Abstract
Objective
Psychiatric impact of COVID-19 is still explored and previous data suggest potential risks of anxiety, depression and PTSD related to COVID-19. We aimed to explore the predictive value of risk factors during hospitalization (T0) for COVID-19 for anxiety, depression and PTSD and at three months (T1) because they could differ over these two time points.
Methods
We performed a screening of mental suffering in hospitalized patients for COVID-19, as well as specialized care and three months longitudinal follow-up. We evaluated at T0 and at T1 the prevalence of anxiety, depression and PTSD in survivors who benefited from early detection and treatment, and assessed possible risk factors in adults surviving COVID-19 between the 30th March and the 1st of July 2020.
Results
109 patients were screened at T0 and 61 of these were reassessed at T1. At T0, we found 44.9% pathological score on peritraumatic dissociation experiences questionnaire (PDEQ), 85.4% of post-traumatic stress disorder symptoms (PTSS), 14.6% of pathological rate of post-traumatic stress disorder scale 5 (PCL5) and at T1, 86.9% of PTSS, 10.6% of pathological rate of PCL5. Finally, PDEQ score at T0 during hospitalization was positively correlated to PCL-5 score at T1 (β = 0.26, p = 0.01) and that was confirmed in multivariate analysis (β = 0.04, p = 0.02 for the log of PCL-5 per point on the PDEQ).
Conclusion
Screening of psychiatric symptoms during hospitalization for COVID-19 should be systematic, especially peritraumatic dissociation to offer an early treatment and prevent PTSD, which seemed frequent for hospitalized patients for COVID-19 at three months.
Keywords: Coronavirus infections, Pneumonia, Post traumatic, Risk factors, Stress disorders
1. Introduction
Experiencing physical illness may induce stress and cause mental distress. Post-Traumatic Stress Disorder (PTSD), anxiety or depression can occur in contexts of somatic diseases exposing patients to a significant risk of death, such as COVID-19. The SARS-CoV-2 pandemic, unprecedented in several ways, combines several risk factors of psychological decompensation. First, its unpredictable and potentially deadly nature was frequently highlighted by media outlets, particularly in the early days of the pandemic (Rogers et al., 2020). Second, the physical manifestations and dyspnea associated with COVID-19 pneumonia or Acute Respiratory Distress Syndrome (ARDS) could be direct triggers of anxiety. Third, the neurotropism of the virus may further worsen neuropsychiatric symptoms of the disease (Wu et al., 2020). Finally, and central to the management of the SARS-CoV-2 pandemic and infections, the isolation of infected people as well as general lockdowns are required. Such measures are often associated with negative psychological effects like PTSD, anxiety, depression, sometimes with long-lasting effects on quality of life and socio-professional integration (Dubey et al., 2020).
COVID-19 psychiatric sequelae are still being explored. To date, confusion and delirium caused by SARS-CoV-2 in the first stage of the infection have been reported (Rogers et al., 2020). In the first study reports, the occurrence of post-traumatic stress symptoms (PTSS) appeared to be exceptionally prevalent (96.2%), with an evolution to depressive symptoms (Vindegaard and Benros, 2020) and PTSD in 28% of adults surviving COVID-19 (Mazza et al., 2020). In a recent retrospective cohort study, incidence of any psychiatric diagnosis after COVID-19 diagnosis reached 18.1%, regardless of known physical health risk factors for COVID-19 (Taquet et al., 2021). In addition, patients who required admission to the intensive care unit (ICU), that is about 5% of hospitalized patients (Krähenbuhl et al., 2020), are known to be at risk of developing a PTSD.
Several mechanisms of PTSD have been postulated including peritraumatic dissociation. Peritraumatic dissociation symptoms occur during and immediately following a trauma, and include feeling emotionally numb or disconnected from reality. The traumatic memory and mechanisms of dissociation can explain a peritraumatic amnesia and the occurrence of false memories, or impair the encoding of traumatic memory (Bedard-Gilligan and Zoellner 2012). Peritraumatic dissociation is thus identified as an important component for early screening following a traumatic injury, as it predicts an increased risk of developing a PTSD (Ozer et al., 2003). To the best of our knowledge, no prospective study has yet explored peritraumatic dissociation among hospitalized patients with moderate to severe COVID-19.
Inspired by other pandemic experiences, the World Health Organization (WHO) published recommendations (2012) for mental health care in an effort to reduce the risk of a large-scale psychological impact due to the COVID-19 context. The panel proposed (2020) essential mental health care and psychosocial support (SMSPS) for all suspected or confirmed cases of COVID-19 by interviewing and responding to the needs and concerns of these individuals. Early identification of patients at risk of psychological consequences is critical in order to offer them specialized care adapted to their clinical condition (World Health Organization, 2020a, World Health Organization, 2020b, World Health Organization, 2020).
While the predictive factors of progression to PTSD – such as peritraumatic dissociation, a high level of anxiety or depression, or isolation – are well known, there are still no validated standardized management recommendations to reduce the risk of PTSD. Early pharmacological treatment is not associated with a significant reduction of PTSD (Astill et al., 2019), however early psychological intervention such as trauma-focused CBT (CBT-T), brief Eyes Movement Desensitization and Reprocessing (EMDR) and cognitive therapy without exposure would have a clinically important effect (Roberts et al., 2010). Anxiety, depression, and PTSD are less frequent at least three months after a traumatic event in people presenting an acute stress disorder if they have benefited from early CBT-T compared to supportive counselling (Kornor et al., 2008).Given the currently available retrospective studies reporting a high prevalence of anxiety, depression and PTSD among COVID-19 pneumonia survivors (Rogers et al., 2020), we hypothesized that a psychotraumatic process is involved in psychiatric symptoms with a potential high level of peritraumatic dissociation during hospitalization. This study aims to evaluate the prevalence of psychiatric symptoms in COVID-19 patients who benefited from early detection and treatment, during hospitalization and at three months following discharge, and to assess possible risk factors for anxiety, depression and PTSD.
2. Methods
2.1. Implementation of a systematic screening for psychological suffering
Preparing for an influx of COVID-19 patients, the Geneva University Hospitals set up the “CoviCare” (Nehme et al., 2020) program - a coordinated and multidisciplinary strategy, including specialists in internal medicine, pulmonary disease, primary care, infectious diseases and psychiatry. “CoviCare” aimed to provide remote ambulatory follow-up for COVID-19 outpatients and following hospitalization. The main objective was to provide care while ensuring a safe transition when patients were discharged from the hospital, allowing for early discharges when necessary and preventing readmissions and the use of emergency services. The division of liaison psychiatry was specifically mobilized to identify patients at risk of developing psychiatric complications during their hospital stay, so as to initiate an appropriate treatment and reduce the psychiatric risks and consequences in the short, medium and long term. Three months following hospitalization, a second systematic psychiatric assessment was performed to identify patients with signs of mental distress, in order to provide appropriate care.
2.2. Psychiatric intervention
Depending on their mental status, at each of these assessments the psychiatrist provided an intervention. The psychiatrist gave psychoeducation about PTSD and explained dissociative symptoms, if identified. Patients were taught techniques of stress management, and could benefit from early and short psychotherapeutic interventions of trauma-focused CBT, EMDR or cognitive-therapy sometimes combined with pharmacological treatment during hospitalization but also after they left hospital if needed. All participating individuals benefited from feed-back at their three-month assessment, as well as a short psychoeducation intervention. This intervention aimed to increase patients’ awareness of the risks of mental suffering in the short, medium and long term, and provided them with possibility to be referred for further follow-up.
2.3. Study population
We screened all patients 18 years and older, hospitalized at the Geneva University Hospitals (HUG) for COVID-19 pneumonia during the first wave of the COVID-19 outbreak from the March 30th, 2020 to 1st July 2020. Exclusion criteria included non-French speaking individuals, and the inability to fill the questionnaires due to cognitive or physical impairment.
2.4. Measurements
Psychiatric symptoms were assessed using the following self-reported questionnaires in their French version: (1) the peritraumatic dissociative experiences questionnaire (PDEQ), which screens for dissociative symptoms such as depersonalization and derealization (Birmes et al., 2005) during and immediately following a traumatic event (COVID-19 disease in this study), using a cutoff at 15 to identify a high risk of future PTSD; (2) the posttraumatic stress disorder checklist for DSM-5 (PCL-5) which assesses current symptoms of PTSD (Ashbaugh et al., 2016), using a cutoff score of 1 to identify at least one symptom of PTSD and a cutoff score of 31 to identify a PTSD diagnosis; (3) Hospital Anxiety and Depression Scale (HADS) which assesses transdiagnostic symptoms of anxiety and depression in patients with a somatic disorder, using a cutoff total score of 11 for anxiety and for depression (Zigmond and Snaith, 1983). It was specified to patients that the traumatic event to consider was the COVID-19 disease for both PDEQ and PCL5.
2.5. Data collection
At their first assessment during hospitalization (T0), patients completed self-reported questionnaires provided by the liaison psychiatry staff. The questionnaires explored questions about peritraumatic dissociation, PTSD, anxiety and depression (see below 2.4). If the patient had been hospitalized in the ICU, this assessment occurred after the patient was transferred to a regular medicine ward. For the second assessment at three months (T1), a second self-reported set of questionnaires on PTSD, anxiety and depression but not peritraumatic dissociation was sent by e-mail or by postal mail. Patients whose questionnaire scores exceeded the established cut-off (PDEQ>15 or/and HADS-A>11 or/and HADS-D>11 or/and PCL5>31), were referred to a psychiatric consultation by the liaison psychiatry team, consisting of an adapted psychiatric and/or psychotherapeutic intervention. Demographic data including age and sex, and medical data including hospitalization in the intensive care unit (ICU) were collected via the electronic medical record. The presence or absence of a psychiatric follow-up prior prior to hospitalization or not was collected at the 1-month phone follow-up done by the CoviCare team.
2.6. Statistical analysis
Continuous variables were reported as means and standard deviation or median and interquartile range (IQR) in case of non-normal distribution. Differences are compared using Student's t-test or the Wilcoxon rank-sum test, as appropriate. Categorical data are expressed as numbers and frequencies (%) and compared with Pearson's chi-square test. Univariate and multivariate regressive linear model have been built to assess the factors associated with the PCL5 and HADS scales at T0, and T1. We looked for risk factors at the acute phasis of COVID-19 and at the post-illness stage because we considered that psychopathological mechanisms could differ in the acute phasis in comparison with the post-illness stage. For instance, there are data concerning the influence of the type of trauma -considered as a risk factor-on the delay of development of PTSD showing that intentional traumas lead more late symptoms of PTSD than non-intentional traumas (Santiago et al., 2013).
Univariate and a multivariate regressive linear model have been built to assess the factors associated with the PCL5 and HADS scales at T0 and T1. We used the following factors: age, gender, hospitalization in an ICU unit, the presence of a psychiatric follow-up and PDEQ at T0. For the univariate model of HADS and PCL5 at T1, we also included HADS and PCL5 at. Due to the strong correlation between the four scores, only the PDEQ score was included in the final multivariate regression model to avoid a co-linearity effect. This choice was guided by our research hypothesis that peritraumatic dissociation is the main factor involved in the COVID-19 psychotraumatic process. Due to the absence of linear correlation between age and the outcomes, age data were categorized into three groups (≤39, 40 to 64, ≥65 years). To optimize normality and homosedasticity of residues, we chose to use the log of the outcomes in the multivariate models (PCL-5 and HADS scales at T0 and T1). We used STATA 15 for the statistical analyses.
2.7. Ethical considerations
Ethics approval was obtained from the Cantonal Commission for Research Ethics (2020–01241). All participants provided informed consent.
3. Results
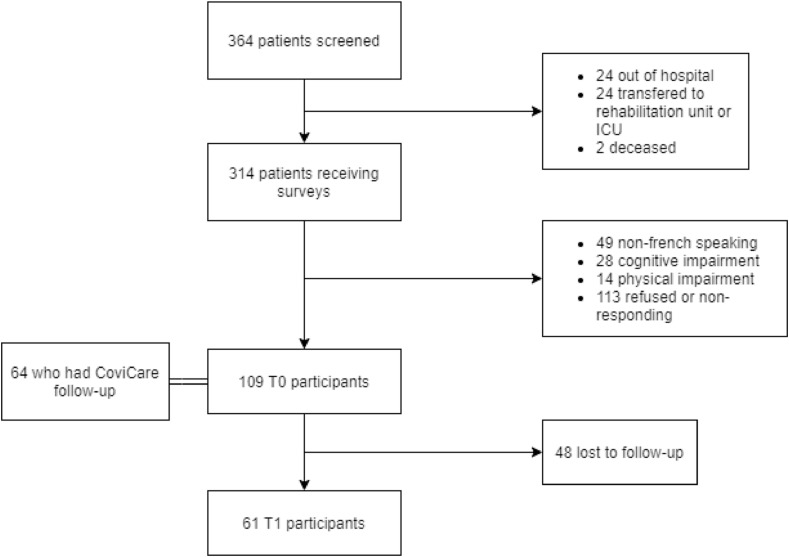
A total of 364 patients were hospitalized between the 30th of March and 1st of July 2020 (Fig. 1 ). Of these, fifty patients did not receive the questionnaires, 92 were subsequently excluded (49 non-French speaking, 28 cognitive impairment, 14 physical impairment) and 113 refused to participate. 109 participants were included at T0, of which 64 benefited from a COVICARE follow-up. Further, 48 patients were lost to follow up between T0 and T1 leading to a total of 61 participants at T1. The mean age was 56.8 years (+/− SD 18–86 years), 61.5% (67) were men and 16.5% (Ashbaugh et al., 2016) required intensive care. Among the 64 participants benefiting from COVICARE follow-up post-hospital discharge, 40.6% reported a psychiatric follow-up one month following hospitalization.
Fig. 1.
Flow chart.
The socio-demographic characteristics, and T0 and T1 results are summarized in Table 1 and Table 2 . A stratified descriptive and univariate analysis by age, gender and ICU hospitalization is available in Table 5 .
Table 1.
Socio-demographic characteristics.
| Age (mean, range) | 56.83 (18–86) |
|---|---|
| Gender | |
| Male | 67 (61.5) |
| Female | 42 (38.5) |
| ICU (N, %) | 18 (16.5) |
| Psychiatric follow-up (N %)a | 26 (40.6) |
For N = 64 patients with CoviCare data.
Table 2.
T0 and T1 PDEQ, PCL5 and HADS for anxiety and depression results (results stratified by age, gender and ICU hospitalization are available in Table 5).
| T0 (N = 109) | T1 (N = 64) | |
|---|---|---|
| PDEQ | ||
| PDEQ score (median, IQR) | 13 (11–23) | NA |
| Positive PDEQ (N, %)) | 49 (45.0%) | NA |
| PCL5 | ||
| PCL5 (median, IQR) | 11 (3–23) | 6 (3–16) |
| Positive PCL5 (N, %) | 15 (14.6%) | 7 (10.6%) |
| ≥1 symptom on PCL5 (N, %) | 88 (85.4%) | 58 (87.9%) |
| HADS-Anxiety | ||
| HADS-A (median, IQR) | 6 (3,5–9) | 4 (2,5–7,5) |
| Positive HADS-A (N,%) | 17 (15.7) | 6 (10.0) |
| HADS-depression | ||
| HADS-D (median, IQR) | 3 (1–8) | 2 (1–6) |
| Positive HADS-dep (N, %) | 20 (18.5) | 6 (10.0) |
Table 5.
Socio-demographic characteristics, T0 and T1 results stratified by age, gender and ICU hospitalization.
| Age category |
Gender |
ICU hospitalization |
Total |
|||||
|---|---|---|---|---|---|---|---|---|
| <40 years (N = 17) | 40–64 years (N = 55) | ≥65 years (N = 37) | Male (N = 67) | Female (N = 42) | No (N = 91) | Yes (N = 18) | N = 109 | |
| Male Gendera | 7 (41.2) | 37 (67.3) | 23 (62.2) | 51 (56) | 16 (88.9) | |||
| ICU hospitalizationa | 0 (0) | 10 (18.2) | 8 (21.6) | 16 (23.9) | 2 (4.8) | |||
| Psychiatric follow-upa | 4 (36.4) | 16 (43.2) | 6 (37.5) | 13 (32.5) | 13 (54.2) | 22 (40) | 4 (44.4) | |
| PDEQ | ||||||||
| PDEQ score at T0b | 13 (11–15) | 15 (11–27) | 12 (10–21) | 14 (11–24) | 13 (10–20) | 12 (10–21) | 21.5 (14–33) | 13 (11–23) |
| Positive PDEQ at T0a | 5 (29.4) | 29 (52.7) | 15 (40.5) | 32 (47.8) | 17 (40.5) | 36 (39.6) | 13 (72.2) | 49 (45.0%) |
| HADS-A | ||||||||
| HADS-A score at T0b | 5 (4–9) | 7 (3–10) | 6 (3–7,5) | 5 (3–8) | 6.5 (5–10) | 6 (4–9) | 5 (3–7) | 6 (3,5–9) |
| Positive HADS-A at T0a | 3 (17.6) | 12 (21.8) | 2 (5.6) | 9 (13.6) | 8 (19) | 16 (17.8) | 1 (5.6) | 17 (15.7) |
| HADS-A score at T1b | 4 (3–8) | 3 (2–6) | 5 (2.5–8.5) | 3.5 (2–6) | 4.5 (3–8) | 4 (3–8) | 2 (1–3) | 4 (2,5–7,5) |
| Positive HADS-A at T1a | 1 (7.7) | 3 (9.7) | 2 (12.5) | 5 (13.9) | 1 (4.2) | 6 (11.8) | 0 (0) | 6 (10.0) |
| HADS-D | ||||||||
| HADS-D score at T0b | 5 (3–11) | 3 (1–10) | 2.5 (1–6) | 2.5 (1–6) | 6 (2–11) | 4 (1–10) | 1 (1–4) | 3 (1–8) |
| Positive HADS-D at T0a | 5 (29.4) | 13 (23.6) | 2 (5.6) | 9 (13.6) | 11 (26.2) | 20 (22.2) | 0 (0) | 20 (18.5) |
| HADS-D score at T1b | 4 (2–9) | 2 (1–6) | 2 (1–4) | 2 (1–6) | 3 (1–5.5) | 2 (1–6) | 1 (1–1) | 2 (1–6) |
| Positive HADS-D at T1a | 1 (7.7) | 3 (9.7) | 2 (12.5) | 5 (13.9) | 1 (4.2) | 6 (11.8) | 0 (0) | 6 (10.0) |
| PCL-5 | ||||||||
| PCL5 score at T0b | 9.5 (4.5–27) | 10 (3–23) | 11 (2–20) | 10 (2–22) | 11.5 (4–23) | 10 (2–23) | 15.5 (6–22) | 11 (3–23) |
| Positive PCL5 at T0a | 2 (12.5) | 9 (17.3) | 4 (11.4) | 11 (16.9) | 4 (10.5) | 12 (14.1) | 3 (16.7) | 15 (14.6) |
| ≥1 symptom on PCL5 at T0a | 14 (87.5) | 43 (82.7) | 31 (88.6) | 55 (84.6) | 33 (86.8) | 70 (82.4) | 18 (100) | 88 (85.4) |
| PCL5 score at T1b | 16 (4–25) | 5 (1–11) | 10 (4.5–18) | 5 (1–11) | 12 (5–20) | 8.5 (3.5–18.5) | 4 (2–5) | 6 (3–16) |
| Positive PCL5 at T1a | 3 (23.1) | 1 (3.1) | 2 (12.5) | 3 (8.3) | 3 (12) | 6 (11.5) | 0 (0) | 7 (10.6) |
| ≥1 symptom on PCL5 at T1a | 12 (92.3) | 26 (81.3) | 15 (93.8) | 30 (83.3) | 23 (92) | 44 (84.6) | 9 (100) | 58 (87.9) |
Expressed by N (%).
Expressed by median (IQR) in bold: significative difference with chi2, wilcoxon or kruskall wallis test when appropriate.
3.1. Psychiatric symptoms in hospitalized patients for COVID-19
There were 44.9% of inpatients with a PDEQ score higher than 15 during hospitalization, with median PDEQ score 13 (IQR 11–23). Patients with pathological levels of anxiety defined by HADS-A>11 amounted to 17% of inpatients and 7% at the three-month follow-up, as well as pathological levels of depression defined by HADS-D >11 in 20% of inpatients and 7% at the three-month follow-up. The medians of HADS-A score and HADS-D score were respectively 6 (IQR 3,5–9) and 3 (IQR 1–8) at T0 and 4 (IQR 2,5–7,5) and 2 (IQR 1–6) at T1. Patients with PTSS defined like at least one positive answer at the PCL5 scale were 85.4% at T0 and 86.9%, whereas patients with a diagnosis of PTSD defined by PCL5 >31reached 14.6% of patients at T0 and 10.6% at T1. The median PCL5 was 11 (IQR 3–23) at T0 and 6 (IQR 3–16) at T1. These data suggest a general improvement in psychiatric related outcomes three months post-hospitalization.
3.2. Risk factors of anxiety, depression and PTSD during hospitalization and at three months
Female gender was associated with higher HADS-D score at T0 in both univariate (Table 5) and multivariate regression models (β = 0.58 for the log of HADS-D, p = 0.019, data not shown, available upon request). Age ≥65 years old was associated with lower HADS for depression at T0 (p = 0.027, Table 5) only in the univariate analysis. Psychiatric follow-up recorded at 1 month following hospitalization and was associated with higher HADS-A score at T1 (β = 0.52, p = 0.028) only in the multivariate analysis.
In univariate analysis, all the four T0 scores (PDEQ, HADS-A, HADS-D and PCL5) were correlated with PCL5 at T1. PCL5, HADS-A and HADS-D at T0 were correlated with both HADS-A and HADS-D at T1 (Table 3).
Table 3.
Univariate analysis results for PCL5, HADS-A and HADS-D at T1.
| PCL5 T1 | HADS A T1 | HADS D T1 | |
|---|---|---|---|
| Age by category | |||
| <40 years | – | – | – |
| 40–64 years | −9.5** | 1.32 | −2.31 |
| ≥65 years | −4.2 | −0.18 | −1.68 |
| Female Gender | 5.34 | 0.61 | 0.35 |
| ICU hospitalization | −8.14 | −3* | −2.48 |
| Presence of a psychiatric follow-up | 5.05 | 2.07 | 2.16 |
| PDEQ at T0 (per point on the score) | 0.4** | 0.02 | 0.09 |
| PCL5 at T0 (per point on the score) | 0.51** | 0.08** | 0.12** |
| HADS-A at T0 (per point on the score) | 1.38** | 0.31** | 0.21 |
| HADS-D at T0 (per point on the score) | 1.88** | 0.32** | 0.51** |
Results shown as regression coefficient β, ** p-value < 0.05.
Patients requiring an ICU stay, compared to those who did not, had significantly higher PDEQ score at T0 (24.1 versus 15.6 p = 0.0014) (Table 5), but a lower HADS-A score at T1 (5.3 vs 2.3, p = 0.01) and a lower HADS-D score at T0 (5.4 vs 2.6, p = 0.01). In the multivariate analysis using the log of the outcomes (Table 4), ICU stay was associated with a lower PCL-5 at T1 (β = −1.24, p = 0.012), a lower HADS-A at T1 (β = −0.77, p = 0.019), and lower HADS-D at T0 (β = −1.03, p = 0.004), and T1 (β-1.06, p = 0.023).
Table 4.
Multivariate regression model analysis results for the log of PCL5, HADS-A and HADS-D at T1.
| PCL5 T1 | HADS A T1 | HADS D T1 | |
|---|---|---|---|
| Age by category | |||
| <40 years | – | – | – |
| 40–64 years | −0.45 | −0.34 | −0,09 |
| ≥65 years | −0.37 | −0.39 | 0,05 |
| Female Gender | 0.58 | 0,04 | 0,03 |
| ICU hospitalization | −1.23** | −0.77** | −1.06** |
| Presence of a psychiatric follow-up | −0.14 | 0.52** | 0.35 |
| PDEQ at T0 (per point on the PDEQ score) | 0,04** | 0,00 | 0,03** |
| R2 | 0.38 | 0.35 | 0.30 |
| F-ratio | 3.2 | 2.92 | 1.97 |
** p-value < 0.05.
Finally, PDEQ score at T0 was positively correlated to PCL5 at T1 (β = 0.26, p = 0.01) and HADS-D at T1 (β = 0.15, p = 0.018). In the multivariate analysis using the log of the outcomes, PDEQ score showed a high correlation with HADS-A, HADS-D and PCL5 at T0 (respectively β = 0.03, p = 0.001, β = 0.03, p = 0.007, β = 0.06, p = 0.000) and also PCL5 at T1 (β = 0.56, p = 0.01).
R2 and F-ratio were rather small in our three multivariate models (Table 4).
3.3. Missing data analysis
Compared to individuals who responded at three months (n = 61), those who did not respond (n = 47) showed during hospitalization significantly higher score of PDEQ (p = 0.04), more pathological score of PDEQ (p = 0.01), more positive PCL5 score (p = 0.04) and more positive HADS-D score (p = 0.03) (Table 6 ).
Table 6.
Missing data analysis.
| Data at T1 |
Missing data at T1 |
p-valuea |
|||
|---|---|---|---|---|---|
| N/Median | %/IQR | N/Median | %/IQR | ||
| Male Gender** | 36 | 59 | 31 | 64.6 | 0.30 |
| ICU hospitalization | 9 | 14.8 | 9 | 18.8 | 0.58 |
| Psychiatric follow up | 20 | 47.6 | 6 | 27.3 | 0.18 |
| PDEQ | |||||
| Positive PDEQ at T0 | 22 | 36.1 | 27 | 56.3 | 0.04 |
| PDEQ score at T0 | 12 | 10–18 | 15.5 | 12–27 | 0.01 |
| PCL-5 | |||||
| PCL-5 score at T0 | 10 | 3–18 | 14.5 | 3–30 | 0.08 |
| Positive PCL-5 at T0 | 5 | 8.2 | 10 | 23.8 | 0.04 |
| HADS for anxiety | |||||
| HADS-A score at T0 | 5 | 3–8 | 6 | 4–9 | 0.19 |
| Positive HADS-A at T0 | 7 | 11.5 | 10 | 21.3 | 0.17 |
| HADS for depression | |||||
| HADS-D score at T0 | 3 | 1–6 | 4 | 1–11 | 0.21 |
| Positive HADS-D at T0 | 7 | 11.5 | 13 | 27.7 | 0.03 |
Using Chi2 or Wilcoxon test.
4. Discussion
Our study confirms that psychiatric disorders are very common in patients hospitalized for COVID-19 pneumonia. The structured longitudinal follow-up demonstrates the persistence of these symptoms for a substantial proportion of patients at three-month follow-up, despite providing a structured psychiatric support. In our study, peritraumatic dissociative symptoms as a risk factor for PTSD were the most frequent pathological score associated with COVID-19 at the acute stage of the illness. Lower PTSD levels were seen at three months in individuals 40–65 years old, possibly because this age group was never considered in the general population as the age group particularly at risk of severe forms of COVID-19, thus potentially reducing the anxiety related to their hospitalization.
Ranking highest at pathological levels was the PDEQ score among inpatients, considered as a risk factor for PTSD at their first assessment (T0), while PCL5 scores where highest at the three-month assessment, followed by high rates of pathological scores of HADS-A and HADS-D. The dissociative experience is impressive and may have a lasting impact on the people who are affected. Many patients testified that the handing over of the PDEQ questionnaire reassured them, because it normalized what they had experienced by legitimizing dissociation symptoms. Our study found a strong association between peritraumatic dissociation and PTSD at three months that confirms the predictive value of peritraumatic dissociation (Ozer et al., 2003). This suggests that the PDEQ score could be predictive of PTSD at three months for hospitalized patients for COVID-19. Our study found a higher rate of PTSD than the prevalence of lifetime PTSD in a general population, recently estimated at 8.3% based on DSM5 criteria (Santiago et al., 2013). The retrospective study of Mazza and coworkers (2020), using the PCL5 scale post hospitalization for patients with COVID-19, found a 15.2% rate of PTSD, comparable to our study with 14.6% of patients with a PTSD diagnosis during hospitalization and 10.6% at three months post hospitalization and peritraumatic dissociation during hospitalization was not assessed. They found a higher rate compared to the rate found with IES-R based on DSM-IV at 28% that was also used in this study and which is known to overestimate PTSD diagnosis (Kilpatrick et al., 2013). In comparison with other infectious diseases, Roger's metaanalysis focusing on previous coronavirus infections, patients admitted to hospital for SARS or MERS, presented high rates of depressive symptoms (32.6%) and anxiety (35.7%) during hospitalization and high depressive symptoms (14.9% %), anxiety (14.8%) and PTSD symptoms rate (32.2%) in the post-illness stage (Rogers et al., 2020). There were no data during hospitalization concerning peritraumatic dissociation.
Our study was not only observational but also interventional at follow-up. In case of detection of psychiatric symptoms during hospitalization, patients preemptively benefited from mental care during their hospital stay. The systematic screening and proactive approach allowed early intervention, which may have contributed to the overall improvement in the mental health state of patients in terms of anxiety, depression and PTSD symptoms between T0 (during hospitalization) and T1 (at three months). This would be in accordance with previous reports demonstrating that specific psychological early intervention after a traumatic injury is associated with a significant reduction of PTSD in case of detection of acute stress symptoms (Roberts et al., 2010; Kornor et al., 2008). All the psychiatric scores (PDEQ, HADS-A, HADS-D, PCL5) of the first assessment during the hospital stay were strongly correlated with PTSD at three months in the univariate analysis. In the absence of a control group however, we cannot conclude that the psychiatric prognosis has been improved in patients who benefited from the liaison psychiatry intervention.
Although we noted more peritraumatic dissociative symptoms during hospitalization for patients who required an ICU stay, these patients paradoxically showed less symptoms of PTSD, depression and anxiety three months following hospitalization. While a protective effect of the ICU experience in itself is unlikely, this may reflect a stronger support and follow-up provided to this population. Indeed, the use of diaries and the continuous care in ICU settings may be involved and potentially associated with a reduction of PTSD incidence (Praker et al., 2015). A second hypothesis is that an ICU stay is associated with more severe physical sequelae (Inoue et al., 2019) which needed a very high level of care provided by a multidisciplinary team including psychiatric care, which later improved psychiatric outcomes for this population. The third hypothesis is that patients who required the ICU had great propensity to dissociate which would explain the low levels of PCL5. However, the low levels of HADS-A and HADS-D scores seen in these patients may not specifically assess dissociative symptoms. These patients would present less emotional symptoms and more functional symptoms like cognitive impairment with attentional and concentration disorders, and less typical PTSD, anxiety or depressive symptoms. The evolution of symptoms in post-ICU patients could hide some longer lasting dissociative symptoms and should be explored in future studies assessing specific dissociative symptoms. Finally, we could consider elements of post-traumatic growth in post-ICU patients.
5. Limitations
Our study has several limitations, including our sample size and the percentage of missing data at the three-month follow-up and the relatively low proportion of patients who needed ICU admission. Our missing data analysis suggests that individuals with more psychiatric symptoms during hospitalization were more likely to be lost to follow-up at three months, thus underestimating the psychiatric impact of COVID-19. We did not have data about other confounding factors as psychiatric history. We did not have a comparison group of hospitalized patients for other medical reasons that could highlight potential specific mechanisms involved in psychiatric complications of COVID-19. We did not collect data about the existence neither the nature of the psychiatric intervention during and after hospitalization for COVID-19 to assess its influence on the development of psychiatric complications, and we just analyzed data on psychiatric follow-up.
6. Conclusion
This study confirmed that COVID-19 pneumonia was associated with a high prevalence of psychiatric symptoms, particularly PTSS and persisting PTSD patterns, three months post-hospital discharge. Depression, anxiety and peritraumatic dissociation during hospitalization were predictive of PTSD at three months. Systematic screening of depression, anxiety and peritraumatic dissociation should be done during hospitalization for all COVID-19 patients. An evaluation of the effectiveness of PTSD prevention strategies for patients hospitalized with COVID-19 remains necessary in future studies as well as longer-term evaluations of these patients.
Contributors
LB made the literature search, the study design, assured the data collection and data interpretation and wrote the manuscript. OB wrote the statistical methodology, assured the data analysis and contributed to write the manuscript, made the tables. VM contributed to data analysis. Dominique Gex contributed to data collection. MN contributed to data collection and writing of the manuscript. SAP contributed to writing of the manuscript. TA contributed to writing and revision of the manuscript. GK contributed to writing of the manuscript. SC contributed to writing of the manuscript. GB contributed to writing of the manuscript. FL contributed to writing and revision of the manuscript.
Author agreement statement
Me, Lamyae Benzakour, the undersigned declare that this manuscript is original, has not been published before and is not currently being considered for publication elsewhere.
I confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed.
I further confirm that the order of authors listed in the manuscript has been approved by all of us.
I understand that the Corresponding Author is the sole contact for the Editorial process. I am responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpsychires.2021.05.031.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Ashbaugh A.R., Houle-Johnson S., Herbert C., El-Hage W., Brunet A. Psychometric validation of the English and French versions of the posttraumatic stress disorder checklist for DSM-5 (PCL-5) PloS One. 2016;11(10) doi: 10.1371/journal.pone.0161645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astill Wright L., Sijbrandij M., Sinnerton R., Lewis C., Roberts N.P., Bisson J.I. Pharmacological prevention and early treatment of post-traumatic stress disorder and acute stress disorder: a systematic review and meta-analysis. Transl. Psychiatry. 2019;9(1):334. doi: 10.1038/s41398-019-0673-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard-Gilligan M., Zoellner L.A. Dissociation and memory fragmentation in post-traumatic stress disorder: an evaluation of the dissociative encoding hypothesis. Memory. 2012;20(3):277–299. doi: 10.1080/09658211.2012.655747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmes P., Brunet A., Benoit M., Defer S., Hatton L., Sztulman H., Schmitt L. Validation of the Peritraumatic Dissociative Experiences Questionnaire self-report version in two samples of French-speaking individuals exposed to trauma. Eur. Psychiatr. 2005;20(2):145–151. doi: 10.1016/j.eurpsy.2004.06.033. [DOI] [PubMed] [Google Scholar]
- Dubey S., Biswas P., Ghosh R., Chatterjee S., Dube y M.J., Chatterjee S., Lahiri D., Lavie C.J. Psychosocial impact of COVID-19. Diabetes Metab Syndr. 2020;14(5):779–788. doi: 10.1016/j.dsx.2020.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue S., Hatakeyama J., Kondo Y., Hifumi T., Sakuramoto H., Kawasaki T., Taito S., Nakamura K., Unoki T., Kawai Y., Kenmotsu Y., Saito M., Yamakawa K., Nishida O. Post-intensive care syndrome: its pathophysiology, prevention, and future directions. Acute Med Surg. 2019;6(3):233–246. doi: 10.1002/ams2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick D.G., Resnick H.S., Milanak M.E., Miller M.W., Keyes K.M., Friedman M.J. National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J. Trauma Stress. 2013;26(5):537–547. doi: 10.1002/jts.21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornør H., Winje D., Ekeberg Ø., Weisaeth L., Kirkehei I., Johansen K., Steiro A. Early trauma-focused cognitive-behavioural therapy to prevent chronic post-traumatic stress disorder and related symptoms: a systematic review and meta-analysis. BMC Psychiatr. 2008;8:81. doi: 10.1186/1471-244X-8-81. 2008 Sep 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krähenbühl M., Oddo M., Piquilloud L., Pantet O. COVID-19: intensive care management. Rev. Med. Suisse. 2020;16(691–2):863–868. [PubMed] [Google Scholar]
- Mazza M.G., De Lorenzo R., Conte C., Poletti S., Vai B., Bollettini I., Melloni E.M.T., Furlan R., Ciceri F., Rovere-Querini P. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav. Immun. 2020;89:594–600. doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehme M., Braillard O., Alcoba G., Aebischer Perone S., Courvoisier D., Chappuis F., Guessous I. COVID-19 symptoms: longitudinal evolution and persistence in outpatient settings. Ann. Intern. Med. 2020;Dec 8:M20–M5926. doi: 10.7326/M20-5926. Epub ahead of print. PMID: 33284676; PMCID: PMC7741180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozer E.J., Best S.R., Lipsey T.L., Weiss D.S. Predictors of posttraumatic stress disorder and symptoms in adults: a meta-analysis. Psychol. Bull. 2003;129(1):52–73. doi: 10.1037/0033-2909.129.1.52. [DOI] [PubMed] [Google Scholar]
- Parker A.M., Sricharoenchai T., Raparla S., Schneck K.W., Bienvenu O.J., Needham D.M. Posttraumatic stress disorder in critical illness survivors: a metaanalysis. Crit. Care Med. 2015;43(5):1121–1129. doi: 10.1097/CCM.0000000000000882. [DOI] [PubMed] [Google Scholar]
- Roberts N.P., Kitchiner N.J., Kenardy J., Bisson J.I. Early psychological interventions to treat acute traumatic stress symptoms. Cochrane Database Syst Rev. Mar. 2010;17(3):CD007944. doi: 10.1002/14651858.CD007944.pub2. [DOI] [PubMed] [Google Scholar]
- Rogers J.P., Chesney E., Oliver D., Pollak T.A., McGuire P., Fusar-Poli P., Zandi M.S., Lewis G., David A.S. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7(7):611–627. doi: 10.1016/S2215-0366(20)30203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago P.N., Ursano R.J., Gray C.L., Pynoos R.S., Spiegel D., Lewis-Fernandez R., Friedman M.J., Fullerton C.S. A systematic review of PTSD prevalence and trajectories in DSM-5 defined trauma exposed populations: intentional and non-intentional traumatic events. PloS One. 2013;8(4) doi: 10.1371/journal.pone.0059236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taquet M., Luciano S., Geddes J.R., Harrison P.J. Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatry. Feb. 2021;8(2):130–140. doi: 10.1016/S2215-0366(20)30462-4. https://doo.org/10.1016/S2215-0366(20)30462-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vindegaard N., Benros . COVID-19 pandemic and mental health consequences: systematic review of the current evidence. Brain Behav. Immun. 2020;89:531–542. doi: 10.1016/j.bbi.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Xu X., Chen Z., Duan J., Hashimoto K., Yang L., Liu C., Yang C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav. Immun. 2020;87:18–22. doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization Interim Briefing Note- Taking into account the psychosocial and mental health aspects of the Covid-19 epidemic. 2020. https://interagencystandingcommittee.org/iasc-reference-group-mental-health-and-psychosocial-support-emergency-settings/interim-briefing (accessed March 17, 2020)
- World Health Organization . 2020. WHO mhGAP Action Programme. Support based on psychological first aid principles in people recently exposed to a traumatic event.www.who.int/mental_health/mhgap/evidence/other_disorders/q6/en/ 13May2020. [Google Scholar]
- World Health Organization . 2020. WHO mhGAP Action Programme. Psychological first aid: a guide for actors in the field.www.who.int/mental_health/publications/guide_field_workers/fr/ 18May2020. [Google Scholar]
- Zigmond A.S., Snaith R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.