Highlights
-
•
UBE2A deficiency syndrome causes X-linked intellectual disability and epilepsy.
-
•
Our patient with super-refractory status epilepticus failed conventional therapy.
-
•
He responded to the ketogenic diet with a long-term favourable outcome.
-
•
Our report should prompt consideration of the ketogenic diet for these conditions.
Keywords: Status Epilepticus, Intensive Care, Neurogenetics
Abstract
The ketogenic diet (KD) may have a role in treating super-refractory status epilepticus (SRSE). Predominantly used in paediatric epilepsy, there are few reports of its use in adults. We describe a 19-year-old man with UBE2A deficiency syndrome, drug resistant generalized epilepsy, and severe intellectual disability, who developed SRSE. Initiation of the KD on day 81 of his intensive care unit stay and achieving a state of ketosis seven days later resulted in SRSE resolution and discharge from hospital and recovery to his normal cognitive state. Initiating the KD required a multidisciplinary team for diet initiation and carer education. The KD requires a prospective study of efficacy for SRSE and this should include adult patients.
1. Introduction
Super-refractory status epilepticus (SRSE) is defined as status epilepticus that continues in spite of standard first and second line anti-seizure treatments, and at least 24 hours of anaesthesia, or upon withdrawal of anesthesia.[1] UBE2A deficiency, first described in 2006[2] causes an X-linked moderate to severe intellectual disability, genetic generalized epilepsy, speech impairment, dysmorphic facial features and skin abnormalities.[3] Ubiquitin conjugating enzyme E2A is involved in the ubiquitin protease pathway, post-replication DNA damage repair and regulation of transcription. How its deficiency causes epilepsy has yet to be elucidated.[4], [5]
2. Case presentation
A 19-year-old man with a background of surgically corrected coarctation of the aorta and a ventricular septal defect, severe learning disability and drug-resistant generalized epilepsy presented with SRSE. During his admission, whole exome sequencing revealed the cause of his intellectual disability and epilepsy to be UBE2A deficiency syndrome caused by a p.Asp101del mutation. At presentation, he had not experienced tonic-clonic seizures for some years and had never experienced status epilepticus before. His usual seizure types were daily absences and myoclonus, a few tonic seizures per week and rare tonic-clonic seizures. He was fully ambulatory but used a wheelchair when outdoors due to his tonic attacks and refusal to wear a helmet. His behavior could be challenging and obstinent. He lived with his family and communicated using signing (Makaton) and single words. He was taking lamotrigine, levetiracetam, clobazam and zonisamide. Sodium valproate had been tried but discontinued because of aggression. He had a vagus nerve stimulator (Model 106, Aspire SR; LivaNova) and used buccal midazolam as a rescue anti-seizure medication. Brain MRI had been performed at 19 months and 18 years. These demonstrated numerous non-progressive subcortical and periventricular white matter T2 hyperintensities, a 1.3 cm complex pineal lesion and progressive cerebral and cerebellar atrophy.
He was admitted to our hospital in generalized tonic-clonic status epilepticus. He was treated in the intensive care unit with intravenous valproate loading, followed by intubation and sedation with midazolam, propofol and later thiopental, (Fig. 1) in order to cause burst-suppression on EEG. Chest x-ray and sputum cultures confirmed a community acquired pneumonia. Extensive investigation, including screening for an alternative infection, brain imaging (both CT and MRI) and CSF analysis were unremarkable.
Fig. 1.
Treatments used during intensive care admission. Anti-seizure medication and anesthetic agents used during intensive care unit admission.
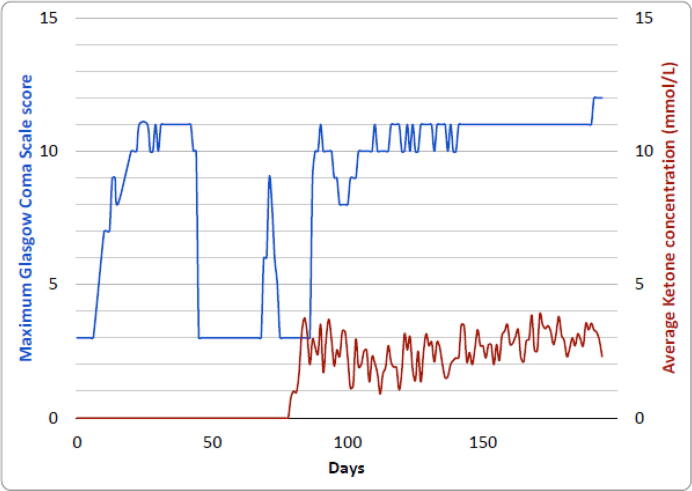
Each time sedation was reduced, he continued to have either generalized tonic or tonic-clonic seizures or generalized epileptiform activity on EEG, fulfilling the definition of SRSE. Phenytoin, rufinamide, acetazolamide and methylprednisolone were tried without success. On day 81 of his admission, as there had been no resolution of his SRSE a classic ketogenic diet (KD) was initiated. Seven days later he reached a state of ketosis; a mean ketone concentration ≥ 2 mmol/L (Fig. 2) and a reduction in seizure frequency was seen clinically and confirmed using EEG. All convulsive seizures ceased though absences and rare tonic seizures remained. Intravenous midazolam infusion was weaned and he regained consciousness. Initiating the KD required a multidisciplinary team to provide prolonged intensive care, specialized nutritional advice, gastrostomy tube placement, care-giver education, and screening of prescription medications for suitability. Weaning from his tracheostomy was prolonged due to acquired critical illness polyneuropathy. On day 196 he was discharged from the intensive care unit to the neurology ward. He was successfully discharged to a supported living facility on the KD and has remained compliant via his gastrostomy. The only long-term consequence was leg weakness due to polyneuropathy. A further episode of pneumonia 18 months later led to clusters of tonic seizures over 72 hours that were rapidly controlled with intravenous valproate. At two years he has remained free of generalized tonic-clonic seizures but had occasional absences persist. He remains on a higher number of anti-seizure medications than at baseline. Attempts to reduce his polypharmacy are planned to be undertaken in future if there is sustained stability. He returned to his baseline cognitive state.
Fig. 2.
Maximum daily Glasgow Coma Score and average ketone concentration during intensive care admission. The maximum Glasgow Coma Score (blue) achieved each day during intensive care admission displayed against the average ketone concentration (red).
3. Discussion
The first published use of the KD in an adult with SRSE was in 2008[6] and following several promising case series, a small (n = 15) prospective multicenter trial was performed in the United States.[7] This showed 79% of those completing the KD achieved resolution of SRSE, although mortality in this group was still 33%. The KD does not contribute to hemodynamic instability seen with anesthetic agents. There may be additional neuroprotective benefits related to improved mitochondrial function due to increased energy reserves combined with decreased production of reactive oxygen species.[8] Contraindications include propofol infusion for anesthesia,[9] carnitine or pyruvate carboxylase deficiency, a deficiency of fatty acid metabolism and porphyria. Relative contraindications include inability to maintain adequate nutrition and a surgical focus identified by neuroimaging.[10] A KD trained dietitian is needed for initiation and long-term monitoring. Input from a pharmacist is required. Many medicines, such as potassium supplements, contain enough carbohydrate to prevent ketosis.
The use of whole exome sequencing detected UBE2A deficiency syndrome in this case. The mechanism that links its deficiency to epilepsy has yet to be elucidated. We speculate that KD may have worked in this case by improving mitochondrial function as UBE2A deficiency impairs the clearance of dysfunctional mitochondria.[4]
The KD is now included in several adult SRSE treatment algorithms [1], [11], [12] despite the current lack of evidence. This uncommon condition has a high mortality and further prospective studies are required to advance our understanding of available treatments. In our case, adjunctive use of the KD led to an immediate and sustained recovery of a critically ill patient with a favourable outcome.
4. Conclusion
The ketogenic diet, as part of polytherapy, successfully treated our patient with UBE2A deficiency syndrome and SRSE. We propose it should be considered as an option for other patients with treatment resistant epilepsy secondary to UBE2A deficiency syndrome. Additional prospective trials may support its efficacy in the treatment of adult convulsive super-refractory status epilepticus associated with UBE2A.
Ethical approval information
Research ethics approval not required.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We would like to thank all members of the clinical team whose hard work contributed to our patient surviving their critical illness.
Funding info
There is no funding to report for this article.
References
- 1.Shorvon S., Ferlisi M. The treatment of super-refractory status epilepticus: a critical review of available therapies and a clinical treatment protocol. Brain: J Neurol. 2011;134(10):2802–2818. doi: 10.1093/brain/awr215. [DOI] [PubMed] [Google Scholar]
- 2.Nascimento R.M.P., Otto P.A., de Brouwer A.P.M., Vianna-Morgante A.M. UBE2A, which encodes a ubiquitin-conjugating enzyme, is mutated in a novel X-linked mental retardation syndrome. Am J Hum Genet. 2006;79(3):549–555. doi: 10.1086/507047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cordeddu V., Macke E.L., Radio F.C., Lo Cicero S., Pantaleoni F., Tatti M. Refinement of the clinical and mutational spectrum of UBE2A deficiency syndrome. Clin Genet. 2020;98(2):172–178. doi: 10.1111/cge.13775. [DOI] [PubMed] [Google Scholar]
- 4.Haddad D., Vilain S., Vos M., Esposito G., Matta S., Kalscheuer V. Mutations in the intellectual disability gene Ube2a cause neuronal dysfunction and impair parkin-dependent mitophagy. Mol Cell. 2013;50(6):831–843. doi: 10.1016/j.molcel.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 5.Bruinsma C.F., Savelberg S.M.C., Kool M.J., Jolfaei M.A., Van Woerden G.M., Baarends W.M. An essential role for UBE2A/HR6A in learning and memory and mGLUR-dependent long-term depression. Hum Mol Genet. 2016;25(1):1–8. doi: 10.1093/hmg/ddv436. [DOI] [PubMed] [Google Scholar]
- 6.Bodenant M., Moreau C., Sejourne C., Auvin S., Delval A., Cuisset J.M. Interest of the ketogenic diet in a refractory status epilepticus in adults. Revue neurologique. 2008;164(2):194–199. doi: 10.1016/j.neurol.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Cervenka M.C., Hocker S., Koenig M., Bar B., Henry-Barron B., Kossoff E.H. Phase I/II multicenter ketogenic diet study for adult superrefractory status epilepticus. Neurology. 2017;88(10):938–943. doi: 10.1212/WNL.0000000000003690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDonald T.J.W., Cervenka M.C. Ketogenic Diets for Adults With Highly Refractory Epilepsy. Epilepsy Currents. 2017;17(6):346–350. doi: 10.5698/1535-7597.17.6.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baumeister F.A.M., Oberhoffer R., Liebhaber G.M., Kunkel J., Eberhardt J., Holthausen H. Fatal propofol infusion syndrome in association with ketogenic diet. Neuropediatrics. 2004;35(4):250–252. doi: 10.1055/s-2004-820992. [DOI] [PubMed] [Google Scholar]
- 10.Kossoff EH, Zupec-Kania BA, Amark PE, Ballaban-Gil KR, Christina Bergqvist AG, Blackford R, et al. Optimal clinical management of children receiving the ketogenic diet: recommendations of the International Ketogenic Diet Study Group. Epilepsia; 2009;50(2):304-17. [DOI] [PubMed]
- 11.Bayrlee A., Ganeshalingam N., Kurczewski L., Brophy G.M. Treatment of Super-Refractory Status Epilepticus. Curr Neurol Neurosci Rep. 2015;15(10):66. doi: 10.1007/s11910-015-0589-2. [DOI] [PubMed] [Google Scholar]
- 12.Holtkamp M. Pharmacotherapy for Refractory and Super-Refractory Status Epilepticus in Adults. Drugs. 2018;78(3):307–326. doi: 10.1007/s40265-017-0859-1. [DOI] [PubMed] [Google Scholar]