be targeted therapeutically—whether it be the parasympathetic,23 the GP as a whole (with radiofrequency ablation),21 the sympathetic,40 the sensory afferent,24 or a combination thereof. Until such therapies are developed, treatment of sleep apnea remains the main strategy to reduce the incidence of AF in the general population and in patients receiving therapies to lower the recurrence of AF.
CONCLUSION
Sleep apnea is highly associated with AF. The cardiac ANS plays an important role in this association, and studies have shown that different components of this system could potentially be therapeutically targeted to decrease AF inducibility in patients with OSA. Cardiac afferents are a crucial component of the cardiac ANS and have therapeutic potential in reducing AF in those with sleep apnea.
KEY POINTS
Sleep apnea is highly associated with atrial fibrillation (AF) and reduces the efficacy of current AF therapies, including catheter ablation.
The precise mechanisms behind this association are unclear, but studies support a prominent role of the cardiac autonomic nervous system (ANS).
Apnea induces hemodynamic and electrophysiological changes that culminate in increased AF susceptibility.
Cardiac afferents are a crucial component of the cardiac ANS and have therapeutic potential in reducing AF in those with sleep apnea.
References
- Peppard P. E., Young T., Barnet J. H., Palta M., Hagen E. W., Hla K. M. American Journal of Epidemiology. 9. Vol. 177. Oxford University Press (OUP); 2013. Increased Prevalence of Sleep-Disordered Breathing in Adults; pp. 1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung P, Anter E. Atrial Fibrillation And Sleep Apnea: Considerations For A Dual Epidemic. J Atr Fibrillation. 2016;8(6):1283–1283. doi: 10.4022/jafib.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga B., Poyares D., Cintra F., Guilleminault C., Cirenza C., Horbach S., Macedo D., Silva R., Tufik S., De Paola A.A.V. Sleep Medicine. 2. Vol. 10. Elsevier BV; 2009. Sleep-disordered breathing and chronic atrial fibrillation; pp. 212–216. [DOI] [PubMed] [Google Scholar]
- Morin D P, Bernard M L, Madias C, Rogers P A, Thihalolipavan S, Estes N A. The State of the Art: Atrial Fibrillation Epidemiology, Prevention, and Treatment. Mayo Clin Proc. 2016;91(12):1778–1810. doi: 10.1016/j.mayocp.2016.08.022. [DOI] [PubMed] [Google Scholar]
- Mehra Reena, Benjamin Emelia J., Shahar Eyal, Gottlieb Daniel J., Nawabit Rawan, Kirchner H. Lester, Sahadevan Jayakumar, Redline Susan. American Journal of Respiratory and Critical Care Medicine. 8. Vol. 173. American Thoracic Society; 2006. Association of Nocturnal Arrhythmias with Sleep-disordered Breathing; pp. 910–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer Friedrich Felix, Lickfett Lars Martin, Mittmann-Braun Erica, Ruland Charlotte, Kreuz Jens, Pabst Stefan, Schrickel Jan, Juergens Uwe, Tasci Selcuk, Nickenig Georg, Skowasch Dirk. Journal of Interventional Cardiac Electrophysiology. 1. Vol. 29. Springer Science and Business Media LLC; 2010. High prevalence of obstructive sleep apnea in patients with resistant paroxysmal atrial fibrillation after pulmonary vein isolation; pp. 37–41. [DOI] [PubMed] [Google Scholar]
- Somers V K, White D P, Amin R. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. J Am Coll Cardiol. 2008;52(8):686–717. doi: 10.1016/j.jacc.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Harmon E K, Stafford P, Ibrahim S. Atrial fibrillation is associated with central sleep apnea in clinic patients undergoing diagnostic polysomnog- raphy. J Arrhythm. 2020;36(6):991–996. doi: 10.1002/joa3.12427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budhiraja R, Budhiraja P, Quan S F. Sleep-disordered breathing and cardiovascular disorders. Respir Care. 2010;55:1322–1354. [PMC free article] [PubMed] [Google Scholar]
- January C T, Wann L S, Alpert J S. AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Prac- tice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):1–76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- Wolf P. A., Dawber T. R., Thomas H. E., Kannel W. B. Neurology. 10. Vol. 28. Ovid Technologies (Wolters Kluwer Health); 1978. Epidemiologic assessment of chronic atrial fibrillation and risk of stroke: The fiamingham Study; pp. 973–973. [DOI] [PubMed] [Google Scholar]
- Krahn Andrew D., Manfreda Jure, Tate Robert B., Mathewson Francis A.L., Cuddy T. Edward. The American Journal of Medicine. 5. Vol. 98. Elsevier BV; 1995. The natural history of atrial fibrillation: Incidence, risk factors, and prognosis in the manitoba follow-up study; pp. 476–484. [DOI] [PubMed] [Google Scholar]
- Ott Alewijn, Breteler Monique M.B., de Bruyne Martine C., van Harskamp Frans, Grobbee Diederick E., Hofman Albert. Stroke. 2. Vol. 28. Ovid Technologies (Wolters Kluwer Health); 1997. Atrial Fibrillation and Dementia in a Population-Based Study; pp. 316–321. [DOI] [PubMed] [Google Scholar]
- Miyasaka Y, Barnes M E, Petersen R C. Risk of dementia in stroke-free patients diagnosed with atrial fibrillation: data from a community-based cohort. Eur Heart J. 2007;28(16):1962–1969. doi: 10.1093/eurheartj/ehm012. [DOI] [PubMed] [Google Scholar]
- Kwok C. S., Loke Y. K., Hale R., Potter J. F., Myint P. K. Neurology. Vol. 76. Ovid Technologies (Wolters Kluwer Health); 2011. Atrial fibrillation and incidence of dementia: A systematic review and meta-analysis; pp. 914–922. [DOI] [PubMed] [Google Scholar]
- Coyne Karin S., Paramore Clark, Grandy Susan, Mercader Marco, Reynolds Matthew, Zimetbaum Peter. Value in Health. Vol. 9. Elsevier BV; 2006. Assessing the Direct Costs of Treating Nonvalvular Atrial Fibrillation in the United States; pp. 348–356. [DOI] [PubMed] [Google Scholar]
- May Anna M., Van Wagoner David R., Mehra Reena. Chest. Vol. 151. Elsevier BV; 2017. OSA and Cardiac Arrhythmogenesis; pp. 225–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewire J, Calkins H. Impact of Obstructive Sleep Apnea on Outcomes of Catheter Ablation of Atrial Fibrillation. J Atr Fibrillation. 2013;5(5):777–777. doi: 10.4022/jafib.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez Alexandra M., Germany Robin, Lozier Matthew R., Schweitzer Michael D., Kosseifi Semaan, Anand Rishi. IJC Heart & Vasculature. Vol. 30. Elsevier BV; 2020. Central sleep apnea and atrial fibrillation: A review on pathophysiological mechanisms and therapeutic implications; pp. 100527–100527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D, Mohanty P, Biase Di L. Safety and efficacy of pulmonary vein antral isolation in patients with obstructive sleep apnea: the impact of continuous positive airway pressure. Circ Arrhythm Electrophysiol. 2010;3(5):445–51. doi: 10.1161/CIRCEP.109.858381. [DOI] [PubMed] [Google Scholar]
- Ghias Muhammad, Scherlag Benjamin J., Lu Zhibing, Niu Guodong, Moers Annerie, Jackman Warren M., Lazzara Ralph, Po Sunny S. Journal of the American College of Cardiology. 22. Vol. 54. Elsevier BV; 2009. The Role of Ganglionated Plexi in Apnea-Related Atrial Fibrillation; pp. 2075–2083. [DOI] [PubMed] [Google Scholar]
- Gao Mei, Zhang Ling, Scherlag Benjamin J., Huang Bing, Stavrakis Stavros, Hou Yue-Mei, Hou Yinglong, Po Sunny S. Heart Rhythm. Vol. 12. Elsevier BV; 2015. Low-level vagosympathetic trunk stimulation inhibits atrial fibrillation in a rabbit model of obstructive sleep apnea; pp. 818–824. [DOI] [PubMed] [Google Scholar]
- Linz Dominik, Schotten Ulrich, Neuberger Hans-Ruprecht, Böhm Michael, Wirth Klaus. Heart Rhythm. 9. Vol. 8. Elsevier BV; 2011. Negative tracheal pressure during obstructive respiratory events promotes atrial fibrillation by vagal activation; pp. 1436–1443. [DOI] [PubMed] [Google Scholar]
- Tavares L, Rodríguez-Mañero M, Kreidieh B. Cardiac Afferent Dener- vation Abolishes Ganglionated Plexi and Sympathetic Responses to Apnea: Implications for Atrial Fibrillation. Circulation Arrhythmia and electrophys- iology. Circ Arrhythm Electrophysiol. 2019;12(6):6942–6942. doi: 10.1161/CIRCEP.118.006942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung P, Levitzky Y S, Wang R. Obstructive and Central Sleep Apnea and the Risk of Incident Atrial Fibrillation in a Community Cohort of Men and Women. J Am Heart Assoc. 2017;6(7):4500–4500. doi: 10.1161/JAHA.116.004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colilla Susan, Crow Ann, Petkun William, Singer Daniel E., Simon Teresa, Liu Xianchen. The American Journal of Cardiology. 8. Vol. 112. Elsevier BV; 2013. Estimates of Current and Future Incidence and Prevalence of Atrial Fibrillation in the U.S. Adult Population; pp. 1142–1147. [DOI] [PubMed] [Google Scholar]
- Lopez-Jimenez F, Kuniyoshi Sert, Gami F H, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. Chest. 2008;133(3):793–804. doi: 10.1378/chest.07-0800. [DOI] [PubMed] [Google Scholar]
- Riaz S, Bhatti H, Sampat P J, Dhamoon A. The Converging Pathologies of Obstructive Sleep Apnea and Atrial Arrhythmias. Cureus. 2020;12(7):e9388–e9388. doi: 10.7759/cureus.9388.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan Lucas M., Kapur Vishesh K. Sleep. 7. Vol. 39. Oxford University Press (OUP); 2016. Prevalence and Characteristics of Central Compared to Obstructive Sleep Apnea: Analyses from the Sleep Heart Health Study Cohort; pp. 1353–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickramasinghe S. Rasika, Patel Vickas V. Circulation. Vol. 128. Ovid Technologies (Wolters Kluwer Health); 2013. Local Innervation and Atrial Fibrillation; pp. 1566–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardell J. Structure and function of mammalian intrinsic cardiac neurons. In: Armour JAA, editor. Neurocardiology. Oxford University Press; New York: 1994. pp. 95–114. [Google Scholar]
- Wake Emily, Brack Kieran. Autonomic Neuroscience. Vol. 199. Elsevier BV; 2016. Characterization of the intrinsic cardiac nervous system; pp. 3–16. [DOI] [PubMed] [Google Scholar]
- Yu L, Li X, Huang B. Atrial Fibrillation in Acute Obstructive Sleep Apnea: Autonomic Nervous Mechanism and Modulation. J Am Heart Assoc. 2017;6(9):6264–6264. doi: 10.1161/JAHA.117.006264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szallasi A., Blumberg P.M. Neuroscience. 2. Vol. 30. Elsevier BV; 1989. Resiniferatoxin, a phorbol-related diterpene, acts as an ultrapotent analog of capsaicin, the irritant constituent in red pepper; pp. 515–520. [DOI] [PubMed] [Google Scholar]
- Walpole Christopher S. J., Bevan Stuart, Bloomfield Graham, Breckenridge Robin, James Iain F., Ritchie Timothy, Szallasi Arpad, Winter Janet, Wrigglesworth Roger. Journal of Medicinal Chemistry. 15. Vol. 39. American Chemical Society (ACS); 1996. Similarities and Differences in the Structure−Activity Relationships of Capsaicin and Resiniferatoxin Analogues; pp. 2939–2952. [DOI] [PubMed] [Google Scholar]
- Caterina Michael J., Schumacher Mark A., Tominaga Makoto, Rosen Tobias A., Levine Jon D., Julius David. Nature. 6653. Vol. 389. Springer Science and Business Media LLC; 1997. The capsaicin receptor: a heat-activated ion channel in the pain pathway; pp. 816–824. [DOI] [PubMed] [Google Scholar]
- Szallasi Arpad, Blumberg Peter M. Neuroscience Letters. 1. Vol. 140. Elsevier BV; 1992. Vanilloid receptor loss in rat sensory ganglia associated with long term desensitization to resiniferatoxin; pp. 51–54. [DOI] [PubMed] [Google Scholar]
- Olah Zoltan, Szabo Tamas, Karai Laszlo, Hough Chris, Fields R. Douglas, Caudle Robert M., Blumberg Peter M., Iadarola Michael J. Journal of Biological Chemistry. 14. Vol. 276. Elsevier BV; 2001. Ligand-induced Dynamic Membrane Changes and Cell Deletion Conferred by Vanilloid Receptor 1; pp. 11021–11030. [DOI] [PubMed] [Google Scholar]
- Zahner Matthew R., Li De‐Pei, Chen Shao‐Rui, Pan Hui‐Lin. The Journal of Physiology. 2. Vol. 551. Wiley; 2003. Cardiac vanilloid receptor 1‐expressing afferent nerves and their role in the cardiogenic sympathetic reflex in rats; pp. 515–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Tang Y, Meng G, et al. Skin sympathetic nerve activity in patients with obstructive sleep apnea. Heart Rhythm. 2020;17(11):1936–1943. doi: 10.1016/j.hrthm.2020.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]


