Abstract
Chronic administration of opioids produces physical dependence and opioid-induced hyperalgesia. Users claim the Thai traditional tea “kratom” and component alkaloid mitragynine ameliorate opioid withdrawal without increased sensitivity to pain. Testing these claims, we assessed the combined kratom alkaloid extract (KAE) and two individual alkaloids, mitragynine (MG) and the analog mitragynine pseudoindoxyl (MP), evaluating their ability to produce physical dependence and induce hyperalgesia after chronic administration, and as treatments for withdrawal in morphine-dependent subjects. C57BL/6J mice (n = 10/drug) were administered repeated saline, or graded, escalating doses of morphine (intraperitoneal; i.p.), kratom alkaloid extract (orally, p.o.), mitragynine (p.o.), or MP (subcutaneously, s.c.) for 5 days. Mice treated chronically with morphine, KAE, or mitragynine demonstrated significant drug-induced hyperalgesia by day 5 in a 48 °C warm-water tail-withdrawal test. Mice were then administered naloxone (10 mg/kg, s.c.) and tested for opioid withdrawal signs. Kratom alkaloid extract and the two individual alkaloids demonstrated significantly fewer naloxone-precipitated withdrawal signs than morphine-treated mice. Additional C57BL/6J mice made physically dependent on morphine were then used to test the therapeutic potential of combined KAE, mitragynine, or MP given twice daily over the next 3 days at either a fixed dose or in graded, tapering descending doses. When administered naloxone, mice treated with KAE, mitragynine, or MP under either regimen demonstrated significantly fewer signs of precipitated withdrawal than control mice that continued to receive morphine. In conclusion, while retaining some liabilities, kratom, mitragynine, and mitragynine pseudoindoxyl produced significantly less physical dependence and ameliorated precipitated withdrawal in morphine-dependent animals, suggesting some clinical value.
Keywords: Opioid, Physical dependence, Kratom, Mitragynine, Withdrawal
Introduction
While opioid agonists of the mu-opioid receptor (MOR) have proven therapeutic advantages in the treatment of pain, MOR activation also produces challenging side effects (Minami and Satoh 1995). For example, repetitive administration of opioids induces hyperalgesia (Hutchinson et al. 2011; Xin et al. 2011), paradoxically increasing sensitivity to pain. Moreover, continual exposure to opioid agonists results in changes of physical homeostatic mechanisms such as adenylyl cyclase activity, factors collectively thought to contribute to undesired effects such as tolerance and physical dependence (Koob et al. 1997). These neuroadaptive mechanisms are theorized to be the body’s defense against life-threatening effects following chronic use of drugs (Koob et al. 1997). Interruption of opioid intake results in clearance of the drug following metabolism (Shafer and Varvel 1991), but adaptations to homeostasis do not reverse rapidly. The absence of the drug after chronic use leads to physical withdrawal symptoms (Hutcheson et al. 2001). Symptoms related to opioid-induced withdrawal include body aches, headache, anxiety, seizures, and/or influenza-like conditions (Khor et al. 2011). Avoidance of opioid withdrawal symptoms is a leading cause of opioid seeking and relapse in abstinent subjects (Khor et al. 2011). The MOR agonist methadone and buprenorphine (a multifunctional MOR partial agonist, KOR/DOR antagonist, and NOP weak agonist) are used to treat opioid use disorder and prevent opioid withdrawal-related symptoms in the clinic (Kosten and O’Connor 2003). However, these opioid agonists potentially produce liabilities of their own, including respiratory depression and substance abuse (Kreek et al. 2002). Thus, identifying new, safer treatments for the symptoms of opioid withdrawal would also potentially help address the urgent problem of opioid substance abuse disorder.
Mitragyna speciosa, or kratom, a natural plant product native to Southeast Asia, has recently gained popularity in regards for its use in self-treatment of opioid addiction and withdrawal syndrome (Boyer et al. 2008; Kruegel and Grundmann 2018; Singh et al. 2020; Chakraborty and Majumdar 2020). Mitragynine, the main active alkaloid in kratom, has been found to make up approximately 66% of the extract content within the plant (Adkins et al. 2011; Hassan et al. 2013). An initial study reported that a single administration of mitragynine reduced behavioral signs of withdrawal in morphine-dependent rats (Hassan et al. 2020). Although a mechanism was not investigated, previous studies with mitragynine show that most of its physiological effects are via the MOR subtype (Yusoff et al. 2014; Matsumoto et al. 1996a; Kruegel et al. 2019). Similarly, the semi-synthetic analog mitragynine pseudoindoxyl (MP) demonstrated potent antinociception with reduced liabilities attributed to its multifunctional MOR biased agonism and DOR antagonism (Váradi et al. 2016). Recent in vitro studies also suggest that mitragynine pseudoindoxyl is a metabolite of mitragynine (Kamble et al. 2020). Lyophilized kratom tea itself has been shown to act via the opioid system (Yusoff et al. 2014; Wilson et al. 2020), mitigate opioid withdrawal syndrome in zebra fish (Khor et al. 2011), and substitute for morphine in rodent discrimination studies (Harun et al. 2015).
From this and the limited clinical reports, we hypothesize that kratom alkaloid extract (KAE) tested presently or its major constituents may be therapeutic candidates for the treatment of opioid withdrawal syndrome. Accordingly, the aim of this study was to assess the ability of KAE, the major alkaloid mitragynine, and the semi-synthetic analog mitragynine pseudoindoxyl for the amelioration of naloxone-precipitated opioid withdrawal syndrome in mice, first comparing them to morphine for their potential to induce opioid-induced hyperalgesia and physical dependence directly after chronic treatment.
Methods
Male C57BL/6J mice (25–35 mg) were used (Jackson Laboratories Bar Harbor, Maine, USA). The mice were housed five per cage on a 12:12 h light/dark cycle (lights off at 7:00 P.M. and on at 7:00 A.M.) with ad libitum access to food and water. All animals were habituated to the testing room for a minimum of 1 h prior to testing. All animal studies were approved and conducted in agreement with the Institutional Animal Care and Use Committees at the University of Florida, in accordance with the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978) and in compliance with the ARRIVE guidelines (Kilkenny et al. 2010).
Chemicals
The kratom alkaloid extract (KAE) used for assays was prepared as described by Sabetghadam et al. (2010); Váradi et al. (2016) and Gutridge et al. (2020) (see also below) from kratom “Red Indonesian Micro Powder,” purchased from Moon Kratom (Austin, TX). Mitragynine pseudoindoxyl was synthesized from mitragynine as previously described (Váradi et al. 2016). Mitragynine itself was extracted from the powdered leaves of M. speciosa (kratom) by following our previously described method (Váradi et al. 2016). Briefly, kratom powder (500 g) was heated to reflux in MeOH 700 mL for 40 min. The suspension was filtered and the methanolic extraction process was repeated three times (3 × 500 mL). The solvent of combined methanolic extract was evaporated under reduced pressure and the content was dried using high vacuum. The dry residue was suspended in 20% acetic acid solution (1 L) and washed with petroleum ether (4 × 500 mL). The aqueous layer was then cooled on an ice bath and basified (pH ~ 9–10) with aqueous NaOH solution (3.5 M. ~ 1 L) slowly. Alkaloids were extracted in EtOAc (4 × 400 mL) from the aqueous layer. The combined EtOAc part was washed with brine (300 mL), dried over anhydrous Na2SO4 and filtered. The solvent was evaporated under reduced pressure, and the residue was dried under high vacuum to obtain kratom extract (11 g). Then, 200 mg of kratom extract was subjected to silica gel column chromatography; using 0–40% EtOAc in hexanes to isolate mitragynine (97.2 mg); paynantheine (11.6 mg), speciogynine (7 mg), and using 0–15% MeOH in DCM to isolate speciociliatine (27.4 mg). This corresponded to approximately 48.6% mitragynine, 5.8% paynantheine, 3.5% speciogynine and 13.7% speciociliatine in the kratom extract along with other minor alkaloids which were below 0.1%.
All drugs and chemicals otherwise used were purchased from Sigma-Aldrich (St. Louis, MO, USA). For experiments, sterile isotonic saline (0.9%) was used to dissolve drugs to desired concentrations for testing.
Hyperalgesic Testing (48 °C Warm-Water Tail-Withdrawal Assay)
The nociceptive stimulus was 48 °C water, with the latency to withdraw the tail taken as the end point (Journigan et al. 2014). The water temperature of 48 °C was selected for this work to ensure a moderate tail-withdrawal response, with a measurable decrease in withdrawal time possible but also a significant temperature for hyperalgesic testing. If the mouse failed to display a tail-flick in 30 s, the tail was removed from the water to minimize tissue damage. Animals showing an initial baseline latency fewer than 4 s or more than 30 s were removed from the study; no mice were so excluded. After determining control latencies on day 1, mice received chronic vehicle (saline), morphine, kratom alkaloid extract, mitragynine, or mitragynine pseudoindoxyl pretreatment for 4 days as described below. Dosing of these compounds was based on the demonstration of equianalgesic efficacy drawn from earlier antinociceptive dose–response and time course studies (Sabetghadam et al. 2010; Váradi et al. 2016; Kruegel et al. 2019; Wilson et al. 2020; see below). Mice were then tested prior to treatment on day 5 for evidence of induced hyperalgesia. Experimentally induced decreases in tail-withdrawal latencies in this assay indicate hyperalgesic effects (Crain and Shen 2001; Journigan et al. 2014). Tail-withdrawal baselines were measured on day 5 prior to opioid or kratom alkaloid administration and subsequent administration and assessment of naloxone-precipitated opioid withdrawal symptoms that are described in experiment design 1.
Naloxone-Precipitated Opioid Withdrawal Assay
Mice were randomly divided into 14 groups: six groups to assess potential physical dependence (experimental design 1, n = 10/group) and eight therapeutic challenge groups (experimental design 2, n = 9–10/group) utilizing a modified dosing regimen (as described below, and in Tables 1 and 2, respectively). Kratom alkaloid extract, mitragynine, or mitragynine pseudoindoxyl (MP) was used to challenge morphine withdrawal. Dosing of these compounds was based on earlier antinociceptive dose–response studies (Sabetghadam et al. 2010; Váradi et al. 2016; Kruegel et al. 2019; Wilson et al. 2020). All daily repeated administrations occurred at 9:00 A.M. and 7:00 P.M., as previously reported (Kamei and Ohsawa 1997; Wilson et al. 2020). On the last day, withdrawal was precipitated with 10 mg/kg naloxone (s.c.) in all groups.
Table 1.
Design of experiment 1: dosing over 5 days to assess potential physical dependence
| Treatment day | Treatment | |||||
|---|---|---|---|---|---|---|
| Saline (i.p.) | Morphine (10–75 mg/kg, i.p.) | Morphine (10–75 mg/kg, i.p.) + clonidine (5 mg/kg, i.p.) | Kratom alkaloid extract (KAE) (30–125 mg/kg, p.o.) | Mitragynine (MG) (10–75 mg/kg, p.o.) | Mitragynine pseudoindoxyl (MP) (1–35 mg/kg, s.c.) | |
| Day 1 am | + | 10 mg/kg | 10 mg/kg | 30 mg/kg KAE | 10 mg/kg MG | 1 mg/kg MP |
| pm | + | 15 mg/kg | 15 mg/kg | 35 mg/kg KAE | 15 mg/kg MG | 3 mg/kg MP |
| Day 2 am | + | 20 mg/kg | 20 mg/kg | 45 mg/kg KAE | 20 mg/kg MG | 3 mg/kg MP |
| pm | + | 30 mg/kg | 30 mg/kg | 60 mg/kg KAE | 30 mg/kg MG | 10 mg/kg MP |
| Day 3 am | + | 50 mg/kg | 50 mg/kg | 100 mg/kg KAE | 50 mg/kg MG | 15 mg/kg MP |
| pm | + | 60 mg/kg | 60 mg/kg | 100 mg/kg KAE | 60 mg/kg MG | 20 mg/kg MP |
| Day 4 am | + | 70 mg/kg | 70 mg/kg | 125 mg/kg KAE | 70 mg/kg MG | 30 mg/kg MP |
| pm | + | 75 mg/kg | 75 mg/kg | 125 mg/kg KAE | 75 mg/kg MG | 35 mg/kg MP |
| Day 5 am | + | 25 mg/kg | 25 mg/kg morphine + 5 mg/kg clonidine | 25 mg/kg KAE | 25 mg/kg MG | 1 mg/kg MP |
| pm | – | – | – | – | – | – |
Table 2.
Design of experiment 2: fixed or tapering dosing to test alkaloid potential to ameliorate precipitated opioid withdrawal
| Treatment day | Treatment | ||||||
|---|---|---|---|---|---|---|---|
| Saline (i.p.) | Morphine (i.p.) | Morphine (d1-4), then Tapering doses of kratom alkaloid extract (KAE) (100–40 mg/kg, p.o.) | Morphine (d1-4), then Fixed mitragynine (MG) (80 mg/kg, p.o.) | Morphine (d1-4) then Tapering mitragynine (MG) (80–25 mg/kg, p.o.) | Morphine (d1-4) then Fixed MP (30 mg/kg, s.c.) | Morphne (d1-4) then Tapering MP (30–1 mg/kg, s.c.) | |
| Day 1 am | + | 10 mg/kg | 10 mg/kg, i.p. | 10 mg/kg, i.p. | 10 mg/kg, i.p. | 10 mg/kg, i.p. | 10 mg/kg, i.p. |
| pm | + | 15 mg/kg | 15 mg/kg, i.p. | 15 mg/kg, i.p. | 15 mg/kg, i.p. | 15 mg/kg, i.p. | 15 mg/kg, i.p. |
| Day 2 am | + | 20 mg/kg | 20 mg/kg, i.p. | 20 mg/kg, i.p. | 20 mg/kg, i.p. | 20 mg/kg, i.p. | 20 mg/kg, i.p. |
| pm | + | 30 mg/kg | 30 mg/kg, i.p. | 30 mg/kg, i.p. | 30 mg/kg, i.p. | 30 mg/kg, i.p. | 30 mg/kg, i.p. |
| Day 3 am | + | 50 mg/kg | 50 mg/kg, i.p. | 50 mg/kg, i.p. | 50 mg/kg, i.p. | 50 mg/kg, i.p. | 50 mg/kg, i.p. |
| pm | + | 60 mg/kg | 60 mg/kg, i.p. | 60 mg/kg, i.p. | 60 mg/kg, i.p. | 60 mg/kg, i.p. | 60 mg/kg, i.p. |
| Day 4 am | + | 70 mg/kg | 70 mg/kg, i.p. | 70 mg/kg, i.p. | 70 mg/kg, i.p. | 70 mg/kg, i.p. | 70 mg/kg, i.p. |
| pm | + | 75 mg/kg | 75 mg/kg, i.p. | 75 mg/kg, i.p. | 75 mg/kg, i.p. | 75 mg/kg, i.p. | 75 mg/kg, i.p. |
| Day 5 am | + | 80 mg/kg | 100 mg/kg KAE | 80 mg/kg MG | 80 mg/kg MG | 30 mg/kg MP | 30 mg/kg MP |
| pm | + | 80 mg/kg | 100 mg/kg KAE | 80 mg/kg MG | 80 mg/kg MG | 30 mg/kg MP | 20 mg/kg MP |
| Day 6 am | + | 80 mg/kg | 80 mg/kg KAE | 80 mg/kg MG | 70 mg/kg MG | 30 mg/kg MP | 15 mg/kg MP |
| pm | + | 80 mg/kg | 70 mg/kg KAE | 80 mg/kg MG | 60 mg/kg MG | 30 mg/kg MP | 10 mg/kg MP |
| Day 7 am | + | 80 mg/kg | 60 mg/kg KAE | 80 mg/kg MG | 50 mg/kg MG | 30 mg/kg MP | 3 mg/kg MP |
| pm | + | 50 mg/kg KAE | 80 mg/kg MG | 40 mg/kg MG | 30 mg/kg MP | 1 mg/kg MP | |
| Day 8 am | + | 25 mg/kg | 40 mg/kg KAE | 25 mg/kg MG | 25 mg/kg MG | 3 mg/kg MP | 1 mg/kg MP |
| pm | – | – | – | – | – | – | – |
Italic text indicates morphine dosing regimen
Experimental design 1 Test of direct physical dependence.
Animals were randomly assigned to one of the following groups: saline (i.p.), morphine (10–75 mg/kg, i.p.), morphine (10–75 mg/kg, i.p.) + acute clonidine (5 mg/kg i.p., on day 5), kratom alkaloid extract (30–125 mg/kg, p.o.), mitragynine (10–75 mg/kg, p.o.), or mitragynine pseudoindoxyl (1–35 mg/kg, s.c.). Dosing for experimental design 1 was performed on a 5-day schedule, with an escalating dose of the compound given twice daily for 4 days, with a final dose administered on the 5th day (see Table 1). Two hours post-treatment on the last day, all mice were administered naloxone (10 mg/kg, s.c.) to precipitate opioid withdrawal symptoms (Kamei and Ohsawa 1997; Özdoğan et al. 2003; Wilson et al. 2020; see also below). For the morphine + clonidine group, clonidine was administered 90 min after the last morphine treatment and 30 min preceding naloxone treatment on day 5 (Özdoğan et al. 2003).
Experimental Design 2 Test of therapeutic potential to ameliorate precipitated withdrawal.
Mice were randomly placed into eight groups and treated repeatedly with saline (i.p.) or morphine (10–75 mg/kg, i.p.) for 4 days (see Table 2). For the next 4 days, groups were administered twice-daily fixed doses of saline (i.p.), morphine (80 mg/kg, i.p.), tapering doses of kratom alkaloid extract (100–30 mg/kg, p.o.), fixed doses of mitragynine (80 mg/kg, p.o.), tapering doses of mitragynine (80–25 mg/kg, p.o.), fixed doses of MP (30 mg/kg, s.c.), or tapering doses of MP (30–1 mg/kg, s.c.). Two hours post-injection on the last day of testing, all mice were injected with naloxone (10 mg/kg, s.c.) to precipitate opioid withdrawal symptoms.
Measurement of Opioid Withdrawal Behavior Signs
During evaluation of opioid withdrawal, animals were observed individually while moving freely in a Plexiglas cylinder for 15 min after naloxone administration using established methods (Shaw-Lutchman et al. 2002, Wilson et al. 2020). In vivo activity was digitally recorded (Noldus EthoVision software), then later evaluated for withdrawal behaviors. Evaluators were blind to the treatment of mice they were scoring. Each animal was assessed for the number of times they demonstrated the following behaviors: forepaw tremors, wet dog shakes, jumping, rearing, and teeth chattering frequencies. Jumping was defined as an attempt to bound and/or leap off the surface. Forepaw tremor was defined as a rapid up/down motion with the front paws that did not include touching of the head or ears. Rearing was defined as repetitive standing on hind paws with front paws not touching any parts of the observation container. Forepaw licking was defined as the mouse licking both of its forepaws at least once without touching head or ears in a grooming way. Wet dog shakes were defined as a spontaneous and quick shaking of the entire head and upper body of the mouse. Straightening was defined as the number times each mouse stretched or elongated their body with all four paws on the touching the bottom of the enclosure. Teeth chattering was defined as repetitive moving up and down of the lower lip portion of the mouth in a “gum chewing” manner. Diarrhea frequency was defined as the number of soft and/or wet excreta pellets.
Statistical Analysis
All data are plotted as mean ± S.E.M. and were analyzed using Prism 8.0 software (GraphPad Software, La Jolla, California, USA). Normality and equal variance were confirmed statistically and justified using parametric analysis. Significant differences in behavioral data were analyzed by ANOVA (one- or two-way with or without repeated measures as appropriate) with significant results further analyzed with Sidak or Tukey post hoc tests for significant pairwise comparisons within and between groups. All significance was set at p ≤ 0.05.
Results
Assessment of Treatment-Induced Hyperalgesia
Mice (n = 10/treatment) were treated twice daily for 4 days with escalating doses of saline (i.p.), morphine (i.p.), the combined kratom alkaloid extract (p.o.), mitragynine (p.o.), or mitragynine psuedoindoxyl (s.c.) as described in the methods. Tail-withdrawal latencies were collected both prior to the start of treatment (on day 1), and again on the morning of day 5. Each group of mice showed equivalent initial responses on day 1 [F(4,45) = 1.12; p = 0.995; one-way ANOVA; Fig. 1]. Treatment significantly changed this response on day 5 [F(1,45) = 56.3; p < 0.0001; two-way RM ANOVA w/Sidak’s post hoc test], with mice treated with morphine (p < 0.0001), combined kratom alkaloid extract (p = 0.002) and mitragynine (p = 0.002) displaying reduced latencies indicative of induced hyperalgesia (Fig. 1). Neither saline (p = 0.68) nor mitragynine pseudoindoxyl (p = 0.10) displayed significant differences from their initial responses, or each other (p = 0.27, Student’s t-test), suggesting the mitragynine pseudoindoxyl treatment regimen did not induce hyperalgesia.
Fig. 1.
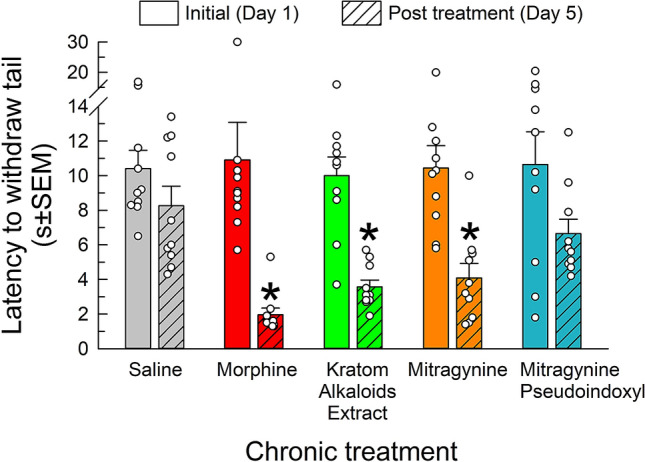
Assessment of treatment-induced hyperalgesia using the 48 °C warm-water tail-withdrawal test. Mice were first tested on day 1 to establish a baseline reading prior to any treatment. Mice were then subjected to a dosing regimen with saline (i.p.), morphine (10–75 mg/kg, i.p.), kratom alkaloid extract (30–125 mg/kg, p.o.), mitragynine (10–75 mg/kg, p.o.), or mitragynine pseudoindoxyl (1–35 mg/kg, s.c.) for 4 days. On day 5, prior to final dosing and withdrawal testing, mice were evaluated a final time in the warm-water tail-withdrawal assay. Data are shown as mean ± SEM of pre- and post-dosing testing. *p < 0.05 versus baseline, Two-way RM ANOVA with Sidak’s multiple comparisons post hoc test. n = 10 mice/treatment
Assessment of Direct Treatment-Induced Physical Dependence
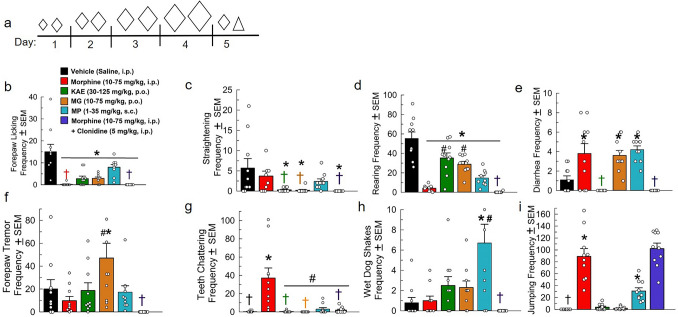
Following chronic twice-daily administration with either saline (i.p.), escalating doses of morphine (10–75 mg/kg, i.p.), or escalating doses of the test compounds, mice on day 5 were administered naloxone (10 mg/kg, s.c.) and precipitated opioid withdrawal signs were assessed. Alone, morphine adminstration significantly reduced forepaw licking [F(5, 54) = 14.03, p < 0.0001; one-way ANOVA with Tukey’s test; Fig. 2b] and rearing [F(5, 54) = 30.29, p < 0.0001; one-way ANOVA with Tukey’s test; Fig. 2c]. Naloxone administered to mice chronically treated with morphine also precipitated increased occurrences of diarrhea [F(5, 54) = 10.74, p < 0.01; one-way ANOVA with Tukey’s test; Fig. 2e], teeth chattering [F(5, 54) = 14.28, p < 0.01; one-way ANOVA with Tukey’s test; Fig. 2g], and jumping [F(5, 54) = 43.5, p < 0.0001; one-way ANOVA with Tukey’s test; Fig. 2i] after the administration of naloxone. No significant effects were observed with assessments of mouse straightening [F(5, 54) = 3.69, p = 0.77; one-way ANOVA with Tukey’s test; Fig. 2c], forepaw tremor [F(5, 54) = 5.6, p = 0.99; one-way ANOVA with Tukey’s test; Fig. 2f], or wet dog shakes [F(5, 54) = 6.7, p > 0.99; one-way ANOVA with Tukey’s test; Fig. 2h].
Fig. 2.
Assessment of direct kratom alkaloid extract, mitragynine, and mitragynine pseudoindoxyl treatment-induced physical dependence. a Dosing schematic for treatment groups. Increasing diamond (◊) size indicates increased dosage of morphine, KAE, MG or MP. The triangle (△) indicates naloxone treatment. Days 1–4: Treated with (a.m. and p.m.): Group 1: (n = 10) Saline, i.p. × 2/day. Group 2: (n = 10), Morphine × 2/day. Day 1: 10 + 15 mg/kg, i.p. Day 2: 20 + 30 mg/kg, i.p. Day 3: 50 + 60 mg/kg, i.p. Day 4: 70 + 75 mg/kg, i.p. Group 3: (n = 10) kratom alkaloid extract (KAE) × 2/day: day 1: 30 + 35 mg/kg, p.o. Day 2: 45 + 60 mg/kg, p.o. Day 3: 100 + 100 mg/kg, p.o. Day 4: 125 + 125 mg/kg, p.o. Group 4: (n = 10) Mitragynine (MG) × 2/day Day 1: 10 + 15 mg/kg, p.o. Day 2: 20 + 30 mg/kg, p.o. Day 3: 50 + 60 mg/kg, p.o. Day 4: 70 + 75 mg/kg, p.o. Group 5: (n = 10) Mitragynine Pseudoindoxyl (MP) × 2/day: day 1: 1 + 3 mg/kg, s.c. Day 2: 3 + 10 mg/kg, s.c. Day 3: 15 + 20 mg/kg, s.c. Day 4: 30 + 35 mg/kg, s.c. Group 6: (n = 10), Morphine × 2/day. Day 1: 10 + 15 mg/kg, i.p. Day 2: 20 + 30 mg/kg, i.p. Day 3: 50 + 60 mg/kg, i.p. Day 4: 70 + 75 mg/kg, i.p. Day 5: Group 1: (n = 10) Saline, i.p.; Group 2: Morphine (25 mg/kg, i.p.); Group 3: KAE (25 mg/kg, p.o.); Group 4: MG (25 mg/kg, p.o.); Group 5: MP (1 mg/kg, s.c.); Group 6: Morphine (25 mg/kg, i.p.). 90 min post-morphine treatment on day 5, group 6 was additionally administered an acute dose of clonidine (5 mg/kg, i.p.). All groups were administered naloxone (10 mg/kg, s.c.) 120 min post-respective day 5 am treatment then observed for withdrawal behaviors (i.e., forepaw licking (b), mouse straightening (c), rearing (d), frequency of diarrhea (e), forepaw tremor (f), teeth chattering (g), wet dog shakes (h), and jumping (i)) for 15 min. *p < 0.05 versus vehicle control, # p < 0.05 versus morphine, one-way RM ANOVA with Tukey’s multiple comparisons post hoc test. †Mean and SEM lower than 1
The α2-adrenoreceptor agonist clonidine has been shown to ameliorate some sequelae of opioid withdrawal (Jasinski 1985). As a positive control, a set of mice treated chronically 5 days with morphine (10–75 mg/kg, i.p.) received an acute dose of clonidine (5 mg/kg, i.p.) 30 min prior to naloxone administration. Clonidine treatment was unable to attenuate naloxone-precipitated reductions in forepaw licking (p < 0.0001; Fig. 2b), body straightening (p = 0.008; Fig. 2c), and rearing (p = 0.003; Fig. 2d) when compared to the saline control groups. However, compared to the group treated with only morphine, clonidine significantly reduced instances of naloxone-precipitated diarrhea (p < 0.0001, Fig. 2d) and teeth chattering (p < 0.0001, Fig. 2g). Acute administration of clonidine did not reduce naloxone-precipitated jumping, with significant differences compared to the saline control group (p < 0.0001; Fig. 2i), although it should be noted that the mice treated with chronic morphine and acute clonidine displayed immobility and persistent full body trembling behaviors between jumping that was not observed in any other groups. There were no significant effects seen in forepaw tremor (Fig. 2f) and wet dog shakes (Fig. 2h) compared to either the saline- or morphine-only-treated groups.
Chronic escalating treatment with the kratom alkaloid extract (30–125 mg/kg, p.o.) or mitragynine (10–75 mg/kg, p.o.) produced minor withdrawal effects in comparison (Fig. 2). Combined kratom alkaloid extract (p < 0.0001) and mitragynine (p < 0.0001) each produced a significant decrease in forepaw licking (Fig. 2b), mouse straightening (p = 0.01 each, Fig. 2c), and rearing (p = 0.005 and p = 0.0001, respectively; Fig. 2d). The decrease in rearing, however, was also significantly higher in the combined kratom alkaloid extract (p < 0.0001) and mitragynine (p = 0.0003)-treated groups than the effects of the morphine-positive control. Unlike the combined kratom alkaloid extract, the administration of mitragynine alone demonstrated an increase in the occurance of diarrhea (p = 0.02, Fig. 2e) and forepaw tremor (p = 0.008, Fig. 2f). Otherwise, chronic treatment with the combined kratom alkaloid extract and mitragynine resulted in no other significant signs of opioid withdrawal (teeth chattering (Fig. 2g), frequency of wet dog shakes (Fig. 2h), or jumping (Fig. 2i).
Mitragynine pseudoindoxyl (MP), the semi-synthetic kratom alkaloid, demonstrated significant signs of opioid physical dependence after chronic twice-daily administration (1–35 mg/kg, s.c.; Fig. 2). Although forepaw tremors, teeth chattering, and straightening responses remained unchanged (Fig. 2f, g, and c, respectively), mitragynine pseudoindoxyl-treated mice displayed significant decreases in forepaw licking (p < 0.0001; Fig. 2b) and increases in “wet dog shakes” (p = 0.001; Fig. 2h). Moreover, mitragynine pseudoindoxyl treatment showed significant increases in the presence of diarrhea (p = 0.003; Fig. 2e), and jumping (p < 0.01; Fig. 2i), as well as a decrease in rearing frequency (p < 0.0001; Fig. 2d). Collectively, these signs suggest mitragynine pseudoindoxyl displayed a greater magnitude of opioid physical dependence as compared to mitragynine, but far less than morphine under this treatment regimen.
Evaluation of Alkaloid’s Ability to Ameliorate Withdrawal in Subjects Physically Dependent on Morphine
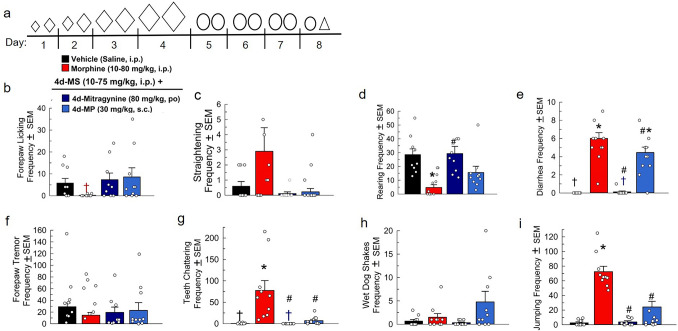
After 4 days of treatment twice daily with escalating doses of morphine (10–75 mg/kg, i.p.), mice were then treated twice daily for three more days with a fixed high dose of either morphine (80 mg/kg, i.p.), mitragynine (80 mg/kg, p.o.), or MP (30 mg/kg, s.c.) prior to a single final treatment on day 8 and the subsequent administration of naloxone (Fig. 3a). Mice treated with the fixed dose of mitragynine still demonstrated a significant naloxone-precipitated increase in the frequency of rearing F(3, 34) = 6.9, p = 0.003, one-way ANOVA with Tukey’s test; Fig. 3d). Fixed doses of both mitragynine and MP demonstrated a significant decrease in the frequency of naloxone-precipitated diarrhea [F(3, 33) = 60.2, p < 0.0001 and p = 0.04, respectively, one-way ANOVA with Tukey’s test; Fig. 3e] compared to the morphine control, although MP-treated mice still experienced increased incidence of diarrhea (p < 0.0001) compared to the saline control. Compared to morphine, both mitragynine and MP significantly decreased naloxone-precipitated teeth chattering [F(3, 32) = 10.8, p < 0.0002 and p = 0.0005, respectively, one-way ANOVA with Tukey’s test; Fig. 3g] and jumping [F(3, 34) = 64.9, p < 0.0001 each, one-way ANOVA with Tukey’s test; Fig. 3i], while showing no significant changes in forepaw licking (Fig. 3b), straightening (Fig. 3c), forepaw tremor (Fig. 3f), or wet dog shakes (Fig. 3h).
Fig. 3.
Evaluation of fixed doses of mitragynine’s and mitragynine pseduoindoxyl’s ability to ameliorate naloxone-precipitated opioid withdrawal symptoms in morphine-dependent mice. a Dosing schematic for treatment groups. Increasing diamond (◊) size indicates increased dosage of morphine. The circles (○) represent fixed doses of either mitragynine or MP. The triangle (△) indicates naloxone treatment. Days 1–4: Treated with (a.m. and p.m.): Group 1: (n = 10) Saline, i.p. × 2/day. Groups 2–4: (n = 9–10/group), Morphine × 2/day. Day 1: 10 + 15 mg/kg, i.p. Day 2: 20 + 30 mg/kg, i.p. Day 3: 50 + 60 mg/kg, i.p. Day 4: 70 + 75 mg/kg, i.p. Days 5–7: Treated with (a.m. and p.m.) Group 1: Saline, i.p. × 2/day; Group 2: Morphine × 2/day: 80 mg/kg, i.p. Group 3: Mitragynine (MG) × 2/day: 80 mg/kg, p.o. Group 4: Mitragynine Pseudoindoxyl (MP) × 2/day: 30 mg/kg,s.c. Day 8: Group 1: (n = 10) Saline, i.p.; Group 2: Morphine (25 mg/kg, i.p.); Group 3: MG (25 mg/kg, p.o.); Group 4: MP (3 mg/kg, s.c.). All groups were administered naloxone (10 mg/kg, s.c.) 120 min post-respective day 5 am treatment then observed for withdrawal behaviors [i.e., forepaw licking (b), mouse straightening (c), rearing (d), frequency of diarrhea (e), forepaw tremor (f), teeth chattering (g), wet dog shakes (h), and jumping (i)] 120 min post-respective day 5 am treatment then observed for withdrawal behaviors [i.e., forepaw licking (b), mouse straightening (c), rearing (d), frequency of diarrhea (e), forepaw tremor (f), teeth chattering (g), wet-dog shakes (h), and jumping (i)] for 15 min. *p < 0.05 versus vehicle control, #p < 0.05 versus morphine, one-way RM ANOVA with Tukey’s multiple comparisons post hoc test. †Mean and SEM lower than 1
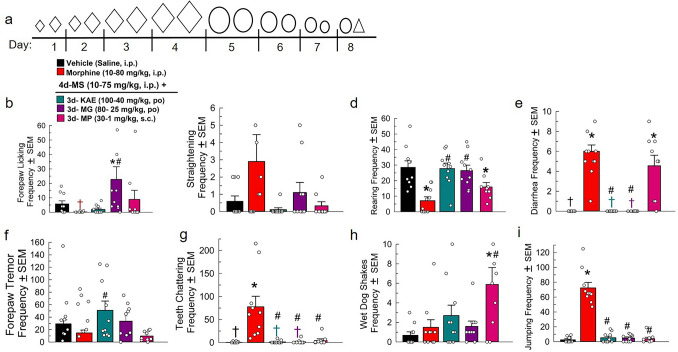
When tested for its abilities to mitigate naloxone-precipitated withdrawal in morphine-dependent mice, 4 days’ treatment with tapering doses of the combined kratom alkaloid extract (100 mg/kg down to 40 mg/kg, p.o.; Fig. 4a) successfully modulated most precipitated withdrawal effects when compared to mice receiving only additional morphine (80 mg/kg, i.p). Mice treated with tapering doses of kratom alkaloid extract or mitragynine displayed significant decreases in the frequency of naloxone-precipitated diarrhea [F(4, 42) = 33.4, p < 0.0001 each, one-way ANOVA with Tukey’s test; Fig. 4e], while MP-treated mice demonstrated an increased frequency of diarrhea (p < 0.0001) as compared to the saline control. Kratom alkaloid extract, mitragynine, and MP-treated mice each showed significant reductions in naloxone-precipitated teeth chattering [F(4, 42) = 11.0, p < 0.0001 each; Fig. 4g] and jumping [F(4, 42) = 115.6, p < 0.0001; Fig. 4i] compared to the morphine control group. Curiously, mice treated with the combined kratom alkaloid extract displayed significantly increased forepaw tremor [F(4, 42) = 2.7, p = 0.04; Fig. 4f] and both kratom alkaloid extract- and mitragynine-treated mice demonstrated increased rearing frequency [F(4, 42) = 11, p < 0.0001; Fig. 4d] compared to the morphine only group. MP-treated mice, however, demonstrated decreased rearing (p = 0.03) following naloxone administration when compared to the saline-treated group. Mice treated with kratom alkaloid extract or MP demonstrated no significant changes in forepaw licking following naloxone administration (Fig. 4b); however, there was a significant increase observed in the group administering tapering doses of mitragynine as compared to responses of both the saline and morphine-treated groups [F(4, 42) = 4.04, p = 0.01, one-way ANOVA with Tukey’s test]. Only mice administered tapering doses of MP presented a significant increase in the frequency of naloxone-precipitated wet dog shakes compared to both the saline and morphine controls [F(4, 42) = 4.7, p = 0.004 and p = 0.02, respectively, one-way ANOVA with Tukey’s test; Fig. 4h]. No tapering treatment regimen produced significant changes in quantitated straightening behavior after naloxone administration [F(4, 42) = 1.6, p = 0.19, one-way ANOVA with Tukey’s test; Fig. 4c].
Fig. 4.
Evaluation of tapering doses of kratom alkaloid extract, mitragyine, and mitragynine pseudoindoxyl’s ability to reduce naloxone-precipitated opioid withdrawal symptoms in morphine-dependent mice. a Dosing schematic for treatment groups. Increasing diamond (◊) size indicates increased dosage of morphine. The decreasing size of the circles (○) represent tapering doses of either kratom alkaloid extract, mitragynine, or MP. The triangle (△) indicates naloxone treatment. Days 1–4: Treated with (a.m. and p.m.): Group 1: (n = 10) Saline, i.p. × 2/day. Groups 2–5: (n = 9–10/group), Morphine × 2/day. Day 1: 10 + 15 mg/kg, i.p. Day 2: 20 + 30 mg/kg, i.p. Day 3: 50 + 60 mg/kg, i.p. Day 4: 70 + 75 mg/kg, i.p. Days 5–7: Treated with (a.m. and p.m.) Group 1: Saline, i.p. × 2/day; Group 2: Morphine × 2/day: 80 mg/kg, i.p. Group 3: Kratom alkaloid extract (KAE) × 2/day: Day 5: 100 mg/kg, p.o. Day 6: 80 + 70 mg/kg, p.o. Day 7: 60 + 50 mg/kg, p.o. Group 4: Mitragynine (MG) × 2/day: Day 5: 80 mg/kg, p.o. Day 6: 70 + 60 mg/kg, p.o. Day 7: 50 + 40 mg/kg, p.o. Group 5: Mitragynine Pseudoindoxyl (MP) × 2/day: Day 5: 30 + 20 mg/kg, s.c. Day 6: 15 + 10 mg/kg, s.c. Day 7: 3 + 1 mg/kg, s.c. Day 8: Group 1: (n = 10) Saline, i.p.; Group 2: Morphine (25 mg/kg, i.p.); Group 3: KAE (40 mg/kg, p.o.); Group 4: MG (25 mg/kg, p.o.); Group 5: MP (1 mg/kg, s.c.). All groups were administered naloxone (10 mg/kg, i.p.) 120 min post-respective day 5 am treatment then observed for withdrawal behaviors [i.e., forepaw licking (b), mouse straightening (c), rearing (d), frequency of diarrhea (e), forepaw tremor (f), teeth chattering (g), wet dog shakes (h), and jumping frequency (i)] 120 min post-respective day 5 am treatment then observed for withdrawal behaviors [i.e., forepaw licking (b), mouse straightening (c), rearing (d), frequency of diarrhea (e), forepaw tremor (f), teeth chattering (g), wet dog shakes (h), and jumping (i)] *p < 0.05 versus vehicle control, #p < 0.05 versus morphine, one-way RM ANOVA with Tukey’s multiple comparisons post hoc test. n = 9–10 mice/treatment. †Mean and SEM lower than 1
Discussion
This study found that kratom alkaloid extract and two component alkaloids, mitragynine and mitragynine pseudoindoxyl, ameliorated varying sequelae of naloxone-precipitated opioid withdrawal syndrome in mice physically dependent on morphine. Moreover, similar to morphine, chronic administration of mitragynine and KAE resulted in significant hyperalgesic effects not produced by mitragynine pseudoindoxyl under the chronic treatment regimen utilized.
Opioid-induced hyperalgesia (OIH) is an increase of sensitivity to pain that develops with repeated exposure to opioids such as morphine (Hutchinson et al. 2011). A significant clinical complication of opioid use to treat chronic pain, OIH been demonstrated in patients experiencing both spontaneous and precipitated withdrawal after acute- or chronic opioid administration (Lipman and Blumenkopf 1989; Devulder et al. 1996; Sun 1998). Studies have also shown the development of hyperalgesia in persons with substance abuse disorder after sudden decreases in the dosage of opioids (Miser et al. 1986; Lipman and Blumenkopf 1989). Previous studies have also demonstrated opioid withdrawal-induced hyperalgesia in rodents (Célèrier et al. 2000; Li et al. 2001; Laulin et al. 2002; Balter and Dykstra 2013). The current study shows that the combined kratom alkaloid extract and its main component, mitragynine, induced hyperalgesia equivalent to that of morphine. In contrast, the semi-synthetic alkaloid mitragynine pseudoindoxyl did not demonstrate significant hyperalgesia under the current treatment conditions. Interestingly, mitragynine pseudoindoxyl was previously shown to retain antinociceptive activity with minimal tolerance under a range of chronic administration conditions (Váradi et al. 2016). The development of opioid-induced hyperalgesia is not well understood, but thought to stem from induced neuroplasticity that enhances the release of excitatory neurotransmitters such as substance P and glutamate from primary afferent fibers in the spinal cord (Jhamandas et al. 1996), potentially as part of the physiological adjustment in homeostasis in response to chronic opioid exposure. Consistent with this, prolonged exposure to MOR agonists is shown to lead to sensitization of the nociceptive system (King et al. 2005). Sufka et al. (1991) also demonstrated that opioid-induced hyperalgesia occurs primarily via MOR, although the same study indicated that kappa opioid receptor (KOR) antagonism may attenuate these effects at high doses. These results are similiar to present observations, in as much as mitragynine pseudoindoxyl was characterized earlier to possess both MOR agonism and DOR antagonism (Váradi et al. 2016), and hyperalgesic effects were not evident in mice treated with MP under the present conditions. However, while the influence of this multifunctional pharmacology on the development of opioid-induced hyperalgesia remains a topic for further study, additional testing of MP at varying higher doses for prolonged periods is warranted in future investigations.
Opioid withdrawal symptoms were precipitated with naloxone in the morphine-treated groups as compared to the saline-treated group. Similar effects were seen with escalating doses of mitragynine pseudoindoxyl, but minimal effects were seen with mice administered the kratom alkaloids extract or mitragynine. While mitragynine pseudoindoxyl (Váradi et al. 2016), the combined kratom alkaloid extract, and mitragynine (Kruegel et al. 2019) each has been shown to produce MOR agonism, reports suggest alternative mechanisms of action between them that may contribute to the differences in opioid withdrawal observed presently. Given that mitragynine pseudoindoxyl is a potent MOR agonist (Váradi et al. 2016), it was not surprising that it demonstrated withdrawal symptoms consistent with (if less than) those produced by the morphine-treated mice when administered naloxone. In contrast, significantly reduced withdrawal effects were seen with the mice chronically treated with combined kratom alkaloid extract or mitragynine alone, despite evidence that these samples produce much of their pharmacological effects via MOR agonism (Gold et al. 1978; Matsumoto et al. 1996b; Boyer et al. 2008; Gowing et al. 2014; Hiranita et al. 2019; Kruegel et al. 2019). The minimal withdrawal symptoms demonstrated by mitragynine-treated mice confirm the finding of Meepong and Sooksawate (2019), which reported the absence of withdrawal behaviors after treatment with either acute or chronic low doses. It has been speculated that mitragynine’s reduced opioid withdrawal syndrome may be due to intrinsic properties of this alkaloid, as intracellular signaling of mitragynine has been shown to be G-protein biased over β-arrestin (Kruegel et al. 2016; Váradi et al. 2016; Gutridge et al. 2020). Substitution with a G-protein-biased MOR agonist was reported to prevent withdrawal in mice made physically dependent on morphine (Grim et al. 2020), but it is possible that higher doses of the compounds than tested here may result in more activation of the β-arrestin pathway and the emergence of physical dependence, although this remains to be examined.
In morphine-dependent animals, treatment with either the combined kratom alkaloid extract or mitragynine alone successfully ameliorated naloxone-precipitated withdrawal symptoms, whereas treatment with mitragynine pseudoindoxyl was less efficacious. These results are consistent with recent demonstrations that treatment with lyophilized kratom tea ameliorated naloxone-precipitated withdrawal in morphine-dependent mice (Wilson et al. 2020), whereas treatment with mitragynine attenuated acute withdrawal signs in morphine-dependent rats undergoing spontaneous morphine abstinence over a period of 28 days (Hassan et al. 2020). These findings are logical, as similar to buprenorphine, mitragynine is reported to possess partial agonism at MOR (Kruegel et al. 2016; Váradi et al. 2016), thought to minimize the physiological changes arising during cessation of morphine treatment. However, both the kratom natural product and mitragynine alkaloid have been shown to act as weak agonists of the α2-adrenergic system (Boyer et al. 2008; Hiranita et al. 2019; Obeng et al. 2020), whereas mitragynine pseudoindoxyl does not (Váradi et al. 2016). Studies have indicated that α2-adrenoreceptor agonists such as clonidine reduce some (primarily noradrenergic-mediated) withdrawal effects in opioid-dependent subjects (Jasinski 1985; Katz 1986; Gowing et al. 2014), a result confirmed presently and that supports an additional possible mechanism by which the kratom alkaloid extract or mitragynine may ameliorate naloxone-precipitated withdrawal in morphine-dependent subjects. In contrast, mitragynine pseudoindoxyl-mediated antinociception was not attenuated by the α2-adrenoreceptor antagonist yohimbine (Váradi et al. 2016), potentially accounting for its significant physical dependence alone and reduced ability presently to mitigate morphine-induced withdrawal effects. Detailed evaluation in human subjects of the kratom alkaloid extract or mitragynine itself against opioid withdrawal symptoms attributed to non-noradrenergic-mediation, such as disruption of sleep or subjective discomfort, would be of potential value in further assessing their utility in the treatment of opioid dependence and withdrawal.
Inpatient treatment with methadone or buprenorphine to ameliorate opioid withdrawal is typically started at higher doses that are tapered to lower doses over 5–7 days (Kleber 2007), but emergent withdrawal symptoms often complicate this transition, sometimes resulting in the prolonged use of a maintenance dose (Srivastava et al. 2020). Accordingly, we compared the therapeutic efficacy of a tapering dose regimen in the current study. Of interest, tapering doses of the combined kratom alkaloid extract or mitragynine over 4 days were as effective as fixed high doses of each treatment in ameliorating naloxone-precipitated withdrawal in morphine physically dependent subjects. The application of pharmaceuticals to reduce and wean opioid-dependent subjects is an ongoing goal of managing clinical pain and physical dependence (Srivastava et al. 2020). The full MOR agonist methadone has been widely used for this purpose for decades (Kreek et al. 2010), but retains a significant risk of misuse and overdose (Kosten and Baxter 2019), while also potentially requiring a much longer taper than other treatment options (Srivastava et al. 2020). While some animal studies (Pinelli et al. 1997) and human case studies suggest the feasibility of this approach with tapering doses of the serotonin receptor antagonist ondansetron (Wakim 2012), larger studies reported ondansetron was unsuccessful in either preventing or reducing withdrawal in patients physically dependent on opioid analgesics (Chu et al. 2017, 2018). Of interest, the α2-adrenergic receptor agonist lofexidine was recently FDA-approved for the treatment of opioid withdrawal (Doughty et al. 2019), but although showing efficacy as compared to placebo, failed to completely suppress withdrawal symptoms (Fishman et al. 2019), much as did clonidine in earlier studies (Jasinski 1985). More widely used is the multifunctional, partial MOR agonist buprenorphine, sometimes in tapering doses (Srivastava et al. 2020). While successful in lessening the severity of symptoms from naltrexone-precipitated opioid withdrawal (Umbricht et al. 1999; Srivastava et al. 2020; Wilson et al. 2020) and potentially safer than methadone treatment, it is notable that buprenorphine still carries some risk of respiratory depression (Dehan et al. 2005) and may itself produce physical dependence (Dum et al. 1981) and reinforcement (Canestrelli et al. 2014). The present demonstration that the kratom alkaloid extract and the alkaloid mitragynine effectively ameliorated morphine withdrawal contributes a controlled animal study to emerging evidence of therapeutic efficacy (Boyer et al. 2008; Toce et al. 2018; Hassan et al. 2020). Currently, the demonstrated results with mitragynine further expand the findings of Hassan et al (2020), showing that even higher doses of mitragynine produce few opioid-related liabilities on their own, while still modulating opioid-induced withdrawal in dependent mice. The current studies also demonstrate mitragynine’s ability to modulate opioid withdrawal with decreasing doses, a demonstration lacking to date within the literature. However, broader therapeutic acceptance of these indole alkaloids will require further evaluation for safety and efficacy in randomized controlled trials with defined, pharmaceutical grade products (as used here).
In conclusion, administration of combined kratom alkaloid extract or mitragynine each demonstrated minimal symptoms of withdrawal alone, suggesting these agents produce less physical dependence than the full MOR agonist morphine. Substitution of the combined kratom alkaloid extract and mitragynine in morphine-dependent mice were able to ameliorate naloxone-precipitated withdrawal symptoms, as was mitragynine pseudoindoxyl to a lesser degree. Similar to morphine, mitragynine and the combined kratom alkaloid extract demonstrated significant hyperalgesic effects that were not produced by chronic treatment with mitragynine pseudoindoxyl. Taken together, these data suggest that while the combined kratom alkaloid extract and mitragynine have confirmed promise for the treatment of opioid physical dependence and withdrawal, they are not without liabilities. Further evaluation of these extracts is warranted to evaluate their usefulness in the clinic.
Author Contributions
SM, JPM and LLW participated in research design and wrote and contributed to the writing of manuscript. TJC, SOE, CAS, HMS and LLW conducted experiments. SM, SC and RU contributed new reagents or analytic tools. JPM and LLW performed data analysis. All authors critically reviewed the content and approved the final version for publication.
Funding
This research was supported by Grants from the National Institute on Drug Abuse RO1DA046487 and R21/R33 DA045884 (to SM), Cancer Center Support Grant P30 CA008748 from the National Cancer Institute (to MSKCC), and funds from the University of Florida (to JPM).
Data Availability
All data needed to evaluate the conclusions in the paper are present in the paper. Data related to this manuscript may be requested from the authors.
Compliance with Ethical Standards
Conflict of interest
The authors have no competing interests.
Ethical Approval
All animal studies were preapproved by the University of Florida (Gainesville, FL, USA) Institutional Animal Care and Use Committee, in accordance with the 2011 National Institute of Health Guide for the Care and Use of Laboratory Animals. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al. 2010). Initial sample sizes were approximated by power analysis, with animals assigned to groups randomly. Drug treatment experiments were conducted in a blinded fashion.
Informed Consent
This manuscript has been approved for publication by all authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adkins JE, Boyer EW, Mccurdy CR (2011) Mitragyna speciosa, a psychoactive tree from Southeast Asia with opioid activity. Curr Top Med Chem 11(9):1165–1175. 10.2174/156802611795371305 [DOI] [PubMed] [Google Scholar]
- Balter RE, Dykstra LA (2013) Thermal sensitivity as a measure of spontaneous morphine withdrawal in mice. J Pharmacol Toxicol Methods 67(3):162–168. 10.1016/j.vascn.2013.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer EW, Babu KM, Adkins JE, Mccurdy CR, Halpern JH (2008) Self-treatment of opioid withdrawal using kratom (Mitragynia speciosa korth). Addiction 103(6):1048–1050. 10.1111/j.1360-0443.2008.02209.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canestrelli C, Marie N, Noble F (2014) Rewarding or aversive effects of buprenorphine/ naloxone combination (Suboxone) depend on conditioning trial duration. Int J Neuropsychopharmacol 17(9):1367–1373. 10.1017/S146114571400025X [DOI] [PubMed] [Google Scholar]
- Célèrier E, Rivat C, Jun Y, Laulin J-P, Larcher A, Reynier P, Simonnet G (2000) Long-lasting hyperalgesia induced by fentanyl in rats. Anesthesiology 92(2):465. 10.1097/00000542-200002000-00029 [DOI] [PubMed] [Google Scholar]
- Chakraborty S, Majumdar S (2020) Natural products for the treatment of pain: chemistry and pharmacology of Salvinorin A, Mitragynine, and Collybolide. Biochemistry. 10.1021/acs.biochem.0c00629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu LF, Sun J, Clemenson A, Erlendson MJ, Rico T, Cornell E, Obasi H, Sayyid ZN, Encisco EM, Yu J, Gamble JG, Carroll I, Clark JD (2017) Ondansetron does not reduce withdrawal in patients with physical dependence on chronic opioid therapy. J Addict Med 11(5):342–349. 10.1097/ADM.0000000000000321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu LF, Rico T, Cornell E, Obasi H, Encisco EM, Vertelney H, Gamble JG, Crawford CW, Sun J, Clemenson A, Erlendson MJ, Okada R, Carroll I, Clark JD (2018) Ondansetron does not prevent physical dependence in patients taking opioid medications chronically for pain control. Drug Alcohol Depend 183:176–183. 10.1016/j.drugalcdep.2017.06.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crain SM, Shen K-F (2001) Acute thermal hyperalgesia elicited by low-dose morphine in normal mice is blocked by ultra-low-dose naltrexone, unmasking potent opioid analgesia. Brain Res 888(1):75–82. 10.1016/s0006-8993(00)03010-9 [DOI] [PubMed] [Google Scholar]
- Dehan A, Yassen A, Bijl H, Romberg R, Sarton E, Teppema L, Olofsen E, Danhof M (2005) Comparison of the respiratory effects of intravenous buprenorphine and fentanyl in humans and rats. Brit J Anesth 94(6):825–834. 10.1093/bja/aei145 [DOI] [PubMed] [Google Scholar]
- Devulder J, Bohyn P, Castille F, Laat MD, Rolly G (1996) A case of uncommon withdrawal symptoms after a short period of spinal morphine administration. Pain 64(3):589–591. 10.1016/0304-3959(95)00187-5 [DOI] [PubMed] [Google Scholar]
- Doughty B, Morgenson D, Brooks T (2019) Lofexidine: a newly FDA-approved, nonopioid treatment for opioid withdrawal. Ann Pharmacother 53:747–753. 10.1177/1060028019828954 [DOI] [PubMed] [Google Scholar]
- Dum J, Bläsig J, Herz A (1981) Buprenorphine: demonstration of physical dependence liability. Eur J Pharmacol 70(3):293–300. 10.1016/0014-2999(81)90163-1 [DOI] [PubMed] [Google Scholar]
- Fishman M, Tirado C, Alam D, Gullo K, Clinch T, Gorodetzky CW (2019) Safety and efficacy of lofexidine for medically managed opioid withdrawal: a randomized controlled clinical trial. J Addict Med 13:169–176. 10.1097/ADM.000000000000074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold M, Redmond D, Kleber H (1978) Clonidine blocks acute opiate-withdrawal symptoms. Lancet 312(8090):599–602. 10.1016/s0140-6736(78)92823-4 [DOI] [PubMed] [Google Scholar]
- Gowing L, Farrell MF, Ali R, White JM (2014) Alpha2-adrenergic agonists for the management of opioid withdrawal. Cochrane Database Syst Rev 3:CD002024. 10.1002/14651858.cd002024.pub4 [Google Scholar]
- Grim TW, Schmid CL, Stahl EL, Pantouli F, Ho J-H, Avevedo-Canabal A, Kennedy NM, Cameron MD, Bannister TD, Bohn LM (2020) A G protein signaling-biased agonist at the μ-opioid receptor reverses morphine tolerance with preventing morphine withdrawal. Neuropsychopharmacology 45(2):416–425. 10.1038/s41386-019-0491-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutridge AM, Robins MT, Cassell RJ, Uprety R, Mores KL, Ko MJ, Pasternak GW, Majumdar S, van Rijn RM (2020) G protein-biased kratom-alkaloids and synthetic carfentanil-amide opioid as potential treatments for alcohol use disorder. Br J Pharmacol 177(7):1497–1513. 10.1111/bph.14913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harun N, Hassan Z, Navaratnam V, Mansor SM, Shoaib M (2015) Discriminative stimulus properties of mitragynine (kratom) in rats. Psychopharmacology 232(13):2227–2238. 10.1007/s00213-015-3866-5 [DOI] [PubMed] [Google Scholar]
- Hassan Z, Muzaimi M, Navaratnam V, Yusoff NH, Suhaimi FW, Vadivelu R, Vicknasingam BK, Amato D, von Hörsten S, Ismail NI, Jayabalan N (2013) From Kratom to mitragynine and its derivatives: Physiological and behavioural effects related to use, abuse, and addiction. Neurosci Biobehav Rev 37(2):138–151. 10.1016/j.neubiorev.2012.11.012 [DOI] [PubMed] [Google Scholar]
- Hassan R, See CP, Sreenivasan S, Mansor SM, Müller CP, Hassan Z (2020) Mitragynine attenuates morphine withdrawal effects in rats—a comparison with methadone and buprenorphine. Front Psychiatry. 10.3389/fpsyt.2020.00411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiranita T, Leon F, Felix JS, Restrepo LF, Reeves ME, Pennington AE, Obeng S, Avery BA, McCurdy CR, McMahon LR, Wilkerson JL (2019) The effects of mitragynine and morphine on schedule-controlled responding and antinociception in rats. Psychopharmacology 236(9):2725–2734. 10.1007/s00213-019-05247-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheson DM, Everitt BJ, Robbins TW, Dickinson A (2001) The role of withdrawal in heroin addiction: enhances reward or promotes avoidance? Nat Neurosci 4(9):943–947. 10.1038/nn0901-943 [DOI] [PubMed] [Google Scholar]
- Hutchinson MR, Shavit Y, Grace PM, Rice KC, Maier SF, Watkins LR (2011) Exploring the neuroimmunopharmacology of opioids: an integrative review of mechanisms of central immune signaling and their implications for opioid analgesia. Pharmacol Rev 63(3):772–810. 10.1124/pr.110.004135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinski DR (1985) Clonidine in morphine withdrawal. Arch Gen Psychiatry 42(11):1063. 10.1001/archpsyc.1985.01790340041006 [DOI] [PubMed] [Google Scholar]
- Jhamandas K, Marsala M, Ibuki T, Yaksh T (1996) Spinal amino acid release and precipitated withdrawal in rats chronically infused with spinal morphine. J Neurosci 16(8):2758–2766. 10.1523/jneurosci.16-08-02758.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Journigan VB, Mésangeau C, Vyas N, Eans SO, Cutler SJ, McLaughlin JP, Mollereau C, McCurdy CR (2014) Nonpeptide small molecule agonist and antagonist original leads for neuropeptide FF1 and FF2 receptors. J Med Chem 57(21):8903–8927. 10.1021/jm500989n [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamble SH, León F, King TI, Berthold EC, Lopera-Londoño C, Siva Rama Raju K, Hampson AJ, Sharma A, Avery BA, McMahon LR, McCurdy CR (2020) Metabolism of a kratom alkaloid metabolite in human plasma increases its opioid potency and efficacy. ACS Pharmacol Transl Sci. 10.1021/acsptsci.0c00075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei J, Ohsawa M (1997) Role of noradrenergic functions in the modification of naloxone-precipitated withdrawal jumping in morphine-dependent mice by diabetes. Life Sci 60(15):PL223-228. 10.1016/s0024-3205(97)00074-x [DOI] [PubMed] [Google Scholar]
- Katz J (1986) Effects of clonidine and morphine on opioid withdrawal in rhesus monkeys. Psychopharmacology. 10.1007/bf00180844 [DOI] [PubMed] [Google Scholar]
- Khor B-S, Jamil MFA, Adenan MI, Shu-Chien AC (2011) Mitragynine attenuates withdrawal syndrome in morphine-withdrawn zebrafish. PLoS ONE. 10.1371/journal.pone.0028340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010) Animal research: reporting in vivo experiments: the ARRIVE guidelines. Brit J Pharmacol 160(7):1577–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King T, Ossipov MH, Vanderah TW, Porreca F, Lai J (2005) Is paradoxical pain induced by sustained opioid exposure an underlying mechanism of opioid antinociceptive tolerance? Neurosignals 14(4):194–205. 10.1159/000087658 [DOI] [PubMed] [Google Scholar]
- Kleber HD (2007) Pharmacologic treatments for opioid dependence: detoxification and maintenance options. Dialogues Clin Neurosci 9:455–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Caine S, Parsons L, Markou A, Weiss F (1997) Opponent process model and psychostimulant addiction. Pharmacol Biochem Behav 57(3):513–521. 10.1016/s0091-3057(96)00438-8 [DOI] [PubMed] [Google Scholar]
- Kosten TR, O'Connor PG (2003) Management of drug and alcohol withdrawal. New Engl J Med 348(18):1786–1795 [DOI] [PubMed] [Google Scholar]
- Kosten TR, Baxter LE (2019) Effective management of opioid withdrawal symptoms: a gateway to opioid dependence treatment. Am J Addict 28:55–62. 10.1111/ajad.12862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreek MJ, Laforge KS, Butelman E (2002) Pharmacotherapy of addictions. Nat Rev Drug Discov 1(11):710–726. 10.1038/nrd897 [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Borg I, Ducat E, Ray B (2010) Pharmacotherapy in the treatment of addiction: methadone. J Addict Dis 29:200–216. 10.1080/10550881003684798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruegel AC, Grundmann O (2018) The medicinal chemistry and neuropharmacology of kratom: a preliminary discussion of a promising medicinal plant and analysis of its potential for abuse. Neuropharmacology 134:108–120. 10.1016/j.neuropharm.2017.08.026 [DOI] [PubMed] [Google Scholar]
- Kruegel AC, Gassaway MM, Kapoor A, Váradi A, Majumdar S, Filizola M, Javitch JA, Sames D (2016) Synthetic and receptor signaling explorations of the mitragyna alkaloids: mitragynine as an atypical molecular framework for opioid receptor modulators. J Am Chem Soc 138(21):6754–6764. 10.1021/jacs.6b00360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruegel AC, Uprety R, Grinnell SG, Langreck C, Pekarskaya EA, Le Rouzic V, Ansonoff M, Gassaway MM, Pintar JE, Pasternak GW, Javitch JA, Majumdar S, Sames D (2019) 7-Hydroxymitragynine is an active metabolite of mitragynine and a key mediator of its analgesic effects. ACS Cent Sci 5(6):992–1001. 10.1021/acscentsci.9b00141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laulin J-P, Maurette P, Corcuff J-B, Rivat C, Chauvin M, Simonnet AG (2002) The role of ketamine in preventing fentanyl-induced hyperalgesia and subsequent acute morphine tolerance. Anesth Analg 94(5):1263–1269. 10.1097/00000539-200205000-00040 [DOI] [PubMed] [Google Scholar]
- Li X, Angst MS, Clark JD (2001) Opioid-induced hyperalgesia and incisional pain. Anesth Analg. 10.1097/00000539-200107000-00040 [DOI] [PubMed] [Google Scholar]
- Lipman JJ, Blumenkopf B (1989) Comparison of subjective and objective analgesic effects of intravenous and intrathecal morphine in chronic pain patients by heat beam dolorimetry. Pain 39(3):249–256. 10.1016/0304-3959(89)90037-7 [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Mizowaki M, Suchitra T, Takayama H, Sakai S-I, Aimi N, Watanabe H (1996a) Antinociceptive action of mitragynine in mice: evidence for the involvement of supraspinal opioid receptors. Life Sci 59(14):1149–1155. 10.1016/0024-3205(96)00432-8 [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Mizowaki M, Suchitra T, Murakami Y, Takayama H, Sakai SI, Aimi N, Watanabe H (1996b) Central antinociceptive effects of mitragynine in mice: contribution of descending noradrenergic and serotonergic systems. Eur J Pharmacol 317(1):75–81. 10.1016/s0014-2999(96)00714-5 [DOI] [PubMed] [Google Scholar]
- Meepong R, Sooksawate T (2019) Mitragynine reduced morphine-induced conditioned place preference and withdrawal in rodents. Thai J Pharm Sci 43(1):21–29 [Google Scholar]
- Minami M, Satoh M (1995) Molecular biology of the opioid receptors: structures, functions and distributions. Neurosci Res 23(2):121–145. 10.1016/0168-0102(95)00933-k [DOI] [PubMed] [Google Scholar]
- Miser AW, Chayt KJ, Sandlund JT, Cohen PS, Dothage JA, Miser JS (1986) Narcotic withdrawal syndrome in young adults after the therapeutic use of opiates. Am J Dis Child 140(6):603–604. 10.1001/archpedi.1986.02140200113039 [DOI] [PubMed] [Google Scholar]
- Obeng S, Kamble SH, Reeves ME, Restrepo LF, Patel A, Behnke M, Chear NJ-Y, Ramanathan S, Sharma A, León F, Hiranita T, Avery BA, McMahon LR, McCurdy CR (2020) Investigation of the adrenergic and opioid binding affinities, metabolic stability, plasma protein binding properties, and functional effects of selected indole-based kratom alkaloids. J Med Chem 63(1):433–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özdoğan ÜK, Lähdesmäki J, Scheinin M (2003) Influence of prazosin and clonidine on morphine analgesia, tolerance and withdrawal in mice. Eur J Pharmacol 460(2–3):127–134. 10.1016/S0014-2999(02)02961-8 [DOI] [PubMed] [Google Scholar]
- Pinelli A, Trivulzio S, Tomasoni L (1997) Effects of ondansetron administration on opioid withdrawal syndrome observed in rats. Eur J Pharmacol 340(2–3):111–119. 10.1016/s0014-2999(97)01349-6 [DOI] [PubMed] [Google Scholar]
- Sabetghadam A, Ramanathan S, Mansor SM (2010) The evaluation of antinociceptive activity of alkaloid, methanolic, and aqueous extracts of Malaysian Mitragyna speciosa Korth leaves in rats. Pharmacogn Res 2(3):181–185. 10.4103/0974-8490.65514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer SL, Varvel JR (1991) Pharmacokinetics, pharmacodynamics, and rational opioid selection. Anesthesiology 74(1):53–63. 10.1097/00000542-199101000-00010 [DOI] [PubMed] [Google Scholar]
- Shaw-Lutchman TZ, Barrot M, Wallace T, Gilden L, Zachariou V, Impey S, Duman RS, Storm D, Nestler EJ (2002) Regional and cellular mapping of cAMP response element-mediated transcription during Naltrexone-precipitated morphine withdrawal. J Neurosci 22(9):3663–3672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh D, Yeou Chear NJ, Narayanan S, Leon F, Sharma A, McCurdy CR, Avery BA, Balasingam V (2020) Patterns and reasons for kratom (Mitragyna speciose) use among current and former opioid poly-drug users. J Ethnopharmacol 249:112462. 10.1016/j.jep.2019.112462 [DOI] [PubMed] [Google Scholar]
- Srivastava AB, Mariani JJ, Levin FR (2020) New directions in the treatment of opioid withdrawal. Lancet 395(10241):1938–1948. 10.1016/S0140-6736(20)30852-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sufka KJ, Hughes RA, Giordano J (1991) Effects of selective opiate antagonists on morphine-induced hyperalgesia in domestic fowl. Pharmacol Biochem Behav 38(1):49–54. 10.1016/0091-3057(91)90588-s [DOI] [PubMed] [Google Scholar]
- Sun HL (1998) Naloxone-precipitated acute opioid withdrawal syndrome after epidural morphine. Anesth Analg 86(3):544–545. 10.1213/00000539-199803000-00019 [DOI] [PubMed] [Google Scholar]
- Toce MS, Chai PR, Burns MM, Boyer EW (2018) Pharmacologic treatment of opioid use disorder: a review of pharmacotherapy, adjuncts, and toxicity. J Med Toxicol 14:306–312. 10.1007/s13181-018-0685-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbricht A, Montoya ID, Hoover DR, Demuth KL, Chiang CT, Preston KL (1999) Naltrexone shortened opioid detoxification with buprenorphine. Drug Alcohol Depend 56:181–190. 10.1016/s0376-8716(99)00033-2 [DOI] [PubMed] [Google Scholar]
- Váradi A, Marrone GF, Palmer TC, Narayan A, Szabó MR, Le Rouzic V, Grinnell SG, Subrath JJ, Warner E, Kalra S, Hunkele A (2016) Mitragynine/corynantheidine pseudoindoxyls as opioid nalgesics with Mu agonism and delta antagonism, which do not recruit β-arrestin-2. J Med Chem 59(18):8381–8397. 10.1021/acs.jmedchem.6b00748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakim JH (2012) Alleviating symptoms of withdrawal from an opioid. Pain Ther 1(4):1–6. 10.1007/s40122-012-0004-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson LL, Harris HM, Eans SO, Brice-Tutt AC, Cirino TJ, Stacy HM, Simons CA, León F, Sharma A, Boyer EW, Avery BA (2020) Lyophilized kratom tea as a therapeutic option for opioid dependence. Drug Alcohol Depend 216:108310. 10.1016/j.drugalcdep.2020.108310 [DOI] [PubMed] [Google Scholar]
- Xin W, Chun W, Ling L, Wei W (2011) Role of melatonin in the prevention of morphine-induced hyperalgesia and spinal glial activation in rats: protein kinase C pathway involved. Int J Neurosci 122(3):154–163. 10.3109/00207454.2011.635828 [DOI] [PubMed] [Google Scholar]
- Yusoff NH, Suhaimi FW, Vadivelu RK, Hassan Z, Rümler A, Rotter A, Amato D, Dringenberg HC, Mansor SM, Navaratnam V, Müller CP (2014) Abuse potential and adverse cognitive effects of mitragynine (kratom). Addict Biol 21(1):98–110. 10.1111/adb.12185 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data needed to evaluate the conclusions in the paper are present in the paper. Data related to this manuscript may be requested from the authors.