Abstract
Background: Metformin, a commonly used antidiabetic medication, is available in both an immediate-release (IR) formulation and a long-acting formulation (metformin extended-release; XR).
Objective: We performed a systematic review to compare the effectiveness, safety, and patient compliance and satisfaction between the metformin IR and XR formulations.
Method: We searched for randomized control trials (RCTs) and observational studies comparing the effectiveness, safety, or patient compliance and satisfaction of metformin XR with metformin IR using the MEDLINE, Embase, and Cochrane Central Register of Controlled Trials databases. Following report screening, data collection, and risk of bias assessment, we separately pooled data from RCTs and observational studies using the Grading of Recommendation Assessment, Development, and Evaluation approach to rate the quality of evidence.
Result: We included five RCTs, comprising a total of 1,662 patients, and one observational study, comprising 10,909 patients. In the meta-analyses, no differences were identified in outcomes of effectiveness and safety between the two forms of metformin (including change in HbA1c: mean difference (MD), 0.04%, 95% confidence interval [CI], −0.05–0.13%, fasting blood glucose: MD, −0.03 mmol/L, 95% CI, −0.22–0.15 mmol/L, postprandial blood glucose: MD, 0.50 mmol/L, 95% CI, −0.71–1.72 mmol/L, adverse events of abdominal pain: relative risk (RR), 1.15, 95% CI, 0.57–2.33, all-cause death (RR, 3.02, 95% CI 0.12–73.85), any adverse events (RR, 1.14, 95% CI 0.97–1.34), any adverse events leading to treatment discontinuation: RR, 1.51, 95% CI, 0.82–2.8, any gastrointestinal adverse events: RR, 1.09, 95% CI, 0.93–1.29, diarrhea: RR, 0.82, 95% CI, 0.53–1.27, flatulence: RR, 0.43, 95% CI, 0.15–1.23, nausea: RR, 0.97, 95% CI, 0.64–1.47, severe adverse events: RR, 0.64, 95% CI, 0.28–1.42, and vomiting: RR, 1.46, 95% CI, 0.6–3.56). Data from both the RCTs and the observational study indicate mildly superior patient compliance with metformin XR use compared with metformin IR use; this result was attributable to the preference for once-daily administration with metformin XR.
Conclusion: Our systematic review indicates that metformin XR and IR formulations have similar effectiveness and safety, but that metformin XR is associated with improved compliance to treatment.
Keywords: metformin immediate-release, metformin extended-release, treatment compliance, once-daily consumption, systematic review, meta-analysis, patient value
Introduction
Type 2 diabetes mellitus was the fourth leading cause of death and disability worldwide in 2017, and has rapidly risen in rank since 1990 (Roth et al., 2018). Approximately 1 in 11 adults worldwide had type 2 diabetes, and those affected can experience life-threatening complications, including cardiovascular and renal diseases (Zheng et al., 2018). Lifestyle intervention and careful pharmacotherapeutic management are critical measures for people living with type 2 diabetes (Amod, 2012; McGuire et al., 2016).
Metformin effectively lowers blood glucose levels and is widely used to treat type 2 diabetes (National Collaborating Centre for Chronic Conditions (UK), 2008; Amod, 2012). Furthermore, metformin use is associated with long-term benefits, including reduced risk of cardiovascular disease and neoplasms (Foretz et al., 2014). Gastrointestinal intolerance is a major concern associated with clinical use of metformin; approximately 10% of adults living with type 2 diabetes are unable to receive metformin treatment because of gastrointestinal intolerance including diarrhea, vomiting, abdominal pain, and constipation (Fujioka et al., 2003; Ji et al., 2018). Long-term treatment with metformin is generally considered safe, except for its association with an increased risk of vitamin B12 deficiency (Reinstatler et al., 2012; Liu et al., 2014). However, the clinical relevance of such a deficiency remains unclear (Zhang et al., 2016).
Metformin tablets are available in an immediate-release (IR) formulation for administration two or three times per day. The need to administer the drug multiple times daily can present challenges for treatment compliance among patients and the maintenance of steady-state pharmacokinetics (Fujioka et al., 2003; Ji et al., 2018). An extended-release (XR) formulation with longer half-life and lower peak drug concentration is also available (Amod, 2012). Adults with type 2 diabetes receiving once-daily metformin XR might have a better treatment experience and are more likely to comply with treatment (Fujioka et al., 2003; Blonde et al., 2004).
The United Kingdom National Institute of Health and Care Excellence (NICE) guidelines recommend the use of metformin XR in adults with type 2 diabetes who cannot tolerate metformin IR (National Collaborating Centre for Chronic Conditions (UK), 2008); however, few studies have examined this recommendation. We previously reported the results of a survey conducted in adults living with type 2 diabetes and their physicians; we found that patients and physicians considered metformin XR to be superior to the IR formulation in terms of both effectiveness and safety (Liu et al., 2021). However, other studies have provided conflicting evidence (Fujioka et al., 2003, Blonde et al., 2004, National Collaborating Centre for Chronic Conditions (UK), 2008, Reinstatler et al., 2012, Foretz et al., 2014, Liu et al., 2014 ,Zhang et al., 2016, Ji et al., 2018). Data from a randomized controlled trial (RCT) of 267 patients in China indicated that the effectiveness and safety of metformin XR and IR were comparable (Ji et al., 2018), and these results have not been confirmed in other RCTs or observational studies (Blonde et al., 2004).
We therefore performed a systematic review comprising a comprehensive search and qualitative analyses to compare the short-term effectiveness and safety of metformin XR and IR in adults with type 2 diabetes.
Methods
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Moher et al., 2011). The protocol of this systematic review was registered in PROSPERO (number: CRD42021226051).
Literature Search
We systematically searched the MEDLINE, EMBASE, and Cochrane Central Register of Controlled Trials (CENTRAL) via OVID from study inception until September 26, 2020 using subject term and free keywords such as metformin XR and long-acting metformin. The supplementary material shows the details of the searching strategy. These searches were supplemented by a search of Clinicaltrials.gov for trials that were complete but not yet published. For duplicate results and studies with overlapping populations, only one report was included. When choosing which to include, we selected the one with the longest follow-up duration or the one with the largest population in cases where the follow-up duration was equal. The search strategy is shown in the supplementary information.
Eligibility Criteria
Searches were limited to RCTs and observational studies comparing long-acting metformin (including metformin XR, controlled release, and sustained release) with metformin IR in adults with type 2 diabetes. We included studies published in English that had a follow-up duration of at least 3 months (or 12 weeks) and reported at least one of the outcomes of interest.
The following studies were excluded: cross-sectional surveys, case series, and case reports; studies in which patients were treated with systematically different doses in the XR and IR groups; and studies in pregnant women.
Outcomes
Outcomes of interest were change from baseline in glycated hemoglobin (HbA1c), fasting plasma glucose (FPG), and postprandial blood glucose. Safety data including all-cause death, number of overall adverse events, adverse events leading to discontinuation, severe adverse events, and gastrointestinal adverse events were included, as were type of gastrointestinal event including vomiting, nausea, abdominal pain, flatulence, and diarrhea. Data on compliance to treatment and patient satisfaction were also included.
Study Selection
Two reviewers (JT and YW) screened the titles and abstracts of publications obtained in the literature search and independently selected the relevant publications. Full-text versions of the remaining publications were assessed for eligibility according to the prespecified eligibility criteria. Discrepancies were addressed through discussion between the two reviewers or, if necessary, by consensus with a third reviewer (SL).
Data Extraction
The following information was collected from included studies: 1) study characteristics and publication details, including first author name, year of publication, study design, region, sample size in each arm, and median follow-up duration; 2) baseline characteristics, including age, sex, body mass index (BMI), HbA1c level, and fasting glucose level; 3) preparation, dose, intervention, and control; and 4) outcomes of interest as stated above.
If a published study did not report the outcome information but the corresponding registry report from ClinicalTrials.gov included the relevant data, data from the registry report were used. The unit of the blood glucose level was expressed in mmol/L; data expressed as mg/dL were converted into mmol/L using the equation 1 mmol/L = 18 mg/dL.
Risk of Bias Assessment
Two reviewers (JT and YW) assessed the risk of bias using the version 2 of the Cochrane risk-of-bias tool for randomized trials (ROB 2) and the Newcastle-Ottawa Scale (NOS) for observational studies, and discrepancies were resolved by discussion with a third reviewer (SL) (Higgins et al., 2011). The ROB 2 tool for RCTs covers five types of bias, including randomization, deviations, missing outcome, measurement of the outcome, and selection of the report bias. The NOS consists of eight items to assess the risk of bias in cohort studies (Guyatt et al., 2008).
Data Synthesis and Statistical Analysis
We evaluated appropriateness for quantitative synthesis for the homogeneity of outcome reporting in the present analysis. For cohort studies, crude and fully adjusted effects were pooled in the quantitative synthesis.
All dichotomous outcomes were measured using relative risks (RRs) in RCTs. We used mean differences (MDs) for continuous outcomes of change from baseline. Gastrointestinal events at the study level were described using both event numbers and proportions, and were pooled when homogeneous. All pooled outcomes were measured using point estimates and corresponding 95% confidence intervals (CIs).
A random effects model using the Mantel-Haenszel method was used to pool study-level effects for RCTs. When the studies involved zero event in one of two groups, the 0.5 correction was used for both groups. We set zero weight for the studies with zero event in both groups in the analysis. Statistical heterogeneity was tested using a χ2 test and was quantified as I2 statistics. Heterogeneity was considered for a p value <0.1 or I2 > 50%.
Subgroup analyses were performed according to the following hypotheses: 1) for duration of follow-up (shorter than 24 vs. 24 weeks or longer): larger effects were hypothesized in studies with longer duration, and 2) risk of bias (high vs. low): larger effects were hypothesized in trials with high risk of bias (with one or more high-risk item).
We used the Grading of Recommendation, Assessment, Development, and Evaluation (GRADE) to assess the quality of evidence (Sun et al., 2012). Publication bias was assessed using funnel plots, Begg’s rank correlations, and Egger’s linear regression in cases where at least 10 studies were available. All data analyses were performed using RStudio (R Pack 3.6.1).
Results
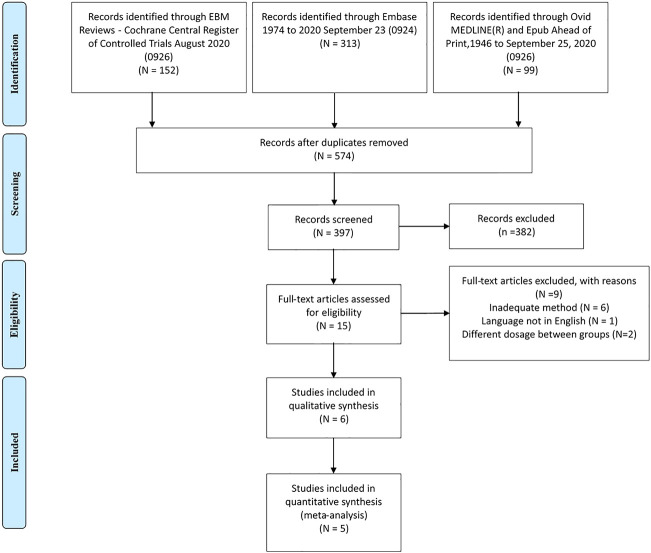
As shown in Figure 1, we included five RCTs comprising 1,662 patients (Schwartz et al., 2006; Gao et al., 2008; Hameed et al., 2017; Aggarwal et al., 2018; Ji et al., 2018) and one observational study from the 574 studies identified during the literature search (Donnelly et al., 2009). The characteristics of included randomized control trials are shown in Table 1, and the baseline data of patients in these studies are shown in Supplementary Table S1. One of the included trials was a global multicenter study, and two of the trials were conducted in China. The observational study was conducted retrospectively using electronic medical records in Scotland. The follow-up duration in the included RCTs ranged from 12 to 24 weeks, and the dose of metformin administered to patients ranged from 1,500 to 2000 mg/d. In the included RCTs, the average patient age was 54.39 years, the average BMI was 30.04 kg/m2, and 54.69% of patients were male. The mean baseline HbA1c and FPG values were 7.89 and 8.96 mmol/L, respectively.
FIGURE 1.
Flow diagram.
TABLE 1.
Characteristics of included randomized control trials.
| Trial | Location | Centers | Funding | Randomized (I/C) (n) | Follow-up | Duration of study treatment | Treatment | Control |
|---|---|---|---|---|---|---|---|---|
| Aggarwal 2017 | North America, Europe, South Africa | In 148 different sites (multicenter) | AstraZeneca | 283/285 a | 24 w | 24 w | Metformin XR 2000 mg QD | Metformin IR 1000 mg bid |
| Gao 2008 | China | 3 lefts in Beijing (multicenter) | National 973 Program of China (2006CB503908), the Natural Science Foundation of Beijing City (7062067) and the National Natural Science Foundation of China (30771032, 30700879) | 75/75 | 12 W | 12 W | 1,500 mg metformin XR once daily after the dinner | Metformin IR 500 mg thrice daily after meals |
| Hameed 2017 | Pakistan | Medical and endocrinology OPDs of Jinnah Hospital Lahore (single center) | NR | 30/30 | 12 W | 12 W | Metformin XR 1000 mg twice daily | Metformin IR 1000 mg twice daily |
| Ji 2017 | China | Multicenter | Merck Serono China Co. Ltd | 265/267 | 18 W | 16 W | Metformin extended-release (XR) tablets, orally QD (starting dose 500 mg, maximum treatment dose 2000 mg) | Metformin immediate release (IR) tablets, orally once daily (QD) (starting dose 500 mg, maximum treatment dose 2000 mg) |
| Schwartz 2006 | United States | 85 centers in United States (multicenter) | Depomed | 178/174 | 24 W | 24 W | 1,500 mg extended-release metformin QD | 1,500 mg immediate-release metformin twice daily (500 mg in the morning and 1,000 mg in the evening) |
29 randomized patients were excluded for study site noncompliance; thus, efficacy endpoints were analyzed in a smaller dataset (268/271).
QD, once a day; bid, twice a day; W, week; XR, extended release; IR, immediate release.
The observational study included 137 patients who were receiving metformin XR at the beginning of the study and 10,772 who had been prescribed metformin IR at the beginning of the study. The mean age of the cohort was 62.7 years, and 53% of patients were male.
Risk of Bias
The risk of bias in the included RCTs is shown in Supplementary Figures S1, S2. Three trials had an open-label design and were evaluated as having a high risk of bias on measurement of the outcome because of insufficient blinding of participants, and the outcome assessments were based on personal judgment (Gao et al., 2008; Hameed et al., 2017; Ji et al., 2018). Furthermore, one trial was rated high risk on deviation bias in the effect of assignment to intervention (Gao et al., 2008).
The observational study had a score of six on the NOS. It lost two stars due to unclear follow-up duration and inadequate adjustment of potential confounders (Donnelly et al., 2009).
Meta-Analysis of Randomized Control Trials
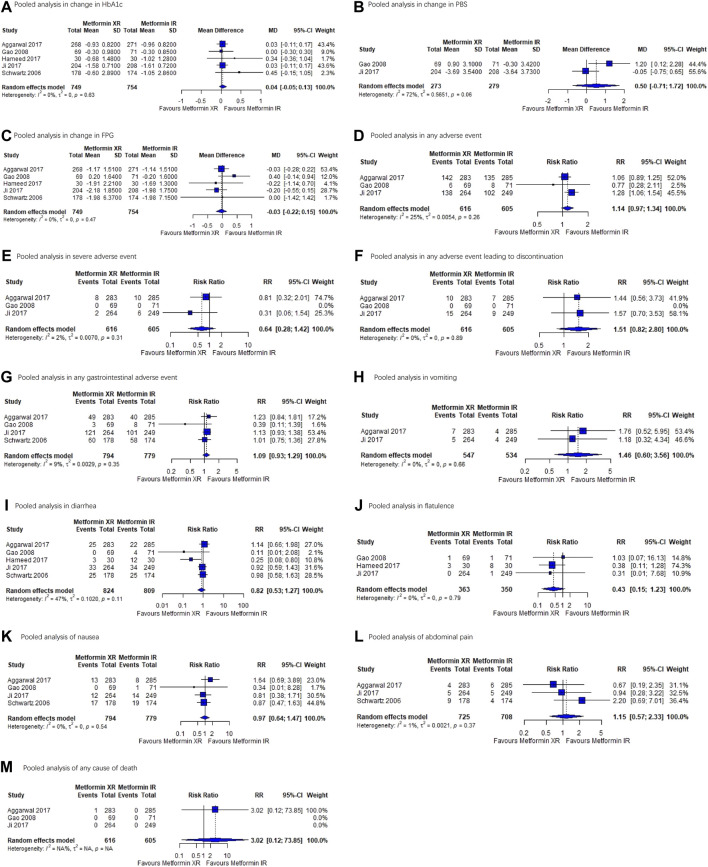
The findings of the quantitative analyses are summarized in Table 2. There was very low– to moderate-level evidence for a lack of difference between metformin XR and IR in adverse events of abdominal pain (RR, 1.15, 95% CI 0.57–2.33), all-cause death (RR, 3.02, 95% CI 0.12–73.85), any adverse events (RR, 1.14, 95% CI 0.97-1.34), any adverse events leading to discontinuation (RR, 1.51, 95% CI 0.82–2.8), any gastrointestinal adverse events (RR, 1.09, 95% CI 0.93–1.29), diarrhea (RR, 0.82, 95% CI 0.53–1.27), flatulence (RR, 0.43, 95% CI 0.15–1.23), nausea (RR, 0.97, 95% CI 0.64–1.47), severe adverse events (RR, 0.64, 95% CI 0.28–1.42), and vomiting (RR, 1.46, 95% CI 0.6–3.56). In the pooled analysis, no statistical difference was observed in change in HbA1c (MD 0.04%, 95% CI −0.05–0.13%), postprandial blood glucose (MD 0.50 mmol/L, 95% CI −0.71–1.72 mmol/L), and FPG (−0.03 mmol/L, 95% CI −0.22–0.15 mmol/L). Forest plots of all endpoints are shown in Figures 2A–M. Our study did not identify any subgroup effects among the outcomes (Supplementary Tables S2, S3).
TABLE 2.
Summary of findings.
| Outcomes | Number of studies | Number of participants | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Others | Event rate in the metformin XR arm | Event rate in the metformin IR arm | Relative risks/mean difference | Quality of evidence |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abdominal pain | 3 | 1,433 | RCTs | Serious risk of bias | No serious inconsistency | No serious indirectness | No serious imprecision | None | 18/725 (2.5%) | 15/708 (2.1%) | 1.15 (0.57–2.33) | Moderate |
| All-cause death | 3 | 1,221 | RCTs | Serious risk of bias | No serious inconsistency | No serious indirectness | Very serious imprecision a | None | 1/616 (0.2%) | 0/605 (0%) | 3.02 (0.12–73.85) | Very low |
| Any adverse events | 3 | 1,221 | RCTs | Serious risk of bias | No serious inconsistency | Serious indirectness b | Serious imprecision | None | 286/616 (46.4%) | 245/605 (40.5%) | 1.14 (0.97–1.34) | Very low |
| Any adverse events leading to discontinuation | 3 | 1,221 | RCTs | Serious risk of bias | No serious inconsistency | Serious indirectness b | No serious imprecision | None | 25/616 (4.1%) | 16/605 (2.6%) | 1.51 (0.82–2.8) | Low |
| Any gastrointestinal adverse events | 4 | 1,573 | RCTs | Serious risk of bias | No serious inconsistency | No serious indirectness | No serious imprecision | None | 233/794 (29.3%) | 207/779 (26.6%) | 1.09 (0.93–1.29) | Moderate |
| Diarrhea | 5 | 1,633 | RCTs | Serious risk of bias | No serious inconsistency | No serious indirectness | No serious imprecision | None | 86/824 (10.4%) | 97/809 (12%) | 0.82 (0.53–1.27) | Moderate |
| Flatulence | 3 | 713 | RCTs | Serious risk of bias | No serious inconsistency | No serious indirectness | No serious imprecision | None | 4/363 (1.1%) | 10/350 (2.9%) | 0.43 (0.15–1.23) | Moderate |
| Nausea | 4 | 1,573 | RCTs | Serious risk of bias | No serious inconsistency | No serious indirectness | No serious imprecision | None | 42/794 (5.3%) | 42/779 (5.4%) | 0.97 (0.64–1.47) | Moderate |
| Severe adverse events | 3 | 1,221 | RCTs | Serious risk of bias | No serious inconsistency | Serious indirectness b | No serious imprecision | None | 10/616 (1.6%) | 16/605 (2.6%) | 0.64 (0.28–1.42) | Low |
| Vomiting | 2 | 1,081 | RCTs | Serious risk of bias | No serious inconsistency | No serious indirectness | No serious imprecision | None | 12/547 (2.2%) | 8/534 (1.5%) | 1.46 (0.6–3.56) | Moderate |
| Change in HbA1c | 5 | 1,503 | RCTs | No serious risk of bias | No serious inconsistency | Serious indirectness b | No serious imprecision | None | — | — | 0.04 (−0.05–0.13) | Moderate |
| Change in PBS (mmol/L) | 2 | 552 | RCTs | No serious risk of bias | No serious inconsistency | Serious indirectness b | No serious imprecision | None | — | — | 0.50 (−0.71–1.72) | Moderate |
| Change in FPG (mmol/L) | 5 | 1,503 | RCTs | No serious risk of bias | No serious inconsistency | Serious indirectness b | No serious imprecision | None | — | — | −0.03 (−0.22–0.15) | Moderate |
RCT, randomized control trial; NA, not available; HbA1c, hemoglobin A1C; PBS, postprandial blood sugar; FPG, fasting plasma glucose; XR, extended release; IR, immediate release.
Imprecision of evidence was downgraded due to wide 95% CI which could not support clinical decision-making.
Indirectness of evidence was downgraded due to composite outcomes or surrogate outcomes which is indirect to the patients.
FIGURE 2.
Forest plots for each outcome. (A) Pooled analysis in change in HbA1c. (B) Pooled analysis in PBS. (C) Pooled analysis in FPG. (D) Pooled analysis in any adverse event. (E) Pooled analysis in severe adverse event. (F) Pooled analysis in adverse effect leading to discontinuation. (G) Pooled analysis in any gastrointestinal adverse event. (H) Pooled analysis in vomiting. (I) Pooled analysis in diarrhea. (J) Pooled analysis of flatulence. (K) Pooled analysis of nausea. (L) Pooled analysis of abdominal pain. (M) Pooled analysis of any cause of death. Abbreviations: HbA1c, hemoglobin A1C; PBS, postprandial blood glucose; FPG, fasting plasma glucose.
Indirectness of evidence was downgraded for any adverse events, any adverse events leading to discontinuation, severe adverse events, change in HbA1c, postprandial blood glucose, and FPG due to the indirectness of composite outcomes or surrogate outcomes. Furthermore, imprecision of evidence was downgraded for all-cause death and any adverse events because of wide 95% CIs that could not be used to support clinical decision-making.
Compliance and Satisfaction
Three of the included RCTs investigated patient compliance to treatment. Quantitative analysis of these assessments was not performed because of heterogeneity in the measurements. In an RCT conducted in 268 patients in 2017, one patient in the metformin XR group discontinued treatment because of noncompliance, whereas no patients discontinued in the metformin IR group (Aggarwal et al., 2018). An RCT conducted in 2008 reported that 97.2% of tablets were taken in the metformin XR group and 93.8% of tablets were taken in the metformin IR group (Gao et al., 2008); however, the investigators did not perform a statistical analysis in that study. Another RCT conducted in 2018 reported that 255 of 264 (96.6%) and 247 of 261 (94.6%) patients achieved good treatment compliance in the metformin XR and metformin IR groups, respectively (Ji et al., 2018). In the included observational study, compliance to metformin XR (80%) was significantly higher than that observed for metformin IR (72%). Patient compliance markedly increased after changing from metformin IR (62%) to metformin XR (81%) (Donnelly et al., 2009). None of the included studies compared patient satisfaction with metformin XR vs. metformin IR.
Discussion
Our systematic review did not identify clear differences in the effectiveness and safety of metformin XR vs. metformin IR with very low to moderate certainty, although metformin XR use was more likely to be associated with treatment compliance. To the best of our knowledge, this is the first systematic review to compare the IR and XR preparations of metformin.
Although both metformin IR and XR are widely used in clinical practice, the rationale for choosing one formulation over the other has not been widely examined. Guidance on the differential use of metformin XR vs. IR is largely absent in clinical practice guidelines for diabetes treatment, with the exception of the NICE guideline, which recommends the use of metformin XR in patients intolerant to metformin IR (McGuire et al., 2016). However, the NICE guideline recommendation is not supported by any specific evidence. Although the findings of some previous studies are in alignment with the NICE recommendation, the results from those studies are not conclusive (Derosa et al., 2017; Henry et al., 2018). In one such RCT, a reduced risk of gastrointestinal intolerance was observed with metformin XR treatment. In that study, the metformin XR dose was systematically lower than that of metformin IR, and serum metformin levels in patients receiving metformin XR were almost 50% less than those in patients receiving metformin IR (Henry et al., 2018). Although the glucose-lowering effect of metformin XR was inferior to that of metformin IR, the results of this trial, in which the ratio of the glucose level decrease to the mean serum level of metformin was calculated, indicate the superior efficacy of metformin XR (Henry et al., 2018). A study conducted in Italy also supported the superior glucose-lowering effect of metformin XR at the maximal tolerated dose compared with metformin IR (Derosa et al., 2017). Of note, metformin XR was associated with significantly fewer adverse events than metformin IR at a 50% lower average maintenance dose (XR 1000 ± 500 mg vs. IR 2000 ± 1,000 mg), meaning that most patients receiving metformin XR stopped up-titration before reaching the maximal dose (Derosa et al., 2017). However, the data from these trials did not clarify whether the superior tolerance observed for metformin XR was attributable to the lower dose. Neither of these trials was included in our systematic review because both featured different doses of metformin XR and IR between the treatment arms.
Our findings support similar effectiveness and safety of metformin XR and IR, indicating that it might not be appropriate to switch from metformin IR to metformin XR for the purpose of improving glucose control or reducing adverse events. Our results highlight the need for a change in clinical practice with respect to the selection of metformin preparation.
Our findings also indicate better compliance with metformin XR treatment due to its once-daily dosing regimen. The results were consistent across the included randomized trials and observational studies, regardless of the population heterogeneity compared with real-world practice (Zhou et al., 2021). We inferred that as the number of doses increases, patients are increasingly likely to miss a dose, leading to noncompliance (Godman et al., 2020). This observation is not unique to metformin therapy in diabetes but is also observed in the treatment of other chronic diseases (Shahiwala, 2011). A 2015 meta-analysis indicated that once-daily administration of antibiotics was associated with better compliance than twice- or thrice-daily treatment (Falagas et al., 2015). Another meta-analysis of antiviral treatment of HIV reported that compliance to a once-daily regimen was slightly better than that to a twice-daily regimen (Nachega et al., 2014). Further, a pilot study among 110 patients reported that once-daily dosage regimens were largely preferred by patients (Witticke et al., 2012). Therefore, clinicians should consider patient preference when deciding on the type of formulation to prescribe and should consider metformin XR for patients who prefer once-daily dosing. The selection of metformin preparation thus represents a good example of patient-centered care, where shared decision-making can be beneficial. Clinicians should also consider which patients will benefit most from once-daily administration. For example, adults in full-time employment may benefit from less frequent dosing, while it may not be necessary to prescribe metformin XR to patients with polypharmacy.
Our previous survey study indicated that most outpatients in China did not have an accurate understanding of why they were receiving the XR formulation of metformin (Liu et al., 2021). The majority (81.2%) believed that metformin XR was more effective and tolerable than metformin IR (Liu et al., 2021). This finding when considered alongside the results of the current systematic review is indicative of shortcomings in the dissemination of evidence to the patients and, potentially, to clinicians. The findings of our systematic review support the education of patients regarding the use of antidiabetic medications.
Our study had some limitations. First, we included only five randomized trials and one observational study; however, the overall sample size of more than 10,000 patients supported the robustness of our results. Second, long-term endpoints were not identified for metformin XR and IR use. Further long-term observational studies are needed to confirm the present findings.
In conclusion, although metformin XR and IR formulations have similar effectiveness and safety, metformin XR is associated with increased treatment compliance. These findings require dissemination to patients and clinicians, and long-term observation of the use of these two formulations is warranted.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
Author Contributions
SL conceived this study. JT and YW performed the literature search, screening, and data extraction. JT, YW, and QS performed the risk of bias assessment. JT, YW, XZ, and YZ performed the statistical analysis. JT, YW, XY, PC, and SL drafted the manuscript. All authors contributed to data analysis and drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This study was supported by grants from the Sichuan Science and Technology Program (grant numbers 2019YFS0305 and 2019YFH0150), and 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (grant numbers ZYGD18022 and 2020HXF011).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.669814/full#supplementary-material
References
- Aggarwal N., Singla A., Mathieu C., Montanya E., Pfeiffer A. F. H., Johnsson E., et al. (2018). Metformin Extended‐release versus Immediate‐release: A N International, Randomized, Double‐blind, Head‐to‐head Trial in Pharmacotherapy‐naïve Patients with Type 2 Diabetes. Diabetes Obes. Metab. 20 (2), 463–467. 10.1111/dom.13104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amod A. (2012). The 2012 SEMDSA Guideline for the Management of Type 2 Diabetes. J. Endocrinol. Metab. Diabetes South Africa 17, 61–62. 10.1080/22201009.2012.10872276 [DOI] [Google Scholar]
- Blonde L., Dailey G. E., Jabbour S. A., Reasner C. A., Mills D. J. (2004). Gastrointestinal Tolerability of Extended-Release Metformin Tablets Compared to Immediate-Release Metformin Tablets: Results of a Retrospective Cohort Study. Curr. Med. Res. Opin. 20 (4), 565–572. 10.1185/030079904125003278 [DOI] [PubMed] [Google Scholar]
- Derosa G., D'Angelo A., Romano D., Maffioli P. (2017). Effects of Metformin Extended Release Compared to Immediate Release Formula on Glycemic Control and Glycemic Variability in Patients with Type 2 Diabetes. Dddt 11, 1481–1488. 10.2147/DDDT.S131670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly L. A., Morris A. D., Pearson E. R. (2009). Adherence in Patients Transferred from Immediate Release Metformin to a Sustained Release Formulation: a Population-Based Study. Diabetes Obes. Metab. 11 (4), 338–342. 10.1111/j.1463-1326.2008.00973.x [DOI] [PubMed] [Google Scholar]
- Falagas M. E., Karagiannis A. K. A., Nakouti T., Tansarli G. S. (2015). Compliance with Once-Daily versus Twice or Thrice-Daily Administration of Antibiotic Regimens: a Meta-Analysis of Randomized Controlled Trials. PLoS One 10 (1), e0116207. 10.1371/journal.pone.0116207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foretz M., Guigas B., Bertrand L., Pollak M., Viollet B. (2014). Metformin: from Mechanisms of Action to Therapies. Cel Metab. 20 (6), 953–966. 10.1016/j.cmet.2014.09.018 [DOI] [PubMed] [Google Scholar]
- Fujioka K., Pans M., Joyal S. (2003). Glycemic Control in Patients with Type 2 Diabetes Mellitus Switched from Twice-Daily Immediate-Release Metformin to a Once-Daily Extended-Release Formulation. Clin. Ther. 25 (2), 515–529. 10.1016/s0149-2918(03)80093-0 [DOI] [PubMed] [Google Scholar]
- Gao H., Xiao W., Wang C., Zhang J., Yang Y., Yang J., et al. (2008). The Metabolic Effects of once Daily Extended-Release Metformin in Patients with Type 2 Diabetes: a Multicentre Study. Int. J. Clin. Pract. 62 (5), 695–700. 10.1111/j.1742-1241.2008.01733.x [DOI] [PubMed] [Google Scholar]
- Godman B., McCabe H., D Leong T., Mueller D., Martin A. P., Hoxha I., et al. (2020). Fixed Dose Drug Combinations - Are They Pharmacoeconomically Sound? Findings and Implications Especially for Lower- and Middle-Income Countries. Expert Rev. Pharmacoeconomics Outcomes Res. 20 (1), 1–26. Epub 2020 Apr 1. 10.1080/14737167.2020.1734456 [DOI] [PubMed] [Google Scholar]
- Guyatt G. H., Oxman A. D., Vist G. E., Kunz R., Falck-Ytter Y., Alonso-Coello P., et al. (2008). GRADE: an Emerging Consensus on Rating Quality of Evidence and Strength of Recommendations. BMJ 336 (7650), 924–926. 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hameed M., Khan K., Salman S., Mehmood N. (2017). Dose Comparison and Side Effect Profile of Metformin Extended Release versus Metformin Immediate Release. J. Ayub Med. Coll. Abbottabad 29 (2), 225–229. [PubMed] [Google Scholar]
- Henry R. R., Frias J. P., Walsh B., Skare S., Hemming J., Burns C., et al. (2018). Improved Glycemic Control with Minimal Systemic Metformin Exposure: Effects of Metformin Delayed-Release (Metformin DR) Targeting the Lower Bowel over 16 Weeks in a Randomized Trial in Subjects with Type 2 Diabetes. PLoS One 13 (9), e0203946. 10.1371/journal.pone.0203946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J. P. T., Altman D. G., Gotzsche P. C., Juni P., Moher D., Oxman A. D., et al. (2011). The Cochrane Collaboration's Tool for Assessing Risk of Bias in Randomised Trials. BMJ 343, d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji L., Liu J., Yang J., Li Y., Liang L., Zhu D., et al. (2018). Comparative Effectiveness of Metformin Monotherapy in Extended Release and Immediate Release Formulations for the Treatment of Type 2 Diabetes in Treatment-Naïve Chinese Patients: Analysis of Results from the CONSENT Trial. Diabetes Obes. Metab. 20 (4), 1006–1013. 10.1111/dom.13190 [DOI] [PubMed] [Google Scholar]
- Liu C., Zhou Y. L., Wang Y., et al. (2021). Knowledge, Attitude and Use of Metformin Hydrochloride Sustained Release Tablets in Outpatients with Type 2 Diabetes Mellitus. Chin. Gen Pract. [Epub ahead of print]. 10.12114/j.issn.1007-9572.2021.00.436 [DOI] [Google Scholar]
- Liu Q., Li S., Quan H., Li J. (2014). Vitamin B12 Status in Metformin Treated Patients: Systematic Review. PLoS One 9 (6), e100379. 10.1371/journal.pone.0100379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire H., Longson D., Adler A., Farmer A., Lewin I. Guideline Development Group (2016). Management of Type 2 Diabetes in Adults: Summary of Updated NICE Guidance. BMJ 353, i1575. 10.1136/bmj.i1575 [DOI] [PubMed] [Google Scholar]
- Moher D., Altman D. G., Liberati A., Tetzlaff J. (2011). PRISMA Statement. Epidemiology 22 (1), 128. 10.1097/EDE.0b013e3181fe7825 [DOI] [PubMed] [Google Scholar]
- Nachega J. B., Parienti J.-J., Uthman O. A., Gross R., Dowdy D. W., Sax P. E., et al. (2014). Lower Pill Burden and Once-Daily Antiretroviral Treatment Regimens for HIV Infection: A Meta-Analysis of Randomized Controlled Trials. Clin. Infect. Dis. 58 (9), 1297–1307. 10.1093/cid/ciu046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Collaborating Centre for Chronic Conditions (UK) (2008). Type 2 Diabetes: National Clinical Guideline for Management in Primary and Secondary Care (Update). LondonUK): Royal College of Physicians. [PubMed] [Google Scholar]
- Reinstatler L., Qi Y. P., Williamson R. S., Garn J. V., Oakley G. P. (2012). Association of Biochemical B12 Deficiency with Metformin Therapy and Vitamin B12 Supplements: The National Health and Nutrition Examination Survey, 1999-2006. Diabetes Care 35 (2), 327–333. 10.2337/dc11-1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth G. A., Abate D., Abate K. H.., Abay S. M., Abbafati C., Abbasi N., et al. (2018). Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 392 (10159), 1736–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S., Fonseca V., Berner B., Cramer M., Chiang Y.-K., Lewin A. (2006). Efficacy, Tolerability, and Safety of a Novel Once-Daily Extended-Release Metformin in Patients with Type 2 Diabetes. Diabetes Care 29 (4), 759–764. 10.2337/diacare.29.04.06.dc05-1967 [DOI] [PubMed] [Google Scholar]
- Shahiwala A. (2011). Formulation Approaches in Enhancement of Patient Compliance to Oral Drug Therapy. Expert Opin. Drug Deliv. 8 (11), 1521–1529. 10.1517/17425247.2011.628311 Epub 2011 Oct 13. [DOI] [PubMed] [Google Scholar]
- Sun X., Briel M., Busse J. W., You J. J., Akl E. A., Mejza F., et al. (2012). Credibility of Claims of Subgroup Effects in Randomised Controlled Trials: Systematic Review. BMJ 344, e1553. 10.1136/bmj.e1553 [DOI] [PubMed] [Google Scholar]
- Witticke D., Seidling H. M., Klimm H. D., Haefeli W. E. (2012). Do we Prescribe what Patients Prefer? Pilot Study to Assess Patient Preferences for Medication Regimen Characteristics. Patient Prefer Adherence 6, 679–684. 10.2147/PPA.S35950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Li S., Li L., Li Q., Ren K., Sun X., et al. (2016). Metformin Treatment and Homocysteine: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 8 (12), 798. 10.3390/nu8120798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Ley S. H., Hu F. B. (2018). Global Aetiology and Epidemiology of Type 2 Diabetes Mellitus and its Complications. Nat. Rev. Endocrinol. 14 (2), 88–98. 10.1038/nrendo.2017.151 [DOI] [PubMed] [Google Scholar]
- Zhou Y.-L., Zhang Y.-G., Zhang R., Zhou Y.-L., Li N., Wang M.-Y., et al. (2021). Population Diversity of Cardiovascular Outcome Trials and Real-World Patients with Diabetes in a Chinese Tertiary Hospital. Chin. Med. J. [Epub ahead of print]. 10.1097/CM9.0000000000001407 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.