Abstract
Background
Biofilm formation and efflux pumps (EPs) correlation play a critical role in the pathogenicity and antibiotic resistance of Pseudomonas aeruginosa. In this study, biofilm formation and EP's collaborative role in clinical isolates of P. aeruginosa infection were investigated.
Methods
Eighty-six (86) P. aeruginosa isolates were collected from different clinical specimens and were confirmed using different biochemical tests. The formation of biofilm was investigated by using a crystal violet assay. Also, EP genes were identified by the PCR method.
Results
Based on the results, gentamicin-resistant (n = 50, 66.29%) and ciprofloxacin-resistant (n = 61, 69.66%) strains were the most frequent and colistin (n = 1, 1.12%) and ceftazidime (n = 12, 7.86%) resistant strains were the least prevalent. Furthermore, 22 isolates (31.42%) were MDR, and 11 isolates (12.35%) were XDR strains. Also, 19 isolates (22.47%) were classified as strong biofilm, 29 isolates (21.34%) as moderate biofilm, and 3 (11.23%) isolates as weak biofilm producers. The distribution of the EP genes was as follows: mexA (n = 44, 34.83%), mexB (n = 33, 32.58%), oprM (n = 59, 29.21%), oprD (n = 61, 30.33%), tetA (n = 22, 25.58%), tetR (n = 19, 22.09%), and emrE (n = 21, 24.41%). However, there was a strong significant association between biofilm formation and EPs in P. aeruginosa. Conclusions. In this study, we suggested that the presence of a multidrug resistance efflux pump, MexEF-OprN, significantly reduced P. aeruginosa pathogenicity. In contrast, the presence of the MexAB-OprM and MexCD-OprJ pumps did not affect virulence.
1. Introduction
Pseudomonas aeruginosa (P. aeruginosa) is a paradigm of an opportunistic clinical pathogen with innate resistance to many antibiotics. In humans, P. aeruginosa is mainly of great concern in severe burns, cancer, and AIDS patients, as well as those people who are immunosuppressive [1, 2]. Other essential infections caused by the organism are pneumonia, endocarditis, endophthalmitis, meningitis, septicemia, and conjunctivitis. The frequency of P. aeruginosa is high in surgical and burn wound infections [3].
In general, P. aeruginosa is naturally less susceptible than other Gram-negative bacilli to many antibiotics. Multidrug-resistant (MDR) P. aeruginosa strains are of particular concern and pose a significant clinical challenge [4, 5]. Separate MDR strains are because there is a synergy between the multidrug efflux system and low outer membrane permeability. One of the organism's crucial inherent characteristics is resistance to various antibiotics and disinfectants, mainly due to the multidrug efflux system and low outer membrane permeability [6, 7].
Efflux pumps (EPs) are membrane transporter proteins representing a significant component of the intrinsic and acquired antibiotic resistance mechanisms in P. aeruginosa [8]. This organism is intrinsically resistant to many structurally unrelated antimicrobial agents because of the low permeability of its outer membrane and the constitutive expression of various EPs with broad substrate specificity [7, 9]. However, four well-known genetically different efflux systems were also had been identified as responsible for multidrug resistance (MDR) in P. aeruginosa, namely, MexAB-DprM, MexCD-OprJ, MexEF-OprN, and MexXY-OprM. Each pump has a preferential set of antimicrobial agent substrates. The EP genes are present in all strains, but they are not expressed at high levels. However, the increased expression can result from a mutation in regulatory genes such as mexR, which controls the mexAB-OprM genes [2, 10].
Various investigations suggest a correlation between EPs and biofilm formation [6]. However, the direct effect of EPs on bacterial pathogenicity and virulence is unclear. Any defect in EP activity can impair biofilm formation. Therefore, inhibiting any of the efflux activity by inhibitors can reduce biofilm formation [11]. P. aeruginosa is an opportunistic pathogen associated with chronic infections. It is one of the leading causes of hospital- and community-acquired infections. Virulent P. aeruginosa is frequently life threatening, and the emergence of multidrug-resistant isolates often presents challenges to treat the patients. The interplay between both resistance and virulence is always considered together. The extent of adaptation of bacteria to many adverse environments is the primary concern among health-care centers. So, the analysis of these essential bacterial characteristics is crucial to management strategies. However, biofilm growth on medical devices and tissue surfaces can lead to biofilm formation and increase the risk of wound and respiratory infections [12, 13]. Also, EPs and biofilm in P. aeruginosa are essential for both clinical and environmental isolates to tolerate desiccation [11].
Therefore, this study is conducted to understand other roles and relationships of EP types with biofilm formation of different clinical isolates of P. aeruginosa in the south of Iran.
2. Materials and Methods
2.1. Study Design and Collection of Isolates
In this descriptive-analytical study, 510 different clinical specimens, including blood, urine, wound, burn wound, catheter, and abscess, were collected from patients admitted to teaching hospitals in Zahedan, Iran, from September 2018 to May 2019. The isolates were then streaked on Luria Bertani (LB) and McConkey agar plates on reaching the laboratory. Their identities were reconfirmed by Gram staining, motility testing, and biochemical reactions, essentially described by Tahmasebi et al. [1]. However, 86 P. aeruginosa isolates were collected. All ethical standards have been respected in preparation for the submitted article, No. IR.ZAMUS.REC.1396.140.
2.2. Determination of the MIC Pattern
An antibiotic susceptibility test by the Disc Diffusion Test (DDT) was carried out for all the biochemically confirmed isolates of P. aeruginosa. However, the isolates were categorized as sensitive, resistant, or intermediate to each antibiotic by measuring the respective zone of inhibition and were finally interpreted following the CLSI guidelines. The DDT is based on using the disc, as meropenem, imipenem, cefepime, ceftazidime, gentamicin, amikacin, ciprofloxacin, colistin, aztreonam, and trimethoprim/sulfamethoxazole (MAST, UK). E-test strips (Liofilchem, Italy) were used for determining colistin-resistant strains.
2.3. Screening of Biofilm Producer Strains
The capacity to form biofilms was assayed in microtiter plates virtually, and the crystal violet method (CVM) was described by Azeredo et al. [14]. In this case, P. aeruginosa ATCC 19606 was used as the positive control, and the culture medium was used as the negative control.
2.4. DNA Extraction
DNA was extracted from all the phenotypically confirmed P. aeruginosa isolates by the boiling method according to Tahmasebi et al.'s study [15].
2.5. Detection of EP Genes
Specific primers (Table 1) were used to amplify EP genes. The PCR reaction was performed in a total volume of 25 µL. Reactions were contained 1 µL of DNA template, 12 µL Master Mix (Fermentas, Waltham, Massachusetts, United States), 1 µM of each primer, and 10 µL deionized water (Sigma-Aldrich, USA). PCR assays were performed (based on Table 1) in a Bio-Rad MJ Mini thermal cycler (T100cyclerBio-Rad, Hemel Hempstead, UK) with a heated lid. On completion of the reaction, tubes with PCR products were held at 4°C. Further analysis and confirmation were carried out by performing analytical agarose gel electrophoresis (1% agarose gel for 95 min at 85 V) [16].
Table 1.
Oligonucleotide sequences used in this study and thermal cycling conditions.
| Gene | Sequence of primers | Thermal cycles | Product size (bp) | Ref |
|---|---|---|---|---|
| mexA | F: CTCGACCCGATCTACGTC | 95°C/5 min; (95°C/1 min, 56°C/30sec, 72°C/45sec) X30; 72°C/5 min | 503 | [8] |
| R: GTCTTCACCTCGACACCC | ||||
|
| ||||
| mexB | F: TGTCGAAGTTTTTCATTGAG | 95°C/5 min; (95°C/1 min, 58°C/45sec, 72°C/45sec) X35; 72°C/5 min | 280 | [8] |
| R: AAGGTCAC GGTGATGGT | ||||
|
| ||||
| oprM | F: GATCCCCGACTACCAGCGCCCCG | 95°C/5 min; (95°C/1 min, 57°C/1 min, 72°C/45sec) X30; 72°C/5 min | 247 | [8] |
| R: ATGCGGTACTGCGCCCGGAAGGC | ||||
|
| ||||
| oprD | F: ATCTACCGCACAAACGATGAG | 95°C/5 min; (95°C/1 min, 59°C/45sec, 72°C/1 min) X30; 72°C/5 min | 156 | [8] |
| R: GCCGAAGCCGATATAATCAAAC | ||||
|
| ||||
| tetA | F: AAGAATCCGCGCGTTCAATCG | 95°C/7 min; (95°C/1 min, 55°C/1 min, 72°C/50sec) X35; 72°C/10 min | 138 | [15] |
| R: GCCCGGCACCGGCATAAT | ||||
|
| ||||
| tetR | F: CCGAATGCGTATGATTCTCC | 95°C/10 min; (95°C/1 min, 57°C/30sec, 72°C/1 min) X30; 72°C/10 min | 84 | [15] |
| R: CGCTTTACTGGCACTTCAGC | ||||
|
| ||||
| emrE | F: CCGCCATGACCAACTATCTC | 95°C/5 min; (95°C/1 min, 58°C/1 min, 72°C/1 min) X30; 72°C/5 min | 249 | [6] |
| R: GCTGGCCGTAGACGAACATC | ||||
2.6. Statistical Analysis
Data were statistically analyzed using GraphPad Prism software version 8 (GraphPad Software, Inc., CA, US) and an appropriate statistical test, i.e., either Student'st-test, Wilcoxon test, chi-square test, or one-way ANOVA.
3. Results
Among 86 isolates of P. aeruginosa, 19 isolates (22.93%) from male patients and 67 isolates (72.04%) from female patients were collected. As shown in Table 2, 15 were from urine (17.44%), 21 from the wound (24.41 %), 11 from indwelling medical devices (12.79%), and 29 from the blood (33.72%). Samples were collected from patients hospitalized in the maternity unit (n = 6), pediatrics (n = 14), internal (n = 13), emergency (n = 17), neurology (n = 7), intensive care unit (ICU) (n = 15), and burns unit (n = 14).
Table 2.
The biofilm-forming capacity of P. aeruginosa and percentages of their efflux pump genes related to antibiotics.
| Biofilm | Efflux pump genes | IMI | MER | GEN | CIP | ATM | CPE | CAZ | C | AMK | TMP/SMX | MDR | XDR | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RND types | MFS types | SMR types | |||||||||||||||||
| MexA | mexB | oprM | oprD | tetA | tetR | emrE | |||||||||||||
| Strong (n = 19, 33.72%) | 12 | 19 | 17 | 19 | 11 | 8 | 7 | 11 | 9 | 19 | 16 | 7 | 6 | 9 | 1 | 16 | 7 | 14 | 9 |
| Moderate (n = 29, 33.72%) | 19 | 7 | 28 | 29 | 3 | 11 | 11 | 6 | 2 | 22 | 28 | 7 | 15 | 0 | 0 | 5 | 12 | 7 | 1 |
| Weak (n = 3, 3.48%) | 9 | 4 | 3 | 3 | 8 | 0 | 3 | 2 | 4 | 4 | 9 | 2 | 9 | 1 | 0 | 8 | 0 | 1 | 1 |
| Clinical isolates | |||||||||||||||||||
| Wound (n = 21, 24.41%) | 15 | 19 | 18 | 20 | 9 | 4 | 10 | 6 | 5 | 13 | 19 | 6 | 14 | 4 | 1 | 3 | 5 | 11 | 4 |
| Urine (n = 15, 17.44%) | 9 | 11 | 14 | 12 | 11 | 14 | 7 | 2 | 1 | 14 | 15 | 1 | 1 | 2 | 0 | 9 | 5 | 5 | 2 |
| Blood (n = 29, 33.72%) | 12 | 3 | 24 | 27 | 2 | 1 | 3 | 11 | 9 | 21 | 24 | 10 | 17 | 6 | 0 | 17 | 8 | 6 | 5 |
| Medical devices (n = 11, 12.79%) | 8 | 1 | 3 | 4 | 0 | 0 | 2 | 0 | 0 | 2 | 3 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 |
| Gender | |||||||||||||||||||
| Male (n = 19, 22.09%) | 19 | 11 | 19 | 13 | 11 | 7 | 6 | 7 | 13 | 17 | 19 | 10 | 8 | 4 | 1 | 17 | 6 | 13 | 5 |
| Female (n = 67, 77.90%) | 25 | 22 | 40 | 48 | 11 | 12 | 15 | 12 | 2 | 33 | 42 | 7 | 24 | 8 | 0 | 12 | 13 | 9 | 6 |
| Hospital sections | |||||||||||||||||||
| Maternity unit (n = 6, 6.97%) | 6 | 3 | 4 | 6 | 5 | 1 | 3 | 0 | 0 | 5 | 6 | 1 | 2 | 0 | 1 | 2 | 0 | 2 | 0 |
| Pediatrics (n = 14, 16.27%) | 3 | 1 | 11 | 11 | 2 | 3 | 1 | 1 | 2 | 3 | 10 | 2 | 4 | 0 | 0 | 2 | 0 | 1 | 0 |
| Internal unit (n = 13, 15.11%) | 6 | 1 | 5 | 9 | 3 | 1 | 1 | 1 | 1 | 1 | 7 | 1 | 3 | 1 | 0 | 1 | 2 | 1 | 1 |
| Emergency (n = 17, 8.13%) | 10 | 9 | 11 | 13 | 1 | 3 | 6 | 3 | 2 | 11 | 10 | 1 | 7 | 0 | 0 | 7 | 1 | 1 | 1 |
| ICU (n = 15, 17.44%) | 7 | 11 | 13 | 9 | 5 | 9 | 2 | 3 | 1 | 15 | 15 | 3 | 5 | 7 | 0 | 6 | 7 | 7 | 3 |
| Burns unit (n = 14, 16.27%) | 12 | 8 | 14 | 13 | 6 | 4 | 8 | 11 | 9 | 14 | 13 | 9 | 13 | 4 | 0 | 11 | 9 | 10 | 6 |
| Neurosurgery (n = 7, 8.13%) | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
IMI: imipenem; MER: meropenem; GEN: gentamycin; CIP: ciprofloxacin; ATM: aztreonam; CPE: cefepime; C: colistin; AMK: amikacin; TMP/SMX: trimethoprim/sulfamethoxazole; ICU: Intensive Care Unit.
3.1. Antibiotic Resistance Pattern
Resistance to ciprofloxacin (70.93%) and gentamicin (58.13%) was the most frequent and followed by cefepime (32.20%), amikacin (33.72%), and aztreonam (19.76%). Resistance to ceftazidime (13.95%) was the least detected among isolates. Also, 22 MDR isolates (31.42%) and 11 XDR isolates (15.71%) were reported (Figure 1).
Figure 1.
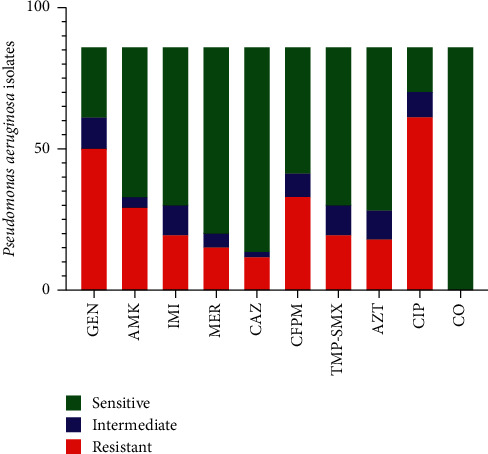
Antibiotic resistance pattern in clinical isolates of P. aeruginosa. IMI: imipenem; MER: meropenem; GEN: gentamycin; CIP: ciprofloxacin; ATM: aztreonam; CPE: cefepime; C: colistin; AMK: amikacin; TMP/SMX: trimethoprim/sulfamethoxazole.
3.2. Biofilm Production
The results for biofilm formation are shown in Table 2. In 86 of P. aeruginosa, 51 isolates (59.30%) were considered biofilm producers, and 35 isolates (40.96%) lacked biofilms. Also, 19 isolates (22.09%) were classified as strong biofilm, 29 isolates (33.72%) as moderate biofilm, and 3 (3.48%) isolates as weak biofilm producers. However, significant correlations between the biofilm formation and the prevalence of antibiotic-resistant bacteria have been observed. Decreases in antibiotic resistance prevalence in biofilm-forming strains were statistically significant except for colistin and aztreonam (p > 0.05).
3.3. Prevalence of EP Genes
According to Figure 2, 59 isolates (68.60%) were oprM and 61 isolates (64%) were oprD, followed by 44 isolates (67.44%), 33 isolates (38.37%), 22 isolates (25.58%), 21 isolates (24.41%), and 19 isolates (22.09%) detected as mexA, mexB, tetA, ermE, and tetR, respectively (Table 2) (Figure 2). However, Figure 3 and Table 3 indicates the statistical association between antibiotic resistance and EPs genes. Also, a strong relationship was observed between biofilm formation and EP genes (p ≤ 0.05). The MexA, mexB, oprM, tetA, and oprD genes were presented significantly more frequently among MDR and XDR isolates and biofilm‐forming isolates. In contrast, the tetR and emrE genes were significantly more frequent among antibiotic-sensitive isolates. There was no statistical association between some of the antibiotics, such as colistin and EP genes (p > 0.05).
Figure 2.
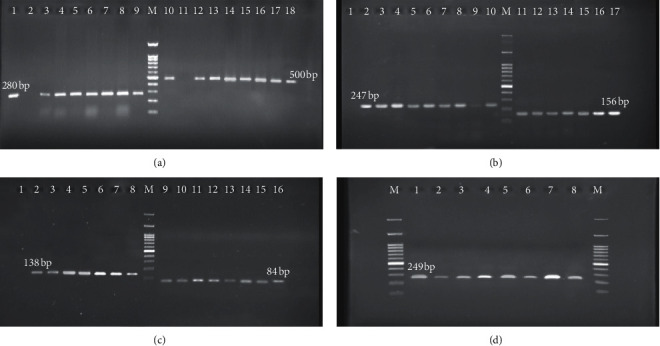
The amplification and gel electrophoresis agarose 2% of EP genes in P. aeruginosa. (a) mexA with 503 bp, mexB with 280 bp; well 1: positive control, wells 3 to 9: positive strains with mexB; well 10: positive control, and wells 11 to 18: positive strains with mexA. (b) oprM with 247 bp, oprD with 156 bp; well 2: positive control, wells 3 to 10: positive strains with oprM; well 11: positive control, and wells 12 to 18: positive strains with oprD. (c) tetA with 138 bp, tetR with 84 bp; well 1: positive control, wells 2 to 7: positive strains with tetA; well 7: positive control, and wells 8 to 14: positive strains with tetR. (d) emrE with 249 bp; well 1: positive control and wells 2 to 8: positive strains with emrE. M Ladder 100 bp.
Figure 3.
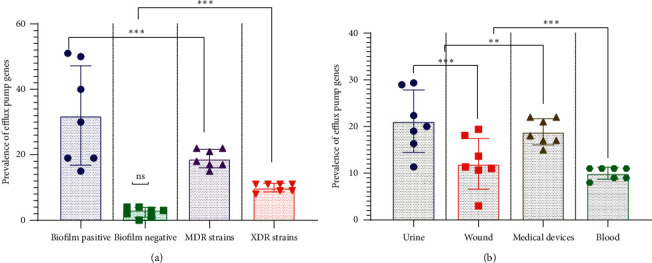
Association between efflux pump genes, antibiotic resistance, biofilm formation, and clinical samples in P. aeruginosa isolates. (a) Association between efflux pump genes, antibiotic resistance, and biofilm formation. (b) Association between efflux pump genes and clinical samples. Each dataset was analyzed using Student's t-test and the two-way ANOVA and was presented as Mean + SEM. ∗p value <0.05; ∗∗p value <0.01; ∗∗∗p value <0.001; and ∗∗∗∗∗p value <0.0001. ns: nonsense.
Table 3.
Correlation between efflux pumps, biofilm formation, separated clinical isolates, and antibiotic resistance in P. aeruginosa.
| Biofilm | Efflux pump families | ||||||
|---|---|---|---|---|---|---|---|
| RND types | MFS types | SMR types | |||||
| MexA | mexB | oprM | oprD | tetA | tetR | emrE | |
| Strong | p = 0.009 | p = 0.001 | p = 0.008 | p = 0.009 | p = 0.039 | p = 0.022 | p = 0.041 |
| Moderate | p = 0.017 | p = 0.050 | p = 0.036 | p = 0.002 | p = 0.075 | p = 0.033 | p = 0.019 |
| Weak | p = 0.043 | p = 0.003 | p = 0.032 | p = 0.017 | p = 0.015 | p = 0.048 | p = 0.028 |
|
| |||||||
| Clinical isolates | |||||||
| Wound | p = 0.040 | p = 0.029 | p = 0.016 | p = 0.048 | p = 0.066 | p = 0.043 | p = 0.051 |
| Urine | p = 0.056 | p = 0.037 | p = 0.044 | p = 0.011 | p = 0.006 | p = 0.005 | p = 0.006 |
| Blood | p = 0.250 | p = 0.089 | p = 0.014 | p = 0.097 | p = 0.25 | p = 0.1 | p = 0.068 |
| Medical devices | p = 0.058 | p = 0.060 | p = 0.084 | p = 0.12 | p = 0.09 | p = 0.049 | p = 0.17 |
|
| |||||||
| Gender | |||||||
| Male | p = 0.31 | p = 0.11 | p = 0.33 | p = 0.47 | p = 0.075 | p = 0.082 | p = 0.059 |
| Female | 0.085 | 0.020 | 0.009 | 0.005 | 0.066 | 0.043 | 0.051 |
| Hospital sections | |||||||
| Maternity unit | p = 0.049 | p = 0.016 | p = 0.055 | p = 0.005 | p = 0.009 | p = 0.045 | p = 0.043 |
| Pediatrics | p = 0.019 | p = 0.062 | p = 0.035 | p = 0.015 | p = 0.009 | p = 0.45 | p = 0.21 |
| Internal unit | p = 0.31 | p = 0.11 | p = 0.33 | p = 0.47 | p = 0.075 | p = 0.082 | p = 0.059 |
| Emergency | 0.025 | 0.015 | 0.084 | 0.001 | 0.075 | 0.033 | 0.019 |
| ICU | p = 0.002 | p = 0.001 | p = 0.072 | p = 0.050 | p = 0.039 | p = 0.044 | p = 0.011 |
| Burns unit | p = 0.019 | p = 0.020 | p = 0.048 | p = 0.053 | p = 0.036 | p = 0.050 | p = 0.018 |
| Neurosurgery | p = 0.094 | p = 0.31 | p = 0.19 | p = 0.020 | p = 0.62 | p = 0. 27 | p = 0.093 |
4. Discussion
This study showed that the ceftazidime- and colistin-susceptible strains were the most frequent, as is described in Figure 1. In the present study, 13.95% of ceftazidime-resistant strains were detected from P. aeruginosa isolates. Also, colistin-resistant strains were not detected in isolates. Dehbashi et al. and Rodulfo et al. reported similar results [1, 17]. However, according to Figure 1, a high rate of gentamycin resistance (58.13%) and ciprofloxacin resistance (70.93%) was observed in isolates. These results agree with the study conducted by Kuti et al. and Ijaz et al. [18, 19].
The ability of P. aeruginosa to resist desiccation and form biofilms allows it to survive for long periods on abiotic surfaces. In the current study, Table 2 displays that 22.47% of isolates were classified as strong biofilm and 21.34% as moderate biofilm. The MDR strains in 95% of strong biofilm isolates and 89/47% of moderate biofilm isolates were reported. Furthermore, XDR strains were detected in 82.44% of strong biofilm and 78.94% of moderate biofilm. Yang et al. examined the effects of biofilm formation on imipenem efficacy. However, they reported that imipenem efficacy was significantly reduced in aged biofilms [20]. Rahimi et al. found that biofilm-producing P. aeruginosa had comparatively higher resistance to amikacin than non-biofilm-forming strains. Also, MDR/XDR strains show a strong relationship between biofilm formation and antibiotic resistance [21].
Investigating the prevalence of RND-type, MFS-type, and SMR-type EP encoding genes, we report that these efflux systems are widely distributed in P. aeruginosa. However, the mexA, mexB, oprM, and oprD genes of RND-EP were present in 51.62%, 38.37%, 68.60%, and 70.93% tested isolates, respectively. Furthermore, the distribution of the SMR-EP gene was observed in 24.42% of P. aeruginosa. However, tetA and tetR MFS-EP genes were detected in 25.58% and 22.09% of isolates, respectively. Murugan et al. reported the same results and demonstrated that RND-EP genes were more abundant in MDR/XDR strains of P. aeruginosa than other EP genes [22].
However, the EP can also alter the pathogenicity of P. aeruginosa and play an important role in biofilm formation. As shown in Table 2, all EP families were characterized by biofilm producer strains. To our knowledge, this is the first report describing the distribution of three EP families in Iran and reporting their associated with biofilm formation in P. aeruginosa. Alav et al. [6] and Rumbo et al. [23] found that isolates with the EP gene had a greater capacity for biofilm formation than strains that lacked the gene. However, Rampioni et al. confirm the relationship between EPs and biofilm formation in P. aeruginosa; it should be noted that this association depends on many factors [24].
Based on Table 2, the strains with efflux systems were the most frequent in wound and urine specimens. Nevertheless, as shown in Table 3 and Figure 3, we found a strong association between the biofilm formation and its EP gene profile. These findings indicated that EPs play an essential role in biofilm formation and the rate of antibiotic resistance in P. aeruginosa. There was also a significant relationship between EPs' family and biofilm formation in P. aeruginosa. In agreement with these findings, Horna et al. and Shigemura et al. reported a significant relationship between the biofilm formation, EPs, and pathogenicity of P. aeruginosa isolated from wound and urine. They also stated that strains with EPs are resistant to treatment and have a more substantial role in causing urinary tract infections [25, 26]. In the current study, there was a significant relationship between EP families and biofilm formation. The frequency of RDN-EP and MFS-EP was higher in biofilm producer strains. This observation is similar to Minagawa et al.'s finding, which suggested that RND-EP plays a more important role in P. aeruginosa [27]. Soto showed that strains with EPs were more prone to biofilm formation. He stated a significant relationship between biofilm formation and EPs in Gram-negative bacteria [28].
Moreover, bacteria with EPs are more pathogenic than bacteria without EPs and play a critical role in wound and urinary tract infections. Hence, the type of clinical samples is an essential factor in this correlation [29, 30]. However, biofilm growth is associated with an increased level of mutations and quorum-sensing-regulated mechanisms. Conventional resistance mechanisms such as chromosomal β-lactamase, upregulated efflux pumps, and mutations in antibiotic target molecules in bacteria also contribute to biofilms' survival [31, 32].
Moreover, the multiple resistance to the most commonly used antibiotics is quite common in P. aeruginosa due to the possession of a high number of virulence factors [33]. A high antibiotic resistance level is attributable to multidrug efflux pumps' concerted action with a chromosomally encoded antibiotic resistance gene and the low permeability of bacterial cellular envelopes and biofilm formation phenomenon [34].
In summary, our study supports the idea of a relationship between EPs and biofilm formations. Biofilm-forming capacity in P. aeruginosa with EPs presumes a vital part in the host-pathogen communications and medical-device-related infection. Also, EPs constitute a significant threat in the clinical wound by acting as reservoirs of multidrug-resistant pathogenic bacteria. P. aeruginosa can become more resistant due to environmental conditions and different physiological states such as biofilms. It is necessary to pay attention to the slight increases in resistance observed in the clinic because this probably indicates the emergence of adaptative resistance during the wound infection and possible treatment troubles. Thus, the resistance status at arrival should ideally be controlled as a potential confounding variable for the association between resistance at the biofilm-forming strains and exposure to antimicrobial drugs. These results suggest that P. aeruginosa has a great tendency to biofilm formation in wound infections, which causes an increase in antibiotic resistance.
Acknowledgments
The authors are grateful to Zahedan University of Medical Sciences (ZAMUS) for their financial support in conducting research. This work was supported by a research grant from Zahedan University of Medical Sciences (Grant/Award No. IR.ZAMUS.REC.1396.140).
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Tahmasebi H., Yousef Alikhani M., Dehbashi S., et al. Investigation of the relationship between the presence of chromosomal and plasmid-encoded ampc genes and type of clinical specimen in pseudomonas aeruginosa. J Babol Univ Med Sci. 2018;20(3):36–43. [Google Scholar]
- 2.Azimi L., Ebrahimzadeh Namvar A., Rastegar Lari A., et al. Comparison of efflux pump involvement in antibiotic resistance among Pseudomonas aeruginosa isolates of burn and non-burn patients. Archives of Pediatric Infectious Diseases. 2016;4(3) doi: 10.5812/pedinfect.36160.e36160 [DOI] [Google Scholar]
- 3.Meskini M., Esmaeili D. The study of formulated Zoush ointment against wound infection and gene expression of virulence factors Pseudomonas aeruginosa. BMC Complementary Medicine and Therapies. 2018;18:p. 185. doi: 10.1186/s12906-018-2251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shahraki Zahedani S., Jahantigh M., Amini Y. Determining a pattern for antibiotic resistance in clinical isolations of pseudomonas aeruginosa. Tehran University Medical Journal. 2018;76(8):517–522. [Google Scholar]
- 5.Tahmasebi H., Dehbashi S., Arabestani M. Prevalence and molecular typing of colistin-resistant Pseudomonas aeruginosa (CRPA) among β-lactamase-producing isolates: a study based on high-resolution melting curve analysis method. Infection and Drug Resistance. 2020;13:2943–2955. doi: 10.2147/idr.s264796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alav I., Sutton J. M., Rahman K. M. Role of bacterial efflux pumps in biofilm formation. Journal of Antimicrobial Chemotherapy. 2018;73(8):2003–2020. doi: 10.1093/jac/dky042. [DOI] [PubMed] [Google Scholar]
- 7.Li X.-Z., Nikaido H. Efflux-Mediated drug resistance in bacteria. Drugs. 2009;69(12):1555–1623. doi: 10.2165/11317030-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su F., Wang J. Berberine inhibits the MexXY-OprM efflux pump to reverse imipenem resistance in a clinical carbapenem-resistant Pseudomonas aeruginosa isolate in a planktonic state. Experimental and Therapeutic Medicine. 2018;15(1):467–472. doi: 10.3892/etm.2017.5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arabestani M. R., Rajabpour M., Yousefi Mashouf R., Alikhani M. Y, Mousavi S. M. Expression of efflux pump MexAB-OprM and OprD of Pseudomonas aeruginosa strains isolated from clinical samples using qRT-PCR. Archives of Iranian Medicine. 2015;18(2):102–108.25644798 [PubMed] [Google Scholar]
- 10.Schindler B. D., Kaatz G. W. Multidrug efflux pumps of Gram-positive bacteria. Drug Resistance Updates. 2016;27:1–13. doi: 10.1016/j.drup.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Virginio C., Yuly L., Estela M., et al. Relationship between biofilm formation and antimicrobial resistance in gram-negative bacteria. Microbial Drug Resistance. 2019;25(1):72–79. doi: 10.1089/mdr.2018.0027. [DOI] [PubMed] [Google Scholar]
- 12.Tahmasebi H., Maleki F., Dehbashi S., et al. Role and function of KPC and MBL enzymes in increasing the pathogenicity of pseudomonas aeruginosa isolated from burn wounds. 2019;21(1):127–134. [Google Scholar]
- 13.Pallett R., Leslie L. J., Lambert P. A., et al. Anaerobiosis influences virulence properties of Pseudomonas aeruginosa cystic fibrosis isolates and the interaction with Staphylococcus aureus. Science Report. 2019;9(1):p. 6748. doi: 10.1038/s41598-019-42952-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Azeredo J., Azevedo N. F., Briandet R., et al. Critical review on biofilm methods. Critical Reviews in Microbiology. 2017;43(3):313–351. doi: 10.1080/1040841x.2016.1208146. [DOI] [PubMed] [Google Scholar]
- 15.Tahmasebi H., Dehbashi S., Alikhani M. Y., Porbaran M., Arabestani M. R. Prevalence and molecular typing of Metallo-β-lactamase-producing Pseudomonas aeruginosa with adhesion factors: a descriptive analysis of burn wounds isolates from Iran. Gene Reports. 2020;21 doi: 10.1016/j.genrep.2020.100853.100853 [DOI] [Google Scholar]
- 16.Møller T. S. B., Overgaard M., Nielsen S. S., et al. Relation between tetR and tetA expression in tetracycline resistant Escherichia coli. BMC Microbiology. 2016;16:p. 39. doi: 10.1186/s12866-016-0649-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodulfo H., Arcia A., Hernandez A., et al. Virulence factors and integrons are associated with MDR and XDR phenotypes in nosocomial strains of Pseudomonas aeruginosa in a Venezuelan university hospital. Revista do Instituto de Medicina Tropical de São Paulo. 2019;61 doi: 10.1590/s1678-9946201961020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ijaz M., Siddique A. B., Rasool M. H., Shafique M. Frequency of multi drug resistant Pseudomonas aeruginosa in different wound types of hospitalized patients. Pakistan Journal of Pharmaceutical Sciences. 2019;32(2):865–870. [PubMed] [Google Scholar]
- 19.Kuti J. L., Wang Q., Chen H., Li H., Wang H., Nicolau D. P. Defining the potency of amikacin against Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter baumannii derived from Chinese hospitals using CLSI and inhalation-based breakpoints. Infection and Drug Resistance. 2018;11:783–790. doi: 10.2147/idr.s161636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang C.-H., Su P.-W., Moi S.-H., Chuang L.-Y. biofilm formation in acinetobacter baumannii: genotype-phenotype correlation. Molecules. 2019;24(10):p. 1849. doi: 10.3390/molecules24101849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rahimi S., Farshadzadeh Z., Taheri B., et al. The relationship between antibiotic resistance phenotypes and biofilm formation capacity in clinical isolates of acinetobacter baumannii. Jundishapur Journal Microbiology. 2018;11(8) doi: 10.5812/jjm.74315.e74315 [DOI] [Google Scholar]
- 22.Murugan N., Malathi J., Therese K. L., Madhavan H. N. Application of six multiplex PCR’s among 200 clinical isolates of Pseudomonas aeruginosa for the detection of 20 drug resistance encoding genes. The Kaohsiung Journal of Medical Sciences. 2018;34(2):79–88. doi: 10.1016/j.kjms.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 23.Rumbo C., Gato E., López M., et al. Contribution of efflux pumps, porins, and β-lactamases to multidrug resistance in clinical isolates of acinetobacter baumannii. Antimicrobial Agents and Chemotherapy. 2013;57(11):5247–5257. doi: 10.1128/aac.00730-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rampioni G., Pillai C. R., Longo F., et al. Effect of efflux pump inhibition on Pseudomonas aeruginosa transcriptome and virulence. Science Report. 2017;7(1):p. 11392. doi: 10.1038/s41598-017-11892-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shigemura K., Osawa K., Kato A., et al. Association of overexpression of efflux pump genes with antibiotic resistance in Pseudomonas aeruginosa strains clinically isolated from urinary tract infection patients. The Journal of Antibiotics. 2015;68(9):568–572. doi: 10.1038/ja.2015.34. [DOI] [PubMed] [Google Scholar]
- 26.Horna G., Amaro C., Palacios A., et al. High frequency of the exoU+/exoS+ genotype associated with multidrug-resistant “high-risk clones” of Pseudomonas aeruginosa clinical isolates from Peruvian hospitals. Science Report. 2019;9(1):p. 10874. doi: 10.1038/s41598-019-47303-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Minagawa S., Inami H., Kato T., et al. RND type efflux pump system MexAB-OprM of pseudomonas aeruginosa selects bacterial languages, 3-oxo-acyl-homoserine lactones, for cell-to-cell communication. BMC Microbiology. 2012;12(1):p. 70. doi: 10.1186/1471-2180-12-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soto S. M. Role of efflux pumps in the antibiotic resistance of bacteria embedded in a biofilm. Virulence. 2013;4(3):223–229. doi: 10.4161/viru.23724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tahmasebi H., Dehbashi S., Arabestani M. R. Co-harboring of mcr-1 and β-lactamase genes in Pseudomonas aeruginosa by high-resolution melting curve analysis (HRMA): molecular typing of superbug strains in bloodstream infections (BSI) Infection, Genetics and Evolution. 2020;85 doi: 10.1016/j.meegid.2020.104518.104518 [DOI] [PubMed] [Google Scholar]
- 30.Pérez A., Gato E., Pérez-Llarena J., et al. High incidence of MDR and XDR Pseudomonas aeruginosa isolates obtained from patients with ventilator-associated pneumonia in Greece, Italy and Spain as part of the MagicBullet clinical trial. Journal of Antimicrobial Chemotherapy. 2019;74(5):1244–1252. doi: 10.1093/jac/dkz030. [DOI] [PubMed] [Google Scholar]
- 31.Kiamco M. M., Atci E., Mohamed A., et al. Hyperosmotic agents and antibiotics affect dissolved oxygen and pH concentration gradients in Staphylococcus aureus biofilms. Applied Environment Microbiology. 2017;83(6) doi: 10.1128/aem.02783-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen B. E. Functional linkage between genes that regulate osmotic stress responses and multidrug resistance transporters: challenges and opportunities for antibiotic discovery. Antimicrobial Agents and Chemotherapy. 2014;58(2):640–646. doi: 10.1128/aac.02095-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malgaonkar A., Nair M. Quorum sensing in Pseudomonas aeruginosa mediated by RhlR is regulated by a small RNA PhrD. Science Report. 2019;9(1):p. 432. doi: 10.1038/s41598-018-36488-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tintino S. R., Souza V. C. A. d., Silva J. M. A. d., et al. Effect of vitamin K3 inhibiting the function of NorA efflux pump and its gene expression on Staphylococcus aureus. Membranes. 2020;10(6):p. 130. doi: 10.3390/membranes10060130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


