Abstract
Glioblastoma is one of the deadliest forms of primary adult tumors, with median survival of 14.6 months post-diagnosis despite aggressive standard of care treatment. This grim prognosis for glioblastoma patients has changed little in the past two decades, necessitating novel treatment modalities. One potential treatment modality is cancer immunotherapy, which has shown remarkable progress in slowing disease progression or even potentially curing certain solid tumors. However, the transport barriers posed by the blood–brain barrier and the immune privileged status of the central nervous system pose drug delivery obstacles that are unique to brain tumors. In this review, we provide an overview of the various physiological, immunological, and drug delivery barriers that must be overcome for effective glioblastoma treatment. We discuss chemical modification strategies to enable nanomedicines to bypass the blood–brain barrier and reach intracranial tumors. Finally, we highlight recent advances in biomaterial-based strategies for cancer immunotherapy that can be adapted to glioblastoma treatment.
Graphical abstract
Keywords: Immunotherapy, Glioblastoma, Nanomedicine, Drug delivery, Blood–brain barrier
Introduction
Glioblastoma (GBM) is the most common primary adult tumor in adults, with 2–3 cases per 100,000 people [1]. Despite aggressive standard of care treatment, which includes surgical resection and radio- or chemo-therapy, median survival is 14.6 months [2]. Unlike many other solid tumors, GBM metastasis outside of the central nervous system (CNS) is infrequent and not the primary cause of morbidity and mortality [3]. Despite the ability to achieve gross total resection of the primary tumor, neoplastic infiltration leads to inevitable recurrence [4]. This makes immunotherapy an attractive option for GBM treatment as immune cells primed to attack infiltrative GBM cells left behind after tumor resection could prevent tumor recurrence and disease progression.
Indeed, there is great interest in harnessing immunotherapy for GBM treatment after recent successes in using immunotherapy to treat other forms of solid tumors. For example, the antigen-specific cell therapy sipuleucel-T was approved by the FDA in 2010 to treat prostate cancer [5] while the immune checkpoint inhibitor ipilimumab was approved in 2011 to treat metastatic melanoma [6]. To date, these treatment strategies have not been effective for GBM, however, as GBM expresses few known tumor-restricted antigens and is considered a largely non-immunogenic tumor type [7]. Furthermore, the brain is an immune privileged space [8], is edema-intolerant [9], and intracranial drug transport is restricted by the blood–brain barrier (BBB) [10]. These challenges necessitate the development of new therapeutics as well as drug delivery systems in order to realize the promise of immunotherapy for GBM treatment.
In this review, we discuss the transport barriers associated with treatment of CNS malignancies and immunological barriers associated with immunotherapy against GBM. We highlight recent advances in drug delivery technology, including chemical modifications of drug delivery vehicles as well as innovative drug administration techniques to facilitate BBB crossing and tumor targeting. Finally, we report recent advances in biomaterial-based cancer immunotherapy strategies. Taken together, these innovations in immunotherapy approaches and drug delivery technologies present promising new treatment modalities for GBM.
Barriers in immunotherapy delivery to GBM
Physical transport barriers: BBB and beyond
The BBB is composed primarily of CNS endothelial cells connected by continuous complex tight junctions with limited vesicular transport [11], and passive transport across the BBB can only be achieved by a small number of low molecular weight (< 400 Da) or lipid-soluble molecules [12]. The BBB is further regulated by interactions with the extracellular matrix, astrocytes, and pericytes [13], which together significantly limit the permeability for most small molecule drugs and nanoparticle drug delivery vehicles. Clinical data have shown that all GBM patients have tumor regions with an intact BBB [14], and a cure for GBM will require drug delivery with effective BBB penetration to target the tumor in these regions and/or stimulation of an endogenous cellular immune response that can reach these regions.
Beyond the BBB, drug delivery to GBM is further limited by transport barriers associated with solid tumors. Historically, design of nanoparticles for drug delivery to solid tumors has been guided by the enhanced permeation and retention (EPR) effect [15], which hypothesized that inter-endothelial gaps form in tumor vasculature as a result of rapid angiogenesis [16]. These gaps could theoretically allow nanoparticles under 1 μm to passively enter the tumor, and poor lymphatic drainage enable them to accumulate in the tumor [17]. There is considerable debate regarding how consistently the EPR effect is recapitulated in human tumors as recent studies have demonstrated that the architecture of tumor vasculature varies greatly in larger species compared to rodents [18, 19]. In addition, a recent study by Sindhwani et al. demonstrated that up to 97% of nanoparticles enter tumors through active transcytosis through endothelial cells, rather than passive extravasation through inter-endothelial gaps in leaky tumor vasculature [20]. Even in tumor regions with leaky vasculature, abnormal blood vessels create high interstitial fluid pressure that hinder convective transport of therapeutics from the blood stream into the tumor tissue; this phenomenon has been dubbed the blood–tumor barrier [21, 22] and presents a further transport challenge. Taken together, these findings challenge our understanding of delivery paradigms in cancer nanomedicine and illustrate the need for more targeted design of therapeutic nanocarriers to solid tumors such as GBM.
Biological challenges associated with GBM immunotherapy
Beyond transport limitations to brain tumors, physiological features of the CNS pose further challenges to GBM immunotherapy. As allografts to the CNS do not elicit typical inflammatory responses, the CNS is generally classified as an immune privileged space [23, 24]. Our understanding of this immune privileged space is evolving as recent studies suggest that immune surveillance of the CNS takes place in meningeal vasculature largely lacking in endothelial tight junctions [25]. Nevertheless, tumor-infiltrating lymphocytes are largely absent from major classes of GBM, making them an immunologically “cold” tumor [26]. HLA class I downregulation [27] and low CD4 T cell counts [28] have been shown to correspond with poor prognosis and shorter survival in GBM patients. This indicates that despite the immunologically distinct status of the CNS, GBM occurs at least partially as a result of immune dysregulation and that invoking a T cell response could modulate GBM outcome. A high degree of heterogeneity in the GBM tumor cell population poses further challenges in GBM treatment. GBM cancer stem cells have been identified as the main drivers behind chemotherapeutic resistance [29] and tumor recurrence [30], and effective therapeutics must be able to target these cell populations in addition to more differentiated cells in the tumor bulk. Finally, the cranial space is inherently edema-intolerant. This poses further limitations for flow rates and pressures associated with bulk transport of therapeutics as well as potential immunotoxicities that could result from immunotherapy-induced cytokine release [9, 31].
Drug Delivery to Intracranial GBM
CED
Direct intracranial administration of therapeutics efficiently bypasses the BBB but requires surgical application and is inherently invasive. Because surgical resection of the tumor is part of the standard of care for GBM, this approach works well for GBM treatment and is the delivery method for Gliadel, a drug-loaded polymer wafer that is one of only two therapeutics to be approved for GBM treatment by the FDA in the past 30 years [32, 33]. Conventional intracranial drug administration methods like placement of Gliadel wafers and direct injection into the tumor rely mostly on diffusion for drug molecules to transport through the tumor bulk. This results in poor penetration even with drugs with ideal characteristics for diffusion, and only a small volume of tissue surrounding the drug source is treated [34, 35]. To address these transport limitations, Bobo et al. developed convection-enhanced delivery (CED), which relies on a pressure gradient generated at the tip of an intracranial infusion catheter to drive bulk flow of the drug through the brain interstitial space (Fig. 1) [36, 37]. Our lab has explored the use of CED and related techniques for the intracranial delivery of a range of biodegradable polymeric nanoparticles carrying nucleic acid drugs to treat brain cancer. We demonstrated that repeat intracranial infusions of nanoparticles encapsulating antitumor microRNAs for epigenetic modulation of GBM stem cells [38] or plasmid DNA encoding an HSV-tk suicide gene [39] significantly extended survival in several orthotopic mouse models of human brain cancer. To improve the clinical translatability of these therapeutics, CED can be performed by implanting an osmotic pump containing a drug depot. Yu et al. have shown that lipopolymeric nanoparticles encapsulating multiple siRNAs infused using such implantable pumps resulted in significant survival benefits in mice without necessitating repeated invasive intracranial procedures [40].
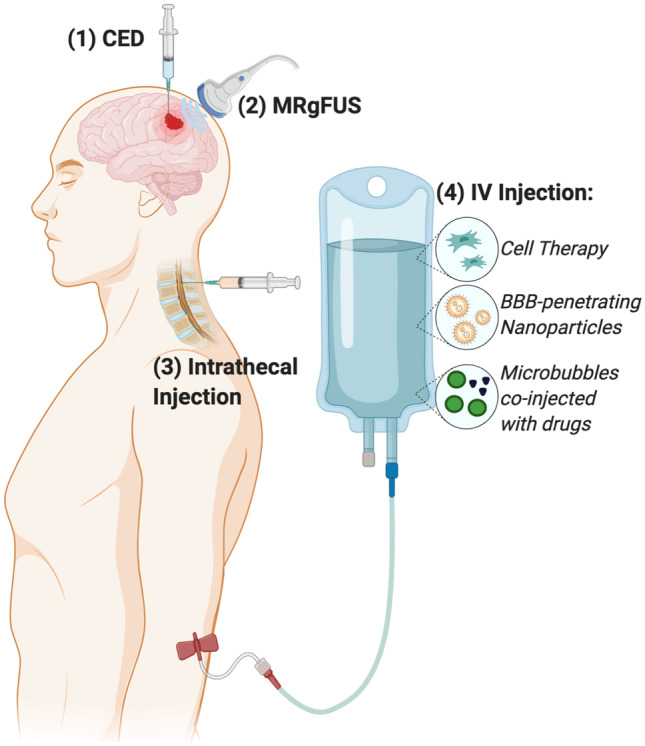
Fig. 1.
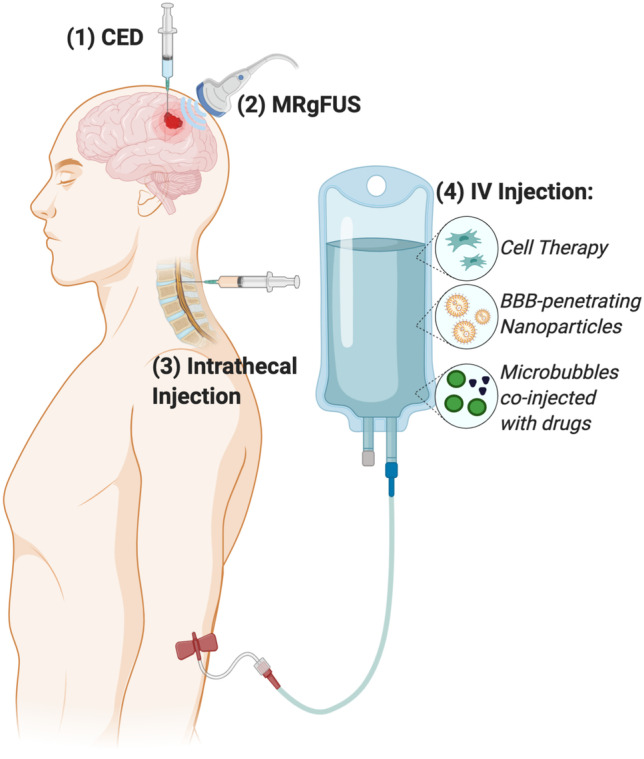
Routes of administration for therapeutic delivery to intracranial GBM. Routes of administration enabling drug delivery across the BBB to the tumor site include (1) direct intratumoral injection or convection enhanced delivery (CED); (2) MRI-guided focused ultrasound to cause transient disruptions in the BBB (MRgFUS); (3) intrathecal injection into the CSF; and (4) intravenous (IV) injection of tumor-homing cell therapies, nanocarriers conjugated with BBB-penetrating ligands, or microbubbles designed to cavitate upon MRgFUS application and allow co-injected drugs to cross the BBB
Intracranial CED has begun to make its way into the clinic. The chemotherapeutic agent paclitaxel [41] and liposomal vectors bearing the HSV-tk suicide gene [42] have been administered via CED in phase I/II clinical trials and generally demonstrated significant reduction in tumor volume in patients with recurrent GBM. However, drug delivery complications including chemical meningitis, peritumoral edema, and inhomogeneity in drug distribution have been reported, suggesting that while CED is a promising route of administration for GBM therapeutics, substantial optimization of delivery vehicles and transport parameters is still needed. A recent study using a skull-mounted transcutaneous port to administer drugs for Parkinson’s disease via CED, while failing to provide clinical benefit, demonstrated putamen-wide delivery and was generally well-tolerated [43]. This study demonstrates the potential for CED methods to safely distribute drugs to a large portion of the brain.
MRI-guided focused ultrasound
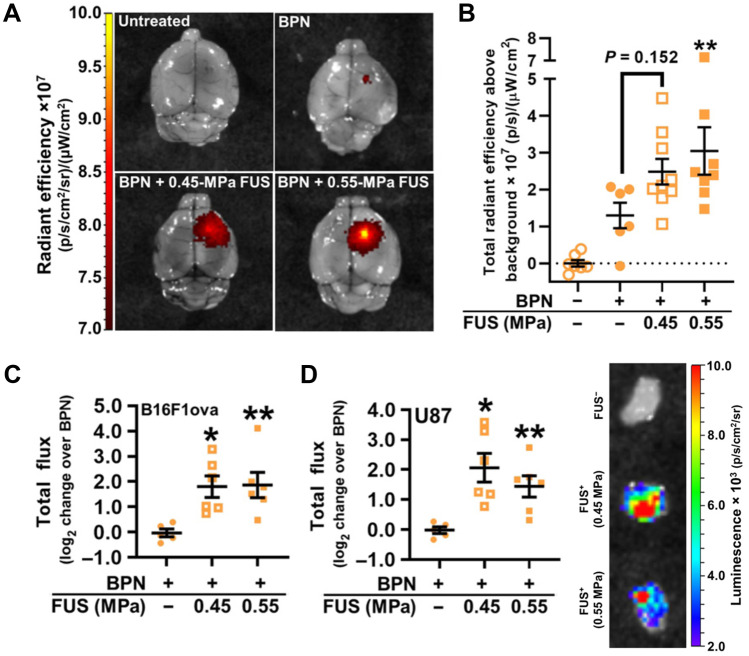
Focused ultrasound techniques concentrate acoustic energy on a focal spot in the brain measuring a few millimeters in diameter and, combined with magnetic resonance imaging (MRI), have been used in the clinic for thermal coagulation of tumors in human patients with real-time monitoring [44, 45]. MRI-guided focused ultrasound (MRgFUS) used to induce cavitation of intravenously (IV) administered microbubbles has been reported to reliably cause temporary physical disruption of the BBB in small animal models and has been used for delivery of BBB-impermeably compounds such as antibody drugs [46, 47]. Airan et al. delivered nanoemulsions encapsulating a BBB-permeable small molecule anesthetic using MRgFUS to enable potent intracranial drug delivery with high spatiotemporal control in an acute rate seizure model without brain parenchymal damage [48], demonstrating the fine level of control that can be exerted with this neuromodulatory technique. Curley et al. showed that MRgFUS-mediated transient BBB opening doubled interstitial flow velocity and increased dispersion of gene delivery nanoparticles through brain tumor tissue by > 100%, resulting in a fourfold increase in transfection of orthotopic U87 and B16F1-ova brain tumors compared to nanoparticle administration alone (Fig. 2) [49]. A first-in-human trial using MRgFUS to induce BBB opening in four amyotrophic lateral sclerosis patients showed successful BBB opening immediately after sonication which normalized after 24 h and reported no serious adverse events [50]. These studies demonstrate that MRgFUS is a viable approach to temporarily permeabilize the BBB with high spatiotemporal control and enable GBM therapeutics to reach the brain.
Fig. 2.
MRgFUS-mediated transient BBB opening enabled gene delivery NPs to accumulate in and transfect intracranial tumors. A Fluorescence images of whole brains with U87 GBM tumors after MRgFUS delivery of intravenously administered nanoparticles encapsulating Cy5-labeled plasmid DNA. BPN, brain-penetrating nanoparticles. B Total fluorescence radiant efficiency in excised U87 tumors. C, D Luciferase expression in intracranial B16F1ova melanoma (C) and U87 (D) tumors 3 days after treatment. Reproduced with permission from Curley et al. [49]
Despite numerous preclinical studies demonstrating the feasibility of using MRgFUS for drug delivery to the brain, the adoption of this technique for clinical utilization has been slow. While an intact BBB is undoubtedly necessary for proper functioning of the brain, numerous studies have shown that temporary opening of the BBB by MRgFUS is not accompanied by adverse side effects such as neuronal damage, inflammation, or infection in the healthy brain [51]. In fact, several studies have shown that using MRgFUS to open the BBB in the absence of drug delivery has actually led to improvements in pathology in rodent models of CNS diseases [52, 53]. However, the complex nature of this technique may be a major obstacle to its clinical translation as parameters such as the frequency of the transmitted ultrasound, duration of ultrasound pulse, and microbubble size and dose all affect the degree of MRgFUS-mediated BBB opening and need to be optimized for each case [51, 54]. Questions also remain regarding the repeatability of the FUS procedure and potential impacts on off-target brain tissue.
Intrathecal administration
Intrathecal administration involves directly injecting therapeutics into the cerebrospinal fluid (CSF) via the spinal canal. Drugs administered using this route have 100% bioavailability in the CSF, allowing significantly lower drug doses to be used compared to other administration routes, and is widely used for applications such as pain management [55]. Intrathecal administration has been successfully used in the clinic to administer BBB-impermeable drugs such as splice-modulating oligonucleotides for the treatment of neurodegenerative diseases [56], including the FDA-approved drug Nusinersen for treatment of spinal muscular atrophy [57]. This method of administration has also been shown to successfully deliver therapeutics to the brain. Gray et al. demonstrated that broad transduction of the brain and spinal cord parenchyma (approximately 2% of the entire brain and spinal cord) could be achieved by injecting adeno-associated viruses (AAV) into the CSF of non-human primates [58]. Furthermore, intrathecal administration had the added benefits of reduced peripheral organ biodistribution and protection from circulating antibodies against AAV, suggesting that this strategy could protect viral delivery vehicles from immune neutralization and allow repeat administrations. Although intrathecal administration has traditionally been used for drug delivery to the spinal cord, several recent studies have highlighted delivery parameters that can be modulated to bias delivery to the brain. Li et al. demonstrated that fast injection of AAV (0.25 μL/s) increased gene expression in the cortex by fourfold compared to a slow injection speed (0.02 μL/s), but this method also led to stronger transduction in peripheral lymph nodes and muscles [59]. Castle et al. reported that placing animals in an inverted or continuously rotating position immediately after intrathecal AAV injection countered the effect of gravitational settling of the CSF and resulted in 15-fold increase in the number of transduced neurons and higher gene expression consistence from animal to animal [60].
Cell-based targeting strategies
IV injected mesenchymal stem cells (MSCs) have been reported to home to sites of traumatic brain injury [61] or brain lesions [62] and have been shown to transmigrate across in vitro models of the BBB through transient inter-endothelial gaps [63]. Jiang et al. exploited the tumor-homing capabilities of MSCs for cell-based therapies against brain cancer by employing ex vivo genetic modification techniques to induce MSCs to express TNF-related apoptosis-inducing ligand (TRAIL) for cancer-specific cell killing. Upon administration in the brain hemisphere contralateral to the tumor, these genetically engineered cells migrated across the corpus collosum to the tumor site, killing infiltrative tumor cells and significantly prolonging animal survival [64]. Mangraviti et al. further demonstrated that human MSCs engineered ex vivo to secrete bone morphogenic protein 4 using non-viral polymeric nanoparticles exhibited brain tropism, crossed the BBB, and significantly improved survival upon IV or intranasal administration [65]. Zhao et al. recently reported a cell-based delivery platform which facilitated the assembly of biologically active nanocomplexes on cell surfaces via a metal-phenolic coordination-mediated interfacial interaction [66]. This strategy can be readily applied to conjugate protein and small molecule drugs onto the surface of MSCs for immunotherapeutic delivery to GBM.
Ongoing exciting immunotherapy research has focused on the use of engineered immune cells for cancer treatment, including for GBM. Chimeric antigen receptor (CAR) T cells are genetically engineered to steer T cells, including CD8 + cytotoxic T lymphocytes, specifically to their target cancer cells. Studies on ex vivo engineered CAR T cells to treat GBM in the clinic are still nascent but have thus far indicated the potential for safety and efficacy [67]. In other complementary pre-clinical studies, macrophages are being genetically engineered in situ with mRNA-containing nanoparticles to become converted from an M2-like phenotype to an M1-like phenotype, and this therapeutic approach shows increased survival in a transgenic mouse model of PDGFβ-driven glioma [68]. Thus, cell therapy with genetically engineered cells, via mesenchymal stem cells, T cells, or macrophages, is a promising future approach to overcome certain delivery challenges in the treatment of brain cancer.
Ligand-mediated strategies for BBB crossing
Conjugating ligands whose receptors are highly expressed on BBB endothelial cells onto drug delivery vehicles is a widely explored strategy to enable therapeutics to transport across the BBB. In this section, we provide an overview of the main active targeting ligands and their use in modifying nanocarriers for drug delivery to GBM (see Table 1 for summary of commonly used BBB-penetrating ligands).
Table 1.
Summary of drug delivery strategies to bypass the BBB
| Method | Description | Notes | Ref | |
|---|---|---|---|---|
| Direct therapeutic administration to the CNS | Convection enhanced delivery (CED) | Infusion catheter inserted intracranially delivers therapeutics directly to the brain | Physically bypasses the BBB but procedure is invasive | [36–39] |
| Intrathecal delivery | Injection of therapeutics into the CSF via the spinal canal | Enables drug delivery to spinal cord as well as brain | [58–60] | |
| Temporary physical disruption of BBB | MRI-guided focused ultrasound (MRgFUS) | Focused ultrasound induces cavitation of IV administered microbubbles and temporarily permeabilizes BBB; MRI enables spatiotemporal control | Has been used in the clinic for thermal coagulation of tumors in human patients | [46, 48, 49] |
| Strategies to cross BBB after IV therapeutic administration and/or to penetrate brain ECM | Cell-based therapeutics | IV injected mesenchymal stem cells (MSCs) have been shown to cross the BBB and home to sites of brain tumor or injury. Engineered CAR T Cells and macrophages can be used to target and treat brain cancer | MSCs can be genetically engineered to secrete therapeutic molecules or molecules can be attached to the surface of MSCs | [61, 64, 65, 67, 68] |
| Ligands targeting transferrin receptor | Transferrin receptor (TfR) is highly expressed by brain capillary endothelial cells, and ligands such as TfR-binding antibodies and the transferrin molecule can enable BBB crossing | Molecules that bind with ultra-high affinity to TfR have been shown to facilitate lysosomal sequestration in BBB endothelial cells and reduce transcytosis to the brain | [69, 73, 75] | |
| Angiopep-2 peptide | Angiopep-2 targets low-density lipoprotein receptor-related protein-1 (LRP1) that is expressed on brain endothelial cells lining the BBB | Angiopep-2 is also overexpressed on GBM and brain metastases from lung and skin cancers, enabling dual targeting of the BBB and cancer cells | [77–79] | |
| Sugar molecules | Glucose transporter protein 1 (GLUT1) on BBB endothelial cells allow drug delivery vehicles displaying ligands such as glucose or galactose to transport across BBB | Surface density of sugar molecules and rapid glycemic intake following fasting play an important role on nanocarrier transcytosis of the BBB | [81–84] |
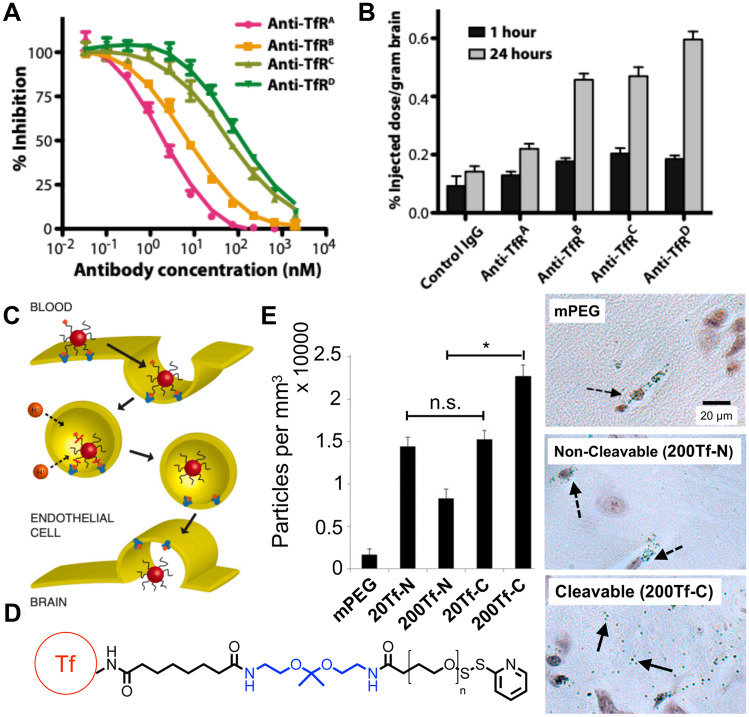
The transferrin receptor (TfR) is highly expressed by brain capillary endothelial cells (BCECs) forming the BBB, and monoclonal antibodies binding to TfR have been well documented to be internalized by BCECs in vivo [69] as well as enhance brain tissue accumulation when conjugated to nanocarriers [70]. Lam et al. reported that transferrin-functionalized liposomes loaded with the drugs temozolomide (TMZ) and JQ1 traversed intact BBB in orthotopic murine GBM models and led to improved survival compared to equivalent doses of free drug [71]. Studies have reported that ligands with ultra-high affinity to TfR facilitate lysosomal sequestration in BCECs and reduce transcytosis to the brain [72]. To address this problem, Yu et al. used protein engineering approaches to reduce the TfR-binding affinity of anti-TfR antibodies by creating a bispecific antibody with low affinity for TfR and high affinity for the enzyme β-secretase (BACE1), an Alzheimer’s disease drug target (Fig. 3). They demonstrated fivefold greater brain accumulation of the lower TfR affinity bispecific antibody compared to the control antibody and significant reduction in brain amyloid-β peptide levels after a single administration [73]. Kariolis et al. used directed evolution to engineer Fc fragments to bind the apical domain of TfR. When conjugated onto Fabs, these engineered Fc fragments enabled nearly 40-fold higher brain uptake than native antibodies upon IV administration in mice [74]. Clark and Davis utilized a materials engineering approach to design acid-labile linkages between a transferrin ligand and gold nanoparticles (NPs) to allow NP release upon endocytosis into acidic vesicles and showed significantly increased NP accumulation in brain parenchyma following transcytosis across BCECs [75]. Interestingly, these linkers did not enable NP trafficking to the brain when high affinity TfR antibodies were used, demonstrating the importance of modulating binding affinity at the ligand-TfR interface.
Fig. 3.
Strategies to reduce binding affinity with transferrin receptors on BBB endothelial cells increased delivery to the brain. A Yu et al. engineered a series of anti-TfR antibodies with varying TfR binding affinities. B Decreasing TfR binding affinity increased drug delivery to the brain especially at 24 h after IV administration of nanoparticles. Reproduced with permission from Yu et al. [73]. C Proposed mechanism for BBB transcytosis of NPs conjugated to holo-transferrin (holo-Tf) ligand via an acid-labile linkage. D Chemical structure of the acid-labile linker used by Clark and Davis to conjugate holo-Tf to NPs; holo-Tf in red, acid-labile linkage in blue. E NPs decorated with holo-Tf via acid cleavable bonds (Tf-C) induced significantly higher levels of NP accumulation in brain parenchyma compared to NPs modified with non-cleavable bonds (Tf-N) at high modification densities (*P < 0.0001). Histology images showed that NPs modified with Tf-N or mPEG were mostly entrapped in blood vessels, but NPs modified with Tf-C dispersed within brain parenchyma (dashed arrows indicate NPs within blood vessels; solid arrows indicate NPs in brain parenchyma). Reproduced with permission from Clark and Davis [75]
The angiopep-2 peptide is another widely used ligand and binds to low-density lipoprotein receptor-related protein-1 (LRP1) expressed on BCECs. NPs coated with angiopep-2 peptide have been shown to bind to LRP1 expressed on BCECs lining the BBB and move across in vitro models of the BBB via an energy-dependent, caveolae- and clathrin-mediated mode of transcytosis [76]. As LRP1 is also overexpressed in GBM and brain metastases from lung and skin cancers, angiopep-2 targeting provides the dual advantage of BBB transcytosis and tumor cell targeting [77]. Qiao et al. designed an angiopep-2-functionalized lipopolymeric NP system encapsulating superparamagnetic iron oxide nanocubes (SPIONs), TMZ, and TGF-β siRNA for immunomodulation of orthotopic GBM tumors. They demonstrated that this theranostic system enabled SPION-mediated MRI tumor imaging and facilitated GBM immunotherapy through TGF-β knockdown and TMZ therapy, resulting in 80% long-term survivors in a GL261 orthotopic mouse glioma model [78]. Similarly, Zheng et al. used angiopep-2 to modify polymeric NPs for combinatorial siRNA therapy and showed significant tumor burden decreases in mice bearing orthotopic U87 human GBM tumors [79]. Joseph et al. conjugated angopep-2 onto the surface of autonomous nano-swimmers to enable transport across the BBB. Using enzymatic reactions encapsulated inside polymersomes with asymmetric surface permeabilities to generate a local gradient, these nano-swimmers demonstrated rapid self-propelled diffusion throughout the parenchyma over mm length scales in rats following intra-arterial perfusion [80].
The brain’s high demand for sugar molecules has also been explored for enabling transport across the BBB. Glucose transporter proteins (GLUT) deliver glucose from circulation to neurons, with GLUT1 being abundantly present in BCECs [81]. Anraku et al. demonstrated that BBB crossing and brain accumulation of a PEG-poly(aspartic acid) decorated with glucose molecules on the surface were boosted by rapid glycemic intake following fasting as GLUT1 in BCECs migrated from the luminal to basal membrane [82]. Fasting for 24 h prior to NP injection followed by intraperitoneal administration of a concentrated glucose solution boosted brain accumulation of NPs by 56-fold compared to the free-feeding group, while the optimal glucose density (25% for this system) resulted in threefold higher brain accumulation than formulations with either higher or lower surface glucose density. PEG-(poly-L-lysine) NPs encapsulating oligonucleotides and decorated with surface glucose molecules were reported to enable nearly 7% accumulation in the whole brain following IV administration with glycemic control [83]. The optimized NP formulation in this study enabled 30% gene knockdown in the whole brain, with nearly 50% knockdown in the cerebral cortex and approximately 30% knockdown in areas such as the midbrain and thalamus/hypothalamus, respectively. Galactose, another sugar molecule shown to bind to GLUT1, has likewise been used to modify polymers for trans-BBB delivery of siRNA targeting BACE1 in a proof-of-concept study for Alzheimer’s disease treatment [84].
One of the main challenges associated with ligand-mediated BBB crossing methods is displaying ligands on NP surfaces in a functional manner. Covalent ligand conjugation is problematic for NPs formed via self-assembly such as polymeric NPs; this is because high molecular weight ligands such as antibodies could result in charge or steric interference that prevents NP formation [85]. This problem could be mitigated by modifying ligands with a charged molecule to enable incorporation into NPs through electrostatic interactions. This strategy was utilized by Smith et al., who modified T cell-targeting antibodies with a negatively charged poly-glutamic acid molecule to enable incorporation into cationic polymeric NPs, which they showed were capable of in situ T cell reprogramming with plasmid DNA encoding chimeric antigen receptor genes [86]. Another challenge is that serum protein coronas have been shown to form rapidly on NPs injected into the bloodstream, which significantly affect NP pathophysiology as well as mask the ability of targeting ligands to interact with cell receptors [87, 88]. This problem can be addressed by adding a hydrophilic stealth molecule such as polyethylene glycol (PEG) onto NP surfaces. PEG molecules incorporated in the form of a PEG linker for ligand conjugation [89] or as a coating molecule to completely saturate the NP surface [90] have been shown to prevent serum protein adsorption and improve NP cell targeting.
Other considerations for intracranial drug delivery
Beyond transport across the BBB, there are several further considerations for effective therapeutic delivery to GBM. This includes design strategies to enable transport through the brain ECM, which has long been believed to require sub-64 nm sizes due to imaging and modeling data showing that brain ECM contains “pores” 38–64 nm in diameter [91]. Nance et al. demonstrated that NPs 114 nm in diameter could diffuse through fresh human and rat brain tissue by using a dense PEG surface coating [92]. The selection of animal models for studying drug delivery to brain tumors has also been shown to be critical. Korangath et al. reported that intratumoral retention of antibody-labeled NPs depended on interactions with innate immune cells and not on antigen–antibody interactions [93]. The observed antitumor effects depended on activation of CD8 + T cells rather than the therapeutic activity of the loaded antibody drugs, highlighting the advantage of tumor models that can be established in immunocompetent animals. More specific to studies on GBM therapy, the method used to inoculate animals with intracranial tumors may be critical to accurately assessing cross-BBB transport and brain accumulation of therapeutic molecules. Wyatt and Davis reported that brain tumors established by intracranial injection of GBM cells enabled free drug and non-targeted NPs to accumulate in tumors and retard tumor growth more significantly compared to brain tumors established via intracardiac or IV administered cells in a model of breast cancer brain metastasis [94]. Thus, a robust test of the transport properties of drug delivery vehicles to GBM should utilize animal models that best preserve the architecture of the BBB, the architecture of the brain ECM, and the completeness of the immune system.
Biomaterials for cancer immunotherapy
Recent advances in therapeutic cancer vaccines
Cancer vaccines are designed to educate the immune system to recognize and eliminate cancer cells and are broken down into two groups, prophylactic and therapeutic. Prophylactic cancer vaccines induce protective immunity against viral pathogens known to cause cancer, such as the human papillomavirus (HPV) [95] and hepatitis B virus (HBV) [96]. Therapeutic vaccines activate immune cells in cancer patients to treat their current disease, and include sipuleucel-T, which uses patient-derived dendritic cells engineered ex vivo to recognize antigens overexpressed by prostate cancer cells [97] and the Bacillus Calmette–Guérin (BCG) vaccine, which is a vaccine against tuberculosis but also acts as a powerful general adjuvant for treatment of early-stage bladder cancer [98].
More recent studies on therapeutic vaccines have focused on stimulating immune responses against tumor neoantigens, which are expressed exclusively by tumor cells and can reduce possible side effects against healthy cells. To this end, Lynn et al. engineered a vaccine delivery system consisting of a single peptide containing the tumor neoantigen, adjuvants, and charge-modifying motifs. These peptides were shown to self-assemble into 20–30 nm nanoparticles regardless of the charge and hydrophobicity of the neoantigen peptide and enabled dose-dependent CD8 T cell responses in non-human primates [99]. Keskin et al. recently reported the use of a multi-epitope personalized neoantigen vaccine in eight patients as part of a phase I/Ib clinical trial of newly diagnosed GBM patients [100]. In the two patients that did not receive dexamethasone during vaccine priming, circulating neoantigen-specific CD4 + and CD8 + T cell responses were observed and an increase in the number of tumor-infiltrating T cells were detected, demonstrating the potential of tumor neoantigen-based cancer vaccines for GBM treatment [100].
One disadvantage to using vaccines based on patient-specific tumor neoantigens is that genetic sequencing of each tumor and synthesis of neoantigen peptides from identified mutations are required for each patient, which can be time consuming, expensive, and can reduce patient accessibility to the treatment. To avoid these problems, Tzeng et al. employed an alternative cancer vaccine approach by using synthetic polymeric nanoparticles encapsulating plasmid DNA encoding a costimulatory molecule (4-1BBL) and an immunostimulatory cytokine (IL-12) to reprogram tumor cells to act as tumor-associated antigen-presenting cells in situ [101]. When used in combination with anti-PD-1 checkpoint blockade, these reprogramming nanoparticles significantly reduced tumor growth and led to long-term survivors with immunological memory against tumor rechallenge in mice bearing syngeneic B16-F10 melanoma tumors and MC38 colorectal carcinoma tumors. Hewitt et al. took a similar approach by using lipid nanoparticles to deliver mRNA encoding the cytokines IL-36γ and IL-23 and the costimulatory molecule OX40L and showed > 90% long-term survival in MC38 colon cancer models and > 40% long-term survival in B16-F10 melanoma models when administered in combination with immune checkpoint blockade [102]. Crucially, these approaches do not require prior knowledge of the neoantigens in a particular patient’s tumor and could be used as an off-the-shelf platform to combat many different solid tumors.
Immunostimulatory effects of biomaterial scaffolds
In addition to acting as delivery vehicles for antigenic cargo, biomaterials have also been used to stimulate the immune system on their own. Recent studies on implantable biomaterial scaffolds to promote wound healing have established that engineered biomaterials can effectively modulate the immune system. Sadtler et al. reported that biomaterial scaffolds induced TH2 guided macrophage polarization to facilitate a local pro-regenerative immune response to facilitate healing of traumatic muscle wounds [103]. Wolf et al. reported that decellularized urinary bladder matrix scaffolds induced a type 2 immune response consisting of IL4-producing TH2 cells, CD206+ macrophages, and eosinophil accumulation which inhibited tumor formation in several murine cancer cell lines [104]. Shah et al. reported that a microporous alginate-based scaffold capable of delivering adjuvants and antigens against acute myeloid leukemia (AML) was able to eradicate established AML even in the absence of a defined vaccine antigen [105]. While the mechanism for this biomaterial-mediated anti-cancer effect is yet to be elucidated, this study provides further evidence that macroscopic materials can provide a unique local immunostimulatory niche with systemic cancer killing effects.
Strategies to overcome GBM immune privilege
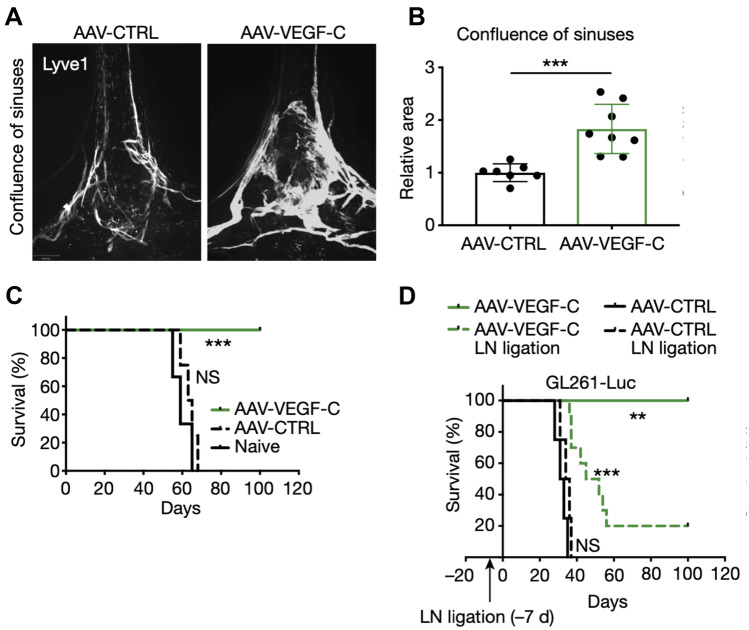
Immune privilege in the CNS is believed to be due to multiple chemical and physical factors including the presence of the BBB and other physical barriers, little to no MHC class II-expressing antigen-presenting cells in normal human brain, the lack of traditional lymphatics, the presence of immunomodulatory proteins, and CNS metabolic needs [24]. To bypass this CNS immune privilege, Song et al. administered nanoparticles containing mRNA encoding for the VEGF-C gene to the CSF to enhance lymphangiogenesis in the brain and increase T cell recruitment to GBM tumors (Fig. 4). The authors demonstrated > 80% long-term survival in mice with orthotopic GL261 tumors when VEGF-C nanoparticle treatment was used in combination with immune checkpoint blockade [106], which was attributed to brain lymphatic remodeling (treated mice showed nearly 100% increase in dural confluence of sinuses), allowing for increased T cell recruitment and drainage of tumor antigens to deep cervical lymph nodes.
Fig. 4.
Upregulation of VEFG-C expression increased lymphatic vasculature confluency and conferred protection against GBM in a draining lymph node-dependent manner. A The dura of mice 6–8 weeks after injection of AAV carrying a control (CTRL) or VEGF-C gene into the CSF; LYVE1 was stained to visualize lymphatic vasculature. B Mice injected with AAV-VEGF-C showed remodeling of lymphatic vasculature and significantly increased confluence compared to the AAV-CTRL group (n 7; ***P = 0.0007). C Mice injected with AAV-VEGF-C 2 months prior to intracranial implantation of GL261 murine brain cancer cells exhibited significantly prolonged survival (n 4; ***P = 0.0004). D Ligation of the deep cervical lymph nodes 1 week prior to tumor inoculation abrogated anti-tumor effects of AAV-VEGF-C injection (n 4; **P = 0.007; ***P < 0.0001). Reproduced with permission from Song et al. [106]
An alternative strategy to overcome the immunologically “cold” GBM tumor microenvironment is to employ viral induction of cytokine expression by tumor cells to promote dendritic cell differentiation and expansion. Specifically, this strategy has involved injecting adenoviral vectors encoding the herpes simplex virus-1 thymidine kinase (HSV-tk) and fms-like tyrosine kinase ligand (Flt3L) directly into intracranial murine glioma tumors. HSV-tk expression sensitizes tumor cells to the drug ganciclovir, causing apoptotic cell death [107]; Flt3l regulates dendritic cell development and mobilizes dendritic cell and T cell responses during inflammation and infection [108]. In a study by Ali et al., HSV-tk/GCV suicide gene therapy was used to kill tumor cells and release tumor antigens, which were subsequently taken up by dendritic cells that were recruited by Flt3L expression. The authors found that administration of either therapeutic modality alone caused tumor expression only in microscopic tumor models (e.g., ≤ 0.3 mm3), but co-expression of both genes prolonged the survival of animals bearing large tumors by over 80% in a macrophage- and CD4 + T cell-dependent manner [109]. A follow-up study showed that this combination gene therapy mediated regression in a secondary untreated tumor growing at a distal site in addition to inducing regression of the primary treated tumor, resulting in 70% long-term survival of animals bearing a single tumor and 50% long-term survival of animals bearing a primary and distal secondary tumor [110].
Finally, recent studies have found that the location of administration of combination therapies is another important consideration specific to GBM immunotherapy. The chemotherapeutic temozolomide that is administered systemically with radiation in standard therapy for newly diagnosed GBM has been shown to cause severe myelotoxicity [111]. This is problematic as the immunosuppressive effects of standard chemotherapy could antagonize the effects of immunotherapies. One strategy to attenuate the adverse effects of systemically administered chemotherapeutics is to load drugs into targeted nanoparticles for accumulation in tumors, which has been shown to be effective for sensitizing tumors to immune checkpoint blockade in combination therapies [112]. Wang et al. took the alternative approach of local chemotherapeutic delivery by synthesizing injectable scaffolds that gelled in situ and released encapsulated gemcitabine as well as anti-PD-L1 blocking antibodies for immune checkpoint blockade [113]. Mathios et al. studied this effect in GBM tumors and demonstrated that systemic administration of the chemotherapeutic Carmustine is immunosuppressive and abrogated anti-PD-1 antitumor effects while local chemotherapy left immune cell populations intact and enhanced the effects of immune checkpoint blockade [114].
Conclusions
The lack of new GBM therapies approved in the past two decades and grim prognosis for GBM patients highlight the need for new treatment strategies. The physiological location of GBM tumors within the CNS pose several formidable transport barriers for GBM therapeutics, including bypassing the BBB and trafficking through brain tissue and ECM to the tumor site. Immunotherapies for GBM face further obstacles of overcoming aspects of CNS immune privilege such as increased metabolism, abnormal lymphatics, and reduced expression of MHC class II by antigen-presenting cells compared to other tissues. Despite these daunting challenges, immunotherapies for GBM are making steady progress from bench to bedside. Physical strategies to enable trans-BBB drug delivery such as CED and MRgFUS have already been used in the clinic to treat other CNS malignancies and are excellent candidates for delivery of GBM immunotherapy. These methods could be first used to deliver FDA-approved small molecule drugs such as TMZ or immune checkpoint inhibitors to establish feasibility of GBM immunotherapy before being used in conjunction with more novel biomaterial-based GBM immunotherapies presented in this review. The recent successful use of lipid NPs to deliver mRNA in COVID-19 vaccines [115] demonstrated the safety and efficacy of using biomaterials to stimulate the immune system. A similar biomaterial system for cancer gene therapy is an exciting candidate for GBM immunotherapy. Currently, many clinical trials using immune checkpoint blockade [116] or monoclonal antibodies [117] are underway. Continued developments in both elucidating cancer biology mechanisms and innovating engineered drug delivery solutions are promising for future immunotherapy treatments for GBM.
Author contribution
YR and JJG wrote the manuscript.
Funding
This study is supported by NIH (R01CA228133, P41EB028239, and F31CA250319).
Declarations
Consent for publication
All authors approve the manuscript for publication.
Conflict of interest
JJG is a co-founder, manager and CTO of Dome Therapeutics and is a scientific advisory board member of Tidal Therapeutics. Any potential conflicts of interests are managed by the Johns Hopkins Committee on Outside Interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chakrabarti I, Cockburn M, Cozen W, Wang YP, Preston-Martin S. A population-based description of glioblastoma multiforme in Los Angeles County, 1974–1999. Cancer. 2005;104:2798–2806. doi: 10.1002/cncr.21539. [DOI] [PubMed] [Google Scholar]
- 2.Ostrom QT, Gittleman H, de Blank PM, Finlay JL, Gurney JG, McKean-Cowdin R, Stearns DS, Wolff JE, Liu M, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS. American Brain Tumor Association Adolescent and Young Adult Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008–2012. Neuro Oncol. 2016;18(Suppl 1):i1–i50. doi: 10.1093/neuonc/nov297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romero-Rojas AE, Diaz-Perez JA, Amaro D, Lozano-Castillo A, Chinchilla-Olaya SI. Glioblastoma metastasis to parotid gland and neck lymph nodes: fine-needle aspiration cytology with histopathologic correlation. Head Neck Pathol. 2013;7:409–415. doi: 10.1007/s12105-013-0448-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jackson CM, Lim M, Drake CG. Immunotherapy for Brain Cancer: Recent Progress and Future Promise. Clin Cancer Res. 2014;20:3651. doi: 10.1158/1078-0432.CCR-13-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anassi E, Ndefo UA. Sipuleucel-T (provenge) injection: the first immunotherapy agent (vaccine) for hormone-refractory prostate cancer. Pharmacy and Therapeutics. 2011;36:197–202. [PMC free article] [PubMed] [Google Scholar]
- 6.Lipson EJ, Drake CG. Ipilimumab: an anti-CTLA-4 antibody for metastatic melanoma. Clin Cancer Res. 2011;17:6958–6962. doi: 10.1158/1078-0432.CCR-11-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brooks WH, Netsky MG, Normansell DE, Horwitz DA. Depressed cell-mediated immunity in patients with primary intracranial tumors characterization of a humoral immunosuppressive factor. J Exp Med. 1972;136:1631–1647. doi: 10.1084/jem.136.6.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barker CF, Billingham RE. Immunologically Privileged Sites. In: Kunkel HG, Dixon FJ (Eds.). Advances in Immunology, Academic Press; 1978. pp. 1–54. [PubMed]
- 9.Tran TT, Jilaveanu LB, Omuro A, Chiang VL, Huttner A, Kluger HM. Complications associated with immunotherapy for brain metastases. Curr Opin Neurol. 2019;32. [DOI] [PMC free article] [PubMed]
- 10.Arvanitis CD, Ferraro GB, Jain RK. The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nat Rev Cancer. 2020;20:26–41. doi: 10.1038/s41568-019-0205-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abbott NJ, Rönnbäck L, Hansson E. Astrocyte–endothelial interactions at the blood–brain barrier. Nat Rev Neurosci. 2006;7:41. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 12.Pardridge WM. Drug transport across the blood–brain barrier. J Cereb Blood Flow Metab. 2012;32:1959–1972. doi: 10.1038/jcbfm.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C. Pericytes regulate the blood–brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 14.Sarkaria JN, Hu LS, Parney IF, Pafundi DH, Brinkmann DH, Laack NN, Giannini C, Burns TC, Kizilbash SH, Laramy JK, Swanson KR, Kaufmann TJ, Brown PD, Agar NYR, Galanis E, Buckner JC, Elmquist WF. Is the blood-brain barrier really disrupted in all glioblastomas? A critical assessment of existing clinical data. Neuro Oncol. 2018;20:184–191. doi: 10.1093/neuonc/nox175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol. 2010;7:653. doi: 10.1038/nrclinonc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hashizume H, Baluk P, Morikawa S, McLean JW, Thurston G, Roberge S, Jain RK, McDonald DM. Openings between Defective Endothelial Cells Explain Tumor Vessel Leakiness. Am J Pathol. 2000;156:1363–1380. doi: 10.1016/S0002-9440(10)65006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 18.Danhier F. To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine? J Control Release. 2016;244:108–121. doi: 10.1016/j.jconrel.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 19. Hansen AE, Petersen AL, Henriksen JR, Boerresen B, Rasmussen P, Elema DR, Rosenschöld PMa, Kristensen AT, Kjær A, Andresen TL. Positron emission tomography based elucidation of the enhanced permeability and retention effect in dogs with cancer using copper-64 liposomes. ACS Nano. 2015;9:6985–6995. [DOI] [PubMed]
- 20.Sindhwani S, Syed AM, Ngai J, Kingston BR, Maiorino L, Rothschild J, MacMillan P, Zhang Y, Rajesh NU, Hoang T, Wu JLY, Wilhelm S, Zilman A, Gadde S, Sulaiman A, Ouyang B, Lin Z, Wang L, Egeblad M, Chan WCW. The entry of nanoparticles into solid tumours. Nat Mater. 2020;19:566–575. doi: 10.1038/s41563-019-0566-2. [DOI] [PubMed] [Google Scholar]
- 21.van Tellingen O, Yetkin-Arik B, de Gooijer MC, Wesseling P, Wurdinger T, de Vries HE. Overcoming the blood–brain tumor barrier for effective glioblastoma treatment. Drug Resist Updat. 2015;19:1–12. doi: 10.1016/j.drup.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Stine CA, Munson JM. Convection-Enhanced Delivery: Connection to and Impact of Interstitial Fluid Flow. Front Oncol. 2019;9:966. doi: 10.3389/fonc.2019.00966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Medawar PB. Immunity to homologous grafted skin; the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br J Exp Pathol. 1948;29:58–69. [PMC free article] [PubMed] [Google Scholar]
- 24.Forrester JV, McMenamin PG, Dando SJ. CNS infection and immune privilege. Nat Rev Neurosci. 2018;19:655–671. doi: 10.1038/s41583-018-0070-8. [DOI] [PubMed] [Google Scholar]
- 25.Rustenhoven J, Kipnis J. Bypassing the blood-brain barrier. Science. 2019;366:1448. doi: 10.1126/science.aay0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rutledge WC, Kong J, Gao J, Gutman DA, Cooper LAD, Appin C, Park Y, Scarpace L, Mikkelsen T, Cohen ML, Aldape KD, McLendon RE, Lehman NL, Miller CR, Schniederjan MJ, Brennan CW, Saltz JH, Moreno CS, Brat DJ. Tumor-Infiltrating Lymphocytes in Glioblastoma Are Associated with Specific Genomic Alterations and Related to Transcriptional Class. Clin Cancer Res. 2013;19:4951. doi: 10.1158/1078-0432.CCR-13-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yeung JT, Hamilton RL, Ohnishi K, Ikeura M, Potter DM, Nikiforova MN, Ferrone S, Jakacki RI, Pollack IF, Okada H. LOH in the HLA class I region at 6p21 is associated with shorter survival in newly diagnosed adult glioblastoma. Clin Cancer Res. 2013;19:1816–1826. doi: 10.1158/1078-0432.CCR-12-2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grossman SA, Ye X, Lesser G, Sloan A, Carraway H, Desideri S, Piantadosi S, Consortium NC Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res. 2011;17:5473–5480. doi: 10.1158/1078-0432.CCR-11-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mazor G, Levin L, Picard D, Ahmadov U, Carén H, Borkhardt A, Reifenberger G, Leprivier G, Remke M, Rotblat B. The lncRNA TP73-AS1 is linked to aggressiveness in glioblastoma and promotes temozolomide resistance in glioblastoma cancer stem cells. Cell Death Dis. 2019;10:1–14. doi: 10.1038/s41419-019-1477-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orzan F, De Bacco F, Crisafulli G, Pellegatta S, Mussolin B, Siravegna G, D'Ambrosio A, Comoglio PM, Finocchiaro G, Boccaccio C. Genetic evolution of glioblastoma stem-like cells from primary to recurrent tumor. Stem Cells. 2017;35:2218–2228. doi: 10.1002/stem.2703. [DOI] [PubMed] [Google Scholar]
- 31.Guha-Thakurta N, Wierda WG. Cerebral edema secondary to chimeric antigen receptor T-cell immunotherapy. Neurology. 2018;91:843. doi: 10.1212/WNL.0000000000006436. [DOI] [PubMed] [Google Scholar]
- 32.Brem H, Piantadosi S, Burger PC, Walker M, Selker R, Vick NA, Black K, Sisti M, Brem S, Mohr G. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. Lancet. 1995;345:1008–1012. doi: 10.1016/S0140-6736(95)90755-6. [DOI] [PubMed] [Google Scholar]
- 33.Ashby LS, Smith KA, Stea B. Gliadel wafer implantation combined with standard radiotherapy and concurrent followed by adjuvant temozolomide for treatment of newly diagnosed high-grade glioma: a systematic literature review. World J Surg Oncol. 2016;14:225. doi: 10.1186/s12957-016-0975-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kroin JS, Penn RD. Intracerebral chemotherapy: chronic microinfusion of cisplatin. Neurosurgery. 1982;10:349–354. doi: 10.1227/00006123-198203000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Sendelbeck SL, Urquhart J. Spatial distribution of dopamine, methotrexate and antipyrine during continuous intracerebral microperfusion. Brain Res. 1985;328:251–258. doi: 10.1016/0006-8993(85)91036-4. [DOI] [PubMed] [Google Scholar]
- 36.Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci. 1994;91:2076. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mehta AM, Sonabend AM, Bruce JN. Convection-Enhanced Delivery. Neurotherapeutics. 2017;14:358–371. doi: 10.1007/s13311-017-0520-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lopez-Bertoni H, Kozielski KL, Rui Y, Lal B, Vaughan H, Wilson DR, Mihelson N, Eberhart CG, Laterra J, Green JJ. Bioreducible polymeric nanoparticles containing multiplexed cancer stem cell regulating miRNAs inhibit glioblastoma growth and prolong survival. Nano Lett. 2018;18:4086–4094. doi: 10.1021/acs.nanolett.8b00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi J, Rui Y, Kim J, Gorelick N, Wilson DR, Kozielski K, Mangraviti A, Sankey E, Brem H, Tyler B, Green JJ, Jackson EM. Nonviral polymeric nanoparticles for gene therapy in pediatric CNS malignancies, Nanomedicine: Nanotechnology. Biol Med. 2020;23:102115. doi: 10.1016/j.nano.2019.102115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu D, Khan OF, Suvà ML, Dong B, Panek WK, Xiao T, Wu M, Han Y, Ahmed AU, Balyasnikova IV, Zhang HF, Sun C, Langer R, Anderson DG, Lesniak MS. Multiplexed RNAi therapy against brain tumor-initiating cells via lipopolymeric nanoparticle infusion delays glioblastoma progression. Proc Natl Acad Sci. 2017;114:E6147. doi: 10.1073/pnas.1701911114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zvi L, Yael M, Tali J, Raphael P, Meir F, Dvora N, Moshe H, Zvi R. Convection-enhanced delivery of paclitaxel for the treatment of recurrent malignant glioma: a Phase I/II clinical study. J Neurosurg. 2004;100:472–479. doi: 10.3171/jns.2004.100.3.0472. [DOI] [PubMed] [Google Scholar]
- 42.Voges J, Reszka R, Gossmann A, Dittmar C, Richter R, Garlip G, Kracht L, Coenen HH, Sturm V, Wienhard K, Heiss WD, Jacobs AH. Imaging-guided convection-enhanced delivery and gene therapy of glioblastoma. Ann Neurol. 2003;54:479–487. doi: 10.1002/ana.10688. [DOI] [PubMed] [Google Scholar]
- 43.Whone A, Luz M, Boca M, Woolley M, Mooney L, Dharia S, Broadfoot J, Cronin D, Schroers C, Barua NU, Longpre L, Barclay CL, Boiko C, Johnson GA, Fibiger HC, Harrison R, Lewis O, Pritchard G, Howell M, Irving C, Johnson D, Kinch S, Marshall C, Lawrence AD, Blinder S, Sossi V, Stoessl AJ, Skinner P, Mohr E, Gill SS. Randomized trial of intermittent intraputamenal glial cell line-derived neurotrophic factor in Parkinson's disease. Brain. 2019;142:512–525. doi: 10.1093/brain/awz023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hynynen K, Pomeroy O, Smith DN, Huber PE, McDannold NJ, Kettenbach J, Baum J, Singer S, Jolesz FA. MR Imaging-guided Focused Ultrasound Surgery of Fibroadenomas in the Breast: A Feasibility Study. Radiology. 2001;219:176–185. doi: 10.1148/radiology.219.1.r01ap02176. [DOI] [PubMed] [Google Scholar]
- 45.Tempany CMC, Stewart EA, McDannold N, Quade BJ, Jolesz FA, Hynynen K. MR imaging–guided focused ultrasound surgery of uterine leiomyomas: a feasibility study. Radiology. 2003;226:897–905. doi: 10.1148/radiol.2271020395. [DOI] [PubMed] [Google Scholar]
- 46.Kinoshita M, McDannold N, Jolesz FA, Hynynen K. Targeted delivery of antibodies through the blood–brain barrier by MRI-guided focused ultrasound. Biochem Biophys Res Commun. 2006;340:1085–1090. doi: 10.1016/j.bbrc.2005.12.112. [DOI] [PubMed] [Google Scholar]
- 47.Kinoshita M, McDannold N, Jolesz FA, Hynynen K. Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood–brain barrier disruption. Proc Natl Acad Sci. 2006;103:11719. doi: 10.1073/pnas.0604318103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Airan RD, Meyer RA, Ellens NPK, Rhodes KR, Farahani K, Pomper MG, Kadam SD, Green JJ. Noninvasive targeted transcranial neuromodulation via focused ultrasound gated drug release from nanoemulsions. Nano Lett. 2017;17:652–659. doi: 10.1021/acs.nanolett.6b03517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Curley CT, Mead BP, Negron K, Kim N, Garrison WJ, Miller GW, Kingsmore KM, Thim EA, Song J, Munson JM, Klibanov AL, Suk JS, Hanes J, Price RJ. Augmentation of brain tumor interstitial flow via focused ultrasound promotes brain-penetrating nanoparticle dispersion and transfection. Sci Adv. 2020;6:eaay1344. [DOI] [PMC free article] [PubMed]
- 50.Abrahao A, Meng Y, Llinas M, Huang Y, Hamani C, Mainprize T, Aubert I, Heyn C, Black SE, Hynynen K, Lipsman N, Zinman L. First-in-human trial of blood–brain barrier opening in amyotrophic lateral sclerosis using MR-guided focused ultrasound. Nat Commun. 2019;10:4373. doi: 10.1038/s41467-019-12426-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burgess A, Shah K, Hough O, Hynynen K. Focused ultrasound-mediated drug delivery through the blood-brain barrier. Expert Rev Neurother. 2015;15:477–491. doi: 10.1586/14737175.2015.1028369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jordão JF, Thévenot E, Markham-Coultes K, Scarcelli T, Weng YQ, Xhima K, O'Reilly M, Huang Y, McLaurin J, Hynynen K. Amyloid-β plaque reduction, endogenous antibody delivery and glial activation by brain-targeted, transcranial focused ultrasound. Exp Neurol. 2013;248:16–29. doi: 10.1016/j.expneurol.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scarcelli T, Jordão JF, O'Reilly MA, Ellens N, Hynynen K, Aubert I. Stimulation of hippocampal neurogenesis by transcranial focused ultrasound and microbubbles in adult mice. Brain Stimul. 2014;7:304–307. doi: 10.1016/j.brs.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang S, Samiotaki G, Olumolade O, Feshitan JA, Konofagou EE. Microbubble type and distribution dependence of focused ultrasound-induced blood-brain barrier opening. Ultrasound Med Biol. 2014;40:130–137. doi: 10.1016/j.ultrasmedbio.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abs R, Verhelst J, Maeyaert J, Van Buyten JP, Opsomer F, Adriaensen H, Verlooy J, Van Havenbergh T, Smet M, Van Acker K. Endocrine Consequences of Long-Term Intrathecal Administration of Opioids. J Clin Endocrinol Metab. 2000;85:2215–2222. doi: 10.1210/jcem.85.6.6615. [DOI] [PubMed] [Google Scholar]
- 56.Kim J, Hu C, Moufawad El Achkar C, Black LE, Douville J, Larson A, Pendergast MK, Goldkind SF, Lee EA, Kuniholm A, Soucy A, Vaze J, Belur NR, Fredriksen K, Stojkovska I, Tsytsykova A, Armant M, DiDonato RL, Choi J, Cornelissen L, Pereira LM, Augustine EF, Genetti CA, Dies K, Barton B, Williams L, Goodlett BD, Riley BL, Pasternak A, Berry ER, Pflock KA, Chu S, Reed C, Tyndall K, Agrawal PB, Beggs AH, Grant PE, Urion DK, Snyder RO, Waisbren SE, Poduri A, Park PJ, Patterson A, Biffi A, Mazzulli JR, Bodamer O, Berde CB, Yu TW. Patient-customized oligonucleotide therapy for a rare genetic disease. N Engl J Med. 2019;381:1644–1652. doi: 10.1056/NEJMoa1813279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mercuri E, Darras BT, Chiriboga CA, Day JW, Campbell C, Connolly AM, Iannaccone ST, Kirschner J, Kuntz NL, Saito K, Shieh PB, Tulinius M, Mazzone ES, Montes J, Bishop KM, Yang Q, Foster R, Gheuens S, Bennett CF, Farwell W, Schneider E, De Vivo DC, Finkel RS. Nusinersen versus sham control in later-onset spinal muscular atrophy. N Engl J Med. 2018;378:625–635. doi: 10.1056/NEJMoa1710504. [DOI] [PubMed] [Google Scholar]
- 58. Gray SJ, Kalburgi SN, McCown TJ, Samulski RJ. Global CNS gene delivery and evasion of anti-AAV-neutralizing antibodies by intrathecal AAV administration in non-human primates. Gene Ther. 20;2013:450–459. [DOI] [PMC free article] [PubMed]
- 59.Li D, Liu C, Yang C, Wang D, Wu D, Qi Y, Su Q, Gao G, Xu Z, Guo Y. Slow Intrathecal Injection of rAAVrh10 Enhances its Transduction of Spinal Cord and Therapeutic Efficacy in a Mutant SOD1 Model of ALS. Neuroscience. 2017;365:192–205. doi: 10.1016/j.neuroscience.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Castle MJ, Cheng Y, Asokan A, Tuszynski MH. Physical positioning markedly enhances brain transduction after intrathecal AAV9 infusion. Sci Adv. 2018;4:eaau9859. [DOI] [PMC free article] [PubMed]
- 61. Menge T, Zhao Y, Zhao J, Wataha K, Gerber M, Zhang J, Letourneau P, Redell J, Shen L, Wang J. Mesenchymal stem cells regulate blood-brain barrier integrity through TIMP3 release after traumatic brain injury. Sci Transl Med. 4;2012:161ra150–161ra150. [DOI] [PMC free article] [PubMed]
- 62.Song CH, Honmou O, Ohsawa N, Nakamura K, Hamada H, Furuoka H, Hasebe R, Horiuchi M. Effect of transplantation of bone marrow-derived mesenchymal stem cells on mice infected with prions. J Virol. 2009;83:5918. doi: 10.1128/JVI.00165-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Matsushita T, Kibayashi T, Katayama T, Yamashita Y, Suzuki S, Kawamata J, Honmou O, Minami M, Shimohama S. Mesenchymal stem cells transmigrate across brain microvascular endothelial cell monolayers through transiently formed inter-endothelial gaps. Neurosci Lett. 2011;502:41–45. doi: 10.1016/j.neulet.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 64.Jiang X, Fitch S, Wang C, Wilson C, Li J, Grant GA, Yang F. Nanoparticle engineered TRAIL-overexpressing adipose-derived stem cells target and eradicate glioblastoma via intracranial delivery. Proc Natl Acad Sci. 2016;113:13857. doi: 10.1073/pnas.1615396113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mangraviti A, Tzeng SY, Gullotti D, Kozielski KL, Kim JE, Seng M, Abbadi S, Schiapparelli P, Sarabia-Estrada R, Vescovi A, Brem H, Olivi A, Tyler B, Green JJ, Quinones-Hinojosa A. Non-virally engineered human adipose mesenchymal stem cells produce BMP4, target brain tumors, and extend survival. Biomaterials. 2016;100:53–66. doi: 10.1016/j.biomaterials.2016.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhao Z, Pan DC, Qi QM, Kim J, Kapate N, Sun T, Shields IV CW, Wang LLW, Wu D, Kwon CJ, He W, Guo J, Mitragotri S. Engineering of living cells with polyphenol-functionalized biologically active nanocomplexes. Adv Mater. 2020;32(49):2003492. [DOI] [PubMed]
- 67.Akhavan D, Alizadeh D, Wang D, Weist MR, Shepphird JK, Brown CE. CAR T cells for brain tumors: Lessons learned and road ahead. Immunol Rev. 2019;290:60–84. doi: 10.1111/imr.12773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang F, Parayath NN, Ene CI, Stephan SB, Koehne AL, Coon ME, Holland EC, Stephan MT. Genetic programming of macrophages to perform anti-tumor functions using targeted mRNA nanocarriers. Nat Commun. 2019;10:3974. doi: 10.1038/s41467-019-11911-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bourassa P, Alata W, Tremblay C, Paris-Robidas S, Calon F. Transferrin Receptor-Mediated Uptake at the Blood-Brain Barrier Is Not Impaired by Alzheimer’s Disease Neuropathology. Mol Pharm. 2019;16:583–594. doi: 10.1021/acs.molpharmaceut.8b00870. [DOI] [PubMed] [Google Scholar]
- 70.Huwyler J, Wu D, Pardridge WM. Brain drug delivery of small molecules using immunoliposomes. Proc Natl Acad Sci. 1996;93:14164. doi: 10.1073/pnas.93.24.14164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lam FC, Morton SW, Wyckoff J, Vu Han TL, Hwang MK, Maffa A, Balkanska-Sinclair E, Yaffe MB, Floyd SR, Hammond PT. Enhanced efficacy of combined temozolomide and bromodomain inhibitor therapy for gliomas using targeted nanoparticles. Nat Commun. 2018;9:1991. [DOI] [PMC free article] [PubMed]
- 72.Bien-Ly N, Yu YJ, Bumbaca D, Elstrott J, Boswell CA, Zhang Y, Luk W, Lu Y, Dennis MS, Weimer RM, Chung I, Watts RJ. Transferrin receptor (TfR) trafficking determines brain uptake of TfR antibody affinity variants. J Exp Med. 2014;211:233–244. doi: 10.1084/jem.20131660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yu YJ, Zhang Y, Kenrick M, Hoyte K, Luk W, Lu Y, Atwal J, Elliott JM, Prabhu S, Watts RJ, Dennis MS. Boosting brain uptake of a therapeutic antibody by reducing its affinity for a transcytosis target. Sci Transl Med. 2011;3:84ra44. [DOI] [PubMed]
- 74.Kariolis MS, Wells RC, Getz JA, Kwan W, Mahon CS, Tong R, Kim DJ, Srivastava A, Bedard C, Henne KR, Giese T, Assimon VA, Chen X, Zhang Y, Solanoy H, Jenkins K, Sanchez PE, Kane L, Miyamoto T, Chew KS, Pizzo ME, Liang N, Calvert MEK, DeVos SL, Baskaran S, Hall S, Sweeney ZK, Thorne RG, Watts RJ, Dennis MS, Silverman AP, Zuchero YJY. Brain delivery of therapeutic proteins using an Fc fragment blood-brain barrier transport vehicle in mice and monkeys. Sci Transl Med. 2020;12:eaay1359. [DOI] [PubMed]
- 75.Clark AJ, Davis ME. Increased brain uptake of targeted nanoparticles by adding an acid-cleavable linkage between transferrin and the nanoparticle core. Proc Natl Acad Sci. 2015;112:12486. doi: 10.1073/pnas.1517048112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xin H, Sha X, Jiang X, Chen L, Law K, Gu J, Chen Y, Wang X, Fang X. The brain targeting mechanism of Angiopep-conjugated poly (ethylene glycol)-co-poly (ɛ-caprolactone) nanoparticles. Biomaterials. 2012;33:1673–1681. doi: 10.1016/j.biomaterials.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 77.Demeule M, Currie JC, Bertrand Y, Ché C, Nguyen T, Régina A, Gabathuler R, Castaigne J-P, Béliveau R. Involvement of the low-density lipoprotein receptor-related protein in the transcytosis of the brain delivery vector Angiopep-2. J Neurochem. 2008;106:1534–1544. doi: 10.1111/j.1471-4159.2008.05492.x. [DOI] [PubMed] [Google Scholar]
- 78.Qiao C, Yang J, Shen Q, Liu R, Li Y, Shi Y, Chen J, Shen Y, Xiao Z, Weng J, Zhang X. Traceable Nanoparticles with Dual Targeting and ROS Response for RNAi-Based Immunochemotherapy of Intracranial Glioblastoma Treatment. Adv Mater. 2018;30:1705054. doi: 10.1002/adma.201705054. [DOI] [PubMed] [Google Scholar]
- 79.Zheng M, Liu Y, Wang Y, Zhang D, Zou Y, Ruan W, Yin J, Tao W, Park JB, Shi B. ROS-responsive polymeric siRNA nanomedicine stabilized by triple interactions for the robust glioblastoma combinational RNAi therapy. Adv Mater. 2019;31:1903277. doi: 10.1002/adma.201903277. [DOI] [PubMed] [Google Scholar]
- 80.Joseph A, Contini C, Cecchin D, Nyberg S, Ruiz-Perez L, Gaitzsch J, Fullstone G, Tian X, Azizi J, Preston J, Volpe G, Battaglia G. Chemotactic synthetic vesicles: Design and applications in blood-brain barrier crossing. Sci Adv. 2017;3:e1700362. doi: 10.1126/sciadv.1700362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Simpson IA, Carruthers A, Vannucci SJ. Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cereb Blood Flow Metab. 2007;27:1766–1791. doi: 10.1038/sj.jcbfm.9600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Anraku Y, Kuwahara H, Fukusato Y, Mizoguchi A, Ishii T, Nitta K, Matsumoto Y, Toh K, Miyata K, Uchida S, Nishina K, Osada K, Itaka K, Nishiyama N, Mizusawa H, Yamasoba T, Yokota T, Kataoka K. Glycaemic control boosts glucosylated nanocarrier crossing the BBB into the brain. Nat Commun. 2017;8:1001. doi: 10.1038/s41467-017-00952-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Min HS, Kim HJ, Naito M, Ogura S, Toh K, Hayashi K, Kim BS, Fukushima S, Anraku Y, Miyata K, Kataoka K. Systemic brain delivery of antisense oligonucleotides across the blood–brain barrier with a glucose-coated polymeric nanocarrier. Angew Chem Int Ed. 2020;59:8173–8180. doi: 10.1002/anie.201914751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zhou Y, Zhu F, Liu Y, Zheng M, Wang Y, Zhang D, Anraku Y, Zou Y, Li J, Wu H, Pang X, Tao W, Shimoni O, Bush AI, Xue X, Shi B. Blood-brain barrier–penetrating siRNA nanomedicine for Alzheimer’s disease therapy. Sci Adv. 2020;6:eabc7031. [DOI] [PMC free article] [PubMed]
- 85.Guo YY, Huang L, Zhang ZP, Fu DH. Strategies for precise engineering and conjugation of antibody targeted-nanoparticles for cancer therapy. Current Medical Science. 2020;40:463–473. doi: 10.1007/s11596-020-2200-6. [DOI] [PubMed] [Google Scholar]
- 86.Smith TT, Stephan SB, Moffett HF, McKnight LE, Ji W, Reiman D, Bonagofski E, Wohlfahrt ME, Pillai SPS, Stephan MT. In situ programming of leukaemia-specific T cells using synthetic DNA nanocarriers. Nat Nanotechnol. 2017;12:813–820. doi: 10.1038/nnano.2017.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Salvati A, Pitek AS, Monopoli MP, Prapainop K, Bombelli FB, Hristov DR, Kelly PM, Åberg C, Mahon E, Dawson KA. Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nat Nanotechnol. 2013;8:137–143. doi: 10.1038/nnano.2012.237. [DOI] [PubMed] [Google Scholar]
- 88.Tenzer S, Docter D, Kuharev J, Musyanovych A, Fetz V, Hecht R, Schlenk F, Fischer D, Kiouptsi K, Reinhardt C, Landfester K, Schild H, Maskos M, Knauer SK, Stauber RH. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat Nanotechnol. 2013;8:772–781. doi: 10.1038/nnano.2013.181. [DOI] [PubMed] [Google Scholar]
- 89.Kumar S, Aaron J, Sokolov K. Directional conjugation of antibodies to nanoparticles for synthesis of multiplexed optical contrast agents with both delivery and targeting moieties. Nat Protoc. 2008;3:314–320. doi: 10.1038/nprot.2008.1. [DOI] [PubMed] [Google Scholar]
- 90.Dai Q, Walkey C, Chan WC. Polyethylene glycol backfilling mitigates the negative impact of the protein corona on nanoparticle cell targeting. Angew Chem Int Ed Engl. 2014;53:5093–5096. doi: 10.1002/anie.201309464. [DOI] [PubMed] [Google Scholar]
- 91.Thorne RG, Nicholson C. In vivo diffusion analysis with quantum dots and dextrans predicts the width of brain extracellular space. Proc Natl Acad Sci U S A. 2006;103:5567–5572. doi: 10.1073/pnas.0509425103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nance EA, Woodworth GF, Sailor KA, Shih TY, Xu Q, Swaminathan G, Xiang D, Eberhart C, Hanes J. A dense poly(ethylene glycol) coating improves penetration of large polymeric nanoparticles within brain tissue. Sci Transl Med. 2012;4:149ra119. [DOI] [PMC free article] [PubMed]
- 93.Korangath P, Barnett JD, Sharma A, Henderson ET, Stewart J, Yu SH, Kandala SK, Yang CT, Caserto JS, Hedayati M, Armstrong TD, Jaffee E, Gruettner C, Zhou XC, Fu W, Hu C, Sukumar S, Simons BW, Ivkov R. Nanoparticle interactions with immune cells dominate tumor retention and induce T cell–mediated tumor suppression in models of breast cancer. Sci Adv. 2020;6:eaay1601. [DOI] [PMC free article] [PubMed]
- 94.Wyatt EA, Davis ME. Method of establishing breast cancer brain metastases affects brain uptake and efficacy of targeted, therapeutic nanoparticles. Bioengineering & Translational Medicine. 2019;4:30–37. doi: 10.1002/btm2.10108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ault KA, Future IISG. Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet. 2007;369:1861–1868. doi: 10.1016/S0140-6736(07)60852-6. [DOI] [PubMed] [Google Scholar]
- 96.Chang MH, Chen CJ, Lai MS, Hsu HM, Wu TC, Kong MS, Liang DC, Shau WY, Chen DS. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. N Engl J Med. 1997;336:1855–1859. doi: 10.1056/NEJM199706263362602. [DOI] [PubMed] [Google Scholar]
- 97.Cheever MA, Higano CS. PROVENGE (Sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine. Clin Cancer Res. 2011;17:3520. doi: 10.1158/1078-0432.CCR-10-3126. [DOI] [PubMed] [Google Scholar]
- 98.Lamm DL. Bacillus Calmette-Guerin immunotherapy for bladder cancer. J Urol. 1985;134:40–46. doi: 10.1016/S0022-5347(17)46972-2. [DOI] [PubMed] [Google Scholar]
- 99. Lynn GM, Sedlik C, Baharom F, Zhu Y, Ramirez-Valdez RA, Coble VL, Tobin K, Nichols SR, Itzkowitz Y, Zaidi N. Peptide–TLR-7/8a conjugate vaccines chemically programmed for nanoparticle self-assembly enhance CD8 T-cell immunity to tumor antigens. Nat Biotechnol. 2020;1–13. [DOI] [PMC free article] [PubMed]
- 100.Keskin DB, Anandappa AJ, Sun J, Tirosh I, Mathewson ND, Li S, Oliveira G, Giobbie-Hurder A, Felt K, Gjini E, Shukla SA, Hu Z, Li L, Le PM, Allesøe RL, Richman AR, Kowalczyk MS, Abdelrahman S, Geduldig JE, Charbonneau S, Pelton K, Iorgulescu JB, Elagina L, Zhang W, Olive O, McCluskey C, Olsen LR, Stevens J, Lane WJ, Salazar AM, Daley H, Wen PY, Chiocca EA, Harden M, Lennon NJ, Gabriel S, Getz G, Lander ES, Regev A, Ritz J, Neuberg D, Rodig SJ, Ligon KL, Suvà ML, Wucherpfennig KW, Hacohen N, Fritsch EF, Livak KJ, Ott PA, Wu CJ, Reardon DA. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature. 2019;565:234–239. doi: 10.1038/s41586-018-0792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tzeng SY, Patel KK, Wilson DR, Meyer RA, Rhodes KR, Green JJ. In situ genetic engineering of tumors for long-lasting and systemic immunotherapy. Proc Natl Acad Sci. 2020;117:4043. doi: 10.1073/pnas.1916039117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Hewitt SL, Bai A, Bailey D, Ichikawa K, Zielinski J, Karp R, Apte A, Arnold K, Zacharek SJ, Iliou MS, Bhatt K, Garnaas M, Musenge F, Davis A, Khatwani N, Su SV, MacLean G, Farlow SJ, Burke K, Frederick JP. Durable anticancer immunity from intratumoral administration of IL-23, IL-36γ, and OX40L mRNAs. Sci Transl Med. 2019;11:eaat9143. [DOI] [PubMed]
- 103.Sadtler K, Estrellas K, Allen BW, Wolf MT, Fan H, Tam AJ, Patel CH, Luber BS, Wang H, Wagner KR, Powell JD, Housseau F, Pardoll DM, Elisseeff JH. Developing a pro-regenerative biomaterial scaffold microenvironment requires T helper 2 cells. Science. 2016;352:366. doi: 10.1126/science.aad9272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wolf MT, Ganguly S, Wang TL, Anderson CW, Sadtler K, Narain R, Cherry C, Parrillo AJ, Park BV, Wang G, Pan F, Sukumar S, Pardoll DM, Elisseeff JH. A biologic scaffold–associated type 2 immune microenvironment inhibits tumor formation and synergizes with checkpoint immunotherapy. Sci Transl Med. 2019;11:eaat7973. [DOI] [PMC free article] [PubMed]
- 105.Shah NJ, Najibi AJ, Shih TY, Mao AS, Sharda A, Scadden DT, Mooney DJ. A biomaterial-based vaccine eliciting durable tumour-specific responses against acute myeloid leukaemia. Nat Biomed Eng. 2020;4:40–51. doi: 10.1038/s41551-019-0503-3. [DOI] [PubMed] [Google Scholar]
- 106.Song E, Mao T, Dong H, Boisserand LSB, Antila S, Bosenberg M, Alitalo K, Thomas JL, Iwasaki A. VEGF-C-driven lymphatic drainage enables immunosurveillance of brain tumours. Nature. 2020;577:689–694. doi: 10.1038/s41586-019-1912-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Freeman SM, Abboud CN, Whartenby KA, Packman CH, Koeplin DS, Moolten FL, Abraham GN. The “bystander effect”: tumor regression when a fraction of the tumor mass is genetically modified. Can Res. 1993;53:5274. [PubMed] [Google Scholar]
- 108.Guermonprez P, Helft J, Claser C, Deroubaix S, Karanje H, Gazumyan A, Darasse-Jèze G, Telerman SB, Breton G, Schreiber HA, Frias-Staheli N, Billerbeck E, Dorner M, Rice CM, Ploss A, Klein F, Swiecki M, Colonna M, Kamphorst AO, Meredith M, Niec R, Takacs C, Mikhail F, Hari A, Bosque D, Eisenreich T, Merad M, Shi Y, Ginhoux F, Rénia L, Urban BC, Nussenzweig MC. Inflammatory Flt3l is essential to mobilize dendritic cells and for T cell responses during Plasmodium infection. Nat Med. 2013;19:730–738. doi: 10.1038/nm.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ali S, King GD, Curtin JF, Candolfi M, Xiong W, Liu C, Puntel M, Cheng Q, Prieto J, Ribas A, Kupiec-Weglinski J, van Rooijen N, Lassmann H, Lowenstein PR, Castro MG. Combined immunostimulation and conditional cytotoxic gene therapy provide long-term survival in a large glioma model. Can Res. 2005;65:7194. doi: 10.1158/0008-5472.CAN-04-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.King GD, Muhammad AKMG, Curtin JF, Barcia C, Puntel M, Liu C, Honig SB, Candolfi M, Mondkar S, Lowenstein PR, Castro MG. Flt3L and TK gene therapy eradicate multifocal glioma in a syngeneic glioblastoma model. Neuro Oncol. 2008;10:19–31. doi: 10.1215/15228517-2007-045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Lombardi G, Rumiato E, Bertorelle R, Saggioro D, Farina P, Della Puppa A, Zustovich F, Berti F, Sacchetto V, Marcato R, Amadori A, Zagonel V. Clinical and genetic factors associated with severe hematological toxicity in glioblastoma patients during radiation plus temozolomide treatment: a prospective study. Am J Clin Oncol. 2015;38. [DOI] [PubMed]
- 112.Kuai R, Yuan W, Son S, Nam J, Xu Y, Fan Y, Schwendeman A, Moon JJ. Elimination of established tumors with nanodisc-based combination chemoimmunotherapy. Sci Adv. 2018;4:eaao1736. [DOI] [PMC free article] [PubMed]
- 113.Wang C, Wang J, Zhang X, Yu S, Wen D, Hu Q, Ye Y, Bomba H, Hu X, Liu Z, Dotti G, Gu Z. In situ formed reactive oxygen species–responsive scaffold with gemcitabine and checkpoint inhibitor for combination therapy. Sci Transl Med. 2018;10:eaan3682. [DOI] [PubMed]
- 114. Mathios D, Kim JE, Mangraviti A, Phallen J, Park CK, Jackson CM, Garzon-Muvdi T, Kim E, Theodros D, Polanczyk M. Anti–PD-1 antitumor immunity is enhanced by local and abrogated by systemic chemotherapy in GBM. Sci Transl Med. 2016;8:370ra180. [DOI] [PMC free article] [PubMed]
- 115. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–15. [DOI] [PMC free article] [PubMed]
- 116.Maxwell R, Jackson CM, Lim M. Clinical trials investigating immune checkpoint blockade in glioblastoma. Curr Treat Options Oncol. 2017;18:51. doi: 10.1007/s11864-017-0492-y. [DOI] [PubMed] [Google Scholar]
- 117.Chekhonin I, Gurina O. Trends in malignant glioma monoclonal antibody therapy. Curr Med Chem. 2015;11:102–118. [Google Scholar]