Abstract
Objective
To summarize overall patterns of the impact of neighborhood socioeconomic status (nSES) on stroke incidence and uncover potential gaps in the literature, we conducted a systematic review of studies examining the association between nSES and stroke incidence, independent of individual SES.
Methods
Four electronic databases and reference lists of included articles were searched, and corresponding authors were contacted to locate additional studies. A keyword search strategy included the 3 broad domains of neighborhood, SES, and stroke. Eight studies met our inclusion criteria (e.g., nSES as an exposure, individual SES as a covariate, and stroke incidence as an outcome). We coded study methodology and findings across the 8 studies.
Results
The results provide evidence for the overall nSES and stroke incidence association in Sweden and Japan, but not within the United States. Findings were inconclusive when examining the nSES-stroke incidence association stratified by race. We found evidence for the mediating role of biological factors in the nSES-stroke incidence association.
Conclusions
Higher neighborhood disadvantage was found to be associated with higher stroke risk, but it was not significant in all the studies. The relationship between nSES and stroke risk within different racial groups in the United States was inconclusive. Inconsistencies may be driven by differences in covariate adjustment (e.g., individual-level sociodemographic characteristics and neighborhood-level racial composition). Additional research is needed to investigate potential intermediate and modifiable factors of the association between nSES and stroke incidence, which could serve as intervention points.
Although medical advances and prevention efforts have decreased stroke incidence over the long term, contemporary social disparities in stroke incidence remain marked.1 In the United States, a decrease in stroke incidence is more notable among White Americans than Black Americans.2,3 In addition, in the United States and European countries, adults from lower socioeconomic status (SES) have a higher risk of stroke incidence than those from higher SES.4-7 Widening social disparities in stroke incidence may be related to contextual and structural factors.
Neighborhood SES (nSES) is an important predictor of individual health. Neighborhoods with low nSES often have fewer health-promoting amenities, such as community centers, physical activity facilities, and access to healthy groceries, as well as less social capital. Health-promoting environments may be associated with healthy behaviors and subsequent health outcomes, including stroke.8-11 As neighborhood-health research has become more prominent over the past 2 decades, recent empirical studies have tested the association between nSES and stroke.6,12-18
Review studies19,20 have documented the association between SES, both at the individual and neighborhood levels, and stroke incidence. However, the reviews included studies that examined the role of nSES in stroke incidence without controlling for individual-level SES. Omitting individual-level SES is likely to lead to biased estimates of the true relationship between nSES and stroke risk because individual-level SES is positively related to nSES and inversely related to stroke risk.21,22 In addition, there has been no review study investigating mediators and moderators of the nSES-stroke incidence association, which could serve as points of policy and program interventions.
We conducted a systematic review to answer the following research questions: (1) is nSES associated with stroke incidence independent of individual SES? and (2) which moderating and mediating factors have been tested and found to be significant in the nSES-stroke incidence association?
Methods
A systematic review was conducted to identify and synthesize all studies that had examined the effects of nSES on stroke incidence independent of individual SES. Preferred Reporting Items for Systematic Reviews and Meta-Analyses reporting guidelines for systematic reviews were followed and fulfilled.23 We predetermined specific inclusion and exclusion criteria, a comprehensive and explicit search strategy, a data extraction form, and a tool for quality assessment.24-26
Inclusion/Exclusion Criteria
We included published and unpublished studies in English that met search criteria. Inclusion criteria were the following: (1) nSES as an exposure, (2) individual SES as a covariate, (3) stroke incidence (hemorrhagic or ischemic) as an outcome, (4) quantitative study, and (5) peer-reviewed article, dissertation, or government report. Exclusion criteria were the following: (1) articles focusing on a non–high-income country (defined by the World Bank)27 because neighborhood effects may not be comparable to countries in different socioeconomic contexts,28 (2) articles using only TIA as an outcome (because TIA represents a heterogeneous outcome often with mixed diagnosis and believed to be underreported in the population because of individuals missing the signs and symptoms of a TIA and, therefore, not getting a diagnosis of TIA), (3) articles examining associations within the stroke population (i.e., stroke incidence is not an outcome, but the population of interest), (4) review articles, or (5) ecological studies.
Search Strategy
A total of 4 databases (i.e., ProQuest Dissertation & Theses, PsychInfo, PubMed, and Web of Science) were used to search for studies. Citations were searched and retrieved from all 4 databases on February 11, 2019, and May 19, 2020. A keyword search strategy included 3 broad domains of neighborhood, SES, and stroke. For example, the search strategy for PubMed consisted of the following: (Neighbor* OR Communit* OR Environ* OR “Residence Characteristics”) AND (“Socioeconomic Factors” OR socioeconomic OR SES OR poverty OR impoverished OR unemploy* OR income OR affluen* OR advantaged OR advantage OR deprivation OR occupation* OR “Standard of Living” OR “Living Standard” OR “living standards” OR inequality OR wealth) AND (“stroke” OR stroke OR strokes OR “Cerebrovascular Accident” OR “cerebrovascular accidents” OR CVA OR “Cerebrovascular Apoplexy” OR “Vascular Accident” OR “vascular accidents” OR “Apoplexy” OR “Cerebral hemorrhage” OR “Subarachnoid hemorrhage”). The PubMed search strategy was translated to other research databases by (1) inputting our search strategy into a polyglot tool for translation (sr-accelerator.com/#/polyglot), (2) subjecting the search strategy to independent review and revision by authors Y. Kim and E. Twardzik, and (3) comparing individual review and translation of the search strategy by Y. Kim and E. Twardzik; when disagreement arose, the authors would come to a consensus.
First, 2 authors independently reviewed titles/abstracts. A study was included for full-text review in cases in which inclusion/exclusion could not be determined from its title or abstract. Second, the authors independently reviewed the full text of all studies selected in the first step. If a study used nSES as a covariate and did not report the effect estimate within the article, the corresponding author for the study was contacted, and additional information was requested. When there were disagreements about a particular study, the authors discussed its eligibility until a consensus was reached. In addition, reference lists from included studies and review studies on neighborhood and stroke were manually searched for further relevant publications. Finally, we contacted through e-mail the corresponding authors of studies included in this review to find whether they could identify any other studies meeting our inclusion criteria and (if relevant statistical findings were not fully reported) to receive additional results or clarification. None of the studies that the corresponding authors recommended met our inclusion criteria. We received additional results for 1 study and confirmation of our interpretation for 3 studies.
Data Extraction and Quality Assessment
For each study, 2 authors independently extracted and recorded data on research methodology and statistical results. Disagreement was resolved through discussion. Cohen kappa for an interreviewer agreement was 0.827 (p < 0.001) across all categories. In addition, an adapted version of the Newcastle-Ottawa Assessment Scale was used to assess the risk of bias of the included studies.
Data Availability
Data are available to investigators on request from the first author.
Results
Identified Studies
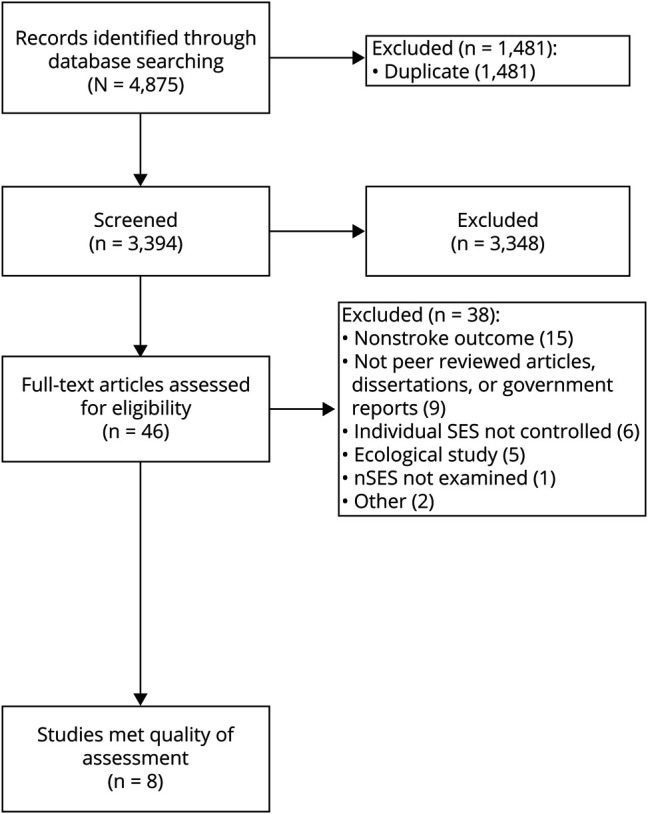
Figure 1 shows the number of articles identified, included, and excluded, and the reasons for exclusion within this study. On screening the title, abstract, and full text, a total of 10 studies met the inclusion criteria; however, 2 studies were excluded because of missing effect estimate information for the nSES-stroke incidence relationship.29,30 As a result, 8 studies were included in the final sample.
Figure 1. PRISMA Flow Diagram for Study Selection.
Description of Included Studies
All studies were published after January 2007, and 5 of 8 studies were conducted in the United States, as shown in table 1. Quality assessment of the study demonstrated satisfactory study quality and low risk of bias in ensuring a sufficient sample size of data (n = 8), clearly describing a measure of nSES (n = 8), controlling for important individual-level confounders (n = 8), clearly describing statistical testing and findings (n = 8), using a representative sample (n = 7), and assessing stroke incidence using record linkage or medical record review (n = 7). The only source of potential bias was no description of the response rate or a low response rate in the data (n = 4).
Table 1.
Characteristics of the 8 Included Studies
Data
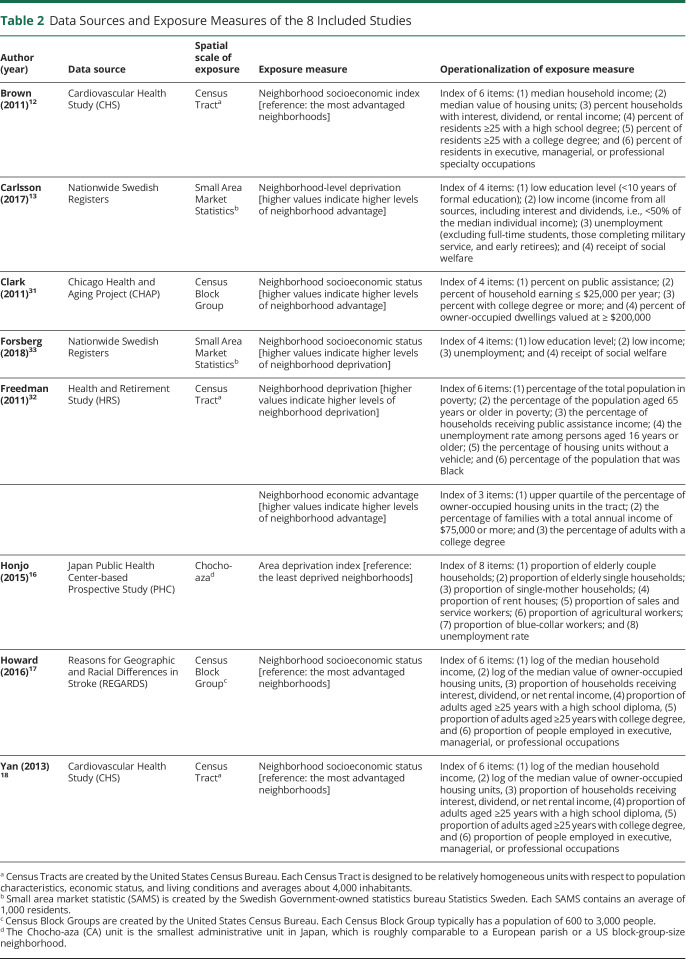
Table 2 presents the data source and exposure measures of included studies. The data sets used within each study varied. Two studies in the United States used the data from the Cardiovascular Health Study (CHS).12,18 Three US studies each used the Chicago Health and Aging Project,31 Health and Retirement Study,32 and REasons for Geographic and Racial Differences in Stroke (REGARDS).17 Two studies in Sweden used data from the Nationwide Swedish Registers13,33: the Total Population Register and the Patient Register in the study by Carlsson et al.13 and the Hospital Discharge Register and the Outpatient Register in the study by Forsberg et al.33 One study in Japan16 used the data from the Japan Public Health Center–based Prospective Study.
Table 2.
Data Sources and Exposure Measures of the 8 Included Studies
nSES Measure
All studies used an administrative entity created by governments to represent a neighborhood. Five studies in the United States12,17,18,31,32 defined neighborhoods using a census unit aggregation, including census tracts (n = 3) and block groups (n = 2). Census tracts and block groups are designed to be relatively homogeneous areas with respect to sociodemographic characteristics of the population. Block groups contain between 600 and 3,000 individuals, whereas census tracts contain about 4,000 individuals. Two studies in Sweden measured neighborhoods based on small area market statistics, which contain an average of 1,000 residents. One study in Japan16 defined neighborhoods based on Chocho-Aza units, a comparable size to a US block group.
All studies created a composite index of nSES using several indicators of neighborhood characteristics. However, the operationalization of nSES varied in the utilization of items. For example, 3 studies12,17,18 derived an nSES index using a factor analysis with 6 items representing education, income, occupation, and housing values. Two studies13,33 created an index of nSES using a principal components analysis with 4 items representing education, income, and employment, with higher values indicating higher levels of deprivation. One study31 conducted a confirmatory factor analysis by including similar items as those above into indices. The remaining 2 studies16,32 included items that do not directly indicate nSES conditions along with other items that directly measure nSES (e.g., a percentage of the total population in poverty, an unemployment rate, and a proportion of blue-collar workers) to create an nSES index; specifically, Honjo et al.16 included a proportion of elderly couples or single households along with nSES items to create an area deprivation index, and Freedman et al.32 included a percentage of the population that was Black along with nSES items when conducting an exploratory factor analysis.
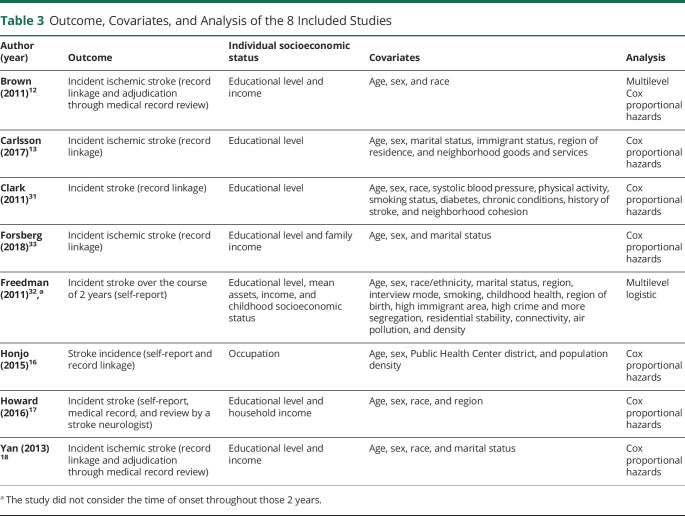
Outcome Measure
Table 3 shows outcome measures, covariates, and analysis of included studies. The primary outcome was measured as follows: stroke incidence in 4 studies16,17,31,32 and ischemic stroke incidence in 4 studies.12,13,18,33 Of note, although 7 studies12,13,16-18,31,33 used a time to event outcome, Freedman et al.32 did not consider the time of onset throughout a 2-year study period. Four studies identified stroke cases within their study population using a single measure of stroke, with 3 studies using record linkage13,31,33 and 1 study using self-reported stroke.32 Three studies identified stroke cases using 2 measures of stroke. Honjo et al.16 used self-reported stroke and linked this report back to records, and 2 studies used record linkage and adjudication of the stroke through medical record review.12,18 Only 1 study used 3 measures of stroke incidence.17 Howard et al.17 had participants self-report stroke incidence through follow-up calls every 6 months, obtained medical records from the stroke event, and confirmed stroke cases through review of participant medical records by a stroke neurologist.
Table 3.
Outcome, Covariates, and Analysis of the 8 Included Studies
Individual SES and Covariates
Studies included a variable of individual SES to examine the nSES-stroke incidence associations, theoretically independent of individual SES. Of 8 studies, 4 studies12,17,18,33 controlled for both education and income, 2 studies13,31 controlled only for education, 1 study32 controlled for education, income, and household wealth, and the other study16 controlled only for occupation. All included studies controlled for individual SES, age, and sex as covariates within the analysis. Beyond these 3 variables, covariate adjustment varied by individual study (column 4, table 3).
Analysis Methods
Seven studies conducted Cox proportional hazards13,16-18,31,33 or multilevel Cox proportional hazards12 using a time-to-event outcome. On the other hand, the remaining study, which measured whether the respondents had stroke during a 2-year study period, conducted a multilevel logistic analysis.32
Findings of Multivariate Analysis
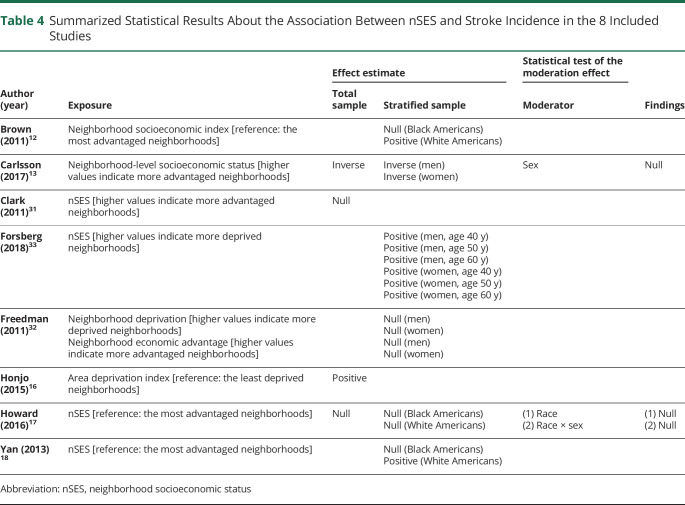
Table 4 and figure 2 present summarized findings of the included studies. Below these results are summarized among studies examining (1) an overall effect within their study sample, (2) effects within subgroups, and (3) mediating mechanisms.
Table 4.
Summarized Statistical Results About the Association Between nSES and Stroke Incidence in the 8 Included Studies
Figure 2. Summarized Findings of 6 Included Studies.
(A) Presents (1) the coefficients of least advantaged neighborhoods (vs. moderately advantaged neighborhoods) from the article by Carlsson et al.,13 (2) the coefficients of most deprived neighborhoods (vs least deprived neighborhoods) from Honjo et al.,16 and (3) the coefficients of least advantaged neighborhoods (vs most advantaged neighborhoods) from Howard et al.,17 Brown et al.,12 and Yan et al.18 From the studies by Freedman et al.32 and Forsberg et al.,33 we presented coefficients of a continuous variable of neighborhood disadvantage, and its higher scores indicate more disadvantaged neighborhoods. (B) Presents (1) the coefficients of the most advantaged neighborhoods (vs moderately advantaged neighborhoods) from the article by Carlsson et al.13 and (2) the coefficients of a continuous variable of neighborhood advantage, and its higher scores indicate more advantaged neighborhoods. From the study by Clark et al.,31 we presented coefficients of a continuous variable of neighborhood advantage, and its higher scores indicate more advantaged neighborhoods.
Overall Effects
Four of the 8 studies tested and presented an overall effect of nSES on stroke incidence within their full study sample.13,16,17,31 Although the 4 studies13,16,17,31 showed the expected direction of effect, namely a positive effect of nSES disadvantage on stroke risk, this relationship was statistically significant in only the 2 studies conducted in non-US countries.13,16 Specifically, a higher risk of ischemic stroke incidence was associated with increasing nSES disadvantage (Carlsson et al.13: Hazard Ratio (HR) = 1.19 [95% Confidence Interval (CI) = 1.08–1.30]; Honjo et al.16: HR = 1.19 [95% CI = 1.01–1.41]), after controlling for education,13 and occupation16 as indicators of individual SES. The other 2 studies conducted in the United States showed an insignificant nSES-stroke incidence association after controlling for individual SES (education31 and both education and income17).
Effects Within Subgroups
The fourth column of table 4 presents summarized findings of multivariate analyses stratified by sex, age, and/or race. Six of the 8 studies12,13,17,18,32,33 examined the effects of nSES on stroke risk within subgroups of sex (n = 2), sex and age (n = 1), and race (n = 3). Of the 6 studies, 3 studies13,32,33 stratified the data by sex. Carlsson et al.13 reported that neighborhood disadvantage increased stroke risk among females (HR = 1.31 [95% CI = 1.14–1.51]) but not among males (HR = 1.09 [95% CI = 0.97–1.24]) but that neighborhood advantage decreased stroke risk among males (HR = 0.87 [95% CI = 0.76–0.99]) but not among females (HR = 0.87 [95% CI = 0.74–1.01]). The study by Forsberg et al.33 stratified the data by sex and age groups of 40, 50, and 60 years to examine the nSES-stroke incidence association within age subgroups. The study found the same direction of association between nSES deprivation and stroke incidence across all the age groups and for both sexes with a greater risk of stroke incidence with greater nSES deprivation (men: HRs = 1.02–1.04; women: HRs = 1.04–1.07). Freedman et al.32 observed no significant relationship between nSES disadvantage and stroke incidence among men (OR = 1.02 [95% CI = 0.85–1.22]) or women (OR = 1.11 [95% CI = 0.97–1.27]). Of note, Carlsson et al.13 and Forsberg et al.33 controlled only for individual-level sociodemographic characteristics and/or region of residence, whereas Freedman et al.32 controlled for several neighborhood measures (i.e., immigrant concentration, crime, segregation, residential stability, street connectivity, air pollution, and population density) along with individual-level demographic and socioeconomic characteristics. The neighborhood-level covariates might serve as mediators in the nSES-stroke incidence association and subsequently make nSES insignificant in the nSES-stroke incidence association. Overall, these results suggest that across these studies, there was no significant effect modification by sex in the relationship between nSES effects and stroke incidence.
Three studies12,17,18 used the same indicators to measure nSES and stratified the data by race. Figure 2 shows that 2 studies12,18 found that the nSES-stroke incidence association was in a different direction for Black and White Americans within each study, whereas the other17 found the same direction observed among Black and White Americans. Specifically, Brown et al.12 and Yan et al.18 reported a significantly positive association between nSES deprivation and ischemic stroke incidence among White Americans (Brown et al.12: HR = 1.32 [95% CI = 1.01–1.72]; Yan et al.18: HR = 1.32 [95% CI = 1.01–1.73]) but reported an insignificant inverse association between nSES deprivation and ischemic stroke incidence among Black Americans (Brown et al.12: HR = 0.60 [95% CI = 0.33–1.07]; Yan et al.18: HR = 0.60 [95% CI = 0.35–1.05]). Of note, these 2 studies used the same data (the CHS) and had a smaller sample size for Black Americans (n = 785) than White Americans (n = 3,834), which may have led to the insignificant association in Black Americans. On the other hand, Howard et al.17 reported an insignificant positive association between neighborhood disadvantage and stroke incidence among Black (HR = 1.17 [95% CI = 0.76–1.80]) and White Americans (HR = 1.26 [95% CI = 0.94–1.70]). Of note, in the study by Howard et al.,17 the sample size for Black Americans was 10,274, and there was considerable variability in nSES within the study sample.
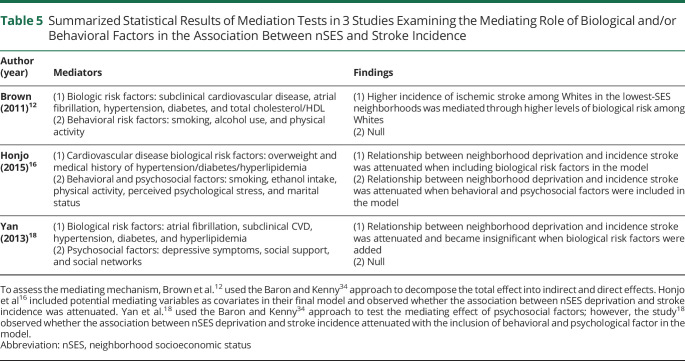
Mediating Mechanisms
Table 5 shows summarized findings of mediation tests. Three studies12,16,18 examined the mediation effect of biological factors. The studies12,16,18 found that biological factors (e.g., subclinical cardiovascular disease) mediated the relationship between nSES and stroke risk. Of note, Brown et al.12 used the method of Baron and Kenny34 to decompose the total effect into indirect and direct effects, whereas Honjo et al.16 and Yan et al.18 assessed mediation through the inclusion of biological risk factors as covariates in the model and observing whether the association between nSES deprivation and stroke incidence was attenuated, which is a less desirable method of testing the mediation effect.
Table 5.
Summarized Statistical Results of Mediation Tests in 3 Studies Examining the Mediating Role of Biological and/or Behavioral Factors in the Association Between nSES and Stroke Incidence
All 3 studies12,16,18 also examined the mediation effect of behavioral factors (n = 1), psychological factors (n = 1), or both (n = 1). Brown et al.12 and Yan et al.,18 who used the method of Baron and Kenny,34 found no significant mediation effect of behavioral or psychological factors. Honjo et al.16 found a possibility of the mediation effect of behavioral and psychological factors. Of note, as described above, Honjo et al.16 assessed mediation by observing whether the association between nSES deprivation and stroke incidence was attenuated with the inclusion of behavioral and psychological factors in the model.
Discussion
This review summarizes empirical evidence examining the relationship between neighborhood socioeconomic conditions and stroke incidence, along with mediating and moderating mechanisms of this relationship. Overall, neighborhood socioeconomic disadvantage is associated with increased stroke risk; however, the association was not significant in all the studies.13,16,17,31 Differences in significant findings are likely driven by differences in the sample size and socioenvironmental contexts. For example, there were large differences in the sample size between studies finding a significant association (N = 1,153,451 for Carlsson et al.13 and 90,843 for Honjo et al.16) and studies finding a nonsignificant association (N = 24,875 for Howard et al.17 and 5,789 for Clark et al.31). It may be that the studies with larger numbers of participants were better powered to find a significant result. In addition, Carlsson et al.13 and Honjo et al.16 conducted their studies in the socioenvironmental contexts of Sweden and Japan, respectively, where all residents have health insurance coverage under a universal health care system. On the other hand, Clark et al.31 and Howard et al.17 conducted their studies in the US socioenvironmental contexts, where health insurance is made up by a complex market of public and private providers. Different health insurance coverage and utilization of health services may be uncontrolled covariates in the United States, which should be considered in future research.
Numerous studies included in this review presented stratified results, presenting differences in the impact of nSES on stroke incidence by sex,13,32,33 sex and age,33 and race.12,17,18 Overall, the studies included found no differential effect by sex and age. Regarding racial differences, findings across studies are inconsistent. Studies12,18 using CHS data found racial differences in the nSES-stroke incidence association, whereas a study17 using REGARDS data found no racial difference in the association. All 3 of these studies used the same operationalization of nSES exposure but differed in the spatial scale in which it was measured and the data set it was examined within. Brown et al.12 and Yan et al.18 both measured nSES at the census tract level and observed that less advantaged neighborhoods increased stroke risk among Whites but not Black Americans in the CHS. Howard et al.17 measured nSES at the block group level and did not observe differential effects by race in the REGARDS study. Although previous studies suggested the differences in census tract and block groups measures to be relatively small,35,36 the role of spatial scale in this relationship is still largely unknown. Further investigation is needed to determine whether the relationship between nSES and stroke incidence is differential by race.
The inconsistency in findings by race could be further explored by considering the role of various features of neighborhood environments in the racial disparity in stroke incidence. The literature demonstrated neighborhood racial/ethnic composition as a potential explanation for racial disparities in health.37 For example, Bond Huie et al.38 found that neighborhood racial/ethnic composition was a stronger driver of Black-White disparity in mortality than neighborhood socioeconomic characteristics. In addition, the literature suggests that systemic racism and stress from daily experiences of racism increase the risk of adverse health outcomes and racial disparities in health.39,40 Thus, the direct effects of neighborhood racial/ethnic composition and racially discriminatory experiences warrant further examination.
This review identified methodological limitations in the 8 studies and suggestions for future research. Measurement of nSES was inconsistent across studies. Studies used different composite measures of nSES identified through factor analysis,12,17,18 principal components analysis,13,33 confirmatory factor analysis,31 exploratory factor analysis,32 or an author-derived index.16 In addition, there were nSES indices that included measures from area-level indicators that do not directly indicate or represent nSES conditions (e.g., age16 or race32 of resident) along with other items that directly measure nSES. Finally, all 8 studies captured absolute measures of nSES resource, rather than a relative nSES measure such as within-neighborhood income inequality and between-neighborhood income segregation. A relative nSES measure assesses the distribution of socioeconomic resource opportunities41-44; thus, examining relative nSES measures may capture a different aspect of nSES conditions relative to an absolute nSES measure, such as psychological stress from highly unequal resource distribution.
Considering the residential mobility of survey participants after the baseline (i.e., moving to a new home) is warranted. Statistics showed that 18% of US adults aged 55–74 years45 and 18–24% of Swedish adults aged 57–66 years46 moved at least once in 5 years. However, all studies included in this review did not account for individuals' residential mobility and, thus, implicitly assumed equal neighborhood effects for the differential durations lived in the baseline neighborhood.47 For example, Brown et al.12 and Yan et al.18 obtained nSES information at the baseline (1989–1993) and examined its effect on stroke incidence collected in the follow-ups (until 2006, every 6 months). Thus, a longitudinal lens on neighborhood circumstances is warranted to estimate neighborhood impacts on stroke incidence.
More than half of the studies relied on 1 measure of stroke incidence.12,13,31-33 In particular, 1 study relied solely on self-report stroke incidence.32 Self-reported stroke may result in information bias of the outcome, whereby those with stroke-like symptoms may self-report stroke, resulting in a false-positive report. In addition, participants who experience a stroke may have severe cognitive impairment and could be lost to follow-up or report stroke status incorrectly.48 Because of these potential sources of bias, we urge additional caution when interpreting the effect estimates relying solely on self-report stroke incidence. Finally, some demographic groups have not been investigated. Future research needs to examine diverse demographic subgroups at risk such as Hispanics and transgender individuals.
This review has several strengths. First, we used several strategies to identify eligible studies: a search of 4 databases, an extensive search of reference lists, and contacting the corresponding authors of studies included in this review. Second, we reviewed a wide range of nSES factors from education, income, and occupation to welfare receipt and wealth. We also examined the nSES-stroke incidence association independent of individual SES and its mediating and moderating mechanism, which have not been explored in previous review studies.19,20
However, our study is not without limitations. First, we included only quantitative studies. Qualitative research can give a better understanding of how people perceive and experience nSES affecting their stroke risk. Study publication bias and outcome reporting bias might have occurred. In addition, this review focused on nSES; however, there are other important attributes of neighborhood environments affecting individual health, such as racial/ethnic composition, racial residential segregation, and built and social environments. Finally, we were unable to conduct a meta-analysis because the included studies measured nSES using different items, adjusted for a different set of covariates, and conducted different analyses ranging from multilevel logistic regression to Cox proportional hazards. As we move the field forward, it is advantageous for researchers to have consistency in the measurement of the neighborhood socioeconomic context. Because of the lack of consistency in study methodology (e.g., measurement and covariate adjustment), we were unable to explain differences between study findings. To facilitate comparisons in the United States in future studies, we recommend that research on nSES uses the 6-item measure at the census tract level validated through factor analysis.49 Testing the validity of the 6-item measure outside the United States context is also warranted. Consistency in operationalization will allow for summarization of effect estimates across studies. Furthermore, we suggest a step-wise model building strategy that includes covariate adjustment for age, sex, and race/ethnicity, followed by adjustment for individual SES and, finally, inclusion of the 6-item nSES measure. Finally, we suggest future studies to use Cox proportional hazards and present an overall effect estimate in the full sample, which would allow for comparisons of effect estimates across studies.
In conclusion, this review summarized 8 articles examining the nSES-stroke risk association with adjustment for individual SES. The association between neighborhood disadvantage and stroke risk was found above and beyond the individual SES, but it was not significant in all the studies.13,16,17,31 Research examining the moderating effect of race on the nSES-stroke risk association was inconsistent.12,17,18 Future research needs to use a standard operational definition of neighborhood and nSES measures to allow for better comparability across studies. Furthermore, additional research is needed to investigate other neighborhood characteristics and biological, behavioral, and psychological factors as potential intermediate and modifiable mediators of the nSES-stroke risk relationship that could serve as policy and program interventions.
Glassary
- CHS
Cardiovascular Health Study
- nSES
neighborhood SES
- REGARDS
REasons for Geographic and Racial Differences in Stroke
- SES
socioeconomic status
Appendix. Authors
Footnotes
Editorial, page 879
Study Funding
Research reported in this publication was supported by the National Institute of Neurological Disorders and Stroke of the NIH under award number 1R01NS092706 and the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the NIH under award number F31HD098870. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Disclosure
Y. Kim reports funding from the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the US NIH (R03HD101752). E. Twardzik reports funding from the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the NIH (F31HD098870, E. Twardzik, PI). S.E. Judd reports funding from the US NIH (1 U01 HL146382, 2 U01 NS041588, 1R01NS09270 1AG 057540, and 5R01HL136666) and the Centers for Disease Control and Prevention 1U01DP006302-01. N. Colabianchi reports funding from the US NIH (R03HD101752, R01HL137731, R01NS092706, R01AG057540, and R01 HL132979). Go to Neurology.org/N for full disclosures.
References
- 1.Marshall IJ, Wang Y, Crichton S, McKevitt C, Rudd AG, Wolfe CD. The effects of socioeconomic status on stroke risk and outcomes. Lancet Neurol 2015;14:1206–1218. [DOI] [PubMed] [Google Scholar]
- 2.Howard VJ, Kleindorfer DO, Judd SE, et al. Disparities in stroke incidence contributing to disparities in stroke mortality. Ann Neurol 2011;69:619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kleindorfer D, Khoury J, Moomaw CJ, et al. Stroke incidence is decreasing in whites, but not in blacks: a population-based estimate of temporal trends in stroke incidence from the Greater Cincinnati/Northern Kentucky stroke study. Stroke 2010;41:1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agyemang C, van Oeffelen AA, Norredam M, et al. Socioeconomic inequalities in stroke incidence among migrant groups: analysis of nationwide data. Stroke 2014;45:2397–2403. [DOI] [PubMed] [Google Scholar]
- 5.Avendano M, Kawachi I, Van Lenthe F, et al. Socioeconomic status and stroke incidence in the US elderly: the role of risk factors in the EPESE study. Stroke 2006;37:1368–1373. [DOI] [PubMed] [Google Scholar]
- 6.Cesaroni G, Agabiti N, Forastiere F, Perucci CA. Socioeconomic differences in stroke incidence and prognosis under a universal healthcare system. Stroke 2009;40:2812–2819. [DOI] [PubMed] [Google Scholar]
- 7.Bray BD, Paley L, Hoffman A, et al. Socioeconomic disparities in first stroke incidence, quality of care, and survival: a nationwide registry-based cohort study of 44 million adults in England. Lancet Public Health 2018;3:e185–e193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowling A, Stafford M. How do objective and subjective assessments of neighbourhood influence social and physical functioning in older age? Findings from a British survey of ageing. Soc Sci Med 2007;64:2533–2549. [DOI] [PubMed] [Google Scholar]
- 9.Franzini L, Caughy M, Spears W, Esquer MEF. Neighborhood economic conditions, social processes, and self-rated health in low-income neighborhoods in Texas: a multilevel latent variables model. Soc Sci Med 2005;61:1135–1150. [DOI] [PubMed] [Google Scholar]
- 10.Sampson RJ, Morenoff JD, Gannon-Rowley T. Assessing “neighborhood effects”: social processes and new directions in research. Annu Rev Sociol 2002;28:443–478. [Google Scholar]
- 11.Wen M, Browning CR, Cagney KA. Poverty, affluence, and income inequality: neighborhood economic structure and its implications for health. Soc Sci Med 2003;57:843–860. [DOI] [PubMed] [Google Scholar]
- 12.Brown AF, Liang L-J, Vassar SD, et al. Neighborhood disadvantage and ischemic stroke: the cardiovascular health study (CHS). Stroke 2011;42:3363–3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carlsson AC, Li X, Holzmann MJ, et al. Neighborhood socioeconomic status at the age of 40 years and ischemic stroke before the age of 50 years: a nationwide cohort study from Sweden. Int J Stroke 2017;12:815–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grimaud O, Béjot Y, Heritage Z, et al. Incidence of stroke and socioeconomic neighborhood characteristics: an ecological analysis of Dijon stroke registry. Stroke 2011;42:1201–1206. [DOI] [PubMed] [Google Scholar]
- 15.Heeley EL, Wei JW, Carter K, et al. Socioeconomic disparities in stroke rates and outcome: pooled analysis of stroke incidence studies in Australia and New Zealand. Med J Aust 2011;195:10–14. [DOI] [PubMed] [Google Scholar]
- 16.Honjo K, Iso H, Nakaya T, et al. Impact of neighborhood socioeconomic conditions on the risk of stroke in Japan. J Epidemiol 2015;25:254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howard VJ, McClure LA, Kleindorfer DO, et al. Neighborhood socioeconomic index and stroke incidence in a national cohort of blacks and whites. Neurology 2016;87:2340–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan T, Escarce JJ, Liang LJ, et al. Exploring psychosocial pathways between neighbourhood characteristics and stroke in older adults: the cardiovascular health study. Age and ageing 2013;42:391–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Addo J, Ayerbe L, Mohan KM, et al. Socioeconomic status and stroke: an updated review. Stroke 2012;43:1186–1191. [DOI] [PubMed] [Google Scholar]
- 20.Backholer K, Peters SA, Bots SH, Peeters A, Huxley RR, Woodward M. Sex differences in the relationship between socioeconomic status and cardiovascular disease: a systematic review and meta-analysis. J Epidemiol Community Health 2017;71:550–557. [DOI] [PubMed] [Google Scholar]
- 21.Hedman L, van Ham M. Understanding Neighbourhood Effects: Selection Bias and Residential Mobility. Springer Netherlands; 2011:79–99. [Google Scholar]
- 22.Diez Roux AV. Investigating neighborhood and area effects on health. Am J Public Health 2001;91:1783–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. Bmj 2015;350:g7647. [DOI] [PubMed] [Google Scholar]
- 24.Higgins JP, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions: John Wiley & Sons, 2019. [Google Scholar]
- 25.Littell JH, Corcoran J, Pillai V. Systematic Reviews and Meta-Analysis: Oxford University Press; 2008. [Google Scholar]
- 26.Slavin RE. Best evidence synthesis: an intelligent alternative to meta-analysis. J Clin Epidemiol 1995;48:9–18. [DOI] [PubMed] [Google Scholar]
- 27.World Bank. World Bank Country and Lending Groups [online]. Available at: data.worldbank.org/about/country-and-lending-groups#High_income. Accessed May 19, 2020. [Google Scholar]
- 28.Minh A, Muhajarine N, Janus M, Brownell M, Guhn M. A review of neighborhood effects and early child development: how, where, and for whom, do neighborhoods matter? Health place 2017;46:155–174. [DOI] [PubMed] [Google Scholar]
- 29.Calling S, Li X, Kawakami N, Hamano T, Sundquist K. Impact of neighborhood resources on cardiovascular disease: a nationwide six-year follow-up. BMC public health 2016;16:634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kulick ER, Wellenius GA, Boehme AK, Sacco RL, Elkind MS. Residential proximity to major roadways and risk of incident ischemic stroke in NOMAS (The Northern Manhattan Study). Stroke 2018;49:835–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clark CJ, Guo H, Lunos S, et al. Neighborhood cohesion is associated with reduced risk of stroke mortality. Stroke 2011;42:1212–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freedman VA, Grafova IB, Rogowski J. Neighborhoods and chronic disease onset in later life. Am J Public Health 2011;101:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forsberg P-O, Ohlsson H, Sundquist K. Causal nature of neighborhood deprivation on individual risk of coronary heart disease or ischemic stroke: a prospective national Swedish co-relative control study in men and women. Health Place 2018;50:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baron RM, Kenny DA. The moderator–mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Personal Soc Psychol 1986;51:1173. [DOI] [PubMed] [Google Scholar]
- 35.Krieger N, Chen JT, Waterman PD, Rehkopf DH, Subramanian SV. Race/ethnicity, gender, and monitoring socioeconomic gradients in health: a comparison of area-based socioeconomic measures—the public health disparities geocoding Project. Am J Public Health (1971) 2003;93:1655–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lovasi GS, Moudon AV, Smith NL, et al. Evaluating options for measurement of neighborhood socioeconomic context: evidence from a myocardial infarction case–control study. Health place 2008;14:453–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anderson NB, Bulatao RA, Cohen B, on Race P, Council NR. What makes a place healthy? Neighborhood influences on racial/ethnic disparities in health over the life course. In: Critical Perspectives on Racial and Ethnic Differences in Health in Late Life: National Academies Press (US); 2004. [PubMed] [Google Scholar]
- 38.Huie B, Hummer RA, Rogers RG. Individual and contextual risks of death among race and ethnic groups in the United States. J Health Soc Behav 2002:359–381. [PubMed] [Google Scholar]
- 39.Shaw G. It's a public health crisis: how systemic racism can Be neurotoxic for black Americans. Neurol Today 2020;20:1–24. [Google Scholar]
- 40.Phelan JC, Link BG. Is racism a fundamental cause of inequalities in health? Annu Rev Sociol 2015;41:311–330. [Google Scholar]
- 41.Bernstein M, Crosby F. An empirical examination of relative deprivation theory. J Exp Soc Psychol 1980;16:442–456. [Google Scholar]
- 42.Guimond S, Dubé-Simard L. Relative deprivation theory and the Quebec nationalist movement: the cognition–emotion distinction and the personal–group deprivation issue. J Personal Soc Psychol 1983;44:526. [Google Scholar]
- 43.Podder N. Relative deprivation, envy and economic inequality. Kyklos 1996;49:353–376. [Google Scholar]
- 44.Yitzhaki S. Relative deprivation and the gini coefficient. Q J Econ 1979;93:321–324. [Google Scholar]
- 45.U.S. Bureau of the Census. Geographical Mobility: 2010 to 2015 [data File]; 2018. [Google Scholar]
- 46.Andersson E, Abramsson M. Changing residential mobility rates of older people in Sweden. Ageing Soc 2012;32:963. [Google Scholar]
- 47.Sheehan CM, Cantu PA, Powers DA, Margerison-Zilko CE, Cubbin C. Long-term neighborhood poverty trajectories and obesity in a sample of California mothers. Health place 2017;46:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kalaria RN, Akinyemi R, Ihara M. Stroke injury, cognitive impairment and vascular dementia. Biochim Biophys Acta Mol basis Dis 2016;1862:915–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Diez-Roux AV, Merkin SS, Arnett D, et al. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med 2001;345:99–106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available to investigators on request from the first author.