Abstract
Objective
Transport of Ca2+ into pancreatic β cell mitochondria facilitates nutrient-mediated insulin secretion. However, the underlying mechanism is unclear. Recent establishment of the molecular identity of the mitochondrial Ca2+ uniporter (MCU) and associated proteins allows modification of mitochondrial Ca2+ transport in intact cells. We examined the consequences of deficiency of the accessory protein MICU2 in rat and human insulin-secreting cells and mouse islets.
Methods
siRNA silencing of Micu2 in the INS-1 832/13 and EndoC-βH1 cell lines was performed; Micu2−/− mice were also studied. Insulin secretion and mechanistic analyses utilizing live confocal imaging to assess mitochondrial function and intracellular Ca2+ dynamics were performed.
Results
Silencing of Micu2 abrogated GSIS in the INS-1 832/13 and EndoC-βH1 cells. The Micu2−/− mice also displayed attenuated GSIS. Mitochondrial Ca2+ uptake declined in MICU2-deficient INS-1 832/13 and EndoC-βH1 cells in response to high glucose and high K+. MICU2 silencing in INS-1 832/13 cells, presumably through its effects on mitochondrial Ca2+ uptake, perturbed mitochondrial function illustrated by absent mitochondrial membrane hyperpolarization and lowering of the ATP/ADP ratio in response to elevated glucose. Despite the loss of mitochondrial Ca2+ uptake, cytosolic Ca2+ was lower in siMICU2-treated INS-1 832/13 cells in response to high K+. It was hypothesized that Ca2+ accumulated in the submembrane compartment in MICU2-deficient cells, resulting in desensitization of voltage-dependent Ca2+ channels, lowering total cytosolic Ca2+. Upon high K+ stimulation, MICU2-silenced cells showed higher and prolonged increases in submembrane Ca2+ levels.
Conclusions
MICU2 plays a critical role in β cell mitochondrial Ca2+ uptake. β cell mitochondria sequestered Ca2+ from the submembrane compartment, preventing desensitization of voltage-dependent Ca2+ channels and facilitating GSIS.
Keywords: Mitochondrial calcium uniporter, Voltage-dependent calcium channels, Bioenergetics, Knockout mice, Stimulus-secretion coupling
Graphical abstract
Highlights
-
•
MICU2 deficiency impairs glucose-stimulated insulin secretion.
-
•
MICU2 deficiency abrogates mitochondrial calcium uptake.
-
•
Depolarization-evoked cytosolic calcium increases are lower in MICU2-deficient cells.
-
•
Mitochondria clear calcium from the subplasma membrane region, maintaining calcium influx.
1. Introduction
While the canonical model of glucose-stimulated insulin secretion (GSIS) from pancreatic β cells with a central role of the mitochondrion has been established for more than 30 years, many details remain unclear. Extensive literature links mitochondrial Ca2+ uptake to facilitation of insulin secretion [[1], [2], [3], [4], [5], [6]]. However, it is unclear whether the reported effects of elevated matrix-free Ca2+ on insulin secretion are due to the bioenergetic consequences of the activation of citric acid cycle enzymes [7], the facilitation of plasma membrane oscillations [8], the generation of so-called coupling factors [5,9], or additional currently unrecognized mechanisms. The molecular identification of the components of the mitochondrial Ca2+ uniporter with the ability to selectively ablate one or more components allows the consequences of disrupted mitochondrial Ca2+ transport to be investigated in β cells.
The uniporter complex comprises the pore-forming subunit MCU plus up to six regulatory subunits [10]. MCU forms the complex's central pore-forming subunit, while MCU regulatory subunit b (MCUb), essential MCU regulator (EMRE), and mitochondrial uptake 1 and 2 (MICU1 and MICU2) form the accessory components [11]. As part of the Ca2+ uniporter holocomplex, MICU1 and MICU2 heterodimerize and act to maintain a threshold for mitochondrial Ca2+ uptake, upholding the mitochondrial Ca2+-buffering capacity [[12], [13], [14]]. It has been demonstrated in INS-1 832/13 insulinoma cells that MCU and MICU1 play a role in metabolic coupling; reduced expression of either decreases mitochondrial Ca2+ uptake and inhibits insulin secretion [8]. Furthermore, β cell-specific Mcu knockout mice display reduced first-phase insulin release in vivo and impaired mitochondrial Ca2+ uptake, glucose-induced ATP production, and insulin secretion in vitro [15]. However, the function of MICU2 in stimulus-secretion coupling in pancreatic β cells has not been examined. The situation in other cell types is somewhat unclear. In HeLa cells, silencing of Micu2 leads to increased mitochondrial Ca2+ uptake [16,17], but only at cytosolic levels lower than 5 μM [17]. A similar phenotype is also observed in HEK-293T cells [13,18], while silencing of Micu2 in mouse liver leads to impaired Ca2+ uptake kinetics [19]. To resolve the role of Ca2+ uptake, we examined the impact of MICU2 deficiency on insulin-secreting cells and mouse islets as well as in HEK-293T cells.
2. Materials and methods
2.1. Cell culture and model systems
INS-1 832/13 cells were cultured as previously described [20]. EndoC-βH1 cells [21] were grown on Matrigel-fibronectin-coated (100 μg/mL and 2 μg/mL, respectively, Sigma–Aldrich) culture vessels in DMEM containing 5.6 mM of glucose, 2% BSA fraction V (Roche Diagnostics), 10 mM of nicotinamide (Calbiochem), 50 μM of 2-mercaptoethanol, 5.5 μg/mL of transferrin, 6.7 ng/mL of sodium selenite (Sigma–Aldrich), 100 U/mL of penicillin, and 100 μg/mL of streptomycin (PAA). Both cell lines were cultured at 37 °C with 5% CO2. Unless otherwise stated, EndoC-βH1 cells were seeded at 2.3 × 105 cells/cm2 and INS-1 832/13 cells at 1.5 × 105 cells/cm2 in 24-well plates (Matrigel-fibronectin coated or uncoated) and transfected either with siRNA specific to MICU2 (50 nM) or a scrambled siRNA (50 nM). Forty-eight h after knockdown, the growth media were changed to an overnight starvation medium containing 5.6 mM of glucose for INS-1 832/13 cells and 1 mM of glucose for EndoC-βH1 cells. HEK-293T cells were cultured in DMEM containing 1 g/L of d-glucose and supplemented with 10% heat-inactivated FBS, 100 U/mL of penicillin, and 100 μg/mL of streptomycin (PAA) [22]. The cells were plated at 1.5 × 105 cells/cm2 in 24-well plates and transfected with either siRNA specific to MICU2 or a scrambled siRNA. All of the assays were performed 48–72 h after knockdown.
2.2. Micu2 knockout mice
We purchased a Micu2 gene trap from the Texas A&M Institute of Genomic Medicine (College Station, TX, USA), bred the mice, and validated the disruption of the Micu2 gene using standard procedures. The Micu2 knockout mice had a B57BL/6J background and were extensively backcrossed. This mouse was previously characterized [23]. A combination of male and female mice was used with no significant differences between the two sexes.
2.3. Insulin secretion assay
INS-1 832/13 cells were incubated in HEPES-balanced salt solution (HBSS; 115 mM of NaCl, 5 mM of KCl, 1 mM of MgSO4, 1 mM of CaCl2, 25.5 mM of NaHCO3, 20 mM of HEPES, and 0.2% BSA at a pH of 7.2) containing 2.8 mM of glucose for 2 h followed by 1 h of stimulation with HBSS containing 2.8 or 16.7 mM of glucose or 15 min of stimulation with HBSS containing 36 mM of KCl and 2.8 mM of glucose [20]. Insulin secretion was measured with a rat insulin ELISA kit (Mercodia A/B, Sweden) according to the manufacturer's instructions. EndoC-βH1 cells were starved overnight in 1 mM of glucose-containing growth medium 1 day before the experiment. On the day of the experiment, the growth medium was removed and replaced with 1 mM of glucose-containing HBSS for 2 h followed by 1 h of stimulation with HBSS containing 1 or 20 mM of glucose [24]. Insulin secretion was measured with a human insulin ELISA (Mercodia A/B, Sweden) according to the manufacturer's instructions.
2.4. RNA isolation and quantitative real-time PCR
Total RNA was extracted from EndoC-βH1 cells, INS-1 832/13, and HEK-293T cells using an RNeasy Mini kit (Qiagen) according to the manufacturer's protocol. RNA concentrations were determined using a NanoDrop Spectrophotometer (Thermo Fisher Scientific). Then 1000 ng of total RNA were reverse transcribed using a RevertAid First-Strand cDNA synthesis kit (Fermentas, Vilnius, Lithuania). Quantitative real-time PCR (qPCR) was performed using TaqMan gene expression assays (EFHA1/Micu2 rat: s235193; EFHA1/Micu2 human: s47976; Hprt1 rat: Rn01527840; Hprt1 human: Hs99999909; Mcu rat: Rn01433739; Micu1 rat: Rn01644475; Cacna1a rat, Rn00563825; Cacna1c rat, Rn00709287; and INS1 mouse, Mm01950294, Applied Biosystems, Life Technologies, Carlsbad, CA, USA) using a 7900HT Fast Real-Time System (Applied Biosystems). qPCR was carried out as follows. Five ng of cDNA were mixed with 0.5 μL of gene assay and 5 μL of TaqMan Master Mix and ddH2O to a final volume of 10 μL and qPCR was performed using the following program: 50 °C for 2 min, 95 °C for 10 min, 40 cycles of 95 °C for 15 s, and 60 °C for 1 min. In all of the runs, a 10x dilution series was produced of one of the samples and used to generate a calibration curve as well as quality control for amplification efficiency. Gene expression was relatively quantified using the calibration curve. The amount of mRNA was calculated relative to the amount of hypoxanthine-guanine phosphoribosyl transferase (HPRT1) as a reference gene.
2.5. SDS-PAGE and western blotting
Cells were harvested in RIPA buffer followed by lysis by shaking the samples for 30 min at 950 rpm and 4 °C for 30 min. This was followed by centrifugation at 16,000g and 4 °C for 20 min to remove cell debris. Supernatants/lysates were kept at −80 °C until use. Then 10–20 μg of protein were separated on mini-PROTEAN TGX pre-stained gels (Bio-Rad) followed by activation of pre-stained Bio-Rad MP imager. The proteins were blotted onto Trans-Blot Turbo Transfer Packs (Bio-Rad), using Bio-Rad semidry blotter, and total protein for loading control was determined from the pre-stains. Membranes were blocked with 10% BSA or 5% skim milk (for mitochondrial complexes I–V experiments) in TRIS-buffered saline (TBS) unless otherwise stated and hybridized with antibodies for MICU2/EFHA1 (Abcam, ab101465), β-tubulin (Abcam, ab6046), α-tubulin (Abcam, ab7291), and mitochondrial complexes I–V (Abcam, ab110413).
2.6. Single-cell live cytosolic ATP and ATP/ADP ratio measurements using perceval and perceval HR
Single-cell cytosolic ATP/ADP ratio measurements were carried out in INS-1 832/13 cells using the genetically encoded biosensor Perceval HR [25]. The cells were seeded on poly-d-lysine-coated 8-well-chambered cover glasses (Lab-Tek, Thermo Fisher Scientific) at a density of 70,000 cells/cm2. Twenty-four h after seeding, the cells were co-transfected with siRNA and 1 μg of plasmid-encoding Perceval HR (Addgene ID: 49,083) at approximately 50% cell confluency. The cells were further grown for 48–72 h before measurements. On the day of imaging, the cells were pre-incubated at 37 °C in 400 μL of buffer P (135 mM of NaCl, 3.6 mM of KCl, 1.5 mM of CaCl2, 0.5 mM of MgSO4, 0.5 mM of Na2HPO4, 10 mM of HEPES, and 5 mM of NaHCO3 at a pH of 7.4) containing 2.8 mM glucose. After 1.5 h of incubation, the cells were imaged at 488 nm excitation and 505–535 nm emission recorded on a Zeiss LSM510 inverted confocal fluorescence microscope. Mouse islets were transduced with adenovirus expressing the similar ATP biosensor Perceval [26,27]. Poly-d-lysine-coated 8-well-chambered cover glasses (Lab-Tek, Thermo Fisher Scientific) containing mouse islets were incubated for 2 h with RPMI with 10% FBS, 100 U of penicillin, and 100 mg/mL of streptomycin containing the Perceval adenovirus. Then this medium was replaced with fresh RPMI medium with 10% FBS, 100 U of penicillin, and 100 mg/mL of streptomycin and incubated overnight. On the day of imaging, the cells were pre-incubated at 37 °C in 400 μL of buffer P (135 mM of NaCl, 3.6 mM of KCl, 1.5 mM of CaCl2, 0.5 mM of MgSO4, 0.5 mM of Na2HPO4, 10 mM of HEPES, and 5 mM of NaHCO3 at a pH of 7.4) containing 2.8 mM of glucose. After 1.5 h of incubation, the cells were imaged as described for clonal cell lines at 488 nm excitation and emission recorded at 505–530 nm on a Zeiss LSM510 inverted confocal fluorescence microscope using a 40x/0.75 objective. Images were recorded at a frequency of 0.25 Hz (scan time = 3.93 s). Equimolarity was accounted for in the 36 mM KCl solution.
2.7. Single-cell live cytoplasmic-free Ca2+ measurements
Cells were seeded onto poly-d-lysine coated 8-well-chambered cover glasses (Lab-Tek, Naperville, IL, USA) at a density of 70,000 cells/cm2 per well. Twenty-four h after seeding, the cells were transfected with siRNA and incubated for further 48–72 h prior to imaging. On the day of imaging, the cells were pre-incubated at 37 °C in 400 μL of buffer P containing 2.8 mM of glucose. After 1.5 h of incubation, based on variable Ca2+ affinities, INS-1 832/13 and HEK-293T cells were loaded with 2 μM of Fluo4 AM and Fluo5F AM, respectively. Incubation was continued for an additional 30 min. Immediately prior to imaging, the pre-incubation buffer was exchanged with 400 μL of buffer P. Fluo4 AM and Fluo5F AM were excited at 488 nm and emission recorded using a 505 nm emission filter on a Zeiss LSM510 inverted confocal fluorescence microscope using a 40x/0.75 objective. Images were recorded at a frequency of 0.64 Hz (scan time = 1.57 s) using a pinhole diameter of 463 μm. Single-cell-free cytoplasmic Ca2+ traces were displayed in arbitrary fluorescent units.
For experiments performed with the line scan configuration, INS-1 832/13 cells and EndoC-βH1 cells were plated, transfected, and loaded as previously described, except Fluo8 AM (2 μM) used instead in EndoC-βH1 cells. Imaging was again performed using a Zeiss LSM510 inverted confocal fluorescence microscope with a 100x/1.45 objective with Fluo4/Fluo8 excited at 488 nm and emission recorded using a 505 nm emission filter. For each recording, a single line was drawn through the center of 1–3 cells. This was the only part of the field of view that was measured during the recording. The pinhole diameter was reduced to 256 μm to minimize signals from outside the focal plane (1.4 μm section). This allowed the Ca2+ signals to be tracked as they propagated from the edge to the center of the cells with minimal interference from Ca2+ diffusing from above or below the focal plane. Recordings were performed at a frequency of 333 Hz (scan time = 3 ms) and a duration of 6000 cycles.
2.8. Single-cell live mitochondrial Ca2+ measurements
Single-cell [Ca2+]mito measurements were carried out using genetically encoded mitochondrial-targeted Ca2+ biosensor mito-case12 (Evrogen cat# FP992.). INS-1 832/13 and HEK-293T cells were seeded onto poly-d-lysine-coated 8-well-chambered cover glasses (Lab-Tek, Thermo Fisher Scientific) at a cell density of 70,000 cells/cm2. Twenty-four h after seeding, the cells were co-transfected with siRNA and 1 μg of plasmid encoding mito-case12 at approximately 50% cell confluency. The cells were further grown for 48–72 h before measurements. On the day of imaging, the cells were pre-incubated at 37 °C in 400 μL of buffer P containing 2.8 mM of glucose. After 1.5 h, the cells were imaged using a 488 nm laser and emission recorded at 505–530 nm on a Zeiss LSM510 inverted confocal fluorescence microscope using a 100x/1.45 objective. Images were recorded at a frequency of 0.25 Hz (scan time = 3.93 s).
2.9. Live cell mitochondrial-free Ca2+ measurements using Rhod2 AM
EndoC-βH1 cells were seeded onto poly-d-lysine-coated 8-well-chambered cover glasses at a density of 70,000 cells/cm2 (Lab-Tek, Naperville, IL, USA). The day after seeding, the cells were transfected with siRNA and incubated for 72 h prior to imaging. On the day of imaging, the cells were pre-incubated at 37 °C in 400 μL of buffer P containing 2.8 mM of glucose. After 1.5 h of incubation, 2 μM of Rhod-2 AM (Abcam) was loaded for 30 min at 37 °C in buffer P. The cells were then washed once with buffer P to remove excess dye prior to imaging. Excitation of Rhod-2 AM was performed at 543 nm and emission recorded at 590–615 nm on a Zeiss LSM510 inverted confocal fluorescence microscope. For single mouse pancreatic islet mitochondrial-free Ca2+ measurements, poly-d-lysine-coated 8-well-chambered cover glasses containing single complete or partially digested mouse pancreatic islets pre-incubated at 37 °C in 400 μL of buffer P containing 2.8 mM of glucose. After 1.5 h of incubation, 2 μM of Rhod-2 AM (Abcam) was loaded for 30 min at 37 °C in buffer P. The cells were then washed once with buffer P to remove excess dye prior to imaging. Excitation of Rhod-2 AM was performed at 543 nm and emission recorded at 565–615 nm. The examination of the effect of glucose on [Ca2+]mito was performed on a Zeiss LSM510 inverted confocal fluorescence microscope using a 100x/1.45 objective. Images were recorded at a frequency of 0.13 Hz (scan time = 7.86 s).
2.10. Simultaneous measurement of cell cytoplasmic-free Ca2+ and cell mitochondrial-free Ca2+
For experiments measuring the effect of 36 mM of KCl on [Ca2+]c and [Ca2+]mito in EndoC- βH1, the protocol was the same except that after 1.5 h of incubation, 2 μM of Rhod-2 AM (Abcam) and 2 μM of Fluo8 AM were loaded for 30 min at 37 °C in buffer P. Imaging was performed on a Zeiss LSM510 inverted confocal fluorescence microscope using a 40x/0.75 objective with images recorded at a frequency of 0.30 Hz (scan time = 3.36 s).
2.11. Single-cell live mitochondrial membrane potential (Δψm) measurements
INS-1 832/13 cells were seeded onto poly-d-lysine-coated 8-well-chambered cover glasses (Lab-Tek, Naperville, IL, USA) at a density of 70,000 cells/cm2. Twenty-four h after seeding, the cells were transfected with siRNA and incubated for 48–72 h prior to imaging. For Δψm measurements, the cells were pre-incubated for 2 h in buffer P with 100 nM of TMRM (Invitrogen) and 2.5 μM of cyclosporin A. After incubation, the cells were washed once with buffer P and then imaging was performed. Under these conditions, Δψm measurements were performed in quench mode [28]. TMRM was present in the medium throughout the experiment. TMRM fluorescence measurements were performed using 543 nm excitation and a 560 nm long pass emission filter on a Zeiss LSM510 inverted confocal fluorescence microscope using a 40x/0.75 objective. Images were recorded at a frequency of 0.64 Hz (scan time = 1.57 s).
2.12. Single-cell live Subplasmalemmal Ca2+ measurements
Single-cell [Ca2+]mem measurements were carried out using PeriCam-based genetically encoded inner plasma membrane-targeted Ca2+ probe mem-case12 (Evrogen cat# FP992.). Specifically, INS-1 832/13 and HEK-293T cells were seeded onto poly-d-lysine-coated 8-well-chambered cover glasses (Lab-Tek, Naperville, IL, USA) at a cell density of 70,000 cells/cm2. After 24 h of seeding, the cells were co-transfected with siRNA and 1 μg of plasmid-encoding mem-case12 at approximately 50% cell confluency. The cells were then further grown for 48–72 h before measurements. On the day of imaging, the cells were pre-incubated at 37 °C in 400 μL of buffer P containing 2.8 mM of glucose. After 1.5 h, the cells were imaged at 488 nm excitation (8%) and emission recorded at 505–535 nm on a Zeiss LSM510 inverted confocal fluorescence microscope using a 100x/1.45 objective. Images were recorded at a frequency of 0.64 Hz (scan time = 1.57 s).
2.13. Mouse islet insulin secretion and insulin content
Islets were picked by hand as previously described [29] under a stereomicroscope and incubated in HBSS containing 2.8 mM of glucose for 2 h. This was followed by 1 h of stimulation with HBSS supplemented with 2.8 or 16.7 mM of glucose. Aliquots of incubation medium were collected and insulin secretion was measured with a mouse insulin ELISA kit (Mercodia A/B, Sweden) according to the manufacturer's instructions. To extract insulin from mouse islets for insulin content measurements, 300 μl of acid ethanol (95% ethanol, HCL (37%), and water) were added to tubes containing 10 islets. The tubes were then sonicated 3 times for 5 s. The samples then underwent 5 cycles of freezing/thawing consisting of 5 min of freezing in ethanol/dry ice followed by 5 min of thawing on ice. The samples were the centrifuged for 5 min at 13.4×g with 50 μl of supernatant removed into a new tube, stored at −20 °C, and then measured using mouse insulin ELISA.
2.14. Glucose and insulin tolerance tests
All tolerance tests were performed in anesthetized mice, using 0.25 mg of midazolam (Midazolam Panpharma, Fougères, France) combined with 0.5 mg of fluanisone and 0.02 mg of fentanyl (Hypnorm, VetPharma, Leeds, UK). For IVGTT, mice were starved for 2 h prior to tail vein injection with 1 g/kg of d-glucose. For ITT, unfasted mice were intraperitoneally injected with 0.75 mU/g of human insulin (Novo Nordisk, Clayton, NC, USA). In each case, blood was collected via retro-orbital bleeding at the indicated time points after injection. Upon completion, the samples were centrifuged and plasma collected and stored at −20 °C. Insulin and glucose concentrations were then determined by a mouse insulin ELISA kit (Mercodia, Uppsala, Sweden) and Infinity Glucose (Oxidase) Reagent (Thermo Fisher Scientific, Waltham, MA, USA), respectively. Glucose measurements for ITT were determined by an Amplex Red Glucose/Glucose Oxidase Assay kit (Thermo Fisher Scientific, Waltham, MA, USA).
2.15. Statistical analyses
All of the experiments were repeated on 3 different days with the exception of ITT, which was performed twice. Data in all of the figures are presented as mean ± SEM, and unless specifically stated; statistical significance was determined by unpaired two-tailed Student's t-tests. For imaging experiments, n in the figures represents the number of cells or regions of interest (ROIs). For islet insulin secretion and IVGT assays, n in the figures represents the number of animals. For all of the statistical comparisons, a p value of less than 0.05 was considered significant.
3. Results
3.1. Functional consequences of MICU2 knockdown in insulin-secreting cells
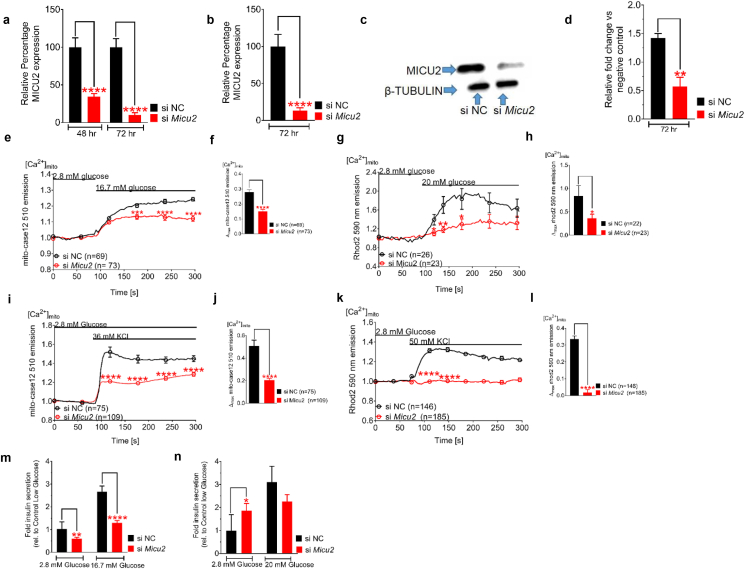
To understand the role of MICU2 in stimulus-secretion coupling in pancreatic β cells, we silenced MICU2 in INS-1 832/13 cells, a rodent-derived insulinoma cell line [20], and in EndoC-βH1 cells, a human embryonic insulin-secreting cell line [21,30]. Our experiments revealed that after 48 or 72 h, small interfering (si) RNA treatment was sufficient to significantly reduce the expression of MICU2 at the mRNA and protein level in both cell lines (Figure 1A–D). Notably, the expression of MCU and MICU1 was unaffected by MICU2 silencing (Supplementary Figs. 1A–B).
Figure 1.
Knockdown of MICU2 reduced mitochondrial Ca2+ uptake and decreased insulin secretion.MICU2 mRNA expression in INS-1 832/13 cells (A) and EndoC-βH1 cells (B) after treatment with scrambled or MICU2 siRNA. MICU2 protein expression (C) and quantification of Western blotting (D) in EndoC-βH1 cells. Mitochondrial Ca2+ ([Ca2+]mito) determined by mito-case12 and Rhod2, respectively, in MICU2-silenced INS-1 832/13 (E and F) and EndoC-βH1 cells (G and H) after glucose stimulation. E and G show average traces while F and H show differences in maximal mitochondrial Ca2+ levels between control and MICU2-silenced cells. [Ca2+]mito by mito-case12 and Rhod2, respectively, in MICU2-silenced INS-1 832/13 (I and J) and EndoC-βH1 cells (K and L) after stimulation with KCl; average traces (I and K) and differences in maximal [Ca2+]mito (J and L) between control and MICU2-silenced cells are shown. Insulin secretion in INS-1 832/13 (M) and EndoC-βH1 cells (N); secretion after 1 h of incubation was normalized to levels in cells treated with scrambled siRNA at 2.8 mM of glucose. Data are from at least three independent experiments 72 h after addition of siRNA unless otherwise stated. Mean ± SEM are given. Comparisons were made with an unpaired two-tailed Student's t-test. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001. See also Fig. S1.
Given the proposed function of MICU2 in the uniporter holocomplex, we examined the uptake of Ca2+ into the mitochondria upon MICU2 knockdown. To this end, we transfected INS-1 832/13 cells with a genetically encoded Ca2+ biosensor targeted to the mitochondrial matrix, mito-case12 [31]. Our experiments revealed that 16.7 mM of glucose raised [Ca2+]mito and that this increase was attenuated by 48% after silencing of MICU2 (p < 0.0001; Figure 1E–F). Similar findings were observed in EndoC-βH1 cells using another reporter, Rhod2, as these cells are difficult to transfect with plasmids [32]. In these cells, 20 mM of glucose triggered a [Ca2+]mito increase that was attenuated by 57% upon MICU2 silencing (p = 0.04; Figure 1G–H). MICU2 knockdown reduced not only the magnitude, but also the rate of [Ca2+]mito elevation in EndoC-βH1 cells (113 s vs 65 s to maximal [Ca2+]mito; p = 0.0202; Supplementary Fig. 1C). Both Rhod2 and mito-case12 showed “punctate” loading reflective of mitochondrial localization (Supplementary Figs. 1D–E). In INS-1 832/13 and EndoC-βH1 cells, evident from unnormalized mito-case12 and Rhod2 fluorescence, respectively, we observed that mitochondrial Ca2+ levels were also lower at basal glucose after Micu2 silencing (Supplementary Figs. 2A–B).
Increasing extracellular K+ to 36 mM depolarized the plasma membrane and activated voltage-dependent Ca2+ channels. This resulted in a robust increase in [Ca2+]mito in control cells, while there was only a minimal response in MICU2-silenced INS-1 832/13 cells (p < 0.0001; Figure 1I–J). Thus, the diminished mitochondrial response to elevated glucose (Figure 1E–H) could not simply be ascribed to decreased coupling between glucose metabolism and plasma membrane depolarization. EndoC-βH1 cells behaved similarly to rodent insulinoma cells, in which the maximal [Ca2+]mito elevation following depolarization with 50 mM of KCl was diminished by 90% in MICU2-silenced cells (p < 0.0001; Figure 1K–L). This recording was performed simultaneously with the measurement of [Ca2+]c shown in Supplementary Fig. 3B.
Our data suggest that MICU2 plays a role in [Ca2+]mito homeostasis in insulin-secreting cell lines, which would be predicted to impact hormone secretion. Indeed, we found that GSIS was reduced by 41% and 51% at 2.8 (p = 0.0023) and 16.7 mM of glucose (p < 0.0001), respectively, in MICU2-deficient INS-1 832/13 cells (Figure 1M). In MICU2-deficient EndoC-βH1 cells, basal insulin secretion at 2.8 mM of glucose increased by 46% (p = 0.0245; Figure 1N). However, secretion at 20 mM of glucose did not increase; accordingly, the net fold change (2.8 mM–20 mM of glucose) in insulin secretion was markedly and significantly reduced (1.4-fold vs 3.1-fold; p = 0.0018).
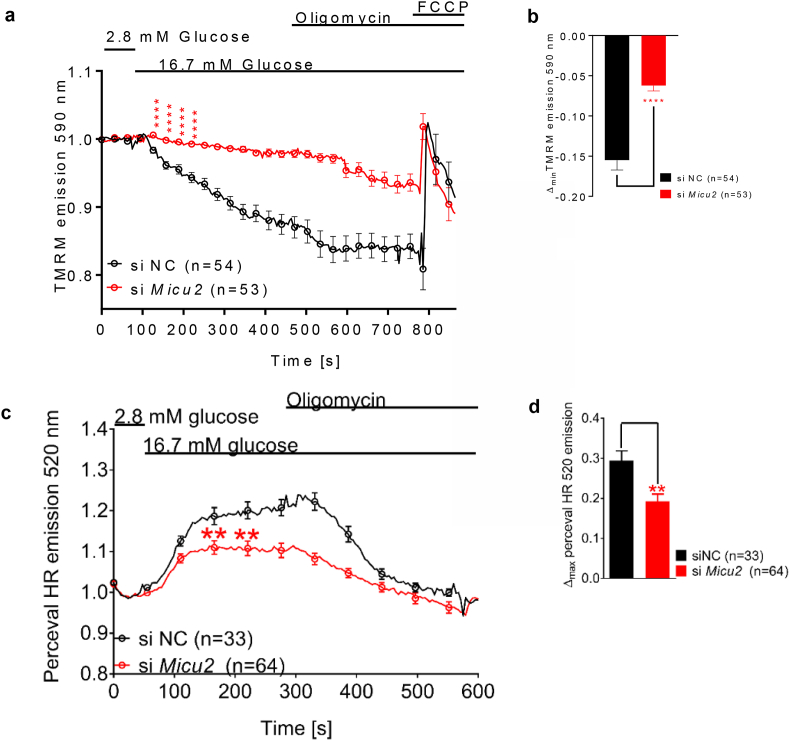
3.2. MICU2 deficiency impaired bioenergetic functions in insulin-secreting cells
The mitochondrial membrane potential Δψm is an essential facet of mitochondrial bioenergetics underlying both ATP synthesis and Ca2+ uptake into the mitochondrial matrix via MCU [33]. The mitochondrial membrane potential is influenced by uptake of Ca2+ into the matrix [34]. Given the potential regulatory role of MICU2 in this process and the observed attenuation of mitochondrial Ca2+ uptake, we monitored the relative changes in Δψm with tetramethylrhodamine methyl ester (TMRM) in quench mode. Indeed, a rise in glucose from 2.8 to 16.7 mM hyperpolarized Δψm in INS-1 832/13 cells and this response decreased by 60% after silencing of MICU2 (p < 0.0001; Figure 2A–B). It therefore appears plausible that decreased TCA cycle activation after silencing was the cause of the decreased hyperpolarization. Under basal conditions, unnormalized TMRM fluorescence was stronger in Micu2-silenced cells, indicating that mitochondria were depolarized to a greater extent than in control cells (Supplementary Fig. 2C).
Figure 2.
Bioenergetic consequences of MICU2 knockdown in INS-1 832/13 cells. Glucose-induced mitochondrial hyperpolarization of the inner mitochondrial membrane upon MICU2 knockdown in INS-1 832/13 cells measured by TMRM (A and B); average traces (A) and difference in maximal hyperpolarization (B) between control and MICU2-silenced cells are shown. The cytosolic ATP/ADP ratio in response to an elevation in glucose from 2.8 to 16.7 mM upon MICU2 knockdown in single INS-1 832/13 cells monitored with Perceval HR (C and D); average traces (C) and difference in the maximal increase in the ATP/ADP ratio (D) between control and MICU2-silenced cells are shown. Data are from at least three independent experiments 72 h after adding siRNA. Mean ± SEM are given. Comparisons were made with an unpaired two-tailed Student's t-test. ∗∗p < 0.01 and ∗∗∗∗p < 0.0001.
In light of this, we examined the effect of MICU2 deficiency on glucose-stimulated changes in the cytosolic ATP/ADP ratio using live cell imaging of INS-1 832/13 cells expressing the fluorescent ATP/ADP reporter Perceval HR [25]. We found that knockdown of MICU2 significantly reduced the increase in the cytosolic ATP/ADP ratio upon stimulation with 16.7 mM of glucose (Figure 2C), with the maximal cytosolic ATP/ADP ratio increase diminished by 36% after stimulation with 16.7 mM of glucose in MICU2-silenced INS-1 832/13 cells (p = 0.0013; Figure 2C–D). However, as previously stated, the decreased [Ca2+]mito response to elevated glucose could not be solely ascribed to decreased bioenergetic coupling, since it was also apparent when KCl was elevated. Basal levels of ATP production also appeared to be diminished as evident from reduced unnormalized Perceval HR fluorescence at 2.8 mM of glucose (Supplementary Fig. 2D).
3.3. MICU2 controlled both mitochondrial and cytosolic Ca2+ entry
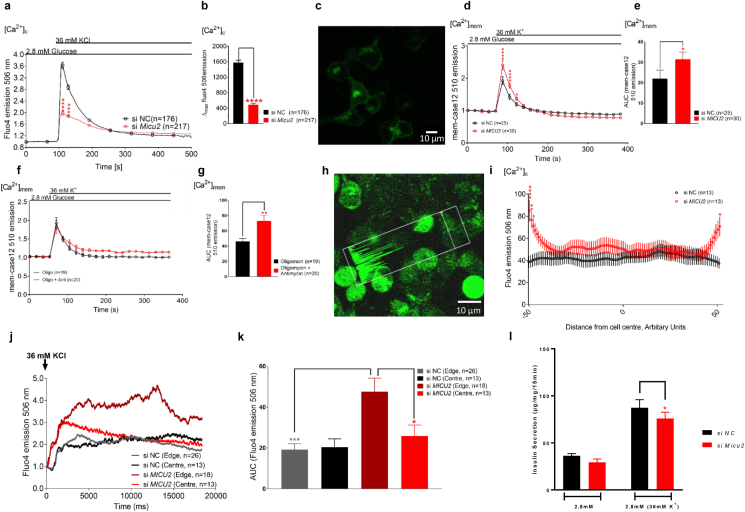
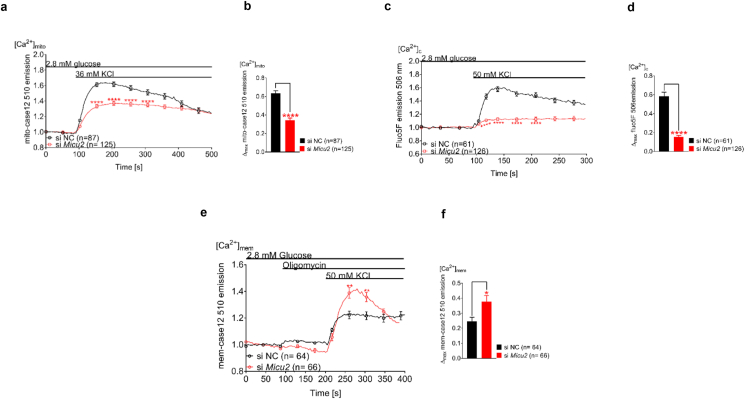
In the next set of experiments, we examined whether MICU2 also influences bulk [Ca2+]c in insulin-secreting cells. Glucose-induced Ca2+ oscillations were reduced in Micu2-silenced INS-1 832/13 cells (Supplementary Fig. 3A). However, as this could be the result of lower ATP production rather than a direct effect of MICU2 on [Ca2+]c dynamics, the cells were then depolarized non-metabolically by adding 36 mM of KCl. We predicted that depolarization-evoked [Ca2+]c increases would be more pronounced in MICU2-silenced cells due to reduced buffering by the mitochondria. Surprisingly, we observed that the maximal [Ca2+]c increase upon stimulation with 36 mM of KCl decreased by 48% in MICU2-silenced INS-1 832/13 cells (p < 0.0001; Figure 3A–B). Similar results were obtained in EndoC-βH1 cells, in which silencing of MICU2 led to a 41% reduction in the maximal [Ca2+]c elevation upon 50 mM of KCl stimulation (p = 0.0019; Supplementary Figs. 3B–C). This implied that the role of MICU2 in this paradoxical regulation of [Ca2+]c was conserved in β cells of both rat and human origin. The measurement of [Ca2+]c was performed simultaneously with the Rhod2 imaging shown in Figure 1K.
Figure 3.
Cytosolic and subplasmalemmal Ca2+ levels in response to KCl stimulation were perturbed in MICU2-silenced INS-1 832/13 cells. Cytosolic Ca2+ levels ([Ca2+]c) in MICU2-silenced INS-1 832/13 cells (and scramble control) upon stimulation with 36 mM of KCl as measured by Fluo4 (A and B). Average traces are shown in A, while B shows differences in maximal Fluo4 signals. (C) Image of mem-Case12 staining, n. b. specific membrane localization. Submembrane Ca2+ levels of [Ca2+]mem in MICU2-silenced INS-1 832/13 cells upon stimulation with 36 mM of KCl measured using mem-case12 (D and E); average traces are shown in D, while E shows iAUC quantification of KCl's effect on [Ca2+]mem. In (F and G), similar experiments are shown but using oligomycin and antimycin to block mitochondrial Ca2+ uptake prior to KCl addition, with oligomycin applied alone as a control. Mean ± SEM are given. (H) Example of line scan selection: the white arrow through the center of the cell is the only part of the field of view that was imaged; the accompanying trace within the white rectangle shows the fluorescence signal for each corresponding point along the arrow. (I) Mean Ca2+ intensity across INS-1 832/13 cells ∼500 ms after initiation of response to 36 mM of KCl. Cells had different sizes, so the data were interpolated to standardize each cell to a set number of points, allowing for comparison. The X axis represents the cell axis moving from one side of the plasma membrane (−50) through the center of the cell (0) to the opposite side of the plasma membrane (50). Points close to the extremities represent the submembrane region. (J) Average trace of cytosolic Ca2+ at cell edge and cell center in MICU2-silenced INS-1 832/13 cells (and scramble control). (K) iAUC quantification of [Ca2+]c at the cell edge and cell center in MICU2-silenced INS-1 832/13 cells (and scramble control). Subplasmalemmal Ca2+ levels ([Ca2+]mem) in MICU2-silenced INS-1 832/13 cells (and scramble control) upon stimulation with 36 mM of KCl. (L) Insulin secretion in control and Micu2-silenced INS-1 832/13 cells after 15 min of incubation in 36 mM of KCl. Comparisons were made with an unpaired two-tailed Student's t-test. In 3L, statistical comparisons were made by a paired two-tailed Student's t-test since variability in short-term incubations was greater. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001.
Given our findings, we hypothesized that the reduced depolarization-evoked [Ca2+]c increases after MICU2 knockdown (and mitochondrial depolarization) were due to reduced net Ca2+ entry across the plasma membrane (diminished entry or enhanced extrusion). To functionally elucidate this, we imaged INS-1 832/13 cells using a genetically encoded Ca2+ biosensor targeted to the inner leaflet of the plasma membrane mem-case12 [[35], [36], [37]]. Figure 3C shows the sensor's plasma membrane localization allowing the subplasmalemmal-free Ca2+ concentration ([Ca2+]mem) to be determined. If mitochondrial Ca2+ sequestration was indeed facilitating net Ca2+ entry into the cell by removing Ca2+ from the subplasma membrane compartment, then it would be predicted that cells with defective mitochondrial Ca2+ transport would show enhanced subplasmalemmal Ca2+ elevation in response to depolarization by KCl. Indeed, the maximal [Ca2+]mem elevation was 20% greater in MICU2-deficient INS-1 832/13 cells than in control cells (p = 0.0118; Figure 3D). The incremental area under the curve (iAUC) was also 43% greater in the MICU2-deficient cells (p = 0.015 vs control; Figure 3E).
To establish whether this effect on [Ca2+]mem could be accounted for by restricting MCU activity, we used an alternative strategy to inhibit mitochondrial Ca2+ transport [38]. In the presence of the ATP synthase inhibitor oligomycin, ATP is maintained by glycolysis and Δψm is retained, or even elevated, allowing mitochondrial Ca2+ transport to occur normally. Further adding an electron transport inhibitor such as antimycin rapidly collapses Δψm with no a priori effect on (glycolysis-maintained) cytosolic ATP production. Mitochondrial Ca2+ transport, however, being dependent upon Δψm, is abolished [38]. We found that although treating control INS-1 832/13 cells with oligomycin/antimycin did not increase the maximal [Ca2+]mem elevation, the Ca2+ concentration remained elevated longer at the membrane, with a delayed return to the basal state (Figure 3F). This was reflected by the iAUC of the effect increasing by 58% compared to oligomycin control (p = 0.0026; Figure 3G). Thus, this treatment had a comparable effect on [Ca2+]mem as MICU2 deficiency. An important proviso is that the capacity of INS-1 832/13 cells to accelerate glycolysis in the presence of oligomycin is limited by their low expression of lactate dehydrogenase [39]; therefore, experiments were performed during the time line before which ATP depletion became significant as indicated by the unperturbed mitochondrial membrane potential during this time (data not shown). Furthermore, using oligomycin alone as a control implied that any effect upon ATP production could not explain the reduced extrusion of [Ca2+]mem in oligomycin/antimycin-treated INS-1 832/13 cells and must therefore have been due to a decrease in mitochondrial Ca2+ uptake.
To further examine whether subplasmalemmal Ca2+ elevations occur when mitochondrial Ca2+ uptake is abrogated, we imaged INS-1 832/13 cells loaded with Fluo4 using the line scan configuration. This allows repeated imaging across a line through a cell axis as opposed to the entire field of view (Figure 3H). Crucially, this method allows the higher temporal resolution required for tracking Ca2+ signal propagation following membrane depolarization. To this end, cell(s) were selected, after which the starvation medium was rapidly replaced with medium containing 36 mM of KCl and the recording started simultaneously. It was expected that MICU2-deficient cells would show enhanced subplasmalemmal Ca2+ elevation in response to depolarization by KCl, reflected by a stronger fluorescence signal intensity at the edge of the line scan. We found that, upon KCl-induced depolarization, Ca2+ accumulated at the subplasma membrane compartment in siMICU2-treated INS-1 832/13 cells (Figure 3I–K) with a 1.85-fold higher Ca2+ signal (measured as iAUC) at the edge of the cell compared to its center (p = 0.0266). Furthermore, there was a 2.5-fold higher Ca2+ response at the submembrane compartment in MICU2-deficient INS-1 832/13 cells compared to controls (p = 0.0006 vs controls; Figure 3K). In contrast, there was no significant difference in the Ca2+ signal between the edge and center in the control cells (p = 0.8027; Figure 3K). Note that when analyzing the line scan data, the nucleus was avoided when selecting the “center” of the cell.
To ensure that the effects on [Ca2+]mem were not the result of off-target effects of Micu2 siRNA on voltage-gated Ca2+ channel expression, expression of L-type and P/Q-type Ca2+ channels in Micu2-silenced INS-1 832/13 cells were determined by qPCR: no difference in expression was observed (Supplementary Fig. 4A). We repeated the line scan experiments in EndoC-βH1 cells but were unable to see any difference between control and Micu2-silenced cells (Supplementary Fig. 4B). Using mem-case12 in Endo C-βH1 cells was not possible due to the cytotoxic effects of the transfection protocols.
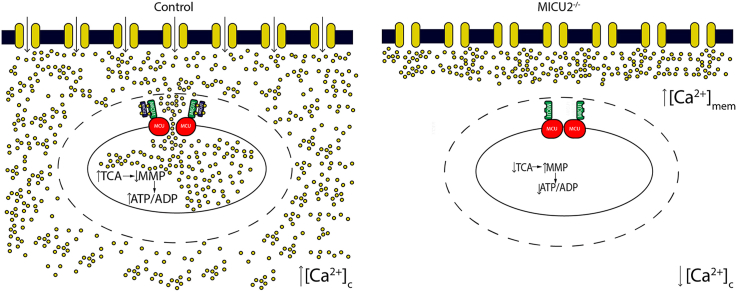
Collectively, these observations support a model in which MICU2-deficient mitochondria in the vicinity of the plasma membrane are less capable of removing Ca2+ from this compartment. Our results thus indicate not only that MICU2 plays a critical role in controlling MCU and Ca2+ homeostasis, but also that mitochondria play a critical role in buffering local subplasmalemmal Ca2+ as previously observed [40], facilitating net Ca2+ entry into the cell. This may occur either by decreasing cytosolic Ca2+-mediated desensitization of voltage-dependent Ca2+ channels in the plasma membrane [41,42] or alternatively by restricting Ca2+ efflux by plasma membrane Ca2+-ATPases [43,44]. The latter was unlikely in the present experimental situation since MICU2 deficiency inhibited an increase in the ATP/ADP ratio, which will limit access to ATP for the ATPases. To assess how this change in Ca2+ dynamics ultimately affects insulin secretion, INS-1 832/13 cells were exposed to 36 mM of KCl for 15 min: insulin secretion was reduced in MICU2-silenced cells (Figure 3L).
3.4. Micu2-deficient mice showed defective stimulus-secretion coupling and insulin secretion
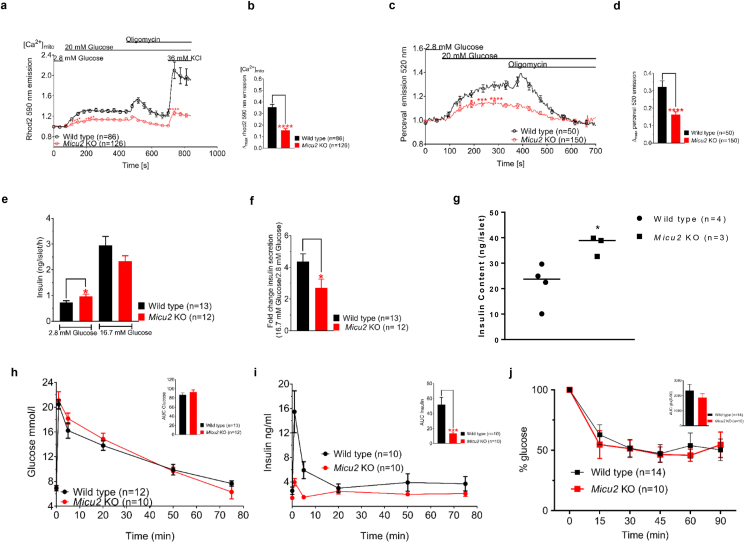
Our experiments suggested that perturbation of MICU2 led to reduced mitochondrial Ca2 uptake, resulting in perturbed Ca2+ entry into the cell and, consequently, defective insulin secretion in both rodent-derived INS-1 832/13 and human EndoC-βH1 cell lines. To understand the consequences of MICU2 deficiency in a more physiological setting, we used a global Micu2 knockout mouse [23]. As previously observed in insulin-secreting cell lines, glucose-stimulated (20 mM) maximal [Ca2+]mito elevation was diminished by 57% in Micu2 knockout islets compared to wild-type islets (p < 0.0001; Figure 4A–B). Note that the KCl response was also diminished (Figure 4A), similar to that observed in the cell lines. These results suggested that MICU2 most likely plays a similar role in maintenance of [Ca2+]mito homeostasis in primary cells to that observed in cell lines.
Figure 4.
Micu2 knockout mice exhibited deficiencies in mitochondrial Ca2+ homeostasis, ATP/ADP ratio, and insulin secretion. Mitochondrial Ca2+ levels [Ca2+]mito in islets isolated from Micu2 knockout (KO) and wild-type mice measured with Rhod2 after stimulation with 20 mM of glucose (A and B). Average traces are shown in (A) while (B) shows differences in maximal Rhod2 signals. A total of 40 islets from 7 wild-type mice and 42 islets from 6 Micu2 KO mice were assayed in response to 20 mM of glucose and 36 mM of KCl. Changes in the cytosolic ATP/ADP ratio in response to 20 mM of glucose in Micu2 KO and wild-type islets were determined by Perceval (C) n = 14 and 27 islets from 4 to 6 wild-type and KO islets, respectively. Average traces are shown in (C) while (D) shows differences in maximal Perceval signals. Insulin secretion from Micu2 KO and wild-type islets at 2.8 and 16.7 mM of glucose after 1 h of incubation (E); (F) shows the fold response of insulin. (G) Insulin content in Micu2 KO and wild-type islets. Plasma glucose (H) and insulin (I) levels following an intravenous glucose tolerance test in Micu2 KO and wild-type mice; n denotes the number of mice. Insets show the area under the curve for glucose (AUC; H) and insulin (J; T = 0–5 min). Data are from at least three independent experiments. (I) Insulin tolerance test (ITT) in Micu2 KO and wild-type mice. Glucose values are expressed as the percentage of glucose in which glucose at 0 min was 100%; n denotes the number of mice. Inset shows the AUC. Mean ± SEM are given. Comparisons of imaging and insulin secretion were made with an unpaired two-tailed Student's t-test (∗p < 0.05 and ∗∗∗∗p < 0.0001) and the Mann–Whitney U-test (∗∗∗p < 0.001) for data from IVGTT.
Furthermore, as [Ca2+]mito homeostasis is critical for cellular bioenergetics, we speculated that the Micu2 knockout might perturb the cytosolic ATP/ADP ratio in primary cells, as was seen in the insulin-secreting cell lines. Therefore, in the next set of experiments, we used an adenovirus expressing the biosensor Perceval, originally described as an ATP/ADP sensor [26], but later shown to report cytoplasmic ATP in mouse islet cells [45]. The maximal Perceval signal was diminished by 50% in Micu2 knockout islets compared with wild-type islets upon stimulation with 20 mM glucose (p < 0.0001; Figure 4C–D). A likely consequence of an abrogated increase in the ATP/ADP ratio in pancreatic β cells is impaired insulin secretion. Batch incubations of Micu2 knockout islets demonstrated that basal (2.8 mM of glucose) insulin secretion was significantly higher compared to that in their wild-type counterparts (p = 0.0427; Figure 4E). However, Micu2 knockout islets failed to release insulin in response to high glucose as efficiently as wild-type islets as illustrated by a 50% reduction in the fold response of insulin secretion (16.7–2.8 mM of glucose; p = 0.0362; Figure 4F). Notably, insulin content was found to be increased in Micu2 knockout islets compared with wild-type islets (p < 0.05; Figure 4G). This effect was most likely due to the accumulation of insulin that was not released since INS1 mRNA expression was similar in wild-type and Micu2 knockout islets (Supplementary Fig. 5A). Supplementary Fig. 5B shows the islet secretion data normalized to the insulin content derived from the analysis shown in Figure 4G: in vitro results shown this way reflect the in vivo observations, in that stimulated insulin secretion was abrogated while basal secretion was unchanged.
To ensure that any effects were not due to changes in the expression of the mitochondrial complexes, we quantified the protein expression of complexes I–V by Western blotting. There were no significant differences between wild-type and Micu2 knockout islets (Supplementary Figs. 5C–D). Our experiments thus suggested that stimulus-secretion coupling in β cells in Micu2 knockout mice was defective and underlied perturbed GSIS, reminiscent of that found in EndoC-βH1 cells (Figure 1N).
In the next set of experiments, we examined the consequences of Micu2 deficiency on whole-body glucose homeostasis. Glucose elimination was not significantly different between the Micu2 knockout and wild-type mice (Figure 4H). However, an intravenous glucose tolerance test (IVGTT) revealed a marked reduction in the initial release of insulin (p = 0.0003; Figure 4I): we observed a 74% and 70% reduction in the plasma insulin levels at 1 and 5 min after glucose administration, respectively, in Micu2 knockout mice compared to wild-type mice. Because insulin secretion was impaired while glucose elimination was unaltered in Micu2 deficiency, we performed an insulin tolerance test (ITT). Both strains of mice responded robustly to insulin, with decreased plasma glucose levels (Figure 4J). These experiments thus demonstrated that the initial release of insulin in Micu2 knockout mice was reduced, likely caused by a stimulus-secretion coupling defect in pancreatic β cells. Hence, Micu2 knockout mice exhibited deficient GSIS both in vitro and in vivo.
3.5. MICU2 regulation of Ca2+ in mitochondria, cytosol, and subplasmalemmal space was conserved between cell types
Previous studies in neuronal cells showed that inhibition of ATP generation and dissipation of mitochondrial membrane potential led to reduced [Ca2+]c upon stimulation with glutamate or 50 mM of KCl [46]. Therefore, having shown the role of MICU2 in regulating [Ca2+]mito and [Ca2+]c in insulin-secreting cells, we asked whether this mechanism may also be observed in other cell types. High-throughput gene expression assays previously demonstrated that the human embryonic kidney-derived HEK-293T cell line robustly expresses levels of MICU2 mRNA [47]. It has been shown that this cell line also expresses voltage-dependent Ca2+ channels and exhibits endogenous Ca2+ currents [48,49]. We therefore used this cell line to address the role of MICU2 in cellular Ca2+ homeostasis.
MICU2 was effectively silenced on the mRNA and protein levels in HEK-293T cells (Supplementary Figs. 6A–C). Measurements of [Ca2+]mito with mito-case12 demonstrated that stimulation with 36 mM of KCl increased [Ca2+]mito in HEK293T cells and that this response was attenuated by 46% after silencing of MICU2 (p < 0.0001; Figure 5A–B). In addition, time to maximal [Ca2+]mito peak in MICU2-silenced cells was 126 s compared to 107 s in controls after 36 mM of KCl stimulation (p = 0.0087; Supplementary Fig. 6D). [Ca2+]c measurements using Fluo5F showed that membrane depolarization by 50 mM KCl triggered a [Ca2+]c increase and the maximal [Ca2+]c elevation was reduced by 74% in MICU2-silenced HEK-293T cells compared to control cells (p < 0.0001; Figure 5C–D). For experiments measuring [Ca2+]mem using mem-case12, oligomycin was added to ensure that any effects of MICU2 silencing were independent from the resultant bioenergetic effects of increased [Ca2+]mito. The same was not done in the experiments with INS-1 832/13 cells due to ATP production in the β cell being finely tuned to the extracellular glucose concentration as a consequence of the low-affinity enzymes GLUT2 and glucokinase and downstream dissipative pathways (high leak current) [50]. This means that at low glucose, metabolic flux and ATP production in INS-1 832/13 cells are low compared to HEK293T cells. Therefore, any effect of increased [Ca2+]mito on ATP production would be unlikely to reach the threshold to affect KATP channel activity, meaning using oligomycin was not necessary. Reminiscent of the findings in INS-1 832/13 cells, [Ca2+]mem elevation upon 50 mM of KCl stimulation increased by 35% in MICU2-silenced HEK293T cells compared to the control cells (p = 0.0103; Figure 5E–F). Thus, these data demonstrate a conservation of the regulation of [Ca2+]mito and [Ca2+]c by MICU2 in different cell types.
Figure 5.
MICU2 played a similar role in Ca2+ homeostasis in HEK-293T cells as observed in insulin-secreting cells. Mitochondrial Ca2+ ([Ca2+]mito) determined by mito-case12 in MICU2-silenced HEK-293T cells stimulated with 36 mM of KCl (A and B); (A) shows average traces while (B) shows differences in the maximal mitochondrial Ca2+ response. Cytosolic Ca2+ levels ([Ca2+]c) determined by Fluo5F in MICU2-silenced HEK-293T cells upon stimulation with 50 mM of KCl (C and D); average traces are shown in (C) while (D) shows differences in maximal Fluo5F signals. Subplasmalemmal Ca2+ levels ([Ca2+]mem) in MICU2-silenced HEK-293T cells upon stimulation with 50 mM of KCl (E and F); average traces are shown in (E) while (F) shows differences in maximal mem-case12 signals. Data are from at least three independent experiments 72 h after adding siRNA. Mean ± SEM are given. Comparisons were made with an unpaired two-tailed Student's t-test. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗∗p < 0.0001. See also Fig. S3.
4. Discussion
It is undisputed that an increase in [Ca2+]c triggers insulin secretion as well as secretion of most other hormones, but it has also been recognized that such a rise in itself does not sustain insulin secretion [51,52]. Cytosolic Ca2+ is further transported into the mitochondrial matrix, and an elevation in free matrix Ca2+ has been considered to trigger additional regulatory factors [53]. Prior to the molecular identification of the components of the uniporter holocomplex [19,54,55], indirect approaches were required to investigate the effect of modulating [Ca2+]mito, none of which were entirely satisfactory. Removal of external Ca2+ [56] affects multiple non-mitochondrial processes, not least exocytosis itself. Moreover, attempts to dampen changes in [Ca2+]mito by increasing the matrix Ca2+-buffering capacity by targeting exogenous Ca2+-binding proteins to the matrix [5] or loading cells with Ca2+-chelator 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA) [57] each succeeded in reducing the bioenergetic response to elevated glucose, but concerns remain about toxicity associated with this treatment [50].
The molecular characterization of the uniporter [19,54,55,58] as well as a number of accessory proteins such as MICU1, 2, 3, and EMRE [19,55,59] opened the possibility of genetic manipulation of the uniporter's components [60]. It was shown that silencing of Mcu abrogates the increase in [Ca2+]mito and the cytosolic ATP/ADP ratio in response to glucose in mouse β cells [61]. Another study demonstrated that silencing of MCU in clonal INS-1-E cells resulted in reduced nutrient-induced hyperpolarization of the inner mitochondrial membrane, ATP production, and GSIS [9]. Moreover, knockdown of MICU1 leads to reductions in [Ca2+]mito, cytosolic ATP levels, and GSIS in clonal insulin-secreting cells [8]. However, the role of MICU2 remains more enigmatic. MICU2 is a peripheral membrane protein associated with the mitochondrial inner membrane and facing the intermembrane space. The absence of MICU2 has been associated with reduced Ca2+ uptake in vivo in mouse liver, though silencing in this system also leads to a decrease in the abundance of the pore-forming subunit MCU and the complex [19]. Observations in other cell systems suggested that the accessory protein may have a regulatory role in Ca2+ uptake into mitochondria [13,16]. MICU1 and MICU2 are thought to heterodimerize via disulfide bridges and thus regulate Ca2+ entry into the mitochondrial matrix via the MCU [62]. Indeed, both MICU1 and MICU2 have been shown to play an important role in setting the threshold for mitochondrial Ca2+ uptake in HeLa cells at variable Ca2+ concentrations [16]. Furthermore, loss of MICU1 or MICU2 in permeabilized HEK-293T cells has also been shown to disrupt this critical threshold for mitochondrial Ca2+ uptake [13,14,63,64].
Herein, using MICU2-deficient rodent and human insulin-producing cell lines as well as Micu2 knockout mice, we were able to show that reduced Ca2+ uptake into mitochondria resulted in inhibition of GSIS. This confirmed observations in previous studies [8,9,61] showing that [Ca2+]mito affects mitochondrial metabolism and insulin secretion. However, our experiments could not resolve how Micu2 knockout mice maintained euglycemia despite reduced early insulin secretion. Insulin tolerance, albeit a rough estimate of insulin action in vivo, was unchanged. In fact, we previously reported that ITT is not sensitive enough to reflect changes in insulin sensitivity in the face of reduced early insulin secretion in vivo [65]. This phenotype is also somewhat similar to what was observed in β cell-specific Mcu knockout mice that exhibit impaired insulin secretion 5 min after intraperitoneal glucose injection with improved glucose tolerance despite normal insulin sensitivity [15]. A hyperinsulinemic euglycemic clamp, which is beyond the scope of the present study, would be required to accurately determine insulin sensitivity. Importantly, MCU and MICU1 expression were unaffected by MICU2 silencing in INS-1 832/13 cells, indicating that MICU2 plays a fundamental non-redundant role in regulating [Ca2+]mito in β cells. However, the mechanisms by which MICU2 and [Ca2+]mito might exert control over GSIS were unexpected and add a new dimension to how mitochondrial Ca2+ transport might regulate β cell function and insulin secretion.
The key observation novel in the context of β cells is that mitochondrial Ca2+ uptake exerts a profound stimulatory effect on the net flux of cations across the plasma membrane, either by enhancing entry or depressing efflux. Insulin-secreting cells have a high concentration of subplasma membrane mitochondria, with roughly 20% of the membrane inner surface associated with mitochondria exerting a strong Ca2+-buffering effect [40]. Mitochondria are dynamic organelles and submembrane Ca2+ buffering is reduced upon plasma membrane depolarization because of Ca2+-dependent remodeling of the cortical F-actin network causing translocation of subplasmalemmal mitochondria to the cell interior [40]. In line with these findings, we demonstrated that restricted mitochondrial Ca2+ uptake resulted in more pronounced depolarization-induced increases in Ca2+ in the submembrane compartment. However, under the same conditions, the global cytoplasmic Ca2+ increases were attenuated.
To rationalize a decreased Ca2+ response to plasma membrane depolarization in both the matrix and cytosolic compartments, the simplest assumption is that net Ca2+ influx across the plasma membrane is inhibited. Increased extrusion of Ca2+ is less likely given the observed decrease in the ATP/ADP ratio as a consequence of MICU2 deficiency and that extrusion is an ATP-dependent process. While the endoplasmic reticulum does take up Ca2+ in response to the elevated ATP/ADP ratio at high glucose, the response is too slow to account for a Ca2+ deficit [66]. In any case, the effect of restricted mitochondrial Ca2+ uptake on bulk [Ca2+]c is seen with elevated K+ as well as with high glucose and is thus not directly dependent on bioenergetics. Had this effect only been observed in response to glucose, then the previously postulated positive effect of mitochondrial Ca2+ uptake on mitochondrial dehydrogenases would have been the most plausible explanation [67].
A paradoxical decrease in [Ca2+]c elevation following inhibited transport of mitochondrial Ca2+ was previously observed in cultured neurons [38,46]. Since these studies predated the molecular identification of the MCU, mitochondrial Ca2+ transport was inhibited by mitochondrial depolarization in the presence of the ATP synthase inhibitor oligomycin together with a rotenone or antimycin electron transport inhibitor. The control was oligomycin alone: both control and mitochondrial depolarized cells maintained similar ATP/ADP ratios supported by glycolysis, but the [Ca2+]c transients induced by Ca2+ entry through NMDA-selective glutamate receptors or voltage-dependent Ca2+ channels drastically reduced. In the present study, treatment with oligomycin + antimycin further confirmed a role of mitochondrial Ca2+ uptake in clearing subplasmalemmal Ca2+ in insulin-secreting cells, and the observations were similar to those in neurons [38,46]. Importantly, the net neuronal accumulation of 45Ca2+ is strongly inhibited by mitochondrial depolarization [38,46], confirming the organelle's ability to control net flux across the plasma membrane. Notably, mitochondrial Ca2+ uptake has also been implicated in store-operated Ca2+ entry, with MCU knockdown in RBL mast cells shown to reduce Ca2+ influx through Ca2+ release-activated channels [68].
It was hypothesized that mitochondria are able to deplete a subplasma membrane layer of elevated Ca2+ [38,46]. Voltage-dependent Ca2+ channels become desensitized when local Ca2+ accumulations are not promptly removed [44,69,70]. In addition, it has been shown in chromaffin cells, neurons, and cardiac muscle that mitochondrial sequestration of subplasmalemmal Ca2+ prevents desensitization of Ca2+ channels and thus might uphold hormone secretion [[71], [72], [73]]. Furthermore, mitochondria play an essential role in the maintenance of [Ca2+]c amplitude wave propagation via feedback regulation of plasma membrane channel activity in diverse cellular systems, including rat cortical astrocytes and Xenopus laevis oocytes [74,75]. The response of the subplasmalemmal-targeted Ca2+ probe mem-case12 and line scan imaging in the present study provided support for the existence of such a layer of elevated Ca2+ [38,46] during β cell depolarization and its depletion by mitochondrial Ca2+ uptake. Notably, in the mem-case12 experiments, there was a difference between the effects on [Ca2+]mem between Micu2 silencing and treatment with oligomycin/antimycin, whereby the former caused an increase in the duration of the subplasmalemmal Ca2+ increase while the latter resulted in an increased magnitude of the response. This may be explained by the different mechanisms by which mitochondrial Ca2+ uptake is inhibited. When MICU2 is deficient, uptake at low Ca2+ is likely to increase as the protein plays an important role in suppressing MCU activity under these conditions [11,13,16,17]. Subsequently, when [Ca2+]c is elevated, the mitochondrial buffering capacity decreases. In contrast, oligomycin and antimycin prevent proton transport from the mitochondrial matrix to the mitochondrial intermembrane space, increasing the matrix pH, subsequently collapsing Δψm and inhibiting Ca2+ entry. These two methods affect the Ca2+ electrochemical gradient differently and as a result the Ca2+ uptake kinetics, potentially explaining the differential effects on [Ca2+]mem.
Regarding line scan imaging, one could argue that due to its relatively low Kd, Fluo4 is unsuitable for measuring [Ca2+]mem, which can reach concentrations of 10–100 μM [76]. This means there is a risk of Fluo4 saturation at the submembrane compartment. This did not seem to occur in the control, where there was little difference in the Fluo4 signal between the edge and center of the cell. In MICU2-silenced cells, however, there was a clear difference between the edge and center of the cell. If saturation was indeed occurring, this would imply that the observed increase in [Ca2+]mem was in fact an underestimation. Moreover, the saturation problems were mitigated against the complementary findings using the lower-affinity mem-case12 probe (Kd = 1 μM) [36].
The lack of a [Ca2+]mem effect on MICU2-silenced Endo C-βH1 cells may lie in differences in the composition of voltage-gated Ca2+ channels between the two cell lines. Notably, the L-type Ca2+ channel current, which is largely responsible for Ca2+ entry upon high K+ stimulation due to the rapid inactivation of other Ca2+ channels [77], only accounts for 20–30% of the Ca2+ current in Endo C-βH1 cells [78] compared to at least 60% of the Ca2+ current in INS-1 832/13 cells [79]. This means the [Ca2+]mem levels attained in EndoC-βH1 cells in the line scan experiment may still be able to be cleared by MICU2-deficient mitochondria. Thus, any buildup of Ca2+ in the subplasmalemmal compartment may occur at a slower rate, evading detection in our experiments. This would also explain the smaller effect of MICU2 silencing on depolarization-evoked [Ca2+]c increases in EndoC-βH1.
This study shows that mitochondrial Ca2+ transport has additional consequences for β cells beyond facilitation of the TCA cycle, which may have varying effects on insulin secretion. On the one hand, attenuation of subplasmalemmal Ca2+ elevations could reduce the triggering of exocytosis in pre-docked vesicles responsible for first-phase insulin release. On the other hand, there is evidence that increased Ca2+ in the cytosol is required to transport reserve vesicles to the release site, at least in neuronal systems [80]. Based on the KCl-induced insulin secretion experiments, the attenuation of mitochondrial Ca2+ transport leads to a reduction in insulin secretion. First-phase insulin release occurs in the first 5–10 min [81]. Therefore, while there may be an increase in first-phase insulin secretion, an attenuation of the second phase likely caused by decreased transport of reserve vesicles means that insulin secretion decreases over the 15 min time course.
[Ca2+]c increases may also occur as a consequence of endoplasmic reticulum Ca2+ release. Indeed, there is evidence in β cells of a basal endoplasmic reticulum Ca2+ leak channeled toward the mitochondria, which stimulates mitochondrial bioenergetics, priming the mitochondria for an increase in glucose [82,83]. This process has been implicated in the generation of cytosolic Ca2+ oscillations and GSIS [83]. Although ablating Micu2 would also abrogate this process, it appears to be distinct from the mechanism observed herein. The reduction in [Ca2+]mito and the associated increase in [Ca2+]mem were more or less instantaneous upon exposure to KCl and plasma membrane depolarization as opposed to the endoplasmic reticulum Ca2+ leak, which seems to occur at a constant rate [82,83].
It was previously demonstrated that dynamic changes in the subplasmalemmal mitochondrial population participate in the regulation of local Ca2+ levels and consequently exocytosis in MIN6 cells [40]. A limitation of our study is that, at this point, we have not obtained sufficient data on whether MICU2 deficiency impacts the localization, morphology, or number of mitochondria in the submembrane compartment. Changes in any of these parameters in MICU2-deficient cells would likely also affect subplasmalemmal and cytosolic Ca2+ dynamics.
Our results may help resolve some of the contradictions in the concept of amplification pathways and KATP-independent mechanisms of insulin secretion. The search for coupling factors responsible for KATP-independent insulin secretion began after the discovery that insulin secretion could be provoked by increasing glucose even when the KATP channel was kept open by diazoxide and the plasma membrane was depolarized by high concentrations of extracellular KCl, thereby opening voltage-dependent Ca2+ channels [51]. Metabolic coupling factors were postulated to account for the insulinotropic effects of glucose under KATP-independent conditions and explain why insulin secretion could not be sustained by an elevation of [Ca2+]c alone [52]. However, at least the latter circumstance can be accounted for by the consequences of mitochondrial Ca2+ uptake that we observed herein: if Ca2+ is not removed from the subplasmalemmal space by mitochondria, voltage-dependent Ca2+ channels will be desensitized. A number of metabolites and metabolic pathways have been convincingly implicated in amplification of GSIS [84]. However, most share a similar feature: it has not been resolved how these factors stimulate exocytosis. Moreover, a number of concerns have also been raised about the bioenergetics of the formation of metabolic coupling factors [50].
5. Conclusions
In summary, we uncovered a crucial role of mitochondria in maintaining β cell excitability involving sequestering of Ca2+ into mitochondria located at the vicinity of the voltage-dependent Ca2+ channels in the plasma membrane that coupled with the well-established bioenergetic effects accounts for the regulatory effect of mitochondrial Ca2+ uptake on insulin secretion. The recent molecular characterization of the mitochondrial Ca2+ uniporter holocomplex holds promise for further elucidating the complexities of β cell stimulus-secretion coupling in which the mitochondria are at center stage.
Author contributions
N.V., A.H., D.G.N., and H.M. designed the study. N.V., A.H., A.B., A.M.B., H.B., Y.S., K.K., P.S., E.C., and M.F. performed the experiments. N.V., A.H., A.M.B., and M.F. analyzed the data. V.M. provided the Micu2 knockout mice. A.T., V.M., D.G.N., and H.M. provided critical insights to the project. N.V., A.H., A.T., V.M., D.G.N., and H.M. wrote the manuscript.
Study approval
All of the experimental animal protocols were approved by the local animal ethical committee of Lund/Malmö and complied with the guidelines of the Federation of European Laboratory Animal Science Association (FELASA).
Acknowledgments
Laila Jacobsson is acknowledged for genotyping the mice. This study was supported by grants from the Swedish Research Council (14196-12-5 and 2017–00956), EFSD/MSD, Novo Nordisk Foundation, Swedish Diabetes Foundation, Swedish Strategic Research Foundation, Crafoord, Hjelt, Lars Hierta's Minne, Fredrik och Ingrid Thuring's, O.E. and Edla Johansson's Vetenskapliga, Åke Wibergs, Director, Albert Påhlssons, and Magnus Bergvalls Foundations, and the Royal Physiographic Society. This study was also supported by equipment grants from the KAW Wallenberg Foundation (2009–0243) and funding from the European Union's Horizon 2020 Research and Innovation Program under grant agreement No. 667191. None of these funding sources were responsible for the study design, collection, analysis, and interpretation of the data or writing the report and decision to submit the article for publication.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2021.101239.
Conflict of interest
None declared.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Kennedy E.D., Wollheim C.B. Role of mitochondrial calcium in metabolism-secretion coupling in nutrient-stimulated insulin release. Diabetes & Metabolism. 1998;24(1):15–24. [PubMed] [Google Scholar]
- 2.Lee B., Miles P.D., Vargas L., Luan P., Glasco S., Kushnareva Y. Inhibition of mitochondrial Na+-Ca2+ exchanger increases mitochondrial metabolism and potentiates glucose-stimulated insulin secretion in rat pancreatic islets. Diabetes. 2003;52(4):965–973. doi: 10.2337/diabetes.52.4.965. [DOI] [PubMed] [Google Scholar]
- 3.Wiederkehr A., Wollheim C.B. Minireview: implication of mitochondria in insulin secretion and action. Endocrinology. 2006;147(6):2643–2649. doi: 10.1210/en.2006-0057. [DOI] [PubMed] [Google Scholar]
- 4.Griffiths E.J., Rutter G.A. Mitochondrial calcium as a key regulator of mitochondrial ATP production in mammalian cells. Biochimica et Biophysica Acta. 2009;1787(11):1324–1333. doi: 10.1016/j.bbabio.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 5.Wiederkehr A., Szanda G., Akhmedov D., Mataki C., Heizmann C.W., Schoonjans K. Mitochondrial matrix calcium is an activating signal for hormone secretion. Cell Metabolism. 2011;13(5):601–611. doi: 10.1016/j.cmet.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 6.Gilon P., Chae H.Y., Rutter G.A., Ravier M.A. Calcium signaling in pancreatic beta-cells in health and in Type 2 diabetes. Cell Calcium. 2014;56(5):340–361. doi: 10.1016/j.ceca.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Tarasov A.I., Semplici F., Li D., Rizzuto R., Ravier M.A., Gilon P. Frequency-dependent mitochondrial Ca(2+) accumulation regulates ATP synthesis in pancreatic beta cells. Pflügers Archiv. 2013;465(4):543–554. doi: 10.1007/s00424-012-1177-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alam M.R., Groschner L.N., Parichatikanond W., Kuo L., Bondarenko A.I., Rost R. Mitochondrial Ca2+ uptake 1 (MICU1) and mitochondrial ca2+ uniporter (MCU) contribute to metabolism-secretion coupling in clonal pancreatic beta-cells. Journal of Biological Chemistry. 2012;287(41):34445–34454. doi: 10.1074/jbc.M112.392084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quan X., Nguyen T.T., Choi S.K., Xu S., Das R., Cha S.K. Essential role of mitochondrial Ca2+ uniporter in the generation of mitochondrial pH gradient and metabolism-secretion coupling in insulin-releasing cells. Journal of Biological Chemistry. 2015;290(7):4086–4096. doi: 10.1074/jbc.M114.632547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cui C., Yang J., Fu L., Wang M., Wang X. Progress in understanding mitochondrial calcium uniporter complex-mediated calcium signalling: a potential target for cancer treatment. British Journal of Pharmacology. 2019;176(9):1190–1205. doi: 10.1111/bph.14632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamer K.J., Mootha V.K. The molecular era of the mitochondrial calcium uniporter. Nature Reviews Molecular Cell Biology. 2015;16(9):545–553. doi: 10.1038/nrm4039. [DOI] [PubMed] [Google Scholar]
- 12.Ahuja M., Muallem S. The gatekeepers of mitochondrial calcium influx: MICU1 and MICU2. EMBO Reports. 2014;15(3):205–206. doi: 10.1002/embr.201438446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamer K.J., Mootha V.K. MICU1 and MICU2 play nonredundant roles in the regulation of the mitochondrial calcium uniporter. EMBO Reports. 2014;15(3):299–307. doi: 10.1002/embr.201337946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamer K.J., Grabarek Z., Mootha V.K. High-affinity cooperative Ca(2+) binding by MICU1-MICU2 serves as an on-off switch for the uniporter. EMBO Reports. 2017;18(8):1397–1411. doi: 10.15252/embr.201643748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Georgiadou E., Haythorne E., Dickerson M.T., Lopez-Noriega L., Pullen T.J., da Silva Xavier G. The pore-forming subunit MCU of the mitochondrial Ca(2+) uniporter is required for normal glucose-stimulated insulin secretion in vitro and in vivo in mice. Diabetologia. 2020;63(7):1368–1381. doi: 10.1007/s00125-020-05148-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patron M., Checchetto V., Raffaello A., Teardo E., Vecellio Reane D., Mantoan M. MICU1 and MICU2 finely tune the mitochondrial Ca2+ uniporter by exerting opposite effects on MCU activity. Molecular Cell. 2014;53(5):726–737. doi: 10.1016/j.molcel.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matesanz-Isabel J., Arias-del-Val J., Alvarez-Illera P., Fonteriz R.I., Montero M., Alvarez J. Functional roles of MICU1 and MICU2 in mitochondrial Ca(2+) uptake. Biochimica et Biophysica Acta. 2016;1858(6):1110–1117. doi: 10.1016/j.bbamem.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 18.Payne R., Hoff H., Roskowski A., Foskett J.K. MICU2 restricts spatial crosstalk between InsP3R and MCU channels by regulating threshold and gain of MICU1-mediated inhibition and activation of MCU. Cell Reports. 2017;21(11):3141–3154. doi: 10.1016/j.celrep.2017.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plovanich M., Bogorad R.L., Sancak Y., Kamer K.J., Strittmatter L., Li A.A. MICU2, a paralog of MICU1, resides within the mitochondrial uniporter complex to regulate calcium handling. PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0055785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hohmeier H.E., Mulder H., Chen G., Henkel-Rieger R., Prentki M., Newgard C.B. Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes. 2000;49(3):424–430. doi: 10.2337/diabetes.49.3.424. [DOI] [PubMed] [Google Scholar]
- 21.Ravassard P., Hazhouz Y., Pechberty S., Bricout-Neveu E., Armanet M., Czernichow P. A genetically engineered human pancreatic beta cell line exhibiting glucose-inducible insulin secretion. Journal of Clinical Investigation. 2011;121(9):3589–3597. doi: 10.1172/JCI58447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pear W.S., Nolan G.P., Scott M.L., Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proceedings of the National Academy of Sciences of the U S A. 1993;90(18):8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bick A.G., Wakimoto H., Kamer K.J., Sancak Y., Goldberger O., Axelsson A. Cardiovascular homeostasis dependence on MICU2, a regulatory subunit of the mitochondrial calcium uniporter. Proceedings of the National Academy of Sciences of the U S A. 2017;114(43):E9096–E9104. doi: 10.1073/pnas.1711303114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scharfmann R., Pechberty S., Hazhouz Y., von Bulow M., Bricout-Neveu E., Grenier-Godard M. Development of a conditionally immortalized human pancreatic beta cell line. Journal of Clinical Investigation. 2014;124(5):2087–2098. doi: 10.1172/JCI72674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tantama M., Martinez-Francois J.R., Mongeon R., Yellen G. Imaging energy status in live cells with a fluorescent biosensor of the intracellular ATP-to-ADP ratio. Nature Communications. 2013;4:2550. [Google Scholar]
- 26.Berg J., Hung Y.P., Yellen G. A genetically encoded fluorescent reporter of ATP:ADP ratio. Nature Methods. 2009;6(2):161–166. doi: 10.1038/nmeth.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J., Shuai H.Y., Gylfe E., Tengholm A. Oscillations of sub-membrane ATP in glucose-stimulated beta cells depend on negative feedback from Ca(2+) Diabetologia. 2013;56(7):1577–1586. doi: 10.1007/s00125-013-2894-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nicholls D.G., Ward M.W. Mitochondrial membrane potential and neuronal glutamate excitotoxicity: mortality and millivolts. Trends in Neurosciences. 2000;23(4):166–174. doi: 10.1016/s0166-2236(99)01534-9. [DOI] [PubMed] [Google Scholar]
- 29.Fex M., Nitert M.D., Wierup N., Sundler F., Ling C., Mulder H. Enhanced mitochondrial metabolism may account for the adaptation to insulin resistance in islets from C57BL/6J mice fed a high-fat diet. Diabetologia. 2007;50(1):74–83. doi: 10.1007/s00125-006-0464-4. [DOI] [PubMed] [Google Scholar]
- 30.Andersson L.E., Valtat B., Bagge A., Sharoyko V.V., Nicholls D.G., Ravassard P. Characterization of stimulus-secretion coupling in the human pancreatic EndoC-betaH1 beta cell line. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0120879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhdanov A.V., Waters A.H., Golubeva A.V., Dmitriev R.I., Papkovsky D.B. Availability of the key metabolic substrates dictates the respiratory response of cancer cells to the mitochondrial uncoupling. Biochimica et Biophysica Acta. 2014;1837(1):51–62. doi: 10.1016/j.bbabio.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 32.Logan C.V., Szabadkai G., Sharpe J.A., Parry D.A., Torelli S., Childs A.M. Loss-of-function mutations in MICU1 cause a brain and muscle disorder linked to primary alterations in mitochondrial calcium signaling. Nature Genetics. 2014;46(2):188–193. doi: 10.1038/ng.2851. [DOI] [PubMed] [Google Scholar]
- 33.Ward M.W., Rego A.C., Frenguelli B.G., Nicholls D.G. Mitochondrial membrane potential and glutamate excitotoxicity in cultured cerebellar granule cells. Journal of Neuroscience. 2000;20(19):7208–7219. doi: 10.1523/JNEUROSCI.20-19-07208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicholls D.G. Calcium transport and porton electrochemical potential gradient in mitochondria from Guinea-pig cerebral cortex and rat heart. Biochemical Journal. 1978;170(3):511–522. doi: 10.1042/bj1700511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagai T., Sawano A., Park E.S., Miyawaki A. Circularly permuted green fluorescent proteins engineered to sense Ca2+ Proceedings of the National Academy of Sciences of the U S A. 2001;98(6):3197–3202. doi: 10.1073/pnas.051636098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Souslova E.A., Belousov V.V., Lock J.G., Stromblad S., Kasparov S., Bolshakov A.P. Single fluorescent protein-based Ca2+ sensors with increased dynamic range. BMC Biotechnology. 2007;7:37. doi: 10.1186/1472-6750-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skene J.H., Virag I. Posttranslational membrane attachment and dynamic fatty acylation of a neuronal growth cone protein, GAP-43. The Journal of Cell Biology. 1989;108(2):613–624. doi: 10.1083/jcb.108.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Budd S.L., Nicholls D.G. A reevaluation of the role of mitochondria in neuronal Ca2+ homeostasis. Journal of Neurochemistry. 1996;66(1):403–411. doi: 10.1046/j.1471-4159.1996.66010403.x. [DOI] [PubMed] [Google Scholar]
- 39.Malmgren S., Nicholls D.G., Taneera J., Bacos K., Koeck T., Tamaddon A. Tight coupling between glucose and mitochondrial metabolism in clonal beta-cells is required for robust insulin secretion. Journal of Biological Chemistry. 2009;284(47):32395–32404. doi: 10.1074/jbc.M109.026708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Griesche N., Sanchez G., Hermans C., Idevall-Hagren O. Cortical mitochondria regulate insulin secretion by local Ca(2+) buffering in rodent beta cells. Journal of Cell Science. 2019;132(9) doi: 10.1242/jcs.228544. [DOI] [PubMed] [Google Scholar]
- 41.Navedo M.F., Amberg G.C., Votaw V.S., Santana L.F. Constitutively active L-type Ca2+ channels. Proceedings of the National Academy of Sciences of the U S A. 2005;102(31):11112–11117. doi: 10.1073/pnas.0500360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DeCaen P.G., Liu X., Abiria S., Clapham D.E. Atypical calcium regulation of the PKD2-L1 polycystin ion channel. Elife. 2016;5 doi: 10.7554/eLife.13413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Groten C.J., Rebane J.T., Hodgson H.M., Chauhan A.K., Blohm G., Magoski N.S. Ca2+ removal by the plasma membrane Ca2+-ATPase influences the contribution of mitochondria to activity-dependent Ca2+ dynamics in Aplysia neuroendocrine cells. Journal of Neurophysiology. 2016;115(5):2615–2634. doi: 10.1152/jn.00494.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Demaurex N., Poburko D., Frieden M. Regulation of plasma membrane calcium fluxes by mitochondria. Biochimica et Biophysica Acta. 2009;1787(11):1383–1394. doi: 10.1016/j.bbabio.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 45.Li J., Yu Q., Ahooghalandari P., Gribble F.M., Reimann F., Tengholm A. Submembrane ATP and Ca2+ kinetics in alpha-cells: unexpected signaling for glucagon secretion. The FASEB Journal. 2015;29(8):3379–3388. doi: 10.1096/fj.14-265918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Castilho R.F., Hansson O., Ward M.W., Budd S.L., Nicholls D.G. Mitochondrial control of acute glutamate excitotoxicity in cultured cerebellar granule cells. Journal of Neuroscience. 1998;18(24):10277–10286. doi: 10.1523/JNEUROSCI.18-24-10277.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zolg D.P., Wilhelm M., Schnatbaum K., Zerweck J., Knaute T., Delanghe B. Building ProteomeTools based on a complete synthetic human proteome. Nature Methods. 2017;14(3):259–262. doi: 10.1038/nmeth.4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berjukow S., Doring F., Froschmayr M., Grabner M., Glossmann H., Hering S. Endogenous calcium channels in human embryonic kidney (HEK293) cells. British Journal of Pharmacology. 1996;118(3):748–754. doi: 10.1111/j.1476-5381.1996.tb15463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang V., Davis D.A., Haque M., Huang L.E., Yarchoan R. Differential gene up-regulation by hypoxia-inducible factor-1 alpha and hypoxia-inducible factor-2 alpha in HEK293T cells. Cancer Research. 2005;65(8):3299–3306. doi: 10.1158/0008-5472.CAN-04-4130. [DOI] [PubMed] [Google Scholar]
- 50.Nicholls D.G. The pancreatic beta-cell: a bioenergetic perspective. Physiological Reviews. 2016;96(4):1385–1447. doi: 10.1152/physrev.00009.2016. [DOI] [PubMed] [Google Scholar]
- 51.Gembal M., Detimary P., Gilon P., Gao Z.Y., Henquin J.C. Mechanisms by which glucose can control insulin release independently from its action on adenosine triphosphate-sensitive K+ channels in mouse B-cells. Journal of Clinical Investigation. 1993;91(3):871–880. doi: 10.1172/JCI116308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jonas J.C., Li G.D., Palmer M., Weller U., Wollheim C.B. Dynamics of Ca2+ and guanosine 5'-[gamma-Thio]Triphosphate action on insulin-secretion from alpha-toxin-permeabilized hit-T15 cells. Biochemical Journal. 1994;301:523–529. doi: 10.1042/bj3010523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rutter G.A., Theler J.M., Murgia M., Wollheim C.B., Pozzan T., Rizzuto R. Stimulated Ca2+ influx raises mitochondrial free Ca2+ to supramicromolar levels in a pancreatic beta-cell line. Possible role in glucose and agonist-induced insulin secretion. Journal of Biological Chemistry. 1993;268(30):22385–22390. [PubMed] [Google Scholar]
- 54.Baughman J.M., Perocchi F., Girgis H.S., Plovanich M., Belcher-Timme C.A., Sancak Y. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476(7360) doi: 10.1038/nature10234. 341-U111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perocchi F., Gohil V.M., Girgis H.S., Bao X.R., McCombs J.E., Palmer A.E. MICU1 encodes a mitochondrial EF hand protein required for Ca(2+) uptake. Nature. 2010;467(7313):291–296. doi: 10.1038/nature09358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Balke C.W., Egan T.M., Wier W.G. Processes that remove calcium from the cytoplasm during excitation-contraction coupling in intact rat heart cells. The Journal of Physiology. 1994;474(3):447–462. doi: 10.1113/jphysiol.1994.sp020036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tanaka T., Nagashima K., Inagaki N., Kioka H., Takashima S., Fukuoka H. Glucose-stimulated single pancreatic islets sustain increased cytosolic ATP levels during initial Ca2+ influx and subsequent Ca2+ oscillations. Journal of Biological Chemistry. 2014;289(4):2205–2216. doi: 10.1074/jbc.M113.499111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.De Stefani D., Raffaello A., Teardo E., Szabo I., Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476(7360):336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sancak Y., Markhard A.L., Kitami T., Kovacs-Bogdan E., Kamer K.J., Udeshi N.D. EMRE is an essential component of the mitochondrial calcium uniporter complex. Science. 2013;342(6164):1379–1382. doi: 10.1126/science.1242993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jhun B.S., Mishra J., Monaco S., Fu D., Jiang W., Sheu S.S. The mitochondrial Ca2+ uniporter: regulation by auxiliary subunits and signal transduction pathways. American Journal of Physiology - Cell Physiology. 2016;311(1):C67–C80. doi: 10.1152/ajpcell.00319.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tarasov A.I., Semplici F., Ravier M.A., Bellomo E.A., Pullen T.J., Gilon P. The mitochondrial Ca2+ uniporter MCU is essential for glucose-induced ATP increases in pancreatic beta-cells. PLoS One. 2012;7(7) doi: 10.1371/journal.pone.0039722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsai C.W., Wu Y., Pao P.C., Phillips C.B., Williams C., Miller C. Proteolytic control of the mitochondrial calcium uniporter complex. Proceedings of the National Academy of Sciences of the U S A. 2017;114(17):4388–4393. doi: 10.1073/pnas.1702938114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kamer K.J., Jiang W., Kaushik V.K., Mootha V.K., Grabarek Z. Crystal structure of MICU2 and comparison with MICU1 reveal insights into the uniporter gating mechanism. Proceedings of the National Academy of Sciences of the U S A. 2019;116(9):3546–3555. doi: 10.1073/pnas.1817759116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xing Y., Wang M., Wang J., Nie Z., Wu G., Yang X. Dimerization of MICU proteins controls Ca(2+) influx through the mitochondrial Ca(2+) uniporter. Cell Reports. 2019;26(5):1203–1212. doi: 10.1016/j.celrep.2019.01.022. e1204. [DOI] [PubMed] [Google Scholar]
- 65.Fex M., Haemmerle G., Wierup N., Dekker-Nitert M., Rehn M., Ristow M. A beta cell-specific knockout of hormone-sensitive lipase in mice results in hyperglycaemia and disruption of exocytosis. Diabetologia. 2009;52(2):271–280. doi: 10.1007/s00125-008-1191-9. [DOI] [PubMed] [Google Scholar]
- 66.Ravier M.A., Daro D., Roma L.P., Jonas J.C., Cheng-Xue R., Schuit F.C. Mechanisms of control of the free Ca2+ concentration in the endoplasmic reticulum of mouse pancreatic beta-cells: interplay with cell metabolism and [Ca2+]c and role of SERCA2b and SERCA3. Diabetes. 2011;60(10):2533–2545. doi: 10.2337/db10-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wiederkehr A., Wollheim C.B. Impact of mitochondrial calcium on the coupling of metabolism to insulin secretion in the pancreatic beta-cell. Cell Calcium. 2008;44(1):64–76. doi: 10.1016/j.ceca.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 68.Samanta K., Bakowski D., Amin N., Parekh A.B. The whole-cell Ca2+ release-activated Ca2+ current, ICRAC , is regulated by the mitochondrial Ca2+ uniporter channel and is independent of extracellular and cytosolic Na. The Journal of Physiology. 2020;598(9):1753–1773. doi: 10.1113/JP276551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Taylor J.T., Huang L., Keyser B.M., Zhuang H., Clarkson C.W., Li M. Role of high-voltage-activated calcium channels in glucose-regulated beta-cell calcium homeostasis and insulin release. American Journal of Physiology. Endocrinology and Metabolism. 2005;289(5):E900–E908. doi: 10.1152/ajpendo.00101.2005. [DOI] [PubMed] [Google Scholar]
- 70.Schiff M.L., Siderovski D.P., Jordan J.D., Brothers G., Snow B., De Vries L. Tyrosine-kinase-dependent recruitment of RGS12 to the N-type calcium channel. Nature. 2000;408(6813):723–727. doi: 10.1038/35047093. [DOI] [PubMed] [Google Scholar]
- 71.Montero M., Alonso M.T., Carnicero E., Cuchillo-Ibanez I., Albillos A., Garcia A.G. Chromaffin-cell stimulation triggers fast millimolar mitochondrial Ca2+ transients that modulate secretion. Nature Cell Biology. 2000;2(2):57–61. doi: 10.1038/35000001. [DOI] [PubMed] [Google Scholar]
- 72.David G., Barrett E.F. Mitochondrial Ca2+ uptake prevents desynchronization of quantal release and minimizes depletion during repetitive stimulation of mouse motor nerve terminals. The Journal of Physiology. 2003;548(Pt 2):425–438. doi: 10.1113/jphysiol.2002.035196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sanchez J.A., Garcia M.C., Sharma V.K., Young K.C., Matlib M.A., Sheu S.S. Mitochondria regulate inactivation of L-type Ca2+ channels in rat heart. The Journal of Physiology. 2001;536(Pt 2):387–396. doi: 10.1111/j.1469-7793.2001.0387c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Boitier E., Rea R., Duchen M.R. Mitochondria exert a negative feedback on the propagation of intracellular Ca2+ waves in rat cortical astrocytes. The Journal of Cell Biology. 1999;145(4):795–808. doi: 10.1083/jcb.145.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jouaville L.S., Ichas F., Holmuhamedov E.L., Camacho P., Lechleiter J.D. Synchronization of calcium waves by mitochondrial substrates in Xenopus laevis oocytes. Nature. 1995;377(6548):438–441. doi: 10.1038/377438a0. [DOI] [PubMed] [Google Scholar]
- 76.Parekh A.B. Ca2+ microdomains near plasma membrane Ca2+ channels: impact on cell function. The Journal of Physiology. 2008;586(13):3043–3054. doi: 10.1113/jphysiol.2008.153460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gerencser A.A., Mulder H., Nicholls D.G. Calcium modulation of exocytosis-linked plasma membrane potential oscillations in INS-1 832/13 cells. Biochemical Journal. 2015;471(1):111–122. doi: 10.1042/BJ20150616. [DOI] [PubMed] [Google Scholar]
- 78.Hastoy B., Godazgar M., Clark A., Nylander V., Spiliotis I., van de Bunt M. Electrophysiological properties of human beta-cell lines EndoC-betaH1 and -betaH2 conform with human beta-cells. Scientific Reports. 2018;8(1):16994. doi: 10.1038/s41598-018-34743-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nitert M.D., Nagorny C.L., Wendt A., Eliasson L., Mulder H. CaV1.2 rather than CaV1.3 is coupled to glucose-stimulated insulin secretion in INS-1 832/13 cells. Journal of Molecular Endocrinology. 2008;41(1):1–11. doi: 10.1677/JME-07-0133. [DOI] [PubMed] [Google Scholar]
- 80.Koenig J.H., Yamaoka K., Ikeda K. Calcium-induced translocation of synaptic vesicles to the active site. Journal of Neuroscience. 1993;13(6):2313–2322. doi: 10.1523/JNEUROSCI.13-06-02313.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rorsman P., Renstrom E. Insulin granule dynamics in pancreatic beta cells. Diabetologia. 2003;46(8):1029–1045. doi: 10.1007/s00125-003-1153-1. [DOI] [PubMed] [Google Scholar]
- 82.Klec C., Madreiter-Sokolowski C.T., Stryeck S., Sachdev V., Duta-Mare M., Gottschalk B. Glycogen synthase kinase 3 beta controls presenilin-1-mediated endoplasmic reticulum Ca(2)(+) leak directed to mitochondria in pancreatic islets and beta-cells. Cellular Physiology and Biochemistry. 2019;52(1):57–75. doi: 10.33594/000000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Klec C., Madreiter-Sokolowski C.T., Ziomek G., Stryeck S., Sachdev V., Duta-Mare M. Presenilin-1 established ER-Ca(2+) leak: a follow up on its importance for the initial insulin secretion in pancreatic islets and beta-cells upon elevated glucose. Cellular Physiology and Biochemistry. 2019;53(3):573–586. doi: 10.33594/000000158. [DOI] [PubMed] [Google Scholar]
- 84.Gooding J.R., Jensen M.V., Newgard C.B. Metabolomics applied to the pancreatic islet. Archives of Biochemistry and Biophysics. 2016;589:120–130. doi: 10.1016/j.abb.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.