Abstract
Background
To date, there is no clearly defined association between plasma selenium levels and first stroke. We aimed to investigate the association between baseline plasma selenium and first stroke risk in a community-based Chinese population.
Methods
Using a nested case-control study design, a total of 1255 first stroke cases and 1255 matched controls were analyzed. Participant plasma selenium concentrations were measured by inductively coupled plasma mass spectrometry (ICP-MS), and the association of plasma selenium with first stroke risk was estimated by conditional logistic regression models.
Results
Overall, a non-linear negative association between plasma selenium and first total stroke and first ischemic stroke risks was found in males but not in females. Compared with participants with lower selenium levels (tertile 1–2, < 94.1 ng/mL), participants with higher selenium levels (tertile 3, ≥ 94.1 ng/mL) had significantly lower risks of first total stroke (OR 0.63; 95% CI 0.48, 0.83) and first ischemic stroke (OR 0.61; 95% CI 0.45, 0.83) in males but not in females with first total stroke (OR 0.92; 95% CI 0.69, 1.22) and first ischemic stroke (OR 0.89; 95% CI 0.65, 1.22). Furthermore, a stronger association between plasma selenium and first total stroke was found in males with higher vitamin E levels (≥ 13.5 μg/mL vs. < 13.5 μg/mL P-interaction = 0.007). No significant association was observed between plasma selenium and first hemorrhagic stroke risk in either males or females.
Conclusion
Our study indicated a significant, non-linear, negative association between plasma selenium and first stroke in males but not in females.
Trial registration
Supplementary Information
The online version contains supplementary material available at 10.1186/s13293-021-00383-2.
Keywords: Selenium, First stroke, First ischemic stroke, First hemorrhagic stroke, Vitamin E
Highlights
This study is the first work to find a significant non-linear, inverse association between baseline plasma selenium and first total stroke and first ischemic stroke risks in males but not in females. The present study is also the first to indicate a stronger non-linear negative relationship between plasma selenium and first stroke in male participants with higher plasma vitamin E levels (≥ 13.5 μg/mL) than in those with lower plasma vitamin E levels (< 13.5 μg/mL). This study found no significant association between plasma selenium and first hemorrhagic stroke risk in either males or females.
Introduction
Stroke is a leading cause of mortality and disability worldwide. Accounting for almost one-third of stroke mortality worldwide, China bears the heaviest stroke burden in the world [1, 2]. Since control of risk factors for stroke helps decrease stroke burden [3, 4], the identification of novel risk factors is urgent to further lower stroke risk. The potential effects of nutritional determinants on stroke have attracted increasing attention for controlling the occurrence of stroke. Appropriate intake of electrolyte minerals, one type of micronutrient, plays a vital role in maintaining brain health. There was an inverse association between potassium intake and stroke risk, whereas higher sodium intake was associated with a higher risk of stroke [5, 6]. We previously found that there was a significant inverse association between plasma zinc and first hemorrhagic stroke [7], whereas excess zinc also led to neurotoxicity [8]. Thus, appropriate intake of nutritional determinants and maintaining their reasonable levels play important roles in controlling stroke risk. Although accumulating evidence has indicated that trace elements might exert effects on stroke [9, 10], there is still limited evidence of dietary supplementation for stroke prevention [11], suggesting that much more evidence is needed to clarify this issue.
Selenium (Se), an essential trace element, acts as the active center of selenoproteins or selenoenzymes (e.g., glutathione peroxidases), which have many important biological functions, including antioxidant, anti-inflammatory, and immunoregulatory roles [12–14]. Insufficient or excessive Se intake may be associated with many adverse health outcomes [15–17]. Se overexposure might have toxic effects, which should not be ignored, as high Se levels have been reported to be positively associated with type 2 diabetes, and this association might exists only in females [18–21], whereas Se deficiency is mainly positively associated with Keshan disease and Kashin-Beck disease [22, 23]. Low Se status was also reported to be related to an increased risk of thyroid disease, including autoimmune thyroiditis, subclinical hypothyroidism, and hypothyroidism, and Se deficiency might constitute a risk factor for hyperthyroidism, especially in males [24, 25]. Low serum Se was an independent predictor of cancer mortality only in male participants with a median plasma Se concentration of 75.9 ng/mL, while another nested case-control study with a mean plasma Se concentration of 84.0 ng/mL from the European Prospective Investigation into Cancer and Nutrition indicated that Se status is suboptimal in many Europeans (with low Se status) and suggested an inverse association between colorectal cancer risk and higher serum Se status, which was more evident in females [26, 27]. Previous studies from two prominent Se intervention trials in North America (with currently high selenium status) observed conflicting results with regard to Se intake and cancer risk, which indicated that an interaction of Se status and cancer risk could be found in the Nutritional Prevention of Cancer Trial (NPC) [28] with a median plasma Se concentration of 115 ng/mL at baseline but not in Selenium and Vitamin E Cancer Prevention Trial (SELECT) [29] with a median plasma Se concentration of 136 ng/mL at baseline. High Se status was also positively associated with cognitive performance in males only among the population of the Nation Health and Nutrition Survey (NHANES, 2011–2014), with a median whole blood Se concentration of 196.7 ng/mL [30]. Thus, there was an interaction of Se with diabetes, thyroid disease, cancers, and cognitive performance, which seemed to be associated with sex differences and needs to be further understood. Different baseline Se levels and any other poorly defined confounders might affect these complex associations. In particular, health problems caused by Se deficiency need to be given great attention in China, a Se-deficient country where it is estimated that over 105 million people potentially face adverse health impacts due to Se deficiency [31, 32]. Based on the aforementioned conclusions, more studies are still needed to identify the appropriate level of Se that should be considered safe to address the uncertainties regarding the health risks of Se exposure. Although cross-sectional epidemiologic studies have indicated inverse associations between Se levels and stroke risk [33–35], previous prospective epidemiologic studies have reported inconsistent findings on the associations between Se concentrations and stroke risk [36–38]. Moreover, few studies have thoroughly analyzed the potential modifiers affecting this association; in particular, whether this relationship was associated with sex differences remains unclear. Therefore, the prospective relationship between plasma Se and the risk of first stroke remains inconclusive and deserves further investigation.
To narrow the knowledge gap mentioned above, we performed a nested case-control study to investigate the association between baseline plasma Se levels and the risk of first total stroke and stroke subtypes (ischemic stroke and hemorrhagic strokes) and examined any possible effect modifiers using data from a community-based population in China.
Methods
Study population and design
Our present study is a subset of the China H-type Hypertension Registry Study (CHHRS; URL: http://www.chictr.org.cn; Unique identifier: ChiCTR1800017274), which is an ongoing community-based non-intervention, prospective, observational, multicenter, real-world registry study and was mainly conducted in Rongcheng County, Shandong Province, and Lianyungang, Jiangsu Province, China. It was designed to establish a national registry of patients with hypertension, to investigate the prevalence and treatment of H-type hypertension in China and the related factors affecting its prognosis, and finally to construct a risk prediction model of cardio-cerebral and renal vascular diseases. Eligible participants were men and women aged ≥ 18 years with essential hypertension, defined as seated systolic blood pressure (SBP) ≥ 140 mmHg and/or seated diastolic blood pressure (DBP) ≥ 90 mmHg at the screening visit. The boundaries for elevated blood pressure (140 and 90 mmHg, respectively) were applied irrespective of age. Participants were excluded if they had psychological or nervous system impairment resulting in an inability to demonstrate informed consent or were unable to be followed-up according to the study protocol. The trial consisted of two stages: screening and recruitment and a 3-year observation follow-up period. Participants were scheduled for follow-up every 3 months. At each visit, blood pressure, heart rate, the usage of medications, adverse events, and study outcome events were measured and recorded. The primary outcome was the first composite of cardiovascular events consisting of non-fatal stroke, myocardial infarction, vascular death, and all-cause death.
The current nested case-control study utilized data from the CHHRS, which was conducted in Rongcheng, a coastal area of Shandong Province, China. This study matched stroke cases with an equal number of controls (patients without stroke) by age ± 1 year, sex, and village. Patients with stroke data from the Chinese Centers for Disease Control and Prevention (CDC, 2016-2018) who had complete records (physical exam, questionnaire, and biological samples) were selected as cases. The initial sample consisted of 1401 incident cases and 1401 matched controls, both of which were recruited from the same trial population. Next, we excluded participants with missing serum selenium values (n = 287) and unpaired individuals (n = 5). Based on the inclusion and exclusion criteria, 1255 stroke cases and 1255 matched controls with complete Se measurements were selected for final data analysis (Supplementary Fig. 1).
Ethics
The present study was approved by the Ethics Committee of the Institute of Biomedicine, Anhui Medical University, Hefei, China. All participants signed an approved written consent form after the study protocol was thoroughly explained to them.
Outcomes
The primary outcome of the present study was a first non-fatal or fatal stroke. Secondary outcomes included first ischemic stroke (fatal and non-fatal) and first hemorrhagic stroke (fatal and non-fatal).
Information on the incidence of first stroke for all participants was obtained via the Centers for Disease Control and Prevention of Rongcheng County and checked against the national health insurance system with electronic linkage to all hospitalizations or ascertained through active follow-up. Diseases were coded according to the International Classification of Diseases, 10th Revision (ICD-10). Secondary outcomes included first ischemic stroke (I63) and first hemorrhagic stroke (I60-I61). The primary outcome (first non-fatal or fatal stroke) included first ischemic stroke (I63), first hemorrhagic stroke (I60-I61), and no type stroke (I64).
According to government regulations, local authorities from medical institutions are required to report all new cases of stroke to the local Centers for Disease Control and Prevention. A report card that includes information on demographics, diagnostic basis and date of stroke is required to be submitted on the 28th of each month. Quality control, including finding and deleting repeated cases, error checking, and determining any missed cases, is completed by trained officials. Furthermore, the staff from the local Centers for Disease Control and Prevention double checked these information and were responsible for deleting repeated cases and finding logistical errors and missed cases. In addition, 5% of all uploaded cases were randomly chosen for further confirmation by phone or door-to-door interviews.
Laboratory assays
Baseline serum total homocysteine (tHcy), fasting glucose levels, and lipids were measured using automatic clinical analyzers (Beckman Coulter, AU680) at the Shenzhen Tailored Medical Laboratory in Shenzhen, China. Estimated glomerular filtration rates (eGFRs) were estimated by the Chronic Kidney Disease Epidemiology Collaboration equation. Baseline plasma vitamin E concentrations were measured using liquid chromatography–tandem quadrupole mass spectrometry (LC-MS/MS), and plasma selenium (Se) concentrations were measured by inductively coupled plasma mass spectrometry (ICP-MS) using a Thermo Fisher iCAP Q ICP-MS in a commercial laboratory (Beijing DIAN Medical Laboratory, China). In the present study, the intra-assay CV for Se ranged from 1.02 to 7.93%, while the inter-assay CV for Se ranged from 2.79 to 3.51%. According to a previous study [39], the reference value (50–120 ng/mL) for plasma Se levels was used in this study.
Statistical analysis
Baseline characteristics are presented as the means ± SDs for continuous variables and as frequencies (%) for categorical variables. Differences in baseline characteristics between males and females and cases and controls were compared using chi-square tests for categorical variables and t tests for continuous variables. Differences in population characteristics according to Se tertiles were compared using ANOVA tests, or chi-square tests.
Variables that are known as traditional or suspected risk factors for stroke [40] and matched variables or variables that showed significant differences between cases and controls were adjusted for in the models. Odds ratios (ORs) and 95% confidence intervals (95% CIs) for first stroke, first ischemic stroke, and first hemorrhagic stroke were calculated by modeling plasma Se as tertiles using conditional logistic regression, without and with adjustment for matched variables (sex and age), body mass index (BMI), baseline systolic blood pressure (SBP), baseline diastolic blood pressure (DBP), smoking status, alcohol consumption, labor intensity, baseline total homocysteine (tHcy), plasma vitamin E, fasting glucose, estimated glomerular filtration rate (eGFR), anti-platelet drugs, lipoprotein-lowering drugs, glucose-lowering drugs, anti-hypertensive drugs, self-reported hypertension, self-reported diabetes, self-reported atrial fibrillation, and self-reported hyperlipidemia. A generalized additive model (GAM) and smooth curve fitting (penalized spline method) were evaluated to further characterize the shape of the association between serum Se and first stroke and its subtypes. As additional exploratory analyses, possible modifications of the association between plasma Se (tertile 3, ≥ 94.1 vs. tertile 1–2, < 94.1 ng/mL) and first total stroke in male and female participants were also assessed for variables including age (< 70 vs. ≥ 70 years), BMI (< 24 vs. ≥ 24 kg/m2), current smoking (yes vs. no), current alcohol consumption (yes vs. no), baseline SBP (< 140 vs. ≥ 140 mmHg), fasting glucose (< 6.1 vs. ≥ 6.1 mmol/L or diabetes), total cholesterol (< 5.8 [median] vs. ≥ 5.8 mmol/L), triglycerides (< 1.2 [median] vs. ≥ 1.2 mmol/L), estimated glomerular filtration rate (< 90 vs. ≥ 90 mL/min/1.73 m2), total homocysteine (< 12.5 [median] vs. ≥ 12.5 μmol/L), and vitamin E (< 13.5 [median] vs. ≥ 13.5 μmol/L) using multivariate logistic regression models. Diabetes was defined as fasting serum glucose ≥ 7.0 mmol/L, self-reported use of anti-diabetic medications, or physician-diagnosed diabetes. Potential interactions were examined by including the interaction terms in those logistic regression models with the greatest number of confounding variables.
A 2-tailed P < 0.05 was considered to be statistically significant in all analyses. R software version 3.4.3 (www.R-project.org) and Empower version 2.17.8 (www.empowerstats.com, X&Y Solutions, Inc.) were used for all statistical analyses.
Results
Study participants and baseline characteristics
A total of 1255 first stroke cases (1079 cases of first ischemic stroke, 171 cases of first hemorrhagic stroke, and 5 cases of first uncertain type of stroke) and 1255 matched controls were included in this analysis. The mean age of all participants at baseline was 70.8 years (SD, 8.1), 49.5% of the participants were male, and the mean Se level was 87.2 ng/mL (SD, 18.6). The baseline characteristics of the male and female participants are shown in Table 1. The reference value of 50–120 ng/mL for serum Se concentration was used in this study. The detailed plasma Se concentration distribution of subjects is listed in Supplemental Table 1, and 95.1% of the participants were within the normal range of Se levels with respect to the reference value (50–120 ng/mL) for plasma Se concentrations. As shown in Table 1, male participants had non-significantly higher Se levels (87.7 ± 18.6 vs. 86.8 ± 18.6 ng/mL; P = 0.230) and significantly lower vitamin E (12.9 ± 3.3 vs. 15.2 ± 4.3 ng/mL; P < 0.001) than females. The distribution graph of serum Se and vitamin E levels in males versus females showed similar results (Supplemental Fig. 2A-B). Males also tended to be older, were more likely to be current smokers and current drinkers, had higher DBP and tHcy levels, as well as lower BMI, SBP, TC, TG, and glucose levels at baseline and a lower frequency of lipid-lowering, glucose-lowering, and anti-hypertensive drug use, and were less likely to be hypertensive patients or self-reported diabetic and self-reported hyperlipidemia patients compared with female participants.
Table 1.
Baseline characteristics of male and female participantsa
| Characteristics | Total | Male | Female | P value |
|---|---|---|---|---|
| N | n = 2510 | n=1242 | n = 1268 | |
| Age, years | 70.8 ± 8.1 | 71.4 ± 8.1 | 70.1 ± 8.0 | < 0.001 |
| BMI, kg/m2 | 26.2 ± 4.1 | 25.3 ± 3.6 | 27.0 ± 4.4 | < 0.001 |
| Current smoking, n (%) | 551 (22.0) | 547 (44.0) | 4 (0.3) | < 0.001 |
| Current alcohol drinking, n (%) | 612 (24.4) | 599 (48.2) | 13 (1.0) | < 0.001 |
| Baseline SBP, mmHg | 153.3 ± 23.1 | 150.8 ± 22.3 | 155.7 ± 23.6 | < 0.001 |
| Baseline DBP, mmHg | 85.3 ± 12.3 | 86.2 ± 12.5 | 84.3 ± 12.0 | < 0.001 |
| Self-reported hypertension, n (%) | 1312 (52.3) | 553 (44.5) | 759 (59.9) | < 0.001 |
| Self-reported diabetes, n (%) | 424 (16.9) | 161 (13.0) | 263 (20.7) | < 0.001 |
| Self-reported hyperlipidemia, n (%) | 264 (10.5) | 108 (8.7) | 156 (12.3) | 0.003 |
| Self-reported atrial fibrillation, n (%) | 46 (1.8) | 26 (2.1) | 20 (1.6) | 0.335 |
| Hypertension, n (%)b | 2016 (80.3) | 940 (75.7) | 1076 (84.9) | < 0.001 |
| Labor intensity, n (%) | < 0.001 | |||
| Mild | 1885 (75.1) | 887 (71.4) | 998 (78.7) | |
| Moderate | 488 (19.4) | 280 (22.5) | 208 (16.4) | |
| Severe | 137 (5.5) | 75 (6.0) | 62 (4.9) | |
| Medication use, n (%) | ||||
| Anti-platelet drugs | 83 (3.3) | 47 (3.8) | 36 (2.8) | 0.186 |
| Lipid-lowering drugs | 44 (1.8) | 15 (1.2) | 29 (2.3) | 0.039 |
| Glucose-lowering drugs | 301 (12.0) | 112 (9.0) | 189 (14.9) | < 0.001 |
| Anti-hypertensive drugs | 1152 (45.9) | 476 (38.3) | 676 (53.3) | < 0.001 |
| Laboratory results | ||||
| TC, mmol/L | 5.9 ± 1.2 | 5.6 ± 1.1 | 6.1 ± 1.3 | < 0.001 |
| TG, mmol/L | 1.4 ± 0.9 | 1.2 ± 0.7 | 1.6 ± 0.9 | < 0.001 |
| HDL-C, mmol/L | 1.6 ± 0.4 | 1.6 ± 0.4 | 1.6 ± 0.4 | 0.558 |
| Glucose, mmol/L | 6.3 ± 2.3 | 6.1 ± 2.1 | 6.4 ± 2.5 | < 0.001 |
| tHcy, μmol/L | 13.9 ± 7.2 | 15.3 ± 8.6 | 12.5 ± 5.0 | < 0.001 |
| eGFR, mL/min/1.73 m2 | 92.5 ± 14.4 | 92.3 ± 15.1 | 92.7 ± 13.8 | 0.428 |
| Vitamin E, μg/mL | 14.0 ± 4.0 | 12.9 ± 3.3 | 15.2 ± 4.3 | < 0.001 |
| Selenium, ng/mL | 87.2 ± 18.6 | 87.7 ± 18.6 | 86.8 ± 18.6 | 0.230 |
Abbreviations: BMI body mass index, SBP systolic blood pressure, DBP diastolic blood pressure, TC total cholesterol, TG triglycerides, HDL-C high-density lipoprotein-cholesterol, tHcy total homocysteine, eGFR estimated glomerular filtration rate
aVariables are presented as the mean ± SD or n (%)
bHypertension was defined as a self-reported history of hypertension, use of anti-hypertensive drugs, SBP ≥ 140 mmHg, or DBP ≥ 90 mmHg
The baseline characteristics of cases and control participants by sex are shown in Table 2. For stroke cases, male stroke cases had non-significantly lower Se levels than females (86.2 ± 16.8 vs. 87.0 ± 18.4 ng/mL; P = 0.436). Male stroke cases also tended to be older, were more likely to be current smokers and current drinkers, had higher DBP and tHcy levels, as well as lower BMI, SBP, TC, TG, glucose, and vitamin E levels at baseline and a lower frequency of glucose-lowering and anti-hypertensive drug use, and were less likely to be hypertensive patients or self-reported diabetic and self-reported hyperlipidemia patients compared with female stroke cases. For non-stroke controls, male non-stroke controls had significantly higher Se levels than females (89.1 ± 20.1 vs. 86.6 ± 18.7 ng/mL; P = 0.020). Male non-stroke controls also tended to be older, were more likely to be current smokers, current drinkers and self-reported atrial fibrillation patients, had higher DBP and tHcy levels, as well as lower BMI, SBP, TC, TG, and vitamin E levels at baseline and a lower frequency of glucose-lowering and anti-hypertensive drug use, and were less likely to be hypertensive patients or self-reported diabetic patients compared with female non-stroke controls.
Table 2.
Baseline characteristics of cases and control participants by sexa
| Characteristics | First stroke cases | P value | Non-stroke controls | P value | ||
|---|---|---|---|---|---|---|
| Males | Females | Males | Females | |||
| N | n = 621 | n = 634 | n = 621 | n = 634 | ||
| Age, years | 71.4 ± 8.1 | 70.1± 8.0 | 0.004 | 71.4 ± 8.1 | 70.1 ± 8.0 | 0.003 |
| BMI, kg/m2 | 25.7 ± 3.6 | 27.3 ± 4.9 | < 0.001 | 25.0 ± 3.5 | 26.7 ± 3.8 | < 0.001 |
| Current smoking, n (%) | 293 (47.2) | 3 (0.5) | < 0.001 | 254 (40.9) | 1 (0.2) | < 0.001 |
| Current alcohol drinking, n (%) | 291 (46.9) | 4 (0.6) | < 0.001 | 308 (49.6) | 9 (1.4) | < 0.001 |
| Baseline SBP, mmHg | 154.8 ± 23.1 | 159.5 ± 24.2 | < 0.001 | 146.8 ± 20.8 | 151.8 ± 22.2 | < 0.001 |
| Baseline DBP, mmHg | 88.0 ± 13.4 | 86.4 ± 12.2 | 0.030 | 84.4 ± 11.3 | 82.2 ± 11.4 | < 0.001 |
| Self-reported hypertension, n (%) | 319 (51.4) | 427 (67.4) | < 0.001 | 234 (37.7) | 332 (52.4) | < 0.001 |
| Self-reported diabetes, n (%) | 97 (15.6) | 164 (25.9) | < 0.001 | 64 (10.3) | 99 (15.6) | 0.005 |
| Self-reported hyperlipidemia, n (%) | 46 (7.4) | 83 (13.1) | < 0.001 | 62 (10.0) | 73 (11.5) | 0.382 |
| Self-reported atrial fibrillation, n (%) | 13 (2.1) | 16 (2.5) | 0.612 | 13 (2.1) | 4 (0.6) | 0.025 |
| Hypertension, n (%)b | 511 (82.3) | 564 (89.0) | < 0.001 | 429 (69.1) | 512 (80.8) | < 0.001 |
| Labor intensity, n (%) | < 0.001 | 0.006 | ||||
| Mild | 457 (73.6) | 521 (82.2) | 430 (69.2) | 477 (75.2) | ||
| Moderate | 125 (20.1) | 96 (15.1) | 155 (25.0) | 112 (17.7) | ||
| Severe | 39 (6.3) | 17 (2.7) | 36 (5.8) | 45 (7.1) | ||
| Medication use, n (%) | ||||||
| Anti-platelet drugs | 37 (6.0) | 23 (3.6) | 0.053 | 10 (1.6) | 13 (2.1) | 0.561 |
| Lipid-lowering drugs | 8 (1.3) | 14 (2.2) | 0.214 | 7 (1.1) | 15 (2.4) | 0.095 |
| Glucose-lowering drugs | 72 (11.6) | 121 (19.1) | < 0.001 | 40 (6.4) | 68 (10.7) | 0.007 |
| Anti-hypertensive drugs | 281 (45.2) | 383 (60.4) | < 0.001 | 195 (31.4) | 293 (46.2) | < 0.001 |
| Laboratory results | ||||||
| TC, mmol/L | 5.6 ± 1.1 | 6.1 ± 1.3 | < 0.001 | 5.7 ± 1.1 | 6.1 ± 1.3 | < 0.001 |
| TG, mmol/L | 1.3 ± 0.8 | 1.7 ± 1.0 | < 0.001 | 1.1 ± 0.7 | 1.5 ± 0.8 | < 0.001 |
| HDL-C, mmol/L | 1.6 ± 0.4 | 1.6 ± 0.4 | 0.935 | 1.7 ± 0.4 | 1.7 ± 0.4 | 0.376 |
| Glucose, mmol/L | 6.3 ± 2.2 | 6.8 ± 2.8 | 0.001 | 5.9 ± 1.9 | 6.1 ± 2.2 | 0.051 |
| tHcy, μmol/L | 15.8 ±9.5 | 12.8 ± 5.7 | < 0.001 | 14.7 ± 7.5 | 12.2 ± 4.3 | < 0.001 |
| eGFR, mL/min/1.73 m2 | 91.4 ± 15.9 | 92.0 ± 14.7 | 0.485 | 93.1 ± 14.1 | 93.4 ± 12.7 | 0.685 |
| Vitamin E, μg/mL | 13.0 ± 3.4 | 15.2 ± 4.3 | < 0.001 | 12.8 ± 3.1 | 15.1 ± 4.3 | < 0.001 |
| Selenium, ng/mL | 86.2 ± 16.8 | 87.0 ± 18.4 | 0.436 | 89.1 ± 20.1 | 86.6 ± 18.7 | 0.020 |
Abbreviations: BMI body mass index, SBP systolic blood pressure, DBP diastolic blood pressure, TC total cholesterol, TG triglycerides, HDL-C high-density lipoprotein-cholesterol, tHcy total homocysteine, eGFR estimated glomerular filtration rate
aVariables are presented as the mean ± SD or n (%)
bHypertension was defined as a self-reported history of hypertension, use of anti-hypertensive drugs, SBP ≥ 140 mmHg, or DBP ≥ 90 mmHg
In addition, plasma Se was positively associated with BMI, current smoking, current alcohol consumption, self-reported diabetes, a higher frequency of glucose-lowering drug use, TC, high-density lipoprotein cholesterol, fasting glucose, and vitamin E levels and was inversely associated with labor intensity and tHcy levels at baseline (Supplemental Table 2).
Association between plasma selenium concentration and first stroke in all participants
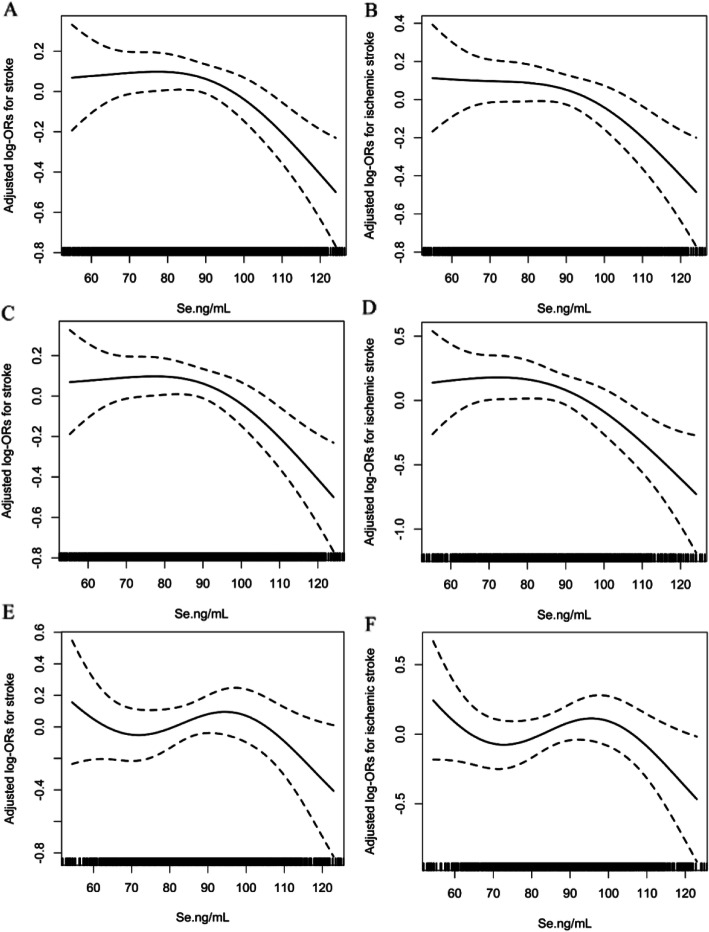
Overall, there was a non-linear negative association between plasma Se levels and the risk of first total stroke and first ischemic stroke (Fig. 1A, B) but not with the risk of first hemorrhagic stroke (Supplemental Fig. 3A) in all participants. Consistently, when plasma Se was assessed as tertiles that were calculated among the whole population, significantly lower risks of first total stroke (Model 2, OR 0.77; 95% CI 0.61, 0.97) and first ischemic stroke (model 2, OR 0.78; 95% CI 0.60, 0.99) were found in participants in tertile 3 (≥ 94.1 ng/mL) than in those in tertile 1 (< 79.1 ng/mL) (Table 3). It is worth noting that non-significantly higher risks of first total stroke (model 2, OR 1.02; 95% CI 0.82, 1.26) and first ischemic stroke (model 2, OR 1.05; 95% CI 0.83, 1.33) were found in participants in tertile 2 (79.1 to < 94.1 ng/mL) than in those in tertile 1 (< 79.1 ng/mL) (Table 3). Due to the similar first total stroke and first ischemic stroke prevalence in participants with Se levels in tertile 1 and tertile 2 (Table 3), we combined these two groups into one group called tertile 1–2. Compared with participants with lower Se levels in tertile 1–2 (< 94.1 ng/mL), significantly lower risks of first total stroke (model 2, OR 0.76; 95% CI 0.63, 0.93) and first ischemic stroke (model 2, OR 0.75; 95% CI 0.61, 0.93) were found in those with higher Se levels in tertile 3 (≥ 94.1 ng/mL) (Table 3). However, no significant association was found between plasma Se concentrations and first hemorrhagic stroke (Table 3).
Fig. 1.
The association between baseline plasma selenium and first total stroke and first ischemic stroke risks. Odds ratios for first total stroke in the (A) total population, (C) males, and (E) females, and for first ischemic stroke in the (B) total population, (D) males, and (F) females by plasma selenium levels. In addition to the matching factors (age and sex), the splines also adjusted for BMI, baseline SBP, baseline DBP, smoking status, alcohol consumption, labor intensity, baseline total homocysteine, vitamin E, fasting glucose, estimated glomerular filtration rate (eGFR), anti-platelet drugs, lipoprotein-lowering drugs, glucose-lowering drugs, anti-hypertensive drugs, self-reported hypertension, self-reported diabetes, self-reported atrial fibrillation, and self-reported hyperlipidemia
Table 3.
Risk of first stroke (total and subtypes) associated with plasma selenium concentrations in all participantsa
| Selenium, ng/mL | Cases/controls | Model 1 | Model 2 | ||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| First total stroke | |||||
| Tertiles | |||||
| T1 (< 79.1) | 420/417 | Ref. | Ref. | ||
| T2 (79.1 to < 94.1) | 434/402 | 1.07 (0.88, 1.30) | 0.516 | 1.02 (0.82, 1.26) | 0.891 |
| T3 (≥ 94.1) | 401/436 | 0.90 (0.73, 1.11) | 0.317 | 0.77 (0.61, 0.97) | 0.027 |
| Categories | |||||
| T1–T2 (< 94.1) | 854/819 | Ref. | Ref. | ||
| T3 (≥ 94.1) | 401/436 | 0.87 (0.73, 1.04) | 0.119 | 0.76 (0.63, 0.93) | 0.007 |
| Ischemic stroke | |||||
| Tertiles | |||||
| T1 (< 79.1) | 360/363 | Ref. | Ref. | ||
| T2 (79.1 to < 94.1) | 372/340 | 1.10 (0.89, 1.35) | 0.376 | 1.05 (0.83, 1.33) | 0.672 |
| T3 (≥ 94.1) | 347/376 | 0.92 (0.74, 1.14) | 0.444 | 0.78 (0.60, 0.99) | 0.045 |
| Categories | |||||
| T1–T2 (< 94.1) | 732/703 | Ref. | Ref. | ||
| T3 (≥ 94.1) | 347/376 | 0.87 (0.72, 1.06) | 0.163 | 0.75 (0.61, 0.93) | 0.009 |
| Hemorrhagic stroke | |||||
| Tertiles | |||||
| T1 (< 79.1) | 58/54 | Ref. | Ref. | ||
| T2 (79.1 to < 94.1) | 60/61 | 0.88 (0.50, 1.57) | 0.673 | 0.76 (0.38, 1.55) | 0.455 |
| T3 (≥ 94.1) | 53/56 | 0.85 (0.47, 1.54) | 0.587 | 0.72 (0.35, 1.48) | 0.371 |
| Categories | |||||
| T1–T2 (< 94.1) | 118/115 | Ref. | Ref. | ||
| T3 (≥ 94.1) | 53/56 | 0.92 (0.57, 1.47) | 0.718 | 0.85 (0.48, 1.50) | 0.577 |
a ORs of first stroke (total), first ischemic, and hemorrhagic stroke in relation to plasma selenium (tertiles) were analyzed using conditional logistic regression models. Model 1 is conditioned on the matching factors of age and sex; model 2 is conditioned on the matching factors of age and sex, as well as adjusted for BMI, baseline SBP, baseline DBP, smoking status, alcohol consumption, labour intensity, baseline total homocysteine, vitamin E, fasting glucose, estimated glomerular filtration rate (eGFR), anti-platelet drugs, lipoprotein-lowering drugs, glucose-lowering drugs, anti-hypertensive drugs, self-reported hypertension, self-reported atrial fibrillation, self-reported diabetes, and self-reported hyperlipidemia.
Association between plasma selenium concentrations and first stroke by sex
Given the differences in plasma Se levels between male and female participants (87.7 ± 18.6 vs. 86.8 ± 18.6 ng/mL), we further investigated the possible effect of sex on the Se-first stroke association. Overall, there was a non-linear negative association between plasma Se levels and the risks of first total stroke and first ischemic stroke in males (Fig. 1C, D) but not in females (Fig. 1E, F). Furthermore, there was no significant association between plasma Se and first hemorrhagic stroke in either sex (Supplemental Fig. 3B-C). Consistently, when the plasma Se of males and females was assessed as tertiles according to the tertile cut-off value of the whole population, the highest tertile (T3, ≥ 94.1 ng/mL) of plasma Se was associated with a lower first total stroke risk in males (model 2, OR 0.67; 95% CI 0.48, 0.93, P = 0.017) but not in females (model 2, OR 0.85; 95% CI 0.61, 1.19, P = 0.353) compared with the lowest tertile (T1, < 79.1 ng/mL) of plasma Se (Table 4). It is also worth noting that non-significantly higher risks of first total stroke (model 2, OR 1.11; 95% CI 0.80, 1.54) and first ischemic stroke (model 2, OR 1.17; 95% CI 0.82, 1.66) were found in male participants in tertile 2 (79.1 to < 94.1 ng/mL) than in those in tertile 1 (< 79.1 ng/mL) (Table 3). Accordingly, higher Se levels in tertile 3 (≥ 94.1 ng/mL) were associated with a lower first total stroke risk in males (model 2 OR 0.63; 95% CI 0.48, 0.83, P = 0.001), but not in females (model 2, OR 0.92; 95% CI 0.69, 1.22, P = 0.563) compared with lower Se levels in tertile 1–2 (< 94.1 ng/mL) (Table 4).
Table 4.
The association of plasma selenium with the risk of first stroke (total and subtypes) by sexa
| bMale participants | Female participants | |||||
|---|---|---|---|---|---|---|
| Cases/controls | Model 1 | Model 2 | Cases/controls | Model 1 | Model 2 | |
| OR (95% CI) P value | OR (95% CI) P value | OR (95% CI) P value | OR (95% CI) P value | |||
| First total stroke | ||||||
| Selenium tertiles | ||||||
| T1 (< 79.1 ng/mL) | 206/193 | Ref. | Ref. | 214/224 | Ref. | Ref. |
| T2 (79.1 to < 94.1) | 218/183 | 1.12 (0.84, 1.49) 0.452 | 1.11 (0.80, 1.54) 0.519 | 216/219 | 1.04 (0.80, 1.36) 0.764 | 0.88 (0.65, 1.19) 0.408 |
| T3 (≥ 94.1) | 197/245 | 0.73 (0.55, 0.98) 0.034 | 0.67 (0.48, 0.93) 0.017 | 204/191 | 1.14 (0.85, 1.54) 0.380 | 0.85 (0.61, 1.19) 0.353 |
| Selenium Categories | ||||||
| T1–T2 (< 94.1) | 424/376 | Ref. | Ref. | 430/443 | Ref. | Ref. |
| T3 (≥ 94.1) | 197/245 | 0.69 (0.54, 0.89) 0.003 | 0.63 (0.48, 0.83) 0.001 | 204/191 | 1.12 (0.86, 1.44) 0.399 | 0.92 (0.69, 1.22) 0.563 |
| First ischemic stroke | ||||||
| Selenium tertiles | ||||||
| T1 (< 79.1 ng/mL) | 179/171 | Ref. | Ref. | 181/192 | Ref. | Ref. |
| T2 (79.1 to < 94.1) | 191/157 | 1.16 (0.85, 1.57) 0.343 | 1.17 (0.82, 1.66) 0.387 | 181/183 | 1.05 (0.79, 1.40) 0.716 | 0.90 (0.64, 1.25) 0.514 |
| T3 (≥ 94.1) | 168/210 | 0.74 (0.55, 1.01) 0.058 | 0.67 (0.47, 0.95) 0.027 | 179/166 | 1.17 (0.85, 1.60) 0.330 | 0.84 (0.58, 1.21) 0.347 |
| Selenium categories | ||||||
| T1–T2 (< 94.1) | 370/328 | Ref. | Ref. | 362/375 | Ref. | Ref. |
| T3 (≥ 94.1) | 168/210 | 0.69 (0.53, 0.90) 0.006 | 0.61 (0.45, 0.83) 0.001 | 179/166 | 1.14 (0.86, 1.50) 0.362 | 0.89 (0.65, 1.22) 0.479 |
| First hemorrhagic stroke | ||||||
| Selenium tertiles | ||||||
| T1 (< 79.1 ng/mL) | 26/22 | Ref. | Ref. | 32/32 | Ref. | Ref. |
| T2 (79.1 to < 94.1) | 26/26 | 0.81 (0.33, 2.00) 0.646 | 0.88 (0.25, 3.04) 0.840 | 34/35 | 0.98 (0.46, 2.12) 0.962 | 0.48 (0.17, 1.37) 0.172 |
| T3 (≥ 94.1) | 29/33 | 0.72 (0.32, 1.60) 0.420 | 0.80 (0.25, 2.58) 0.714 | 24/23 | 1.05 (0.43, 2.57) 0.919 | 0.58 (0.17, 1.92) 0.368 |
| Selenium categories | ||||||
| T1–T2 (< 94.1) | 52/48 | Ref. | Ref. | 66/67 | Ref. | Ref. |
| T3 (≥ 94.1) | 29/33 | 0.80 (0.41, 1.54) 0.506 | 0.86 (0.33, 2.26) 0.761 | 24/23 | 1.06 (0.54, 2.10) 0.862 | 0.95 (0.36, 2.46) 0.910 |
Abbreviations: T tertile, OR odds ratio, CI confidence interval
aModel 1 is conditioned on the matching factors of age and sex; model 2 is conditioned on the matching factors of age and sex, as well as adjusted for BMI, baseline SBP, baseline DBP, smoking status, alcohol consumption, labor intensity, baseline total homocysteine, vitamin E, fasting glucose, estimated glomerular filtration rate (eGFR), anti-platelet drugs, lipoprotein-lowering drugs, glucose-lowering drugs, anti-hypertensive drugs, self-reported hypertension, self-reported diabetes, self-reported atrial fibrillation, and self-reported hyperlipidemia
b Adjusted P-interaction between sex and plasma selenium (T3, ≥ 94.1 ng/mL vs. T1–2, < 94.1 ng/mL) on first total stroke = 0.029
Similar effects of sex on the Se-first ischemic stroke association were also observed and are displayed in Table 4. However, no significant association was found between plasma Se and first hemorrhagic stroke risk among either males or females (Table 4).
Stratified analysis by potential effect modifiers in male and female participants
Stratified analyses were conducted to explore potential modifiers affecting the association between plasma Se (tertile 3, ≥ 94.1 vs. tertile 1–2, < 94.1 ng/mL) and first total stroke risk among male participants (Table 5). A stronger non-linear negative association between baseline plasma Se and first total stroke was found among males with higher vitamin E levels than among those with lower vitamin E levels (≥ 13.5 μg/mL; OR 0.39; 95% CI 0.25, 0.60; vs. < 13.5 μg/mL; OR 0.85; 95% CI 0.62, 1.17; P for interaction = 0.007). None of the other variables, including age (< 70 vs. ≥ 70 years), BMI (< 24 vs. ≥ 24 kg/m2), current smoking (yes vs. no), current alcohol consumption (yes vs. no), baseline SBP (< 140 vs. ≥ 140 mmHg), fasting glucose (< 6.1 vs. ≥ 6.1 mmol/L or diabetes), TC (< 5.8 [median] vs. ≥ 5.8 mmol/L), TG (< 1.2 [median] vs. ≥ 1.2 mmol/L), eGFR (< 90 vs. ≥ 90 mL/min/1.73 m2), and total homocysteine (< 12.5 [median] vs. ≥ 12.5 μmol/L), were found to modify the association between plasma Se (tertile 3, ≥ 94.1 vs. tertile 1–2, < 94.1 ng/mL) and the risk of first stroke in males (P for all interactions > 0.05).
Table 5.
Stratified analysis of the association between plasma selenium concentrations (T3, ≥ 94.1 ng/mL vs. T1–2, < 94.1 ng/mL) and incident risk of first total stroke in males
| Subgroups | No. of cases/no. of controls | aAdjusted model | P for interaction | |
|---|---|---|---|---|
| Selenium ≥ 94.1 ng/mL | Selenium < 94.1 ng/mL | OR (95% CI) | ||
| Age, years | 0.288 | |||
| < 70 | 98/110 | 173/161 | 0.73 (0.50, 1.08) | |
| ≥ 70 | 99/135 | 251/215 | 0.54 (0.38, 0.76) | |
| Body mass index, kg/m2 | 0.067 | |||
| < 24 | 44/89 | 151/152 | 0.39 (0.24, 0.63) | |
| ≥ 24 | 153/156 | 273/224 | 0.75 (0.55, 1.02) | |
| Current smoking | 0.739 | |||
| No | 95/136 | 233/231 | 0.72 (0.51, 1.01) | |
| Yes | 102/109 | 191/145 | 0.54 (0.36, 0.80) | |
| Current alcohol drinking | 0.230 | |||
| No | 98/106 | 232/207 | 0.74 (0.51, 1.06) | |
| Yes | 99/139 | 192/169 | 0.53 (0.37, 0.77) | |
| SBP, mmHg | 0.759 | |||
| < 140 | 46/92 | 124/156 | 0.60 (0.38, 0.96) | |
| ≥ 140 | 151/153 | 300/220 | 0.64 (0.47, 0.88) | |
| Glucose, mmol/L | 0.898 | |||
| < 6.1 | 108/168 | 274/284 | 0.66 (0.48, 0.90) | |
| ≥ 6.1 or diabetesb | 89/77 | 150/92 | 0.66 (0.42, 1.02) | |
| TC, mmol/L | 0.402 | |||
| < 5.8 | 95/107 | 262/236 | 0.77 (0.54, 1.10) | |
| ≥ 5.8 | 102/138 | 162/140 | 0.53 (0.36, 0.78) | |
| TG, mmol/L | 0.611 | |||
| < 1.2 | 115/160 | 246/255 | 0.71 (0.52, 0.98) | |
| ≥ 1.2 | 82/85 | 178/121 | 0.55 (0.36, 0.84) | |
| eGFR, mL/min/1.73 m2 | 0.753 | |||
| < 90 | 64/68 | 173/141 | 0.71 (0.45, 1.10) | |
| ≥ 90 | 133/177 | 251/235 | 0.61 (0.45, 0.83) | |
| tHcy, μmol/L | 0.117 | |||
| < 12.5 | 89/105 | 129/142 | 0.81 (0.54, 1.22) | |
| ≥ 12.5 | 108/140 | 293/234 | 0.55 (0.39, 0.76) | |
| Vitamin E, μg/mL | 0.007 | |||
| < 13.5 | 117/132 | 270/277 | 0.85 (0.62, 1.17) | |
| ≥ 13.5 | 80/113 | 154/99 | 0.39 (0.25, 0.60) | |
Abbreviations: TC, total cholesterol, T tertile, OR odds ratio, CI confidence interval
aORs of first total stroke in relation to serum selenium levels were calculated using multivariate logistic regression models. Each subgroup analysis was adjusted, if not stratified, for age, BMI, baseline SBP, baseline DBP, smoking status, alcohol consumption, labor intensity, baseline total homocysteine, vitamin E, fasting glucose, estimated glomerular filtration rate (eGFR), anti-platelet drugs, lipoprotein-lowering drugs, glucose-lowering drugs, anti-hypertensive drugs, self-reported hypertension, self-reported diabetes, self-reported atrial fibrillation, and self-reported hyperlipidemia
bDiabetes was defined as a self-reported history of diabetes mellitus, use of anti-diabetic medications, or fasting glucose ≥ 7.0 mmol/L
Furthermore, none of the above variables significantly modified the association of plasma Se and the risk of first total stroke in female participants (P for all interactions > 0.05) (Supplemental Table 3).
Discussion
This nested case-control study demonstrates that higher baseline plasma selenium (Se) is associated with lower risks of first total stroke and first ischemic stroke in males but not in females. Plasma vitamin E levels significantly modified the association between plasma Se and first total stroke in males. Furthermore, no significant association was found between plasma Se and first hemorrhagic stroke risk in either male or female participants.
Conflicting findings of the association between plasma Se and the risk of stroke have been reported by previous studies. A nested case-control study [38] enrolling 1304 stroke cases with a median plasma Se concentration of 64.9 ng/mL found that higher plasma Se levels were significantly associated with a lower risk of hemorrhagic stroke, but not ischemic stroke; the odds ratios (ORs) of hemorrhagic and ischemic stroke were 0.68 (95% CI 0.51, 0.91) and 0.92 (95% CI 0.82, 1.05) in the higher Se level tertile 3 (compared with tertile 1). One case-control study [35] including 1277 ischemic stroke patients with a median plasma Se concentration of 81.1 ng/mL indicated that higher plasma Se levels were associated with a decreased risk of ischemic stroke, where the OR for those with higher Se levels in quartile 4 (compared with quartile 1) was 0.10 (95% CI 0.06, 0.17). Moreover, the Canadian Health Measures Survey (CHMS 2007–2011) enrolling 7065 adult subjects with a median whole blood Se concentration of 184 ng/mL and the National Health and Nutrition Examination Study (NHANES 2011–2012) enrolling 5030 adult subjects with a median whole blood Se concentration of 181 ng/mL found inverse, cross-sectional associations between whole blood Se and the prevalence of stroke, and the Inuit Health Survey (IHS) enrolling 49 stroke cases with a median whole blood Se concentration of 260 ng/mL indicated a reverse relation of whole blood and dietary Se levels with stroke but revealed an L-shaped relationship [33, 34]. However, the Reasons for Geographic and Racial Differences in Stroke Study (REGARDS) [36] revealed that higher environmental Se levels were associated with increased stroke risk; the hazard ratio (HR) for those in quartile 4 (0.45–2.20 ppm) of environmental Se exposure (compared with quartile 1, 0.10–0.30 ppm) was 1.33 (95% CI 1.09, 1.62). It is noteworthy that all of these studies used different sources (plasma, whole blood, diet, and environment) of Se levels to assess the association between Se levels and stroke, which might be one reason for the discrepancy in these findings. Different Se statuses at baseline might be another important reason for the discrepancy in these findings.
Several studies have also explored the association between Se and stroke mortality specifically. A cohort study [37] enrolling 23 stroke death cases among 1100 Finnish males with a mean plasma Se concentration of 55.4 ng/mL found that low Se selenium (< 45 μg/L) was associated with a higher risk of stroke mortality, reporting an adjusted relative risk of 3.7 (95% CI 1.0, 13.1). The NHANES III cohort study [41] including 13,887 participants with a mean plasma Se concentration of 125.6 ng/mL found that the association curve for Se and stroke mortality had a reversed U-shape. However, another cohort study including 1103 Chinese participants with a mean plasma Se concentration of 73 ng/mL found no significant association of plasma Se levels and stroke mortality [42]. Notably, these studies focused on stroke mortality, and these findings might not represent the association of plasma Se levels and first stroke risk. Similarly, prospective associations between Se status/intake and cardiovascular outcomes remain inconclusive [43–45]. The Selenium and Vitamin E Cancer Prevention Trial (SELECT) with a median plasma Se concentration of 136 ng/mL at baseline and the Nutritional Prevention of Cancer Trial (NPC) with a median plasma Se concentration of 115 ng/mL at baseline found no beneficial effects of Se supplementation on cardiovascular outcomes in relatively similar North American populations. However, an interaction of Se status and cancer risk could be found in NPC but not in SELECT. A previous meta-analysis enrolling 16 prospective studies indicated that the Se level was negatively related to cardiovascular risk within a narrow Se range of 55–145 ng/mL [46]. Therefore, the findings of the studies mentioned above suggested that baseline Se status was an important factor affecting the association between Se and human health. The median serum Se level in the present study was 86.7 ng/mL, which was slightly higher than those in European populations (German, mean Se level of 73.9 ng/mL; Sweden, mean serum Se level of 67.1 ng/mL) but significantly lower than those in the American population (mean serum Se level of 137.1 ng/mL). Several studies found an inverse relationship between the Se level and cardiovascular disease among some Chinese and European populations with low Se levels, which was not observed in other studies performed among some American populations with high Se levels [35, 37, 47]. Thus, the present study found a negative association between serum Se and stroke risk at a relatively low level of Se at baseline, which further suggested that baseline Se status should be taken into account when evaluating the association between Se and stroke risk. None of the previous studies reported a sex difference in the association between plasma Se and stroke risk, and the results of these studies remain inconclusive. The present study provides an opportunity to explore the possible relationship between plasma Se and first stroke and to examine the potential effect modifiers in a community-based Chinese population.
Our current study provides three new insights into the field. First, to the best of our knowledge, this is the first study to find a significant non-linear, inverse association between plasma Se and first total stroke and first ischemic stroke risks in males but not in females. The differences in the primary outcome (first stroke) between the sexes in our study may be explained by the differences in the way Se is metabolized between the male and female reproductive systems. The retention rate for Se is highly efficient in the testes, while it appears that the female reproductive system does not retain significant levels of Se as efficiently [48–50]. The interaction of Se with the thyroid axis may be another reason for the differences. Wang et al. [25] proved strong sex-specific differences in the risk and development of hyperthyroidism in relation to baseline Se intake. Se deficiency might constitute a risk factor for hyperthyroidism in males, but no substantial association was found between hyperthyroidism prevalence and Se status in females. Hyperthyroidism has been reported to be associated with a 2- to 3-fold increased risk of ischemic and non-ischemic stroke [51]. Therefore, we speculate that high Se levels may reduce the adverse effects in males due to Se deficiency, which may explain why the non-linear inverse association between serum Se and first stroke was mainly found among males. Further prospective studies are needed to verify this differential association by sex.
Second, we observed a sharp decline in the risk of first stroke when plasma Se was over 94.1 ng/mL, suggesting that this value might serve as a high plasma Se cut-off point marking a decreased risk of first stroke or a low plasma Se cut-off point marking an increased risk of first stroke. This cut-off value agrees with a previous study that reported that plasma Se > 90 μg/L was sufficient to optimize the functions of selenoproteins [52], which are believed to carry out the functions of Se in its role of Se compounds. Schomburg Lutz et al. [53] also reported that deficiency of selenoprotein P, the main carrier of Se to target organs and reduces tissue oxidative stress both directly and by delivering Se to protective selenoproteins, was associated with an increased risk of stroke in a North European population without a history of cardiovascular disease. Se concentrations approximately 110–125 ng/mL and 90–100 ng/mL are needed to maximize the expression of selenoprotein P and GPX3, respectively, both of which are biomarkers for a replete Se status [54, 55]. From this perspective, it is reasonable that no significant inverse relationship between serum selenium level and stroke risk was observed in the American populations with baseline selenium above 130 ng/mL because the Se level is replete for the populations, whereas a significant negative association between Se level and stroke risk was found in the present study with baseline selenium of approximately 86.66 ng/mL because the Se level is inadequate for this population. The reference value of serum Se concentration used in the present study was 50–120 ng/mL, which was based on the evaluation of data from the literature summarized by the Human Biomonitoring Commission, 2002 [56]. However, it should also be noted that the cut-off value of Se in the present study is still within the normal range for human plasma Se (50–120 ng/mL) [39], and our findings were found mainly among a population with normal Se levels, with a prevalence of plasma Se < 50, 50 to 120, and > 120 ng/mL of 1.2%, 95.1%, and 3.7% in this study (Supplemental Table 1). Therefore, the use of the cut-off value and normal range for human plasma Se (50–120 ng/mL) among stroke patients needs careful consideration. Our results, if further confirmed, might have vital clinical and public health implications for community residents in China.
Third, our study is the first to indicate a stronger non-linear negative relationship between plasma Se and first stroke in male participants with higher plasma vitamin E levels (≥ 13.5 μg/mL) than in those with lower plasma vitamin E levels (<13.5 μg/mL). This finding suggests that higher plasma Se and higher plasma vitamin E levels may jointly decrease the first stroke risk. A previous meta-analysis demonstrated a significant inverse association between dietary vitamin E intake and stroke risk, where a higher dietary vitamin E intake was associated with a lower risk of stroke [57]. The exact mechanisms underlying a high Se × high vitamin E interaction remain unclear. One plausible biological explanation for the interaction may be that both Se and vitamin E belong to the vital antioxidants and participate in protecting against brain oxidative stress [58], one of the hallmarks of stroke. Accordingly, high plasma vitamin E and Se levels may share some cellular and molecular mechanisms with the pathogenesis of stroke, which could cause the interaction in the non-linear negative relationship between plasma Se and first stroke in males. Further studies are warranted to verify this hypothesis.
While the mechanisms underlying the effect of Se on first stroke remain inconclusive, an association seems reasonable due to several vital biological functions of Se. Hosnedlova et al. [59] demonstrated that Se mainly exerts a protective effect against oxidative lipid damage in the brain and modulates neurotoxicity and oxidative stress in nervous tissue. Furthermore, modulation of inflammatory and metabolic signaling, as well as preservation of mitochondrial function, may also be involved in the protective role of Se in stroke [60, 61]. Se deficiency in heart failure patients was independently associated with impaired exercise tolerance and a 50% higher mortality rate and impaired mitochondrial function in vitro in human cardiomyocytes [62]. Ishrap et al. [63] reported that pharmacological Se supplementation might have an unexpected ability to drive adaptive transcription to counter ferroptosis and protect neurons after stroke both in vitro and in vivo in animal models. Further studies are needed to illuminate the mechanisms underlying the association between plasma Se and stroke.
Several possible limitations in this study should be mentioned. First, the plasma Se concentrations only represent the baseline Se levels of all participants; more frequent measurements during the follow-up would have strengthened the accuracy of our results. Second, only plasma Se concentrations were used as the biomarker of Se levels in our study; other biomarkers, including whole blood and urinary Se concentrations, should also be considered when performing a sensitivity analysis to confirm our findings. Third, this was a nested, case-control study, not a cohort study, with a relatively small sample size from a community-based population, and all stratified analyses were not prespecified; thus, this work was a product of hypothesis generation. Although the use of first stroke in this study may be reasonable to avoid reverse causation, it cannot entirely clarify the prospective relationship between Se and first stroke, and further larger-scale cohort studies are needed to verify this issue. Fourth, the findings were observed among subjects with H-type hypertension, which might not be entirely representative of the general population; thus, the findings of this study might not be applied to other populations. Finally, since Se is renally eliminated under the influence of diuretics, we adjusted for all anti-hypertensive drugs together and did not analyze the effects of diuretics separately on the association; thus, further analysis is needed to clarify this issue.
In summary, we found a significant non-linear, inverse association between baseline plasma Se and the risks of first stroke and first ischemic stroke in males but not in females. Plasma vitamin E level was a potential modifier affecting this association. In addition, no significant association between plasma Se and first hemorrhagic stroke was found among either sex.
Perspectives and significance
Data from the present study show that there was a sex difference in the association between plasma Se concentration and first stroke risk. This relationship existed among males but not in females. Thus, our findings suggest that we should take sex differences into account and stratify the data by sex when evaluating the association between plasma Se and first stroke risk in the future. If further confirmed, our findings may provide important data for clinical and nutritional guidelines on the primary prevention of first stroke among males by taking plasma Se into account to serve as a potentially modifiable risk factor and a possible biomarker for the purposes of monitoring and intervention. Furthermore, one future direction of this work is to clarify the potential mechanisms of sex disparities in plasma Se and first stroke risk.
Supplementary Information
Additional file 1: Supplemental Table 1. Distributions of plasma selenium concentrations. Supplemental Table 2. Characteristics of study participants by tertiles of baseline plasma selenium concentrationsa. Supplemental Table 3. Stratified analysis of the association between plasma selenium concentrations (T3, ≥94.1 ng/mL vs. T1-2, <94.1 ng/mL) and incident risk of first total stroke in females. Supplemental Figure 1. Flow chart of the study participants using a nested case-control design. *1401 controls were individually matched with 1401 cases by age (within 1 year), sex and village at a 1:1 ratio. Abbreviations: CHHRS: China H-type Hypertension Registry Study. Supplemental Figure 2. Distributions of plasma selenium (A) and vitamin E (B) levels by sex. Supplemental Figure 3. The association between baseline plasma selenium and the risk of first hemorrhagic stroke. Odds ratios for first hemorrhagic stroke in the (A) total population, (B) males, and (C) females by plasma selenium levels. In addition to the matching factors (age and sex), the splines also adjusted for BMI, baseline SBP, baseline DBP, smoking status, alcohol consumption, labor intensity, baseline total homocysteine, vitamin E, fasting glucose, estimated glomerular filtration rate (eGFR), anti-platelet drugs, lipoprotein-lowering drugs, glucose-lowering drugs, anti-hypertensive drugs, self-reported hypertension, self-reported diabetes, self-reported atrial fibrillation, and self-reported hyperlipidemia.
Acknowledgements
We acknowledge the contribution of all staff who participated in the present study as well as the study participants who shared their time with us.
Abbreviations
- CHHRS
China H-type Hypertension Registry Study
- CDC
Chinese Centers for Disease Control and Prevention
- OR
Odds ratio
- CI
Confidence intervals
- BMI
Body mass index
- SBP
Systolic blood pressure
- DBP
Diastolic blood pressure
- tHcy
Total homocysteine
- eGFR
Estimated glomerular filtration rate
- TC
Total cholesterol
- TG
Triglycerides
- HDL-C
High-density lipoprotein-cholesterol
Authors’ contributions
Concept and design of this study: Dr. Ping Li and Xiping Xu. Manuscript composition: Dr. Huan Hu and Ping Li. Data acquisition and collation: Lishun Liu. Statistical analysis: Huan Hu, Ping Wang, and Ziyi Zhou. Reviewed and revised the manuscript: Ping Li, Xiao Huang, Huihui, Bao, and Xiping Xu. The other authors coordinated this analysis. All authors read and approved the final manuscript.
Funding
The study was supported by funding from the following: the National Key Research and Development Program [2016YFE0205400, 2018ZX09739010, 2018ZX09301034003], the Science and Technology Planning Project of Guangzhou, China [201707020010]; the Science, Technology and Innovation Committee of Shenzhen [GJHS20170314114526143, JSGG20180703155802047]; the Economic, Trade and Information Commission of Shenzhen Municipality [20170505161556110, 20170505160926390]; the National Natural Science Foundation of China [81960074, 81860058, 81500233, 81560079]; the Jiangxi Outstanding Person Foundation [20192BCBL23024], the Major Projects of the Science and Technology Department, Jiangxi [20171BAB205008, 20152ACB20022, 20202ACBL206004], the Funding Scheme for Academic and Technical Leaders of Major Disciplines, Jiangxi [20172BCB22027], and Special Funds for Guiding Local Scientific and Technological Development by the Central Government of China (S2019CXSFG0016).
Availability of data and materials
All data are available from the corresponding author upon request.
Declarations
Ethics approval and consent to participate
The protocol of the present study was approved by the Ethics Committee of the Institute of Biomedicine, Anhui Medical University, Hefei, China. All participants signed an approved written consent form.
Competing interests
Dr. Xiping Xu reports grants from the National Key Research and Development Program [2016YFE0205400, 2018ZX09739010, 2018ZX09301034003), the Science and Technology Planning Project of Guangzhou, China (201707020010), the Science, Technology and Innovation Committee of Shenzhen [GJHS20170314114526143, JSGG20180703155802047), and the Economic, Trade and Information Commission of Shenzhen Municipality [20170505161556110, 20170505160926390]. Dr. Xiao Huang reports grants from the National Natural Science Foundation of China [81960074, 81500233], the Jiangxi Outstanding Person Foundation [20192BCBL23024], and Major projects of the Science and Technology Department, Jiangxi [20171BAB205008]. Dr. Ping Li reports grants from the National Natural Science Foundation of China [81560079, 81860058], Major Projects of the Science and Technology Department, Jiangxi, [20152ACB20022, 20202ACBL206004], Funding Scheme for Academic and Technical Leaders of Major Disciplines, Jiangxi [20172BCB22027], and Special Funds for Guiding Local Scientific and Technological Development by the Central Government of China (S2019CXSFG0016). No other disclosures were reported. The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhou M, Wang H, Zeng X, Yin P, Zhu J, Chen W, Li X, Wang L, Wang L, Liu Y, Liu J, Zhang M, Qi J, Yu S, Afshin A, Gakidou E, Glenn S, Krish VS, Miller-Petrie MK, Mountjoy-Venning WC, Mullany EC, Redford SB, Liu H, Naghavi M, Hay SI, Wang L, Murray CJL, Liang X. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;394(10204):1145–1158. doi: 10.1016/S0140-6736(19)30427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang W, Jiang B, Sun H, Ru X, Sun D, Wang L, Wang L, Jiang Y, Li Y, Wang Y, Chen Z, Wu S, Zhang Y, Wang D, Wang Y, Feigin VL, NESS-China Investigators Prevalence, incidence, and mortality of stroke in China: results from a nationwide population-based survey of 480 687 adults. Circulation. 2017;135(8):759–771. doi: 10.1161/CIRCULATIONAHA.116.025250. [DOI] [PubMed] [Google Scholar]
- 3.Wu S, Wu B, Liu M, Chen Z, Wang W, Anderson CS, Sandercock P, Wang Y, Huang Y, Cui L, Pu C, Jia J, Zhang T, Liu X, Zhang S, Xie P, Fan D, Ji X, Wong KSL, Wang L, Wu S, Wu B, Liu M, Chen Z, Wang W, Anderson CS, Sandercock P, Wang Y, Huang Y, Cui L, Pu C, Jia J, Zhang T, Liu X, Zhang S, Xie P, Fan D, Ji X, Wong KSL, Wang L, Wei C, Wang Y, Cheng Y, Liu Y, Li X, Dong Q, Zeng J, Peng B, Xu Y, Yang Y, Wang Y, Zhao G, Wang W, Xu Y, Yang Q, He Z, Wang S, You C, Gao Y, Zhou D, He L, Li Z, Yang J, Lei C, Zhao Y, Liu J, Zhang S, Tao W, Hao Z, Wang D, Zhang S. Stroke in China: advances and challenges in epidemiology, prevention, and management. Lancet Neurol. 2019;18(4):394–405. doi: 10.1016/S1474-4422(18)30500-3. [DOI] [PubMed] [Google Scholar]
- 4.Lackland DT, Roccella EJ, Deutsch AF, Fornage M, George MG, Howard G, et al. Factors influencing the decline in stroke mortality: a statement from the American Heart Association/ American Stroke Association. Stroke. 2014;45:315–353. doi: 10.1161/01.str.0000437068.30550.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vinceti M, Filippini T, Crippa A, de Sesmaisons A, Wise LA, Orsini N. Meta-analysis of potassium intake and the risk of stroke. J Am Heart Assoc. 2016;5:e004210. doi: 10.1161/JAHA.116.004210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jayedi A, Ghomashi F, Zargar MS, Shab-Bidar S. Dietary sodium, sodium-to-potassium ratio, and risk of stroke: A systematic review and nonlinear dose-response meta-analysis. Clin Nutr. 2019;38(3):1092–1100. doi: 10.1016/j.clnu.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 7.Zhang J, Cao J, Zhang Y, Li H, Zhang H, Huo Y, Li J, Liu X, Wang X, Qin X, Xu X. Baseline plasma zinc and risk of first stroke in hypertensive patients: a nested case-control study. Stroke. 2019;50(11):3255–3258. doi: 10.1161/STROKEAHA.119.027003. [DOI] [PubMed] [Google Scholar]
- 8.Morris DR, Levenson CW. Neurotoxicity of Zinc. Adv Neurobiol. 2017;18:303–312. doi: 10.1007/978-3-319-60189-2_15. [DOI] [PubMed] [Google Scholar]
- 9.Uesugi S, Ishihara J, Iso H, Sawada N, Takachi R, Inoue M, et al. Dietary intake of antioxidant vitamins and risk of stroke: the Japan Public Health Center-based Prospective Study. Eur J Clin Nutr. 2017;71(10):1179–1185. doi: 10.1038/ejcn.2017.71. [DOI] [PubMed] [Google Scholar]
- 10.Mohammadifard N, Humphries KH, Gotay C, Mena-Sánchez G, Salas-Salvadó J. Esmaillzadeh. A, et al. Trace minerals intake: Risks and benefits for cardiovascular health. Crit Rev Food Sci Nutr. 2019;59(8):1334–1346. doi: 10.1080/10408398.2017.1406332. [DOI] [PubMed] [Google Scholar]
- 11.Jenkins DJA, Spence JD, Giovannucci EL, Kim YI, Josse R, Vieth R, Blanco Mejia S, Viguiliouk E, Nishi S, Sahye-Pudaruth S, Paquette M, Patel D, Mitchell S, Kavanagh M, Tsirakis T, Bachiri L, Maran A, Umatheva N, McKay T, Trinidad G, Bernstein D, Chowdhury A, Correa-Betanzo J, del Principe G, Hajizadeh A, Jayaraman R, Jenkins A, Jenkins W, Kalaichandran R, Kirupaharan G, Manisekaran P, Qutta T, Shahid R, Silver A, Villegas C, White J, Kendall CWC, Pichika SC, Sievenpiper JL. Supplemental vitamins and minerals for CVD prevention and treatment. J Am Coll Cardiol. 2018;71(22):2570–2584. doi: 10.1016/j.jacc.2018.04.020. [DOI] [PubMed] [Google Scholar]
- 12.Rayman MP. Selenium and human health. Lancet. 2012;379(9822):1256–1268. doi: 10.1016/S0140-6736(11)61452-9. [DOI] [PubMed] [Google Scholar]
- 13.Roman M, Jitaru P, Barbante C. Selenium biochemistry and its role for human health. Metallomics. 2014;6(1):25–54. doi: 10.1039/C3MT00185G. [DOI] [PubMed] [Google Scholar]
- 14.Zoidis E, Seremelis I, Kontopoulos N, Danezis GP. Selenium-dependent antioxidant enzymes: actions and properties of selenoproteins. Antioxidants (Basel) 2018;7:66. doi: 10.3390/antiox7050066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loscalzo J. Keshan disease, selenium deficiency, and the selenoproteome. N Engl J Med. 2014;370(18):1756–1760. doi: 10.1056/NEJMcibr1402199. [DOI] [PubMed] [Google Scholar]
- 16.Fang LQ, Goeijenbier M, Zuo SQ, Wang LP, Liang S, Klein SL, Li XL, Liu K, Liang L, Gong P, Glass G, van Gorp E, Richardus J, Ma JQ, Cao WC, de Vlas S. The association between hantavirus infection and selenium deficiency in mainland China. Viruses. 2015;7(1):333–351. doi: 10.3390/v7010333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang XL, Yang TB, Wei J, Lei GH, Zeng C. Association between serum selenium level and type 2 diabetes mellitus: a non-linear dose-response meta-analysis of observational studies. Nutr J. 2016;15:48–56. doi: 10.1186/s12937-016-0169-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Li H, Lin T, Guo H, Jiang C, Xie L, Li Y, Zhou Z, Song Y, Wang B, Liu C, Liu L, Li J, Zhang Y, Wang G, Liang M, Cui Y, Huo Y, Yang Y, Ling W, Yang J, Wang X, Zhang H, Qin X, Xu X. Plasma selenium levels and risk of new-onset diabetes in hypertensive adults. J Trace Elem Med Biol. 2019;56:6–12. doi: 10.1016/j.jtemb.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Liao XL, Wang ZH, Liang XN, Liang J, Wei XB, Wang SH, Guo WX. The association of circulating selenium concentrations with diabetes mellitus. Diabetes Metab Syndr Obes. 2020;13:4755–4761. doi: 10.2147/DMSO.S284120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vinceti M, Filippini T, Wise LA. Environmental Selenium and Human Health: an Update. Curr Environ Health Rep. 2018;5(4):464–485. doi: 10.1007/s40572-018-0213-0. [DOI] [PubMed] [Google Scholar]
- 21.Vinceti M, Filippini T, Rothman KJ. Selenium exposure and the risk of type 2 diabetes: a systematic review and meta-analysis. Eur J Epidemiol. 2018;33(9):789–810. doi: 10.1007/s10654-018-0422-8. [DOI] [PubMed] [Google Scholar]
- 22.Liu X, Wang Y, Han S, Zhang Y, Zou Y, Su S, et al. A spatial ecological study on serum selenium and Keshan disease in Heilongjiang Province. China. Biol Trace Elem Res. 2020;7. 10.1007/s12011-020-02478-0. [DOI] [PubMed]
- 23.Ning Y, Wang X, Zhang P, Anatoly SV, Prakash NT, Li C, Zhou R, Lammi M, Zhang F, Guo X. Imbalance of dietary nutrients and the associated differentially expressed genes and pathways may play important roles in juvenile Kashin-Beck disease. J Trace Elem Med Biol. 2018;50:441–410. doi: 10.1016/j.jtemb.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 24.Wu Q, Rayman MP, Lv H, Schomburg L, Cui B, Gao C, Chen P, Zhuang G, Zhang Z, Peng X, Li H, Zhao Y, He X, Zeng G, Qin F, Hou P, Shi B. Low population selenium status is associated with increased prevalence of thyroid disease. J Clin Endocrinol Metab. 2015;100(11):4037–4047. doi: 10.1210/jc.2015-2222. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Zhao F, Rijntjes E, Wu L, Wu Q, Sui J, et al. Role of selenium intake for risk and development of Hyperthyroidism. J Clin Endocrinol Metab. 2019;104(2):568–580. doi: 10.1210/jc.2018-01713. [DOI] [PubMed] [Google Scholar]
- 26.Kornitzer M, Valente F, De Bacquer D, Neve J, De Backer G. Serum selenium and cancer mortality: a nested case-control study within an age- and sex-stratified sample of the Belgian adult population. Eur J Clin Nutr. 2004;58(1):98–104. doi: 10.1038/sj.ejcn.1601754. [DOI] [PubMed] [Google Scholar]
- 27.Hughes DJ, Fedirko V, Jenab M, Schomburg L, Méplan C, Freisling H, Bueno-de-Mesquita HB, Hybsier S, Becker NP, Czuban M, Tjønneland A, Outzen M, Boutron-Ruault MC, Racine A, Bastide N, Kühn T, Kaaks R, Trichopoulos D, Trichopoulou A, Lagiou P, Panico S, Peeters PH, Weiderpass E, Skeie G, Dagrun E, Chirlaque MD, Sánchez MJ, Ardanaz E, Ljuslinder I, Wennberg M, Bradbury KE, Vineis P, Naccarati A, Palli D, Boeing H, Overvad K, Dorronsoro M, Jakszyn P, Cross AJ, Quirós JR, Stepien M, Kong SY, Duarte-Salles T, Riboli E, Hesketh JE. Selenium status is associated with colorectal cancer risk in the European prospective investigation of cancer and nutrition cohort. Int J Cancer. 2015;136(5):1149–1161. doi: 10.1002/ijc.29071. [DOI] [PubMed] [Google Scholar]
- 28.Clark LC, Combs GF, Turnbull BW, Slate EH, Chalker DK, Chow J, et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA. 1996;276:1957–1963. [PubMed] [Google Scholar]
- 29.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, Parnes HL, Minasian LM, Gaziano JM, Hartline JA, Parsons JK, Bearden JD, Crawford ED, Goodman GE, Claudio J, Winquist E, Cook ED, Karp DD, Walther P, Lieber MM, Kristal AR, Darke AK, Arnold KB, Ganz PA, Santella RM, Albanes D, Taylor PR, Probstfield JL, Jagpal TJ, Crowley JJ, Meyskens FL, Baker LH, Coltman CA. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301(1):39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cardoso RB, Hare DJ, Macpherson H. Sex-dependent association between selenium status and cognitive performance in older adults. Eur J Nutr. 2021;60(2):1153–1159. doi: 10.1007/s00394-020-02384-0. [DOI] [PubMed] [Google Scholar]
- 31.Xu ZC, Shao HF, Li S, Zheng C. Relationships between the selenium content in flue-cured tobacco leaves and the selenium content in soil in ENSHI, China tobacco-growing area. Pak J Bot. 2012;44:1563–1568. [Google Scholar]
- 32.Dinh QT, Cui Z, Huang J, Tran TAT, Wang D, Yang W, Zhou F, Wang M, Yu D, Liang D. Selenium distribution in the Chinese environment and its relationship with human health: A review. Environ Int. 2018;112:294–309. doi: 10.1016/j.envint.2017.12.035. [DOI] [PubMed] [Google Scholar]
- 33.Hu XF, Stranges S, Chan LHM. Circulating selenium concentration is inversely associated with the prevalence of stroke: results from the Canadian Health Measures Survey and the National Health and Nutrition Examination Survey. J Am Heart Assoc. 2019;8:e012290. doi: 10.1161/JAHA.119.012290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu XF, Sharin T, Chan HM. Dietary and blood selenium are inversely associated with the prevalence of stroke among Inuit in Canada. J. Trace. Elem. Med. Biol. 2017;44:322–330. doi: 10.1016/j.jtemb.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 35.Wen Y, Huang S, Zhang Y, Zhang H, Zhou L, Li D, Xie C, Lv Z, Guo Y, Ke Y, Wu T, Cheng J. Associations of multiple plasma metals with the risk of ischemic stroke: A case-control study. Environ. Int. 2019;125:125–134. doi: 10.1016/j.envint.2018.12.037. [DOI] [PubMed] [Google Scholar]
- 36.Merrill PD, Ampah SB, He K, Rembert NJ, Brockman J, Kleindorfer D, McClure LA. Association between trace elements in the environment and stroke risk: The reasons for geographic and racial differences in stroke (REGARDS) study. J Trace Elem Med Biol. 2017;42:45–49. doi: 10.1016/j.jtemb.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Virtamo J, Valkeila E, Alfthan G, Punsar S, Huttunen JK, Karvonen MJ. Serum selenium and the risk of coronary heart disease and stroke. Am J Epidemiol. 1985;122(2):276–282. doi: 10.1093/oxfordjournals.aje.a114099. [DOI] [PubMed] [Google Scholar]
- 38.Xiao Y, Yuan Y, Liu Y, Yu Y, Jia N, Zhou L, Wang H, Huang S, Zhang Y, Yang H, Li X, Hu FB, Liang L, Pan A, Zhang X, He M, Cheng J, Wu T. Circulating multiple metals and incident stroke in Chinese adults. Stroke. 2019;50(7):1661–1668. doi: 10.1161/STROKEAHA.119.025060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilhelm M, Ewers U, Schulz C. Revised and new reference values for some trace elements in blood and urine for human biomonitoring in environmental medicine. Int J Hyg Environ Health. 2004;207(1):69–73. doi: 10.1078/1438-4639-00260. [DOI] [PubMed] [Google Scholar]
- 40.Meschia JF, Bushnell C, Boden-Albala B, Braun LT, Bravata DM, Chaturvedi S, Creager MA, Eckel RH, Elkind MS, Fornage M, Goldstein LB, Greenberg SM, Horvath SE, Iadecola C, Jauch EC, Moore WS, Wilson JA, American Heart Association Stroke Council. Council on Cardiovascular and Stroke Nursing. Council on Clinical Cardiology. Council on Functional Genomics and Translational Biology. Council on Hypertension Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(12):3754–3832. doi: 10.1161/STR.0000000000000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bleys J, Navas-Acien A, Guallar E. Serum selenium levels and all-cause, cancer, and cardiovascular mortality among US adults. Arch Intern Med. 2008;168(4):404–410. doi: 10.1001/archinternmed.2007.74. [DOI] [PubMed] [Google Scholar]
- 42.Wei WQ, Abnet CC, Qiao YL, Dawsey SM, Dong ZW, Sun XD, Fan JH, Gunter EW, Taylor PR, Mark SD. Prospective study of serum selenium concentrations and esophageal and gastric cardia cancer, heart disease, stroke, and total death. Am J Clin Nutr. 2004;79(1):80–85. doi: 10.1093/ajcn/79.1.80. [DOI] [PubMed] [Google Scholar]
- 43.Stranges S, Navas-Acien A, Rayman MP, Guallar E. Selenium status and cardiometabolic health: state of the evidence. Nutr Metab Cardiovasc Dis. 2010;20(10):754–760. doi: 10.1016/j.numecd.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 44.Khan SU, Khan MU, Riaz H, Valavoor S, Zhao D, Vaughan L, Okunrintemi V, Riaz IB, Khan MS, Kaluski E, Murad MH, Blaha MJ, Guallar E, Michos ED. Effects of nutritional supplements and dietary interventions on cardiovascular outcomes: an umbrella review and evidence map. Ann Intern Med. 2019;171(3):190–198. doi: 10.7326/M19-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rees K, Hartley L, Day C, Flowers N, Clarke A, Stranges S. Selenium supplementation for the primary prevention of cardiovascular disease. Cochrane. Database Syst Rev. 2013;31:CD009671. doi: 10.1002/14651858.CD009671.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang X, Liu C, Guo J, Song Y. Selenium status and cardiovascular diseases: meta-analysis of prospective observational studies and randomized controlled trials. Eur J Clin Nutr. 2016;70(2):162–9. [DOI] [PubMed]
- 47.Salvini S, Hennekens CH, Morris JS, Willett WC, Stampfer MJ. Plasma levels of the antioxidant selenium and risk of myocardial infarction among U.S. physicians. Am J Cardiol. 1995;76(17):1218–21. [DOI] [PubMed]
- 48.Brown DG, Burk RF. Selenium retention in tissues and sperm of rats fed a Torula yeast diet. J Nutr. 1973;103(1):102–108. doi: 10.1093/jn/103.1.102. [DOI] [PubMed] [Google Scholar]
- 49.Hardy G, Hardy I. Selenium: the Se-XY nutraceutical. Nutrition. 2004;20(6):590–593. doi: 10.1016/j.nut.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 50.Hatfield DL, Schweizer U, Tsuji PA, Gladyshev VN, Schomburg L, et al. Sex-specific differences in biological effects and metabolism of selenium. In: Hatfifield DL, et al., editors. Selenium - Its Molecular Biology and Role in Human Health. 4. New York: Springer; 2016. pp. 214–220. [Google Scholar]
- 51.Dekkers OM, Horváth-Puhó E, Cannegieter SC, Vandenbroucke JP, Sørensen HT, Jørgensen JOL. Acute cardiovascular events and all-cause mortality in patients with hyperthyroidism: a population-based cohort study. Eur J Endocrinol. 2017;176(1):1–9. doi: 10.1530/EJE-16-0576. [DOI] [PubMed] [Google Scholar]
- 52.Xia Y, Hill KE, Li P, Xu J, Zhou D, Motley AK, Wang L, Byrne DW, Burk RF. Optimization of selenoprotein P and other plasma selenium biomarkers for the assessment of the selenium nutritional requirement: a placebo-controlled, double-blind study of selenomethionine supplementation in selenium-deficient Chinese subjects. Am J Clin Nutr. 2010;92(3):525–531. doi: 10.3945/ajcn.2010.29642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schomburg L, Orho-Melander M, Struck J, Bergmann A, Melander O, et al. Selenoprotein-P deficiency predicts cardiovascular disease and death. Nutrients. 2019;11:1852. doi: 10.3390/nu11081852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duffield AJ, Thomson CD, Hill KE, Williams S. An estimation of selenium requirements for New Zealanders. Am J Clin Nutr. 1999;70(5):896–903. doi: 10.1093/ajcn/70.5.896. [DOI] [PubMed] [Google Scholar]
- 55.Hurst R, Armah CN, Dainty JR, Hart DJ, Teucher B, Goldson AJ. Establishing optimal selenium status: results of a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2010;91(4):923–931. doi: 10.3945/ajcn.2009.28169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.HBC–Human Biomonitoring Commission (Kommission Human-Biomonitoring des Umweltbundesamtes), Stellungnahme der Kommission. Bekanntmachung des UmweltbundesamtesSelen und Human-Biomonitoring. Bundesgesundheitsbl Gesundheitsforsch Gesundheitsschutz. 2002;45:190–5. 10.1007/s00103-001-0357-0. [DOI] [PubMed]
- 57.Cheng P, Wang L, Ning S, Liu Z, Lin H, Chen S, Zhu J. Vitamin E intake and risk of stroke: a meta-analysis. Br J Nutr. 2018;120(10):1181–1188. doi: 10.1017/S0007114518002647. [DOI] [PubMed] [Google Scholar]
- 58.Beytut E, Yilmaz S, Aksakal M, Polat S, et al. The possible protective effects of vitamin E and selenium administration in oxidative stress caused by high doses of glucocorticoid administration in the brain of rats. J Trace Elem Med Biol. 2018;45:131–135. doi: 10.1016/j.jtemb.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 59.Hosnedlova B, Kepinska M, Skalickova S, Fernandez C, Ruttkay-Nedecky B, Malevu TD, Sochor J, Baron M, Melcova M, Zidkova J, Kizek R. A summary of new findings on the biological effects of selenium in selected animal species-a critical review. Int J Mol Sci. 2017;18(10):2209. doi: 10.3390/ijms18102209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Amani H, Habibey R, Shokri F, Hajmiresmail SJ, Akhavan O, Mashaghi A, Pazoki-Toroudi H. Selenium nanoparticles for targeted stroke therapy through modulation of inflammatory and metabolic signaling. Sci Rep. 2019;9(1):6044. doi: 10.1038/s41598-019-42633-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mehta SL, Kumari S, Mendelev N, Li PA. Selenium preserves mitochondrial function, stimulates mitochondrial biogenesis, and reduces infarct volume after focal cerebral ischemia. BMC Neurosci. 2012;13(1):79. doi: 10.1186/1471-2202-13-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bomer N, Beverborg NG, FHoes M, WStreng K, Vermeer M, Dokter MM, et al. Selenium and outcome in heart failure. Eur J Heart Fail. 2020;22(8):1415–1423. doi: 10.1002/ejhf.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alim I, Caulfield JT, Chen Y, Swarup V, Geschwind DH, Ivanova E, Seravalli J, Ai Y, Sansing LH, Ste.Marie EJ, Hondal RJ, Mukherjee S, Cave JW, Sagdullaev BT, Karuppagounder SS, Ratan RR. Selenium Drives a Transcriptional Adaptive Program to Block Ferroptosis and Treat Stroke. Cell. 2019;177(5):1262–1279. doi: 10.1016/j.cell.2019.03.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplemental Table 1. Distributions of plasma selenium concentrations. Supplemental Table 2. Characteristics of study participants by tertiles of baseline plasma selenium concentrationsa. Supplemental Table 3. Stratified analysis of the association between plasma selenium concentrations (T3, ≥94.1 ng/mL vs. T1-2, <94.1 ng/mL) and incident risk of first total stroke in females. Supplemental Figure 1. Flow chart of the study participants using a nested case-control design. *1401 controls were individually matched with 1401 cases by age (within 1 year), sex and village at a 1:1 ratio. Abbreviations: CHHRS: China H-type Hypertension Registry Study. Supplemental Figure 2. Distributions of plasma selenium (A) and vitamin E (B) levels by sex. Supplemental Figure 3. The association between baseline plasma selenium and the risk of first hemorrhagic stroke. Odds ratios for first hemorrhagic stroke in the (A) total population, (B) males, and (C) females by plasma selenium levels. In addition to the matching factors (age and sex), the splines also adjusted for BMI, baseline SBP, baseline DBP, smoking status, alcohol consumption, labor intensity, baseline total homocysteine, vitamin E, fasting glucose, estimated glomerular filtration rate (eGFR), anti-platelet drugs, lipoprotein-lowering drugs, glucose-lowering drugs, anti-hypertensive drugs, self-reported hypertension, self-reported diabetes, self-reported atrial fibrillation, and self-reported hyperlipidemia.
Data Availability Statement
All data are available from the corresponding author upon request.