Abstract
The rust fungi (Pucciniales) with 7000+ species comprise one of the largest orders of Fungi, and one for which taxonomy at all ranks remains problematic. Here we provide a taxonomic framework, based on 16 years of sampling that includes ca. 80 % of accepted genera including type species wherever possible, and three DNA loci used to resolve the deeper nodes of the rust fungus tree of life. Pucciniales are comprised of seven suborders – Araucariomycetineae subord. nov., Melampsorineae, Mikronegeriineae, Raveneliineae subord. nov., Rogerpetersoniineae subord. nov., Skierkineae subord. nov., and Uredinineae – and 18 families – Araucariomycetaceae fam. nov., Coleosporiaceae, Crossopsoraceae fam. nov., Gymnosporangiaceae, Melampsoraceae, Milesinaceae fam. nov., Ochropsoraceae fam. & stat. nov., Phakopsoraceae, Phragmidiaceae, Pileolariaceae, Pucciniaceae, Pucciniastraceae, Raveneliaceae, Rogerpetersoniaceae fam. nov., Skierkaceae fam. & stat. nov., Sphaerophragmiaceae, Tranzscheliaceae fam. & stat. nov., and Zaghouaniaceae. The new genera Araucariomyces (for Aecidium fragiforme and Ae. balansae), Neoolivea (for Olivea tectonae), Rogerpetersonia (for Caeoma torreyae), and Rossmanomyces (for Chrysomyxa monesis, Ch. pryrolae, and Ch. ramischiae) are proposed. Twenty-one new combinations and one new name are introduced for: Angiopsora apoda, Angiopsora chusqueae, Angiopsora paspalicola, Araucariomyces balansae, Araucariomyces fragiformis, Cephalotelium evansii, Cephalotelium neocaledoniense, Cephalotelium xanthophloeae, Ceropsora weirii, Gymnotelium speciosum, Lipocystis acaciae-pennatulae, Neoolivea tectonae, Neophysopella kraunhiae, Phakopsora pipturi, Rogerpetersonia torreyae, Rossmanomyces monesis, Rossmanomyces pryrolae, Rossmanomyces ramischiae, Thekopsora americana, Thekopsora potentillae, Thekopsora pseudoagrimoniae, and Zaghouania notelaeae. Higher ranks are newly defined with consideration of morphology, host range and life cycle. Finally, we discuss the evolutionary and diversification trends within Pucciniales.
Citation: Aime MC, McTaggart AR (2020). A higher-rank classification for rust fungi, with notes on genera. Fungal Systematics and Evolution 7: 21–47. doi: 10.3114/fuse.2021.07.02
Keywords: host alternation, life cycles, Uredinales, Urediniomycetes, 37 new taxa
INTRODUCTION
Rust fungi (Pucciniomycotina, Pucciniales) comprise one of the largest orders in Fungi, containing ca. 25 % of described Basidiomycota. All are obligate pathogens of plants and at ca. 7 000+ accepted species (Kirk et al. 2008) form the most species-rich group of plant pathogens. Diseases caused by rust fungi have impacted human agriculture and history through time. Rusts likely caused the earliest recognized diseases of agricultural plants (Carefoot & Sprott 1967), and have continued to impact anthropogenic ecosystems through epidemics and localized host extinctions (Carnegie & Pegg 2018). The Green Revolution in the mid to late 20th century that heralded the era of host resistance breeding targeted rust fungi (Philips 2013).
Pucciniales has a suite of characteristics that are rare or unique within Fungi, including alternation of generations with separate gametothalli (spermogonia and aecia) and sporothalli (uredinia and telia) that may infect unrelated hosts (heteroecious); and the production of up to five different morphs within the life cycle. These characteristics, together with many instances of convergent evolution within morphs, repeated evolution of derived life cycle variants, and varying taxonomic emphases on different morph characteristics, have contributed to the development of numerous classification schemes for rust fungi (Fig. S1). Further taxonomic confusion within Pucciniales at the species rank has been shaped by separate naming systems under prior nomenclatural codes for sexual and asexual morphs. For instance, prior to the use of molecular data to link morphs, only through painstaking inoculation studies could complete life cycles be elucidated (e.g., Cummins 1978). Consequently, many asexual morphs were unplaceable within a sexual morph-based classification system. Recent changes to the nomenclatural code now allow the placement of taxa within natural genera, regardless of morph (McNeill et al. 2012, Turland et al. 2018). Although most asexual genera have been reduced to synonymy (Aime et al. 2018b), some, such as Uredo and Aecidium contain species that occur in over 50 sexual genera, and it will be non-trivial to assign these to natural genera.
Generic-rank classification, even for sexual morph species, is similarly difficult. At least 334 generic names have been described in Pucciniales; most researchers accept ca. 130 of these (e.g., Cummins & Hiratsuka 2003). Studies have shown that many diagnostic characters are homoplasious, such as the number of cells per teliospore (Aime 2006, Maier et al. 2007, van der Merwe et al. 2007, Yun et. al. 2008, Beenken & Wood 2015). As a result, most taxon-rich genera – the largest being Puccinia (ca. 4 000 species), Uromyces (ca. 800 species), and Ravenelia (ca. 200 species) – are polyphyletic and will need thoughtful re-evaluation for how to reassign these species into monophyletic genera.
At the higher ranks, classification of rust fungi has varied through time as well (Fig. S1). Rust fungi were initially classified into families by characteristics of basidia and teliospores (e.g., Cunningham 1931). This approach divided rusts into three (or four) families, Melampsoraceae, (Coleosporiaceae), Pucciniaceae and Zaghouaniaceae (Sydow & Sydow 1915, Cunningham 1931). Arthur (1907–1931), Sydow & Sydow (1915) and Dietel (1928) further classified rusts in subfamilies or tribes based on morphology of telia. Other workers, such as Hiratsuka & Cummins (1963) placed greater emphasis on the gametothallus, especially spermogonial morphology, resulting in conflicting taxonomic hypotheses. This approach was later combined with teliospore morphology (Cummins & Hiratsuka 1983, 2003) to achieve a 13-family classification that became the most broadly applied in the pre-molecular era.
The first molecular systematic study to test the familial classification of Cummins & Hiratsuka (2003) subdivided the rust fungi into three major radiations, Mikronegeriineae, Melampsorineae, and Uredinineae, that mostly correspond to the earlier three-family approach of Cunningham (1931) (Aime 2006). Within these radiations were (i) several lineages more or less corresponding to families circumscribed by Cummins & Hiratsuka (2003), such as Coleosporiaceae, Melampsoraceae, Zaghouaniaceae (as Mikronegeriaceae), Phragmidiaceae, Pileolariaceae, Pucciniaceae, Pucciniastraceae and Raveneliaceae; (ii) families, such as Chaconiaceae and Phakopsoraceae that were comprised of polyphyletic assemblages that could not be effectively resolved without data from type species; and (iii) several so-called “orphan” genera that could not confidently be assigned to families (Aime 2006).
Numerous subsequent studies have focused on resolution of single families, e.g., Sphaerophragmiaceae (Beenken 2017); polyphyletic genera, e.g., segregation of Neophysopella from Phakopsora (Ji et al. 2019); as well as conservation efforts to stabilize use of generic names (e.g., Aime et al. 2018b, 2019a, b). Despite these efforts, a stable and resolved higher-rank classification for the rust fungi has not been achieved. A major bottleneck has been limited sampling of taxa that represent the type species of genera, especially for genera with convergent morphologies, that are polyphyletic, and/or contain species with multiple competing names for different morphs.
The purpose of the present study is to provide a stable higher-rank classification for Pucciniales that will serve as a framework for future systematic studies. We have assembled a dataset over the last 16 years that includes exemplars from 113 (ca. 80 %) rust genera, including 108 that are represented by sequences from type species (86) or type species proxies (22). Our phylogenetic hypotheses are based on DNA data from three loci (nuclear large subunit and small subunit rDNA, and Cytochrome-c-oxidase subunit 3) with varying evolutionary rates across Pucciniales (e.g., Aime 2006, Vialle et al. 2009, Feau et al. 2011, Aime et al. 2018a, McTaggart & Aime 2018). We propose a natural classification for Pucciniales based on combined evidence from morphology, life cycles, hosts, and phylogenetic data. Several new suborders, families, genera, and combinations are proposed, and suborders and families are redefined. Finally, we discuss the evolutionary trends that led to diversification within Pucciniales and highlight unresolved areas of the rust family tree for future research.
MATERIALS AND METHODS
Taxon selection
Priority was given to species that represent generic types of rust fungi. If type species were unavailable, wherever possible two congeneric species similar to the type in respect to host genus, morphology, and geography were chosen as proxies (e.g., Skierka, Tranzschelia, Uredopeltis and Uropyxis). At least one exemplar was included for every major lineage of Pucciniales that had been previously identified (e.g., Aime 2006e.g., Aime 2018a, Beenken 2017). Additional genera were targeted (i) from families that appeared polyphyletic in prior studies (e.g., Chaconiaceae, Phakopsoraceae); (ii) from previously undersampled families, e.g., Uropyxidaceae; and (iii) to broaden sampling of endocyclic species (e.g., Baeodromus, Chardoniella, Cionothrix, Dietelia, Pucciniosira). If possible, more than one species was included for genera (i) previously determined as orphaned taxa sensu Aime (2006) (e.g., Gymnosporangium, Prospodium, Ochropsora, and Tranzschelia) or incertae sedis sensu Cummins & Hiratsuka (2003) (Elateraecium, Masseeëlla); and (ii) previously demonstrated as polyphyletic (e.g., Maravalia, Phakopsora, Pucciniastrum, Ravenelia). Additional taxa were also included for genera if complete data at the three sequenced loci were available (e.g., Gymnosporangium, Hamaspora, Melampsora, Neophysopella, and Phragmidium). An initial dataset of 130 rust taxa and three loci (Table 1) was used to determine the familial placement of genera and the relationships between families in an overview tree. The overview tree was rooted with Eocronartium muscicola, from the sister order to Pucciniales (Aime et al. 2006).
Table 1.
Collection and accession data for sequences used in Pucciniales overview tree (Fig. 1).
| Taxon | Type statusa | Voucher number (Collection number)b | 28S | 18S | CO3 | Host | Source |
|---|---|---|---|---|---|---|---|
| Achrotelium ichnocarpi | T | BRIP 55685 | KT199393 | KT199381 | KT199404 | Ichnocarpus frutescens | McTaggart et al. (2016) |
| Aecidium kalanchoe | BPI 843633 (U18, HOLOTYPE) | AY463163 | DQ354524 | NA | Kalanchoe blossfeldiana | Hernandez et al. (2004) | |
| Allodus podophylli | T | BPI 842277 (U2, NEOTYPE):28S,18S; PUR N16753:CO3 | DQ354543 | DQ354544 | MG907270 | Podophyllum peltatum | Aime (2006); Aime et al. (2018a) |
| Angiopsora paspalicola | * | BRIP 55625 | MW049243 | NA | MW036496 | Paspalum sp. | this paper |
| Aplopsora nyssae | T | BPI 877823 (U1191) | MW049244 | NA | NA | Nyssa sylvatica | this paper |
| Araucariomyces fragiformis | T | BRIP 68996 | MW049245 | MW049292 | MW036497 | Agathis robusta | this paper |
| Austropuccinia psidii | T | BRIP 58164 | KF318449 | KF318457 | KT199419 | Rhodamnia angustifolia | Pegg et al. (2014); McTaggart et al. (2016) |
| Baeodromus eupatorii | * | PUR N16312 (U1386) | MW049246 | NA | NA | Ageratina sp. | this paper |
| Bibulocystis pulcherrima | T | BRIP 58450 | MW049247 | NA | MW036498 | Daviesia latifolia | this paper |
| Blastospora smilacis | T | PUR N270 | DQ354568 | DQ354567 | NA | Smilax sieboldii | Aime (2006) |
| Bubakia argentinensis (as Phakopsora argentinensis) | * | ZT:RB 8248 | KF528009 | NA | NA | Croton cf. anisodontus | Beenken (2014) |
| Calyptospora goeppertiana | T | BPI 882188 (U866) | MW147023 | NA | NA | Abies balsamea | this paper |
| Catenulopsora flacourtiae | T | PUR N13865 (U669) | MW049248 | MW049293 | NA | Flacourtia indica | this paper |
| Cephalotelium macowaniana (as Ravenelia macawaniana) | T | PREM 61222 | MG946007 | NA | NA | Vachellia karroo | Ebinghaus et al. (2018a) |
| Cephalotelium neocaledoniense (as Ravenelia neocaledoniensis) | BRIP 56908 | KJ862348 | NA | KJ862460 | Vachellia farnesiana | McTaggart et al. (2015) | |
| Ceratocoma jacksoniae | T | BRIP 57717 | KT199394 | KT199382 | KT199405 | Davesia sp. | McTaggart et al. (2016) |
| Ceropsora weirii (as Chrysomyxa weirii) | * | 916CHWPCGSG8 | FJ666465 | NA | NA | n.d. | Vialle et al. (2009) |
| Chaconia ingae | * | BPI 863575 (GUY74) | MW049249 | NA | NA | Inga sp. | this paper |
| Chardoniella gynoxidis | T | R15 | MW049250 | NA | NA | Gynoxys sp. (cf.) | this paper |
| Chrysocelis lupini | T | PUR N11562 (U1570) | MW049251 | NA | NA | Lupinus sp. | this paper |
| Chrysomyxa arctostaphyli | CUW CFB 22246 | AF522163 | AY657009 | NA | n.d. | Matheny et al. unpublished | |
| Cionothrix praelonga | T | PUR 90104 | MW049252 | NA | NA | Eupatorium sp. | this paper |
| Coleopuccinia sinensis | T | BJFC R02506 | MF802285 | NA | NA | Cotoneaster microphyllus | Cao et al. (2018) |
| Coleosporium senecionis | T | PDD 98309 | KJ716348 | KJ746818 | NA | Senecio sp. | Padamsee & McKenzie (2014) |
| Cronartium flaccidum | T | PUR N16561 (MCA4165) | MW049253 | MW049294 | NA | Vincetoxicum hirundinaria | this paper |
| Cronartium harknessii (≡Endocronartium harknessii) | (T) | CFB22250 | AF522175 | AY665785 | NA | Pinus sp. | Szaro & Bruns unpublished; Matheny et al. unpublished |
| Crossopsora fici | BRIP 58118 | MH047207 | MH047212 | MH047204 | Ficus virens var. sublanceolata | this paper | |
| Crossopsora ziziphi | T | BPI 877877 (U904) | MG744558 | NA | NA | Ziziphus mucronata | Souza et al. (2018) |
| Cumminsiella mirabilissima | T | BPI 871101 (U480) | DQ354531 | DQ354530 | NA | Mahonia aquifolium | Aime (2006) |
| Dasyspora gregaria | T | ZT Myc 3397 | JF263477 | JF263502 | JF263518 | Xylopia cayennensis | Beenken et al. (2012) |
| Desmella aneimiae | T | BRIP 60995 | KM249867 | NA | NA | Nephrolepis hirsutula | McTaggart et al. (2014) |
| Diaphanopellis purpurea | * | BJFC R02448 | MK874622 | NA | NA | Picea brachytyla | Yang & Wang unpublished |
| Didymopsora solani-argentei | T | PUR N3728 | MW049254 | NA | NA | Solanum argentum | this paper |
| Dietelia codiaei | * | PUR N16488 | MW049255 | NA | NA | Codiaeum variegatum | this paper |
| Dipyxis mexicana | T | BPI 871906 | MW049256 | NA | NA | Adenocalymna sp. | this paper |
| Edythea quitensis | T | QCAM6453 | MG596499 | NA | NA | Berberis hallii | Barnes & Ordonez unpublished |
| Elateraecium salaciicola | T | PUR F17677 | MW049257 | MW049295 | NA | Salacia sp. | this paper |
| Endophylloides portoricensis (as Dietelia portoricensis) | T | BPI 844288 (U322):28S; n.d.:18S | DQ354516 | AY125389 | NA | Mikania micrantha | Aime (2006); Wingfield et al. (2004) |
| Endophyllum cassiae | BPI 871369 (U525) | MW049258 | NA | NA | Cassia obtusifolia | this paper | |
| Endophyllum circumscriptum | BPI 872271 | MW049259 | NA | NA | Cissus sp. | this paper | |
| Endoraecium acaciae | T | BPI 871098 (MCA2957) | DQ323916 | DQ323917 | NA | Acacia koa | Scholler & Aime (2006) |
| Eocronartium muscicola | MIN796447:28S; DUKE:DAH(e1):18S | AF014825 | DQ241438 | NA | NA | Bruns & Szaro unpublished; Henk & Vilgalys (2007) | |
| Gerwasia rubi | T | BRIP 58440 | KT199397 | NA | KT199408 | Rubus sp. | McTaggart et al. (2016) |
| Gymnoconia interstitialis | T | BPI 747600 | JF907677 | DQ521422 | NA | Rubus allegheniensis | Yun et al. (2011); Matheny et al. unpublished |
| Gymnosporangium clavariiforme (≡Podisoma clavariiforme) | (T) | BRIP 59471 | MW049261 | MW049296 | MW036499 | Crataegus sp. | this paper |
| Gymnosporangium sabinae | T | TNM F0030477 | KY964764 | KY964764 | NA | Pyrus communis | Shen et al. (2018) |
| Gymnotelium blasdaleanum | * | PUR N10018 (U1469) | MG907218 | MG907206 | MG907269 | Amelanchier alnifolia | Aime et al. (2018a) |
| Hamaspora acutissima | BRIP 56949 | KT199398 | KT199385 | KT199409 | Rubus moluccanus | McTaggart et al. (2016) | |
| Hamaspora longissima | T | BPI 871506 (U305) | MW049262 | MW049297 | NA | Rubus ludwigii | this paper |
| Hapalophragmium derridis | T | PUR N16494 | MW049263 | NA | NA | unidentified Fabaceae | this paper |
| Hemileia vastatrix | T | BRIP 61233 | KT199399 | DQ354565 | KT199410 | Coffea robusta | McTaggart et al. (2016); Aime (2006) |
| Hyalopsora aspidiotus | T | PUR N4641 | MW049264 | NA | NA | Gymnocarpium dryopteris | this paper |
| Kernkampella breyniae | * | BRIP 56909 | KJ862346 | KJ862428 | KJ862459 | Breynia cernua | McTaggart et al. (2015) |
| Kuehneola uredinis | T | BPI 871104 (MCA2830) | DQ354551 | DQ092919 | NA | Rubus argutus | Aime (2006); Matheny & Hibbett unpublished |
| Kweilingia bambusae | T | PUR F18200 | MW147026 | NA | NA | Bambusa sp. | this paper |
| Lipocystis acaciae-pennatulae (as Ravenelia acaciae-pennatulae) | BPI 864189 (U115) | MG907213 | MG907204 | MG907264 | Vachellia pennatula | Aime et al. (2018a) | |
| Lipocystis caesalpiniae | T | BPI 863966 | MW049265 | NA | NA | Mimosa ceratonia | this paper |
| Macruropyxis fraxini | T | ZT Myc 56551 | KP858145 | KP858144 | NA | Fraxinus platypoda | Beenken & Wood (2015) |
| Maravalia limoniformis | * | BRIP 59649 | MW049266 | NA | MW036500 | Austrosteenisia blackii | this paper |
| Masseeëlla capparis | T | BRIP 56844 | JX136798 | NA | KT199413 | Flueggea virosa | McTaggart et al. (2016) |
| Melampsora euphorbiae | T | BPI 863501 (U138) | DQ437504 | DQ789986 | MW036501 | Euphorbia macroclada | Aime (2006); this paper |
| Melampsora laricis-populina | strain 98AG31 | NW6768836 | NW6768836 | NW6768836 | Populus sp. | Duplessis et al. unpublished | |
| Melampsorella caryophyllacearum | T | PUR ex-MPPD-40507 | MG907233 | NA | NA | Cerastium fontanum | Aime et al. (2018a); this paper |
| Melampsoridium betulinum | T | BPI 871107 (MCA2884):28S; n.d.: 18S | DQ354561 | AY125391 | NA | Alnus sp. | Aime (2006); Wingfield et al. (2004) |
| Mikronegeria fagi | T | PUR N16373 | MW049267 | MW049298 | NA | Nothofagus obliqua | this paper |
| Mikronegeria fuchsiae | PDD 101517 | KJ716350 | KJ746826 | NA | Phyllocladus trichomanoides | Padamsee & McKenzie (2014) | |
| Milesia polypodii (as Milesina polypodii) | T | KRM0043190 | MK302190 | NA | NA | Polypodium vulgare | Bubner et al. (2019) |
| Milesina kriegeriana | T | KRM0048480 | MK302207 | NA | NA | Dryopteris dilatata | Bubner et al. (2019) |
| Miyagia pseudosphaeria | * | BPI 842230 (U63):28S; n.d.: 18S | DQ354517 | AY125411 | NA | Sonchus oleraceus | Aime (2006); Wingfield et al. (2004) |
| Naohidemyces vaccinii | T | BPI 871754 (MCA2780) | DQ354563 | DQ354562 | NA | Vaccinium ovatum | Aime (2006) |
| Neoolivea tectonae | T | PUR N15331 (MCA6480) | MW049282 | MW049307 | MW036507 | Tectona grandis | this paper |
| Neophysopella ampelopsidis (as Phakopsora ampelopsidis) | T | IBA 8597 | AB354738 | NA | NA | Ampelopsis brevipedunculata | Chatasiri & Ono (2008) |
| Neophysopella kraunhiae | PUR N15073 | MW049242 | NA | NA | Wisteria floribunda | this paper | |
| Neophysopella meliosmae-myrianthae | BRIP 58404 | MW049270 | NA | NA | Vitus sp. | this paper | |
| Newinia heterophragmatis | T | PUR N16505 | MW049271 | NA | NA | Kigelia cf. africana | this paper |
| Nothoravenelia japonica | T | HMJAU8598 | MK296509 | NA | NA | n.d. | Ji unpublished |
| Nyssopsora echinata | T | KR-0012164 (U1022):28S; ESS244:18S | MW049272 | U77061 | NA | Meum athamanticum | this paper; Swann & Taylor (1995) |
| Ochropsora ariae | T | KR-0015027 (U1036) | MW049273 | NA | NA | Anemone nemorosa | this paper |
| Olivea capituliformis | T | BPI 863670 | MW049274 | NA | NA | Alchornea latifolia | this paper |
| Peridiopsora mori | PUR N11676 (MCA4685) | MW147025 | NA | MW166323 | Morus alba | this paper | |
| Phakopsora crucis-filii | T | ZT Myc 48990 | KF528016 | KF528041 | KF528049 | Annona paludosa | Beenken (2014) |
| Phakopsora fici | BRIP 59463 | MH047210 | MW049299 | MW036502 | Ficus carica | this paper | |
| Phakopsora pachyrhizi | T | BRIP 56941 | KP729475 | MW049300 | MW036503 | Neonotonia wightii | Maier et al. (2016); this paper |
| Phragmidium mucronatum | T | BRIP 60097 | MW049275 | NA | NA | Rosa rubiginosa | this paper |
| Phragmidium tormentillae (≡Frommeëlla tormentillae) | (T) | BPI 843392 (U3) | DQ354553 | DQ354552 | MG907265 | Potentilla canadensis | Aime (2006); Aime et al. (2018a) |
| Pileolaria brevipes | PUR N16525 (MCA3477):28S, CO3; BPI 877989 (MCA3223):18S | MG907216 | MW049301 | MG907267 | Toxicodendron sp. | Aime et al. (2018a); this paper | |
| Pileolaria shiraiana | BRIP 58344 | KJ651957 | NA | NA | Rhus japonica | Doungsa-ard et al. (2018) | |
| Pileolaria terebinthi | T | PUR N11686 (U1282) | KY796222 | NA | NA | Pistacia terebinthus | Ishaq et al. (2019) |
| Porotenus biporus | * | ZT Myc 3414 | JF263494 | JF263510 | NA | Memora flavida | Beenken et al. (2012) |
| Prospodium appendiculatum | T | BPI 879956 (U753) | MW049276 | NA | NA | Tecoma stans | this paper |
| Prospodium lippiae | BPI 843901 (U152) | DQ354555 | DQ831024 | NA | Aloysia polystachya | Aime (2006) | |
| Prospodium tuberculatum | BRIP 57630 | KJ396195 | KJ396196 | MW036504 | Lantana camara | Pegg et al. (2014); this paper | |
| Puccinia graminis | T | BRIP 60137 | KM249852 | MW049302 | MW036505 | Glyceria maxima | McTaggart et al. (2016); this paper |
| Pucciniastrum epilobii | T | PUR N11088 (MCA5308) | MW049277 | NA | NA | Epilobium angustifolium | this paper |
| Pucciniastrum minimum | BRIP 57654 | KC7633401 | KT199391 | KT199422 | Vaccinium corymbosum | McTaggart et al. (2016) | |
| Pucciniosira pallidula | * | BPI 863541 (U282) | DQ354534 | MW049303 | NA | Triumfetta semitriloba | Aime (2006); this paper |
| Pucciniosira solani | n.d. | EU851137 | NA | NA | Solanum aphyodendron | Zuluaga et al. unpublished | |
| Puccorchidium polyalthiae | T | ZT HeRB 251 | JF263493 | JF263509 | JF263525 | Polyalthia longifolia | Beenken & Wood (2015) |
| Ravenelia sp. | * | PUR F19717 | MW147024 | MW166323 | MW166322 | Tephrosia sp. | this paper |
| Rogerpetersonia torreyae (as Caeoma torryeyae) | T | BPI 877825 (U1168):28S,CO3; BPI 877824 (U808):18S | MG907207 | MG907197 | MG907254 | Torreya californica | Aime et al. (2018a) |
| Rossmanomyces pyrolae (as Chrysomyxa pyrolae) | T | 390CHPPCGVF1 | FJ666456 | NA | NA | n.d. | Vialle et al. (2009) |
| Skierka diploglottidis | * | BRIP 59646 | MW049278 | MW049304 | MW036506 | Dictyoneura obtusa | this paper |
| Skierka robusta | * | BPI 879954 (U747) | MW049279 | MW049305 | NA | Rhoicissus rhomboidea | this paper |
| Sorataea arayatensis | U416 | MW049280 | NA | NA | Derris elliptica | this paper | |
| Sphaerophragmium acaciae | T | BRIP 56910 | KJ862350 | KJ862429 | KJ862462 | Albizzia sp. | McTaggart et al. (2015) |
| Sphenorchidium xylopiae | T | n.d. | KM217355 | KM217372 | NA | Xylopia aethiopica | Beenken & Wood (2015) |
| Sphenospora kevorkianii | BPI 863558 (U10) | DQ354521 | DQ354520 | NA | Stanhopea candida | Aime (2006) | |
| Stereostratum corticioides | T | BPI 842314 (U27) | MW049281 | MW049306 | NA | Bambusa sp. | this paper |
| Stomatisora psychotriicola | * | PREM 60886 | NG059953 | NA | NA | Psychotria capensis | Wood et al. (2014) |
| Tegillum scitulum (as Olivea scitula) | * | BPI 871108 (U668) | DQ354541 | DQ354540 | NA | Vitex doniana | Aime (2006) |
| Thekopsora areolata | T | n.d. | KJ546894 | NA | NA | Picea engelmannii | Kaitera et al. unpublished |
| Trachyspora intrusa | T | BPI 84328 (MCA2384) | DQ354550 | DQ354549 | MW036508 | Alchemilla vulgaris | Aime (2006); this paper |
| Tranzschelia discolor | * | BRIP 57662 | KR995082 | KR994969 | KR995082 | Prunus persica | Doungsa-ard et al. (2018) |
| Tranzschelia mexicana | * | KR-M-0040855 | KP308391 | NA | NA | Prunus salicifolia | Blomquist et al. (2015) |
| Triphragmium ulmariae | T | BPI 881364 (MCA2378):28S; n.d.:18S | JF907676 | AY125401 | NA | Filipendula ulmaria | Yun et al. (2011); Wingfield et al. (2004) |
| Uredinopsis filicina | T | WM112 | AF426237 | NA | NA | Phegopteris connectilis | Maier et al. (2003) |
| Uredo cryptostegiae (as Maravalia cryptostegiae) | BRIP 56898 | KT199401 | KT199387 | KT199412 | Cryptostegia grandiflora | McTaggart et al. (2016) | |
| Uredo elephantopodis | BRIP 58415 | MW049283 | NA | MW036509 | Elephantopus scaber | this paper | |
| Uredo hiulca | BRIP 53244 | MW049284 | NA | MW036510 | Dioscorea transversa | this paper | |
| Uredo trichosanthis | PUR N3445 | MW049285 | MW049309 | NA | Trichosanthes bracteata | this paper | |
| Uredopeltis atrides | * | PUR N13866 (U454) | MW049286 | NA | NA | Grewia flavescens | this paper |
| Uredopeltis chevalieri | * | BRIP 56924 | MW049287 | NA | NA | Grewia retusifolia | this paper |
| Uromyces appendiculatus | T | BRIP 60020 | KM249870 | DQ354510 | KX999933 | Phaseolus vulgaris | Aime (2006); McTaggart et al. (2014) |
| Uromycladium simplex | T | BRIP 59214 | KJ632990 | KJ633029 | KJ639078 | Acacia pycnantha | Doungsa-ard et al. (2014) |
| Uropyxis daleae | * | BPI 910337 | KY798364 | NA | NA | Dalea pringlei | Demers & Castlebury unpublished |
| Uropyxis diphysae | * | BPI 864148 | MW049288 | NA | NA | Diphysa americana | this paper |
| Xenodochus carbonarius | T | PUR N15566 (U1534) | MW049289 | NA | NA | Sanguisorba officinalis | this paper |
| Xenostele litseae | * | BRIP 53335 | MW049290 | MW049310 | NA | Neolitsea dealbata | this paper |
| Ypsilospora tucumanensis | * | BPI 863688 | MW049291 | NA | NA | Inga sp. | this paper |
| Zaghouania notelaeae (as Cystopsora notelaeae) | * | BRIP 58325 | KT199396 | KT199384 | KT199407 | Notelaea microcarpa | McTaggart et al. (2016) |
aType Status: T = type species for the genus; * = proxy for generic type (see methods for explanation).
bnumbers in parentheses are collection numbers, preceded by herbarium accession numbers. When sequences from more than one collection are used, data are separated by a /.
n.d. = no data.
NA = not applicable.
bold = new sequences generated for this paper.
With the overview tree as a guide, we divided the data into three subsets, Melampsorineae (73 species), Raveneliineae (77 species) and Uredinineae (164 species), for additional sampling and analyses (Table S1). In expanded sampling, we included taxa only sequenced for one of the three loci in order to broaden both generic representation and species representation for polyphyletic genera. Trees were rooted from the sister lineage as shown by the overview tree, or, in the case of Raveneliineae, midpoint rooted.
DNA extraction, PCR and sequencing
DNA was extracted from fresh or herbarium material with the UltraClean Plant DNA Isolation Kit (MoBio Laboratories Inc., Solana Beach, CA, U.S.A.). The nuclear large subunit (28S) region of the ribosomal DNA repeat was amplified with Rust2INV (Aime 2006)/LR6 or LR7 (Vilgalys & Hester 1990) and, for weak products, nested with Rust28SF (Aime et al. 2018a)/LR5 or LR6 (Vilgalys & Hester 1990) following the protocols of Aime et al. 2018a. The small subunit (18S) region of the ribosomal DNA repeat was amplified with NS1 (White et al. 1990)/Rust 18S-R (Aime 2006) and nested with RustNS2-F (Aime et al. 2018a)/NS6 (White et al. 1990) following the protocols of Aime et al. (2018a). Cytochrome-c-oxidase subunit 3 (CO3) of the mitochondrial DNA was amplified with CO3_F1/CO3_R1 (Vialle et al. 2009) following the protocols of Vialle et al. (2009). PCR products were cleaned and sequenced with the amplification primers by Macrogen (Seoul, Korea) or Beckman Coulter Sequencing (Danvers, Massachusetts, USA). Sequences were edited in Sequencher v. 4.5–5.4 (Gene Codes Corp., Ann Arbor, Michigan, USA) and verified by BLASTn against the NCBI database (Altschul et al. 1990). Sequence accession numbers are provided in Tables 1 and S1.
Phylogenetic analyses
The 28S, 18S and CO3 sequences were aligned in four datasets, (i) Pucciniales overview, (ii) Melampsorineae, (iii) Raveneliineae, and (iv) Uredinineae with the GUIDANCE2 webserver (Sela et al., 2015; available at http://guidance.tau.ac.il/ver2/credits.php) (alignments are available from TreeBASE, study TB2:S27114). The aligned loci were concatenated and run as partitioned datasets with maximum likelihood (ML). We searched for the most likely tree in IQTree v. 1.7 beta (Nguyen et al. 2015) with a GTR gamma FreeRate heterogeneity model of evolution and a different rate for each partition (command -spp -m GTR+R), 10 000 ultrafast bootstraps (Hoang et al. 2018), an approximate likelihood ratio test with 10 000 replicates (Guindon et al. 2010) and genealogical concordance factors calculated from each locus (Minh et al. 2018).
We used the concatenated three-locus alignment of the familial-overview dataset to estimate the divergence dates of genera with BEAST v. 2.5 (Bouckaert et al. 2019). We calibrated the most recent common ancestor of the Pucciniales at 175 M yr and the Melampsorineae at 91 M yr based on Aime et al. (2018a). The dating analyses were constrained to the topology of the ML tree, and run for 150 M generations, with a BEAST model test for each partition and a relaxed log normal clock. Convergence of all priors was visualised in Tracer v. 1.7 (Rambaut et al. 2018) and 135 001 trees were summarised with TreeAnnotator, part of the BEAST v. 2.5 package.
We attempted to provide better resolution of genera and families within Raveneliineae by multiple means including removal of incongruent (rogue) taxa, constructing alignments with stricter and weaker gap opening penalties, pruning taxa with missing sequence data, removal of 18S and CO3 loci, and rooting with different outgroups from the Melampsorineae and Uredinineae. The 28S data of Raveneliineae were analysed with SplitsTree4 (Huson & Bryant 2005) to visualize the evolution as a network in order to determine if groups were supported when not constrained by dichotomous evolution as imposed by ML analyses.
RESULTS
Phylogenetic analyses
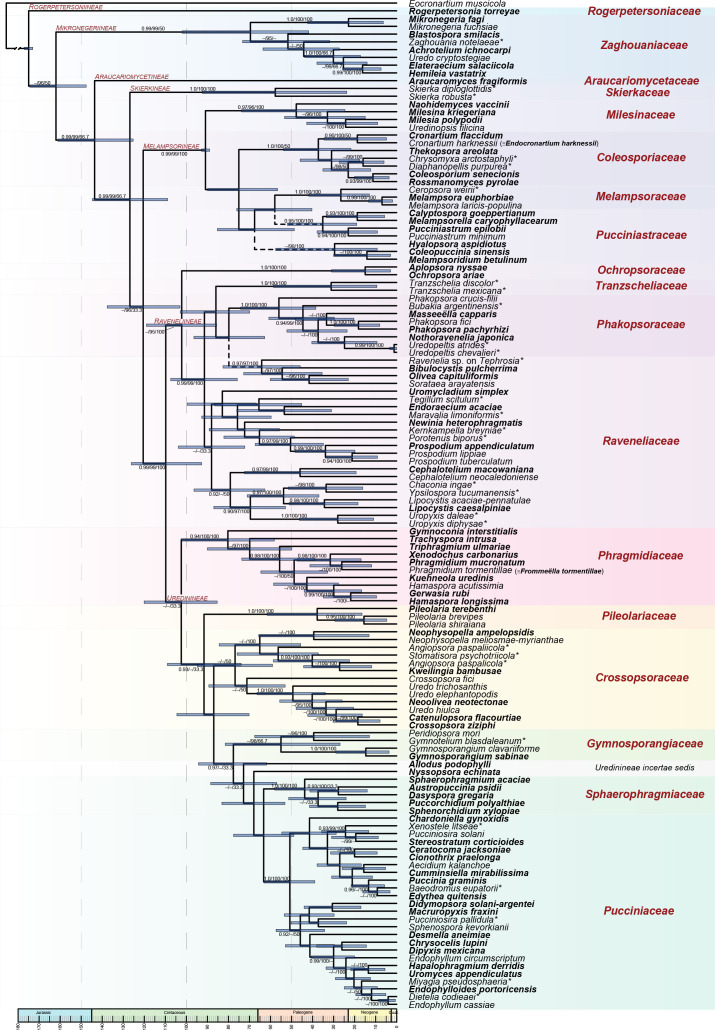
The ML tree based on three concatenated loci (Fig. 1) was mostly congruent with prior studies of more limited taxon and locus sampling (Aime 2006, Beenken & Wood 2015, McTaggart et al. 2016, Aime et al. 2017, Aime et al. 2018a, Beenken 2017, Souza et al. 2018). Sampled trees constrained to the ML topology in the dating analyses converged after 150 M generations, supported by all effective sample size values over 200. We recovered support for placement of previously unsupported or unplaceable taxa such as Tranzschelia. Newly sequenced taxa resolved include the rust fungi on Agathis, genera such as Elateraecium, Masseeëlla, and Skierka, and most of the endocyclic Pucciniosiraceae. Despite numerous attempts with different alignments and taxon selection, some families/genera could not be confidently resolved, namely: Pucciniastrum and Pucciniastraceae; Raveneliaceae; and Allodus, Neopuccinia, and Nyssopsora within Uredinineae. SplitsTree analysis of Raveneliineae recovered a star-shaped pattern of reticulate edges indicative of multiple competing hypotheses of evolution for this lineage (Fig. S2).
Fig. 1.
Pucciniales. Phylogram obtained from BEAST constrained to a ML topology from three concatenated loci (28S, 18S, and CO3). The tree is rooted with Eocronartium muscicola. Families are indicated by coloured blocks; dashed lines indicate uncertainty at the referenced nodes. Genera represented by types are indicated in bold; genera represented by type proxies (as explained in methods) are indicated by *. Support for nodes is provided from an approximate likelihood ratio test (≥ 0.90), ultrafast bootstraps (≥ 95 %) and genealogical concordance factors for the three loci at each node as aLRT/UFBoot/gCF.
Taxonomy
Families and sub-orders treated here show strong support at their most recent common ancestor, with the exception of Pucciniastraceae and Raveneliaceae (Figs 1–3). We propose four new suborders (Araucariomycetineae, Raveneliineae, Rogerpetersoniineae, and Skierkineae), seven new families (Araucariomycetaceae, Crossopsoraceae, Milesinaceae, Ochropsoraceae, Rogerpetersoniaceae, Skierkaceae, and Tranzscheliaceae) and four new genera (Araucariomyces, Neoolivea, Rogerpetersonia, and Rossmanomyces); 21 new combinations and one new name are made for species. Suborders and families are arranged from earliest diverging to more recently derived (Fig. 1). We use the terms gametothallus and sporothallus as applied by Berndt (2018) and use the notation 0-I [for spermogonial (0) and aecial (I) stages] to denote the gametothallus, and II-III [for uredinial (II) and telial (III) stages] to denote the sporothallus. We follow the ontogenic system for sorus terminology, which emphasizes function in the life cycle and the nuclear cycle over morphology, as refined by Hiratsuka (1973). Morphological terms for spermogonia follow Hiratsuka & Cummins (1963); terms for aecial and uredinial sori follow the descriptions for asexual genera in Cummins & Hiratsuka (2003) but are indicated in lowercase, non-italics, to delineate use as descriptive terms from generic names.
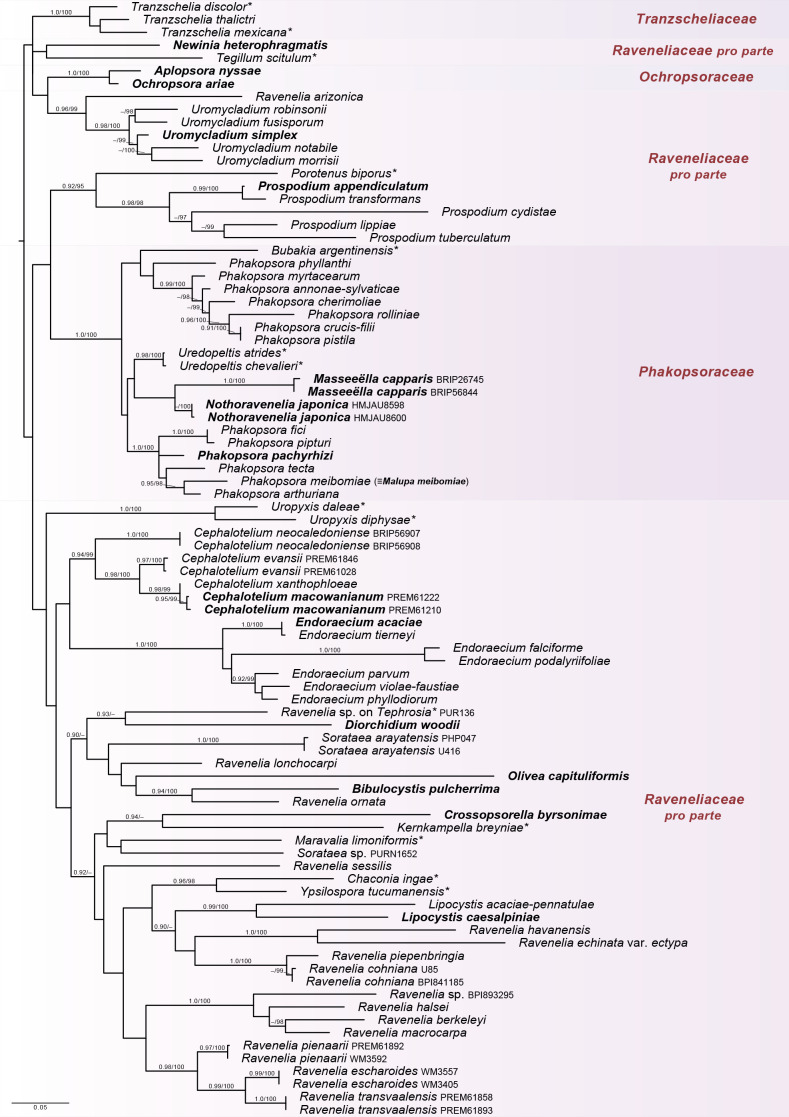
Fig. 3.
Raveneliineae. ML topography generated from 28S with expanded taxon sampling. The tree is mid-point rooted. Families are indicated by colour blocks; Raveneliaceae is not resolved. Only 26 of the estimated 45+ genera in this suborder are represented by types (indicated in bold) and type proxies (indicated by *), and poor resolution may be attributable to missing data (both locus and taxon sampling), combined with long branch lengths (Fig. S2) in this lineage. Support for nodes is provided from an approximate likelihood ratio test (≥ 0.90) and ultrafast bootstraps (≥ 95 %) as aLRT/UFBoot.
Rogerpetersoniineae Aime & McTaggart, subord. nov. MycoBank MB836604.
Type family: Rogerpetersoniaceae Aime & McTaggart, this paper.
Diagnosis: Differs from all other Pucciniales in that gametothalli are formed on Taxaceae.
Description: With the characteristics of Rogerpetersoniaceae.
Included family: Rogerpetersoniaceae.
Rogerpetersoniaceae Aime & McTaggart, fam. nov. MycoBank MB836605.
Type genus: Rogerpetersonia Aime & McTaggart, this paper.
Diagnosis: Differs from all other Pucciniales in that gametothalli are formed on Taxaceae.
Description: With the characteristics of Rogerpetersonia.
Included genus: Rogerpetersonia.
Host family: Taxaceae (0-I); II-III unknown.
Rogerpetersonia Aime & McTaggart, gen. nov. MycoBank MB836606.
Type species: Rogerpetersonia torreyae (Bonar) Aime & McTaggart, this paper.
Etymology: In honour of Roger Peterson, botanist, ecologist, mycologist and plant pathologist, who pioneered studies on Southern Hemisphere conifer rusts.
Diagnosis: Differs from all other rust fungi in forming gametothalli on Taxaceae (Torreya).
Description: With the characteristics of Rogerpetersonia torreyae.
Rogerpetersonia torreyae (Bonar) Aime & McTaggart, comb. nov. MycoBank MB836608.
Basionym: Caeoma torreyae Bonar, Mycologia 43: 62. 1951.
Description: Rogerpetersonia torreyae is described and illustrated as C. torreyae in Peterson (1974). Spermogonia are deep-seated, periphysate, otherwise similar to Group III (type 12). Aecia petersonia-like, i.e., without peridium or intercalary cells. Sporothallus unknown.
Notes: Caeoma, as typified by C. berberidis, is a synonym of Puccinia (Aime et al. 2018b), necessitating a new name for the only known rust fungus that infects Torreya. Peterson (1974) hypothesized that R. torreyae belonged to an undescribed early diverging lineage of Pucciniales. Subsequent analyses have shown that R. torreyae is the earliest diverging extant rust sequenced to date and holds an isolated position within Pucciniales (Aime 2006, Aime et al. 2018a) (Fig. 1). No alternate host is known for this rust and it is likely that R. torreyae has adapted to cause systemic infections in the gametothallus host in order to compensate for loss of a sporothallus.
Mikronegeriineae Aime, Mycoscience 47: 120. 2006.
Description: With the characteristics of the family.
Included family: Zaghouaniaceae.
Zaghouaniaceae P. Syd. & Syd., Monogr. Uredin. (Lipsiae) 3(3): 586. 1915. emend. Aime & McTaggart
Synonyms: Hemileieae Dietel, Uredinales in Engler and Prantl., Naturl.: 51. 1928.
Mikronegeriaceae Cummins & Y. Hirats. (as ‘Micronegeriaceae’), Illustr. Gen. Rust Fungi, rev. Edn (St. Paul): 13. 1983.
Type genus: Zaghouania Pat., Bull. Soc. mycol. Fr. 17: 187. 1901.
Description: Spermogonia most often Group III (type 12) (deep seated and non-periphysate), but periphyses noted for some; aecia most commonly of the petersonia-type, i.e., without peridium or intercalary cells, however in Elateraecium accompanied with specialized elaters; uredinia most often uredo-type, in Elateraecium with a weakly developed peridium in young sori; teliospores without dormancy, germinating externally by apical growth, or internally (Achrotelium). Blastospora and Mikronegeria are heteroecious and macrocyclic, Elateraecium and Zaghouania are autoecious and macro- or demi-cyclic; complete life cycles unknown for Achrotelium, Botryorhiza and Hemileia.
Included genera: Achrotelium, Blastospora, Botryorhiza, Elateraecium (= Hiratsukamyces), Hemileia, Mikronegeria, Zaghouania (= Cystopsora); likely includes Desmosorus.
Host families: Araucariaceae, Betulaceae (0-I heteroecious species); Apocynaceae, Araliaceae, Capparidaceae, Celastraceae, Cupressaceae, Dioscoreaceae, Euphorbiaceae, Fagaceae, Oleaceae, Orchidaceae, Rubiaceae, Smilacaceae, Verbenaceae (II-III and autoecious species).
Notes: The family Mikronegeriaceae accommodated the heteroecious rust genera Mikronegeria, Blastospora, and Chrysocelis, which have thin-walled basidia that germinate externally without dormancy (Cummins & Hiratsuka 2003). Hemileia and some Maravalia species formerly placed in Chaconiaceae that share the feature of substomatal sori without paraphyses or peridium, also belong here (Aime 2006). Two additional genera, Achrotelium and Zaghouania (as Cystopsora), were included by McTaggart et al. (2016). Zaghouaniaceae, long considered a synonym for Pucciniaceae (e.g., Kirk et al. 2008), has priority over Mikronegeriaceae and the family is now referred to by the earlier name. The current study adds Elateraecium (syn. Hiratsukamyces; Aime et al. 2018b), whereas Chrysocelis is resolved within the Pucciniaceae (Fig. 4). The formation of basidia is primarily external by apical growth and spermogonia are primarily deep-seated Group III (type 12), or if periphysate, similar to Group V (type 4).
Fig. 4.
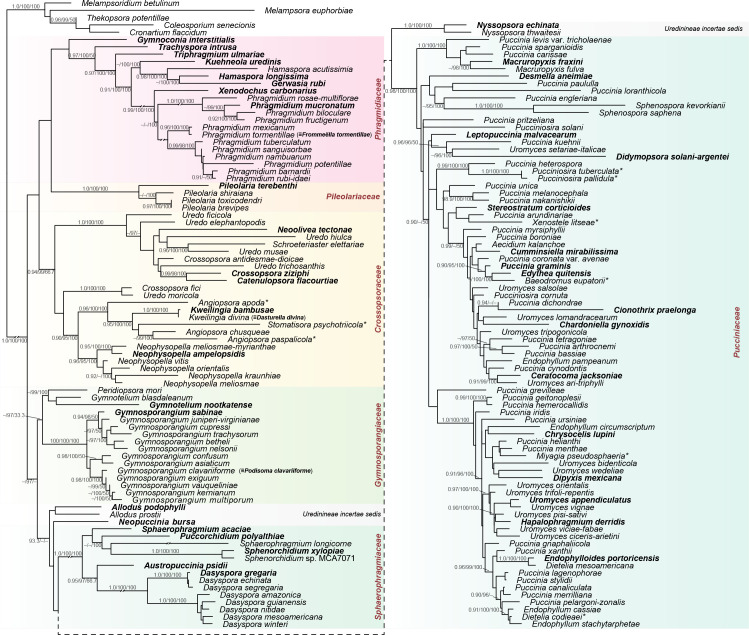
Uredinineae. ML topography generated from three concatenated loci (28S, 18S, and CO3) with expanded taxon sampling. The tree is rooted with Melampsorineae. Six families are resolved and indicated by coloured blocks; three genera are unresolved to family and indicated as incertae sedis. Genera represented by types are indicated in bold; genera represented by type proxies (as explained in methods) are indicated by *. Support for nodes is provided from an approximate likelihood ratio test (≥ 0.90), ultrafast bootstraps (≥ 95 %) and genealogical concordance factors for the three loci at each node as aLRT/UFBoot/gCF.
Uredo cryptostegiae (syn. Maravalia cryptostegiae; Scopella cryptostegiae), which has been used as a biocontrol agent for rubber-vine (Cryptostegia grandiflora) is placed in Zaghouaniaceae (Fig. 1). Cummins (1950) transferred M. cryptostegiae to Scopella, while hypothesizing that the rust might belong to Hemileia. Most later workers considered Scopella and Maravalia congeneric and Scopella fell out of use. The type of Maravalia, M. pallida occurs on Fabaceae and is now placed in Raveneliineae. The type of Scopella, S. echinulata, is a subepidermal rust on Sapotaceae (Mains 1939a). Uredo cryptostegiae, which is not congeneric with Maravalia (as represented by M. limoniformis, Fig. 1), is most appropriately retained in Uredo until type data from S. echinulata is obtained.
Zaghouania notelaeae (Syd.) Aime & McTaggart, comb. nov. MycoBank MB836655.
Basionym: Cystopsora notelaeae Syd., Annls mycol. 35: 351. 1937.
Notes: Zaghouania contains two other species of rust fungi on Oleaceae with pale-walled teliospores that germinate without dormancy (Cummins & Hiratsuka 2003). There is little to differentiate Cystopsora and Zaghouania (Thirumalachar 1945, Cummins & Hiratsuka 2003) and we treat Cystopsora as a synonym of Zaghouania.
Araucariomycetineae Aime & McTaggart, subord. nov. MycoBank MB836623.
Type family: Araucariomycetaceae Aime & McTaggart, this paper.
Diagnosis: Differs from all other Pucciniales in forming gametothalli on Agathis.
Description: With the characteristics of Araucariomycetaceae.
Included family: Araucariomycetaceae.
Araucariomycetaceae Aime & McTaggart, fam. nov. MycoBank MB836624.
Type genus: Araucariomyces Aime & McTaggart, this paper.
Diagnosis: Differs from all other Pucciniales in forming gametothalli on Agathis.
Description: With the characteristics of Araucariomyces.
Included genus: Araucariomyces.
Host family: Araucariaceae (0-I); II-III unknown.
Araucariomyces Aime & McTaggart, gen. nov. MycoBank MB836625.
Type species: Araucariomyces fragiformis (Ces.) McTaggart, R.G. Shivas & Aime, this paper.
Entomology: From the host family, Araucariaceae.
Diagnosis: Differs from all other rust genera in forming the gametothallus on species of Agathis (Araucariaceae).
Description: These species are described and illustrated in Peterson (1966). Spermogonia amphigenous, intra-epidermal becoming sub-epidermal as they break through host walls, convex hymenium; similar to Group 1 (type 1) but with scant periphyses not visible in all mounts, similar to Rogerpetersonia. Aecia peridiate, aecidium-type, deep-set within swollen host tissues. Sporothallus unknown. On leaves of Agathis (Araucariaceae). Two known species.
Notes: Two rust fungi with cupulate aecia on Agathis spp., formerly placed in the form-genus Aecidium, belong here. Our analyses consistently place these in a lineage separate from all other sequenced Pucciniales (Fig. 1). Despite over a decade of sampling rust fungi from Australia and Southeast Asia on hosts co-distributed with Agathis species, we have been unable to locate a telial state for these rusts. Peterson (1968) ruled out the possibility that Araucariomyces represents an endocyclic form, because aeciospores of Ar. balansae germinate to produce germ tubes rather than basidia. As is conjectured with Rogerpetersonia, the life cycle may not produce a sporothallus, and instead has adapted to systemically infect their hosts possibly including a cryptic sexual or parasexual cycle.
Araucariomyces balansae (Cornu) McTaggart, R.G. Shivas & Aime, comb. nov. MycoBank MB836626.
Basionym: Aecidium balansae Cornu., Bull. Soc. mycol. Fr. 3: 173. 1887.
Synonym: Peridermium balansae (Cornu) Sacc., Syll. Fung. 9: 326. 1891.
Araucariomyces fragiformis (Ces.) McTaggart, R.G. Shivas & Aime, comb. nov. MycoBank MB836627.
Basionym: Aecidium fragiforme Ces., Atti Accad. Sci. fis. mat. Napoli 8: 26. 1879.
Skierkineae Aime & McTaggart, subord. nov. MycoBank MB836628.
Type family: Skierkaceae Aime & McTaggart, this paper.
Diagnosis: Differs from all other rust fungi in that sporothalli sori are deep-seated and subepidermal with mature uredinio- and teliospores single-celled and non-catenulate, these forced through a narrow sorus opening by the production of new spores from sporogenous cells from which they are detached before extrusion.
Description: With the characters of Skierkaceae.
Included family: Skierkaceae.
Skierkaceae (Arthur) Aime & McTaggart, fam. & stat. nov. MycoBank MB836629.
Basionym: Skierkatae Arthur, North American Flora 7(10): 704. 1926.
Type genus: Skierka Racib., Parasit. Alg. Pilze Javas (Jakarta) 2: 30. 1900.
Diagnosis: Differs from all other rust fungi in that sporothalli sori are deep-seated and subepidermal with mature uredinio- and teliospores single-celled and non-catenulate, these forced through a narrow sorus opening by the production of new spores from sporogenous cells from which they are detached before extrusion.
Description: With the characteristics of Skierka as described and illustrated in Mains (1939b). Spermogonia deep-seated with convex hymenium, subepidermal, periphysate; aecia and uredinia uredo-type; teliospores strongly adherent, extruded in hair-like columns, germination external, without dormancy. Autoecious and macrocyclic.
Included genus: Skierka.
Host families: Burseraceae, Euphorbiaceae, Sapindaceae.
Notes: Skierka species are tropical and autoecious (Mains 1939c, Cummins & Hiratsuka 2003). All sori are subepidermal and deep-seated; non-catenulate teliospores are extruded in hair-like columns. Skierka has long held an isolated placement within Pucciniales. Arthur (1907–1931) and Dietel (1928) placed Skierka in a separate subfamily or tribe, respectively, in the Pucciniaceae; Cummins & Hiratsuka (2003) treat it as incertae sedis within the rusts. Mains (1939c) hypothesised that Skierka represented an intermediate taxon between the Melampsoraceae and Pucciniaceae (equivalent to the subordinal ranks Melampsorineae and Raveneliineae/Uredinineae, under the present classification), a position largely congruent with our placement (Fig. 1).
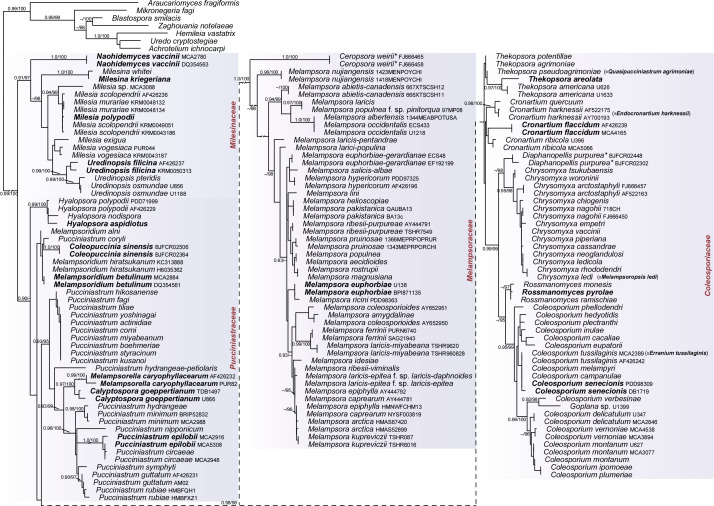
Melampsorineae Aime, Mycoscience 47: 120. 2006.
Type family: Melampsoraceae Dietel, in Engler & Prantl, Nat. Pflanzenfam. 1(1): 38. 1897.
Description: Mostly macrocyclic and heteroecious, forming the gametothallus on species of Pinaceae. Teliospores germinate after a period of dormancy.
Included families: Coleosporiaceae, Melampsoraceae, Milesinaceae, Pucciniastraceae.
Milesinaceae Aime & McTaggart, fam. nov. MycoBank MB836630.
Type genus: Milesina Magnus, Ber. Deutsch. Bot. Ges. 27: 325. 1909.
Diagnosis: Similar to other Melampsorineae, differing in either production of colourless urediniospores in species that infect ferns, or in production of milesia-type aecia in species that infect Ericaceae.
Description: With typically colourless sori, although urediniospores of Naohidemyces are orange, otherwise similar to Pucciniastraceae. Spermogonia Group I (mostly type 1, also type 2 and 3); aecia peridermium-type, milesia-type in Naohidemyces; uredinia milesia-type. Teliospores with dormant germination, 1- to many-celled, barely differentiated, sometimes laterally adherent, typically formed within host epidermal cells. Most species macrocyclic and heteroecious with sporothalli on ferns (excepting Naohidemyces on Ericaceae), and gametothalli on Pinaceae.
Included genera: Milesia, Milesina, Naohidemyces, Uredinopsis.
Host families: Pinaceae (Abies, Tsuga) (0-I); Ericaceae and some ferns in Polypodiales and Lygodium (II-III).
Notes: Early workers considered rust fungi on early diverging plant hosts (i.e., ferns) to be the “ancestral” Pucciniales. Several molecular phylogenetic studies have shown this not to be the case (e.g., Sjamsuridzal et al. 1999). However, the fern rusts are among the earliest diverging members of Melampsorineae (Fig. 2), the second major radiation of the rust fungi, and belong to the two earliest families in this suborder (Milesinaceae and Pucciniastraceae). Most of the species in Milesinaceae form sporothalli on fern species, except for Naohidemyces, which alternates between Tsuga and Vaccinium.
Fig. 2.
Melampsorineae. ML topography generated from three concatenated loci (28S, 18S, and CO3) with expanded taxon sampling. The tree is rooted with Araucariomycetaceae and Zaghouaniaceae. Four families are indicated by coloured blocks; Pucciniastraceae is recovered as a grade in these analyses. Genera represented by types are indicated in bold; genera represented by type proxies (as explained in methods) are indicated by *. Support for nodes is provided from an approximate likelihood ratio test (≥ 0.90) and ultrafast bootstraps (≥ 95 %) as aLRT/UFBoot.
Aime et al. (2018b) recommended protecting the name Milesina Magnus over Milesia F.B. White. However, our data show that the type of Milesina, M. kriegeriana (Magnus) Magnus, is not congeneric with the type of Milesia, M. polypodii F.B. White (Fig. 2), thus we recommend retaining both genera at this time. Should future work demonstrate that Uredinopsis is polyphyletic, then disposition of these taxa will need revision.
Coleosporiaceae Dietel, In: Engler & Prantl, Nat. Pflanzenfam., Teil. I (Leipzig) 1: 548. 1900. emend. Aime & McTaggart
Synonym: Cronartiaceae Dietel, in Engler and Prantl, Nat. Pflanzenfam. 1(1) (Suppl.): 548. 1900.
Type genus: Coleosporium Lév., Ann. Sci. Nat. Bot. III, Ser. 8: 373. 1847.
Description: Spermogonia Group I (type 2 or 3) (but Group II, type 9 in Cronartium); aecia of peridermium-type; uredinia either of caeoma-type or milesia-type. Teliospores packed to loosely adherent, often extruded in columns and/or gelatinous; not dormant, with external germination. Most are heteroecious and macrocyclic, with some derived microcyclic or endocyclic species.
Included genera: Chrysomyxa, Coleosporium, Cronartium, Diaphanopellis, Rossmanomyces, Thekopsora (= Quasi-pucciniastrum).
Host families: Pinaceae (primarily Pinus) (0-I); various, including Apocynaceae, Asteraceae, Campanulaceae, Convolvulaceae, Ericaceae, Lamiaceae, Ranunculaceae, Rosaceae, Rutaceae, Violaceae (II-III).
Notes: Coleosporiaceae was shown to include Cronartiaceae (Aime 2006) as well as Thekopsora s.s. (Aime et al. 2018a). Aecia are peridermium-type in contrast to most Milesinaceae. Telial states show variable morphology but tend to form the sporothallus on herbaceous rather than woody plants (cf. Pucciniastraceae) or ferns. Dietel (1900) established both Coleosporiaceae and Cronartiaceae in the same publication. We follow Sydow & Sydow (1915) in applying Coleosporiaceae over Cronartiaceae, which is discussed in Aime (2006). Endocronartium is a later synonym of Cronartium (Aime et al. 2018b).
Rossmanomyces Aime & McTaggart, gen. nov. MycoBank MB836632.
Type species: Rossmanomyces pyrolae (Rostr.) Aime & McTaggart, this paper.
Etymology: In honour of Amy Rossman, biologist, mycologist, plant pathologist, and mentor.
Diagnosis: Similar to Chrysomyxa but differs in forming a systemic sporothallus; differs from all other rust fungi in forming sporothalli on Moneses and Orthilia (Ericaceae).
Description: See Saville (1950) and Feau et al. (2011). Gametothalli systemic in cones of Picea species; sporothalli systemic in Moneses, Orthilia, and Pyrola species.
Rossmanomyces monesis (Ziller) Aime & McTaggart, comb. nov. MycoBank MB836633.
Basionym: Chrysomyxa monesis Ziller, Canad. J. Bot. 32: 435. 1954.
Rossmanomyces pyrolae (Rostr.) Aime & McTaggart, comb. nov. MycoBank MB836634.
Basionym: Chrysomyxa pyrolae Rostr., Botan. Zbl. 5: 127. 1881.
Rossmanomyces ramischiae (Lagerh.) Aime & McTaggart, comb. nov. MycoBank MB836635.
Basionym: Chrysomyxa ramischiae Lagerh., Svensk bot. Tidskr. 3: 26. 1909.
Notes: Chrysomyxa is typified by C. abietis, a microcyclic species for which there are no sequence data. In our analyses (Fig. 2) most species of Chrysomyxa were monophyletic, excluding C. weirii now placed in Ceropsora, and species that infect wintergreens, now placed in Rossmanomyces. The species of Rossmanomyces are the only known rust species that form sporothalli on species of Moneses and Orthilia, and the only Coleosporiaceae that form sporothalli on species of Pyrola. The gametothalli are produced on Picea and are systemic within the cones, in contrast to gametothalli of Chrysomyxa species, which infect needles.
Thekopsora americana (Farl.) Aime & McTaggart, comb. et stat. nov. MycoBank MB836637.
Basionym: Pucciniastrum arcticum var. americanum Farl., Rhodora 10: 16. 1908.
Synonym: Pucciniastrum americanum (Farl.) Arthur, Bull. Torrey bot. Club 47: 468. 1920.
Thekopsora potentillae (Korn.) Aime & McTaggart, comb. nov. MycoBank MB836636.
Basionym: Pucciniastrum potentillae Korn., in Jaczewski et al., Fungi Rossiae Exsicc. fasc. 7: 327. 1900 [1899].
Notes: Delimitation between Thekopsora and Pucciniastrum has never been satisfactory (e.g., Hiratsuka 1958, Sato et al. 1993). While prior works mostly consider these confamilial or even congeneric, Thekopsora s.s., as typified by T. areolata, belongs to Coleosporiaceae (Aime et al. 2018a; Fig. 2). New combinations are proposed for ex-Pucciniastrum species. Other former Thekopsora species, such as P. minima and P. rubiae are placed in Pucciniastraceae (Fig. 2).
Thekopsora pseudoagrimoniae Aime & McTaggart, nom. nov. MycoBank MB836638.
Basionym: Quasipucciniastrum agrimoniae X.H. Qi et al., Mycology 10(3): 145. 2019.
Description: See Qi et al. (2019).
Notes: The recently described monotypic Quasipucciniastrum based on Q. agrimoniae is congeneric with Thekopsora (Fig. 2). In addition to the phylogenetic data, Quasipucciniastrum shares key morphological features, ecology, and hosts with Thekopsora. This paper highlights the importance of including type species and adequate sampling in phylogenetic studies of known polyphyletic genera. The name Thekopsora agrimoniae Dietel is already in use, thus a new name is proposed for this taxon. However, there is little to differentiate T. pseudoagrimoniae from T. agrimoniae and the two may be conspecific.
Pucciniastraceae Gäum. ex Leppik, Ann. bot. fenn. 9: 139. 1972. emend. Aime & McTaggart
Type genus: Pucciniastrum G.H. Otth, Mitt. Naturforsch. Ges. Bern 1861: 71. 1861.
Description: Similar to Milesinaceae, but most species with cytoplasmic pigmentation, at least within urediniospores. Spermogonia Group I (type 2 or 3). Aecia peridermium-type; uredinia milesia-type. Telia undergo dormancy with external germination; either formed within epidermal cells, or as a subepidermal crust, which is gelatinous in Coleopuccinia. Most species heteroecious, macrocyclic; Calyptospora is demicyclic, Coleopuccinia is microcylic, producing only teliospores.
Included genera: Calyptospora, Coleopuccinia, Hyalopsora, Melampsorella, Melampsoridium, Pucciniastrum.
Host families: Pinaceae (Abies, Larix, Picea, Tsuga) (0-I); Aceraceae, Betulaceae, Caryophyllaceae, Ericaceae, Fagaceae, Onagraceae, Rosaceae, Rubiaceae and some ferns in the Polypodiales (II-III).
Notes: Most species of Pucciniastraceae produce spores with pigmented cytoplasm and telia that may be subepidermal, in contrast to Milesinaceae. Hyalopsora is the only genus in Pucciniastraceae that infects ferns. Coleopuccinia is known only from teliospores (Cao et al. 2018). Pucciniastraceae s.l. has been difficult to resolve and appears polyphyletic with varying degrees of support in earlier studies (e.g., Maier et al. 2003, Aime 2006, Aime et al. 2016a, Ji et al. 2019). In this work, we find weak support for Pucciniastraceae in some analyses (data not shown) but not all (e.g., Fig. 1). In nearly all analyses Pucciniastraceae is resolved into two groups: (i) Calyptospora, Melampsorella, and Pucciniastrum; and (ii) Coleopuccinia, Hyalopsora, and Melampsoridium. These often form a grade (Fig. 2) and may or may not represent separate family-rank lineages. Pending additional analyses, we broadly define Pucciniastraceae to include both groups. Pucciniastrum is also difficult to resolve with confidence, and is most likely paraphyletic, even after removing the ex-Pucciniastrum elements that were reassigned to Thekopsora (Fig. 2). We retain Coleopuccinia, Calyptospora, and Melampsorella at this time, although future work may show that the latter two are synonyms for Pucciniastrum.
Melampsoraceae Dietel, in Engler & Prantl, Nat. Pflanzenfam., Teil. I (Leipzig) 1: 38. 1897.
Type genus: Melampsora Castagne, Obs. Plantes Acotylédonées Fam. Urédinié 2: 18. 1843.
Description: Spermogonia Group I (type 2 or 3). Aecia mostly caeoma-type; uredinia uredo-type. Teliospores subepidermal, laterally adherent in crusts, 1-celled, often with a sterile basal cell; germination external or semi-external (Ceropsora). Most species heteroecious, macrocyclic; Ceropsora species are microcyclic.
Included genera: Melampsora; likely includes Ceropsora.
Host families: Primarily Pinaceae (0-I); primarily Salicaceae, also Apocynaceae, Asteraceae, Euphorbiaceae, Flacourtiaceae, Hypericaceae, Linaceae, Passifloraceae, Saxifragaceae, Scrophulariaceae, Thymelaeaceae (II-III)
Ceropsora weirii (H.S. Jacks.) Aime & McTaggart, comb. nov. MycoBank MB836631.
Basionym: Chrysomyxa weirii H.S. Jacks., Phytopathology 7: 353. 1917.
Notes: Most of the ca. 30 species of Chrysomyxa are heteroecious with gametothalli on Pinaceae and are allied within Coleosporiaceae (Fig. 2). Chrysomyxa weirii, an autoecious microcyclic pathogen of Picea species, is unique among described Chrysomyxa in forming laterally adherent teliospores that act as diaspores, are adapted for dispersal in water, and germinate to produce 2-celled basidia (Crane 2000, Crane et al. 2000). Crane et al. (2000) conjectured that Ch. weirii is not a true Chrysomyxa, which is supported with molecular data (Feau et al. 2011, Aime et al. 2018a, Fig. 2). The type and only other species of Ceropsora, C. picea, is a teliospore-only species infecting Picea in India (Bakshi & Singh 1960). While we have been unable to sequence a representative of the type species, C. weirii and C. picea are both microcyclic producing telia on Picea species. In both species, the telia contain some thin-walled sterile cells on the sides that have been interpreted as remnants of a peridermium. And in both, teliospores are subtended by sterile basal cells forming initially adherent crusts that separate at dispersal; germination is semi-external (Bakshi & Singh 1960, Crane et al. 2000).
Raveneliineae Aime & McTaggart subord. nov. MycoBank MB836639.
Type family: Raveneliaceae Leppik, Ann. Bot. Fenn. 9(3): 139. 1972.
Diagnosis: Similar to Uredinineae differing in that the majority of species form Group VI spermogonia whereas the majority of Uredinineae form Group V spermogonia.
Description: With the characteristics of the included families. Most species form Group VI spermogonia; many species form elaborate, multi-celled teliospores.
Included families: Ochropsoraceae, Phakopsoraceae, Raveneliaceae, Tranzscheliaceae.
Notes: The Raveneliineae is the most challenging suborder in which to resolve families due to: (i) a pattern of multiple, parallel radiations in this lineage (Fig. S2); (ii) multiple instances of convergent morphologies; (iii) polyphyly; and (iv) incomplete sampling and missing data in our analyses. Raveneliineae is the second richest suborder in terms of taxonomic diversity, with ca. 45 accepted genera, of which we were only able to sample representatives from about half and most of these with incomplete locus data.
Host range may be an informative character to place taxa of Raveneliineae in families. For example, Savile (1989) predicted Maravalia sensu Ono (1984) was polyphyletic, and hypothesised that species on Fabaceae belonged to Raveneliaceae, supported here with the placement of the Fabaceae-infecting M. limoniformis within Raveneliineae (Figs 1, 3) and the Apocynaceae-infecting U. cryptostegiae (syn. M. cryptostegiae) within Zaghouaniaceae (Fig. 1). Likewise, Triphragmium Link has evolved elaborate teliospores similar to those in some Raveneliaceae where it has been allied in the past (e.g., Cummins & Hiratsuka 2003); Triphragmium species are now known to belong to Phragmidiaceae with other Rosaceae-infecting rusts (Aime 2006).
We treat four families within Raveneliineae, taking into account life cycle and host data, and have taken a conservative approach to assigning genera within families and species to genera until data from type species and/or exemplars from key missing taxa as well as additional loci can be obtained.
Ochropsoraceae (Arthur) Aime & McTaggart, fam. & stat. nov. MycoBank MB836640.
Basionym: Ochropsoratae Arthur, Rés. Sci. Congr. Int. Bot. Vienne: 336. 1906.
Type genus: Ochropsora Dietel, Ber. Dtsch. Bot. Ges. 13: 401. 1895.
Description: Spermogonia Group VI (type 7). Aecia aecidium-type; uredinia malupa-type; aecial states systemic overwintering as mycelium; telia forming crusts, 1-cell deep, at first subepidermal, then erumpent; teliospores germinate without dormancy, either internally (Ochropsora) or externally (Aplopsora). Species likely macrocyclic and heteroecious, although gametothallus not known for Aplopsora.
Included genera: Aplopsora, Ochropsora; likely includes Ceraceopsora.
Host families: Ranunculaceae (0-I); Rosaceae, Cornaceae (II-III)
Notes: A monophyletic Ochropsoraceae as the earliest diverging lineage of Raveneliineae was recovered in all of our analyses. Aplopsora and Ochropsora were previously treated within the artificial Chaconiaceae (Cummins & Hiratsuka 2003) where they shared the convergent character of teliospore germination without dormancy.
Tranzscheliaceae (Arthur) Aime & McTaggart, fam. & stat. nov. MycoBank MB836641.
Basionym: Tranzschelieae Arthur, Rés. Sci. Congr. Int. Bot. Vienne: 340. 1906.
Type genus: Tranzschelia Arthur, Rés. Sci. Congr. Int. Vienne: 340. 1906.
Description: Spermogonia Group VI (type 7). Aecia aecidium-type; uredinia uredo-type. Teliospores 2-celled, pedicellate, produced from sterile basal cells. Species are macrocyclic and heteroecious, with some derived microcyclic species.
Included genera: Leucotelium, Tranzschelia.
Host families: Ranunculaceae (0-I and autoecious species); Rosaceae (II-III in heteroecious species).
Notes: Tranzschelia has held an isolated position within Pucciniales in prior molecular studies (Aime 2006) and appears as an independent lineage of Raveneliineae in this work (Fig. 1). Leucotelium has been treated as a synonym of Sorataea (Cummins & Hiratsuka 2003) but retained by Thirumalachar & Cummins (1940) due to the presence of a sterile basal cell layer from which the teliospores develop that is lacking in Sorataea. Two non-type species of Sorataea were included in our analyses and are referable to Raveneliaceae (Fig. 3). Leucotelium is the sister genus to Tranzschelia (Scholler et al. 2019), with which it shares a similar host range and teliospore production from sterile sporogenous cells (Thirumalachar & Cummins 1940, López-Franco & Hennen 1990). Many species of Tranzschelia are microcyclic on Ranunculaceae in accordance with Tranzschel’s Law (Scholler et al. 2019).
Phakopsoraceae Cummins & Y. Hirats., Illustr. Gen. Rust Fungi, rev. Edn (St. Paul): 13. 1983. emend. Aime & McTaggart
Type genus: Phakopsora Dietel, Ber. Deutsch. Bot. Ges. 13: 333. 1895.
Description: Spermogonia Group VI (type 7). Aecia caeoma-type, some Masseeëlla with aecidium-type aecia; uredinia lecythea- or uredo-type. Teliospores 1-celled. Bubakia, Masseeëlla and Nothoravenelia species are autoecious and macrocyclic. The majority of Phakopsora and Uredopeltis species are only known from the sporothallus.
Included genera: Bubakia, Masseeëlla, Nothoravenelia, Phakopsora, Uredopeltis; likely includes Arthuria, Cerotelium, Dicheirinia, Monosporidium, Phragmidiella, Pucciniostele, Scalarispora.
Host families: Annonaceae, Bignoniaceae, Burseraceae, Commelinaceae, Euphorbiaceae, Fabaceae, Myrtaceae, Rubiaceae, Urticaceae (0-III).
Notes: Both Phakopsora and the Phakopsoraceae are known to be polyphyletic (e.g., Aime 2006), with more than 100 species currently classified in Phakopsora s.l. However, lack of data and differing interpretations of the type have hampered taxonomic progress. The recent designation of a new type species for Phakopsora, P. pachyrhizi (Aime et al. 2019a, b), has stabilized use of the name as applied here, for those genera and species that share a common ancestor with P. pachyrhizi. Phakopsora remains poorly resolved with our data and consists of two supported clades, one containing P. pachyrhizi and its allies and the other containing most of the Annonaceae-infecting species, which may represent a separate genus, but were recovered as monophyletic in some analyses (not shown).
The name Bubakia is often treated as a synonym of Phakopsora (e.g., Cummins & Hiratsuka 2003). Our study shows that Bubakia argentinensis belongs to a distinct lineage within Phakopsoraceae (Figs 1, 3). Further, B. argentinensis shares similar hosts (Croton spp.) and characteristics with the type, B. crotonis, and we accept Bubakia for these species (Mundkur 1943). Masseeëlla has previously been treated as incertae sedis within Pucciniales (Cummins & Hiratsuka 2003), but our data place it within Phakopsoraceae (Figs 1, 3).
The majority of Phakopsora and Uredopeltis species are known only from sporothalli. It is unknown whether gametothalli occur on an alternate host, or whether these species are autoecious. Sporothalli have been described for a few Phakopsora species, i.e., P. breyniae, P. innata, P. phyllanthi-discoidei, and P. stratosa, which are all autoecious (Berndt & Wood 2012, Ono 2015b), although it is unclear whether these should be retained in Phakopsora s.s. or are allied with one of the segregate ex-Phakopsora genera.
Phakopsora pipturi (Syd.) Aime & McTaggart, comb. nov. MycoBank MB836642.
Basionym: Pucciniastrum pipturi Syd., Annls mycol. 29(3/4):171. 1931.
Synonym: Uredo pipturi (Syd.) Hirats. f., Trans. Mycol. Soc. Japan 5: 4. 1957.
Raveneliaceae Leppik, Ann. bot. fenn. 9: 139. 1972. emend. Aime & McTaggart
Synonyms: Chaconiaceae Cummins & Y. Hirats., Illustr. Gen. Rust Fungi, rev. Edn (St. Paul): 14. 1983.
Uropyxidaceae Cummins & Y. Hirats., Illustr. Gen. Rust Fungi, rev. Edn (St. Paul): 14. 1983.
Type genus: Ravenelia Berk., Gard. Chron. 13:132. 1853.
Description: Spermogonia Group VI (type 5 or 7); aecia uredo- (rarely aecidium-, caeoma-, or lecythea-) type; uredinia uredo-type. Teliospores 1- to many-celled, some species with elaborate compound or multi-celled teliospores. Majority of species autoecious and macrocyclic, with a few derived microcyclic species; many species on mimosoid (Caesalpinioideae) hosts.
Included genera: Bibulocystis, Cephalotelium, Crossopsorella, Diorchidium, Endoraecium, Kernkampella, Lipocystis, Newinia, Olivea, Porotenus, Prospodium, Ravenelia, Sorataea, Uromycladium, Uropyxis, Ypsilospora; likely includes Allotelium, Anthomyces, Anthomycetella, Apra, Atelocauda, Chaconia, Cystomyces, Diabole, Diochordiella, Esalque, Hennenia, Maravalia, Mimema, Phragmopyxis, Spumula, Tegillum.
Host families: Bignoniaceae, Euphorbiaceae, Fabaceae, Ranunculaceae, Rosaceae, Sapotaceae, Verbenaceae (0-III).
Notes: Leppik (1972) limited Melampsoraceae to rust species that are heteroecious and temperate, reassigning the autoecious and tropical species to a new family, Raveneliaceae. Savile (1989) provided an in-depth study of Raveneliaceae and hypothesised that the most recent common ancestor of Raveneliaceae was heteroecious, but that the family diversified as autoecious species on mimosoid (Caesalpinioideae) hosts after an environmental event severed their association with the initial sporothallus host. This hypothesis finds support in our work, which shows that the two early diverging families of Raveneliineae, Ochropsoraceae and Tranzscheliaceae (Fig. 1), are heteroecious with sporothalli hosts in Ranunculaceae.
Chaconia, which we place within Raveneliaceae, has been placed variously in the Melampsoraceae or with other rust genera in the artificial Chaconiaceae. This and prior works have shown Chaconiaceae, and most of the genera therein, as polyphyletic. The morphological character on which they were based, specifically thin-walled, pale teliospores that germinate without dormancy, was derived multiple times within Pucciniales (Aime, 2006, Aime et al. 2018a), as a result of convergent morphologies in species adapted to tropical climates that do not need to overwinter (Savile 1989).
Uropyxidaceae consists of an artificial assemblage of rust fungi combining (mostly) 2-celled, transversely septate teliospores and Group VI (type 5) spermogonia (where present). In this study, we sampled nearly all genera of Uropyxidaceae as circumscribed by Cummins & Hiratsuka (1983, 2003), most of which had not been previously sequenced; from our results the family is clearly polyphyletic. Many of the genera placed in Uropyxidaceae by Cummins & Hiratsuka (1983, 2003) were once considered allied within Pucciniaceae due to similarities in teliospore morphology. Our analysis shows that several of these, i.e., Desmella, Dipyxis, Edythea, Macruropyxis, belong to Pucciniaceae (Fig. 4). Dasyspora is allied in Sphaerophragmiaceae and Tranzschelia in Tranzscheliaceae (Fig. 1). The remaining genera – Newinia, Porotenus, Prospodium, Sorataea, and Uropyxis – are included within a broadly defined Raveneliaceae (Fig. 3).
Raveneliaceae is not resolved in our analyses, with strong support for some genera with multiple sampling, but almost no support for infra-familial nodes (Figs 1, 3, S2). Branch lengths for species of Raveneliaceae are comparatively long (Figs 3, S2) and may indicate an accelerated evolutionary rate in this family. 28S data alone can be informative for other Pucciniales lineages (e.g., Ji et al. 2019), but are inadequate for resolving relationships of genera, and in many cases even species, within Raveneliaceae (Fig. S2).
No sequence data are available for the generic type, R. glandulosa, a Western Hemisphere rust of Tephrosia. Ravenelia sp. (PUR F19717, Fig. 3) shares a host with R. glandulosa and may be congeneric with the type. Maravalia s.s. as represented by M. limoniformis (Figs 1, 3) is likely to belong here.
The genus Olivea, as circumscribed in the past, contains a polyphyletic assemblage of species that form a hymenial layer of probasidia that germinate via apical extension. Three species formerly placed in Olivea were included in our analyses: (i) O. capituliformis, the type for the genus; (ii) O. scitula; and (iii) O. tectonae, none of which are related to each other (Figs 3 & S2). Neoolivea tectonae (syn. O. tectonae) is placed in the Crossopsoraceae and discussed there. Olivea scitula was considered by Mains (1940) as most similar to Tegillum fimbriatum, and we apply the name T. scitulum to this species, although further work is necessitated to determine if it is, indeed, congeneric with the type species, T. fimbriatum. Olivea capituliformis is the only described species in this complex that infects hosts in Euphorbiaceae; the ex-Olivea species that we treat infect hosts in Verbenaceae (Ono & Hennen 1983).
Cephalotelium evansii (Syd. & P. Syd.) Aime & McTaggart, comb. nov. MycoBank MB836643.
Basionym: Ravenelia evansii Syd. & P. Syd. Annls mycol. 10: 440. 1912.Synonym: Dendroecia evansii (Syd. & P. Syd.) Syd., Annls mycol. 19: 165. 1921.
Cephalotelium neocaledoniense (B. Huguenin) Aime & McTaggart, comb. nov. MycoBank MB837616.
Basionym: Ravenelia neocaledoniensis B. Huguenin, Bull. trimest. Soc. mycol. Fr. 82: 263 (1966).
Cephalotelium xanthophloeae (M. Ebinghaus et al.) Aime & McTaggart, comb. nov. MycoBank MB836644.
Basionym: Ravenelia xanthophloeae M. Ebinghaus et al., MycoKeys 43: 11. 2018.
Notes: Of the ca. 200 species currently placed in Ravenelia, our data consistently resolved as congeneric those we now refer to Cephalotelium (Figs 3, S2). These species were also strongly supported as one of two monophyletic groups in Ravenelia s.l. by Ebinghaus et al. (2018b). Cephalotelium macowanianum (syn. Ravenelia macowanianum) is the type of Cephalotelium. The formation of telial galls is sometimes induced by infection of Ravenelia species, but not by Cephalotelium species. In contrast, C. evansii, C. macowanianum and C. xanthophloeae induce aecial gall formation in host tissues, which is a trait that appears to be confined to the Cephalotelium lineage (Ebinghaus et al. 2018a, b). Cephalotelium species infect members of Vachellia (Caesalpinioideae) in the Eastern Hemisphere (Sydow 1921). Cephalotelium is possibly a later synonym for Dendroecium, however, the type, D. farlowiana, occurs on Senegalia (Caesalpinioideae) species in the Western Hemisphere (Dietel 1894).
Lipocystis acaciae-pennatulae (Dietel) Aime & McTaggart, comb. nov. MycoBank MB836645.
Basionym: Ravenelia acaciae-pennatulae Dietel, Beih. bot. Zbl., Abt. 2 20: 373. 1906.
Notes: Lipocystis with the type species L. caesalpiniae was described as a monotypic genus for a rust on Mimosa from the West Indies. A second species, Lipocystis acaciae-pennatulae, infects Acacia species in Central America and is congeneric with L. caesalpiniae (Figs 1, 3, S2).
Uredinineae Engl., Syllabus der Vorlesungen über spezielle und medizinisch-pharmazeutische Botanik: 36. 1892. emend. Aime & McTaggart
Synonym: Pucciniineae Doweld, Index Fungorum 77: 1. 2014.
Type family: Pucciniaceae Chevall.
Description: With the characteristics of the included families. Most species form Group V but also Group VI spermogonia and 1- or 2-celled teliospores but multi-celled telia formed in some or most Nyssopsora, Phragmidiaceae, and Sphaerophragmiaceae.
Included families: Crossopsoraceae, Gymnosporangiaceae, Phragmidiaceae, Pileolariaceae, Pucciniaceae, Sphaero-phragmiaceae.
Notes: Uredinineae is the largest suborder in both species numbers and generic diversity. Pucciniineae is a superfluous name for the older Uredinineae. We were able to sample types or type representatives for 50 of the ca. 70 genera placed here as well as several species currently assigned to form-genera.
We were unable to resolve the placement for three genera: Allodus, Neopuccinia, and Nyssopsora. Allodus was long considered a synonym of Puccinia due to its pedicellate, 2-celled teliospores. Minnis et al. (2012) resurrected Allodus as an orphan genus of uncertain placement. Our analyses occasionally resolved Allodus as sister to Peridiopsora mori with weak support (not shown). Only a single 28S sequence is available for the newly described Neopuccinia, which shares many similarities with Kimuromyces (Dianese et al. 1995). Connections between Nyssopsora and Sphaerophragmium have been noted by Lohsomboon et al. (1994). Nyssopsora was recovered as sister to Sphaerophragmiaceae in some but not all of our analyses (Figs 1, 4) and may represent a separate family lineage.
Phragmidiaceae Corda Icon. fung. (Prague) 1: 6. 1837.
Type genus: Phragmidium Link, Mag. Ges. Naturfr. Freunde Berlin 7: 30. 1816.
Description: Spermogonia of Group IV (various types); aecia variable, caeoma-, petersonia- or uredo-type; uredinia lecythea- or uredo-type. Teliospores mostly multi-celled, usually by transverse septa. Species autoecious on Rosoideae subfamily of Rosaceae.
Included genera: Gerwasia, Gymnoconia, Hamaspora, Kuehneola, Phragmidium, Trachyspora, Triphragmium, Xenodochus; likely includes Joerstadia.
Host family: Rosaceae (0-III).
Notes: Convergence in teliospore morphology between some genera of Phragmidiaceae and Raveneliaceae has been previously noted (e.g., Cummins & Hiratsuka 2003); Aime (2006) showed that Phragmidiaceae species are confined almost exclusively to the Rosoideae in contrast to Raveneliaceae.
Pileolariaceae (Arthur) Cummins & Y. Hirats., llustr. Gen. Rust Fungi, rev. Edn (St. Paul): 14. 1983. emend. Aime & McTaggart
Type genus: Pileolaria Castagne, Obs. Plantes Acotylédonées Fam. Urédinées 1: 22. 1842.
Description: Spermogonia Group VI (type 7). Aecia and uredinia uredo-type. Teliospores 1-celled with characteristic sculpted appearance; germination external after dormancy. Species mostly macrocyclic and autoecious.
Included genus: Pileolaria.
Host family: Anacardiaceae (0-III).
Notes: Pileolariaceae was established for autoecious rusts in Pileolaria, Uromycladium and Endoraecium (Arthur 1906, Cummins & Hiratsuka 2003). The latter two have been resolved within Raveneliaceae, while Pileolaria holds an isolated position within Pucciniales (Aime 2006, Scholler & Aime 2006, Figs 1, 4). Pileolaria species are autoecious on Anacardiaceae, with very characteristic sculpted discoid teliospores.
Crossopsoraceae Aime & McTaggart, fam. nov. MycoBank MB836646.
Type genus: Crossopsora Syd. & P. Syd., Annls mycol. 16(3/6): 243. 1919.
Diagnosis: Similar to Phakopsoraceae, differing in that the majority of sporothalli infect Poaceae, Vitaceae, Lamiaceae, and Rhamnaceae with none known on Annonaceae and Euphorbiaceae and that some species are known to be heteroecious.
Description: Spermogonia Group VI (type 7) where known; aecia aecidium-type where known; uredinia typically paraphysate, malupa-type; teliospores germinate externally, with or without dormancy, 1-celled, compact, often produced in catenulate chains of a few to many cells. Most species only known from the sporothallus; Neophysopella is macrocyclic and heteroecious, as may be other species in this family.
Included genera: Angiopsora, Catenulopsora, Crossopsora, Kweilingia (= Dasturella), Neoolivea, Neophysopella, Stomatisora.
Host families: Papaveraceae, Sabiaceae, Rubiaceae (0-I); Lamiaceae, Fabaceae, Poaceae, Rhamnaceae, Rubiaceae, Salicaceae, Vitaceae (II-III).
Notes: Phakopsoraceae sensu Cummins & Hiratsuka (2003) is a polyphyletic family, with multiple polyphyletic genera (Aime 2006, Aime et al. 2018a, 2019a, b, Ji et al. 2019). The phakopsoroid fungi share a convergent suite of characters including pale, subepidermal sori, and 1-celled, sessile teliospores with external germination, which have long been the subject of taxonomic debate, especially concerning application of the names Angiopsora, Bubakia, Phakopsora, and Physopella. Phakopsoraceae s.s. is now confined to species within Raveneliineae on various hosts including Annonaceae, Euphorbiaceae and Fabaceae. The remaining ex-Phakopsoraceae are now placed in Crossopsoraceae (Figs 1, 4), including the species that have radiated on Poaceae and Vitaceae. Some species in both families form teliospores in extruded columns, but these are produced in catenulate chains in Crossopsoraceae, versus tightly packed individual cells in Phakopsoraceae. Life cycles are unknown for many species. However, Neophysopella is heteroecious, alternating either between Sabiaceae and Vitaceae species (Ji et al. 2019) or Papaveraceae and Fabaceae (N. kraunhiae, Hiratsuka & Kaneko 1978); K. divina alternates between Rubiaceae (gametothallus) and Poaceae (sporothallus) (Thirumalachar et al. 1947).
Physopella Arthur (1906), although often applied to the species now assigned to Neophysopella and others, is a later homonym of Physopella G. Poirault 1905 and is therefore an illegitimate name (Xi et al. 2019). Our work (Fig. 4) shows that Kweilingia (type K. bambusae, syn. Chrysomyxa bambusae) and Dasturella (type D. divina, syn. Angiopsora divina) are congeneric; Kweilingia 1940 has priority over Dasturella 1943 and we retain these species in the former genus.
The phakopsoroid species that form teliospore chains are difficult to diagnose and classify, as exemplified by the complex taxonomic histories of Crossopsora, Cerotelium, Catenulopsora, and Kuehneola (e.g., Ono 2015a). In this work, Crossopsora and Catenulopsora are assigned to Crossopsoraceae. Cerotelium most likely belongs to Phakopsoraceae s.s.; the type, C. canavaliae parasitizes Fabaceae and the uredinia are peridiate in contrast to C. fici with paraphysate uredinia (Cummins 1941). Nonetheless, these genera still appear to be polyphyletic with little support for generic lineages and resolution will require additional taxon and locus sampling (Fig. 4).
Numerous Uredo species assigned to Crossopsoraceae, especially within the Crossopsora/Catenulopsora complex, could not be placed in other genera and we have retained use of names in anamorphic form-genera for these. Crossopsora fici and U. moricola form a distinct lineage within Crossopsoraceae and may require a new genus, pending examination of other critical types including those of Mehtamyces, Phragmidiella, Pucciniostele, and Scalarispora.
Neophysopella kraunhiae (Dietel) Aime & McTaggart, comb. nov. MycoBank MB837747.
Basionym: Phakopsora kraunhiae Dietel, Hedwigia 41: 178. 1902.
Synonyms: Ochropsora kraunhiae (Dietel) Dietel., Bot. Jahrb. 37: 106. 1905.
Aecidium corydalinum Syd. & P. Syd., Monogr. Ured. 4: 235.1923.
Notes: Neophysopella kraunhiae is heteroecious and produces gametothalli on Corydalis incisa (Papaveraceae) and sporothalli on Wisteria floribunda (Fabaceae) (Hiratsuka & Kaneko 1978). Our data support its classification in the newly circumscribed Neophysopella, a genus that contains most other known heteroecious species in Crossopsoraceae.
Neoolivea Aime & McTaggart, gen. nov. MycoBank MB837748.
Type species: Neoolivea tectonae (T.S. Ramakr. & K. Ramakr.) Aime & McTaggart, this paper.
Etymology: New genus segregated from Olivea.
Diagnosis: Similar to Olivea and Tegillum but differs in having subglobose to ellipsoid, non-angular urediniospores with inconspicuous germ pores, and waxy telia.
Description: With characteristics of the type species Neoolivea tectonae.
Neoolivea tectonae (Racib.) Aime & McTaggart, comb. nov. MycoBank MB837749.
Basionym: Uredo tectonae Racib, Parasit. Alg. Pilze Java’s (Jakarta) 1: 28. 1900.
Synonyms: Olivea tectonae (Racib.) Thirum., Curr. Sci. 18: 176. 1949.
Tegillum tectonae (Racib.) Doweld, Index Fungorum 36: 1. 2013.
Chaconia tectonae T.S. Ramakr. & K. Ramakr., Indian Phytopath. 2: 19. 1949.
Olivea tectonae (T.S. Ramakr. & K. Ramakr.) R.L. Mulder, CMI
Descriptions of Pathogenic Fungi and Bacteria 37: no. 365. 1973.
Olivea neotectonae Buriticá & Salazar-Yepes, Revista Fac. Nac. Agron. Medellín 60(1): 3652. 2007.
Tegillum neotectonae (Buriticá & Salazar-Yepes) Doweld, Index Fungorum 36: 1. 2013.
Notes: Neoolivea tectonae causes leaf rust on teak (Tectona grandis, Tectoneae, Verbenaceae). It is described in Ono & Hennen (1983) and illustrated in Ramakrishnan & Ramakrishnan (1949). Our data show that N. tectonae is unrelated to the type of Olivea (O. capituliformis), which belongs to the Raveneliaceae (Figs 1, 3, 4). Tegillum was established for Olivea-like species, with the type T. fimbriatum (Mains 1940). While no type data exist for T. fimbriata, the species is most similar to T. scitulum (Mains 1940), sharing characteristics such as lobed or angular urediniospores with germ pores residing in the lobes and hosts in the Vitex group of Verbenaceae (Ono & Hennen 1983), which is placed in Raveneliineae (Fig. 1). Neoolivea tectonae differs from other described Olivea and Tegillum species in producing rounded urediniospores with inconspicuous germ pores, as well as waxy, orange telia (Ono & Hennen 1983, Osorio et al. 2019).
Angiopsora apoda (Har. & Pat.) Aime & McTaggart, comb. nov. MycoBank MB836647.
Basionym: Puccinia apoda Har. & Pat., Bull. Mus. natn. Hist. nat., Paris 15:199. 1909.
Synonym: Phakopsora apoda (Har. & Pat.) Mains, Mycologia 30: 45. 1938.
Angiopsora chusqueae (Pardo-Card.) Aime & McTaggart, comb. nov. MycoBank MB836648.
Basionym: Uredo chusqueae Pardo-Card., Revta Acad. colomb. cienc. exact. fis. nat. 20: 205. 1996.
Angiopsora paspalicola (Henn.) Aime & McTaggart, comb. nov. MycoBank MB836649.
Basionym: Uredo paspalicola Henn., Hedwigia 44: 57. 1905.
Synonyms: Puccinia paspalicola (Henn.) Arthur, Manual Rusts U.S. & Canada. Purdue Res. Found.: 127. 1934.
Physopella paspalicola (Henn.) Buriticá & Hennen, Buriticá Rev. I. C. N. E. (Medellín) 5: 179. 1994.
Puccinia compressa Arthur & Holway, Proc. American Phil. Soc. 64:257. 1925.
Angiopsora compressa (Arthur & Holway) Mains, Mycologia 26: 29. 1934.
Physopella compressa (Arthur & Holway) Cummins & Ramachar, Mycologia 50: 742. 1958.
Phakopsora compressa (Arthur & Holway) Buriticá & Hennen, Buriticá, Rev. I. C. N. E. (Medellín) 5: 179. 1994.
Notes: Mains (1934) established Angiopsora for Poaceae-infecting ex-Pucciniaceae species with similarities to Phakopsora. Although the name has been considered a synonym for Phakopsora (e.g., Cummins & Hiratsuka 2003), we find that it is applicable to numerous former Phakopsora species on grasses (Fig. 4).
Gymnosporangiaceae P. Zhou & L. Cai, Persoonia 45: 79. 2020. emend. Aime & McTaggart
Synonym: Gymnosporangieae Dietel, In: Engler & Prantl, Naturlichen Pflanzenfamilien Band 6: 73. 1938.
Type genus: Gymnosporangium R. Hedw. ex DC., In: Lamarck & de Candolle, Fl. franç., Edn 3 (Paris) 2: 216. 1805.
Description: Spermogonia Group V (type 4). Aecia roestelia-type (Gymnosporangium) or less frequently aecidium-type (Gymnotelium). Teliospores mostly 2-celled, germinating without dormancy via external basidia. Life cycles mostly demicyclic and heteroecious (Gymnosporangium).
Included genera: Gymnosporangium, Gymnotelium; likely includes Peridiopsora.
Host families: Rosaceae, Hydrangeaceae, Myricaceae (0-I); Cupressaceae (II-III); autoecious species on Berberidaceae, Cupressaceae, Liliaceae, Moraceae.
Notes: The genus Gymnosporangium is unusual in that temperate species form teliospores in the spring and aecia in the summer, in contrast to most other temperate rust species (Kern 1960). Most species of Gymnosporangium form sporothalli on Juniperus spp. and gametothalli on Maloideae (Rosaceae). Although traditionally placed in Pucciniaceae, Gymnosporangium has been treated as an “orphan” genus in molecular phylogenetic studies (Maier 2003, Aime 2006, Aime et al. 2018a) and recently established as a monotypic family of unresolved placement (Zhao et al. 2020). Although the older name Gymnosporangieae Dietel exists for this lineage, priority only applies within rank, thus we retain Gymnosporangiaceae P. Zhou & L. Cai for the family. In addition to the puccinioid character of 2-celled, pedicellate teliospores, most species are demicyclic. Gymnosporangium has been conserved against the older name Roestelia Rebent. (Aime et al. 2018b).
Peridiopsora mori (syn. Caeoma mori, Aecidium mori) causes a well-known disease in mulberries. It is unusual in having a true hemicyclic life cycle, wherein the mitospores appear to function as urediniospores although likely are derived from the aecial part of the life cycle (Mordue 1991). Spermogonia and teliospores are not known. Peridiopsora was erected to accommodate an unusual asexual rust that persisted as cyclical urediniospores, producing uredinia in peridiate sori that lack paraphyses, the latter being typical of aecia but rare in uredinia (Kamat & Sathe 1969) and which we interpret as uredinioid aecia. Whether P. mori will prove to be congeneric with the type, P. adelocaryi, or will be placed within Gymnotelium, remains uncertain.
Gymnotelium speciosum (Peck) Aime & McTaggart, comb. nov. MycoBank MB836652.
Basionym: Gymnosporangium speciosum Peck, Bot. Gaz. (Crawfordsville) 4 (10): 217. 1879.
Synonym: Tremella speciosa (Peck) Arthur, Proc. Indiana Acad. Sci. 1900: 135. 1901.
Notes: Gymnotelium was erected for Gyt. nootkatense, a macrocyclic (rather than demicyclic) species with a gametothallus host (Chamaecyparis) not within the usual Maloideae/Juniperus range of Gymnosporangium. Arthur (1929) considered the diagnostic character of aecidium-like rather than rostrate aecia as more important than presence of uredinia, transferring an additional two species, Gyt. blasdaleanum (syn. G. libocedri) and Gyt. myricatum (syn. G. ellisii) to Gymnotelium. In later works Arthur (1934) treated Gymnotelium as a subgenus of Gymnosporangium, including a fourth species, G. speciosum. Novick (2008) showed that G. nootkatense, G. blasdaleanum (as G. libocedri), G. myricatum (as G. elisii), and G. speciosum are the earliest diverging members of Gymnosporangium. The cupulate aecia of Gymnotelium bear a strong resemblance to those of P. mori, and our work resolves these together as the sister lineage to Gymnosporangium (Figs 1, 4). In addition to producing cupulate (rather than rostrate) aecia, Gymnotelium species produce their sporothallus on hosts other than Maloideae spp., and/or the sporothallus on hosts other than Juniperus spp. (Kern 1960).
Sphaerophragmiaceae Cummins & Y. Hirats., Illustr. Gen. Rust Fungi, rev. Edn (St. Paul): 15. 1983. emend. Aime & McTaggart
Synonym: Dasysporatae Arthur, North American Flora 7: 807. 1926.
Type genus: Sphaerophragmium Magnus Ber. dt. bot. Ges 9: 121. 1891.
Description: Spermogonia mostly lacking, Group V (type 4) in Sphenorchidium. Aecia aecidium-type; uredinia resembling aecia, lecythea-type in Sphenorchidium. Teliospores 2- to multi-celled. Species autoecious with variable life cycles.
Included genera: Austropuccinia, Dasyspora, Puccorchidium, Sphaerophragmium, Sphenorchidium.
Host families: Annonaceae, Fabaceae, Myrtaceae.
Notes: Sphaerophragmium has been hypothesized as belonging to Raveneliaceae based on similarities in teliospores and host (Cummins & Hiratsuka 2003). More recently Sphaerophragmium was shown to belong to a monophyletic lineage (Beenken & Wood 2015) for which the oldest available name is Dasysporatae, but for which Sphaerophragmiaceae has been recently applied (e.g., McTaggart et al. 2016, Beenken 2017). Because priority only applies within rank, we retain the more widely used name for this family. Austropuccinia psidii, causes an economically important epidemic disease of Myrtaceae and was recently demonstrated to be autoecious and macrocyclic but lacking spermogonia (I-IV) and with uredinioid aecia (McTaggart et al. 2018, 2020).
Pucciniaceae Chevall., Fl. gén. env. Paris (Paris) 1: 413. 1826. emend. Aime & McTaggart
Synonyms: Pucciniosiraceae (Dietel) Cummins & Y. Hirats., Illustrated Genera of Rust Fungi: 15. 1983.
Endophyllaceae Dietel, In: Engler & Prantl, Nat. Pflanzenfam., Teil. I (Leipzig) 1: 35. 1897.
Type genus: Puccinia Pers., Synopsis methodica fungorum: 225. 1801.
Description: Spermogonia Group V (type 4). Aecia aecidium-type; uredinia mostly uredo-type. Teliospores borne singly, mostly pedicellate, typically with 1 or 2 cells. Basidia external. Most species macrocyclic but many endocyclic and microcyclic species as well; heteroecious or autoecious.
Included genera: Baeodromus, Ceratocoma, Chardoniella, Chrycocelis, Cionothrix, Cumminsiella, Desmella, Didymopsora, Dietelia, Dipyxis, Edythea, Endophylloides, Endophyllum, Hapalophragmium, Leptopuccinia, Macruropyxis, Miyagia, Puccinia, Pucciniosira, Sphenospora, Stereostratum, Uromyces, Xenostele; likely includes Cerradoa, Chrysella, Chrysocyclus, Chrysopsora, Cleptomyces, Corbulopsora, Kernella, Polioma, Trichopsora.
Host families: various including, Berberidaceae, Ranunculaceae (0-I); various including Asteraceae, Euphorbiaceae, Fabaceae, Malvaceae, Orchidaceae, Poaceae, Solanaceae (II-III and autoecious species).
Notes: As with Raveneliineae, infra-familial relationships in Pucciniaceae are difficult to resolve at least within the context of currently circumscribed genera (Fig. 4). However, whereas Raveneliineae represents a lineage with multiple parallel radiations and differing rates of evolution, Pucciniaceae represents a recent and rapidly radiating lineage that has undergone multiple losses of teliospore septa and morphs. The majority of rust species and the two most speciose genera, Puccinia and Uromyces, belong here. Taxonomic changes that reflect natural genera will depend on sequencing of additional loci as well as representative types from genera such as Cerradoa, Cleptomyces, Corbulopsora, Didymopsora, Polioma, Kernella, Ramakrishnania, and Trichopsora. Pucciniaceae includes many endocyclic derivatives, such as those once included in the family Pucciniosiraceae, as hypothesized by Berndt (2018).
Species of Pucciniaceae can be roughly circumscribed into three radiations (Figs 1, 4). One, represented by P. graminis, the type of Puccinia, includes primarily species with 2-celled teliospores that are macrocyclic and heteroecious, and includes economically important species on Poaceae and Asteraceae. The second, represented by U. appendiculatus, the type of Uromyces, includes many species with 1-celled teliospores that have radiated on Euphorbiaceae and Fabaceae, and are primarily autoecious. A third, weakly supported in Fig. 1, but not in Fig. 4, includes, among others, the fern- and orchid-infecting species of Pucciniaceae.
DISCUSSION
The proposed classification of rust fungi includes seven suborders and 18 families. Although we treat approximately the same number of families as Cummins & Hiratsuka (2003), the disposition of many genera varies considerably between our and earlier classifications (Fig. S1). We have excluded demonstrably artificial families such as Chaconiaceae, Pucciniosiraceae, and Uropyxidaceae, while establishing new families for cohorts that have been repeatedly shown in our and other works to be strongly supported entities (e.g., Crossopsoraceae, Milesinaceae, Rogerpetersoniaceae). While our three-locus approach has enabled resolution of most major lineages of Pucciniales, providing a stable framework for future studies, resolution remains poor in some areas, especially in the Pucciniastraceae and Raveneliaceae, which will require additional strategic sampling of both taxa and loci. Additionally, three of our sampled genera – Allodus, Neopuccinia, and Nyssopsora – still cannot be placed to family and remain incertae sedis within Uredinineae; placement of these is sensitive to taxon selection within analyses. For example, in our initial analyses (not shown) Allodus was weakly supported within Gymnosporangiaceae, however, inclusion of Nyssopsora (Fig. 1) and Neopuccinia (Fig. 4) results in uncertain placement for all three genera and underscores the need for appropriate taxon selection in reconstructing phylogenetic hypotheses in Pucciniales. The necessity of including type species and adequate sampling when dealing with rust taxonomy, especially in polyphyletic and/or convergent genera and families, cannot be overemphasized.
The classification of rust fungi has undergone several epochs, each with emphasis on a different suite of characters including teliospores (e.g., Cunningham 1931) and telia (e.g., Dietel 1928), aecia (e.g., Leppik 1953), and spermogonia (Hiratsuka & Cummins 1963, Hiratsuka & Hiratsuka 1980) in attempts to circumscribe natural groups (Fig. S1). Our work shows that sporothallus characteristics can be useful at the generic and sometimes family ranks. Ontogenic characters, such as the presence of hymenial-like sporogenous cells from which teliospores develop, which distinguishes Tranzscheliaceae from Ochropsoraceae, may be useful for diagnosing some families. However, in general telial and uredinial characters are among the most homoplasious in rust taxonomy.
The asexual genera – At least 34 generic names for asexual rust morphs have been introduced. Of these, ca. 13 were in wide use (Cummins & Hiratsuka 2003) prior to changes in the nomenclatural code that now eliminate the use of dual nomenclature (McNeill et al. 2012, Turland et al. 2018). Most of these genera are recognized as later synonyms for sexual-morph genera (e.g., Canasta = Prospodium, Endocronartium = Cronartium, Pelastoma = Blastospora) or in cases where the asexual name has priority, the sexual name has been conserved (e.g., Gymnosporangium over Roestelia; Melampsorella over Peridermium) (Aime et al. 2018b). In many cases, species only known from an asexual morph can be recombined into sexual genera by a combination of host, morphology, and/or DNA sequence data. But for the largest asexual genera, Aecidium and Uredo, there remain hundreds of species that cannot reliably be recombined at this time, and the process to place these within natural genera will take painstaking work, even with DNA data. In this paper we were unable to assign several species (e.g., A. kalanchoes, U. cryptostegiae, U. elephantopodis, U. hiulca Cummins, U. trichosanthes) to sexual genera and recommend use of form-genera names for these species until they can be confidently reassigned.The endocyclic rusts—Species with endocyclic life cycles, i.e., reduced autoecious life cycles in which the aeciospores function as teliospores, were once treated collectively in heterogeneous families such as Pucciniosiraceae and Endophyllaceae (Cummins & Hiratsuka 2003, Buriticá 1991). Perhaps not surprisingly we find that these are derived multiple times within Pucciniales as predicted by Berndt (2018) (e.g., Baeodromus, Ceratocoma, Chardoniella, Cionothrix, Didymopsora, Dietelia, Endophyllum and Pucciniosira, Fig. 4). Interestingly, we see little evidence for expanded radiations of endocyclic rusts. Coupled with the fact that the majority of these species are found within the most recent radiation (Pucciniaceae), this life history strategy may represent an evolutionary dead-end for Pucciniales.
The chaconiaceous rusts—Classification of the primarily tropical rust species that produce thin-walled teliospores and germinate without dormancy has not received consensus in the past. Genera such as Chaconia, Goplana, Hemileia, Maravalia, Ochropsora, and Olivea were often treated as a single family, Chaconiaceae, hypothesized to represent the earliest diverging lineage of rusts by some urediniologists (e.g., Cummins & Hiratsuka 1983, Hiratsuka 1983). Our and prior works (e.g., Aime 2006, Aime et al. 2018a) show that these characters represent a syndrome, most likely as an adaptive response to tropical climates where teliospore dormancy or overwintering is not necessary, and that even the species within genera (with the probable exception of Hemileia) do not share a common recent ancestor (Figs 1–4).
Rust evolution—The study of rust fungi has been fertile ground for evolutionary theory. Researchers have posited co-evolution to explain rust success (Savile 1971, McTaggart et al. 2015), or alternatively host jumps (Hart 1988, McTaggart et al. 2016). However, most studies fail to take the heteroecious nature of many rust species into account. The most likely explanation for the success, in terms of species, of Pucciniales proposes a combination of both factors in a more complex interplay between the forces of biological specialization and biogenic radiation (Leppik 1953, van der Merwe et al. 2008, Aime et al. 2018a). A pattern of host jumps followed by rapid radiation to related or ecologically co-distributed host species, or biogenic radiation, is the best explanation for the relationship between rusts and their sporothallus hosts (van der Merwe et al. 2008, Aime et al. 2018). In contrast, a pattern of co-evolution or biological specialization, best explains the relationship between rust fungi and their gametothallus hosts (Aime et al. 2018a). This pattern becomes more evident in light of spermogonial evolution. The earliest rusts (Rogerpetersoniaceae and Zaghouaniaceae) produce spermogonia of Group III, deep-seated with an exit canal, convex hymenium, and indeterminate growth. Loss of the exit canal occurred approximately 145 mya coinciding with a gametothallus jump to Pinaceae hosts. These initially produced subepidermal convex hymenia [Group I (type 1); Araucariomyces and Skierkaceae], becoming flat and eventually subcuticular [Group I (types 2 & 3)] within the Melampsorineae. Well-developed periphyses (Group VI) are apparent approximately 115 mya, coinciding with a gametothallus jump to angiosperms. Group VI spermogonia have a flat hymenium and are retained in Raveneliineae and the earlier diverging Uredinineae. A well-defined bounding structure surrounding a convex hymenium (Group V) evolved last, ca. 85–90 mya, coinciding with the major angiosperm radiation, in the crown rust radiation that includes Gymnosporangiaceae, Sphaerophragmiaceae, and Pucciniaceae.
The complexities of the macrocyclic rust life cycle and its derivations is difficult to explain in an evolutionary context. One explanation, Tranzschel’s law (Arthur 1929, Jackson 1931), posits that autoecious-microcyclic rusts are derived from heteroecious-macrocyclic life cycles that have been restricted to the gametothallus host. Evidence of this has been found at the scale of recently diverging, correlated species (Scholler et al. 2019). The authors posit that this plasticity of life cycle provides the template for multiple avenues of speciation, not just on the gametothallus host as demonstrated, but hypothetically from the sporothallus host as well (Scholler et al. 2019). At a larger scale, Raveneliineae is hypothesized as an initially heteroecious and macrocyclic lineage (Savile 1989) and the earliest diverging families in the suborder, Ochropsoraceae and Tranzscheliaceae, share this strategy, with gametothallus hosts in Ranunculaceae. Loss of the original gametothallus host may have occurred ca. 80 mya, driving the evolution of autoecious and macrocyclic lineages on the sporothallus hosts, with several contemporaneous radiations into what are now recognizable as the Phakopsoraceae and Raveneliaceae (Fig. 1).
Lineages restricted due to extinction or other forces to gametothallus hosts compensate by becoming microcyclic (e.g., microcyclic Tranzschelia species), endocyclic (e.g., endocronartium-type species of Cronartium) or systemic infections (as hypothesized for Rogerpetersonia and Araucariomyces), but these show constraints, at least in terms of species diversification on that host and seem incapable of regaining all five spore stages. In contrast, lineages that have been restricted on the sporothallus hosts, e.g., Raveneliaceae, appear capable of more expansive radiation and in recovering lost spore stages.
In conclusion – We provide a rust tree of life resolved at the deeper nodes and use this framework to redefine the higher rank (suborder and family) classification for Pucciniales. The complexities of rust fungal biology that includes alternation of generations, heteroecism, and five developmental stages is mirrored in the taxonomic complexities encountered in this group. Taxonomy is often confounded by multiple parallel radiations, convergent morphologies, and the previous application of dual nomenclature making the necessity of consulting type species in taxonomic revision an imperative.
Our data support a model of rust evolution in which: 1) heteroecism favours diversity by allowing different speciation processes to act on different parts of the life cycle (biogenic radiation on the sporothallus, biologic specialization on the gametothallus); 2) the five developmental stages of a macrocyclic rust provide the templates for multiple avenues of speciation; and, 3) both unique features were present in the earliest extant rust radiation (Mikronegeriineae). There is evidence that the highly reduced endocylic rusts on gametothallus hosts may represent an evolutionary dead-end but that rusts confined to the sporothallus host can regain lost spore stages. The heteroecious macrocyclic strategy is unique to Pucciniales and may help to explain the tremendous diversity in form and in species found in the rusts.
ACKNOWLEDGEMENTS
MCA acknowledges the U.S. National Science Foundation, the U.S. Department of Agriculture, The Mellon Foundation, the Louisiana Board of Regents, and the Indiana Academy of Sciences for funding in support of various parts of this project. ARM acknowledges the University of Queensland Development Fellowships (UQFEL1718905) and support from the Department of the Environment and Energy under the Australian Biological Resources Study (grant numbers RG18-43 and RFL212-33). We are extremely grateful to Reinhard Berndt and Amy Rossman for numerous discussions on rust fungus classification and taxonomy, to Roger Shivas for his contributions of specimens and mentorship to ARM along the journey, to Amy Rossman and Roger Shivas for critical review of earlier versions of this manuscript, and to two anonymous reviewers for pointing out errors and providing helpful comments. Herbaria PUR, BPI, and BRIP provided loans of material without which this study would not have been possible.
Footnotes
Conflict of interest: The authors declare that there is no conflict of interest.
Supplementary Material: http://fuse-journal.org/
Collection and accession data for additional sequences used in Melampsorineae, Raveneliineae, and Uredinineae analyses (Figs 2–4 & S2).
Alluvial plot tracking generic placement at familial and subfamilial rank. Each colour represents the taxonomic hypotheses of an author. Tracks for genera with conflicting familial and subfamilial placement from multiple authors are dashed. The plot was made in R with the ggalluvial package.
Raveneliineae. Network analysis generated with SplitsTree from 28S data. Generic types are indicated in bold, type proxies by *.
REFERENCES
- Aime MC. (2006). Toward resolving family-level relationships in rust fungi (Uredinales). Mycoscience 47: 112–122. [Google Scholar]
- Aime MC, Bell CD, Wilson AW. (2018a). Deconstructing the evolutionary complexity between rust fungi (Pucciniales) and their plant hosts. Studies in Mycology 89: 143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aime MC, Castlebury LA, Abbasi M, et al. (2018b). Competing sexual and asexual generic names in Pucciniomycotina and Ustilaginomycotina (Basidiomycota) and recommendations for use. IMA Fungus 9: 75–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aime MC, Matheny PB, Henk DA, et al. (2006). An overview of the higher-level classification of Pucciniomycotina based on combined analyses of nuclear large and small subunit rDNA sequences. Mycologia 98: 896–905. [DOI] [PubMed] [Google Scholar]
- Aime MC, McTaggart AR, Mondo SJ, et al. (2017). Phylogenetics and phylogenomics of rust fungi. Advances in Genetics 100: 267–307. [DOI] [PubMed] [Google Scholar]
- Aime MC, Rossman AY, Ono Y, et al. (2019a). (2688) Proposal to conserve the name Phakopsora (Basidiomycota, Pucciniales) with a conserved type. Taxon 68: 592. [Google Scholar]
- Aime MC, Rossman AY, Ono Y, et al. (2019b). (2689–2690) Proposals to conserve the names Phakopsora pachyrhizi against Uredo erythrinae and U. sojae (Malupa sojae) and Physopella meibomiae (Phakopsora meibomiae) against Aecidium crotalariicola, U. teramni, and U. vignae (M. vignae) (Basidiomycota, Pucciniales). Taxon 68: 593–594. [Google Scholar]
- Altschul SF, Gish W, Miller W, et al. (1990). Basic local alignment search tool. Journal of Molecular Biology 215: 403–410. [DOI] [PubMed] [Google Scholar]
- Arthur JC. (1906). Résultats scientifiques du Congrés international de Botanique Wien 1905: 331–348. [Google Scholar]
- Arthur JC. (1907–1931). Uredinales. North American Flora 7: 83–969. [Google Scholar]
- Arthur JC. (1929). The Plant Rusts (Uredinales). John Wiley & Sons, New York, USA. [Google Scholar]
- Arthur JC. (1934). Manual of the rusts in the US and Canada. Purdue Res. Foundation, USA. [Google Scholar]
- Bakshi BK, Singh S. (1960). A new genus in plant rusts. Canadian Journal of Botany 38: 259–262. [Google Scholar]
- Beenken L. (2014). Pucciniales on Annona (Annonaceae) with special focus on the genus Phakopsora. Mycological Progress 13: 791–809. [Google Scholar]
- Beenken L. (2017). Austropuccinia: a new genus name for the myrtle rust Puccinia psidii placed within the redefined family Sphaerophragmiaceae (Pucciniales). Phytotaxa 297: 53–61. [Google Scholar]
- Beenken L, Wood AR. (2015). Puccorchidium and Sphenorchidium, two new genera of Pucciniales on Annonaceae related to Puccinia psidii and the genus Dasyspora. Mycological Progress 14: 1–13. [Google Scholar]
- Beenken L, Zoller S, Berndt R. (2012). Rust fungi on Annonaceae II: the genus Dasyspora Berk. & M.A. Curtis. Mycologia 104: 659–681. [DOI] [PubMed] [Google Scholar]
- Berndt R. (2018). The Pucciniosiraceae: taxonomy of a polyphyletic family of rust fungi (Uredinales). In: Biodiversity and Ecology Fungi, Lichens and Mosses. Österreichische Akademie der Wissenschaften, Germany: 245–269. [Google Scholar]
- Berndt R, Wood AR. (2012). Additions to the rust fungi of South Africa. Mycological Progress 11: 483–497. [Google Scholar]
- Blomquist CL, Scholler M, Scheck HJ. (2015). Detection of rust caused by Tranzschelia mexicana on Prunus salicifolia in the United States. Plant Disease 99: 1856. [Google Scholar]
- Bouckaert R, Vaughan TG, Barido-Sottani J, et al. (2019). BEAST 2.5: An advanced software platform for Bayesian evolutionary analysis. PLOS Computational Biology 15: e1006650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns TD, Vilgalys R, Barns SM, et al. (1992). Evolutionary relationships within the fungi: Analyses of nuclear small subunit rRNA sequences. Molecular Phylogenetics and Evolution 1: 231–241. [DOI] [PubMed] [Google Scholar]
- Bubner B, Buchheit R, Friedrich F, et al. (2019). Species identification of European forest pathogens of the genus Milesina (Pucciniales) using urediniospore morphology and molecular barcoding including M. woodwardiana sp. nov. MycoKeys 48: 1–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buriticá P. (1991). Familias del orden Uredinales con ciclo de vida completamente reducio. Revista de la Academia Colombiana de Ciencias Exactas, Físicas y Naturales 18: 131–148. [Google Scholar]
- Carefoot GL, Sprott ER. (1967). Famine on the wind: plant diseases and human history. Rand McNally & Company, USA. [Google Scholar]
- Cao B, Tao S, Tian C, et al. (2018). Coleopuccinia in China and its relationship to Gymnosporangium. Phytotaxa 347: 235–242. [Google Scholar]
- Carnegie AJ, Pegg GS. (2018). Lessons from the incursion of myrtle rust in Australia. Annual Review of Phytopathology 56: 457–478. [DOI] [PubMed] [Google Scholar]
- Chatasiri S, Ono Y. (2008). Phylogeny and taxonomy of the Asian grapevien leaf rust fungus, Phakopsora euvitis, and its allies (Uredinales). Mycoscience 49: 66–74. [Google Scholar]
- Crane PE. (2000). Systematics and Biology of the Genus Chrysomyxa. Ph.D. thesis. University of Alberta, Canada. [Google Scholar]
- Crane PE, Hiratsuka Y, Currah RS. (2000). Reproductive biology and evidence for water dispersal of teliospores in Chrysomyxa weirii, a microcyclic spruce needle rust. Mycologia 92: 745–763. [Google Scholar]
- Cummins G. (1950). The genus Scopella of the Uredinales. Bulletin of the Torrey Botanical Club 77: 204–213. [Google Scholar]
- Cummins G. (1978). J.C. Arthur: the man and his work. Annual Review of Phytopathology 16: 19–30. [Google Scholar]
- Cummins GB, Hiratsuka Y. (1983). Illustrated genera of rust fungi. Rev. ed. APS Press, USA. [Google Scholar]
- Cummins GB, Hiratsuka Y. (2003). Illustrated genera of rust fungi. 3rd ed. APS Press, USA. [Google Scholar]
- Cunningham GH. (1931). The rust fungi of New Zealand, together with the biology, cytology and therapeutics of the Uredinales. John McIndoe, NZ. [Google Scholar]
- Dietel P. (1928). Hemibasidii (Ustilaginales und Uredinales). A Engler, K Prantl: Die natülichen Pflanzenfamilien, Germany. [Google Scholar]
- Doungsa-ard C, McTaggart AR, Geering ADW, et al. (2014). Uromycladium falcatarium sp. nov., the cause of gall rust of Paraserianthes falcataria in south-east Asia. Australasian Plant Pathology 44: 25–30. [Google Scholar]
- Doungsa-ard C, McTaggart AR, Geering ADW, et al. (2018). Diversity of gall-forming rusts (Uromycladium, Pucciniales) on Acacia in Australia. Persoonia 40: 221–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebinghaus M, Maier W, Wingfield MJ, et al. (2018a). New host associations and a novel species for the gall-inducing acacia rust genus Ravenelia in South Africa. MycoKeys 43: 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebinghaus M, Wingfield MJ, Begerow D, et al. (2018b). The genus Ravenelia (Pucciniales) in South Africa. Mycological Progress 19: 259–290. [Google Scholar]
- Feau N, Vialle A, Allaire M, et al. (2011). DNA barcoding in the rust genus Chrysomyxa and its implications for the phylogeny of the genus. Mycologia 103: 1250–1266. [DOI] [PubMed] [Google Scholar]
- Gäumann E. (1964). Die Pilze. Grundzüge ihrer Entwicklungsgeschichte und Morphologie. Birkhäuser, Basel. [Google Scholar]
- Guindon S, Dufayard JF, Lefort V, et al. (2010). New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Systematic Biology 59: 307–321. [DOI] [PubMed] [Google Scholar]
- Hart JA. (1988). Rust fungi and host plant coevolution: do primitive hosts harbor primitive parasites? Cladistics 4: 339–366. [DOI] [PubMed] [Google Scholar]
- Henk DA, Vilgalys R. (2007). Molecular phylogeny suggests a single origin of insect symbiosis in the Pucciniomycetes with support for some relationships within genus Septobasidium. American Journal of Botany 94: 1515–1526. [DOI] [PubMed] [Google Scholar]
- Hernández J, Aime MC, Newbry B. (2004). Aecidium kalanchoe sp. nov., a new rust on Kalanchoe blossfeldiana (Crassulaceae). Mycological Research 108: 846–848. [DOI] [PubMed] [Google Scholar]
- Hibbett DS, Blackwell M, James TY, et al. (2018). Phylogenetic taxon definitions for Fungi, Dikarya, Ascomycota and Basidiomycota. IMA Fungus 9: 291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiratsuka N. (1958). Revision of taxonomy of the Pucciniastreae. Kasai Publ. Print Co., Tokyo. [Google Scholar]
- Hiratsuka N, Kaneko S. (1978). Heteroecism of the wisteria rust, Ochropsora kraunhiae (Diet.) Dietel. Proceedings of the Japan Academy, Series B 54: 300–303. [Google Scholar]
- Hiratsuka Y, Cummins GB. (1963). Morphology of the spermogonia of the rust fungi. Mycologia 55: 487–507. [Google Scholar]
- Hiratsuka Y. (1983). The nuclear cycle and terminology of spore states in Uredinales. Mycologia 65: 432–443. [Google Scholar]
- Hiratsuka Y, Hiratsuka N. (1980). Morphology of spermogonia and taxonomy of rust fungi. Reports of the Tottori Mycological Institute 18: 257–268. [Google Scholar]
- Hoang DT, Chernomor O, von Haeseler A, et al. (2018). UFBoot2: Improving the ultrafast bootstrap approximation. Molecular Biology and Evolution 35: 518–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huson DH, Bryant D. (2005). Application of phylogenetic networks in evolutionary studies. Molecular Biology and Evolution 23: 254–267. [DOI] [PubMed] [Google Scholar]
- Ishaq A, Aime MC, Karleson-Ayaloa E, et al. (2019). First report of Asian pistachio rust (Pileolaria pistachiae) in Pakistan. Canadian Journal of Plant Pathology 42: 210–217. [Google Scholar]
- Jackson HS. (1931). Present evolution tendencies and the origin of life cycles in the Uredinales. Memoirs of the Torrey Botanical Club 18: 1–108. [Google Scholar]
- Ji J-X, Li Z, Li Y, et al. (2019). Life cycle of Nothoravenelia japonica and its phylogenetic position in Pucciniales, with special reference to the genus Phakopsora. Mycological Progress 18: 855–864. [Google Scholar]
- Kern FD. (1960). Changing concepts of Gymnosporangium. Mycologia 52: 837–844. [Google Scholar]
- Kirk PM, Cannon PF, Minter DW, et al. (2008). Dictionary of the Fungi. 10th Ed. CABI, UK. [Google Scholar]
- Leppik EE. (1953). Some viewpoints on the phylogeny of rust fungi. I. Coniferous rusts. Mycologia 45: 46–74. [Google Scholar]
- Leppik EE. (1965). Some viewpoints on the phylogeny of rust fungi. V. Evolution of biological specialization. Mycologia 57: 6–22. [Google Scholar]
- Leppik EE. (1972). Evolutionary specialization of rust fungi (Uredinales) on the Leguminosae. Annales Botanici Fennici 9: 135–148. [Google Scholar]
- Lopéz-Franco RM, Hennen JF. (1990). The genus Tranzschelia (Uredinales) in the Americas. Systematic Botany 15: 560–591. [Google Scholar]
- Maier W, Begerow D, Weiss M, et al. (2003). Phylogeny of the rust fungi: an approach using nuclear large subunit ribosomal DNA sequences. Canadian Journal of Botany 81: 12–23. [Google Scholar]
- Mains EB. (1934). Angiopsora, a new genus of rusts on grasses. Mycologia 26: 122–132. [Google Scholar]
- Mains EB. (1939a). Studies in the Uredinales, the genus Maravalia. Bulletin of the Torrey Botanical Club 66: 173–179. [Google Scholar]
- Mains EB. (1939b). Scopella gen. nov. of the Pucciniaceae. Annales Mycologici 37: 57–60. [Google Scholar]
- Mains EB. (1939c). The genera Skierka and Ctenoderma. Mycologia 31: 175–190. [Google Scholar]
- Mains EB. (1940). Tegillum a new genus of Uredinales. Bulletin of the Torrey Botanical Club 67: 705–709. [Google Scholar]
- McNeill J, Barrie FR, Buck WR, et al. (2012). International Code of Nomenclature for algae, fungi and plants (Melbourne Code) adopted by the Eighteenth International Botanical Congress Melbourne, Australia, July 2011. Koeltz Scientific Books, Germany. [Google Scholar]
- McTaggart AR, Aime MC. (2018). The species of Coleosporium (Pucciniales) on Solidago in North America. Fungal Biology 122: 800–809. [DOI] [PubMed] [Google Scholar]
- McTaggart AR, Doungsa-ard C, Geering ADW, et al. (2015). A co-evolutionary relationship exists between Endoraecium (Pucciniales) and its Acacia hosts in Australia. Persoonia 35: 50–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTaggart AR, du Plessis E, Roux J, et al. (2020). Sexual reproduction in populations of Austropuccinia psidii. European Journal of Plant Pathology 156: 537–545. [Google Scholar]
- McTaggart AR, Geering ADW, Shivas RG. (2012). Uredinopsis pteridis and Desmella aneimiae, the first rust fungi (Pucciniales) reported on ferns (Pteridophyta) in Australia. Australasian Plant Disease Notes 9: 149. [Google Scholar]
- McTaggart AR, Shivas RG, van der Nest MA, et al. (2016). Host jumps shaped the diversity of extant rust fungi (Pucciniales). New Phytologist 209: 1149–1158. [DOI] [PubMed] [Google Scholar]
- McTaggart AT, Shuey LS, Granados GM, et al. (2018). Evidence that Austropuccinia psidii may complete its sexual life cycle on Myrtaceae. Plant Pathology 67: 729–734. [Google Scholar]
- Minh BQ, Hahn M, Lanfear R. (2018). New methods to calculate concordance factors for phylogenomic datasets. bioRxiv: 487801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnis AM, McTaggart A, Rossman A, et al. (2012). Taxonomy of mayapple rust: the genus Allodus resurrected. Mycologia 104: 942–950. [DOI] [PubMed] [Google Scholar]
- Minnis AM, Yun HY, Rossman AY. (2008). (1852) Proposal to conserve the name Olivea tectonae (T.S. Ramakr. & K. Ramakr.) R.L. Mulder against Olivea tectonae (Racib.) Thirum. (Basidiomycota). Taxon 57: 1355–1356. [Google Scholar]
- Moore RT. (1990). Order Platygloeales ord. nov. Mycotaxon 39: 245–248. [Google Scholar]
- Mordue JEM. (1991). Aecidium mori. C.M.I. Descriptions of Pathogenic Fungi and Bacteria 1031: 1–2. [Google Scholar]
- Mundkur BB. (1943). Indian species of Phakopsora and Bubakia. Mycologia 35: 538–545. [Google Scholar]
- Novick RS. (2008). Phylogeny, taxonomy, and life cycle evolution in the cedar rust fungi (Gymnosporangium). PhD Thesis, Yale University, New Haven, Connecticut, USA. [Google Scholar]
- Nguyen L-T, Schmidt HA, von Haeseler A, et al. (2015). IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Molecular Biology and Evolution 32: 268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono Y. (1984). A monograph of Maravalia (Uredinales). Mycologia 76: 892–911. [Google Scholar]
- Ono Y. (2015a). Kuehneola species (Phragmidiaceae, Pucciniales) on Vitaceae plants. Mycological Progress 14: 50. [Google Scholar]
- Ono Y. (2015b). Phakopsora breyniae (Phakopsoraceae, Pucciniales), an autoecious macrocyclic species on Breynia cernua from Australia. Mycological Progress 14: 117. [Google Scholar]
- Ono Y, Hennen J. (1983). Taxonomy of the chaconiaceous genera (Uredinales). Transactions of the Mycological Society of Japan 24: 369–402. [Google Scholar]
- Padamsee M, McKenzie EHC. (2014). A new species of rust fungus on the New Zealand endemic plant Myosotidium from the isolated Chatham Islands. Phytotaxa 174: 223–230. [Google Scholar]
- Paradis E, Schliep K. (2018). Ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 35: 526–528. [DOI] [PubMed] [Google Scholar]
- Pegg GS, Giblin FR, McTaggart AR, et al. (2014). Puccinia psidii in Queensland, Australia: disease symptoms, distribution and impact. Plant Pathology 63: 1005–1021. [Google Scholar]
- Persoon CH. (1801). Synopsis Methodica Fungorum. Göttingen, Germany. [Google Scholar]
- Peterson RS. (1968). Rust fungi on Araucariaceae. Mycopathologia et mycologia applicata 34: 17–26. [Google Scholar]
- Peterson RS. (1974). Rust fungi with Caeoma-like sori on conifers. Mycologia 66: 242–255. [Google Scholar]
- Peterson RS, Oehrens E. (1978) Mikronegeria alba (Uredinales). Mycologia 70: 321–331. [Google Scholar]
- Qi X-H, Cai L, Zhao P. (2019). Quasipucciniastrum agrimoniae, gen. et sp. nov. on Agrimonia (Rosaceae) from China. Mycologia 10: 141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R_Core_Team (2014). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Austria. [Google Scholar]
- Ramakrishnan TS, Ramakrishnan K. (1949). Chaconia tectonae Ramakrishnan T.S. & K. sp. nov. on teak. Indian Phytopathology 2: 17–19. [Google Scholar]
- Rambaut A, Drummond AJ, Xie D, et al. (2018). Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Systematic Biology 67: 901–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S, Katsuya K, Hiratsuka Y. (1993). Morphology, taxonomy and nomenclature of Tsuga-Ericaceae rusts. Transactions of the Mycological Society of Japan 34: 47–62. [Google Scholar]
- Savile DBO. (1950). North American species of Chrysomyxa. Canadian Journal of Botany 28: 318–330. [Google Scholar]
- Savile DBO. (1971). Co-evolution of the rust fungi and their hosts. The Quarterly Review of Biology 46: 211–218. [Google Scholar]
- Savile DBO. (1989). Raveneliaceae revisited. Canadian Journal of Botany 67: 2983–2994. [Google Scholar]
- Scholler M, Aime MC. (2006). On some rust fungi (Uredinales) collected in an Acacia koa-Metrosideros polymorpha woodland, Mauna Loa Road, Big Island, Hawaii. Mycoscience 47: 159–165. [Google Scholar]
- Scholler M, Lutz M, Aime MC. (2019). Repeated formation of correlated species in Tranzschelia (Pucciniales). Mycological Progress 18: 295–303. [Google Scholar]
- Sela I, Ashkenazy H, Katoh K, Pupko T. (2015). GUIDANCE2: accurate detection of unreliable alignment regions accounting for the uncertainty of multiple parameters. Nucleic Acids Research 43: W7–W14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen YM, Chung WH, Huang TC, et al. (2018). Unveiling Gymnosporangium corniforme, G. unicorne, and G. niitakayamense sp. nov. in Taiwan. Mycoscience 59: 218–228. [Google Scholar]
- Sjamsuridzal W, Nishida H, Ogawa H, et al. (1999). Phylogenetic positions of rust fungi parasitic on ferns: evidence from 18S rDNA sequence analysis. Mycoscience 40: 21–27. [Google Scholar]
- Souza ESC, Aime MC, Elias SG, et al. (2018). Crossopsorella, a new tropical genus of rust fungi. Phytotaxa 375: 189–202. [Google Scholar]
- Swann EC, Taylor JW. (1995). Phylogenetic perspectives on basidiomycete systematics: evidence from the 19S rRNA gene. Canadian Journal of Botany 73: 862–868. [Google Scholar]
- Sydow P, Sydow H. (1915). Monographia Uredinearum seu Specierum Omnium ad hunc usque Diem Descriptio et Adumbratio Systematica. Fratres Borntraeger, Germany. [Google Scholar]
- Sydow H. (1921). Die Verwertung der Verwandtschaftsverhältnisse und des gegenwärtigen Entwicklungsganges zur Umgrenzung der Gattungen bei den Uredineen. Annales Mycologici 19: 161–175. [Google Scholar]
- Sydow H. (1937). Novae fungorum species. XXV. Annals Mycologici 35: 244–286. [Google Scholar]
- Thirumalachar MJ. (1945). Development of spore-forms and nuclear cycle in the autoecious opsis rust Cystopsora oleae. Botanical Gazette 107: 74–86. [Google Scholar]
- Thirumalachar MJ, Cummins GB. (1948). Status of Allopuccinia, Leucotelium, Edythea, and Ypsilospora. Mycologia 40: 417–422. [Google Scholar]
- Thirumalachar MJ, Narasimhan MJ, Gopalkrishnan KS. (1947). Morphology of spore forms and heteroecism of the giant bamboo rust, Dasturella divina. Botanical Gazette 108: 371–379. [Google Scholar]
- Turland NJ, Wiersema JH, Barrie FR, et al. (2018). International Code of Nomenclature for algae, fungi, and plants (Shenzhen Code) Regnum Vegetabile 159. Koeltz Botanical Books, Germany. [Google Scholar]
- Van der Merwe M, Ericson L, Walker J, et al. (2007). Evolutionary relationships among species of Puccinia and Uromyces (Pucciniaceae, Uredinales) inferred from partial protein coding gene phylogenies. Mycological Research 111: 163–175. [DOI] [PubMed] [Google Scholar]
- Vialle A, Feau N, Allaire M, et al. (2009). Evaluation of mitochondrial genes as DNA barcode for Basidiomycota. Molecular Ecology Resources 9: 99–113. [DOI] [PubMed] [Google Scholar]
- Vilgalys R, Hester M. (1990). Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TJ, Bruns TD, Lee S, et al. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protocols: A Guide to Methods and Applications. (Innis MA, Gelfand DH, Sninsky JJ, et al. eds). Academic Press Inc., USA: 315–322. [Google Scholar]
- Wingfield BD, Ericson L, Szaro T, et al. (2004). Phylogenetic patterns in the Uredinales. Australasian Plant Pathology 33: 327–335. [Google Scholar]
- Wood AR, Lutz M, Bauer R, et al. (2014). Morphology and phylogenetics of Stomatisora, including Stomatisora psychotriicola sp. nov. Mycological Progress 13: 1097–1104. [Google Scholar]
- Yun HY, Minnis AM, Kim YH, et al. (2011). The rust genus Frommeëlla revisited: a later synonym of Phragmidium after all. Mycologia 103: 1451–1463. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Collection and accession data for additional sequences used in Melampsorineae, Raveneliineae, and Uredinineae analyses (Figs 2–4 & S2).
Alluvial plot tracking generic placement at familial and subfamilial rank. Each colour represents the taxonomic hypotheses of an author. Tracks for genera with conflicting familial and subfamilial placement from multiple authors are dashed. The plot was made in R with the ggalluvial package.
Raveneliineae. Network analysis generated with SplitsTree from 28S data. Generic types are indicated in bold, type proxies by *.