Abstract
Mechanical forces are conducted through myofibers and into nuclei to regulate muscle development, hypertrophy, and homeostasis. We hypothesized that nuclei in aged muscle have changes in the nuclear envelope and associated proteins, resulting in altered markers of mechano-signaling.
METHODS:
YAP/TAZ protein expression and gene expression of downstream targets, Ankrd1 and Cyr61, were evaluated as mechanotransduction indicators. Expression of proteins in the nuclear lamina and the nuclear pore complex (NPC) were assessed, and nuclear morphology was characterized by electron microscopy. Nuclear envelope permeability was assessed by uptake of 70 kDa fluorescent dextran.
RESULTS:
Nuclear changes with aging included a relative decrease of lamin β1 and Nup107, and a relative increase in Nup93, which could underlie the aberrant nuclear morphology, increased nuclear leakiness, and elevated YAP/TAZ signaling.
CONCLUSION:
Aged muscles have hyperactive nuclear-cytoplasmic signaling, indicative of altered nuclear mechanotransduction. These data highlight a possible role for the nucleus in aging-related aberrant mechano-sensing.
Keywords: Muscles, mice, sarcopenia, biology of aging
1. Introduction
Skeletal muscles show decline in muscle cell (myofiber) size with age (1), resulting in a loss of muscle mass (sarcopenia). This deficit, together with increased susceptibility to injury (2), predisposes the risk of falls and related injuries, which are linked to morbidity and mortality (3). The precise cause of the gradual decline in muscle mass remains to be determined, but several mechanisms have been put forth including apoptosis (4), oxidative stress (5), chronic low-level inflammation (6), reduced satellite cell function (7), and blunted anabolic responses (8). Here we propose an additional factor that contributes to sarcopenia: aberrant nuclear-cytoplasmic transport in aging nuclei.
Myofibers are multinucleated, and to support the large cytoplasm in the skeletal myofiber, the nuclei are positioned to maximize the distance between each other (9), thereby minimizing transport distances (10). Each nucleus contributes to the organization of the cytoskeleton, but importantly the nuclei throughout a single fiber also act as cellular mechano-sensors (11). The nuclear lamina, a dense meshwork of nuclear lamins underneath the inner nuclear envelope, along with the chromatin, are the primary determinants of nuclear stiffness (12, 13). The nuclear lamina is integral in facilitating mechanical signals to the nucleus and plays an important role in DNA organization and repair, and transcriptional regulation (reviewed in (14). Changes in nuclear shape can result in conformational changes in chromatin structure, directly affecting transcriptional regulation (15), and ultimately altering cell function (16).
In addition to its role in mechanotransduction, the nuclear envelope is critical for the physical separation of the nuclear genome from the cytoplasm. That is, improperly compartmentalized nucleic acids arising from dysfunctional nuclear pore complexes (NPCs) are harmful to the cell, including aberrant nuclear entry of signaling molecules without regulation (17). The NPC is a small multiprotein channel that mediates molecular traffic across the nuclear envelope, i.e. the bidirectional exchange of proteins and mRNA between the nucleus and cytoplasm. Each NPC is composed of ~30 different proteins, called nucleoporins (aka ‘Nups’). Loss or deterioration of nucleoporins have been found to contribute to the aberrant regulation of gene expression, cell-cycle progression, maintenance of nuclear envelope integrity, and chromosome segregation (18). In neurons, processes such as oxidative stress can result in age-dependent NPC deterioration, which is associated with a loss of nuclear-cytoplasmic compartmentalization. Such findings have led to the notion that ‘leaky’ nuclei (via NPCs) could therefore be a major driver of aging in post-mitotic cells such as neurons (18, 19). Here, we adopt similar methods to interrogate whether nuclei in adult skeletal muscle show analogous changes with aging.
Mechanotransduction, the process by which mechanical force is translated into a biochemical signal to activate downstream cellular responses, is crucial to myofiber function. Although much research has focused on how protein complexes at the sarcolemma and within the fiber respond to mechanical stress, the nucleus has emerged as a crucial organelle for the cell to perceive and respond to changes in its mechanical environment. YAP (Yes-associated protein) and its paralog TAZ (transcriptional coactivator with PDZ-binding motif) have been touted as nuclear relays of mechanical signals (20). In response to mechanical load, unphosphorylated YAP/TAZ translocates to the nucleus and binds to a host of transcription factors (primarily TEAD1-4 (21)), inducing the transcription of many downstream genes. Although nuclear translocation of YAP/TAZ appears to be dependent on force transmission, the effect of aging on YAP/TAZ signaling has not been examined.
We used muscles from young and aged mice to study the nuclear envelope and associated structures. We tested the hypothesis that compared to nuclei in young muscle, nuclei in aged muscle have changes in the nuclear lamina and NPC proteins, with parallel changes of altered nucleo-cytoplasmic transport and mechano-signaling indicators in skeletal muscle.
2. Materials And Methods
2.1. Animals
We used young (2–5 months) and aged (24–28 months) male mice from the C57BL6 strain (purchased from The Jackson Laboratory, Bar Harbor, ME, and obtained through the NIA Aged Rodent Colonies). A total of 21 mice were used for these studies, with specific numbers per experiment listed below. Mice were maintained under a 12-hour light/12-hour dark schedule with ad libitum access to food and water. All experimental procedures were approved by the University of Maryland Institutional Animal Care & Use Committee.
2.2. Western blotting
Quadriceps muscles (N = 3 for young, N = 3 for aged mice were homogenized in RIPA buffer (Cell Signaling Technologies, 9806S, supplemented with 1% SDS and Protease/Phosphatase inhibitor, Invitrogen, A32961) with sonication. BCA protein assay was used to measure protein concentration in homogenates (Thermo Fisher Scientific, Waltham, MA), and 30 μg of protein were loaded and separated in 4–12% gradient gels. Separated proteins were transferred to a nitrocellulose membrane, with membranes blocked in 5% milk. Membranes were incubated in primary antibodies against Lamin A/C (Cell Signaling Technology, Danvers, MA, 2032, 1:1000 dilution), total-YAP/TAZ (Santa Cruz Biotechnology, Dallas, TX, sc-101199, 1:500) and phospho-YAP/TAZ (Cell Signaling Technology, 4911S, 1:500 dilution). 60 μg of protein were also loaded, separated in 4–12% gradient gels and transferred to nitrocellulose membranes. Membranes were also blocked in 5% milk and incubated in primary antibodies against Lamin β1 (Cell Signaling Technology, 13435S, 1:1000 dilution), Nup107 (Proteintech, Rosemont, IL, 19217-1-AP, 1:500), Nup93 (Proteintech, 13077-1-AP, 1:500), Nup88 (Abcam, ab79785, 1:500), Nup98 (Cell Signaling Technology, 2598S, 1:500), KPNA4 (Proteintech, 12463-1-AP, 1:500), CRM1 (Cell Signaling Technology, 66763-1-AP, 1:1000), RAN (BD Biosciences, 610341, 1:1000) and RCC1 (Proteintech, 22142-1-AP, 1:500). Membranes were visualized after incubation with HRP-conjugated rabbit and mouse antibodies and ECL substrate (Thermo Fisher Scientific). Ponceau S staining was performed to ensure proper loading and to monitor the quality of protein transfer to the membrane. Bands were quantified using ImageJ software and normalized to GAPDH (Santa Cruz, 6C5, 1:1000). Protein expression was normalized to levels in young samples for obtaining the fold change in expression. Changes were not altered even with densitometry measures normalized to Ponceau stain.
2.3. Quantitative RT-PCR
For real time (RT)-PCR detection of YAP and downstream targets of YAP/TAZ, three young and three aged quadriceps muscle were snap frozen and then homogenized in Trizol reagent (Invitrogen, Carlsbad, CA). Total RNA was extracted according to manufacturer’s instruction, and reverse transcribed. Quantitative RT-PCR was performed as described previously, with an ABI 7300 Sequence Detection System (Applied Biosystems, Foster City, CA) using SYBR green. Relative expression was determined by comparison to housekeeping gene, GAPDH, using the geNorm software (v3.5, Ghent University Hospital, Ghent, Belgium). No significant changes (p > 0.05) were observed between young and aged muscles for cycle threshold values for GAPDH. Transcripts for YAP (Yap), and its downstream targets (Ankrd1 and Cyr61) were assessed (Primer sets described in S1A).
2.4. Myofiber isolation
After mice were euthanized, the flexor digitorum brevis (FDB) muscles were harvested bilaterally. To obtain single myofibers by enzymatic dissociation, muscles were placed into DMEM with 10% Fetal Bovine Serum (FBS), 1 μl/ml gentamicin, and 1 mg/ml type A collagenase (Roche, Basel, Switzerland, 11099793001) overnight at 37°C. Myofibers were plated on extracellular matrix (ECM, Sigma E1270)-coated imaging dishes (MatTek Corporation, Ashland, MA, P34G-1.0, 0-14-C) before fixation. The small size of the mouse FDB muscle makes it ideal for performing these enzymatic dissociations to obtain isolated, live myofibers, which are challenging to perform with the larger size of the mouse quadriceps muscle.
2.5. Examination of ultrastructure with electron microscopy
Fixed single myofibers were isolated from FDB muscles as above, and were stored at 4°C before processing (N=3 mice for young and N=4 mice for aged animals). Fixed cultures were washed and post-fixed with 1% osmium tetroxide and 0.75% potassium ferrocyanide in 0.1M PIPES, PH 7 for 1 hour at 4oC. After osmication, specimens were washed in water, stained en bloc with 1% (w/v) uranyl acetate for 1 hour and dehydrated using 30%, 50%, 70%, 90% and 100% ethanol in series. After dehydration, specimens were infiltrated and embedded in Araldite-Epoxy resin (Araldite, Embed 812, Electron Microscopy Sciences, PA) following manufacturer’s recommendations. Ultrathin sections were cut at ~70 nm thickness on a Leica UC6 ultramicrotome (Leica Microsystems, Inc., Bannockburn, IL) and examined in a Tecnai T12 transmission electron microscope (Thermo Fisher Scientific) operated at 80KeV. Digital images were acquired by using an AMT bottom mount CCD camera (Advanced Microscopy Techniques, Woburn, MA) and AMT600 software. ImageJ software (NIH, Bethesda, MD) was used to determine the number of invaginations, the length of invaginations, and the length of the nuclear envelope gap in young and aged nuclei.
2.6. Nuclear isolation and leakiness assessment
To isolate intact nuclei, we used a protocol described by Cutler et al (22). All steps were carried out at 4 °C. Whole quadriceps and gastrocnemius muscles (N = 3 young, N = 5 aged) were dissected, minced, and suspended in 10 mL homogenization buffer 1 (10 mM HEPES, 60 mM KCL, 0.5 mM spermidine, 0.15 mM spermine tetrahydrochloride, 2 mM EDTA, 0.5 mM EGTA, 300 mM sucrose, 5 mM MgCl2, 2 mM dithiothreitol (DTT), and 5% complete mini protease inhibitors (Roche)). Muscles were then homogenized with approximately 50 strokes using a Dounce homogenizer, filtered (40 μm filter) and centrifuged at 1000g for 10 min, yielding a crude nuclear pellet. The pellet was resuspended in homogenization buffer and loaded over a sucrose gradient: 2.8 M sucrose and 2.0 M sucrose in 50 mM HEPES, 25 mM KCl, and 5 mM MgCl2. The sample was centrifuged for 4 h at 27500 RPM in a SW28 rotor in a Beckman Optima L Series ultracentrifuge.
After ultracentrifugation, the nuclei concentrated at the interface between the 2.0 M and 2.8 M sucrose layers were collected in conical tubes pretreated with 1% BSA, diluted with resuspension buffer (20 mM HEPES, 10 mM KCl, 1.5 mM MgCl2, 0.5 mM spermidine, 0.15 mM spermine, and 0.2 mM EDTA), mixed thoroughly by inverting and pelleted at 1000g for 10 min. The remaining pellet was washed in 1% BSA, diluted with resuspension buffer and pelleted again at 800g for 10 min. The pellet was resuspended with 1% BSA, diluted with resuspension buffer. For assessing nuclear leakiness, isolated nuclei were stained with 1 μg mL−1 DAPI and 60 μg mL−1 70 kDa fluorescein isothiocyanate (FITC)-conjugated dextran (Sigma-Aldrich) in 1% BSA, diluted in resuspension buffer and examined by digital images obtained with Zeiss LSM Duo microscope (Oberkochen, Germany, 40x objective). Average intensity of FITC-conjugated dextran in the area outlined by DAPI labeling, and the corresponding background intensity of the image area was measured in z-stacked images using ImageJ. The nuclear leakiness (leakiness was indicated by normalized intensity of FITC-conjugated dextran greater than 1) was measured for more than 450 nuclei for each group, and was averaged for each animal (18, 22).
2.7. Statistical analysis
Statistical analyses were performed using SigmaStat 3.5 (San Rafael, CA). Data are presented as mean ± SD unless otherwise noted. Statistical significance was assessed using a t-test with p < 0.05. Assumptions of normality and equal variance were confirmed. If the assumptions of normality or equal variance were not met, a Mann Whitney U-test was performed. Scatter plots were produced using RStudio (RStudio: Integrated Development for R. RStudio, PBC, Boston, MA) and open source ggplot2 3.1.0 package (23).
3. Results
3.1. Nuclear mechano-signaling
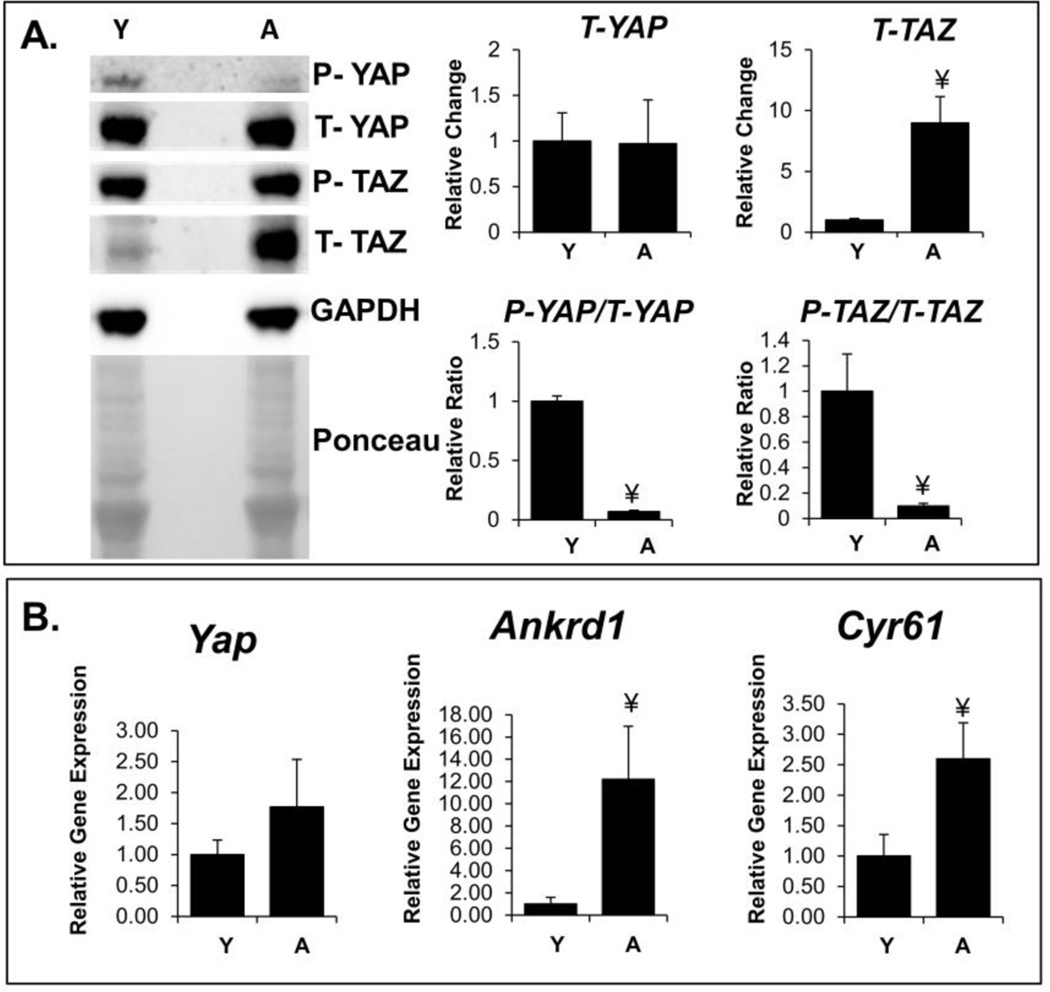
Changes in YAP/TAZ phosphorylation reflect nuclear mechano-signaling. The phospho/total ratio of YAP/TAZ is an indicator of this nuclear localization, as only unphosphorylated YAP/TAZ can translocate to the nucleus. Thus, a decrease in the phospho/total ratio, as seen in aged muscle for both YAP and TAZ, (Figure 1A) indicates increased nuclear signaling. We found an increase in total TAZ protein content in aged muscle, although we did not see a corresponding change in the total YAP protein content. Gene expression of downstream targets of YAP/TAZ signaling, Ankrd1 and Cyr61, was increased in aged muscle (Figure 1B). Elevated YAP/TAZ signaling supports the notion of altered nuclear mechanotransduction in aging muscles.
Figure 1:
Elevated YAP/TAZ signaling in aged muscle. A: Phosphorylated (P) and total (T) YAP/TAZ protein expression determined using western blots. The cropped blot and Ponceau stain in each panel are from a single gel and single exposure of two contiguous lanes. Densitometry measures were normalized to GAPDH expression. Densitometry measures were further normalized to levels in young samples for obtaining the fold change in expression. B: Relative gene expression of Yap and downstream targets of YAP/TAZ, Ankrd1 and Cyr61. Both results indicate increased YAP/TAZ activity in aged muscle.
¥, p-value < 0.05 compared to young muscles; Y indicates 2–5 months old; A indicates 24–28 months old.
3.2. Nuclear Structure and Transport
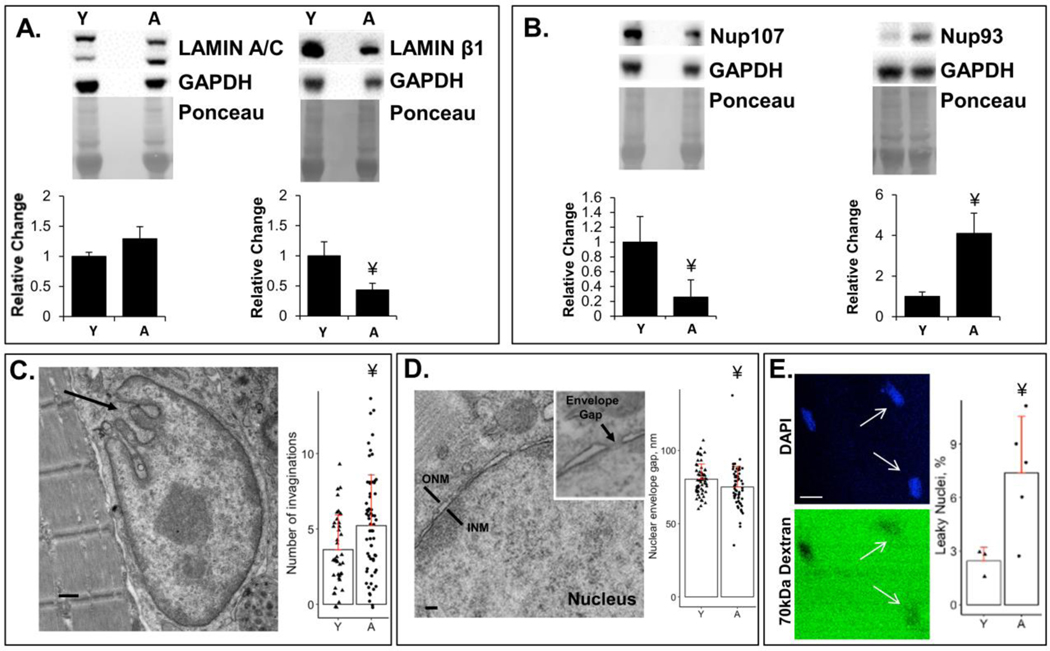
Although protein expression of lamin A/C was unchanged in aged muscle, lamin β1was decreased in aged muscle (Figure 2A). These nuclear envelope proteins are known to play a key role in nuclear morphology, which was examined using electron microscopy in nuclei from isolated myofibers (Figure 2C). We found an increased number of invaginations in nuclei of aged muscles, although the lengths of these invaginations were not different between young and aged muscles (S1F). Protein levels for nucleoporins were also assessed by western blot (Figure 2B and S1H). We found differential protein expression of nucleoporins in nuclei between young versus aged muscles, with decreased expression of Nup107, increased expression of Nup93 and no change in protein expression of Nups88 and 98. The gap for the NPC in the nuclear envelope (Figure 2D) measured with EM was smaller in aged muscles. Fluorescent dextran was used to detect influx via the NPC into the nucleus (18, 22). NPCs typically exclude 70 kDa dextran. The average fluorescence was not significantly different between young and aged nuclei (S1G). We used a quantitative measure to define leakiness (normalized nuclear intensity of FITC-conjugated dextran greater than the background fluorescent intensity). A higher percentage of nuclei in aged muscle are leaky, with the NPCs incapable of excluding 70 kDa dextrans (Figure 2E). It has been shown previously that dextran influx assays can detect a single defective pore, making it a more sensitive indicator than measurement of transport through the NPC (18, 24).
Figure 2:
Alterations to the nuclear structure and transport with aging. A: Expression of nuclear lamina proteins, lamin A/C and lamin β1, determined using western blots. Lamin β1 is significantly reduced with aging. B: Expression of nuclear pore complex proteins Nup107 and Nup93 were determined using western blots. Aged muscles have altered expression of nuclear pore complex proteins. The cropped blot and Ponceau stain in each panel are from a single gel and single exposure of two contiguous lanes. Densitometry measures were normalized to GAPDH expression. Densitometry measures were further normalized to levels in young samples for obtaining the fold change in expression. C: Representative EM image of an aged nucleus showing example of invaginations (arrow). Number of invaginations per nucleus were measured in young and aged nuclei in isolated muscle fibers. Scale bar = 500 nm. D: Representative EM image of the cross-section of the nuclear envelope gap in which the NPC resides. Scale bar = 100 nm. E: Representative image of DAPI (blue) and corresponding 70 kDa fluorescent dextran (green) in isolated nuclei from aged muscle. 70 kDa dextran, which typically cannot enter through the nuclear pore, freely enters some nuclei from aged muscle, indicating aberrant (“leaky”) nuclear transport (white arrows). Aged muscles have significantly higher percentage of leaky nuclei than young muscles (see Methods). Scale bar = 10 μm.
¥, p-value < 0.05 compared to young muscles; Y indicates 2–5 months old; A indicates 24–28 months old.
4. Discussion
Myofibers are constantly subjected to mechanical forces. Proper structure and levels of proteins in the nuclear lamina and NPCs are required for proper mechano-signaling (15, 25). Extracellular and cytoplasmic forces are transmitted across the nuclear envelope to the nuclear interior, where they can cause deformation of chromatin and nuclear bodies (26–28). Gene expression is dependent on chromatin organization (29), thus, unstable regulation of this pathway in response to mechanical stress could drive sarcopenia (30–32). Our results also revealed age-dependent changes in the lamina (specifically a reduction in lamin-β1) and in the NPC (specifically a reduction in Nup107 and an increase in Nup93). These findings could provide a possible molecular explanation, such as destabilized nucleocytoplasmic transport (i.e. ‘leaky nuclei’) and aberrant mechano-signaling (i.e. elevated YAP/TAZ signaling) underlying sarcopenia.
Although an increase in YAP/TAZ signaling typically drives hypertrophy, when constitutively active, YAP/TAZ paradoxically leads to atrophy and degeneration (33). We and others have observed increased YAP/TAZ signaling in diseased muscle (34, 35). YAP and TAZ are molecular indicators of nuclear mechanotransduction (20), and are altered with changes in expression of nuclear envelope and cytoskeletal proteins. The anabolic response to exercise is attenuated with aging, and recent work confirms minimal increases in nuclear signaling with loading in aged skeletal muscle (36). This attenuated response to loading in aged skeletal muscle could be due to the already elevated baseline of YAP/TAZ signaling (both decreased phospho/total protein ratio and increased gene expression of downstream targets) we describe here.
We further observed a relative increase in total TAZ protein expression, but not in total YAP protein expression. YAP and TAZ have common roles in skeletal muscle (e.g. both enhance proliferation), however they also have distinct roles (e.g. only TAZ enhances differentiation) (37, 38). TAZ increases dramatically during skeletal muscle differentiation, while YAP remains relatively constant. Others have reported differences in total YAP between young and aged muscle, however this could be due to the use of a different muscle (gastrocnemius) (39).
Lamins govern numerous biological functions, both biophysical and biochemical. This includes determination of nuclear size, shape, stiffness (25), regulation of transcription factors (40) and chromatin (41), and control of cell polarization and migration (42). Indeed, mechano-sensing by the nuclear lamina is thought to be important for protection against nuclear rupture and damage (43). Laminopathies, which include a subset of muscular dystrophies resulting from genetic mutations in lamins, have disrupted nuclear mechanotransduction (14). Our results indicate a decrease in protein expression of lamin-β1, but not lamin A/C, with aging. In non-muscle cells, lamin-β1 appears to be a marker of cellular senescence and various forms of cellular stress (44). Such findings suggest that defects in the nuclear lamina can impair the ability of cells to respond appropriately to mechanical forces.
Previous studies have observed alterations in nuclear shape and chromatin organization in aged muscle (9, 31, 45), which have been linked to changes in lamins. While other studies have observed invaginations in aging nuclei, there have been no quantitative measures. Previous studies on nuclei with lamin mutations have also observed nuclear invaginations (46, 47). Abnormal nuclear shape, such as our finding of increased number of invaginations with aging, could also affect transcription (48), further implicating abnormal nuclear function in aging.
The loss of lamin-β1 in neurons has been associated with altered nucleo-cytoplasmic transport consequent to changes in NPCs (49). Previous studies on nuclei in neurons have also reported loss of lamin-β1, increase in the fraction of ‘leaky’ nuclei, and altered expression of Nups (50, 51). Our finding that increased nuclear influx (‘leaky nuclei’) occurs in nuclei of aged skeletal muscle, and that a subset of nucleoporins is altered in aged myofibers, is consistent with the notion that accumulation of damage at the NPC might be a crucial aging event, as has been postulated in aging neurons (18). We examined nucleoporins in different regions of the NPC (Figure 3A) to examine the impact of aging. We found changes only in some Nups, such as: Nup107 (nuclear and cytoplasmic ring), Nup93 (central channel scaffold), but not in other such as Nup88 and Nup98 (connecting the NPC to both the cytoplasm filaments and the nuclear ring). These nucleoporins were examined not only to sample from different regions of the NPC, but also because of evidence that they change with aging in other tissues. For example, Nup98 and Nup93 are reduced in nuclei of aged neurons (50), and Nup107 is increased in isolated skeletal muscle nuclei with aging (52). Nup88 is a key nucleoporin that can bind to lamin A (53), supports nuclear export, and helps regulate neuromuscular junction formation and acetylcholine receptor clustering (54), which is impaired in aging skeletal muscle. Interestingly, we found no differences between young and aged muscle in the nucleo-cytoplasmic transport machinery we examined (RAN, RCC, CRM1 and KPNA4) (Figure S1 D).
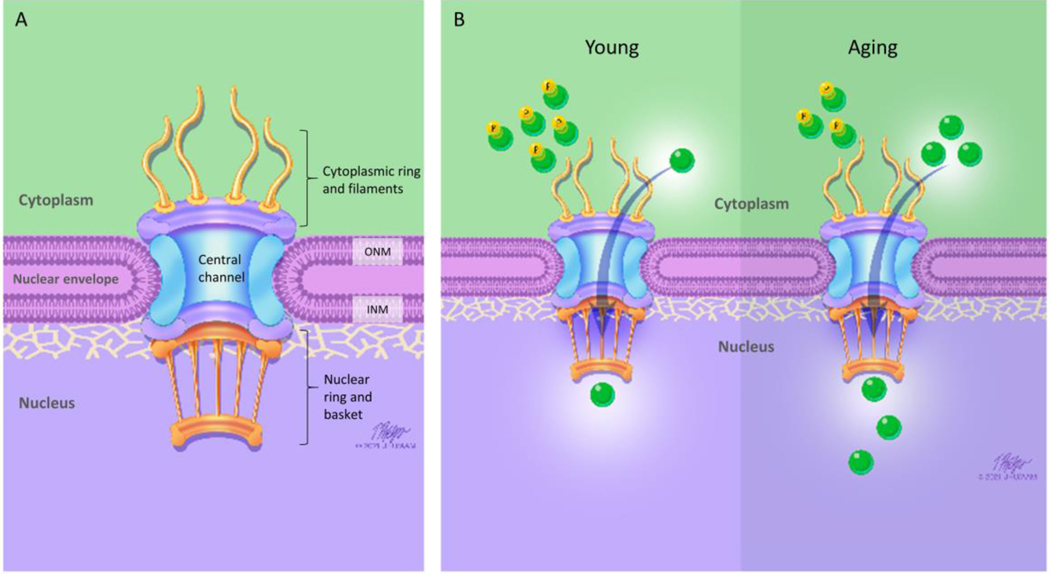
Figure 3:
Nuclear Pore Complex and Aging. A: Schematic shows structure of the nuclear pore complex (NPC). The nuclear envelope serves as a permeability barrier, with the NPC mediating movement of molecules across the nuclear envelope. ONM: outer nuclear membrane, INM: inner nuclear membrane. B: Young (left side): YAP/TAZ (green circles) localization reflects nuclear mechano-signaling. When phosphorylated (P), YAP/TAZ remains in the cytoplasm of the myofiber. In response to mechanical load, unphosphorylated YAP/TAZ can translocate (arrow) into the nucleus to regulate gene expression. Aging (right side): We found a decrease in the phospho/total ratio for both YAP and TAZ in aged muscle (end result is increased YAP/TAZ nucleocytoplasmic transport into the nucleus). This heightened mechano-signaling (i.e. increased YAP/TAZ signaling) might contribute to sarcopenia.
In non-muscle tissues, it has been suggested that NPCs deteriorate with time, losing nucleoporins responsible for maintaining the pore diffusion barrier (19, 22). Indeed, nuclei of old rat neurons show an increased permeability, and the age-dependent nuclear pore deterioration is associated with a loss of cell nuclear-cytoplasmic compartmentalization (18). Mislocalized NPC proteins could result in the impairment of various cellular functions, such as chromatin organization and gene expression (55). It is unclear whether the stable scaffold of the NPC is turned over by replacing the entire scaffold at once, or whether NPCs are maintained by piecemeal replacement of subunits, resulting in mosaic complexes of polypeptides with vastly different ages (56).
While Nup107, Nup93 and Nup88 are long-lived nucleoporins, Nup98 is a dynamic nucleoporin. The long-lived nucleoporins are primarily in the scaffold, and are frequently associated with oxidative damage, potentially yielding malfunctional NPCs (18). Here, we observed a decrease in Nup107 expression and an increase in Nup93 expression in skeletal muscle with aging. Both Nup107 and Nup93 bind chromatin (57–59). Previous studies have reported an increase in Nup107 in aged skeletal muscle nuclei, but no change in aged brain nuclei (18, 22). In fact, a loss-of-function mutation in Nup107 rescues the neurodegeneration in drosophila models of amyotrophic lateral sclerosis (60). However, more work is needed to characterize the role of these nucleoporins in skeletal muscle.
While neurons are postmitotic cells, satellite cells in skeletal muscle can divide and produce new myofiber nuclei. Muscle fiber nuclei are post-mitotic and cannot replace themselves. However, skeletal muscle is maintained by repair and regeneration, due to the presence of satellite cells, which divide to replace nuclei. The turnover of nuclei in rodent muscle is at most between 1–2% per week (61). Studies of human skeletal muscle suggest nuclei in healthy adults can age as much as 15 years (62), making skeletal muscle a low-turnover and relatively post-mitotic tissue (63). In addition, aged skeletal muscle has a depletion of satellite cell number and function (64–66), which could potentially result in primarily older nuclei with accrued defects in NPCs and other structures. Previous studies on aged nuclei in neurons have also found significantly higher percentage of ‘leaky nuclei’ without any significant change to normalized dextran fluorescence, similar to our study (51), further implying accrued defects in skeletal muscle aging nuclei. Moreover, aberrant nuclear-cytoplasmic transport has also been implicated in age-related diseases related to nerve degeneration (60). Our finding that there is more NPC leakiness in aged nuclei, despite no increase in length of the nuclear envelope gap, could indicate altered function at the nuclear pore. Dysfunction of the NPC could be due to oxidative damage of nucleoporins in the NPC (18) or disrupted NPC assembly (19, 56), but either way such findings have led to the notion that ‘leaky’ nuclei (via NPCs) could be a major driver of aging (17–19). Although we did not study changes across the mouse life-span, changes in the nuclear lamina, the NPCs, and YAP/TAZ signaling might contribute to the decline in skeletal muscle function. When phosphorylated, YAP/TAZ remains in the cytoplasm of the myofiber. Unphosphorylated YAP/TAZ can translocate to the nucleus, forming a complex with TEAD transcription factors to regulate gene expression (Figure 3B). However, the link between our findings on nuclear changes with age and sarcopenia is speculation at this point, and more work is needed to identify how specific changes in morphology and NPC structure/function might contribute to sarcopenia. Human and animal studies have demonstrated that aged muscles maintain the ability to undergo hypertrophy, but the capacity to sense and subsequently respond to the mechanical stimuli is diminished (67). The elderly have a blunted response to exercise/resistance training (68), and recovery of muscle function following contraction-induced damage severely diminished in aging muscle (69). Perhaps this blunted response is due, at least in part, to the already heightened YAP/TAZ signaling, which cannot respond further to mechanical load (34).
Understanding the mechanisms of impaired mechanotransduction in aging muscle is likely to shed light on strategies designed to prevent and treat aging-associated skeletal muscle dysfunction. Because pathways that regulate muscle mass may represent a potential therapeutic avenue for interventions, it would also be interesting if future studies were conducted to determine whether pharmacological interventions targeting YAP/TAZ signaling can help ameliorate age-dependent weakness and susceptibility to injury. Aberrant mechano-signaling in aged muscles, secondary to alterations in nuclear structure and transport, likely contributes to sarcopenia.
Supplementary Material
Acknowledgements
The authors wish to thank the UMB Electron Microscopy Facility Core for assistance with EM and the UMB Confocal Microscopy Core for assistance with confocal microscopy.
Funding
This work was supported by grants to RML from the University of Maryland Claude D. Pepper Older Americans Independence Center (UM-OAIC) and the National Institutes of Health (R56AR073193), to SRI from the National Institutes of Health (K01AR074048) and the Muscular Dystrophy Association development grant (MDA 577897). This work utilized an EM sample preparation instrument that was purchased with funding from a National Institute of Health SIG grant (S10RR26870-1)
Footnotes
Conflict of interest
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nilwik R, Snijders T, Leenders M, Groen BB, van KJ, Verdijk LB, et al. The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type II muscle fiber size. ExpGerontol. 2013;48:492–498. [DOI] [PubMed] [Google Scholar]
- 2.Faulkner JA, Brooks SV, Zerba E. Muscle atrophy and weakness with aging: contraction-induced injury as an underlying mechanism. JGerontolA BiolSciMedSci. 1995;50 Spec No:124–129. [DOI] [PubMed] [Google Scholar]
- 3.Stevens JA, Corso PS, Finkelstein EA, Miller TR. The costs of fatal and non-fatal falls among older adults. InjPrev. 2006;12:290–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marzetti E, Leeuwenburgh C. Skeletal muscle apoptosis, sarcopenia and frailty at old age. Exp Gerontol. 2006;41:1234–1238.DOI: 10.1016/j.exger.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Meng SJ, Yu LJ. Oxidative stress, molecular inflammation and sarcopenia. Int J Mol Sci. 2010;11:1509–1526.DOI: 10.3390/ijms11041509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beyer I, Mets T, Bautmans I. Chronic low-grade inflammation and age-related sarcopenia. Curr Opin Clin Nutr Metab Care. 2012;15:12–22.DOI: 10.1097/MCO.0b013e32834dd297. [DOI] [PubMed] [Google Scholar]
- 7.Alway SE, Myers MJ, Mohamed JS. Regulation of satellite cell function in sarcopenia. Front Aging Neurosci. 2014;6:246.DOI: 10.3389/fnagi.2014.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hildebrandt W, et al. Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol. 2009;587:211–217.DOI: 10.1113/jphysiol.2008.164483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruusgaard JC, Liestol K, Gundersen K. Distribution of myonuclei and microtubules in live muscle fibers of young, middle-aged, and old mice. J ApplPhysiol (1985). 2006;100:2024–2030. [DOI] [PubMed] [Google Scholar]
- 10.Blau HM, Pavlath GK, Rich K, Webster SG. Localization of muscle gene products in nuclear domains: does this constitute a problem for myoblast therapy? AdvExpMedBiol. 1990;280:167–172. [DOI] [PubMed] [Google Scholar]
- 11.Cho S, Irianto J, Discher DE. Mechanosensing by the nucleus: From pathways to scaling relationships. J Cell Biol. 2017;216:305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PC, Pinter J, et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science. 2013;341:1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stephens AD, Banigan EJ, Adam SA, Goldman RD, Marko JF. Chromatin and lamin A determine two different mechanical response regimes of the cell nucleus. Mol Biol Cell. 2017;28:1984–1996.DOI: 10.1091/mbc.E16-09-0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gruenbaum Y, Foisner R. Lamins: nuclear intermediate filament proteins with fundamental functions in nuclear mechanics and genome regulation. AnnuRevBiochem. 2015;84:131–164. [DOI] [PubMed] [Google Scholar]
- 15.Dahl KN, Ribeiro AJ, Lammerding J. Nuclear shape, mechanics, and mechanotransduction. CircRes. 2008;102:1307–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Auld AL, Folker ES. Nucleus-dependent sarcomere assembly is mediated by the LINC complex. MolBiolCell. 2016;27:2351–2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lusk CP, King MC. The nucleus: keeping it together by keeping it apart. Curr Opin Cell Biol. 2017;44:44–50.DOI: 10.1016/j.ceb.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D’Angelo MA, Raices M, Panowski SH, Hetzer MW. Age-dependent deterioration of nuclear pore complexes causes a loss of nuclear integrity in postmitotic cells. Cell. 2009;136:284–295.DOI: 10.1016/j.cell.2008.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rempel IL, Crane MM, Thaller DJ, Mishra A, Jansen DP, Janssens G, et al. Age-dependent deterioration of nuclear pore assembly in mitotic cells decreases transport dynamics. Elife. 2019;8.DOI: 10.7554/eLife.48186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. [DOI] [PubMed] [Google Scholar]
- 21.Stein C, Bardet AF, Roma G, Bergling S, Clay I, Ruchti A, et al. YAP1 Exerts Its Transcriptional Control via TEAD-Mediated Activation of Enhancers. PLoS Genet. 2015;11:e1005465.DOI: 10.1371/journal.pgen.1005465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cutler AA, Dammer EB, Doung DM, Seyfried NT, Corbett AH, Pavlath GK. Biochemical isolation of myonuclei employed to define changes to the myonuclear proteome that occur with aging. Aging Cell. 2017;16:738–749.DOI: 10.1111/acel.12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wickham H Elegant Graphics for Data Analysis. New York: Springer-Verlag; 2016. [Google Scholar]
- 24.Lenart P, Rabut G, Daigle N, Hand AR, Terasaki M, Ellenberg J. Nuclear envelope breakdown in starfish oocytes proceeds by partial NPC disassembly followed by a rapidly spreading fenestration of nuclear membranes. J Cell Biol. 2003;160:1055–1068.DOI: 10.1083/jcb.200211076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dahl KN, Kahn SM, Wilson KL, Discher DE. The nuclear envelope lamina network has elasticity and a compressibility limit suggestive of a molecular shock absorber. J Cell Sci. 2004;117:4779–4786.DOI: 10.1242/jcs.01357. [DOI] [PubMed] [Google Scholar]
- 26.Maniotis AJ, Chen CS, Ingber DE. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. ProcNatlAcadSciUSA. 1997;94:849–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guilluy C, Osborne LD, Van LL, Sharek L, Superfine R, Garcia-Mata R, et al. Isolated nuclei adapt to force and reveal a mechanotransduction pathway in the nucleus. NatCell Biol. 2014;16:376–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lombardi ML, Jaalouk DE, Shanahan CM, Burke B, Roux KJ, Lammerding J. The interaction between nesprins and sun proteins at the nuclear envelope is critical for force transmission between the nucleus and cytoskeleton. J BiolChem. 2011;286:26743–26753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nava MM, Miroshnikova YA, Biggs LC, Whitefield DB, Metge F, Boucas J, et al. Heterochromatin-Driven Nuclear Softening Protects the Genome against Mechanical Stress-Induced Damage. Cell. 2020;181:800–817 e822.DOI: 10.1016/j.cell.2020.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phillip JM, Aifuwa I, Walston J, Wirtz D. The Mechanobiology of Aging. Annu Rev Biomed Eng. 2015;17:113–141.DOI: 10.1146/annurev-bioeng-071114-040829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malatesta M, Perdoni F, Muller S, Zancanaro C, Pellicciari C. Nuclei of aged myofibres undergo structural and functional changes suggesting impairment in RNA processing. EurJ Histochem. 2009;53:97–106. [DOI] [PubMed] [Google Scholar]
- 32.Lacavalla MA, Cisterna B, Zancanaro C, Malatesta M. Ultrastructural immunocytochemistry shows impairment of RNA pathways in skeletal muscle nuclei of old mice: A link to sarcopenia? Eur J Histochem. 2021;65.DOI: 10.4081/ejh.2021.3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Judson RN, Gray SR, Walker C, Carroll AM, Itzstein C, Lionikas A, et al. Constitutive expression of Yes-associated protein (Yap) in adult skeletal muscle fibres induces muscle atrophy and myopathy. PLoSOne. 2013;8:e59622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iyer SR, Shah SB, Ward CW, Stains JP, Spangenburg EE, Folker ES, et al. Differential YAP nuclear signaling in healthy and dystrophic skeletal muscle. Am J Physiol Cell Physiol. 2019;317:C48–C57.DOI: 10.1152/ajpcell.00432.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hulmi JJ, Oliveira BM, Silvennoinen M, Hoogaars WM, Ma H, Pierre P, et al. Muscle protein synthesis, mTORC1/MAPK/Hippo signaling, and capillary density are altered by blocking of myostatin and activins. AmJ Physiol EndocrinolMetab. 2013;304:E41–E50. [DOI] [PubMed] [Google Scholar]
- 36.Parkington JD, LeBrasseur NK, Siebert AP, Fielding RA. Contraction-mediated mTOR, p70S6k, and ERK1/2 phosphorylation in aged skeletal muscle. J Appl Physiol (1985). 2004;97:243–248.DOI: 10.1152/japplphysiol.01383.2003. [DOI] [PubMed] [Google Scholar]
- 37.Sun C, De Mello V, Mohamed A, Ortuste Quiroga HP, Garcia-Munoz A, Al Bloshi A, et al. Common and Distinctive Functions of the Hippo Effectors Taz and Yap in Skeletal Muscle Stem Cell Function. Stem Cells. 2017;35:1958–1972.DOI: 10.1002/stem.2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Setiawan I, Sanjaya A, Lesmana R, Yen PM, Goenawan H. Hippo pathway effectors YAP and TAZ and their association with skeletal muscle ageing. J Physiol Biochem. 2021.DOI: 10.1007/s13105-021-00787-z. [DOI] [PubMed] [Google Scholar]
- 39.Yoshida N, Endo J, Kinouchi K, Kitakata H, Moriyama H, Kataoka M, et al. (Pro)renin receptor accelerates development of sarcopenia via activation of Wnt/YAP signaling axis. Aging Cell. 2019;18:e12991.DOI: 10.1111/acel.12991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Osmanagic-Myers S, Dechat T, Foisner R. Lamins at the crossroads of mechanosignaling. Genes Dev. 2015;29:225–237.DOI: 10.1101/gad.255968.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harr JC, Luperchio TR, Wong X, Cohen E, Wheelan SJ, Reddy KL. Directed targeting of chromatin to the nuclear lamina is mediated by chromatin state and A-type lamins. J Cell Biol. 2015;208:33–52.DOI: 10.1083/jcb.201405110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davidson PM, Lammerding J. Broken nuclei--lamins, nuclear mechanics, and disease. Trends Cell Biol. 2014;24:247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cho S, Vashisth M, Abbas A, Majkut S, Vogel K, Xia Y, et al. Mechanosensing by the Lamina Protects against Nuclear Rupture, DNA Damage, and Cell-Cycle Arrest. Dev Cell. 2019;49:920–935 e925.DOI: 10.1016/j.devcel.2019.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tran JR, Chen H, Zheng X, Zheng Y. Lamin in inflammation and aging. Curr Opin Cell Biol. 2016;40:124–130.DOI: 10.1016/j.ceb.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cristea A, Qaisar R, Edlund PK, Lindblad J, Bengtsson E, Larsson L. Effects of aging and gender on the spatial organization of nuclei in single human skeletal muscle cells. Aging Cell. 2010;9:685–697. [DOI] [PubMed] [Google Scholar]
- 46.Vergnes L, Peterfy M, Bergo MO, Young SG, Reue K. Lamin B1 is required for mouse development and nuclear integrity. Proc Natl Acad Sci U S A. 2004;101:1042810433.DOI: 10.1073/pnas.0401424101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dechat T, Pfleghaar K, Sengupta K, Shimi T, Shumaker DK, Solimando L, et al. Nuclear lamins: major factors in the structural organization and function of the nucleus and chromatin. Genes Dev. 2008;22:832–853.DOI: 10.1101/gad.1652708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim DH, Li B, Si F, Phillip JM, Wirtz D, Sun SX. Volume regulation and shape bifurcation in the cell nucleus. JCell Sci. 2015;128:3375–3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Giacomini C, Mahajani S, Ruffilli R, Marotta R, Gasparini L. Lamin B1 protein is required for dendrite development in primary mouse cortical neurons. Mol Biol Cell. 2016;27:35–47.DOI: 10.1091/mbc.E15-05-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gillon A, Nielsen K, Steel C, Cornwall J, Sheard P. Exercise attenuates age-associated changes in motoneuron number, nucleocytoplasmic transport proteins and neuromuscular health. Geroscience. 2018;40:177–192.DOI: 10.1007/s11357-018-0020-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gillon A, Steel C, Cornwall J, Sheard P. Increased nuclear permeability is a driver for age-related motoneuron loss. Geroscience. 2020;42:833–847.DOI: 10.1007/s11357-020-00155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Apirakkan O, Frinculescu A, Shine T, Parkin MC, Cilibrizzi A, Frascione N, et al. Analytical characterization of three cathinone derivatives, 4-MPD, 4F-PHP and bk-EPDP, purchased as bulk powder from online vendors. Drug Test Anal. 2018;10:372–378.DOI: 10.1002/dta.2218. [DOI] [PubMed] [Google Scholar]
- 53.Lussi YC, Hugi I, Laurell E, Kutay U, Fahrenkrog B. The nucleoporin Nup88 is interacting with nuclear lamin A. Mol Biol Cell. 2011;22:1080–1090.DOI: 10.1091/mbc.E10-050-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bonnin E, Cabochette P, Filosa A, Juhlen R, Komatsuzaki S, Hezwani M, et al. Biallelic mutations in nucleoporin NUP88 cause lethal fetal akinesia deformation sequence. PLoS Genet. 2018;14:e1007845.DOI: 10.1371/journal.pgen.1007845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raices M, D’Angelo MA. Nuclear pore complexes and regulation of gene expression. Curr Opin Cell Biol. 2017;46:26–32.DOI: 10.1016/j.ceb.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Toyama BH, Arrojo EDR, Lev-Ram V, Ramachandra R, Deerinck TJ, Lechene C, et al. Visualization of long-lived proteins reveals age mosaicism within nuclei of postmitotic cells. J Cell Biol. 2019;218:433–444.DOI: 10.1083/jcb.201809123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ibarra A, Benner C, Tyagi S, Cool J, Hetzer MW. Nucleoporin-mediated regulation of cell identity genes. Genes Dev. 2016;30:2253–2258.DOI: 10.1101/gad.287417.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brown CR, Kennedy CJ, Delmar VA, Forbes DJ, Silver PA. Global histone acetylation induces functional genomic reorganization at mammalian nuclear pore complexes. Genes Dev. 2008;22:627–639.DOI: 10.1101/gad.1632708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gozalo A, Duke A, Lan Y, Pascual-Garcia P, Talamas JA, Nguyen SC, et al. Core Components of the Nuclear Pore Bind Distinct States of Chromatin and Contribute to Polycomb Repression. Mol Cell. 2020;77:67–81 e67.DOI: 10.1016/j.molcel.2019.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chou CC, Zhang Y, Umoh ME, Vaughan SW, Lorenzini I, Liu F, et al. TDP-43 pathology disrupts nuclear pore complexes and nucleocytoplasmic transport in ALS/FTD. Nat Neurosci. 2018;21:228–239.DOI: 10.1038/s41593-017-0047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schmalbruch H, Lewis DM. Dynamics of nuclei of muscle fibers and connective tissue cells in normal and denervated rat muscles. Muscle Nerve. 2000;23:617–626.DOI:. [DOI] [PubMed] [Google Scholar]
- 62.Collins CA, Partridge TA. Self-renewal of the adult skeletal muscle satellite cell. Cell Cycle. 2005;4:1338–1341.DOI: 10.4161/cc.4.10.2114. [DOI] [PubMed] [Google Scholar]
- 63.Spalding KL, Bhardwaj RD, Buchholz BA, Druid H, Frisen J. Retrospective birth dating of cells in humans. Cell. 2005;122:133–143.DOI: 10.1016/j.cell.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 64.Chakkalakal JV, Jones KM, Basson MA, Brack AS. The aged niche disrupts muscle stem cell quiescence. Nature. 2012;490:355–360.DOI: 10.1038/nature11438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Day K, Shefer G, Shearer A, Yablonka-Reuveni Z. The depletion of skeletal muscle satellite cells with age is concomitant with reduced capacity of single progenitors to produce reserve progeny. Dev Biol. 2010;340:330–343.DOI: 10.1016/j.ydbio.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shefer G, Van de Mark DP, Richardson JB, Yablonka-Reuveni Z. Satellite-cell pool size does matter: defining the myogenic potency of aging skeletal muscle. Dev Biol. 2006;294:50–66.DOI: 10.1016/j.ydbio.2006.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu M, Fannin J, Rice KM, Wang B, Blough ER. Effect of aging on cellular mechanotransduction. Ageing Res Rev. 2011;10:1–15.DOI: 10.1016/j.arr.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lavin KM, Roberts BM, Fry CS, Moro T, Rasmussen BB, Bamman MM. The Importance of Resistance Exercise Training to Combat Neuromuscular Aging. Physiology (Bethesda). 2019;34:112–122.DOI: 10.1152/physiol.00044.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brooks SV, Faulkner JA. Contraction-induced injury: recovery of skeletal muscles in young and old mice 3. AmJPhysiol. 1990;258:C436–C442. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.