Abstract
Backgroud
Tissue engineering using cells, scaffolds, and bioactive molecules can promote the repair and regeneration of injured tissues. Copper is an essential element for the human body that is involved in many physiological activities and in recent years, copper has been used increasingly in tissue engineering.
Methods
The current advances of copper-based biomaterial for bone and cartilage tissue engineering were searched on PubMed and Web of Science.
Results
Various forms of copper-based biomaterials, including pure copper, copper ions, copper nanoparticles, copper oxides, and copper alloy are introduced. The incorporation of copper into base materials provides unique properties, resulting in tuneable porosity, mechanical strength, degradation, and crosslinking of scaffolds. Copper also shows promising biological performance in cell migration, cell adhesion, osteogenesis, chondrogenesis, angiogenesis, and antibacterial activities. In vivo applications of copper for bone and cartilage tissue engineering are discussed.
Conclusion
This review focuses on copper’s physiochemical and biological effects, and its applications in bone and cartilage tissue engineering. The potential limitations and future perspectives are also discussed.
Translational potential of this article
This review introduces the recent advances in copper-based biomaterial for bone and cartilage tissue engineering. This revie could guide researchers to apply copper in biomaterials, improving the generation of bone and cartilages, decrease the possibility of infection and shorten the recovery time so as to decrease medical costs.
Keywords: Copper, Tissue engineering, Bone, Cartilage, Biomaterials
1. Introduction
Bone tissue comprises hard connective tissue that constitutes a major part of the musculoskeletal system. The bone extracellular matrix (ECM) contains both organic and inorganic components. The organic matter mainly includes matrix proteins, proteoglycans, and various types of collagen fibres, and the inorganic matter mainly comprises hydroxyapatite. Cells within bones include bone progenitor cells, osteoblasts, osteoclasts, and osteocytes [1]. Bone defects caused by trauma, tumours, infection, or bone diseases are the most common types of injury seen in the clinic. Currently, more than 1 million people are suffering from bone defects in the United States and with the aging population, this number is expected to double in the near future [2]. Severe bone defects usually lead to patient disability, which affects their living and working abilities, and causes a severe burden on society and the economy.
Bone defects are divided into small bone defects and large bone defects (i.e (critical-size defects, with a range exceeding 1.5 times the diameter of the long bones). Small bone defects without infection can heal spontaneously via debridement, while large bone defects need to be treated surgically. Currently, the main treatment methods for large bone defects are autologous, allogeneic, or bone graft substitutes [3]. However, the disadvantages of complex treatment steps and high graft-rejection rates, mean that practical applications remain limited.
Articular cartilage is a kind of supportive connective tissue that covers the epiphyseal surface of the articulating bones and acts to reduce friction between adjacent bones. The main components of cartilage ECM are water and mucin, which is composed of polysaccharides and proteins. Polysaccharides include acid glycosaminoglycan (GAG), chondroitin sulphate A, chondroitin sulphate C, and keratan sulphate. The fibres comprise collagen fibrils, mainly type II collagen [4]. GAG and type II collagen help to maintain cartilage elasticity, resist abrasion, and reduce friction of joints. Cartilage does not contain blood vessels and lymphatic vessels, which limits cartilage's intrinsic repair capacity. Articular cartilage is one of the most frequently damaged tissues from aging and joint injury. In a research reviewing over 30,000 arthroscopic procedures, about 60% of patients were verified to have cartilage defects, with lesion depths involving 50% or more of the cartilage surface [5]. According to the depth of the defects, articular cartilage defects are divided into partial-thickness defects, full-thickness defects, and osteochondral defects. Among them, partial-thickness and full-thickness defects are limited to the cartilage layer.
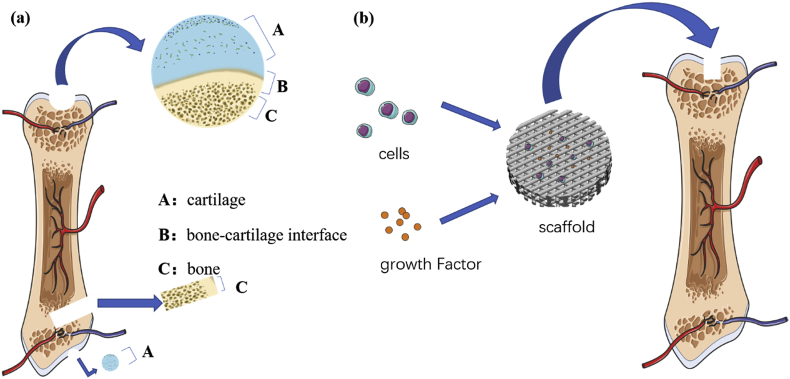
Currently available cartilage repair methods include subchondral bone drilling, cartilage transplantation, chondrocyte transplantation, and mesenchymal cell transplantation [6]. However, despite of the temporary relief from the symptoms of pain and swelling associated with cartilage defects, all the above methods might contribute to the formation of fibrous repair tissue or fibrocartilage, which lacks the full load-bearing properties and durability of healthy articular cartilage [7]. Moreover, if the injuries involve both bone and cartilage (i.e., osteochondral defects), the treatment plan needs to consider both bone and cartilage repair (Fig. 1a). However, the differences between the physicochemical and biological properties of cartilage and subchondral bone make it very difficult to treat them simultaneously [8].
Fig. 1.
(a) Schematic diagram of cartilage defects and bone defects. (b) Schematic diagram of tissue engineering strategies.
In recent years, tissue engineering, as an alternative approach to repair defects, has received increased attention. Tissue engineering involves the use of cells, scaffolds, and growth factors to guide bone and cartilage formation. Scaffolds made of different biomaterials, as a base substance to load cells and growth factors, are implanted into bone or cartilage defects to stimulate the regeneration of bones and cartilage (Fig. 1b). Various materials have been used in bone and cartilage tissue engineering, such as polymers, bioglasses, bioceramics, hydrogels, metals, and alloys [9]. With the development of metal-based biomaterials and alloys, many studies have explored the unique properties of metals for bone and cartilage tissue engineering.
The healthy growth of the human body relies on various metal elements that play vital roles in skeleton health, such as calcium, phosphorus, iron, magnesium, and copper. Table 1 shows the metal elements and their effects on the human body.
Table 1.
Metal elements and their effects on human body [127].
| Element | Symbol | Atomic percent (%) | Properties |
|---|---|---|---|
| Major Elements | |||
| Sodium | Na | 0.037 | Regulation of osmotic pressure, “sodium pump” action, acid-base balance, and transmission of neural information |
| Potassium | K | 0.033 | Prosthetic groups that stabilize internal cell structure and activate certain internal enzymes |
| Magnesium | Mg | 0.007 | Internal structure stabilizers and cofactors of intracellular enzymes, assisting in DNA replication and protein biosynthesis, maintaining bone mechanical strength |
| Calcium | Ca | 0.22 | One of the main components of bones, teeth, and cell walls; maintaining the permeability of blood capillary and cytomembrane and the excitability of neuromuscular |
| Trace Elements | |||
| Iron | Fe | 6.7 × 10−4 | Hemoglobin, a component of myoglobin, aiding mitochondrial electron to transfer and oxidative phosphorylation |
| Copper | Cu | 1.04 × 10−5 | An important cofactor for cytochrome oxidases, an important cofactor for the cross-linking effect of elastic fibers and collagen in connective tissue, having an antibacterial function |
| Zinc | Zn | 3.1 × 10−4 | The main component of zincase, aiding in forming collagen and healing wounds |
| Manganese | Mn | 1.5 × 10−6 | Assisting in the synthesis of important factors such as mucopolysaccharide, lipopolysaccharide, glycoprotein, hyaluronic acid, chondroitin sulfate, and maintains the integrity of connective tissue structure and function |
| Cobalt | Co | 3.0 × 10−7 | One of the essential components of vitamin B12, stimulating the production of RBC, promoting the release of bradykinin to expand blood vessels |
| Molybdenum | Mo | 4.5 × 10−8 | Participating in the mutual reaction between sulfur, iron and copper, is the main component of molybdenum-containing enzymes |
| Chromium | Cr | 8.9 × 10−8 | Maintaining normal glucose tolerance and blood lipid metabolism |
| Tin | Sn | 6.0 × 10−7 | Promoting body growth and development |
| Vanadium | V | 1.2 × 10−8 | Large doses of vanadium can inhibit ATP hydrolase, thereby affecting the metabolism of bone and sugar |
| Nickel | Ni | 1.5 × 10−6 | Composition of Cu2 + binding site and albumin amino-terminal binding site |
Copper (Cu) is the third most abundant essential trace element in the body of animals and humans, and it plays an important role in maintaining bone volume and increasing the rate of wound healing [10]. Furthermore, the copper affects many physiological processes, including enzymatic reactions, nucleic acid synthesis, antioxidant defence, and immune function [11]. In addition, copper is a strong antioxidant that can eliminate free radicals and prevent cell damage, and has anti-cancer effects [12]. Copper insufficiency is associated with certain diseases, such as anaemia, neutropoenia (copper deficiency or hypocupraemia) and abnormalities of bone health [13]. On the other hand, excessive copper is associated with adverse health effects, including damaged lungs, renal, and liver function, and cellular toxicity via oxidative damage [14]. In conclusion, copper is very important for the normal function of the human body.
In the field of tissue engineering, copper has been proven to have biological properties of stimulating endothelial cell proliferation during wound healing by upregulating vascular endothelial growth factor (VEGF) gene expression [15], promoting mesenchymal stem cells (MSCs) to differentiate into the osteogenic lineage, and avoiding infections via its antibacterial property [16]. Moreover, copper has certain physicochemical properties, such as improving porosity, enhancing mechanical strength, and improving crosslinking. Copper is therefore a promising metal that could be applied to endow functional properties for bone and cartilage repair by directing cell behaviours as well as modifying the physicochemical properties of biomaterials.
Hence, the aim of this review is to illustrate the recent advances of copper-based biomaterials for bone and cartilage tissue engineering. We firstly introduce the different forms of copper materials and then elaborate the physicochemical and biological properties of copper. Finally, we introduce the applications of copper-based materials in bone and cartilage tissue engineering.
2. Different forms of copper-based biomaterials
In nature, copper mostly exists in the form of compounds; however, natural copper is of low purity [17]. To better exert its biological roles, it is mainly used in the form of pure copper, copper ions, copper nanoparticles, copper oxide, copper alloys, and other copper compounds for tissue engineering applications (Fig. 2).
Fig. 2.
Different formats of copper. (a) Copper simple substance; (b) Copper ions; (c) Copper oxide powders; (d) Copper alloy; (e) Copper nanoparticles.
Here, we list the previously reported copper-containing scaffolds used in tissue engineering applications, including copper ions, copper nanocomposites, and copper alloy (Table 2).
Table 2.
Various copper-containing scaffolds.
| Base Materials | Copper Formats | Properties | Ref. |
|---|---|---|---|
| Mesoporous bioactive glass | copper ions | Osteogenesis, angiogenesis, antibacterial, drug loading | [23] |
| Bioactive glass nanoparticles (Nbg) and alginate | copper ions | Osteogenesis, angiogenesis, cross-linking, promote the production of BMSCs, sustained release | [128] |
| Boron-containing bioactive glass-based scaffolds coated with alginate | copper ions | Osteogenesis, angiogenesis | [25] |
| Chitosan/hydroxyapatite composite scaffolds | copper ions | Osteogenesis, angiogenesis, antibacterial, anti-inflammatory, sustained release | [82] |
| Electrospun Bioactive Glass Nanofibers | copper ions | Osteogenesis, angiogenesis, antibacterial | [129] |
| mesoporous bioactive glass and nanofibrillated cellulose | copper ions | Angiogenesis | [130] |
| Borosilicate glasses | copper ions | Osteogenesis, angiogenesis, sustained release, structural stability | [20] |
| Alginate | copper ions | Angiogenesis, auxiliary cross-linking | [55] |
| Quercetin | copper ions | Osteogenesis, angiogenesis | [131] |
| Calcium phosphate | copper ions | Osteogenesis, angiogenesis, antibacterial | [132] |
| Bioactive glass (BG) scaffolds | CuFeSe2 | Osteogenesis, photothermal effect, anti-cancer | [40] |
| CaP scaffold with graphene oxide | Copper Nanocomposite | Angiogenesis, osteogenesis | [29] |
| Ti–Cu sintered alloy | Ti–Cu sintered alloy | Antibacterial | [41] |
| Calcium polyphosphate scaffolds | copper carbonate | Osteogenesis, angiogenesis, improvement of mechanical strength | [26] |
| Alginate hydrogels | CuSO4 | Chondrogenesis, antibacterial | [56] |
| Cylindrical collagen-based scaffold. | CuSO4 | Angiogenesis | [133] |
| Bio-composite scaffolds | Cu–Zn alloy nanoparticles | Osteogenesis, antibacterial, porosity | [70] |
| Carboxymethyl Chitosan/Alginate Scaffolds | Cu Nanoparticles | Osteogenesis, antibacterial, angiogenesis, auxiliary cross-linking | [30] |
| PDLLA scaffolds with Cu- and Zn-doped bioactive glasses | CuO | Osteogenesis, angiogenesis, antibacterial | [134] |
| Copper-bearing stainless steel | Cu-SS alloy | Osteogenesis, cell adhesion, biocompatibility, anti-inflammatory | [80] |
| Calcium phosphate cement | Cu-TCP powders | Osteogenesis, angiogenesis, osteogenic differentiation of BMSCs | [135] |
2.1. Pure copper
Pure copper refers to copper metal, which is a soft, malleable, and ductile metal with a pinkish-orange colour. Copper tends to slowly react with oxygen to form a layer of brown-black copper oxide [17].
Copper is one of the few metals that occurs in nature in a directly useable metallic form. It is usually extracted from mines and then purified to obtain copper simple substance [17]. However, copper, as a heavy metal, has notable toxicity if used in high doses [18], and pure copper is easily oxidized [19], which restricts its direct application in tissue engineering.
2.2. Copper ions
Copper ions are the free form of copper, including Cu2+ and Cu+. Compared with other forms, copper ions are relatively easier to obtain and apply. For example, copper chloride, copper sulphate, and certain other copper salts can be dissolved in water to obtain solutions with copper ions in a controllable concentration. To mix copper ions into base materials, it is most common to dissolve copper minerals into water or ethanol then dry the copper-doped materials.
Many studies have fabricated scaffolds using copper ions. For example, CuCl2 was dissolved in ethanol and then mixed into a mesoporous bioactive glass (MBG) scaffold [18]; copper ions released from CuSO4·5H2O were crosslinked with alginate to produce a copper-containing alginate scaffold [19]; CuCl2 and Cu(NO3)2 were also used to fabricate bioglass scaffolds [[20], [21], [22]]; Cu(NO3)2 was dissolved in ethanol to produce copper-doped mesoporous silica nanospheres [16].
However, it is hard to control the release kinetics of copper ions, which may result in irregular crosslinking or excessive cytotoxicity. Thus, copper ions tend to be mixed with some base materials to acquire synergetic properties and reduce cytotoxicity [20]. Various copper-containing materials have been fabricated [21], such as Cu doping into calcium phosphates [22], into silicate glasses [23], into phosphate-based glasses [24], and incorporated with polymer coatings in bioactive glass scaffolds [25] (Table 2). Wang et al. fabricated bioglass (BG)–Cu scaffolds, allowing a more stable release of copper, a lower degradation ability, and better angiogenic properties [20]. Li et al. dissolved copper carbonate in phosphoric acid and mixed it with calcium polyphosphate to fabricate a copper-doped calcium polyphosphate scaffold [26].
2.3. Copper nanoparticles
Copper nanoparticles (CuNPs) are a unique format of biomaterials, referring to a material ranging from 1 to 100 nm in diameter [27]. They have unique properties that differ from large particles, such as a fine microstructure [28]. CuNPs include pure copper nanoparticles, CuO/Cu2O nanoparticles [29], and copper-containing compound nanoparticles. Commercial CuNPs and CuO NPs can be purchased directly from companies, such as Sigma [30] and Merck [13,30]. Some other methods of producing CuNPs have been applied, such as the chemical reduction approach [31] and the Cu-polyethylenimine method [32].
Traditionally, the format of copper added into biomaterials are copper ions. Despite of their convenience and inexpensiveness, some materials mixed with copper ions directly might undergo copper burst release because of their unstable structure, leading to cytotoxicity and fast degradation. In contrast to copper ions, CuNPs have shown more advantages. Firstly, CuNPs could stabilize the physiochemical structure of scaffolds. Copper nanoparticles are more chemically stable than copper ions when added into another material and can release Cu2+ ions in a gradual and stable manner [33], which maintains the stable crosslinking of the base materials, leading to a uniform structure and enhanced stability. Lu et al. demonstrated that the gradual release of Cu2+ from CuNPs could control the cross-linking process of the polymer mixtures, turning the scaffold into an interconnected well-distributed porous structure [30]. Moreover, CuNPs could directly change the structure of the scaffolds. Kumari et al. found that the CuNPs with an average size of 5.31 nm are very well dispersed and that the CuNP layer completely filled the pores of the scaffold [31]. Zhang et al. reported that CuNPs could cover the scaffold surface and fill the gaps of the original material [34], thereby enhancing cell adhesion.
Secondly, nanoparticles show more controllable and stable release of copper ions than copper salts because of their ordered structure, leading to less cytotoxicity than that elicited by copper ions. Some studies showed that scaffolds containing CuNPs showed more stable degradation and stable release of copper ions, leading to lower cytotoxicity [[29], [30],[29], [30]]. However, the ion release mechanism of nanoparticles has not been fully studied, and their release kinetics require further investigation.
2.4. Copper oxide
Inorganic metallic elements or their oxides are often applied in biomaterials to improve their biological properties and physicochemical or mechanical performance [35,36]. Common copper oxides include Copper (II) oxide (CuO) and Copper(I) oxide (Cu2O). Cu2O has good antibacterial properties but has high cytotoxicity; therefore, it is rarely used in medicine. In contrast, CuO is more cytocompatibility and shows some advantages, including its lower price, easy mixture with various polymers, and relative stability in terms of its chemical and physical properties [37].
As a common metal oxide, the production of copper oxide is easy and the output is high. Copper oxide can be produced by heating copper with oxygen and can be obtained by decomposing copper nitrate, copper hydroxide, and basic copper carbonate.
Some methods have been tried to add CuO into scaffolds, such as the robotic deposition (robocasting) method [38] and doping method [39]; however, different materials have different abilities to combine copper oxide, thus the content and concentration of CuO needs to be adjusted according to the base materials. For example, the addition of CuO can decrease the degradation rate of the 13–93 bioglass scaffolds while increase that of the 45S5 bioglasses [38,39].
Copper oxide plays a role in improving the physical structures and biological activity of biomaterials. Ali et al. verified that CuO could increase the compressive strength, flexural strength, and elastic modulus of scaffolds [16]. Zhang et al. designed a graphene oxide-copper nanocomposite-coated porous calcium phosphate, and reported the prolonged release of copper ions, and the enhanced adhesion and stronger osteogenic ability of rat bone mesenchymal stem cells (BMSCs), without obvious cytotoxicity [29].
In addition to the direct use of CuO, CuO nanoparticles are also a research hotspot. Sahmani et al. combined natural hydroxyapatite (n-HA) with different weight fractions of CuO nanoparticles to investigate its effects on bone tissue engineering, and achieved great synergetic properties of apatite formation, enhanced compressive strength and structural stability, and increased osteogenesis [13].
2.5. Copper alloys
Copper alloys are another major category of copper-containing composites. An alloy is a combination of metals combined with one or more other elements such as copper-zinc alloy, copper-gold alloy, and copper-titanium alloy. Copper is often combined with other metals by sintering. For example, red gold is the combination of gold and copper, and sterling silver is the combination of silver and copper. Some new and unique properties can be imparted to the new alloy material.
In past researches, various copper alloy scaffolds have been fabricated such as CuFeSe2 alloy for the photothermal treatment of tumours, which also showed great antibacterial, osteogenic, chondrogenic, and angiogenic properties [40]. Ti–10Cu sintered alloy showed strong anti-infective properties toward Staphylococcus aureus in rabbit back muscles [41]. Ti–Cu alloy also showed in vitro osteogenic and anti-infectious properties in MG-63 cells [42]. Moreover, copper-bearing alloy (Ti6Al4V–6Cu) displayed anti-inflammatory and angiogenic properties for human umbilical vein endothelial cells (HUVECs) [43]. In addition, Mg–Cu alloys showed enhanced mechanical properties, long-lasting antibacterial activity against S. aureus, and osteogenic effects in MC3T3-E1 cells and HUVECs [44]. Although in vitro tests have clarified the value of Cu alloys, more in vivo experiments need to be carried out. At the same time, the degradation, compatibility, and mechanical strength of the alloy still need to be explored.
3. Properties of copper as a biomaterial
3.1. Porosity
The porosity and pore size of scaffolds are important for cell proliferation, cell differentiation, ECM secretion, and neo-tissue formation [45]. Many studies have focused on the interaction of copper with scaffold pores. Tripathi et al. found that Cu–Zn particles added into scaffolds could distribute uniformly, resulting in a porous architecture [70]. Shikha et al. demonstrated that the addition of copper nanoparticles into the scaffold increased the pore size to provide a better strength with enhanced stiffness [31]. This might be caused by the aggregation of nanoparticles on the surface of the scaffold and an increase in the compactness of the scaffold. Thus, particles attached to the surface might induce a change in biological activity because of their effects on scaffold morphology. Moreover, the addition of copper has been proven to make the pores of scaffolds distribute more uniformly. Li et al. demonstrated that the addition of copper into calcium polyphosphate scaffolds generated more uniformly-distributed pores compared with the copper-free scaffolds [26]. Lu et al. found that the porous structure in the base scaffolds was not as well distributed as that of scaffolds mixed with copper [30].
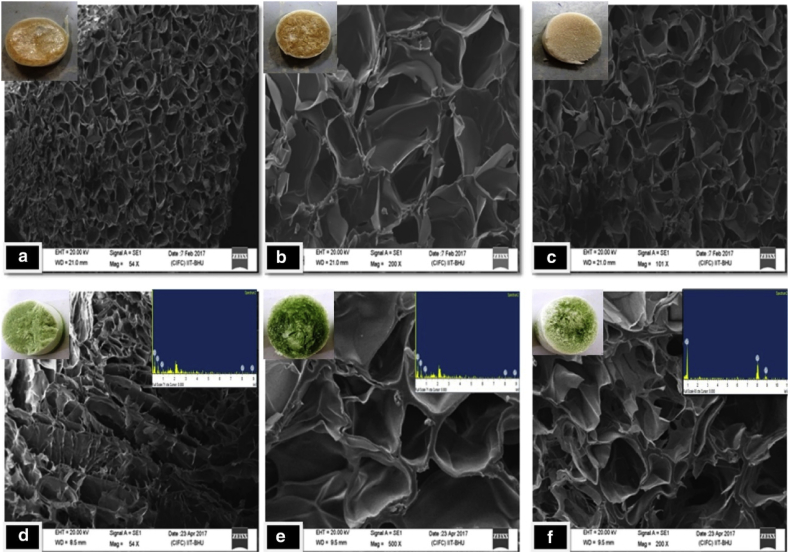
Uniform and even pores are beneficial for cell penetration, bone formation, and vessel formation. However, this effect might be related to the copper concentration. Kargozar et al. found the mesoporous bioactive glasses containing 2 mol% of copper showed a higher exposed surface area and pore volume than those containing 5 mol% of Cu [46]. Fig. 3 shows that different copper concentrations result in different pores of scaffolds, leading to different mechanical properties. Kumari et al. fabricated scaffolds with copper nanoparticles and proved that they provided better strength to the scaffolds, with increased stiffness and firmness [31].
Fig. 3.
Scanning electron microscopy micrographs of scaffolds without and with copper nanoparticles: (a) 50% chitosan + 50% Gelatin +0% copper, (b) 25% chitosan + 75% Gelatin +0% copper, (c) 75% chitosan + 25% Gelatin +0% copper, (d) 50% chitosan + 50% Gelatin +0.01% copper, (e) 25% chitosan + 75% Gelatin +0.02% copper, (f) 75% chitosan + 25% Gelatin +0.03% copper.
3.2. Mechanical strength
Mechanical strength often involves indicators such as stiffness, brittleness, tensile strength, compressive mechanical strength, and elasticity [47].Many studies have shown that copper in biomaterials affects the stiffness and elasticity of scaffolds. Makris et al. demonstrated that neo-cartilage in culture medium containing copper sulphate showed improved compressive properties because of enhanced crosslinking and decreased water content caused by copper [48]. Broomell et al. observed increased hardness and modulus with the addition of copper ions into nereid jaws, and hypothesised that copper would displace a significant amount of water and bind with matrix proteins [49]. Li et al. observed that 0.1% copper-doped calcium polyphosphate scaffolds showed better compressive mechanical strength than control copper-free calcium polyphosphate scaffolds control group. Copper may make the crystal grains of calcium polyphosphate connect more tightly, thus improving the compressive strength [26]. CuO [17] and copper nanoparticles [31] added into scaffolds have been shown to increase compressive strength, such as stiffness and elastic modulus.
Although many studies have reported that the incorporation of copper could improve the mechanical properties of scaffolds, there is still a lack of systematic evaluation. Different copper formats, copper concentrations, and combination methods need to be further investigated to maximize the role of copper in improving the mechanical properties of scaffolds.
3.3. Degradation
Degradation of scaffolds is very important for tissue regeneration. The degradation performance of scaffolds is not only related to the mechanical properties of the scaffold itself, but also to the stability of the repairing environment. Too fast degradation will lead to instability of the scaffolds, making it difficult to provide sufficient mechanical support; while too slow degradation will hinder new tissue ingrowth and cause chronic inflammatory response [50].
Research has proven that the incorporation of copper into biomaterials will affect their degradation rate. The degradation rate of some scaffolds increased, such as copper-containing calcium polyphosphate [28], while some decreased [38]. Kumari observed that after the addition of copper nanoparticles, the rate of biodegradation gradually decreased, thereby providing better stability to the cells [31]. However, the degradation rate is also affected by the copper concentration. For example, Li et al. found that a low dose of copper in scaffolds did not change the degradation rate significantly, but a higher copper content did increase the scaffolds’ degradation rate [28].
Copper can indeed change the degradation rate; however, different materials have different effects. When constructing new materials, the degradation rate must be measured, and the stability of the scaffolds and the release trend of copper ions must be evaluated to ensure stability and safety.
3.4. Cross-linking
Copper ions are able to act as cross-linking agents to cross-link polymers immediately and spontaneously during the preparation of natural polymeric materials [30]. Cations of copper, iron, and zinc can, by coordinating with multiple ligands, function as noncovalent cross-linkers of polymers [51]and protein scaffolds [52]. As divalent cations, Cu2+ could play an important role in the cross-linking reaction, and the cross-linking effect of Cu2+ ions might occur in mannuronic residues (M-blocks) and the mannuronic residues-guluronic acid (MG-blocks), in a similar way as was described for Zn2+ ions [53]. Ions with a larger radius are expected to fill a larger space between the blocks of alginate polymer and collagen, resulting in a tighter structure [54].
Caccavo et al. thought that copper's stimulation of crosslinking is weaker than that of calcium; however, Diego et al. stated the process should last longer for copper compared with that for calcium [55]. They also demonstrated that increasing the ionic strength during crosslinking in an alginate solution could enhance the mechanical properties. Ele et al. showed that copper sulphate was able to significantly increase both the number of cross-links as well as the tensile properties of the tissue [48]. This might be because activation of lysyl oxidase (LOX) by copper sulphate can catalyse the formation of pyridinium (PYR) cross-links.
Some factors can affect the crosslinking process. The higher the concentration of copper ions, the more intensive is the cross-linking process, resulting in the release of structural water from the alginate hydrogel, with a consequent decrease in hydrogel dimensions and porosity [56]. Lu et al. found that adding the Cu2+ solution directly to the anionic polymer solution led to immediate spontaneous cross-linking of the natural polymers [30]; however, the distribution of Cu2+ was hard to control, which was consistent with previous findings that it was difficult to prepare natural polymer scaffolds with a homogeneous distribution of Cu2+ ions [57]. Different factors, such as pH value, temperature, and the microenvironment could affect the role of copper in crosslinking.
Therefore, copper can promote the cross-linking of alginate, collagen, and other materials to change their mechanical properties; however, the interaction between copper and base materials, such as cross-linking speed and cross-linking structure, is still difficult to control.
4. Biological performance in vitro
4.1. Cytotoxicity
In tissue engineering, biocompatibility is the top priority for the in vivo repair of defects. There is a general consensus that the cytotoxicity of copper is attributed to high concentration of Cu2+ ions and their associated oxidative pathways [58], [59], [60], [61]. A previous study demonstrated that the best concentration of Cu2+ ions for maintaining high bioactive efficacy, but low cytotoxicity toward mammalian cells, is 10−5 to 10−4 mol/L [62].
However, Saeid et al. found that 1 mmol/L copper nanoparticles exhibited a combination of very low cytotoxicity and no haemolysis to human red blood cells, but showed strong antibacterial properties in vitro [46]. Nanoparticles could control the release of copper ions, leading to this phenomenon. In a human squamous cell culture study, Akher et al. demonstrated cellular proliferation and viability were enhanced after the addition of 3 mol% CuO into the glass scaffolds [16], indicating that using an appropriate concentration of CuO did not show cytotoxicity. However, Singh et al. reported that the cell the proliferation rate decreased with increasing copper concentration [63]. Therefore, it is clear that a possible burst release and a higher concentration of copper could be cytotoxic and provide unfavourable conditions for cell attachment and growth.
Cytotoxicity will damage the cellular environment and should be restricted within a reasonable range. The copper releasing ability varies in different copper-containing materials; therefore, when fabricating a novel scaffold, the copper concentration, the form of copper, and the form of the basic materials should considered.
4.2. Cell migration
Cell migration is a comprehensive multi-step process involving the coordination of complex biochemical and biomechanical signals to rearrange or aggregate similar cells to enhance their biological effects [64]. As an important part of tissue engineering, the migration ability on the scaffold plays a great role in tissue repair.
Heavy metals like copper show cytotoxicity at high concentrations; however, copper can also improve cell migration under controlled dosage. Alizadeh et al. explored the influence of copper nanoparticles on cell migration, concluding that copper nanoparticles could enhance the quantity and speed of migration of human dermal fibroblasts (HDFs), human embryonic kidney (HEK) cells, and HUVECs. However, this improvement depends on copper's concentration and size [65]. Dang et al. combined a CuFeSe2 alloy with bioglasses (BGs) and found that the scaffold could improve the migration of rabbit BMSCs with more cellular pseudopodia than those on BG-only scaffolds [40]. In addition, copper-doped borate bioactive glass [73] allowed faster migration and better potential to form tubules of HUVECs. Copper-containing mesoporous glass could promote the cell migration of bovine aortic endothelial cells [74], and Mg–Cu alloy showed better cell migration of HUVECs and MC3T3-E1 cells than pure Mg [44].
Some researchers have explored the effect and mechanism of copper on cell migration. Chen et al. demonstrated that the migration of adipose-derived stem cells was promoted in standard culture medium supplemented with 20 μM copper, an effect caused by the regulation of the activity of cytoskeletal proteins, which then activated the Rac-PAK signalling pathway [66]. Chen et al. demonstrated that copper ions could enhance the migration of rat BMSCs in complete medium and they thought that this effect was mediated in part by the stimulation of the hypoxia inducible factor 1 subunit alpha (HIF1α)-Rho family GTPase 3 (RND3, also known as RhoE) pathway [67]. Moreover, Milewska et al. showed that copper promotes the migration and recruitment of human adipose derived MSCs by stimulating the production of stromal cell-derived factor-1α (SDF-1α) [68,69].
Although copper could enhance cellular migration, the concentration of copper should be tailored to avoid cytotoxicity. Proper cell migration will help cell homing and cell proliferation. However, there are few studies on bone and cartilage tissue engineering, and the effect of copper on cell migration in these fields should be explored.
4.3. Cell adhesion
Cell adhesions, including cell–cell and cell-environment, are essential for tissue formation [30]. Tripathi et al. demonstrated that the amounts of cells adhered to a copper-containing scaffold were significantly increased, without toxicity, compared with the copper-free scaffolds [70]. In addition, Mg–Cu alloy showed better cell attachment and spreading of HUVECs and MC3T3-E1 cells than pure Mg [44]. CuFeSe2 alloy could sustain the attachment and proliferation of rabbit BMSCs, and further induced the formation of new bone in bone defects, even after photothermal treatment [40]. It has been reported that increased cell adhesion would lead to better cell matrix interaction and cell distribution on the scaffolds. Lu et al. found more typical filopodia among MC3T3-E1 cells and higher expression of filamentous actin, which proved that the cell adhesion and cell morphology of copper-containing scaffolds were better than those without copper [71].
Some studies have explored the mechanism of copper's improvement in cell adhesion. As has been reported, Integrin α/β subunit genes are responsible for the early adhesion of cells [72]. The known receptors of integrins and their ligand can be bound by metal ions such as Cu2+. Lu et al. found that the expression of integrin on copper-containing scaffolds occurred earlier than that on the copper-free scaffold, followed by proliferation and migration of the attached cells, thus activating the adhesion and actin cytoskeleton signalling pathway genes (FAK (focal adhesion kinase), PXN (Paxillin), and VCL (vinculin)) [30,73]. The proteins encoded by these genes can increase focal adhesion formation, regulate the actin cytoskeleton structure, and promote cell spreading [74].
4.4. Osteogenesis
Copper is essential for the metabolism of the skeleton, and copper deficiency leads to bone abnormalities [75]. Copper deficiency can reduce the activities of monoamine oxidase and LOX, leading to increases in the solubility of bone collagen and damage to the connection of peptide chains, which in turn damages the stability of bone collagen and reduces bone strength [76,77].
Some researchers reported the pro-osteogenic effect of Cu2+ ions on BMSCs and preosteoblasts [23,78]. Copper has been shown to regulate the proliferation and osteogenic differentiation of human MSCs [85]. However, Rodriguez et al. indicated that osteogenic differentiation was enhanced, while cell proliferation was decreased, when human MSCs were cultured in a copper-supplemented medium [79]. This decrease was concentration dependent.
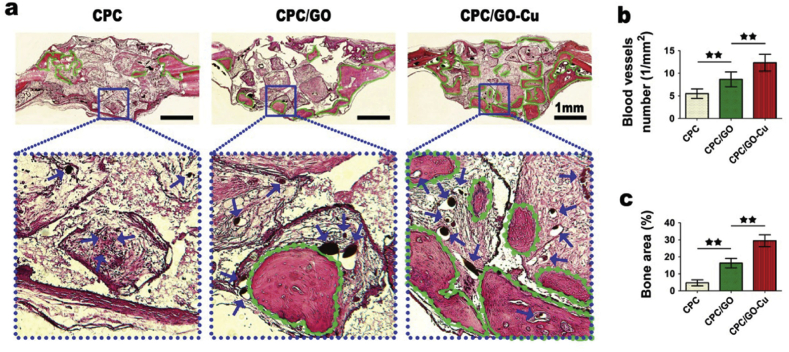
Zhang et al. fabricated an oxide-copper coated scaffold and showed that the osteogenic gene expression of ALP (alkaline phosphatase) and OCN (osteocalcin) in the copper-containing group was highest compared with that in the control groups. The results indicated that the Cu coatings facilitated cell adhesion and enhanced growth and osteogenic inductive capacity on BMSCs (Fig. 4.) [29]. In addition, copper-containing mesoporous bioglass scaffolds cultured with human BMSCs [18], copper-containing alginate scaffolds and copper-containing chitosan scaffolds [23], and copper-containing stainless steel cultured with MC3T3-E1 cells all showed high expression of osteogenesis-related genes, enhanced calcium deposition, and increased bone formation and mineralization. Copper has also been proven to stimulate ALP activity and osteogenic gene expression in vitro, as well as enhance new bone formation around the implants in vivo by improving the adhesion and proliferation of osteoblasts on the steel surface [80].
Fig. 4.
Histological analysis of newly formed bone tissues. (a) Eight weeks after surgery, the longitudinal sections were imaged to display the new bones, shown as green lines, in the defect regions. Blue arrows indicate blood vessels perfused with Microfil. (b) The statistics of the blood vessels number (n = 6; ∗∗represents p < 0.01). (c) The statistics of the percentage of new bone area in the defect regions (n = 6; ∗∗represents p < 0.01).
Obviously, copper can promote the adhesion and proliferation of osteoblasts as well as osteogenic differentiation of MSCs; however, the underlying mechanisms are not fully understood. It has been reported that HIF-1α might be a critical mediator of copper in accelerating bone regeneration by regulating both angiogenesis and osteogenesis [81]. Zhang et al. demonstrated that the Cu-containing group activated the expression of HIF-1α, VEGF and bone morphogenetic protein 2 (BMP-2) by inhibiting Von Hippel-Lindau (VHL) tumor suppressor and activating the phosphorylation of extracellular signal-regulated kinase (ERK)1/2 [82]. Li et al. concluded that copper regulates the HIF-1α signal pathway and then mediates VEGF expression to stimulate osteogenesis and angiogenesis [26]. In addition, the osteogenic protein expression of Runx2 can be enhanced via stimulating Akt cell signalling pathway, which was observed in 316L-Cu SS [83]. Zhao et al. showed that osteoblast activity was also related to the Integrin β1/FAK/ERK pathway [84].
In conclusion, copper has shown great in vitro osteogenic properties; however, how to combine copper with materials to make them suitable for the ECM and to play a role in osteogenesis in vivo remains to be further studied.
4.5. Chondrogenesis
In addition to promoting osteogenic differentiation, some studies have found that copper also promotes chondrogenic proliferation and differentiation. In cellular medium with copper ions, copper plays a significant role in maintaining the formation of proteoglycan and collagen II of cartilage [85,104,105], which are important components of the cartilaginous matrix [86,87]. Madz et al. demonstrated that copper ions at 60 μmol/L in a copper-alginate hydrogel could enhance the production of glycosaminoglycan and the proliferation of chondrocytes, promoting the chondrogenic differentiation of MSCs and the maturation of chondrocytes [56]. Makris et al. demonstrated in mediums supplemented with copper sulphate, the activity of LOX to form collagen cross-links was enhanced, increasing the strength and integrity of neo-cartilage [48]. Lin et al. cultured copper-incorporated bioactive glass-ceramics extracts with chondrocytes and observed increased chondrocyte proliferation, enhanced expression of chondrocyte-specific genes (COL2 (collagen type 2), SOX9 (SRY-box transcription factor 9), ACAN (aggrecan)), and elevated levels of type II collagen protein compared with those in the copper-free group.
Copper plays a significant role in cartilage metabolism by promoting the synthesis of insulin-like growth factor 1 (IGF-1), which is the major growth factor in chondrogenesis [89]. The release of IGF-1 promotes chondrocyte growth and proliferation [90], and the expression of cartilaginous genes [91], via autocrine and paracrine mechanisms, thus contributing to cartilage regeneration. Besides, some studies indicated that copper could promote chondrocyte proliferation, differentiation and synthesis of the cartilage matrix by promoting the secretions of IGF-1, IGF-binding protein-3 [92], and transforming growth factor-β (TGF-β) [93]. In addition, the enhanced LOX activity stimulated by copper upregulates the proliferative effect of hydroxylysine, leading to the formation of GAG and collagen [48]. In addition, the copper could also enhance the expression level of HIF-1α, activate the HIF-1α pathway and elevate the expression of type II collagen protein and NCAD protein [94]. Further studies are needed to determine the precise underlying mechanisms of copper's improvement on chondrocyte maturation and chondrogenic differentiation.
4.6. Angiogenesis
New vessels act as conduits to provide nutrients, immune cells, and growth factors to help repair bone defects. Studies have demonstrated the ability of copper ions to promote in vitro endothelial cell proliferation and to stimulate VEGF gene expression to promote angiogenesis [95,96]. Previous research suggested that a lack of copper could suppress the formation of blood vessels by downregulating the expression of certain copper-binding proteins [97,98].
Copper ions are particularly involved in cell growth and angiogenesis [99]. Barralet et al. found that copper-loaded bioceramic scaffolds not only provided directional vascularization, but also improved wound healing compared with that of copper-free scaffolds [100]. More recently, Gérard et al. showed copper ions added to a 3D culture system could significantly promote vascularisation [96]. Zhang et al. demonstrated that scaffolds with oxide-copper nanocomposites had a better angiogenic effect than the copper-free scaffolds in rat cranial bone defects, indicating an in vivo angiogenic effect [29]. Lu et al. added copper into alginate scaffolds, and observed more collagen with small blood vessels in the copper-containing scaffolds [30]. The appropriate amount of copper can stimulate collagen cross-linking and promote angiogenesis; however, it does not increase the load-bearing capabilities of blood vessels significantly [101].
The concentration of copper affects the angiogenesis process. Erol et al. demonstrated that low doses of copper sulphate (0.56 mg/mL) enhanced angiogenesis on silicate scaffolds and a 10-fold dose (5.6 mg/mL) enhanced the growth of wound tissue [25]. Therefore, an appropriate concentration should be determined to achieve the maximum effect of copper ions on angiogenesis. The concentration of copper ions also affects the capillary formation and length. Diego et al. showed that the higher the concentration of copper, the longer the formed capillaries because of copper's effects on the crosslinking of vessels [55].
The mechanism that copper promotes angiogenesis is mainly via the HIF-1α pathway. In a study of Cu-containing mesoporous bioactive glass (MBG), both Cu-MBG scaffolds and their ionic extracts stimulated the expression of HIF-1α and VEGF in human BMSCs [23]. Zhang et al. demonstrated that copper promoted the activation of HIF-1α via the ERK1/2 signalling pathway [29]. Lukas et al. concluded that copper could stimulate eukaryotic cells by inducing the overexpression of the VEGF and CCND1 (cyclin D1) genes, stimulating angiogenesis and cell proliferation [82]. Although most studies show that copper enhances angiogenesis mainly through the HIF-1α pathway, Li et al. thought that this effect is not VEGF-dependent, but is endothelial nitrous oxide synthase (eNOS)-dependent in a HUVEC medium with copper sulphate [12]. Hence, the mechanism of copper's promotion of angiogenesis remains to be further determined.
4.7. Antibacterial effects
A major problem after implanting biomaterials in the human body is infection, which could cause implantation failure. How to minimize the possibility of infection is a major point of consideration. All forms of copper, such as copper ions, nanoparticles, and alloys, have antibacterial properties. Studies have shown that the effective antibacterial ingredient of copper-containing materials are mainly copper ions released from compounds [102].
Several studies provided evidence of the remarkable antibacterial effect of copper ions. Kim et al. produced copper-containing hydroxyapatite (HA) and reported that it exhibited a strong antibacterial effect on Escherichia coli and S. aureus [103]. Higher concentrations of copper ions on the surface of HA nanoparticles allowed faster diffusion of toxic Cu ions to the surrounding medium, penetrating and killing bacterial cells [104]. Rau et al. added 5 wt % of CuO to 45S5 bioactive glass and demonstrated effective activity against the gram-negative bacteria [105].
A high concentration of copper ions might have a strong antibacterial effect; however, it also has obvious cytotoxicity, thus the appropriate concentration should be determined. In addition, the slow and stable release of Cu ions is conducive to long-lasting repair and antibacterial effect. When exploring the suitable antibacterial concentration, a sustained and slow release of copper should be maintained to avoid the cytotoxicity induced by the burst release of copper ions.
The antibacterial effect of copper correlates with its concentration in extracellular environment. It was reported that in vitro cytotoxic concentration is 10−4 mol/L and the minimum inhibitory concentration (MIC) is 10−5 mol/L for copper ions [88], [89], [90]. Within this window of concentrations, it can be anticipated that the material will be antibacterial without being cytotoxic. However, Lu et al. found that 1 mmol/L Cu nanoparticles exhibited a combination of very low cytotoxicity and a high antibacterial effect (above 99% bacterial reduction) in vitro. This might be related to the unique properties of the nanoparticles, which release copper ions at a slow and stable rate; therefore, the actual copper concentration of the extracting solution should be monitored.
The mechanism of copper's anti-bacterial effects is not completely understood. Generally, metal ions exert antibacterial effects by damaging bacterial proteins and nucleic acids [107], disrupting the ultra-structures of bacteria-cell binding and promoting DNA cleavage [108]. Cu2+ released from the materials strongly binds to thiol groups through the electrostatic forces imparted by Cu2+ in the active sites of surface proteins in bacteria. This damages the functional groups of proteins, and blocks enzyme activities by denaturing proteins and damaging DNA, thereby destroying the constituents of the cell membrane, such as lipopolysaccharide, and interrupting transport protein activity and ion permeability [[82], [109], [110],109,110]. However, some study reported that Cu2+ was observed to interfere with the replication 16 S rRNA genes, but without damage to DNA [111]. Therefore, whether copper damages DNA directly remains to be determined.
Other studies have shown that copper ions are involved in the formation of reactive oxygen species (ROS) [82], which can produce free radicals that have detrimental effects on cell respiration [30]. Studies also reported that Cu exerts its antibacterial function by alternating between the redox states of Cu+ and Cu2+ ions [112,113]. Moreover, through redox cycling between Cu2+ and Cu+, copper can catalyse the production of ROS and facilitate the attack of free radicals on amino acids, thus leading to substantial protein alterations and protein cleavage [109].
5. Applications of copper biomaterials
5.1. Bone tissue engineering
When developing a new type of biomaterial, in vivo studies are required to evaluate its suitability for specific applications. The animals used for bone and cartilage tissue engineering research are mainly mice, rats, rabbits, sheep, pigs, and monkeys. Monkeys, with similar physiological and anatomical characteristics to humans, are the most ideal model; however, the cost is too high and the number of monkeys is small, making their wider use difficult. Large animals, such as pigs and goats, are rarely used because of the high cost and difficulty in feeding. Histological evaluation with imaging data, such as BMD (bone mineral density) and TBV (total bone volume) can reflect new bone formation. In addition, certain commonly used staining methods, like haematoxylin and eosin (HE) staining and Safranin O staining, could show the status of tissue repair, and the formation of neo-bone, neo-cartilage, and other tissues.
Mice and Rats are widely used because of their low cost and easy access to surgery. Copper-containing scaffolds implanted into rodents showed good osteogenesis and angiogenesis abilities. Copper also showed good biocompatibility and inflammatory properties [23,34]. Moreover, copper-containing biomaterials not only promoted osteogenesis in the callus, but also upregulated the callus remodelling efficiency, leading to an early and mechanically stable recovery of the fractured femur [114]. However, the effects on tissue regeneration might be concentration-dependent. Lin et al. observed enhanced bone regeneration along with blood vessels and fibrous tissue in bioactive glass doped with 2.0 wt.% CuO; however, the 0.4 and 0.8 wt.% groups did not show much angiogenesis in the neo-bone [38].
Rabbits are of moderate size, with proper femoral condyle of the knee joint for surgery, which is suitable to establish bone and cartilage defects. Prinz et al. applied copper-containing nails to fix tibia fractures of rabbits, which demonstrated enhanced bone formation together with significant antibacterial performance [115]. In addition, copper-bioglass scaffolds also showed great osteogenic properties in a rabbit femoral condyle osteochondral defect model [94].
Among large mammals, goats are frequently used for research. Li et al. demonstrated that copper-containing bioactive glass nanocoating on polyethylene terephthalate (PET) artificial ligaments (Cu-BG/PET) could promoted osteogenesis and angiogenesis in the repair of bone defects in goat femurs and tibias [116].
In conclusion, the application of copper in bone tissue engineering has been reported by many studies. Copper-containing scaffolds can promote bone repair and regeneration because of its pro-osteogenic, pro-angiogenic, and antibacterial properties. However, current research mostly uses small animals (such as mice and rats), and there are still few large animal studies. To stimulate the human body structure, more large animal studies are encouraged.
5.2. Cartilage and osteochondral tissue engineering
The cartilage lacks a blood supply and is difficult to regenerate under natural repair processes. There is much in vitro evidence for the role of copper in promoting cartilage regeneration. Copper affects cartilage matrix remodelling [86] and evidence suggests that copper deficiency can lead to defects in collagen cross-linking of neo-cartilage [87]. However, few in vivo experiments have been conducted and the mechanism is unclear. Given the significant pro-osteogenic and chondrogenic properties of copper in vitro, studies were conducted to verify its property of repairing osteochondral defects. Copper-containing scaffolds could not only enhance the formation of fibrous tissues and new cartilage in rats [114], but also promote the production of new hyaline-like cartilage tissues in rabbits. In addition, a smooth and well-integrated interface between the newly-formed cartilage and subchondral bone was found in copper-bioglass scaffolds, and the tide mark closely resembled to the surrounding natural tissue [94]. Xu et al. reported copper-induced promotion of MSC chondrogenesis and observed enhanced formation of new cartilage tissue with the deposition of GAG, together with elevated expression of cartilage-related genes such as SOX9, ACAN, and COL2, which confirmed copper-induced improvement of chondrogenesis [117].
As revealed in previous reports, chondrocytes are in a hypoxic environment and HIF1α plays an important role in the hypoxic environment [23,118,119]. The copper ions released by copper-containing materials promoted the activation of the HIF signalling pathway, thereby promoting the expression of SOX9, COL2, and ACAN [120]. Further experiments showed that Cu2+ released from Cu-bioactive glass ceramic (BGC) scaffolds played an important role in promoting the differentiation of chondrocytes and the repair of cartilage by activating the HIF pathway, which then further promoted the anti-inflammatory M2 phenotype and elevated the secretion of anti-inflammatory cytokines in macrophages to reduce the damage to cartilage tissue [106].
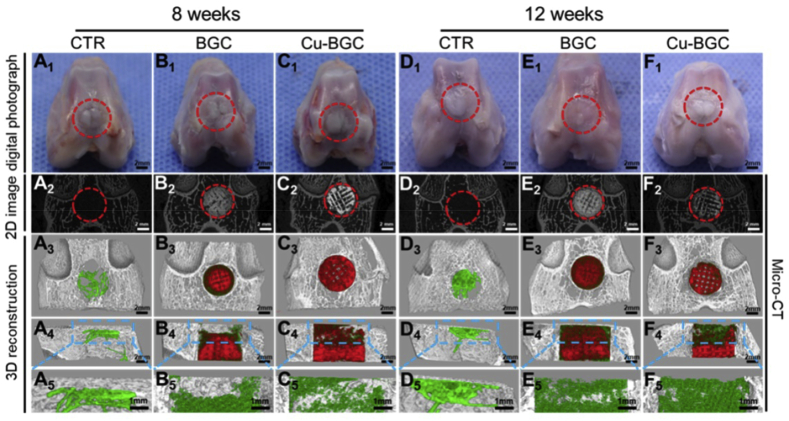
Osteochondral defects involve cartilage layer and subchondral bone layer defects, which have different physiochemical and biological characteristics. To achieve simultaneous repair of subchondral bone and cartilage, the scaffold is required to be multifunctional and have osteogenic and chondrogenic properties. Lin et al. revealed that the defects of Cu-BGC groups were most covered with newly formed bone tissue and fibrous tissues, while the osteochondral defects still existed in the blank control (Fig. 5). They demonstrated that Cu-BGC possesses the ability to facilitate the regeneration of osteochondral tissue [94].
Fig. 5.
The gross morphology and Micro-computed tomography images of the samples at 8 and 12 weeks of post-surgery (A1-F1) Digital photographs of the samples (A2-F2) 2-dimensional projection images of the defects (A3-F3) and (A4-F4) show the transverse view and sagittal view of 3-dimensional (3D) reconstruction images, respectively (A5-F5) Images of new bone in the upper part of the defects. In 3D reconstruction images, off-white, green, and red indicate primary bone, new bone, and scaffolds, respectively. As compared with control group and bioactive glass ceramic (BGC) groups, Cu-BGC group displayed a considerable amount of neo-bone tissue in the defect region at 12 weeks.
5.3. Antibacterial and anti-infection effects
Copper has been proved to have remarkable antibacterial and anti-infectious effects for long time and many copper-containing materials have been applied for medical use. In 2008, with enough scientific evidence, copper was registered by the U.S. Environmental Protection Agency (EPA) as the first and only metal with antibacterial properties [121]. Some novel alloys containing copper have been synthesized such as Mg–Cu alloy [44],Ti–Cu alloy [122], Ti–Mo–Cu alloy [123] and Ti6Al4V–6Cu alloy [43], which show prospective medical application in bone implantation, preventing postoperative infection.
Copper has been added into many kinds of mature medical alloy implants to play an extra role in preventing bacterial infections. Liu et al. showed that copper-bearing titanium alloy has great in vitro and in vivo antibacterial effects for dental application and reduce the infectious rate associated with dental implants, preventing the bone resorption induced by peri-implantitis [122]. Xu et al. added copper into Ti–Mo alloy, showing excellent bacterial inhibitory property, avoiding the failure of implantation and revision surgery [123]. Ti6Al4V, a kind of common alloy applied in bone implantation, showed better anti-bacterial effect after the combination with copper, providing a theoretical evidence for the clinical application [124]. In addition, Mg-based metals have been investigated widely for clinical application, Liu et al. added copper into magnesium and showed that Mg–Cu alloy could inhibit bacteria growth while stimulating bone formation [44]. To fix prothesis and repair bone defects, Poly (methyl methacrylate) (PMMA)-based bone cements have been applied in orthopaedic field. De Santis et al. demonstrated that copper doped PMMA bone cement can both help repair bone defects and prevent infections, which is promising in clinical application [125].
In the field of prothesis design, various coatings are added in order to impart better properties to bone implants like osteogenic and anti-infectious properties. Copper could be used as a coating to enhance the anti-bacterial properties of implants. Rivera et al. modified the antibacterial property of metallic implant by coating copper onto its surface, significantly reducing the infectious rate from joint pathogens [126].
As has been cited above, traditional metallic biomaterials and bone cements have been widely applied in orthopaedic field, but infections often occur. The incorporation of copper can possibly avoid bacterial infections and speed up the repair process, which is a promising approach for bone regeneration.
6. Conclusions and future perspectives
In this review, we summarized the physicochemical and biological properties of copper and copper-containing biomaterials, and their applications in bone and cartilage tissue engineering. The incorporation of copper could enhance the porosity, mechanical strength, and crosslinking of scaffolds, as well as influencing their degradation behaviours. Copper could not only enhance cell migration and adhesion, but also improves osteogenesis, chondrogenesis, and angiogenesis, and exerts antibacterial activity. In vivo animal studies show that copper is beneficial for the repair of bone, cartilage, and blood vessels. Further studies are needed using more clinically relevant large animal models to fully elucidate the effect of copper biomaterials on in situ bone and cartilage regeneration. The detailed mechanisms of copper-induced improvement of the osteogenic and chondrogenic properties should be explored in future studies. In addition, systematic research should be performed to determine the appropriate forms and concentration of copper used in specific tissue engineering of bone, cartilage, or other tissues.
Declaration of competing interest
The author(s) have no conflicts of interest relevant to this article.
Acknowledgements
This work was supported by the National Natural Science Foundation of China [grant numbers 81771985, 81702205, 81901903]; the National Key Research and Development Program of China [grant number 2018YFC1105201/204]; the Key Research Program of Science & Technology Support Program of Jiangsu Province [grant number BE2016763]; the Natural Science Foundation of Jiangsu Province [grant number BK20190356]; and the Postgraduate Research and Innovation Project of Jiangsu Province [grant number KYCX20_1432].
Contributor Information
Wei Zhang, Email: zhang.wei@seu.edu.cn.
Qingqiang Yao, Email: yaoqingqiang@126.com.
References
- 1.Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol: CJASN. 2008;3(Suppl 3):S131–S139. doi: 10.2215/CJN.04151206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amini A.R., Laurencin C.T., Nukavarapu S.P. Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng. 2012;40(5):363–408. doi: 10.1615/critrevbiomedeng.v40.i5.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van der Stok J., Van Lieshout E.M.M., El-Massoudi Y. Bone substitutes in The Netherlands - a systematic literature review. Acta Biomater. 2011;7(2):739–750. doi: 10.1016/j.actbio.2010.07.035. [DOI] [PubMed] [Google Scholar]
- 4.Sophia Fox A.J., Bedi A., Rodeo S.A. The basic science of articular cartilage: structure, composition, and function. Sport Health. 2009;1(6):461–468. doi: 10.1177/1941738109350438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curl W.W., Krome J., Gordon E.S. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthrosc: J Arthrosc Related Surg: Off Publ Arthrosc Assoc North America Int Arthrosc Assoc. 1997;13(4):456–460. doi: 10.1016/s0749-8063(97)90124-9. [DOI] [PubMed] [Google Scholar]
- 6.Karuppal R. Current concepts in the articular cartilage repair and regeneration. J Orthop. 2017;14(2):A1–A3. doi: 10.1016/j.jor.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahmoudifar N., Doran P.M. Chondrogenesis and cartilage tissue engineering: the longer road to technology development. Trends Biotechnol. 2012;30(3):166–176. doi: 10.1016/j.tibtech.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Goldring M.B., Goldring S.R. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann N Y Acad Sci. 2010;1192:230–237. doi: 10.1111/j.1749-6632.2009.05240.x. [DOI] [PubMed] [Google Scholar]
- 9.Wubneh A., Tsekoura E.K., Ayranci C. Current state of fabrication technologies and materials for bone tissue engineering. Acta Biomater. 2018;80:1–30. doi: 10.1016/j.actbio.2018.09.031. [DOI] [PubMed] [Google Scholar]
- 10.Tomaszewska E., Dobrowolski P., Kwiecien M. The influence of the dietary Cu-Glycine complex on the histomorphology of cancellous bone, articular cartilage, and growth plate as well as bone mechanical and geometric parameters is dose dependent. Biol Trace Elem Res. 2017;178(1):54–63. doi: 10.1007/s12011-016-0894-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uriu-Adams J.Y., Keen C.L. Copper, oxidative stress, and human health. Mol Aspect Med. 2005;26(4–5):268–298. doi: 10.1016/j.mam.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 12.Li S., Xie H., Li S. Copper stimulates growth of human umbilical vein endothelial cells in a vascular endothelial growth factor-independent pathway. Exp Biol Med. 2012;237(1):77–82. doi: 10.1258/ebm.2011.011267. [DOI] [PubMed] [Google Scholar]
- 13.Sahmani S., Shahali M., Ghadiri Nejad M. Effect of copper oxide nanoparticles on electrical conductivity and cell viability of calcium phosphate scaffolds with improved mechanical strength for bone tissue engineering. Eur Phys J Plus. 2019;134(1):7. [Google Scholar]
- 14.Abdellahi M., Najafinezhad A., Ghayour H. Preparing diopside nanoparticle scaffolds via space holder method: simulation of the compressive strength and porosity. J Mech Behav Biomed Mater. 2017;72:171–181. doi: 10.1016/j.jmbbm.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Shi M., Chen Z., Farnaghi S. Copper-doped mesoporous silica nanospheres, a promising immunomodulatory agent for inducing osteogenesis. Acta Biomater. 2016;30:334–344. doi: 10.1016/j.actbio.2015.11.033. [DOI] [PubMed] [Google Scholar]
- 16.Ali A., Ershad M., Vyas V.K. Studies on effect of CuO addition on mechanical properties and in vitro cytocompatibility in 1393 bioactive glass scaffold. Mater Sci Eng C, Mater Biol Appl. 2018;93:341–355. doi: 10.1016/j.msec.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 17.Barceloux D.G. Copper. J Toxicol Clin Toxicol. 1999;37(2):217–230. doi: 10.1081/clt-100102421. [DOI] [PubMed] [Google Scholar]
- 18.Scheiber I., Dringen R., Mercer J.F.B. Copper: effects of deficiency and overload. Metal Ions Life Sci. 2013;13:359–387. doi: 10.1007/978-94-007-7500-8_11. [DOI] [PubMed] [Google Scholar]
- 19.Duffy J., Paunovic M., Zeblisky R. PHYSICAL-PROPERTIES OF electroless copper. Plat Surf Finish. 1983;70(11) 45–45. [Google Scholar]
- 20.Wang H., Zhao S., Xiao W. Influence of Cu doping in borosilicate bioactive glass and the properties of its derived scaffolds. Mater Sci Eng C, Mater Biol Appl. 2016;58:194–203. doi: 10.1016/j.msec.2015.08.027. [DOI] [PubMed] [Google Scholar]
- 21.Barralet J., Gbureck U., Habibovic P. Angiogenesis in calcium phosphate scaffolds by inorganic copper ion release. Tissue Eng. 2009;15(7):1601–1609. doi: 10.1089/ten.tea.2007.0370. [DOI] [PubMed] [Google Scholar]
- 22.Ewald A., Käppel C., Vorndran E. The effect of Cu(II)-loaded brushite scaffolds on growth and activity of osteoblastic cells. J Biomed Mater Res. 2012;100(9):2392–2400. doi: 10.1002/jbm.a.34184. [DOI] [PubMed] [Google Scholar]
- 23.Wu C., Zhou Y., Xu M. Copper-containing mesoporous bioactive glass scaffolds with multifunctional properties of angiogenesis capacity, osteostimulation and antibacterial activity. Biomaterials. 2013;34(2):422–433. doi: 10.1016/j.biomaterials.2012.09.066. [DOI] [PubMed] [Google Scholar]
- 24.Stähli C., Muja N., Nazhat S.N. Controlled copper ion release from phosphate-based glasses improves human umbilical vein endothelial cell survival in a reduced nutrient environment. Tissue Eng. 2013;19(3–4):548–557. doi: 10.1089/ten.tea.2012.0223. [DOI] [PubMed] [Google Scholar]
- 25.Erol M.M., Mourino V., Newby P. Copper-releasing, boron-containing bioactive glass-based scaffolds coated with alginate for bone tissue engineering. Acta Biomater. 2012;8(2):792–801. doi: 10.1016/j.actbio.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 26.Li Y., Wang J., Wang Y. Transplantation of copper-doped calcium polyphosphate scaffolds combined with copper (II) preconditioned bone marrow mesenchymal stem cells for bone defect repair. J Biomater Appl. 2018;32(6):738–753. doi: 10.1177/0885328217739456. [DOI] [PubMed] [Google Scholar]
- 27.Lewinski N., Colvin V., Drezek R. Cytotoxicity of nanoparticles. Small. 2008;4(1):26–49. doi: 10.1002/smll.200700595. [DOI] [PubMed] [Google Scholar]
- 28.Tang L., Zhu L., Tang F. Mild synthesis of copper nanoparticles with enhanced oxidative stability and their application in antibacterial films. Langmuir: ACS J Surf Colloids. 2018;34(48):14570–14576. doi: 10.1021/acs.langmuir.8b02470. [DOI] [PubMed] [Google Scholar]
- 29.Zhang W., Chang Q., Xu L. Graphene oxide-copper nanocomposite-coated porous CaP scaffold for vascularized bone regeneration via activation of hif-1α. Adv Healthcare Mater. 2016;5(11):1299–1309. doi: 10.1002/adhm.201500824. [DOI] [PubMed] [Google Scholar]
- 30.Lu Y., Li L., Zhu Y. Multifunctional copper-containing carboxymethyl chitosan/alginate scaffolds for eradicating clinical bacterial infection and promoting bone formation. ACS Appl Mater Interfaces. 2018;10(1):127–138. doi: 10.1021/acsami.7b13750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumari S., Singh B.N., Srivastava P. Effect of copper nanoparticles on physico-chemical properties of chitosan and gelatin-based scaffold developed for skin tissue engineering application. 3 Biotech. 2019;9(3):102. doi: 10.1007/s13205-019-1624-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nath J., Dror I., Landa P. Synthesis and characterization of isotopically-labeled silver, copper and zinc oxide nanoparticles for tracing studies in plants. Environ Pollut. 2018;242:1827–1837. doi: 10.1016/j.envpol.2018.07.084. [DOI] [PubMed] [Google Scholar]
- 33.Midander K., Cronholm P., Karlsson H.L. Surface characteristics, copper release, and toxicity of nano- and micrometer-sized copper and copper(II) oxide particles: a cross-disciplinary study. Small. 2009;5(3):389–399. doi: 10.1002/smll.200801220. [DOI] [PubMed] [Google Scholar]
- 34.Zhang W., Jiang P., Chen W. Genotoxicity of copper oxide nanoparticles with different surface chemistry on rat bone marrow mesenchymal stem cells. J Nanosci Nanotechnol. 2016;16(6):5489–5497. doi: 10.1166/jnn.2016.11753. [DOI] [PubMed] [Google Scholar]
- 35.Vyas V.K., Kumar A.S., Prasad S. Bioactivity and mechanical behaviour of cobalt oxide-doped bioactive glass. Bull Mater Sci. 2015;38(4):957–964. [Google Scholar]
- 36.Ershad M., Vyas V.K., Prasad S. Effect of Sm2O3 substitution on mechanical and biological properties of 45S5 bioactive glass. J Australian Ceram Soc. 2018;54(4):621–630. [Google Scholar]
- 37.Ren G., Hu D., Cheng E.W.C. Characterisation of copper oxide nanoparticles for antimicrobial applications. Int J Antimicrob Agents. 2009;33(6):587–590. doi: 10.1016/j.ijantimicag.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 38.Lin Y., Xiao W., Bal B.S. Effect of copper-doped silicate 13-93 bioactive glass scaffolds on the response of MC3T3-E1 cells in vitro and on bone regeneration and angiogenesis in rat calvarial defects in vivo. Mater Sci Eng C, Mater Biol Appl. 2016;67:440–452. doi: 10.1016/j.msec.2016.05.073. [DOI] [PubMed] [Google Scholar]
- 39.Wang H., Zhao S., Zhou J. Evaluation of borate bioactive glass scaffolds as a controlled delivery system for copper ions in stimulating osteogenesis and angiogenesis in bone healing. J Mater Chem B. 2014;2(48):8547–8557. doi: 10.1039/c4tb01355g. [DOI] [PubMed] [Google Scholar]
- 40.Dang W., Li T., Li B. A bifunctional scaffold with CuFeSe2 nanocrystals for tumor therapy and bone reconstruction. Biomaterials. 2018;160:92–106. doi: 10.1016/j.biomaterials.2017.11.020. [DOI] [PubMed] [Google Scholar]
- 41.Wang X., Dong H., Liu J. In vivo antibacterial property of Ti-Cu sintered alloy implant. Mater Sci Eng C, Mater Biol Appl. 2019;100:38–47. doi: 10.1016/j.msec.2019.02.084. [DOI] [PubMed] [Google Scholar]
- 42.Liu R., Ma Z., Kunle Kolawole S. In vitro study on cytocompatibility and osteogenesis ability of Ti-Cu alloy. J Mater Sci Mater Med. 2019;30(7):75. doi: 10.1007/s10856-019-6277-z. [DOI] [PubMed] [Google Scholar]
- 43.Xu X., Lu Y., Li S. Copper-modified Ti6Al4V alloy fabricated by selective laser melting with pro-angiogenic and anti-inflammatory properties for potential guided bone regeneration applications. Mater Sci Eng C, Mater Biol Appl. 2018;90:198–210. doi: 10.1016/j.msec.2018.04.046. [DOI] [PubMed] [Google Scholar]
- 44.Liu C., Fu X., Pan H. Biodegradable Mg-Cu alloys with enhanced osteogenesis, angiogenesis, and long-lasting antibacterial effects. Sci Rep. 2016;6:27374. doi: 10.1038/srep27374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Griffon D.J., Sedighi M.R., Schaeffer D.V. Chitosan scaffolds: interconnective pore size and cartilage engineering. Acta Biomater. 2006;2(3):313–320. doi: 10.1016/j.actbio.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 46.Kargozar S., Montazerian M., Hamzehlou S. Mesoporous bioactive glasses: promising platforms for antibacterial strategies. Acta Biomater. 2018;81:1–19. doi: 10.1016/j.actbio.2018.09.052. [DOI] [PubMed] [Google Scholar]
- 47.Roylance D. Wiley; New York: 1996. Mechanics of materials[M] [Google Scholar]
- 48.Makris E.A., MacBarb R.F., Responte D.J. A copper sulfate and hydroxylysine treatment regimen for enhancing collagen cross-linking and biomechanical properties in engineered neocartilage. Faseb J: Off Publ Feder Am Soc Exp Biol. 2013;27(6):2421–2430. doi: 10.1096/fj.12-224030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Broomell C.C., Zok F.W., Waite J.H. Role of transition metals in sclerotization of biological tissue. Acta Biomater. 2008;4(6):2045–2051. doi: 10.1016/j.actbio.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 50.O'Brien F.J. Biomaterials & scaffolds for tissue engineering. Mater Today. 2011;14(3):88–95. [Google Scholar]
- 51.Kramer R., Lehn J.M., Marquis-Rigault A. Self-recognition in helicate self-assembly: spontaneous formation of helical metal complexes from mixtures of ligands and metal ions. Proc Natl Acad Sci Unit States Am. 1993;90(12):5394–5398. doi: 10.1073/pnas.90.12.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vaccaro E., Waite J.H. Yield and post-yield behavior of mussel byssal thread: a self-healing biomolecular material. Biomacromolecules. 2001;2(3):906–911. doi: 10.1021/bm0100514. [DOI] [PubMed] [Google Scholar]
- 53.Yang C.H., Wang M.X., Haider H. Strengthening alginate/polyacrylamide hydrogels using various multivalent cations. ACS Appl Mater Interfaces. 2013;5(21):10418–10422. doi: 10.1021/am403966x. [DOI] [PubMed] [Google Scholar]
- 54.Bajpai S.K., Sharma S. Investigation of swelling/degradation behaviour of alginate beads crosslinked with Ca2+ and Ba2+ ions. React Funct Polym. 2004;59(2):129–140. [Google Scholar]
- 55.Caccavo D., Strom A., Larsson A. Modeling capillary formation in calcium and copper alginate gels. Mater Sci Eng C, Mater Biol Appl. 2016;58:442–449. doi: 10.1016/j.msec.2015.08.040. [DOI] [PubMed] [Google Scholar]
- 56.Madzovska-Malagurski I., Vukasinovic-Sekulic M., Kostic D. Towards antimicrobial yet bioactive Cu-alginate hydrogels. Biomed Mater. 2016;11(3) doi: 10.1088/1748-6041/11/3/035015. [DOI] [PubMed] [Google Scholar]
- 57.D'Mello S., Elangovan S., Hong L. Incorporation of copper into chitosan scaffolds promotes bone regeneration in rat calvarial defects. J Biomed Mater Res B Appl Biomater. 2015;103(5):1044–1049. doi: 10.1002/jbm.b.33290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang H., Ji Z., Xia T. Use of metal oxide nanoparticle band gap to develop a predictive paradigm for oxidative stress and acute pulmonary inflammation. ACS Nano. 2012;6(5):4349–4368. doi: 10.1021/nn3010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Studer A.M., Limbach L.K., Van Duc L. Nanoparticle cytotoxicity depends on intracellular solubility: comparison of stabilized copper metal and degradable copper oxide nanoparticles. Toxicol Lett. 2010;197(3):169–174. doi: 10.1016/j.toxlet.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 60.Jo H.J., Choi J.W., Lee S.H. Acute toxicity of Ag and CuO nanoparticle suspensions against Daphnia magna: the importance of their dissolved fraction varying with preparation methods. J Hazard Mater. 2012;227–228:301–308. doi: 10.1016/j.jhazmat.2012.05.066. [DOI] [PubMed] [Google Scholar]
- 61.Sowjanya J.A., Singh J., Mohita T. Biocomposite scaffolds containing chitosan/alginate/nano-silica for bone tissue engineering. Colloids Surf B Biointerfaces. 2013;109:294–300. doi: 10.1016/j.colsurfb.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 62.Ning C., Wang X., Li L. Concentration ranges of antibacterial cations for showing the highest antibacterial efficacy but the least cytotoxicity against mammalian cells: implications for a new antibacterial mechanism. Chem Res Toxicol. 2015;28(9):1815–1822. doi: 10.1021/acs.chemrestox.5b00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Singh R.P., Kumar S., Nada R. Evaluation of copper toxicity in isolated human peripheral blood mononuclear cells and it's attenuation by zinc: ex vivo. Mol Cell Biochem. 2006;282(1–2):13–21. doi: 10.1007/s11010-006-1168-2. [DOI] [PubMed] [Google Scholar]
- 64.Rikitake Y., Takai Y. Elsevier; 2011. Directional cell migration[M]//international review of cell and molecular biology; pp. 97–143. [DOI] [PubMed] [Google Scholar]
- 65.Alizadeh S., Seyedalipour B., Shafieyan S. Copper nanoparticles promote rapid wound healing in acute full thickness defect via acceleration of skin cell migration, proliferation, and neovascularization. Biochem Biophys Res Commun. 2019;517(4):684–690. doi: 10.1016/j.bbrc.2019.07.110. [DOI] [PubMed] [Google Scholar]
- 66.Chen M., Li R., Yin W. Copper promotes migration of adipose-derived stem cells by enhancing vimentin-Ser39 phosphorylation. Exp Cell Res. 2020;388(2):111859. doi: 10.1016/j.yexcr.2020.111859. [DOI] [PubMed] [Google Scholar]
- 67.Chen X., Hu J.-G., Huang Y.-Z. Copper promotes the migration of bone marrow mesenchymal stem cells via Rnd3-dependent cytoskeleton remodeling. J Cell Physiol. 2020;235(1):221–231. doi: 10.1002/jcp.28961. [DOI] [PubMed] [Google Scholar]
- 68.Milewska M., Burdzińska A., Zielniok K. Copper does not induce tenogenic differentiation but promotes migration and increases lysyl oxidase activity in adipose-derived mesenchymal stromal cells. Stem Cell Int. 2020;2020:9123281. doi: 10.1155/2020/9123281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Du L., Yang P., Ge S. Stromal cell-derived factor-1 significantly induces proliferation, migration, and collagen type I expression in a human periodontal ligament stem cell subpopulation. J Periodontol. 2012;83(3):379–388. doi: 10.1902/jop.2011.110201. [DOI] [PubMed] [Google Scholar]
- 70.Tripathi A., Saravanan S., Pattnaik S. Bio-composite scaffolds containing chitosan/nano-hydroxyapatite/nano-copper-zinc for bone tissue engineering. Int J Biol Macromol. 2012;50(1):294–299. doi: 10.1016/j.ijbiomac.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 71.Cukierman E., Pankov R., Stevens D.R. Taking cell-matrix adhesions to the third dimension. Science. 2001;294(5547):1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 72.Oxboel J., Schjoeth-Eskesen C., El-Ali H.H. Cu-NODAGA-c(RGDyK) is a promising new angiogenesis PET tracer: correlation between tumor uptake and integrin expression in human neuroendocrine tumor xenografts. Int J Mol Imag. 2012;2012:1–11. doi: 10.1155/2012/379807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ren N., Li J., Qiu J. Nanostructured titanate with different metal ions on the surface of metallic titanium: a facile approach for regulation of rBMSCs fate on titanium implants. Small. 2014;10(15):3169–3180. doi: 10.1002/smll.201303391. [DOI] [PubMed] [Google Scholar]
- 74.Carragher N.O., Frame M.C. Focal adhesion and actin dynamics: a place where kinases and proteases meet to promote invasion. Trends Cell Biol. 2004;14(5):241–249. doi: 10.1016/j.tcb.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 75.Strain J.J. A reassessment of diet and osteoporosis--possible role for copper. Med Hypotheses. 1988;27(4):333–338. doi: 10.1016/0306-9877(88)90016-3. [DOI] [PubMed] [Google Scholar]
- 76.Liu X., Zhao Y., Gao J. Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat Genet. 2004;36(2):178–182. doi: 10.1038/ng1297. [DOI] [PubMed] [Google Scholar]
- 77.Jalkanen S. New embo MEMBER’S review: cell surface monoamine oxidases: enzymes in search of a function. EMBO J. 2001;20(15):3893–3901. doi: 10.1093/emboj/20.15.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rodriguez J.A., Valentine J.S., Eggers D.K. Familial amyotrophic lateral sclerosis-associated mutations decrease the thermal stability of distinctly metallated species of human copper/zinc superoxide dismutase. J Biol Chem. 2002;277(18):15932–15937. doi: 10.1074/jbc.M112088200. [DOI] [PubMed] [Google Scholar]
- 79.Rodriguez J.P., Rios S., Gonzalez M. Modulation of the proliferation and differentiation of human mesenchymal stem cells by copper. J Cell Biochem. 2002;85(1):92–100. [PubMed] [Google Scholar]
- 80.Ren L., Wong H.M., Yan C.H. Osteogenic ability of Cu-bearing stainless steel. J Biomed Mater Res B Appl Biomater. 2015;103(7):1433–1444. doi: 10.1002/jbm.b.33318. [DOI] [PubMed] [Google Scholar]
- 81.Wan C., Gilbert S.R., Wang Y. Activation of the hypoxia-inducible factor-1alpha pathway accelerates bone regeneration. Proceed Nat Acad Sci U S A. 2008;105(2):686–691. doi: 10.1073/pnas.0708474105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gritsch L., Maqbool M., Mouriño V. Chitosan/hydroxyapatite composite bone tissue engineering scaffolds with dual and decoupled therapeutic ion delivery: copper and strontium. J Mater Chem B. 2019;7(40):6109–6124. doi: 10.1039/c9tb00897g. [DOI] [PubMed] [Google Scholar]
- 83.Yuan Y., Jin S., Qi X. Osteogenesis stimulation by copper-containing 316L stainless steel via activation of akt cell signaling pathway and Runx2 upregulation. J Mater Sci Technol. 2019;35(11):2727–2733. [Google Scholar]
- 84.Zhao Q., Yi L., Hu A. Antibacterial and osteogenic activity of a multifunctional microporous coating codoped with Mg, Cu and F on titanium. J Mater Chem B. 2019;7(14):2284–2299. doi: 10.1039/c8tb03377c. [DOI] [PubMed] [Google Scholar]
- 85.Chung M.H., Kesner L., Chan P.C. Degradation of articular cartilage by copper and hydrogen peroxide. Agents Actions. 1984;15(3–4):328–335. doi: 10.1007/BF01972367. [DOI] [PubMed] [Google Scholar]
- 86.Knight D.A., Weisbrode S.E., Schmall L.M. The effects of copper supplementation on the prevalence of cartilage lesions in foals. Equine Vet J. 1990;22(6):426–432. doi: 10.1111/j.2042-3306.1990.tb04310.x. [DOI] [PubMed] [Google Scholar]
- 87.Hurtig M., Green S.L., Dobson H. Correlative study of defective cartilage and bone growth in foals fed a low-copper diet. Equine Vet J. 2010;25(S16):66–73. [Google Scholar]
- 88.Yuan X., Wang J., Zhu X. Effect of copper on levels of collagen and alkaline phosphatase activity from chondrocytes in newborn piglets in vitro. Biol Trace Elem Res. 2011;144(1–3):597–605. doi: 10.1007/s12011-011-9151-5. [DOI] [PubMed] [Google Scholar]
- 89.Wang J., Zhu X., Li X. Effects of copper on proliferation and autocrine secretion of insulin-like growth factor-1 (IGF-1) and IGF-binding protein-3 (IGFBP-3) in chondrocytes from newborn pigs in vitro. Biol Trace Elem Res. 2011;144(1–3):588–596. doi: 10.1007/s12011-011-9033-x. [DOI] [PubMed] [Google Scholar]
- 90.Olney R.C., Wang J., Sylvester J.E. Growth factor regulation of human growth plate chondrocyte proliferation in vitro. Biochem Biophys Res Commun. 2004;317(4):1171–1182. doi: 10.1016/j.bbrc.2004.03.170. [DOI] [PubMed] [Google Scholar]
- 91.McQuillan D.J., Handley C.J., Campbell M.A. Stimulation of proteoglycan biosynthesis by serum and insulin-like growth factor-I in cultured bovine articular cartilage. Biochem J. 1986;240(2):423–430. doi: 10.1042/bj2400423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang J., Zhu X., Li X. Effects of copper on proliferation and autocrine secretion of insulin-like growth factor-1 (IGF-1) and IGF-binding protein-3 (IGFBP-3) in chondrocytes from newborn pigs in vitro. Biol Trace Elem Res. 2011;144(1–3):588–596. doi: 10.1007/s12011-011-9033-x. [DOI] [PubMed] [Google Scholar]
- 93.Zhu X., Wang J., Xie G. Effect of copper on the expression of TGF-beta in incubated chondrocytes of newborn pigs. Biol Trace Elem Res. 2011;143(3):1461–1469. doi: 10.1007/s12011-011-8966-4. [DOI] [PubMed] [Google Scholar]
- 94.Lin R., Deng C., Li X. Copper-incorporated bioactive glass-ceramics inducing anti-inflammatory phenotype and regeneration of cartilage/bone interface. Theranostics. 2019;9(21):6300–6313. doi: 10.7150/thno.36120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sen C.K., Khanna S., Venojarvi M. Copper-induced vascular endothelial growth factor expression and wound healing. Am J Physiol Heart Circ Physiol. 2002;282(5):H1821–H1827. doi: 10.1152/ajpheart.01015.2001. [DOI] [PubMed] [Google Scholar]
- 96.Gérard C., Bordeleau L.-J., Barralet J. The stimulation of angiogenesis and collagen deposition by copper. Biomaterials. 2010;31(5):824–831. doi: 10.1016/j.biomaterials.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 97.Raju K.S., Alessandri G., Ziche M. Ceruloplasmin, copper ions, and angiogenesis. J Nat Canc Inst. 1982;69(5):1183–1188. [PubMed] [Google Scholar]
- 98.Raju K.S., Alessandri G., Ziche M. Ceruloplasmin, copper ions, and angiogenesis. J Nat Canc Inst. 1982;69(5):1183–1188. [PubMed] [Google Scholar]
- 99.Bar-Or D., Thomas G.W., Yukl R.L. Copper stimulates the synthesis and release of interleukin-8 in human endothelial cells: a possible early role in systemic inflammatory responses. Shock. 2003;20(2):154–158. doi: 10.1097/01.shk.0000068318.49350.3a. [DOI] [PubMed] [Google Scholar]
- 100.Barralet J., Gbureck U., Habibovic P. Angiogenesis in calcium phosphate scaffolds by inorganic copper ion release. Tissue Eng. 2009;15(7):1601–1609. doi: 10.1089/ten.tea.2007.0370. [DOI] [PubMed] [Google Scholar]
- 101.Dahl S.L.M., Rucker R.B., Niklason L.E. Effects of copper and cross-linking on the extracellular matrix of tissue-engineered arteries. Cell Transplant. 2005;14(10):861–868. [PubMed] [Google Scholar]
- 102.Mitra D., Li M., Kang E.-T. Transparent copper-based antibacterial coatings with enhanced efficacy against Pseudomonas aeruginosa. ACS Appl Mater Interfaces. 2019;11(1):73–83. doi: 10.1021/acsami.8b09640. [DOI] [PubMed] [Google Scholar]
- 103.Kim T.N., Feng Q.L., Kim J.O. Antimicrobial effects of metal ions (Ag+, Cu2+, Zn2+) in hydroxyapatite. J Mater Sci Mater Med. 1998;9(3):129–134. doi: 10.1023/a:1008811501734. [DOI] [PubMed] [Google Scholar]
- 104.Li Y., Ho J., Ooi C.P. Antibacterial efficacy and cytotoxicity studies of copper (II) and titanium (IV) substituted hydroxyapatite nanoparticles. Mater Sci Eng C. 2010;30(8):1137–1144. [Google Scholar]
- 105.Rau J.V., Curcio M., Raucci M.G. Cu-releasing bioactive glass coatings and their in vitro properties. ACS Appl Mater Interfaces. 2019;11(6):5812–5820. doi: 10.1021/acsami.8b19082. [DOI] [PubMed] [Google Scholar]
- 106.Moolman M.C., Tiruvadi Krishnan S., Kerssemakers J.W.J. The progression of replication forks at natural replication barriers in live bacteria. Nucleic Acids Res. 2016;44(13):6262–6273. doi: 10.1093/nar/gkw397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Urie R., Ghosh D., Ridha I. Inorganic nanomaterials for soft tissue repair and regeneration. Annu Rev Biomed Eng. 2018;20:353–374. doi: 10.1146/annurev-bioeng-071516-044457. [DOI] [PubMed] [Google Scholar]
- 108.Wu G.-Y., Shi X., Phan H. Efficient self-assembly of heterometallic triangular necklace with strong antibacterial activity. Nat Commun. 2020;11(1):3178. doi: 10.1038/s41467-020-16940-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Borkow G., Gabbay J. Copper as a biocidal tool. Curr Med Chem. 2005;12(18):2163–2175. doi: 10.2174/0929867054637617. [DOI] [PubMed] [Google Scholar]
- 110.Avery S.V., Howlett N.G., Radice S. Copper toxicity towards Saccharomyces cerevisiae: dependence on plasma membrane fatty acid composition. Appl Environ Microbiol. 1996;62(11):3960–3966. doi: 10.1128/aem.62.11.3960-3966.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gritsch L., Lovell C., Goldmann W.H. Fabrication and characterization of copper(II)-chitosan complexes as antibiotic-free antibacterial biomaterial. Carbohydr Polym. 2018;179:370–378. doi: 10.1016/j.carbpol.2017.09.095. [DOI] [PubMed] [Google Scholar]
- 112.Neel E.A.A., Ahmed I., Pratten J. Characterisation of antibacterial copper releasing degradable phosphate glass fibres. Biomaterials. 2005;26(15):2247–2254. doi: 10.1016/j.biomaterials.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 113.Applerot G., Lellouche J., Lipovsky A. Understanding the antibacterial mechanism of CuO nanoparticles: revealing the route of induced oxidative stress. Small. 2012;8(21):3326–3337. doi: 10.1002/smll.201200772. [DOI] [PubMed] [Google Scholar]
- 114.Wang L., Li G., Ren L. Nano-copper-bearing stainless steel promotes fracture healing by accelerating. Int J Nanomed. 2017;12:8443–8457. doi: 10.2147/IJN.S146866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Prinz C., Elhensheri M., Rychly J. Antimicrobial and bone-forming activity of a copper coated implant in a rabbit model. J Biomater Appl. 2017;32(2):139–149. doi: 10.1177/0885328217713356. [DOI] [PubMed] [Google Scholar]
- 116.Li H., Li J., Jiang J. An osteogenesis/angiogenesis-stimulation artificial ligament for anterior cruciate ligament reconstruction. Acta Biomater. 2017;54:399–410. doi: 10.1016/j.actbio.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 117.Xu C., Chen J., Li L. Promotion of chondrogenic differentiation of mesenchymal stem cells by copper: implications for new cartilage repair biomaterials. Mater Sci Eng C, Mater Biol Appl. 2018;93:106–114. doi: 10.1016/j.msec.2018.07.074. [DOI] [PubMed] [Google Scholar]
- 118.Bouaziz W., Sigaux J., Modrowski D. Interaction of HIF1α and β-catenin inhibits matrix metalloproteinase 13 expression and prevents cartilage damage in mice. Proceed Nat Acad Sci U S A. 2016;113(19):5453–5458. doi: 10.1073/pnas.1514854113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Goldring M.B., Goldring S.R. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann N Y Acad Sci. 2010;1192:230–237. doi: 10.1111/j.1749-6632.2009.05240.x. [DOI] [PubMed] [Google Scholar]
- 120.Agarwal S., Loder S., Brownley C. Inhibition of Hif1α prevents both trauma-induced and genetic heterotopic ossification. Proceed Nat Acad Sci U S A. 2016;113(3):E338–E347. doi: 10.1073/pnas.1515397113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Prado J.V., Vidal A.R., Durán T.C. Application of copper bactericidal properties in medical practice. Rev Med Chile. 2012;140(10):1325–1332. doi: 10.4067/S0034-98872012001000014. [DOI] [PubMed] [Google Scholar]
- 122.Liu R., Tang Y., Zeng L. In vitro and in vivo studies of anti-bacterial copper-bearing titanium alloy for dental application. Dent Mater: Ocff Publ Acad Dental Mater. 2018;34(8):1112–1126. doi: 10.1016/j.dental.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 123.Xu W., Hou C., Mao Y. Characteristics of novel Ti-10Mo-xCu alloy by powder metallurgy for potential biomedical implant applications. Bioact Mater. 2020;5(3):659–666. doi: 10.1016/j.bioactmat.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yang J., Qin H., Chai Y. Molecular mechanisms of osteogenesis and antibacterial activity of Cu-bearing Ti alloy in a bone defect model with infection in vivo. J Orthopaed Translat. 2021;27:77–89. doi: 10.1016/j.jot.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.De Santis R., Russo T., Rau J.V. Design of 3D additively manufactured hybrid structures for cranioplasty. Materials. 2021;14(1) doi: 10.3390/ma14010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rivera L.R., Cochis A., Biser S. Antibacterial, pro-angiogenic and pro-osteointegrative zein-bioactive glass/copper based coatings for implantable stainless steel aimed at bone healing. Bioact Mater. 2021;6(5):1479–1490. doi: 10.1016/j.bioactmat.2020.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zoroddu M.A., Aaseth J., Crisponi G. The essential metals for humans: a brief overview. J Inorg Biochem. 2019;195:120–129. doi: 10.1016/j.jinorgbio.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 128.Cattalini J.P., Hoppe A., Pishbin F. Novel nanocomposite biomaterials with controlled copper/calcium release capability for bone tissue engineering multifunctional scaffolds. J R Soc Interface. 2015;12(110) doi: 10.1098/rsif.2015.0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Weng L., Boda S.K., Teusink M.J. Binary doping of strontium and copper enhancing osteogenesis and angiogenesis of bioactive glass nanofibers while suppressing osteoclast activity. ACS Appl Mater Interfaces. 2017;9(29):24484–24496. doi: 10.1021/acsami.7b06521. [DOI] [PubMed] [Google Scholar]
- 130.Wang X., Cheng F., Liu J. Biocomposites of copper-containing mesoporous bioactive glass and nanofibrillated cellulose: biocompatibility and angiogenic promotion in chronic wound healing application. Acta Biomater. 2016;46:286–298. doi: 10.1016/j.actbio.2016.09.021. [DOI] [PubMed] [Google Scholar]
- 131.Vimalraj S., Rajalakshmi S., Raj Preeth D. Mixed-ligand copper(II) complex of quercetin regulate osteogenesis and angiogenesis. Mater Sci Eng C, Mater Biol Appl. 2018;83:187–194. doi: 10.1016/j.msec.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 132.Yang L., Perez-Amodio S., Barrere-de Groot F.Y.F. The effects of inorganic additives to calcium phosphate on in vitro behavior of osteoblasts and osteoclasts. Biomaterials. 2010;31(11):2976–2989. doi: 10.1016/j.biomaterials.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 133.Gerard C., Bordeleau L.-J., Barralet J. The stimulation of angiogenesis and collagen deposition by copper. Biomaterials. 2010;31(5):824–831. doi: 10.1016/j.biomaterials.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 134.Bejarano J., Detsch R., Boccaccini A.R. PDLLA scaffolds with Cu- and Zn-doped bioactive glasses having multifunctional properties for bone regeneration. J Biomed Mater Res. 2017;105(3):746–756. doi: 10.1002/jbm.a.35952. [DOI] [PubMed] [Google Scholar]
- 135.Zhang J., Wu H., He F. Concentration-dependent osteogenic and angiogenic biological performances of calcium phosphate cement modified with copper ions. Mater Sci Eng C, Mater Biol Appl. 2019;99:1199–1212. doi: 10.1016/j.msec.2019.02.042. [DOI] [PubMed] [Google Scholar]