Abstract
Objective
To evaluate whether a retinal spectral-domain optical coherence tomography (SD-OCT) assessment at baseline is associated with long-term disability worsening in people with multiple sclerosis (PwMS), we performed SD-OCT and Expanded Disability Status Scale (EDSS) assessments among 132 PwMS at baseline and at a median of 10 years later.
Methods
In this prospective, longitudinal study, participants underwent SD-OCT, EDSS, and visual acuity (VA) assessments at baseline and at follow-up. Statistical analyses were performed using generalized linear regression models, adjusted for age, sex, race, multiple sclerosis (MS) subtype, and baseline disability. We defined clinically meaningful EDSS worsening as an increase of ≥2.0 if baseline EDSS score was <6.0 or an increase of ≥1.0 if baseline EDSS score was ≥6.0.
Results
A total of 132 PwMS (mean age 43 years; 106 patients with relapsing-remitting MS) were included in analyses. Median duration of follow-up was 10.4 years. In multivariable models excluding eyes with prior optic neuritis, relative to patients with an average baseline ganglion cell + inner plexiform layer (GCIPL) thickness ≥70 µm (the mean GCIPL thickness of all eyes at baseline), an average baseline GCIPL thickness <70 µm was associated with a 4-fold increased odds of meaningful EDSS worsening (adjusted odds ratio [OR] 3.97, 95% confidence interval [CI] 1.24–12.70; p = 0.02) and an almost 3-fold increased odds of low-contrast VA worsening (adjusted OR 2.93, 95% CI 1.40–6.13; p = 0.04).
Conclusions
Lower baseline GCIPL thickness on SD-OCT is independently associated with long-term disability worsening in MS. Accordingly, SD-OCT at a single time point may help guide therapeutic decision-making among individual PwMS.
Classification of Evidence
This study provides Class I evidence that lower baseline GCIPL thickness on SD-OCT is independently associated with long-term disability worsening in MS.
Multiple sclerosis (MS) is an autoimmune demyelinating disorder of the CNS, in which neuroaxonal degeneration constitutes the principal substrate of disability progression.1-3 The MS disease course is difficult to predict and as such, biomarkers that accurately quantify neurodegeneration and help identify future disability are needed. Because occult optic neuropathy is essentially ubiquitous in MS, the retina represents an opportune window into MS-related neurodegeneration.4
Optical coherence tomography (OCT) is a rapid, noninvasive, high-resolution imaging technique that uses near-infrared light to quantify retinal layers. The evolution of fourth-generation spectral-domain OCT (SD-OCT) has facilitated accurate quantification of composite ganglion cell + inner plexiform layer (GCIPL) thickness measurement, which demonstrates superior reliability and reproducibility than conventional OCT measures, including peripapillary retinal nerve fiber layer (pRNFL) thickness.5-9 Relative to pRNFL thickness, GCIPL thickness exhibits superior structure–function relationships, is strongly correlated with visual acuity (VA) and global disability scores, and mirrors whole brain and gray matter atrophy in MS.8-11 Therefore, GCIPL thickness measures likely reflect optic neuropathy processes recapitulating global aspects of MS pathobiology.
Recent studies have suggested that retinal thickness measures derived from older time-domain OCT (TD-OCT) may predict long-term disability in people with multiple sclerosis (PwMS), but the utility of modern SD-OCT measurements in predicting disability has not been examined beyond 5 years of follow-up.12-15 The objective of this study was to investigate whether SD-OCT assessment at a single time point may help predict disability worsening over 10 years in PwMS.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
The study was approved by the Johns Hopkins Institutional Review Board. All participants provided written informed consent.
Study Design and Participants
PwMS were recruited in a nonconsecutive manner, using convenience sampling, from the Johns Hopkins MS Center, and were prospectively studied between September 2008 and March 2020. MS diagnosis was confirmed by the treating neurologist based on the 2005 McDonald criteria, with participants classified as having relapsing-remitting MS (RRMS), secondary progressive MS (SPMS), or primary progressive MS (PPMS).16,17 Given the small number of patients with SPMS and PPMS, these groups were combined into a single progressive MS (PMS) cohort. History of optic neuritis (ON) was determined by the patient's treating neurologist. Participants underwent SD-OCT scan within 6 months of baseline Expanded Disability Status Scale (EDSS) assessment, with a repeated EDSS assessment performed at least 7 years later (median 10.4 years). A subset of participants, recruited by convenience sampling, underwent baseline and follow-up assessments of VA at 100%, 2.5%, and 1.25% contrast. Similarly, an additional subset underwent repeat SD-OCT assessment at least 7 years following their initial SD-OCT scan. In keeping with consensus standards, patients with glaucoma, uncontrolled hypertension, diabetes, or other neurologic or ophthalmologic disorders were not enrolled.18 Additional exclusion criteria included prior ocular surgery or trauma, refractive errors greater than ±6 diopters, and history of ON within 6 months of baseline OCT. Follow-up OCT and VA data from eyes that developed ON during the course of the study were excluded.
Demographic and clinical data were recorded at baseline and follow-up, including disease-modifying treatment (DMT) use throughout the duration of follow-up. In accordance with previously published studies, we classified glatiramer acetate, teriflunomide, and interferon-β as low-potency DMTs, fingolimod and dimethyl fumarate as intermediate-potency DMTs, and natalizumab, rituximab, and ocrelizumab as high-potency DMTs.19
Optical Coherence Tomography
Retinal imaging was performed using SD-OCT (Cirrus HD-OCT, Carl Zeiss Meditec, Dublin, CA), as previously described.11 Briefly, peripapillary and macular scans were obtained utilizing the Optic Disc Cube 200 × 200 protocol and Macular Cube 512 × 128 protocol, respectively. Scans with signal strength <7/10 or with artifact were excluded, in accordance with OSCAR-IB criteria.18,20 Thickness values for the pRNFL were generated by conventional Cirrus HD-OCT software, as described elsewhere.11 Segmentation of the GCIPL, inner nuclear layer (INL), and outer nuclear layer (ONL) was performed utilizing an open-source, validated segmentation algorithm, as previously described.6 Average thicknesses of the GCIPL, INL, and ONL were calculated within an annulus, centered on the fovea, with an inner diameter of 1 mm and an outer diameter of 5 mm. Qualitative review of all macular cube scans and segmentations was performed to assess for incidental pathology and to confirm the accuracy of the segmentation. OCT methods and results are reported in accordance with consensus APOSTEL recommendations.21
Additional Procedures
EDSS assessments were performed by Neurostatus-qualified raters. Raters were blinded to the patient's prior OCT measurements and EDSS scores.
Standardized visual function testing was performed using retroilluminated eye charts in a darkened room prior to OCT assessment; 100% high-contrast VA (HCVA) was assessed with Early Treatment of Diabetic Retinopathy Study charts at 4 m, and 2.5% and 1.25% low-contrast VA (LCVA) were assessed using Sloan letter charts at 2 meters. Patients used their habitual glasses/contact lenses when applicable. Results were recorded as the number of letters correctly identified.
Statistical Analyses
Demographic and clinical characteristics of study participants were compared using a cutoff for baseline GCIPL thickness of 70 µm; this cutoff was chosen as it was the mean GCIPL thickness of all eyes included at baseline. Participants were categorized into 1 of 2 groups based on whether the average GCIPL thickness of both eyes was ≥70 µm or <70 µm, excluding eyes with a prior history of ON, as disproportionate localized tissue degeneration following remote ON may potentially obscure relationships between retinal thickness and global CNS pathology.10 Among patients with a history of ON in one eye, only the fellow non-ON eye was included. Patients with prior ON in both eyes (n = 12), or with prior unilateral ON and no useable OCT data from the fellow non-ON eye (n = 3), were not included in comparisons of demographic/clinical characteristics. Comparisons between groups at baseline were performed using generalized linear models or nonparametric tests, as appropriate. With respect to DMT use during the study period, we calculated patient-years on low-potency, intermediate-potency, and high-potency DMTs for each group; comparisons between groups were performed using the χ2 test.
Comparisons of baseline SD-OCT, EDSS, and binocular VA measures were performed across groups, using the same dichotomized value for average baseline GCIPL thickness of <70 µm vs ≥70 µm (as the baseline mean), excluding eyes with prior ON. We used hierarchical linear regression models with robust variance (to account for multiple eyes per person), adjusting for age, sex, race, MS subtype, disease duration (as measured from symptom onset), and patient history of ON.
The same groups were compared in our primary longitudinal analyses, assessing change in EDSS over the duration of follow-up. We used multivariable logistic regression models with robust variance, adjusted for age, sex, race, disease duration, MS subtype, and patient history of ON, to assess the relationship between baseline GCIPL thickness (<70 µm vs ≥70 µm) and odds of clinically meaningful EDSS worsening, which we defined as an increase of ≥2.0 if baseline EDSS score was <6.0, or an increase of ≥1.0 if baseline EDSS score was ≥6.0. We did not adjust for baseline EDSS in statistical models analyzing EDSS worsening, as the definition of worsening inherently accounts for baseline EDSS. We also performed analyses to assess the odds of meaningful EDSS worsening among the following prespecified subgroups: patients with RRMS, patients with disease duration <9 years (the mean disease duration of all patients at baseline), and patients with an EDSS score ≤2 (the median score of all patients at baseline). We performed similar analyses of individual eyes, including (1) all eyes, with and without a history of ON, adjusting for ON history in each eye; (2) only eyes with a history of ON; and (3) only the eye with the lowest baseline GCIPL value for each patient.
Comparisons of change in monocular VA and retinal layer thicknesses over time were performed between groups of individual eyes with baseline GCIPL thickness <70 µm vs ≥70 µm. We used hierarchical linear regression models with robust variance, accounting for intereye correlation, adjusted for age, sex, race, MS subtype, disease duration, ON history, and baseline VA/retinal layer thickness. Similar to models analyzing EDSS change, multivariable logistic regression models were used to assess the odds of VA worsening, defined as a decrease in monocular letter acuity of ≥5 letters for 100% contrast, and of ≥7 letters for monocular 2.5% and 1.25% contrast.22,23 We also considered retinal thicknesses as continuous variables in linear regression models assessing their association with disability outcomes.
Analyses were based on a priori established research hypotheses, and consequently, adjustment for multiple comparisons was not performed. Statistical analyses were performed using Stata version 15 (StataCorp, College Station, TX). Statistical significance was defined as p < 0.05. The figure was made using GraphPad Prism version 8.4.2 (GraphPad Software, La Jolla, CA; graphpad.com).
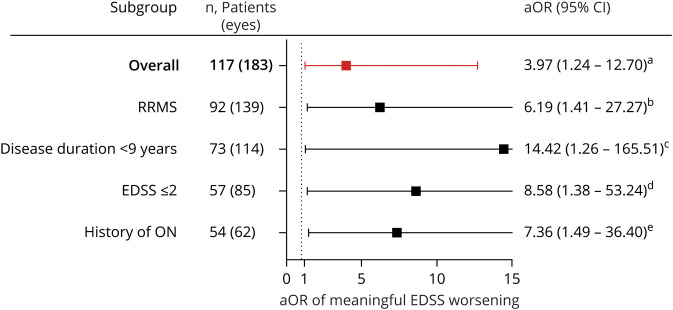
Figure. Adjusted Odds Ratios (aORs) of Meaningful Expanded Disability Status Scale (EDSS) Worsening.
aORs of clinically meaningful EDSS worsening over 10 years of follow-up for patients with an average baseline ganglion cell + inner plexiform layer (GCIPL) thickness <70 µm, as compared to patients with an average baseline GCIPL thickness ≥70 µm, excluding eyes with a history of optic neuritis (ON) (comparisons of ON eyes only were performed using individual eye GCIPL thicknesses). aLogistic regression model with robust variance, adjusted for age at baseline optical coherence tomography (OCT), sex, race (Black or non–Black), multiple sclerosis (MS) subtype, disease duration, and patient history of ON. bLogistic regression model with robust variance, adjusted for age at baseline OCT, sex, race (Black or non–Black), disease duration, and patient history of ONs, restricted to patients with a diagnosis of relapsing-remitting multiple sclerosis (RRMS) at baseline. cLogistic regression model with robust variance, adjusted for age at baseline OCT, sex, race (Black or non–Black), MS subtype, and patient history of ON, restricted to patients with disease duration <9 years at baseline (the mean among all patients). dLogistic regression model with robust variance, adjusted for age at baseline OCT, sex, race (Black or non–Black), MS subtype, disease duration, and ON history, restricted to patients with an EDSS score ≤2.0 at baseline (the median among all patients). eLogistic regression model with robust variance, accounting for intereye correlation, adjusted for age at baseline OCT, sex, race (Black or non–Black), MS subtype, and disease duration, restricted to eyes with a history of ON. Figure made using GraphPad Prism version 8.4.2 (GraphPad Software, La Jolla, CA; graphpad.com). CI = confidence interval.
Data Availability
Anonymized data used for this study are available from the corresponding author upon reasonable request with the proper data sharing agreements in place.
Results
Characteristics of the Study Population
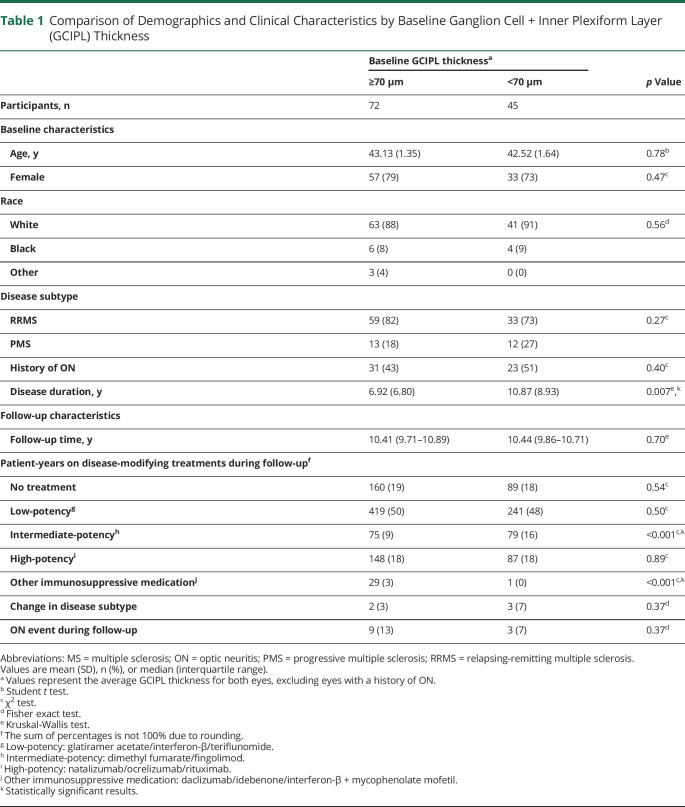
The study population included 132 PwMS with a median follow-up duration of 10.4 ± 0.9 years. Demographic and clinical characteristics are presented in table 1. At baseline, there were no significant differences in age, sex, race, MS subtype, or patient history of ON in PwMS with an average baseline GCIPL thickness <70 µm as compared to ≥70 µm. However, patients with an average GCIPL thickness <70 µm were more likely to have a longer disease duration than those with an average thickness ≥70 µm (10.9 ± 8.9 vs 6.9 ± 6.8 years, respectively; p = 0.007). Follow-up time was similar between the groups. With respect to DMTs, among patients with an average GCIPL thickness <70 µm, we observed a greater proportion of follow-up on intermediate-potency DMTs relative to patients with an average thickness ≥70 µm (16% vs 9%; p < 0.001), but no differences between groups in proportion of patient-years on low-potency (48% vs 50%; p = 0.50) or high-potency DMTs (18% for both groups; p = 0.89).
Table 1.
Comparison of Demographics and Clinical Characteristics by Baseline Ganglion Cell + Inner Plexiform Layer (GCIPL) Thickness
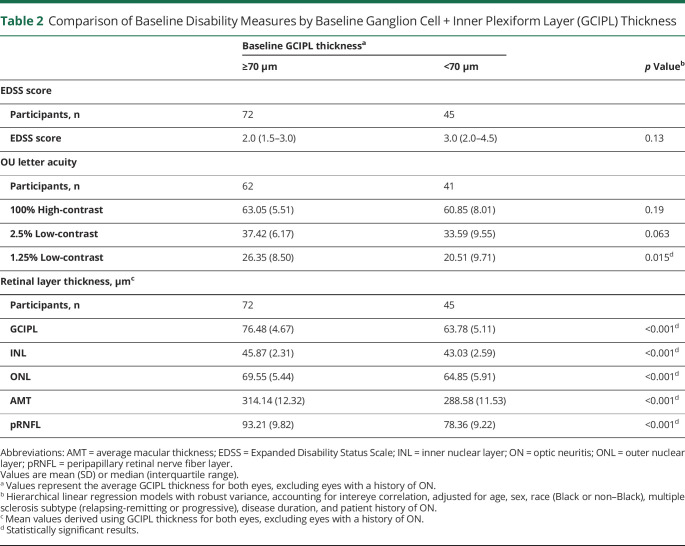
Baseline disability measures, excluding eyes with prior ON, are presented in table 2. Baseline comparisons included OCT and EDSS data from 117 patients (177 eyes) and VA data from 103 patients (151 eyes). Patients with an average baseline GCIPL thickness <70 µm had lower baseline thicknesses of all other retinal layers (pRNFL, INL, ONL, average macular thickness [AMT]; p < 0.001 for all), and lower binocular VA at 1.25% contrast (20.5 ± 9.7 vs 26.4 ± 8.5 letters; p = 0.015) relative to the those with a GCIPL thickness ≥70 µm. Median baseline EDSS scores did not significantly differ between groups dichotomized according to the mean 70 µm GCIPL thickness cutoff (2.0 vs 3.0, respectively; p = 0.13).
Table 2.
Comparison of Baseline Disability Measures by Baseline Ganglion Cell + Inner Plexiform Layer (GCIPL) Thickness
Association Between Baseline SD-OCT Measures and 10-Year EDSS
In our primary analyses, using an average GCIPL thickness of both eyes, excluding eyes with prior ON, a greater proportion of patients with an average baseline GCIPL thickness <70 µm (the mean thickness at baseline) experienced EDSS worsening over the duration of follow-up (14 of 45 patients [31%]), as compared to patients with an average thickness ≥70 µm (7 of 72 patients [10%]; p = 0.006). In multivariable analyses adjusted for age, sex, race, MS subtype, disease duration, and patient history of ON, patients with an average GCIPL thickness <70 µm were at an almost 4-fold increased odds of EDSS worsening over time, relative to individuals with an average thickness ≥70 µm (n = 117 patients; adjusted odds ratio [aOR] 3.97, 95% confidence interval [CI] 1.24–12.70; p = 0.02; figure). The odds of meaningful EDSS worsening were even higher when these analyses were restricted to only RRMS (n = 92 patients; aOR 6.19, 95% CI 1.41–27.27; p = 0.016), patients with a disease duration <9 years at baseline (n = 73 patients; aOR 14.42, 95% CI 1.26–165.51; p = 0.032), and patients with a baseline EDSS score ≤2 (n = 57 patients; aOR 8.58, 95% CI 1.38–53.24; p = 0.021). The highest odds of meaningful EDSS worsening for patients with an average baseline GCIPL thickness <70 µm vs ≥70 µm were observed in further analyses restricted to the subgroup of patients with both RRMS and disease duration <9 years at baseline (aOR 15.10, 95% CI 1.60–142.83; p = 0.018).
In secondary analyses of raw EDSS scores, adjusted in addition for baseline EDSS and follow-up duration, we observed that patients with an average GCIPL thickness <70 µm at baseline trended towards having higher EDSS scores at study end, as compared to those with thickness ≥70 µm; however, this difference did not reach statistical significance (β 0.54, 95% CI −0.01 to 1.10; p = 0.054). A statistically significant difference was observed in multivariable analyses of annualized change in raw EDSS scores: patients with average baseline GCIPL thickness <70 µm demonstrated greater annualized increase in raw EDSS scores during follow-up, relative to patients with baseline GCIPL thickness ≥70 µm (β 0.06; 95% CI 0.002–0.11; p = 0.043). We also assessed the association of nondichotomized (i.e., continuous) baseline OCT measures with EDSS and did not find that lower average baseline retinal layer thicknesses were significantly associated with EDSS worsening (data not shown).
We performed additional analyses of individual eyes, adjusting for age, sex, race, MS subtype, disease duration, and ON history in each eye. In analyses including all eyes (rather than average measures from both eyes), a baseline GCIPL thickness <70 µm was similarly associated with a 4.2-fold increased odds of meaningful EDSS worsening, relative to eyes with a baseline thickness ≥70 µm (n = 239 eyes; aOR 4.19, 95% CI 1.50–11.72; p = 0.006). The odds of meaningful EDSS worsening were higher when these analyses were restricted to eyes with a previous history of ON (n = 62 eyes; aOR 7.36, 95% CI 1.49–36.40; p = 0.014). In analyses restricted to only the lowest GCIPL thickness measure from either eye (rather than the average of both), patients in whom the GCIPL thickness was ≤70 µm in the lowest eye demonstrated a similar over 4-fold increased odds of EDSS worsening, relative to patients with a GCIPL thickness ≥70 µm in the lowest eye (n = 132 eyes; aOR 4.10, 95% CI 1.21–13.91; p = 0.023). Finally, in an effort to better understand how to clinically use baseline GCIPL thickness for predicting clinical course in PwMS, we further categorized PwMS into 1 of 3 groups: patients with a baseline GCIPL thickness <70 µm in both eyes (n = 41 patients); patients with a baseline GCIPL thickness <70 µm in one eye and ≥70 µm in the fellow eye (n = 21 patients); and patients with a baseline GCIPL thickness ≥70 µm in both eyes (n = 45 patients). Relative to patients with baseline GCIPL thickness ≥70 µm in both eyes, those with thickness <70 µm in both eyes were at an over 5-fold increased odds of EDSS worsening (aOR 5.03, 95% CI 1.27–19.83; p = 0.021), while no difference was observed relative to the group with only one eye with thickness <70 µm (aOR 1.45, 95% CI 0.18–11.34; p = 0.73). Therefore, quantifying the number of eyes with a baseline GCIPL thickness <70 µm enabled stratification of PwMS into discrete groups to assist in predicting risk of disability progression at the patient level.
Association Between Baseline SD-OCT and Other Disability Outcomes at 10 Years
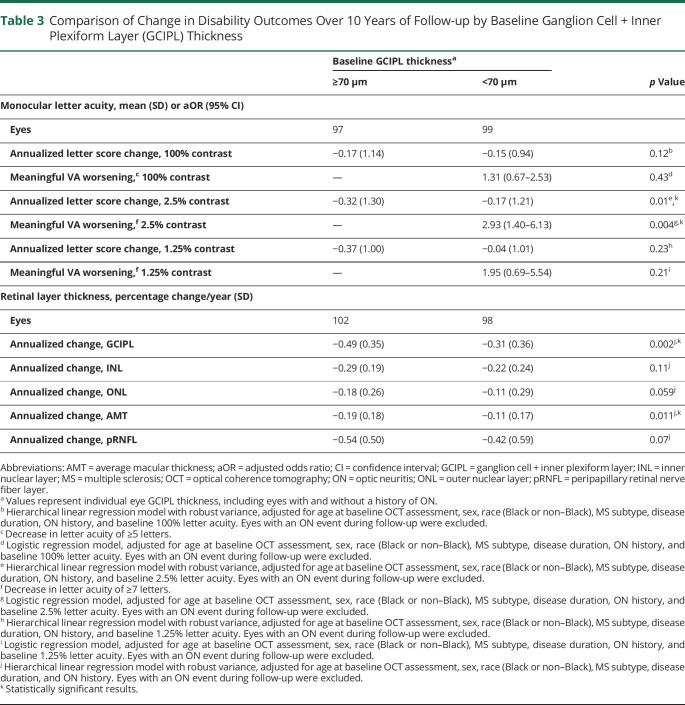
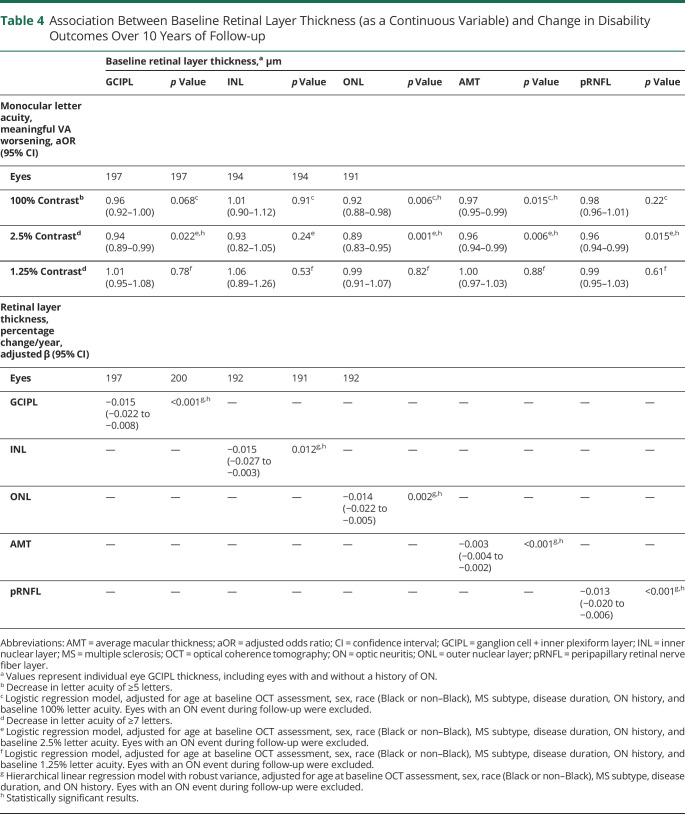
In multivariable logistic regression analyses adjusted for age, sex, race, MS subtype, disease duration, ON history, and baseline 2.5% VA, individual eye GCIPL thickness <70 µm at baseline was associated with an almost 3-fold increased odds of 2.5% VA worsening relative to eyes with thickness ≥70 µm (aOR 2.93, 95% CI 1.40–6.13; p = 0.004; table 3). In multivariable analyses with retinal thicknesses included as continuous variables, each 1 µm higher baseline retinal layer thickness was associated with reduced odds of 2.5% VA worsening for the GCIPL (aOR 0.94, 95% CI 0.89–0.99; p = 0.022), ONL (aOR 0.89, 95% CI 0.83–0.95; p = 0.001), AMT (aOR 0.96, 95% CI 0.94–0.99; p = 0.006), and pRNFL (aOR 0.96, 95% CI 0.94–0.99; p = 0.015; table 4). A similar relationship was observed between 100% VA worsening and each 1 µm higher ONL (aOR 0.92, 95% CI 0.88–0.98; p = 0.006), and AMT thickness (aOR 0.97, 95% CI 0.95–0.99; p = 0.015). Retinal layer thickness was not associated with 1.25% VA worsening.
Table 3.
Comparison of Change in Disability Outcomes Over 10 Years of Follow-up by Baseline Ganglion Cell + Inner Plexiform Layer (GCIPL) Thickness
Table 4.
Association Between Baseline Retinal Layer Thickness (as a Continuous Variable) and Change in Disability Outcomes Over 10 Years of Follow-up
In multivariable analyses of eyes with baseline and follow-up SD-OCT data, each 1 µm lower baseline thickness was associated with lower annualized percentage change for all retinal layers analyzed (GCIPL: β = −0.015, 95% CI −0.022 to −0.008; p < 0.001; INL: β = −0.015, 95% CI −0.027 to −0.003; p = 0.012; ONL: β = −0.014, 95% CI −0.022 to −0.005; p = 0.002; AMT: β = −0.003, 95% CI −0.004 to −0.002; p < 0.001; pRNFL: β = −0.013, 95% CI −0.020 to −0.006; p < 0.001; table 4).
Classification of Evidence
The primary research question was to evaluate whether SD-OCT assessment at a single time point may help predict disability worsening over 10 years in PwMS. This study provides Class I evidence that lower baseline GCIPL thickness is independently associated with long-term disability worsening among PwMS, as an average baseline GCIPL thickness <70 µm was associated with a 4-fold increased odds of clinically meaningful EDSS worsening, relative to patients with an average baseline GCIPL thickness ≥70 µm, excluding eyes with prior ON (aOR 3.97, 95% CI 1.24–12.70; p = 0.02).
Discussion
Our study demonstrates that baseline retinal GCIPL thickness, as measured by SD-OCT, is independently associated with EDSS worsening over a median of 10 years later in PwMS. The relationship between baseline GCIPL thickness and long-term EDSS worsening was strongest among patients with a shorter disease duration and those with little cumulative disability at baseline. Our findings provide strong support for the utility of OCT measures, and in particular GCIPL thickness, as clinically useful biomarkers that provide valuable insight regarding global aspects of the MS disease process, which help to predict long-term clinical course in PwMS, and in turn, identify patients who may be at risk of developing significant disability. MS is clinically heterogeneous, and predicting individual disease course in PwMS has remained an ongoing challenge, particularly during the early disease stage, making appropriate DMT selection from an increasing armamentarium of treatments (some with potentially life-threatening complications) challenging.24 Current imaging, clinical, demographic, and serologic prognostic biomarkers in MS are limited, and moreover only have modest predictive values. Our study therefore helps to address gaps in MS risk stratification, thereby underpinning the importance and relevance of our study findings. Whereas the significance of our cohort-level findings may be difficult to interpret in isolation at an individual patient level, baseline GCIPL thickness, in conjunction with established MS biomarkers such as MRI, and relatively new biomarkers such as serum neurofilament light chain may play a role in facilitating more individualized therapeutic decision-making in PwMS. As a rapid, noninvasive, and inexpensive office-based imaging tool, SD-OCT can potentially be readily incorporated into routine practice in the MS clinic.
Our findings corroborate and expand upon previous studies that have reported relationships between baseline OCT measurements and future disability in MS. Rothman et al12 reported an approximately 3.5-fold increased odds of EDSS worsening over 10 years among PwMS in the lowest tertile of baseline total macular volume (of which the GCIPL constitutes a substantial proportion). This study used TD-OCT, which is older and less reliable than modern SD-OCT.11 Martinez-Lapiscina et al,14 using SD-OCT, observed an almost 4-fold increased risk of MS disability progression over 3–5 years of follow-up using a cutoff of 87–88 µm (depending on the OCT device used) for baseline pRNFL thickness. However, neither of these studies evaluated GCIPL thickness, which can be readily quantified using segmentation techniques applied to SD-OCT measures.6 As discussed previously, GCIPL thickness demonstrates superior reliability, reproducibility, and structure–function relationships than pRNFL thickness in MS, potentially due to lack of confounding from astrogliosis (which mainly occurs in the RNFL) and swelling (RNFL, but not GCIPL, swelling occurs during optic nerve inflammation).7-9,25,26 Intervisit intraclass correlation coefficients for GCIPL thickness have been shown to be as high as 0.99.11 However, few studies have evaluated GCIPL thickness as a biomarker for predicting MS disability worsening in the medium to long term. Knier et al15 reported a 2.4-fold increased risk for disability worsening over 5 years among a limited cohort of PwMS with a median disease duration of <36 months and low baseline GCIPL volumes (≤2.00 mm3) relative to patients with higher GCIPL volumes. A key finding of our study was that baseline GCIPL thickness of <70 µm was associated with a 4-fold increased odds of EDSS worsening at a median of 10 years later among the entire cohort of PwMS (with a median disease duration of 6 years at baseline).
It is recognized that neuroaxonal degeneration is the primary pathophysiologic process underpinning disability progression in MS. Whereas ON occurs in approximately 50% of PwMS at some point during their disease course, up to 99% of patients demonstrate demyelinating plaques within their optic nerves postmortem.4,27 Occult optic neuropathy in MS, as demonstrated in vivo by retinal thinning on OCT in the absence of overt ON, may provide insight regarding neurodegeneration occurring elsewhere in the CNS, potentially related to smoldering inflammation. Our findings could potentially support the concept of a threshold of neuroaxonal injury, after which further CNS damage translates to sustained disability progression. Interestingly, and in keeping with previous observations for both pRNFL and GCIPL thickness, in analyses restricted to eyes with a prior history of ON, a GCIPL thickness <70 µm was associated with an over 7-fold increased odds of EDSS worsening, relative to eyes with a GCIPL thickness ≥70 µm.14,28,29 This may suggest a critical threshold following localized axonal injury, below which there is an increased propensity towards more widespread neurodegeneration. Alternatively, a linear relationship may exist between GCIPL thickness and risk of EDSS worsening; however, our analyses were likely underpowered to detect such a relationship across the entire cohort included in this study.
Several studies have demonstrated correlation of OCT measures, in particular GCIPL and pRNFL, with VA. Our observation that GCIPL, ONL, AMT, and pRNFL thicknesses were associated with 2.5% VA 10 years later is therefore not unexpected; however, its clinical relevance should not be underestimated, as reductions in LCVA, while not always noticeable to patients, correlate with vision-specific quality of life assessments, even after accounting for HCVA.30 Whereas no significant associations were observed between OCT measures and 1.25% VA worsening, this was potentially due to a floor effect, as baseline letter acuity of <7 letters cannot subsequently meet the definition of meaningful worsening.31 We observed faster atrophy rates for all retinal layers analyzed for each 1 µm higher baseline thickness. These findings are in accordance with prior studies that demonstrated that baseline retinal thicknesses are among the strongest predictors of rates of retinal atrophy, with faster atrophy rates observed where there is a larger reservoir of tissue to be lost.10
Our study has several limitations. For our primary analyses of the association of baseline SD-OCT with disability outcomes, we chose to focus predominantly on the average GCIPL thickness between eyes, excluding eyes with a history of ON. This approach is in keeping with previous similar studies, and was also chosen because EDSS is a patient-level outcome.12,14 However, this approach does not account for eyes with prior ON, or for patients with a history of ON in both eyes. In order to address this, we performed several additional analyses. First, we repeated our logistic regression analyses, including all individual eyes rather than averages between eyes, adjusting for ON history in each eye. Separate analyses included only individual eyes with a history of ON. In each of these analyses, GCIPL thickness <70 µm was associated with increased odds of EDSS worsening, relative to eyes with thickness ≥70 µm. Although this approach does not account for patients in whom GCIPL thickness is <70 µm in one eye and ≥70 µm in the fellow eye, these findings nevertheless support the utility of GCIPL thickness for stratifying risk of disability worsening in MS, irrespective of ON history. Additional analyses, including only the eye with lowest baseline GCIPL thickness for each patient, again showed an approximately 4-fold increased odds of EDSS worsening among patients with a GCIPL thickness <70 µm in the lowest eye, vs ≥70 µm. Finally, we categorized patients into 1 of 3 groups based on the number of eyes with baseline GCIPL thickness <70 µm, as described previously, in turn identifying PwMS with a bilateral GCIPL thickness <70 µm as potentially being at higher risk of worse disability outcomes.
EDSS, our primary outcome measure, remains the most commonly used MS-related disability measure. Despite this, EDSS has well-described weaknesses including interrater variability and low sensitivity to change, particularly at higher scores, where scoring depends predominantly on ambulatory disability.32 EDSS assessments at follow-up were completed at a single time point, rather than repeated at 3–6 months to confirm disability progression. Therefore, in accordance with a similar study performed by our group, we selected a more stringent definition of meaningful EDSS worsening than that which is conventionally used in clinical trials, and did not include EDSS assessments performed within 3 months of a clinical relapse, in order to mitigate concerns that EDSS change may be due in part to relapse activity or inherent variability of the outcome measure.12 In addition, our models did not adjust for DMT use; this was primarily due to difficulties in appropriately accounting for variation among individuals in the length of time on various DMTs, as the majority of patients switched between categories of DMT potency during the follow-up period. However, separate analyses comparing groups of baseline GCIPL thickness demonstrated only minor differences in patient-years on different categories of DMT potency, and no differences were observed between groups with respect to patient-years on high-potency DMTs. Finally, with regard our study population, although our cohort of PwMS was heterogeneous with varying disease duration and disease subtypes, our cohort was small relative to previous similar studies, and as such, our findings may not be generalizable to a larger MS population.12,14 Our open cohort design may introduce a selection bias, as patients may have been lost to follow-up secondary to very high levels of physical disability, or other factors that may affect disability outcomes, such as race, socioeconomic status, and the presence of comorbidities.33-35
Our study provides robust evidence that SD-OCT assessment performed at a single time point is independently associated with long-term disability worsening in PwMS. Lower baseline GCIPL thickness is associated with increased odds of meaningful EDSS worsening and LCVA worsening over a median of 10 years later in PwMS. Our findings strongly support the utility of OCT measures as powerful surrogates of neurodegeneration, helping to predict clinically meaningful long-term outcomes in PwMS that may influence treatment decisions in the clinical setting.
Glossary
- aOR
adjusted odds ratio
- CI
confidence interval
- DMT
disease-modifying treatment
- EDSS
Expanded Disability Status Scale
- GCIPL
ganglion cell + inner plexiform layer
- HCVA
high-contrast visual acuity
- INL
inner nuclear layer
- LCVA
low-contrast visual acuity
- MS
multiple sclerosis
- OCT
optical coherence tomography
- ON
optic neuritis
- ONL
outer nuclear layer
- PMS
progressive multiple sclerosis
- PPMS
primary progressive multiple sclerosis
- pRNFL
peripapillary retinal nerve fiber layer
- PwMS
people with multiple sclerosis
- RRMS
relapsing-remitting multiple sclerosis
- SD-OCT
spectral-domain optical coherence tomography
- SPMS
secondary progressive multiple sclerosis
- TD-OCT
time-domain optical coherence tomography
- VA
visual acuity
Appendix. Authors
Footnotes
Editorial, page 731
Class of Evidence: NPub.org/coe
Study Funding
This study was funded by MIH (5R01NS082347-02 [to P.A.C.]), National Multiple Sclerosis Society (TR 3760-A-3 [to P.A.C.] and RG-1606-08,768 [to S.S.]) and Race to Erase MS (to S.S.).
Disclosure
Dr. Lambe, Dr. Fitzgerald, Dr. Murphy, Dr. Filippatou, Dr. Kalaitzidis, Dr. Vasileiou, N. Pellegrini, E. Ogbuokiri, B. Toliver, N.J. Luciano, S. Davis, N. Fioravante, O. Kwakyi, H. Risher, and Dr. Crainiceanu report no disclosures. Dr. Sotirchos has served received speaker honoraria from Viela Bio and has served on scientific advisory boards for Viela Bio and Genentech. Dr. Prince is a founder of Sonovex, Inc. and serves on its Board of Directors, has received consulting fees from JuneBrain LLC, and is PI on a research grant to Johns Hopkins from Biogen. Dr. Newsome has received consultant fees for scientific advisory boards from Biogen, Genentech, Celgene, EMD Serono, Novartis, a clinical adjudication committee member for a MedDay Pharmaceuticals clinical trial, and has received research funding (paid directly to institution) from Biogen, Novartis, Genentech, National MS Society, Department of Defense, and Patient Centered Outcomes Institute. Dr. Mowry has grants from Biogen and Genzyme, is site PI for studies sponsored by Biogen, has received free medication for a clinical trial from Teva, and receives royalties for editorial duties from UpToDate. Dr. Saidha has received consulting fees from Medical Logix for the development of CME programs in neurology and has served on scientific advisory boards for Biogen, Genzyme, Genentech Corporation, EMD Serono, and Celgene; is the PI of investigator-initiated studies funded by Genentech Corporation and Biogen Idec; received support from the Race to Erase MS Foundation; has received equity compensation for consulting from JuneBrain LLC, a retinal imaging device developer; and is the site investigator of a trial sponsored by MedDay Pharmaceuticals. Dr. Calabresi has received consulting fees from Disarm and Biogen and is PI on grants to JHU from Biogen and Annexon. Go to Neurology.org/Nhttps://n.neurology.org/lookup/doi/10.1212/WNL.0000000000011788 for full disclosures.
References
- 1.Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–1517. [DOI] [PubMed] [Google Scholar]
- 2.Fisniku LK, Chard DT, Jackson JS, et al. . Gray matter atrophy is related to long-term disability in multiple sclerosis: GM atrophy and disability in MS. Ann Neurol. 2008;64(3):247–254. [DOI] [PubMed] [Google Scholar]
- 3.Reich DS, Lucchinetti CF, Calabresi PA. Multiple sclerosis. N Engl J Med. 2018;378(2):169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ikuta F, Zimmerman HM. Distribution of plaques in seventy autopsy cases of multiple sclerosis in the United States. Neurology. 1976;26(6):26–28. [DOI] [PubMed] [Google Scholar]
- 5.Chen TC, Cense B, Pierce MC, et al. . Spectral domain optical coherence tomography: ultra-high speed, ultra-high resolution ophthalmic imaging. Arch Ophthalmol. 2005;123(12):1715–1720. [DOI] [PubMed] [Google Scholar]
- 6.Lang A, Carass A, Hauser M, et al. . Retinal layer segmentation of macular OCT images using boundary classification. Biomed Opt Express. 2013;4(7):1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Syc SB, Saidha S, Newsome SD, et al. . Optical coherence tomography segmentation reveals ganglion cell layer pathology after optic neuritis. Brain. 2012;135(2):521–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saidha S, Syc SB, Durbin MK, et al. . Visual dysfunction in multiple sclerosis correlates better with optical coherence tomography derived estimates of macular ganglion cell layer thickness than peripapillary retinal nerve fiber layer thickness. Mult Scler. 2011;17(12):1449–1463. [DOI] [PubMed] [Google Scholar]
- 9.Walter SD, Ishikawa H, Galetta KM, et al. . Ganglion cell loss in relation to visual disability in multiple sclerosis. Ophthalmology. 2012;119(6):1250–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saidha S, Al-Louzi O, Ratchford JN, et al. . Optical coherence tomography reflects brain atrophy in multiple sclerosis: a four-year study. Ann Neurol. 2015;78(5):801–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Syc SB, Warner CV, Hiremath GS, et al. . Reproducibility of high-resolution optical coherence tomography in multiple sclerosis. Mult Scler. 2010;16(7):829–239. [DOI] [PubMed] [Google Scholar]
- 12.Rothman A, Murphy OC, Fitzgerald KC, et al. . Retinal measurements predict 10-year disability in multiple sclerosis. Ann Clin Transl Neurol. 2019;6(2):222–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bsteh G, Hegen H, Teuchner B, et al. . Peripapillary retinal nerve fibre layer as measured by optical coherence tomography is a prognostic biomarker not only for physical but also for cognitive disability progression in multiple sclerosis. Mult Scler. 2019;25(2):196–203. [DOI] [PubMed] [Google Scholar]
- 14.Martinez-Lapiscina EH, Arnow S, Wilson JA, et al. . Retinal thickness measured with optical coherence tomography and risk of disability worsening in multiple sclerosis: a cohort study. Lancet Neurol. 2016;15(6):574–584. [DOI] [PubMed] [Google Scholar]
- 15.Knier B, Leppenetier G, Wetzlmair C, et al. . Association of retinal architecture, intrathecal immunity, and clinical course in multiple sclerosis. JAMA Neurol. 2017;74(7):847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polman CH, Reingold SC, Edan G, et al. . Diagnostic criteria for multiple sclerosis: 2005 revisions to the McDonald Criteria. Ann Neurol. 2005;58(6):840–846. [DOI] [PubMed] [Google Scholar]
- 17.Lublin FD, Reingold SC, Cohen JA, et al. . Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83(3):278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tewarie P, Balk L, Costello F, et al. . The OSCAR-IB consensus criteria for retinal OCT quality assessment. PLoS One. 2012;7(4):e34823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Filippatou AG, Lambe J, Sotirchos ES, et al. . Association of body mass index with longitudinal rates of retinal atrophy in multiple sclerosis. Mult Scler. 2020;26(7):843–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schippling S, Balk L, Costello F, et al. . Quality control for retinal OCT in multiple sclerosis: validation of the OSCAR-IB criteria. Mult Scler. 2015;21(2):163–170. [DOI] [PubMed] [Google Scholar]
- 21.Cruz-Herranz A, Balk LJ, Oberwahrenbrock T, et al. . The APOSTEL recommendations for reporting quantitative optical coherence tomography studies. Neurology. 2016;86(24):2303–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Talman LS, Bisker ER, Sackel DJ, et al. . Longitudinal study of vision and retinal nerve fiber layer thickness in MS. Ann Neurol. 2010;67(6):749–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balcer LJ, Baier ML, Pelak VS, et al. . New low-contrast vision charts: reliability and test characteristics in patients with multiple sclerosis. Mult Scler. 2000;6(3):163–171. [DOI] [PubMed] [Google Scholar]
- 24.Hart FM, Bainbridge J. Current and emerging treatment of multiple sclerosis. Am J Manag Care. 2016;22(6):s159–170. [PubMed] [Google Scholar]
- 25.Green AJ, McQuaid S, Hauser SL, et al. . Ocular pathology in multiple sclerosis: retinal atrophy and inflammation irrespective of disease duration. Brain. 2010;133(6):1591–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagelhus EA, Veruki ML, Torp R, et al. . Aquaporin-4 water channel protein in the rat retina and optic nerve: polarized expression in Müller cells and fibrous astrocytes. J Neurosci. 1998;18(7):2506–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arnold AC. Evolving management of optic neuritis and multiple sclerosis. Am J Ophthalmol. 2005;139(6):1101–1108. [DOI] [PubMed] [Google Scholar]
- 28.Albrecht P, Fröhlich R, Hartung H-P, et al. . Optical coherence tomography measures axonal loss in multiple sclerosis independently of optic neuritis. J Neurol. 2007;254(11):1595–1596. [DOI] [PubMed] [Google Scholar]
- 29.González-López JJ, Rebolleda G, Leal M, et al. . Comparative diagnostic accuracy of ganglion cell-inner plexiform and retinal nerve fiber layer thickness measures by cirrus and Spectralis optical coherence tomography in relapsing-remitting multiple sclerosis. Biomed Res Int. 2014;2014:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mowry EM, Loguidice MJ, Daniels AB, et al. . Vision related quality of life in multiple sclerosis: correlation with new measures of low and high contrast letter acuity. J Neurol Neurosurg Psychiatry. 2009;80(7):767–772. [DOI] [PubMed] [Google Scholar]
- 31.Balcer LJ, Miller DH, Reingold SC, Cohen JA. Vision and vision-related outcome measures in multiple sclerosis. Brain. 2015;138(1):11–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meyer-Moock S, Feng Y-S, Maeurer M, et al. . Systematic literature review and validity evaluation of the expanded disability status scale (EDSS) and the multiple sclerosis functional composite (MSFC) in patients with multiple sclerosis. BMC Neurol. 2014;14(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caldito NG, Saidha S, Sotirchos ES, et al. . Brain and retinal atrophy in African-Americans versus Caucasian-Americans with multiple sclerosis: a longitudinal study. Brain. 2018;141(11):3115–3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harding KE, Wardle M, Carruthers R, et al. . Socioeconomic status and disability progression in multiple sclerosis: a multinational study. Neurology. 2019;92(13):e1497–506. [DOI] [PubMed] [Google Scholar]
- 35.Marrie RA. Comorbidity in multiple sclerosis: implications for patient care. Nat Rev Neurol. 2017;13(6):375–382. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data used for this study are available from the corresponding author upon reasonable request with the proper data sharing agreements in place.