Abstract
The development of practical C−H/C−H coupling reactions remains a challenging yet appealing synthetic venture because it circumvents the need to prefunctionalize both coupling partners for the generation of C−C bonds. Herein, we report a cyclative C(sp3)−H/C(sp2)−H coupling reaction of free aliphatic acids enabled by a cyclopentane-based mono-N-protected β-amino acid ligand. This reaction uses inexpensive sodium percarbonate (Na2CO3·1.5H2O2) as the sole oxidant, generating water as the only byproduct. A range of biologically important scaffolds, including tetralins, chromanes, and indanes, could be easily prepared by this protocol. Finally, the synthetic application of this methodology is demonstrated by the concise total synthesis of (±)-russujaponol F in a four-step sequence starting from readily available phenylacetic acid and pivalic acid through the sequential functionalizations of four C−H bonds.
Graphical abstract
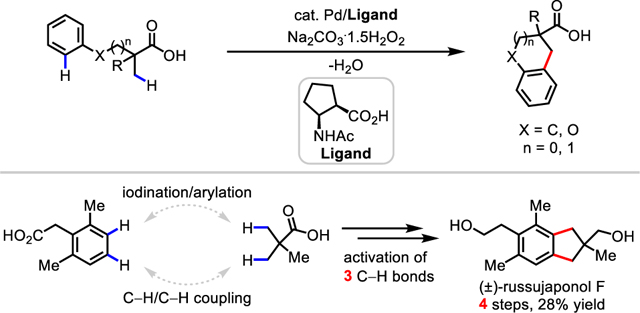
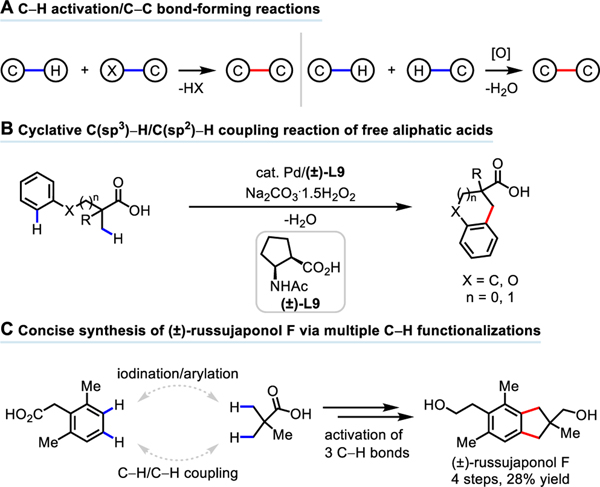
Carbon-carbon (C−C) bond formation constitutes one of the most important classes of reactions in organic synthesis. Owing to its potential to shorten synthesis, the past two decades have witnessed rapid developments in using C−H activation strategies for the construction of C−C bonds.1 While most coupling methods require prefunctionalized coupling partners (e.g. organoborons and organohalides), C−H/C−H coupling reactions offer a complementary strategy to construct a C−C bond directly from two simple C−H bonds (Scheme 1A).2 Compared to traditional coupling methods, this green and atom-economical approach is highly attractive because water is potentially the sole stoichiometric byproduct of this process (Scheme 1A). To date, extensive studies have focused on the coupling of two relatively reactive C(sp2)−H bonds for biaryl synthesis,3 whereas only a few reactions have been reported for the construction of more challenging C(sp3)−C(sp2) bonds. Because these existing reaction protocols require exogenous directing groups (DGs) to promote cyclometallation, additional steps to install and remove the DG are necessary.5,6 Additionally, reported methods pose practical limitations, such as the stoichiometric use of precious silver salts4b,c,5,6b,c and harsh conditions4b,c,5a,b,6 — with temperatures as high as 160 °C being reported. Moreover, current methods for C(sp3)−H/C(sp2)−H coupling initiated by C(sp3)−H activation are largely limited to more reactive heterocyclic C(sp2)−H bonds.5a,b,6 Despite the great value that C−H/C−H coupling reactions might have for organic synthesis, the development of C(sp3)−H/C(sp2)−H coupling reactions that use both a practical oxidant and native substrates remains a significant challenge.
Scheme 1.
C−H Activation/C−C Bond-Forming Reactions
Recent advances in C−H functionalization have provided chemists with creative and strategic retrosynthetic disconnections that are otherwise difficult to achieve using traditional methods.7 However, for C−H functionalization strategies to truly improve the overall efficiency of synthesis, three criteria should be met: (1) the ability to use a wide range of simple starting materials to enable the synthesis of diverse natural product families; (2) the use of native functionalities as the DG; (3) the site-selectivity of C−H functionalization reactions should be precisely controllable. Given the ubiquitous nature of C−H bonds in organic molecules, synthetic sequences that incorporate multiple C−H functionalizations are particularly attractive for the efficient synthesis of natural products. However, approaches that meet these aforementioned criteria are challenging to execute and so uncommon in literature.7a,8
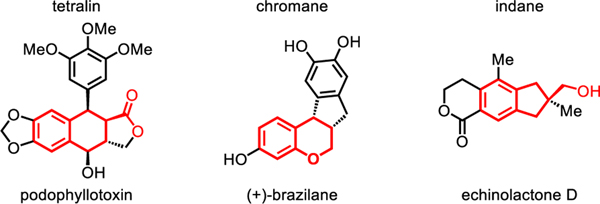
To address these challenges, we herein report a cyclative C(sp3)−H/C(sp2)−H coupling reaction using a native free carboxylic acid as the DG (Scheme 1B). The use of a cyclopentane-based mono-N-protected β-amino acid ligand and a practical and inexpensive oxidant sodium percarbonate (Na2CO3·1.5H2O2) proved crucial to the success of this reaction. Tetralins, chromanes, or indanes, common frameworks in natural products could be readily prepared by this protocol (Figure 1). The synthetic application of this methodology is further demonstrated by a concise total synthesis of (±)-russujaponol F (the shortest and highest yielding to date) via multiple C−H functionalizations in four steps from readily available phenylacetic acid and pivalic acid (Scheme 1C), demonstrating the potential of C−H activation disconnections to enhance the ideality of synthesis9.
Figure 1.
Biologically significant natural products containing tetralin, chromane, or indane frameworks.
Aliphatic carboxylic acids are ubiquitous and synthetically versatile motifs and are often inexpensive reagents in organic chemistry; as such, they are privileged substrates for C−H activation reactions.10 Following our recent disclosure of the β-C(sp3)−H lactonization10i and acyloxylation10j of free carboxylic acids using tert-butyl hydrogen peroxide (TBHP) as the sole oxidant, we initiated our investigation of cyclative C(sp3)−H/C(sp2)−H coupling reactions by selecting TBHP as the bystanding oxidant and aliphatic acid 1a as a model substrate. Under the optimal conditions of the aforementioned β-acyloxylation reaction10j, we were delighted to observe a 50% 1H NMR yield of the desired product 2a without forming competing reductive elimination products, such as the β-lactone or β-hydroxy acid (see Supporting Information, Table S1). Further investigation of the bystanding oxidants and bases revealed that the combination of Na2CO3·1.5H2O2 and LiOAc could further improve the yield to 57% (see Supporting Information, Table S1−S2). The yields using LiOAc are generally better than those using NaOAc as the metal additives under the optimized conditions (see Supporting Information, Table S4). The use of sodium percarbonate, one of the cheapest and most easily handled oxidants,11 potentially renders this protocol practical and scalable. In light of recent advances in ligand-accelerated Pd(II)-catalyzed C−H activation,12 we next searched for ligands that could substantially improve the reactivity of the catalyst. Guided by mono-N-protected amino acid (MPAA) ligand-enabled C(sp3)−H activation reactions of free carboxylic acids10c,d,g,i,j, we tested a series of commercially available MPAA ligands (L1−L4): β-amino acid ligand L4 showed superior reactivity over α-amino acid ligands L1−L3 (57% vs. 19−45%), as was also observed in other C(sp3)−H functionalization reactions of free acids via Pd(II)/Pd(IV) catalytic cycles10d,i,j. Through systematic modifications to the backbone of the β-amino acid ligand (L5−L10), we found that cis-cyclopentane-based ligand (±)-L9 gave the optimal reactivity (78% isolated yield). The superior reactivity of (±)-L9 might be attributed to the more rigid conformation enforced by the cyclopentane linkage. Control experiments showed that the yields were low in the absence of the ligand or in the presence of the γ-amino acid ligand (L11) (23% or 20%, respectively), indicating the importance of six-membered chelation by the ligand for reactivity.
With the optimal ligand and reaction conditions in hand, we evaluated the scope of the cyclative C(sp3)−H/C(sp2)−H coupling reaction (Table 2). A wide range of tertiary aliphatic acids bearing a single α-methyl group (1a−1e and 1h) or an α-gem-dimethyl group (1f and 1g) were all compatible, affording the tetralin products in moderate to good yields (52−78%). The reaction could also be conducted on a 2.0 mmol scale, delivering 2a in 69% yield. The attempted desymmetrization of the α-gem-dimethyl group of 1f using enantioenriched L9 resulted in racemic product. Less reactive free carboxylic acids containing α-hydrogens (1i−1l) also reacted in synthetically useful yields (35−65%). Among these, a variety of functionalities on the aryl rings such as methyl (2b), methoxy (2j and 2k), fluoro (2c, 2g, and 2l), and chloro (2d) as well as naphthyl (2e) were tolerated, with the halogen moiety (2d) serving as a useful synthetic handle for subsequent derivatization. This protocol could also be successfully extended to the synthesis of biologically important chromane products. β-Phenoxy carboxylic acids containing an α-gem-dimethyl group (1m−1r) or α-hydrogens (1s, from Roche ester) were all reactive substrates. While a range of electron-donating (methoxy, tert-butyl, cyclohexyl, and benzyl) (2s and 2n−2p) groups on the aryl ring were well tolerated to afford the desired products in good yields (70−85%), aliphatic acids containing electron-withdrawing (bromo and trifluoromethyl) groups (2q and 2r) showed comparatively low reactivity (31% and 23%), likely due to the sluggish nature of C(sp2)−H activations of electron-deficient arenes. Although intermolecular KIE experiments of electron-rich 1m and 1m-d5 (kH/kD = 1.1) suggest that C(sp2)−H activation is not the rate-determining step (see Supporting Information, KIE experiments section), the possibility of C(sp2)−H activation with electron-deficient substrates as the rate-determining step cannot be ruled out. It is noteworthy that high regioselectivity was observed for the aliphatic acids containing meta-methoxy groups (1j and 1s), while the substrate bearing a tert-butyl group (1n) afforded a mixture of regioisomers (2n/2n’ = 3/1). Considering the previously observed high para-selectivity of Pd(IV)-mediated C−H coupling reactions of anisoles3g,5c,22, it is likely that the alkyl-Pd(II) intermediate is oxidized to Pd(IV) prior to C−H activation. Under the present conditions, the carboxylic acid 1t containing either NBoc or NTs groups failed to deliver tetrahydroisoquinoline (THIQ) product 2t. This cyclative C−H/C−H coupling reaction was also amenable to the syntheses of indane scaffolds (2u−2w). Notably, an [F+] oxidant3g,13 (1-fluoro-2,4,6-trimethylpyridinium tetrafluoroborate) showed superior reactivity for tertiary aliphatic acids containing an α-gem-dimethyl group (2v and 2w) (see Supporting Information, Table S5).
Table 2.
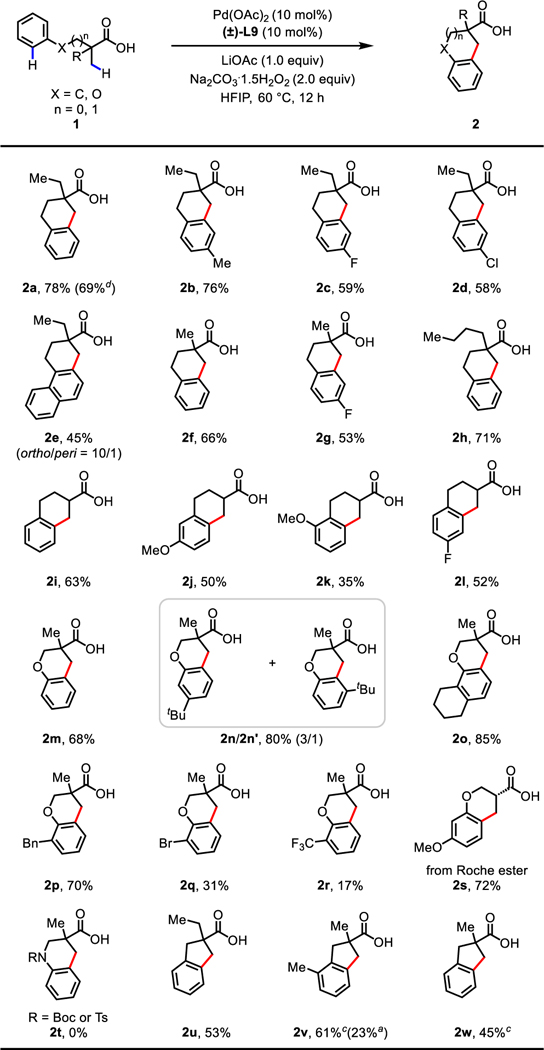 |
Conditions A: 1 (0.1 mmol), Pd(OAc)2 (10 mol%), (±)-L9 (10 mol%), LiOAc (1.0 equiv), Na2CO3·1.5H2O2 (2.0 equiv), HFIP (1.0 mL), 60 °C, 12 h.
Isolated yields.
Conditions B: 1 (0.1 mmol), Pd(CH3CN)4(BF4)2 (10 mol%), Ag2CO3 (1.0 equiv), 1-fluoro-2,4,6-trimethylpyridinium tetrafluoroborate (2.0 equiv), HFIP (1.0 mL), 90 °C, 12 h. dOn 2.0 mmol scale.
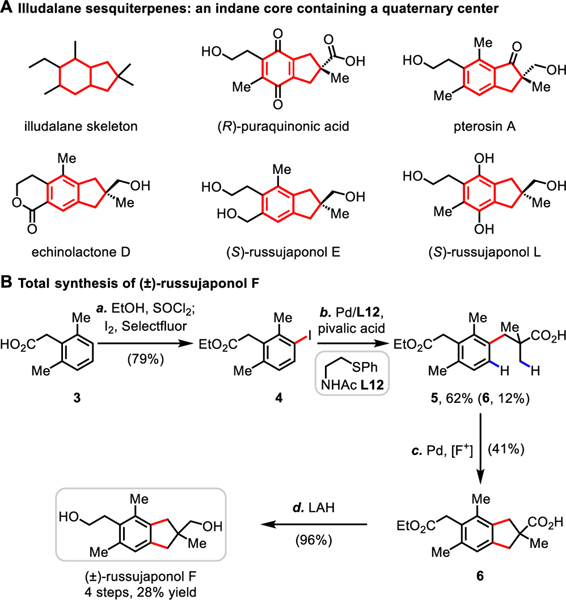
Illudalane sesquiterpenes comprise a large family of natural products, which typically feature an indane core (for which various oxidation states are possible) bearing a challenging all-carbon quaternary center (Scheme 2A).14 Owing to their promising biological activities, tremendous efforts have been devoted to the total syntheses of these targets.15,16 Given the power of this methodology for the construction of indane scaffolds, we embarked on the total synthesis of (±)-russujaponol F via multiple C−H functionalizations (Scheme 2B). Baudoin’s group reported the first total synthesis of russujaponol F in racemic and enantioselective forms based on a C(sp3)−H arylation strategy in 13 steps (26% yield) and 15 steps (12% yield) respectively.15 Beginning with phenylacetic acid 3 that is commercially available or synthesized through ortho-C−H methylation17, we were able to prepare aryl iodide 4 by esterification and subsequent mono-iodination18 of 3 using I2 and Selectfluor in 79% yield. Investigation of the C−H arylation of pivalic acid indicated that, with ligand L1210f,19, the mono-arylated product 5 could be obtained in 62% yield, along with 12% of the cyclative C−H/C−H coupling product 6 (see Supporting Information, Table S6). The formation of 6 under these conditions might be attributed to a second arylation of 5 with additional aryl iodide serving as the bystanding oxidant.20 The cyclative C−H/C−H coupling was then performed under the standard conditions using an [F+] oxidant to give the desired product 6 in 41% yield. Finally, global reduction of 6 using LAH cleanly delivered (±)-russujaponol F in 96% yield, completing the total synthesis in four steps and 28% overall yield: the shortest and highest yielding total synthesis of russujaponol F to date.
Scheme 2.
Total Synthesis of (±)-Russujaponol Fa
aConditions: (a) SOCl2, EtOH, reflux, overnight; I2 (0.5 equiv), Selectfluor (0.5 equiv), CH3CN, 60 °C, 3 h. (b) Pd(OAc)2 (10 mol%), L12 (10 mol%), pivalic acid (3.0 equiv), CsOAc (1.0 equiv), Ag2CO3 (2.0 equiv), HFIP, 80 °C, 12 h. (c) Pd(CH3CN)4(BF4)2 (10 mol%), Ag2CO3 (1.0 equiv), 1-fluoro-2,4,6-trimethylpyridinium tetrafluoroborate (2.0 equiv), HFIP, 90 °C, 12 h. (d) LAH (3.0 equiv), THF, 0 °C to rt, overnight.
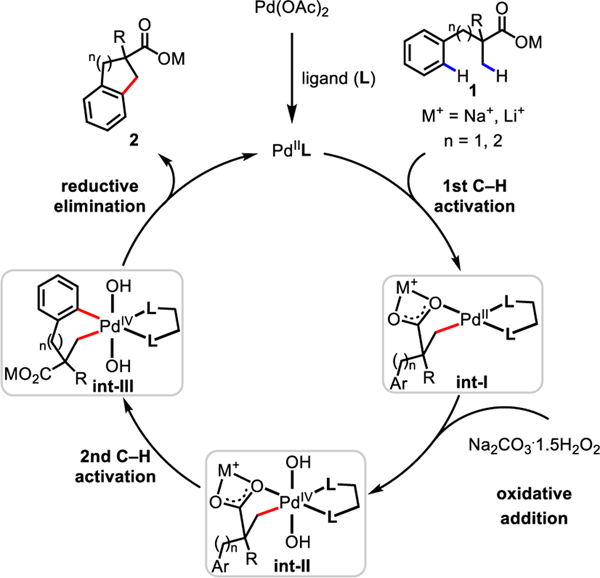
On the basis of literature precedents3−5 and our recent work on the C−H activation of free acids10i,j, we propose that this cyclative C(sp3)−H/C(sp2)−H coupling reaction proceeds via a Pd(II)/Pd(IV) catalytic cycle as outlined in Scheme 3. First, coordination of Pd(OAc)2 to an MPAA ligand generates the active LPd(II) species. After coordination of the acid substrate 1 to Pd, both the countercation Na+ or Li+ and the MPAA ligand accelerate the cyclopalladation of the β-C(sp3)−H bond to form int-I. Next, oxidative addition of the hydrogen peroxide occurs to produce int-II, a process established in previous studies on the oxidation of Pd(II) to Pd(IV) by benzoyl peroxide21a, tert-butyl peroxyacetate21b, or hydrogen peroxide21c,d. In the previously reported β-lactonization10i or acetoxylation10j reactions, selective reductive elimination yields β-lactone or β-acetoxylated carboxylic acid. In this case, a reactive phenyl group on the side chain of the substrate undergoes a second C(sp2)−H activation of int-II to deliver int-III via a seven or six-membered palladacycle, enabled by the facile dissociation of the weakly coordinating free acid.22 However, it is also possible that the Pd(II) intermediate int-I performs the second C−H activation prior to the oxidative addition of hydrogen peroxide that generates int-III. Finally, reductive elimination of int-III generates the cyclative C−H/C−H coupling product 2 and regenerates the LPd(II) species.
Scheme 3.
Proposed Mechanism for Cyclative C(sp3)−H/C(sp2)−H Coupling Reaction
In summary, we have realized a Pd(II)-catalyzed cyclative C(sp3)−H/C(sp2)−H coupling reaction enabled by a cyclopentane-based mono-N-protected β-amino acid ligand. The use of inexpensive sodium percarbonate as the sole oxidant and native free carboxylic acids as the directing group renders this reaction highly practical and potentially amenable to large-scale manufacturing. A range of biologically significant scaffolds, including tetralins, chromanes, and indanes, could be readily prepared by this protocol. The synthetic application of this methodology was demonstrated by a concise total synthesis of (±)-russujaponol F via multiple C−H functionalizations in four steps from readily available phenylacetic acid and pivalic acid.
Supplementary Material
Table 1.
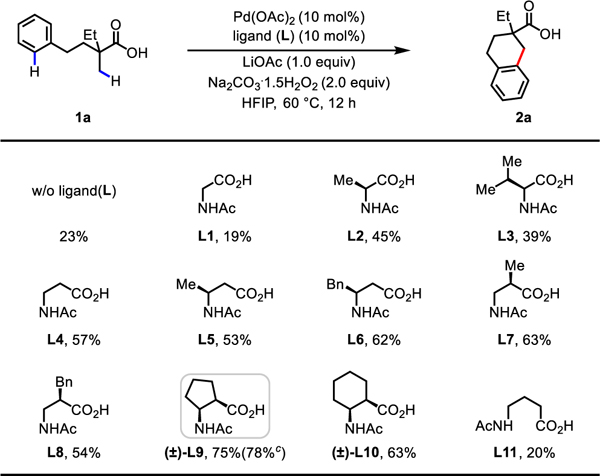
|
Conditions: 1a (0.1 mmol), Pd(OAc)2 (10 mol%), ligand (L) (10 mol%), LiOAc (1.0 equiv), Na2CO3·1.5H2O2 (2.0 equiv), HFIP (1.0 mL), 60 °C, 12 h.
The yields were determined by 1H NMR analysis of the crude product using CH2Br2 as the internal standard.
Isolated yield.
ACKNOWLEDGMENT
We gratefully acknowledge the NIH (NIGMS, R01GM084019), the NSF under the CCI Center for Selective C−H Functionalization (CHE-1700982), and The Scripps Research Institute for financial support. Z.Z. thanks Dr. Nelson Y. S. Lam for proofreading.
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website.
Full experimental details and characterization of new compounds (PDF)
The authors declare no competing financial interests.
REFERENCES
- (1).For reviews on C−H activation/C−C bond-forming reactions, see: [Google Scholar]; (a) Chen X.; Engle KM; Wang D-H; Yu J-Q Palladium(II)-catalyzed C−H activation/C−C cross-coupling reactions: versatility and practicality. Angew. Chem., Int. Ed. 2009, 48, 5094–5115. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Daugulis O.; Roane J.; Tran LD Bidentate, monoanionic auxiliary-directed functionalization of carbon−hydrogen bonds. Acc. Chem. Res. 2015, 48, 1053–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) He G.; Wang B.; Nack WA; Chen G. Syntheses and transformations of α-amino acids via palladium-catalyzed auxiliary directed sp3 C−H Functionalization. Acc. Chem. Res. 2016, 49, 635–645. [DOI] [PubMed] [Google Scholar]
- (2).For reviews on C−H/C−H coupling reactions, see: [Google Scholar]; (a) Yeung CS; Dong VM Catalytic dehydrogenative cross-coupling: forming carbon-carbon bonds by oxidizing two carbon-hydrogen bonds. Chem. Rev. 2011, 111, 1215–1292. [DOI] [PubMed] [Google Scholar]; (b) Girard SA; Knauber T.; Li C-J The cross-dehydrogenative coupling of C(sp3)−H bonds: a versatile strategy for C−C bond formations. Angew. Chem., Int. Ed. 2014, 53, 74–100. [DOI] [PubMed] [Google Scholar]; (c) Liu C.; Yuan J.; Gao M.; Tang S.; Li W.; Shi R.; Lei A. Oxidative coupling between two hydrocarbons: an update of recent C−H functionalizations. Chem. Rev. 2015, 115, 12138–12204. [DOI] [PubMed] [Google Scholar]
- (3).For early examples of C(sp2)−H/C(sp2)−H coupling reaction, see: [Google Scholar]; (a) Stuart DR; Fagnou K. The catalytic cross-coupling of unactivated arenes. Science 2007, 316, 1172–1175. [DOI] [PubMed] [Google Scholar]; (b) Xia J-B; You S-L Carbon−carbon bond formation through double sp2 C−H activations: synthesis of ferrocenyl oxazoline derivatives. Organometallics 2007, 26, 4869–4871. [Google Scholar]; (c) Hull KL; Sanford MS Catalytic and highly regioselective cross-coupling of aromatic C−H substrates. J. Am. Chem. Soc. 2007, 129, 11904–11905. [DOI] [PubMed] [Google Scholar]; (d) Brasche G.; García-Fortanet J.; Buchwald SL Twofold C−H functionalization: palladium-catalyzed ortho arylation of anilides. Org. Lett. 2008, 10, 2207–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Cho SH; Hwang SJ; Chang S. Palladium-catalyzed C−H functionalization of pyridine N-oxides: highly selective alkenylation and direct arylation with unactivated arenes. J. Am. Chem. Soc. 2008, 130, 9254–9256. [DOI] [PubMed] [Google Scholar]; (f) Zhao X.; Yeung CS; Dong VM Palladium-catalyzed ortho-arylation of O-phenylcarbamates with simple arenes and sodium persulfate. J. Am. Chem. Soc. 2010, 132, 5837–5844. [DOI] [PubMed] [Google Scholar]; (g) Wang X.; Leow D.; Yu J-Q Pd(II)-catalyzed para-selective C−H arylation of monosubstituted arenes. J. Am. Chem. Soc. 2011, 133, 13864–13867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).For Pd-catalyzed C(sp3)−H/C(sp2)−H coupling reactions initiated by C(sp2)−H activation, see: [Google Scholar]; (a) Liégault B.; Fagnou K. Palladium-catalyzed intramolecular coupling of arenes and unactivated alkanes in air. Organometallics 2008, 27, 4841–4843. [Google Scholar]; (b) Pierre C.; Baudoin O. Intramolecular PdII-catalyzed dehydrogenative C(sp3)−C(sp2) coupling: an alternative to Pd0-catalyzed C(sp3)−H arylation from aryl halides? Tetrahedron 2013, 69, 4473–4478. [Google Scholar]; (c) Shi J-L; Wang D.; Zhang X-S; Li X-L; Chen Y-Q; Li Y-X; Shi Z-J Oxidative coupling of sp2 and sp3 carbon−hydrogen bonds to construct dihydrobenzofurans. Nat. Commun. 2017, 8, 238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).For Pd-catalyzed C(sp3)−H/C(sp2)−H coupling reactions initiated by C(sp3)−H activation, see: [Google Scholar]; (a) Jiang Y.; Deng G.; Zhang S.; Loh T-P Directing group participated benzylic C(sp3)−H/C(sp2)−H cross-dehydrogenative coupling (CDC): synthesis of azapolycycles. Org. Lett. 2018, 20, 652–655. [DOI] [PubMed] [Google Scholar]; (b) Sun W-W; Liu J-K; Wu B. Practical synthesis of polysubstituted unsymmetric 1,10-phenanthrolines by palladium catalyzed intramolecular oxidative cross coupling of C(sp2)−H and C(sp3)−H bonds of carboxamides. Org. Chem. Front. 2019, 6, 544–550. [Google Scholar]; (c) Hao H-Y; Mao Y-J; Xu Z-Y; Lou S-J; Xu D-Q Selective cross-dehydrogenative C(sp3)−H arylation with arenes. Org. Lett. 2020, 22, 2396–2402. [DOI] [PubMed] [Google Scholar]
- (6).For other metal-enabled C(sp3)−H/C(sp2)−H coupling reactions, see: [Google Scholar]; (a) Wu X.; Zhao Y.; Ge H. Pyridine-enabled copper-promoted cross dehydrogenative coupling of C(sp2)−H and unactivated C(sp3)−H bonds. Chem. Sci. 2015, 6, 5978–5983. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Tan G.; You J. Rhodium(III)-catalyzed oxidative cross-coupling of unreactive C(sp3)−H bonds with C(sp2)−H bonds. Org. Lett. 2017, 19, 4782–4785. [DOI] [PubMed] [Google Scholar]; (c) Wang X.; Xie P.; Qiu R.; Zhu L.; Liu T.; Li Y.; Iwasaki T.; Au C-T; Xu X.; Xia Y.; Yin S-F; Kambe N. Nickel-catalysed direct alkylation of thiophenes via double C(sp3)−H/C(sp2)−H bond cleavage: the importance of KH2PO4. Chem. Commun. 2017, 53, 8316–8319. [DOI] [PubMed] [Google Scholar]; (d) Tan G.; Zhang L.; Liao X.; Shi Y.; Wu Y.; Yang Y.; You J. Copper- or nickel-enabled oxidative cross-coupling of unreactive C(sp3)−H bonds with azole C(sp2)−H bonds: rapid access to β-azolyl propanoic acid derivatives. Org. Lett. 2017, 19, 4830–4833. [DOI] [PubMed] [Google Scholar]
- (7).For reviews on C−H functionalization for natural product synthesis, see: [Google Scholar]; (a) Baudoin O. Multiple catalytic C−H bond functionalization for natural product synthesis. Angew. Chem., Int. Ed. 2020, 59, 17798–17809. [DOI] [PubMed] [Google Scholar]; (b) Lam NYS; Wu K.; Yu J-Q Advancing the logic of chemical synthesis: C−H activation as strategic and tactical disconnections for C−C bond construction. Angew. Chem., Int. Ed. 2020, 59, DOI: 10.1002/anie.202011901. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Gutekunst WR; Baran PS C−H functionalization logic in total synthesis. Chem. Soc. Rev. 2011, 40, 1976–1991. [DOI] [PubMed] [Google Scholar]; (d) Abrams DJ; Provencher PA; Sorensen EJ Recent applications of C−H functionalization in complex natural product synthesis. Chem. Soc. Rev. 2018, 47, 8925–8967. [DOI] [PubMed] [Google Scholar]
- (8).For selected examples of total synthesis using multiple C−H functionalizations, see: [Google Scholar]; (a) Wang D-H; Yu J-Q Highly convergent total synthesis of (+)-lithospermic acid via a late-stage intermolecular C−H olefination. J. Am. Chem. Soc. 2011, 133, 5767–5769. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Gutekunst WR; Baran PS Total synthesis and structural revision of the piperarborenines via sequential cyclobutane C−H arylation. J. Am. Chem. Soc. 2011, 133, 19076–19079. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Rosen BR; Simke LR; Thuy-Boun PS; Dixon DD; Yu J-Q; Baran PS C−H functionalization logic enables synthesis of (+)-hongoquercin A and related compounds. Angew. Chem., Int. Ed. 2013, 52, 7317–7320. [DOI] [PubMed] [Google Scholar]; (d) Hong B.; Li C.; Wang Z.; Chen J.; Li H.; Lei X. Enantioselective total synthesis of (−)-incarviatone A. J. Am. Chem. Soc. 2015, 137, 11946–11949. [DOI] [PubMed] [Google Scholar]; (e) Dailler D.; Danoun G.; Ourri B.; Baudoin O. Divergent synthesis of aeruginosins based on a C(sp3)−H activation strategy. Chem. Eur. J. 2015, 21, 9370–9379. [DOI] [PubMed] [Google Scholar]; (f) Wu F.; Zhang J.; Song F.; Wang S.; Guo H.; Wei Q.; Dai H.; Chen X.; Xia X.; Liu X.; Zhang L.; Yu J-Q; Lei X. Chrysomycin A derivatives for the treatment of multi-drug-resistant tuberculosis. ACS Cent. Sci. 2020, 6, 928–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Gaich T.; Baran PS Aiming for the ideal synthesis. J. Org. Chem. 2010, 75, 4657–4673. [DOI] [PubMed] [Google Scholar]
- (10).(a) Giri R.; Maugel N.; Li J-J; Wang D-H; Breazzano SP; Saunder LB; Yu J-Q Palladium-catalyzed methylation and arylation of sp2 and sp3 C−H bonds in simple carboxylic acids. J. Am. Chem. Soc. 2007, 129, 3510–3511. [DOI] [PubMed] [Google Scholar]; (b) Chen G.; Zhuang Z.; Li G-C; Saint-Denis TG; Hsiao Y.; Joe CL; Yu J-Q Ligand-enabled β-C−H arylation of α-amino acids without installing exogenous directing groups. Angew. Chem., Int. Ed. 2017, 56, 1506–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Zhu Y.; Chen X.; Yuan C.; Li G.; Zhang J.; Zhao Y. Pd-catalysed ligand-enabled carboxylate-directed highly regioselective arylation of aliphatic acids. Nat. Commun. 2017, 8, 14904. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Ghosh KK; van Gemmeren M. Pd-catalyzed β-C(sp3)−H arylation of propionic acid and related aliphatic acids. Chem. Eur. J. 2017, 23, 17697–17700. [DOI] [PubMed] [Google Scholar]; (e) Shen P-X; Hu L.; Shao Q.; Hong K.; Yu J-Q Pd(II)-catalyzed enantioselective C(sp3)−H arylation of free carboxylic acids. J. Am. Chem. Soc. 2018, 140, 6545–6549. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Zhuang Z.; Yu C-B; Chen G.; Wu Q-F; Hsiao Y.; Joe CL; Qiao JX; Poss MA; Yu J-Q Ligand-enabled β-C(sp3)−H olefination of free carboxylic acids. J. Am. Chem. Soc. 2018, 140, 10363–10367. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Hu L.; Shen P-X; Shao Q.; Hong K.; Qiao JX; Yu J-Q PdII-catalyzed enantioselective C(sp3)−H activation/cross-coupling reactions of free carboxylic acids. Angew. Chem., Int. Ed. 2019, 58, 2134–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Ghosh KK; Uttry A.; Koldemir A.; Ong M.; van Gemmeren M. Direct β-C(sp3)−H acetoxylation of aliphatic carboxylic acids. Org. Lett. 2019, 21, 7154–7157. [DOI] [PubMed] [Google Scholar]; (i) Zhuang Z.; Yu J-Q Lactonization as a general route to β-C(sp3)−H functionalization. Nature 2020, 577, 656–659. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Zhuang Z.; Herron AN; Fan Z.; Yu J-Q Ligand-enabled monoselective β-C(sp3)−H acyloxylation of free carboxylic acids using a practical oxidant. J. Am. Chem. Soc. 2020, 142, 6769–6776. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Ghiringhelli F.; Uttry A.; Ghosh KK; van Gemmeren M. Direct β- and γ-C(sp3)−H alkynylation of free carboxylic acids. Angew. Chem., Int. Ed. 2020, 59, 23127–23131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).(a) McKillop A.; Sanderson WR Sodium perborate and sodium percarbonate: Cheap, safe and versatile oxidising agents for organic synthesis. Tetrahedron Lett. 1995, 51, 6145. [Google Scholar]; (b) Muzart J. Sodium perborate and sodium percarbonate in organic synthesis. Synthesis 1995, 1325. [Google Scholar]
- (12).For reviews, see: [Google Scholar]; (a) He J.; Wasa M.; Chan KSL; Shao Q.; Yu J-Q Palladium-catalyzed transformations of alkyl C−H bonds. Chem. Rev. 2017, 117, 8754–8786. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Shao Q.; Wu K.; Zhuang Z.; Qian S.; Yu J-Q From Pd(OAc)2 to chiral catalysts: the discovery and development of bifunctional mono-N-protected amino acid ligands for diverse C−H activation reactions. Acc. Chem. Res. 2020, 53, 833–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Engle KM; Mei T-S; Wang X.; Yu J-Q Bystanding F+ oxidants enable selective reductive elimination from high-valent metal centers in catalysis. Angew. Chem., Int. Ed. 2011, 50, 1478–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).(a) Yoshikawa K.; Kaneko A.; Matsumoto Y.; Hama H.; Arihara S. Russujaponols A−F, illudoid sesquiterpenes from the fruiting body of Russula japonica. J. Nat. Prod. 2006, 69, 1267–1270. [DOI] [PubMed] [Google Scholar]; (b) Yoshikawa K.; Matsumoto Y.; Hama H.; Tanaka M.; Zhai H.; Fukuyama Y.; Arihara S.; Hashimoto T. Russujaponols G−L, illudoid sesquiterpenes, and their neurite outgrowth promoting activity from the fruit body of Russula japonica. Chem. Pharm. Bull. 2009, 57, 311–314. [DOI] [PubMed] [Google Scholar]; (c) Becker U.; Erkel G.; Anke T.; Sterner O. Puraquinonic acid, a novel inducer of differentiation of human HL-60 promyelocytic leukemia cells from Mycena pura (Pers. Ex Fr.). Nat. Prod. Lett. 1997, 9, 229–236. [Google Scholar]; (d) Kuroyanagi M.; Fukuoka M.; Yoshihira K.; Natori S. The absolute configurations of pterosins, 1-indanone derivatives from bracken, Pteridium aquilinum var. latiusculum. Chem. Pharm. Bull. 1974, 22, 723–726. [Google Scholar]; (e) Suzuki S.; Murayama T.; Shiono Y. Echinolactones C and D: two illudalane sesquiterpenoids isolated from the cultured mycelia of the fungus Echinodontium japonicum. Z. Naturforsch., B 2006, 61, 1295–1298. [Google Scholar]
- (15).(a) Melot R.; Craveiro M.; Bürgi T.; Baudoin O. Divergent enantioselective synthesis of (nor)illudalane sesquiterpenes via Pd0-catalyzed asymmetric C(sp3)−H activation. Org. Lett. 2019, 21, 812–815. [DOI] [PubMed] [Google Scholar]; (b) Melot R.; Craveiro MV; Baudoin O. Total synthesis of (nor)illudalane sesquiterpenes based on a C(sp3)−H activation strategy. J. Org. Chem. 2019, 84, 12933–12945. [DOI] [PubMed] [Google Scholar]
- (16).For recent examples, see: [Google Scholar]; (a) Tiong EA; Rivalti D.; Williams BM; Gleason JL A concise total synthesis of (R)-puraquinonic acid. Angew. Chem., Int. Ed. 2013, 52, 3442–3445. [DOI] [PubMed] [Google Scholar]; (b) Elmehriki AAH; Gleason JL A spiroalkylation method for the stereoselective construction of α-quaternary carbons and its application to the total synthesis of (R)-puraquinonic acid. Org. Lett. 2019, 21, 9729–9733. [DOI] [PubMed] [Google Scholar]; (c) Zeng Z.; Zhao Y.; Zhang Y. Divergent total syntheses of five illudalane sesquiterpenes and assignment of the absolute configuration. Chem. Commun. 2019, 55, 4250–4253. [DOI] [PubMed] [Google Scholar]; (d) Xun MM; Bai Y.; Wang Y.; Hu Z.; Fu K.; Ma W.; Yuan C. Synthesis of four illudalane sesquiterpenes utilizing a one-pot Diels−Alder/oxidative aromatization sequence. Org. Lett. 2019, 21, 6879–6883. [DOI] [PubMed] [Google Scholar]
- (17).Thuy-Boun PS; Villa G.; Dang D.; Richardson P.; Su S.; Yu J-Q Ligand-accelerated ortho-C−H alkylation of arylcarboxylic acids using alkyl boron reagents. J. Am. Chem. Soc. 2013, 135, 17508–17513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Stavber S.; Kralj P.; Zupan M. Selective and effective iodination of alkyl-substituted benzenes with elemental iodine activated by SelectfluorTM F-TEDA-BF4. Synlett 2002, 598–600. [Google Scholar]
- (19).For examples of C−H activation reactions using L12, see: [Google Scholar]; (a) Le KKA; Nguyen H.; Daugulis O. 1-Aminopyridinium ylides as monodentate directing groups for sp3 C−H bond functionalization. J. Am. Chem. Soc. 2019, 141, 14728–14735. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zhuang Z.; Yu J-Q Pd(II)-catalyzed enantioselective γ-C(sp3)−H functionalizations of free cyclopropylmethylamines. J. Am. Chem. Soc. 2020, 142, 12015–12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).(a) Sun W-W; Cao P.; Mei R-Q; Li Y.; Ma Y-L; Wu B. Palladium-catalyzed unactivated C(sp3)−H bond activation and intramolecular amination of carboxamides: a new approach to β‑lactams. Org. Lett. 2014, 16, 480–483. [DOI] [PubMed] [Google Scholar]; (b) Zhang S-J; Sun W-W; Cao P.; Dong X-P; Liu J-K; Wu B. Stereoselective synthesis of diazabicyclic β‑lactams through intramolecular amination of unactivated C(sp3)−H Bonds of carboxamides by palladium catalysis. J. Org. Chem. 2016, 81, 956–968. [DOI] [PubMed] [Google Scholar]; (c) Tong H-R; Zheng W.; Lv X.; He G.; Liu P.; Chen G. Asymmetric synthesis of β-lactam via palladium-catalyzed enantioselective intramolecular C(sp3)−H amidation. ACS Catal. 2020, 10, 114–120. [Google Scholar]; (d) Zhou T.; Jiang M-X; Yang X.; Yue Q.; Han Y-Q; Ding Y.; Shi B-F Synthesis of chiral β-lactams by Pd-catalyzed enantioselective amidation of methylene C(sp3)−H bonds. Chin. J. Chem. 2020, 38, 242–246. [Google Scholar]
- (21).(a) Canty AJ; Jin H.; Skelton BW; White AH Oxidation of complexes by (O2CPh)2 and (ER)2 (E = S, Se), including structures of Pd(CH2CH2CH2CH2)(SePh)2(bpy) (bpy = 2,2’-bipyridine) and MMe2(SePh)2(L2) (M = Pd, Pt; L2 = bpy, 1,10-phenanthroline) and C···O and C···E bond formation at palladium(IV). Inorg. Chem. 1998, 37, 3975–3981. [DOI] [PubMed] [Google Scholar]; (b) Giri R.; Liang J.; Lei J-G; Li J-J; Wang D-H; Chen X.; Naggar IC; Guo C.; Foxman BM; Yu J-Q Pd-catalyzed stereoselective oxidation of methyl groups by inexpensive oxidants under mild conditions: a dual role for carboxylic anhydrides in catalytic C−H bond oxidation. Angew. Chem., Int. Ed. 2005, 44, 7420–7424. [DOI] [PubMed] [Google Scholar]; (c) Oloo W.; Zavalij PY; Zhang J.; Khaskin E.; Vedernikov AN Preparation and C−X reductive elimination reactivity of monoaryl PdIV−X complexes in water (X = OH, OH2, Cl, Br). J. Am. Chem. Soc. 2010, 132, 14400–14402. [DOI] [PubMed] [Google Scholar]; (d) Abada E.; Zavalij PY; Vedernikov AN Reductive C(sp2)−N elimination from isolated Pd(IV) amido aryl complexes prepared using H2O2 as oxidant. J. Am. Chem. Soc. 2017, 139, 643–646. [DOI] [PubMed] [Google Scholar]
- (22).For examples of C−H activation of arenes at Pd(IV), see: [Google Scholar]; (a) Rosewall CF; Sibbald PA; Liskin DV; Michael FE Palladium-catalyzed carboamination of alkenes promoted by N-fluorobenzenesulfonimide via C−H activation of arenes. J. Am. Chem. Soc. 2009, 131, 9488–9489. [DOI] [PubMed] [Google Scholar]; (b) Sibbald PA; Rosewall CF; Swartz RD; Michael FE Mechanism of N-fluorobenzenesulfonimide promoted diamination and carboamination reactions: divergent reactivity of a Pd(IV) species. J. Am. Chem. Soc. 2009, 131, 15945–15951. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.