Abstract
Dirofilaria immitis, also known as heartworm, is a major parasitic threat for dogs and cats around the world. Because of its impact on the health and welfare of companion animals, heartworm disease is of huge veterinary and economic importance especially in North America, Europe, Asia and Australia. Within the animal health market many different heartworm preventive products are available, all of which contain active components of the same drug class, the macrocyclic lactones. In addition to compliance issues, such as under-dosing or irregular treatment intervals, the occurrence of drug-resistant heartworms within the populations in the Mississippi River areas adds to the failure of preventive treatments. The objective of this review is to provide an overview of the disease, summarize the current disease control measures and highlight potential new avenues and best practices for treatment and prevention.
Keywords: Heartworm disease, Dirofilaria immitis, Macrocyclic lactones, Mechanism of action, Anthelmintic resistance, Animal health
Graphical abstract
1. Introduction
Companion animals, specifically dogs and cats, are hosts to a variety of external and internal parasites (Selzer and Epe, 2021). One of the most important endoparasites in companion animal health, from both pathologic and an economic perspective, is Dirofilaria immitis, the filarial nematode parasite that causes heartworm disease. In dogs, the disease is caused by young adult and adult parasites provoking pathology in the pulmonary arteries. Canines act as the definitive host, so sexual reproduction occurs in the pulmonary arteries and microfilariae are released into the circulatory system (McCall et al., 2008b; Bowman and Atkins, 2009; Selzer and Epe, 2021). D. immitis infections also occur in cats, and the disease is usually more severe in this atypical host. In cats, severe disease, or even death, can be caused by just a few developing immature or adult filariae. The adult worms are often shorter than those found in dogs and rarely produce microfilariae (Venco et al., 2015). The life cycle of D. immitis is similar to the pathogenic filarial parasites of humans, Onchocerca volvulus, Brugia malayi, and Wuchereria bancrofti, in that they are all transmitted by arthropod vectors (Tahir et al., 2019). D. immitis is distributed across the globe, being endemic in countries on six continents (Simón et al., 2012). From an animal health perspective, dogs and to a lesser extend cats are the most important animals amongst the numerous mammalian hosts that can be infected by D. immitis (Simón et al., 2009; Moroni et al., 2020; Selzer and Epe, 2021). Thus, this review will focus primarily on heartworm disease in dogs and include relevant background on cats where appropriate. Other filarial parasite species have also been reported in dogs including Acanthocheilonema reconditum, Cercopithifilaria bainae, and Onchocerca lupi. Among these, O. lupi is of clinical significance, and has been reported not only in dogs, but also in cats and humans in North America (Dantas-Torres and Otranto, 2020).
Treatment of an established D. immitis infection in dogs requires a prolonged regimen of drug treatment, exercise restriction and sometimes even surgery (Bowman and Atkins, 2009; Carretón et al., 2019). Therefore, the current practice is to control heartworm disease through prevention, which currently utilizes a single class of drugs, the macrocyclic lactones (MLs), including e.g. ivermectin, milbemycin oxime and moxidectin (Wolstenholme et al., 2016). Ivermectin is considered an essential medicine by the World Health Organization (World Health Organization, 2019) based on its efficacy against human filarial parasites. The drug has been successfully applied in the Americas (Lakwo et al., 2020) and has led to the elimination of onchocerciasis in four out of six endemic countries (Sauerbrey et al., 2018). Ivermectin has and is still contributing substantially in mass drug administration campaigns in Africa to control human pathogenic filarial parasites (Kim et al., 2015; Lakwo et al., 2020). However, successful elimination of onchocerciosis might be endangered by ivermectin-resistant O. volvolus populations (Osei-Atweneboana et al., 2011; Nana-Djeunga et al., 2014). Similarly, successful prevention of heartworm disease in dogs may be compromised by ML-resistant D. immitis (Hampshire, 2005; Bourguinat et al., 2015).
In this review, we give an overview on heartworm diseases, outline the industry's perspective on its control, the currently available treatments, their potential mode of action and the issues involved that lead to selection of resistance. In addition to the general distribution of D. immitis, reasons for both the increasing and decreasing prevalence in certain geographic areas will be discussed. Advances in the diagnostic capabilities, as well as the potential for the discovery of novel treatments based on new technology platforms will also be addressed.
As a note for the reader and in accordance with the World Association for the Advancement of Veterinary Parasitology (WAAVP, https://www.waavp.org) we used the term “dirofilariosis” (Kassai et al., 1988). However, in many publications the disease is also referred to as “dirofilariasis” or occasionally also as “dirofilarosis”, which is important to consider when reviewing the literature (Ashford, 2001; Kassai, 2006).
2. Heartworm biology
2.1. Taxonomy and lifecycle
Within the phylum of Nematoda, Dirofilaria immitis (Fig. 1, Fig. 2) belongs to the superfamily Filarioidea, commonly known as filarial parasites or filariae. The definite host of the filarial parasites are always vertebrates and the intermediate hosts are arthropods, often mosquitos. The Filarioidea are grouped into three families, the Filariidae, Setariidae, and Onchocercidae. The Onchocercidae include the human pathogenic species O. volvulus, causing onchocerciosis also known as river blindness, as well as W. bancrofti and B. malayi, both causative agents of lymphatic filariosis (Deplazes et al., 2016; Mehlhorn, 2016). The Onchocercidae also contain D. immitis and Dirofilaria repens, both causing dirofilariosis in carnivores, a major global threat to companion animal health and welfare (Bowman and Atkins, 2009; Capelli et al., 2018). Other animal pathogenic species of the Filarioidea are Parafilaria multipapillosa (Filariidae) causing hemorrhagic subcutaneous nodules – equine summer bleeding – and Setaria digitata (Setariidae), a pathogenic parasite of cattle and water buffalo in Asia (Deplazes et al., 2016; Mehlhorn, 2016). The larval stage of S. digitata can cause fatal cerebrospinal nematodosis in goats, sheep and horses (Perumal et al., 2016) and infections in humans can cause abscesses, allergic reactions, enlarged lymph nodes, eye lesions, and lung inflammation (Rodrigo et al., 2014).
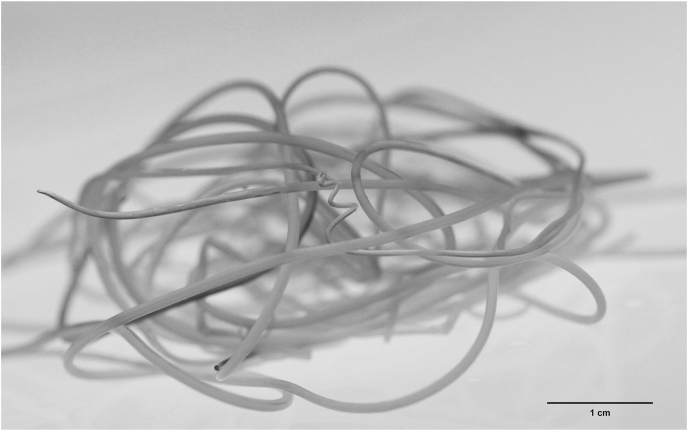
Fig. 1.
Adult D. immitis parasite. Photograph of a mass of adult D. immitis worms removed from a dog at necropsy. Most are female worms, but the coiled posterior end of a male worm can be seen in the middle of the mass.
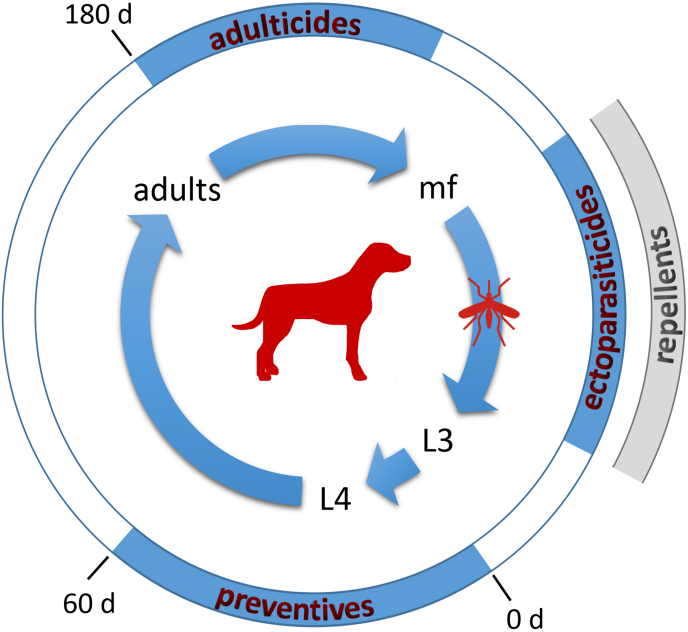
Fig. 2.
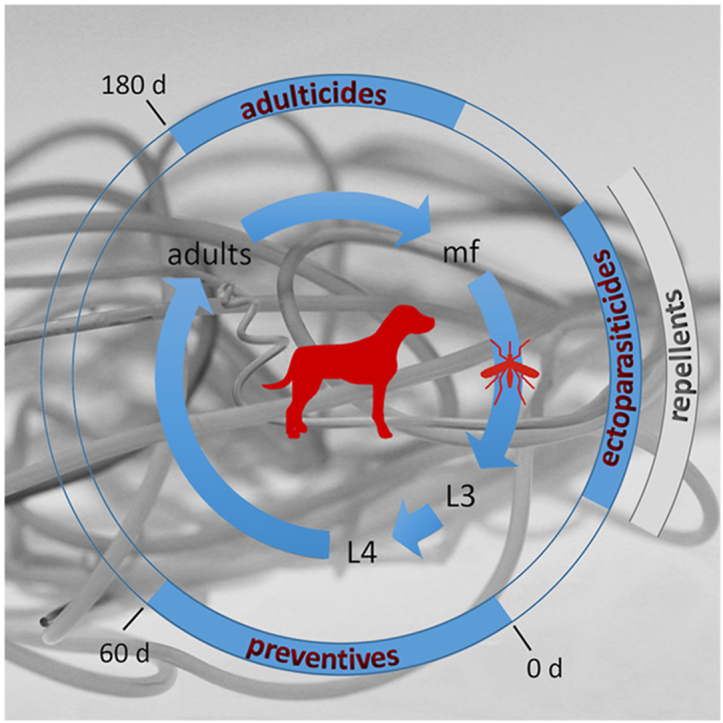
Dirofilaria immitis life cycle and chemical intervention periods. The inner circle represents the life cycle of D. immitis within the mammalian host (dog) and the vector (mosquito). The length of the arrows approximately reflects the development time of each stage. During a blood meal on an infected dog, microfilariae (mf) circulating in the blood are ingested by a mosquito. In the vector they develop into the larval (L) stages from L1 to infective L3, which can be transmitted during another blood meal to a mammalian host. Within the host they develop rapidly into L4 and finally to adult female or male parasites, which reside and mate in the heart (vascular and cardio pulmonary system, right heart chamber). The outer circle shows prevention and treatment options depending on the stage of development of the parasite. MLs are used as preventive treatment up to 60 days (d) post infection (0 d) against D. immitis L3 and L4 larval stages. After that period, efficacy of the MLs is no longer 100% (Bowman and Drake, 2017). Melarsomine, the only registered heartworm adulticide, is efficacious against adult D. immitis which can be diagnosed around 180 days post infection. Melarsomine administered in 2 doses 24 h apart also has substantial efficacy against juveniles, as shown in controlled studies (2017). However, melarsomine is not recommended to be used as a preventive or prior to definitive diagnosis of heartworm infection, but only for treatment of adult heartworms (https://capcvet.org/guidelines/heartworm/accessed November 27th, 2020). Ectoparasiticides or repellents can be used to prevent mosquitos from feeding on dogs and cats, reducing the potential for infection.
Like the majority of filariae, D. immitis has no free-living stages and exhibits a complex lifecycle involving multiple developmental stages in both, the definitive mammalian host and the mosquito vector (Fig. 2). The adult worms (macrofilariae) live as obligate endoparasites mainly in the lobar arteries and main pulmonary artery of the canine hosts. In dogs with high worm burden, adult D. immitis can also be found in the right ventricle. Females measure up to 30 cm and males around 18 cm. Females are ovoviviparous; they release sheathless microfilariae in the blood which are 250–300 μm in size. Various mosquito species can acquire the D. immitis microfilarial stage in a bloodmeal from an infected host. Once ingested by a mosquito, the microfilariae migrate within hours from the midgut to the Malpighian tubules where they will morphologically change into various “sausage” forms representing first stage larvae (Sawyer and Weinstein, 1963, Shang Kuan and Prichard, 2020). However, reports for D. immitis described these changes using in vitro cultivated microfilariae which were unable to develop in those systems beyond the L1 stage (Kuan and Prichard, 2020). Nevertheless, the principle development stages of D. immitis within the mosquitos are confirmed by observations on the related B. malayi (Erickson et al., 2009). Filariae harvested from infected mosquitos at various time intervals could be distinctly differentiated into microfilariae, which migrate and develop within hours into intracellular L1 stages. These shorter and non-feeding L1 stages undergo a first molt resulting in intracellular remaining L2 stages. They molt for a second time to become finally infective B. malayi L3 stages (Erickson et al., 2009), which can be transmitted to another vertebrate host. Development of D. immitis and migration in the canine host, as well as the host immune response, are largely uncharacterized until adults appear in the pulmonary arteries approximately 6 months after infection (Bowman and Atkins, 2009; Simón et al., 2012; Deplazes et al., 2016; Mehlhorn, 2016).
Cats are less suitable hosts for D. immitis than dogs (McCall et al., 2008b) and most worms in cats do not develop to the adult stage. Those cats with adult D. immitis usually harbor 1–3 worms only. These worms are also smaller than those found in dogs and they rarely produce microfilariae (Simón et al., 2012; Venco et al., 2015). The fact that cats rarely have infections that produce microfilariae also complicates diagnosis (Bowman et al., 2002), and therefore, cats are infected but remain often undiagnosed (American Heartworm Society Heartworm Basics https://www.heartwormsociety.org/pet-owner-resources/heartworm-basics accessed November 25th, 2020).
2.2. The disease
Young adult and adult D. immitis heartworms cause disease of dogs, initially a vascular disease that can progress to impaired blood flow, eventually affecting the vascular and pulmonary system, and in severe cases the right heart chambers. First symptoms include a mild persistent cough and reluctance to exercise. Dogs may manifest decreased appetite and become exercise intolerant. Eventually, the damage to the pulmonary endothelium and vascular occlusion from worm death will reduce cardiac output. The resulting pulmonary hypertension may lead to compensatory right-side heart enlargement and progress to right heart failure. A less common sequelae occurs when a sudden obstruction of blood flow through the lungs caused by large numbers of adults in the pulmonary arteries, reduces the flow to the point where worms migrate and become aberrantly located in the right atrium, ventricle and often in the vena cava (Fig. 3). This form of blockage is called caval syndrome and will cause a life-threatening form of heart failure (Bowman and Atkins, 2009; Simón et al., 2012; Ames and Atkins, 2020).
Fig. 3.
Necropsy view of dog euthanized due to caval syndrome. D. immitis are indicated by arrows and clearly visible in the incised anterior vena cava (+). The lung lobe (*) is reflected back to the upper left, the trachea (x) is visible above the vena cava, and the pericardium and heart (#) are in the lower left of the image.
Cats are much less immunologically tolerant of heartworm infections, and as a result manifest clinical signs different from dogs. In cats, even the death of immature D. immitis in the pulmonary arteries can cause severe, pulmonary symptoms (Atkins and Litster, 2006). This condition has been named heartworm associated respiratory disease (HARD). Clinical signs manifest as chronic coughing, labored breathing, vomiting and even sudden death, with no other apparent clinical signs. It has been experimentally verified that as early as 70-90-day-old D. immitis infections resulted in induced severe pulmonary airway, interstitial, and arterial lung lesions (Dillon et al., 2017). In contrast to dogs, cats have pulmonary intravascular macrophages, which can be modulated by parasite products and may contribute to the different outcome of the disease in cats (Dillon et al., 2008). Many of these symptoms mimic those of asthma in cats, requiring differential diagnosis because treatment and prognosis of these two diseases is different (Garrity et al., 2019).
Dirofilaria immitis has zoonotic potential and thus could be considered a Public Health issue. Humans can be accidently infected with D. immitis, and in many cases, these infections will progress, developing through the tissue stages, and reaching the pulmonary vasculature. In the pulmonary vasculature of humans the young adult worms will die, resulting in development of pulmonary nodules, but will remain asymptomatic. Thirty-three cases of such pulmonary dirofilariosis were reported in Europe (Simón et al., 2012). However, the exposure of humans to D. immitis is likely to be higher, as analysis of 250 serum samples from northern Spain for specific IgG antibodies against D. immitis revealed a seroprevalence of 11.6% (Morchón et al., 2010). Similarly, 6.1% of 668 investigated people in Portugal were found to be seropositive for D. immitis (Fontes-Sousa et al., 2019). Although D. immitis infections in humans are typically asymptomatic, they can cause great concern as radiographically, the resulting coin lesions are indistinguishable from lung cancer (Simón et al., 2012).
2.3. Vectors
Heartworm abundance and distribution are closely linked with mosquito vector biology and ecology. Any mosquito species that allows for the successful development of microfilariae into the infective L3 stage and subsequent migration to the proboscis can be a competent vector of D. immitis (Ledesma and Harrington, 2011). Over 60 species of mosquitoes are capable of supporting the development of L3 D. immitis (Ludlam et al., 1970). More than 20 different species were detected with infective L3 in various field studies (Scoles, 1998; Ward, 2005; Bowman and Atkins, 2009; Ledesma and Harrington, 2011). Nine species have been identified as major potential vectors, all with well-known developmental and biological parameters allowing for the development of national forecast models for canine heartworm risk (Brown et al., 2012). These species are Aedes aegypti, Ae. albopictus, Ae. canadensis, Ae. sierrensis, Ae. trivitattus, Ae. vexans, Anopheles punctipennis, A. quadrimaculatus, and Culex quinquefasciatus. Mosquito interspecies competition, which depends on various factors such as humidity, vegetation, and urbanization, can lead to a distribution of transmission competent species. Vector and environmental maps have been investigated for the occurrence of these species, suggesting that presence of C. quinquefasciatus, Ae. sierrensis, A. punctipennis, A. quadrimaculatus, or Ae. canadensis is associated with higher heartworm prevalence while other mosquito species are estimated to decrease prevalence rates (Wang et al., 2014). Like many filariae, D. immitis microfilariae exhibit a circadian rhythm, which will have an impact on the vector capacity and thus, on the epidemiology of heartworm, because chance for transmission of parasites increases with higher overlap of the mosquito activity pattern and microfilaremia peak in the host (Ionică et al., 2017; Evans et al., 2017a).
In Europe, several species of mosquitoes have been found infected with D. immitis including C. pipiens in Italy (Cancrini et al., 2006), Spain (Morchón et al., 2007), and Turkey (Yildirim et al., 2011); C. theileri on the island of Madeira, Portugal (Santa-Ana et al., 2006), and on the Canary Islands, Spain (Morchón et al., 2012); Ae. vexans in Turkey (Yildirim et al., 2011) and Ae. albopictus, A. caspius, A. maculipennis, and Coquillettidia richiardii in Italy (Cancrini et al., 2003, 2006). More recently, Montarsi et al. (2015) identified Ae. (Finlaya) koreicus as a new vector for D. immitis in Europe. In most areas of Europe, the activity of these species is limited to the period of time between spring and summer. An uptick of D. immitis infections in dogs has been projected into currently heartworm-free areas, as has already been observed for D. repens. This is possibly due to climatic changes favourable for survival and spread of D. immitis in mosquito vectors (Genchi et al., 2011).
While many mosquito species can transmit D. immitis, in Australia the primary vector is Ae. notoscriptus (Russell and Geary, 1996). In Japan, C. pipiens pallens and C. tritaeniorhynchus are the major vectors for Dirofilaria transmission. In all, at least 16 mosquito species are known to play a role in heartworm transmission in Japan (Akao, 2011). The primary vector species in Brazil are Ae. taeniorhynchus (73.9%), Ae. scapularis (20%) and C. quinquefasciatus (2.5%). However, it was suggested that in contrast to the analysis performed for the USA (Wang et al., 2014), the composition of the mosquito population in the investigated area is not such a critical factor in the distribution of heartworm infections in South America (Labarthe and Guerrero, 2005).
2.4. Wolbachia
Most filarial species harbor and depend on bacterial endosymbionts. This is also the case for D. immitis (Sironi et al., 1995; Bandi et al., 1999) and D. repens (Grandi et al., 2008) as both rely on the rickettsia-like endosymbiont Wolbachia for embryogenesis, development and survival (Ferri et al., 2011; Taylor et al., 2013). Genome analysis from Brugia malayi and their Wolbachia endosymbiont population showed that the Wolbachia genome encodes genes sufficient for the biosynthetic pathway of purines and pyrimidines, heme and riboflavin, none of which are completely encoded in the Brugia genome (Foster et al., 2005; Ghedin et al., 2007). Those findings were confirmed by genome analysis of the Wolbachia population of D. immitis. These Wolbachia also encode enzymes that are missing in the D. immitis genome like biosynthesis of heme, purine and pyrimidines. Additionally, the Wolbachia population from B. malayi contains genes necessary for folate synthesis (Godel et al., 2012). The essential metabolic contributions of Wolbachia renders them a valid drug target to control filariae (Slatko et al., 2010; McCall et al., 2014b; Landmann, 2019; Turner et al., 2020).
3. Prevalence and diagnosis
3.1. Prevalence
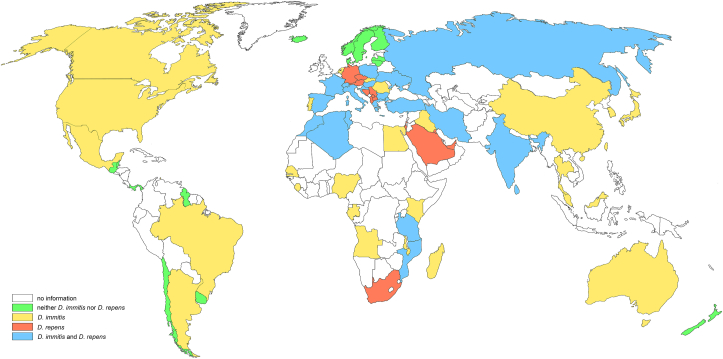
Dirofilaria immitis infections in dogs and cats have been identified throughout the world in tropical and temperate regions (Simón et al., 2012; Genchi and Kramer, 2019) while the occurrence of D. repens is restricted to the Old World (Fig. 4). A few anecdotal reports suggest the presence of D. repens in Mexico and Chile as well (López et al., 2012; Ramos-Lopez et al., 2016). Two opposing phenomena currently influence the prevalence and spreading of dirofilariosis. The prevalence of Dirofilaria infections appears to be increasing worldwide mainly due to climate changes and the accompanying spread of competent mosquito species such as Ae. albopictus and Ae. koreicus (Montarsi et al., 2015). In contrast, heartworm prevalence is decreasing in some regions including Japan (Oi et al., 2014) and Northern Italy, likely due to a higher awareness and intensified control of the disease (Genchi and Kramer, 2019; Mendoza-Roldan et al., 2020). The latter observation may indicate that the distribution and thus the risk of heartworm infection could be reduced by a higher awareness of veterinary practitioners and a concomitant increase in preventive treatment of dogs.
Fig. 4.
Presence of D. immitis and D. repens infections throughout the world. Analyzing the number of dogs at risk for Dirofilaria infection, in Asia approx. 148 million dogs are at risk, in Latin America and Europe approx. 98 million dogs each, in North America approx. 80 million, in Africa approx. 50 million, and in Oceania approx. 6 million (López et al., 2012, Simón et al., 2012, Ramos-Lopez et al., 2016, Genchi and Kramer, 2019; Boehringer Ingelheim internal analysis).
However, a decreasing heartworm prevalence, particularly in the Mediterranean, may also be due to the overall reduction of the mosquito population. The general correlation of mosquito abundance and risk of disease transmission is well established for malaria (Kitron and Spielman, 1989) and it was demonstrated that depending on local conditions for mosquito populations, there is less or more risk of malaria transmission (Parham and Michael, 2010). Efforts to eliminate malaria in Europe and the Mediterranean date back as far as the late 19th century with a widespread achievement of elimination in the 20th century (Feachem et al., 2010). Today, an additional important effect is the ongoing reduction of insect populations due to industrialization, urbanization and application of insecticides (Goulson et al., 2015). Thus, heartworm prevalence may continue to decline in some endemic areas because of reduction of vector populations in these regions. In contrast, climate changes will lead to a northward spread of mosquito vectors, leading to higher disease risk in presently non-endemic areas (Medlock et al., 2012; Cella et al., 2019; Hertig, 2019). This forecast spread of Anopheles spp. and as a result malaria could also be applicable to the prevalence of dirofilariosis, assuming the mosquito migration includes species which are competent intermediate hosts for Dirofilaria spp.
The prevalence of D. immitis in cats is estimated to be 5–20 fold lower than in dogs (Montoya-Alonso et al., 2017; Garrity et al., 2019). In a heartworm endemic area in northern Italy, a prevalence in dogs of 29% and in cats of 4.7% was shown (Venco et al., 2011). A similar ratio of prevalence rates was detected in central Italy with 5.6% in dogs and 1.6% in cats (Traversa et al., 2010).
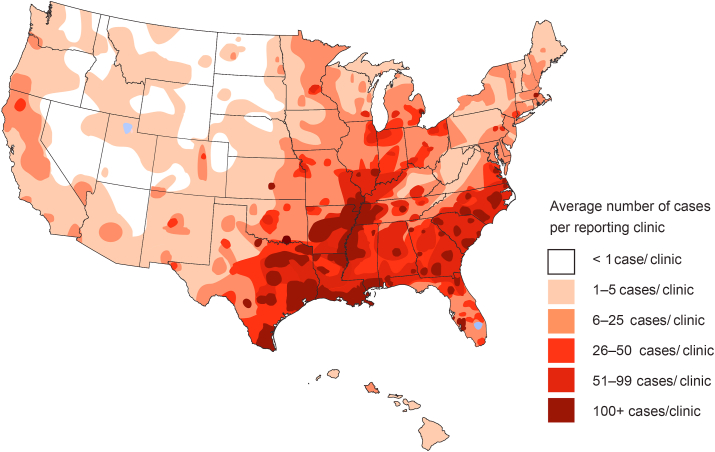
In dogs, D. immitis is prevalent in all the Americas with a few exceptions such as Chile where no heartworm cases were found in surveys (Labarthe and Guerrero, 2005; Simón et al., 2012; Maggi and Krämer, 2019; Dantas-Torres and Otranto, 2020). In the USA, mean prevalence rates are generally between 1% and 12% (Lee et al., 2010; Little et al., 2014, 2021), but can be locally quite high. Florida, the most southeastern state in the USA, exhibits a 28% prevalence rate (Hays et al., 2020), and rates as high as 48% were observed in the Gulf Coast regions (McCall et al., 2008b; Bowman et al., 2009). These reports have been confirmed by more recent surveys of the American Heartworm Society (https://www.heartwormsociety.org/in-the-news/558-ahs-announces-findings-of-2019-heartworm-incidence-survey, accessed November 25th, 2020), which revealed that the top five states in heartworm incidence in 2019 were in the southeast (Mississippi, Louisiana, South Carolina, Arkansas and Alabama) (Fig. 5), similar to the report of Little et al. (2021), which showed highest incidence in Mississippi, Louisiana, Arkansas, Alabama, and Texas. The general distribution has not changed compared to 2016. No state is free of D. immitis infections (Little et al., 2021). Prevalence rates are much lower in regions with colder climates. In Canada, for example, a study conducted in 1993 determined the prevalence as 0.24% (Slocombe and Villeneuve, 1993). A more recent study revealed a prevalence of 3.9% in shelter dogs in Ontario, Canada (Jacobson et al., 2020). Climate change may also drive an increase in heartworm prevalence in previously preclusive cold regions (Bowman et al., 2016).
Fig. 5.
2019 heartworm incidence survey, American heartworm society. Heartworm incidence as shown in this map is based on the average number of cases per reporting clinic in 2019. Some remote regions of the United States lack veterinary clinics; therefore, no cases were reported from these areas. Used with permission from the American Heartworm Society https://www.heartwormsociety.org/.
No detailed European surveys on the distribution of D. immitis have been reported, but surveys conducted at national or regional level are available. A few surveys have also focused on D. repens in Europe due to the zoonotic potential in humans, even though the infection in dogs is often asymptomatic (Sałamatin et al., 2013; Moskvina and Ermolenko, 2018). Furthermore, a substantial decrease of D. immitis infections has been observed in some endemic areas such as Northern Italy and the Canary Islands (Spain) (Genchi and Kramer, 2019). In contrast to the reduction of prevalence in those areas, increased transmission in Central and Northern Europe has been observed and may be attributed to climate changes (Simón et al., 2012; Capelli et al., 2018; Széll et al., 2020). Even in areas as far north as Finland, Estonia and Siberia, autochthonous cases have been reported (Jokelainen et al., 2016; Pietikäinen et al., 2017; Genchi and Kramer, 2019). An additional factor contributing to the spread of dirofilariosis is the movement of positive dogs from endemic countries to formerly heartworm-free countries like Germany (Genchi et al., 2014). In addition, increasing occurrence and climate-change driven spread of reservoir hosts in wildlife, e.g. the golden jackal (Canis aureus), seem to play a significant role, too (Széll et al., 2020). A special case appears to be Austria, where only recently the introduction of D. repens was confirmed in mammals and in the mosquito vectors A. algeriensis and A. maculipennis (Fuehrer et al., 2016). Most cases of dirofilariosis were imported cases, but climate analysis indicates that D. immitis also has the capacity to establish itself in the lowland regions of Austria, given that the host and a number of competent culicid vectors are present (Fuehrer et al., 2016).
In Australia, D. immitis has been reported in all states with historical prevalence up to 100% in the Northern Territory (Welch et al., 1979). Today, prevalence is considered to be low throughout Australia (Nguyen et al., 2016). Dirofilariosis is also present in the near and far East and in Asia. In China prevalence ranges from 2% to 15% (Liu et al., 2013). Interestingly, a novel Dirofilaria species, Candidatus D. hongkongensis, was identified in Hong Kong (Yilmaz et al., 2016, 2019) which also occurs in India (Pradeep et al., 2019). In Japan, heartworm prevalence decreased within a decade by about half in shelter dogs, from 46% in 1999–2001 to 23% in 2009–2011 (Oi et al., 2014). Notably, no D. immitis was detected in Israel while it was observed in other Middle East countries (Genchi and Kramer, 2019). Information on the prevalence of dirofilariosis in Africa is limited (McCall et al., 2008b). However, reports on the presence of D. immitis and D. repens are increasing in recent years. Dirofilariosis has been observed in Tunisia, Algeria, Tanzania and Mozambique, although the more dominant filarial species in dogs appears to be Acanthocheilonema dracunculoides (Genchi and Kramer, 2019).
There are more than 470 million dogs and 370 million cats worldwide (https://www.statista.com/statistics/1044386/dog-and-cat-pet-population-worldwide/accessed October 10th, 2020), and the populations continue growing. More than 200 million dogs live in North America, Europe, Australia and Japan where prophylaxis and treatment probability are expected to be high (Boehringer Ingelheim internal analysis), making heartworm prevention a highly attractive market segment.
3.2. Diagnosis
The Companion Animal Parasite Council (CAPC, https://capcvet.org/) and the American Heartworm Society (https://heartwormsociety.org) recommend that all dogs, including those on heartworm prevention, should be tested annually using both antigen and microfilarial tests. Different parasitological, serological or molecular tests are available to detect different life stages of heartworm taking into account that not all life stages may be present in an infected dog at any given time point (Little et al., 2018). Microscopic diagnosis for microfilariae can be performed utilizing direct blood smears or from concentrated blood (modified Knott's test) and enables differential diagnosis to other filariae, particularly Acanthocheilonema spp and D. repens (Magnis et al., 2013). However, this technique may not be suitable for mixed filarial infections, and is, compared to other diagnostic tests, rather insensitive, and a test should not be defined as negative unless at least 1 ml of blood has been investigated (American Heartworm Society, 2020; Panarese et al., 2020).
The knowledge of any circadian rhythm can have an impact on diagnostic reliability when diagnosis is based on identification of microfilariae. However, D. immitis microfilaremia rhythm does not show a clear pattern. While in Romania microfilaremia peaks during night (Ionică et al., 2017), another study could not detect any consistent pattern even under standardized environmental conditions (Lovis et al., 2017). In a smaller study with experimentally infected dogs, a 24-h periodicity that decreased in magnitude as the dog and infection aged was apparent, adding the age of the infection(s) as another factor contributing to the complexity of interpreting potential circadian rhythms (Evans et al., 2017a).
Several serological test based on enzyme-linked immunosorbent assays (ELISA) or immunochromatographic (ICT) tests have been developed for the detection of D. immitis adult antigens for in-clinic use (Barr et al., 2011; Lee et al., 2011; Henry et al., 2018; Little et al., 2018). The available highly specific antigen tests (98%–100%) detect only mature infections about 7 months post infection (McCall et al., 2001; Carmichael et al., 2017; Henry et al., 2018). At least one female D. immitis is needed and infections with very low worm burden may not be detected (Courtney and Zeng, 2001; Atkins, 2003). However, current commercial antigen tests were found to reliably detect infections in which more than one female adult D. immitis was present (Genchi et al., 2018). Furthermore, antigens may not be detected in some dogs, unless the samples are heat pretreated to release antigens from immune complexes (Little et al., 2018). Heat pretreatment is generally not recommended, with the notable exception of patients manifesting clinical signs and living in endemic areas which have not been under consistent preventive use (Little et al., 2018). Further development of assays resulted in tests suitable for diagnosis of multiple infections at the same time. Two of such rapid in-clinic tests, the FLEX4 assay (Abaxis, Union City, CA) and the SNAP assay (IDEXX Laboratories, Inc., Westbrook, ME) were recently evaluated for the detection of various Anaplasma, Borrelia and Ehrlichia species, and for D. immitis (Liu et al., 2018). While specificities for both rapid assays ranged from 98% to 100%, their sensitivities differed substantially for various pathogens and were 88.2% (FLEX4) versus 94.1% (SNAP) for D. immitis (Liu et al., 2018).
Molecular diagnosis is particularly appropriate for dogs suspected of being infected, but with microfilaria-negative results. It was demonstrated that in samples with only four microfilariae per ml, a multiplex PCR assay was able to give reliable positive results and to distinguish between D. immitis and D. repens (Gioia et al., 2010; Ferreira et al., 2017). Radiography and echocardiography are additional test methods for confirming the diagnosis and moreover, to assess the severity of heartworm disease (American Heartworm Society, 2020), particularly in cats (Berdoulay et al., 2004; Garrity et al., 2019).
4. Disease control
4.1. Macrocyclic lactones
Starting with the introduction of ivermectin in the canine market in 1987 (Heartgard®), until today, heartworm prevention is achieved almost solely through regular administration of active pharmaceutical ingredients (APIs) from the same chemical class, the macrocyclic lactones (MLs). The first MLs active against parasites – the avermectins, fermentation products of Streptomyces avermitilis – were discovered in 1975 from a soil sample collected in Japan (Burg et al., 1979; Campbell, 2012). Subsequently, in 2015, half of the Nobel Prize in Physiology or Medicine was awarded jointly to Campbell and Ōmura for their outstanding contribution to the discovery of the avermectins (Van Voorhis et al., 2015).
The MLs can be divided into two groups, the avermectins (abamectin, ivermectin, eprinomectin, and selamectin) and the milbemycins (milbemycin oxime and moxidectin) (Shoop et al., 1995; Vercruysse and Rew, 2002). All contain a common 16-member ML ring. The main structural difference between both classes resides at C13 of the macrocyclic ring: avermectins contain sugar residues, whereas milbemycins are protonated (Fig. 6). For further details on classification of the different MLs, for example the differences between avermectin 1- and 2-subsets or A and B series, please refer to Shoop et al. (1995) and Prichard and Geary (2019).
Fig. 6.
Structure of MLs marketed against heartworm infections. The macrocyclic core ring structure (top) indicates the regions where the MLs differ from each other. C13 is marked and highlighted in bold, where the main difference between the two major classes of MLs resides. Residues specific for each individual ML are visualized in blue. Modified following Prichard and Geary (2019).
4.1.1. Ivermectin
The soil-dwelling bacterium Streptomyces avermitilis was first discovered by Satoshi Ōmura in a soil sample from Kawana on the southeast coast of Honshu, Japan in 1973. As anecdotes are told, Ōmura always carried plastic bags with him to collect specimen and found his most promising sample in the woods next to a golf course (Van Voorhis et al., 2015). Extracts from cultures from this strain were sent to Merck laboratories to be tested in anthelmintic screening in 1974, where it showed promising activity against nematodes and many ectoparasites (Burg et al., 1979; Chabala et al., 1980; Ōmura, 2008; Campbell, 2012). William C. Campbell had the active components purified and identified the avermectins in 1975. The more effective chemical derivative ivermectin was subsequently commercialized, entering the Animal Health market in 1981 initially for livestock (Ōmura and Crump, 2014; Van Voorhis et al., 2015; Laing et al., 2017). The drug's potential in human health to fight onchocerciosis was confirmed a few years later and it was registered in 1987 and immediately provided free of charge for control of river blindness (branded as Mectizan®) (Molyneux and Ward, 2015; Ashour, 2019; http://www.mectizan.org/resources/2014-annual-highlights accessed November 27th, 2020). The World Health Organization lists ivermectin amongst the essential medicines (World Health Organization, 2019), and mass drug administration campaigns in Africa rely on its efficacy to control human filarial parasites (Kim et al., 2015).
Ivermectin is a chemically modified, dihydro derivative of naturally produced avermectin B1, composed of >80% 22,23-dihydro-avermectin B1a and <20% 22,23-dihydro-avermectin B1b at initial launch (Fig. 6) (Campbell, 1981; Campbell et al., 1983), whereas today's ratio is 90 to 10% (https://online.uspnf.com DocID: GUID-2506EE29-023C-4689-BE0C–392C296F1803_4_en-US). It shows activity against a broad spectrum of parasitic nematodes after both oral and parenteral administration, but not against cestodes or trematodes. In addition, it has activity against different arthropods like fleas, lice, mites and some tick species (McKellar and Benchaoui, 1996; Martin et al., 2020). While it is effective against microfilariae, L3 and L4 stages, it is not efficacious against adult but does reduce fertility (Martin et al., 2020). Ivermectin is marketed as oral, topical and injectable formulations, including long-acting injectables and boluses against endo- and ectoparasites of animals (Soll et al., 1988; Prichard et al., 2012).
While the antiparasitic activity of ivermectin is dependent on glutamate-gated chloride channels of endo-as well as ectoparasites (Arena et al., 1995; Wolstenholme and Rogers, 2005; Wolstenholme, 2012; Weber and Selzer, 2016), this API may target many more processes within different organisms. This includes modulation of other types of ligand-gated channels (Wolstenholme and Rogers, 2005), diverse modes of action in cancer treatment (e.g. Sharmeen et al., 2010; Yin et al., 2015; Nambara et al., 2017; Wang et al., 2018; Intuyod et al., 2019; Jiang et al., 2019; Zhang et al., 2019; Tang et al., 2020), inhibition of viral replication in several flaviviruses (Mastrangelo et al., 2012), regulation of metabolism through the farnesoid receptor in diabetic mice (Jin et al., 2013), and improvement of allergic skin inflammation by reducing activation of allergen-specific T cells (Ventre et al., 2017). Very recently, activity against SARS-CoV-2, the causative virus for COVID-19, has also been hypothesized for ivermectin based on observations in in vitro assays (Caly et al., 2020) and in hospitalized patients (Rajter et al., 2020). Although ivermectin is very safe, it is not without toxicity in mammals. While filariae and other nematodes are exquisitely sensitive to the drug, micromolar doses are often associated with activity in mammalian cell culture experiments. Therefore, it seems unlikely to reach effective and safe doses for antiviral therapy in humans (Schmith et al., 2020).
4.1.2. Eprinomectin
Eprinomectin or 4″-epi-acetylamino-4″-deoxyavermectin B1 was developed exclusively for veterinary medicine use as the first topical endectocide for all cattle, including lactating animals (Shoop et al., 1996a, 1996b). It is a semi-synthetic derivative of avermectin B1 or abamectin, consisting of two homologs, B1a (not less than 90%) and B1b (not more than 10%), which differ by a methylene group. Eprinomectin first entered the market in a topical formulation against internal and external parasites of cattle including lactating cows (Shoop et al., 1996b; Holste et al., 1997, 1998; Pitt et al., 1997; Williams et al., 1997; Rehbein et al., 2005). As it showed good bioavailability and systemic activity also in cats following topical application (Kvaternick et al., 2014), it was included in a topical endectoparasiticide combination product together with fipronil, (S)-methoprene, and praziquantel for cats (Broadline®) (Baker et al., 2014; Rehbein et al., 2014).
4.1.3. Abamectin
Abamectin remains the only ML that is used in both animal health and crop protection (Bai and Ogbourne, 2016). It consists of avermectin B1a (>90%) and avermectin B1b (<10%). Abamectin is approved in Australia for preventive use against heartworm in dogs, however only in endoparasiticide combination products that include oxibendazole and praziquantel (https://portal.apvma.gov.au/pubcris accessed November 27th, 2020).
4.1.4. Selamectin
Selamectin is a semisynthetic monosaccharide oxime derivative of doramectin (25-cyclohexyl-25-de(1-methylpropyl)-5-deoxy-22,23-dihydro-5-(hydroxyimino)-avermectin B1 monosaccharide). Doramectin has been identified as the most potent nematicide in a series of new avermectins prepared by mutational biosynthesis, having a cyclohexyl group in the C25 position of the avermectin ring (Dutton et al., 1991; Goudie et al., 1993). Selamectin was selected for its efficacy against D. immitis, gastrointestinal nematodes, fleas and ticks in 1999 (Banks et al., 2000; Bishop et al., 2000; Prichard and Geary, 2019). It is available as topical formulation for dogs and cats, while doramectin is marketed for ruminants and swine only.
4.1.5. Milbemycin oxime
The milbemycins were initially isolated in 1967 as fermentation products from Streptomyces hygroscopicus, and subsequently from Streptomyces cyaneogriseus in 1983, that displayed very high acaricidal activity. (Prichard et al., 2012; Prichard and Geary, 2019). Structure elucidation, in 1972 by Sankyo scientists, revealed the 16-membered ML structure of the active compound family of milbemycins, from which the anthelmintic milbemycin oxime (6R, 25R)-5-demethoxy-28-deoxy-6, 28-epoxy-5-hydroxyimino-25-ethyl/methyl milbemycin) was derived (Takiguchi et al., 1980, 1983; Prichard et al., 2012). Milbemycin oxime is available in an oral formulation, consisting of a mixture of 70–80% milbemycin A4 oxime and 30-20% milbemycin A3 oxime, for heartworm prevention in dogs and cats. In addition, milbemycin oxime shows efficacy against immature and adult stages of other parasitic roundworms, hookworms, whipworms, and lungworms, and mites (Garfield and Reedy, 1992; Holm, 2003; Prichard et al., 2012).
4.1.6. Moxidectin
Exploration of fermentation products from S. cyaneogriseus in 1983 revealed not only a new source of milbemycin, but also the new ML nemadectin (F-29249α) (Carter et al., 1987, 1988). Addition of a methoxime moiety at C-23 and a substituted olefinic side chain at the 25-position to nemadectin yields moxidectin (Ranjan et al., 1992). Heartworm prophylaxis products administer moxidectin orally, topically, or as injectable. Moxidectin has been approved for human use against river blindness (https://www.fda.gov/drugs/drug-approvals-and-databases/drug-trials-snapshots-moxidectin accessed November 25th, 2020).
Compared to the avermectins, moxidectin inhibits Pgp-mediated rhodamine123 transport with 10 times lower potency (Lespine et al., 2007). Moxidectin is very lipophilic and has a long half-life, which makes it particularly suitable for long-acting injectable formulations, e.g., ProHeart-6® and ProHeart-12® preventives in canines, and Cydectin® LA for prevention and treatment of gastrointestinal nematodes in cattle (Prichard et al., 2012; Prichard and Geary, 2019). Topical moxidectin products obtained FDA approval for elimination of microfilariae in heartworm-positive dogs, diminishing adverse reactions, which occur normally due to high microfilarial counts in infected dogs (McCall et al., 2014a). A combination product containing moxidectin, sarolaner and pyrantel to obtain protection against endo- and ectoparasites has been marketed recently (Simparica® Trio) (Kryda et al., 2019; Becskei et al., 2020).
4.2. Non-macrocyclic lactone treatments
4.2.1. Diethylcarbamazine citrate
Diethylcarbamazine citrate (DEC) (Fig. 7) is the oldest heartworm preventive, discovered in 1947 as a derivative of piperazine. It shows both microfilaricidal and adulticidal activity, presumably by increasing filarial susceptibility to innate immune attack (Sutton et al., 1985; El-Shahawi et al., 2010). It was first used to control human filariosis around the world (Hawking, 1962), and made it to the animal health market in products for heartworm prophylaxis in the 1960s (Pailet et al., 1968; Prescott et al., 1978). In contrast to other preventives, it has to be given daily (https://apvma.gov.au accessed December 2nd, 2020).
Fig. 7.
Structures of Non-ML heartworm APIs.
As diethylcarbamazine citrate is on the World Health Organization's List of Essential Medicines (World Health Organization, 2019) for treatment of filariosis including lymphatic filariosis, tropical pulmonary eosinophilia, and loiosis (Chitkara and Sarinas, 1997), its use in animal health has been limited, with only a few products still marketed.
4.2.2. Adulticide treatment using arsenamide sodium and melarsomine dihydrochloride
The adulticide arsenamide (thiacetarsamide) sodium (Caparsolate®) (Fig. 7) was used for treatment of adult D. immitis since the 1940s. Treatment needed to be administered intravenously, and dogs had to be hospitalized during initial treatment to handle possible hepatotoxic and nephrotoxic side effects (Raynaud, 1992). In the 1990s, melarsomine dihydrochloride (Immiticide®) (Fig. 7) supplanted thiacetarsamide as an adulticide, as it provided easier administration as well as increased safety and efficacy (Raynaud, 1992; Rawlings et al., 1993a; McTier et al., 1994; Maksimowich et al., 1997). Still today, melarsomine dihydrochloride is the first line adulticide treatment for heartworm infections. Even though this therapy reduces the need for hospitalization of dogs, strict exercise restriction is required to limit thromboembolic effects (Raynaud, 1992; Rawlings et al., 1993b; Bowman and Drake, 2017; American Heartworm Society, 2020). Treated dogs should be carefully monitored for adverse effects such as increase in pulmonary pressure due to dying or dead worms, as well as intense inflammatory reactions against the parasite or its Wolbachia endosymbionts (Kramer et al., 2008; Ames et al., 2020).
To achieve complete elimination of adult heartworm infections, both the American Heartworm Society and the European Society of Dirofilariosis and Angiostrongylosis propose protocols with 2–3 month pre-treatment with an ML combined with an antibiotic against Wolbachia (such as doxycycline, see below) prior to the administration of three doses of Immiticide® (European Society of Dirofilariosis and Angiostrongylosis, 2017; American Heartworm Society, 2020). The reason for this pre-treatment is to help close the susceptibility gap in the efficacy of MLs and Immiticide® against some heartworm stages (Bowman and Drake, 2017). The combined delayed treatment provides efficacy by initially using MLs to eliminate susceptible larvae and prevent new infections, while allowing older worms to develop further and become susceptible to melarsomine dihydrochloride. In addition, the risk of severe pulmonary thromboembolism is reduced due to the staged killing of the adult parasites (Carretón et al., 2014; American Heartworm Society, 2020). As some studies provided evidence that melarsomine dihydrochloride may be effective already against worms from two to four months of age, the adulticidal treatment protocol might be improved further (McCall, 2005; McCall et al., 2010; Bowman and Drake, 2017; American Heartworm Society, 2020; Carretón et al., 2019).
Adulticide therapy using melarsomine is not considered safe for cats, as worm death in cats is associated with a high risk of pulmonary thromboembolism and anaphylactic reactions (Pennisi et al., 2020). Surgery known as worm embolectomy is an alternative to relying on self-cure, which can occur within 18–48 months, and carefully monitoring disease progression (European Society of Dirofilariosis and Angiostrongylosis, 2017; Pennisi et al., 2020). Surgical extraction of adult heartworms in dogs remains the only solution for heavily infected dogs manifesting clinical signs of caval syndrome, as dying or dead adult heartworms obstruct blood flow and degrading worms cause inflammatory reactions (Glaus et al., 1995; Lee et al., 2008; Bové et al., 2010).
4.2.3. Doxycycline for supportive treatment
Doxycycline-mediated clearance of Wolbachia in Onchocerca and Dirofilaria infections demonstrated that Wolbachia are required for filarial larval development, embryogenesis and long-term viability (Turner et al., 2020). Treatment with doxycycline (Fig. 7) successfully killed third- and fourth-stage heartworm larvae in experimentally infected dogs (McCall et al., 2011). However, a major draw-back of doxycycline is the long treatment duration needed to eliminate the required 90% of Wolbachia for a sustainable effect (Turner et al., 2020). In addition, long-term application of doxycycline in dogs is often associated with low tolerability and severe gastro-intestinal side effects (Savadelis et al., 2018). Nevertheless, for adulticidal treatment, a combination of doxycycline with monthly doses of ivermectin showed a superior microfilaricidal and adulticidal efficacy compared to the drugs given alone (Bazzocchi et al., 2008; McCall et al., 2008a). Moreover, doxycycline seems to enable a shorter treatment regimen by eliminating Wolbachia prior to the first adulticidal dose of melarsomine dihydrochloride (Carretón et al., 2019). Reducing the burden of Wolbachia in D. immitis prior to adulticide treatment proved to be more efficacious with fewer inflammatory reactions and lower risk of fatal pulmonary thromboembolisms (Bazzocchi et al., 2008; Kramer et al., 2008; Nelson et al., 2017). The combination of doxycycline, ivermectin and melarsomine significantly reduced the severity of arterial lesions and thrombi (Kramer et al., 2008). The American Heartworm Society (2020) recommends a therapy including ivermectin or moxidectin, doxycycline and melarsomine.
4.3. The mode of action of macrocyclic lactones
At a biochemical level it is well established that MLs are potent allosteric agonists of some nematode glutamate gated chloride channels (GluCl, Fig. 8) (Duce and Scott, 1985; Scott and Duce, 1986; Arena et al., 1992; Cully et al., 1994; Yates and Wolstenholme, 2004), with GluCl sequence diversity within nematode genomes providing both sensitive and insensitive subunits (Wolstenholme 2012). These channels belong to a large superfamily of cys-loop ligand gated ion channels characterized by a conserved extracellular region of amino acids that form a loop, closed by disulfide bonded cysteine residues (Connolly and Wafford, 2004). Genetic analysis has revealed that GluCl occur in many nematodes and arthropods including filariae but the size and composition vary between species (Williamson et al., 2007). While GluCl are limited to invertebrates (Wolstenholme, 2012; Buckingham et al., 2020), members of this superfamily span vertebrate and invertebrate lineages and include diverse receptors with distinct ligand specificity, such as the vertebrate glycine- and 5-HT-receptors (Lynch 2004; Barnes et al., 2009). MLs are promiscuous allosteric modulators of these related channels, for example ML can act as agonist on human glycine receptors (Shan et al., 2001) and both as agonist and inhibitor on various insect and human gamma-amino-butyric acid gated channels (Lees et al., 2014; Estrada-Mondragon and Lynch, 2015). However, because of GluCl's exquisite ML sensitivity, therapeutic relevance of other channels is most likely irrelevant for heartworm disease therapy. Nicotinic acetylcholine receptors as well may be potentiated or attenuated by MLs (Raymond et al., 2000). Despite the channel promiscuity of MLs, a strong line of evidence from multiple experimental systems and species implicate GluCl as the relevant nematode targets, perhaps the most compelling data coming from resistance mutagenesis studies in Caenorhabditis elegans (Dent et al., 2000). Indeed, the pentameric ligand gated ion channels are well-established molecular targets for nematodes and arthropods that have provided for a spectrum of veterinary parasiticides that act at distinct binding sites (Weber and Selzer, 2016).
Fig. 8.
Illustration of the binding site and opening of LGCC in response to IVM. (A) Five GluCl subunits, each consisting of four alpha helical structures (M1-M4) and an extracellular domain not shown here, adopt a pentameric tertiary structure. The transmembrane region is arranged with each subunit perpendicular to the plane of the membrane and the M2 helices (orange cylinders) of each subunit lining the interior channel. Chloride ions (green circles) flow down the concentration gradient (indicated by the green arrow) when IVM (yellow/orange wedge) binds at the interface of two GluCl subunits, inducing a shift in the helices and tilting of the M2 helices away from the center of the channel, effectively widening the extracellular portion of the channel. (B) An illustration of the electrophysiological response of GluCl to IVM. The closed state is represented by a static electrical potential (flat line) and circle with narrow pore, precluding chloride ion flow. Addition of IVM (yellow/orange arrow) induces an irreversible open channel conformation, illustrated by the right part of the trace and the circle with a large pore.
Observations in a homopentameric GluCl from C. elegans indicate the allosteric signal to adopt an open channel conformation is induced when MLs bind to a site created by the interface of the first (M1) and third (M3) transmembrane helices of adjacent subunits (Hibbs and Gouaux, 2011) (Fig. 8). Subunit heterogeneity can create structural diversity in the interfacial ML-binding site that can, in turn, result in differential GluCl sensitivity, as well as differential agonized or inhibited response of the ligand-gated chloride channel (LGCC) to ML (Estrada-Mondragon and Lynch, 2015; Atif et al., 2019). Indeed, within parasitic nematodes, GluCl sequences can be divided into alpha- and beta-subunits, with alpha-subunits generally displaying sensitivity to IVM and beta-subunits presenting glutamate but not IVM-gated channels (Wolstenholme and Rogers 2005). With respect to ML structure, the benzofuran group fused to the 16-membered macrocyclic lactone ring participates directly in binding at the subunit interfaces (Hibbs and Gouaux, 2011). The structurally variable spiroketal group may mediate ML-affinity by applying spatial constraints on site occupation, providing a plausible explanation for the different potencies displayed by structurally variable MLs (Martin et al., 2020). The binding site is buried in the hydrophobic region of the phospholipid bilayer and is in fact normally occupied by phospholipids (Althoff et al., 2014). Accordingly, it has been demonstrated that ivermectin first partitions into the phospholipid bilayer and diffuses laterally in the membrane before displacing lipids from and binding to GluCl (Atif et al., 2019). Closed channels adopt a structure in which the second transmembrane helix (M2) of each subunit lines the pore and aligns parallel to other subunit M2 helices and perpendicular to the plane of the membrane (Althoff et al., 2014). In the ML-bound state the M2 helices tilt away from the center of the pore resulting in a larger diameter opening at the extracellular membrane surface (Hibbs and Gouaux, 2011; Althoff et al., 2014). Prolonged opening of GluCl results in neuronal hyperpolarization, which precludes action potential propagation essentially silencing the associated neuron.
MLs evoke an array of phenotypes from various nematode species. Inhibition of pharyngeal pumping, reduction of oviposition, and decreased motility are all well characterized outcomes of treating nematodes in vitro (Wolstenholme and Rogers, 2005). In alignment with these observations, ML-sensitive GluCl localize to tissues and cells that mediate the respective phenotypes. For example, GluCl-subunits localize to the pharynx in C. elegans, Haemonchus contortus and Ascaris suum, others are expressed in motor neurons and motor neuron commissures in C. elegans and H. contortus, and the amphids in H. contortus (Wolstenholme and Rogers, 2005). However, a direct link between these phenotypes and the in vivo mode of action in ML-based prevention of heartworm disease remains hypothetical. In some cases, evidence suggests that such phenotypes as motility are not relevant to the in vivo MoA for heartworm disease prevention (Wolstenholme et al., 2016; Evans et al., 2017b). Indeed, the concentration of ML required to elicit paralysis in vitro (4.6 μM) (Evans et al., 2013) is several orders of magnitude higher than an extrapolated tissue concentration sufficient to provide 100% protection against heartworm disease (3.4 nM) (Daurio et al., 1992). Moreover, the in vitro EC50 of eprinomectin and ivermectin against susceptible and resistant strains of D. immitis in motility-based assays are not significantly different (4.56 μM susceptible versus 2.21 μM resistant, p-value = 0.35) (Evans et al., 2017b), as would have been expected if motility inhibition played a role in vivo. These lines of evidence suggest that ML evoke phenotypes in vitro that are, particularly in the case of filarial parasites, incongruous with ML-mediated parasite neutralization in vivo.
In an effort to connect the biochemical, in vitro and in vivo data, for filarial parasites, Moreno et al. (2010) looked deeper into GluCl biology in B. malayi. Immunolocalization of GluCl subunits in B. malayi microfilariae revealed that the channel is associated with a specialized cellular apparatus at the excretory/secretory pore, that facilitates release of proteins, and presumably nucleic acids and other bioactive excretory/secretory products (ES), into the host environment (Fig. 9). Based upon localization to this apparatus, the authors reasoned that disrupting GluCl signaling with MLs should have a measurable effect on the function of this specialized region. Indeed, treatment of microfilariae with ivermectin resulted in significant decreases of secreted protein (Moreno et al., 2010). Importantly, the profile and relative abundance of proteins remains unchanged by ivermectin treatment, consistent with disruption of a ubiquitous mechanism underlying protein secretion. Postulating that ES play a critical role in immune evasion, a mechanism is proposed in which ML engage GluCl at the excretory/secretory apparatus, halting the cellular processes that mediate secretion, which in turn causes loss of ability to manipulate the host and ultimately neutralization of the parasite by the immune system (Moreno et al., 2010, 2021; Carithers, 2017). While in vitro evidence in support of this hypothesis has been generated (Tritten and Geary, 2018), including ivermectin-mediated inhibition of extracellular vesicle secretion (Harischandra et al., 2018), and ML-mediated peripheral blood immune cell adhesion to D. immitis larva (Berrafato et al., 2019), the effect of ML on ES inhibition in target L4 stages remains to be evaluated and in vivo experimental approaches remain to be conducted.
Fig. 9.
Illustration of the putative components of D. immitis host pathogen interactions. A testable model for the ML in vivo mode of action can be constructed from the hypothetical interaction of ML, D. immitis, ES (variously colored circles), and the canine immune system (illustrated as various peripheral circulating morphonuclear cells). Microfilariae, and potentially L3 and L4 stages, broadcast a milieu of bioactive molecules that may specifically interact with host immune cells, abrogating a successful immune response that would clear the parasite. In the presence of MLs GluCls are engaged, neuronal signaling is silenced and the function of the ES apparatus (marked brown) shuts down. The resulting loss of ability to manipulate host immune signaling pathways results in clearance by a functional immune response.
4.4. Drug resistance in canine filarial chemotherapy
Failure of preventive treatment with MLs may be due to lack of owner compliance – such as under-dosing or dosing at irregular treatment intervals – and/or due to drug-resistant heartworm populations. From 1998 onwards “Lack of Efficacy” (LoE) complaints have been reported, and in 2002 the volume of complaints exceeded 1000 (Hampshire, 2005). Failure of compliance has played a substantial role in a large number of LoE cases as elucidated by a review of medical records from 2004 to 2011 (Atkins et al., 2014). This analysis revealed that in 80.7% of LoE cases, insufficient heartworm preventive drugs were purchased, suggesting a period in which dogs were not protected. However, already then, in 1.7% of the LoE cases, drug-resistance was the most likely explanation (Atkins et al., 2014). Within the last decade studies have demonstrated that ML-resistant D. immitis populations have contributed to treatment failures. Several resistant D. immitis isolates were identified and characterized (Bourguinat et al., 2015; Pulaski et al., 2014; Ballesteros et al., 2018), and the occurrence of drug resistance in heartworm has been accepted by the American Heartworm Society (https://www.heartwormsociety.org) and CAPC (https://capcvet.org). The scientific characterization of those isolates started with an infected dog, which originated in New Orleans and was transferred to Canada after the Katrina hurricane (Bourguinat et al., 2011a). Microfilariae in that dog could not be eliminated with repeated treatments of ivermectin and milbemycin oxime, indicating that those parasites showed resistance to normally efficacious doses of MLs. Moreover, genetic differences were elucidated between D. immitis sensitive isolates and isolates expressing a substantially reduced sensitivity to MLs based on in vitro measurements (Bourguinat et al., 2011b). These initial findings were substantiated when the sensitivity of D. immitis isolates was determined in dogs applying more field related protocols of preventive treatment (Snyder et al., 2011; Blagburn et al., 2011; Pulaski et al., 2014; Bourguinat et al., 2015). All of these well characterized isolates, including the MP3, Td2008 and Jd2009 isolate, were resistant to all tested MLs, particularly ivermectin and milbemycin oxime. The resistance pattern was demonstrated in dogs under various treatment protocols starting from one dose only (Snyder et al., 2011) up to monthly treatments (Bourguinat et al., 2015) and was independent of the application route (Pulaski et al., 2014). The Td2008 and Jd2009 D. immitis isolates were able to develop to adult heartworms in dogs despite of 5 or 9 monthly repeated ivermectin treatments following experimental infection (Bourguinat et al., 2015). Importantly, moxidectin still showed efficacy against strains that showed resistance to other MLs. The sensitivity of one of these drug-resistant D. immitis isolates, the MP3 isolate, was evaluated against various MLs in dogs with the result that only moxidectin was 100% effective (Blagburn et al., 2011). The reasons for this observation have not been elucidated yet but may be a higher intrinsic potency of moxidectin particularly against filariae, a significantly different pharmacokinetic profile, or a different mechanism of resistance (Prichard and Geary, 2019). However, moxidectin resistance was also reported to occur in four ML-resistant D. immitis isolates (JYD-34, ZoeMO, ZoeLA and AMAL) (McTier et al., 2017) which could be partially overcome by increasing dose and treatment frequency (McTier et al., 2019). With the documented evidence, there is no doubt that there is genuine ML resistance present in the field. Several important questions remain: How prevalent are such parasite populations, and what is the risk of further distribution of drug resistance in other areas? What are the mechanisms behind resistance, and could resistance be diagnosed on an epidemiological level? However, for the majority of heartworm prophylaxis, todays ML-based drugs are still of good value, because resistance appears to be present mainly in the Mississippi River areas.
4.5. Mdr1 mutations in collies and related breeds
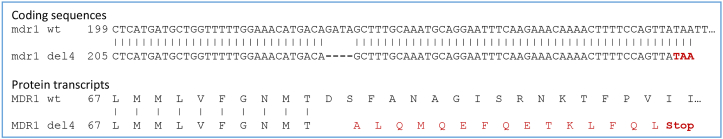
While in general MLs are considered to be safe for most mammals, some dog breeds including collies and shepherds are prone to moderate to severe neurological effects. The genetic reason underlying this susceptibility is a 4 base pair deletion mutation leading to a frameshift in the multi-drug resistant (mdr1) transporter gene (nt230 (del4) mdr1 mutation; Fig. 10) (Mealey et al., 2001; Geyer et al., 2005). The mdr1 (del4) mutation in these dogs can be traced back to a common ancestor in Great Britain around 1873, before formal breeds were registered and genetically isolated (Neff et al., 2004; Gramer et al., 2011).
Fig. 10.
Local alignment of mdr1 wt and mutant mdr1 del4. Mdr1 wt from Canis lupus familiaris (Genebank accession number DQ068953.1) and mdr1 nt230 (del4) (Genebank accession number AJ419568.1) coding sequences as well as their transcripts were aligned. The nt230(del4) mdr1 deletion leads to a frame shift at amino acid position 75, resulting in a truncated, non-functional MDR1 protein due to the premature stop codon (TAA marked in red) after amino acid position 91.
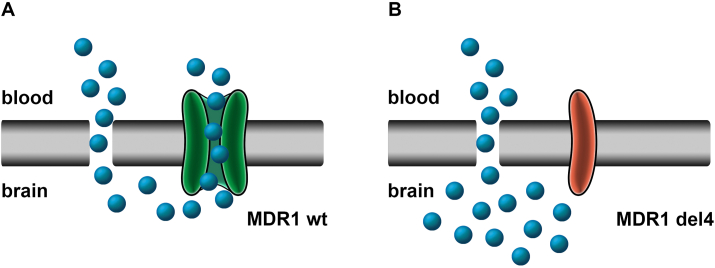
The P-glycoprotein MDR1 belongs to a family of membrane-bound ATP-binding cassette transporters (ABC transporters) (Dean et al., 2001) and acts as a drug efflux pump across the blood-brain barrier. MDR1 plays an important role in elimination of many drugs from the mammalian central nervous system, including humans (Fig. 11) (Mealey et al., 2008). The channel was first isolated and characterized from Chinese hamster ovary cells that had developed resistance to chemotherapy drugs by overexpression of MDR1 (Juliano and Ling, 1976). While mice with a deficient mdr1 gene showed no obvious phenotype in general, all mice of a colony infested with mites showed enhanced drug sensitivity and subsequently died after treatment with ivermectin (Schinkel et al., 1994). Not only MLs, but also many structurally unrelated drugs, toxins, and xenobiotics can also be substrates for MDR1, with distinct affinities and binding modes for different classes of substrates (Schinkel et al., 1996; Srikant and Gaudet, 2019).
Fig. 11.
Consequence of mdr1 mutation for drug exposure in the central nervous system. (A) functional MDR1 receptor actively lowers concentration of APIs within the central nervous system, while (B) mdr1 mutation nt230 (del4), leading to a non-functional, truncated MDR1 channel, results in accumulation of APIs within brain cells, thus increasing susceptibility to neurotoxic side effects.
Sensitivity to ivermectin is increased already for heterozygous mdr1 (+/−) but especially for homozygous mdr1 (−/−) dogs, lacking expression of functional MDR1 (Mealey et al., 2008; Merola and Eubig, 2012). Although all marketed products consider this fact and therefore provide ML doses for heartworm prevention that are well tolerated by mdr1 (del4) deficient dogs, it is advised to genetically pre-test collies, shepherds and related breeds for mdr1 (del4) mutations before treatment with MLs (Geyer and Janko, 2012; Stiedl and Weber, 2017). The prevalence of at least one mdr1 (del4) allele, either as mdr1 (+/−) or mdr1 (−/−), can be as high as 75% for Collies (Table 1), but is almost nonexistent for other breeds, including breeds which share some history with the affected dog breads (e.g., Bearded Collie, Anatolian Shepherd Dog, Greyhound, Belgian Tervuren). It is estimated that about 1–2% of all dogs in the northern hemisphere carry such a mutation (Neff et al., 2004; Geyer et al., 2005; Mealey et al., 2005; Gramer et al., 2011; Tappin et al., 2012; Monobe et al., 2015; Mizukami et al., 2016; Dekel et al., 2017; Marelli et al., 2020). Across geographies, the prevalence of MDR1 deficiency in collies is comparable (Geyer and Janko, 2012).
Table 1.
Allelic frequency of mdr1 (del4) mutation in dog breeds worldwide.
| Dog Breed | Range of mdr1 (del4) allelic frequency (%) |
|---|---|
| Collie | 48–75 |
| Longhaired Whippet | 24–45 |
| Shetland Sheepdog | 7–36 |
| (Miniature) Australian Shepherd | 16–54 |
| Australian Shepherd | 17–46 |
| White Swiss Shepherd | 7–16 |
| Old English Sheepdog | 0–11 |
| English Shepherd | 7 |
| German Shepherd | 0–6 |
| Border Collie | 0–4 |
Data retrieved from (Gramer et al., 2011; Geyer and Janko, 2012; Tappin et al., 2012; Monobe et al., 2015; Firdova et al., 2016; Marelli et al., 2020; Soussa et al., 2020). As data availability and sample size differ widely, frequencies have not been listed for all breeds.
Today, with further technological advancement and the rise of an integrated health management, it is rather straightforward to test dogs for this mutation using genetic tests on cells obtained by cheek swabbing or a blood sample (e.g. Stiedl and Weber, 2017; Lee et al., 2019; Silvestro et al., 2019). It is not only reasonable to know the risk of one's own dog before treatment with MLs, but this information is also used by many dog breeders, selecting for mdr wt dogs to increase the value of the pups and thus, hopefully outbreeding the mdr1 (del4) mutation in the future.
In addition to the nt230 (del4) mdr1 mutation, more than 30 single nucleotide polymorphisms have been identified in the canine mdr1 gene which might affect the transport function or expression level (Geyer and Janko, 2012). One can speculate that these polymorphisms might be the reason for increased drug sensitivity of some dogs that lack the deletion.
4.6. Market products
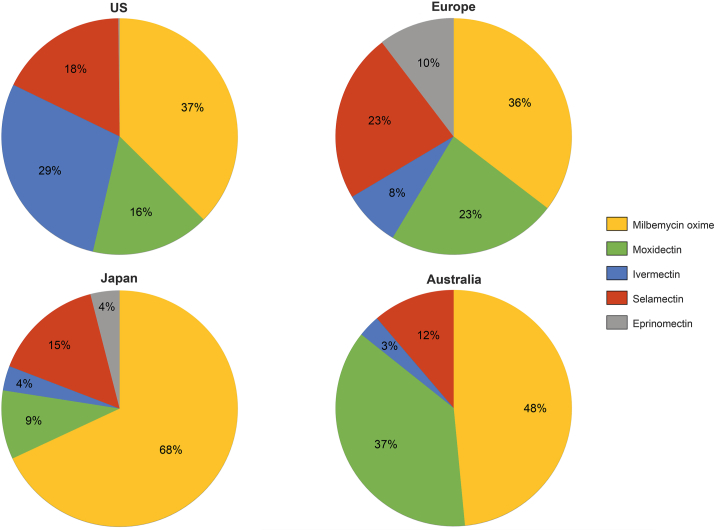
Most heartworm preventives today are available as oral formulations, while only few topicals and two injectable formulations are marketed. To illustrate distinctions in differently regulated markets, we focus on marketed products in the USA, Europe, Japan and Australia. In these geographies, heartworm active APIs take different market shares – either based on local registrations, differences in marketing, or customer preferences (Fig. 12).
Fig. 12.
Market shares of products based on specific APIs differ across geographies. As not all approved products are marketed anymore or in all geographies or might be marketed via channels not captured for this analysis, the API market analysis might be slightly skewed. One example are products containing besides MLs diethylcarbamazine citrate, a rather old API which has been replaced in more modern products. Data based on Boehringer Ingelheim internal market analysis, AH market 2019.
4.6.1. USA
International Cooperation on Harmonisation of Technical Requirements for Registration of Veterinary Medicinal Products (VICH) guidelines for the registration of anthelmintic products have been largely adopted by the U.S. Food & Drug Administration (FDA) in its Guidance for Industry (GFI) system, e.g., GFI #90 (VICH GL7) Efficacy of Anthelmintics: General Requirements, GFI #111 (VICH GL19) Efficacy of Anthelmintics: Specific Recommendations for Canine, and GFI #113 (VICH GL20) Efficacy of Anthelmintics: Specific Recommendations for Feline (https://www.fda.gov/animal-veterinary/guidance-regulations/guidance-industry accessed November 30th, 2020). At present, two laboratory dose confirmation studies and one multisite field safety and effectiveness study must be conducted to demonstrate heartworm preventive efficacy, following the principles of Good Clinical Practice (GCP) as described in GFI #85 (VICH GL9) “Good Clinical Practice.” The FDA has historically required 100% efficacy in these studies for registration. The Center for Veterinary Medicine (CVM) is currently evaluating alternative approaches for the design of studies conducted to show effectiveness (FDA, 2018).
While there are several approved heartworm preventives for dogs and cats, only one has been approved for ferrets to date (Advantage Multi for Cats). Table 2 summarizes the products approved for use in the USA.
Table 2.
APIs and products approved in the USA for prevention of heartworm disease or treatment of heartworm infections.
| Heartworm active API | Species | Route of application | Trade names | Combination product with | Company |
|---|---|---|---|---|---|
| Diethylcarbamazine citrate | dog, cat | oral | Carbam Diro-Form Dirocide/Filaribits/Pet-Dec Filban Nemacide |
Bimeda AH Lloyd Zoetis Intervet Cronus Pharma |
|
| dog | oral | Filaribits Plus | Oxibendazole | Zoetis | |
| Eprinomectin | cat | topical | Centragard | Praziquantel | Boehringer lngelheim AH |
| Ivermectin | dog, cat | oral | Heartgard generics thereof: Iverhart Ivermectin |
Boehringer lngelheim AH Virbac AH Cronus Pharma |
|
| dog | topical | Advantage DUO | Imidacloprid | Elanco | |
| dog | oral | Heartgard Plus generics thereof: Iverhart Plus Tri-Heart Plus |
Pyrantel Pamoate | Boehringer lngelheim AH Virbac AH Heska |
|
| dog | oral | Panacur Plus | Praziquantel, Fenbendazole, | Intervet | |
| dog | oral | Iverhart Max | Praziquantel, Pyrantel pamoate |
Virbac AH | |
| Milbemycin oxime | dog, cat | oral | Interceptor generic thereof: MilbeGuard |
Elanco Ceva Sante Animale |
|
| dog | oral | Sentinel | Lufenuron | Intervet | |
| dog | oral | Sentinel Spectrum | Lufenuron, Praziquantel |
Intervet | |
| dog | oral | Interceptor Plus | Praziquantel | Elanco | |
| dog | oral | Trifexis | Spinosad | Elanco | |
| Moxidectin | dog | oral s.c. topical |
ProHeart Proheart 6/12 Coraxis |
Zoetis Elanco |
|
| dog, cat, ferret | topical | Advantage Multi generic thereof: Imoxi |
Imidacloprid | Elanco Vetoquinol |
|
| cat | topical | Bravecto Plus | Fluralaner | Intervet | |
| dog | oral | Simparica Trio | Sarolaner, Pyrantel pamoate | Zoetis | |
| Selamectin | dog, cat | topical | Revolution generic therof: Revolt, Selarid, Senergy |
Zoetis Aurora Pharmaceutical, Norbrook Laboratories, Chanelle Pharmaceuticals |
|
| cat | topical | Revolution Plus | Sarolaner | Zoetis | |
| Arsenamide sodium | dog | i.v. | Caparsolate Sodium not marketed anymore |
Boehringer lngelheim AH | |
| Melarsomine dihydrochloride | dog | i.m. | Immiticide generic thereof: Diroban |
Boehringer lngelheim AH Anzac AH |
|
Preventives dominate the market; only three products are approved for adulticidal treatment (see last two rows of table). Data retrieved from: U.S. Food & Drug Administration https://animaldrugsatfda.fda.gov/adafda/views/#/search, accessed November 30th, 2020; AH – Animal Health.
4.6.2. European Union
EU Regulation 2019/6 currently governs the centralized marketing authorization procedure for both human and veterinary medicines (amending EU Regulation 726/2004 relating to authorization and supervision of veterinary medicines) (https://eur-lex.europa.eu/eli/reg/2019/6/oj accessed November 30th, 2020), while national registrations can be requested from the respective national authorities. In addition, the respective VICH guidelines have to be followed: VICH GL7 Efficacy of Anthelmintics: General Requirements, VICH GL19 Efficacy of Anthelmintics: Specific Recommendations for Canine, and VICH GL20 Efficacy of Anthelmintics: Specific Recommendations for Feline (https://vichsec.org/en/guidelines/pharmaceuticals/pharma-efficacy/anthelmintics accessed November 30th, 2020). In general, most new, innovative medicines are submitted to EMA for centralized authorization, while most generics and over-the-counter medicines use national marketing authorization. Expansion of marketing authorization to other EU member states can be obtained by either a mutual recognition procedure or a centralized procedure. Nevertheless, data requirements and standards for authorization of medicines in the EU are the same, irrespective of the authorization route. Combination products dominate the European market for heartworm prevention (Table 3).
Table 3.
APIs and products approved in Europe for prevention of heartworm disease or treatment of heartworm infections.
| Heartworm active API |
Species | Route of application | Trade names | Combination product with | Company |
|---|---|---|---|---|---|
| Eprinomectin | cat | topical | NexGard Combo | Esafoxolaner, Praziquantel | Boehringer Ingelheim AH |
| cat | topical | Broadline | Fipronil, Praziquantel, (S)-Methoprene | Boehringer Ingelheim AH | |
| Ivermectin | dog | s.c. | Guardian inj. | Elanco | |
| dog | oral | Heartgard/Cardotek plus | Pyrantel | Boehringer Ingelheim AH | |
| Milbemycin oxime | dog | oral | Program plus | Lufenuron | Elanco |
| dog, cat | oral | Milbemax generics thereof: Milbactor, Milprazon, Milquantel Milpro |
Praziquantel | Elanco Krka Virbac |
|
| dog | oral | Nexgard Spectra | Afoxolaner | Boehringer Ingelheim AH | |
| dog | oral | Trifexis | Spinosad | Elanco | |
| Moxidectin | dog | s.c. | Afilaria | Support Pharma, Fatro | |
| dog, cat, ferret | topical | Advocate/Prinovox | Imidacloprid | Bayer AH | |
| cat | topical | Bravecto Plus | Fluralaner | Intervet | |
| dog | oral | Simparica Trio | Sarolaner, Pyrantel embonate | Zoetis | |
| Selamectin | dog, cat | topical | Stronghold generic thereof: Chanhold, Evicto |
Zoetis Chanelle Pharmaceuticals, Virbac AH |
|
| cat | topical | Stronghold Plus Felisecto Plus |
Sarolaner | Zoetis | |
| Melarsomine dihydrochloride | dog | i.m. | Immiticide | Boehringer Ingelheim AH | |
Preventives dominate the market; only one product is approved for adulticidal treatment (see last row of table). Data retrieved from: EMA Europa Veterinary https://www.ema.europa.eu/en/medicines/field_ema_web_categories%253Aname_field/Veterinary, HMA Heads of Medicines Agencies https://mri.cts-mrp.eu/veterinary/and CIMAVET https://cimavet.aemps.es/cimavet/publico/home.html accessed November 30th, 2020; AH – Animal Health.
4.6.3. Japan
In Japan, the Ministry of Agriculture, Forestry and Fisheries (MAFF) holds jurisdiction over affairs concerning veterinary medicinal products. Regulations include the Law and the Enforcement Ordinance of the Law for Ensuring the Quality, Efficacy, and Safety of Drugs and Medical Devices, (Enforcement Ordinance No. 11, 1961), and the Control Regulations of Veterinary Medical Products (Control Regulations, Ministerial Ordinance No. 107, 2004). Besides local guidelines established for registration studies by MAFF, further globally harmonized VICH guidelines on quality, safety, and efficacy have to be considered for registration (http://www.maff.go.jp/nval/accessed November 30th, 2020). Preventive treatment using oral products containing only one API dominate the market, including many generics (Table 4). Adulticidal products based on melarsomine dihydrochloride (Immiticide and generics thereof) have been authorized, but are no longer available in Japan, as their production and sales have been discontinued.
Table 4.
APIs and products approved in Japan for prevention of heartworm disease or treatment of heartworm infections.
| Heartworm active API | Species | Route of application | Trade name | Combination product with | Company |
|---|---|---|---|---|---|
| Eprinomectin | cat | topical | Broadline | Fipronil, Praziquantel, (S)-Methoprene | Boehringer Ingelheim AH |
| Ivermectin | dog | oral | Cardomec generics thereof: Azavasuca Heartmectin Panamectin |
Boehringer Ingelheim AH Nissin Pharmaceutical ASKA AH Meiji Seika Pharma |
|
| dog | oral | Cardomec P generics thereof: Iverguard P Ivermec DSP, PI Panamectin |
Pyrantel pamoate | Boehringer Ingelheim AH Kyoritsu Seiyaku Fujita Pharmaceutical Meiji Seika Pharma |
|
| Milbemycin oxime | dog | oral | Milbemycin A generics thereof: Milbeguard Milbejelly Milbemycin |
Elanco SAMPO Pharm Meiji Seika Pharma Fujita Pharmaceutical |
|
| dog | oral | Interceptor S | Praziquantel | Elanco | |
| dog, cat | oral | Milbemax | Praziquantel | Elanco | |
| dog | oral | Nexgard Spectra | Afoxolaner | Boehringer Ingelheim AH | |
| dog | oral | Panoramis | Spinosad | Elanco | |
| dog | oral | Systec | Lufenuron | Elanco | |
| Moxidectin | dog | oral | Moxidec generics thereof: Moxiguard Moxiheart |
Zoetis SAMPO Pharm Fujita Pharmaceutical |
|
| dog | s.c. | Proheart-12 | Zoetis | ||
| dog, cat | topical | Advocate | Imidacloprid | Bayer Yakuhin | |
| Selamectin | dog, cat | topical | Revolution | Zoetis | |
| cat | topical | Revolution plus | Sarolaner | Zoetis | |
| Melarsomine dihydrochloride | dog | i.m. | Immiticide | Boehringer Ingelheim AH Kyoritsu Pharmaceutical |
|
Preventives dominate the market; only one product is approved for adulticidal treatment (see last row of table). Data retrieved from: MAFF National Veterinary Assay Laboratory http://www.maff.go.jp/nval/accessed November 30th, 2020; AH – Animal Health.
4.6.4. Australia
All agricultural and veterinary chemical products sold in Australia have to be registered by the Australian Pesticides and Veterinary Medicines Authority (APVMA). The “Efficacy and target animal safety general guideline (Part 8)” in conjunction with the adopted VICH guidelines as well as the World Association for the Advancement of Veterinary Parasitology (WAAVP) guidelines have to be followed. More than 95% efficacy against D. immitis is required for registration.
While preventive products containing abamectin and diethylcarbamazine citrate as well as melarsomine treatment are available for dogs only, all other products are also approved for use in cats. Some moxidectin-based products are also approved for use in ferrets, while some selamectin-based products can be used in rabbits, too. For adulticide treatment, only melarsomine dihydrochloride as Immiticide is registered (Table 5).
Table 5.
APIs and products approved in Australia for prevention of heartworm disease or treatment of heartworm infections.
| Heartworm active API |
Species | Route of application | Trade names | Combination product with | Company |
|---|---|---|---|---|---|
| Abamectin | dog | oral | Virbac Canimax Purina Total Care Heartwormer and Allwormer |
Praziquantel Oxibendazole |
Virbac Nestle Purina Petcare |
| Diethylcarbamazine citrate | dog | oral | Aristopet/Vitapet Heartworm Dimmitrol |
Aristopet Mavlab |
|
| Ivermectin | dog | oral | Exelpet EZY - Heartworm Heartgard 30 Heartworm Soluble I Love My Pet Heartworm Chewables Nuheart Saint Bernard Petcare Soluble Heartworm Valuheart Vetafarm Heart Gold |
Exelpet Products/Mars Boehringer Ingelheim AH Arkolette My Pet Products Australia Bocko/Flexsky in partnership Australian Pharmavet Contract Manufacturing Jurox & Zoo Pets Vetafarm |
|
| Exelpet EZY - Heartwormer + Intestinal All-Wormer Guardian Complete Popantel Allwormer Plus Heartworm |
Oxantel embonate Pyrantel embonate Praziquantel |
Exelpet Products/Mars Intervet Jurox |
|||
| Heartgard 30 Plus Startgard Plus for Puppies (Heartgard 30 Plus + Frontline Plus combi pack) |
Pyrantel embonate | Boehringer Ingelheim AH | |||
| cat | oral | Heartgard 30 FX Startgard Plus for Kittens (Heartgard 30 FX + Frontline plus combi pack) |
Boehringer Ingelheim AH | ||
| Milbemycin oxime | dog | oral | NexGard Spectra | Afoxolaner | Boehringer Ingelheim AH |
| Interceptor Spectrum, Purina Total Care Heartwormer & Allwormer |
Praziquantel | Elanco | |||
| Purina Total Care Heartwormer, Allwormer & Flea Control, Sentinel Spectrum |
Praziquantel, Lufenuron, | Elanco | |||
| Panoramis, Trifexis | Spinosad | Elanco | |||
| dog, cat | oral | Milbemax Milpro |
Praziquantel | Elanco Virbac |
|
| Moxidectin | dog | oral | Proheart | Zoetis | |
| dog | s.c. | Proheart SR-12 Injection | Zoetis | ||
| dog | topical | Vets Choice for Fleas, Heartworm and Worms | Imidacloprid | Elanco | |
| dog, cat, ferret | topical | Advantage Advocate Exelpet Vet Series Flea, Intestinal & Heartworm Exi-Flea Plus Moxiclear |
Imidacloprid | Elanco Abbey Laboratories Norbrook Laboratories |
|
| dog, cat | topical | Aristopet AH fleas, heartworm and worms Neovet Wagg & Purr Fleas, Heartworm & Worms |
Imidacloprid | Aristopet Shanghai Neway AH Avet Health |
|
| cat | topical | Bravecto Plus | Fluralaner | Intervet | |
| dog | oral | Simparica Trio | Sarolaner, Pyrantel embonate | Zoetis | |
| Selamectin | dog, cat, rabbit | topical | Evicto Neovela Purevet/Revolution Selapro Wagg & Purr Fleas & Heartworm |
Virbac Shanghai Neway AH Zoetis Norbrook Laboratories Avet Health |
|
| cat | topical | Revolution Plus | Sarolaner | Zoetis | |
| Melarsomine dihydrochloride | Immiticide Canine Heartworm Treatment | Boehringer Ingelheim AH | |||
Preventives dominate the market; only one product is approved for adulticidal treatment (see last row of table). Data retrieved from: Australian Pesticides and Veterinary Medicines Authority https://apvma.gov.au accessed November 30th, 2020; AH – Animal Health.
5. The way forward
5.1. Drug discovery
The potential for an economic return on investment in the heartworm disease area will certainly continue to drive anti-filarial drug discovery and development. Novel non-ML compounds suitable for preventive treatment are needed in order to control or delay the development of ML-resistant D. immitis populations. A comprehensive discovery approach includes phenotypic screening, mode of action and molecular target assessment, target based screening, medicinal chemistry, and advanced in vitro and in vivo profiling (Selzer and Epe, 2021). The availability of automated phenotypic screening assays with multiple endpoint readings enables a substantial increase of the number of compounds to be evaluated (Preston et al., 2015; Cornaglia et al., 2017; Partridge et al., 2018). In addition, rapid technological improvements in molecular biology, biochemistry, and related technologies allow detailed insight in the molecular mechanisms of drug action. The combination of phenotypic and molecular studies brings anti-filarial drug discovery to an innovative new level (Selzer and Epe, 2021). The introduction of advanced models to assess compounds against the larval stages of Dirofilaria in vitro and moreover in vivo will accelerate that approach and overcome the presently time-consuming study periods due to the long development cycle of D. immitis. Immunocompromised rodents have recently been employed to model the infection of D. immitis in canines (Abraham et al., 2018; Mills et al., 2020). Other qualities of these models include facilitating more routine access to the L4 stages from an in vivo environment. In addition to the discovery of novel actives, it may be worthwhile to revisit the mechanism of action and the potential of derivatives of known anthelmintics such as emodepside and monepantel (Harder and von Samson-Himmelstjerna, 2002; Hess et al., 2016; Kuesel, 2016). Beyond the scientific and technical challenges for a new heartworm preventive, FDA registration has traditionally required a new drug to reach 100% efficacy. However, it has been suggested that the statistical and scientific requirements for registration need to be re-evaluated (Vidyashankar et al., 2017).
5.2. Genomics
Technology advancements of the life sciences in the last three decades have been so innovative that personalized or precision human medicine is possible today (Selzer et al., 2018; Johnson et al., 2020). One example of this breakthrough technology is seen in human cancer treatment, where CRISPR-Cas has positively impacted genetic research and gene therapy (Uddin et al., 2020; Zhang, 2020). In strong contrast, genomics, transcriptomics or gene manipulation in helminths and especially in filarial parasites is still in its infancy. The currently incomplete mapping of the D. immitis genome does not support rapid advancement of target-based approaches to drug discovery but applying advanced omics approaches may allow discovery of D. immitis or filariae specific drug targets in the coming years (Grote et al., 2017; Wheeler et al., 2020). The first draft genome sequence of a filarial nematode parasite – representing the first helminth parasite genome – was that of B. malayi in 2007 (Ghedin et al., 2007) nine years after the elucidation of the C. elegans genome (C. elegans Sequencing Consortium, 1998). Like many other parasites, B. malayi has undergone a genomic reduction in the course of evolution. While C. elegans has around 19,000 protein-coding genes (C. elegans Sequencing Consortium, 1998), B. malayi has only about 11,000 protein-coding genes in the draft genome (Ghedin et al., 2007), nearly completed by Tracey et al. (2020). Most interestingly, with the deciphering of the draft genome of B. malayi it became obvious that many filarial parasites harbor the endosymbiont Wolbachia (Scott et al., 2012). The first genome sequence of a filarial nematode important in animal health was that of D. immitis (Godel et al., 2012). As with most filarial parasites, D. immitis also harbors the endosymbiont Wolbachia, as analyzed throughout for the different life cycle stages and by tissue specific transcriptomics (Luck et al., 2014, 2015). Currently, many helminth genomes have been sequenced (Zarowiecki and Berriman, 2015) and most data are provided at the ‘WormBase ParaSite’ database (http://parasite.wormbase.org) (Howe et al., 2017). More recently, genome sequencing and stage specific transcriptomic analysis of D. repens has been provided (Cafarelli et al., 2019). Additionally, extensive comparative genomics analyses have been performed for a total of 81 roundworms and flatworms, elucidating gene families relevant to parasitism including host parasite interaction, modulation of immune response or parasite migration, to name only a few (Coghlan et al., 2019). In this regard, the draft, unassembled state of the D. immitis genome has presented a major hurdle for target-based drug discovery. One particular approach that may offer the chance to accelerate maturation/assembly of the genome is long-read RNA sequencing technology (Doyle et al., 2020; Wheeler et al., 2020). Longer sequence reads offer the ability to better define the organization of the genome and illuminate isoform diversity (Doyle et al., 2020). The latter attribute in particular will greatly inform target-based drug discovery in D. immitis with high confidence gene models. Regardless of the genome assembly mechanism, better quality genomes will also facilitate identification and characterization of small RNAs that play a role in host-pathogen interactions. These interactions may offer new landscapes in which novel preventive targets present themselves.
5.3. Anti-Wolbachia drugs
As elucidated above, the symbiotic gram-negative Wolbachia is considered a valid drug target to control filariae due to the essential metabolic contributions of Wolbachia to D. immitis (Slatko et al., 2010; Landmann, 2019). The viability of that approach was shown by screening of the predicted Wolbachia proteome of D. immitis which revealed antibiotic drug targets such as Fts proteins essential for cell division (Fts - filamentous temperature-sensitive mutant), and Sec proteins involved in directed transport of proteins across membranes (Sec - protein-secreting ATPase complex) (Godel et al., 2012). This was demonstrated experimentally in studies using the tetracycline antibiotic doxycycline, which cleared Wolbachia from D. immitis and led to gradual reduction of microfilariae and adult nematodes (Bandi et al., 1999; McCall et al., 2014b). Doxycycline was also successfully employed in humans as an anti-Wolbachia therapy against O. volvulus in combination with ivermectin (Hoerauf et al., 2003). However, the required long-treatment periods represent a major draw-back of this approach for veterinary filariosis (Savadelis et al., 2018). While the positive effects of anti-Wolbachia drugs in adulticidal therapy have been demonstrated, the necessity of a long administration period makes them unsuitable as preventive drugs for dogs. Novel anti-Wolbachia drugs with shorter treatment regimens would be desired as outlined by the “anti-Wolbachia (A-WOL) Consortium” (Taylor et al., 2014). More recently, other anti-Wolbachia drugs like minocycline (Papich, 2017) and next-generation anti-Wolbachia candidates discovered through phenotypic screening (Turner et al., 2020) were evaluated for efficacy against D. immitis. Various novel anti-Wolbachia candidates are currently under investigation. Advanced high throughput cell-based phenotypic screens have revealed a number of potent anti-Wolbachia small molecules with unknown mode of action (Bakowski and McNamara, 2019). Moreover, a tylosin analog microfilaricide showed a potent in vitro anti-Wolbachia activity in insect cells, performed well in preclinical safety studies, and showed efficacy in in vivo mouse and gerbil models (Taylor et al., 2019).
5.4. Other approaches
The application of insecticides to block transmission of D. immitis to dogs was demonstrated in a study with topically applied imidacloprid and permethrin (Hayasaki and Saeki, 2009). The principle of using insecticides to prevent pathogen transmission including nematodes was reviewed by Beugnet and Franc (2012). More recently, dinotefuran-permethrin-pyriproxyfen (DPP) in a topical formulation was 95% effective in blocking transmission of D. immitis in two controlled laboratory studies (McCall et al., 2017a). If translatable to field conditions, that would most likely lead to effective population control of the mosquitoes, resulting in a reduction of the infection pressure. In addition, it was demonstrated that a combination treatment with DPP and milbemycin oxime was 100% efficacious against the ML-resistant Dirofilaria JYD-34 isolate while neither the insecticide nor the milbemycin oxime alone showed a 100% efficacy (McCall et al., 2017b). Thus, the authors argued for a double defense protocol to provide effective prevention of heartworm disease in dogs. While repellents or insecticides would reduce transmission of D. immitis, they might not be completely effective as a monotherapy for prevention, but the synergistic activity in combination with a ML could provide a more complete prevention. However, these findings need to be verified under field conditions. Deltamethrin or imidacloprid and flumethrin containing collars (Scalibor®, Serestro®) claim to prevent predominantly ticks and fleas, and possibly also heartworm transmitting mosquitoes, but are not recommended for a monotherapy for prevention. The more recently introduced isoxazolines are not effective until ingested, thus do not act quickly enough to prevent transmission of D. immitis (Schorderet-Weber et al., 2017).
A safe and efficacious vaccine against D. immitis has the potential to substantially impact the established heartworm market. It would provide veterinarians and pet owners with an alternative to the current use of regularly administered MLs. However, at present there are only two anthelmintic vaccines commercialized: The lungworm vaccine Dictol® has been on the market for more than 50 years to protect cattle against Dictyocaulus viviparus infections (Jarrett et al., 1960). This attenuated vaccine is still used despite the threefold booster process and the requirement for a cold chain distribution process. More recently, a recombinant subunit vaccine demonstrated protective capability in cattle but does not provide a sterilizing efficacy (Strube et al., 2015). The Barbervax® vaccine for sheep against H. contortus (also known as barber-pole worm) utilizes biochemically purified gut proteins from harvested worms from infected sheep (Scarff et al., 2020). The vaccine lasts for only 6 weeks after the sheep have been primed by a series of 3–6 vaccinations but aids in the control of H. contortus under high infection pressure (Bassetto et al., 2018) and has been commercialized in Australia since 2014 (Maxwell, 2015).
A vaccine against heartworm would need to fulfill a number of stronger requirements than those necessary for vaccines against ruminant GI-nematodes. Because the FDA set efficacy requirements for chemical preventives to 100%, it can be assumed that the efficacy of a heartworm vaccine would also need to approach or be 100%, which would be difficult to achieve. Possible alternatives to a mono vaccine prevention approach could be a combination of a vaccine with a ML. The vaccine would add an alternative mode of prevention and thus, such combination may be superior to protect against ML resistant heartworm isolates than MLs alone. However, beside the difficulties to develop an effective vaccine, acceptance of a vaccine-drug combination by dog owners would depend on additional parameters like the frequency of immunization boosters and the period of protection. A vaccine would only provide an advantage over current preventives if it protects dogs and potentially cats for an entire heartworm season, which in many areas would mean year-round. A genetically or even biochemically engineered vaccine would be superior to an attenuated D. immitis based product. The further advancement of the ‘omics’, and together with the rise of deep data analyses in multiple fields and other technologically advanced tools like mRNA or microRNA technologies (Britton et al., 2014; Sahin et al., 2014; Kis et al., 2019; Tombácz et al., 2021), may enable next-generation vaccine development against D. immitis in the future. Current successful examples for an RNA-based vaccine approach are seen with the RNA vaccine candidates for global SARS-CoV-2 pandemic (Bloom et al., 2020).
6. Conclusion
Heartworm disease is a serious threat for dogs and cats in many parts of the world. Because it is unlikely that heartworm prevention can be achieved solely by improved vector control, preventive treatment based on actives of the ML class remains the mainstay of control. However, ML-resistant populations have been reported from the Mississippi River areas in the USA and have been characterized for their sensitivity status. Fortunately, for the majority of regions, todays available drugs are still of high value. With confirmed resistance to all MLs it is evident that new control methods are urgently needed. Continuous progression in technology and science enables new innovative approaches to search for novel drugs affecting filarial-specific targets. For example, focused assays have been developed to discover anti-Wolbachia compounds with an indirect but finally lethal effect on D. immitis. Apart from the search for novel APIs, the time and technology seem also ripe to discover highly effective vaccines. Anti-filarial vaccines would have the potential to change heartworm control substantially. Such a vaccine may become part of a more holistic heartworm control when combined with preventive drugs like MLs or yet to be discovered actives. Finally, it is important to emphasize compliance with veterinarians and pet owners in order to provide sufficient protection of the animals and to delay development of drug resistant D. immitis populations. Therefore, ideal novel products should be sustainable and highly effective, and should possess a convenient route of administration to encourage owner compliance with required dosing regimens. Covering a broader range of endo- and ectoparasites within one product is a desired add-on.
Declaration of competing interest
SN, JH, DSC and PMS are employees of Boehringer Ingelheim Animal Health, an organization with commercial interest in the animal health market. RK is an independent consultant of Boehringer Ingelheim Animal Health.
Acknowledgement
We are very grateful to Hiroki Maeda, Charles Q. Meng and Cara A. Noack for their technical support in preparing tables and figures. We thank Frédéric Beugnet and Steffen Rehbein for helpful and constructive discussions.
References
- Abraham D., Hess J., Bondesen B.A., Harrington J.M. 2018. In Vivo Model for Parasitic Worm Infection Uses Immunocompromized Rodent and Methods for Evaluating Antiparasitic Compounds, Including Compounds Active against Canine Heartworm. WO2018148392. World Intellectual Property Organization. [Google Scholar]
- Akao N. Human dirofilariasis in Japan. Trop. Med. Health. 2011;39:65–71. doi: 10.2149/tmh.39-1-suppl_2-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Althoff T., Hibbs R.E., Banerjee S., Gouaux E. X-ray structures of GluCl in apo states reveal a gating mechanism of Cys-loop receptors. Nature. 2014;512:333–337. doi: 10.1038/nature13669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Heartworm Society . American Heartworm Society; 2020. Current canine guidelines for the prevention, diagnosis, and management of heartworm (Dirofilaria immitis) infection in dogs.https://d3ft8sckhnqim2.cloudfront.net/images/pdf/2020_AHS_Canine_Guidelines_Summary_11_12.pdf?1605556516 accessed November 25th, 2020. [Google Scholar]
- Ames M.K., Atkins C.E. Treatment of dogs with severe heartworm disease. Vet. Parasitol. 2020;283:109131. doi: 10.1016/j.vetpar.2020.109131. [DOI] [PubMed] [Google Scholar]
- Ames M.K., VanVranken P., Evans C., Atkins C.E. Non-Arsenical heartworm adulticidal therapy using topical moxidectin-imidacloprid and doxycycline: a prospective case series. Vet. Parasitol. 2020;282:109099. doi: 10.1016/j.vetpar.2020.109099. [DOI] [PubMed] [Google Scholar]
- Arena J.P., Liu K.K., Paress P.S., Schaeffer J.M., Cully D.F. Expression of a glutamate-activated chloride current in Xenopus oocytes injected with Caenorhabditis elegans RNA: evidence for modulation by avermectin. Brain research. Mol. Brain Res. 1992;15:339–348. doi: 10.1016/0169-328x(92)90127-w. [DOI] [PubMed] [Google Scholar]
- Arena J.P., Liu K.K., Paress P.S., Frazier E.G., Cully D.F., Mrozik H., Schaeffer J.M. The mechanism of action of avermectins in Caenorhabditis elegans: correlation between activation of glutamate-sensitive chloride current, membrane binding, and biological activity. J. Parasitol. 1995;81:286–294. [PubMed] [Google Scholar]
- Ashford R.W. Current usage of nomenclature for parasitic diseases, with special reference to those involving arthropods. J. Med. Entomol. 2001;15:121–125. doi: 10.1046/j.0269-283x.2001.00306.x. [DOI] [PubMed] [Google Scholar]
- Ashour D.S. Ivermectin: from theory to clinical application. Int. J. Antimicrob. Agents. 2019;54:134–142. doi: 10.1016/j.ijantimicag.2019.05.003. [DOI] [PubMed] [Google Scholar]
- Atif M., Smith J.J., Estrada-Mondragon A., Xiao X., Salim A.A., Capon R.J., Lynch J.W., Keramidas A. GluClR-mediated inhibitory postsynaptic currents reveal targets for ivermectin and potential mechanisms of ivermectin resistance. PLoS Pathog. 2019;15 doi: 10.1371/journal.ppat.1007570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins C.E. Comparison of results of three commercial heartworm antigen test kits in dogs with low heartworm burdens. J. Am. Vet. Med. Assoc. 2003;222:1221–1223. doi: 10.2460/javma.2003.222.1221. [DOI] [PubMed] [Google Scholar]
- Atkins C.E., Litster A.L. Heartworm disease. In: August J.R., editor. Consultations in Feline Internal Medicine. ElsevierSaunders; Philadelphia, PA: 2006. pp. 323–330. [Google Scholar]
- Atkins C.E., Murray M.J., Olavessen L.J., Burton K.W., Marshall J.W., Brooks C.C. Heartworm 'lack of effectiveness' claims in the Mississippi delta: computerized analysis of owner compliance--2004-2011. Vet. Parasitol. 2014;206:106–113. doi: 10.1016/j.vetpar.2014.08.013. [DOI] [PubMed] [Google Scholar]
- Bai S.H., Ogbourne S. Eco-toxicological effects of the avermectin family with a focus on abamectin and ivermectin. Chemosphere. 2016;154:204–214. doi: 10.1016/j.chemosphere.2016.03.113. [DOI] [PubMed] [Google Scholar]
- Baker C.F., Tielemans E., Pollmeier M.G., McCall J.W., McCall S.D., Irwin J., Chester S.T., Carithers D.S., Rosentel J.K. Efficacy of a single dose of a novel topical combination product containing eprinomectin to prevent heartworm infection in cats. Vet. Parasitol. 2014;202:49–53. doi: 10.1016/j.vetpar.2014.02.039. [DOI] [PubMed] [Google Scholar]
- Bakowski M.A., McNamara C.W. Advances in antiwolbachial drug discovery for treatment of parasitic filarial worm infections. Trav. Med. Infect. Dis. 2019;4(3):108. doi: 10.3390/tropicalmed4030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesteros C., Pulaski C.N., Bourguinat C., Keller K., Prichard R.K., Geary T.G. Clinical validation of molecular markers of macrocyclic lactone resistance in Dirofilaria immitis. Int. J. Parasitol. Drugs Drug Resist. 2018;8:596–606. doi: 10.1016/j.ijpddr.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandi C., McCall J.W., Genchi C., Corona S., Venco L., Sacchi L. Effects of tetracycline on the filarial worms Brugia pahangi and Dirofilaria immitis and their bacterial endosymbionts Wolbachia. Int. J. Parasitol. 1999;29:357–364. doi: 10.1016/s0020-7519(98)00200-8. [DOI] [PubMed] [Google Scholar]
- Banks B.J., Bishop B.F., Evans N.A., Gibson S.P., Goudie A.C., Gration K.A., Pacey M.S., Perry D.A., Witty M.J. Avermectins and flea control: structure-activity relationships and the selection of selamectin for development as an endectocide for companion animals. Bioorg. Med. Chem. 2000;8:2017–2025. doi: 10.1016/s0968-0896(00)00120-6. [DOI] [PubMed] [Google Scholar]
- Barnes N.M., Hales T.G., Lummis S.C., Peters J.A. The 5-HT3 receptor--the relationship between structure and function. Neuropharmacology. 2009;56:273–284. doi: 10.1016/j.neuropharm.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr M.C., Boynton E.P., Schmidt P.L., Bossong F., Johnston G.R. Diagnosis of canine heartworm infection. Today’s Veterinary Practice Jul/Aug. 2011:30–37. [Google Scholar]
- Bassetto C.C., Almeida F.A., Newlands G.F.J., Smith W.D., Castilhos A.M., Fernandes S., Siqueira E.R., Amarante A.F.T. Trials with the Haemonchus vaccine, Barbervax®, in ewes and lambs in a tropical environment: nutrient supplementation improves protection in periparturient ewes. Vet. Parasitol. 2018;264:52–57. doi: 10.1016/j.vetpar.2018.11.006. [DOI] [PubMed] [Google Scholar]
- Bazzocchi C., Mortarino M., Grandi G., Kramer L.H., Genchi C., Bandi C., Genchi M., Sacchi L., McCall J.W. Combined ivermectin and doxycycline treatment has microfilaricidal and adulticidal activity against Dirofilaria immitis in experimentally infected dogs. Int. J. Parasitol. 2008;38:1401–1410. doi: 10.1016/j.ijpara.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Becskei C., Thys M., Doherty P., Mahabir S.P. Efficacy of orally administered combination of moxidectin, sarolaner and pyrantel (Simparica Trio™) for the prevention of experimental Angiostrongylus vasorum infection in dogs. Parasites Vectors. 2020;13:64. doi: 10.1186/s13071-020-3948-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdoulay P., Levy J.K., Snyder P.S., Pegelow M.J., Hooks J.L., Tavares L.M., Gibson N.M., Salute M.E. Comparison of serological tests for the detection of natural heartworm infection in cats. J. Am. Anim. Hosp. Assoc. 2004;40:376–384. doi: 10.5326/0400376. [DOI] [PubMed] [Google Scholar]
- Berrafato T., Coates R., Reaves B.J., Kulke D., Wolstenholme A.J. Macrocyclic lactone anthelmintic-induced leukocyte binding to Dirofilaria immitis microfilariae: influence of the drug resistance status of the parasite. Int. J. Parasitol. Drugs Drug Resist. 2019;10:45–50. doi: 10.1016/j.ijpddr.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beugnet F., Franc M. Insecticide and acaricide molecules and/or combinations to prevent pet infestation by ectoparasites. Trends Parasitol. 2012;28:267–279. doi: 10.1016/j.pt.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Bishop B.F., Bruce C.I., Evans N.A., Goudie A.C., Gration K.A., Gibson S.P., Pacey M.S., Perry D.A., Walshe N.D., Witty M.J. Selamectin: a novel broad-spectrum endectocide for dogs and cats. Vet. Parasitol. 2000;91:163–176. doi: 10.1016/s0304-4017(00)00289-2. [DOI] [PubMed] [Google Scholar]
- Blagburn B.L., Dillon A.R., Arther R.G., Butler J.M., Newton J.C. Comparative efficacy of four commercially available heartworm preventive products against the MP3 laboratory strain of Dirofilaria immitis. Vet. Parasitol. 2011;176:188–194. doi: 10.1016/j.vetpar.2010.12.049. [DOI] [PubMed] [Google Scholar]
- Bloom K., van den Berg F., Arbuthnot P. Self-amplifying RNA vaccines for infectious diseases. Gene Ther. 2020:1–13. doi: 10.1038/s41434-020-00204-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguinat C., Keller K., Bhan A., Peregrine A., Geary T., Prichard R. Macrocyclic lactone resistance in Dirofilaria immitis. Vet. Parasitol. 2011;181:388–392. doi: 10.1016/j.vetpar.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Bourguinat C., Keller K., Prichard R.K., Geary T.G. Genetic polymorphism in Dirofilaria immitis. Vet. Parasitol. 2011;176:368–373. doi: 10.1016/j.vetpar.2011.01.023. [DOI] [PubMed] [Google Scholar]
- Bourguinat C., Lee A.C., Lizundia R., Blagburn B.L., Liotta J.L., Kraus M.S., Keller K., Epe C., Letourneau L., Kleinman C.L., Paterson T., Gomez E.C., Montoya-Alonso J.A., Smith H., Bhan A., Peregrine A.S., Carmichael J., Drake J., Schenker R., Kaminsky R., Bowman D.D., Geary T.G., Prichard R.K. Macrocyclic lactone resistance in Dirofilaria immitis: failure of heartworm preventives and investigation of genetic markers for resistance. Vet. Parasitol. 2015;210:167–178. doi: 10.1016/j.vetpar.2015.04.002. [DOI] [PubMed] [Google Scholar]
- Bové C.M., Gordon S.G., Saunders A.B., Miller M.W., Roland R.M., Achen S.E., Drourr L.T., Boggess M.M. Outcome of minimally invasive surgical treatment of heartworm caval syndrome in dogs: 42 cases (1999-2007) J. Am. Vet. Med. Assoc. 2010;236:187–192. doi: 10.2460/javma.236.2.187. [DOI] [PubMed] [Google Scholar]
- Bowman D.D., Atkins C.E. Heartworm biology, treatment, and control. Vet. Clin. North. Am. Small Anim. Pract. 2009;39:1127–1158. doi: 10.1016/j.cvsm.2009.06.003. vii. [DOI] [PubMed] [Google Scholar]
- Bowman D.D., Drake J. Examination of the "susceptibility gap" in the treatment of canine heartworm infection. Parasites Vectors. 2017;10:513. doi: 10.1186/s13071-017-2433-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman D.D., Hendrix C.M., Lindsay D.S., Barr S.C. first ed. Wiley-Blackwell; 2002. Feline Clinical Parasitology. [Google Scholar]
- Bowman D., Little S.E., Lorentzen L., Shields J., Sullivan M.P., Carlin E.P. Prevalence and geographic distribution of Dirofilaria immitis, Borrelia burgdorferi, Ehrlichia canis, and Anaplasma phagocytophilum in dogs in the United States: results of a national clinic-based serologic survey. Vet. Parasitol. 2009;160:138–148. doi: 10.1016/j.vetpar.2008.10.093. [DOI] [PubMed] [Google Scholar]
- Bowman D.D., Liu Y., McMahan C.S., Nordone S.K., Yabsley M.J., Lund R.B. Forecasting United States heartworm Dirofilaria immitis prevalence in dogs. Parasites Vectors. 2016;9:540. doi: 10.1186/s13071-016-1804-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton C., Winter A.D., Gillan V., Devaney E. microRNAs of parasitic helminths - identification, characterization and potential as drug targets. Int. J. Parasitol. Drugs Drug Resist. 2014;4:85–94. doi: 10.1016/j.ijpddr.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H.E., Harrington L.C., Kaufman P.E., McKay T., Bowman D.D., Nelson C.T., Wang D., Lund R. Key factors influencing canine heartworm, Dirofilaria immitis, in the United States. Parasites Vectors. 2012;5:245. doi: 10.1186/1756-3305-5-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham S.D., Mann H.J., Hearnden O.K., Sattelle D.B. 2020. Turning a Drug Target into a Drug Candidate: A New Paradigm for Neurological Drug Discovery? BioEssays: News and Reviews in Molecular, Cellular and Developmental Biology. [DOI] [PubMed] [Google Scholar]
- Burg R.W., Miller B.M., Baker E.E., Birnbaum J., Currie S.A., Hartman R., Kong Y.L., Monaghan R.L., Olson G., Putter I., Tunac J.B., Wallick H., Stapley E.O., Oiwa R., Omura S. Avermectins, new family of potent anthelmintic agents: producing organism and fermentation. Antimicrob. Agents Chemother. 1979;15:361–367. doi: 10.1128/aac.15.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- C. elegans Sequencing Consortium Genome sequence of the nematode C. elegans: a platform for investigating biology. Science. 1998;282:2012–2018. doi: 10.1126/science.282.5396.2012. [DOI] [PubMed] [Google Scholar]
- Cafarelli C., Russo G., Mathis A., Silaghi C. De novo genome sequencing and comparative stage-specific transcriptomic analysis of Dirofilaria repens. Int. J. Parasitol. 2019;49:911–919. doi: 10.1016/j.ijpara.2019.04.008. [DOI] [PubMed] [Google Scholar]
- Caly L., Druce J.D., Catton M.G., Jans D.A., Wagstaff K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020;178:104787. doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell W.C. An introduction to the avermectins. N. Z. Vet. J. 1981;29:174–178. doi: 10.1080/00480169.1981.34836. [DOI] [PubMed] [Google Scholar]
- Campbell W.C. History of avermectin and ivermectin, with notes on the history of other macrocyclic lactone antiparasitic agents. Curr. Pharmaceut. Biotechnol. 2012;13:853–865. doi: 10.2174/138920112800399095. [DOI] [PubMed] [Google Scholar]
- Campbell W.C., Fisher M.H., Stapley E.O., Albers-Schönberg G., Jacob T.A. Ivermectin: a potent new antiparasitic agent. Science. 1983;221:823–828. doi: 10.1126/science.6308762. [DOI] [PubMed] [Google Scholar]
- Cancrini G., Frangipane di Regalbono A., Ricci I., Tessarin C., Gabrielli S., Pietrobelli M. Aedes albopictus is a natural vector of Dirofilaria immitis in Italy. Vet. Parasitol. 2003;118:195–202. doi: 10.1016/j.vetpar.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Cancrini G., Magi M., Gabrielli S., Arispici M., Tolari F., Dell'Omodarme M., Prati M.C. Natural vectors of Dirofilariasis in rural and urban areas of the Tuscan region, central Italy. J. Med. Entomol. 2006;43:574–579. doi: 10.1603/0022-2585(2006)43[574:nvodir]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Capelli G., Genchi C., Baneth G., Bourdeau P., Brianti E., Cardoso L., Danesi P., Fuehrer H.-P., Giannelli A., Ionică A.M., Maia C., Modrý D., Montarsi F., Krücken J., Papadopoulos E., Petrić D., Pfeffer M., Savić S., Otranto D., Poppert S., Silaghi C. Recent advances on Dirofilaria repens in dogs and humans in Europe. Parasites Vectors. 2018;11:663. doi: 10.1186/s13071-018-3205-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carithers D.S. Examining the role of macrolides and host immunity in combatting filarial parasites. Parasites Vectors. 2017;10:182. doi: 10.1186/s13071-017-2116-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael J., McCall S., DiCosty U., Mansour A., Roycroft L. Evaluation of Dirofilaria immitis antigen detection comparing heated and unheated serum in dogs with experimental heartworm infections. Parasites Vectors. 2017;10:486. doi: 10.1186/s13071-017-2445-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carretón E., Morchón R., Simón F., Juste M.C., González-Miguel J., Montoya-Alonso J.A. Evaluation of cardiopulmonary biomarkers during classic adulticide treatment versus the American Heartworm Society recommended treatment protocol in dogs infected by Dirofilaria immitis. Vet. Parasitol. 2014;206:55–59. doi: 10.1016/j.vetpar.2014.08.015. [DOI] [PubMed] [Google Scholar]
- Carretón E., Falcón-Cordón Y., Falcón-Cordón S., Morchón R., Matos J.I., Montoya-Alonso J.A. Variation of the adulticide protocol for the treatment of canine heartworm infection: can it be shorter? Vet. Parasitol. 2019;271:54–56. doi: 10.1016/j.vetpar.2019.06.006. [DOI] [PubMed] [Google Scholar]
- Carter G.T., Nietsche J.A., Goodman J.J., Torrey M.J., Dunne T.S., Borders D.B., Testa R.T. LL-F42248 alpha, a novel chlorinated pyrrole antibiotic. J. Antibiot. (Tokyo) 1987;40:233–236. doi: 10.7164/antibiotics.40.233. [DOI] [PubMed] [Google Scholar]
- Carter G.T., Nietsche J.A., Hertz M.R., Williams D.R., Siegel M.M., Morton G.O., James J.C., Borders D.B. LL-F28249 antibiotic complex: a new family of antiparasitic macrocyclic lactones. Isolation, characterization and structures of LL-F28249 alpha, beta, gamma, lambda. J. Antibiot. (Tokyo) 1988;41:519–529. doi: 10.7164/antibiotics.41.519. [DOI] [PubMed] [Google Scholar]
- Cella W., Baia-da-Silva D.C., Melo G.C., Tadei W.P., Sampaio V.S., Pimenta P., Lacerda M.V.G., Monteiro W.M. Do climate changes alter the distribution and transmission of malaria? Evidence assessment and recommendations for future studies. Rev. Soc. Bras. Med. Trop. 2019;52 doi: 10.1590/0037-8682-0308-2019. [DOI] [PubMed] [Google Scholar]
- Chabala J.C., Mrozik H., Tolman R.L., Eskola P., Lusi A., Peterson L.H., Woods M.F., Fisher M.H., Campbell W.C., Egerton J.R., Ostlind D.A. Ivermectin, a new broad-spectrum antiparasitic agent. J. Med. Chem. 1980;23:1134–1136. doi: 10.1021/jm00184a014. [DOI] [PubMed] [Google Scholar]
- Chitkara R.K., Sarinas P.S. Dirofilaria, visceral larva migrans, and tropical pulmonary eosinophilia. Semin. Respir. Infect. 1997;12:138–148. [PubMed] [Google Scholar]
- Coghlan A., Tyagi R., Cotton J.A., Holroyd N., Rosa B.A., Tsai I.J., Laetsch D.R., Beech R.N., Day T.A., Hallsworth-Pepin K., Ke H.-M., Kuo T.-H., Lee T.J., Martin J., Maizels R.M., Mutowo P., Ozersky P., Parkinson J., Reid A.J., Rawlings N.D., Ribeiro D.M., Swapna L.S., Stanley E., Taylor D.W., Wheeler N.J., Zamanian M., Zhang X., Allan F., Allen J.E., Asano K., Babayan S.A., Bah G., Beasley H., Bennett H.M., Bisset S.A., Castillo E., Cook J., Cooper P.J., Cruz-Bustos T., Cuéllar C., Devaney E., Doyle S.R., Eberhard M.L., Emery A., Eom K.S., Gilleard J.S., Gordon D., Harcus Y., Harsha B., Hawdon J.M., Hill D.E., Hodgkinson J., Horák P., Howe K.L., Huckvale T., Kalbe M., Kaur G., Kikuchi T., Koutsovoulos G., Kumar S., Leach A.R., Lomax J., Makepeace B., Matthews J.B., Muro A., O'Boyle N.M., Olson P.D., Osuna A., Partono F., Pfarr K., Rinaldi G., Foronda P., Rollinson D., Samblas M.G., Sato H., Schnyder M., Scholz T., Shafie M., Tanya V.N., Toledo R., Tracey A., Urban J.F., Wang L.-C., Zarlenga D., Blaxter M.L., Mitreva M., Berriman M., International Helminth Genomes C. Comparative genomics of the major parasitic worms. Nat. Genet. 2019;51:163–174. doi: 10.1038/s41588-018-0262-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly C.N., Wafford K.A. The Cys-loop superfamily of ligand-gated ion channels: the impact of receptor structure on function. Biochem. Soc. Trans. 2004;32:529–534. doi: 10.1042/BST0320529. [DOI] [PubMed] [Google Scholar]
- Cornaglia M., Lehnert T., Gijs M.A.M. Microfluidic systems for high-throughput and high-content screening using the nematode Caenorhabditis elegans. Lab Chip. 2017;17:3736–3759. doi: 10.1039/c7lc00509a. [DOI] [PubMed] [Google Scholar]
- Courtney C.H., Zeng Q. Comparison of heartworm antigen test kit performance in dogs having low heartworm burdens. Vet. Parasitol. 2001;96:317–322. doi: 10.1016/s0304-4017(01)00374-0. [DOI] [PubMed] [Google Scholar]
- Cully D.F., Vassilatis D.K., Liu K.K., Paress P.S., Van der Ploeg L.H., Schaeffer J.M., Arena J.P. Cloning of an avermectin-sensitive glutamate-gated chloride channel from Caenorhabditis elegans. Nature. 1994;371:707–711. doi: 10.1038/371707a0. [DOI] [PubMed] [Google Scholar]
- Dantas-Torres F., Otranto D. Overview on Dirofilaria immitis in the Americas, with notes on other filarial worms infecting dogs. Vet. Parasitol. 2020;282:109113. doi: 10.1016/j.vetpar.2020.109113. [DOI] [PubMed] [Google Scholar]
- Daurio C.P., Cheung E.N., Jeffcoat A.R., Skelly B.J. Bioavailability of ivermectin administered orally to dogs. Vet. Res. Commun. 1992;16:125–130. doi: 10.1007/BF01839009. [DOI] [PubMed] [Google Scholar]
- Dean M., Rzhetsky A., Allikmets R. The human ATP-binding cassette (ABC) transporter superfamily. Genome Res. 2001;11:1156–1166. doi: 10.1101/gr.184901. [DOI] [PubMed] [Google Scholar]
- Dekel Y., Machluf Y., Stoler A., Aderet A., Baumel D., Kellerman E., Plotsky Y., Noked Partouche O., Elhalal G., Ben-Shlomo I., Bercovich D. Frequency of canine nt230(del4) MDR1 mutation in prone pure breeds, their crosses and mongrels in Israel - insights from a worldwide comparative perspective. BMC Vet. Res. 2017;13:333. doi: 10.1186/s12917-017-1251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent J.A., Smith M.M., Vassilatis D.K., Avery L. The genetics of ivermectin resistance in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U.S.A. 2000;97:2674–2679. doi: 10.1073/pnas.97.6.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deplazes P., Eckert J., Mathis A., von Samson-Himmelstjerna G., Zahner H. Wageningen Academic Publishers; The Netherlands: 2016. Parasitology in Veterinary Medicine. [DOI] [Google Scholar]
- Dillon A.R., Warner A.E., Brawner W., Hudson J., Tillson M. Activity of pulmonary intravascular macrophages in cats and dogs with and without adult Dirofilaria immitis. Vet. Parasitol. 2008;158:171–176. doi: 10.1016/j.vetpar.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Dillon A.R., Blagburn B.L., Tillson M., Brawner W., Welles B., Johnson C., Cattley R., Rynders P., Barney S. Heartworm-associated respiratory disease (HARD) induced by immature adult Dirofilaria immitis in cats. Parasites Vectors. 2017;10:514. doi: 10.1186/s13071-017-2452-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle S.R., Tracey A., Laing R., Holroyd N., Bartley D., Bazant W., Beasley H., Beech R., Britton C., Brooks K., Chaudhry U., Maitland K., Martinelli A., Noonan J.D., Paulini M., Quail M.A., Redman E., Rodgers F.H., Sallé G., Shabbir M.Z., Sankaranarayanan G., Wit J., Howe K.L., Sargison N., Devaney E., Berriman M., Gilleard J.S., Cotton J.A. Genomic and transcriptomic variation defines the chromosome-scale assembly of Haemonchus contortus, a model gastrointestinal worm. Commun. Biol. 2020;3:656. doi: 10.1038/s42003-020-01377-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duce I.R., Scott R.H. Actions of dihydroavermectin B1a on insect muscle. Br. J. Pharmacol. 1985;85:395–401. doi: 10.1111/j.1476-5381.1985.tb08874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton C.J., Gibson S.P., Goudie A.C., Holdom K.S., Pacey M.S., Ruddock J.C., Bu'Lock J.D., Richards M.K. Novel avermectins produced by mutational biosynthesis. J. Antibiot. (Tokyo) 1991;44:357–365. doi: 10.7164/antibiotics.44.357. [DOI] [PubMed] [Google Scholar]
- El-Shahawi G.A., Abdel-Latif M., Saad A.H., Bahgat M. Setaria equina: in vivo effect of diethylcarbamazine citrate on microfilariae in albino rats. Exp. Parasitol. 2010;126:603–610. doi: 10.1016/j.exppara.2010.06.022. [DOI] [PubMed] [Google Scholar]
- Erickson S.M., Xi Z., Mayhew G.F., Ramirez J.L., Aliota M.T., Christensen B.M., Dimopoulos G. Mosquito infection responses to developing filarial worms. PLoS Neglected Trop. Dis. 2009;3:e529. doi: 10.1371/journal.pntd.0000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada-Mondragon A., Lynch J.W. Functional characterization of ivermectin binding sites in α1β2γ2L GABA(A) receptors. Front. Mol. Neurosci. 2015;8:55. doi: 10.3389/fnmol.2015.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Society of Dirofilariosis and Angiostrongylosis . 2017. Guidelines for Clinical Management of Canine Heartworm Disease.https://www.esda.vet/wp-content/uploads/2017/11/GUIDELINES-FOR-CLINICAL-MANAGEMENT-OF-CANINE-HEARTWORM-DISEASE.pdf accessed November 25th, 2020. [Google Scholar]
- Evans C.C., Moorhead A.R., Storey B.E., Wolstenholme A.J., Kaplan R.M. Development of an in vitro bioassay for measuring susceptibility to macrocyclic lactone anthelmintics in Dirofilaria immitis. Int. J. Parasitol. Drugs Drug Resist. 2013;3:102–108. doi: 10.1016/j.ijpddr.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans C.C., Burkman E.J., Dzimianski M.T., Moorhead A.R., Savadelis M.D., Angenendt C., Zymny S., Kulke D. Periodicity of Dirofilaria immitis in longterm infections. Parasitol. Res. 2017;116:75–80. doi: 10.1007/s00436-017-5493-z. [DOI] [PubMed] [Google Scholar]
- Evans C.C., Moorhead A.R., Storey B.E., Blagburn B.L., Wolstenholme A.J., Kaplan R.M. Evaluation of the larval migration inhibition assay for detecting macrocyclic lactone resistance in Dirofilaria immitis. Vet. Parasitol. 2017;246:76–81. doi: 10.1016/j.vetpar.2017.09.003. [DOI] [PubMed] [Google Scholar]
- FDA . 2018. The FDA Is Seeking Input on the Evaluation of Approaches to Demonstrate Effectiveness of Heartworm Preventatives for Dogs.https://www.fda.gov/animal-veterinary/cvm-updates/fda-seeking-input-evaluation-approaches-demonstrate-effectiveness-heartworm-preventatives-dogs accessed November 23rd, 2020. [Google Scholar]
- Feachem R.G., Phillips A.A., Hwang J., Cotter C., Wielgosz B., Greenwood B.M., Sabot O., Rodriguez M.H., Abeyasinghe R.R., Ghebreyesus T.A., Snow R.W. Shrinking the malaria map: progress and prospects. Lancet. 2010;376:1566–1578. doi: 10.1016/S0140-6736(10)61270-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira C., Afonso A., Calado M., Maurício I., Alho A.M., Meireles J., Madeira de Carvalho L., Belo S. Molecular characterization of Dirofilaria spp. circulating in Portugal. Parasites Vectors. 2017;10:250. doi: 10.1186/s13071-017-2180-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri E., Bain O., Barbuto M., Martin C., Lo N., Uni S., Landmann F., Baccei S.G., Guerrero R., de Souza Lima S. New insights into the evolution of Wolbachia infections in filarial nematodes inferred from a large range of screened species. PloS One. 2011;6 doi: 10.1371/journal.pone.0020843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firdova Z., Turnova E., Bielikova M., Turna J., Dudas A. The prevalence of ABCB1:c.227_230delATAG mutation in affected dog breeds from European countries. Res. Vet. Sci. 2016;106:89–92. doi: 10.1016/j.rvsc.2016.03.016. [DOI] [PubMed] [Google Scholar]
- Fontes-Sousa A.P., Silvestre-Ferreira A.C., Carretón E., Esteves-Guimarães J., Maia-Rocha C., Oliveira P., Lobo L., Morchón R., Araújo F., Simón F., Montoya-Alonso J.A. Exposure of humans to the zoonotic nematode Dirofilaria immitis in Northern Portugal. Epidemiol. Infect. 2019;147:e282. doi: 10.1017/S0950268819001687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J., Ganatra M., Kamal I., Ware J., Makarova K., Ivanova N., Bhattacharyya A., Kapatral V., Kumar S., Posfai J., Vincze T., Ingram J., Moran L., Lapidus A., Omelchenko M., Kyrpides N., Ghedin E., Wang S., Goltsman E., Joukov V., Ostrovskaya O., Tsukerman K., Mazur M., Comb D., Koonin E., Slatko B. The Wolbachia genome of Brugia malayi: endosymbiont evolution within a human pathogenic nematode. PLoS Biol. 2005;3:e121. doi: 10.1371/journal.pbio.0030121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuehrer H.P., Auer H., Leschnik M., Silbermayr K., Duscher G., Joachim A. Dirofilaria in humans, dogs, and vectors in Austria (1978-2014) - from imported pathogens to the endemicity of Dirofilaria repens. PLoS Neglected Trop. Dis. 2016;10 doi: 10.1371/journal.pntd.0004547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfield R.A., Reedy L. The use of oral milbemycin oxime, (Interceptor) in the treatment of chronic generalized canine demodicosis. Vet. Dermatol. 1992;3:231–235. [Google Scholar]
- Garrity S., Lee-Fowler T., Reinero C. Feline asthma and heartworm disease: clinical features, diagnostics and therapeutics. J. Feline Med. Surg. 2019;21:825–834. doi: 10.1177/1098612X18823348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genchi C., Kramer L.H. The prevalence of Dirofilaria immitis and D. repens in the old world. Vet. Parasitol. 2019;280:108995. doi: 10.1016/j.vetpar.2019.108995. [DOI] [PubMed] [Google Scholar]
- Genchi C., Mortarino M., Rinaldi L., Cringoli G., Traldi G., Genchi M. Changing climate and changing vector-borne disease distribution: the example of Dirofilaria in Europe. Vet. Parasitol. 2011;176:295–299. doi: 10.1016/j.vetpar.2011.01.012. [DOI] [PubMed] [Google Scholar]
- Genchi C., Bowman D., Drake J. Canine heartworm disease (Dirofilaria immitis) in Western Europe: survey of veterinary awareness and perceptions. Parasites Vectors. 2014;7:206. doi: 10.1186/1756-3305-7-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genchi M., Mangia C., Ferrari N., Loukeri S. Evaluation of a rapid immunochromatographic test for the detection of low burden Dirofilaria immitis (heartworm) in dogs and cats. Parasitol. Res. 2018;117:31–34. doi: 10.1007/s00436-017-5709-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer J., Janko C. Treatment of MDR1 mutant dogs with macrocyclic lactones. Curr. Pharmaceut. Biotechnol. 2012;13:969–986. doi: 10.2174/138920112800399301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer J., Döring B., Godoy J.R., Leidolf R., Moritz A., Petzinger E. Frequency of the nt230 (del4) MDR1 mutation in Collies and related dog breeds in Germany. J. Vet. Pharmacol. Therapeut. 2005;28:545–551. doi: 10.1111/j.1365-2885.2005.00692.x. [DOI] [PubMed] [Google Scholar]
- Ghedin E., Wang S., Spiro D., Caler E., Zhao Q., Crabtree J., Allen J.E., Delcher A.L., Guiliano D.B., Miranda-Saavedra D., Angiuoli S.V., Creasy T., Amedeo P., Haas B., El-Sayed N.M., Wortman J.R., Feldblyum T., Tallon L., Schatz M., Shumway M., Koo H., Salzberg S.L., Schobel S., Pertea M., Pop M., White O., Barton G.J., Carlow C.K.S., Crawford M.J., Daub J., Dimmic M.W., Estes C.F., Foster J.M., Ganatra M., Gregory W.F., Johnson N.M., Jin J., Komuniecki R., Korf I., Kumar S., Laney S., Li B.-W., Li W., Lindblom T.H., Lustigman S., Ma D., Maina C.V., Martin D.M.A., McCarter J.P., McReynolds L., Mitreva M., Nutman T.B., Parkinson J., Peregrín-Alvarez J.M., Poole C., Ren Q., Saunders L., Sluder A.E., Smith K., Stanke M., Unnasch T.R., Ware J., Wei A.D., Weil G., Williams D.J., Zhang Y., Williams S.A., Fraser-Liggett C., Slatko B., Blaxter M.L., Scott A.L. Draft genome of the filarial nematode parasite Brugia malayi. Science. 2007;317:1756–1760. doi: 10.1126/science.1145406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioia G., Lecová L., Genchi M., Ferri E., Genchi C., Mortarino M. Highly sensitive multiplex PCR for simultaneous detection and discrimination of Dirofilaria immitis and Dirofilaria repens in canine peripheral blood. Vet. Parasitol. 2010;172:160–163. doi: 10.1016/j.vetpar.2010.04.027. [DOI] [PubMed] [Google Scholar]
- Glaus T.M., Jacobs G.J., Rawlings C.A., Watson E.D., Calvert C.A. Surgical removal of heartworms from a cat with caval syndrome. J. Am. Vet. Med. Assoc. 1995;206:663–666. [PubMed] [Google Scholar]
- Godel C., Kumar S., Koutsovoulos G., Ludin P., Nilsson D., Comandatore F., Wrobel N., Thompson M., Schmid C.D., Goto S., Bringaud F., Wolstenholme A., Bandi C., Epe C., Kaminsky R., Blaxter M., Maser P. The genome of the heartworm, Dirofilaria immitis, reveals drug and vaccine targets. Faseb. J. 2012;26:4650–4661. doi: 10.1096/fj.12-205096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudie A.C., Evans N.A., Gration K.A., Bishop B.F., Gibson S.P., Holdom K.S., Kaye B., Wicks S.R., Lewis D., Weatherley A.J. Doramectin - a potent novel endectocide. Vet. Parasitol. 1993;49:5–15. doi: 10.1016/0304-4017(93)90218-c. [DOI] [PubMed] [Google Scholar]
- Goulson D., Nicholls E., Botías C., Rotheray E.L. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science. 2015;347:1255957. doi: 10.1126/science.1255957. [DOI] [PubMed] [Google Scholar]
- Gramer I., Leidolf R., Döring B., Klintzsch S., Krämer E.M., Yalcin E., Petzinger E., Geyer J. Breed distribution of the nt230(del4) MDR1 mutation in dogs. Vet. J. 2011;189:67–71. doi: 10.1016/j.tvjl.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Grandi G., Morchon R., Kramer L., Kartashev V., Simon F. Wolbachia in Dirofilaria repens, an agent causing human subcutaneous Dirofilariasis. J. Parasitol. 2008;94:1421–1423. doi: 10.1645/GE-1575.1. [DOI] [PubMed] [Google Scholar]
- Grote A., Lustigman S., Ghedin E. Lessons from the genomes and transcriptomes of filarial nematodes. Mol. Biochem. Parasitol. 2017;215:23–29. doi: 10.1016/j.molbiopara.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire V.A. Evaluation of efficacy of heartworm preventive products at the FDA. Vet. Parasitol. 2005;133:191–195. doi: 10.1016/j.vetpar.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Harder A., von Samson-Himmelstjerna G. Cyclooctadepsipeptides – a new class of anthelmintically active compounds. Parasitol. Res. 2002;88:481–488. doi: 10.1007/s00436-002-0619-2. [DOI] [PubMed] [Google Scholar]
- Harischandra H., Yuan W., Loghry H.J., Zamanian M., Kimber M.J. Profiling extracellular vesicle release by the filarial nematode Brugia malayi reveals sex-specific differences in cargo and a sensitivity to ivermectin. PLoS Neglected Trop. Dis. 2018;12 doi: 10.1371/journal.pntd.0006438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawking F. A review of progress in the chemotherapy and control of filariasis since 1955. Bull. World Health Organ. 1962;27:555–568. [PMC free article] [PubMed] [Google Scholar]
- Hayasaki M., Saeki H. Inhibition and prevention efficacy against mosquito bloodsucking and Dirofilaria immitis infection by administration of topical insecticide. J. Vet. Med. Sci. 2009;71:1049–1052. doi: 10.1292/jvms.71.1049. [DOI] [PubMed] [Google Scholar]
- Hays K.M., Rodriguez J.Y., Little S.E., Litster A.L., Mwacalimba K.K., Sundstrom K.D., Amodie D.M., Serrano M.A., Guerios S.D., Lane J.N., Levy J.K. Heartworm prevalence in dogs versus cats: multiple diagnostic modalities provide new insights. Vet. Parasitol. X. 2020;4:100027. doi: 10.1016/j.vpoa.2020.100027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry L.G., Brunson K.J., Walden H.S., Wenzlow N., Beachboard S.E., K L.B., Long M.T. Comparison of six commercial antigen kits for detection of Dirofilaria immitis infections in canines with necropsy-confirmed heartworm status. Vet. Parasitol. 2018;254:178–182. doi: 10.1016/j.vetpar.2018.02.037. [DOI] [PubMed] [Google Scholar]
- Hertig E. Distribution of Anopheles vectors and potential malaria transmission stability in Europe and the Mediterranean area under future climate change. Parasites Vectors. 2019;12:18. doi: 10.1186/s13071-018-3278-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess J., Patra M., Rangasamy L., Konatschnig S., Blacque O., Jabbar A., Mac P., Jorgensen E.M., Gasser R.B., Gasser G. Organometallic derivatization of the nematocidal drug monepantel leads to promising antiparasitic drug candidates. Chemistry. 2016;22:16602–16612. doi: 10.1002/chem.201602851. [DOI] [PubMed] [Google Scholar]
- Hibbs R.E., Gouaux E. Principles of activation and permeation in an anion-selective Cys-loop receptor. Nature. 2011;474:54–60. doi: 10.1038/nature10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoerauf A., Mand S., Fischer K., Kruppa T., Marfo-Debrekyei Y., Debrah A.Y., Pfarr K.M., Adjei O., Büttner D.W. Doxycycline as a novel strategy against bancroftian filariasis-depletion of Wolbachia endosymbionts from Wuchereria bancrofti and stop of microfilaria production. Med. Microbiol. Immunol. 2003;192:211–216. doi: 10.1007/s00430-002-0174-6. [DOI] [PubMed] [Google Scholar]
- Holm B.R. Efficacy of milbemycin oxime in the treatment of canine generalized demodicosis: a retrospective study of 99 dogs (1995–2000) Vet. Dermatol. 2003;14:189–195. doi: 10.1046/j.1365-3164.2003.00339.x. [DOI] [PubMed] [Google Scholar]
- Holste J.E., Smith L.L., Hair J.A., Lancaster J.L., Lloyd J.E., Langholff W.K., Barrick R.A., Eagleson J.S. Eprinomectin: a novel avermectin for control of lice in all classes of cattle. Vet. Parasitol. 1997;73:153–161. doi: 10.1016/s0304-4017(97)00063-0. [DOI] [PubMed] [Google Scholar]
- Holste J.E., Colwell D.D., Kumar R., Lloyd J.E., Pinkall N.P., Sierra M.A., Waggoner J.W., Langholff W.K., Barrick R.A., Eagleson J.S. Efficacy of eprinomectin against Hypoderma spp in cattle. Am. J. Vet. Res. 1998;59:56–58. [PubMed] [Google Scholar]
- Howe K.L., Bolt B.J., Shafie M., Kersey P., Berriman M. WormBase ParaSite - a comprehensive resource for helminth genomics. Mol. Biochem. Parasitol. 2017;215:2–10. doi: 10.1016/j.molbiopara.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intuyod K., Hahnvajanawong C., Pinlaor P., Pinlaor S. Anti-parasitic drug ivermectin exhibits potent anticancer activity against gemcitabine-resistant cholangiocarcinoma In vitro. Anticancer Res. 2019;39:4837–4843. doi: 10.21873/anticanres.13669. [DOI] [PubMed] [Google Scholar]
- Ionică A.M., Zittra C., Wimmer V., Leitner N., Votýpka J., Modrý D., Mihalca A.D., Fuehrer H.P. Mosquitoes in the Danube Delta: searching for vectors of filarioid helminths and avian malaria. Parasites Vectors. 2017;10:324. doi: 10.1186/s13071-017-2264-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson L.S., Ward A.K., Lacaden A.B., Hornak A.T. Prevalence of heartworm in relocated, local and outreach clinic dogs: a Canadian sheltering perspective. Vet. Parasitol. 2020;283:109081. doi: 10.1016/j.vetpar.2020.109081. [DOI] [PubMed] [Google Scholar]
- Jarrett W.F., Jennings F.W., Mc I.W., Mulligan W., Urquhart G.M. Immunological studies on Dictyocaulus viviparus infection; immunity produced by the administration of irradiated larvae. Immunol. 1960;3:145–151. [PMC free article] [PubMed] [Google Scholar]
- Jiang L., Wang P., Sun Y.-J., Wu Y.-J. Ivermectin reverses the drug resistance in cancer cells through EGFR/ERK/Akt/NF-κB pathway. J. Exp. Clin. Canc. Res. 2019;38:265. doi: 10.1186/s13046-019-1251-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L., Feng X., Rong H., Pan Z., Inaba Y., Qiu L., Zheng W., Lin S., Wang R., Wang Z., Wang S., Liu H., Li S., Xie W., Li Y. The antiparasitic drug ivermectin is a novel FXR ligand that regulates metabolism. Nat. Commun. 2013;4:1937. doi: 10.1038/ncomms2924. [DOI] [PubMed] [Google Scholar]
- Johnson K.B., Wei W.Q., Weeraratne D., Frisse M.E., Misulis K., Rhee K., Zhao J., Snowdon J.L. Precision medicine, AI, and the future of personalized health care. Clin. Trans. Sci. 2020;14:86–93. doi: 10.1111/cts.12884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokelainen P., Mõtsküla P.F., Heikkinen P., Ülevaino E., Oksanen A., Lassen B. Dirofilaria repens microfilaremia in three dogs in Estonia. Vector Borne Zoonotic Dis. 2016;16:136–138. doi: 10.1089/vbz.2015.1833. [DOI] [PubMed] [Google Scholar]
- Juliano R.L., Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim. Biophys. Acta. 1976;455:152–162. doi: 10.1016/0005-2736(76)90160-7. [DOI] [PubMed] [Google Scholar]
- Kassai T. The impact on database searching arising from inconsistency in the nomenclature of parasitic diseases. Vet. Parasitol. 2006;138:358–361. doi: 10.1016/j.vetpar.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Kassai T., Cordero del Campillo M., Euzeby J., Gaafar S., Hiepe T., Himonas C.A. Standardized nomenclature of animal parasitic diseases (SNOAPAD) Vet. Parasitol. 1988;29:299–326. doi: 10.1016/0304-4017(88)90148-3. [DOI] [PubMed] [Google Scholar]
- Kim Y.E., Remme J.H., Steinmann P., Stolk W.A., Roungou J.B., Tediosi F. Control, elimination, and eradication of river blindness: scenarios, timelines, and ivermectin treatment needs in Africa. PLoS Neglected Trop. Dis. 2015;9 doi: 10.1371/journal.pntd.0003664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kis Z., Shattock R., Shah N., Kontoravdi C. Emerging technologies for low-cost, rapid vaccine manufacture. Biotechnol. J. 2019;14 doi: 10.1002/biot.201800376. [DOI] [PubMed] [Google Scholar]
- Kitron U., Spielman A. Suppression of transmission of malaria through source reduction: antianopheline measures applied in Israel, the United States, and Italy. Rev. Infect. Dis. 1989;11:391–406. doi: 10.1093/clinids/11.3.391. [DOI] [PubMed] [Google Scholar]
- Kramer L., Grandi G., Leoni M., Passeri B., McCall J., Genchi C., Mortarino M., Bazzocchi C. Wolbachia and its influence on the pathology and immunology of Dirofilaria immitis infection. Vet. Parasitol. 2008;158:191–195. doi: 10.1016/j.vetpar.2008.09.014. [DOI] [PubMed] [Google Scholar]
- Kryda K., Six R.H., Walsh K.F., Holzmer S.J., Chapin S., Mahabir S.P., Myers M., Inskeep T., Rugg J., Cundiff B., Pullins A., Ulrich M., McCall J.W., McTier T.L., Maeder S.J. Laboratory and field studies to investigate the efficacy of a novel, orally administered combination product containing moxidectin, sarolaner and pyrantel for the prevention of heartworm disease (Dirofilaria immitis) in dogs. Parasites Vectors. 2019;12:445. doi: 10.1186/s13071-019-3702-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuesel A.C. Research for new drugs for elimination of onchocerciasis in Africa. Internat. J. Parasitol. Drugs Drug Resist. 2016;6:272–286. doi: 10.1016/j.ijpddr.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvaternick V., Kellermann M., Knaus M., Rehbein S., Rosentel J. Pharmacokinetics and metabolism of eprinomectin in cats when administered in a novel topical combination of fipronil, (S)-methoprene, eprinomectin and praziquantel. Vet. Parasitol. 2014;202:2–9. doi: 10.1016/j.vetpar.2014.02.031. [DOI] [PubMed] [Google Scholar]
- Labarthe N., Guerrero J. Epidemiology of heartworm: what is happening in South America and Mexico? Vet. Parasitol. 2005;133:149–156. doi: 10.1016/j.vetpar.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Laing R., Gillan V., Devaney E. Ivermectin - old drug, new tricks? Trends Parasitol. 2017;33:463–472. doi: 10.1016/j.pt.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakwo T., Oguttu D., Ukety T., Post R., Bakajika D. Onchocerciasis elimination: progress and challenges. Res. Rep. Trop. Med. 2020;11:81–95. doi: 10.2147/RRTM.S224364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmann F. The Wolbachia endosymbionts. Microbiol. Spectr. 2019;7 doi: 10.1128/microbiolspec.BAI-0018-2019. [DOI] [PubMed] [Google Scholar]
- Ledesma N., Harrington L. Mosquito vectors of dog heartworm in the United States: vector status and factors influencing transmission efficiency. Top. Companion Anim. Med. 2011;26:178–185. doi: 10.1053/j.tcam.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Lee S.G., Moon H.S., Hyun C. Percutaneous heartworm removal from dogs with severe heart worm (Dirofilaria immitis) infestation. J. Vet. Sci. 2008;9:197–202. doi: 10.4142/jvs.2008.9.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A.C., Montgomery S.P., Theis J.H., Blagburn B.L., Eberhard M.L. Public health issues concerning the widespread distribution of canine heartworm disease. Trends Parasitol. 2010;26:168–173. doi: 10.1016/j.pt.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Lee A.C., Bowman D.D., Lucio-Forster A., Beall M.J., Liotta J.L., Dillon R. Evaluation of a new in-clinic method for the detection of canine heartworm antigen. Vet. Parasitol. 2011;177:387–391. doi: 10.1016/j.vetpar.2010.11.050. [DOI] [PubMed] [Google Scholar]
- Lee J.-J., Lin H.-Y., Chen C.-A., Lin C.-S., Wang L.-C. Development of an oligonucleotide microarray for simultaneous detection of two canine MDR1 genotypes and association between genotypes and chemotherapy side effects. J. Vet. Sci. 2019;20:27–33. doi: 10.4142/jvs.2019.20.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees K., Musgaard M., Suwanmanee S., Buckingham S.D., Biggin P., Sattelle D. Actions of agonists, fipronil and ivermectin on the predominant in vivo splice and edit variant (RDLbd, I/V) of the Drosophila GABA receptor expressed in Xenopus laevis oocytes. PloS One. 2014;9 doi: 10.1371/journal.pone.0097468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lespine A., Martin S., Dupuy J., Roulet A., Pineau T., Orlowski S., Alvinerie M. Interaction of macrocyclic lactones with P-glycoprotein: structure-affinity relationship. Eur. J. Pharmaceut. Sci. 2007;30:84–94. doi: 10.1016/j.ejps.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Little S.E., Beall M.J., Bowman D.D., Chandrashekar R., Stamaris J. Canine infection with Dirofilaria immitis, Borrelia burgdorferi, Anaplasma spp., and Ehrlichia spp. in the United States, 2010-2012. Parasites Vectors. 2014;7:257. doi: 10.1186/1756-3305-7-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little S., Saleh M., Wohltjen M., Nagamori Y. Prime detection of Dirofilaria immitis: understanding the influence of blocked antigen on heartworm test performance. Parasites Vectors. 2018;11:186. doi: 10.1186/s13071-018-2736-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little S., Braff J., Place J., Buch J., Dewage B.G., Knupp A., Beall M. Canine infection with Dirofilaria immitis, Borrelia burgdorferi, Anaplasma spp., and Ehrlichia spp. in the United States, 2013–2019. Parasites Vectors. 2021;14:10. doi: 10.1186/s13071-020-04514-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Yang N., He J., Yang M., Sun M. Prevalence of Dirofilaria immitis in dogs in shenyang, northeastern China. Kor. J. Parasitol. 2013;51:375–377. doi: 10.3347/kjp.2013.51.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Drexel J., Andrews B., Eberts M., Breitschwerdt E., Chandrashekar R. Comparative evaluation of 2 in-clinic assays for vector-borne disease testing in dogs. Top. Companion Anim. Med. 2018;33:114–118. doi: 10.1053/j.tcam.2018.09.003. [DOI] [PubMed] [Google Scholar]
- López J., Valiente-Echeverría F., Carrasco M., Mercado R., Abarca K. [Morphological and molecular identification of canine filariae in a semi-rural district of the Metropolitan Region in Chile] Rev. Chilena Infectol. 2012;29:248–289. doi: 10.4067/S0716-10182012000300006. [DOI] [PubMed] [Google Scholar]
- Lovis L., Grandjean M., Overney L., Seewald W., Sager H. Seasonality and circadian variation of microfilaremia in dogs experimentally infected with Dirofilaria immitis. Vet. Parasitol. 2017;243:235–241. doi: 10.1016/j.vetpar.2017.07.010. [DOI] [PubMed] [Google Scholar]
- Luck A.N., Evans C.C., Riggs M.D., Foster J.M., Moorhead A.R., Slatko B.E., Michalski M.L. Concurrent transcriptional profiling of Dirofilaria immitis and its Wolbachia endosymbiont throughout the nematode life cycle reveals coordinated gene expression. BMC Genom. 2014;15:1041. doi: 10.1186/1471-2164-15-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck A.N., Anderson K.G., McClung C.M., VerBerkmoes N.C., Foster J.M., Michalski M.L., Slatko B.E. Tissue-specific transcriptomics and proteomics of a filarial nematode and its Wolbachia endosymbiont. BMC Genom. 2015;16:920. doi: 10.1186/s12864-015-2083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlam K.W., Jachowski L.A., Jr., Otto G.F. Potential vectors of Dirofilaria immitis. J. Am. Vet. Med. Assoc. 1970;157:1354–1359. [PubMed] [Google Scholar]
- Lynch J.W. Molecular structure and function of the glycine receptor chloride channel. Physiol. Rev. 2004;84:1051–1095. doi: 10.1152/physrev.00042.2003. [DOI] [PubMed] [Google Scholar]
- Maggi R.G., Krämer F. A review on the occurrence of companion vector-borne diseases in pet animals in Latin America. Parasites Vectors. 2019;12:145. doi: 10.1186/s13071-019-3407-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnis J., Lorentz S., Guardone L., Grimm F., Magi M., Naucke T.J., Deplazes P. Morphometric analyses of canine blood microfilariae isolated by the Knott's test enables Dirofilaria immitis and D. repens species-specific and Acanthocheilonema (syn. Dipetalonema) genus-specific diagnosis. Parasites Vectors. 2013;6:48. doi: 10.1186/1756-3305-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maksimowich D.S., Bell T.G., Williams J.F., Kaiser L. Effect of arsenical drugs on in vitro vascular responses of pulmonary artery from heartworm-infected dogs. Am. J. Vet. Res. 1997;58:389–393. [PubMed] [Google Scholar]
- Marelli S.P., Polli M., Frattini S., Cortellari M., Rizzi R., Crepaldi P. Genotypic and allelic frequencies of MDR1 gene in dogs in Italy. Vet. Rec. Open. 2020;7 doi: 10.1136/vetreco-2019-000375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R.J., Robertson A.P., Choudhary S. Ivermectin: an anthelmintic, an insecticide, and much more. Trends Parasitol. 2020;37:48–64. doi: 10.1016/j.pt.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrangelo E., Pezzullo M., De Burghgraeve T., Kaptein S., Pastorino B., Dallmeier K., de Lamballerie X., Neyts J., Hanson A.M., Frick D.N., Bolognesi M., Milani M. Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: new prospects for an old drug. J. Antimicrob. Chemother. 2012;67:1884–1894. doi: 10.1093/jac/dks147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell D. 2015. Barbervax? - a Vaccine to Protect against Barber's Pole Worm.http://www.wormboss.com.au/sheep-goats/news/articles/nonchemical-management/barbervaxa-vaccine-to-protect-against-barbers-pole-worm.php accessed November 25th, 2020. [Google Scholar]
- McCall J.W. The safety-net story about macrocyclic lactone heartworm preventives: a review, an update, and recommendations. Vet. Parasitol. 2005;133:197–206. doi: 10.1016/j.vetpar.2005.04.005. [DOI] [PubMed] [Google Scholar]
- McCall J.W., Supakorndej N., Donoghue A.R., Turnbull R.K., Radecki S.V. Evaluation of the performance of canine heartworm antigen test kits licensed for use by veterinarians and canine heartworm antigen tests conducted by diagnostic laboratories. In: Seward R.L., editor. Recent Advances in Heartworm Disease: Symposium 2001. American heartworm society; USA, Batavia, IL: 2001. pp. 97–104. [Google Scholar]
- McCall J.W., Genchi C., Kramer L., Guerrero J., Dzimianski M.T., Supakorndej P., Mansour A.M., McCall S.D., Supakorndej N., Grandi G., Carson B. Heartworm and Wolbachia: therapeutic implications. Vet. Parasitol. 2008;158:204–214. doi: 10.1016/j.vetpar.2008.09.008. [DOI] [PubMed] [Google Scholar]
- McCall J.W., Genchi C., Kramer L.H., Guerrero J., Venco L. Heartworm disease in animals and humans. Adv. Parasitol. 2008;66:193–285. doi: 10.1016/S0065-308X(08)00204-2. [DOI] [PubMed] [Google Scholar]
- McCall J., Kramer L., Genchi C., Guerrero J., Dzimianski M., Supakorndej P., Mansour A., McCall S., Supakorndej N., Grandi G. Proceedings of the American Heartworm Society Triennial Meeting. Memphis; Poster: 2010. Effects of melarsomine dihydrochloride on two-month-old infections of Dirofilaria immitis and Brugia pahangi in dogs with dual infections; p. 8. [Google Scholar]
- McCall J.W., Kramer L., Genchi C., Guerrero J., Dzimianski M.T., Supakorndej P., Mansour A., McCall S.D., Supakorndej N., Grandi G., Carson B. Effects of doxycycline on early infections of Dirofilaria immitis in dogs. Vet. Parasitol. 2011;176:361–367. doi: 10.1016/j.vetpar.2011.01.022. [DOI] [PubMed] [Google Scholar]
- McCall J.W., Arther R., Davis W., Settje T. Safety and efficacy of 10% imidacloprid + 2.5% moxidectin for the treatment of Dirofilaria immitis circulating microfilariae in experimentally infected dogs. Vet. Parasitol. 2014;206:86–92. doi: 10.1016/j.vetpar.2014.09.011. [DOI] [PubMed] [Google Scholar]
- McCall J.W., Kramer L., Genchi C., Guerrero J., Dzimianski M.T., Mansour A., McCall S.D., Carson B. Effects of doxycycline on heartworm embryogenesis, transmission, circulating microfilaria, and adult worms in microfilaremic dogs. Vet. Parasitol. 2014;206:5–13. doi: 10.1016/j.vetpar.2014.09.023. [DOI] [PubMed] [Google Scholar]
- McCall J., Hodgkins E., Varloud M., Mansour A., DiCosty U. Blocking the transmission of heartworm (Dirofilaria immitis) to mosquitoes (Aedes aegypti) by weekly exposure for one month to microfilaremic dogs treated once topically with dinotefuran-permethrin-pyriproxyfen. Parasites Vectors. 2017;10:511. doi: 10.1186/s13071-017-2439-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall J., Varloud M., Hodgkins E., Mansour A., DiCosty U., McCall S., Carmichael J., Carson B., Carter J. Shifting the paradigm in Dirofilaria immitis prevention: blocking transmission from mosquitoes to dogs, using repellents/insecticides and macrocyclic lactone prevention as part of a multi-modal approach. Parasites Vectors. 2017;10:525. doi: 10.1186/s13071-017-2438-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKellar Q.A., Benchaoui H.A. Avermectins and milbemycins. J. Vet. Pharmacol. Therapeut. 1996;19:331–351. doi: 10.1111/j.1365-2885.1996.tb00062.x. [DOI] [PubMed] [Google Scholar]
- McTier T.L., McCall J.W., Dzimianski M.T., Raynaud J.P., Strickland J.E. Use of melarsomine dihydrochloride (RM 340) for adulticidal treatment of dogs with naturally acquired infections of Dirofilaria immitis and for clinical prophylaxis during reexposure for 1 year. Vet. Parasitol. 1994;55:221–233. doi: 10.1016/0304-4017(93)00643-D. [DOI] [PubMed] [Google Scholar]
- McTier T.L., Six R.H., Pullins A., Chapin S., McCall J.W., Rugg D., Maeder S.J., Woods D.J. Efficacy of oral moxidectin against susceptible and resistant isolates of Dirofilaria immitis in dogs. Parasites Vectors. 2017;10:482. doi: 10.1186/s13071-017-2429-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTier T.L., Six R.H., Pullins A., Chapin S., Kryda K., Mahabir S.P., Woods D.J., Maeder S.J. Preventive efficacy of oral moxidectin at various doses and dosage regimens against macrocyclic lactone-resistant heartworm (Dirofilaria immitis) strains in dogs. Parasites Vectors. 2019;12:444. doi: 10.1186/s13071-019-3685-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mealey K.L., Bentjen S.A., Gay J.M., Cantor G.H. Ivermectin sensitivity in collies is associated with a deletion mutation of the mdr1 gene. Pharmacogenetics. 2001;11:727–733. doi: 10.1097/00008571-200111000-00012. [DOI] [PubMed] [Google Scholar]
- Mealey K.L., Munyard K.A., Bentjen S.A. Frequency of the mutant MDR1 allele associated with multidrug sensitivity in a sample of herding breed dogs living in Australia. Vet. Parasitol. 2005;131:193–196. doi: 10.1016/j.vetpar.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Mealey K.L., Greene S., Bagley R., Gay J., Tucker R., Gavin P., Schmidt K., Nelson F. P-glycoprotein contributes to the blood-brain, but not blood-cerebrospinal fluid, barrier in a spontaneous canine p-glycoprotein knockout model. Drug Metab. Dispos. 2008;36:1073–1079. doi: 10.1124/dmd.107.018978. [DOI] [PubMed] [Google Scholar]
- Medlock J.M., Hansford K.M., Schaffner F., Versteirt V., Hendrickx G., Zeller H., Van Bortel W. A review of the invasive mosquitoes in Europe: ecology, public health risks, and control options. Vector Borne Zoonotic Dis. 2012;12:435–447. doi: 10.1089/vbz.2011.0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlhorn H. fourth ed. ed. Springer Berlin Heidelberg; 2016. Encyclopedia of Parasitology. [Google Scholar]
- Mendoza-Roldan J., Benelli G., Panarese R., Iatta R., Furlanello T., Beugnet F., Zatelli A., Otranto D. Leishmania infantum and Dirofilaria immitis infections in Italy, 2009-2019: changing distribution patterns. Parasites Vectors. 2020;13:193. doi: 10.1186/s13071-020-04063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merola V.M., Eubig P.A. Toxicology of avermectins and milbemycins (macrocyclic lactones) and the role of P-glycoprotein in dogs and cats. Vet. Clin. North Am. Small Anim. Pract. 2012;42:313–vii. doi: 10.1016/j.cvsm.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills B.J., McTier T.L., Knauer C.S., Woods D.J. World Intellectual Property Organization; 2020. Anthelmintic Laboratory Animal Model for Heartworm. WO2020146338. [Google Scholar]
- Mizukami K., Yabuki A., Kohyama M., Kushida K., Rahman M.M., Uddin M.M., Sawa M., Yamato O. Molecular prevalence of multiple genetic disorders in Border collies in Japan and recommendations for genetic counselling. Vet. J. 2016;214:21–23. doi: 10.1016/j.tvjl.2016.05.004. [DOI] [PubMed] [Google Scholar]
- Molyneux D.H., Ward S.A. Reflections on the Nobel prize for medicine 2015--the public health legacy and impact of avermectin and artemisinin. Trends Parasitol. 2015;31:605–607. doi: 10.1016/j.pt.2015.10.008. [DOI] [PubMed] [Google Scholar]
- Monobe M.M., Junior J.P.A., Lunsford K.V., Silva R.C., Bulla C. Frequency of the MDR1 mutant allele associated with multidrug sensitivity in dogs from Brazil. Vet. Med. 2015;6:111–117. doi: 10.2147/VMRR.S72373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montarsi F., Ciocchetta S., Devine G., Ravagnan S., Mutinelli F., Frangipane di Regalbono A., Otranto D., Capelli G. Development of Dirofilaria immitis within the mosquito Aedes (Finlaya) koreicus, a new invasive species for Europe. Parasites Vectors. 2015;8:177. doi: 10.1186/s13071-015-0800-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya-Alonso J.A., Morchón R., Falcón-Cordón Y., Falcón-Cordón S., Simón F., Carretón E. Prevalence of heartworm in dogs and cats of Madrid, Spain. Parasites Vectors. 2017;10:354. doi: 10.1186/s13071-017-2299-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morchón R., Bargues M.D., Latorre J.M., Melero-Alcíbar R., Pou-Barreto C., Mas-Coma S., Simón F. Haplotype H1 of Culex pipiens implicated as natural vector of Dirofilaria immitis in an endemic area of Western Spain. Vector Borne Zoonotic Dis. 2007;7:653–658. doi: 10.1089/vbz.2007.0124. [DOI] [PubMed] [Google Scholar]
- Morchón R., Moya I., González-Miguel J., Montoya M.N., Simón F. Zoonotic Dirofilaria immitis infections in a province of Northern Spain. Epidemiol. Infect. 2010;138:380–383. doi: 10.1017/S0950268809990434. [DOI] [PubMed] [Google Scholar]
- Morchón R., Carretón E., González-Miguel J., Mellado-Hernández I. Heartworm disease (Dirofilaria immitis) and their vectors in Europe - new distribution trends. Front. Physiol. 2012;3:196. doi: 10.3389/fphys.2012.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno Y., Nabhan J.F., Solomon J., Mackenzie C.D., Geary T.G. Ivermectin disrupts the function of the excretory-secretory apparatus in microfilariae of Brugia malayi. Proc. Natl. Acad. Sci. U. S. A. 2010;107:20120–20125. doi: 10.1073/pnas.1011983107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno Y., Geary T.G., Tritten L. When secretomes meet anthelmintics: lessons for therapeutic interventions. Trends Parasitol. 2021 doi: 10.1016/j.pt.2021.01.007. doi.org/10.1016/j.pt.2021.01.007 1. [DOI] [PubMed] [Google Scholar]
- Moroni B., Rossi L., Meneguz P.G., Orusa R., Zoppi S., Robetto S., Marucco F., Tizzani P. Dirofilaria immitis in wolves recolonizing northern Italy: are wolves competent hosts? Parasites Vectors. 2020;13:482. doi: 10.1186/s13071-020-04353-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskvina T.V., Ermolenko A.V. Dirofilariasis in Russian Federation: a big problem with large distribution. Russ. Open Med. J. 2018;7 [Google Scholar]
- Nambara S., Masuda T., Nishio M., Kuramitsu S., Tobo T., Ogawa Y., Hu Q., Iguchi T., Kuroda Y., Ito S., Eguchi H., Sugimachi K., Saeki H., Oki E., Maehara Y., Suzuki A., Mimori K. Antitumor effects of the antiparasitic agent ivermectin via inhibition of Yes-associated protein 1 expression in gastric cancer. Oncotarget. 2017;8:107666–107677. doi: 10.18632/oncotarget.22587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nana-Djeunga H.C., Bourguinat C., Pion S.D., Bopda J., Kengne-Ouafo J.A., Njiokou F., Prichard R.K., Wanji S., Kamgno J., Boussinesq M. Reproductive status of Onchocerca volvulus after ivermectin treatment in an ivermectin-naïve and a frequently treated population from Cameroon. PLoS Neglected Trop. Dis. 2014;8 doi: 10.1371/journal.pntd.0002824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff M.W., Robertson K.R., Wong A.K., Safra N., Broman K.W., Slatkin M., Mealey K.L., Pedersen N.C. Breed distribution and history of canine mdr1-1Delta, a pharmacogenetic mutation that marks the emergence of breeds from the collie lineage. Proc. Natl. Acad. Sci. U. S. A. 2004;101:11725–11730. doi: 10.1073/pnas.0402374101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C.T., Myrick E.S., Nelson T.A. Clinical benefits of incorporating doxycycline into a canine heartworm treatment protocol. Parasites Vectors. 2017;10:515. doi: 10.1186/s13071-017-2446-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen C., Koh W.L., Casteriano A., Beijerink N., Godfrey C., Brown G., Emery D., Šlapeta J. Mosquito-borne heartworm Dirofilaria immitis in dogs from Australia. Parasites Vectors. 2016;9:535. doi: 10.1186/s13071-016-1821-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oi M., Yoshikawa S., Ichikawa Y., Nakagaki K., Matsumoto J., Nogami S. Prevalence of Dirofilaria immitis among shelter dogs in Tokyo, Japan, after a decade: comparison of 1999-2001 and 2009-2011. Parasite. 2014;21:10. doi: 10.1051/parasite/2014008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osei-Atweneboana M.Y., Awadzi K., Attah S.K., Boakye D.A., Gyapong J.O., Prichard R.K. Phenotypic evidence of emerging ivermectin resistance in Onchocerca volvulus. PLoS Neglected Trop. Dis. 2011;5:e998. doi: 10.1371/journal.pntd.0000998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pailet A., Abadie S.H., Smith M.W., Gonzalez R.R. Chemotherapeutic heartworm control--the use of diethylcarbamazine in the control of Dirofilaria immitis infection in dogs in clinical trials. Vet. Med. Small Anim. Clin. 1968;63:691–693. [PubMed] [Google Scholar]
- Panarese R., Iatta R., Mendoza-Roldan J.A., Szlosek D., Braff J., Liu J., Beugnet F., Dantas-Torres F., Beall M.J., Otranto D. 2020. Comparison of diagnostic tools for the detection of Dirofilaria immitis infection in Dogs. Pathogens 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papich M.G. Considerations for using minocycline vs doxycycline for treatment of canine heartworm disease. Parasites Vectors. 2017;10:493. doi: 10.1186/s13071-017-2449-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parham P.E., Michael E. Modelling climate change and malaria transmission. Adv. Exp. Med. Biol. 2010;673:184–199. doi: 10.1007/978-1-4419-6064-1_13. [DOI] [PubMed] [Google Scholar]
- Partridge F.A., Brown A.E., Buckingham S.D., Willis N.J., Wynne G.M., Forman R., Else K.J., Morrison A.A., Matthews J.B., Russell A.J., Lomas D.A., Sattelle D.B. An automated high-throughput system for phenotypic screening of chemical libraries on C. elegans and parasitic nematodes. Int. J. Parasitol. Drugs Drug Resist. 2018;8:8–21. doi: 10.1016/j.ijpddr.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennisi M.G., Tasker S., Hartmann K., Belák S., Addie D., Boucraut-Baralon C., Egberink H., Frymus T., Hofmann-Lehmann R., Hosie M., Lloret A., Marsilio F., Thiry E., Truyen U., Möstl K. Dirofilarioses in cats: European guidelines from the ABCD on prevention and management. J. Feline Med. Surg. 2020;22:442–451. doi: 10.1177/1098612X20917601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perumal A.N.I., Gunawardene Y.I.N.S., Dassanayake R.S. Setaria digitata in advancing our knowledge of human lymphatic filariasis. J. Helminthol. 2016;90:129–138. doi: 10.1017/S0022149X15000309. [DOI] [PubMed] [Google Scholar]
- Pietikäinen R., Nordling S., Jokiranta S., Saari S., Heikkinen P., Gardiner C., Kerttula A.M., Kantanen T., Nikanorova A., Laaksonen S., Lavikainen A., Oksanen A. Dirofilaria repens transmission in southeastern Finland. Parasites Vectors. 2017;10:561. doi: 10.1186/s13071-017-2499-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt S.R., Langholff W.K., Eagleson J.S., Rehbein S. The efficacy of eprinomectin against induced infections of immature (fourth larval stage) and adult nematode parasites in cattle. Vet. Parasitol. 1997;73:119–128. doi: 10.1016/s0304-4017(97)00035-6. [DOI] [PubMed] [Google Scholar]
- Pradeep R.K., Nimisha M., Pakideery V., Johns J., Chandy G., Nair S., Chandrasekhar L., Ajithkumar K.G., Deepa C.K., Varghese A., Ravindran R. Whether Dirofilaria repens parasites from South India belong to zoonotic Candidatus Dirofilaria hongkongensis (Dirofilaria sp. hongkongensis)? Infect. Genet. Evol. 2019;67:121–125. doi: 10.1016/j.meegid.2018.10.019. [DOI] [PubMed] [Google Scholar]
- Prescott C.W., O'Grady A.S., English P.B. Dose rate of diethylcarbamazine for heartworm prophylaxis. Aust. Vet. J. 1978;54:404–405. doi: 10.1111/j.1751-0813.1978.tb02522.x. [DOI] [PubMed] [Google Scholar]
- Preston S., Jabbar A., Nowell C., Joachim A., Ruttkowski B., Baell J., Cardno T., Korhonen P.K., Piedrafita D., Ansell B.R., Jex A.R., Hofmann A., Gasser R.B. Low cost whole-organism screening of compounds for anthelmintic activity. Int. J. Parasitol. 2015;45:333–343. doi: 10.1016/j.ijpara.2015.01.007. [DOI] [PubMed] [Google Scholar]
- Prichard R.K., Geary T.G. Perspectives on the utility of moxidectin for the control of parasitic nematodes in the face of developing anthelmintic resistance. Int. J. Parasitol. Drugs Drug Resist. 2019;10:69–83. doi: 10.1016/j.ijpddr.2019.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prichard R., Ménez C., Lespine A. Moxidectin and the avermectins: consanguinity but not identity. International J for Parasitol. Drugs Drug Resist. 2012;2:134–153. doi: 10.1016/j.ijpddr.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulaski C.N., Malone J.B., Bourguinat C., Prichard R., Geary T., Ward D., Klei T.R., Guidry T., Smith G., Delcambre B., Bova J., Pepping J., Carmichael J., Schenker R., Pariaut R. Establishment of macrocyclic lactone resistant Dirofilaria immitis isolates in experimentally infected laboratory dogs. Parasites Vectors. 2014;7:494. doi: 10.1186/s13071-014-0494-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajter J.C., Sherman M.S., Fatteh N., Vogel F., Sacks J., Rajter J.J. Use of Ivermectin is associated with lower mortality in hospitalized patients with Coronavirus disease 2019: the Ivermectin in COVID Nineteen Study. Chest. 2020;159:85–92. doi: 10.1016/j.chest.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Lopez S., León-Galván M.F., Salas-Alatorre M., Lechuga-Arana A.A., Valencia-Posadas M., Gutiérrez-Chávez A.J. First molecular identification of Dirofilaria repens in a dog blood sample from guanajuato, Mexico. Vector Borne Zoonotic Dis. 2016;16:734–736. doi: 10.1089/vbz.2016.1948. [DOI] [PubMed] [Google Scholar]
- Ranjan S., Trudeau C., Prichard R.K., von Kutzleben R., Carrier D. Efficacy of moxidectin against naturally acquired nematode infections in cattle. Vet. Parasitol. 1992;41:227–231. doi: 10.1016/0304-4017(92)90082-k. [DOI] [PubMed] [Google Scholar]
- Rawlings C.A., Raynaud J.P., Lewis R.E., Duncan J.R. Pulmonary thromboembolism and hypertension after thiacetarsamide vs melarsomine dihydrochloride treatment of Dirofilaria immitis infection in dogs. Am. J. Vet. Res. 1993;54:920–925. [PubMed] [Google Scholar]
- Rawlings C.A., Tonelli Q., Lewis R.E., Duncan J.R. Semiquantitative test for Dirofilaria immitis as a predictor of thromboembolic complications associated with heartworm treatment in dogs. Am. J. Vet. Res. 1993;54:914–919. [PubMed] [Google Scholar]
- Raymond V., Mongan N.P., Sattelle D.B. Anthelmintic actions on homomer-forming nicotinic acetylcholine receptor subunits: chicken alpha7 and ACR-16 from the nematode Caenorhabditis elegans. Neuroscience. 2000;101:785–791. doi: 10.1016/s0306-4522(00)00279-7. [DOI] [PubMed] [Google Scholar]
- Raynaud J.P. Thiacetarsamide (adulticide) versus melarsomine (RM 340) developed as macrofilaricide (adulticide and larvicide) to cure canine heartworm infection in dogs. Ann. Rech. Vet. 1992;23:1–25. [PubMed] [Google Scholar]
- Rehbein S.E., Winter R.E., Visser M.E., Maciel A.E., Marley S.E. Chorioptic mange in dairy cattle: treatment with eprinomectin pour-on. Parasitol. Res. 2005;98:21–25. doi: 10.1007/s00436-005-0005-y. [DOI] [PubMed] [Google Scholar]
- Rehbein S., Capári B., Duscher G., Keidane D., Kirkova Z., Petkevičius S., Rapti D., Wagner A., Wagner T., Chester S.T., Rosentel J., Tielemans E., Visser M., Winter R., Kley K., Knaus M. Efficacy against nematode and cestode infections and safety of a novel topical fipronil, (S)-methoprene, eprinomectin and praziquantel combination product in domestic cats under field conditions in Europe. Vet. Parasitol. 2014;202:10–17. doi: 10.1016/j.vetpar.2014.02.032. [DOI] [PubMed] [Google Scholar]
- Rodrigo W.W., Dassanayake R.S., Weerasena S.J., Silva Gunawardene Y.I. Novel parasitic nematode-specific protein of bovine filarial parasite Setaria digitata displays conserved gene structure and ubiquitous expression. Trop. Biomed. 2014;31:514–524. [PubMed] [Google Scholar]
- Russell R.C., Geary M.J. The influence of microfilarial density of dog heartworm Dirofilaria immitis on infection rate and survival of Aedes notoscriptus and Culex annulirostris from Australia. J. Med. Entomol. 1996;10:29–34. doi: 10.1111/j.1365-2915.1996.tb00078.x. [DOI] [PubMed] [Google Scholar]
- Sahin U., Karikó K., Türeci Ö. mRNA-based therapeutics--developing a new class of drugs. Nat. Rev. Drug Discov. 2014;13:759–780. doi: 10.1038/nrd4278. [DOI] [PubMed] [Google Scholar]
- Santa-Ana M., Khadem M., Capela R. Natural infection of Culex theileri (Diptera: Culicidae) with Dirofilaria immitis (Nematoda: Filarioidea) on Madeira island, Portugal. J. Med. Entomol. 2006;43:104–106. doi: 10.1093/jmedent/43.1.104. [DOI] [PubMed] [Google Scholar]
- Sauerbrey M., Rakers L.J., Richards F.O. Progress toward elimination of onchocerciasis in the Americas. Int. Health. 2018;10:i71–i78. doi: 10.1093/inthealth/ihx039. [DOI] [PubMed] [Google Scholar]
- Savadelis M.D., Day K.M., Bradner J.L., Wolstenholme A.J., Dzimianski M.T., Moorhead A.R. Efficacy and side effects of doxycycline versus minocycline in the three-dose melarsomine canine adulticidal heartworm treatment protocol. Parasites Vectors. 2018;11:671. doi: 10.1186/s13071-018-3264-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer T.K., Weinstein P.P. The in vitro development of microfilariae of the dog heartworm Dirofilaria immitis to the "sausage-form. J. Parasitol. 1963;49:218–224. [PubMed] [Google Scholar]
- Sałamatin R.V., Pavlikovska T.M., Sagach O.S., Nikolayenko S.M., Kornyushin V.V., Kharchenko V.O., Masny A., Cielecka D., Konieczna-Sałamatin J., Conn D.B., Golab E. Human Dirofilariasis due to Dirofilaria repens in Ukraine, an emergent zoonosis: epidemiological report of 1465 cases. Acta Parasitol. 2013;58:592–598. doi: 10.2478/s11686-013-0187-x. [DOI] [PubMed] [Google Scholar]
- Scarff C.A., Thompson R.F., Newlands G.F.J., Jamson A.H., Kennaway C., da Silva V.J., Rabelo E.M., Song C.F., Trinick J., Smith W.D., Muench S.P. Structure of the protective nematode protease complex H-gal-GP and its conservation across roundworm parasites. PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1008465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinkel A.H., Smit J.J.M., van Tellingen O., Beijnen J.H., Wagenaar E., van Deemter L., Mol C.A.A.M., van Der Valk M.A., Robanus-Maandag E.C., Te Riele H.P.J., Berns A.J.M., Borst P. Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell. 1994;77:491–502. doi: 10.1016/0092-8674(94)90212-7. [DOI] [PubMed] [Google Scholar]
- Schinkel A.H., Wagenaar E., Mol C.A., van Deemter L. P-glycoprotein in the blood-brain barrier of mice influences the brain penetration and pharmacological activity of many drugs. J. Clin. Invest. 1996;97:2517–2524. doi: 10.1172/JCI118699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmith V.D., Zhou J.J., Lohmer L.R.L. The approved dose of ivermectin alone is not the ideal dose for the treatment of COVID-19. Clin. Pharmacol. Ther. 2020;108:762–765. doi: 10.1002/cpt.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorderet-Weber S., Noack S., Selzer P.M., Kaminsky R. Blocking transmission of vector-borne diseases. International J for Parasitol. Drugs Drug Resist. 2017;7:90–109. doi: 10.1016/j.ijpddr.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoles G. Vectors of canine heartworm in the United States: a review of the literature including new data from Indiana, Florida, and Louisiana, Recent Advances in Heartworm Disease: symposium’98. Proceedings of the American Heartworm Society. 1998:21–36. 1–3 May 1998. [Google Scholar]
- Scott R.H., Duce I.R. Anion selectivity of gamma-aminobutyric acid (GABA) 22,23-dihydroavermectin B1a (DHAVM)-induced conductance changes on locust muscle. Neurosci. Lett. 1986;68:197–201. doi: 10.1016/0304-3940(86)90141-2. [DOI] [PubMed] [Google Scholar]
- Scott A.L., Ghedin E., Nutman T.B., McReynolds L.A., Poole C.B., Slatko B.E., Foster J.M. Filarial and Wolbachia genomics. Parasite Immunol. 2012;34:121–129. doi: 10.1111/j.1365-3024.2011.01344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selzer P.M., Epe C. Antiparasitics in animal health: Quo Vadis? Trends Parasitol. 2021;37:77–89. doi: 10.1016/j.pt.2020.09.004. [DOI] [PubMed] [Google Scholar]
- Selzer P.M., Marhöfer R.J., Koch O. Springer; Cham: 2018. A Short History of Bioinformatics. Applied Bioinformatics; pp. IX–XII. [Google Scholar]
- Shan Q., Haddrill J.L., Lynch J.W. Ivermectin, an unconventional agonist of the glycine receptor chloride channel. J. Biol. Chem. 2001;276:12556–12564. doi: 10.1074/jbc.M011264200. [DOI] [PubMed] [Google Scholar]
- Shang Kuan T.C., Prichard R.K. Developmental regulation of Dirofilaria immitis microfilariae and evaluation of ecdysone signaling pathway transcript level using droplet digital PCR. Parasites Vectors. 2020;13:614. doi: 10.1186/s13071-020-04480-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharmeen S., Skrtic M., Sukhai M.A., Hurren R., Gronda M., Wang X., Fonseca S.B., Sun H., Wood T.E., Ward R., Minden M.D., Batey R.A., Datti A., Wrana J., Kelley S.O., Schimmer A.D. The antiparasitic agent ivermectin induces chloride-dependent membrane hyperpolarization and cell death in leukemia cells. Blood. 2010;116:3593–3603. doi: 10.1182/blood-2010-01-262675. [DOI] [PubMed] [Google Scholar]
- Shoop W.L., Mrozik H., Fisher M.H. Structure and activity of avermectins and milbemycins in animal health. Vet. Parasitol. 1995;59:139–156. doi: 10.1016/0304-4017(94)00743-v. [DOI] [PubMed] [Google Scholar]
- Shoop W.L., DeMontigny P., Fink D.W., Williams J.B., Egerton J.R., Mrozik H., Fisher M.H., Skelly B.J., Turner M.J. Efficacy in sheep and pharmacokinetics in cattle that led to the selection of eprinomectin as a topical endectocide for cattle. Int. J. Parasitol. 1996;26:1227–1235. doi: 10.1016/s0020-7519(96)00122-1. [DOI] [PubMed] [Google Scholar]
- Shoop W.L., Egerton J.R., Eary C.H., Haines H.W., Michael B.F., Mrozik H., Eskola P., Fisher M.H., Slayton L., Ostlind D.A., Skelly B.J., Fulton R.K., Barth D., Costa S., Gregory L.M., Campbell W.C., Seward R.L., Turner M.J. Eprinomectin: a novel avermectin for use as a topical endectocide for cattle. Int. J. Parasitol. 1996;26:1237–1242. doi: 10.1016/s0020-7519(96)00123-3. [DOI] [PubMed] [Google Scholar]
- Silvestro C.A., Soria L.A., Conte A., Marrube G. Two methods for genotyping a 4-base deletion in the canine ABCB1 gene. J. Vet. Diagn. Invest. 2019;31:889–892. doi: 10.1177/1040638719887374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simón F., Morchón R., González-Miguel J., Marcos-Atxutegi C., Siles-Lucas M. What is new about animal and human dirofilariosis? Trends Parasitol. 2009;25:404–409. doi: 10.1016/j.pt.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Simón F., Siles-Lucas M., Morchón R., González-Miguel J., Mellado I., Carretón E., Montoya-Alonso J.A. Human and animal Dirofilariasis: the emergence of a zoonotic mosaic. Clin. Microbiol. Rev. 2012;25:507–544. doi: 10.1128/CMR.00012-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sironi M., Bandi C., Sacchi L., Di Sacco B., Damiani G., Genchi C. Molecular evidence for a close relative of the arthropod endosymbiont Wolbachia in a filarial worm. Mol. Biochem. Parasitol. 1995;74:223–227. doi: 10.1016/0166-6851(95)02494-8. [DOI] [PubMed] [Google Scholar]
- Slatko B.E., Taylor M.J., Foster J.M. The Wolbachia endosymbiont as an anti-filarial nematode target. Symbiosis. 2010;51:55–65. doi: 10.1007/s13199-010-0067-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slocombe J.O., Villeneuve A. Heartworm in dogs in Canada in 1991. Can. Vet. J. 1993;34:630–633. [PMC free article] [PubMed] [Google Scholar]
- Snyder D.E., Wiseman S., Cruthers L.R., Slone R.L. Ivermectin and milbemycin oxime in experimental adult heartworm (Dirofilaria immitis) infection of dogs. J. Vet. Intern. Med. 2011;25:61–64. doi: 10.1111/j.1939-1676.2010.0657.x. [DOI] [PubMed] [Google Scholar]
- Soll M.D., Carmichael I.H., Harvey R.G. Prophylactic efficacy of sustained-release ivermectin against induced nematode infestations in cattle. J. S. Afr. Vet. Assoc. 1988;59:9–11. [PubMed] [Google Scholar]
- Soussa R.W., Woodward A., Marty M., Cannon C.M. Breed is associated with the ABCB1-1Δ mutation in Australian dogs. Aust. Vet. J. 2020;98:79–83. doi: 10.1111/avj.12896. [DOI] [PubMed] [Google Scholar]
- Srikant S., Gaudet R. Mechanics and pharmacology of substrate selection and transport by eukaryotic ABC exporters. Nat. Struct. Mol. Biol. 2019;26:792–801. doi: 10.1038/s41594-019-0280-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiedl C.P., Weber K. Fast and simple detection methods for the 4-base pair deletion of canine MDR1/ABCB1 gene by PCR and isothermal amplification. J. Vet. Diagn. Invest. 2017;29:176–180. doi: 10.1177/1040638716683213. [DOI] [PubMed] [Google Scholar]
- Strube C., Haake C., Sager H., Schorderet Weber S., Kaminsky R., Buschbaum S., Joekel D., Schicht S., Kremmer E., Korrell J., Schnieder T., von Samson-Himmelstjerna G. Vaccination with recombinant paramyosin against the bovine lungworm Dictyocaulus viviparus considerably reduces worm burden and larvae shedding. Parasites Vectors. 2015;8:119. doi: 10.1186/s13071-015-0733-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton R.H., Atwell R.B., Boreham P.F. Liver changes, following diethylcarbamazine administration, in microfilaremic dogs infected with Dirofilaria immitis. Vet. Pathol. 1985;22:177–183. doi: 10.1177/030098588502200213. [DOI] [PubMed] [Google Scholar]
- Széll Z., Bacsadi Á., Szeredi L., Nemes C., Fézer B., Bakcsa E., Kalla H., Tolnai Z., Sréter T. Rapid spread and emergence of heartworm resulting from climate and climate-driven ecological changes in Hungary. Vet. Parasitol. 2020;280:109067. doi: 10.1016/j.vetpar.2020.109067. [DOI] [PubMed] [Google Scholar]
- Tahir D., Davoust B., Parola P. Vector-borne nematode diseases in pets and humans in the Mediterranean Basin: an update. Vet. World. 2019;12:1630–1643. doi: 10.14202/vetworld.2019.1630-1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takiguchi Y., Mishima H., Okuda M., Terao M., Aoki A., Fukuda R. Milbemycins, a new family of macrolide antibiotics: fermentation, isolation and physico-chemical properties. J. Antibiot. (Tokyo) 1980;33:1120–1127. doi: 10.7164/antibiotics.33.1120. [DOI] [PubMed] [Google Scholar]
- Takiguchi Y., Ono M., Muramatsu S., Ide J., Mishima H., Terao M. Milbemycins, a new family of macrolide antibiotics. Fermentation, isolation and physico-chemical properties of milbemycins D, E, F, G. and H. J Antibiot (Tokyo) 1983;36:502–508. doi: 10.7164/antibiotics.36.502. [DOI] [PubMed] [Google Scholar]
- Tang M., Hu X., Wang Y., Yao X., Zhang W., Yu C., Cheng F., Li J., Fang Q. Ivermectin, a potential anticancer drug derived from an antiparasitic drug. Pharmacol. Res. 2020;163:105207. doi: 10.1016/j.phrs.2020.105207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tappin S.W., Goodfellow M.R., Peters I.R., Day M.J., Hall E.J., Mealey K.L. Frequency of the mutant MDR1 allele in dogs in the UK. Vet. Rec. 2012;171:72. doi: 10.1136/vr.100633. [DOI] [PubMed] [Google Scholar]
- Taylor M.J., Voronin D., Johnston K.L., Ford L. Wolbachia filarial interactions. Cell Microbiol. 2013;15:520–526. doi: 10.1111/cmi.12084. [DOI] [PubMed] [Google Scholar]
- Taylor M.J., Hoerauf A., Townson S., Slatko B.E., Ward S.A. Anti-Wolbachia drug discovery and development: safe macrofilaricides for onchocerciasis and lymphatic filariasis. Parasitology. 2014;141:119–127. doi: 10.1017/S0031182013001108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M.J., von Geldern T.W., Ford L., Hübner M.P., Marsh K., Johnston K.L., Sjoberg H.T., Specht S., Pionnier N., Tyrer H.E., Clare R.H., Cook D.A.N., Murphy E., Steven A., Archer J., Bloemker D., Lenz F., Koschel M., Ehrens A., Metuge H.M., Chunda V.C., Ndongmo Chounna P.W., Njouendou A.J., Fombad F.F., Carr R., Morton H.E., Aljayyoussi G., Hoerauf A., Wanji S., Kempf D.J., Turner J.D., Ward S.A. Preclinical development of an oral anti-Wolbachia macrolide drug for the treatment of lymphatic filariasis and onchocerciasis. Sci. Transl. Med. 2019;11 doi: 10.1126/scitranslmed.aau2086. [DOI] [PubMed] [Google Scholar]
- Tombácz I., Weissman D., Pardi N. Vaccination with messenger RNA: a promising alternative to DNA vaccination. In: Sousa Â., editor. DNA Vaccines: Methods and Protocols. Springer US; New York, NY: 2021. pp. 13–31. [DOI] [PubMed] [Google Scholar]
- Tracey A., Foster J.M., Paulini M., Grote A., Mattick J., Tsai Y.-C., Chung M., Cotton J.A., Clark T.A., Geber A., Holroyd N., Korlach J., Libro S., Lustigman S., Michalski M.L., Rogers M.B., Twaddle A., Dunning Hotopp J.C., Berriman M., Ghedin E. Nearly complete genome sequence of Brugia malayi strain FR3. Microbiol Resour Announc. 2020;9 doi: 10.1128/MRA.00154-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traversa D., Aste G., Milillo P., Capelli G., Pampurini F., Tunesi C., Santori D., Paoletti B., Boari A. Autochthonous foci of canine and feline infections by Dirofilaria immitis and Dirofilaria repens in central Italy. Vet. Parasitol. 2010;169:128–132. doi: 10.1016/j.vetpar.2009.12.034. [DOI] [PubMed] [Google Scholar]
- Tritten L., Geary T.G. Helminth extracellular vesicles in host-parasite interactions. Curr. Opin. Microbiol. 2018;46:73–79. doi: 10.1016/j.mib.2018.08.002. [DOI] [PubMed] [Google Scholar]
- Turner J.D., Marriott A.E., Hong D., N P.O., Ward S.A., Taylor M.J. Novel anti-Wolbachia drugs, a new approach in the treatment and prevention of veterinary filariasis? Vet. Parasitol. 2020;279:109057. doi: 10.1016/j.vetpar.2020.109057. [DOI] [PubMed] [Google Scholar]
- Uddin F., Rudin C.M., Sen T. CRISPR gene therapy: applications, limitations, and implications for the future. Front. Oncol. 2020;10:1387. doi: 10.3389/fonc.2020.01387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venco L., Genchi M., Genchi C., Gatti D., Kramer L. Can heartworm prevalence in dogs be used as provisional data for assessing the prevalence of the infection in cats? Vet. Parasitol. 2011;176:300–303. doi: 10.1016/j.vetpar.2011.01.013. [DOI] [PubMed] [Google Scholar]
- Venco L., Marchesotti F., Manzocchi S. Feline heartworm disease: a 'Rubik's-cube-like' diagnostic and therapeutic challenge. J. Vet. Cardiol. 2015;17(Suppl. 1):S190–S201. doi: 10.1016/j.jvc.2015.08.004. [DOI] [PubMed] [Google Scholar]
- Ventre E., Rozières A., Lenief V., Albert F., Rossio P., Laoubi L., Dombrowicz D., Staels B., Ulmann L., Julia V., Vial E., Jomard A., Hacini-Rachinel F., Nicolas J.F., Vocanson M. Topical ivermectin improves allergic skin inflammation. Allergy. 2017;72:1212–1221. doi: 10.1111/all.13118. [DOI] [PubMed] [Google Scholar]
- Vercruysse J., Rew R.S. CABI Pub; 2002. Macrocyclic Lactones in Antiparasitic Therapy. [Google Scholar]
- Vidyashankar A.N., Jimenez Castro P.D., Kaplan R.M. A statistical approach for evaluating the effectiveness of heartworm preventive drugs: what does 100% efficacy really mean? Parasites Vectors. 2017;10:516. doi: 10.1186/s13071-017-2440-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Voorhis W.C., Hooft van Huijsduijnen R., Wells T.N.C. Profile of William C. Campbell, Satoshi Ōmura, and Youyou tu, 2015 Nobel laureates in Physiology or medicine. Proc. Natl. Acad. Sci. U. S. A. 2015;112:15773–15776. doi: 10.1073/pnas.1520952112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Bowman D.D., Brown H.E., Harrington L.C., Kaufman P.E., McKay T., Nelson C.T., Sharp J.L., Lund R. Factors influencing U.S. canine heartworm (Dirofilaria immitis) prevalence. Parasites Vectors. 2014;7:264. doi: 10.1186/1756-3305-7-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Xu Y., Wan H., Hu J. Antibiotic ivermectin selectively induces apoptosis in chronic myeloid leukemia through inducing mitochondrial dysfunction and oxidative stress. Biochem. Biophys. Res. Commun. 2018;497:241–247. doi: 10.1016/j.bbrc.2018.02.063. [DOI] [PubMed] [Google Scholar]
- Ward R.D. In: second ed. Darsie R.F. Jr., Ward R.A., editors. vol. 131. Parasitology; 2005. p. 416. (Identification and Geographical Distribution of the Mosquitoes of North America, North of Mexico). 580-580. [Google Scholar]
- Weber T., Selzer P.M. Isoxazolines: a novel chemotype highly effective on ectoparasites. ChemMedChem. 2016;11:270–276. doi: 10.1002/cmdc.201500516. [DOI] [PubMed] [Google Scholar]
- Welch J.S., Dobson C., Freeman C. Distribution and diagnosis of Dirofilariasis and toxocariasis in Australia. Aust. Vet. J. 1979;55:265–274. doi: 10.1111/j.1751-0813.1979.tb00393.x. [DOI] [PubMed] [Google Scholar]
- Wheeler N.J., Airs P.M., Zamanian M. Long-read RNA sequencing of human and animal filarial parasites improves gene models and discovers operons. PLoS Neglected Trop. Dis. 2020;14 doi: 10.1371/journal.pntd.0008869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J.C., Stuedemann J.A., Bairden K., Kerboeuf D., Ciordia H., Hubert J., Broussard S.D., Plue R.E., Alva-Valdes R., Baggott D.G., Pinkall N., Eagleson J.S. Efficacy of a pour-on formulation of eprinomectin (MK-397) against nematode parasites of cattle, with emphasis on inhibited early fourth-stage larvae of Ostertagia spp. Am. J. Vet. Res. 1997;58:379–383. [PubMed] [Google Scholar]
- Williamson S.M., Walsh T.K., Wolstenholme A.J. The cys-loop ligand-gated ion channel gene family of Brugia malayi and Trichinella spiralis: a comparison with Caenorhabditis elegans. Invertebr. Neurosci. 2007;7:219–226. doi: 10.1007/s10158-007-0056-0. [DOI] [PubMed] [Google Scholar]
- Wolstenholme A.J. Glutamate-gated chloride channels. J. Biol. Chem. 2012;287:40232–40238. doi: 10.1074/jbc.R112.406280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme A.J., Rogers A.T. Glutamate-gated chloride channels and the mode of action of the avermectin/milbemycin anthelmintics. Parasitology. 2005;131(Suppl. l):S85–S95. doi: 10.1017/S0031182005008218. [DOI] [PubMed] [Google Scholar]
- Wolstenholme A.J., Maclean M.J., Coates R., McCoy C.J., Reaves B.J. How do the macrocyclic lactones kill filarial nematode larvae? Invertebr. Neurosci. 2016;16:7. doi: 10.1007/s10158-016-0190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . World Health Organization; 2019. World Health Organization Model List of Essential Medicines: 21st List 2019.https://apps.who.int/iris/bitstream/handle/10665/325771/WHO-MVP-EMP-IAU-2019.06-eng.pdf accessed June 18th, 2020. [Google Scholar]
- Yates D.M., Wolstenholme A.J. An ivermectin-sensitive glutamate-gated chloride channel subunit from Dirofilaria immitis. Int. J. Parasitol. 2004;34:1075–1081. doi: 10.1016/j.ijpara.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Yildirim A., Inci A., Duzlu O., Biskin Z., Ica A., Sahin I. Aedes vexans and Culex pipiens as the potential vectors of Dirofilaria immitis in Central Turkey. Vet. Parasitol. 2011;178:143–147. doi: 10.1016/j.vetpar.2010.12.023. [DOI] [PubMed] [Google Scholar]
- Yilmaz E., Fritzenwanker M., Pantchev N., Lendner M., Wongkamchai S., Otranto D., Kroidl I., Dennebaum M., Le T.H., Anh Le T., Ramünke S., Schaper R., von Samson-Himmelstjerna G., Poppert S., Krücken J. The mitochondrial genomes of the zoonotic canine filarial parasites Dirofilaria (nochtiella) repens and Candidatus Dirofilaria (nochtiella) Honkongensis provide evidence for presence of cryptic species. PLoS Neglected Trop. Dis. 2016;10 doi: 10.1371/journal.pntd.0005028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz E., Wongkamchai S., Ramünke S., Koutsovoulos G.D., Blaxter M.L., Poppert S., Schaper R., von Samson-Himmelstjerna G., Krücken J. High genetic diversity in the Dirofilaria repens species complex revealed by mitochondrial genomes of feline microfilaria samples from Narathiwat, Thailand. Transbound. Emerg. Dis. 2019;66:389–399. doi: 10.1111/tbed.13033. [DOI] [PubMed] [Google Scholar]
- Yin J., Park G., Lee J.E., Choi E.Y., Park J.Y., Kim T.-H., Park N., Jin X., Jung J.-E., Shin D., Hong J.H., Kim H., Yoo H., Lee S.-H., Kim Y.-J., Park J.B., Kim J.H. DEAD-box RNA helicase DDX23 modulates glioma malignancy via elevating miR-21 biogenesis. Brain. 2015;138:2553–2570. doi: 10.1093/brain/awv167. [DOI] [PubMed] [Google Scholar]
- Zarowiecki M., Berriman M. What helminth genomes have taught us about parasite evolution. Parasitology. 2015;142(Suppl. 1):S85–S97. doi: 10.1017/S0031182014001449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B. CRISPR/Cas gene therapy. J. Cell. Physiol. 2020;236:2459–2481. doi: 10.1002/jcp.30064. [DOI] [PubMed] [Google Scholar]
- Zhang P., Zhang Y., Liu K., Liu B., Xu W., Gao J., Ding L., Tao L. Ivermectin induces cell cycle arrest and apoptosis of HeLa cells via mitochondrial pathway. Cell Prolif. 2019;52:e12543. doi: 10.1111/cpr.12543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ōmura S. Ivermectin: 25 years and still going strong. Int. J. Antimicrob. Agents. 2008;31:91–98. doi: 10.1016/j.ijantimicag.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Ōmura S., Crump A. Ivermectin: panacea for resource-poor communities? Trends Parasitol. 2014;30:445–455. doi: 10.1016/j.pt.2014.07.005. [DOI] [PubMed] [Google Scholar]