Abstract
In many low- and middle-income countries, providers without formal training are an important source of antibiotics, but may provide these inappropriately, contributing to the rising burden of drug resistant infections. Informal providers (IPs) who practise allopathic medicine are part of India's pluralistic health system legacy. They outnumber formal providers but operate in a policy environment of unclear legitimacy, creating unique challenges for antibiotic stewardship. Using a systems approach we analysed the multiple intrinsic (provider specific) and extrinsic (community, health and regulatory system and pharmaceutical industry) drivers of antibiotic provision by IPs in rural West Bengal, to inform the design of community stewardship interventions. We surveyed 291 IPs in randomly selected village clusters in two contrasting districts and conducted in-depth interviews with 30 IPs and 17 key informants including pharmaceutical sales representatives, managers and wholesalers/retailers; medically qualified private and public doctors and health and regulatory officials. Eight focus group discussions were conducted with community members. We found a mosaic or bricolage of informal practices conducted by IPs, qualified doctors and industry stakeholders that sustained private enterprise and supplemented the weak public health sector. IPs' intrinsic drivers included misconceptions about the therapeutic necessity of antibiotics, and direct and indirect economic benefits, though antibiotics were not the most profitable category of drug sales. Private doctors were a key source of IPs' learning, often in exchange for referrals. IPs constituted a substantial market for local and global pharmaceutical companies that adopted aggressive business strategies to exploit less-saturated rural markets. Paradoxically, the top-down nature of regulations produced a regulatory impasse wherein regulators were reluctant to enforce heavy sanctions for illegal sales, fearing an adverse impact on rural healthcare, but could not implement enabling strategies to improve antibiotic provision due to legal barriers. We discuss the implications for a multi-stakeholder antibiotic stewardship strategy in this setting.
Keywords: Informal private providers, India, Rural, Antibiotic provision, Antibiotic stewardship, Community settings, Drivers, Antimicrobial resistance
Highlights
-
•
IPs are a key source of healthcare and antibiotics in rural India.
-
•
Informal antibiotic practices cut across IPs, doctors and pharmaceutical industry.
-
•
Multiple intrinsic and extrinsic drivers influence IPs' antibiotic practices.
-
•
Regulations are difficult to enforce but create barriers for stewardship solutions.
-
•
Efforts to strengthen antibiotic stewardship must target multiple stakeholders.
1. Background
Many low- and middle-income countries (LMICs) face a high burden of infectious diseases (GBD, 2018) along with high rates of antimicrobial resistance (AMR) (Klein et al., 2019). Though per capita antibiotic consumption in LMICs is lower than in high income countries-suggesting that many people lack access to essential antibiotics – between 2000 and 2015 antibiotic consumption increased by 77% in LMICs while it decreased by 4% in high income countries (Klein et al., 2018). Over-use and misuse of antibiotics is a major driver of AMR (Holmes et al., 2016), a grave public health threat that could reverse the gains in mortality and morbidity of the last century and increase the costs of healthcare (WHO, 2015), with significant social and economic implications for LMICs. It is estimated that 700,000 people die of drug resistant infections every year, and this could increase to 10 million by 2050 if the rise in drug resistance continues (O'Neill, 2016). In addition, by 2050, annual global gross domestic product (GDP) could fall by 3.8%, with poorer countries being worst affected due to higher infectious disease prevalence and greater declines in economic growth (World Bank, 2017).
Antibiotic regulations restrict their prescription to qualified medical prescribers (WHO, 2018a), but many people across LMICs obtain antibiotics without a prescription from pharmacies, family and friends (Morgan et al., 2011) or a variety of ‘informal’ sources (Bloom et al., 2015). In South Asia a key component of these ‘informal’ providers are practitioners who provide consultation services in small ‘clinics’, and dispense and/or prescribe allopathic medicines (Gautham et al., 2013). Various terms are used for these practitioners including rural medical practitioners in India (Nahar et al., 2016) and ‘village doctors’ in Bangladesh (Mahmood et al., 2010). The term informal provider can also be used to refer to a wider range of actors, including unregistered pharmacies, itinerant drug sellers and traditional healers (Sudhinaraset et al., 2013), but in this study we focus on informal rural medical practitioners in India.
While the informal provider (IP) term is widely used within India and in global health discourse more broadly, we acknowledge its problematic nature. The notion of informality as a group of people or organisations within a given sector or territory (e.g. slum populations), or that lacks state authorization, has been challenged by scholars in urban studies (Roy, 2005; McFarlane, 2012). They have argued for a broader definition of informality as forms of practice that are irregular and illegal, whoever these practices are conducted by. For example, McFarlane explains that different hybrids of informal and formal practices enable urban development, like violation of land laws by the legal elite to build townships. Further, Roy argues that the state's own planning apparatus produces informality through its processes of formalization as these can displace and make illegitimate the most vulnerable and powerless. In the health sector, the institutionalization of India's healthcare and medical education system in the 1940s de-institutionalized and made illegitimate large numbers of medical licentiates and indigenous practitioners (predecessors of the present day IPs) without creating an alternative legal, pedagogical or service delivery architecture to meet the country's health needs (Gautham and Shyamprasad, 2010).
So-called ‘IPs’ have therefore continued to exist and meet primary healthcare needs over several decades (Leslie, 1976; Rohde JE and Vishwanathan, 1995). In rural India, they are frequently the first port of call for healthcare and medicines, where access to medically qualified doctors and pharmacies is very limited (Gautham et al., 2013; Sabde et al., 2011).
It is difficult to estimate the total number of IPs in India, though they are known to vastly outnumber medically qualified doctors (Rao et al., 2016). A WHO health workforce analysis (Anand and Fan, 2016) found that 57% of 632,434 self-defined “allopathic doctors” all over India, and 81% in rural areas, did not have a medical qualification. Das et al.‘s survey of 1519 villages across 19 states is the largest scale survey of rural providers in India. They surveyed 3473 providers of whom 68% were IPs, 24% were AYUSH providers (collective term for practitioners qualified in Ayurveda/Yoga and Naturopathy/Unani/Siddha or Homeopathy) and only 8% had an MBBS degree (the undergraduate medical degree in India) (Das et al., 2020). Data on care seeking from smaller studies show that IPs were the first providers sought by 91% patients in Uttarakhand, 82% in Andhra Pradesh and 43% in Karnataka, for acute illnesses such as diarrhoea, fevers and respiratory tract infections (Gautham et al., 2013; George and Iyer, 2013). Primary care currently comprises 41% of private out of pocket household expenditure on health in India (42% is on secondary and 16% on tertiary care) (GOI, 2019) and an overall 68% of outpatient care in rural areas and 74% in urban, is sought in the private sector (GOI, 2020).
IPs operate in an environment of unclear legitimacy, reflecting the ambiguous and contradictory laws in this area. According to the Indian Medical Council Act, 1956 (GOI, 1956) IPs are illegal. However, the Indian Drugs and Cosmetics Act (GOI, 2016) distinguishes between prescription drugs (Schedules H and H1) and non-prescription drugs that can be sold over-the-counter. It could be argued that IPs could legally provide the latter, and in fact a few non-governmental organisations offer courses in primary care restricted to non-prescription drugs (IRMA, 2020; CIPSACADEMY, 2020). It is generally clear that it is illegal for IPs to prescribe or dispense antibiotics, all of which are prescription-only medicines. However, the practice of dispensing a variety of antibiotics, especially broad-spectrum ones, is common to IPs in different parts of India (Nair et al., 2019a; Khare et al., 2019) and is of particular concern for AMR containment in community settings.
A few programmes engaging with IPs through training and social franchising have been evaluated, demonstrating improvements in knowledge (Mohanan et al., 2017) and case management (Das et al., 2016), but not in inappropriate antibiotic dispensing. To address this, an in-depth understanding of the drivers of these practices is required. While there is plentiful evidence that qualified physicians' prescribing practices are driven by multiple factors including deficits in diagnostic knowledge, prescribing cultures, patients' expectations, financial incentives from drugs and the influence of pharmaceutical representatives (Xue et al., 2019; Charani et al., 2013; Broom et al., 2014; Kotwani et al., 2010; Om et al., 2016; Li et al., 2012), there is very limited evidence about IPs’ antibiotic use (Nair et al., 2019a, 2019b).
We address this knowledge gap by conducting a comprehensive analysis of the intrinsic and extrinsic drivers of IPs’ antibiotic provision in rural West Bengal. We expand our enquiry beyond the IPs themselves to include the perspectives of the communities they serve, actors in the formal health and regulatory system, and pharmaceutical industry stakeholders. Our intention is to provide a firm basis for the design of antibiotic stewardship interventions at the community level.
2. Methods
2.1. Conceptual framework
AMR has been described as a health systems problem, arising from and in turn affecting health systems adversely (Tomson and Vlad, 2014; Ahmad et al., 2019). We agree that a health systems approach, that emphasizes a holistic view of the system and its constituents, is fundamental to understanding the drivers of IPs' antibiotic use. We draw on the framework developed by Rodrigues et al. (2013) which distinguishes between intrinsic drivers or factors (coming from the provider themselves) and extrinsic factors (external influences on the provider). Intrinsic factors include physicians' sociodemographic characteristics, knowledge, and attitudes such as fear of complications and of losing patients if they are not cured. Extrinsic factors include patient-related factors (like patients' expectations and presenting clinical signs and symptoms), healthcare system-related factors (like policies, guidelines and diagnostic support), and the influence of pharmaceutical companies including financial incentives. The framework also emphasizes interactions within and between the factors, such as the influence of pharmaceutical marketing on physician's knowledge that may shape their attitudes.
2.2. Setting
West Bengal is India's fourth most populous state with 91 million inhabitants (GOI, 2011) and a per capita net state domestic product of US$1,333, slightly lower than the average for India (RBI, 2018). We selected two contrasting districts: Birbhum, a landlocked district in the north, and South 24Parganas (S24P) that extends south of Kolkata and includes remote villages in the tidal mangrove forests of the Sundarbans. 87% of Birbhum's 3.5 million population and 74% of S24P's 8.2 million population was rural (GoWB, 2014a; GoWB, 2014b). S24P ranked above Birbhum on a state level human development ranking (GOWB, 2009a; GOWB, 2009b). There were 91 public sector primary (first level at which a medically qualified doctor with an MBBS degree should be available) and secondary facilities in S24P and 77 in Birbhum (GOI, 2017), giving a population per facility of 66,371 in S24P and 39,572 in Birbhum. India's norm is 1 primary facility for 30,000 population (GOI, 2014-15).
2.3. Data collection
We used a sequential, explanatory mixed methods approach (Creswell et al., 2003), starting with focus group discussions (FGDs) with community members and a structured survey with IPs, followed by in-depth interviews (IDIs) with IPs and other stakeholders. The results primarily draw on the qualitative data from IDIs and FGDs and these are presented in a qualitative style, supplemented by quantitative survey data to characterise the providers and their antibiotic provision.
As there was no existing sampling frame of IPs we adopted a census approach by surveying every IP within randomly selected clusters (O'Connell et al., 2013). A cluster was an administrative grouping of villages called ‘gram-panchayat’, of which there were 310 in S24P and 167 in Birbhum with an estimated 15 IPs per gram-panchayat in S24P and 7–8 in Birbhum. A sample size of 150 IPs per district was calculated, conservatively assuming 50% prevalence of indicators of interest at 95% confidence interval and 10% margin of error, with a design effect of 1.5. To obtain this sample size, 11 grampanchayats in S24P and 7 adjacent pairs of gram panchayats in Birbhum were randomly selected. IPs were defined as unqualified providers who provided consultation services and dispensed/prescribed drugs. Retail pharmacies and government community health workers were excluded. We consulted IP associations and village key informants to identify all IPs in the study sites.
The survey tool explored IPs’ demographic characteristics, education and training, service provision, knowledge of antibiotics and resistance, patterns of antibiotic provision, patient characteristics and interactions with the pharmaceutical industry, health department and medically qualified allopathic doctors (we use the term doctor to refer to both graduate (with an MBBS degree) and post graduate (with an MD degree) physicians in either the public or private sector). The tool (in Bengali) was piloted twice and the survey conducted during February to May 2017 by trained researchers supervised by the PI and study coordinator.
IDIs were conducted with 15 IPs per district (30 in total), purposively sampled to represent variation in their antibiotic provision, geographical location, and mode of dispensing and/or prescribing drugs. The IDIs explored IPs’ motivations for antibiotic provision and details of their interactions with pharmaceutical, regulatory and formal sector actors. The topic guide was piloted, and interviews conducted from May to August 2017. IDIs were also conducted with 17 other stakeholders, identified through snowballing: five public and private doctors, eight pharmaceutical representatives including managers, sales representatives and wholesalers who supplied antibiotics to IPs, three regulatory and health department officials and one leader of an IP association. Community perceptions were obtained through eight FGDs (four in each district: two with men and two with women), with 8–12 participants each. FGDs and IDIs were conducted in private spaces to promote confidentiality and each lasted 60–90 min.
2.4. Data management and analysis
Survey data were entered in Excel and analysed descriptively using Stata IC 14.2. IDIs and FGDs were audio-recorded, except for seven IDIs where consent to audio-recording was not granted; detailed notes were taken for these. Transcripts and field notes were translated into English. The PI and study coordinator developed a coding tree reflecting the intrinsic and extrinsic thematic areas (a deductive approach) as well as themes emerging from the data (an inductive approach) (Creswell et al., 2003). To strengthen validity, data were coded separately by pairs of researchers and then compared, and initial findings were shared with small groups of IPs, pharmaceutical representatives and health department officials for feedback. Key themes, concepts and emergent categories were analysed using the framework approach, and interpreted in the broader health systems context (Spencer et al., 2003).
Ethical approval was obtained from the London School of Hygiene and Tropical Medicine the Ethics Committee and the Institutional Review Board of the Centre for Media Studies (CMS-IRB), New Delhi, India. Signed consent was obtained from survey and IDI interviewees and verbal consent audio-recorded for FGDs.
3. Results
3.1. Overview of IPs and their antibiotic provision
We identified 326 IPs in the 25 grampanchayats: 151 in Birbhum and 175 in S24P. All were invited to participate in the survey, with eight declining to be interviewed and 15 unavailable. Of the 303 surveyed, 291 (96%) practised allopathic medicine and 12 (4%) practiced only homeopathy. Our antibiotics survey data were collected from the 291 allopathy practitioners, of whom 55% only practised allopathy while the rest practised a blend of allopathic and traditional medical systems, including Ayurveda (30%), homeopathy (6%) and Unani (2%) (Table 1). Of the 291, 75% had paramedical certifications such as certificates of Rural Medical Practice, Community Medical Service, diplomas in ‘alternative medicine’ and others. Six had a certificate in pharmacy, four had completed lab assistants' courses and three dental assistants' courses.
Table 1.
IPs’ background and services.
| Characteristics | N = 291 | |
|---|---|---|
| Gender | Male | 98% |
| Age | ≤35 years | 21% |
| 36–45 years | 32% | |
| 46–55 years | 24% | |
| >55 years | 23% | |
| Religion | Hindu | 65% |
| Muslim | 35% | |
| School education | Up to class 10 | 31% |
| Up to class 12 | 35% | |
| Graduate or postgraduate | 34% | |
| Any health certification | 75% | |
| Worked as a compounder to formal doctors | 84% | |
| Years of practice | ≤10 years | 29% |
| 10.1–20 years | 36% | |
| >20 years | 35% | |
| Operate out of a small clinic | 99% | |
| Practice in more than one clinic | 20% | |
| Other source of income | 37% | |
| Dispense antibiotics | 95% | |
| Prescribe antibiotics |
88% |
|
| System of medicine practised | ||
| Only allopathy | 55% | |
| Allopathy and Ayurveda | 30% | |
| Allopathy and Homeopathy | 6% | |
| Allopathy and Unani | 2% | |
| Allopathy, Ayurveda, Homeopathy | 3% | |
| Allopathy, Ayurveda, Unani | 2% | |
| Allopathy, Ayurveda, Unani, Homeopathy |
2% |
|
| Health services | ||
| Outpatient care | 97% | |
| Inpatient care | 16% | |
| Diabetes | 66% | |
| Hypertension | 90% | |
| Dental care | 91% | |
| Eye care | 86% | |
| Wound suturing | 89% | |
| Small surgeries (e.g. draining an abscess) | 78% | |
| Piles | 6% | |
| Delivery care | 23% | |
| Abortions | 19% | |
| Animal healthcare (mainly cattle and poultry) | 34% | |
| Mean number of patients out of every ten who belong to the lowest socio-economic groups (daily wage workers) | 7/10 | |
| Mean number of patients out of every ten who come from villages within 5 kms | 8/10 | |
Nearly all IPs surveyed (98%) were male (Table 1). Around 70% had over 10 years of schooling and 34% reported sufficient literacy to read medical books. None had yet received the state government's training that was launched just before this study, but 18% had been trained through a precursor training by a local organisation. Ninety-nine percent practised out of small clinics that differed in room space and building material, but resembled the clinics of formal doctors in that they were equipped with a table, chair and a patient's stool, a curtained off examination area, a waiting area with benches, equipment like a stethoscope and blood pressure monitor and medicines displayed in a cabinet. For the majority, this was their sole source of income, but 37% had other sources including agriculture. Ninety-seven percent of IPs reported providing outpatient care for fever, diarrhoea and cold/cough but 16% also provided inpatient care. Many reported providing services for hypertension (90%), diabetes (66%), dental care (91%) and eye care (86%). They saw a daily average of 32 patients in Birbhum and 22 in S24P. In both districts on average IPs reported that seven out of every ten patients were from the lowest socio-economic groups and engaged in daily wage labour. Eight out of every ten patients came from nearby villages, from within a distance of 5 kms.
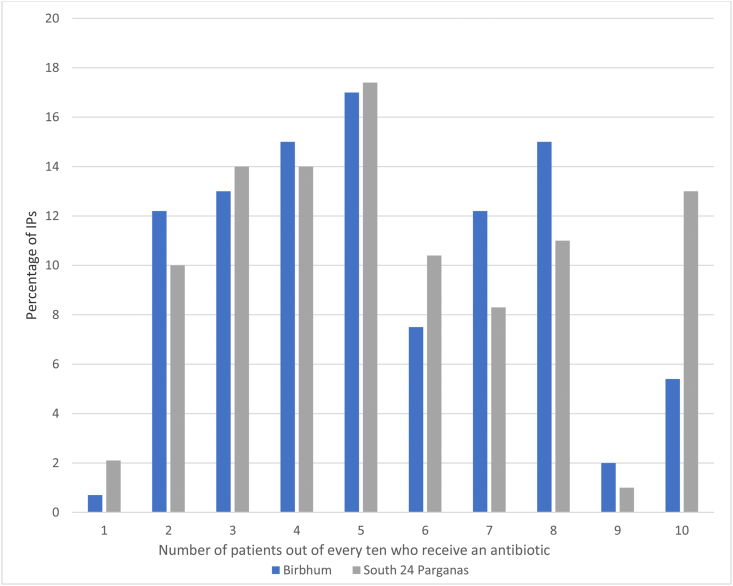
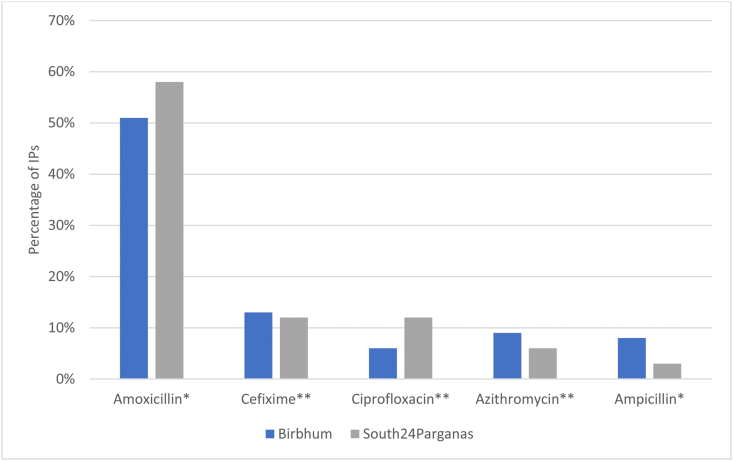
IPs typically dispensed antibiotics (reported by 95%) and charged for these, rather than the consultation alone; 88% also prescribed. From the qualitative interviews we learned that IPs prescribed when they had no stock or when the patient could not pay for the full course (in which case a partial amount was dispensed and the rest prescribed). A majority (75%) of the surveyed IPs dispensed at least one antibiotic to more than three patients out of every ten (Fig. 1), and an average of five patients out of every ten. Amoxicillin was dispensed most frequently, followed by cefixime, ciprofloxacin, azithromycin and ampicillin (Fig. 2). By WHO's AWARE classification of antibiotics (ACCESS, WATCH, RESERVE) (WHO, 2019), amoxicillin and ampicillin are ACCESS antibiotics that should be widely available. The others belong to the WATCH category and should be monitored due to their higher risk of selection for resistance. Two IPs had tried linezolid, a RESERVE category antibiotic, on patients not responding to other drugs.
Fig. 1.
Number of patients out of every ten daily patients who receive an antibiotic.
Fig. 2.
Most commonly dispensed/prescribed antibiotics by IPs (self-reported). *ACCESS antibiotics **WATCH antibiotics (WHO, 2019).
Both the survey and IDIs suggested that on average, two to three-day courses of antibiotics were dispensed, usually as tablets and sometimes as injectables for severe cases or for those who did not respond to oral drugs.
3.2. Intrinsic drivers
Qualitative findings from the IDIs suggested that IPs' intrinsic drivers stemmed from an interplay between limited knowledge about antibiotics and their economic interests. Believing that antibiotics could cure most illnesses, they considered them indispensable for primary care. The choice and dosage of antibiotics was based on IPs' experiences of the effectiveness of different antibiotics for specific symptoms. They felt confident with some degree of experimentation. For example, one IP said that if a patient had visited another provider before coming to him, he would give him a ‘double antibiotic’ (two different antibiotics) for quick recovery. Some said they might extend the course to 10 or 15 days if a shorter course did not work, and others said they would change the antibiotic if required:
If I see that a medicine is not working, I twist it and give it to the patient. Twisting means if I see that amoxicillin is not working, I give ciprofloxacin. If ciprofloxacin doesn't work, I give ofloxacin... (IPS11010102)
Behind this arbitrary antibiotic use lay several knowledge gaps. For example, the survey data showed that only 30% of IPs knew that antibiotics cannot cure viral infections and only 35% correctly associated resistance with bacteria rather than the human body (see Supplementary Table S1). Their understanding of resistance was limited to simple inferences like ‘antibiotics are becoming the food of the bacteria instead of killing them’ (IPS08050303) or ‘patients stopping antibiotics makes them resistant’ (IPS08050303). Only 41% were aware of any laws about the sale and use of antibiotics. In the IDIs, most spoke openly about using antibiotics and saw nothing wrong or unlawful about this ‘basic’ practice: ‘I provide basic treatment. In case of fever with shivering, or normal fever we give medicine for two three days…amoxicillin or cc efixime’ (IPB02130501). Many IPs spoke proudly about their knowledge of a variety of antibiotics.
Believing antibiotics to be a therapeutic necessity, IPs feared that ‘without antibiotics, patients will not be cured, and they will not visit me again’ (IPS06080405), which would adversely affect their patient footfall and income. In the IDIs, IPs estimated that antibiotic sales contributed 20%–30% to their incomes, through the margins between the retail price (paid by patients) and the price paid by IPs to wholesalers/retailers. The most expensive branded antibiotics and the cheapest ones were both said to provide high profit margins. However, many IPs said that the most expensive were beyond the paying capacity of patients, while the cheapest products could be poor quality and ineffective, damaging their reputation amongst patients. Hence, they preferred modestly priced brands even if they were less profitable, in relative or absolute terms. Antibiotics however were said to be less profitable than some other medicines such as vitamins, antacids and analgesics.
3.3. Extrinsic drivers
3.3.1. Patients’ physical and economic needs
Community members participating in FGDs displayed mixed awareness of antibiotics, indeed some had not heard of them, and we did not find much evidence of people demanding antibiotics when ill. However, most people did need to recover quickly due to the precarious daily wage nature of their work, and at a cost that was affordable. They relied on IPs as a primary source of healthcare and antibiotics: ‘Because Siuiri [town] is too far for me (30 kms) and you cannot travel that long with your sick child…we visit this ‘kaku’ [uncle-an IP] for primary treatment’ (Women's FGD2, Birbhum).
IPs had to live up to more than expectations of effective treatment; they also had to be responsive to each patient's paying capacity, which in turn determined the choice of antibiotic and how much of the course was dispensed, resulting frequently in sub-optimal courses. However, some FGD participants said they preferred to buy antibiotics from IPs and pharmacies rather than receive them free at government facilities:
‘It is a belief that whatever (medicines) we get free of cost or at lower cost from government ‘fair price shop’ [public-private partnership pharmacies at bigger government health facilities]… are less effective than those we purchase from the market or from ‘local doctors’ (IPs).’ (Men's FGD2, S24P)
Other informal practices reported by community members included self-medication and arbitrary dosing (by those who knew a little about antibiotics) and not completing the full course. Several female and male FGD participants said they stopped taking an antibiotic when they started feeling better, though they had heard antibiotics should be taken for 5–7 days. Some women said that while their children and husbands often completed the full course, they themselves did not, in order to save money. Other reasons for stopping antibiotics mid-course included side-effects such as nausea and loss of appetite. Two FGD participants explained that allopathic medicines generated heat in the body, a perception consistent with traditional, non-biomedical explanatory models of illnesses linked to different types of foods and seasonality (Payyappallimana and Venkatasubramanian, 2016).
3.3.2. Economic and social relationships with medically qualified doctors
IDIs revealed that medically qualified doctors, with MBBS as well as post graduate specialisations and especially private ones, were an important influence on IPs' treatment practices, reflecting their economic interdependence. IPs received lifelong informal training from their doctor mentors whom they referred to as their ‘gurus’ [teachers]For example, 84% of IPs surveyed reported having worked as a doctor's compounder or assistant, after which the doctor continued to mentor them. Some IPs said that when in doubt, they consulted doctors they knew, who advised them ‘which medicine to give after what and what should be the dosage based on patient's details' (IPS11010102). For example, one IP received this advice for treating a patient with high fever who was not responding to an anti-malarial:
‘The doctor Sir told me to give small amount of Monocef [3rd generation cephalosporin antibiotic] injection on the layer of his skin to check whether the patient has any allergy or not. If it is found okay, then apply the full dosage.’ (IPS06080101)
IP associations invited local doctors to lecture to their association members, and doctors attached to private hospitals in urban areas were said to train on emerging noncommunicable conditions, including cancers, heart conditions and diabetes. One IP said:
‘They said… If you see that the BP [blood pressure] is high, give necessary medicine… which a doctor can only give, but if we see a patient with nausea, high blood pressure, myocardial infarction.. we are allowed to give atorvastatin, clopidogrel, amlodipine…in order to save the patient's life...if the blood pressure is not becoming normal you must transfer the case to us.’ (IPS09070303)
IPs also learned indirectly from doctors by following their prescriptions brought by patients. While one might expect these interactions to enhance IP practices, our IDIs with several stakeholders revealed that qualified doctors also commonly engaged in non-standardised antibiotic use themselves, attributing this to economic incentives, lack of suitable guidelines, lack of diagnostic facilities in rural areas and regulatory challenges. One public sector official saw this as primarily a private sector problem:
‘We do not have any control on private practicing formal doctors. We know they have been using unnecessary antibiotics randomly. We cannot take any action against them till we get any complaint from patient or any other end. Actually, there is no guideline of antibiotic use for formal doctors.’ (Senior government official, BGOKII01)
On the other hand, there were also examples of the positive influence of doctors as mentors. A few IPs who had reported lesser antibiotic use than others in the survey, in the IDIs fondly described their ‘guru’, meaning teacher or mentor, who had cautioned them to be careful with antibiotic provision:
‘I was in Rakhhaskhali for 3 years with a rural health practitioner…in that time period I used painkillers and antibiotics hugely. After that I met Dr. X (a medically qualified doctor). I was with him as a compounder. Dr. X used antibiotic less than less. He doesn't even prescribe a painkiller..only paracetamol. I became habituated...’ (IPS10040502)
These relationships were not without mutual gains. Private doctors were said to have an unspoken understanding with IPs about sharing medical knowledge in exchange for patient referrals; however a few doctors whom we interviewed in-depth also attributed this to the lack of formal healthcare in rural areas and the fact that IPs were ‘always available, for 24 h, anytime of the day’ (Private doctor, SPFDKII01). One doctor said that IPs should be allowed to use some antibiotics for this reason:
‘We tell them to provide general medicine...but diarrhoea can be a problem…in that case we ask them to use Norflox [norfloxacin] in order to control the situation. This much we can allow them… because in the rural areas people are not able to bear that many expenses (to come to town)’. (Private doctor, BPFDKII01)
Hence, relationships between IPs and doctors in this setting were based on more than just economic gains, extending to concern for providing practical solutions for primary healthcare provision in rural areas.
3.3.3. Expanding the pharmaceutical industry's market
IDIs further revealed that IPs represented a significant market for many pharmaceutical industry actors, local as well as global, with considerable drug promotion and marketing aimed at IPs.
Pharmaceutical companies organised educational programmes for IPs that were inclusive of meals and professionally conducted by local medically qualified doctors: ‘Now they show everything on a giant screen following which the formal doctors explain to us about the topic and medicines’ (IPS08050303). Some IPs and pharmaceutical sales representatives referred to these as Continuing Medical Education (CME) programmes, reflecting medical professionals' vocabulary. The aim was to train IPs and to promote the products:
‘They do not come to meet us only for the trainings. They also want their products to be sold in the market. They tell us to prescribe their products and give our feedback of the product regarding its efficiency.’ (IPS06080405)
Training topics included chronic obstructive pulmonary disease, respiratory diseases, fever and diarrhoea, medicines, root canal therapy, treatment of mouth cancers, fungal infections and skin care.
Pharmaceutical sales representatives, better known as medical representatives (MRs), offered various incentives to IPs including free drug samples and offers such as ‘if you buy 10 strips of that molecule product from us you get one strip for free’ (MR, BMRKII02). In the survey, 70% of IPs reported visits by MRs in the last month and 60% had received free samples. Free samples typically included a few tablets, for example a 2-day course of an antibiotic. IPs said they liked these samples because they were of good quality, and they could dispense them free or at low cost to poor patients, notwithstanding that these were not full treatment courses.
Some pharmaceutical company interviewees reported that antibiotics were the number one market in drug sales in West Bengal and all over India. The market was intensely competitive and all companies including global players were said to have rural divisions that focused on marketing in rural areas because ‘if they don't, they won't be able to exist’ (Wholesaler, BWSKII01). In fact, four IPs reported that in the last five years they had attended 8–10 trainings organised by global and local pharmaceutical companies. The IDIs highlighted that IPs were a major commercial segment in the rural pharmaceutical market:
‘One MR is working from Kolkata to Kakdip (distance of 88kms). His monthly target is rupees 100,000. Rupees 80,000 comes from [selling antibiotics to] the RMPs [local term for informal providers] only. Rest of the rupees 20,000 is earned from the formal doctors.’ (Pharmaceutical manager, SMRKII01)
MRs were said to be under constant pressure to meet sales targets that were reviewed every quarter. They were increasingly trained to analyse provider behaviour and in aggressive marketing techniques:
‘Earlier they used to train the MRs about in-depth product knowledge, human anatomy, physiology and the medicines that the company offers. Now, they focus on marketing and train the MRs on how to convert a doctor.’ (MR, BMRKII02)
Besides pharmaceutical manufacturing and marketing companies, the market included an elaborate drug distribution network of stockists and distributors, wholesalers and retailers. Business models in this supply chain followed a demand generation strategy or ‘pushing sell’ in the words of a small stockist who supplied IPs and retail outlets in villages, with discounts on the drug's maximum retail price built into each rung of the supply chain right down to the IPs.
There were also some reports of cheap and possibly sub-standard products available in wholesale shops in different parts of the state, and a category of unregulated middlemen called ‘daily passengers of medicine’ who purchased drugs and free samples that MRs give to physicians, and sold them mainly to IPs and small retail pharmacies in rural areas.
3.3.4. The public health sector's reliance on IPs
IPs were seen as playing an important role in supplementing the government health system in rural areas. For example, in one remote block (with 250,000 population) about 100 kms from Kolkata, there was only one resident government doctor and two visiting NGO doctors, and 2500 IPs (800 IPs for every doctor). Even in our less remote sites, IPs were seen as an important part of the local healthcare system: ‘They are the main component of rural healthcare delivery system. We cannot exclude them..’ (Government official, BGOKII02).
Public sector interviewees were willing to discuss IPs with an openness we had not anticipated. One government doctor said that the state government's policy to train IPs had made it easier for them to engage more openly with IPs. Some primary health centres invited their local IPs for regular meetings in order to improve their role in community healthcare, reduce inappropriate drug use and increase timeliness of referrals. The public sector's dependence on IPs was different from the one-on-one relationships between IPs and private doctors. A senior government stakeholder acknowledged that if IPs were constrained or arrested, the rural health system would be adversely affected.
‘We don't catch the informal providers because we know that if we do, we won't be able to balance the health condition in the society. The situation of the rural health system will deteriorate.’ (Senior government official, SGOKII03)
3.3.5. Regulatory challenges
Several gaps in the interpretation and enforcement of medical and drug related regulations created a regulatory impasse in terms of IP engagement.
As noted in the introduction, only medically qualified providers can legally prescribe antibiotics. The national government also allows public sector Auxiliary Nurse Midwives to administer gentamycin and amoxicillin for infants with pneumonia (GoWB, 2014c), and the West Bengal government allows qualified AYUSH (Ayurveda, Unani, Siddha and Homeopathy) practitioners to use selected antibiotics (Singhania, 2017). However, with respect to IPs, there were varied perceptions of what was allowed. One senior district official said that IPs can use some antibiotics: ‘They are allowed to use co-trimoxazole, amoxicillin, ranitidine, metronidazole, paracetamol, etc. which are also available in PHC [Primary Health Centre]’ (Government official, BGOKII02). On the other hand, a state official said that IPs were not permitted to use antibiotics and it would need a national government order to permit them.
The regulatory authorities for the manufacture and sales of drugs were aware of illegal, over-the-counter sales of antibiotics but said their department was under-resourced, with only 150 inspectors to monitor the 50,000 registered pharmacies in West Bengal, much less the countless unlicensed pharmacies and IPs. Besides, drugs inspectors were reluctant to take any action unless they had higher orders, and as noted above, the higher health and regulatory authorities did not necessarily want IPs to be sanctioned or prosecuted, due to concerns about restricting access to healthcare and to antibiotics and the political consequences of this.
However, opposition from medical professionals at the state and national level prevented the government and local authorities from relaxing the laws or including selected antibiotic provision in IP training.
‘..The main problem is MCI [Medical Council of India]. They are not allowing any short course (for mid-level providers) for the informal providers…. the fact is that everyone is concerned about their own boundaries and interests.’ (Senior government official, SGOKII03)
This had created a paradoxical situation where it was easier for health authorities to overlook unrestricted antibiotic use than take practical steps towards improving IPs’ antibiotic dispensing by allowing them to use a few essential antibiotics in a regulated manner.
4. Discussion
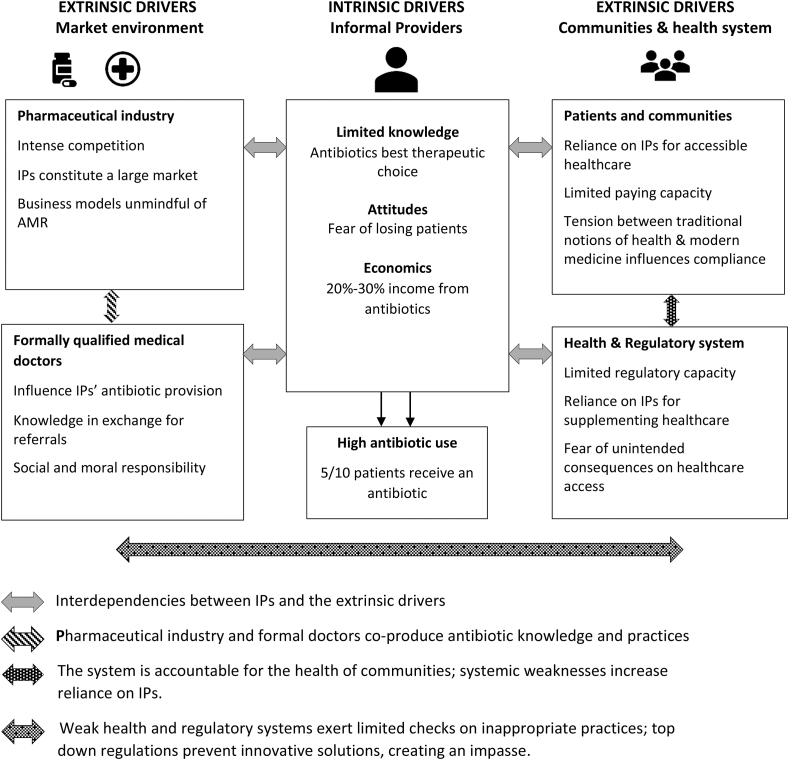
Compared with WHO's prescription indicators that 20%–30% of clinical encounters in LMICs may need an antibiotic (Isah et al., 2002; WHO, 2006), antibiotic provision in our study was excessive at an estimated 50%. Although our estimation was based on self-reported data which can be biased (Kruger and Dunning, 1999), one might expect this if anything to be an under-estimate of true rates. Indeed, Khare et al. reported that 75% of IP prescriptions in Madhya Pradesh included antibiotics and these were prescribed more often than other drugs (Khare et al., 2019). Our findings are consistent with general trends in antibiotic prescribing across India and Africa (Kenya) where around half of all patients in primary care settings receive an antibiotic (Sulis et al., 2020). Our study also revealed haphazard patterns of antibiotic selection and dosing, with little systematic relation to standard treatment guidelines or regulatory rules. The frequency, extent and patterns of antibiotic consumption are a concern in this setting as antibiotic overuse and misuse is a primary driver of AMR (Holmes et al., 2016). A strong association exists between the number of antibiotic courses prescribed over 12 months and bacterial resistance to those antibiotics in an individual (Costelloe et al., 2010). By interviewing a wide range of key stakeholders, we provide a holistic analysis of the intrinsic and extrinsic drivers of antibiotic provision by rural IPs and how these interact, between and amongst themselves, to co-produce a culture of informal antibiotic practices (Fig. 3). This analysis allows us to add more depth and new insights to existing evidence.
Fig. 3.
Drivers of antibiotic provision by informal providers.
Compared to the study in Madhya Pradesh (Khare et al., 2019) that reported higher ciprofloxacin (a WATCH antibiotic) use, IPs in our study most frequently usd amoxicillin, an ACCESS antibiotic. Notwithstanding that all antibiotics are prescription-only, this is more in line with WHO's recommendations (WHO, 2019). The absence of RESERVE antibiotics like carbapenems and colistin, in our study and in Madhya Pradesh, was notable. One reason could be that carbapenems are contained in the H1 Schedule of the Drugs and Cosmetics Act, which is taken more seriously than Schedule H by pharmacies and regulatory authorities (Miller et al., 2018) as it incurs a more severe punishment. However, the H1 schedule is currently not aligned with the AWARE categorisation and this could be a reason why Linezolid, a RESERVE antibiotic, but not in the H1 Schedule, was being promoted in our sites. India's drug regulatory authorities could consider aligning the H and H1 antibiotics with WHO's categories.
We found that IPs' intrinsic drivers resulted from an interplay between misconceived notions of antibiotics being a therapeutic necessity and their economic needs, contributing about 20%–30% to IPs' incomes. This is partly in line with Nair et al.‘s finding that IPs perceive antibiotics as essential for patient retention and indirectly ensuring their livelihoods (Nair et al., 2019a), and with a study from Vietnam where antibiotics accounted for 24% and 18% of revenues in urban and rural pharmacies (Nga et al., 2014). Interestingly, in both Vietnam and West Bengal, antibiotics were not the drugs with the highest profit-margins. IPs in our study said they chose antibiotic brands to fit with affordability for patients and they customized sub-optimal packages that depended on the paying capacity of patients. Economic constraints and perceptions of harm from medicine use also led patients to stop antibiotics mid-course.
While the role of patient demand for antibiotics has been emphasised in the literature (Nair et al., 2019a; Wilkinson et al., 2018) mainly in the context of qualified prescribers, our findings indicate that this may be less relevant in this context with community FGD participants having limited knowledge about antibiotics and not specifically asking for them. They wanted value for money: the best quality treatment at an affordable price. This interplay between knowledge/perceptions and economic drivers for both IPs and patients is critical from a stewardship perspective, because addressing one without the other would likely lead to limited impact.
Besides patients, the main extrinsic drivers of IPs' antibiotic provision stemmed from the pharmaceutical industry, and the formal health and regulatory sectors. Nair et al.'s study has emphasised the role of MRs in unethical drug promotion and marketing (Nair et al., 2019a). Our findings show that MRs are just the visible face of an industry that has aggressive business strategies. Our interviewees described the significance of IPs for corporate revenues and said that companies needed a ‘rural marketing division’ to survive. Market research data reveal that the Indian pharmaceutical industry is one of the largest in the world and among the fastest growing (IBEF, 2019). Almost half its turnover is from the domestic market which is projected to increase 9–12% in the coming five years, with much of this growth expected from increasing penetration of sales in rural areas as urban markets become saturated. The industry is large and fragmented with an estimated 10,500 manufacturers (IBEF, 2019), intensifying domestic competition and increasing regulatory challenges.
IPs' economic and mentoring relationships with medically qualified private doctors supported and sustained their antibiotic use through exchange of knowledge for referrals. The mentorship role of doctors is arguably a key driver, as their antibiotic prescribing has been found to be 55% higher than that of IPs (Sulis et al., 2020), and even doctors more likely to provide correct treatment are equally likely to give an unnecessary antibiotic (Das et al., 2020). Studies confirm the relationships between doctors and IPs for antibiotics (Nair et al., 2019a) and for other health services as well (Nahar et al., 2016; Chandra and Bhattacharya, 2019). However, the public health sector's reliance on IPs is not well articulated in literature, even less its implications for the implementation of regulations. Our study shows that IPs tacitly supplement the public health system in rural areas, raising practical and ethical dilemmas for regulators responsible for enforcing top-down regulations. Laws related to antibiotic sales added another twist as they prevented the design of practical solutions. It was easier for regulators to ignore regulatory infringements than allow regulatory leeway for some antibiotics to be sold without a prescription so the rest could be monitored effectively. Poor regulatory enforcement in India has been attributed to weak capacity and ambiguity of laws (Sheikh et al., 2013; Chandra and Bhattacharya, 2019); in addition we found that a disconnect between top-down regulations and people's health needs had created a regulatory impasse that acted as a barrier for antibiotic stewardship and effective IP solutions.
Drawing on the expanded notion of informality as forms of irregular practice rather than a specific set of providers, our findings reveal a mosaic of informal practices in this health market by IPs, community members, medically qualified doctors, pharmaceutical companies and public health authorities. Multiple actors have improvised a practical system of rural healthcare with its own informal service delivery architecture, knowledge and referral pathways and supply chains. Akin to the bricolage of urban planning (McFarlane, 2012), this hybrid of informal and formal practices also sustains an expanding pharmaceutical industry and private healthcare in urban India, and supplements a weak public health sector in rural areas.
The generalisability of our findings to other states and urban India is not without limitations, though the main themes are likely to be common, given the health system weaknesses across India. Despite greater physical accessibility of qualified health providers, the urban poor also seek care from IPs (Priya et al., 2019), reflecting considerations of affordability, comfort and trust (Ergler et al., 2011). It is also likely that during the Covid-19 pandemic use of both IPs and antibiotics may have increased.
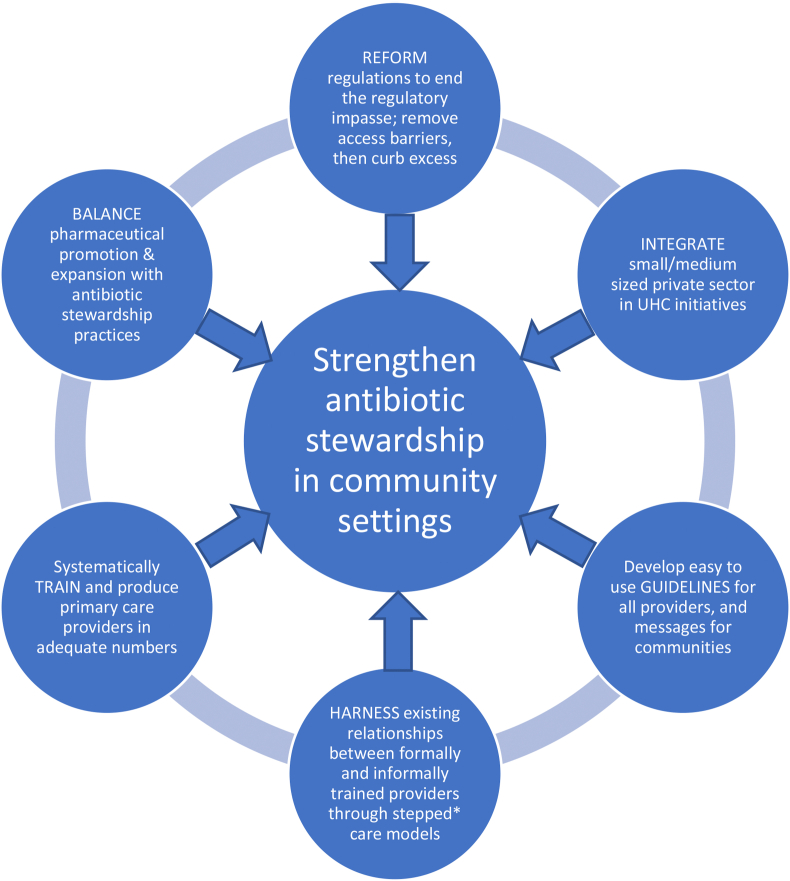
Our findings emphasise that an effective antibiotic stewardship strategy must consider IPs as one actor within the wider rural healthcare bricolage. The number of extrinsic drivers influencing IP behaviours, and their inter-relationships, suggest that a wide range of regulatory, educational and behaviour change interventions, targeting multiple stakeholders, are needed over the short and long terms. We present a conceptual framework (with the acronym BRIGHT-see Fig. 4) to depict these broad health system and antibiotic stewardship interventions needed across multiple actors. These will involve community/public behaviour change interventions that are very limited at present in LMICs (Batura et al., 2018). First however, interventions are needed at the opposite end of the supply chain, to address the marketing strategies of large pharmaceutical companies, and balance pharmaceutical expansion with antibiotic stewardship. As the actions of pharmaceutical MRs are driven by the business models of corporate giants, appropriate boundaries need to be defined for the marketing of antibiotics by companies, supported by an enhanced role of public health bodies in public and provider education (Edwards et al., 2018). We need to engage with the corporates, starting with those that have expressed commitments to tackle AMR (AMF, 2020), and further research is needed to understand the distribution of incentives for stakeholders throughout pharmaceutical value chains.
Fig. 4.
BRIGHT: a framework for conceptualising interconnected interventions in the primary healthcare, regulatory and pharmaceutical systems that are required to address antibiotic stewardship in community settings in a sustainable way. Balance; Reform; Integrate; Guidelines; Harness; Train (see explanations provided in the respective circles in the figure) *stepped care: a healthcare delivery model developed for mental healthcare where lay health workers deliver basic care at the first level and primary care physicians and specialists provide advanced care at higher levels (Patel et al., 2010).
Large scale and sustained antibiotic stewardship in these settings will require addressing the legitimacy of IPs within the overall health system. IPs' illegitimacy is an historical artefact of the state's formalization of the medical profession in India that displaced many rural providers without creating suitable alternatives. Laws designed to regulate the medical profession criminalise IPs without providing suitable frameworks for regulating their services and antibiotic provision. While regulation is seen as a solution to inappropriate antibiotic use, in this context, top-down regulations are creating a barrier for both expanding healthcare coverage and AMR containment (Porter et al., 2020). Addressing this impasse would allow for innovative approaches like ‘smart’ regulation - that incorporate a range of instruments and actors to optimise win-win outcomes (ibid)- to be used to design suitable frameworks for provision of a limited range of ACCESS antibiotics by IPs through collaborative stepped-care models (Patel et al., 2010) with medically qualified doctors and the broader public health system.
India stands committed to Universal Health Coverage (UHC) but global UHC tracer indicators focus on vertical conditions (like coverage of family planning, immunization, care for tuberculosis and HIV) and on health workers qualified as per western standards (physicians, surgeons, psychiatrists) (Abiiro and De Allegri, 2015; Hogan et al., 2018); these could limit real expansion of primary care. However, the global UHC and primary care discourse now encompasses the private health sector including the informal sector (WHO, 2018b) and this can enable countries like India adopt a decolonized lens to recognise the potential of a pluralistic health workforce for improving universal coverage. The Indian government's hospitalisation insurance programme for 500 million poor households, under its Ayushman Bharat Scheme (GOI, 2021) includes the private secondary and tertiary sectors. Plans to strengthen rural primary care include upgrading the public sector's 119,628 sub-centres and 25,743 primary health centres as Health and Wellness centres (NHM, 2020) with mid-level providers delivering primary care. However, even when fully functional, these numbers may be inadequate for India's 800 million rural population. Smaller solo private practitioners and IPs could be concurrently integrated into UHC initiatives to improve healthcare and AMR stewardship and improve the efficiency of private out of pocket expenditure on primary care (41%). The latter could perhaps be addressed through alternative pre-payment methods like micro-health insurance (Habib et al., 2016).
In high income countries, educational strategies combined with prescription restrictions and separation of dispensing from prescribing have helped control economic incentives for physician's prescribing (Lim et al., 2019), but such a separation might be difficult to enforce in India given current business models. In LMICs, successful strategies with formal providers and drug sellers include knowledge enhancement, antibiotic guidelines and decision support, audit and review, peer supervision and performance-based incentives (Bagonza et al., 2020; Wilkinson et al., 2018). Private sector literature also highlights the role of supply side interventions that address provider quality and incentives. Social franchising models for example, offer provider training and social marketing of commodities, to encourage consumer behaviour change by stimulating demand for public health products like contraceptives, Oral Rehydration Solution (ORS) and zinc (for diarrhoea), antimalarials and bed-nets (Montagu and Goodman, 2016). These have had mixed results in India, with one donor funded initiative leading to increased ORS and zinc use in one state but not in another (Lam et al., 2019), while another social franchising and telemedicine programme failed to improve IPs' performance (Mohanan et al., 2017). Programme designs may have been weak, or lacked appeal for IPs with established practices who saw no added benefits of joining social franchises. The public sector in India has experimented with pre-packaged drug kits for management of sexually transmitted diseases, but ensuring uninterrupted supplies is challenging (Jha, 2014). With some limitations, these examples might offer lessons for marketing of antibiotic kits for IPs, supported by guidelines, targeted training and peer review and supervisory systems for antibiotic behaviour change across all providers types. In sum, given the multi-stakeholder influences on antibiotic use in this setting, we recommend a multi-stakeholder approach for designing and implementing crosscutting interventions.
Authorship statement
MG prepared the first draft of the manuscript and was the study PI responsible for the study design, implementation and data analysis and interpretation. CG and NS contributed extensively to study conceptualization and implementation, data analysis and interpretation and to the development of the manuscript. SC participated in the study implementation, data collection and analysis and critically reviewed the manuscript.
Declaration of competing interest
None.
Acknowledgements
Financial support for this study was through a Health Systems Research Initiative grant (Ref: MR/P004512/1) jointly funded by the Medical Research Council, the Economic and Social Research Council, the Foreign, Commonwealth and Development Office and Wellcome Trust, UK. This study is also one of UK Research and Innovation's Global Challenges Research Fund projects. The funders had no role in the study design or in the collection, analysis and interpretation of data or in the writing of this article. We are grateful to all our field investigators and to our study participants including informal providers, community members and other key stakeholders for sharing their valuable insights with us.
Contributor Information
Meenakshi Gautham, Email: Meenakshi.gautham@lshtm.ac.uk.
Neil Spicer, Email: Neil.Spicer@lshtm.ac.uk.
Soumyadip Chatterjee, Email: soumyadipch@gmail.com.
Catherine Goodman, Email: Catherine.goodman@lshtm.ac.uk.
References
- Abiiro G.A., De Allegri M. Universal health coverage from multiple perspectives: a synthesis of conceptual literature and global debates. BMC Int. Health Hum. Right. 2015;15:17. doi: 10.1186/s12914-015-0056-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad R., Zhu N.J., Leather A.J.M. Strengthening strategic management approaches to address antimicrobial resistance in global human health: a scoping review. BMJ Global Health. 2019;4 doi: 10.1136/bmjgh-2019-001730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AMF . Access to Medicine Foundation; Amsterdam: 2020. 2020 Antimicrobial Resistance Benchmark. [Google Scholar]
- Anand S., Fan V. World Health Organization; Geneva, Switzerland: 2016. THE HEALTH WORKFORCE IN INDIA Human Resources for Health Observer Series No. 16. [Google Scholar]
- Bagonza A., Wamani H., Peterson S. Peer supervision experiences of drug sellers in a rural district in East-Central Uganda: a qualitative study. Malar. J. 2020;19:270. doi: 10.1186/s12936-020-03343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batura N., Cuevas C., Khan M. How effective and cost-effective are behaviour change interventions in improving the prescription and use of antibiotics in low-income and middle-income countries? A protocol for a systematic review. BMJ Open. 2018;8 doi: 10.1136/bmjopen-2018-021517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom G., Wilkinson A., Tomson G. Pluralistic Health Systems. STEPS Working Paper 84. STEPS Centre; Brighton: 2015. Addressing resistance to antibiotics. [Google Scholar]
- Broom A., Broom J., Kirby E. Cultures of resistance? A Bourdieusian analysis of doctors' antibiotic prescribing. Soc. Sci. Med. 2014;110(2014):81–88. doi: 10.1016/j.socscimed.2014.03.030. [DOI] [PubMed] [Google Scholar]
- Chandra S., Bhattacharya S. Unqualified medical practitioners: their illegal but indispensable role in primary healthcare. Econ. Polit. Wkly. 2019;LIV(5):36–44. [Google Scholar]
- Charani E., Castro-Sanchez E., Sevdalis N. Understanding the determinants of antimicrobial Prescribing Within hospitals: the role of “prescribing etiquette”. Clin. Infect. Dis. 2013;57(2):188–196. doi: 10.1093/cid/cit212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CIPSACADEMY. (2020) http://www.cipsacademy.in/paramedical-course-community-medical-service-and-essential-drugs.php (accessed 16 May, 2020).
- Costelloe C., Metcalfe C., Lovering A. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ. 2010;340:c2096. doi: 10.1136/bmj.c2096. [DOI] [PubMed] [Google Scholar]
- Creswell J.W., Plano Clark V.L., Gutmann M.L. Advanced mixed methods research designs. In: Tashakkori A., Teddlie C., editors. Handbook of Mixed Methods in Social and Behavioral Research. Sage; Thousand Oaks, CA: 2003. [Google Scholar]
- Das J., Chowdhury A., Hussam R. The impact of training informal health care providers in India: a randomized controlled trial. Science. 2016;354(6308) doi: 10.1126/science.aaf7384. [DOI] [PubMed] [Google Scholar]
- Das J., Daniels B., Ashok M. Two Indias: the structure of primary health care markets in rural Indian villages with implications for policy. Soc. Sci. Med. 2020 doi: 10.1016/j.socscimed.2020.112799. (Article (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S.E., Morel C.M., Busse R. Combatting antibiotic resistance together: how can we enlist the help of industry? Antibiotics. 2018;7:111. doi: 10.3390/antibiotics7040111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ergler C.R., Sakdapolrak P., Bohle H.-G. Entitlements to health care: why is there a preference for private facilities among poorer residents of Chennai, India? Soc. Sci. Med. 2011;72:327–337. doi: 10.1016/j.socscimed.2010.09.042. [DOI] [PubMed] [Google Scholar]
- Gautham M., Shyamprasad K. The ‘basic’ doctor for rural India: a failed promise? Econ. Polit. Wkly. 2010;xlv(38):25–29. [Google Scholar]
- Gautham M., Shyamprasad K., Singh R. Informal rural healthcare providers in North and South India. Health Pol. Plann. 2013;2013:1–10. doi: 10.1093/heapol/czt050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George A., Iyer A. Unfree markets: socially embedded informal health providers in northern Karnataka, India. Soc. Sci. Med. 2013;96(2013):297–304. doi: 10.1016/j.socscimed.2013.01.022. [DOI] [PubMed] [Google Scholar]
- GOI . Government of India; 1956. The Indian Medical Council Act.https://old.mciindia.org/acts/Complete-Act-1.pdf [Google Scholar]
- GOI . Census of India, Government of India; 2011. West Bengal Population 2011-2018 Census.https://www.census2011.co.in/census/state/west+bengal.html Available at: [Google Scholar]
- GOI . Ministry of Health and Family Welfare, Statistics Division. Government of India; New Delhi: 2014-15. Rural Health Statistics. [Google Scholar]
- GOI . Ministry of Health and Family Welfare; New Delhi: 2016. The Indian Drugs and Cosmetics Act, 1940 (As Amended Upto 31st December, 2016) [Google Scholar]
- GOI Rural health statistics. 2017. https://data.gov.in/resources/district-wise-availability-health-centres-india-31st-march-2017
- GOI . National Health Systems Resource Centre, Ministry of Health and Family Welfare, Government of India; New Delhi: 2019. National Health Accounts Estimates for India 2016-17. [Google Scholar]
- GOI . Government of India; New Delhi: 2020. Health in India. NSS 75th Round July 2017 - June 2018. National Statistical Office, Ministry of Statistics and Programme Implementation. [Google Scholar]
- GOI About pradhan mantri jan arogya Yojana (PM-JAY) 2021. https://pmjay.gov.in/about/pmjay Available at:
- GOWB . Development & Planning Department, Government of W. Bengal; Kolkata: 2009. District Human Development Report: Birbhum. 2009. [Google Scholar]
- GOWB . Development and Planning Department, Government of West Bengal; Kolkata: 2009. District Human Development Report: South 24 Parganas. [Google Scholar]
- GoWB . Bureau of Applied Economics & Statistics, Department of Statistics & Programme Implementation; Govt. of West Bengal: 2014. District Statistical Handbook Birbhum. [Google Scholar]
- GoWB . Bureau of Applied Economics & Statistics, Department of Statistics & Programme Implementation; Govt. of West Bengal: 2014. District Statistical Handbook South 24-Parganas. [Google Scholar]
- GoWB . Department of Health and Family Welfare, GoWB, Swasthya Bhavan; Kolkata: 2014. Memorandum No. H/SFWB/26A-01-2014/3680. [Google Scholar]
- Habib S.S., Perveen S., Khuwaja H.M.A. The role of micro health insurance in providing financial risk protection in developing countries- a systematic review. BMC Publ. Health. 2016;16:281. doi: 10.1186/s12889-016-2937-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan D.R., Stevens G.A., Hosseinpoor A.R. Monitoring universal health coverage within the Sustainable Development Goals: development and baseline data for an index of essential health services. The Lancet Global Health. 2018;6:e152–e168. doi: 10.1016/S2214-109X(17)30472-2. [DOI] [PubMed] [Google Scholar]
- Holmes A., Moore L., Sundsfjord A. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet. 2016;387:176–187. doi: 10.1016/S0140-6736(15)00473-0. [DOI] [PubMed] [Google Scholar]
- IBEF . India Brand Equity Foundation; New Delhi: 2019. Indian Pharmaceutical Industry Report June 2019.https://www.ibef.org/download/Pharmaceuticals-June-2019.pdf [Google Scholar]
- IRMA Indian rural medical association. 2020. http://indianruralmedicalassociation.co.in/
- Isah A., Laing R., Quick J. The development of reference values for the WHO health facility core prescribing indicators. W. Afr. J. Pharmacol. Drug Res. 2002;18 2002:6–11. [Google Scholar]
- Jha D.N. Hospitals have no drug kits for STDs. Times of India. 2014. https://timesofindia.indiatimes.com/city/delhi/hospitals-have-no-drug-kits-for-stds/articleshow/31360165.cms
- Khare S., Purohit M., Sharma M. Antibiotic prescribing by informal healthcare providers for common illnesses: a repeated cross-sectional study in rural India. Antibiotics. 2019;8:139. doi: 10.3390/antibiotics8030139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein E., Tseng K., Pant S. Tracking global trends in the effectiveness of antibiotic therapy using the Drug Resistance Index. BMJ Glob Health. 2019;4 doi: 10.1136/bmjgh-2018-001315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein E., Van Boeckel T., Martineza E. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. Unit. States Am. 2018;115(15):E3463–E3470. doi: 10.1073/pnas.1717295115. www.pnas.org/cgi/doi/10.1073/pnas.1717295115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotwani A., Wattal C., Katewa S. Factors influencing primary care physicians to prescribe antibiotics in Delhi India. Fam. Pract. 2010;27:684–690. doi: 10.1093/fampra/cmq059. [DOI] [PubMed] [Google Scholar]
- Kruger J., Dunning D. Unskilled and unaware of it: how difficulties in recognizing one's own incompetence lead to inflated self-assessments. J. Pers. Soc. Psychol. 1999;77:1121–1134. doi: 10.1037//0022-3514.77.6.1121. [DOI] [PubMed] [Google Scholar]
- Lam F., Pro G., Agrawal S. Effect of enhanced detailing and mass media on community use of oral rehydration salts and zinc during a scale-up program in Gujarat and Uttar Pradesh. Journal of Global Health. 2019;9 doi: 10.7189/jogh.09.010501. 010501-010501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie C. University of California Press, 1976; California: 1976. Asian Medical Systems: A Comparative Study. [Google Scholar]
- Li Y., Xu J., Wang F. Overprescribing in China, driven by financial incentives, results in very high use of antibiotics, injections, and corticosteroids. Health Aff. 2012;31(5):1075–1082. doi: 10.1377/hlthaff.2010.0965. (2012) [DOI] [PubMed] [Google Scholar]
- Lim J.M., Singh S.R., Duong M.C. Impact of national interventions to promote responsible antibiotic use: a systematic review. J. Antimicrob. Chemother. 2019;75:14–29. doi: 10.1093/jac/dkz348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood S., Iqbal M., Hanifi M. Are 'village doctors' in Bangladesh a curse or a blessing? BMC Int. Health Hum. Right. 2010;10:18. doi: 10.1186/1472-698X-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane C. Rethinking informality: politics, crisis, and the city. Plann. Theor. Pract. 2012;13(1):89–108. [Google Scholar]
- Miller R., Hutchinson E., Goodman C. ‘A smile is most important.’ Why chains are not currently the answer to quality concerns in the Indian retail pharmacy sector. Soc. Sci. Med. 2018;212:9–16. doi: 10.1016/j.socscimed.2018.07.001. [DOI] [PubMed] [Google Scholar]
- Mohanan M., Giardili S., Das V. Evaluation of a social franchising and telemedicine programme and the care provided for childhood diarrhoea and pneumonia, Bihar, India. Bull. World Health Organ. 2017;95:343–352E. doi: 10.2471/BLT.16.179556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagu D., Goodman C. Prohibit, constrain, encourage, or purchase: how should we engage with the private health-care sector? Lancet. 2016;388:613–621. doi: 10.1016/S0140-6736(16)30242-2. [DOI] [PubMed] [Google Scholar]
- Morgan D., Okeke I., Laxminarayan R. Non-prescription antimicrobial use worldwide: a systematic review. Lancet Infect. Dis. 2011;11(9):692–701. doi: 10.1016/S1473-3099(11)70054-8. 2011 September. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahar P., Kannuri N., Mikkilineni S. At the margins of biomedicine: the ambiguous position of ‘Registered Medical Practitioners’ in rural Indian healthcare. Sociol. Health Illness. 2016;39(4):614–628. doi: 10.1111/1467-9566.12521. 2017. [DOI] [PubMed] [Google Scholar]
- Nair M., Tripathi S., Mazumdar S. “Without antibiotics, I cannot treat”: a qualitative study of antibiotic use in Paschim Bardhaman district of West Bengal, India. PloS One. 2019;14(6) doi: 10.1371/journal.pone.0219002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair M., Tripathi S., Mazumdar S. Knowledge, attitudes, and practices related to antibiotic use in Paschim Bardhaman District: a survey of healthcare providers in West Bengal, India. PloS One. 2019;14(5) doi: 10.1371/journal.pone.0217818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nga D., Chuc N., Hoa N. Antibiotic sales in rural and urban pharmacies in northern Vietnam: an observational study. BMC Pharmacology and Toxicology. 2014;15:6. doi: 10.1186/2050-6511-15-6. http://www.biomedcentral.com/2050-6511/15/1/6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NHM . National Health Mission, Ministry of Health and Family Welfare, Government of India; New Delhi: 2020. Towards Universal Health Coverage: Ayushman Bharat Health and Wellness Centres. A Compendium of Health and Wellness Centres Operationalization. [Google Scholar]
- O'Neill J. UK Government; 2016. The Review on Antimicrobial Resistance: Tackling Drug-Resistant Infections Globally. [Google Scholar]
- O'Connell K., Poyer S., Solomon T. Methods for implementing a medicine outlet survey: lessons from the anti-malarial market. Malar. J. 2013;12:52. doi: 10.1186/1475-2875-12-52. http://www.malariajournal.com/content/12/1/52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Om C., Daily F., Vlieghe E. “If it's a broad spectrum, it can shoot better”: inappropriate antibiotic prescribing in Cambodia. Antimicrob. Resist. Infect. Contr. 2016;5:58. doi: 10.1186/s13756-016-0159-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V., Weiss H.A., Chowdhary N. Effectiveness of an intervention led by lay health counsellors for depressive and anxiety disorders in primary care in Goa, India (MANAS): a cluster randomised controlled trial. Lancet. 2010;376:2086–2095. doi: 10.1016/S0140-6736(10)61508-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payyappallimana U., Venkatasubramanian P. Exploring ayurvedic knowledge on food and health for providing innovative solutions to contemporary healthcare. Frontiers in Public Health. 2016;4 doi: 10.3389/fpubh.2016.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter G., Joshi J., Bhullar L. Using ‘smart regulation’ to tackle antimicrobial resistance in low-income and middle-income countries. BMJ Global Health. 2020;5 doi: 10.1136/bmjgh-2019-001864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priya R., Singh R., Das S. Health implications of diverse visions of urban spaces: bridging the formal-informal divide. Front. Public Health. 2019;7:239. doi: 10.3389/fpubh.2019.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao K., Shahrawat R., Bhatnagar A. Composition and distribution of the health workforce in India: estimates based on data from the National Sample Survey. WHO South-East Asia Journal of Public Health. 2016;5(2):133–140. doi: 10.4103/2224-3151.206250. 2016. [DOI] [PubMed] [Google Scholar]
- RBI . Handbook of Statistics on Indian Economy 2017-18. Reserve Bank of India; New Delhi: 2018. Per capita net state domestic product - statewise (at constant prices)https://www.rbi.org.in/Scripts/PublicationsView.aspx?id=18475 [Google Scholar]
- Rodrigues A., Roque F., Falcão A. Understanding physician antibiotic prescribing behaviour: a systematic review of qualitative studies. Int. J. Antimicrob. Agents. 2013;41(2013):203–212. doi: 10.1016/j.ijantimicag.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Rohde J.E., Vishwanathan H. Oxford University Press; Delhi: 1995. The Rural Private Practitioner. 1995. [Google Scholar]
- Roy A. Urban informality toward an epistemology of planning. Journal of the American Planning Associarion. 2005;71(2) Spring 2005. [Google Scholar]
- Sabde Y.D., Diwan V., Saraf V.S. Mapping private pharmacies and their characteristics in Ujjain district, Central India. BMC Health Serv. Res. 2011;11:351. doi: 10.1186/1472-6963-11-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh K., Saligram P., Hort K. What explains regulatory failure? Analysing the architecture of health care regulation in two Indian states. Health Pol. Plann. 2013;30 doi: 10.1093/heapol/czt095. [DOI] [PubMed] [Google Scholar]
- Singhania M. West Bengal allows AYUSH practitioners to practice modern medicine including antibiotics, 4 sept, 2017. 2017. https://medicaldialogues.in/west-bengal-allows-ayush-practitioners-to-practice-modern-medicine-including-antibiotics
- Spencer L., Ritchie J., O'Connor W. Analysis: practices, principles and processes. In: Ritchie J., Lewis J., editors. Qualitative Research Practice: A Guide for Social Science Students and Researchers. Sage Publications Ltd; London: 2003. pp. 200–257. [Google Scholar]
- Sudhinaraset M., Ingram M., Lofthouse H. What is the role of informal healthcare providers in developing countries? A systematic review. PloS One. 2013;8(2) doi: 10.1371/journal.pone.0054978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulis G., Daniels B., Kwan A. Antibiotic overuse in the primary health care setting: a secondary data analysis of standardised patient studies from India, China and Kenya. BMJ Global Health. 2020;5 doi: 10.1136/bmjgh-2020-003393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomson G., Vlad I. The need to look at antibiotic resistance from a health systems perspective. Ups. J. Med. Sci. 2014;119:117–124. doi: 10.3109/03009734.2014.902879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . World Health Organization; Geneva: 2006. Using Indicators to Measure Country Pharmaceutical Situations: Fact Book on WHO Level I and Level II Monitoring Indicators. [Google Scholar]
- WHO . World Health Organization; Geneva, Switzerland: 2015. Global Action Plan on Antimicrobial Resistance. [Google Scholar]
- WHO . 2018. Monitoring Global Progress on Addressing Antimicrobial Resistance: Analysis Report of the Second Round of Results of AMR Country Self-Assessment Survey 2018. World Health Organization (WHO), Food and Agriculture Organization of the United Nations (FAO) and World Organisation for Animal Health (OIE): Geneva, Switzerland. [Google Scholar]
- WHO . World Health Organization; Geneva: 2018. The Private Health Sector: Universal Health Coverage and Primary Health Care. [Google Scholar]
- WHO . World Health Organization; Geneva: 2019. The 2019 WHO AWaRe Classification of Antibiotics for Evaluation and Monitoring of Use.https://www.who.int/medicines/news/2019/WHO_releases2019AWaRe_classification_antibiotics/en/ 2019. (WHO/EMP/IAU/2019.11) [Google Scholar]
- Wilkinson A., Ebata A., MacGregor H. Interventions to reduce antibiotic prescribing in LMICs: a scoping review of evidence from human and animal health systems. Antibiotics. 2018;8:2. doi: 10.3390/antibiotics8010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Bank . World Bank; Washington, DC: 2017. Drug-Resistant Infections: A Threat to Our Economic Future. [Google Scholar]
- Xue H., Shi Y., Huang L. Diagnostic ability and inappropriate antibiotic prescriptions: a quasi-experimental study of primary care providers in rural China. J. Antimicrob. Chemother. 2019;74:256–263. doi: 10.1093/jac/dky390. [DOI] [PubMed] [Google Scholar]