Abstract
It is very challenging to visualize implantable medical devices made of biodegradable polymers in deep tissues. Herein, we designed a novel macromolecular contrast agent with ultrahigh radiopacity (iodinate content > 50%) via polymerizing an iodinated trimethylene carbonate monomer into the two ends of poly(ethylene glycol) (PEG). A set of thermosensitive and biodegradable polyester-PEG-polyester triblock copolymers with varied polyester compositions synthesized by us, which were soluble in water at room temperature and could spontaneously form hydrogels at body temperature, were selected as the demonstration materials. The addition of macromolecular contrast agent did not obviously compromise the injectability and thermogelation properties of polymeric hydrogels, but conferred them with excellent X-ray opacity, enabling visualization of the hydrogels at clinically relevant depths through X-ray fluoroscopy or Micro-CT. In a mouse model, the 3D morphology of the radiopaque hydrogels after injection into different target sites was visible using Micro-CT imaging, and their injection volume could be accurately obtained. Furthermore, the subcutaneous degradation process of a radiopaque hydrogel could be non-invasively monitored in a real-time and quantitative manner. In particular, the corrected degradation curve based on Micro-CT imaging well matched with the degradation profile of virgin polymer hydrogel determined by the gravimetric method. These findings indicate that the macromolecular contrast agent has good universality for the construction of various radiopaque polymer hydrogels, and can nondestructively trace and quantify their degradation in vivo. Meanwhile, the present methodology developed by us affords a platform technology for deep tissue imaging of polymeric materials.
Keywords: Thermosensitive hydrogels, Radiopacity, Block copolymers, In vivo degradation, Non-invasive deep tissue imaging
Graphical abstract
Highlights
-
•
A universal macromolecular contrast agent with ultrahigh radiopacity was prepared for construction of radiopaque hydrogels.
-
•
The location, morphology and volume of the hydrogels in deep tissues could be non-invasively achieved by CT imaging.
-
•
The degradation curve of radiopaque hydrogel confirmed by Micro-CT could truly reflect the in vivo fate of virgin hydrogel.
-
•
The present methodology is easily extended to other polymer devices for in vivo monitoring at clinically relevant depths.
1. Introduction
Non-invasive in vivo bioimaging techniques have emerged a vital impact on modern medical practice and greatly promote advances in a variety of biomedical applications, such as in vivo cell labeling, early disease diagnosis, image-guided treatment and post-operative medical devices follow-up [[1], [2], [3], [4]]. In particular, X-ray fluoroscopy has been utilized in clinic for more than a century and has the advantages of low-cost, high spatial resolution and unrestricted depth of tissue penetration [5,6]. X-ray computed tomography (CT) imaging is also based on the absorption of X-rays and has the ability to visualize three dimensional (3D) morphology of implanted objects/devices [5,7].
Since the beginning of the new century, many permanent implantable devices for various disease treatments have been gradually replaced by biodegradable devices made of polymeric materials [[8], [9], [10], [11]]. Nevertheless, polymeric materials are usually transparent to X-rays due to the atomistic composition of C, H, O, and N atoms. To nondestructively locate these devices/materials, trace morphology changes, and quantify the in situ degradation profiles, contrast agents are generally required.
One conventional approach to endow polymers with X-ray opacity is to blend X-ray absorbing additives, such as metal powders, barium salts, zirconium dioxide, organic iodine compounds, etc [[12], [13], [14], [15]]. However, this method suffers from some important defects: (1) the physical, chemical and mechanical properties of polymeric materials are often influenced due to the incorporation of fillers [15]; (2) it is not easy to blend small-molecule contrast agents evenly with polymers; (3) small-molecule contrast agents easily leach and penetrate into body fluids, resulting in the loss of X-ray opacity and causing systemic toxicities [7,13,14]. An attractive alternative is to develop macromolecular radiographic contrast agents by covalently modifying a radiocontrast probe, typically an iodine-containing moiety, to the polymer skeleton, thereby overcoming the drawbacks of small-molecule contrast agents [6,16,17].
In-situ-forming injectable hydrogels as implantable biomaterials/devices have attracted tremendous attention because of their minimally invasive administration mode [[18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30]]. In particular, thermosensitive and biodegradable hydrogels comprised of copolymers of hydrophobic polyesters, such as poly(lactic acid) (PLA), poly(ε-caprolactone) (PCL), poly(lactic acid-co-glycolic acid) (PLGA), poly(ε-caprolactone-co-glycolic acid) (PCGA), poly(ε-caprolactone-co-lactic acid) (PCLA), and hydrophilic poly(ethylene glycol) (PEG) are free-flowing polymer aqueous solutions at low or ambient temperature; however, they exhibit sol-gel transitions in response to the changes in temperature [[31], [32], [33], [34], [35], [36]]. Bioactive substances and live cells are then easily loaded by blending them with a low viscous polymer solution and this loading process thoroughly avoids contact with organic solvents. After injection into the target site of a warmblooded animal, a substance/cell-containing hydrogel is spontaneously formed via the temperature-triggered gelation, acting as a reservoir of drugs or a cell-growing matrix. Meanwhile, in-situ gelation also affords a perfect match for filling a complex defect. In virtue of the characteristics of facile synthesis, good biocompatibility and adjustable biodegradability, thermosensitive PEG/polyester copolymer hydrogels have suggested for various applications in biomedical fields, such as drug delivery [35,[37], [38], [39], [40], [41], [42]], wound repair [43], tissue regeneration [44,45] and post-surgical anti-adhesion [34,46].
As a kind of implantable and biodegradable biomaterials, nondestructive and real-time monitoring of thermosensitive hydrogels at the injection sites as well as acquirement of information on in vivo behaviors of degradation are of significance for their fundamental research and following clinical translation. However, there are few reports on the PEG/polyester thermosensitive hydrogels for X-ray imaging modalities so far. The first PEG/polyester polymer thermosensitive hydrogel with X-ray opacity was developed by our group [47], where 2,3,5-triiodobenzoic acid (TIB) as a radiocontrast dye was covalently modified to the hydrophobic end of methoxy poly(ethylene glycol) (mPEG)-PLA diblock copolymer and the aqueous solution of TIB-capped mPEG-PLA with a very narrow PEG/PLA ratio window could form a temperature-responsive hydrogel. This methodology could not be employed to obtain other types of radiopaque and thermosensitive hydrogels. For example, temperature-responsive hydrogels composed of ABA-type polyester-PEG-polyester or BAB-type mPEG-polyester-mPEG triblock copolymers are more popular compared with thermosensitive mPEG-polyester diblock copolymers. However, due to thermosensitive polyester-PEG-polyester triblock copolymers featuring a subtle end-group effect [48,49] plus the very strong hydrophobicity of TIB group, their TIB-capped derivatives only precipitated in water and failed to form a thermosensitive hydrogel [47]. As to thermosensitive mPEG-polyester-mPEG triblock copolymers, TIB cannot be covalently modified due to the lack of reactive groups.
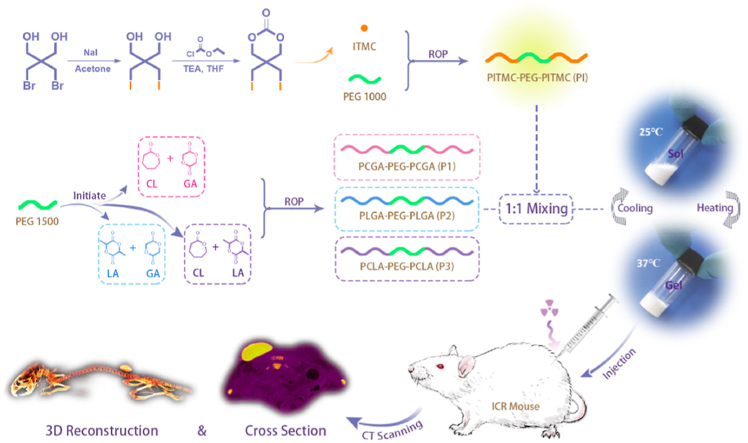
Is there a method free of post-modification? We have taken this into account and made efforts to address this challenge. In this study, we designed an iodinated polycarbonate-based universal macromolecular contrast agent, poly(5,5-bis(iodomethyl)-1,3-dioxan-2-one)-co-poly(ethylene glycol)-co-poly(5,5-bis(iodomethyl)-1,3-dioxan-2-one) (PITMC-PEG-PITMC, PI), with ultrahigh radiopacity, as illustrated in Fig. 1. The molecular design of PI was mainly based on the following two considerations. First, since thermosensitive PEG/polyester copolymers that exhibit temperature-responsive sol-gel transitions in water are attributed to their amphiphilic nature [[50], [51], [52]], the macromolecular radio-opacifier should be amphiphilic to facilitate mixing with them to form a homogeneous system and maintain the capacity of thermogelation. Second, to ensure the adequate iodine content in macromolecular radio-opacifier, the molecule weight (MW) of PEG should not be very high and eventually PEG with MW 1000 Da was selected by us. Meanwhile, a set of thermosensitive hydrogels comprised of polyester-PEG-polyester polymers with different, but familiar polyester compositions were chosen as the demonstration materials to validate the universality of macromolecular contrast agent for the fabrication of radiopaque and thermosensitive hydrogels, as presented in Fig. 1. The effect of the PI introduction on their thermogelation properties was evaluated. The imaging capacity of radiopaque hydrogels in deep tissues was tested in vitro and in vivo. Finally, the feasibility to nondestructively monitor and quantify hydrogel degradation in vivo was demonstrated.
Fig. 1.
Schematic presentation of design of macromolecular contrast agent, construction of radiopaque and thermosensitive PEG/polyester copolymer hydrogels, and their nondestructive imaging in vivo via Micro-CT. CL: ε-caprolactone, GA: glycolide, ITMC: 5,5-bis(iodomethyl)-1,3-dioxan-2-one, LA: d,l-lactide, PEG: poly(ethylene glycol), ROP: ring-opening polymerization, TEA: triethylamine, THF: tetrahydrofuran.
2. Results and discussion
2.1. Synthesis and characterization of macromolecular contrast agent PI and polyester-PEG-polyester polymers
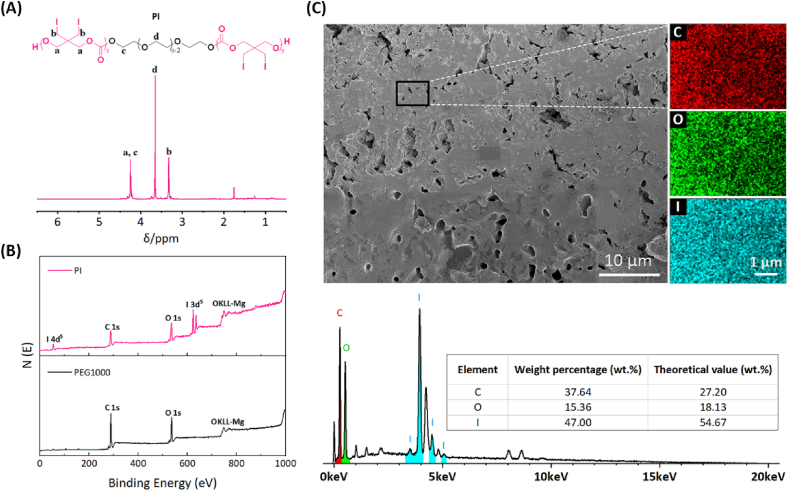
5,5-bis(iodomethyl)-1,3-dioxan-2-one (ITMC), a diiodinated carbonate monomer, was synthesized via iodine exchange reaction in the presence of 2,2-bis(bromomethyl)-1,3-propanediol and subsequently cyclization with ethyl chloroformate using triethylamine (TEA) as an acid scavenger, as presented in Fig. 1, and nuclear magnetic resonance hydrogen spectroscopy (1H NMR) analysis confirmed the successful acquirement of ITMC monomer (Fig. S1). Then, a novel macromolecular contrast agent PI with excellent radiopacity was obtained via initiating macromolecular initiator PEG1000 to conduct ring-opening polymerization (ROP) of ITMC in the presence of zinc bis[bis(trimethylsilyl)amide] (Zn(HMDS)2) as the catalyst. The chemical structure and composition of PI were analyzed by 1H NMR, X-ray photoelectron spectroscopy (XPS), energy-dispersive X-ray spectroscopy (EDS) and gel permeation chromatography (GPC). As displayed in Fig. 2A, the characteristic proton peaks belonging to PI were clearly assicribed, and the average number of ITMC repeating units in each PI calculated by its 1H NMR spectrum was 14.5. Namely, the iodine content in PI reached amazing 56.3 wt%, which is significantly superior to the known iodinated polyesters/polycarbonates [6,16,[53], [54], [55]], to the best of our knowledge. Different from PEG, except for the signals of carbon and oxygen elements, the strong signal of iodine element was also sucessfully detected on the surface of PI by XPS and EDS analysis (Fig. 2B and C and S2). The GPC trace of PI presented a unimodal pattern, as diplayed in Fig. S3. All these features demonstrated that the ROP was successfully conducted and the desired macromolecular contrast agent PI was harvested.
Fig. 2.
Characterization of PI, the novel macromolecular contrast agent. (A) 1H NMR spectrum of PI in CDCl3. (B) Full XPS survey spectra for PI and PEG1000. (C) SEM image, EDS distribution maps and analysis results (wt%) for carbon (red dots), oxygen (green dots) and iodine (blue dots) of PI.
Meanwhile, three thermosensitive PEG/polyester triblock copolymers, poly(ε-caprolactone-co-glycolic acid)-b-poly(ethylene glycol)-b-poly(ε-caprolactone-co-glycolic acid) (PCGA-PEG-PCGA, P1), poly(lactic acid-co-glycolic acid)-b-poly(ethylene glycol)-b-poly(lactic acid-co-glycolic acid) (PLGA-PEG-PLGA, P2), poly(ε-caprolactone-co-lactic acid)-b-poly(ethylene glycol)-b-poly(ε-caprolactone-co-lactic acid) (PCLA-PEG-PCLA, P3), were prepared by ROP of different monomers using stannous octoate as the catalyst and PEG1500 as the macroinitiator (Fig. 1). As shown in Fig. S4 and S5, the chemical compositions and structures of P1, P2 and P3 were confirmed by 1H NMR and GPC. Molecular parameters of PI, P1, P2 and P3 copolymers are summarized in Table 1.
Table 1.
Molecular parameters of PI, P1, P2 and P3 polymers in this study.
| Sample | Mna | [M1]/[M2]b | Mnc | ĐMc |
|---|---|---|---|---|
| PI | 2770-1000-2770 | – | 3870 | 1.64 |
| P1 | 1800-1500-1800 | 2.3/1 (CL/GA) | 5240 | 1.35 |
| P2 | 1790-1500-1790 | 1.9/1 (LA/GA) | 4270 | 1.24 |
| P3 | 1820-1500-1820 | 2.5/1 (CL/LA) | 5350 | 1.33 |
Mn calculated from 1H NMR.
Repeating unit ratio in mol/mol calculated from 1H NMR.
Mn and ĐM measured via GPC.
2.2. Sol-gel transitions of aqueous polymer solutions
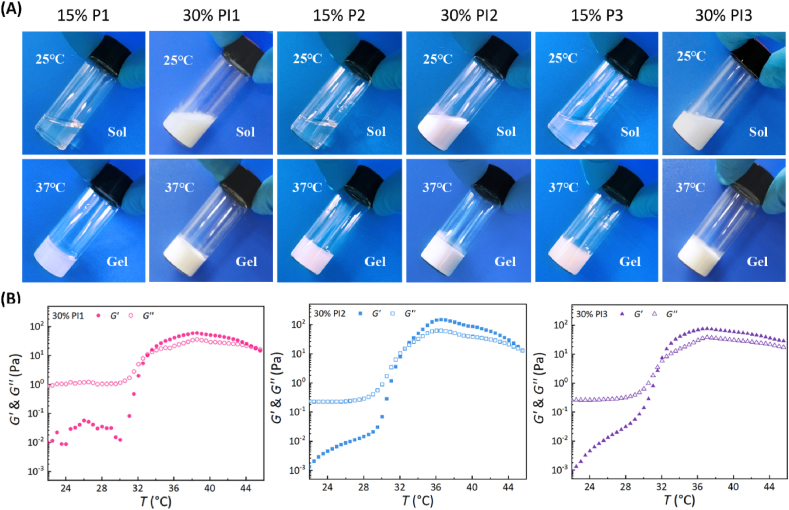
All the three triblock copolymers, P1, P2 and P3, synthesized by our group could be dissolved in water to form low viscous sols at low or room temperature and exhibited a state transformation of sol-to-gel with responding to the environmental temperature increase when the polymer concentration was higher than the critical gel concentration (> 5 wt%). As illustrated in Fig. 3a, the 15 wt% aqueous polymer solutions were transparent at room temperature and formed opaque gels at body temperature. Our previous studies and others have demonstrated that amphiphilic PEG/polyester copolymers easily self-assemble in water to form core-corona micelles, which have a good capacity for solubilization of hydrophobic small-molecules or macromolecules [37,38,40,44,50,51]. In fact, the macromolecular contrast agent PI was so hydrophobic that it was only partially soluble in water. Fortunately, the aqueous solutions of thermosensitive P1, P2 and P3 polymers with suspended core-corona-like micelles were able to significantly solubilize the hydrophobic PI. After solubilization, the PI-loaded aqueous polymer solutions containing 30 wt% solute turned into a milky white state, but they were still uniform and easy to flow at room temperature and formed semi-solid gels at 37 °C (Fig. 3A), indicating that the incorporation of PI did not compromise the sol-gel transitions of the polymer solutions.
Fig. 3.
Thermogelation behaviors of aqueous polymer solutions with or without the macromolecular contrast agent PI. (A) Photographs showing sol (25 °C) and gel (37 °C) states of the various aqueous polymer solutions. (B) Storage modulus G′ and loss modulus G″ of the aqueous polymer solutions containing PI as a function of temperature. PI1 means the 1/1 (w/w) mixture of PI and P1, and the same is for PI2 and PI3.
In order to further quantitatively evaluate the effect of PI introduction, changes in the storage modulus G′ and loss modulus G″ of aqueous polymer solutions in the presence or absence of PI were observed as a function of temperature (Fig. 3B and S6). G′ and G″ represent the elastic component and viscous component, respectively, of the system. Generally speaking, G' < G″ suggests the sol state of the system, while G' > G″ reflects the gel state [56,57]. Along with the elevation of temperature, both the G′ and G″ of aqueous polymer solutions gradually increased and eventually the G′ exceeded the G''. The temperature corresponding to the crossover point where G′ and G″ are equal has be considered as the sol-gel transition temperature (Tgel) [56,57]. As shown in Fig. S6, all the three PI-free polymer aqueous solutions formed gels at about 32 °C due to their similar MWs, while the Tgels of aqueous polymer solutions containing PI also remained around 32 °C (Fig. 3B). This finding further confirmed that the introduction of PI maintained the thermogelation properties of the virgin polymer solutions well.
2.3. In vitro X-ray opacity
First, to explore the relationship between iodine content and X-ray opacity, a series of aqueous solutions of iohexol, a commercially available iodine contrast agent, with indicated concentrations were prepared and then subjected to Micro-CT scanning. As shown in Fig. 4A, the grayscale indices and Hounsfield units of iohexol aqueous solutions were linearly positively correlated with the iodine content in the iohexol solution. The linear fitting equations were as follows:
| (1) |
| (2) |
where Y1 represents the grayscale index, Y2 means the Hounsfield unit, and x represents the iodine content (wt%).
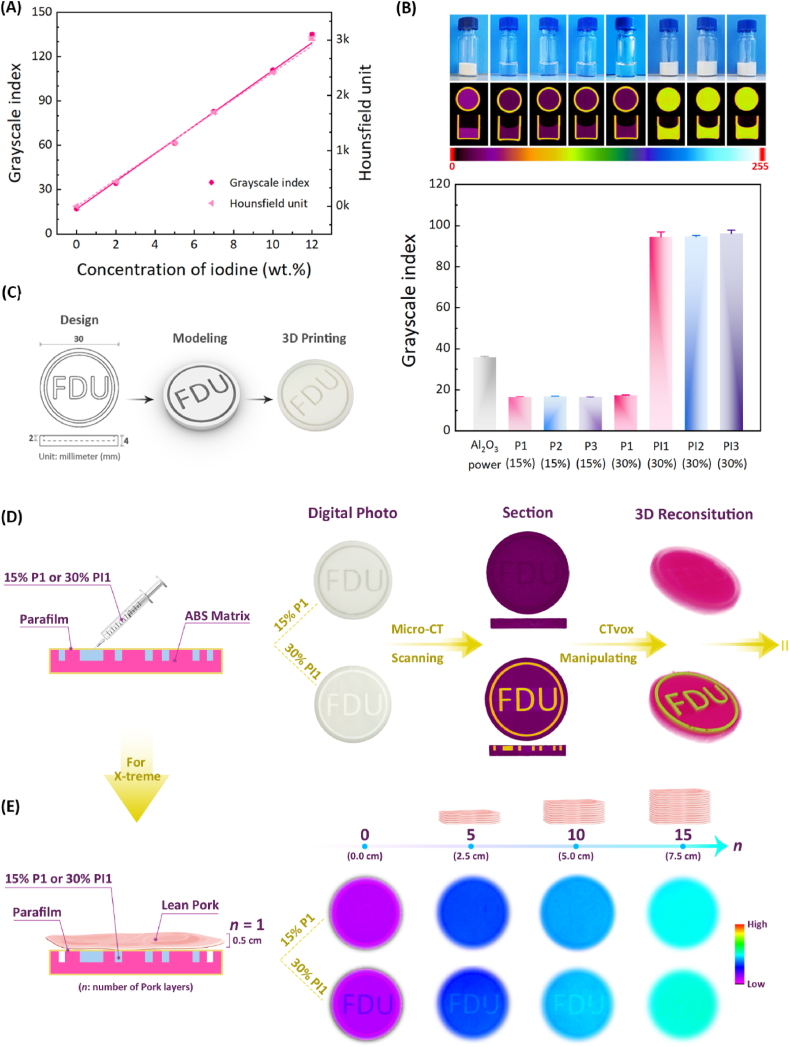
Fig. 4.
X-ray opacity of various aqueous polymer solutions in vitro. (A) The linear relationship between the average grayscale indices/Hounsfield units of iohexol aqueous solutions and their iodine amounts (n = 3, Pmax = 0.1). (B) The optical photographs, the corresponding Micro-CT images on the x-axis and the radiopacities of the indicated samples using the grayscale unit. The results are expressed as mean ± standard deviation (SD) (n = 3, Pmax = 0.1). (C) Design, modeling and 3D printing of “FDU” pattern. (D) The X-ray opacity of “FDU” pattern containing P1 or PI1 solution using Micro-CT. (E) The X-ray opacity of “FDU” pattern containing P1 or PI1 solution covered with different layers of pork using X-treme.
According to Equations (1), (2)), the grayscale index and Hounsfield unit of a system can be conveniently calculated via its iodine content, and vice versa. It is worth pointing out that the difference in the preview of maximum absorption (Pmax) can affect the grayscale index of the same sample. The larger the Pmax is, the smaller the grayscale index of the material is, that is, Pmax is inversely proportional to the grayscale index of the system. In contrast, the Hounsfield unit of a specimen is a fixed value.
In vitro X-ray opacities of the different aqueous polymer solutions were determined by Micro-CT. Due to the only presence of light atomic mass elements like C, H and O in P1–P3 polymers, their grayscale indices of aqueous solutions were similar regardless of their structural composition and concentration, as presented in Fig. 4B. In contrast, the aqueous polymer solutions containing PI showed significantly enhanced radiopacity, and their grayscale indices increased to about 95, equivalent to the Hounsfield unit value of 2003, and were almost six times larger than those of the PI-free polymer solutions. Meanwhile, the radiopacities of the PI-loaded aqueous polymer solutions were also superior to that of the alumina powder as the control group and their grayscale indices were approximately 2.5 times that of alumina powder. The Hounsfield unit values of mineralized tissues such as bone are approximately 1000 [58]. Obviously, the radiopacities of the PI-loaded systems were twice those of mineralized tissues. In addition, the uniform distribution of grayscale as presented in Fig. 4B indicated that PI was effectively solubilized into the aqueous polymer solutions. Also, based on Equation (1) and the known grayscale indices of PI-loaded aqueous polymer solutions, the iodine content of PI could be calculated to be 55.4 wt%, equivalent to 13.3 ITMC repeating units in each PI, which was very close to the calculation result obtained from 1H NMR.
To further visually demonstrate the X-ray opacity of PI-loaded aqueous polymer solutions, a 3D mold with “FDU” (abbreviation of Fudan University) pattern was designed and manufactured by 3D printing, whose detailed size parameters are illustrated in Fig. 4C. As shown in Fig. 4D, the “FDU” pattern filled with P1 solution was invisible under Micro-CT, while the pattern filled with PI1 solution could be clearly observed via Micro-CT imaging and the 3D reconstructed image intuitively reflected the morphology of the “FDU” pattern, indicating the outstanding X-ray opacity of PI1 system.
Similarly, the “FDU” pattern, which was filled with P1 solution, was not visualized under X-ray of X-treme, as shown in Fig. 4E. By contrast, due to the ultrahigh radiopacity of PI, the “FDU” pattern containing PI1 solution was still obviously visible through X-treme even when it was covered with 0.5 cm thick pork layer by layer to the 10th layer, and did not appear until the 15th layer was covered. It is worth noting that the X-ray imaging depth is proportional to the thickness and iodine content of the sample. The current “FDU” pattern contained only PI1 solution of 2 mm thickness, and the imaging depth has exceeded 5 cm. This finding affirms that the radiopaque PI1 system has an excellent capacity for deep tissue imaging. Of course, if the PI content in the polymer solution decreases, the imaging depth of the sample will decrease correspondingly.
2.4. In vitro cytotoxicity
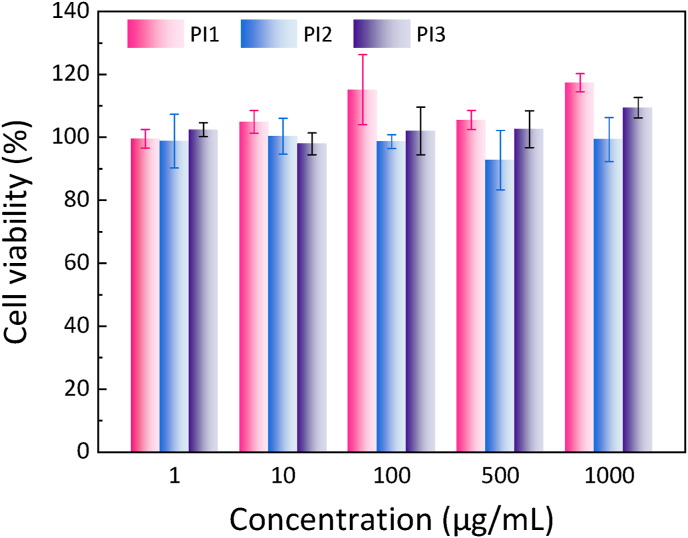
The in vitro cytocompatibility of the polymers were tested by cell counting kit-8 (CCK-8) assay using normal mouse embryo osteoblast precursor cells (MC3T3-E1). As shown in Fig. 5, the viabilities of cells receiving the treatment of polymer mixtures, PI1, PI2 and PI3, for 24 h were as high as 90% at all the tested concentrations, which were similar to those treated with pure P1, P2 or P3 polymers (Fig. S7), indicating that the macromolecular contrast agent PI and the three PEG/polyester polymers have good cell compatibilities.
Fig. 5.
In vitro cytotoxicity of the polymer mixtures, PI1, PI2 and PI3, as a function of polymer concentration against MC3T3-E1 cells. The viability of cells with the treatment of culture medium only was set as 100% and each point is represented as the mean ± SD (n = 4).
2.5. In vivo Micro-CT imaging
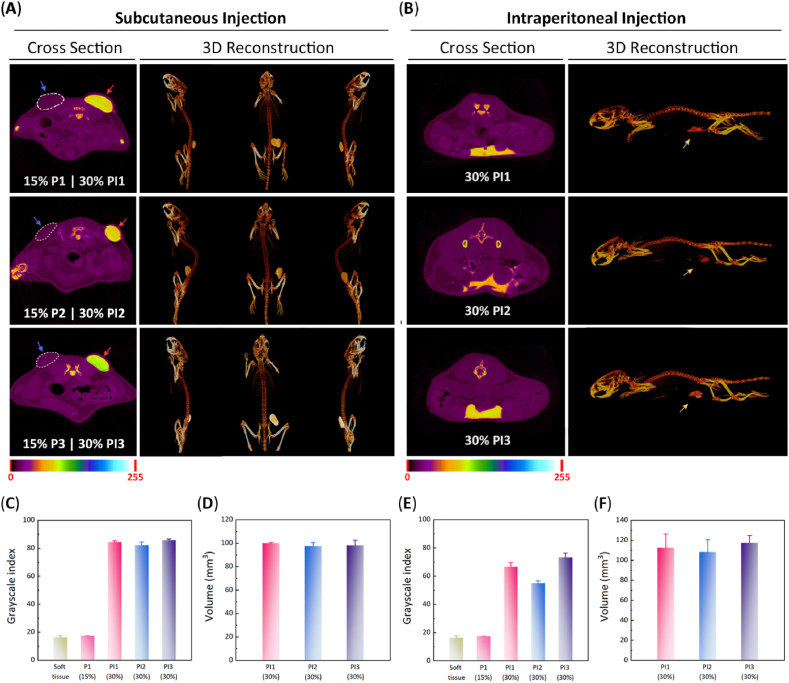
The different aqueous polymer solutions were injected subcutaneously into ICR mice through a conventional 1-mL syringe, and the in situ-forming hydrogels were obtained within 30 s via body temperature-triggered gelation, reflecting the appearance of elliptic protrusions at the administration sites. Subsequently, the in vivo X-ray opacities of in situ-forming hydrogels were evaluated by Micro-CT. Since the X-ray absorption capacity of pure polymer hydrogels without PI contrast agent was similar to that of soft tissue, it is impossible to distinguish them from the surrounding soft tissues using Micro-CT imaging, as shown in Fig. 6A. By contrast, with the mediation of excellent X-ray opacity of PI, the PI-loaded hydrogels could be visualized via Micro-CT with clear boundaries between the hydrogels and the adjacent soft tissues. Furthermore, the 3D morphologies of PI-loaded polymer hydrogels were intuitively observed by 3D reconstruction of Micro-CT images (Fig. 6A and Video S1). The quantitative results of grayscale value and injection volume were also harvested. As presented in Fig. 6C and D, the three PI-loaded hydrogels had a similar grayscale index and injection volume. Their mean grayscale index was far greater than that of soft tissue; however, there was no obvious discrimination between the PI-free polymer hydrogels and the surrounding tissues. Meanwhile, the volume of PI-loaded hydrogels obtained from Micro-CT well coincided with the actual injection volume.
Fig. 6.
(A) Cross-sectional views and 3D reconstructed Micro-CT images of the indicated samples 30 min after subcutaneous injection into female ICR mice. The dotted coils represent the PI-free hydrogels. (B) Cross-sectional views and 3D reconstructed Micro-CT images of the indicated samples 30 min after intraperitoneal injection into female ICR mice. (C) Grayscale indices of the indicated samples after subcutaneous injection. (D) Volume of the indicated samples after subcutaneous injection. (E) Grayscale indices of the indicated samples after intraperitoneal injection. (F) Volume of the indicated samples after intraperitoneal injection. (n = 3, Pmax = 0.1).
The three thermosensitive and radiopaque hydrogels were also intraperitoneally injected into ICR mice and then measured by Micro-CT. Fig. 6B displays their Micro-CT images on the z-axis and reconstructed 3D images. Likewise, these hydrogels were visible with the help of Micro-CT and exhibited an irregular shape, indicating that they were located in the space between the abdominal organs. The quantitative data validated that these hydrogels had prominently strong radiopacity compared with the adjacent abdominal organs (Fig. 6E). It should be noted that the intensity of X-ray opacity is related to the shape and injection site of the object tested. Owing to the more dispersed morphology of PI2 hydrogel in abdominal cavity compared with the other two hydrogels, its grayscale index was slightly lower than that of PI1 or PI3 hydrogel. Also, because the intraperitoneal injection site was deeper, the gray indices of these hydrogels were relatively smaller than those of subcutaneous injection. Moreover, these hydrogels had similar injection volume calculated by Micro-CT (Fig. 6F). These results indicate that PI is suitable as a universal macromolecular contrast agent for in vivo visualized detection of various thermosensitive hydrogels.
2.6. Nondestructive monitoring of in vivo hydrogel degradation
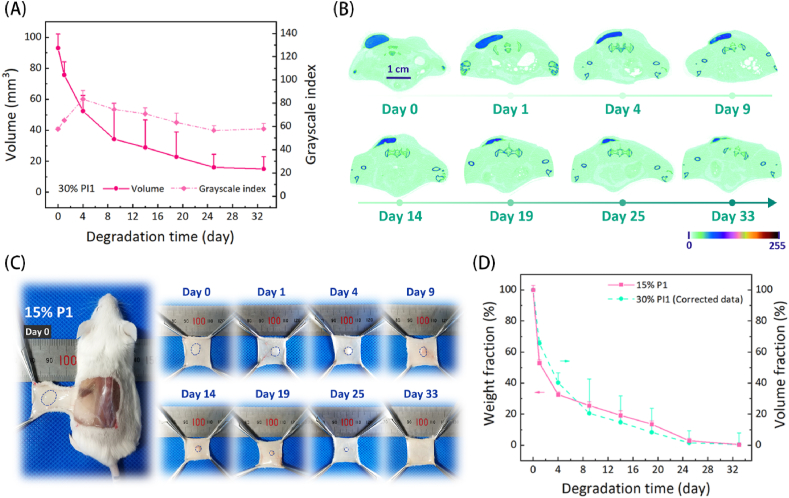
Considering that the medical applications of injectable thermosensitive hydrogels after subcutaneous injection are much more than those of intraperitoneal injection, the subcutaneous degradation process of PI1 hydrogel was nondestructively monitored by Micro-CT for example. Fig. 7A shows the volume and grayscale index of the residual PI1 hydrogel as a function of degradation time, and the morphological change of residual PI1 hydrogel over time is presented in Fig. 7B. The volume of the PI1 hydrogel gradually decreased as the time elapsed, whereas the remaining volume tended to be unchanged from day 24. The grayscale index of residual PI1 hydrogel showed a tendency to increase first and then slow decrease over time. In general, thermosensitive PEG/polyester copolymer hydrogels suffered from a rapid surface erosion by body fluids at the first stage after subcutaneous injection, reflected by a significant reduction in gel vol/wt [32]. This feature was also observed in the current PI1 hydrogel. Because the macromolecular contrast agent PI was far more hydrophobic than thermosensitive P1 polymers, P1 polymers were more easily dissolved through body fluids in the first few days, resulting in an initial increase in the radiopacity of residual PI1 hydrogel.
Fig. 7.
The in vivo degradation of polymer hydrogels with or without the macromolecular contrast agent PI. (A) Changes in the volume and grayscale index of PI1 hydrogel over time after subcutaneous injection into female ICR mice. (B) Representative cross-sectional views of the remaining PI1 hydrogels at the predetermined time points. (C) Representative photographs of the remaining hydrogels in ICR mice that received the subcutaneous administration of 15 wt% P1 hydrogel. (D) Changes in the weight fraction of 15 wt% P1 hydrogel and the corrected volume fraction of 30 wt% PI1 hydrogel over time. The results are expressed as mean ± SD (n = 3).
To affirm that the degradation curve of the PI-mediated hydrogel did truly reflect the degradation process of PI-free polymeric hydrogel, a 15 wt% P1 aqueous solution was injected subcutaneously into ICR mice and then dissected for observation of degradation at the predetermined time points. As shown in Fig. 7C and D, the volume of the P1 hydrogel continuously decreased with the effluxion of time, and no residual gel was detected on day 33 post-injection, implying that it was almost completely degraded. Compared with the PI1 hydrogel, the pure P1 hydrogel without the macromolecular contrast agent PI seems to have a faster degradation rate. The previous studies have confirmed that the degradation rate of carbonate bonds is far lower than that of ester bonds [[59], [60], [61]]. Also, if the C–I bonds in PI break, iodide ions would be released, which should be diluted away immediately by body fluids. However, the residual PI1 hydrogel maintained strong radiopacity during the whole examination period, as demonstrated in Fig. 7A, indicating that the C–I bonds in PI should be stable and the remaining substance in the PI1 hydrogel on day 33 should belong to the undegradable macromolecular contrast agent PI. Therefore, the remaining volume of PI1 hydrogel on day 33 was used to correct the data of degradation at the previous time points one by one (V33' = V33 − V33; V25' = V25 − V33; V19' = V19 − V33 …) and then a new degradation curve was acquired. As displayed in Fig. 7D, the corrected degradation curve of PI1 hydrogel almost perfectly coincided with that of pure P1 hydrogel determined via the gravimetric approach. This finding convincingly demonstrates that PI as a macromolecular X-ray contrast agent can accurately and nondestructively quantify the in vivo degradation of the thermosensitive P1 hydrogel.
The ICR mice receiving the injection of the PI1 hydrogel were sacrificed, and then the main organs and adjacent tissues at the injection sites were harvested. The representative hematoxylin-eosin (H&E) staining slices of the main organs are shown in Fig. S8. Compared with normal organs, no significant abnormality was observed in the PI1 hydrogel group, suggesting that the injection of PI1 hydrogel and the subsequent degradation in vivo did not cause apparently systemic side effects. Fig. S9 displays the microphotograph of the subcutaneous tissue at the injection site and the corresponding CT scanning image of the paraffin-embedded tissue. A strong radiopaque signal at the injection site was still detected via Micro-CT. As mentioned above, the radiopaque signal should be attributed to the residual macromolecular contrast agent PI, which had not been degraded. Meanwhile, no obvious inflammatory reaction was observed around the residual gel, indicating that the macromolecular CT imaging agent PI has good biocompatibility in vivo.
3. Conclusions
By polymerizing ITMC functional monomers onto PEG, an ultrahigh radiopaque macromolecular contrast agent PI was designed and synthesized, which affords a universal contrast agent for the construction of radiopaque polymer hydrogels. The introduction of PI not only made all the hydrogels maintain temperature-responsive sol-gel transitions, but also provided the ability of radiopacity. The location, morphology and volume of the radiopaque hydrogels at different injection sites could be achieved via Micro-CT imaging nondestructively and accurately. The degradation profile determined by Micro-CT could truly reflect the in vivo fate of virgin polymer hydrogel. Except for the in situ monitoring of the in vivo degradation, the visualization of radiopaque thermosensitive hydrogels can facilitate their medical applications, such as embolization treatment, drug delivery, tissue regeneration, tissue marking, etc. Meanwhile, the current methodology can be easily extended to other implantable polymer devices for in vivo imaging at clinically relevant depths.
4. Experimental section
4.1. Materials
2,2-bis(bromomethyl)-1,3-propanediol, sodium iodide and sodium bisulfite were the products of Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). Ethyl chloroformate was acquired from Wuhan Changcheng Chemical Technology Development Co., Ltd. (Wuhan, China). PEG1000 (number-average MW Mn = 1000 Da), PEG1500 (Mn = 1500 Da), Zn(HMDS)2, stannous octoate and ε-caprolactone (CL) were bought from Sigma-Aldrich. Glycolide (GA) and d,l-lactide (LA) were acquired from Hangzhou Medzone Biotech Ltd. (Hangzhou, China). Iohexol was the product of TCI Development Co., Ltd. (Shanghai, China). Tetrahydrofuran (THF) and TEA were stirred in the presence of calcium hydride (CaH2) for 24 h and then collected by distillation. CCK-8 for mammalian cells was bought from Beyotime Co., Ltd. (Shanghai, China). The other chemical reagents provided by Sinopharm Chemical Reagent Co. (Shanghai, China) were utilized without additional treatment.
4.2. Animals
Female ICR mice weighing approximately 25 g were purchased from Shanghai Lab. Animal Research Center (Shanghai, China). All animal experiments were conducted in accordance with the “Laboratory Animal Care Principles” (NIH Publication # 85–23, revised in 1985) and ratified by the Fudan University Ethics Committee.
4.3. Synthesis of ITMC
In according to our previous work [62], ITMC was synthesized via a two-step reaction. The first step of reaction was to synthesize 2,2-bis(iodomethyl)propane-1,3-diol. 30.0 g of 2,2-bis(bromomethyl)-1,3-propanediol (114.5 mmol), 60.0 g of sodium iodide (400.3 mmol) and 200 mL of acetone were transferred into an eggplant-shaped flask and a heating reflux was carried out for 8 days. Then, the filtrate obtained by filtering the generated sodium bromide, which was insoluble in acetone, was concentrated under reduced pressure to remove the solvent, and the resulting mixture was washed with a 1% (w/v) aqueous sodium bisulfite solution (400 mL) to remove the iodine generated in the reaction. Finally, the desired diiodine-substituted compound was harvested via filtration and drying at 85 °C for 12 h and the yield was 88%. The next step was to synthesize ITMC monomer. In brief, under vigorous stirring, 4.8 g of ethyl chloroformate (44.0 mmol) was added into a flask containing 7.1 g of 2,2-bis(iodomethyl)-1,3-propanediol (20.0 mmol) and 100 mL of THF in an ice bath. Next, 8 mL of THF containing 4.8 g of TEA (48.0 mmol) was added dropwise to the reaction system in 2 h. Then, the ice bath was removed and the reaction was continued at room temperature for 12 h. After that, the reaction system was filtered and the collected filtrate was concentrated by rotary evaporation. The crude product obtained was purified twice via recrystallization in a mixed solvent of THF and ether (1/3, v/v). Finally, the recrystallized product was gathered and dried under vacuum at 35 °C for 24 h to obtain ITMC monomer with a yield of 56%.
4.4. Synthesis of PI
PI was synthesized by ROP of functional monomer ITMC using PEG1000 as the macroinitiator and Zn(HMDS)2 as the catalyst. First, 1.0 g of PEG1000 (1 mmol) and 23 mL of anhydrous toluene were added to a 50 mL eggplant-shaped flask. Then, the residual water in the reaction system was removed by azeotropic distillation at 125 °C. Afterwards, 3.8 g ITMC (10 mmol) and 2 mL of anhydrous toluene containing 386.0 mg Zn(HMDS)2 were added to the flask, and the system was stirred under a blanket of argon at 40 °C for 24 h. After the solvent was removed by a rotary evaporator, the crude product was evenly dispersed in THF and then precipitated in cold ether for 24 h. The final product was separated via dumping the supernatant and a vacuum drying was then conducted at 35 °C for another 24 h.
4.5. Synthesis of P1, P2 and P3
P1, P2 and P3 triblock copolymers were synthesized by stannous octoate-catalyzed bulk ROP of different monomers in the presence of PEG1500 as the macroinitiator [34,63]. Briefly, 15 g of PEG1500 (10 mmol) was added to a 250 mL three-necked flask, and the system was mechanically stirred at 120 °C under vacuum for 2 h to remove water from the raw materials. After that, a specified amount of monomers and stannous octoate were added to the flask at 80 °C under an argon atmosphere. After three gas substitutions, the system was in the state of argon atmosphere and heated at 150 °C for 12 h with mechanical stirring. After the completion of polymerization, heating and stirring were continued under vacuum at 120 °C for 1 h to remove unreacted monomers and low MW products. Further, the crude product was washed three times with 200 mL of water at 80 °C and then freeze-dried for about 3 days to obtain the final product.
4.6. 1H NMR
The structures and compositions of ITMC and polymers were characterized by a 400 MHz Fourier Transform Nuclear Magnetic Resonance Spectrometer (AVANCE III HD, Bruker). All samples were dissolved in DMSO-d6 or CDCl3 to make 10 mg mL−1 solutions for scanning (scanning times, 16; temperature, 298 K).
4.7. GPC
The MWs and malar mass dispersity (ĐM) values of synthetic specimens were confirmed using a GPC system (Agilent 1260), which equipped with a differential detector (G1362A) and a Polargel-L column (7.5 mm × 300 mm). The polymers were dissolved in chromatographic grade THF and the samples (20 μL) were then injected into the GPC system. THF was selected as the mobile phase with 1.0 mL min−1 flow rate at 35 °C. The standard curve was calibrated with monodisperse polystyrenes.
4.8. XPS
The surface elements of the samples were analyzed by XPS (PHI 5300). The Mg anode target (hν = 1253.6 eV) was selected as X-ray excitation source with 250 W of power and 14 kV of voltage. Peak data were processed by software AugerScan 3.3, and the obtained binding energy spectrum peaks were corrected with C 1s peak (Eb = 284.6 eV) as the standard.
4.9. Cryo-field emission scanning electron microscopy-energy dispersion spectroscopy (Cryo-FESEM-EDS)
The surface morphology and element distributions of polymers were scanned and analyzed by a Cryo-FESEM-EDS system (AZtec X-Max Extreme EDS). Polymers were evenly spread on the sample table with conductive glue and then sprayed with a 2 nm-thickness gold coating (10 mA, 120 s) to enhance the conductivity of samples. Polymers were scanned for 2 min at 20 kV of acceleration voltage.
4.10. Preparation of aqueous polymer solutions
The preparation procedure of various aqueous polymer solutions was similar. 15% P1 and 30% PI1 aqueous solutions were prepared as examples. For 15% P1 aqueous solution, 1.5 g of P1 and 8.5 g of normal saline (NS) were added to a 25 mL vial and stirred in refrigerator at 4 °C for 3 days to obtain 15% P1 solution. As to 30% PI1 aqueous solution, 1.5 g of P1 and 7.0 g of NS were added to a 25 mL vial and stirred in refrigerator at 4 °C until the system became uniform. Then, 1.5 g of PI was added into the system and stirred at 4 °C. 2 days later, the system became uniform and 30% PI1 aqueous solution was obtained.
4.11. Dynamic rheological measurement
The rheological behaviors of the prepared aqueous polymer solutions upon heating were measured using a stress-controlled rheometer Kinexus Pro (Malvern Instrument Inc., UK). A cone plate with a diameter of 60 mm and a cone angle of 1° was selected. The distance between the upper and lower plates was set to 0.03 mm 1.5 mL of aqueous polymer solution was added dropwise on the lower plate, and a thin layer of silicone oil was covered on the edge of the cone plate to reduce the evaporation of water. Test parameters: temperature range, 15–50 °C; heating rate, 0.5 °C min−1; frequency, 1.59 Hz.
4.12. In vitro Micro-CT imaging
Iohexol was diluted with deionized water to obtain a series of iohexol aqueous solutions with specified iodine content. Alumina powder and deionized water were selected as the control and blank groups, respectively. The above samples were packed into 2 mL (d = 7 mm) vials and their grayscale indices and Hounsfield units were obtained by Micro-CT (Bruker, Skyscan1176) to verify the correlation between iodine content and grayscale index/Hounsfield unit. The specific parameters used for scanning were set as follows: 1 mm aluminum filter; X-ray tube voltage, 65 kV; tube current, 370 μA; pixel space size, 35 μm; rotation step length, 0.7° s−1; scanning angle range, 180°; Pmax, 0.1. Meanwhile, 0.5 mL aqueous polymer solutions were added to 2 mL vials (d = 7 mm) and placed at 4 °C for 12 h to eliminate air bubbles generated during addition. Subsequently, Micro-CT scanning was performed to confirm the X-ray radiopacity of various aqueous polymer solutions. The grayscale indices were obtained by analyzing data with CTAn software. The specific parameters used for scanning were the same as above. The Hounsfield unit value of a system was calculated via the following equation:
| (3) |
where and are the attenuation coefficients of a system and water, respectively.
4.13. “FDU” mold and imaging
A cylinder model with “FDU” pattern (d = 30 mm, h = 4 mm) was designed by the software ironCAD 2018, and the “FDU” mold made of ABS resin was then obtained by 3D printing. 0.2 mL of aqueous polymer solution was injected into the “FDU” pattern, and the surface of the mold was sealed with parafilm film to prevent water evaporation. The “FDU” mold was scanned by Micro-CT. The scanning parameters were the same as above. Meanwhile, 0.2 mL of aqueous polymer solution was injected into another “FDU” mold, and the surface of the mold was also sealed with parafilm film to prevent water volatilization. Then, the surface was further covered with different layers of 0.5 cm thick pork and scanned by X-treme (Bruker, In Vivo Xtreme). X-treme scanning parameters: X-ray resolution, 18 Ip/mm; exposure time, 1.2 s; voltage, 40 keV; filter, 0.4 mm Al; fstop, 1.1.
4.14. In vitro cytotoxicity
The in vitro cytotoxicity of the synthetic polymers was evaluated by CCK-8 assay. The used cell type was MC3T3-E1 cells (Shanghai Cell Bank, Chinese Academy of Sciences). First, MC3T3-E1 cells were incubated and then seeded in 96-well plates with a cell density of 5 × 103 per well. The cell culture medium containing 10% fetal bovine serum was added to the culture plate, and then cells were incubated for another 12 h to allow cells to adhere fully. Next, cell culture medium in the well plate was replaced with a fresh culture solution containing polymers with a specified concentration, and the incubation was conducted for another 24 h. Finally, the absorbance of each well at 450 nm wavelength was measured with a microplate reader (ELx808, Biotech). The cell viability of blank group that did not receive any treatment of polymers was defined as 100%. In vitro cytotoxicity of the sample was evaluated by the relative cell viability. Equation (4) is the calculation equation for the relative cell viability.
| (4) |
where ODexp and ODblank are the absorbance values of the experimental and blank groups, respectively.
4.15. In vivo Micro-CT imaging
Subcutaneous injection. Female ICR mice were randomly divided into three groups, A, B, and C, with three mice in each group. Group A was injected subcutaneously with 15% P1 and 30% PI1 aqueous solutions on both sides of the spine, respectively; group B was injected subcutaneously with 15% P2 and 30% PI2 aqueous solutions, respectively; and group C was injected subcutaneously with 15% P3 and 30% PI3 aqueous solutions, respectively. The injection volume of aqueous polymer solution was 0.1 mL. Before injection, each mouse received a standard anesthesia. 30 min post-injection, all mice were scanned by Micro-CT.
4.15.1. Intraperitoneal injection
Similar to subcutaneous injection, female ICR mice were randomly divided into three groups, a, b, and c, with three mice in each group. After general anesthesia, the mice in the group a were intraperitoneally injected with 0.1 mL of 30% PI1 solution; the mice in the group b were intraperitoneally injected with 0.1 mL of 30% PI2 solution; and the mice in the group c were intraperitoneally injected with 0.1 mL of 30% PI3 solution. 30 min later, Micro-CT was used to scan all mice, and the gel volume was analyzed and calculated by 3D reconstruction. The specific parameters used for in vivo Micro-CT-scanning were set as follows: 1 mm aluminum filter; X-ray tube voltage, 65 kV; tube current, 370 μA; pixel space size, 35 μm; rotation step length, 0.7° s−1; scanning angle range, 180°; Pmax, 0.1.
4.16. In vivo hydrogel degradation
The mice receiving the subcutaneous injection of 30% PI1 system were subjected to Micro-CT scanning on day 0, 1, 4, 9, 14, 19, 25, and 33 post-injection, and the residual amount of gel was analyzed by 3D reconstruction. The specific parameters used for scanning were the same as in vivo Micro-CT scanning except Pmax was 0.15. In addition, another 16 female ICR mice were subcutaneously injected with 15% P1 aqueous solution on both sides of the back spine and the injection volume of each site was 0.1 mL. Then, two mice were randomly euthanized and dissected on day 0, 1, 4, 9, 14, 19, 25 and 33 post-injection, and the subcutaneous residual gel was stripped and weighed for quantitative analysis.
4.17. In vivo biocompatibility
The mice treated with the 30% PI1 hydrogel were euthanized on day 49 post-injection and their heart, liver, spleen, lung kidney and subcutaneous tissues were harvested and stored in tissue fixative. All the collected tissues were embedded in paraffin, sectioned, and stained by hematoxylin-eosin (H&E). The slides were observed using an inverted microscope (Eclipse LV100ND, Nikon). The paraffin-embedded subcutaneous tissue containing the residual gel was also scanned by Micro-CT. The specific parameters used for scanning were the same as above except Pmax was 0.25.
CRediT authorship contribution statement
Xiaohui Wu: Writing – original draft, Methodology, Investigation. Xin Wang: Investigation, Writing – review & editing. Xiaobin Chen: Investigation, Writing – review & editing. Xiaowei Yang: Investigation, Writing – review & editing. Qian Ma: Methodology. Guohua Xu: Funding acquisition, Validation. Lin Yu: Conceptualization, Funding acquisition, Supervision, Writing – review & editing. Jiandong Ding: Funding acquisition, Writing – review & editing.
Declaration of competing interest
There are no conflicts of interest to declare.
Acknowledgements
Authors acknowledge funding from the National Natural Science Foundation of China (grant Nos. 51773043, 81772363 and 21975045) and the National Key R&D Program of China (grant Nos. 2020YFC1107102 and 2016YFC1100300).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bioactmat.2021.05.013.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Janib S.M., Moses A.S., MacKay J.A. Imaging and drug delivery using theranostic nanoparticles. Adv. Drug Deliv. Rev. 2010;62(11):1052–1063. doi: 10.1016/j.addr.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu R., Zhang Y., Tao H., Zhou L., Li H., Chen T., Zhang P., Lu Y., Chen S. Gadolinium-hyaluronic acid nanoparticles as an efficient and safe magnetic resonance imaging contrast agent for articular cartilage injury detection. Bioact. Mater. 2020;5(4):758–767. doi: 10.1016/j.bioactmat.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li D., Lin L., Fan Y., Liu L., Shen M., Wu R., Du L., Shi X. Ultrasound-enhanced fluorescence imaging and chemotherapy of multidrug-resistant tumors using multifunctional dendrimer/carbon dot nanohybrids. Bioact. Mater. 2021;6(3):729–739. doi: 10.1016/j.bioactmat.2020.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu H., Ma B., Jiang J., Xiao S., Peng R., Zhuang W., Li G., Wang Y. Integrated prodrug micelles with two-photon bioimaging and pH-triggered drug delivery for cancer theranostics. Regen Biomater. 2020;7(2):171–180. doi: 10.1093/rb/rbz035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lei K., Ma Q., Yu L., Ding J. Functional biomedical hydrogels for in vivo imaging. J. Mater. Chem. B. 2016;4(48):7793–7812. doi: 10.1039/c6tb02019d. [DOI] [PubMed] [Google Scholar]
- 6.Samuel R., Girard E., Chagnon G., Dejean S., Favier D., Coudane J., Nottelet B. Radiopaque poly(ε-caprolactone) as additive for X-ray imaging of temporary implantable medical devices. RSC Adv. 2015;5(102):84125–84133. [Google Scholar]
- 7.Datta S., Jana S., Das A., Chakraborty A., Chowdhury A.R., Datta P. Bioprinting of radiopaque constructs for tissue engineering and understanding degradation behavior by use of Micro-CT. Bioact. Mater. 2020;5(3):569–576. doi: 10.1016/j.bioactmat.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yin J., Luan S. Opportunities and challenges for the development of polymer-based biomaterials and medical devices. Regen Biomater. 2016;3(2):129–135. doi: 10.1093/rb/rbw008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao D.Y., Zhu T.T., Li J., Cui L.G., Zhang Z.Y., Zhuang X.L., Ding J.X. Poly(lactic-co-glycolic acid)-based composite bone-substitute materials. Bioact. Mater. 2021;6(2):346–360. doi: 10.1016/j.bioactmat.2020.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu L., Yang Z., Gao C., Li H., Yuan Z., Wang F., Sui X., Liu S., Guo Q. Advances and prospects in biomimetic multilayered scaffolds for articular cartilage regeneration. Regen Biomater. 2020;7(6):527–542. doi: 10.1093/rb/rbaa042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohamed M.A., Shahini A., Rajabian N., Caserto J., El-Sokkary A.M.A., Akl M.A., Andreadis S.T., Cheng C. Fast photocurable thiol-ene elastomers with tunable biodegradability, mechanical and surface properties enhance myoblast differentiation and contractile function. Bioact. Mater. 2021;6(7):2120–2133. doi: 10.1016/j.bioactmat.2020.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X., Ji X., Chen K., Ullah M.W., Li B., Cao J., Xiao L., Xiao J., Yang G. Immobilized thrombin on X-ray radiopaque polyvinyl alcohol/chitosan embolic microspheres for precise localization and topical blood coagulation. Bioact. Mater. 2021;6(7):2105–2119. doi: 10.1016/j.bioactmat.2020.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang H., Lei K., Zhou F., Yang X., An Q., Zhu W., Yu L., Ding J. Injectable PEG/polyester thermogel: a new liquid embolization agent for temporary vascular interventional therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2019;102:606–615. doi: 10.1016/j.msec.2019.04.075. [DOI] [PubMed] [Google Scholar]
- 14.Pariente J.L., Bordenave L., Bareille R., Ohayon-Courtes C., Baquey C., Le Guillou M. In vitro cytocompatibility of radio-opacifiers used in ureteral endoprosthesis. Biomaterials. 1999;20(6):523–527. doi: 10.1016/s0142-9612(98)00198-7. [DOI] [PubMed] [Google Scholar]
- 15.Vallo C.I., Cuadrado T.R., Frontini P.M. Mechanical and fracture behaviour evaluation of commercial acrylic bone cements. Polym. Int. 1997;43(3):260–268. [Google Scholar]
- 16.Olsen T.R., Davis L.L., Nicolau S.E., Duncan C.C., Whitehead D.C., Van Horn B.A., Alexis F. Non-invasive deep tissue imaging of iodine modified poly(caprolactone-co-1-4-oxepan-1,5-dione) using X-ray. Acta Biomater. 2015;20:94–103. doi: 10.1016/j.actbio.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 17.Sandker M.J., Petit A., Redout E.M., Siebelt M., Muller B., Bruin P., Meyboom R., Vermonden T., Hennink W.E., Weinans H. In situ forming acyl-capped PCLA-PEG-PCLA triblock copolymer based hydrogels. Biomaterials. 2013;34(32):8002–8011. doi: 10.1016/j.biomaterials.2013.07.046. [DOI] [PubMed] [Google Scholar]
- 18.Lv Q., Yu S., Quan F., He C., Chen X. Thermosensitive polypeptide hydrogels Co-loaded with two anti-tumor agents to reduce multi-drug resistance and enhance local tumor treatment. Adv. Ther. 2020;3(3):1900165. [Google Scholar]
- 19.Ftiervoet L.A.L., Zhang H., van Groesen E., Fortuin K., Duin N.J.C.B., Remaut K., Schiffelers R.M., Hennink W.E., Vermonden T. Local release of siRNA using polyplex-loaded thermosensitive hydrogels. Nanoscale. 2020;12(18):10347–10360. doi: 10.1039/d0nr03147j. [DOI] [PubMed] [Google Scholar]
- 20.Lv Q., He C., Quan F., Yu S., Chen X. DOX/IL-2/IFN-gamma co-loaded thermo-sensitive polypeptide hydrogel for efficient melanoma treatment. Bioact. Mater. 2018;3(1):118–128. doi: 10.1016/j.bioactmat.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ilochonwu B.C., Urtti A., Hennink W.E., Vermonden T. Intravitreal hydrogels for sustained release of therapeutic proteins. J. Contr. Release. 2020;326:419–441. doi: 10.1016/j.jconrel.2020.07.031. [DOI] [PubMed] [Google Scholar]
- 22.Patel M., Lee H.J., Park S., Kim Y., Jeong B. Injectable thermogel for 3D culture of stem cells. Biomaterials. 2018;159:91–107. doi: 10.1016/j.biomaterials.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Yu S., Wang C., Yu J., Wang J., Lu Y., Zhang Y., Zhang X., Hu Q., Sun W., He C., Chen X., Gu Z. Injectable bioresponsive gel depot for enhanced immune checkpoint blockade. Adv. Mater. 2018;30(28):1801527. doi: 10.1002/adma.201801527. [DOI] [PubMed] [Google Scholar]
- 24.Zhong G., Yao J., Huang X., Luo Y., Wang M., Han J., Chen F., Yu Y. Injectable ECM hydrogel for delivery of BMSCs enabled full-thickness meniscus repair in an orthotopic rat model. Bioact. Mater. 2020;5(4):871–879. doi: 10.1016/j.bioactmat.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng Y., Wu G., Chen L., Zhang Y., Luo Y., Zheng Y., Hu F., Forouzanfar T., Lin H., Liu B. Neuro-regenerative imidazole-functionalized GelMA hydrogel loaded with hAMSC and SDF-1 alpha promote stem cell differentiation and repair focal brain injury. Bioact. Mater. 2021;6(3):627–637. doi: 10.1016/j.bioactmat.2020.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma X., Agas A., Siddiqui Z., Kim K., Iglesias-Montoro P., Kalluru J., Kumar V., Haorah J. Angiogenic peptide hydrogels for treatment of traumatic brain injury. Bioact. Mater. 2020;5(1):124–132. doi: 10.1016/j.bioactmat.2020.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu Y., Chang T., Chen W., Wang X., Li J., Chen Y., Yu Y., Shen Z., Yu Q., Zhang Y. Release of VEGF and BMP9 from injectable alginate based composite hydrogel for treatment of myocardial infarction. Bioact. Mater. 2021;6(2):520–528. doi: 10.1016/j.bioactmat.2020.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang C., Wang M., Xia K., Wang J., Cheng F., Shi K., Ying L., Yu C., Xu H., Xiao S., Liang C., Li F., Lei B., Chen Q. A bioactive injectable self-healing anti-inflammatory hydrogel with ultralong extracellular vesicles release synergistically enhances motor functional recovery of spinal cord injury. Bioact. Mater. 2021;6(8):2523–2534. doi: 10.1016/j.bioactmat.2021.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cipriani F., Arino Palao B., Gonzalez de Torre I., Vega Castrillo A., Aguado Hernandez H.J., Alonso Rodrigo M., Alvarez Barcia A.J., Sanchez A., Garcia Diaz V., Lopez Pena M., Rodriguez-Cabello J.C. An elastin-like recombinamer-based bioactive hydrogel embedded with mesenchymal stromal cells as an injectable scaffold for osteochondral repair. Regen Biomater. 2019;6(6):335–347. doi: 10.1093/rb/rbz023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong Y., Saeed A.O., Hassan W., Keigher C., Zheng Y., Tai H., Pandit A., Wang W. One-step" preparation of thiol-ene clickable PEG-based thermoresponsive hyperbranched copolymer for in situ crosslinking hybrid hydrogel. Macromol. Rapid Commun. 2012;33(2):120–126. doi: 10.1002/marc.201100534. [DOI] [PubMed] [Google Scholar]
- 31.Petit A., Muller B., Bruin P., Meyboom R., Piest M., Kroon-Batenburg L.M., de Leede L.G., Hennink W.E., Vermonden T. Modulating rheological and degradation properties of temperature-responsive gelling systems composed of blends of PCLA-PEG-PCLA triblock copolymers and their fully hexanoyl-capped derivatives. Acta Biomater. 2012;8(12):4260–4267. doi: 10.1016/j.actbio.2012.07.044. [DOI] [PubMed] [Google Scholar]
- 32.Chen X., Zhang J., Wu K., Wu X., Tang J., Cui S., Cao D., Liu R., Peng C., Yu L., Ding J. Visualizing the in vivo evolution of an injectable and thermosensitive hydrogel using tri-modal bioimaging. Small Methods. 2020;4(9):2000310. [Google Scholar]
- 33.Lee H.J., Jeong B. ROS-sensitive degradable PEG-PCL-PEG micellar thermogel. Small. 2019;16(12):1903045. doi: 10.1002/smll.201903045. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Z., Ni J., Chen L., Yu L., Xu J.W., Ding J.D. Biodegradable and thermoreversible PCLA-PEG-PCLA hydrogel as a barrier for prevention of post-operative adhesion. Biomaterials. 2011;32(21):4725–4736. doi: 10.1016/j.biomaterials.2011.03.046. [DOI] [PubMed] [Google Scholar]
- 35.Zhou X., He X., Shi K., Yuan L., Yang Y., Liu Q., Ming Y., Yi C., Qian Z. Injectable thermosensitive hydrogel containing erlotinib-loaded hollow mesoporous silica nanoparticles as a localized drug delivery system for NSCLC therapy. Adv. Sci. 2020;7(23):2001442. doi: 10.1002/advs.202001442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y., Yang H.Y., Lee D.S. Advances in biodegradable and injectable hydrogels for biomedical applications. J. Contr. Release. 2021;330:151–160. doi: 10.1016/j.jconrel.2020.12.008. [DOI] [PubMed] [Google Scholar]
- 37.Chen X.B., Wang M.L., Yang X.W., Wang Y.B., Yu L., Sun J., Ding J.D. Injectable hydrogels for the sustained delivery of a HER2-targeted antibody for preventing local relapse of HER2+breast cancer after breast-conserving surgery. Theranostics. 2019;9(21):6080–6098. doi: 10.7150/thno.36514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang X.W., Chen X.B., Wang Y.B., Xu G.H., Yu L., Ding J.D. Sustained release of lipophilic gemcitabine from an injectable polymeric hydrogel for synergistically enhancing tumor chemoradiotherapy. Chem. Eng. J. 2020;396:125320. [Google Scholar]
- 39.Buwalda S.J., Vermonden T., Hennink W.E. Hydrogels for therapeutic delivery: current developments and future directions. Biomacromolecules. 2017;18(2):316–330. doi: 10.1021/acs.biomac.6b01604. [DOI] [PubMed] [Google Scholar]
- 40.Ma H.C., He C.L., Cheng Y.L., Li D.S., Gong Y.B., Liu J.G., Tian H.Y., Chen X.S. PLK1shRNA and doxorubicin co-loaded thermosensitive PLGA-PEG-PLGA hydrogels for osteosarcoma treatment. Biomaterials. 2014;35(30):8723–8734. doi: 10.1016/j.biomaterials.2014.06.045. [DOI] [PubMed] [Google Scholar]
- 41.Yang F., Shi K., Hao Y., Jia Y., Liu Q., Chen Y., Pan M., Yuan L., Yu Y., Qian Z. Cyclophosphamide loaded thermo-responsive hydrogel system synergize with a hydrogel cancer vaccine to amplify cancer immunotherapy in a prime-boost manner. Bioact. Mater. 2021;6(10):3036–3048. doi: 10.1016/j.bioactmat.2021.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xue B., Lei M., Shi K., Wang M., Hao Y., Xiao Y., Yuan L., Peng J., Qian Z. Intratumoral injection of norcantharidin-loaded poly(D, L-lactide)-b-poly(ethylene glycol)-b-Poly(D,L-lactide) thermosensitive hydrogel for the treatment of primary hepatocellular carcinoma. J. Biomed. Nanotechnol. 2019;15(10):2025–2044. doi: 10.1166/jbn.2019.2822. [DOI] [PubMed] [Google Scholar]
- 43.Xu W.K., Tang J.Y., Yuan Z., Cai C.Y., Chen X.B., Cui S.Q., Liu P., Yu L., Cai K.Y., Ding J.D. Accelerated cutaneous wound healing using an injectable teicoplanin-loaded PLGA-PEG-PLGA thermogel dressing. Chin. J. Polym. Sci. 2019;37(6):548–559. [Google Scholar]
- 44.Li X., Ding J., Zhang Z., Yang M., Yu J., Wang J., Chang F., Chen X. Kartogenin-incorporated thermogel supports stem cells for significant cartilage regeneration. ACS Appl. Mater. Interfaces. 2016;8(8):5148–5159. doi: 10.1021/acsami.5b12212. [DOI] [PubMed] [Google Scholar]
- 45.Ni P., Ding Q., Fan M., Liao J., Qian Z., Luo J., Li X., Luo F., Yang Z., Wei Y. Injectable thermosensitive PEG-PCL-PEG hydrogel/acellular bone matrix composite for bone regeneration in cranial defects. Biomaterials. 2014;35(1):236–248. doi: 10.1016/j.biomaterials.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 46.Lei K.W., Chen Y.P., Wang J.Y., Peng X.C., Yu L., Ding J.D. Non-invasive monitoring of in vivo degradation of a radiopaque thermoreversible hydrogel and its efficacy in preventing post-operative adhesions. Acta Biomater. 2017;55:396–409. doi: 10.1016/j.actbio.2017.03.042. [DOI] [PubMed] [Google Scholar]
- 47.Lei K., Shen W., Cao L., Yu L., Ding J. An injectable thermogel with high radiopacity. Chem. Commun. 2015;51(28):6080–6083. doi: 10.1039/c5cc00049a. [DOI] [PubMed] [Google Scholar]
- 48.Yu L., Chang G., Zhang H., Ding J. Temperature-induced spontaneous sol-gel transitions of poly(D,L-lactic acid-co-glycolic acid)-b-poly(ethylene glycol)-b-poly(D,L-lactic acid-co-glycolic acid) triblock copolymers and their end-capped derivatives in water. J. Polym. Sci., Part A: Polym. Chem. 2007;45(6):1122–1133. [Google Scholar]
- 49.Yu L., Zhang H., Ding J. A subtle end-group effect on macroscopic physical gelation of triblock copolymer aqueous solutions. Angew. Chem. Int. Ed. 2006;45(14):2232–2235. doi: 10.1002/anie.200503575. [DOI] [PubMed] [Google Scholar]
- 50.Luan J.B., Cui S.Q., Wang J.T., Shen W.J., Yu L., Ding J.D. Positional isomeric effects of coupling agents on the temperature-induced gelation of triblock copolymer aqueous solutions. Polym. Chem. 2017;8(17):2586–2597. [Google Scholar]
- 51.Cui S., Yu L., Ding J. Thermogelling of amphiphilic block copolymers in water: ABA type versus AB or BAB type. Macromolecules. 2019;52(10):3697–3715. [Google Scholar]
- 52.Cui S., Chen L., Yu L., Ding J. Synergism among polydispersed amphiphilic block copolymers leading to spontaneous physical hydrogelation upon heating. Macromolecules. 2020;53(18):7726–7739. [Google Scholar]
- 53.El Habnouni S., Blanquer S., Darcos V., Coudane J. Aminated PCL-based copolymers by chemical modification of poly(α-iodo-ε-caprolactone-co-ε-caprolactone) J. Polym. Sci., Part A: Polym. Chem. 2009;47(22):6104–6115. [Google Scholar]
- 54.Nottelet B., Coudane J., Vert M. Synthesis of an X-ray opaque biodegradable copolyester by chemical modification of poly (epsilon-caprolactone) Biomaterials. 2006;27(28):4948–4954. doi: 10.1016/j.biomaterials.2006.05.032. [DOI] [PubMed] [Google Scholar]
- 55.Van Horn B.A., Davis L.L., Nicolau S.E., Burry E.E., Bailey V.O., Guerra F.D., Alexis F., Whitehead D.C. Synthesis and conjugation of a triiodohydroxylamine for the preparation of highly X-ray opaque poly(ε-caprolactone) materials. J. Polym. Sci., Part A: Polym. Chem. 2017;55(5):787–793. [Google Scholar]
- 56.Hong J.H., Lee H.J., Jeong B. Injectable polypeptide thermogel as a tissue engineering system for hepatogenic differentiation of tonsil-derived mesenchymal stem cells. ACS Appl. Mater. Interfaces. 2017;9(13):11568–11576. doi: 10.1021/acsami.7b02488. [DOI] [PubMed] [Google Scholar]
- 57.Patel M., Lee H.J., Son S., Kim H., Kim J., Jeong B. Iron ion-releasing polypeptide thermogel for neuronal differentiation of mesenchymal stem cells. Biomacromolecules. 2020;21(1):143–151. doi: 10.1021/acs.biomac.9b01096. [DOI] [PubMed] [Google Scholar]
- 58.Lusic H., Grinstaff M.W. X-ray-computed tomography contrast agents. Chem. Rev. 2013;113(3):1641–1666. doi: 10.1021/cr200358s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu K.J., Hendren R.W., Jensen K., Pitt C.G. Synthesis, properties, and biodegradation of poly(1,3-trimethylene carbonate) Macromolecules. 1991;24(8):1736–1740. [Google Scholar]
- 60.Albertsson A.-C., Eklund M. Influence of molecular structure on the degradation mechanism of degradable polymers: in vitro degradation of poly(trimethylene carbonate), poly(trimethylene carbonate-co-caprolactone), and poly(adipic anhydride) J. Appl. Polym. Sci. 1995;57(1):87–103. [Google Scholar]
- 61.Zhang Z., Kuijer R., Bulstra S.K., Grijpma D.W., Feijen J. The in vivo and in vitro degradation behavior of poly(trimethylene carbonate) Biomaterials. 2006;27(9):1741–1748. doi: 10.1016/j.biomaterials.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 62.Ma Q., Lei K., Ding J., Yu L., Ding J. Design, synthesis and ring-opening polymerization of a new iodinated carbonate monomer: a universal route towards ultrahigh radiopaque aliphatic polycarbonates. Polym. Chem. 2017;8(43):6665–6674. [Google Scholar]
- 63.Zhuang Y., Yang X., Li Y., Chen Y., Peng X., Yu L., Ding J. Sustained release strategy designed for lixisenatide delivery to synchronously treat diabetes and associated complications. ACS Appl. Mater. Interfaces. 2019;11(33):29604–29618. doi: 10.1021/acsami.9b10346. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.