Abstract
Uncontrolled bleeding associated with trauma and surgery is the leading cause of preventable death. Batroxobin, a snake venom-derived thrombin-like serine protease, has been shown to clot fibrinogen by cleaving fibrinopeptide A in a manner distinctly different from thrombin, even in the presence of heparin. The biochemical properties of batroxobin and its effect on coagulation have been well characterized in vitro. However, the efficacy of batroxobin on hemostatic clot formation in vivo is not well studied due to the lack of reliable in vivo hemostasis models. Here, we studied the efficacy of batroxobin and slounase, a batroxobin containing activated factor X, on hemostatic clot composition and bleeding using intravital microcopy laser ablation hemostasis models in micro and macro vessels and liver puncture hemostasis models in normal and heparin-induced hypocoagulant mice. We found that prophylactic treatment in wild-type mice with batroxobin, slounase and activated factor X significantly enhanced platelet-rich fibrin clot formation following vascular injury. In heparin-treated mice, batroxobin treatment resulted in detectable fibrin formation and a modest increase in hemostatic clot size, while activated factor X had no effect. In contrast, slounase treatment significantly enhanced both platelet recruitment and fibrin formation, forming a stable clot and shortening bleeding time and blood loss in wild-type and heparin-treated hypocoagulant mice. Our data demonstrate that, while batroxobin enhances fibrin formation, slounase was able to enhance hemostasis in normal mice and restore hemostasis in hypocoagulant conditions via the enhancement of fibrin formation and platelet activation, indicating that slounase is more effective in controlling hemorrhage.
Keywords: hemostasis, platelet, fibrin, coagulation, bleeding, batroxobin
Introduction
Life-threatening bleeding due to traumatic injury or surgical procedures is the leading cause of preventable death in the world.1 Excessive blood loss accounts for around 40% of the deaths associated with trauma.2 Hemostasis is a complex physiological process, which arrests bleeding and involves blood cells and plasma as well as extracellular and matrix proteins. Platelet accumulation and activation of the coagulation system are two key mechanisms required for hemostatic clot formation to stop bleeding.3,4 Platelet adhesion and subsequent platelet activation and aggregation at the site of vascular injury are vital steps in initiating the hemostatic process to form platelet plugs (primary hemostasis) and prevent blood loss.3,5 It has been shown that von Willebrand factor (VWF) and fibrinogen (Fg) are the two molecules required for platelet adhesion and aggregation in the event of vascular injury.3,5,6 Interestingly, platelet adhesion and aggregation still persist in mice lacking both VWF and Fg, even after further depletion of plasma fibronectin (pFn).7–9 We have shown that both plasma Fn and vitronectin (Vn) inhibit platelet aggregation in their soluble forms, however, the insoluble or cellular forms of pFN and Vn uniquely support platelet aggregation and promote hemostasis, demonstrating the complexities of the platelet aggregation process.9–11 Formation of a stable hemostatic clot also requires the activation of the coagulation cascade through a series of enzymatic reactions, leading to the generation of thrombin.12 Thrombin is the most potent platelet agonist known to amplify platelet activation. Thrombin generation ultimately leads to the formation of fibrin, further enhancing the platelet aggregation process and platelet-fibrin clot formation (secondary hemostasis) to effectively seal blood leakage.13–15 It is well documented that thrombin converts soluble fibrinogen to fibrin I monomers by releasing fibrinopeptide A and B from the NH2-terminal domains of the alpha- and beta-chains of fibrinogen.16 Exposed NH2 termini on fibrin monomers will initiate the fibrin polymerization at the site of vascular injury and further enhance the formation of a stable hemostatic clot.17,18 There are many interactions between the primary and secondary mechanisms of hemostasis. Activated platelets provide procoagulant cell surface membranes to activate the coagulation cascade and enhance thrombin generation. Conversely, thrombin generation also leads to further platelet activation, amplifying platelet recruitment into the growing platelet fibrin clot under shear conditions. Thus, the procoagulant activities of platelets and the formation of fibrin are crucial for effective hemostasis in order to limit bleeding. In pathological conditions, such as when the integrity of the vessel wall is disrupted by the rupturing of an atherosclerotic plaque, however, those same processes can also lead to occlusive thrombi and vessel occlusion.3,19
Impairment of hemostasis and excessive bleeding associated with trauma is primarily due to blood loss from injury in addition to subsequent activation of coagulation, hyperfibrinolysis, consumption of platelets, coagulation factors, and hemodilution from aggressive resuscitation.20,21 The existence of underlying medical conditions, such as congenital or acquired bleeding disorders, can also increase the risk of excessive bleeding in the event of vascular injury.22,23 While current anti-thrombotic therapies, including both anti-platelet and anti-coagulation therapies, may reduce or prevent the thrombotic complication of cardiovascular disease and possible mortality, it’s associated with the increased risk of bleeding due to the inhibition of key elements required for normal hemostasis.24 Despite the existence of a variety of antithrombotic agents that are clinically available, cessation of uncontrolled bleeding is mostly achieved by surgical interventions and systematic pharmacological reagents targeted at restoring hemostasis, especially in hypocoagulant conditions, are very limited.25 Furthermore, the effects of available hemostatic reagents on hemostatic clot composition in vivo are not well characterized due to the lack of reliable in vivo hemostasis models.
Snake venom serine proteinases have long been known to affect various physiological functions including blood coagulation and fibrinolysis.26–28 Snake venom toxins have been extensively studied as potential drug targets, and several toxin-based antithrombotic drugs are currently in use or under development for trials, including our previous work in the development of Anfibatide, the first-in-class anti-GPbα antagonist.29–31 Batroxobin is a thrombin-like serine protease from the venom of Bothrops atrox and is the most intensively studied active pharmaceutical ingredient from snake venom. In contrast to the cleavage of Fg by thrombin, batroxobin releases only FpA resulting in the polymerization of fibrin monomers or acting as a defibrase.32,33 This process is not inhibited by antithrombin or heparin cofactor II.34 Therefore, batroxobin can induce clot formation in platelet-rich plasma without affecting platelet function in vitro in the presence of heparin, making it a viable heparin-insensitive diagnostic test in parallel with thrombin time.35 Most recently, batroxobin has been shown to bind fibrin(ogen) with higher affinity than thrombin and promotes greater clot expansion in vitro. 36 Notably, the ability of batroxobin to polymerize fibrin makes it a potential hemostatic agent and it has been successfully isolated for development as a medical adhesive hemostatic drug.34,35,37 Snake venoms that contain both batroxobin and factor X activator have been reported to have a higher efficacy in promoting intravascular coagulation.38 This indicates that the cleavage of fibrinogen by batroxobin coupled with the promotion of pro-coagulant activity by factor X, the first enzyme in the common pathway of the coagulation cascade, might have a better hemostatic efficacy than either alone. While the findings from in vitro studies are promising, they may not fully reflect hemostatic clot formation in vivo, especially the dynamics of hemostatic clot composition, stability of the clot and the associated risk of bleeding. Therefore, further in vivo studies on the efficacy of snake venom-derived drugs on hemostatic clot formation and bleeding are warranted.
Slounase is a batroxobin extracted from the venom of viperidae containing activated factor X and formulated for small volume intravenous injections.39 In this study, we determine the effect of slounase on hemostatic clot composition and bleeding in vivo using intravital microcopy laser ablation hemostasis models in micro and macro vessels and liver puncture hemostasis models in normal and heparin-induced hypocoagulant mice. Furthermore, we assess the hemostatic potential of slounase in platelet recruitment and fibrin formation in growing thrombi in vivo in response to vascular injury in parallel with batroxobin and activated factor X and provide the mechanistic insights of these hemostatic reagents in the hemostatic process.
Materials and Methods
Reagents
Slounase, batroxobin and activated factor X were obtained from Lee’s Pharmaceutical Holdings Limited (Hong Kong China). Unfractionated Heparin was acquired from SAGENT Pharmaceuticals (IL, USA). DyLight 488 anti-GPIbβ was obtained from Emfret Analytics (Eibelstadt, Germany). Anti-mouse fibrin antibody was a kind gift from Dr. R. Camire (Children’s Hospital of Philadelphia) and was fluorescently labeled using an Alexa Flour 647 antibody labeling kit (Thermo Fisher).
Experimental Animals
All experimental procedures in this study were approved by the Institutional Animal Care and Use Committee at the University of Michigan. C57BL/6 wild-type (WT) mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA) and housed in the research facility at the University of Michigan.
Laser-Ablation Cremaster Arteriole Rupture Hemostasis Model
Adult male mice (10-12 weeks old) were anesthetized by an intraperitoneal injection of ketamine/xylazine (100 and 10 mg/kg, respectively) and the cremaster arteriole was surgically prepared and superfused with preheated bicarbonate saline buffer throughout the experiment as described.11,40 The mice were intravenously administered with DyLight 488-conjugated rat anti-mouse platelet GP1bβ antibody (0.1 μg/g; EMFRET Analytics) and Alexa Fluor 647-conjugated anti-fibrin (0.3 μg/g) via a jugular vein cannula prior to vascular injury, and cremaster microcirculation was monitored and recorded under multichannel intravital microscopy. Mice were intravenously injected in bolus with control buffer (normal coagulant control mice) or 1000 U/kg of unfractionated heparin to inhibit coagulation (hypocoagulant mice). Hemostatic reagents of batroxobin, activated factor X, or slounase (0.1 and 1U/kg) were administered intravenously by a jugular vein catheter prior to vascular injury. The cremaster muscle arteriole (30-50 µm in diameter) was exposed to a high intensity 532-nm laser pulse (70 lJ; 100 Hz; for about 7 ns) in order to puncture a hole through the vessel wall, which resulted in red blood cell (RBC) extravasation as visualized by RBC leakage (bleeding) from the vessel.
The entire process of RBC extravasation and formation of the platelet-fibrin hemostatic plug at the site of injury, which resulted in the cessation of RBC extravasation, was recorded in real-time using a Zeiss Axio Examiner Z1 intravital fluorescent microscope equipped with a solid laser launch system under a 63X water-immersion objective. The dynamics of platelet accumulation and fibrin formation within the hemostatic clot were analyzed by the changes in the mean fluorescent intensity over time using the Slidebook 6.0 program. The time required for the cessation of RBC extravasation following the rupture of the vessel wall was determined by reviewing single-frame still images under a bright field. Arterial bleeding time was defined as the time from laser pulse injury until cessation of RBC leakage from the vessel.
Laser Ablation Saphenous Vein Hemostasis Model
The laser ablation saphenous vein hemostasis model was performed as described in detail.41,42 Briefly, adult mice (10-12 weeks old) were anesthetized as described above and intravenously administered fluorescently labeled antibodies against platelets and fibrin as described above. The saphenous vein was surgically exposed under an intravital microscope and superfused throughout the experiment with preheated bicarbonate-buffered saline. Mice were intravenously injected in bolus with 1000U/Kg of unfractionated heparin alone or followed by 1 U/kg of batroxobin, activated factor X, or slounase, separately 10 minutes prior to vascular injury. Saphenous vein blood flow was visualized under a 20X water immersion objective using a Zeiss Axio Examiner Z1 fluorescent microscope. The saphenous vascular wall was exposed to two maximum-strength 532-nm laser pulses (70 lJ; 100 Hz; for about 7 ns, 10 ms intervals) to puncture a hole (48 to 65 µm in diameter) through the vessel wall, which resulted in bleeding visualized by the escape of fluorescent platelets to the extravascular space. The laser injury was performed at 30 seconds and repeated at 5- and 10-minute intervals at the same site of injury to assess the platelet-fibrin hemostat clot formation. The dynamics of platelet accumulation and fibrin deposition within the clot were recorded in real-time and analyzed as described above using the Slidebook 6.0 program.
Hepatic-Pricking Injury Bleeding Model
Adult mice (8-10 weeks old) were anesthetized as described above and were intravenously bolus injected with 1000 U/kg of heparin alone, followed by intravenous treatment of 1 U/kg of batroxobin, activated factor X, slounase or control buffer via the tail vein. The amount of blood loss following the injury was assessed using a modified hepatic-prick injury-bleeding model as described.37 Briefly, the anesthetized mice were placed in a supine position on a heating pad and the left lobe of the liver was exteriorized on parafilm via a midline incision through the abdominal wall in order to prevent the absorption of body fluids. Then, a penetrative pricking injury was made in the thickest area in the middle of the left liver lobe using a 16G needle. Immediately after making the injury, the pre-weighed filter paper and parafilm were placed under the lobe, allowing the filter paper to absorb blood for 10 minutes as the heating board was tilted at 45 degrees. The parafilm was used to prevent the absorption of body fluids other than blood exiting the wound. The filter paper with blood was measured by a microscale to evaluate the total amount of blood loss by injury in each mouse.
Statistical Analysis
Experimental results were analyzed by unpaired and paired two-tailed student t-tests as well as a two-way analysis of variance (ANOVA) between experimental groups with the Prism 7.0 software (GraphPad). Differences with a P-value less than 0.05 were considered statistically significant. Data is expressed as mean ± standard error of the mean (SEM).
Results
Slounase Treatment does Not Induce Platelet Adhesion or Aggregation in the Absence of Vascular Injury In Vivo
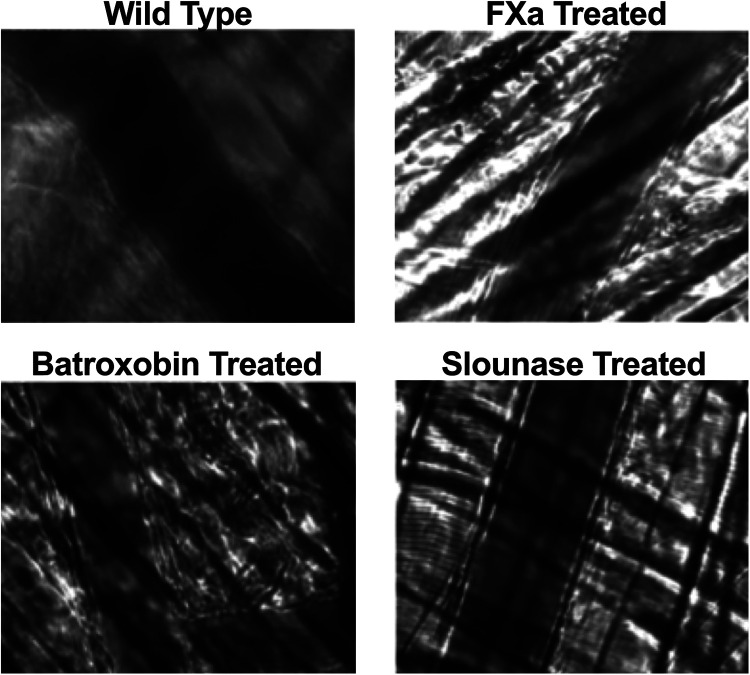
In order to determine if intravenous (IV) administration of slounase, batroxobin, or activated factor X results in spontaneous platelet adhesion, aggregation, or thrombus formation, blood flow in the cremaster microvessels was continuously monitored under brightfield and fluorescent channels with a real-time intravital microscope during the intravenous injection of slounase, batroxobin and activated factor X as well as 90 minutes post-treatment. No platelet interaction, adhesion, aggregate formation or fibrin formation was detected in respective channels in cremaster arterioles, venules and capillaries in the absence of vascular injury. Intravenous injection of 1 U/kg of slounase, batroxobin or activated factor X did not cause fluorescently labeled platelets to adhere to or form any visible platelet aggregates on the vessel wall, similar to mice treated with control buffer (5 mice per group). Intravenous treatment of these agents did not alter blood flow or spontaneously cause any detectable platelet-fibrin thrombosis in cremaster microcirculation in the extended 90 min recording period, indicating that slounase does not cause intravascular thrombosis in the absence of vascular injury (Figure 1).
Figure 1.
Intravenous administration of slounase, batroxobin or activated factor X did not cause detectable platelet adhesion or platelet aggregate formation in the absence of vascular injury in vivo. Blood flow in cremaster microcirculation of WT mice was monitored in real-time and recorded under the intravital microscope. Circulating platelets were fluorescently labeled as green and detection of fibrin was achieved by injecting fluorescently conjugated anti-fibrin antibody as red in vivo. 1 U/kg of activated factor X, batroxobin, slounase, or control buffer were intravenously injected while real-time recording and continuously monitored for an extended period of time up to 90 minutes without inducing vascular injury. No platelet adhesion, aggregation or fibrin formation was detected in cremaster microcirculation prior to, during, or up to 90 minutes post injection. Representative pictures of overlaid bright field with fluorescent channels after subtracting fluorescent backgrounds (10 min post injection) show no fluorescent platelet adherence, aggregation, or fibrin formation on the cremaster arterioles vessel wall in the absence of injury in vivo following the injection of slounase (bottom right), batroxobin (bottom left), or activated factor X (top right) respectively.
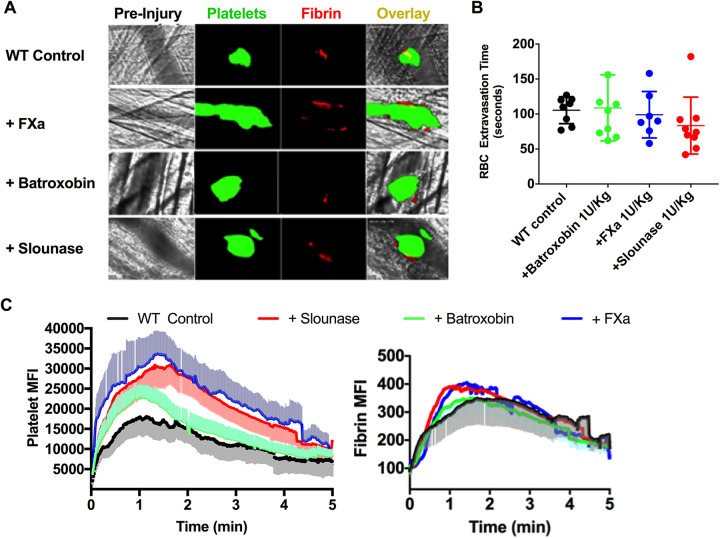
Slounase Treatment Enhances Platelet-Fibrin Hemostatic Clot Formation in Laser-Ablation Cremaster Arteriole Puncture Hemostasis Model in Wild-Type Mice
In order to confirm the effects of batroxobin, activated factor X, and slounase on hemostatic clot formation and bleeding in response to penetrative vascular injury in vivo, WT mice were intravenously administered 1 U/kg of batroxobin, activated factor X, or slounase 10 minutes prior to initiating a laser-induced puncture to the wall of the cremaster arteriole in the intravital microscopy laser-ablation cremaster arteriole hemostasis model, as we characterized in our previous study.40,42 In control mice, immediately upon cremaster arteriole vessel wall rupture induced by laser, red blood cells (RBCs) and blood components extravasated from the ruptured section of the cremaster arteriole, and the bleeding was monitored under a bright field (Figure 2A). The process of hemostatic clot formation at the site of arterial wall rupture was recorded under fluorescent channels as platelets accumulated at the point of vessel injury along with fibrin formation, leading to the formation of a hemostatic clot, but did not result in vessel occlusion. Formation of the platelet-rich fibrin clot at the site of arteriole rupture resulted in diminished RBC extravasation from the arteriole and led to the complete cessation of RBC leakage into the extravascular space. The hemostatic response to vascular injury was significantly enhanced in mice pre-treated with batroxobin, activated factor X, or slounase when compared to buffer controls (Figure 2A). As expected, the hemostatic response was robustly increased in mice treated with activated factor X as both platelet and fibrin clot formation were significantly enhanced at the site of vascular injury, resulting in vessel occlusion and interruption of blood flow. Enhancement of the platelet-fibrin clot by slounase was modest and limited to the site of vascular injury when compared to treatment with activated factor X, but seemed stronger than batroxobin treatment. Overall, all three reagents strongly enhanced hemostatic clot formation in vivo compared to WT controls (supplemental movies 1A-D). Analysis of the single frames of images over time in the bright field indicated a trend toward a reduction in RBC extravasation time in both the activated factor X and slounase conditions when compared to the batroxobin and control treatments, but was not statistically significant in WT mice in normal coagulant stasis (Figure 2B, Mean RBC extravasation time: control = 105.5 ± 6 seconds; batroxobin= 108.8 ± 15 seconds, activated factor X=99 ± 12 seconds and slounase= 83.4 ± 13 seconds, n = 8 per group P > 0.05). Quantitative analysis of the dynamics of platelet accumulation and fibrin formation by florescent intensity shows a notable increase in platelet recruitment and fibrin formation by slounase and activated factor X (platelet: P < 0.0001; fibrin: P < 0.001), while the effect of batroxobin on platelet fibrin within the clot was modest (Figure 2C).
Figure 2.
Hemostatic clot formation was enhanced in WT normal coagulant mice pretreated with activated factor X, batroxobin or slounase. WT mice were pretreated with 1 U/kg of slounase, batroxobin, activated factor X or control buffer respectively and hemostatic response and bleeding were assessed by a laser-ablation puncture to the cremaster arterioles as described. (A) Representative images of hemostatic clot formation in response to laser-induced cremaster arteriole wall rupture. Platelet accumulation is shown in green, fibrin formation is shown in red and composite images of hemostatic clot formation are shown in yellow. (B) The time required for the cessation of RBC extravasation from arterioles in WT control mice and WT mice treated with 1U/kg of slounase, batroxobin, or activated factor X (Data from 2-3 independent injuries per mouse, 3 mice in each group. P < 0.001). (C) Dynamics of mean fluorescent intensity of platelets (left) and fibrin (right) in a hemostatic clot plotted as a function of time. WT mice were pretreated with slounase, batroxobin, activated factor X or control buffer and fluorescent intensity was recorded over 5 minutes. The shaded regions are representative of the standard error (SEM).
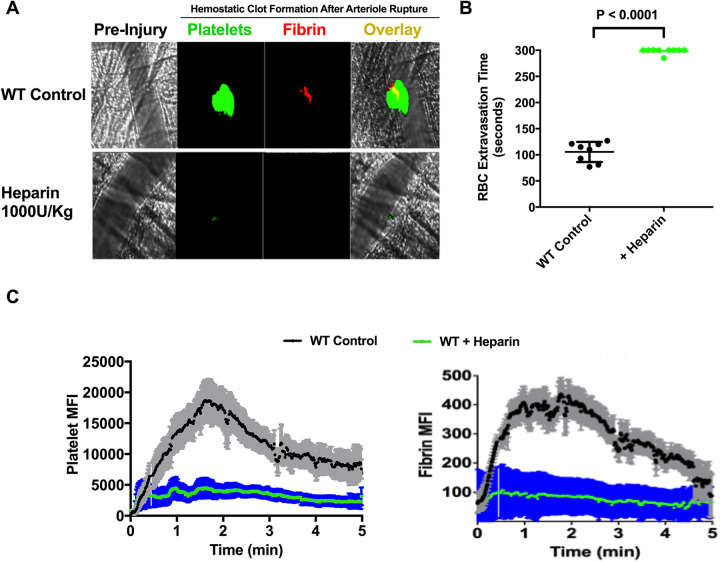
Hypocoagulant Activity Induced by Prophylactic Heparin Pretreatment Resulted in Impaired Clot Formation and Extended Bleeding in Arteriole Rupture Model
As the conversion of Fg to fibrin in blood by batroxobin occurs in the presence of heparin, hypocoagulant activity in mice was induced by heparin treatment and impairment of hemostasis was characterized using the intravital microscopy laser-ablation cremaster arteriole puncture hemostasis model in vivo. WT mice were injected with a high dose of heparin (1000 U/kg) to inhibit thrombin generation and to create a hypocoagulant condition in the mice. The impairment of hemostatic clot formation and bleeding associated with the hypocoagulant condition was characterized in vivo using a laser-ablation cremaster arteriole puncture model of hemostasis under intravital microscopy. As expected, heparin-treatment in mice resulted in a strong inhibition of platelet-fibrin clot formation and the inability to form a hemostatic clot in response to vascular injury, which resulted in extended bleeding. Analysis of the dynamics of mean fluorescent intensity for platelets and fibrin within the clot showed a complete inhibition of both platelet recruitment and fibrin formation and prevented the formation of a hemostatic clot at the site of vascular injury following the arteriole wall rupture when compared to controls (Figure 3A and C, platelet: P < 0.001, fibrin: P < 0.001). Although some transient platelet clot formation was observed immediately following the vessel rupture, the thrombi were unstable and unable to seal the site of injury during the recorded period of time (5 min). Due to the strong inhibition of clot formation by heparin, RBCs and other blood components continuously extravasated from the injury site during the recording period. Arterial bleeding time was significantly prolonged and failed to cease for the 5 minutes recorded (P < 0.0001, n = 8 per group; Figure 3B).
Figure 3.
Heparin treatment in mice potently inhibited platelet accumulation and fibrin formation at the site of vascular injury in vivo and impaired hemostasis, resulting in prolonged bleeding in the laser-ablation cremaster arteriole puncture hemostasis model. WT mice were intravenously injected with saline (control) or 1000 U/kg heparin to inhibit coagulation in vivo then the hemostatic response and bleeding were assessed by laser-ablation puncture to the cremaster arterioles as described. The cremaster muscle arteriole wall was exposed to a high-intensity laser pulse to puncture a hole, and bleeding was monitored by RBC extravasation from the cremaster arterioles wall. The subsequent formation of a platelet-fibrin hemostatic clot was recorded in real-time under intravital microscopy. (A) Representative images of hemostatic clot formation in WT control mice (upper panel) and WT mice pretreated with heparin in response to laser-induced cremaster arteriole wall rupture. Platelet accumulation is shown in green, fibrin formation is shown in red and composite images are shown in yellow. (B) The time required for the cessation of RBC extravasation from arterioles in WT control mice (black) and WT mice treated with heparin (green) was analyzed by reviewing sequential images offline. (Data from 2 independent injuries per mouse, 4 mice in each group. P < 0.0001). (C) Dynamics of platelet accumulation and fibrin formation in response to injury were assesses by the changes in the fluorescent intensity of platelets (left) and fibrin (right) in a hemostatic clot in WT control mice (black) and WT mice treated with heparin (green). The shaded regions are representative of the standard error (SEM).
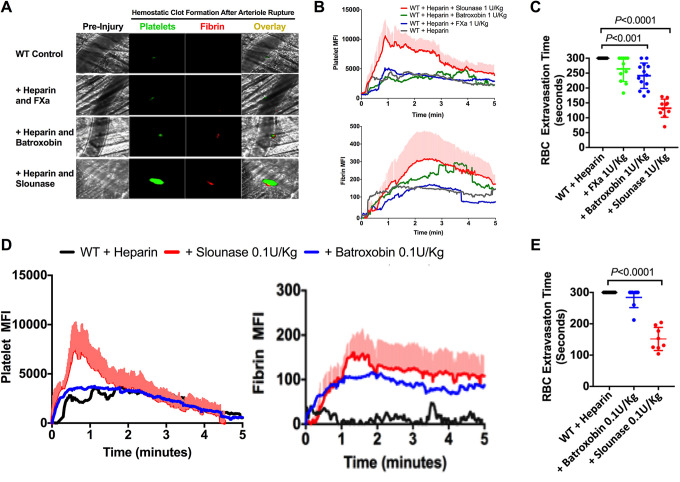
Slounase Restores Platelet Fibrin Clot Formation and Limits Bleeding in Heparin-Treated Mice
The effects of slounase, batroxobin, and activated factor X on platelet recruitment and fibrin formation, the two primary components of the hemostatic clot, were studied in heparin-induced hypocoagulant mouse as characterized above. The restoration of blood clotting in hypocoagulant conditions and reversal of bleeding was assessed in intravital microscopy laser-ablation cremaster arteriole puncture hemostasis model in vivo. In heparin-treated control mice (with buffer treatment), there was a loss of the hemostatic response as is evidenced by the complete inhibition of fibrin formation and platelet recruitment into the clot in the laser-ablation cremaster arteriole puncture model of hemostasis (Figure 4A). Fibrin formation is detectable at the site of vascular rupture in vivo in mice that were administered 1 U/kg of batroxobin in the presence of heparin, confirming that batroxobin indeed converts Fg to fibrin, independent of thrombin in vivo in the presence of heparin. Despite the detectable fibrin clot formation in the presence of batroxobin, there was no notable enhancement of platelet recruitment into the clot as is shown by the dynamics of florescent intensity of the platelets (Figure 4A). 1 U/kg of activated factor X failed to enhance clot formation as the heparin–anti-thrombin complex strongly inhibits activated factor X.43 Contrastingly, 1 U/kg of slounase significantly enhanced both platelet recruitment and fibrin formation and partially restored clot formation in the presence of heparin, which resulted in a significant shortening of bleeding time, demonstrating that slounase has much a much better hemostatic effect than either batroxobin or activated factor X alone (Figure 4A and B and supplemental movies 2A-D). Additionally, the effect of slounase was detectable in a 10-fold lower dose (0.1 U/kg) than batroxobin with no effect on platelet-fibrin clot formation (Figure 4D). The time required to stop the RBC extravasation was shorter following the administration of slounase at 1 and 0.1 U/kg (P < 0.001) compared to batroxobin. Administration of batroxobin at 0.1 U/kg showed no shortening in arterial bleeding time (n-6 in each group. P > 0.05) (Figure 4C and E).
Figure 4.
Slounase treatment restored hemostatic clot formation and shortened arterial bleeding time in heparin-induced hypocoagulant mice. WT mice were intravenously injected with 1000U/kg of heparin to induce a hypocoagulant condition. After confirming the absence of hemostatic response to injury using a laser-ablation puncture to the cremaster arteriole, mice were given intravenous treatment with 0.1 or 1 U/kg of activated factor X, batroxobin or slounase. Then, the hemostatic response to injury was continuously evaluated through RBC extravasation and hemostatic clot formation in real time under a microscope in response to the laser-ablation cremaster arteriole puncture as described. (A) Representative images of hemostatic clot formation in heparinized WT mice treated with control buffer or 1U/kg of activated factor X, batroxobin, or slounase, respectively. (B) Dynamics of fluorescent intensity of platelets (top) and fibrin (bottom) in a hemostatic clot in heparinized WT mice treated with saline or 1U/kg of activated factor X, batroxobin or slounase (P < 0.001). The shaded regions are representative of the standard error (SEM). (C) The time required for the cessation of RBC extravasation from arterioles in heparinized WT mice treated with saline or 1 U/kg (left) of activated factor X, batroxobin or slounase. (Data from 2 independent injuries per mouse, 3 mice in each group. P<0.0001). (D) Dynamics of fluorescent intensity of platelets (left) and fibrin (right) in a hemostatic clot in heparinized WT mice treated with saline or 0.1 U/kg of activated factor X, batroxobin or slounase. The shaded regions are representative of the standard error (SEM). (E) The time required for the cessation of RBC extravasation from arterioles in heparinized WT mice treated with saline or 0.1 U/kg of activated factor X, batroxobin or slounase. (Data from 2 independent injuries per mouse, 3 mice in each group. P < 0.0001).
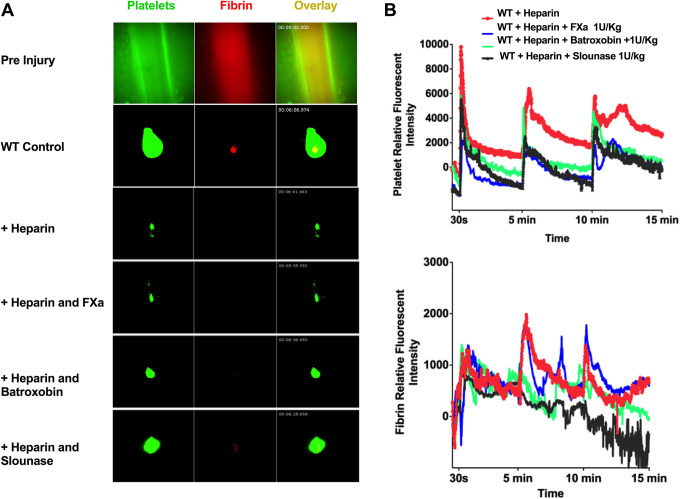
Slounase Enhances Hemostatic Clot Formation in Large Vessels in Heparin-Treated Mice
The effect of slounase on hemostatic clot formation in large vessel injury was assessed in heparin-treated, hypocoagulant mice using the intravital microscopy saphenous vein hemostasis model with repeated penetrative vascular injury at 0, 5, and 10 minutes as described.41 Induction of a penetrative injury on the saphenous vein vessel wall by a high intensity laser resulted in the immediate accumulation of platelets at the site of injury along with the formation of a fibrin ring that surrounded the edges of the vascular injury to stabilize the clot. Applying the repeated laser injury on the saphenous vein vessel wall resulted in the formation of a serially larger and more robust, stable platelet-fibrin hemostatic clot (Figure 5A and B). Fibrin formation was strongly inhibited in response to injury in heparin-treated mice. However, unlike the cremaster arteriole puncture hemostasis model, platelet accumulation was diminished, but not abolished despite the high dose of heparin treatment. Platelet accumulation was observed shortly after vein injury, but was transient, unstable, and easily embolized, as is evidenced by the sharp drop in platelet intensity (Figure 5B). Consistent with the cremaster hemostatic model, activated factor X administration did not significantly enhanced platelet-fibrin clot formation at the site of injury. Batroxobin treatment restored some fibrin formation at the site of vascular injury, but did not enhance platelet recruitment. Slounase treatment significantly enhanced platelet recruitment and fibrin formation, leading to a more stable hemostatic clot in response to repeated vascular injury (N = 6, n = 8 per group, P < 0.01. Figure 5B).
Figure 5.
Slounase treatment enhances hemostatic plug formation in the saphenous vein of hypocoagulant mice. WT mice were intravenously injected with control buffer or 1000 U/kg of heparin to induce a hypocoagulant condition. Subgroups of heparin-treated mice were also were given intravenous treatment with 1 U/kg of activated factor X, batroxobin and slounase. Hemostatic plug formation in the saphenous vein wall was assessed using laser-ablation cremaster arteriole hemostasis model under intravital microscopy as described. (A) Representative images of the saphenous vein in multi-channel prior to vascular injury (with a fluorescent background) are shown in the top panel. Representative images of hemostatic clot formation after vascular injury (2nd laser injury after the subtraction of the fluorescent background) in control WT mice, WT mice treated with heparin alone, or further treated with 1 U/kg of activated factor X, batroxobin or slounase, are shown below as indicated. Within the hemostatic plug that formed at the site of injury on the saphenous vessel wall, platelet accumulation is shown in green, fibrin formation is shown in red and composite images are shown in yellow. (B) Quantitative analysis of platelet accumulation and fibrin formation in the hemostatic clot with repetitive vascular injury to the saphenous vein. Times of vascular injury are indicated at 30 seconds and repeated at 5 and 10 minutes. The kinetic curves represent the relative platelet (top) and fibrin (bottom) fluorescent intensity (n = 6; 2 independent injuries in each mouse, 3 mice in each group). Slounase treatment in heparinized WT mice partially restored clot formation by enhancing platelet adhesion and accumulation as well as fibrin formation (P < 0.05) in the saphenous vein when compared with batroxobin and activated factor X treatment in heparin-treated hypocoagulant mice.
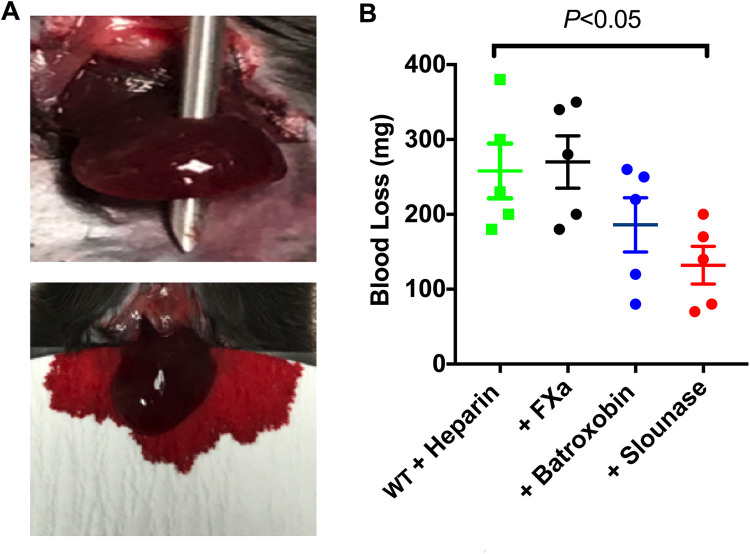
Slounase Treatment Reduces Blood Loss in Liver Injury in Heparin-Treated Hypocoagulant Mice
Intravital microscopic in vivo hemostasis models are advantageous in their ability to image and compare clot formation and bleeding time. However, it is difficult to evaluate the amount of blood loss following a severe vascular injury in these models. Therefore, the hemostatic effect of slounase on blood loss was assessed in a liver injury model in heparin-treated, hypocoagulant mice. Total blood loss from a severe liver injury was significantly reduced in slounase-treated mice, which is consistent with the results from the other hemostasis models tested (Figure 6A and B; P < 0.05). No significant change in blood loss was observed in the activated factor X treatment group under the same experimental conditions (5 mice/group, P > 0.05). While a trend toward decreased blood loss was observed in the presence of batroxobin, this was not observed to be statistically significant (P > 0.05).
Figure 6.
Slounase treatment reduces total blood loss in the liver wound model. (A) Mice were treated with 1000U/kg of heparin followed by an intravenous injection with 1U/kg of slounase, batroxobin, or activated factor X respectively. Blood loss in mice was assessed using a pre-weighed filter paper following a needle injury to the left lobe of the mouse liver as described (top). Bleeding in hypocoagulant mice induced by heparin following the injury is shown (bottom). (B) Total amount of blood lost in mice treated with heparin alone or further treated with activated factor X, batroxobin or slounase. Slounase treatment effectively reduced the blood loss when compared to control buffer treatment in the hypocoagulant condition induced by heparin treatment (n = 5 mice /group, P < 0.05).
Discussion
Rapid response to vascular injury resulting in stable hemostasis is a key element in minimizing morbidity and mortality due to excessive blood loss following traumatic injury or surgery. Uncontrolled bleeding is associated with serious adverse outcomes including shock, blood transfusions, extended surgery time, impaired wound healing, longer hospital stays, and death.1,25 The prevalence and clinical burden of hemorrhage remains considerably high, as bleeding is associated with more than one third of deaths as a result of trauma in the hospital setting.44 Achieving the prompt cessation of bleeding is critical in maintaining hemodynamic stability, ensuring oxygen delivery to vital tissues, and preventing organ failure. Despite advances in the understanding of the roles of the platelet and coagulation pathways in thrombosis and hemostasis, the availability of effective, reliable, and safe hemostatic agents is still limited. There is an urgent, unmet need for effective systemic pharmacological hemostatic agents, especially for patients in hypocoagulant conditions at risk of bleeding. Snake venom serum proteinases are known to alter the hemostatic balance of the various key factors in platelets and the coagulation/fibrinolysis cascade.26 The biochemical properties and effects of batroxobin coagulation have been well characterized. The hemostatic benefits of batroxobin contribute to its ability to form fibrin in a thrombin independent manner.36 However, its effect on hemostatic clot composition and platelet procoagulant activity in vivo is not yet studied due to the lack of reliable in vivo hemostasis models. In this study, we used intravital microscopy-based micro- and macro-vascular in vivo hemostasis models to quantitatively assess the hemostatic effect of slounase compared to batroxobin and activated factor X. Notably, we investigated the mechanistic insights of their effect on platelet recruitment and fibrin formation, the two key components of clots, in an in vivo setting in both normal and hypocoagulant mice to explore an effective strategy to enhance hemostasis.
Snake venoms toxins contain a variety of components that exert procoagulant, anticoagulant, pro-platelet and anti-platelet functions, as well as fibrinolytic activators and hemorrhaging.45,46 The efficacy and safety of hemostatic agents purified from snake venom for the treatment of hemostatic disorders largely depend on the biochemical properties and purity of derivates of snake venom during the manufacturing process. Targeting the key steps to enhance hemostasis may be associated with a higher risk of thrombosis or bleeding. Our results from real-time monitoring of microcirculation under intravital microcopy confirm that an intravenous injection of slounase, batroxobin or activated factor X into mice did not cause any detectable adhesion or aggregation of fluorescently labeled platelets or spontaneously induce fibrin formation in the absence of vascular injury, demonstrating the safety profile of all three treatments in vivo. Modification of the existing cremaster arteriole thrombotic hemostasis model with a penetrative vascular injury by laser under intravital microscopic imaging allowed us to real-time monitor and quantitatively assess both hemostatic clot composition and bleeding in vivo without causing vessel occlusion. Consistent with the reports from published studies, our results show that batroxobin treatment indeed promotes platelet-fibrin clot formation by enhancing fibrin formation at the site of vascular rupture in normal WT mice.36,47 As expected, the hemostatic response at the site of injury to vascular rupture was robustly enhanced and caused massive intravascular clotting, resulting in vessel occlusion in WT mice pretreated with a high dose of activated factor X.
Our results indicate that a high dose of activated factor X could increase the risk of vessel occlusion and may not be a safe approach for maintaining hemostasis. Nevertheless, inhibiting the activation of factor X remains an attractive anti-thrombotic approach. In contrast, slounase treatment in WT mice in vivo resulted in a modest enhancement in both platelet and fibrin clot formation in a cremaster arteriole rupture model, supporting slounase as exhibiting a better safety profile for hemostasis compared to activated factor X. Remarkably, the limited amount of activated factor X contained in slounase seems to lead to the enhancement of platelet recruitment into the fibrin clot but does not result in vessel occlusion as activated factor X is known to enhance thrombin generation.38,48 This result indicates that the modest promotion of platelet recruitment along with enhanced fibrin formation might be a viable hemostatic approach.
In order to imitate hypocoagulant conditions and increased bleeding risk in bleeding disorders in vivo, mice were treated with heparin to inhibit thrombin generation and activation of coagulation. As we expected, heparin treatment resulted in the inhibition of fibrin formation and platelet accumulation, ultimately extending bleeding time in response to vascular injury in the cremaster arterioles and saphenous vein models. Batroxobin treatment in mice under these hypocoagulant conditions was shown to promote the formation of a fibrin clot at the site of injury, shortening the arterial bleeding time despite the presence of heparin. This result confirms that batroxobin induces fibrin clot formation and promotes hemostasis in vivo independent of thrombin. However, platelet accumulation associated with enhanced fibrin formation in batroxobin treated mice was not significant, indicating batroxobin alone may not sufficiently promote platelet procoagulant activity. Activated factor X failed to enhance clot formation when administered alone in the presence of heparin. This was expected as it has been previously shown that the heparin–anti-thrombin complex strongly inhibits activated factor X.43 In contrast with results from activated factor X treatment in heparin-treated mice, we observed that slounase was able to enhance clot formation by promoting both fibrin formation and platelet recruitment in the presence of heparin. The ability of the activated factor X contained in slounase to promote platelet procoagulant activity could be a result of a modification leading to the stabilization of activated factor X through its interaction with batroxobin, or possibly due to the local availability and concentration of heparin present at the site of vascular injury. The effects and implications of this interaction could be a topic of future study. Nevertheless, our data strongly indicate that slounase exhibited a more desirable hemostatic profile by enhancing both platelet accumulation and fibrin formation, the two primary components required for clot formation in micro and macro vascular injury models, even under hypocoagulant conditions. Our results are further supported by the decreased blood loss in slounase-treated mice in the liver injury model.
Interestingly, the observation that slounase effectively enhanced platelet-fibrin clot formation in the presence of heparin demonstrates its ability to bypass coagulation. The use of bypassing agents in a clinical setting has provided a useful method of circumventing defects in the clotting cascade.49,50 By inhibiting thrombin using heparin, we are able to show that slounase has the ability to bypass coagulation, leading to the formation of a platelet-fibrin clot in a thrombin-independent manner.51 However, future investigations into the effect of slounase on other hypocoagulent conditions, such as hemophilia and the loss of platelet coagulation factor due to trauma, as well as in the presence of other anti-platelet and anti-coagulant therapies could reveal more potential clinical applications. Our study results suggest that slounase may represent an effective bypassing hemostatic agent and could be beneficial in treating defects in the coagulation pathway.
In summary, our study results strongly indicate that slounase enhances hemostasis in normocoagulable conditions and restores hemostasis by shortening bleeding time and blood loss in the event of vascular injury in hypocoagulant stasis. Notably, our study results show that slounase improves hemostasis by both enhancing fibrin formation and platelet procoagulant activity, while batroxobin mainly enhances fibrin formation in vivo. Findings from this study provide important mechanistic insight into hemostatic clot formation in vivo and demonstrate the potential benefit of developing therapeutic approaches to achieve better hemostasis for the treatment of bleeding disorders.
Supplemental Materials
Supplemental Material, sj-docx-1-cat-10.1177_10760296211018510 for Slounase, a Batroxobin Containing Activated Factor X Effectively Enhances Hemostatic Clot Formation and Reducing Bleeding in Hypocoagulant Conditions in Mice by Reheman Adili, Madeline Jackson, Livia Stanger, Xiangrong Dai, Mandy Li, Benjamin Xiaoyi Li and Michael Holinstat in Clinical and Applied Thrombosis/Hemostasis
Acknowledgments
We would like to thank Dr. R. Camire from the Children’s Hospital of Philadelphia for providing us with anti-mouse fibrin antibody.
Authors’ Note: The Institutional Animal Care and Use Committee at the University of Michigan approved all experimental procedures in this study. Access to any underlying research materials, including data, samples or models can be requested by contacting the corresponding author.
Author Contributions: R.A. conceived of the idea and designed the study, performed the experiments, conducted the data analysis supervised the research and wrote the manuscript. M.J. and L.S carried out the experiments, conducted the data analysis and assisted in preparing the manuscript. X.D., M.L. and B.X.L. provided critical reagents needed for the study, contributed to the interpretation of the results and assisted in preparing the manuscript. M.H. designed the study, contributed to the interpretation of the results and assisted in preparing the manuscript. All authors provided critical feedback and helped shape the research, analysis and manuscript and have read and agreed to the published version of the manuscript.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: This work was supported by Lee’s Pharmaceutical Holdings limited. Dai is an employee of Zhaoke Pharmaceutical Co. Ltd. M. Li and B. X. Li is employer of Lee’s Pharmaceutical Holdings limited, the company which holds the patent for slounase.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by research grant from Lee’s Pharmaceutical Holdings Limited, Hong Kong (RA). This work was funded in part by the National Institutes of Health; grant number R01 GM105671 (MH) and R01 HL114405 (MH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ORCID iD: Reheman Adili  https://orcid.org/0000-0002-1973-9695
https://orcid.org/0000-0002-1973-9695
Michael Holinstat  https://orcid.org/0000-0001-5100-1933
https://orcid.org/0000-0001-5100-1933
Supplemental Materials: Supplemental material for this article is available online.
References
- 1. Rossaint R, Bouillon B, Cerny V, et al. The European guideline on management of major bleeding and coagulopathy following trauma: fourth edition. Crit Care. 2016;20:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60(6 Suppl):S3–11. [DOI] [PubMed] [Google Scholar]
- 3. Ruggeri ZM. Platelets in atherothrombosis. Nat Med. 2002;8(11):1227–1234. [DOI] [PubMed] [Google Scholar]
- 4. Ni H, Freedman J. Platelets in hemostasis and thrombosis: role of integrins and their ligands. Transfus Apher Sci. 2003;28(3):257–264. [DOI] [PubMed] [Google Scholar]
- 5. Jackson SP. The growing complexity of platelet aggregation. Blood. 2007;109(12):5087–5095. [DOI] [PubMed] [Google Scholar]
- 6. Wang Y, Andrews M, Yang Y, et al. Platelets in thrombosis and hemostasis: old topic with new mechanisms. Cardiovasc Hematol Disord Drug Targets. 2012;12(2):126–132. [DOI] [PubMed] [Google Scholar]
- 7. Ni H, Denis CV, Subbarao S, et al. Persistence of platelet thrombus formation in arterioles of mice lacking both von Willebrand factor and fibrinogen. J Clin Invest. 2000;106(3):385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yang H, Reheman A, Chen P, et al. Fibrinogen and von Willebrand factor-independent platelet aggregation in vitro and in vivo. J Thromb Haemost. 2006;4(10):2230–2237. [DOI] [PubMed] [Google Scholar]
- 9. Reheman A, Yang H, Zhu G, et al. Plasma fibronectin depletion enhances platelet aggregation and thrombus formation in mice lacking fibrinogen and von Willebrand factor. Blood. 2009;113(8):1809–1817. [DOI] [PubMed] [Google Scholar]
- 10. Wang Y, Reheman A, Spring CM, et al. Plasma fibronectin supports hemostasis and regulates thrombosis. J Clin Invest. 2014;124(10):4281–4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Reheman A, Gross P, Yang H, et al. Vitronectin stabilizes thrombi and vessel occlusion but plays a dual role in platelet aggregation. J Thromb Haemost. 2005;3(5):875–883. [DOI] [PubMed] [Google Scholar]
- 12. Wolberg AS. Determinants of fibrin formation, structure, and function. Curr Opin Hematol. 2012;19(5):349–356. [DOI] [PubMed] [Google Scholar]
- 13. Monroe DM, Hoffman M, Roberts HR. Platelets and thrombin generation. Arterioscler Thromb Vasc Biol. 2002;22(9):1381–1389. [DOI] [PubMed] [Google Scholar]
- 14. Berndt MC, Metharom P, Andrews RK. Primary haemostasis: newer insights. Haemophilia. 2014;20(Suppl 4):15–22. [DOI] [PubMed] [Google Scholar]
- 15. Adili R, Hawley M, Holinstat M. Regulation of platelet function and thrombosis by omega-3 and omega-6 polyunsaturated fatty acids. Prostaglandins Other Lipid Mediat. 2018;139:10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Blombäck B, Hessel B, Hogg D, Therkildsen L. A two-step fibrinogen–fibrin transition in blood coagulation. Nature. 1978;275(5680):501–505. [DOI] [PubMed] [Google Scholar]
- 17. Mosesson MW. Fibrinogen and fibrin structure and functions. J Thromb Haemost. 2005;3(8):1894–1904. [DOI] [PubMed] [Google Scholar]
- 18. Mullin JL, Gorkun OV, Binnie CG, Lord ST. Recombinant fibrinogen studies reveal that thrombin specificity dictates order of fibrinopeptide release. J Biol Chem. 2000;275(33):25239–25246. [DOI] [PubMed] [Google Scholar]
- 19. Badimon L, Vilahur G. Thrombosis formation on atherosclerotic lesions and plaque rupture. J Intern Med. 2014;276(6):618–632. [DOI] [PubMed] [Google Scholar]
- 20. Hess JR, Brohi K, Dutton RP, et al. The coagulopathy of trauma: a review of mechanisms. J trauma. 2008;65(4):748–754. [DOI] [PubMed] [Google Scholar]
- 21. Frith D, Goslings JC, Gaarder C, et al. Definition and drivers of acute traumatic coagulopathy: clinical and experimental investigations. J Thromb Haemost. 2010;8(9):1919–1925. [DOI] [PubMed] [Google Scholar]
- 22. Inherited and Acquired Coagulation Disorders. Practical Transfusion Medicine. 2017:264–278. [Google Scholar]
- 23. Cattaneo M. Inherited platelet-based bleeding disorders. J Thromb Haemost. 2003;1(7):1628–1636. [DOI] [PubMed] [Google Scholar]
- 24. Cavender MA, Rao SV. Bleeding associated with current therapies for acute coronary syndrome: what are the mechanisms? J Thromb Thrombolysis. 2010;30(3):332–339. [DOI] [PubMed] [Google Scholar]
- 25. Rossaint R, Bouillon B, Cerny V, et al. Management of bleeding following major trauma: an updated European guideline. Crit Care. 2010;14(2):R52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Serrano SM. The long road of research on snake venom serine proteinases. Toxicon. 2013;62:19–26. [DOI] [PubMed] [Google Scholar]
- 27. Thakur R, Mukherjee AK. Pathophysiological significance and therapeutic applications of snake venom protease inhibitors. Toxicon. 2017;131:37–47. [DOI] [PubMed] [Google Scholar]
- 28. Lu Q, Clemetson JM, Clemetson KJ. Snake venoms and hemostasis. J Thromb Haemost. 2005;3(8):1791–1799. [DOI] [PubMed] [Google Scholar]
- 29. Lei X, Reheman A, Hou Y, et al. Anfibatide, a novel GPIb complex antagonist, inhibits platelet adhesion and thrombus formation in vitro and in vivo in murine models of thrombosis. Thromb Haemost. 2014;111(2):279–289. [DOI] [PubMed] [Google Scholar]
- 30. Chang CH, Chung CH, Tu YS, et al. Trowaglerix venom polypeptides as a novel antithrombotic agent by targeting immunoglobulin-like domains of glycoprotein VI in platelet. Arterioscler Thromb Vasc Biol. 2017;37(7):1307–1314. [DOI] [PubMed] [Google Scholar]
- 31. Kini RM. Toxins in thrombosis and haemostasis: potential beyond imagination. J Thromb Haemost. 2011;9 Suppl 1:195–208. [DOI] [PubMed] [Google Scholar]
- 32. Davie EW, Fujikawa K, Kurachi K, Kisiel W. The role of serine proteases in the blood coagulation cascade. Adv Enzymol Relat Areas Mol Biol. 1979;48:277–318. [DOI] [PubMed] [Google Scholar]
- 33. Vilca-Quispe A, Ponce-Soto LA, Winck FV, Marangoni S. Isolation and characterization of a new serine protease with thrombin-like activity (TLBm) from the venom of the snake Bothrops marajoensis. Toxicon. 2010;55(4):745–753. [DOI] [PubMed] [Google Scholar]
- 34. Aronson DL. Comparison of the actions of thrombin and the thrombin-like venom enzymes ancrod and batroxobin. Thromb Haemost. 1976;36(1):9–13. [PubMed] [Google Scholar]
- 35. You WK, Choi WS, Koh YS, Shin HC, Jang Y, Chung KH. Functional characterization of recombinant batroxobin, a snake venom thrombin-like enzyme, expressed from Pichia pastoris. FEBS Lett. 2004;571(1-3):67–73. [DOI] [PubMed] [Google Scholar]
- 36. Vu TT, Stafford AR, Leslie BA, Kim PY, Fredenburgh JC, Weitz JI. Batroxobin binds fibrin with higher affinity and promotes clot expansion to a greater extent than thrombin. J Biol Chem. 2013;288(23):16862–16871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. You KE, Koo MA, Lee DH, et al. The effective control of a bleeding injury using a medical adhesive containing batroxobin. Biomed Mater. 2014;9(2):025002. [DOI] [PubMed] [Google Scholar]
- 38. Hofmann H, Bon C. Blood coagulation induced by the venom of Bothrops atrox. 2. Identification, purification, and properties of two factor X activators. Biochemistry. 1987;26(3):780–787. [DOI] [PubMed] [Google Scholar]
- 39. Slounase®. Lee’s Pharmaceutical Holdings. Published 2005. Updated January 15, 2021. Accessed January 15, 2021. http://www.leespharm.com
- 40. Yeung J, Tourdot BE, Adili R, et al. 12(S)-HETrE, a 12-lipoxygenase oxylipin of dihomo-gamma-linolenic acid, inhibits thrombosis via Galphas signaling in platelets. Arterioscler Thromb Vasc Biol. 2016;36(10):2068–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Getz TM, Piatt R, Petrich BG, Monroe D, Mackman N, Bergmeier W. Novel mouse hemostasis model for real-time determination of bleeding time and hemostatic plug composition. J Thromb Haemost. 2015;13(3):417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Adili R, Tourdot BE, Mast K, et al. First selective 12-LOX inhibitor, ML355, impairs thrombus formation and vessel occlusion in vivo with minimal effects on hemostasis. Arterioscler Thromb Vasc Biol. 2017;37(10):1828–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yang L, Manithody C, Rezaie AR. Heparin-activated antithrombin interacts with the autolysis loop of target coagulation proteases. Blood. 2004;104(6):1753–1759. [DOI] [PubMed] [Google Scholar]
- 44. Zimmerman LH. Causes and consequences of critical bleeding and mechanisms of blood coagulation. Pharmacotherapy. 2007;27(9 Pt 2):45S–56S. [DOI] [PubMed] [Google Scholar]
- 45. Kini RM. Serine proteases affecting blood coagulation and fibrinolysis from snake venoms. Pathophysiol Haemost Thromb. 2005;34(4-5):200–204. [DOI] [PubMed] [Google Scholar]
- 46. Kini RM. Anticoagulant proteins from snake venoms: structure, function and mechanism. Biochem J. 2006;397(3):377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Braud S, Bon C, Wisner A. Snake venom proteins acting on hemostasis. Biochimie. 2000;82(9-10):851–859. [DOI] [PubMed] [Google Scholar]
- 48. Bunce MW, Toso R, Camire RM. Zymogen-like factor Xa variants restore thrombin generation and effectively bypass the intrinsic pathway in vitro. Blood. 2011;117(1):290–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hoffman M, Dargaud Y. Mechanisms and monitoring of bypassing agent therapy. J Thromb Haemost. 2012;10(8):1478–1485. [DOI] [PubMed] [Google Scholar]
- 50. George LA, Thalji NK, Raffini LJ, Gimotty PA, Camire RM. Correction of human hemophilia A whole blood abnormalities with a novel bypass agent: zymogen-like FXa(I16 L). J Thromb Haemost. 2015;13(9):1694–1698. [DOI] [PubMed] [Google Scholar]
- 51. Hirsh J. Heparin. N Engl J Med. 1991;324(22):1565–1574. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-docx-1-cat-10.1177_10760296211018510 for Slounase, a Batroxobin Containing Activated Factor X Effectively Enhances Hemostatic Clot Formation and Reducing Bleeding in Hypocoagulant Conditions in Mice by Reheman Adili, Madeline Jackson, Livia Stanger, Xiangrong Dai, Mandy Li, Benjamin Xiaoyi Li and Michael Holinstat in Clinical and Applied Thrombosis/Hemostasis