Abstract
Background and objectives:
Extreme cardiovascular reactions to psychological stress have been associated with traumatic life experiences. Previous studies have focused on the occurrence or frequency of abuse rather than type of abuse. We examined how occurrence, frequency, and the type of abuse history are related to cardiovascular reactivity (CVR) to acute psychological stress.
Design:
The study consisted of between group and continuous analyses to examine the association between occurrence, type, and frequency of abuse with cardiovascular reactions to acute psychological stress.
Methods:
Data from 64 participants were collected. Heart rate, systolic blood pressure, and diastolic blood pressure were measured at baseline and during a standard mental arithmetic stress task.
Results:
Individuals who experienced abuse showed diminished CVR to acute psychological stress; this was driven specifically by the history of sexual abuse. Frequency of abuse did not relate to stress reactions.
Conclusions:
These findings accord with previous work suggesting a relationship between traumatic life experience and hypoarousal in physiological reactivity and extend previous findings by suggesting the relationship may be driven by sexual abuse.
Keywords: Physical trauma, emotional trauma, diminished reactivity, adversity, cardiovascular reactivity(CVR)
The literature examining psychophysiological responses associated with traumatic exposures or adversity is equivocal, some cite over arousal (Heim et al., 2000; Kendall-Tackett, 2000) and others report under arousal (Carpenter et al., 2007; Lovallo, Farag, Sorocco, Cohoon, & Vincent, 2012). Some studies have shown a dose-dependent negative relationship between the number of adverse childhood experiences and cardiovascular reactivity (CVR) (Lovallo et al., 2012; Voellmin et al., 2015), while others have found no association (Carpenter, Shattuck, Tyrka, Geracioti, & Price, 2010). The changes in the functioning of the cardiovascular system are thought to be a reflection of changes in the neural circuitry of the brain, conferring vulnerability onto the individual and pre-disposing them to adverse health outcomes. Yet, the reasoning behind this physiological differentiation in trauma victims (i.e., experiencing exaggerated or diminished reactivity) is still poorly understood (Banihashemi, Sheu, Midei, & Gianaros, 2014). While the aforementioned studies contribute substantially to the trauma literature, most measure exposure to traumatic life experiences as a binary variable of occurrence (Fagundes & Way, 2014) or as a function of frequency (Lovallo et al., 2012). Fagundes and Way (2014) argue for the importance of moving away from binary categorization of abuse history and moving toward developing investigations that emphasize more descriptive and qualitative aspects of abuse for the purpose of better understanding, how the type of abuse associates with pathology.
The aim of the current study is to (1) re-examine associations between cardiovascular stress reactivity and abuse, occurring at any age, as a both a binary occurrence and as a function of frequency, (2) examine if a specific type of abuse (e.g., physical and emotional) is driving the associations between abuse history and cardiovascular responses to stress. We hypothesized, based on the balance of previous findings, that the occurrence of abuse would be associated with diminished responses to stress and that those who experienced the highest frequency of abuse would have the most diminished responses to acute psychological stress. We hypothesized that these results would be driven by the occurrence of physical abuse (Carpenter et al., 2010).
Methods
Selection of participants
Undergraduate students (N = 125) were recruited via flyers and screened using the Revised Stressful Life Events Screening Questionnaire (SLESQ-R) (Green, Chung, Daroowalla, Kaltman, & DeBenedictis, 2006). The SLESQ-R is a 13-item self-report that identifies lifetime exposure to traumatic events including physical and emotional abuse, illness or injury, and frightening experiences (Goodman, Corcoran, Turner, Yuan, & Green, 1998). It has cultural validity (Green et al., 2006), high internal consistency (Cohen’s κ = 0.89) (Goodman et al., 1998), and relatively high ecological validity (Cohen’s κ = 0.77) (Goodman et al., 1998).
A disproportionate number of participants reported no abuse or trauma (n = 63), to keep sample size in the groups consistent, a random number generator was used to select 16 participants to attend the laboratory session for the no abuse group. There were no significant differences (p’s > .50) in age, gender, or BMI between participants with no history of trauma who were randomly selected to attend the laboratory session and those who were not. A subsample of participants (N = 64, age, M = 19.7, SD = 1.03, 81% female) were invited to attend a laboratory session based on SLESQ-R responses. Four distinct and independent groups based on the participants’ experience: no abuse or trauma history (NA; n = 16), physical abuse (PA; n = 17), emotional abuse (EA; n = 17), and physical plus emotional abuse (PPEA; n = 14). Items used to define physical and emotional abuse are reported in Table 1 (Goodman et al., 1998). Participants who did not endorse “Yes” to any question on the SLESQ-R were identified as the NA group. Participants who endorsed items on the SLESQ-R were identified as the EA (“yes” answer to the emotional abuse item), PA (“yes” answer to any physical abuse item), and PPEA groups (“yes” answer in both categories). All participants provided informed voluntary consent and the study was approved by Allegheny College’s Institutional Review Board.
Table 1.
Endorsement items for physical and emotional abuse categorization according to the Revised Stressful Life Events Screening Questionnaire (SLESQ; Goodman et al., 1998).
| Physical abuse |
|---|
|
| Emotional abuse |
|
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Laboratory procedure
The laboratory session consisted of a two-minute adaptation period, a six-minute baseline period, and a six-minute stress period. Heart rate (HR), systolic blood pressure (SBP), and diastolic blood pressure (DBP) were measured semi-automatically at two-minute intervals using a Dinamap Vital Signs Monitor (Model 1846SX, Critikon, Tampa, FL) via a brachial cuff placed on the non-dominant arm. Participants completed a widely used, mental arithmetic task to induce psychological stress (Edens, Larkin, & Abel, 1992; Ginty & Conklin, 2011; Kamarck, Manuck, & Jennings, 1990; Matthews, Woodall, & Allen, 1993). During the stress period, participants completed serial subtraction by 17s from a random four-digit number generated from random.org; new numbers were introduced at two-minute intervals, with additional numbers being provided if the participant failed to continue counting. Participants were encouraged to increase their speed and monitor their accuracy (Al’Absi et al., 1997).
Data analysis
Separate chi-square and ANOVA were used to assess differences in demographic characteristics and baseline cardiovascular activity between groups. Repeated measures ANOVAs were conducted between baseline and stress phases to ensure that the stress task significantly perturbed the cardiovascular system. Reactivity was calculated by creating residual change scores; using residual change scores allows baseline cardiovascular activity to be taken into account. Residual change scores were calculated separately for each cardiovascular parameter (HR, SBP, and DBP) using regression analyses with baseline cardiovascular activity as the independent variable and stress cardiovascular activity as the dependent variable. One-way ANOVA was used to examine differences in CVR based on binary occurrence of abuse. A cumulative abuse occurrence variable was created by multiplying the total types of abuse that the participant experienced (physical or emotional) by the number of times each abuse was experienced. Correlation analyses between the cumulative abuse occurrence variable and each of the CVR variables were conducted among those participants who reported at least one occurrence of abuse. Group differences in CVR for the four different types of abuse (NA, EA, PA, and PPEA) were assessed using a series of one-way ANOVAs. Based on recent evidence suggesting that sexual abuse may have a distinct impact on stress physiology (Schalinski, Elbert, Steudte-Schmiedgen, & Kirschbaum, 2015), post hoc sensitivity ANOVA analyses for each cardiovascular parameter was conducted with three groups: no abuse history, sexual abuse history, and any other type of abuse. A recent study found that children who experienced physical and/or sexual abuse displayed a significantly more attenuated cortisol response to stress compared to children who had experienced other types of abuse (Trickett, Gordis, Peckins, & Susman, 2014); however, the study did not distinguish between physical and/or emotional abuse. All tests used Greenhouse–Geisser correction and Bonferroni post hoc analyses. Statistical analyses were completed using SPSS, version 19, Chicago, IL.
Results
There were no significant differences in gender composition, age, BMI, or baseline cardiovascular activity between the groups, all p’s > .05 (Table 2). A repeated measures ANOVA confirmed that stress phase means for HR, F(1,63) = 89.84, p < .001, pη2 = 0.59, SBP, F(1,63) = 54.08, p < .001, pη2 = 0.46, and DBP, F(1, 63) = 177.70, p < .001, pη2 = 0.74, were significantly higher than baseline means. There were significant differences between participants who had experienced abuse and those who had not in HR reactivity, F(1,62) = 7.41, p = .008, pη2 = 0.107, and SBP reactivity, F(1,62) = 7.61, p = .008, pη2 = 0.109. Those who had experienced abuse had diminished cardiovascular responses to stress compared to those who did not. There were no significant differences between groups in DBP reactivity. Among those who had experienced abuse, there were no significant associations between cumulative abuse exposure and CVR for HR, SBP, or DBP (p’s > .34). There were significant differences in HR reactivity, F(3,60) = 2.94, p = .040, pη2 = 0.13, and SBP reactivity, F(3,60) = 3.40, p = .024, pη2 = 0.145, between abuse type groups (NA, PA, EA, and PPEA), but no significant difference in DBP reactivity (p = .42). Post hoc analyses using the Bonferroni correction indicated that the PPEA and PA groups had diminished HR and SBP reactivity compared to the NA group (see Figure 1). Sensitivity analyses creating three groups: no abuse (n = 16), sexual abuse (n = 18)1, and any other type of abuse (n = 30) demonstrated significant differences between groups in HR reactivity, F(2,61) = 4.33, p = .017, pη2 = 0.124, and SBP reactivity, F(2,61) = 4.12, p = .021, pη2 = 0.119, but not for DBP reactivity or any of the demographic variables. Post hoc analyses indicated that individuals had experienced sexual abuse had significantly diminished SBP and HR reactivity compared to those who had no history of abuse.
Table 2.
Descriptive statistics and demographics of participants.
| No abuse (n = 16) | Physical abuse (n = 17) | Emotional abuse (n = 17) | Physical + emotional abuse (n = 14) | p | |||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | ||
| Sex | .783 | ||||||||
| Male | 4 | 25 | 2 | 12 | 3 | 18 | 3 | 21 | |
| Female | 12 | 75 | 15 | 88 | 14 | 82 | 11 | 79 | |
| M | SD | M | SD | M | SD | M | SD | ||
| Age | 19.9 | 1.15 | 19.5 | 1.18 | 19.9 | 0.86 | 19.6 | 0.94 | .588 |
| BMI | 24.1 | 4.58 | 23.3 | 4.33 | 23.6 | 3.45 | 24.3 | .30 | .881 |
| SLESQ-R | 0.0 | 0.00 | 1.9 | 1.27 | 1.4 | 0.94 | 4.4 | 1.22 | *.000 |
| Baseline | |||||||||
| HR | 72.2 | 11.18 | 69.2 | 7.88 | 76.4 | 11.8 | 72.8 | .63 | .303 |
| SBP | 110.1 | 15.17 | 106.2 | 10.46 | 106.0 | 11.75 | 108.2 | 14.50 | .779 |
| DBP | 57.2 | 7.24 | 56.2 | 4.75 | 60.0 | 7.00 | 58.2 | 6.80 | .376 |
Note: A body mass index (BMI) from 18.5 to 24.9 is a normal weight. SLESQ-R, Revised Stressful Life Events Screening Questionnaire, mean number of events reported; systolic blood pressure (SBP) and diastolic blood pressure (DBP) reported in mmHg; heart rate (HR) reported as beats per minute (bpm).
p < .05.
Figure 1.
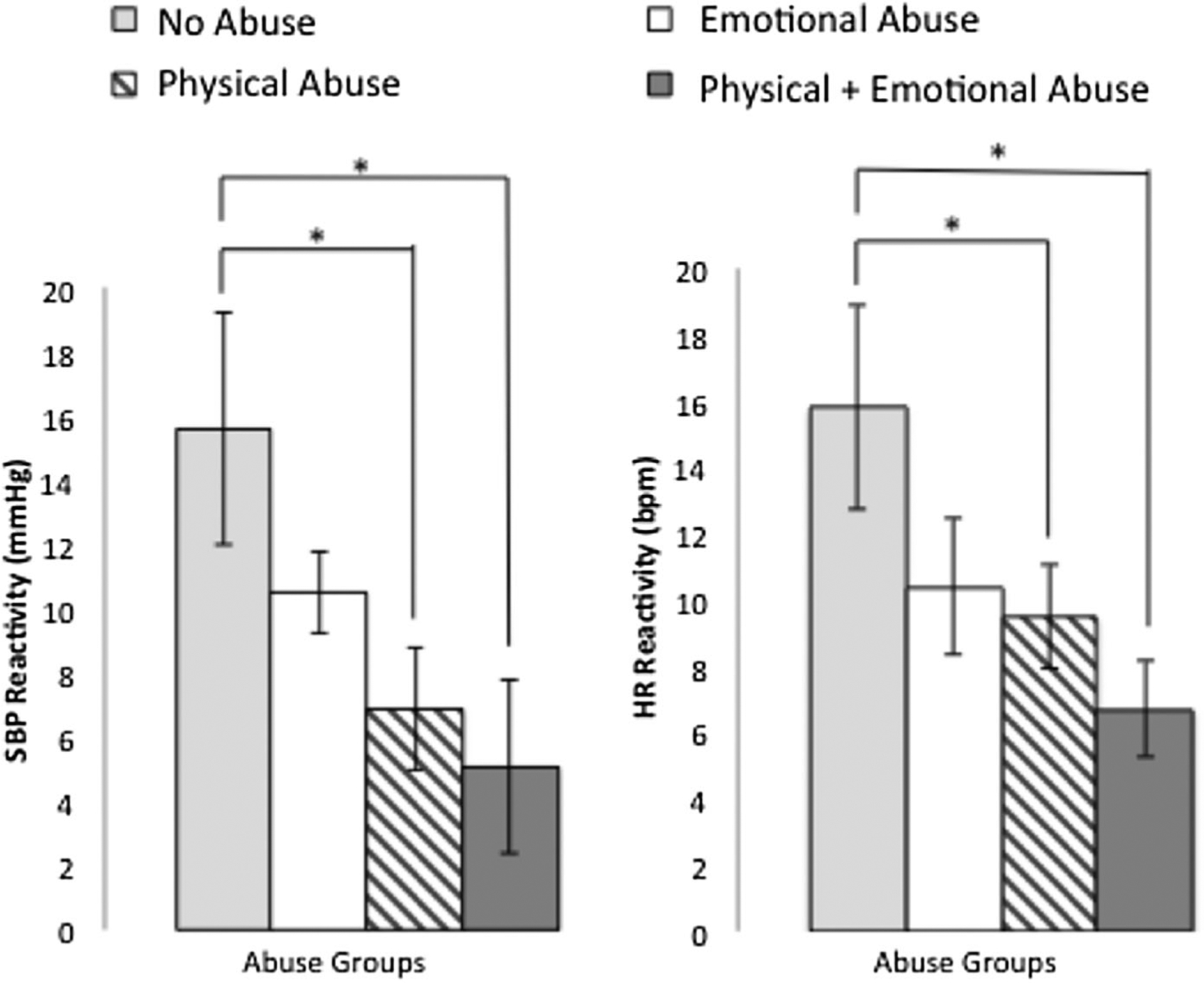
Cardiovascular reactivity to the acute psychological stress task by abuse groups. Reactivity values represent absolute change rather than residual change scores.
Discussion
The current study extended the literature by examining group differences in cardiovascular responses to acute psychological stress between those who experienced physical abuse, emotional abuse, or both physical and emotional abuse. Individuals who experienced PA or PPEA abuse exhibited diminished reactivity in response to psychological stress compared to those who had no history of abuse. Post hoc sensitivity analyses examining the effects of those who had exposure to sexual abuse compared to those who had either no abuse exposure or exposure to non-sexual abuse indicated that the associations between abuse and diminished CVR were being driven by exposure to sexual abuse. The study also re-examined associations between abuse history as a binary occurrence and as a frequency of occurrence with CVR. Results indicated that participants who had a history of abuse experienced diminished cardiovascular responses to stress compared to those without a history of abuse. However, there was no evidence of a relationship between cumulative exposure to abuse and CVR.
Results support previous findings showing a diminished biological response to stress among those who experience abuse or trauma (Carpenter et al., 2007; D’Andrea, Pole, DePierro, Freed, & Wallace, 2013; Lovallo et al., 2012; MacMillan et al., 2009; Voellmin et al., 2015), but are in contrast with other literature showing exaggerated biological responses among those exposed to traumatic events (Heim et al., 2000; Kendall-Tackett, 2000). Results accord with some previous work showing no relationship between number of types of abuse and reactivity (Carpenter et al., 2010), but are at odds with other literature showing an inverse dose-dependent relationship (Lovallo et al., 2012). In the current study, cumulative abuse was measured by creating a variable that took into account both number of types of abuse and also number of times each type of abuse occurred.
It would appear that experiencing physical abuse, whether alone or paired with emotional abuse, is marked by diminished cardiovascular responses to stress. However, these findings could also be indicative of the increased likelihood of comorbidity between physical and emotional abuse (Mullen, Martin, Anderson, Romans, & Herbison, 1996) meaning that individuals who reported only experiences of physical abuse are inherently experiencing double abuse due to its inseparable emotional component. A recent study found that individuals exposed to physical abuse had diminished responses compared to those not exposed to physical abuse (Carpenter et al., 2010). However, only two participants in that study were exposed to just physical abuse making it difficult to interpret if the diminished responses were a result of the physical abuse or a combination of different types of abuse with physical abuse. The current study extended this work by creating separate and distinct categories to allow examination of the effects of those exposed to only physical abuse versus those exposed to both physical and emotional abuse (Carpenter et al., 2010).
In a study of over 400 children, those who were exposed to maltreatment displayed attenuated responses to stress (Trickett et al., 2014). Further analysis indicated that those who had experienced physical and/or sexual maltreatment had the most attenuated responses. Evidence suggests that sexual abuse can lead to diminished biological responses to stress among women who have experienced significant trauma and subsequently developed trauma-related disorders (Schalinski et al., 2015). Post hoc sensitivity analyses in the present study found that sexual abuse was driving the relationship between abuse history and diminished reactions to stress in generally healthy, young adults. Thus, it is reasonable to conclude the type of abuse one is exposed to, rather than whether or not one experienced any type of abuse, plays an important role in altering biological responses to stress.
Adolescents who are exposed to violence are at an increased risk for developing both eating disorders and depression (Ackard & Nuemark-Sztainer, 2002; Close, 2005; Silverman, Raj, Mucci, & Hathaway, 2001) and are more likely to engage in risky behaviors such as substance abuse and sexual promiscuity (Close, 2005; Schiff & Zeria, 2005). Diminished reactivity could act as a marker for this risky trajectory, as it has been associated with impulsivity (Bennett, Blissett, Carroll, & Ginty, 2014), conduct disorder (Oritz & Raine, 2004), and a dysregulation in areas of the brain associated with motivated behavior (Ginty, Gianaros, Derbyshire, Phillips, & Carroll, 2013). This biological dysregulation in children who experience violence can lead to maladaptive emotional perception, including assigning negative valence to neutral stimuli (Phillips, Ginty, & Hughes, 2013), suspecting hostile motives in others (Repetti, Taylor, & Seeman, 2002), and a tendency to show emotional detachment and antisocial behavior (Graham, Kimonis, Wasserman, & Kline, 2012).
The present study is not without limitations. First, the sample size within each group is relatively small, increasing the potential of a type II error. However, the sample size of this study is in line with other studies examining group differences in stress reactivity (Carroll, Phillips, & Balanos, 2009; Ginty, Phillips, Higgs, Heaney & Carroll, 2012; Heim et al., 2000). Second, there were no measures assessing the participant’s level of effort, nor his or her perception of the difficulty level of the task; however, studies have shown that diminished reactivity is not associated with a lack of effort on the part of the participant (Salomon, Bylsma, White, Panaite, & Rottenberg, 2013). Third, the study did not use an established subscale to classify participants; however, the items selected from the SLESQ-R relate to definitions of physical and emotional abuse and align with items in the subscale of the Childhood Trauma Short Form Questionnaire (CTQ-SF), which has been shown to have ecological validity within adolescent populations (Bernstein et al., 2003). Fourth, the study did not control for important variables associated with abuse, such as depression and substance use. However, previous work has shown the associations between diminished reactivity and abuse withstand adjustment for potential confounding variables such as post-traumatic stress disorder and major depressive disorder (MacMillan et al., 2009). The significant differences observed in SBP and HR reactivity, but not DBP reactivity suggest that the differences are being driven from reductions in β-adrenergic activity (Mills & Dimsdale, 1991). However, the current study measured only HR and BP reactivity. Future research should extend this work by adding a more comprehensive assessment of hemodynamics. Fifth, participants were not asked to limit caffeine intake, smoking, or exercise prior to the study. Lastly, the current study relied on retrospective reports of abuse and did not include factors such as self-report neglect. This could result in some reporting bias. Future research should aim to obtain more concrete records of abuse.
In summary, in a sample of healthy young adults, individuals who experienced abuse have diminished cardiovascular responses to stress compared to those who have not experienced abuse. Results suggested that it was the type of experience, rather than the cumulative total of abuse experienced that was associated with these diminished reactions. Individuals who experienced physical abuse or physical and emotional abuse show diminished cardiovascular response to acute psychological stress compared to those who have no history of abuse. Further analyses indicated that sexual abuse specifically was driving the association between diminished reactivity and abuse history.
Acknowledgements
The authors thank Deborah Dickey and Professor Douglas Carroll for their valuable feedback on this manuscript.
Funding
This research was conducted at Allegheny College with funding provided by the Allegheny College Neuroscience Program. Annie Ginty is supported by the National Heart, Lung, and Blood Institute under Grant [T32 HL07560].
Footnotes
Of the 18 participants who reported sexual abuse: 10 reported sexual abuse only; 1 reported sexual and another kind of physical abuse; 1 reported sexual and emotional abuse; and 6 reported sexual, another kind of physical, and emotional abuse.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Ackard DM, & Nuemark-Sztainer D (2002). Date violence and date rape among adolescents: Associations with disordered eating behaviors and psychological health. Child Abuse & Neglect, 26, 455–473. doi: 10.1016/S0145-2134(02)00322-8 [DOI] [PubMed] [Google Scholar]
- Al’Absi M, Bongard S, Buchanan T, Pincomb GA, Licinio J, & Lovallo WR (1997). Cardiovascular and neuroendocrine adjustment to public speaking and mental arithmetic stressors. Psychophysiology, 34, 266–275. doi: 10.1111/j.1469-8986.1997.tb02397.x [DOI] [PubMed] [Google Scholar]
- Banihashemi L, Sheu L, Midei AJ, & Gianaros PJ (2014). Childhood physical abuse predicts stressor-evoked activity within central visceral control regions. Social Cognitive and Affective Neuroscience, 10, 474–485. doi: 10.1093/scan/nsu073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett C, Blissett J, Carroll D, & Ginty AT (2014). Rated and measured impulsivity in children is associated with diminished cardiac reactions to acute psychological stress. Biological Psychology, 102, 68–72. doi: 10.106/j.biopsycho.2014.07.009 [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, Walker EW Pogge D, Taruna A, … Zule W (2003). Development and validation of a brief screening version of the childhood trauma questionnaire. Child Abuse and Neglect, 27, 169–190. doi: 10.1016/S0145-2134(02)00541-0 [DOI] [PubMed] [Google Scholar]
- Carroll D, Phillips AC, & Balanos GM (2009). Metabolically exaggerated cardiac reactions to acute psychological stress revisited. Psychophysiology, 46, 270–275. doi: 10.1111/j.1469-8986.2008.00762.x [DOI] [PubMed] [Google Scholar]
- Carroll D, Smith GD, Sheffield D, Shipley MJ, & Marmot MG (1995). Pressor reactions to psychological stress and prediction of future blood pressure: Data from the Whitehall II study. British Medical Journal, 310, 771–776. doi: 10.113/bmj.310.6982.771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LM, Carvalho JP, Tyrka AR, Wier LM, Mello AF, Mello MF, … Price LH (2007). Decreased adreno-corticotropic hormone and cortisol responses to stress in healthy adults reporting childhood maltreatment. Clinical Psychology, 62, 1080–1087. doi:10.1016.j.biopsych.2007.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LM, Shattuck TT, Tyrka AR, Geracioti TD, & Price LH (2010). Effect of childhood physical abuse on cortisol stress response. Psychopharmacology, 214, 367–375. doi: 10.1007/s00213-010-2007-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close SM (2005). Dating violence prevention in middle school and high school youth. Journal of Child and Adolescent Psychiatric Nursing, 18, 2–9. doi: 10.1111/j.1744-6171.2005.00003.x [DOI] [PubMed] [Google Scholar]
- D’Andrea W, Pole N, DePierro J, Freed S, & Wallace DB (2013). Heterogeneity of defensive responses after exposure to trauma: Blunted autonomic reactivity in response to startling sounds. International Journal of Psychophysiology, 90, 80–89. doi: 10.1016/j.ijpsycho.2013.07.008 [DOI] [PubMed] [Google Scholar]
- Edens JL, Larkin KT, & Abel JL (1992). The effect of social support and physical touch on cardiovascular reactions to mental stress. Journal of Psychosomatic Research, 36, 371–381. doi: 10.1037/0278-6133.17.5.436 [DOI] [PubMed] [Google Scholar]
- Fagundes CP & Way B (2014). Early-life stress and adult inflammation. Current Directions in Psychological Science, 23, 277. doi: 10.1177/0963721414535603 [DOI] [Google Scholar]
- Ginty AT, & Conklin SM (2011). High perceived stress in relation to life events is associated with blunted cardiac reactivity. Biological Psychology, 86, 383–385. doi:1.1016/j.biopsycho.2011.01.002 [DOI] [PubMed] [Google Scholar]
- Ginty AT, Gianaros PJ, Derbyshire SWG, Phillips AC, & Carroll D (2013). Blunted cardiac stress reactivity relates to neural hypoactivation. Psychophysiology, 50, 219–229. doi: 10.1111/psyp,12017 [DOI] [PubMed] [Google Scholar]
- Ginty AT, Phillips AC, Higgs S, Heaney JLJ, & Carroll D (2012). Disordered eating behavior is associated with blunted cortisol and cardiovascular reactions to acute psychological stress. Psychoneuroendocrinology, 37, 715–724. doi: 10.1016/j.psyneuen.2011.09.004 [DOI] [PubMed] [Google Scholar]
- Goodman LA, Corcoran C, Turner K, Yuan N, & Green BL (1998). Assessing traumatic event exposure: General issues and preliminary findings for the stressful life events screening questionnaire. Journal of Traumatic Stress, 11, 521–542. doi: 10.1023/A:1024456713321 [DOI] [PubMed] [Google Scholar]
- Graham N, Kimonis ER, Wasserman AL, & Kline SM (2012). Associations among childhood abuse and psychopathy facets in male sexual offenders. Personality Disorders, 3, 66–75. doi: 10.1037/a0025605 [DOI] [PubMed] [Google Scholar]
- Green BL, Chung JY Daroowalla A, Kaltman S, & DeBenedictis C (2006). Evaluating the cultural validity of the stressful life events screening questionnaire. Violence Against Women, 12, 1191–1213. doi: 10.1177/1077801206294534 [DOI] [PubMed] [Google Scholar]
- Heim C, Newport J, Heit S, Graham YP, Wilcox M, Bonsall R, … Nemeroff CB (2000). Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA, 284, 592–597. doi: 10.1001/jama.284.5.592 [DOI] [PubMed] [Google Scholar]
- Kamarck TW, Manuck SB, & Jennings JR (1990). Social support reduces cardiovascular reactivity to a psychological challenge: A laboratory model. Psychosomatic Medicine, 52, 42–48. doi: 10.1097/00006824-1990010000-00004 [DOI] [PubMed] [Google Scholar]
- Kendall-Tackett KA (2000). Physiological correlates of child abuse: Chronic hyperarousal in PTSD, depression and irritable bowel syndrome. Child Abuse & Neglect, 24, 799–810. doi: 10.1016/S0145-2134(00)00136-8 [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Farag NH, Sorocco KH, Cohoon AJ, & Vincent AS (2012). Lifetime adversity leads to blunted stress axis reactivity: Studies from the Oklahoma family health patterns project. Biological Psychiatry, 71, 344–349. doi: 10.1016/j.biopsych.2011.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMillan HL, Georgiades K, Duku EK, Shea A, Steiner M, Niec A, … Schmidt LA (2009). Cortisol response to stress in female youth exposed to childhood maltreatment: Results of the youth mood project. Biological Psychiatry, 66, 62–68. doi: 10.1016/j.biopsych.2008.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews KA, Woodall KL, & Allen MT (1993). Cardiovascular reactivity to stress predicts future blood pressure status. Hypertension, 22, 479–485. doi: 10.1161/01.HYP.22.4.479 [DOI] [PubMed] [Google Scholar]
- Mills PJ, & Dimsdale JE (1991). Cardiovascular reactivity to psychosocial stressors: a review of the effects of beta-block-ade. Psychosomatics, 32, 209–220. [DOI] [PubMed] [Google Scholar]
- Mullen PE, Martin JL, Anderson JC, Romans SE, & Herbison GP (1996). The long term impact of the physical, emotional, and sexual abuse of children: A community study. Child Abuse & Neglect, 20, 7–21. doi: 10.1016/0145-2134(95)00112-3 [DOI] [PubMed] [Google Scholar]
- Oritz J, & Raine A (2004). Heart rate level and antisocial behavior in children and adolescents: A meta-analysis. Journal of the American Academy of Child and Adolescent Psychiatry, 43, 154–162. doi: 10.1097/00004583-200402000-00010 [DOI] [PubMed] [Google Scholar]
- Phillips AC, Ginty AT, & Hughes BM (2013). The other side of the coin: Blunted cardiovascular and cortisol activity are associated with negative health outcomes. International Journal of Psychology, 90, 1–7. doi: 10.1016/j.ijpsycho.2013.02.002 [DOI] [PubMed] [Google Scholar]
- Repetti R, Taylor SE, & Seeman T (2002). Risky families: Family social environments and the mental and physical health of offspring. Psychological Bulletin, 128, 330–366. doi: 10.1037/0033-2909.128.2.330 [DOI] [PubMed] [Google Scholar]
- Salomon K, Bylsma LM, White KE, Panaite V, & Rottenberg J (2013). Is blunted cardiovascular reactivity in depression mood-state dependent? A comparison of major depressive disorder remitted depression and healthy controls. International Journal of Psychophysiology, 90, 50–57. doi: 10.1016/j.ijpsycho.2013.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalinski I, Elbert T, Steudte-Schmiedgen SS, & Kirschbaum C (2015). The cortisol paradox of trauma-related disorders: Lower phasic responses but higher toner levels of cortisol are associated with sexual abuse in childhood. PLoS One. doi: 10.1371/journal.pone.0136921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff M, & Zeria A (2005). Dating violence and sexual risk behaviors in a sample of at-risk Israeli youth. Child Abuse and Neglect, 29, 1949–1263. doi: 10.1016/j.chiabu.2005.04.007 [DOI] [PubMed] [Google Scholar]
- Silverman JG, Raj A, Mucci LA, & Hathaway JE (2001). Dating violence against adolescent girls and associated substance use, unhealthy weight control, sexual risk behavior, pregnancy, and suicidality. Journal of the American Medical Association, 286, 572–579. doi: 10.1001/jama.286.5.572 [DOI] [PubMed] [Google Scholar]
- Trickett PK, Gordis E, Peckins MK, & Susman EJ (2014). Stress reactivity in maltreated and comparison male and female young adolescents. Child Maltreatment, 19, 27–37. doi: 10.1177/1077559513520466 [DOI] [PubMed] [Google Scholar]
- Voellmin A, Winzeler K, Hug E, Wilhelm FH, Schaefer V, Gaab J, … Bader K (2015). Blunted endocrine and cardiovascular reactivity in young healthy women reporting a history of childhood adversity. Psychoneuroendocrinology, 51, 58–67. doi: 10.1016/j.psyneuen.2014.09.008 [DOI] [PubMed] [Google Scholar]


