Abstract
Potentiometric biosensors are incredibly versatile tools with budding uses in industry, security, environmental safety, and human health. This mini-review on recent (2018–2020) advances in the field of potentiometric biosensors is intended to give a general overview of the main types of potentiometric biosensors for novices while still providing a brief but thorough summary of the novel advances and trends for experienced practitioners. These trends include the incorporation of nanomaterials, graphene, and novel immobilization materials, as well as a strong push towards miniaturized, flexible, and self-powered devices for in-field or at-home use.
Keywords: Potentiometry, Biosensors, Field effect transistors, Flexible sensors, Biofuel cells
1. Introduction
Biosensors are very popular tools for applications from environmental sensing, to clinical diagnoses, forensics, security, and more, coming in an incredible variety of forms. Most of these forms are outside of the scope of this review, but an interested reader can find a thorough history on the field of biosensors and their forms here [1].
Electrochemistry is a popular technique used to manufacture biosensors as it is versatile, rapid, inexpensive, highly sensitive, and selective. These Preadvantageshaveledto an immense variety of electrochemical sensors existing today, with the commercial glucose meter being one of the most widely-known and used examples. While the vast majority of electrochemical biosensors are either voltammetric or amperometric, there is a growing number of potentiometric sensors being designed due to its advantages. Potentiometry is an electrochemical technique where negligible bias current (on the order of 10-15 A) flows as the potential between a working electrode and a reference electrode is measured across some interface. This technique is advantageous in that it is simple, compact, and requires little power [2]. Additionally, the negligible current flow means the technique should be more resistant to interferent effects [3] and ohmic drop considerations compared to voltammetric or amperometric sensors. Finally, potentiometry has shown to be relatively insensitive to electrode size [4], meaning that miniaturization without loss of sensitivity is achievable.
This mini-review aims to highlight the types of potentiometric biosensors that exist today, and the novel advances in the field from the past few years.
2. Main Text
2.1. Ion Selective Electrodes
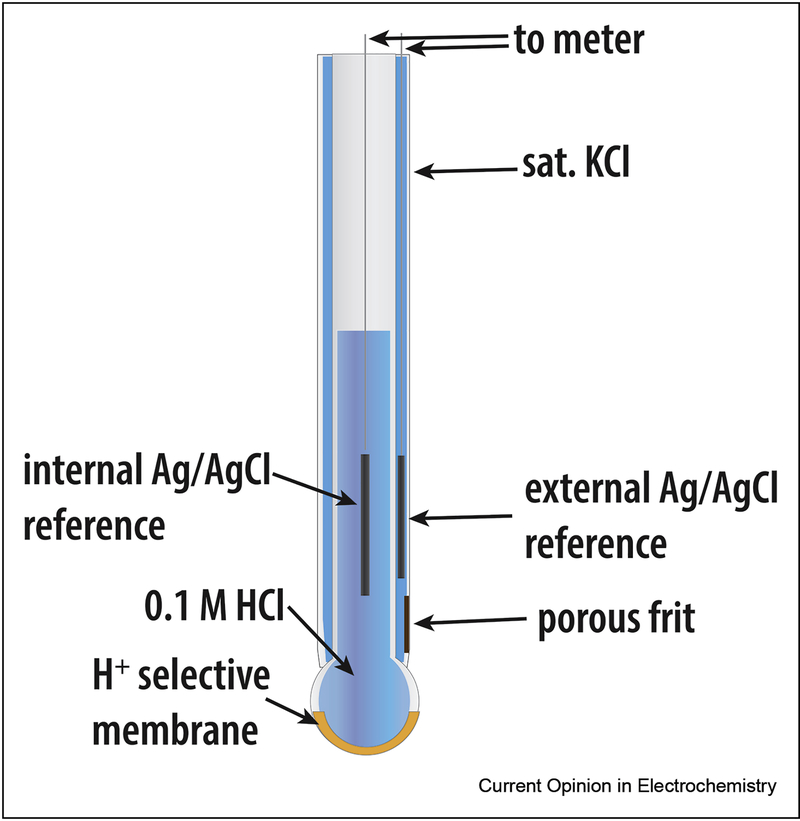
One of the most widely used forms of potentiometric sensors are ion-selective electrodes (ISEs), where an ion transport or exchange at a selective membrane causes a change in membrane potential (Figure 1). Traditionally, ISEs have been used to measure pH and electrolyte concentrations, but clever membrane formations have led to an expansion of analytes that can be detected with these electrodes. For a thorough review on ISEs, the reader is encouraged to view [2, 5, 6].
Figure 1.
Schematic of a conventional ISE for analysis of solution pH.
More recent advances in this field have allowed for the measurement of neurotransmitters [7, 8], proteins [9], bacteria [10, 11], small molecules [12–14], and toxins [15]. These advances have occurred through innovative use of biomimetics as the ion exchanger in the polymeric membrane [7], the addition of analyte-selective enzymes to some part of the biosensor to form ions that an ISE membrane is selective for [8, 12, 13, 15], and multiple molecule sandwiches integrated into the polymeric membrane made of antibodies [9], DNA [10], proteins [11], or aptamers [14]. Other advances have included the design of a solid-state ISE designed for simple miniaturization and mass fabrication [15], the use of a combination ISE and FET (see section 2.3) [8], and simultaneous potentiometric detection of two different analytes [10].
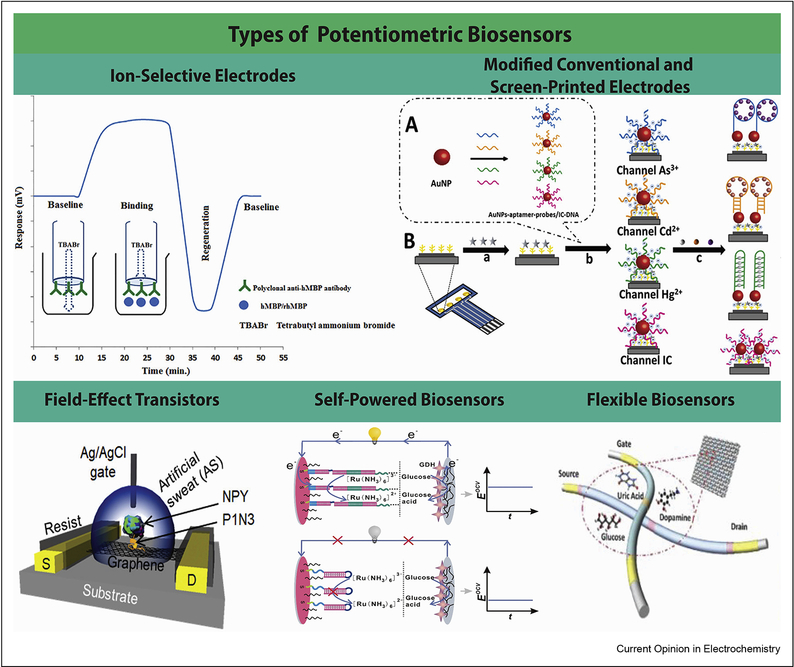
Of particular interest is an ISE that can detect recombinant human myelin basic protein (rhMBP) with a limit of detection of 50 ng/mL [9]. Polyclonal rhMBP antibodies were adsorbed to a PVC-COOH membrane, so that the presence of rhMBP creates a build-up of TBABr (tetrabutylammonium bromide) in the inner filling solution and thus changes the measured potential (Figure 4).
Figure 4.
Summary of the types of potentiometric biosensors, with an example schematic of each type: Ion-selective electrode for the detection of recombinant human myelin basic protein [9], a modified screen-printed electrode for the simultaneous detection of Hg2+, Cd2+, and As3+ [23], a graphene-based field effect transistor for the detection of neuropeptide Y [34], a self-powered biosensor for the detection of a single nucleotide polymorphism [38], and a flexible, fabric-based biosensor for the detection of glucose [32].
2.2. Conventional potentiometric sensors with new materials
Another way to design a potentiometric biosensor is to immobilize the biorecognition element(s) on the surface of a conventional electrode (where metal or carbon rods or disks are encased in an insulating sheath of plastic or glass) or an array of screen-printed electrodes. This immobilization can be done is a huge variety of ways, including the formation of self-assembled monolayers, hydrogels, polymeric membranes, and more. Recent advances in this type of biosensor have focused on the discovery of new materials or the creative use of old materials to improve biosensor selectivity, sensitivity, and stability. Some groups have investigated different polymer materials for biorecognition element immobilization [16, 17], including Nafion [16, 18], Aquivion [17], chitosan and synthetic polymer blends [16, 19, 20], and 4-mercaptobenzoic acid as an alternative to the common thiol-bonding methods [21]. Others have inserted metal oxides, such as zinc oxide [18] and aluminum-doped zinc oxide [18], or nanomaterials [21] into the immobilization matrix to improve sensor performance. While many of these biosensors use oxidase enzymes to detect small molecules like glucose [16–18, 20], some groups are exploring the use of direct electron transfer enzymes [22].
Other advances have included the use of metal ion-specific aptamers bound to gold nanoparticles to monitor the concentrations of three metal ions simultaneously [23]. These nanoparticles are immobilized using thiol-chemistry on the surface of screen-printed carbon electrodes modified with reduced graphene oxide and dendritic gold nanostructures (Figure 4). Additional aptamer-coated nanoparticles acted as internal standards, allowing for limits of detection as low as 2.0 pM for Hg2+, 0.62 pM for Cd2+, and 0.17 pM for As3+. An amperometric sensor for Hg2+, on the other hand, has a limit of detection of 10 nM [24].
Additionally, antibody-conjugated liposomes have been designed to split open and begin a novel precipitation reaction at the electrode surface in the presence of the analyte [25], and an enzyme-based reference electrode was designed to mitigate drift problems that are known to occur when metal salt references are used in long-term in vivo studies [21].
2.3. Field-Effect Transistors (FETs)
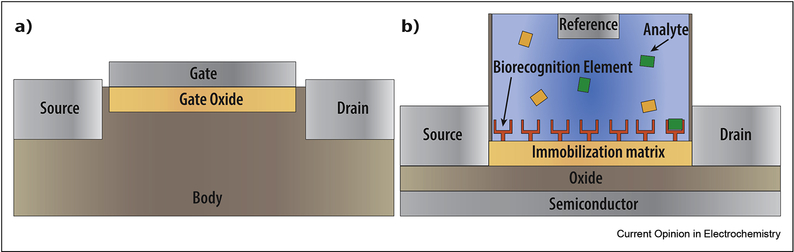
In order to achieve incredibly high sensitivities (aM [26] to nM [27]), many groups are moving towards the use of various field effect transistors (FETs), which operate based on the principle that a change of the voltage at the gate electrode leads to a change in the FET’s conductance (Figure 2a). Biologically coupled FETs (Bio-FETs) are designed so that binding of the analyte to the biorecognition element leads to a change in the charge distribution of the semiconductor layer underneath, and therefore a change to the overall device conductance (Figure 2b). BioFETs have large linear ranges, incredible sensitivities, and can be readily and inexpensively miniaturized. For an in-depth review on BioFETs, the reader is encourage to read [28].
Figure 2.
Schematic of a a) field effect transistor, where changes in the gate voltage cause either holes or electron carries to move between the source and the drain, resulting in a change to the device’s conductance and a b) Bio-FET, where binding of the analyte to the biorecognition element leads to a change in charge distribution of the semiconducting layer, driving a change in overall conductance of the device.
These BioFETs have been used to detect small molecules [26, 29–32], proteins [33, 34], bacteria [35], lipids [26], neurotransmitters [8, 26, 36], and pH [37]. These molecules have been detected by binding enzymes [29–32], peptides [33, 34], DNA probes [35], and aptamers [26, 36] to the gate electrode. Groups have explored the use of novel materials for immobilization of biorecognition elements on the gate electrode, including: zeolites [29], polyaniline films [30], nanoporous gold [35], and layered double hydroxides [8]. Others have investigated alternative materials for increasing the charge transfer rate of the gate, such as graphene [34]. In this paper, a peptide specific to the biomarker neuropeptide Y was adsorbed to the graphene-covered FET (Figure 4), creating a highly specific sensor with a dynamic range of 1 pM to 10 μM.
Other advances have included the use of extended gate electrodes [27, 33, 35, 37], which separates the biorecognition portion of the biosensor from the rest of the FET, and the design of a polymeric nanofilter to prevent small molecular weight molecules from interfering with the measurement [27]. Some groups are exploring FETs based off of different principles, such as the junctionless nanowire FET [33] or the organic electrochemical transistors [31, 32, 36] in order to achieve the desired levels of performance.
2.4. Transition to Field-Deployable Biosensors
One of the biggest pushes in the field of biosensors is to create sensors that are field-deployable. To do this, the sensors have to be either very low-powered or self-powered, portable, inexpensive to manufacture, and robust. To tackle the power issue, potentiometry has inherent advantages as having low power needs to begin with. However, in many field locations even potentiometry’s low power needs are too much, so some groups have been working on designing self-powered sensors. Other groups have been working on making their sensors incredibly robust and flexible for real-time analyses.
2.4.1. Self-powered biosensors
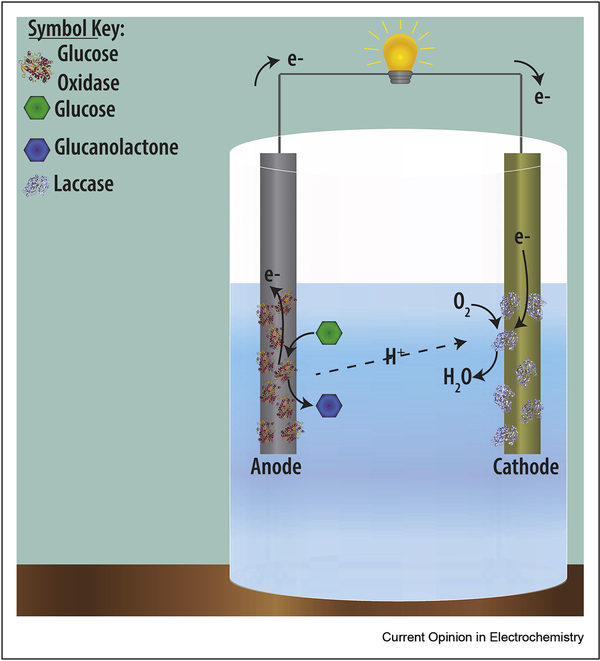
Self-powered biosensors often take the form of biofuel cells (BFCs), where both the anode and the cathode are modified in such a way that the reactions occurring provide all of the power needed for the biosensor to function (Figure 3). Other advantages of these systems are that they are relatively easy to make, inexpensive, and portable.
Figure 3.
Schematic of a biofuel cell, where the energy to power the lightbulb is made by the reactions occurring at the cathode-glucose oxidase turning over glucose and releasing an electrode into the anode-and laccase using that electron to turn dissolved oxygen into water.
These BFCs have been used to detect single nucleotide polymorphisms [38], microRNA [39, 40], glucose [31, 41, 42], lactate [42], and organophosphate pesticides [43]. For many of these, the electrode material is either made of or functionalized with nanomaterials such as gold, silica, iron oxide nanoparticles [32, 38–40] or carbon nanotubes [38, 39, 43] in order to adsorb or bind to the biorecognition element(s), which usually either an enzyme [31, 41–43] or DNA capture probe [38–40]. However, one novel work uses covalent organic frameworks to immobilize both the enzyme and the electron mediator to stabilize them and mitigate their loss over extended use [41].
Often, the reaction occurring at the electrode opposite to the one where the biorecognition element is immobilized is the conversion of oxygen to water. This is achieved through a variety of materials, including bilirubin oxidase [43] and platinum black [42]. In other biosensors, the electrode is modified by an enzyme specific to glucose, and the binding event between the biorecognition element and the analyte on the other electrode causes a mediator to be released from inside a porous particle [39, 40] or moved towards the electrode [38] to become reduced by the enzymatic reaction occurring as glucose turned over.
One ingenious BFC uses a combination of DNA strand displacement reactions and hybridization chain reaction such that in the presence of the target DNA sequence, a mediator is extended towards the anode, where the enzymatic oxidation of glucose reduces the mediator and alters the measured potential (Figure 4). This BFC has a limit of detection of 20 aM, and is capable of differentiating the target sequence from nearly-identical sequences [38].
2.4.2. Flexible Biosensors
Biosensors geared towards use in the field and everyday life have been experiencing a move towards flexible sensors, as they can provide comfortable, inexpensive, robust, easy-to-use real-time analyses. Many of these devices are intended as point-of-care monitoring devices for small molecules that can warn users about potential harm ahead of time, such as diabetes, or for sweat analysis during exercise. If interested in more detail than can be provided here, the reader is directed towards the recent reviews on of flexible biosensors [44], wearable electrochemical sensors [45], and challenges associated with developing wearable sensors [46].
One of the main types of flexible biosensors are wearable sensors designed for the analysis of sweat during exercise. For example, a recent publication describes a glucose and lactate sensor made on polydimethylsiloxane that is not only self-powered, but is also very flexible and can use the analysis of electrolyte concentrations and pH of the sweat to correct the glucose and lactate concentrations measured [42].
The other main type of flexible sensor is designed to monitor for indicators of a particular disease state. A flexible array made on polyethylene terephthalate and modified with lactate dehydrogenase and glucose oxidase allowed for the detection of glucose and lactate in blood as markers for disease [47]. A similar paper-based device was created to measure galactose by immobilizing galactose oxidase on platinized paper in order to screen for galactosemia in infants [48]. A textile-based biosensor was created to monitor glucose and uric acid by modifying organic electrochemical transistors with the appropriate enzyme and then weaving nylon fibers around the sensor (Figure 4). The glucose fibers had a detection limit of 30 nM and were woven into a fabric square inside of a diaper to monitor glucose levels in urine [32].
3. Conclusions and Future Outlooks
The main challenge with potentiometric biosensing is achieving selectivity. There has been a great deal of recent advancement in biosensing, from the novel use of materials both old and new to improved potentiometric sensors that expand the range of available analytes to the use of new sensing devices to drastically improve sensitivity. An important trend has emerged in the push towards portable, self-powered, and wearable biosensors for use in technologically limited locations or for point-of-care devices. Though there have been many exciting leaps, an overview of which is illustrated in Figure 4, many of these biosensors are not quite yet refined enough for commercial or wide-scale use. Further work is needed to get many of these devices to market, but we anticipate that it will not be much longer before potentiometric biosensors are commonplace.
Highlights.
Potentiometric biosensors are versatile tools with a wide variety of applications
New advances push the limits of detection, sensitivity, and selectivity
Biosensing is moving towards portable and flexible sensors for in-field use
Acknowledgements
Research reported in this publication was supported by the National Institute Of General Medical Sciences of the National Institutes of Health under Award Number R35GM138133. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. AIC acknowledges the Russian Foundation for Basic Research (17–54-33003) and Ministry of Science and Higher Education of the Russian Federation (FZMW-2020–0007). The authors also acknowledge Dr. Koji Sode and David Probst for helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of competing interest
The authors declare no conflicts of interest.
References
Papers of particular interest, published within the period of review, have been highlighted as
• of special interest
•• of outstanding interest
- [1].Turner APF: Biosensors: sense and sensibility. Chem. Soc. Rev 2013, 42: 3184–3196. [DOI] [PubMed] [Google Scholar]
- [2].Din J, Qin W: Recent advances in potentiometric biosensors. Trends Anal. Chem 2020, 124: 115803. [Google Scholar]
- [3].Smith LA, Glasscott MW, Vannoy KJ, Dick JE: Enzyme Kinetics via Open Circuit Potentiometry. Anal. Chema 2020, 92: 2266–2273. [DOI] [PubMed] [Google Scholar]
- [4].Park JH, Zhou H, Percival SJ, Zhang Bo, Fan FRF, Bard AJ: Open Circuit (Mixed) Potential Changes Upon Contact Between Different Inert Electrodes-Size and Kinetic Effects. Anal. Chem 2013, 85: 964–970. [DOI] [PubMed] [Google Scholar]
- [5].Zdrachek E, Bakker E: Potentiometric Sensing. Anal. Chem 2019, 91: 2–26. [DOI] [PubMed] [Google Scholar]
- [6].Bobacka J, Ivaska A, Lewenstam A: Potentiometric Ion Sensors. 2008, Chemical Reviews, vol. 108 (2): 329–351. [DOI] [PubMed] [Google Scholar]
- [7].Mousavi MPS, Abd El-Rahman MK, Mahmoud AM, Abdelsalam RM, Bühlmann P: In Situ Sensing of the Neurotransmitter Acetylcholine in a Dynamic Range of 1 nM to 1 mM. ACS Sensors 2018, 3: 2581–2589. [DOI] [PubMed] [Google Scholar]
- [8].Hidouri S, Errachid AH, Baussels J, Korpan YI, Ruiz-Sanchez O, M Baccar Z: Potentiometric sensing of histamine using immobilized enzymes on layered double hydroxides. J. Food Sci Technol 2020: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Al-Ghobashy MA, Nadim AH, El-Sayed GM, Nebsen M: Label-Free Potentiometric Ion Flux Immunosensor for Determination of Recombinant Human Myelin Basic Protein: Application to Downstream Purification from Transgenic Milk. ACS Sens 2019, 4: 413–420. [DOI] [PubMed] [Google Scholar]
- [10].Ding J, Yu N, Wang X, Qin W: Sequential and Selective Detection of Two Molecules with a Single Solid-Contact Chronopotentiometric Ion-Selective Electrode. Anal. Chem 2018, 90: 1734–1739. [DOI] [PubMed] [Google Scholar]
- [11].Lv E, Ding J, Qin W: Potentiometric Detection of Listeria monocytogenes via a Short Antimicrobial Peptide Pair-Based Sandwich Assay. Anal. Chem 2018, 90: 13600–13606. [DOI] [PubMed] [Google Scholar]
- [12].Liu Y, Cánovas R, Crespo GA, Cuartero M: Thin-Layer Potentiometry for Creatinine Detection in Undiluted Human Urine Using Ion-Exchange Membranes as Barriers for Charged Interferences. Anal. Chem 2020, 92: 3315–3323. [DOI] [PubMed] [Google Scholar]
- [13].Sihombing K, Tamba MC, Marbun WS, Situmorang M: Urease immobilized potentiometric biosensor for determination of urea. Ind. J. Chem 2018, 57A: 175–180. [Google Scholar]
- [14].Lv E, Ding J, Qin W: Potentiometric Aptasensing of Small Molecules Based on Surface Charge Change. Sens. Actuat. B 2018, 259: 463–466. [Google Scholar]
- [15].Goud KY, Teymourian H, Sandhu SS, Tostado N, Mishra RK, Moore LC, Harvey SP, Wang J: OPAA/fluoride biosensor chip towards field detection of G-type nerve agents. Sens. Actuat. B Chem 2020, 320: 128344. [Google Scholar]
- [16].Guadarrama-Fernández L, Novell M, Blondeau P, Andrade FJ: A disposable, simple, fast and low-cost paper-based biosensor and its application to the determination of glucose in commercial orange juices. Food Chem 2018, 265: 64–69. [DOI] [PubMed] [Google Scholar]
- [17].Cánovas R, Blondeau P, Andrade FJ: Modulating the mixed potential for developing biosensors: Direct potentiometric determination of glucose in whole, undiluted blood. Biosens. Bioelectron 2020, 163: 112302. [DOI] [PubMed] [Google Scholar]
- [18].Chou JC, Lin SH, Lai TY, Kuo PY, Lai CH, Nien YH, Su TY: A Facile Fabrication of a Potentiometric Arrayed Glucose Biosensor Based on Nafion-GOx/GO/AZO. Sensors 2020, 20: 964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kushwaha CS, Singh P, Abbas NS, Shukla SK: Self-activating zinc oxide encapsulated polyaniline-grafted chitosan composite for potentiometric urea sensor. J. Mater. Sci.: Mater. Electron 2020, 31: 1187–11896. [Google Scholar]
- [20].Walker NL, Dick JE. Oxidase-Loaded Hydrogels for Versatile Potentiometric Metabolite Sensing. Biosens. Bioelectron 2021, 178: 112997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ramašauskas L, Meskys R, Ratautas D. Real-time glucose monitoring system containing enzymatic sensor and enzymatic reference electrodes. Biosens. Bioelectron 2020, 164: 112338. [DOI] [PubMed] [Google Scholar]
- [22].Lee I, Loew N, Tsugawa W, Ikebukuro K, Sode K: Development of a third-generation glucose sensor based on the open circuit potential for continuous glucose monitoring. Biosens. Bioelectron 2019, 124: 216–223. [DOI] [PubMed] [Google Scholar]
- [23].Tang W, Wang Z, Yu J, Zhang F, He P: Internal Calibration Potentiometric Aptasensors for Simultaneous Detection of Hg2+, Cd2+, and As3+ Based on a Screen-Printed Carbon Electrodes Array. Anal. Chem 2018, 90: 8337–8344.••The authors were able to simultaneously and specifically detect 3 analytes using different aptamers, and included another aptamer as an internal calibration standard.
- [24].Vyas G, Bhatt S, Paul P: Synthesis of Calixarene-Capped Silver Nanoparticles for Co lorimetric and Amperometric Detection of Mercury (HgII,Hg0). ACS Omega, 2019,4 (2):3860–3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cao Y, Zheng M, Cai W, Wang Z: Enzyme-loaded liposome with biocatalytic precipitation for potentiometric immunoassay of thyroid-stimulating hormone in thyroid carcinoma. Chin. Chem. Lett 2019, 31: 463–467. [Google Scholar]
- [26].Nakatsuka N, Yang KA, Abendroth JM, Cheung KM, Xu X, Yang H, Zhao C, Zhu B, Rim YS, Yang Y, Weiss PS, Stojanovic MN, Andrews AN: Aptamer–field-effect transistors overcome Debye length limitations for small-molecule sensing. Science 2018, 362: 319–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Nishitani S, Sakata T: Polymeric Nanofilter Biointerface for Potentiometric Small-Biomolecule Recognition. ACS Appl. Mater. Interfaces 2019, 11: 5561–5569. [DOI] [PubMed] [Google Scholar]
- [28].Sakata T: Biologically Coupled Gate Field-Effect Transistors Meet in Vitro Diagnostics. ACS Omega 2019, 4: 11852–11862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Soldatkina OV, Kucherenko IS, Soldatkin OO, Pyeshkova1 VM, Dudchenko OY, Akata Kurç A, Dzyadevych SV: Development of electrochemical biosensors with various types of zeolites. Appl. Nanosci 2018, 9: 737–747. [Google Scholar]
- [30].Mello HJMPD, Mulato M: Enzymatically functionalized polyaniline thin films produced with one-step electrochemical immobilization and its application in glucose and urea potentiometric biosensors. Biomed. Microdev 2020, 22: 22. [DOI] [PubMed] [Google Scholar]
- [31].Ohayon D, Nikiforidis G, Savva A, Giugni A, Wustoni S, Palanisamy T, Chen X, Petruta Maria I, Di Fabrizio E, Costa PMFJ, McCulloch I, Inal S: Biofuel powered glucose detection in bodily fluids with an n-type conjugated polymer. Nature Mater 2020, 19: 456–463. [DOI] [PubMed] [Google Scholar]
- [32].Yang A, Li Y, Yang C, Fu Y, Wang N, Li L, Yan F: Fabric Organic Electrochemical Transistors for Biosensors. Adv. Mater 2018, 30: 1800051.•• The authors made very durable, inexpensive sensing fibers for a variety of analytes that could be easily woven into useable textiles.
- [33].Chen CW, Lin RZ, Chiang LC, Pan FM, Sheu JT: Junctionless gate-all-around nanowire field-effect transistors with an extended gate in biomolecule detection. Jap. J. Appl. Phys 2019, 58: 027001. [Google Scholar]
- [34].Islam AE, Martineau R, Crasto CM, Kim H, Rao RS, Maruyama B, Kim SS, Drummy LF: Graphene-Based Electrolyte-Gated Field-Effect Transistors for Potentiometrically Sensing Neuropeptide Y in Physiologically Relevant Environments. ACS Appl. Nano Mater 2020, 3: 5088–5097. [Google Scholar]
- [35].Purwidyantri A, Kamajaya L, Chen CH, Luo JD, Chiou CC, Tian YC, Lin CY, Yang CM, Lai CS: A Colloidal Nanopatterning and Downscaling of a Highly Periodic Au Nanoporous EGFET Biosensor. J. Electrochem. Soc 2018, 165: H3170–H3177.• The authors created a highly periodic nanoporous gold film on an extended gate FET that can be easily modified with oligonuecleotides to specifically detect the complimentary sequence from the bacteria of choice over a very wide linear range.
- [36].Liang Y, Guo T, Zhou L, Offenhausser A, Mayer D: Label-Free Split Aptamer Sensor for Femtomolar Detection of Dopamine by Means of Flexible Organic Electrochemical Transistors. Materials 2020, 13: 2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kajisa T, Yanagimoto Y, Saito A, Sakata T: Biocompatible Poly(catecholamine)-Film Electrode for Potentiometric Cell Sensing. ACS Sens 2018, 3: 476–483. [DOI] [PubMed] [Google Scholar]
- [38].Gu C, Kong X, Liu X, Gai P, Li F: Enzymatic Biofuel-Cell-Based Self-Powered Biosensor Integrated with DNA Amplification Strategy for Ultrasensitive Detection of Single-Nucleotide Polymorphism. Anal. Chem 2019, 91: 8697–8704.• The authors made a self-powered biosensor capable of selectively detecting a particular single nucleotide polymorphism down to 0.1pM, even in biological samples.
- [39].Gai P, Gu C, Hou T, Li F: Integration of Biofuel Cell-Based Self-Powered Biosensing and Homogeneous Electrochemical Strategy for Ultrasensitive and Easy- To-Use Bioassays of MicroRNA. ACS Appl. Mater. Interfaces 2018, 10: 9325–9331. [DOI] [PubMed] [Google Scholar]
- [40].Zhang T, Chai H, Meng F, Guo Z, Jiang Y, Miao P: DNA-Functionalized Porous Fe3O4 Nanoparticles for the Construction of Self-Powered miRNA Biosensor with Target Recycling Amplification. ACS Appl. Mater. Interfaces 2018, 10: 36796–36804. [DOI] [PubMed] [Google Scholar]
- [41].Su D, Feng B, Xu P, Zeng Q, Shan B, Song Y: Covalent organic frameworks and electron mediator-based open circuit potential biosensor for in vivo electrochemical measurements. Anal. Methods 2018, 10: 4320–4328.• The authors used covalent organic frameworks to improve the stability of enzyme and electron mediator loading for in vivo glucose detection.
- [42].Bandodkar AJ, Gutruf P, Choi J, Lee KH, Sekine Y, Reeder JT, Jeang WJ, Aranyosi AJ, Lee SP, Model JB, Ghaffari R, Su CJ, Leshock JP, Ray T, Verrillo A, Thomas K, Krishnamurthi V, Han S, Kim J, Krishnan S, Hang T, Rogers JA: Battery-free, skin-interfaced microfluidic/electronic systems for simultaneous electrochemical, colorimetric, and volumetric analysis of sweat. Sci. Adv 2019, 5: 3294.• The authors made a combination colorimetric and electrochemical wearable sensor that makes highly accurate measurements of small molecules and electrolyte concentrations in human sweat inreal time.
- [43].Gai P, Zhang S, Yu W, Li H, Li F:Light-driven self-powered biosensor for ultra sensitive organophosphate pesticide detection via integration of the conjugated polymer-sensitized CdS and enzyme inhibition strategy. J. Mater. Chem. B 2018, 6: 6842–6847. [DOI] [PubMed] [Google Scholar]
- [44].Yang A, Yan F: Flexible electrochemical biosensors for health monitoring. ACS Appl. Electron. Mater 2020, X: XXX-XXX. [Google Scholar]
- [45].Min J, Sempionatto JR, Teymourian H, Wang J, Gao W: Wearable electrochemical biosensors in North America. Biosens. Bioelectron 2021, 172: 112750. [DOI] [PubMed] [Google Scholar]
- [46].Fan R, Andrew TL: Perspective—Challenges in Developing Wearable Electrochemical Sensors for Longitudinal Health Monitoring. J. Electrochem. Soc 2020, 167 (3): 037542. [Google Scholar]
- [47].Chou JC, Yan SJ, Liao YH, Lai CH, Wu YX, Wu CY: Remote Detection for Glucose and Lactate Based on Flexible Sensor Array. IEEE Sens. J 2018, 18: 3467–3474. [Google Scholar]
- [48].Bouri M, Zuaznabar-Gardona JC, Novell M, Blondeau P, Andrade FJ: Paper-based Potentiometric Biosensor for Monitoring Galactose in Whole Blood. Electroanalysis 2020, 32: 1–10. [Google Scholar]